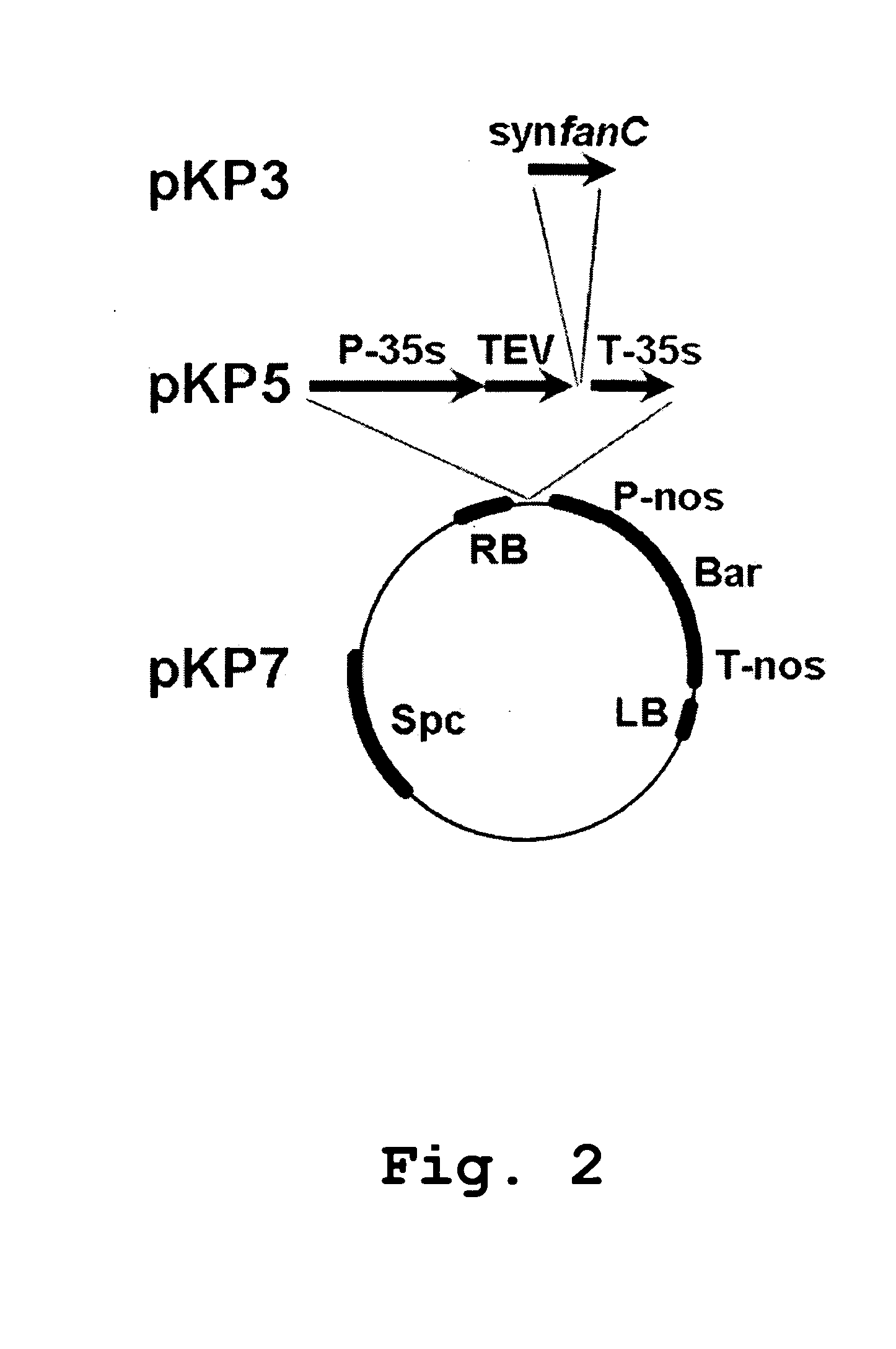
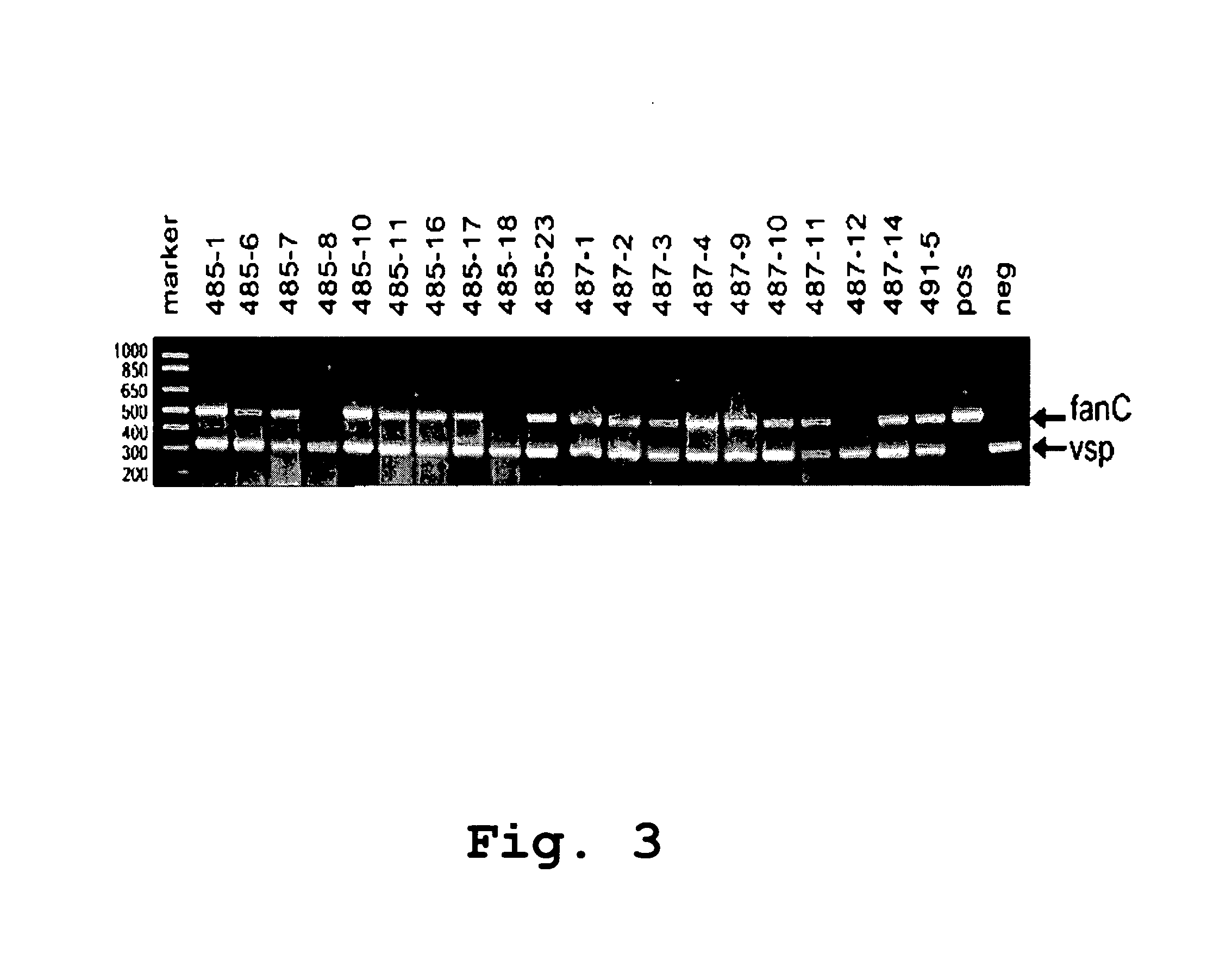
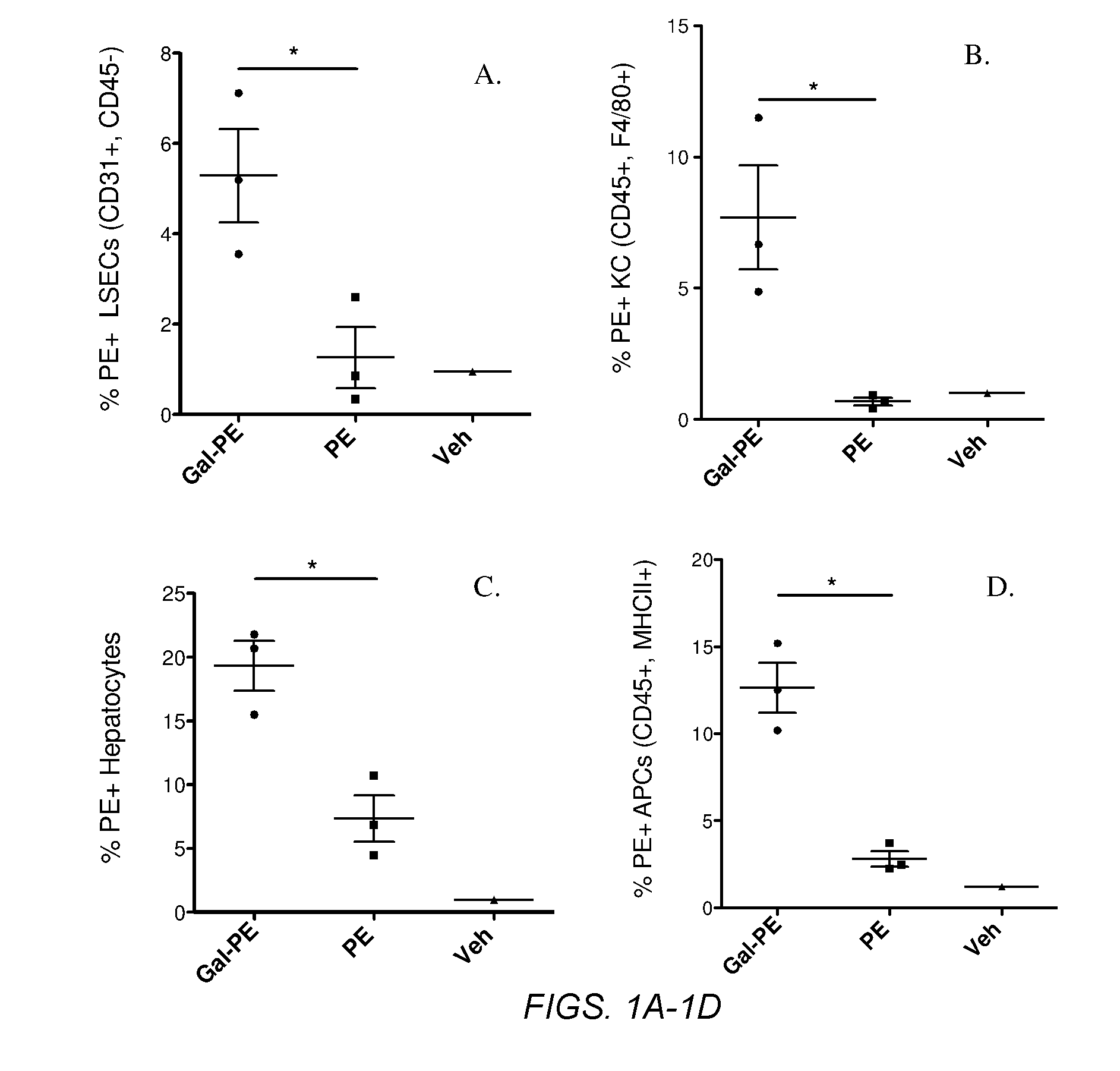
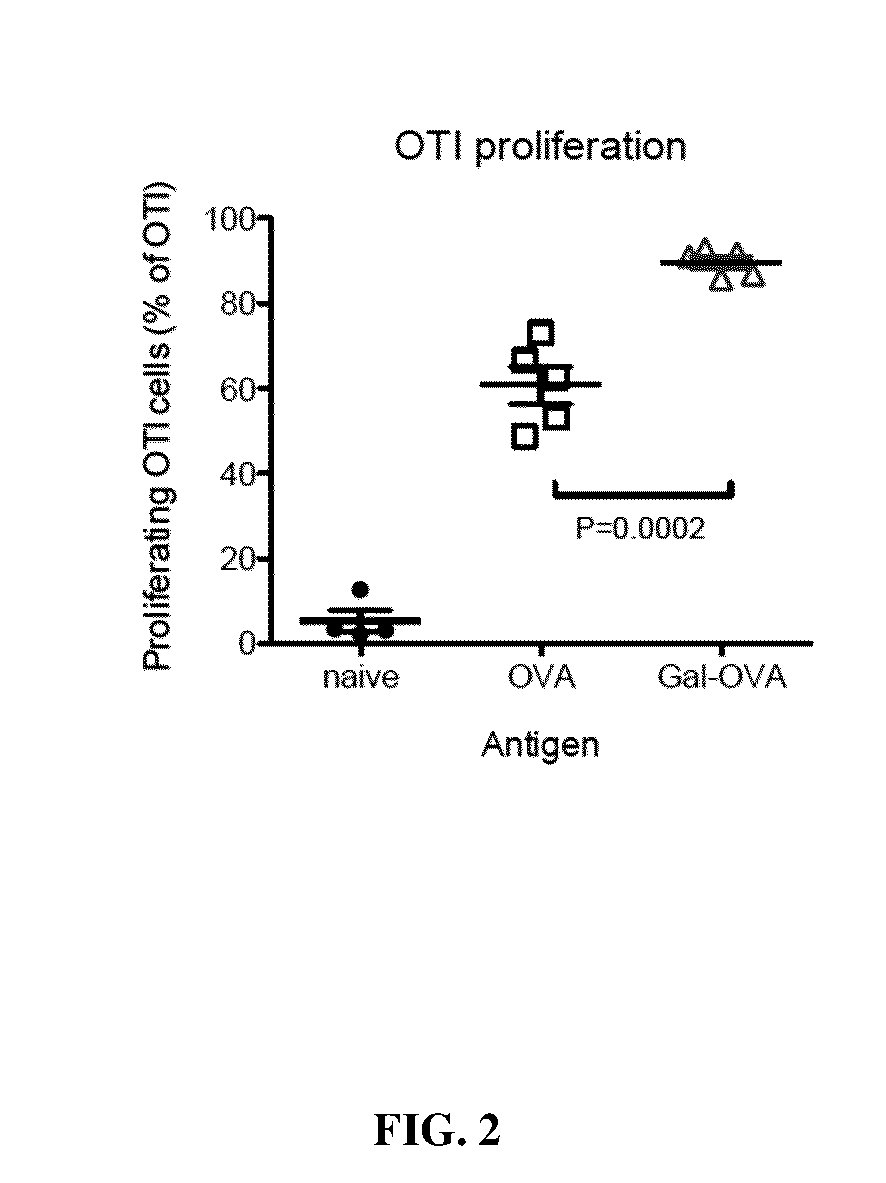
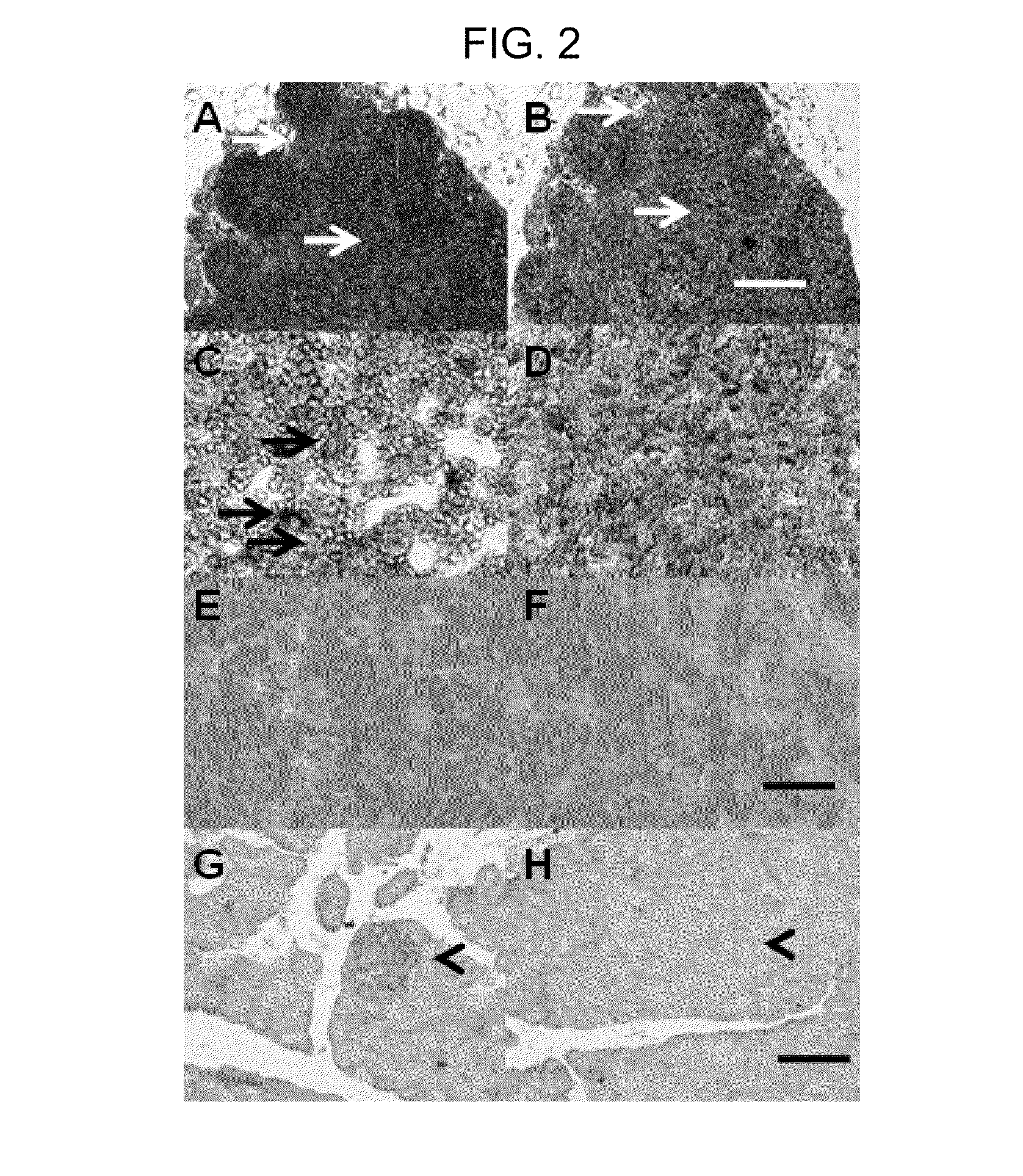Patents
Literature
42results about How to "Induce tolerance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Regulatory T cells and their use in immunotherapy and suppression of autoimmune responses
ActiveUS20050196386A1Enhanced growthInduce toleranceBiocideMammal material medical ingredientsImmunosuppressionSuppressor
Based upon a strong correlation between regulator T cells (Treg cells) and suppressing or preventing a cytotoxic T cell response, provided are methods for the production of ex vivo activated and culture-expanded isolated CD4+CD25+ suppressor Treg cells for the prevention or suppression of immune reactions in a host, particularly in a human host, and including autoimmune responses. The resulting ex vivo culture-expanded Treg cells provide a sufficient amount of otherwise low numbers of such cells, having long term suppressor capability to permit therapeutic uses, including the preventing, suppressing, blocking or inhibiting the rejection of transplanted tissue in a human or other animal host, or protecting against graft vs host disease. Also provided are therapeutic and immunosuppressive methods utilizing the ex vivo culture-expanded Treg cells for human treatment, and high efficiency methods for research use.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
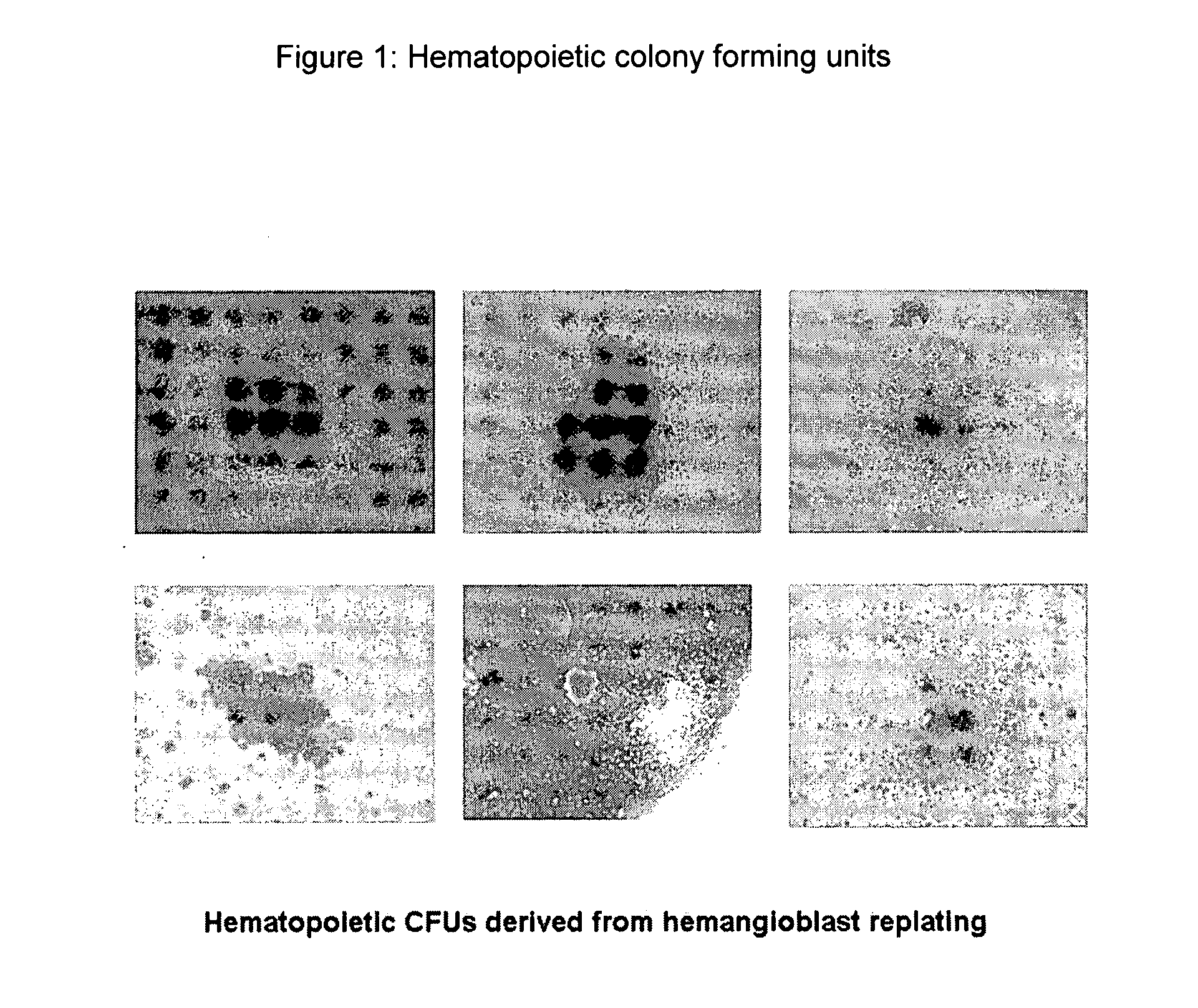
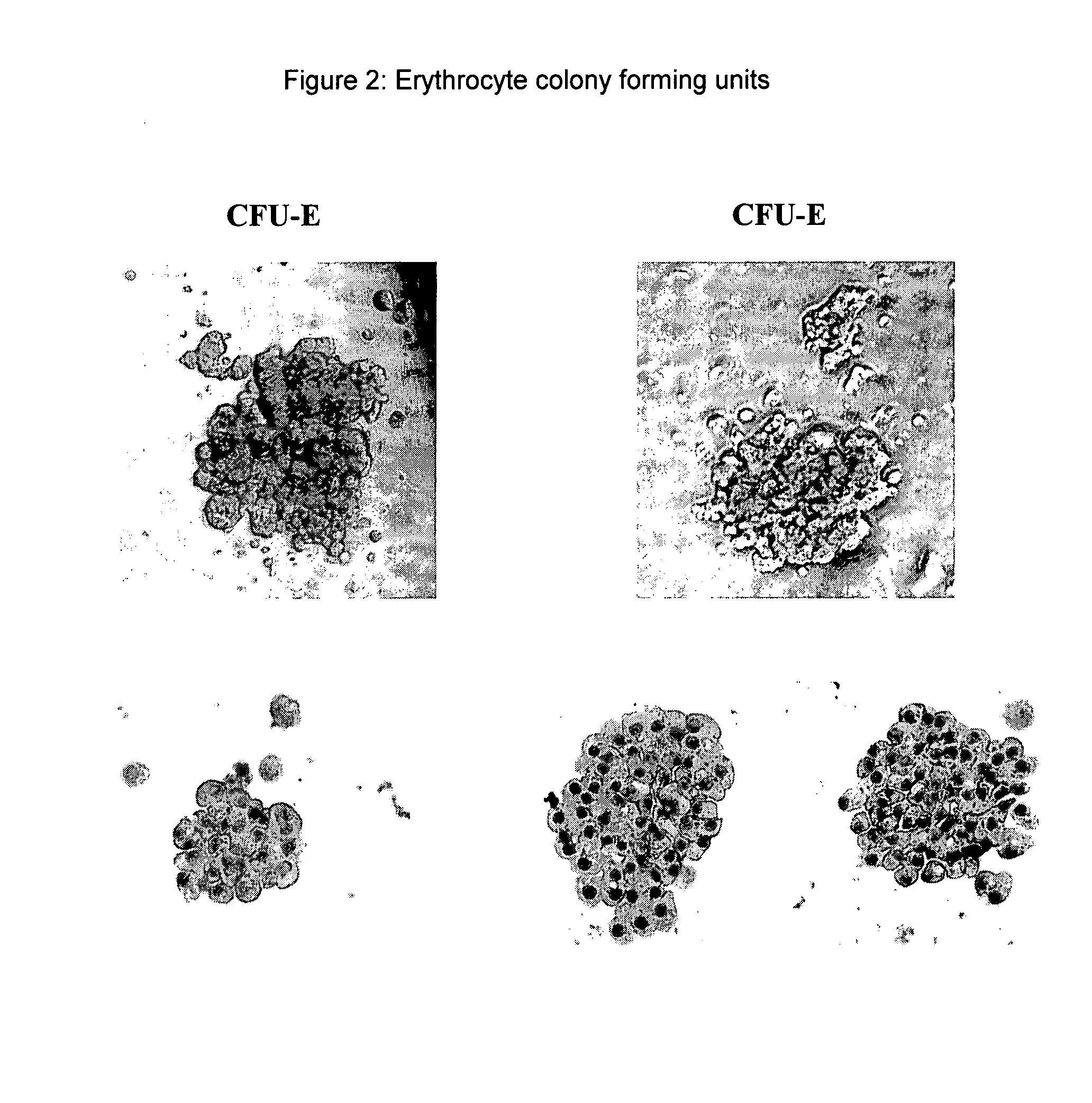
Hemangio-colony forming cells
ActiveUS20080014180A1Reduce riskInduce toleranceBiocideBlood/immune system cellsHematopoietic cellCell biology
Methods of generating and expanding human hemangio-colony forming cells in vitro and methods of expanding and using such cells are disclosed. The methods permit the production of large numbers of hemangio-colony forming cells as well as derivative cells, such as hematopoietic and endothelial cells. The cells obtained by the methods disclosed may be used for a variety of research, clinical, and therapeutic applications.
Owner:ADVANCED CELL TECH INC
Hemangio-colony forming cells
ActiveUS8017393B2Reduce riskInduce toleranceCulture processArtificial cell constructsHematopoietic cellCell biology
Methods of generating and expanding human hemangio-colony forming cells in vitro and methods of expanding and using such cells are disclosed. The methods permit the production of large numbers of hemangio-colony forming cells as well as derivative cells, such as hematopoietic and endothelial cells. The cells obtained by the methods disclosed may be used for a variety of research, clinical, and therapeutic applications.
Owner:ADVANCED CELL TECH INC
CTLA4-Cgamma4 fusion proteins
InactiveUS20020114814A1Reduce complement activating abilityReduced effector functionBacteriaPeptide/protein ingredientsAntigenAutoimmune responses
CTLA4-immunoglobulin fusion proteins having modified immunoglobulin constant region-mediated effector functions, and nucleic acids encoding the fusion proteins, are described. The CTLA4-immunoglobulin fusion proteins comprise two components: a first peptide having a CTLA4 activity and a second peptide comprising an immunoglobulin constant region which is modified to reduce at least one constant region-mediated biological effector function relative to a CTLA4-IgG1 fusion protein. The nucleic acids of the invention can be integrated into various expression vectors, which in turn can direct the synthesis of the corresponding proteins in a variety of hosts, particularly eukaryotic cells. The CTLA4-immunoglobulin fusion proteins described herein can be administered to a subject to inhibit an interaction between a CTLA4 ligand (e.g., B7-1 and / or B7-2) on an antigen presenting cell and a receptor for the CTLA4 ligand (e.g. CD28 and / or CTLA4) on the surface of T cells to thereby suppress an immune response in the subject, for example to inhibit transplantation rejection graft versus host disease or autoimmune responses.
Owner:GRAY GARY S +4
Edible vaccines expressed in soybeans
The present invention relates to vaccines that are made in transgenic soybeans for use in humans, animals of agricultural importance, pets, and wildlife. These vaccines are used as vaccines against viral, bacterial, fungal, parasitic or prion related diseases, cancer antigens, toxins, and autologous or self proteins. The transgenic soybeans of the instant invention also can be used for inducing tolerance to allergens or tolerance to autoimmune antigens, wherein an individual shows hypersensitivity to said allergen or has developed autoimmunity to autologous or self proteins, respectively. The invention also relates to prophylatically treating individuals and / or populations prior to showing hypersensitivity to allergens. Other aspects of the invention include using the transgenic soybeans as an oral contraceptive, and the expression of protein adjuvants in transgenic soybeans.
Owner:SOYMEDS
Glycotargeting therapeutics
ActiveUS20170007708A1Reduce immune responseReduce symptomPolypeptide with localisation/targeting motifPeptide/protein ingredientsAutoimmune diseaseTolerability
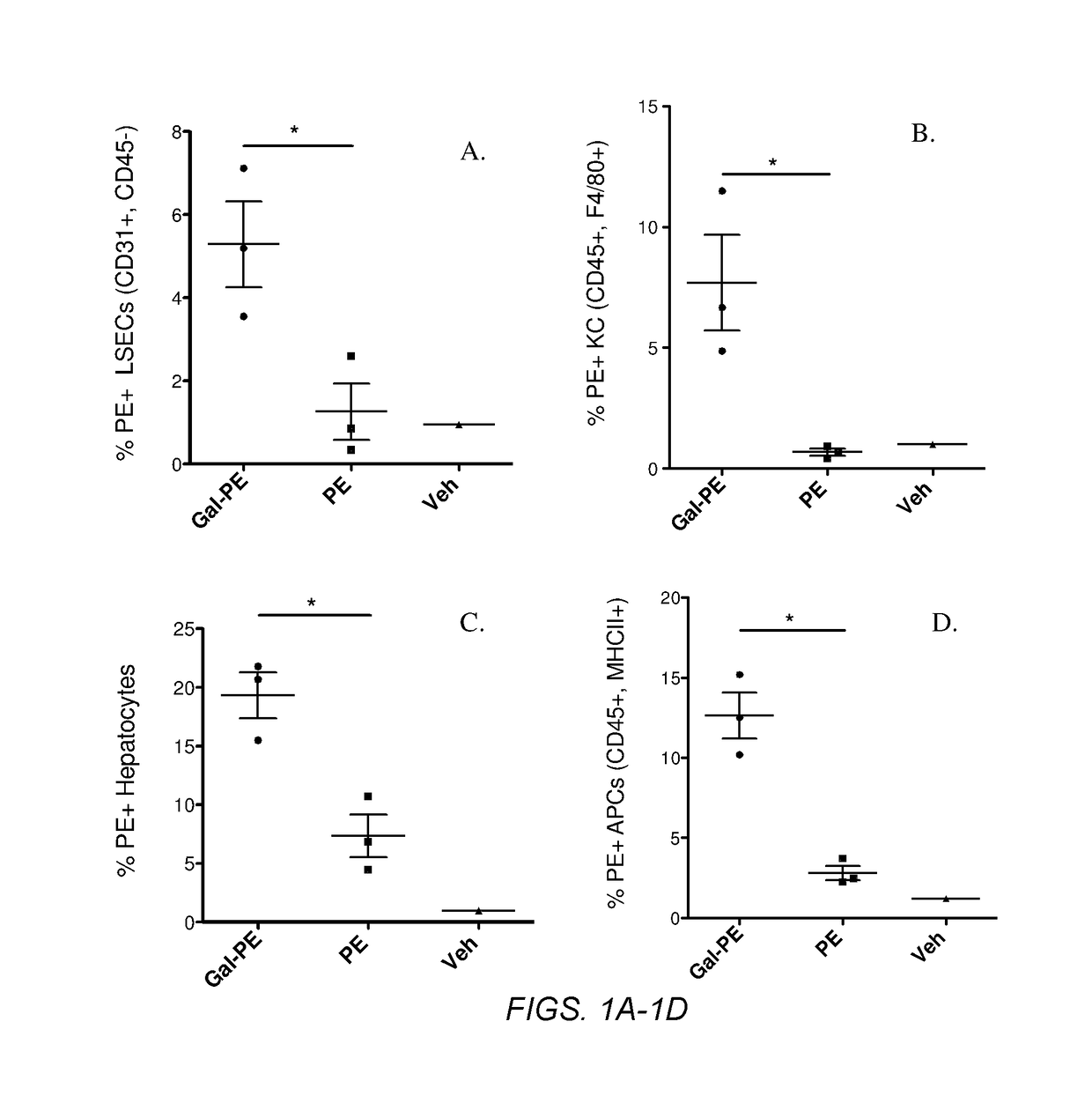
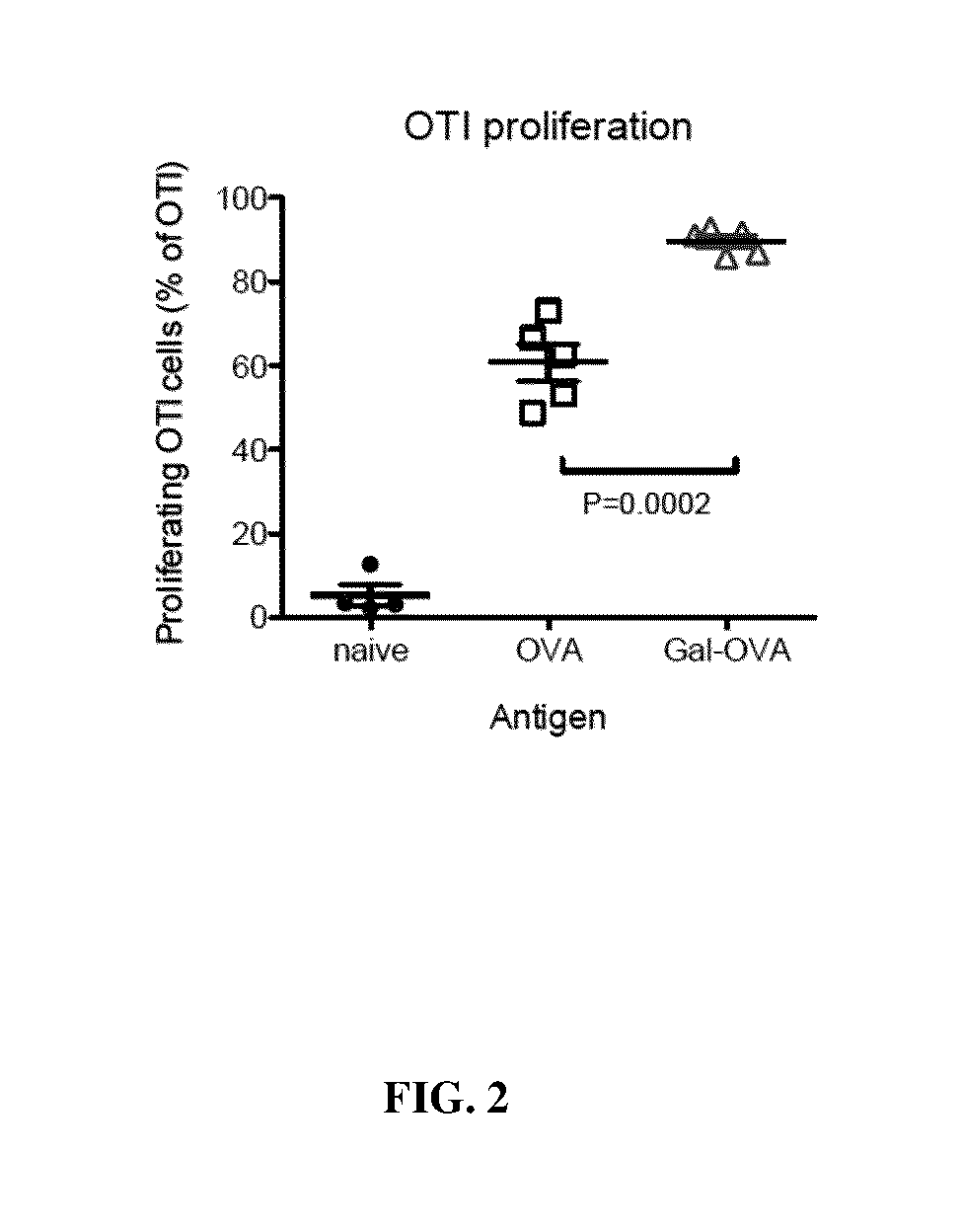
Several embodiments of the present disclosure relate to glycotargeting therapeutics that are useful in the treatment of transplant rejection, autoimmune disease, food allergy, and immune response against a therapeutic agent. In several embodiments, the compositions are configured to target the liver and deliver antigens to which tolerance is desired. Methods and uses of the compositions for induction of immune tolerance are also disclosed herein.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Therapeutic Compositions and Vaccines By Glycosyl-Phosphatidylinositol (Gpi)-Anchored Cytokines and Immunostimulatory Molecules
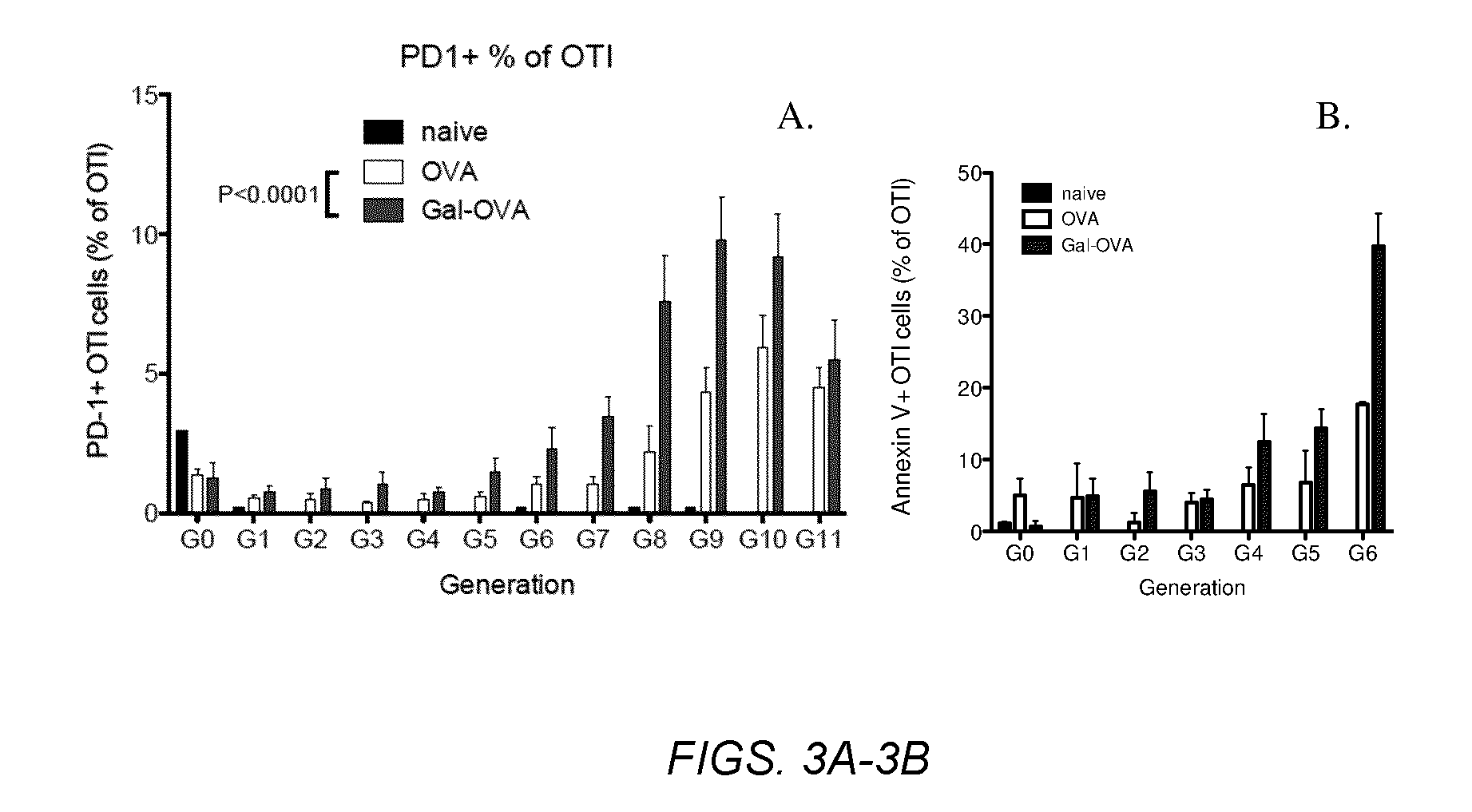
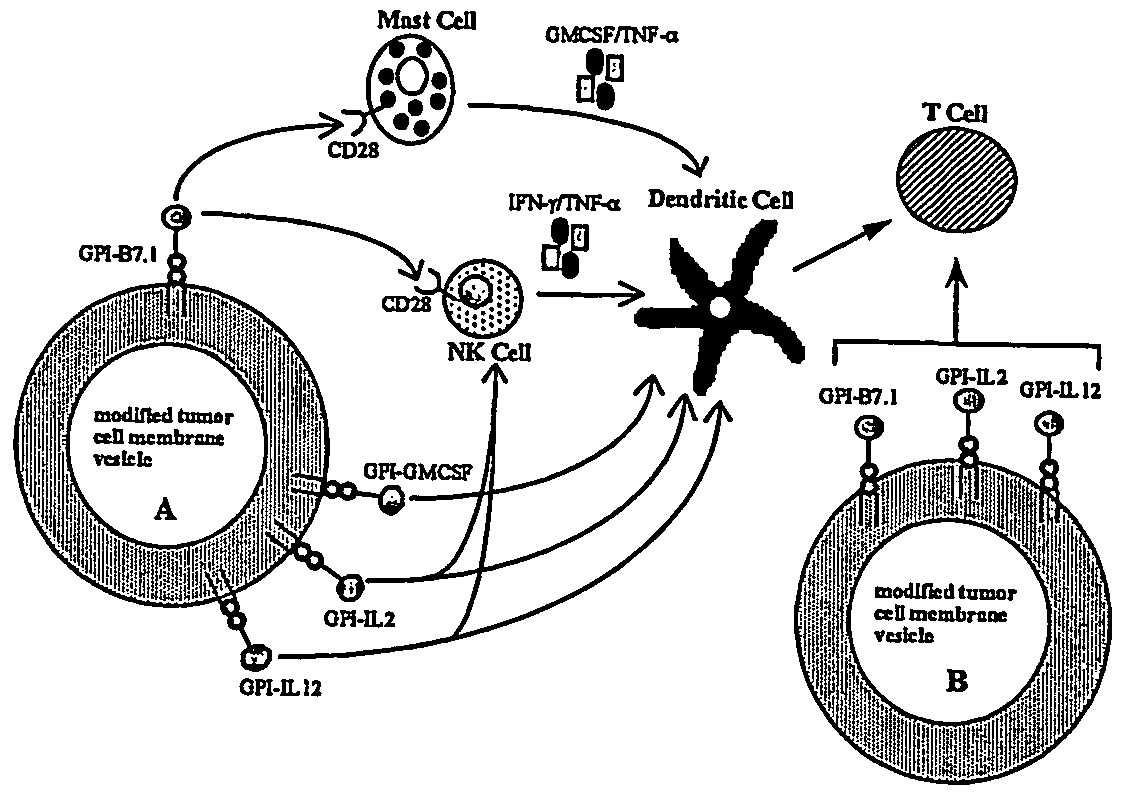
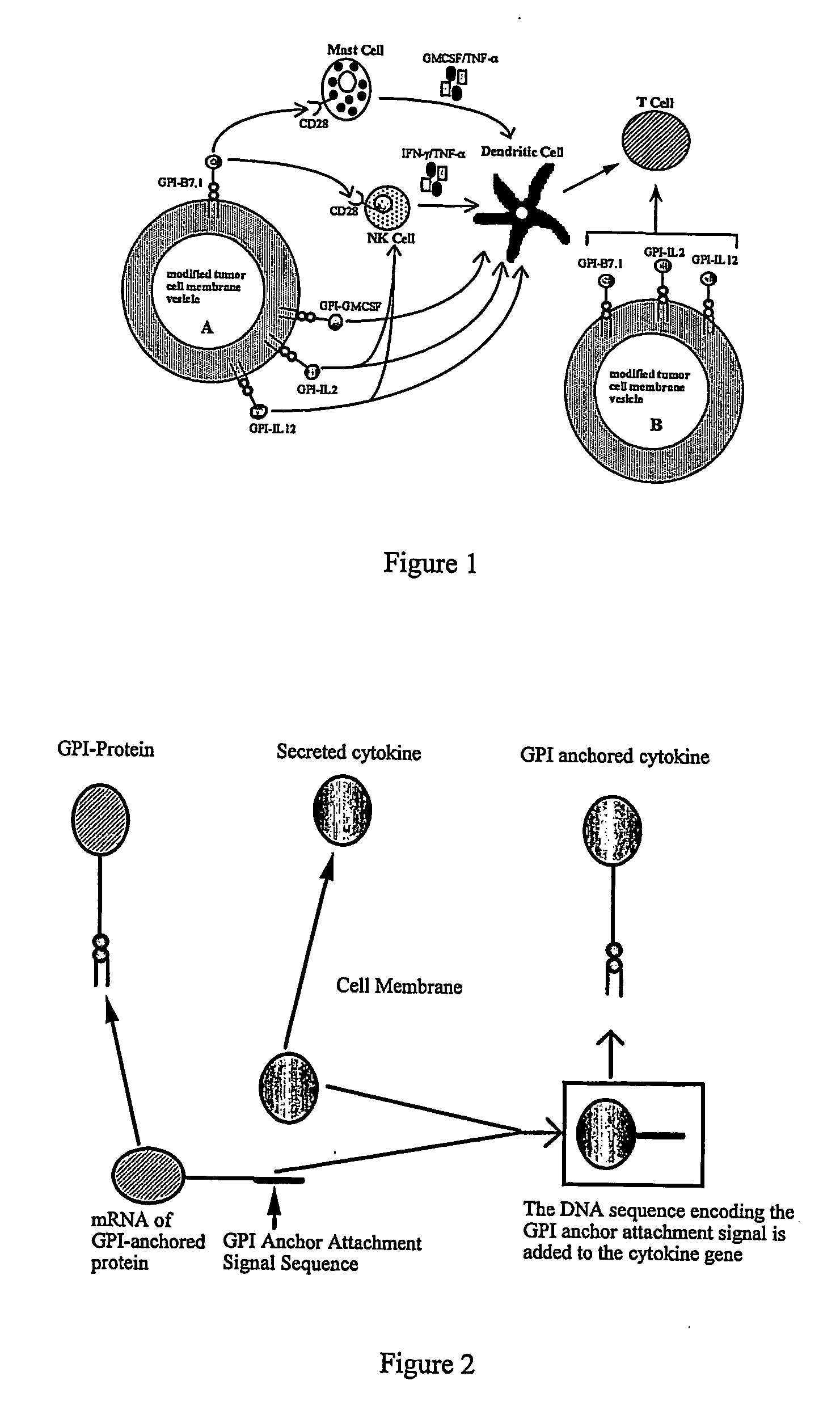
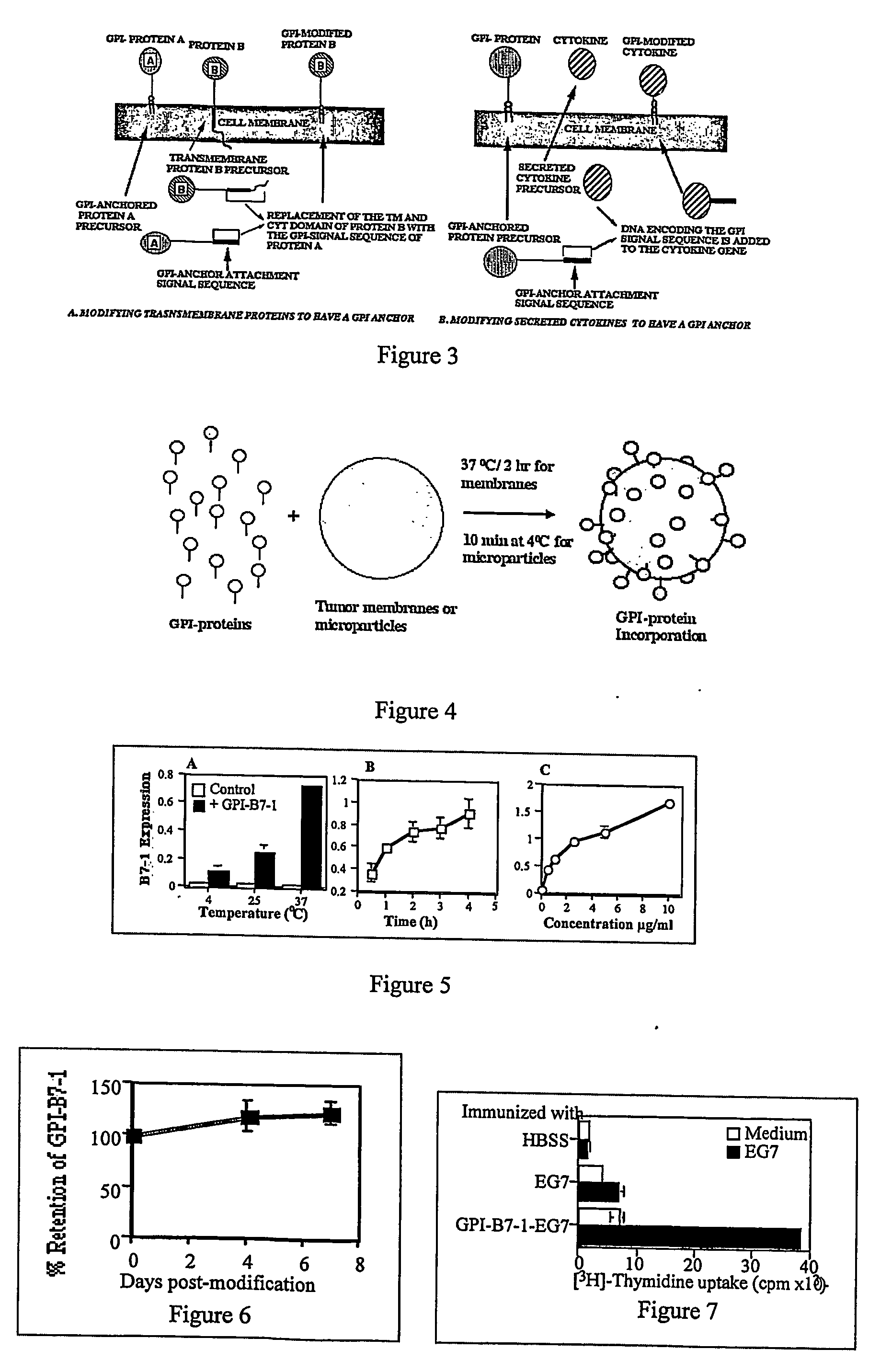
InactiveUS20070243159A1Induce toleranceSuppress immunityViral antigen ingredientsCancer antigen ingredientsAbnormal tissue growthGlycosyl-Phosphatidylinositol
A therapeutic composition or a vaccine comprising tumor membrane-anchored cytokines or other immunostimulatory or costimulatory molecules are provided. The therapeutic composition or a tumor vaccine can be used for treating a tumor or other disease such as autoimmune disorder, viral diseases, bacterial diseases, parasitic diseases, and transplant rejection.
Owner:IRM +1
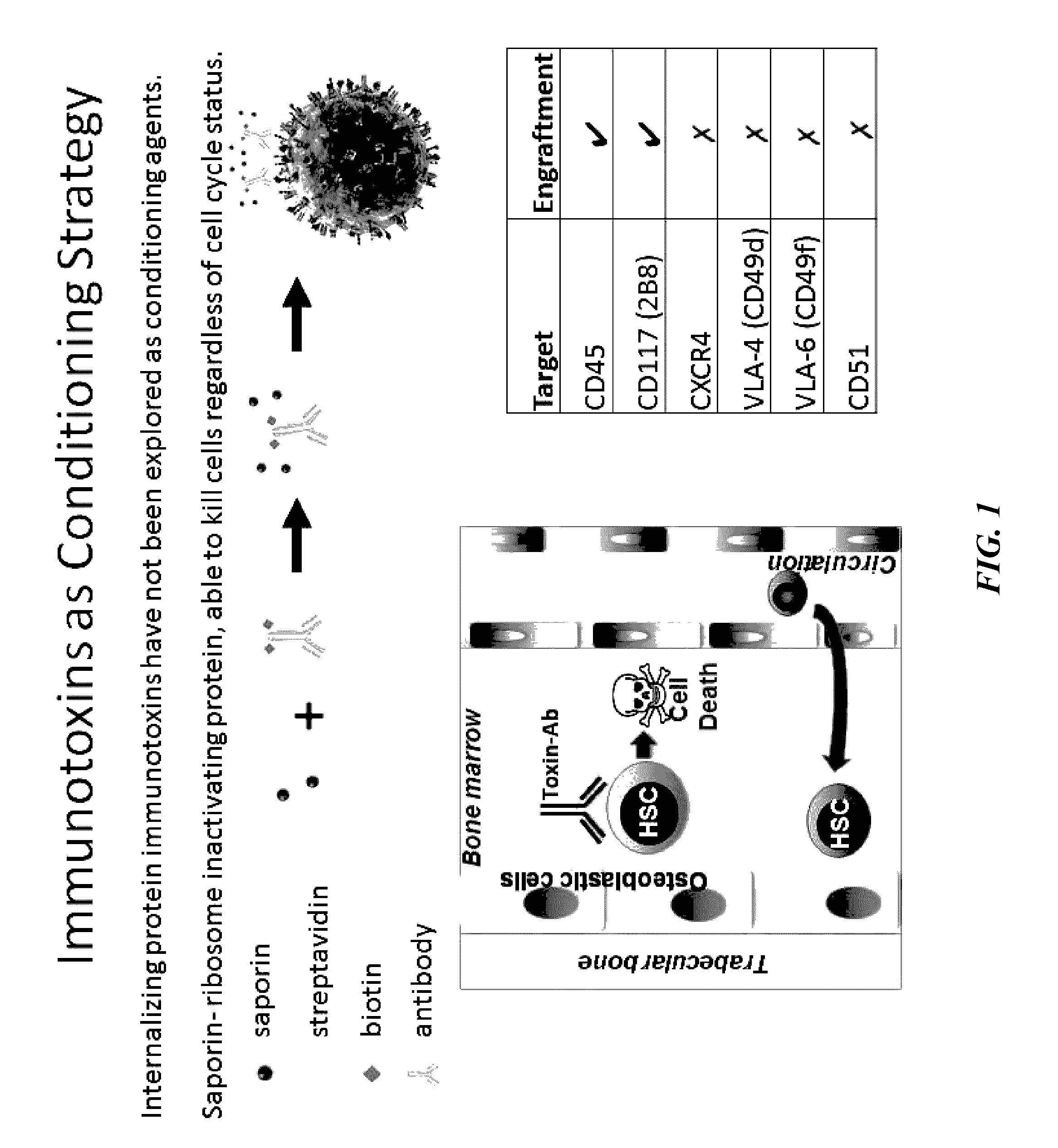
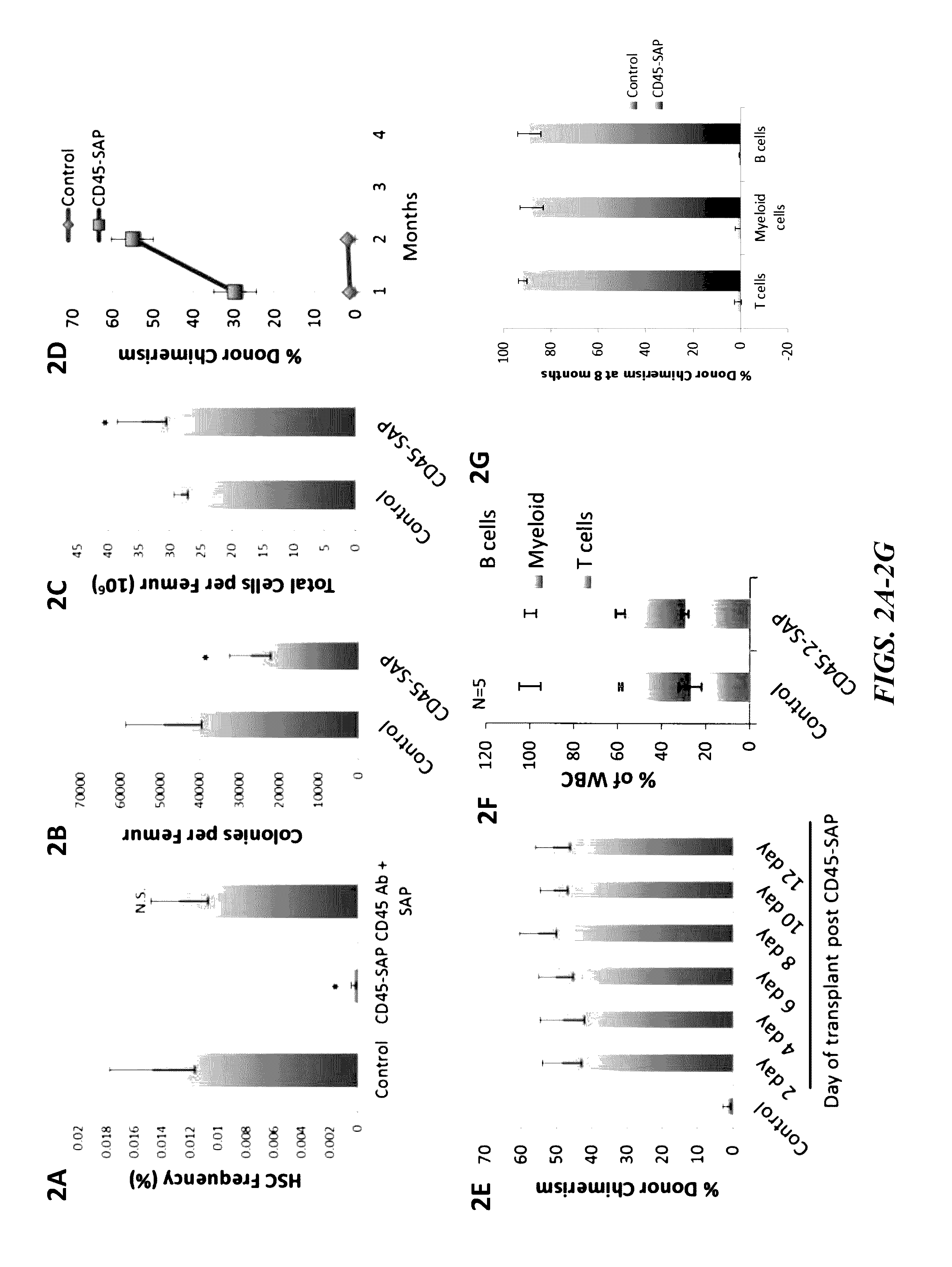
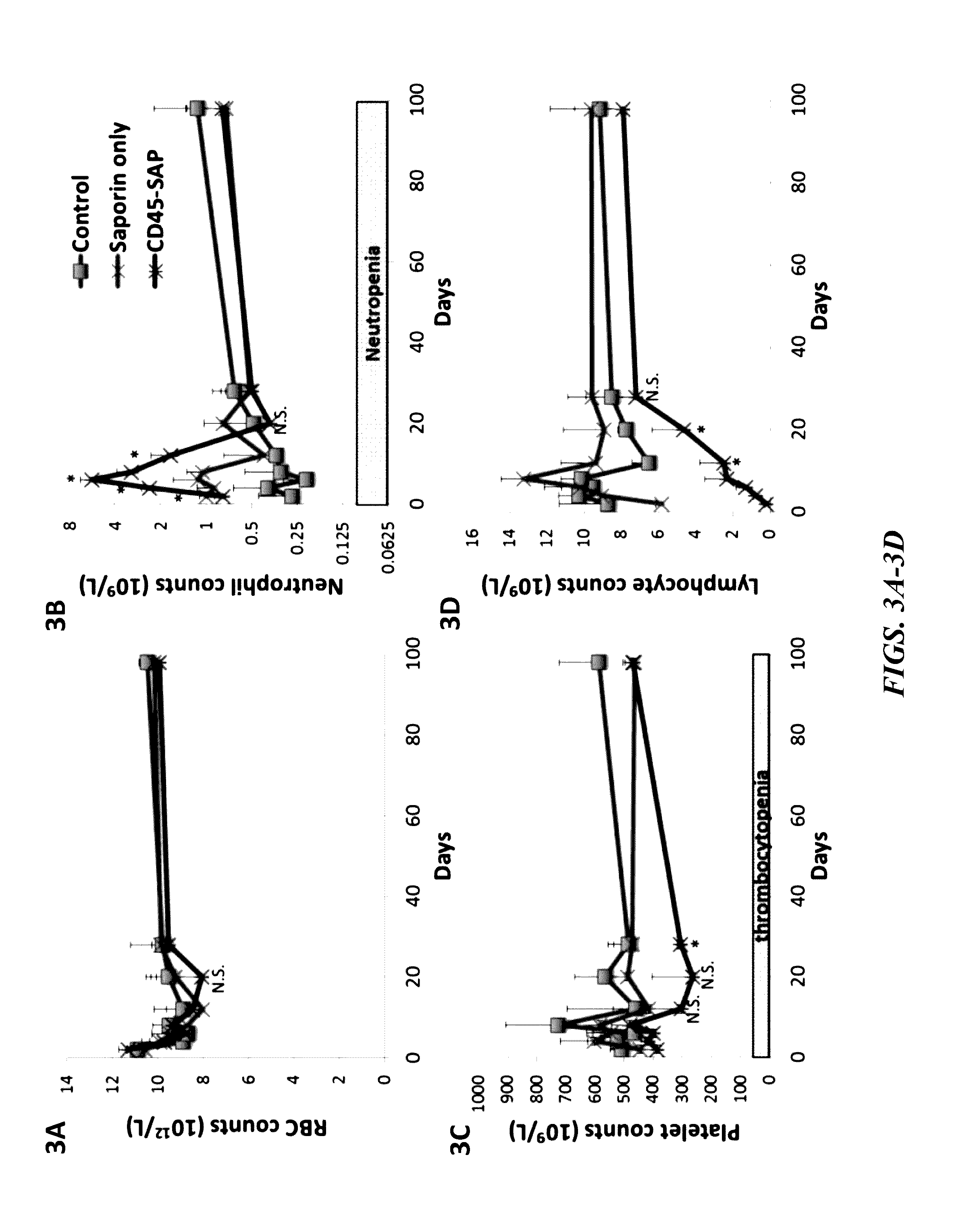
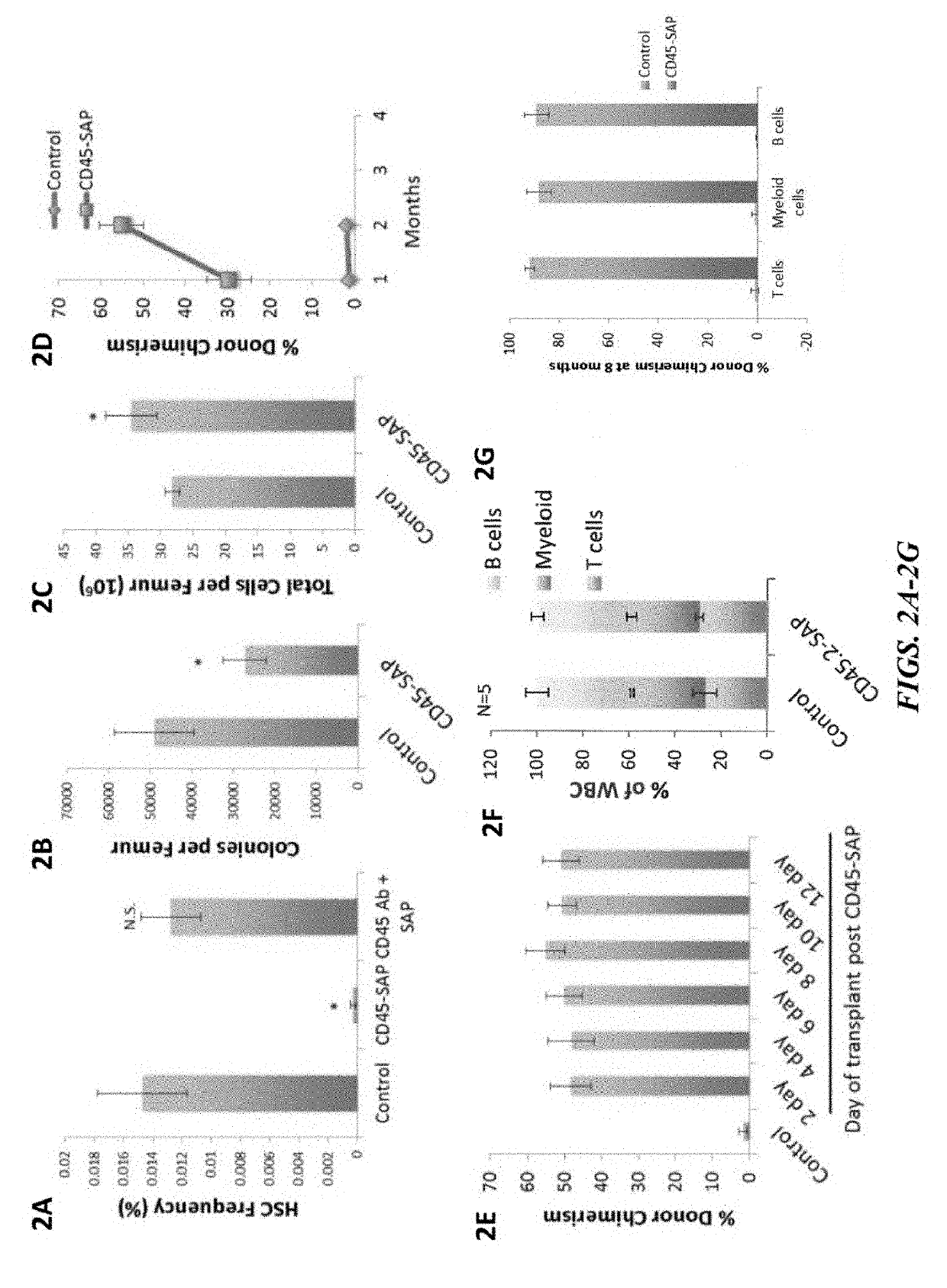
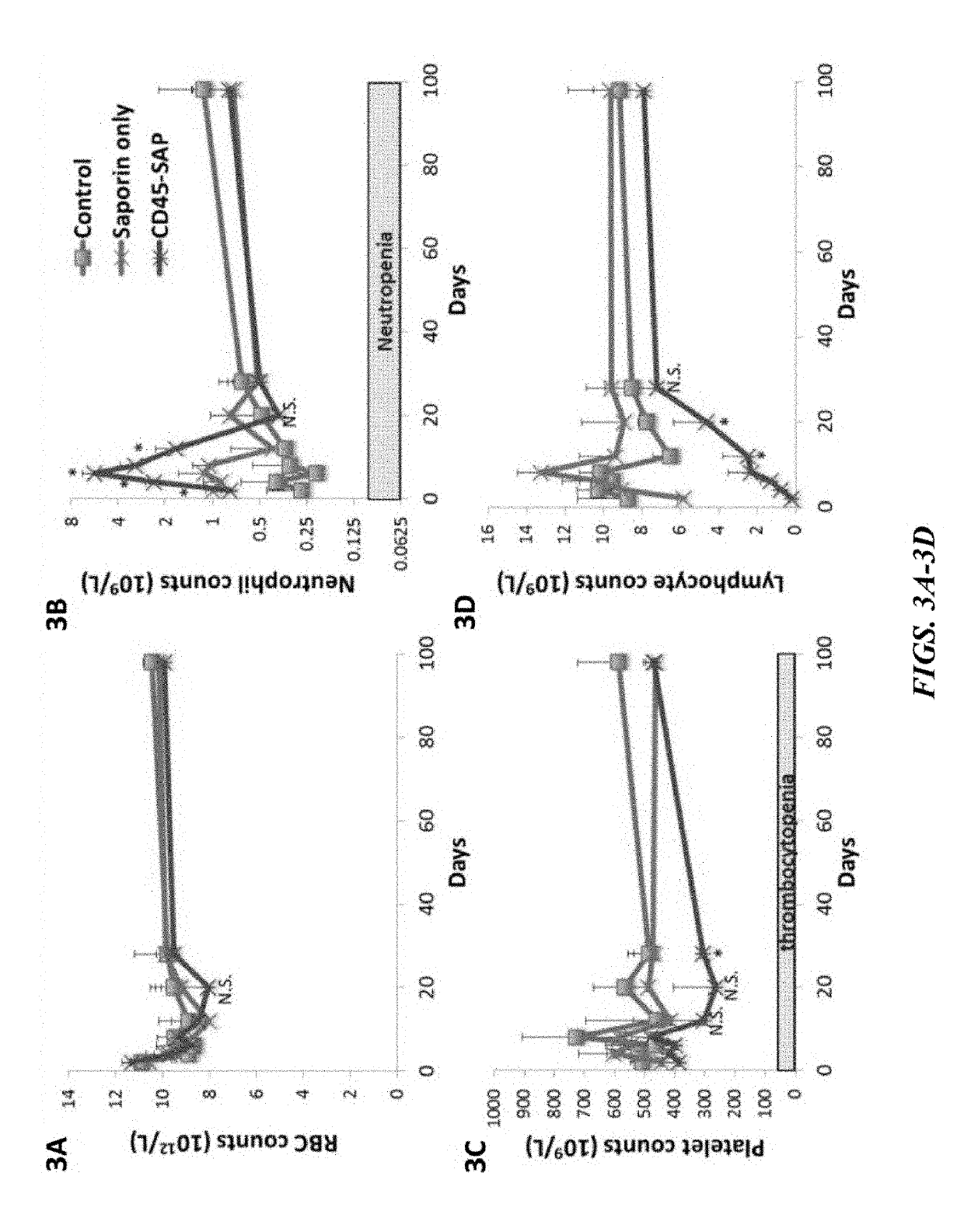
Compositions and methods for non-myeloablative conditioning
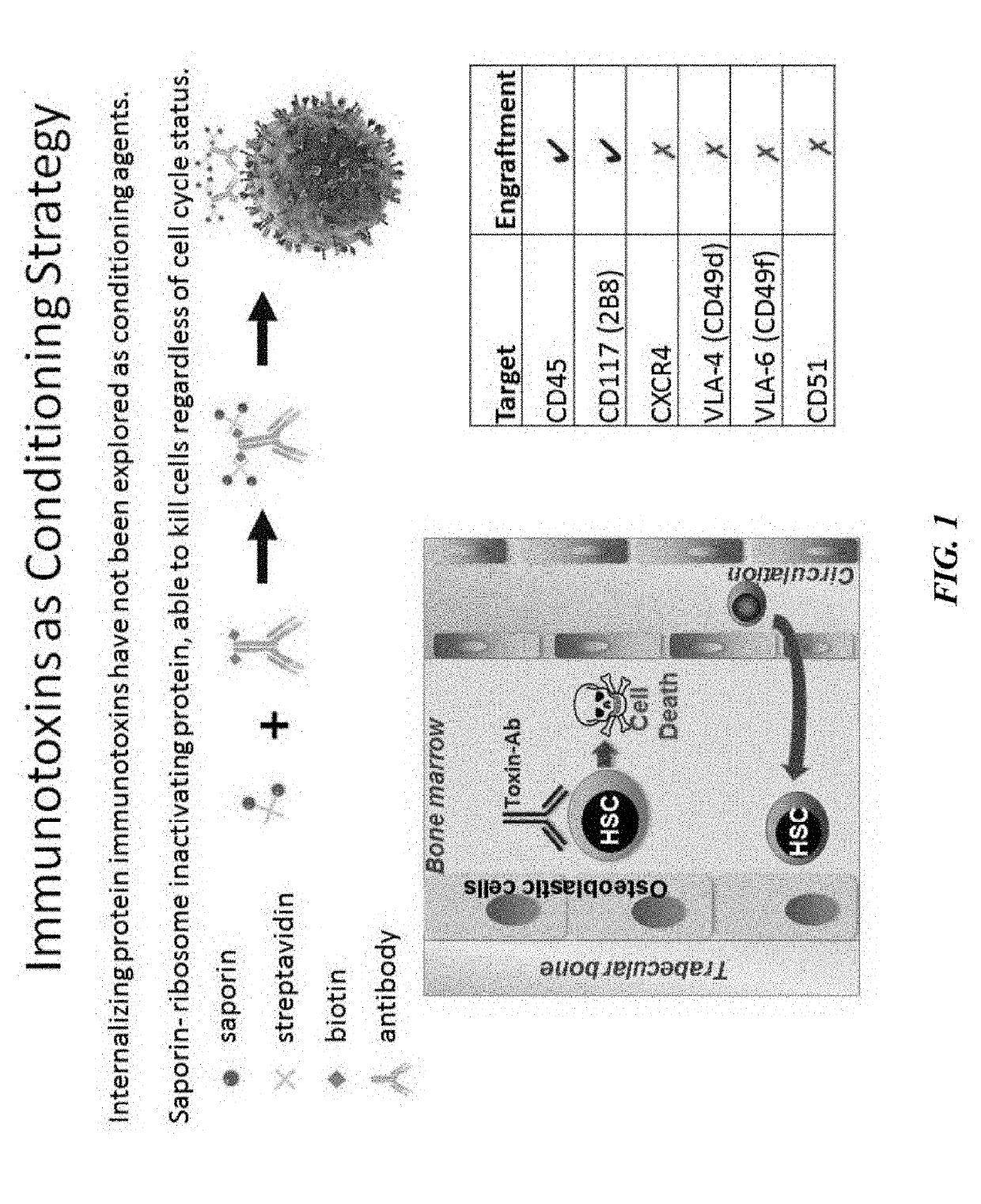
ActiveUS20160324982A1InduceImprove efficiencyNervous disorderPeptide/protein ingredientsSurface markerNon myeloablative
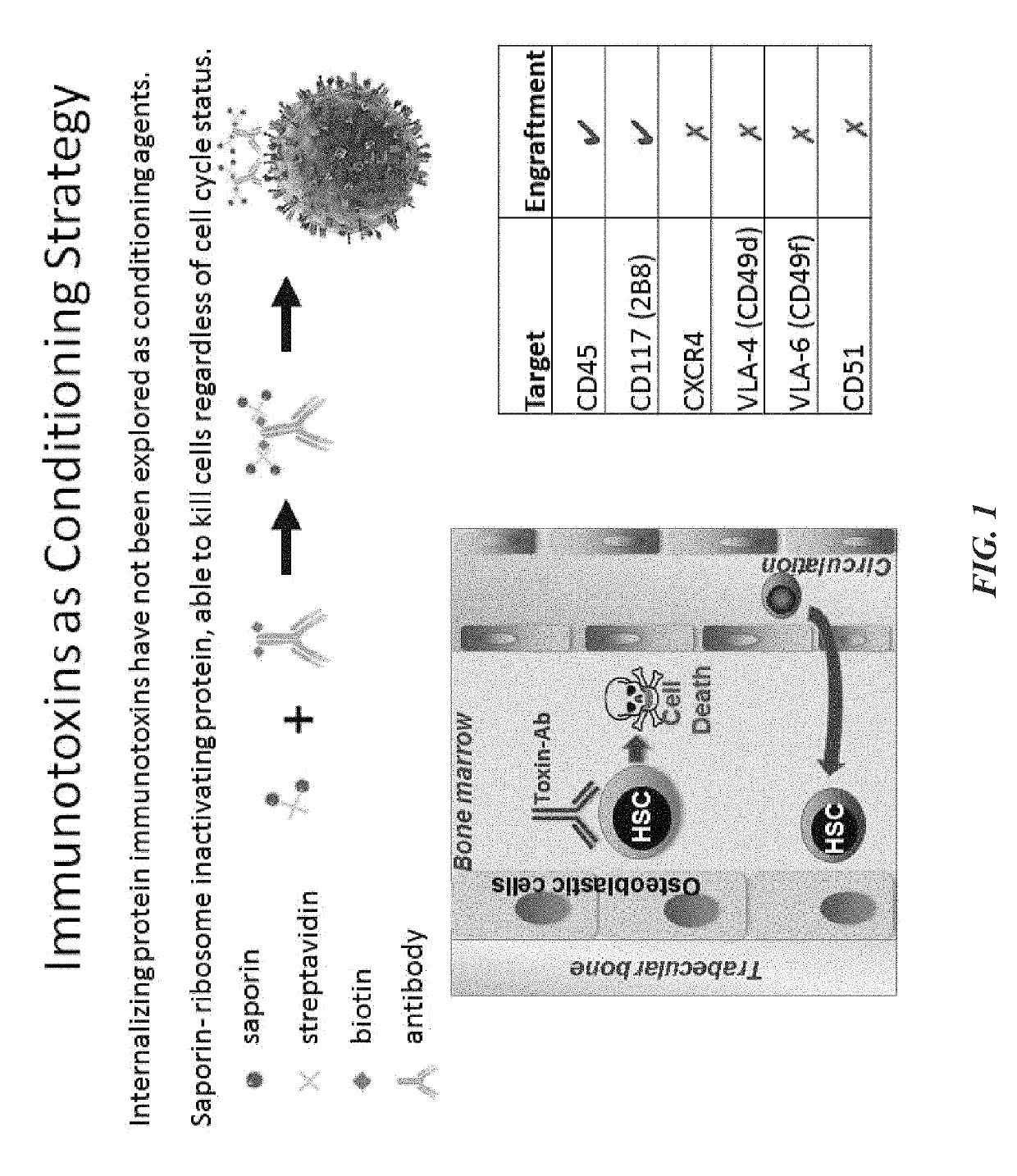
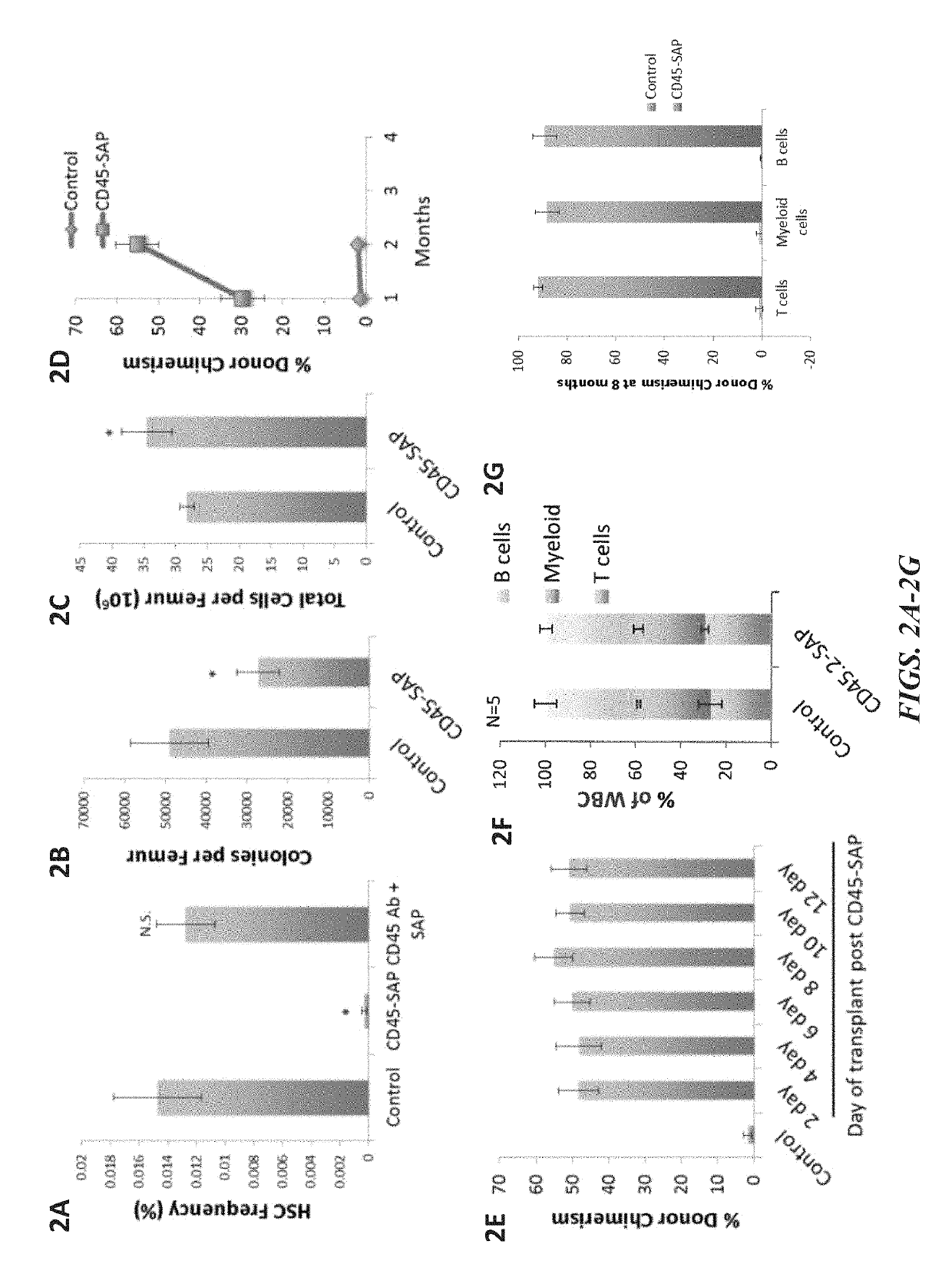
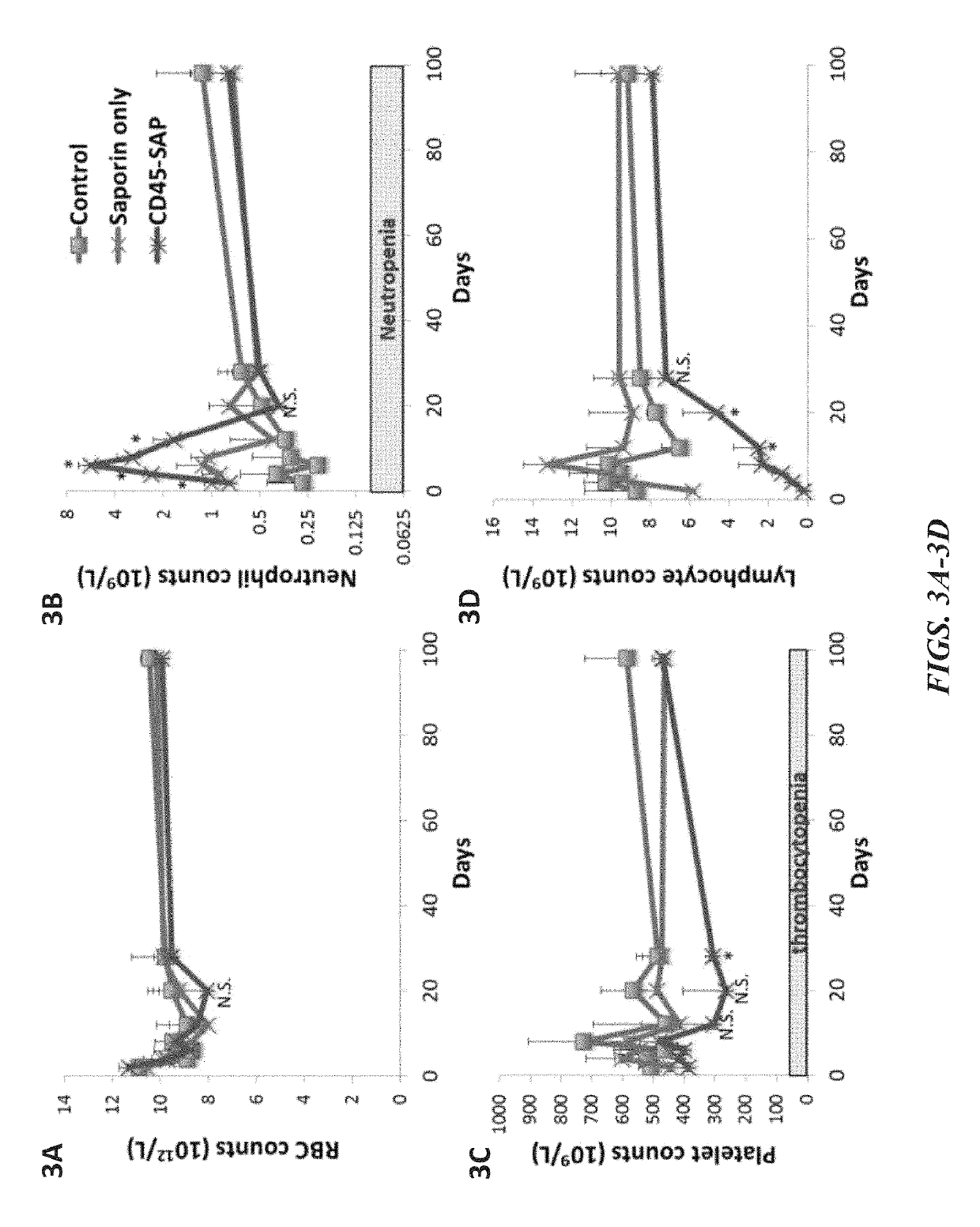
Disclosed herein are non-myeloablative antibody-toxin conjugates and compositions that target cell surface markers, such as the CD34, CD45 or CD117 receptors, and related methods of their use to effectively conditioning a subject's tissues (e.g., bone marrow tissue) prior to engraftment or transplant. The compositions and methods disclosed herein may be used to condition a subject's tissues in advance of, for example, hematopoietic stem cell transplant and advantageously such compositions and methods do not cause the toxicities that are commonly associated with traditional conditioning methods.
Owner:THE GENERAL HOSPITAL CORP +2
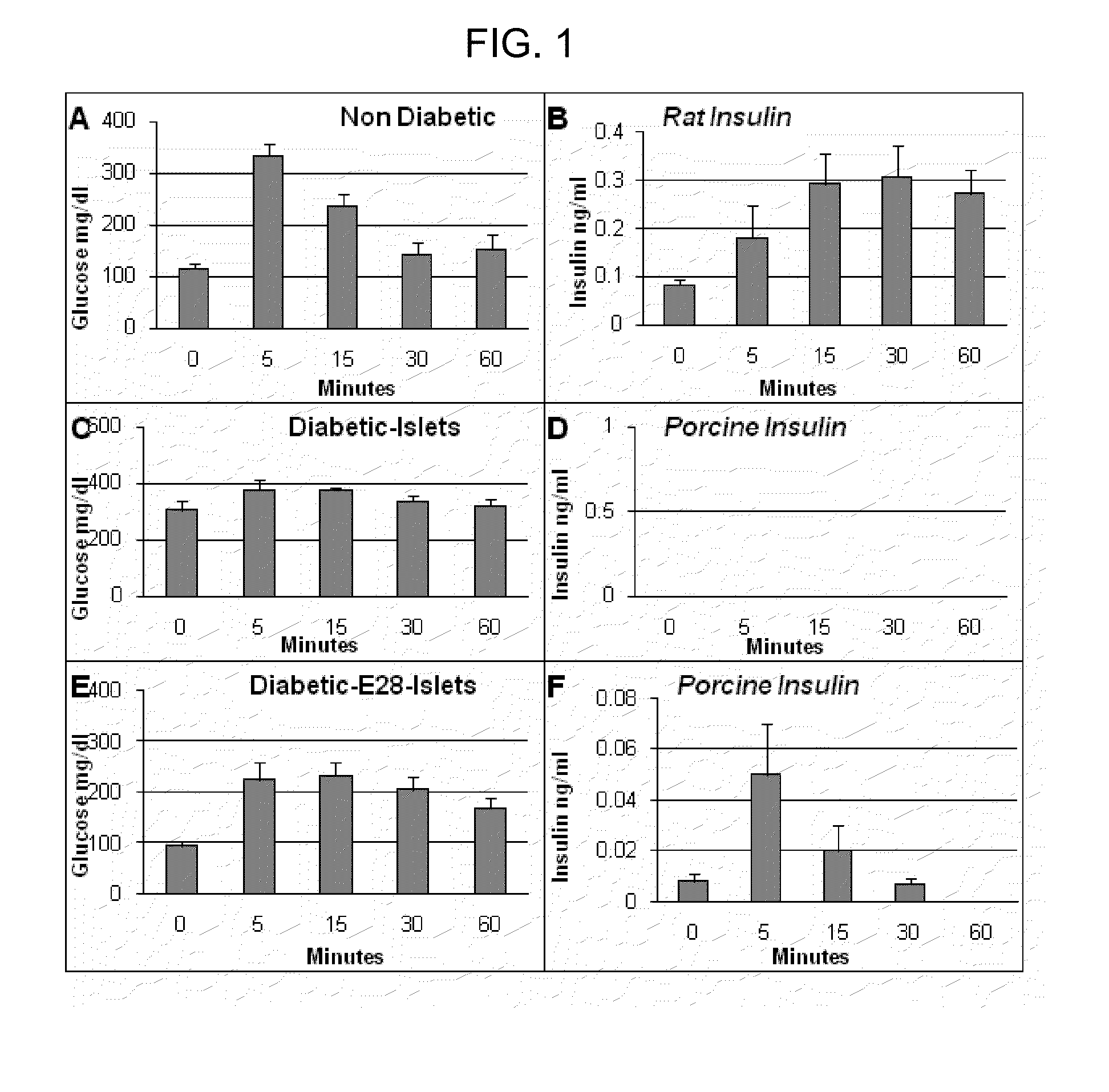
Inducement of organogenetic tolerance for pancreatic xenotransplant
InactiveUS20110020294A1Induce toleranceRequirement for immunosuppression is reduced and eliminatedBiocideMetabolism disorderIslet cellsPancreas
Provided herein is an approach to establish organogenetic tolerance via prior transplantation of pig embryonic pancreas, thereby enabling subsequent implantation of porcine islets in a subject without the need for immune-suppression. In one aspect of the invention, porcine pancreatic primordia are implanted into a mammalian subject, and after a period of time sufficient to induce tolerance, porcine islet cells are implanted in the subject.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Glycotargeting therapeutics
ActiveUS10046056B2Reduced responseRelieve symptomsPolypeptide with localisation/targeting motifPeptide/protein ingredientsAntigenAutoimmune condition
Several embodiments of the present disclosure relate to glycotargeting therapeutics that are useful in the treatment of transplant rejection, autoimmune disease, food allergy, and immune response against a therapeutic agent. In several embodiments, the compositions are configured to target the liver and deliver antigens to which tolerance is desired. Methods and uses of the compositions for induction of immune tolerance are also disclosed herein.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Compositions containing combinations of bioactive molecules derived from microbiota for treatment of disease
InactiveUS10130695B2Induce toleranceEfficaciousAntibacterial agentsBacterial antigen ingredientsAutoimmune ReactionsIntestinal mucosal barrier
Compositions consisting of bioactive molecules derived from the microbiota of a mammal are provided herein. When administered orally with a colonic delivery system, the compositions are useful for the prophylaxis and treatment of diseases, in particular inflammatory, autoimmune and infectious diseases. The compositions comprise combinations of small molecules and bacterial antigens formulated in colonic delivery systems. Use of the compositions results in any or all of: induction of immune tolerance; strengthening of the gut mucosal barrier integrity; reduction of inflammation; and amelioration of a disease state caused by inflammation, an autoimmune reaction or an infectious agent.
Owner:RIKEN +1
Attaching substances to microorganisms
InactiveUS20050169937A1Avoid potential risksEnhance immune responseAntibacterial agentsFungiSurface displayFungal microorganisms
The invention relates to surface display of proteins on microorganisms via the targeting and anchoring of heterologous proteins to the outer surface of cells such as yeast, fungi, mammalian, plant cells, and bacteria. The invention provides a proteinaceous substance comprising a reactive group and at least one attaching peptide including a stretch of amino acids having a sequence corresponding to at least a part of the consensus amino acid sequence listed in FIG. 10 and further includes a method for attaching a proteinaceous substance to the cell wall of a microorganism comprising the use of the attaching peptide.
Owner:BUIST GIRBE +3
Compositions and methods for non-myeloablative conditioning
ActiveUS20190100593A1Improve efficiencyPreserve thymic integrityNervous disorderPeptide/protein ingredientsSurface markerNon myeloablative
Disclosed herein are non-myeloablative antibody-toxin conjugates and compositions that target cell surface markers, such as the CD34, CD45 or CD117 receptors, and related methods of their use to effectively conditioning a subject's tissues (e.g., bone marrow tissue) prior to engraftment or transplant. The compositions and methods disclosed herein may be used to condition a subject's tissues in advance of, for example, hematopoietic stem cell transplant and advantageously such compositions and methods do not cause the toxicities that are commonly associated with traditional conditioning methods.
Owner:THE GENERAL HOSPITAL CORP +2
Inducement of organogenetic tolerance for pancreatic xenotransplant
InactiveUS8709400B2Induce toleranceRequirement for immunosuppression is reduced and eliminatedBiocideMetabolism disorderTolerabilityMammal
Owner:WASHINGTON UNIV IN SAINT LOUIS
Compositions and methods for non-myeloablative conditioning
ActiveUS10280225B2Improve efficiencyPreserve thymic integrityNervous disorderPeptide/protein ingredientsSurface markerNon myeloablative
Disclosed herein are non-myeloablative antibody-toxin conjugates and compositions that target cell surface markers, such as the CD34, CD45 or CD117 receptors, and related methods of their use to effectively conditioning a subject's tissues (e.g., bone marrow tissue) prior to engraftment or transplant. The compositions and methods disclosed herein may be used to condition a subject's tissues in advance of, for example, hematopoietic stem cell transplant and advantageously such compositions and methods do not cause the toxicities that are commonly associated with traditional conditioning methods.
Owner:THE GENERAL HOSPITAL CORP +2
Edible vaccines expressed in soybeans
The present invention relates to vaccines that are made in transgenic soybeans for use in humans, animals of agricultural importance, pets, and wildlife. These vaccines are used as vaccines against viral, bacterial, fungal, parasitic or prion related diseases, cancer antigens, toxins, and autologous or self proteins. The transgenic soybeans of the instant invention also can be used for inducing tolerance to allergens or tolerance to autoimmune antigens, wherein an individual shows hypersensitivity to said allergen or has developed autoimmunity to autologous or self proteins, respectively. The invention also relates to prophylatically treating individuals and / or populations prior to showing hypersensitivity to allergens. Other aspects of the invention include using the transgenic soybeans as an oral contraceptive, and the expression of protein adjuvants in transgenic soybeans.
Owner:SOYMEDS
Nutritional composition for infants delivered via caesarean section
InactiveUS20110117077A1Fast colonisationInduce toleranceOrganic active ingredientsPeptide/protein ingredientsBiotechnologyPhysiology
The present invention relates to methods for feeding of infants delivered via caesarean section and to compositions to be administered to infants delivered via caesarean section and in particular to the use of a product obtained by fermentation of milk, whey, whey protein, whey protein hydrolysate, casein, casein hydrolysate and / or lactose by lactic acid producing bacteria. Thereby it is possible to stimulate a fast colonisation of the intestinal microbiota of said infants.
Owner:NV NUTRICIA
Optimized dosing with anti-CD4 antibodies for tolerance induction in primates
InactiveUS20080112949A1Lower immune responseInduce toleranceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTolerance induction
The present invention is based, at least in part, on the finding that tolerance can be induced by inhibition of CD4+ cells (and optionally CD8+ cells). Accordingly, the optimized dosing methods of the invention are useful in treating a primate, e.g., a human, by inhibiting CD4+ T cells to induce tolerance to at least one antigen, e.g., self or foreign, such as for inducting tolerance in a primate against a soluble or a cell bound antigen (e.g., an allogeneic or xenogeneic transplanted antigen).
Owner:TOLERX INC
Use of cytokines and mitogens to inhibit graft versus host disease
InactiveUS7381563B2InhibitionAvoiding and minimizingBiocidePeptide/protein ingredientsFactor iiCytokine
The field of the invention is generally related to pharmaceutical agents useful in treating graft-versus-host disease (GVHD) in patents that have received allogenic bone marrow transplants.
Owner:UNIV OF SOUTHERN CALIFORNIA
Gene vector for inducing transgene-specific immune tolerance
ActiveUS20110218234A1Prevents and reduces expressionImprove toleranceOrganic active ingredientsVectorsHematopoietic lineageImmune tolerance
A gene vector adapted for transient expression of a transgene in a peripheral organ cell comprising a regulatory sequence operably linked to a transgene wherein the regulatory sequence prevents or reduces expression of said transgene in hematopoietic lineage cells.
Owner:FOND AZIONE TELETHON +1
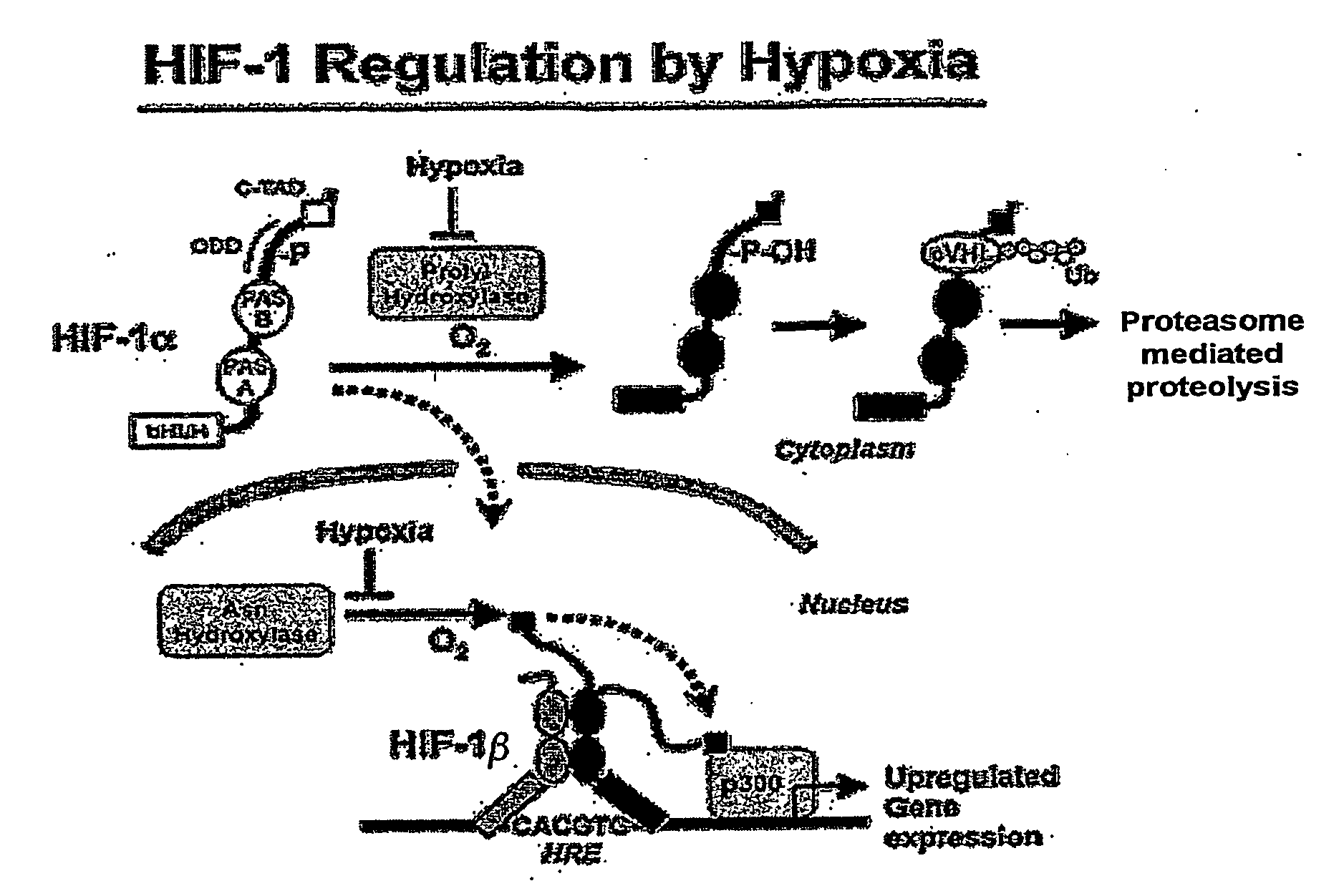
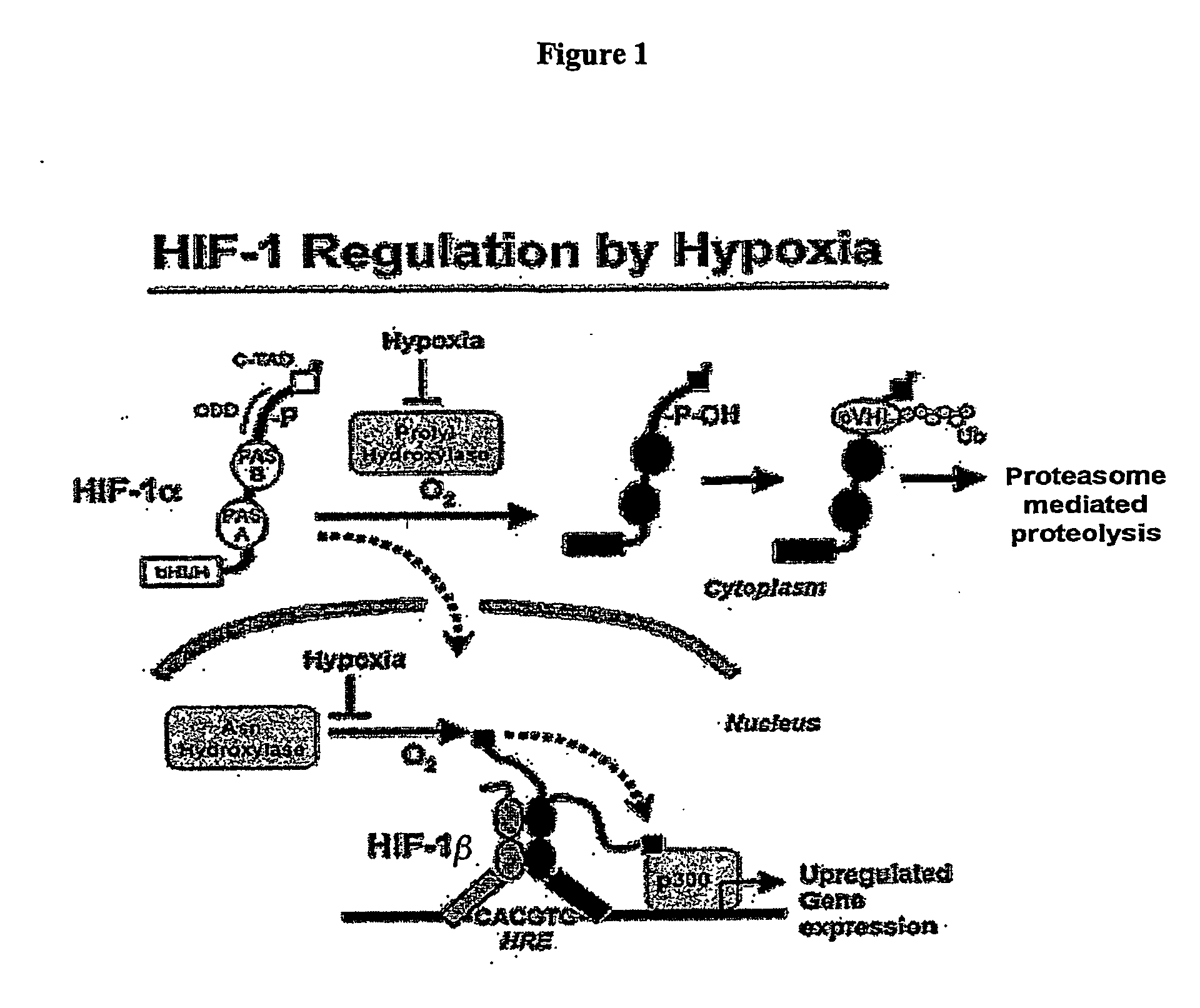
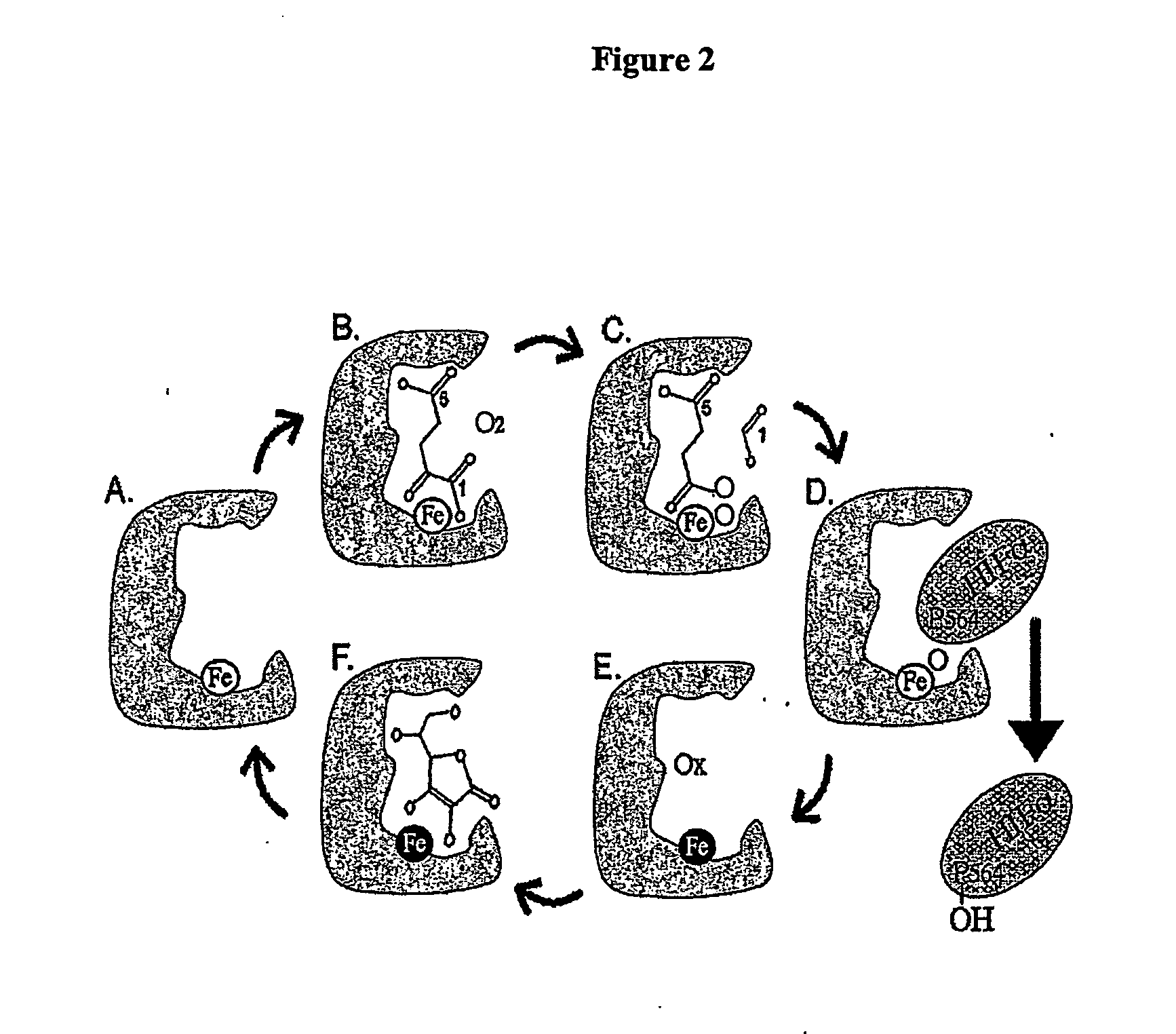
Activation of hypoxia-inducible gene expression
InactiveUS20070173545A1Induce vascularizationTreat anemiaBiocideNervous disorderOrgan transplantationBiological activation
The present invention relates to the elucidation of specific molecular features of endogenous 2-oxoacid molecules and their derivatives for activating hypoxia-inducible gene expression by inactivating hypoxia-inducible factor hydroxylating enzymes. This invention identifies agents that can be used to induce tissue vascularization, treat anemias, induce tolearance to stroke and heart attacks, improve tissue healing and improve organ transplantation.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC +1
Novel uses of PPAR modulators and professional APCs manipulated by the same
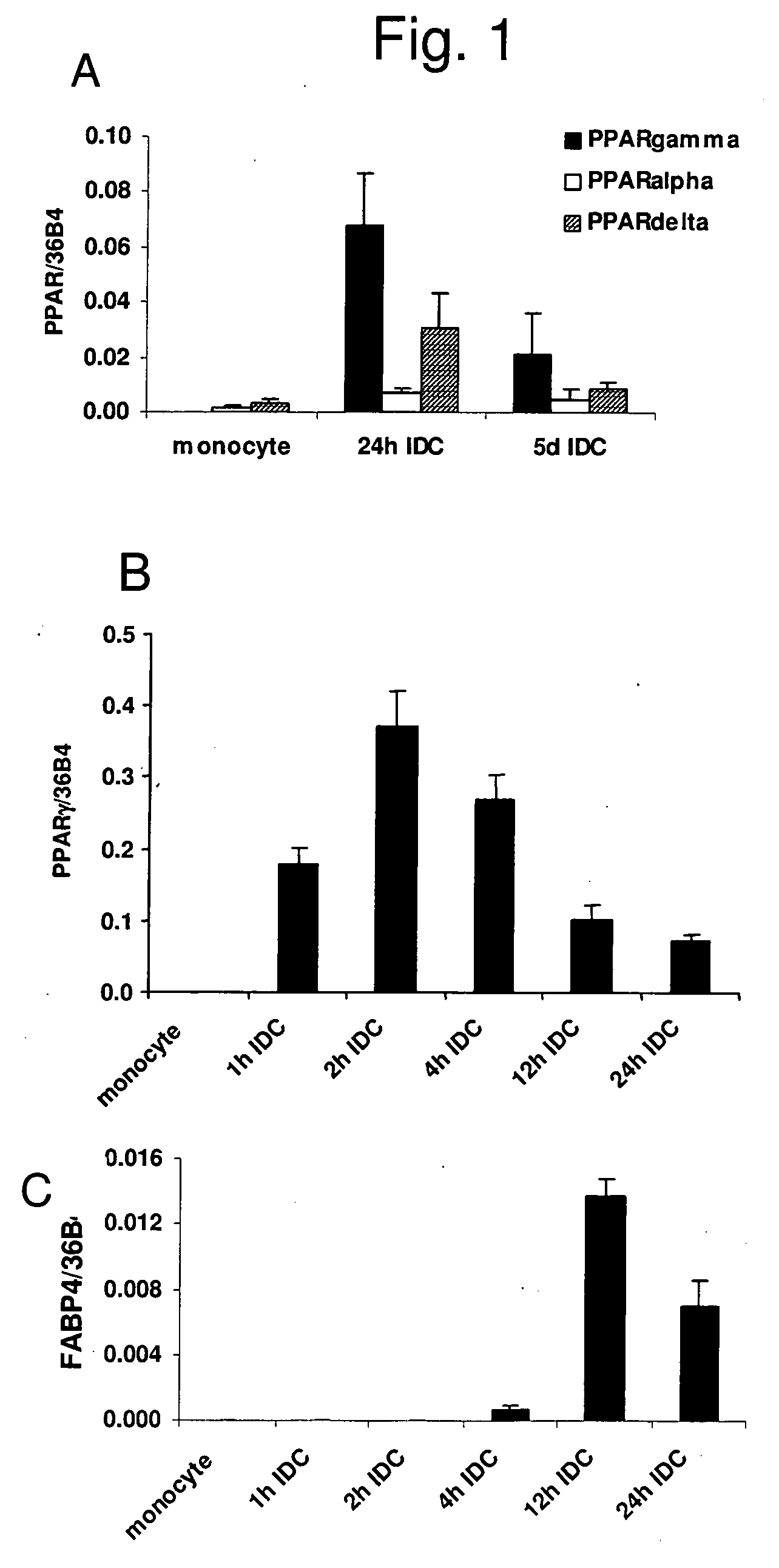
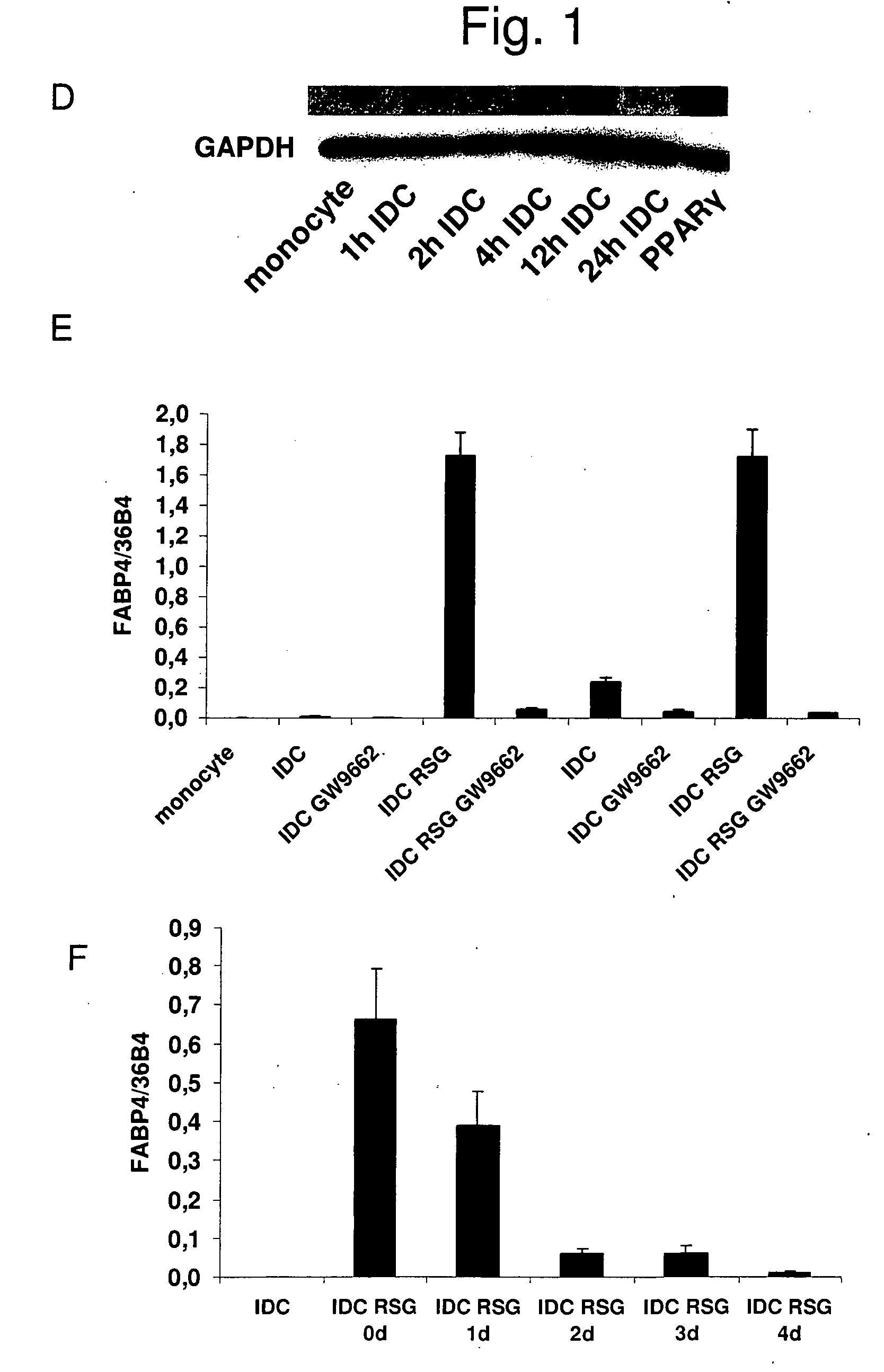
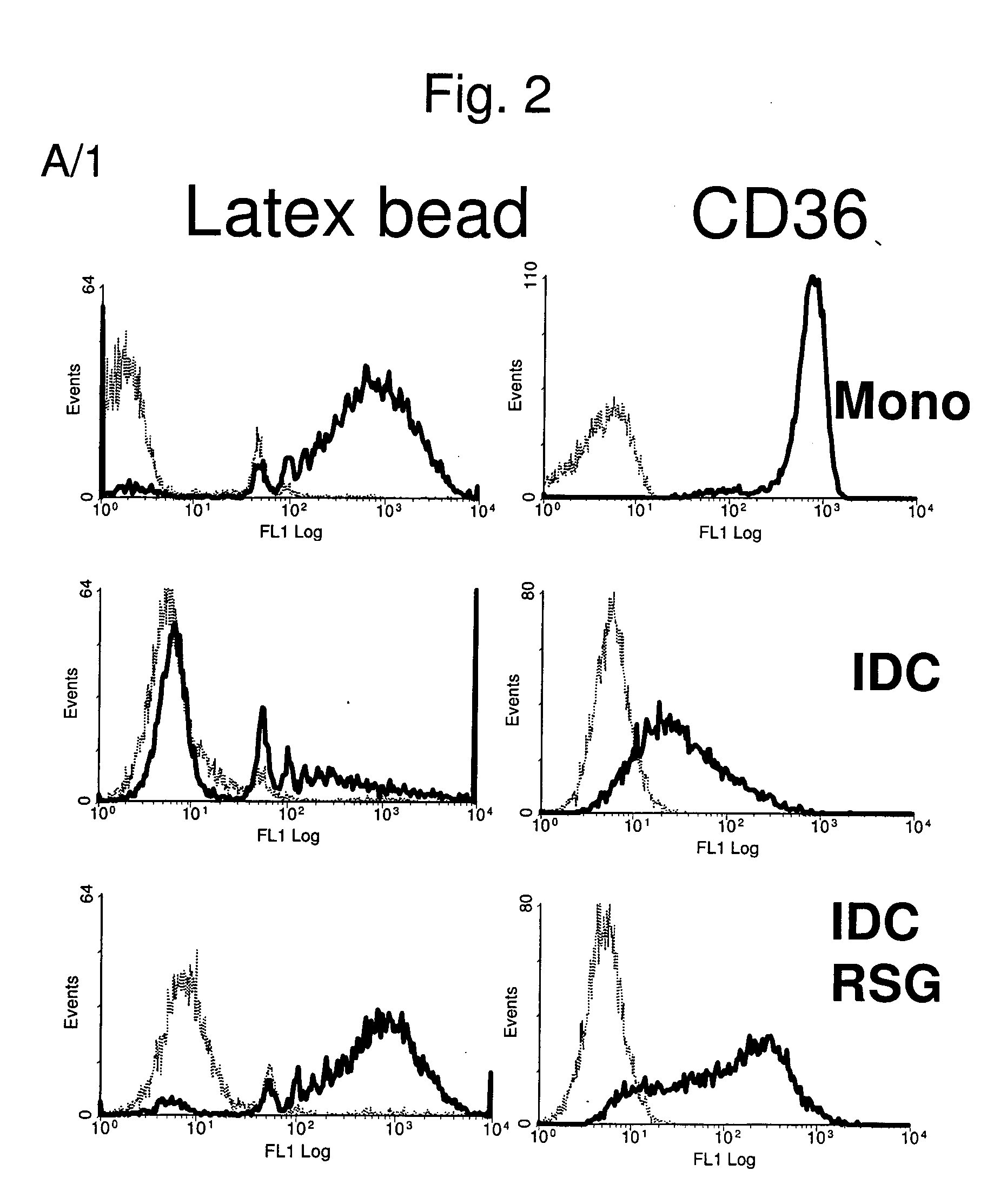
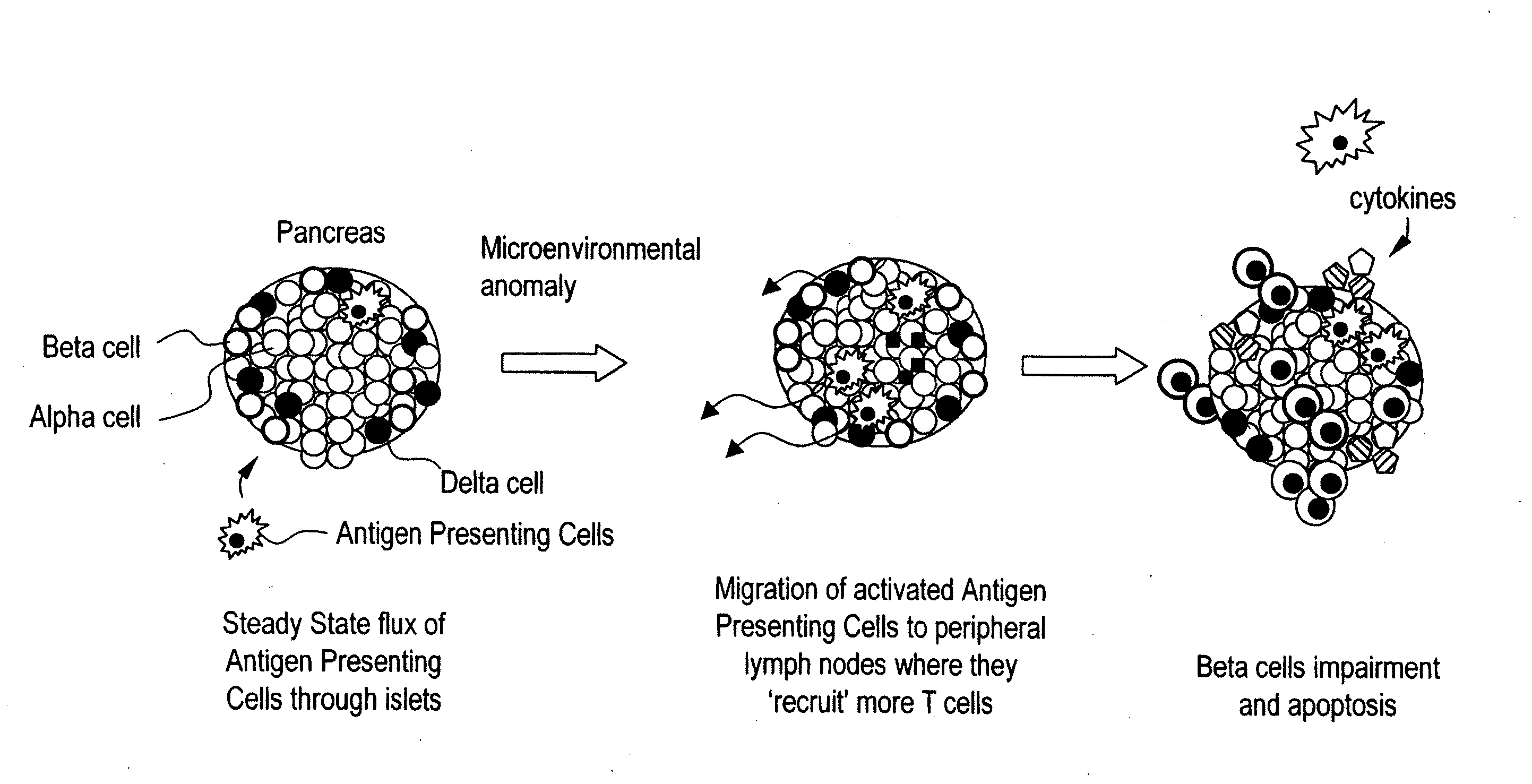
InactiveUS20060159669A1Ameliorate any conditionAltered phenotypeBiocideGenetic material ingredientsAntigenAutoimmune condition
The invention relates to a manipulated professional antigen presenting cell (APC) having increased expression of a CD1 type II molecule, preferably at least a CD1d molecule, relative to a control non manipulated cell. The invention further relates to the use of PPARg modulators in the preparation of a pharmaceutical composition or kit for the treatment of a disease treatable by activation of CD1d restricted T-cells, e.g. in autoimmune diseases, allergies, post-transplant conditions or infectious diseases, or in the treatment of a neoplastic disease, e.g. skin cancer, hematological tumors, colorectal carcinoma, and therapeutic compositions and kits therefor. Furthermore, the invention relates to methods of manipulating professional APCs and kits therefor.
Owner:UNIVERSITY OF DEBRECEN
Combinations of antigen and mucosal binding component for inducing specific immunological tolerance
InactiveUS7097845B2Reduce riskInduce toleranceBacterial antigen ingredientsPeptide/protein ingredientsAntigenGanglioside
This invention provides combinations of a tolerance-inducing antigen such as insulin and a mucosal binding component that preferably binds ganglioside GM1. The components are present in a non-covalent arrangement. When administered to a mucosal surface, the combinations are effective in inducing specific immunological tolerance at a 10-fold lower dose than antigen alone. Tolerance is sustained for a number of weeks without the necessity of booster administrations. The compositions and procedures of this invention are of benefit for the prevention or amelioration of conditions attributable to an unwanted immunological response.
Owner:JACOB STEN PETERSEN
Delivery of as-oligonucleotide microspheres to induce dendritic cell tolerance for the treatment of autoimmune type 1 diabetes
InactiveUS20100260855A1Avoid accessInduce toleranceOrganic active ingredientsPowder deliveryTolerabilityDendritic cell
AS-oligonucleotides are delivered in microsphere form in order to induce dendritic cell tolerance, particularly in the non-obese-diabetic (NOD) mouse model. The microspheres incorporate antisense (AS) oligonucleotides. A process includes using an antisense approach to prevent an autoimmune diabetes condition in NOD mice in vivo and in situ. The oligonucleotides are targeted to bind to primary transcripts CD40, CD80, CD86 and their combinations.
Owner:UNIVERSITY OF PITTSBURGH +2
Modulation of plasma membrane human leukocyte elastase
InactiveUS20020009788A1Modulating responseEfficient modulationPeptide/protein ingredientsChemical treatment enzyme inactivationDiseaseAutoimmune disease
A method for modulation of plasma membrane associated Human Leukocyte Elastase (HLE) to inflammatory states by interaction of HLE with an antagonist to inhibit HLE and thereby interruption in plasma associated events (e.g. HIV disease progression, bacterial infections and autoimmune diseases), which are responsive / sensitive to such inflammation. The antagonist suitable for use in this invention is designed to interact with each of the catalytic triad of the HLE plasma membranes protein and the lipid interactive amino acids of the HLE plasma membrane protein.
Owner:BRISTOW CYNTHIA L
Prevention of allergy at weaning
ActiveUS20110021428A1Efficient transferReduce riskVitamin food ingredientsPeptide/protein ingredientsNutritional compositionBiology
A nutritional composition comprising a partially hydrolysed milk protein having a degree of hydrolysis between 15 and 25% and 50 to 1000 nanograms of TGF-β per 100 ml of ready to consume composition and methods for the primary prevention of allergic reactions to newly introduced dietary protein at weaning and the prevention of development of atopic diseases in a young mammal at weaning comprising feeding to the young mammal a therapeutic amount of the composition are disclosed.
Owner:SOC DES PROD NESTLE SA
Use of cytokines and mitogens to inhibit graft versus host disease
InactiveUS20090061514A1InhibitionAvoiding and minimizingEnzymologyArtificial cell constructsT cellCytokine
A method for inducing T cell tolerance in peripheral blood mononuclear cells (PBMCs) comprising adding a suppressive composition to said cells.
Owner:UNIV OF SOUTHERN CALIFORNIA
Method of acquiring immunological tolerance
InactiveUS7276234B1Avoid immune responseEfficient self-tolerance of T lymphocytesBiocideGenetic material ingredientsHuman animalTherapeutic effect
The aim of the present invention is to provide a method of acquiring immunological tolerance to a foreign DNA or its expression product whereby the foreign DNA such as a vector carrying a foreign gene incorporated thereinto or its expression product can be recognized not as non-self but as self; a method of sustaining a gene therapeutic effect whereby a rejection to a foreign DNA such as a vector carrying a foreign gene incorporated thereinto or its expression product can be avoided; and a non-human animal which has acquired immunological tolerance to a foreign DNA such as a vector carrying a foreign gene incorporated thereinto or its expression product. Fetal immature T lymphocytes transferred with a foreign DNA, such as a foreign gene-incorporated viral vector, are introduced into thymus and said foreign DNA is expressed in the thymus organ. The methods of transferring said foreign DNA into a fetal immature T lymphocyte include, for example, co-cultivating the fetal immature T lymphocytes with viral vector-infected virus producer cells.
Owner:JAPAN SCI & TECH CORP
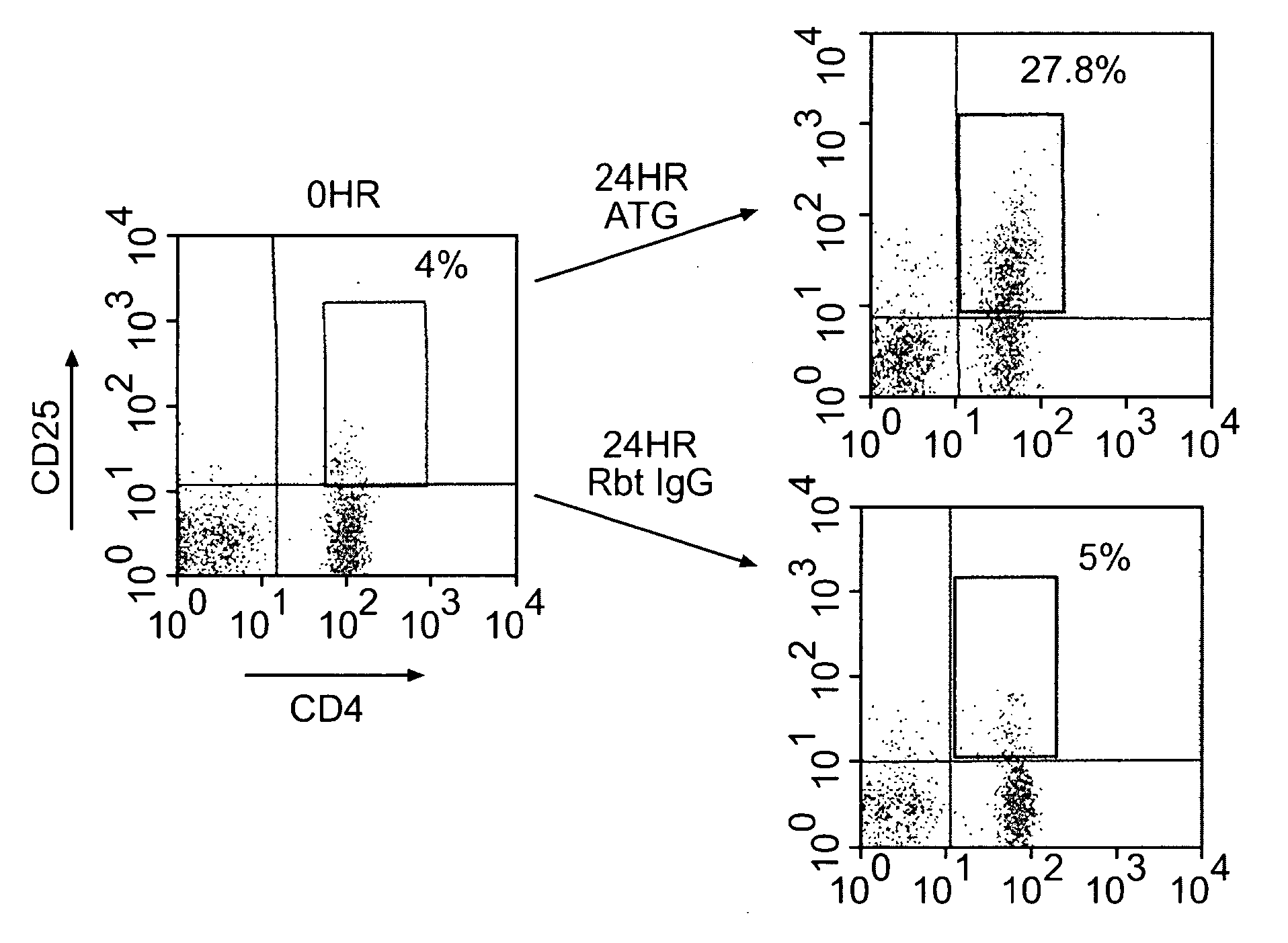
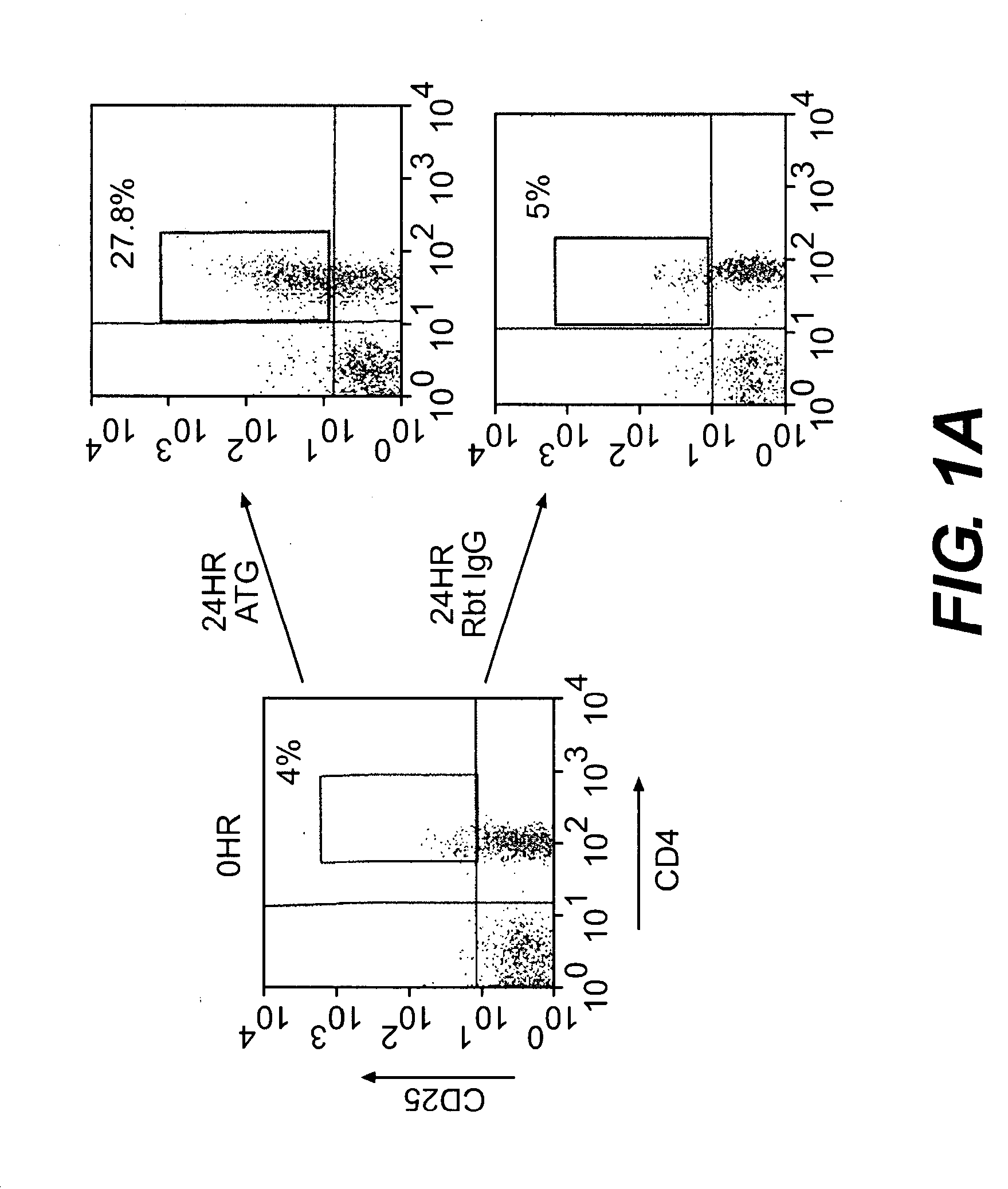
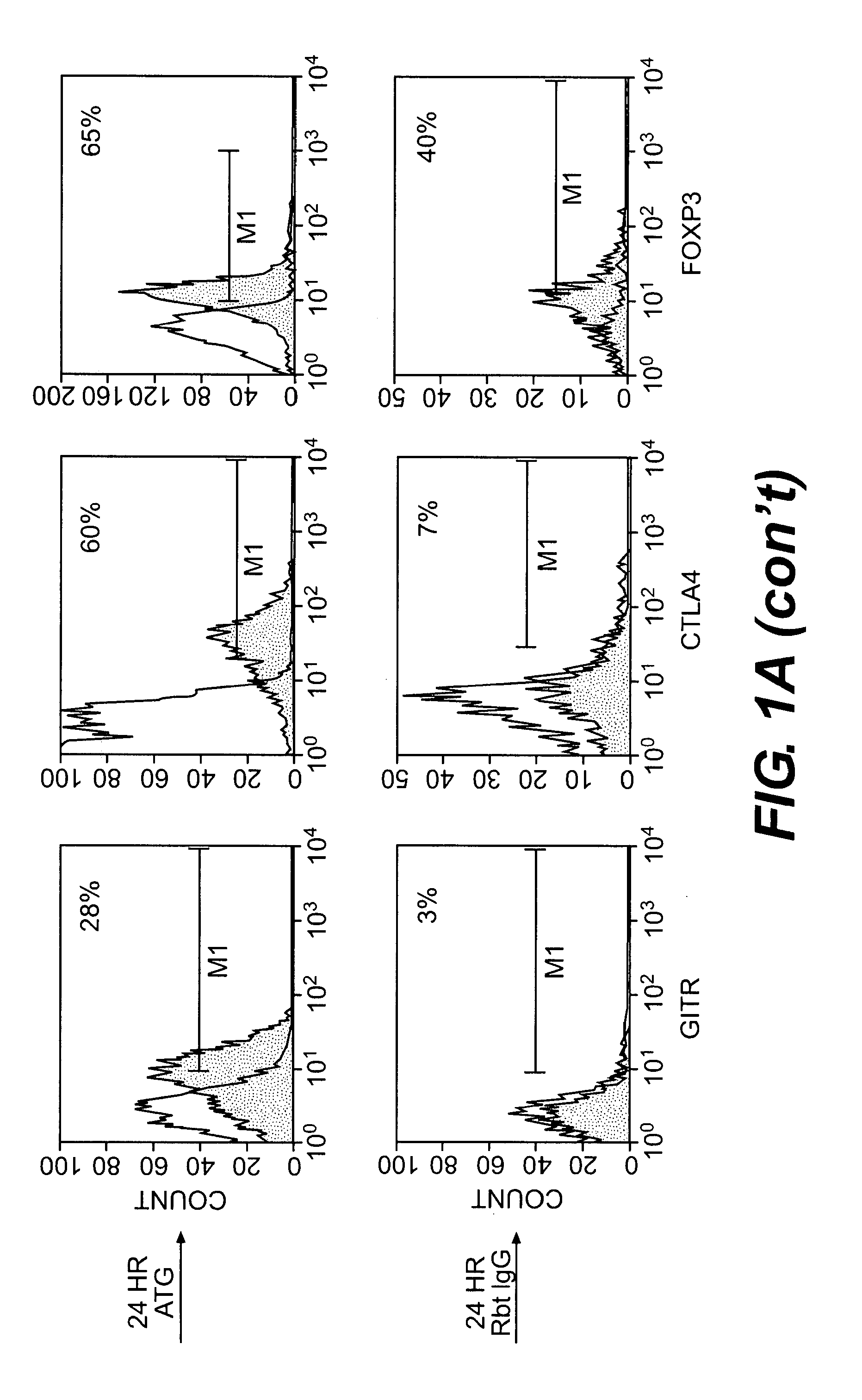
Methods of Using Anti-Thymocyte Globulin and Related Agents
InactiveUS20100034782A1Promote generationSuppress abnormal immune responseBiocidePeptide/protein ingredientsAutoimmune conditionRegulatory T cell
Uses for anti-thymocyte globulin (ATG, e.g., Thymoglobulin®) and related compositions are described. In one aspect, ATG and, optionally, TGF-beta are used for in vitro generation of regulatory T cells, which are useful for cell therapy of immune-mediated conditions. In another aspect, ATG is directly administered to a subject at a low dose (e.g., less than 1 mg / kg per day) to treat an immune-mediated condition. The immune-mediated conditions include, for example, transplant rejection, graft-versus-host disease, and autoimmune diseases.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
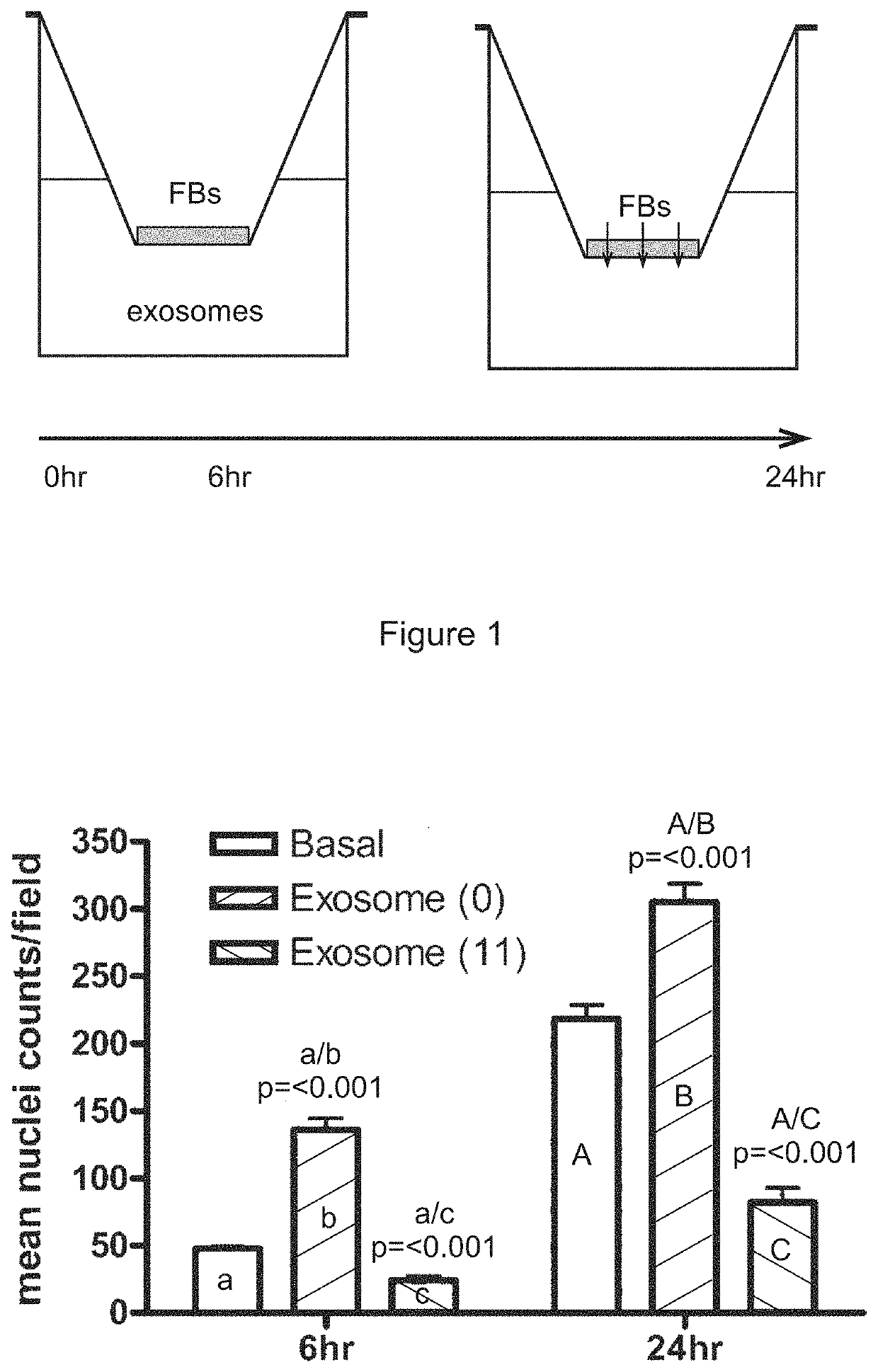
STEM CELL MICROPARTICLES AND miRNA
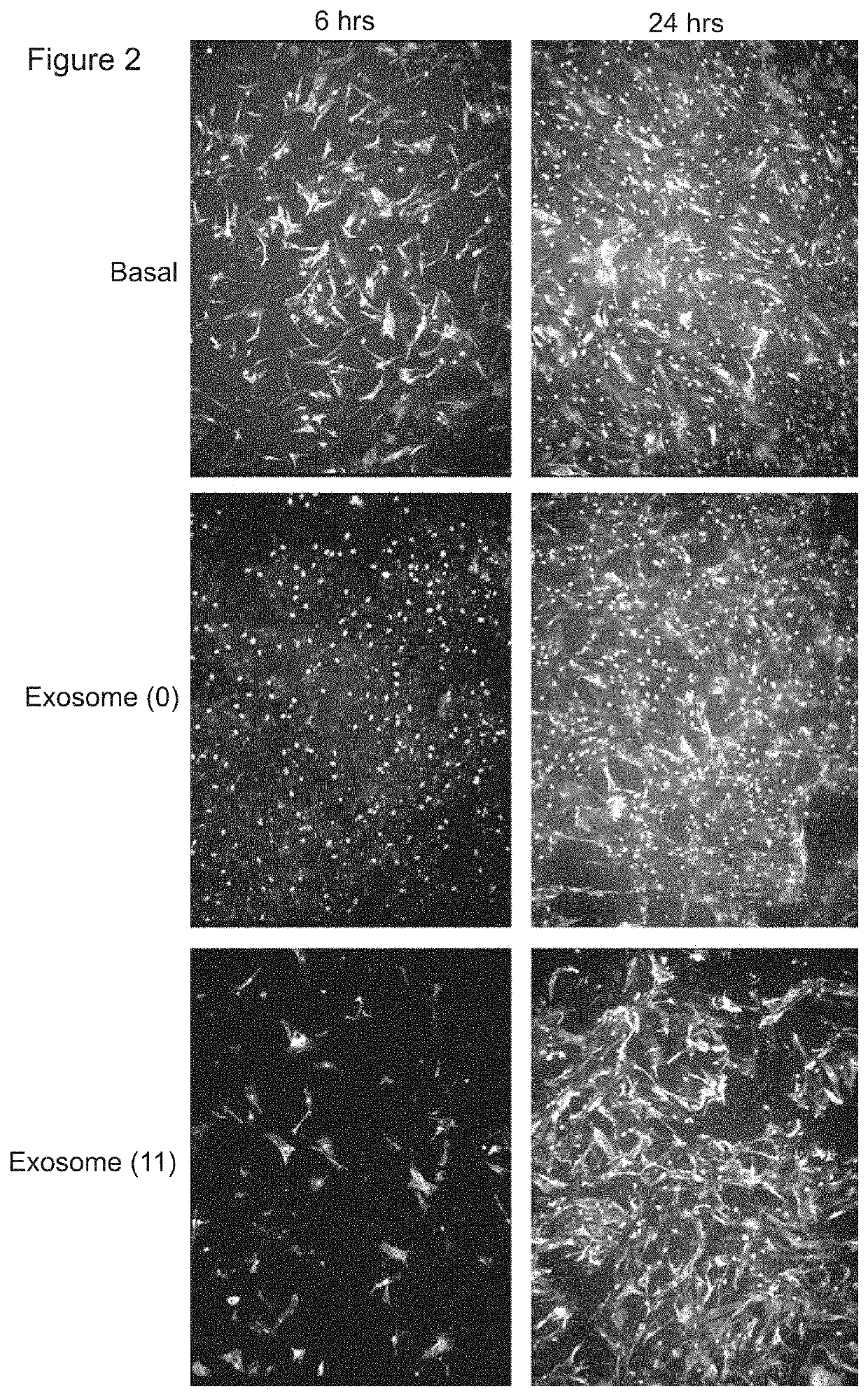
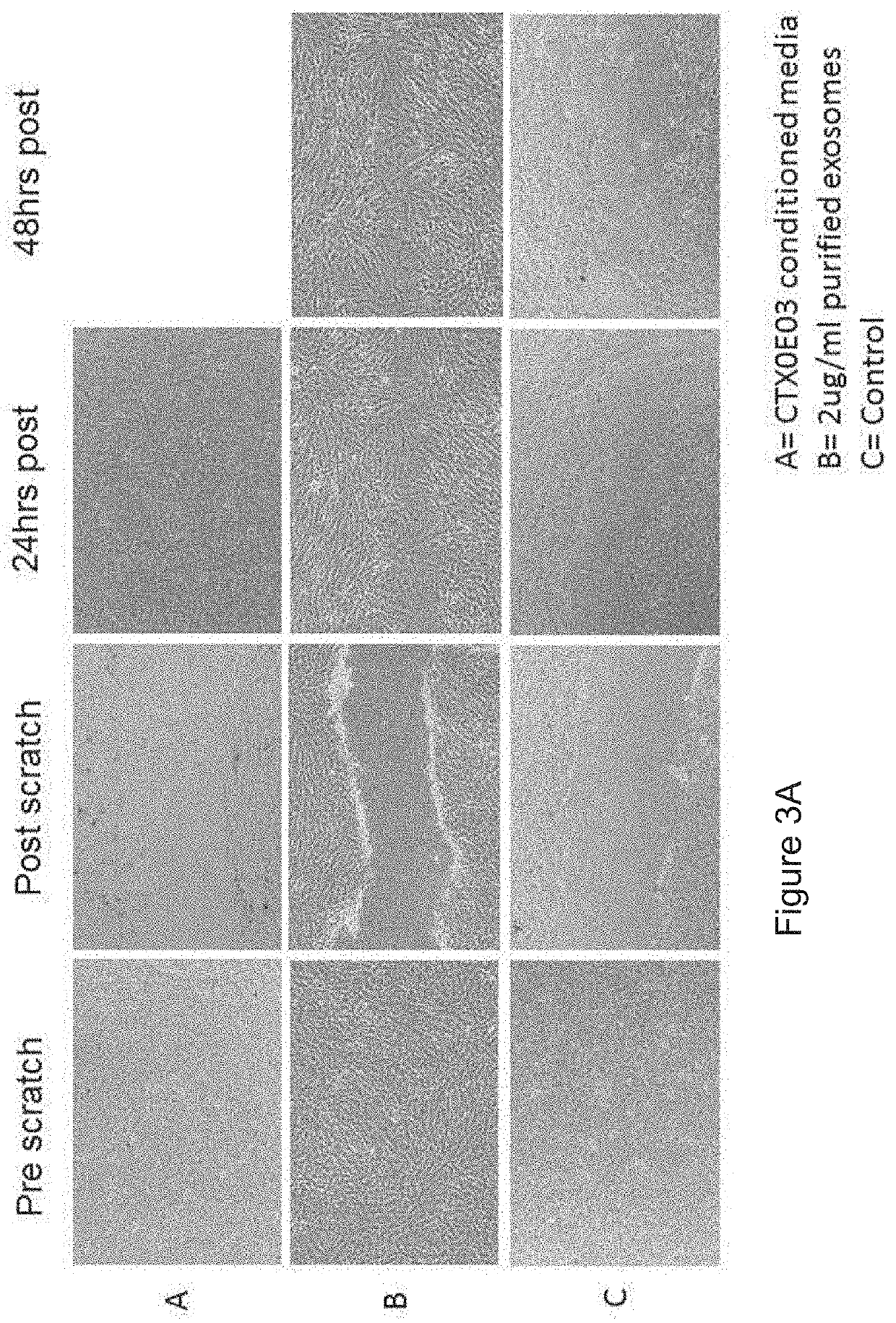
PendingUS20190381105A1Dramatic and effective ablationReduce tumor volumePharmaceutical delivery mechanismNervous system cellsMelanomaMedicine
This invention relates to stem cell microparticles and miRNA isolated from these microparticles, their use and production thereof, in particular neural stem cell microparticles and their use in therapy of cancer, typically a nestin-positive cancer. The cancer may be glioma, melanoma, breast cancer, pancreatic cancer or prostate cancer. The stem cell microparticle is typically an exosome or microvesicle and may be derived from a neural stem cell line. The neural stem cell line may be a conditionally-immortalised stem cell line such as CTX0E03 (deposited at the ECACC with Accession No. 04091601).
Owner:RENEURON LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com