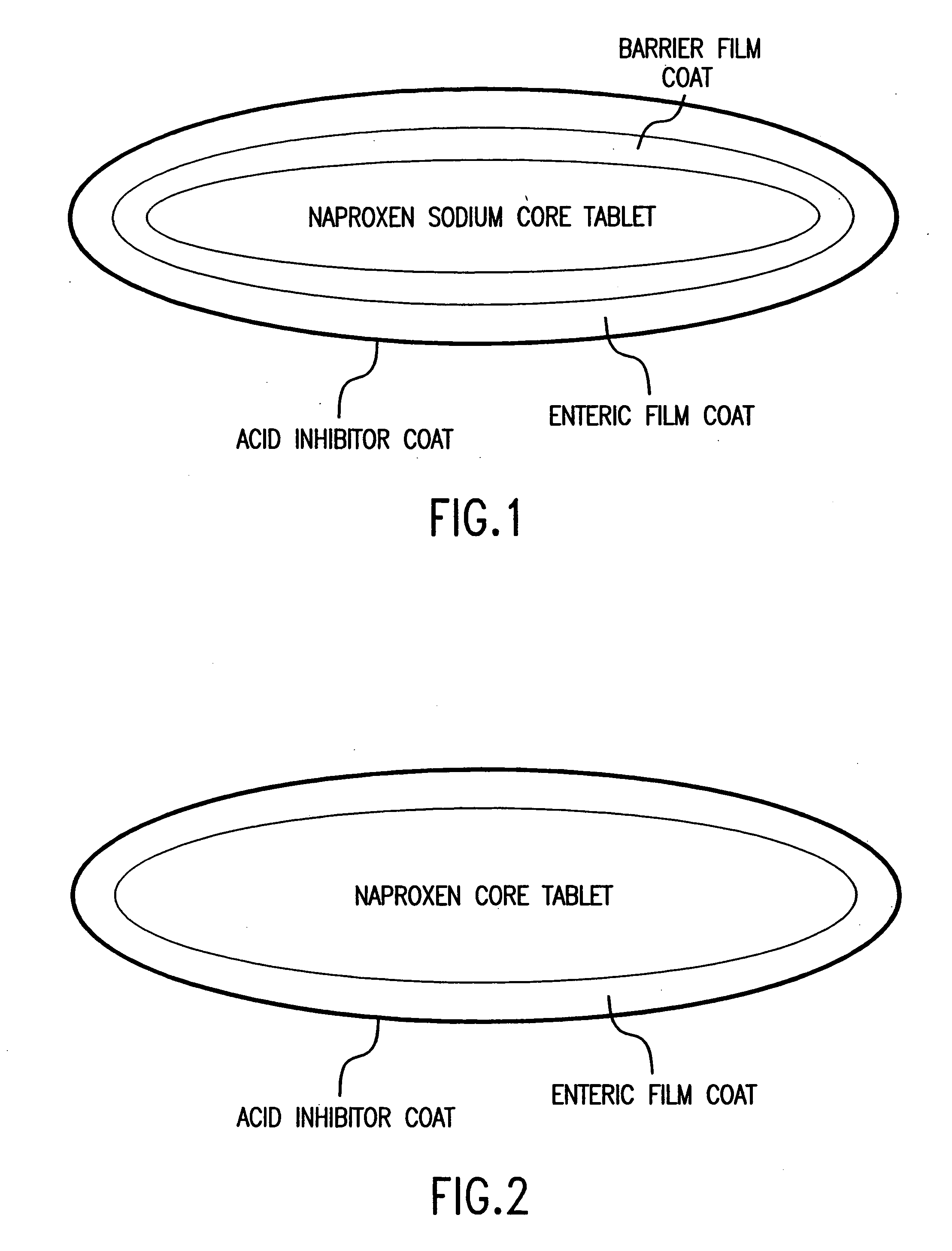
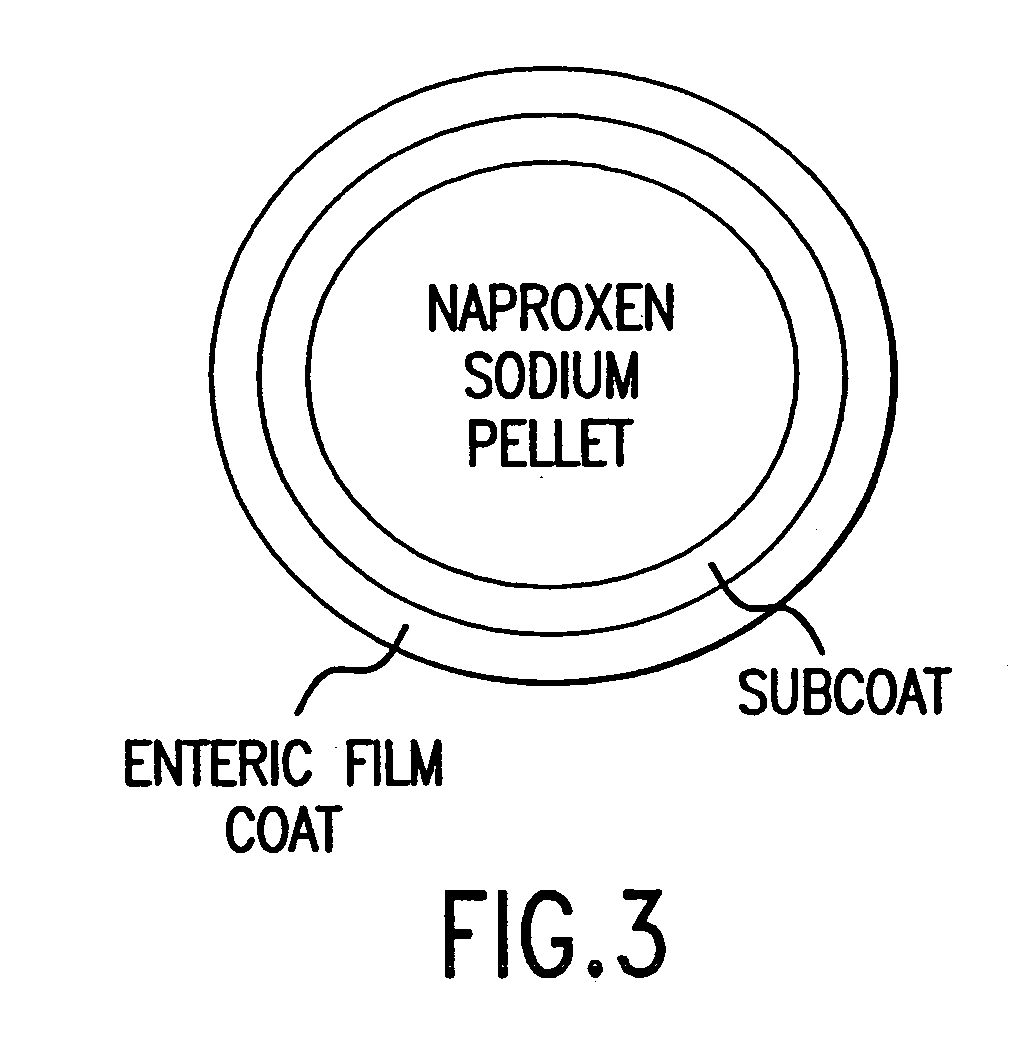
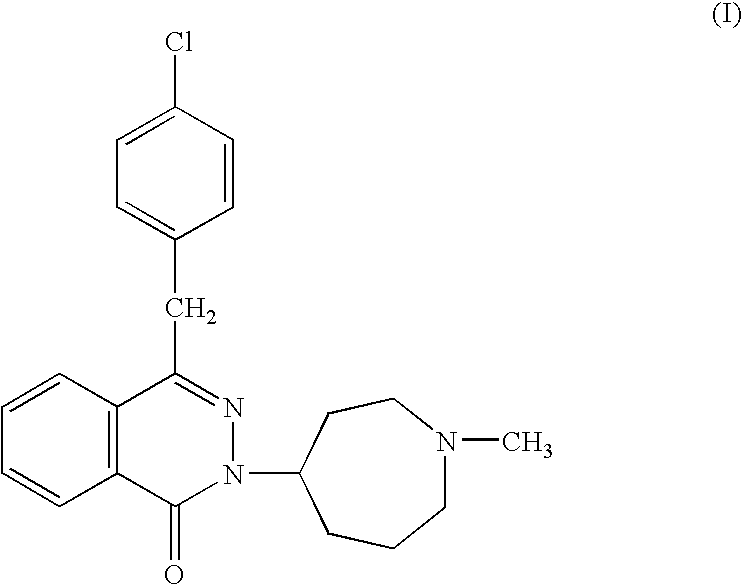
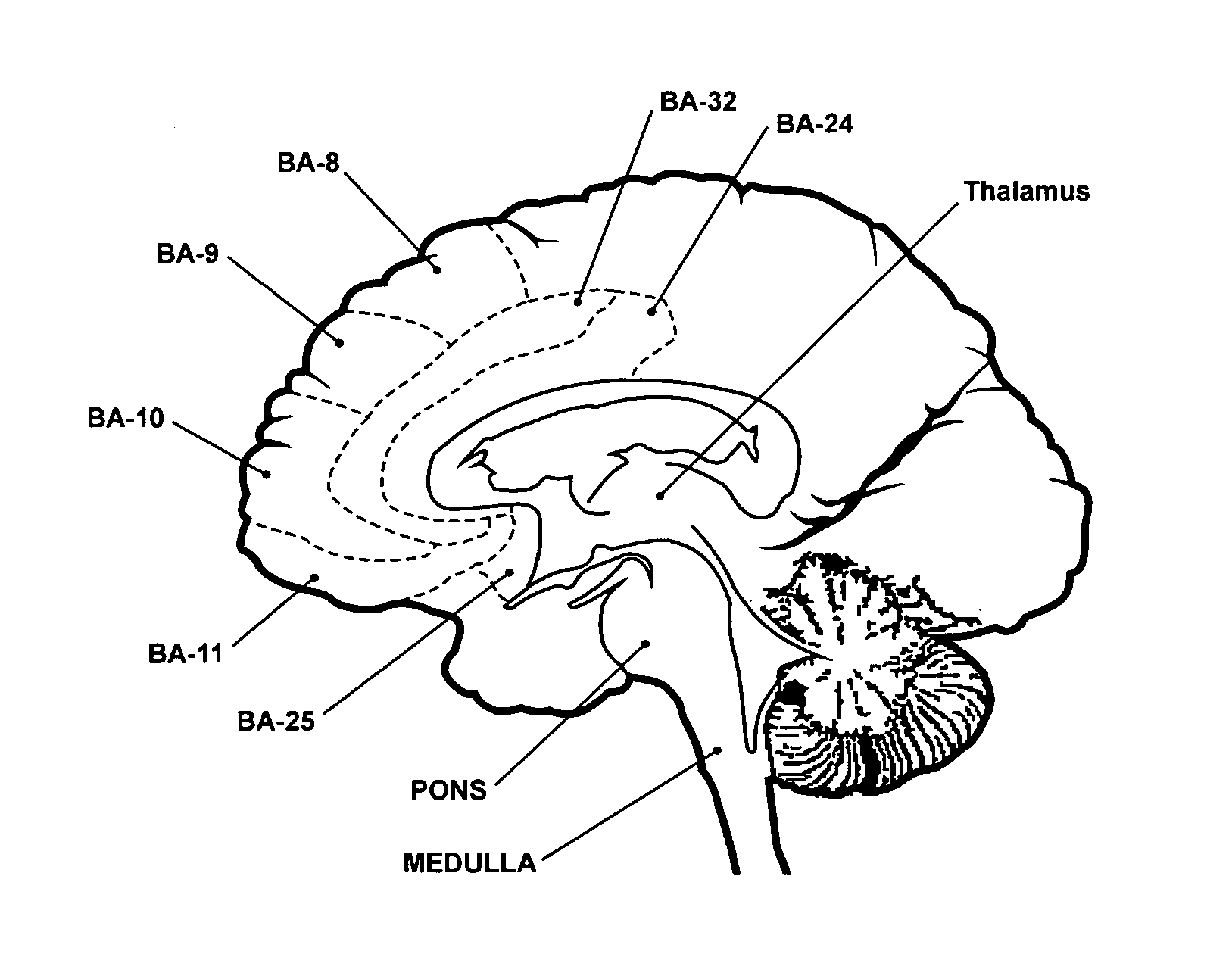
Patents
Literature
78 results about "Symptomatic relief" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
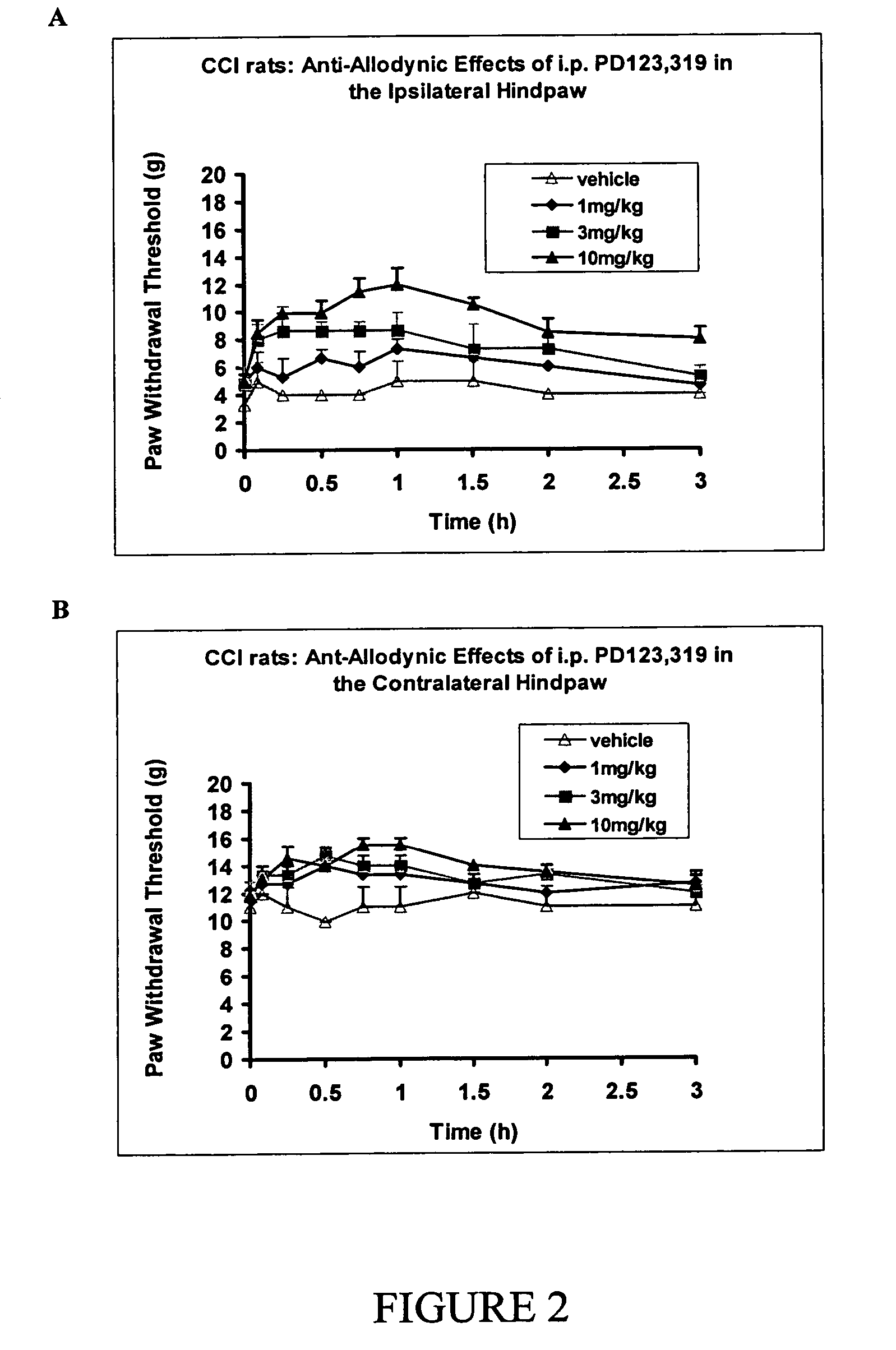
Symptomatic relief medications are drugs used to relieve symptoms associated with headaches, including the pain of a headache or the nausea and vomiting associated with migraine; these may include simple analgesics, ibuprofen, acetaminophen, antiemetics, or sedatives. SOURCES: Mayo Clinic.
Selective neurostimulation for treating epilepsy
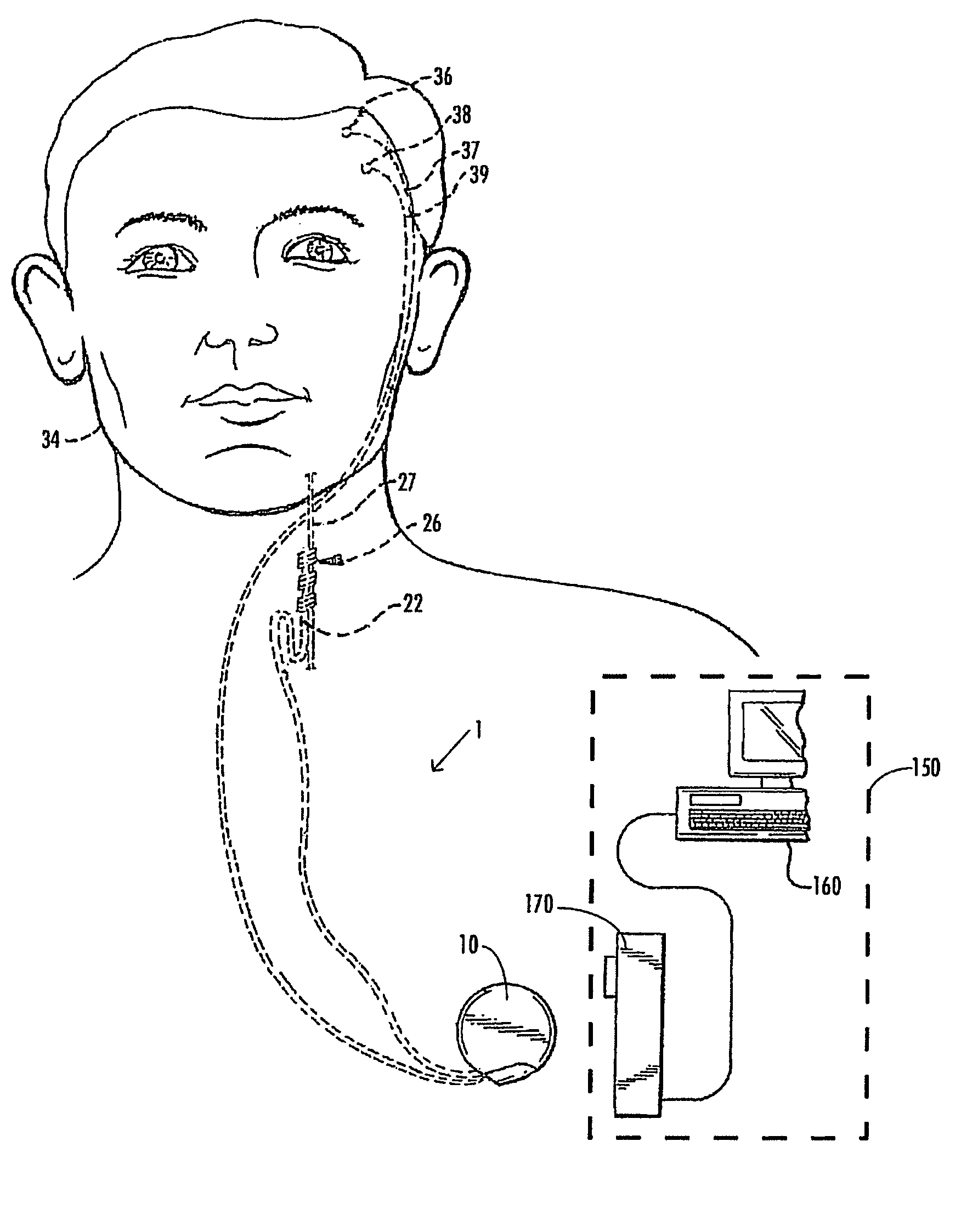
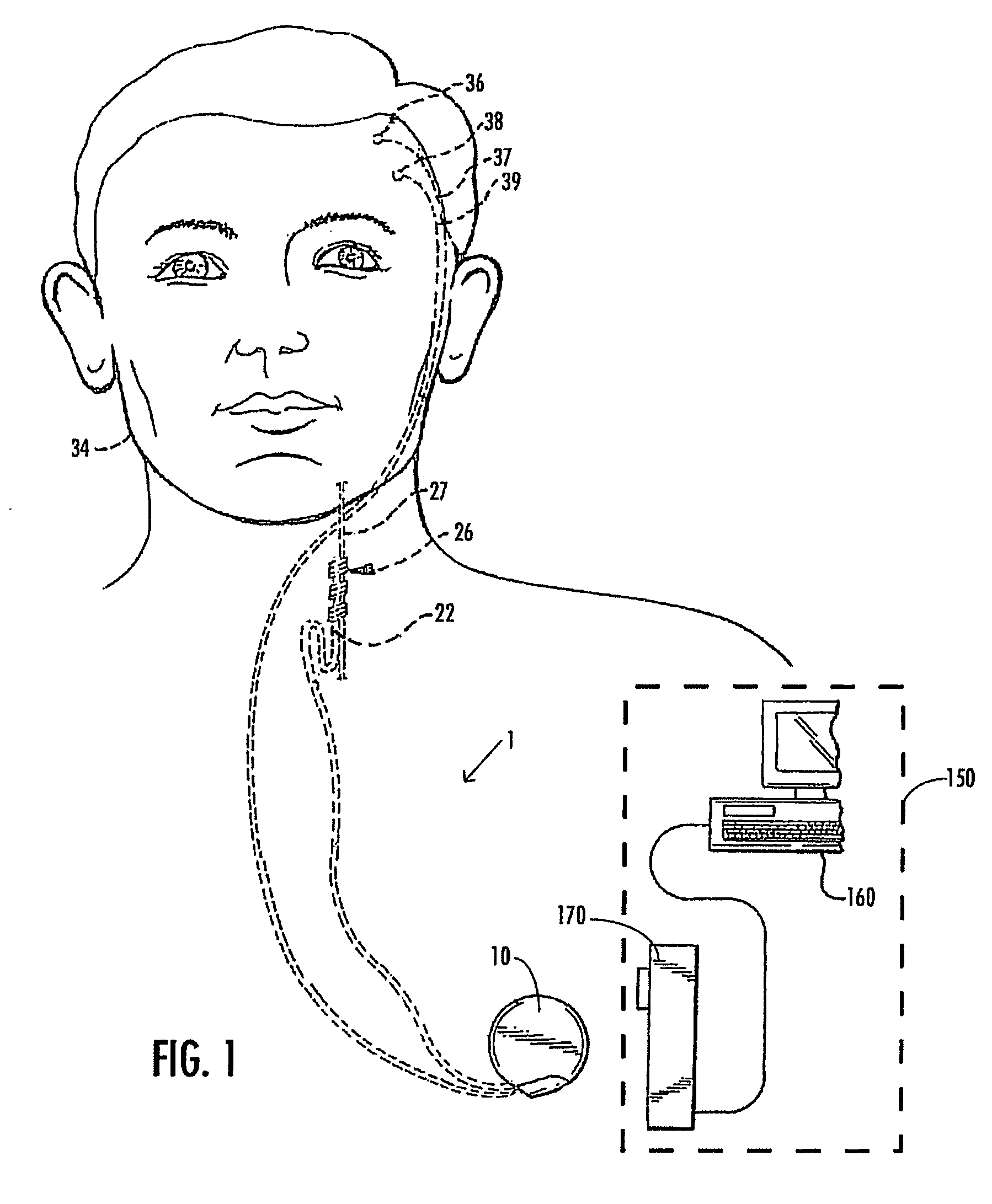
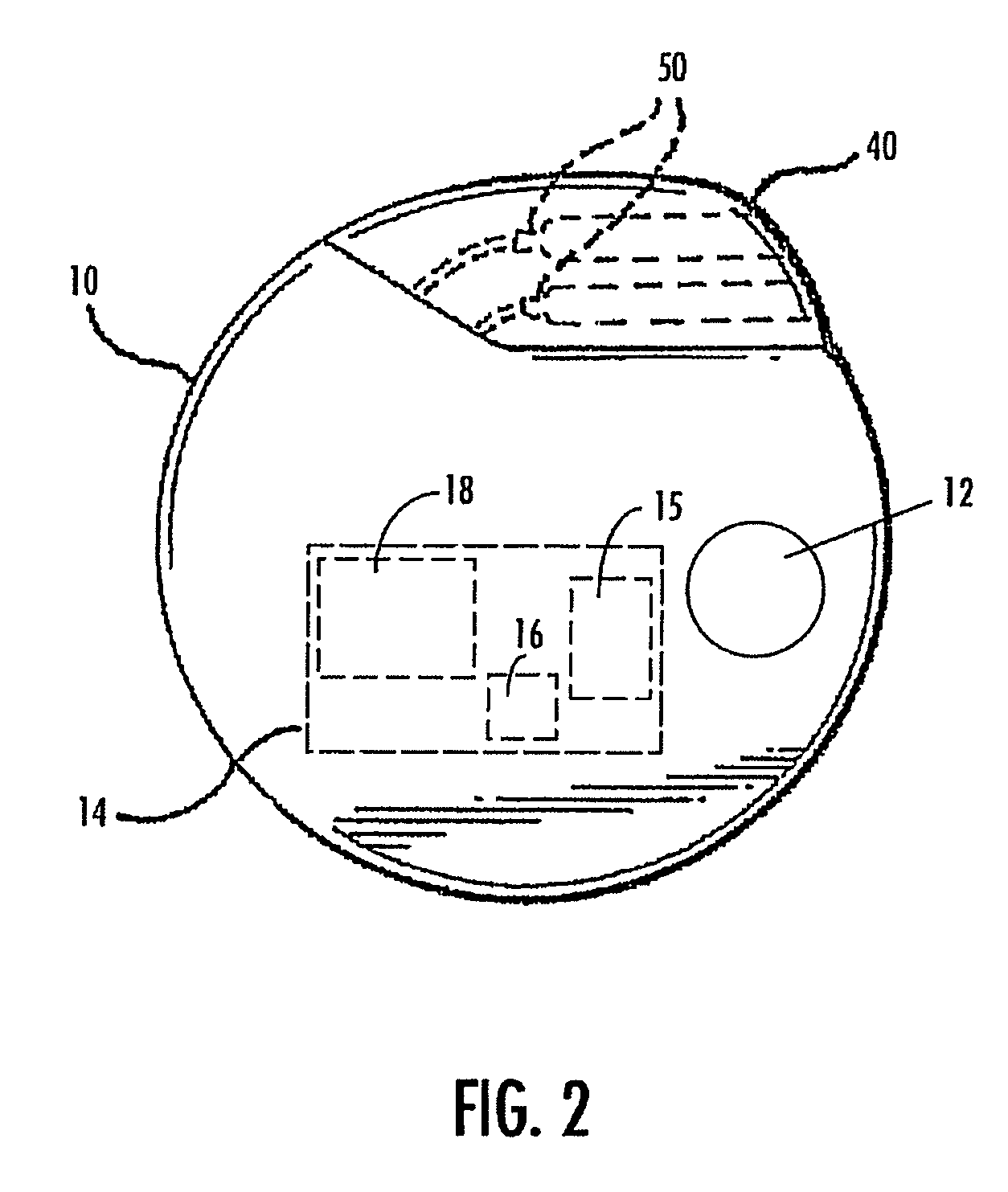
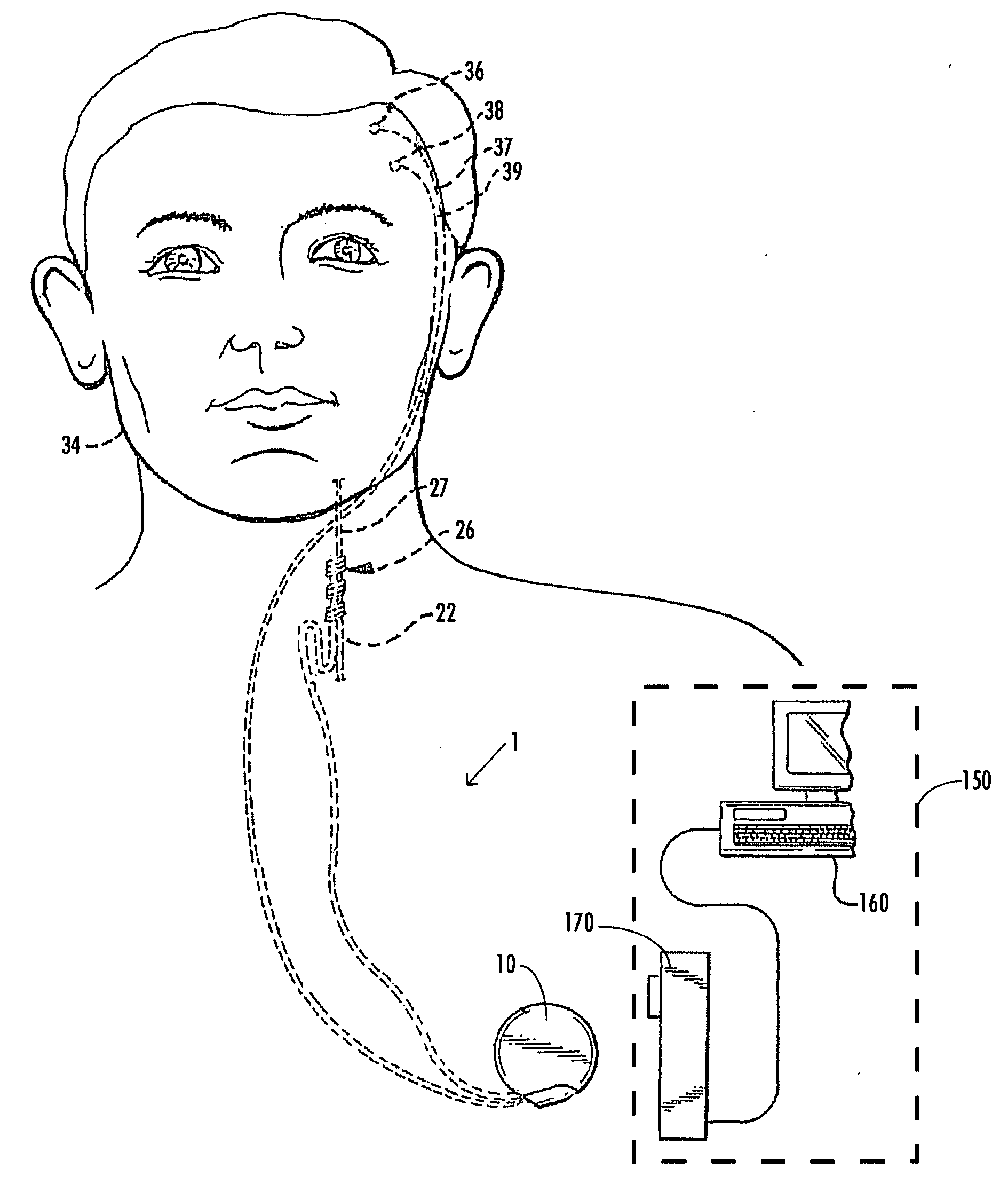
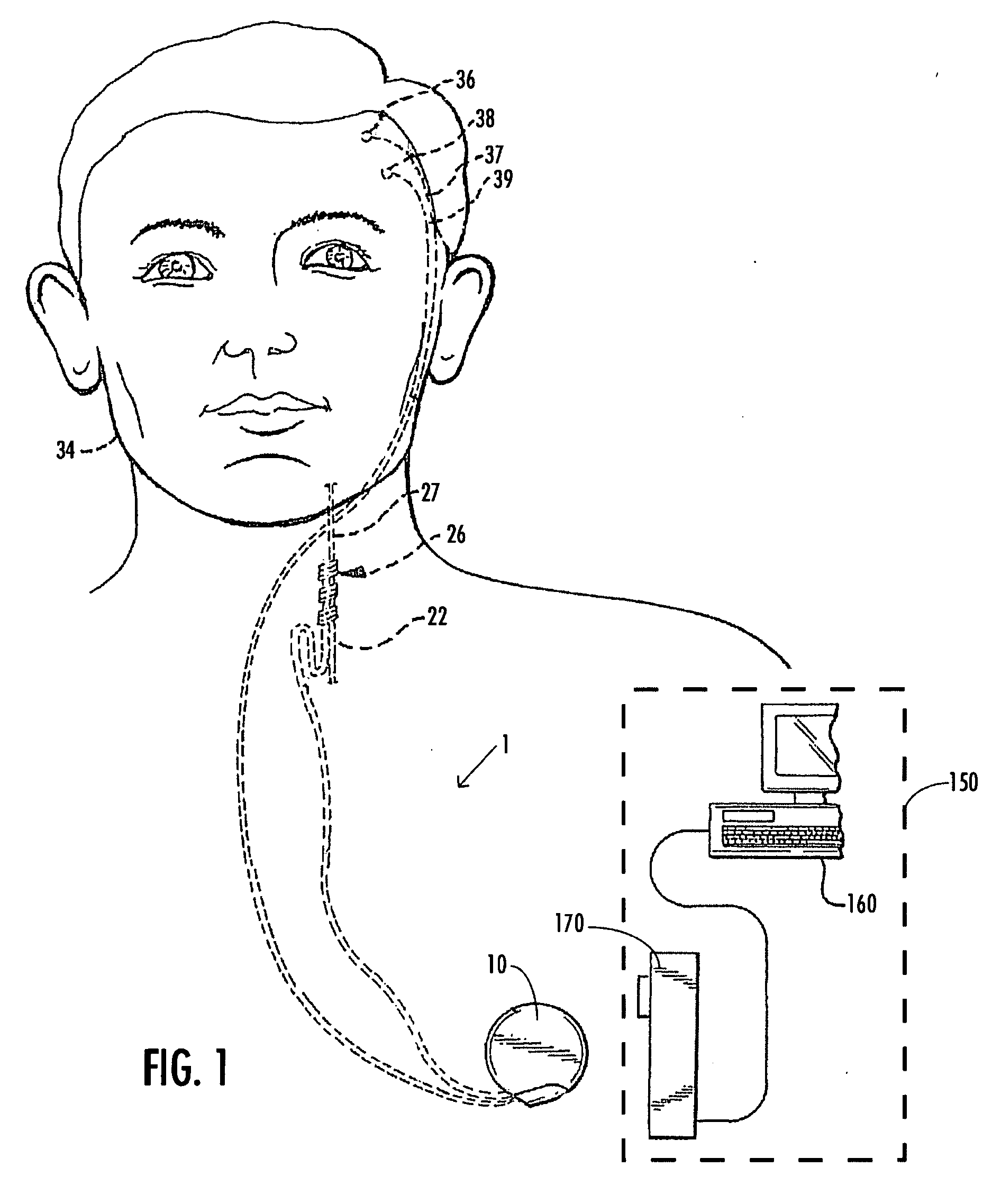
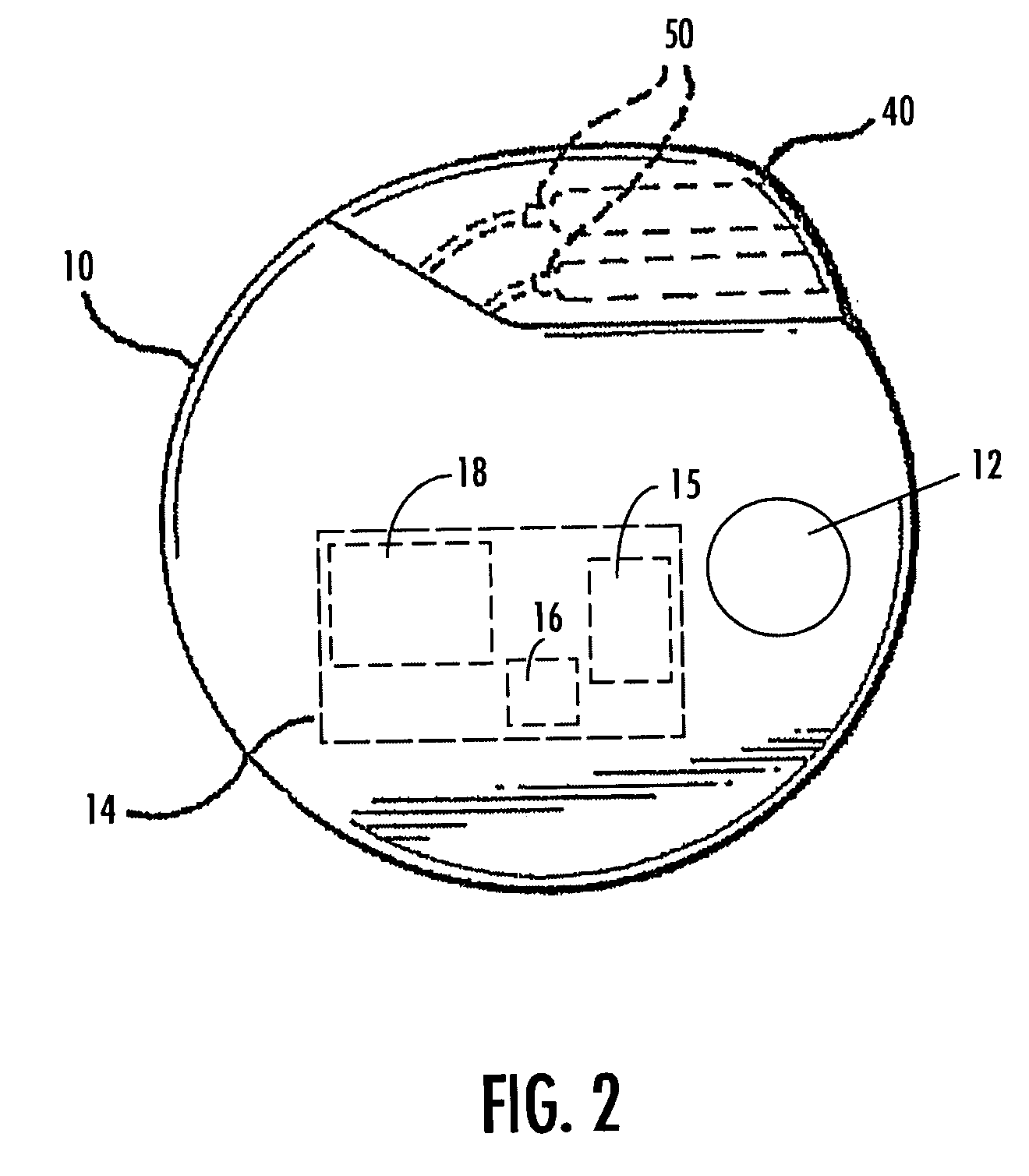
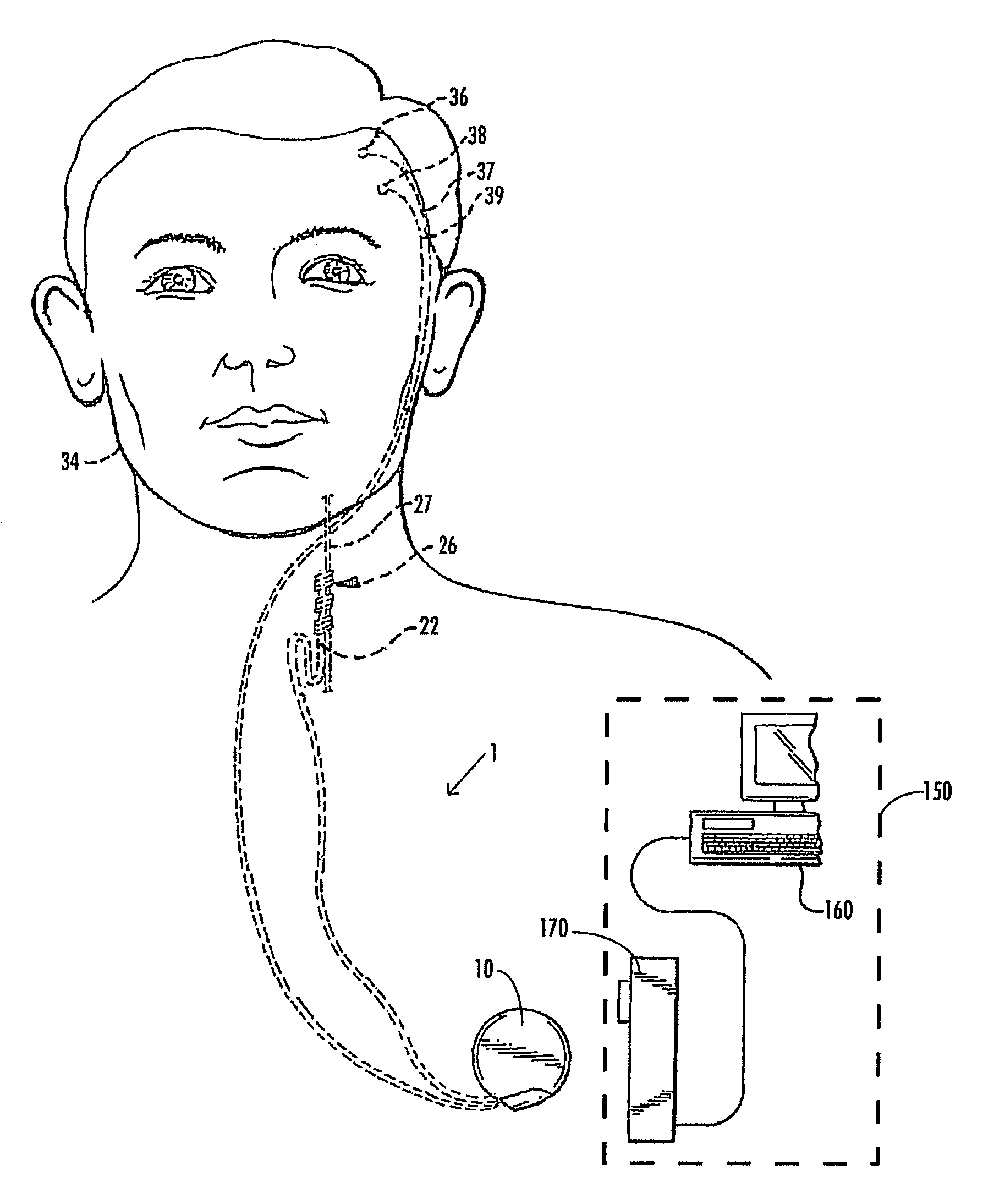
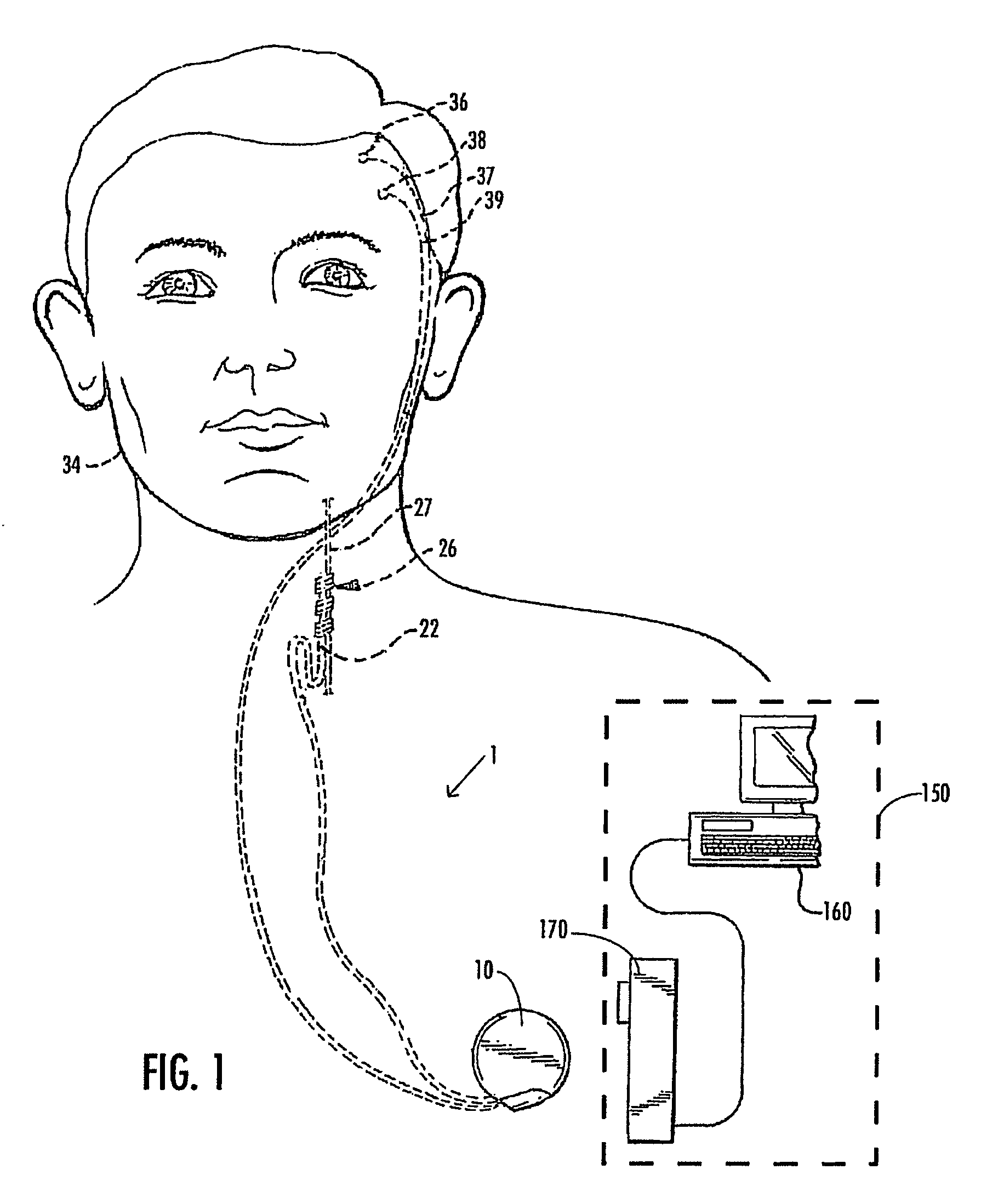
A method and device for treating epilepsy are disclosed which provide for electrical, chemical or magnetic stimulation of certain areas of the brain to modulate neuronal activity of areas associated with symptoms of epilepsy. Deep brain stimulation is combined with vagus nerve stimulation to enhance symptomatic relief of the disorder. Some embodiments also employ a sensing capability to optimize the therapeutic treatment regimen.
Owner:LIVANOVA USA INC
Selective neurostimulation for treating mood disorders
ActiveUS20070027500A1Relieve symptomsAlter modulation of neuronal activityHead electrodesDiseaseRegimen
A method and device for treating a mood and / or anxiety disorder are disclosed which comprise electrical, chemical or magnetic stimulation of certain areas of the brain to modulate neuronal activity of areas associated with symptoms of mood disorders. In certain embodiments, deep brain stimulation is combined with cranial nerve stimulation to enhance symptomatic relief of the disorder. Certain embodiments also employ a sensing capability to optimize the therapeutic treatment regimen.
Owner:LIVANOVA USA INC
Selective neurostimulation for treating epilepsy
A method and device for treating epilepsy are disclosed which provide for electrical, chemical or magnetic stimulation of certain areas of the brain to modulate neuronal activity of areas associated with symptoms of epilepsy. Deep brain stimulation is combined with vagus nerve stimulation to enhance symptomatic relief of the disorder. Some embodiments also employ a sensing capability to optimize the therapeutic treatment regimen.
Owner:LIVANOVA USA INC
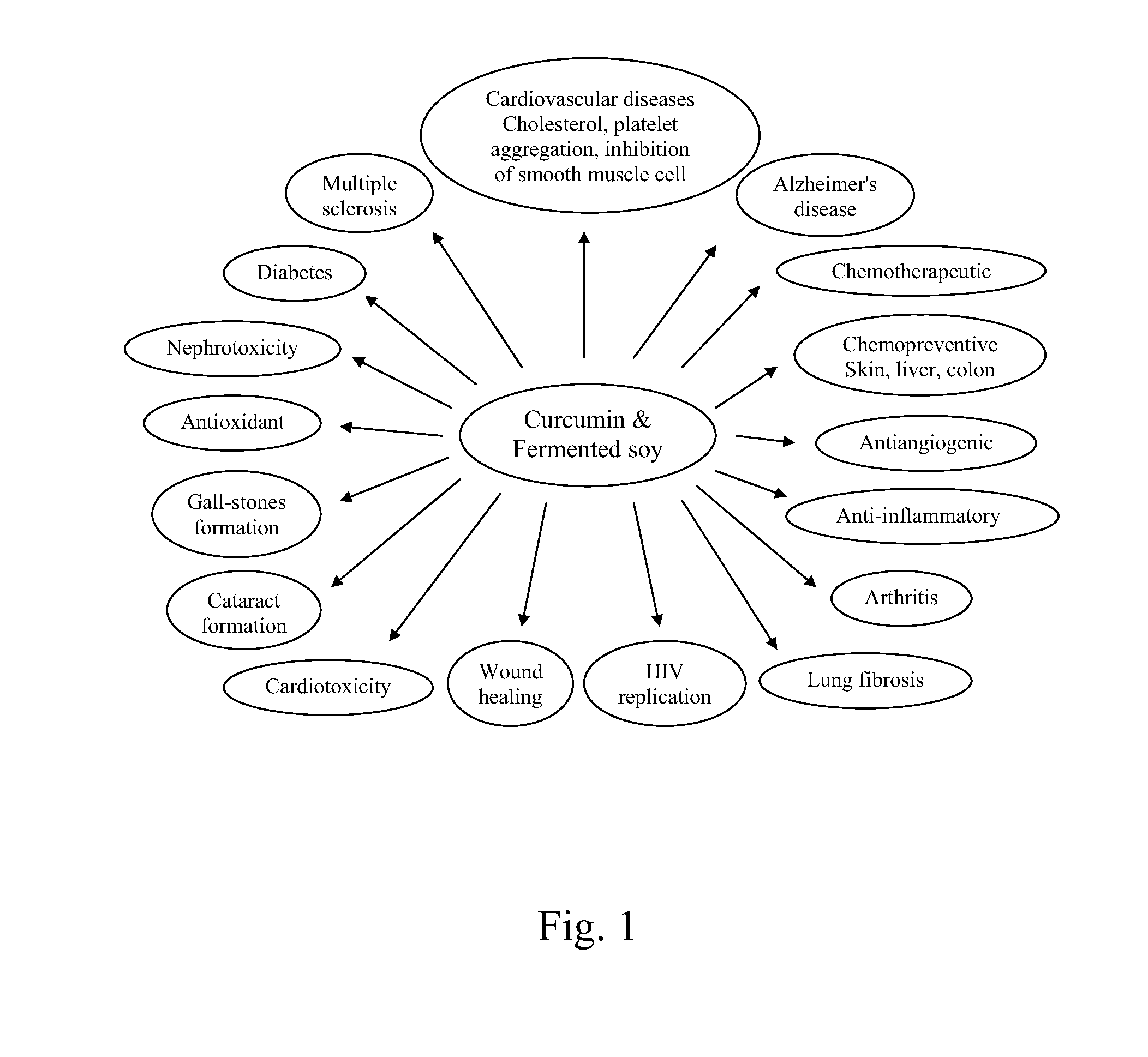
Fermented soy nutritional supplements including mushroom components
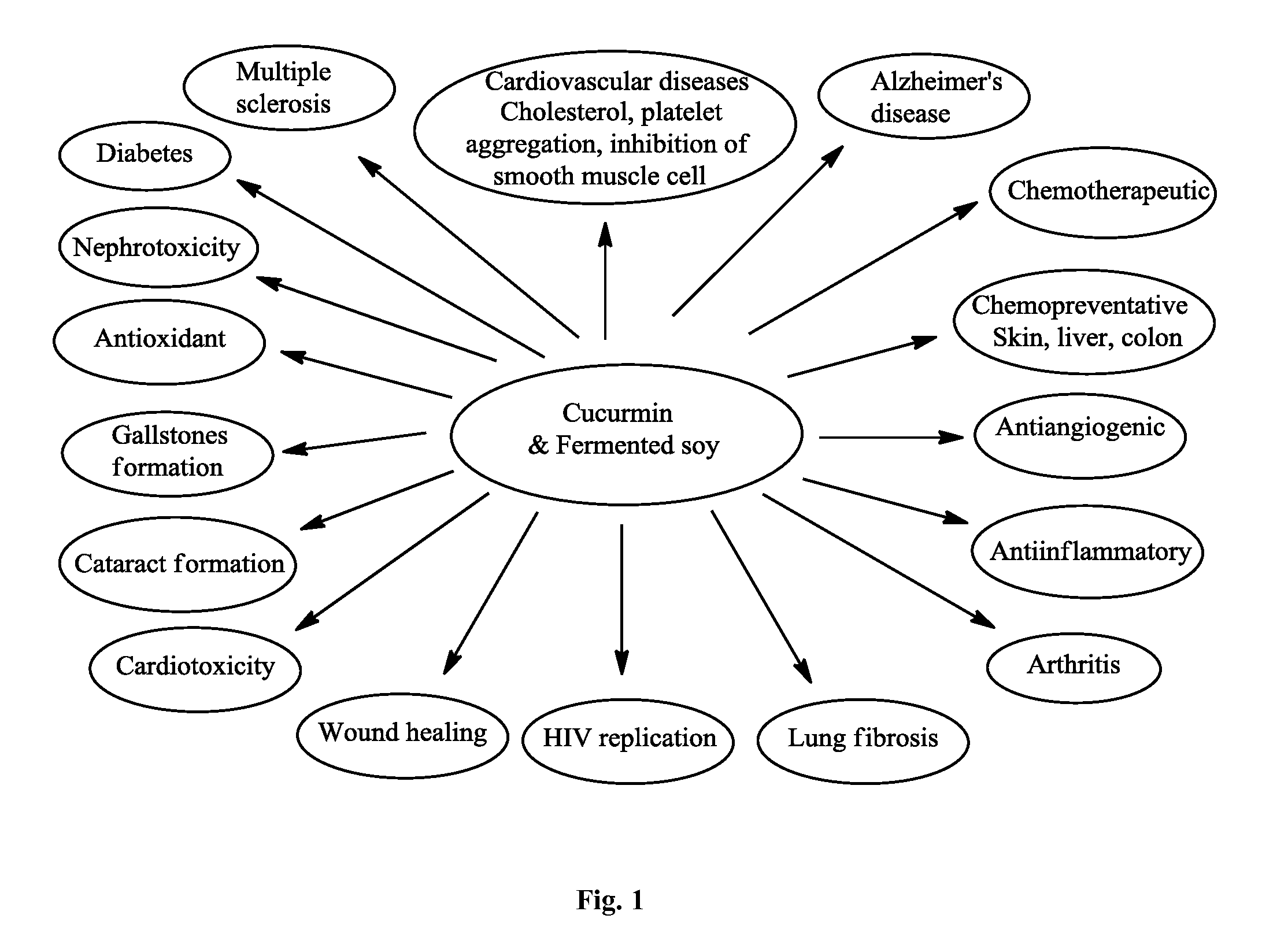
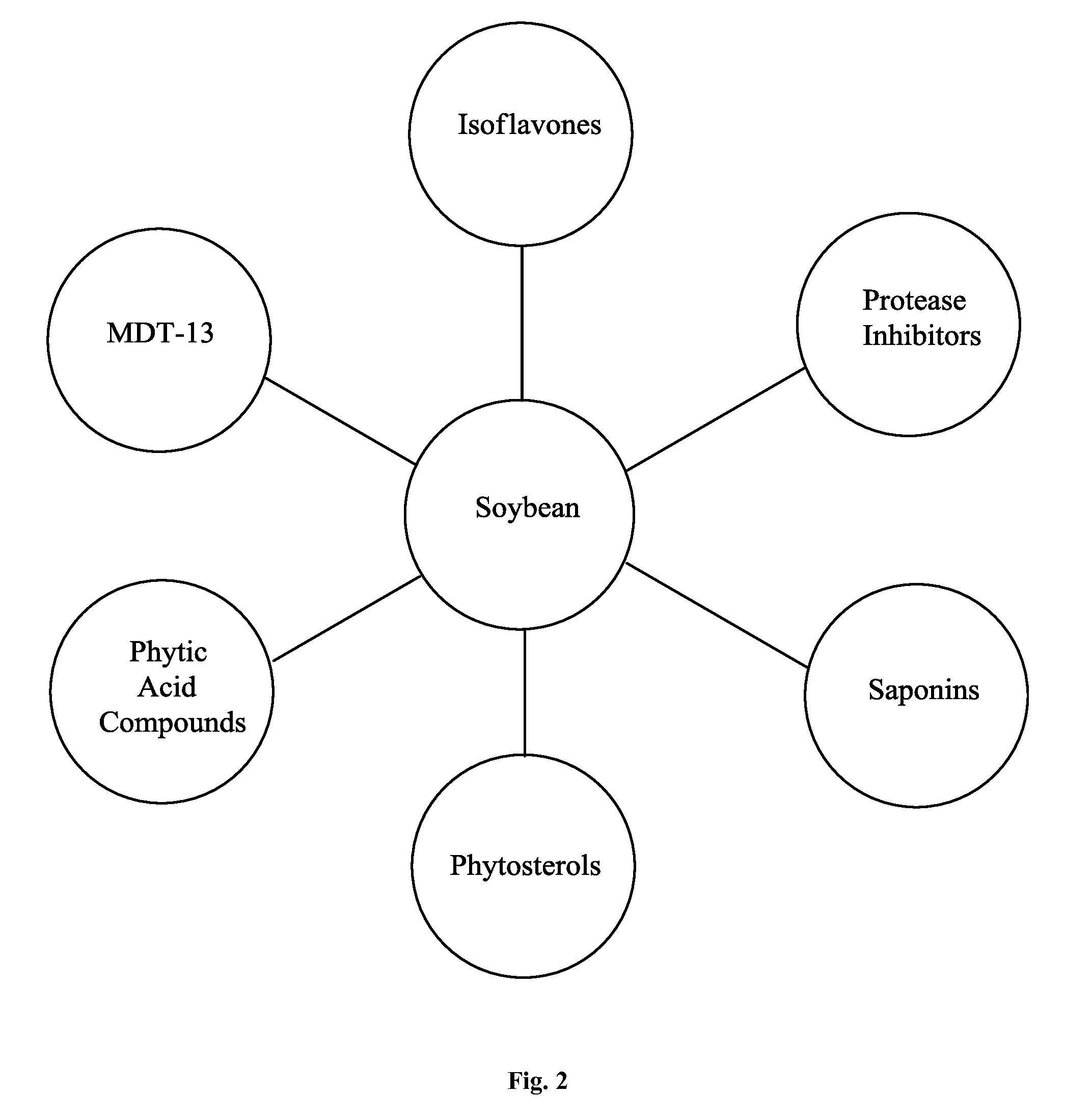
Dietary, health, and / or nutritional supplements provide various formulations of mushrooms grown in fermented soy, with or without rice flour, optionally in combination with sweetener(s), curcumin, and various other herbs and spices. Certain embodiments or compositions may be in liquid, beverage, solid, paste or powder forms. In certain embodiments the supplements contain mushroom species grown in fermented soy in the presence of certain bacterial species and optionally rice or rice flour. In some embodiments, other ingredients that may be present include one or more of curcumin, desmethoxycurcumin and bis-desmethoxycurcumin or all three curcumins. Certain embodiments may be used to treat or provide symptomatic relief from a variety of maladies ranging from malnutrition to mood related disorders to metabolic support and other more severe conditions as described herein.
Owner:NAIR VIJAYA
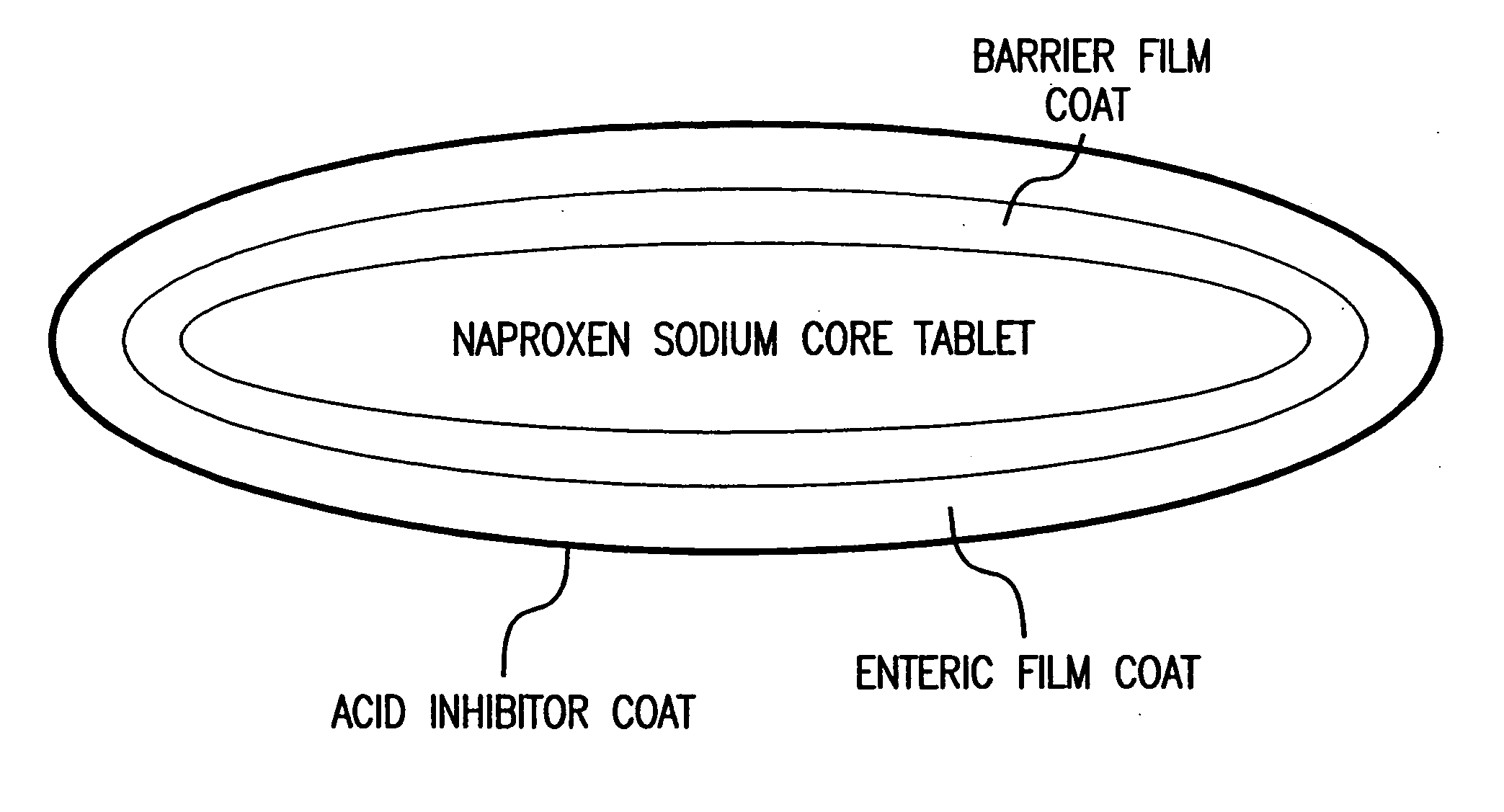
Pharmaceutical compositions for the coordinated delivery of NSAIDs
InactiveUS20050249811A1Improve complianceReduce in quantityBiocideAntipyreticGastrointestinal InjuryArthritis
The present invention is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The invention also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO +1
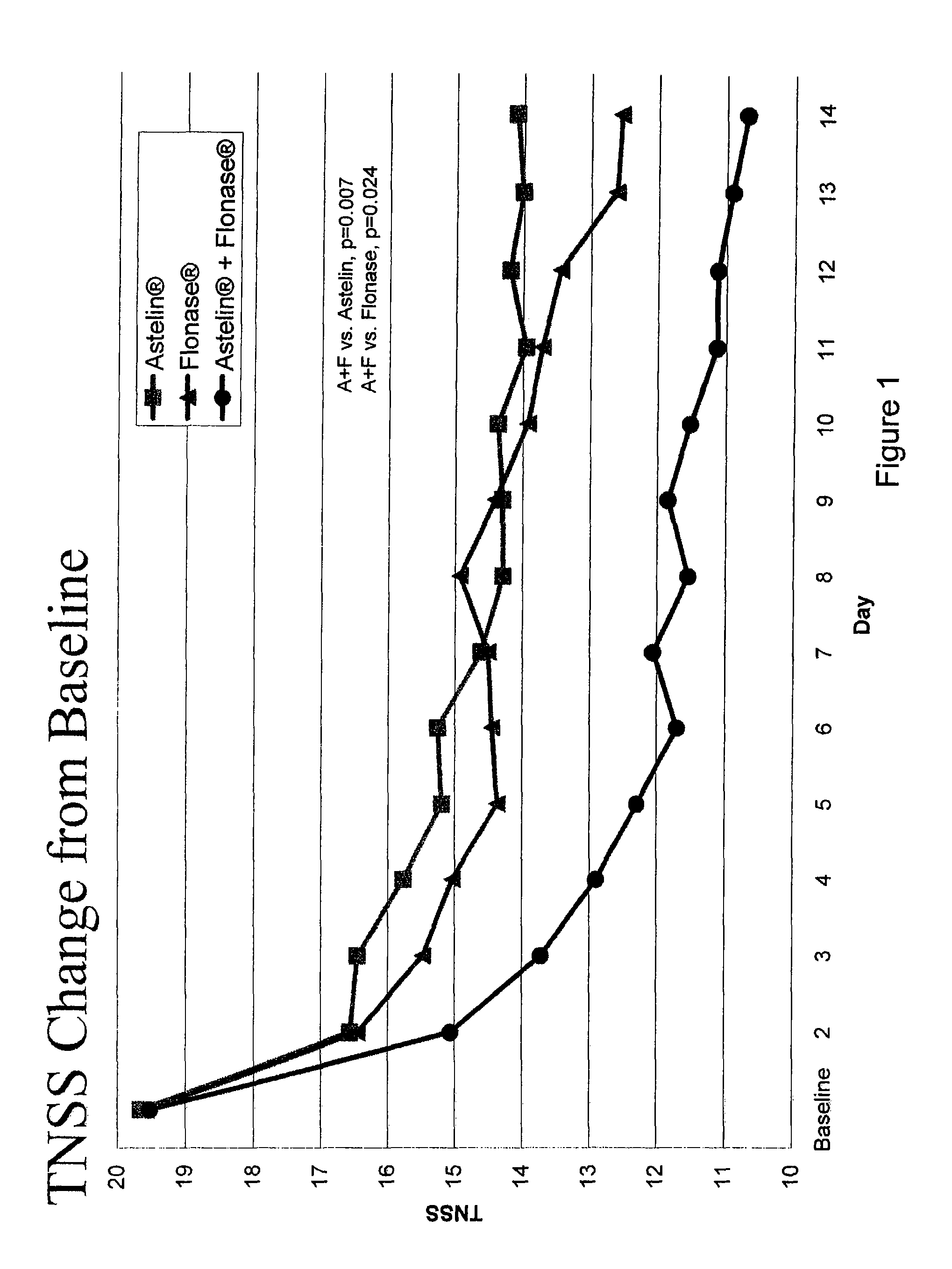
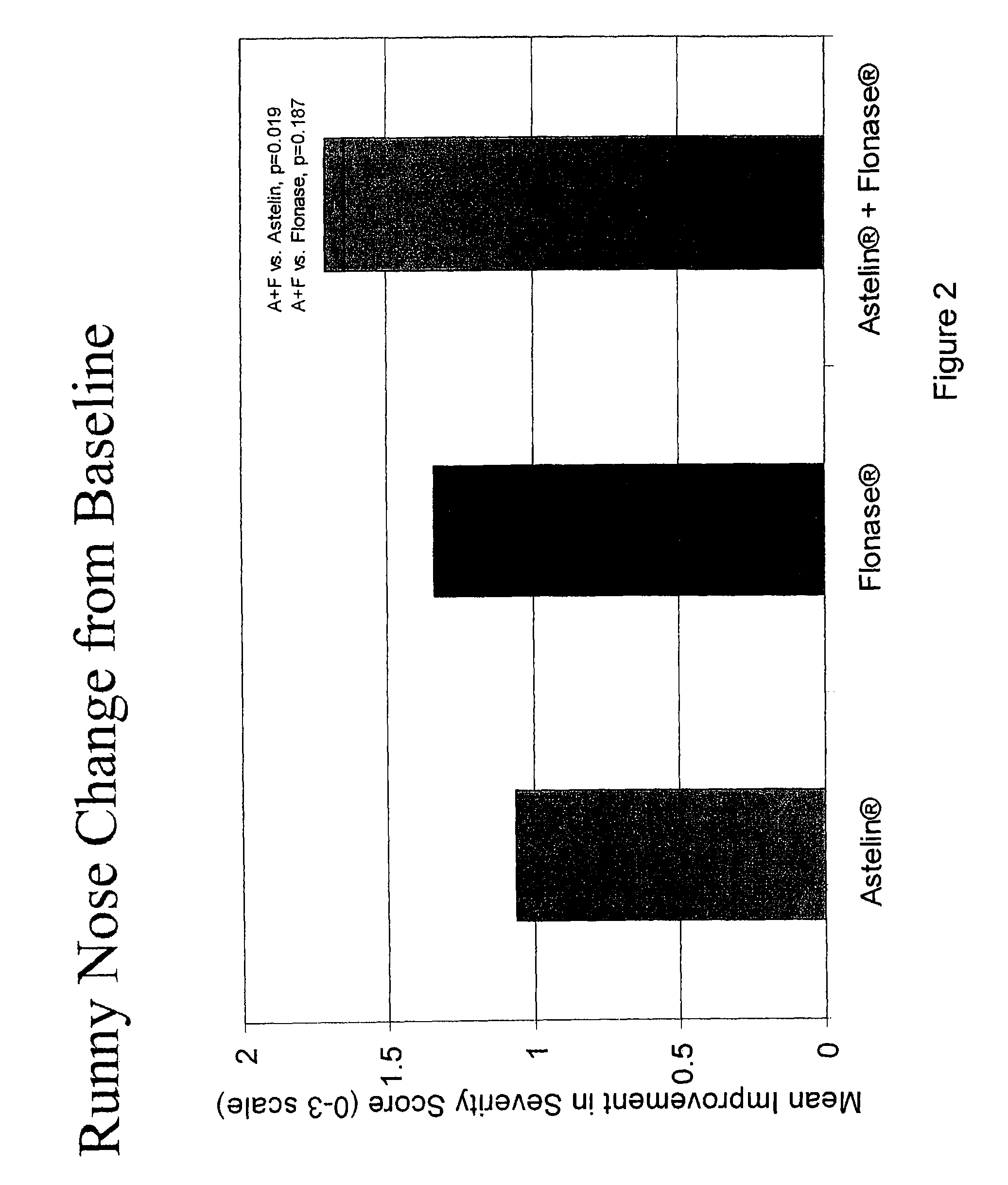
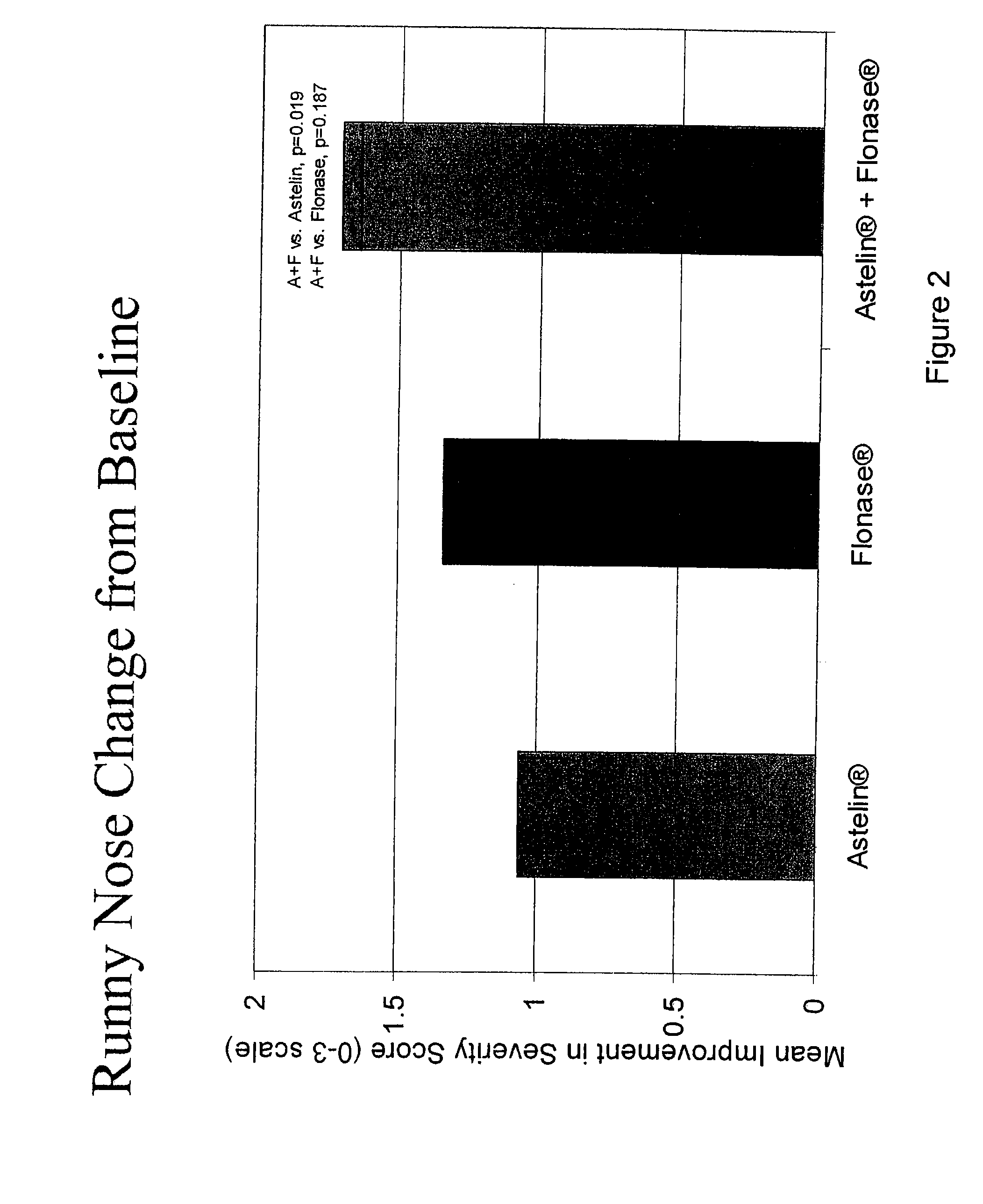
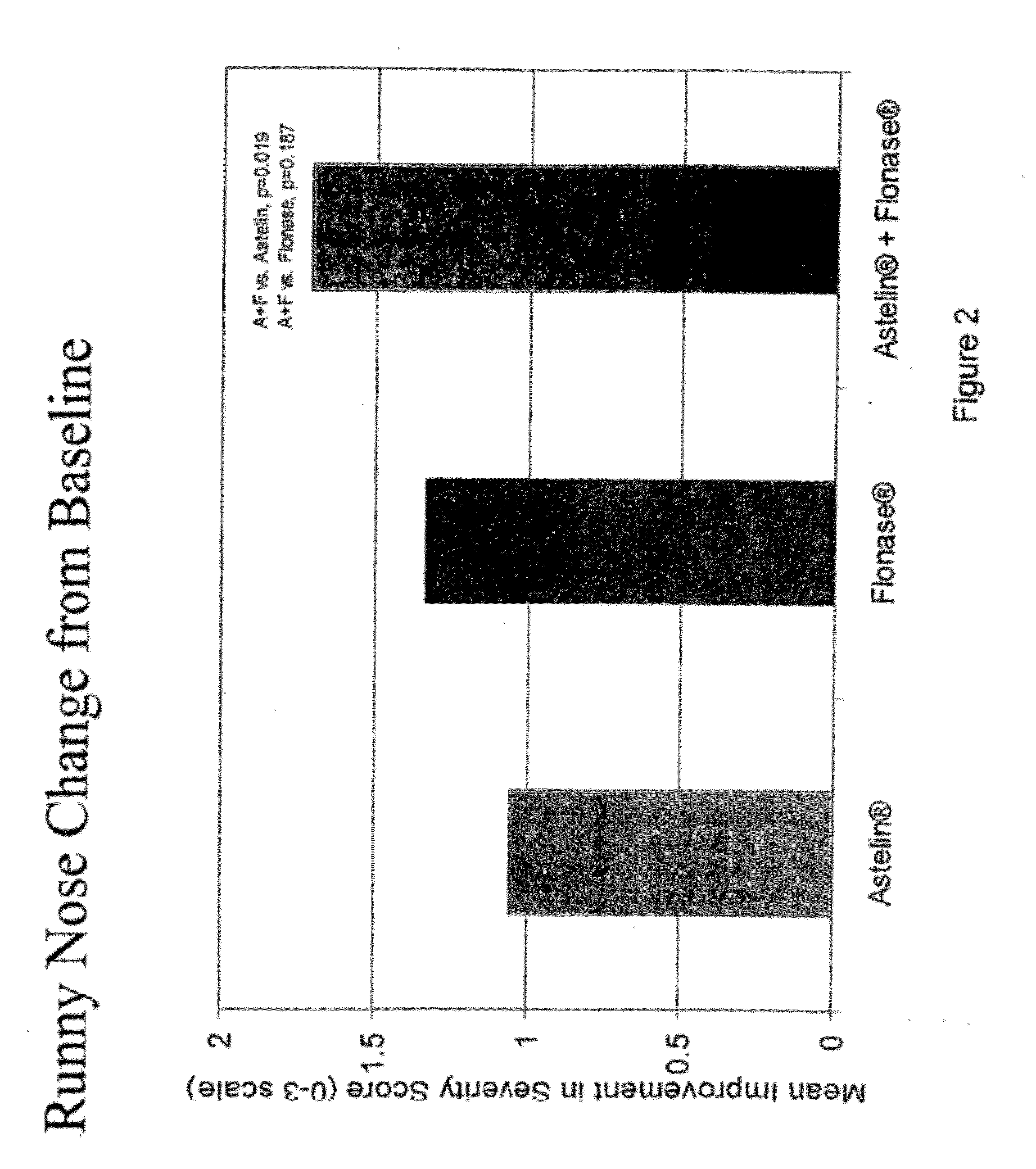
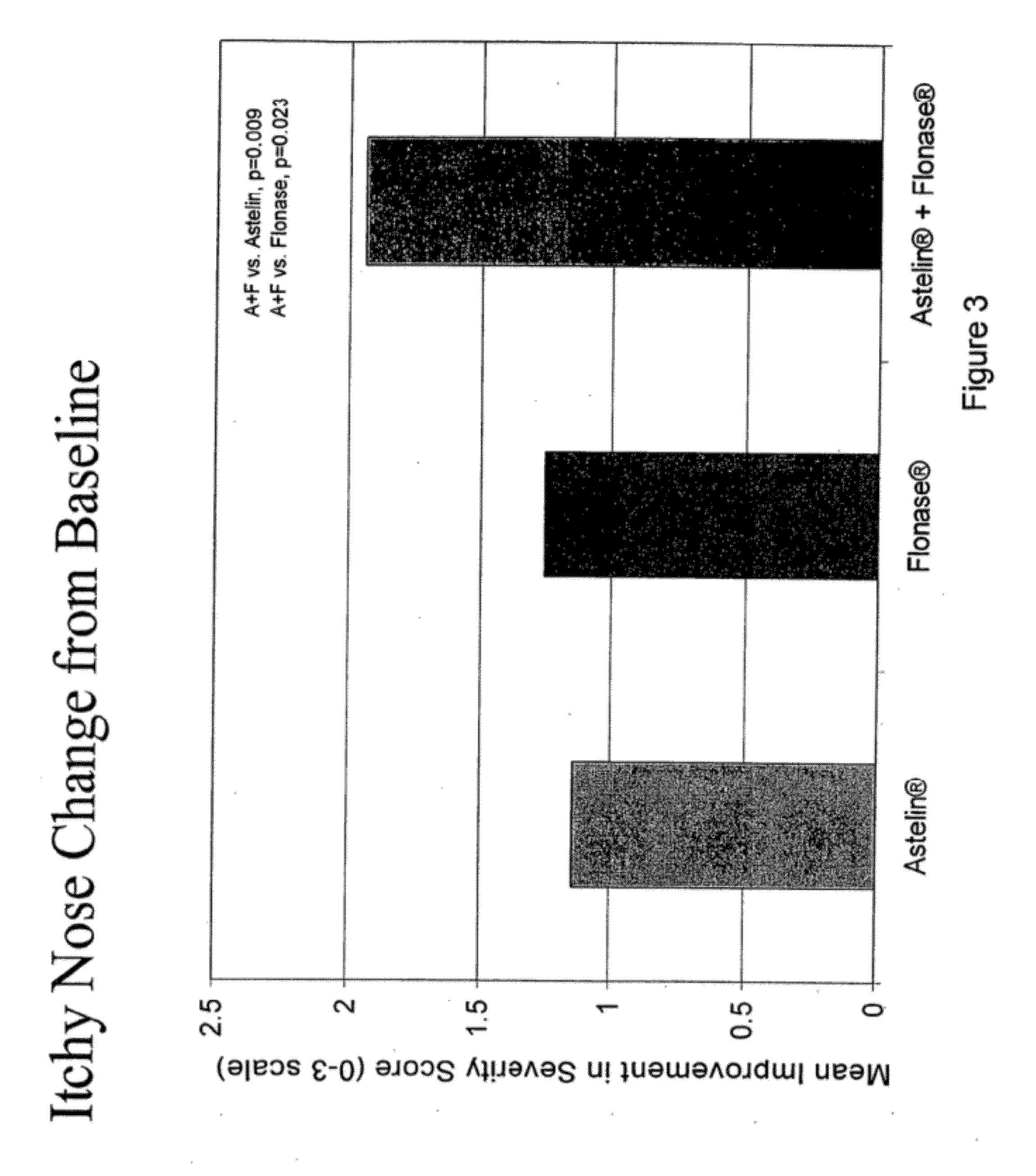
Compositions comprising azelastine and methods of use thereof
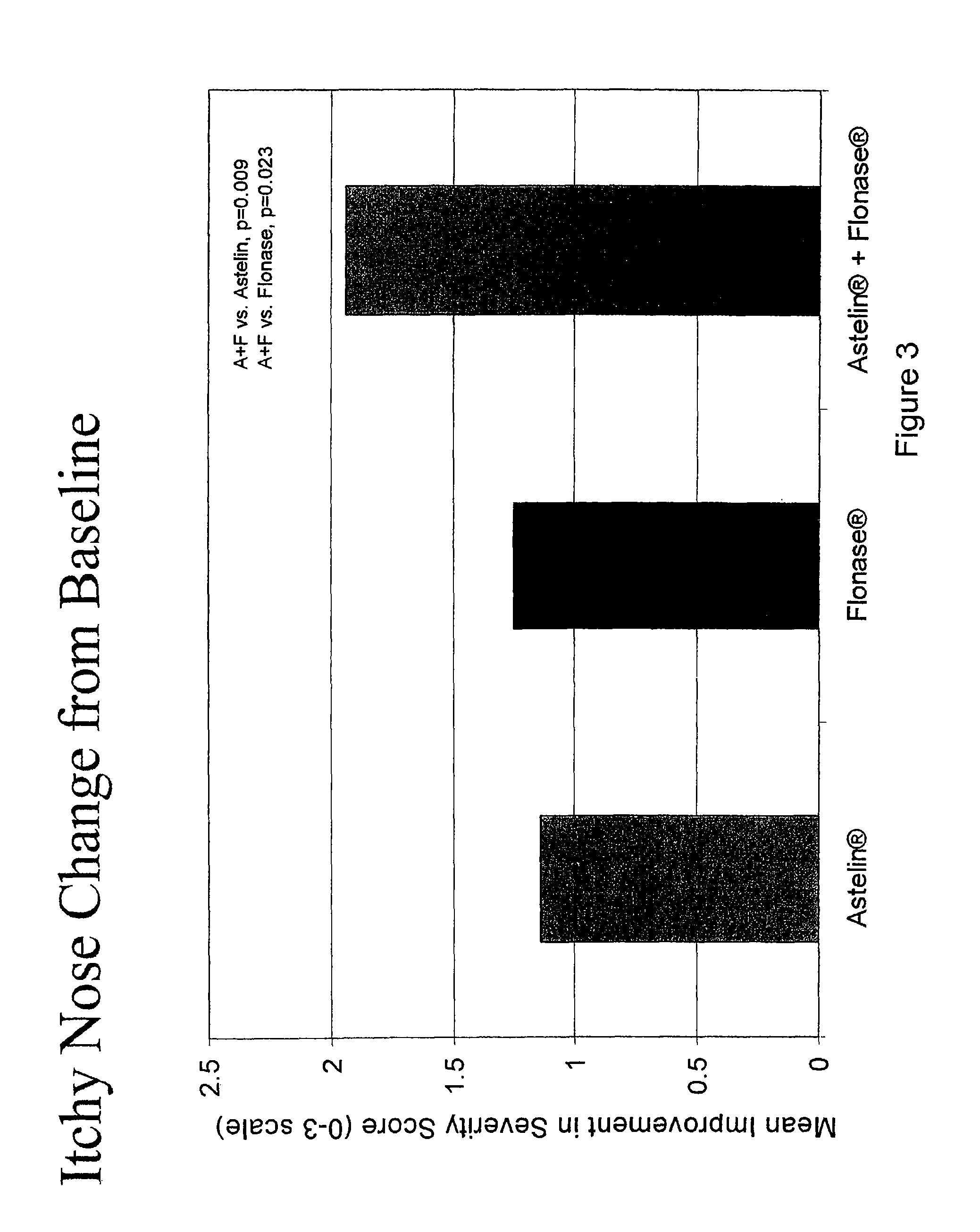
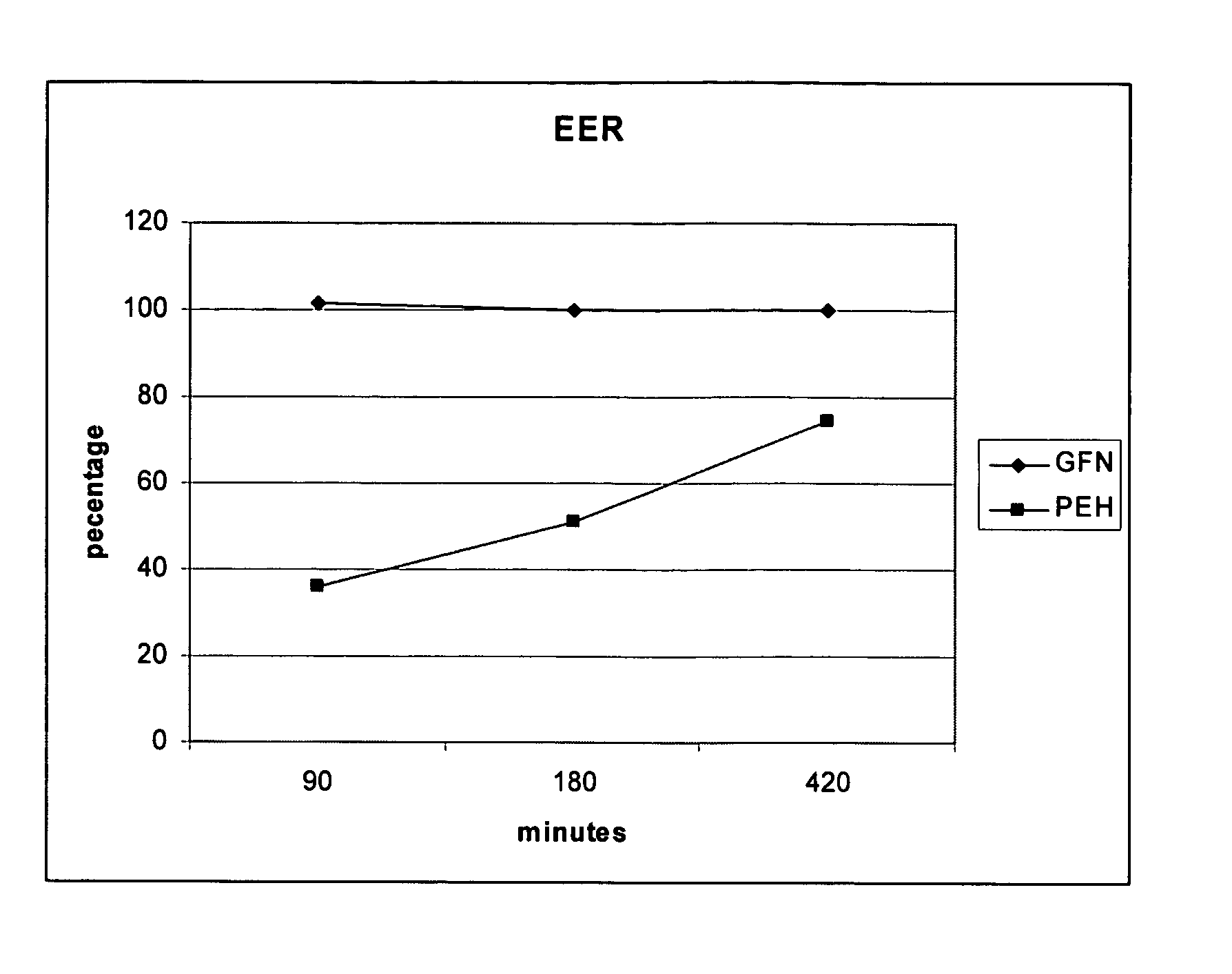
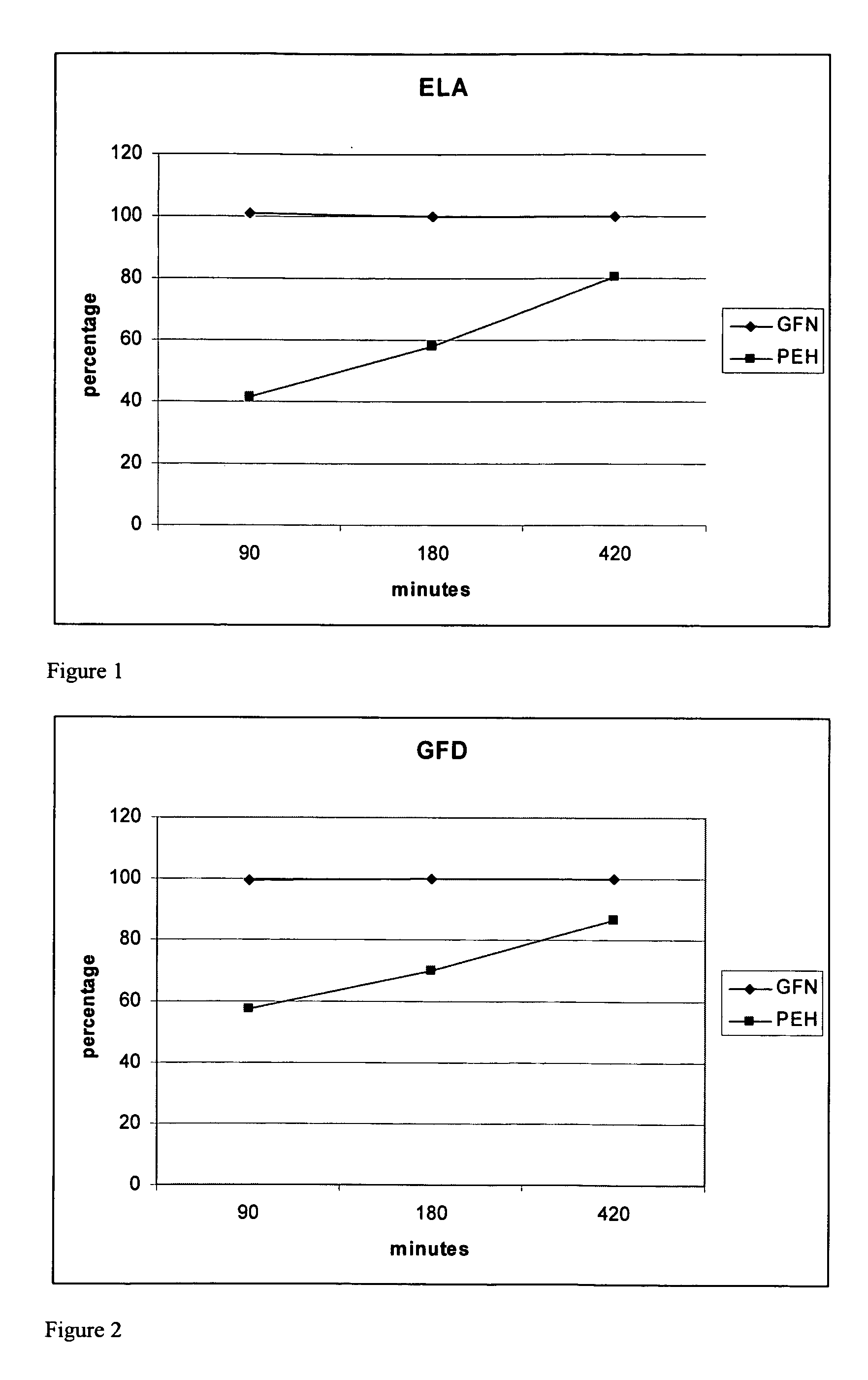
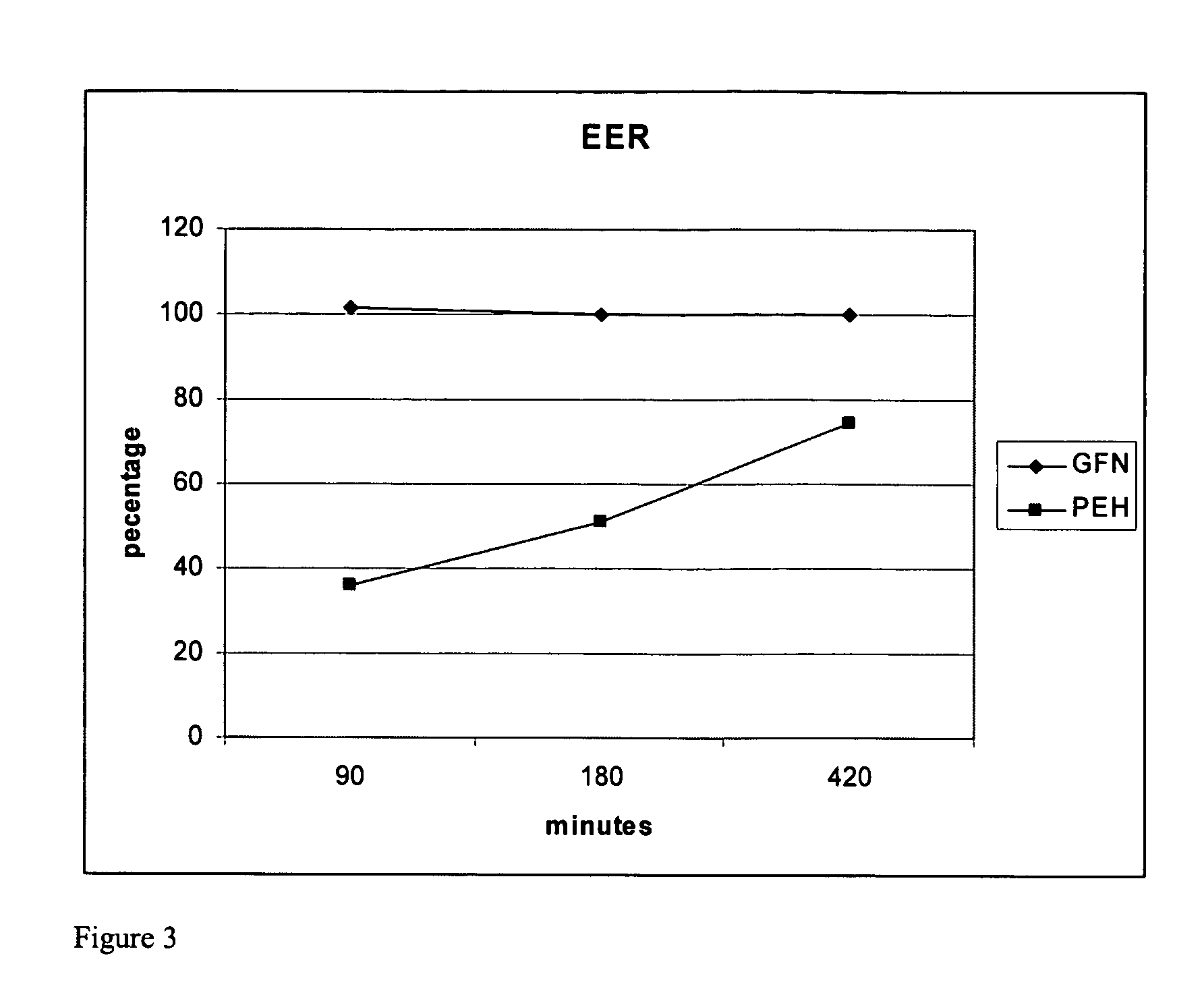
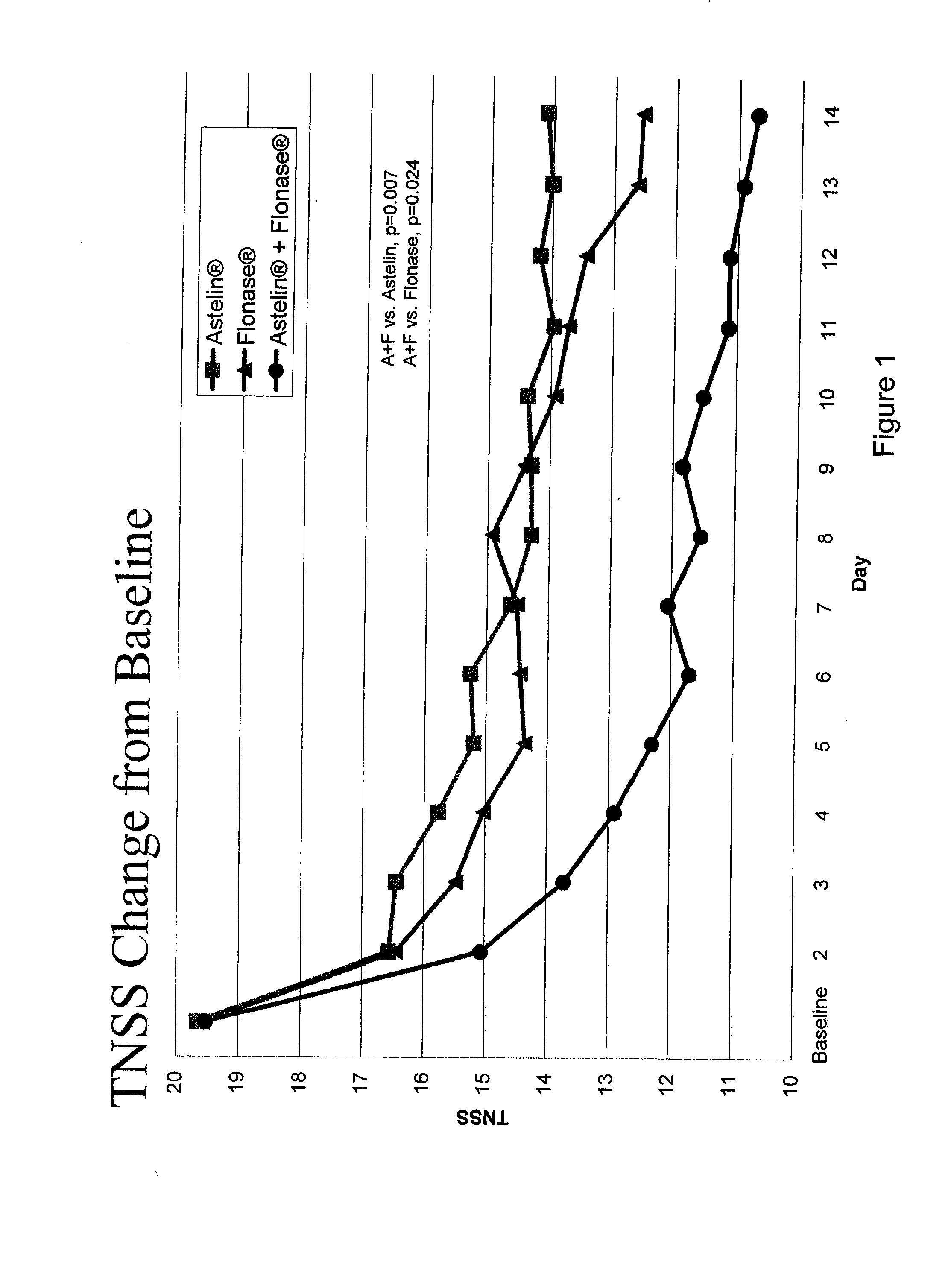
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Neurostimulation device for treating mood disorders
A device and method for treating a mood and / or anxiety disorder are disclosed which provide for stimulation of certain areas of the brain to modulate neuronal activity of areas associated with symptoms of mood disorders. In certain embodiments, brain stimulation is combined with cranial nerve stimulation for enhancing symptomatic relief of the disorder. Certain embodiments also employ a sensing capability for optimizing the therapeutic treatment regimen.
Owner:LIVANOVA USA INC
Dietary regimen of nutritional supplements for relief of symptoms of arthritis
InactiveUS6136795AReduce inflammationPermits healingBiocideAcidic food ingredientsDocosahexaenoic acidRegimen
This invention is directed to a dietary regimen and a unique combination of nutritional supplements and a method. More specifically, this invention is directed to a unique combination of nutritional supplements which provides symptomatic relief from arthritis. The unique combination of nutritional supplements of this invention is believed to function by both increasing the available (effective blood level) of anti-inflammatory agents and promotion of the healing / regenerative process in the effected joints, thus, producing unexpected and lasting symptomatic relief from the debilitating effects of both osteoarthritis and rheumatoid arthritis. The essential nutritional supplements of the dietary regimen of this invention are as follows: (a) gamma linolenic acid (unrefined), hereinafter "GLA"(b) a mixture of eicosapentaenoic acid and docosahexaneoic acid, hereinafter collectively "EPA"(c) a mixture of chondroitin sulfate, N-acetyl glucosamine sulfate, glucosamine sulfate and manganese aspartate, hereinafter collectively "CHONDROX"The regimen is adjusted based upon the weight of the individual, and once symptomatic relief is achieved, the individual remains essentially free from the debilitating effects of arthritis so as long the daily regimen is faithfully followed.
Owner:IRWIN NATURALS 4HEALTH INC +1
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Dynamic variable release
InactiveUS20050152967A1Improve efficiencyReduce in quantityBiocideOrganic active ingredientsDiseaseCommon cold
The present invention relates to novel mixed release pharmaceutical formulations that include a expectorant available for immediate release and a decongestant for extended release that provide for the symptomatic relief of cough associated with respiratory tract conditions such as the common cold, bronchial asthma, acute and chronic bronchitis.
Owner:NEOS THERAPEUTICS LP
Method of treatment and/or prophylaxis
InactiveUS20030199424A1Easy to prepareHigh viscosityHeavy metal active ingredientsBiocideVertebrate AnimalsPsychiatry
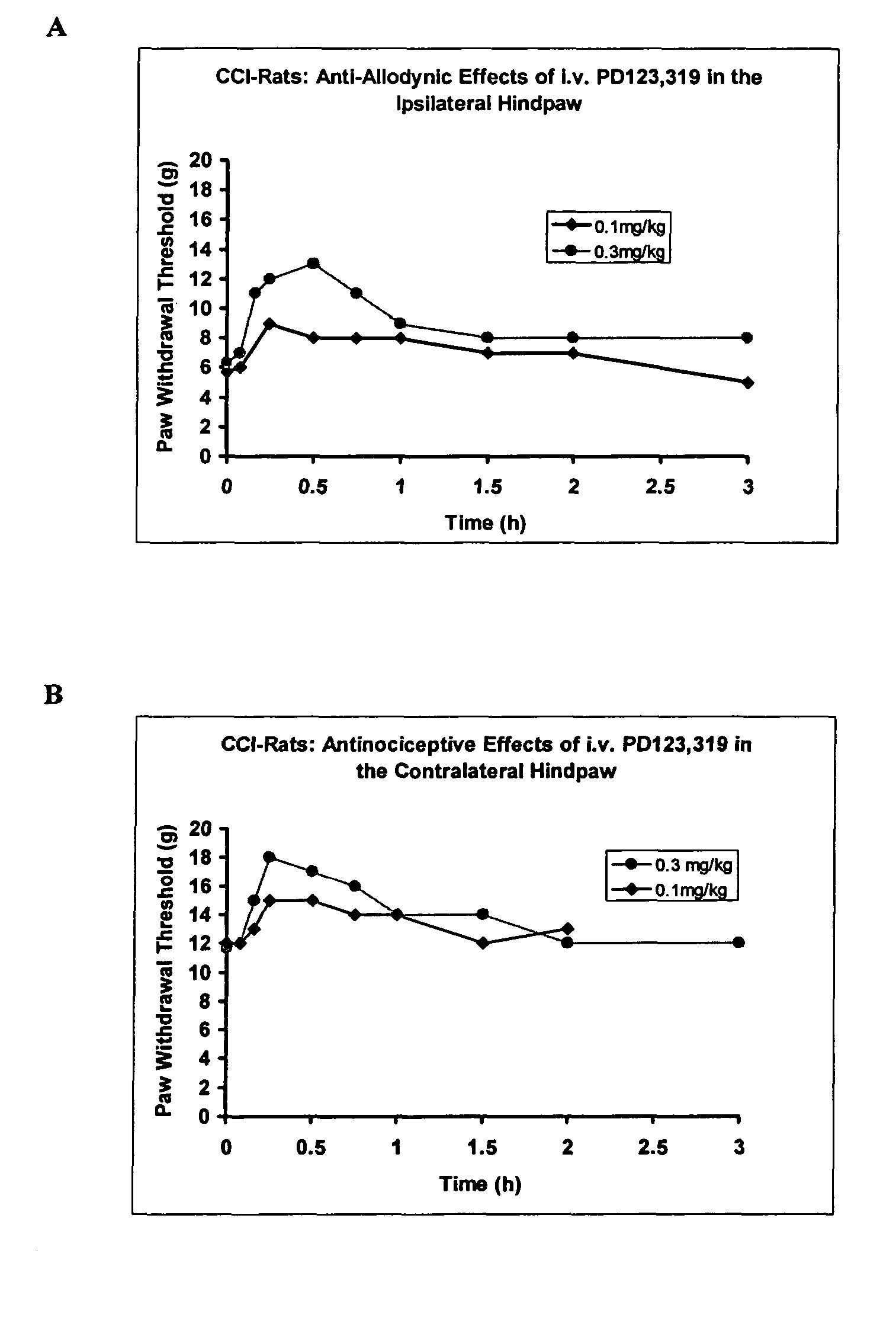
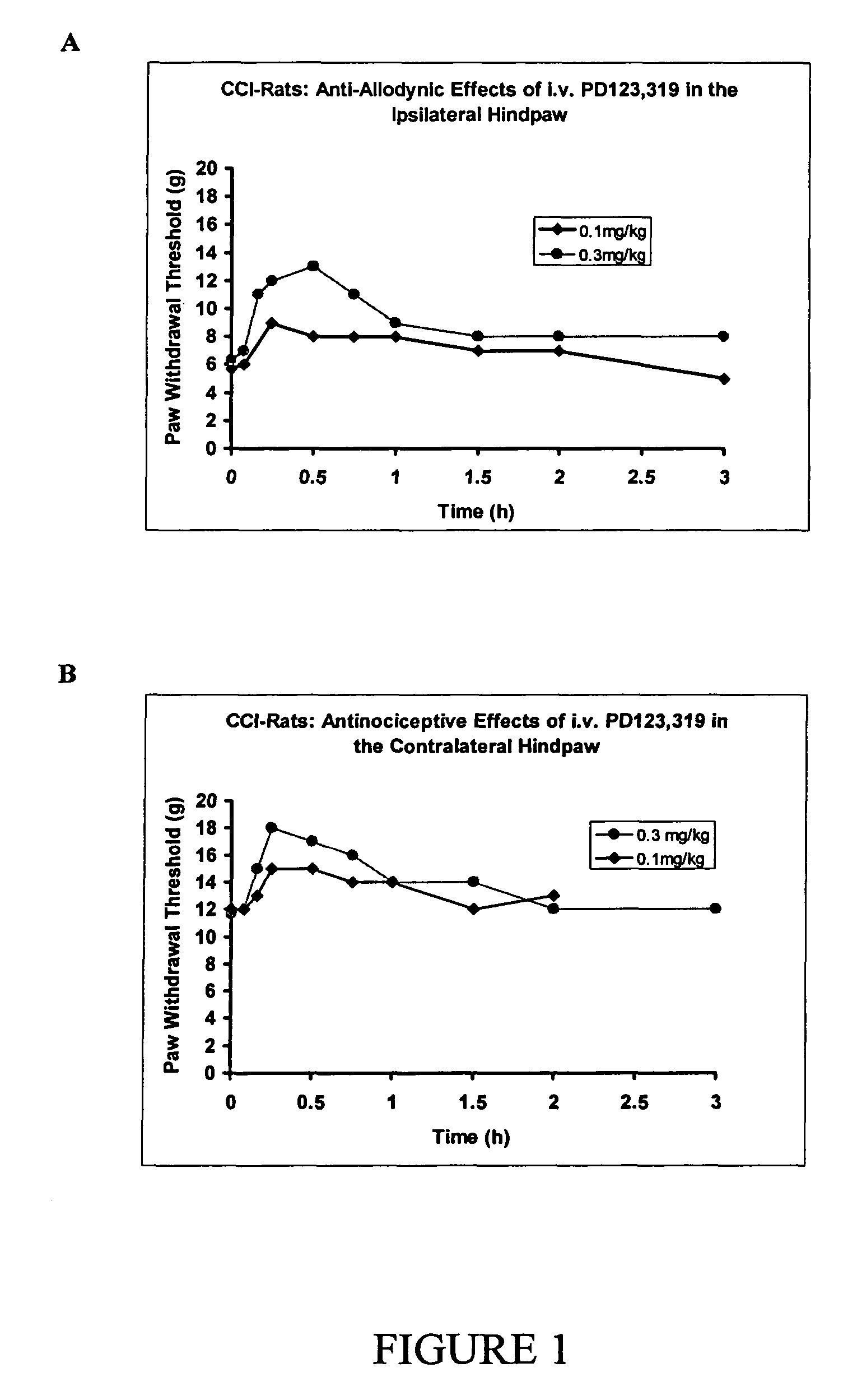
The present invention is directed to the use of angiotensin II receptor I (AT1 receptor) antagonists for the treatment, prophylaxis, reversal and / or symptomatic relief of a neuropathic condition, especially a peripheral neuropathic condition such as painful diabetic neuropathy, in vertebrate animals and particularly in human subjects. The present invention also discloses the use of AT1 receptor antagonists for preventing, attenuating or reversing the development of reduced opioid sensitivity, and more particularly reduced opioid analgesic sensitivity, in individuals and especially in individuals having, or at risk of developing, a neuropathic condition.
Owner:QUEENSLAND UNIV OF
Compositions and methods for symptomatic relief
InactiveUS20070041993A1Relieve painIncrease valueBiocideLichen medical ingredientsDiseaseTrichosanthes kirilowii
An herbal formula provides symptomatic relief for various diseases and especially for patients diagnosed with AIDS / ARC. Particularly preferred formulae include at least part of at least four plants selected from the group consisting of Panax ginseng, Angelica sinensis, Astralagus membranaceus, Ligustrum lucidum, Sophora flavescens, Trichosanthes kirilowii, Agrimonia pilosa, Ganoderma lucidum, Rehmannia glutinosa, Cordiceps sinensis, Oldenlandia diffusea, Isatis spec., Polyporus umbellatus, Pogostemon cablin, Solanum nigrum, Atractylodis macrocephalae, Clematis spec., and Glycyrrhiza spec., and further include at least one of calculus bovis, concha pteriae powder, and borneolum syntheticum.
Owner:HOLCOMB HALSTEAD TERRI
Selective neurostimulation for treating mood disorders
A method and device for treating a mood and / or anxiety disorder are disclosed which comprise electrical, chemical or magnetic stimulation of certain areas of the brain to modulate neuronal activity of areas associated with symptoms of mood disorders. In certain embodiments, deep brain stimulation is combined with cranial nerve stimulation to enhance symptomatic relief of the disorder. Certain embodiments also employ a sensing capability to optimize the therapeutic treatment regimen.
Owner:LIVANOVA USA INC
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Selective neurostimulation for treating epilepsy
A method and device for treating epilepsy are disclosed which provide for electrical, chemical or magnetic stimulation of certain areas of the brain to modulate neuronal activity of areas associated with symptoms of epilepsy. Deep brain stimulation is combined with vagus nerve stimulation to enhance symptomatic relief of the disorder. Some embodiments also employ a sensing capability to optimize the therapeutic treatment regimen.
Owner:LIVANOVA USA INC
Methods of using cgrp for cardiovascular and renal indications
InactiveUS20090023644A1Minimizing and attenuating deleterious effectHormone peptidesDepsipeptidesDiseaseMortality rate
Owner:VASOGENIX PHARMA
Antibiotic and combinations of antibiotic and symptomatic relief agent formulations
Disclosed herein are antibiotic formulations and combinations of antibiotic and symptomatic relief agent formulations. The combinations are suitable to treat a variety of diseases, including an infection, while treating the symptoms associated with the disease. Also disclosed are methods of treating a disease or an infection and its symptoms, as well as pharmaceutical kits containing such formulations.
Owner:MUTUAL PHARMA CO INC
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Pharmaceutical combinations of cox-2 inhibitors and opiates
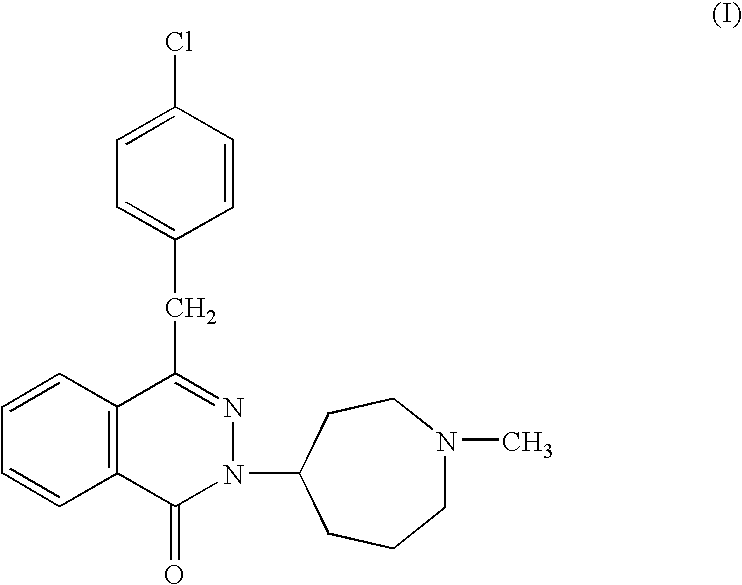
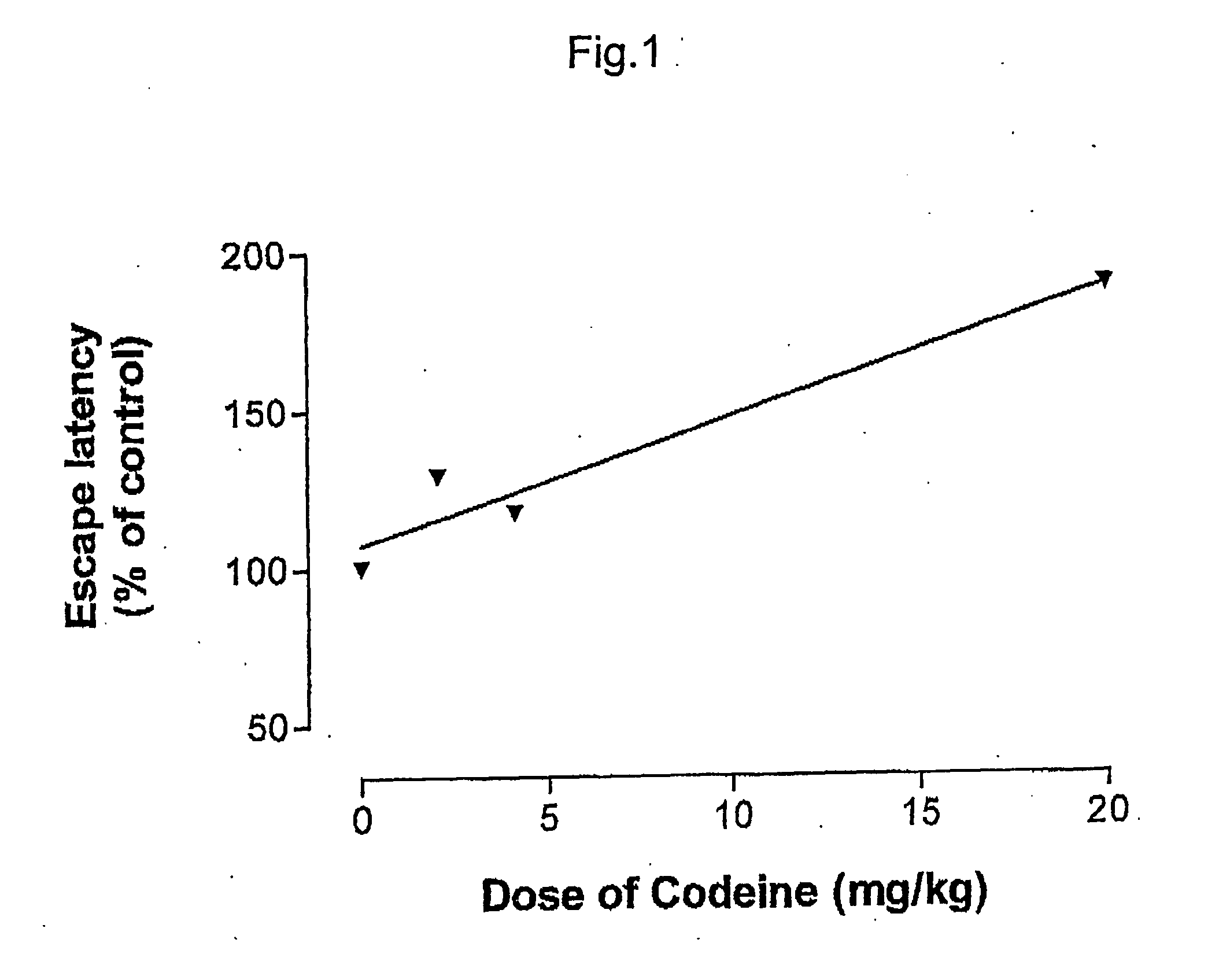
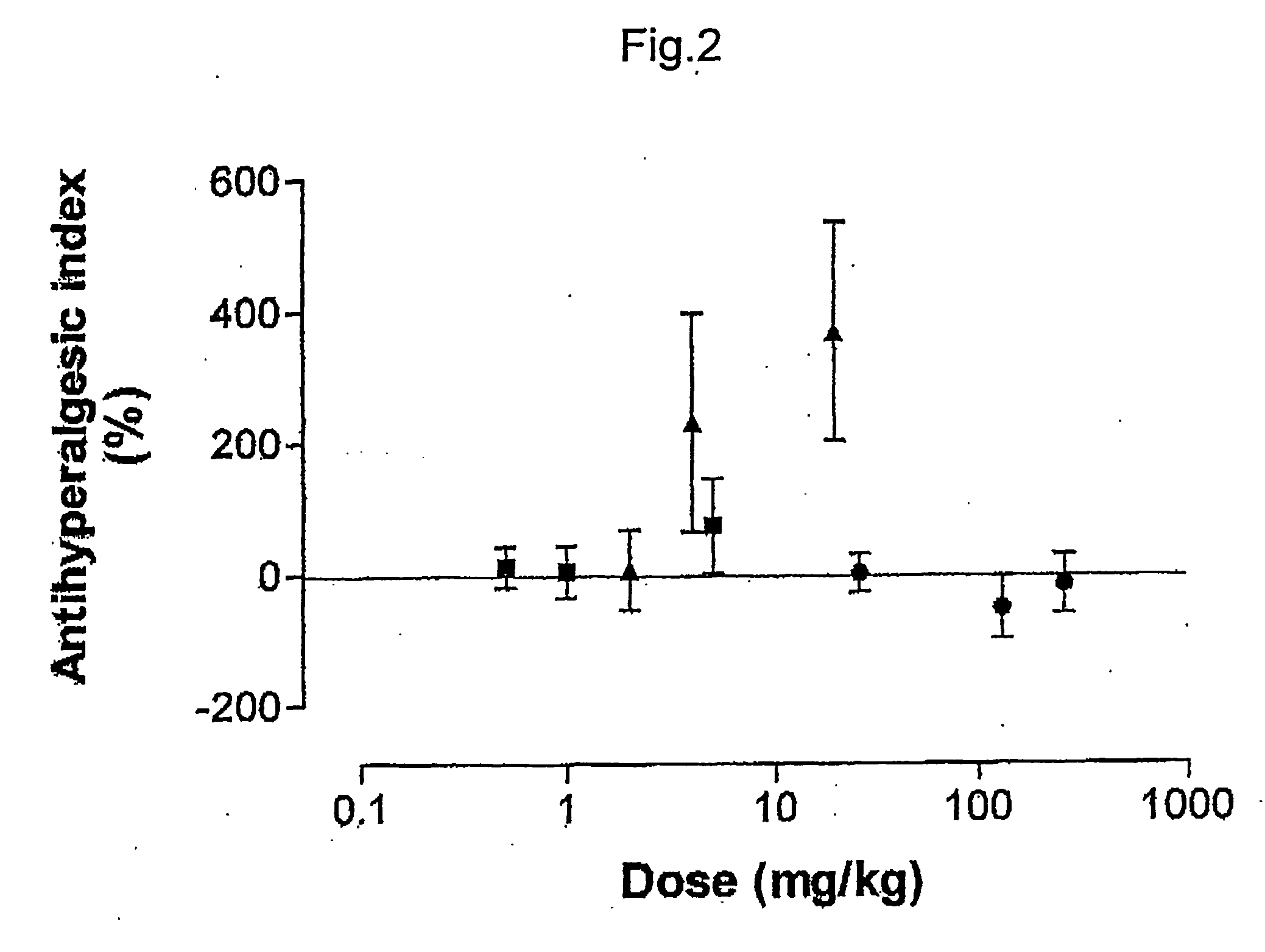
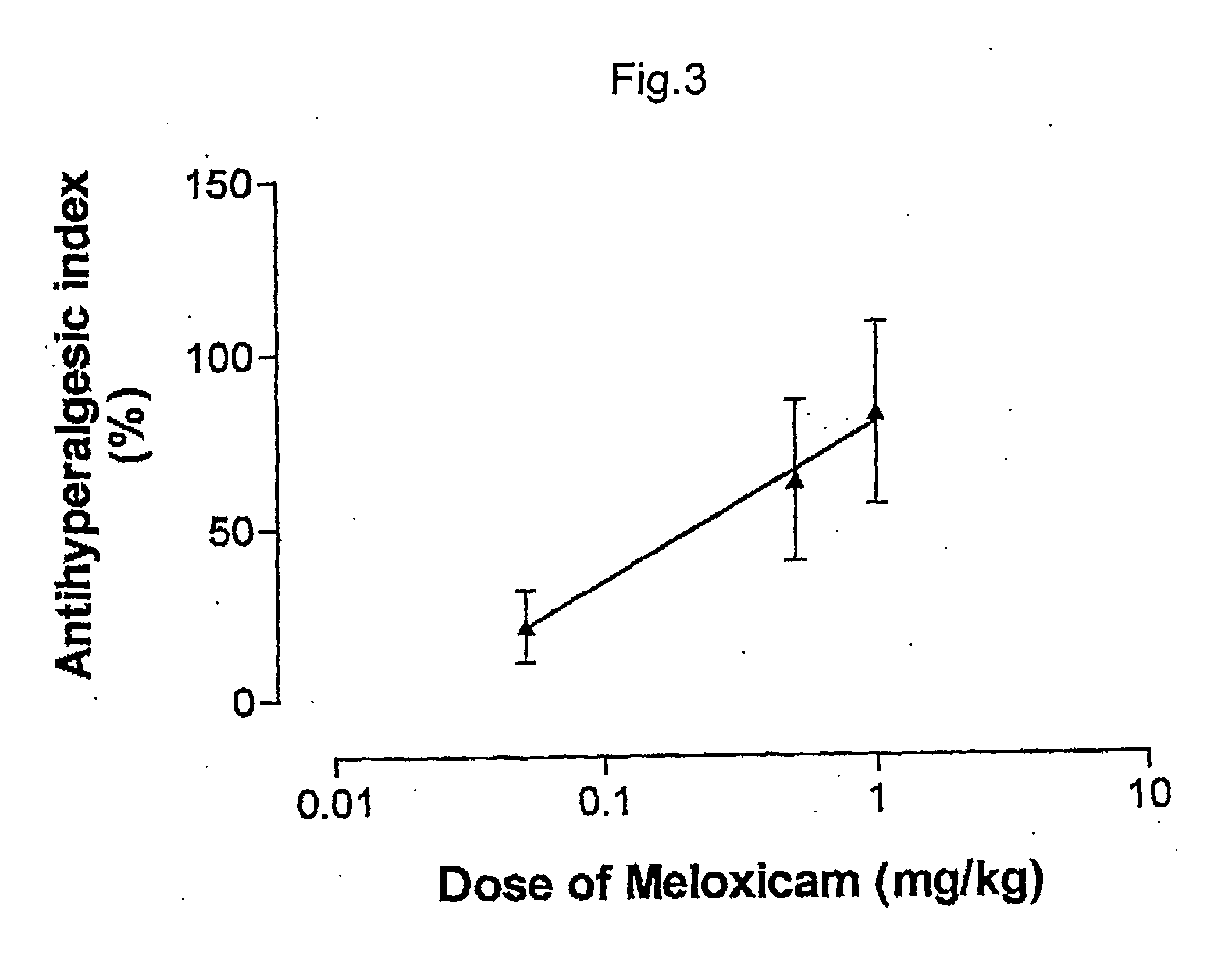
ABSTRACTA pharmaceutical composition comprises a combination of a selective or specific COX 2 inhibitor or a pharmaceutically acceptable salt or derivative thereof and an opiate or a pharmaceutically acceptable salt or derivative thereof, for example a combination of meloxicam and codeine, as active ingredients, and a pharmaceutically acceptable carrier. It may include a centrally-acting cyclo-oxygenase inhibitor such as paracetamol or its pharmaceutically acceptable salts or derivatives. The pharmaceutical compositions are used in methods of providing symptomatic relief or treatment of pain, in an algesic and / or hyperalgesic state, with or without fever, in particular that associated with inflammation such as that associated with trauma, osteoarthritis, rheumatoid arthritis, non-inflammatory myalgia or dysmenorrhoea
Owner:ADCOCK INGRAM LTD
Fermented soy nutritional supplements including mushroom components
Dietary, health, and / or nutritional supplements provide various formulations of mushrooms grown in fermented soy, with or without rice flour, optionally in combination with sweetener(s), curcumin, and various other herbs and spices. Certain embodiments or compositions may be in liquid, beverage, solid, paste or powder forms. In certain embodiments the supplements contain mushroom species grown in fermented soy in the presence of certain bacterial species and optionally rice or rice flour. In some embodiments, other ingredients that may be present include one or more of curcumin, desmethoxycurcumin and bis desmethoxycurcumin or all three curcumins. Certain embodiments may be used to treat or provide symptomatic relief from a variety of maladies ranging from malnutrition to mood related disorders to metabolic support and other more severe conditions as described herein.
Owner:NAIR VIJAYA
Self-contained electronic musculoskeletal stimulation apparatus and method of use
InactiveUS20090216294A1Securely placedOptimizationElectrotherapyEducational modelsSequential stimulationBandage
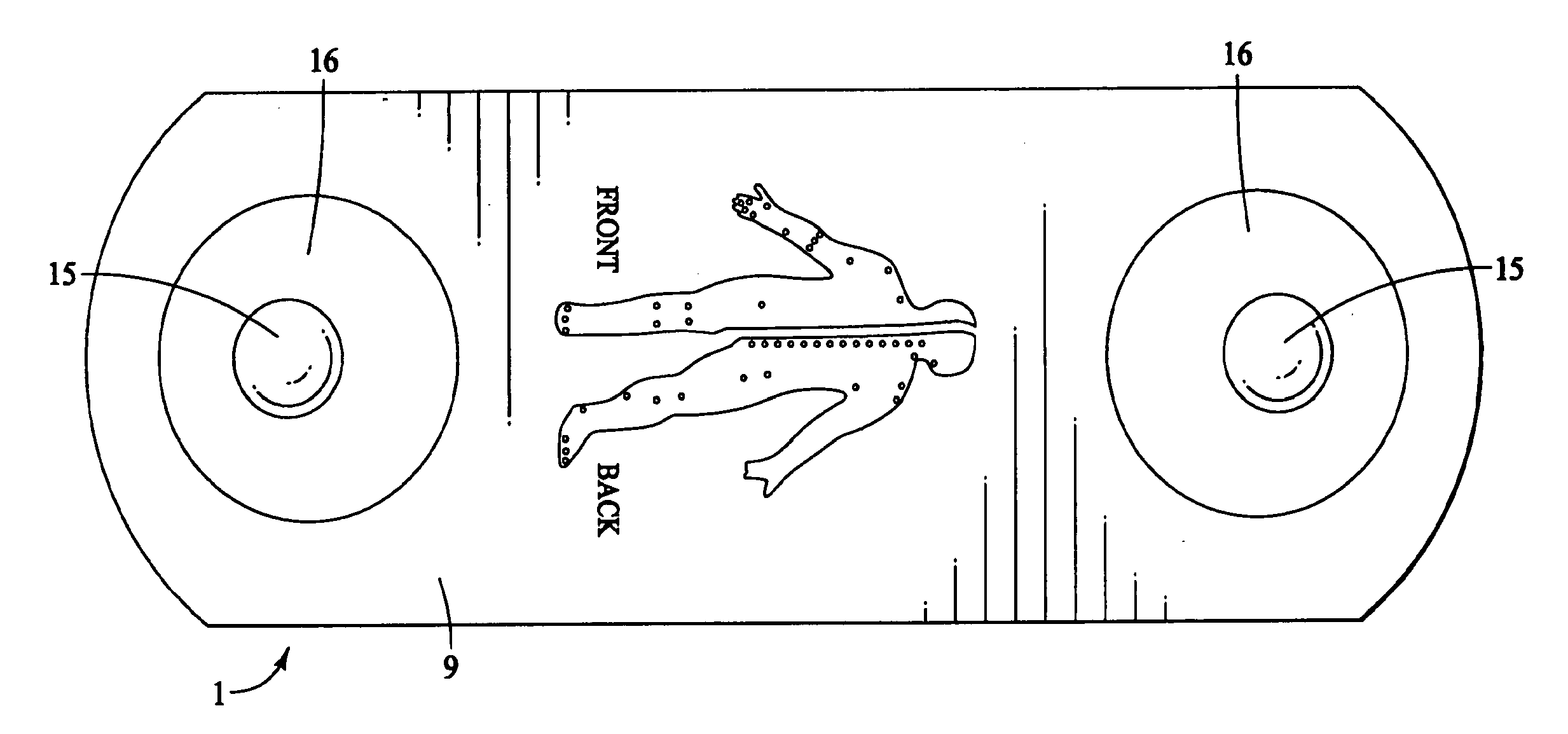
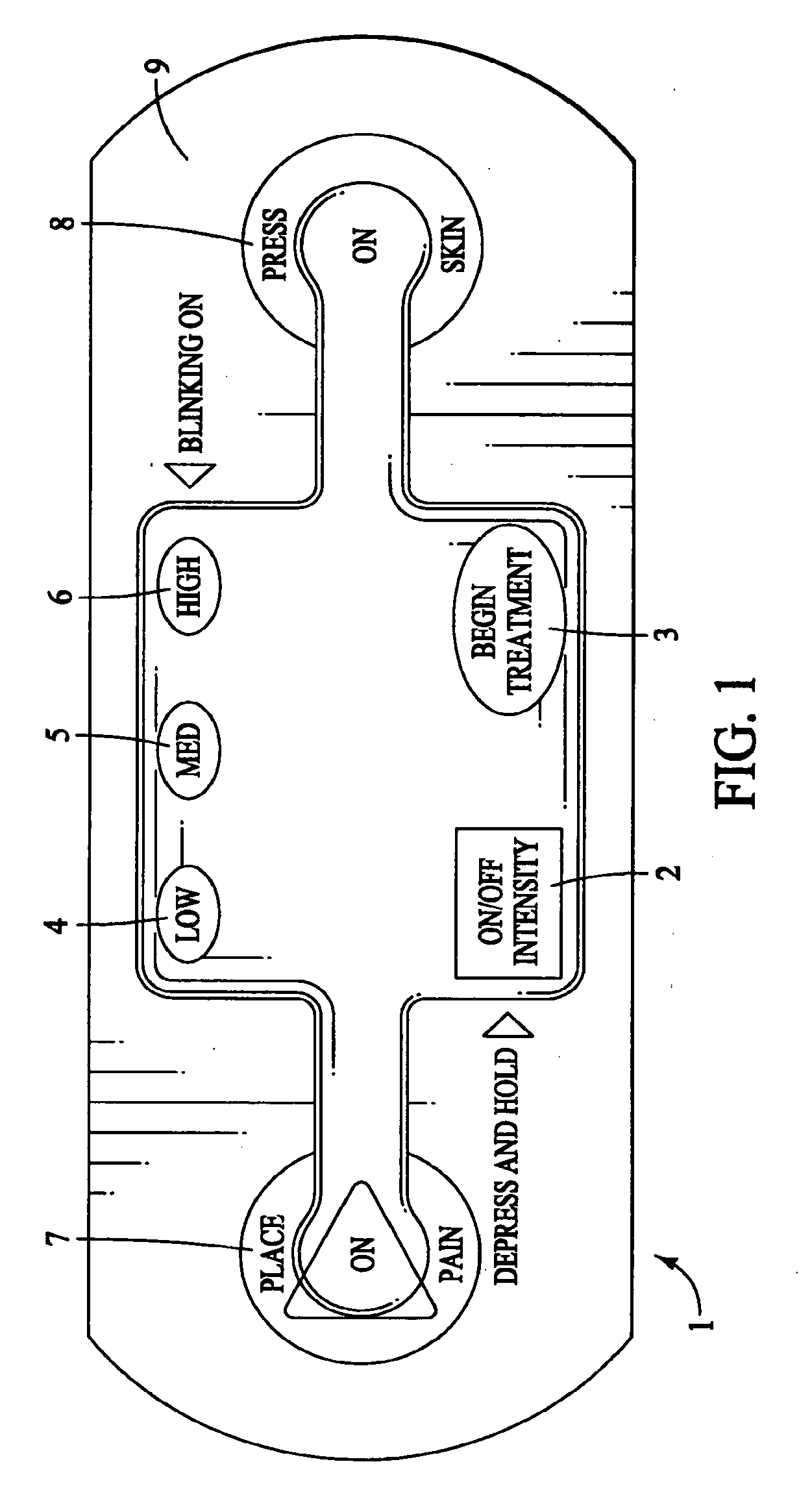
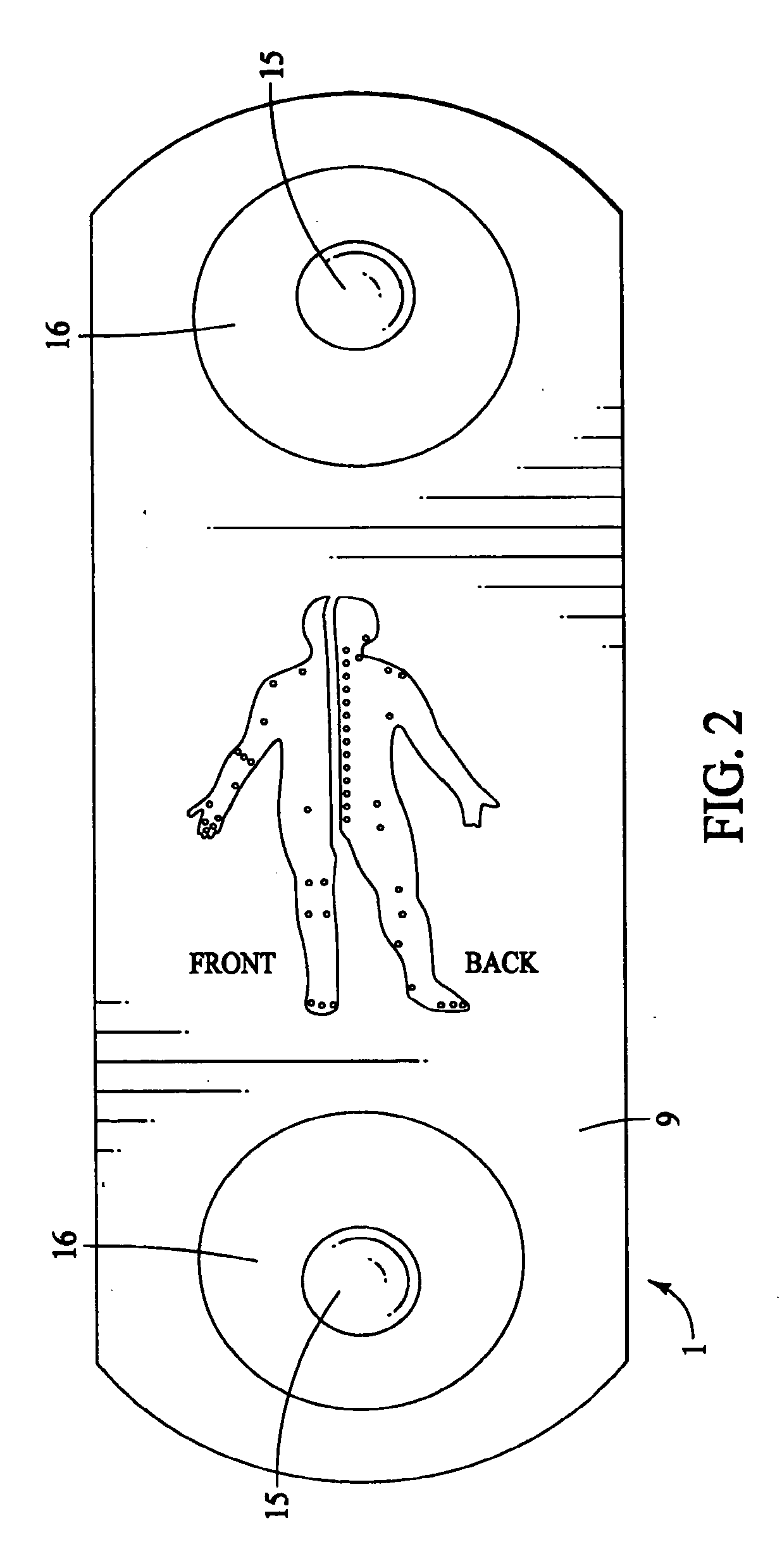
The present invention provides a self-contained electronic musculoskeletal stimulation apparatus that is a battery operated device that applies electronic stimulation to a human with a pre-programmed treatment stimulation protocol to introduce pain relieving electronic stimulation to the body for immediate, symptomatic relief of minor, chronic and acute musculoskeletal aches and pains and mild muscle tension. This invention also provides a method of using a self-contained electronic musculoskeletal stimulation apparatus whereby pain relieving electronic stimulation is applied to the body on predetermined, sequential stimulation points with electronic stimulation being activated at each consecutive stimulation point. Further, this invention provides a method of applying pain relieving electronic stimulation to a body using a self-contained reusable electronic musculoskeletal stimulation bandage with a preprogrammed treatment stimulation protocol. Also provided is a stimulation apparatus that transmits apparatus and patient information by a wireless signal, so the number of times the apparatus was used and intensity level for each use of the apparatus can be determined.
Owner:EWING DONALD P
Method for treating urinary disorders
The present invention relates to a method, preferable an oral method, for treating urinary disorders, such as unstable or overactive bladder, while minimizing the occurrences of dry mouth, dyspepsia and reduced stream of tears. The methods of the present invention comprise orally administering to a mammal, preferably a human, a pharmaceutically effective dose of an antimuscarinic agent, such as tolterodine, when needed, whereby a symptomatic relief of urgency and / or frequency is achieved.
Owner:PHARMACIA CORP
Use of composition comprising Formoterol and Budesonide for prevention or treatment of acute condition of asthma
InactiveCN1305380AOffset deteriorationIncrease inflammationPowder deliveryHydroxy compound active ingredientsChronic asthmaMedicine
Owner:ASTRAZENECA AB
Ophthalmic solutions for glaucoma and conjunctivitis treatment
This invention generally relates to an ophthalmic solution comprising cannabinoids for the treatment of glaucoma. Also disclosed is an ophthalmic solution comprising cannabinoids for symptomatic relief of conjunctival inflammation. Cannabinoids are selected to achieve the specific purpose of the respective ophthalmic solution.
Owner:APIRX PHARMA USA LLC
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders.
Owner:MEDA PHARMA INC
Method of treatment or prophylaxis
InactiveUS7795275B2Good curative effectBiocideCompound screeningMechanical HyperalgesiaNeuropathic pain
The present invention is directed to methods and agents that are useful in the prevention and amelioration of signs and symptoms associated with neuropathic conditions. More particularly, the present invention discloses the use of angiotensin II receptor 2 (AT2 receptor) antagonists for the treatment, prophylaxis, reversal and / or symptomatic relief of neuropathic pain, including mechanical hyperalgesia, thermal or mechanical allodynia, diabetic pain and entrapment pain, in vertebrate animals and particularly in human subjects. The AT2 receptor antagonists may be provided alone or in combination with other compounds such as those that are useful in the control of neuropathic conditions.
Owner:NOVARTIS AG
Antihistaminic/Decongestant/Anticholinergic Compositions and Methods of Use
Compositions comprising essentially of methscopolamine in combination with tannate compounds which are effective when administered orally for the symptomatic relief of symptoms associated with upper respiratory tract conditions such as the common cold, sinusitis, allergic rhinitis, and other upper respiratory tract conditions.
Owner:REVOGENEX IRELAND
Diphenhydramine tannate solid dose compositions and methods of use
Pharmaceutical compositions consisting of diphenhydramine tannate in solid dosage form which are effective when administered for the symptomatic relief of sneezing, itchy, watery eyes, itchy nose or throat and runny nose due to hay fever (allergic rhinitis) or other respiratory allergies are disclosed.
Owner:KIEL LAB
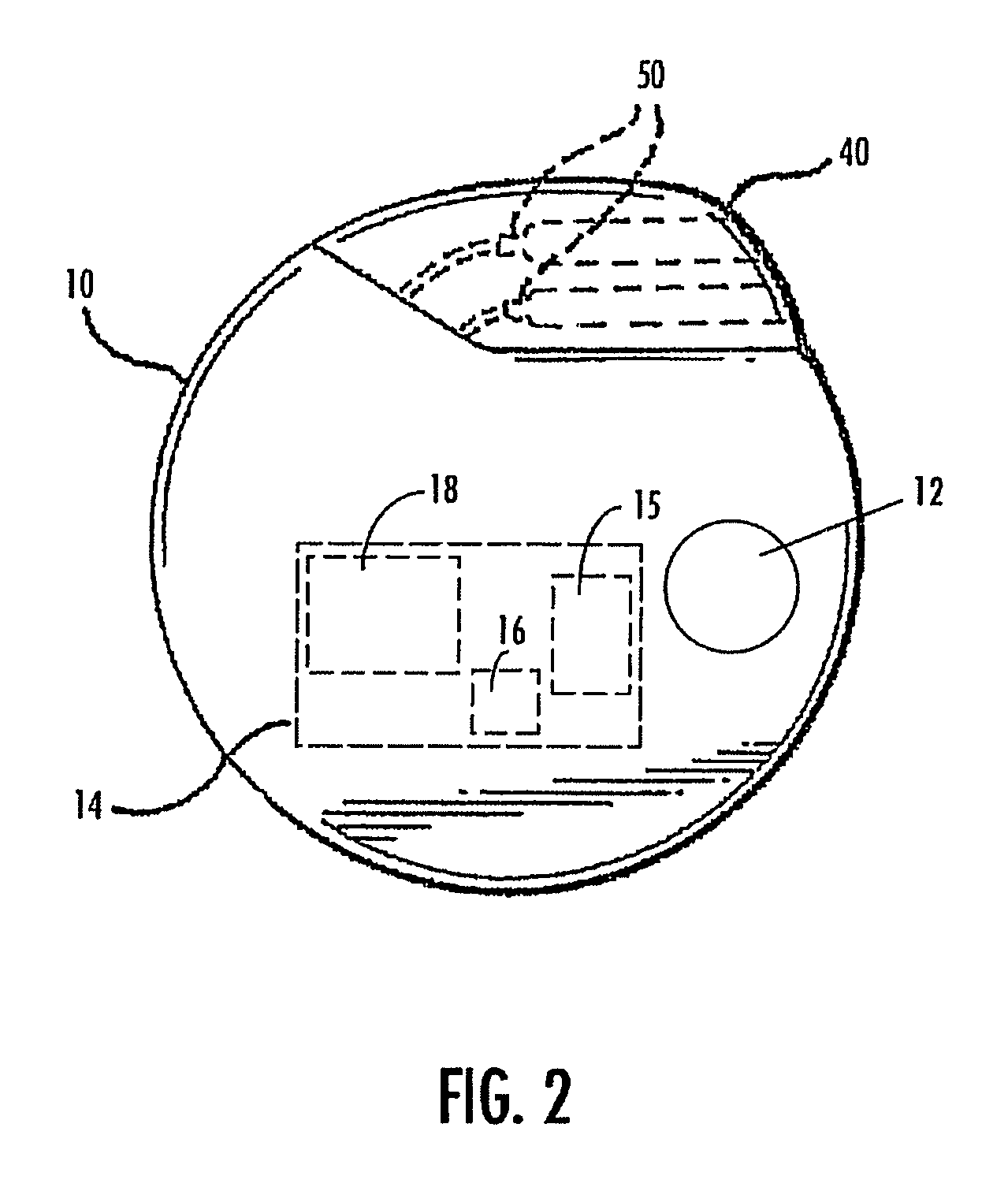
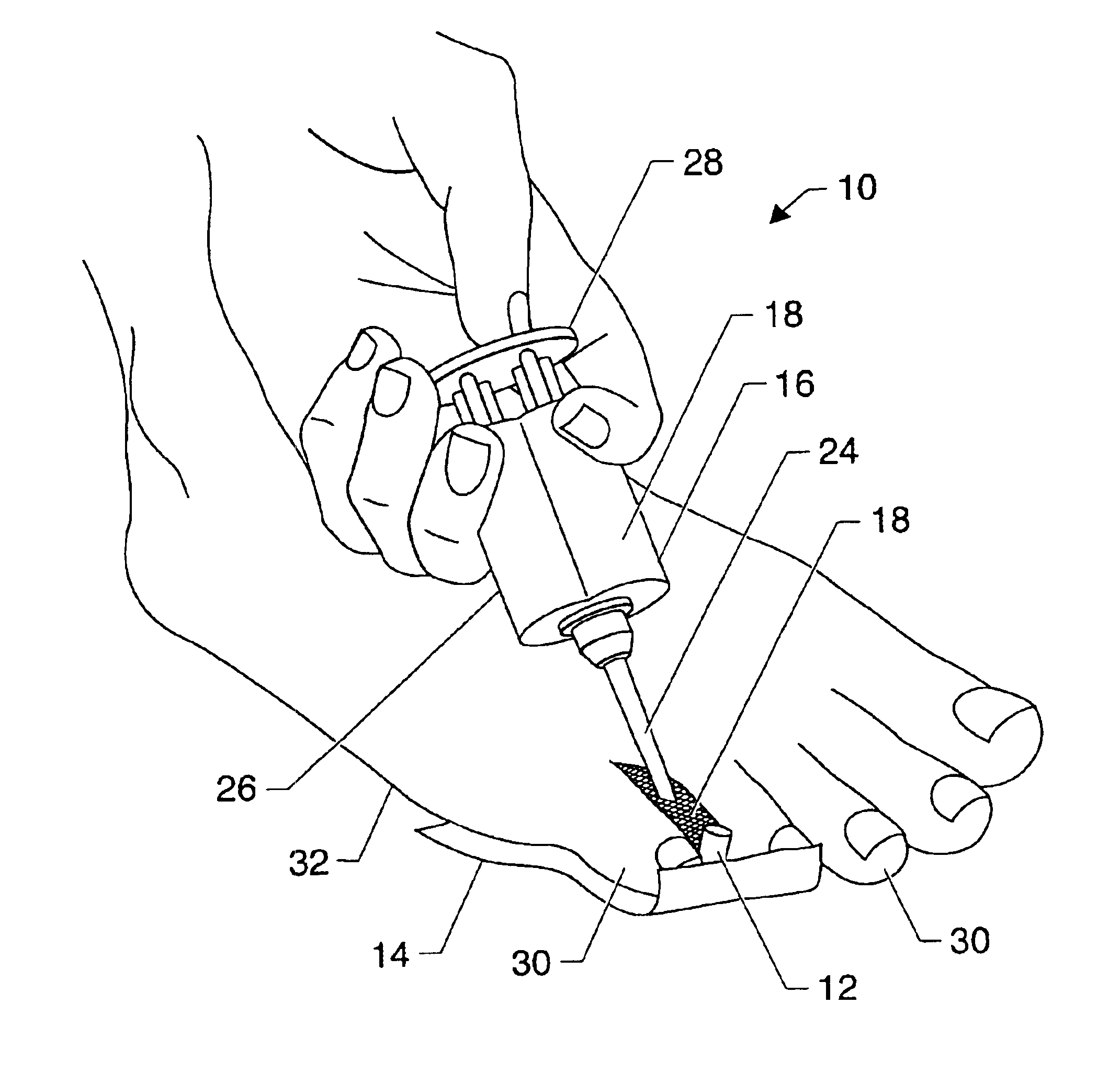
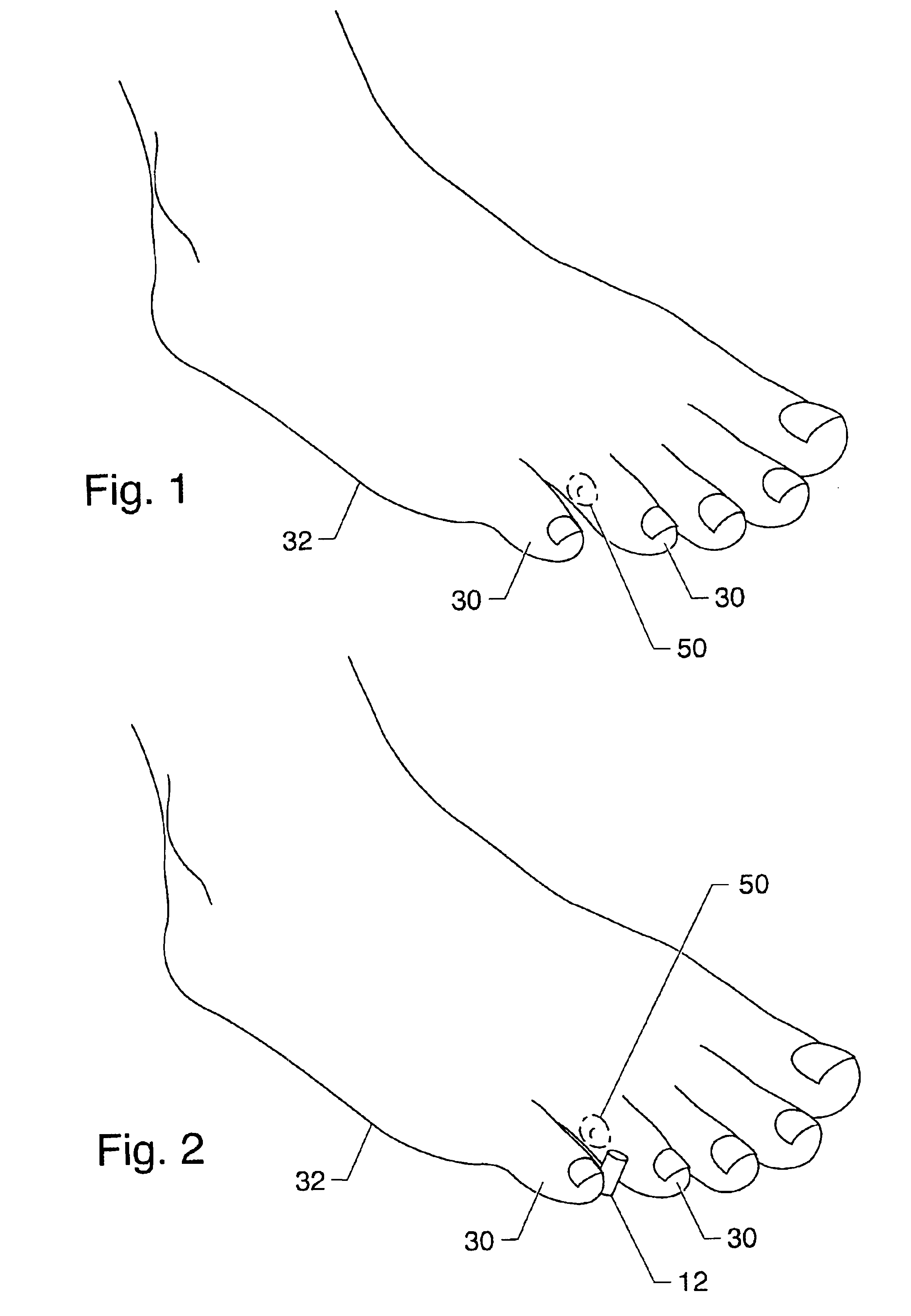
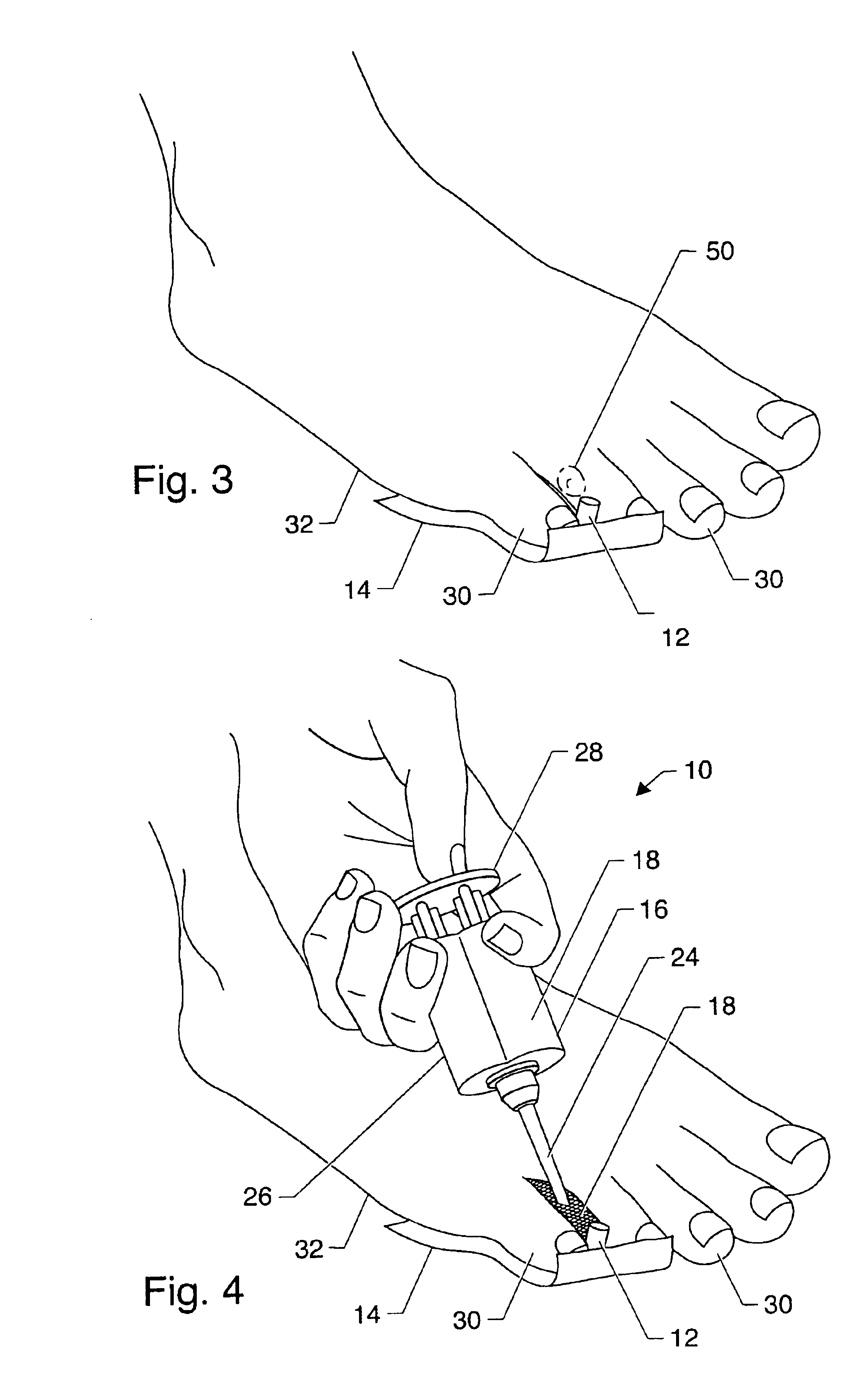
Symptomatic relief for soft corns
InactiveUS6881196B2Increasing putative surface areaRelief the painDiagnosticsSurgeryBiomedical engineeringHigh definition
A system, method and device for the symptomatic relief for soft corns by injecting a medical grade molding substance between the toes between which a corn is located, forming a molded-in-place, custom-shaped high definition mold, unique to the individual's physical conformation and which reduces friction and eliminates pressure points on the corn.
Owner:CRUNKLETON JAMES A
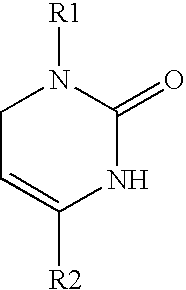
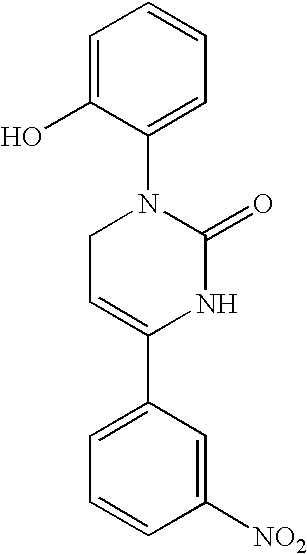
1,2,3,6-Tetrahydropyrimidine-2-one compositions, articles and therapeutic methods for upper airway breathing disorders
A therapeutic composition is provided that comprises a 1-R1-phenyl, 4-R2-phenyl substituted 1,2,3,6-tetrahydropyrimidine-2-one cold receptor agonist in a therapeutically effective amount. The cold receptor agonist may be represented by the general formula 1-[R1-phenyl]-4-[R2-phenyl]-1,2,3,6 -tetrahydropyrimidine-2-one wherein: R1 is -hydroxy, -chloro, -fluoro, -alkyl, -acetoxy, -trifluoromethyl; and R2 is -nitro, -chloro, -fluoro, -alkyl, -trifluoromethyl. Therapeutic compositions of the invention when formulated for delivery to the mucous membranes of the nose and throat alleviate the sensations of airway obstruction and provide symptomatic relief of upper airway breathing disorders.
Owner:CRAGMONT PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com