Patents
Literature
114 results about "Diphenhydramine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
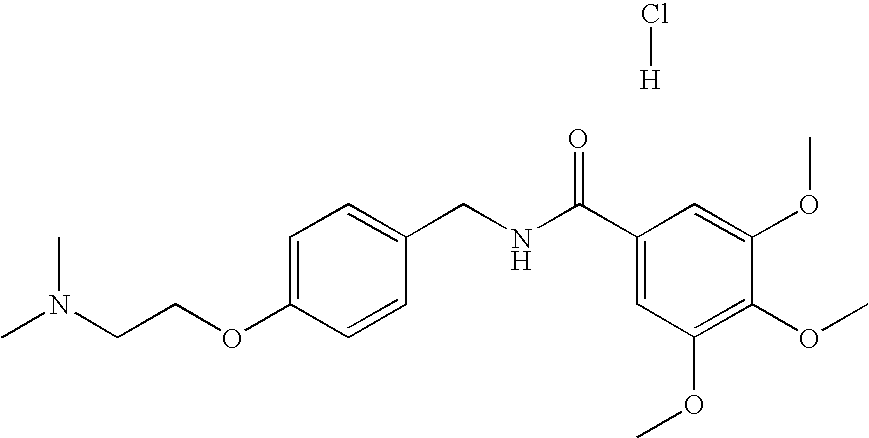
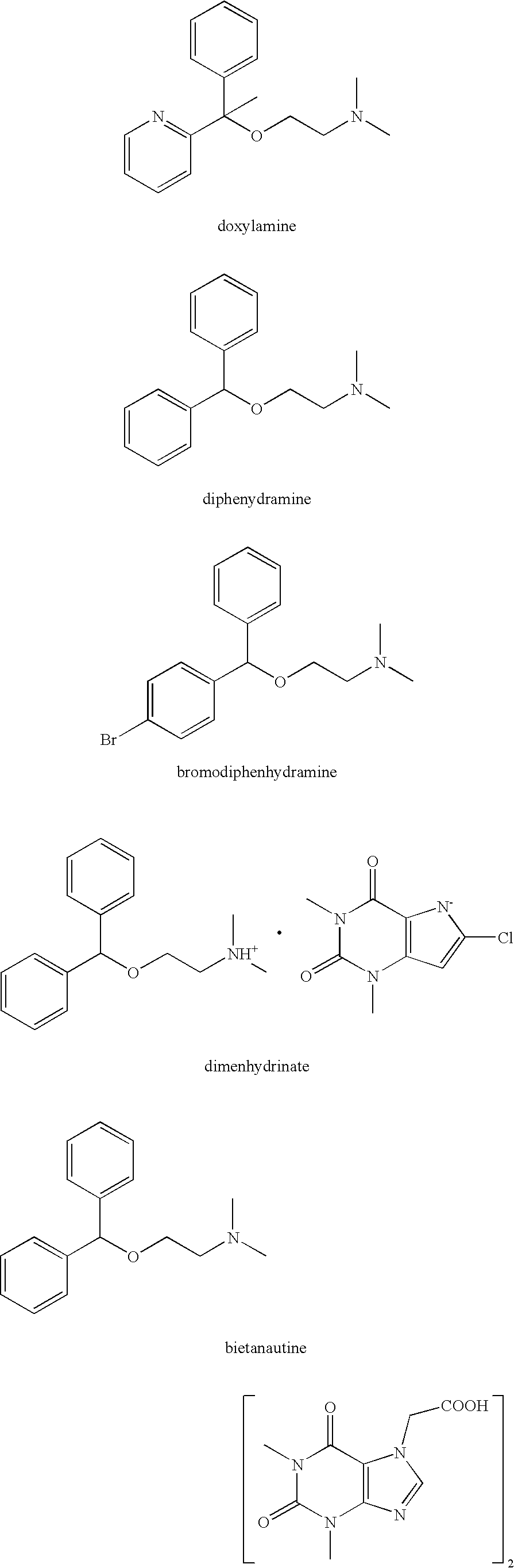
Diphenhydramine is an antihistamine used to relieve symptoms of allergy, hay fever, and the common cold.
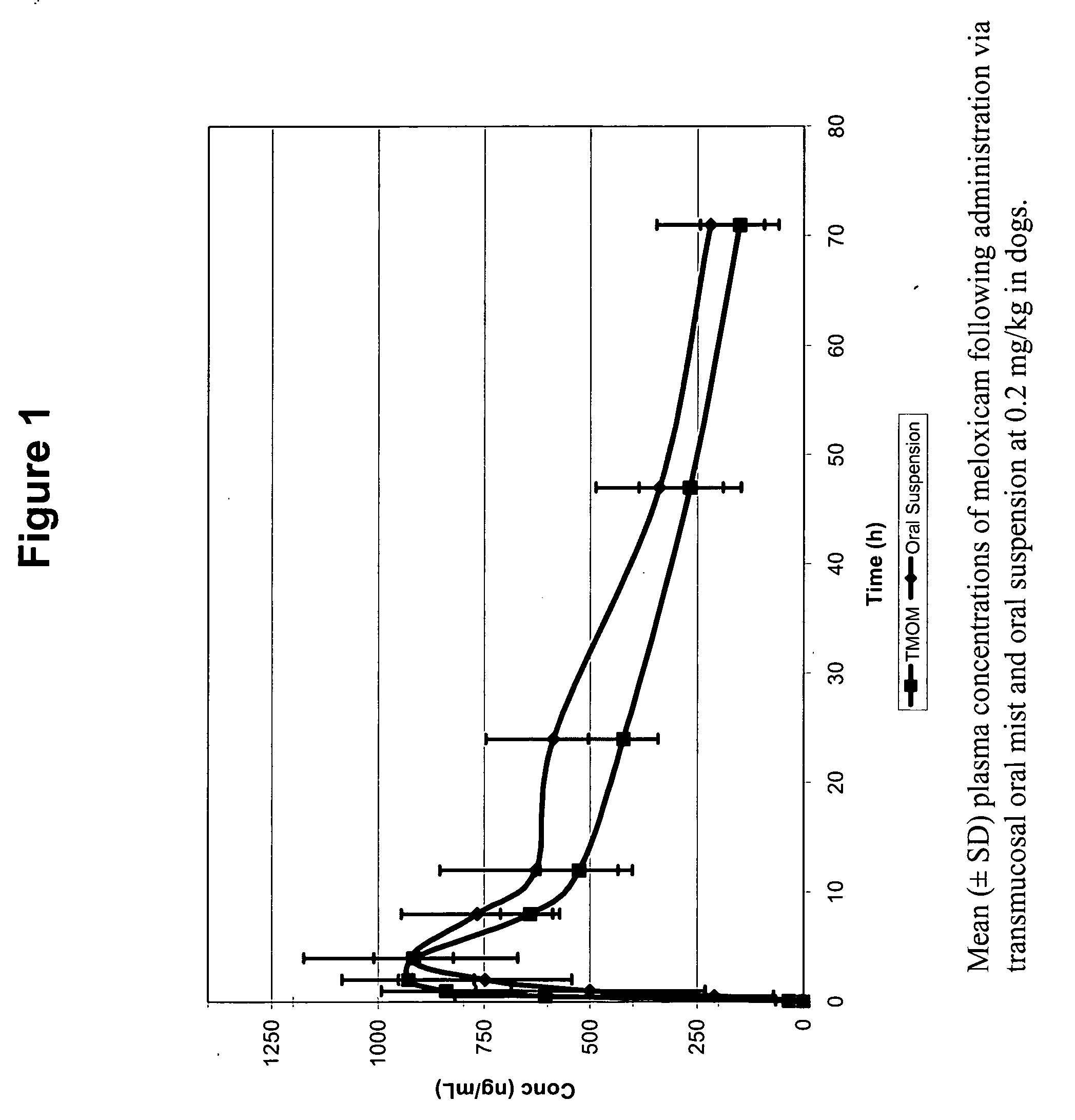
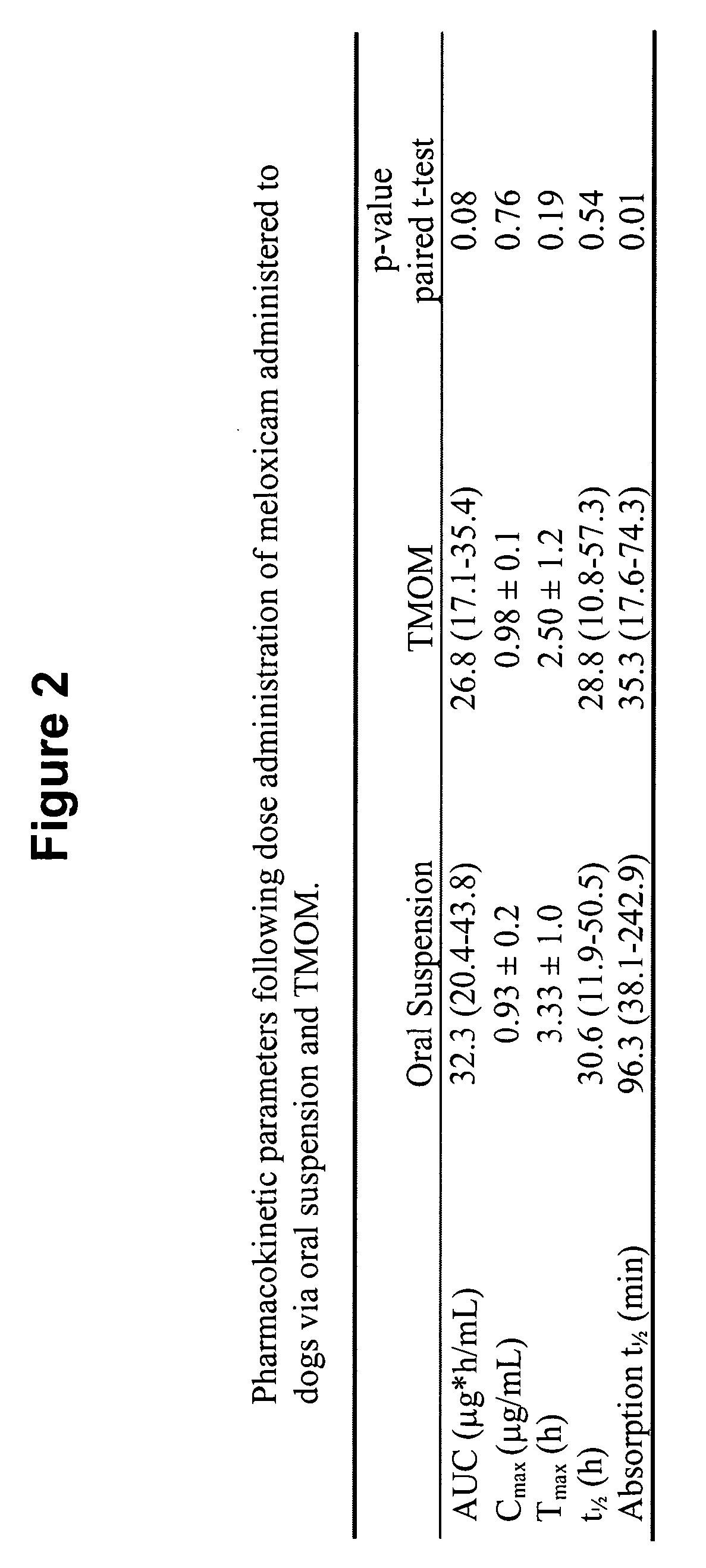
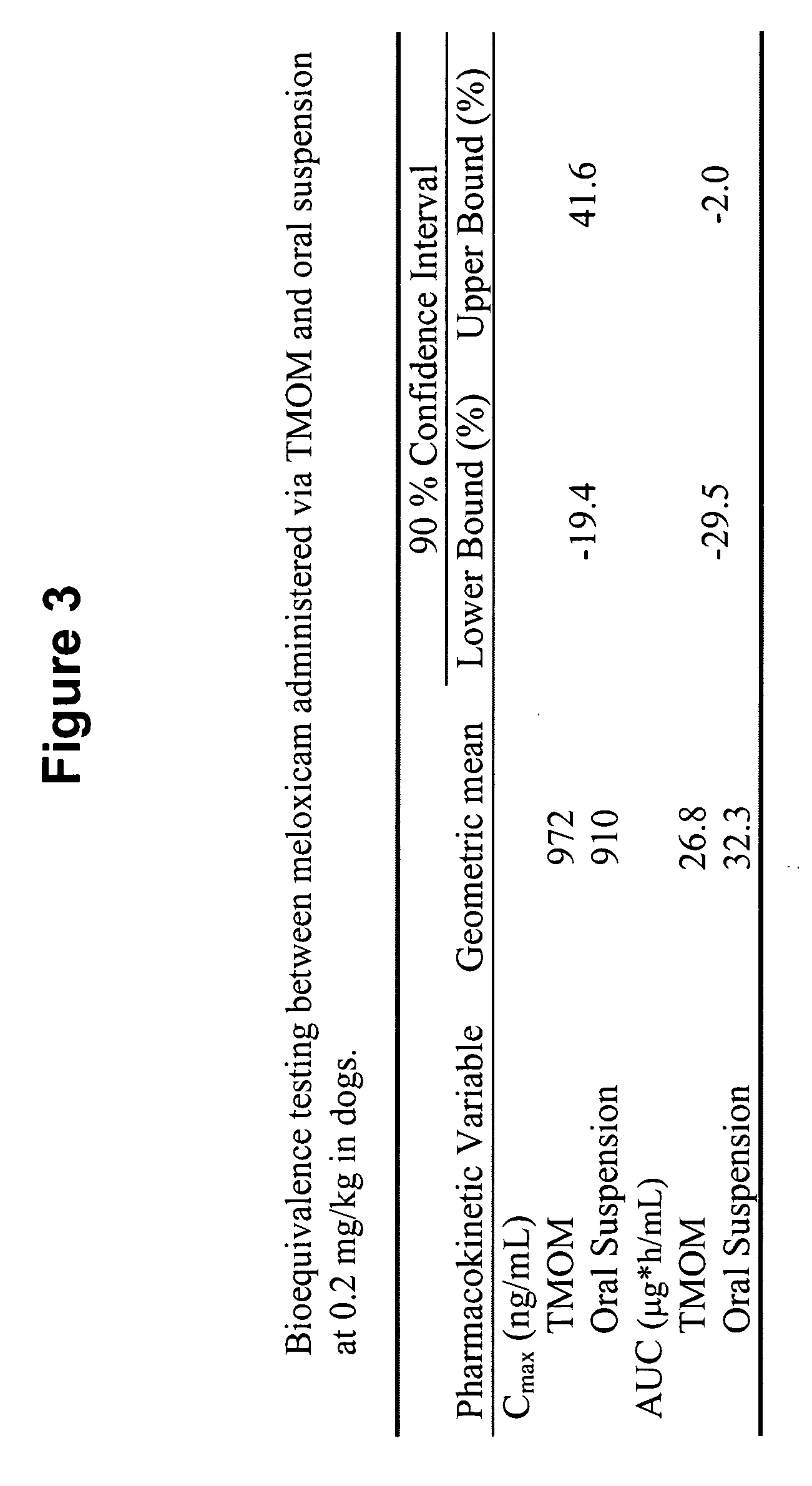
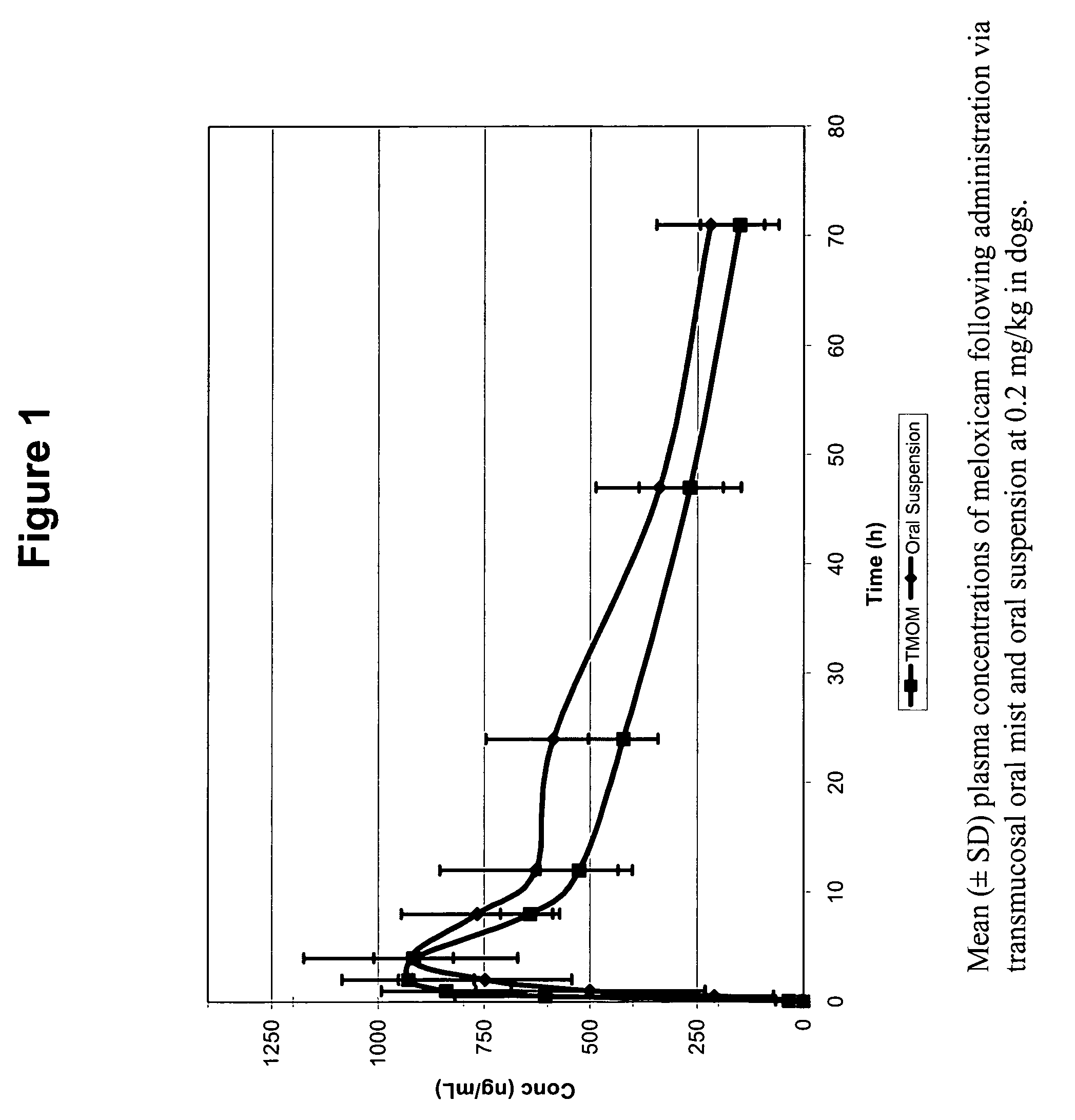
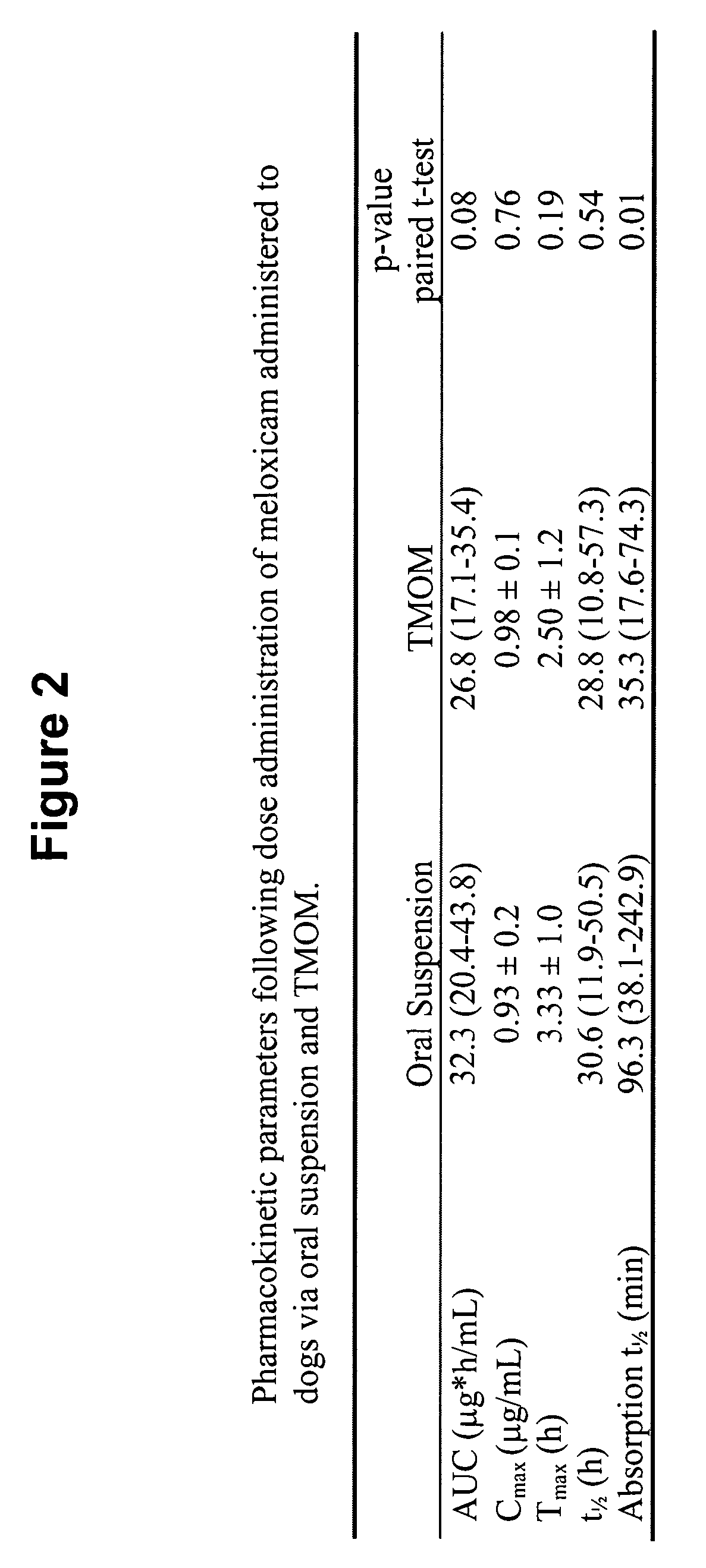
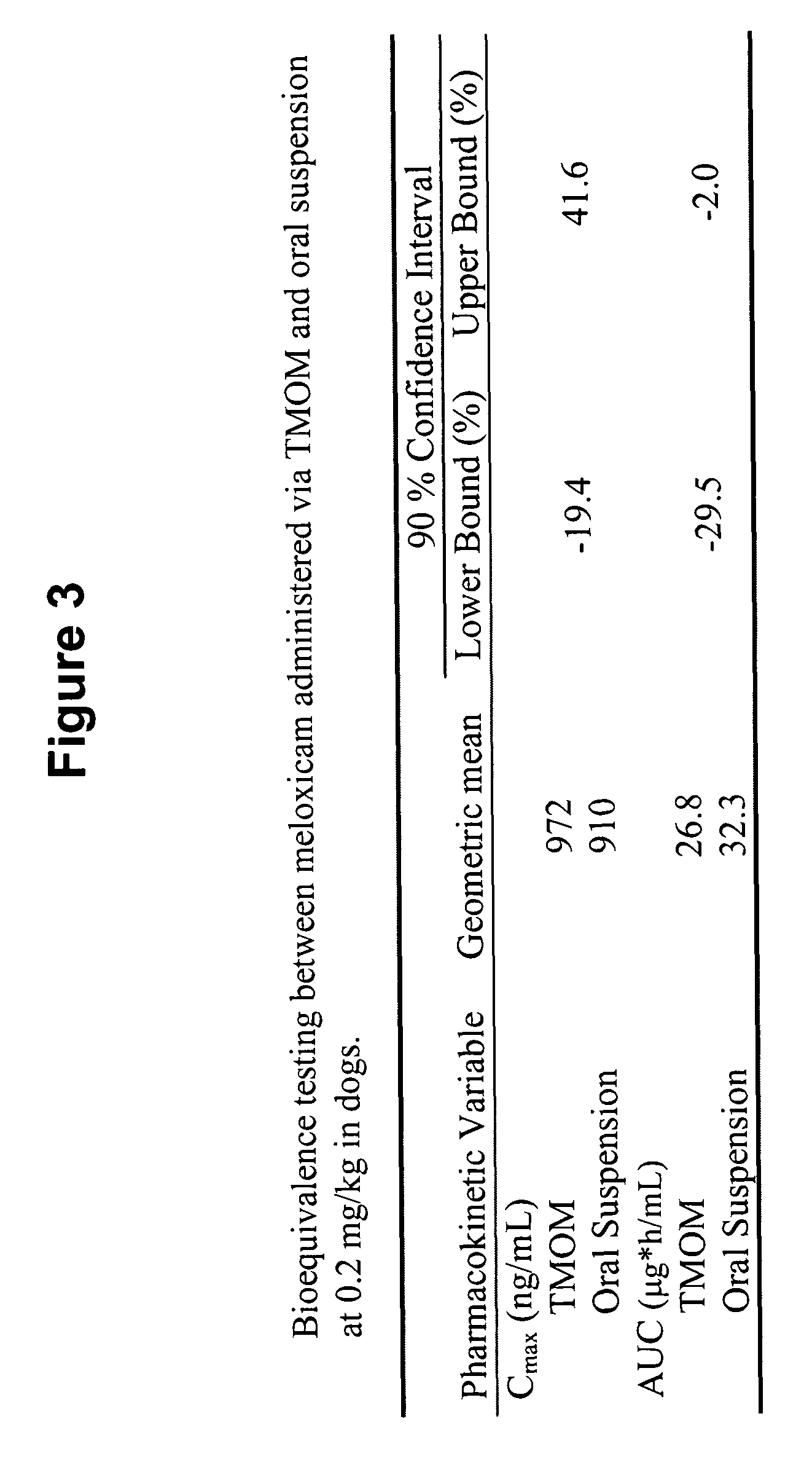
Transmucosal administration of drug compositions for treating and preventing disorders in animals
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
Methods and composition for treatment of migraine and symptoms thereof
Compositions, methods and kits are provided for the treatment of migraines. The compositions, methods and kits include an effective dose of trimethobenzamide and an ethanolamine antihistamine that, when administered to an individual suffering from migraine headaches, will alleviate symptoms associated with the migraine headaches. Compositions, methods, and kits for the treatment of migraines include pharmaceutical compositions of trimethobenzamide and diphenhydramine.
Owner:SALEHANI FOAD
Orally disintegrating tablets comprising diphenhydramine
The compositions of the present invention comprise a therapeutically effective amount of particles consisting of diphenhydramine or pharmaceutically acceptable salts thereof, optionally in combination with another drug such as pseudoephedrine, or phenylephrine and hydrocodone, in combination with rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol and / or a saccharide. These compositions are useful in treating the symptoms of one or more diseases or conditions in which diphenhydramine (alone or in combination with one or two other drugs) is a therapeutically effective, e.g. allergic rhinitis, sinusitis, upper respiratory tract infections, motion sickness, Parkinson's disease, insomnia, the common cold, and nighttime pain management, particularly for subjects or patients with dysphagia, and people ‘on the move’.
Owner:ADARE PHARM INC
Psycho-pharmaceuticals
InactiveUS20110034562A1Improve cognitive abilityRelieve symptomsOrganic active ingredientsBiocidePsychoactive drugAgonist
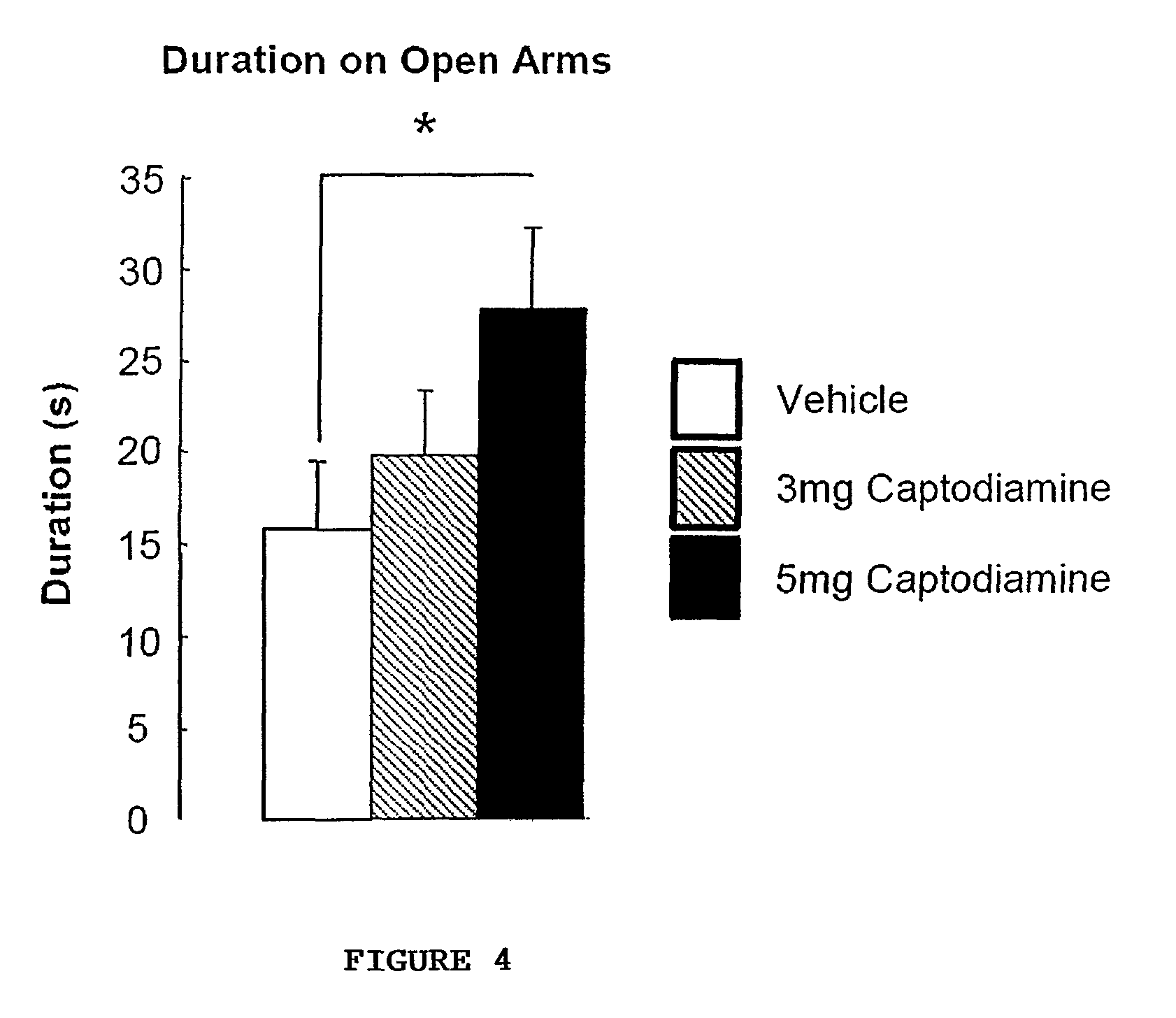
The invention provides a selective Sigma 1 or Dopamine D3 receptor agonist or a 5HT2c receptor ligand for use in the treatment of symptoms of anxiety and / or depression associated with an affective disorder and / or symptoms associated with cognitive impairment disorder. Particularly useful are diphenhydramine derivatives of Formula (I) and particularly (2-[(4-butylsulfanylphenyl)-phenyl-methyl]sulfanyl-N,N-dimethyl-ethanamin) (Captodiamine).
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN
Synergistic proteoglycan compositions for inflammatory conditions
Compositions with synergistic anti-inflammatory effect in inflammatory diseases resulting from activation and consequent degranulation of mast cell and followed by secretion of inflammatory biomolecules from the activated mast cells, composed of a heavily sulfated, non-bovine proteoglycan such as chondroitin sulfate C and one or more of a hexosamine sulfate such as D-glucosamine sulfate, a flavone such as quercetin, a special organic extra virgin kernel seed olive oil, S-adenosylmethionine and diphenhydramine.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
Transmucosal administration of meloxicam compositions for treating and preventing disorders in non-human domesticated animals
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
Taste masking spill-resistant formulation
InactiveUS20050143471A1Suppress bitternessIncrease sweetnessOrganic active ingredientsBiocidePolyethylene glycolFood flavor
The invention relates to a spill-resistant pharmaceutical composition, comprising a spill-resistant formulation with taste masking concentrations of polyethylene glycol (PEG) and diphenhydramine, which is less bitter, sweeter and has better overall flavor than current pharmaceutical compositions, while maintaining advantageous spill-resistant properties.
Owner:TARO PHARMA US INC
Optical activity di(heteto)aryl methanol and asymmetric synthesis method thereof
InactiveCN106831550AEasy to synthesizeLow priceOrganic compound preparationHydroxy compound preparationIridiumSynthesis methods
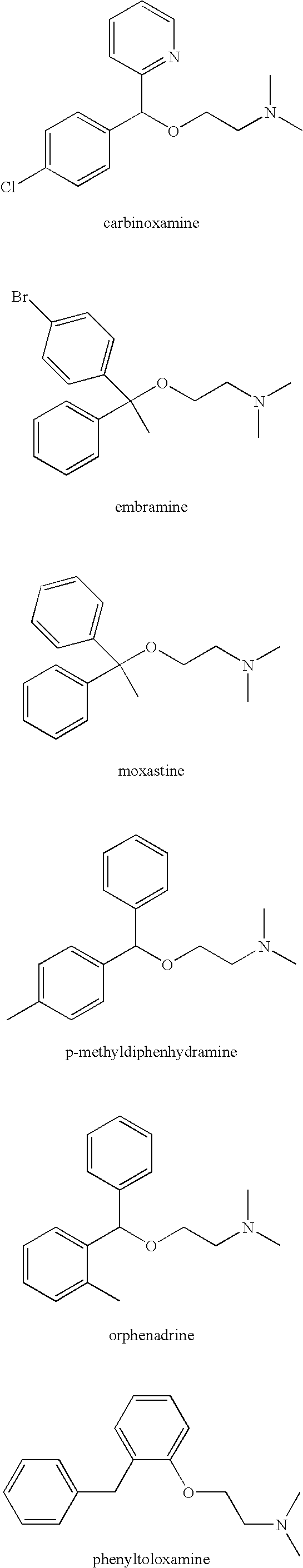
The invention relates to optical activity di(heteto)aryl methanol and an asymmetric synthesis method thereof. The method comprises the following steps: taking mono-sulfonyl chiral diamine and a complex of metals ruthenium, rhodium and iridium as catalysts, taking sodium formate or formic acid / triethylamine or isopropanol as a hydrogen source, carrying out an asymmetric transfer hydrogenation reaction of di(heteto)aryl ketone first, thereby obtaining the optical activity di(heteto)aryl methanol. The method is mild in reaction conditions, easy and convenient to operate, readily available in raw materials, wide in substrate application range and high in enantioselectivity and has important application prospects in the aspects of synthesis of antihistamine chiral drugs such as diphenhydramine, methyldiphenhydramine, carbinoxamine and bepotastine.
Owner:CHINA THREE GORGES UNIV
Composition containing albendazole for agricultural control of plant root-knot nematode
InactiveCN102283226ADoes not increase the risk of insecurityThe dosage space is largeBiocideDisinfectantsTriazofosBacillus thuringiensis
The invention discloses an agricultural composition containing albendazole for preventing and controlling plant root-knot nematodes. It is a solid preparation or a liquid preparation, and its components include 1% to 49.7% of albendazole and 0.3% to 0.3% of auxiliary active ingredients. 49%, functional additives 1% to 20%, solid preparations include the rest of the filler, liquid preparations include the rest of the solvent, and the auxiliary active ingredients are selected from abamectin, emamectin benzyl ester One or two of salt, thiazophos, triazophos, chlorazophos, phoxim, Bacillus thuringiensis, and diphenhydramine. The composition of the invention has the advantages of obvious insecticidal effect, high activity, good safety, broad spectrum and high efficiency, and the like.
Owner:湖南大方农化股份有限公司
Psycho-pharmaceuticals
The invention provides a selective Sigma 1 or Dopamine D3 receptor agonist or a 5HT2c receptor ligand for use in the treatment of symptoms of anxiety and / or depression associated with an affective disorder and / or symptoms associated with cognitive impairment disorder. Particularly useful are diphenhydramine derivatives of Formula (I) and particularly (2-[(4-butylsulfanylphenyl)-phenyl-methyl]sulfanyl-N,N-dimethyl-ethanamin) (Captodiamine).
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN
Lozenge for delivery of dextromethorphan
InactiveUS20050238695A1Powder deliveryOrganic active ingredientsDextromethorphan+diphenhydramineEthylmorphine
The present invention provides an organoleptically pleasing lozenge containing an antitussive selected from the group consisting of dextromethorphan, diphenhydramine, caramiphen, carbapentane, ethylmorphine, noscapine, codeine, and mixtures thereof, complexed with an ion exchange resin wherein the particle size of the resin is 38 μm or less in diameter. Also provided is a process for producing the lozenge and methods of administering the lozenge.
Owner:MCNEIL PPC INC +1
Paddy rice rice blast-resistant selenium-rich yield-increasing agent and preparation method thereof
InactiveCN103641592AGood for balanced growthIncrease productionFertilizer mixturesSodium metasilicateBetaine
The invention discloses a paddy rice rice blast-resistant selenium-rich yield-increasing agent and a preparation method thereof. The paddy rice rice blast-resistant selenium-rich yield-increasing agent comprises the following raw materials by weight: 30-50 parts of ammonium biphosphate; 45-65 parts of monopotassium phosphate; 6-12 parts of boron fertilizer; 8-12 parts of selenium fertilizer; 3-6 parts of humic acid; 3-6 parts of lithium sulfate monohydrate; 10-20 parts of sodium metasilicate pentahydrate; 0.3-1.2 parts of diclofenac sodium; 0.1-0.3 parts of glycyrrhizic acid; 1-3 parts of p-chloro-m-xylenol; 0.5-1.5 parts of flucytosine; 6-9 parts of betaine; 0.2-0.4 parts of diphenhydramine; and 0.1-0.4 parts of cromolyn sodium. The paddy rice rice blast-resistant selenium-rich yield-increasing agent of the invention is strong in pertinency, solves various prominent problems in paddy rice plantation, and has various effects of rice blast resistance, seed setting rate increasing, thousand seed weight increasing, yield increasing, polished rice rate increasing, rice protein content increasing, rice selenium content increasing, and the like.
Owner:新疆久业富硒农业科技开发有限公司
Pharmaceutical composition
InactiveUS20100015248A1Reduce severityShorten the durationBiocideHydroxy compound active ingredientsVitamin CMedicine
A pharmaceutical composition for the treatment of colds and influenza. The pharmaceutical composition is a mixture of: acetaminophen, diphenhydramine, dextromethorphan, arabinogalactan, vitamin C, zinc, olive leaf extract, resveratrol and elderberry extract.
Owner:VANTERPOOL ELAINE A
Externally applied compound medicinal tincture for treating intractable tinea and dermatitis and method for preparing same
InactiveCN1965971AQuick resultsShort course of treatmentAmphibian material medical ingredientsOrganic active ingredientsDiseasePinellia
The invention relates to an externally-used medicinal preparation for treating tinea and dermitis and process for its preparation, wherein the raw materials include hibiscus bark, Sichuan aconite root, Kusnezoff monkshood root, jack-in-the-pulpit tuber, pinellia tuber, toad skin, snake slough, asaryl, sulfur, orpiment, white alum, zanthoxylum piperitum, flavescent sophora root, corktree bark, Chinese mugwort leafmulberry leaf, toasted garlic, cow-bezoar, musk ketone, oxytetracycline powder, diphenhydramine powder, chlorpheniramine and vinegar.
Owner:闫玉国
Hyperostosis cold compress paste and preparation method thereof
InactiveCN107296852AGuaranteed respiratory metabolismIncrease contact surfaceHydroxy compound active ingredientsSkeletal disorderDihydroxyaluminum aminoacetateIrritation
The invention discloses a hyperostosis cold compress paste which comprises a backing layer, a gel drug layer and an anti-bonding layer, wherein the gel drug layer contains the following raw materials: sodium polyacrylate, carbomer, glycerol, dihydroxyaluminum aminoacetate, tartaric acid, purified water, medical borneol, camphor, diclofenac sodium, menthol, atropa belladonna fluid extract, clove oil, propylene glycol, glycerol, medical azone, diphenhydramine, oleum menthae and capsaicin. The invention also discloses a preparation method for the hyperostosis cold compress paste. The method comprises the following steps: preparing a gel drug and then coating the backing layer with the gel drug by machine so as to form a gel drug layer; covering the gel drug layer with the anti-bonding layer; cutting, puncturing and slicing, thereby acquiring the hyperostosis cold compress paste. No organic solvent is added into the bruise cold compress paste disclosed by the invention; the cold compress paste can be directly coated and does not need to be heated; the technology is simple and no chemical residue exists; the cold compress paste is free from irritability and stimulation, is high in drug loading capacity and moisture retention, has high compatibility with skin, is anti-ageing, has excellent weather fastness and lasting viscidity and can be repeatedly uncovered and pasted.
Owner:四川利佰生物科技有限公司
Diphenhydramine tannate solid dose compositions and methods of use
Pharmaceutical compositions consisting of diphenhydramine tannate in solid dosage form which are effective when administered for the symptomatic relief of sneezing, itchy, watery eyes, itchy nose or throat and runny nose due to hay fever (allergic rhinitis) or other respiratory allergies are disclosed.
Owner:KIEL LAB
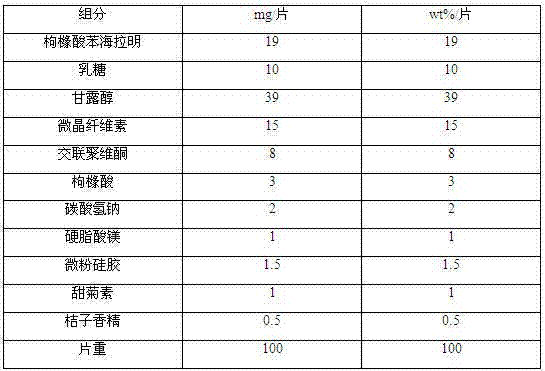
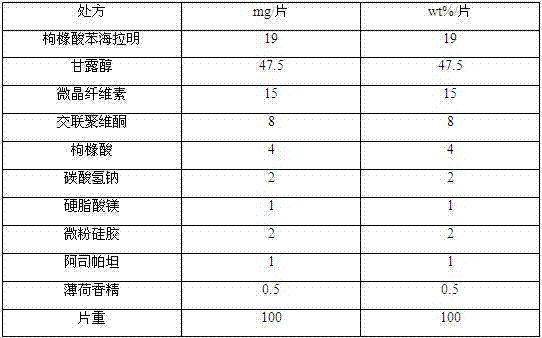
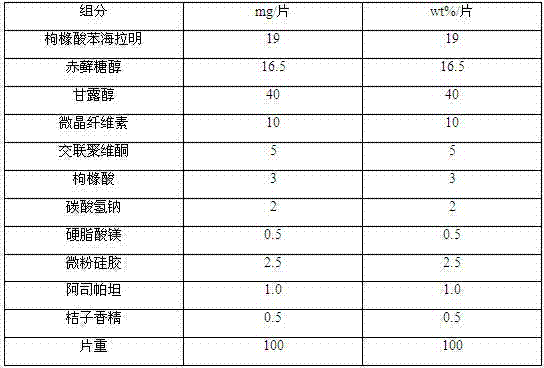
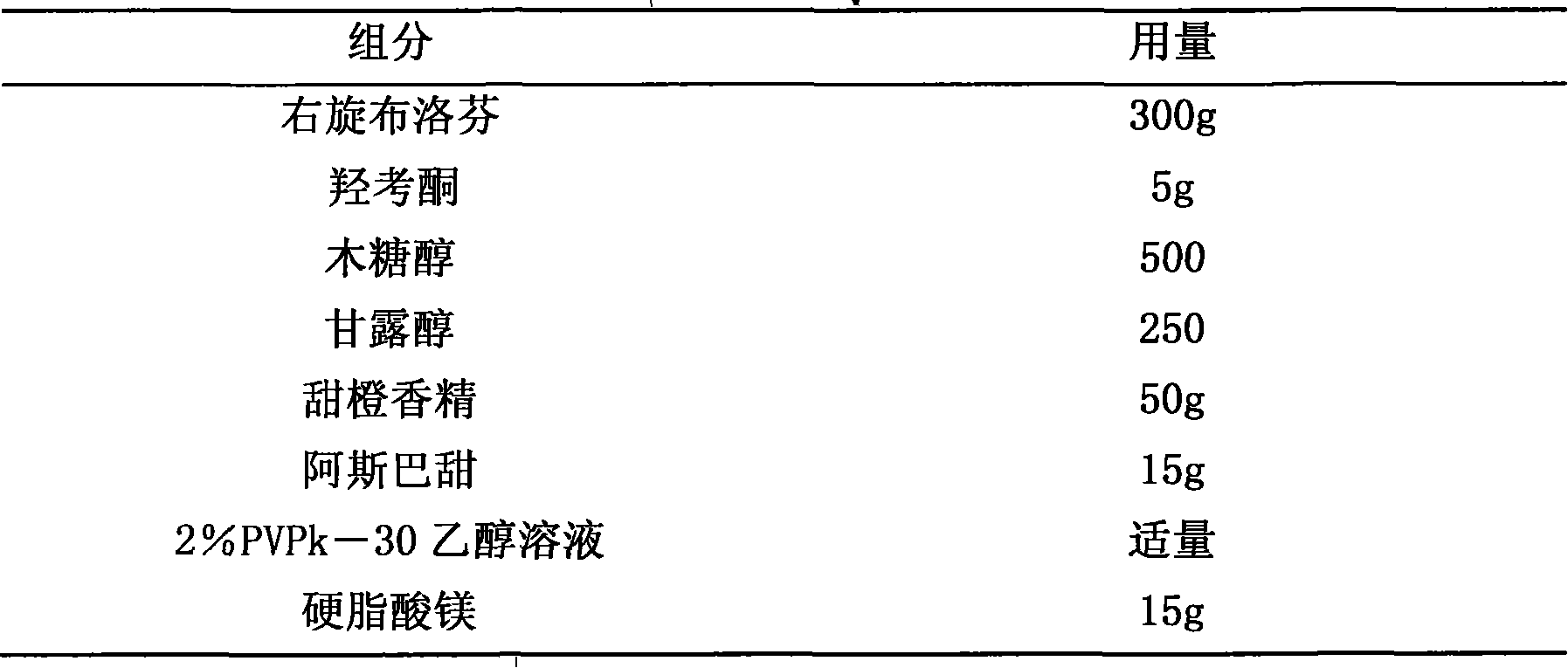
Diphenhydramine citrate orally disintegrating tablet and preparation method thereof
InactiveCN102440973AMeet quality requirementsReasonable prescription designOrganic active ingredientsNervous disorderSodium bicarbonateOrally disintegrating tablet
The invention discloses a diphenhydramine citrate orally disintegrating tablet, the prescription is composed by the following components in mass percent: 19% of diphenhydramine citrate, 45 swung dash 58% of filler, 15 swung dash 25% of disintegrating agent, 4 swung dash 8% of effervescent disintegrant, 0.5 swung dash 1% of lubricant, 1 swung dash 3% glidant, 1 swung dash 2% of sweetner, 0.2swung dash 0.6% aromatic and 0 swung dash 0.5% of surfactant, wherein the filler uses mannitol, or mannitol and lactose or erythritol, the disintegrating agent uses any one of polyplasdone, crosslinking sodium carboxy methyl cellulose and low substitution hydroxyl propyl cellulose together with microcrystalline cellulose, the effervescent disintegrant comprises citric acid and sodium bicarbonate which have the mass ratio of 1 swung dash 2 / 1, the lubricant is magnesium stearate, the glidant is aerosil or talcum powder, the sweetner is aspartame or stevia rebaudianum, the aromatic is the pharmaceutically acceptable essence, and the surfactant is lauryl sodium sulfate; and the invention further discloses a preparation method of the orally disintegrating tablet, which is simple to operate, the cost is low, the obtained product is in accordance with the quality requirement of the orally disintegrating tablet, and has attractive appearance, good taste and stable quality.
Owner:SOUTHWEST UNIV
Rhinitis plaster and preparation method thereof
InactiveCN102552535ANo side effectsHeals fastHydroxy compound active ingredientsRespiratory disorderMethyl salicylateLanolin
The invention relates to a medicament for treating rhinitis and a preparation method, and in particular relates to an external plaster for treating rhinitis and a preparation method of same. The rhinitis plaster comprises major materials and auxiliary materials, wherein the major materials include 20 g to 40 g of dandelion, 15 g to 30 g of Cortex Phellodendri Chinensis, 20 g to 40 g of Gardenia jasminoides, 20 g to 40 g of Radix Saposhnikoviae, 15 g to 30 g of Flos Farfarae, 20 g to 40 g of Aster tataricus, 15 g to 30 g of Radix Trichosanthis, 1 g to 5 g of menthol, 0.5 g to 4 g of artificial musk, 1 g to 5 g of borneol, 1 g to 5 g of diphenhydramine, 1 g to 5 g of camphor, 3 g to 6 g of methyl salicylate and 3 g to 7 g of belladonna liquid extract; and the auxiliary materials include 0.5 g to 4 g of rubber, 1 g to 5 g of zinc oxide, 1 g to 5 g of rosin, 1 g to 5 g of vaseline and 1 g to 5 g of lanolin liquid wax. The preparation method of the rhinitis plaster comprises the following steps: extracting effective components by a percolation method and uniformly spreading the effective components on elastic fabric or white flat cloth. The rhinitis plaster acts rapidly and prevents recurrence after one course of treatment.
Owner:王晓书
Coated solid hyponotic
The invention provides a preparation which comprises diphenhydramine or its acid addition salt as the pharmaceutical component of hypnotic / sedative action, has no discoloration, is stable, masks bitterness in administration and causes quick and secure effect. The coated solid hypnotic preparation is obtained by coating a solid agent comprising diphenhydramine or its acid addition salt as the pharmaceutical component having hypnotic / sedative action with a coating film containing a lightproof substance and a water-soluble polymer.
Owner:SS PHARMA CO LTD
Diphenhydramine tannate liquid and semi-solid compositions and methods of use
Pharmaceutical compositions consisting of diphenhydramine tannate which are effective when administered for the symptomatic relief of sneezing, itchy, watery eyes, itchy nose or throat and runny nose due to hay fever (allergic rhinitis) or other respiratory allergies are disclosed.
Owner:KIEL LAB
Topical medications for bruises and burns
ActiveUS20160022676A1Reduce physical impactBiocideHydroxy compound active ingredientsTransdermal patchPhenylephrine hcl
Topical medications to treat the physical effects of bruises and burns. For bruises, the topical medication preferably includes therapeutically effective amounts of vasoconstrictor, such as phenylephrine HCL USP, an anti-inflammatory, such as Arica, and compounding agents. In one embodiment, the compounding agents are a Versabase® gel which includes water, ammonium acryloyldimethyltaurate copolymer, aloe barbadensis leaf juice powder, allatoin, disodium EDTA, methylchloroisothiazolinone and methylisothiazolinone. For burns, the topical medication preferably combines therapeutically effective amounts of an active anti-bacterial compound, such as silver sulfadiazine, an anesthetic, such as lidocaine, and a histamine blocker, such as diphenhydramine, with suitable compounding agents, such as Versabase® gel. Both the anti-bruise and anti-burn medications of the present invention can advantageously be formulated in solutions, lotions, creams, ointments, gels, foams or transdermal patches.
Owner:MERIDIAN RES & DEV
Anti-freckle whitening emulsion
InactiveCN103385838AEasy to buyLow priceCosmetic preparationsToilet preparationsImidazolidinePalmitates
The invention provides an anti-freckle whitening emulsion. The anti-freckle whitening emulsion is produced through the following steps of proportionally mixing a compound emulsifier 9122, squalane, vitamin E, azone, kojic dipalmitate, vitamin C, magnesium phosphate, ditertbutyl-p-cresol, propyl hydroxybenzoate, dimeticone, isopropyl myristate, 1,3-butanediol, an amino acid wetting agent, allantoin, methylparaben, diphenhydramine, ethylene diamine tetraacetic acid disodium salt, purified water, licoflavone, propyleneglycol, 0.5% hyaluronicacid, aloe extract, imidazolidinylurea and an essence together, and then performing an emulsifying process. Compared with the prior art, the anti-freckle whitening emulsion has the following advantages that 1, as no original Chinese herbal medicine raw materials are used in the formula, the product obtained according to the invention is tasteless and colorless; 2, as the strong penetrating agent azone and the antihistamine diphenhydramine are selected in the formula, the product is quick to take effect and non-allergic; 3, as the raw materials are convenient to buy and low in price and the production process is simple, the product is low in production cost.
Owner:AESTHETIC TECH BEIJING +2
Drug composition for treating rhinitis
InactiveCN103585373AGood for rhinitisNo side effectsHydroxy compound active ingredientsUnknown materialsSide effectMethyl salicylate
The invention belongs to a drug composition for treating rhinitis. The drug composition comprises the following raw materials by weight: 20-40 g of dandelion, 15-30 g of golden cypress, 20-40 g of ligusticum wallichii, 20-40 g of dried orange peels, 15-30 g of pinellia ternate, 20-40 g of aster, 15-30 g of radices trichosanthis, 1-5 g of menthol, 0.5-4 g of muscone, 1-5 g of borneol, 1-5 g of diphenhydramine, 1-5 g of camphor, 3-6 g of methyl salicylate and 3-7 g of a belladonna liquid extract. The raw materials also can be prepared into ointments according to a conventional preparation method. The drug composition is used for treating the rhinitis, has a good effect and is free from toxic and side effects.
Owner:钱家美
Acne-removing and anti-allergic cosmetic cream and preparation method thereof
ActiveCN103099756AImproves Allergy ToleranceImprove skin toneCosmetic preparationsToilet preparationsCosmetic creamSkin sensitization
The invention discloses acne-removing and anti-allergic cosmetic cream comprising the following components in parts by weight of: 5-10 parts of glycerin monostearate, 5-10 parts of cod liver oil, 5-10 parts of isopropyl palmitate, 1-10 parts of glycerol, 1-10 parts of benzoyl peroxide, 1-10 parts of stearic acid, 2-8 parts of diphenhydramine, 1-10 parts of sulfonated shale oil, 1-10 parts of tea tree oil, 0.1-5 parts of angelica sinensis extracted liquid, 0.1-10 parts of ethylparaben, 0.1-5 parts of vitamin E, 0.1-5 parts of vitamin C and 1 part of potassium hydroxide. The invention further discloses a preparation method of the acne-removing and anti-allergic cosmetic cream. The acne-removing and anti-allergic cosmetic cream disclosed by the invention can eliminate acnes on the face and improve the allergic tolerance of the skins, is safe and has no toxic side effects, so that the acne-removing and anti-allergic cosmetic cream has a wide applicable crowd.
Owner:广州市白云区天芳化妆品厂
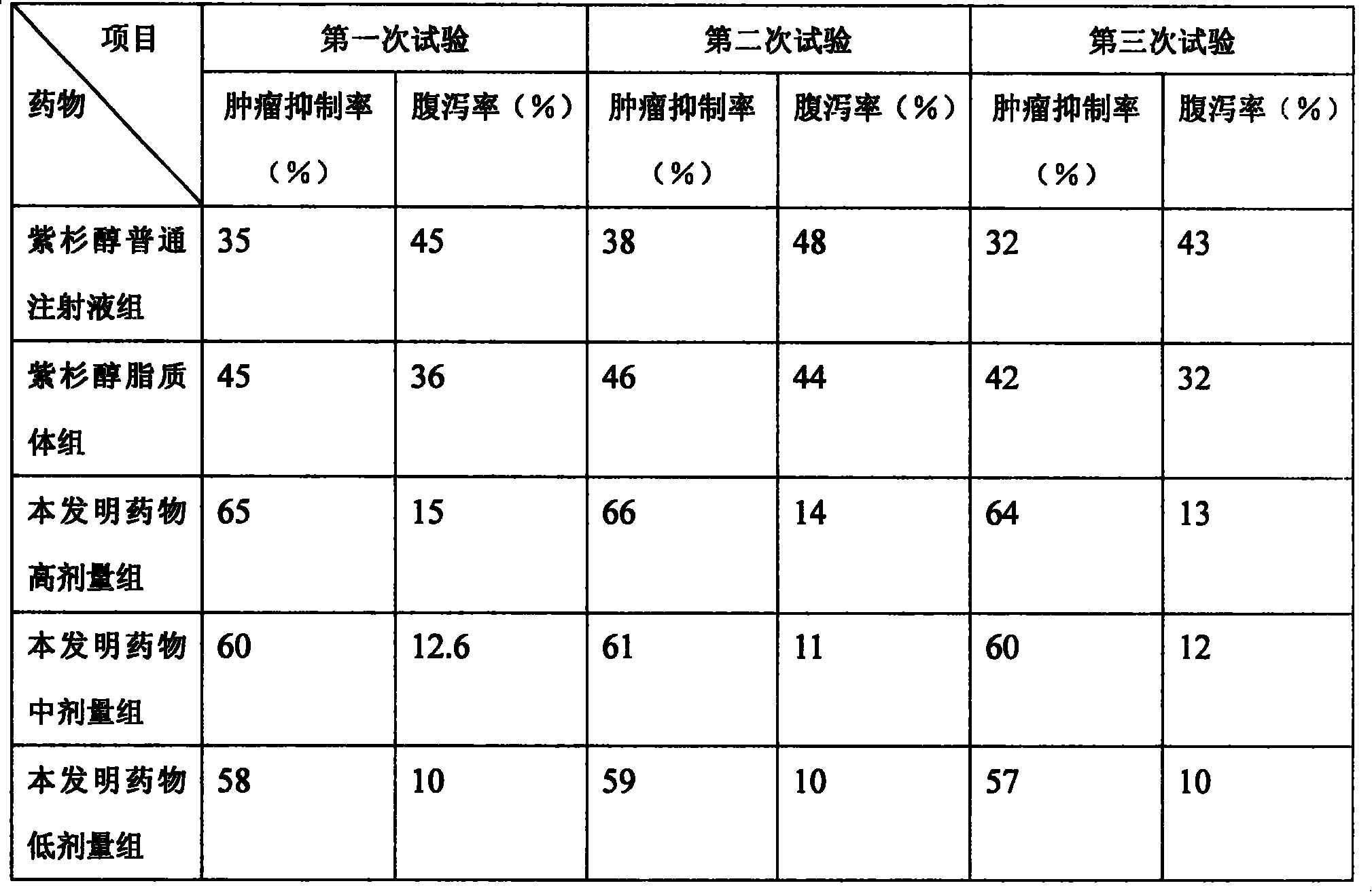
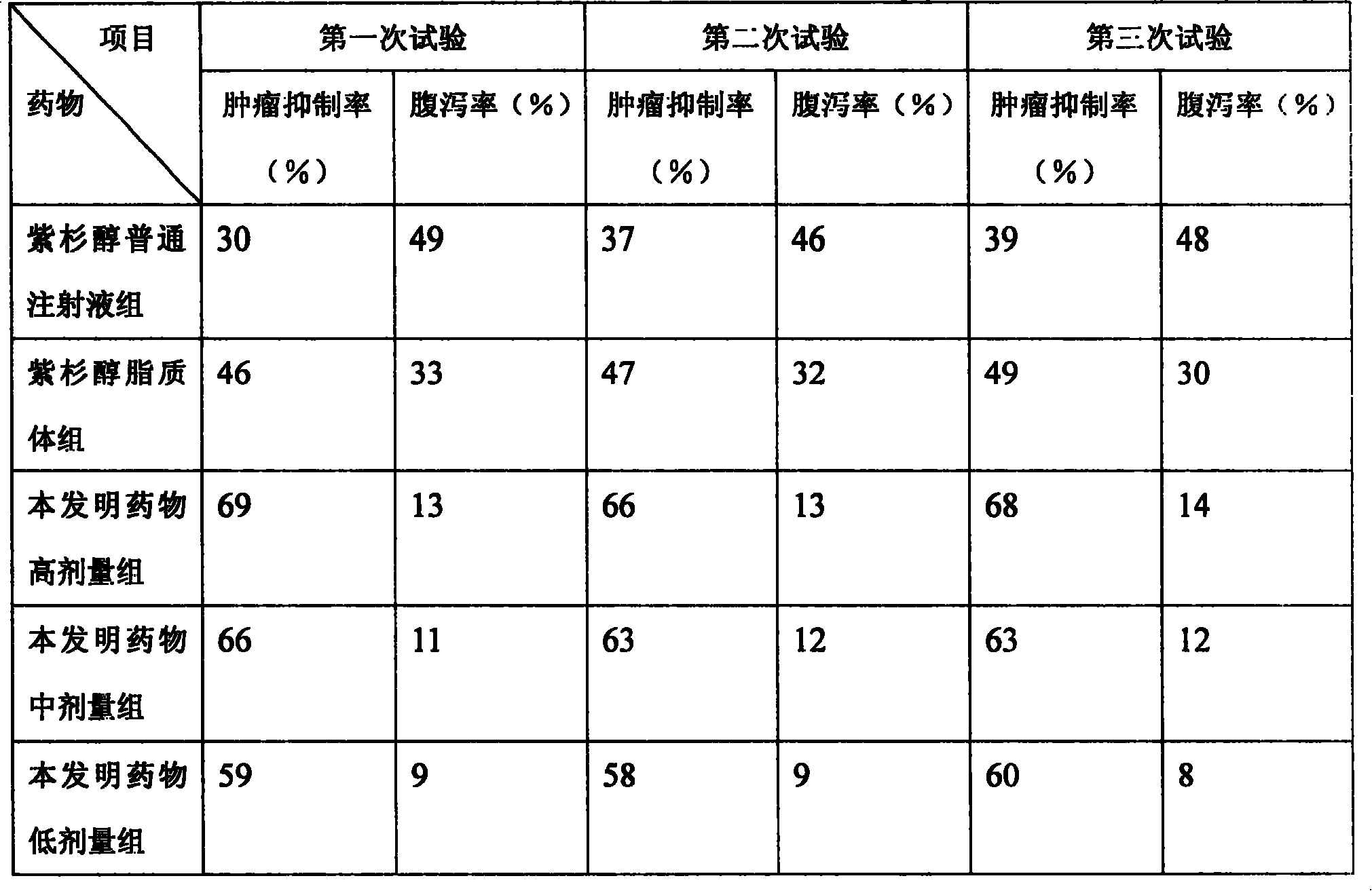
Paclitaxel/multialkene paclitaxel liposome composite medicine and preparation method thereof
InactiveCN101176719ASmall doseSmall toxicityOrganic active ingredientsAntineoplastic agentsSide effectVitamin C
The invention relates to a liposome drug combination and a preparation method of the drug combination. The weights of the effective drug components are listed as follows: the weight of the admixture for hydrogenated soybean phosphatide and sheep brain lecithin with a weight ratio of 2:1 is 300 to 550; the weight of the admixture for cholesterol, stearamide and beta-sitosterol with a weight ratio of 1:1:0.1 is 200 to 400; the weight of the admixture for tiopronin and vitamin E with a weight ratio of 4:1 is 100 to 200; the weight of the admixture for polyethylene glycol-4000 and polyethylene glycol-6000 with a discretional weight ratio is 150 to 200; the weight of vitamin C is 150 to 180; the weight of the admixture for tert-butyl alcohol and anhydrous alcohol with a weight ratio of 1.5 to 2.2:0.01 to 0.05 is 4000 to 7000; the weight of the admixture for paclitaxel and polyene paclitaxel with a discretional weight ratio is 20 and 40; the weight of the admixture for diphenhydramine and cimetidine with a weight ratio of 1:6 is 350 to 500. The invention has the advantages of reducing the drug dosage by nearly one time, increasing the curative effect by over 15 percent, and greatly reducing the side effect of the drugs.
Owner:HUNAN KANGDU PHARMA
Medicinal composition for diminishing inflammation and ease pain and preparation and use thereof
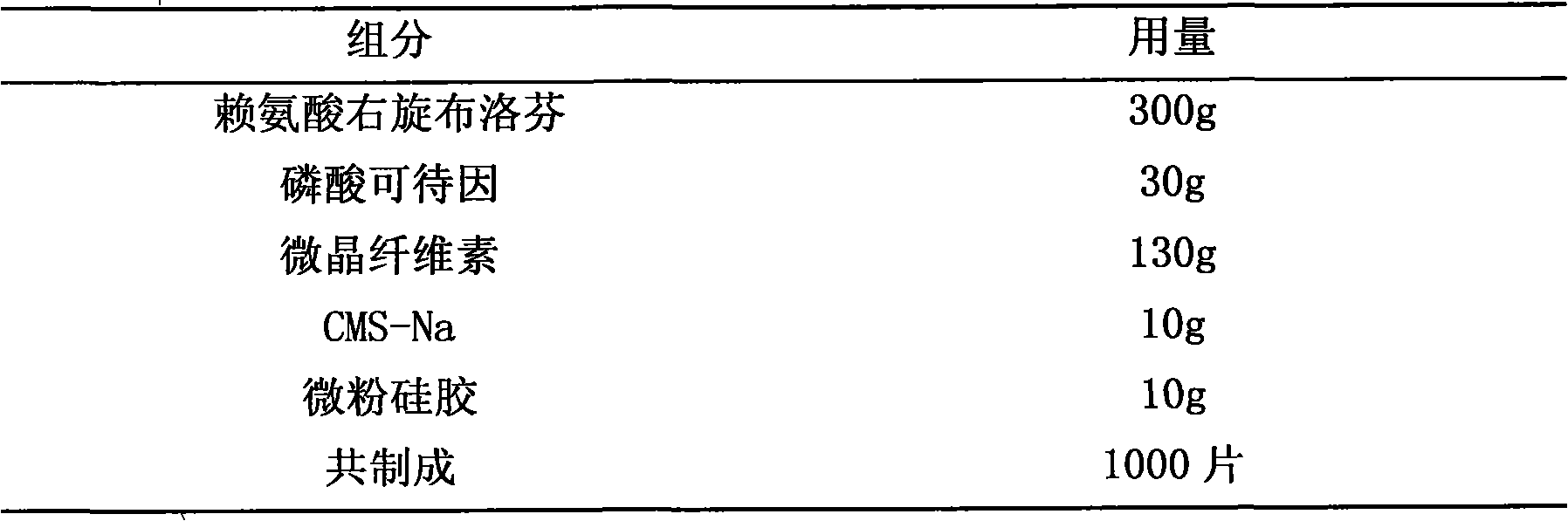
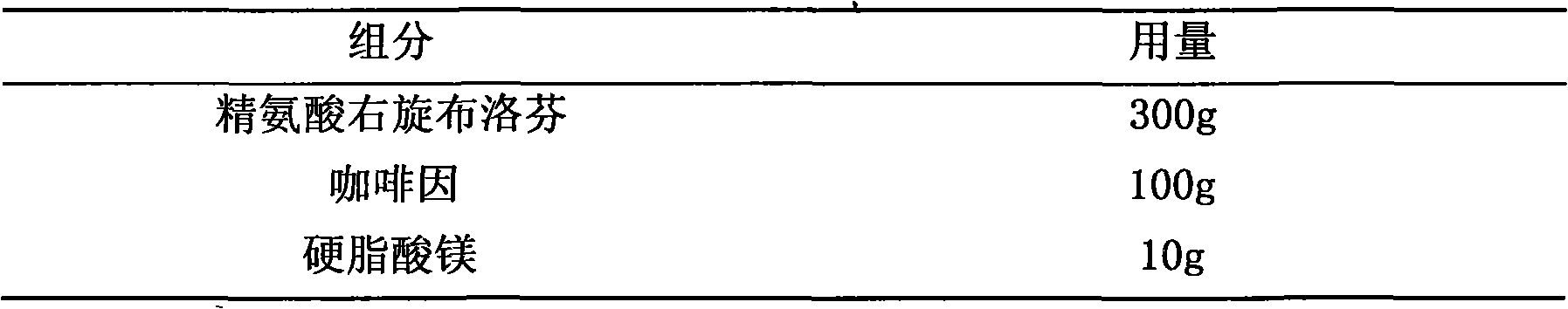
The invention provides a medicine composition for inflamination diminishing and pain easing, which is a composition formed by an active component and a medicinal carrier, and the active component is formed by one of right hand ibuprofen, ibuprofen or amino acid salt of right hand ibuprofen, right hand ketoprofen, ketoprofen or amino acid salt of right hand ketoprofen and another backbone pain easing medicine or quill. The preferred composition way: lysine right hand ibuprofen and phosphoric acid codeine, arginine right hand ibuprofen and caffeine, right hand ibuprofen and oxycodone, lysine right hand ketoprofen and hydrocodone, arginine right hand ketoprofen and diphenhydramine. The invention is prepared to various oral preparations to acquire better coordination effect.
Owner:FUKANGREN BIO PHARMA
Emulsifiable paste for treating skin disease and formula thereof
InactiveCN103356742AEasy to makeHigh utilization rate of economic valueSalicyclic acid active ingredientsHydroxy compound active ingredientsBenzoic acidDisease
The invention discloses an emulsifiable paste for treating skin disease and a formula of the emulsifiable paste. According to the formula, the emulsifiable paste comprises the following components: 4-5g of camphor, 3-6g of menthol, 12-14g of benzoic acid, 6-8g of salicylic acid, 1-2g of zinc oxide, 3-4g of sublimed sulfur, 1-2g of methyl salicylate, 1-2g of borneol, 0.3-0.5g of glycyrrhetinic acid, 0.2-0.4g of chlorhexidine gluconate and 1-1.5g of diphenhydramine. The preparation process comprises the following steps of: first, grinding camphor, menthol and borneol into fine powder; then, adding benzoic acid, salicylic acid, zinc oxide, sublimed sulfur, methyl salicylate, glycyrrhetinic acid, chlorhexidine gluconate and diphenhydramine and grinding into paste; and packaging by using small containers for later use. The emulsifiable paste disclosed by the invention is simple to prepare and has the advantages of reliable curative effect, high economic value and high utilization ratio.
Owner:刘洪东
Rheumatism cold compress paste and preparation method thereof
InactiveCN107296951AGuaranteed respiratory metabolismIncrease contact surfaceAnthropod material medical ingredientsHydroxy compound active ingredientsRheumatismGlycerol
The invention discloses a rheumatism cold compress paste which comprises a backing layer, a gel drug layer and an anti-bonding layer, wherein the gel drug layer contains the following raw materials: sodium polyacrylate, carbomer, glycerol, dihydroxyaluminum aminoacetate, tartaric acid, purified water, medical borneol, camphor, diclofenac sodium, menthol, atropa belladonna liquid extract, ginger oil, propylene glycol, glycerol, medical azone, diphenhydramine, oleum menthae and capsaicin. The invention also discloses a preparation method for the rheumatism cold compress paste. The method comprises the following steps: firstly, preparing a gel drug, and then coating the backing layer with the gel drug by machine so as to form the gel drug layer, coating the gel drug layer with the anti-bonding layer, cutting, puncturing and slicing, thereby acquiring the bruise cold compress paste. No organic solvent is added into the rheumatism cold compress paste disclosed by the invention; the rheumatism cold compress paste can be directly used for coating and does not need to be heated; the technology is simple and no chemical residue exists; the rheumatism cold compress paste is free from irritability and stimulation, is high in drug loading capacity and moisture retention, has high compatibility with skin, is anti-ageing, has excellent weather fastness and lasting viscidity and can be repeatedly uncovered and pasted; the production cost is lower.
Owner:四川利佰生物科技有限公司
Cold compression patch for traumatic injury and preparation method of cold compression patch
InactiveCN107375527AGuaranteed respiratory metabolismIncrease contact surfaceHydroxy compound active ingredientsAntipyreticDiclofenac SodiumRaw material
The invention discloses a cold compression patch for traumatic injury. The cold compression patch comprises a back lining layer, a gel medicine layer and an anti-sticking layer, wherein the gel medicine layer comprises the following raw materials: sodium polyacrylate, Carbomer, glycerin, aluminium glycinate, tartaric acid, purified water, medical borneol, camphor, diclofenac sodium, menthol, belladonna liquid extract, cinnamon oil, propylene glycol, medical azone, diphenhydramine and peppermint oil. The invention further discloses a preparation method of the cold compression patch for traumatic injury. A gel medicine is prepared firstly and then applied to the back lining layer by a machine, the gel medicine layer is formed and covered with the anti-sticking layer, and the cold compression patch for traumatic injury is obtained after slitting, punching and slicing. The cold compression patch contains no additive, is directly applied without heating, adopts a simple process, has no chemical residue, is non-allergic and non-irritant, is high in drug loading capacity and moisture retention, well compatible with skin, resistant to aging and weather, lasting in adhesion and lower in production cost, can be torn and pasted repeatedly and facilitates popularization.
Owner:四川利佰生物科技有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com