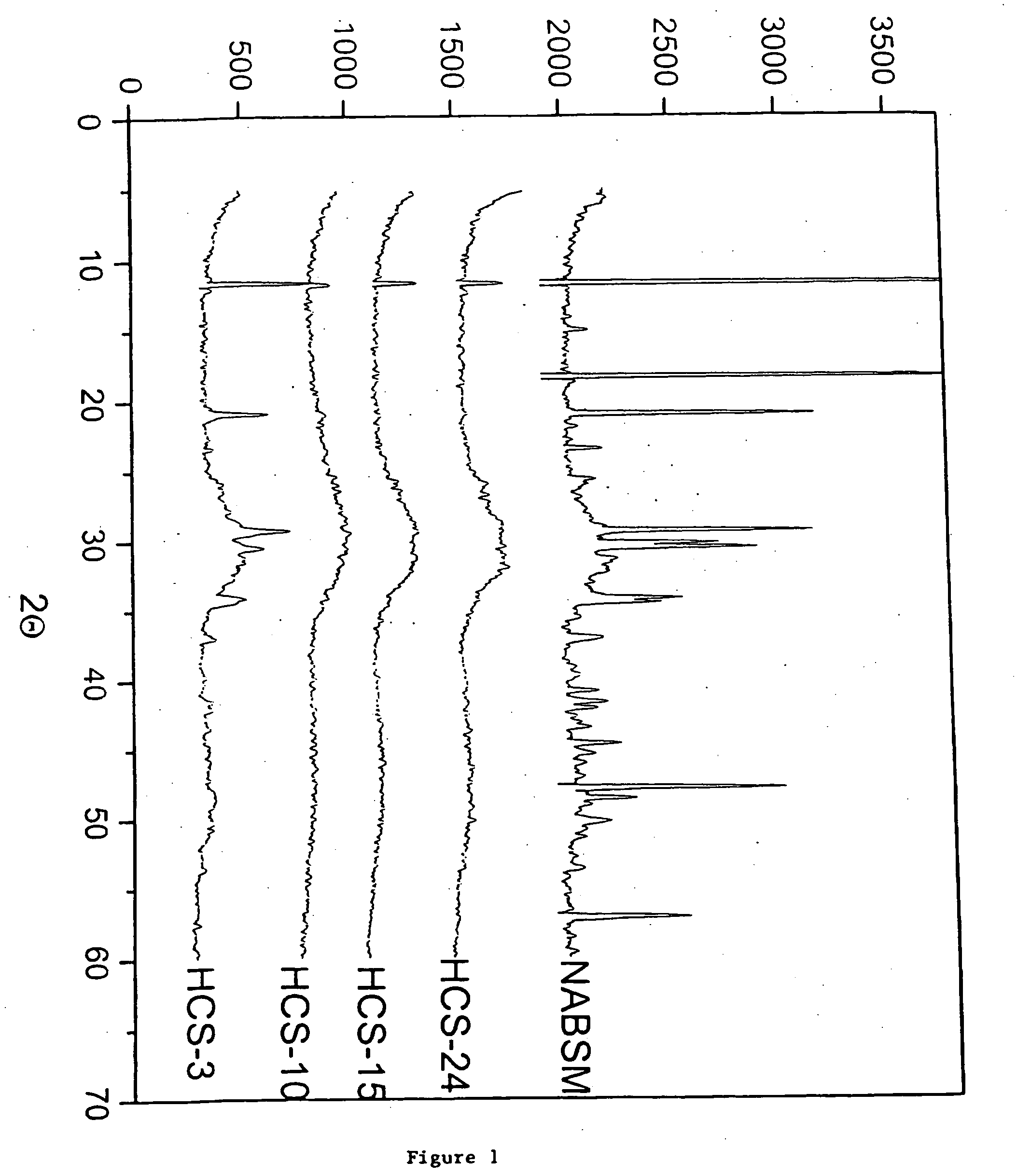
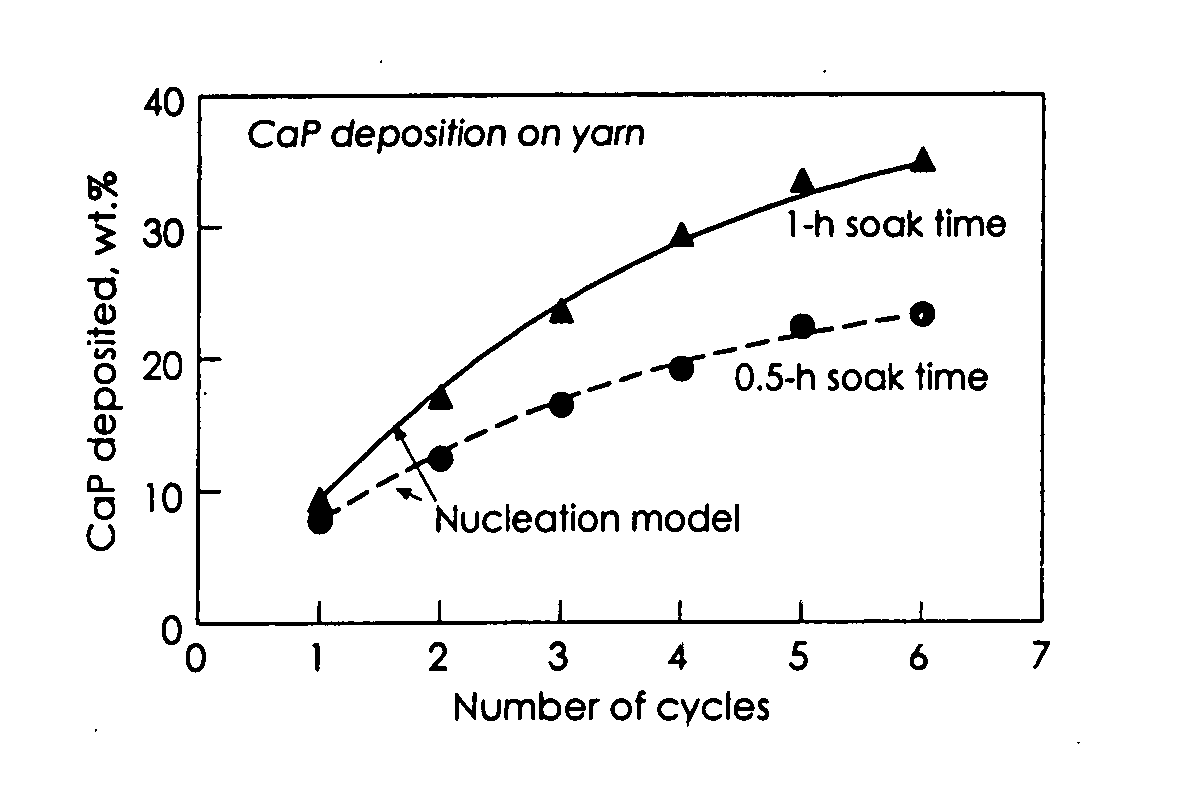
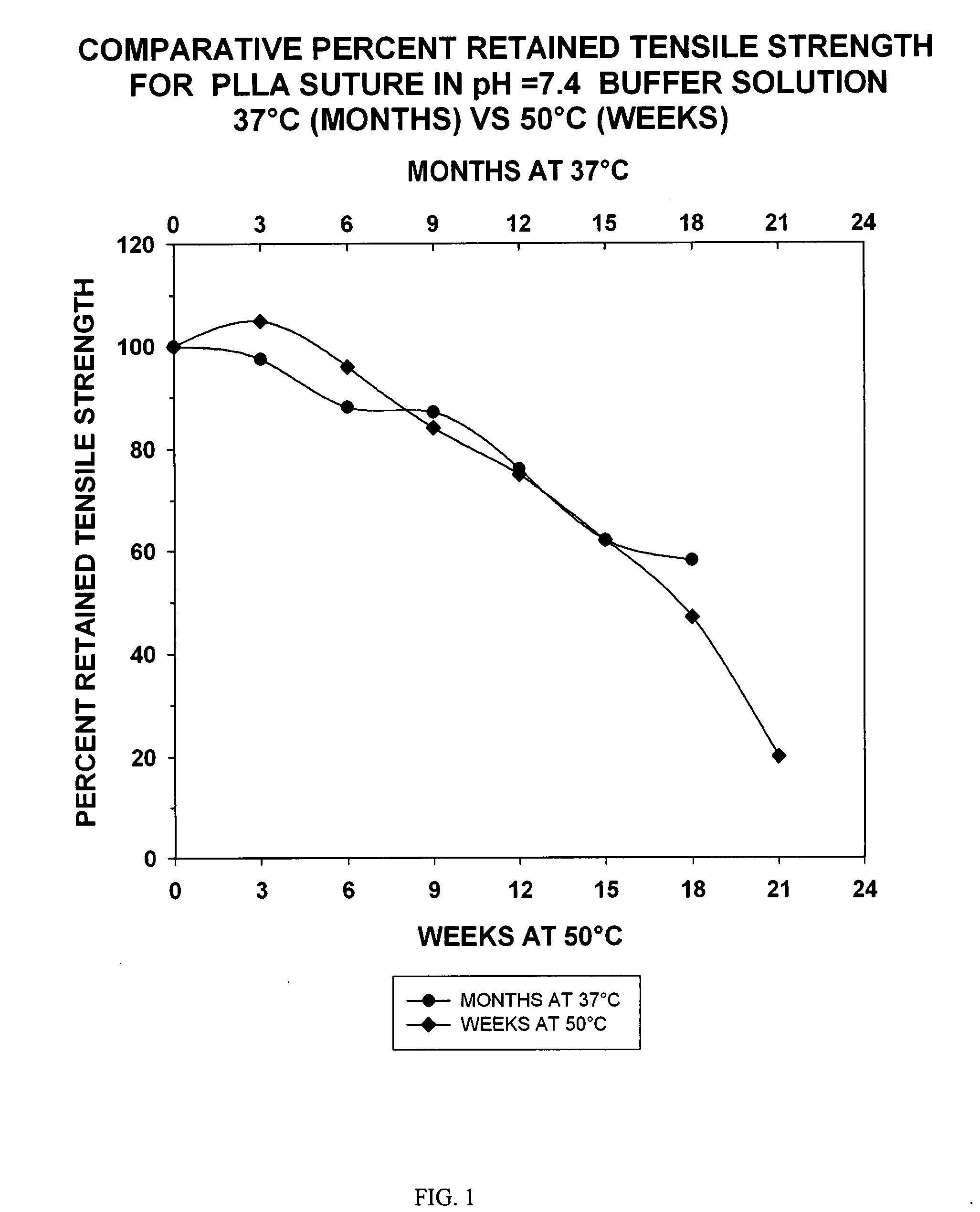
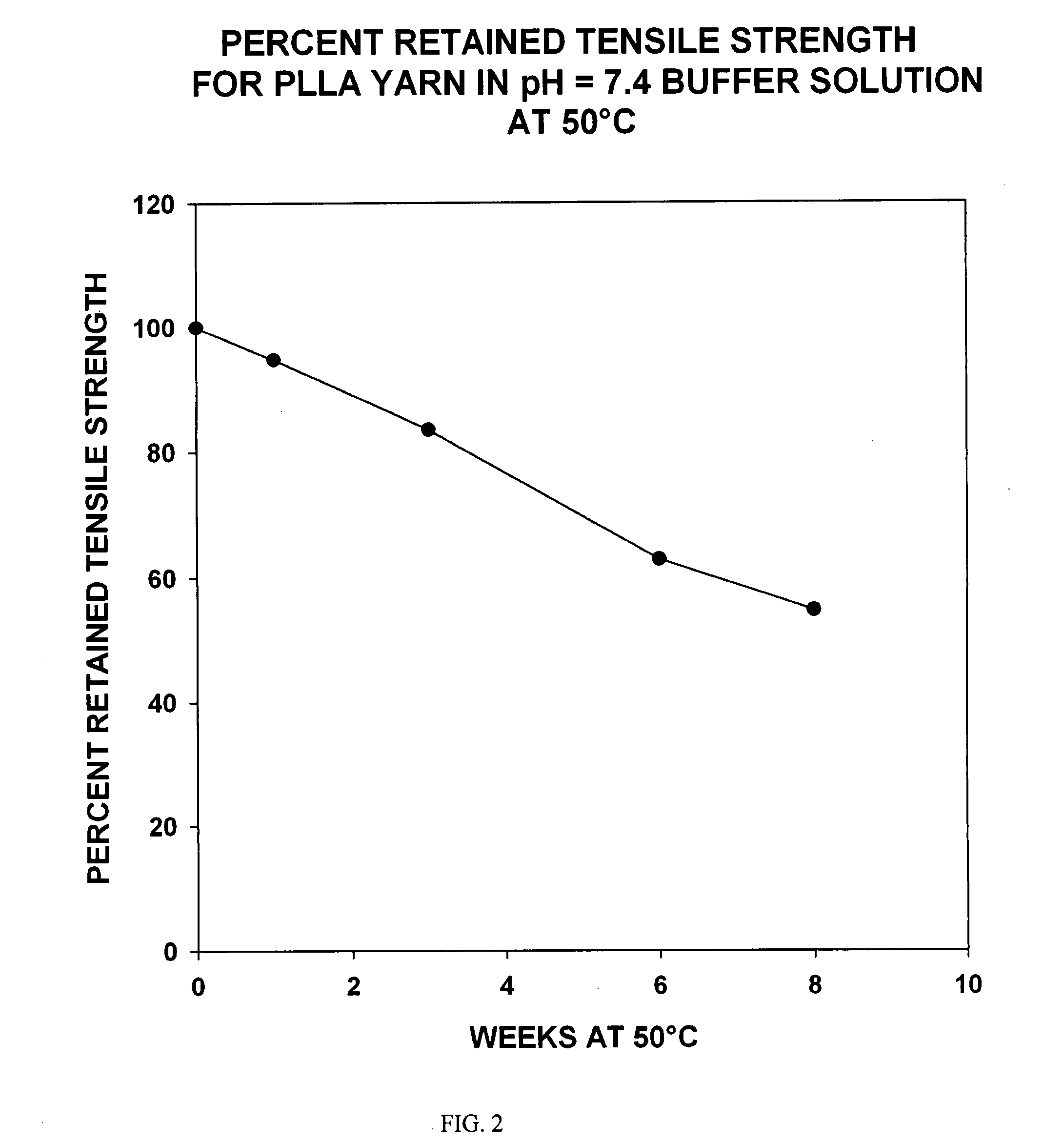
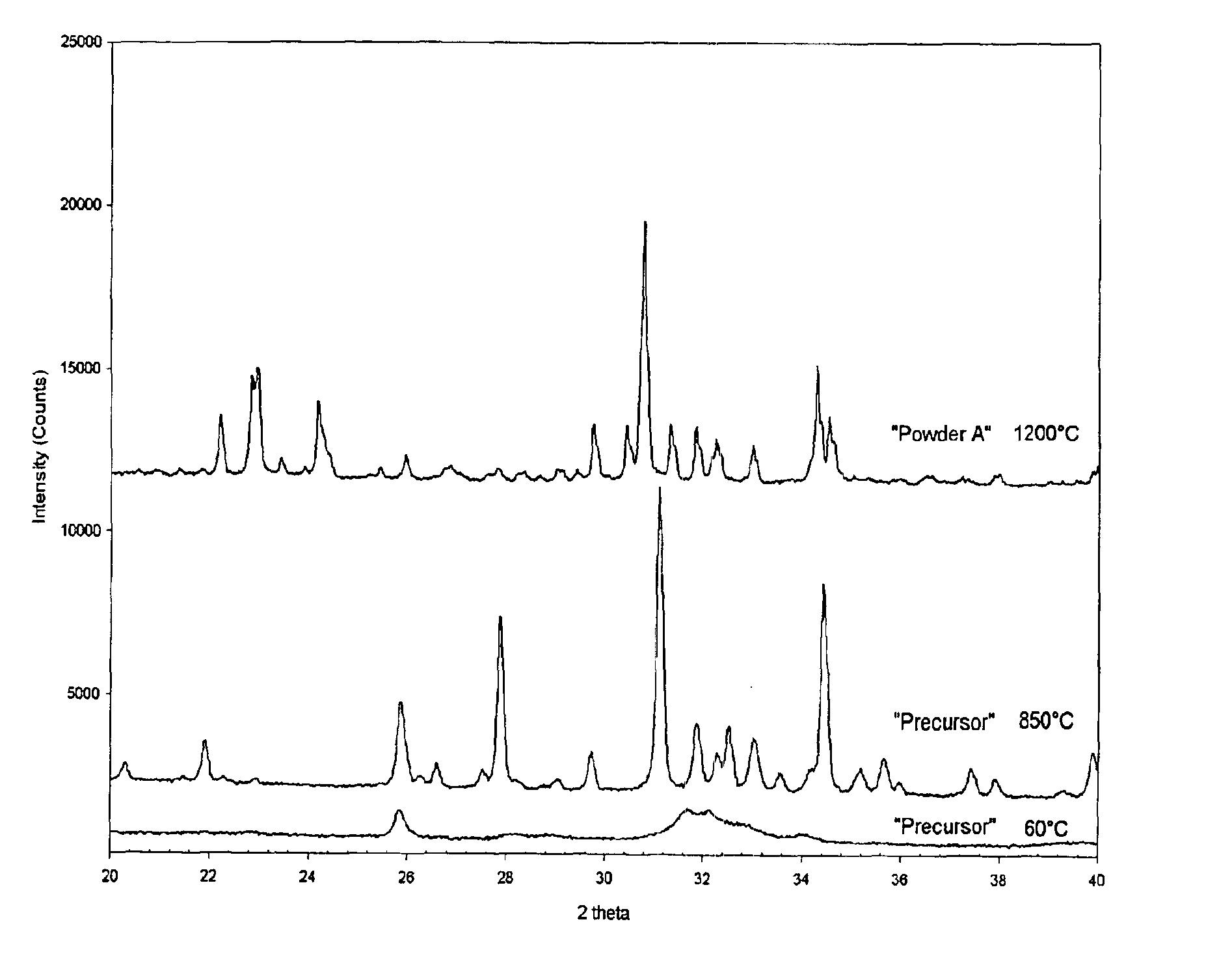
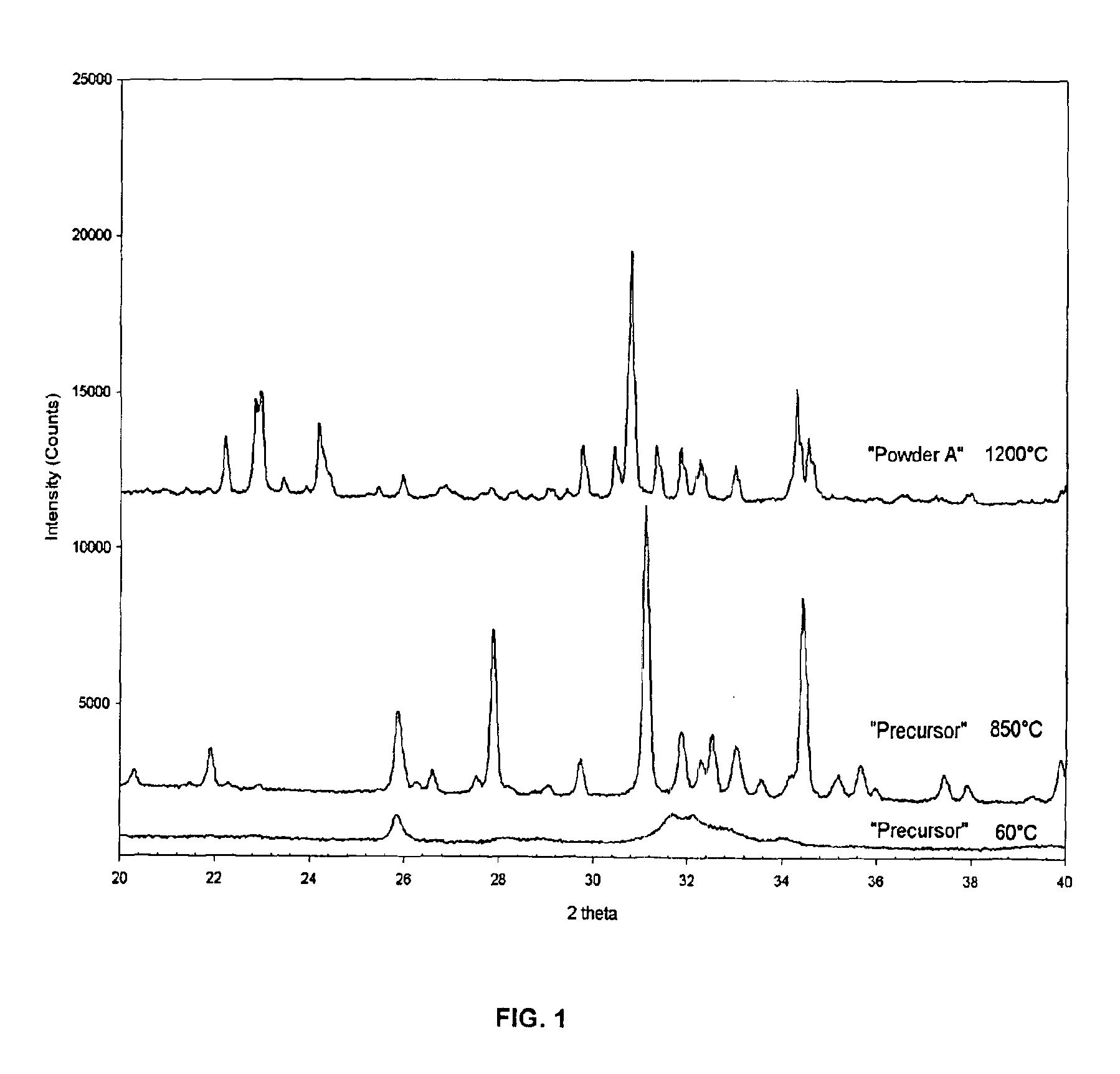
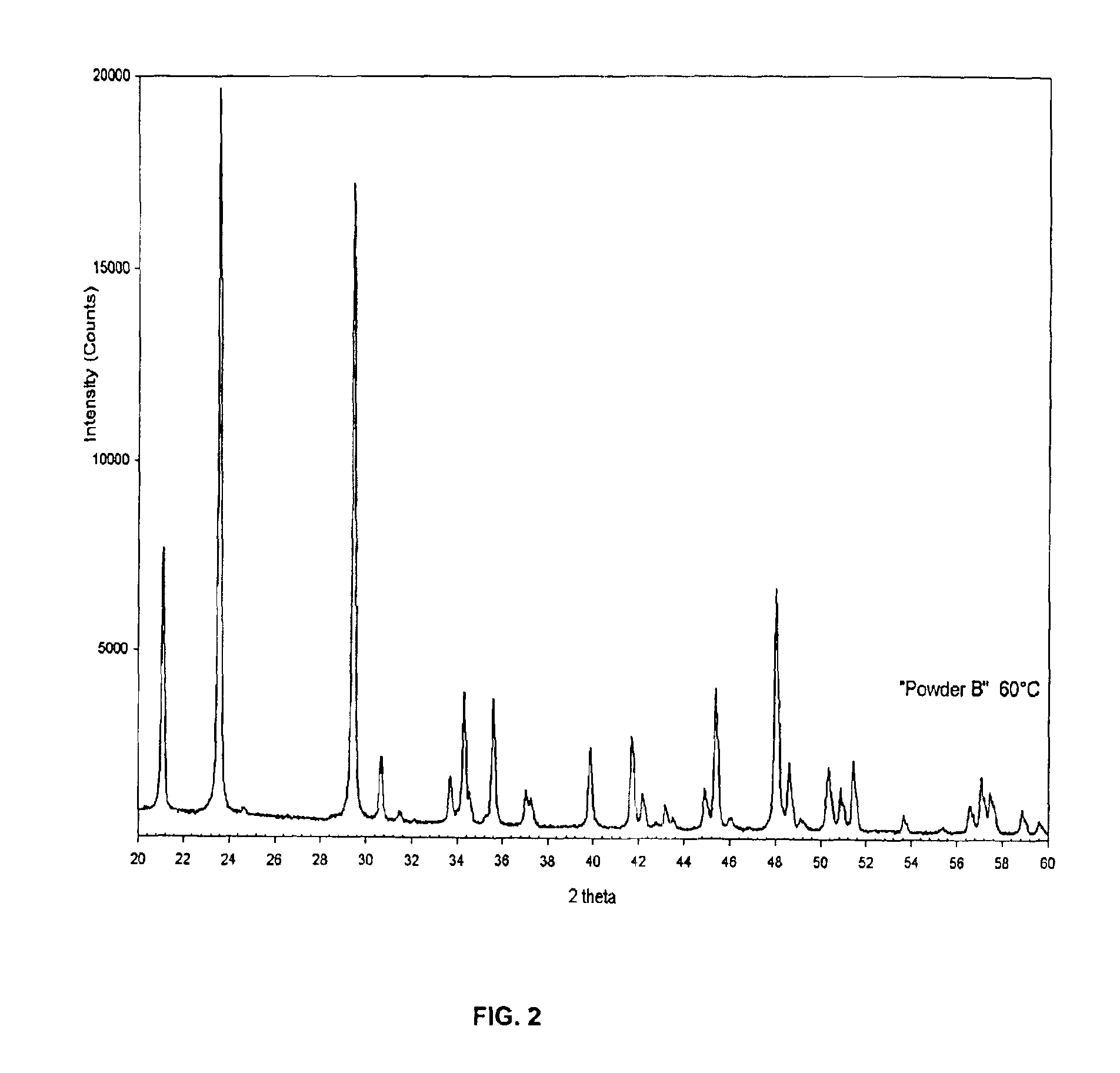
Patents
Literature
5339 results about "Calcium biphosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Calcium phosphate is a chemical compound. Its chemical formula is Ca3(PO4)2, although there are other formulas, like Ca2HPO4 (dicalcium phosphate) and Ca(H2PO4)2 (monocalcium phosphate). It is made of calcium and phosphate ions.
Methods and devices for the preparation, storage and administration of calcium phosphate cements
InactiveUS6149655AStable reductionPromote resultsLiquid surface applicatorsShaking/oscillating/vibrating mixersCalcium biphosphatePhosphoric acid
A system is provided for the storage, preparation and administration of calcium phosphate cements. The subject invention provides a storage means for storing a two component calcium phosphate cement having a liquid component and a dry component. Also provided is a preparation means for combining the two components of the cement while present in the storage means. The subject invention further provides a means for administering the prepared cement to a physiological site. The subject devices and methods find use in a variety of applications where the introduction of a flowable material capable of setting to a solid calcium phosphate mineral to a physiological site is desired, including dental and orthopedic applications.
Owner:NORIAN CORP
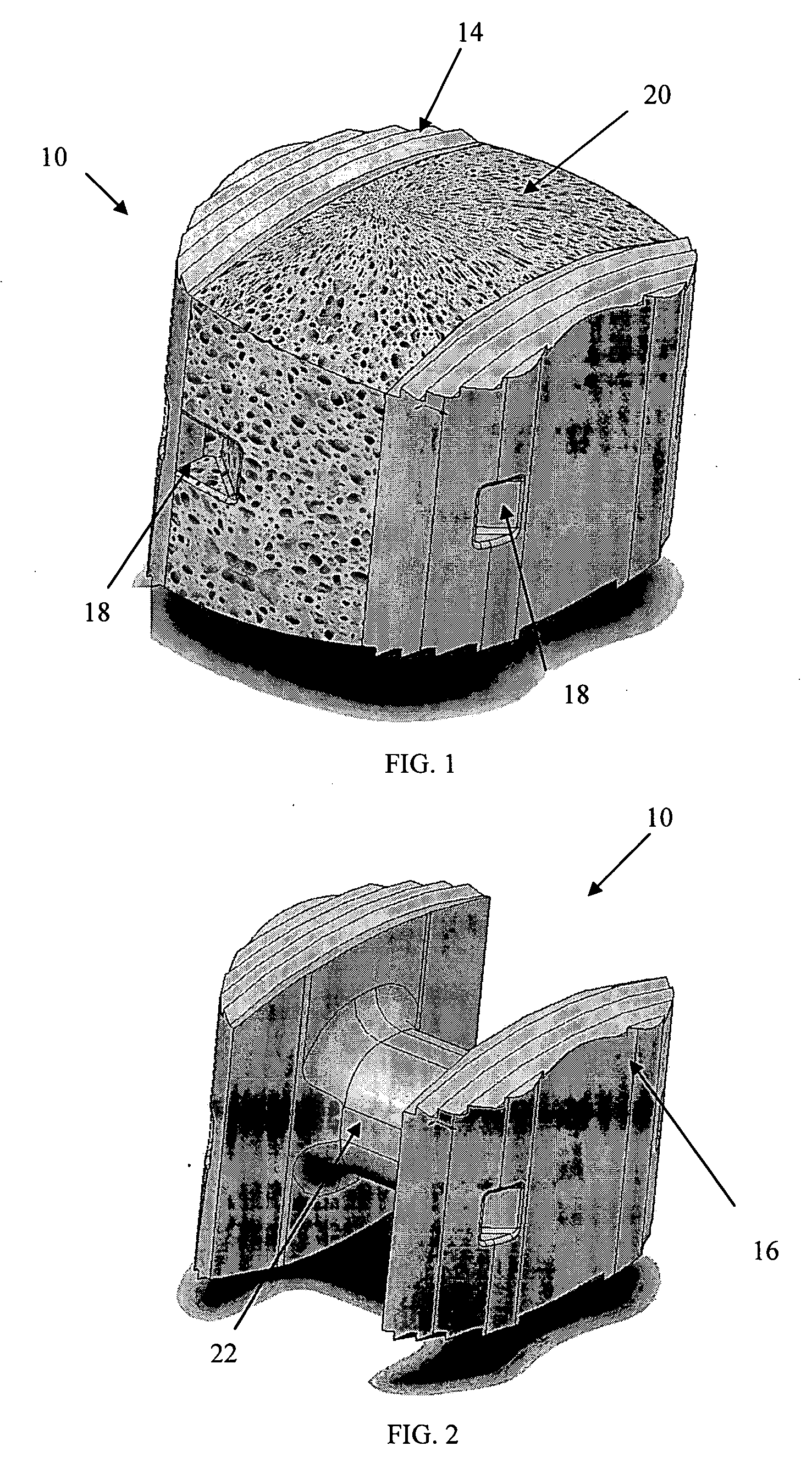
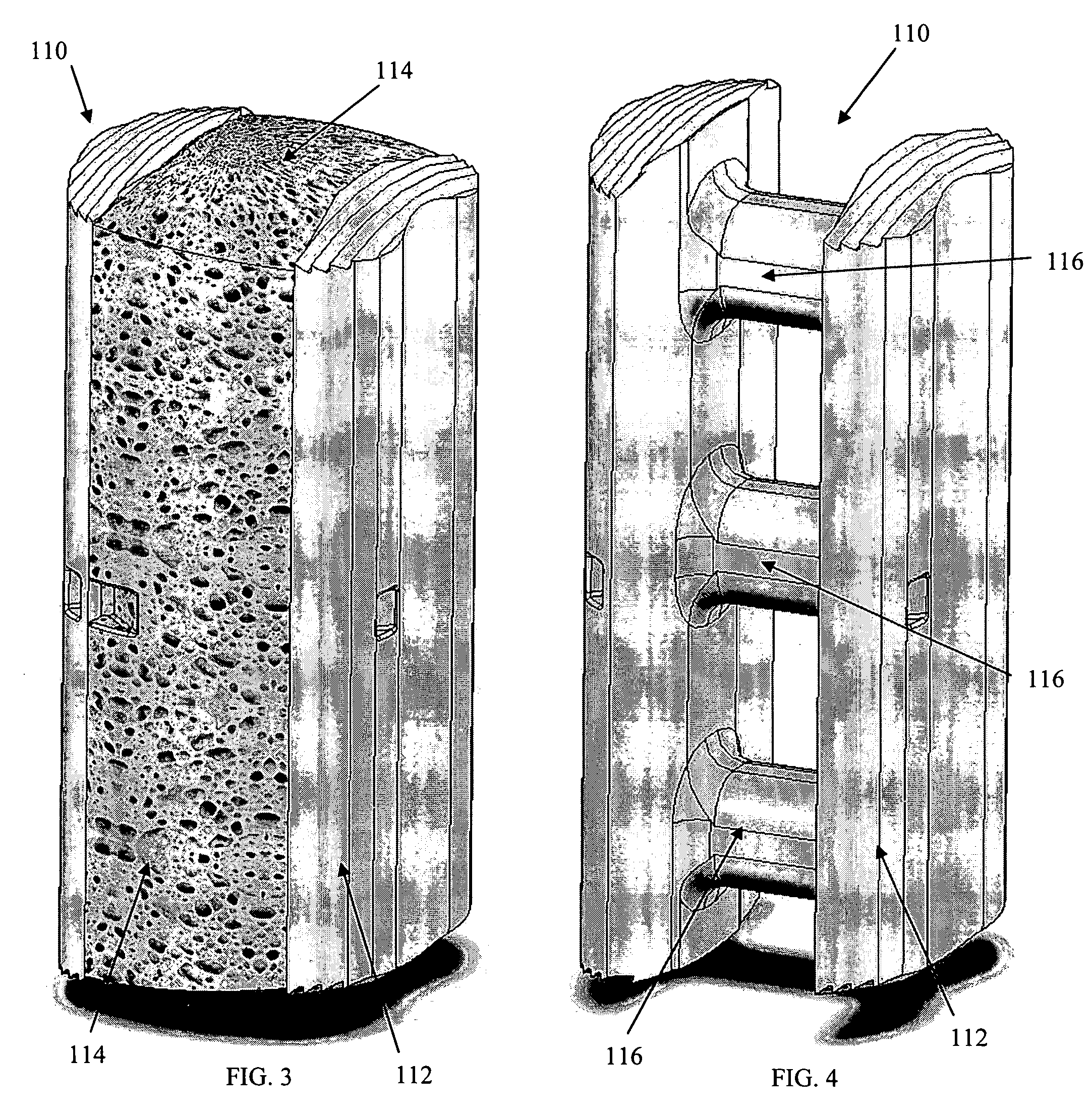
Radiolucent bone graft
An improved bone graft is provided for human implantation, bone graft includes a substrate block of high strength biocompatible material having a selected size and shape to fit the anatomical space, and a controlled porosity analogous to natural bone. The substrate block may be coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone ingrowth and enhanced bone fusion. Upon implantation, the bone graft provides a spacer element having a desired combination of mechanical strength together with osteoconductivity and osteoinductivity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The bone graft may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:AMEDICA A DELAWARE
Composite shaped bodies and methods for their production and use
InactiveUS6863899B2Overcome the lack of robustnessNovel featuresCosmetic preparationsDental implantsCalcium biphosphatePlastic surgery
Shaped, composite bodies are provided. One portion of the shaped bodies comprises an RPR-derived porous inorganic material, preferably a calcium phosphate. Another portion of the composite bodies is a different solid material, preferably metal, glass, ceramic or polymeric. The shaped bodies are especially suitable for orthopaedic and other surgical use.
Owner:ORTHOVITA INC
Biofunctional hydroxyapatite coatings and microspheres for in-situ drug encapsulation
InactiveUS20020155144A1Improve relationshipImprove interface strengthPowder deliveryOrganic active ingredientsGene deliverySide effect
Owner:THE UNIV OF BRITISH COLUMBIA
Functionally graded biocompatible coating and coated implant
ActiveUS20060210494A1Degree of reductionDental implantsCosmetic preparationsCalcium biphosphateCalcium phosphate coating
The present invention provides a biocompatible coating comprising calcium phosphate that is functionally graded across the thickness of the coating. The coating, which preferably includes hydroxyapatite, is particularly useful for coating implants, such as dental or orthopedic implants. The functionally graded coating is generally crystalline near the interface with the surface of the implant, with crystallinity and crystal diameter decreasing toward the outer layer of the coating. The invention further provides methods for preparing a coated implant comprising a functionally graded calcium phosphate coating thereon.
Owner:NORTH CAROLINA STATE UNIV
Bioceramic compositions
InactiveUS6972130B1Good biocompatibilityEasy to processBiocideInorganic phosphorous active ingredientsDiseaseDelivery vehicle
The present invention provides a synthetic, poorly crystalline apatite (PCA) calcium phosphate containing a biologically active agent and / or cells (preferably tissue-forming or tissue-degrading cells). The compositions provided by the present invention are useful for a variety of in vivo and in vitro applications, including drug delivery (for example, to bony sites, the central nervous system, intramuscular sites, subcutaneous sites, interperitoneal sites, and occular sites) tissue growth (preferably bone or cartilage) osseous augmentation, and methods of diagnosing disease states by assaying tissue forming potential of cells isolated from a host. The invention also provides methods of preparing delivery vehicles, of altering delivery vehicle characteristics, and of delivering biologically active agents to a site. The invention further provides in vitro cell culture systems and cell encapsulation materials. The invention is useful for both medical and veterinary applications.
Owner:LIFE SCI ENTERPRISES
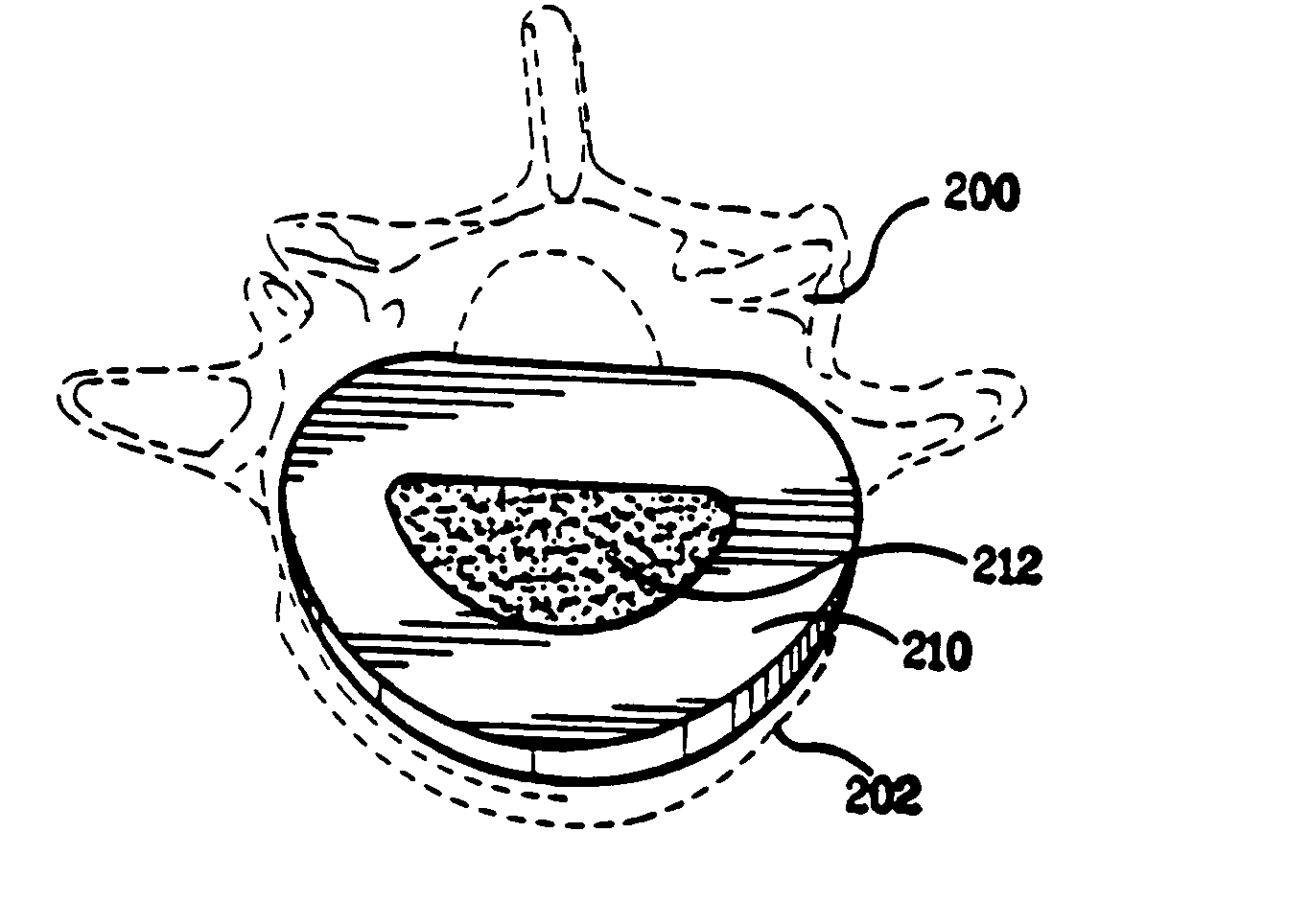
Spinal cage insert, filler piece and method of manufacturing
A spinal cage insert for a spinal cage is provided. The spinal cage insert has a shape suitable to be inserted into and fit closely in an interior of the spinal cage. The insert may comprise a member of the calcium phosphate family. The spinal cage insert may be made to a desired shape of porous ceramic, and it may include channels and / or surface features. Various shapes of filler pieces are also provided, wherein the filler pieces may be suitable to augment external regions of vertebrae which have been fused to each other so as to promote build-up of bone. The spinal cage insert and / or the filler pieces may be osteoconductive and may also contain osteoinductive substances or material. The articles may also contain cavities suitable for containing particles of demineralized bone matrix (DBM). Methods of use and methods of manufacturing the spinal cage insert and filler pieces are also provided.
Owner:THERICS
Porous calcium phosphate bone material
ActiveUS20070128245A1Improved controlled releaseExtend posting timeAntibacterial agentsPeptide/protein ingredientsCalcium biphosphateChemical composition
Porous calcium phosphate implant compositions that approximate the chemical composition of natural bone mineral are provided. In addition to calcium phosphate, the compositions include an effervescent agent to promote the formation of interconnected pores and a cohesiveness agent to maintain the shape and hardness of the hardened composition. When introduced at an implant site, the calcium phosphate compositions are remodeled into bone. Methods for using the calcium phosphate compositions, e.g., to repair or replace bone, are also provided.
Owner:ETEX
Calcium phosphate polymer composite and method
A bone-repair composite includes a core and a sheath. The core is a first primary unit including a combination of a first set of yarns coated with a calcium phosphate mineral layer. The first set of yarns being made from a first group of one ore more polymers. The sheath is a second primary unit a combination of a second set of yarns or one or more polymer coatings. The second set of yarns being made from a second group of one or more polymers, wherein the composite is made by covering the core with the sheath, and the composite is compression molded to allow the sheath to bond to the core. The bone-repair composite has a bending modulus comparable to that of a mammalian bone, such that the ratio of the core to the sheath is provided to maximize the mechanical strength of the bone-repair composite to mimic the mammalian bone.
Owner:TELEFLEX MEDICAL INC +1
Self-hardening calcium phosphate materials with high resistance to fracture, controlled strength histories and tailored macropore formation rates
InactiveUS6955716B2Strong and tough self-hardeningHigh strengthPhosphatesOther chemical processesFiberHigh resistance
A bone replacement material and therapy comprises the combination of calcium phosphate compounds and two or more soluble fillers in the form of fibers, mesh or other materials which have the dual functions of reinforcing an in vivo implant while dissolving at a programmed rate to form macropores capable of receiving natural bone ingrowth.
Owner:ADA FOUND
Radiolucent spinal fusion cage
InactiveUS20050049706A1Improve carrying capacityHigh porosityBone implantJoint implantsPorosityBone growth
An improved bone graft is provided for human implantation, particularly such as a spinal fusion cage for implantation into the inter-vertebral space between two adjacent vertebrae. The improved spinal fusion cage includes a substrate block of high strength biocompatible material having a selected size and shape to fit the anatomical space, and a controlled porosity analogous to natural bone. The substrate block may be coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone in growth and enhanced bone fusion. Upon implantation, the fusion cage provides a spacer element having a desired combination of mechanical strength together with osteoconductivity and osteoinductivity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The fusion cage may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:AMEDICA A DELAWARE
Methods for treating dental conditions using tissue scaffolds
The invention provides methods, apparatus and kits for regenerating dental tissue in vivo that are useful for treating a variety of dental conditions, exemplified by treatment of caries. The invention uses tissue scaffold wafers, preferably made of PGA, PLLA, PDLLA or PLGA dimensioned to fit into a hole of corresponding sized drilled into the tooth of subject to expose dental pulp in vivo. In certain embodiments the tissue scaffold wafer further comprises calcium phosphate and fluoride. The tissue scaffold wafer may be secured into the hole with a hydrogel, a cement or other suitable material. Either the wafer or the hydrogel or both contain a morphogenic agent, such as a member encoded by the TGF-β supergene family, that promotes regeneration and differentiation of healthy dental tissue in vivo, which in turn leads to remineralization of dentin and enamel. The tissue scaffold may further include an antibiotic or anti-inflammatory agent.
Owner:IVOCLAR VIVADENT INC
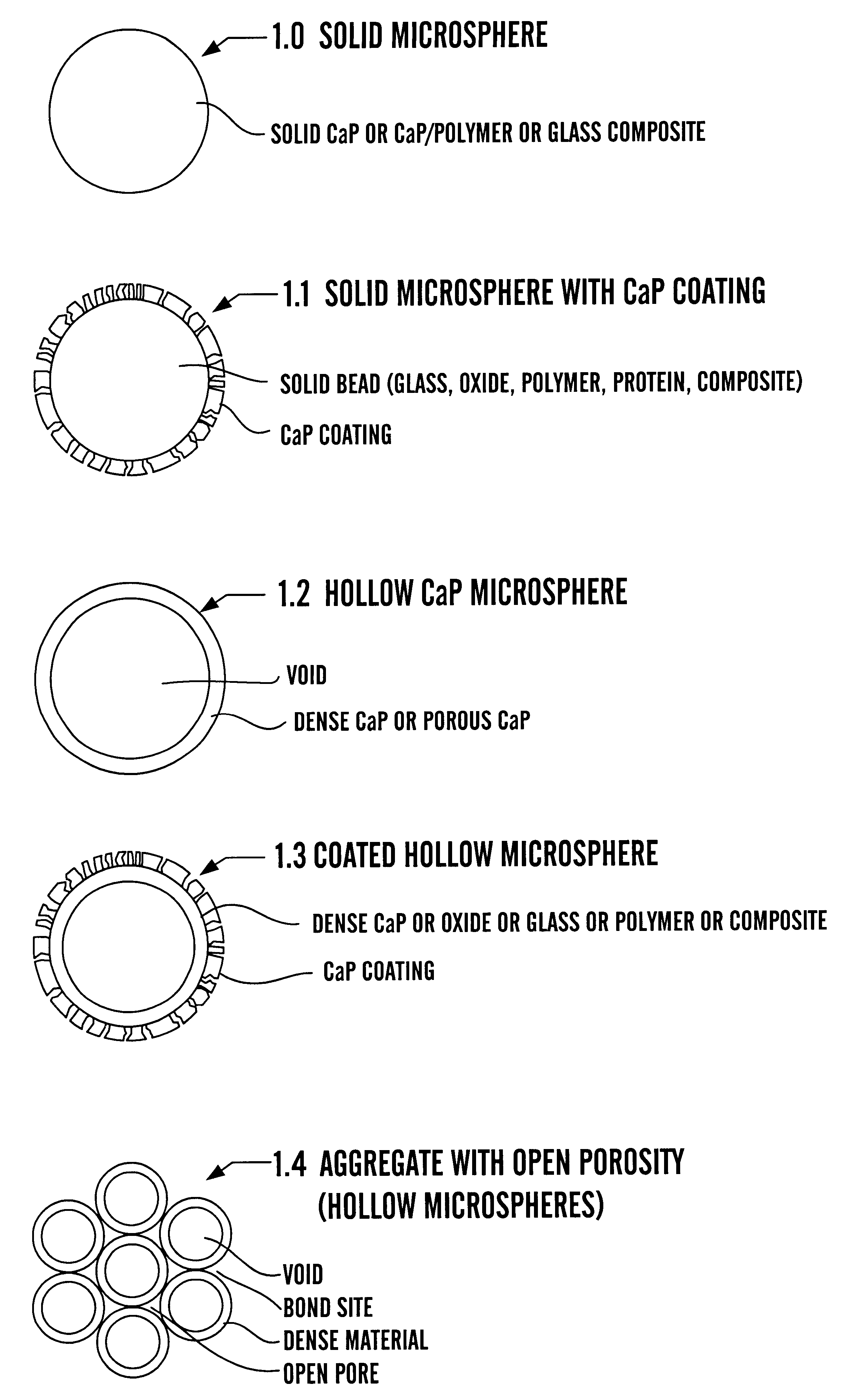
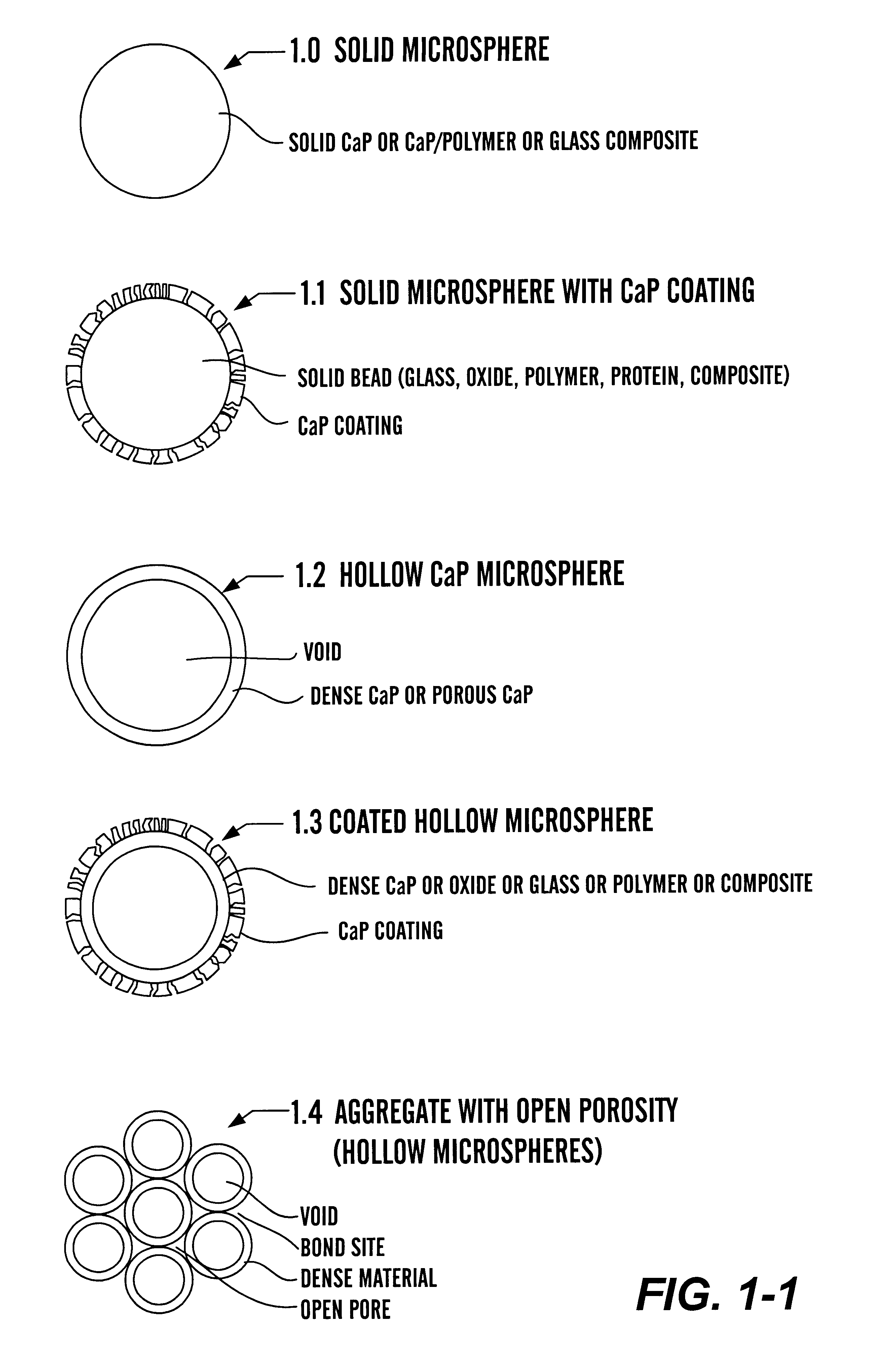
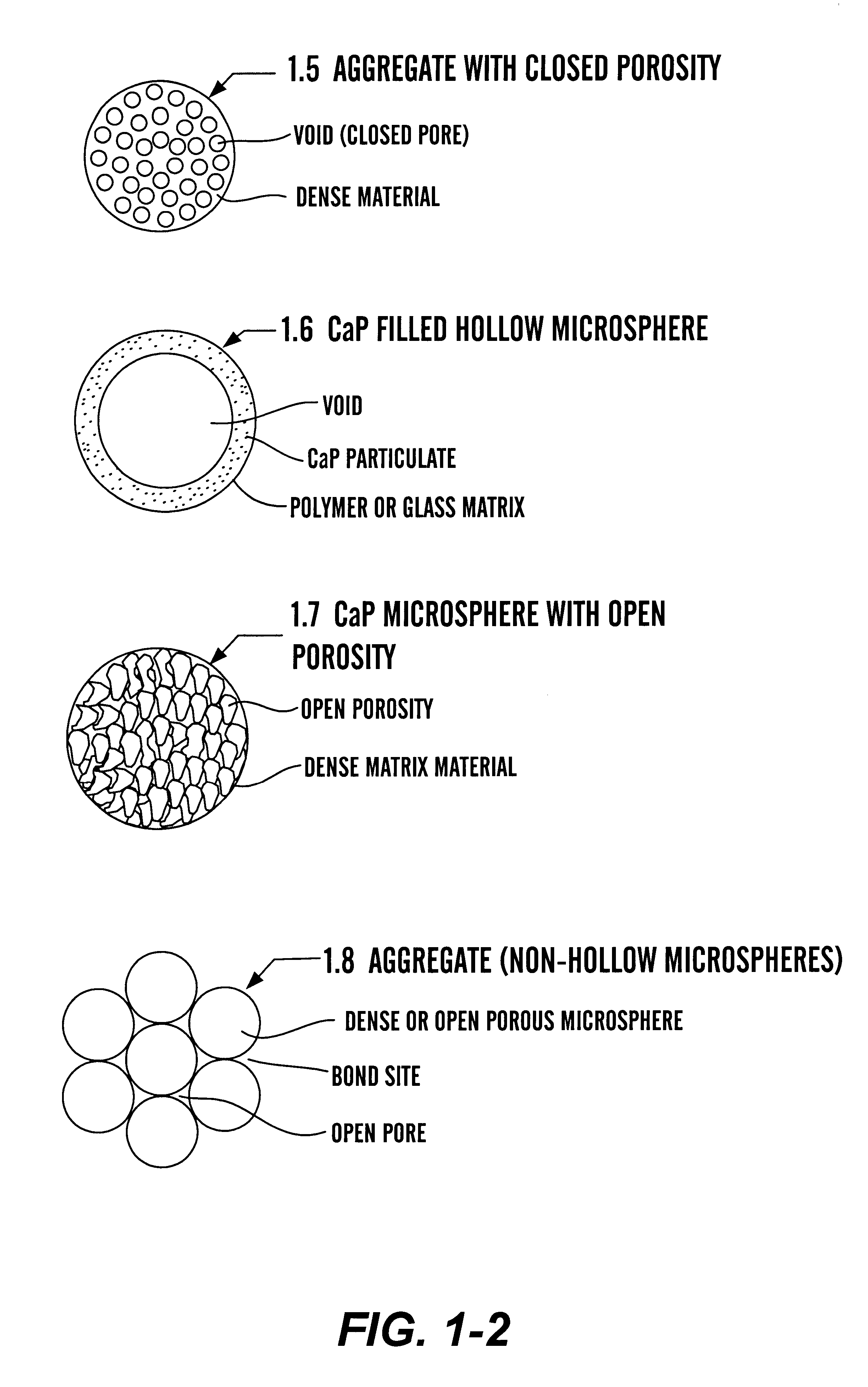
Calcium phosphate microcarriers and microspheres
The present invention provides calcium phosphate-based (CaP) microcarriers and their use, for example, in cell culturing systems, chromatography, and implantable biomedical materials.
Owner:CAP BIOTECH
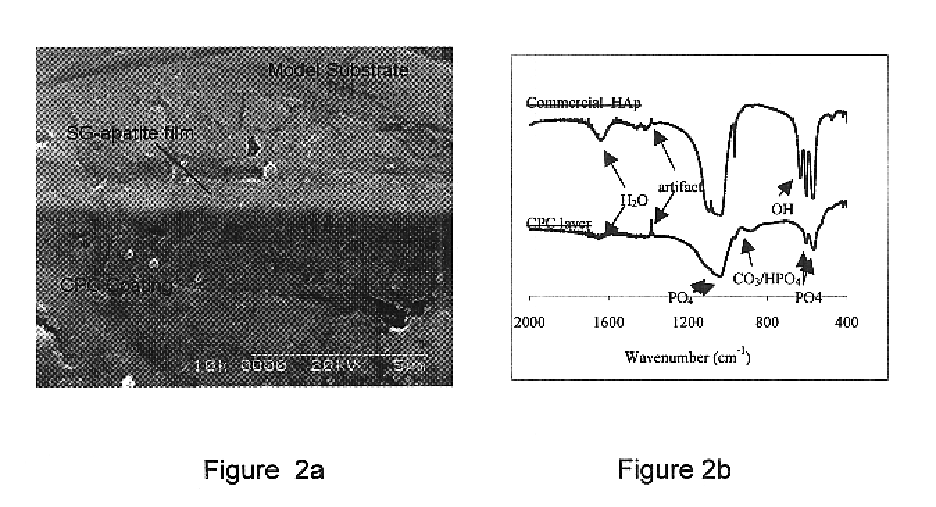
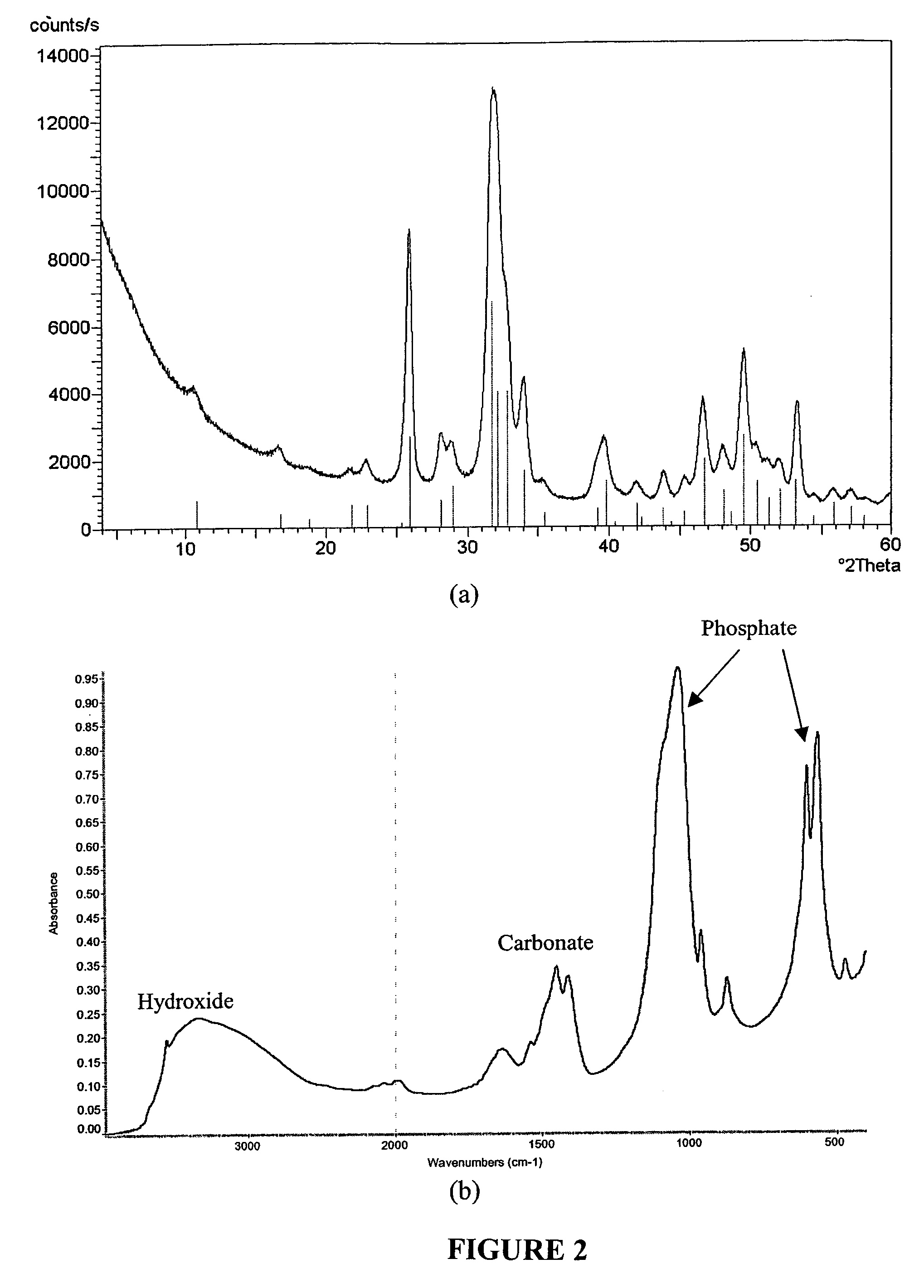
Biofunctional hydroxyapatite coatings and microspheres for in-situ drug encapsulation
InactiveUS6730324B2High strengthEasy to makePowder deliveryOrganic active ingredientsGene deliverySide effect
This invention relates to novel room-temperature process for obtaining calcium phosphate, in particular hydroxyapatite, coatings and microspheres that encapsulate drugs, proteins, genes, DNA for therapeutical use. The coatings and microspheres are designed to perform a defined biological function related to drug delivery, such as gene therapy through gene delivery. A novel method for encapsulation, and subsequent controlled release of therapeutically active agents from such biofunctional coatings and microspheres is disclosed. Such coatings and microspheres are useful for side-effects free, long-term, targeted, controlled release and delivery of drugs, proteins, DNA, and other therapeutic agents.
Owner:THE UNIV OF BRITISH COLUMBIA
Suspension of calcium phosphate particulates for local delivery of therapeutic agents
InactiveUS20060257358A1Reduce adverse effectsAvoid the needBiocidePeptide/protein ingredientsCalcium biphosphateParticulates
Disclosed herein are methods for preparing and using porous, crystalline biomimetic bioactive compositions of calcium phosphate with at least one therapeutic agent. The bioactive composition has strong adsorption properties for therapeutic agents which adsorb to the calcium phosphate with a high affinity. The bioactive composition also provides a sustained release implant that can be used for localized delivery of therapeutic agents. This localized delivery of therapeutic agents, promotes repair, healing, or regeneration of hard and soft tissues.
Owner:DEPUY PROD INC
Osteoconductive spinal fixation system
InactiveUS20060276788A1High strengthHigh mechanical strengthSuture equipmentsInternal osteosythesisPorositySpinal column
An improved spinal fixation system is provided for human implantation, including a set of screws with interconnecting rods for implantation into the pedicle and between two adjacent vertebrae or a plate with screws for fixating two adjacent vertebrae. The screws, rods, and plates include a substrate portion of high strength biocompatible material and a controlled porosity analogous to natural bone. The substrate portion may be coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone ingrowth and enhanced bone fusion. Upon implantation, the fixation system provides a desired combination of mechanical strength together with osteoconductivity and bio-activity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The fixation system may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:AMEDICA A DELAWARE
Calcium phosphate cement composition and a method for the preparation thereof
ActiveUS6929692B2Good water solubilityIncreased formationOther chemical processesBone implantChemical synthesisCalcium biphosphate
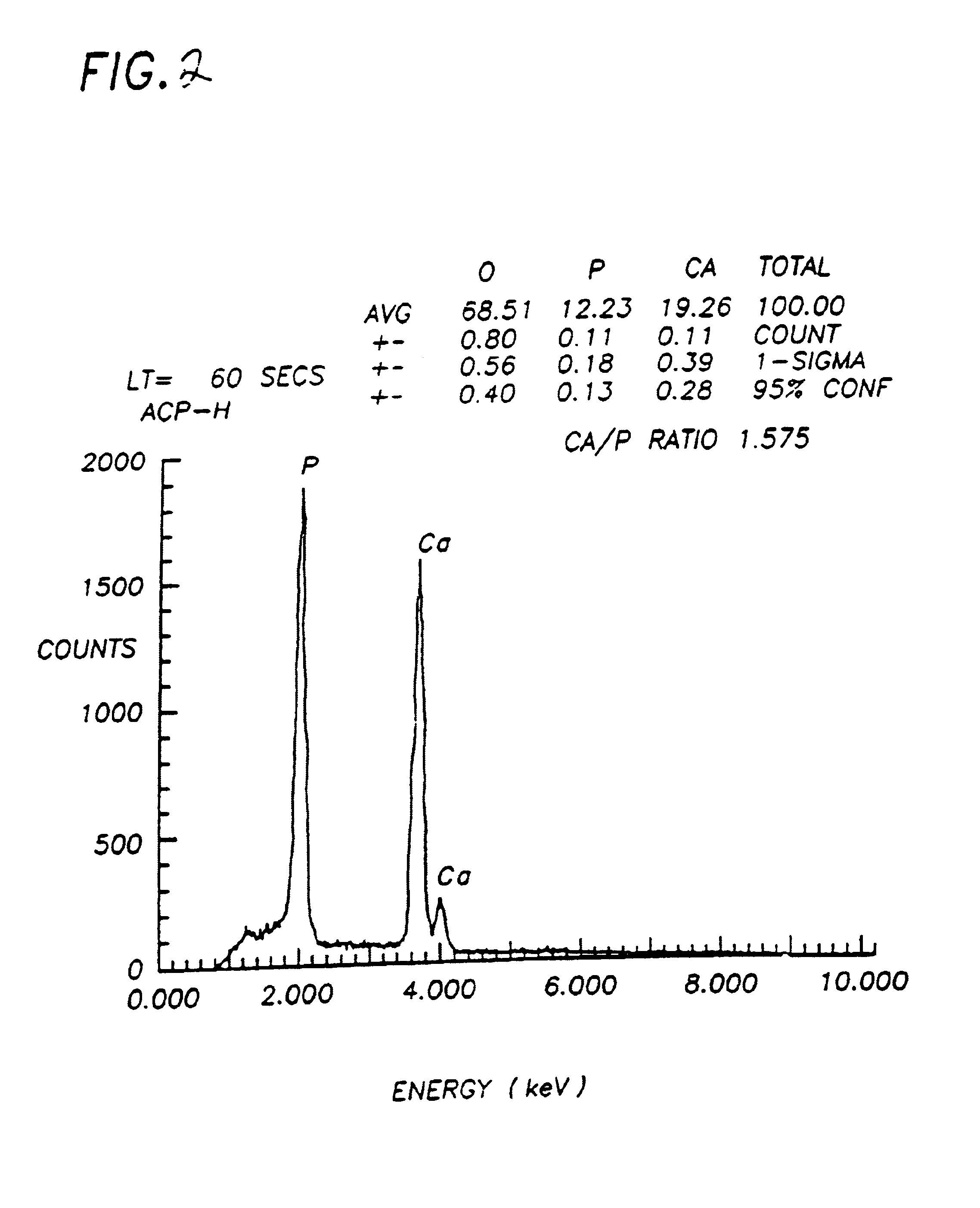
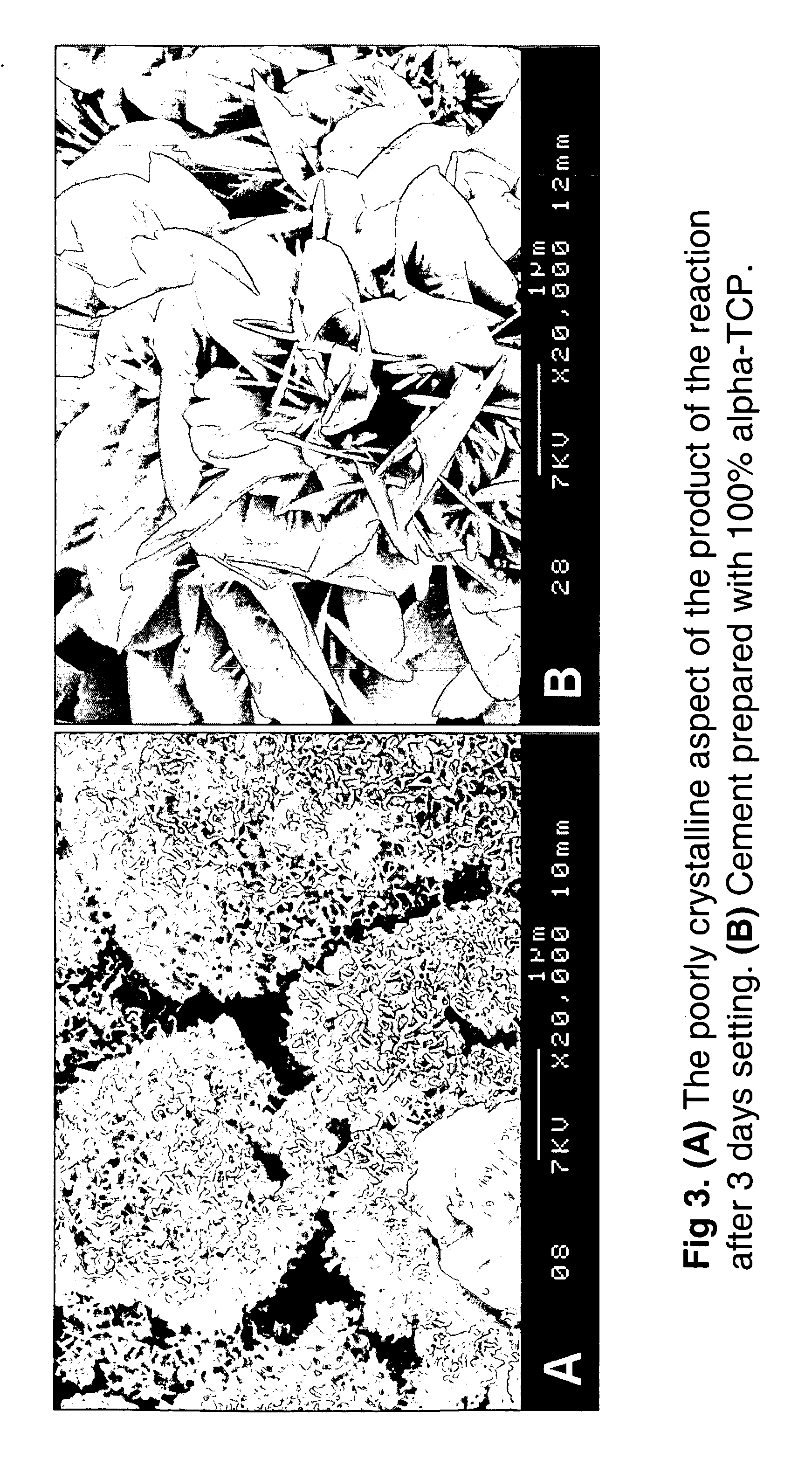
The invention describes a new calcium phosphate cement powder, whose composition can best be described over the Ca / P molar ratio range of 1.35 to 1.40, most preferably 1.39, and whose two components were prepared by wet chemical synthesis procedures. One component is chemically-synthesized, bi-phasic alpha-TCP (Ca3(PO4)2, 95 wt %)+HA (Ca10(PO4)6(OH)2, 5 wt %) powder, while the second component is again a chemically-synthesized, single-phase DCPD (CaHPO4·2H2O) powder. A setting solution (Na2HPO4·2H2O) is used to form a self-setting calcium phosphate cement from the powder mixture. This cement can be used as bone filler or bone substitute in applications, which require higher rates of resorption.
Owner:DR AHMET CUNEYT TAS
Composite shaped bodies and methods for their production and use
InactiveUS20050042288A1Increase productionLow process temperatureDental implantsPowder deliveryCalcium biphosphatePlastic surgery
Shaped, composite bodies are provided. One portion of the shaped bodies comprises an RPR-derived porous inorganic material, preferably a calcium phosphate. Another portion of the composite bodies is a different solid material, preferably metal, glass, ceramic or polymeric. The shaped bodies are especially suitable for orthopaedic and other surgical use.
Owner:ORTHOVITA INC
Deposition of calcium-phosphate (CaP) and calcium-phosphate with bone morphogenic protein (CaP+BMP) coatings on metallic and polymeric surfaces
InactiveUS20080097618A1Reduce the amount of solutionHigh densityImpression capsBone implantPolymeric surfaceSolubility
The invention is a medical implantable device which is coated by the method according to the invention. The surface of the substrate used for the implantable device, in the raw condition, following a cleaning regime and physiochemical pretreatments, is coated using a biomimetic process in a supersaturated calcium phosphate solution (SCPS) to obtain the desired coating coverage and morphology maintaining a ratio of calcium to phosphorus pH, as well as solution temperature plays a major role in yielding precipitation of the proper phase of CaP so that composition, morphologies, crystal structures, and solubility characteristics are optimal for the deposition process. The biomimetic coating adds the attribute of osteoconductivity to the implant device. To maximize bone growth, the implant must also induce bone growth, or possess the attribute of osteoinductivity. This attribute is acquired by the use of therapeutic agents, i.e. bone morphogenic proteins (BMP), growth factors, stem cells, etc. The preparation of the SCPS solution is slightly altered so that during the immersion of the implant in the SCPS, the therapeutic agents are co-precipitated and bonded with the CaP directly on the underlying surface of the implant device. A final dipping process into a BMP solution provides an initial burst of cellular activity. For delivering stem and / or progenitor cell, after drying the dipped solution of BMP, the cells are cultured on the surface of the implant.
Owner:HERKOWITZ HARRY N
Calcium phosphate coated implantable medical devices and processes for making same
InactiveUS20060134160A1Reduce porosityControlled release rateBiocideInorganic phosphorous active ingredientsCalcium phosphate coatingCardiovascular stent
This invention relates to novel calcium phosphate-coated implantable medical devices and processes of making same. The calcium-phosphate coatings are designed to minimize the immune response to the implant (e.g. restenosis in stenting procedures) and can be used to store and release a medicinally active agent in a controlled manner. Such coatings can be applied to any implantable medical devices and are useful for a number of medical procedures including (but not limited to) balloon angioplasty in cardiovascular stenting, ureteral stenting and catheterisation. The calcium phosphate coatings can be applied to a substrate as one or more coatings by a sol-gel deposition process, an aerosol-gel deposition process, a biomimetic deposition process, a calcium phosphate cement deposition process, an electro-phoretic deposition process or an electrochemical deposition process. The coating can contain and elude a drug in an engineered manner.
Owner:THE UNIV OF BRITISH COLUMBIA
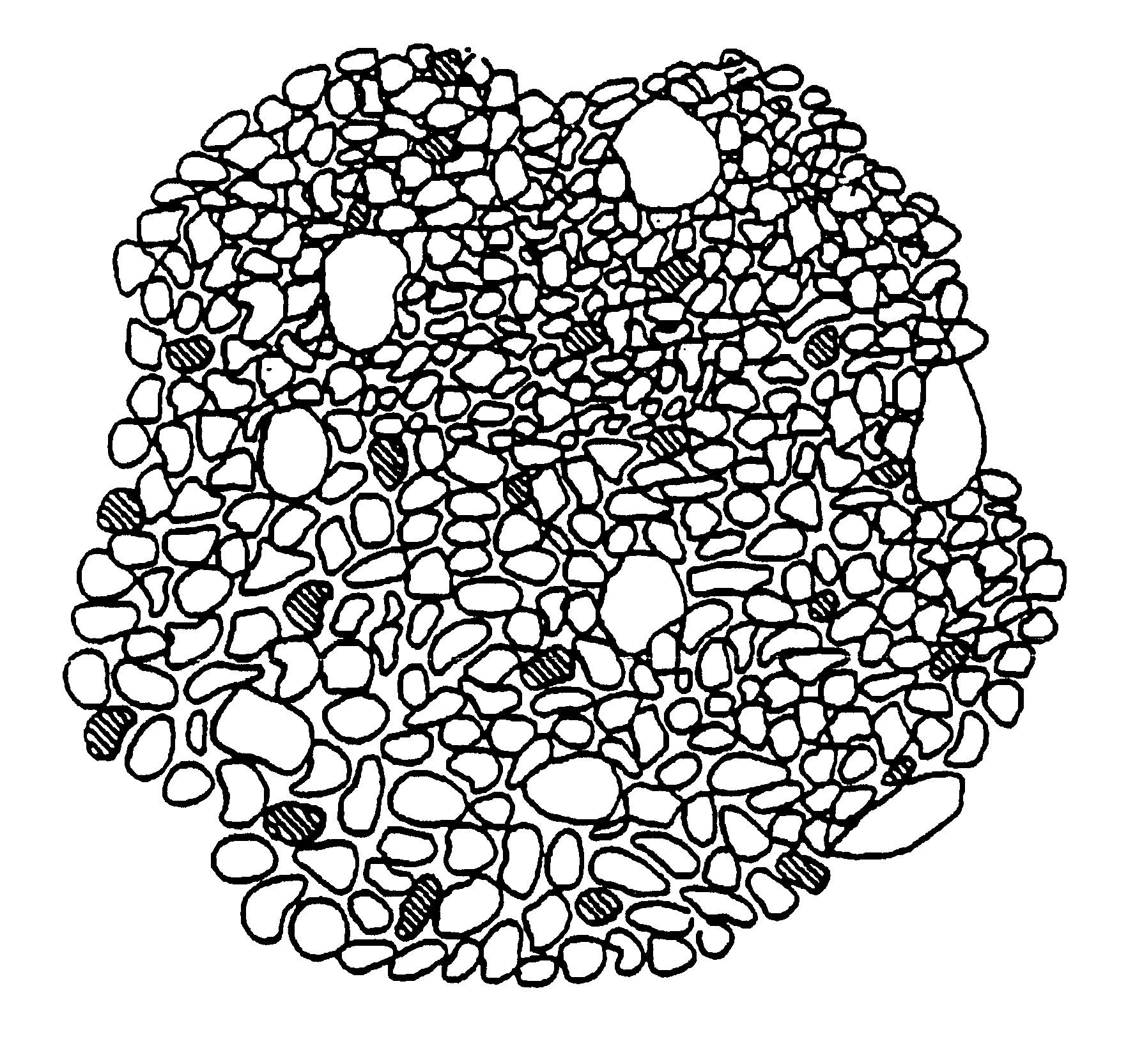
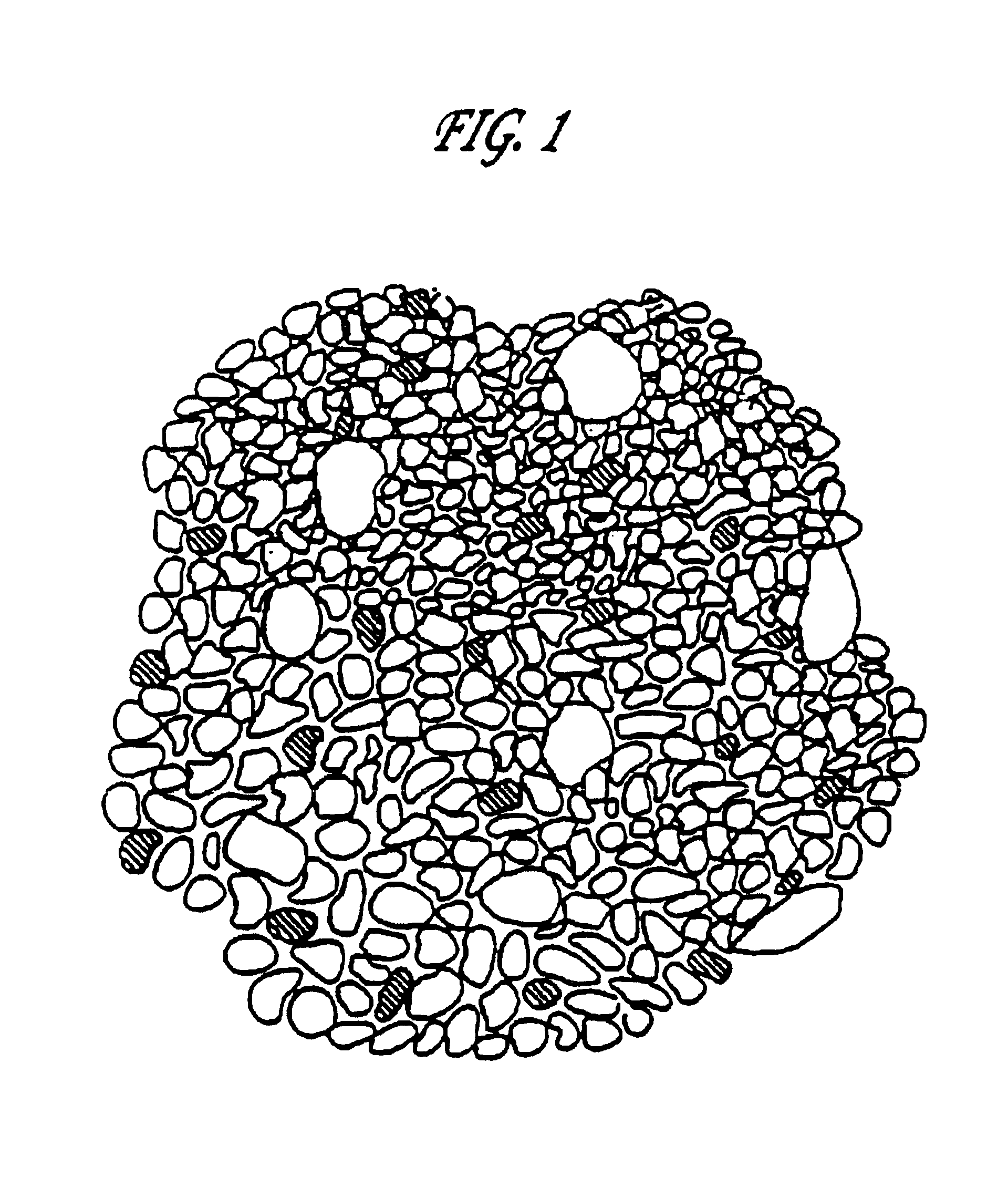
Biomaterial
InactiveUS20020022885A1Cell infiltration into an inner surface is very fastBone implantCalcium biphosphatePore diameter
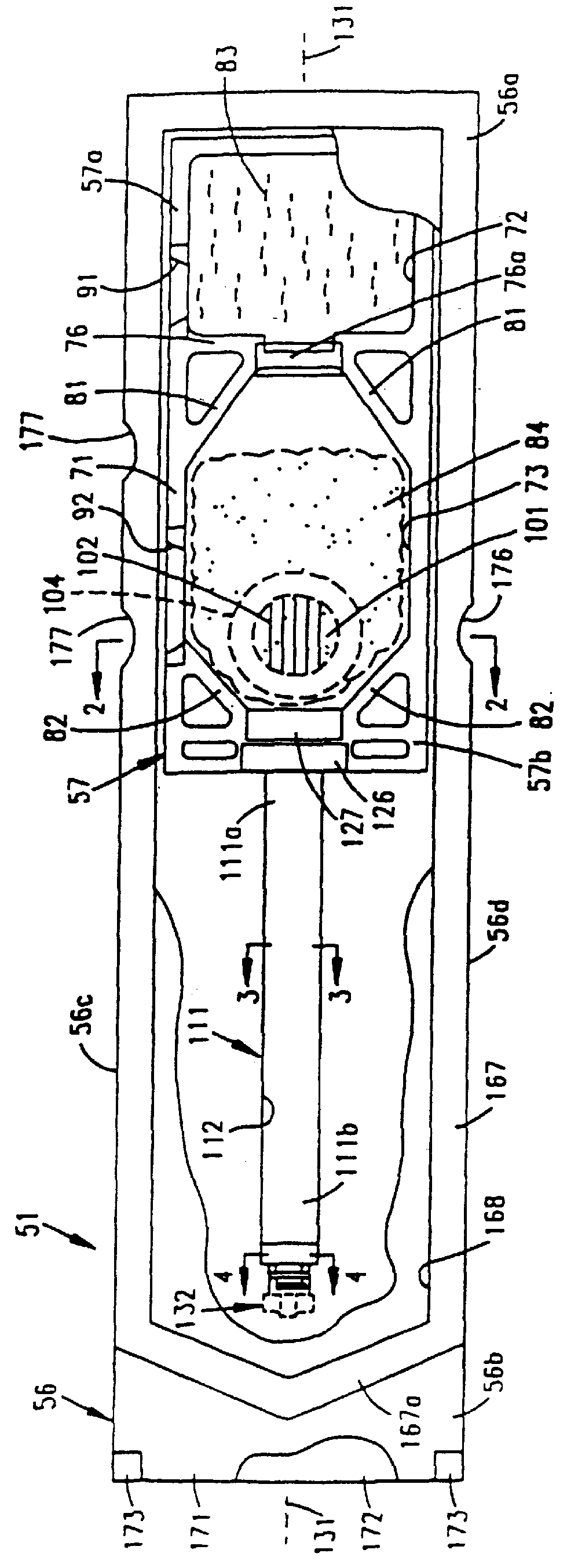
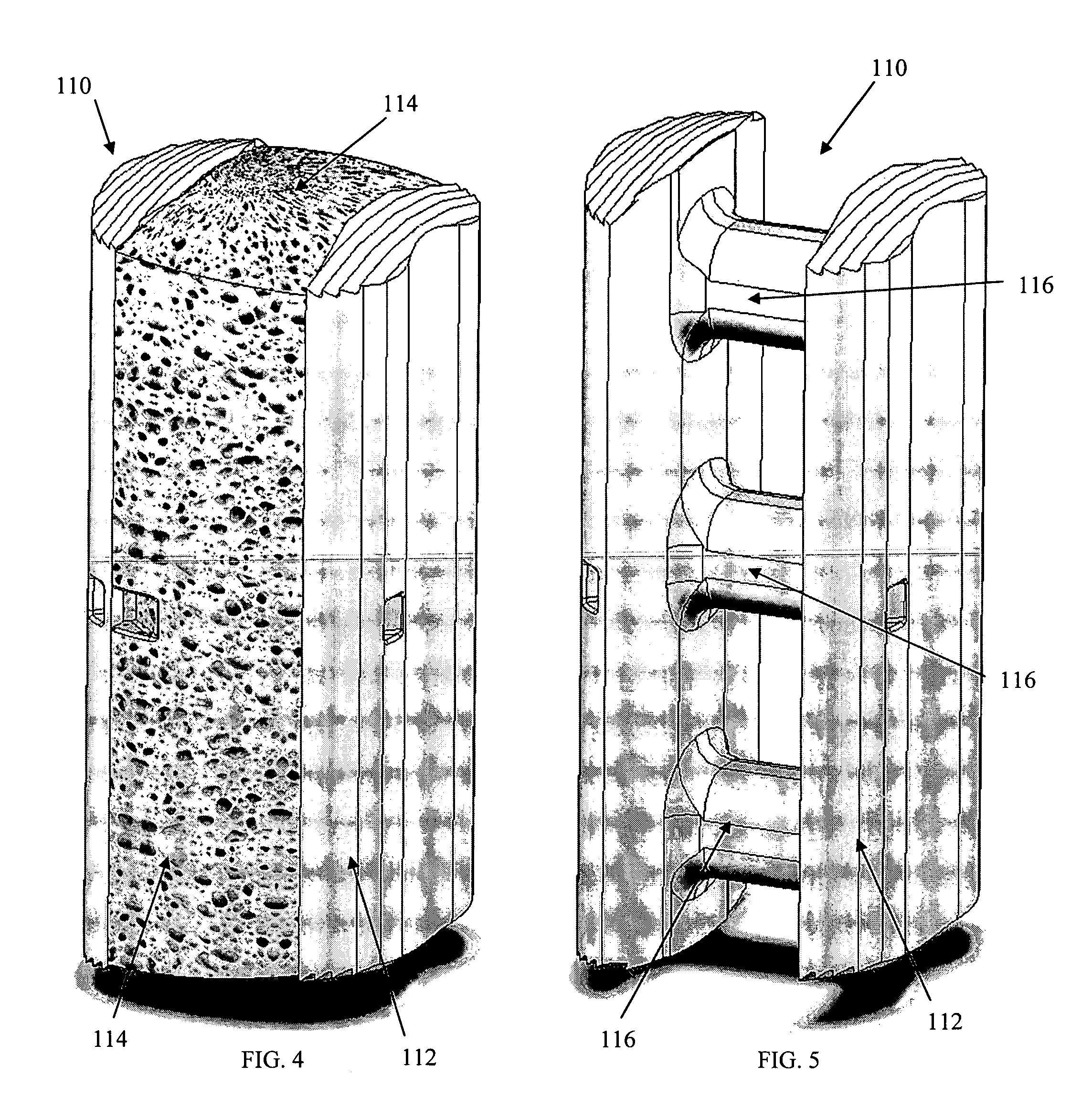
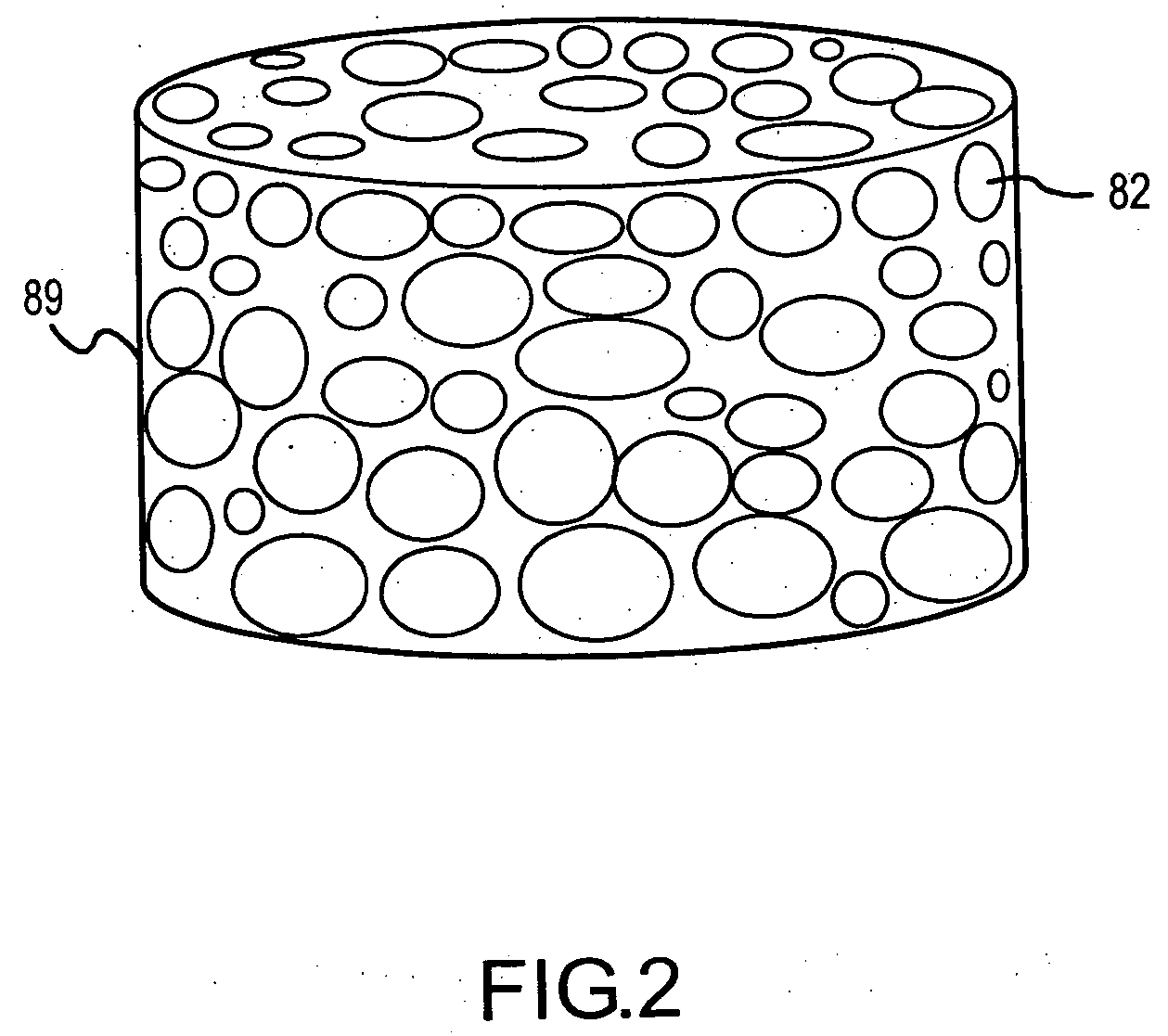
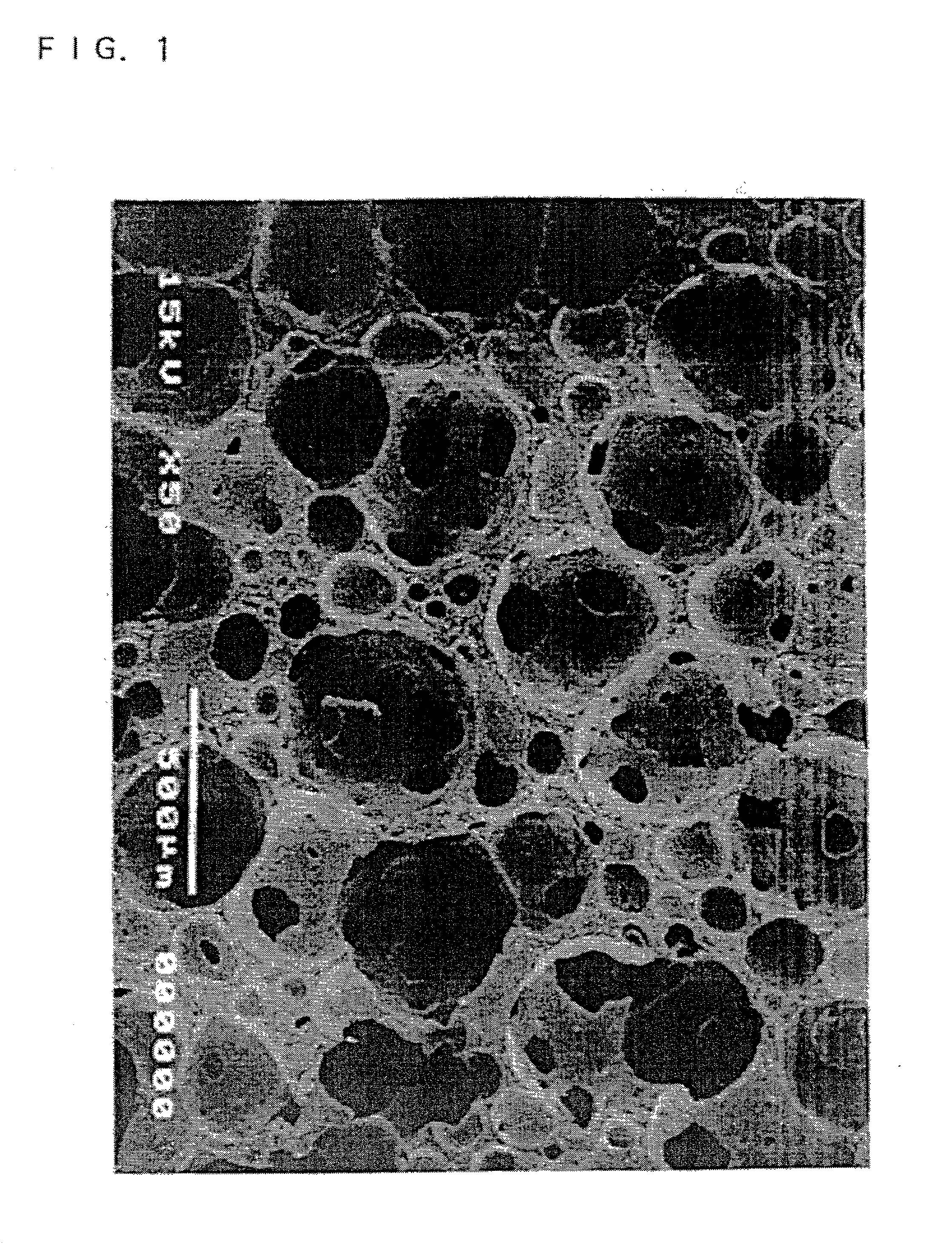
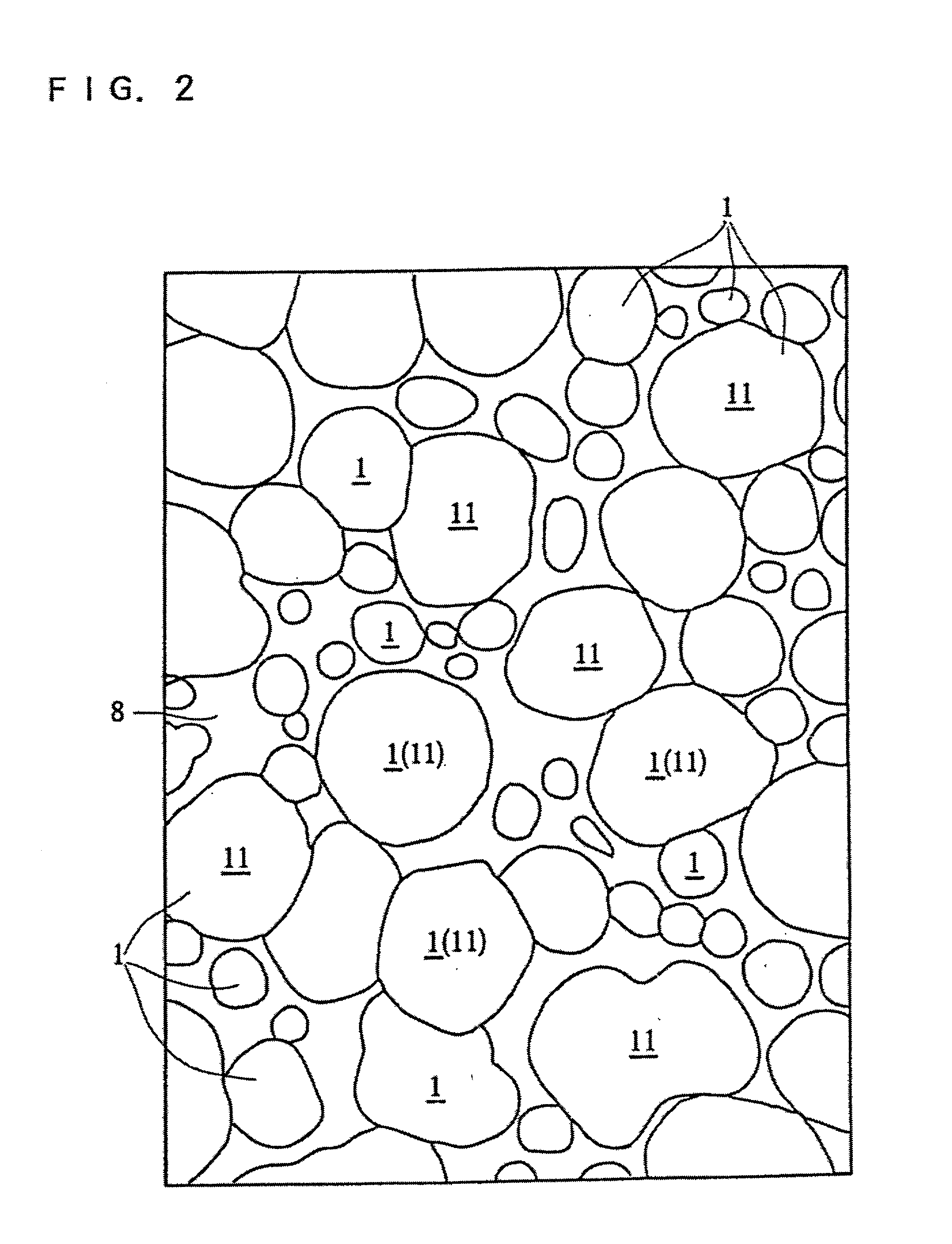
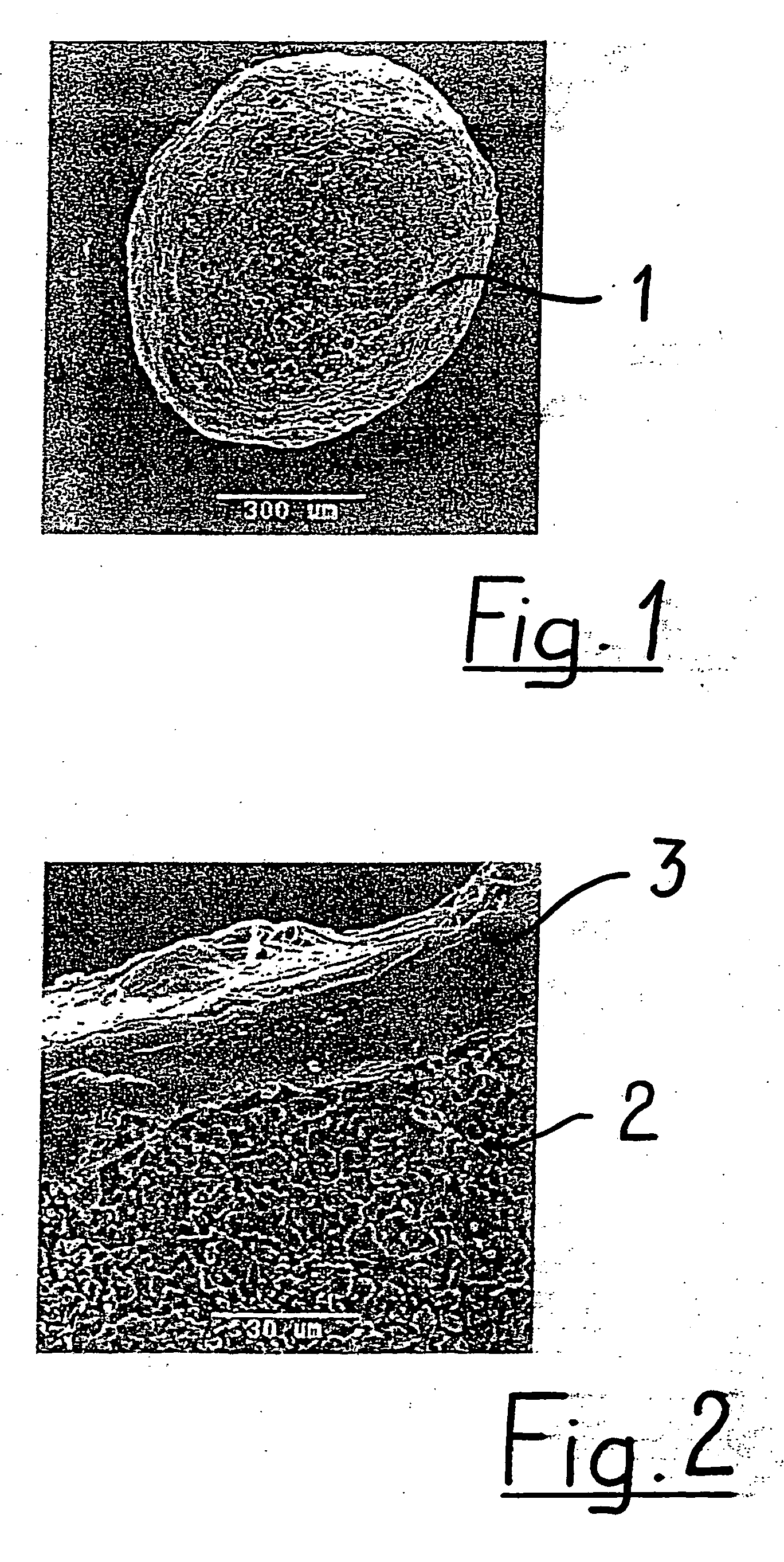
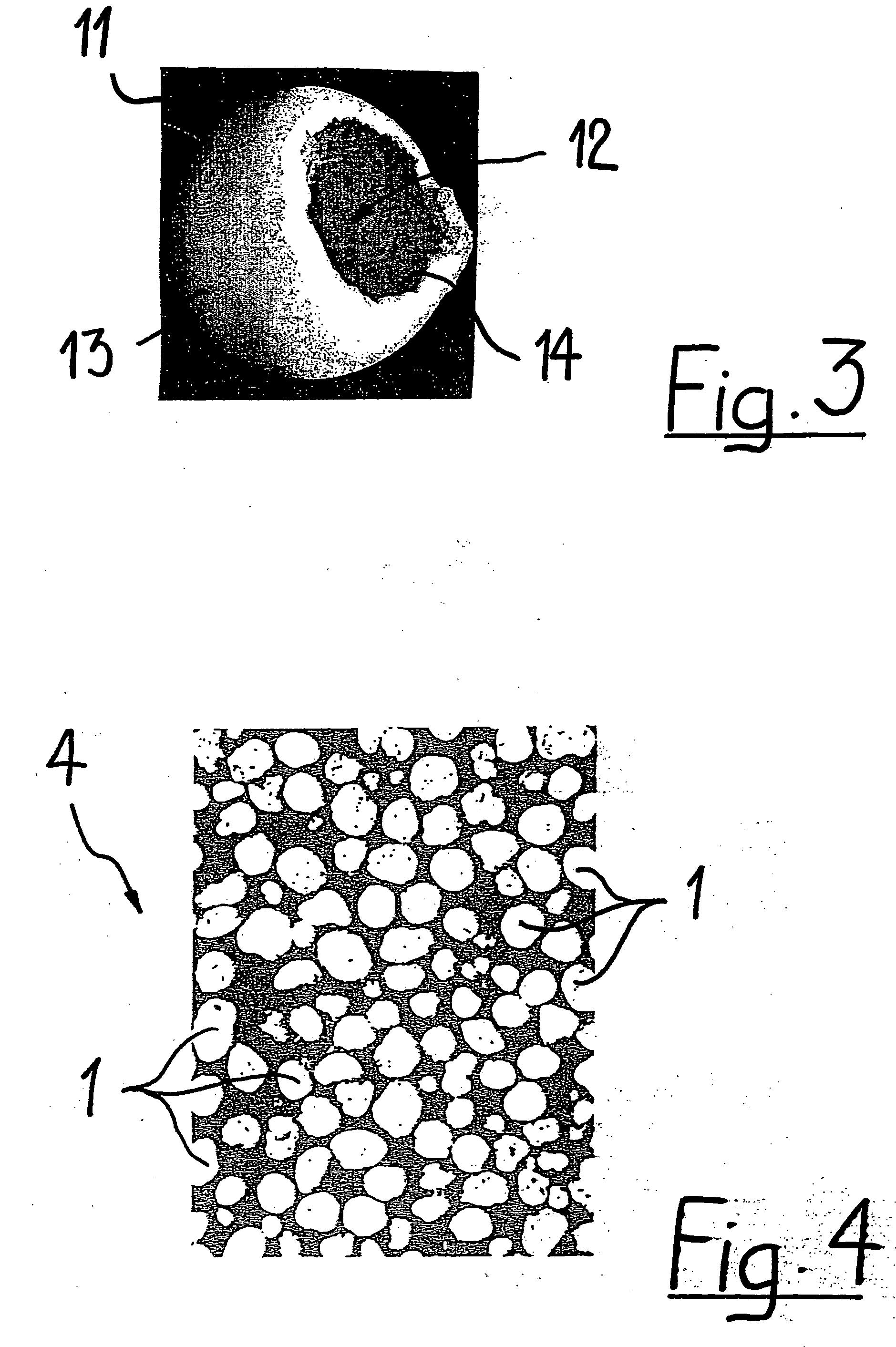
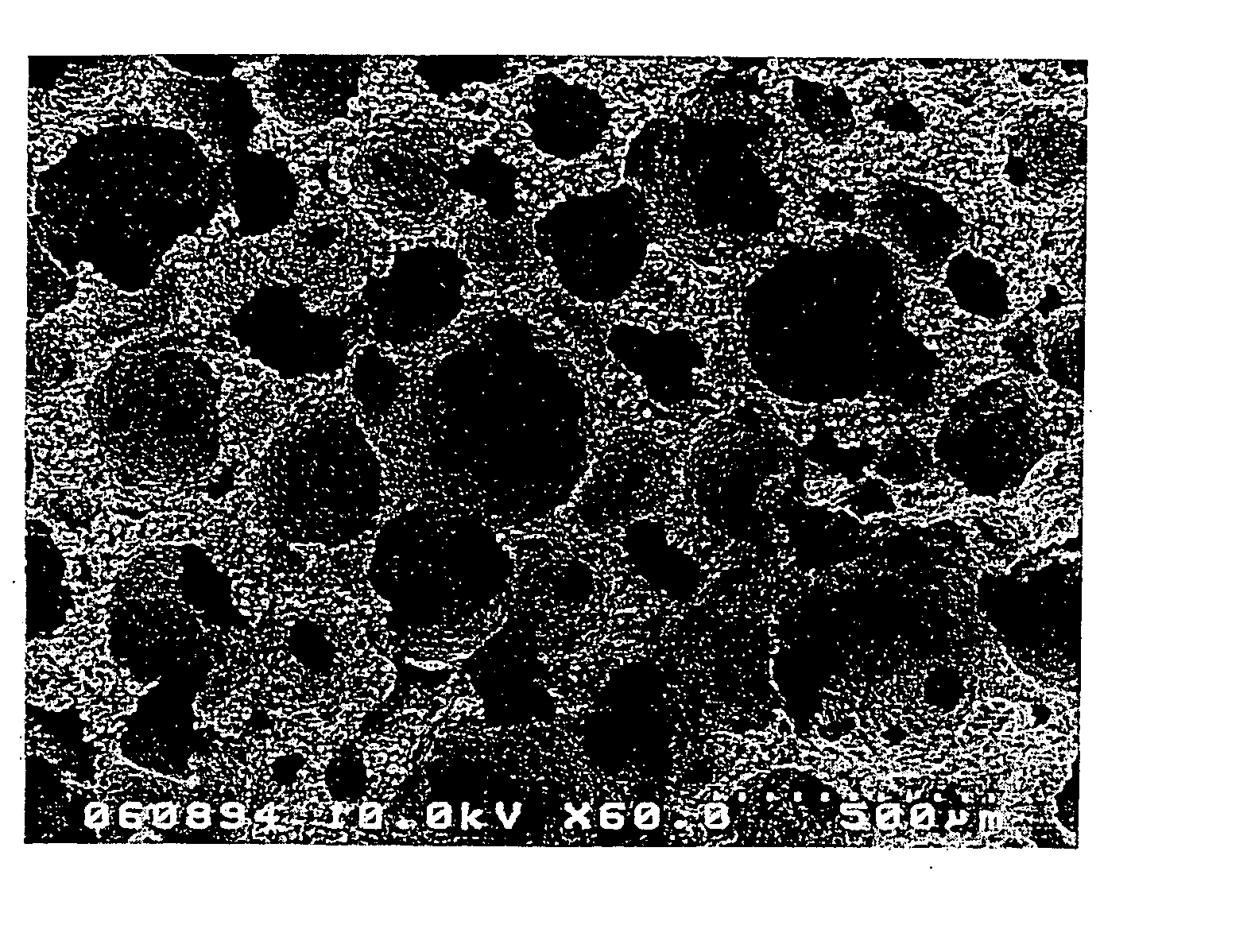
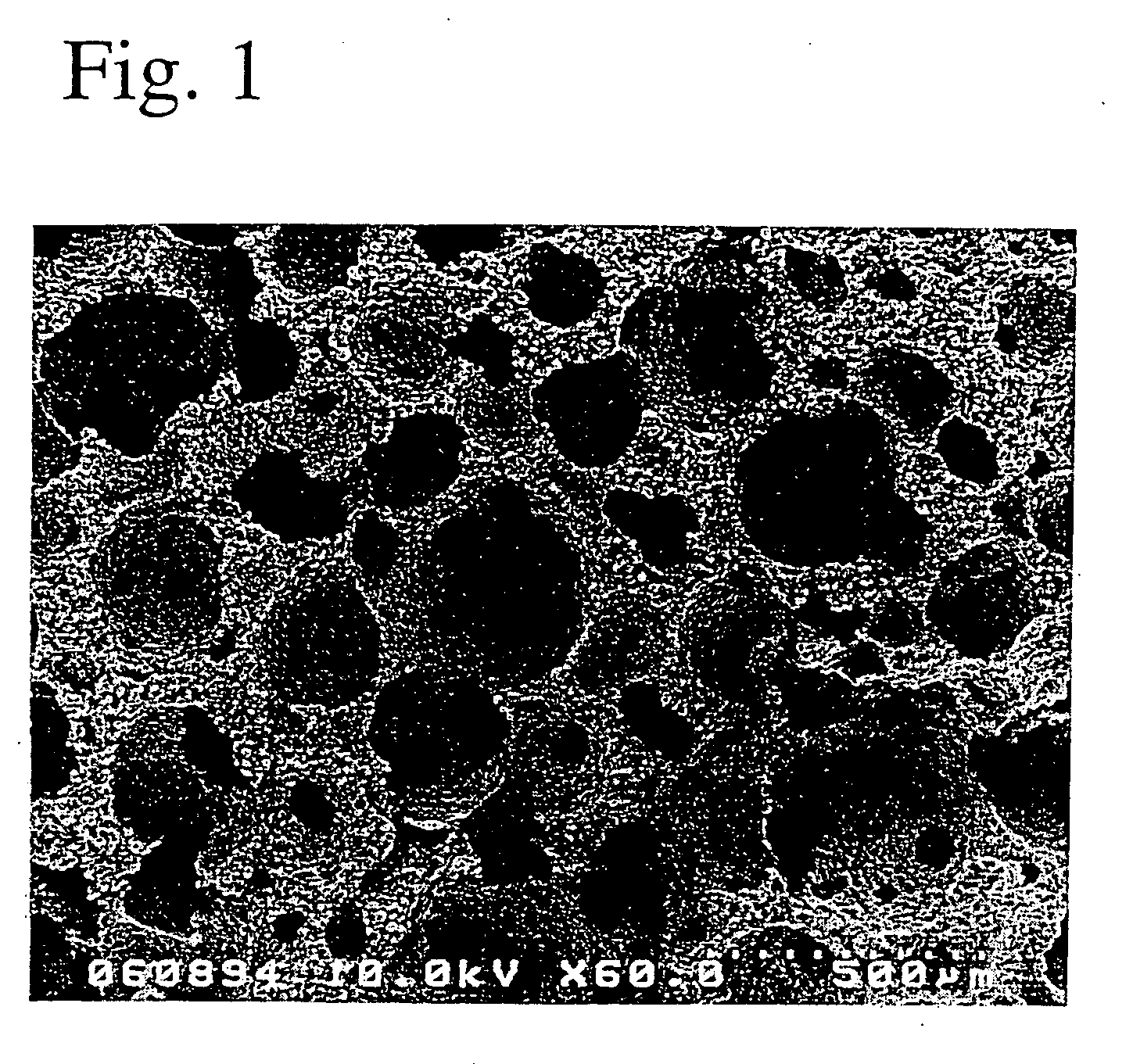
A porous body is a calcium phosphates sintered body having a number of substantially globular pores 1. A porosity is not less than 55% and not more than 85%, and simultaneously, and a mean pore diameter is not less than 50 mum and not more than 800 mum. A pore 11 having a size larger than the mean pore diameter has at least three communicating pores 2 having a diameter of not less than 5 mum, on the average, and simultaneously, a pore having at least the three communicating pores 2 has at least one communicating pore 2 having a diameter of not less than 25 mum, on the average. The total opening area of the communicating pore 2 which is possessed by the pore 11 having a size larger than the mean pore diameter occupies the ratio of not more than 50% of the pore surface area. In a dry state, it is possible to wet the whole the porous body by dropping water and blood.
Owner:OCHI TAKAHIRO +2
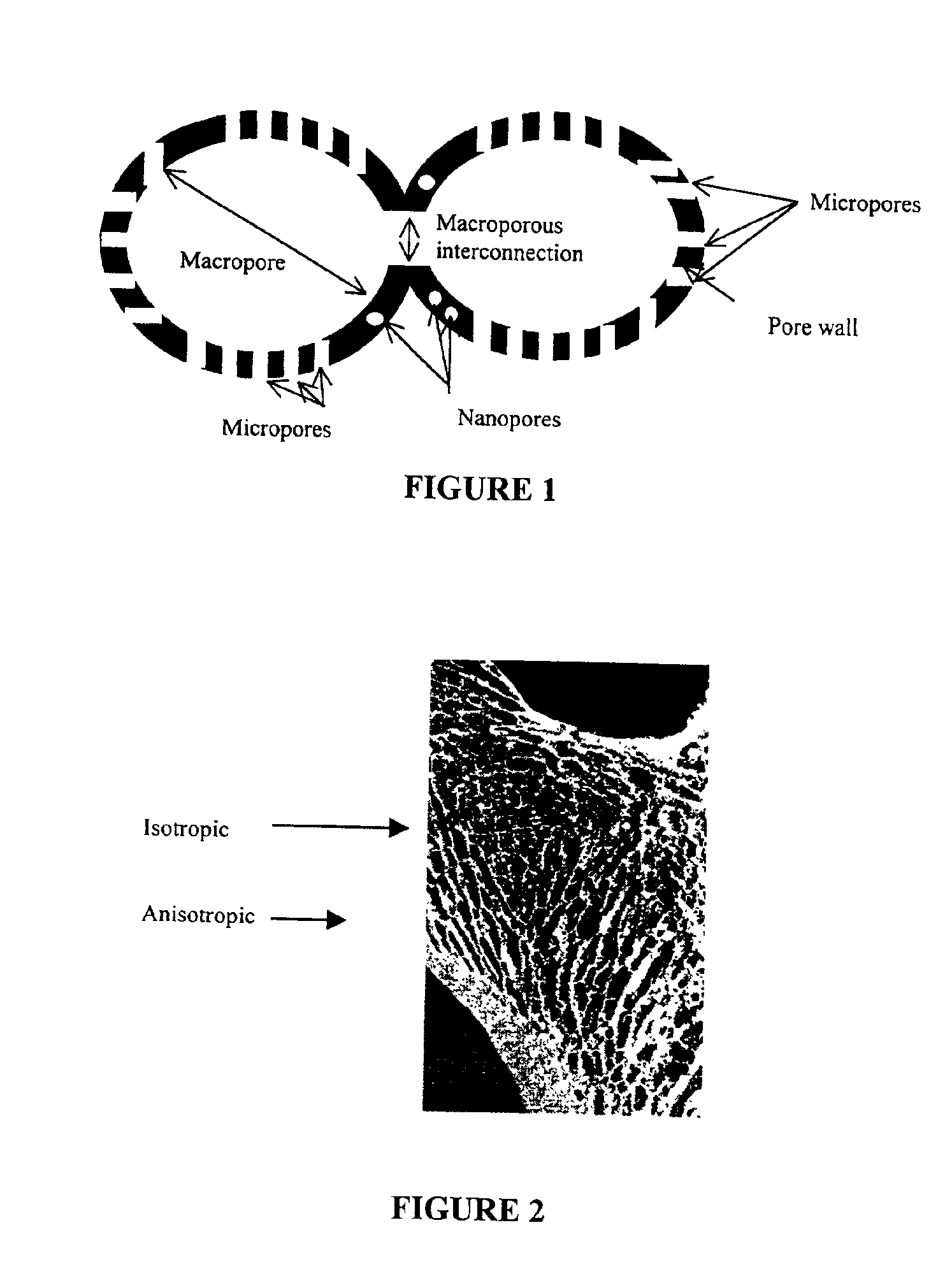
Macroporous, resorbable and injectible calcium phosphate-based cements (MCPC) for bone repair, augmentation, regeneration, and osteoporosis treatment
ActiveUS20050199156A1Easy to controlSurgical adhesivesOther chemical processesApatitePhosphoric acid
A composition and method for producing interconnective macroporous, resorbable and injectable calcium phosphate-based cements (MICPCs). The composition of the invention sets to poorly crystalline apatitic calcium phosphate after mixing a powder component and an aqueous solution. The multiphasic calcium phosphate components in the cement resorb at different rates allowing the timely replacement by new bone. The interconnected macroporosity in the cement allows for vascularization, entrapment of growth factors, cell colonization and tissue ingrowth. This MICPC can be used for dental and medical applications relating to bone repair, augmentation, reconstruction, regeneration, and osteoporosis treatment, and also for drug delivery, and as scaffolds for tissue engineering.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Method of preparing a poorly crystalline calcium phosphate and methods of its use
InactiveUS7517539B1Readily injectableHigh strengthBiocideSurgical adhesivesOsteoporotic boneIntervertebral spaces
The present invention provides a novel process for producing a calcium phosphate cement or filler which hardens in a temperature dependent fashion in association with an endothermic reaction. In the reaction a limited amount of water is mixed with dry calcium phosphate precursors to produce a hydrated precursor paste. Hardening of the paste occurs rapidly at body temperature and is accompanied by the conversion of one or more of the reactants to poorly crystalline apatitic calcium phosphate. The hardened cements, fillers, growth matrices, orthopedic and delivery devices of the invention are rapidly resorbable and stimulate hard tissue growth and healing. A composite material is provided including a strongly bioresorbable, poorly crystalline apatitic calcium phosphate composite and a supplementary material. The supplementary material is in intimate contact with the hydroxyapatite material in an amount effective to impart a selected characteristic to the composite. The supplemental material may be biocompatible, bioresorbable or non-resorbable. A method for treating a bone defect also is provided by identifying a bone site suitable for receiving an implant, and introducing a strongly resorbable, poorly crystalline apatitic calcium phosphate at the implant site, whereby bone is formed at the implant site. The implant site may be a variety of sites, such as a tooth socket, non-union bone, bone prosthesis, an osteoporotic bone, an intervertebral space, an alveolar ridge or a bone fracture.
Owner:LIFE SCI ENTERPRISES
Porous biocompatible implant material and method for its fabrication
a biocompatible and biodegradable implant for a cavity in a bone of a living organism is made of a biocompatible and biodegradable granules which are selected from the group including biopolymers, bioglasses, bioceramics preferably calcium sulfate, calcium phosphate such as monocalcium phosphate monohydrate, monocalcium phosphate anhydrous, dicalcium phosphate dihydrate, dicalcium phosphate anhydrous, tetracalcium phosphate, calcium orthophosphate phosphate, α-tricalcium phosphate, β-tricalcium phosphate, apatite such as hydroxyapatite, or a mixture thereof. The biocompatible and biodegradable granules are provided with a coating, which comprises at least one layer of a biocompatible and biodegradable polymer which is selected from the group including poly((α-hydroxyesters), poly(orthoesters), polyanhydrides, poly(phosphazenes), poly(propylene fumarate), poly(ester amides), poly(ethylene fumarate), polylactide, polyglycolide, polycaprolactone, poly(glycolide-co-trimethylene carbonate), polydioxanone, co-polymers thereof and blends of those polymers. The biocompatible and biodegradable implants are obtained by fusing together the polymer-coated granules through polymer-linkage of the polymer coatings of neighboring granules.
Owner:COLLAGEN MATRIX
Resorbable bone graft materials
Ceramic materials operable to repair a defect in bone of a human or animal subject comprising a porous ceramic scaffold having a bioresorbable coating, and a carrier comprising denatured demineralized bone. The ceramic may contain a material selected from the group consisting of hydroxyapatite, tricalcium phosphate, calcium phosphates, calcium carbonates, calcium sulfates, and combinations thereof. The compositions may also contain a bone material selected from the group consisting of: bone powder, bone chips, bone shavings, and combinations thereof. The bioresorbable coating may be, for example, demineralized bone matrix, gelatin, collagen, hyaluronic acid, chitosan, polyglycolic acid, polylactic acid, polypropylenefumarate, polyethylene glycol, or mixtures thereof.
Owner:BIOMET MFG CORP
Intraocular delivery compositions and methods
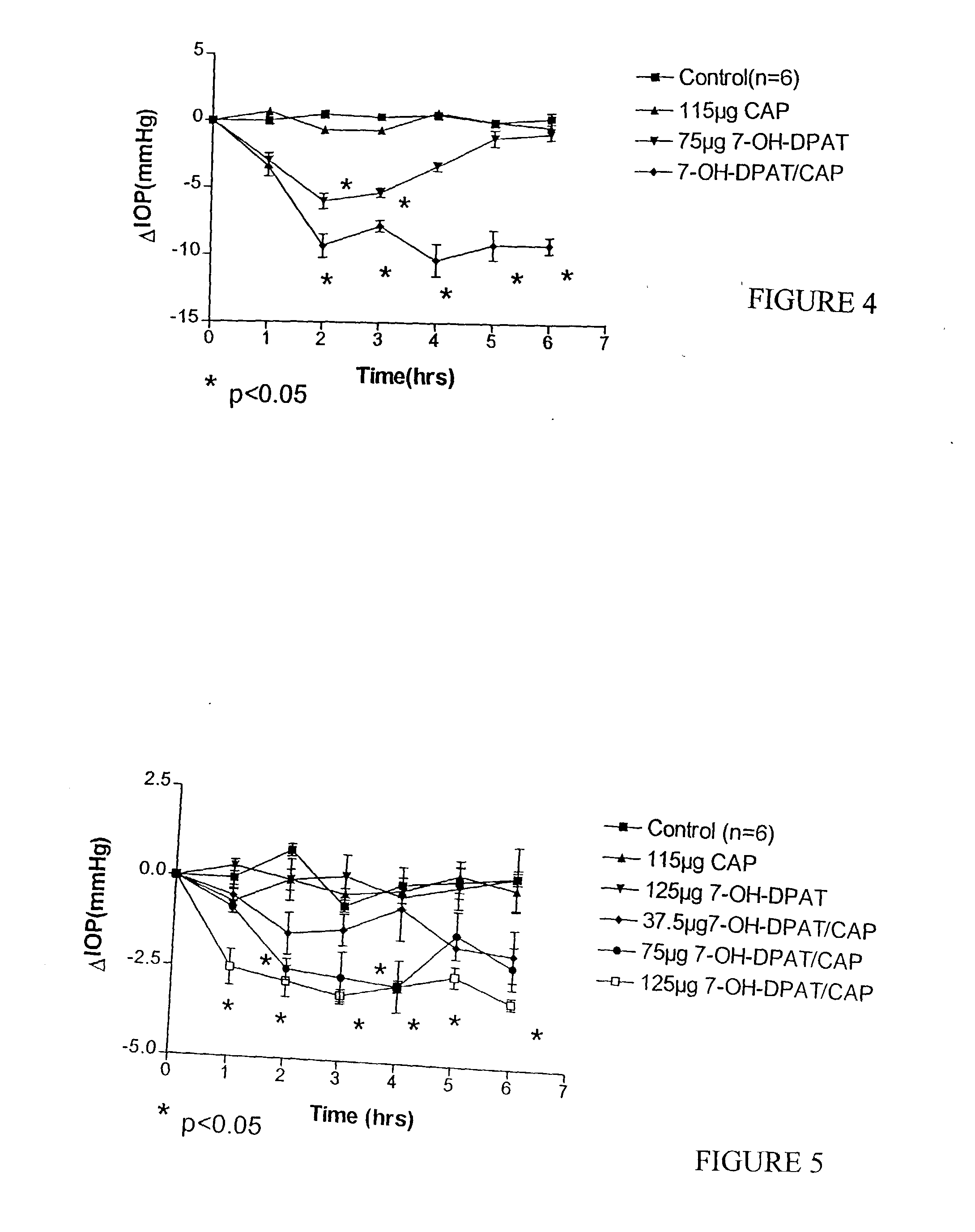
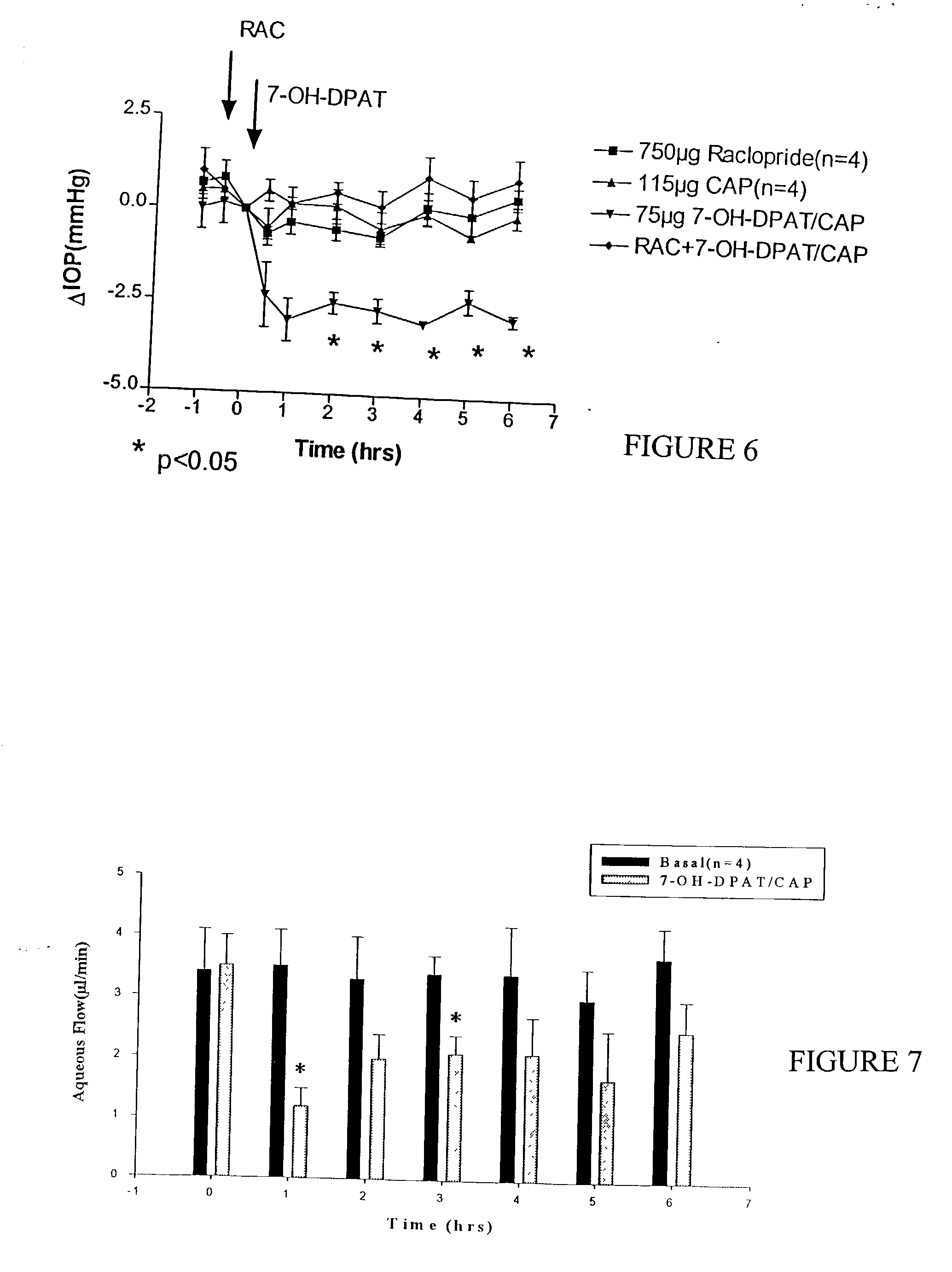
The present invention relates to intraocular drug delivery for treating ocular diseases. Particularly, the invention relates to particles useful for the delivery of certain pharmacologically active agents to treat ocular diseases. The particles contain calcium phosphate core particles, particularly nanoparticles, as delivery agents and adjuvants. The invention also relates to methods of making such particles and to methods of treating ocular disease by delivery of a therapeutic drug to an ocular surface using the particles of this invention. The invention further relates to methods of regulating ocular pressure using certain formulations according to the present invention.
Owner:BIOSANTE PHARMA
Delayed-Setting Calcium Phosphate Pastes
ActiveUS20080028992A1Easy injectionReduce the ratioBiocideData processing applicationsCalcium biphosphateDelivery vehicle
Owner:ETEX
Porous sintered body of calcium phosphate-based ceramic and method for producing same
InactiveUS20040185181A1High porosityEnhance stirringDental implantsLayered productsCalcium biphosphatePorosity
A porous sintered body of a calcium phosphate-based ceramic having a porosity of 80% or more. The porous sintered body is produced by a method comprising the steps of: (1) preparing a slurry comprising a calcium phosphate-based ceramic powder, a water-soluble high molecular compound and a nonionic surface active agent; (2) stirring the slurry vigorously to froth the slurry; (3) solidifying the frothed slurry into a gel; and (4) drying and sintering the gel.
Owner:HOYA CORP
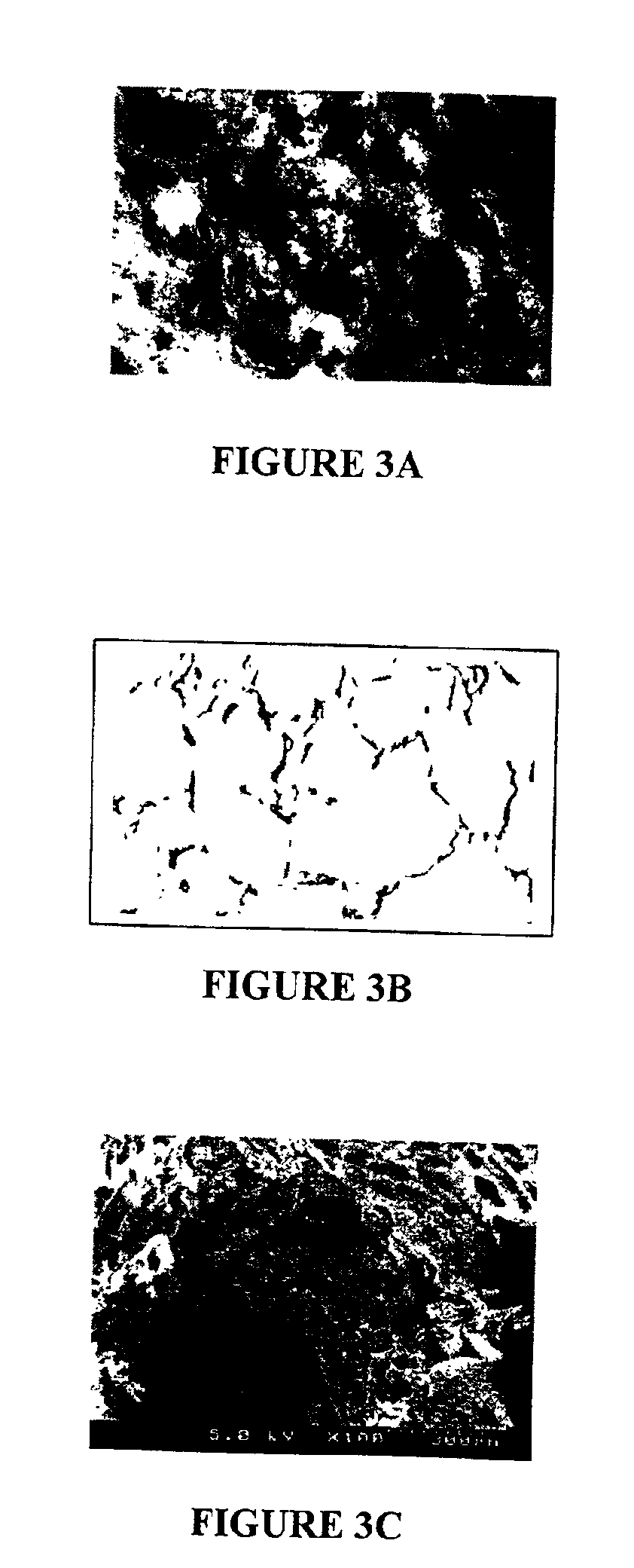
Macroporous polymer scaffold containing calcium phosphate particles
A polymer scaffold is provided comprising an extensively interconnected macroporous network. The polymer scaffold embodies macropores having a diameter in a range of 0.5-3.5 mm, and preferably in a range of about 1.0-2.0 mm. The polymer scaffold is prepared using a novel process which advantageously combines the techniques of particulate leaching and phase inversion to render a process that provides amplified means by which to control the morphology of the resulting polymer scaffold. The polymer scaffold has utility in the area of tissue engineering, particularly as a scaffold for both in vitro and in vivo cell growth. The polymer scaffold may be produced using pure polymer or alternatively a composite material may be formed consisting of a macroporous polymer scaffold and osteoclast-resorbable calcium phosphate particles with a binding agent binding the calcium phosphate particles to the polymer scaffold.
Owner:GUAN LIMIN +3
Implant improving local bone formation
InactiveUS20060188542A1Improve effectivenessBiocideDental implantsChemical LinkageCalcium biphosphate
A bone implant comprises an active agent on at least a portion thereof. The active agent is locally deliverable to bone proximate the implant in at least a two-phased release scheme. A first phase rapidly releases a first quantity of the active agent, and at least a second phase gradually releases a second quantity of the active agent, whereby bone formation stimulated by the active agent is modulated. In one embodiment, a porous implant comprises a porous portion coated with a calcium phosphate compound and which is contacted with a bisphosphonate compound to form a bisphosphonate layer chemically bound to the calcium phosphate at the surface of the porous portion and to form bisphosphonate molecules being non-chemically attached inside the pores of the porous portion. The non-chemically attached bisphosphonate molecules are released in the subject at a rate greater than that of the chemically bound bisphosphonate layer.
Owner:BOBYN JOHN DENNIS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com