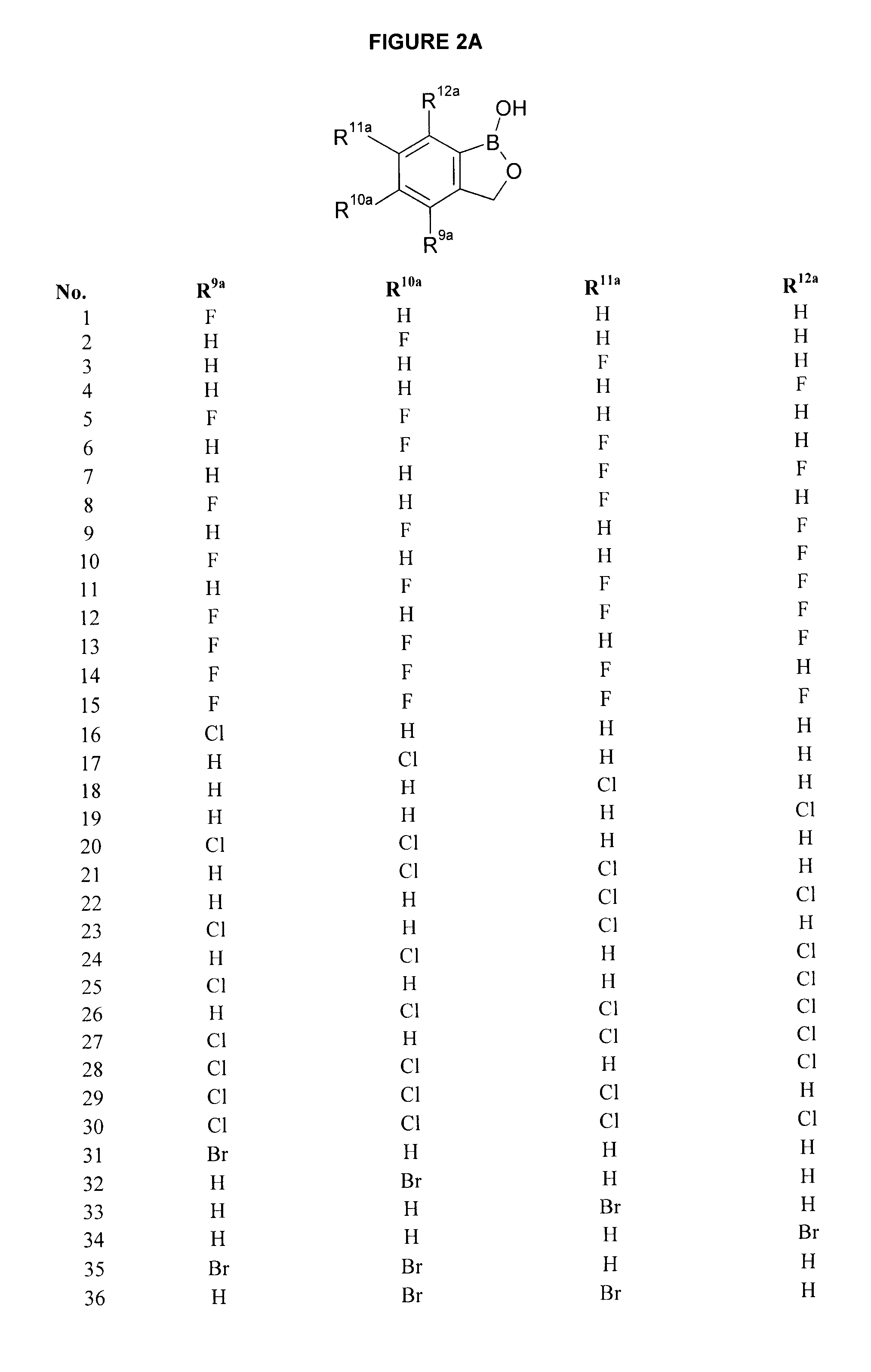Patents
Literature
773 results about "Periodontal disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Personal Care Compositions Comprising An Antimicrobial Blend of Essential Oils or Constituents Thereof
InactiveUS20080253976A1High activitySuppressing growthAntibacterial agentsCosmetic preparationsPersonal careAdditive ingredient
Disclosed are personal care compositions, including compositions for oral, throat and skin care comprising a blend of naturally occurring flavor or perfume ingredients or essential oils containing such ingredients, wherein the blend provides excellent antimicrobial activity and comprises at least two components, a first acyclic component selected from citral, neral, geranial, geraniol and nerol and a second cyclic-containing component selected from eucalyptol, carvacrol and eugenol. Preferably, the blend comprises 3, 4, 5 or more of the above components. Greater synergy in terms of antimicrobial efficacy may be obtained the more different components are blended together. The present compositions are effective in killing, suppressing the growth of and / or altering metabolism of microorganisms including those which cause undesirable oral cavity conditions including plaque, caries, calculus, gingivitis, periodontal disease and malodor. Optionally the blend further comprises additional antimicrobial and / or anti-inflammatory components, preferably naturally-occurring as well.
Owner:THE PROCTER & GAMBLE COMPANY
Morinda citrifolia-based oral care compositions and methods
InactiveUS20050084551A1Reduce penetrationReduce enzyme activityCosmetic preparationsBiocideDiseaseIrritation
Owner:TAHITIAN NONI INT INC
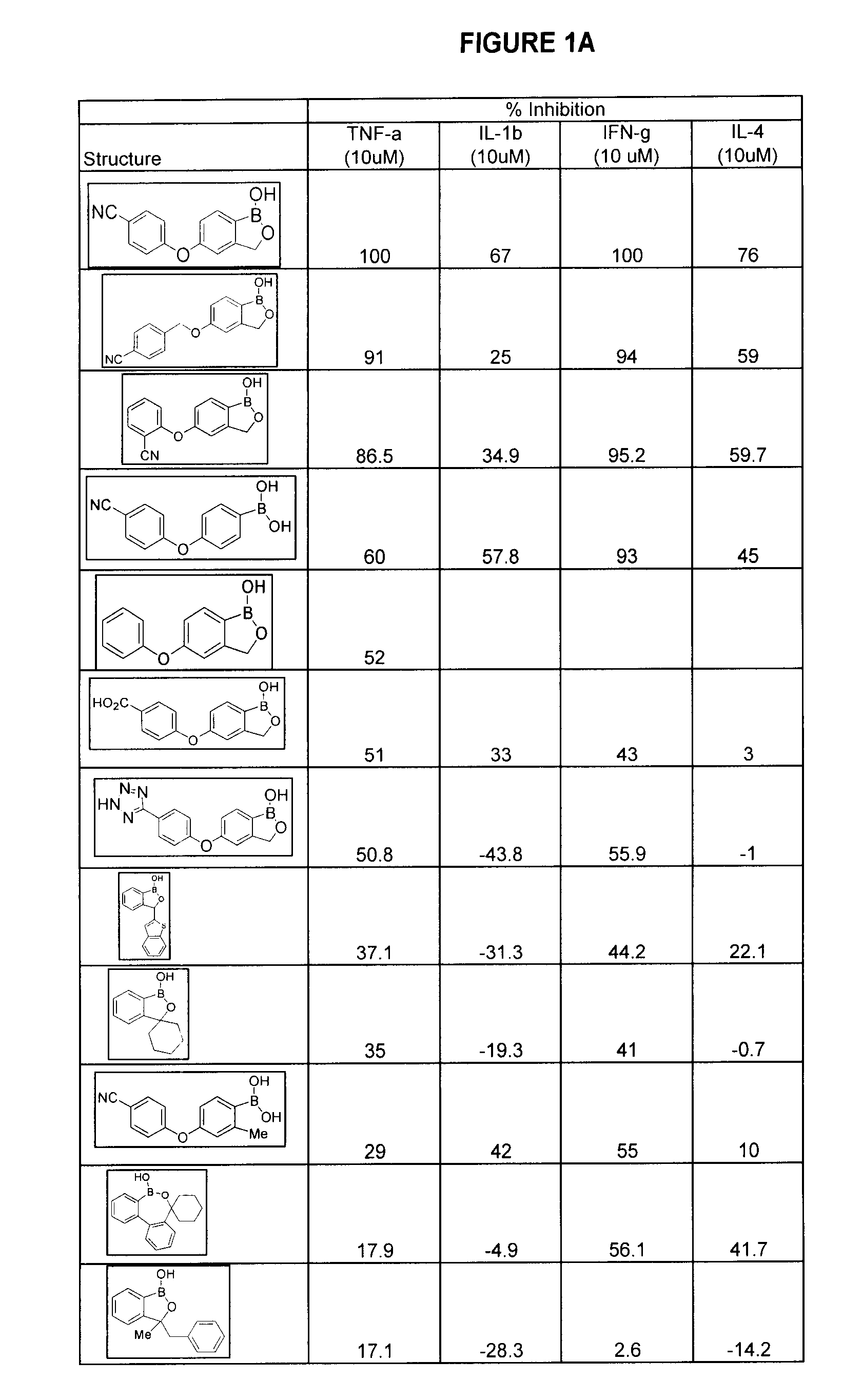
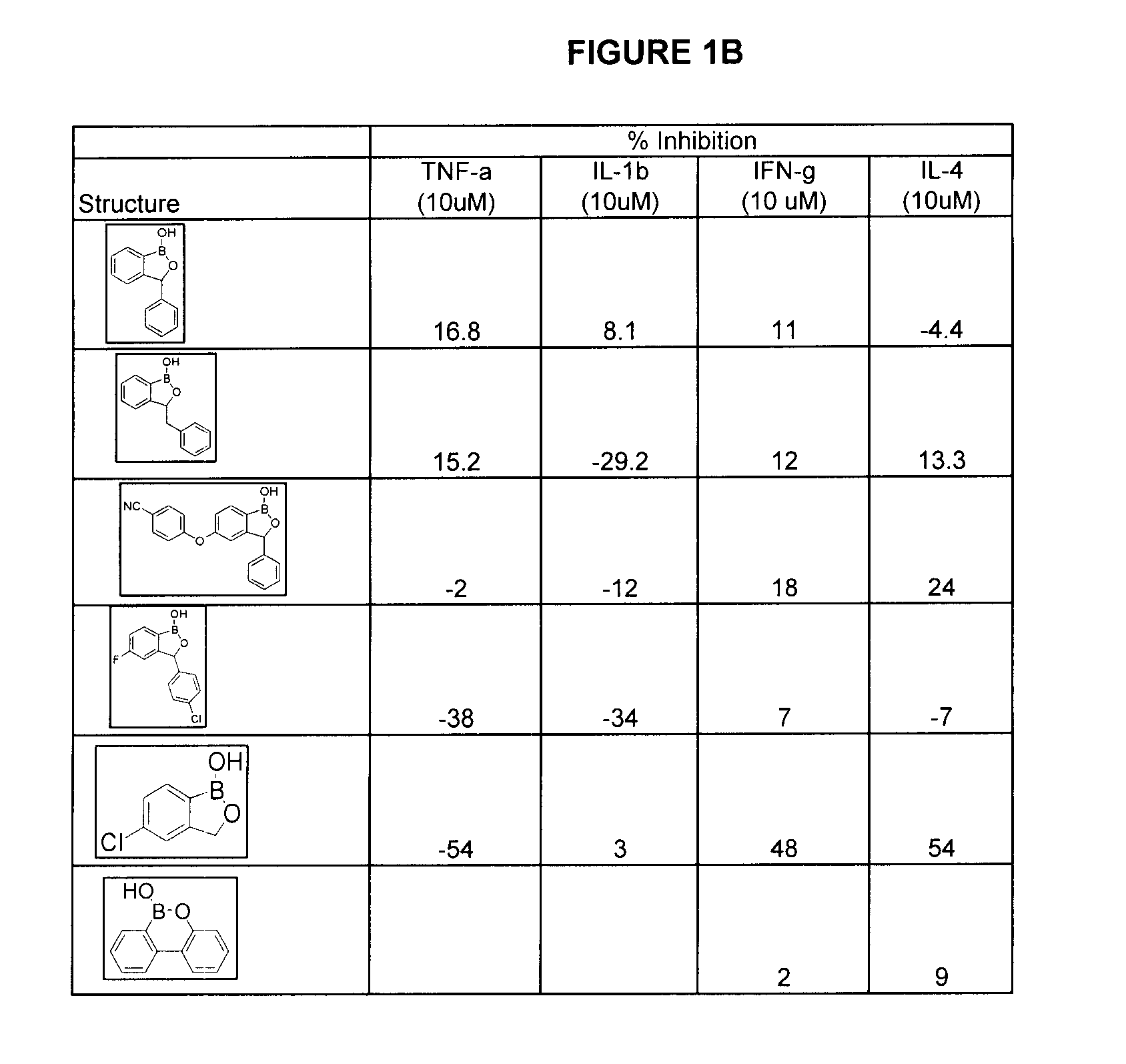
Compounds for the Treatment of Periodontal Disease
Compounds, compositions and methods are provided which are useful in the treatment of periodontal disease.
Owner:ANACOR PHARMA INC
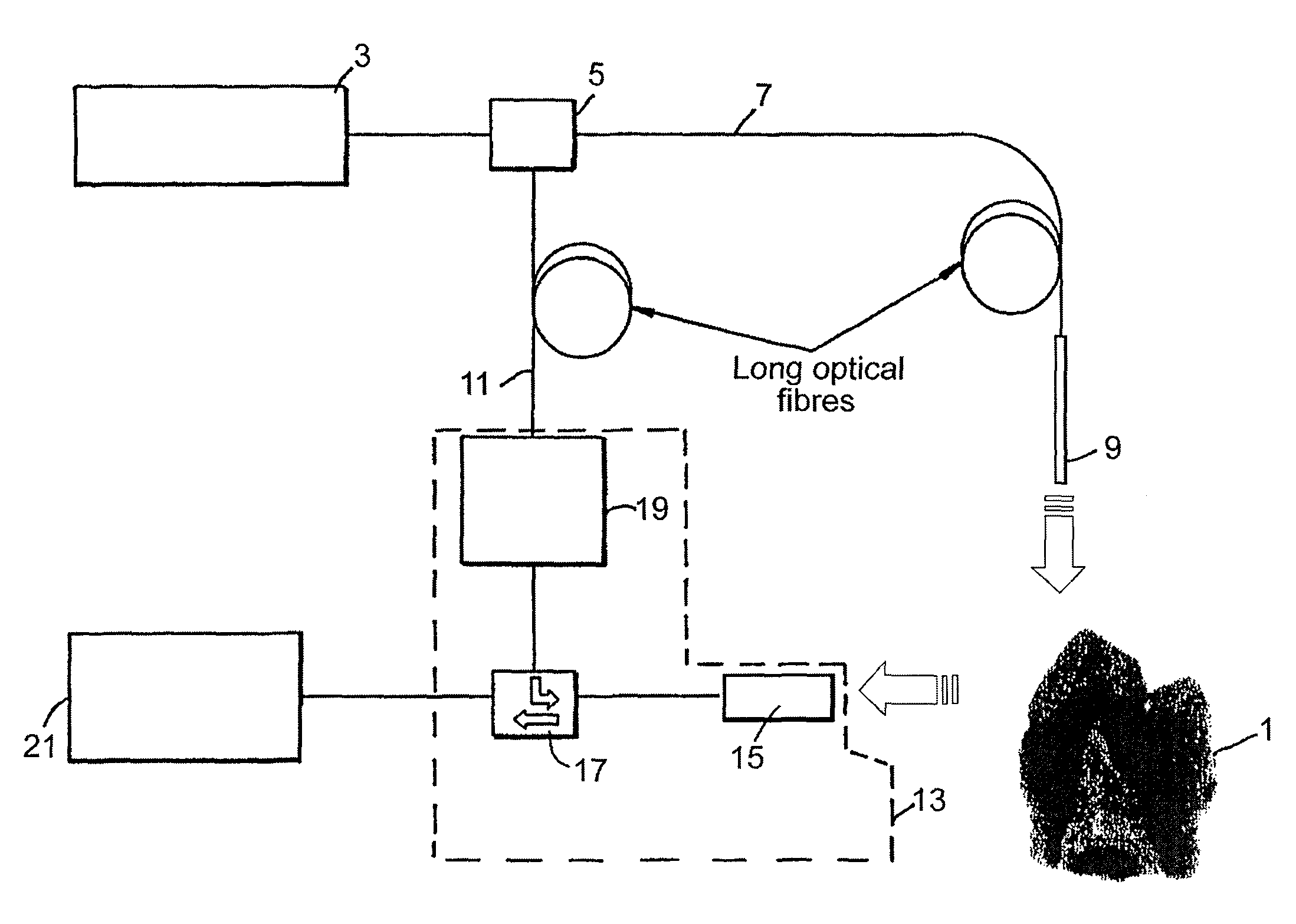
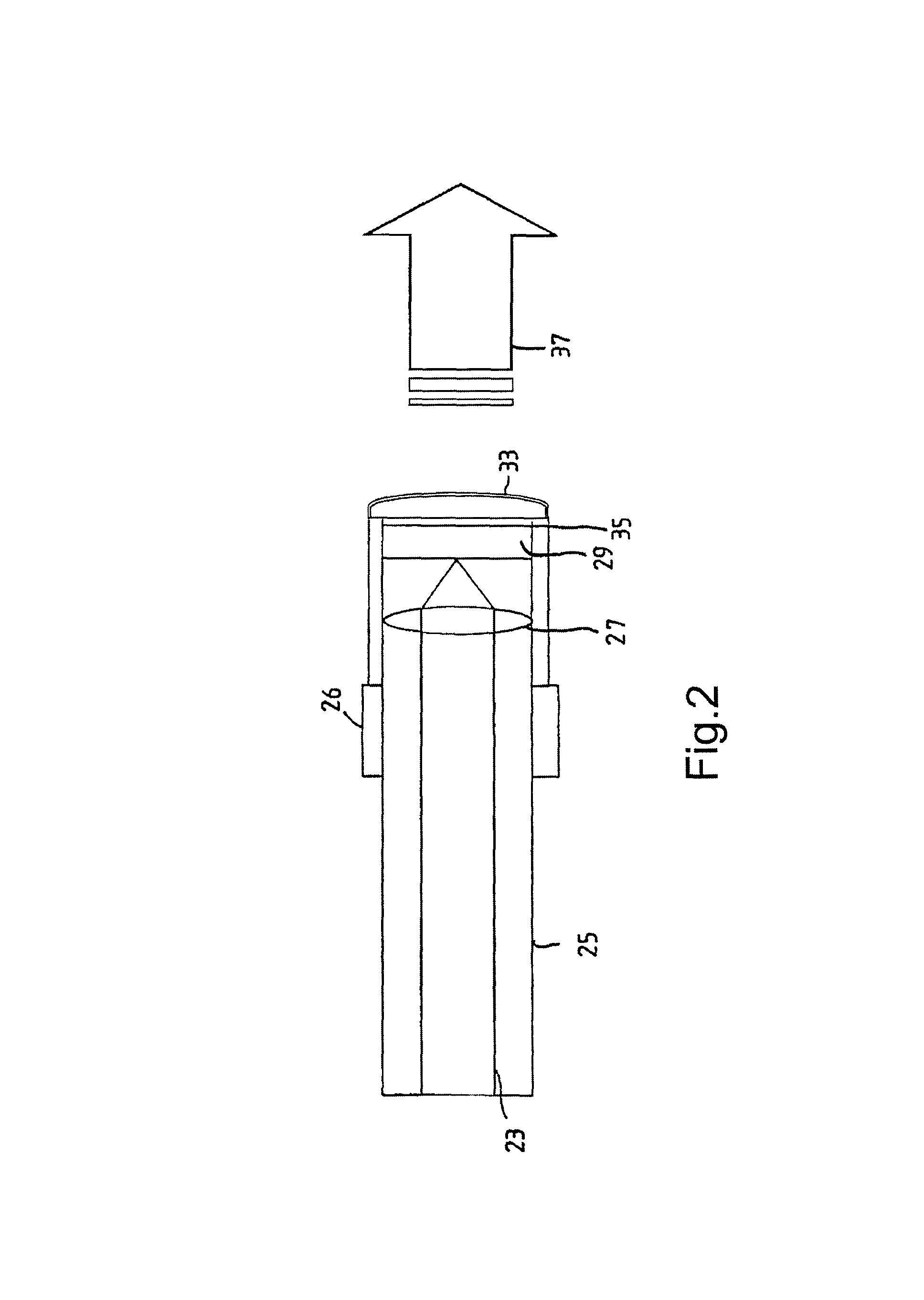
Radiation probe and detecting tooth decay
ActiveUS8027709B2Maximize contrastHigh porosityTeeth fillingSurgeryCementum cariesFrequency conversion
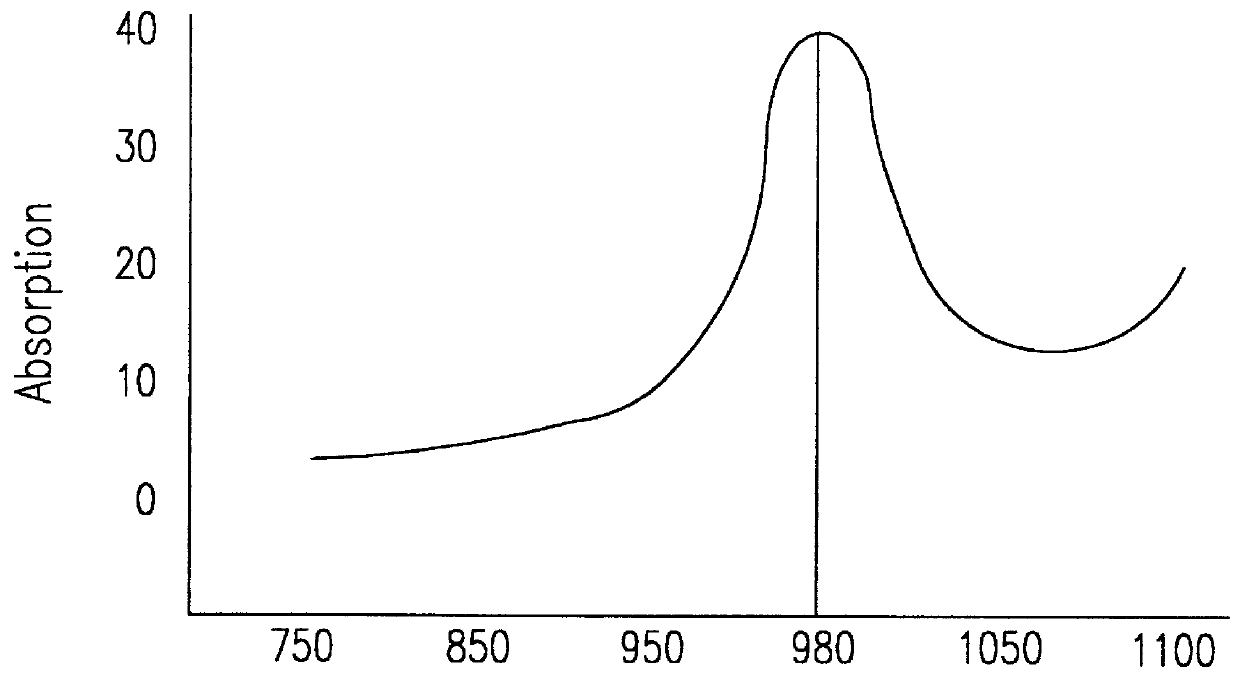
A probe assembly for examining a sample, the assembly including a probe, a fiber optic cable for communicating signals to and / or from the probe, an emitter for emitting radiation to irradiate the sample and an electro-magnetic radiation detector for detecting radiation which is transmitted or reflected from the sample. The emitter includes a frequency conversion member which emits radiation in response to being irradiated with input radiation which has a different frequency to that of the emitted radiation. At least one of the emitter or detector is located in the probe. The probe is particularly for use as an endoscope or for imaging teeth. The invention also extends to a method of imaging teeth, and apparatus for imaging diseased teeth, for example, teeth with caries or suffering from periodontal disease.
Owner:TERAVIEW
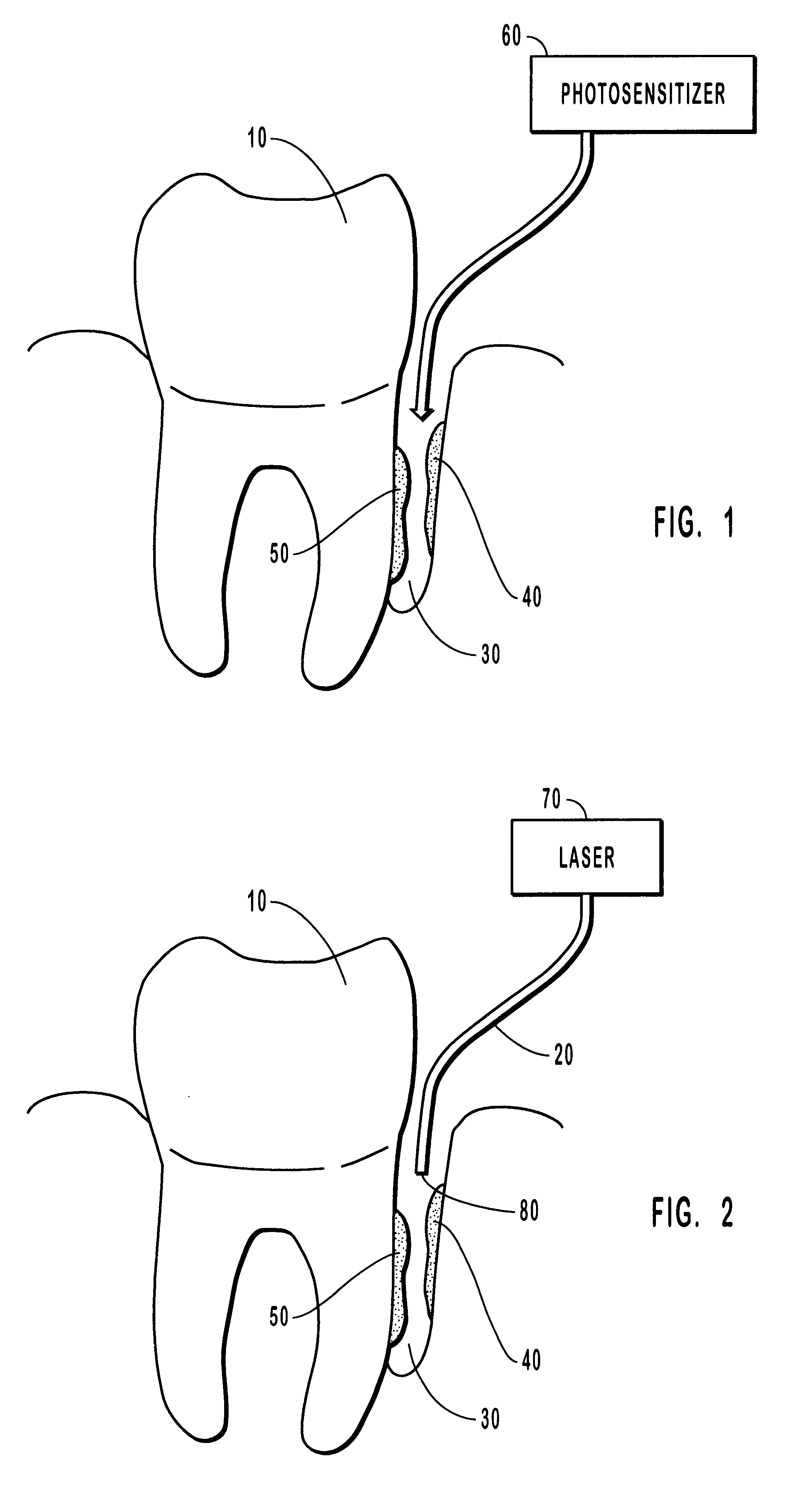
Methods for treating periodontal disease
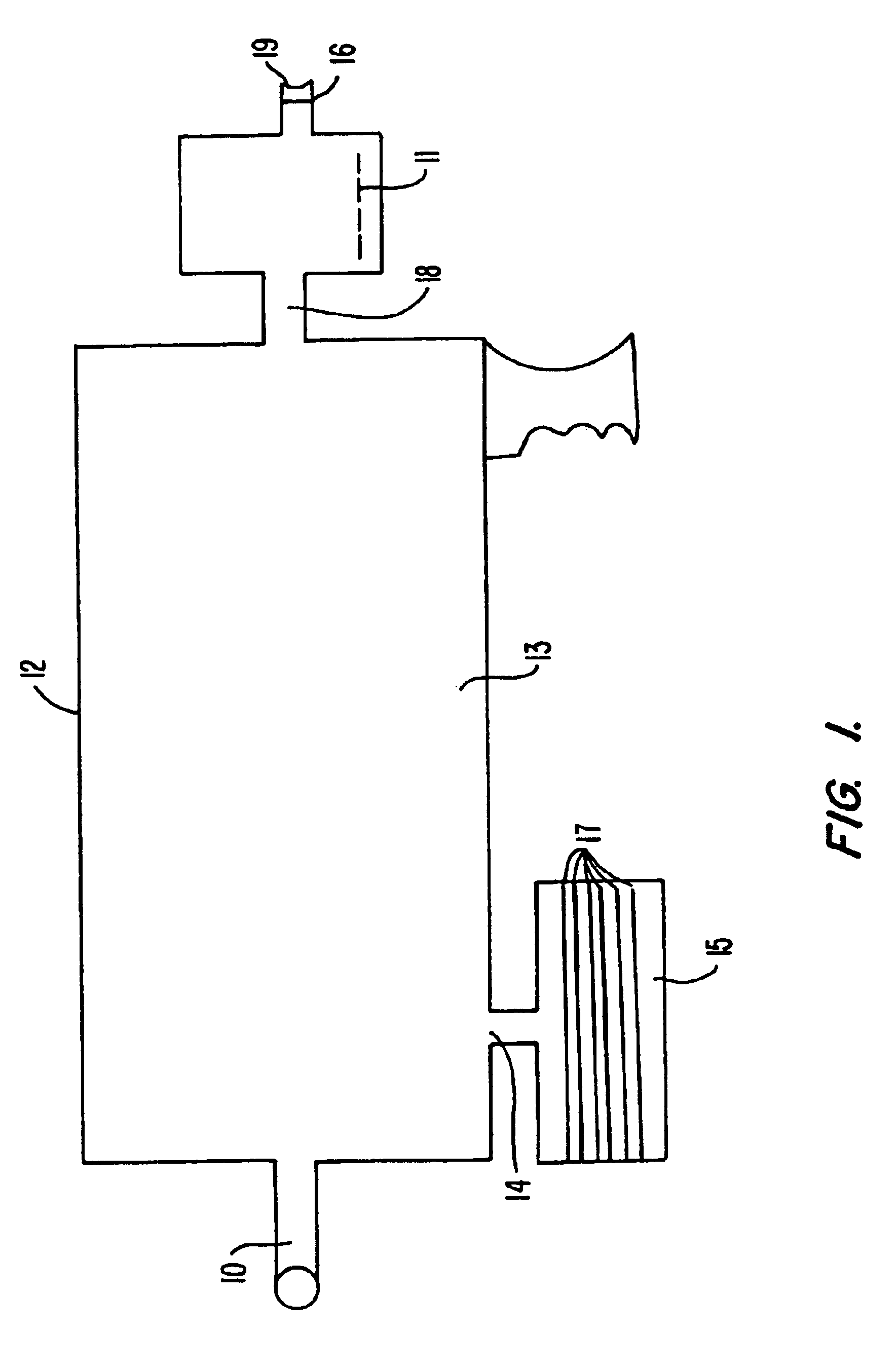
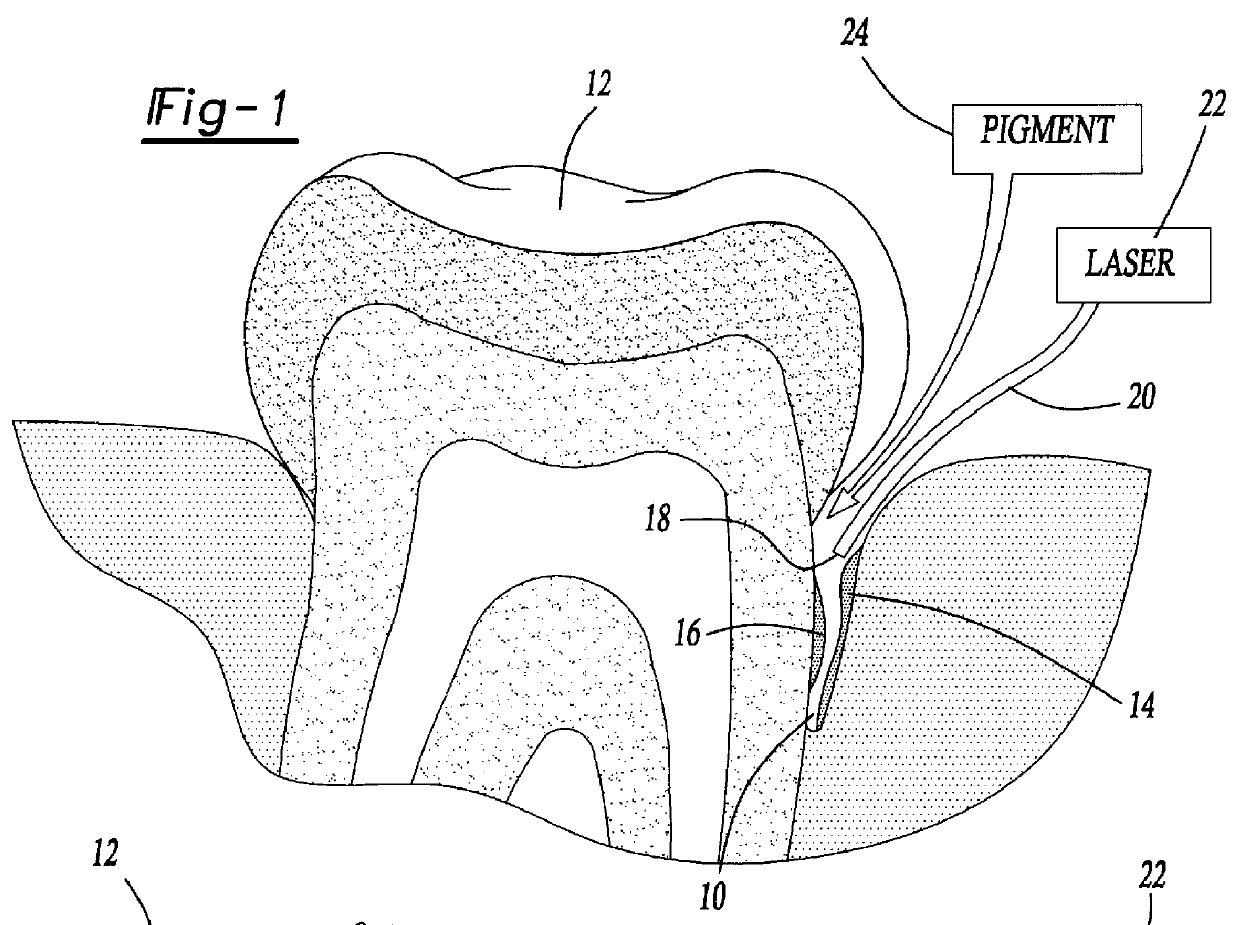
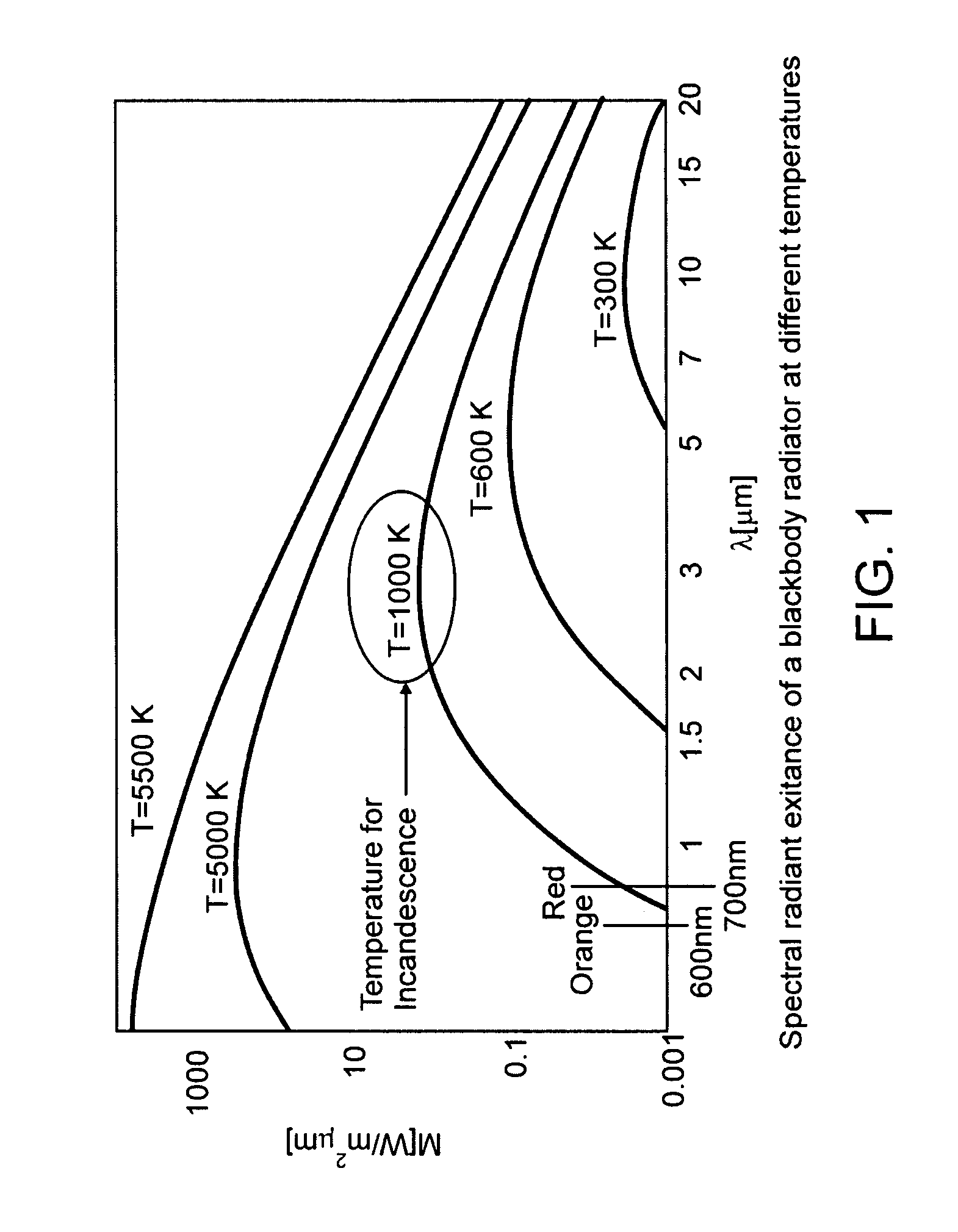
Methods for treating periodontal disease and other diseased tissues that utilize a dye composition and laser energy. The laser energy (typically about 450 nm to about 600 nm) heats and destroys the diseased tissue and bacteria, while the dye composition causes the laser energy to be selectively absorbed by the targeted tissue. An argon gas laser that emits blue-green light may be used in conjunction with a red-orange dye that strongly absorbs light energy emitted by the argon gas laser. An 810 diode laser may be used in conjunction with the argon laser in order to provide additional heating properties.
Owner:ANDERSEN SCOT N +1
Medical applications of artificial olfactometry
InactiveUS6841391B2High sensitivityEasy to useAnalysing fluids using sonic/ultrasonic/infrasonic wavesNanotechAnalyteOlfactometry
The present invention provides methods for detecting the presence of an analyte indicative of various medical conditions, including halitosis, periodontal disease and other diseases are also disclosed.
Owner:SMITHS DETECTION PASADENA INC
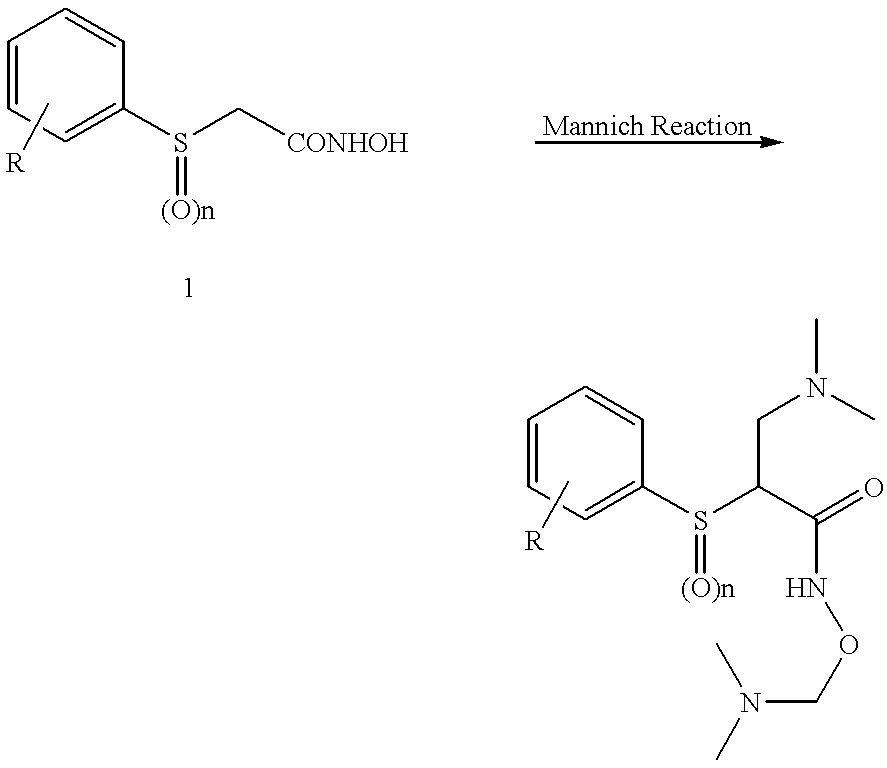
N-hdroxy-2-(alkyl, aryl, or heteroaryl, sulfanyl, sulfinyl or sulfonyl)-3-substituted alkyl, aryl or heteroarylamides as matrix metalloproteinase inhibitors
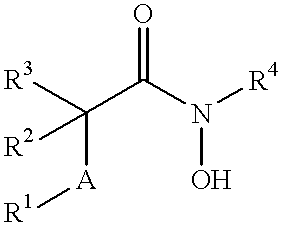
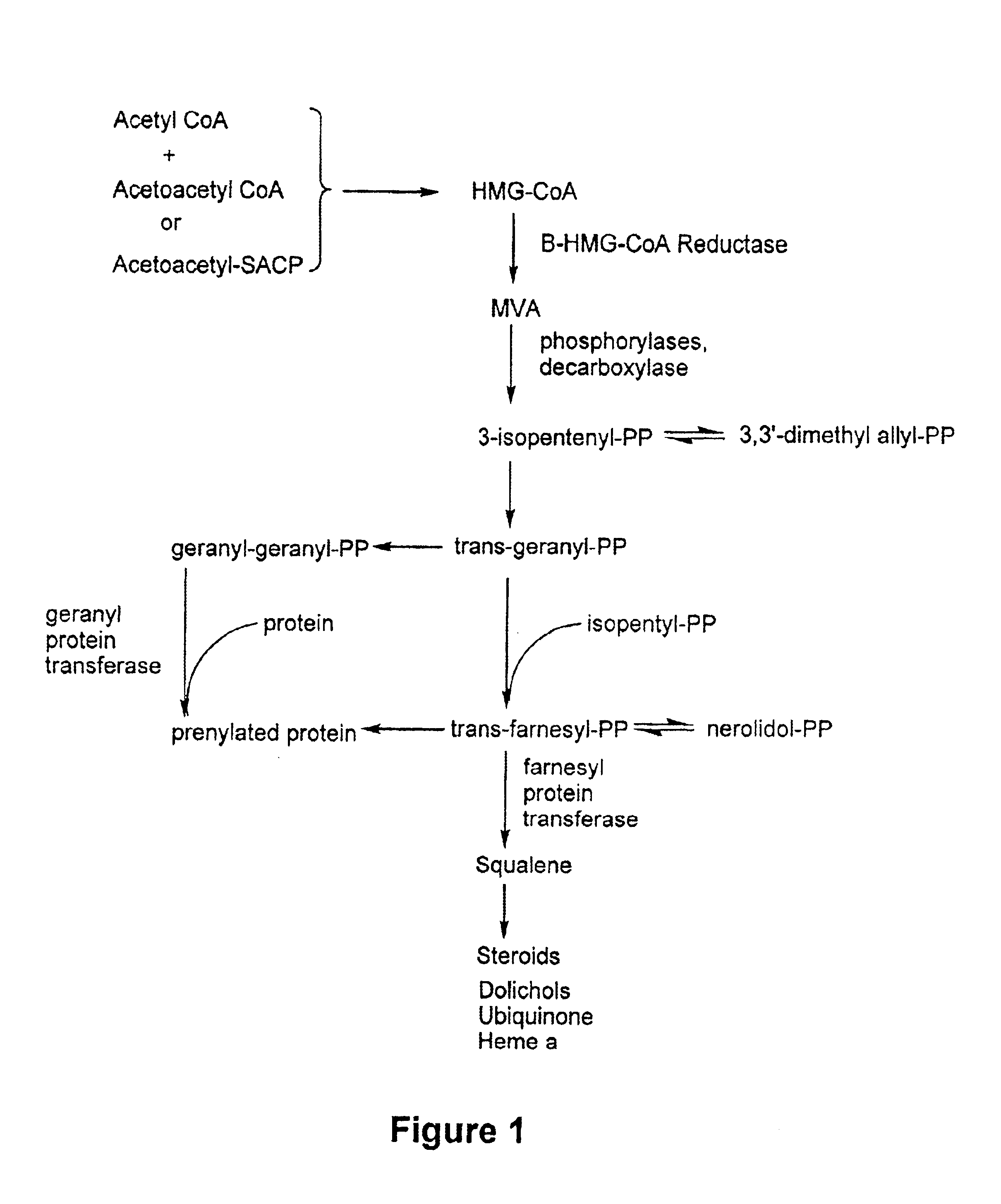
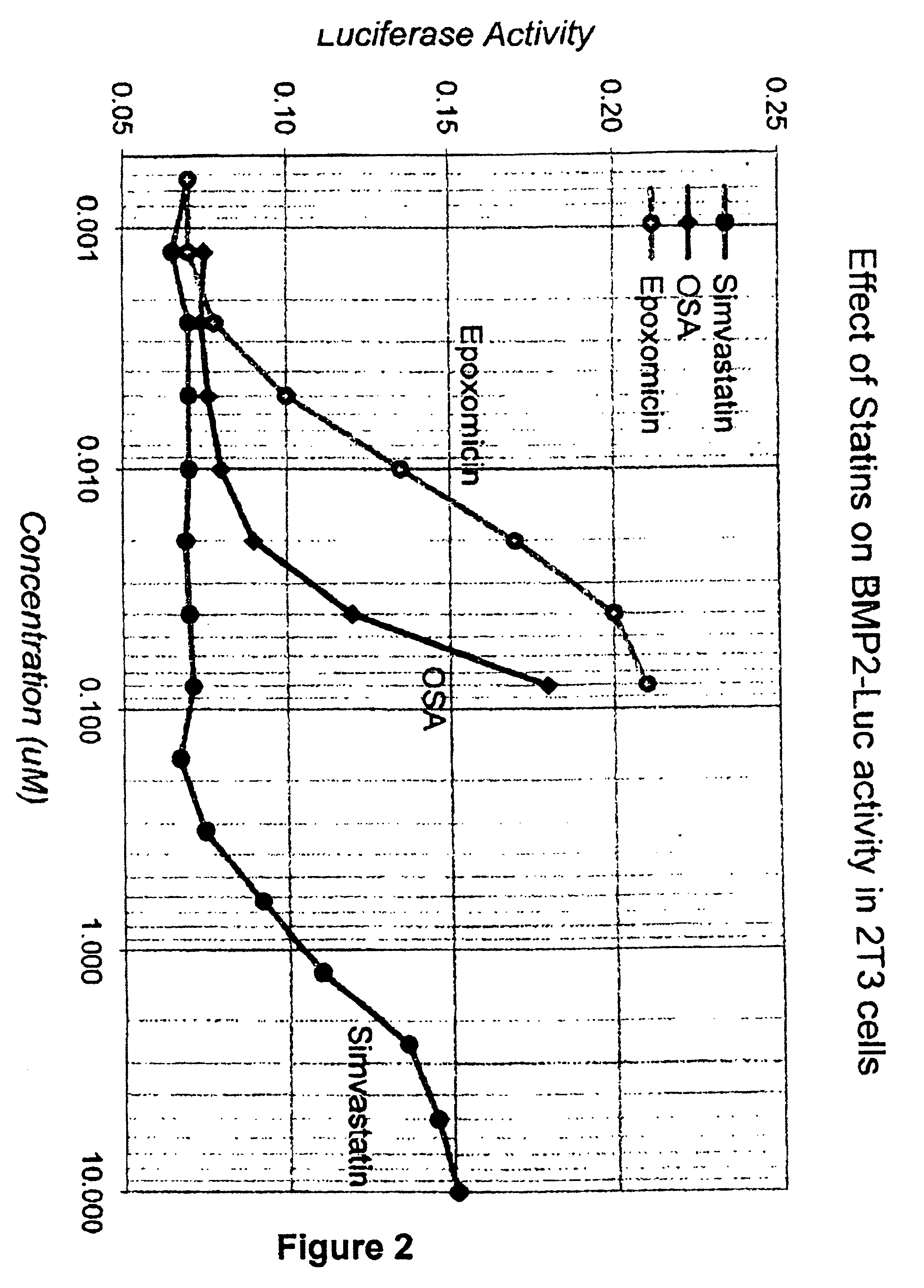
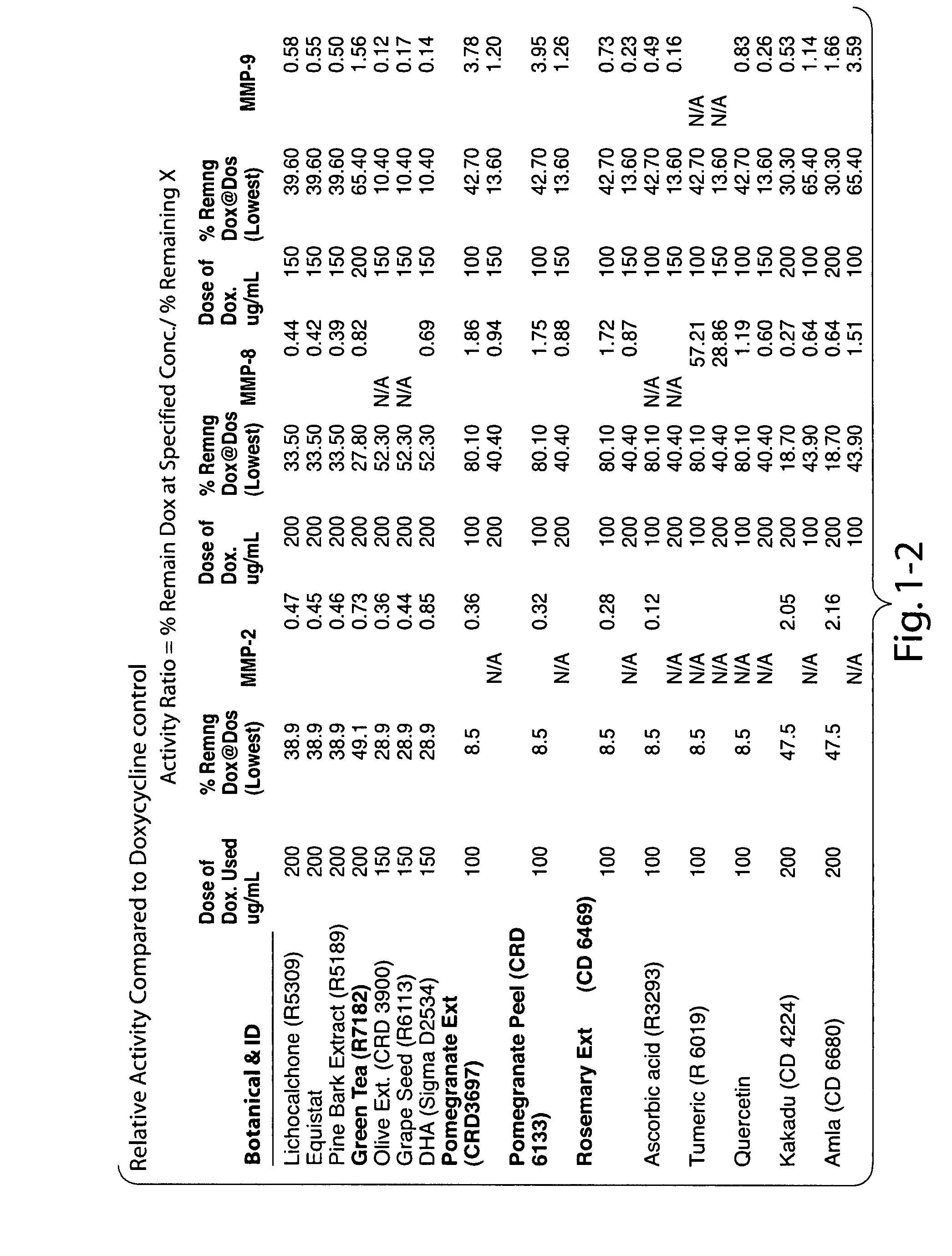
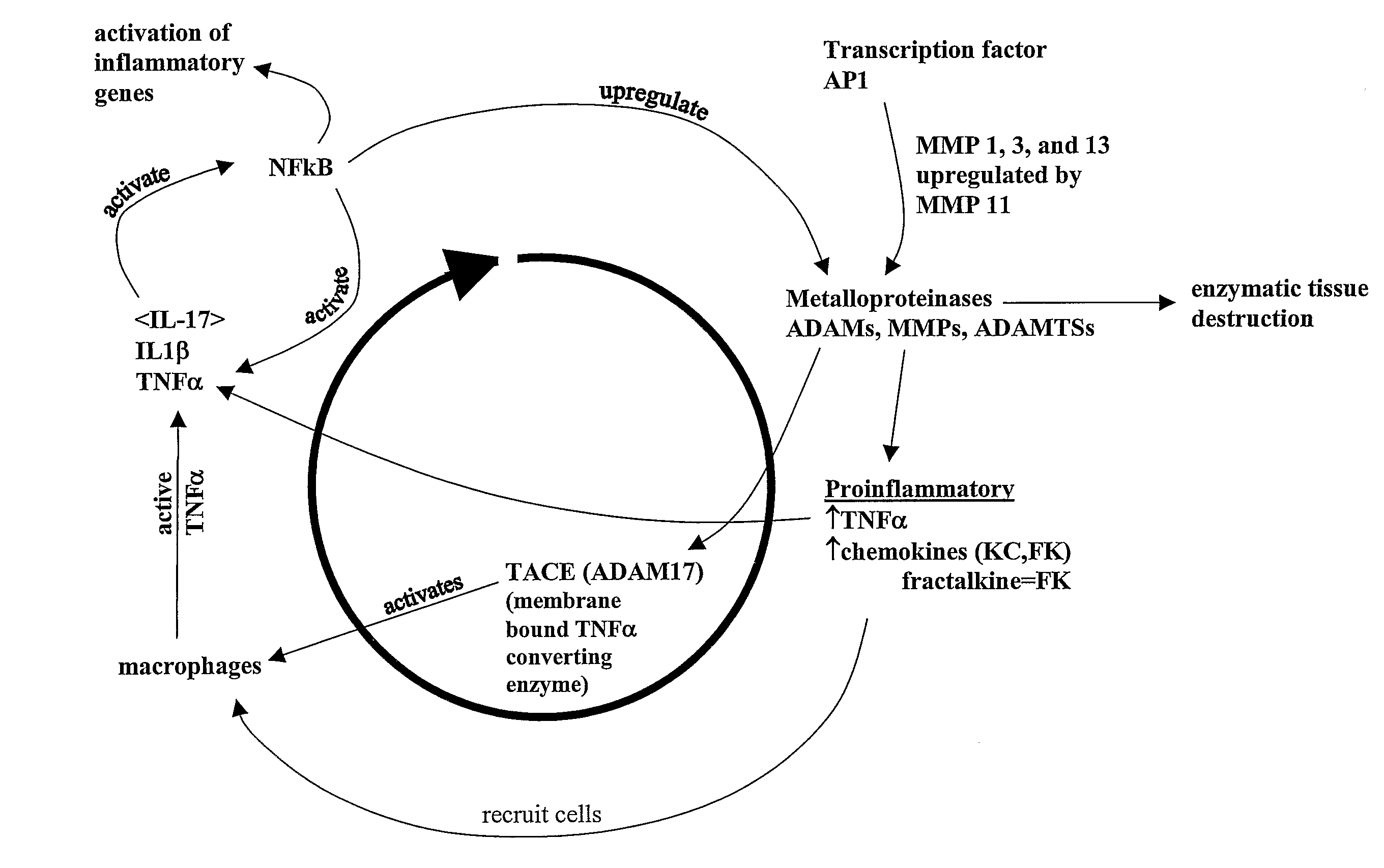
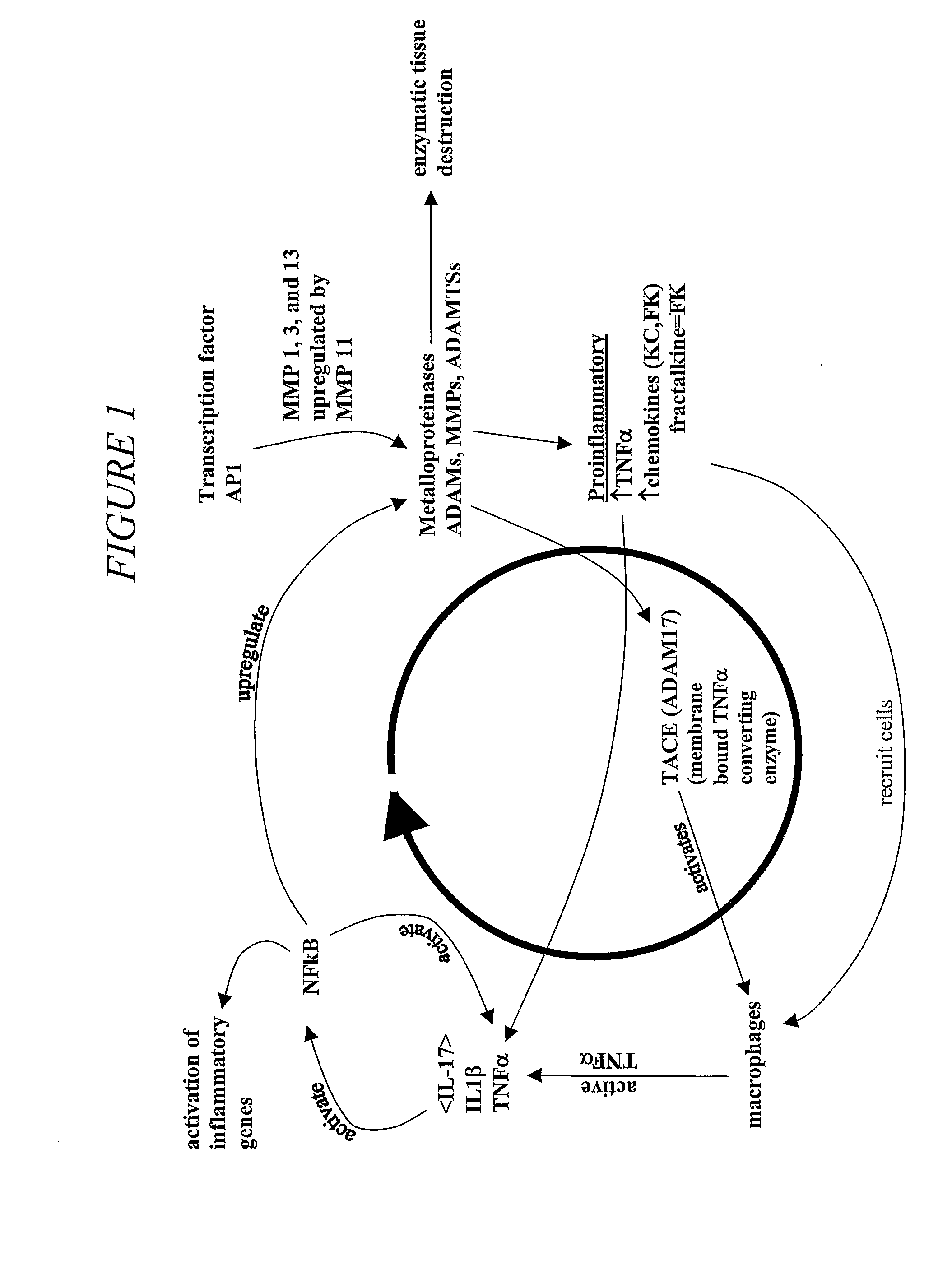
Matrix metalloproteinases (MMPs) are a group of enzymes that have been implicated in the pathological destruction of connective tissue and basement membranes. These zinc containing endopeptidases consist of several subsets of enzymes including collagenases, stromelysins and gelatinases. TNF-alpha converting enzyme (TACE), a pro-inflammatory cytokine, catalyzes the formation of TNF-alpha from membrane bound TNF-alpha precursor protein. It is expected that small molecule inhibitors of MMPs and TACE therefore have the potential for treating a variety of disease states. The present invention provides low molecular weight, non-peptide inhibitors of matrix metalloproteinases (MMPs) and TNF-alpha converting enzyme (TACE) for the treatment of arthritis, tumor metastasis, tissue ulceration, abnormal wound healing, periodontal disease, bone disease, diabetes (insulin resistance) and HIV infection having the formulawherein R2 and R3 form a heterocyclic ring and A is S, S(O), or S(O)2, and R1 and R4 are defined herein.
Owner:WYETH HOLDINGS CORP
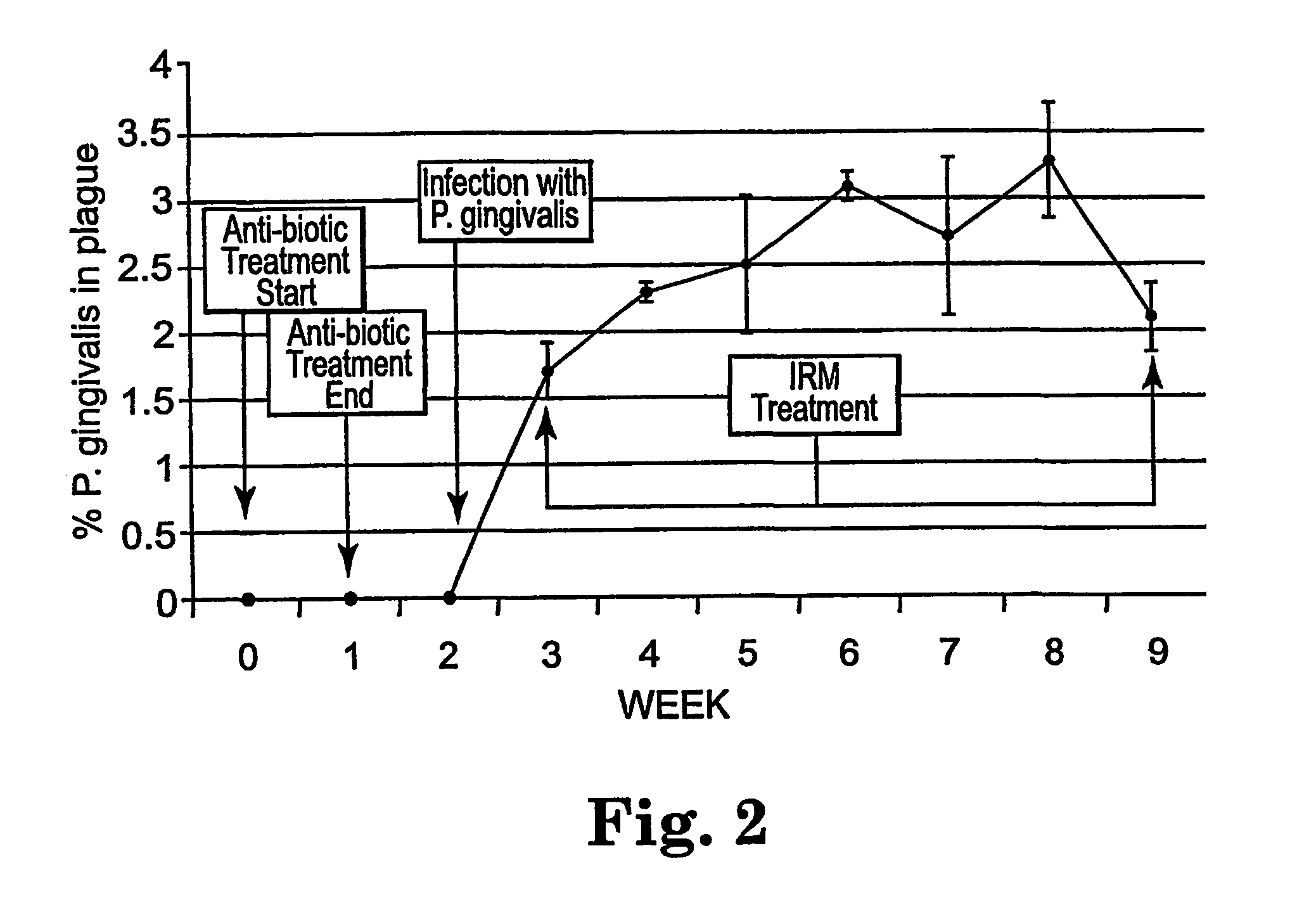
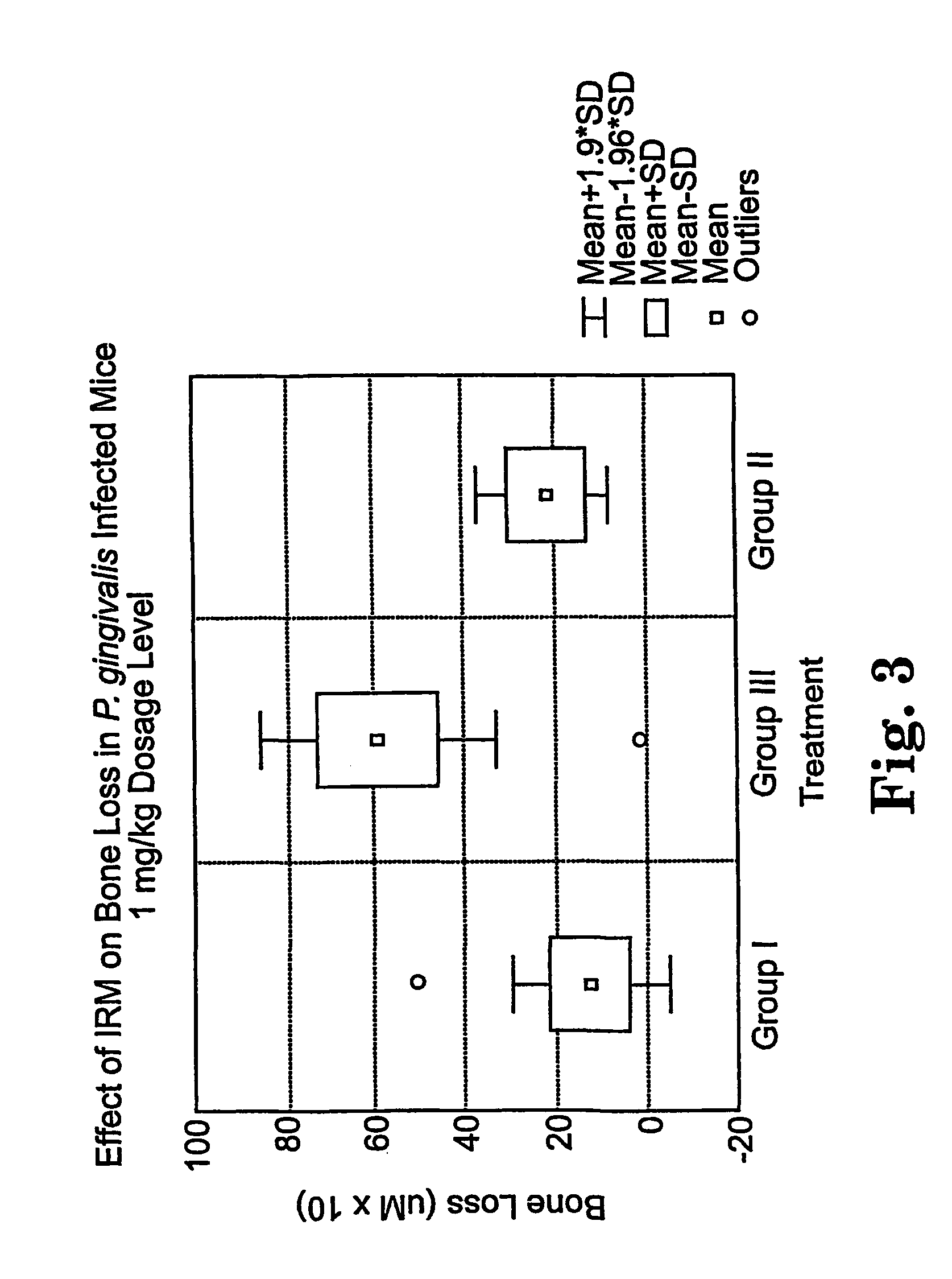
Methods for the treatment of periodontal disease
The disclosure provides methods for the treatment and prevention of periodontal disease. In preferred embodiments, the invention provides for local treatment of periodontal tissues with a pharmaceutical composition including an immune response modifier (IRM) selected from the group of immune response modifiers comprising imidazoquinoline amines, imidazopyridine amines, 6,7-fused cycloalkylimidazopyridine amines, imidazonaphtyridine amines, oxazoloquinoline amines, thiazoloquinoline amines and 1,2-bridged imidazopyridine amines.
Owner:COLEY PHARMA GRP INC
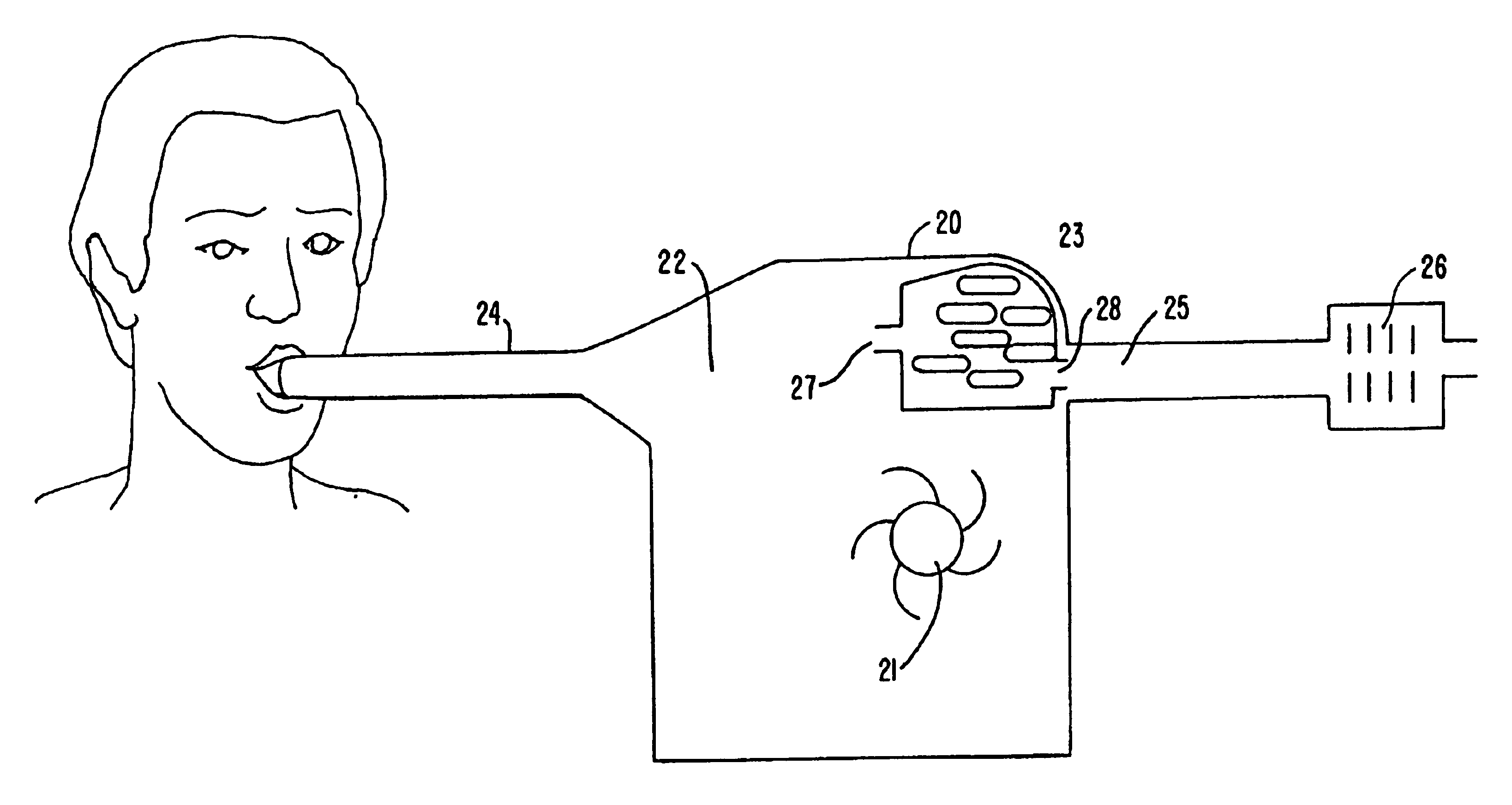
Methods for effecting oral treatment of teeth or gums
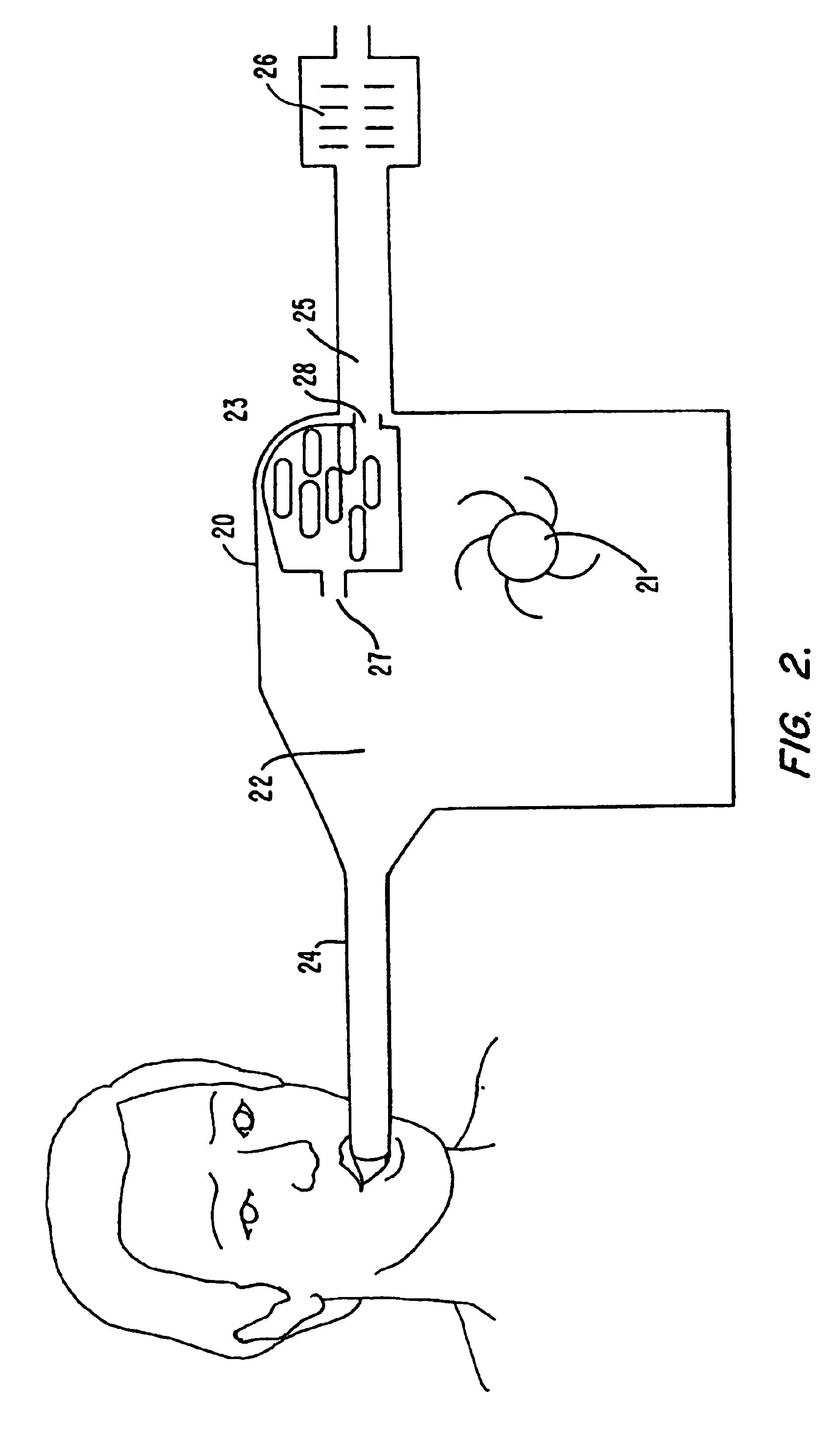
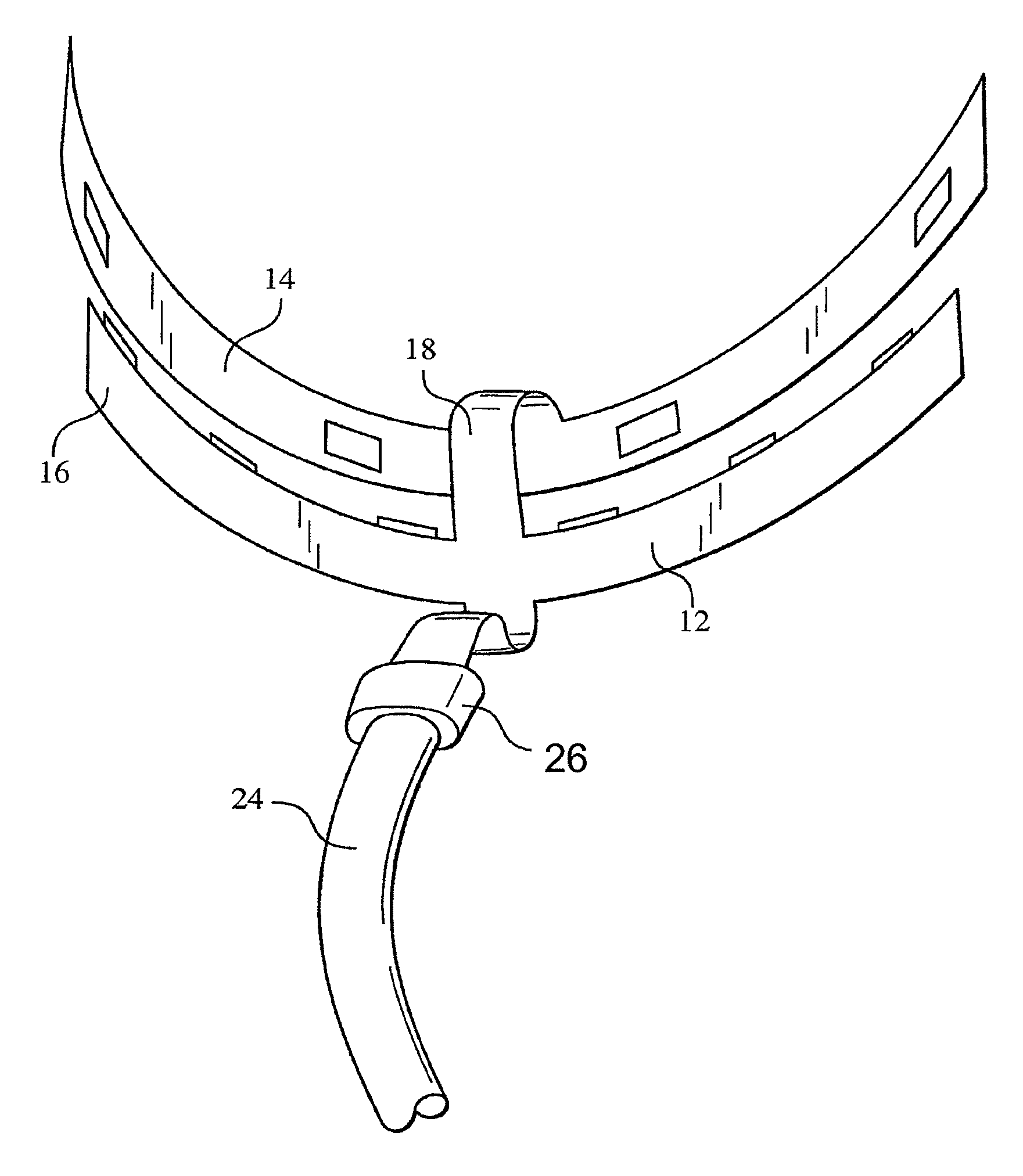
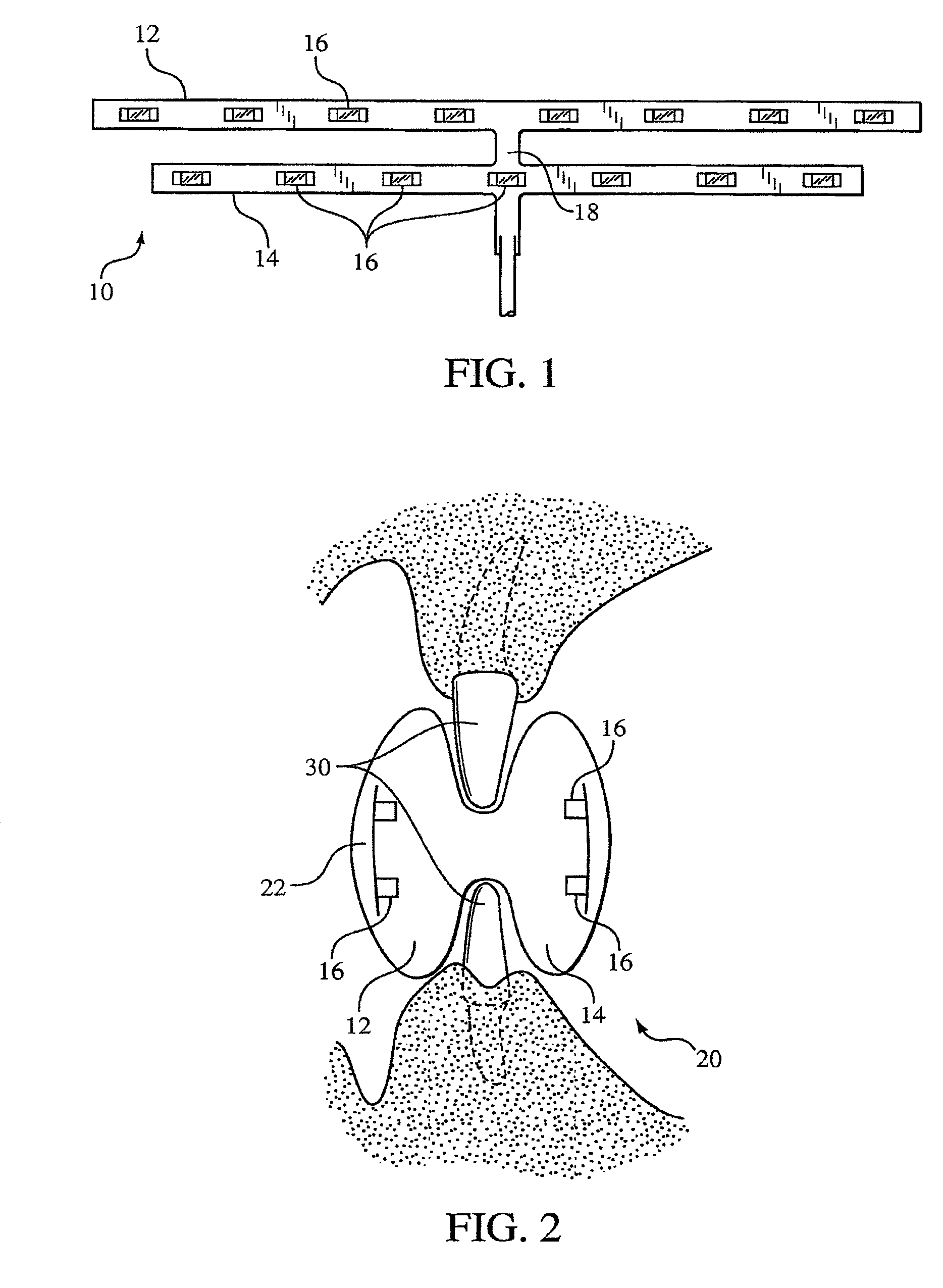
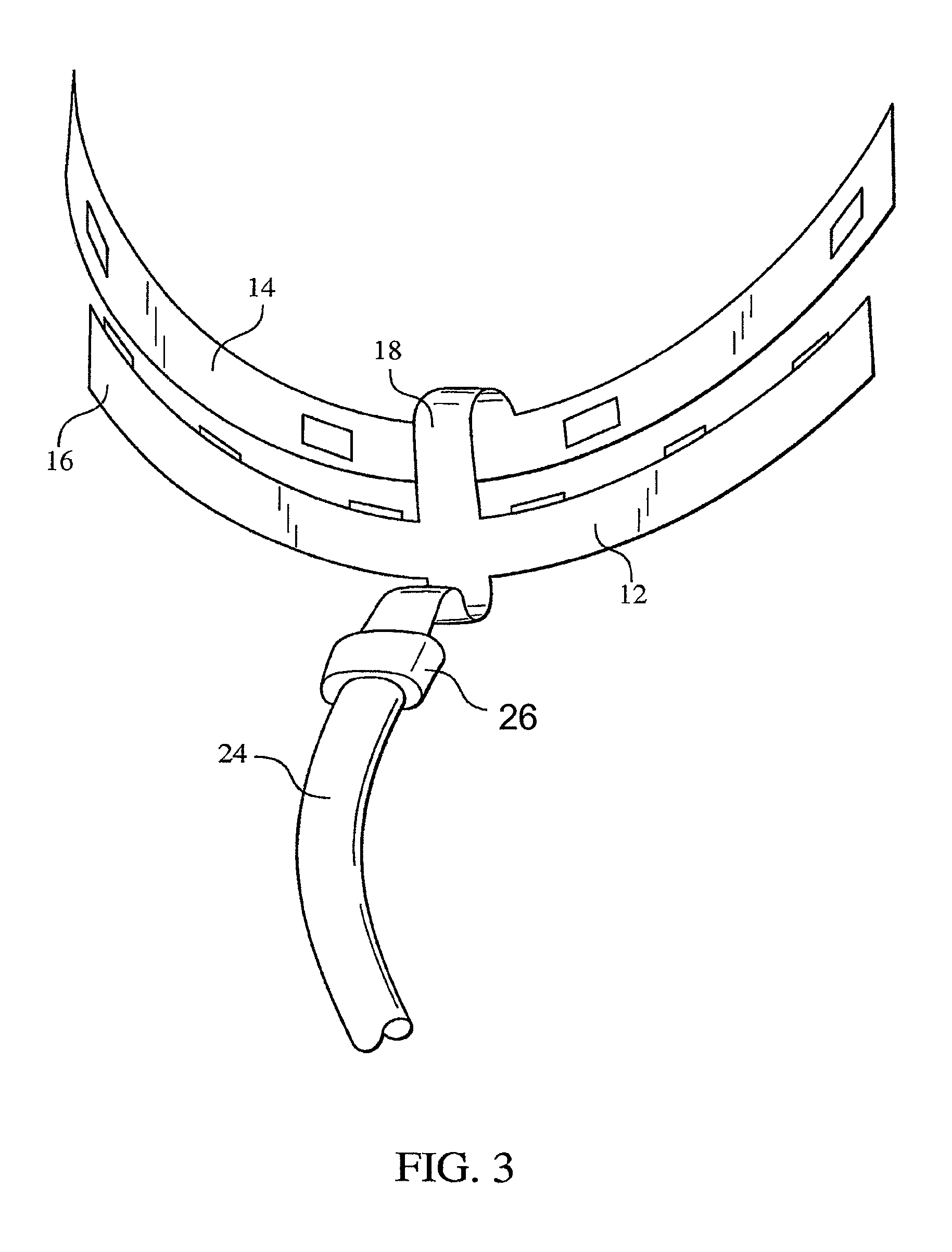
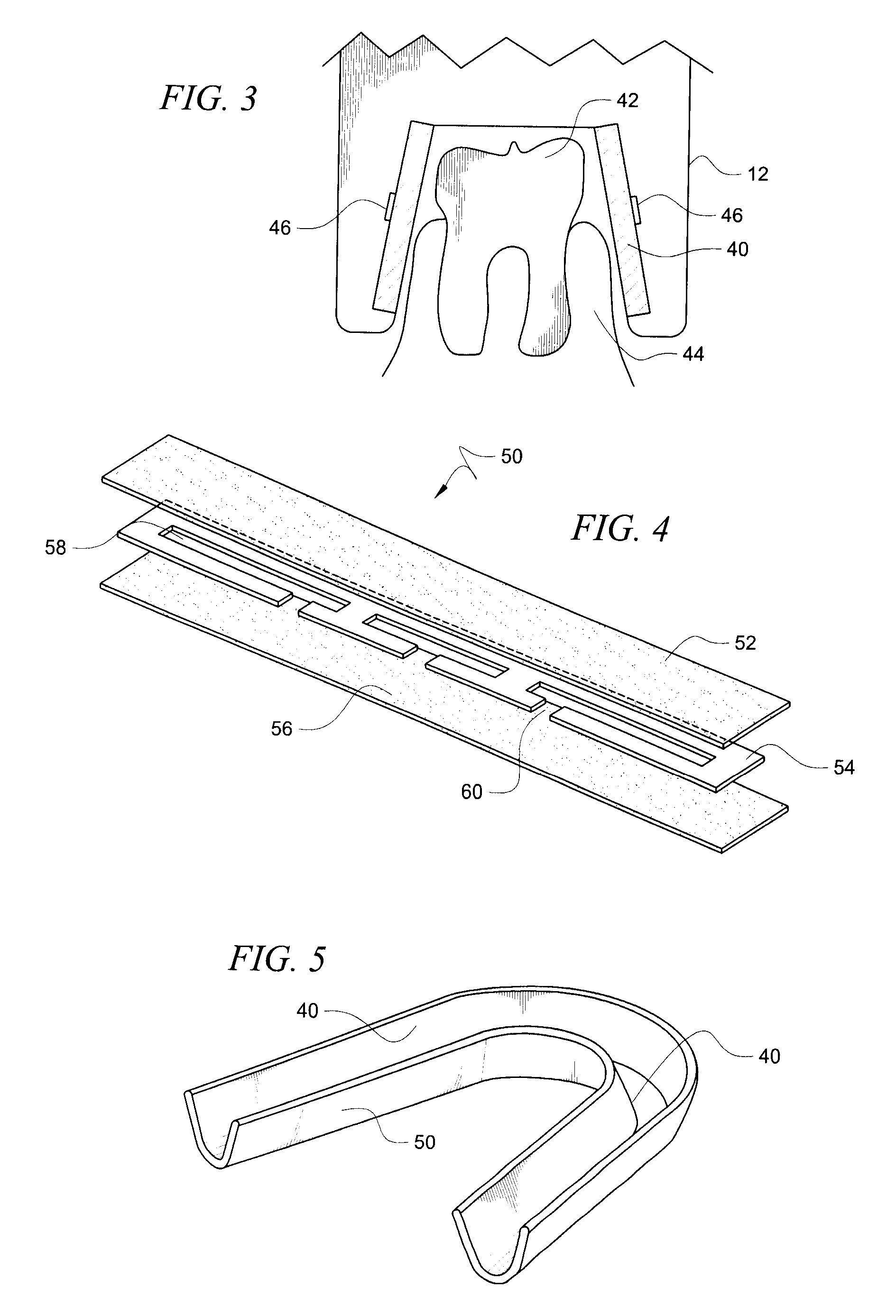
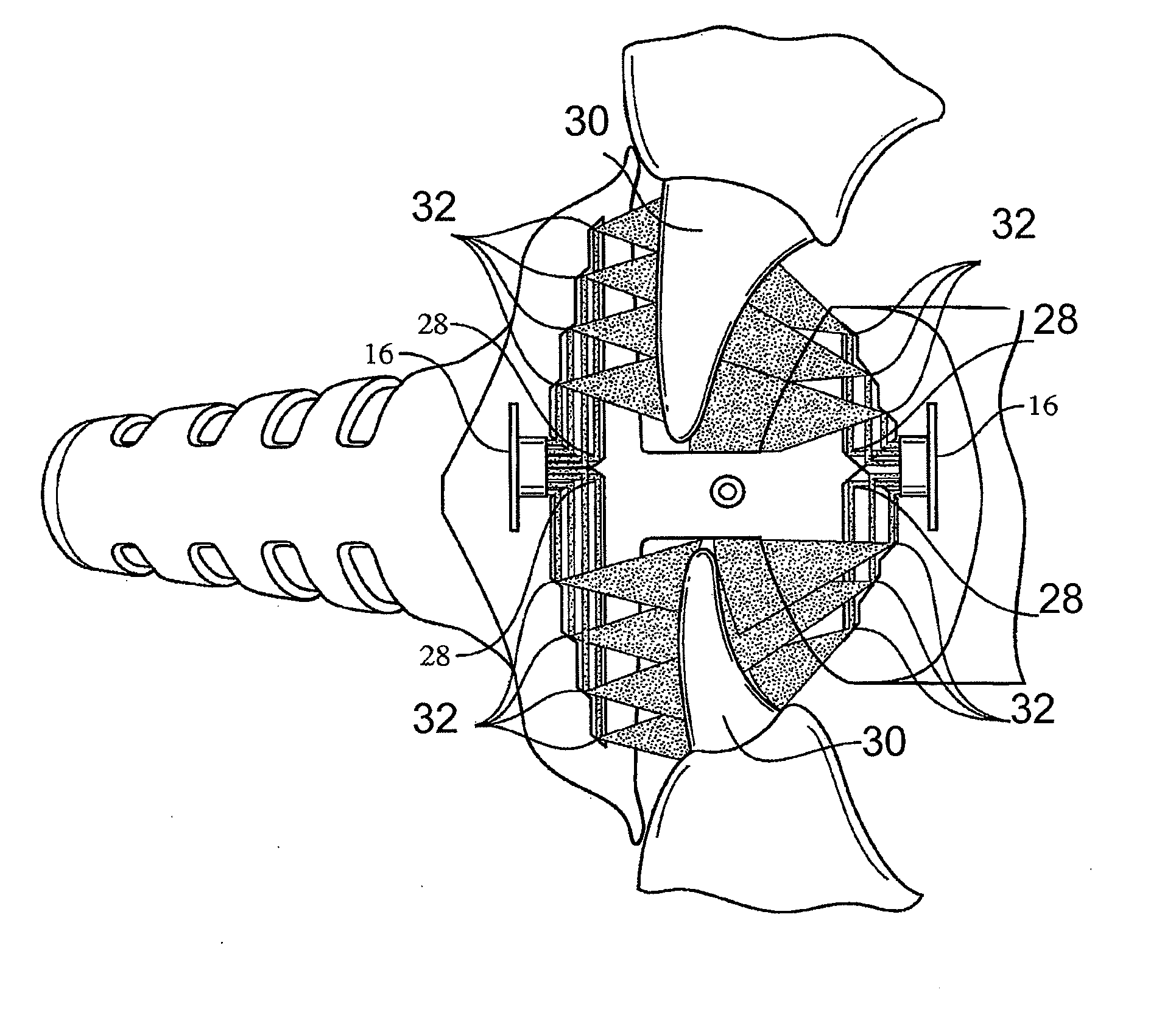
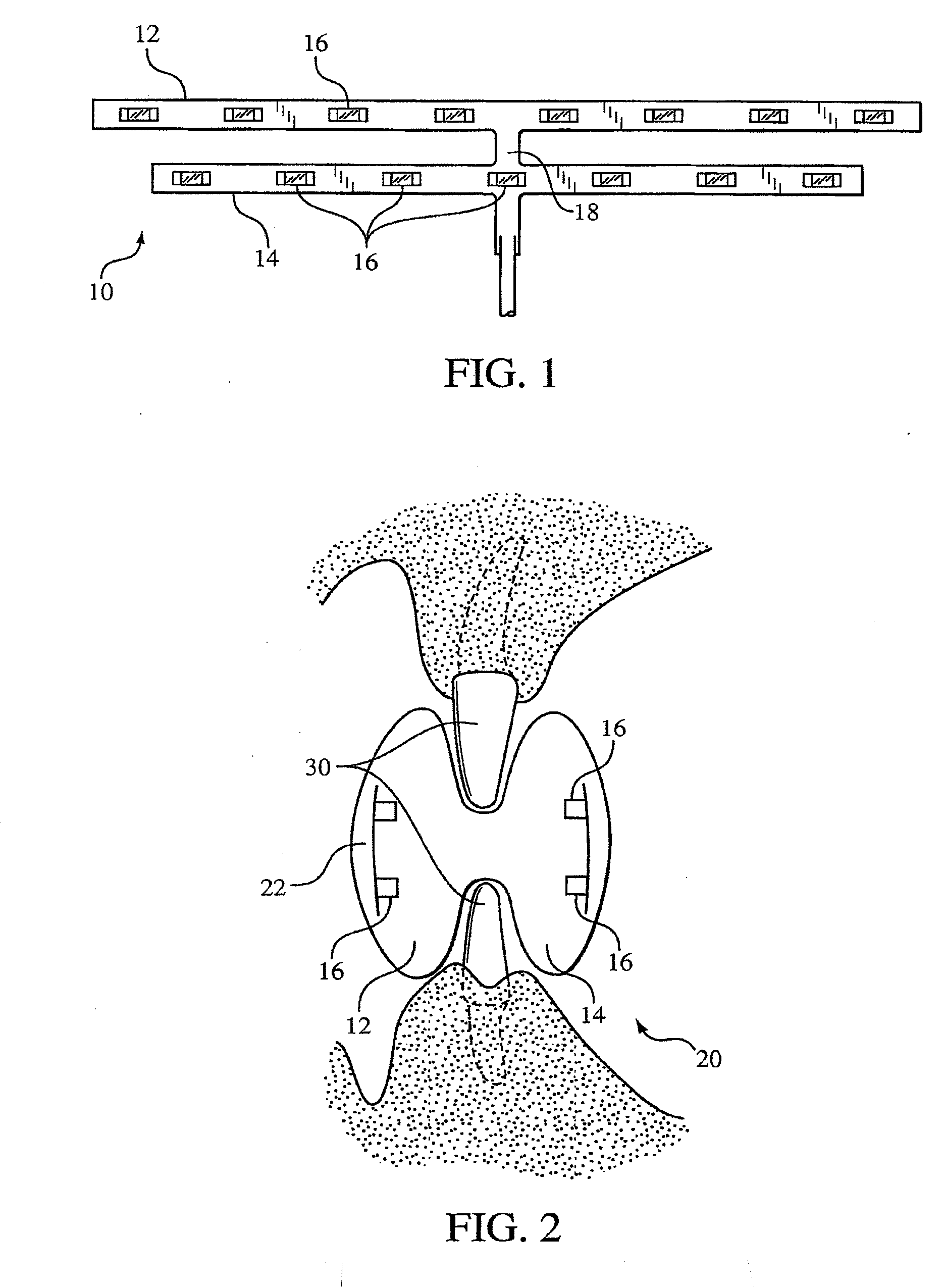
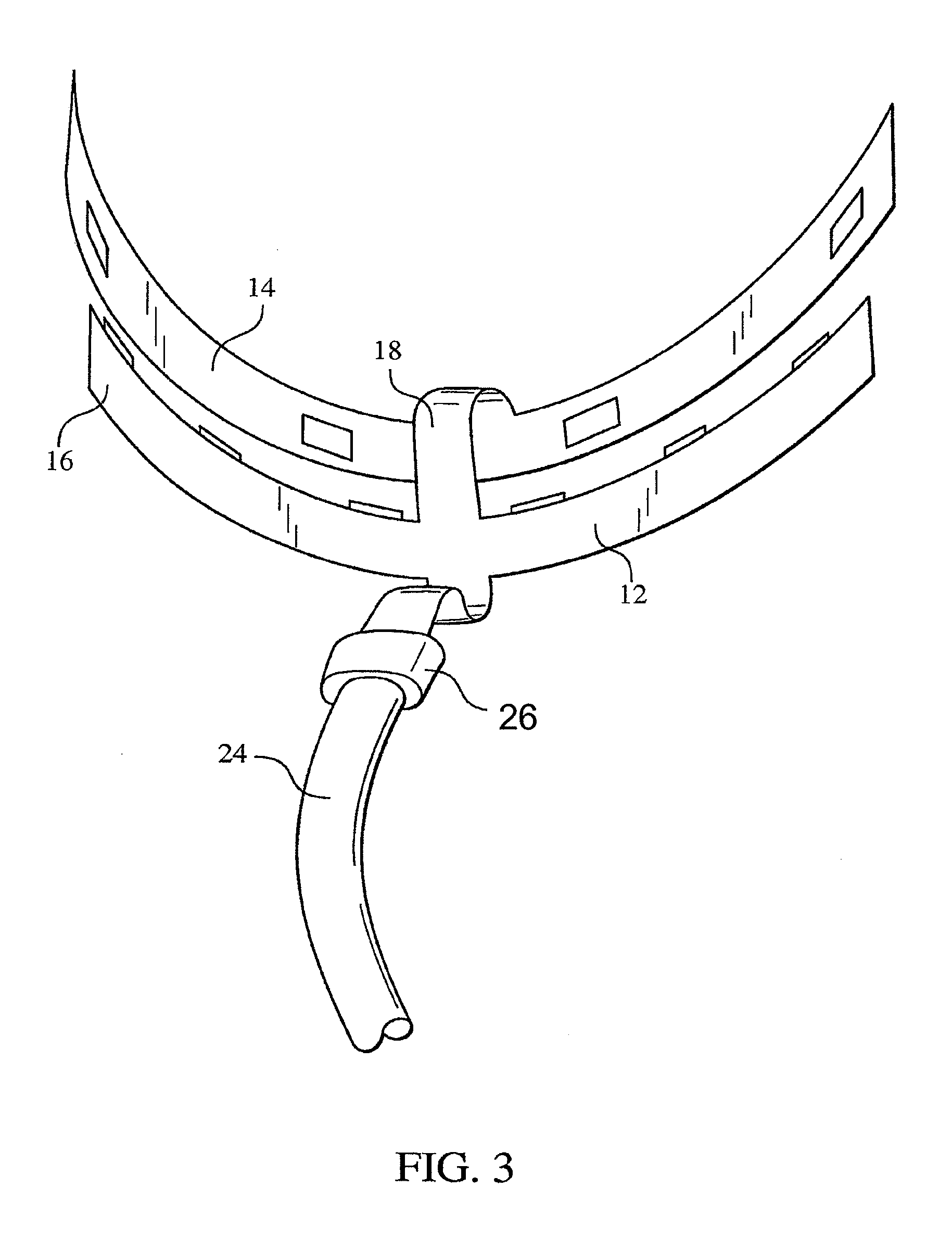
ActiveUS8215954B2Improve reaction speedSafe and effective and convenientTeeth fillingDental toolsOral treatmentFlexible circuits
A method for effecting an oral treatment of teeth and / or gums using an intra-oral device that has a mouthpiece in which is embedded a flexible circuit board and arrays of spaced apart lamps. The mouthpiece has a curvature. The lamps may be light emitting diodes that generate electromagnetic radiation, preferably in the white and blue light spectrum and the infrared and ultraviolet light spectrum. The arrays are positioned to expose the facial and lingual sides of the teeth and / or gums for effecting the treatment when the mouthpiece is positioned to fit upper and lower rows of teeth within accommodating recesses. The flexible circuit board is flexed to exhibit a curvature that follows a curvature of the mouthpiece. Treatments include whitening teeth, desensitizing teeth, and treating gums to prevent periodontal disease.
Owner:GLO SCI INC
Methods, compositions, and kits for the treatment of medical conditions
The invention features methods, compositions, and kits for treating an immunoinflammatory disorder, an ophthalmic disorder, a musculoskeletal disorder or pain associated therewith, a periodontal disease, or a disease or condition associated with an increased serum CRP level.
Owner:THERAOPTICS
System for Imaging Lesions Aligning Tissue Surfaces
ActiveUS20140350395A1Easy to useLow costTelevision system detailsImage enhancementBladder cancerRadiology
Owner:ORLUCENT INC
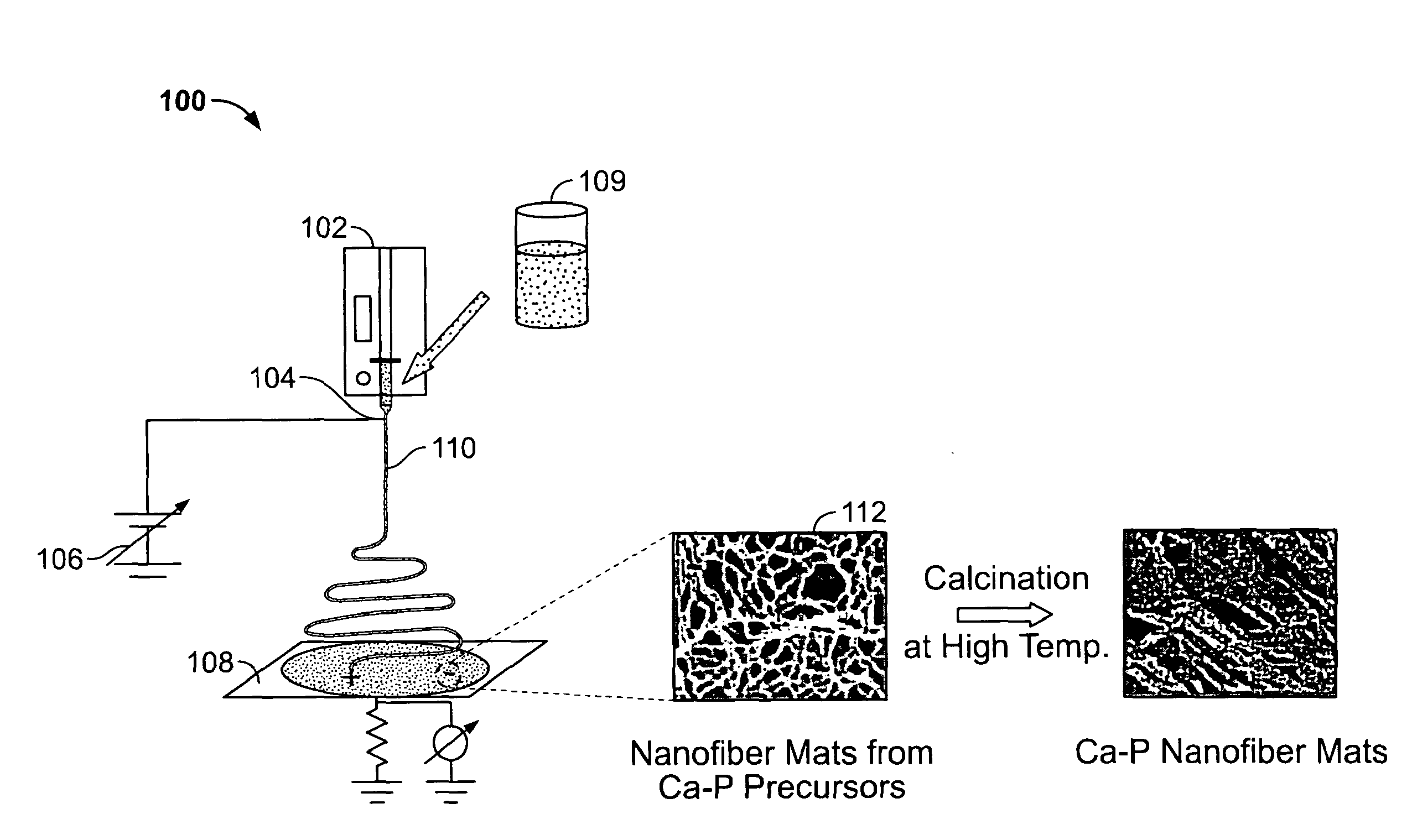
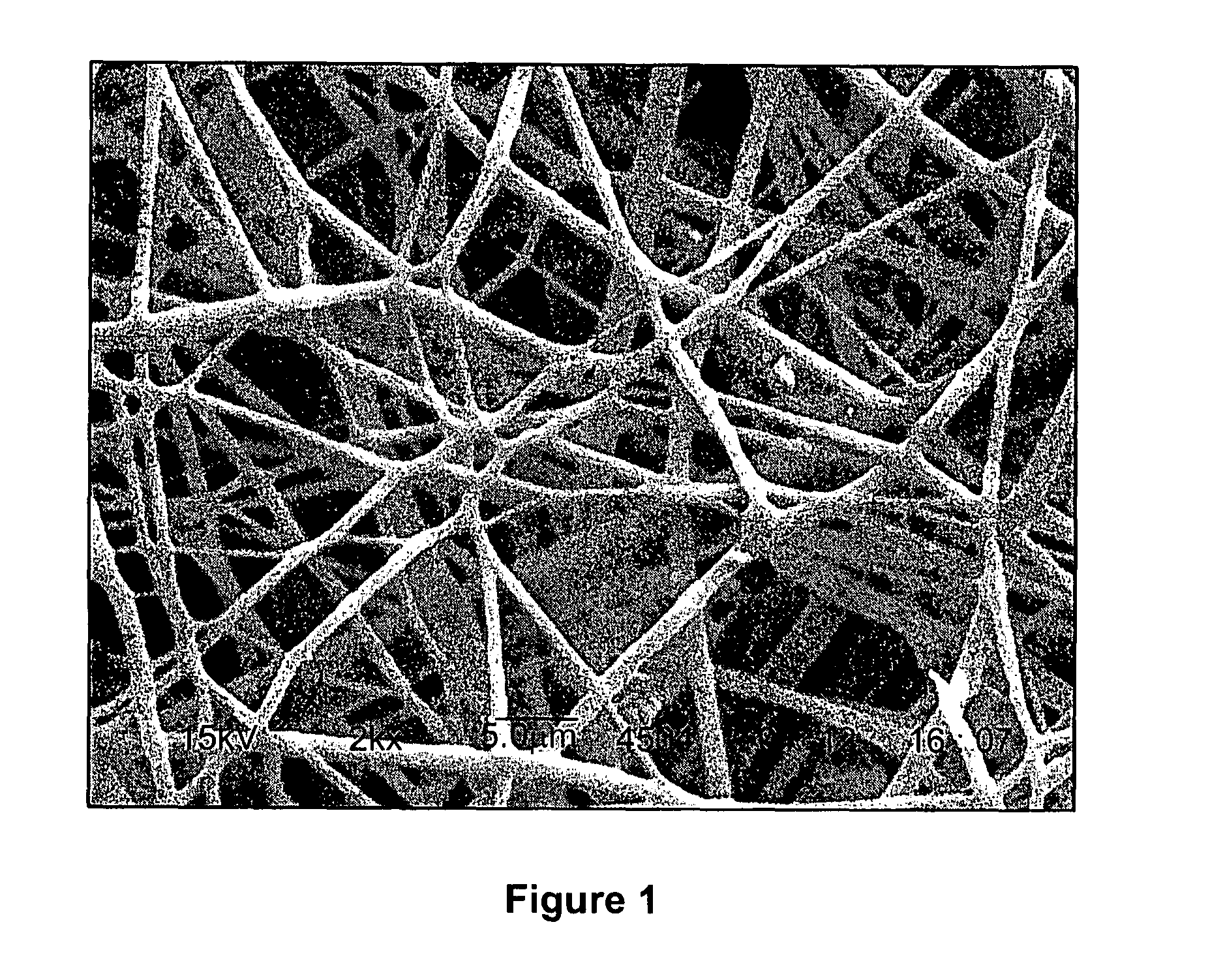
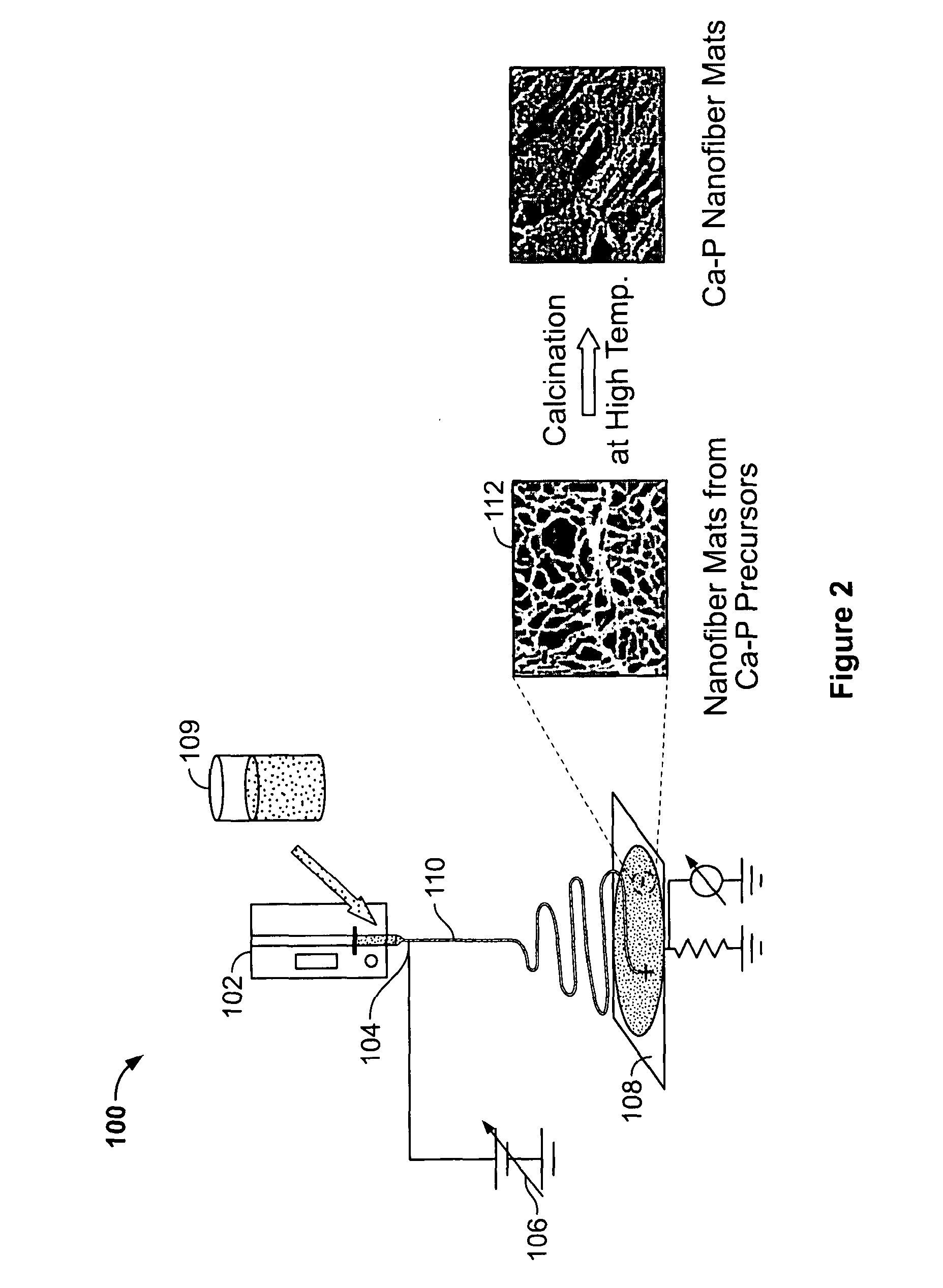
Calcium Phosphate Nanofibers
ActiveUS20090317446A1Suitable environmentMaterial nanotechnologyDental implantsCalcium biphosphateFiber
Calcium-phosphate nanofiber matrices comprising randomly dispersed crystalline calcium-phosphate nanofibers are provided. The nanofibers are synthesized using sol-gel methods combined with electrospinning. The nanofibers may be hollow, solid or may comprise a calcium-phosphate shell surrounding a polymer containing inner core to which biologically functional additives may be added. The nanofiber matrices may be used to culture bone and dental cells, and as implants to treat bone, dental or periodontal diseases and defects.
Owner:CORNELL RES FOUNDATION INC
Device and method to treat oral disease in small animals
InactiveUS6086363ARemove complicationsEliminate chronic symptomTeeth fillingAnimal teeth treatmentOral diseasePretreatment method
A laser system and method are described that will improve dental treatments in small animals, particularly in situations where periodontal disease has progressed to the advanced stages of periodontitis and when dental pulp is exposed by fracture or disease. A laser system is employed to achieve enhanced precision by selectively ablating affected tissue without damaging the collateral tissue. The laser system is also capable of sealing the tubules and eradicating bacteria within the periodontium to significantly reduce the risk of infection. Additional pre-treatment methods may be employed to further enhance the laser therapy.
Owner:BIOLITEC PHARMA MARKETING
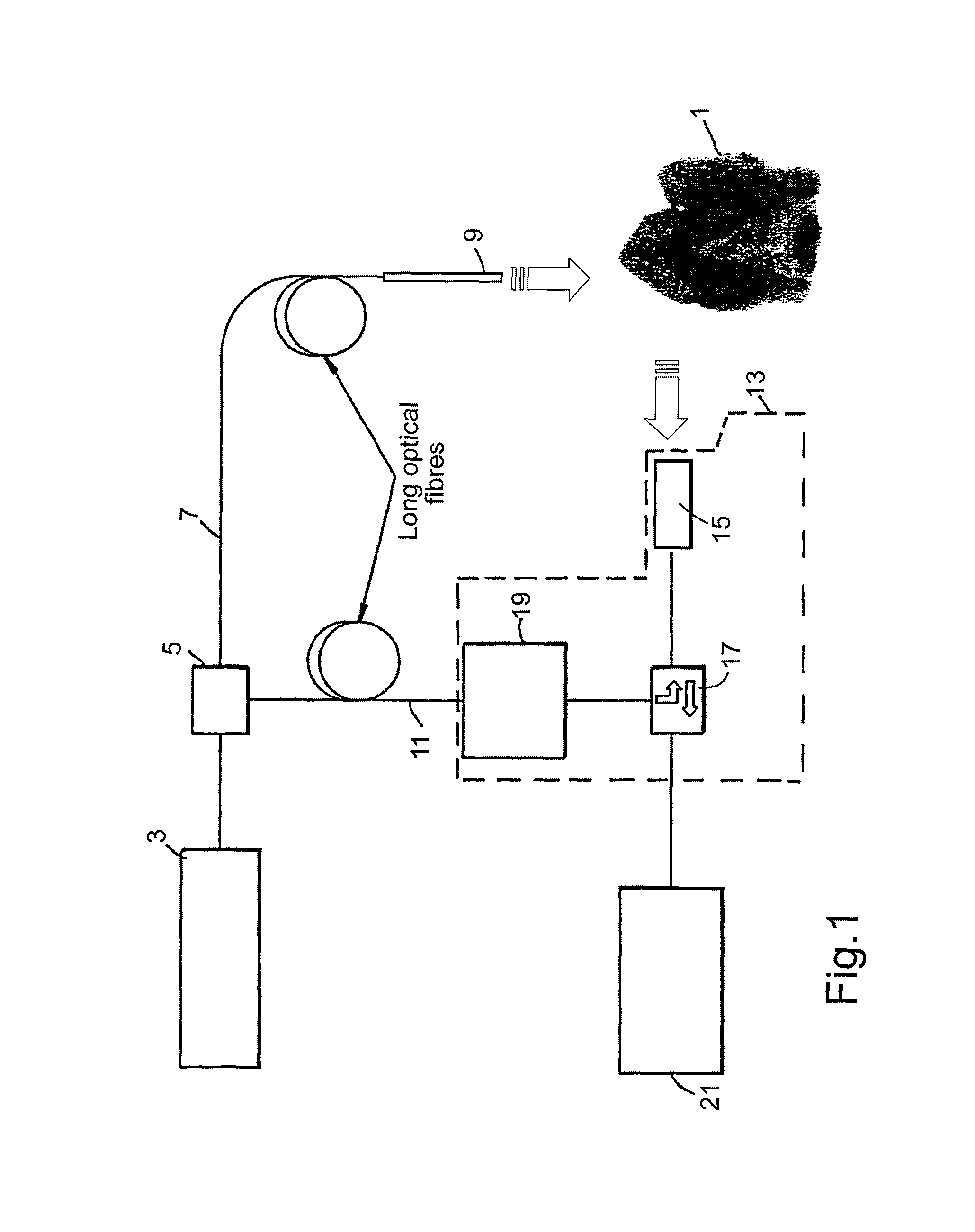
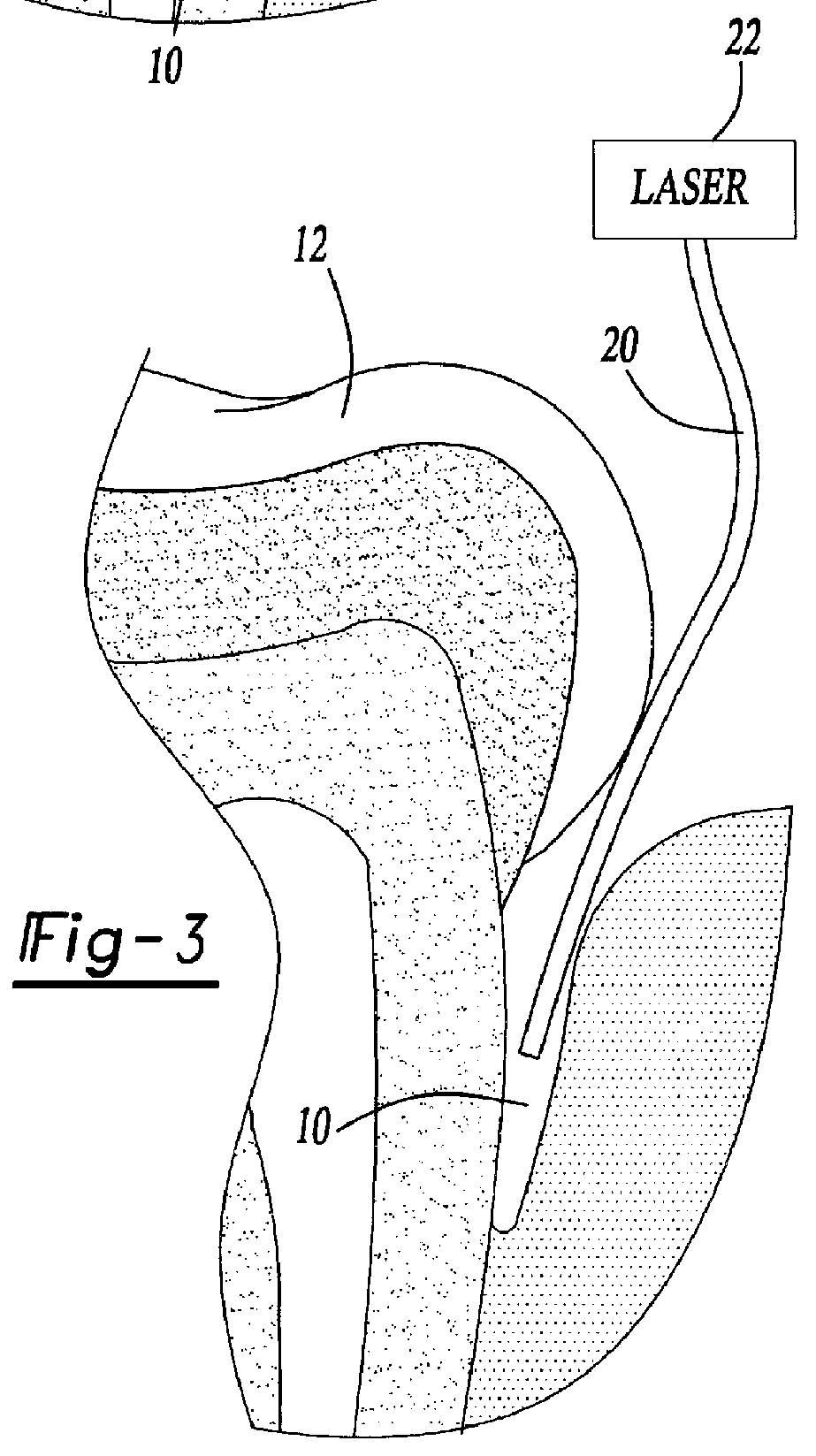
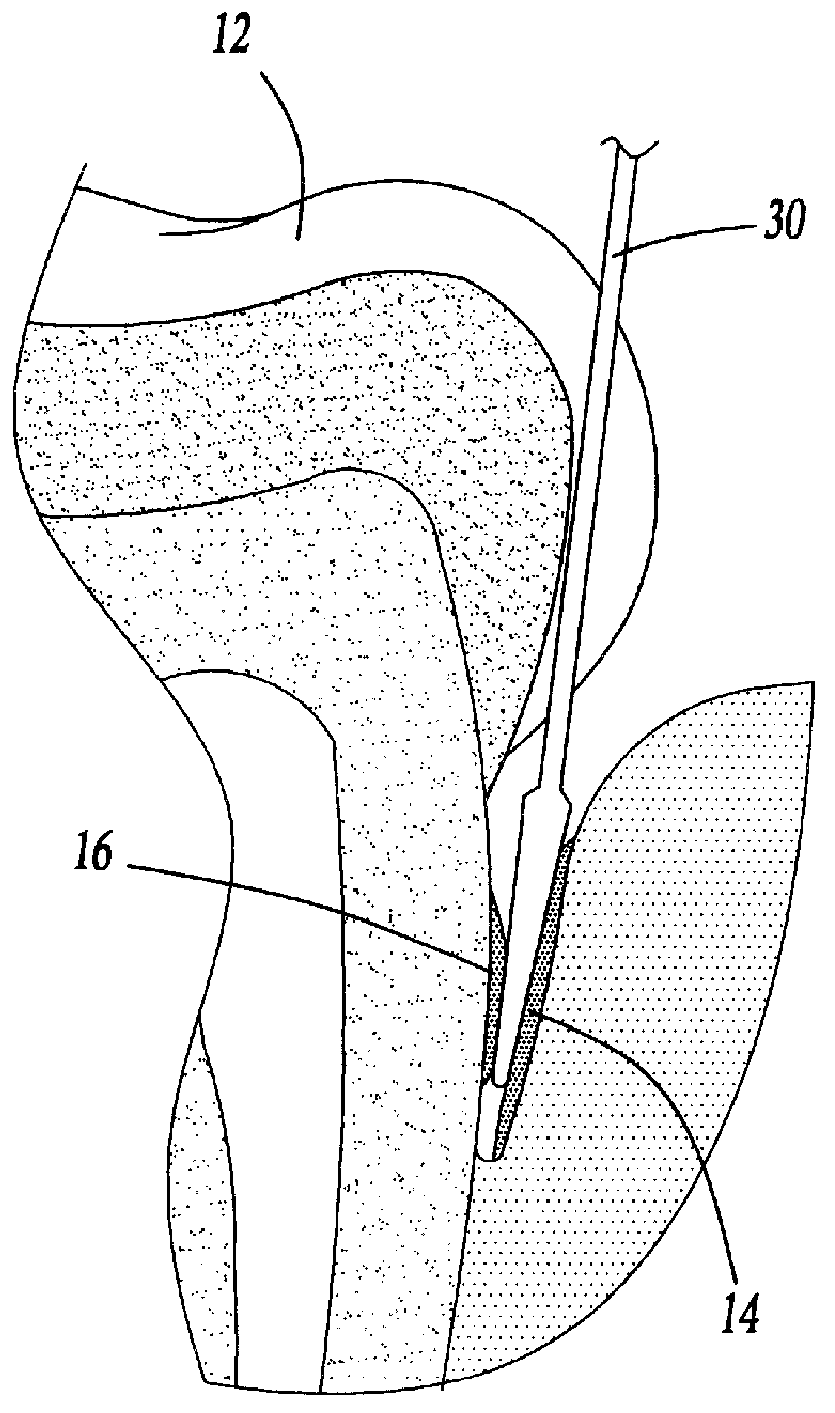
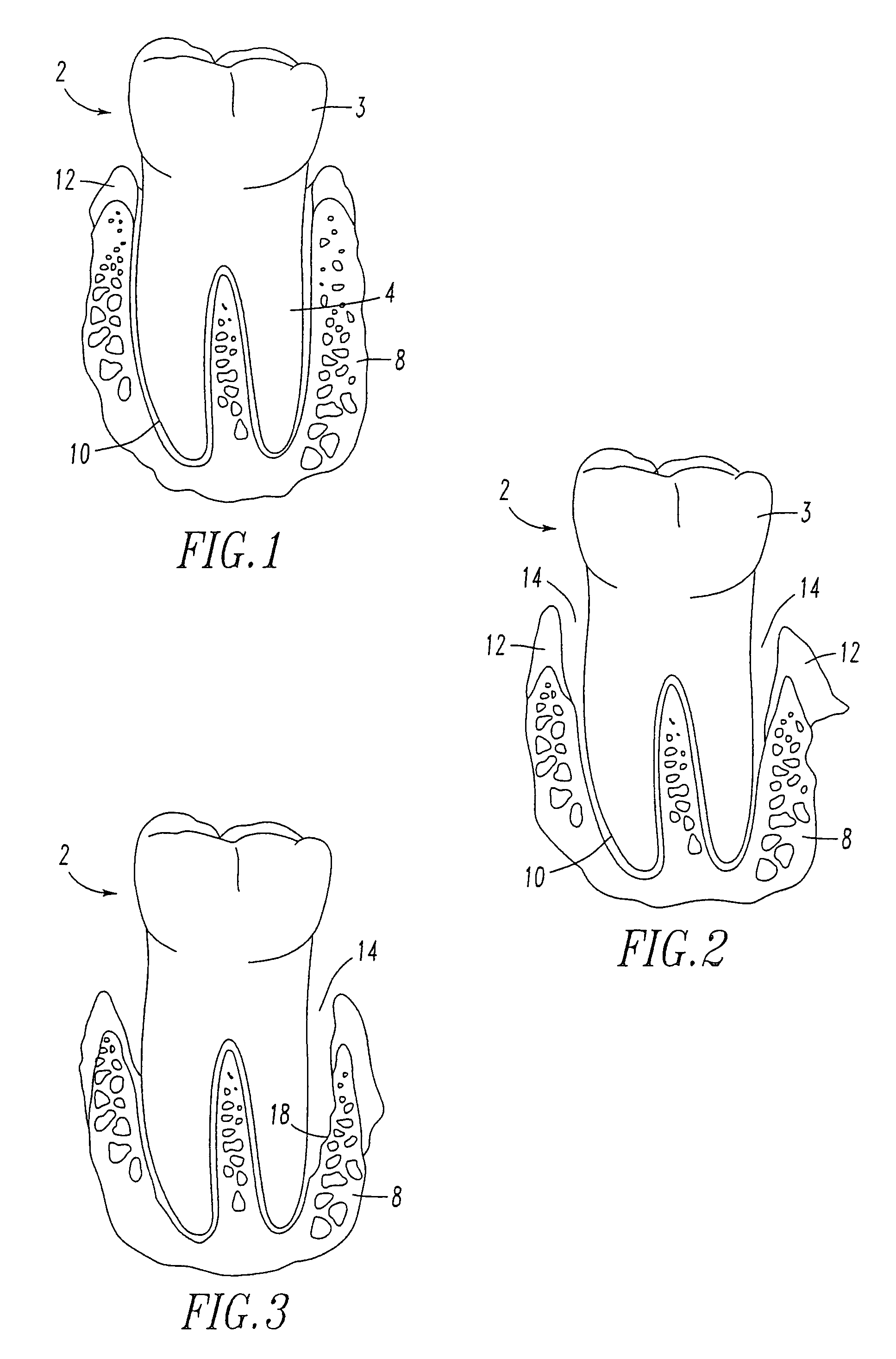
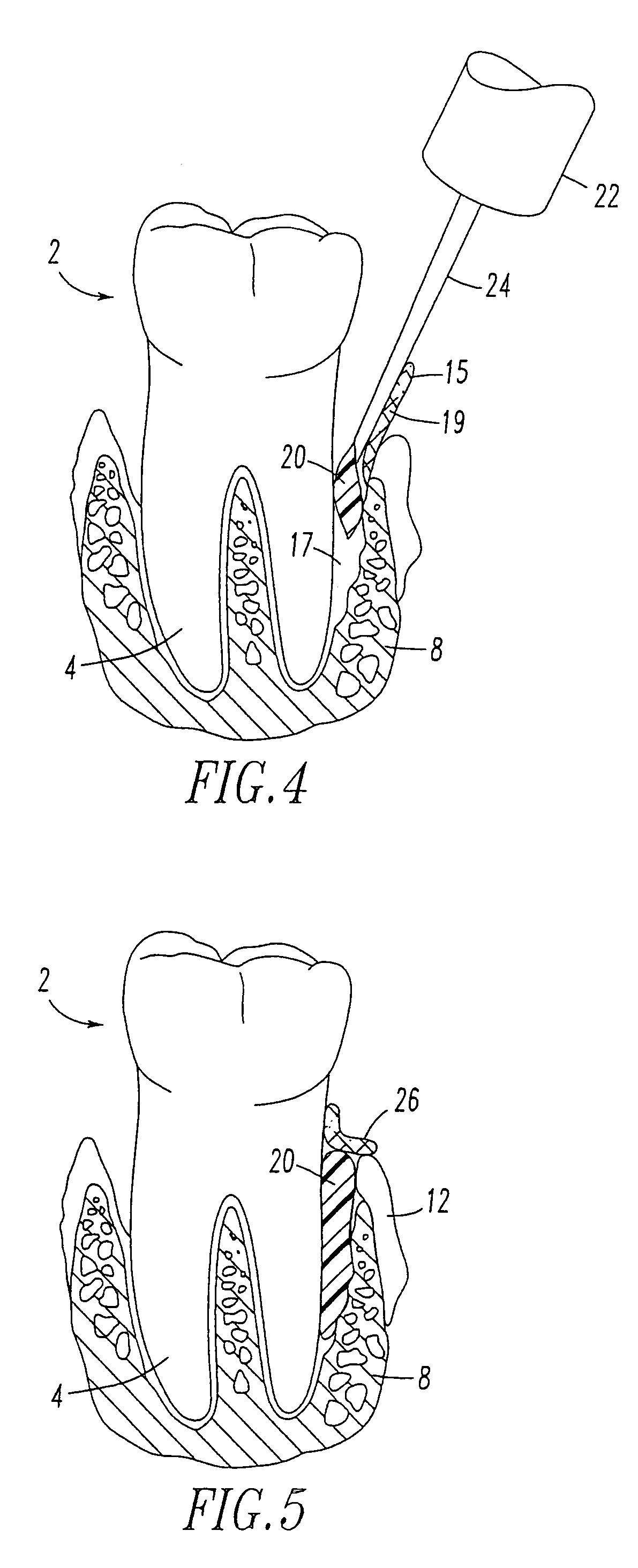
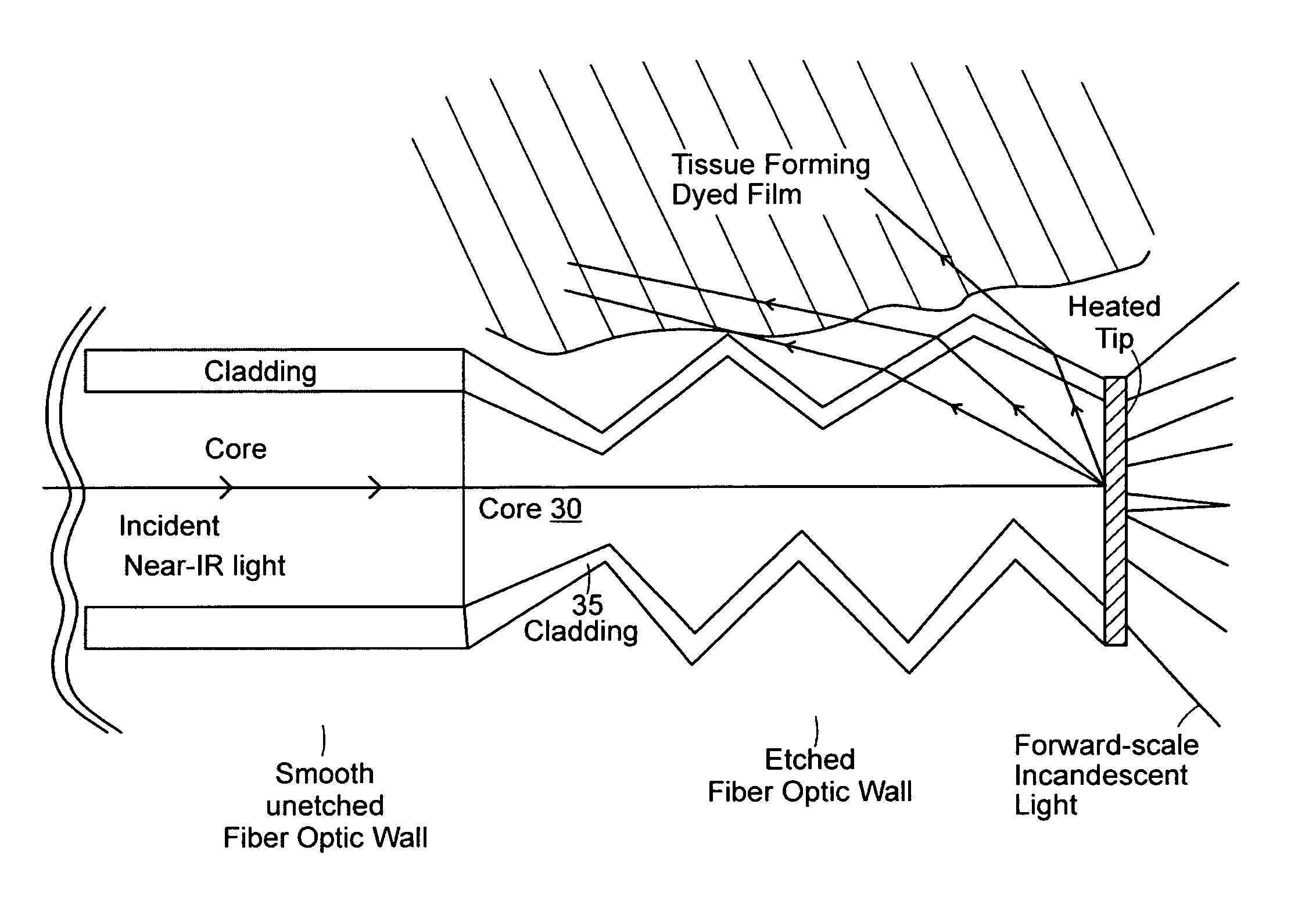
Method for treating periodontal disease
A method for treating periodontal disease in the periodontal pocket and for rendering the periodontal pocket resistant to future bacterial infection. After necrotic soft tissue and plaque is removed from the periodontal pocket by debridement, an optical fiber is inserted into the periodontal pocket. Laser emission is transmitted through the optical fiber into the periodontal pocket to substantially eradicate any remaining bacteria in the periodontal pocket and to also render the periodontal pocket resistant to subsequent bacterial infection. Optionally, the optical fiber is inserted into the periodontal pocket prior to debridement and laser radiation is transmitted through the optical fiber into the periodontal pocket and against both the soft and hard tissue. Such laser radiation not only kills bacteria in the periodontal pocket but also loosens the plaque which is typically present on the tooth and desensitizes the area surrounding the periodontal pocket.
Owner:MYERS TERRY D
Inhibitors of proteasomal activity for stimulating hair growth
Compounds that inhibit the activity of NF-κB or inhibit the activity of the proteasome or both promote bone formation and hair growth and are thus useful in treating osteoporosis, bone fracture or deficiency, primary or secondary hyperparathyrdidism, periodontal disease or defect, metastatic bone disease, osteolytic bone disease, post-plastic surgery, post-prosthetic joint surgery, and post-dental implantation; they also stimulate the production of hair follicles and are thus useful in stimulating hair growth, including hair density, in subject where this is desirable.
Owner:OSTEOSCREEN IP +1
Functional polymorphisms of the interleukin-1 locus affecting transcription and susceptibility to inflammatory and infectious diseases
InactiveUS20030235890A1Effectively prescribeSugar derivativesMicrobiological testing/measurementWhite blood cellInterleukin-1beta
The invention provides methods and reagents for detecting a polymorphism associated with in an upstream region of the interleukin-1 beta (IL-B) gene (IL-1B (-3737)) that affects transcription of the gene and susceptibility to inflammatory and infectious diseases such as periodontal disease and Alzheimer's disease.
Owner:ORIG3N INC
Novel peptide with osteogenic activity
The present invention provides a composition including an isolated or recombinant peptide component that has osteogenic cell proliferative activity. The peptide, which promotes proliferation of osteoblasts, is useful for treatment of fractures, as a filler in deficient sites of bone, for inhibition of decrease in bone substance related to osteoporosis and periodontic diseases, and for prevention of fractures associated with osteoporosis and rheumatoid arthritis. The peptide, or cells that have been genetically engineered to produce the peptide, can be combined with a bone-compatible matrix to facilitate slow release of the peptide to a treatment site and / or provide a structure for developing bone.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Edible compositions capable of removing oral biofilm
InactiveUS20050058744A1Significantly adsorbing oral bacteriaReduce bacterial adhesionCosmetic preparationsToilet preparationsDiseaseCarrageenan
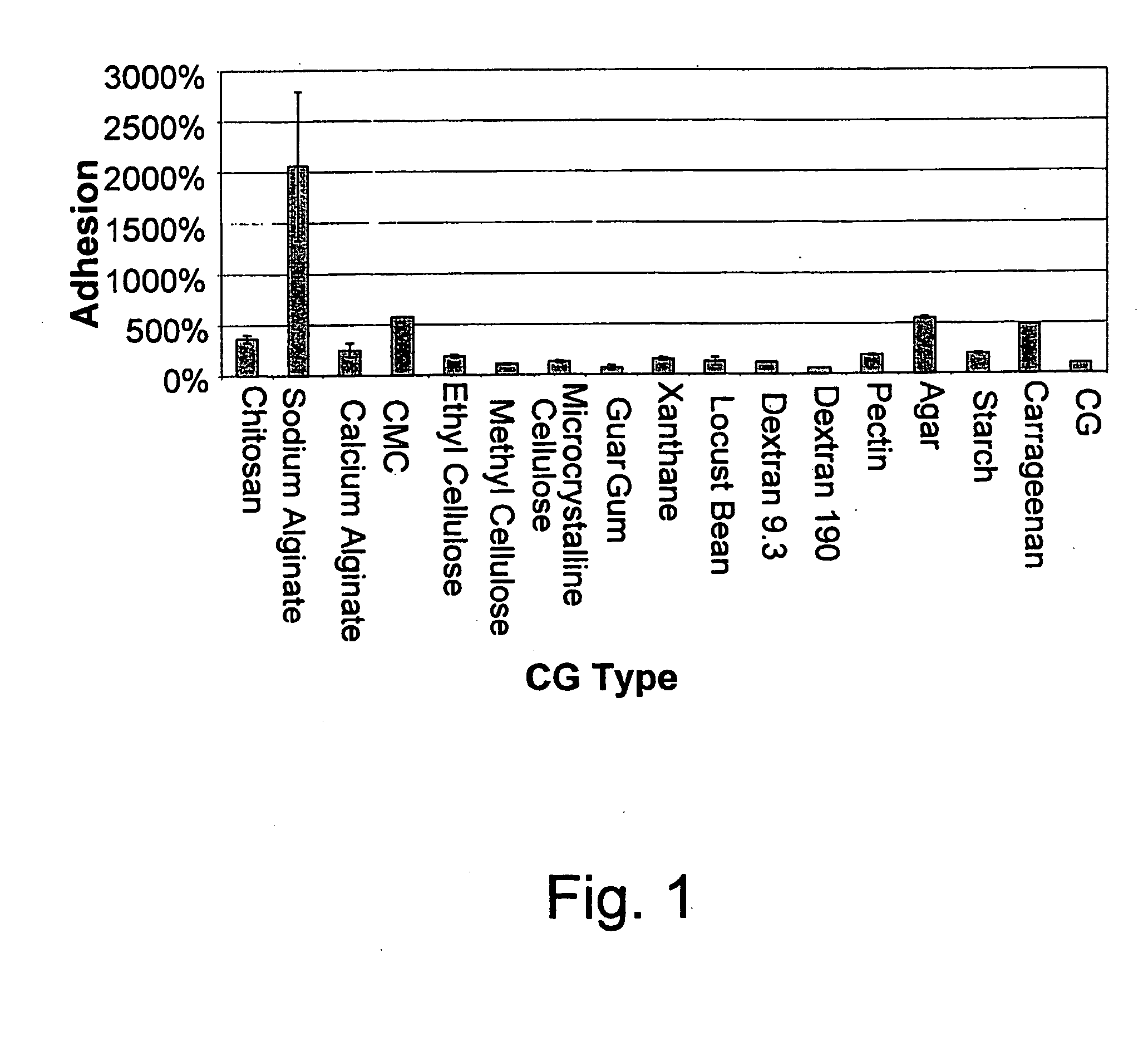
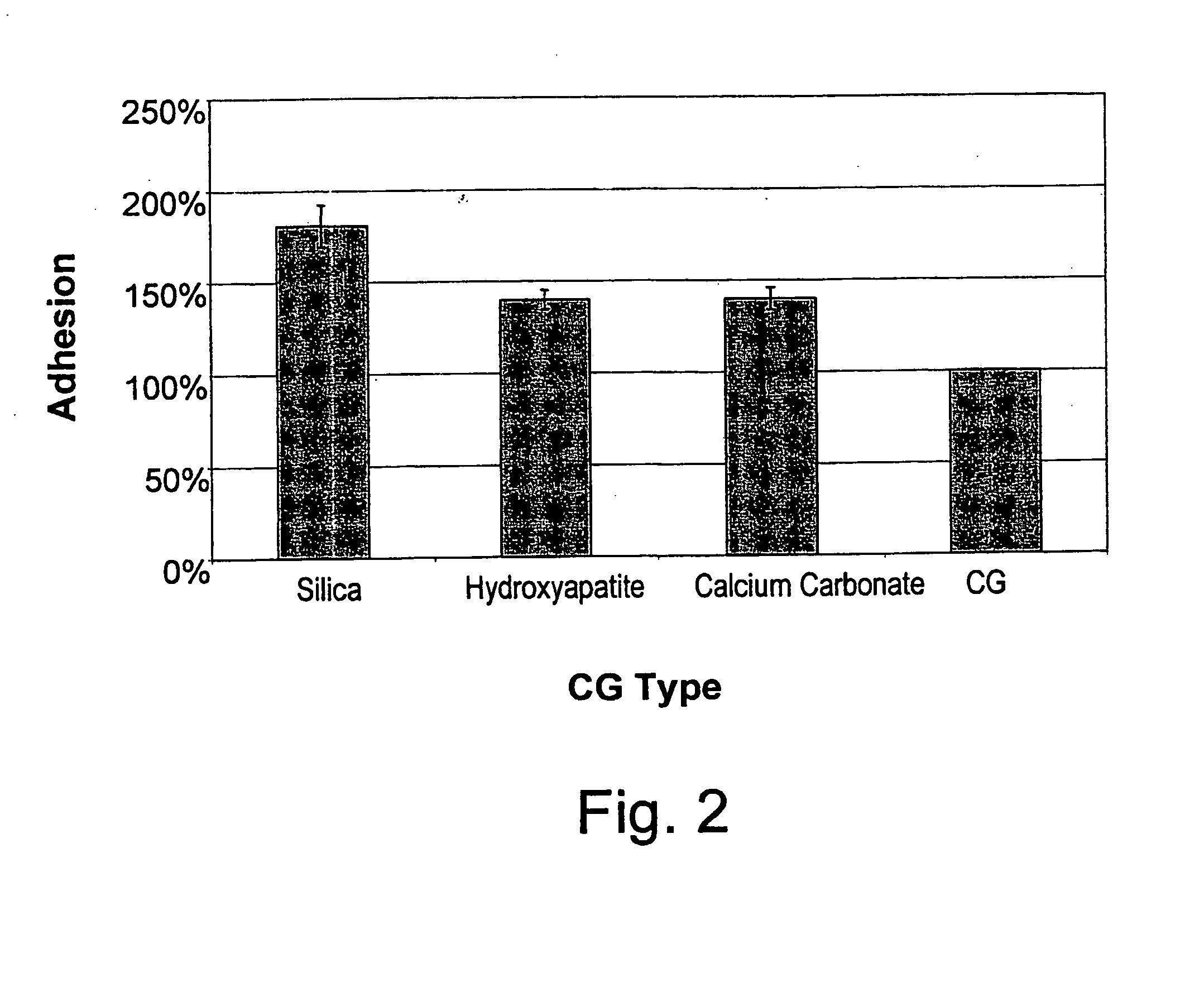
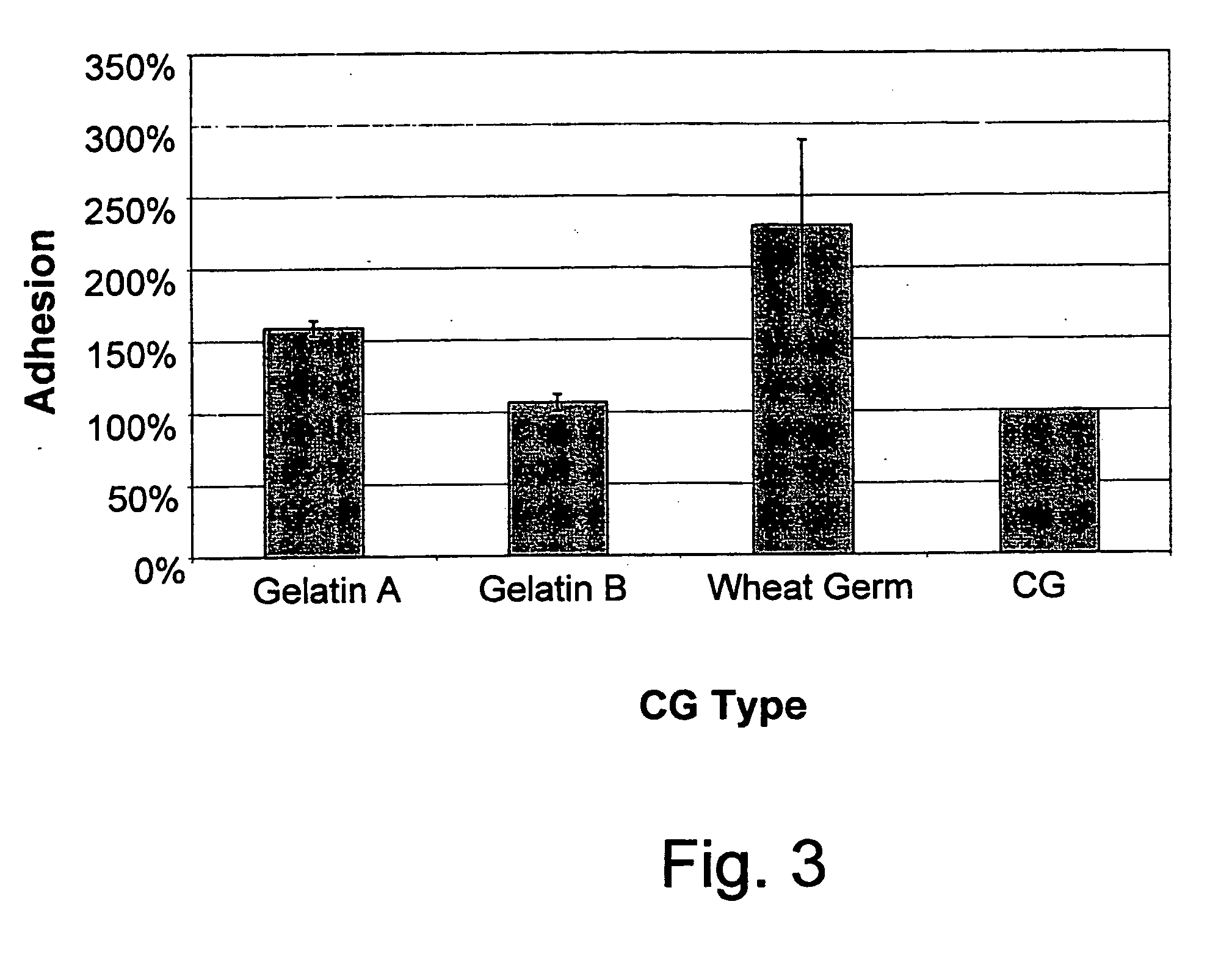
An edible and / or chewable article of manufacture containing at least one food grade substance having adsorption affinity towards at least one dental plaque (biofilm) constituent and capable of reducing and / or removing the oral biofilm while present in the mouth. Particular articles of manufacture are chewing gums, sweets, candies, candy, and other nutritional bars, ice creams, chocolates, confectionery and bakery / pastry products, honey, dairy products and beverages, and oral hygiene products such as tooth pastes, oral gels and mouthwashes. A chewing gum having a conventional gum base and at least one food grade active substance having adsorption affinity towards at least one dental plaque (biofilm) constituent (bacteria and proteins and bacterial cell-free enzymes) and capable of reducing and / or removing the oral biofilm while present in the mouth. Active substances include polysaccharides and non-toxic salts thereof, such as alginates, chitosan, carboxymethylcellulose, agar and carrageenan, inorganic substances such as silica, hydroxyapatite and calcium carbonate and proteins, particularly gelatin and lectin. The article of manufacture removes and / or for prevents or reduces dental plaque (biofilm), and controlling oral, dental and periodontal diseases.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
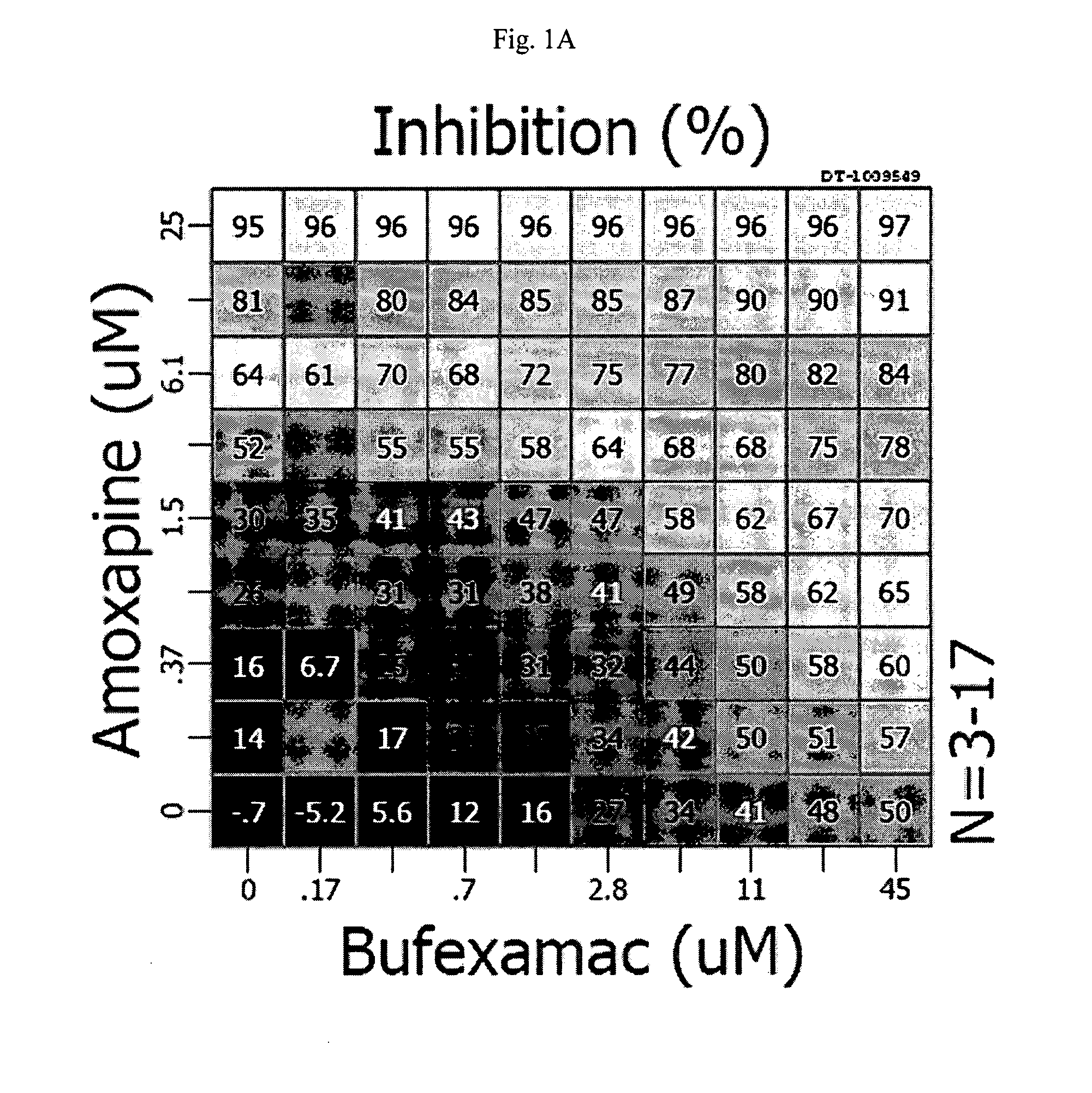
Food Compositions and Methods of Treating Periodontal Disease
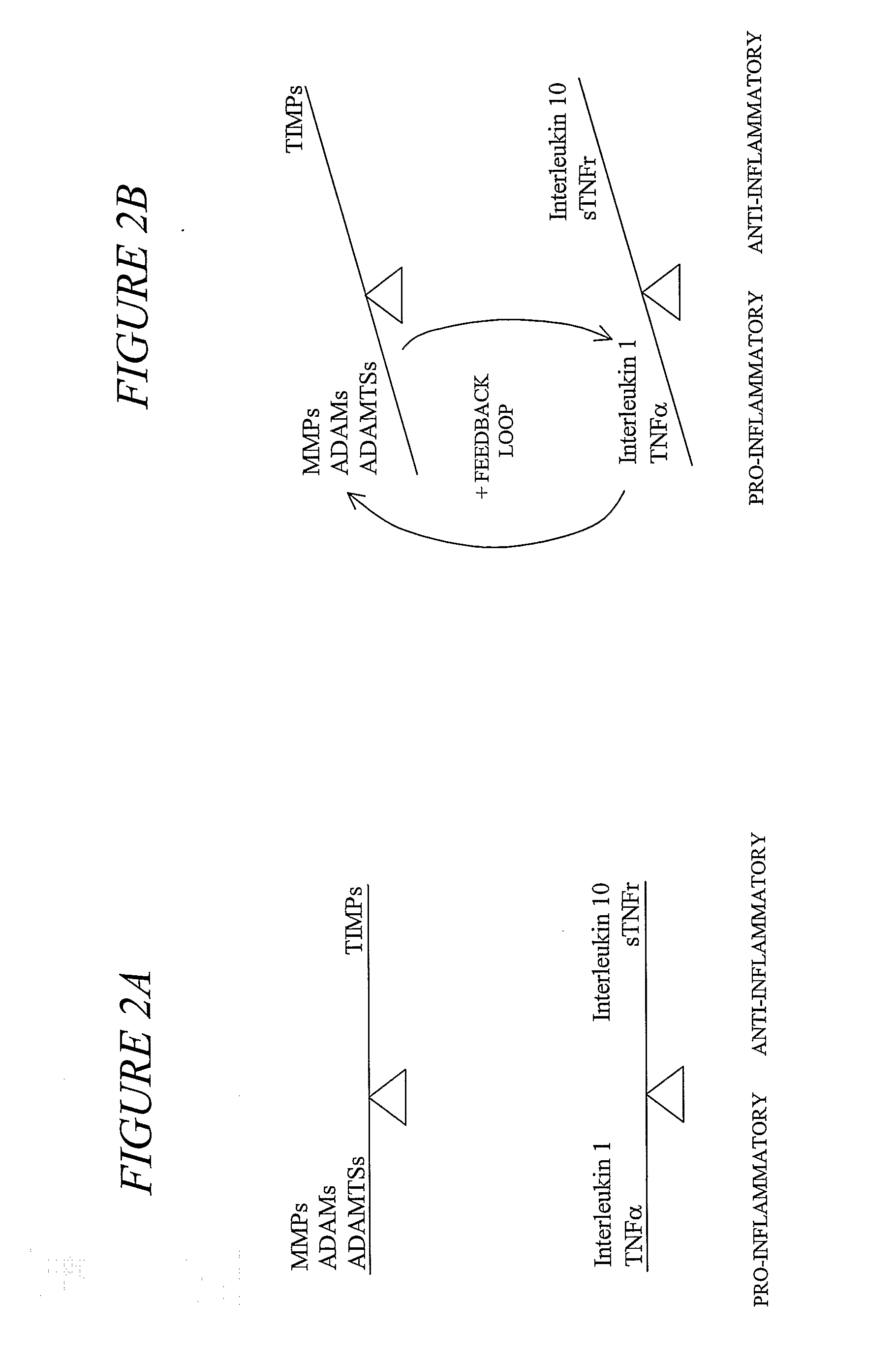
The invention provides a method of alleviating a sign or symptom of periodontal disease in a subject, by administering to the subject a composition containing a natural compound which inhibits matrix metalloproteinase activity and interleukin-1 activity.
Owner:INTERLEUKIN GENETICS
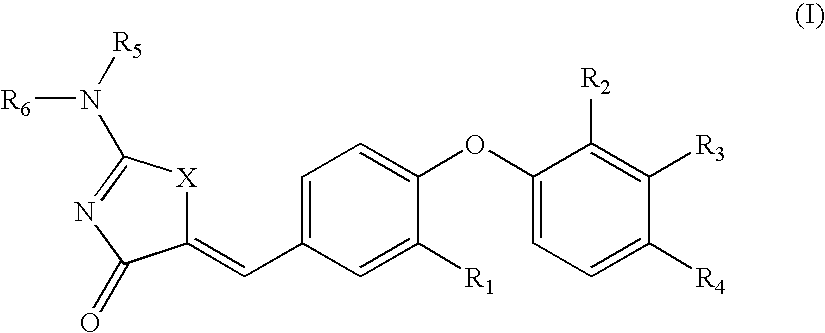
SUBSTITUTED PHENOXY AMINOTHIAZOLONES as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
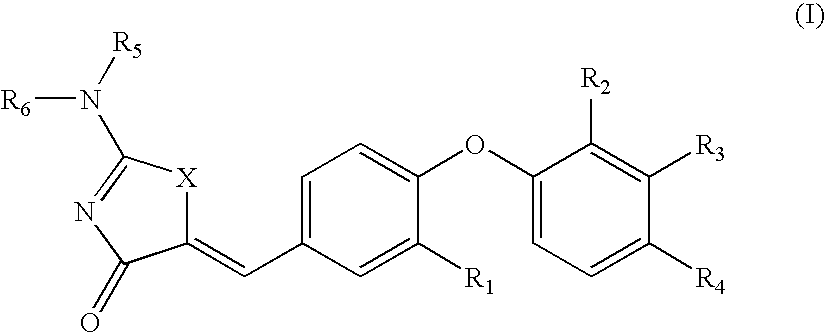
SUBSTITUTED PHENOXY THIAZOLIDINEDIONES AS ESTROGEN RELATED RECEPTOR-alpha MODULATORS
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
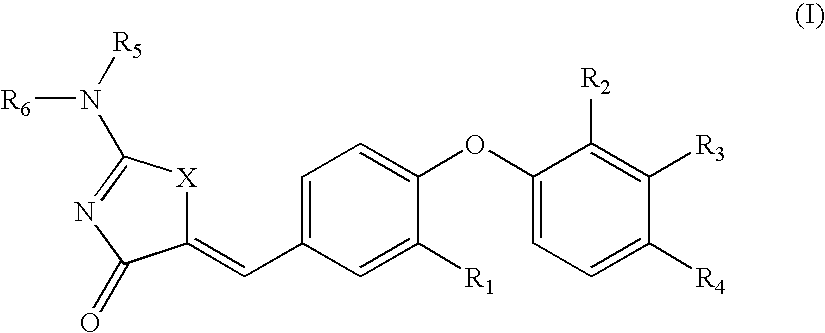
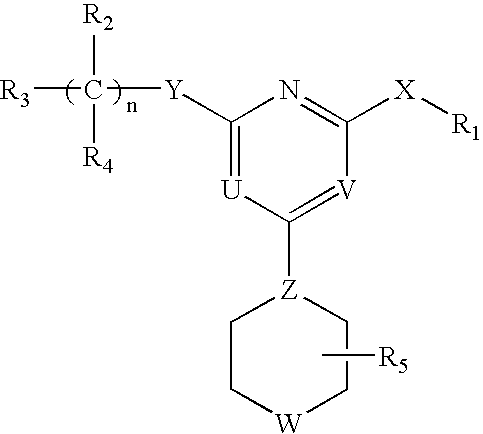
Heterocyclic Compounds For Preventing And Treating Disorders Associated With Excessive Bone Loss
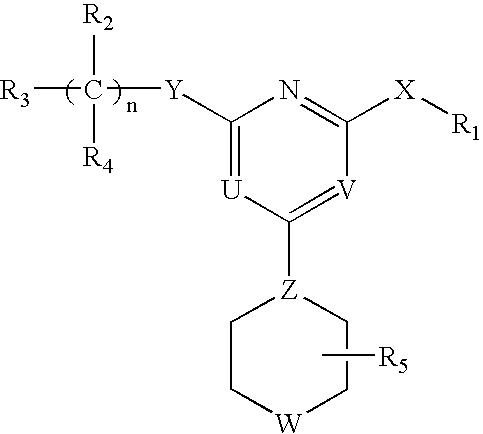
This invention relates to pyrimidine compounds of formula (I), formula (I′), and formula (I″):and pharmaceutically acceptable salts, solvates, clathrates, and prodrugs thereof, wherein R1, R2, R3, R4, R5, U, V, W, X, Y, Z, and n are defined herein. This invention also relates to compositions comprising these compounds and methods for using them. The compounds and compositions of this invention are useful to treat or prevent disorders associated with excessive bone loss, including, without limitation periodontal disease, non-malignant bone disorders (such as osteoporosis, Pagers-disease of bone, osteogenesis imperfecta, fibrous dysplasia, and primary hyperparathyroidism) estrogen deficiency, inflammatory bone loss, bone malignancy, arthritis, osteopetrosis, and certain cancer-related disorders (such as hypercalcemia of malignancy (HCM), osteolytic bone lesions of multiple myeloma and osteolytic bone metastases of breast cancer and other metastatic cancers).
Owner:SYNTA PHARMA CORP
Treatment device and method for treating or preventing periodontal disease through application of heat
A regulated heat source is described that can be applied to the teeth and gums in order to accelerate the death of the bacterial or viral systems known to contribute to periodontal disease. The regulated heat source can use a segmented mouthpiece to facilitate application of the thermal energy to the teeth and gums
Owner:LUMATHERM INC
Programming of cells for tolerogenic therapies
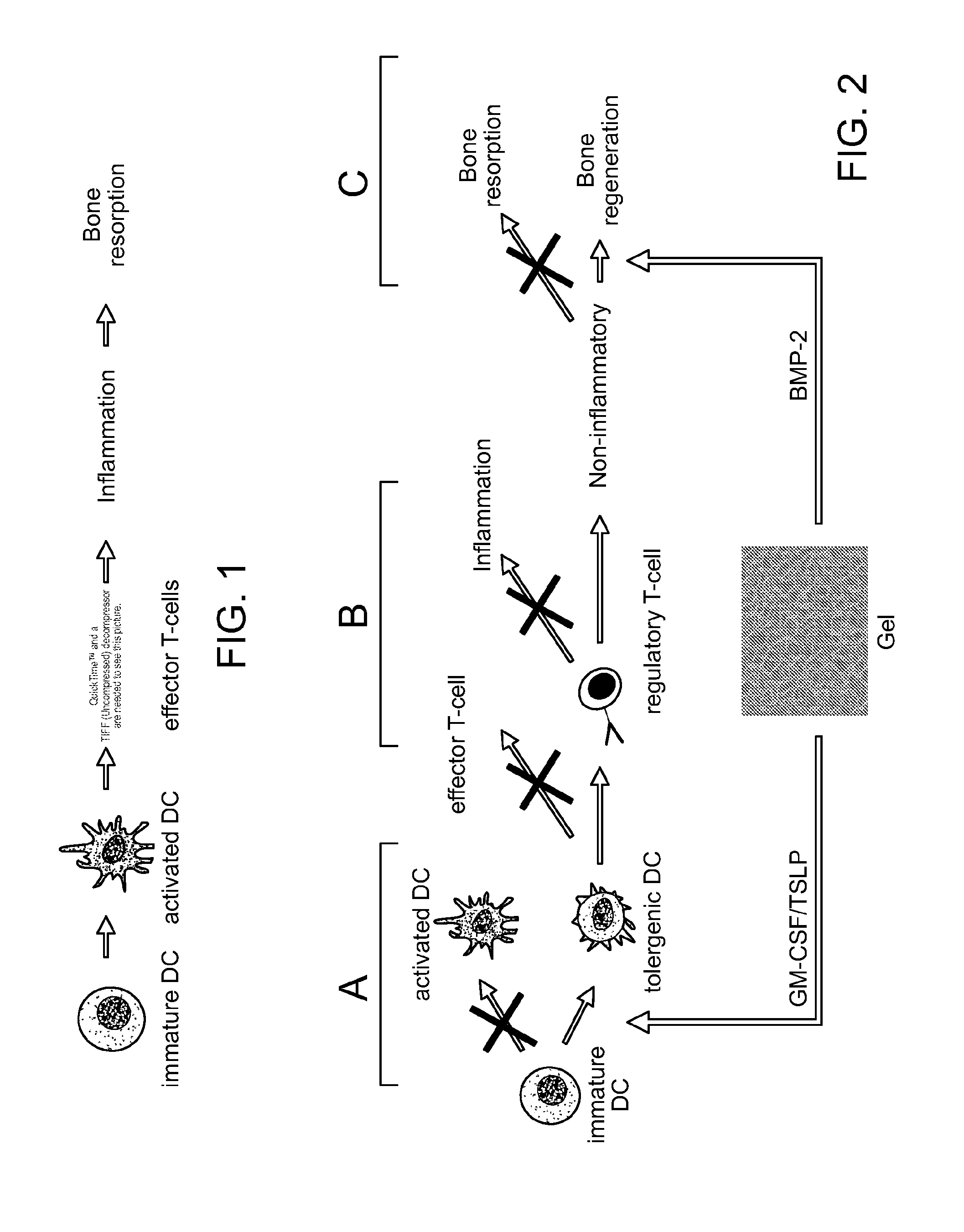
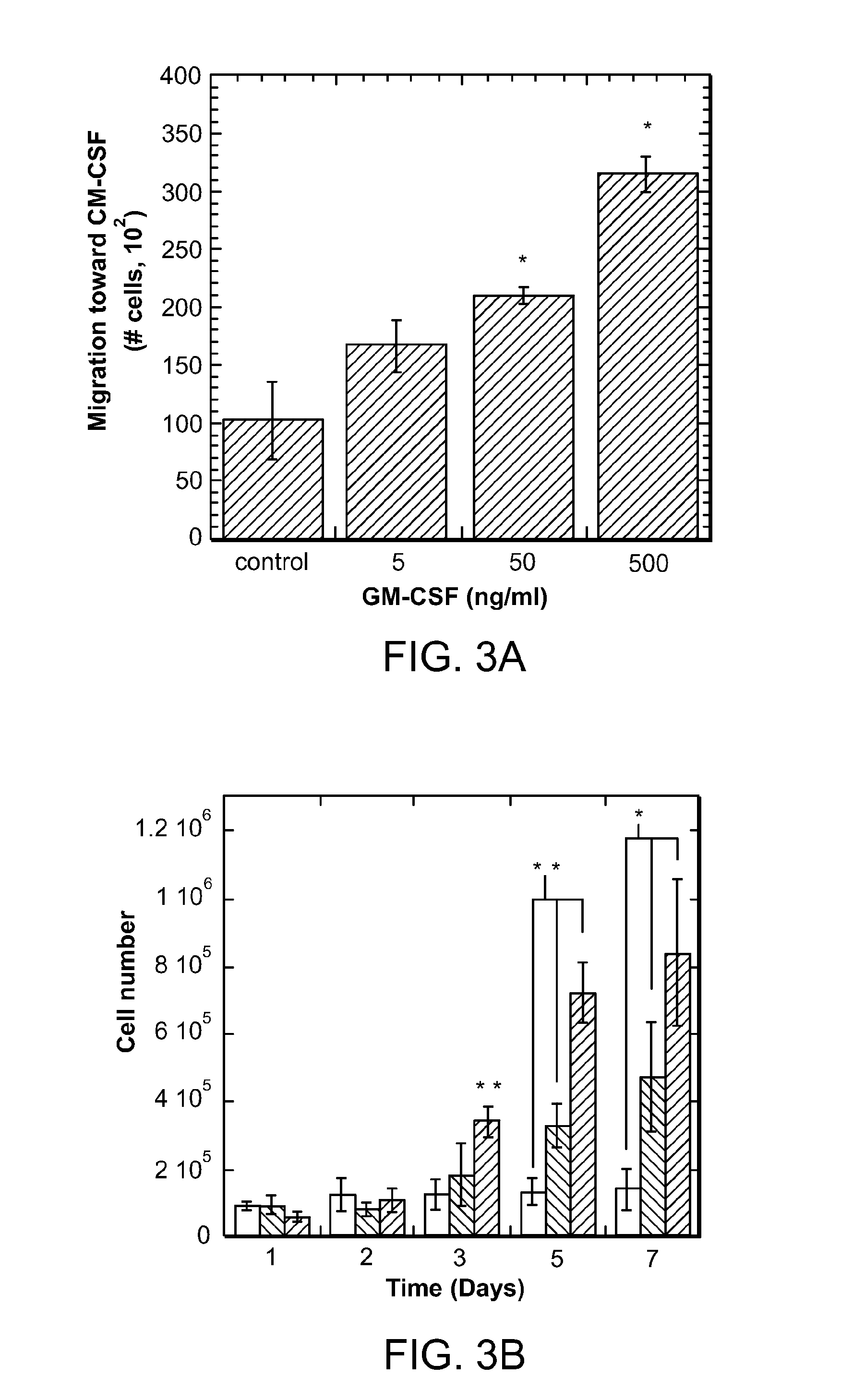
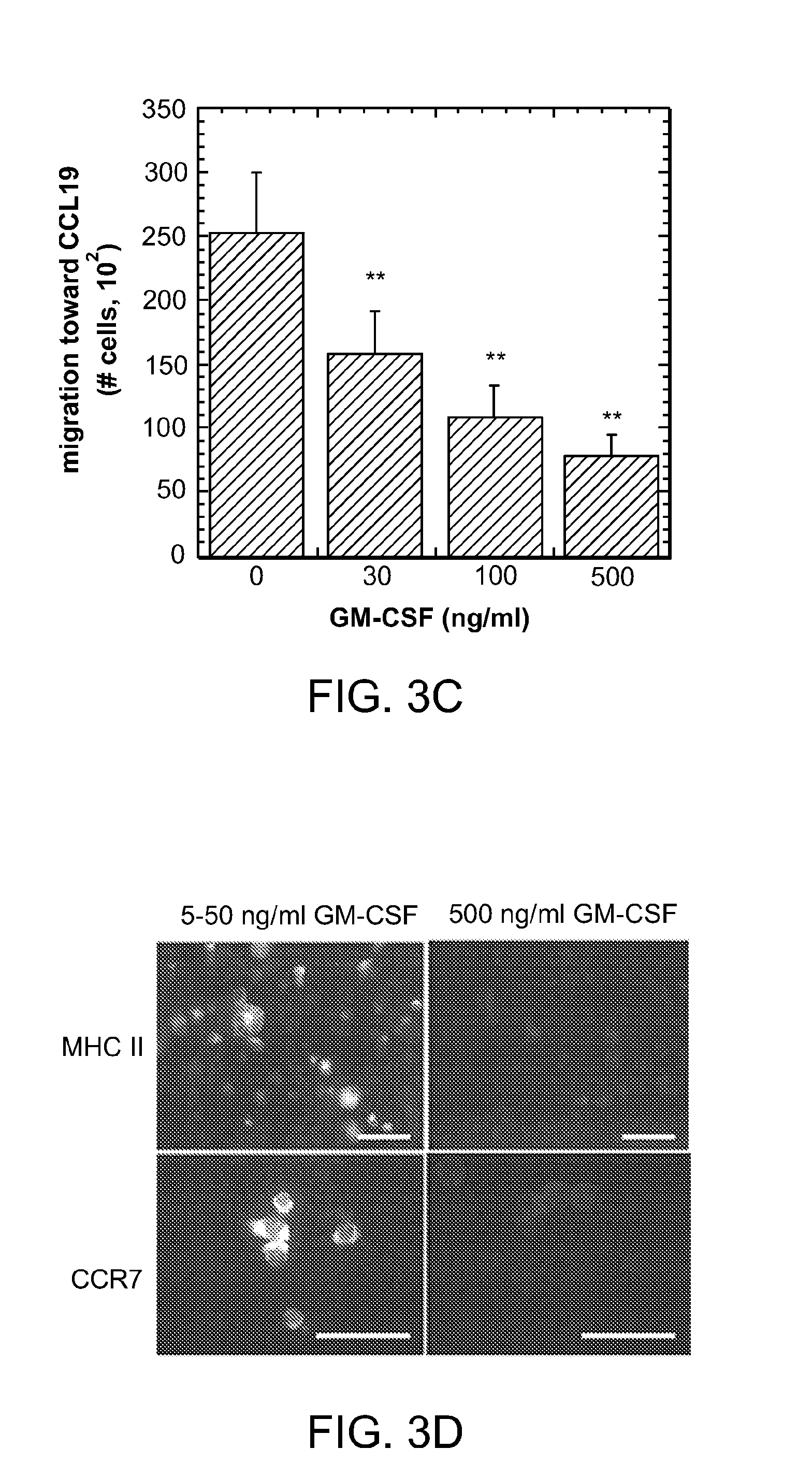
ActiveUS8728456B2Reduce severityReduce attackNervous disorderPeptide/protein ingredientsTolerogenic therapyAutoimmune responses
Biomaterial systems, e.g., gel scaffolds, are used in vivo to recruit immune cells and promote their activation towards a non-inflammatory phenotype, thereby leading suppression of inflammation. The compositions and methods are useful to reduce the severity of autoimmunity, chronic inflammation, allergy, and periodontal disease.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Method of treatment for and prevention of periodontal disease
ActiveUS20060036194A1Increase amplitudeUltrasonic/sonic/infrasonic diagnosticsDental implantsBiofilmAcoustic shock
The method of treatment for a periodontal tissue exhibiting a periodontal disease or periodontal condition in a diagnosed patient is disclosed. The method has the steps of activating an acoustic shock wave generator or source to emit acoustic shock waves; and subjecting the periodontal tissue, or the entire periodontal region of the patient to the acoustic shock waves stimulating said tissue, wherein the tissue is positioned within a path of the emitted shock waves. The method of treatment may further have the steps of administering one or more medicaments prior, during or after subjecting the patient to acoustic shock waves or testing the bacterial count or viability of the treated tissue or region of the diagnosed patient after exposure to one or more acoustic shock wave treatments; or subjecting a tissue or organ to a surgical procedure to remove or repair some or all of any defects or degenerative tissues. The method of treatment is for prevention of periodontal disease and may be used with debridement. The treatment is particularly useful in eradicating and inhibiting periodontal biofilms.
Owner:SOFTWAVE TISSUE REGENERATION TECH LLC
Method of treating periodontal disease using periodontal regeneration composition
Owner:PERIOVANCE INC
Edible compositions which are adapted for use by a companion animal
Disclosed herein are a variety of embodiments of compositions and methods which are each adapted for use by a companion animal. In one embodiment, an edible composition comprising an amount of a soluble mineral component is disclosed, wherein the soluble mineral component comprises one or more minerals selected from the group consisting of zinc, manganese, tin, copper, and mixtures thereof, wherein the amount is an effective amount for use as an oral medicament. In further embodiments, a phosphate component is included. The edible compositions are advantageously companion animal foods or supplements. Further disclosed are methods selected from treating oral cavity tartar, oral cavity plaque, periodontal disease, gingivitis, breath odor and combinations thereof comprising orally administering a described composition to a companion animal.
Owner:MARS INC +1
Use of secondary optical emission as a novel biofilm targeting technology
InactiveUS7621745B2Without harming healthy dental structure and tissueMore processedDental implantsGum massageBiofilmFiber
Owner:NOMIR MEDICAL TECH
Compositions and Methods for Treating and Preventing Inflammatory and/or Degenerative Processes in Humans and Other Animals
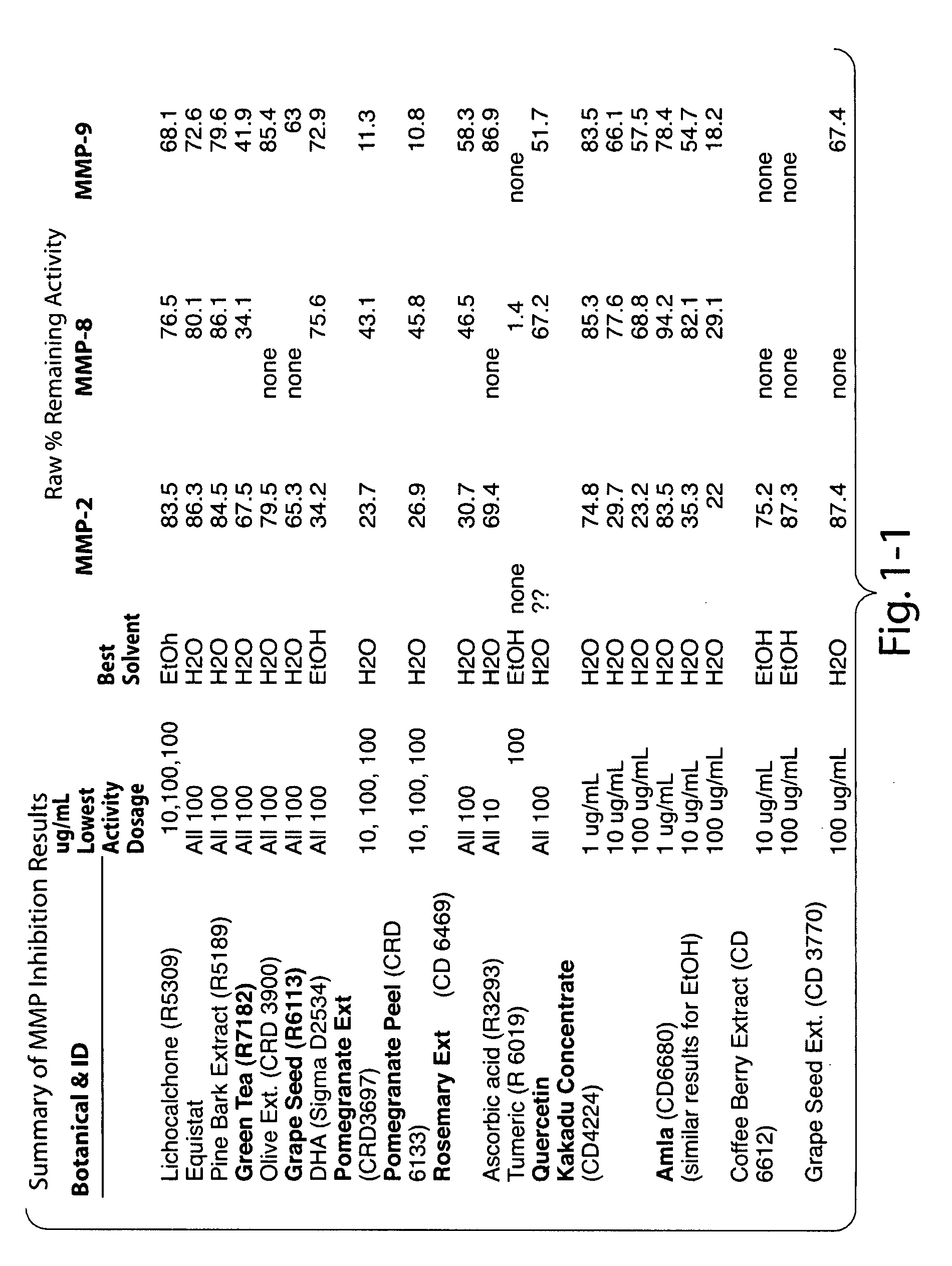
Disclosed are compositions useful for treating Alzheimer's disease, atherosclerosis, arteriosclerosis, osteoarthritis and other degenerative joint diseases, Huntington's chorea, Parkinson's disease, optic atrophy, retinitis pigmentosa, macular degeneration, muscular dystrophy, aging-associated degenerative processes, asthma, dermatitis, laminitis, pemphigoid, pemphigus, reactive airway disease (e.g., COPD, IAD), inflammatory bowel disease (e.g., Crohn's disease, ulcerative colitis), multiple sclerosis, rheumatoid arthritis, periodontal disease, systemic lupus erythematosus, sarcoidosis, psoriasis, type I diabetes, ischemia-reperfusion injury, chronic inflammatory diseases, geriatric wasting, cancer cachexia, cachexia associated with chronic inflammation, sick feeling syndrome, and other inflammatory and / or degenerative diseases, disorders, conditions, and processes in humans and other animals. In one embodiment, the compositions include at least 4 of the following: a MMP1 inhibitor, a MMP2 inhibitor, a MMP3 inhibitor, a MMP7 inhibitor, a MMP9 inhibitor, an ADAMTS-4 inhibitor, a MMP13 inhibitor, and a MMP14 inhibitor. In another embodiment, the compositions include a curcuminoid, a polymethoxylated flavone, a catechin, and a boswellic acid.
Owner:BAKER DONALD J
Methods for effecting oral treatment of teeth or gums
ActiveUS20110104631A1Improve reaction speedSafe and effective and convenientTeeth fillingDental toolsOral treatmentFlexible circuits
A method for effecting an oral treatment of teeth and / or gums using an intra-oral device that has a mouthpiece in which is embedded a flexible circuit board and arrays of spaced apart lamps. The mouthpiece has a curvature. The lamps may be light emitting diodes that generate electromagnetic radiation, preferably in the white and blue light spectrum and the infrared and ultraviolet light spectrum. The arrays are positioned to expose the facial and lingual sides of the teeth and / or gums for effecting the treatment when the mouthpiece is positioned to fit upper and lower rows of teeth within accommodating recesses. The flexible circuit board is flexed to exhibit a curvature that follows a curvature of the mouthpiece. Treatments include whitening teeth, desensitizing teeth, and treating gums to prevent periodontal disease.
Owner:JBL RADICAL INNOVATIONS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com