Patents
Literature
92 results about "Cardiac allograft" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cardiac allograft vasculopathy (CAV) is a common long-term complication of heart transplantation affecting up to half of people within 10 years. It arises when the blood vessels supplying the transplanted heart gradually narrow and restrict its blood flow, subsequently leading to impairment...
Stent systems and methods for spine treatment
InactiveUS20060100706A1Prevent subsidenceRestore body heightInternal osteosythesisSpinal implantsSpinal columnCardiac allograft
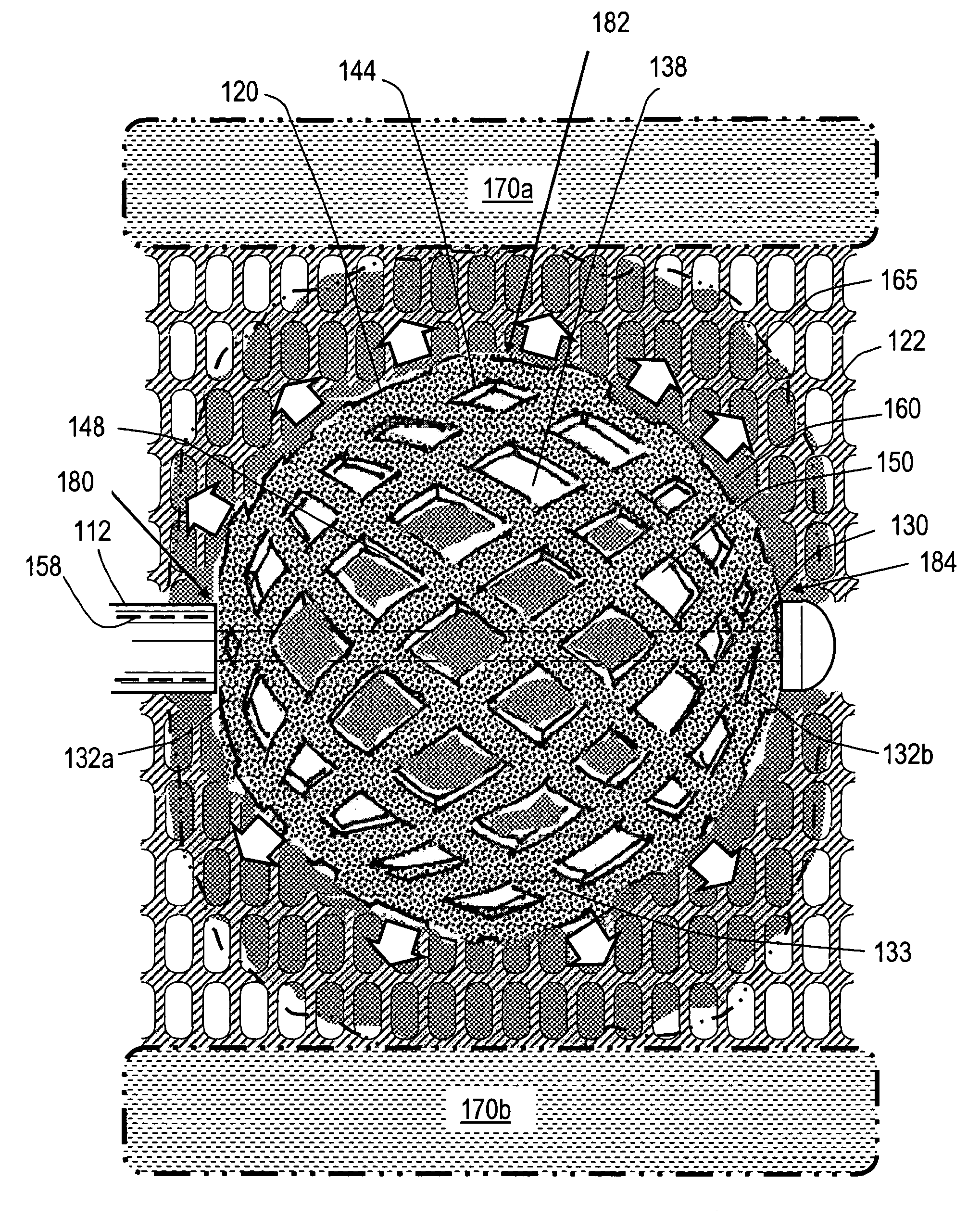
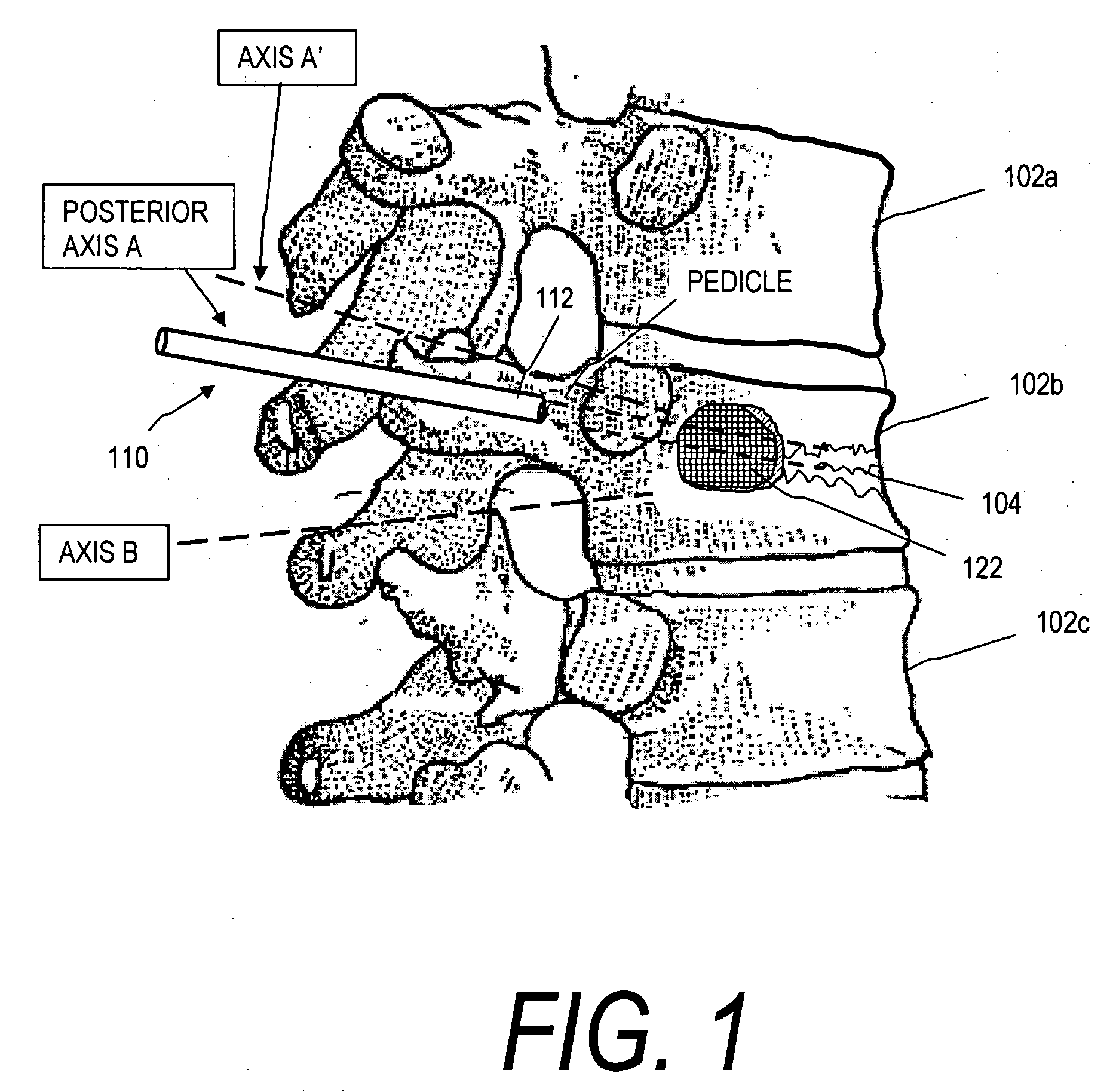
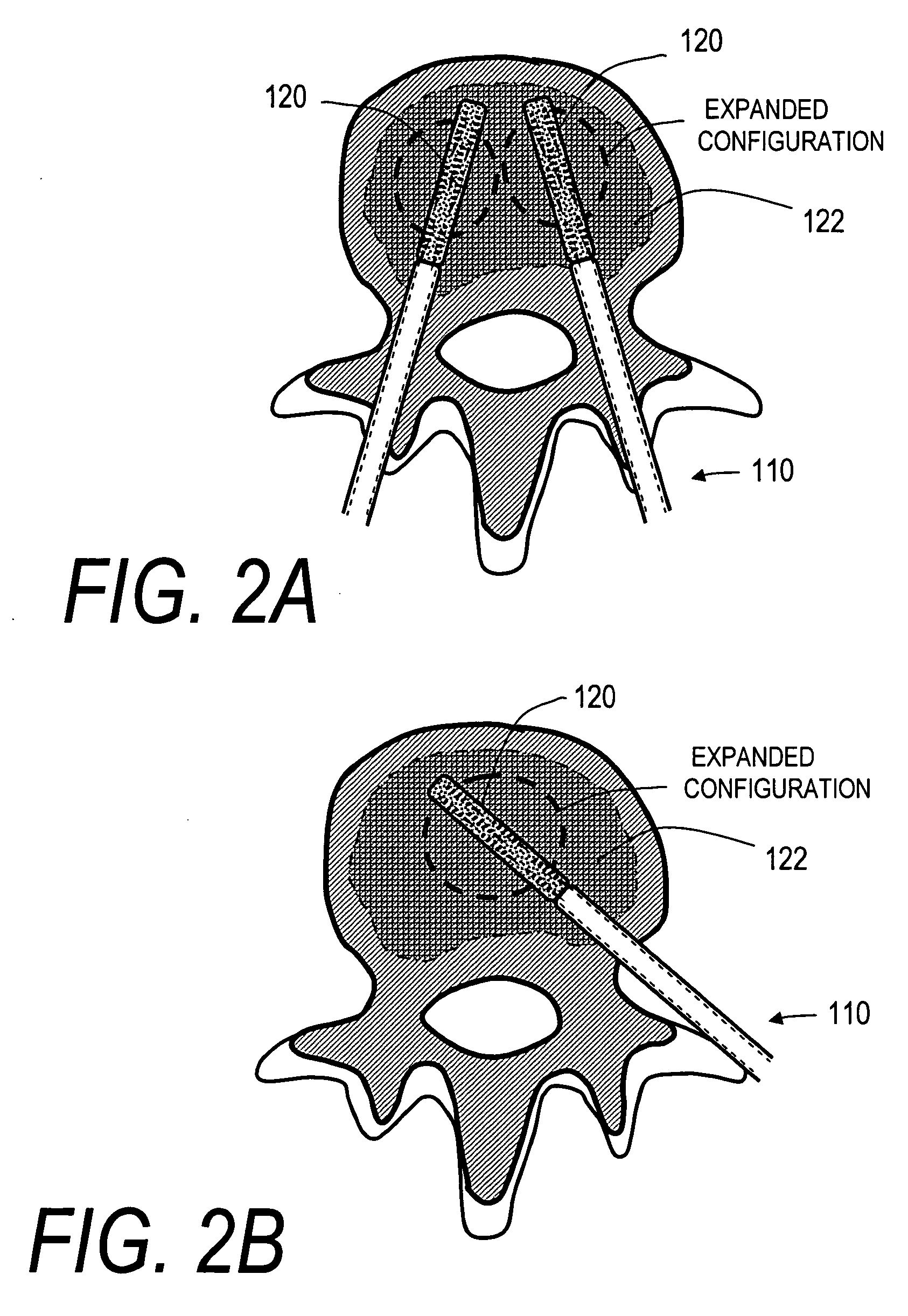
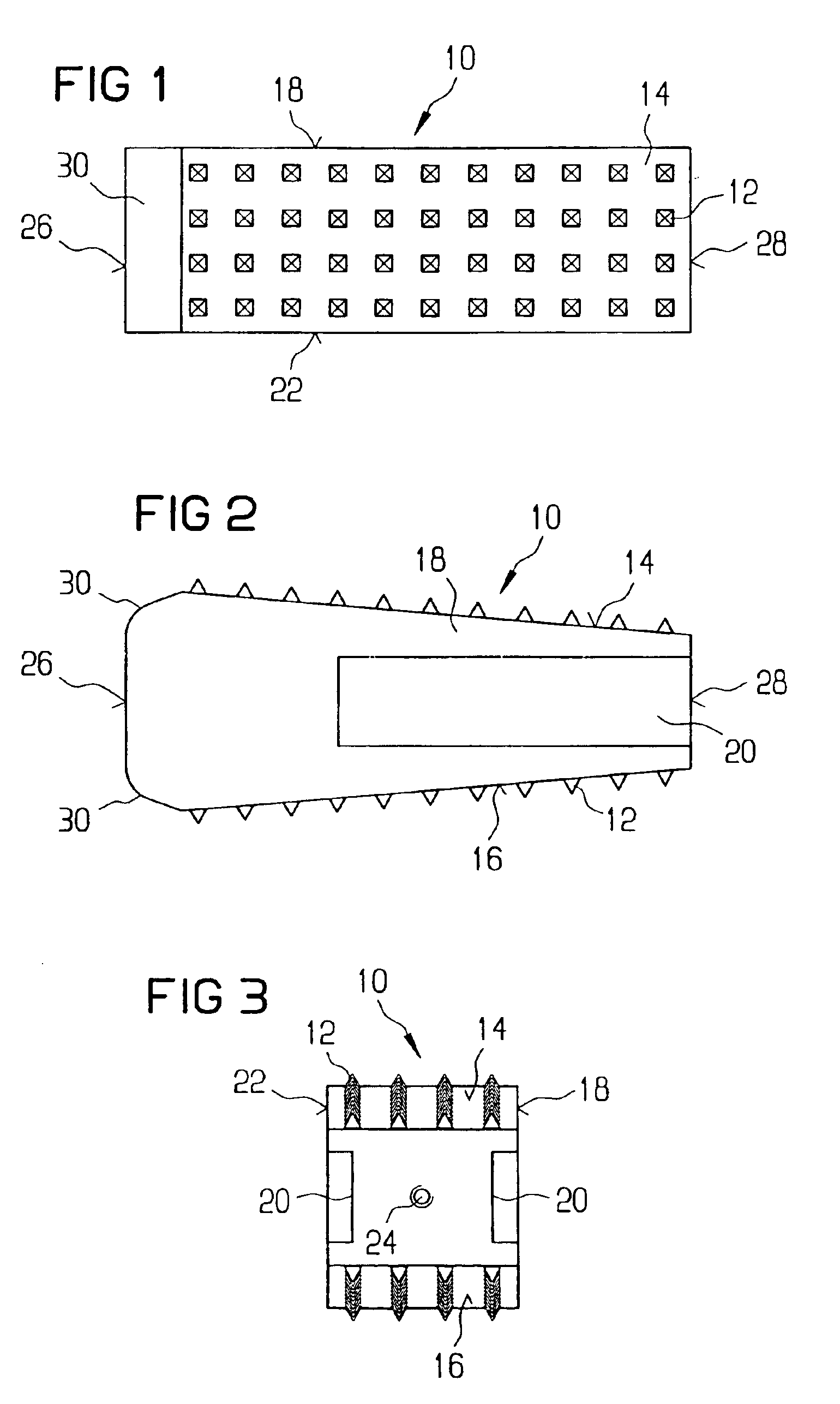
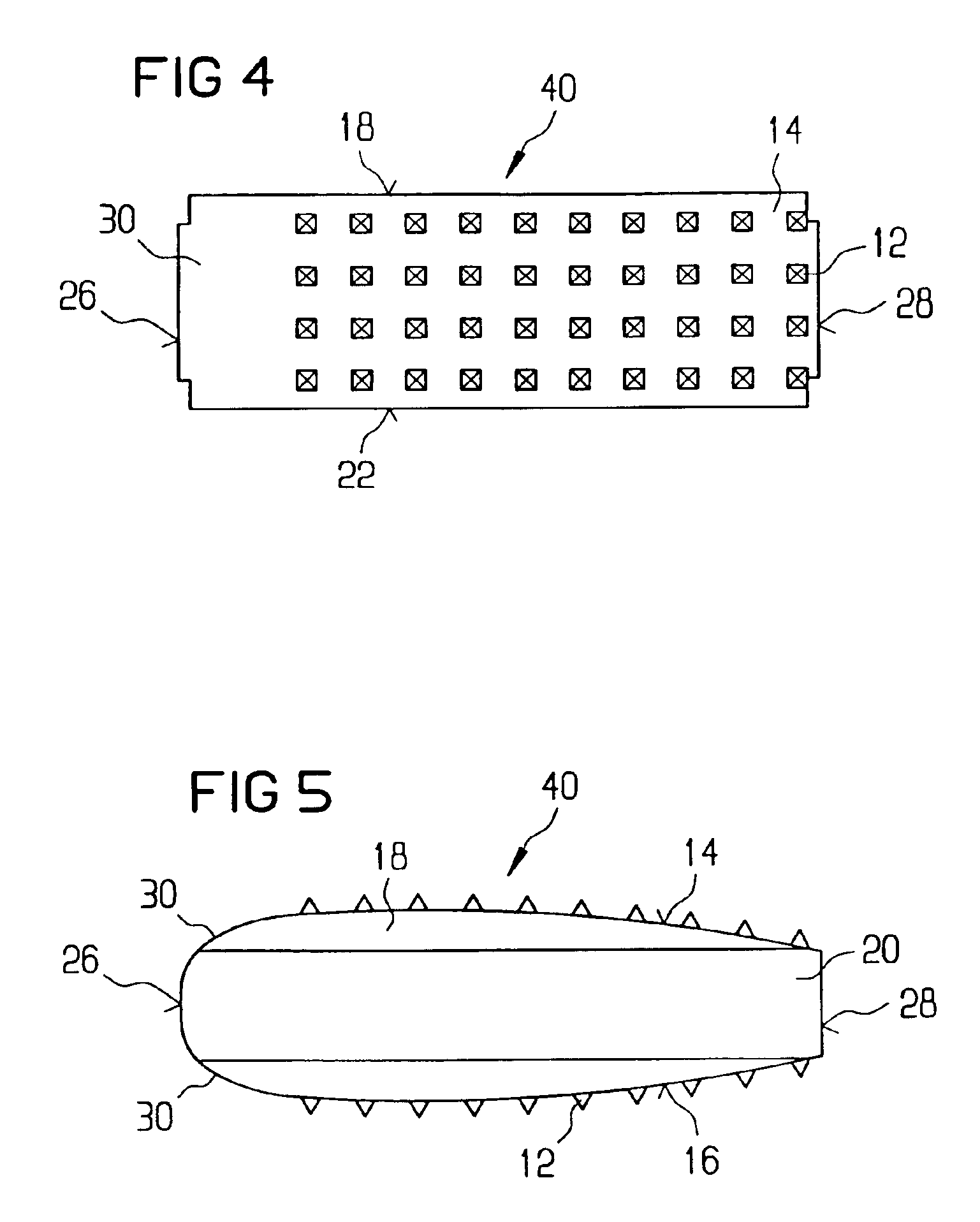
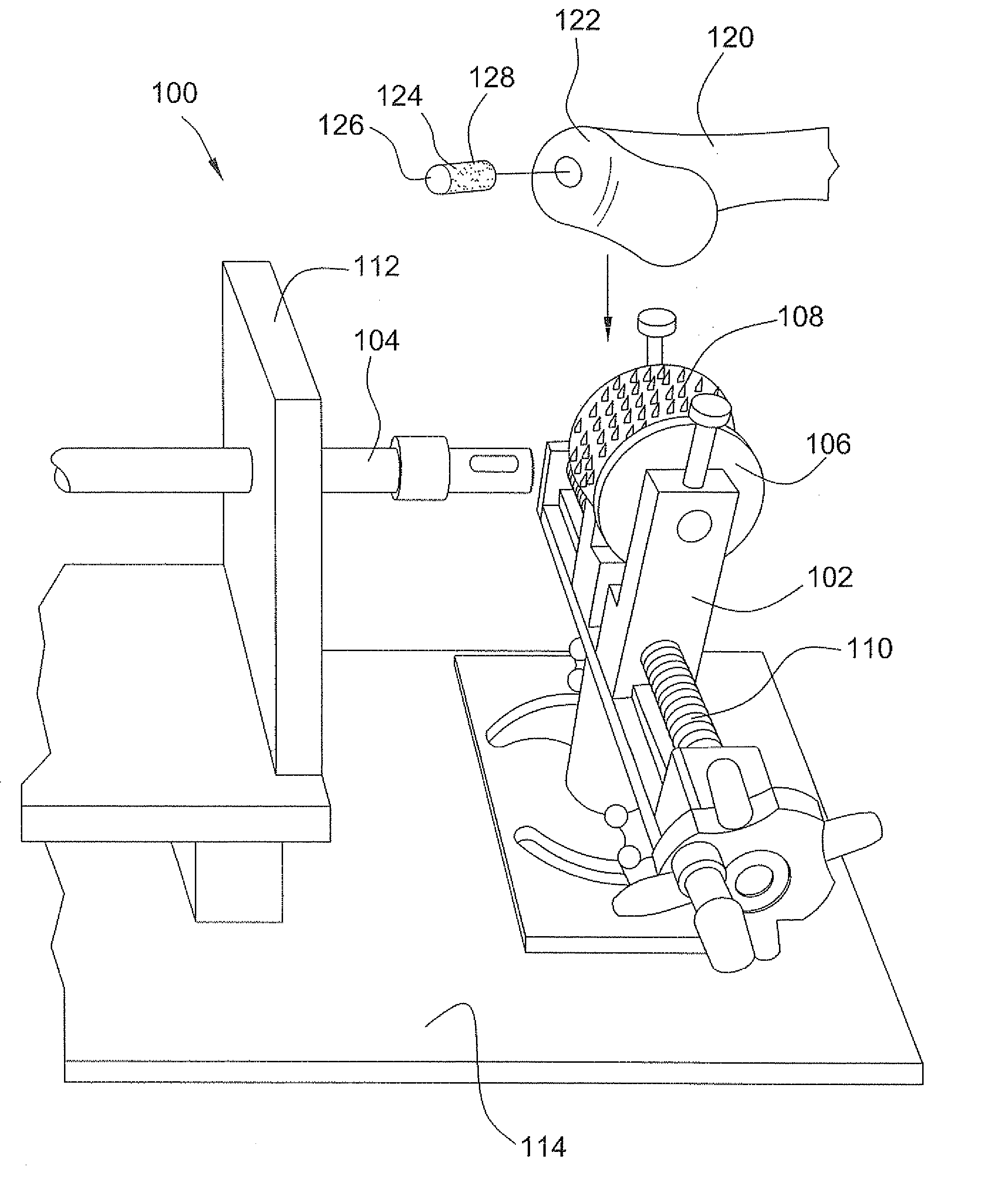
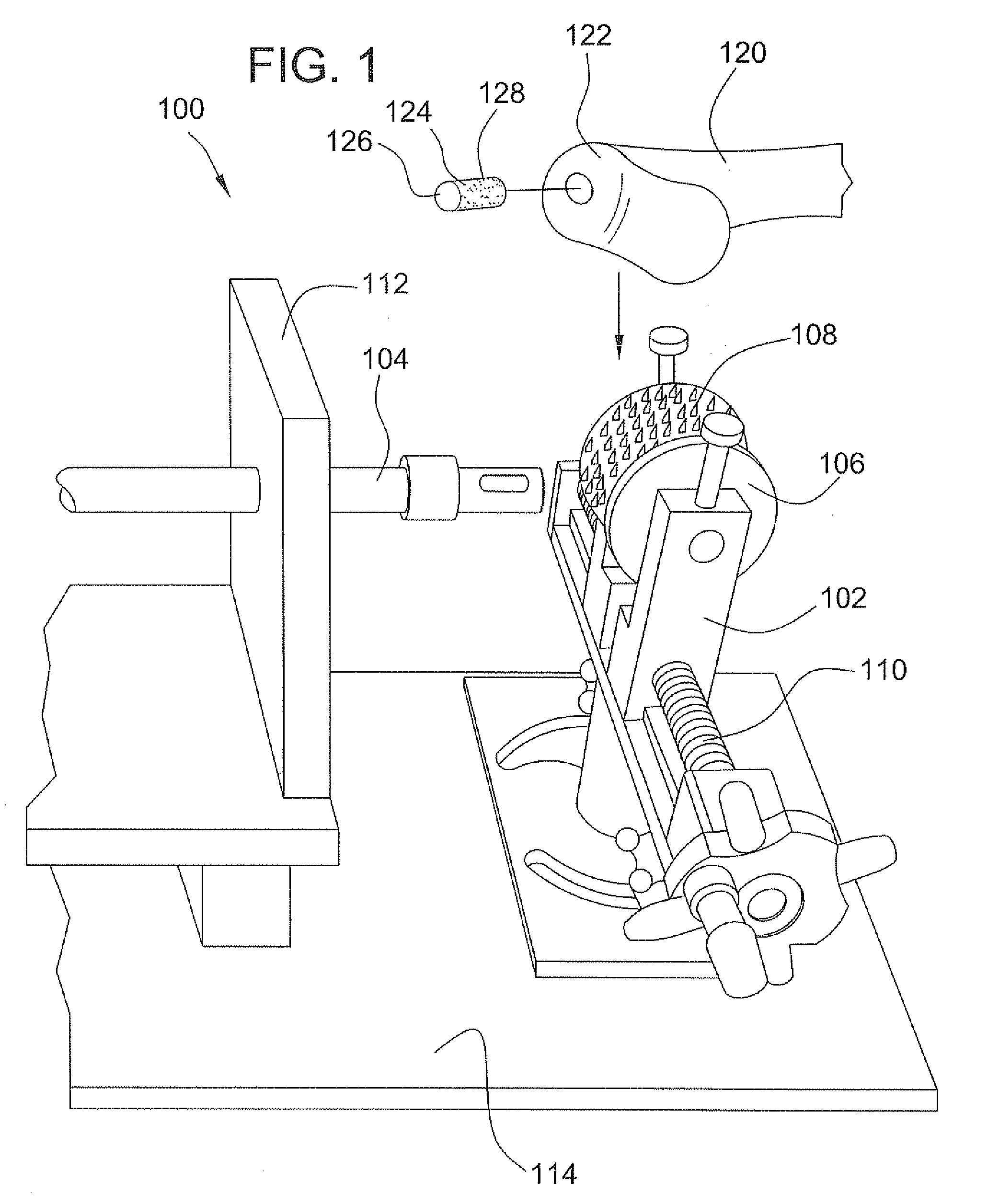
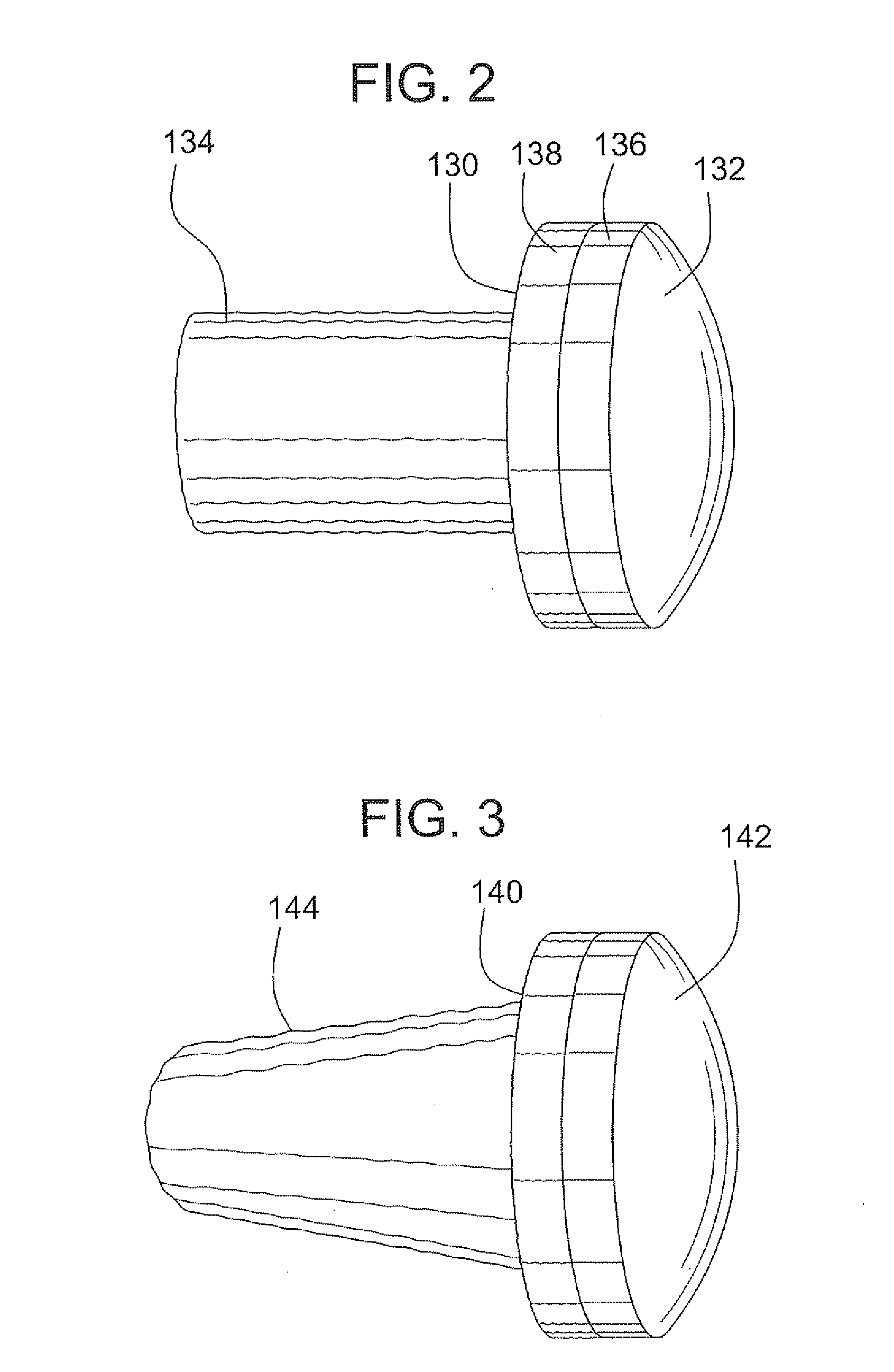
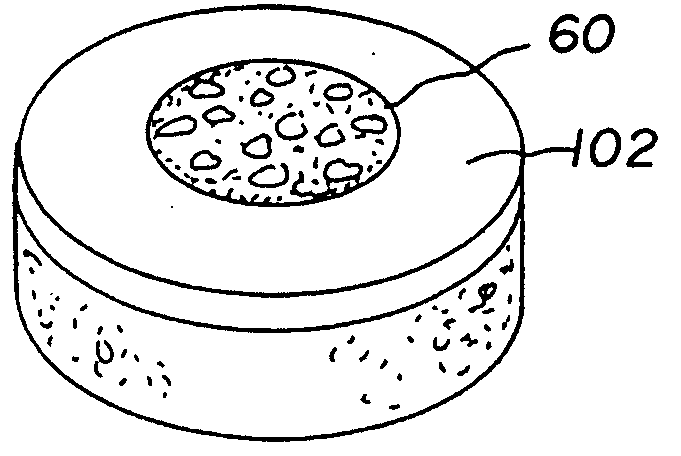
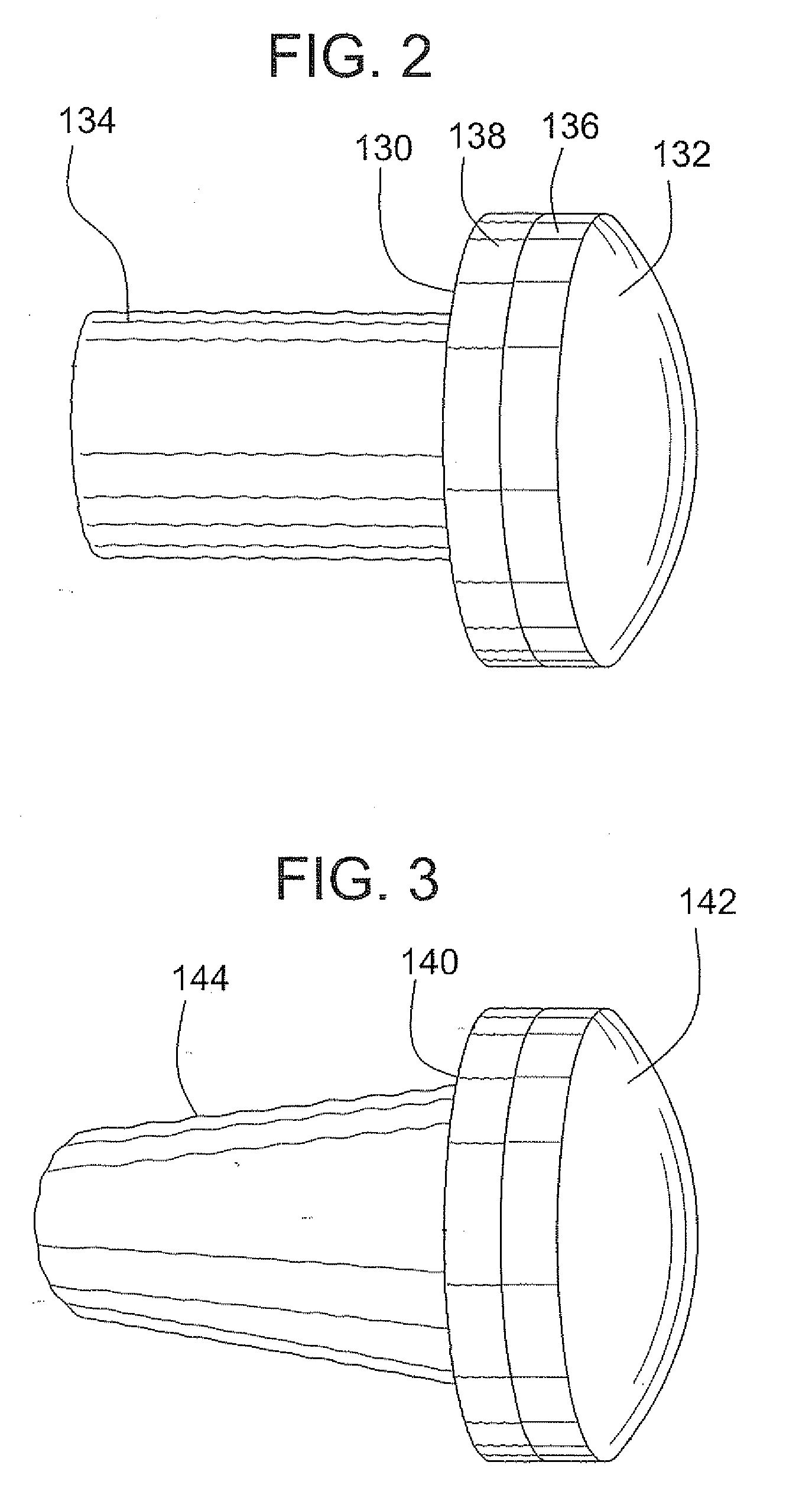
Stent systems and methods for expanding and deploying stents in hard tissue such as bone, more particularly within a vertebral body. One exemplary method includes using a stent body that is coupled to a high speed rotational motor with the stent expandable and detachable from an introducer working end. In one embodiment, the stent is a deformable metal body with zig-zag type struts in an expanded configuration that carries diamond cutting particles bonded to the strut surfaces. The “spin” stent is rotated at high rpm's to remove cancellous bone from the deployment site together with irrigation and aspiration at the end of the probe that carries the stent. The stent may be expanded asymmetrically, such as with first and second balloons or by using an interior restraint, to apply vertical distraction forces to move apart the cortical endplates and support the vertebra in the distracted condition. The cancellous bone about the expanded stent as well as the interior of the stent can be filled with a bone cement, allograft or other bone graft material. In one method of use, the spin stent is designed and adapted for (i) treating a vertebral compression fracture (VCF) or for (ii) reinforcing an osteoporotic vertebral body.
Owner:DFINE INC
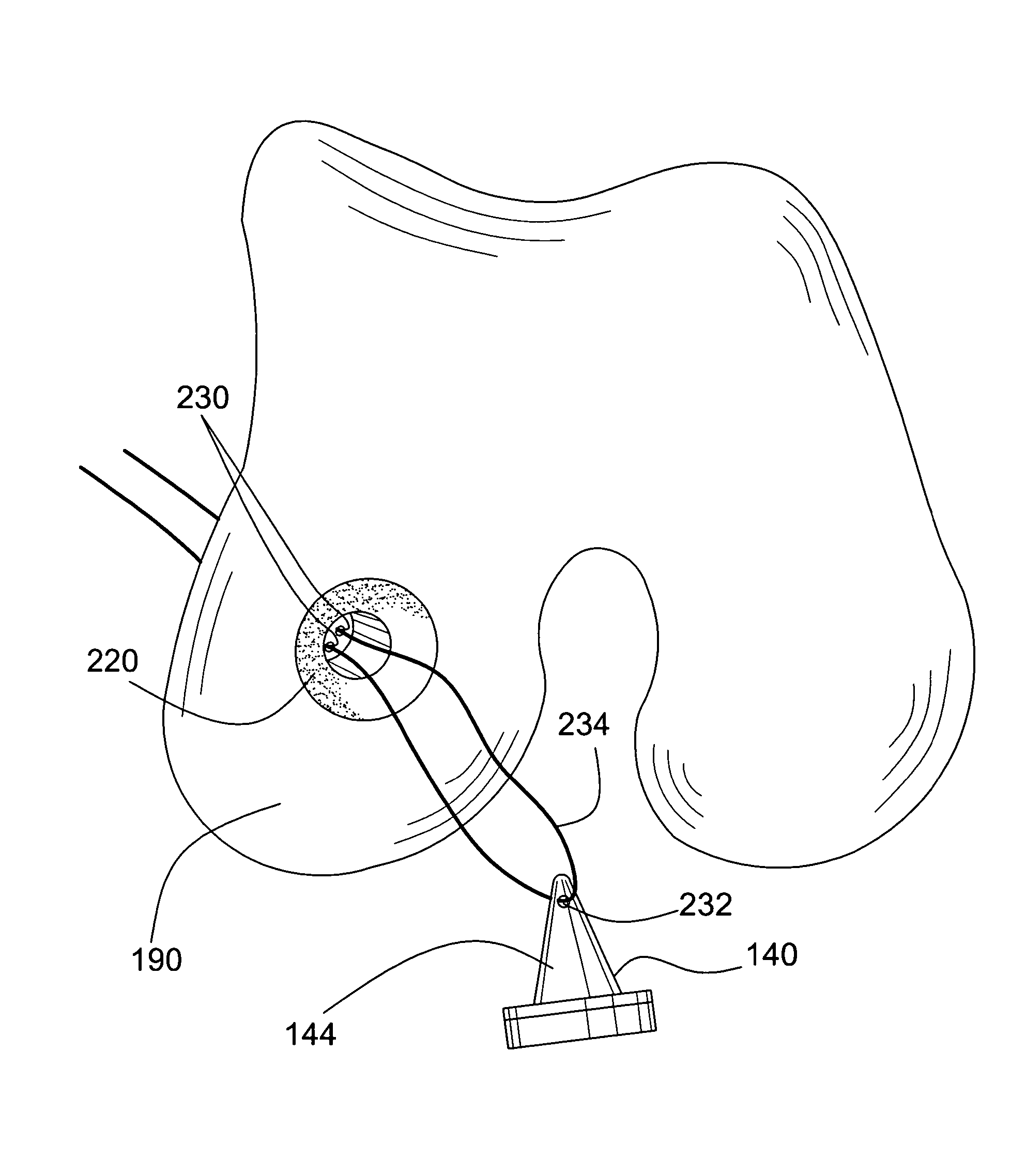
Intervertebral allograft spacer
InactiveUS6986788B2Facilitate implantationEasy to implantBone implantJoint implantsVertebraStress shielding
An allogenic intervertebral implant for fusing vertebrae is disclosed. The implant is a piece of allogenic bone conforming in size and shape with a portion of an end plate of a vertebra. The implant has a wedge-shaped profile to restore disc height and the natural curvature of the spine. The top and bottom surfaces of the implant have a plurality of teeth to resist expulsion and provide initial stability. The implant according to the present invention provides initial stability need for fusion without stress shielding.
Owner:SYNTHES USA
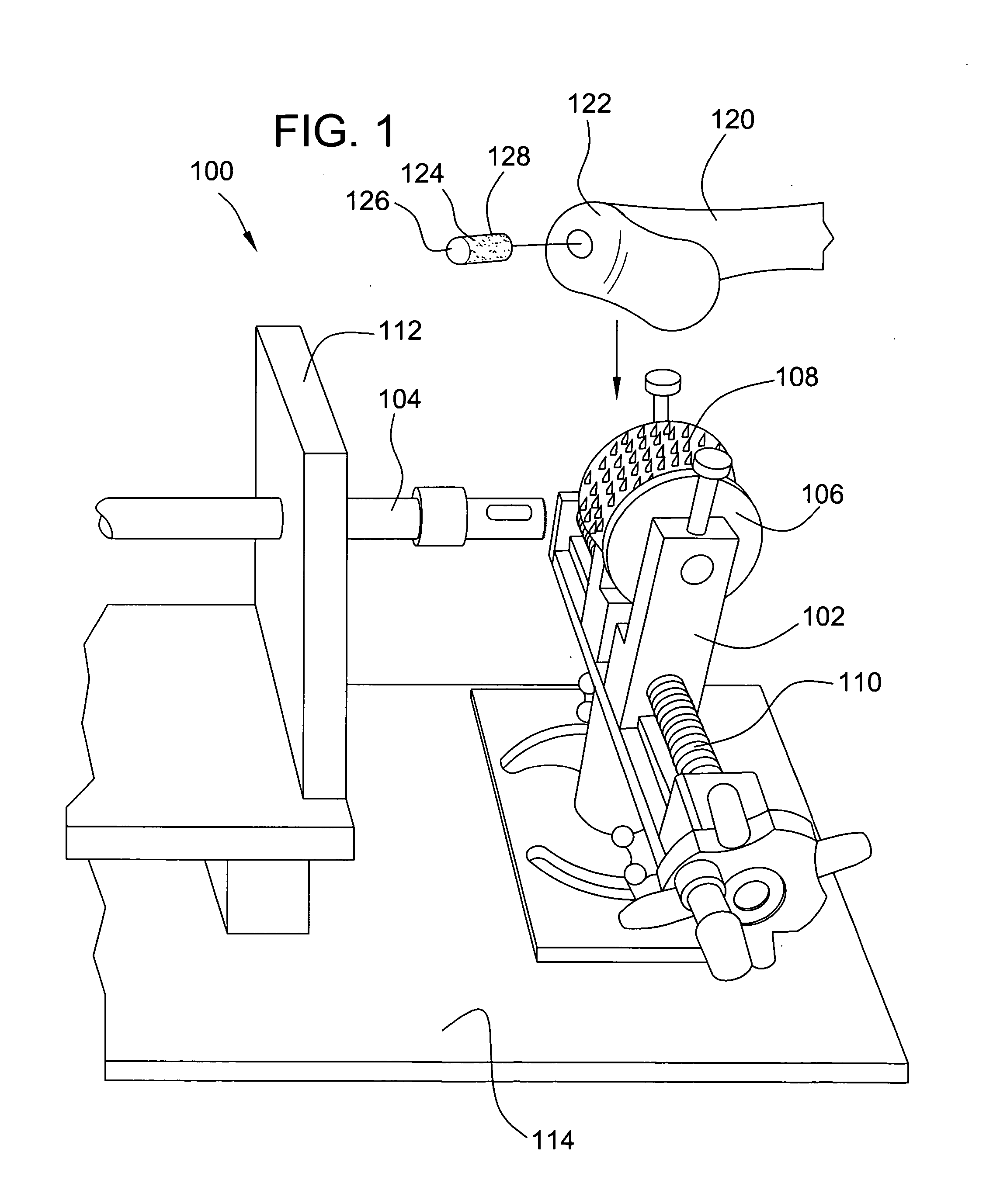
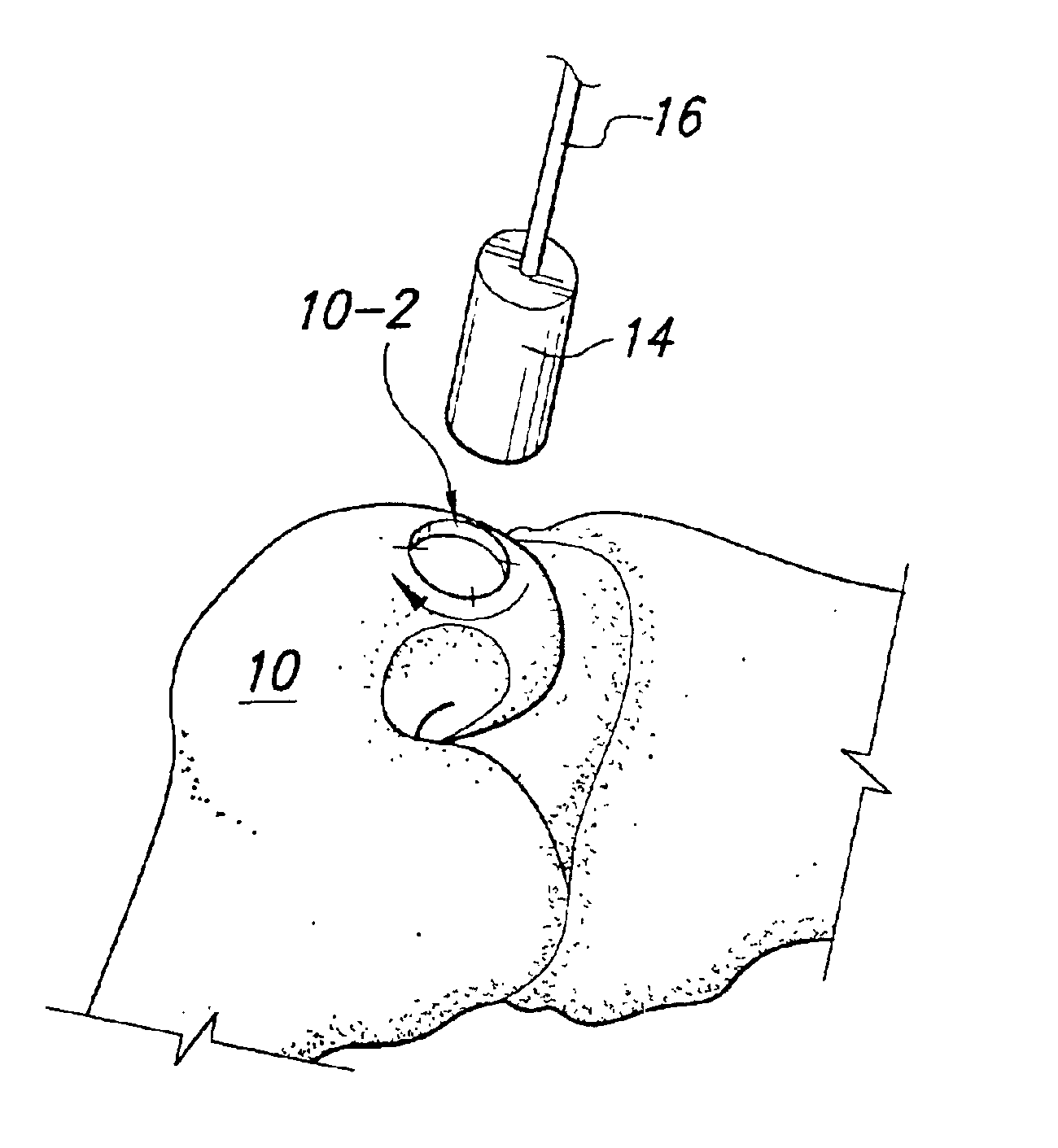
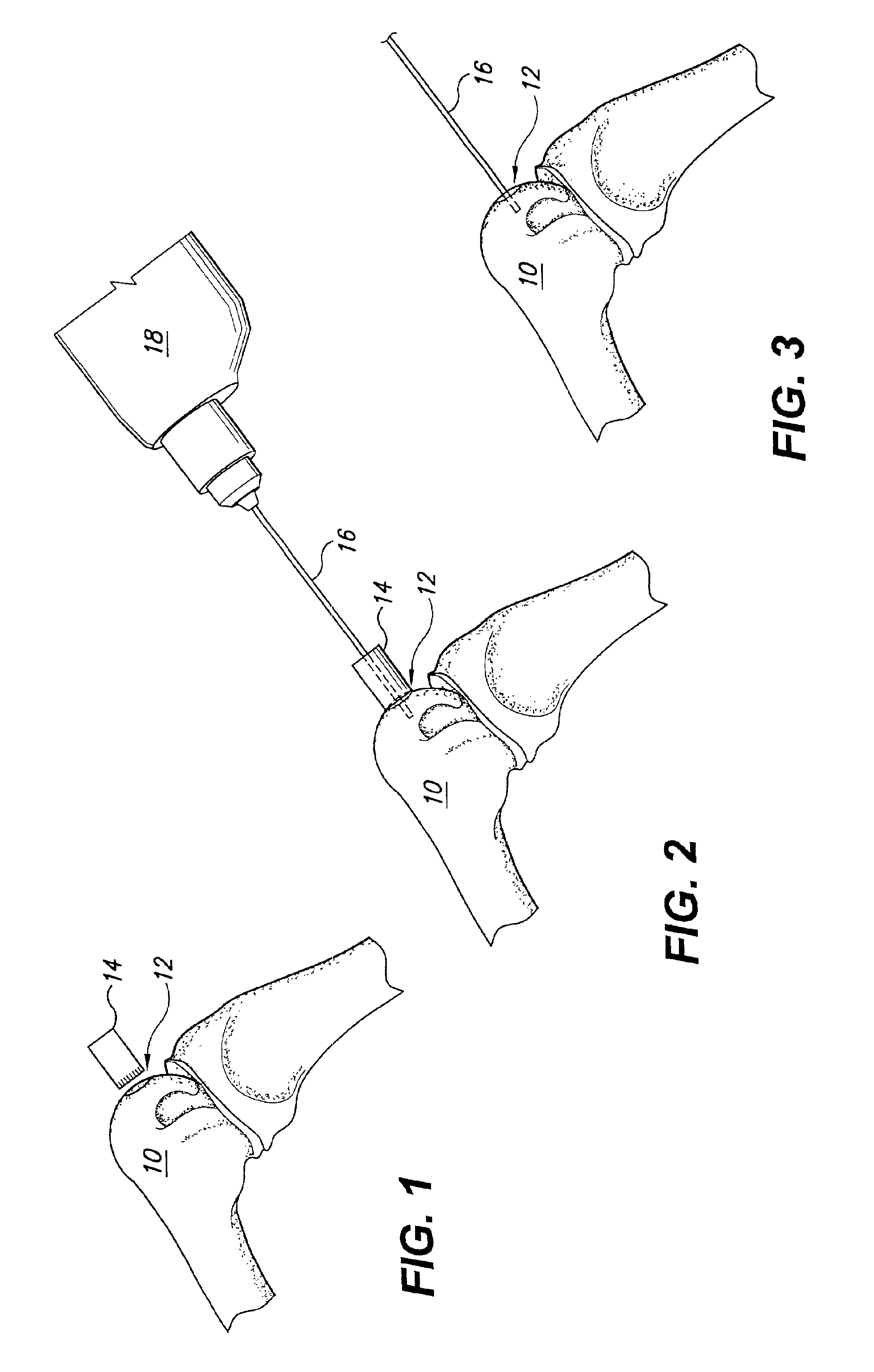
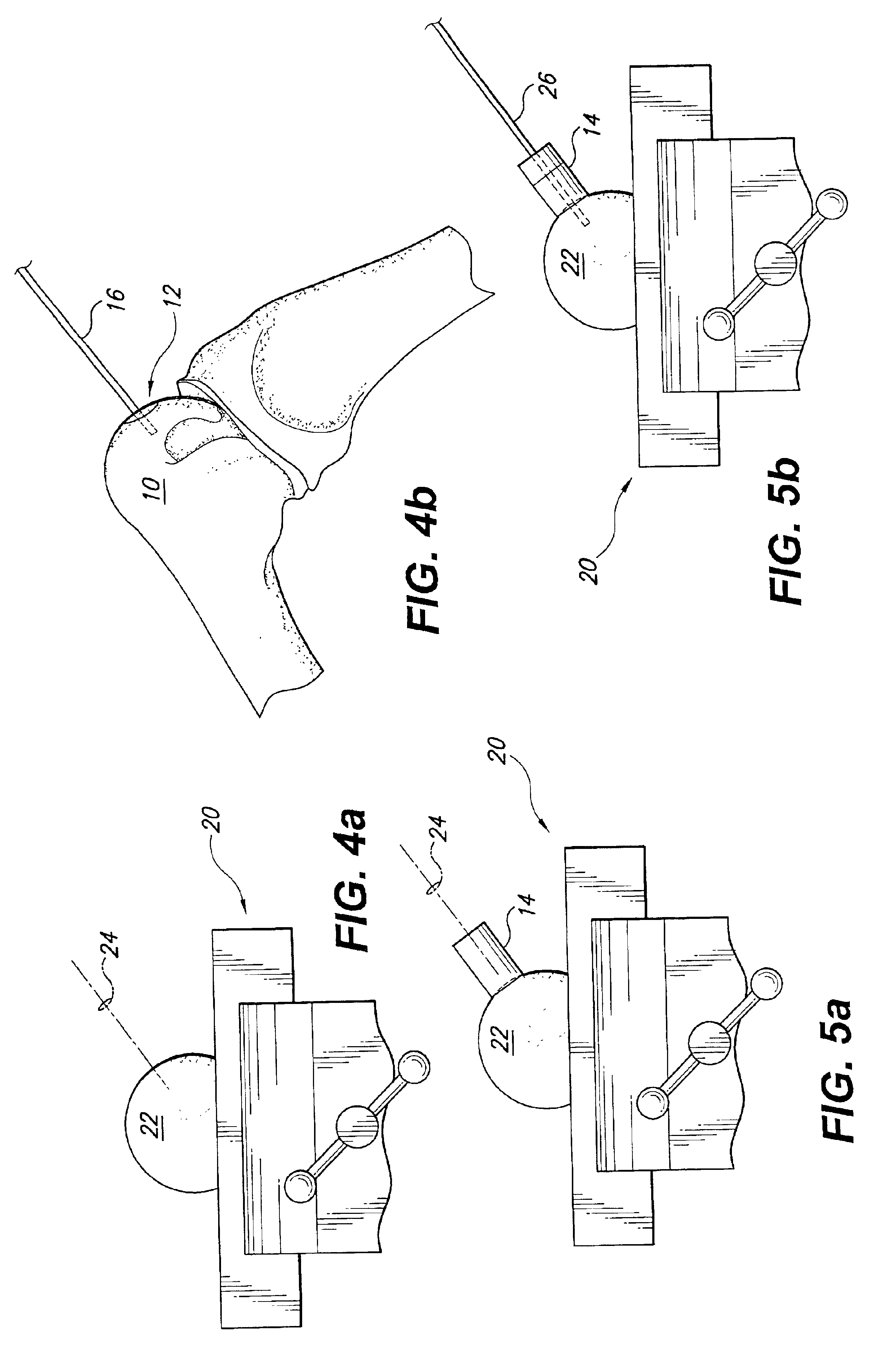
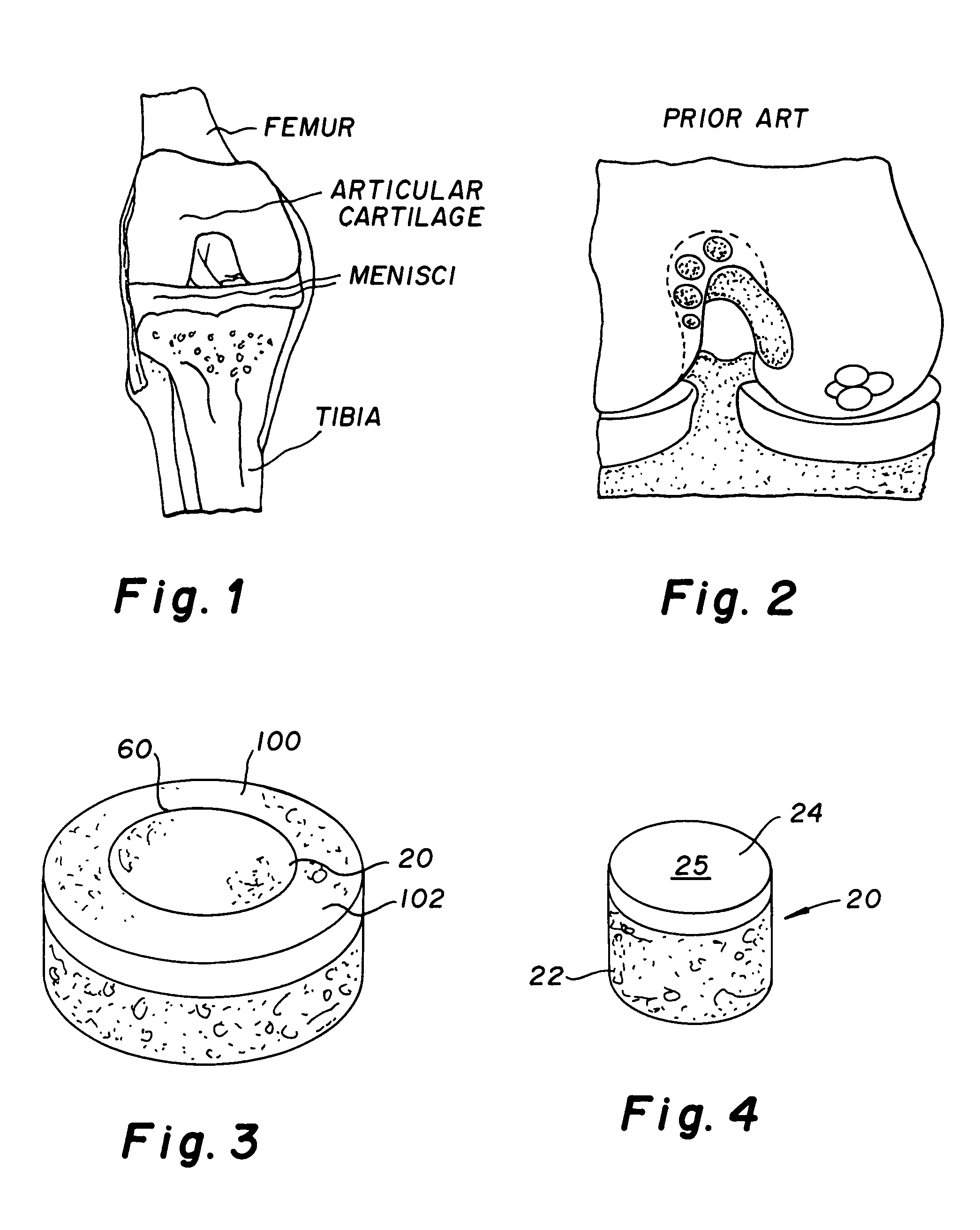
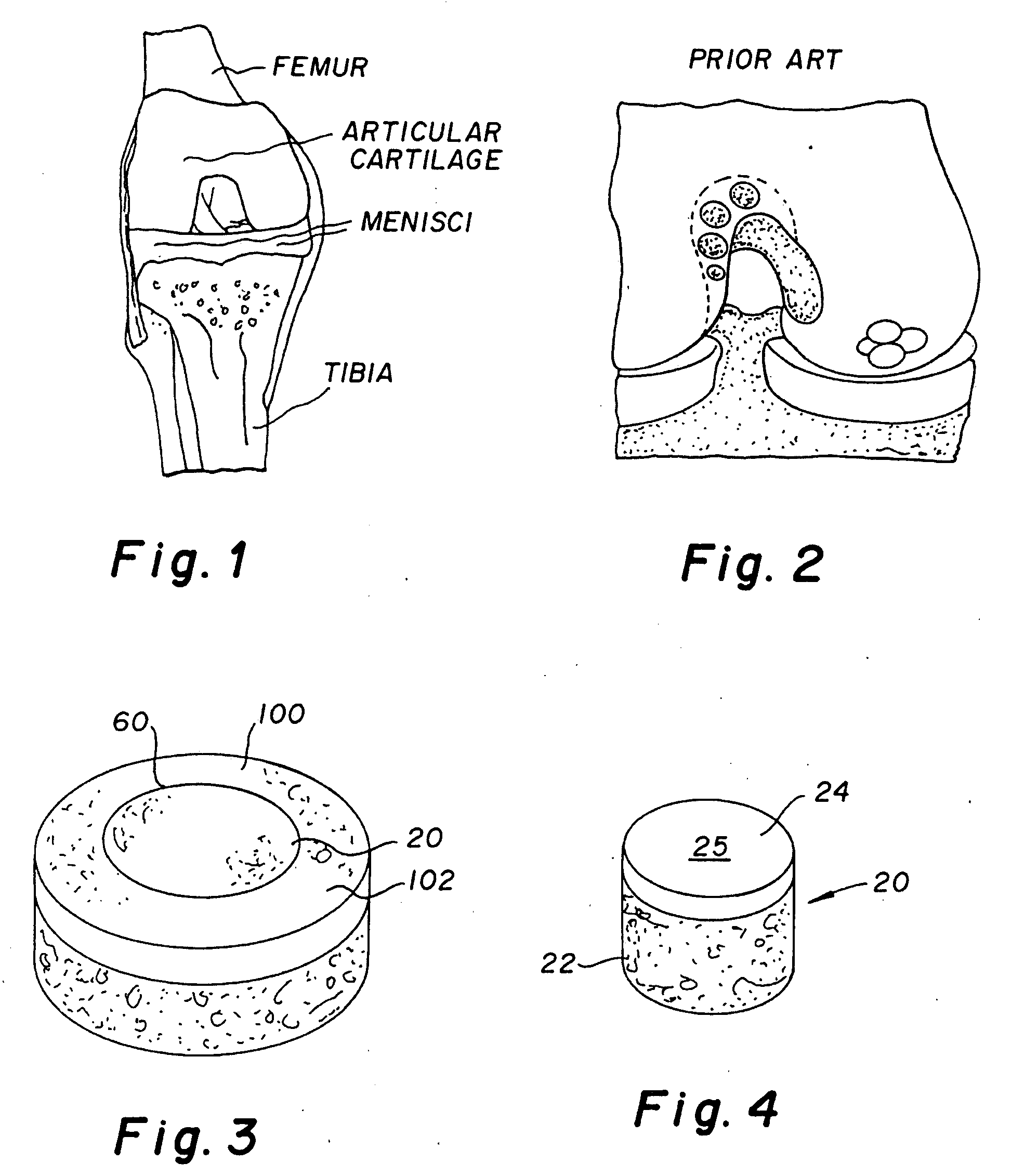
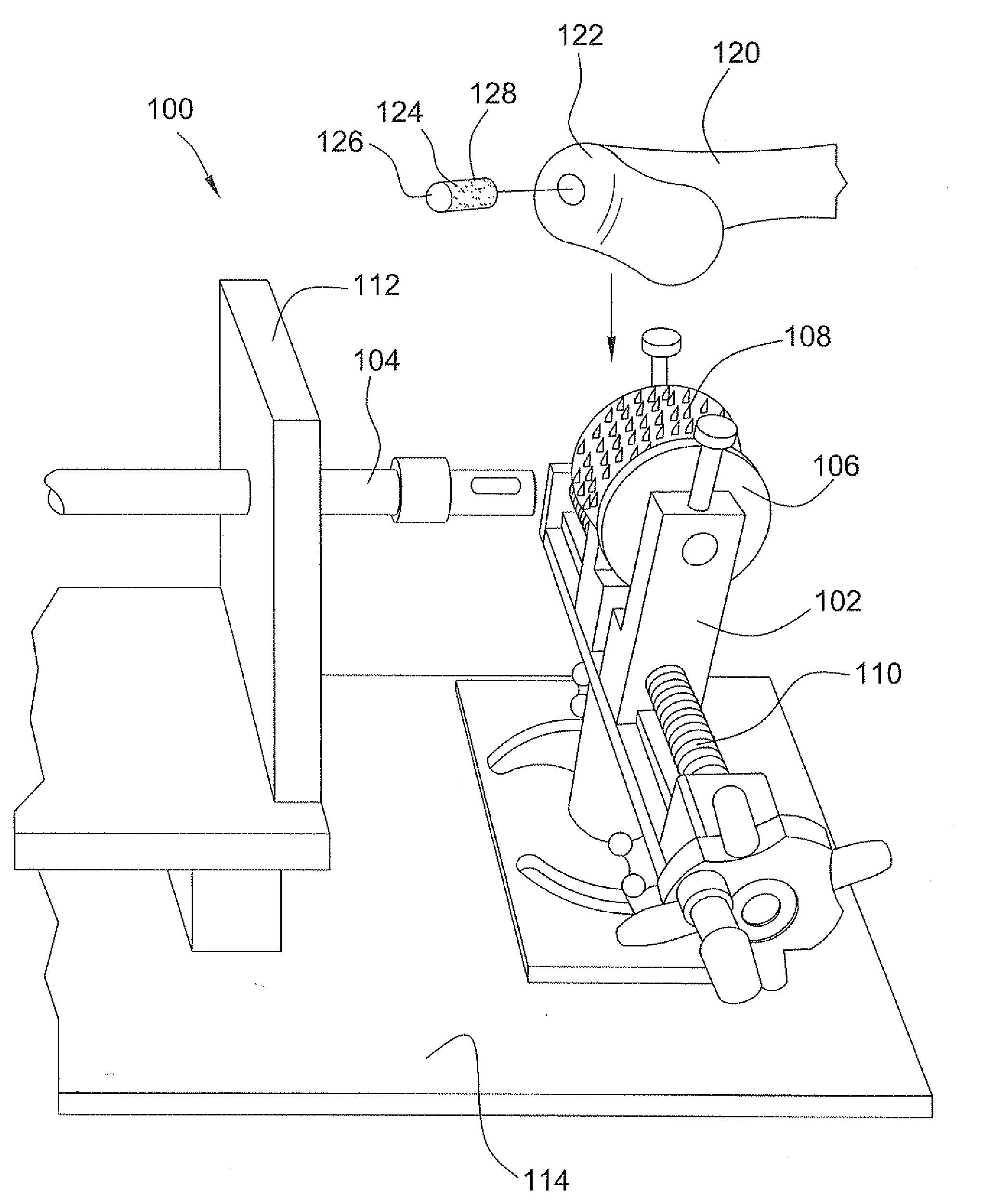
Method and instrumentation for the preparation and transplantation of osteochondral allografts
InactiveUS20070093896A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilnear. At the recipient site of the host bone, a cutout is formed corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
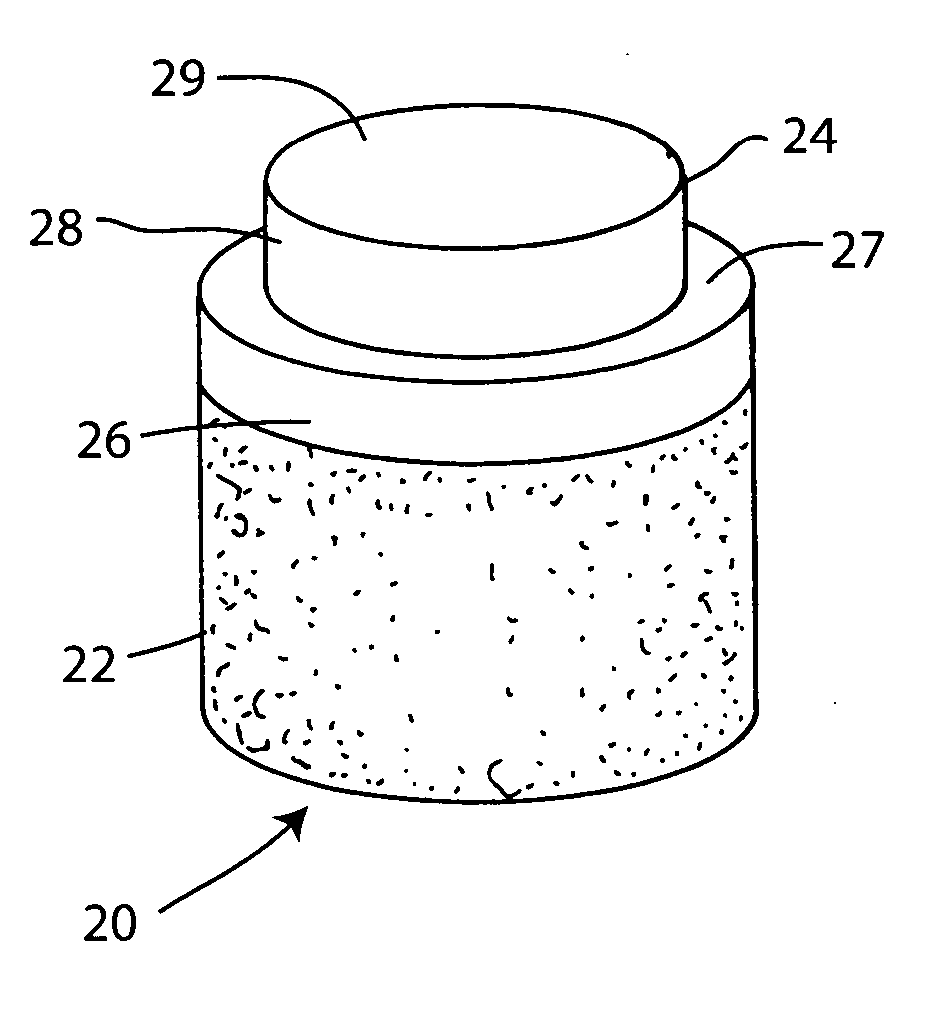
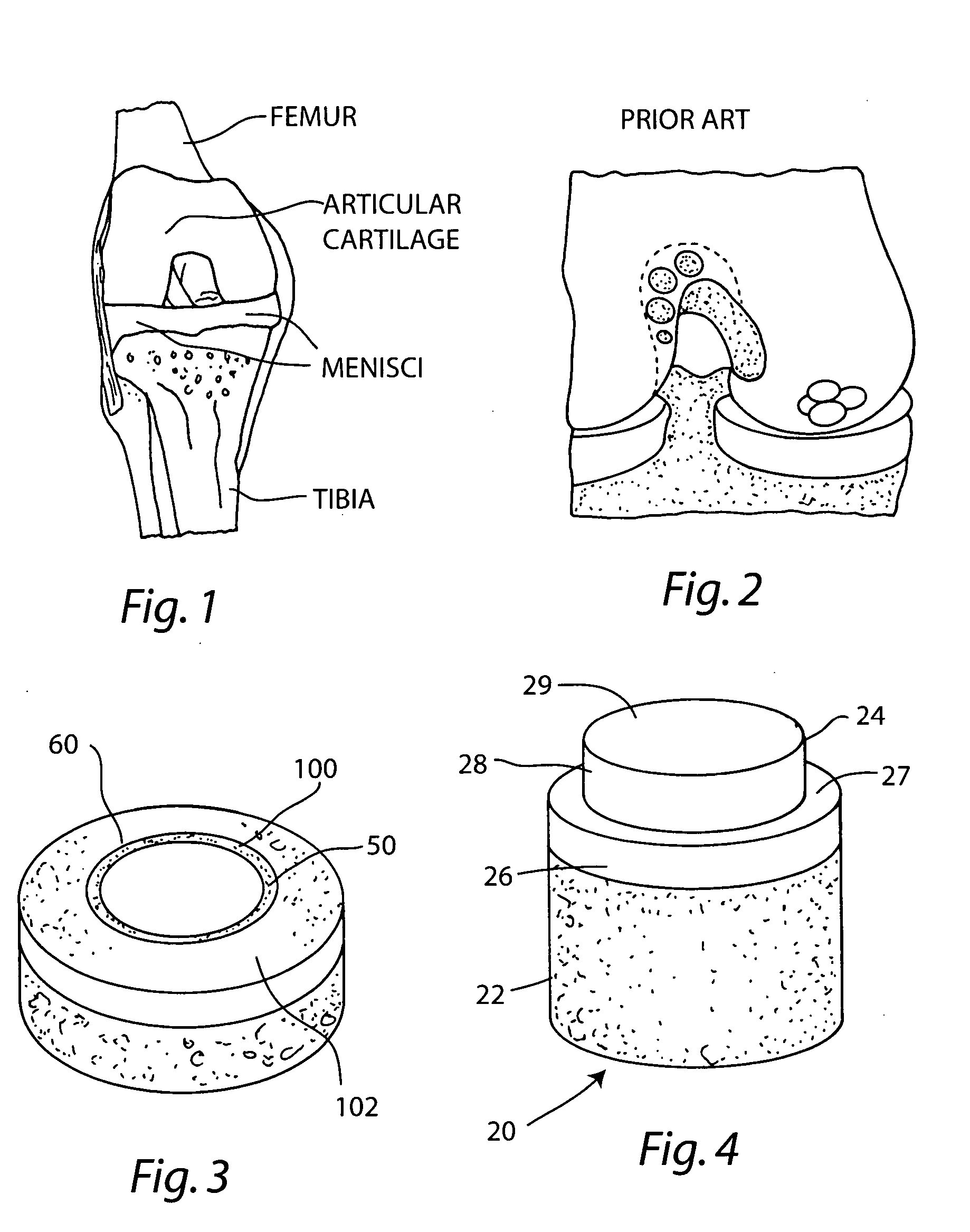
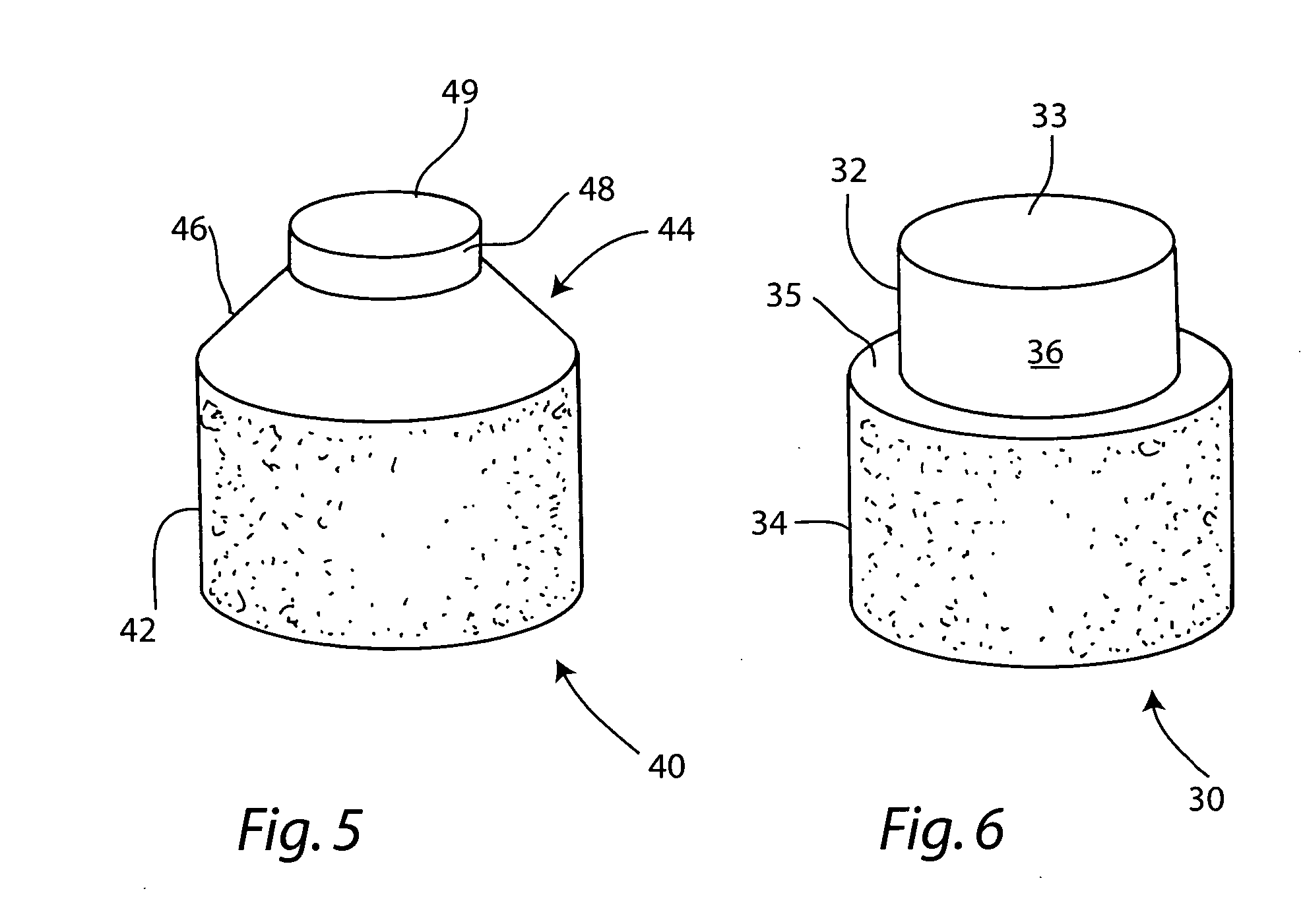
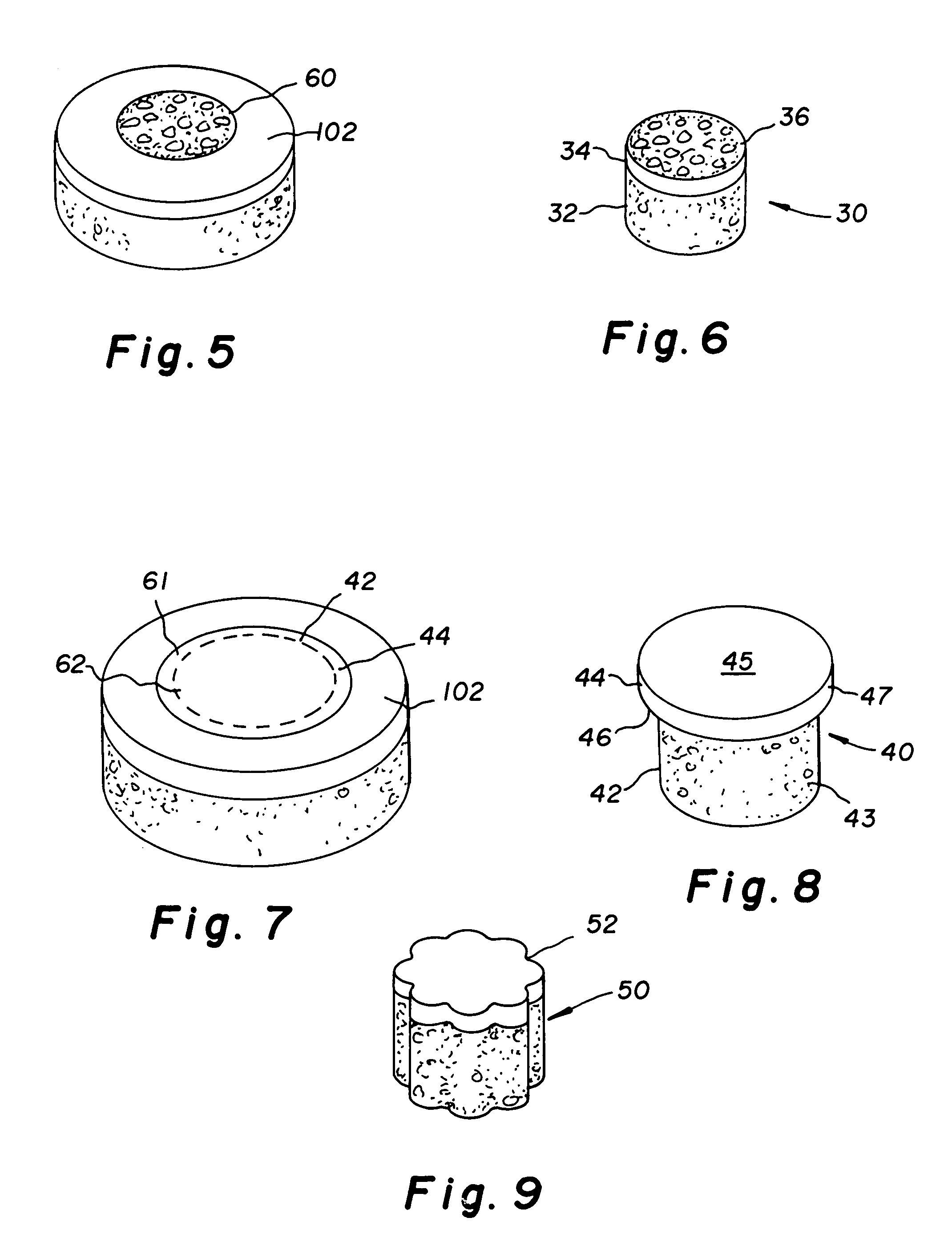
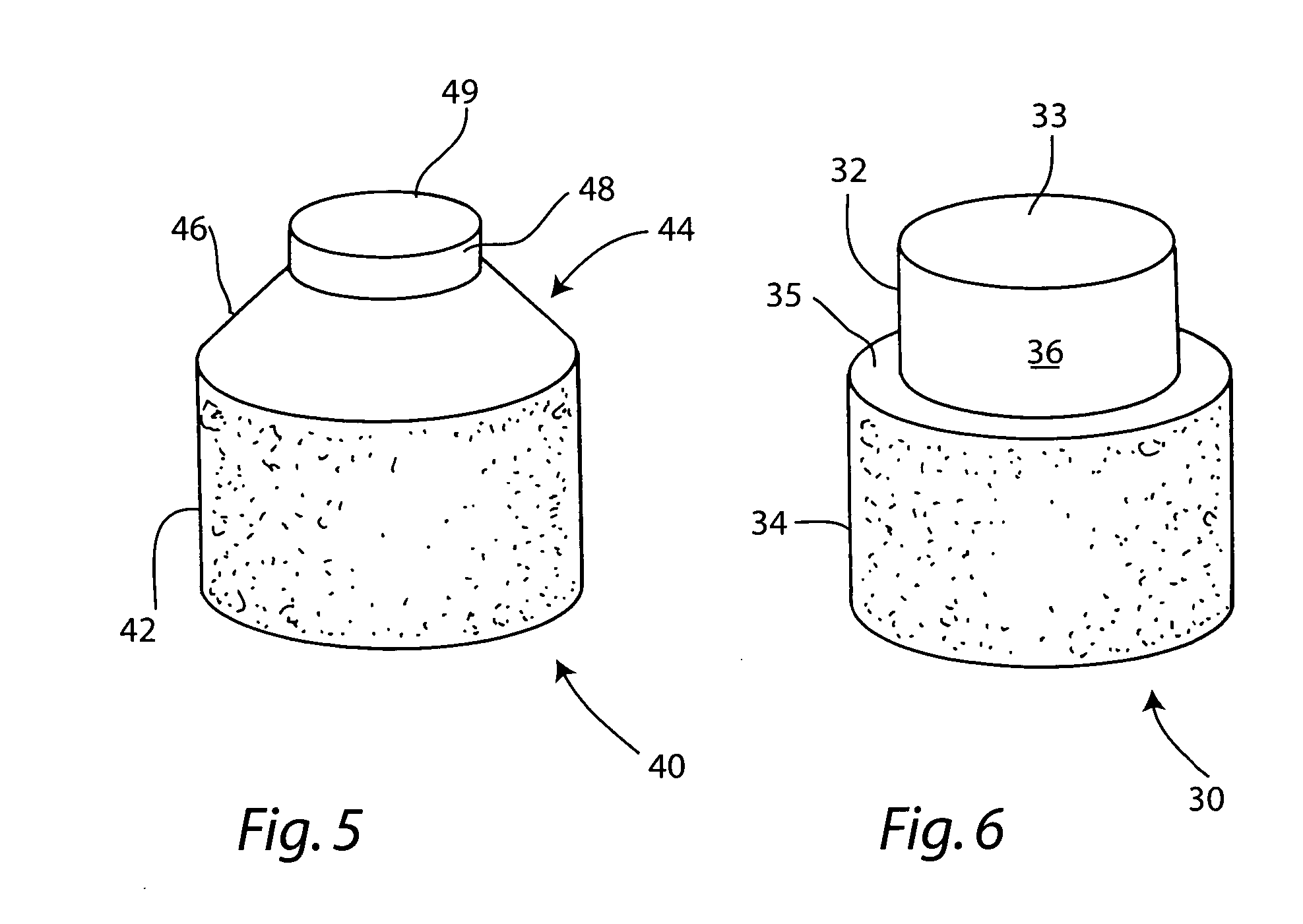
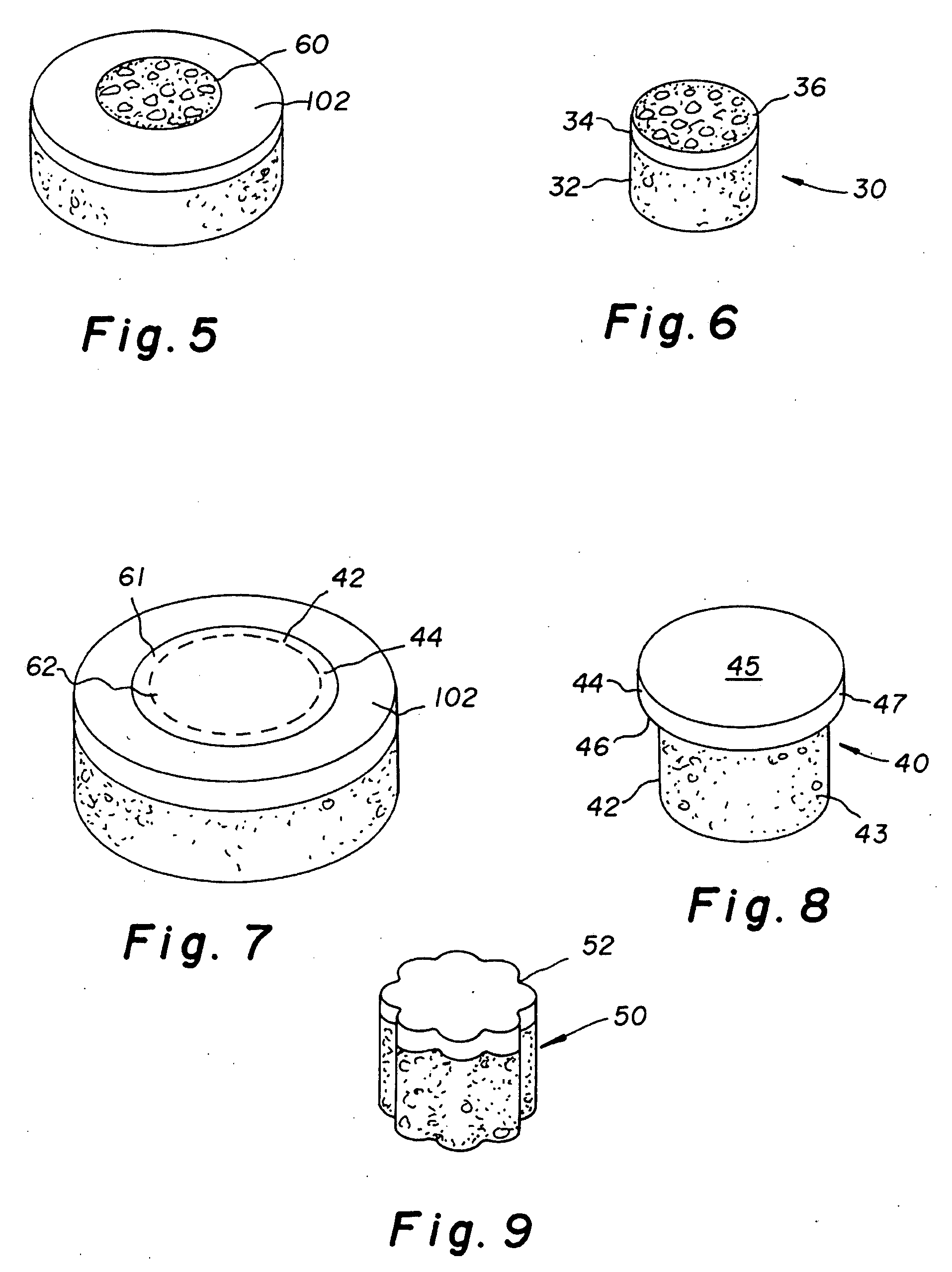
Cartilage allograft plug
InactiveUS20050251268A1Increase chondrocyte migrationIncreased proliferationBone implantLigamentsInterference fitSubchondral bone
The invention is directed toward a cartilage repair assembly comprising a cylindrically shaped allograft structure of subchondral bone with an integral overlying smaller diameter cartilage cap which is treated to remove cellular debris and proteoglycans. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the subchondral bone of the structure engages the side wall of the bone portion of the drilled bore in an interference fit while the cartilage cap is spaced from cartilage portion of the side wall of the drilled bore forming a gap in which a milled cartilage and biocompatible carrier mixture is placed allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Osteochondral transplant techniques
Osteoarticular allografts are transplanted by techniques which ensure substantial surface contour matching. Specifically, surgical techniques are provided whereby a plug from an osteochondral allograft may be transplanted to a cavity site which remains after a condylar defect is removed from a patient's condyle. In this regard, the present invention essentially includes placing an osteochondral allograft in substantially the same orientation as the patient condyle, and then removing the transplantable plug therefrom and forming the cavity site in the patient condyle while maintaining their relative same orientation. In this manner, the surface of the transplanted plug is matched to the contour of the excised osteochondral tissue.
Owner:STAT INSTR
Cartilage allograft plug
InactiveUS7901457B2Promote migrationIncreased proliferationBone implantJoint implantsSubchondral boneInterference fit
The invention is directed toward a cartilage repair assembly comprising a shaped allograft structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled allograft cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that either the shaped bone or the cartilage cap engage the side wall of the drilled bore in an interference fit and is in contact with a milled cartilage and biocompatible carrier mixture allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
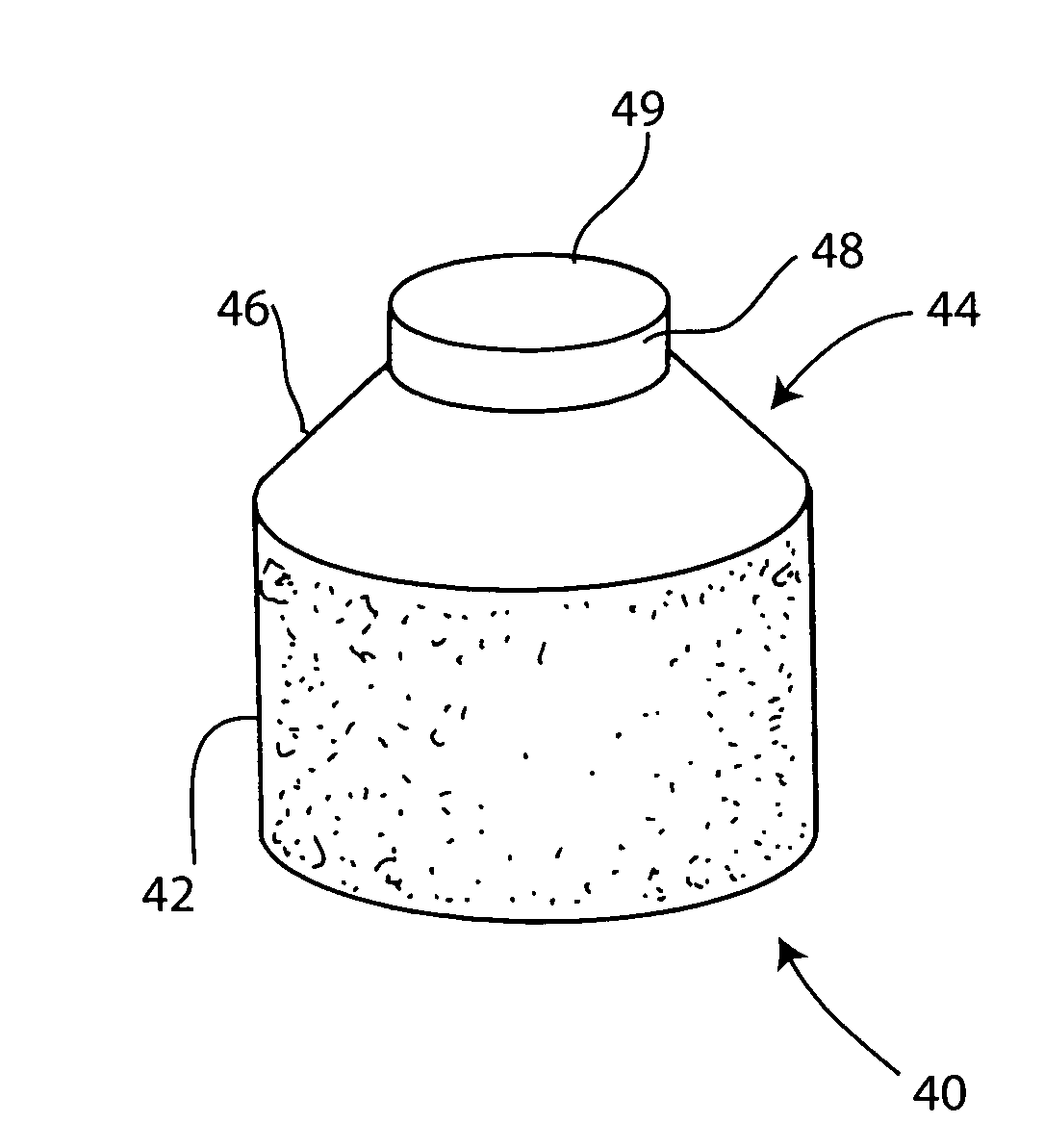
Cartilage allograft plug
InactiveUS7488348B2Guaranteed functionEasily placed in defect areaBone implantLigamentsInterference fitSubchondral bone
The invention is directed toward a cartilage repair assembly comprising a cylindrically shaped allograft structure of subchondral bone with an integral overlying smaller diameter cartilage cap which is treated to remove cellular debris and proteoglycans. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that the subchondral bone of the structure engages the side wall of the bone portion of the drilled bore in an interference fit while the cartilage cap is spaced from cartilage portion of the side wall of the drilled bore forming a gap in which a milled cartilage and biocompatible carrier mixture is placed allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Instrumentation for the preparation and transplantation of osteochondral allografts
ActiveUS20070135918A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilinear. At the recipient site of the host bone, a cutout is forined corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Cartilage allograft plug
ActiveUS20090069901A1Increase chondrocyte migrationIncreased proliferationBone implantJoint implantsSubchondral boneInterference fit
The invention is directed toward a cartilage repair assembly comprising a shaped allograft structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled allograft cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that either the shaped bone or the cartilage cap engage the side wall of the drilled bore in an interference fit and is in contact with a milled cartilage and biocompatible carrier mixture allowing cell transfer throughout the defect area. A method for inserting the shaped allograft structure into a cartilage defect area is also disclosed.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Instrumentation for the preparation and transplantation of osteochondral allografts
ActiveUS20070135917A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilinear. At the recipient site of the host bone, a cutout is formed corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Osteochondral allografts
ActiveUS20070135928A1Reduce the amount requiredLess likely to be rejected by a recipientSuture equipmentsBone implantDonor boneChondral defect
Provided are procedures and instruments for preparing and transplanting osteochondral allograft plugs to a host bone to repair an articular cartilage defect. An allograft bone plug having a cartilage plate and cancellous bone tissue attached thereto is removed from a donor bone. The allograft plug is further shaped by removing or cutting away cancellous bone tissue to form a cancellous stalk extending from the cartilage plate. The formed cancellous stalk can have any suitable shape including cylindrical, conical, and rectilinear. At the recipient site of the host bone, a cutout is formed corresponding in shape to the allograft plug. The allograft plug is inserted into the cutout such that the cancellous stalk is retained in the host bone and the cartilage plate aligns with the condyle surface of the host bone. Aspects of the invention may also be applicable to preparing and transplanting osteochondral autograft plugs.
Owner:BIOMET MFG CORP
Tolerance induction and maintenance in hematopoietic stem cell allografts
InactiveUS20050058641A1Induce toleranceMaintain long-termBiocideGenetic material ingredientsTolerance inductionBiology
Provided are methods and compositions for the induction and maintenance of tolerance in hematopoietic stem cell allografts.
Owner:THE CLEVELAND CLINIC FOUND
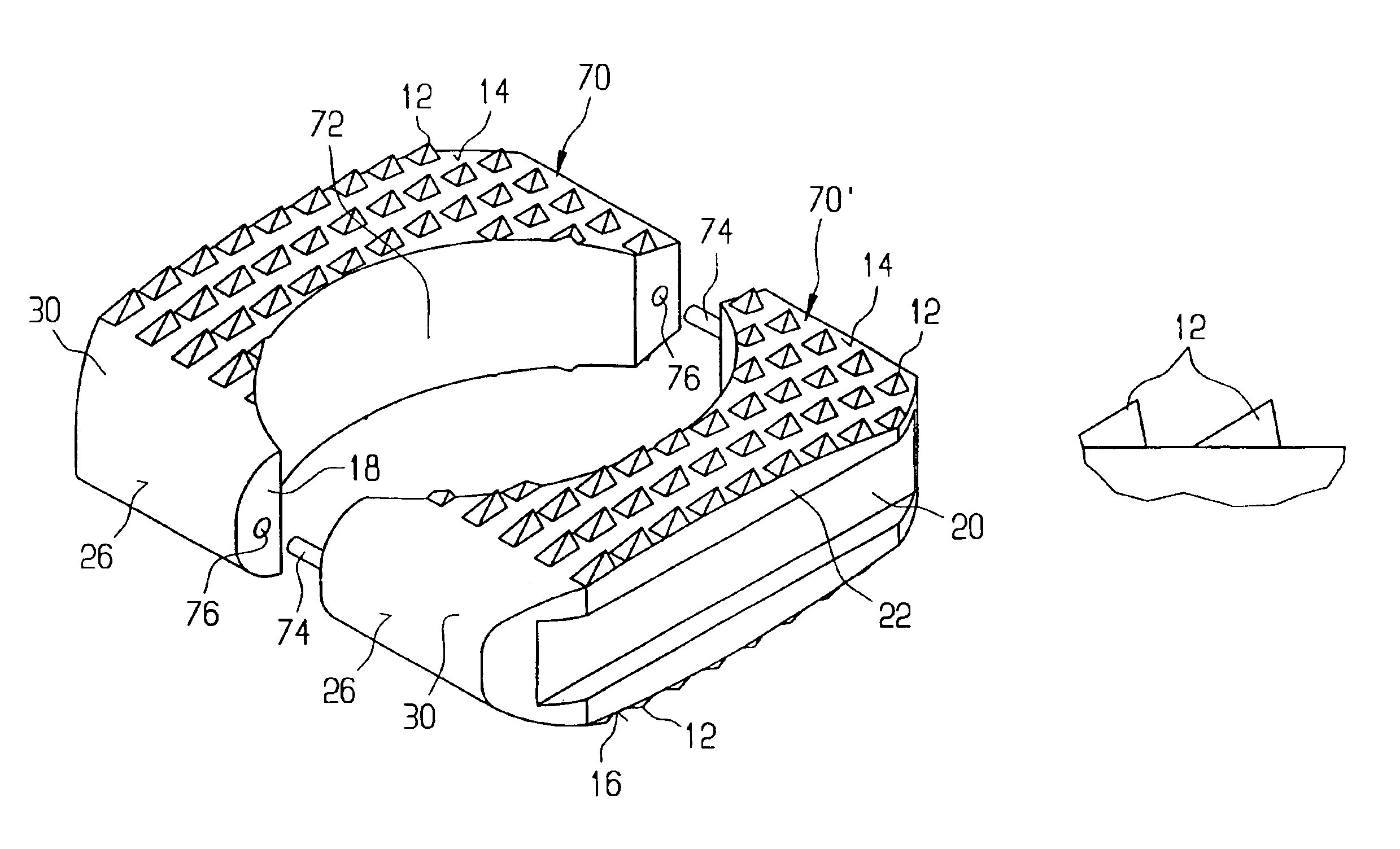
Multiple wafer cortical bone and cancellous bone allograft with cortical pins
InactiveUS20110137417A1Reduce the possibilityRelieve pressureBone implantSpinal implantsHuman bodyAllogeneic graft
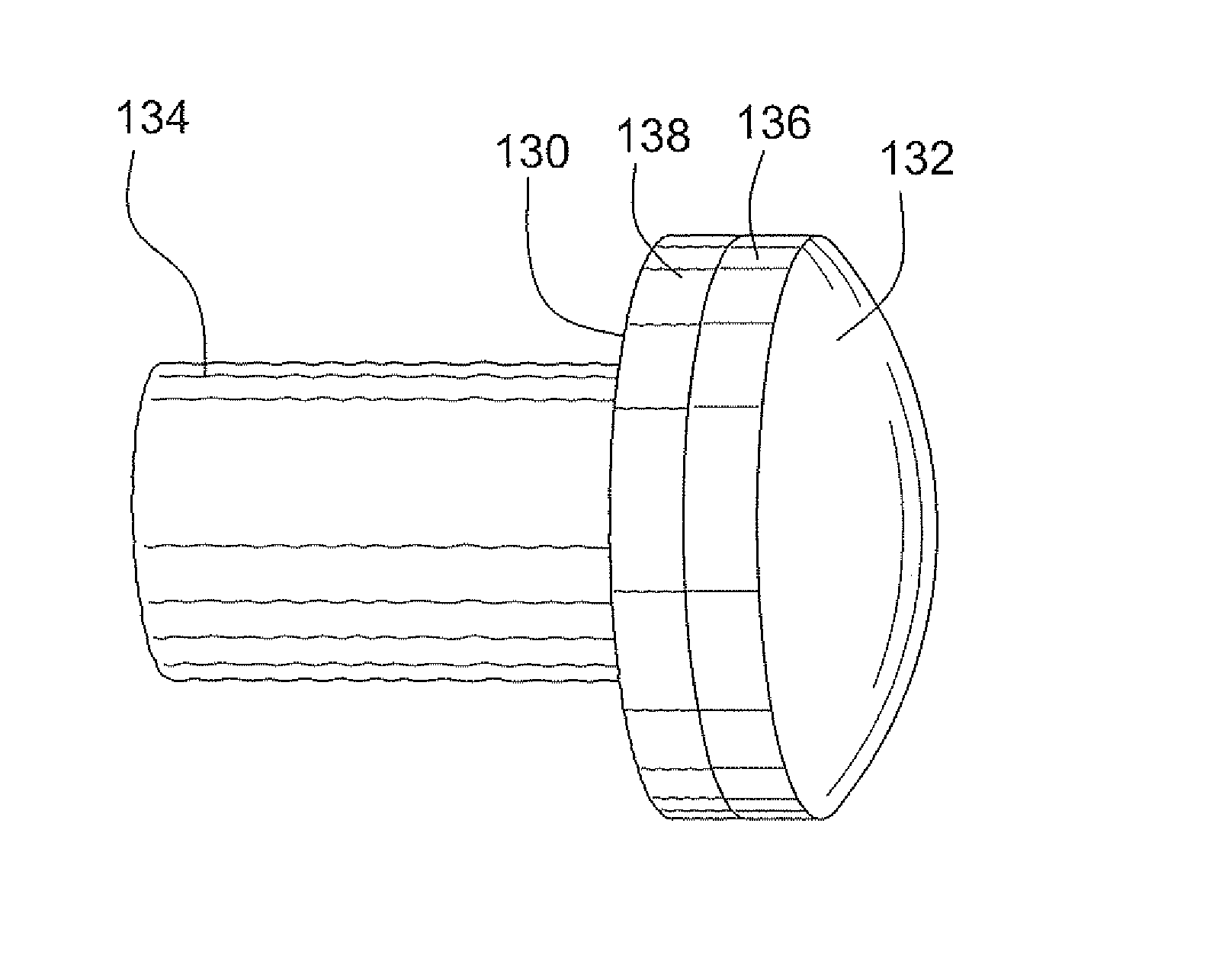
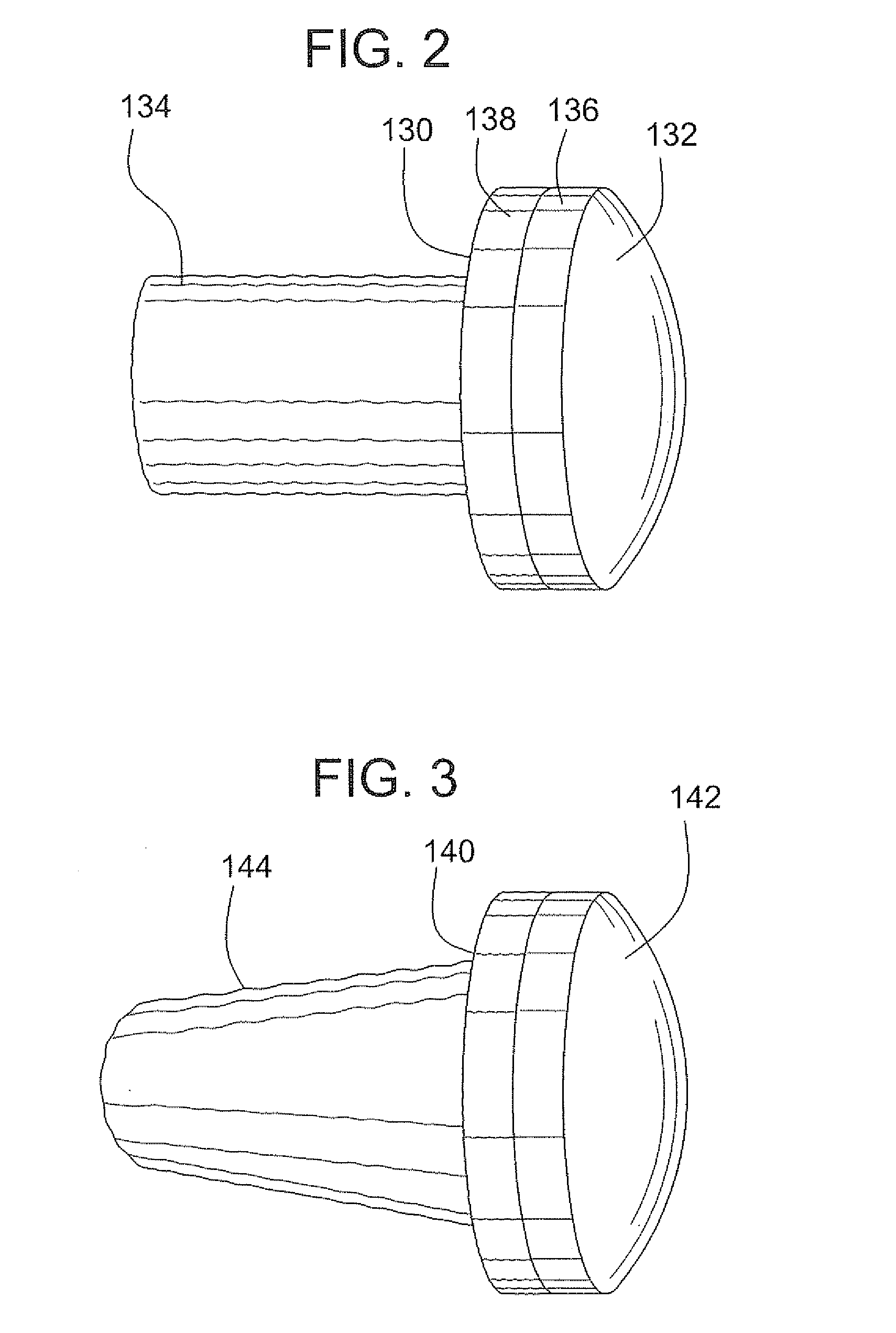
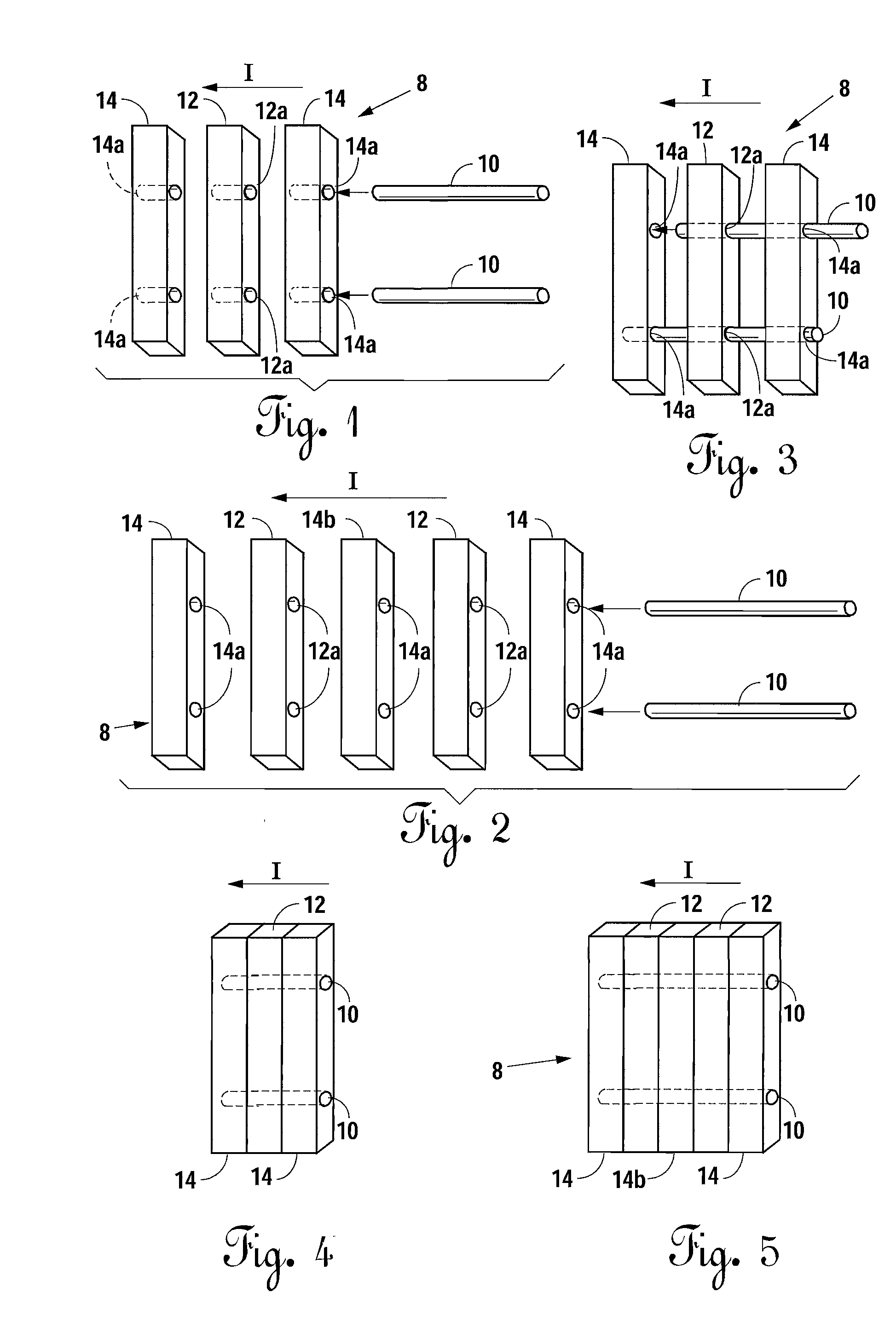
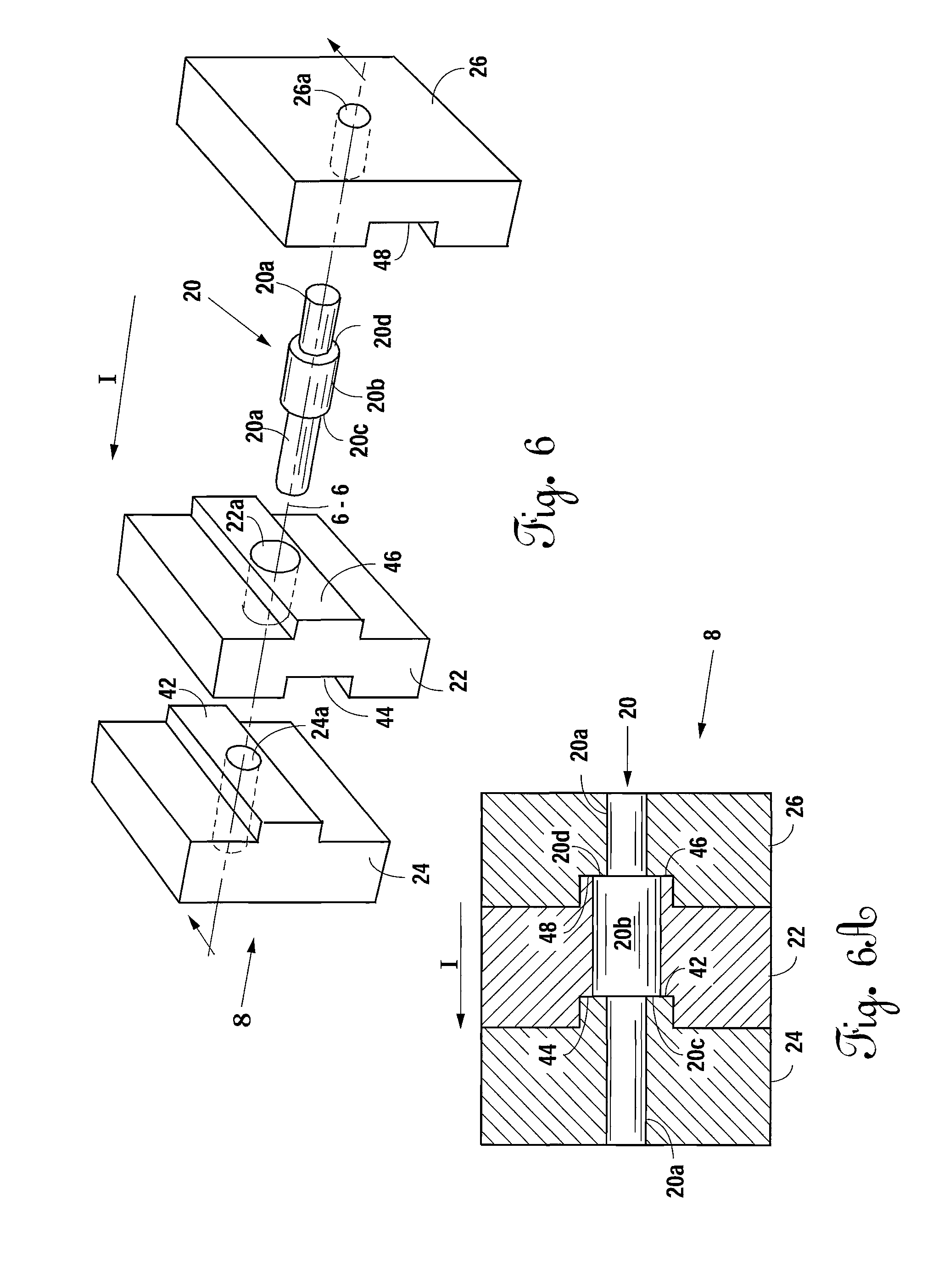
A bone allograft and cortical pin for inserting into a surgically altered site of a human. The cortical pin is cylindrically shaped and has a thick middle diameter surrounded by two smaller end portions. The allograft has two end cortical wafers with small canals to receive the small ends of the cortical pin. At least one cancellous wafer is disposed adjacently between the end cortical bone wafers. A plurality of cancellous and / or cortical wafers may be inserted between the end cortical wafers to form the allograft. The wafers inserted between the end cortical wafers have larger canals to receive the thick diameter of the cortical pin. The size of the allograft may be adjusted as desired by either adding or removing inner wafers, or adjusting the size of the inner wafers of the allograft. The allograft and cortical pin are inserted parallel to the plane of insertion into the human.
Owner:TRANSPLANT TECH OF TEXAS
Modification of reactivity of bone constructs
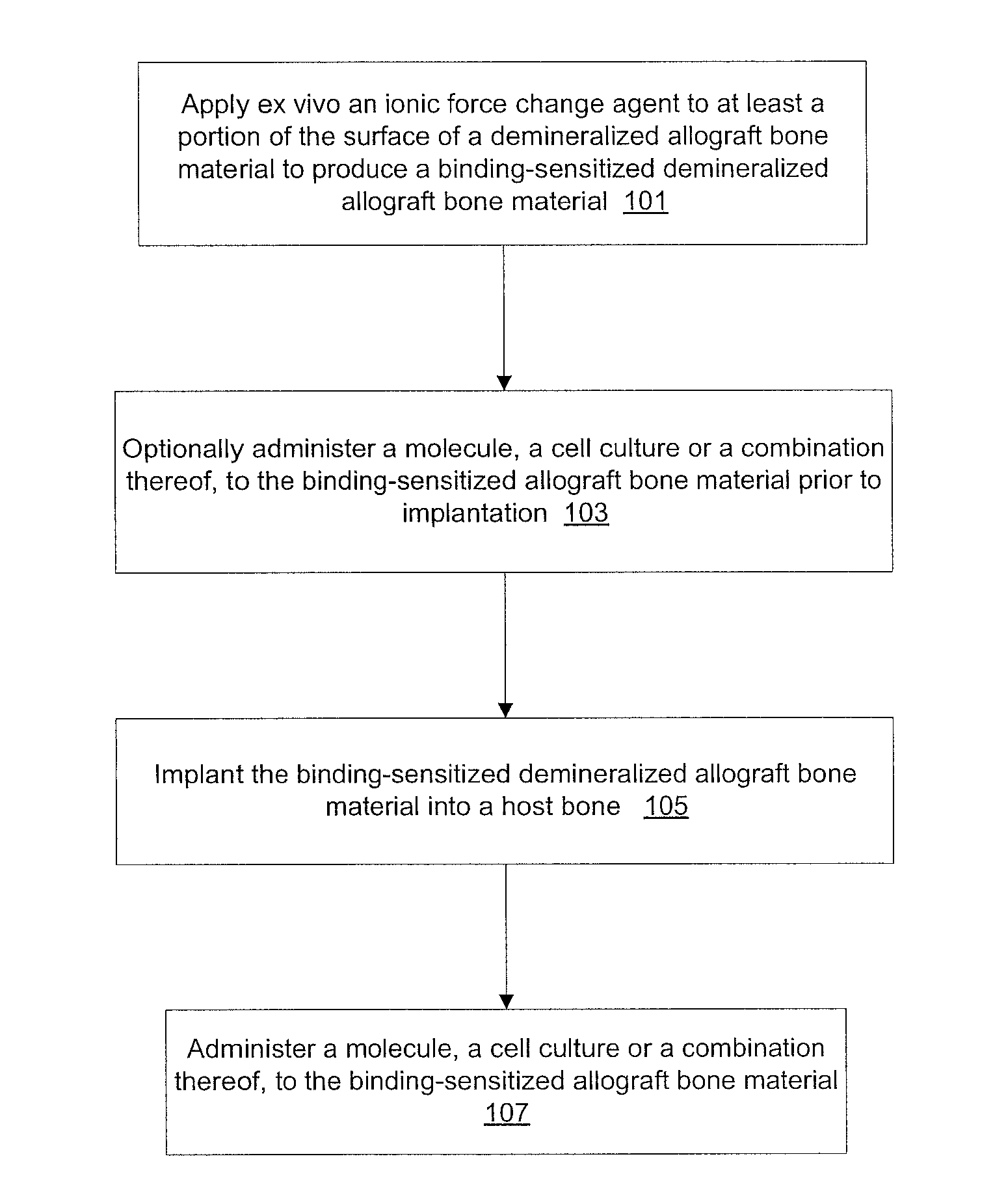
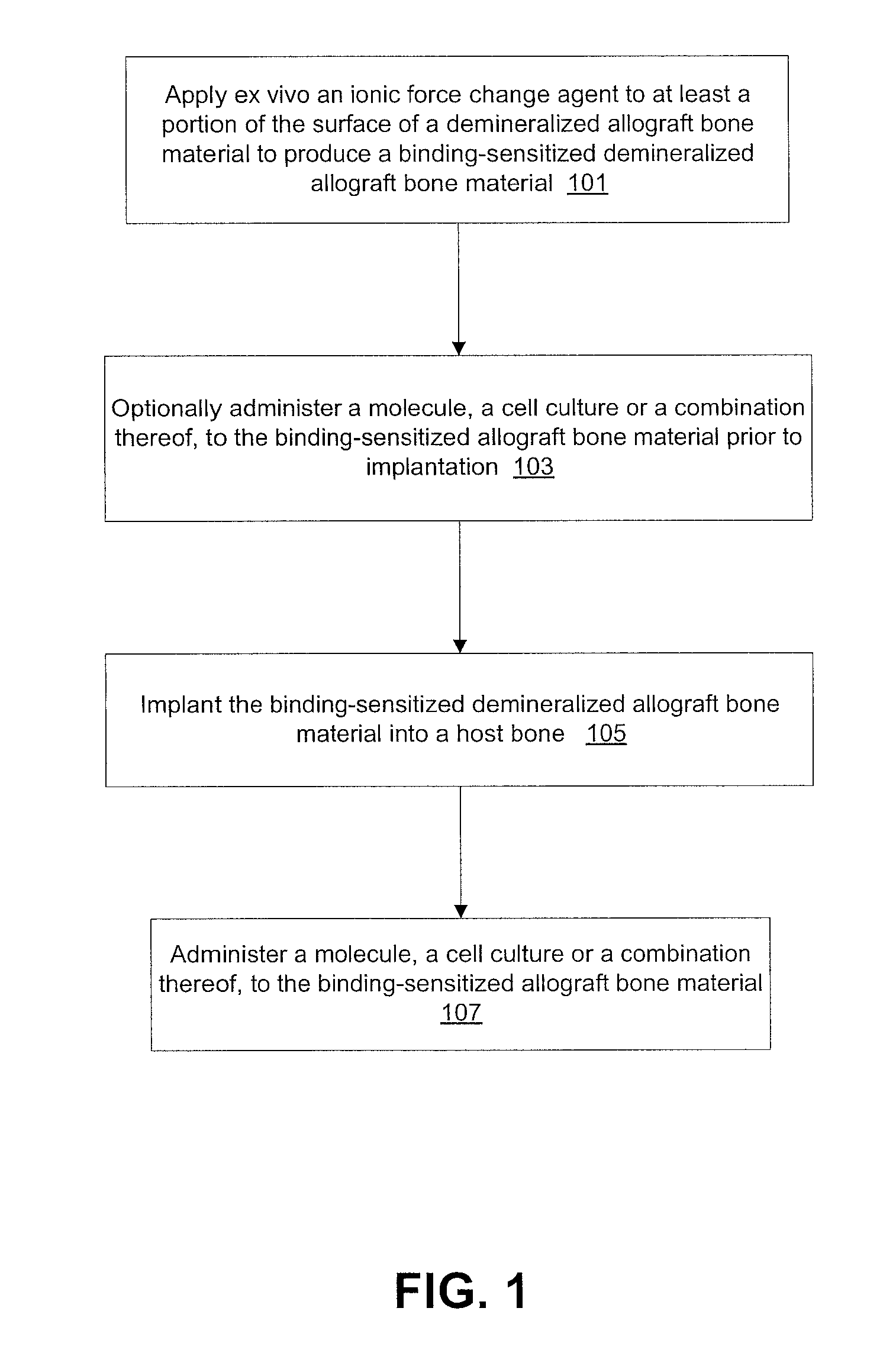
A method of enhancing the binding of growth factors and cell cultures to a demineralized allograft bone material which includes applying ex vivo an effective quantity of an ionic force change agent to at least a portion of the surface of a demineralized allograft bone material to produce a binding-sensitized demineralized allograft bone material and implanting the binding-sensitized demineralized allograft bone material into a host bone. The ionic force change agent may include at least one of enzymes, pressure, chemicals, heat, sheer force, oxygen plasma, supercritical nitrogen, supercritical carbon, supercritical water or a combination thereof. A molecule, a cell culture, or a combination thereof is administered to the binding-sensitized demineralized allograft bone material.
Owner:WARSAW ORTHOPEDIC INC
Osteoinduction of cortical bone allografts by coating with biopolymers seeded with recipient periosteal bone cells
InactiveUS6899107B2Improve clinical outcomesLower immune responseBone implantDiagnosticsBiopolymerBone Cortex
A method by which immune responses to cortical bone grafts and other substrates (e.g., cement, IPN, etc.) can be minimized and at the same time graft osteoinductive potential can be improved, and improved graft substrate materials are disclosed. The method of the invention provides new types of bone grafts that incorporate into host bone more thoroughly and more rapidly, eliminating long-term complications, such as fracture, non-union, infection, and rejection. In the method of the invention, bone grafts or other substrates are modified to have an osteoinductive surface modification that the recipient's body will accept as its own tissue type and therefore will not reject or otherwise cause to fail. The osteoinductive surface modification comprises a biopolymer matrix coating that is seeded with periosteal cells that have been previously harvested either from the graft recipient or from an allogenic or xenogenic donor source.
Owner:DEPUY MITEK INC
Immunogenic peptides and their use in transplantation
ActiveUS20110110964A1Preventing allograft rejectionPeptide/protein ingredientsAntibody mimetics/scaffoldsRedoxAllograft rejection
The present invention relates to the use of immunogenic peptides comprising a T-cell epitope derived from an allograft antigen and a redox motif such as C—(X)2-[CST] or [CST]-(X)2-C in the prevention and / or treatment of allograft rejection and in the manufacture of medicaments therefore.
Owner:IMCYSE
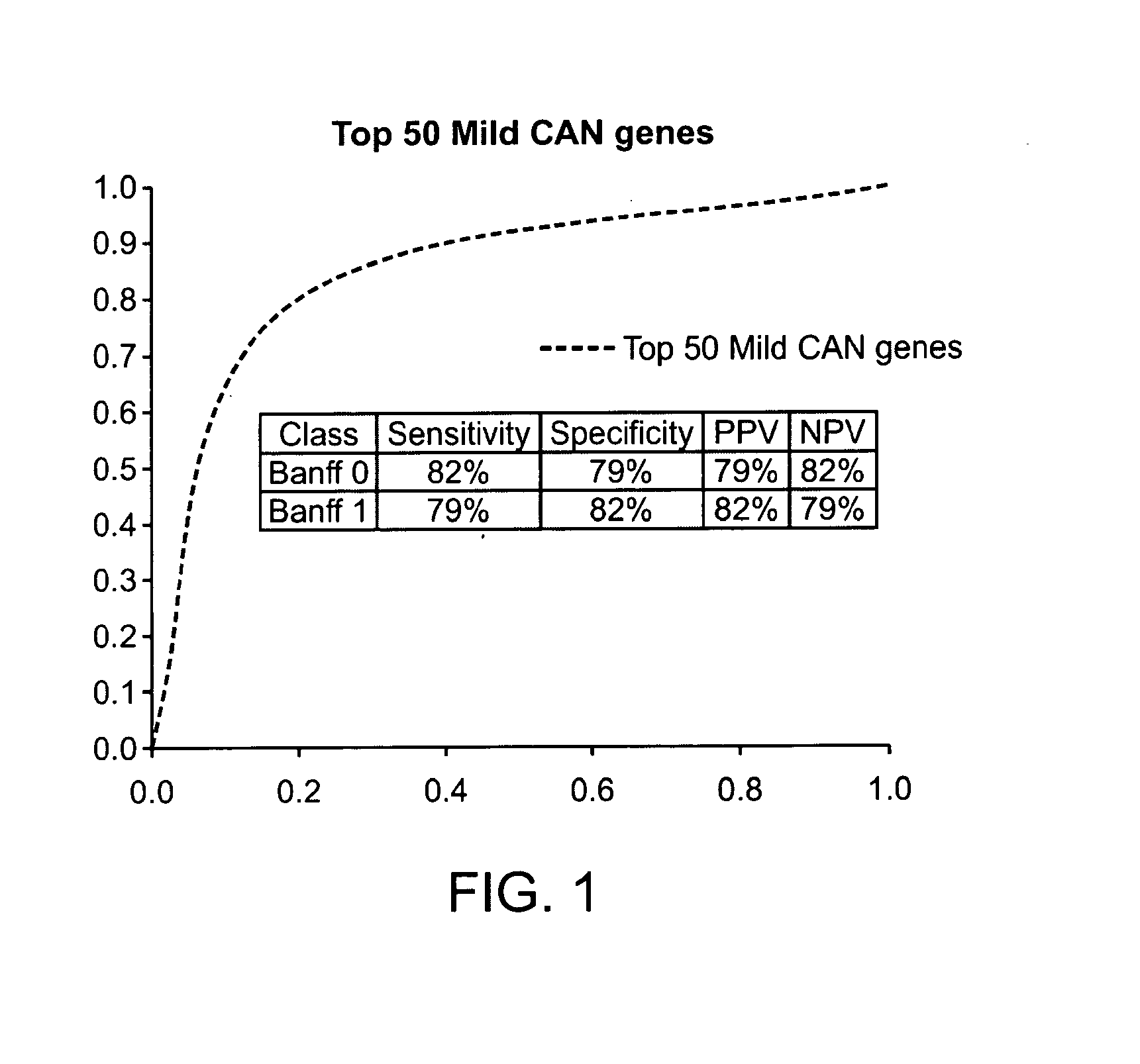
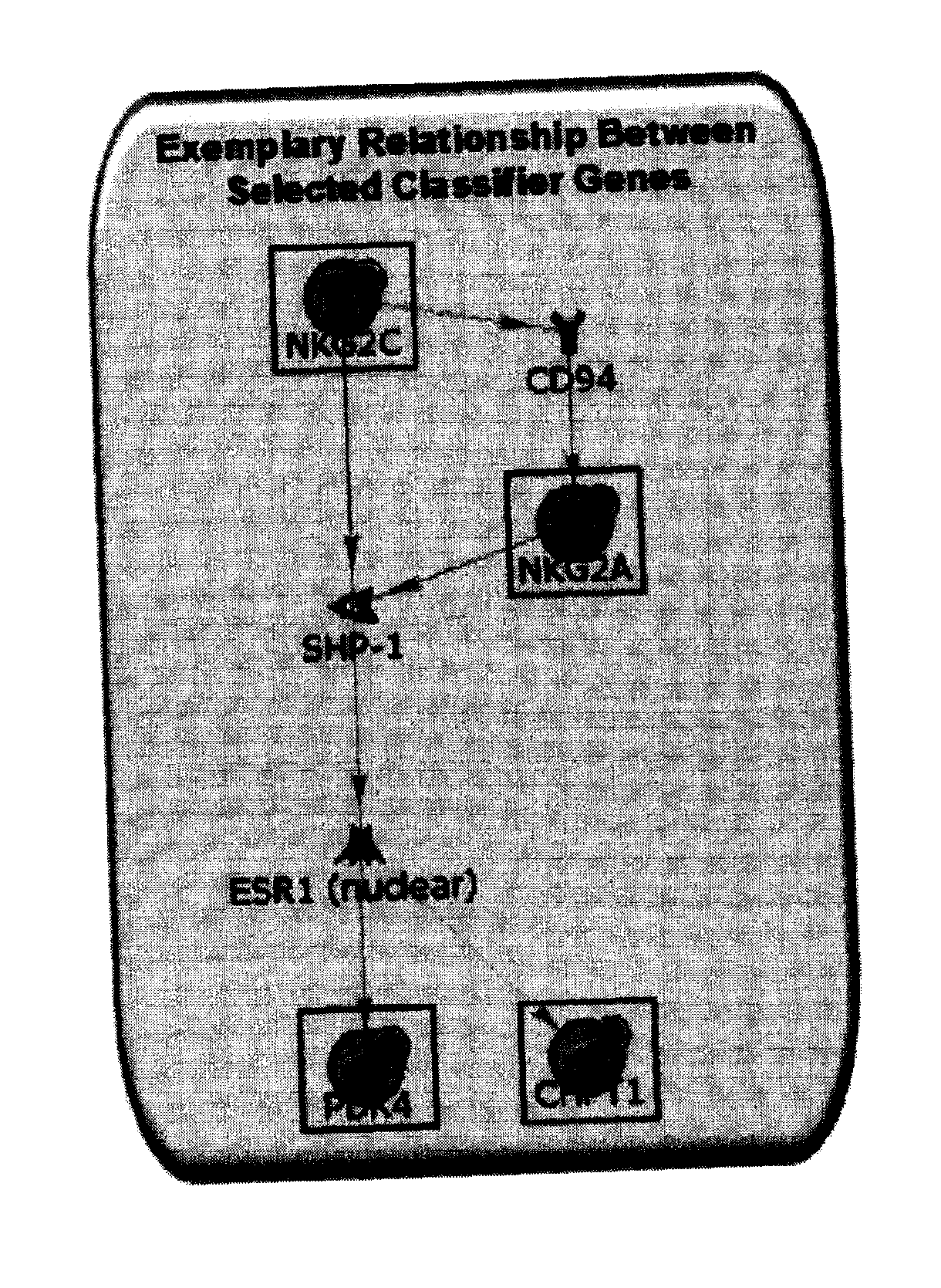
Gene expression profiles associated with chronic allograft nephropathy
ActiveUS20120178642A1Nucleotide librariesMicrobiological testing/measurementChronic allograft nephropathyTubular atrophy
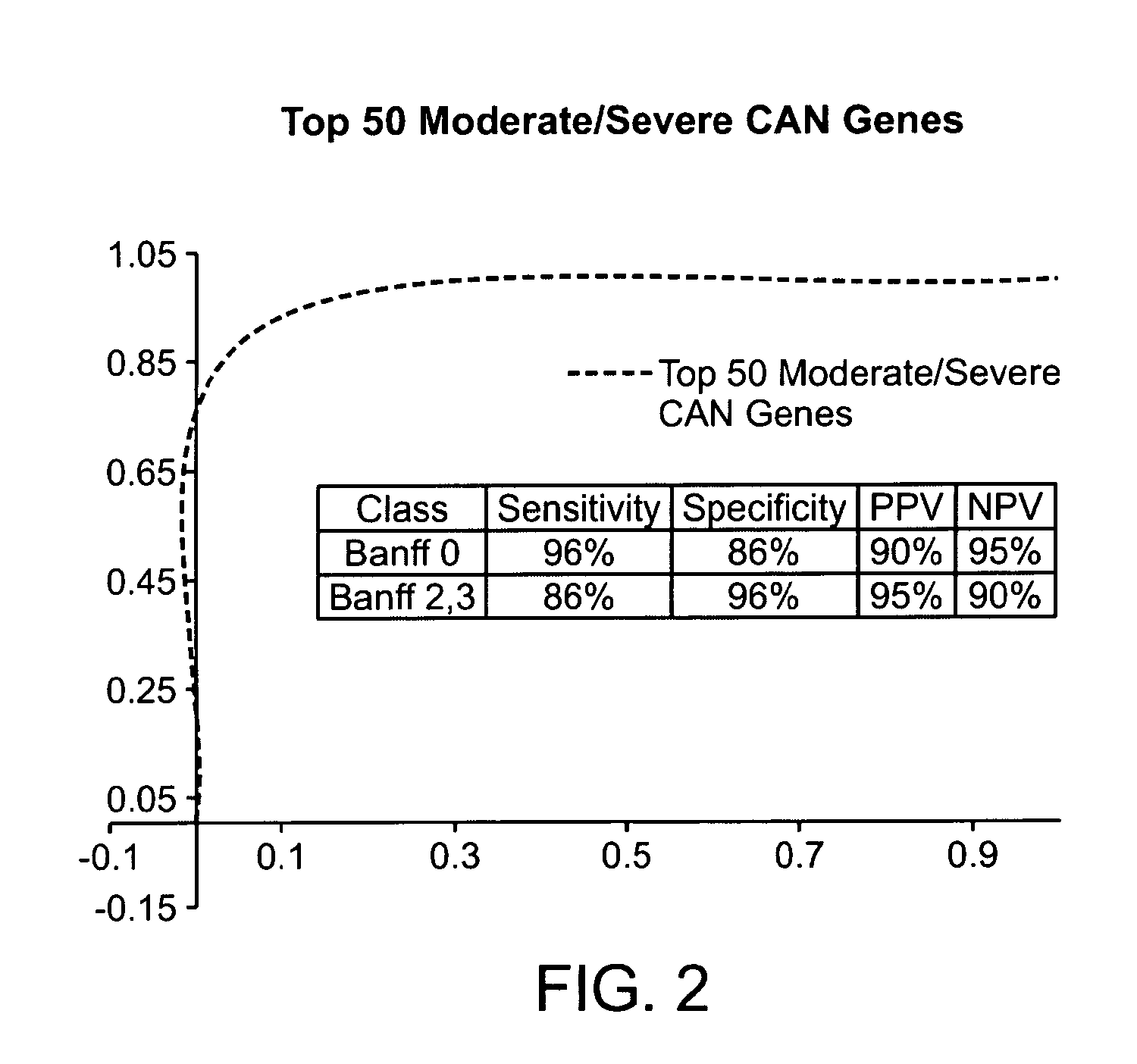
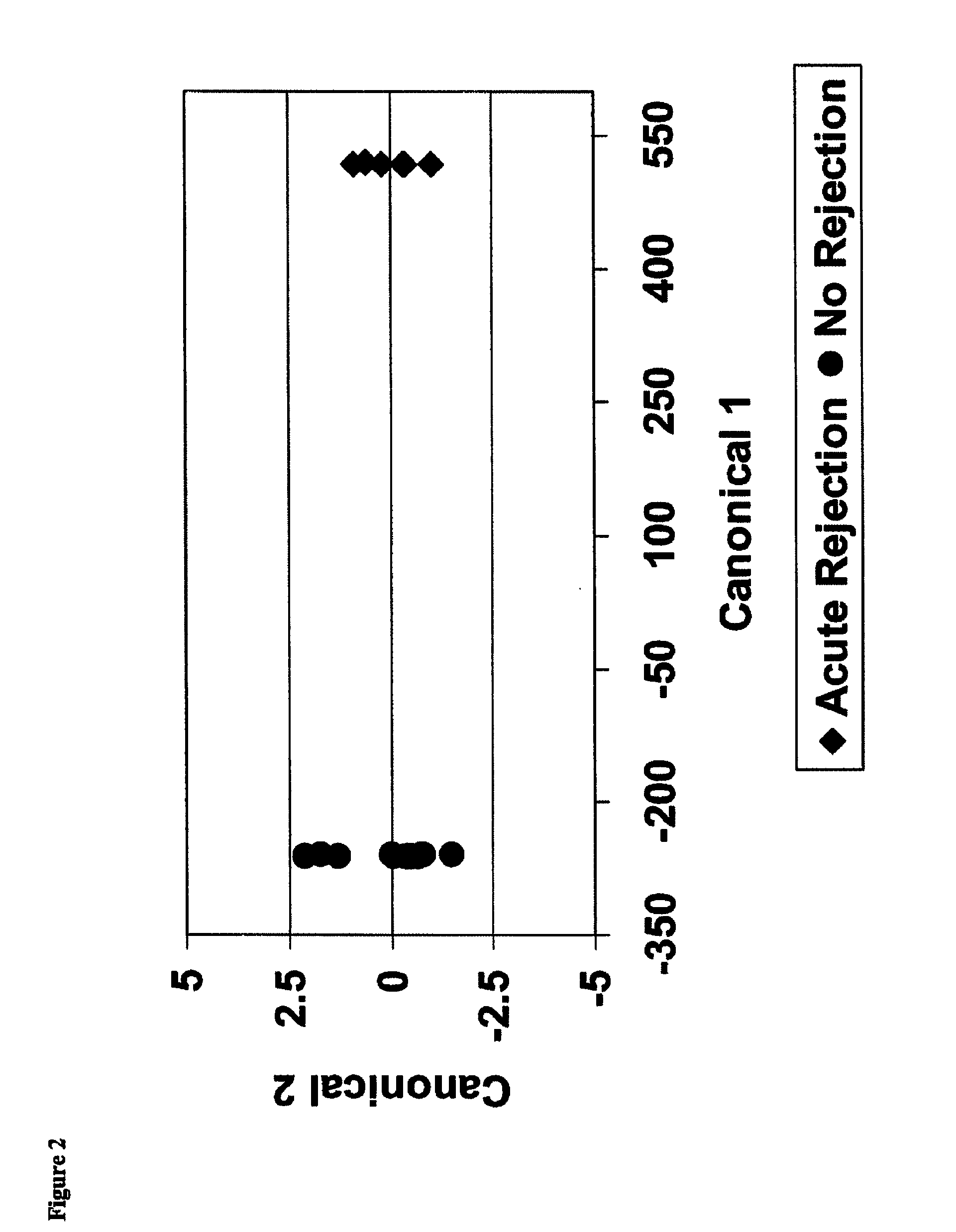
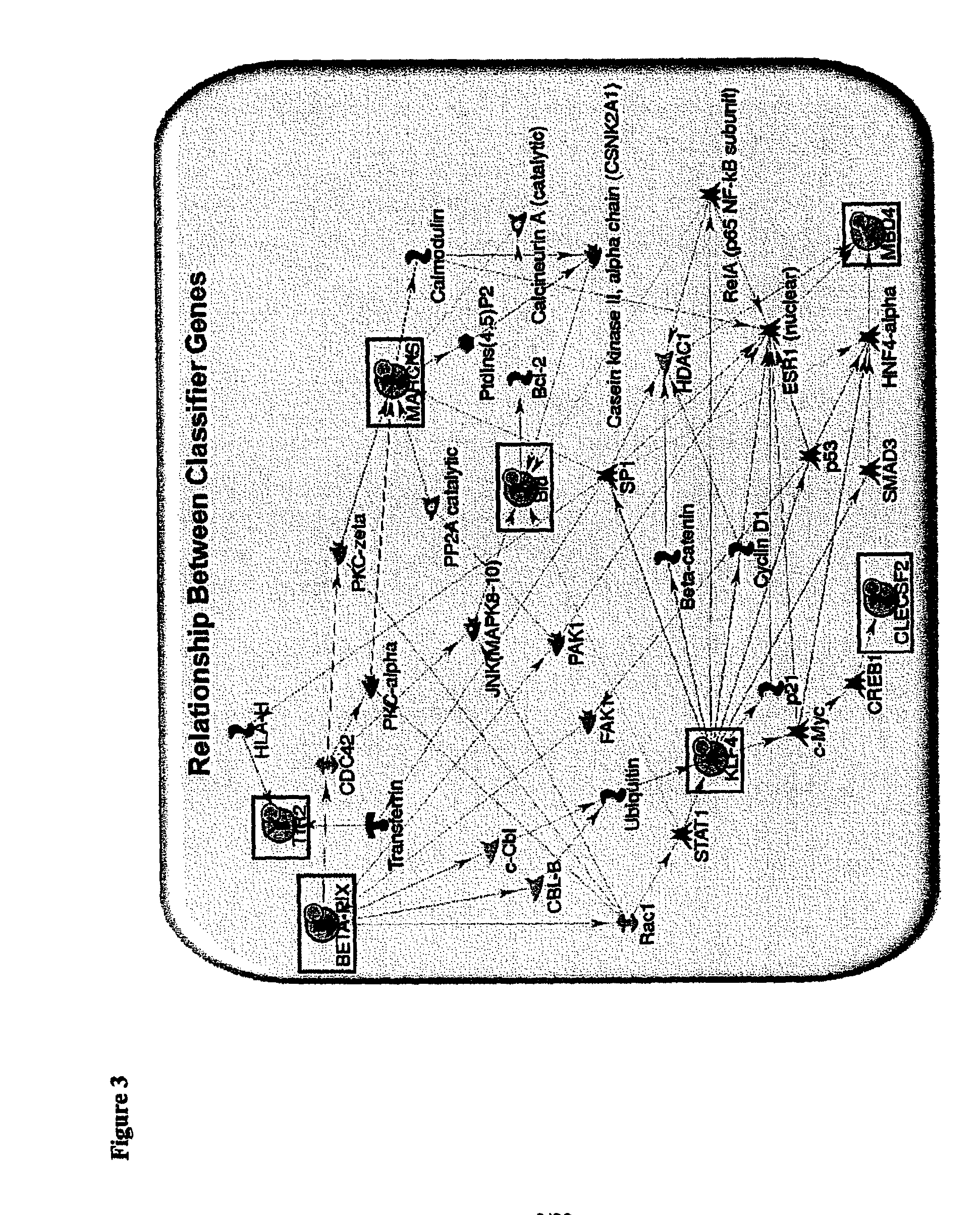
By a genome-wide gene analysis of expression profiles of over 50,000 known or putative gene sequences in peripheral blood, the present inventors have identified a consensus set of gene expression-based molecular biomarkers associated with chronic allograft nephropathy and / or interstitial fibrosis and tubular atrophy CAN / IFTA and subtypes thereof. These genes sets are useful for diagnosis, prognosis, monitoring and / or subtyping of CAN / IFTA.
Owner:THE SCRIPPS RES INST
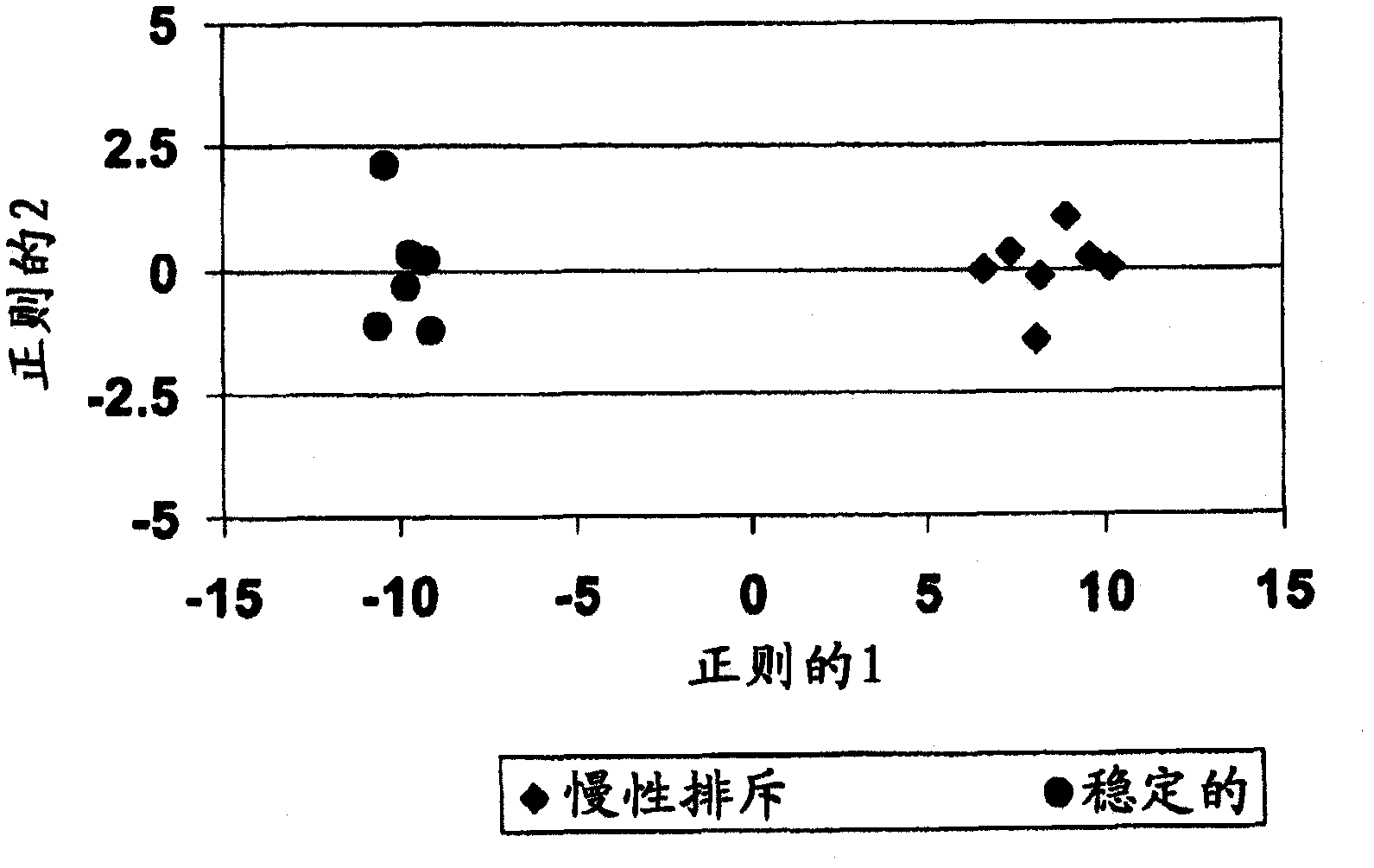
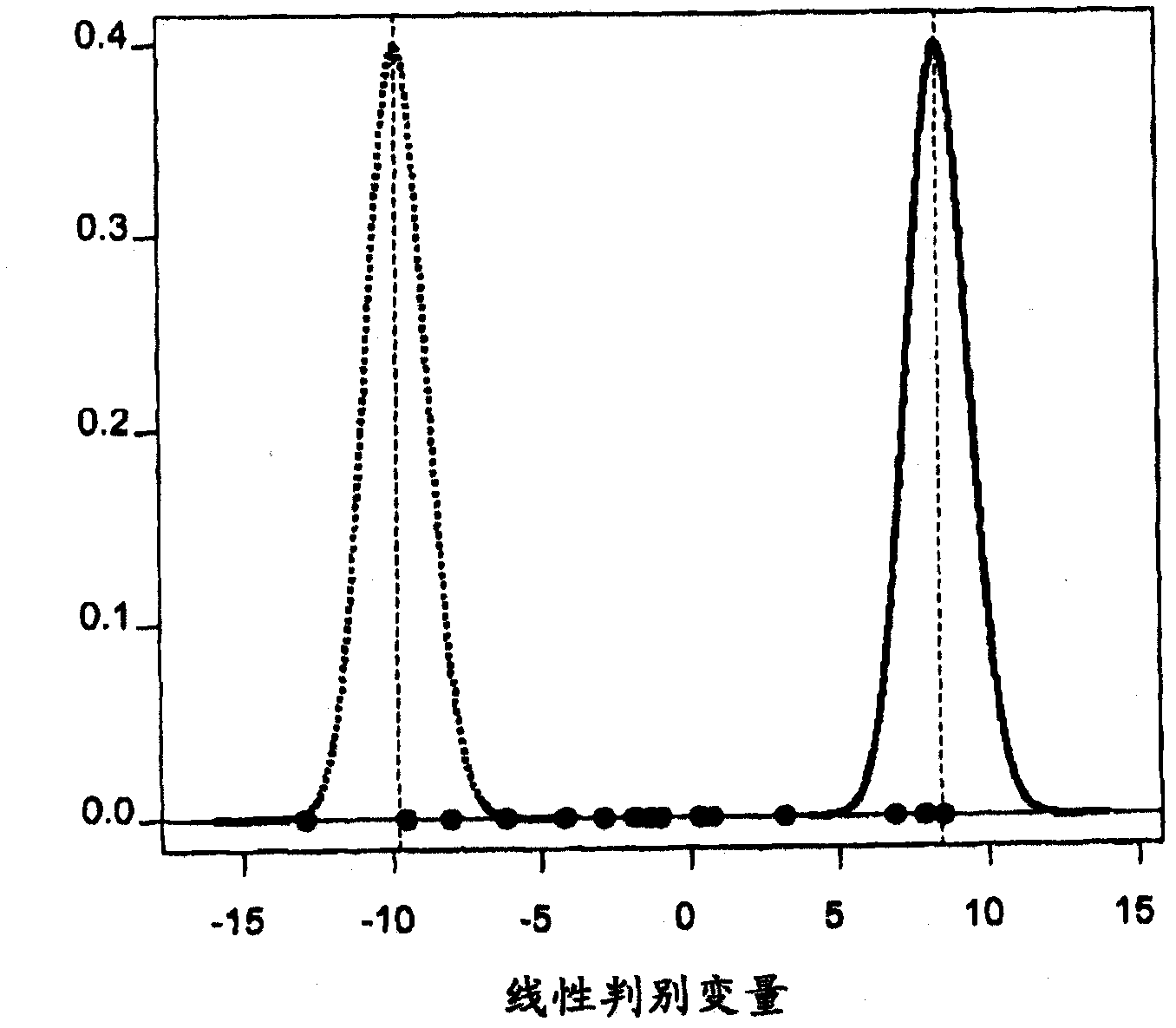
Methods of diagnosing acute cardiac allograft rejection
InactiveUS20110171645A1Acute allograft rejectionMicrobiological testing/measurementIsotope separationCardiac allograftT cell
The present invention relates to methods of diagnosing acute rejection of a cardiac allograft using genomic expression profiling, proteomic expression profiling, metabolite profiling, or alloreactive T-cell genomic expression profiling,
Owner:THE UNIV OF BRITISH COLUMBIA
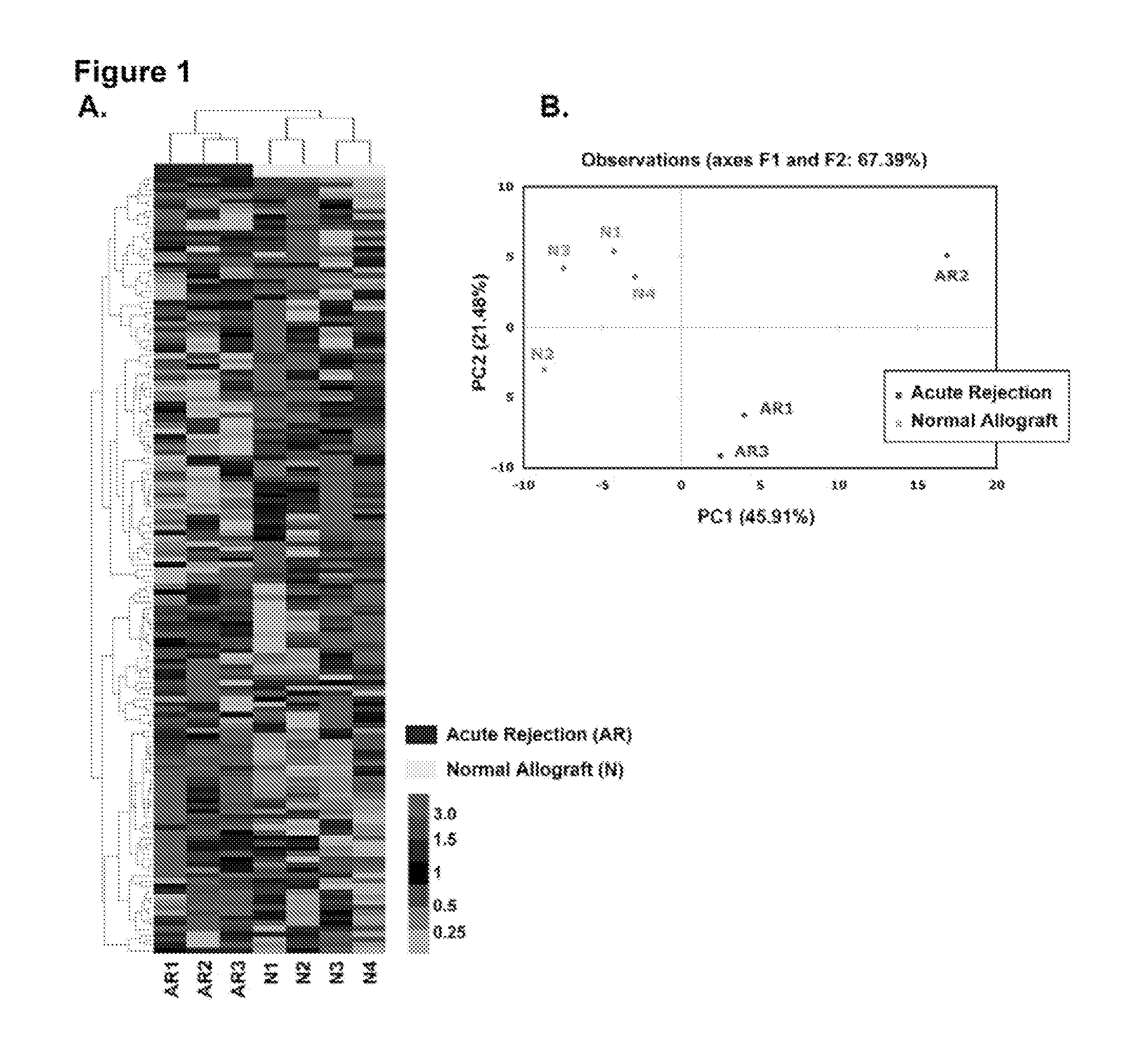
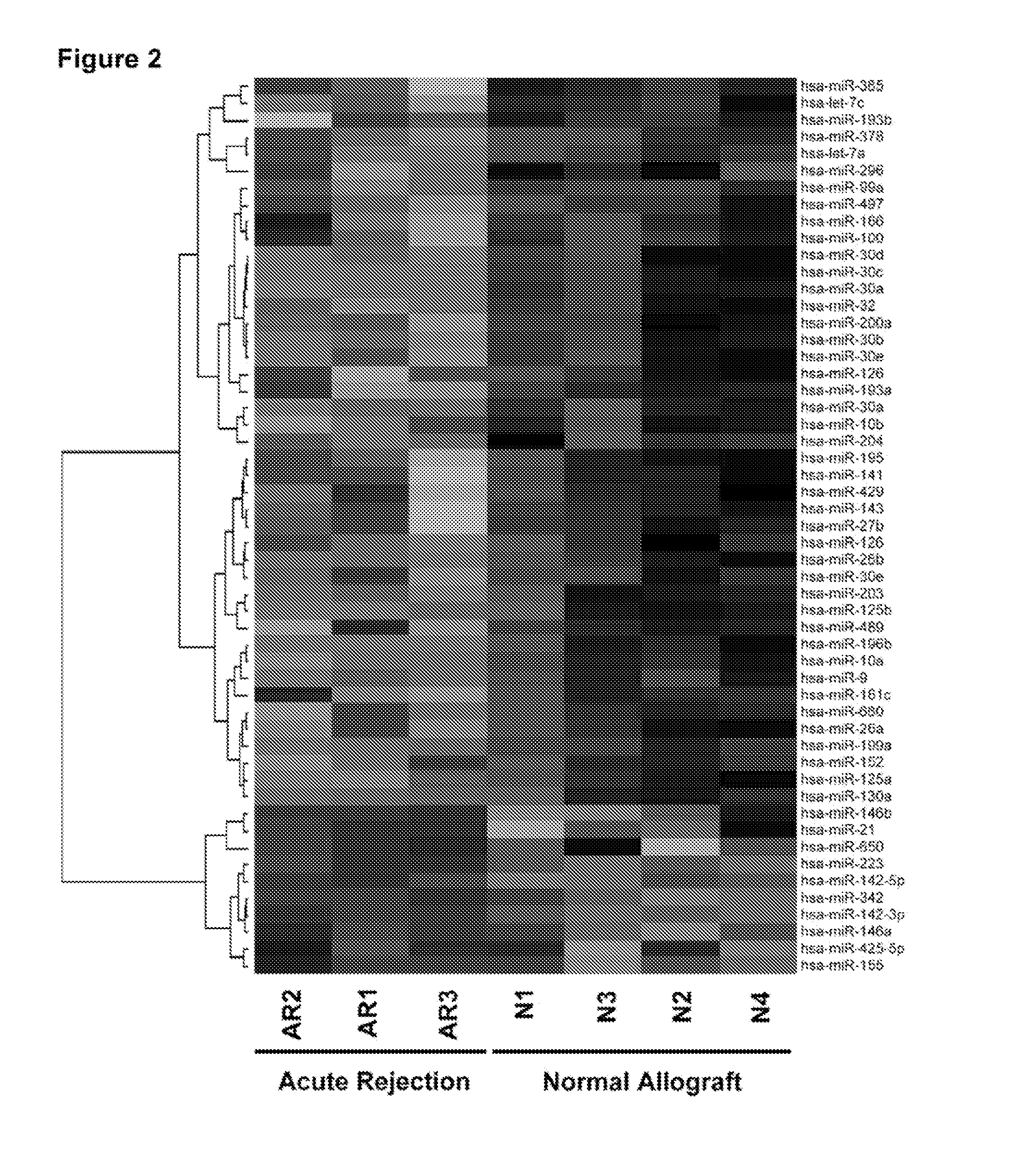
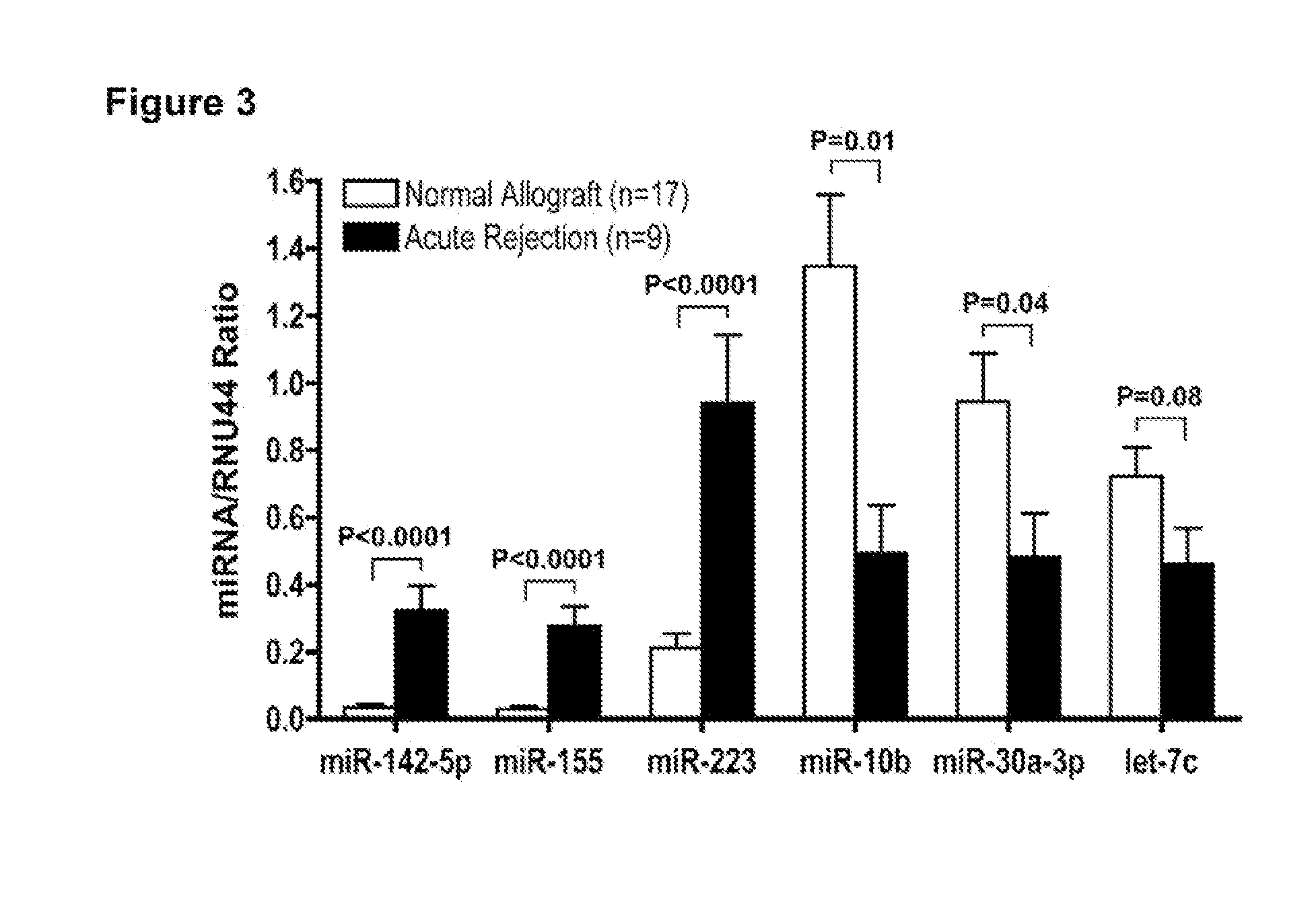
Method to assess human allograft status from microrna expression levels
ActiveUS20120101001A1Increased riskRisk assessmentMicrobiological testing/measurementLibrary screeningMicroRNACvd risk
The invention relates to, among other things, a method for assessing risk of organ rejection in a patient having a transplanted organ. The method includes measuring an amount of expression of a small non-coding marker RNA in a biological sample from the patient. The method further includes comparing the measured amount of expression of the small non-coding marker RNA in the patient to a reference amount of expression of the small non-coding marker RNA. In another aspect, the invention relates to kits for assessing risk of organ rejection in a patient having a transplanted organ.
Owner:CORNELL UNIVERSITY
Immunogenic peptides and their use in transplantation
ActiveUS9248171B2Polypeptide with localisation/targeting motifPeptide/protein ingredientsOxidation-Reduction AgentRedox
The present invention relates to the use of immunogenic peptides comprising a T-cell epitope derived from an allograft antigen and a redox motif such as C-(X)2-[CST] or [CST]-(X)2-C in the prevention and / or treatment of allograft rejection and in the manufacture of medicaments therefore.
Owner:IMCYSE
2-propylene-1-ones as hsp70 inducer
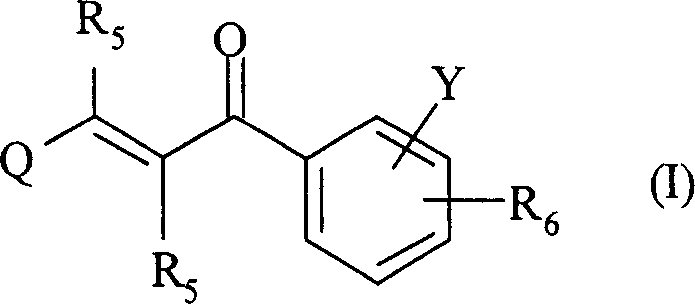
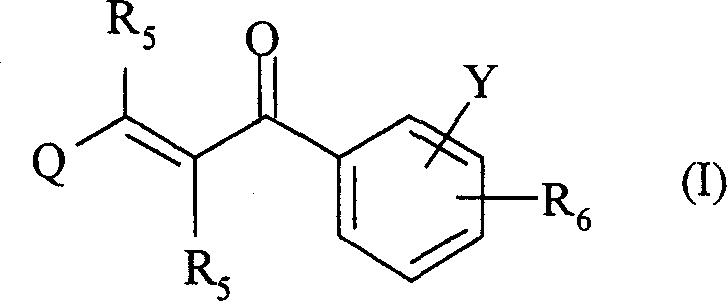
The present invention relates to novel compounds of 2-propene-1-one series, of general formula (I), their derivatives, analogs, tautomeric forms, stereoisomers, polymorphs, pharmaceutically acceptable salts, pharmaceutically acceptable solvates and pharmaceutically acceptable compositions containing them, wherein R5, R6, Q and Y are as defined in the specification. The present invention also relates to a process for preparing such compounds, compositions containing such compounds, and use of such compound and composition in medicine. The compounds of the general formula (I) induce HSP-70 and are useful for the treatment of diseases accompanying pathological stress in a living mammalian organism, including a human being, such as stroke, myocardial infarction, inflammatory disorder, hepatotoxicity, sepsis, diseases of viral origin, allograft rejection, tumourous diseases, gastric mucosal damage, brain haemorrhage, endothelial dysfunctions, diabetic complications, neuro-degenerative diseases, post-traumatic neuronal damage, acute renal failure, glaucoma and aging related skin degeneration.
Owner:TORRENT PHARMA LTD
Preparation and Application of Universal Targeting CD19 Antigen Chimeric Receptor T Cells
ActiveCN110616189AVigorousReduce rejectionAntibody mimetics/scaffoldsMammal material medical ingredientsAllograft rejectionCardiac allograft
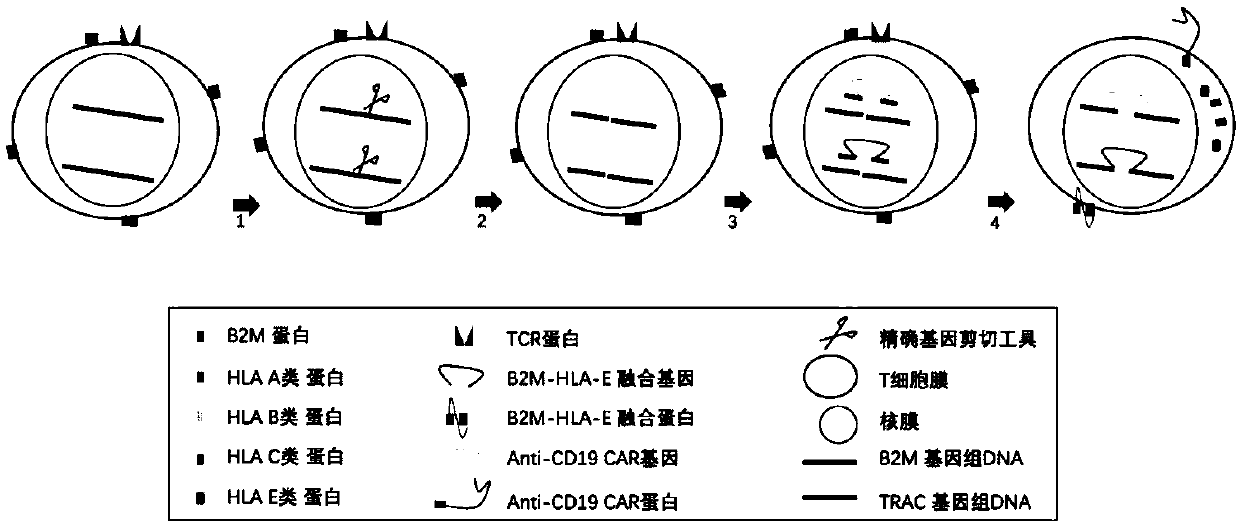
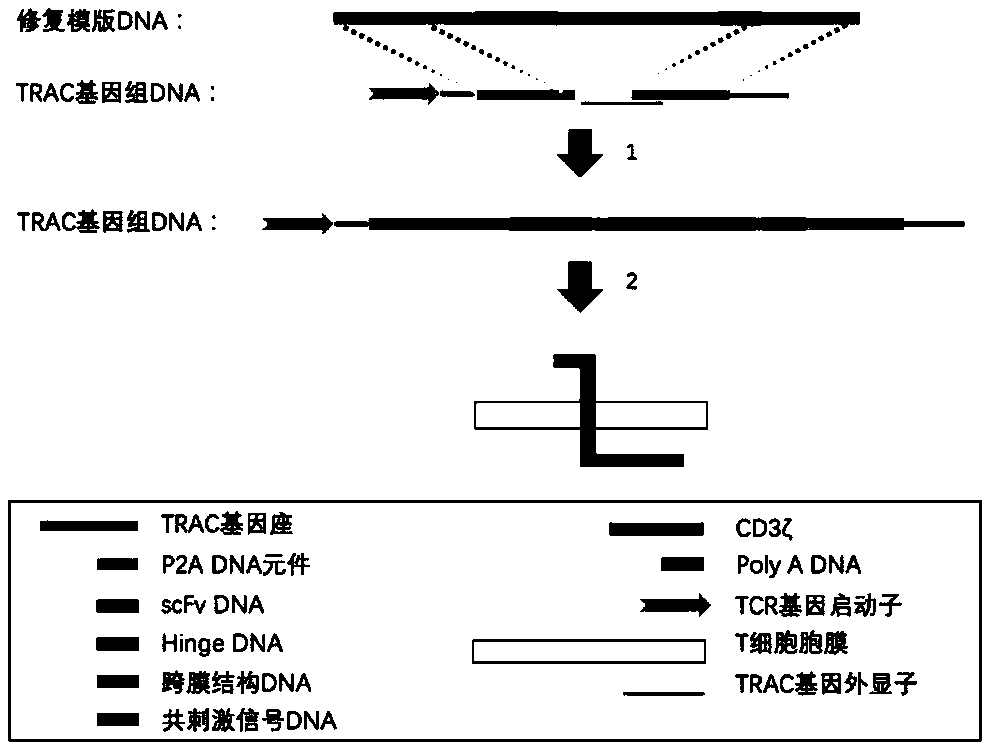
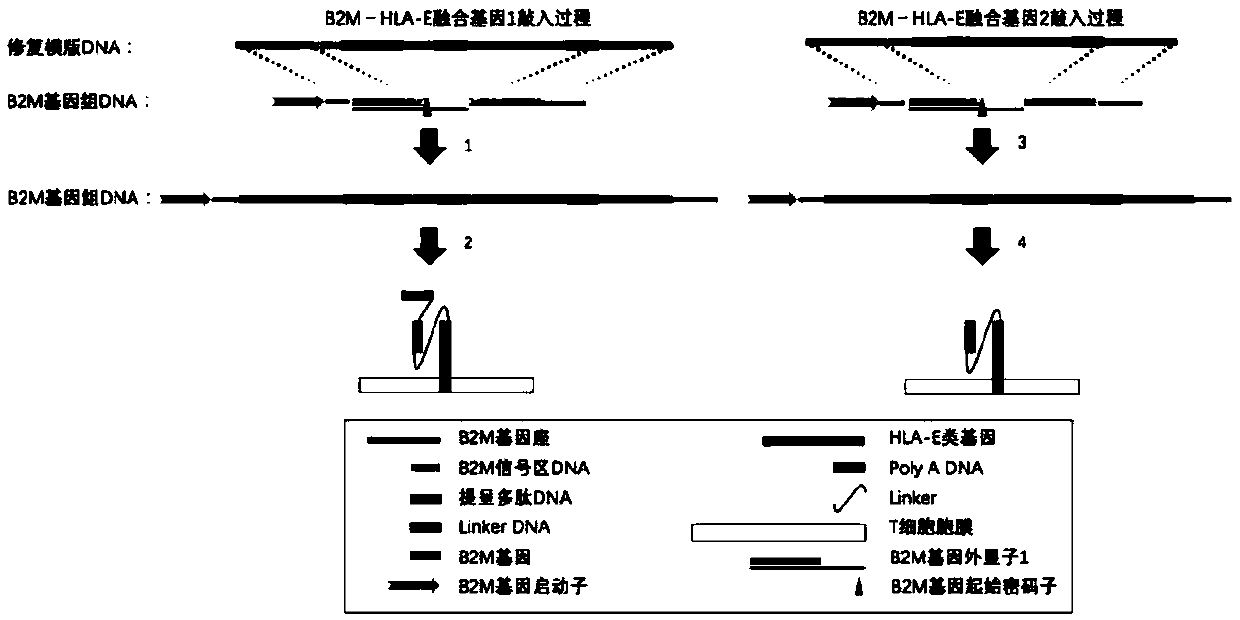
The invention relates to a preparation and an application of universal targeted CD19 antigen chimeric receptor T cells. Concretely, the invention relates to a method for preparing targeted CD19 antigen chimeric receptor T cells. The method comprises delivering a gene editing substance to the T cell to shear TRAC and B2M genes; and cloning the targeted CD19 CAR and B2M-HLA-E fusion gene into a repair template vector to simultaneously introduce targeted CD19 CAR and B2M-HLA-E fusion gene homologous recombination repair templates into T cells. The universal targeted CD19 antigen chimeric receptorT cell has the advantages of small allograft rejection reaction, high yield, high safety, immediate availability, wide application range and the like.
Owner:西安桑尼赛尔生物医药有限公司
Novel Predictors of Transplant Rejection Determined by Peripheral Blood Gene-Expression Profiling
ActiveUS20080274906A1Sugar derivativesMicrobiological testing/measurementProtein profilingTransplant rejection
The present invention provides methods and kits for predicting transplant rejection or tolerance. Methods for predicting the probability of cardiac allograft rejection via profiling of peripheral blood gene expression are also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Preparation method of tissue engineering acellular vascular scaffold
InactiveCN106729979AImprove mechanical propertiesAddressing issues such as aneurysmsTissue regenerationProsthesisCross-linkCell-Extracellular Matrix
The invention discloses a preparation method of a tissue engineering acellular vascular scaffold. Enhanced tissue engineering blood vessels without immunogenicity can be obtained by embedding micron high-molecular polymer scaffold into subcutaneous part or abdominal cavity of a host and performing acellular treatment on the blood vessel scaffold wrapped by utilizing a host immune protection mechanism. The preparation method has the advantages that deficient mechanical performance of the tissue engineering acellular blood vessel can be solved, and the mechanical performance of the original tissue engineering acellular vascular scaffold can be fully improved through a multilayer cross-linked mesh type high-molecular scaffold; the problem of immunogenicity of the tissue engineering blood vessel in allotransplantation can be solved, the scaffold blood vessel formed by migrating cells caused by immunologic mechanism is established in a host animal body, the transplanted immunoreaction can be reduced through acellular treatment, extracellular matrix is fully utilized to provide a good environment for cell regeneration; and different acellular vascular scaffolds can be prepared through designing scaffolds of different shapes and sizes of by utilizing the method, so as to be applied under different blood vessel transplanting conditions.
Owner:NANKAI UNIV
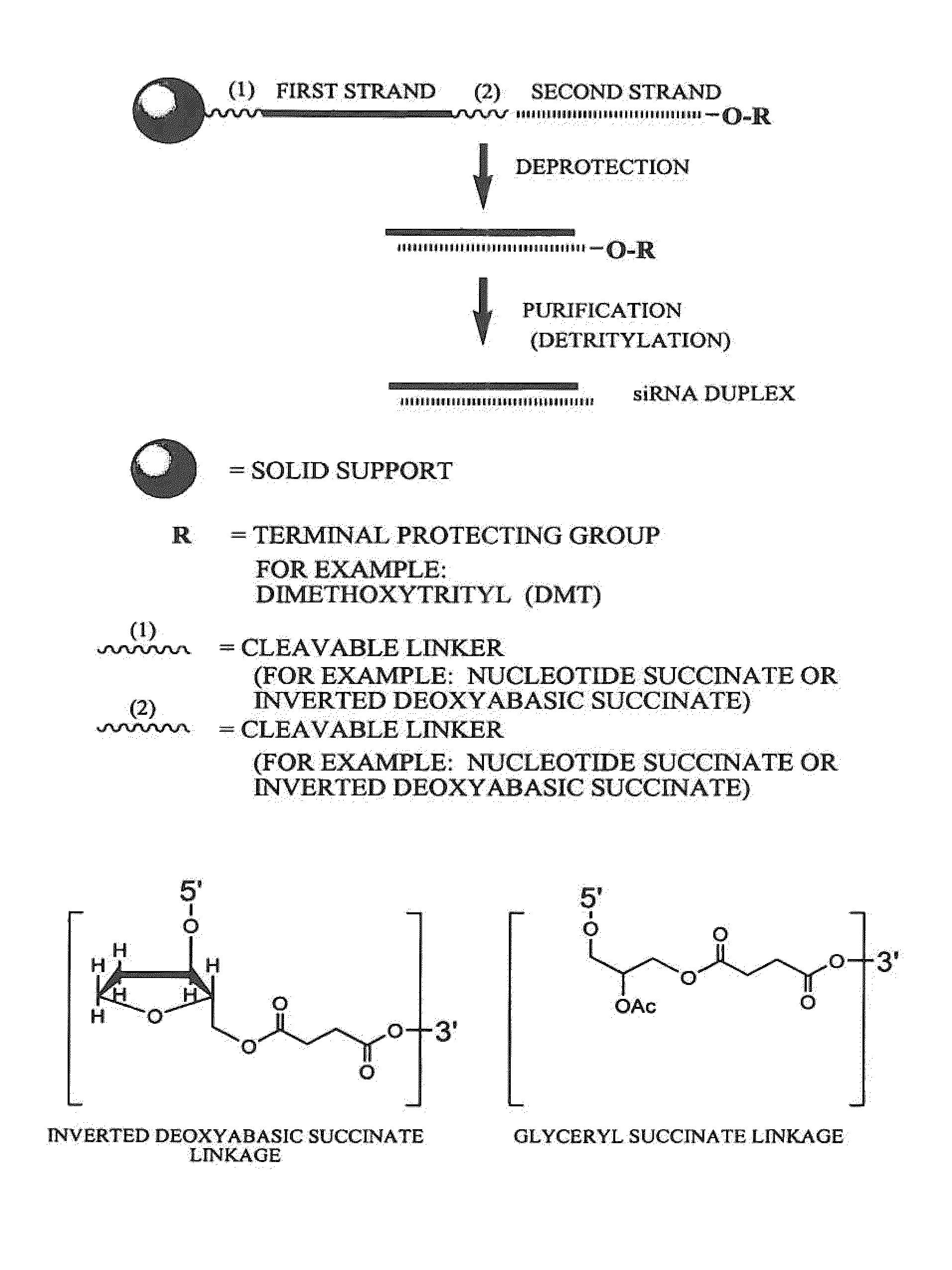
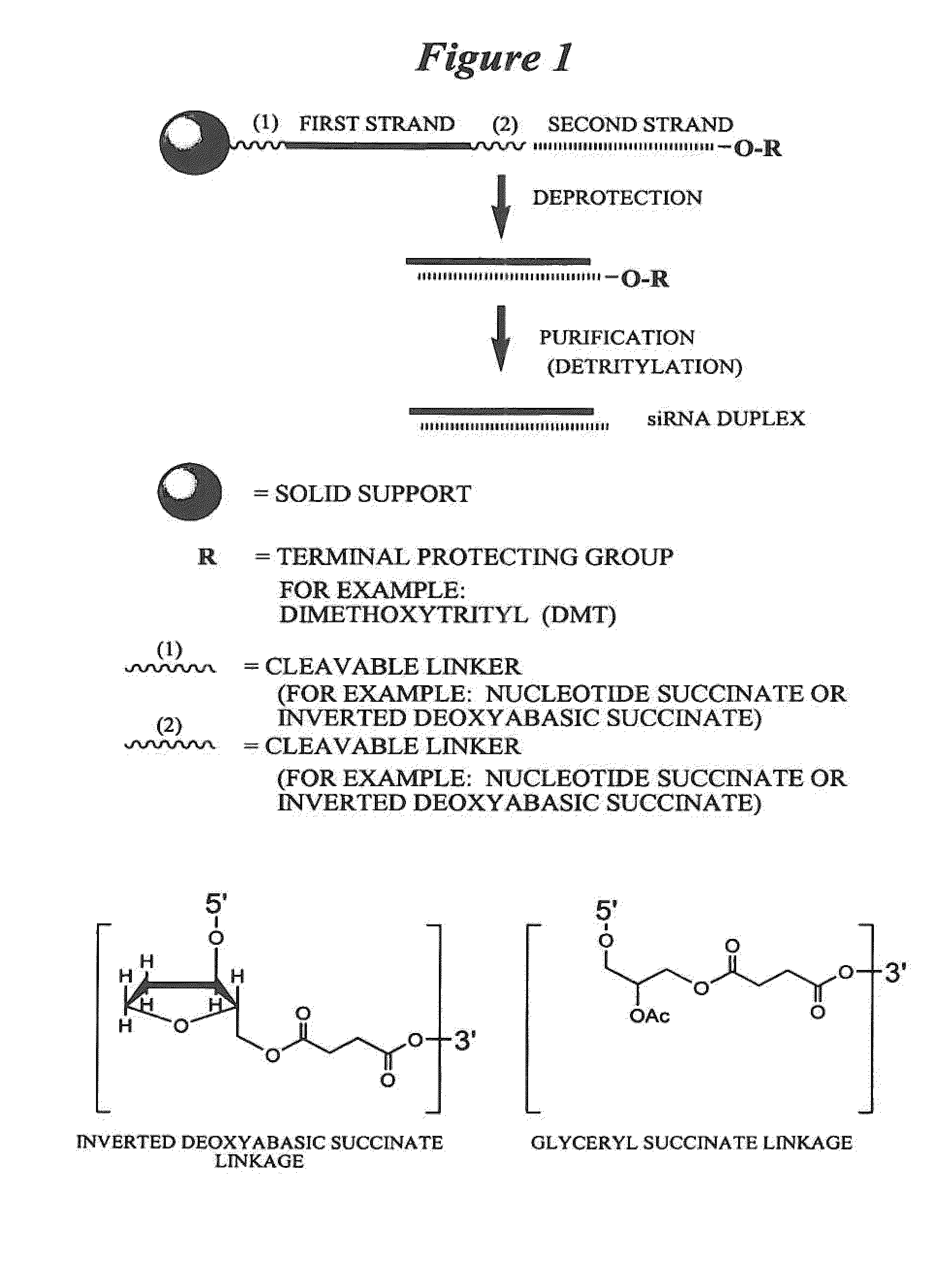
RNA interference mediated inhibition of Fos gene expression using short interfering nucleic acid (siNA)
InactiveUS7910724B2Improves various propertyImprove the immunityOrganic active ingredientsSugar derivativesAutoimmune diseaseAllograft rejection
This invention relates to compounds, compositions, and methods useful for modulating c-Fos gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of c-Fos gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of c-Fos genes. The small nucleic acid molecules are useful in the treatment of cancer, proliferative diseases or conditions, inflammatory diseases or conditions, allergic diseases or conditions, infectious diseases or conditions, autoimmune diseases or conditions, or transplantation / allograft rejection in a subject or organism.
Owner:SIRNA THERAPEUTICS INC
Method for identifying kidney allograft recipients at risk for chronic injury
A method for identifying a renal allograft recipient at risk for chronic allograft damage or interstutial fibrosis and tubular atrophy (IF / TA) by comparing the transcription level of a preselected gene signature set with the transcription level of a comparison standard, and diagnosing the recipient as being at risk for chronic allograft damage if the transcription level of the preselected gene signature set is significantly higher than the transcription level of the comparison standard.
Owner:WESTERN SYDNEY LOCAL HEALTH DISTRICT +1
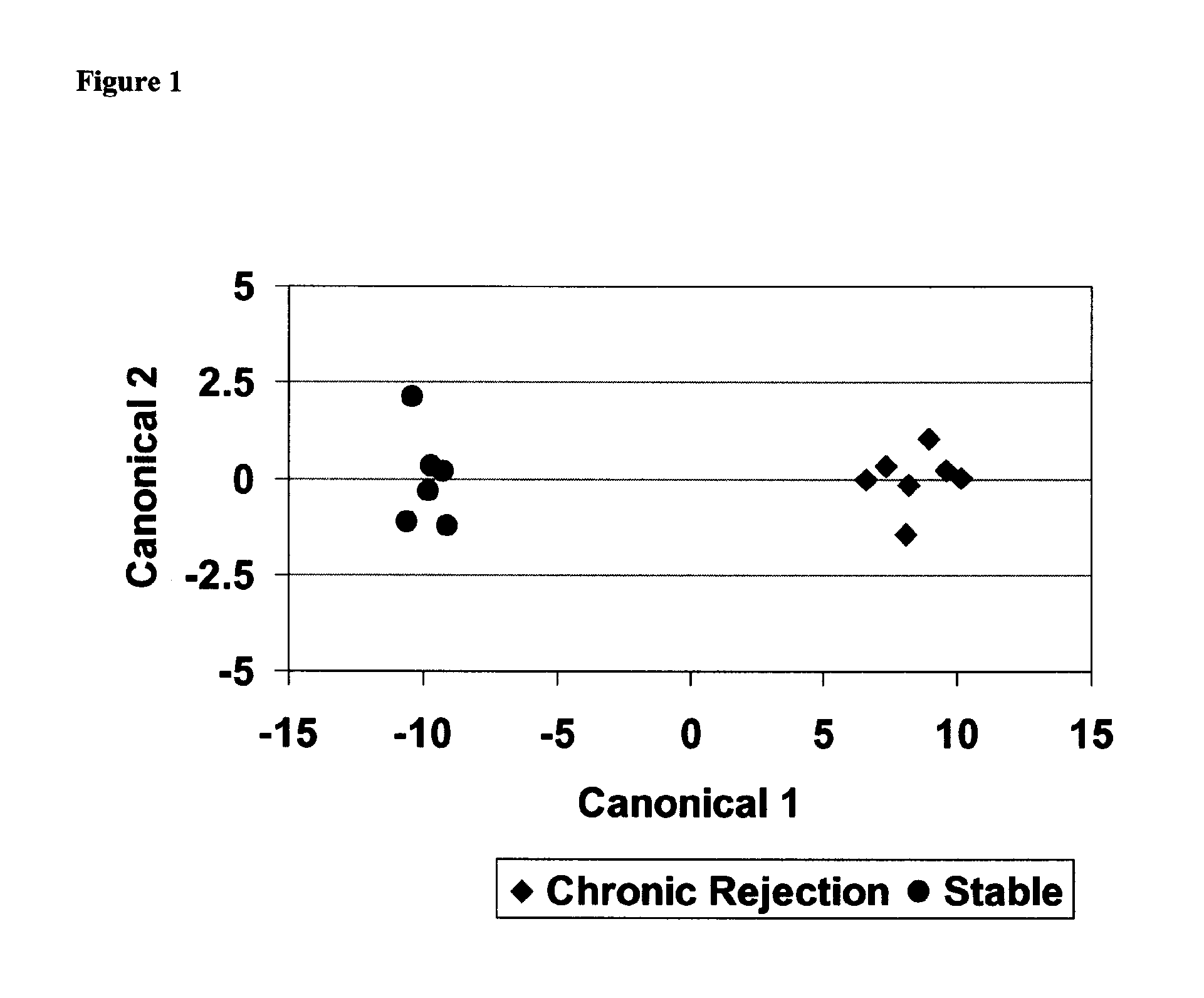
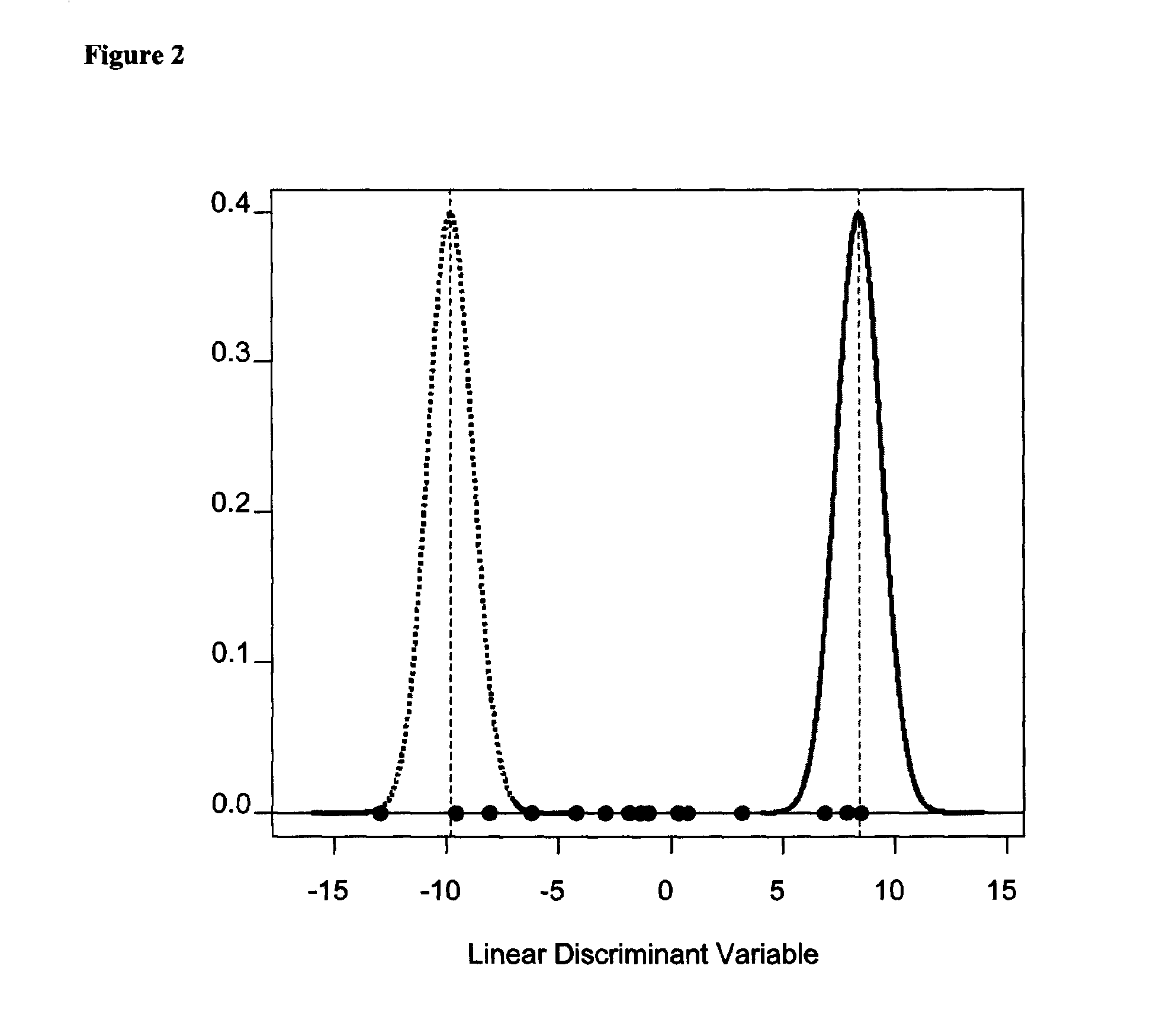
Methods of diagnosing chronic cardiac allograft rejection
The present invention relates to methods of diagnosing chronic rejection of a cardiac allograft using genomic expression profiling, proteomic expression profiling, or a combination of genomic and proteomic expression profiling.
Owner:THE UNIV OF BRITISH COLUMBIA
Targeted delivery of therapeutic agents with lyophilized matrices
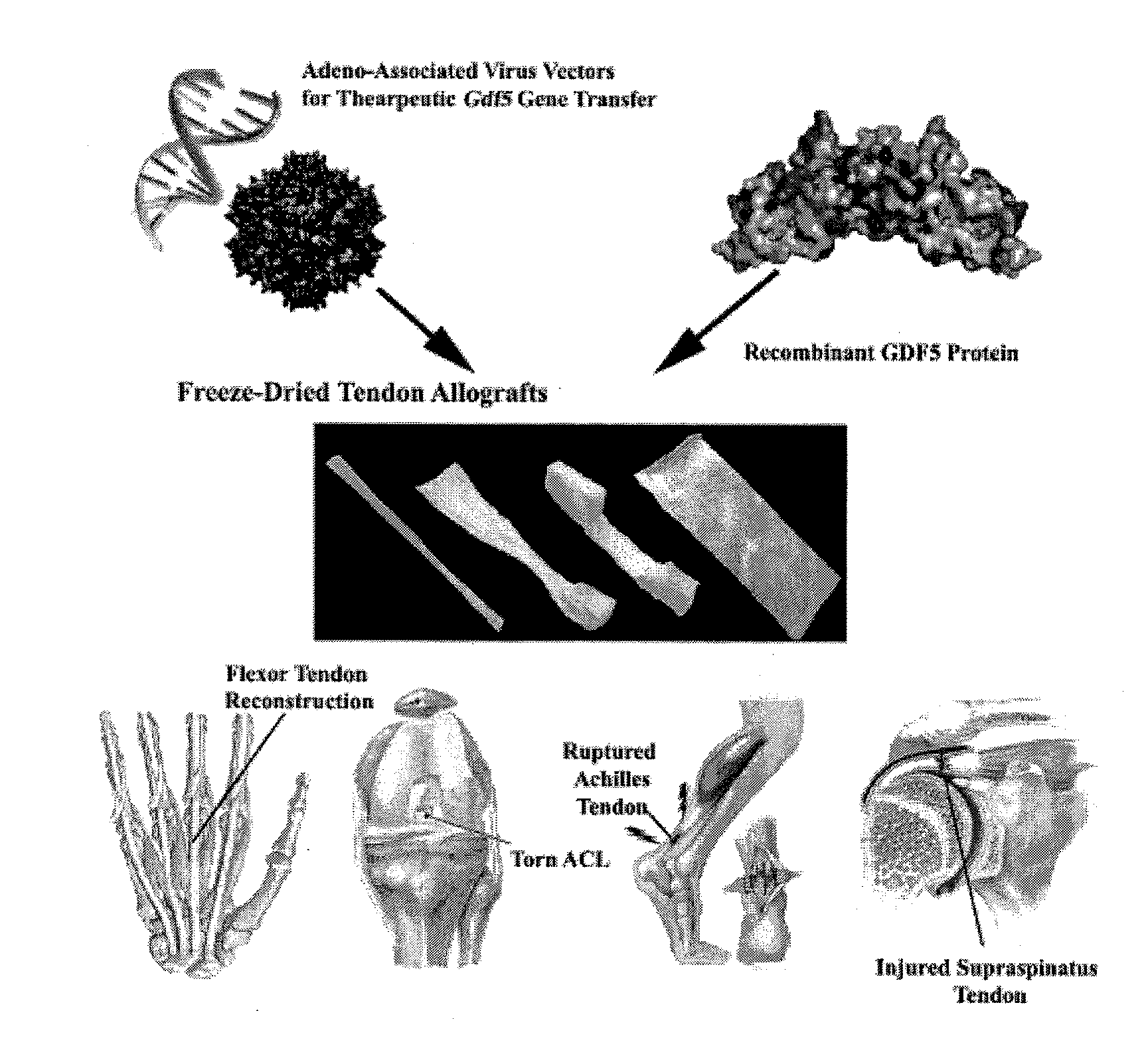
InactiveUS20120093801A1Healing time constantPromote healingBiocideOrganic active ingredientsSurgical GraftTissue repair
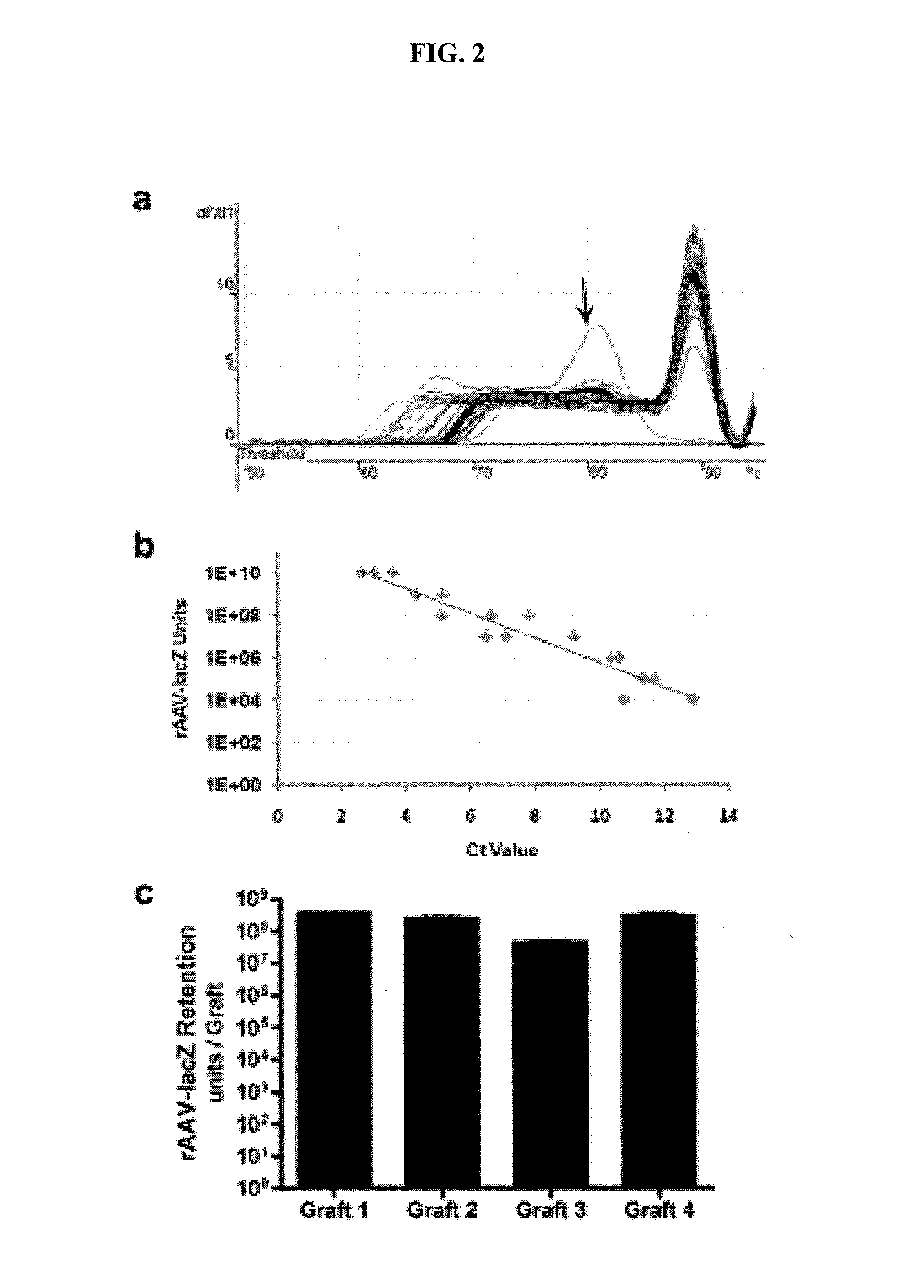
Embodiments of surgical grafts, and methods, for the delivery of therapeutic agents to a target tissue via acellular matrices, are described. In some embodiments, nonviable matrices are successful in preventing or lessening adhesion formation by guiding tissue repair and remodeling, while also providing the target tissue with therapeutic agents that can act as repair and remodeling factors. An exemplary method to modulate flexor tendon healing and provide elimination or reduction of fibrotic adhesions involves loading a freeze-dried flexor digitorum longus allograft with recombinant adeno-associated viral (rAAV) vectors for the targeted and transient expression of growth / differentiation factor 5 (GDF5).
Owner:UNIVERSITY OF ROCHESTER
Methods of diagnosing chronic cardiac allograft rejection
InactiveUS20110190151A1Chronic allograft rejectionMicrobiological testing/measurementLibrary screeningCardiac allograftGenome
The present invention relates to methods of diagnosing chronic rejection of a cardiac allograft using genomic expression profiling, proteomic expression profiling, or a combination of genomic and proteomic expression profiling.
Owner:THE UNIV OF BRITISH COLUMBIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com