Patents
Literature
50 results about "Anatomical space" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
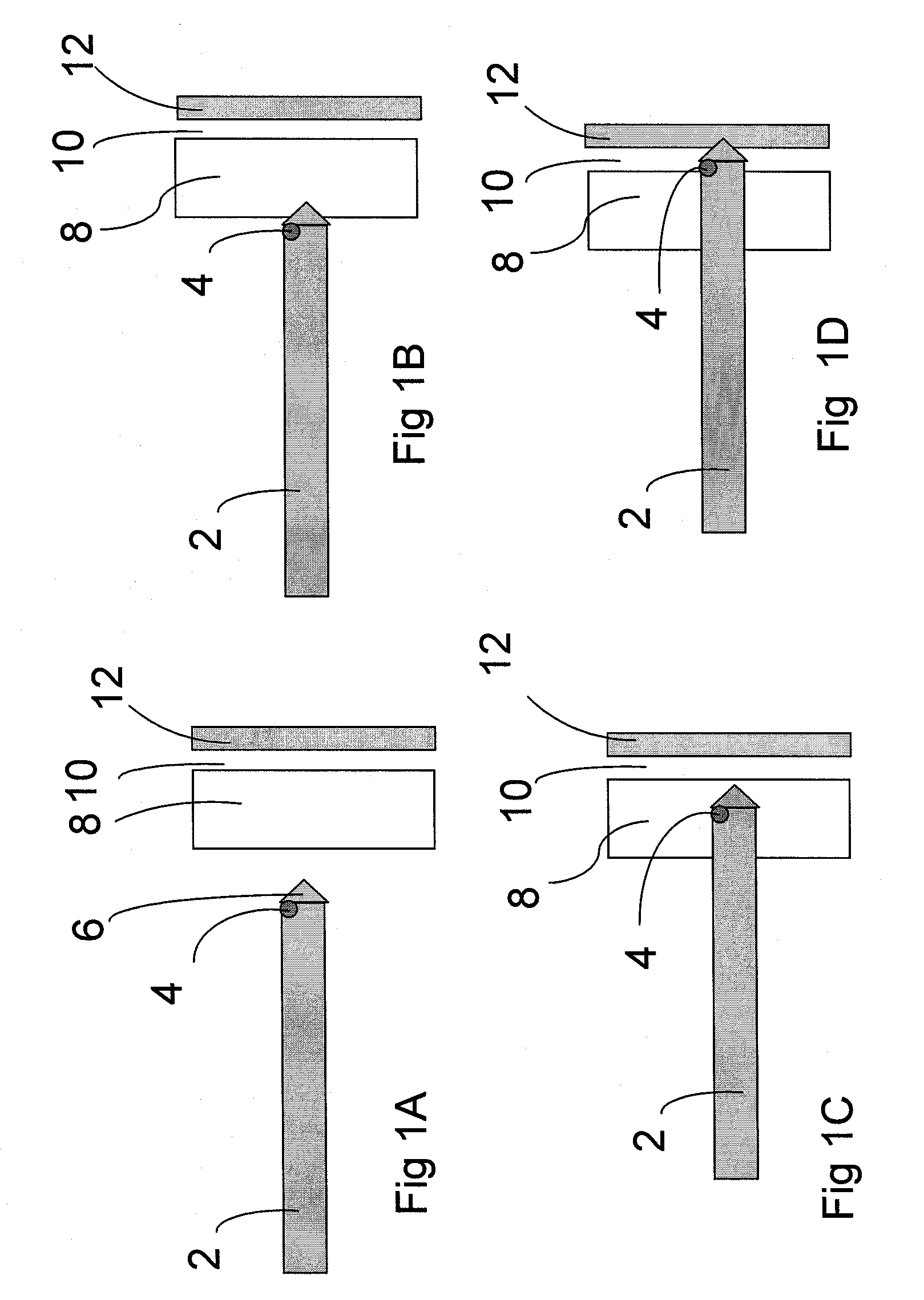
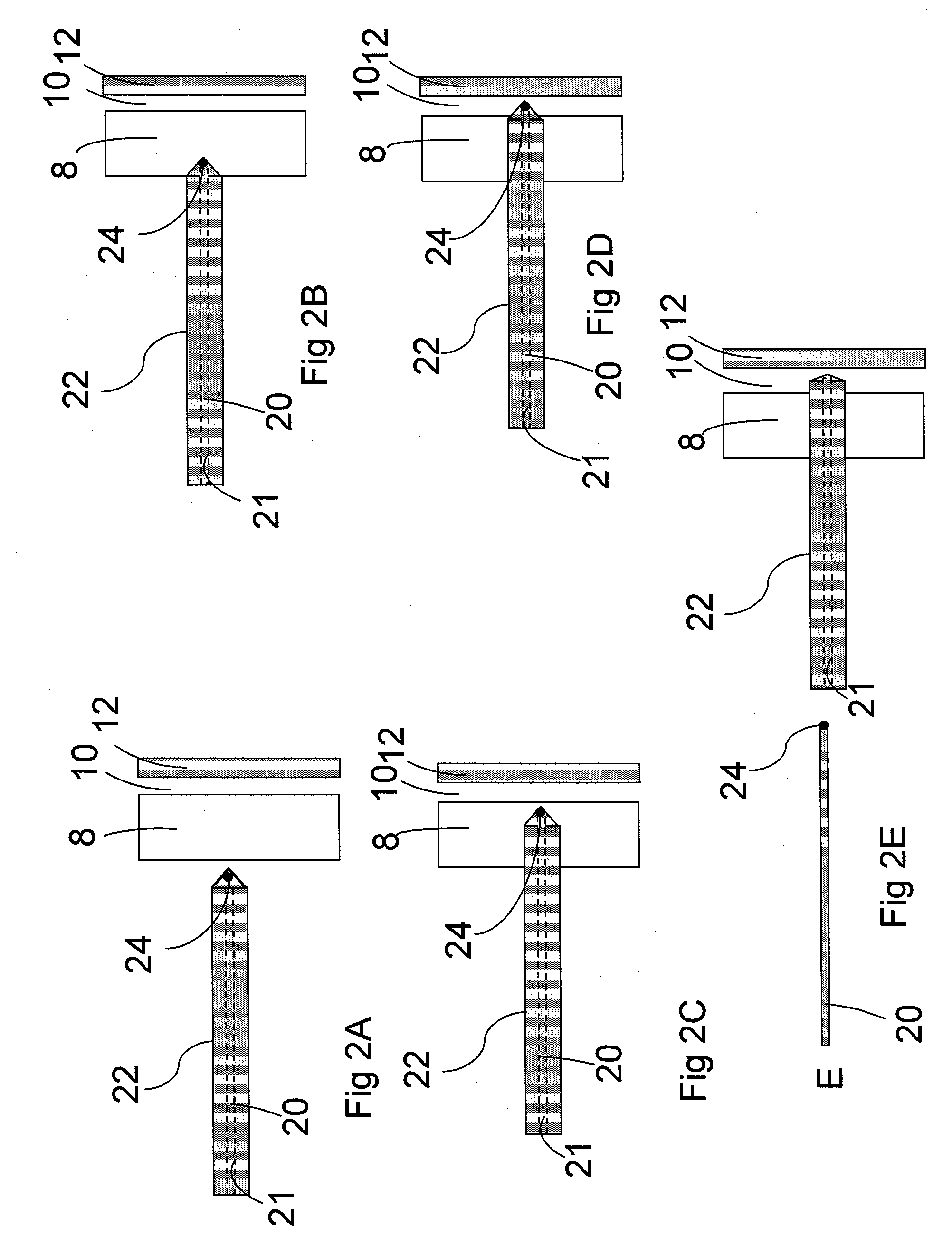
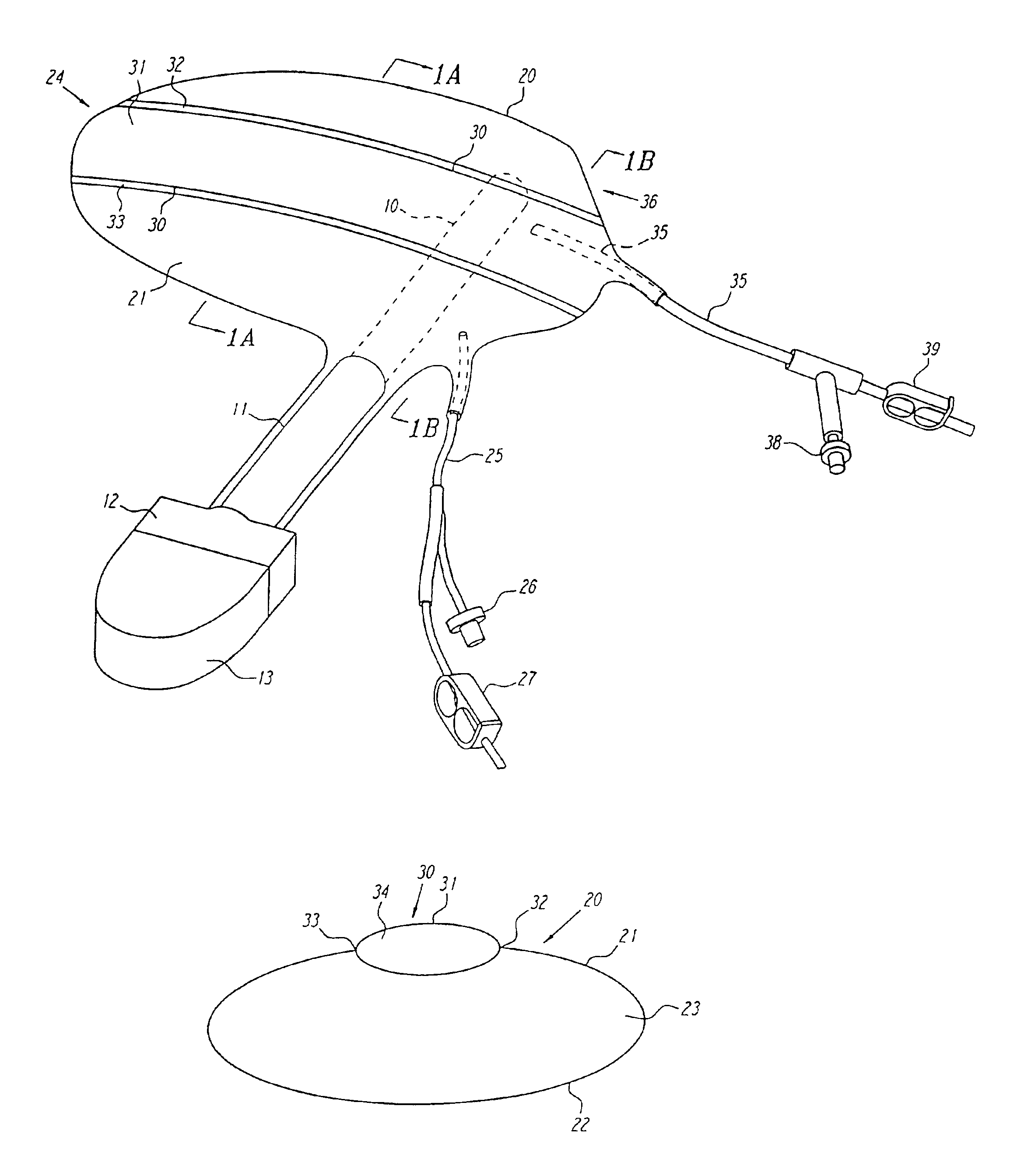
Non-material physical anatomical entity of three dimensions, which is generated by morphogenetic or other physiologic processes; is surrounded by one or more anatomical structures; contains one or more body substances or anatomical structures. Examples: celom, thoracic cavity, lesser sac of peritoneum, cavity of right atrium, lumen of aorta, mediastinum, anterior compartment of forearm, intervertebral foramen.
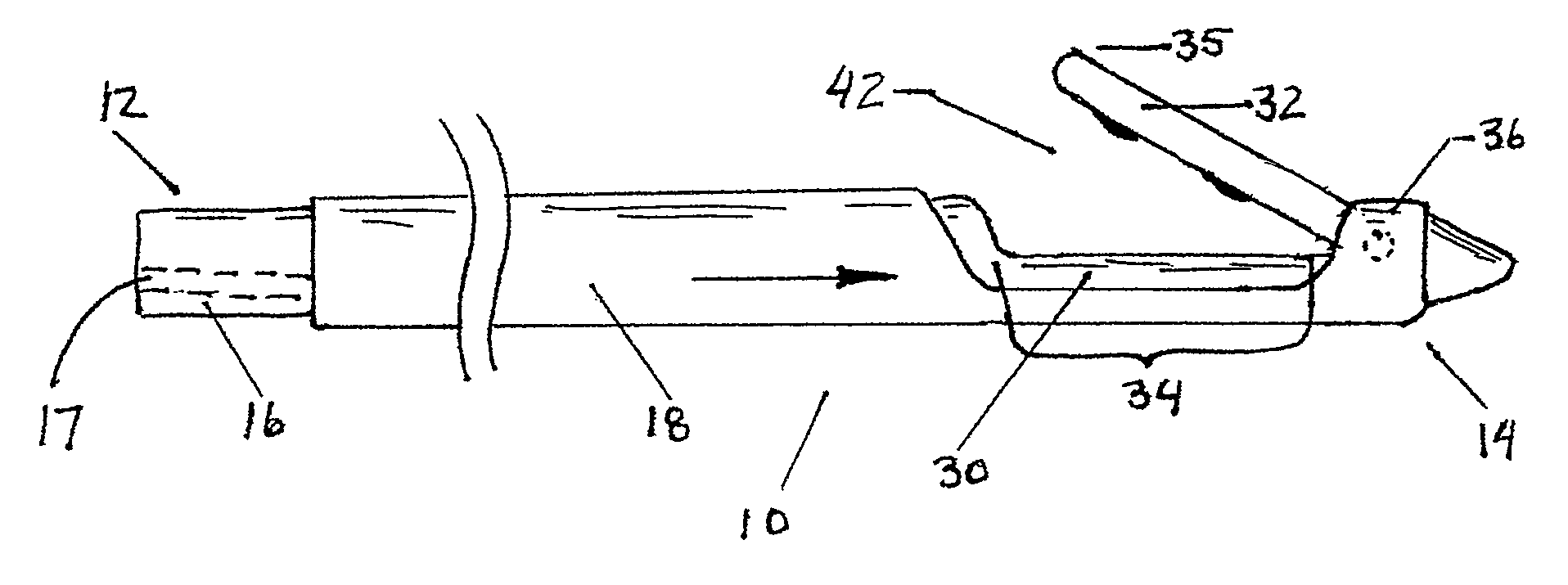
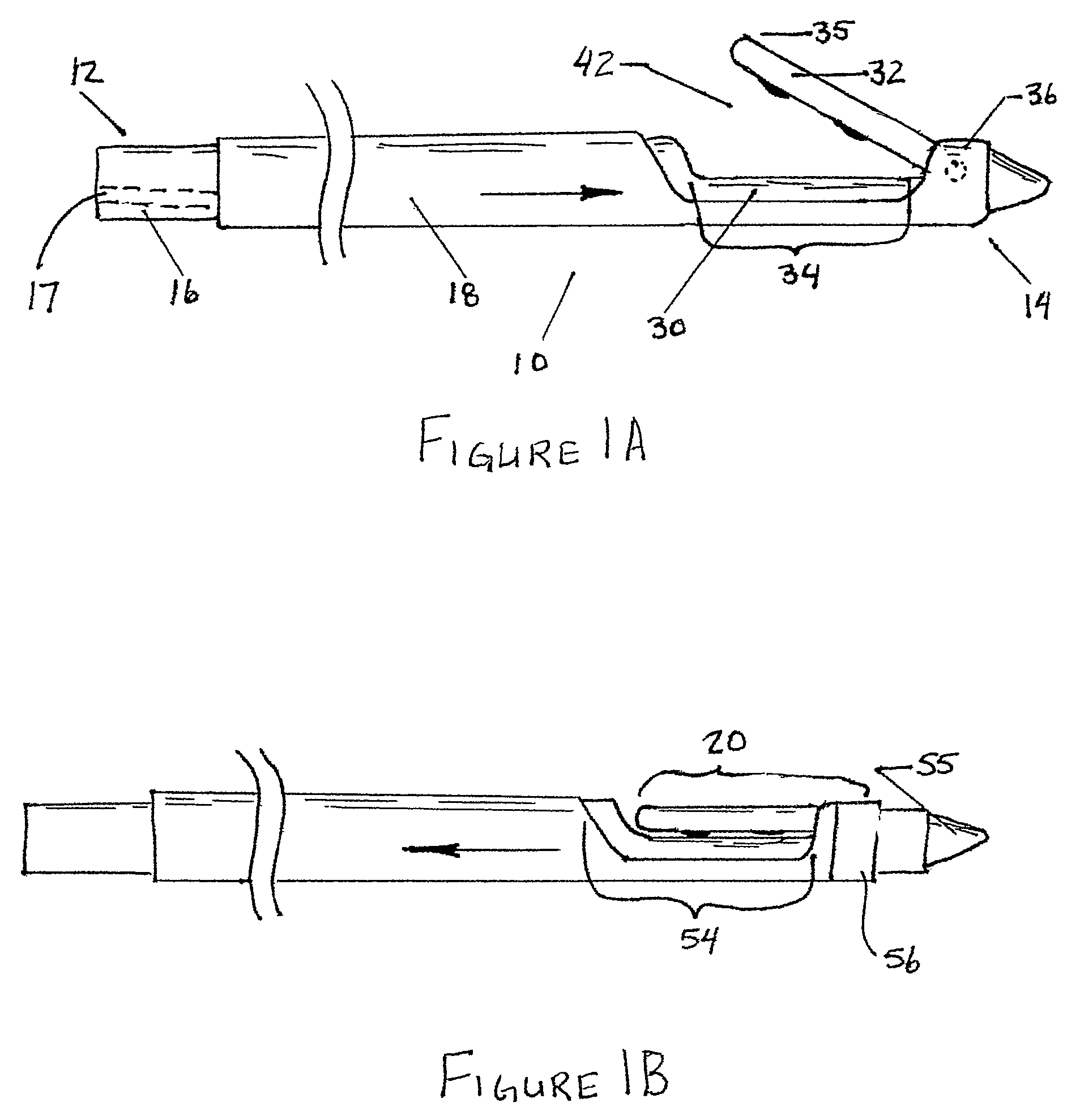
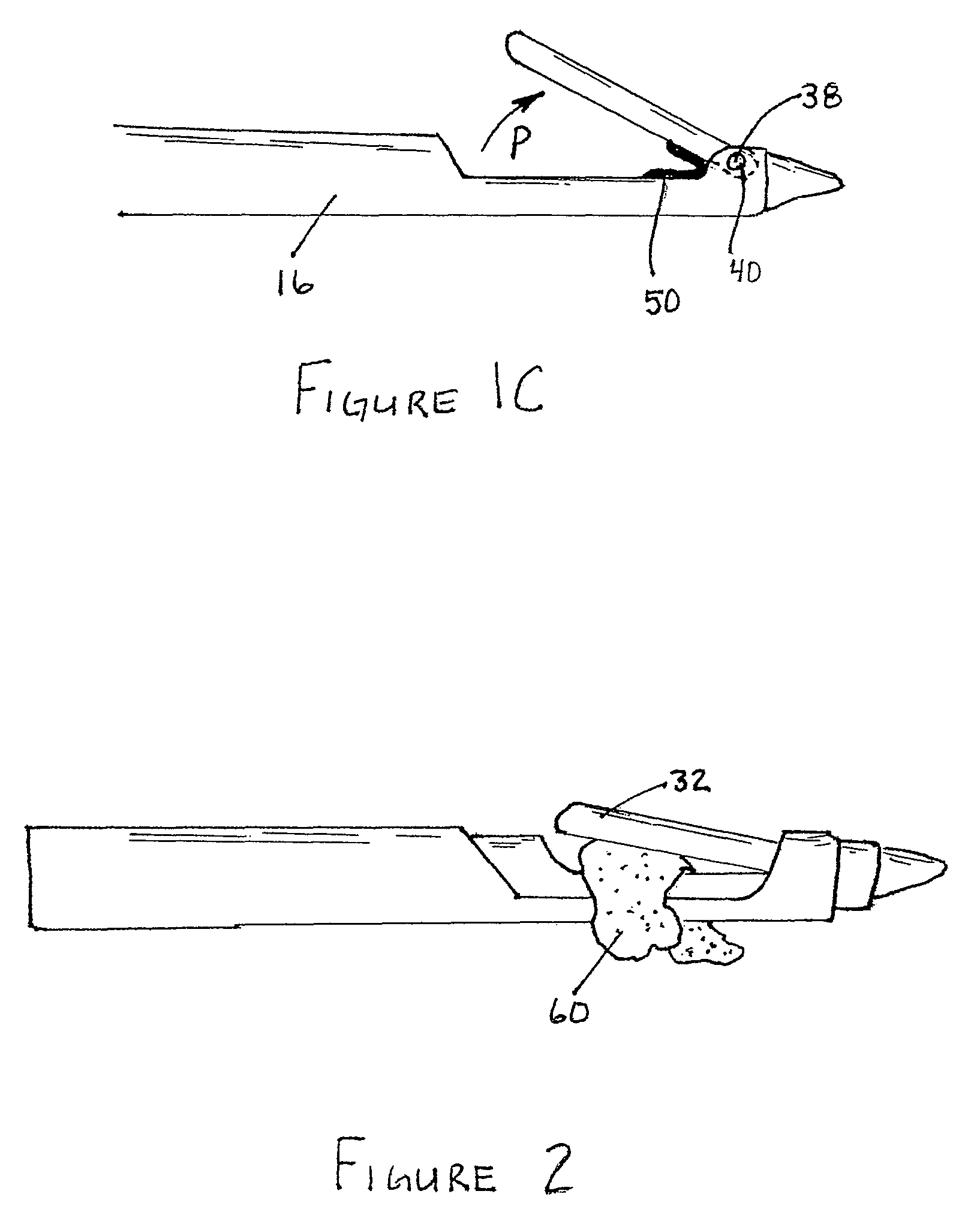
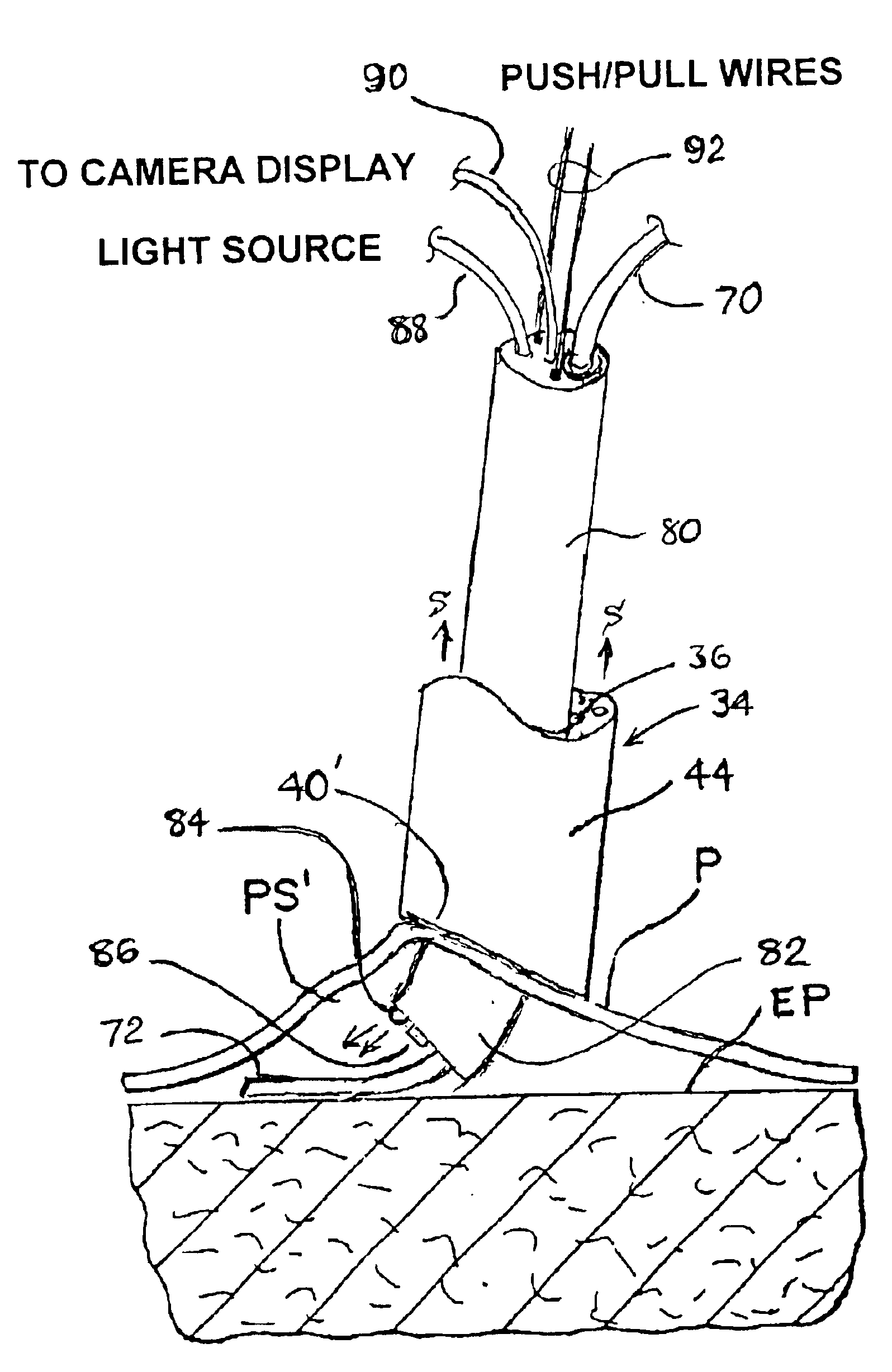
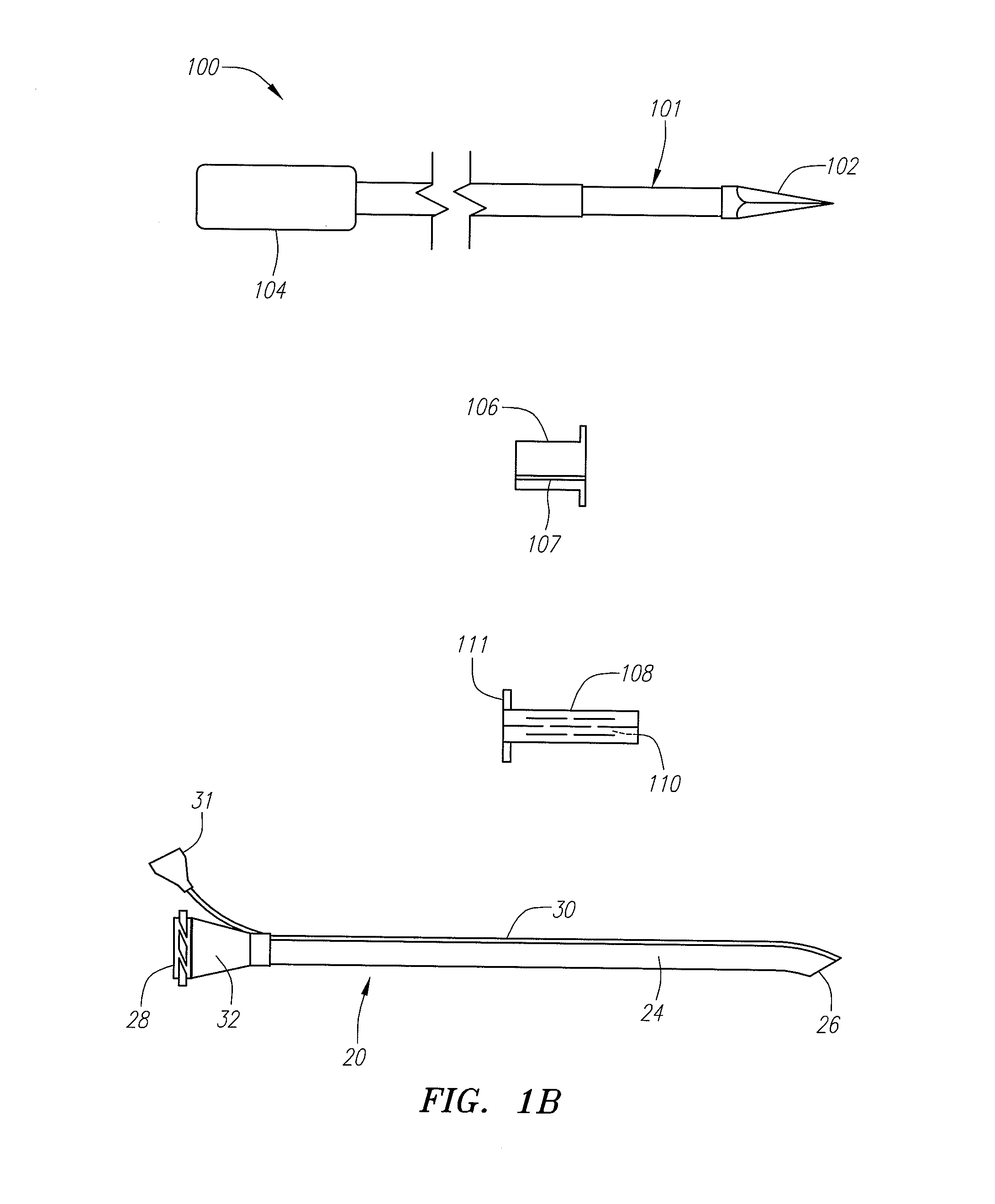
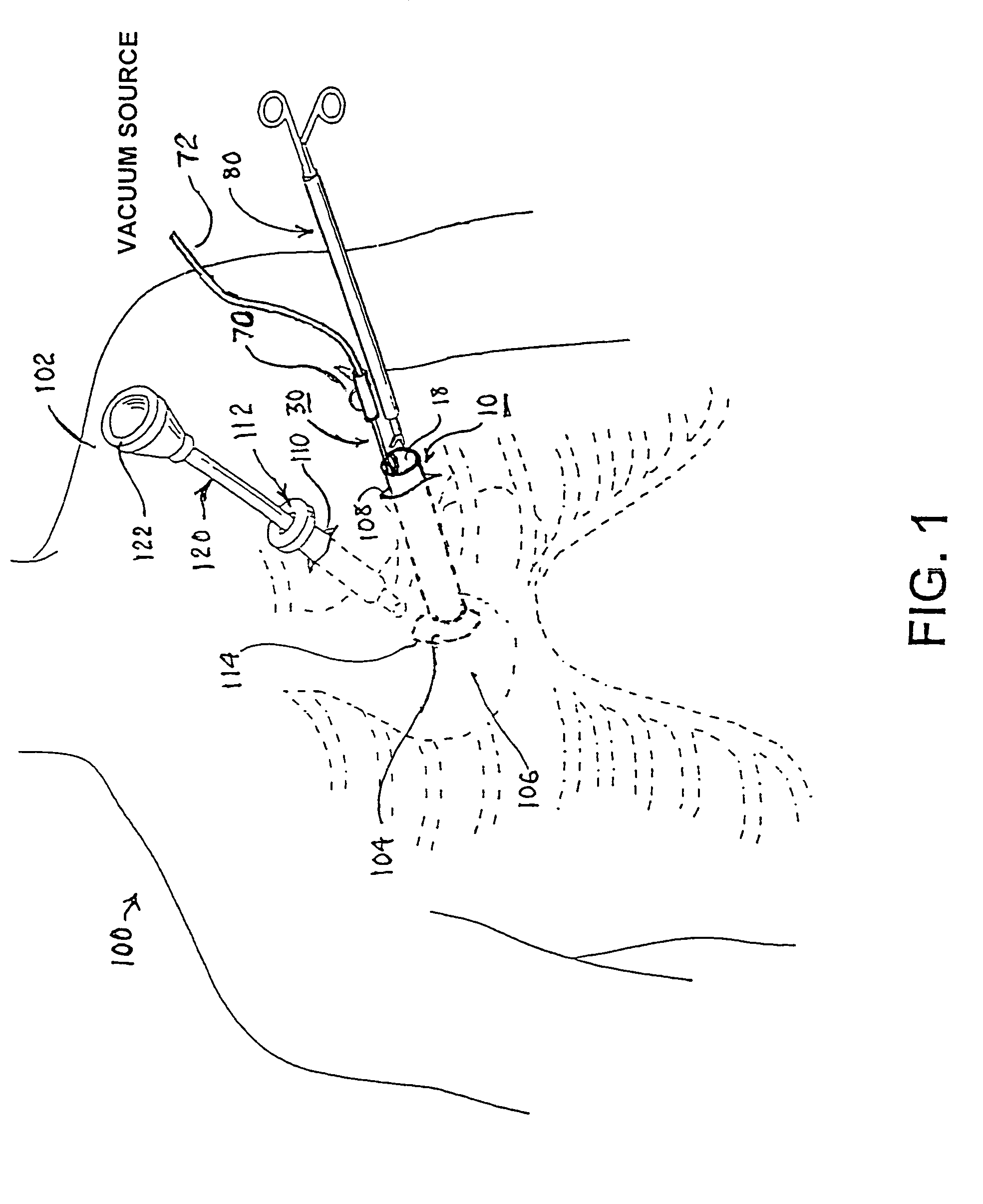
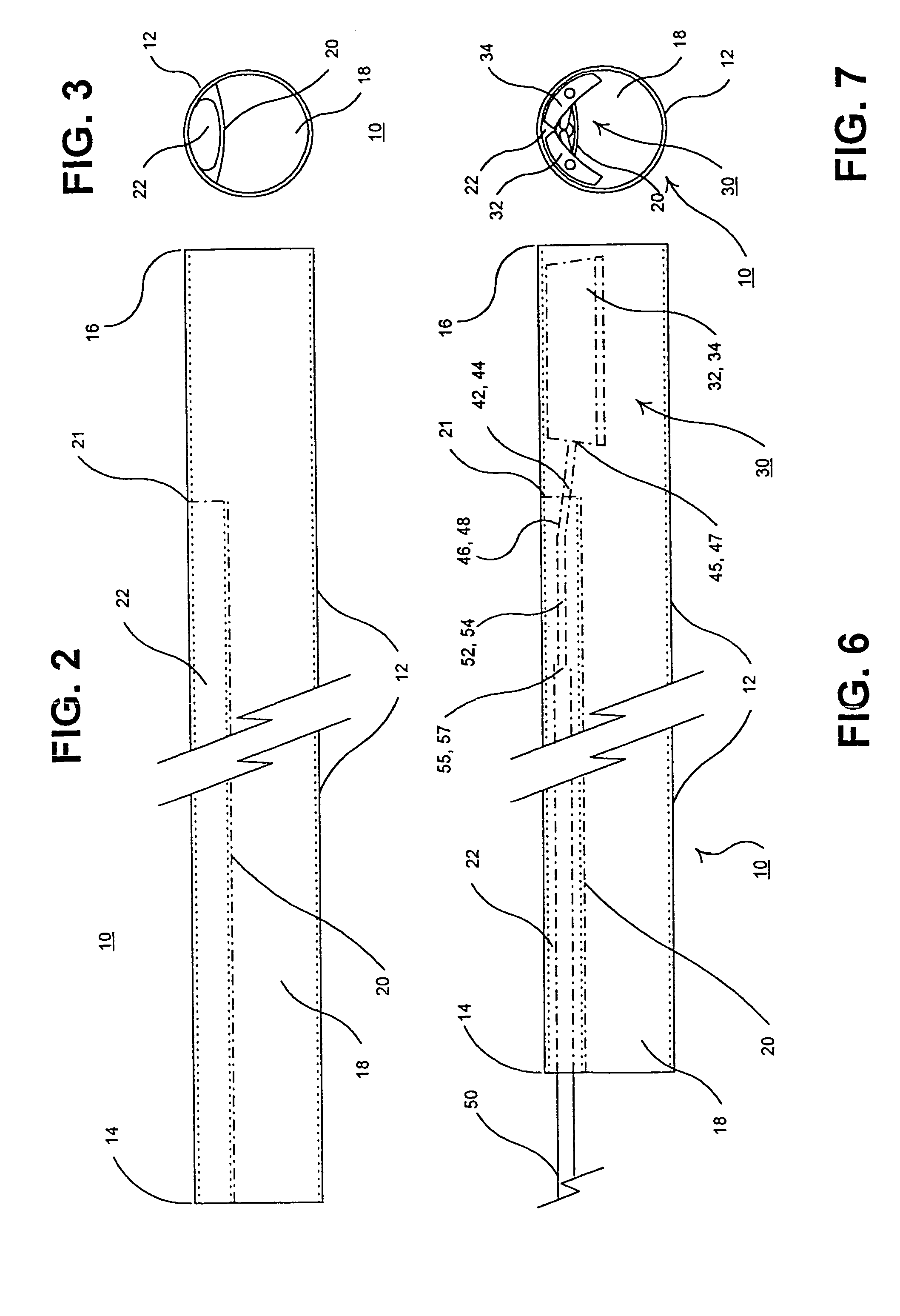
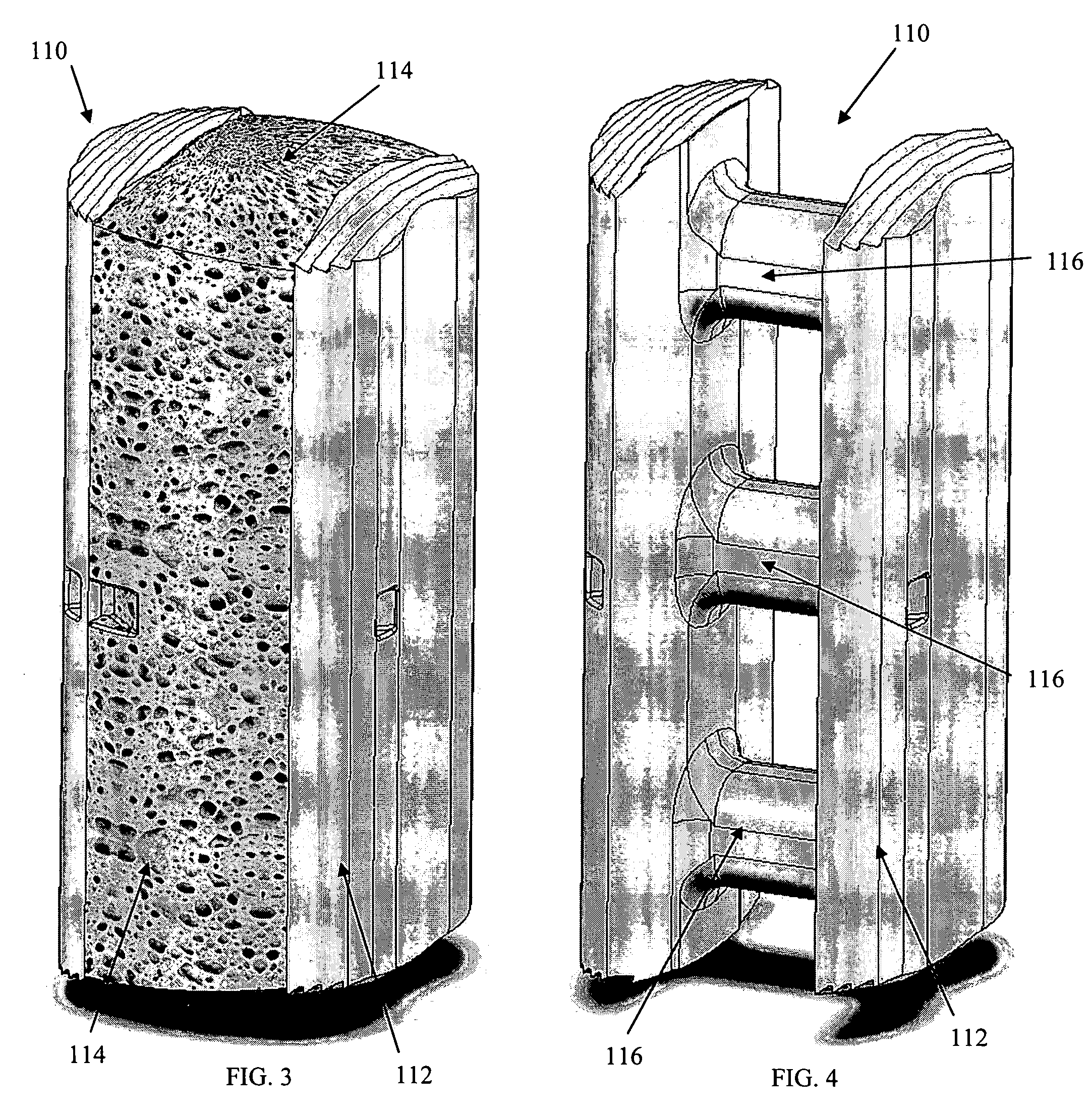
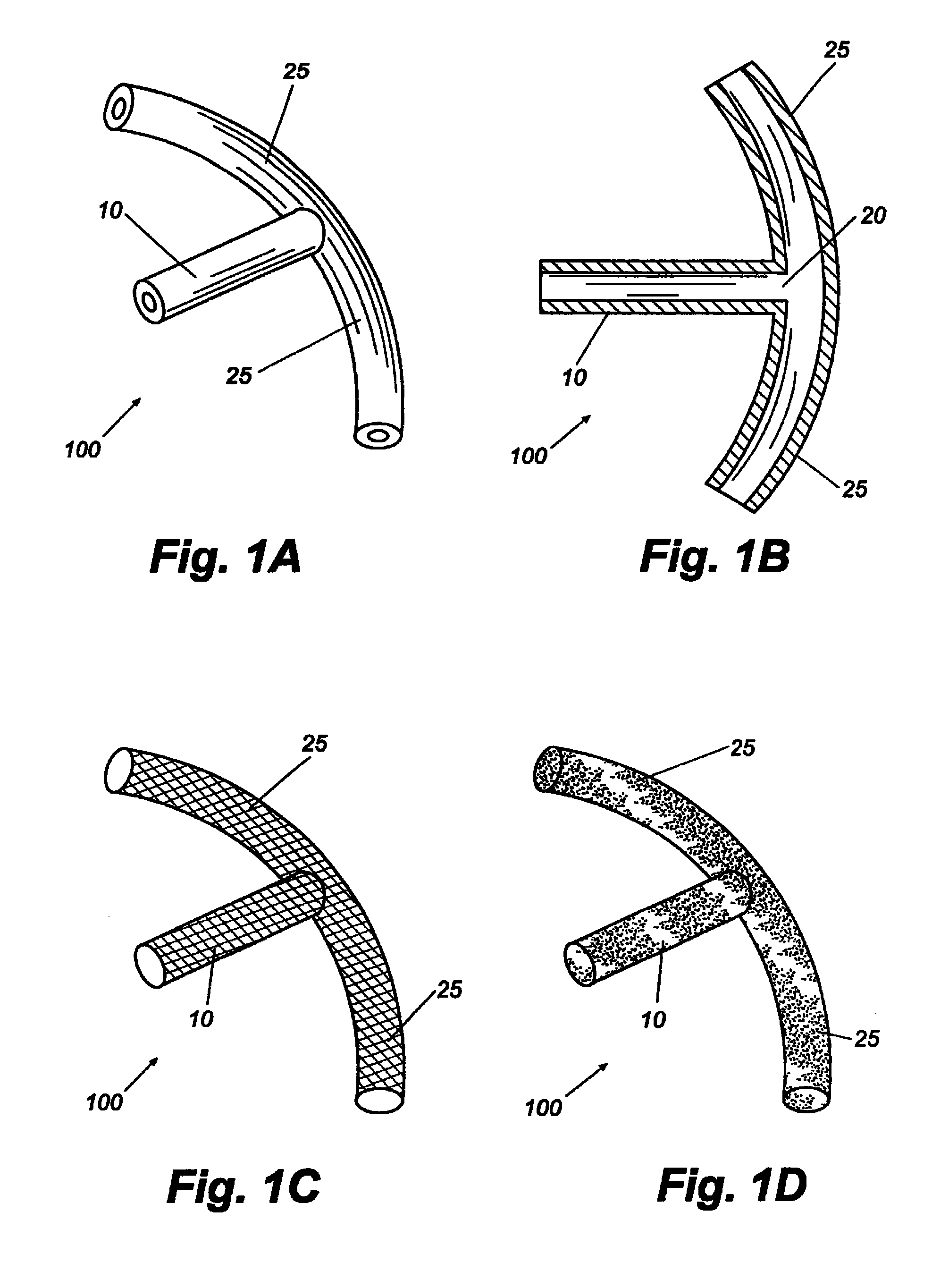
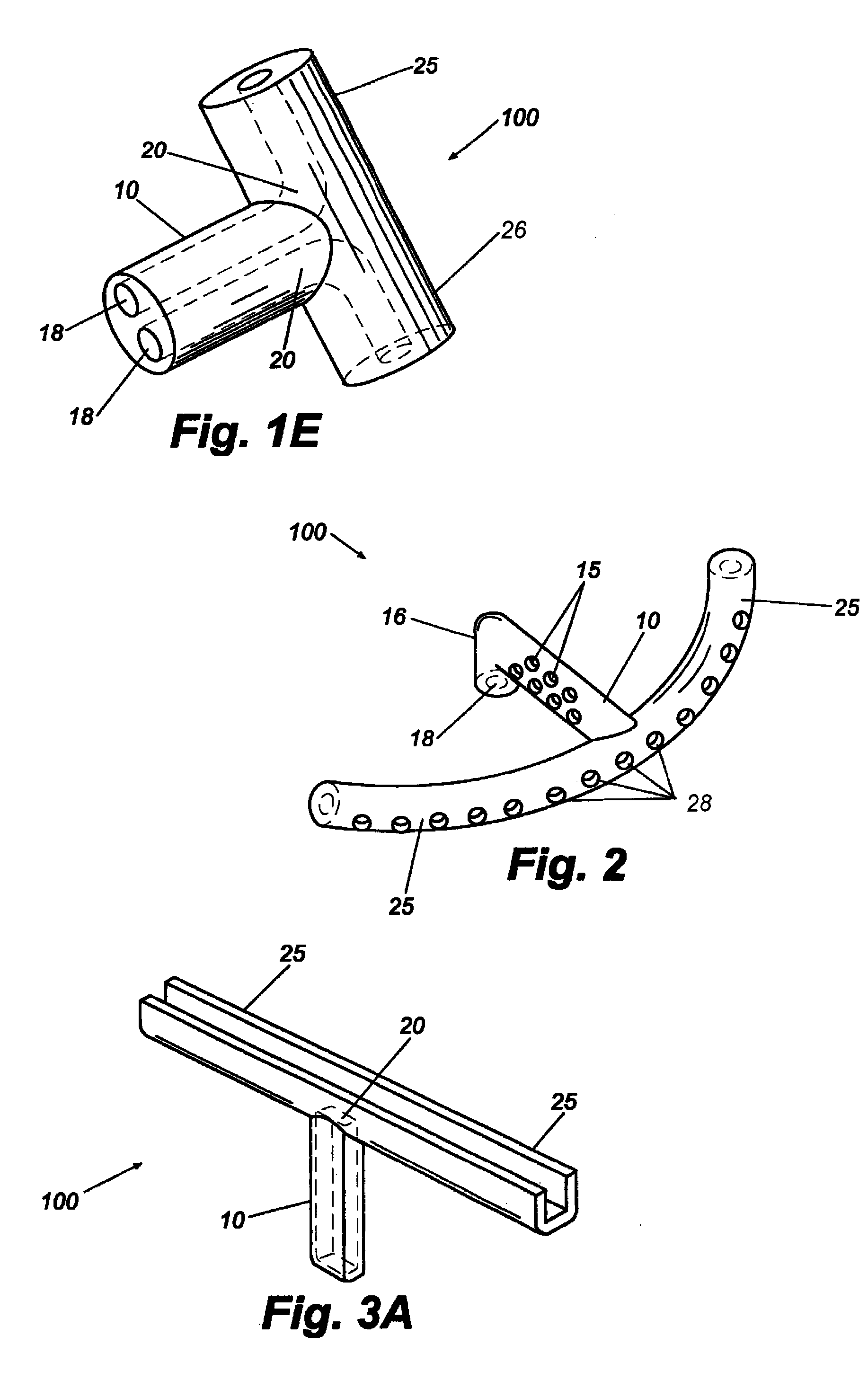
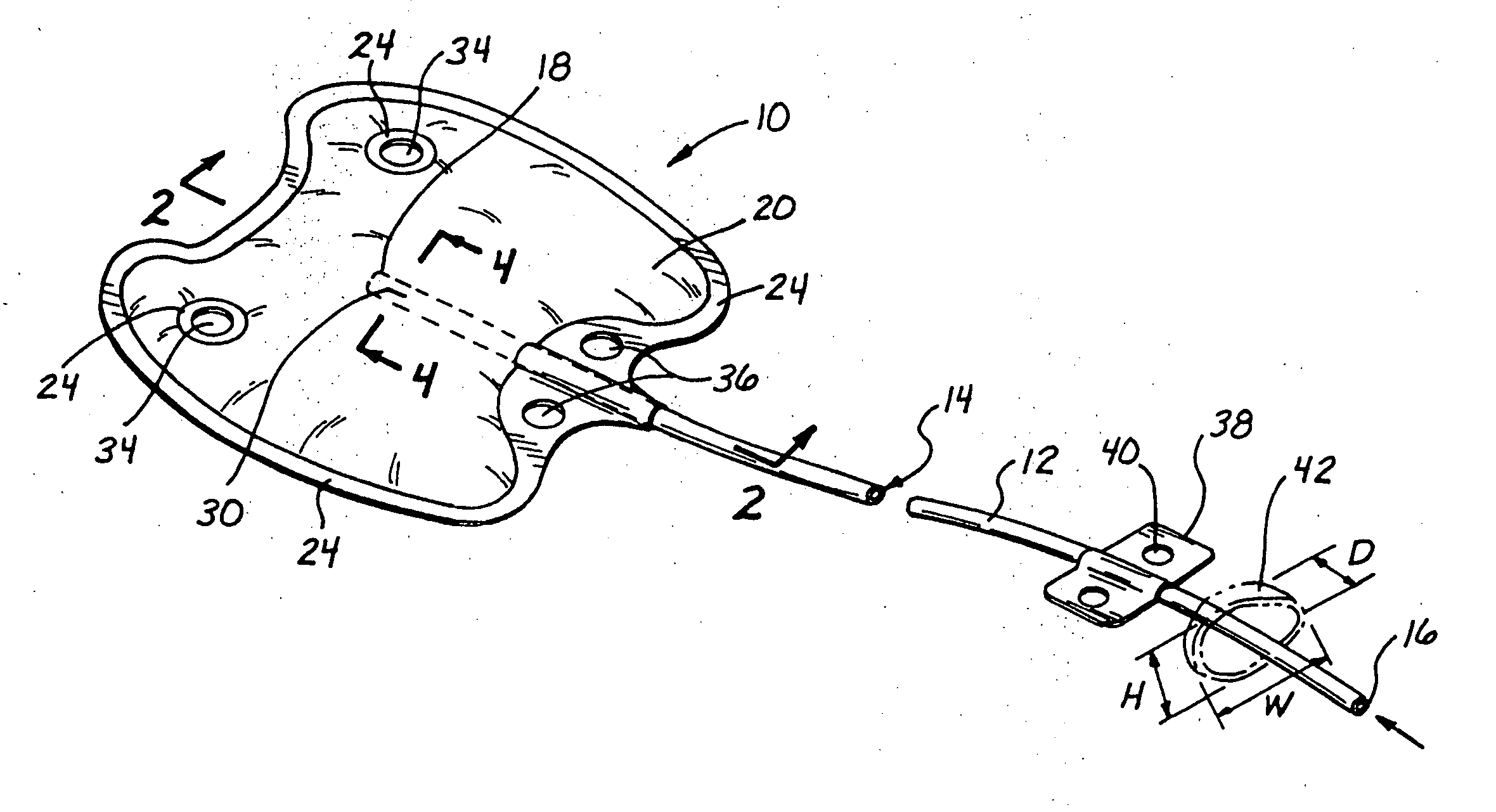
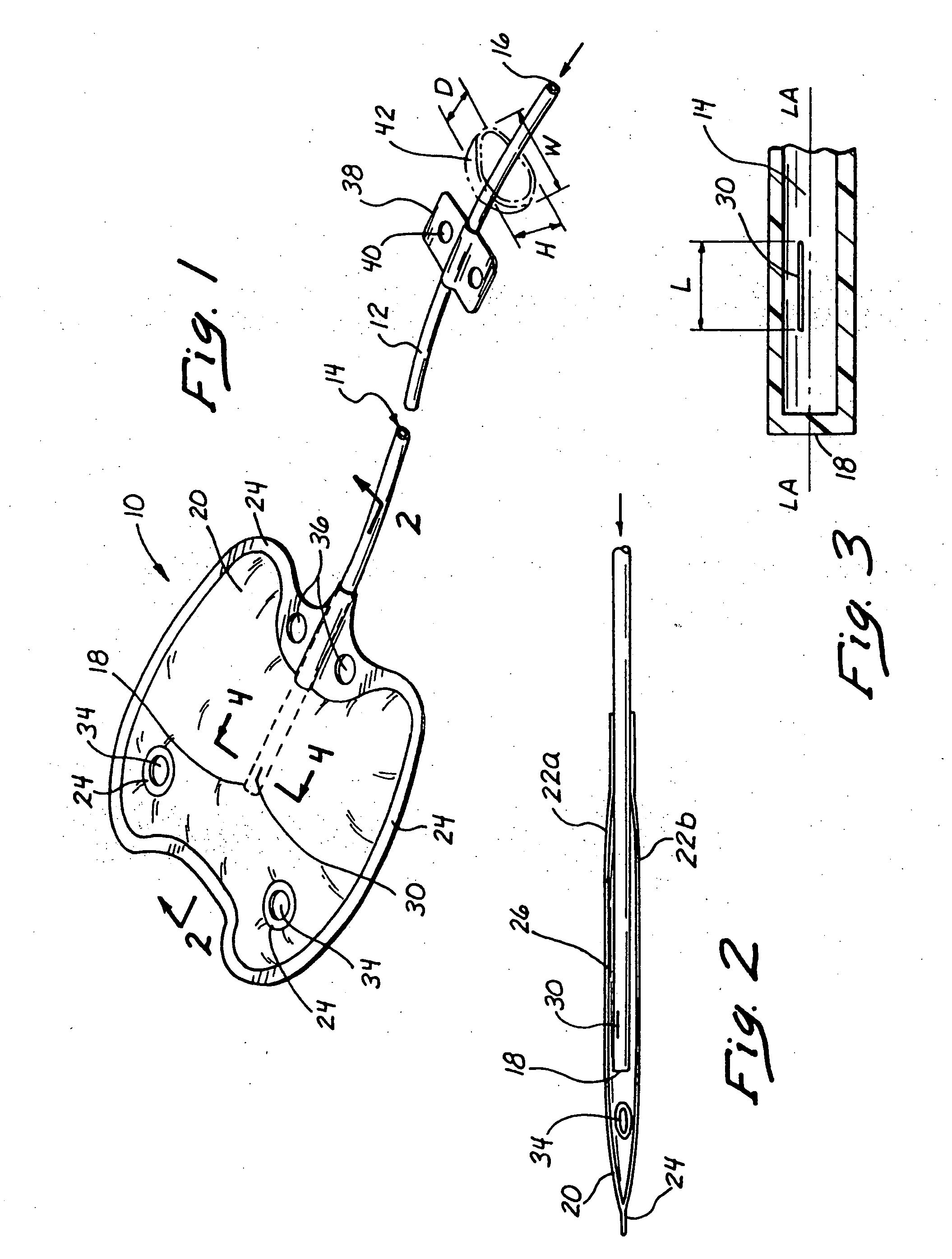
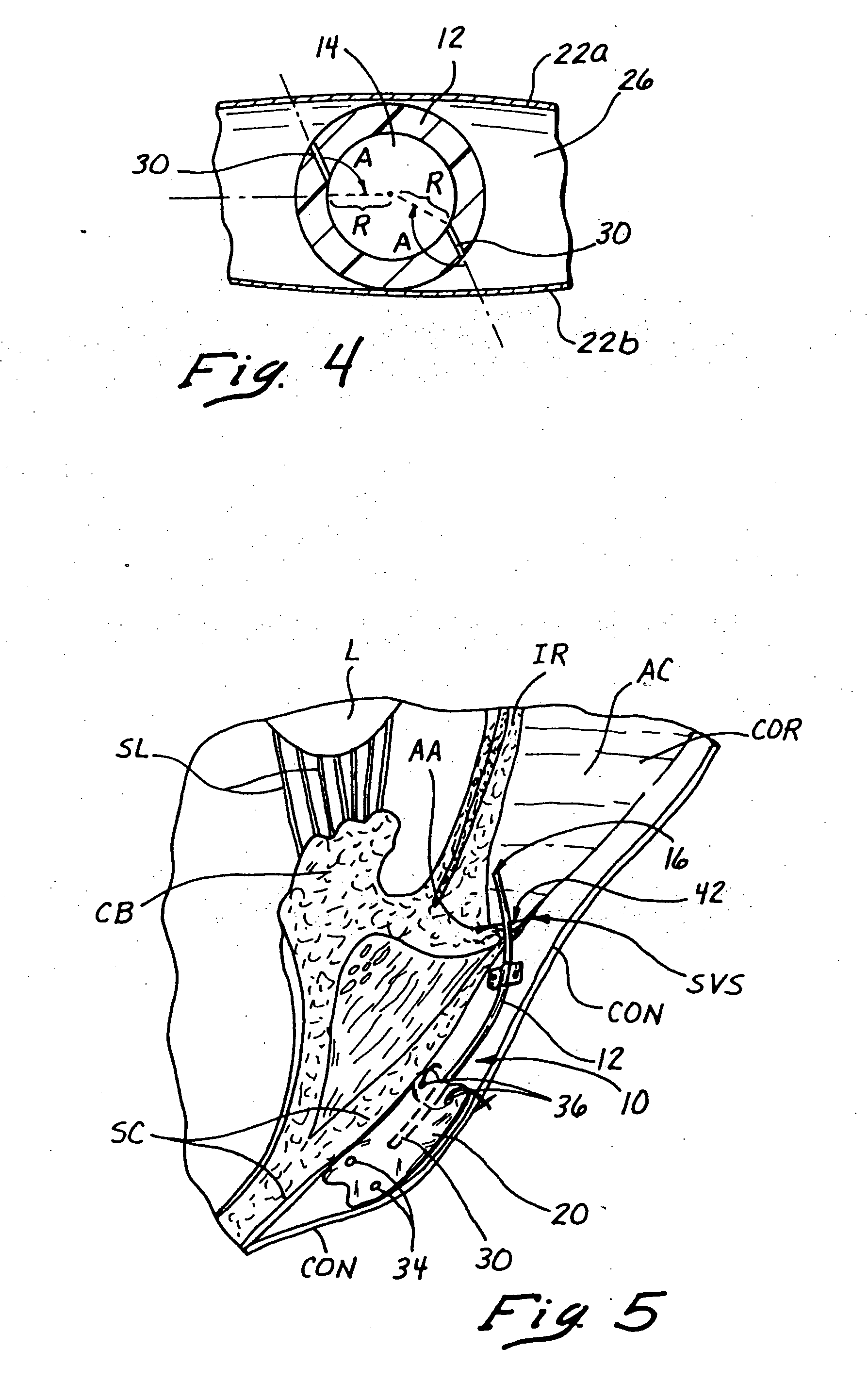
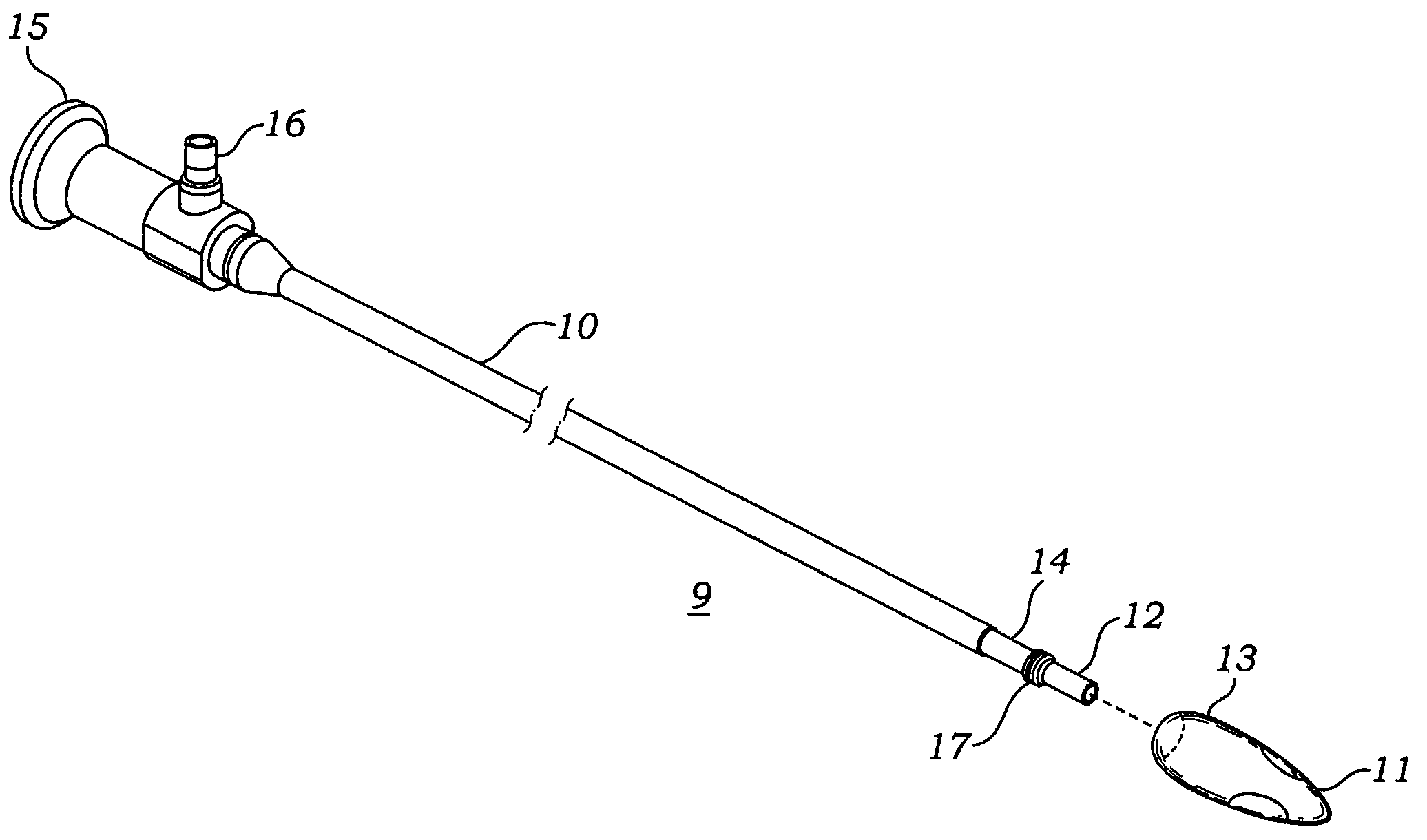
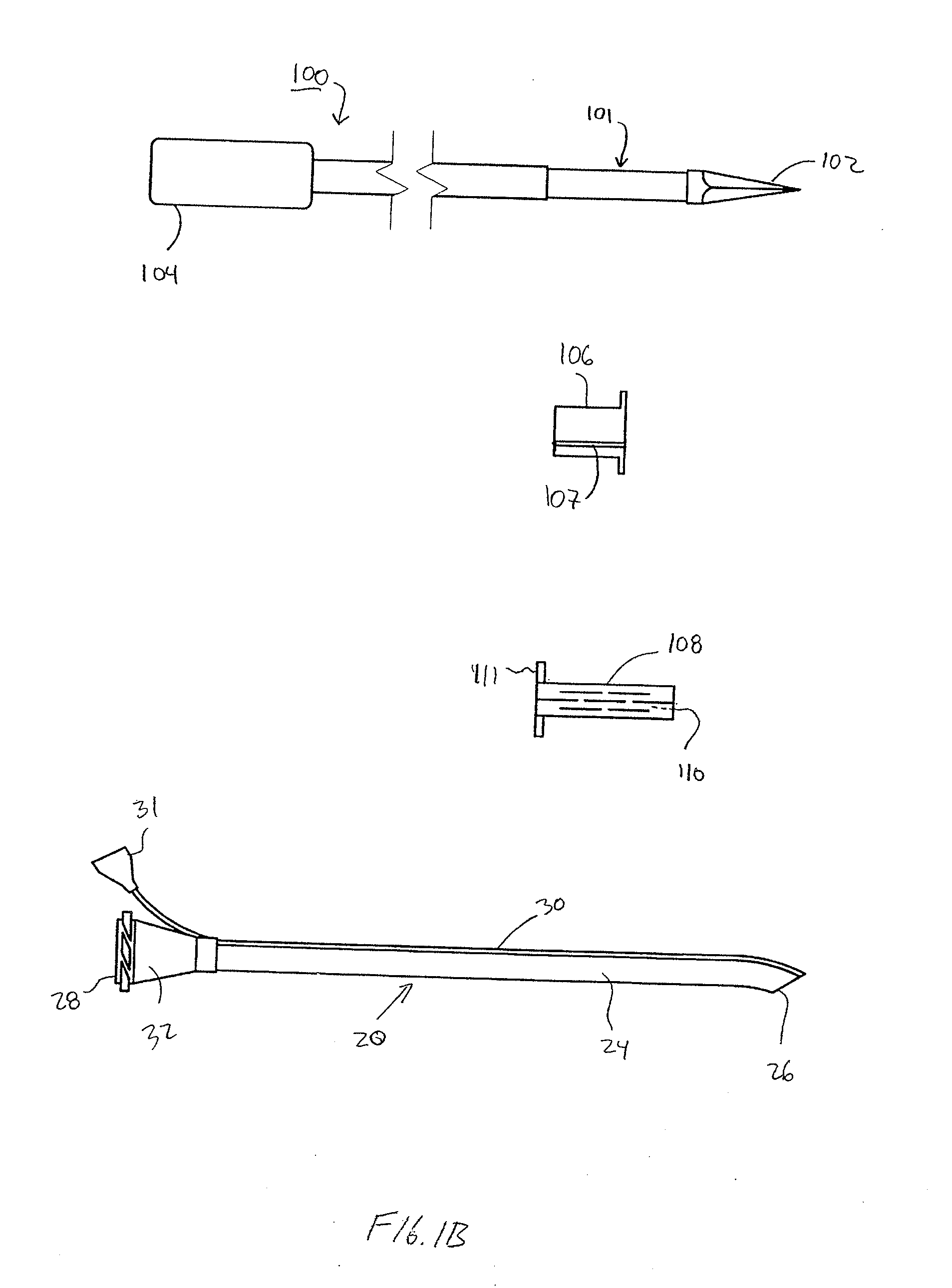
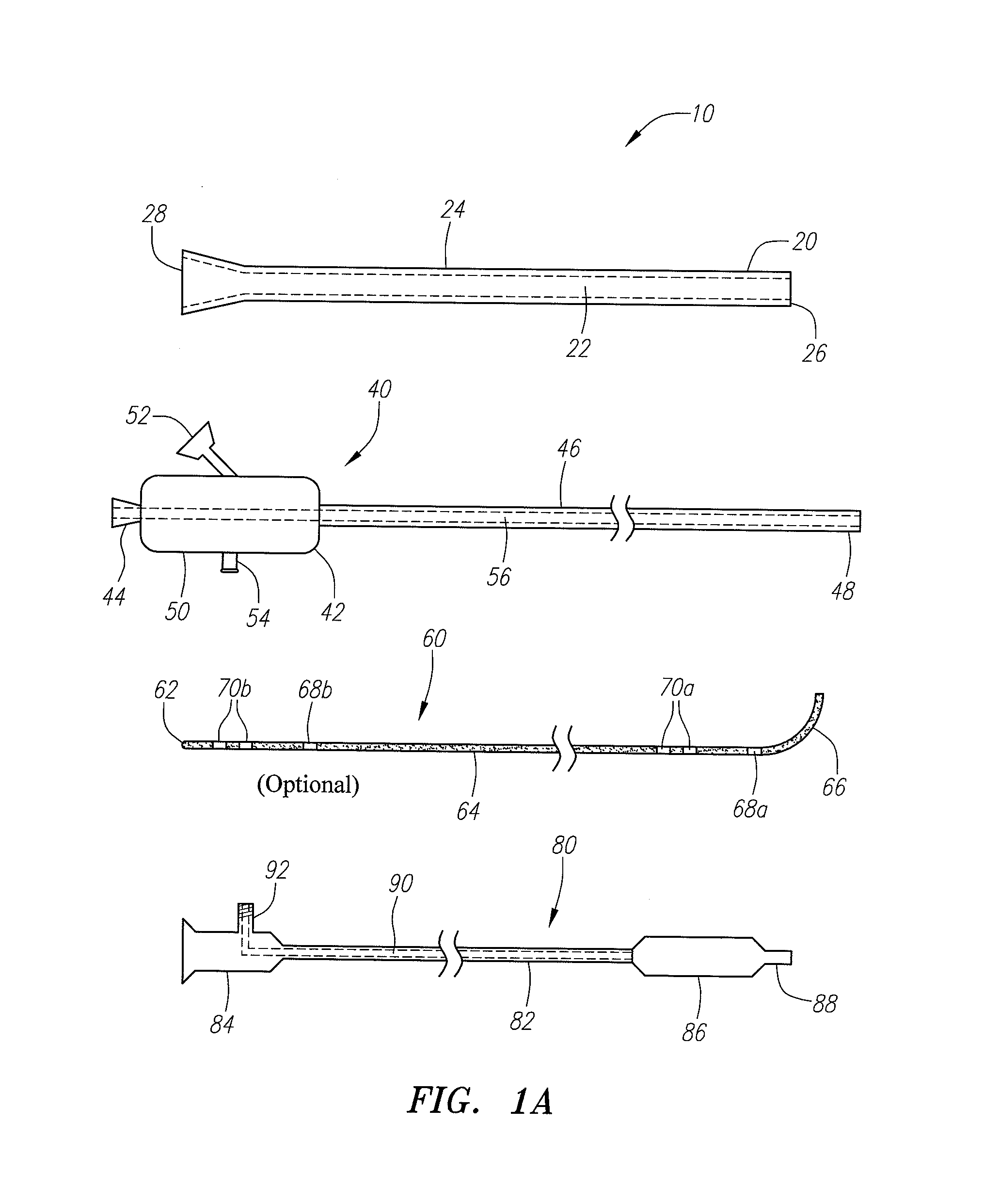
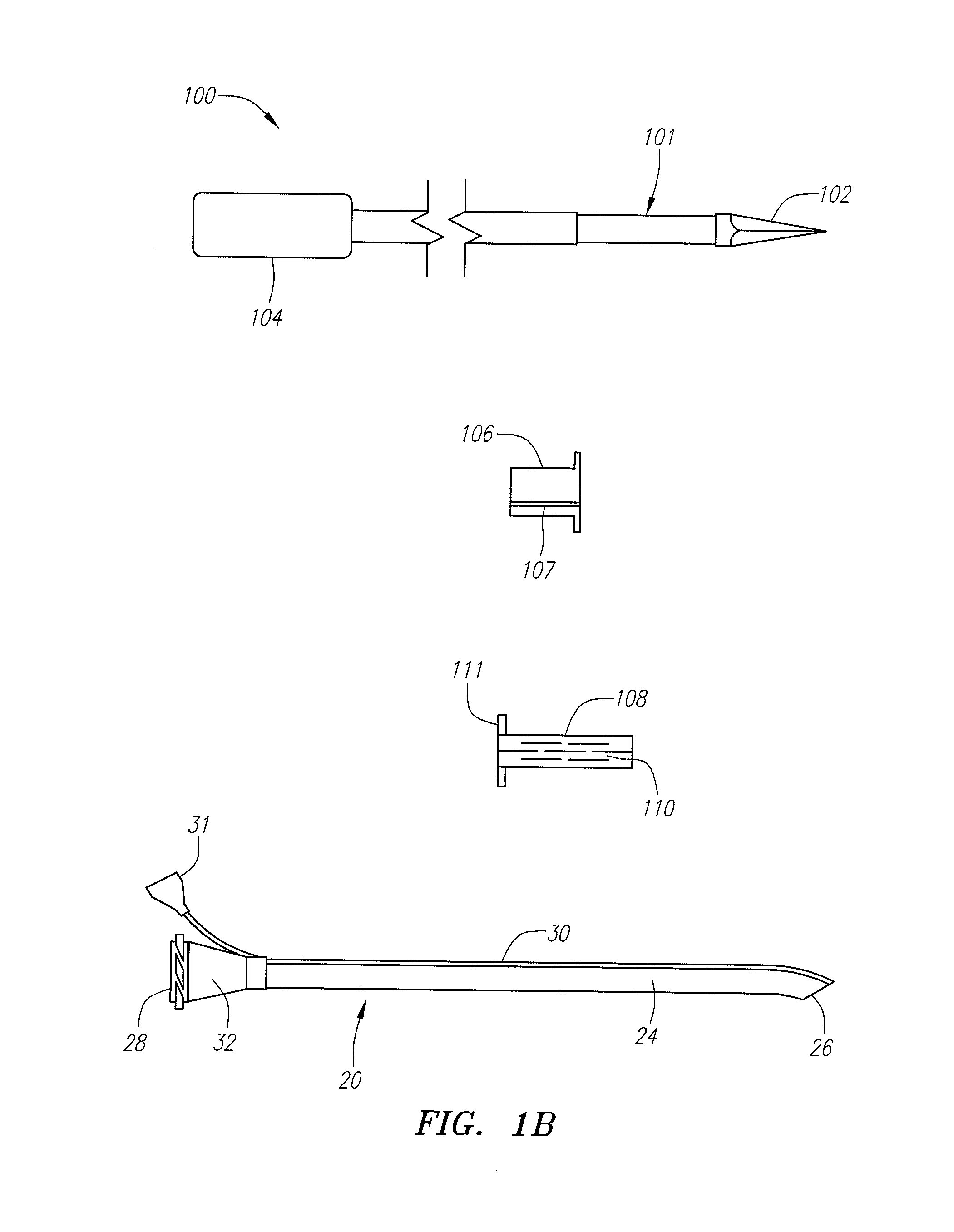
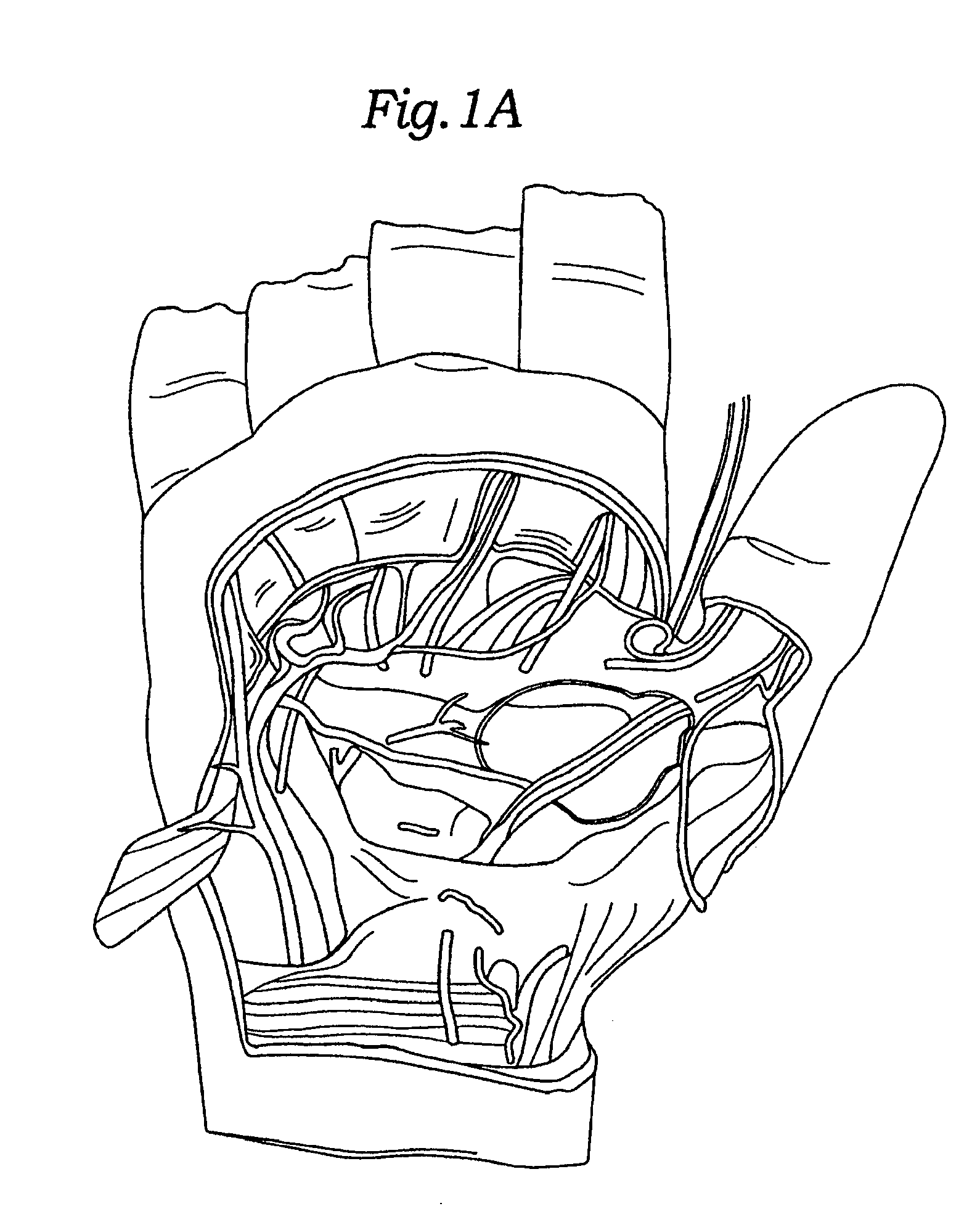
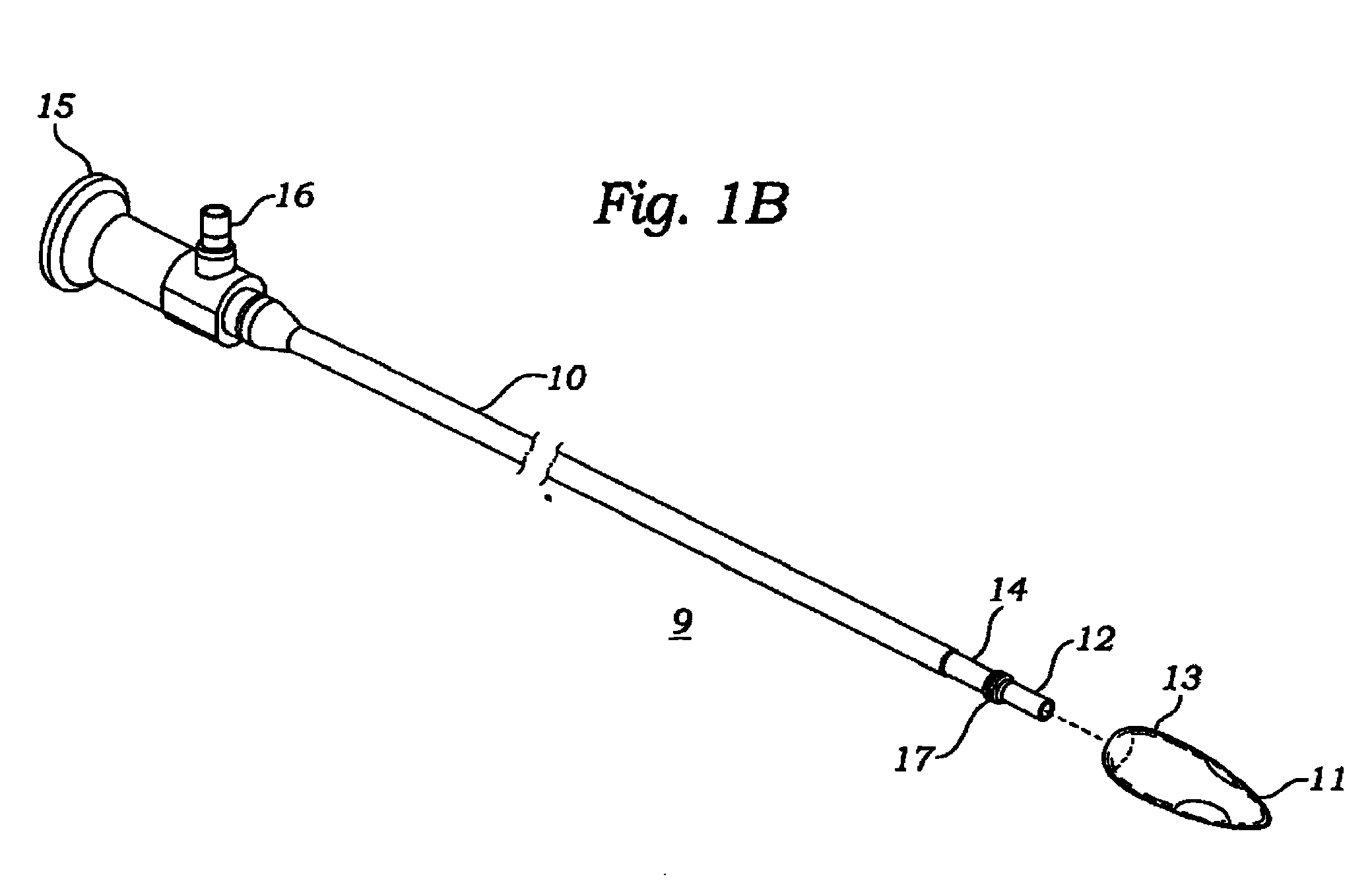
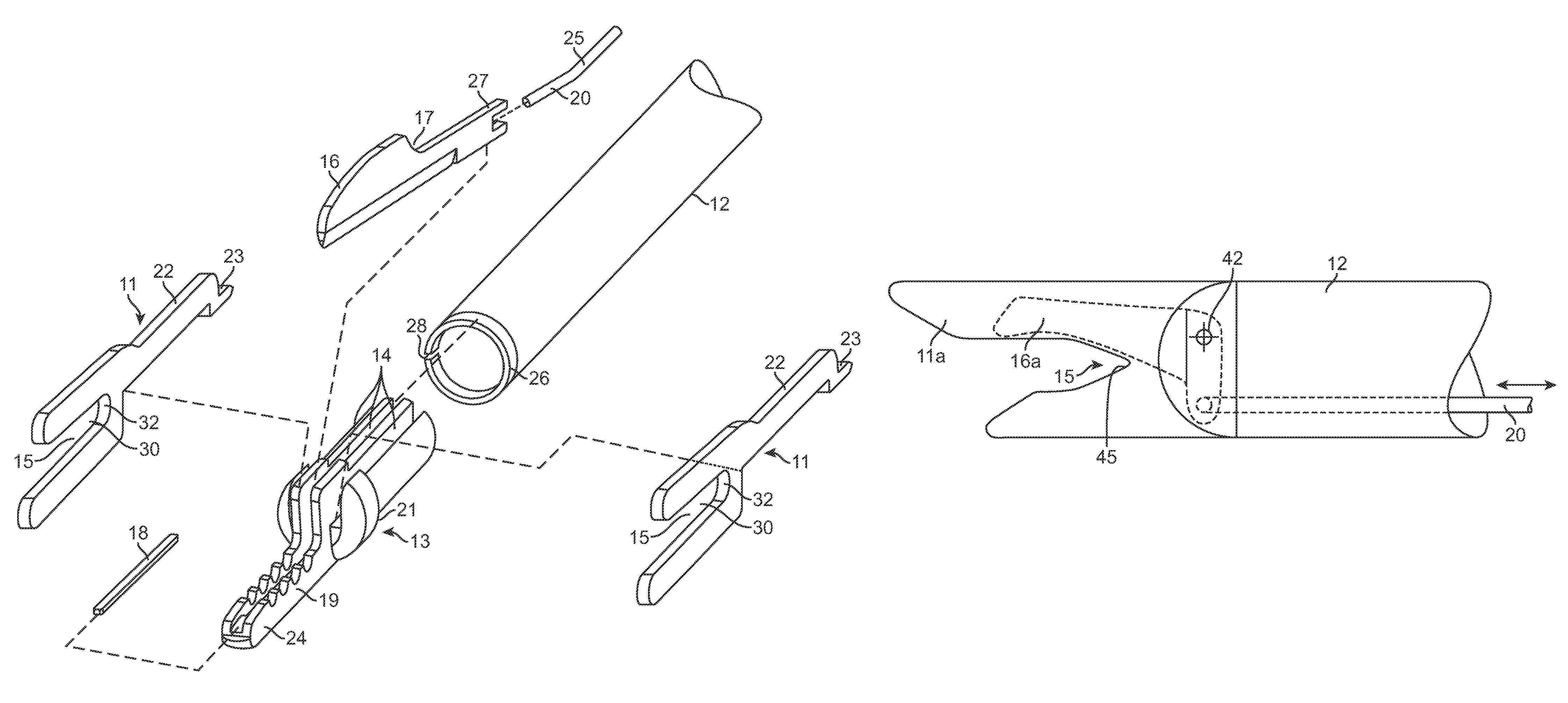
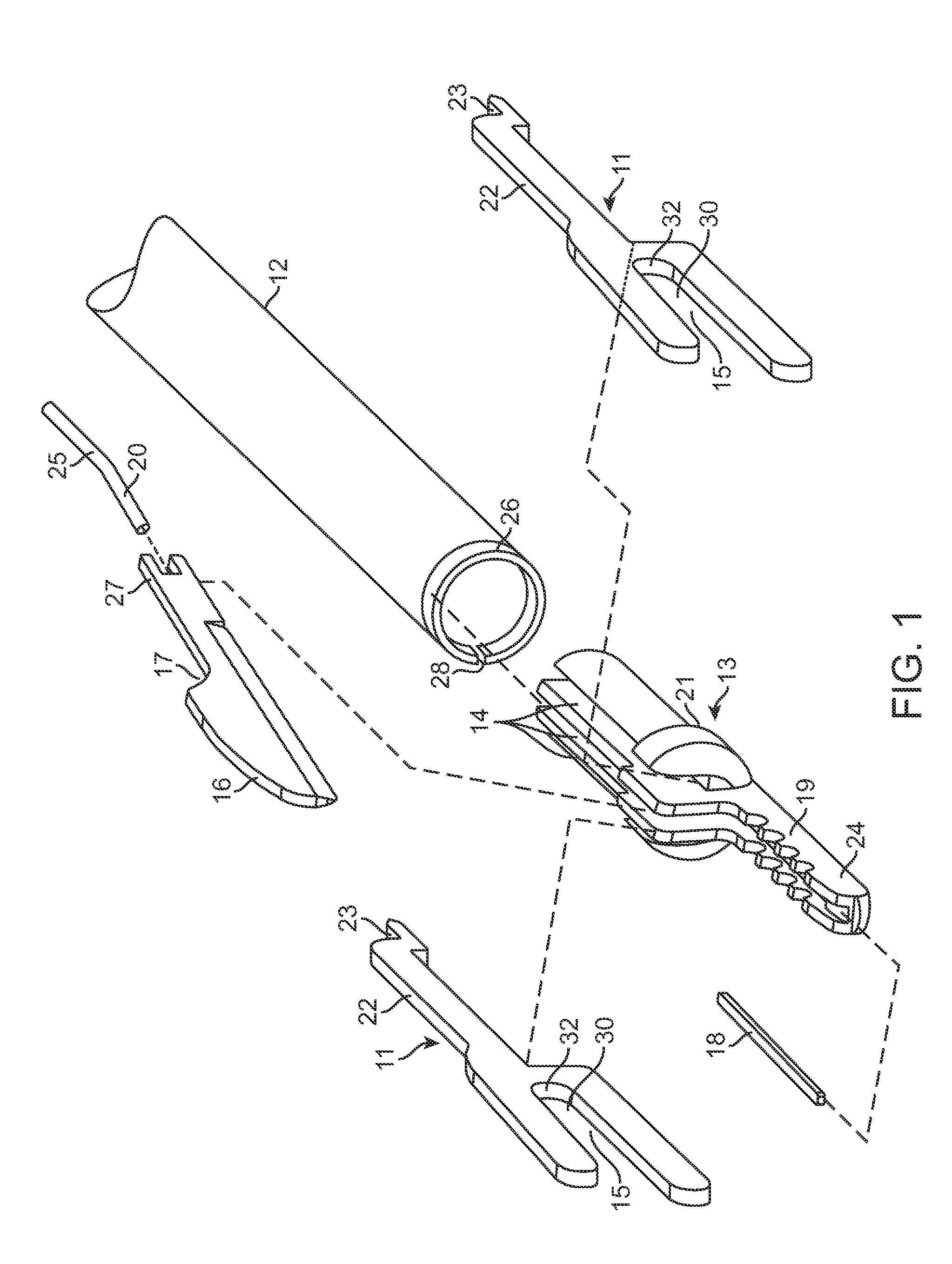
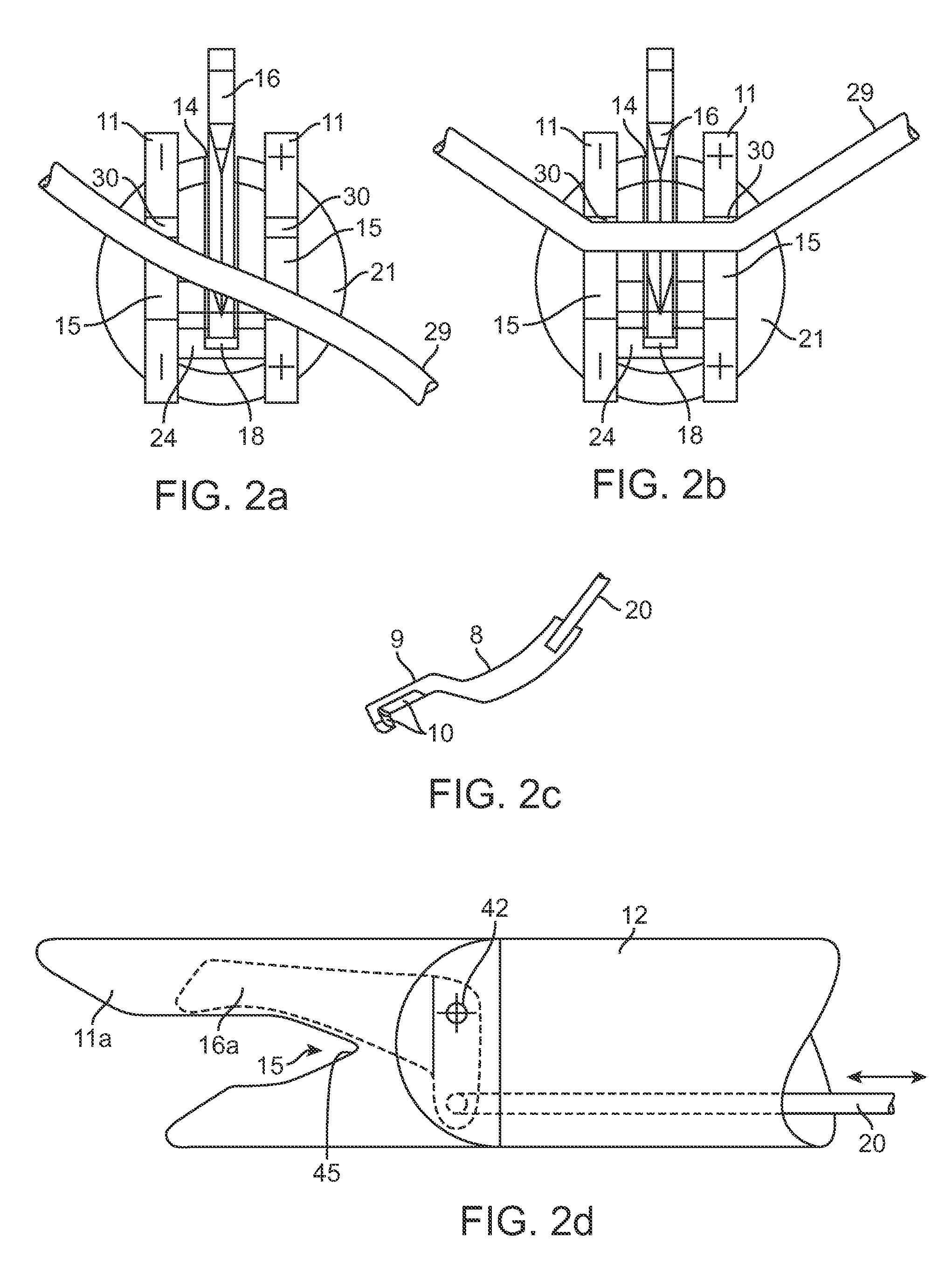
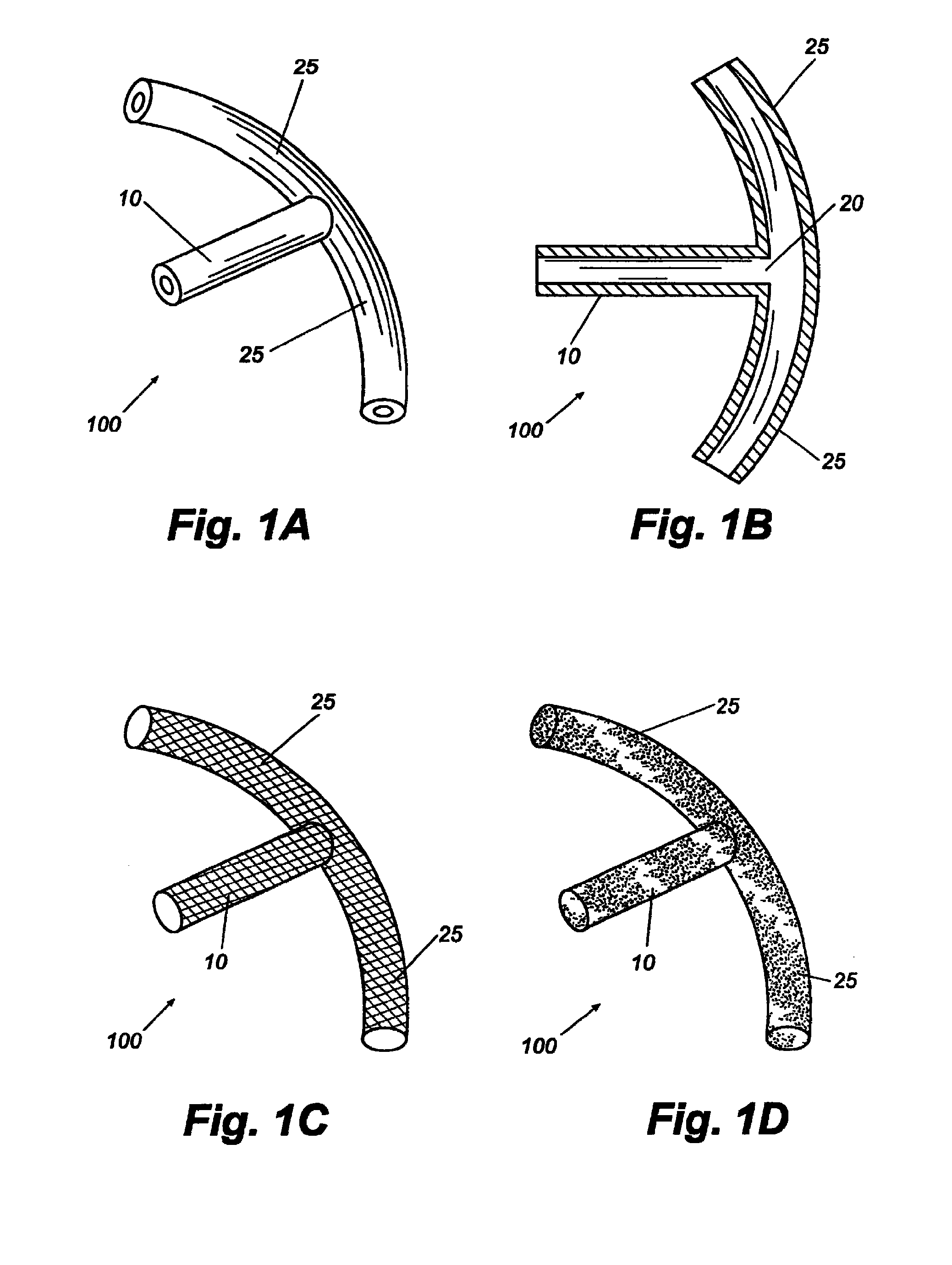
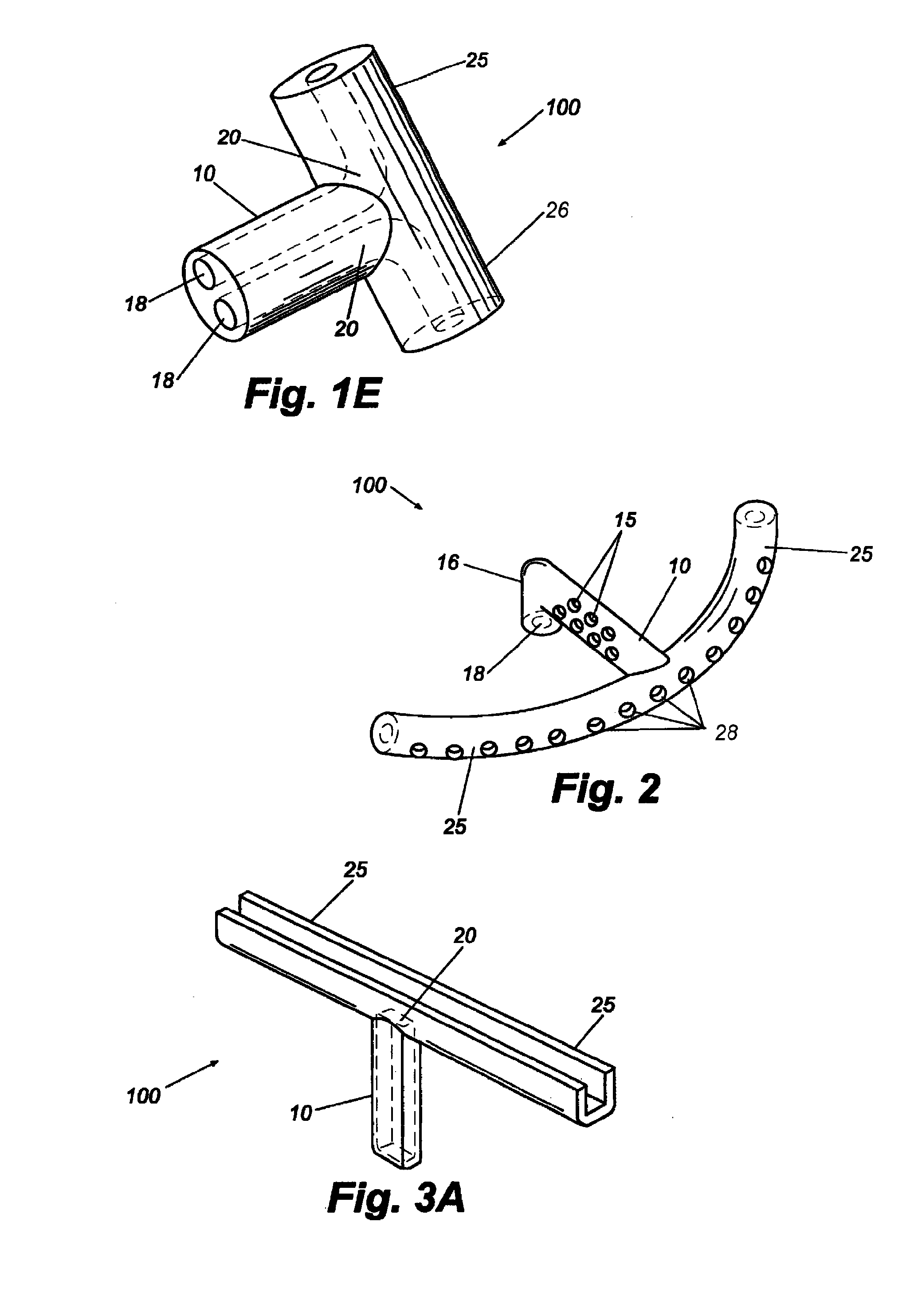
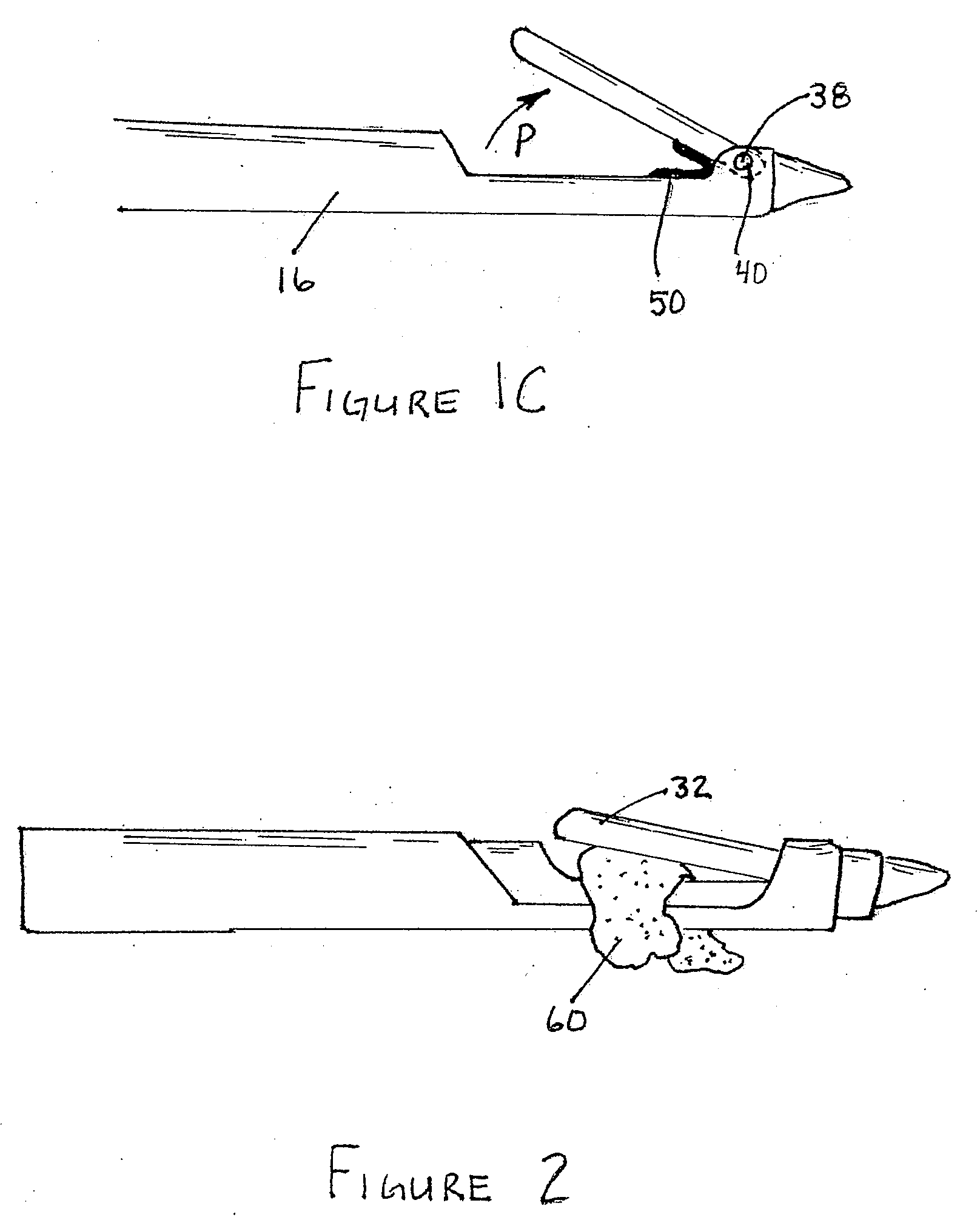
Reverse sealing and dissection instrument
ActiveUS8308725B2Facilitate tissue removalImprove abilitiesSurgical scissorsSurgical instruments for coolingPERITONEOSCOPESurgical department
The invention relates to a device and related method for sealing and / or dissecting body tissue using a reverse action instrument. The devices and methods described permit laparoscopic or natural body orifice access to an anatomical space and facilitate sealing portions of tissue together, dissecting tissue or combinations thereof. A surgical instrument for sealing and dissecting body tissue is described having distal and proximal ends with an elongated body. The body includes a jaw member positioned at the distal end that is defined by a stationary arm and a pivotable arm. A moveable closure sleeve is disposed at least partially about the body and the closure sleeve is configured such that coaxial movement of the sleeve along the longitudinal axis of the body causes the jaw member to open or close.
Owner:TENNESSEE MEDICAL INNOVATIONS INC
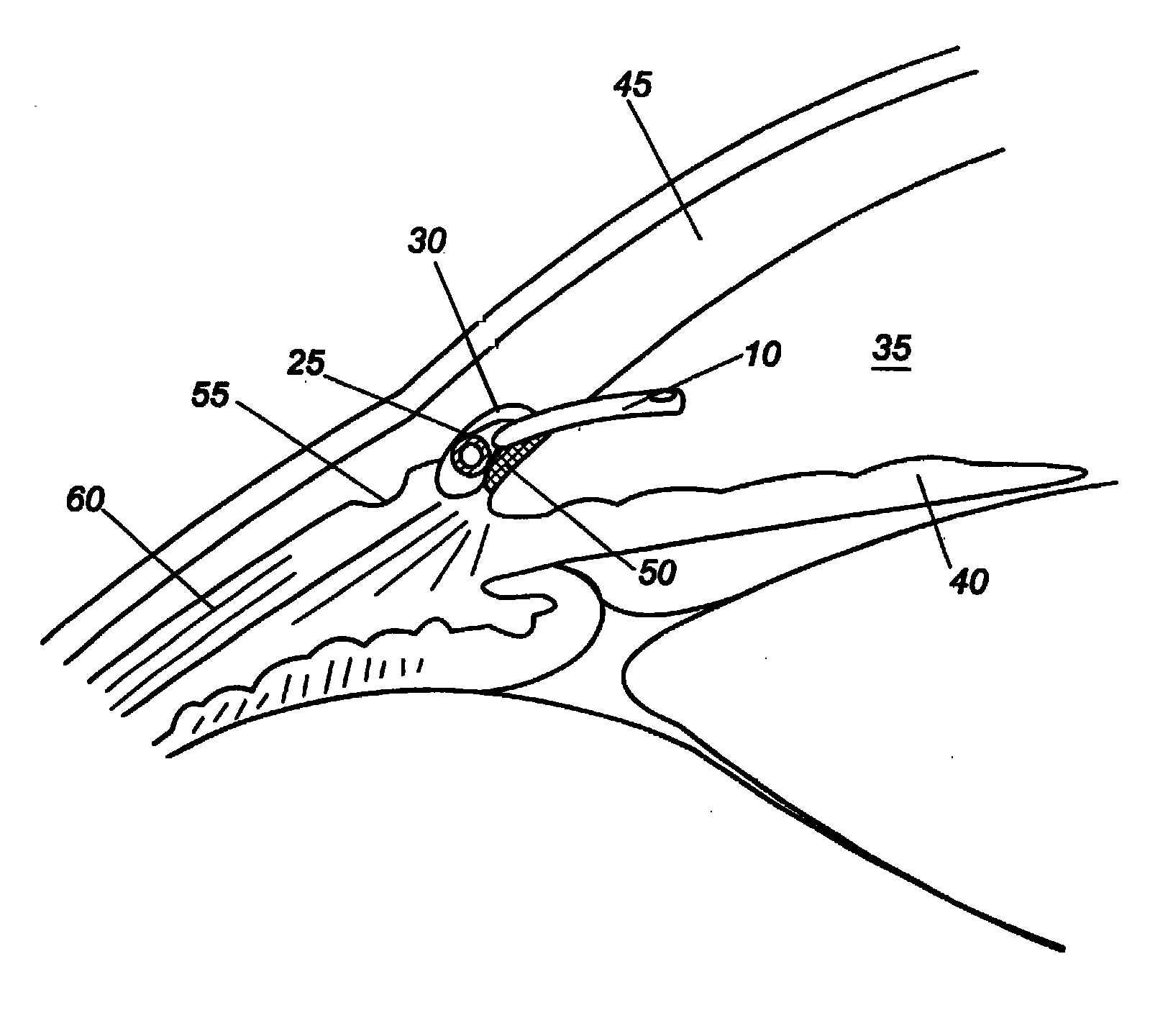
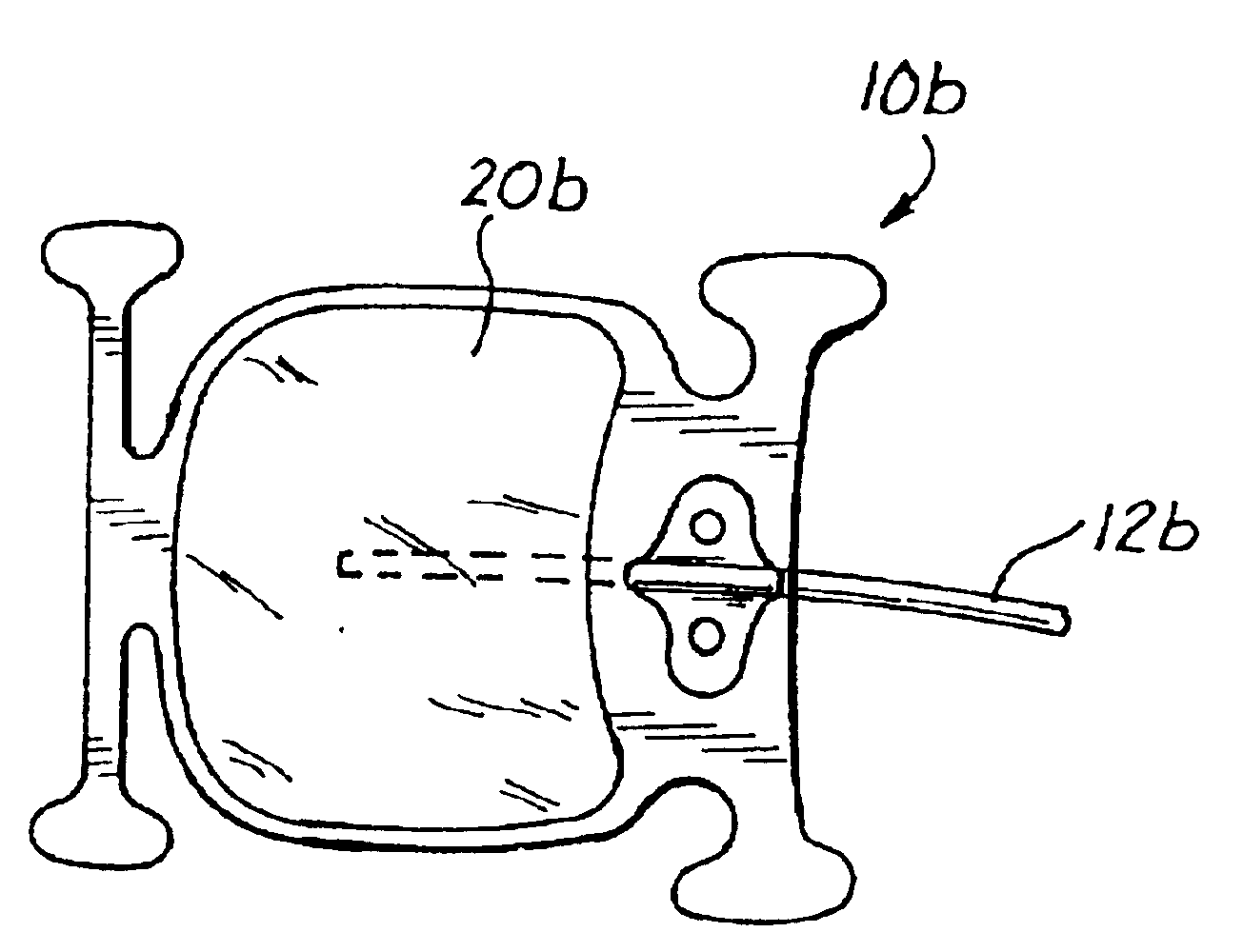
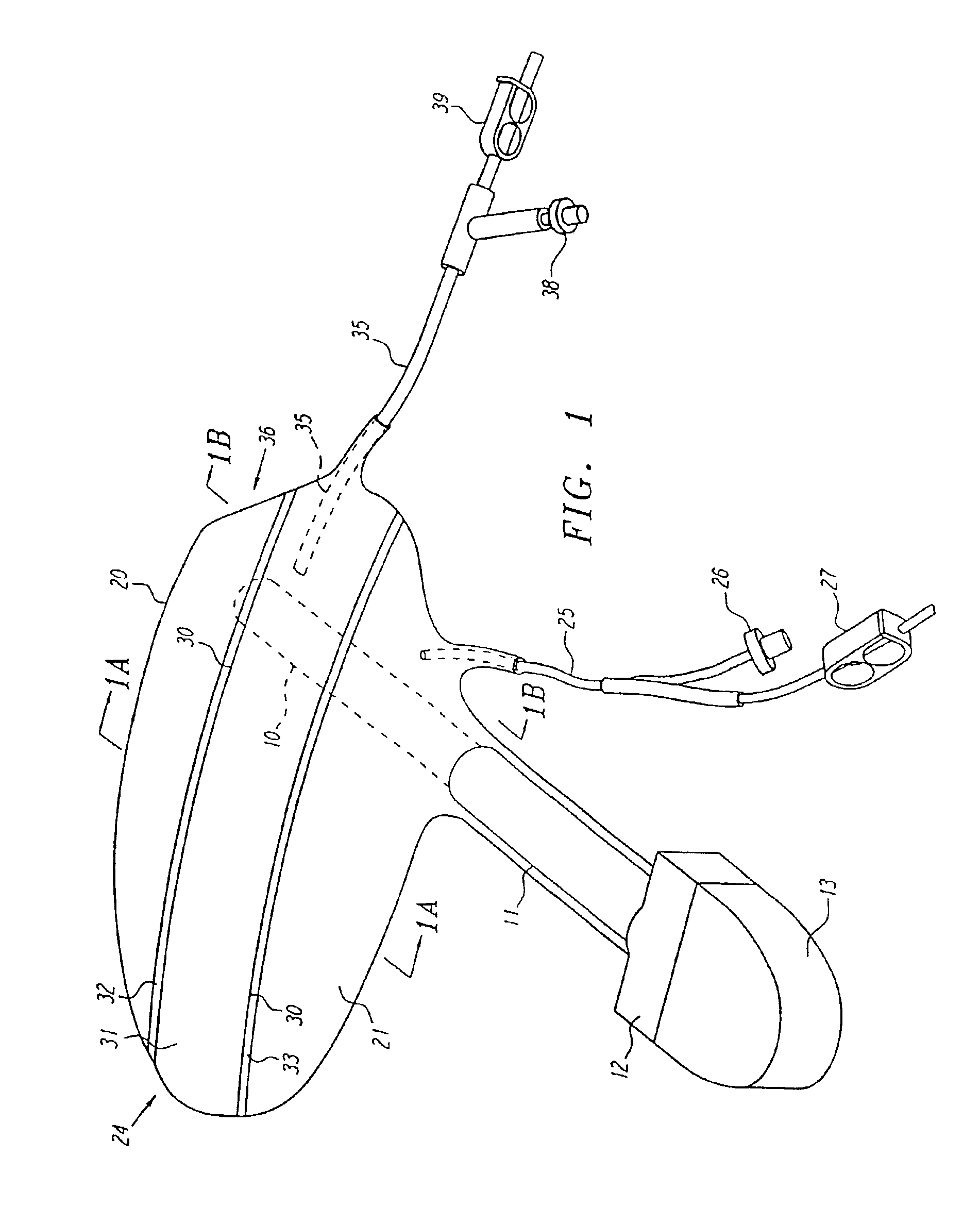
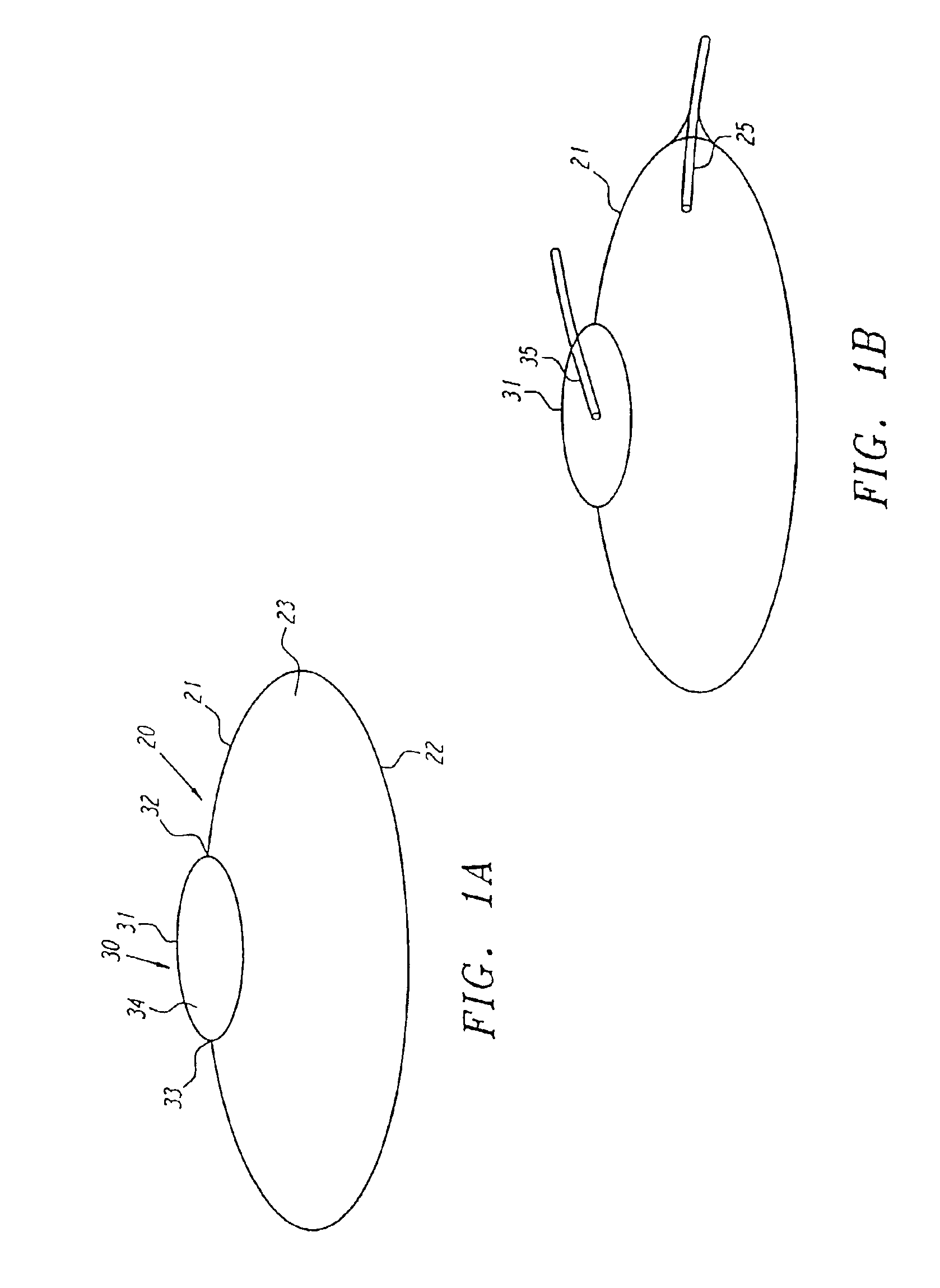
Anatomical space access tools and methods
InactiveUS6890295B2Robust fixationEasy accessCannulasSurgical needlesPericardial spaceMedical device
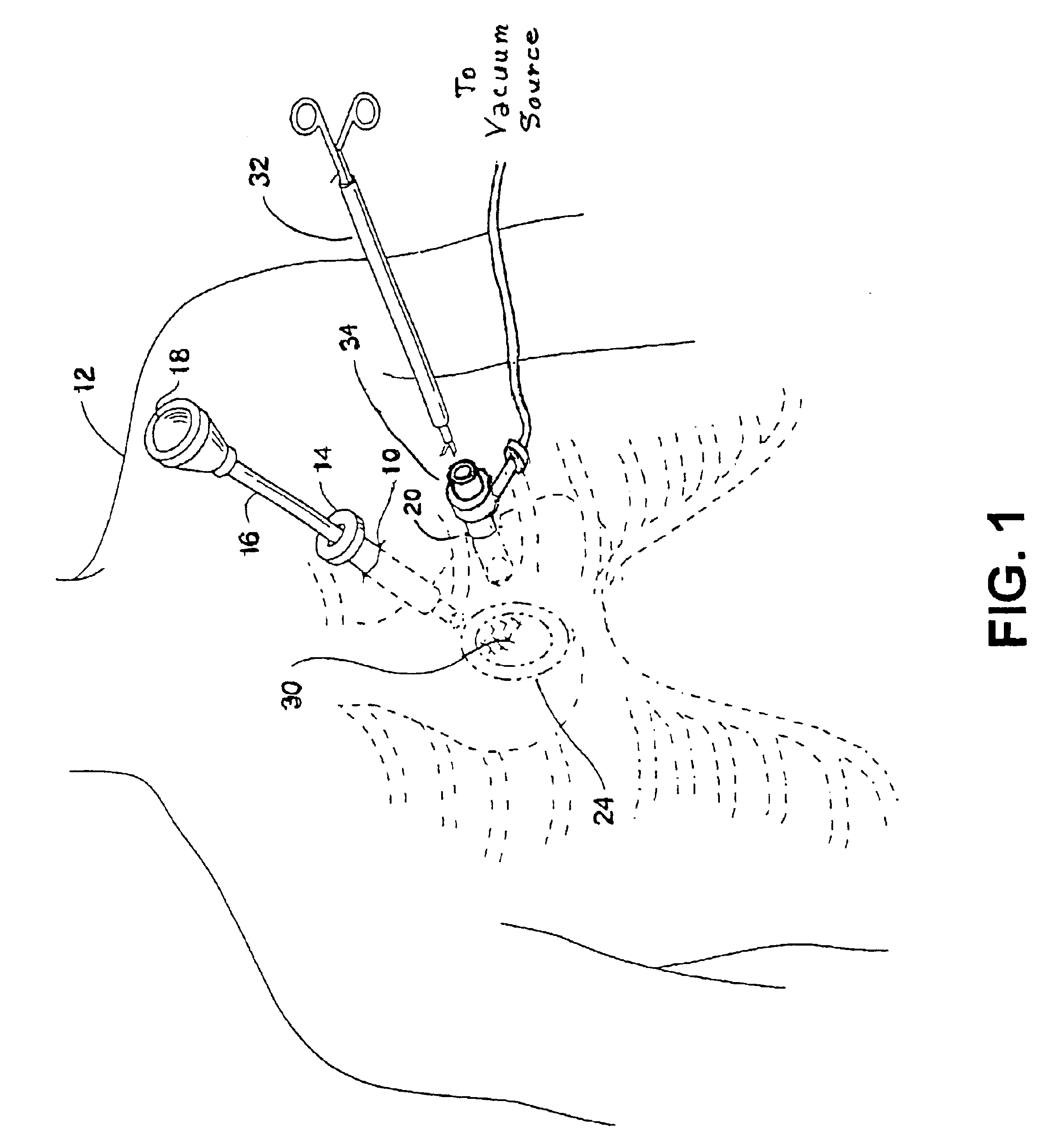
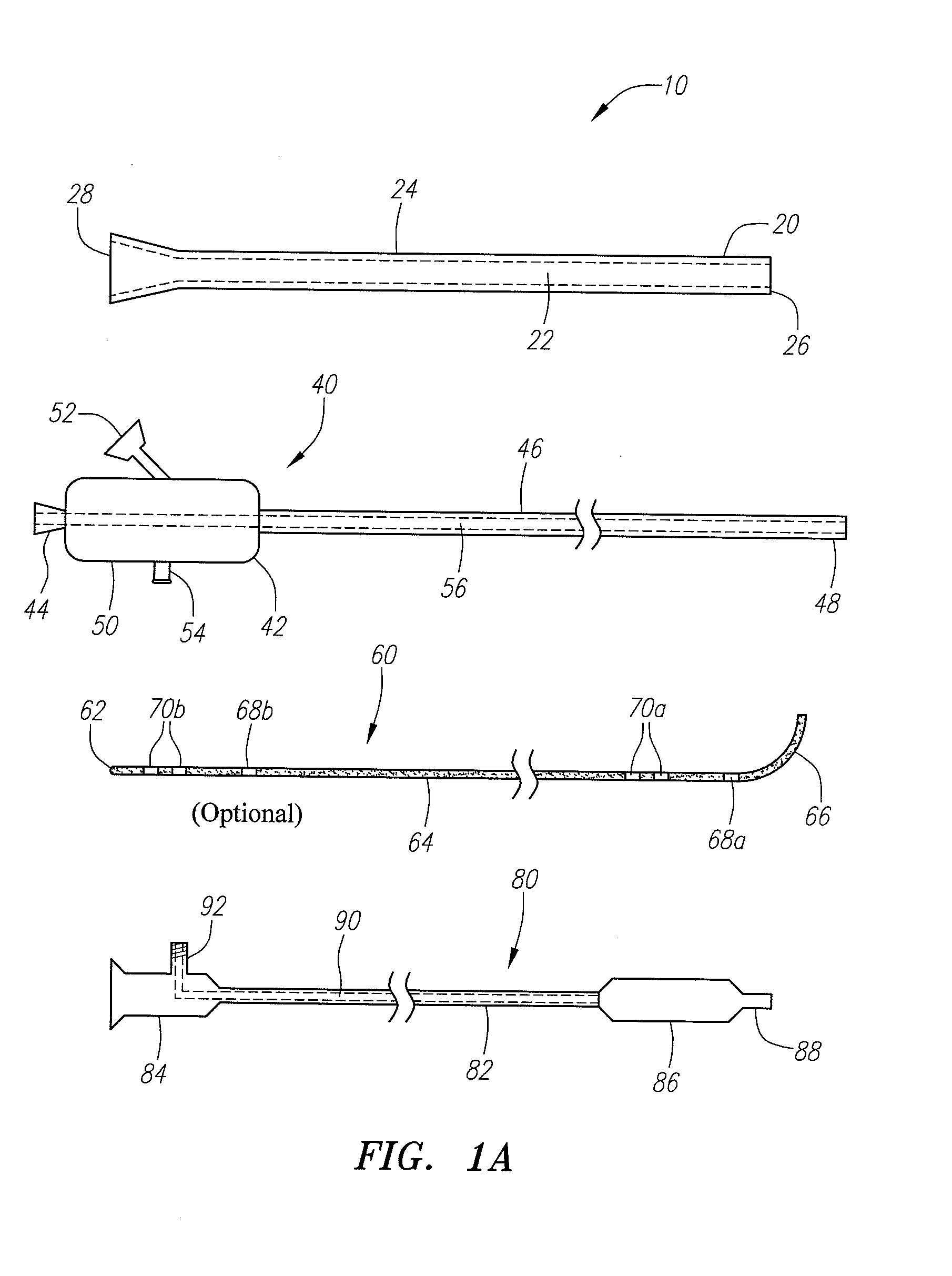
Medical devices and methods for accessing an anatomical space of the body and particularly for penetrating the epicardium to access pericardial space and the epicardial surface of the heart in a minimally invasive manner employing suction are disclosed. The distal end of a tubular access sleeve having a sleeve wall surrounding a sleeve access lumen and extending between a sleeve proximal end and a sleeve distal end having a plurality of suction ports arrayed around the sleeve access lumen distal end opening is applied against an outer tissue layer. Suction is applied through the plurality of suction ports to a plurality of portions of the outer tissue layer. A perforation instrument is introduced through the sleeve access lumen to perforate the outer tissue layer to form an access perforation into the anatomic space while the applied suction stabilizes the outer tissue layer, whereby further treatment drugs and devices can be introduced into the anatomic space.
Owner:MEDTRONIC INC
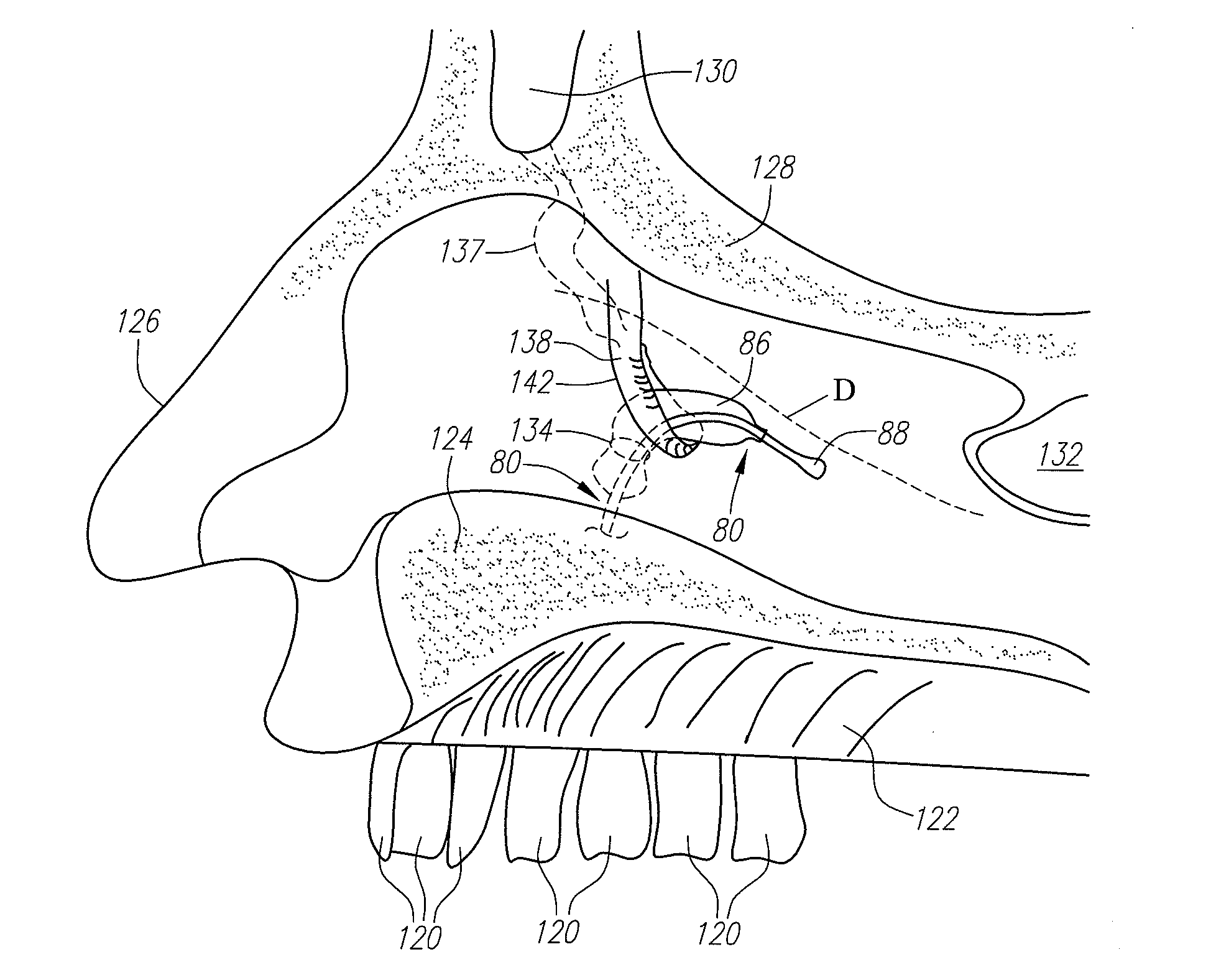
Apparatus and method for treatment of sinusitis
A method of treating a constricted sinus passageway of a patient includes traversing the canine fossa region of the patient so as to form a passageway in the sinus cavity. A cannula is positioned in the passageway. A visualization tool such as an endoscope is passed through a lumen or channel in the cannula to aid in visualization of the anatomical site of interest. A balloon dilation catheter is then deployed through or along the cannula so as to place the balloon within or across the constricted anatomical space (e.g., ostium). The balloon is then expanded so as to expand at least a portion of the constricted anatomical space. Alternative embodiments include the use of an optional guide wire and incorporating a endoscope lumen through the balloon dilation catheter.
Owner:ENTELLUS MEDICAL
Endoscopic surgical access port and method
InactiveUS7276075B1Easy to blowEliminate needCannulasSurgical needlesSurgical siteSurgical department
A sliding gas-tight seal on an access port promotes insufflation of an anatomical space formed in tissue at a surgical site only during insertion of an endoscopic instrument through the access port into the anatomical space, and promotes deflation of the inflated space upon removal of the endoscopic instrument from within the access port. An inflatable balloon disposed about the port near the distal end may be selectively expanded to seal and anchor the access port within an incision through which a surgical procedure with insufflation is to be performed. Multiple resilient seals may be attached to the body of the port, and an auxiliary resilient seal may be inserted within the aperture of a seal attached to the body to accommodate a wide range of endoscopic instruments of various exterior dimensions inserted through the seals.
Owner:MAQUET CARDIOVASCULAR LLC
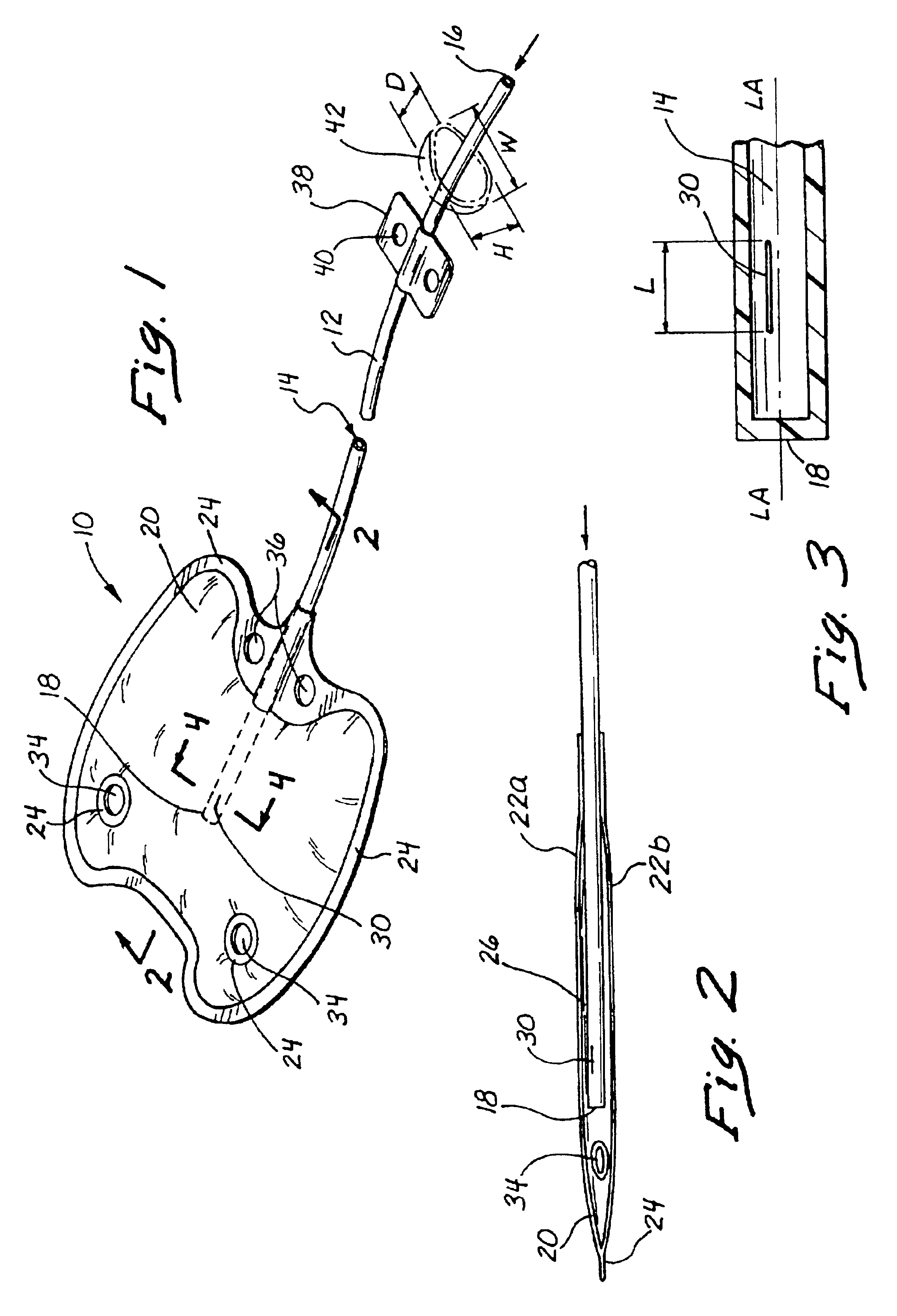
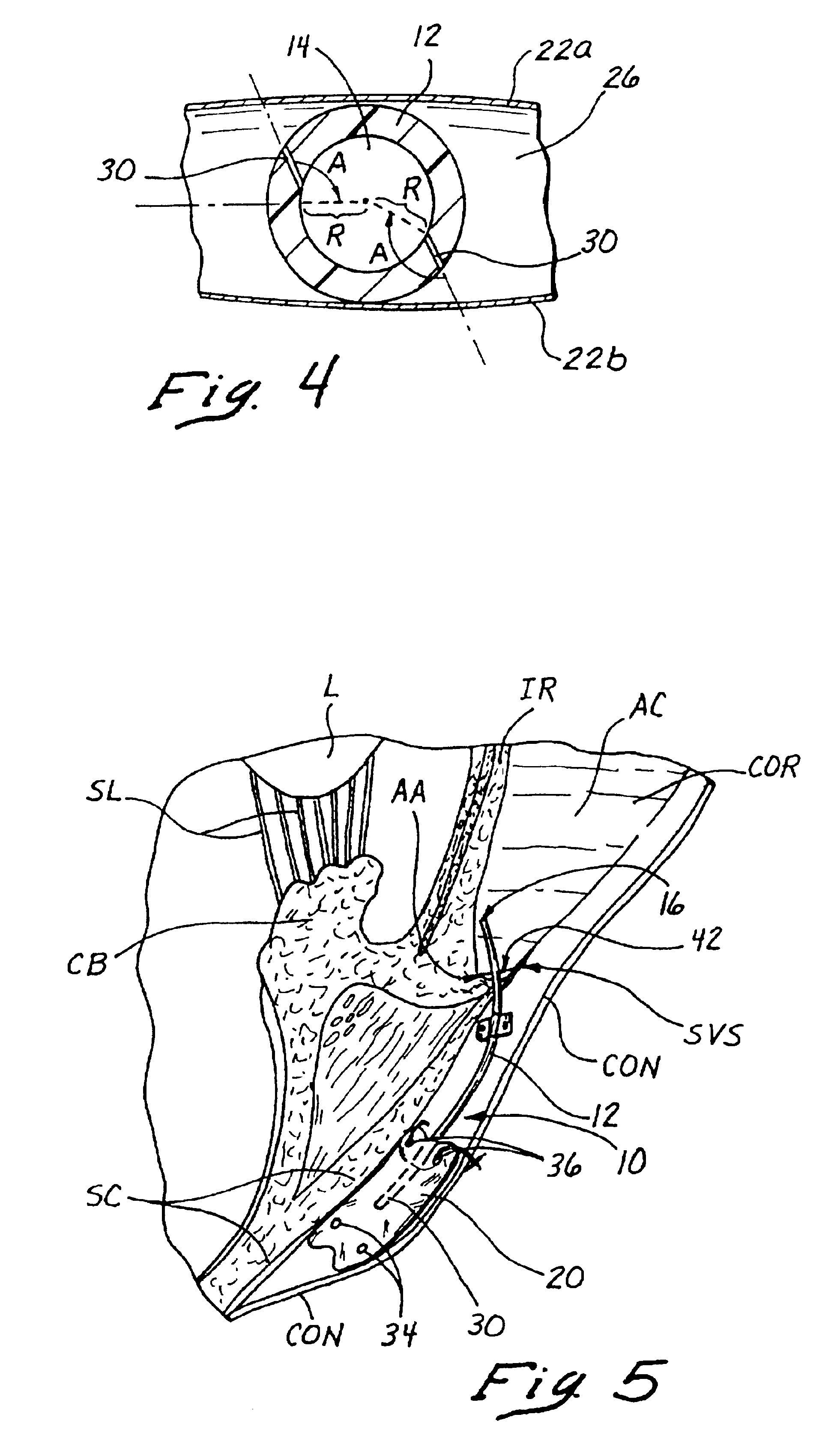
Instruments and methods for accessing an anatomic space
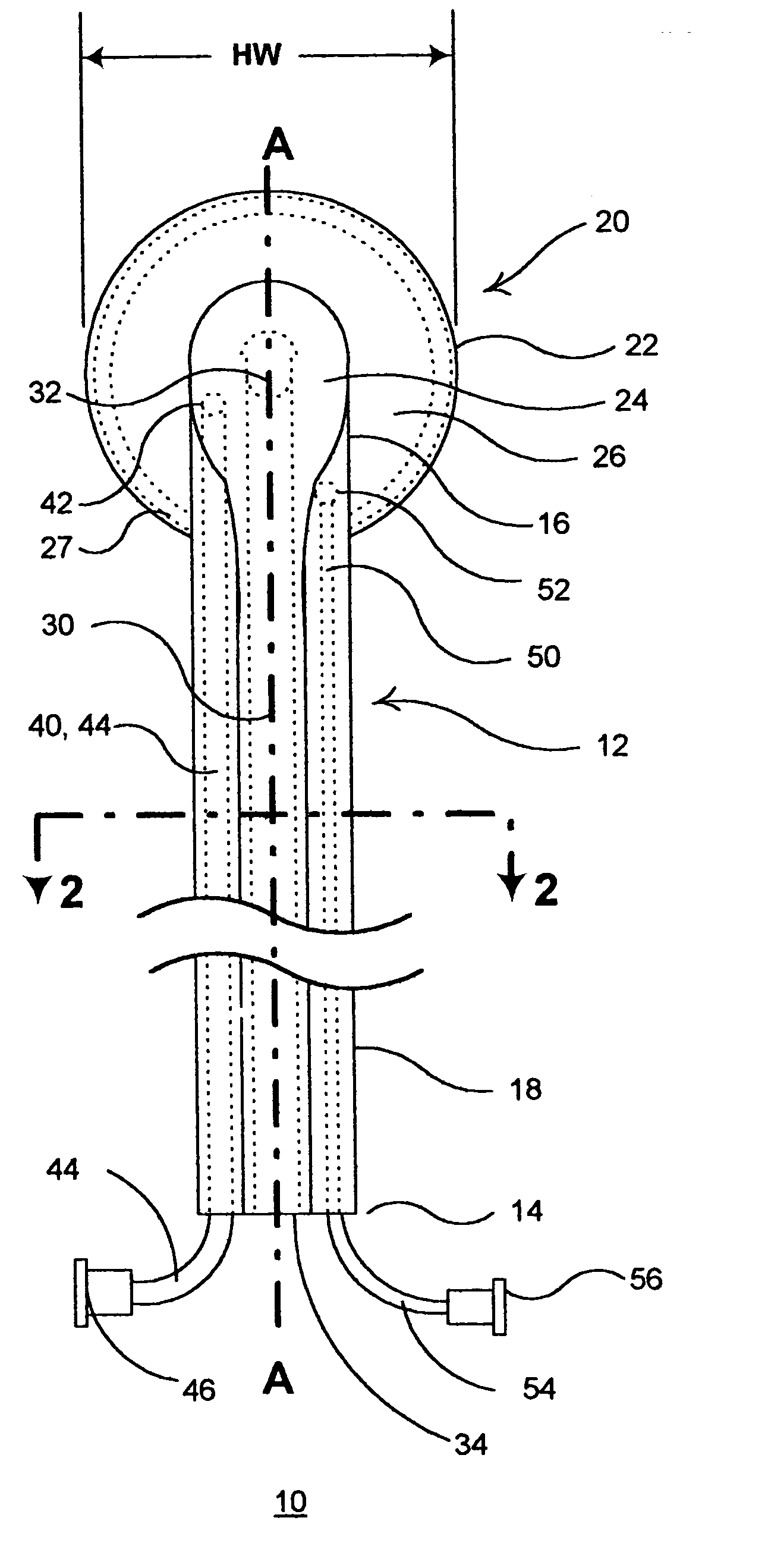
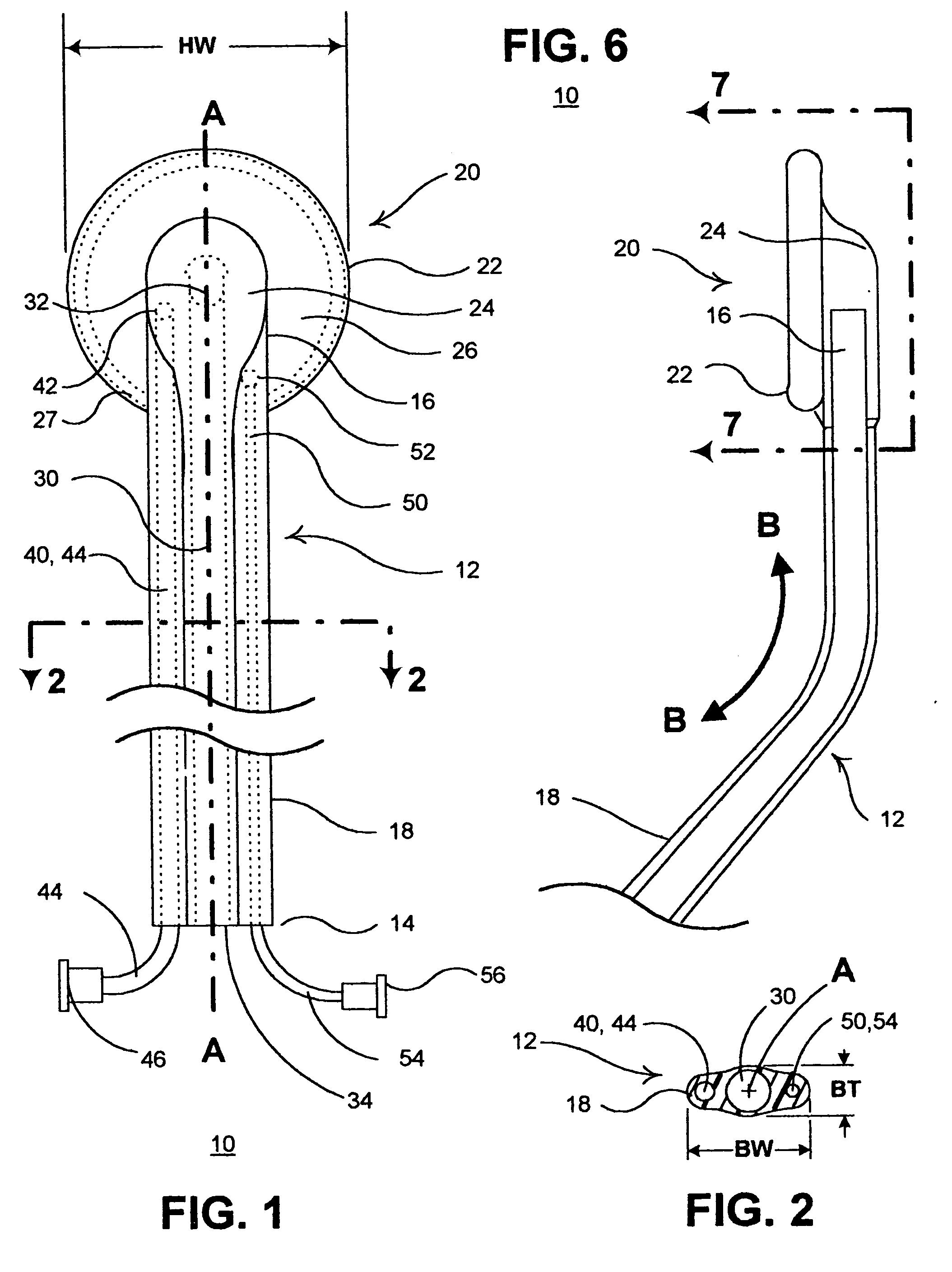
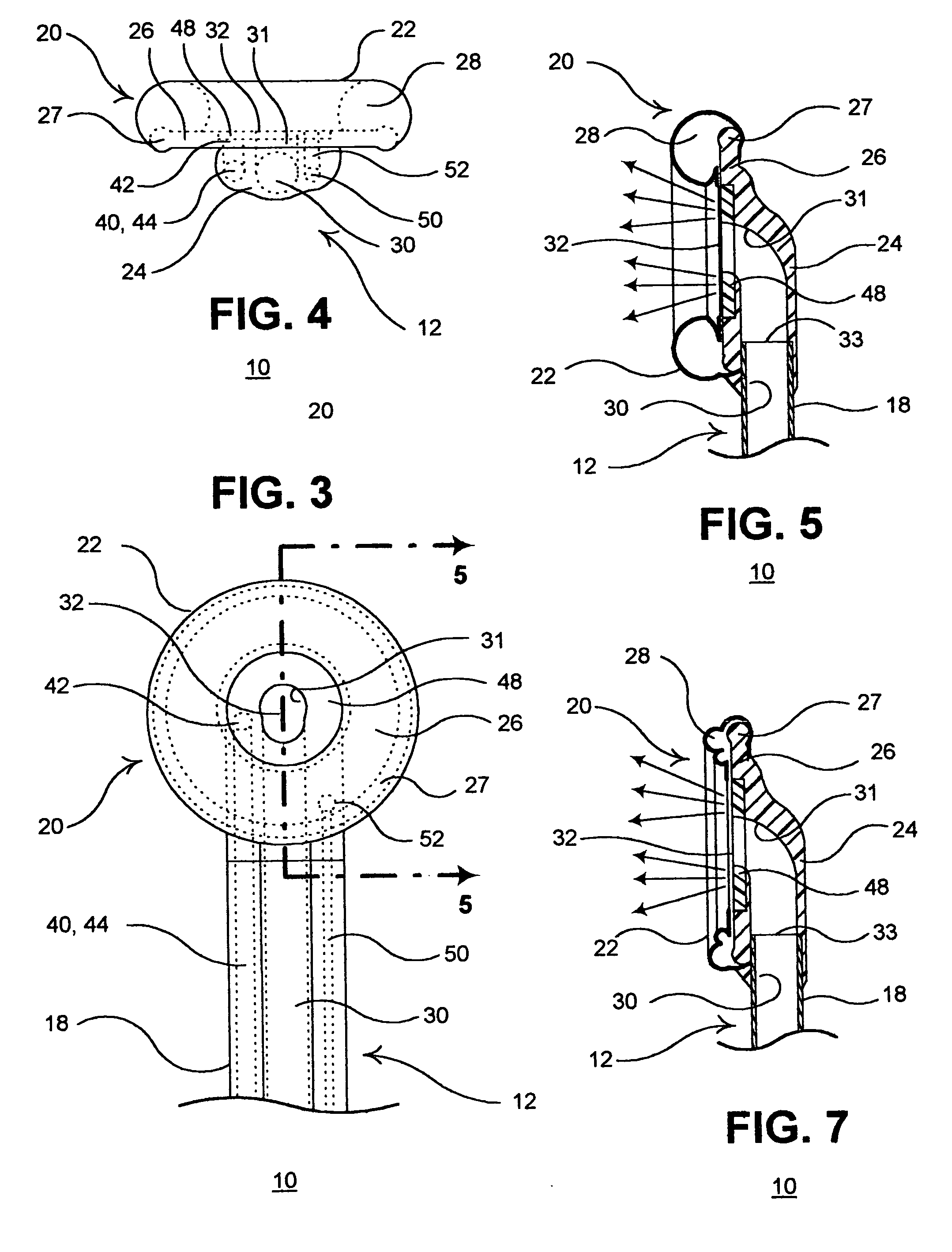
InactiveUS20050182465A1Convenient introductionRigid enoughStentsCannulasPericardial spaceAnatomic Site
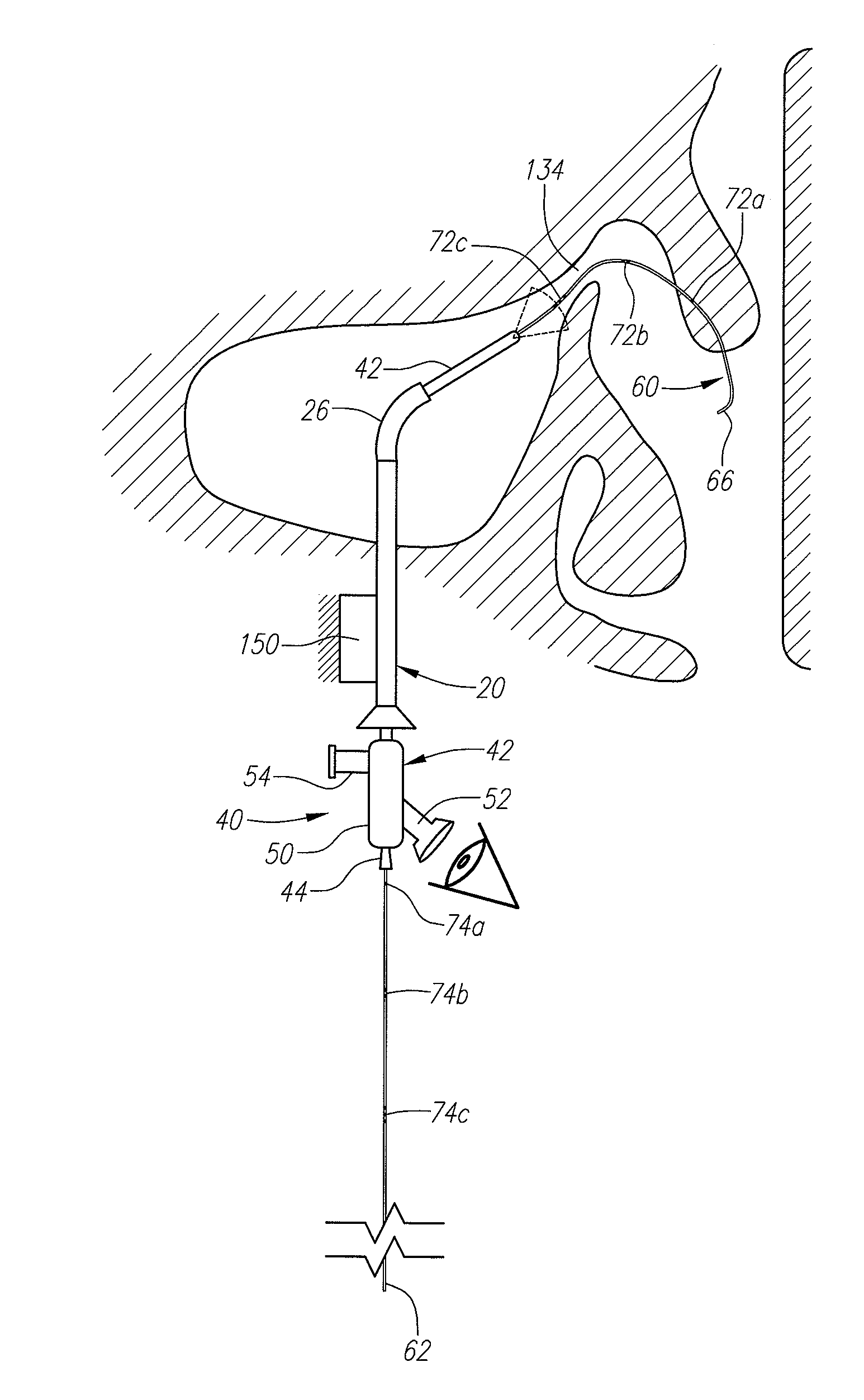
An anatomic space of the body, particularly the pericardial space, is accessed in a minimally invasive manner from a skin incision by an access instrument to facilitate visualization and introduction of devices or drugs or other materials, performance of medical and surgical procedures, and introducing and fixating a cardiac lead electrode to the heart. An elongated access instrument body preferentially bends in one direction and resists bending in a transverse direction, whereby the access instrument body distal end can be directed through a path around body structures to the anatomic site by manipulation of the access instrument body proximal end portion. A distal header formed at the access instrument body distal end extends outward of the access instrument body in the transverse direction and supports an inflatable balloon surrounding a working lumen exit port that is directed toward an anatomic surface when the balloon is inflated by inflation media introduced through an inflation lumen.
Owner:MEDTRONIC INC
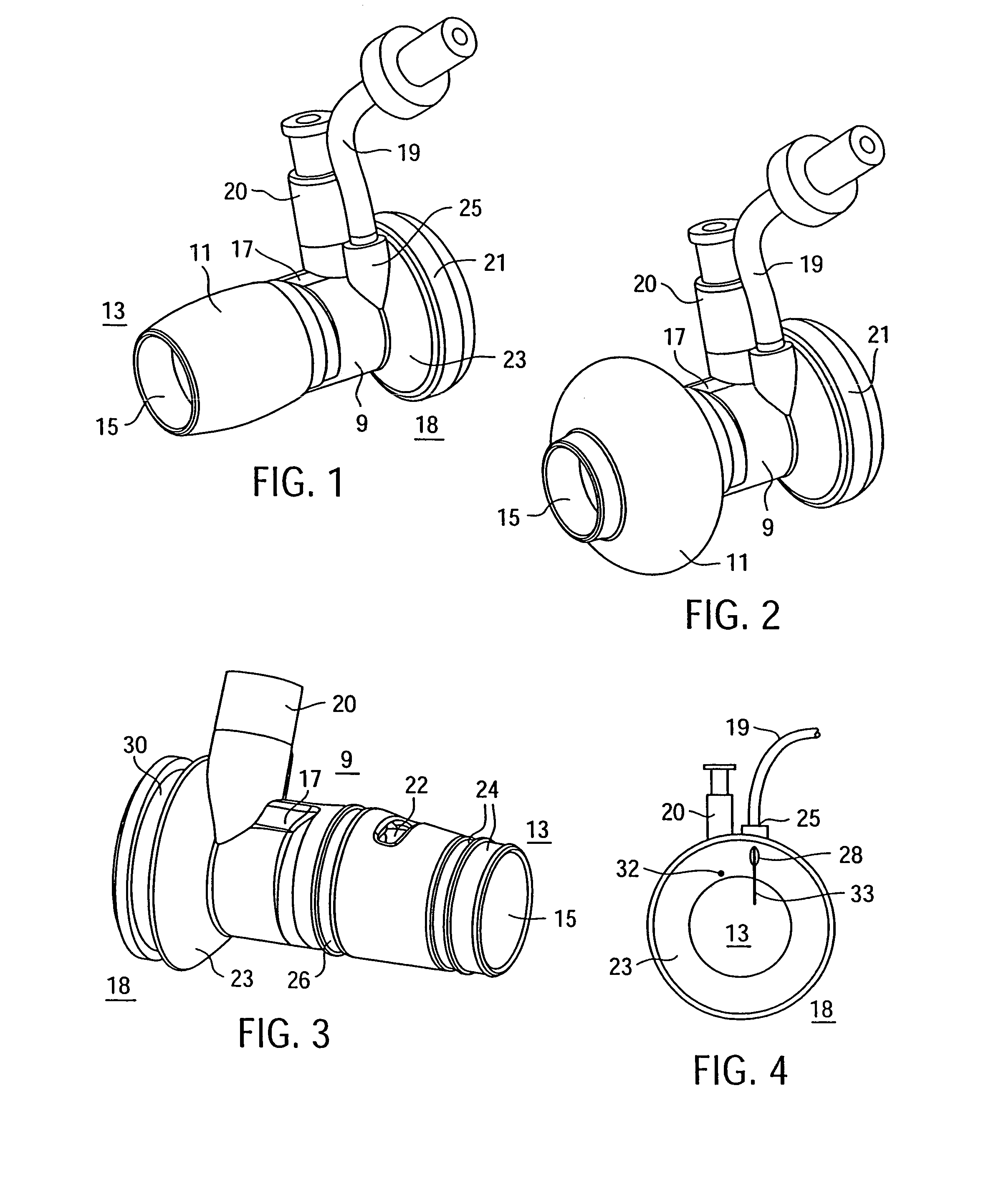
Methods and tools for accessing an anatomic space
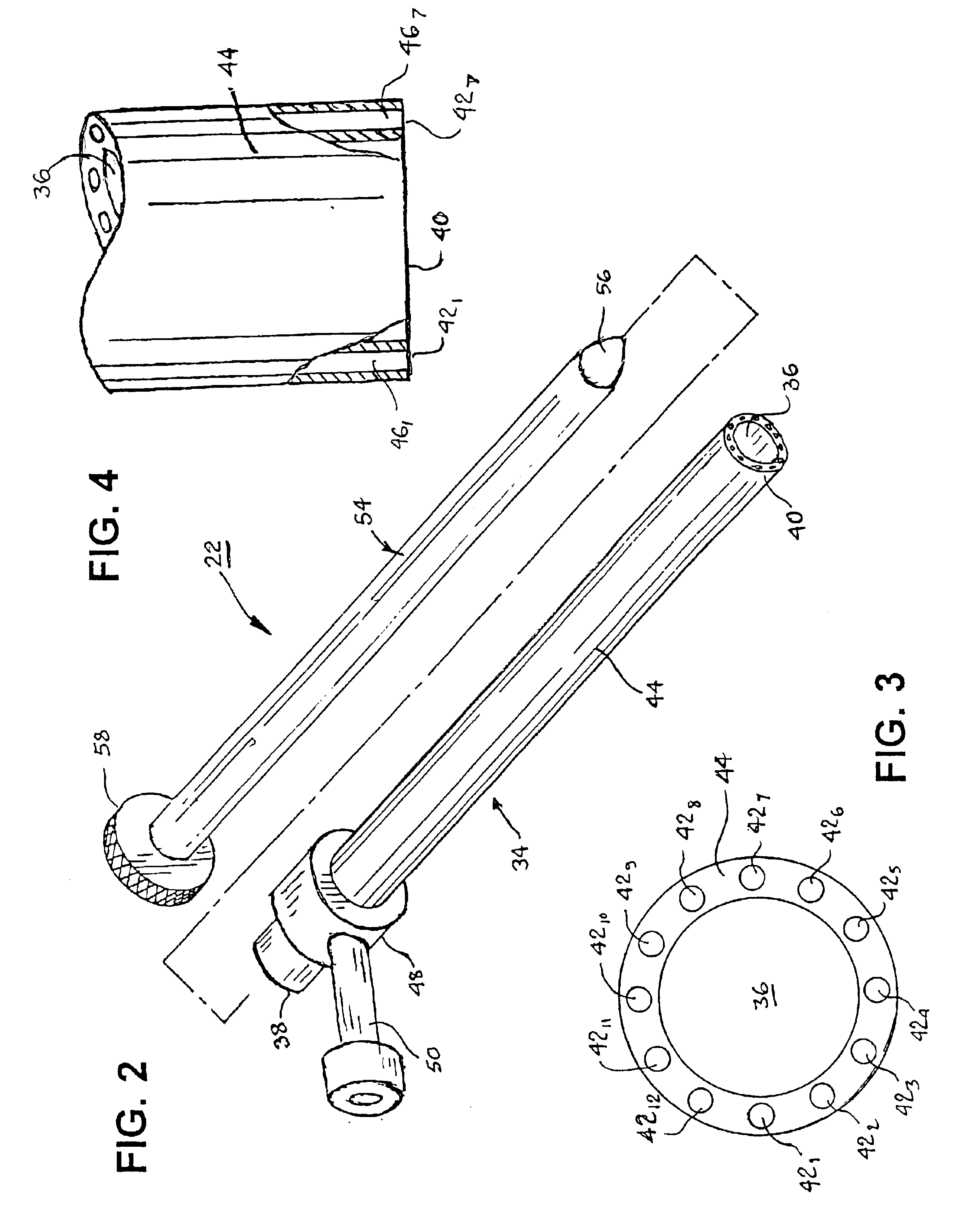
InactiveUS7063693B2Robust fixationReduce layeringEndoscopesSurgical instrument detailsPericardial spaceAnatomic Surface
A tubular access sleeve and suction tool for accessing an anatomic surface or anatomic space and particularly the pericardium to access pericardial space and the epicardial surface of the heart in a minimally invasive manner are disclosed. A suction tool trunk extending through a suction tool lumen of the sleeve is coupled to suction pads at the ends elongated support arms. The suction pads can be retracted into a sleeve working lumen during advancement of the access sleeve through a passage and deployed from the tubular access sleeve lumen and disposed against the an outer tissue layer. Suction can be applied through suction tool lumens to suction ports of the suction pads that fix to the outer tissue layer so as to tension the outer tissue layer and / or pull the outer tissue layer away from an inner tissue layer so that the anatomic space can be accessed by instruments introduced through the working lumen to penetrate the outer tissue layer.
Owner:MEDTRONIC INC
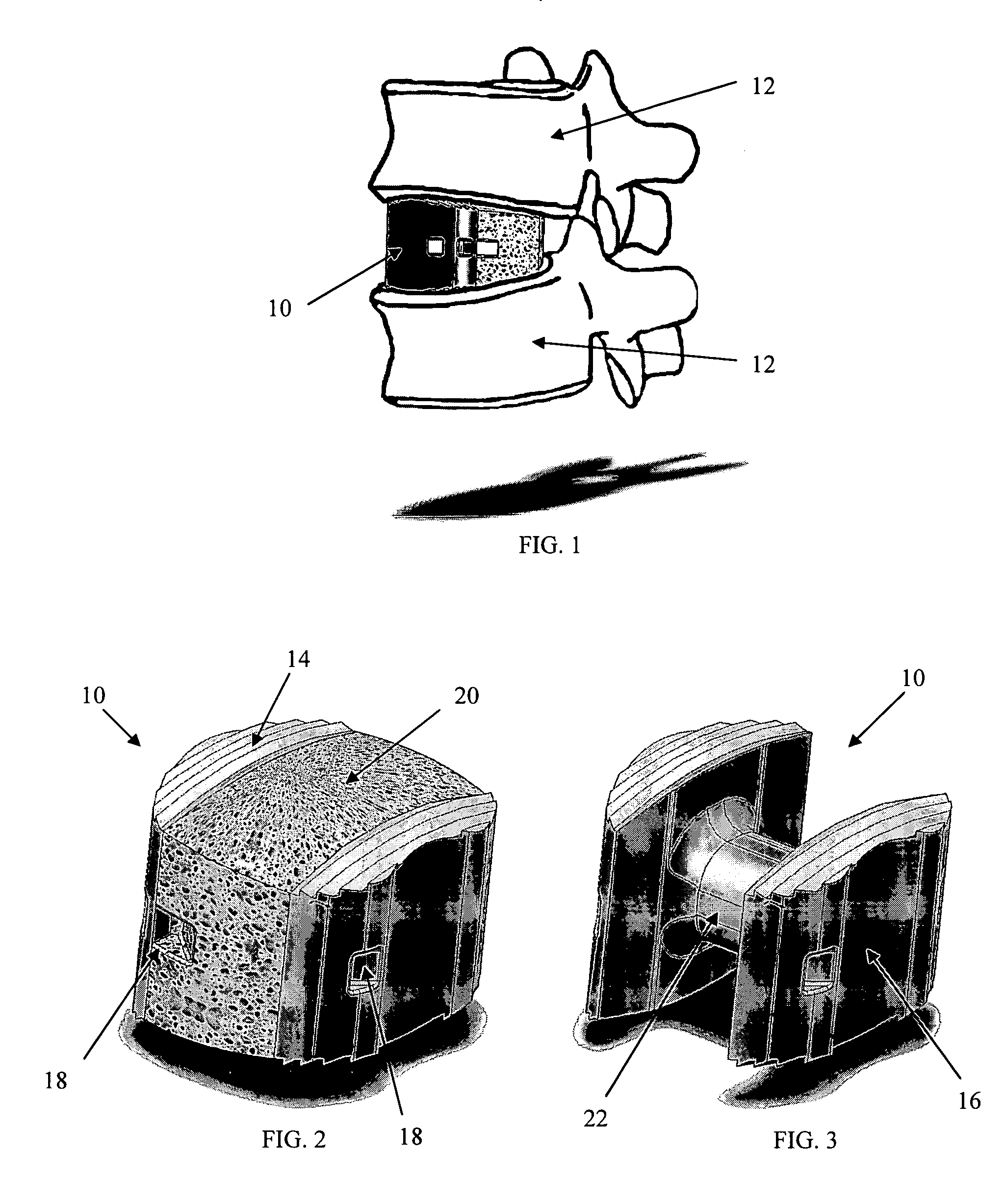
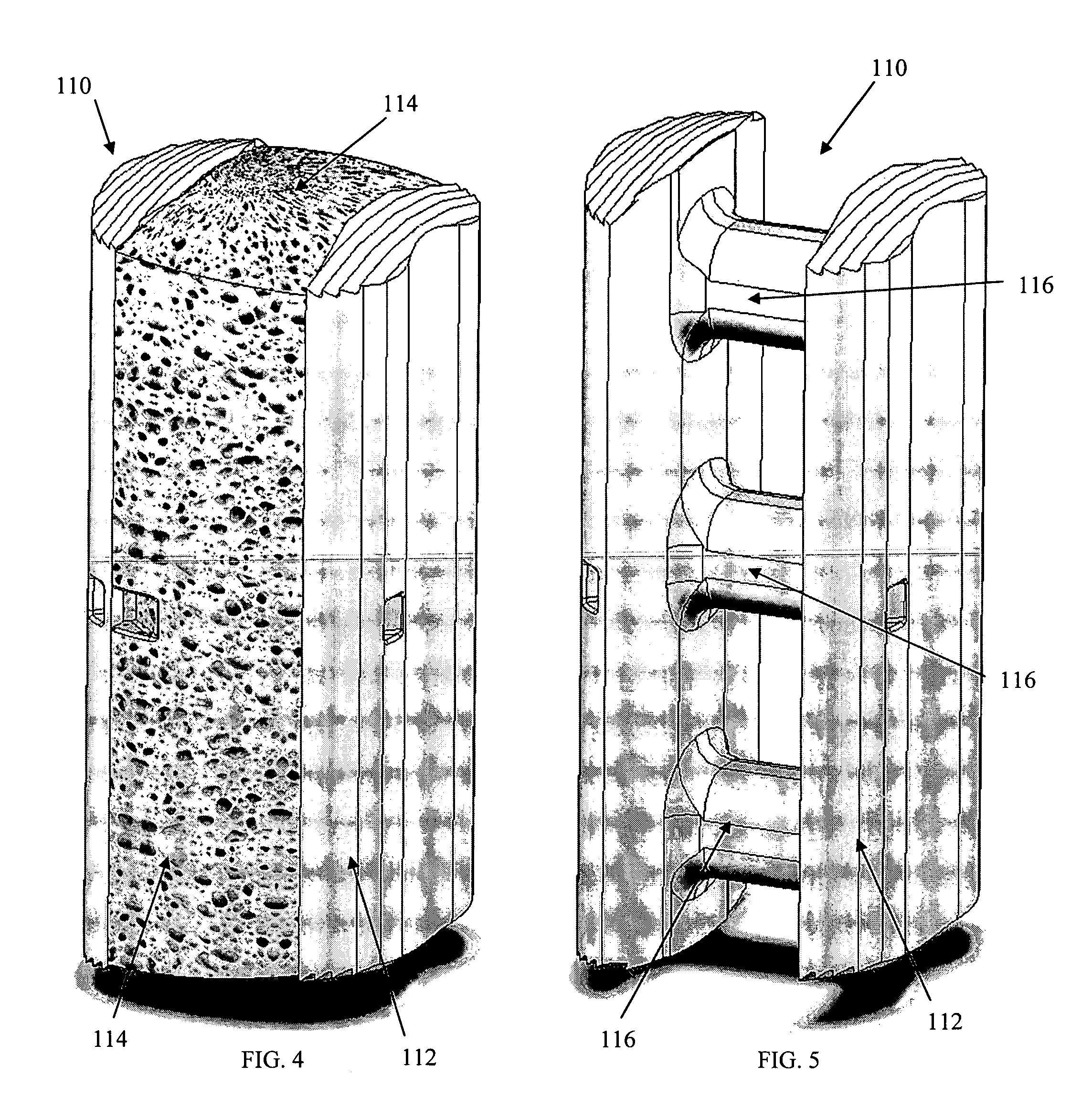
Radiolucent bone graft
An improved bone graft is provided for human implantation, bone graft includes a substrate block of high strength biocompatible material having a selected size and shape to fit the anatomical space, and a controlled porosity analogous to natural bone. The substrate block may be coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone ingrowth and enhanced bone fusion. Upon implantation, the bone graft provides a spacer element having a desired combination of mechanical strength together with osteoconductivity and osteoinductivity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The bone graft may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:AMEDICA A DELAWARE
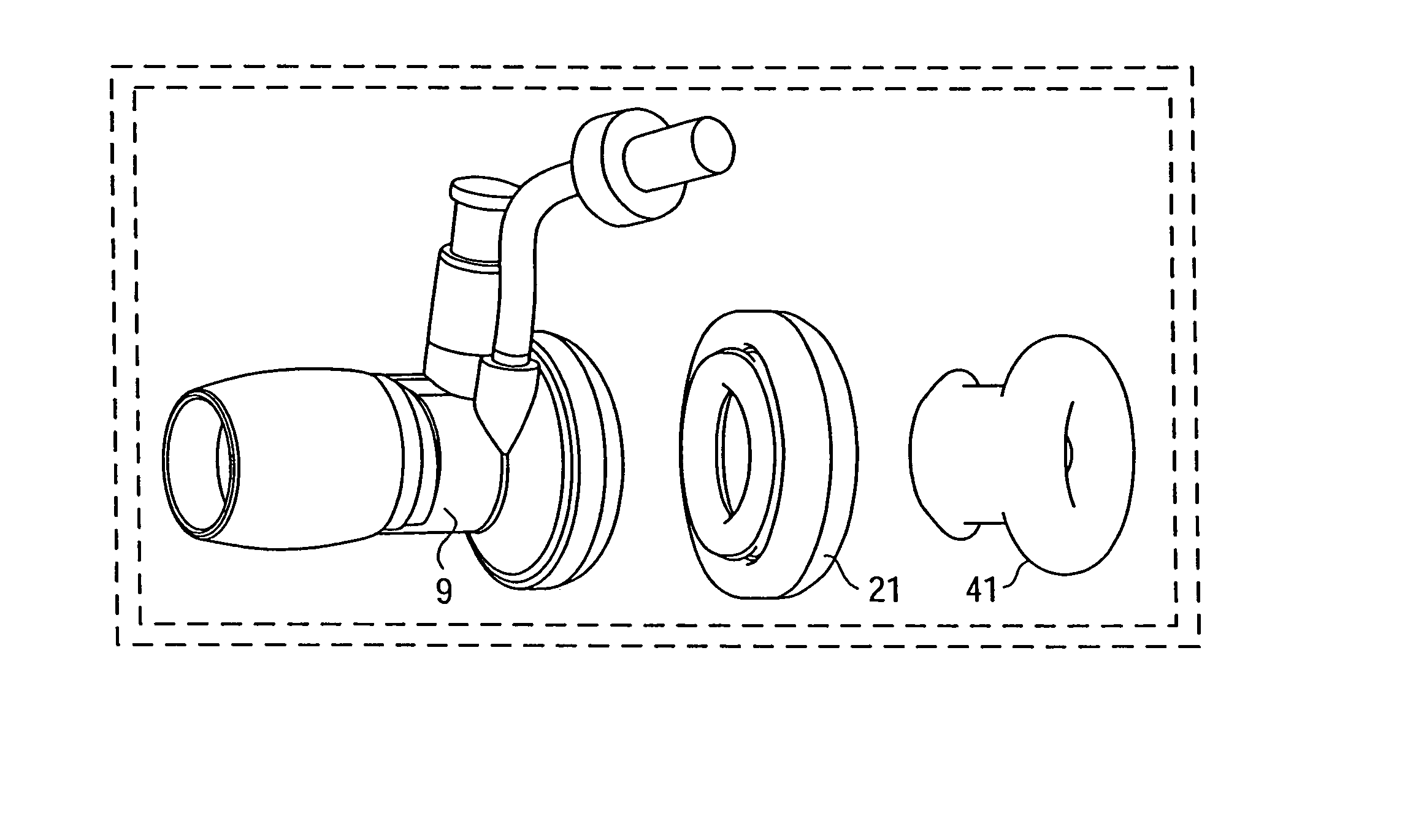
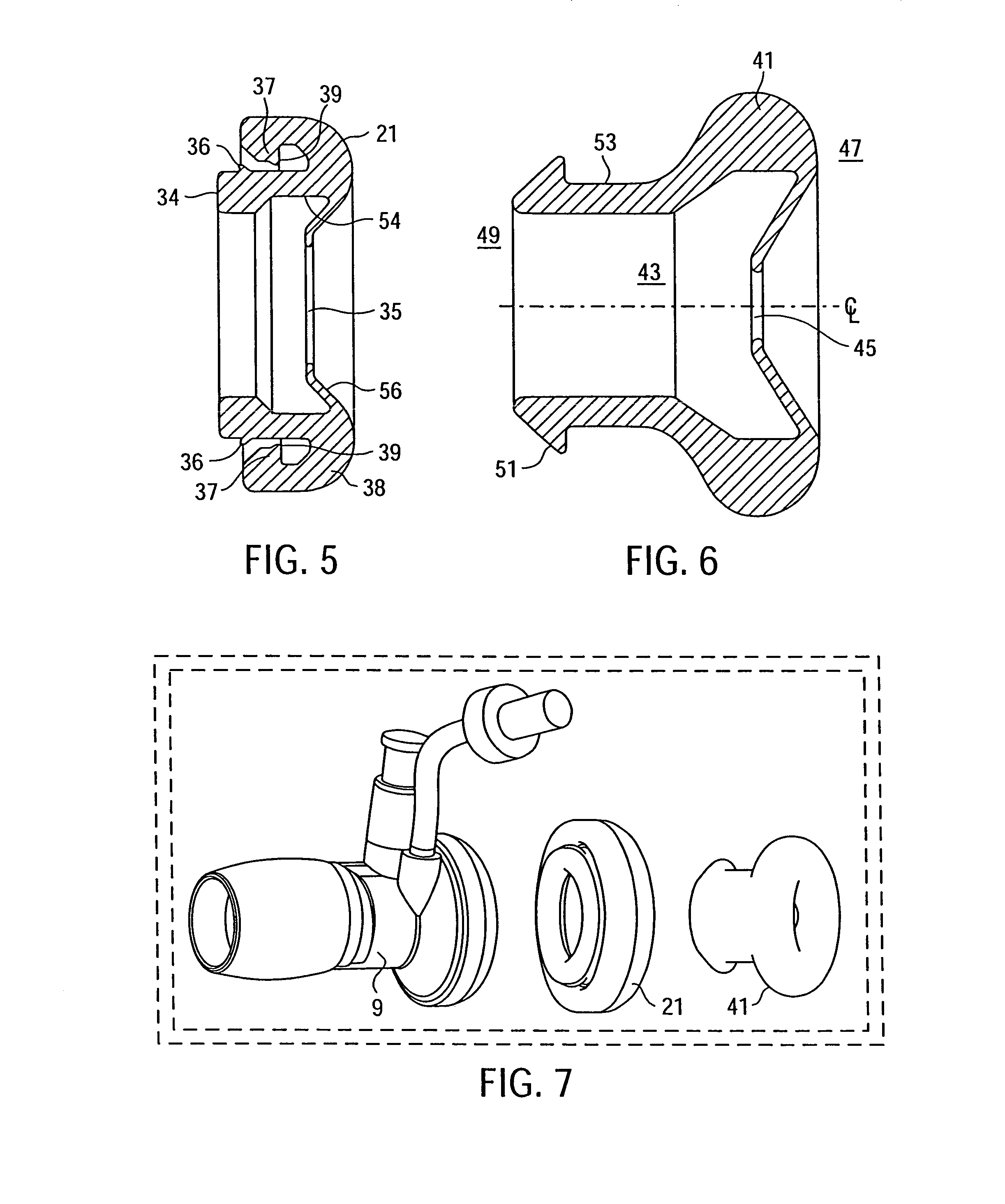
Methods and tools for accessing an anatomic space
ActiveUS20060200002A1Reduce layeringRobust fixationSurgical instrument detailsSurgical pincettesPericardial spacePericardium tissue
Owner:MEDTRONIC INC
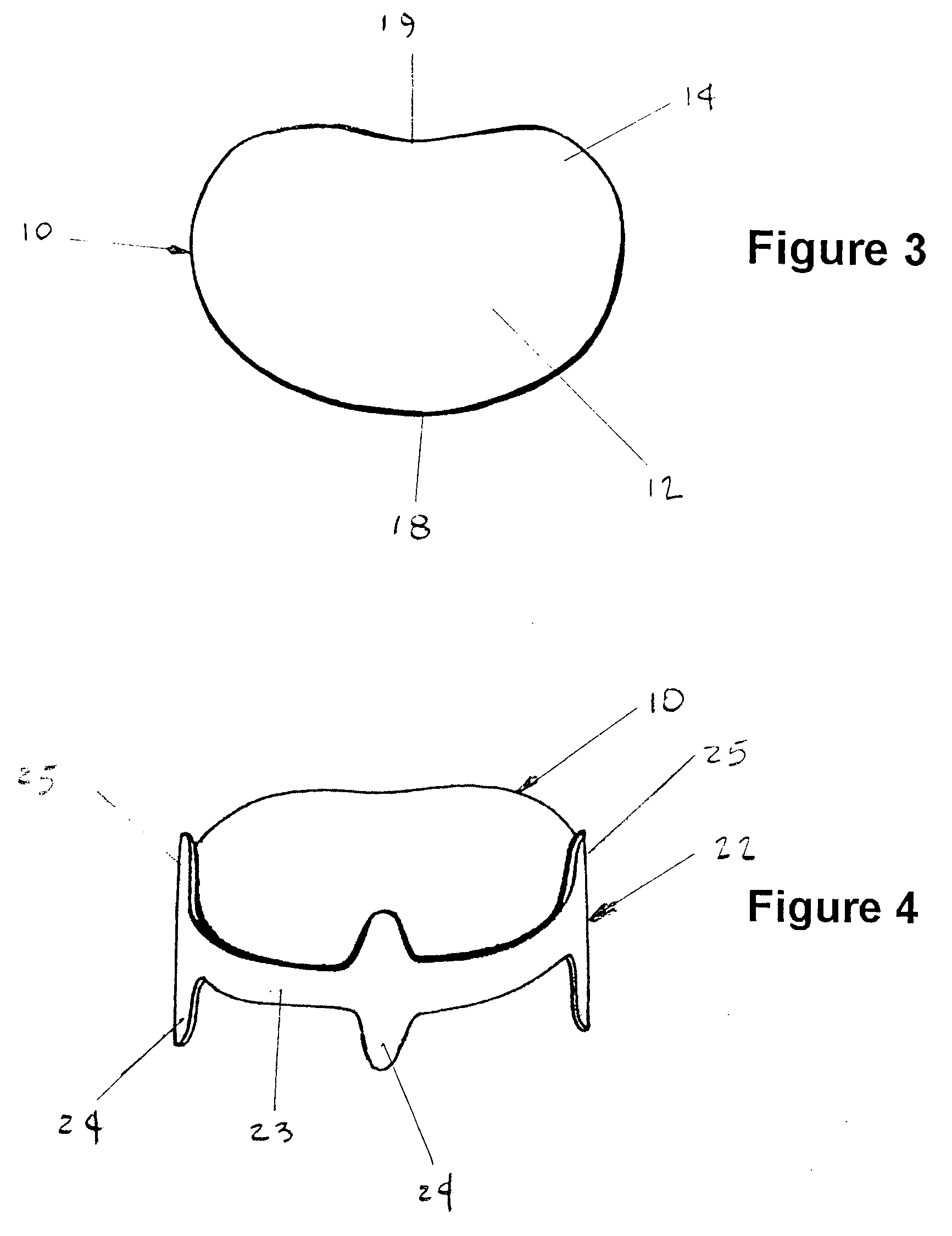
Dual drainage pathway shunt device and method for treating glaucoma
A shunt is provided for the flow of aqueous humor from the anterior chamber of the eye to Schlemm's canal and to other anatomical spaces of the eye. The shunt comprises at least one lumen and optionally has at least one anchor extending from a proximal portion of the shunt to assist in placement and anchoring of the device in the correct anatomic position.
Owner:GLAUKOS CORP
Device and Method for Safe Access to a Body Cavity
InactiveUS20080249467A1Easy accessImprove securitySurgical needlesLaproscopesBody cavityAnatomical space
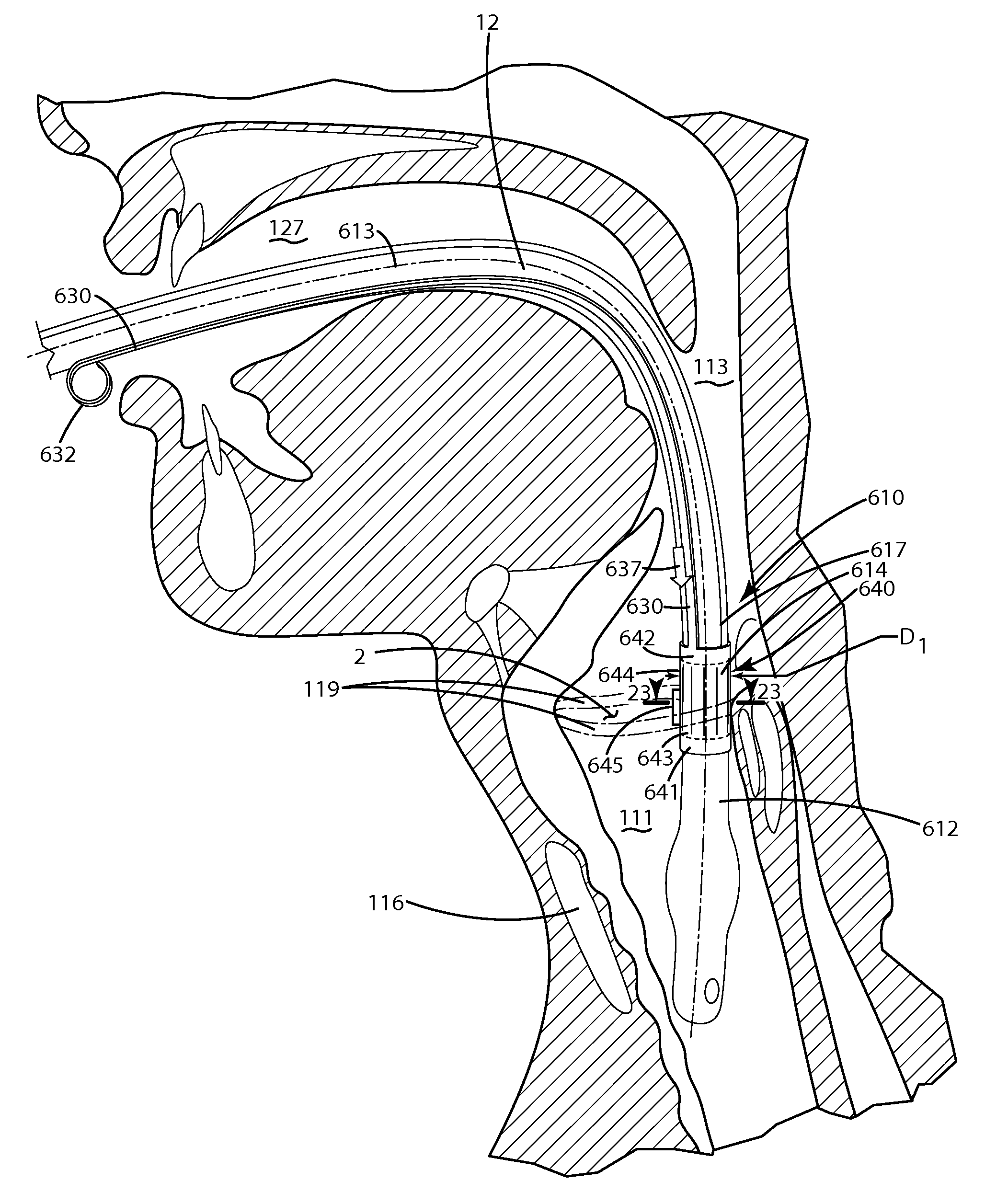
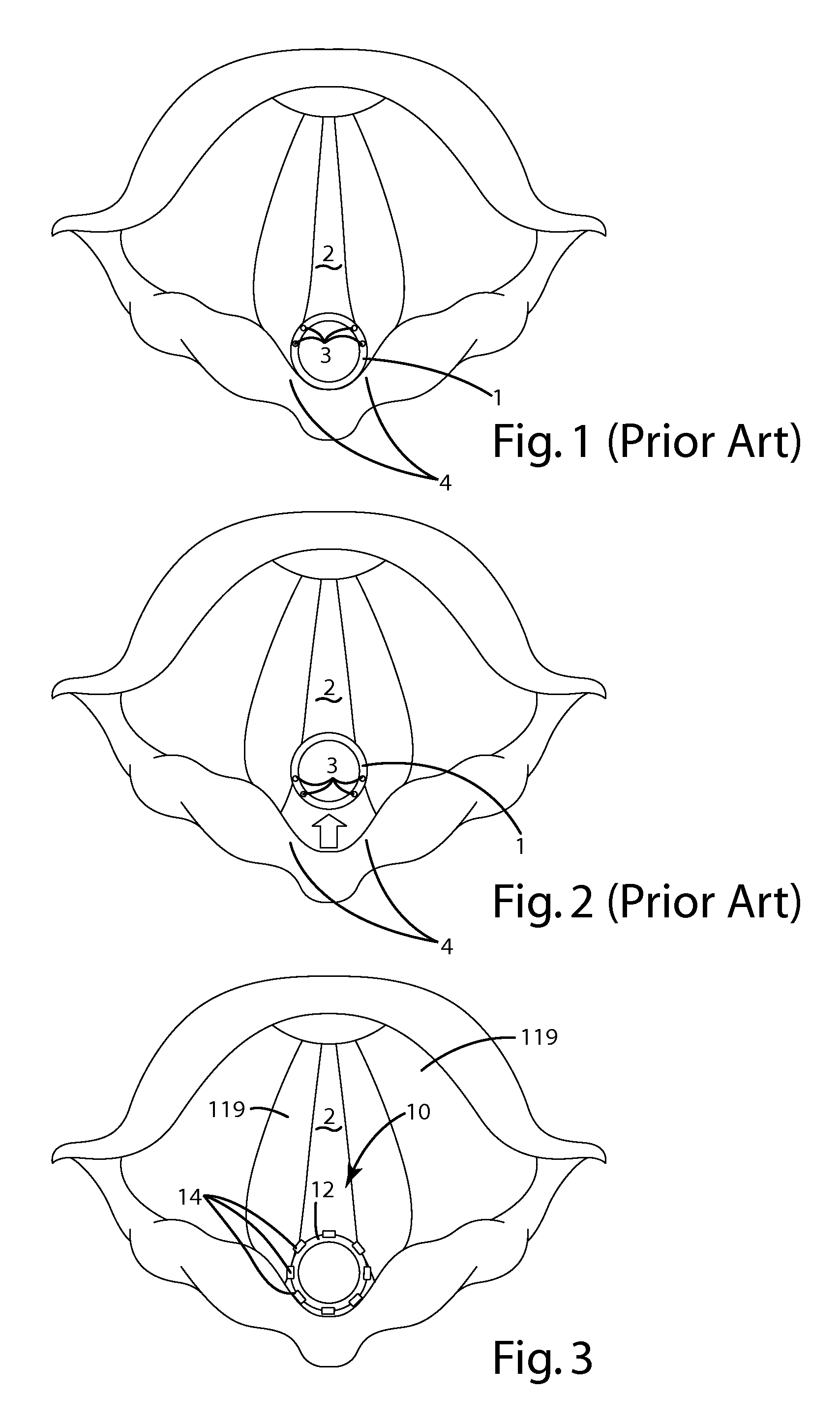
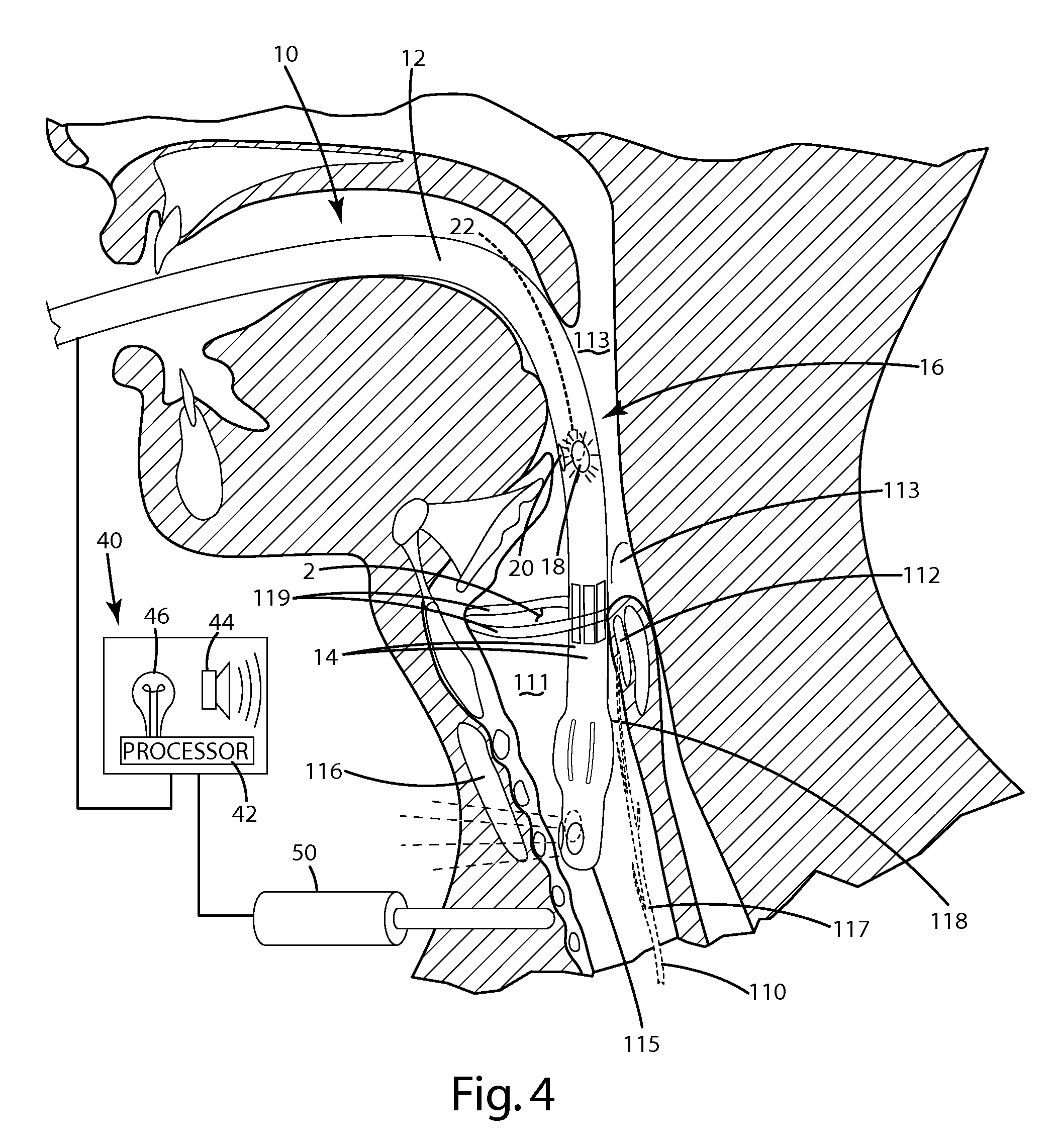
An anatomical space access device having an elongate body; an insertion tip at a distal end of the elongate body; an anatomical space sensor disposed at the distal end of the elongate body, the sensor being adapted to sense a parameter identifying an anatomical space other than a vasculature space and to generate a signal; and an indicator operatively connected to the sensor to receive the signal and to indicate access of the sensor to the anatomical space. The invention also provides a method for providing access to an anatomical space outside of a vasculature space, including the following steps: inserting a distal end of an instrument through a tissue volume into the anatomical space outside of a vasculature space, the instrument comprising an anatomical space sensor; and generating a location indication of the anatomical space sensor.
Owner:THERANOVA LLC
Flexible spinal disc
InactiveUS20050055099A1Allow flexibilityGuaranteed shock absorptionJoint implantsSpinal implantsMedicineIntervertebral space
A medical device and its use are described. The device is useful for replacement or treatment of a diseased or damaged intervertebral spinal disc. The device has volume to occupy space between vertebral bodies, has mechanical elasticity to provide motion between vertebral bodies, and sufficient strength to withstand the forces and loads on the vertebra. The device may have modifications to allow for attachment to the bones of the vertebrae. The device may also contain modifications for ease of placement in the anatomic space between vertebral bodies. The device may be constructed to expand to restore the normal height of the intervertebral space.
Owner:SPINEMEDICA
Sutureless implantable device and method for treatment of glaucoma
InactiveUS6881197B1Easy to installControl pressureEye surgeryWound drainsDiffusion barrierMaximum pressure
Sutureless, implantable fluid shunting devices and associated methods for controlling the pressure of fluids within anatomical spaces or cavities of the body. The device generally comprises a tube having a diffusion barrier (e.g., diffusion chamber) formed on a proximal end thereof. Fluid which flows through the tube will collect within the diffusion chamber and will diffuse outwardly therethrough. However, the presence of the diffusion chamber will prevent microbes, cells or other matter from interfering with or backflowing through the tube. Additionally, the tube may be provided with a pressure-openable aperture through which fluid from the tube may flow into the diffusion chamber. Such pressure-openable aperture will remain closed, until the pressure of fluid within the tube exceeds a predetermined maximum pressure PMAX. In this manner, the pressure-openable aperture will limit the amount of fluid drained from the anatomical space or cavity of the body, thereby avoiding hypotony within such anatomical space or cavity. The diffusion barrier of the device is preferably configured to fit between, and to be engaged by, adjacent recti muscles of the eye. Such engagement of the diffusion barrier with the adjacent recti muscles serves to prevent unwanted migration or post-implantation movement of the device, without the need for suturing of the device to the tissue of the eye.
Owner:REVISION OPTICS
Sutureless implantable device and method for treatment of glaucoma
InactiveUS20050182350A1Easy to installMinimal discomfort to the patientEye surgeryWound drainsDiffusion barrierMaximum pressure
Sutureless, implantable fluid shunting devices and associated methods for controlling the pressure of fluids within anatomical spaces or cavities of the body. The device generally comprises a tube having a diffusion barrier (e.g., diffusion chamber) formed on a proximal end thereof. Fluid which flows through the tube will collect within the diffusion chamber and will diffuse outwardly therethrough. However, the presence of the diffusion chamber will prevent microbes, cells or other matter from interfering with or backflowing through the tube. Additionally, the tube may be provided with a pressure-openable aperture through which fluid from the tube may flow into the diffusion chamber. Such pressure-openable aperture will remain closed, until the pressure of fluid within the tube exceeds a predetermined maximum pressure PMAX. In this manner, the pressure-openable aperture will limit the amount of fluid drained from the anatomical space or cavity of the body, thereby avoiding hypotony within such anatomical space or cavity. The diffusion barrier of the device is preferably configured to fit between, and to be engaged by, adjacent recti muscles of the eye. Such engagement of the diffusion barrier with the adjacent recti muscles serves to prevent unwanted migration or post-implantation movement of the device, without the need for suturing of the device to the tissue of the eye.
Owner:REVISION OPTICS
Radiolucent spinal fusion cage
InactiveUS20050049706A1Improve carrying capacityHigh porosityBone implantJoint implantsPorosityBone growth
An improved bone graft is provided for human implantation, particularly such as a spinal fusion cage for implantation into the inter-vertebral space between two adjacent vertebrae. The improved spinal fusion cage includes a substrate block of high strength biocompatible material having a selected size and shape to fit the anatomical space, and a controlled porosity analogous to natural bone. The substrate block may be coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone in growth and enhanced bone fusion. Upon implantation, the fusion cage provides a spacer element having a desired combination of mechanical strength together with osteoconductivity and osteoinductivity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The fusion cage may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:AMEDICA A DELAWARE
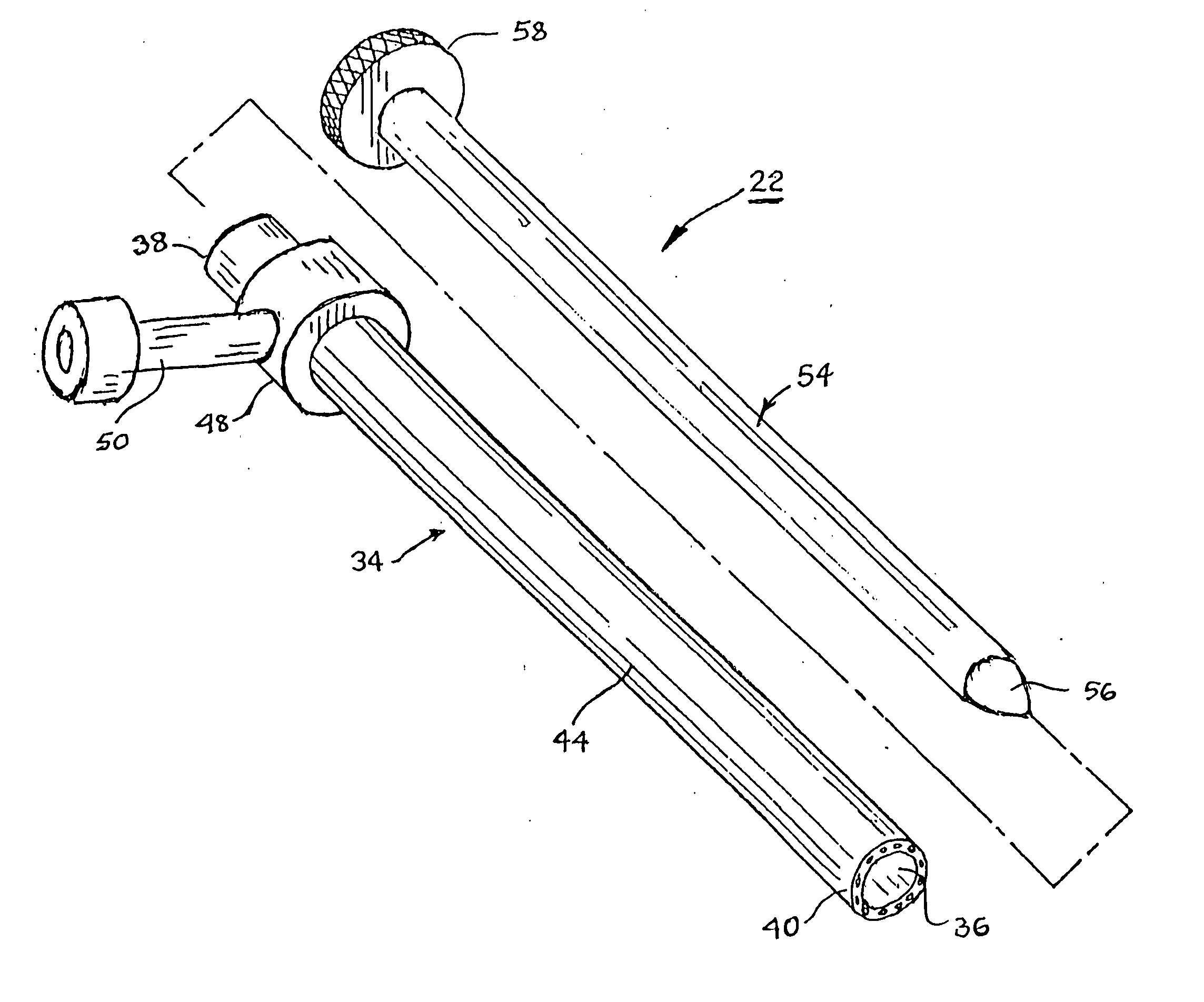
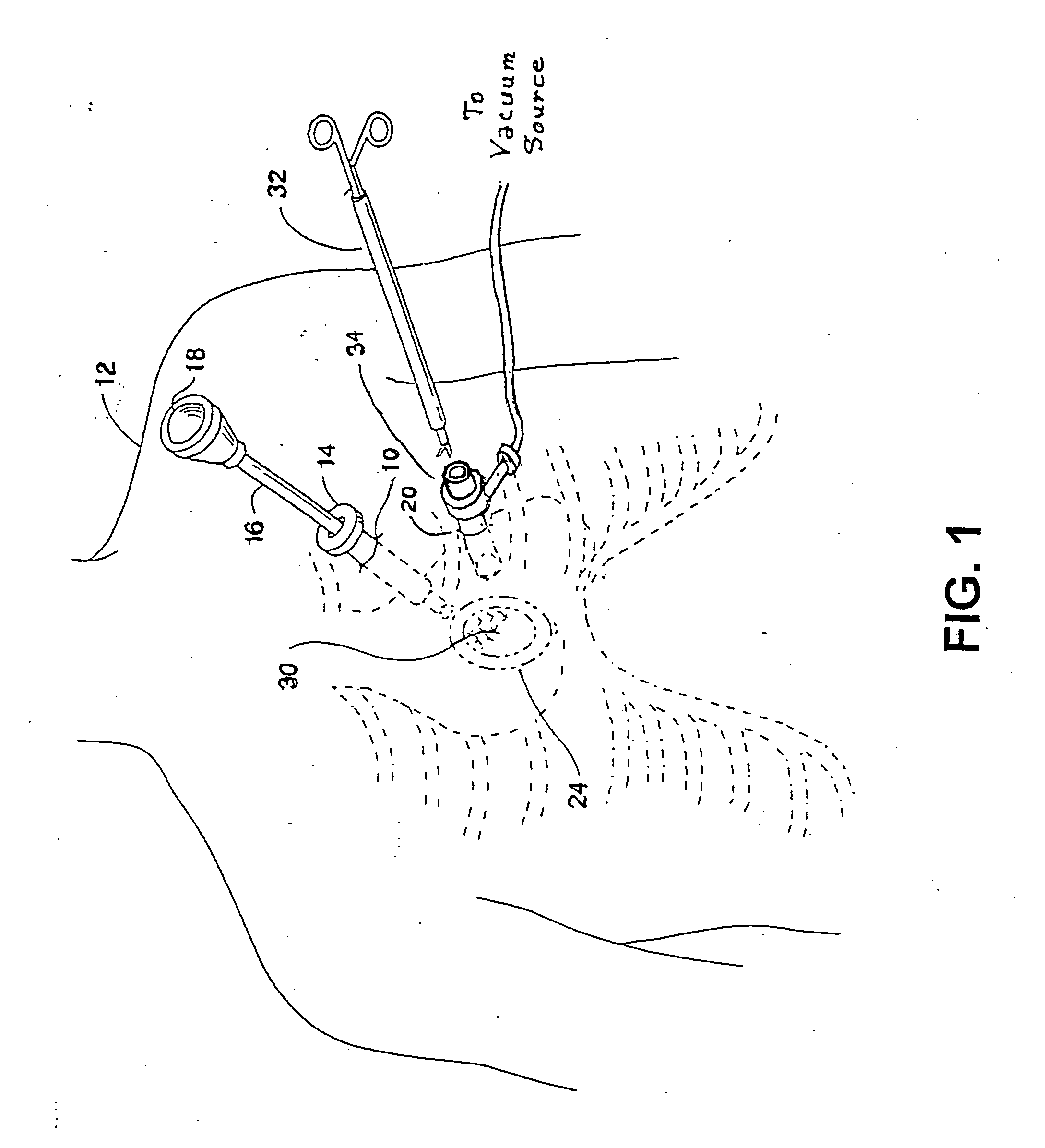
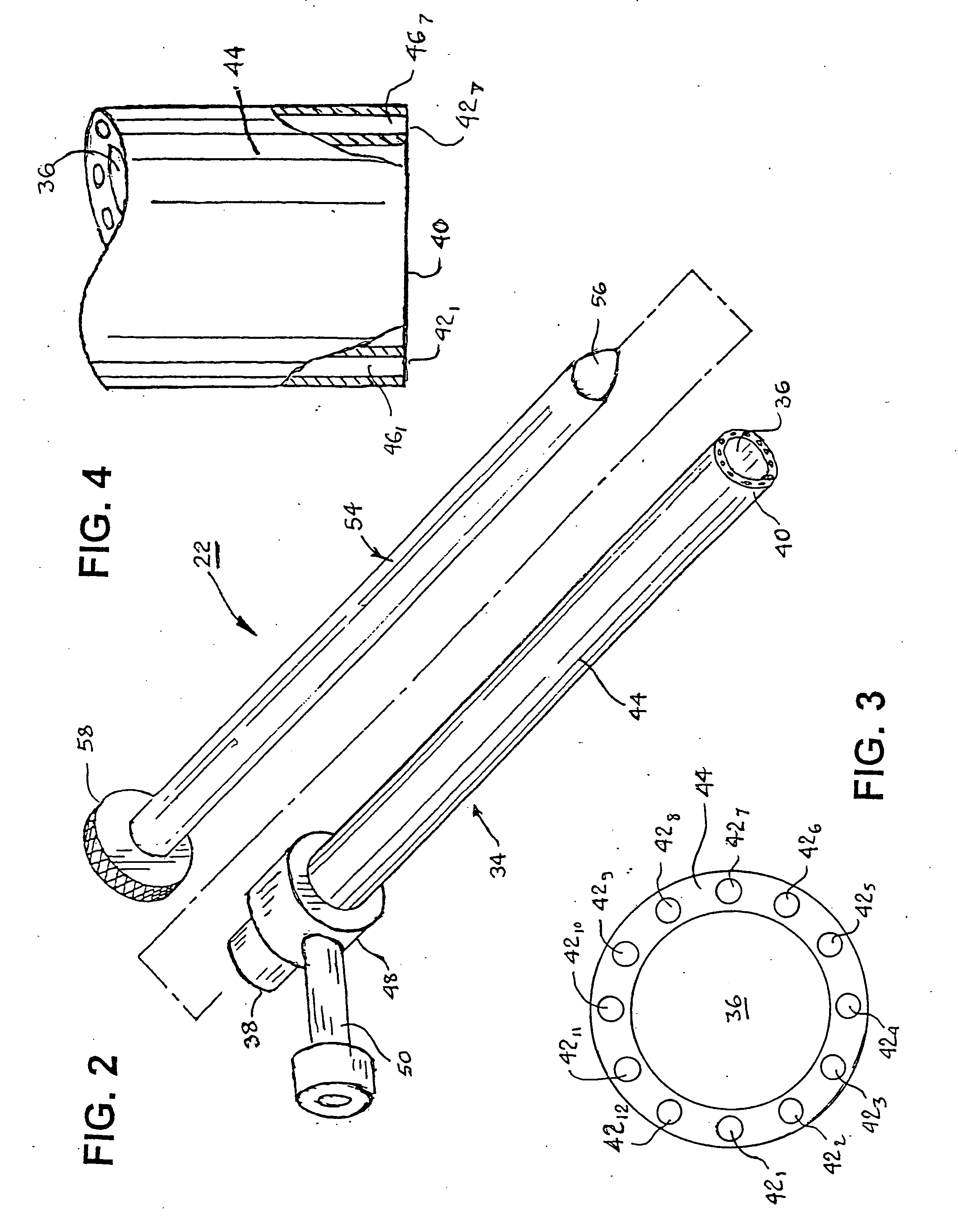
Vessel harvesting apparatus and method
InactiveUS7485092B1Less risk of injuryLess riskIncision instrumentsSurgical furnitureTissue expansionEndoscope
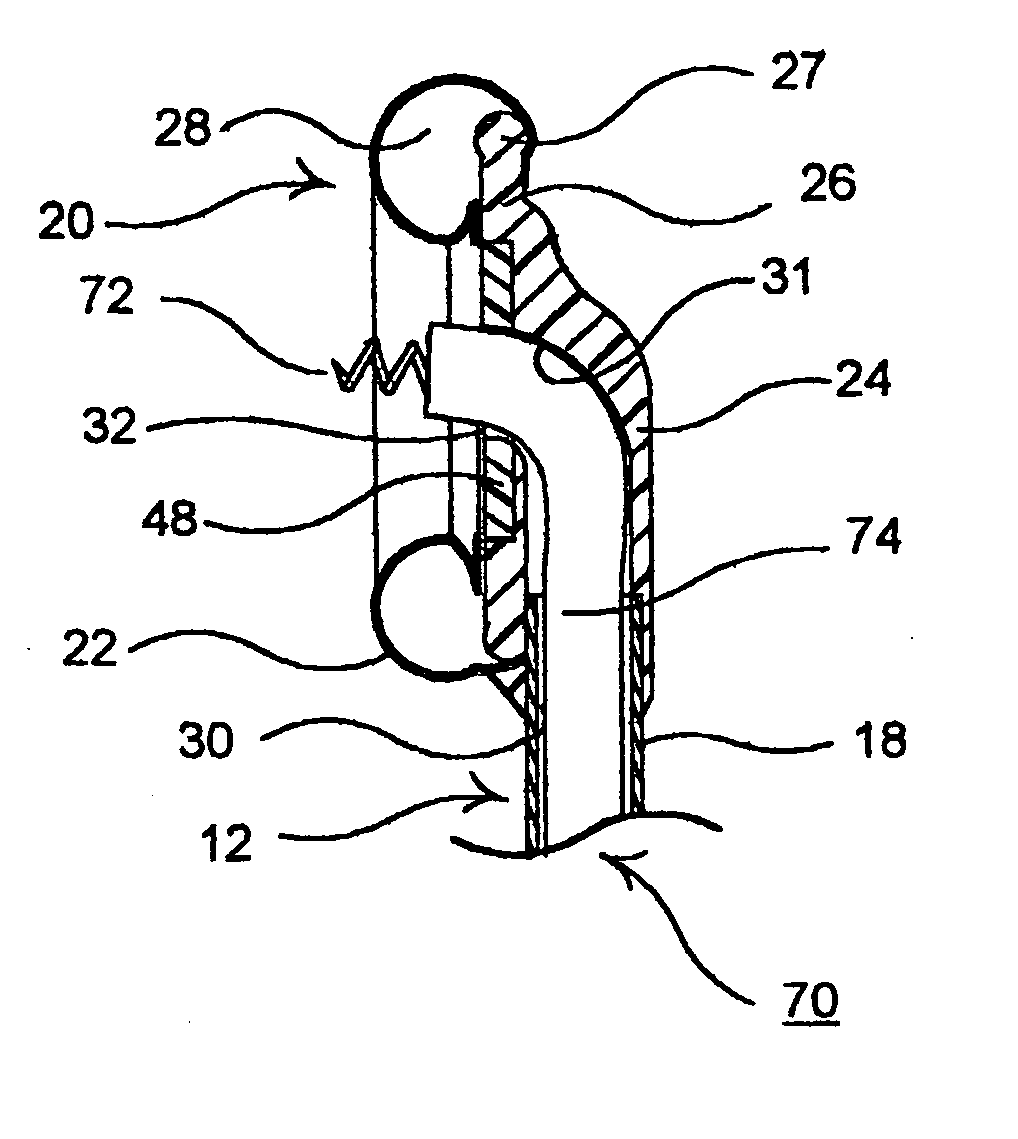
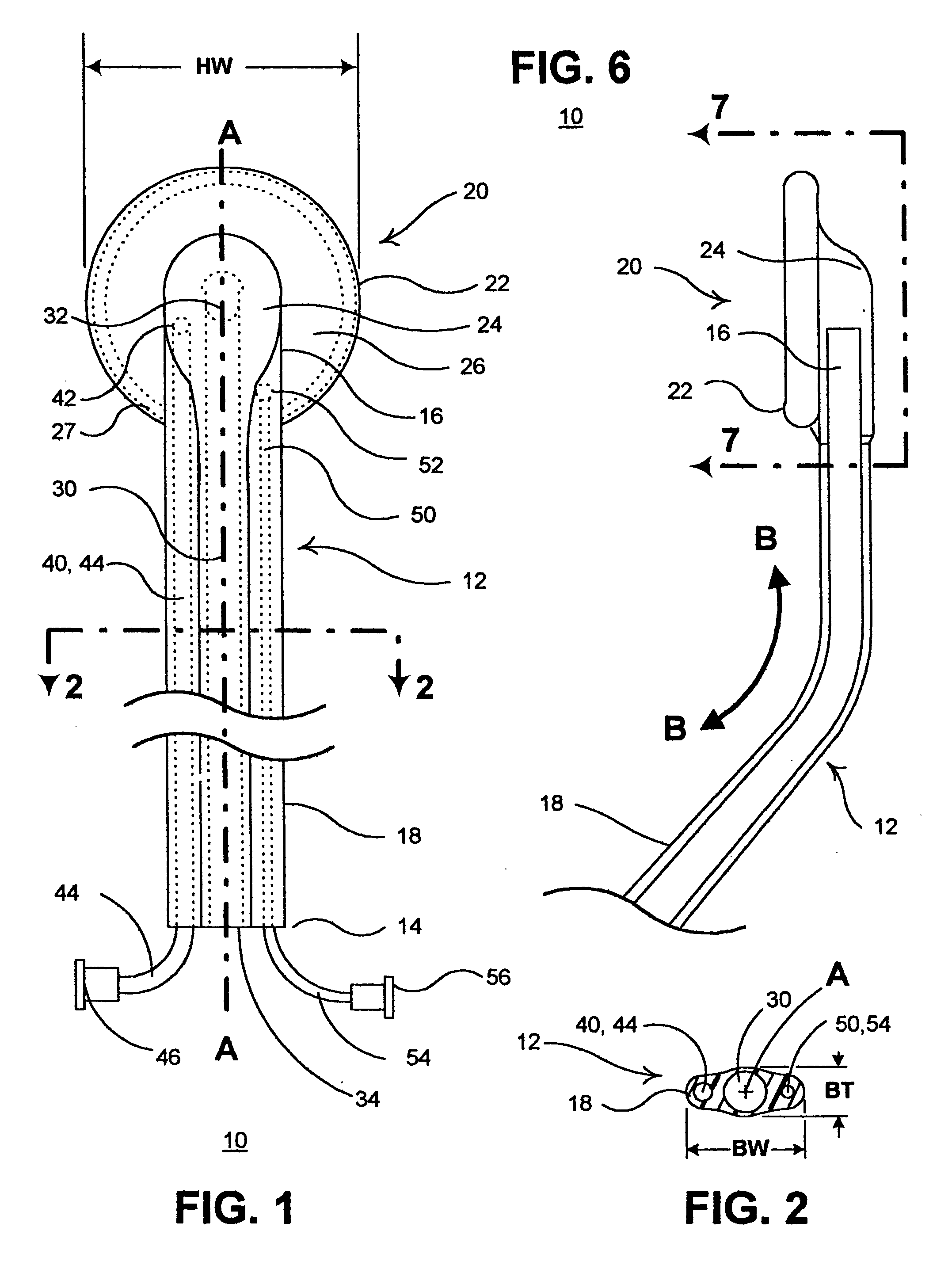
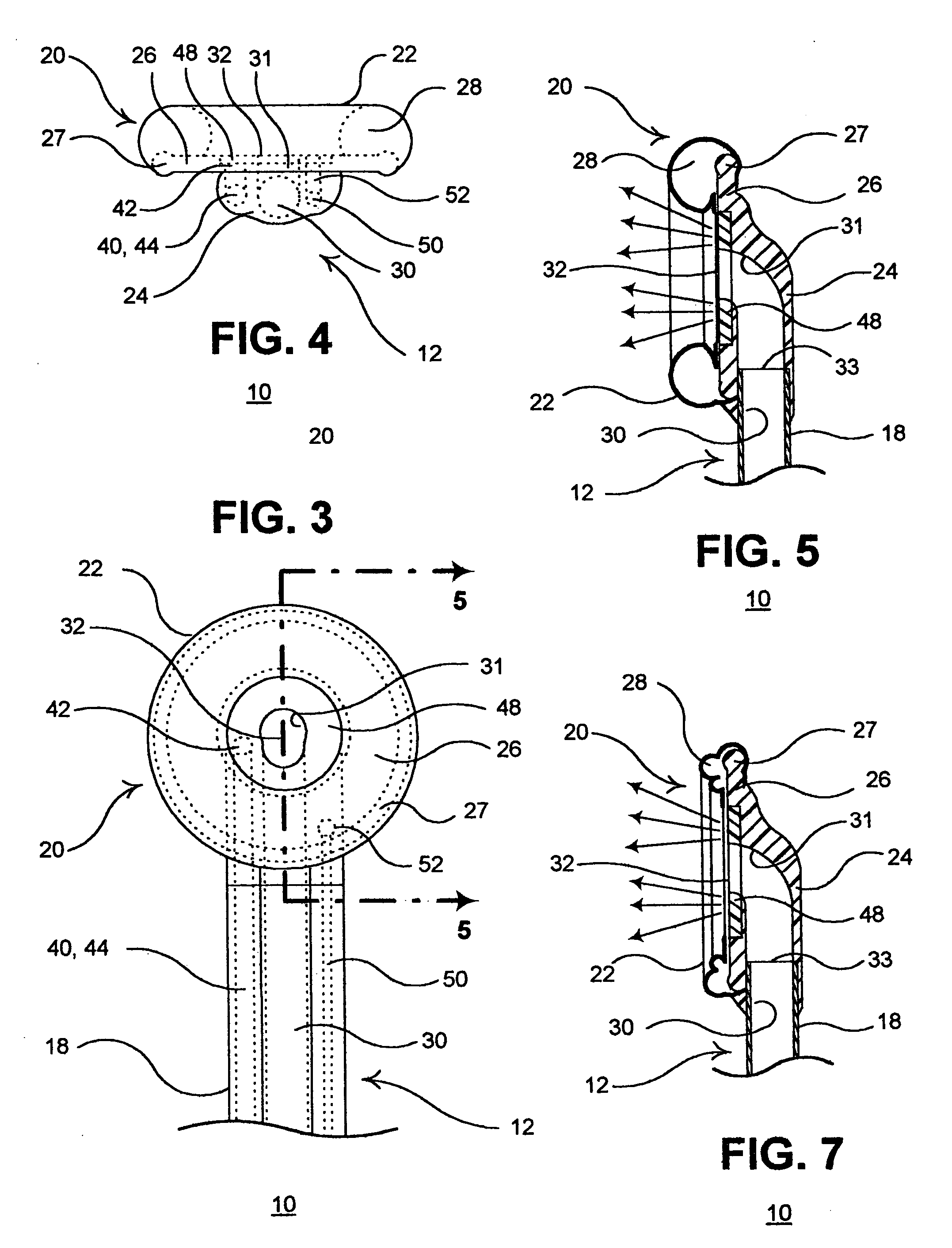
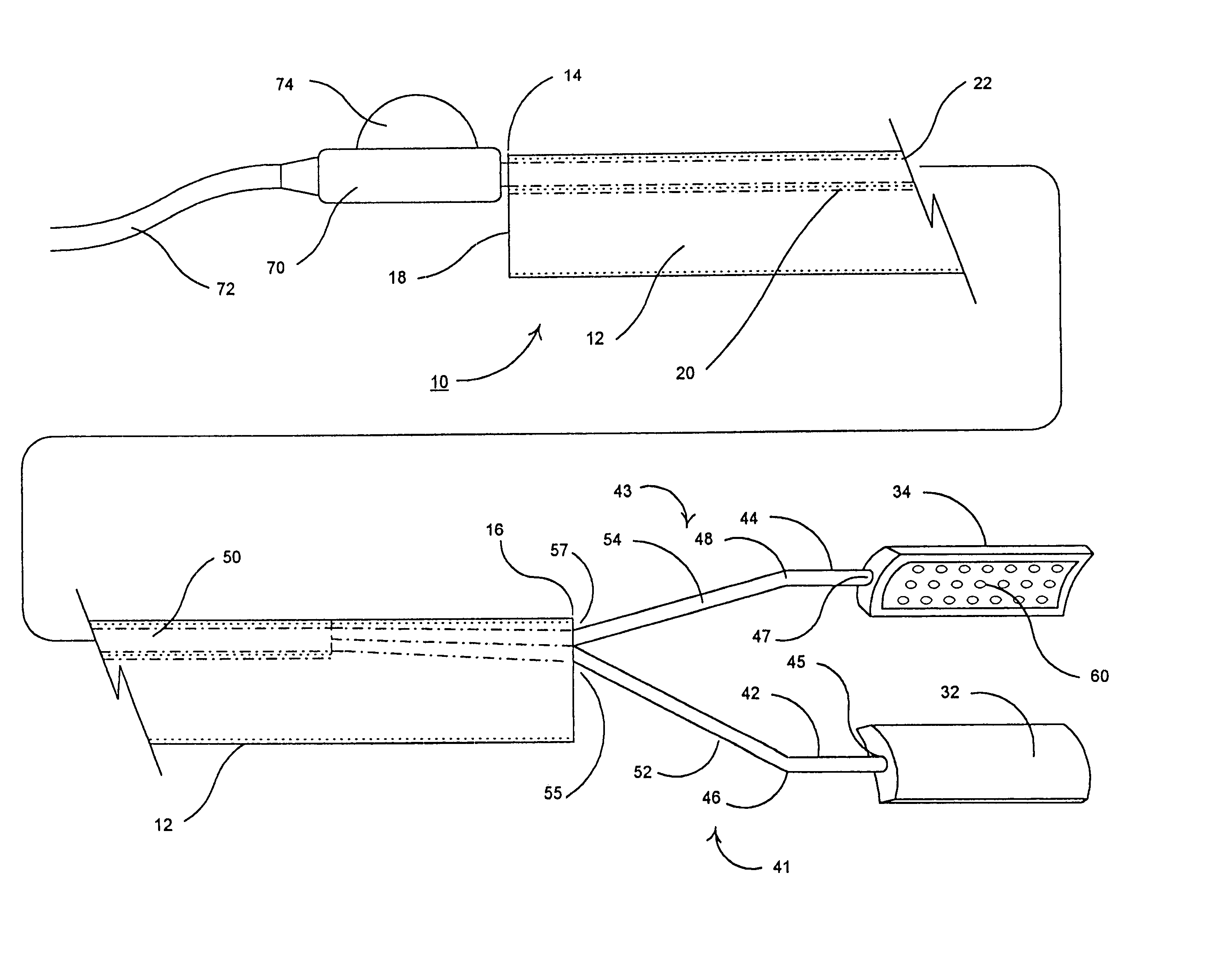
Apparatus and method for harvesting selected vessels in the body of a patient include manual manipulation of a rigid dissecting endoscope and the reconfiguration thereof to facilitate tissue dissection and tissue dilation in the formation of an anatomical space about the vessel within which side-branch vessels may be manipulated in preparation for severance and removal of the vessel from the anatomical space.
Owner:MAQUET CARDIOVASCULAR LLC
Methods of and apparatus for determining fluid volume presence in mammalian tissue
Methods and apparatus (20 ) process noninvasively measured electrical bio-impedence values and perform a technique that indicates whether there exists a change from a homeostatic fluid condition, preferably with respect to blood loss, in mammalian tissue. Analyses can be performed to determine a presence or a change in volume of fluid in an anatomical space of a mammal. A preferred implementation of such technique is embodied in an instrument that carries out a method that may predict an onset of a hemorrhagic shock condition.
Owner:SCHOCK & CO GMBH
Instruments and methods for accessing an anatomic space
InactiveUS20070010708A1Rigid enoughConvenient introductionStentsCannulasPericardial spaceAnatomic Site
An anatomic space of the body, particularly the pericardial space, is accessed in a minimally invasive manner from a skin incision by an access instrument to facilitate visualization and introduction of devices or drugs or other materials, performance of medical and surgical procedures, and introducing and fixating a cardiac lead electrode to the heart. An elongated access instrument body preferentially bends in one direction and resists bending in a transverse direction, whereby the access instrument body distal end can be directed through a path around body structures to the anatomic site by manipulation of the access instrument body proximal end portion. A distal header formed at the access instrument body distal end extends outward of the access instrument body in the transverse direction and supports an inflatable balloon surrounding a working lumen exit port that is directed toward an anatomic surface when the balloon is inflated by inflation media introduced through an inflation lumen.
Owner:MEDTRONIC INC
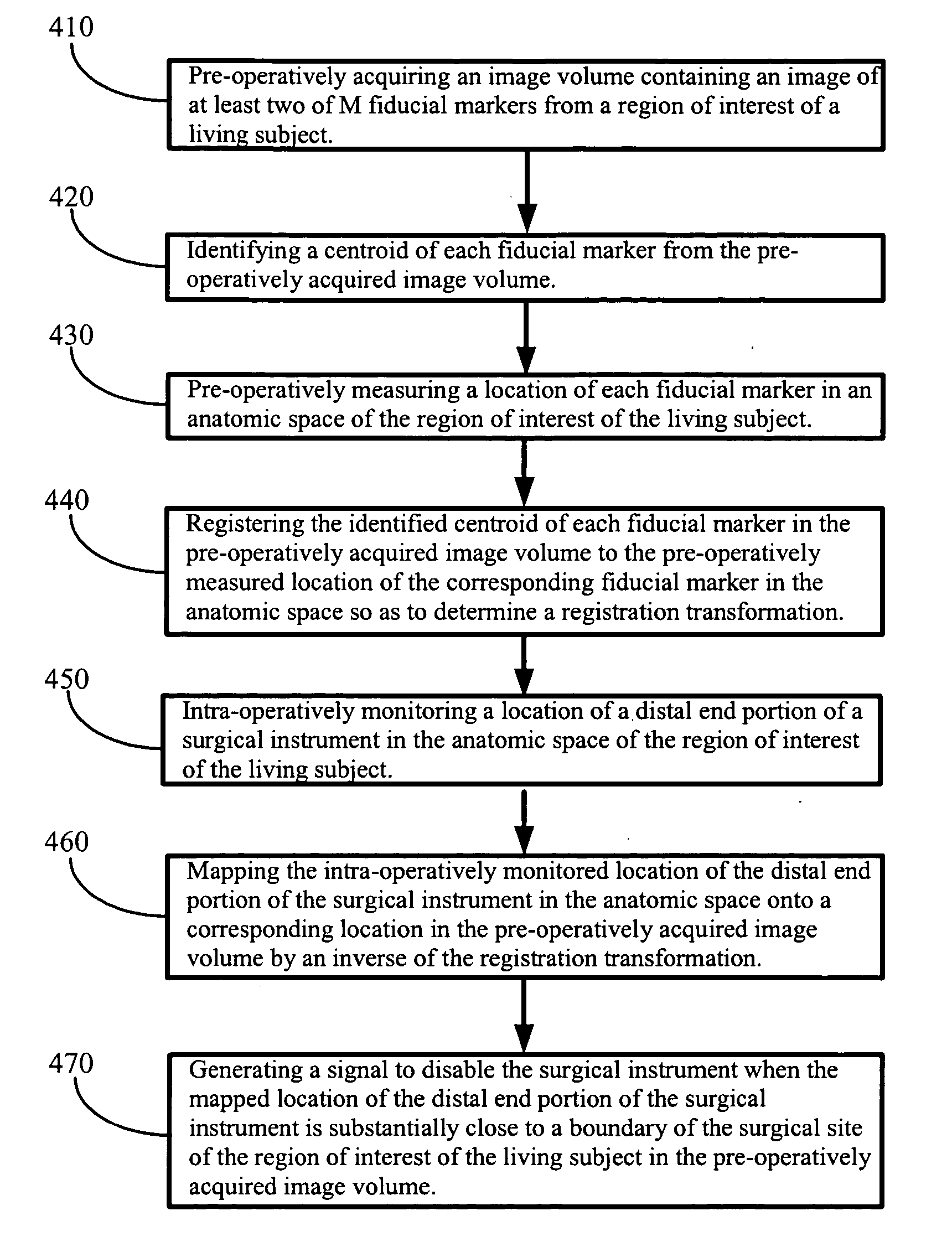
System and method for surgical instrument disablement via image-guided position feedback
InactiveUS20050228256A1Selectively disableError minimizationSurgical navigation systemsCatheterMap LocationSurgical site
A system for selectively disabling a surgical instrument operating in a surgical site of a region of interest of a living subject. In one embodiment, the system includes means for noninvasively placing a number of fiducial markers in an anatomic space of the region of interest of the living subject, means for pre-operatively measuring a location of each fiducial marker in the anatomic space, an imaging acquisition device for pre-operatively acquiring an image volume from the region of interest of the living subject, a probe for intra-operatively monitoring a location of the surgical instrument in the anatomic space, and a controller configured to perform the steps of identifying a centroid of each fiducial marker in the image volume, registering the identified centroid of each fiducial marker in the image volume to the measured location of the corresponding fiducial marker in the anatomic space to determine a registration transformation, mapping the monitored location of the surgical instrument in the anatomic space onto a corresponding location in the image volume by an inverse of the registration transformation, and generating a signal to disable the surgical instrument when the mapped location of the surgical instrument is substantially close to a boundary of the surgical site of the region of interest in the image volume.
Owner:VANDERBILT UNIV
Apparatus and method for treatment of sinusitis
A method of treating a constricted sinus passageway of a patient includes traversing the canine fossa region of the patient so as to form a passageway in the sinus cavity. A cannula is positioned in the passageway. A visualization tool such as an endoscope is passed through a lumen or channel in the cannula to aid in visualization of the anatomical site of interest. A balloon dilation catheter is then deployed through or along the cannula so as to place the balloon within or across the constricted anatomical space (e.g., ostium). The balloon is then expanded so as to expand at least a portion of the constricted anatomical space. Alternative embodiments include the use of an optional guide wire and incorporating a endoscope lumen through the balloon dilation catheter.
Owner:ENTELLUS MEDICAL
Apparatus and method for treatment of sinusitis
A method of treating a constricted sinus passageway of a patient includes traversing the canine fossa region of the patient so as to form a passageway in the sinus cavity. A cannula is positioned in the passageway. A visualization tool such as an endoscope is passed through a lumen or channel in the cannula to aid in visualization of the anatomical site of interest. A balloon dilation catheter is then deployed through or along the cannula so as to place the balloon within or across the constricted anatomical space (e.g., ostium). The balloon is then expanded so as to expand at least a portion of the constricted anatomical space. Alternative embodiments include the use of an optional guide wire and incorporating a endoscope lumen through the balloon dilation catheter.
Owner:ENTELLUS MEDICAL
Nerve monitoring device
ActiveUS20100317956A1Efficiently monitor a variety of nervesTracheal tubesBronchoscopesAnatomic SiteMode transformation
Owner:THE MAGSTIM
Vessel Harvesting
InactiveUS20090023986A1Less risk of injuryLess riskSurgical scissorsBlunt dissectorsTissue expansionEndoscope
Apparatus and method for harvesting selected vessels in the body of a patient include manual manipulation of a rigid dissecting endoscope and the reconfiguration thereof to facilitate tissue dissection and tissue dilation in the formation of an anatomical space about the vessel within which side-branch vessels may be manipulated in preparation for severance and removal of the vessel from the anatomical space.
Owner:STEWART MICHAEL C +6
Anatomical space access tools and methods
InactiveUS20050273129A1Increase spacingRobust fixationCannulasSurgical needlesPericardial spaceMedical device
Owner:MEDTRONIC INC
Nerve monitoring device
ActiveUS8886280B2Efficiently monitor a variety of nervesBronchoscopesLaryngoscopesAnatomic SiteMode transformation
Owner:THE MAGSTIM
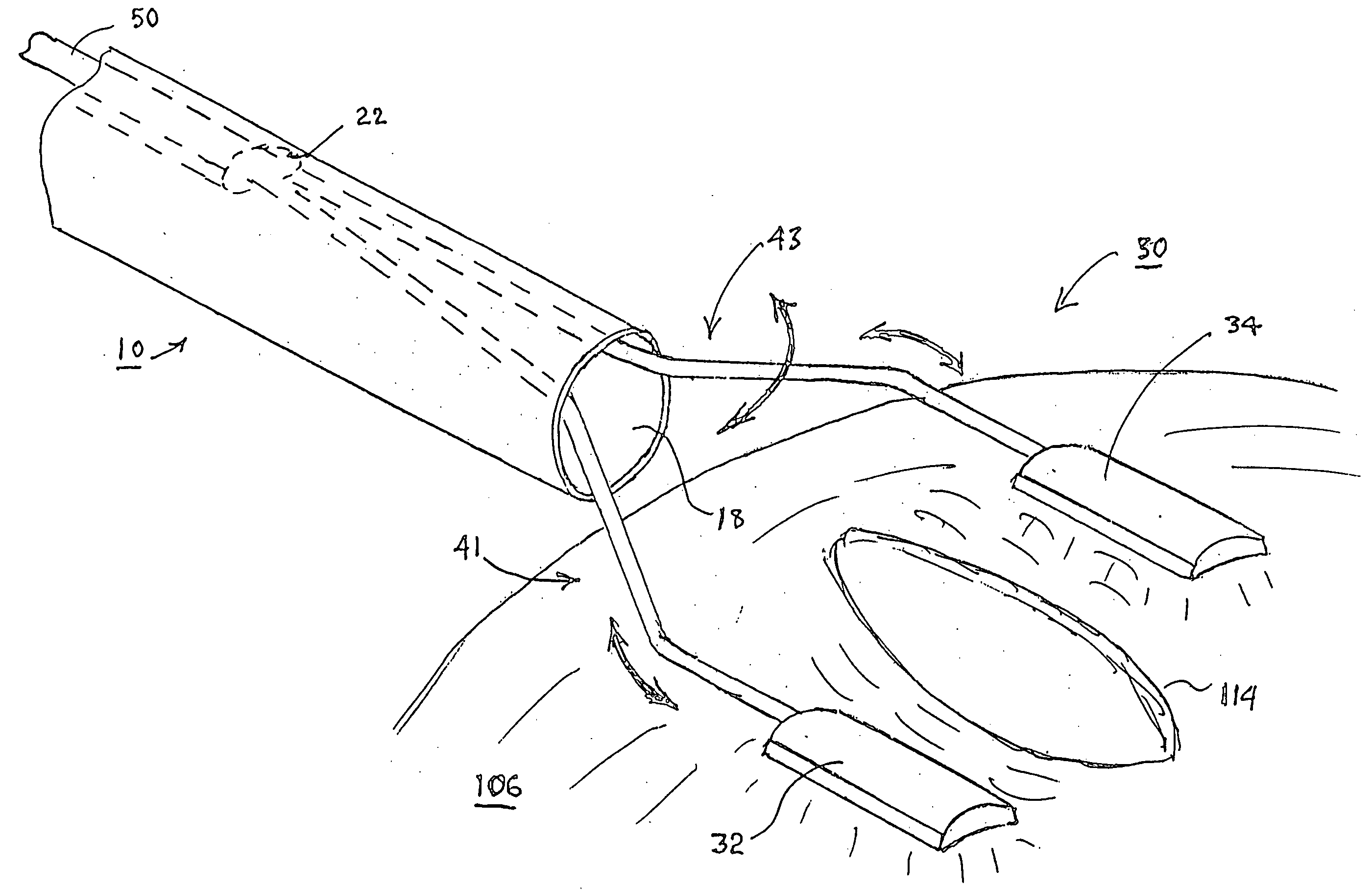
Integrated vessel ligator and transector
Surgical treatment of tissue includes electrocauterization of blood vessels interposed between spaced sets of electrodes of opposite polarity, and includes transection of such tissue by a cutter that is mounted between the spaced sets of electrodes for translational and lateral movement relative to the sets of electrodes. Orientations of the sets of electrodes within a range of angles about an elongated axis of a supporting body are controlled by manual movement of an actuator mounted near a proximal end of the body for movement through a smaller range of angles via linkage connecting the actuator to the electrodes. Tissue dissection with gas insufflation to form an anatomical space in tissue is facilitated by a fluid outlet port located near the tissue-dissecting tip at the distal end of the elongated body that delivers through the tissue-dissecting tip to the dissected tissue a fluid under pressure that is supplied along a lumen within the elongated body.
Owner:MAQUET CARDIOVASCULAR LLC
Dual drainage pathway shunt device
A shunt is provided for the flow of aqueous humor from the anterior chamber of the eye to Schlemm's canal and to other anatomical spaces of the eye. The shunt comprises at least one lumen and optionally has at least one anchor extending from a proximal portion of the shunt to assist in placement and anchoring of the device in the correct anatomic position.
Owner:GLAUKOS CORP
Reverse Sealing And Dissection Instrument
ActiveUS20080312652A1Facilitate tissue removalSuperior dissectionSurgical scissorsChiropractic devicesPERITONEOSCOPEDissection
The invention relates to a device and related method for sealing and / or dissecting body tissue using a reverse action instrument. The devices and methods described permit laparoscopic or natural body orifice access to an anatomical space and facilitate sealing portions of tissue together, dissecting tissue or combinations thereof.A surgical instrument for sealing and dissecting body tissue is described having distal and proximal ends with an elongated body. The body includes a jaw member positioned at the distal end that is defined by a stationary arm and a pivotable arm. A moveable closure sleeve is disposed at least partially about the body and the closure sleeve is configured such that coaxial movement of the sleeve along the longitudinal axis of the body causes the jaw member to open or close.
Owner:TENNESSEE MEDICAL INNOVATIONS INC
Flexible spinal disc
InactiveUS20070164464A1Allow flexibilityGuaranteed shock absorptionMovable spraying apparatusWood working apparatusMedicineIntervertebral space
Owner:SPINEMEDICA
Method and apparatus for combined dissection and retraction
A method and apparatus for dissecting a first layer of tissue from a second layer of tissue and thereafter holding open an anatomic space for the performance of a surgical procedure. The method includes steps of making an incision in a body, introducing a deflated balloon dissector into the incision, inflating the balloon dissector to effect dissection of the first layer of tissue from the second layer of tissue, deploying a retractor within the anatomic space in order to hold open the anatomic space, and optionally deflating or evacuating the balloon dissector to open a cavity for surgical manipulations. The apparatus includes a combined dissector-retractor having a balloon retractor disposed upon the surface of the balloon dissector and integrated therewith.
Owner:GEN SURGICAL INNOVATIONS
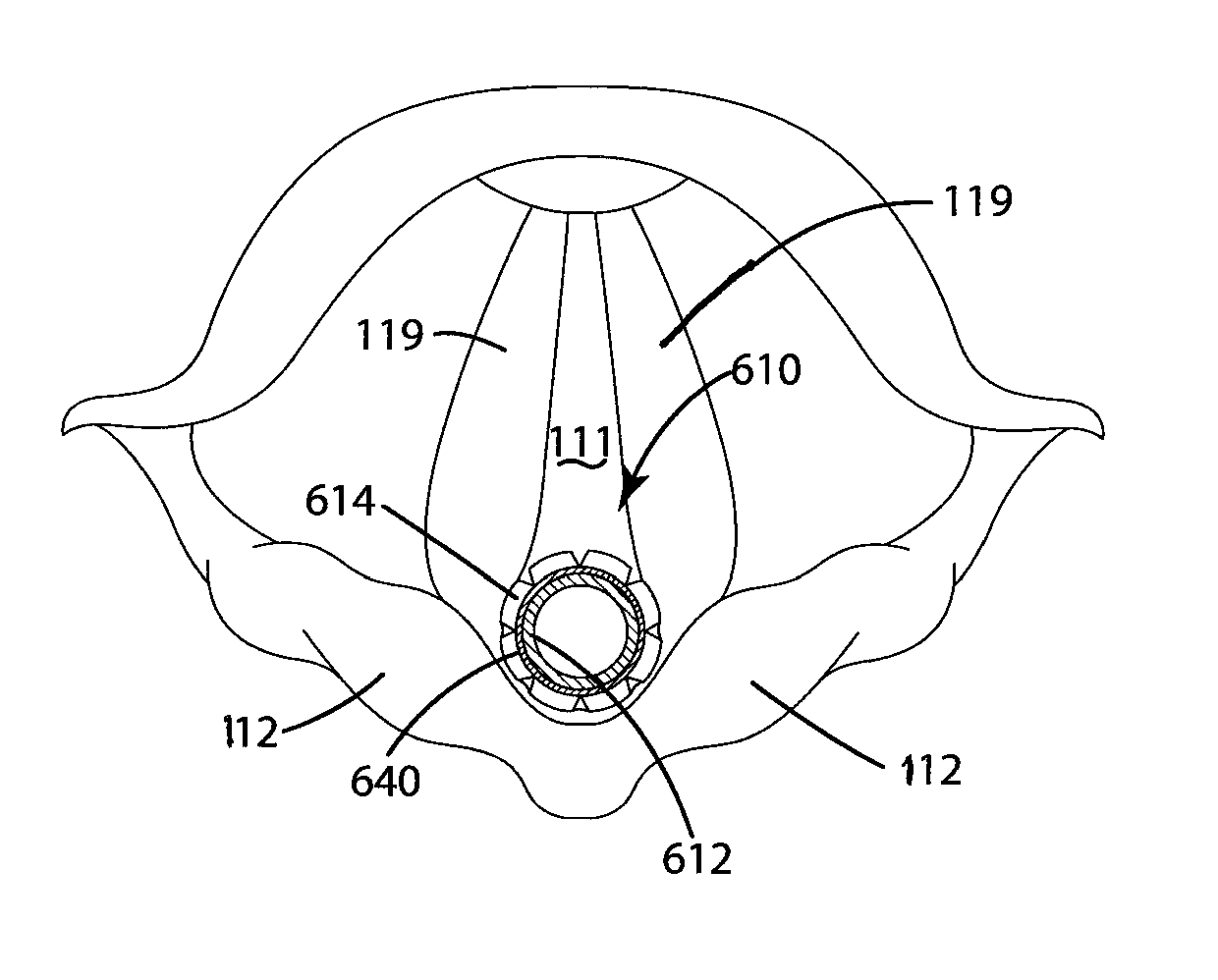
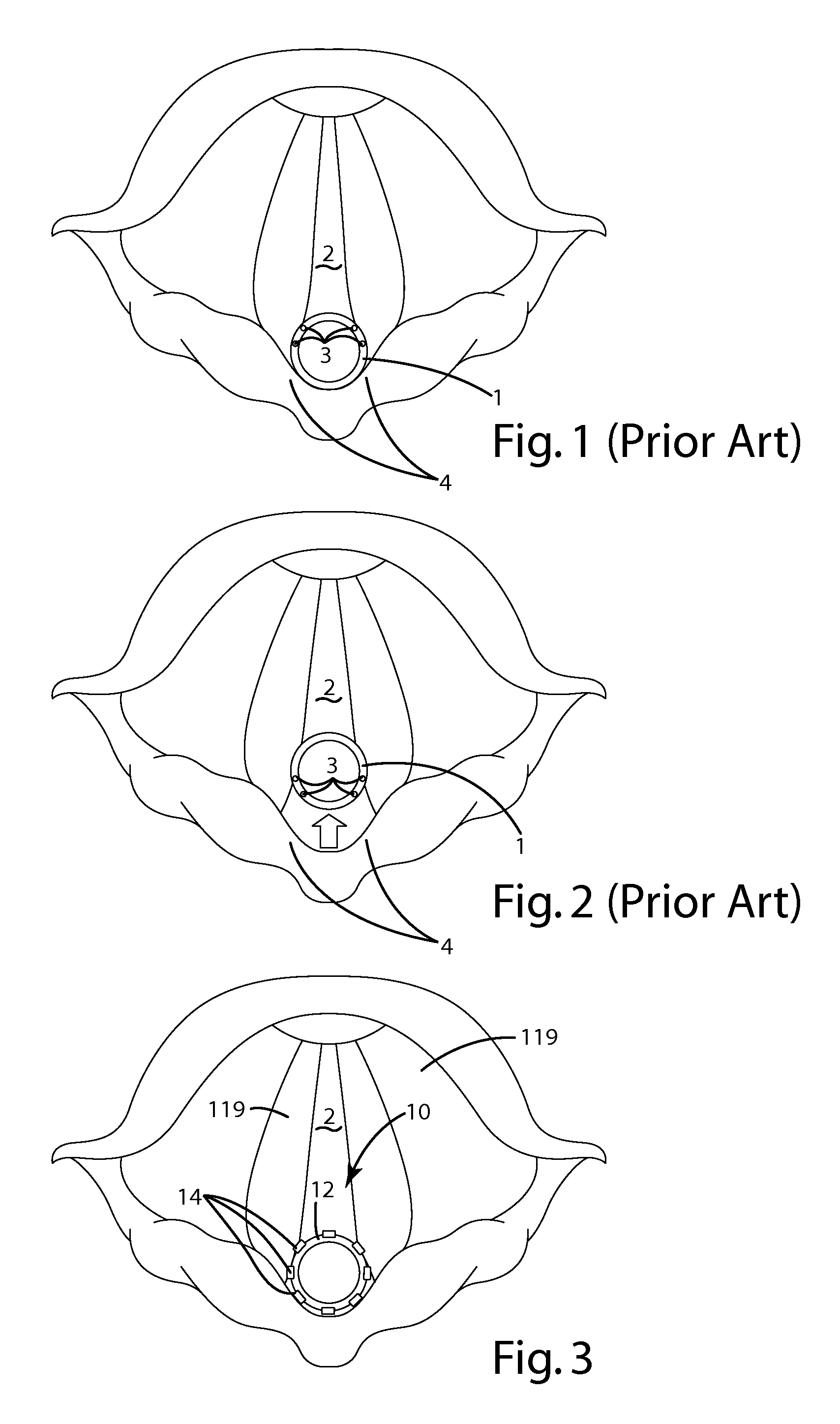
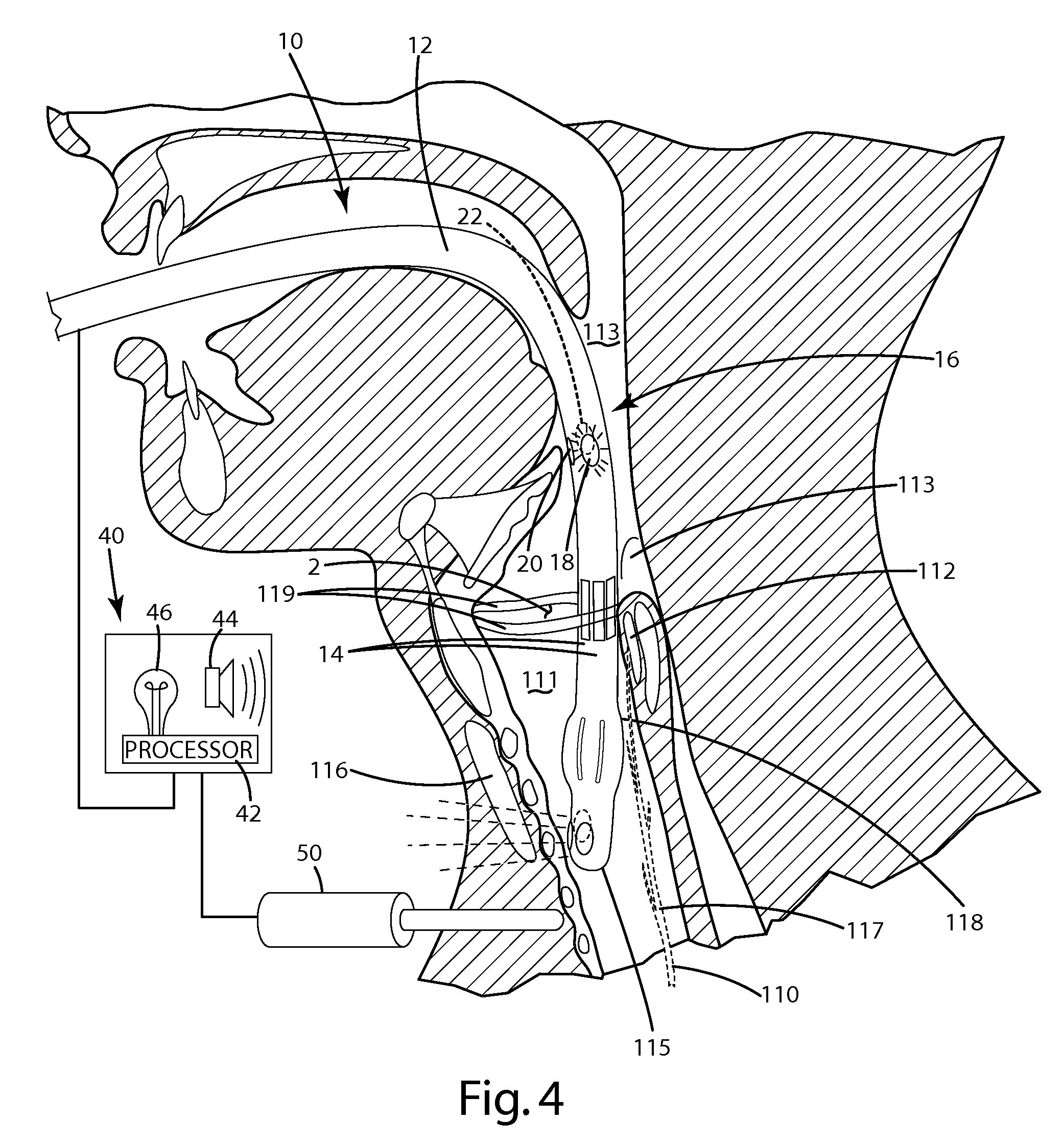
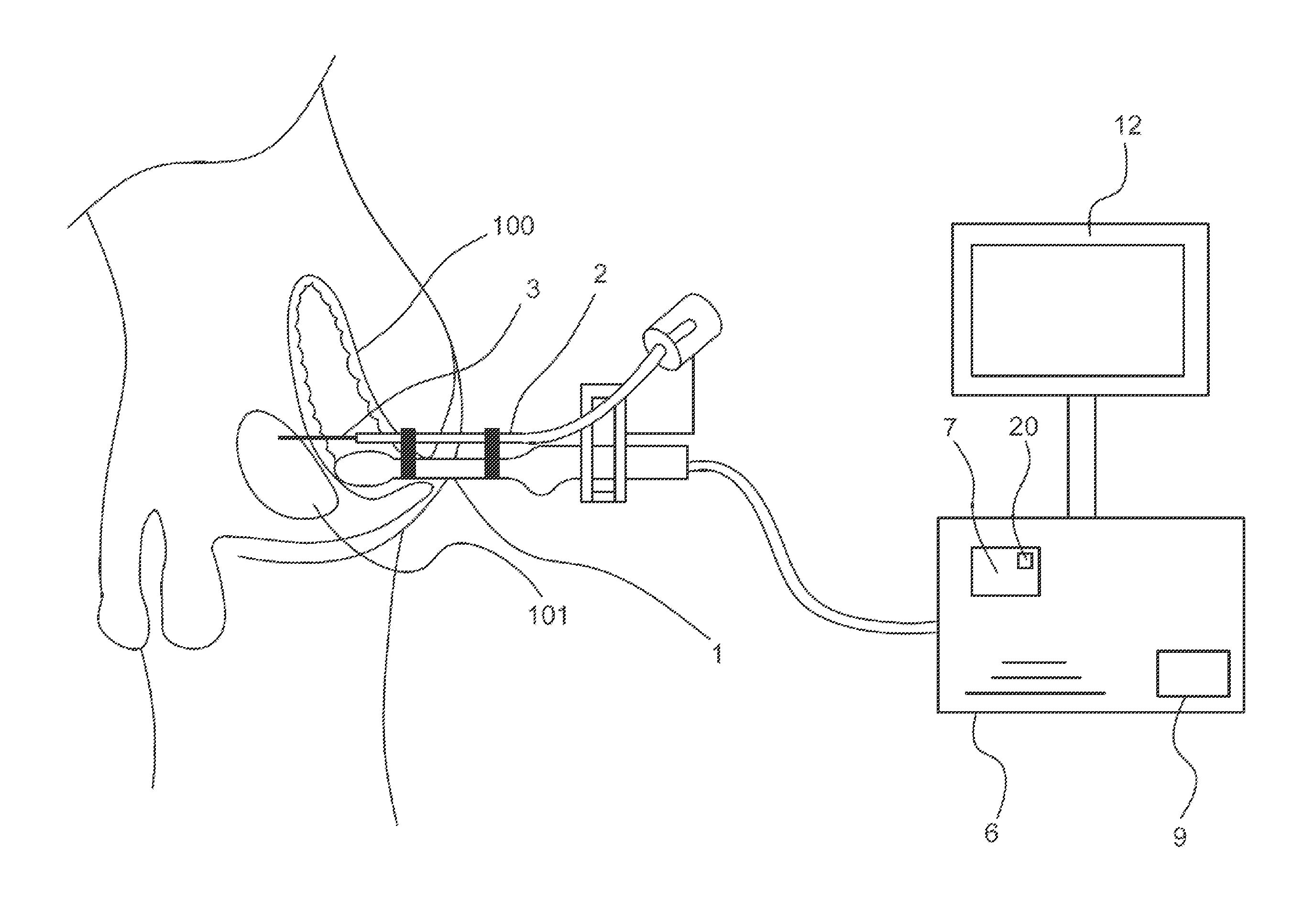
Device for guiding a medical imaging probe and method for guiding such a probe
ActiveUS20150119715A1Process can be speededPrecise positioningOrgan movement/changes detectionSurgical needlesMedical imagingBiomedical engineering
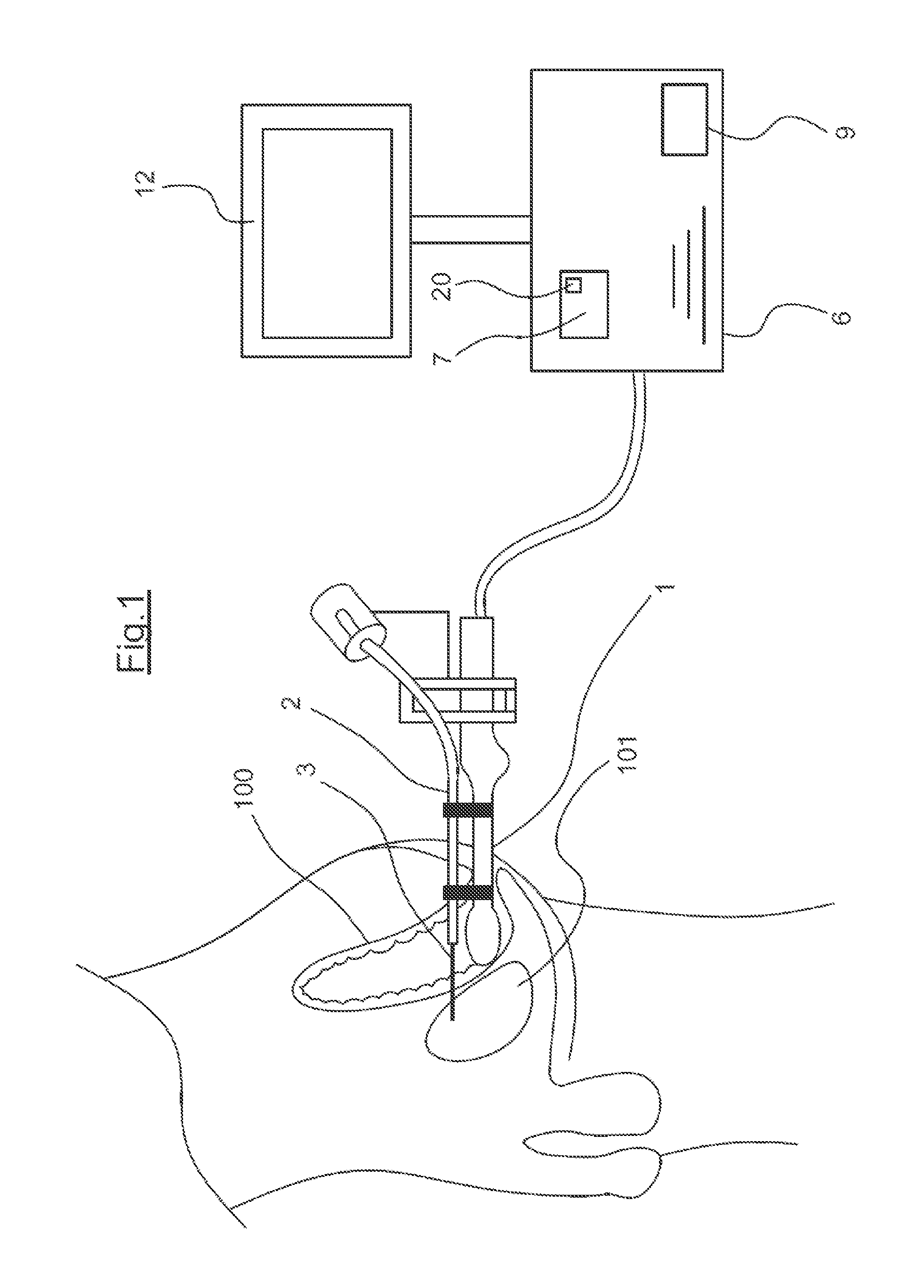
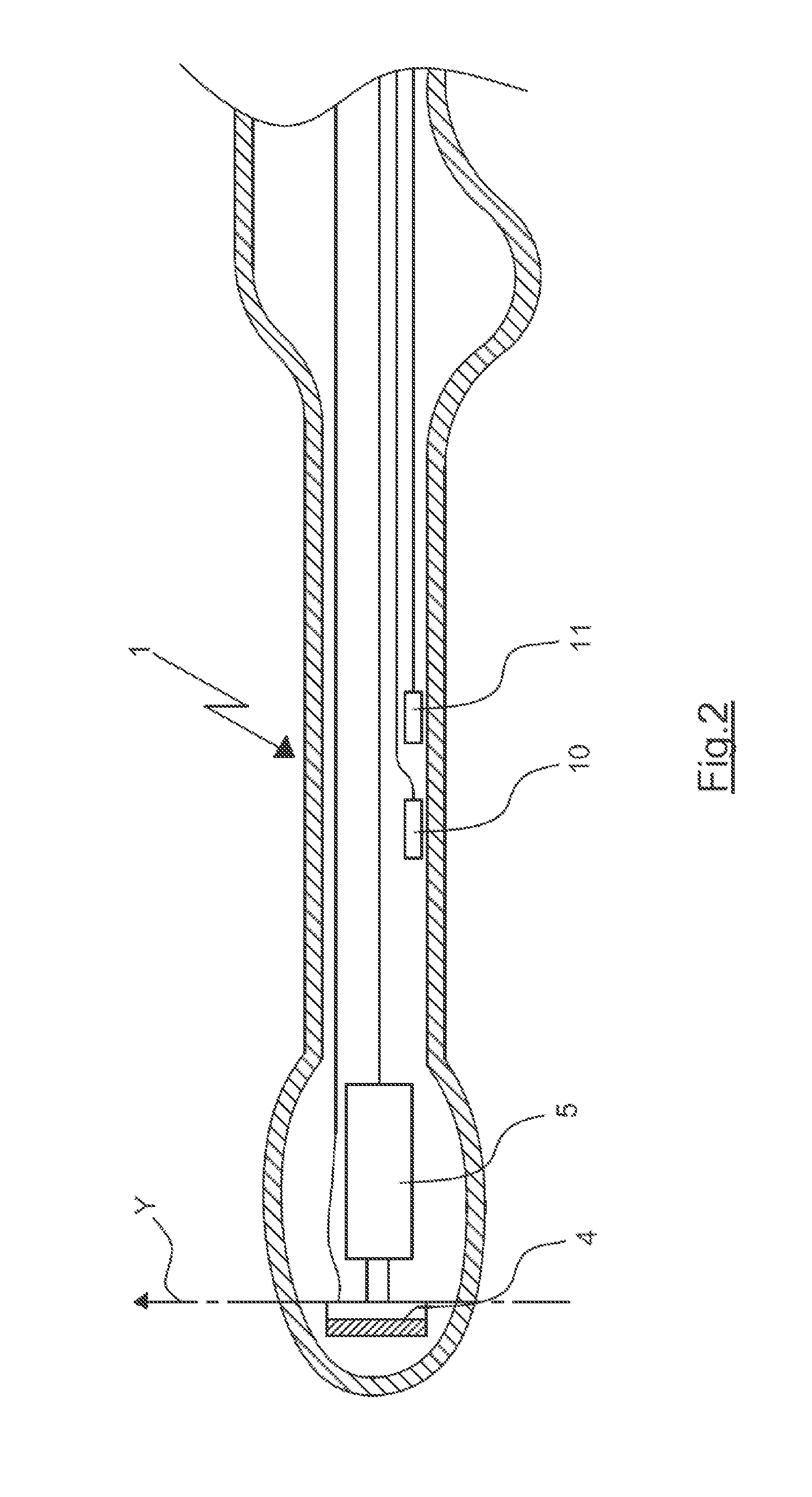
A device for guiding a medical imaging probe in order to move the probe close to an anatomical space, the probe having a mechanism for acquiring images of the anatomical space, and the device having at least one sensor mounted to the probe. The device includes a processing mechanism for deducing a rotation of the probe in the space from the signals generated by the sensor and for estimating a plurality of plausible positions of the probe relative to the anatomical space by deducing the rotation of the probe in the space. The processing mechanism is arranged such as to determine the position of the probe relative to the anatomical space from the plausible positions. Also disclosed is a method for guiding the probe.
Owner:KOELIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com