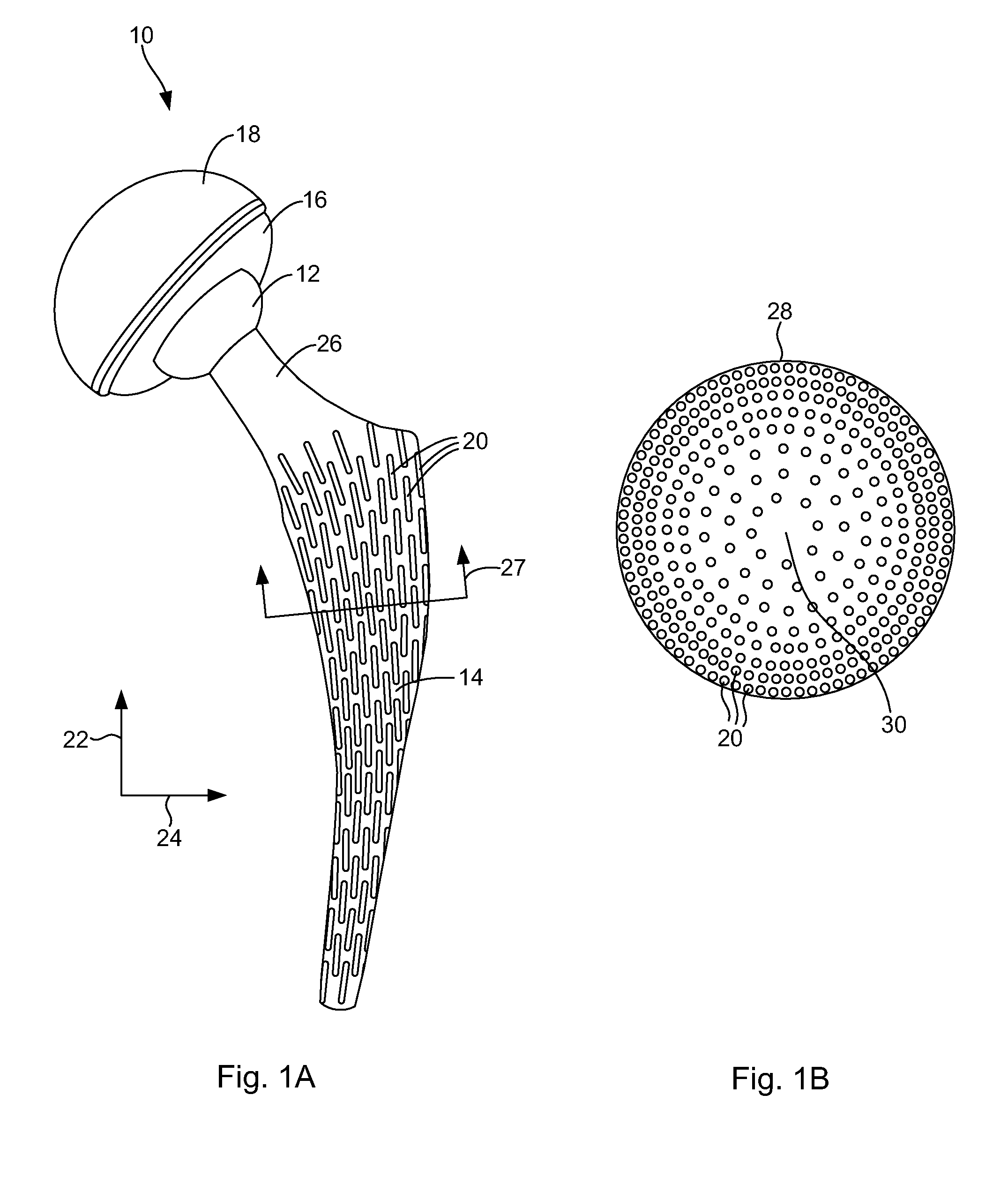
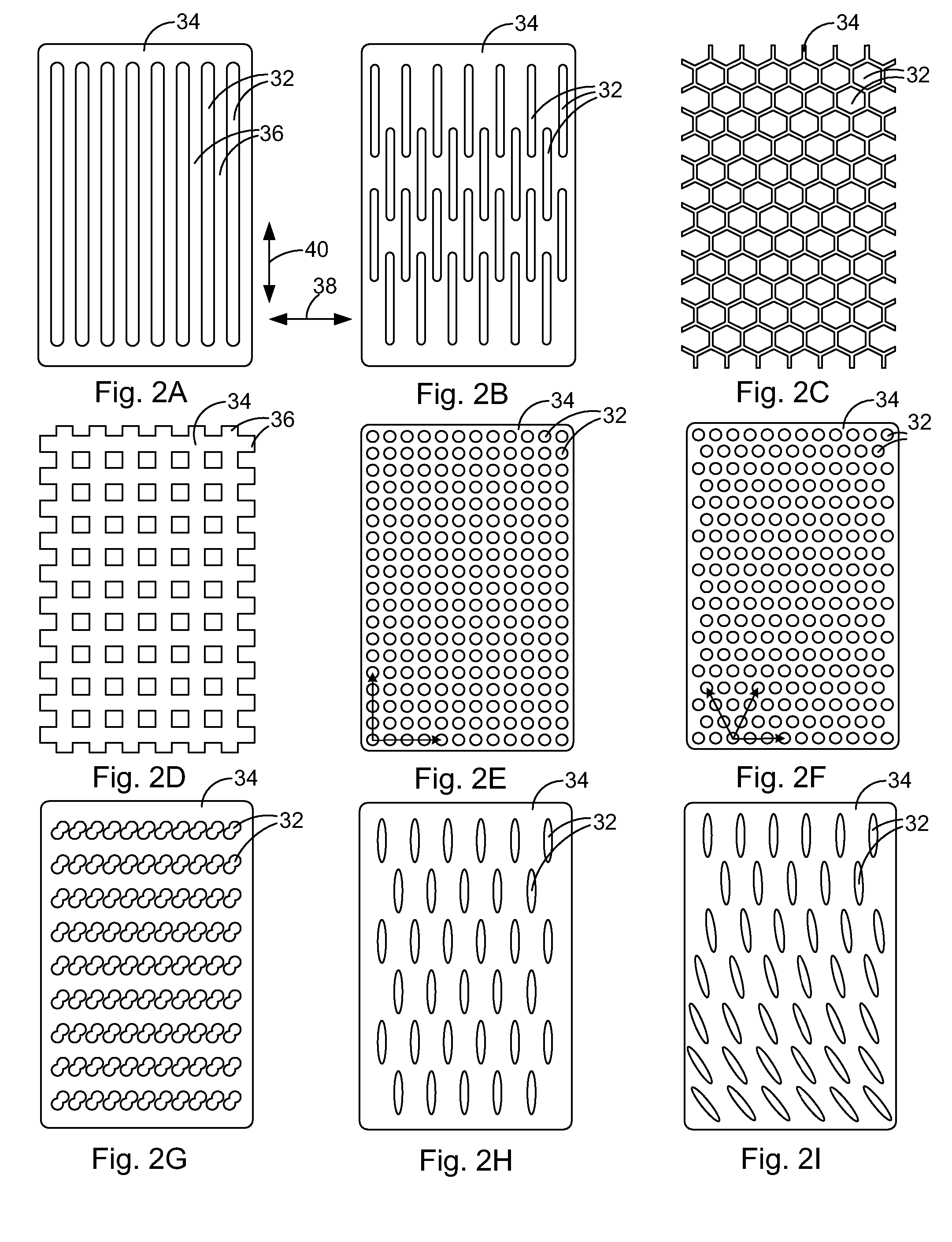
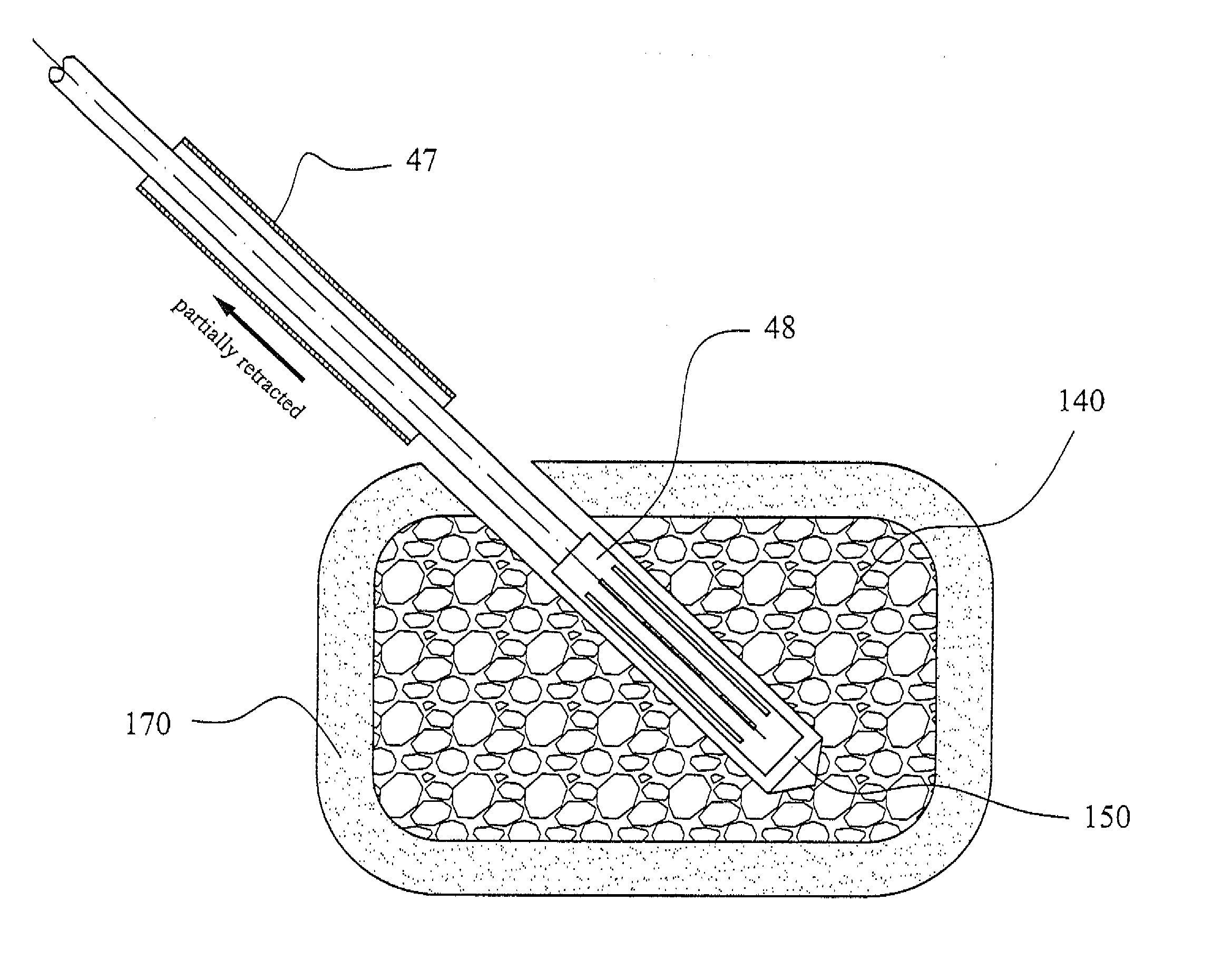
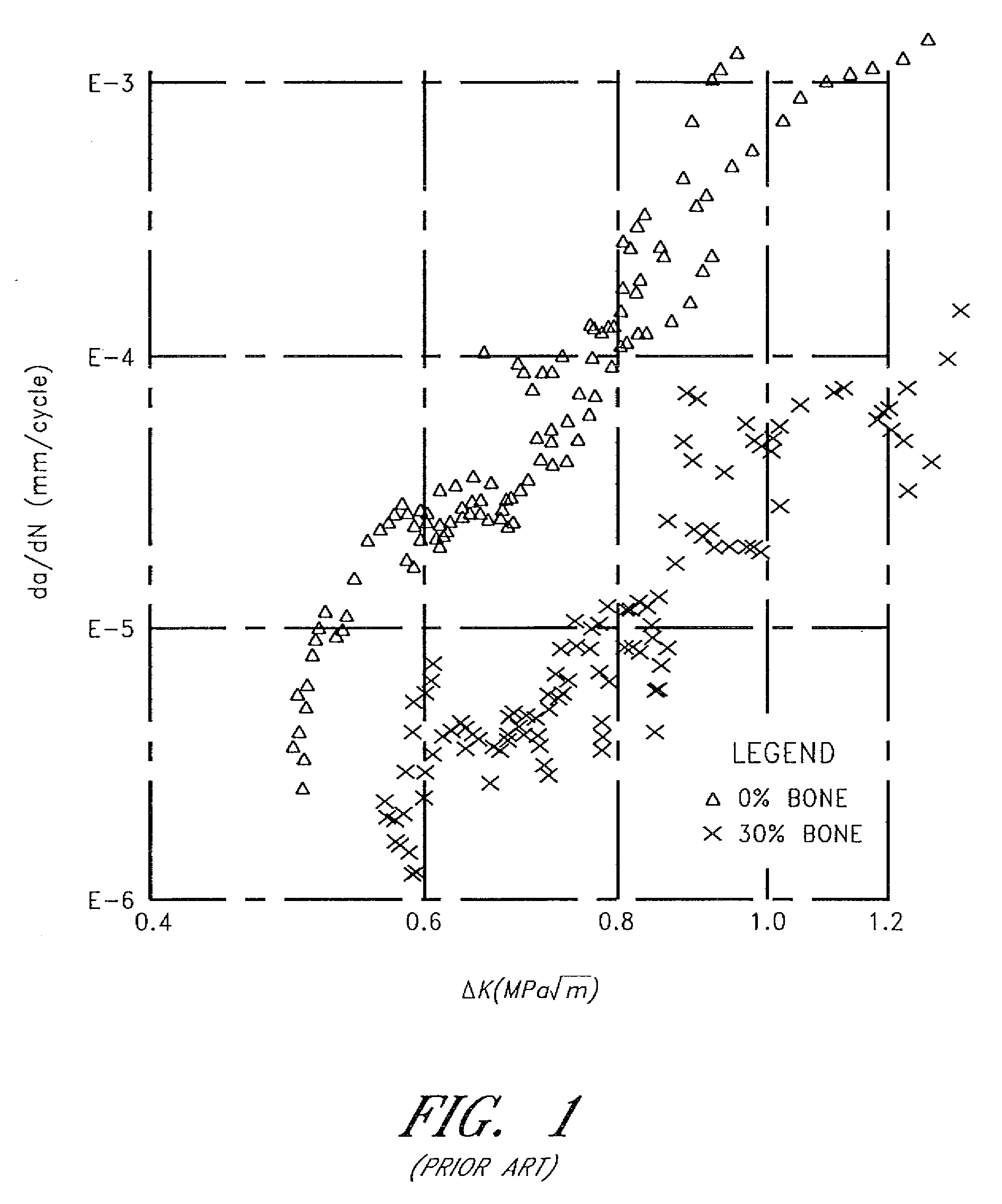
Patents
Literature
367 results about "Bone ingrowth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
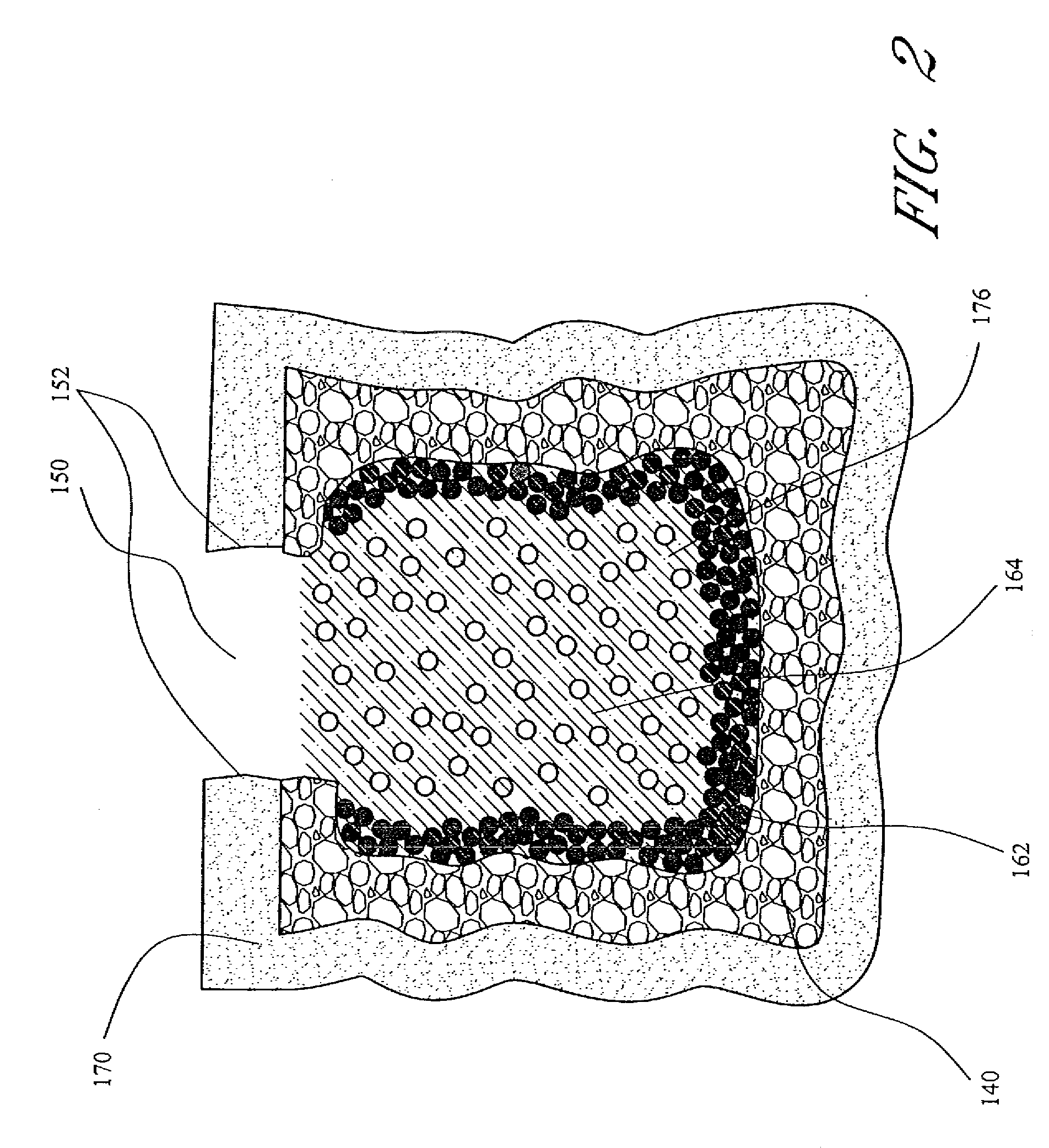
Bone ingrowth refers to the formation of bone within an irregular surface of an implant, which improves the implant’s integration into bone.
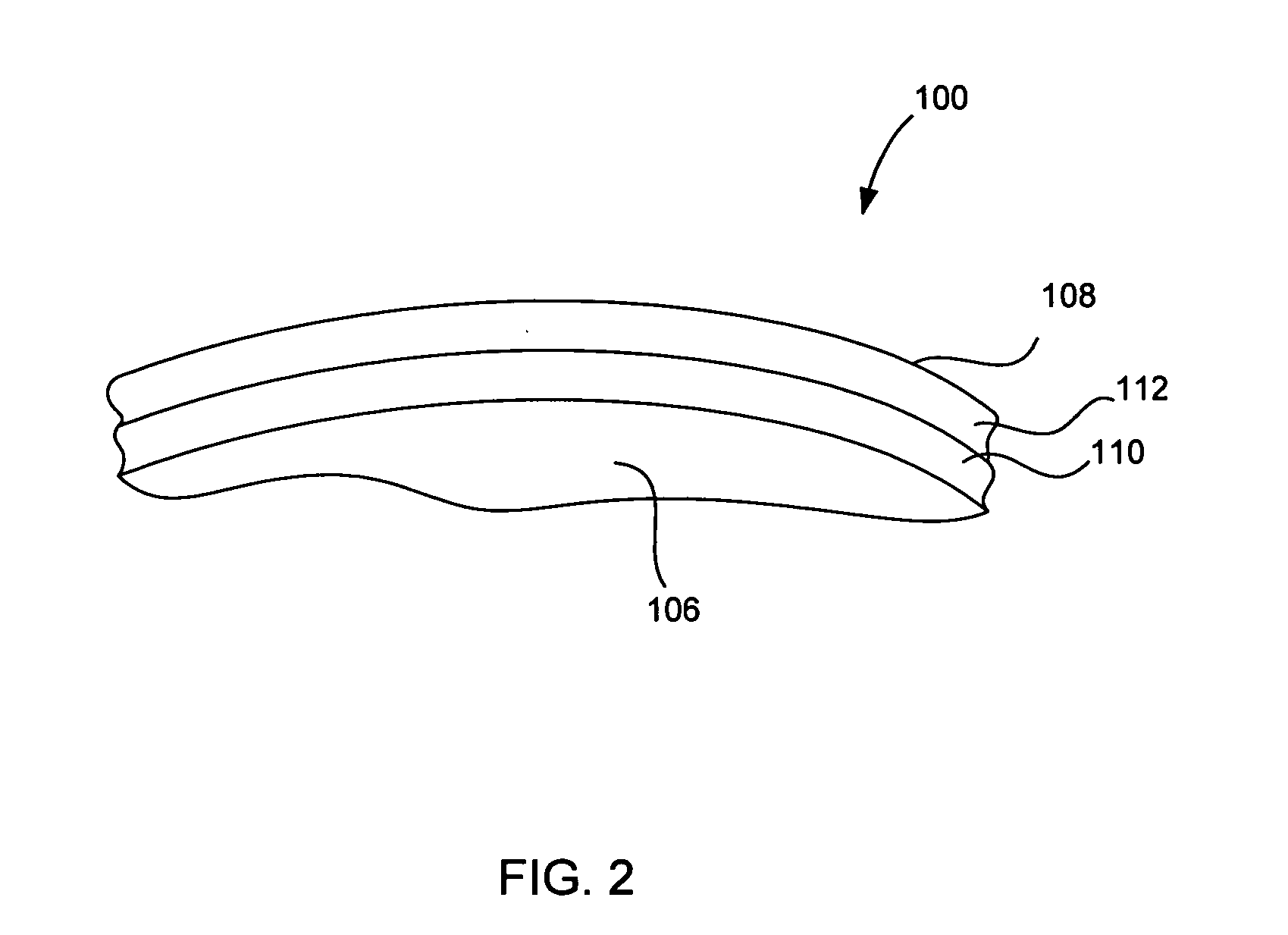
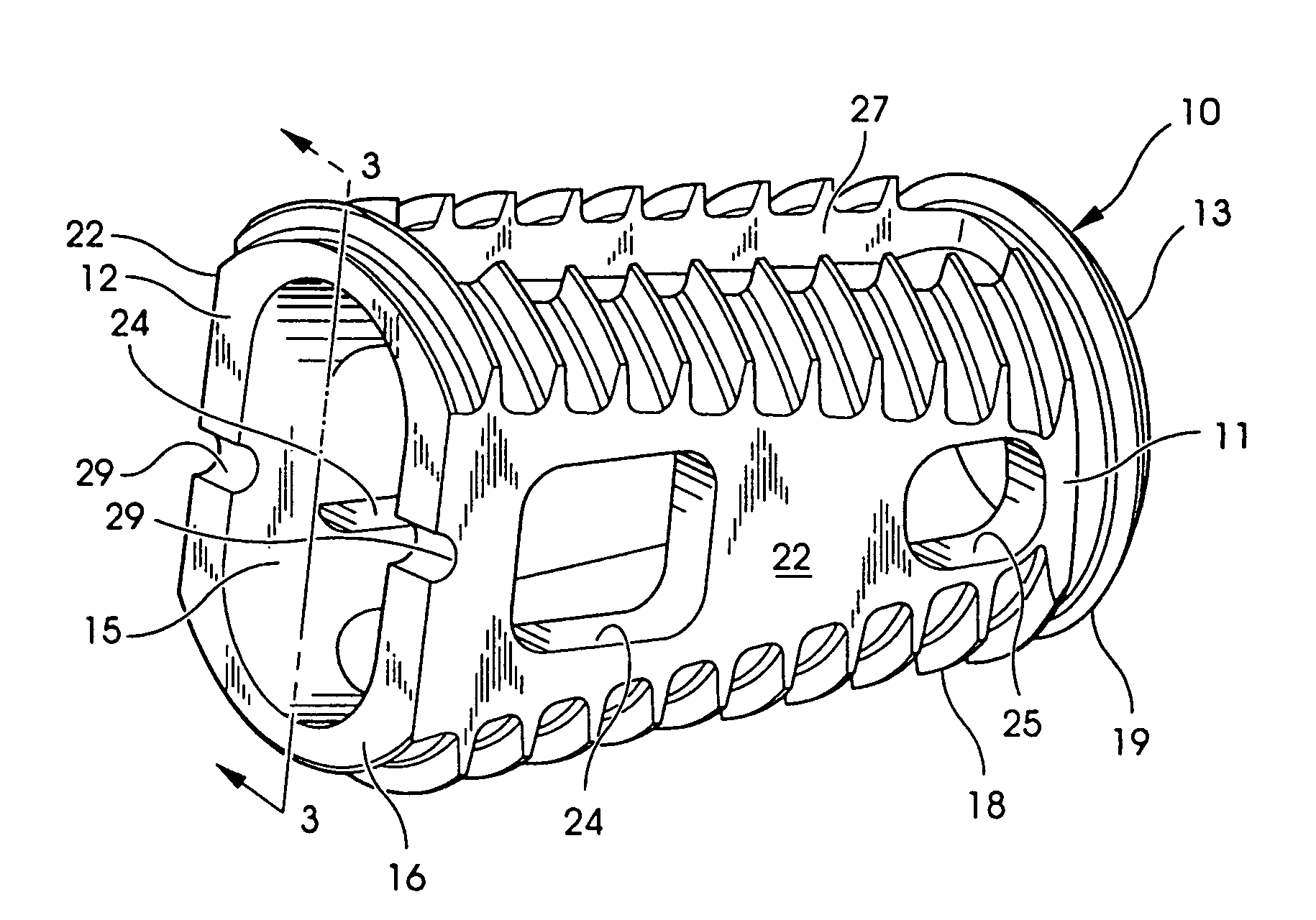
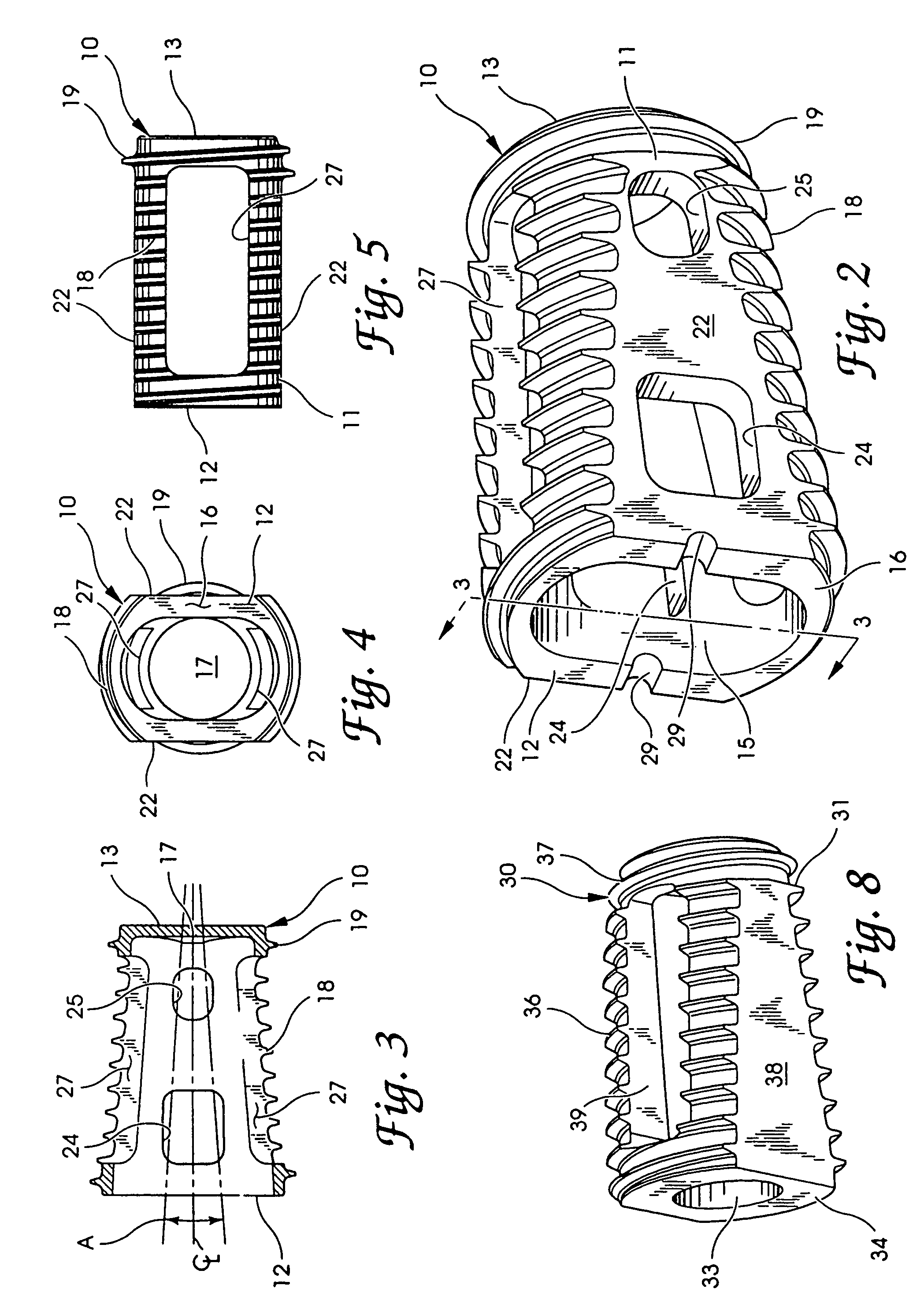
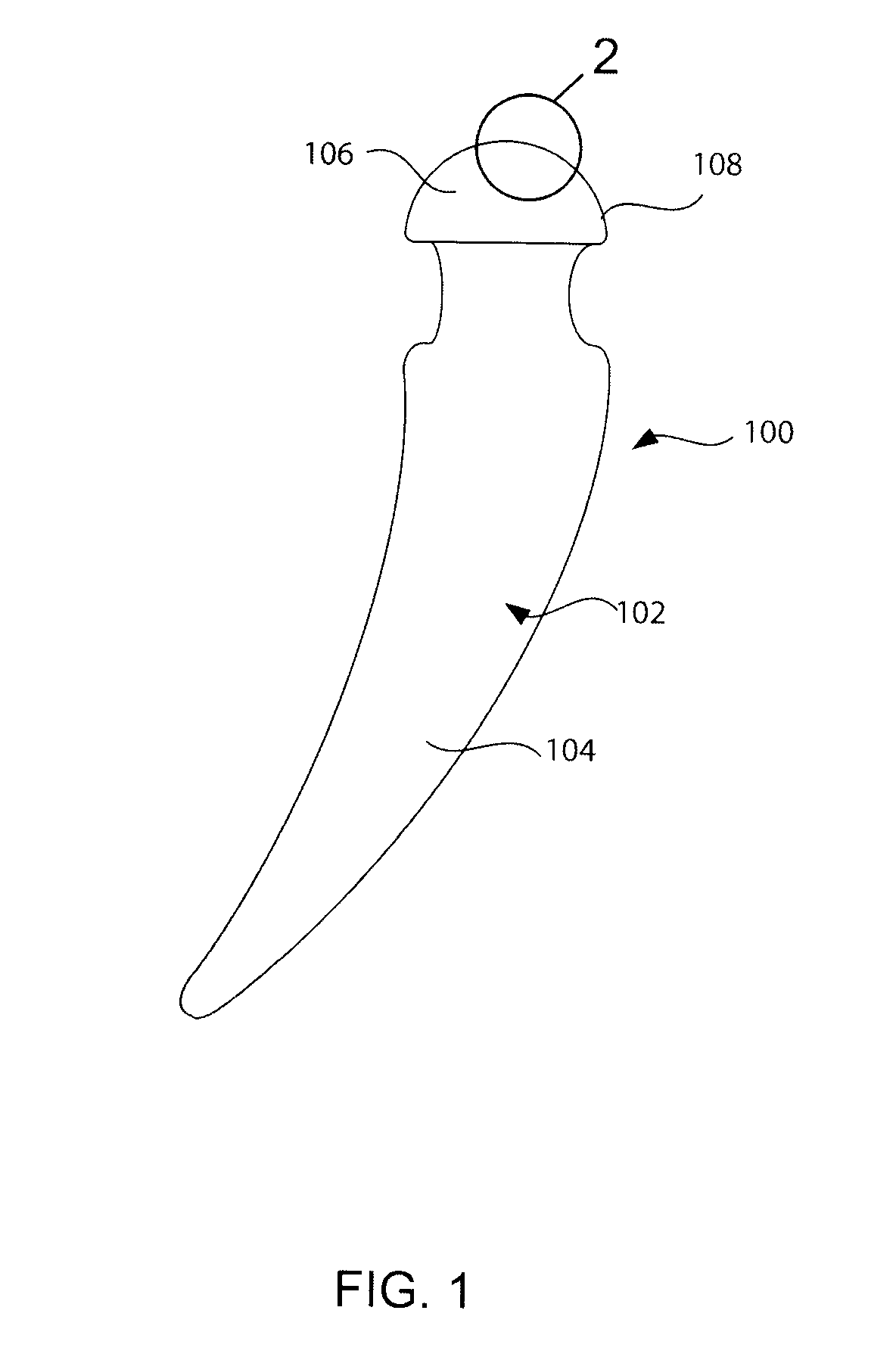
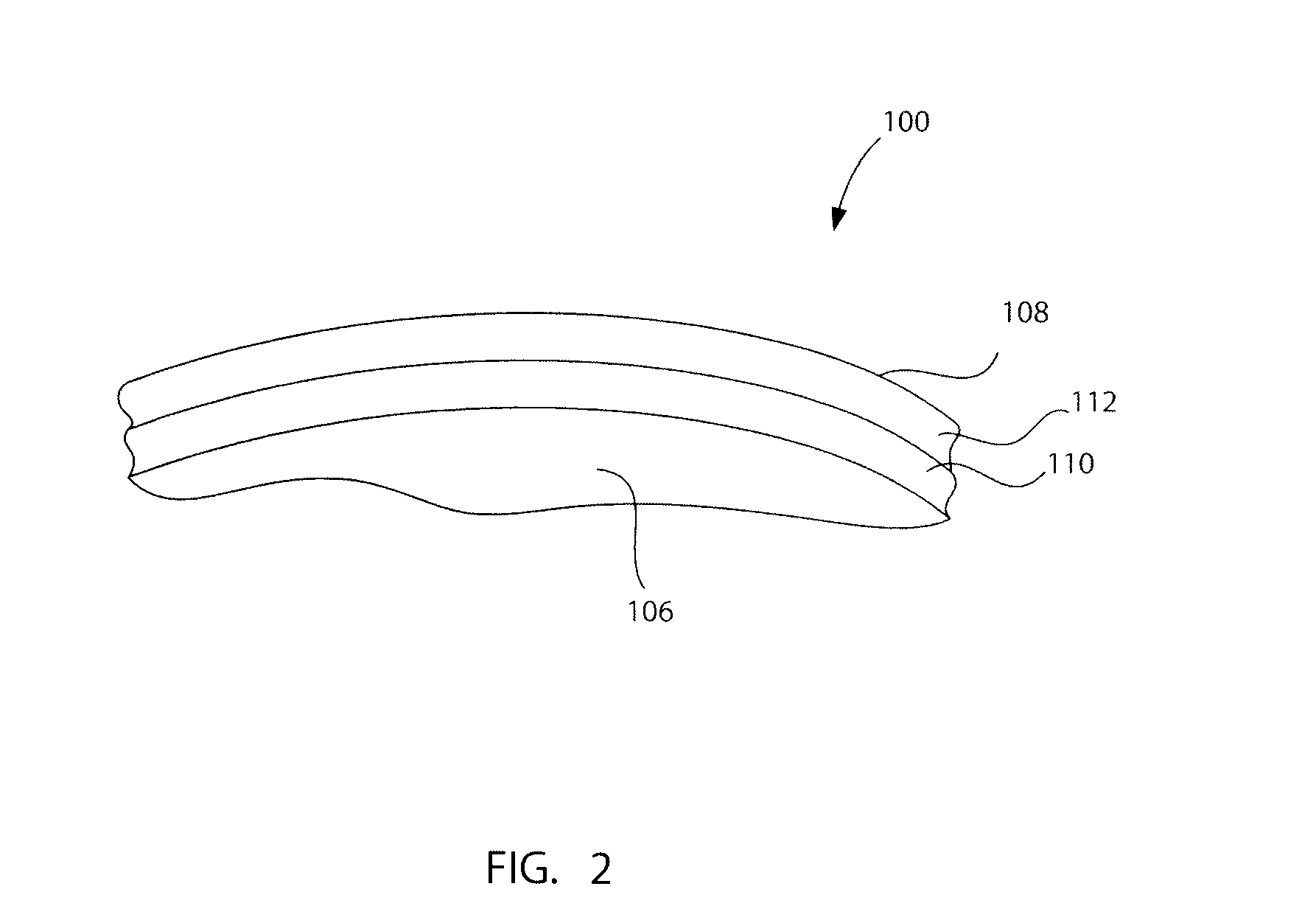
Articulating spinal implant
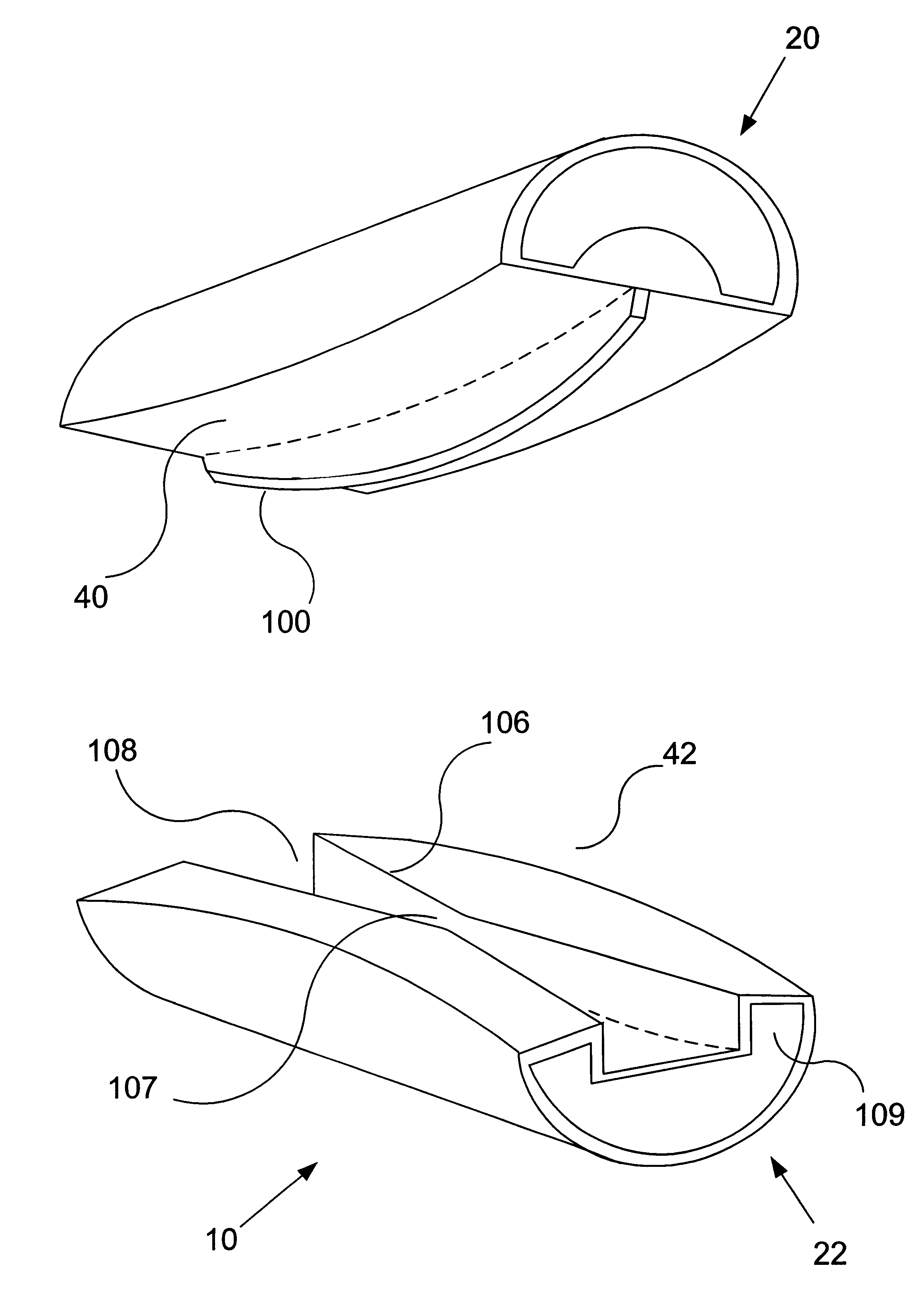
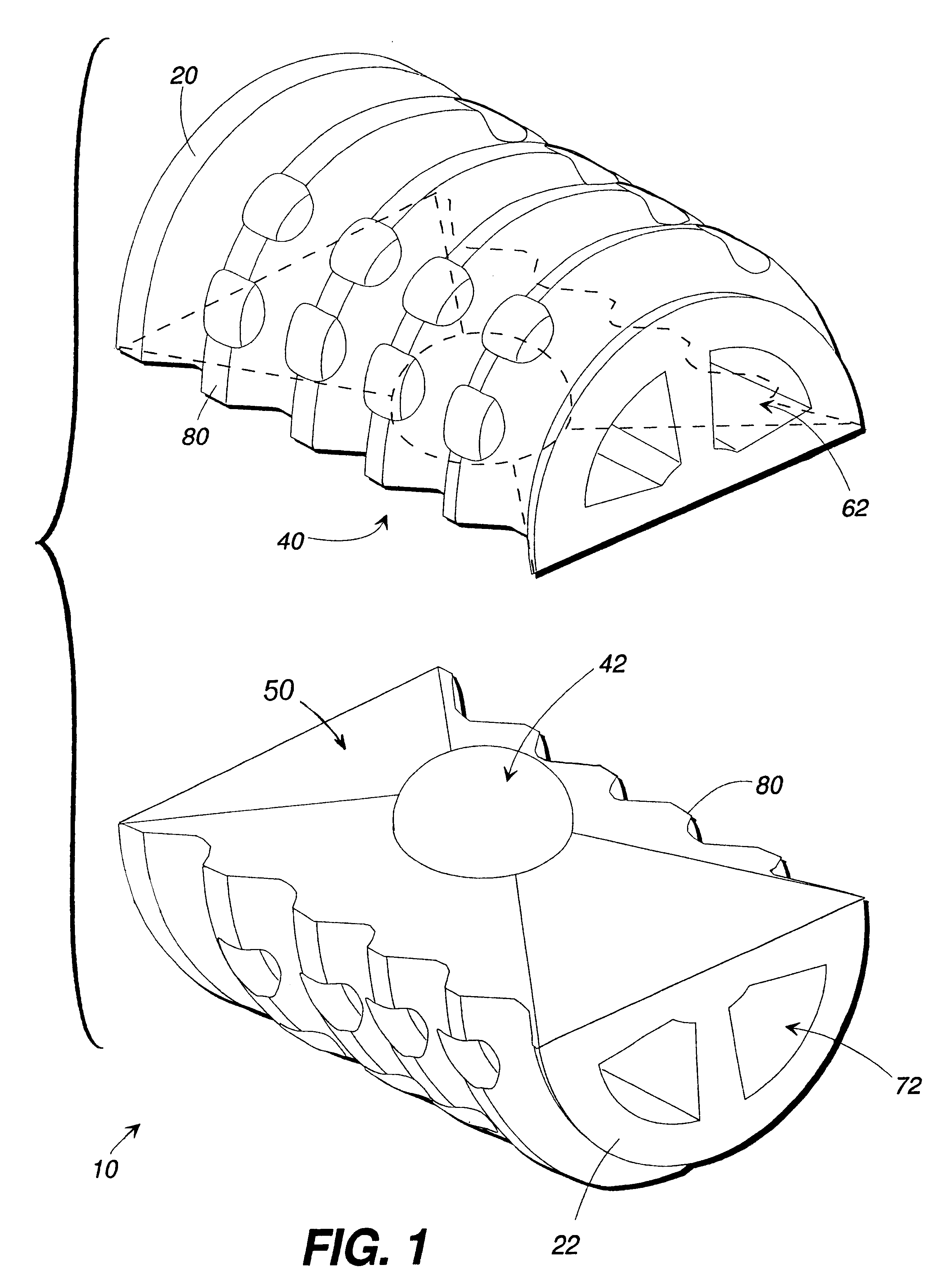
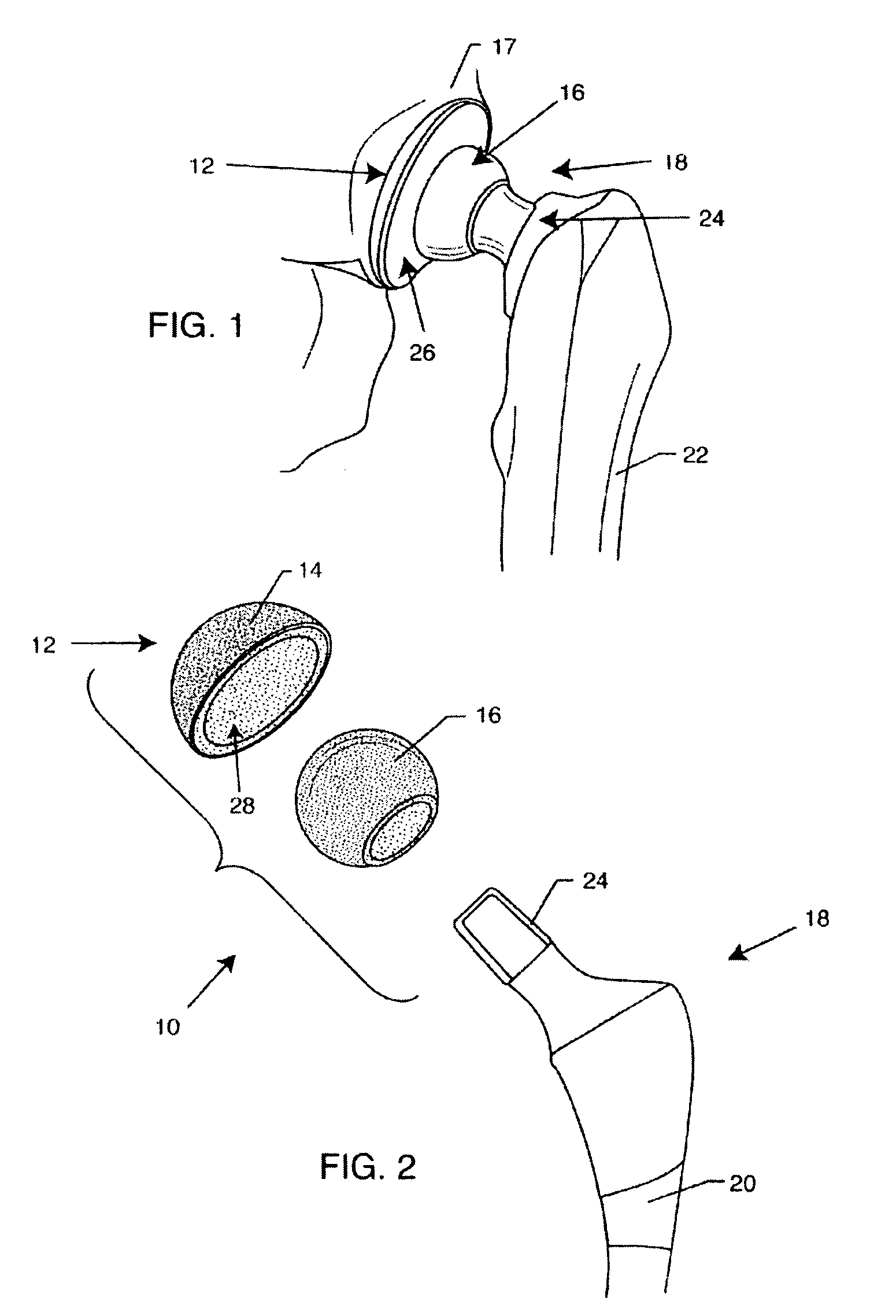
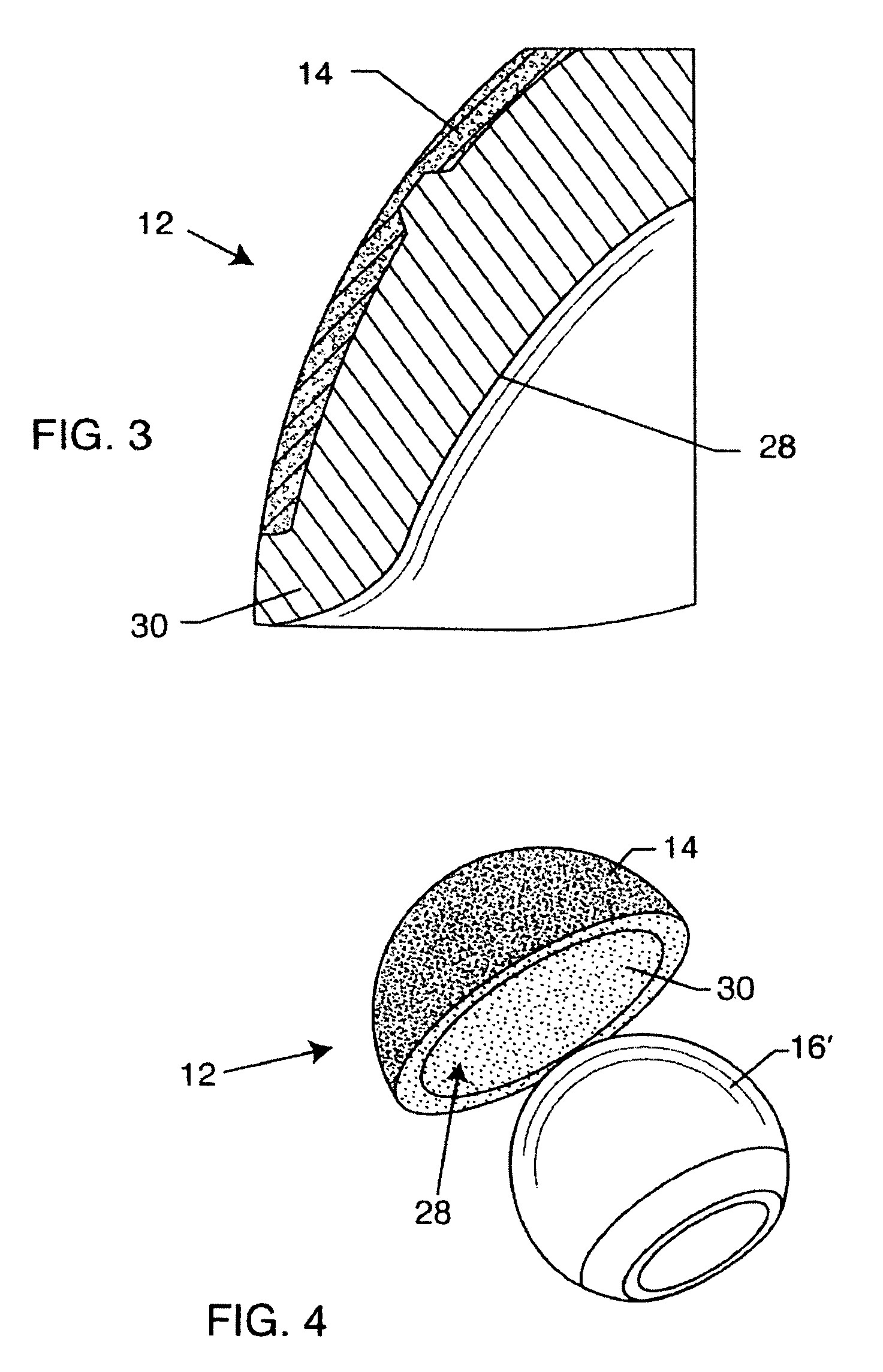
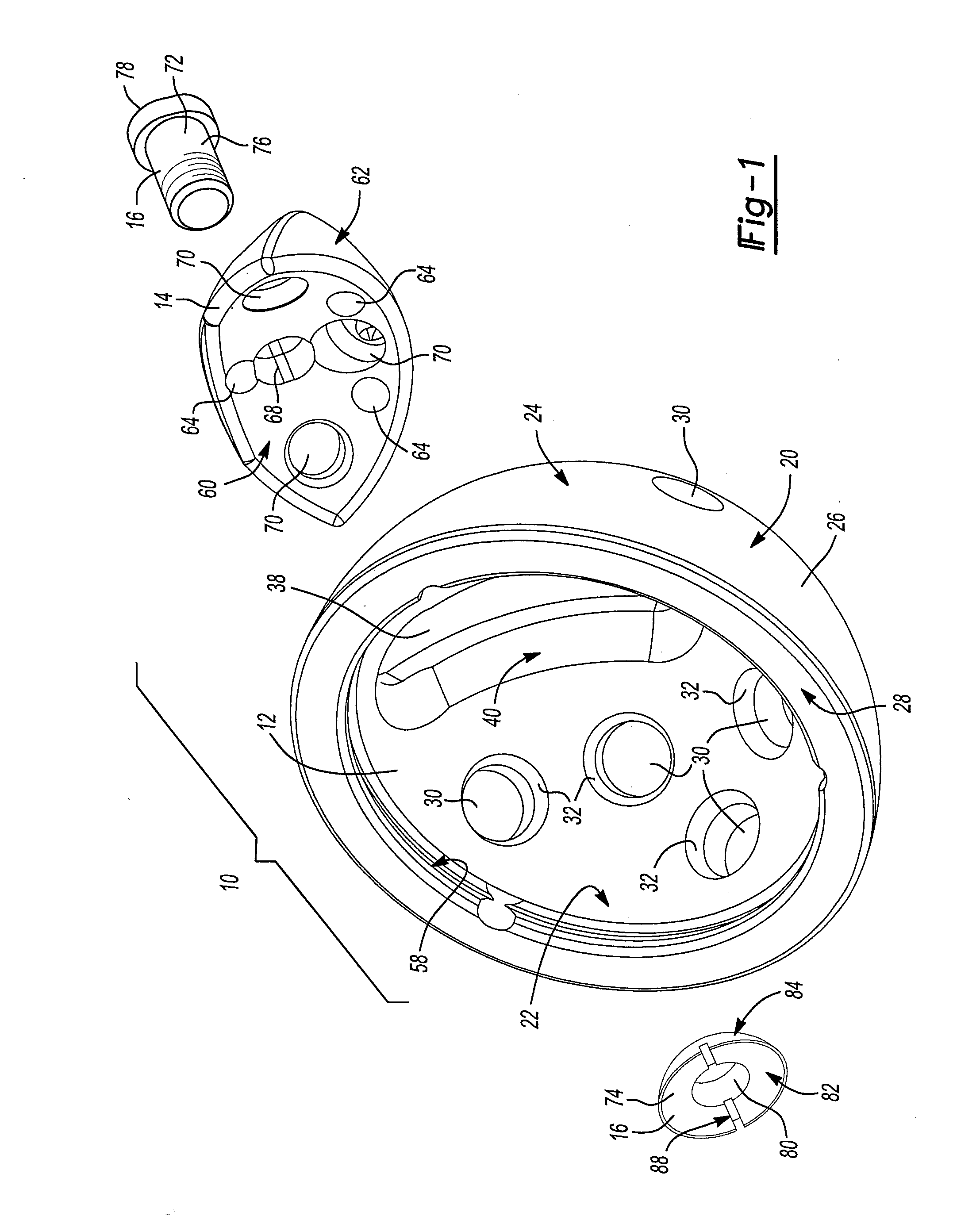
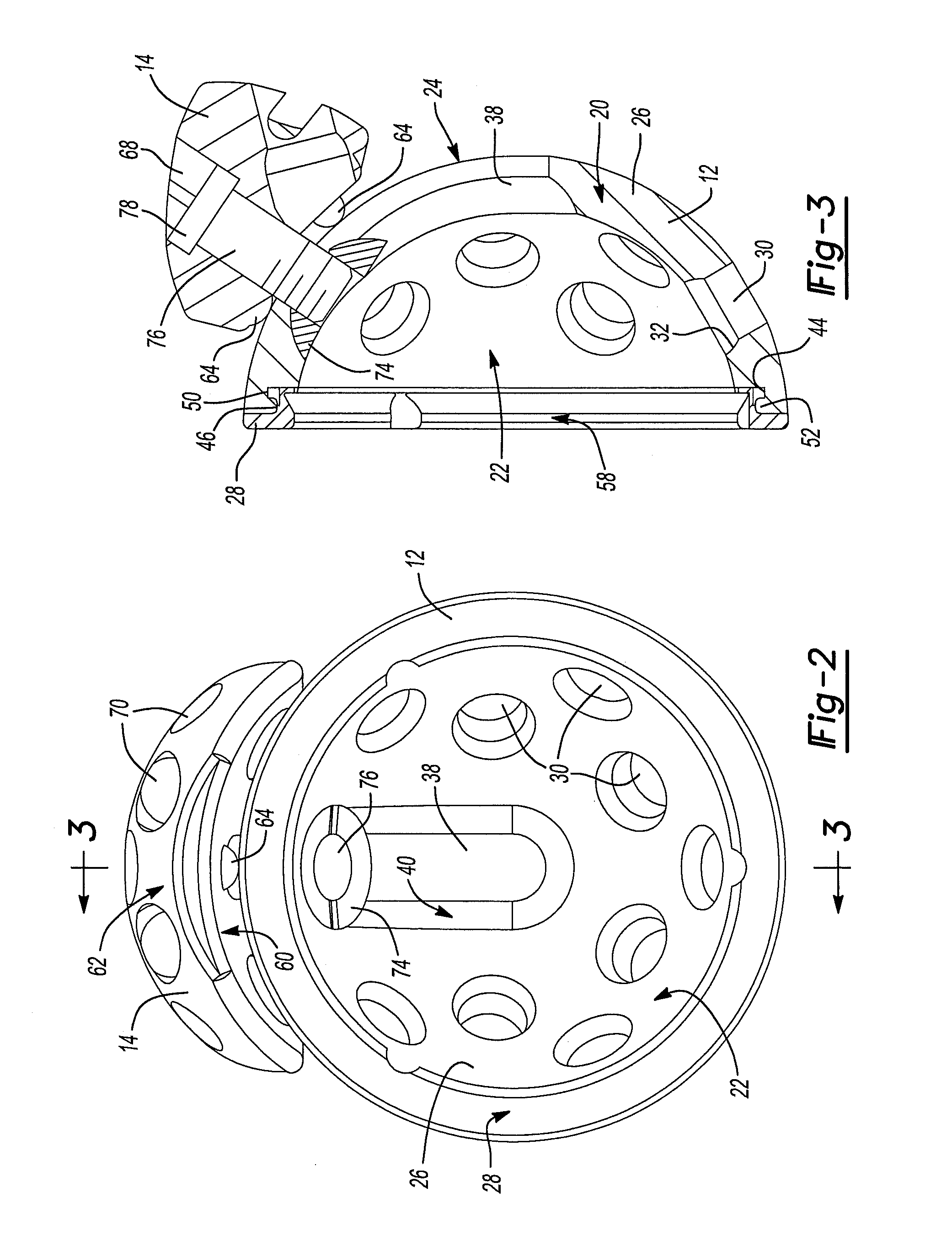
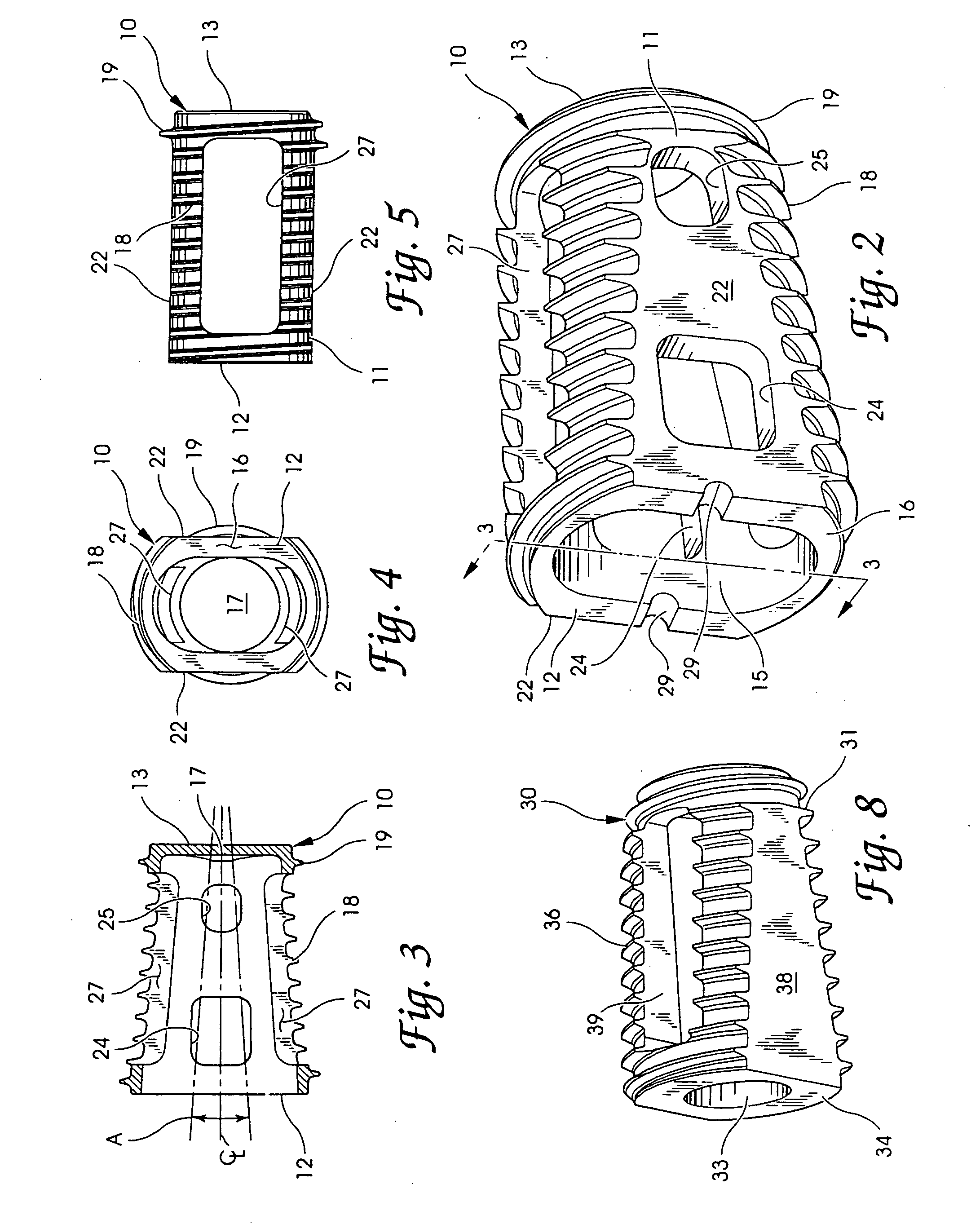
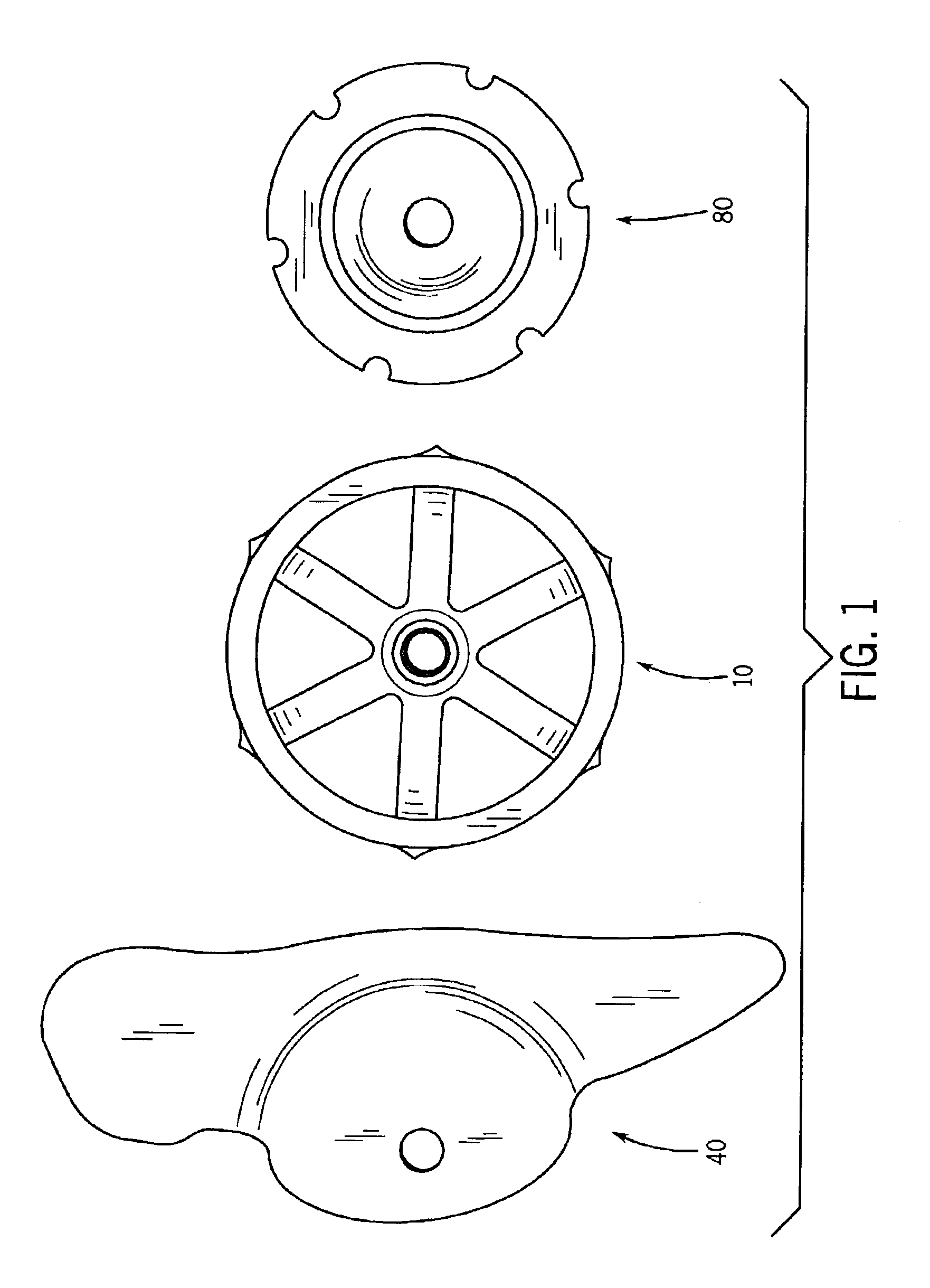
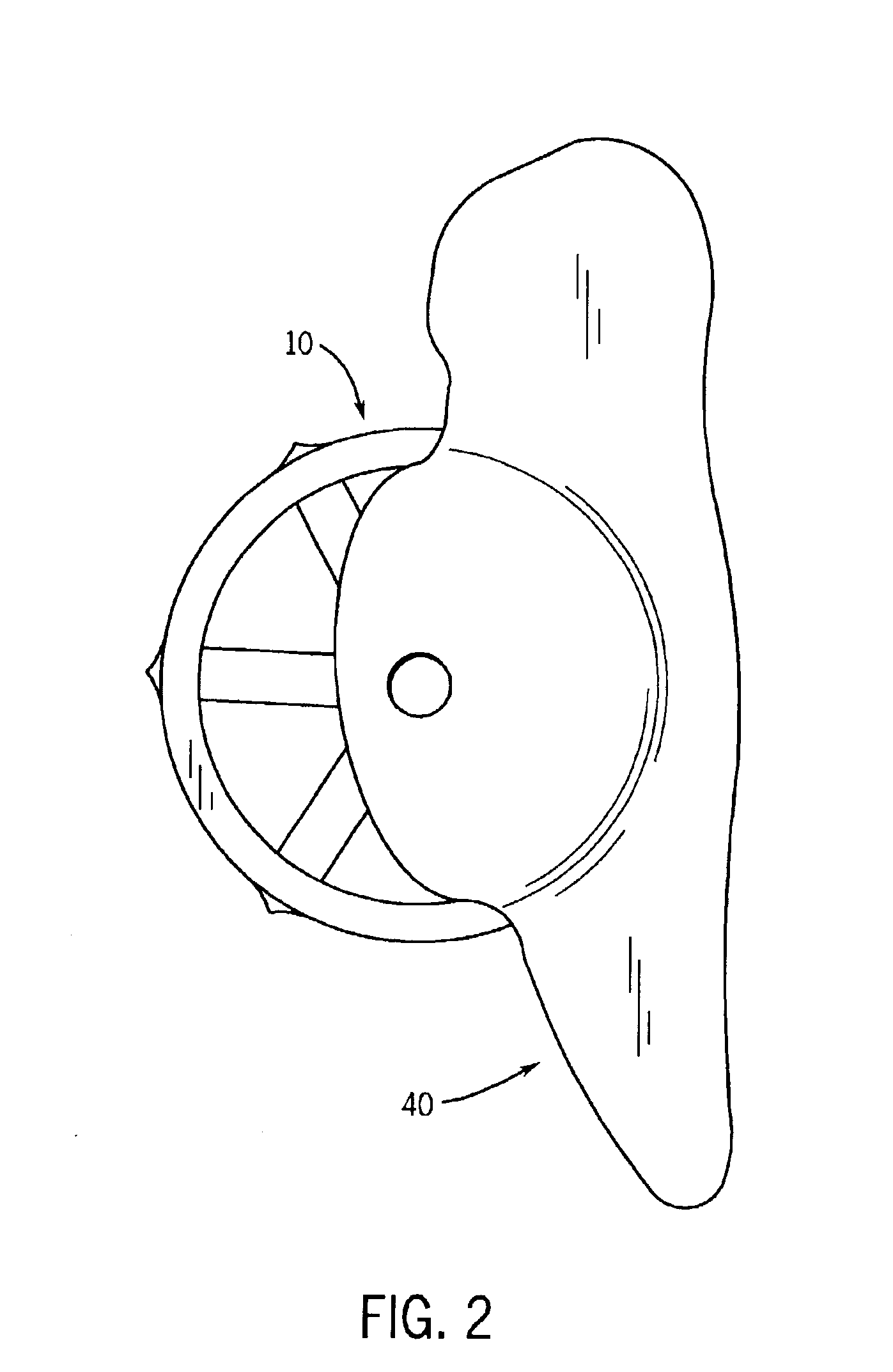
A spinal implant for intervertebral disc replacement. The implant is formed from two hemicylindrical elements, each engaging one of an adjacent pair of vertebrae. An articulating ball-and-socket joint between the two elements resists compression and lateral movement between the vertebra, but allows pivotal movement, thereby preserving mobility. Fusion chambers are provided for allowing bone ingrowth to fuse the elements to the vertebrae. Biocompatible, bioreabsorbable struts, shims, fillers and / or end caps are provided for temporary stabilization of the first and second hemicylindrical elements. Bone chips removed from the vertebrae during implantation or bone growth stimulators can be inserted into the fusion chamber or otherwise applied to the implant to enhance bone ingrowth.
Owner:WARSAW ORTHOPEDIC INC
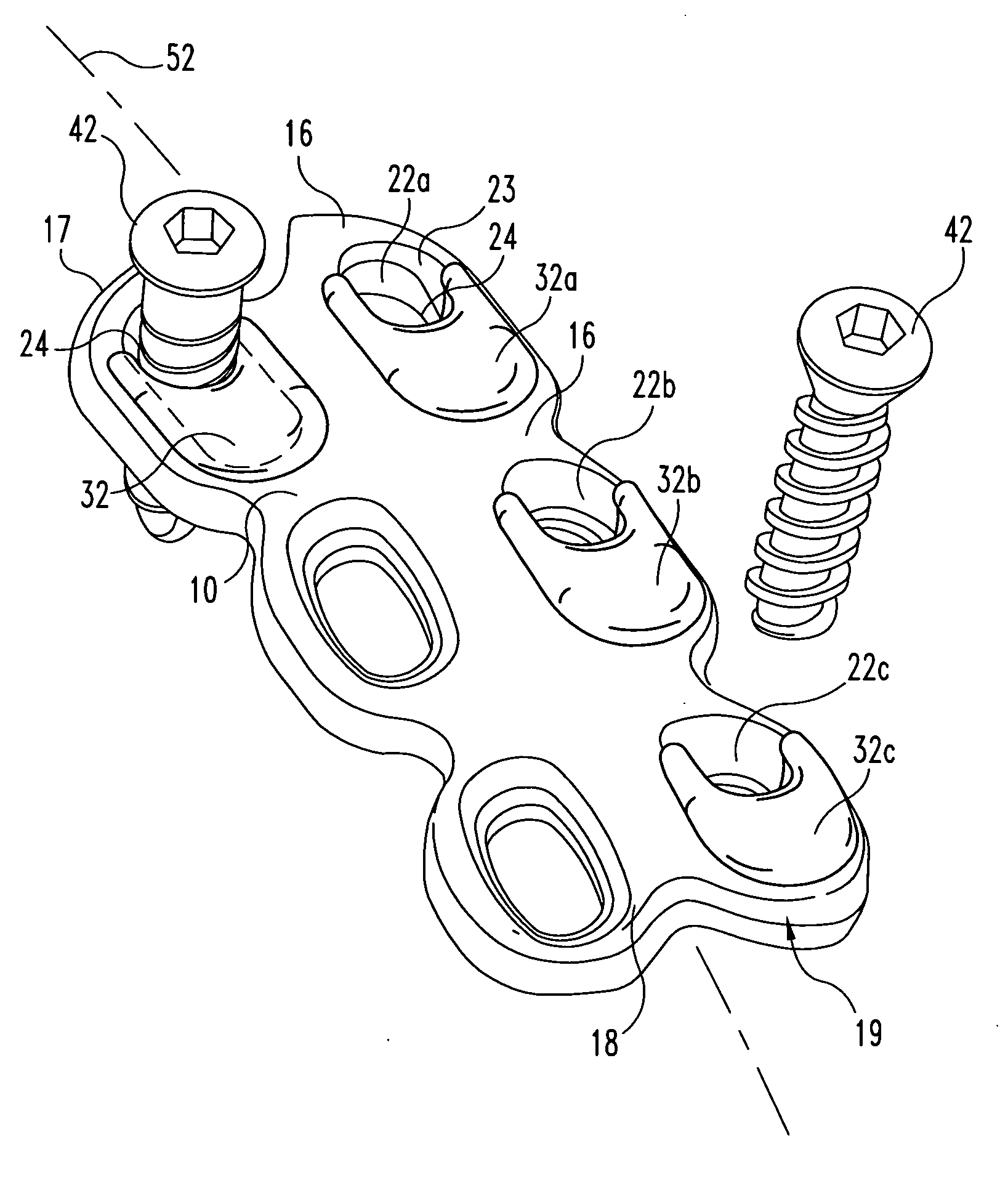
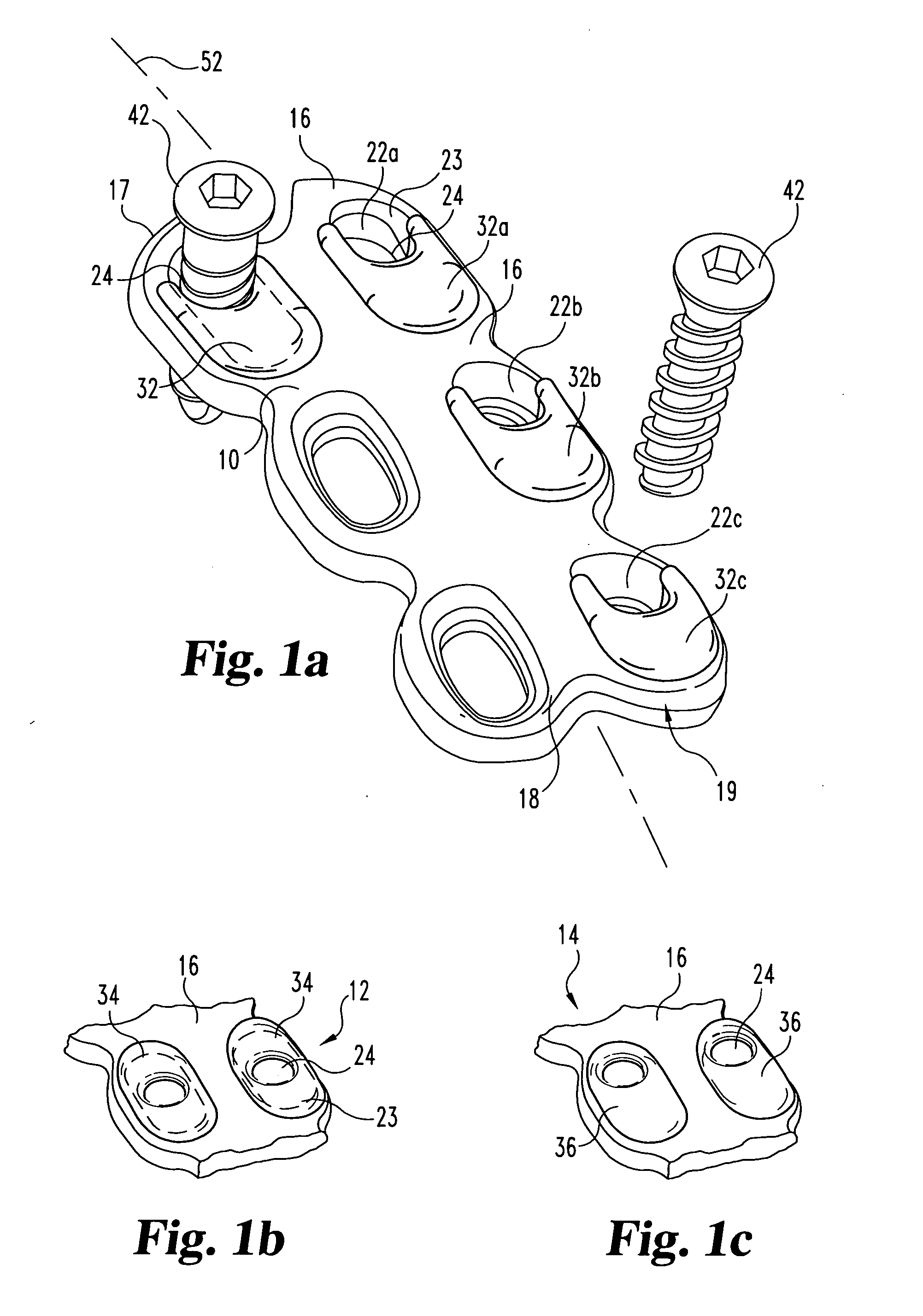
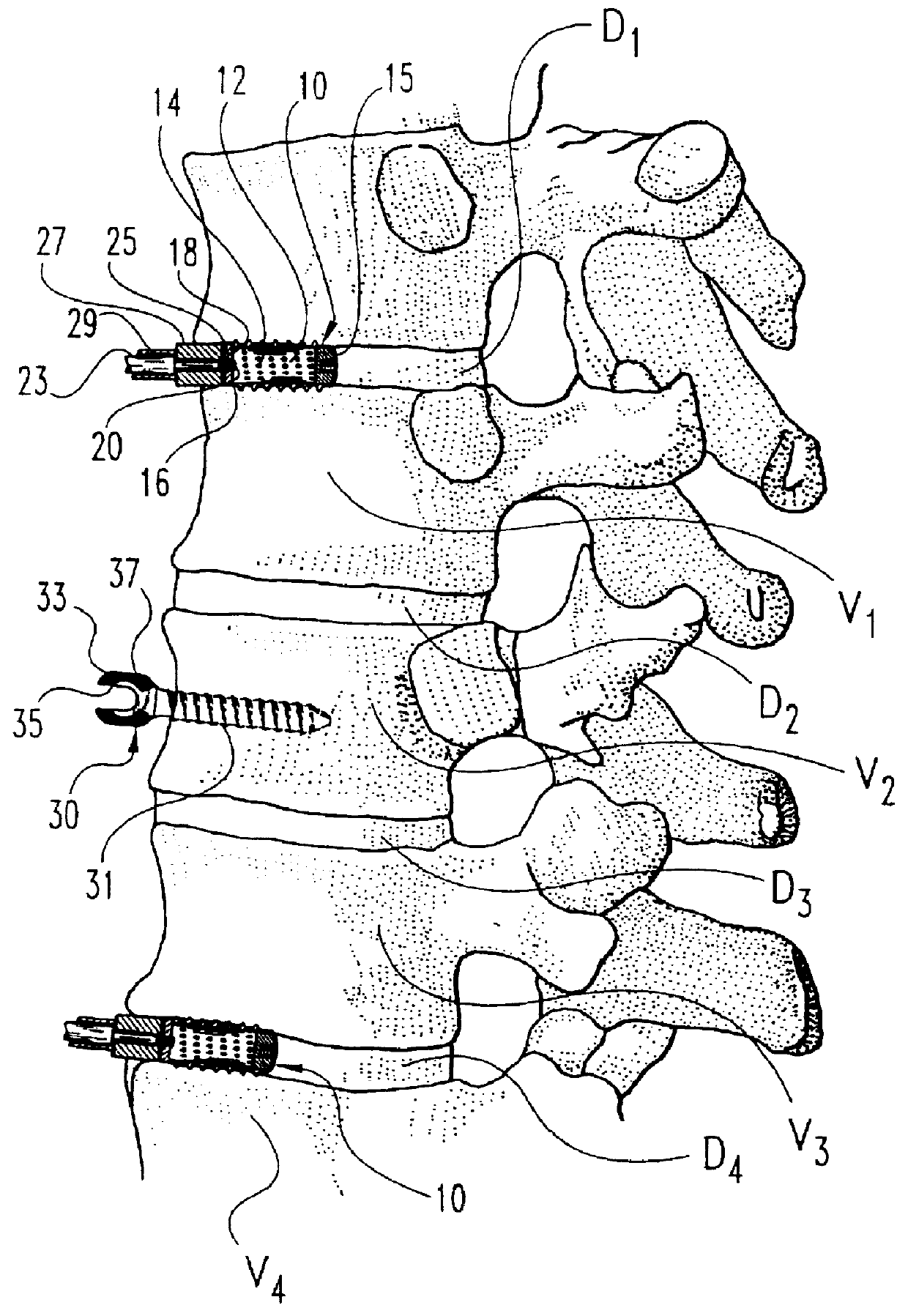
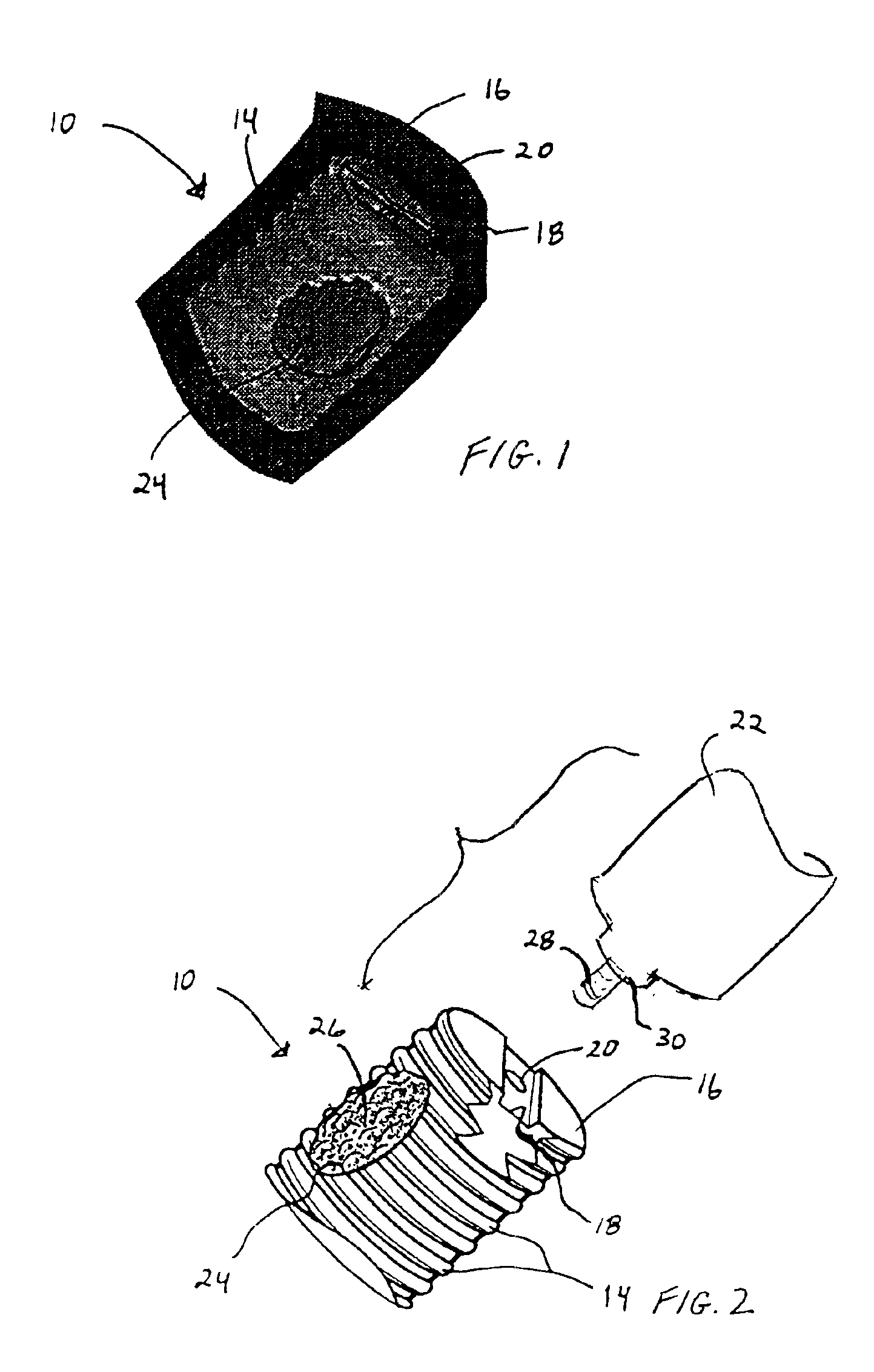
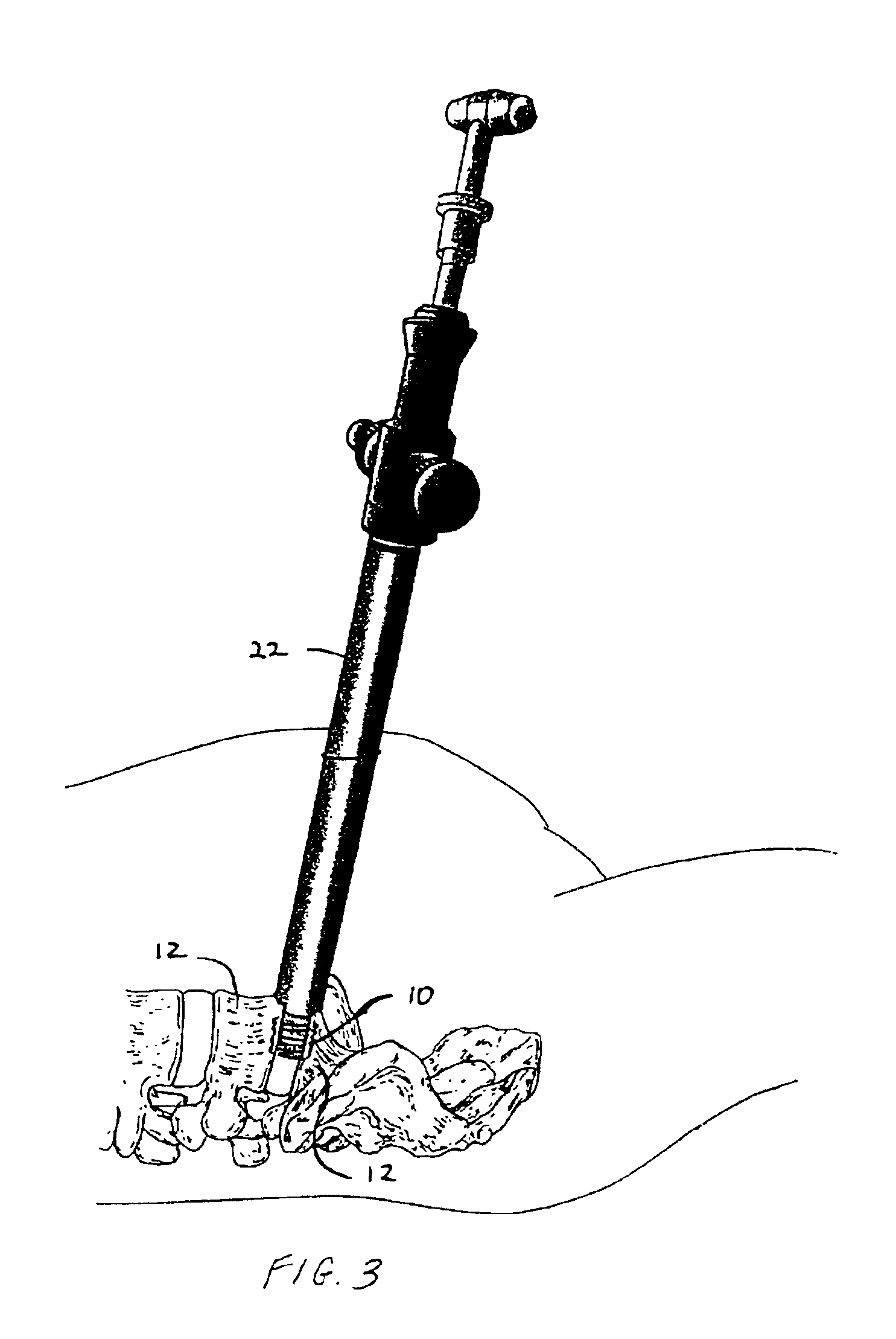
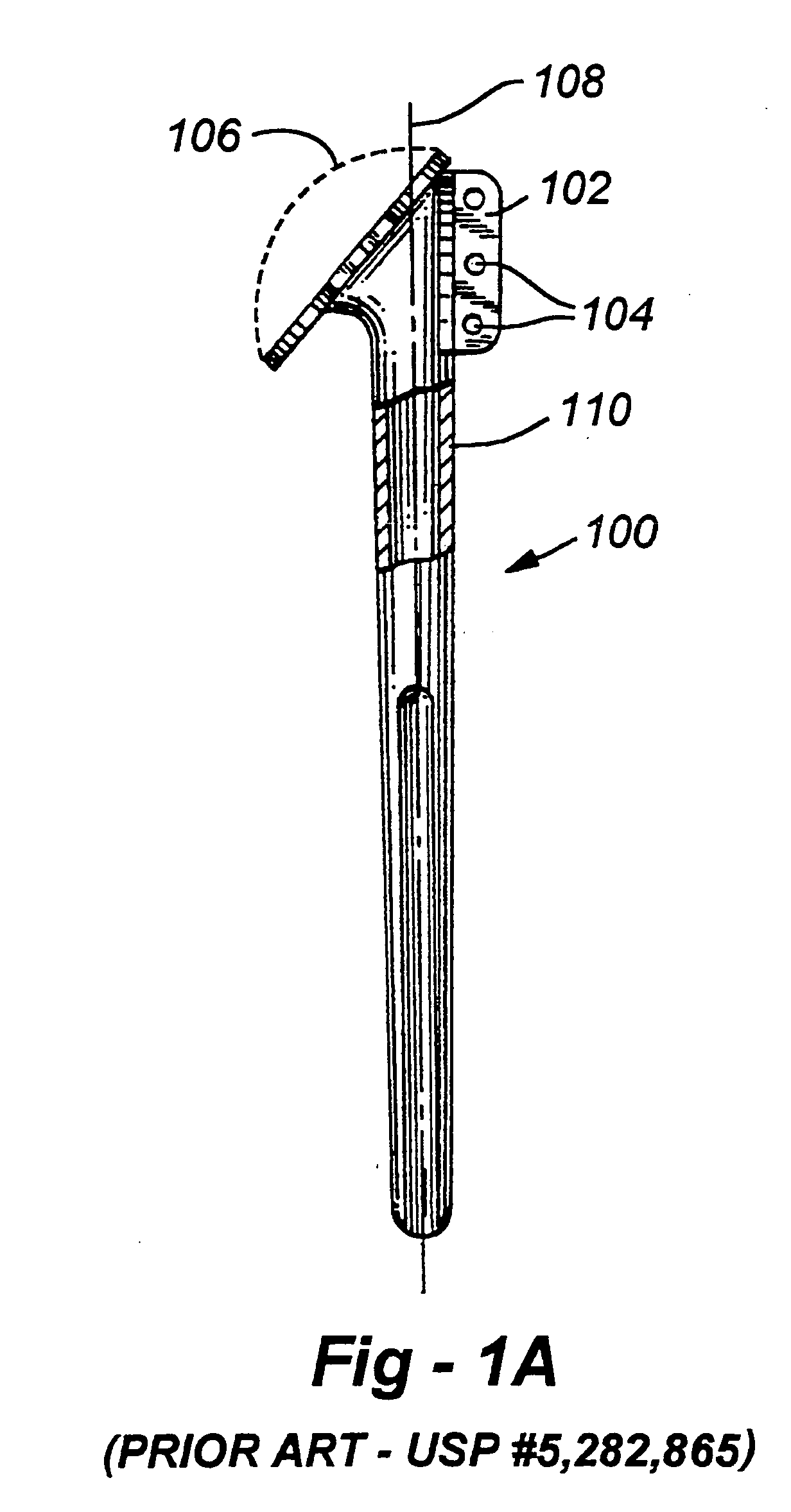
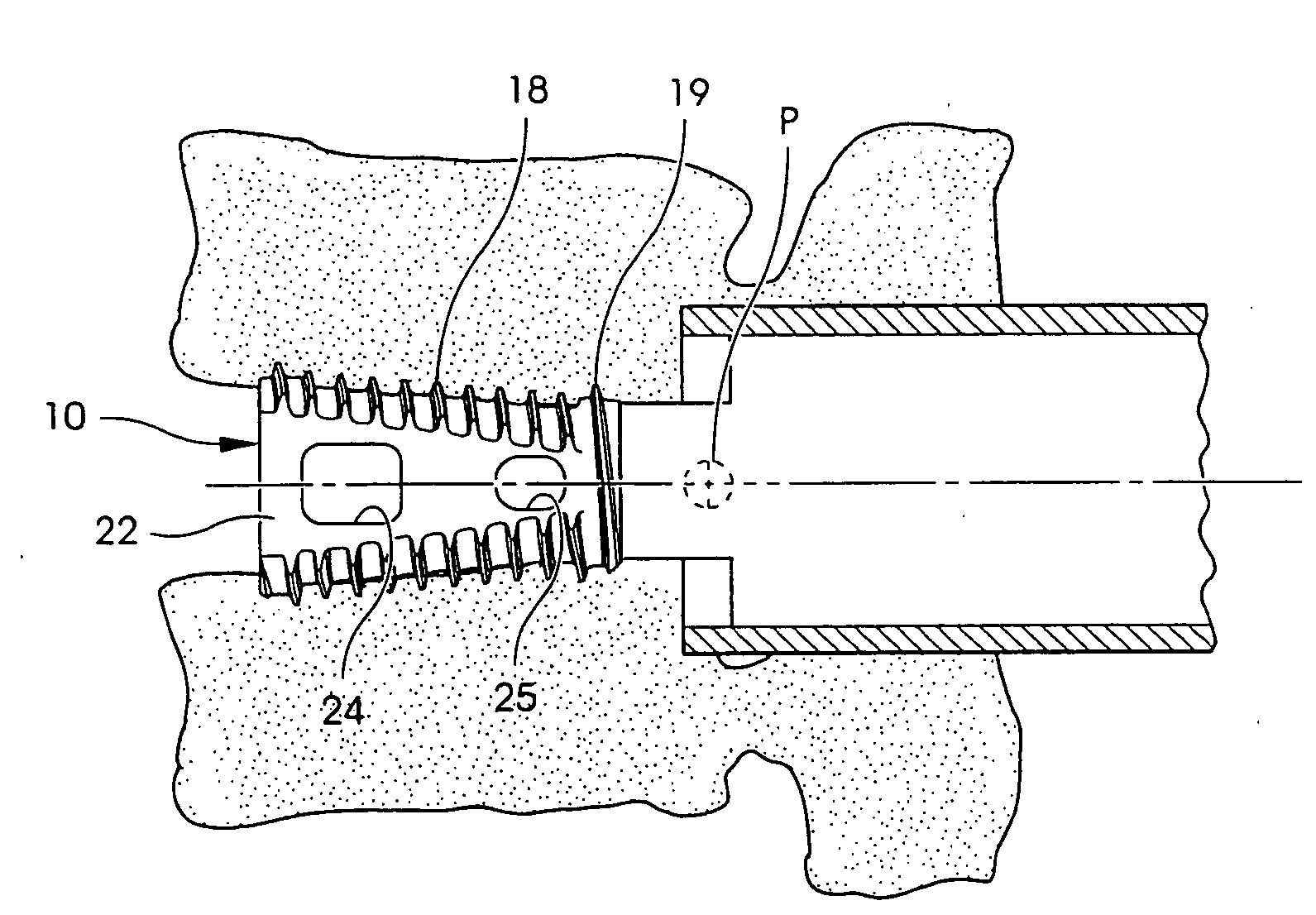
Anterior spinal instrumentation and method for implantation and revision
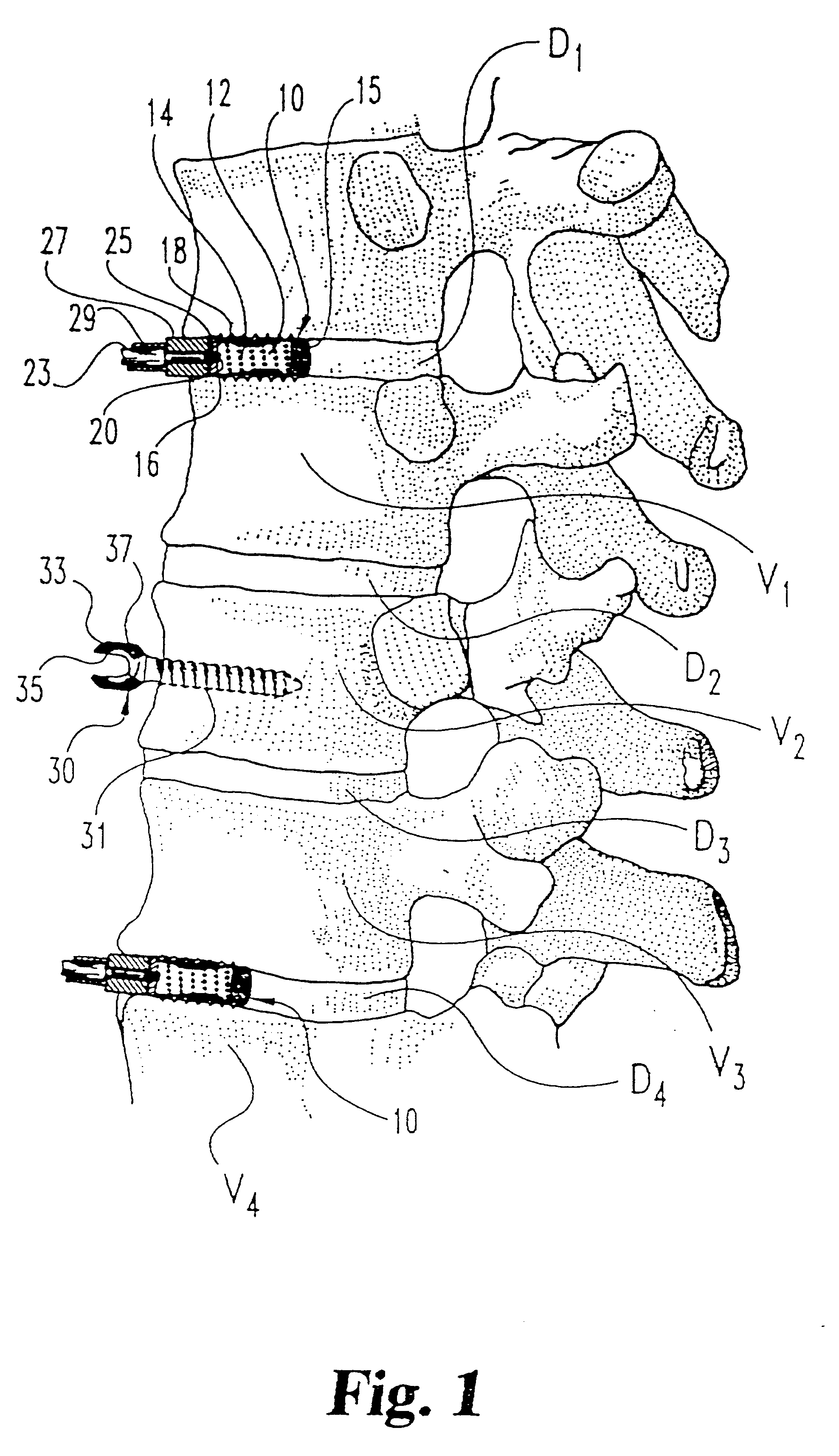
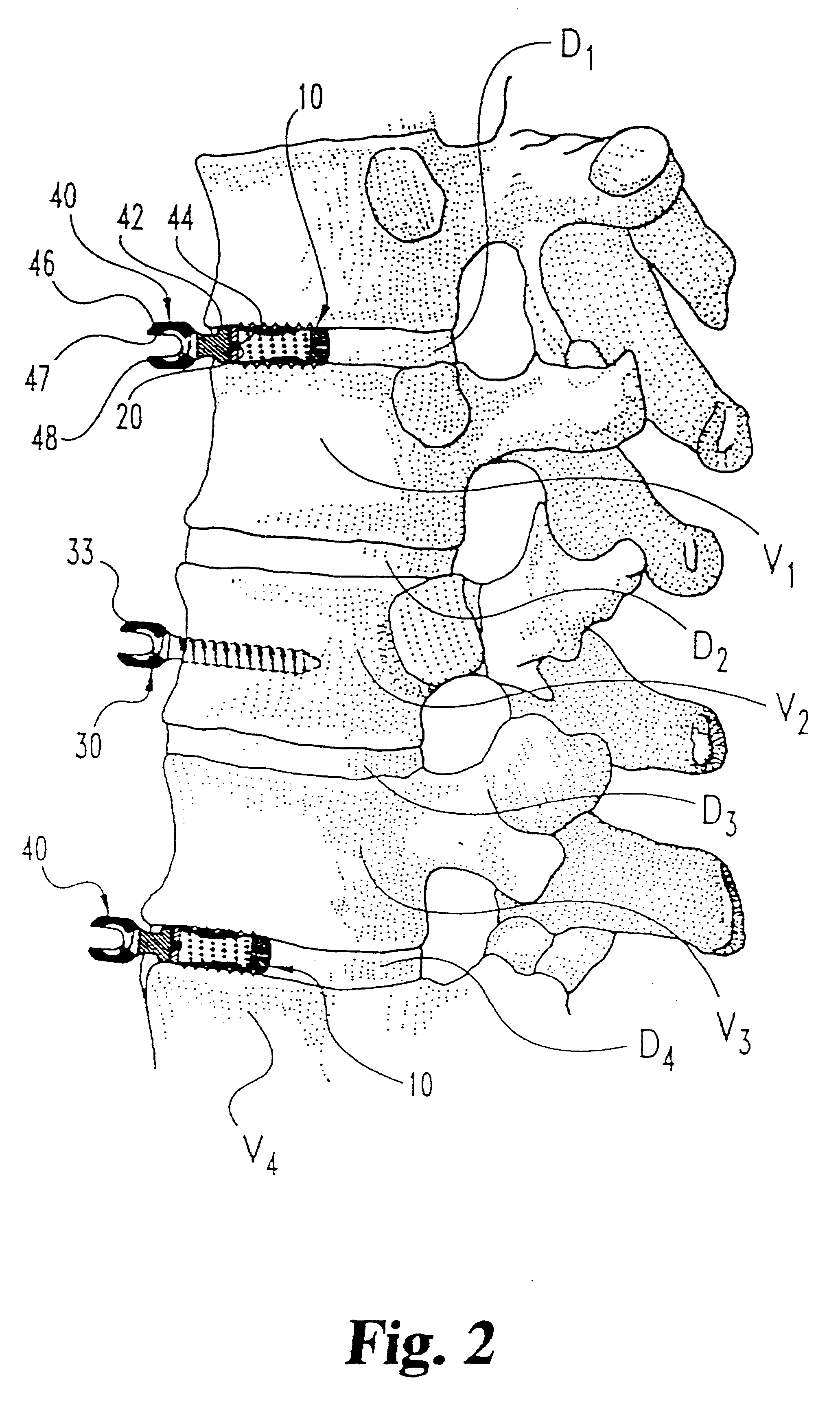
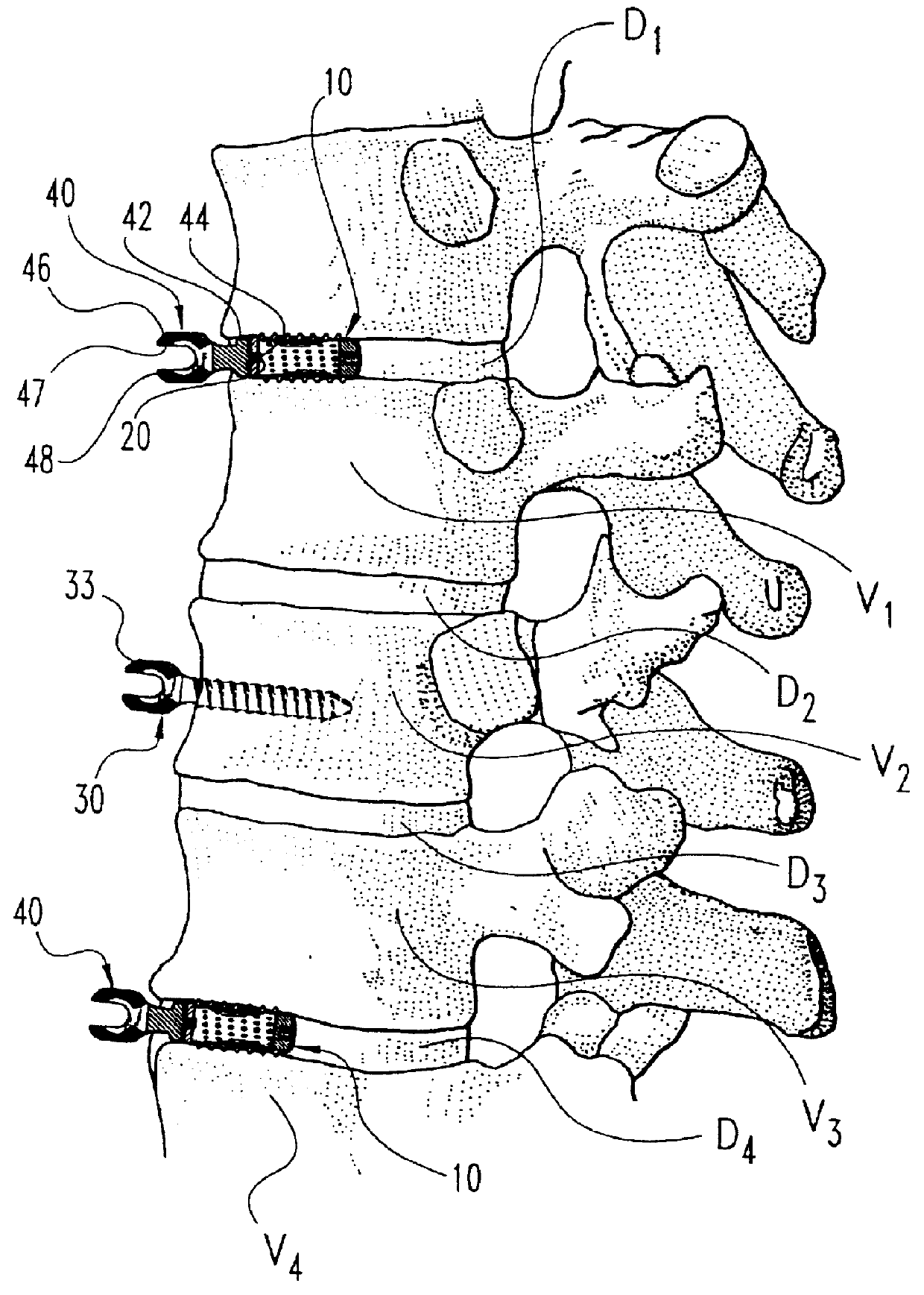
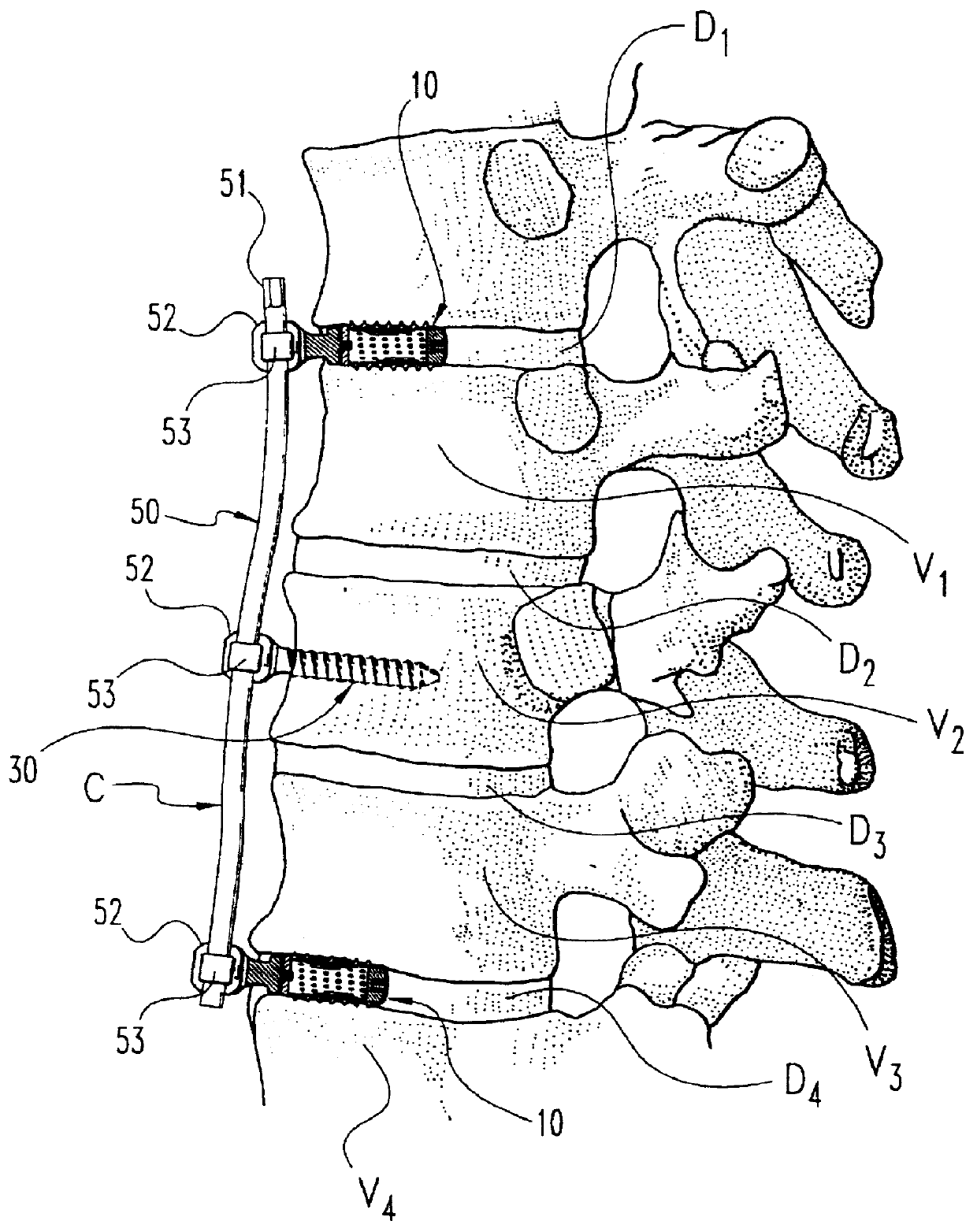
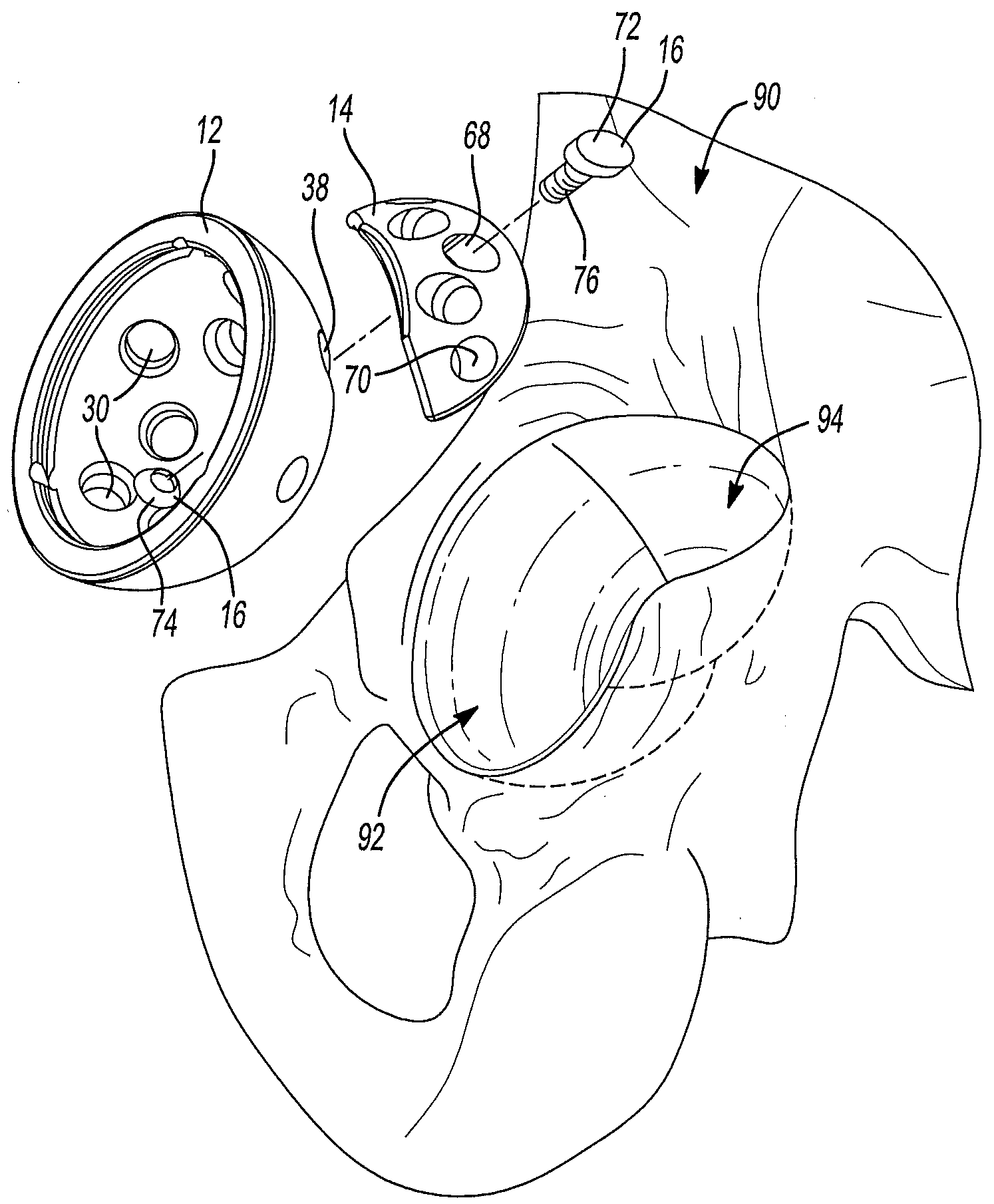
InactiveUSRE37161E1Reliable and decompressionEnhanced rigidity and fixationInternal osteosythesisBone implantSpinal columnBone trephine
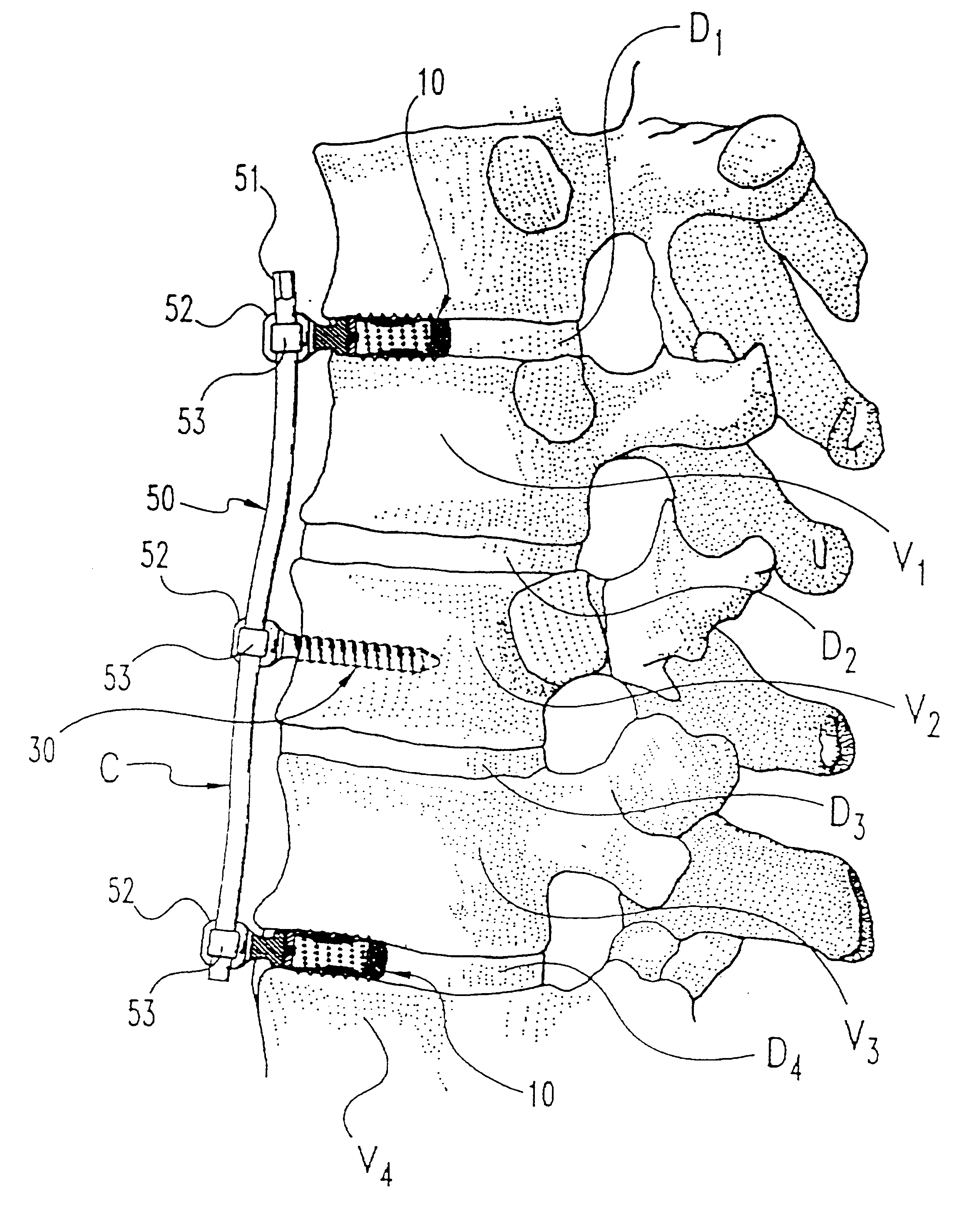
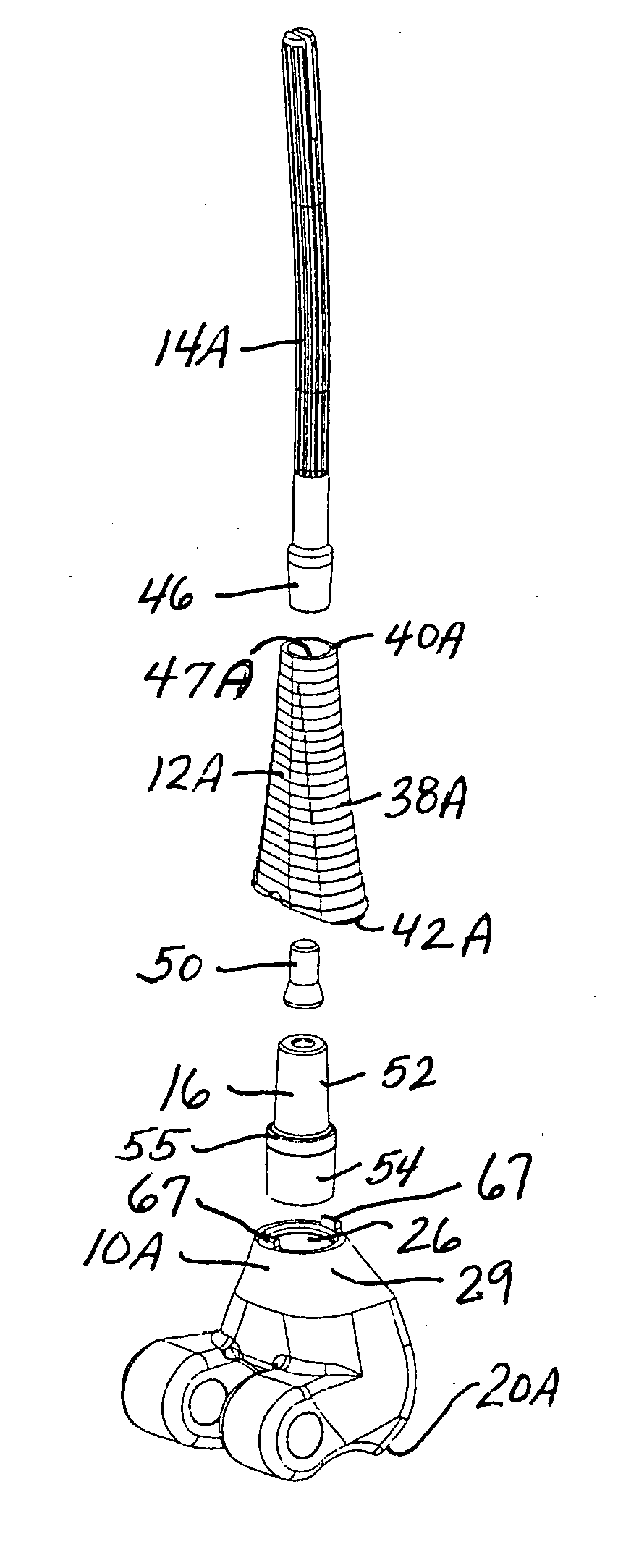
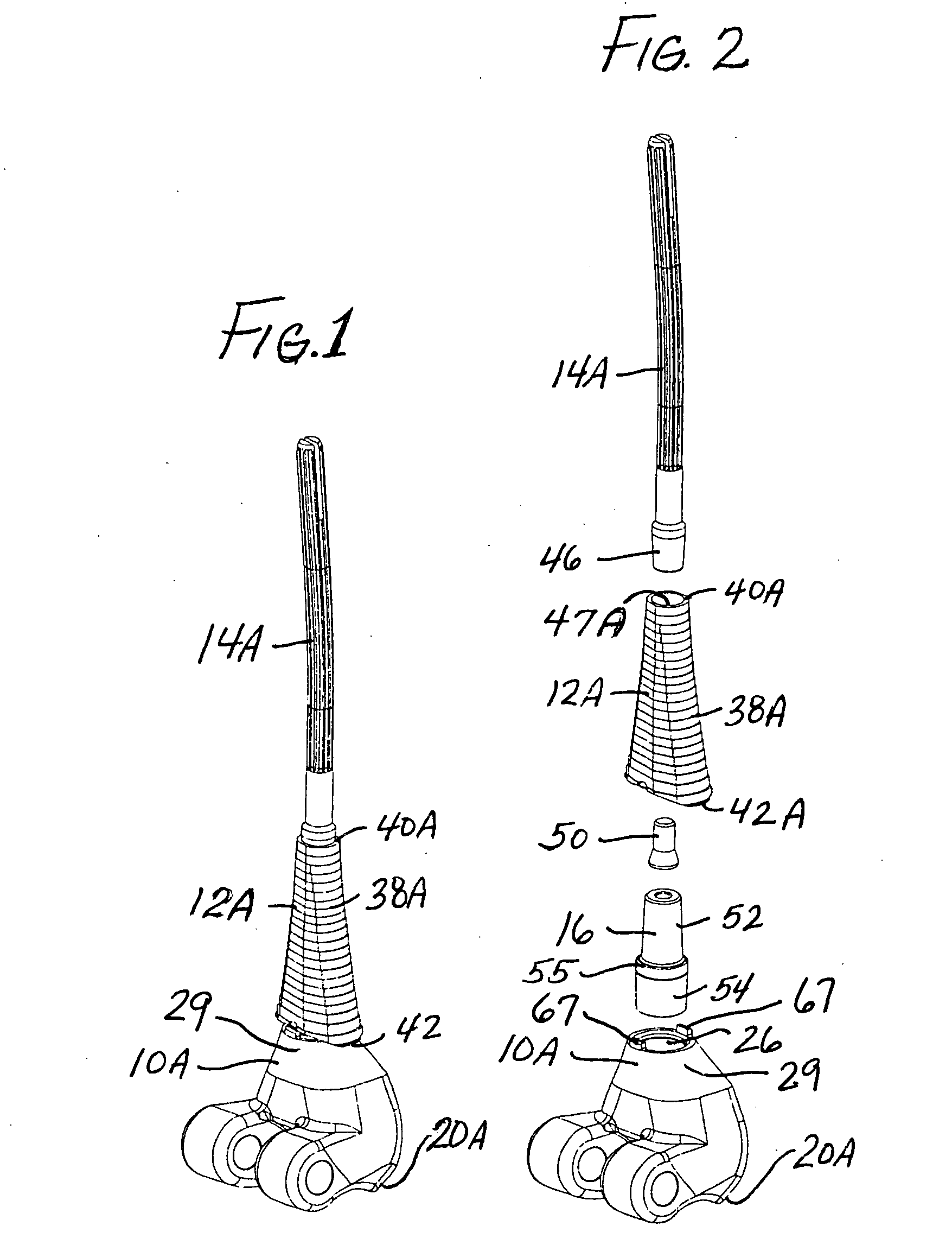
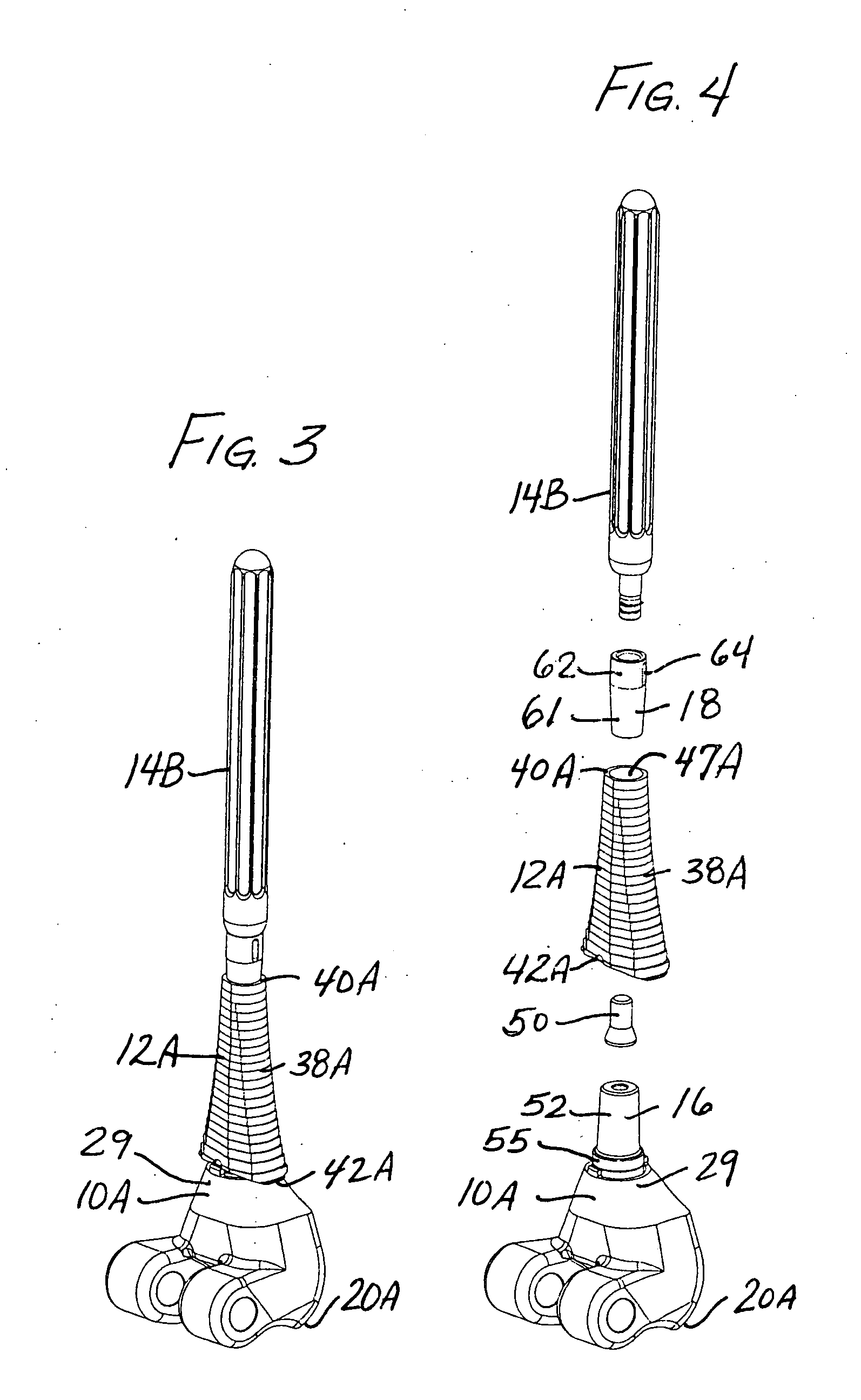
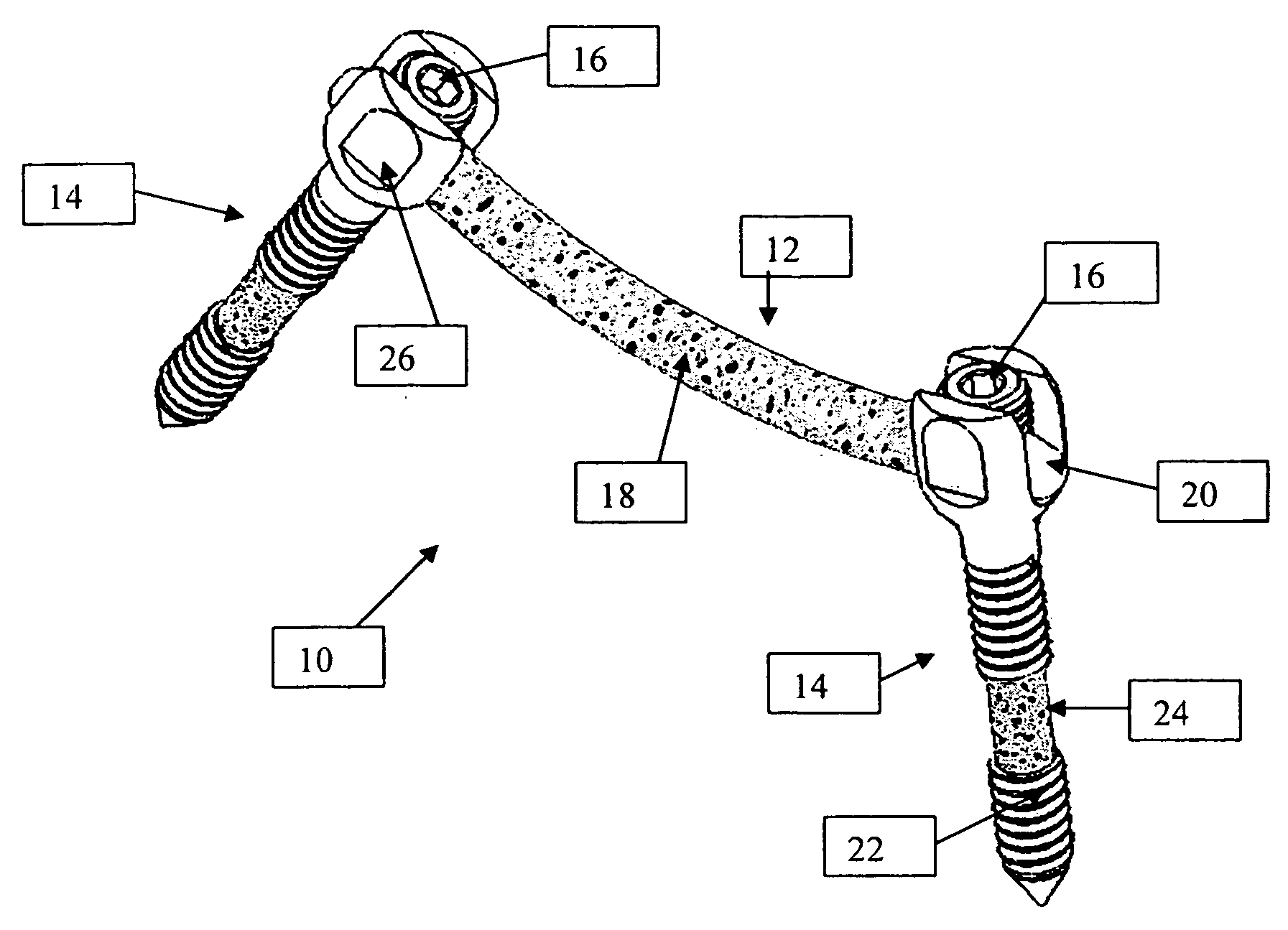
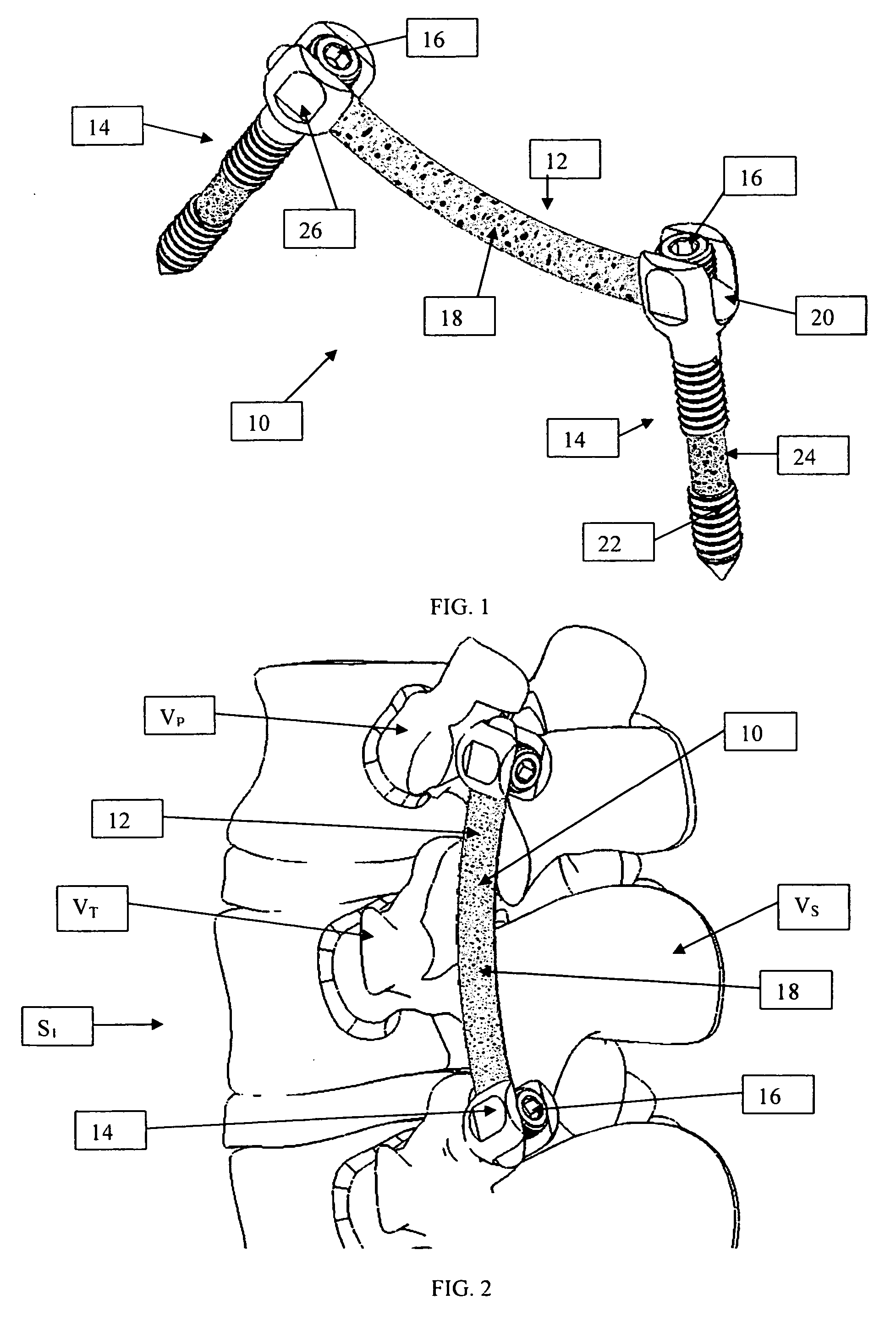
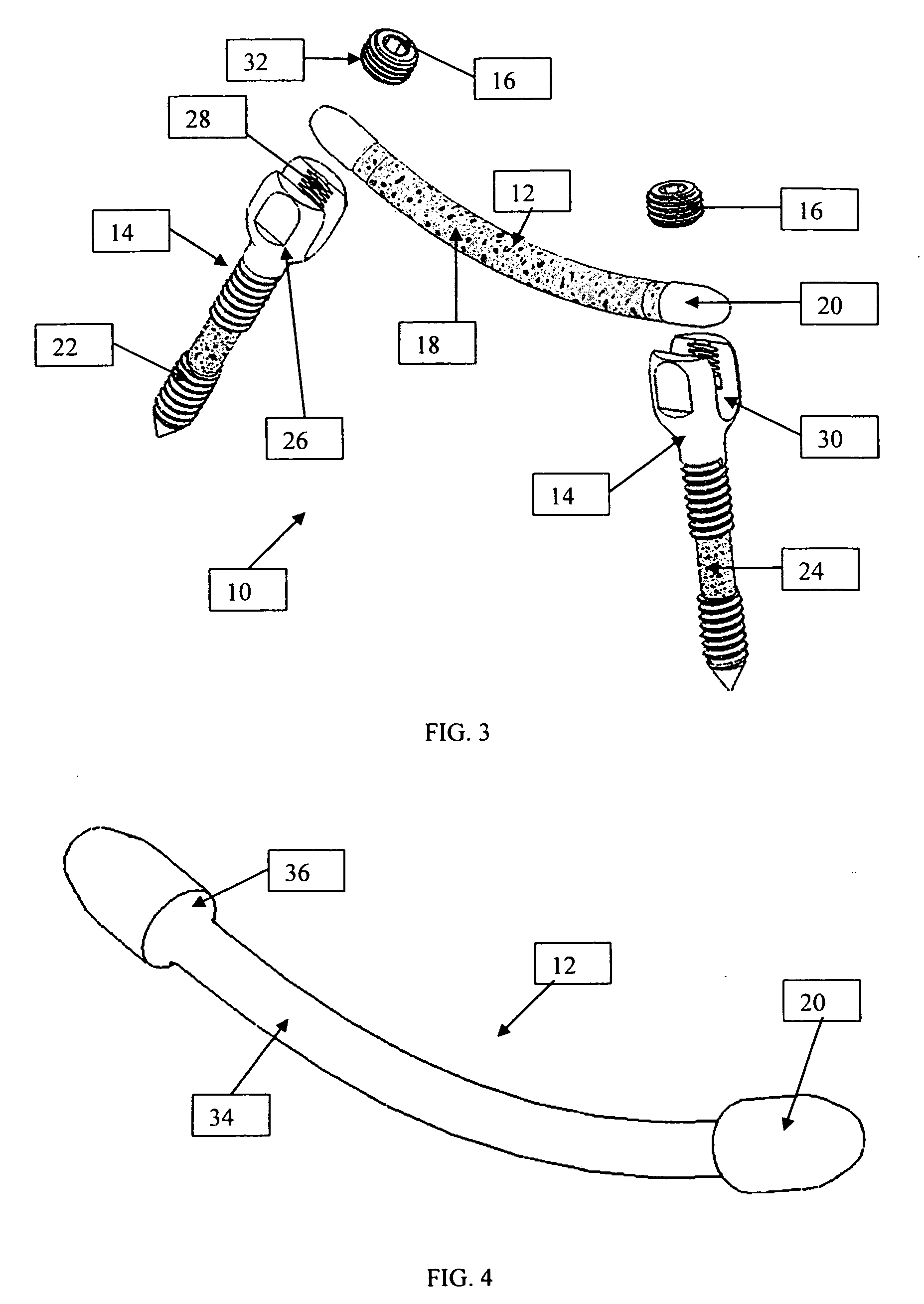
A system and method for anterior fixation of the spine utilizes a cylindrical implant engaged in the intradiscal space at the cephalad and caudal ends of the construct. The implants are cylindrical fusion devices (10) filled with bone material to promote bone ingrowth and fusion of the disc space. An attachment member (40) is connected to each of the fusion devices (10) and bone screws (30) having similar attachment members (33) are engaged in the vertebral bodies of the intermediate vertebrae. A spinal rod (50) is connected to each of the attachment members using an eyebolt assembly (53, 54, 55). In a further inventive method, a revision of the construct is achieved by removing the fusion devices. Each fusion device is engaged by an elongated guide member (62) over which a cylindrical trephine (70) is advanced. The trephine (70) has an inner diameter larger than the diameter of the fusion implant and includes cutting teeth (72) for extracting a core (84) of bone material around the fusion implant. The trephine (70) and guide member (62) are removed along with the bone core (84) containing the fusion implant (10). The trephine (70) is also used to extract a bone dowel from a solid bone mass to be inserted into the space left by the removed bone core (84).
Owner:MICHELSON GARY K +1
Articulating spinal implant
A spinal implant for intervertebral disc replacement. The implant is formed from two hemicylindrical elements, each engaging one of an adjacent pair of vertebrae. An articulating ball-and-socket joint and / or rocker and channel between the two elements resists compression and lateral movement between the vertebra, but allows pivotal movement, thereby preserving mobility. Fusion chambers are provided for allowing bone ingrowth to fuse the elements to the vertebrae. Biocompatible, bioreabsorbable struts, shims, fillers and / or end caps are provided for temporary stabilization of the first and second hemicylindrical elements. Bone chips removed from the vertebrae during implantation or bone growth stimulators can be inserted into the fusion chamber or otherwise applied to the implant to enhance bone ingrowth.
Owner:WARSAW ORTHOPEDIC INC
Apparatus and method for providing dynamizable translations to orthopedic implants
InactiveUS20050085812A1Avoid stress shieldingLimited supportSuture equipmentsInternal osteosythesisBone structureJoint arthrodesis
The present invention generally relates to orthopedic devices and methods for treating bone defects. The orthopedic devices can provide sufficient support to the bone defect while allowing bone ingrowth and minimizing the risk to stress shield and / or pseudo-arthrodesis. The bone fixation devices include a biodegradable material or component that further resists relative motion of attached bones and allows the device to gradually transfer at least some load from the device to the growing bone structure in vivo and permitting an increase in the relative motion of bones attached to the device.
Owner:WARSAW ORTHOPEDIC INC
Modular implant system with fully porous coated sleeve
A modular knee implant system allows a surgeon to select between several different styles of distal femoral implant components and several different styles of stem extensions while also allowing for use of a metaphyseal component. The metaphyseal component can be a universal one that is usable with all of the styles of distal femoral implant components through use of an adapter. A second adapter allows for use of stem extensions with different types of connectors with the metaphyseal component. A separate metaphyseal component could also be provided with a distal Morse taper post to mate with a distal femoral component having a proximal Morse taper bore. The metaphyseal component may have an outer surface that is configured to maximize contact area with the patient's bone, and may have a surface finish over a substantial part of its overall length that is conducive to bone ingrowth.
Owner:DEPUY PROD INC
Hip prosthesis with monoblock ceramic acetabular cup
InactiveUS7695521B2High strengthImprove toughnessBone implantJoint implantsRight femoral headMetallic materials
An improved hip prosthesis includes an acetabular cup bearing component constructed from a relatively hard and high strength ceramic material for articulation with a ball-shaped femoral head component which may be constructed from a compatible ceramic or metal material. In one form, the acetabular cup further includes a ceramic porous bone ingrowth surface adhered thereto for secure ingrowth attachment to natural patient bone.
Owner:AMEDICA A DELAWARE
Radiolucent bone graft
An improved bone graft is provided for human implantation, bone graft includes a substrate block of high strength biocompatible material having a selected size and shape to fit the anatomical space, and a controlled porosity analogous to natural bone. The substrate block may be coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone ingrowth and enhanced bone fusion. Upon implantation, the bone graft provides a spacer element having a desired combination of mechanical strength together with osteoconductivity and osteoinductivity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The bone graft may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:AMEDICA A DELAWARE
Canine acetabular cup
ActiveUS7169185B2Increase flexibilityLife maximizationSurgeryJoint implantsPorous coatingRight femoral head
Owner:IMPACT SCI & TECH
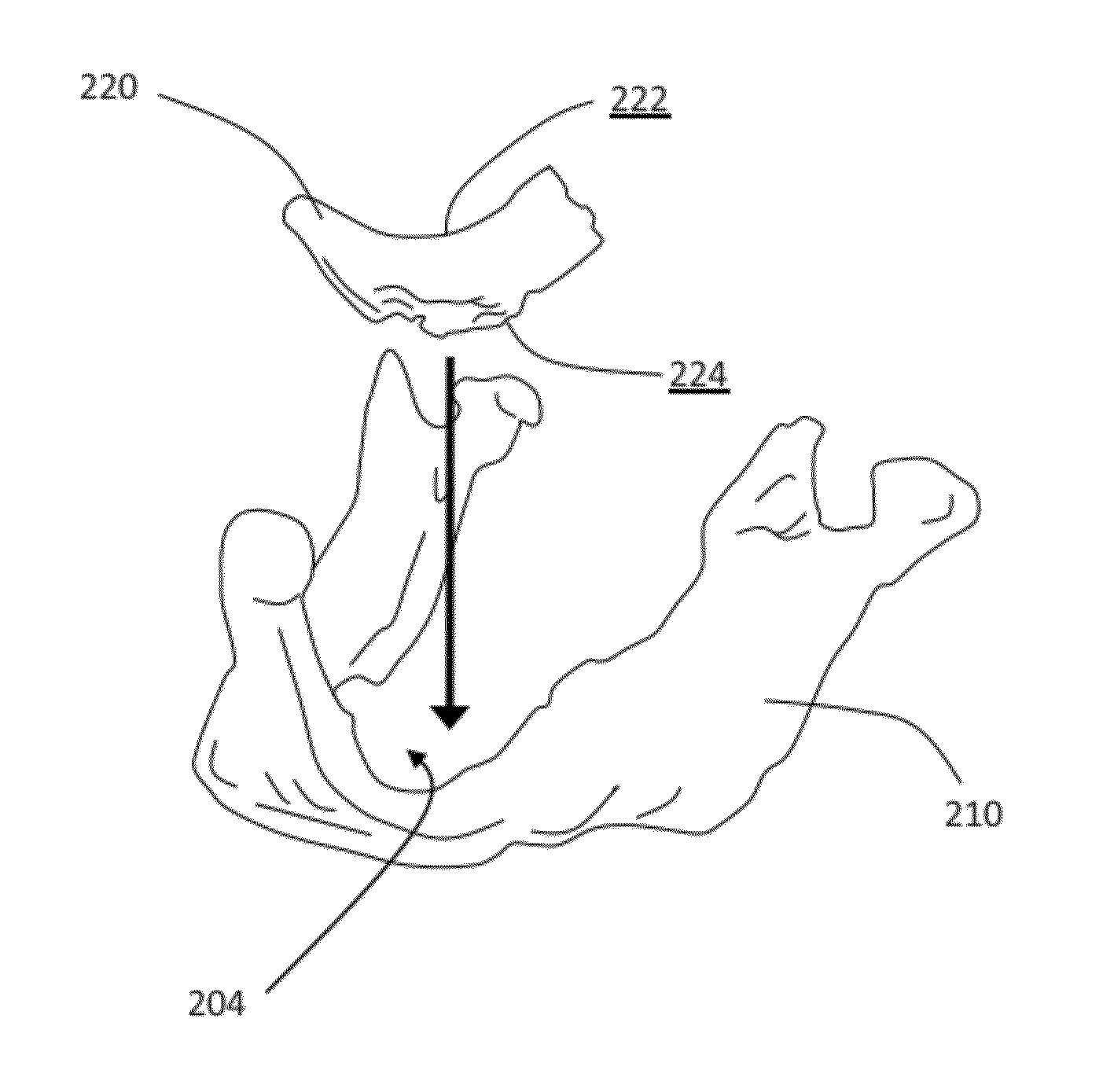
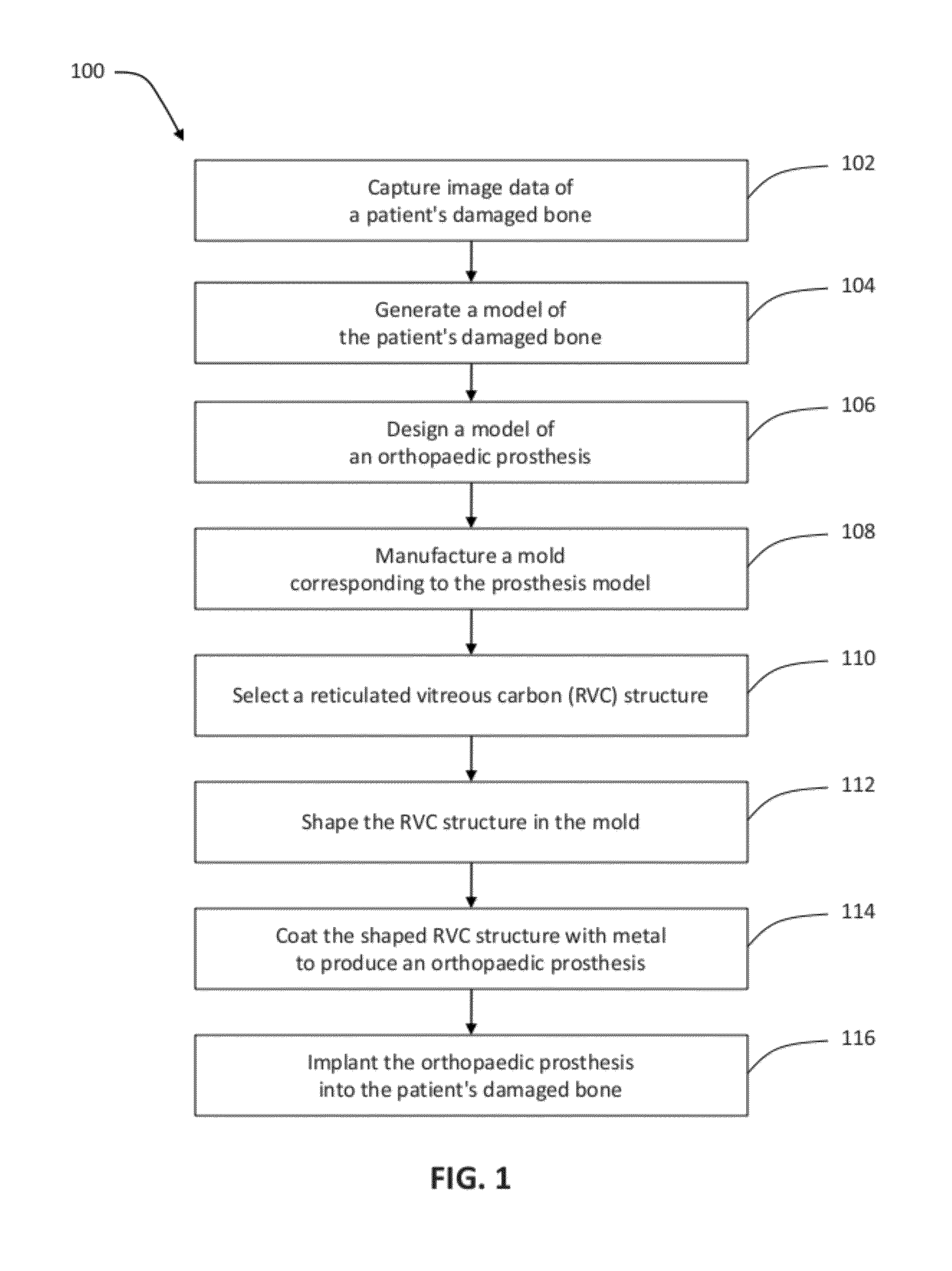
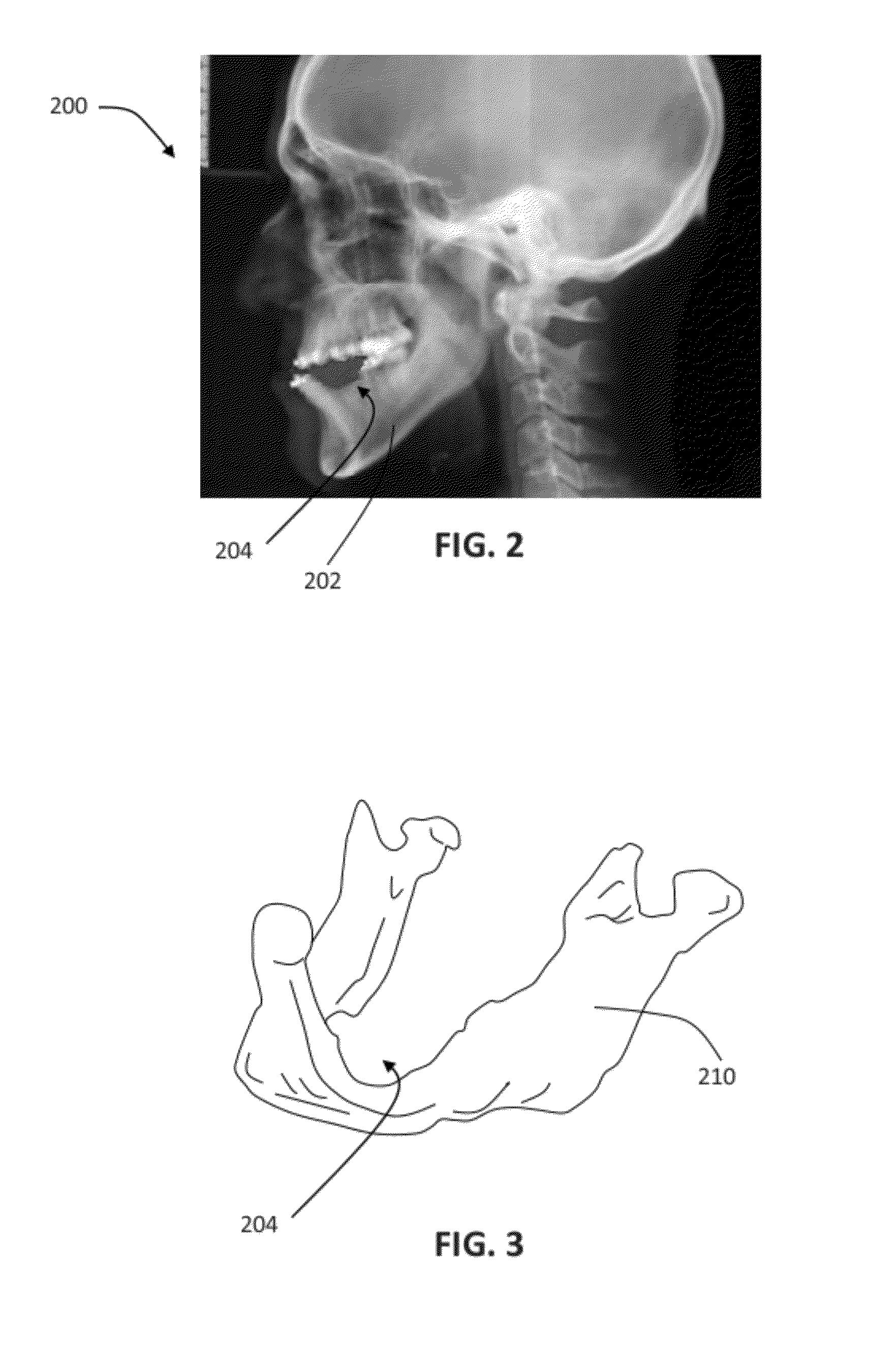
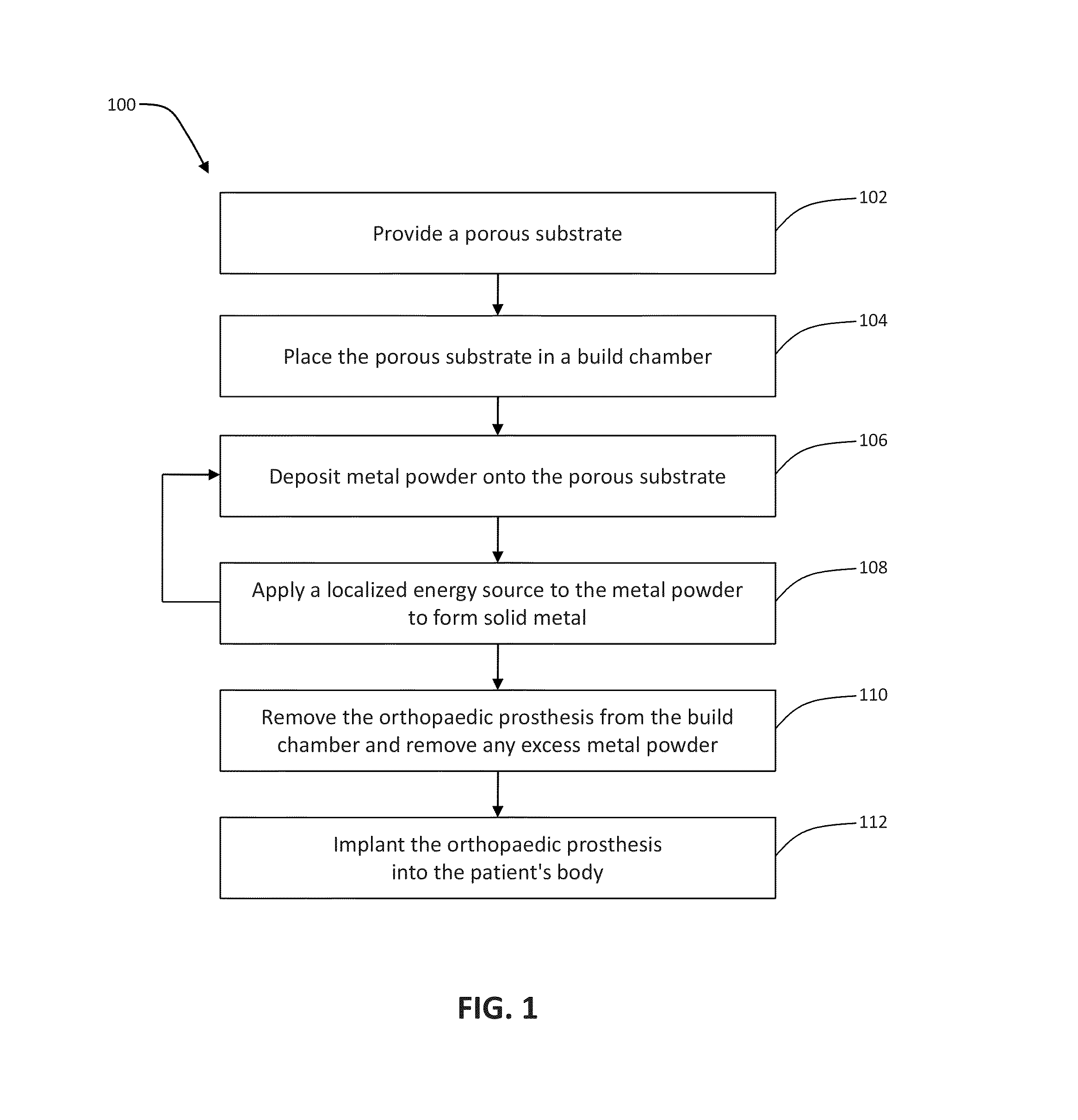
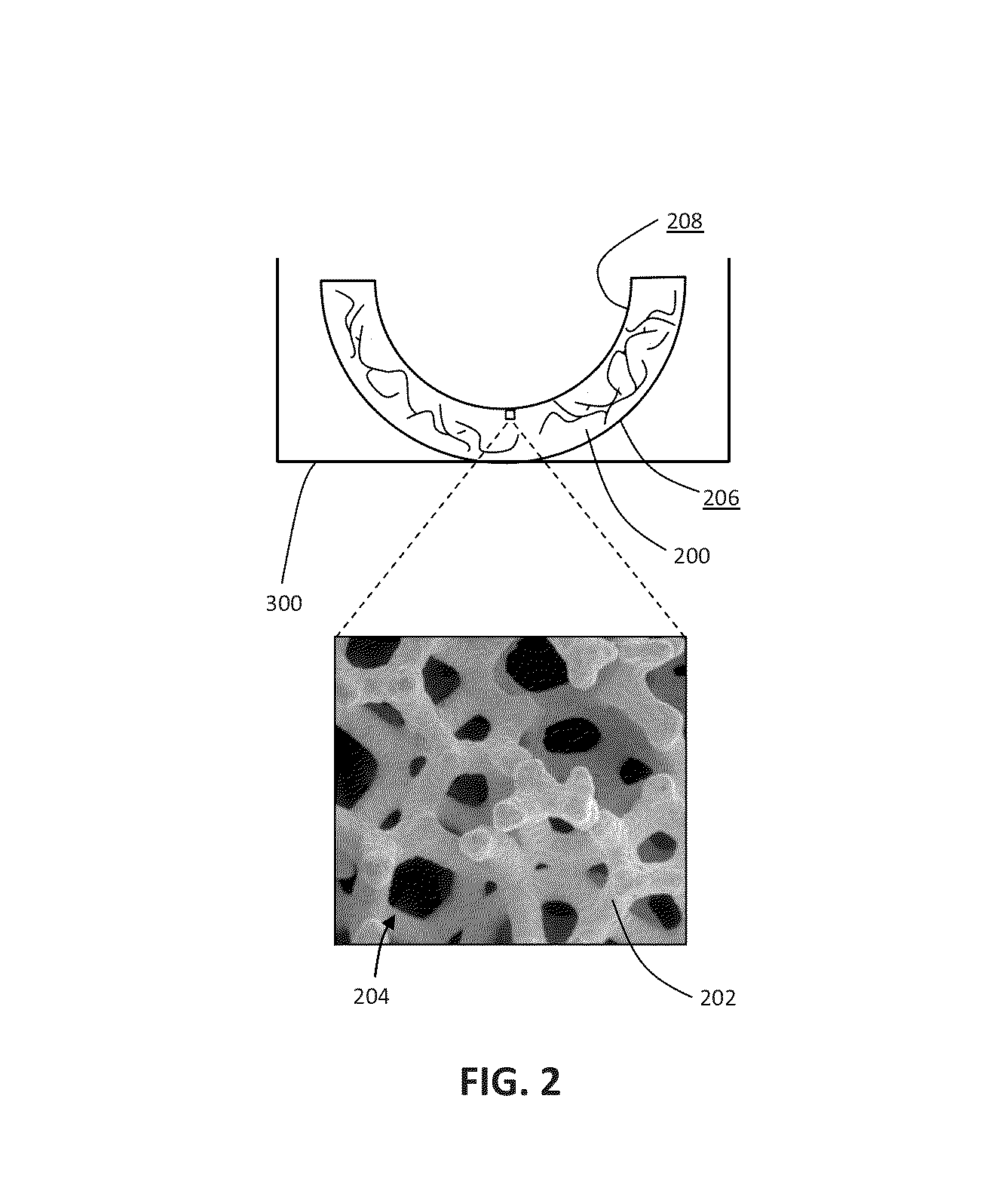
Patient-specific manufacturing of porous metal prostheses
ActiveUS20120310364A1Low efficiencyAvoid substantially changing porosityImpression capsPretreated surfacesProsthesisPlastic surgery
A patient-specific porous metal prosthesis and a method for manufacturing the same are provided. The orthopaedic prosthesis may be metallic to provide adequate strength and stability. Also, the orthopaedic prosthesis may be porous to promote bone ingrowth.
Owner:ZIMMER INC
Vertebral implant for promoting arthrodesis of the spine
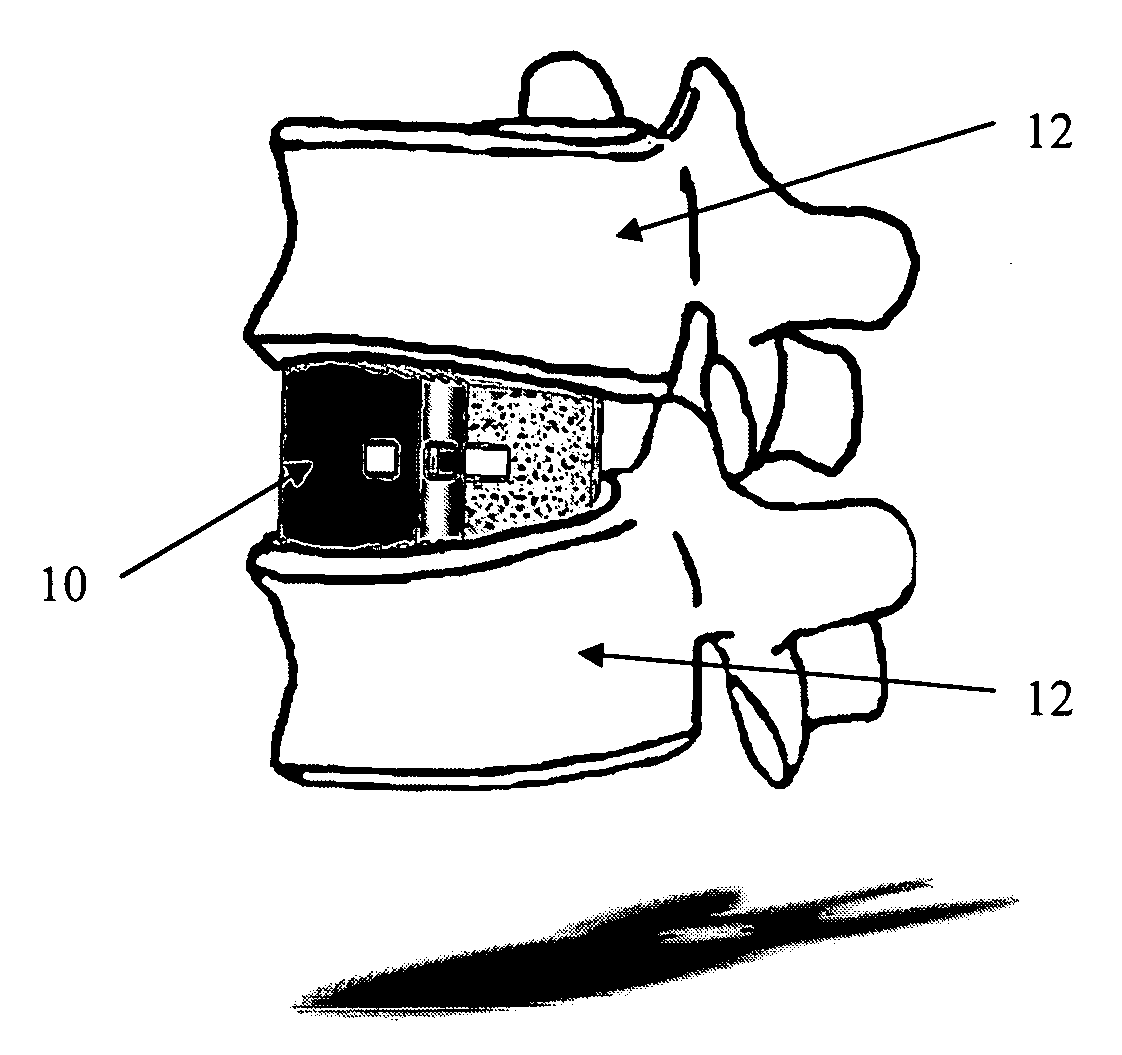
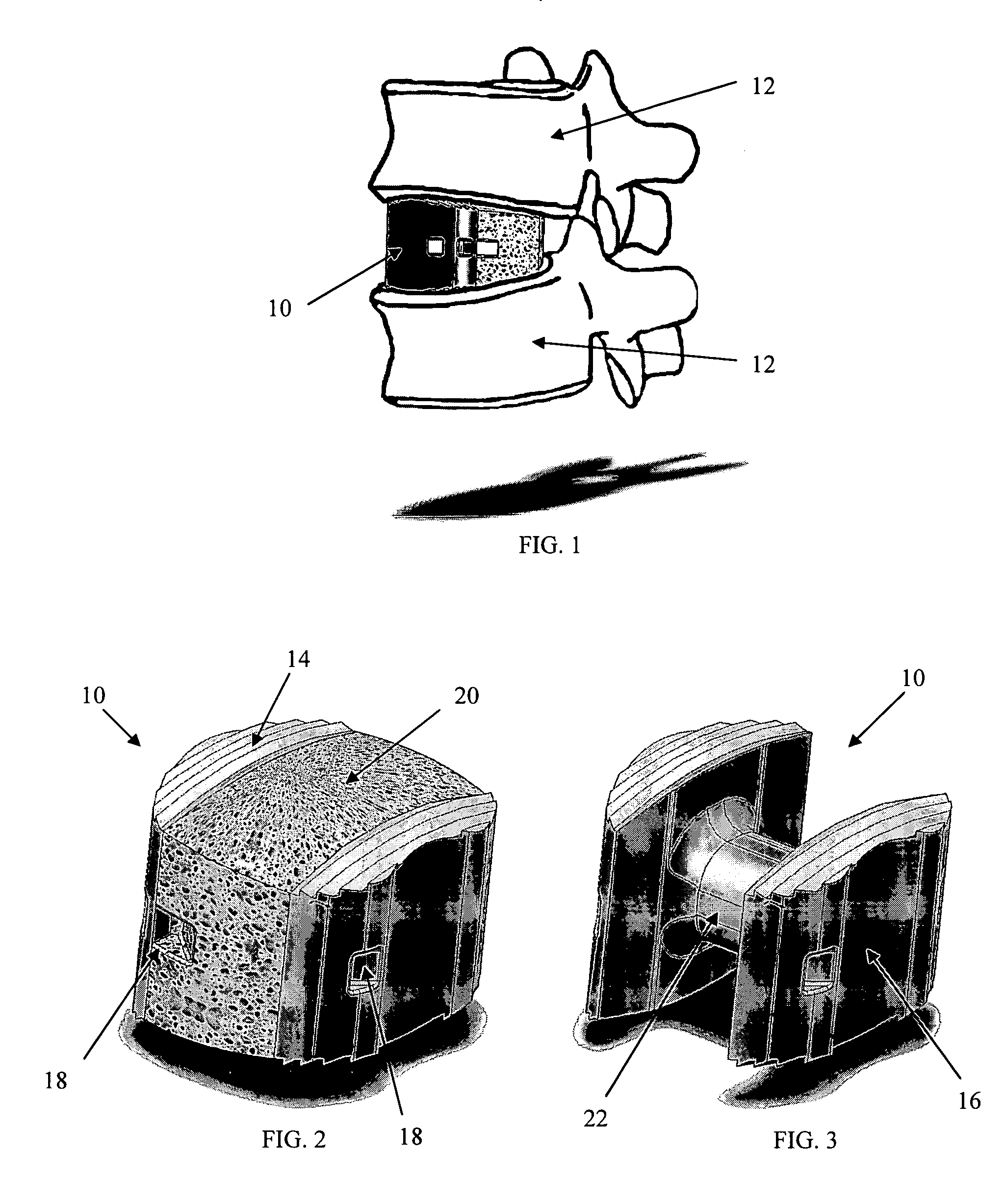
This invention provides a vertebral implant for impaction in a disc space to restore and / or maintain desired disc space height and spinal orientation. The implant has an elongated basis body having a generally lens-shape provided by convex upper and lower surfaces. Bearing surfaces are provided on the cross-edge surfaces of the endwalls. Grooves are provided in the upper and lower surfaces positioned between the bearing surfaces. The implant can be prepared from a wide variety of materials including metallic materials, synthetic materials, polymeric materials, ceramic materials, and composite materials including reinforced materials i.e. glass, fiber, and / or carbon fiber reinforced materials (CFRP). These preferred materials for fabricating implants in the present invention reduce costs, increase service life and provide excellent physiological compatibility. The non-metallic material can be selected to be either a substantially permanent material, a biodegradable material or a bioerodable material. Further, the implant material can be provided to be radio opaque to facilitate monitoring of bone ingrowth both into the implant and between the opposing endplates of the adjacent vertebrae.
Owner:WARSAW ORTHOPEDIC INC
Laser based metal deposition of implant structures
InactiveUS20050123672A1Improved bearing propertyIncrease bone ingrowth propertyDental implantsFinger jointsArtificial jointsBone ingrowth
Owner:MEDICINELODGE
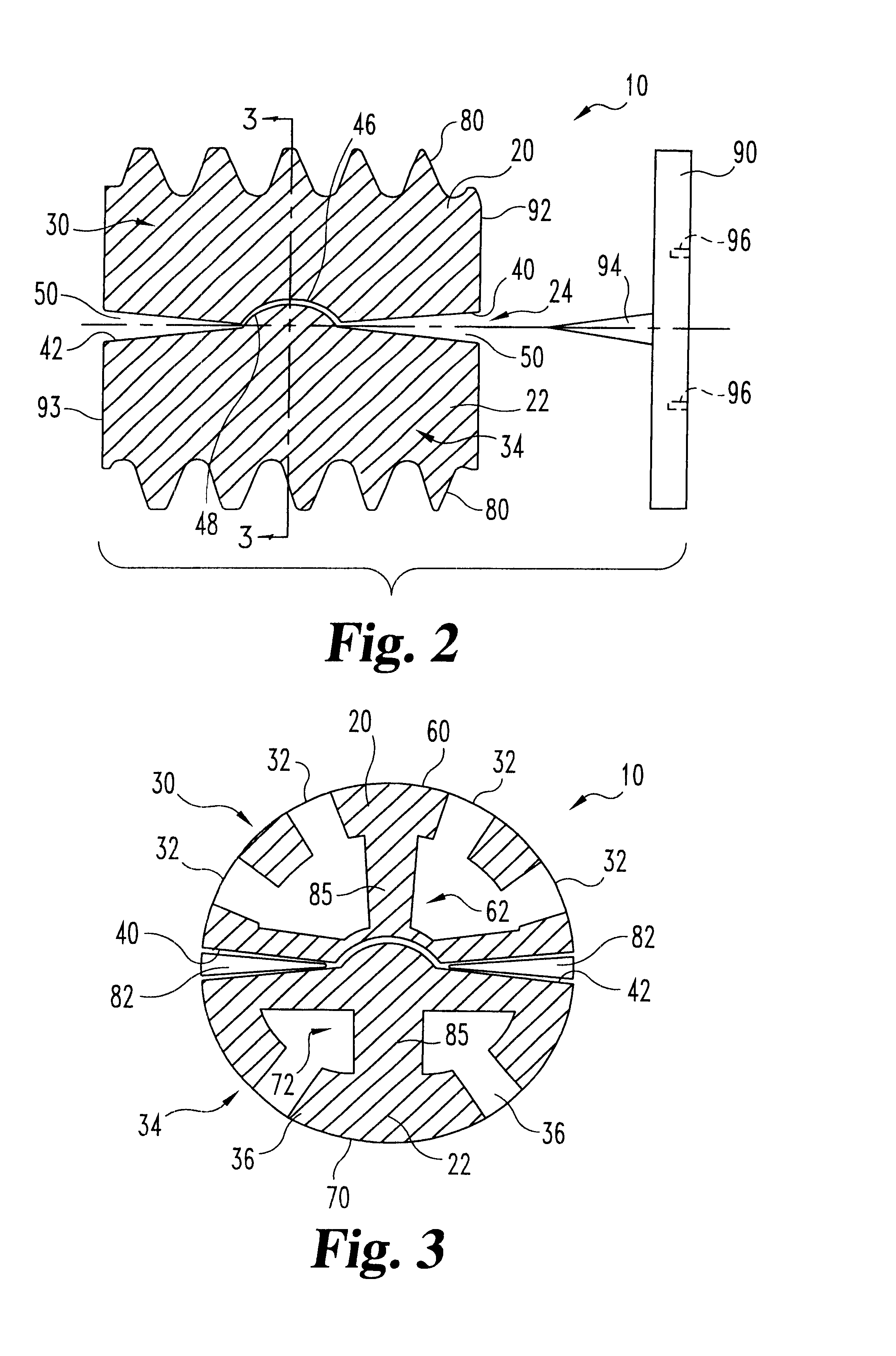
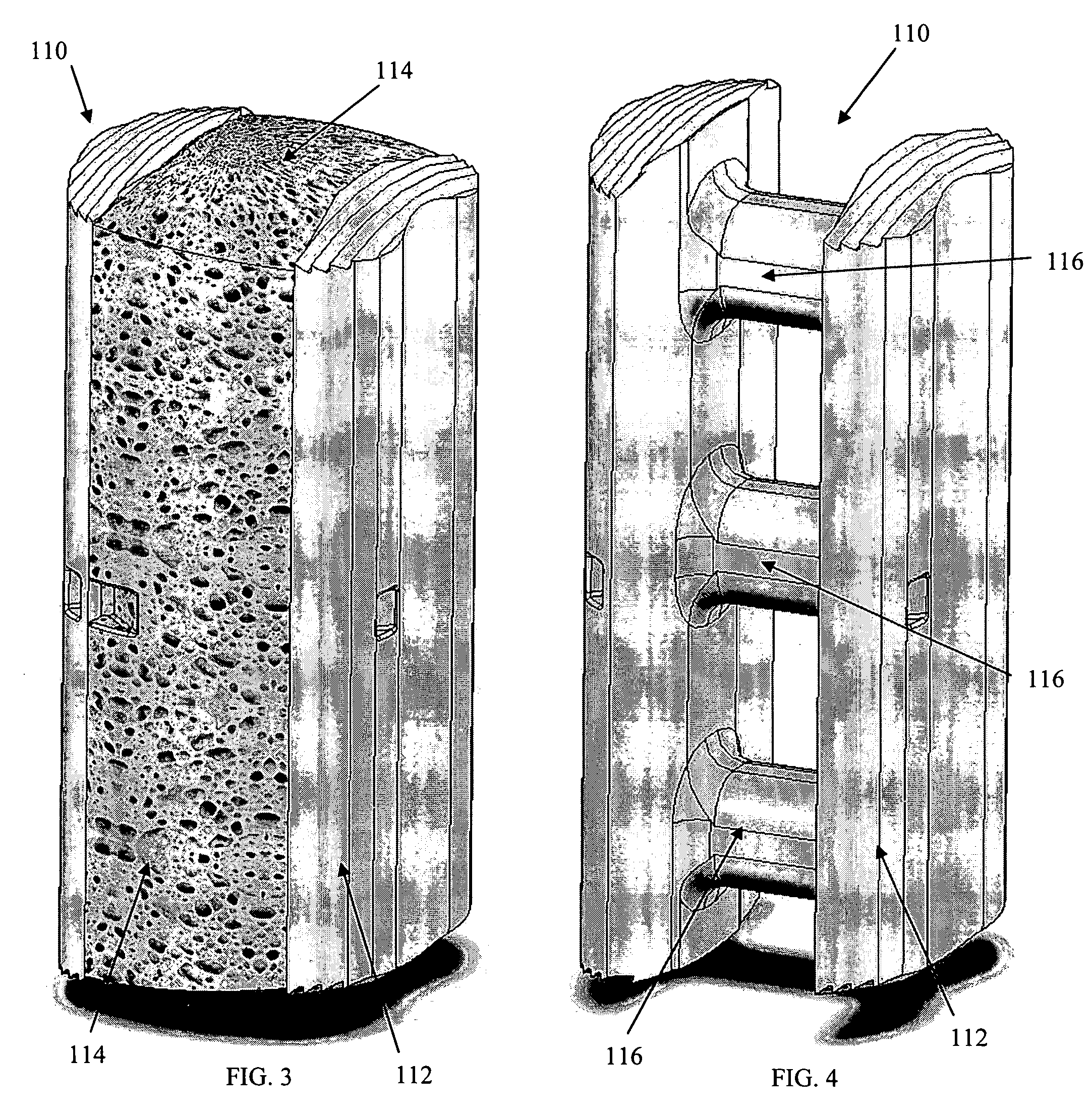
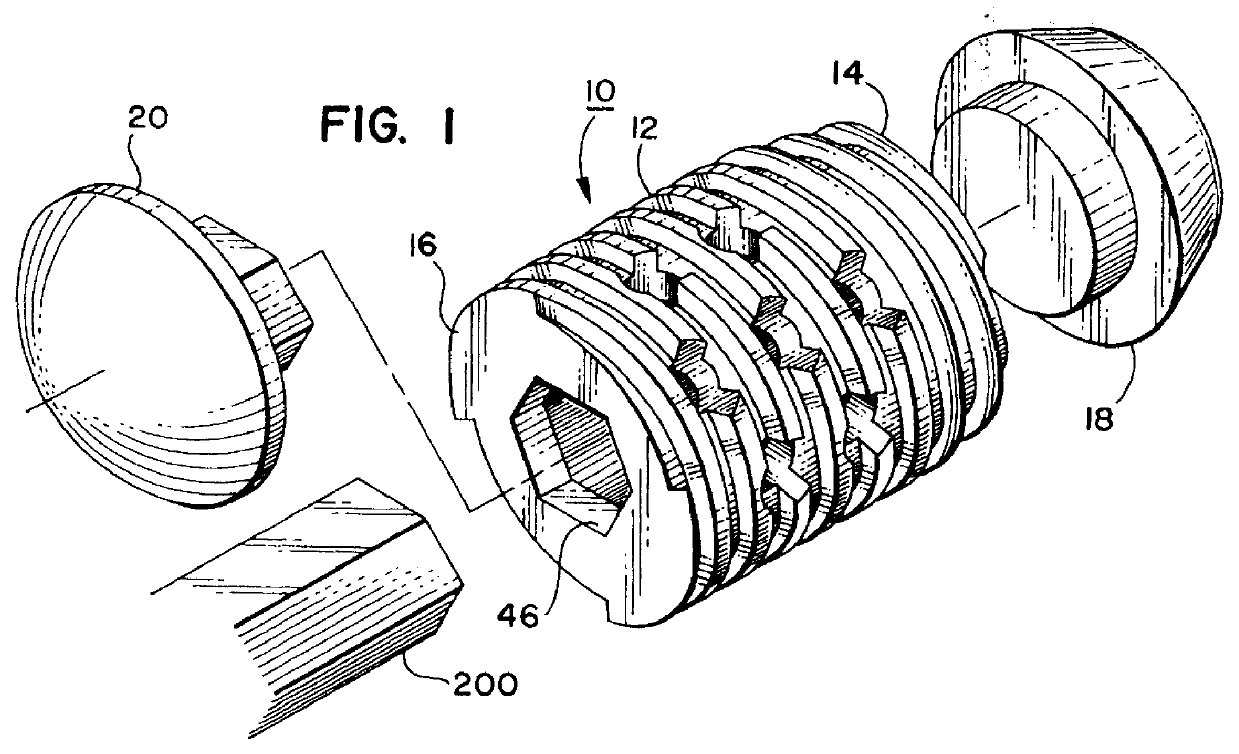
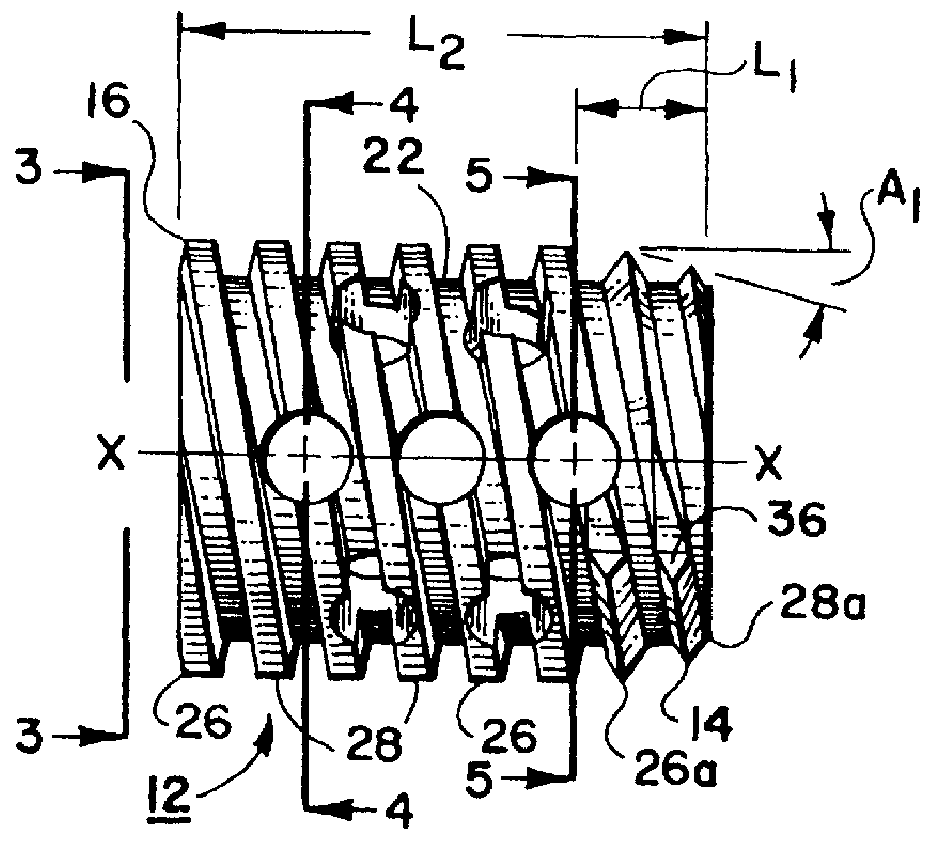
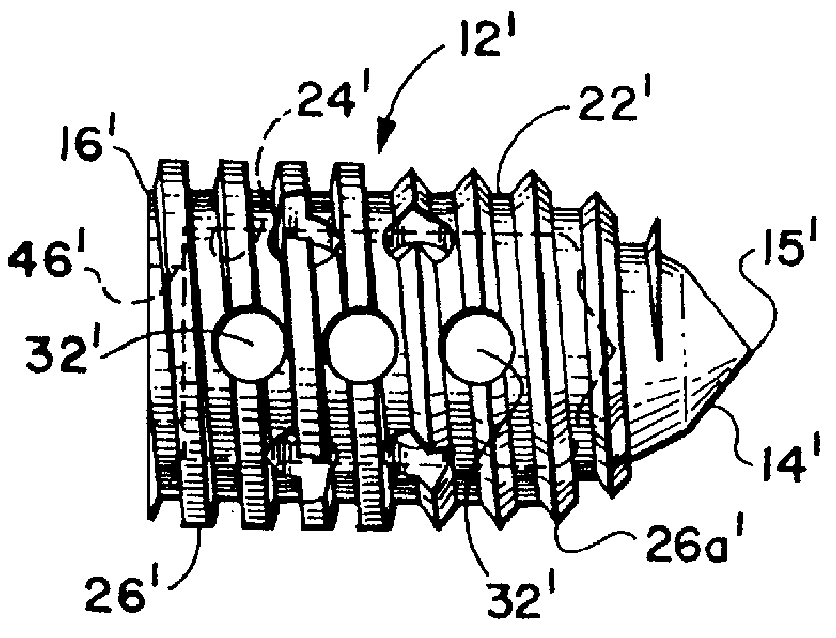
Threaded spinal implant with bone ingrowth openings
An implant is disclosed for use in spinal stabilization. In one preferred embodiment, the implant is described as including a hollow, cylindrical body having external threading and a plurality of openings formed radially through the body in communication with the body interior. The holes are positioned to chip bone into the implant as the implant is rotated.
Owner:ZIMMER SPINE INC
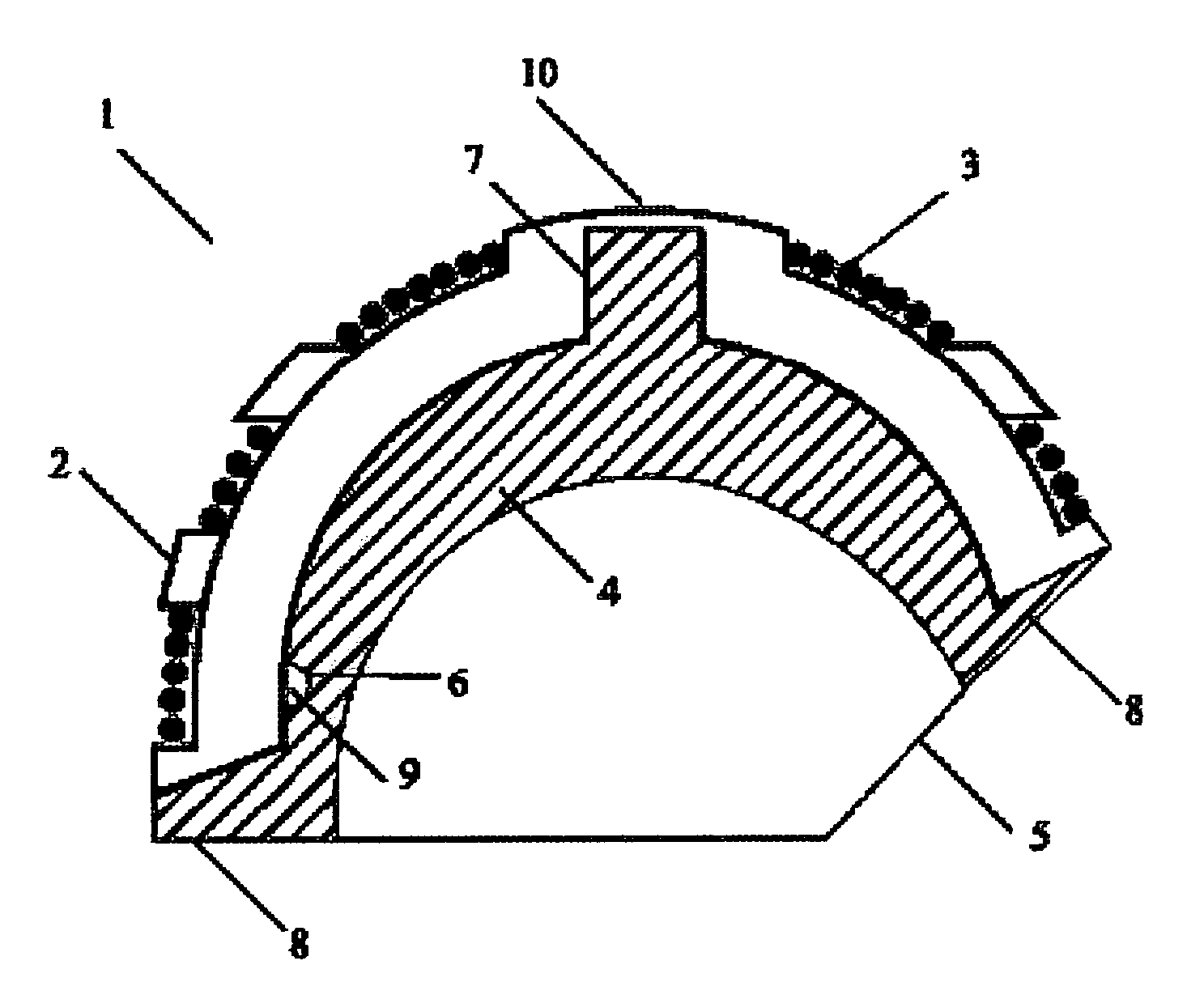
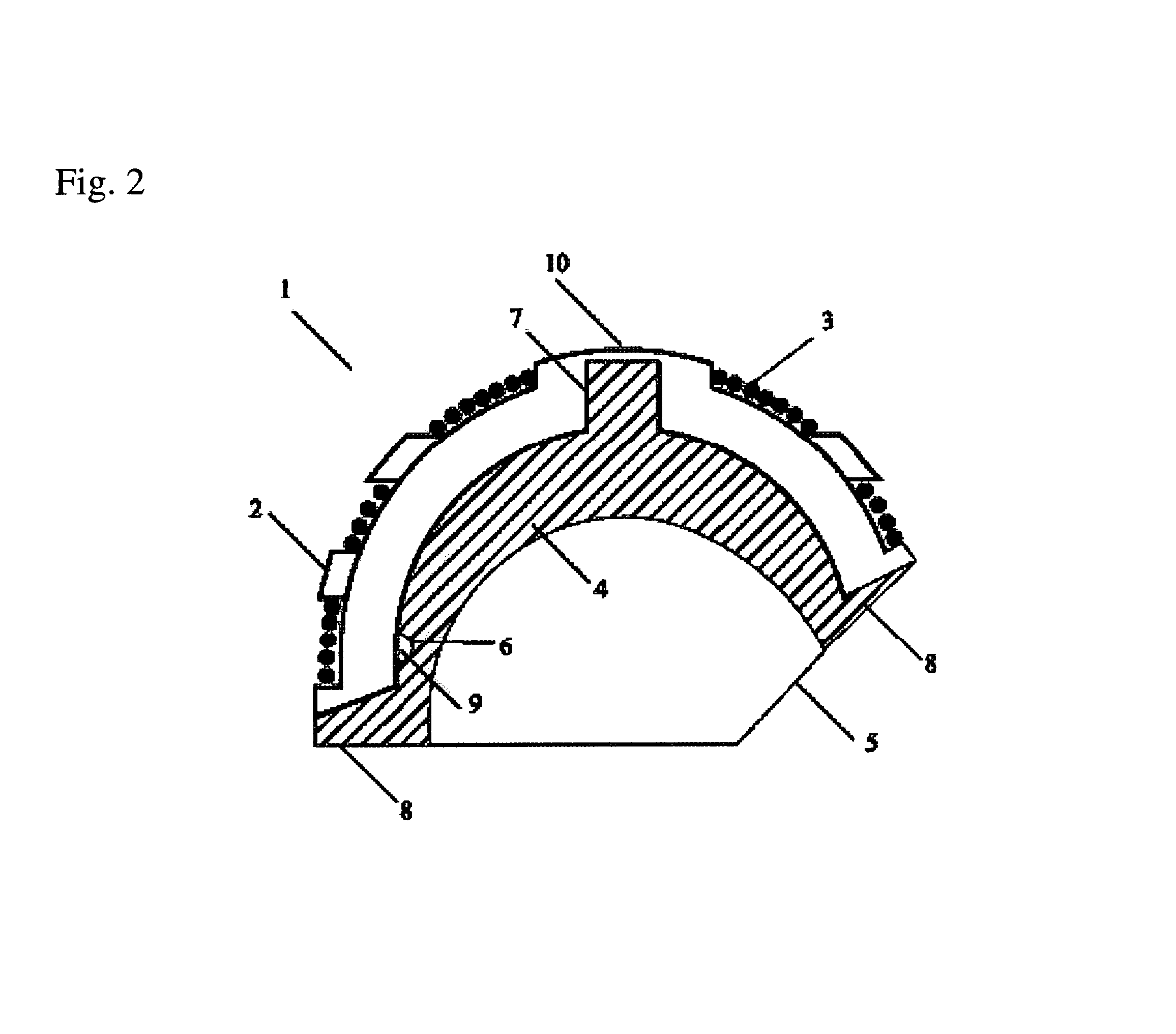
Interbody fusion device and method for restoration of normal spinal anatomy
InactiveUS7238186B2Maintain patency and stabilityRapid and stable arthrodesisInternal osteosythesisBone implantSpinal columnPorous tantalum
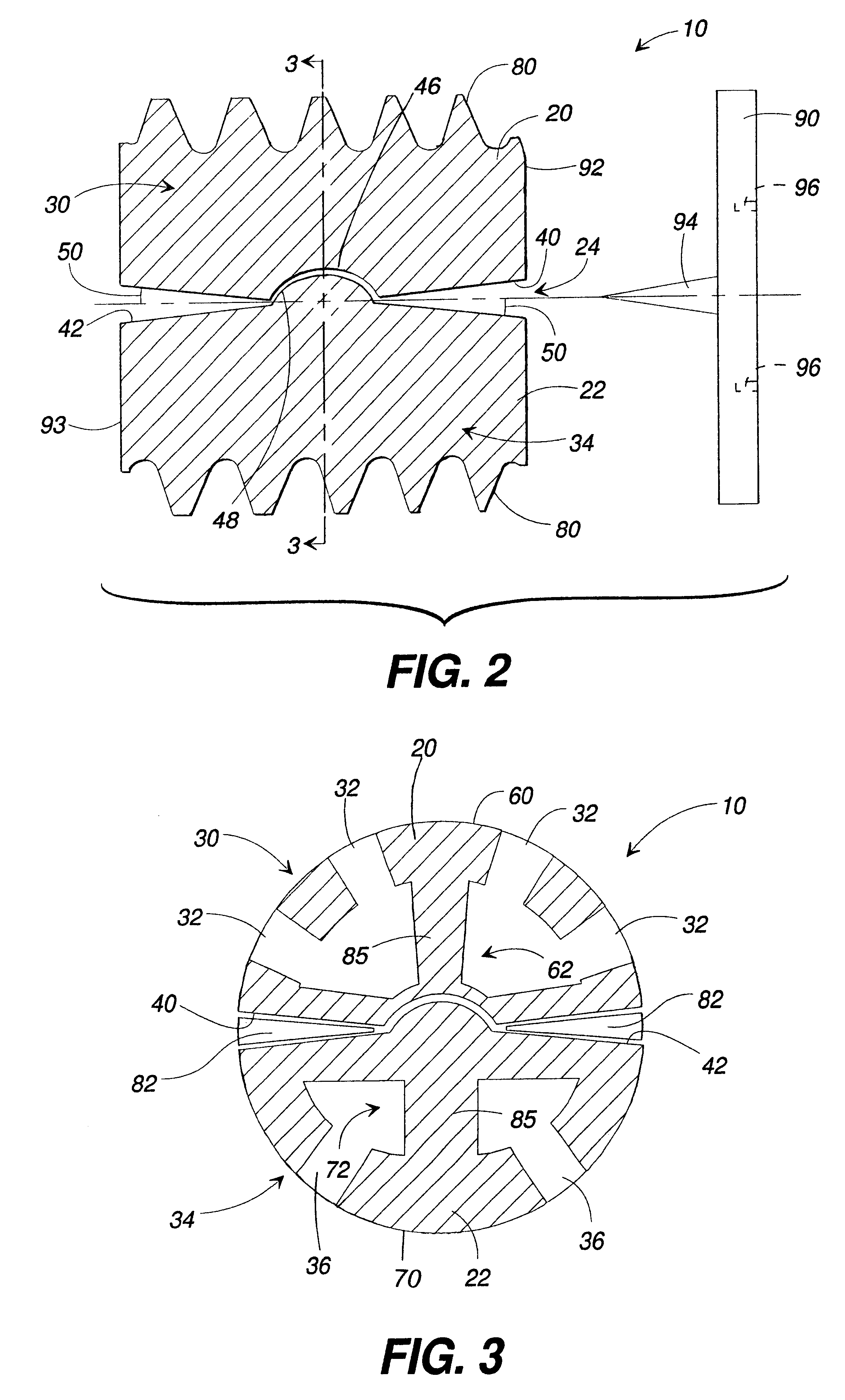
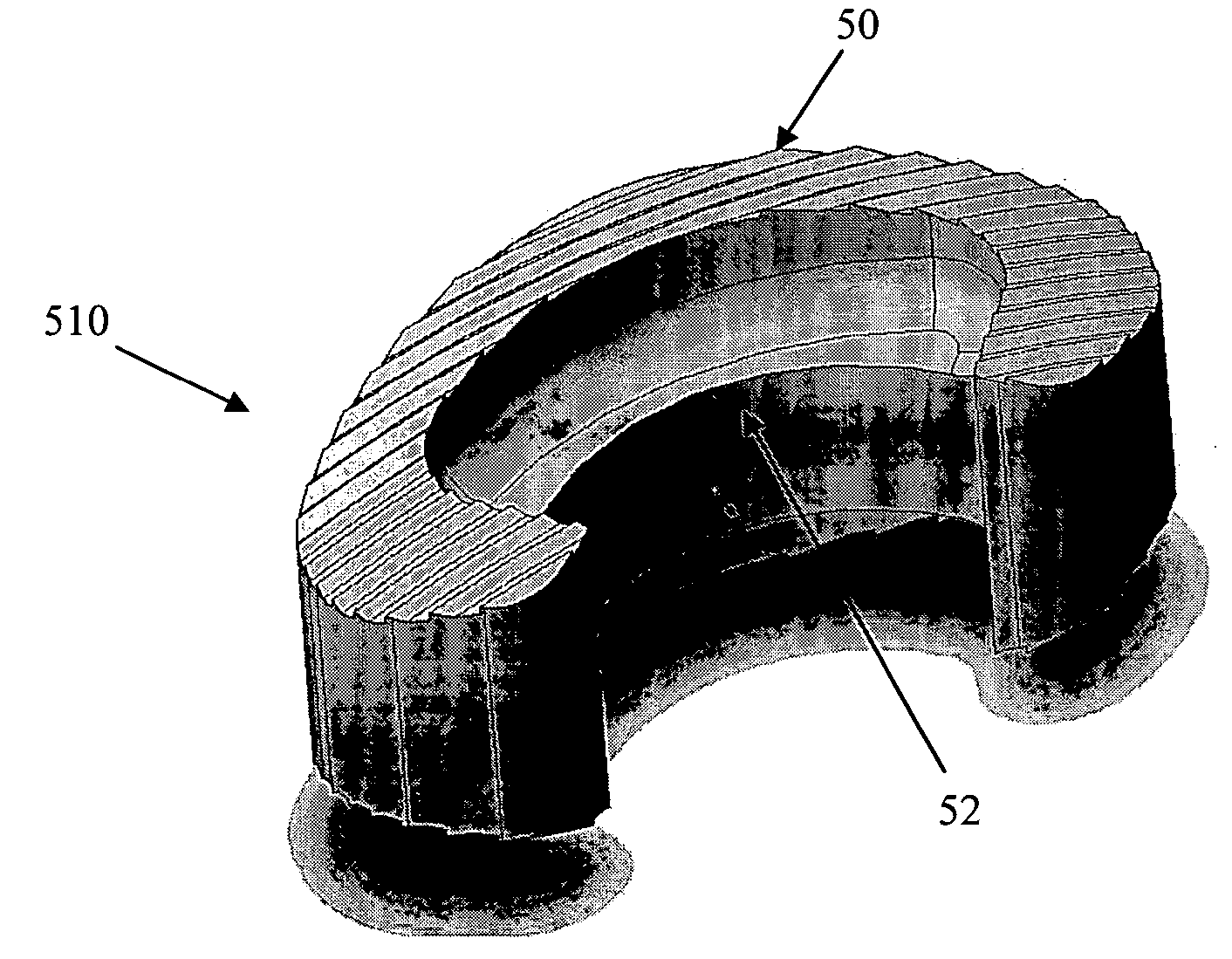
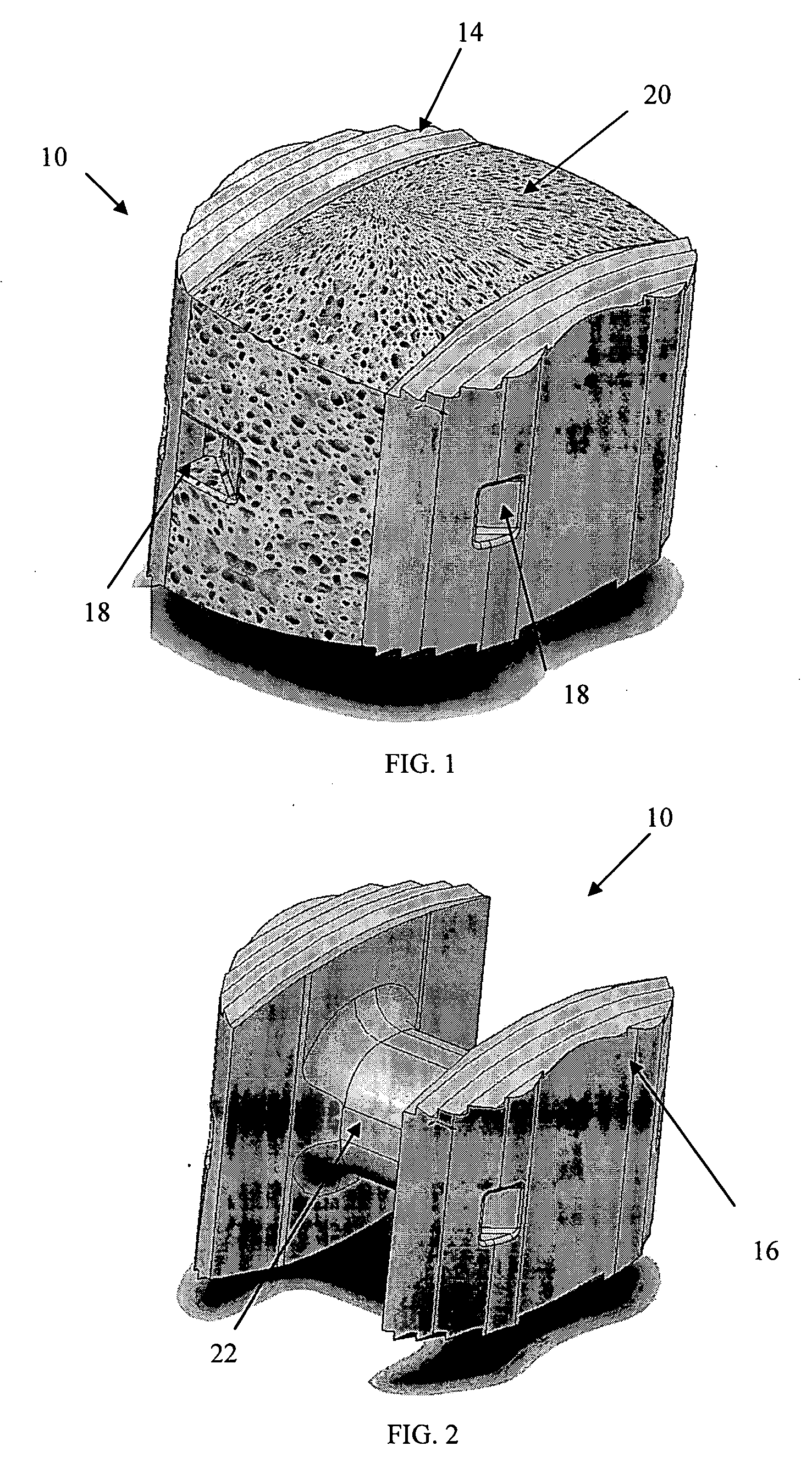
An interbody fusion device in one embodiment includes a tapered body defining a hollow interior for receiving bone graft or bone substitute material. The body defines exterior threads which are interrupted over portions of the outer surface of the device. The fusion device defines truncated side walls so that on end view the body takes on a cylindrical form. The side walls are provided with vascularization openings, and the body wall device includes opposite bone ingrowth slots extending through the interrupted thread portion of the body. In another embodiment, the tapered body is solid and formed of a porous biocompatible material having sufficient structural integrity to maintain the intradiscal space and normal curvature. The material is preferably a porous tantalum having fully interconnected pores to facilitate complete bone tissue ingrowth into the implant. An implant driver is provided which engages the truncated side walls to complete the cylindrical form of the implant at the root diameter of the interrupted threads, to thereby facilitate threaded insertion of the implant to the intra-discal space between adjacent vertebrae. Methods for posterior and anterior insertion of the fusion device are also disclosed.
Owner:WARSAW ORTHOPEDIC INC
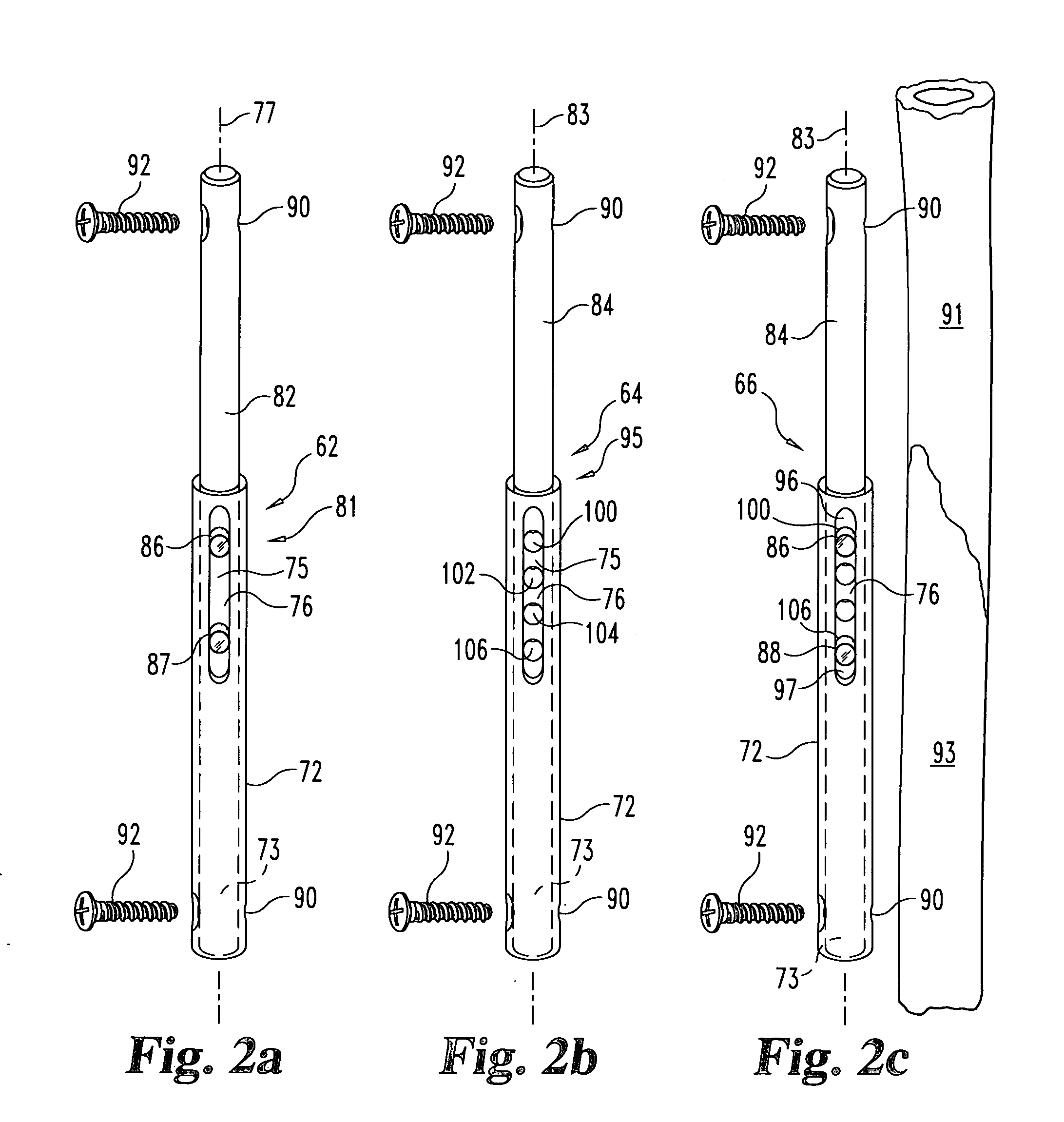
Anterior spinal instrumentation and method for implantation and revision
InactiveUSRE37005E1Reliable and decompressionEnhanced rigidity and fixationInternal osteosythesisBone implantBone trephineIntervertebral disk
A system and method for anterior fixation of the spine utilizes a cylindrical implant engaged in the intradiscal space at the cephalad and caudal ends of the construct. The implants are cylindrical fusion devices (10) filled with bone material to promote bone ingrowth and fusion of the disc space. An attachment member (40) is connected to each of the fusion devices (10) and a spinal rod (50) is connected to each of the attachment members using an eyebolt assembly (53, 54, 55). In a further inventive method, a revision of the construct is achieved by removing the fusion devices. Each fusion device is engaged by an elongated guide member (62) over which a cylindrical trephine (70) is advanced. The trephine (70) is used to extract a core (84) of bone material around the fusion implant, while the guide member (62) is used to remove the bone core (84) containing the fusion implant (10). In another aspect of the invention, a removal insert (90, 90') is provided that engages an implanted fusion device (10). The removal insert (90, 90') can be used to guide the trephine (70) around the fusion device, and is connected to a removal tool (100) once the bone core is created. The removal tool (100) includes a shaft (101) attached to the removal insert (90, 90'), and a slap hammer (104) slidably mounted on the shaft.
Owner:WARSAW ORTHOPEDIC INC
Threaded spinal implant with bone ingrowth openings
An implant is disclosed for use in spinal stabilization. In one preferred embodiment, the implant is described as including a hollow, cylindrical body having external threading and a plurality of openings formed radially through the body in communication with the body interior. The holes are positioned to chip bone into the implant as the implant is rotated.
Owner:ZIMMER SPINE INC
Radiolucent bone graft
InactiveUS6846327B2Enhanced bone ingrowthImprove fusionBone implantJoint implantsBone ingrowthVertebra
An improved ceramic bone graft is provided for human implantation, particularly such as a spinal fusion cage for implantation into the inter-vertebral space between two adjacent vertebrae. The improved spinal fusion cage includes a substrate block of high strength ceramic having a selected size and shape to fit the anatomical space, and a controlled porosity analogous to natural bone. The substrate block is coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone ingrowth and enhanced bone fusion. Upon implantation, the fusion cage provides a spacer element having a desired combination of mechanical strength together with osteoconductivity and osteoinductivity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The fusion cage may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:CTL MEDICAL CORP +1
Acetabular Cup Having An Adjustable Modular Augment
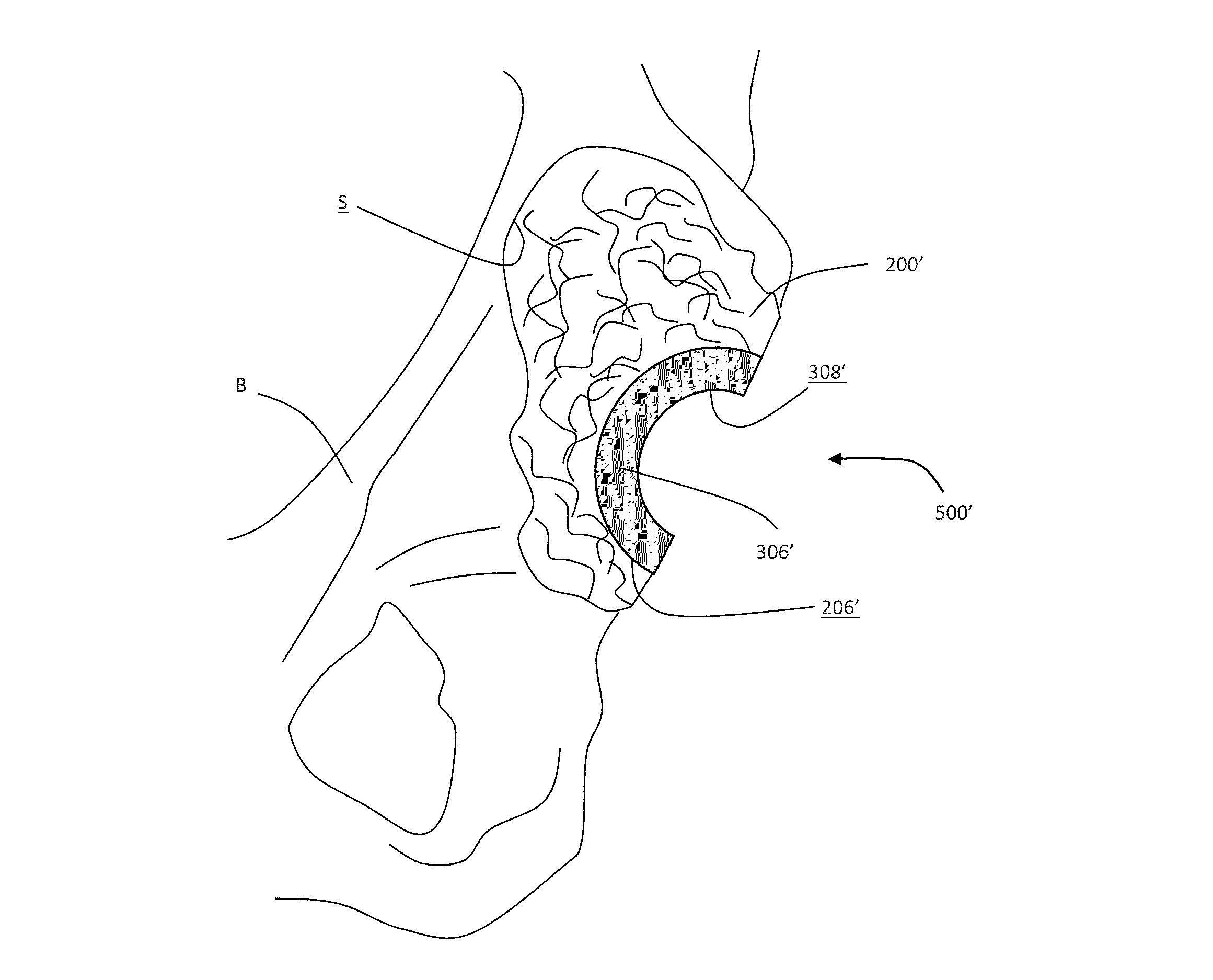
An orthopedic prosthesis for implantation into a bone of a patient can include a shell, an augment and a securing member. The shell can be adapted to be affixed within the bone and have an outer surface adapted to receive bone ingrowth after implantation. The shell can have an inner surface adapted to engage a liner. An elongated slot can extend between the outer and inner surfaces of the shell. The augment can define a body and have a passage therethrough. The securing member can extend through the passage and the slot. The securing member can be movable between a locked position wherein the augment is precluded from relative movement with the shell and an unlocked position wherein the securing member is adapted to slidably traverse along the slot to locate the augment at a plurality of positions relative to the shell.
Owner:BIOMET MFG CORP
Rapid manufacturing of porous metal prostheses
ActiveUS20130018483A1Fast preparationAdditive manufacturing apparatusBone implantProsthesisPlastic surgery
An orthopaedic prosthesis and a method for rapidly manufacturing the same are provided. The orthopaedic prosthesis includes a solid bearing layer, a porous bone-ingrowth layer, and an interdigitating layer therebetween. A laser sintering technique is performed to manufacture the orthopaedic prosthesis.
Owner:ZIMMER INC
Radiolucent spinal fusion cage
InactiveUS20050049706A1Improve carrying capacityHigh porosityBone implantJoint implantsPorosityBone growth
An improved bone graft is provided for human implantation, particularly such as a spinal fusion cage for implantation into the inter-vertebral space between two adjacent vertebrae. The improved spinal fusion cage includes a substrate block of high strength biocompatible material having a selected size and shape to fit the anatomical space, and a controlled porosity analogous to natural bone. The substrate block may be coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone in growth and enhanced bone fusion. Upon implantation, the fusion cage provides a spacer element having a desired combination of mechanical strength together with osteoconductivity and osteoinductivity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The fusion cage may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:AMEDICA A DELAWARE
Bone cement composite containing particles in a non-uniform spatial distribution and devices for implementation
InactiveUS20070185231A1Avoid compressionReduce cavity volumeImpression capsSurgical adhesivesNatural boneControl manner
One embodiment of the invention comprises a differential composite in which bone cement everywhere or substantially everywhere contains at least some non-zero volume fraction of particles, and in which the local volume fraction of particles may vary from place to place in the composite in a controlled manner. The variation may be by identifiable region or may be in the form of a gradient of the local volume fraction of particles. In at least some places, the local volume fraction of particles may be such that the particles act as crack arrestors. Close to the interface with natural bone, the local volume fraction of particles may be greater. In at least some places adjoining natural bone, the local volume fraction of particles may be such as to allow bone ingrowth into appropriate region(s) of the composite, resulting in improved interfacial shear strength. Methods and apparatuses for producing and delivering the composite are also disclosed, which may include use of an introducer and an expandable basket-type device.
Owner:OSSEON THERAPEUTICS
Osteoconductive spinal fixation system
InactiveUS20060276788A1High strengthHigh mechanical strengthSuture equipmentsInternal osteosythesisPorositySpinal column
An improved spinal fixation system is provided for human implantation, including a set of screws with interconnecting rods for implantation into the pedicle and between two adjacent vertebrae or a plate with screws for fixating two adjacent vertebrae. The screws, rods, and plates include a substrate portion of high strength biocompatible material and a controlled porosity analogous to natural bone. The substrate portion may be coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone ingrowth and enhanced bone fusion. Upon implantation, the fixation system provides a desired combination of mechanical strength together with osteoconductivity and bio-activity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The fixation system may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:AMEDICA A DELAWARE
Porous, load-bearing, ceramic or metal implant
A method and apparatus for adjusting the modulus of elasticity, flexural strength, or porosity of metal and ceramic implants is disclosed in one embodiment of the invention as including a green tape comprising metal or ceramic particles, or a combination thereof, for incorporation into a solid implant structure. Apertures are cut in selected regions of the green tape in order to create a desired pore structure in the solid implant structure. This pore structure may be designed to give the solid structure a desired modulus of elasticity, flexural strength, or porosity as well as to promote bone ingrowth. The green tape may then be layered in an orientation that will provide the desired pore structure and the metal or ceramic particles and layers may be fused together to create the solid implant structure.
Owner:CERAMTEC
Vertebroplasty methods with optimized shear strength and crack propagation resistance
InactiveUS20080195112A1Reduce cavity volumeImpression capsSurgical adhesivesNatural boneControl manner
Owner:LIU Y KING +3
Laser based metal deposition of implant structures
InactiveUS7001672B2Minimize adverse effectsFine grain structureFinger jointsDental implantsArtificial jointsWear resistant
Owner:MEDICINELODGE
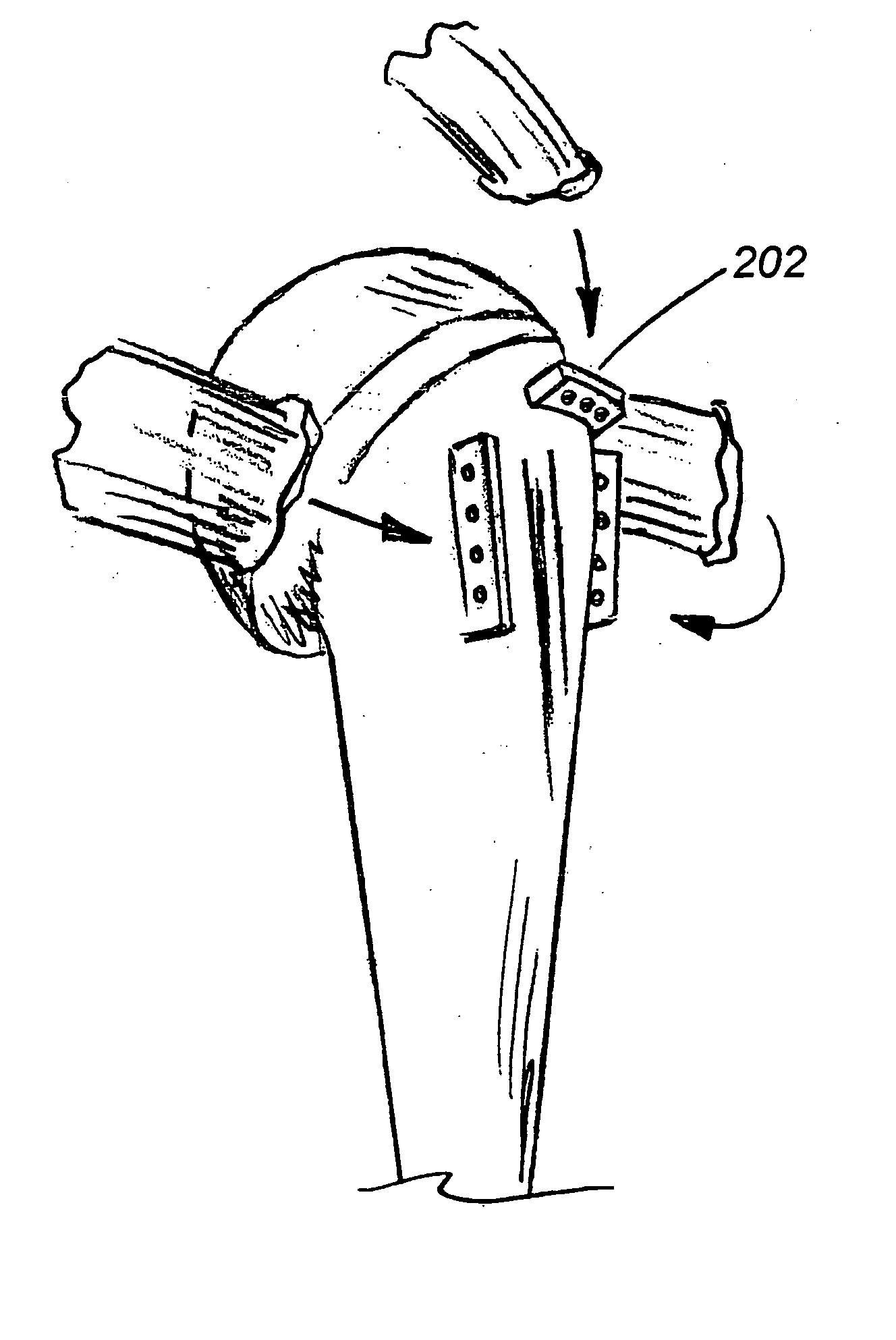
Shoulder prosthesis with anatomic reattachment features
InactiveUS20050090902A1Improved anatomic attachment areaSuture equipmentsSurgical needlesLesser TuberosityBiceps brachii tendon
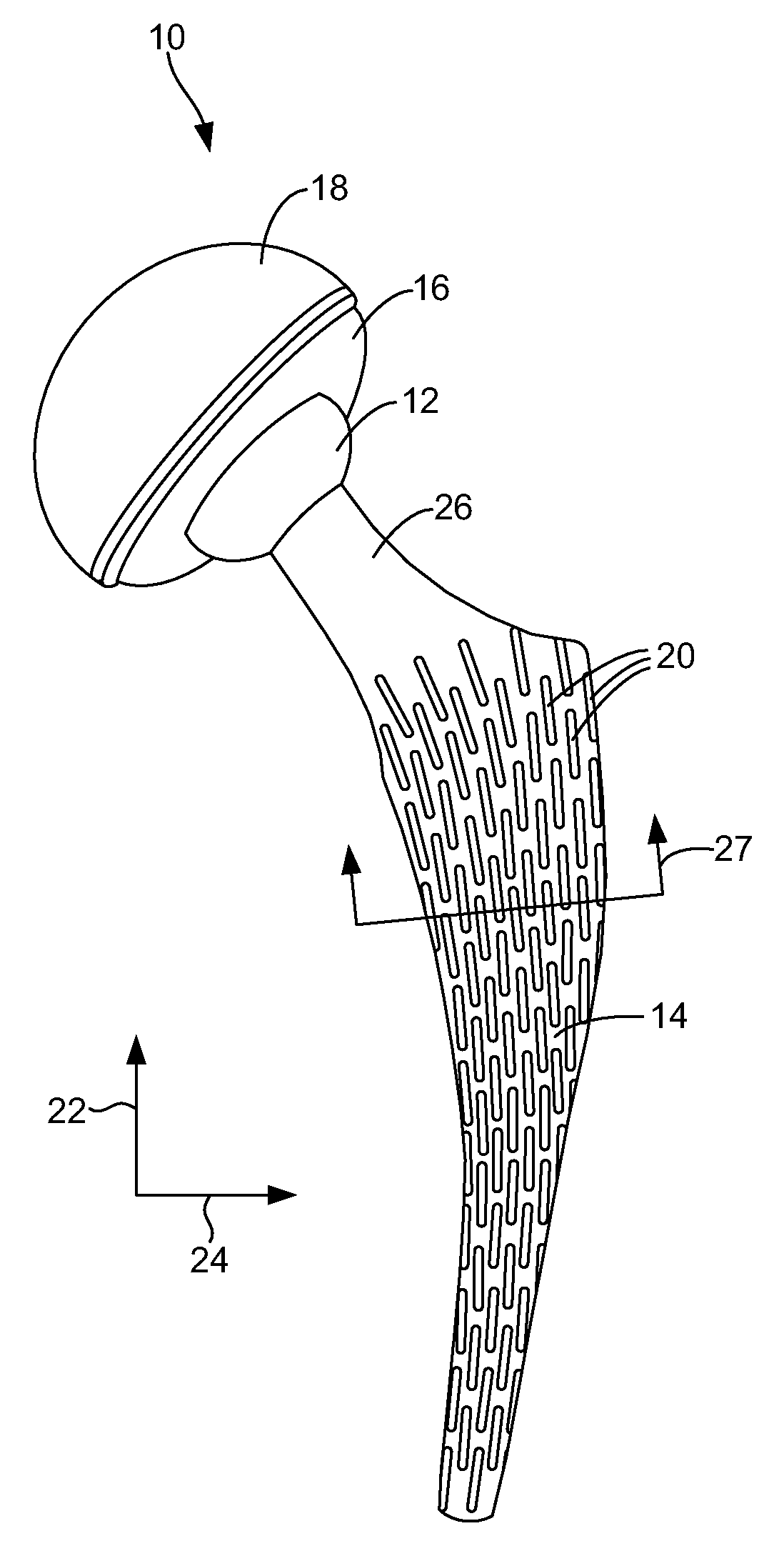
A humeral prosthesis features improved anatomic attachment areas for tendon or bone. In the preferred embodiment, in contrast to existing devices, at least one set of tendon / bone attachment points are provided along a line, at least a portion of which is divergent with respect to the axis of the stem. One or more sets of attachment points may be further be provided along a line which is substantially parallel to the axis of the stem, resulting in a “T”“L” or “U” shape. Alternatively, attachment points having a changing degree of diversion with respect to the axis of the stem may be provided along a common, curved line. The attachment points may simply be apertures formed through the body of the implant though, in the preferred embodiment, the apertures are provided on raised tabs. An area of bone-ingrowth material may be provided adjacent the attachment points, and may include a separate fastening mechanism such as a threaded hole to receive a screw. A groove may also be provided in any embodiment to receive the biceps tendon. Particularly with respect to fractures, including multi- and ‘four-part’ fractures, means specifically intended for the rigid reattachment of the greater or lesser tuberosities may be provided separately or in conjunction with other sets of reattachment configurations.
Owner:MASINI MICHAEL A
Laser based metal deposition (LBMD) of antimicrobials to implant surfaces
InactiveUS20070287027A1Improved bearing propertyImprove propertiesFinger jointsDental implantsWear resistantBearing surface
A method is provided for depositing a hard wear resistant surface onto a porous or non-porous base material of a medical implant. The wear resistant surface of the medical implant device may be formed by a Laser Based Metal Deposition (LBMD) method such as Laser Engineered Net Shaping (LENS). The wear resistant surface may include a blend of multiple different biocompatible materials. Further, functionally graded layers of biocompatible materials may be used to form the wear resistant surface. Usage of a porous material for the base may promote bone ingrowth to allow the implant to fuse strongly with the bone of a host patient. The hard wear resistant surface provides device longevity, particularly when applied to bearing surfaces such as artificial joint bearing surfaces or a dental implant bearing surfaces. An antimicrobial material such as silver may be deposited in combination with a metal to form an antimicrobial surface deposit.
Owner:MEDICINELODGE
Spinal fusion implants and tools for insertion and revision
InactiveUS20090043394A1Avoid expulsionInternal osteosythesisBone implantSpinal columnPorous tantalum
An interbody fusion device in one embodiment includes a tapered body defining a hollow interior or chamber for receiving bone graft or bone substitute material. The body defines exterior threads which are interrupted over portions of the outer surface of the device. The fusion device includes truncated side walls so that on end view the body takes on a cylindrical form. In another embodiment, the tapered body is solid and formed of a porous biocompatible material having sufficient structural integrity to maintain the intradiscal space and normal curvature. The material is preferably a porous tantalum composite having fully interconnected pores to facilitate complete bone tissue ingrowth into the implant. In further embodiments, the fusion devices are provided with osteogenic material to facilitate bone ingrowth. A cap is also provided to block the opening of hollow fusion devices. The cap includes an occlusion body and an elongated anchor. In some embodiments the anchor includes a lip which is engageable to openings in the body wall. Tools are also provided for manipulating caps for interbody fusion devices. In one embodiment the tool includes a pair of prongs each having facing engagement surfaces for engaging the fusion device, and a shaft slidably disposed between the prongs. The shaft has a first end defining a cap-engaging tip for engaging a tool hole in the cap. In one embodiment the cap engaging tip defines threads. In another embodiment the prongs include a pair of releasing members on each of the facing engagement surfaces. The releasing members have a height and a width for being insertable into apertures in a body wall in the fusion device to disengage the elongate anchors from the apertures.
Owner:WARSAW ORTHOPEDIC INC
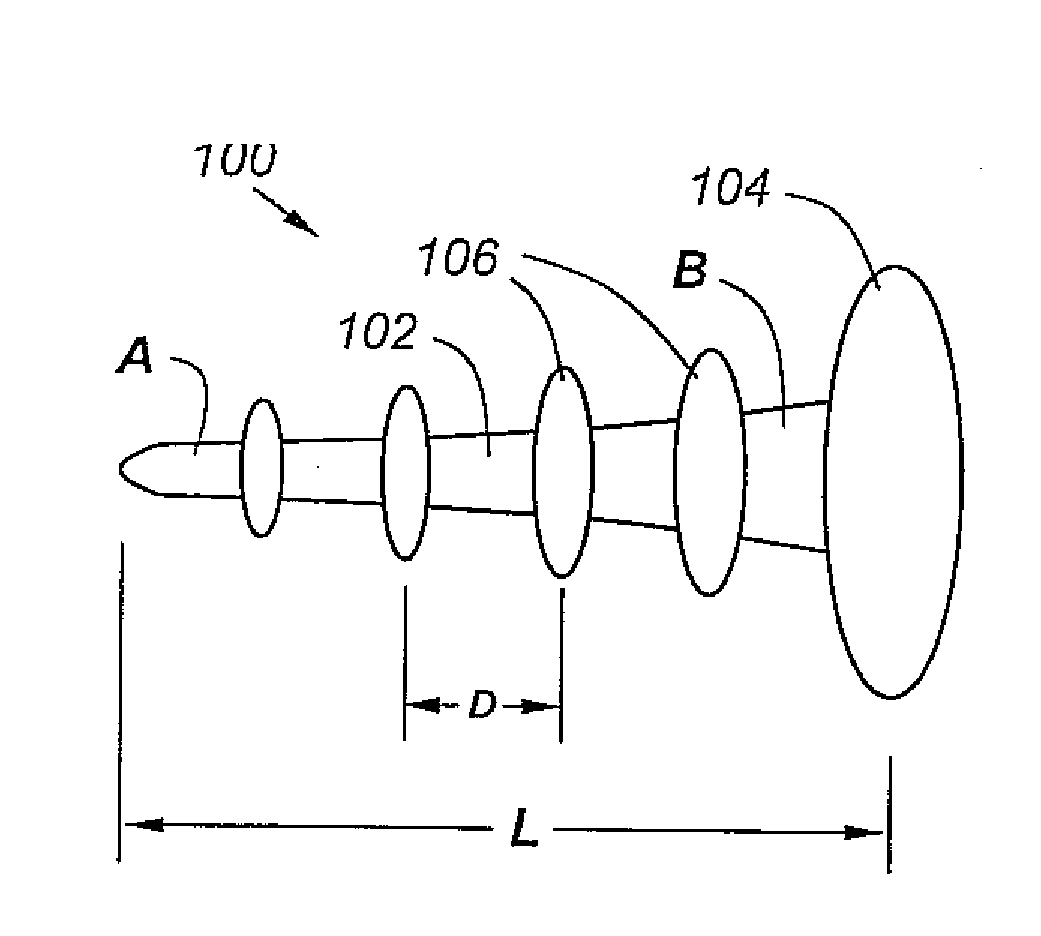
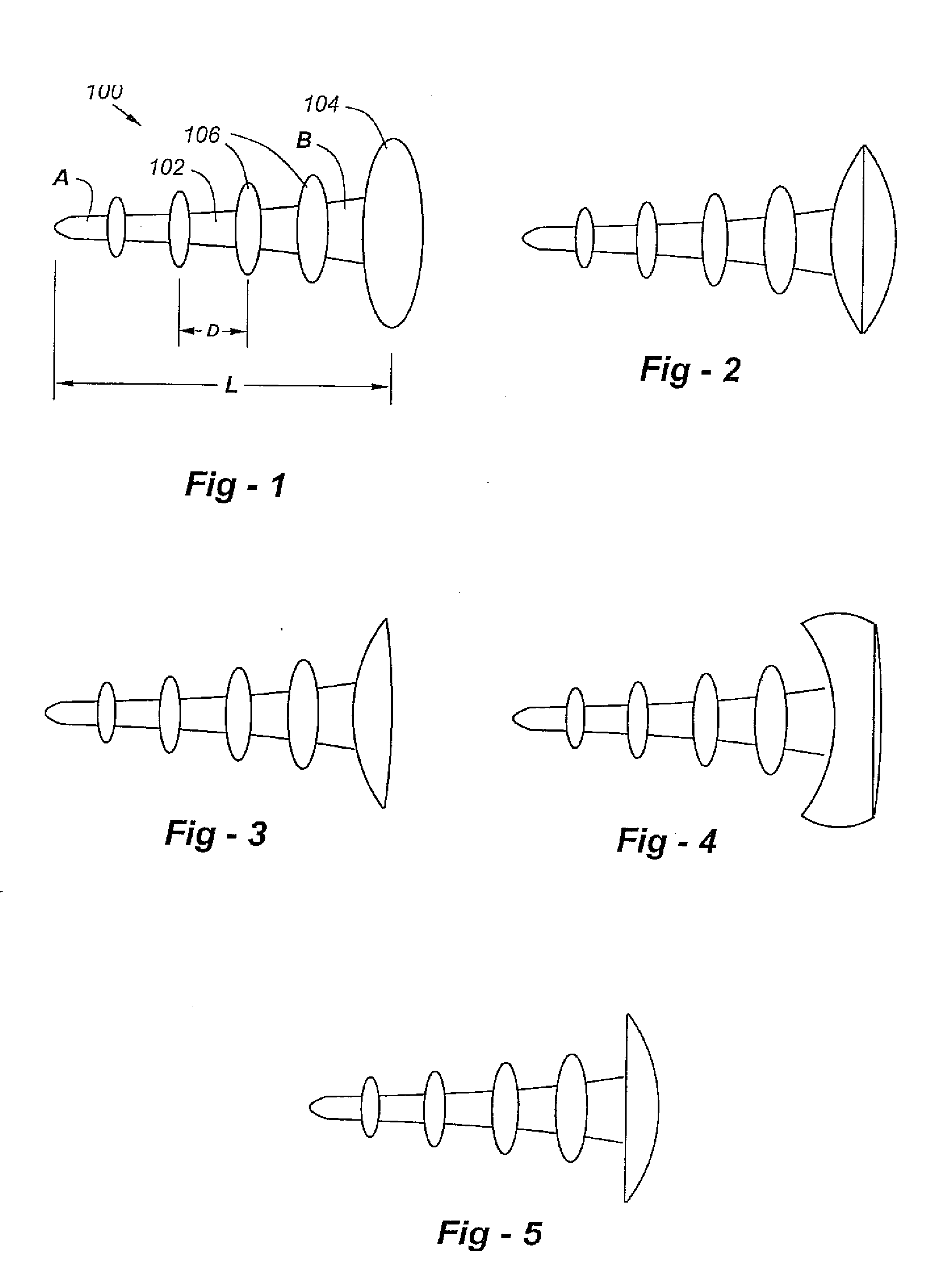
Anti-backout arthroscopic uni-compartmental prosthesis
An improved uni-compartmental implant has a shaft having a proximal end attached to a head and a distal end, and one or more raised portions spaced apart along the shaft to resist back-out. The length between the head and distal end is preferably less than 50 mm, the distal end of the shaft has a diameter on the order of 2 to 3 mm, the proximal end of the shaft has a diameter on the order of 2 to 4 mm, and the head has a diameter ranging from 4 mm or less to 20 mm or more, making the device suitable for knee arthroscopy and other applications. The shaft and / or raised portions may include a bone-ingrowth or bone-ongrowth surface, and the shaft and / or raised portions may be made of a fiber-metal. The head portion is preferably ceramic, though a chrome-cobalt alloy, titanium, or other bio-compatible material may be used. The head portion may have a bi-convex shape, a piano-convex shape, or a concave-convex shape.
Owner:LENKBAR
Modular acetabular anti-protrusio cage and porous ingrowth cup combination
InactiveUS6908486B2Maximum flexibilityInternal osteosythesisJoint implantsAcetabular componentRight femoral head
A modular acetabular anti-protrusio cage and acetabular cup combination includes an acetabular cup having a bone ingrowth-promoting surface and a shaped anti-protrusio cage having one or more fixation flanges. The acetabular cup is attached to acetabular bone, and the anti-protrusio cage is thereafter mounted to the inner surface of the acetabular cup. Alternatively, the anti-protrusio cage can be mounted to the inner surface of the acetabular cup before the acetabular cup is attached to acetabular bone. The flanges of the anti-protrusio cage are joined to the ilium, ischium and / or pubis to secure the acetabular cup and the anti-protrusio cage to the hip bone and to distribute forces away from the medial wall of the acetabulum. After the cup and the cage are mounted in the patient, a bearing insert is secured within the interior of the anti-protrusio cage, and a prosthetic femoral head is positioned in the bearing insert.
Owner:ZIMMER INC
Prosthetic implant assembly
InactiveUS20040117014A1Common problemConvenient for temporary fixationLigamentsJoint implantsTibiaBone tunnel
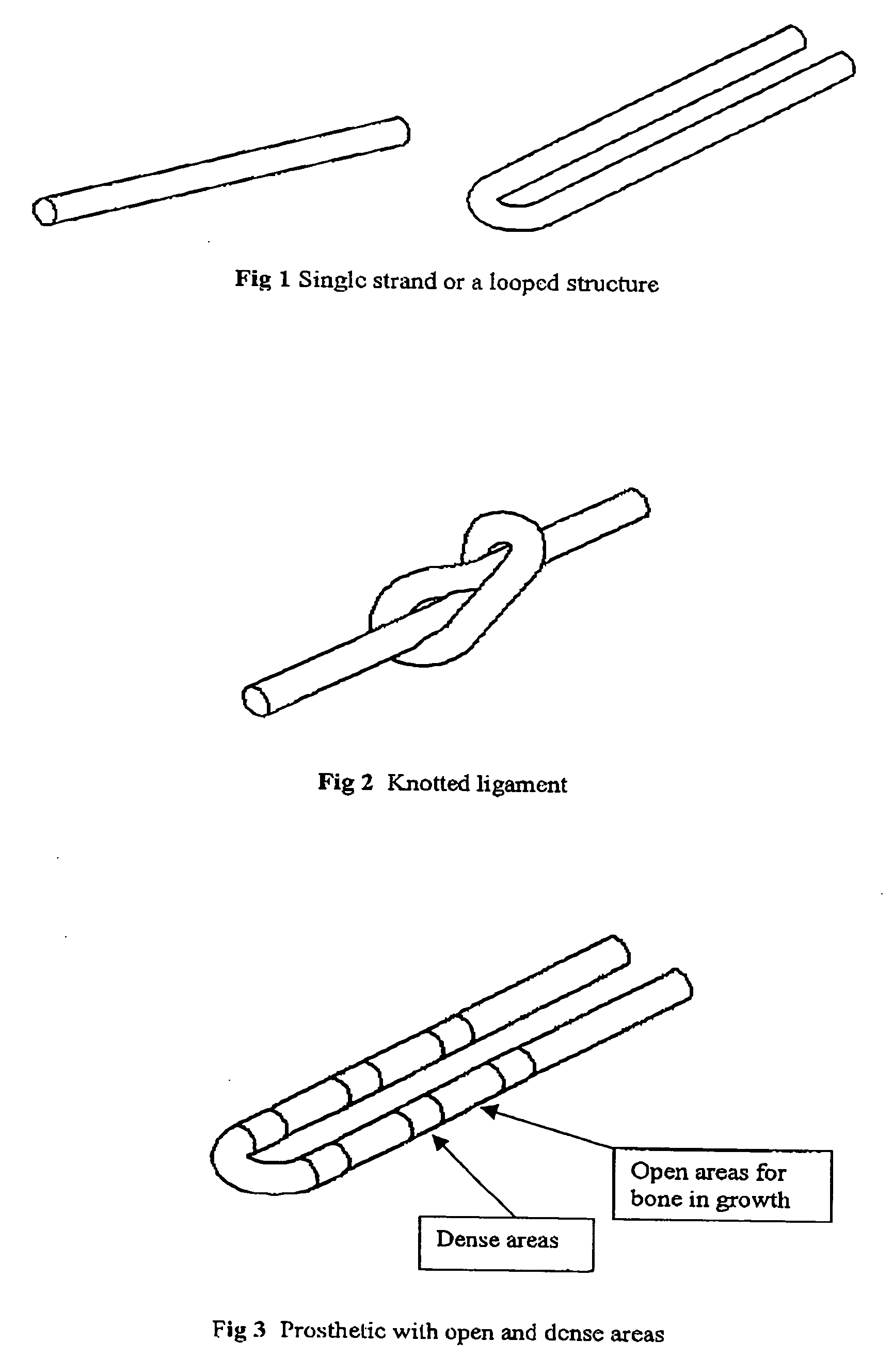
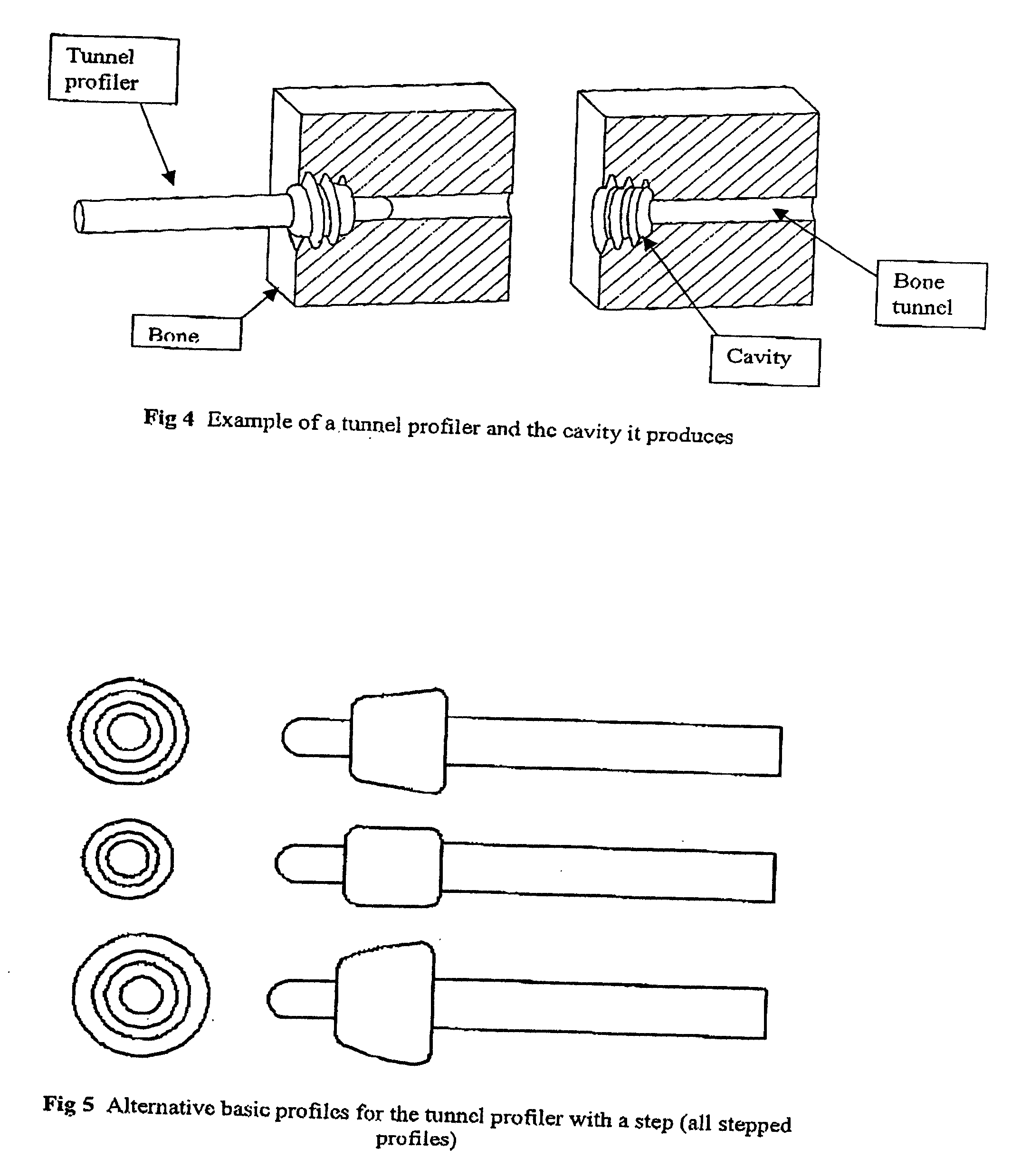
A prosthetic implant kit for implantation in a bone joint between first and second bones, such as the knee joint between a femur and a tibia, such joint having bone tunnels formed in each of the first and second bones, and the implant assembly being intended to be introduced into the bone tunnels and to be fixated therein, and implant kit assembly comprising: a prosthetic implant e.g. a ligament to be fitted in the bone tunnels; a degradable adhesive and / or filler material to be introduced into the bone tunnel to fill the space available between the implant and the wall of the bone tunnel, such maternal being of such a nature that it is capable of setting in order temporarily to anchor the implant in position, but which degrades over time so as to allow natural boney ingrowth to take over the anchoring of the implant; and a tunnel profiler to form an enlarged entry cavity to the bone tunnels to be filled with the material and improve the temporary fixation of the implant.
Owner:BRYANT JULIAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com