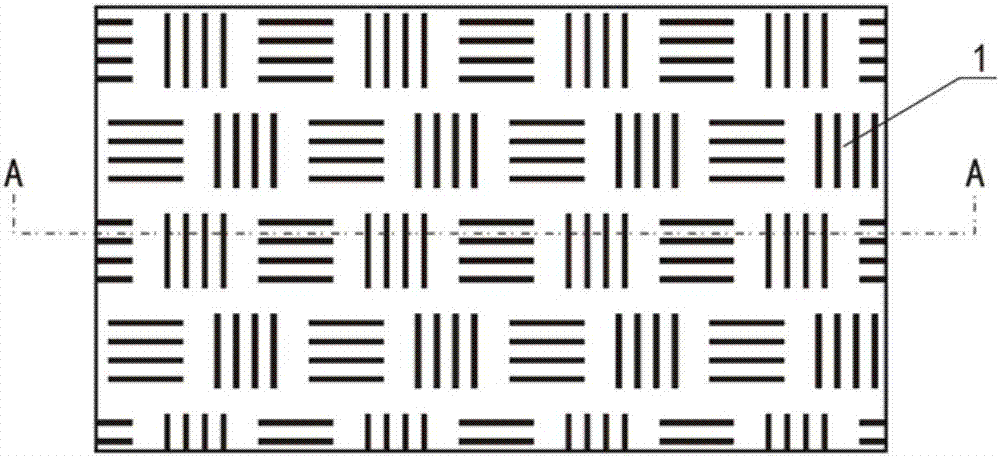
Hyperostosis cold compress paste and preparation method thereof
A technology for bone hyperplasia and mass fraction, which is used in bone diseases, pharmaceutical formulations, cooling appliances for treatment and treatment, etc. Inflammation and purulent spread, increased skin contact, reduced local blood flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
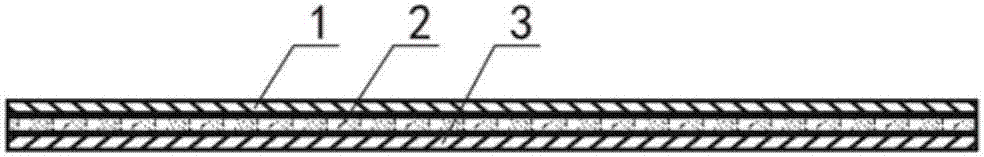
[0037] The gel drug layer 2 includes the following raw materials in parts by mass: 20-50 parts of sodium polyacrylate, 15-30 parts of carbomer, 150-270 parts of glycerin, 1-10 parts of aluminum glycolate, 1-10 parts of tartaric acid, purified 380-450 parts of water, 90-150 parts of medical borneol, 60-100 parts of camphor, 60-100 parts of diclofenac, 30-50 parts of menthol, 30-50 parts of belladonna liquid extract, 30-50 parts of clove oil, propylene glycol 21-35 parts, glycerin 21-35 parts, medical azone 21-35 parts, diphenhydramine 21-35 parts, peppermint oil 15-24 parts, capsaicin 1-1.6 parts.
[0038] The mass parts of the above-mentioned raw materials can be adjusted arbitrarily within the above-mentioned range in specific applications. The principle of adjustment is based on the different degrees of demand of patients for the corresponding medicinal effects of each raw material, as well as the different degrees of demand for viscosity and permeability. The difference in ...
Embodiment 2
[0046] The gel drug layer 2 includes the following raw materials in parts by mass: 20-50 parts of sodium polyacrylate, 15-30 parts of carbomer, 150-270 parts of glycerin, 1-10 parts of aluminum glycolate, 1-10 parts of tartaric acid, purified 380-450 parts of water, 90-150 parts of medical borneol, 60-100 parts of camphor, 60-100 parts of diclofenac, 30-50 parts of menthol, 30-50 parts of belladonna liquid extract, 30-50 parts of clove oil, propylene glycol 21-35 parts, 21-35 parts of glycerin, 21-35 parts of medical azone, 21-35 parts of diphenhydramine, 15-24 parts of peppermint oil, 1-1.6 parts of capsaicin, 50-100 parts of Millennium Health Powder 40-80 parts of Clematis powder, 10-30 parts of Golden Retriever Dog Ridge powder, 10-30 parts of Cnidinia powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com