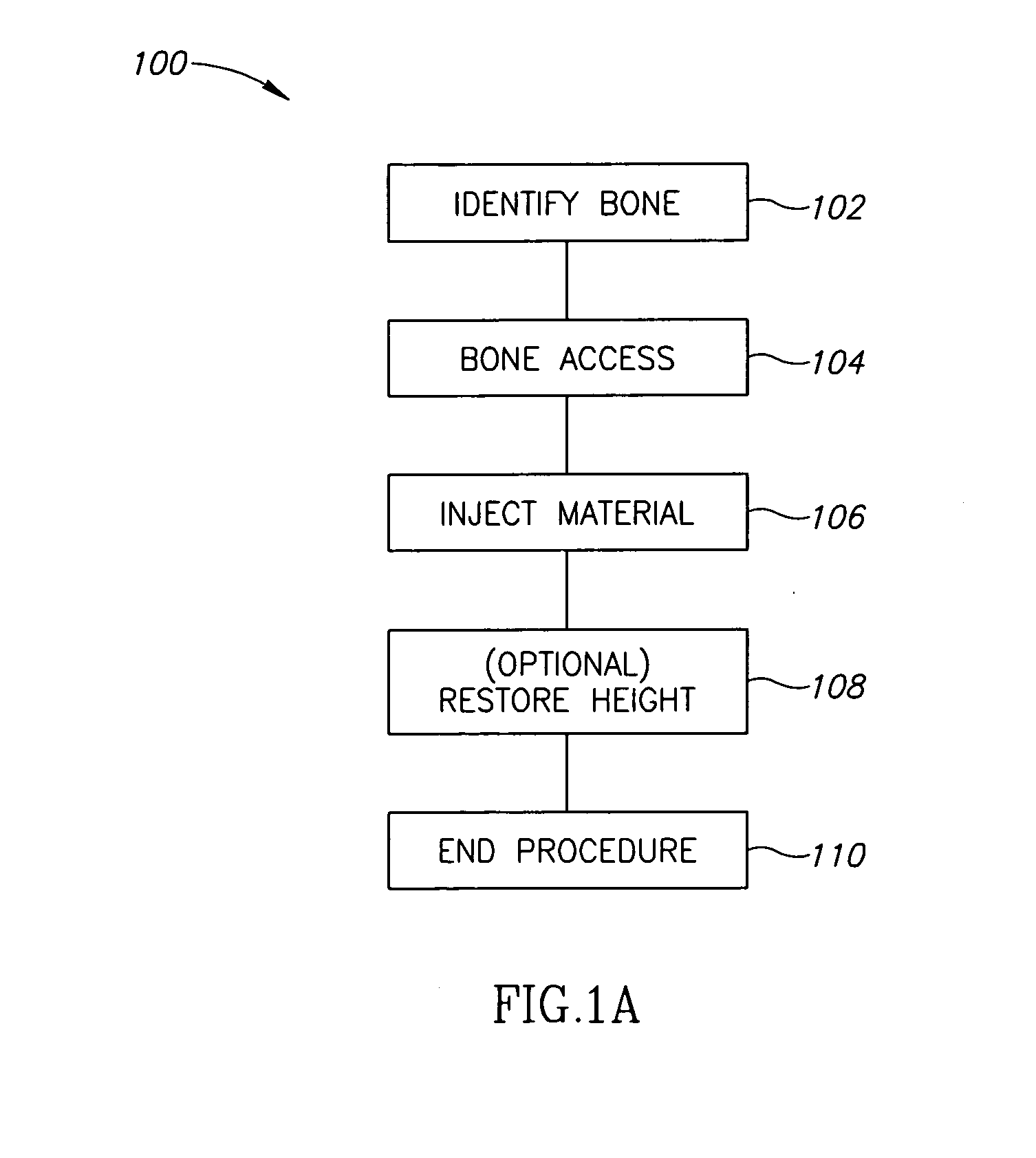
Patents
Literature
1800 results about "Bone cement" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
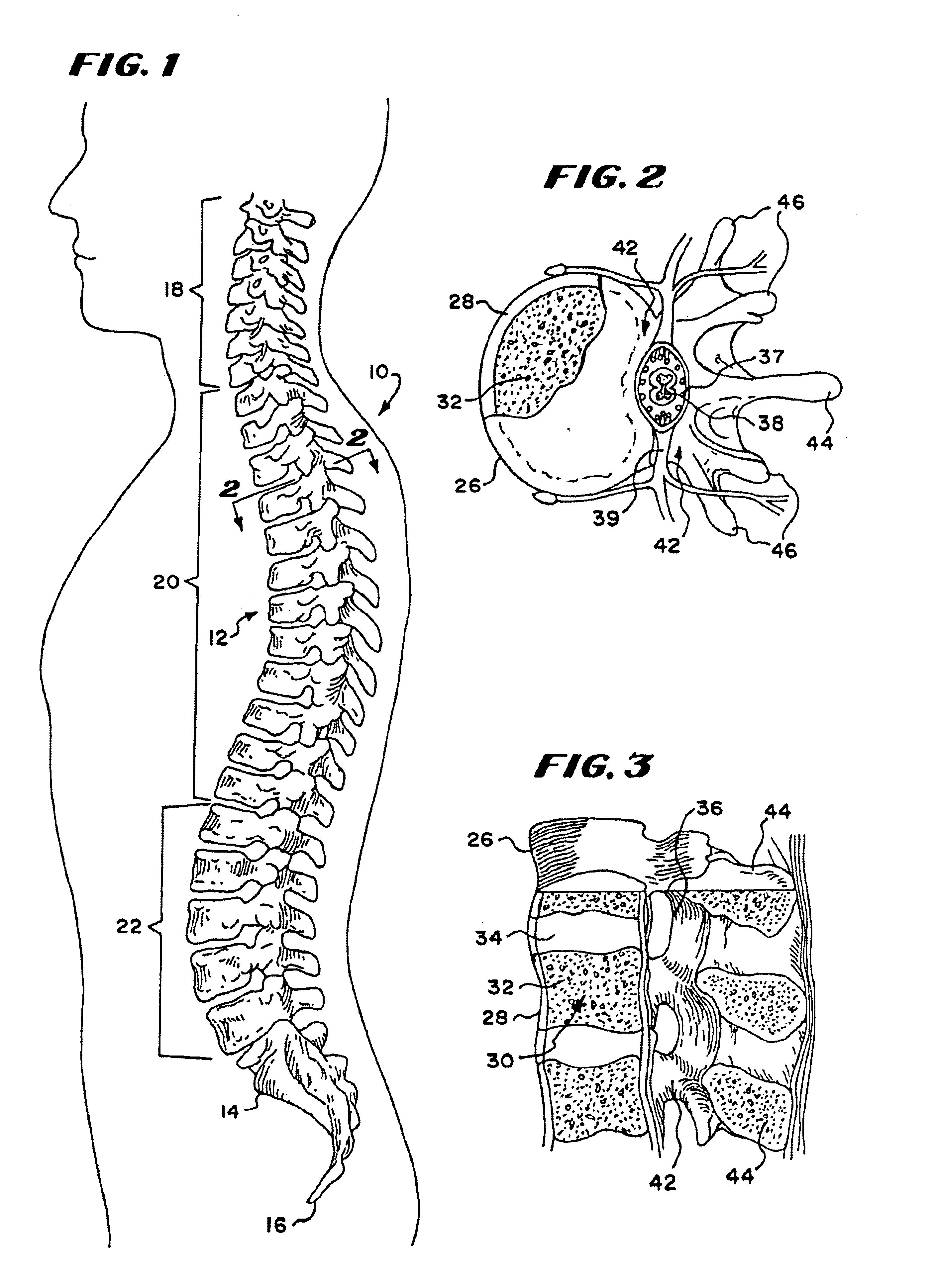
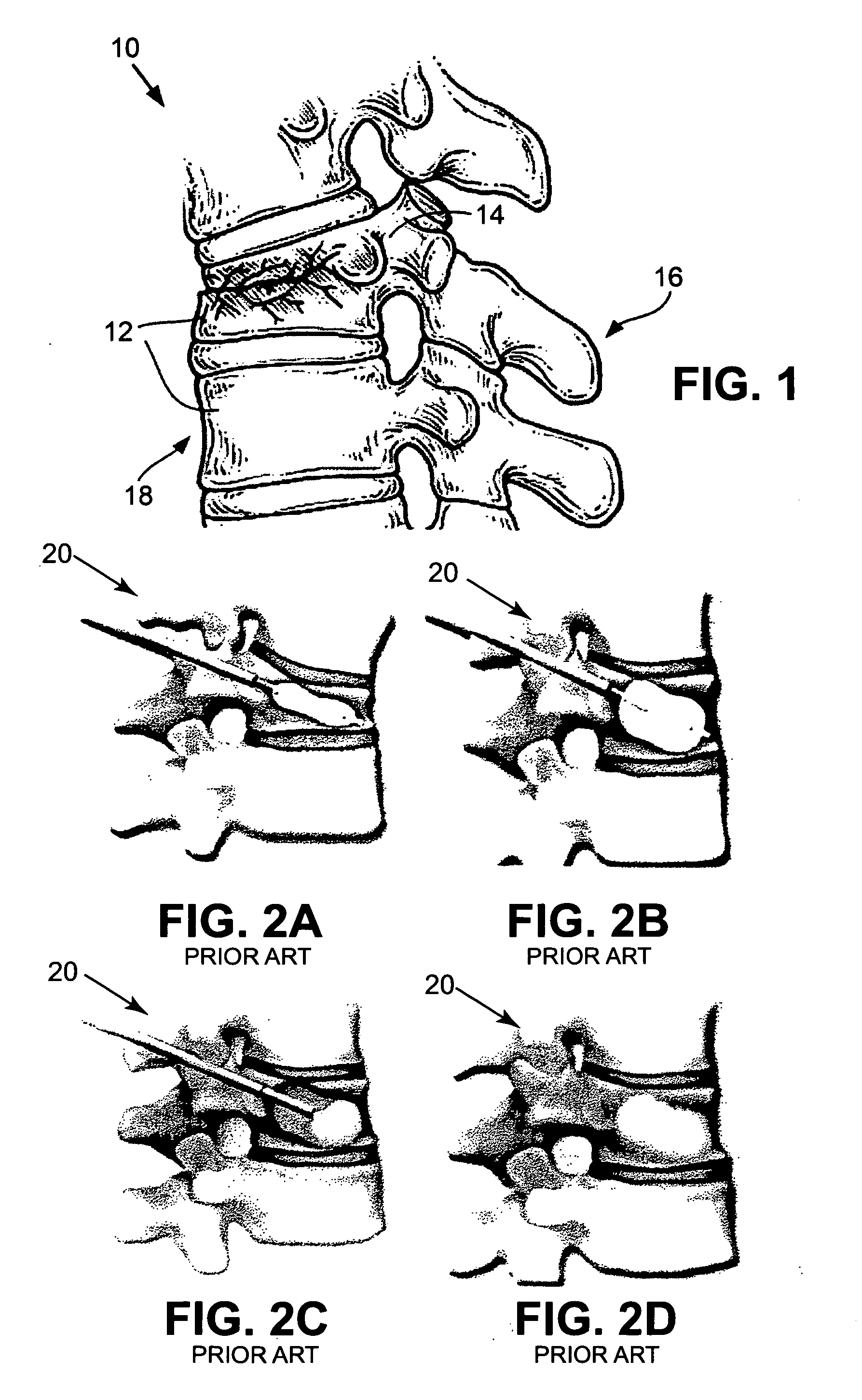
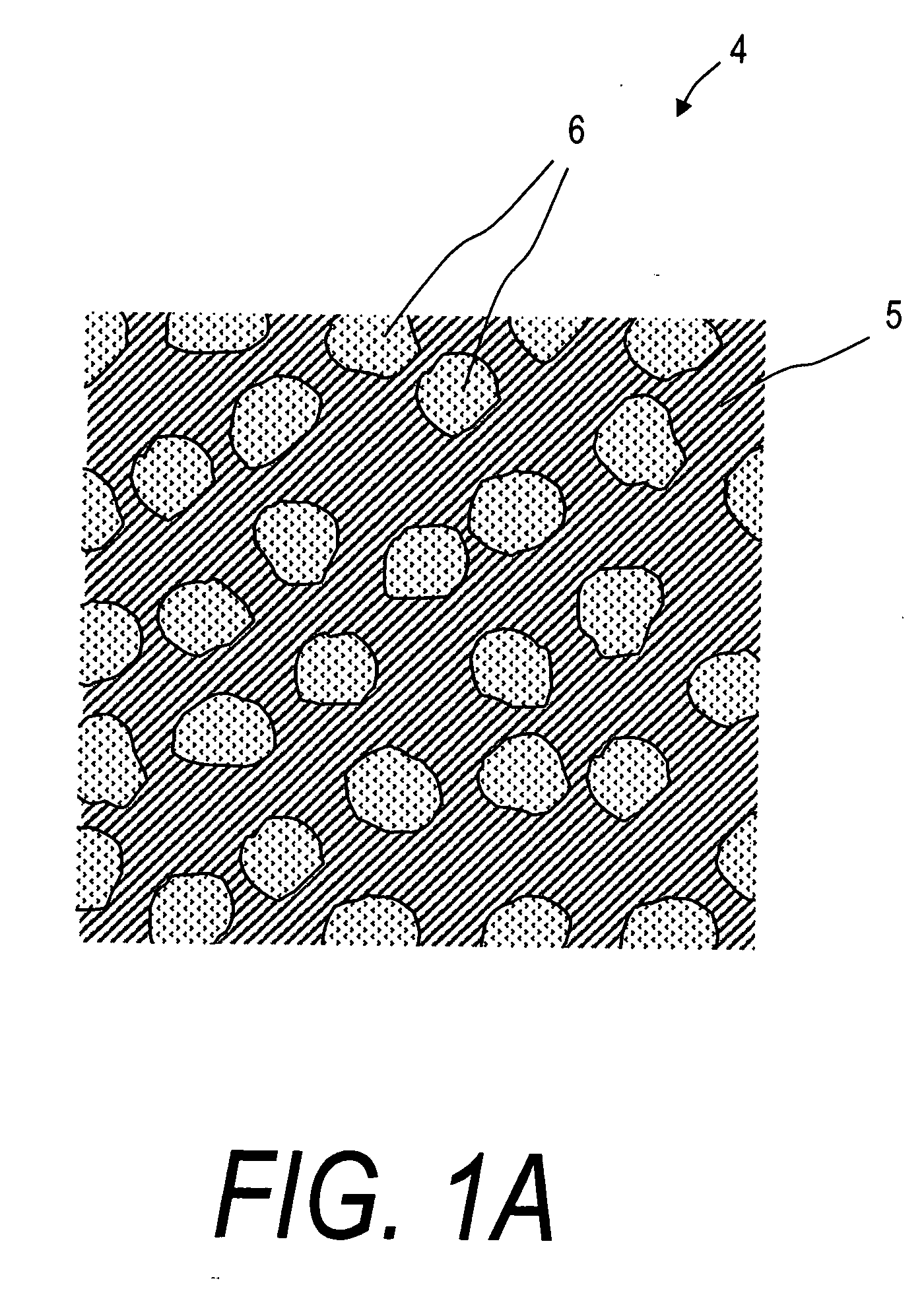
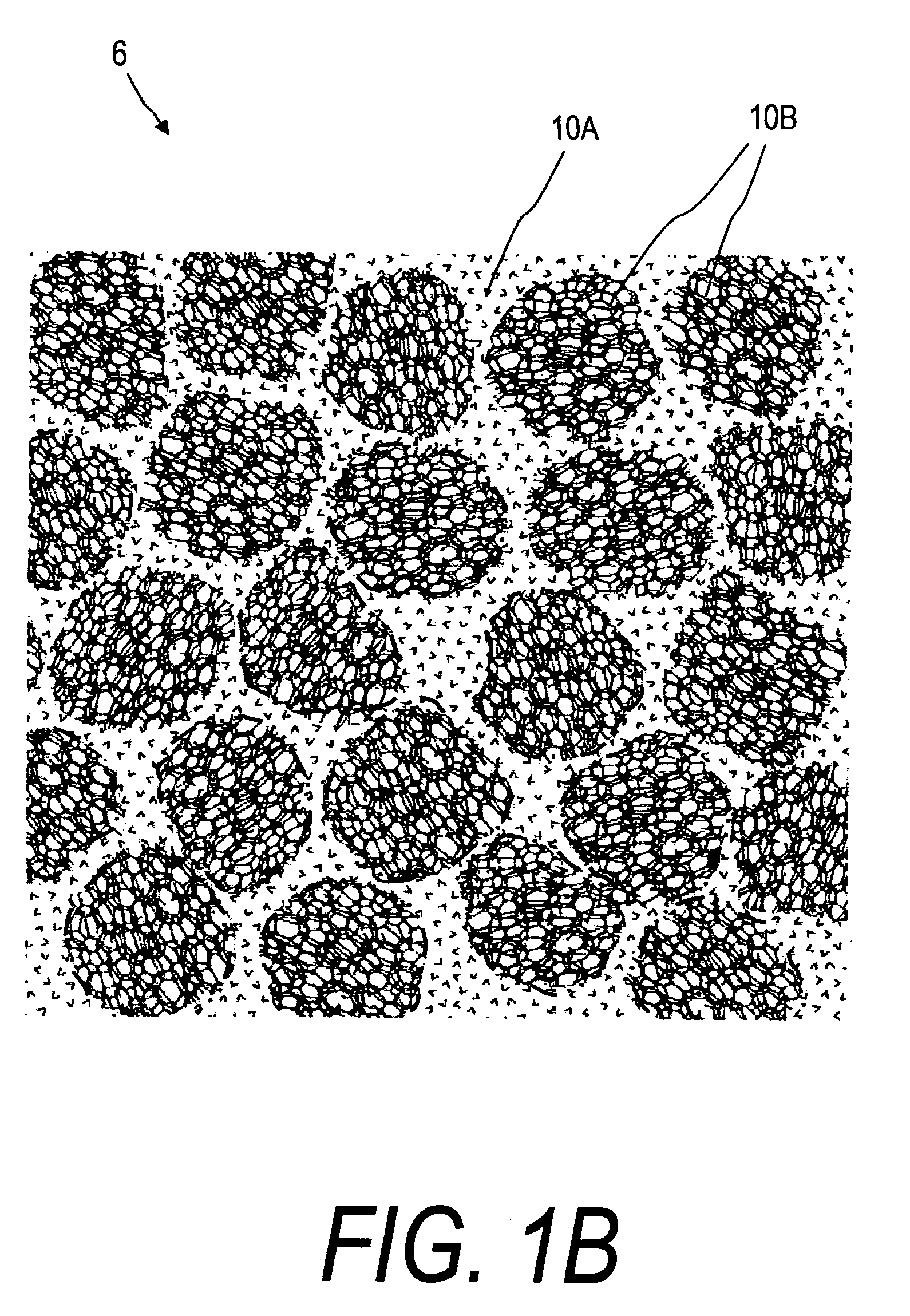
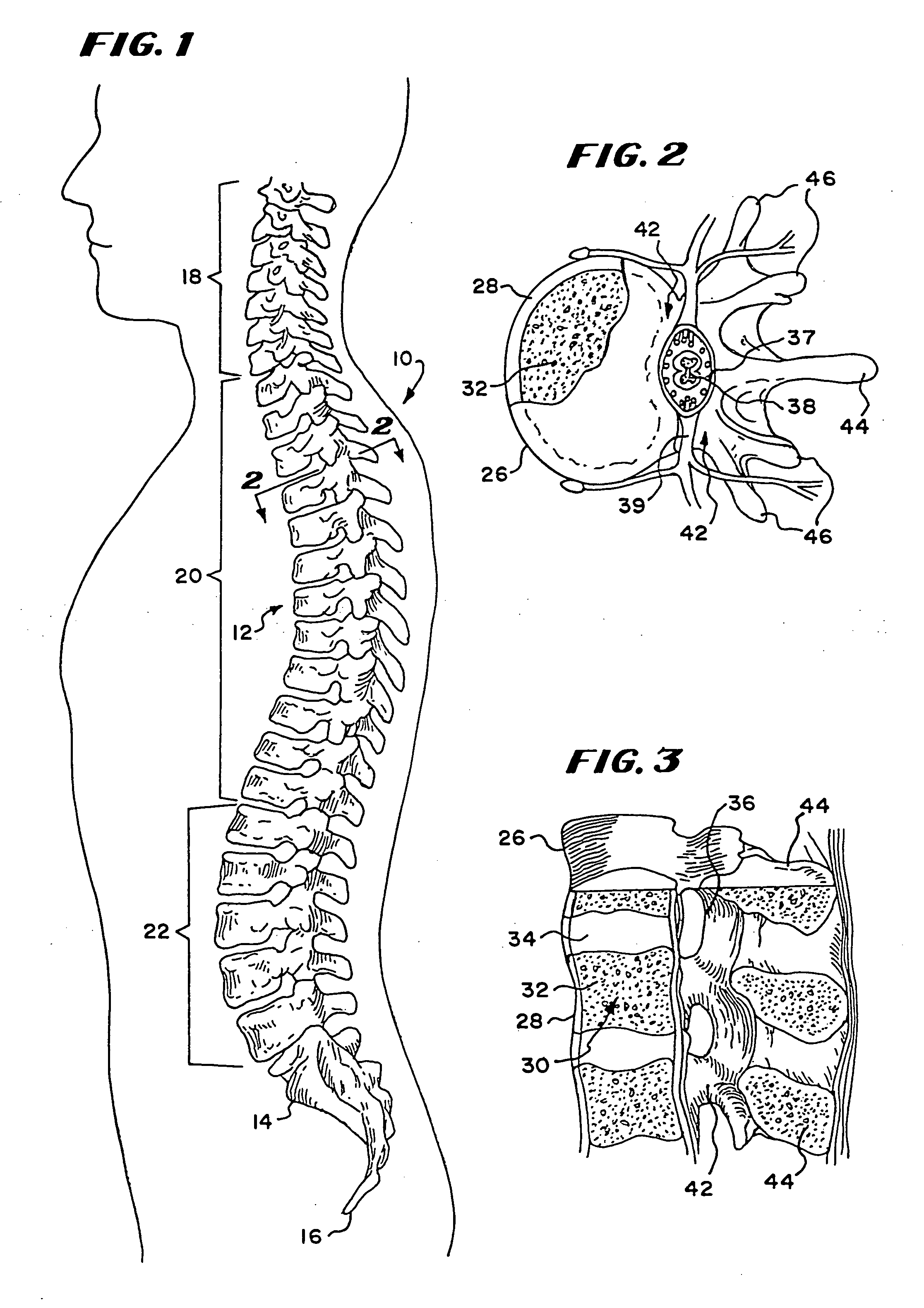
Bone cements have been used very successfully to anchor artificial joints (hip joints, knee joints, shoulder and elbow joints) for more than half a century. Artificial joints (referred to as prostheses) are anchored with bone cement. The bone cement fills the free space between the prosthesis and the bone and plays the important role of an elastic zone. This is necessary because the human hip is acted on by approximately 10-12 times the body weight and therefore the bone cement must absorb the forces acting on the hips to ensure that the artificial implant remains in place over the long term.
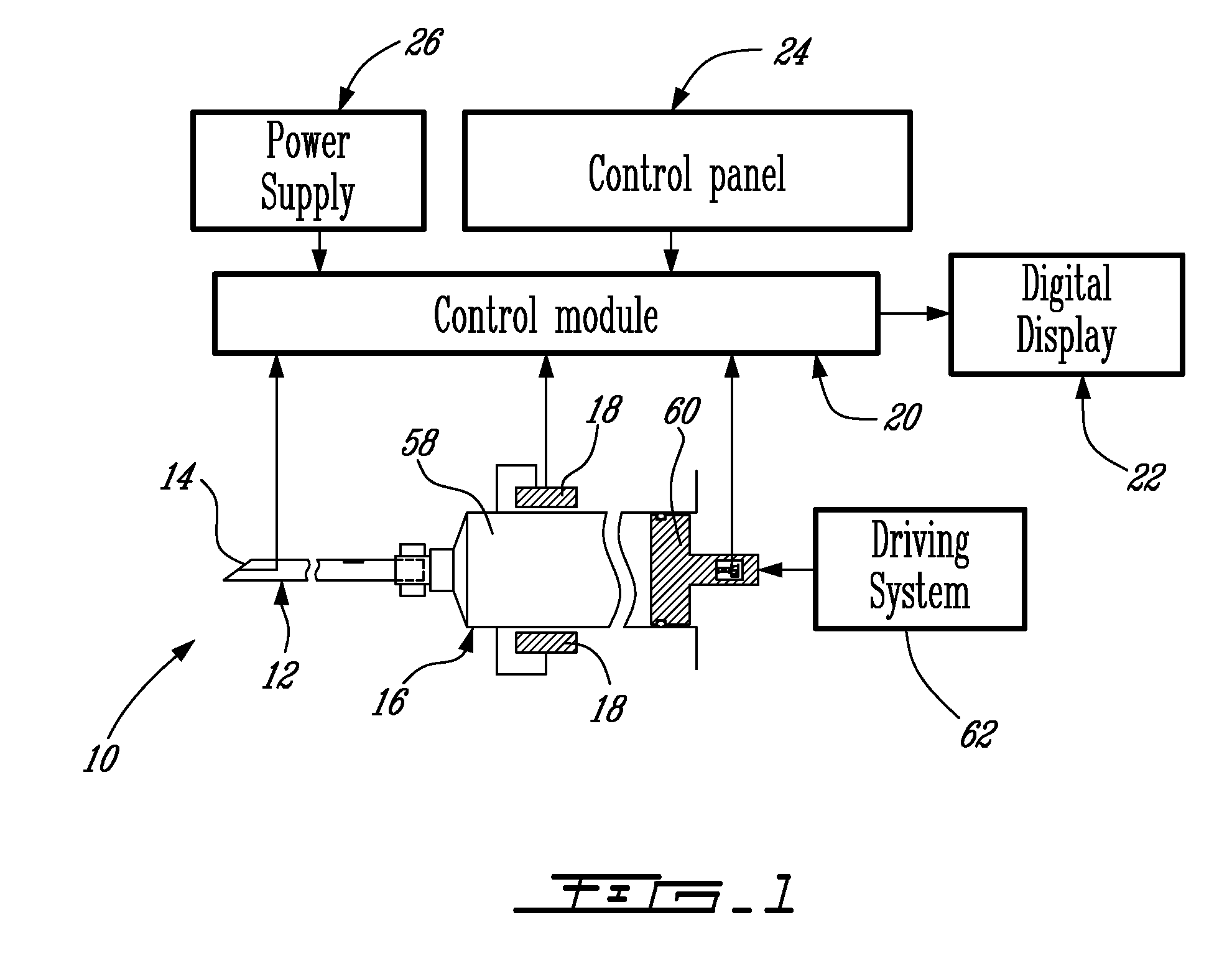
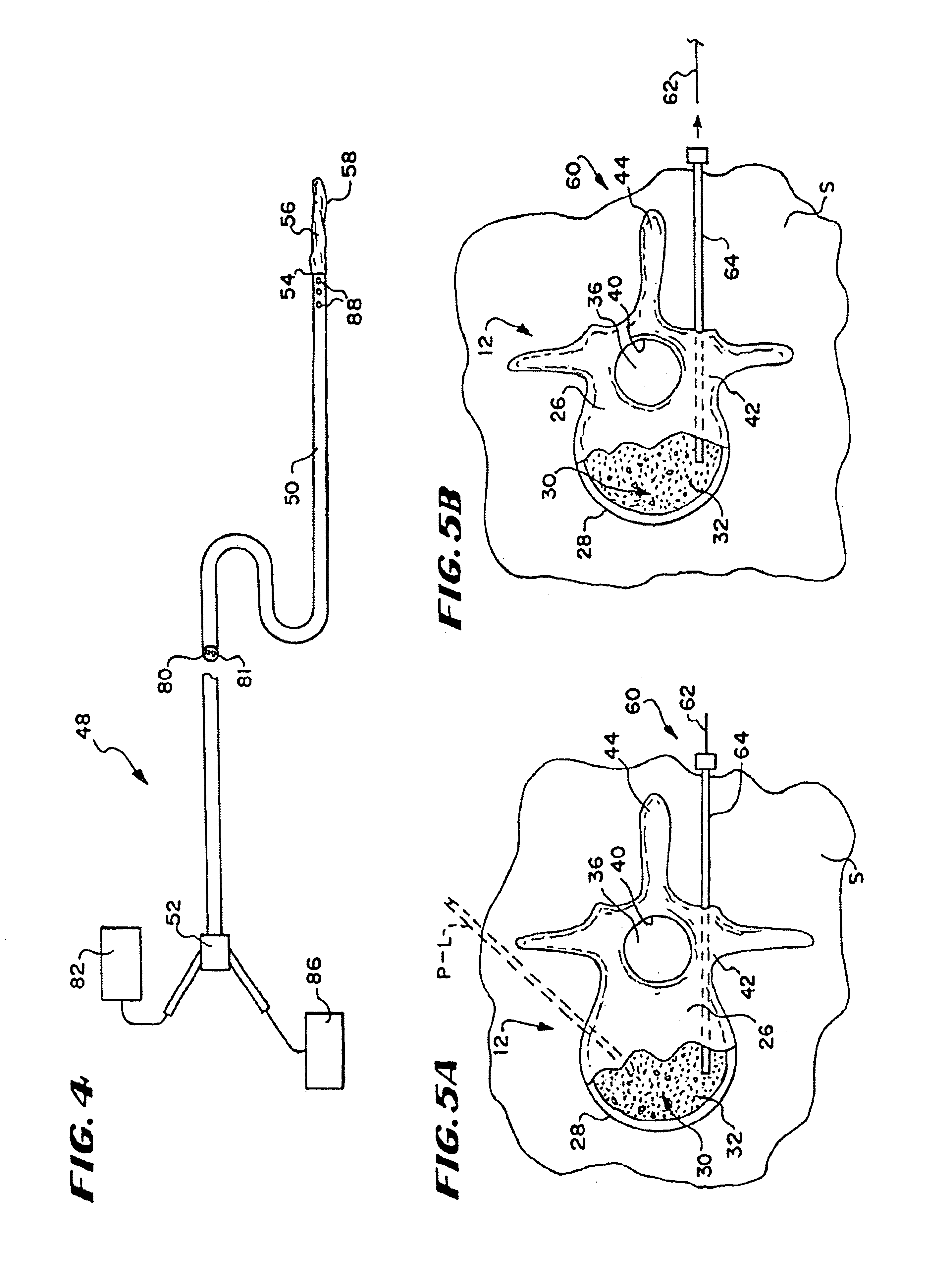
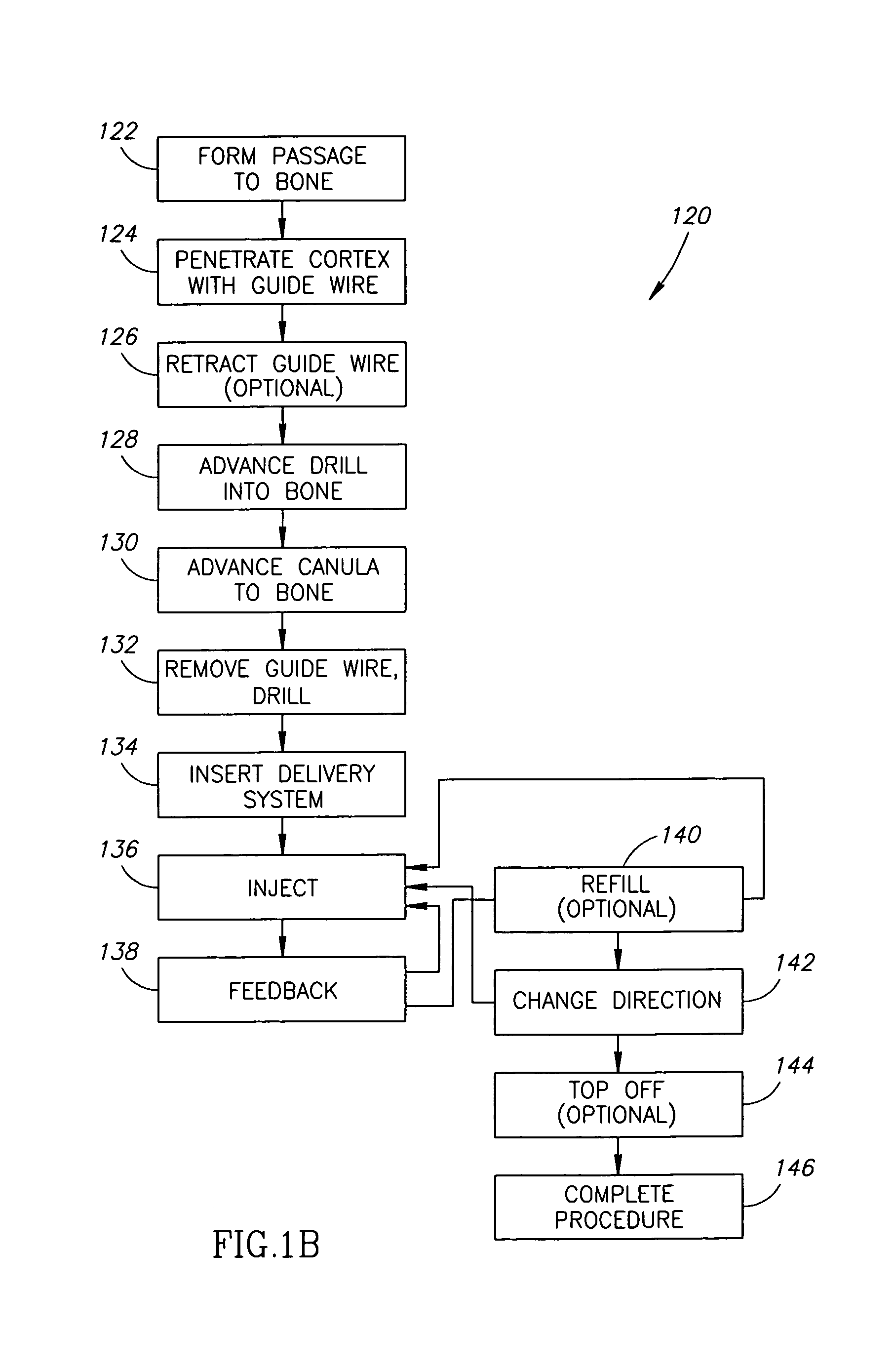
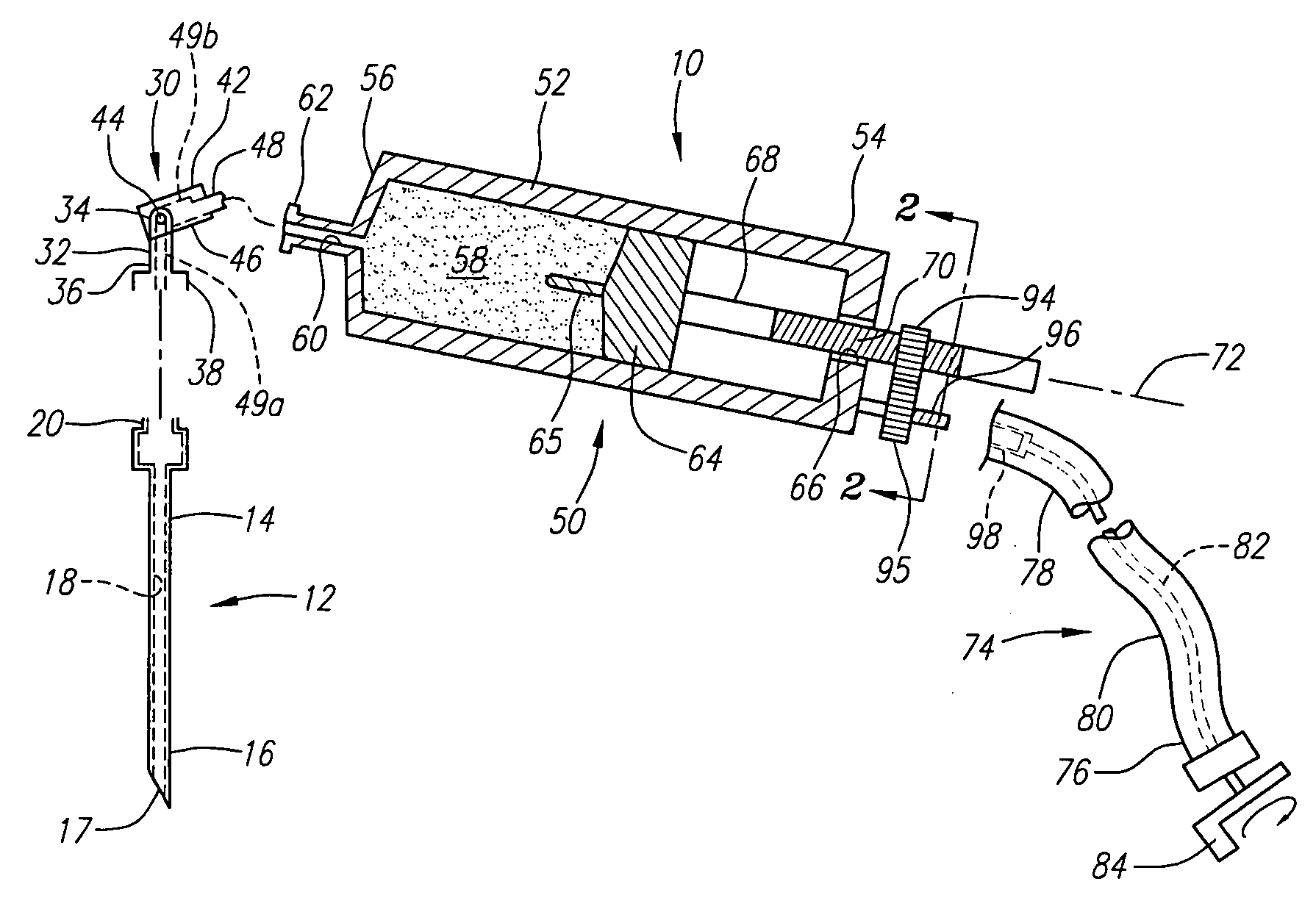
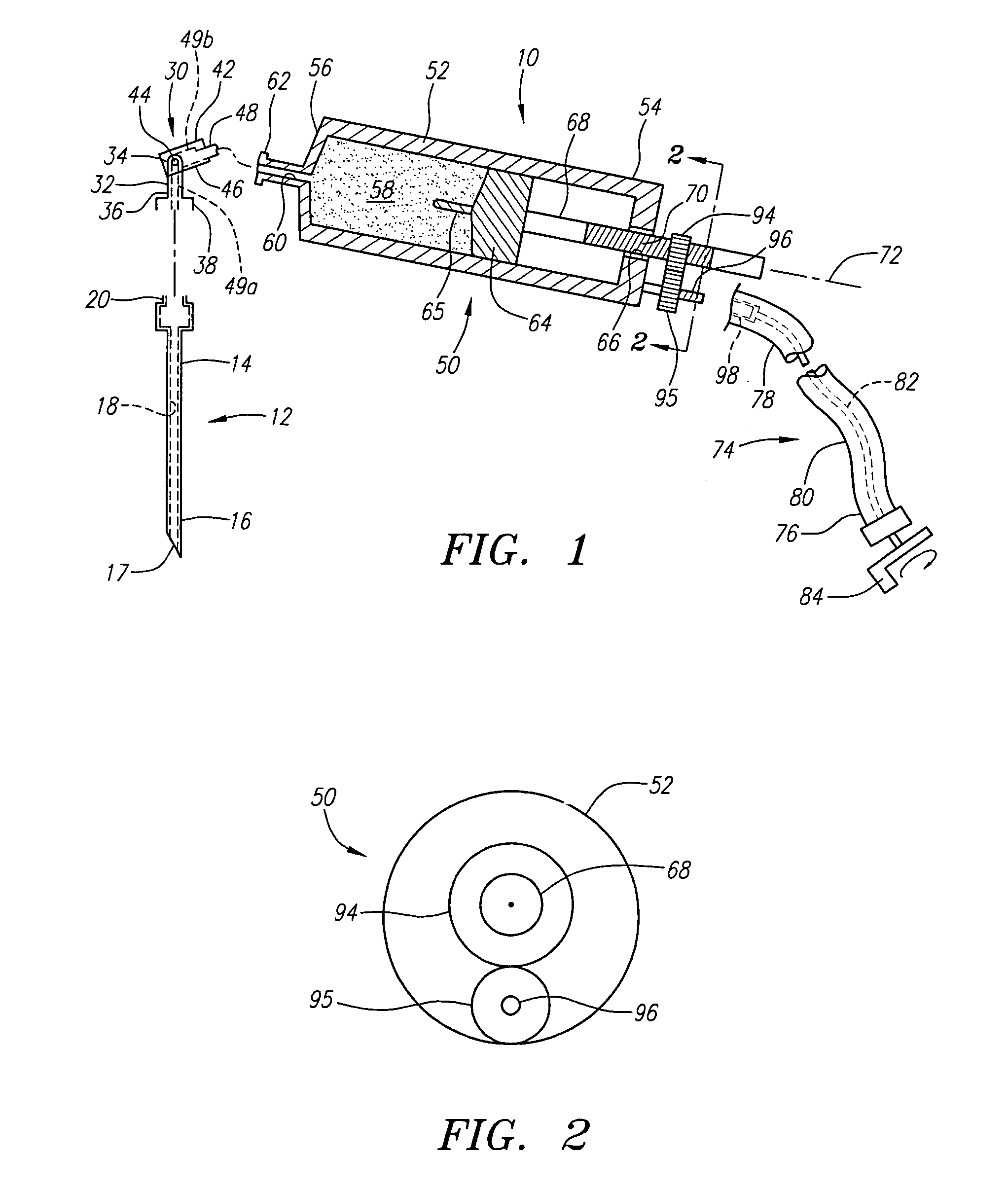
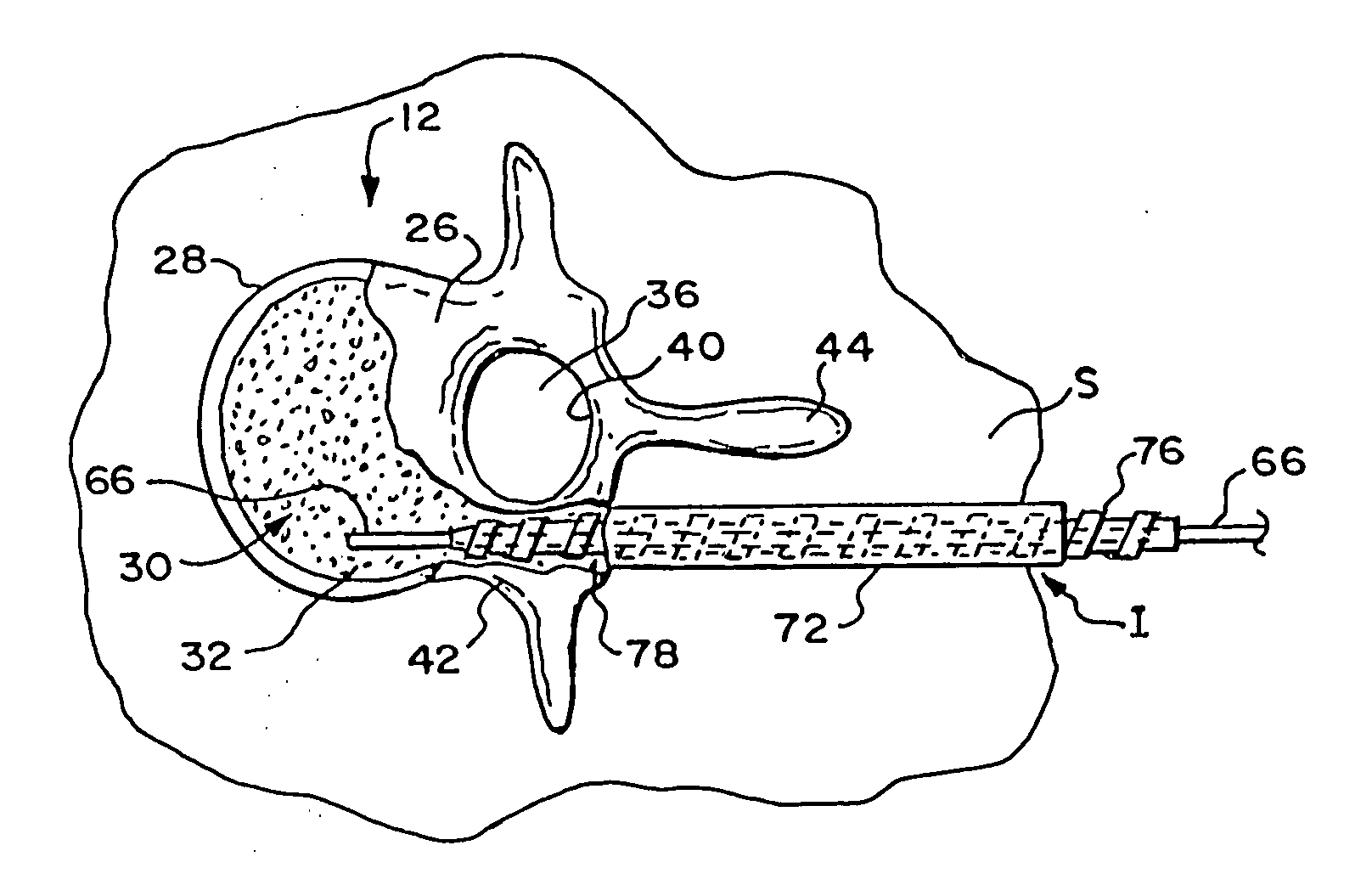
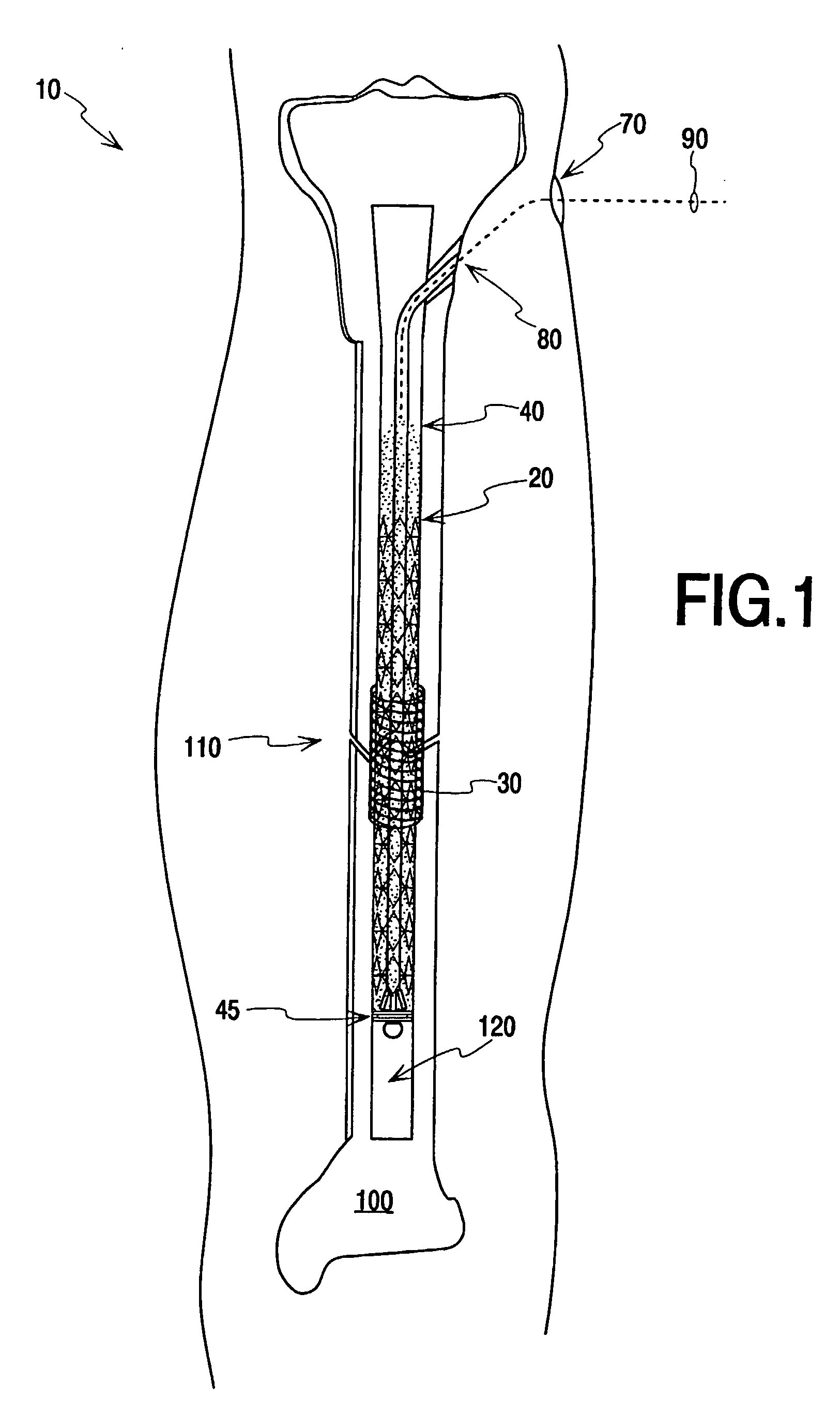
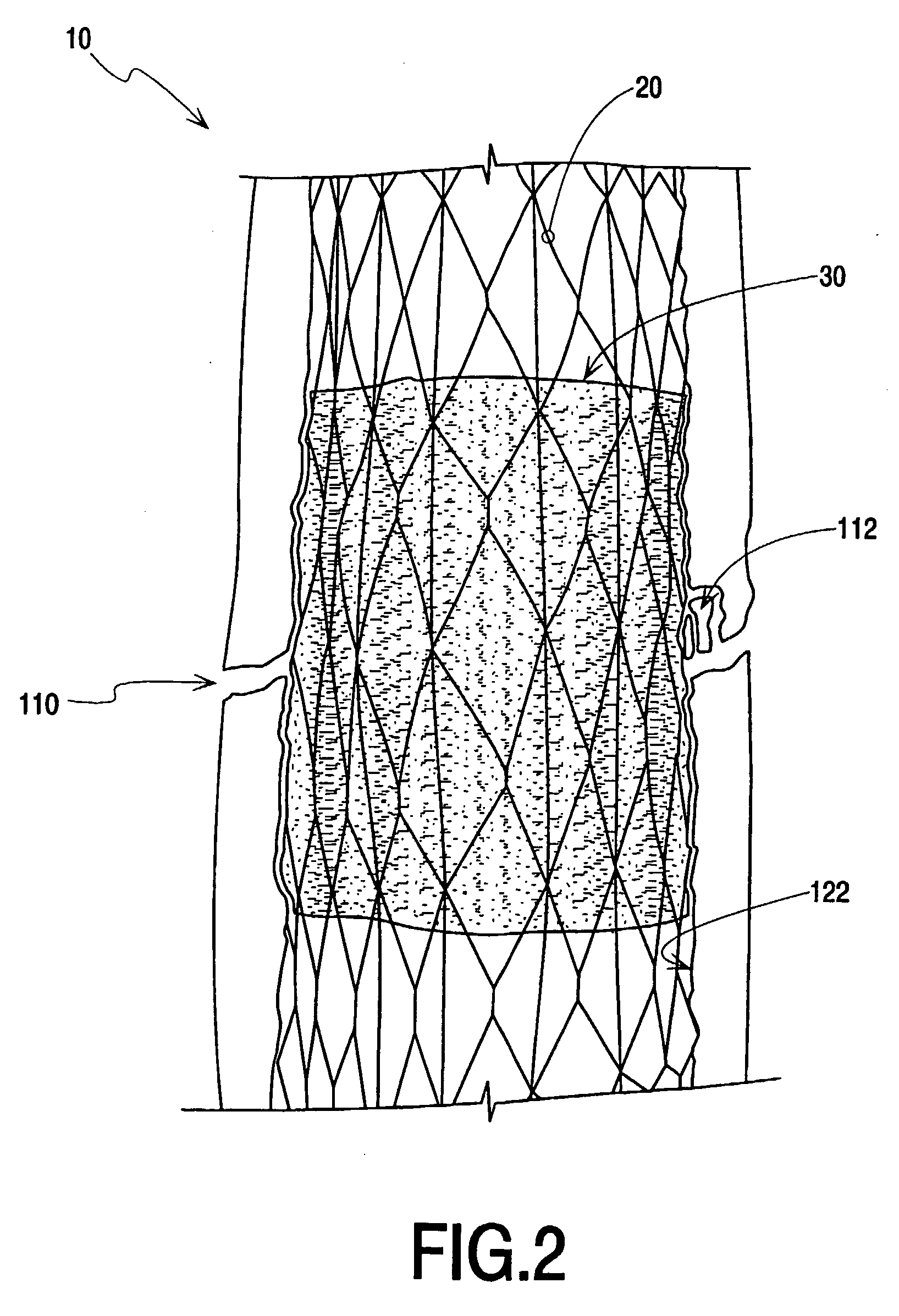
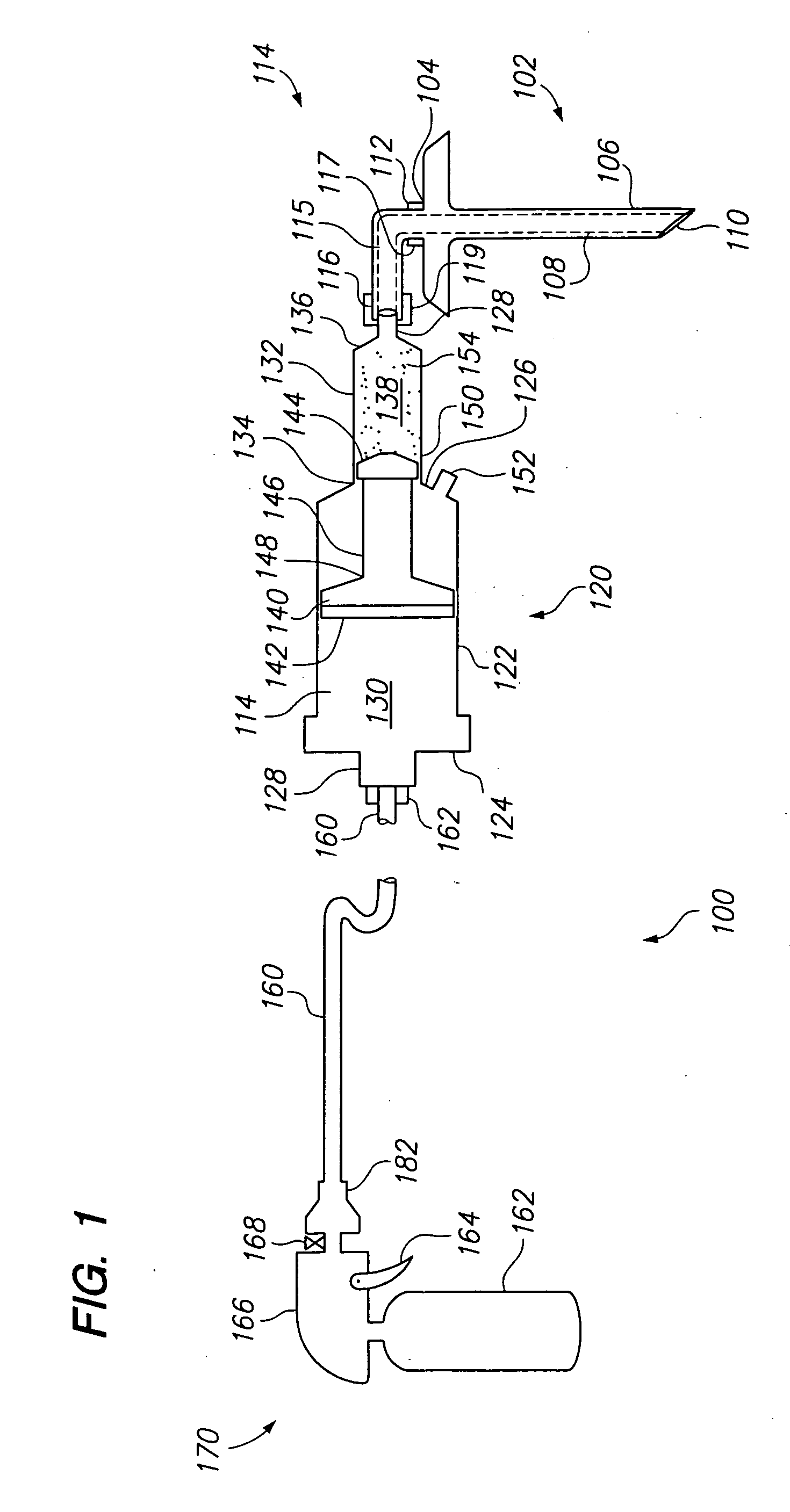
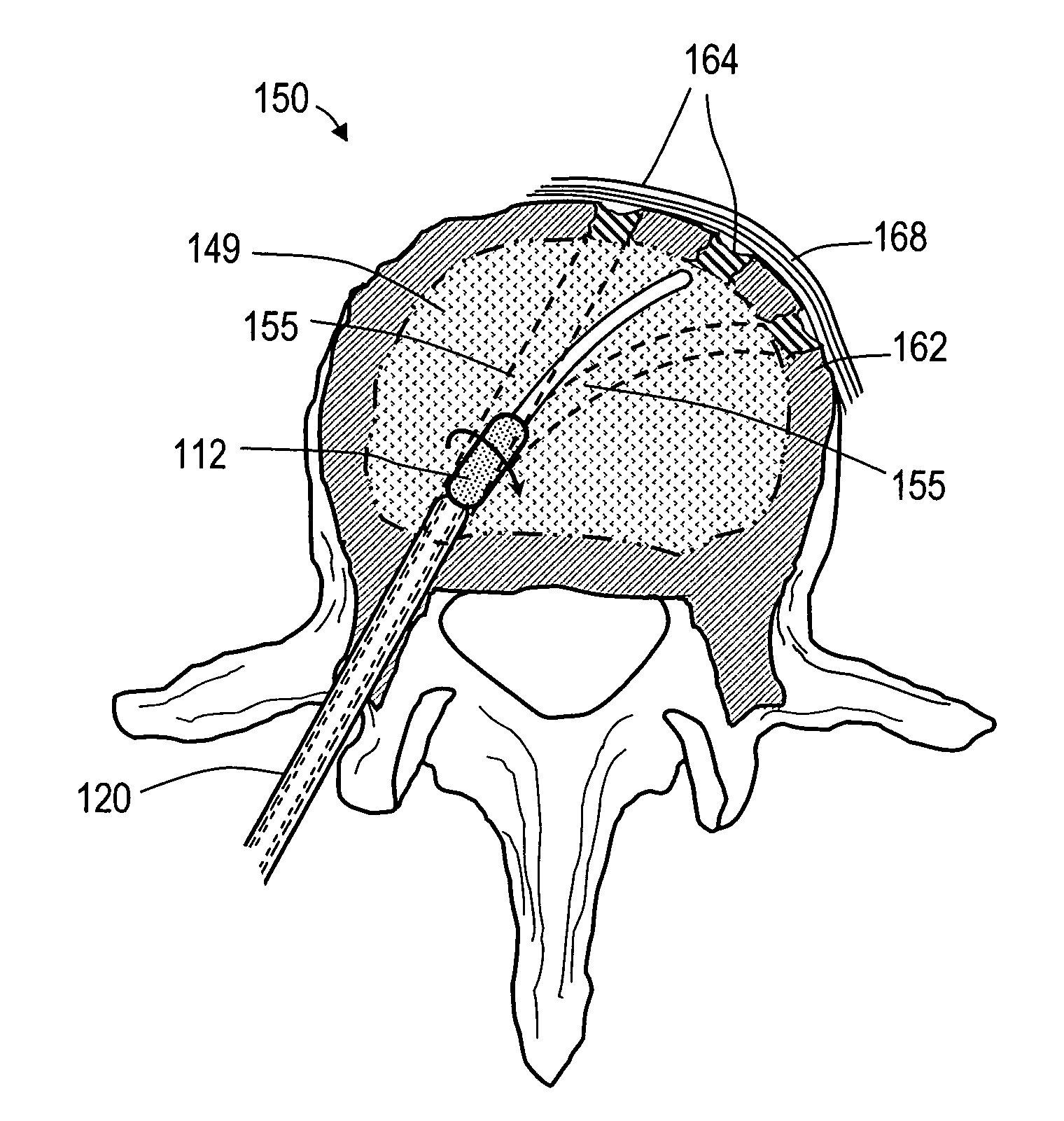
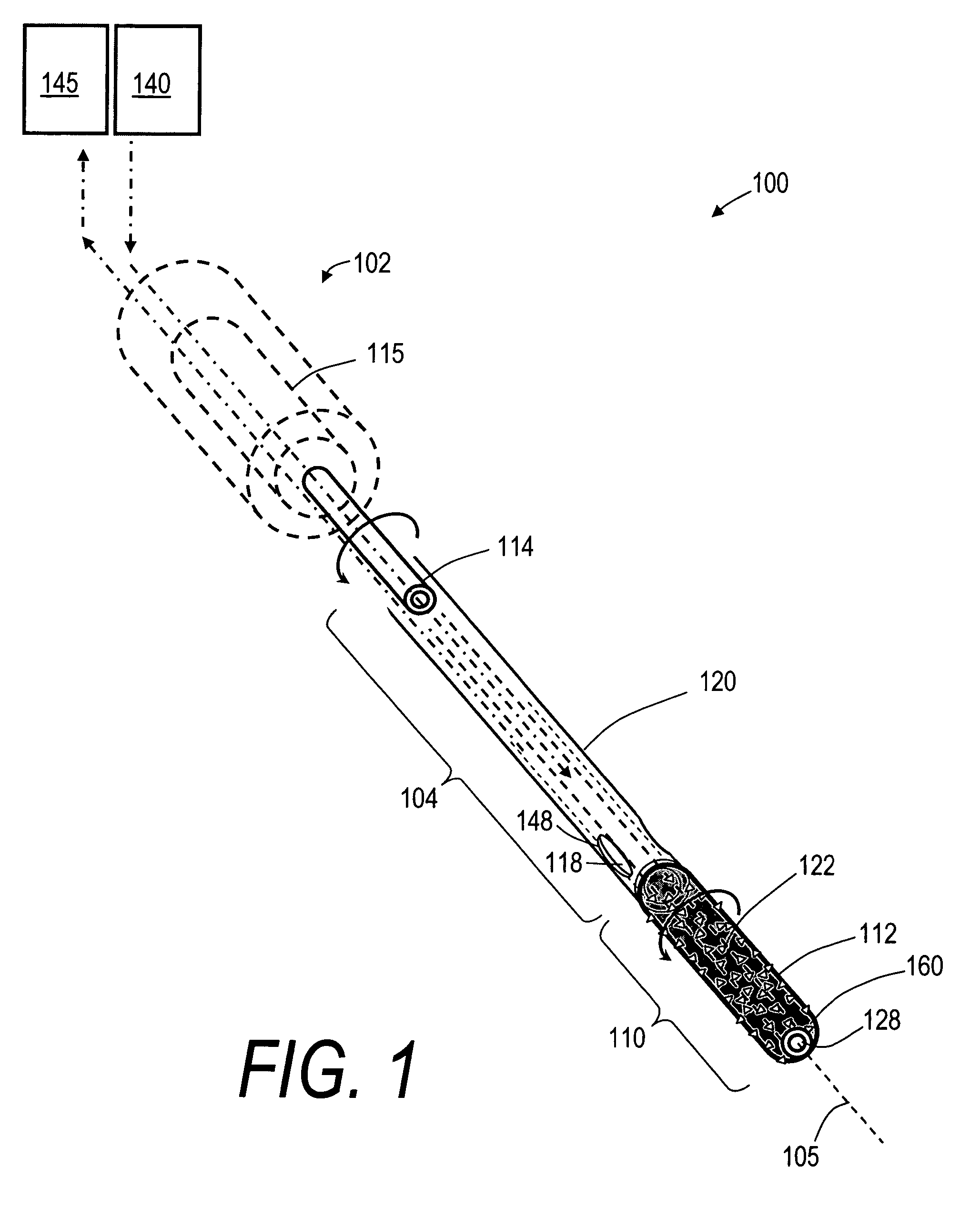
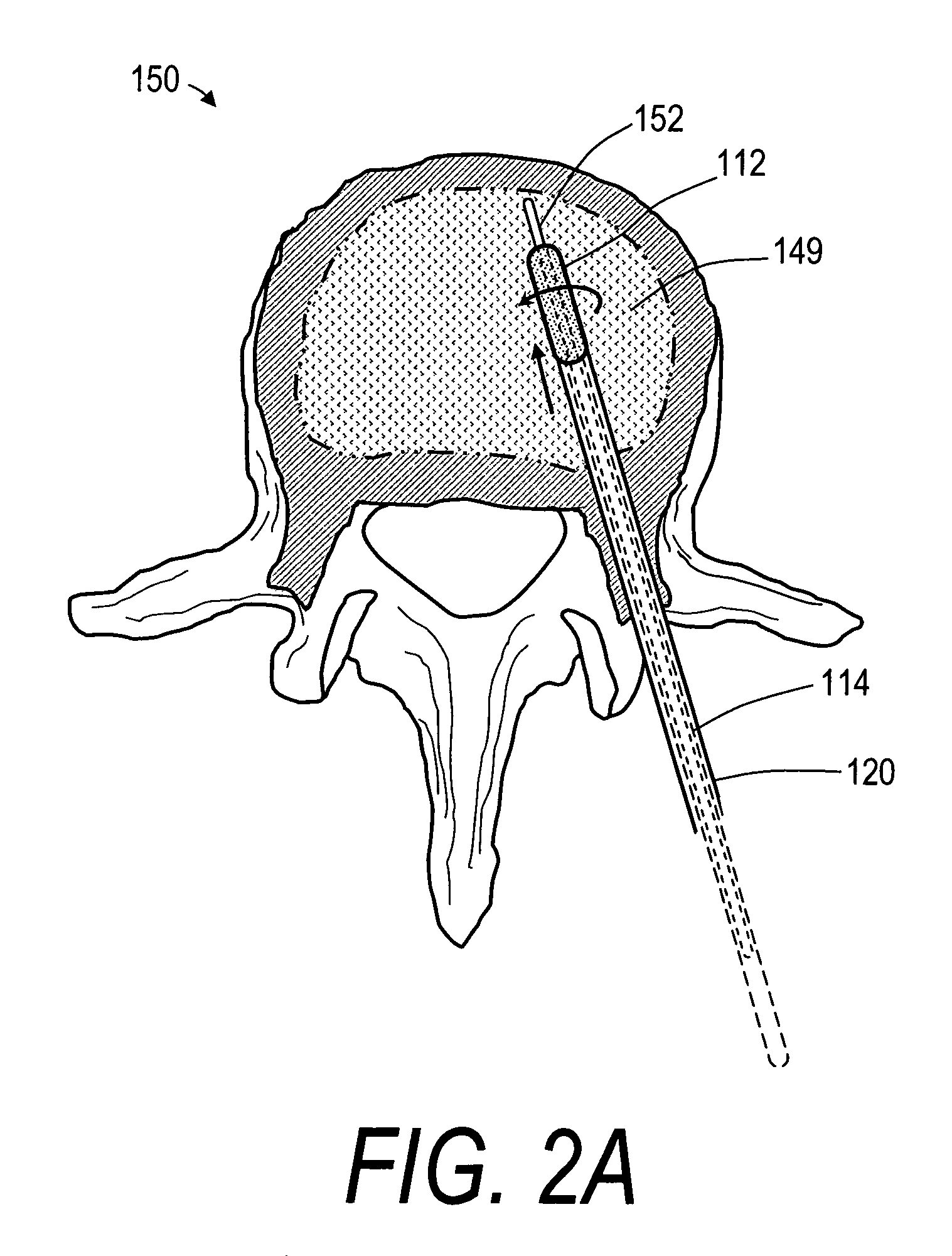
Vertebroplasty injection device
InactiveUS7008433B2Small sizeIncrease pressurePowder deliveryShaking/oscillating/vibrating mixersInjectable biomaterialBone cement
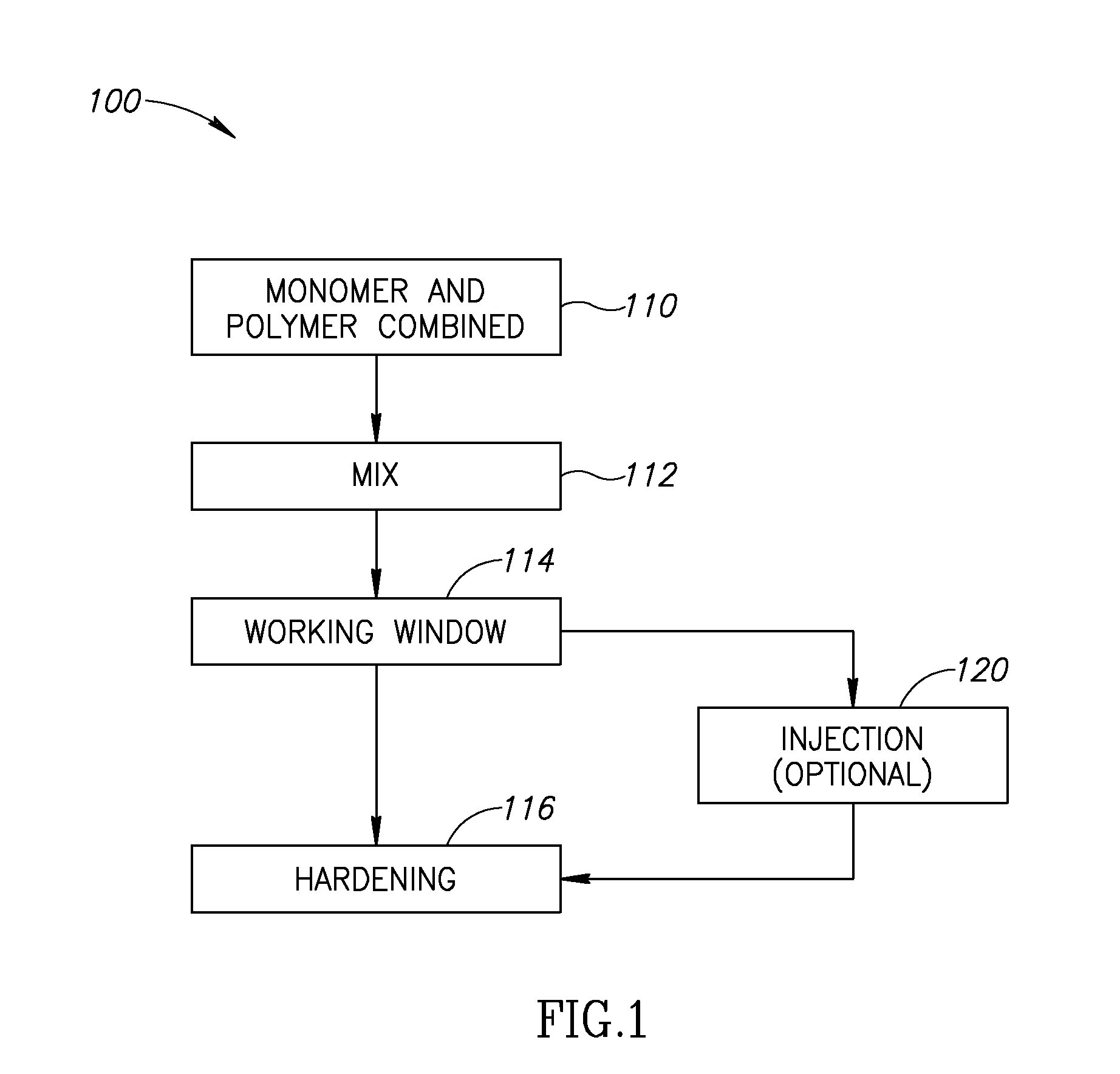
This invention relates to a mixing and delivery device suitable for delivering injectable biomaterials, and to preferred bone cement formulations.
Owner:DEPUY ACROMED INC
Integrated cement delivery system for bone augmentation procedures and methods
A cement delivery system for vertebroplasty including a rigid cannula having a tubular inner wall defining a central conduit to deliver bone cement and a tubular outer wall extending around the inner wall and spaced apart therefrom to define a peripheral conduit for aspirating bone fluids. Aspirating means communicate with a proximal outlet port of the peripheral conduit to create a pressure gradient between the central conduit and the peripheral conduit to provide a hydraulic force guiding the displacement of bone fluid and the flow of cement. Also, a bone cement delivery system including a rigid cannula having a tubular inner wall defining a central conduit, a tubular outer wall extending around the inner wall and spaced apart therefrom to define a peripheral conduit, and a tubular middle wall extending between the inner and peripheral walls and spaced apart therefrom to define a middle conduit around the central conduit.
Owner:BAROUD GAMAL DR
Stent systems and methods for spine treatment
InactiveUS20060100706A1Prevent subsidenceRestore body heightInternal osteosythesisSpinal implantsSpinal columnCardiac allograft
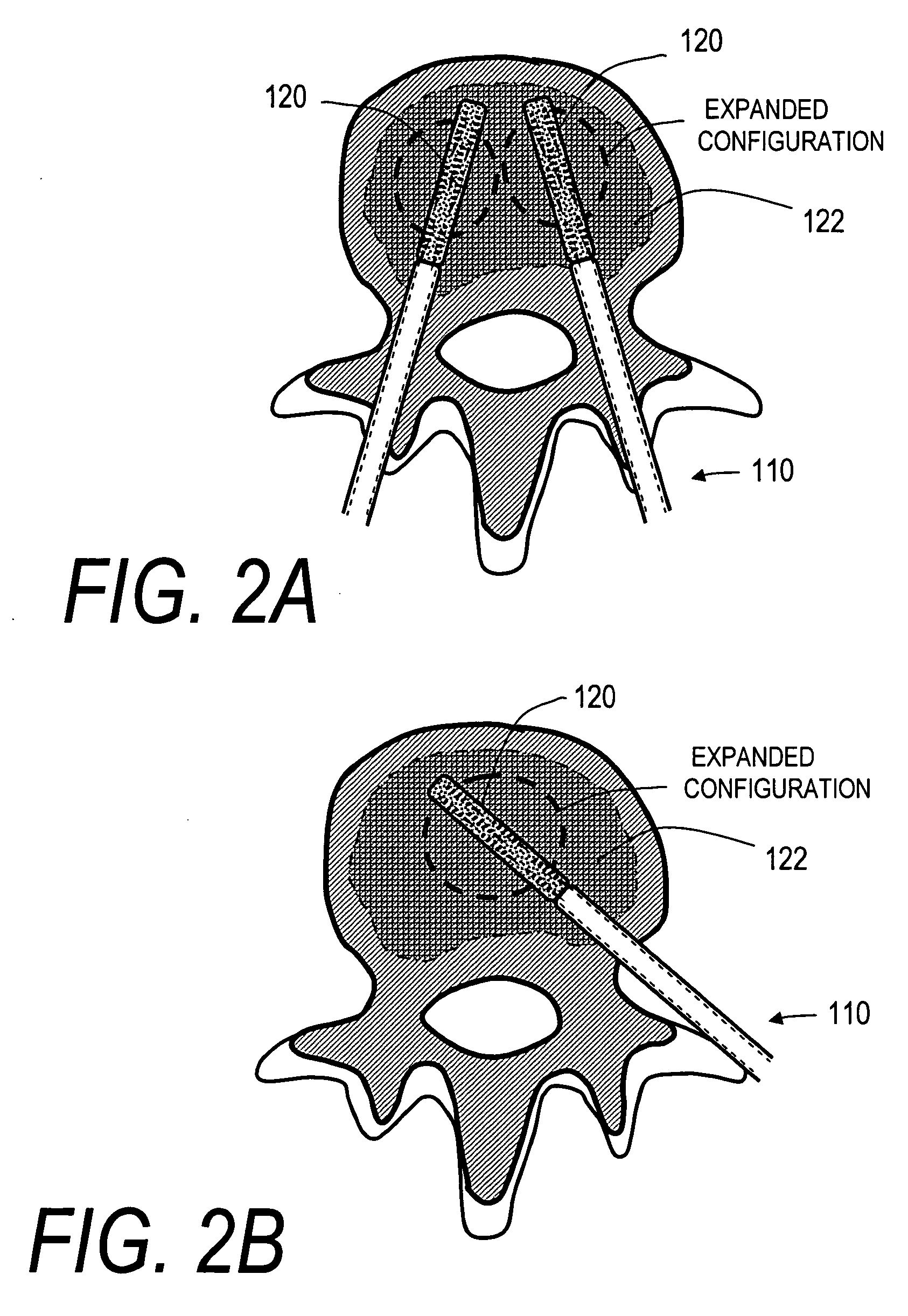
Stent systems and methods for expanding and deploying stents in hard tissue such as bone, more particularly within a vertebral body. One exemplary method includes using a stent body that is coupled to a high speed rotational motor with the stent expandable and detachable from an introducer working end. In one embodiment, the stent is a deformable metal body with zig-zag type struts in an expanded configuration that carries diamond cutting particles bonded to the strut surfaces. The “spin” stent is rotated at high rpm's to remove cancellous bone from the deployment site together with irrigation and aspiration at the end of the probe that carries the stent. The stent may be expanded asymmetrically, such as with first and second balloons or by using an interior restraint, to apply vertical distraction forces to move apart the cortical endplates and support the vertebra in the distracted condition. The cancellous bone about the expanded stent as well as the interior of the stent can be filled with a bone cement, allograft or other bone graft material. In one method of use, the spin stent is designed and adapted for (i) treating a vertebral compression fracture (VCF) or for (ii) reinforcing an osteoporotic vertebral body.
Owner:DFINE INC
Systems and methods for treating fractured or diseased bone using expandable bodies
Systems and methods treat fractured or diseased bone by deploying more than a single therapeutic tool into the bone. In one arrangement, the systems and methods deploy an expandable body in association with a bone cement nozzle into the bone, such that both occupy the bone interior at the same time. In another arrangement, the systems and methods deploy multiple expandable bodies, which occupy the bone interior volume simultaneously. Expansion of the bodies form cavity or cavities in cancellous bone in the interior bone volume.
Owner:ORTHOPHOENIX
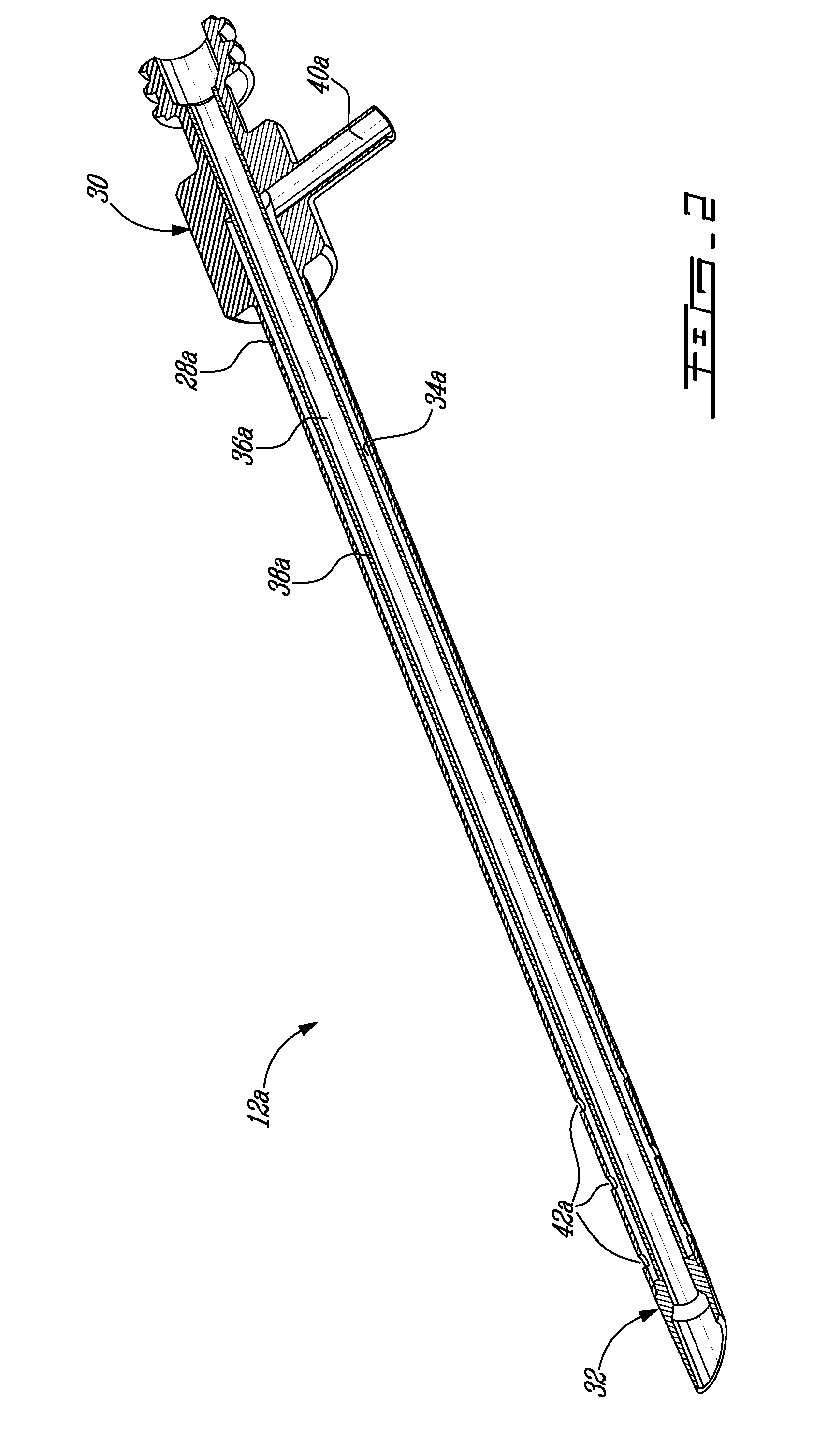
Biocompatible wires and methods of using same to fill bone void
InactiveUS20050015148A1Reduce compression fractureInternal osteosythesisSpinal implantsWire rodBone structure
Devices, kits, and methods are provided for reducing a bone fracture, e.g., a vertebral compression fracture, is provided. The device comprises a plurality of resilient wires composed of a biocompatible material, such as a biocompatible polymer (e.g., polymethylmethacrylate (PMMA)). The wires can be introduced into the cavity of the bone structure to form a web-like arrangement therein. The web-like arrangement can be stabilized by applying uncured bone cement onto the arrangement to connect the wires at their contacts point. The bone cavity can then be filled with a bone growth enhancing medium.
Owner:BOSTON SCI SCIMED INC
Apparatus and methods for treating bone
InactiveUS20070093899A1Minimally augmentationRecovery heightInternal osteosythesisCannulasInsertion stentBone implant
Implants and methods for minimally invasive augmentation and repositioning of vertebrae may comprise one or more expandable members, e.g., stents, implants, surrounding a balloon-tipped catheter or other expansion device, inserted into a vertebral body or other bone. Expansion of the expandable member within the vertebral body or other bone may reposition the fractured bone to a desired height and augment the bone to maintain the desired height. A bone cement or other filler can be added to further augment and stabilize the vertebral body or other bone.
Owner:SYNTHES USA
Methods, materials, and apparatus for treating bone and other tissue
ActiveUS20070027230A1Avoid less flexibilityEasy to useCosmetic preparationsImpression capsMedicineIn vivo
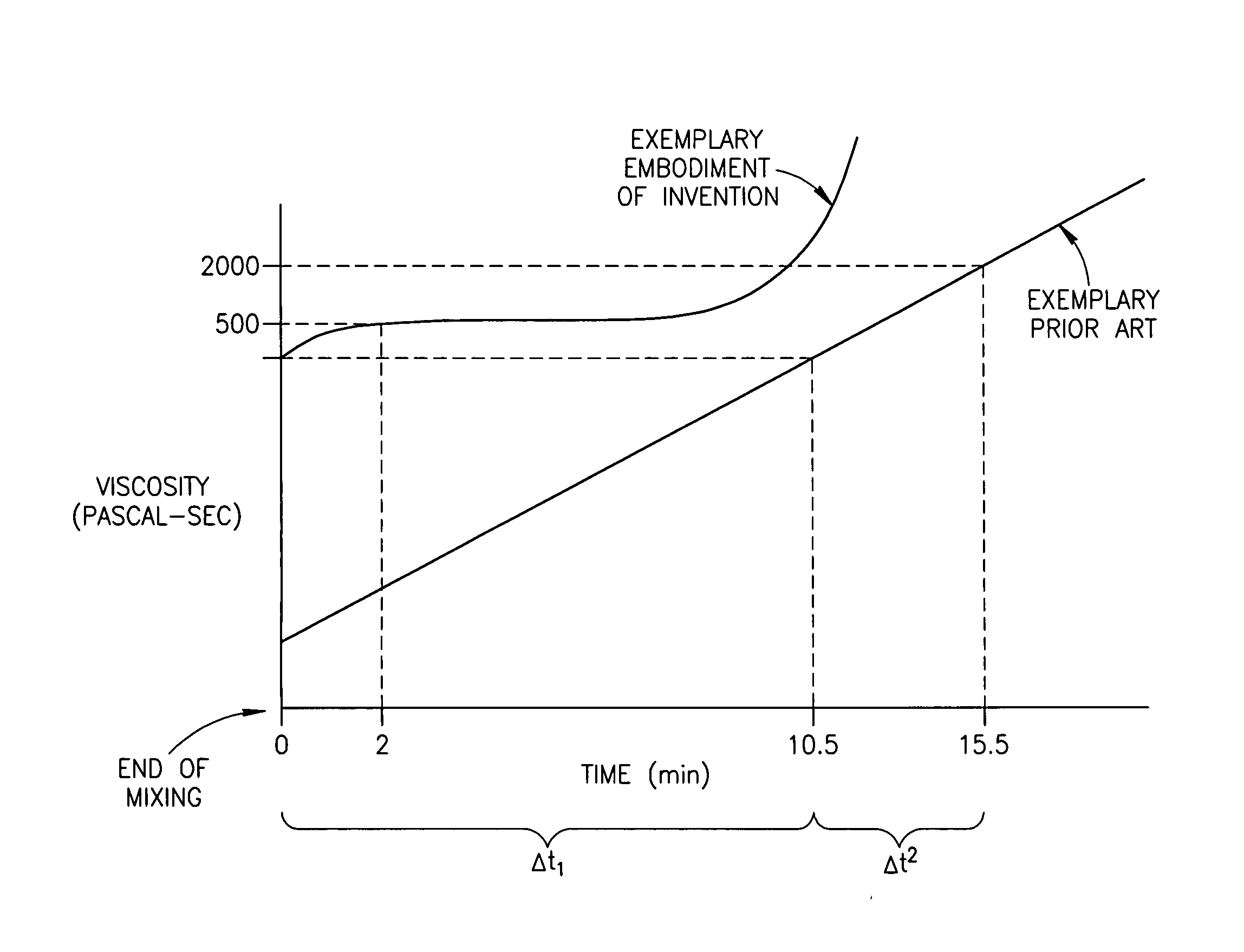
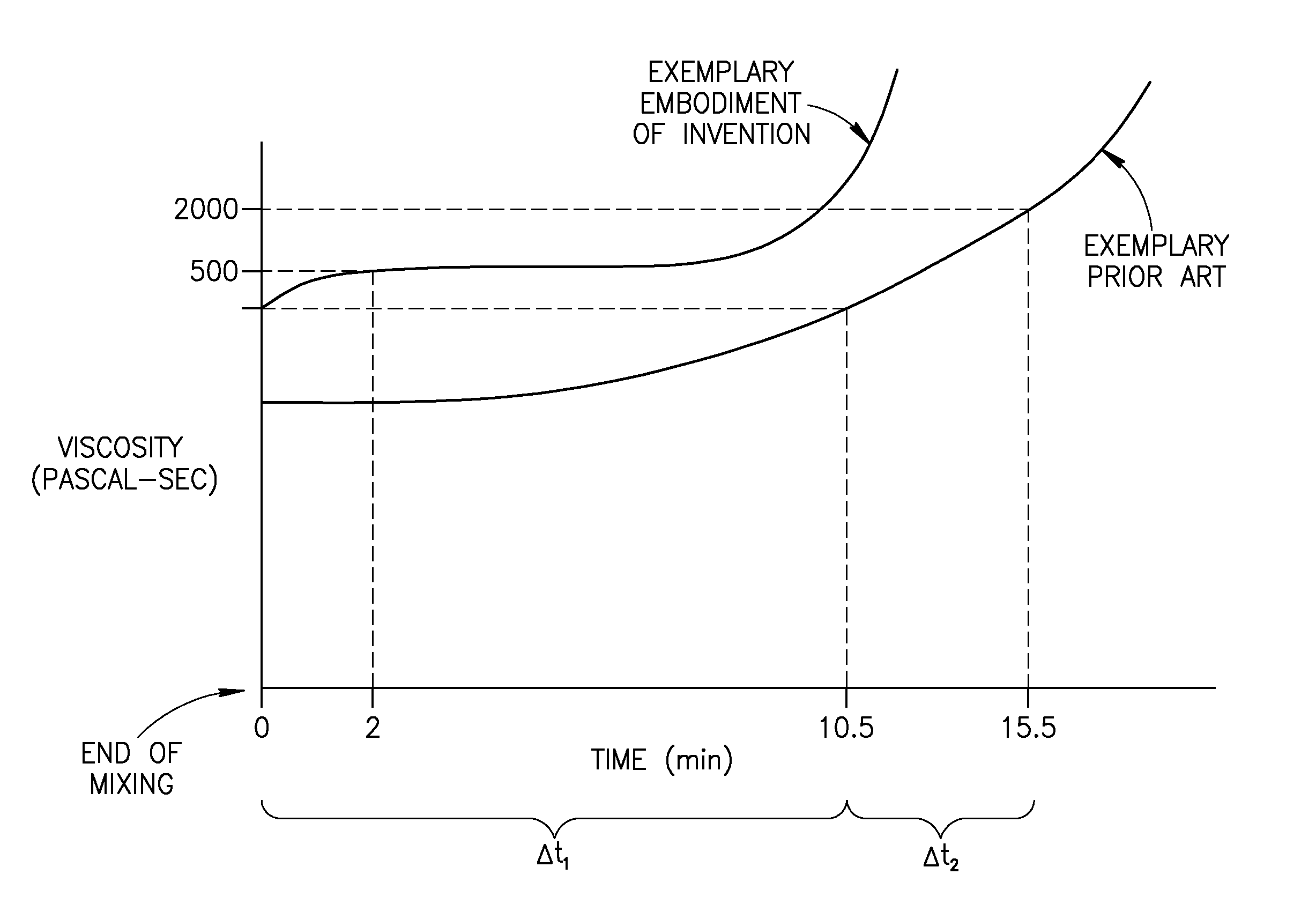
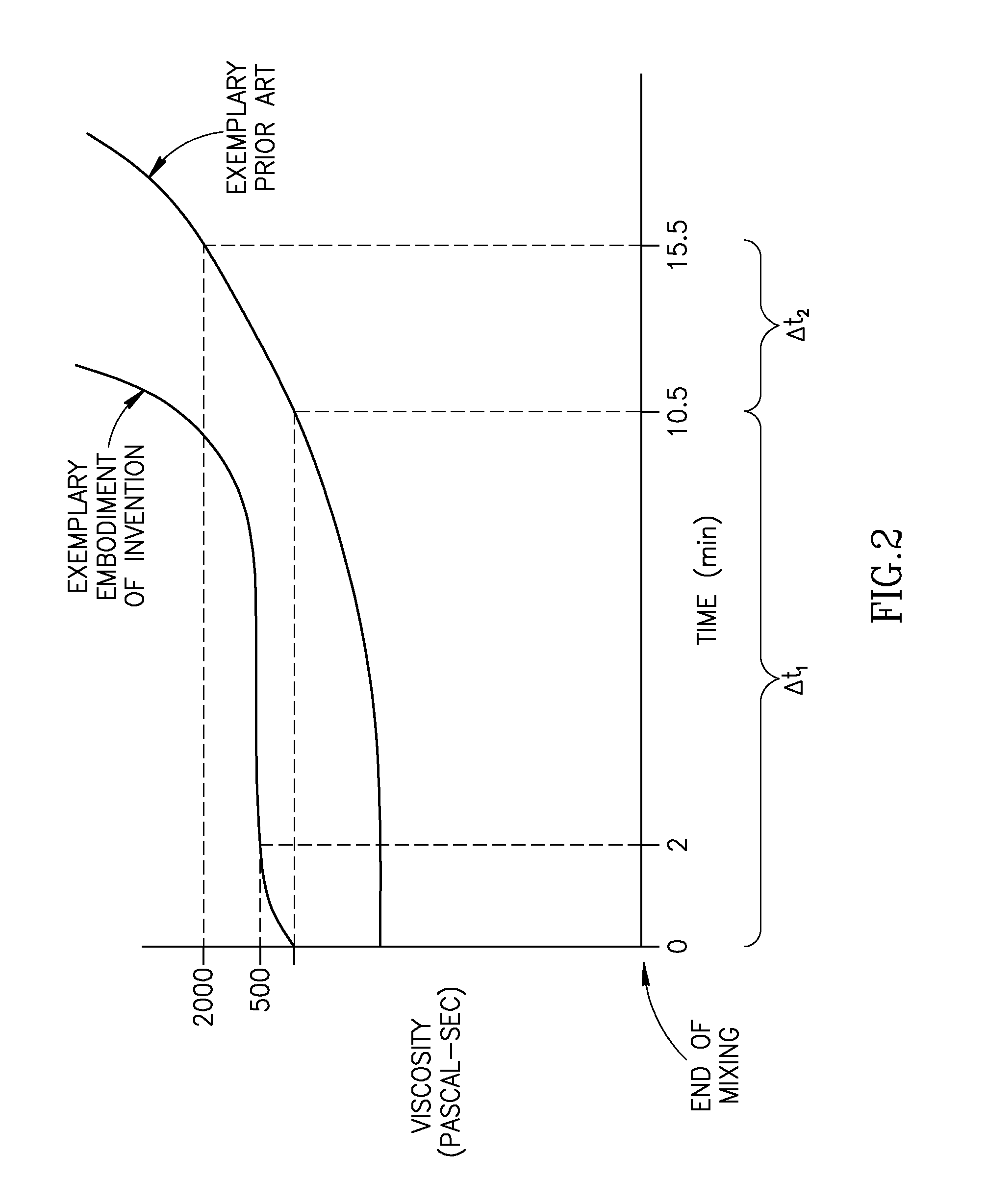
A bone cement comprising a first component and a second component, wherein contacting the first component and the second component produces a mixture which attains a high viscosity an initial period and the viscosity of the mixture remains relatively stable for a working time of at least 5 minutes after the initial setting period, and the mixture is suitable for in-vivo use.
Owner:DEPUY SYNTHES PROD INC
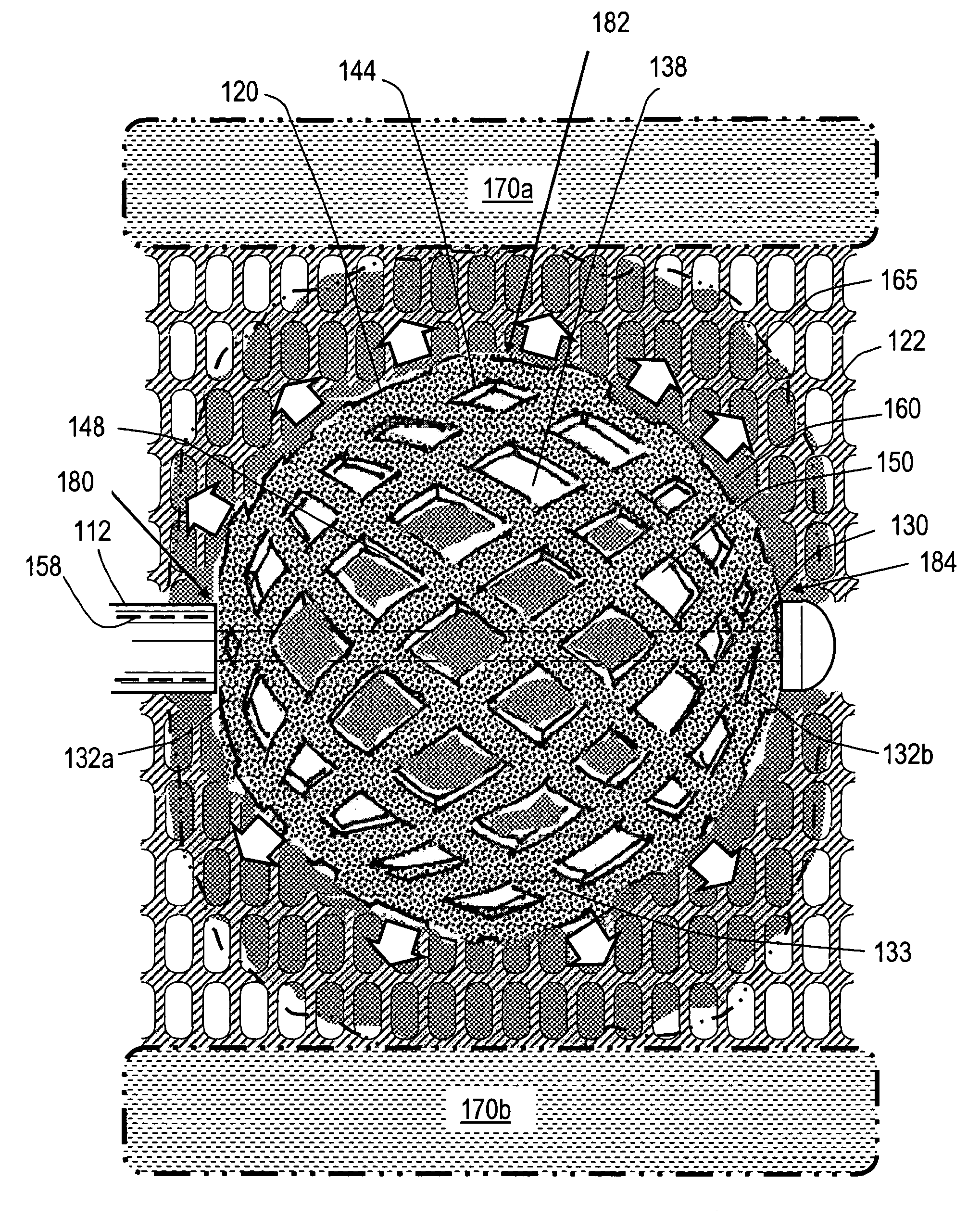
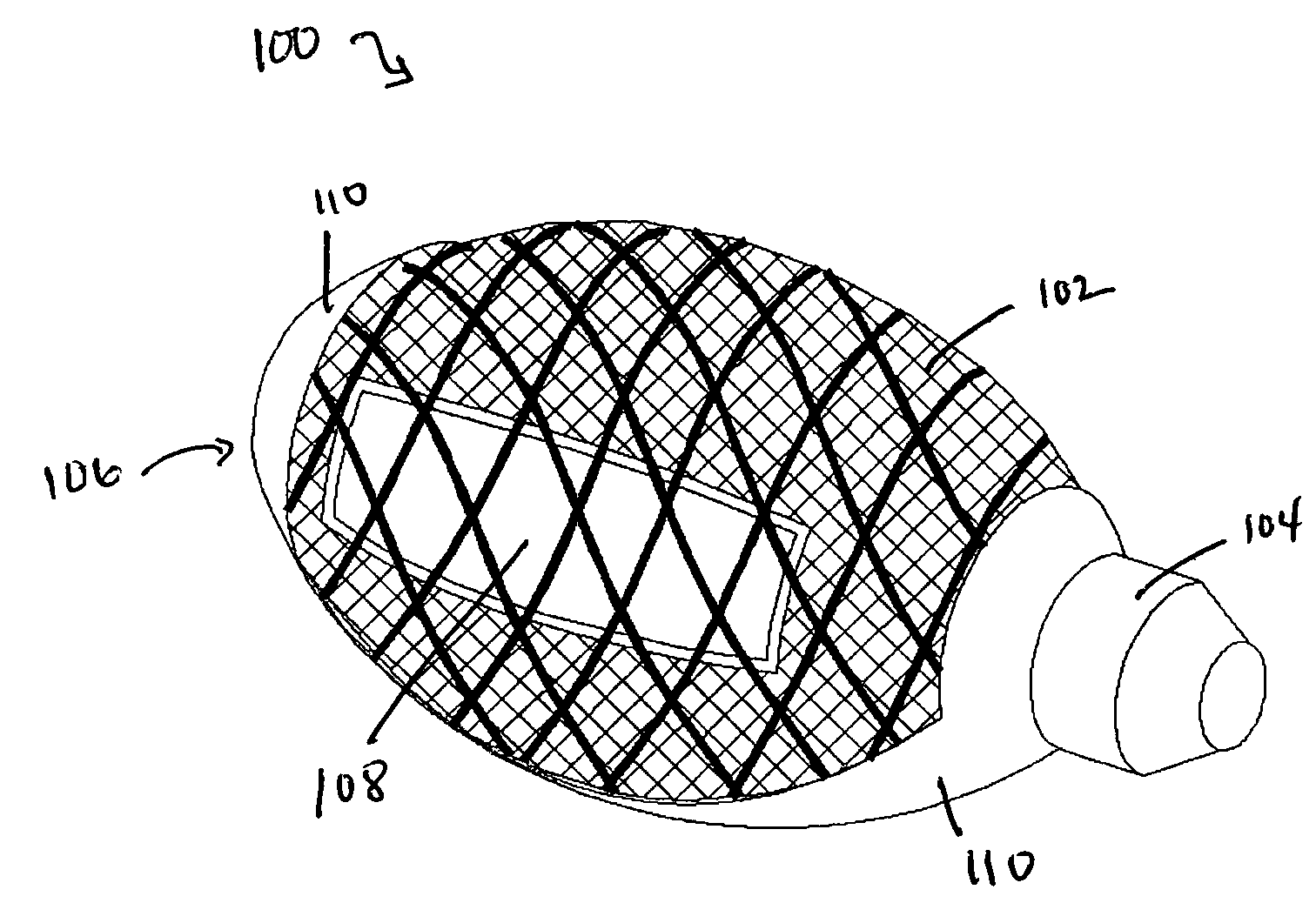
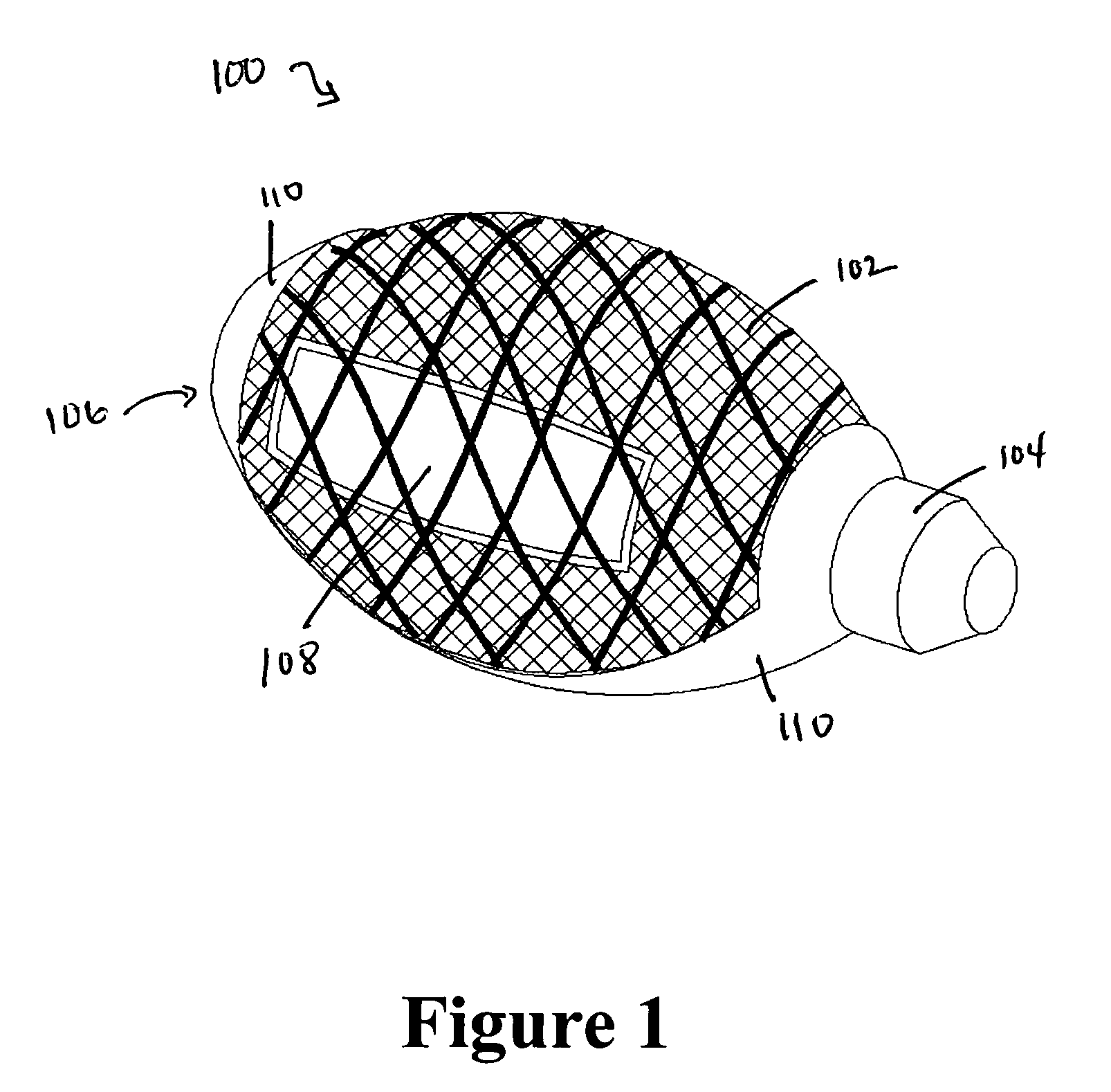
Cement-directing orthopedic implants
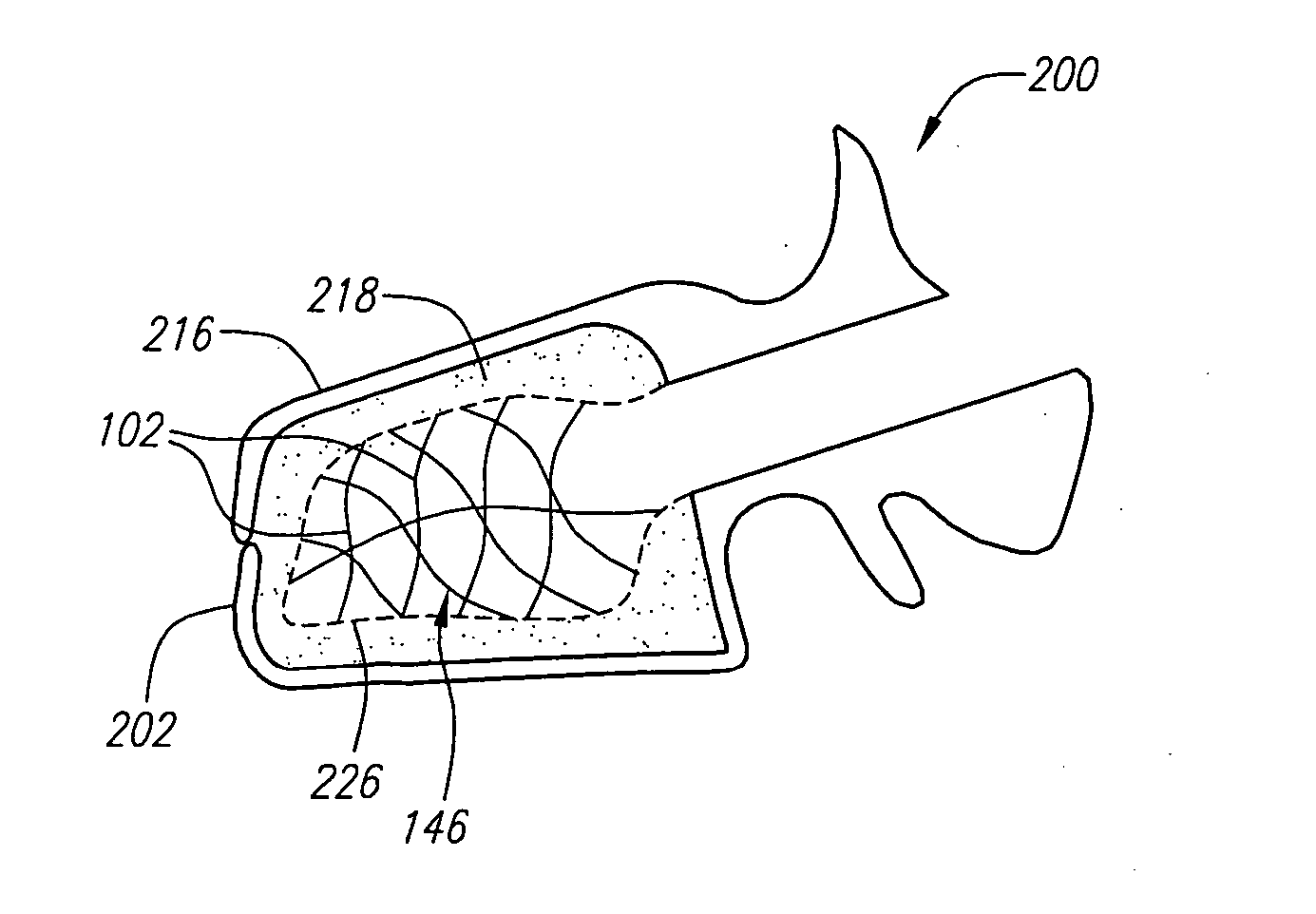
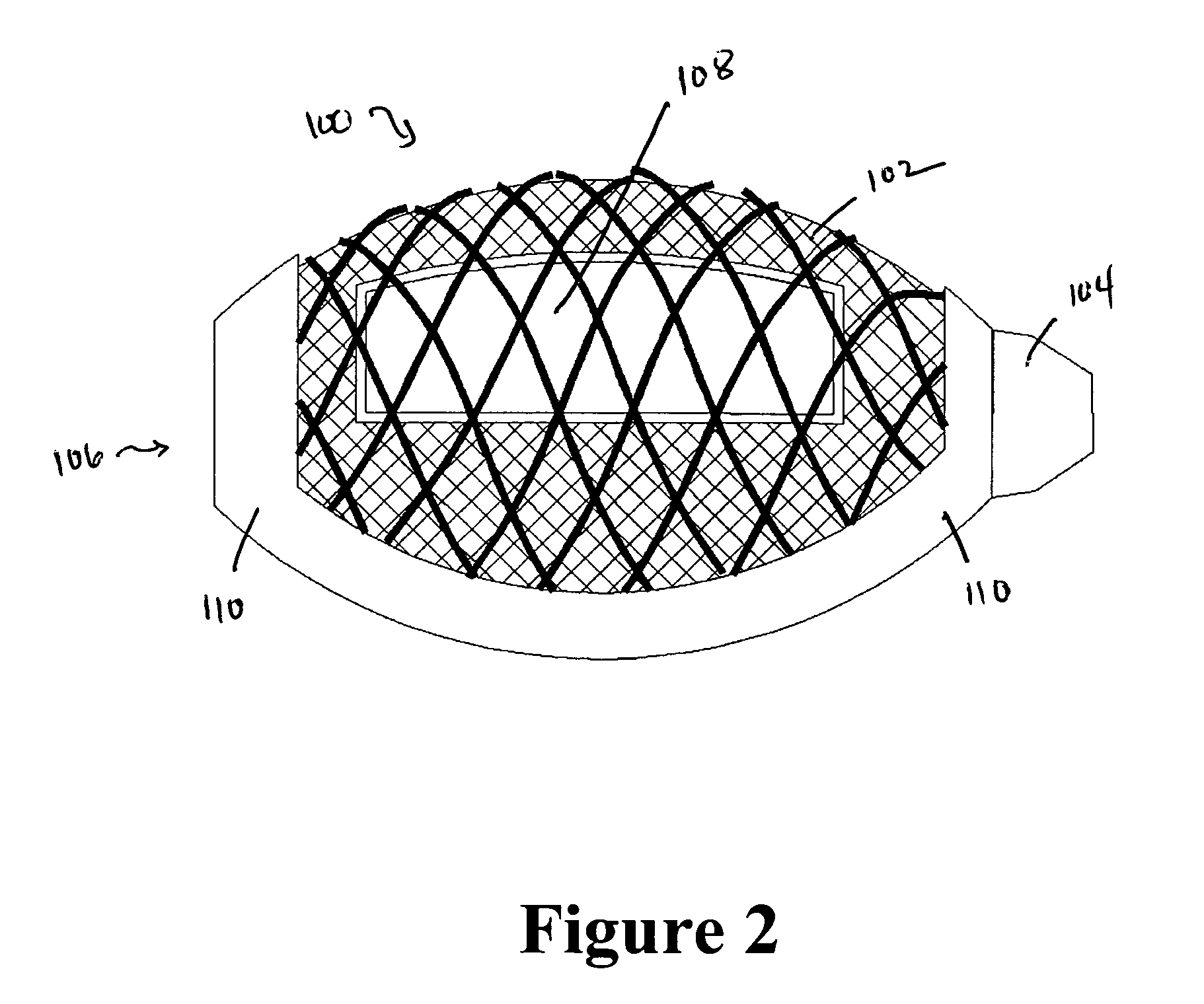
A cement-directing structure for use in cement-injection bone therapy includes a collapsible, self-restoring braided structure with regions of differential permeability to the bone cement. The regions of differential permeability may be provided by areas where the braided mesh density is greater or lesser than surrounding areas and / or by means of a baffle. After the structure is placed in a void within a bony structure, cement is injected into the interior of the structure then oozes out in preferred directions according to the locations of the regions of differential permeability.
Owner:GLOBUS MEDICAL INC
Bone treatment systems and methods
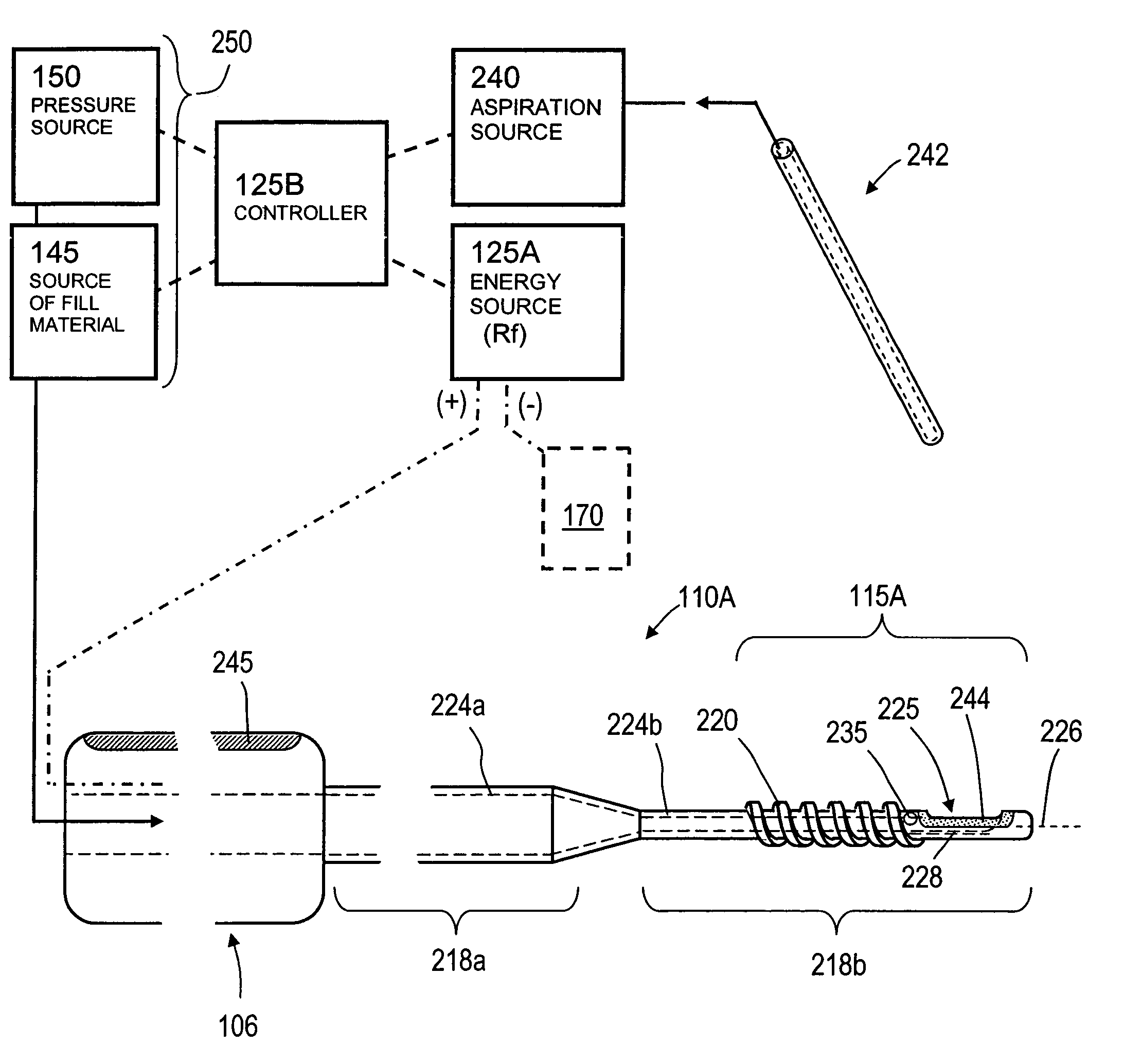
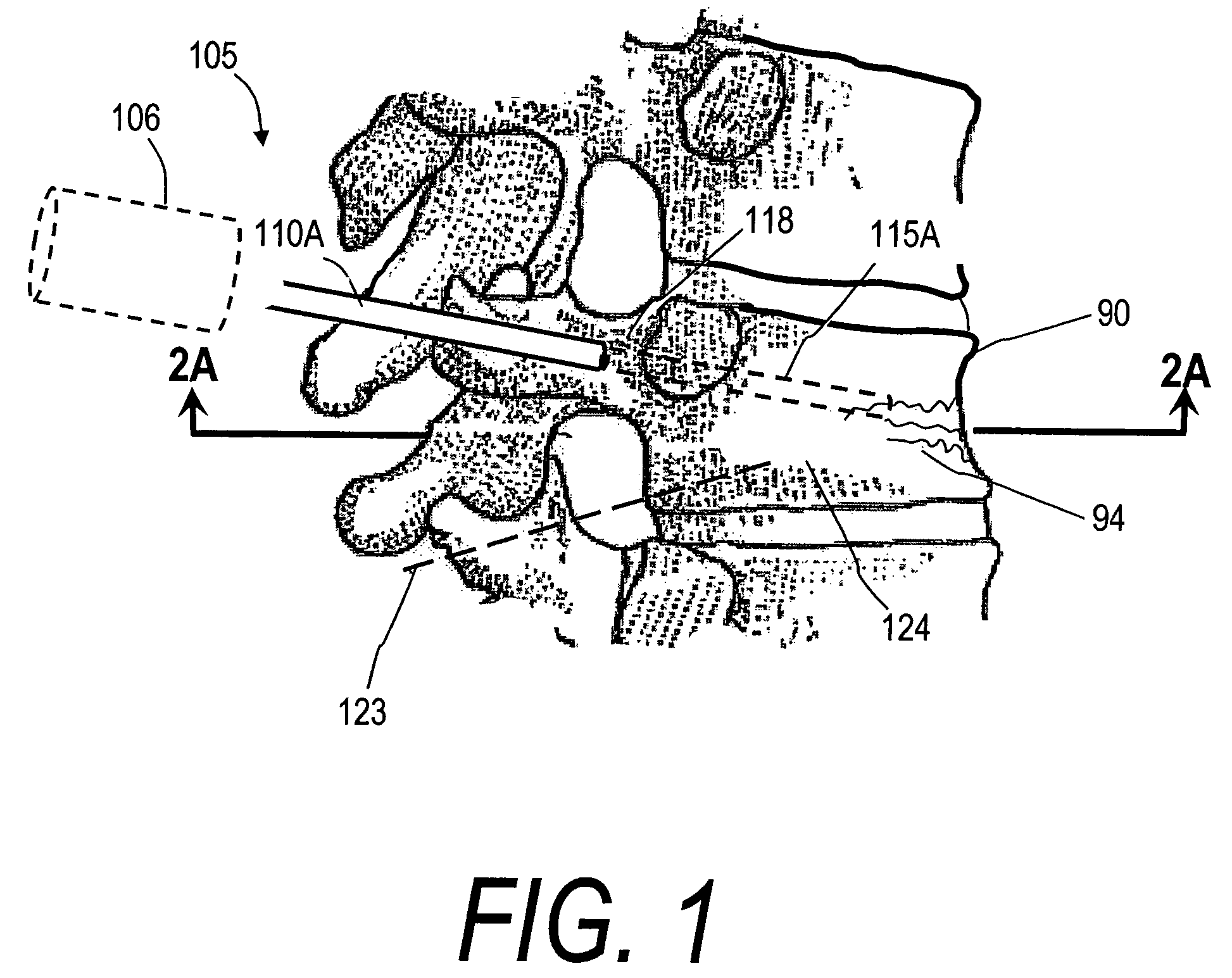
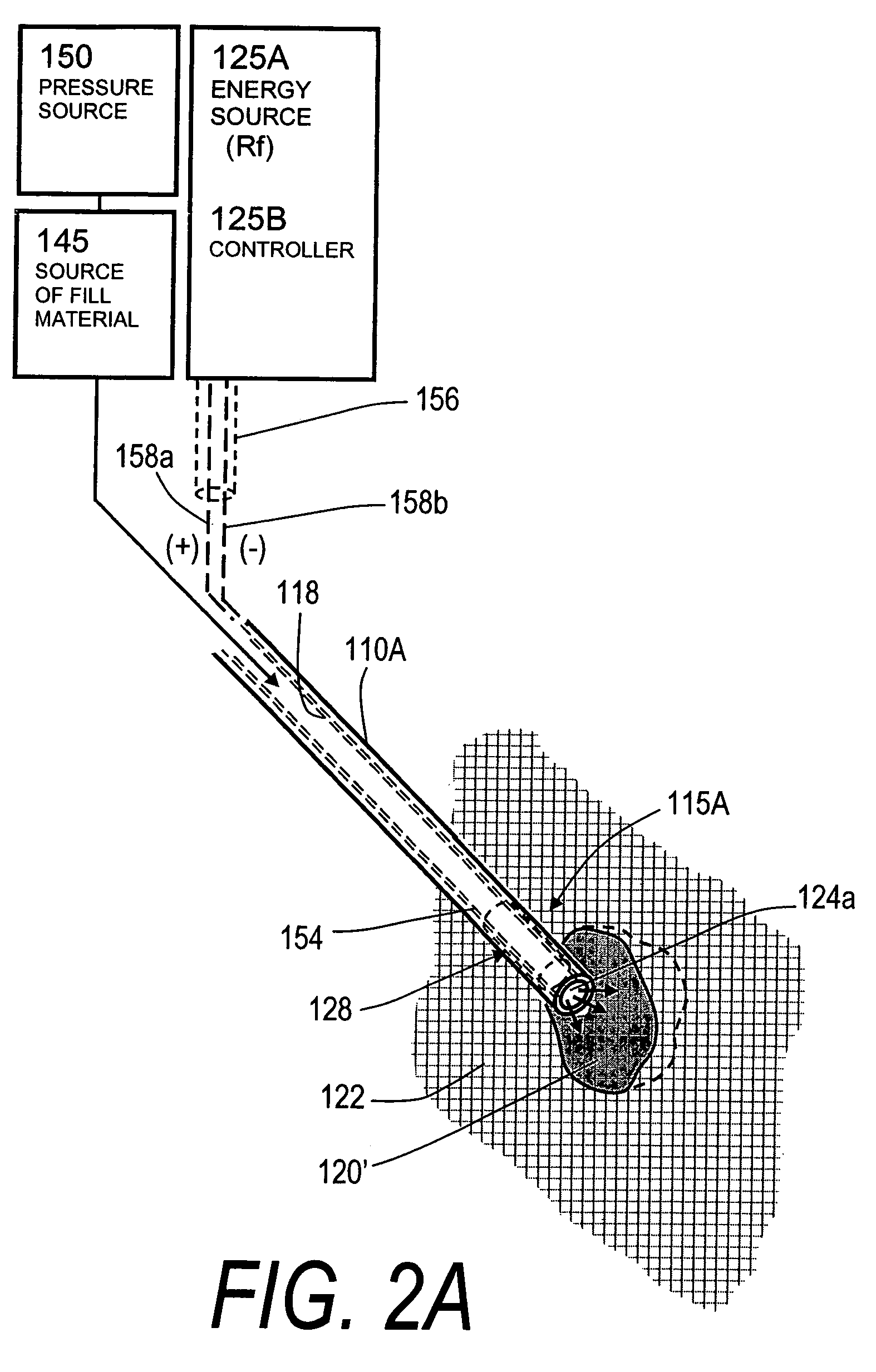
InactiveUS20060106459A1Reduces and eliminates possibilityAvoid thermal damageInternal osteosythesisSpinal implantsFracture reductionHigh pressure
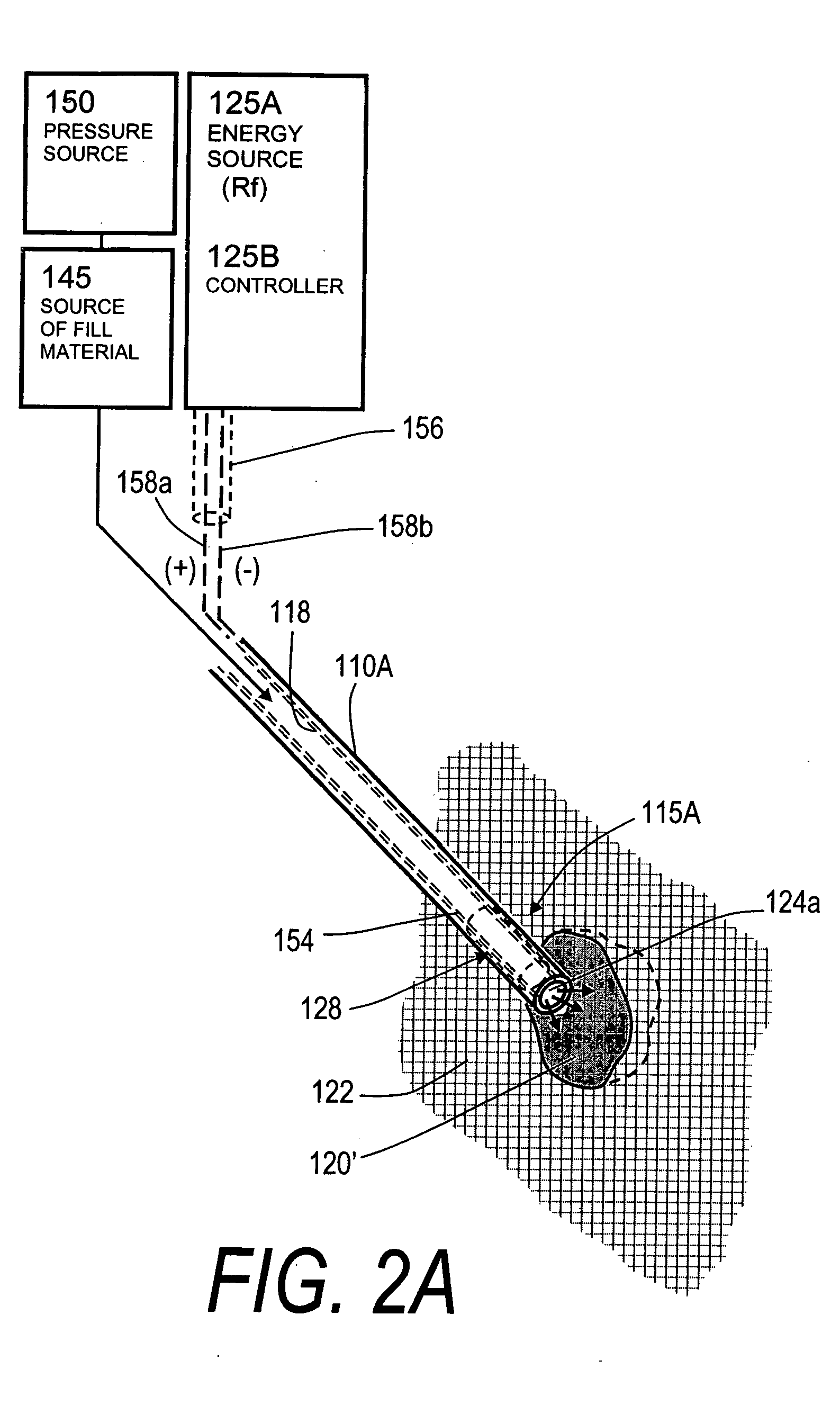
A system for treating an abnormal vertebral body such as a compression fracture. In an exemplary embodiment, the system includes a biocompatible flow-through implant structure configured with a three-dimensional interior web that defines flow openings therein for cooperating with a two-part hardenable bone cement. The flow-through structure is capable of compacted and extended shapes and in one embodiment provides a gradient in flow openings for controlling flow parameters of a bone cement injected under high pressure into the interior thereof. The flow-through implant structure is configured for transducing cement injection forces into a selected direction for moving apart cortical endplates of a vertebra to reduce a fracture. In one embodiment, the flow-through implant structure is coupled to an Rf source for applying Rf energy to a two-part bone cement to accelerate curing of the cement to thereby allow on-demand alterations of cement viscosity. The Rf system allows for control of bone cement polymerization globally or regionally to prevent cement extravasion and to direct forces applied to a vertebra to reduce a fracture.
Owner:DFINE INC
Composites and methods for treating bone
A system and method for treating bone abnormalities including vertebral compression fractures and the like. In one vertebroplasty method, a fill material is injected under high pressures into cancellous bone wherein the fill material includes a flowable bone cement component and an elastomeric polymer component that is carried therein. The elastomer component can further carry microscale or mesoscale reticulated elements. Under suitable injection pressures, the elastomeric component ultimately migrates within the flowable material to alter the apparent viscosity across the plume of fill material to accomplish multiple functions. For example, the differential in apparent viscosity across the fill material creates a broad load-distributing layer within cancellous bone for applying retraction forces to cortical bone endplates. The differential in apparent viscosity also transitions into a flow impermeable layer at the interface of cancellous bone and the flowable material to prevent extravasion of the flowable bone cement component.
Owner:DFINE INC
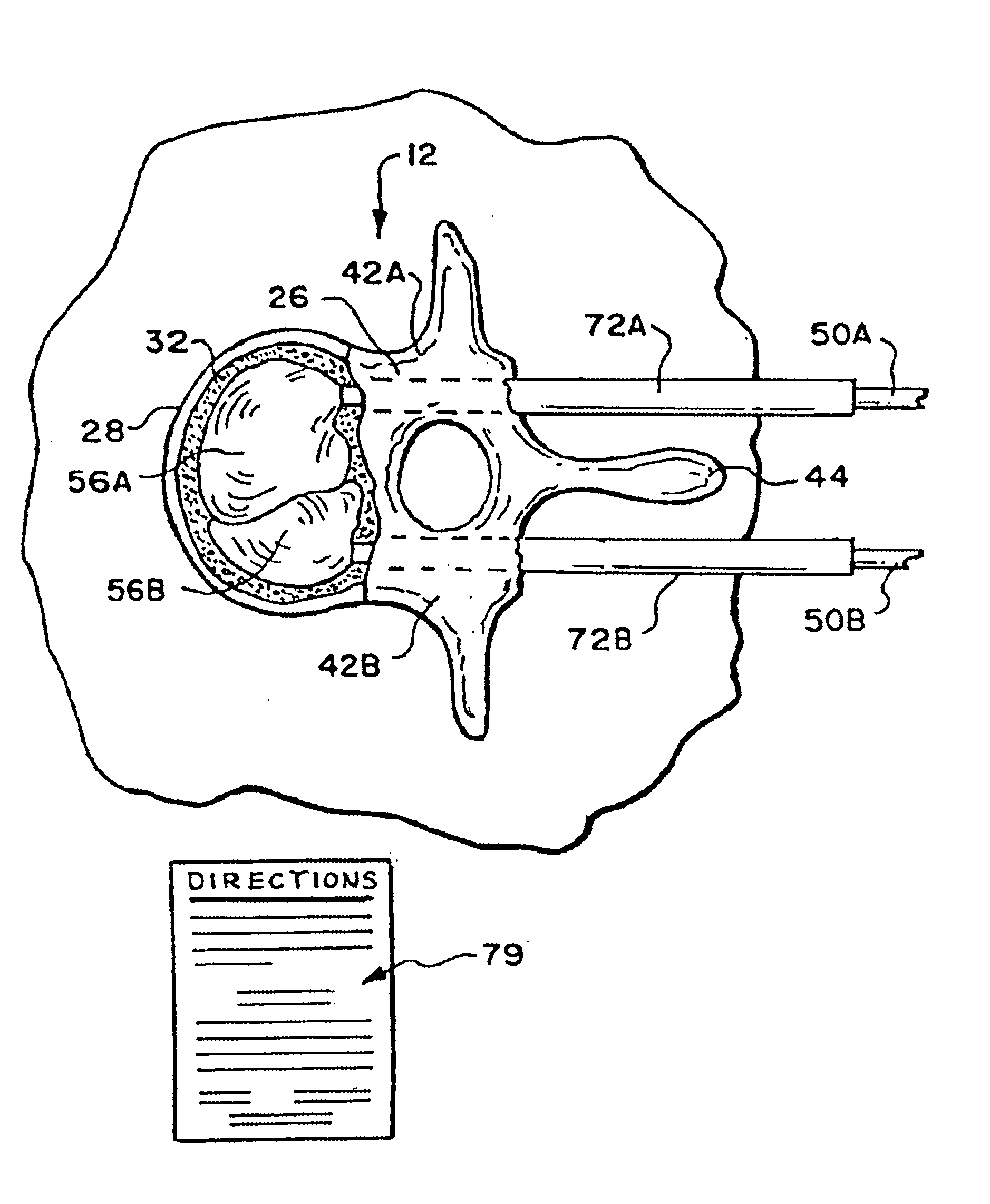
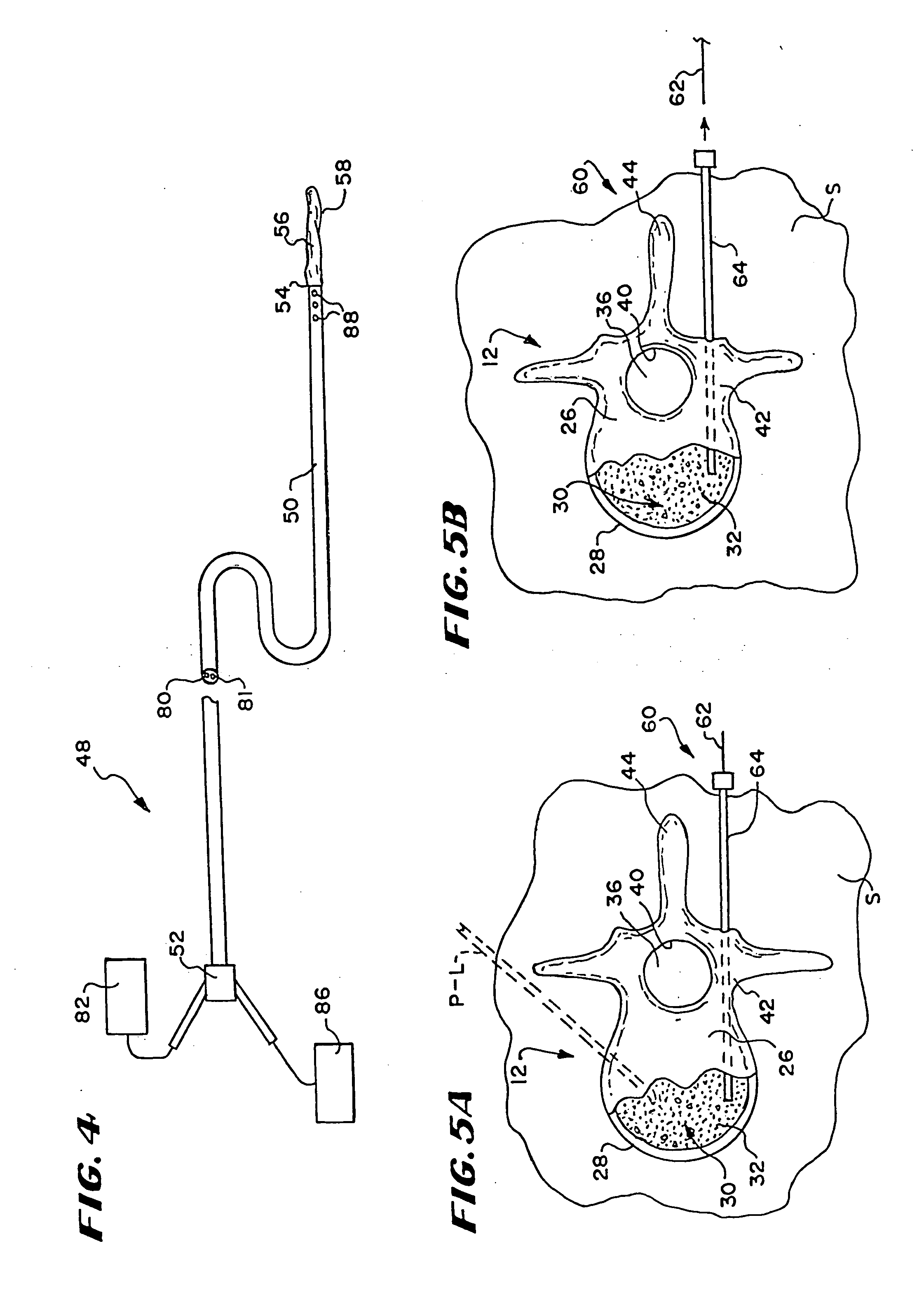
Apparatus and methods for delivering compounds into vertebrae for vertebroplasty
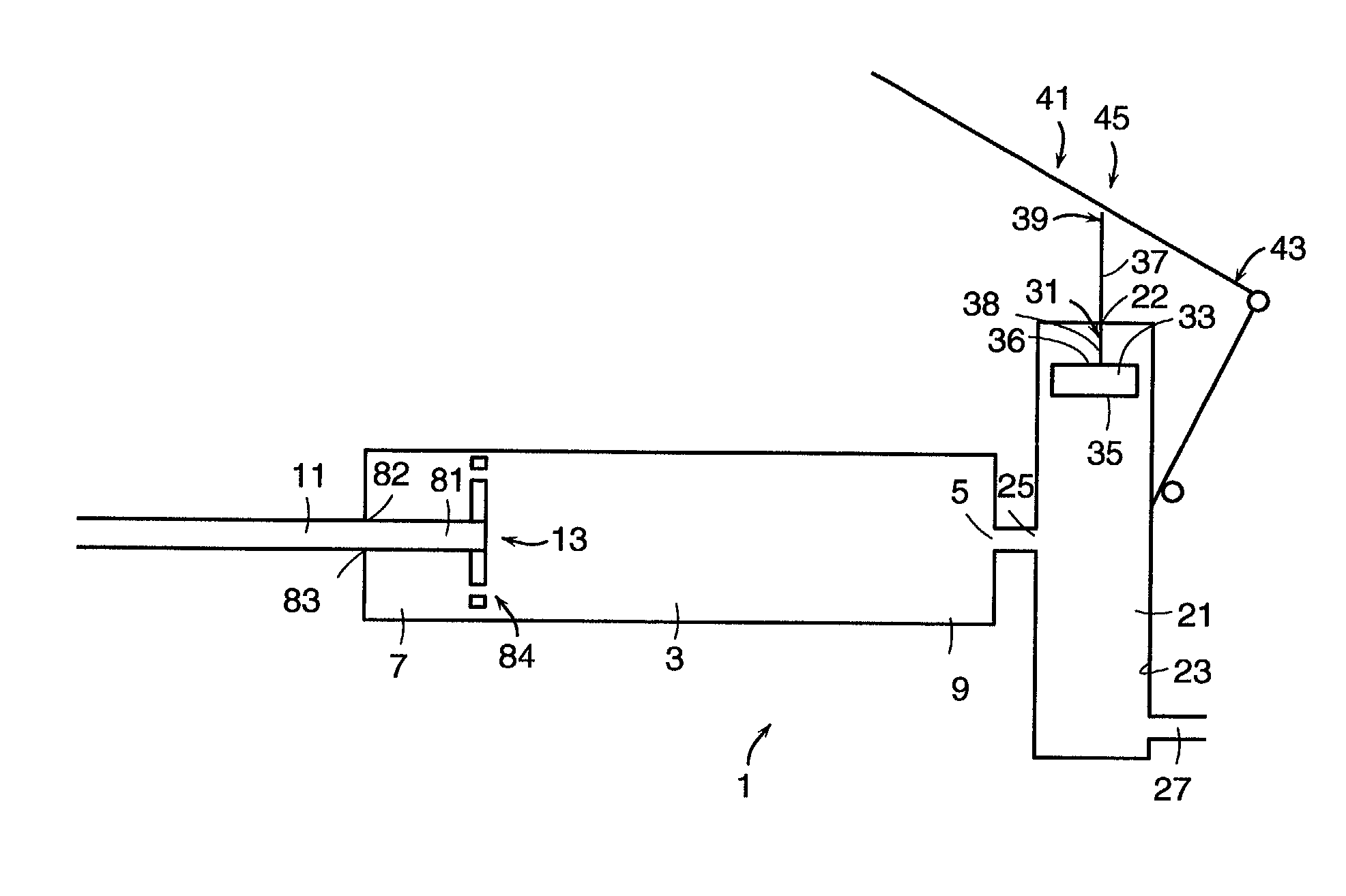
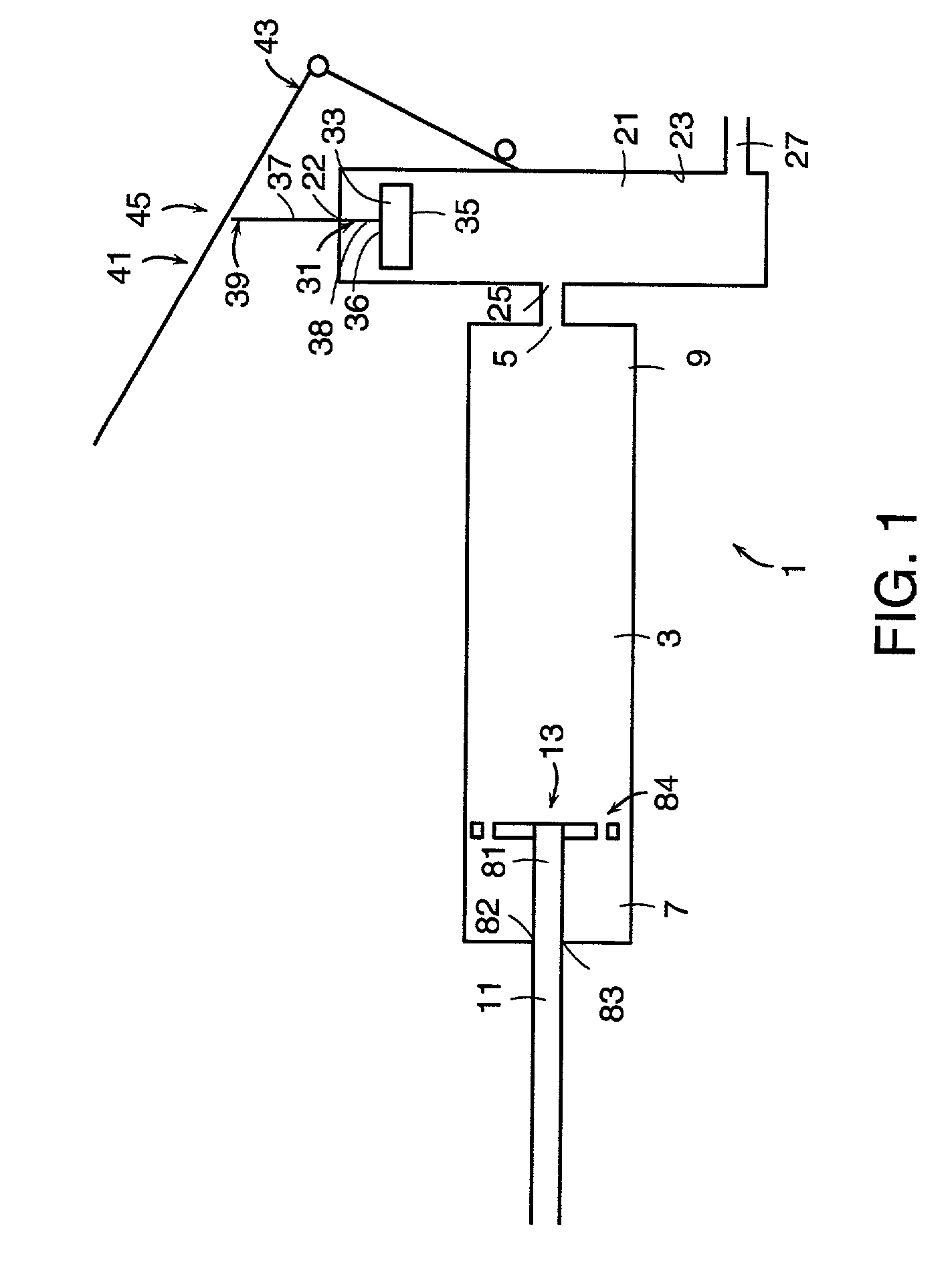
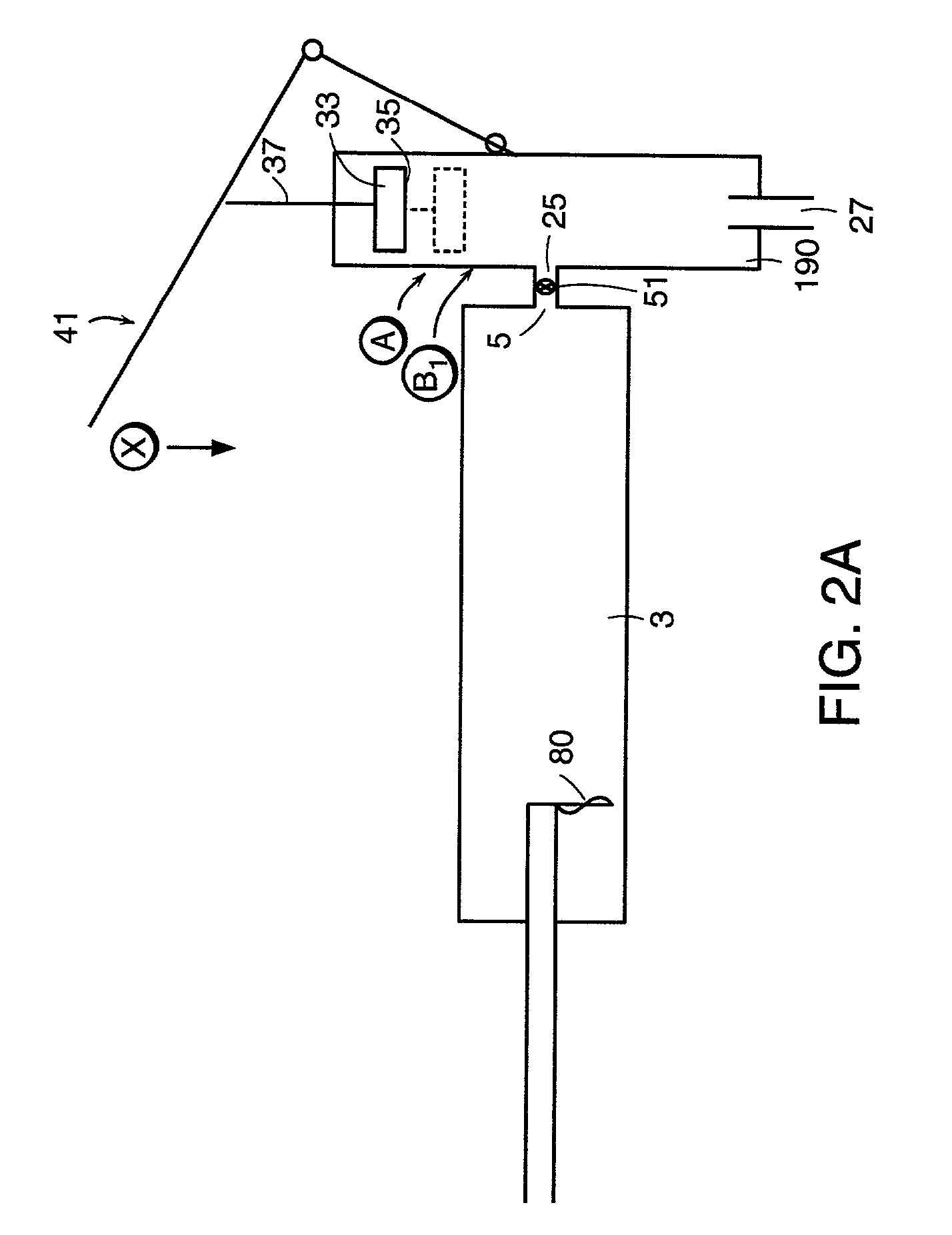
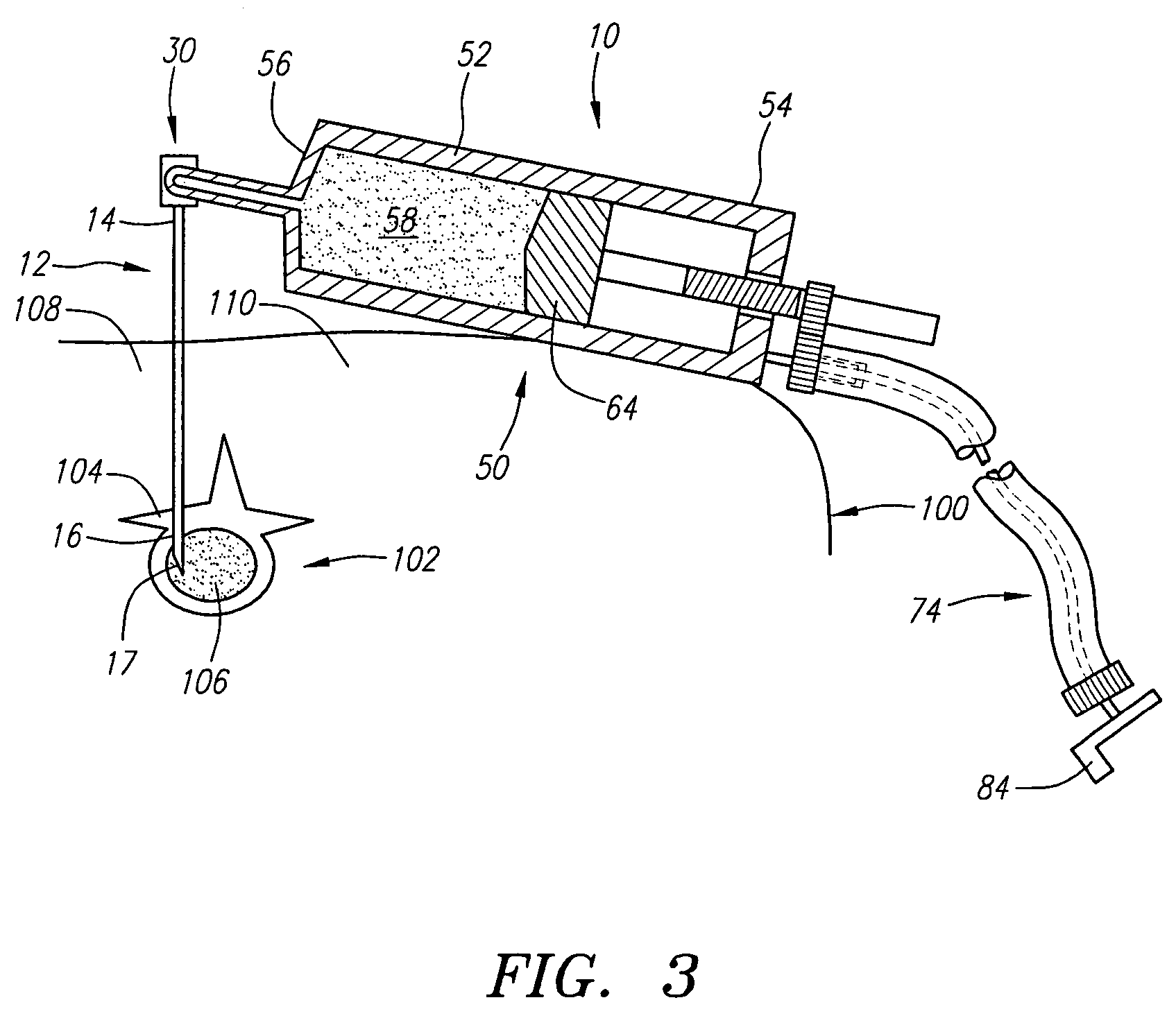
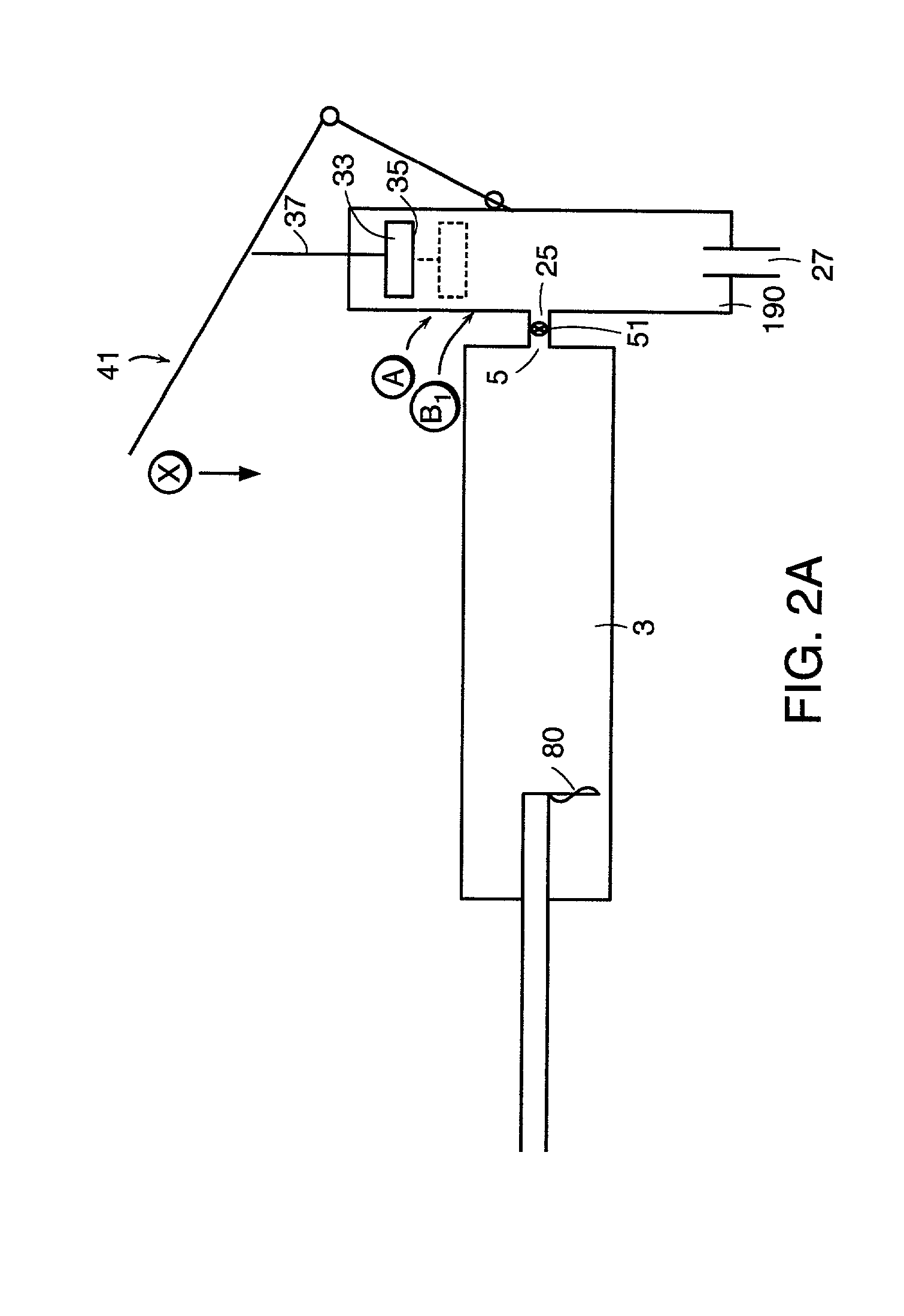
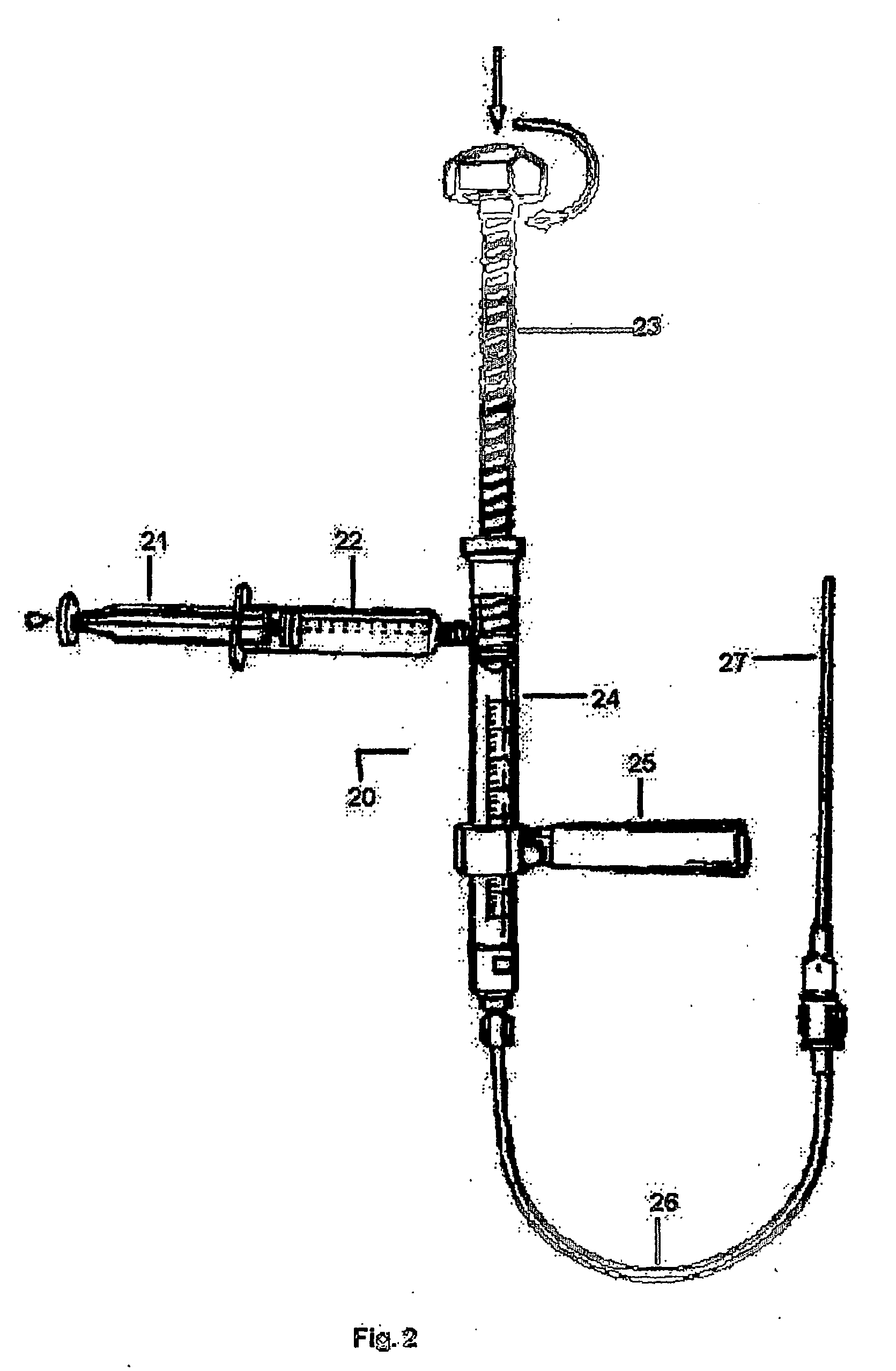
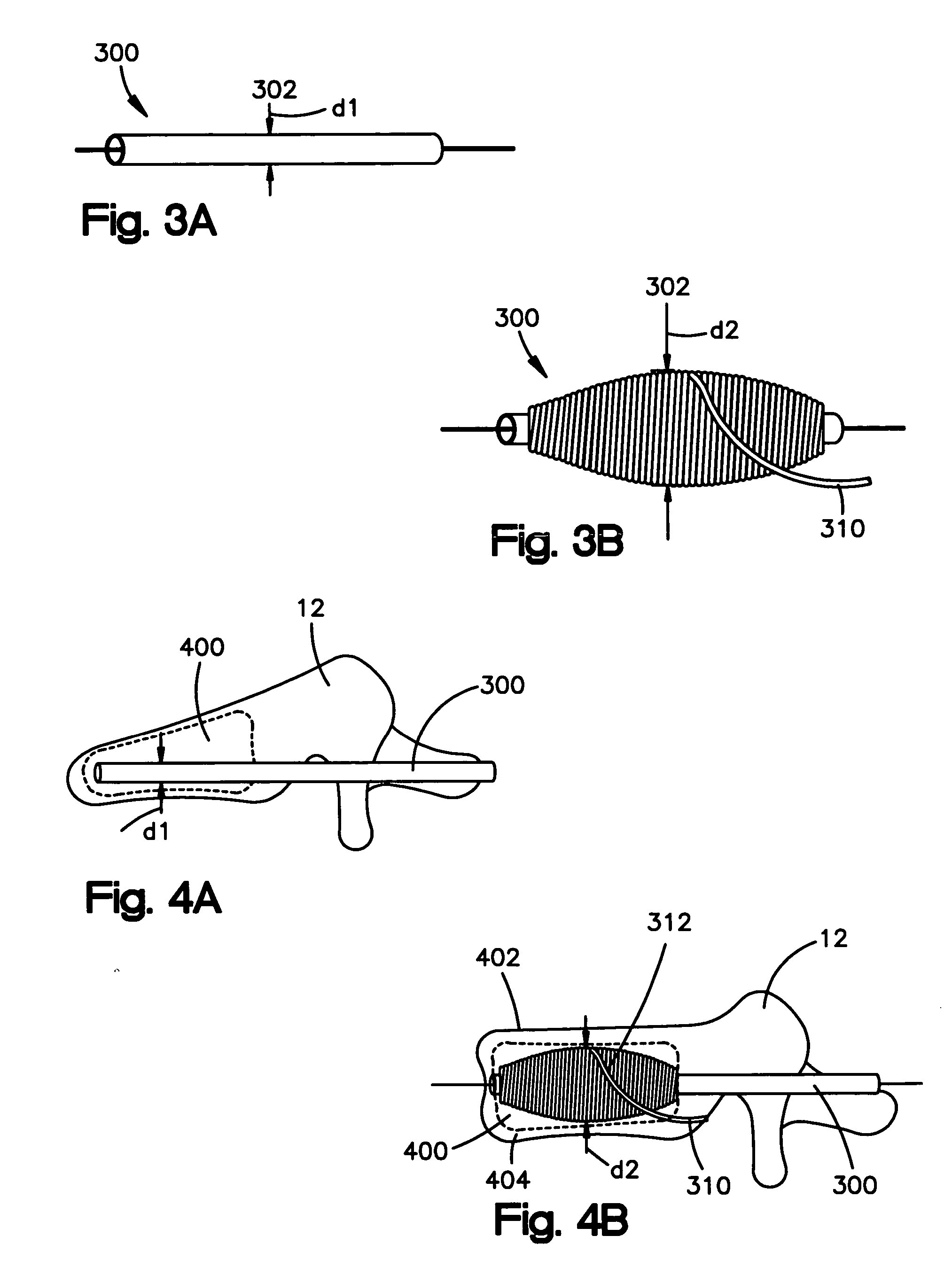
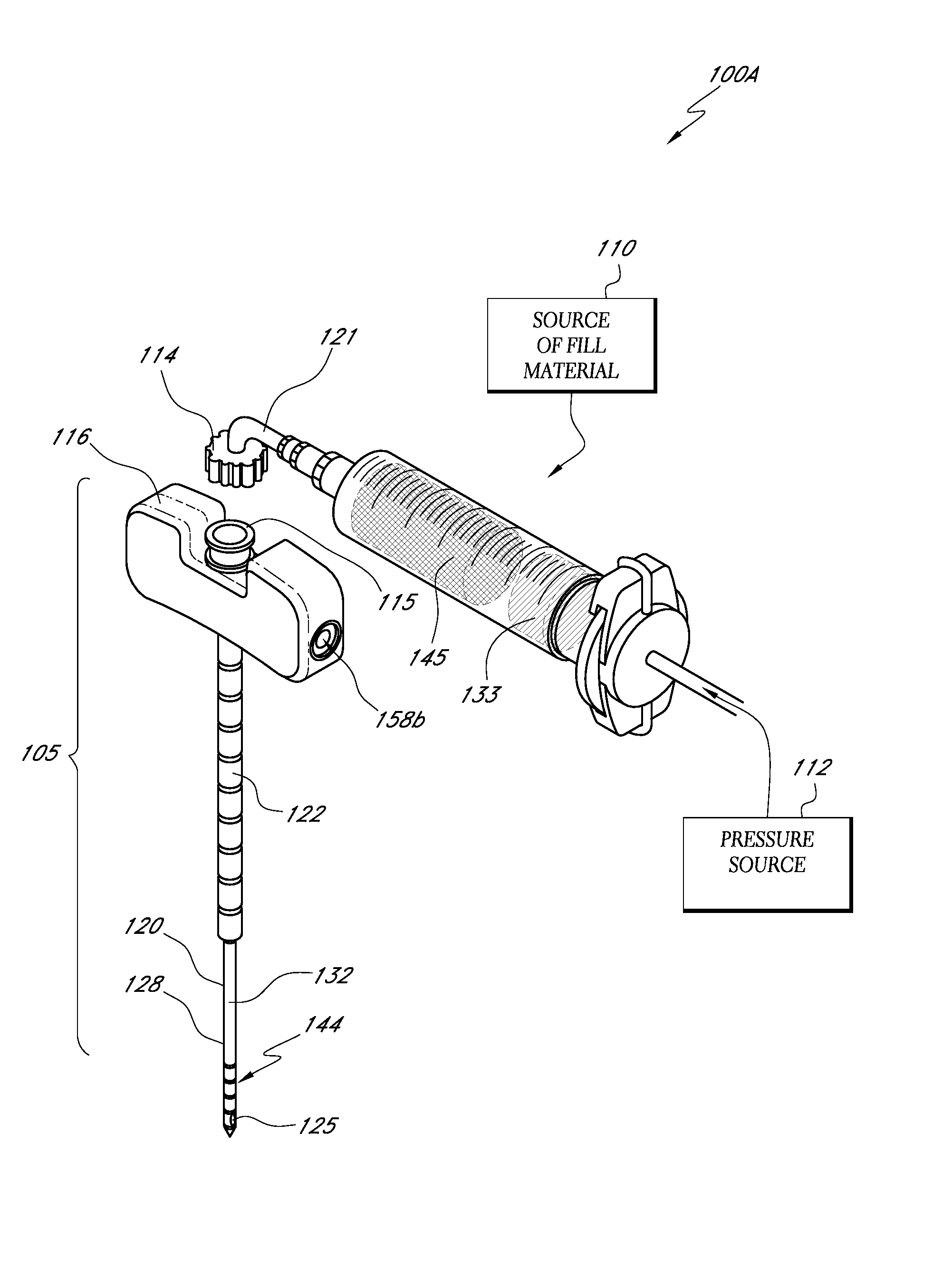
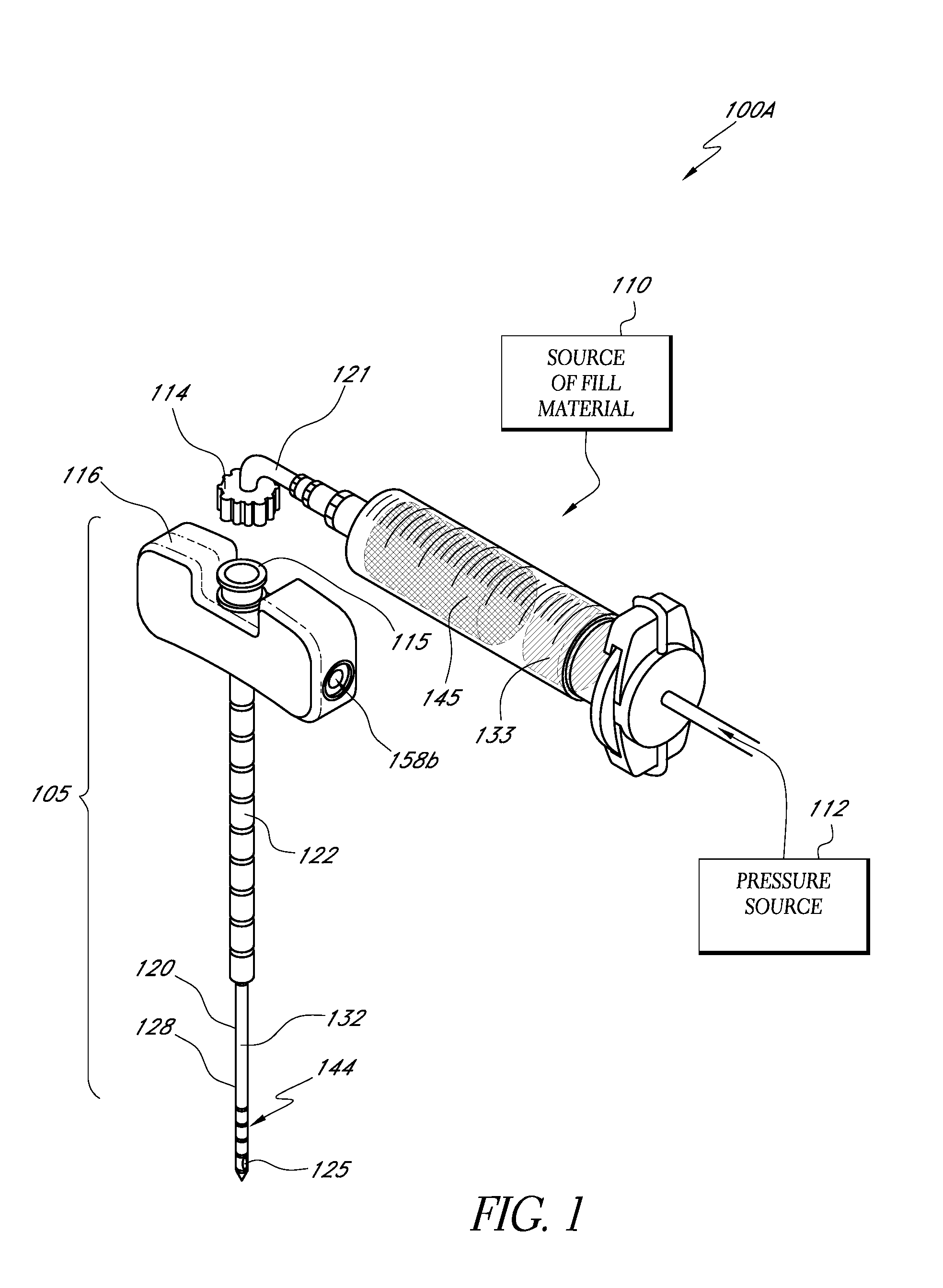
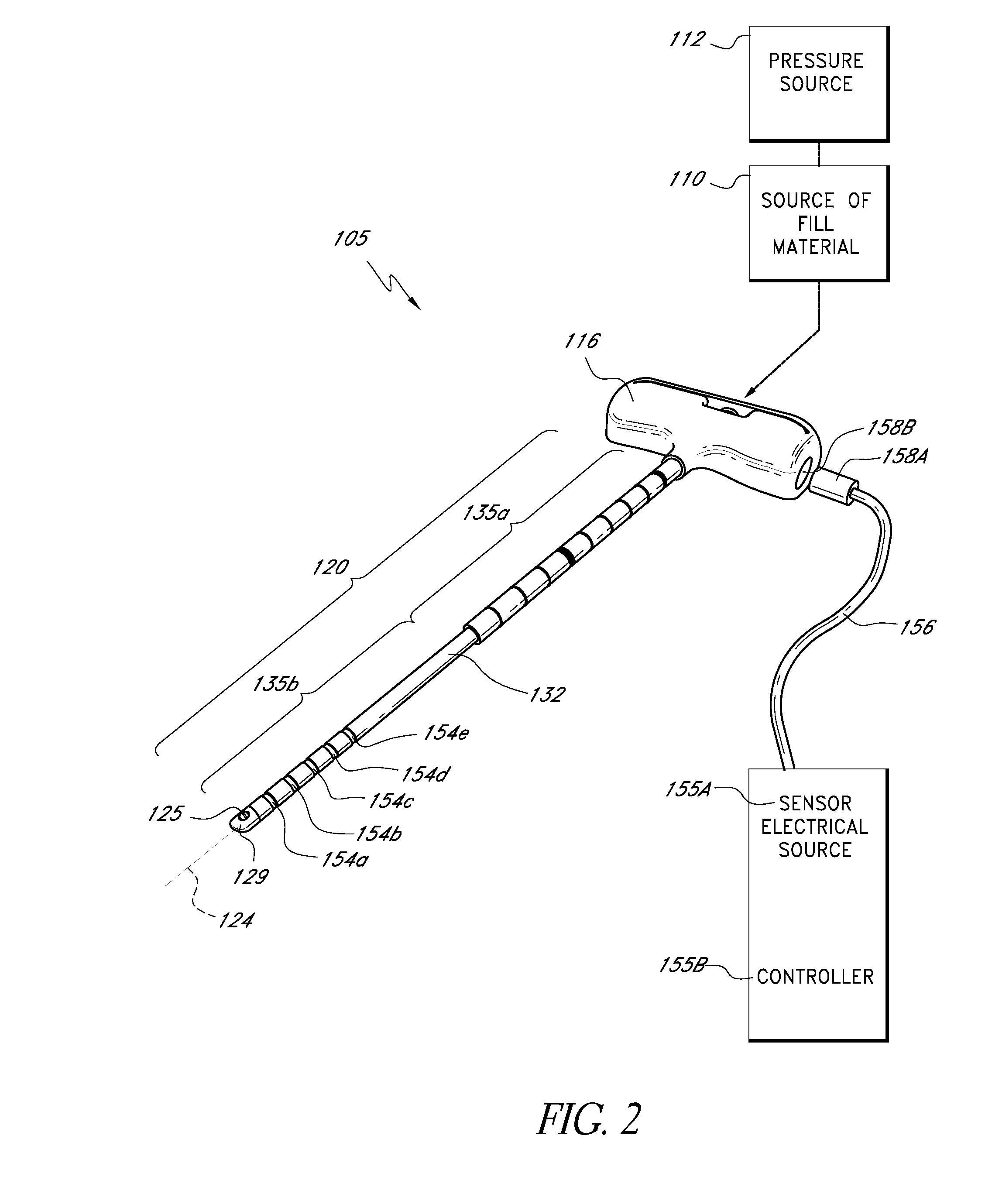
An apparatus for delivering bone cement into a vertebra includes a cannula and a syringe pivotally coupled to the cannula. Preferably, the syringe is pivotally coupled to the cannula by a pivot fitting that may be detachable from one or both of the cannula and the syringe. The syringe includes a piston slidable in a barrel for advancing the bone cement through an outlet communicating with the cannula. A shaft and cable arrangement or a fluid-driven system may be used to advance the piston within the syringe. The cannula is inserted into a vertebra, and is connected to the syringe. The syringe is pivoted to a desired position, and bone cement is delivered from the syringe through the cannula and into the vertebra.
Owner:BOSTON SCI SCIMED INC
Instrumentation and method for prosthetic knee
InactiveUS20050192588A1Shorten study timeImprove usabilityDiagnosticsSurgical sawsTibial boneFemoral bone
Instrumentation for guiding a surgeon in performing a unicompartmental knee replacement includes a tibial block having a guide, and a milling tool adapted to engage the guide, such that the surgeon can mill the desired tibial bone bed by directing the milling tool along the guide. Further including a femoral jig having a cutting slot, a guide, and a milling tool adapted to engage the guide, such that a surgeon can cut a portion of the femur through the slot, and mill the femoral bone bed by directing the milling tool about the guide. A femoral trial removal clamp facilitates in removing the femoral trial prosthesis, and a spreader compression clamp aids in compressing the final prostheses as the bone cement cures.
Owner:GARCIA DANIEL X
Systems and methods for treating fractured or diseased bone using expandable bodies
Systems and methods treat fractured or diseased bone by deploying more than a single therapeutic tool into the bone. In one arrangement, the systems and methods deploy an expandable body in association with a bone cement nozzle into the bone, such that both occupy the bone interior at the same time. In another arrangement, the systems and methods deploy multiple expandable bodies, which occupy the bone interior volume simultaneously. Expansion of the bodies form cavity or cavities in cancellous bone in the interior bone volume.
Owner:ORTHOPHOENIX
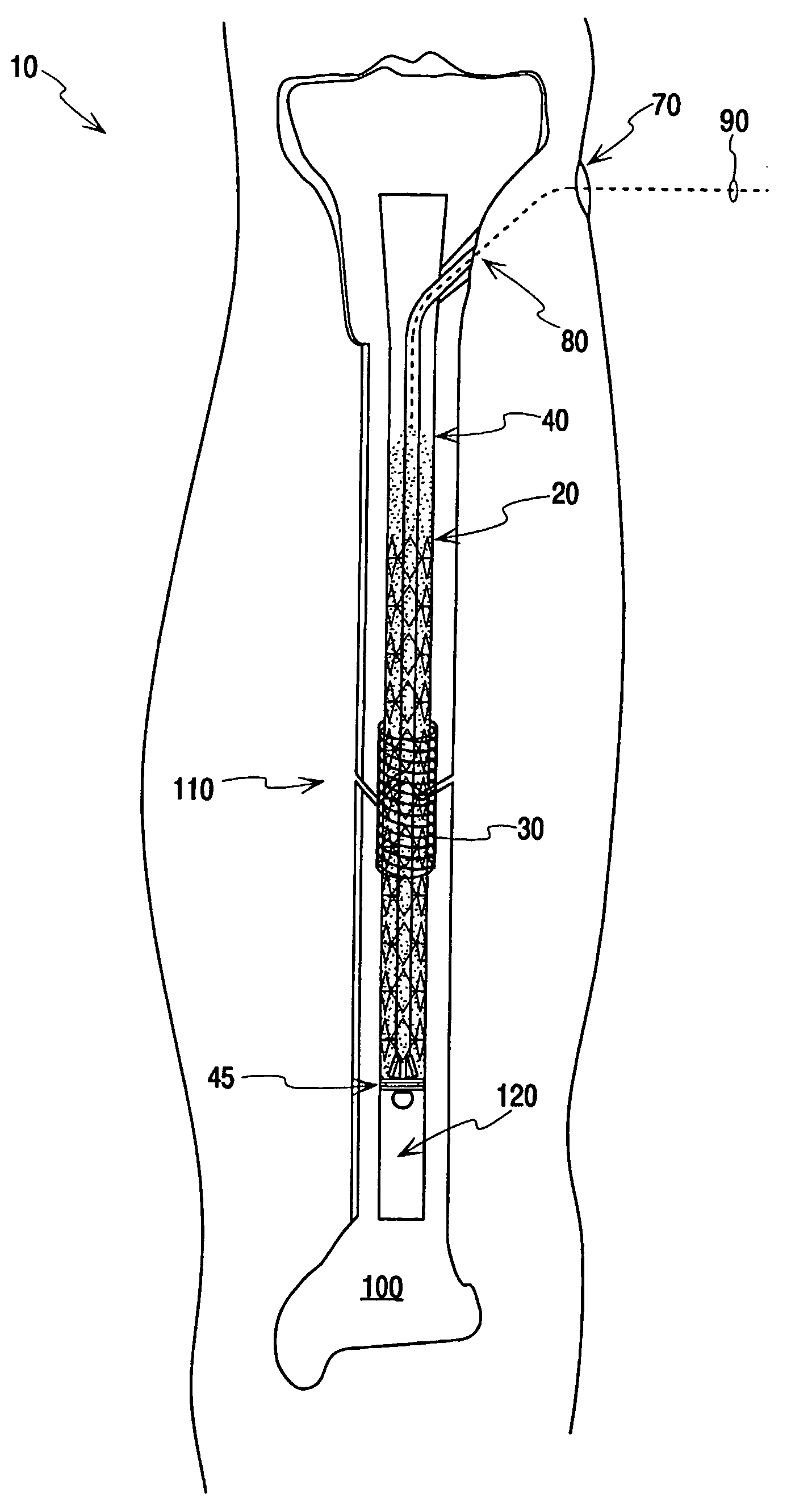
Fracture Fixation and Site Stabilization System
A system for percutaneous fixation and stabilization of a fracture with a spanning, expandable structural frame placed in the intramedullary canal of the bone, comprising a column of surgical fluid such as bone cement, within which the structural frame acts as a reinforcing cage, and a sheath positioned at the fracture site and at least partially surrounding the frame and fluid. The fluid may be supported by a restrictor and may be agitated with a vibrating probe to remove entrapped air. The structural frame may be self-expanding or opened by an internal force, and it may be retrievable. The frame, fluid, or sheath may contain antibiotics, pharmaceuticals, or other therapeutic compounds to be delivered to the fracture site.
Owner:SONOMA ORTHOPEDIC PROD INC +1
Vertebroplasty injection device and bone cement therefor
InactiveUS20020156483A1Small sizeIncrease pressurePowder deliveryShaking/oscillating/vibrating mixersInjectable biomaterialVertebroplasty procedure
This invention relates to a mixing and delivery device suitable for delivering injectable biomaterials, and to preferred bone cement formulations.
Owner:DEPUY ACROMED INC
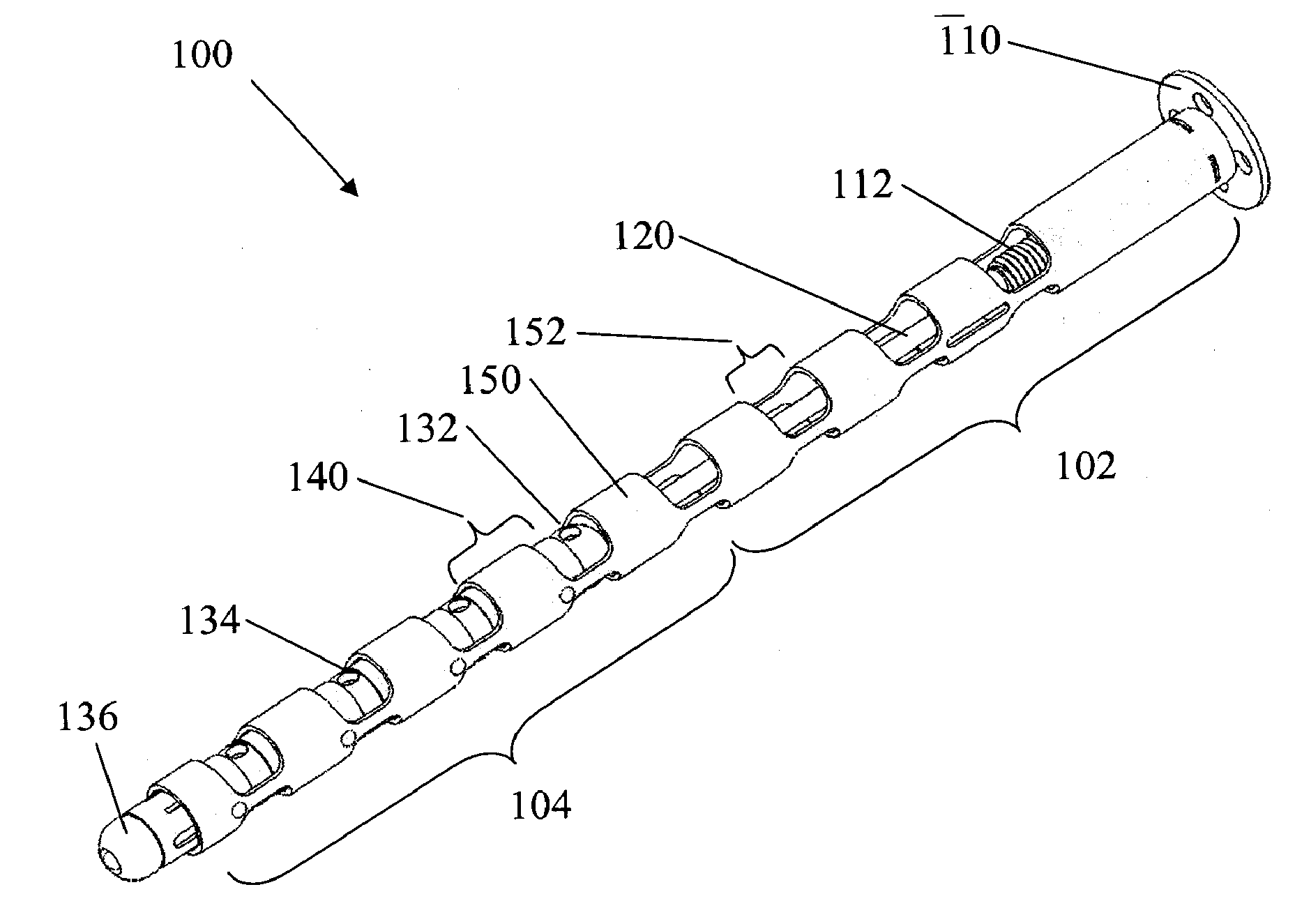
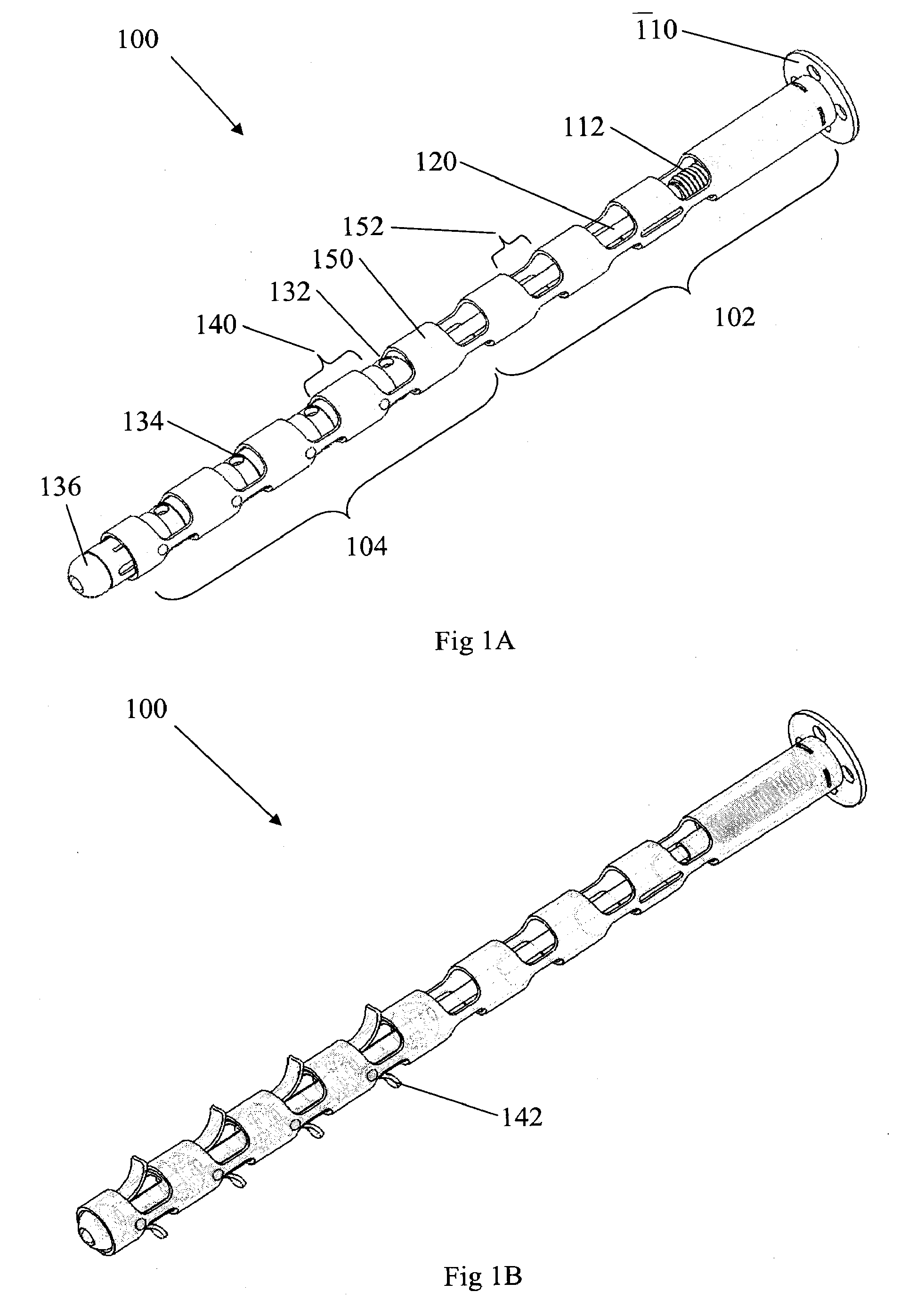
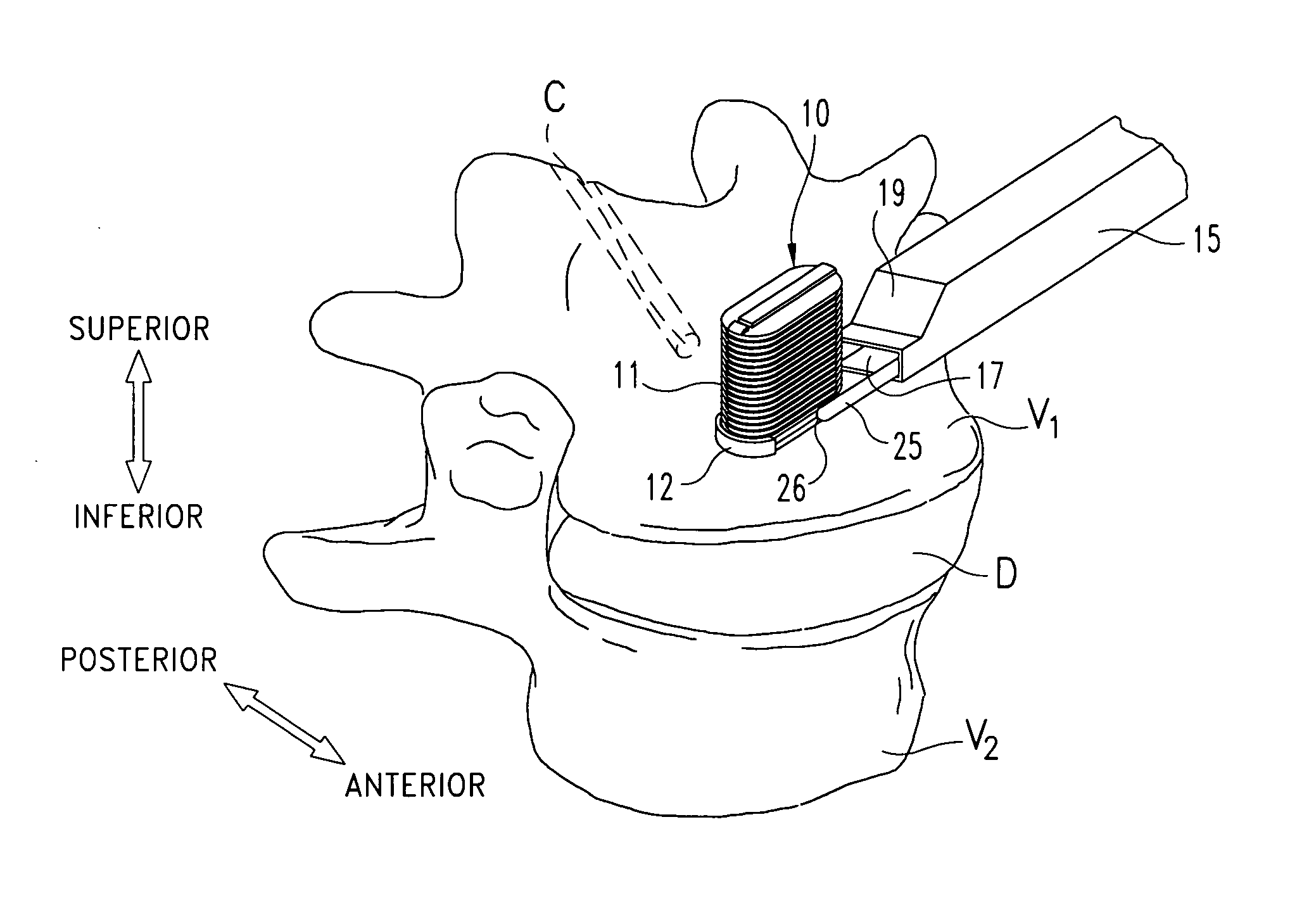
Flexible elongated chain implant and method of supporting body tissue with same
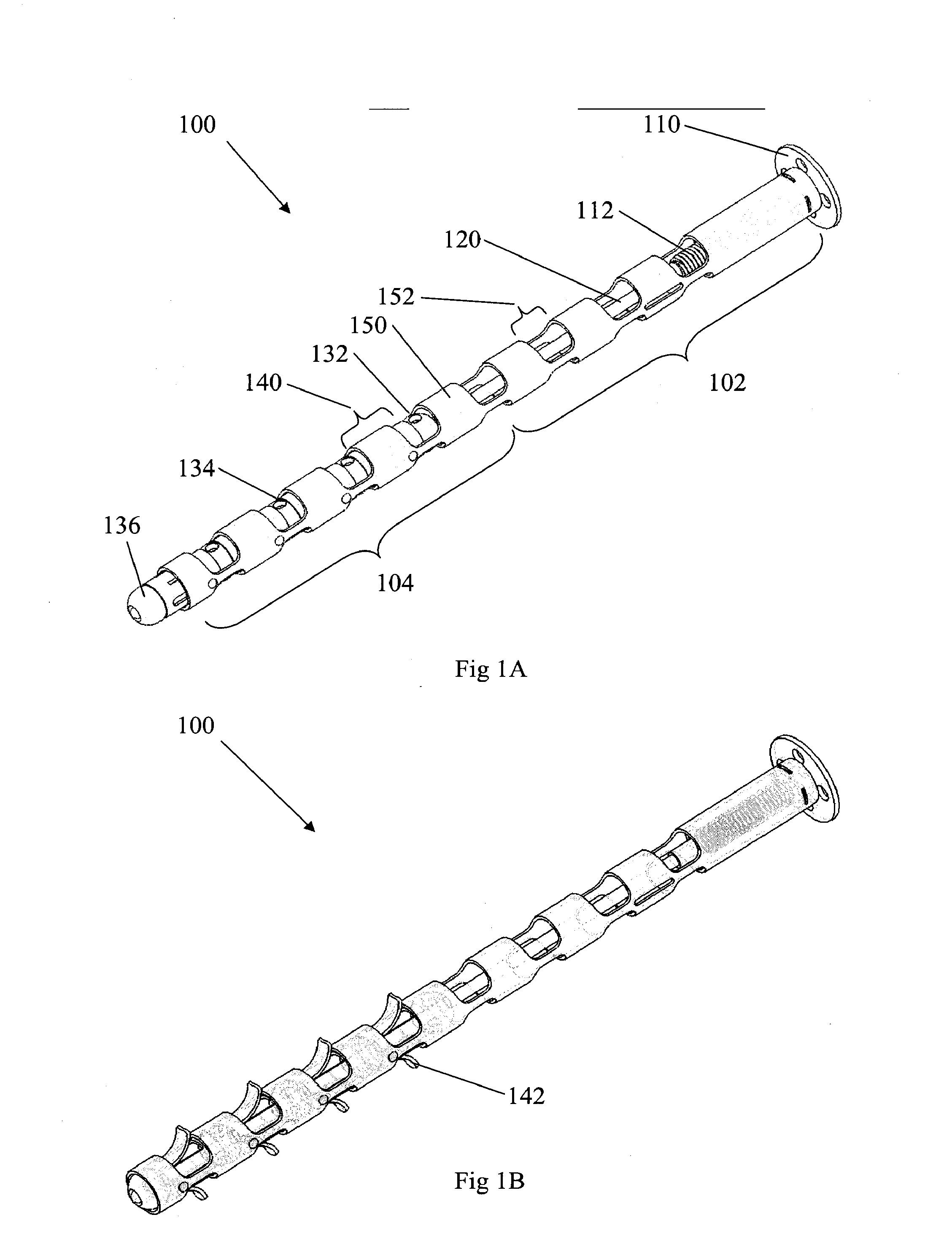
InactiveUS20070162132A1Internal osteosythesisSpinal implantsSurface layerMinimally invasive procedures
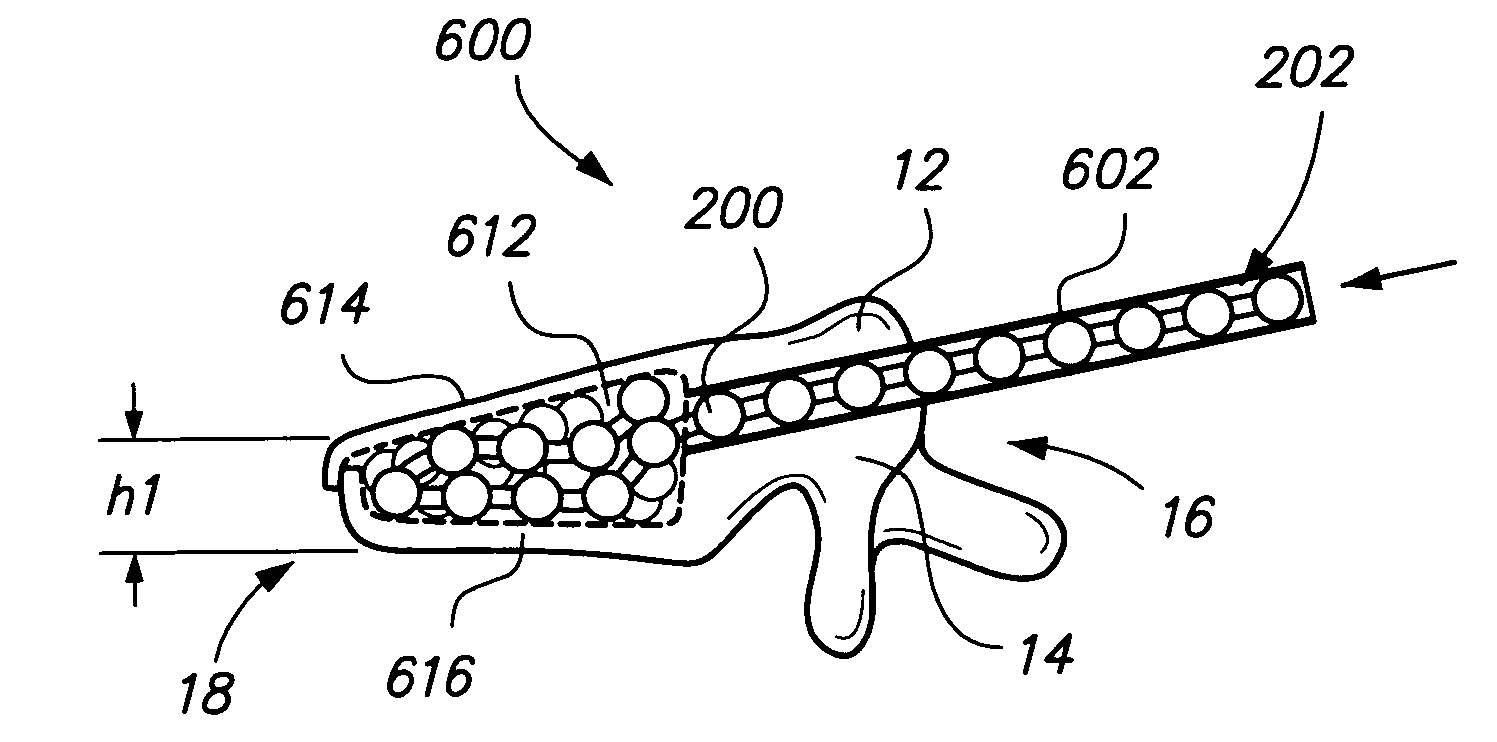
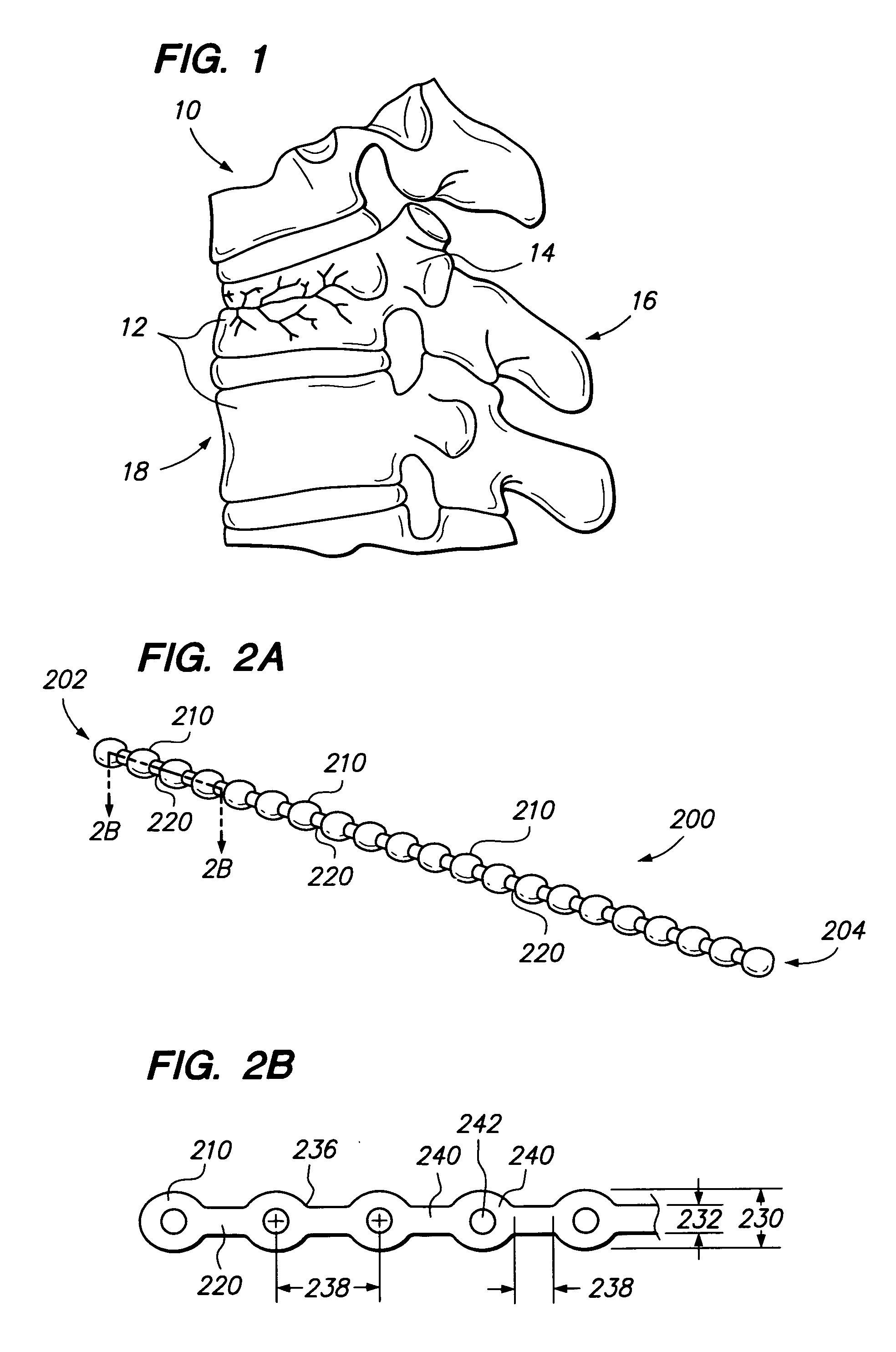
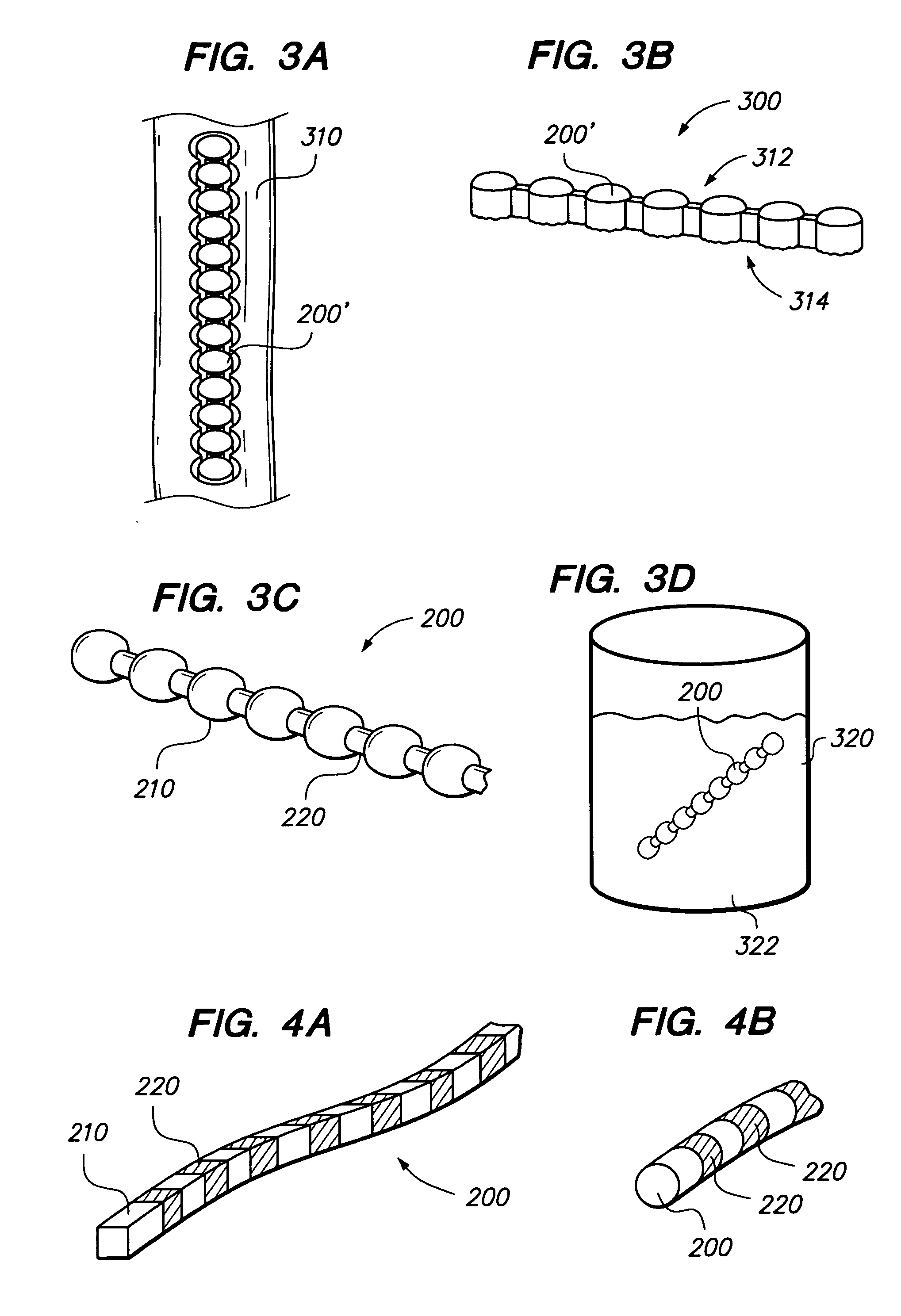
Implants and methods for augmentation, preferably by minimally invasive procedures and means, of body tissue, including in some embodiments repositioning of body tissue, for example, bone and, preferably vertebrae are described. The implant may comprise one or more chain linked bodies inserted into the interior of body tissue. As linked bodies are inserted into body tissue, they may fill a central portion thereof and for example in bone can push against the inner sides of the cortical exterior surface layer, for example the end plates of a vertebral body, thereby providing structural support and tending to restore the body tissue to its original or desired treatment height. A bone cement or other filler can be added to further augment and stabilize the body tissue. The preferred implant comprises a single flexible monolithic chain formed of allograft cortical bone having a plurality of substantially non-flexible bodies connected by substantially flexible links.
Owner:DEPUY SYNTHES PROD INC
Bone treatment systems and methods
ActiveUS20060122625A1Inhibit migrationInternal osteosythesisJoint implantsHigh accelerationVertebra compression fracture
The present invention relates in certain embodiments to medical devices for treating vertebral compression fractures. More particularly, embodiments of the invention relate to instruments and methods for controllably restoring vertebral body height by controlling the flow of bone cement into the interior of a vertebra and the application of forces causes by the cement flow. An exemplary system utilizes Rf energy in combination a conductive bone cement for selectively polymerizing the inflow plume to increase the viscosity of the cement. In one aspect of the invention, the system utilizes a controller to control bone cement flow parameters to either allow or disallow cement interdigitation into cancellous bone. A method of the invention includes pulsing the flows of bone cement wherein high acceleration of the flow pulses can apply expansion forces across the surface of the cement plume to reduce a vertebral fracture.
Owner:DFINE INC
Minimally Invasive Actuable Bone Fixation Devices
ActiveUS20060264950A1Low weight to volumeReduce traumaSuture equipmentsInternal osteosythesisSurgical siteBone fixation devices
Apparatus for bone reinforcement, fixation and treatment of diseased or fractured bones including a supporting structure optionally coated with therapeutic agent is provided. The supporting structure or device may be collapsible upon deployment at the surgical site, and include fixation features such as anchors to securely position in place once deployed. Bone cement or other material may be provided to alternatively secure the positioned supporting structure for treatment. A lockable bone fixation device is described that comprises: a sleeve adapted to be positioned in a space formed in a bone; a guidewire adapted to guide movement of the sleeve; and an actuable lock adapted to secure the sleeve within the space of the bone from an end of the device.
Owner:ARTHREX
Cement-directing orthopedic implants
ActiveUS7465318B2Beneficial interdigitation of bone cementKeep openInternal osteosythesisBone implantVolumetric Mass DensitySurgery
A cement-directing structure for use in cement-injection bone therapy includes a collapsible, self-restoring braided structure with regions of differential permeability to the bone cement. The regions of differential permeability may be provided by areas where the braided mesh density is greater or lesser than surrounding areas and / or by means of a baffle. After the structure is placed in a void within a bony structure, cement is injected into the interior of the structure then oozes out in preferred directions according to the locations of the regions of differential permeability.
Owner:GLOBUS MEDICAL INC
Spine treatment devices and methods
A spine implant device for fusion or dynamic stabilization of a spine segment can include a fixation device with a shaft portion for engaging bone and a proximal end for coupling to a rod that allows for limited flexing of the proximal end relative to the shaft portion. A spine implant system and method utilizing such a bone fixation device can include an energy delivery mechanism for on-demand curing of bone cement delivered through the bone fixation device into a bone.
Owner:DFINE INC
Bone treatment systems and methods
Owner:DFINE INC
Apparatus and methods for delivering compounds into vertebrae for vertebroplasty
An apparatus for delivering bone cement into a vertebra, includes a cannula, a delivery device in communication with the cannula and a pressure delivery device in communication with the delivery device. The pressure delivery device provides an actuating force that acts either directly or through a medium to cause a flowable compound to be delivered from the delivery device to the cannula and into the vertebra. The pressure delivery device causes a pressurized compound to be delivered, the pressurized compound may be liquid or gaseous CO2 or other mediums.
Owner:BOSTON SCI SCIMED INC
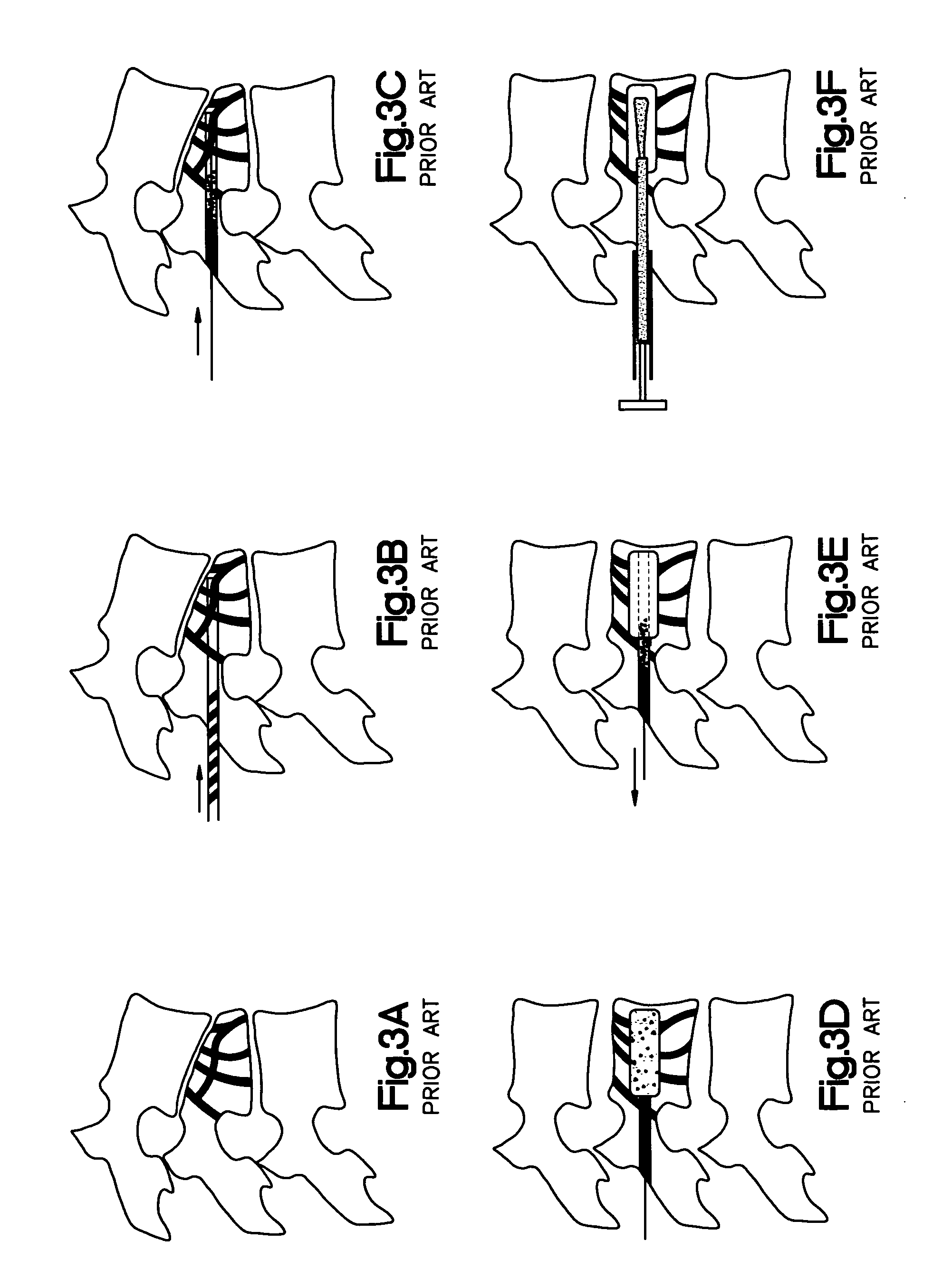
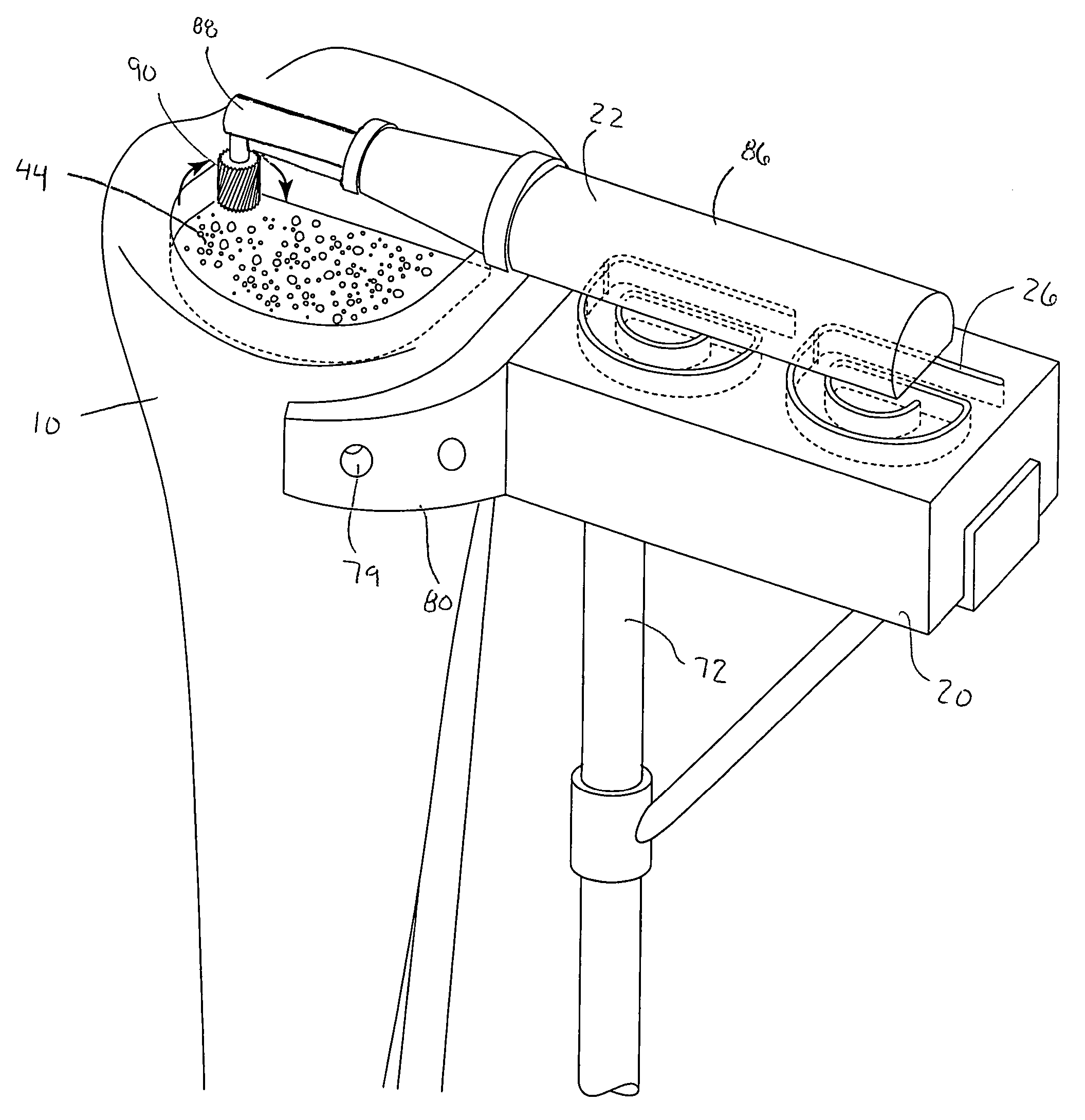
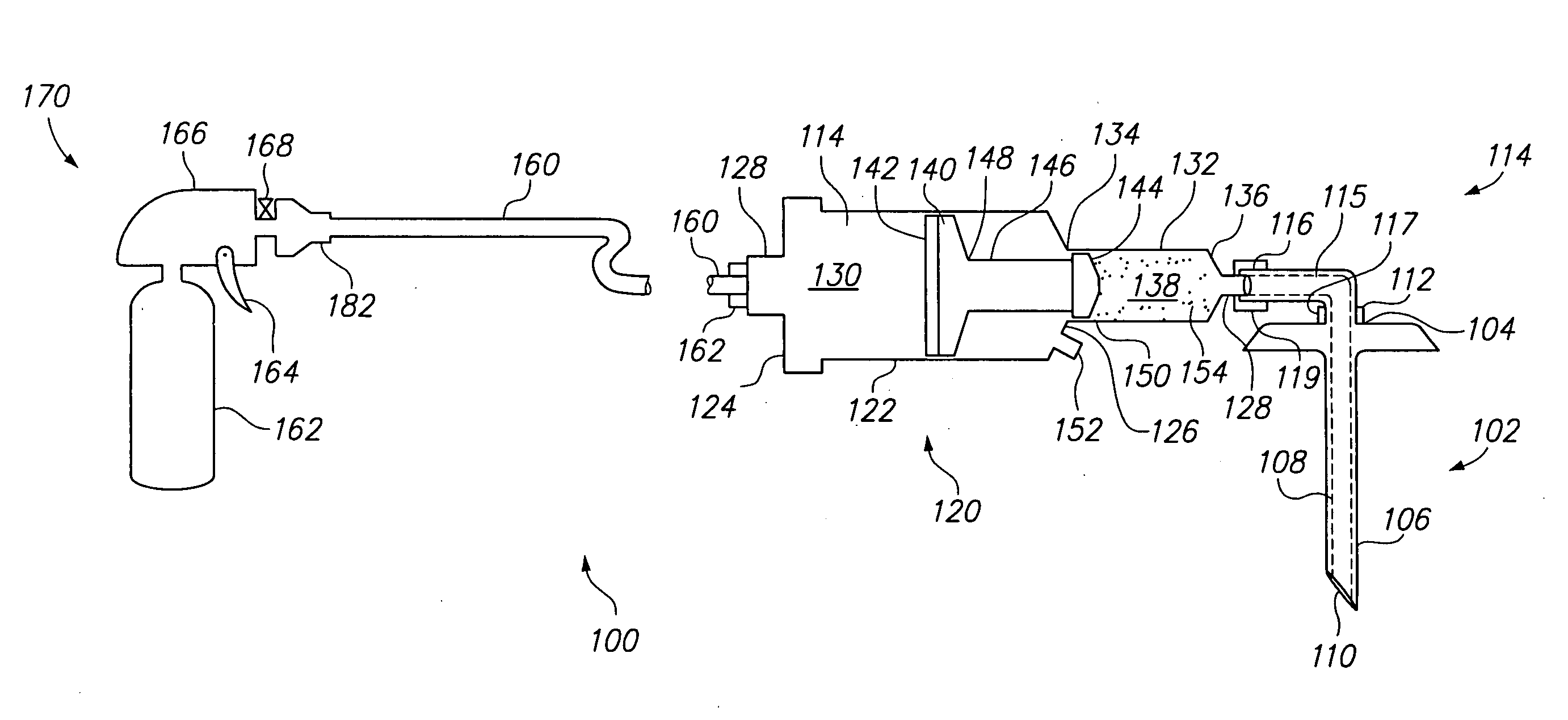
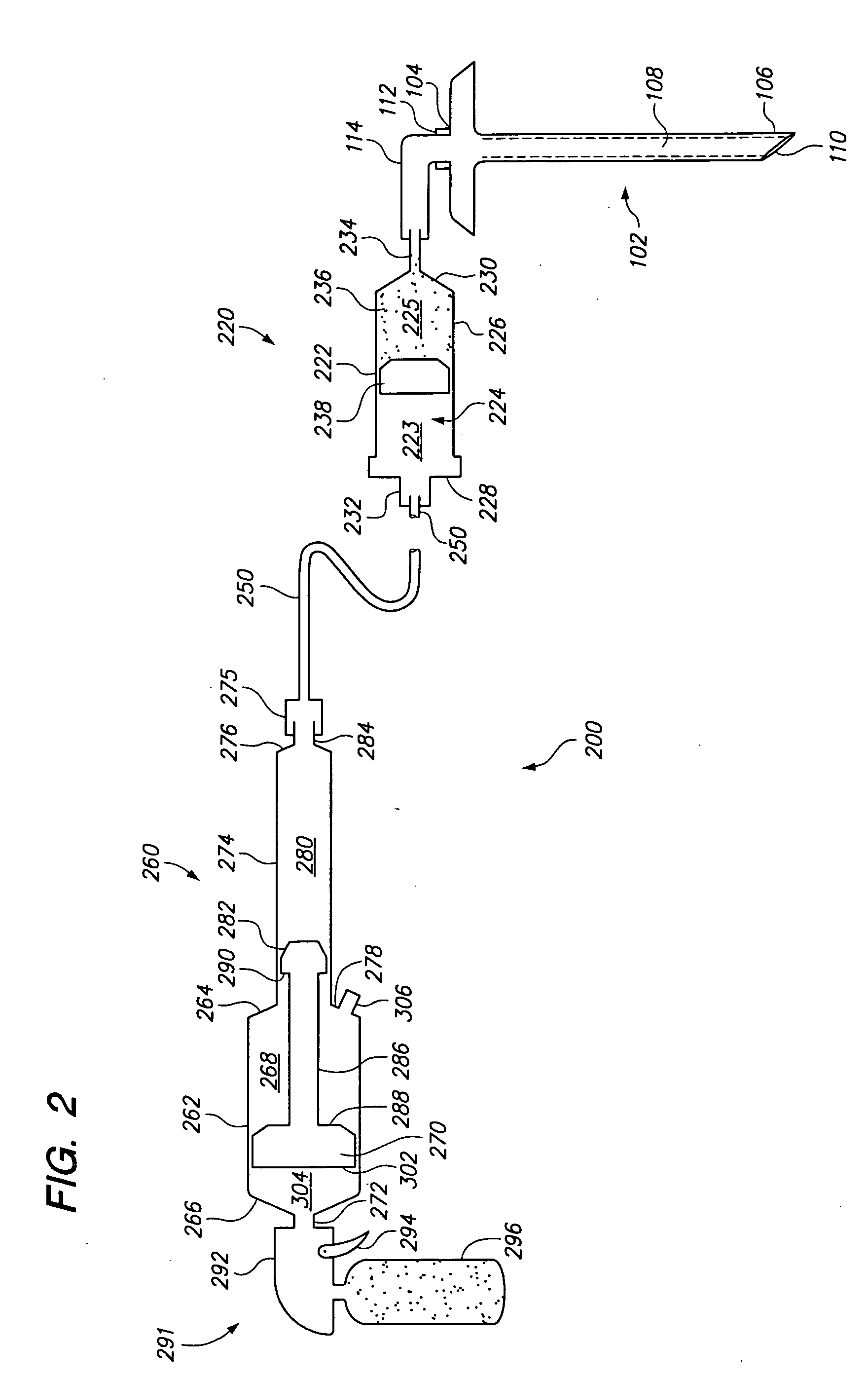
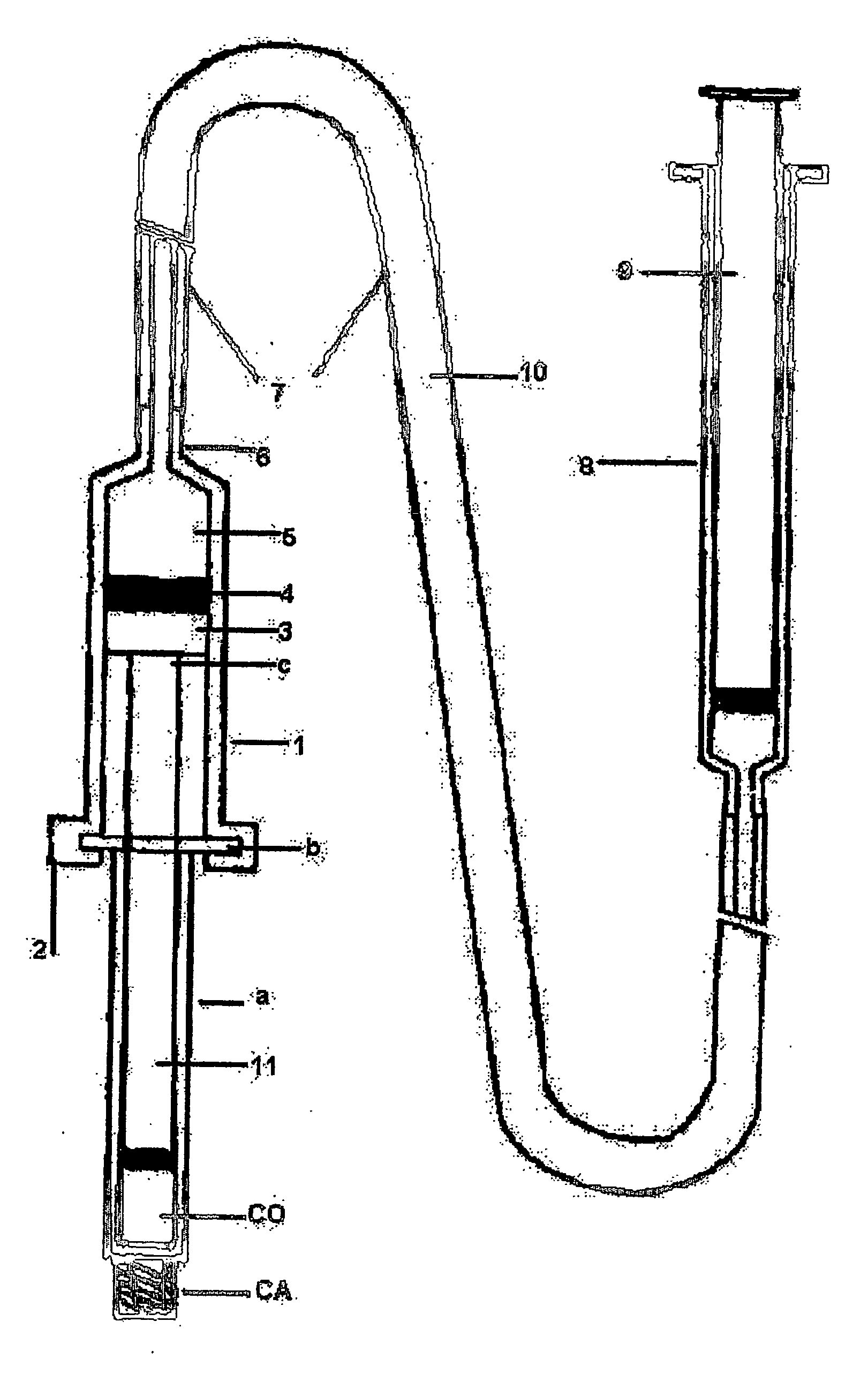
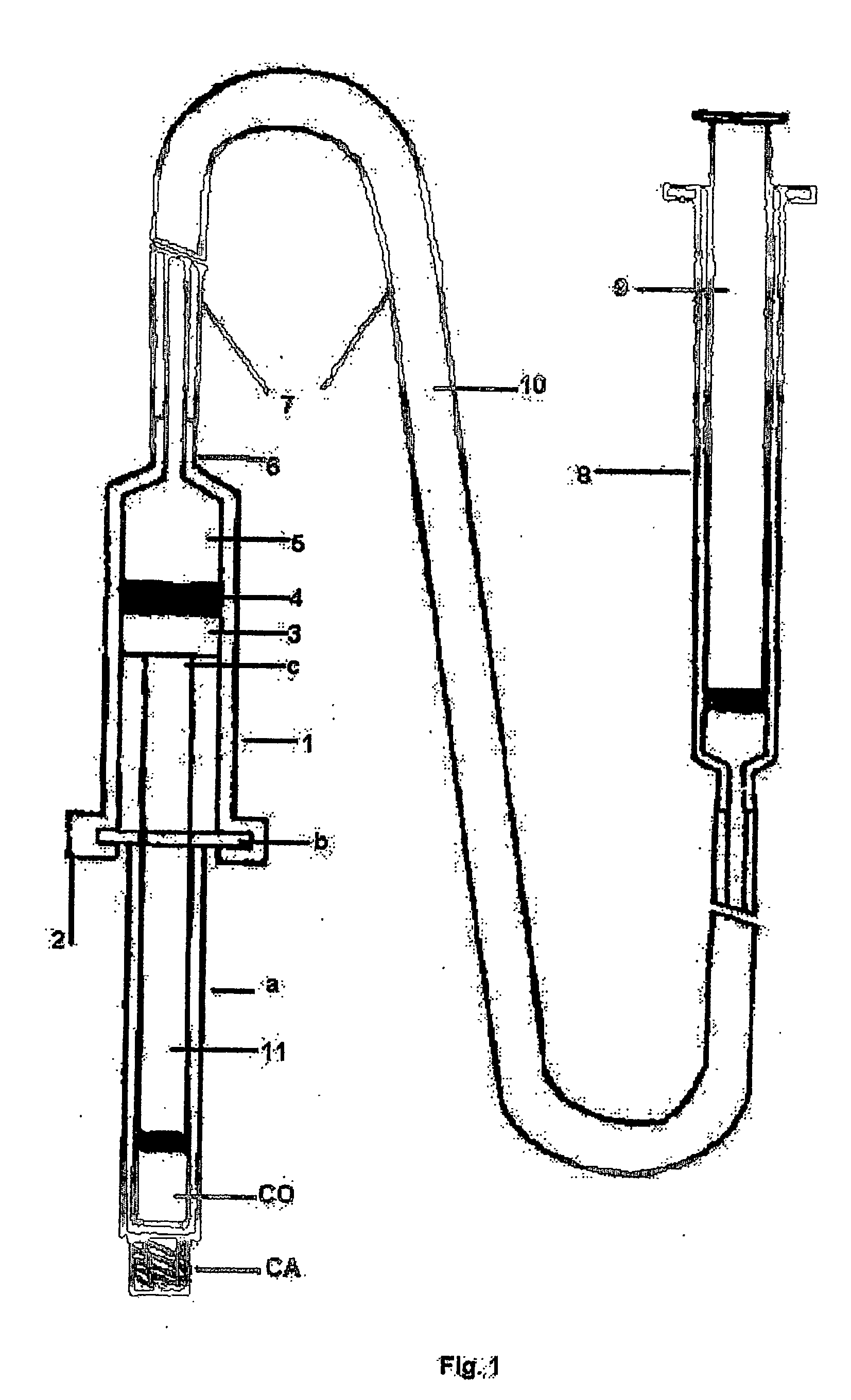
Hydraulic device for the injection of bone cement in percutaneous vertebroplasty
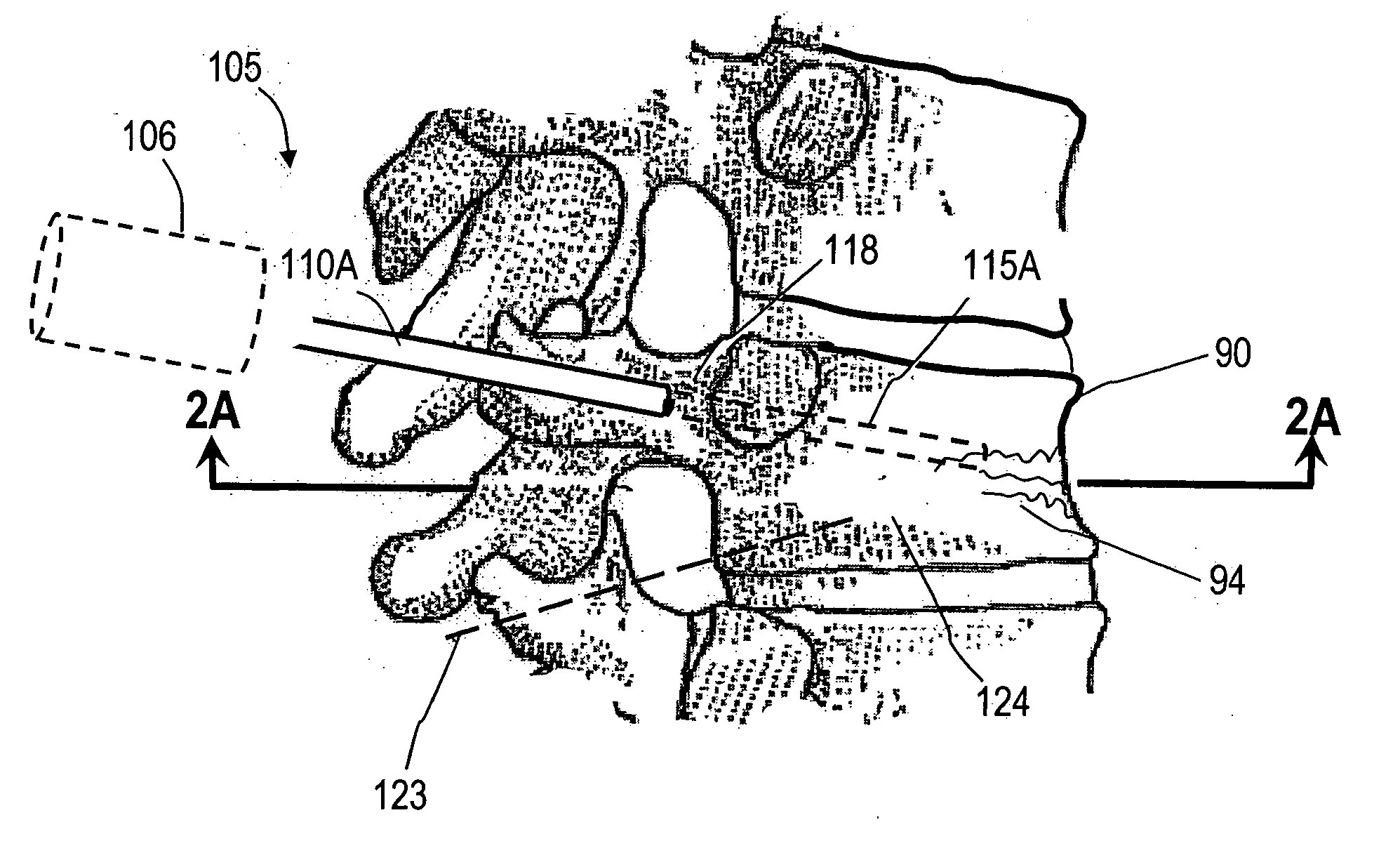
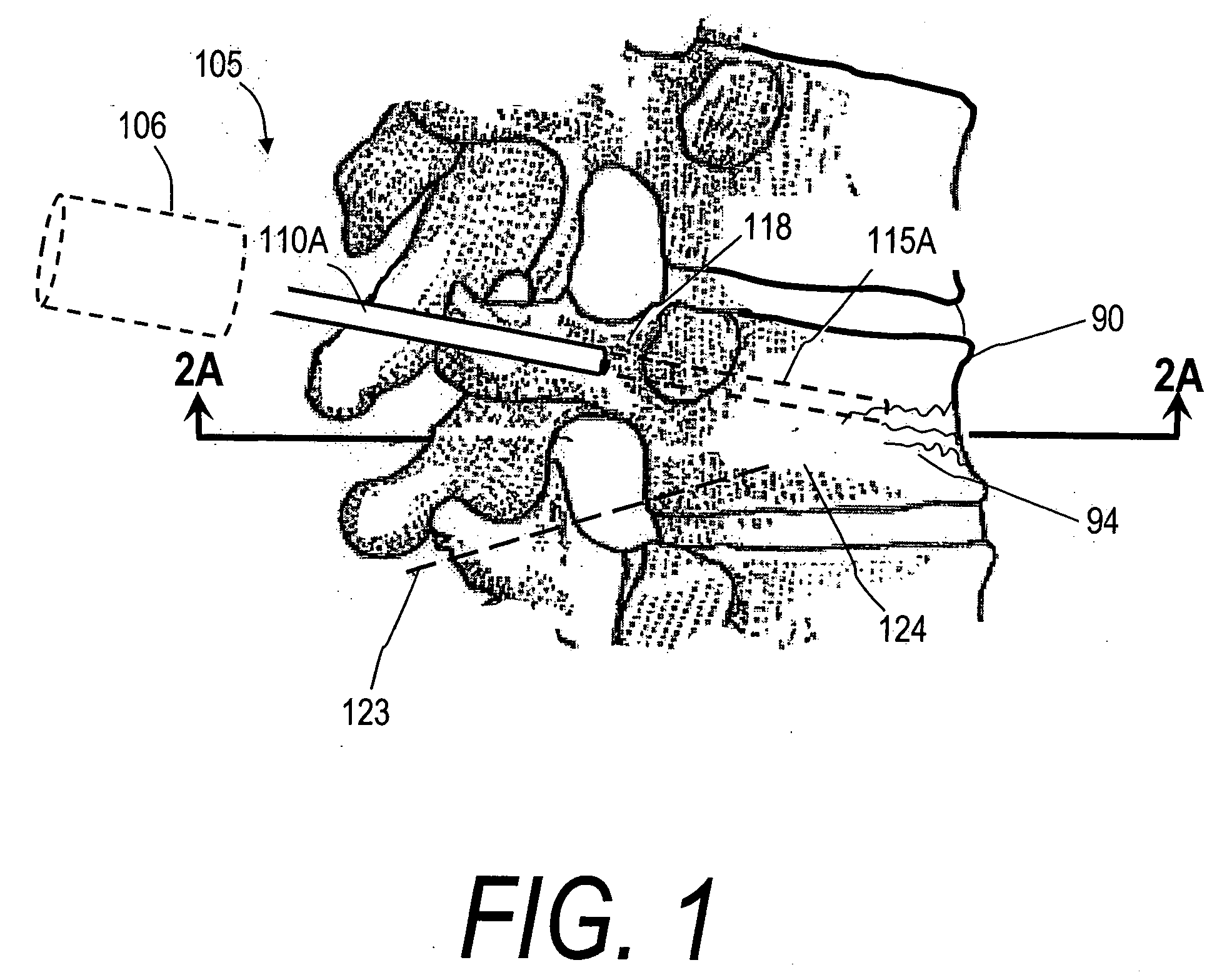
InactiveUS20060264967A1Reduce radiationReduce overexposureJoint implantsIntravenous devicesFluid controlPressure transmission
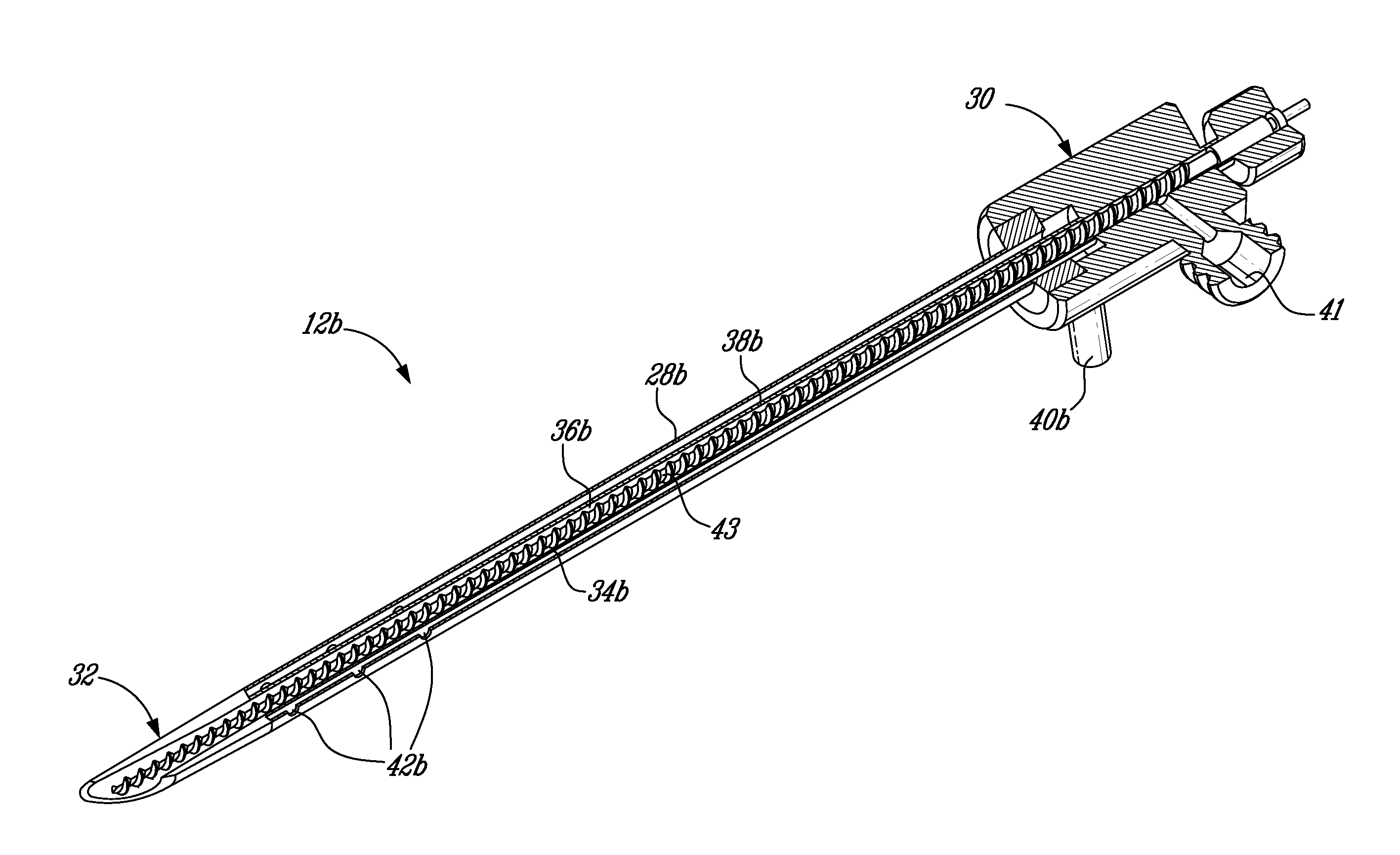
The present invention relates to the medical field, in particular relates to the practice of percutaneous vertebroplasty where a pair of syringes in the distal extreme of a lengthened hydraulic device, are united by a camera of intermediate connection of larger diameter (pressure exerting body) or modified inverted syringe tube with a bolster, a hydraulic connecting tube of flexible material that transmits the pressure of the smaller diameter manual or impulsion syringe in the proximal extreme of the device toward the intermediate cylindrical larger diameter camera (pressure exerting body), this camera is in an inverted position with regard to the first syringe (fluid control), this intermediate camera has a moving piston longitudinal to the axis of the cylinder that is controlled with the first syringe (manual) and in cooperation with the atmospheric pressure. The injecting syringe loaded with bone cement is coupled with the bolster of the body of pressure, and to the needle that drives the cement toward the interior of the bone. The intermediate camera (pressure exerting body) together with the hydraulic tube and the manual syringe form a hydraulic press system (F / A=f / a) that allows to increase in a potential way the pressure exerted in the first syringe and to make the injection of polymethylmethacrylate (PMMA) at an approximate distance of 1.0 m to 1.5 m.
Owner:DEPUY SYNTHES PROD INC
Bone Cement And Methods Of Use Thereof
InactiveUS20070032567A1High unit weightImprove wettabilityImpression capsSurgical adhesivesPolymer scienceBiocompatibility Testing
A bone cement comprising an acrylic polymer mixture. The cement is characterized in that it achieves a viscosity of at least 500 Pascal-second within 180 seconds following initiation of mixing of a monomer component and a polymer component and characterized by sufficient biocompatibility to permit in-vivo use.
Owner:DEPUY SYNTHES PROD INC
Bone treatment systems and methods for introducing an abrading structure to abrade bone
ActiveUS7682378B2Increase the sectionIncrease spacingJoint implantsExcision instrumentsMedicineProphylactic treatment
The invention provides instruments and methods for prophylactic treatment of an osteoporotic vertebral body or for treating a vertebral compression fracture (VCF). In one exemplary method, a probe system uses a high speed rotational elastomeric cutter having an optional expandable abrasive surface for abrading or cutting at least one path or region within vertebral cancellous bone. Irrigation and aspiration sources are included in the probe system for removing abraded bone debris. In one embodiment, the high speed rotational abrader uses a tissue-selective abrading surface that abrades or cuts bone but does not cut soft tissue. In another embodiment, an expandable abrading surface allows the treatment of bone with low pressures to create paths or spaces without explosive expansion forces known in prior art balloon procedures that are designed to crush and compact cancellous bone in a vertebra. After the creation of a path or space, an in-situ hardenable bone cement volume is introduced into each path or space to support the vertebra.
Owner:DFINE INC
Apparatus and method for injecting fluent material at a distracted tissue site
A system and method is provided for distracting opposite surfaces from the interior of a bone, such as a vertebral body. A working channel cannula provides a working channel through which an inserter and an injection cannula can simultaneously pass. The inserter transports a plurality of wafers into the interior of the bone to form a load-bearing stack bearing against the opposite surfaces. The injection cannula is used to inject a fluent material into and / or around the stack. In certain embodiments, the fluent material is a load-bearing or hardenable material, such as bone cement. In other embodiments, the fluent material can be a BMP, HAP, or other osteo-inductive, osteo-conductive, or pharmaceutical compositions. A syringe containing the fluent material is engaged to the injection cannula and is operable to inject the fluent material into the vertebral body under controlled pressure.
Owner:SPINEWAVE
Apparatus and methods for treating bone
ActiveUS20070055274A1Increased radialIncreasing diameter of coilInternal osteosythesisSpinal implantsFiberBobbin
Implants and methods for bone treatment, preferably minimally invasive treatment, including repositioning of vertebrae may comprise insertion of a bobbin having a wire, string, thread or band, coiled around the bobbin. During coiling, the diameter of the bobbing / band complex may increase. Such increase in diameter can push against the inner side of the endplates of the vertebral body, and augment the vertebral body to its original height. The implant may also take the form of a coiled sleeve which when inserted into the vertebral body is uncoiled. The force of the uncoiling sleeve pushes against the inner side o the endplates of the vertebral body, restoring the vertebral body to its original height. The implant may also take the form of fibrous masses comprised of a thread or other relatively thin structure, for example a fiber or strand, of any biocompatible material having desired characteristics, for example a shape memory alloy, titanium, stainless steel, another metal or metal alloy, a ceramic, a composite or any combination thereof. The, strand, thread or other fiber may be coiled, woven, matted, tangled or otherwise formed into a wool-like mass or body having a desired configuration. Expansion of the expandable member within the vertebral body or other bone may reposition the fractured bone to a desired height and augment the bone to maintain the desired height. A bone cement or other filler can be added to further treat and stabilize the vertebral body or other bone.
Owner:SYNTHES USA
Bone treatment systems and methods
InactiveUS20080091207A1Joint implantsSurgical instruments for heatingFilling materialsCancellous bone
Apparatuses, methods, and kits for treating bone (e.g., vertebral compression fractures) includes a shaft with a working end that can be bent into an arc-shaped working end. The shaft can be introduced into a bone (e.g., introduced through a sleeve into cancellous bone) and carries a cutting element that can be actuated across the arc-shaped working end to cut a plane in cancellous bone, which can optionally be filled with a bone fill material (e.g., bone cement).
Owner:DFINE INC
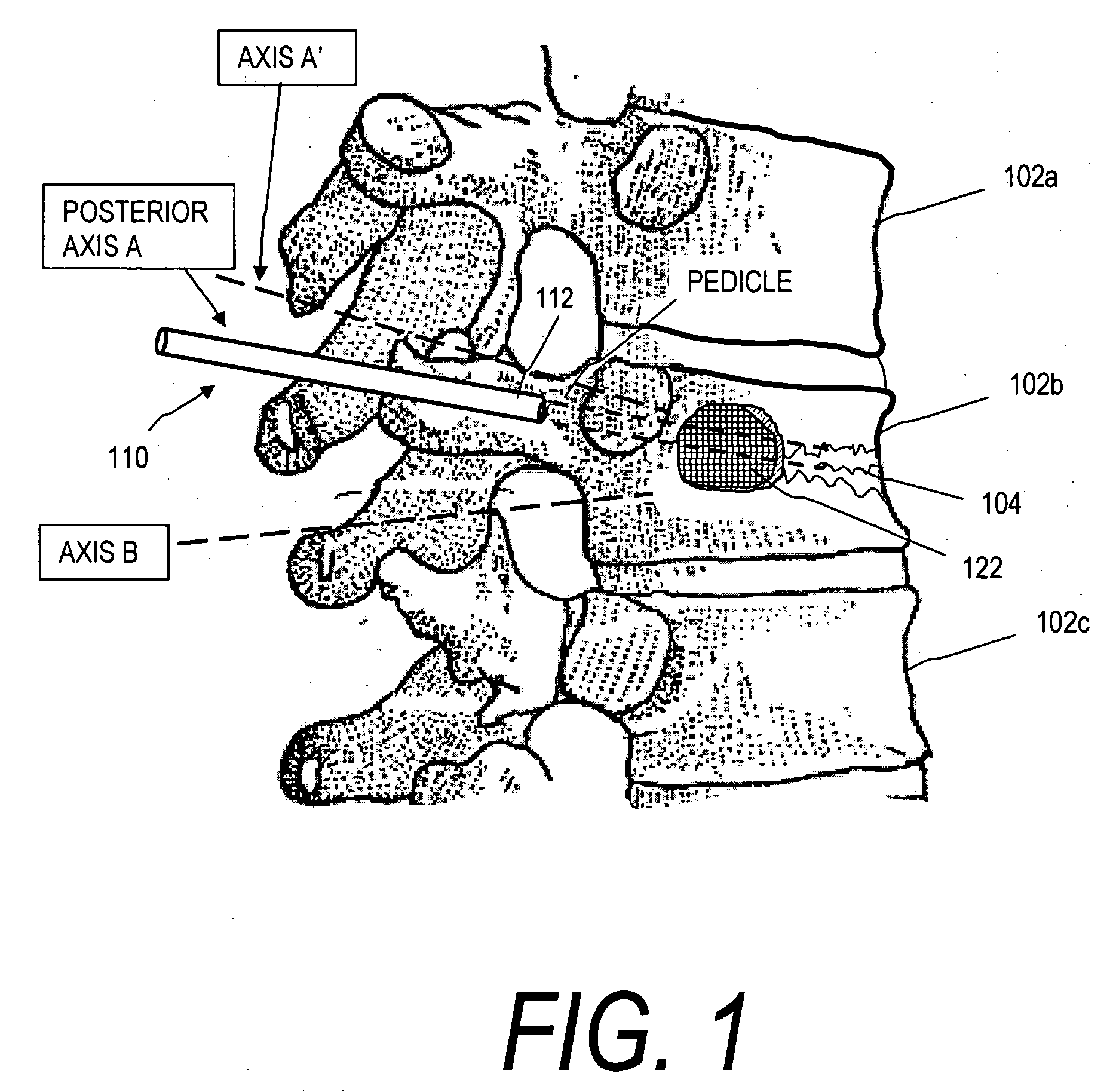
Axial spinal implant and method and apparatus for implanting an axial spinal implant within the vertebrae of the spine
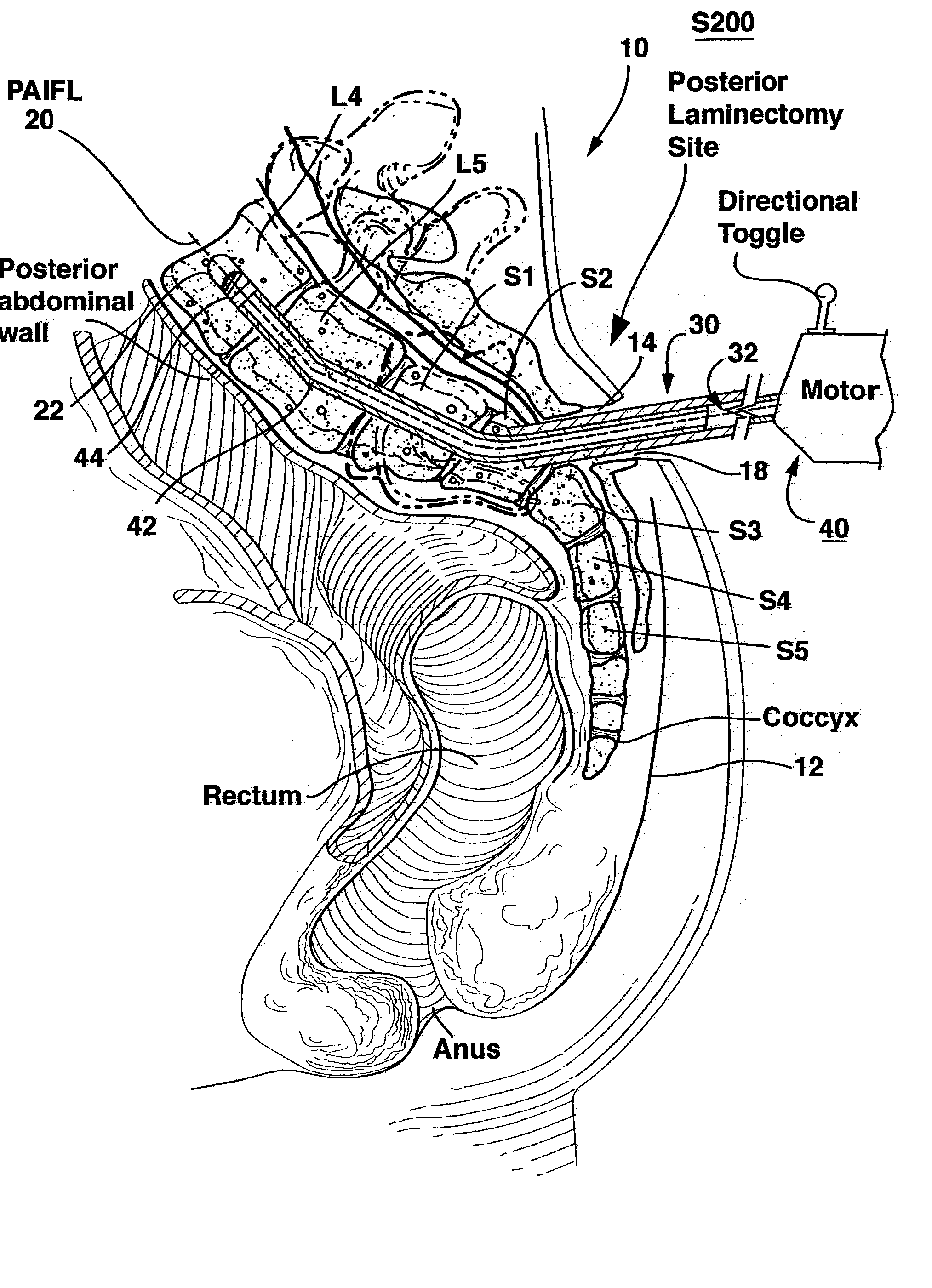
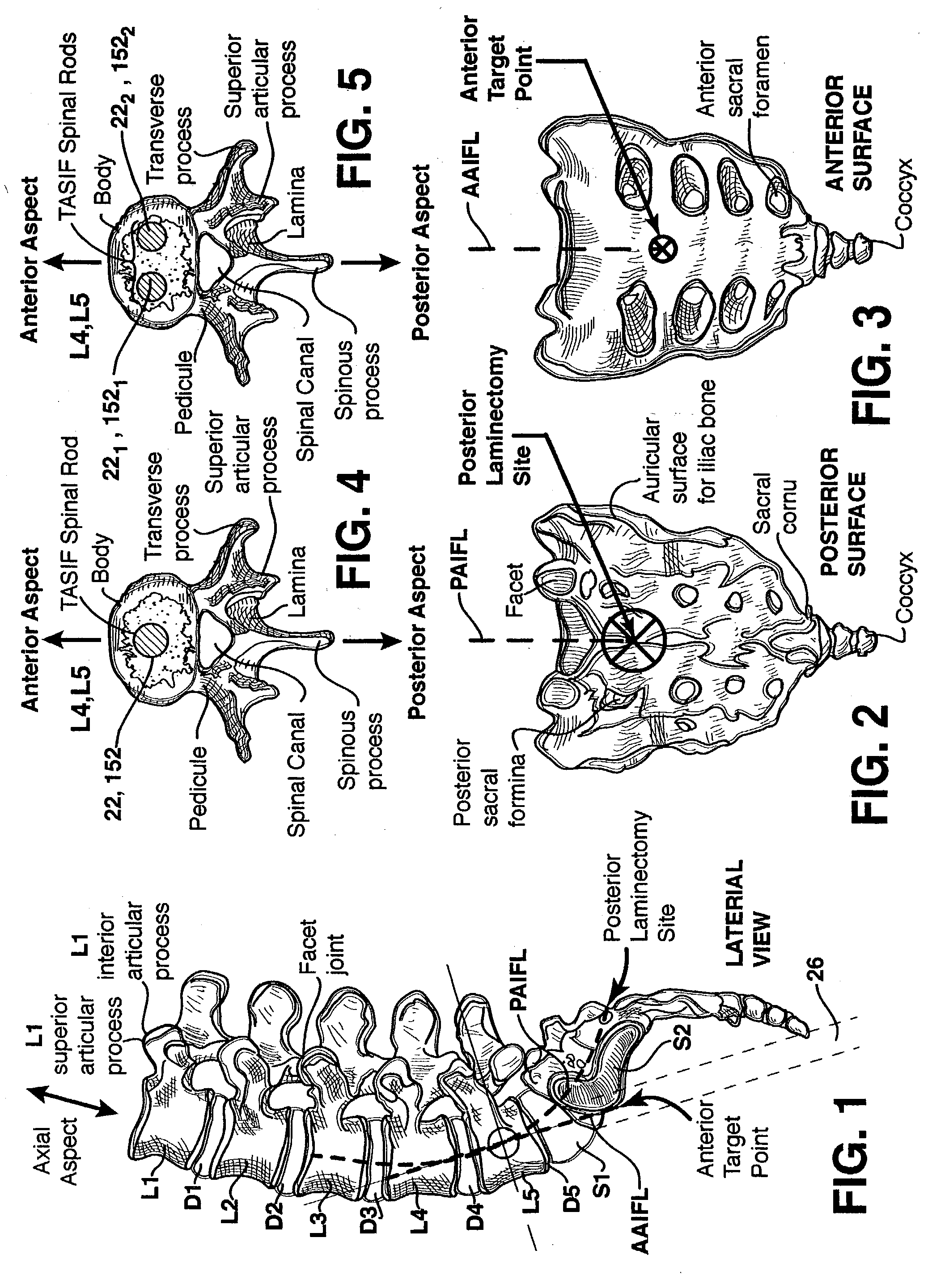
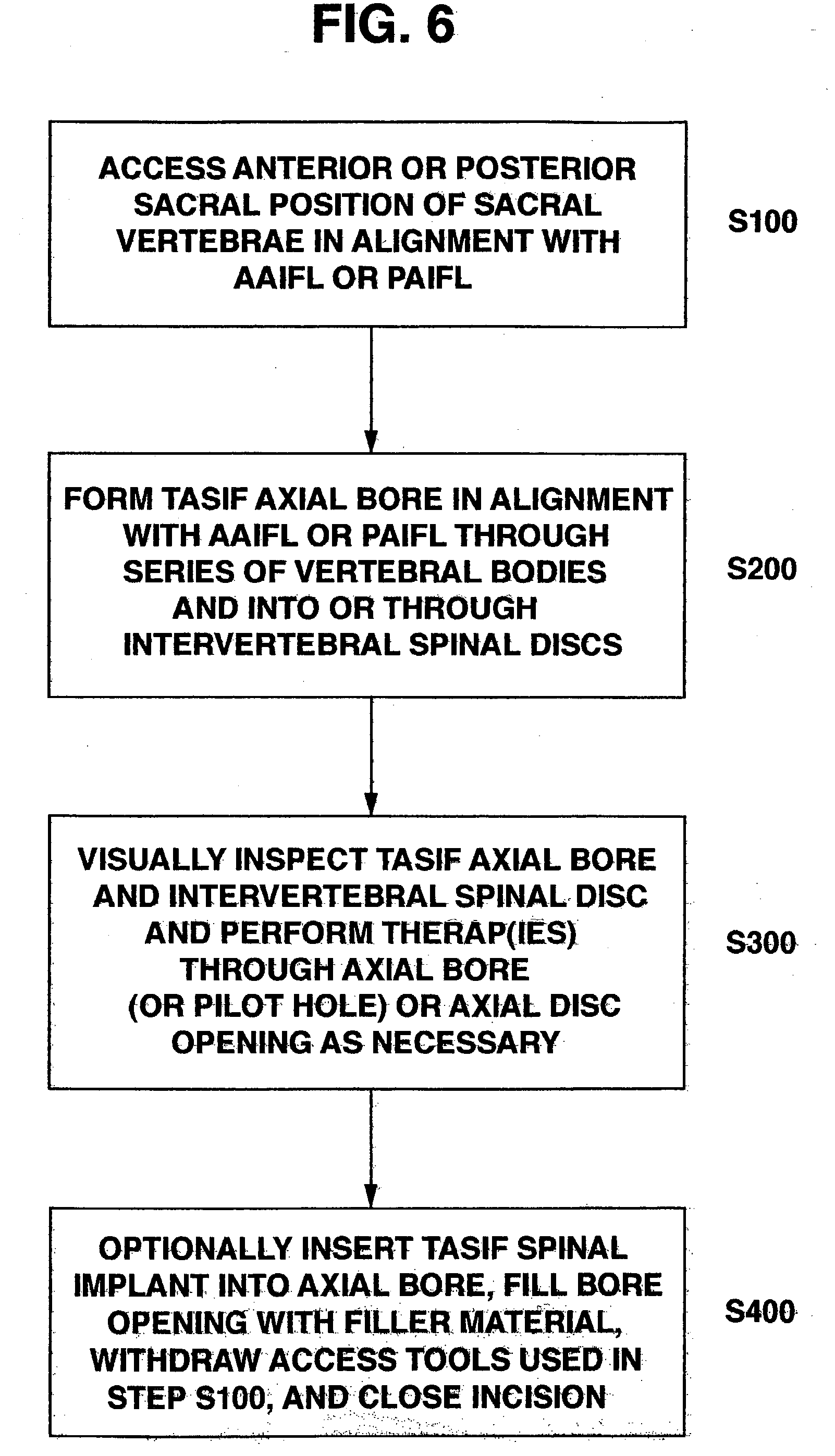
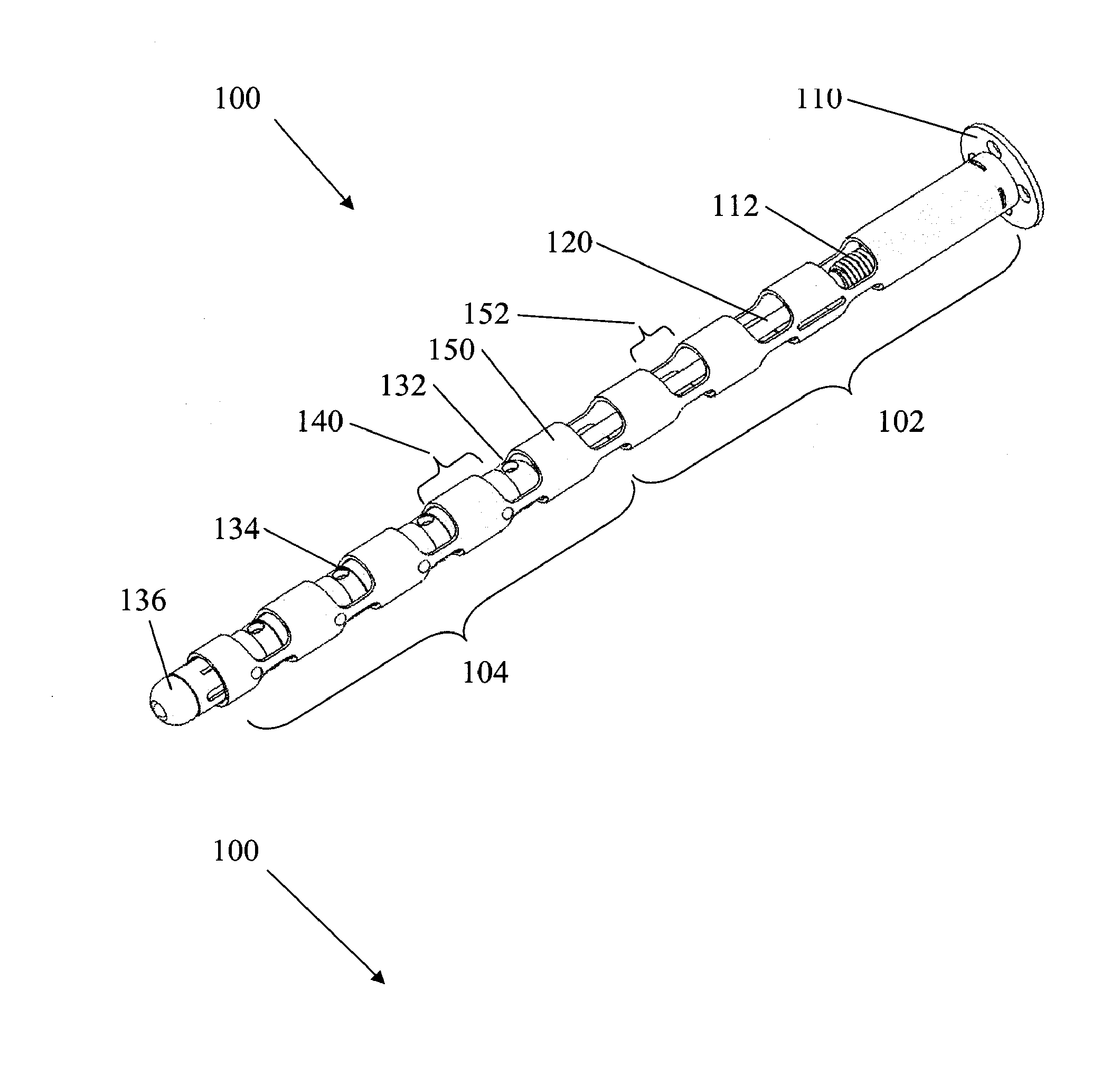
Spinal implants for fusing and / or stabilizing spinal vertebrae and methods and apparatus for implanting one or more of such spinal implants axially within one or more axial bore within vertebral bodies in alignment with a visualized, trans-sacral axial instrumentation / fusion (TASIF) line in a minimally invasive, low trauma, manner are disclosed. Attachment mechanisms are provided that attach or affix or force the preformed spinal implants or rods to or against the vertebral bone along the full length of a TASIF axial bore or bores or pilot holes or at the cephalad end and / or caudal end of the TASIF axial bore or bores or pilot holes. The engagement of the vertebral body is either an active engagement upon implantation of the spinal implant into the TASIF axial bore or a passive engagement of the external surface configuration with the vertebral bone caused by bone growth about the external surface configuration. A plurality of such spinal implants can be inserted axially in the same TASIF axial bore or pilot hole or separately in a plurality of TASIF axial bores or pilot holes that extend axially and in a side-by-side relation through the vertebrae and discs, if present, between the vertebrae. Discectomies and / or vertebroblasty can be performed through the TASIF axial bore or bores or pilot holes prior to insertion of the spinal implants. Vertebroblasty is a procedure for augmentation of collapsed vertebral bodies by pumped-in materials, e.g., bone cement or bone growth materials. Materials or devices can also be delivered into the disc space to separate the adjoining vertebrae and / or into damaged vertebral bodies or to strengthen them.
Owner:TRANSI
Minimally Invasive Actuable Bone Fixation Devices Having a Retractable Interdigitation Process
InactiveUS20060264951A1Low weight to volumeReduce traumaSuture equipmentsInternal osteosythesisSurgical siteBone fixation devices
An apparatus for bone reinforcement, fixation and treatment of diseased or fractured bones including a supporting structure optionally coated with therapeutic agent is provided. The supporting structure or device may be collapsible upon deployment at the surgical site, and include fixation features such as anchors to securely position in place once deployed. Bone cement or other material may be provided to alternatively secure the positioned supporting structure for treatment. The bone fixation device disclosed also comprising: a first sleeve having a retractable interdigitation process at a location along its length adapted to engage a bone; and a second sleeve sized adapted to activate the interdigitation process of the first sleeve.
Owner:SONOMA ORTHOPEDIC PROD INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com