Attaching substances to microorganisms
a technology of substances and microorganisms, applied in the field of surface display of proteins on microorganisms, can solve the problems of limiting the application of organisms containing recombinant nucleic acids, affecting the immune response, so as to avoid potential risks and enhance the immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Experimental Part
Introduction
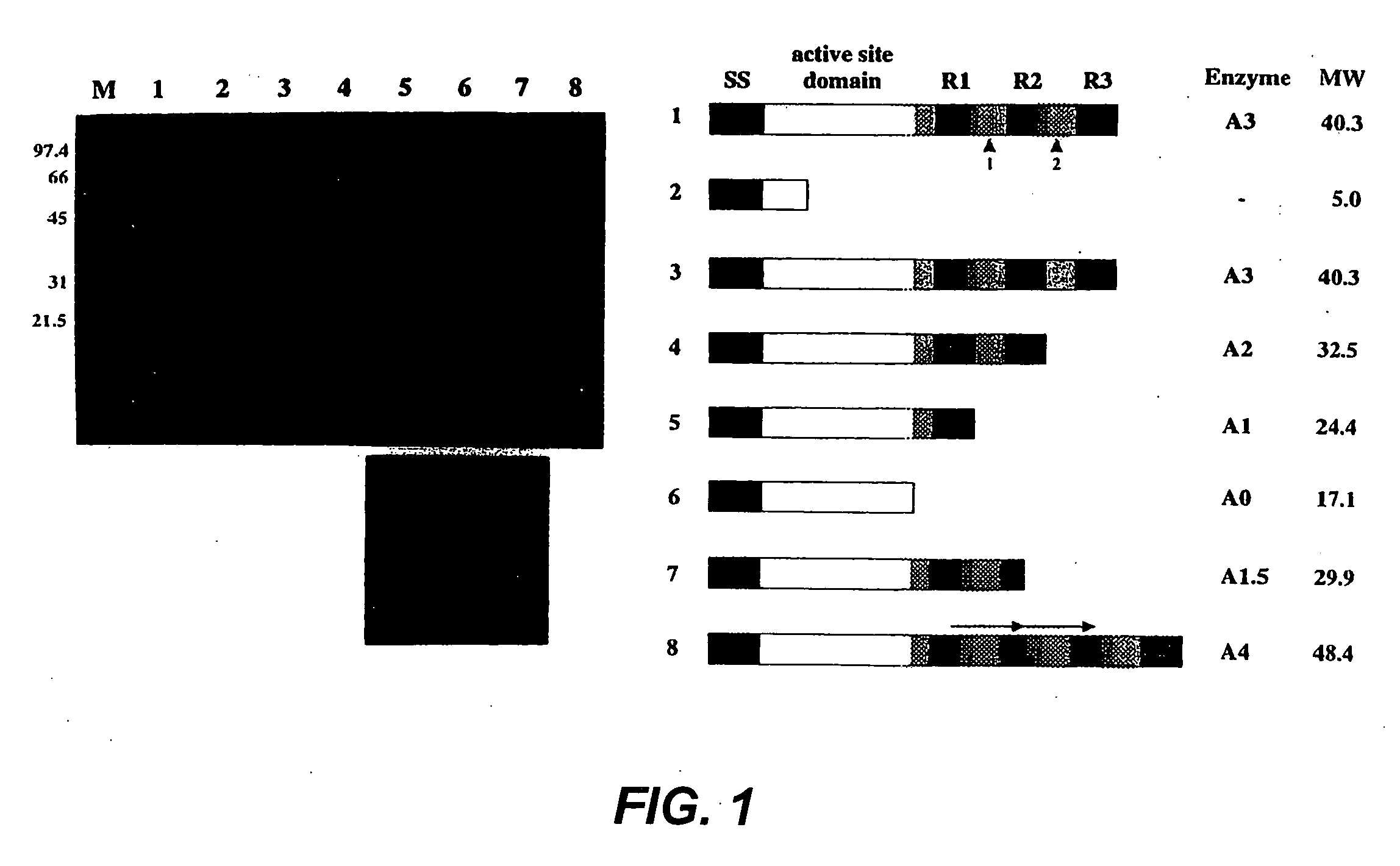
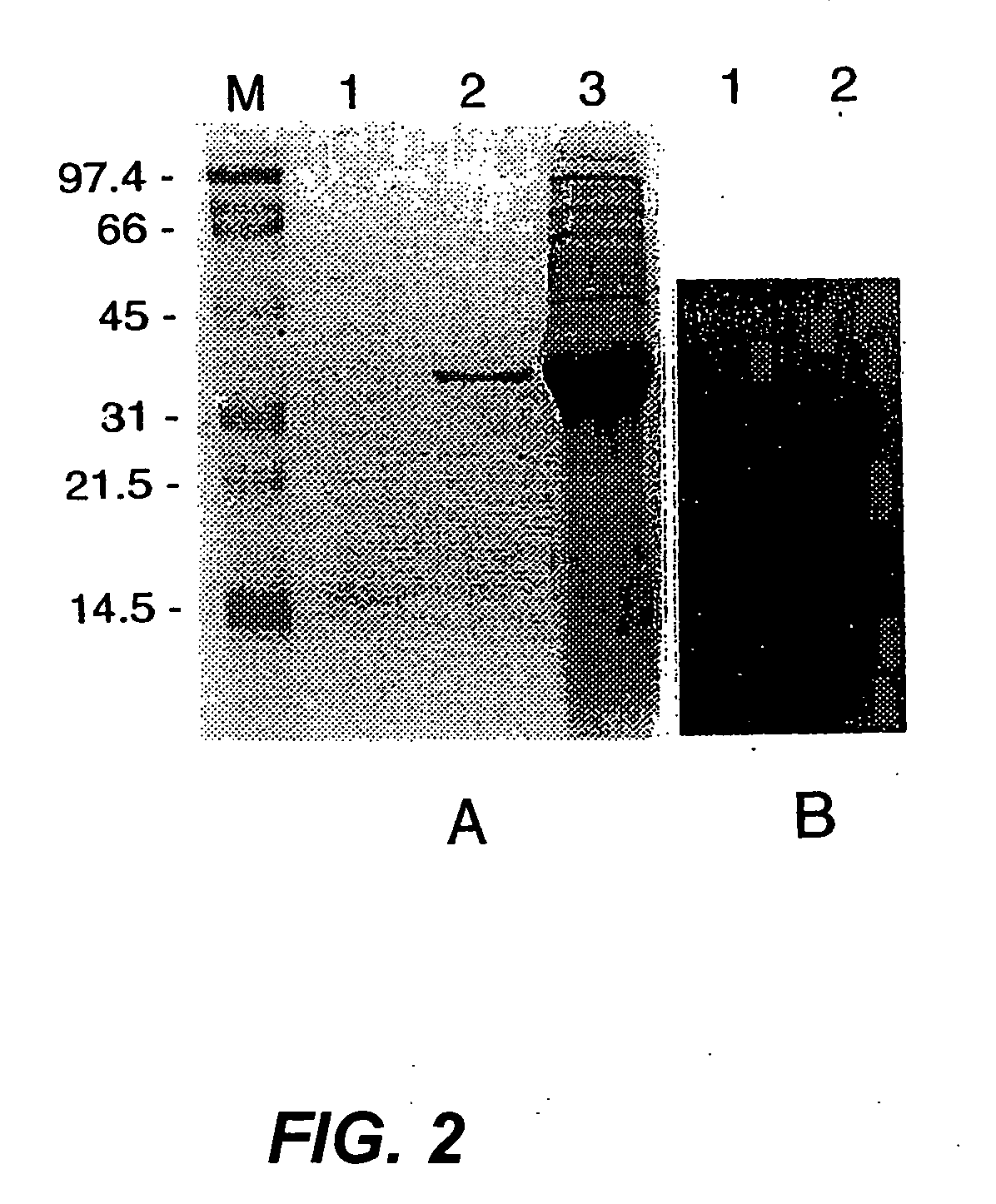
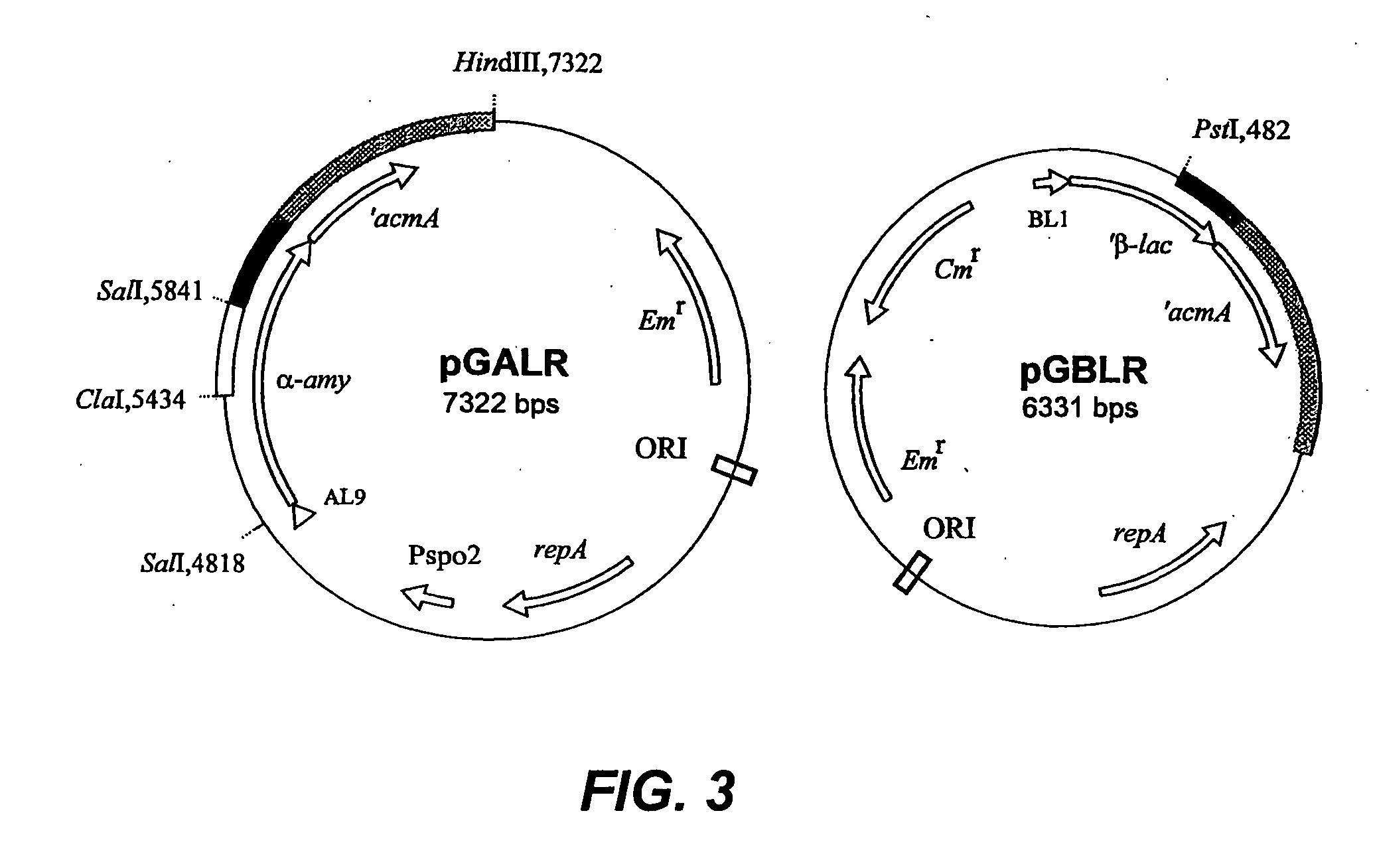
[0032] The major autolysin AcmA of Lactococcus lactis subsp. cremoris MG1363 is an N-acetylmuramidase which is required for cell separation and is responsible for cell lysis during the stationary phase (5, 6). The 40.3-kDa secreted mature protein produces a number of activity bands in a zymogram of the supernatant of a lactococcal culture. Bands as small as that corresponding to a protein of 29 kDa were detected. As no clearing bands are produced by an L. lactis acmA deletion mutant, all bands represent products of AcmA (6). From experimental data and homology studies, it was inferred that AcmA likely consists of three domains: a signal sequence followed by an active site domain and a C-terminal region containing three highly homologous repeats of approximately 45 amino acids which are involved in cell wall binding. As the smallest active protein is 29 kDa, it was suggested that the protein undergoes proteolytic breakdown in the C-terminal portion (5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com