Culture medium used for culturing adipose mesenchymal stem cells, and applications thereof
A technology of basal medium and serum-free medium, which is applied in the direction of culture process, tissue culture, animal cells, etc. It can solve the problems of easy change of cell morphology, cell aging, difficulty in ensuring the consistency of medium batches, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
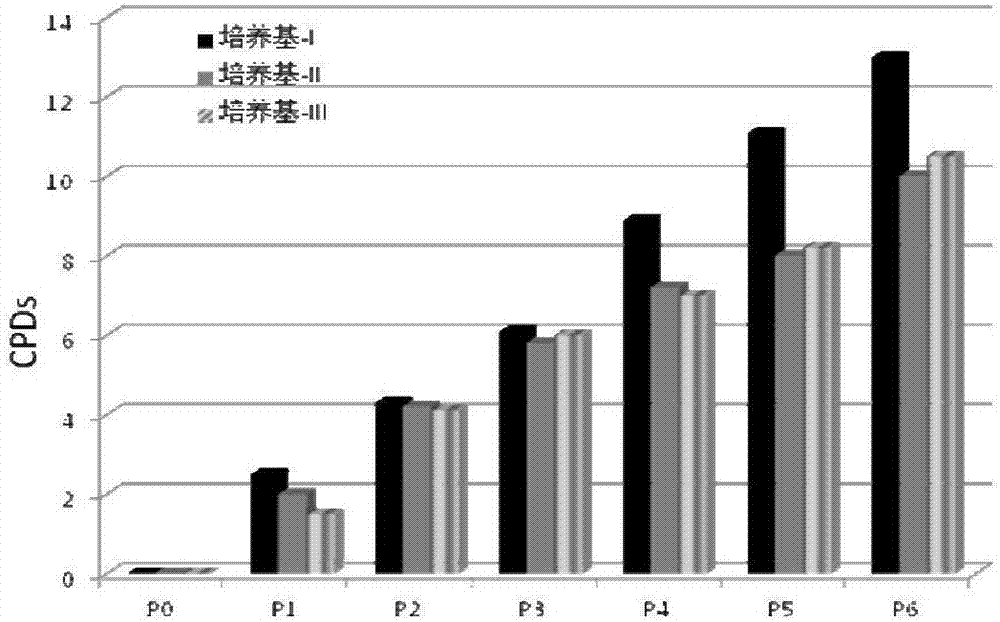
[0055]Embodiment 1, the special culture medium of human adipose-derived mesenchymal stem cells
[0056] The special medium for human adipose-derived mesenchymal stem cells of the present invention is based on a-MEM medium, and a-MEM medium, recombinant human insulin, human serum albumin, transferrin, fibronectin, ascorbic acid , biotin, PDGF (platelet-derived growth factor), bFGF (basic fibroblast growth factor) and TGF-β (transforming growth factor-β) mixed culture medium. Among them, the concentration of recombinant human insulin in the special medium is 5 μg / ml; the concentration of human serum albumin in the special medium is 0.5 mg / ml, the concentration of transferrin in the special medium is 5 μg / ml, fiber The concentration of connexin in the special medium is 5ng / ml, the concentration of ascorbic acid in the special medium is 5μg / ml, the concentration of biotin in the special medium is 3μg / ml, and the concentration of PDGF in the special medium is 5ng / ml, the concentra...
Embodiment 2
[0057] Example 2. Primary isolation and culture of human adipose-derived mesenchymal stem cells and detection of proliferation ability
[0058] 1. Primary isolation of human adipose-derived mesenchymal stem cells
[0059] 1. Aseptically collect the fat extract from the liposuction operation, wash it several times with PBS (GIBCO, catalog number C10010500BT), remove the drugs and blood cells used in the liposuction operation, and obtain adipose tissue.
[0060] 2. Cut up the adipose tissue obtained in step 1, collect it in a 50ml centrifuge tube, add an equal volume of PBS to shake vigorously, leave it at room temperature after shaking until layers appear in the centrifuge tube, and collect the upper layer.
[0061] 3. Wash the upper layer collected in step 2 with PBS, add an equal volume of 0.1% type I collagenase (Sigma, catalog number 9001-12-1) to digest at 37°C for 1 hour, centrifuge at 400g / min for 5min, and draw The fat in the upper layer was mixed evenly, then filtered...
Embodiment 3
[0073] Example 3, detection of biological characteristics of human adipose-derived mesenchymal stem cells
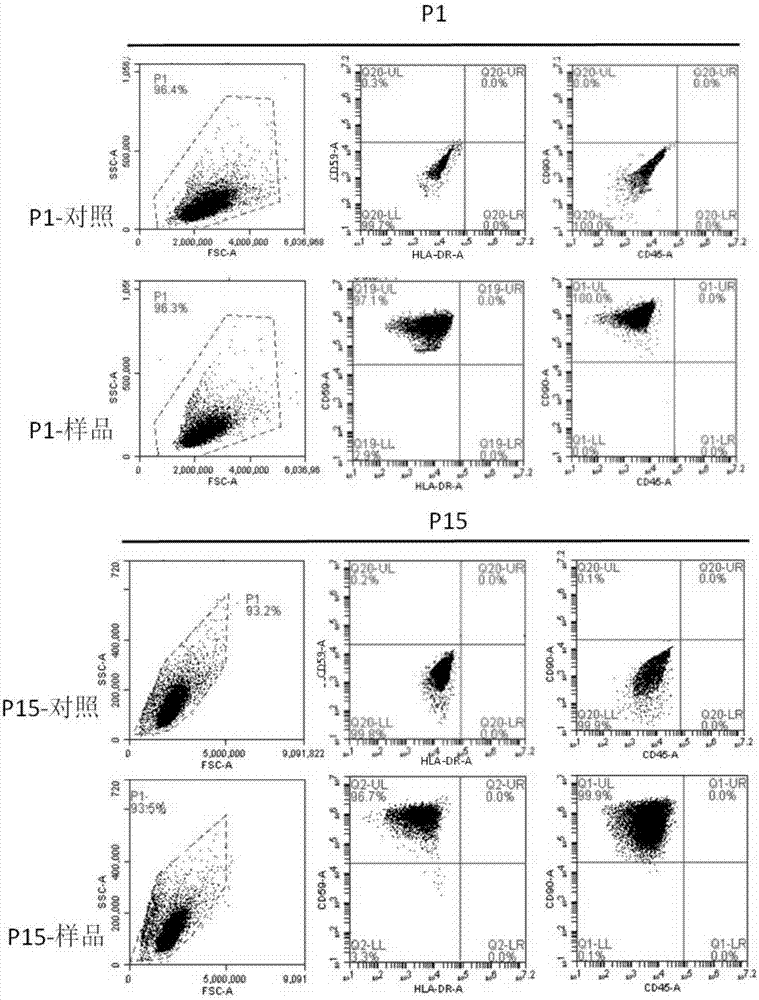
[0074] Take the P1 generation and P15 generation human adipose-derived mesenchymal stem cells obtained in the medium-I culture in step 2 of Example 2, and add 0.25% (v / v) Trypsin and 0.04% (v / v) EDTA for digestion, and then collect the cells, take 1 × 10 6 Transfer cells into a flow tube, wash twice with PBS, then resuspend with PBS, add the following labeled antibodies: CD90, CD45, HLA-DR and CD59, incubate at 4°C for 30 min, and wash twice with PBS , the labeled cells were analyzed with BD's FACSCanto II flow cytometer to detect the expression of surface markers CD90, CD45, HLA-DR and CD59. And no antibody was used as negative control.
[0075] The result is as figure 2 as shown ( figure 2 P1-control and P15-control in both refer to negative control). As can be seen from the figure: when the human adipose-derived mesenchymal stem cells obtained by culturing the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com