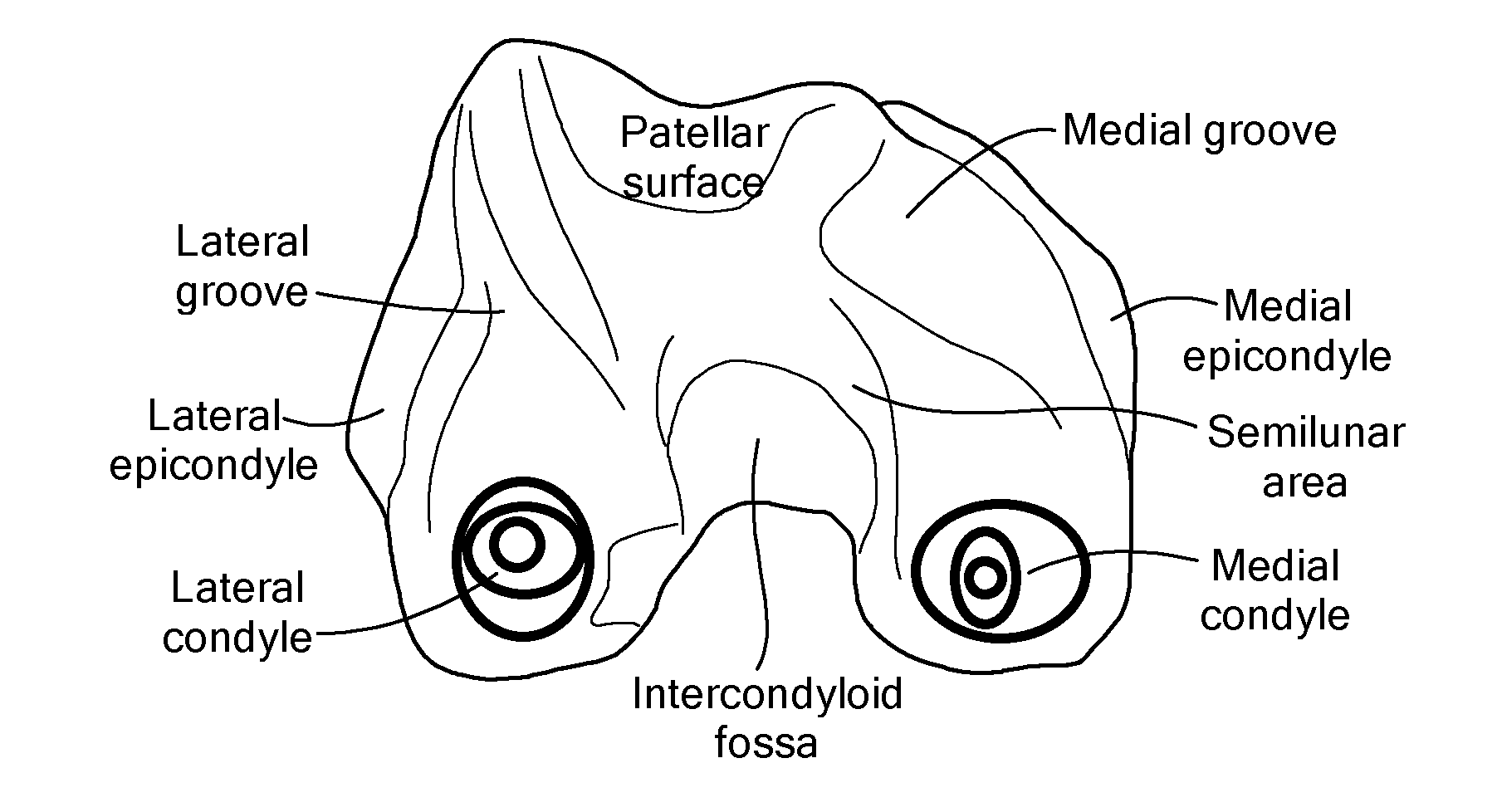
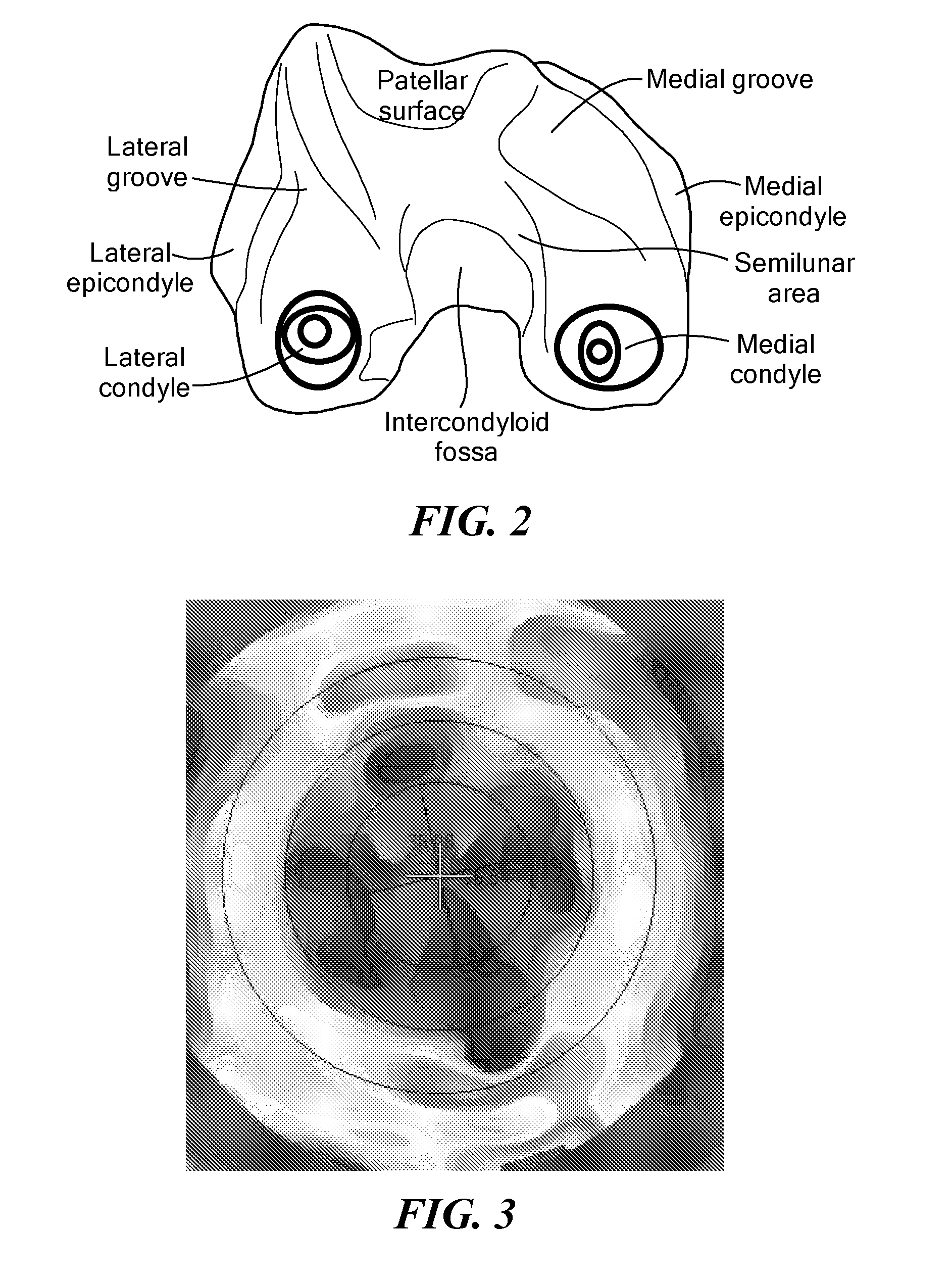
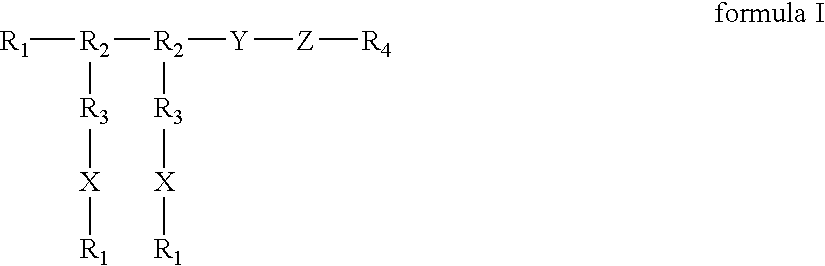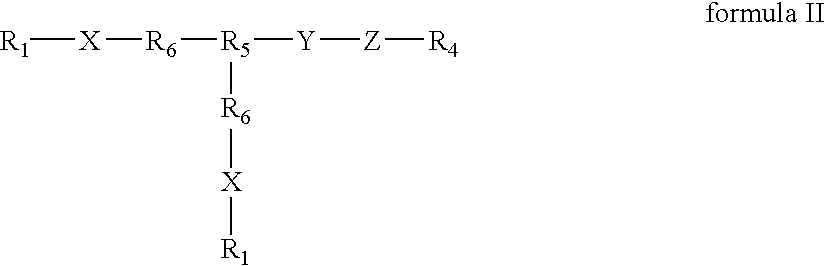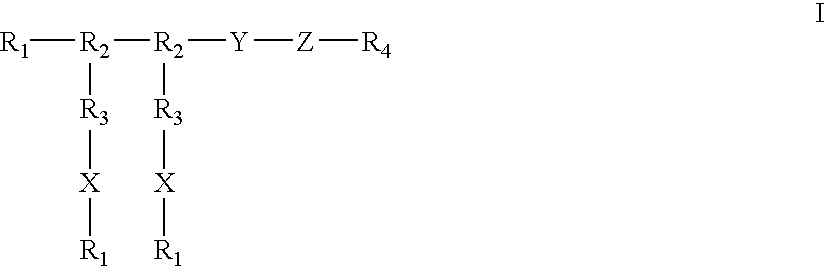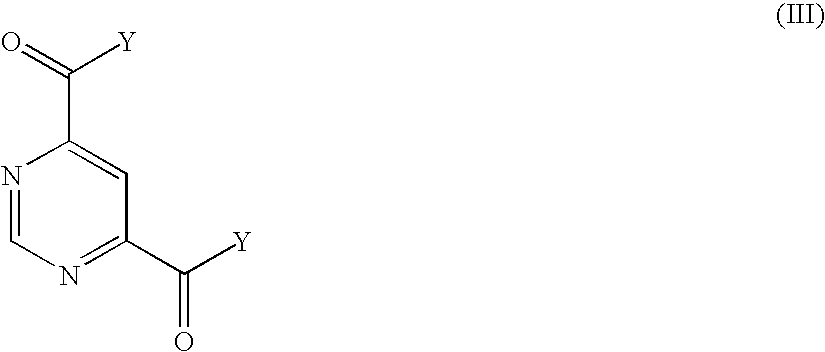Patents
Literature
381 results about "Joint disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method and apparatus for resurfacing an articular surface
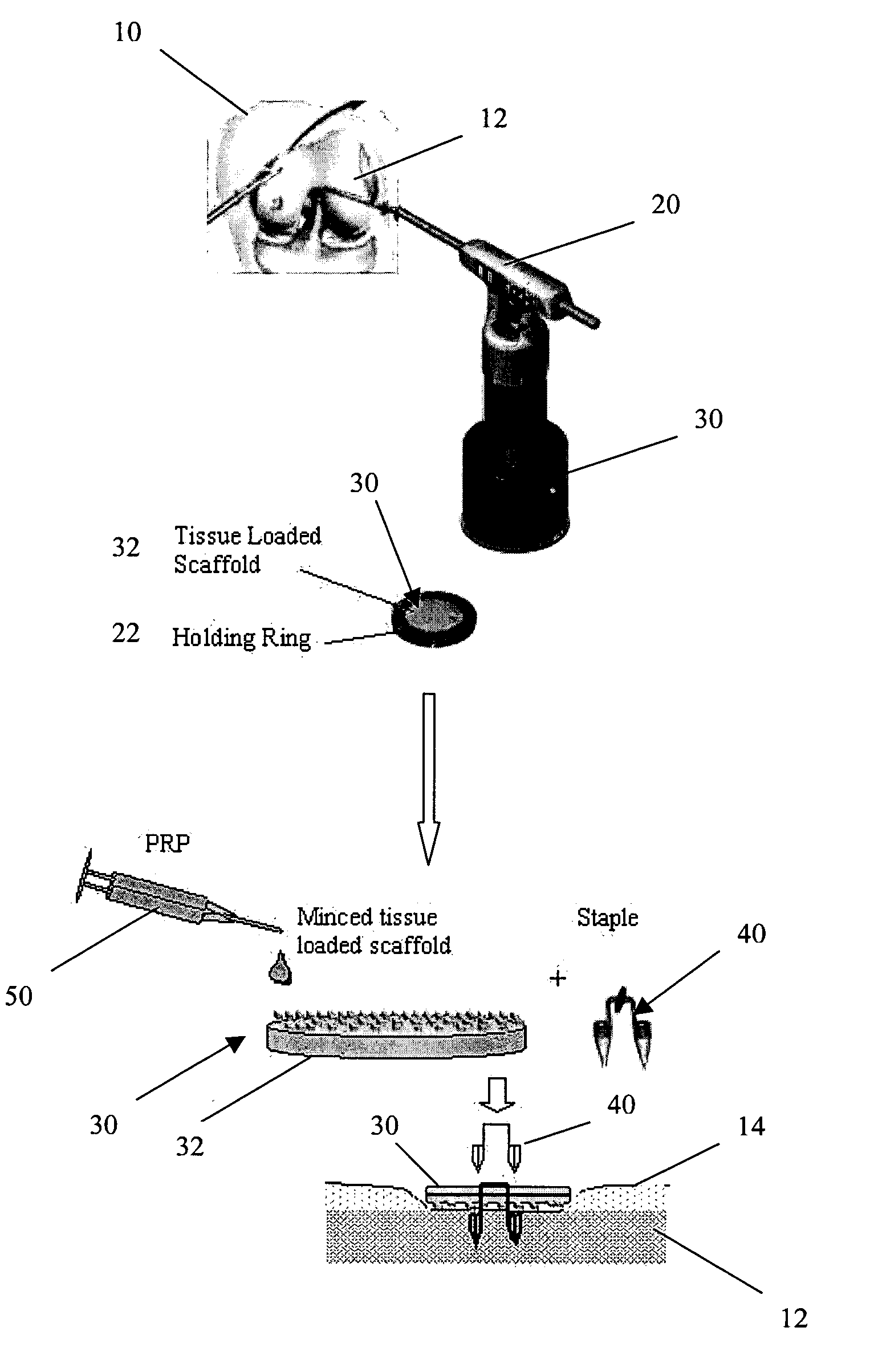
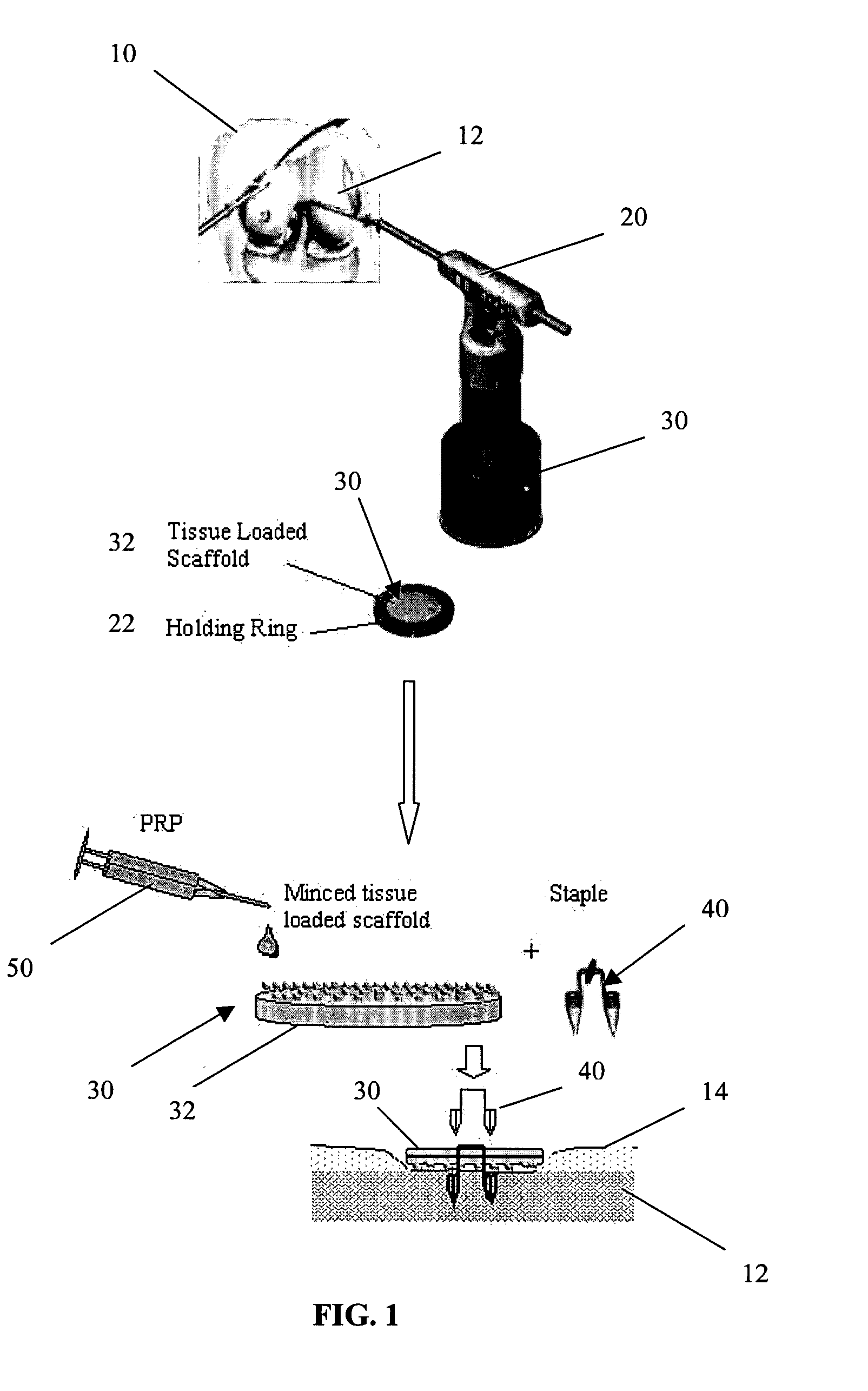
A biocompatible, bioresorbable tissue repair implant or scaffold device is provided for use in repairing a variety of cartilage tissue injuries, and particularly for resurfacing and / or repairing damaged or diseased cartilage. The repair procedures may be conducted with tissue repair implants that contain a biological component that assists in delaying or arresting the progression of degenerative joint diseases and in enhancing tissue healing or repair. The biocompatible, bioresorbable tissue repair implants include a scaffold and particles of viable tissue derived from cartilage tissue, such that the tissue and the scaffold become associated. The particles of living tissue contain one or more viable cells that can migrate from the tissue and populate the scaffold.
Owner:DEPUY SYNTHES PROD INC
Methods for determining meniscal size and shape and for devising treatment
The present invention relates to methods for determining meniscal size and shape for use in designing therapies for the treatment of various joint diseases. The invention uses an image of a joint that is processed for analysis. Analysis can include, for example, generating a thickness map, a cartilage curve, or a point cloud. This information is used to determine the extent of the cartilage defect or damage and to design an appropriate therapy, including, for example, an implant. Adjustments to the designed therapy are made to account for the materials used.
Owner:CONFORMIS
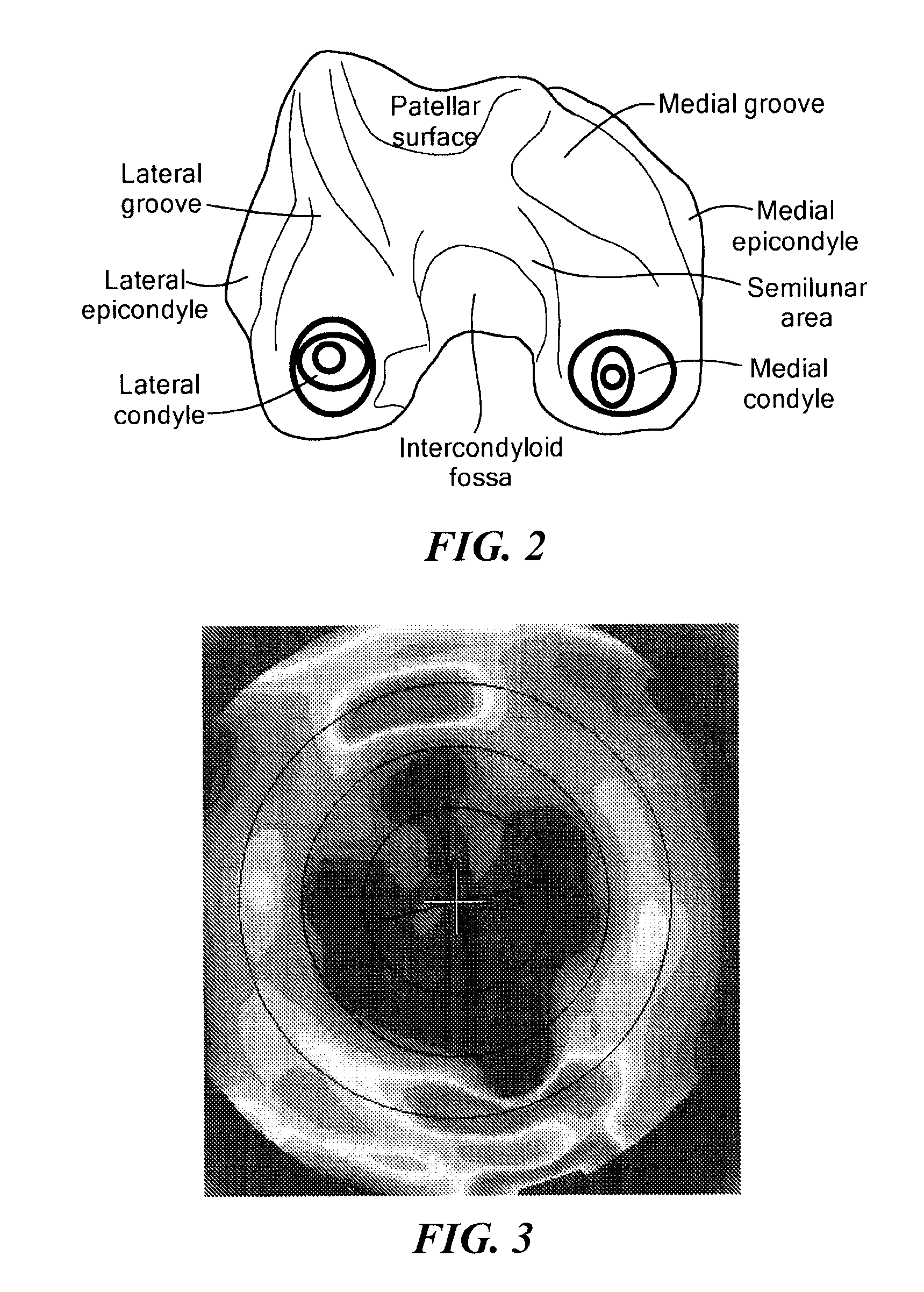
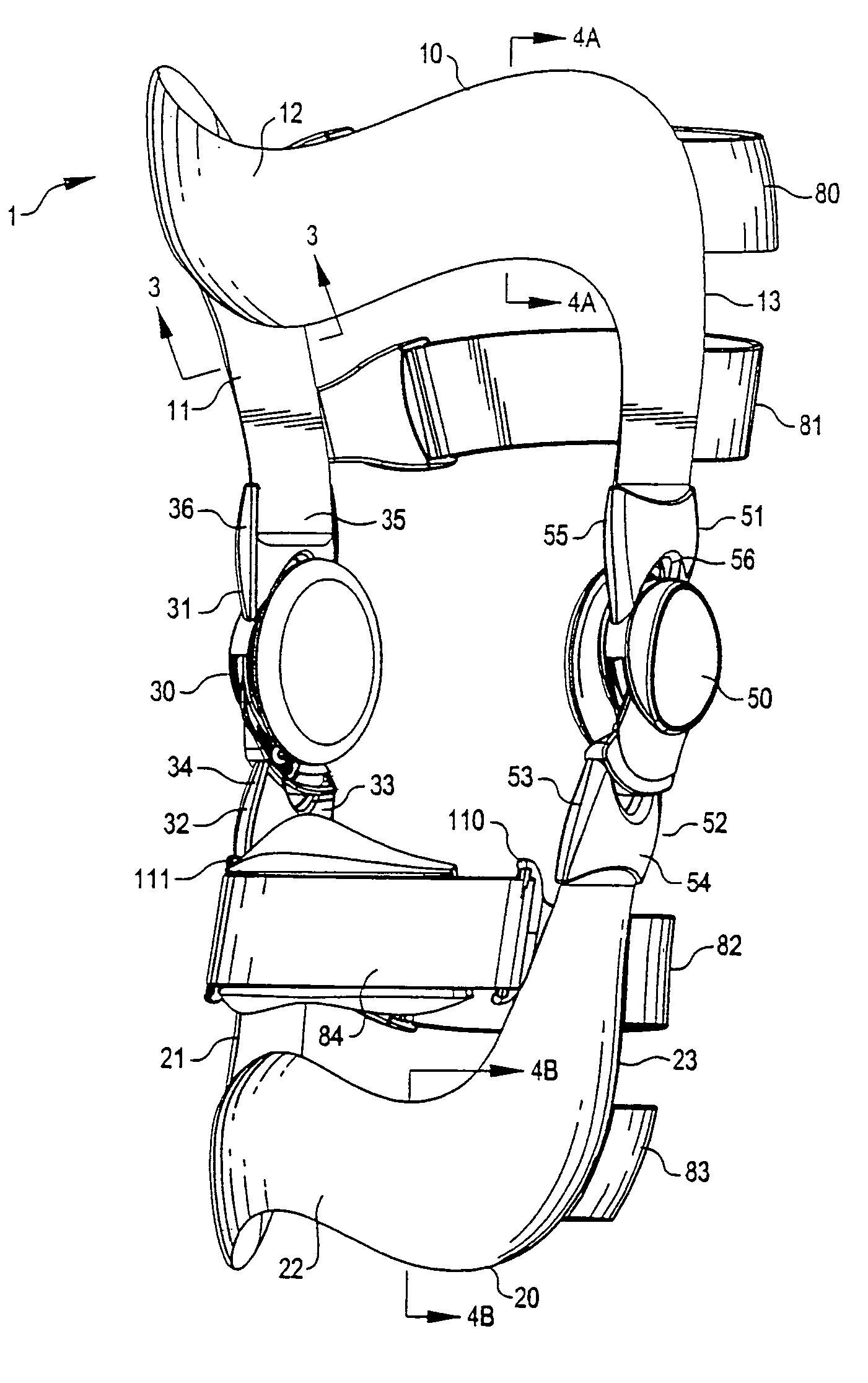
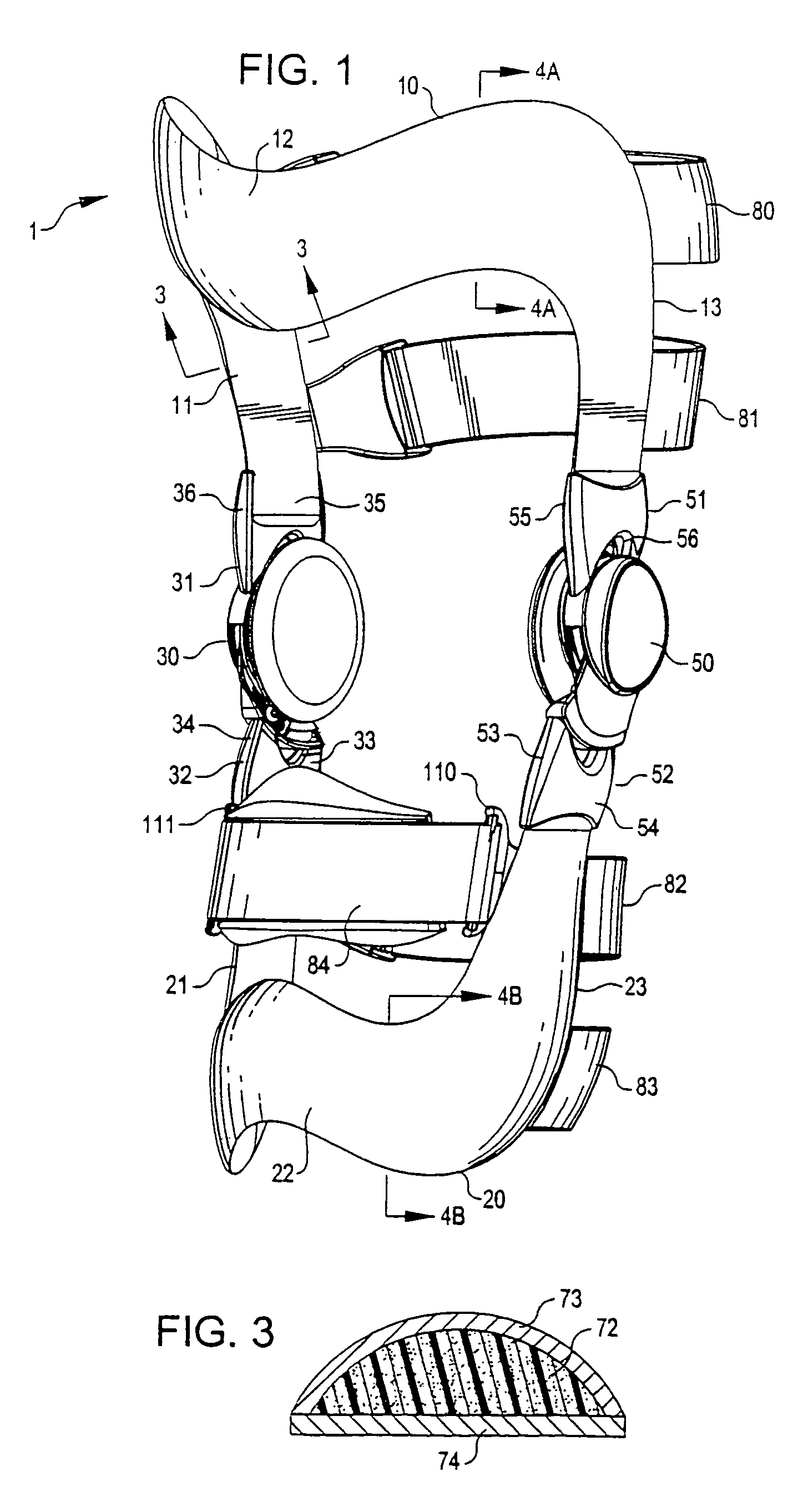
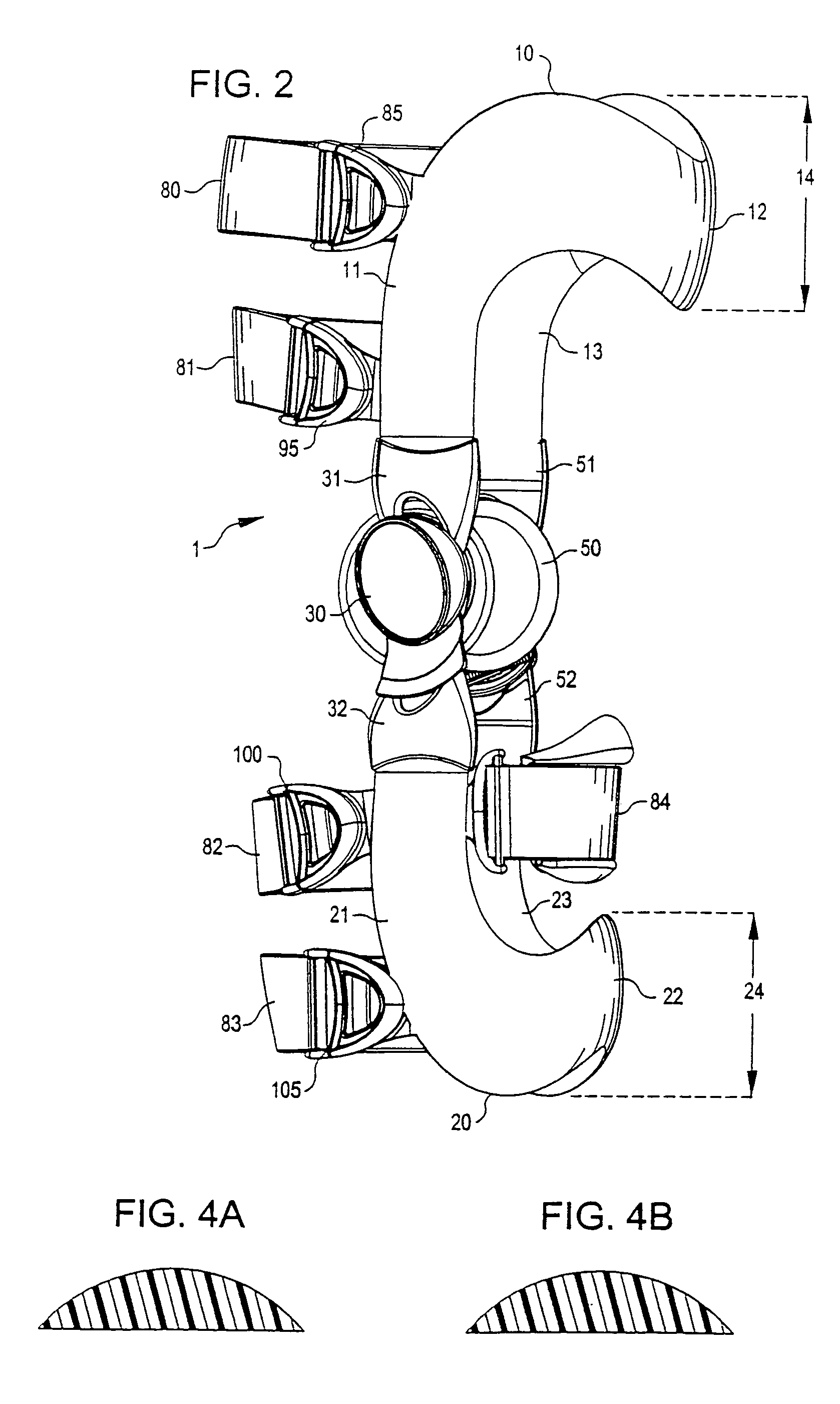
Anatomically designed orthopedic knee brace
InactiveUS7201728B2Novel and effectiveAccurately prescribingNon-surgical orthopedic devicesKnee proceduresTibia
An orthopedic knee brace provides an apparatus for accurately prescribing the anatomical motion of the human knee. The orthopedic knee brace is used for treatment and rehabilitation following surgery to the knee, protection for a surgically repaired knee, and protection for an uninjured knee, among other applications. The orthopedic knee brace actively prescribes asymmetric three-dimensional anatomic motion in six degrees of freedom of the wearer's knee. The rigid connections between the thigh and calf engaging members and the medial and lateral hinges provide the ability of the orthopedic knee brace to prescribe asymmetric three-dimensional anatomic motion in six degrees of freedom by actively prescribing flexion and extension, abduction and adduction, internal / external rotation, anterior / posterior translation, medial / lateral translation, and proximal / distal translation between a femur and a tibia of a wearer's leg. In alternate embodiments a single-hinge brace design is provided for treatment and prevention of osteoarthritis and other joint diseases and conditions.
Owner:OSSUR HF
Methods for Determining Meniscal Size and Shape and for Devising Treatment
The present invention relates to methods for determining meniscal size and shape for use in designing therapies for the treatment of various joint diseases. The invention uses an image of a joint that is processed for analysis. Analysis can include, for example, generating a thickness map, a cartilage curve, or a point cloud. This information is used to determine the extent of the cartilage defect or damage and to design an appropriate therapy, including, for example, an implant. Adjustments to the designed therapy are made to account for the materials used.
Owner:CONFORMIS
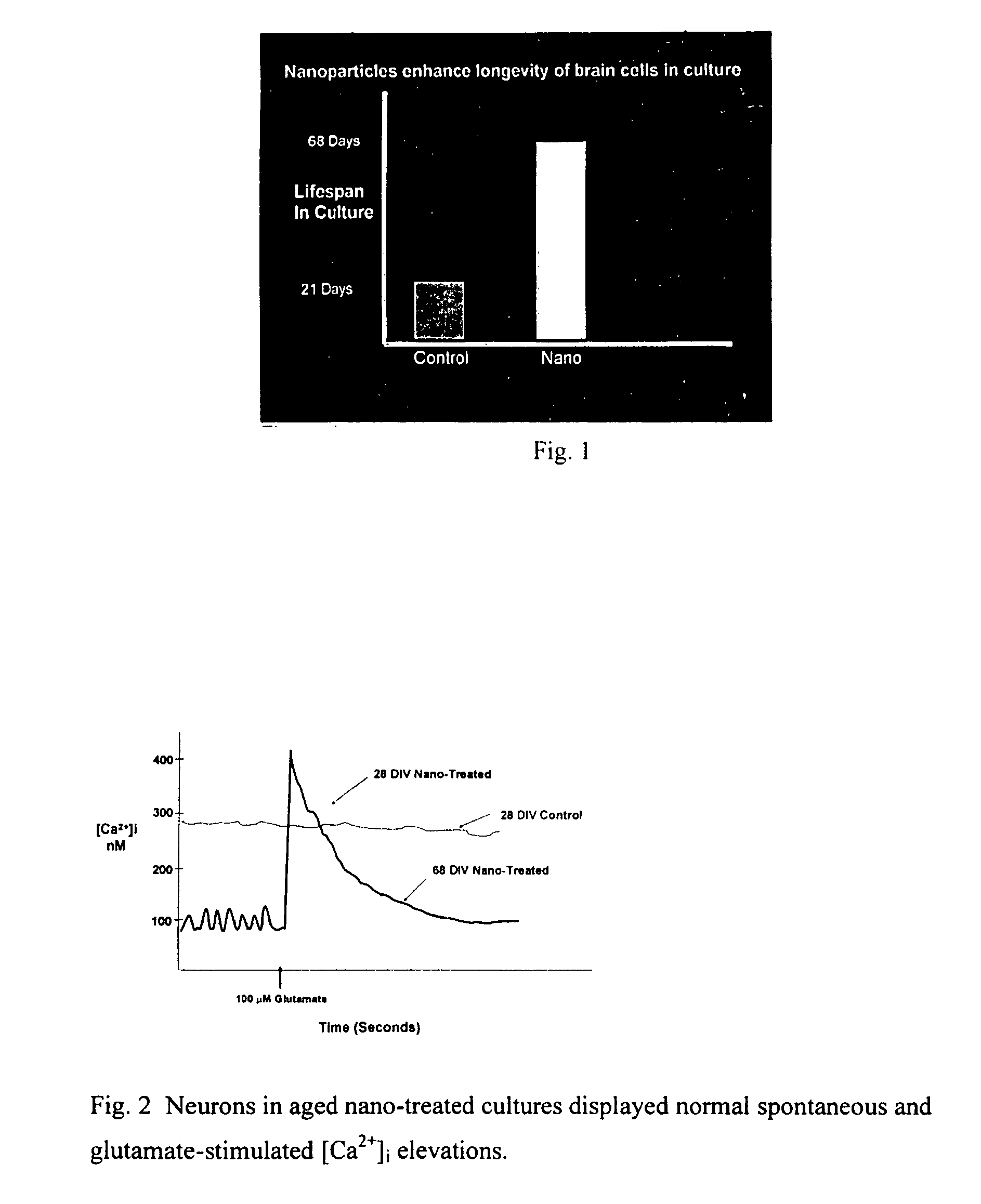
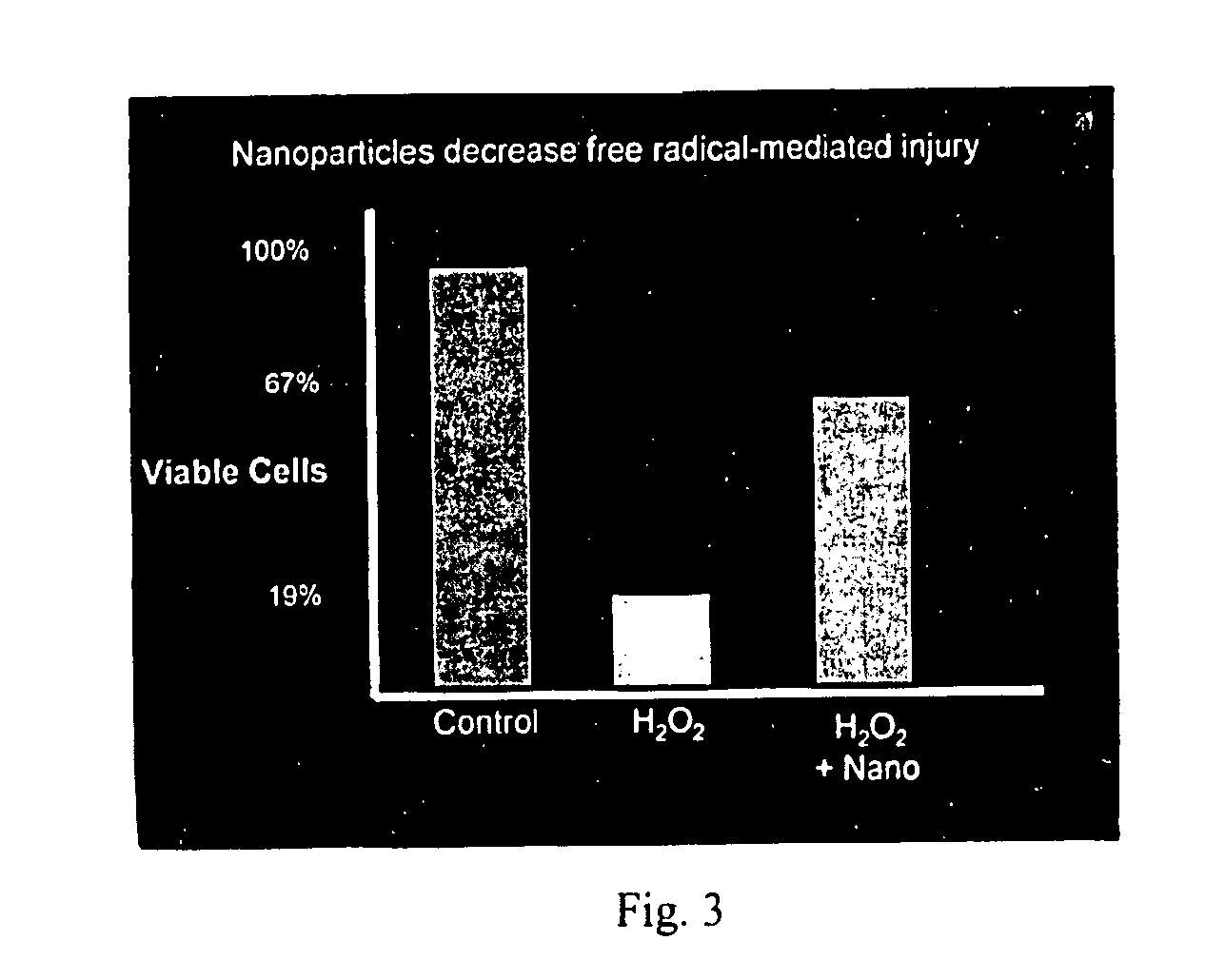
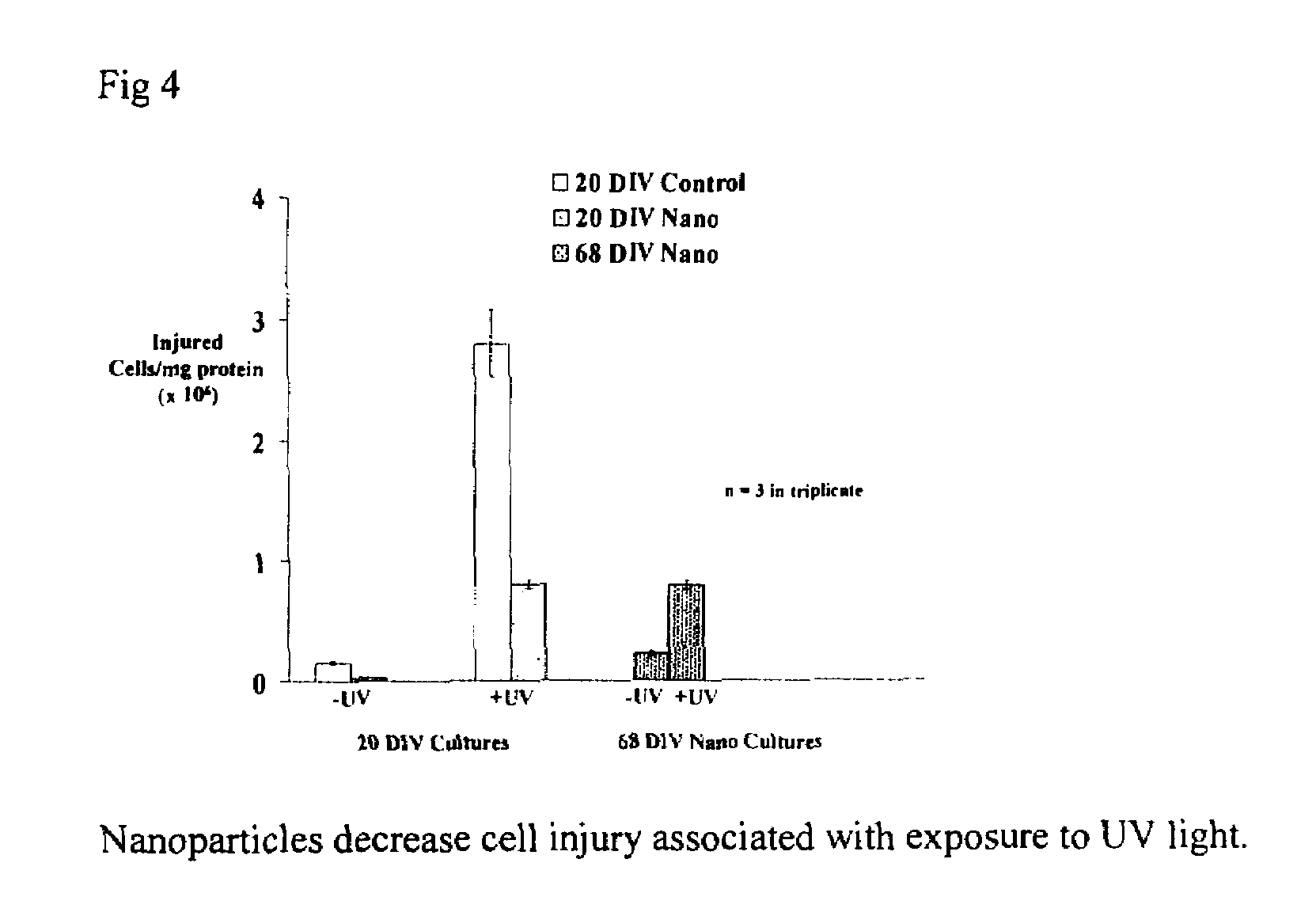
Cerium oxide nanoparticles and use in enhancing cell survivability
Novel, nonagglomerated, engineered, ultra fine Cerium Oxide particles the size of approximately 2 to approximately 10 nm and methods for preparation of the particles. The resultant particles enhance the longevity of cells in culture. Applications of the particles include benefits for wound healing, treating arthritis and joint diseases, anti-aging and the treating of inflammations.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Methods of predicting musculoskeletal disease
ActiveUS7840247B2Predict riskUltrasonic/sonic/infrasonic diagnosticsImage enhancementBiomechanicsMusculoskeletal disease
Methods of predicting bone or joint disease in a subject are presented. The method may include determining one or more micro-structural parameters, one or more macroanatomical parameters or biomechanical parameters of a joint in the subject. At least two of the parameters are combined to predict the risk of bone or articular disease. Additionally, methods of determining the effect of a candidate agent on any subject's risk of developing bone or joint disease are presented.
Owner:IMATX
Spine distraction implant
InactiveUS20100262243A1Increase volumeReduce restrictionsInternal osteosythesisJoint implantsDistractionDilator
Owner:MEDTRONIC EURO SARL
Nutrient composition
InactiveUS20090069217A1Promotes collagen productionPrevent and treat skin agingOrganic active ingredientsHeavy metal active ingredientsVitamin CAdditive ingredient
Provided is a nutrient composition for promoting collagen production, which contains vitamin C, an iron preparation, and collagen as active ingredients, promotes collagen production in a living body such as skin or bone, prevents or treats skin aging or bone and joint diseases, and is an iron preparation-containing composition having stability imparted thereto. Also provided are a food or beverage, a feed, and a medicine each containing the nutrient composition incorporated therein.
Owner:SNOW BRAND MILK PROD CO LTD
Positive modulator of bone morphogenic protein-2
InactiveUS20050196425A1Improve biological activityMaximize bioactivityOrganic active ingredientsPeptide/protein ingredientsDiseaseBone formation
Compounds of the present invention of formula I and formula II are disclosed in the specification and wherein the compounds are modulators of Bone Morphogenic Protein activity. Compounds are synthetic peptides having a non-growth factor heparin binding region, a linker, and sequences that bind specifically to a receptor for Bone Morphogenic Protein. Uses of compounds of the present invention in the treatment of bone lesions, degenerative joint disease and to enhance bone formation are disclosed.
Owner:BROOKHAVEN SCI ASSOCS +1
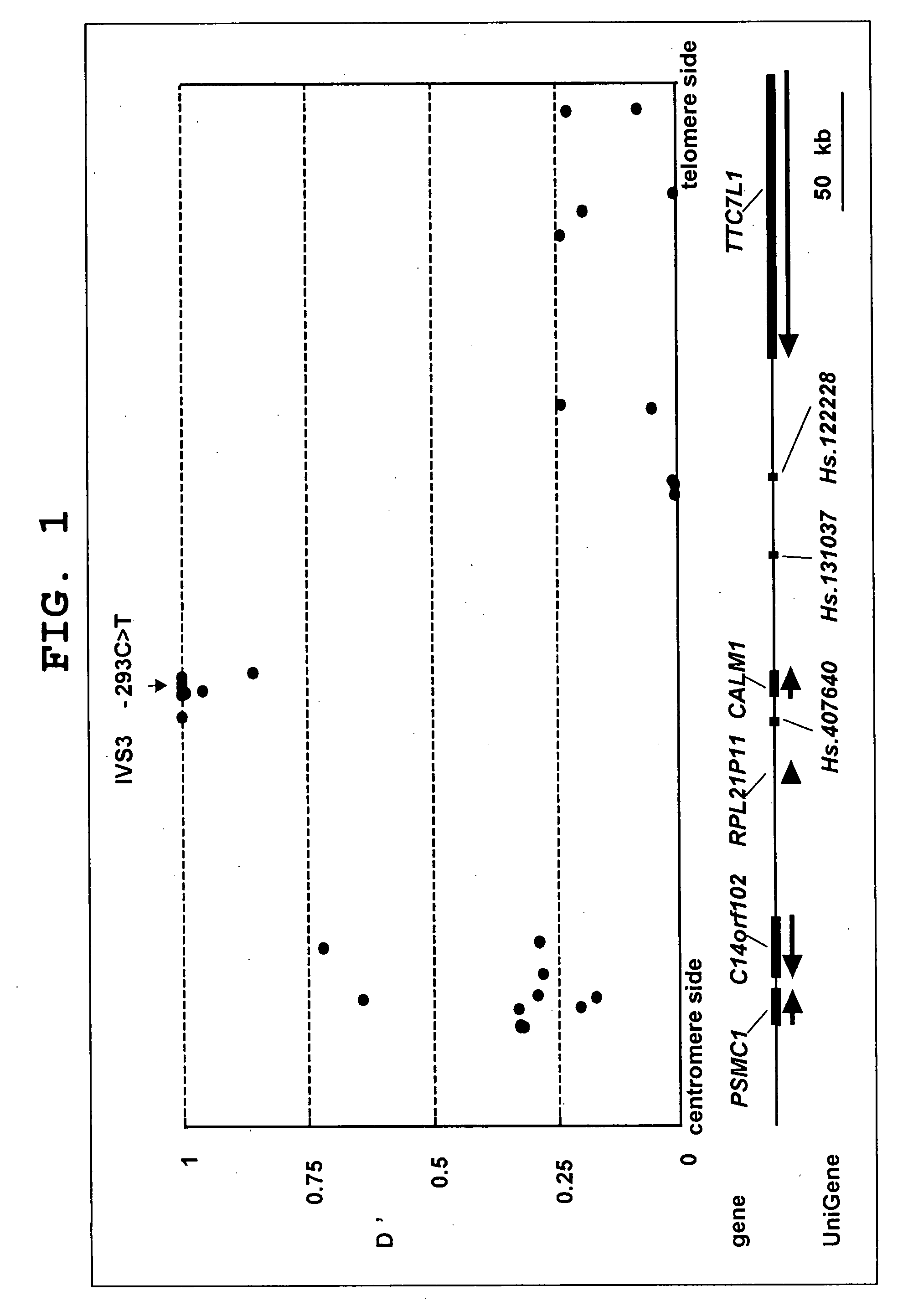
Bone/joint disease sensitivity gene and use thereof
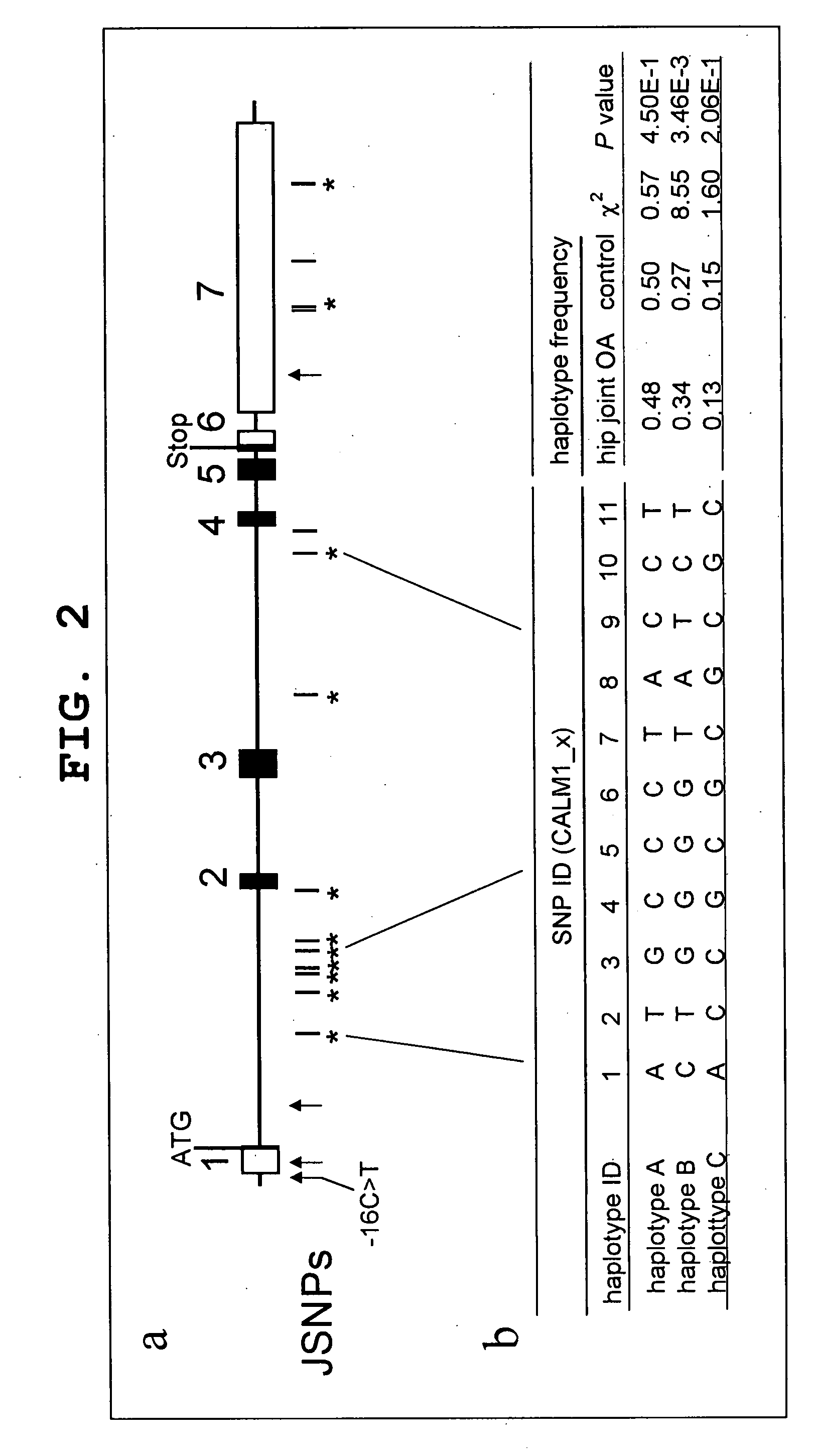
InactiveUS20090075921A1High expressionPromote differentiationOrganic active ingredientsPeptide/protein ingredientsDiseaseCALM1 Gene
The present invention provides the prophylaxis and treatment of bone and joint diseases by regulating the expression or activity of calmodulin, the prophylaxis and treatment of bone and joint diseases by regulating the expression or activity of asporin, and a diagnostic method for genetic susceptibility to bone and joint diseases by detecting polymorphisms in the CALM1 gene and / or the asporin gene, and the like.
Owner:TAKEDA PHARMA CO LTD +1
Composition and method for treatment and prevention of traumatic synovitis and damage to articular cartilage
ActiveUS6979679B2Easy to produceReduce presenceBiocideSkeletal disorderWhole bodyInflammatory arthropathy
Owner:ARTHRODYNAMIC HLDG LLC
Pharmaceutical composition and method for treating a joint-capsule arthropathy
InactiveUS20060122150A1Benefit of efficiencyEfficiency suitabilityBiocideSkeletal disorderIntraarticular InjectionsJoint disease
A pharmaceutical composition for use in treating a joint-capsule arthropathy comprising an effective amount of one or more of a locally administered, optionally encapsulated therapeutic agent in admixture with a hyaluronic acid delivery vehicle and a method for use thereof in treating a joint-capsule arthropathy by intra-articular injection.
Owner:JANSSEN PHARMA NV
Method of Improving Treatments in Rheumatic and Arthritic Diseases
Improved treatments of joint diseases, such as, e.g. osteoarthritis and rheumatoid arthritis, and pain, wherein a strontium-containing compound is administered alone or in combination with one or more second therapeutically and / or prophylactically active substances, selected from the group consisting of bisphosphonates, glucosamine, pallitative agents, analgesic agents, disease modifying anti-rheumatic compounds (DMARDs), selective estrogen receptor modulators (SERMs), aromatase inhibitors, non-steroidal anti-inflammatory agents (NSAIDs), COX-2 inhibitors, COX-3 inhibitors, opioids, inhibitors / antagonists of IL-1, inhibitors / antagonists of TNF-alpha, inhibitors of matrix metallo-proteinases (MMPs), cathepsin K inhibitors, inhibitors / antagonists of RANK-ligand, statins, glucocorticoids, chondroitin sulphate, NMDA receptor antagonists, inhibitors of interleukin-I converting enzyme, Calcitonin gene related peptide antagonists, glycine antagonists, vanilloid receptor antagonists, inhibitors of inducible nitric oxide synthetase (iNOS), N-acetylcholine receptor agonists, neurokinin antagonists, neuroleptic agents, PAR2 receptor antagonists and anabolic growth factors acting on joint tissue components. Pharmaceutical compositions comprising a strontium-containing compound and a second therapeutically and / or prophylactically active substance as defined above.
Owner:OSTEOLOGIX AS
Therapeutics of osteoarthritis and inflammatory joint disease
InactiveUS6787518B1Ameliorating osteoarthritisReduce capacityPeptide/protein ingredientsSkeletal disorderAdditive ingredientInflammatory bowel disease
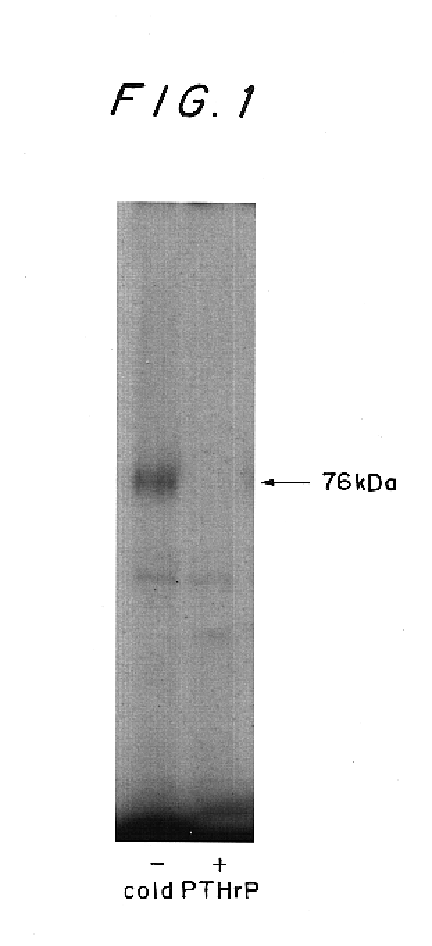
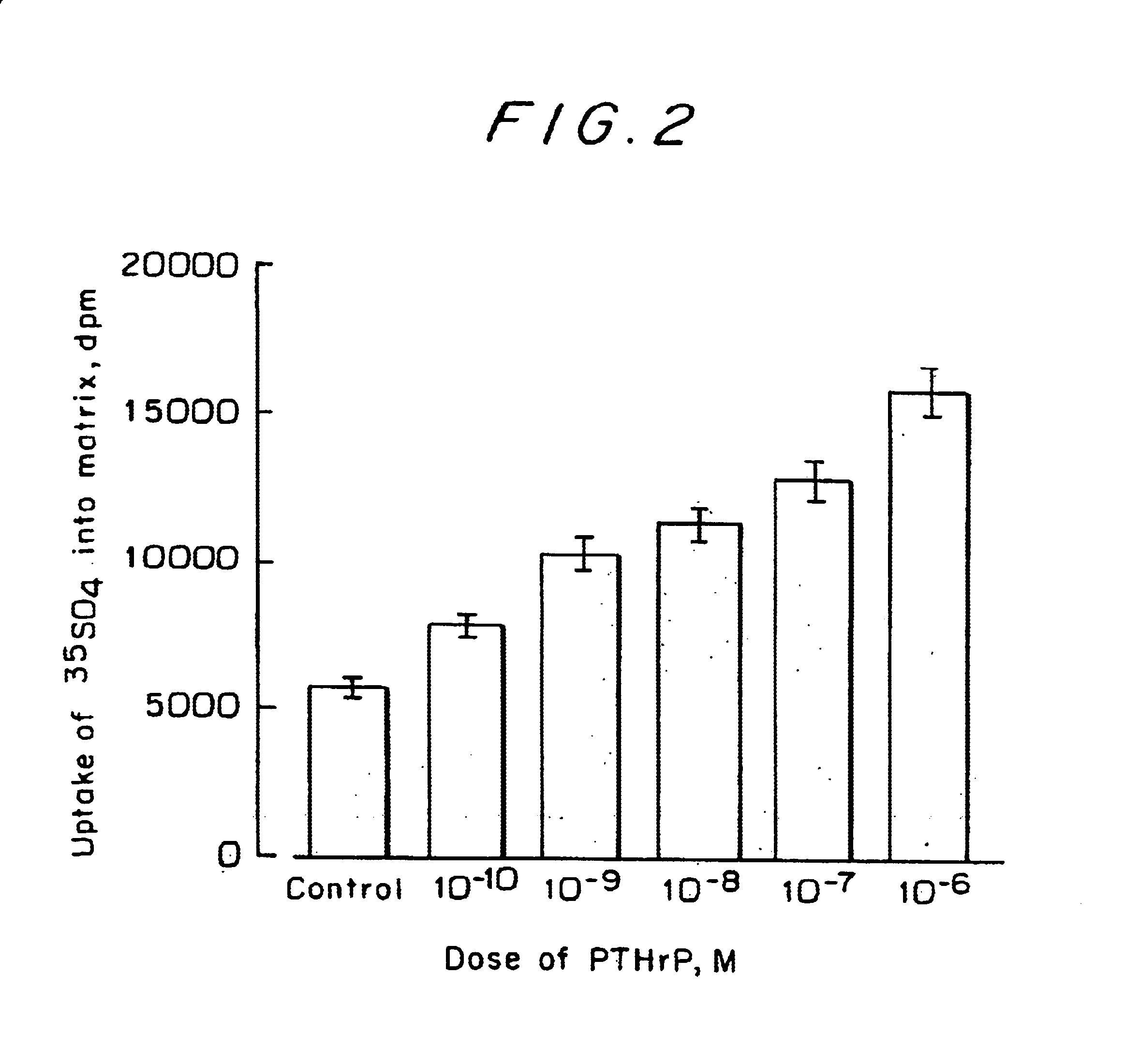
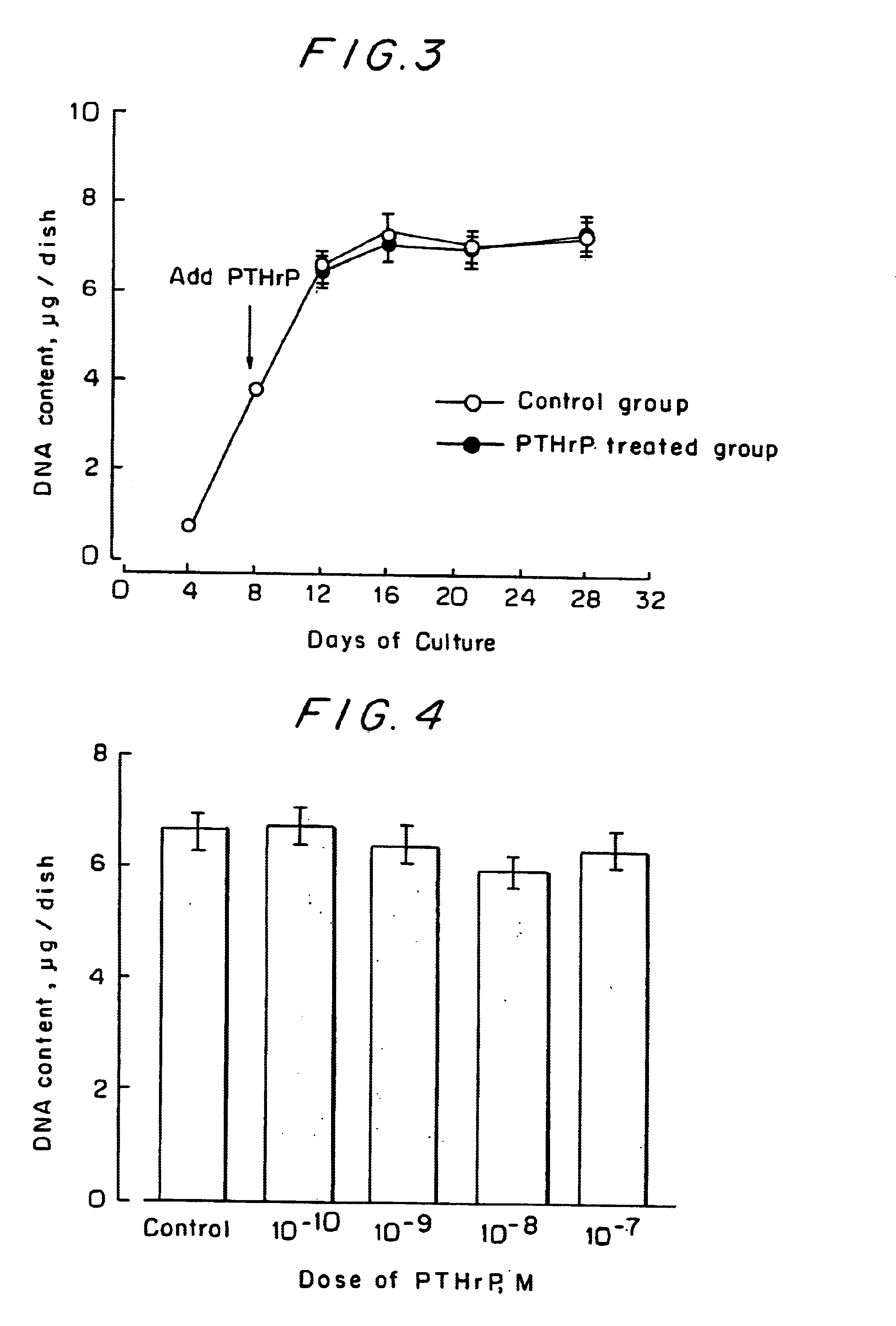
Drugs for preventing or treating diseases that involve the destruction and degeneration of articular cartilage tissue contain a parathyroid hormone related peptide (PTHrP) or a PTHrP derived substance as an effective ingredient.
Owner:KATO +2
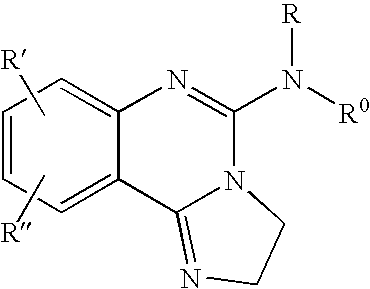
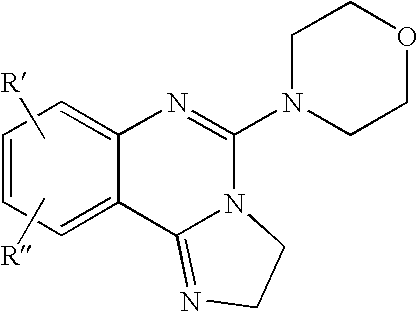
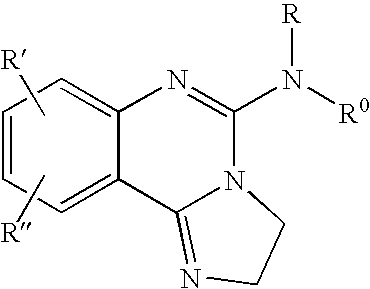
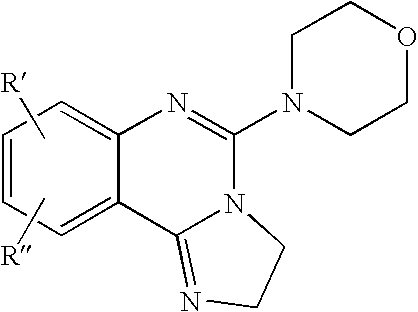
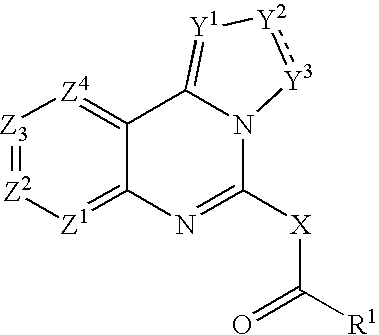
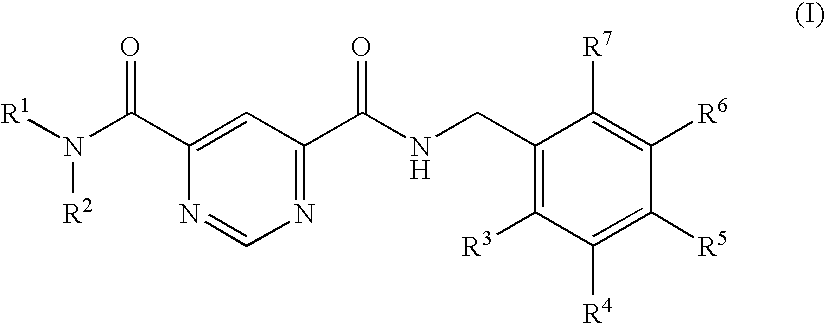
Fused azole-pyrimidine derivatives
Owner:BAYER INTELLECTUAL PROPERTY GMBH +1
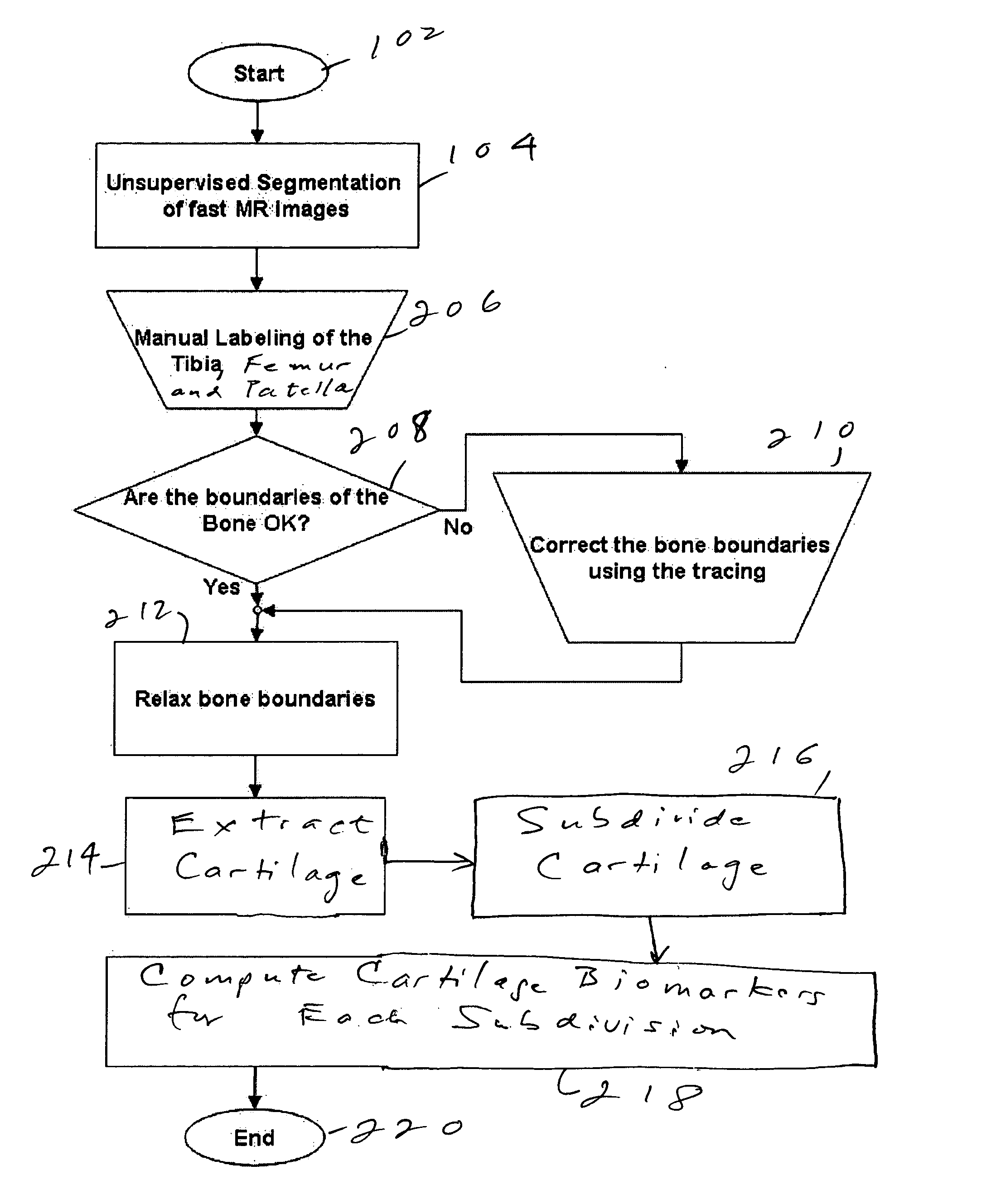
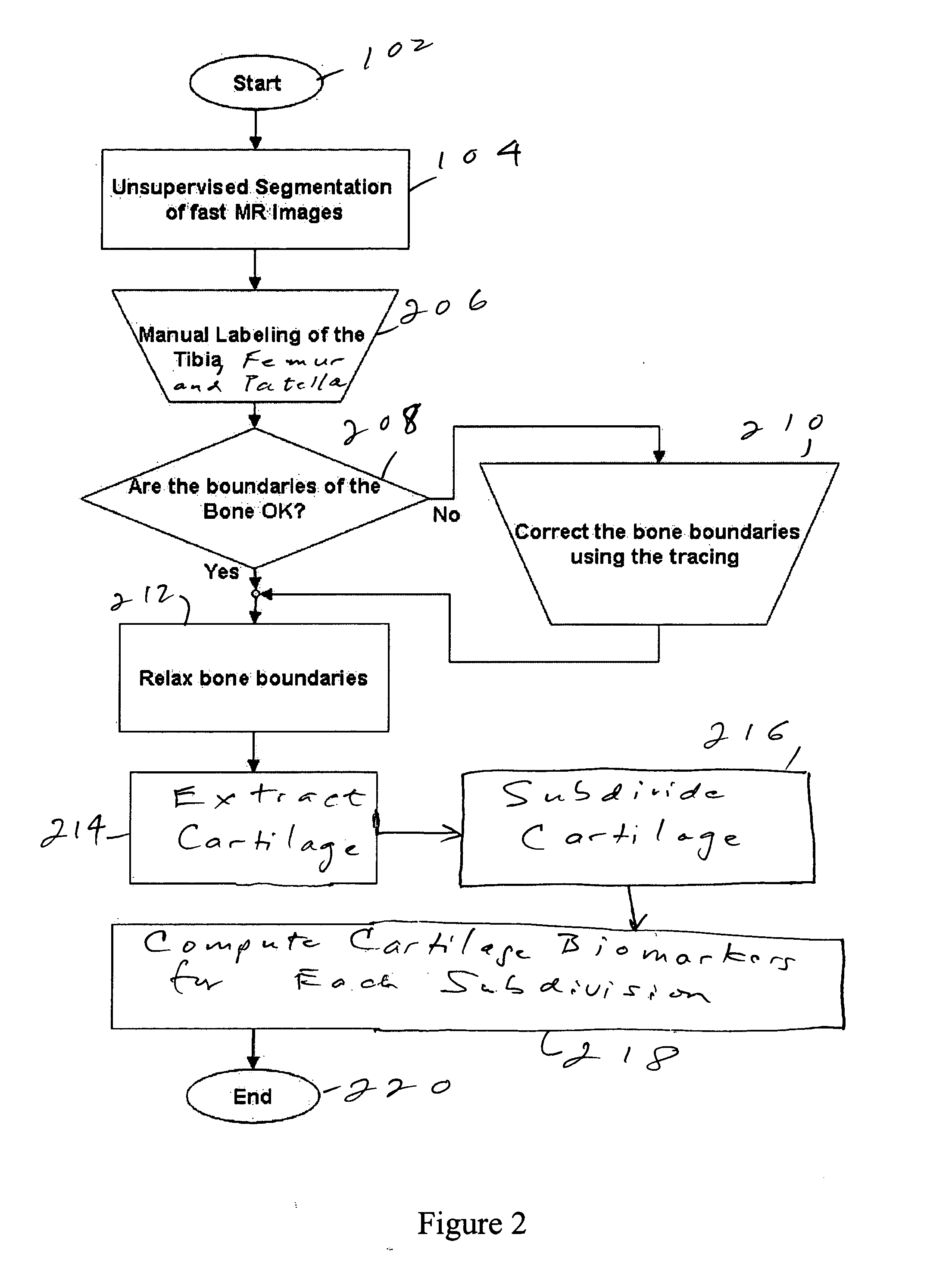
Method and system for automatic extraction of load-bearing regions of the cartilage and measurement of biomarkers
InactiveUS20050113663A1Improve assessmentImprove diagnostic capabilitiesImage enhancementImage analysisDiseaseNuclear medicine
An image is taken of a knee or other region of interest. The cartilage is extracted from the image and is subdivided into load-bearing and non-load-bearing regions. A biomarker is calculated for each of the load-bearing and non-load-bearing regions. The biomarkers can be assessed over time. The biomarkers for the load-bearing and non-load-bearing regions, and their changes, are used to assess the progress of joint disease.
Owner:VIRTUALSCOPICS
Composition and Method for Treating Connective Tissue Damage
ActiveUS20080003257A1Improve efficiencyPrevent degradationSuture equipmentsOrganic active ingredientsJoints inflammationJoint disease
The present invention provides a composition, and a method of use thereof for treating connective tissue damage in man and in animals, which comprises a therapeutically effective amount of chondroitin sulfate, N-acetyl D-glucosamine, and hyaluronan (hyaluronic acid). Particularly, the present invention provides a composition, and a method of use thereof, for treating connective tissue damage including, but not limited to, arthritic disease, osteoarthritis, rheumatoid arthritis, osterochondrosis dessicans, cartilage damage, joint injury, joint inflammation, joint synovitis, degenerative joint disease (DJD), post surgical DJD, traumatic injury, fracture, tendon damage, ligament damage, skeletal damage, musculoskeletal damage, fiber damage, adipose tissue damage, blood cell damage, and plasma damage. Compositions for delivery of the present invention include those for parenteral, oral, and transmucosal delivery and for direct surgical placement onto the affected tissues.
Owner:ARTHRODYNAMIC HLDG LLC
Compositions and Methods for Treating and Preventing Inflammatory and/or Degenerative Processes in Humans and Other Animals
Disclosed are compositions useful for treating Alzheimer's disease, atherosclerosis, arteriosclerosis, osteoarthritis and other degenerative joint diseases, Huntington's chorea, Parkinson's disease, optic atrophy, retinitis pigmentosa, macular degeneration, muscular dystrophy, aging-associated degenerative processes, asthma, dermatitis, laminitis, pemphigoid, pemphigus, reactive airway disease (e.g., COPD, IAD), inflammatory bowel disease (e.g., Crohn's disease, ulcerative colitis), multiple sclerosis, rheumatoid arthritis, periodontal disease, systemic lupus erythematosus, sarcoidosis, psoriasis, type I diabetes, ischemia-reperfusion injury, chronic inflammatory diseases, geriatric wasting, cancer cachexia, cachexia associated with chronic inflammation, sick feeling syndrome, and other inflammatory and / or degenerative diseases, disorders, conditions, and processes in humans and other animals. In one embodiment, the compositions include at least 4 of the following: a MMP1 inhibitor, a MMP2 inhibitor, a MMP3 inhibitor, a MMP7 inhibitor, a MMP9 inhibitor, an ADAMTS-4 inhibitor, a MMP13 inhibitor, and a MMP14 inhibitor. In another embodiment, the compositions include a curcuminoid, a polymethoxylated flavone, a catechin, and a boswellic acid.
Owner:BAKER DONALD J
Fused azole-pyrimidine derivatives
The present invention relates to hovel fused azolepyrimidine derivatives, processes for preparing them and pharmaceutical preparations containing them. The fused azolepyrimidine derivatives of the present invention exhibit enhanced potency for phosphotidylinositol-3-kinase (PI3K) inhibition, especially for PI3K-γ inhibition and can be used for the prophylaxis and treatment of diseases associated with PI3K and particularly with PI3K-γ activity. More specifically, the azole derivatives of the present invention are useful for treatment and prophylaxis of diseases as follows: inflammatory and immunoregulatory disorders, such as asthma, atopic dermatitis, rhinitis, allergic diseases, chronic obstructive pulmonary disease (COPD), septic shock, joint diseases, autoixnmune pathologies such as rheumatoid arthritis, and Graves' disease, cancer, myocardial contractility disorders, heart failure, thromboembolism, ischemia, and atherosclerosis. The compounds of the present invention are also useful for pulmonary hypertension, renal failure, cardiac hypertrophy, as well as neurodegenerative disorders such as Parkinson's disease, Alzheimer's disease, diabetes and focal ischemia, since the diseases also relate to PI3K activity in a human or animal subject.
Owner:BAYER INTELLECTUAL PROPERTY GMBH +1
Composition and method for treating connective tissue damage
Owner:ARTHRODYNAMIC HLDG LLC
Hyaluronic acid modification product
The present invention provides a safe hyaluronic acid base material that is suitable for use in practical hyaluronic acid pharmaceutical preparations capable of distribution at room temperature and having such a low viscosity that injection is easy. The hyaluronic acid pharmaceutical preparations can reside in a joint cavity for a prolonged period of time while exhibiting analgesic effects. More specfically, there is provide a hyaluronic acid modification product in which hyaluronic acid and / or a pharmaceutically acceptable salt thereof is bounded to a block polymer selected from PEO-PPO-PEO, PPO-PEO-PPO, PEO-PLGA-PEO, PLGA-PEO-PLGA, PEO-PLA-PEO and PLA-PEO-PLA. The hyaluronic acid modification product, despite capability of distribution at room temperature and ease in handling because of the low viscosity, can have its viscoelasticity rapidly increased after injection into a living body, so that it is highly useful in treatment of joint diseases, aid in surgical operation, repair of tissue, etc. as a main ingredient of novel and practical hyaluronic acid pharmaceutical preparations.
Owner:CHUGAI PHARMA CO LTD
Nutritional composition beneficial for bone and joint health
InactiveCN106135890AEffective nutritional supplementPromotes joint repairFood preparationNutritional statusPhosphopeptide
The invention provides a nutritional composition suitable for patients suffering from bone and joint diseases. The nutritional composition is prepared from 20%-40% of marine fish oligopeptides, 10%-30% of ossein protein peptides, 1%-5% of organic calcium, 1%-5% of casein phosphopeptides, 0.1%-1% of a saussurea involucrate culture, 1%-5% of shark cartilage powder, 5%-20% of inulin, 5%-30% of oligopeptides, 0.1%-1% of vitamins, 1%-3% of mineral substances and the like. The marine food which is used for special dietary uses, is suitable for being eaten by the patients suffering from the bone and joint diseases and contains the marine fish oligopeptides and the ossein protein peptides is provided according to physiological characteristics, nutritional requirements and conventional diet defects of the patients suffering from the bone and joint diseases, and the food has the effects of improving the nutritional condition of the organism, promoting rehabilitation of bones and joints, assisting in controlling blood glucose and conditioning the intestinal functions of the organism and the like.
Owner:QINGDAO BETTER BIO TECH
Composition and method for treatment of joint damage
ActiveUS7485629B2Facilitating nutrient transferPromote regenerationBiocideCarbohydrate active ingredientsWhole bodyInflammatory arthropathy
The invention provides compositions useful for the treatment and / or prevention of damage to diarthrodial (synovial) joints and, in particular, traumatic synovitis, inflammation of the synovial membrane, and damage to the articular cartilage of the joint. Specifically, provided are compositions specially formulated for intra-articular and / or parenteral use in the treatment and / or prevention of traumaticsynovitis and / or damage to articular cartilage. Compositions adapted specifically for post surgical joint lavage or treatment and / or prevention of inflammatory arthritis, osteoarthritis (OA) and / or degenerative joint disease (DJD) are also provided. Compositions adapted for intra-articular and / or systemic administration comprised of therapeutic amounts of: chondroitin sulfate and hyaluronan (hyaluronic acid) are provided.
Owner:ARTHRODYNAMIC HLDG LLC
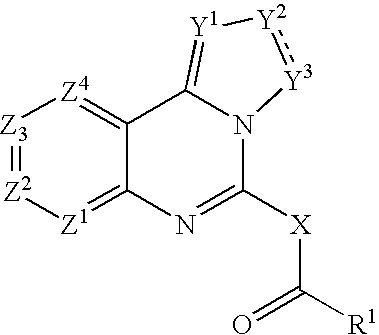
Selective MMP-13 inhibitors
The invention relates to a pyrimidine-4,6-dicarboxylic acid diamide compound, pharmaceutical preparation comprising it, process for preparing it and method for its pharmaceutical use. Particularly, the pyrimidine-4,6-dicarboxylic acid diamide compound is useful for selectively inhibiting collagenase matrix metalloproteinase (MMP) 13, or for treating a degenerative joint disease.
Owner:SANOFI AVENTIS DEUT GMBH
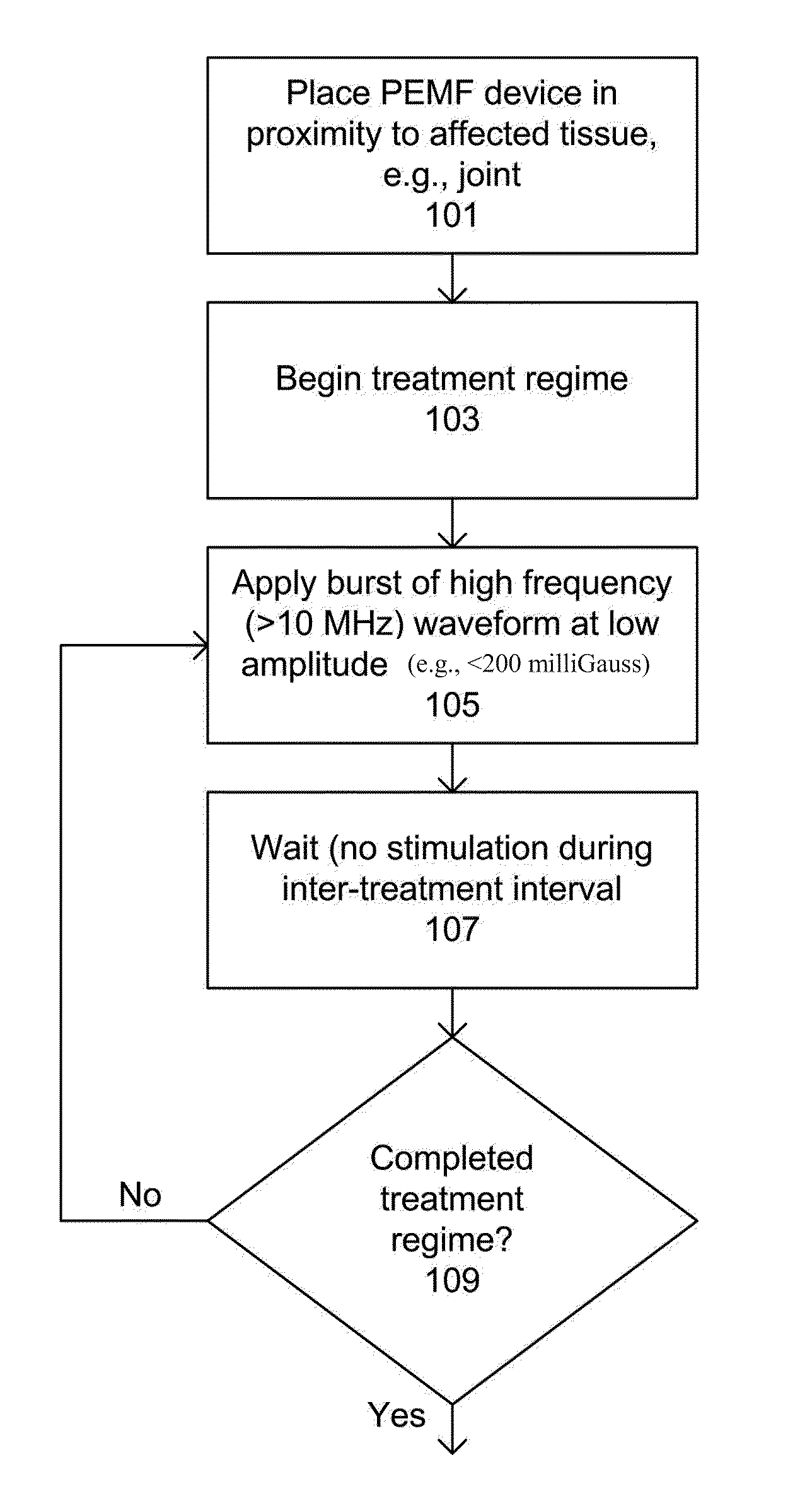
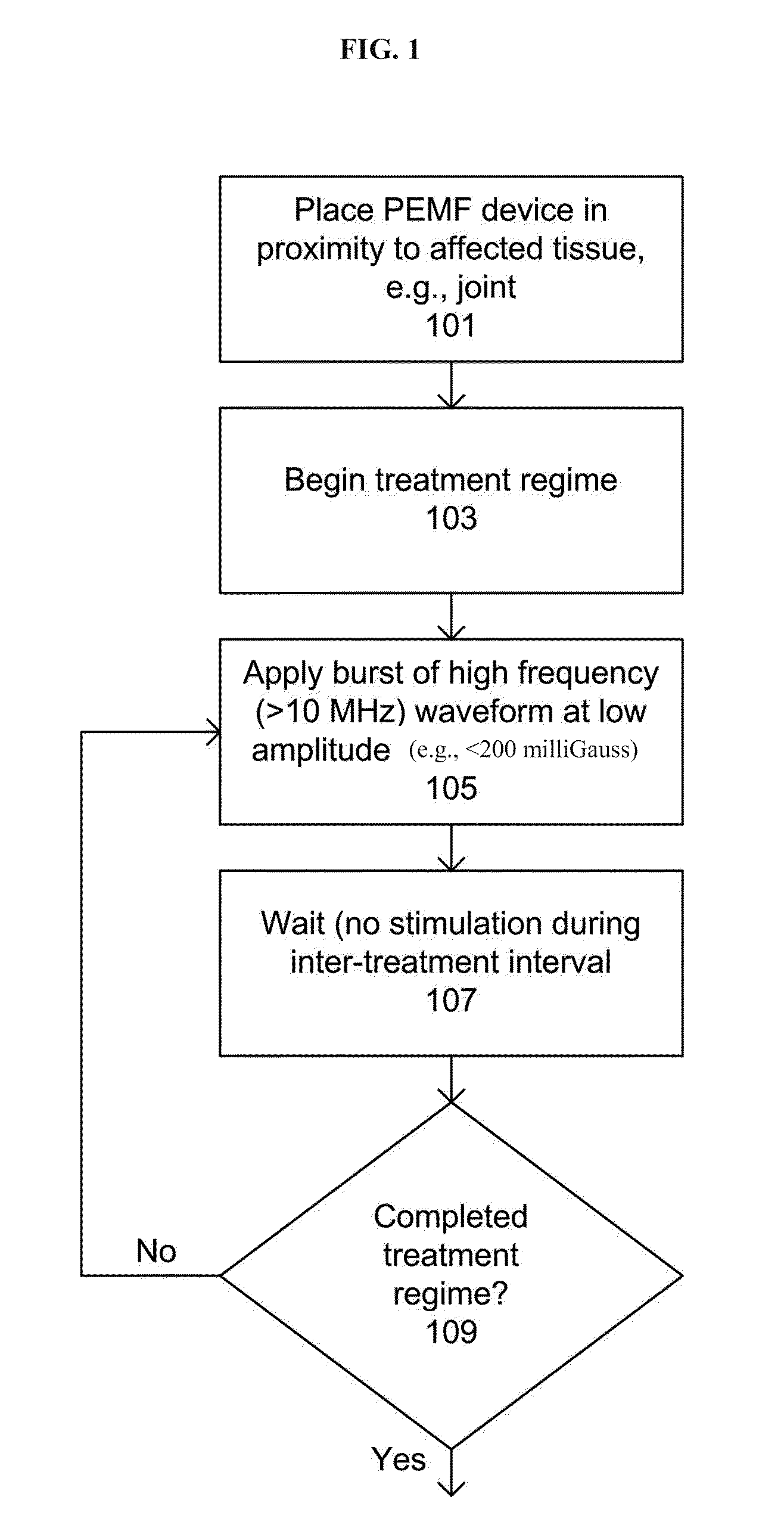
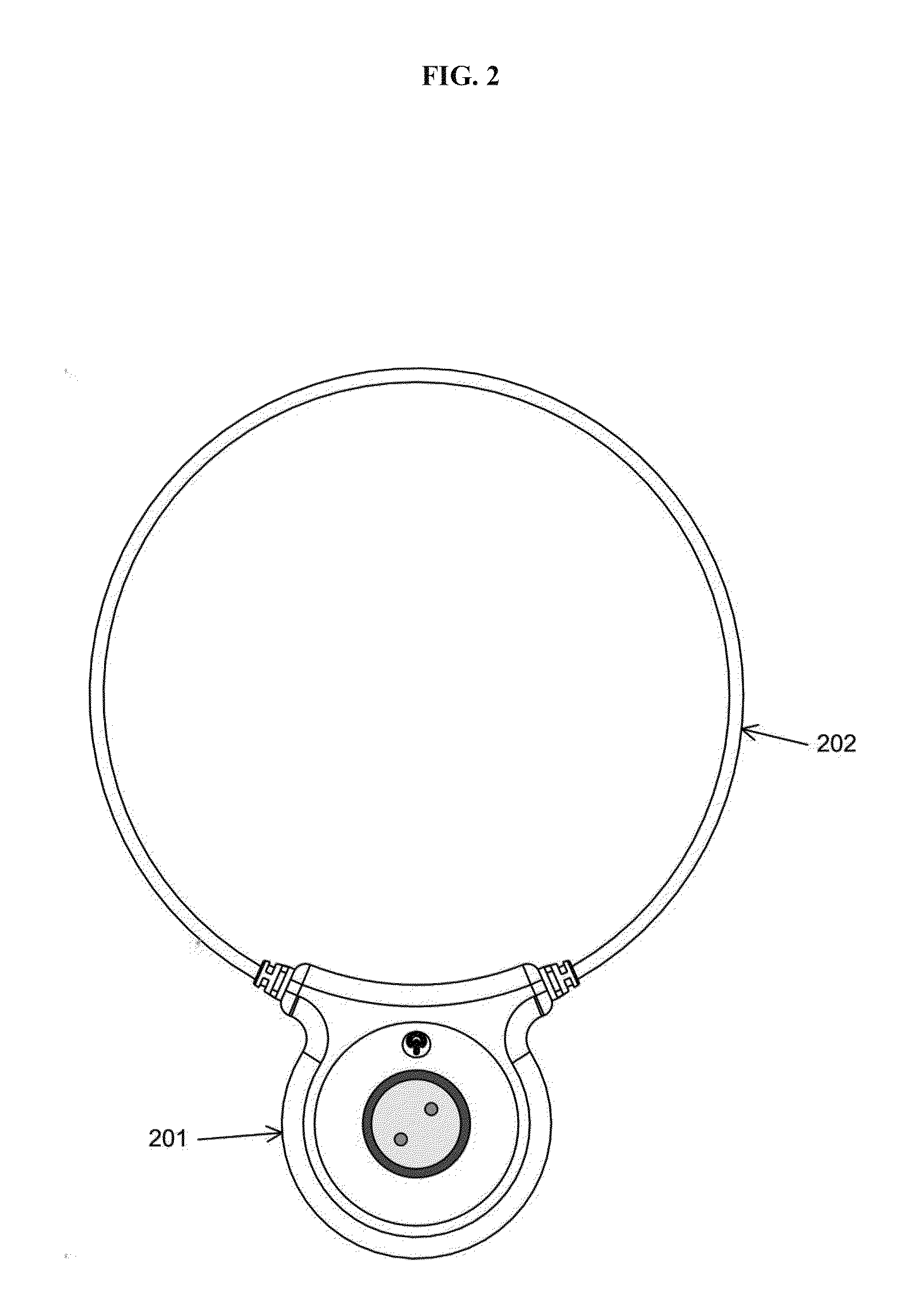
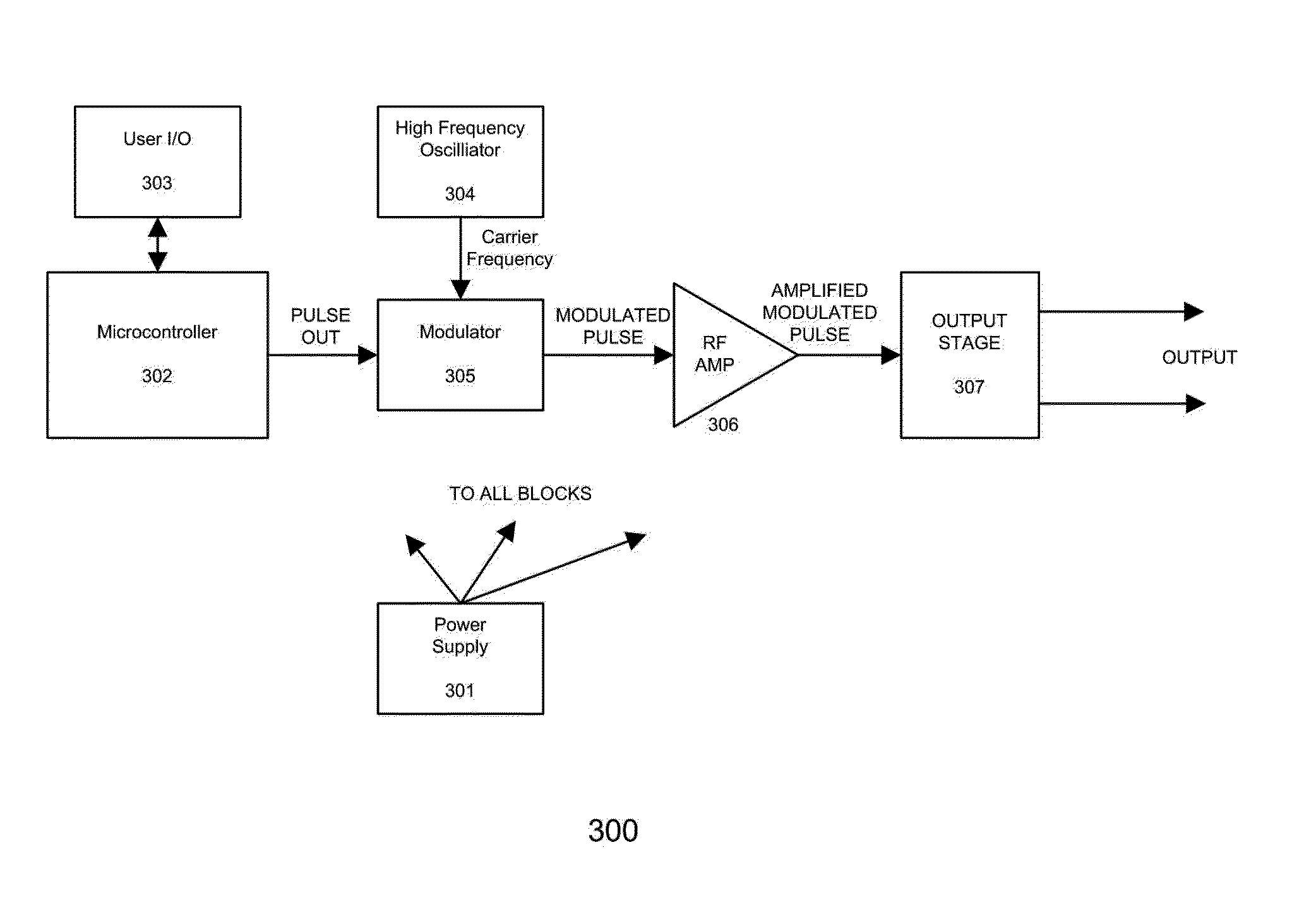
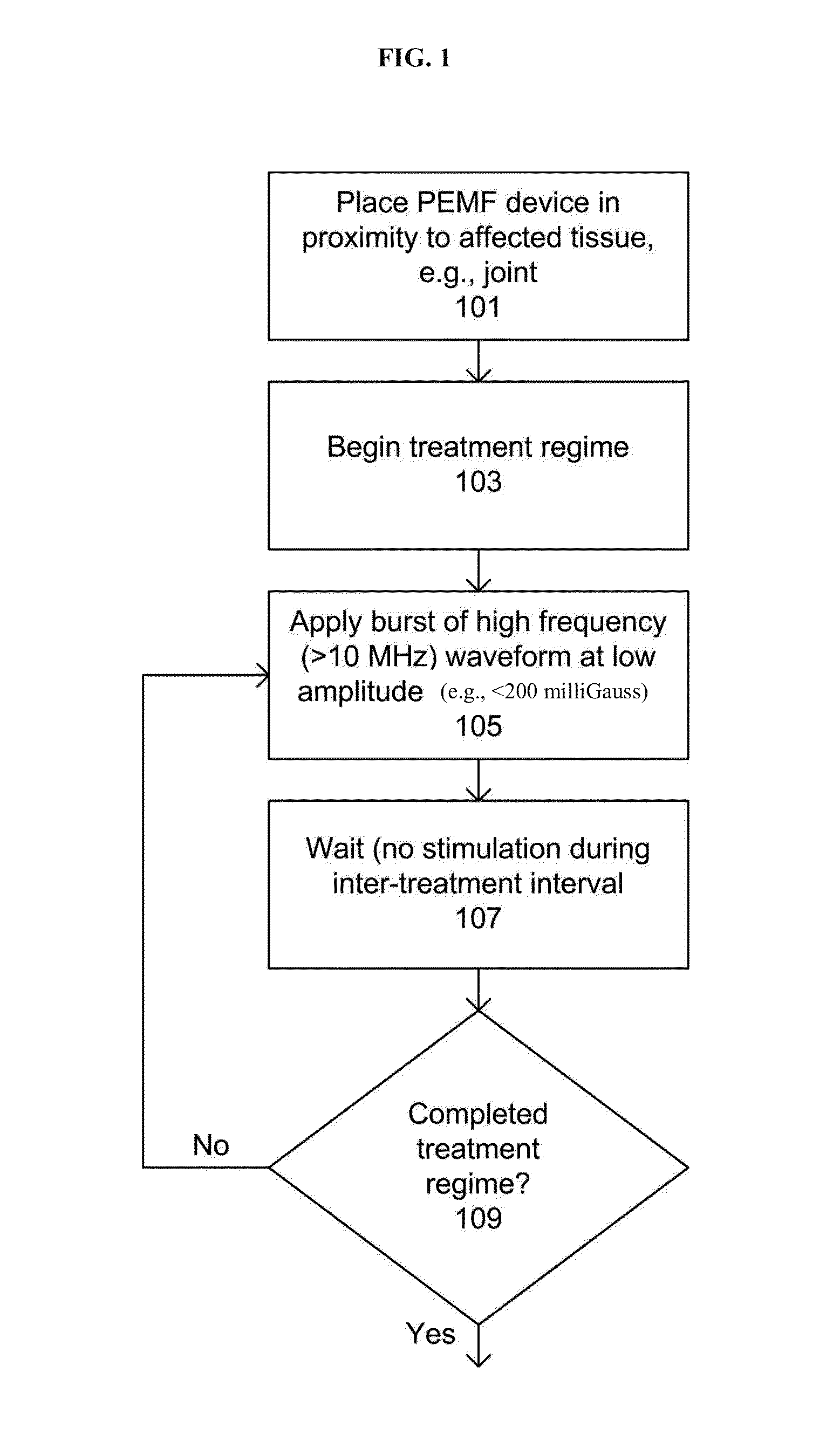
Devices and method for treatment of degenerative joint diseases with electromagnetic fields
InactiveUS20110207989A1Reduce pro-inflammatory cytokine pro-inflammatoryReduce pro-inflammatory other pro-inflammatory pathwayElectrotherapyMagnetotherapy using coils/electromagnetsDiseaseElectromagnetic field
Described herein are devices and methods for treating degenerative joint diseases with electromagnetic fields using one or more waveforms that are configured to modulate Ca2+ binding to calmodulin and thereby modulate calmodulin-dependent nitric oxide signaling within joint and other affected tissue for the purpose of reducing pain and inflammation, as well as enhancing the healing and regeneration of such tissue.
Owner:ENDONOVO THERAPEUTICS INC
Devices and method for treatment of degenerative joint diseases with electromagnetic fields
InactiveUS8961385B2Enhance immune responseEnhanced signalElectrotherapyMagnetotherapy using coils/electromagnetsDiseaseElectromagnetic field
Described herein are devices and methods for treating degenerative joint diseases with electromagnetic fields using one or more waveforms that are configured to modulate Ca2+ binding to calmodulin and thereby modulate calmodulin-dependent nitric oxide signaling within joint and other affected tissue for the purpose of reducing pain and inflammation, as well as enhancing the healing and regeneration of such tissue.
Owner:ENDONOVO THERAPEUTICS INC
Treatment of degenerative joint disease
ActiveUS20130090292A1Improve throughputLimit deliverySkeletal disorderPharmaceutical delivery mechanismAmino acid side chainDiketopiperazines
The invention provides a method of treating a degenerative joint disease. The method comprises administering an effective amount of a pharmaceutical composition comprising a diketopiperazine with amino acid side chains of aspartic acid and alanine (DA-DKP). The invention also provides a pharmaceutical product as well as a kit comprising DA-DKP.
Owner:AMPIO PHARMA
Diagnostic polymorphisms for the ecnos promoter
InactiveUS20050084849A1Eliminate the effects ofDigital data processing detailsMicrobiological testing/measurementValvular diseaseProstate cancer
Disclosed are single nucleotide polymorphisms (SNIps) associated with breast cancer, lung cancer, prostate cancer, non-insulin dependent diabetes, end stage renal disease due to non-insulin dependent diabetes, hypertension, end stage renal disease F due to hypertension, myocardial infarction, colon cancer, hypertension, atherosclerotic peripheral vascular disease due to hypertension, cerebrovascular accident due to hypertension, cataracts due to hypertension, cardiomyopathy with hypertension, myocardial infarction due to hypertension, non-insulin dependent diabetes mellitus, atherosclerotic peripheral vascular disease due to non-insulin dependent diabetes mellitus, cerebrovascular accident due to non-insulin dependent diabetes mellitus, ischemic cardiomyopathy, ischemic cardiomyopathy with non-insulin dependent diabetes mellitus, myocardial infarction due to non-insulin dependent diabetes mellitus, atrial fibrillations without valvular disease, alcohol abuse, anxiety, asthma, chronic obstructive pulmonary disease. cholecystectomy, degenerative joint disease, end stage renal disease and frequent de-clots, end stage renal disease due to focal segmental glomerular sclerosis, end stage renal disease due to insulin dependent diabetes mellitus, or seizure disorder. Also disclosed are methods for using SNPs to determine susceptibility to these diseases; nucleotide sequences containing SNPs; kits for determining the presence of SNPs; and methods of treatment or prophylaxis based on the presence of SNPs.
Owner:VIRAL THERAPEUTICS
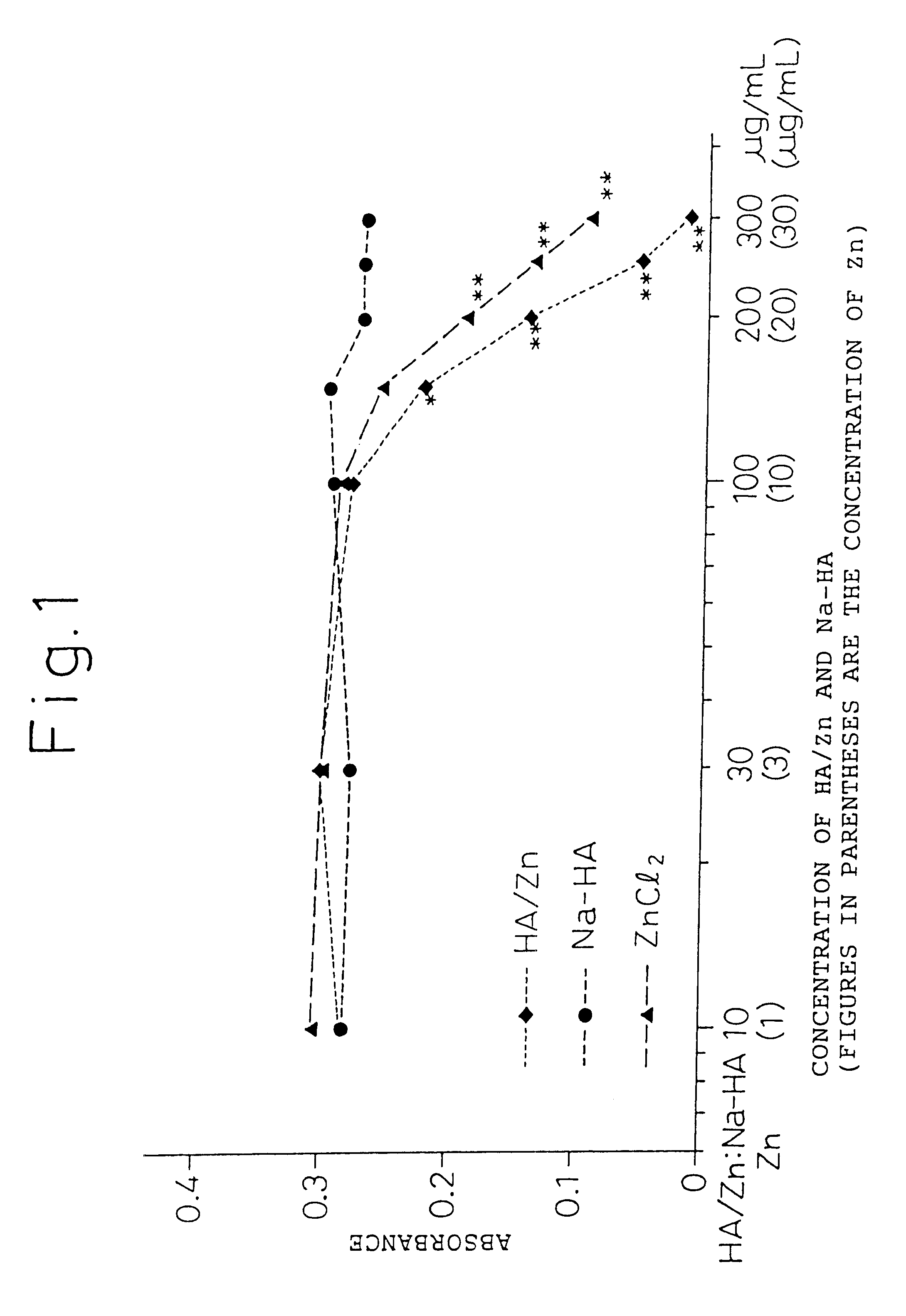
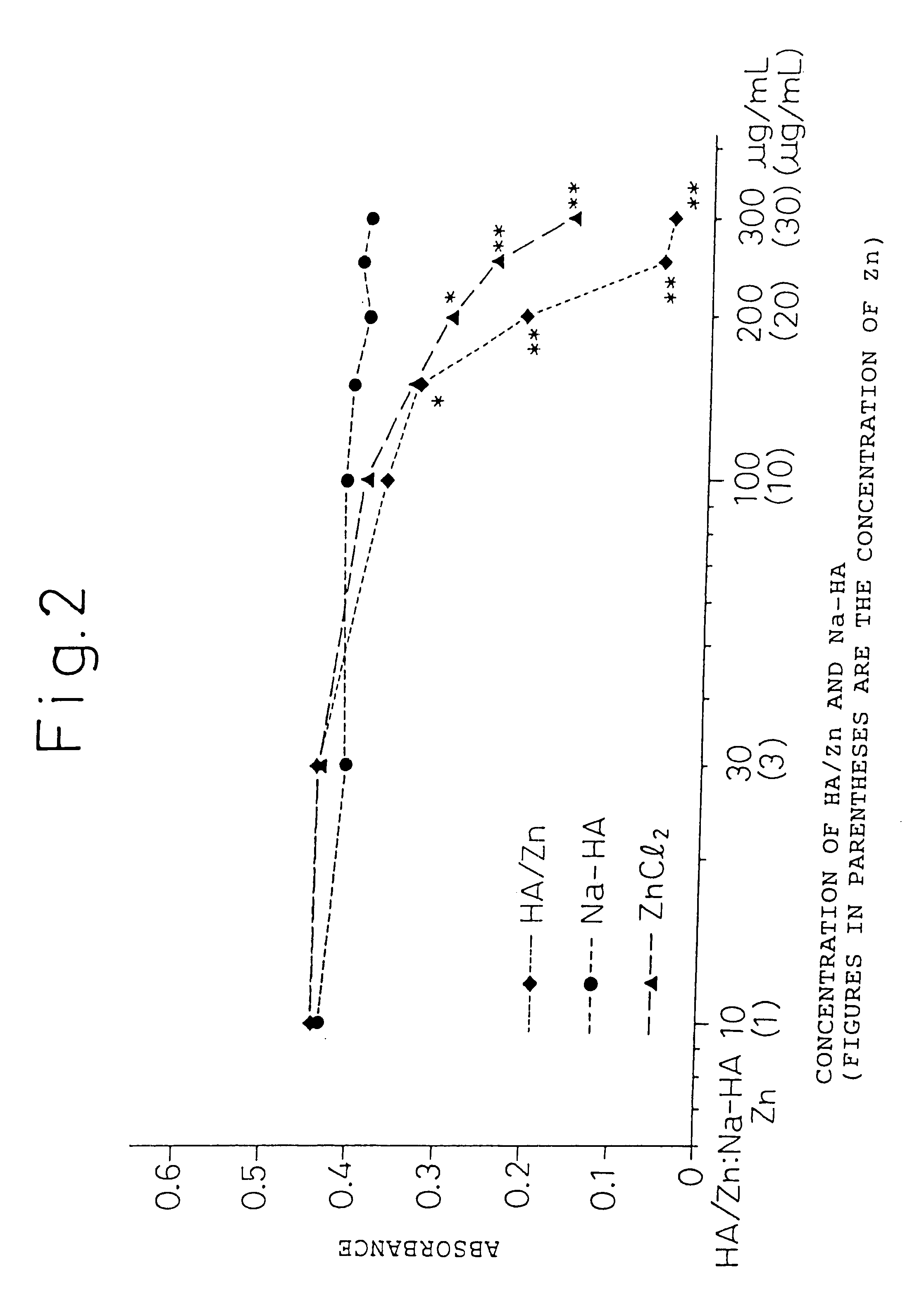
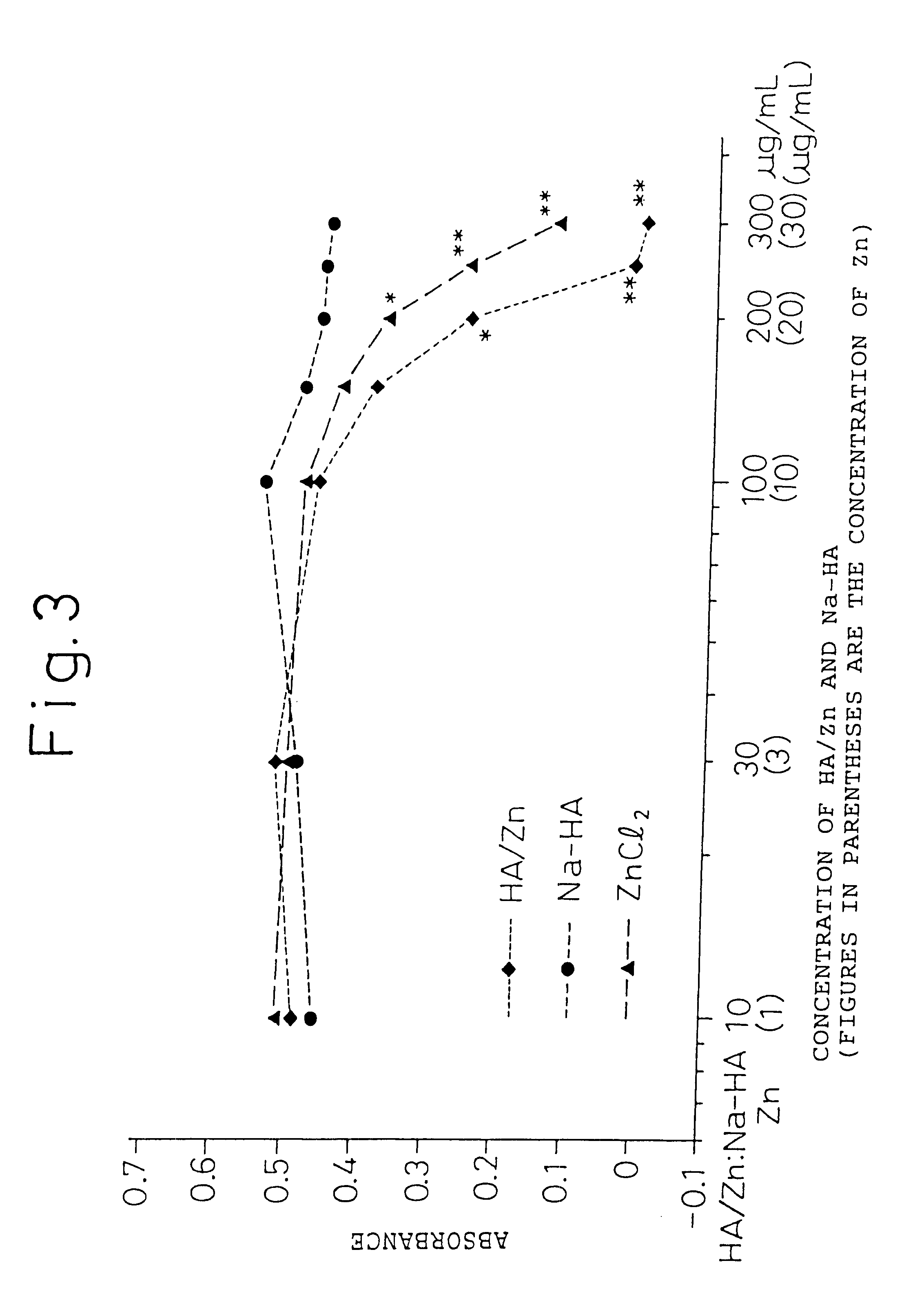
Remedies for joint diseases
InactiveUS6608043B1Effective treatmentSuppressing matrix metalloproteinaseBiocideSenses disorderDiseaseSynovial Cell
The present invention provides an agent for treatment of arthritic diseases such as rheumatoid arthritis that has for its active ingredient a complex of hyaluronic acid and zinc. This complex synergistically inhibits proliferation of synovial cells and suppresses matrix metalloproteinase MMP-9, which is produced by synovial cells, as compared with its constituents, hyaluronic acid and zinc, alone.
Owner:TAKATA SEIYAKU +1
Methods for the treatment of inflammatory joint disease
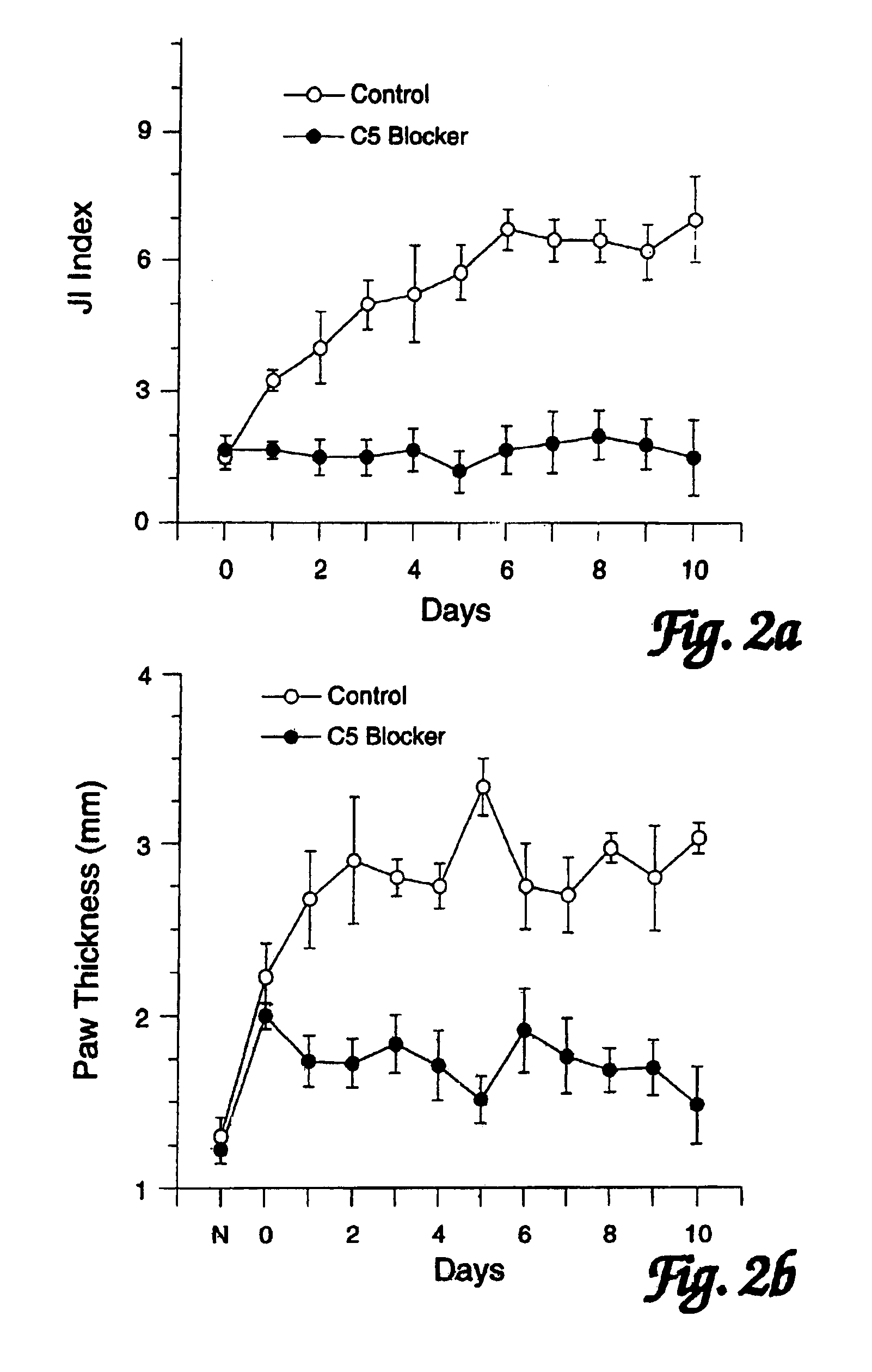
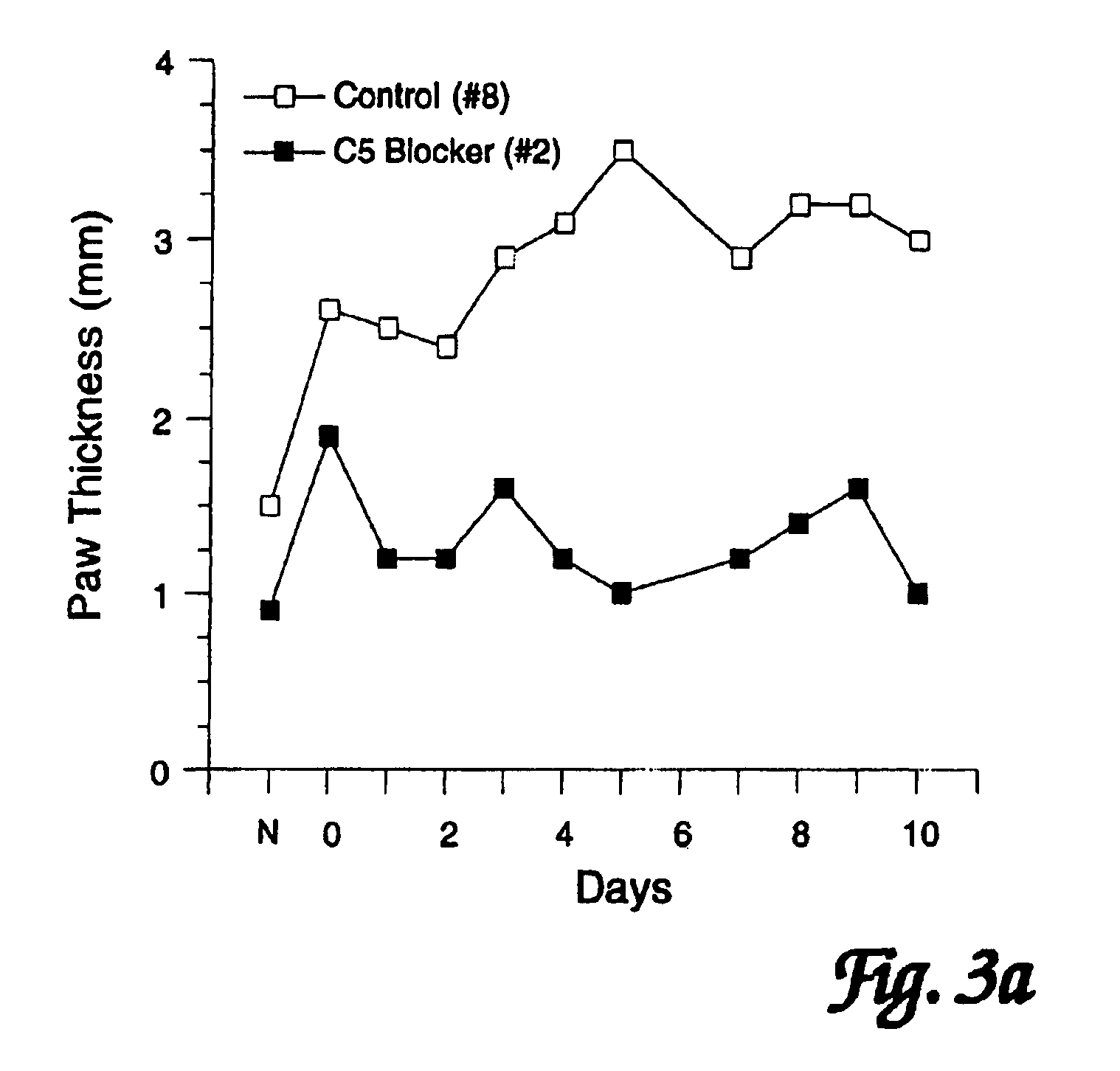
InactiveUS7279158B2Reduction and stabilization of inflammationAvoid spreadingPeptide/protein ingredientsAntipyreticJoints inflammationComplement component C5
The use of compounds that block complement component C5 or its active fragments C5a and / or C5b (such compounds collectively referred to as “C5 blockers”) to treat established joint inflammation (arthritis) is disclosed. Administration of such C5 blockers has been found to: 1) arrest and / or reduce inflammation in joints which are already inflamed, and 2) inhibit the spread of inflammation to unaffected joints.
Owner:ALEXION PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com