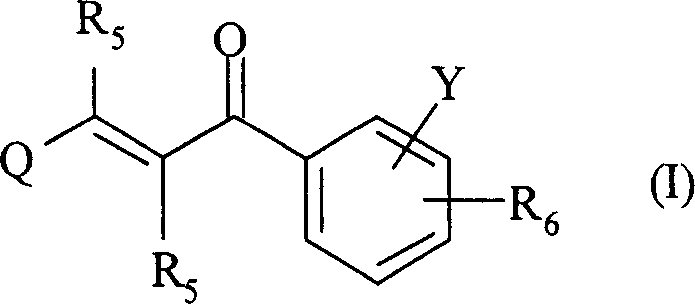
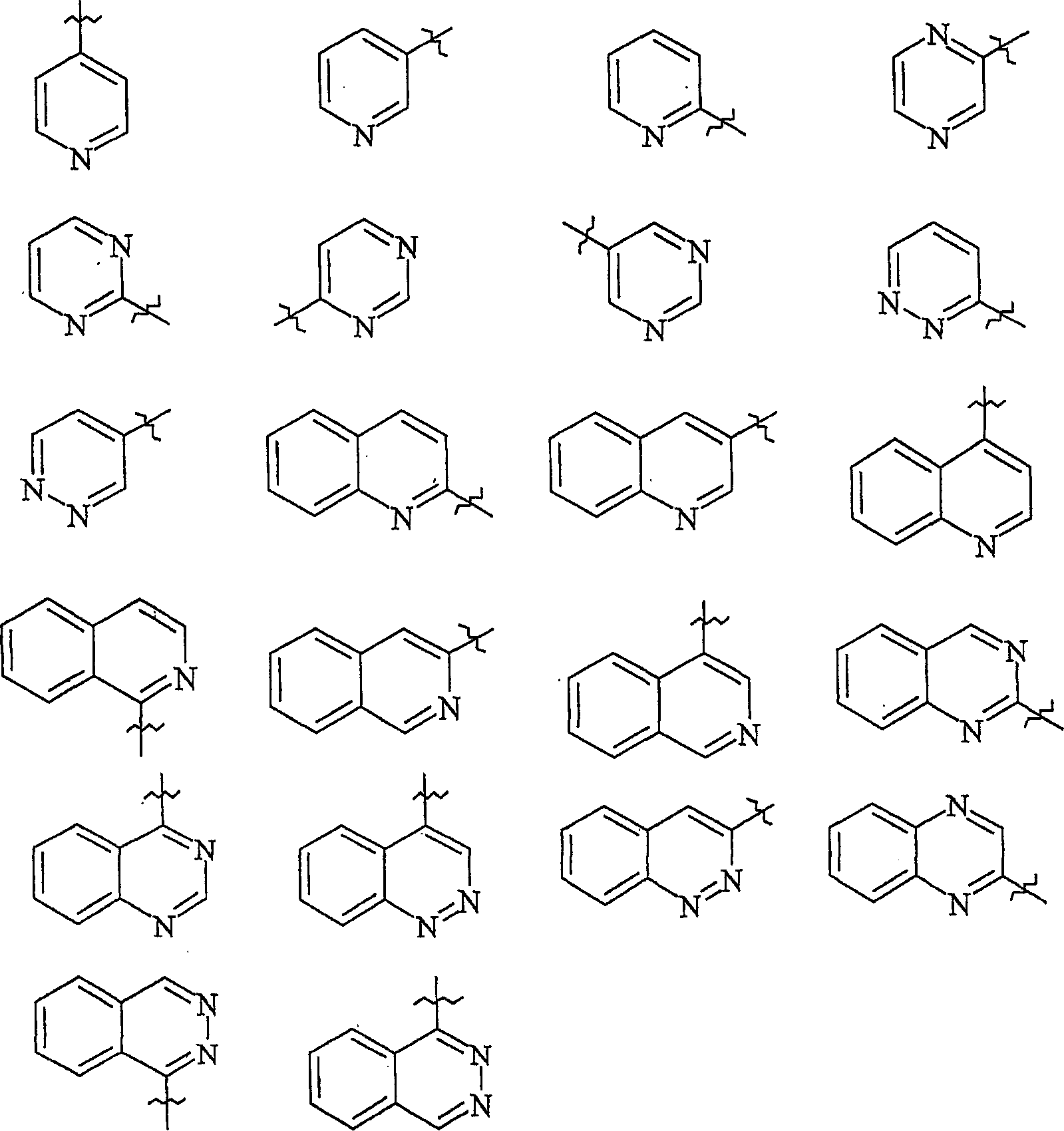
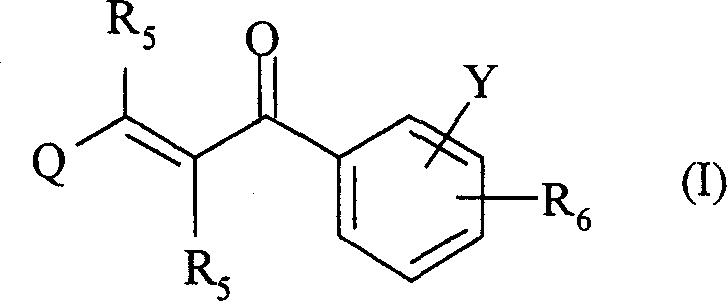
2-propylene-1-ones as hsp70 inducer
A CH2, drug technology, applied in the field of 2-propen-1-one as HSP 70 inducer, can solve the problem of slow wound healing and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0839] 3-(3-Hydroxy-quinoxalin-2-yl)-1-[4-(4-methyl-piperazine-1-carbonyl-phenyl)-propenone (Compound No. 37)
[0840] Step A: Preparation of 3-Hydroxy-quinoxaline-2-carboxaldehyde
[0841] To 3-methyl-quinoxalin-2-ol (1 g, 6.2 mmol) in 1,4-dioxane (30 mL) was added selenium dioxide (2 g, 18.7 mmol) and refluxed for 4 hours. The reaction mixture was then cooled, filtered through celite and partitioned between water and ethyl acetate. The combined organic layers were washed successively with water (50ml x 2) and brine (50ml x 2), dried over anhydrous sodium sulfate and evaporated under vacuum to give 0.58g of the title compound as a brown solid. This compound was used in the next step without purification.
[0842] 1 H NMR (400 MHz, DMSOd 6 ) δ 7.63-7.69 (2H, m), 7.84-8.38 (2H, m), 10.19 (IH, s), 12.84 (IH, bs).
[0843] Step B: Preparation of 4-[3-(3-Hydroxy-quinoxalin-2-ylacryloyl)benzoic acid
[0844] A solution of 0.58 g (3.3 mmol) of the product from Example 1, Step ...
Embodiment 2
[0851] 1-[4-(4-Methyl-piperazine-1-carbonyl)-phenyl-3-(6-trifluoromethyl-quinolin-2-yl)-propenone (compound number 32)
[0852] Step A: Preparation of 2-methyl-6-trifluoromethylquinoline
[0853] Add sodium m-nitrobenzenesulfonate (7g, 31mmol), ferrous sulfate (8.62g, 31mmol) and boric acid ( 7.7 g, 124 mmol). The reaction mixture was refluxed for 1 hour with vigorous stirring. Crotonaldehyde (3.25 g, 46 mmol) was then added dropwise thereto and the mixture was refluxed for 8 hours. After cooling to 60°C, methanol (10ml) was added and filtered through celite. The pH of the filtrate was adjusted to 7 with aqueous sodium hydroxide solution (1 N). Volatile materials were evaporated in vacuo. The reaction mixture was partitioned between water and ethyl acetate. The combined organic layers were washed successively with water (100ml x 2) and brine (50ml x 2), dried over anhydrous sodium sulfate and evaporated under vacuum. The residue was purified by silica gel column chromat...
Embodiment 3
[0866] 2-{3-[4-(3-Dimethylamino-pyrazole-1-carbonyl)-phenyl]-3-oxo-propenyl}-quinoline-6-sulfonamide (Compound No. 46)
[0867] Step A: Preparation of 2-methyl-quinoline-6-sulfonamide (sulphonamide)
[0868]Add m-nitrobenzenesulfonate sodium (1.3g, 5.8mmol), ferrous sulfate (1.6g, 5.8mmol) ) and boronic acid (1.4 g, 23 mmol). The reaction mixture was refluxed for 1 hour with vigorous stirring. Crotonaldehyde (0.7 g, 8.7 mmol) was then added dropwise thereto and the mixture was refluxed for 8 hours. After cooling to 60°C, methanol (2ml) was added and filtered through celite. The pH of the filtrate was adjusted to 7 with 1N aqueous sodium hydroxide solution. The reaction mixture was partitioned between water and ethyl acetate. The combined organic layers were washed successively with water (10ml x 2) and brine (5ml x 2), dried over anhydrous sodium sulfate and evaporated under vacuum. The residue was purified by silica gel column chromatography using 30% ethyl acetate in h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com