Patents
Literature
2259 results about "Bone marrow" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bone marrow is a semi-solid tissue which may be found within the spongy or cancellous portions of bones. In birds and mammals, bone marrow is the primary site of new blood cell production or hematopoiesis. It is composed of hematopoietic cells, marrow adipose tissue, and supportive stromal cells. In adult humans, bone marrow is primarily located in the ribs, vertebrae, sternum, and bones of the pelvis. On average, bone marrow constitutes 4% of the total body mass of humans; in an adult having 65 kilograms of mass (143 lb), bone marrow typically accounts for approximately 2.6 kilograms (5.7 lb).
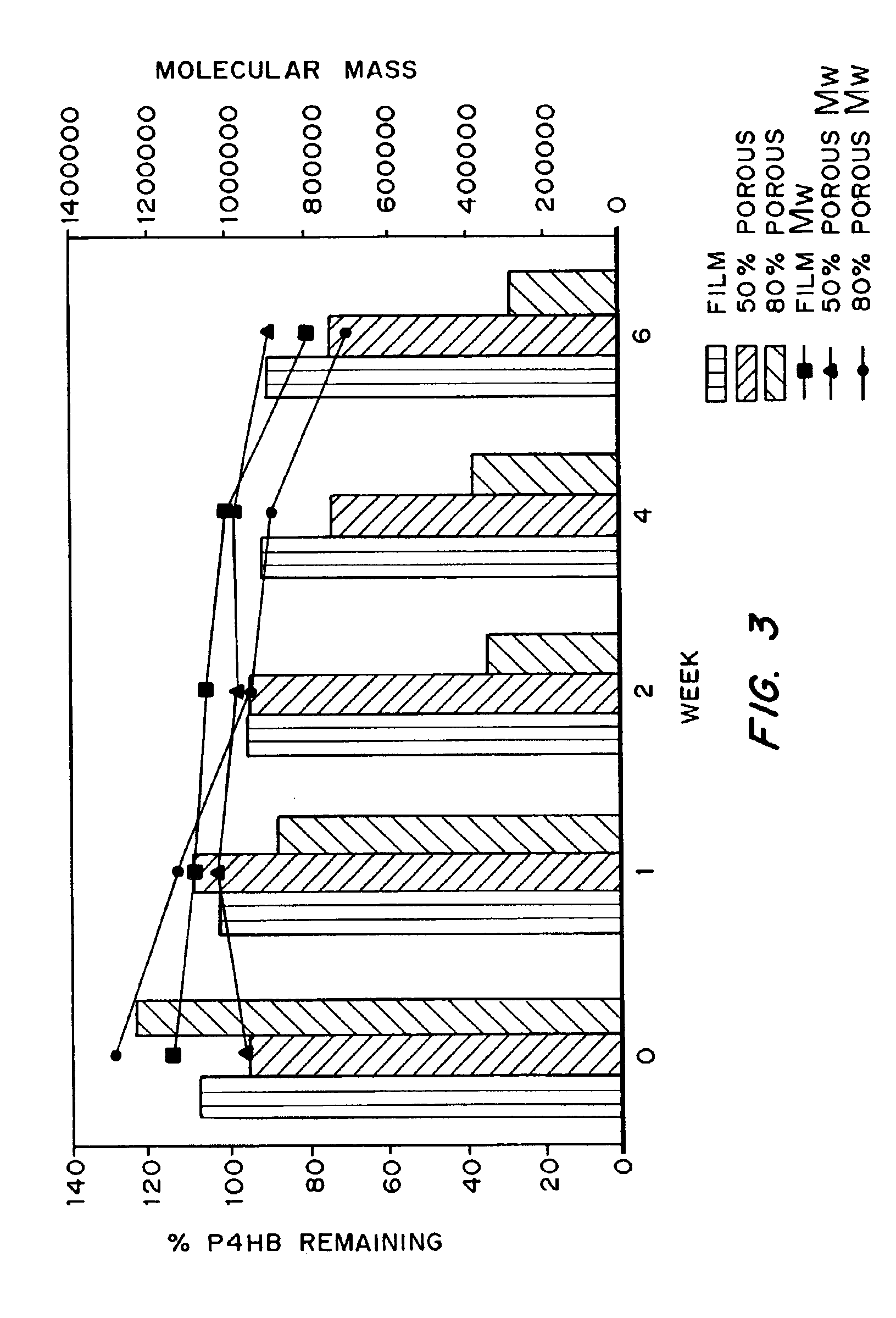
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
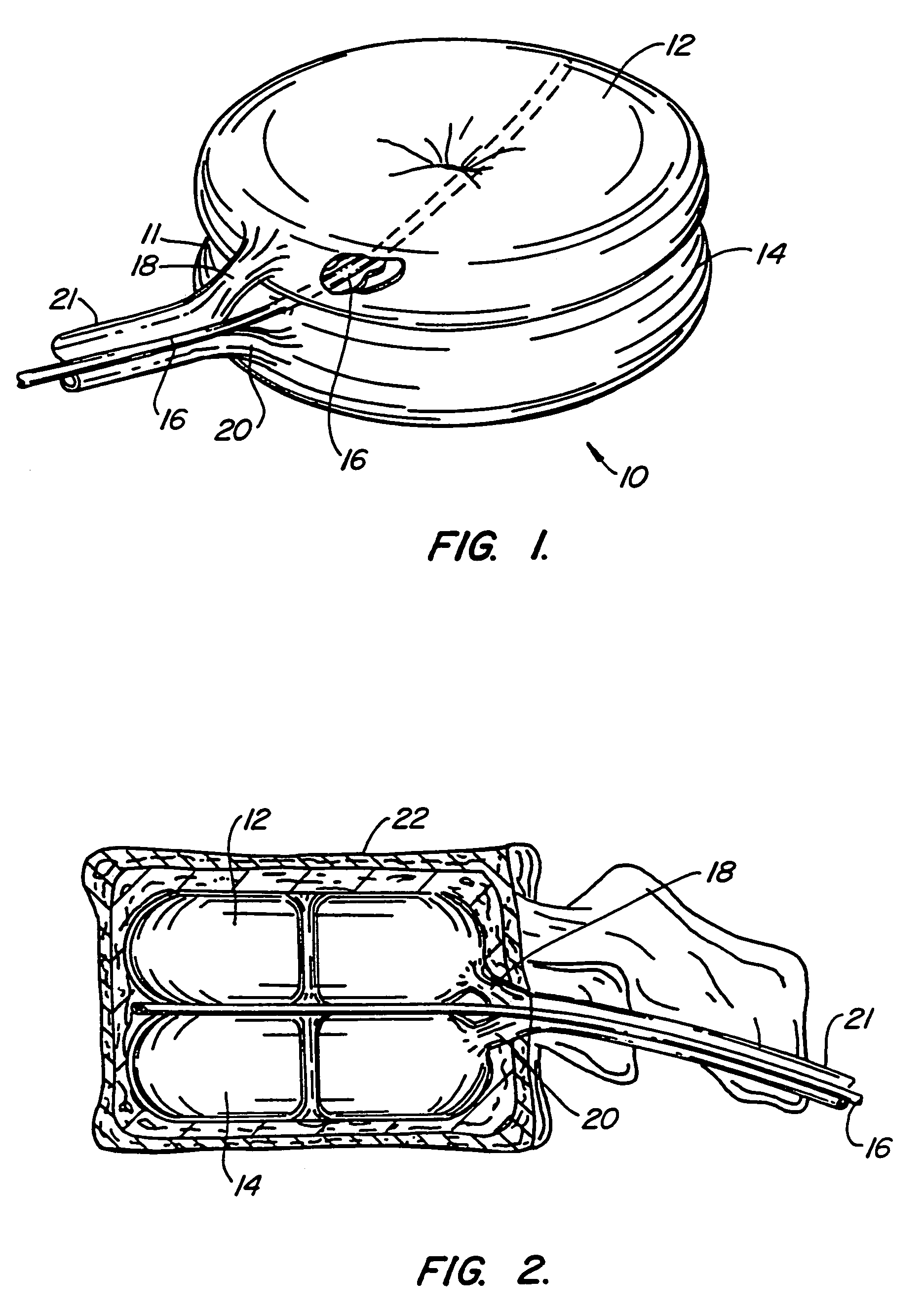
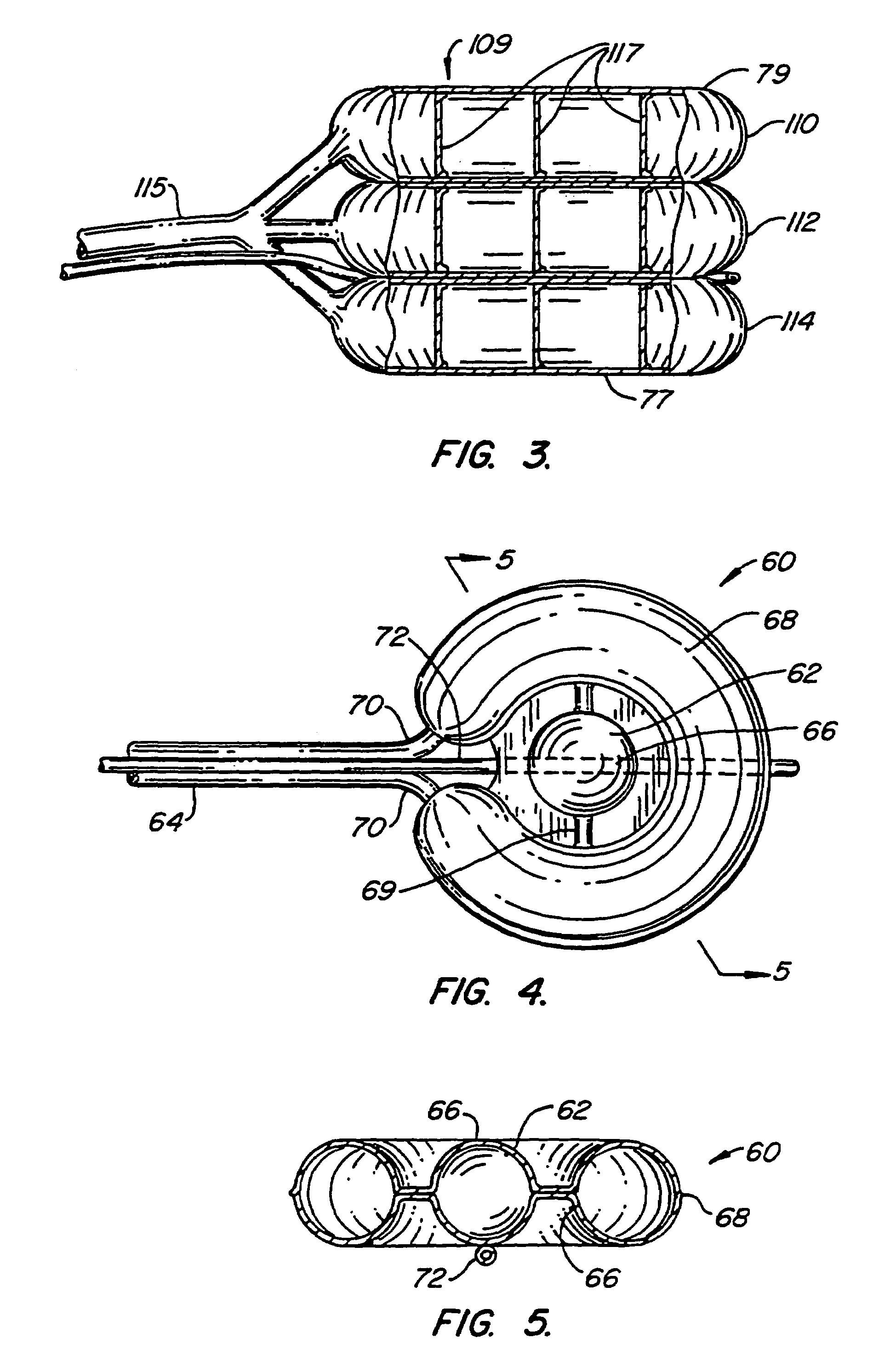
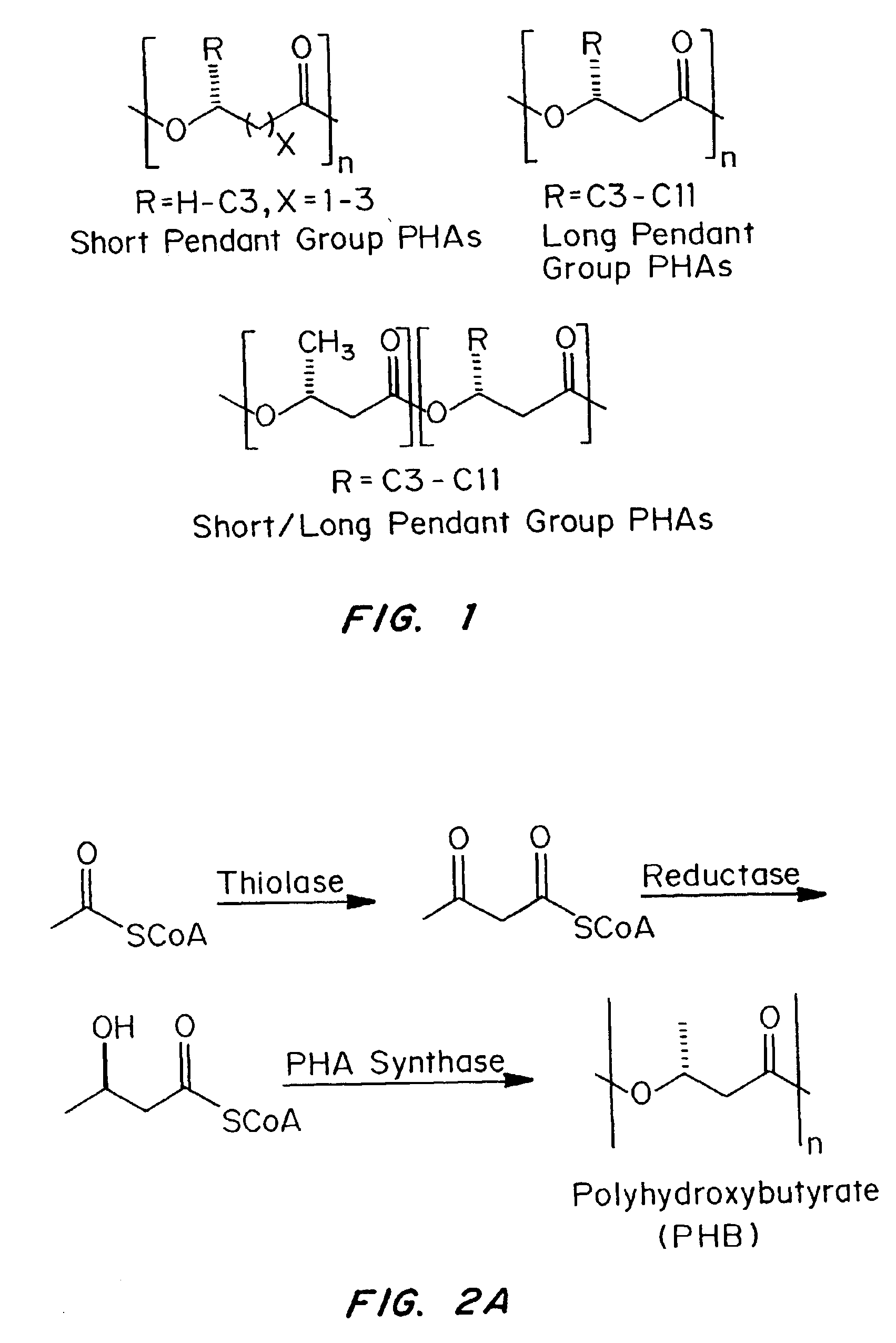
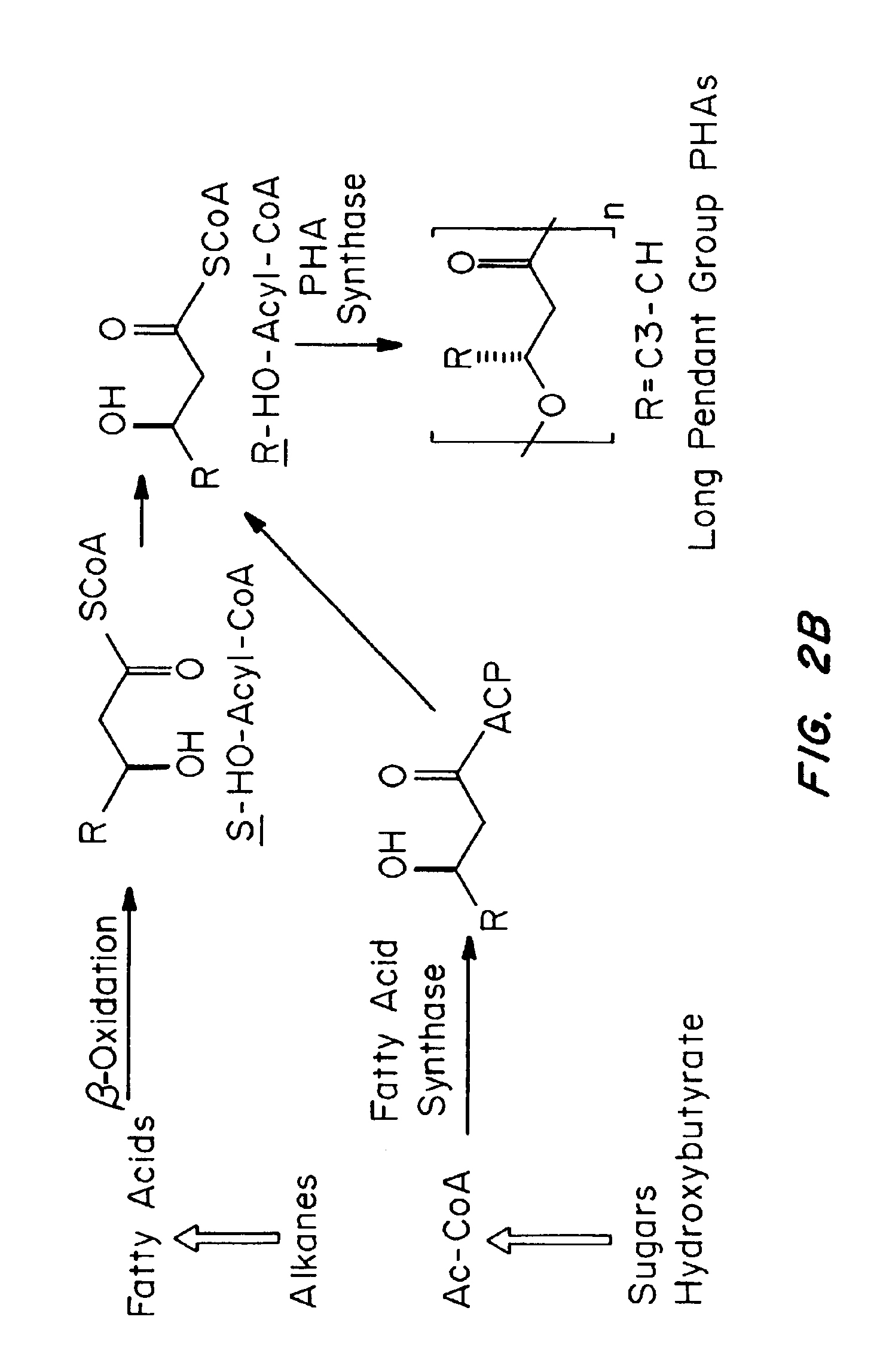
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
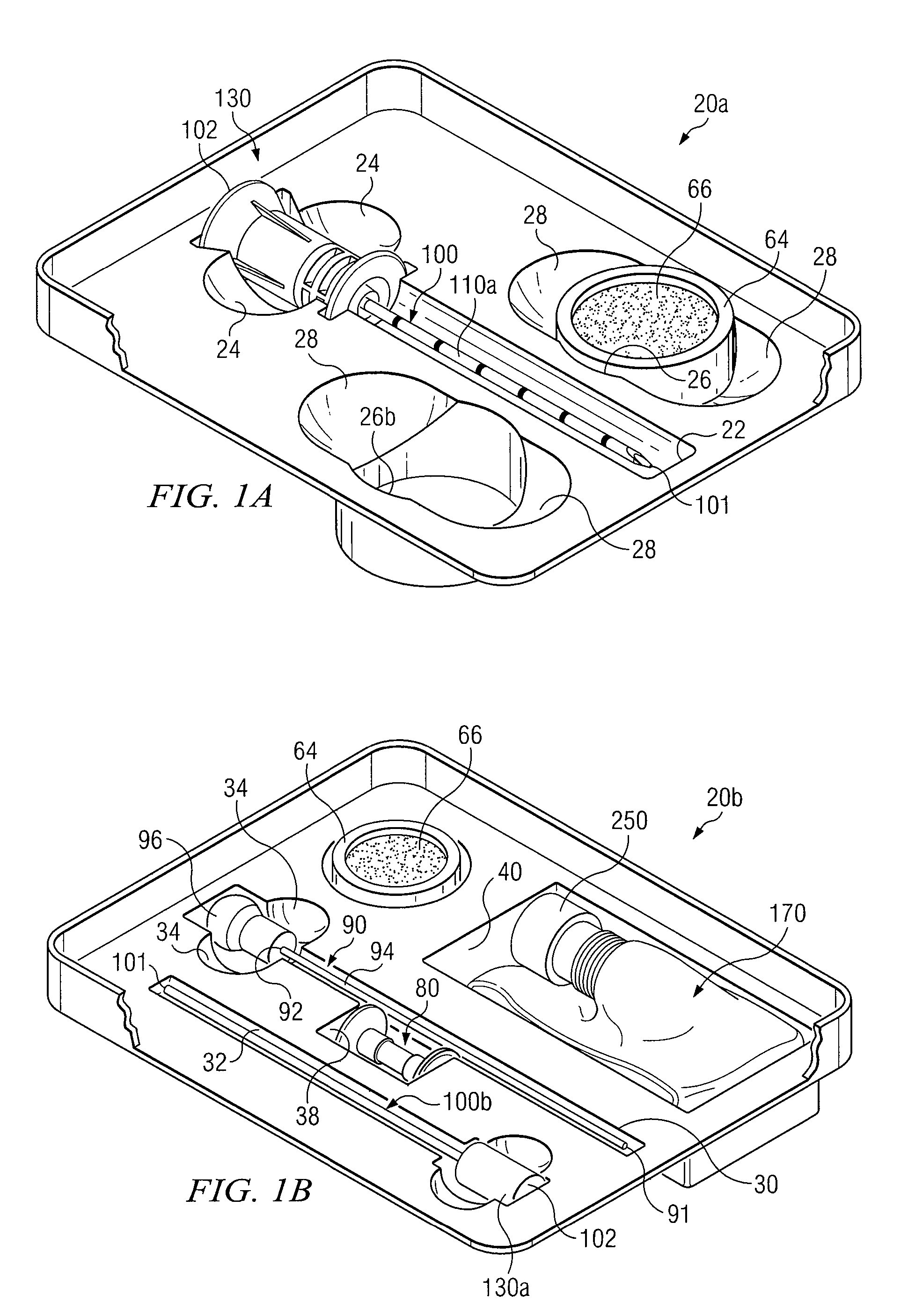
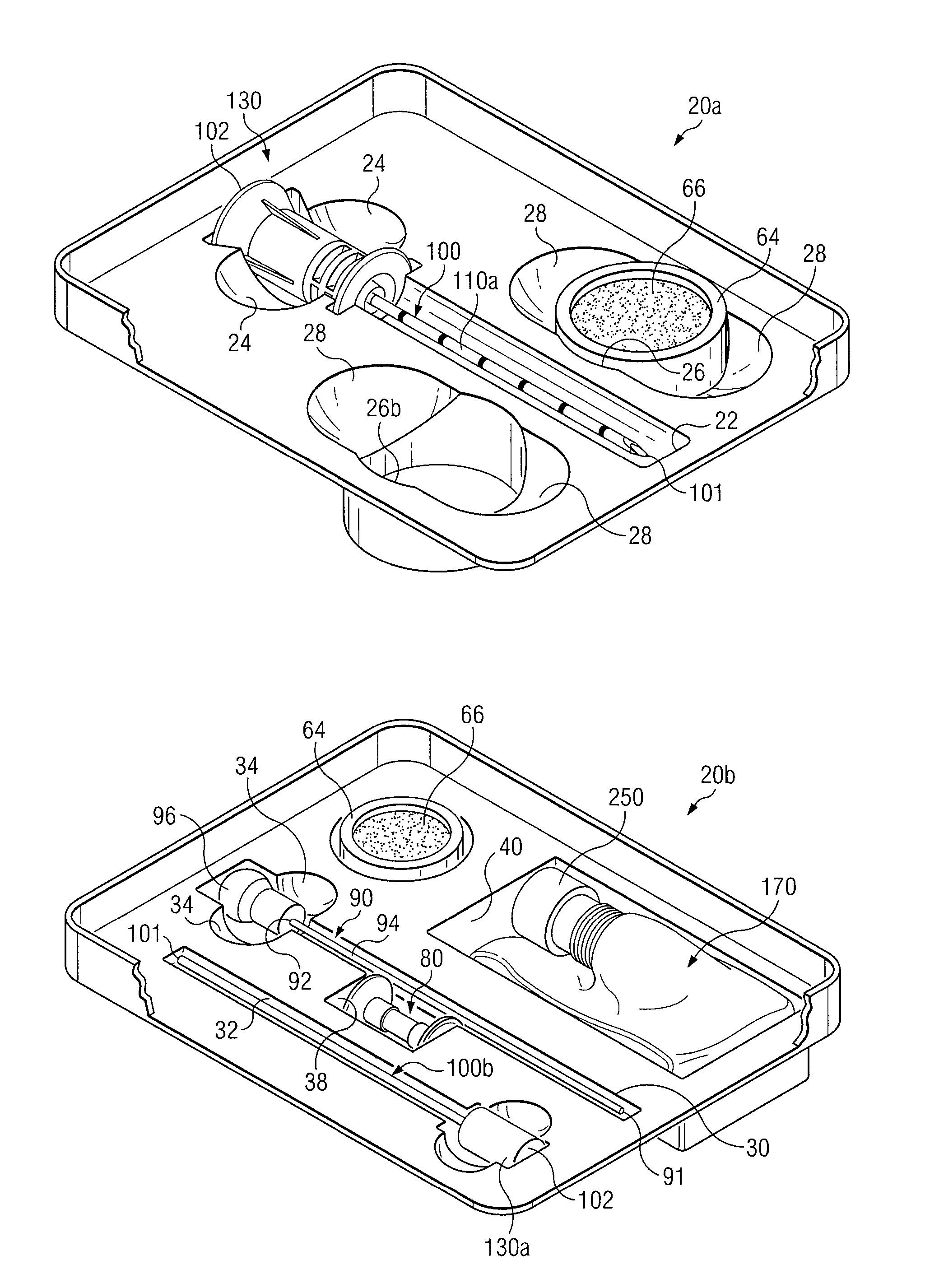
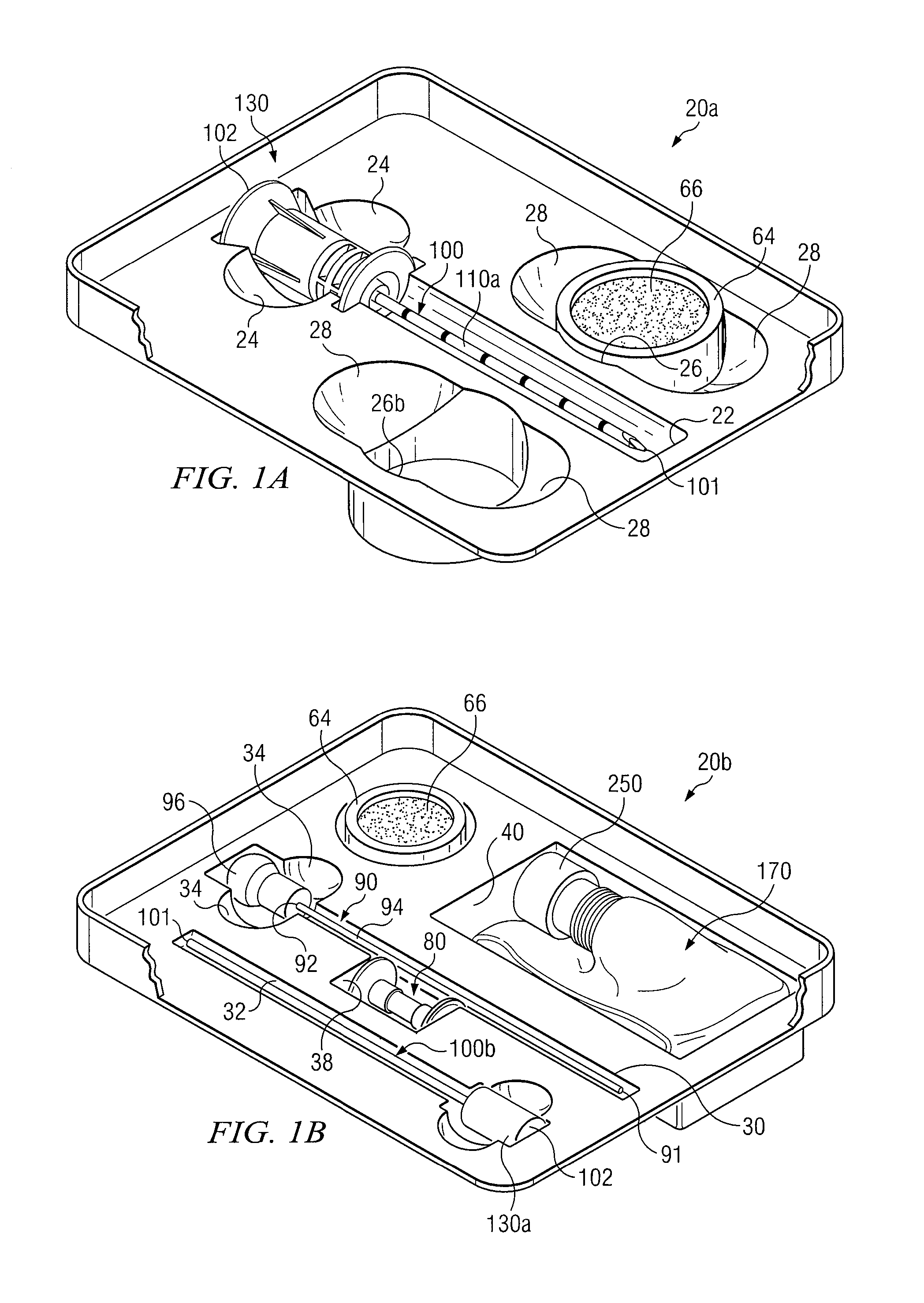
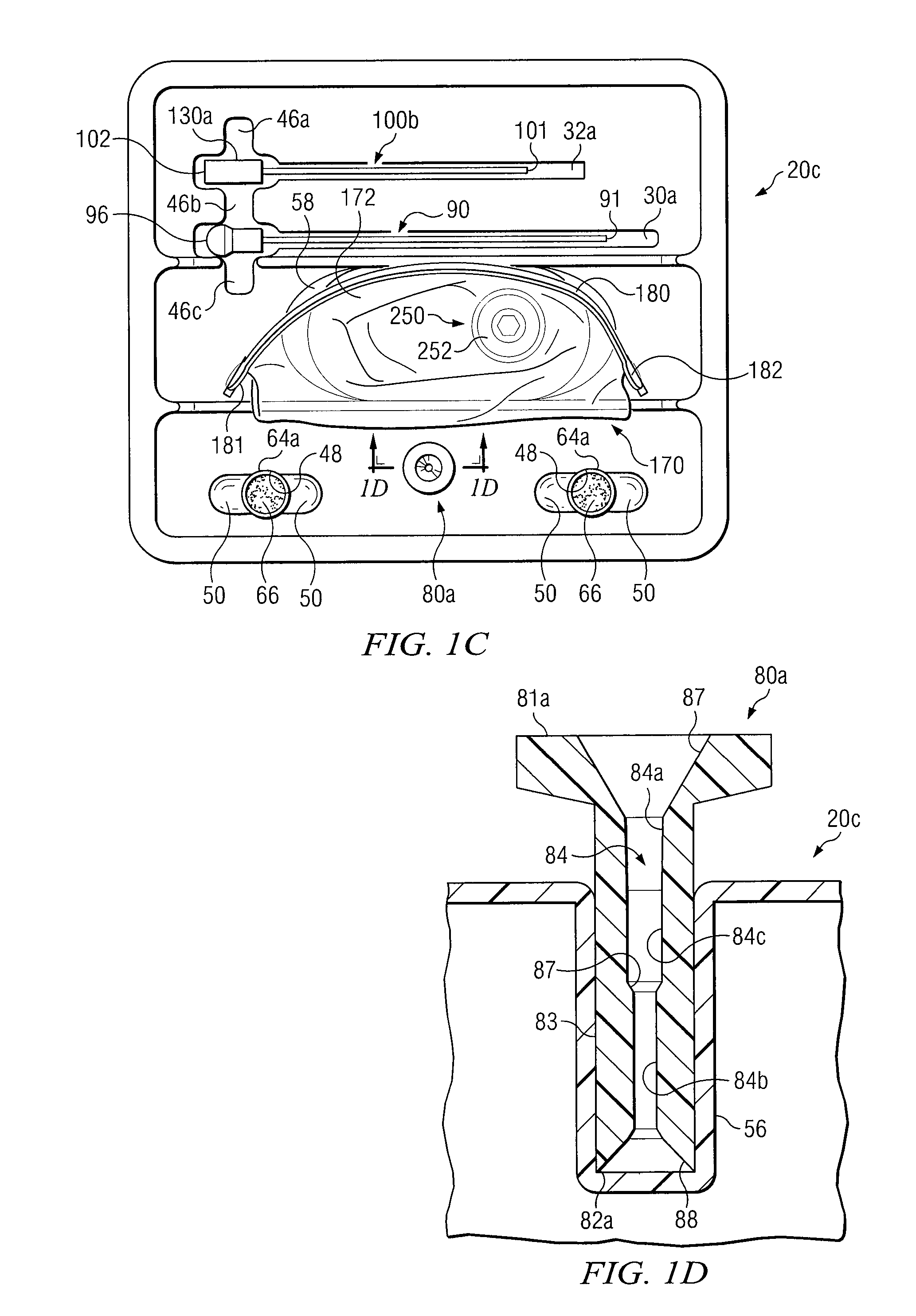
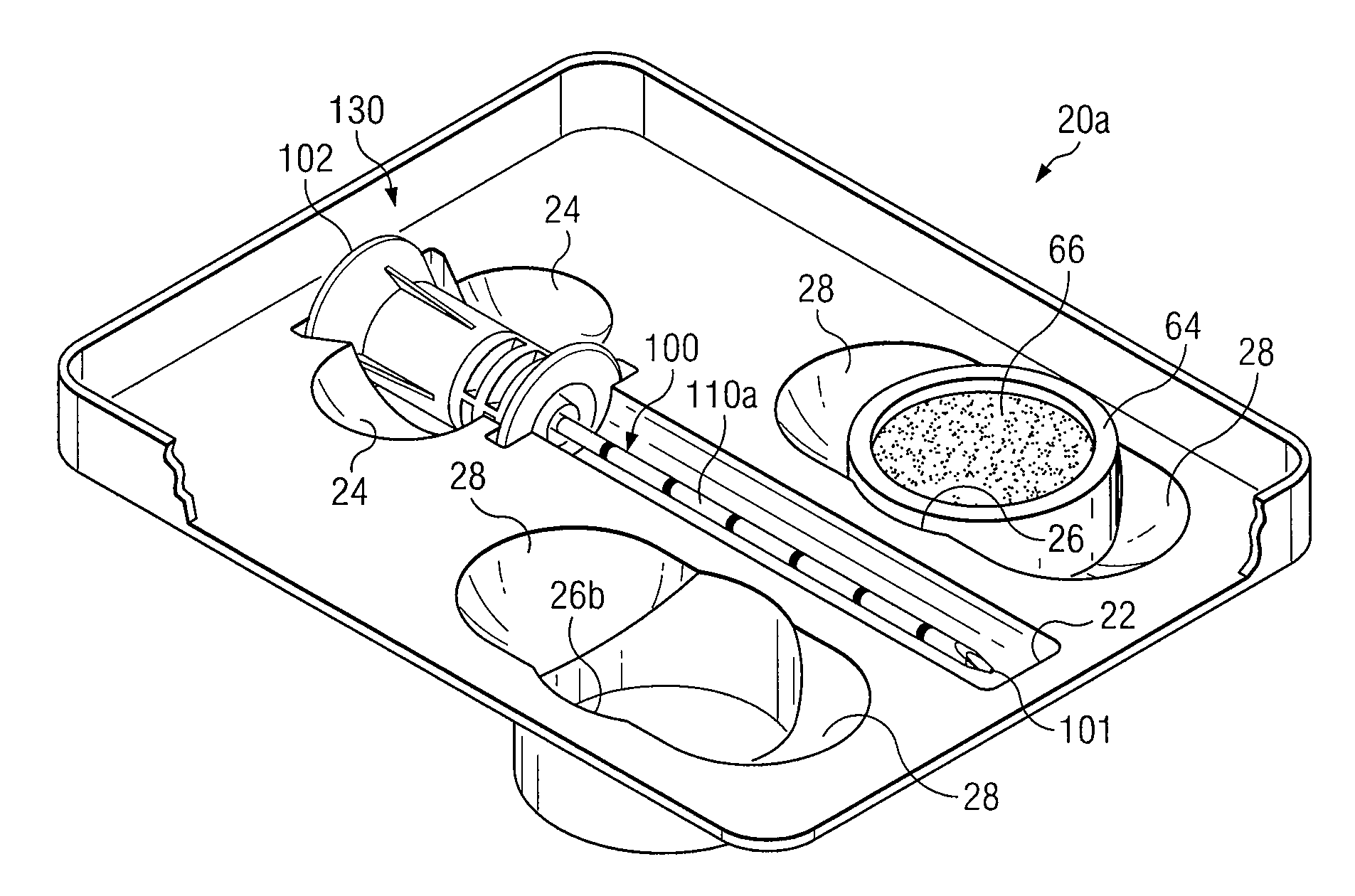
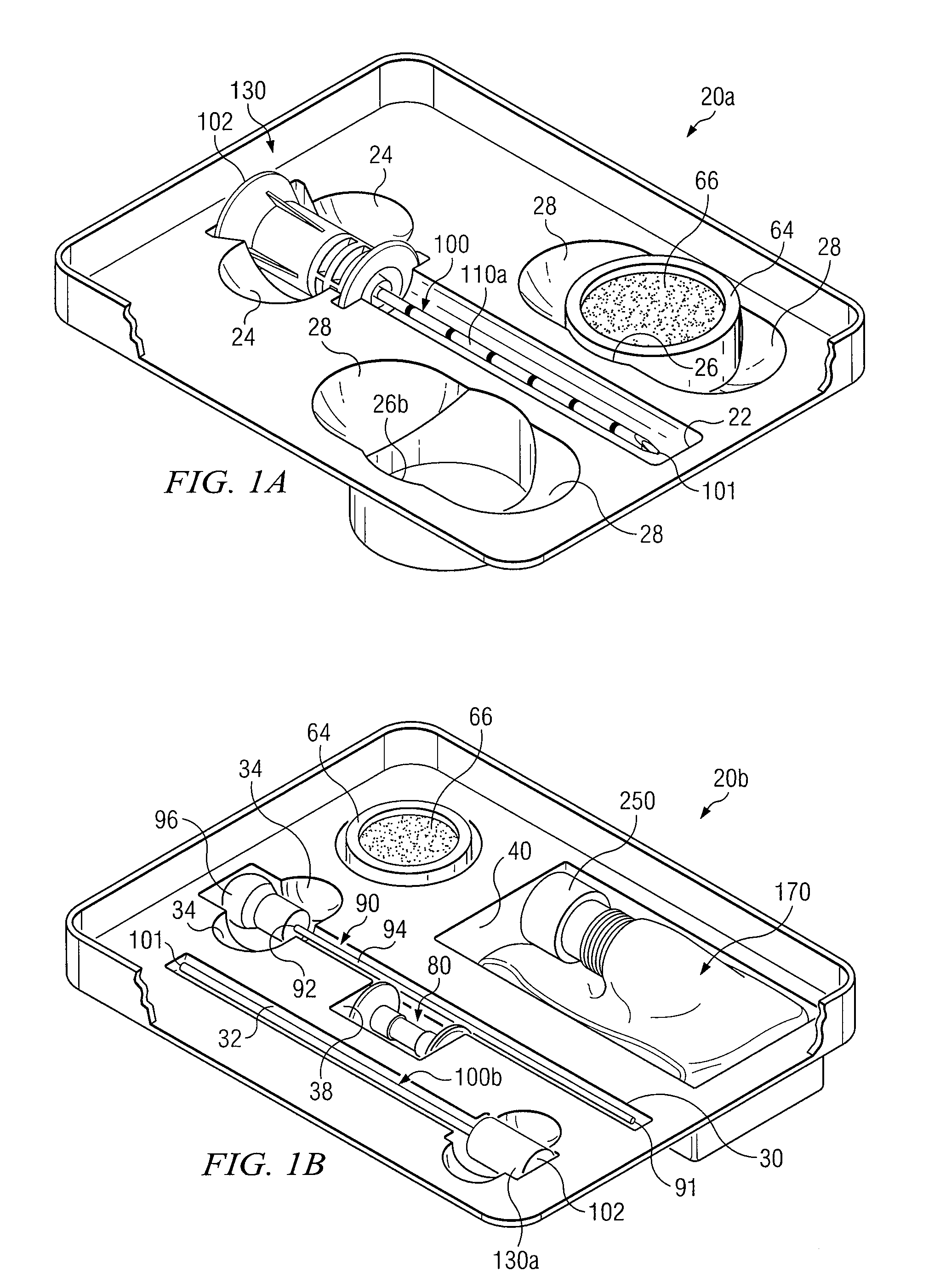
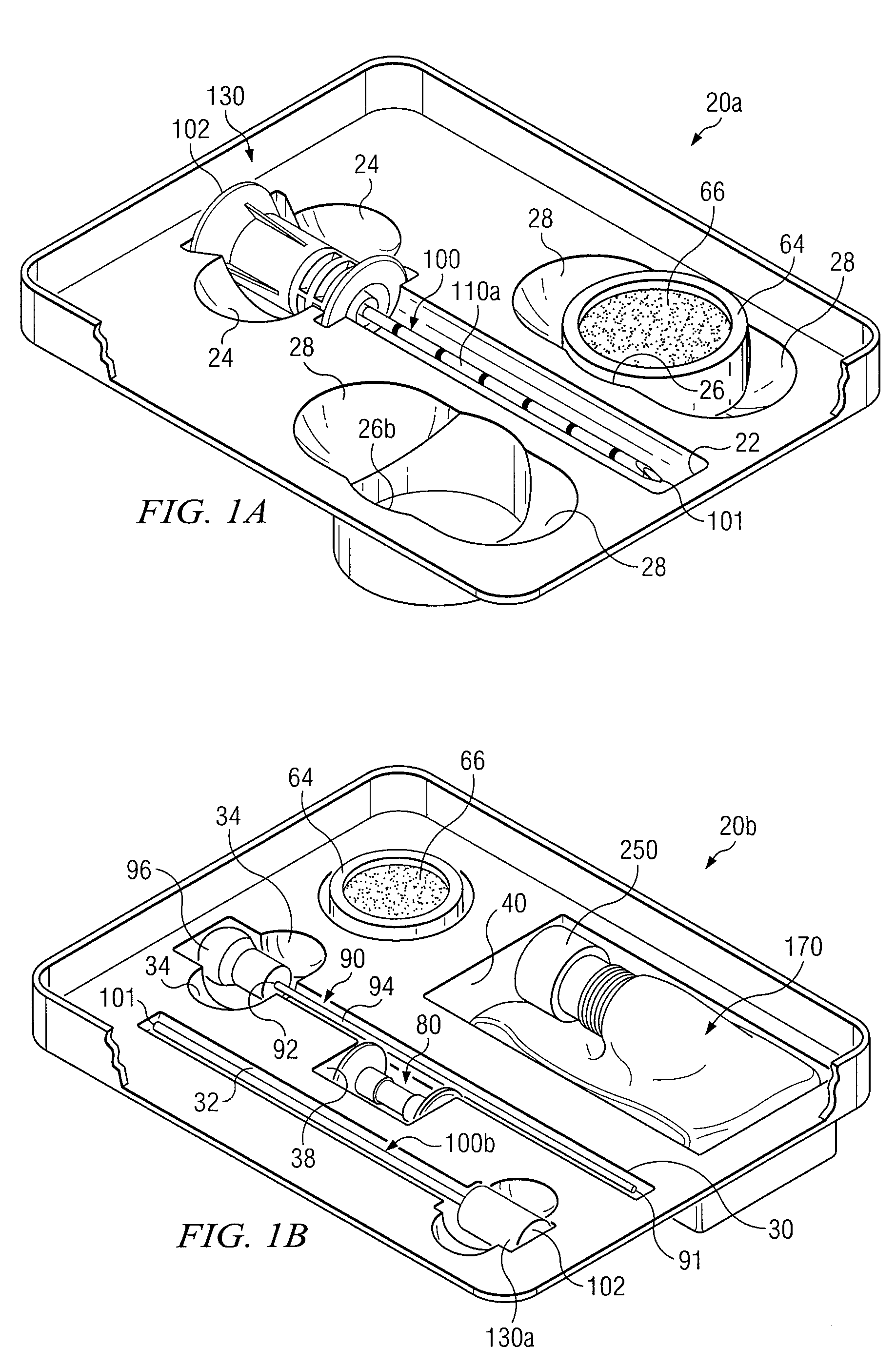
Medical procedures trays and related methods
ActiveUS8656929B2Easy to captureIncrease speedSurgical furnitureDispensing apparatusTransplant ProcedureSurgery
Medical procedure trays and related methods are provided to accommodate joining a first non-sterile medical device with a second sterile medical device and maintaining required sterilization of the second sterile medical device to perform an associated medical procedure. One example of such medical procedures includes biopsy of a bone and / or associated bone marrow using a non-sterile powered drive and a sterile biopsy needle or biopsy needle set. Medical procedure trays and related methods may also be provided for use during aspiration of bone marrow and / or stem cell transplant procedures. Each medical procedure tray may include a containment bag or sterile sleeve. A coupler assembly, one or more sharps protectors, a biopsy sample ejector and / or associated ejector funnel may also be included. Some medical procedure trays may allow engaging a non-sterile powered driver with one end of a coupler assembly and sealing the non-sterile powered driver in a sterile sleeve or containment bag without compromising sterility of other components in the medical procedure tray.
Owner:TELEFLEX LIFE SCI LTD
Inflatable device for use in surgical protocol relating to fixation of bone
InactiveUS7261720B2Easy to compressEasy to foldSurgical furnitureInternal osteosythesisBone CortexTrabecular bone
A balloon for use in compressing cancellous bone and marrow (also known as medullary bone or trabecular bone). The balloon comprises an inflatable balloon body for insertion into said bone. The body has a shape and size to compress at least a portion of the cancellous bone to form a cavity in the cancellous bone and / or to restore the original position of the outer cortical bone, if fractured or collapsed. The balloon desirably incorporates restraints which inhibit the balloon from applying excessive pressure to various regions of the cortical bone. The wall or walls of the balloon are such that proper inflation of the balloon body is achieved to provide for optimum compression of the bone marrow. The balloon can be inserted quickly into a bone. The balloon can be made to have a suction catheter. The balloon can be used to form and / or enlarge a cavity or passage in a bone, especially in, but not limited to, vertebral bodies. Various additional embodiments facilitate directionally biasing the inflation of the balloon.
Owner:ORTHOPHOENIX
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6867247B2Reduce probabilityHigh porositySuture equipmentsStentsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
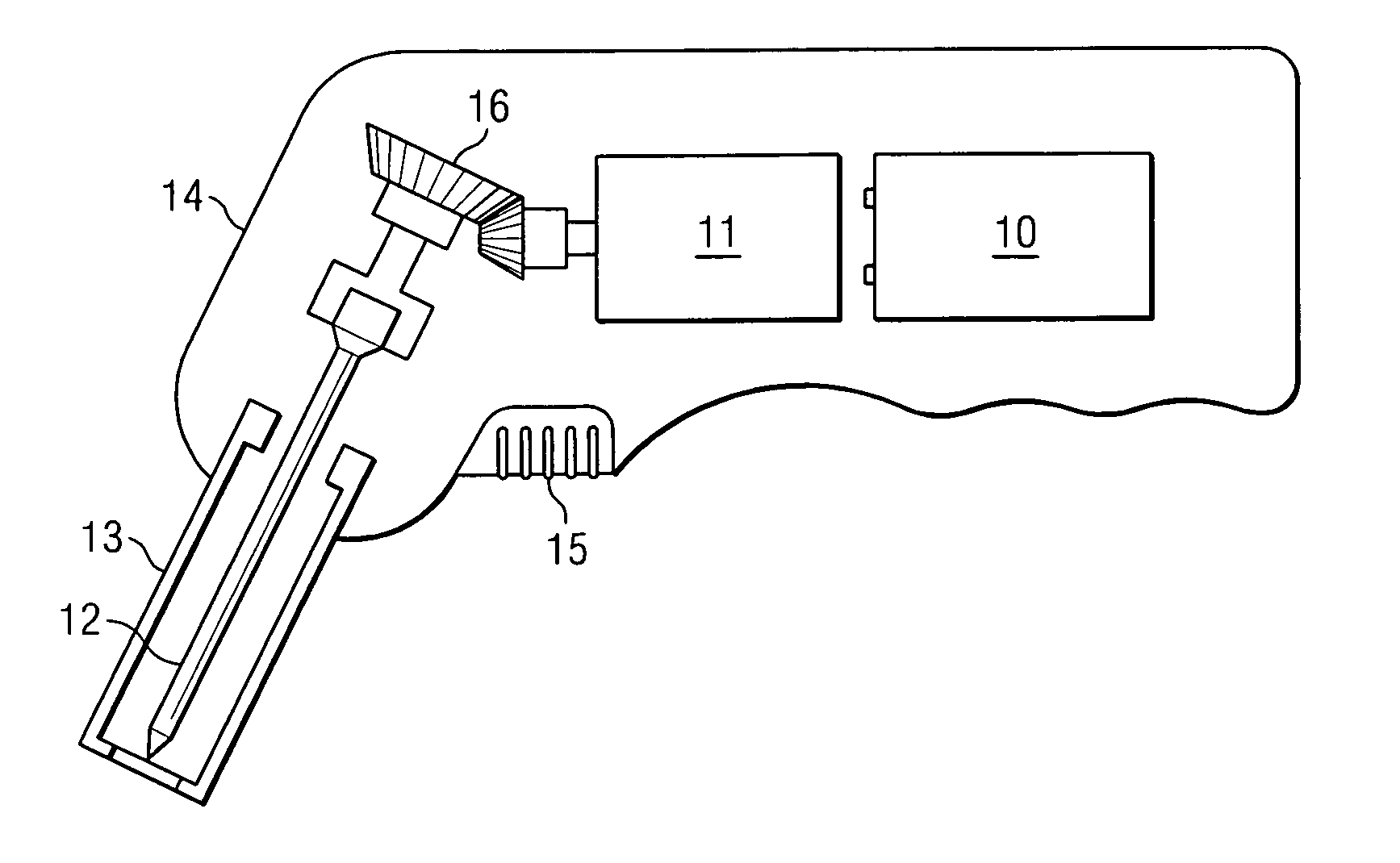
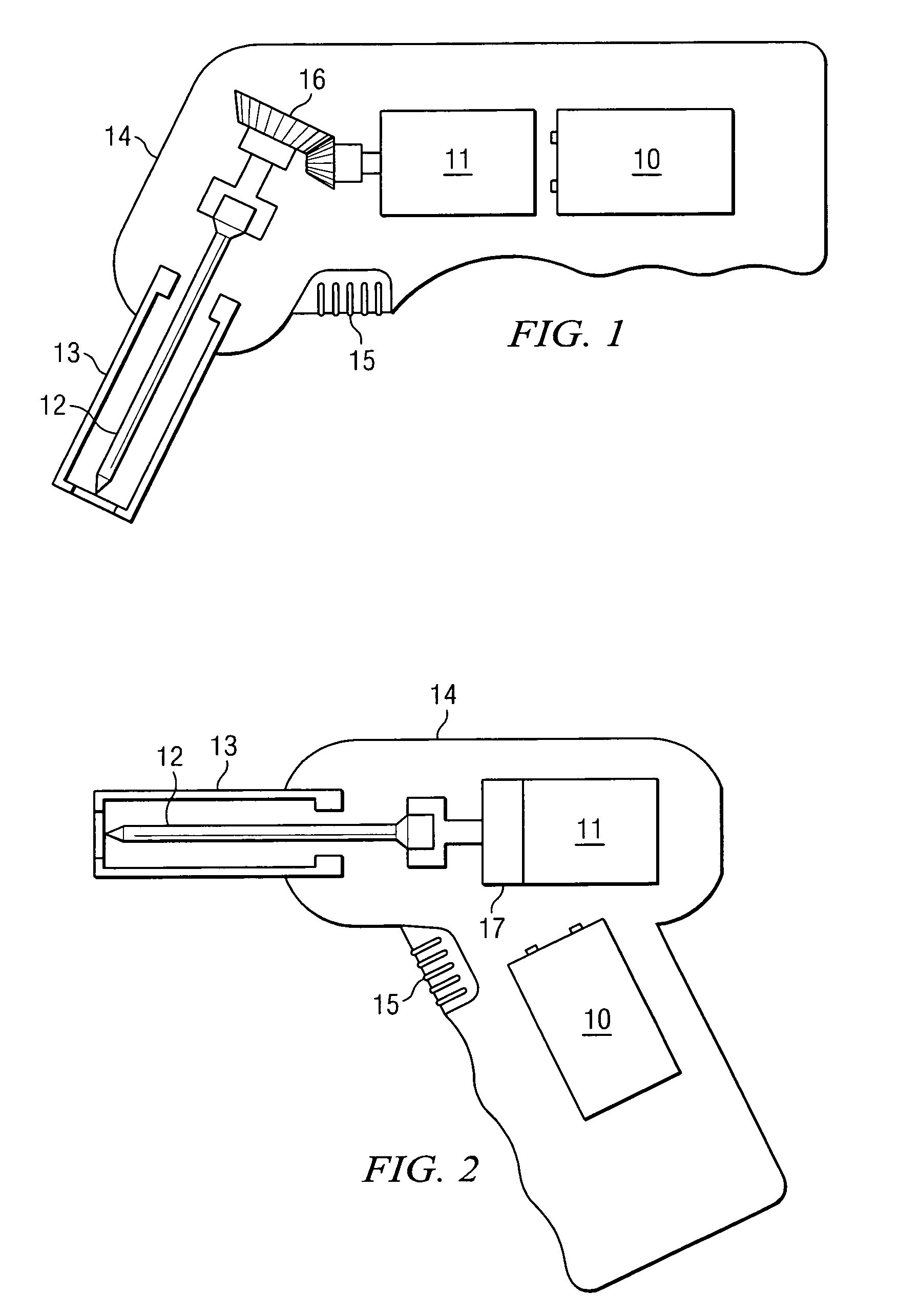
Manual interosseous device
An apparatus for penetrating the bone marrow of a bone is provided. The apparatus includes a handle having a drive shaft, a connector having a first end operable to connect to a drive shaft and a second end operable to connect to a penetrator hub and a penetrator hub having a penetrator that accesses the bone marrow.
Owner:TELEFLEX LIFE SCI LTD
Embryonic-like stem cells derived from post-partum mammalian placenta, and uses and methods of treatment using said cells
Owner:CELULARITY INC
Therapy via targeted delivery of nanoscale particles
InactiveUS20050090732A1Destroying inhibiting vascularityAntibacterial agentsNervous disorderDiseaseProstate cancer
Disclosed are compositions, systems and methods for treating a subject's body, body part, tissue, body fluid cells, pathogens, or other undesirable matter involving the administration of a targeted thermotherapy that comprises a bioprobe (energy susceptive materials that are attached to a target-specific ligand). Such targeted therapy methods can be combined with at least one other therapy technique. Other therapies include hyperthermia, direct antibody therapy, radiation, chemo- or pharmaceutical therapy, photodynamic therapy, surgical or interventional therapy, bone marrow or stem cell transplantation, and medical imaging, such as MRI, PET, SPECT, and bioimpedance. The disclosed therapies may be useful in the treatment of a variety of indications, including but not limited to, cancer of any type, such as bone marrow, lung, vascular, neuro, colon, ovarian, breast and prostate cancer, epitheleoid sarcomas, AIDS, adverse angiogenesis, restenosis, amyloidosis, tuberculosis, cardiovascular plaque, vascular plaque, obesity, malaria, and illnesses due to viruses, such as HIV.
Owner:NANOTX INC
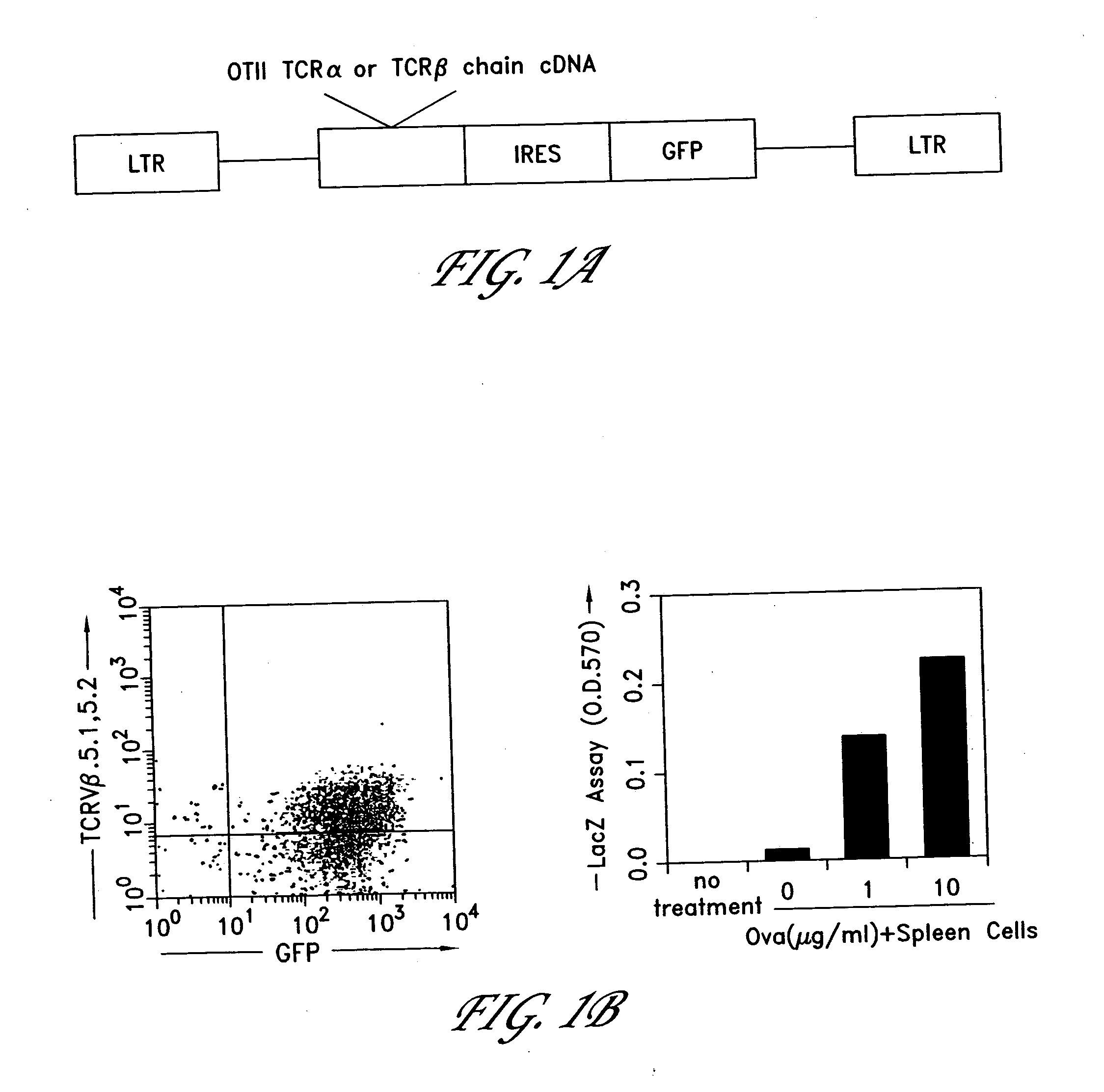
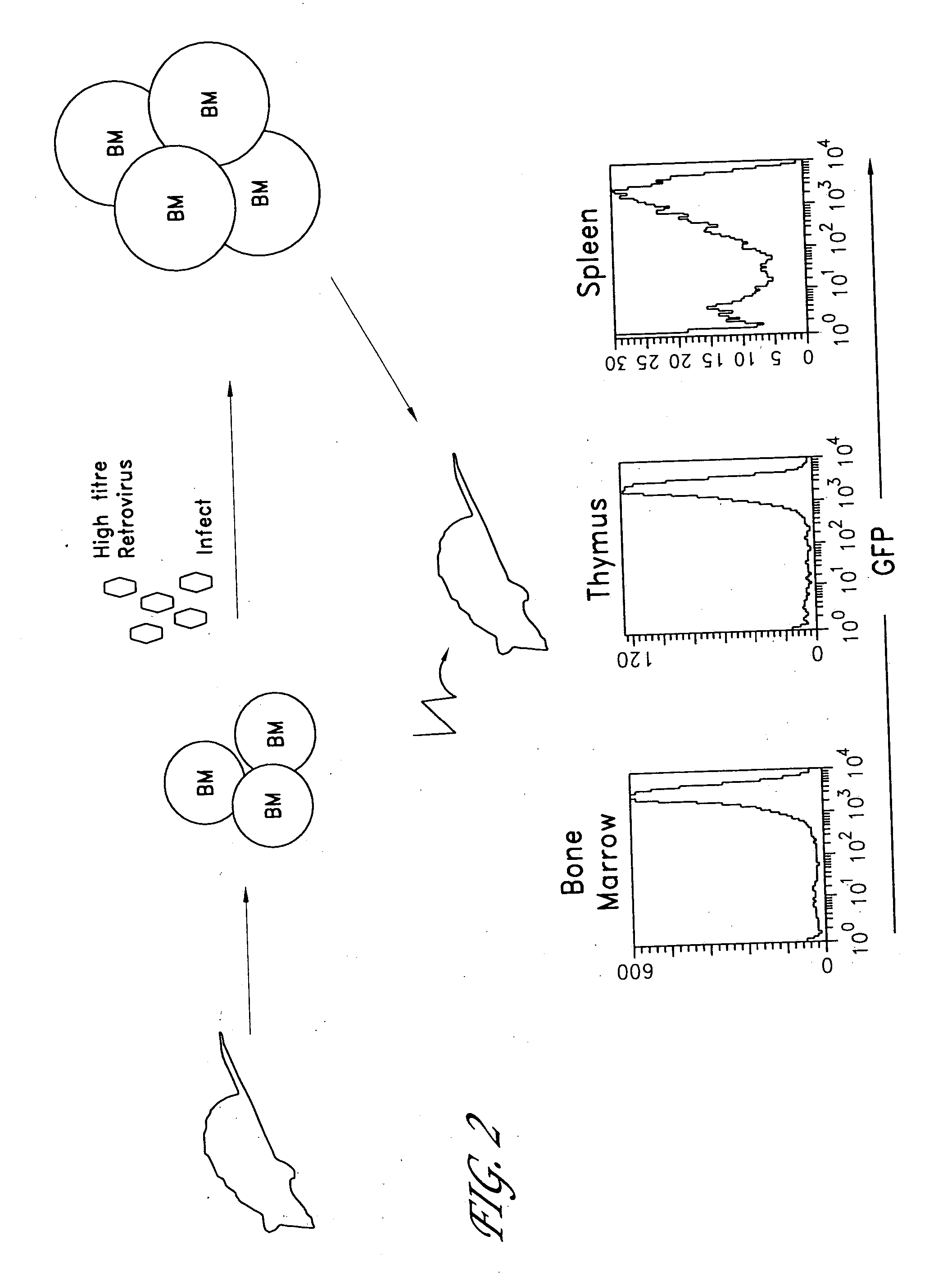
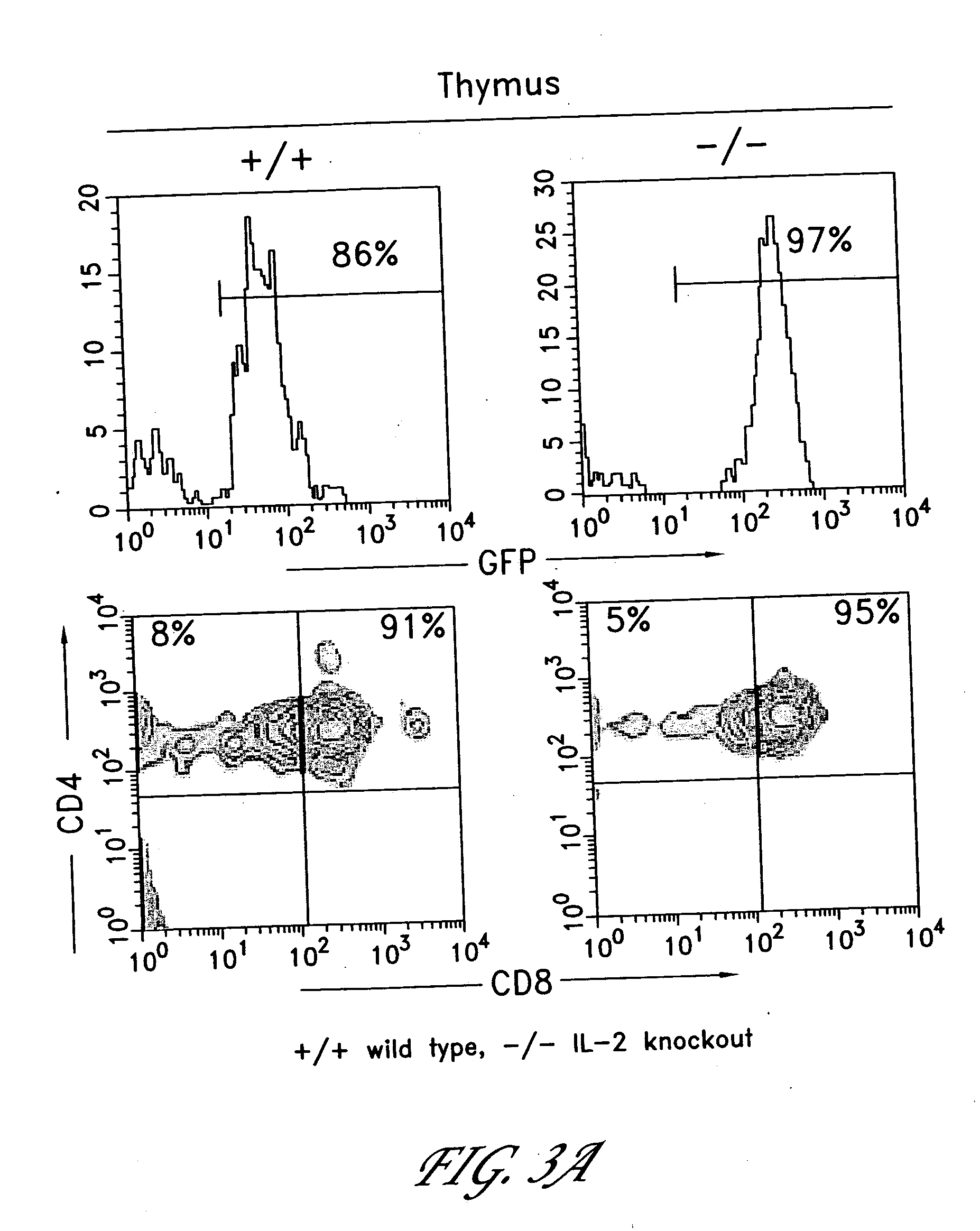
Method for the generation of antigen-specific lymphocytes
InactiveUS20070116690A1Function increaseEnhancing function of T cellBiocideVirusesAutoimmune conditionAutoimmune disease
The invention provides systems and methods for the generation of lymphocytes having a unique antigen specificity. In a preferred embodiment, the invention provides methods of virally infecting cells from bone marrow with one or more viral vectors that encode antigen-specific antibodies for the production of, for example B cells and T cells. In some embodiments, the viral vectors include an IRES or 2A element to promote separation of, for example, the α subunit and β subunit of a T cell receptor (TCR) or heavy and light chains of a B-cell antibody. The resulting lymphocytes, express the particular antibody that was introduced in the case of B cells and TCR in the case of T cells. The lymphocytes generated can be used for a variety of therapeutic purposes including the treatment of various cancers and the generation of a desired immune response to viruses and other pathogens. The resulting cells develop normally and respond to antigen both in vitro and in vivo. We also show that it is possible to modify the function of lymphocytes by using stem cells from different genetic backgrounds. Thus our system constitutes a powerful tool to generate desired lymphocyte populations both for research and therapy. Future applications of this technology may include treatments for infectious diseases, such as HIV / AIDS, cancer therapy, allergy, and autoimmune disease.
Owner:CALIFORNIA INST OF TECH
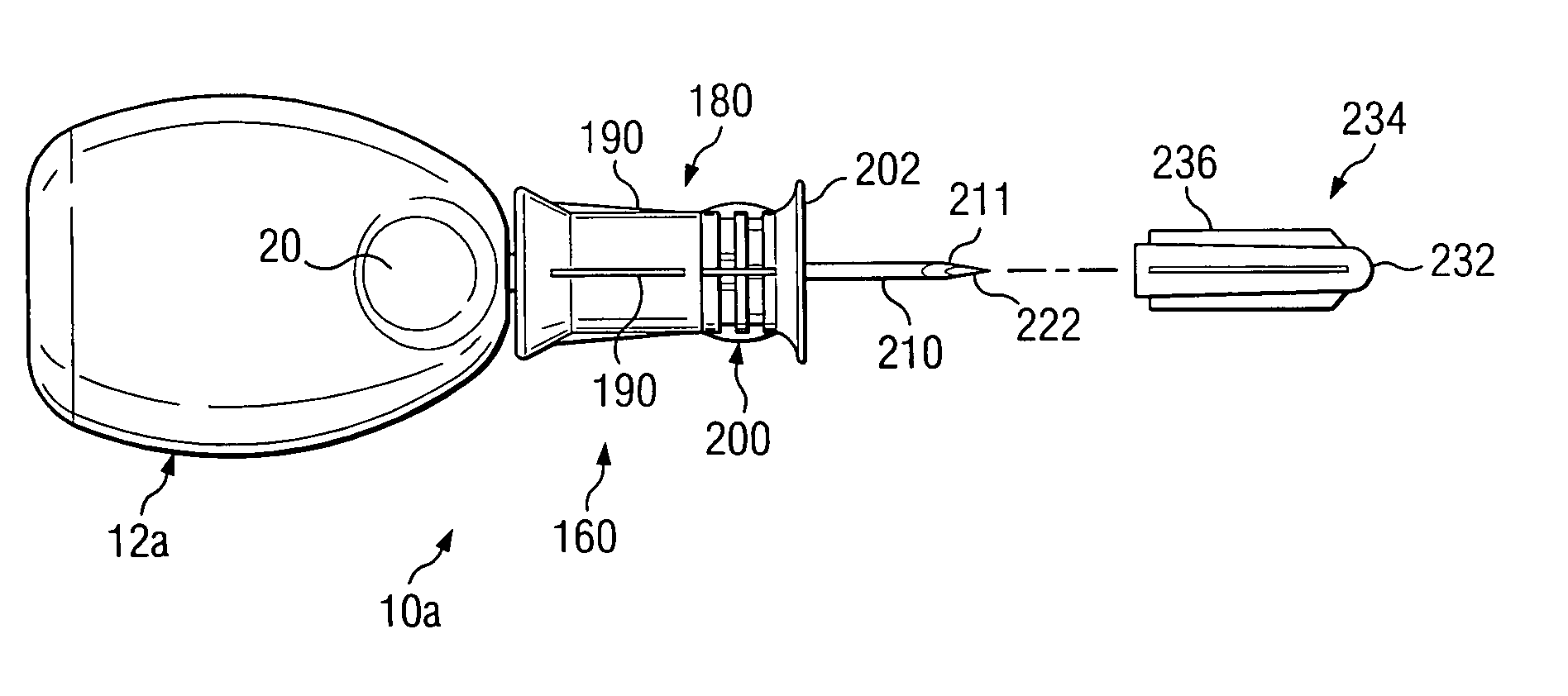
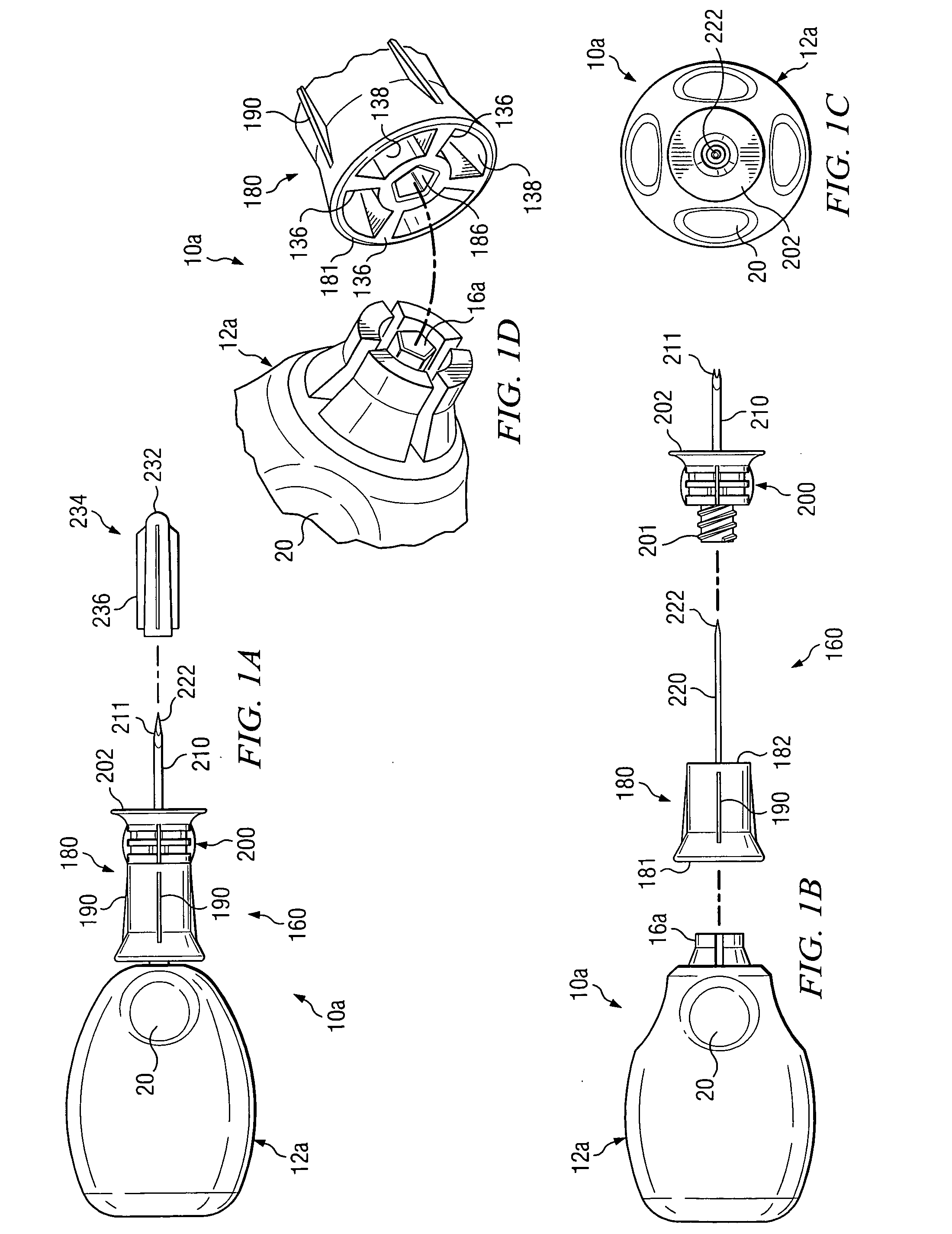
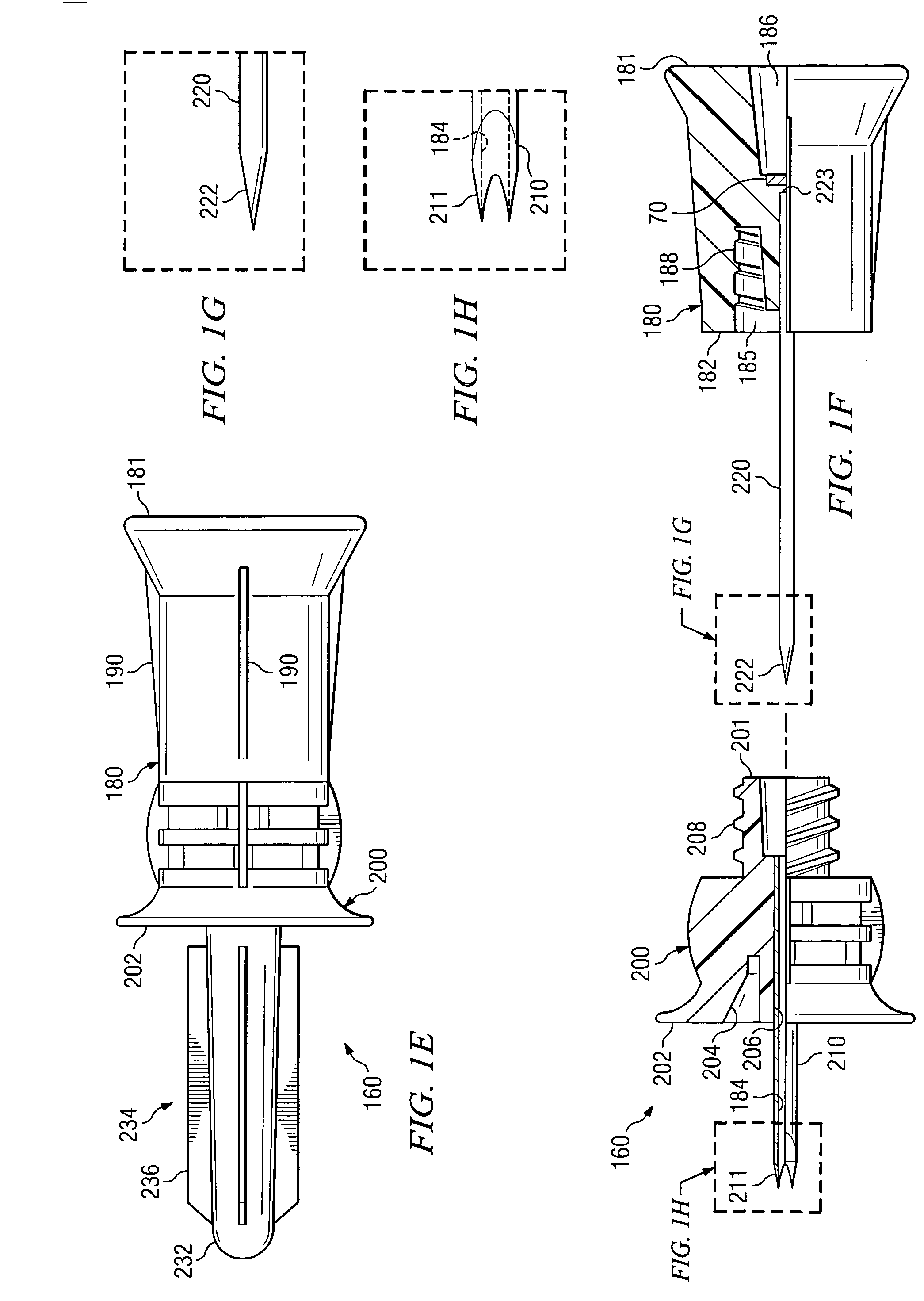
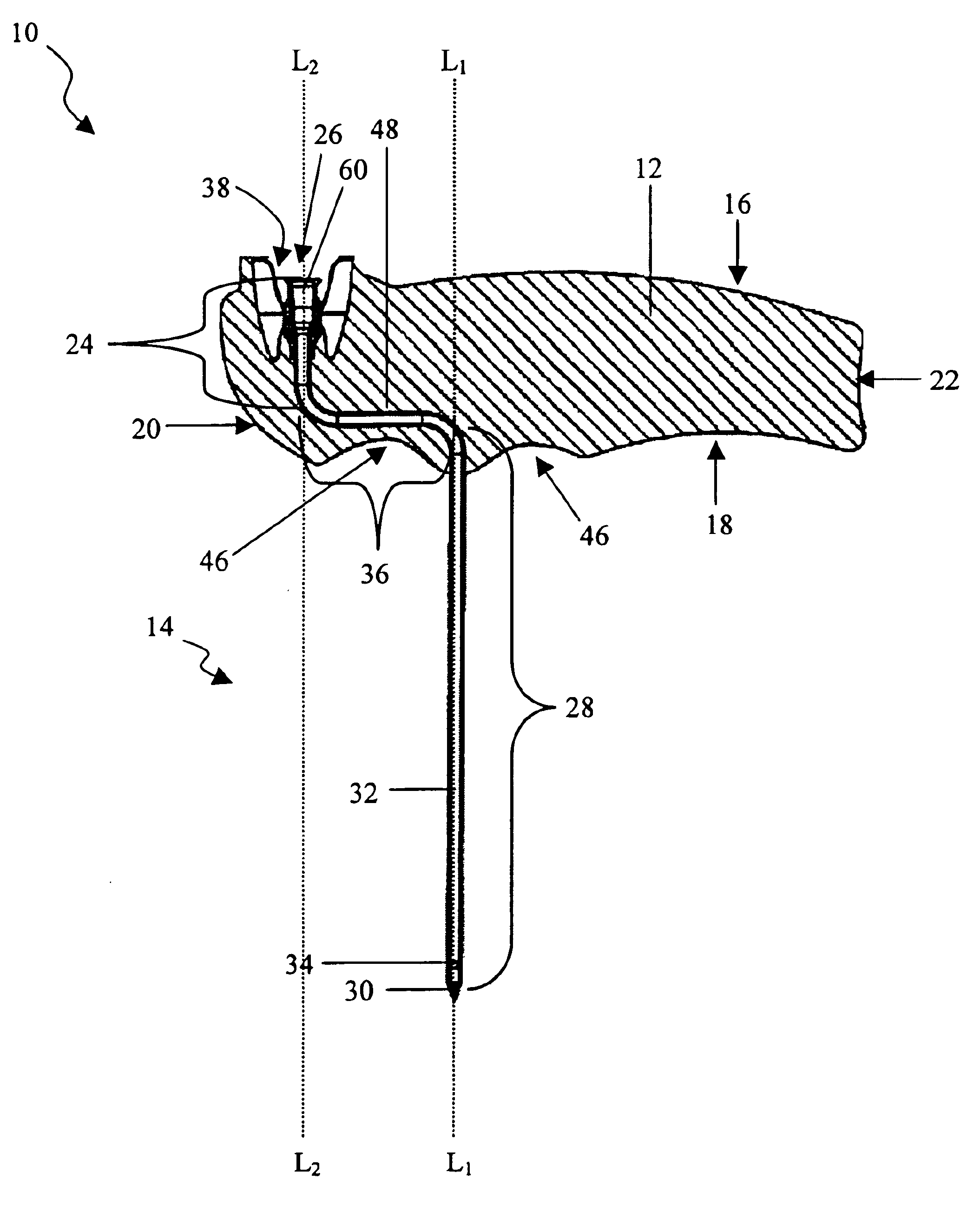
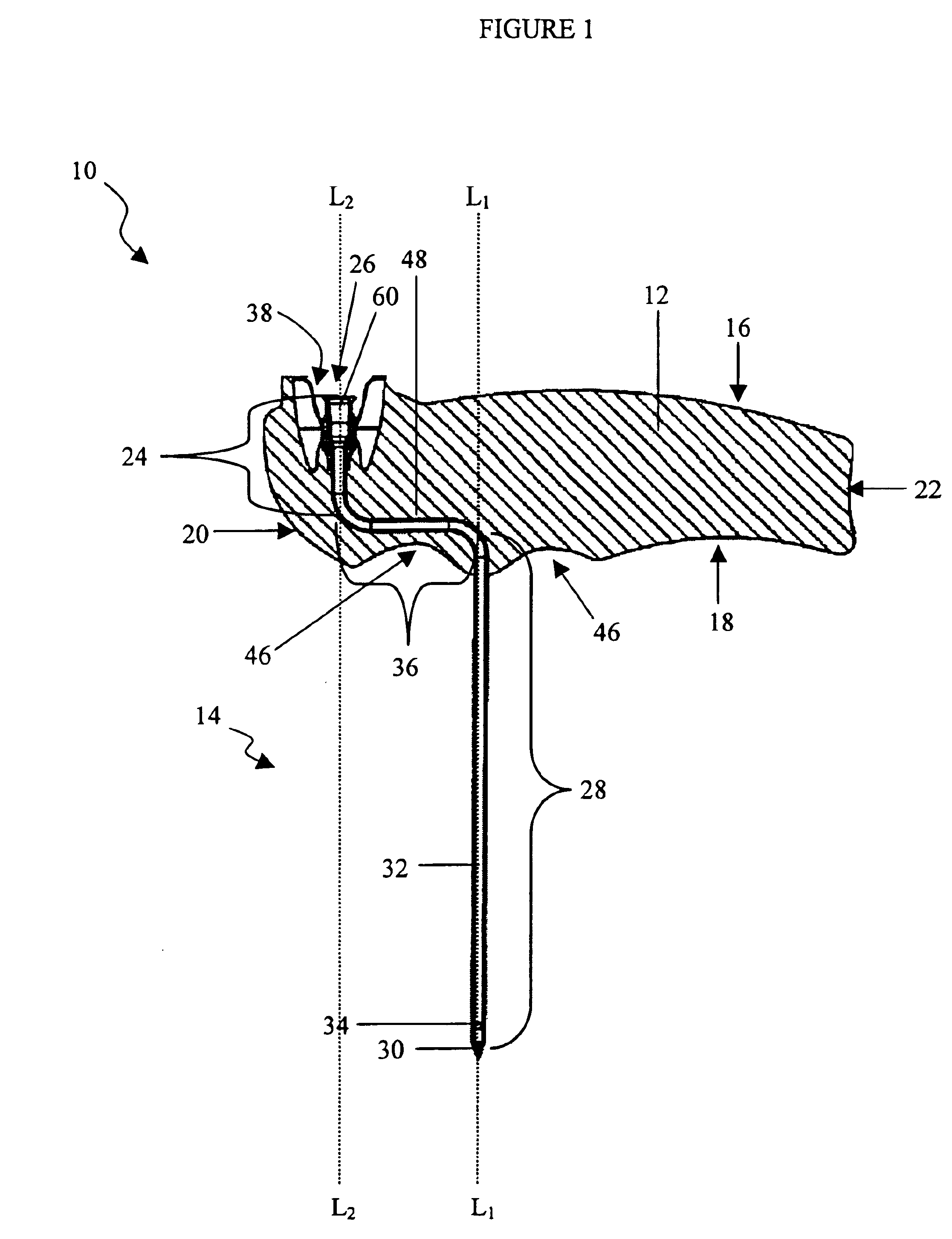
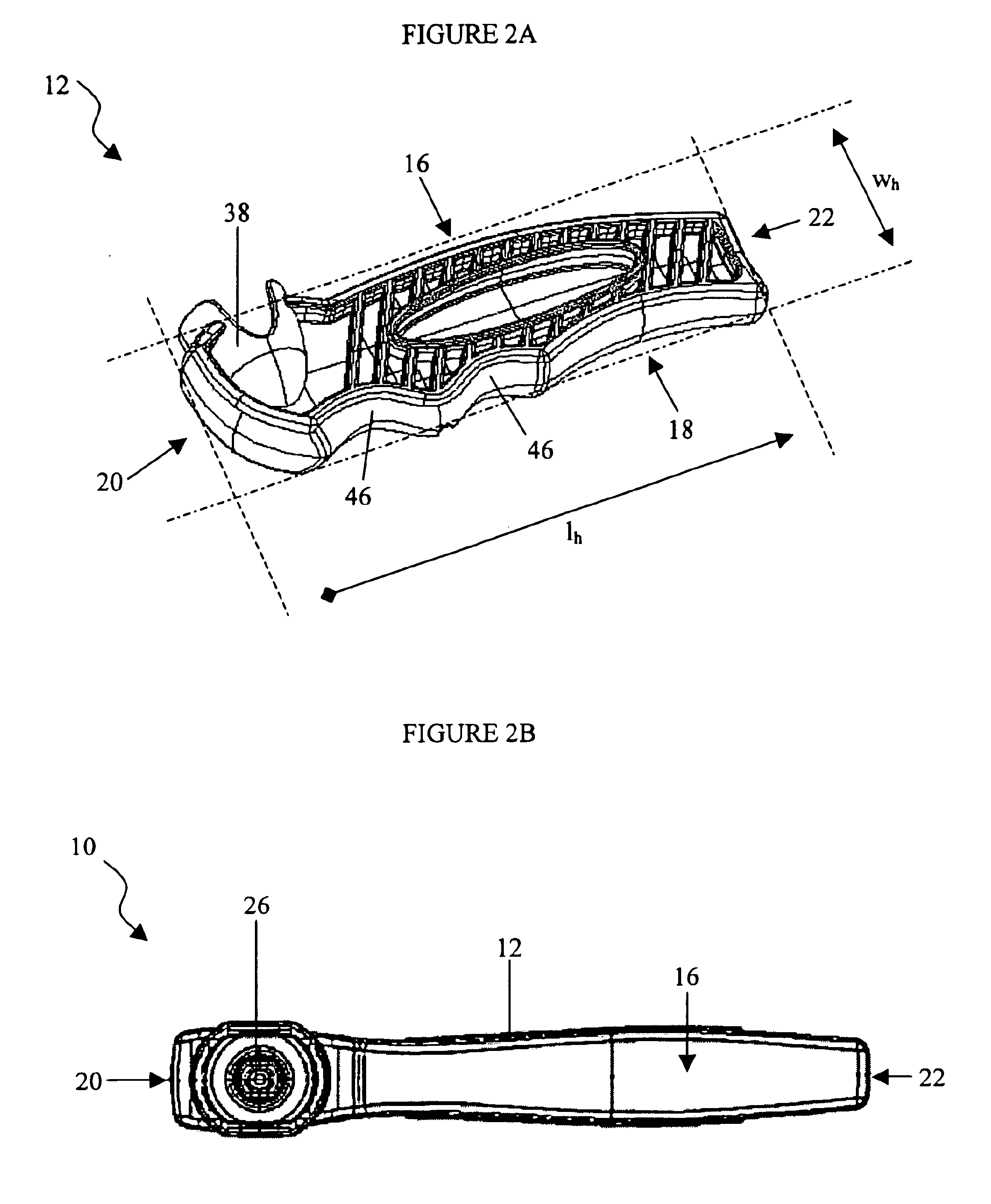
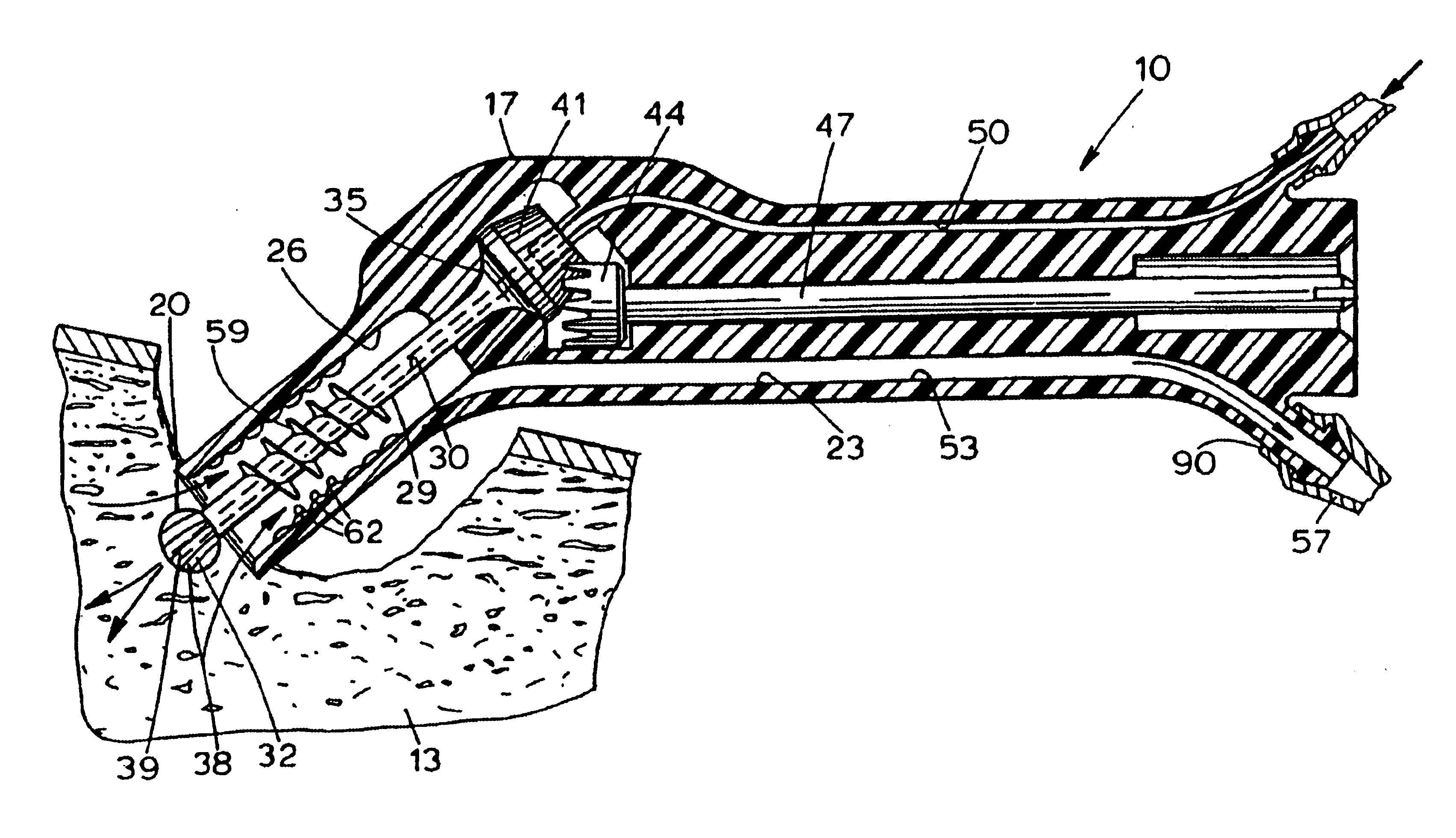
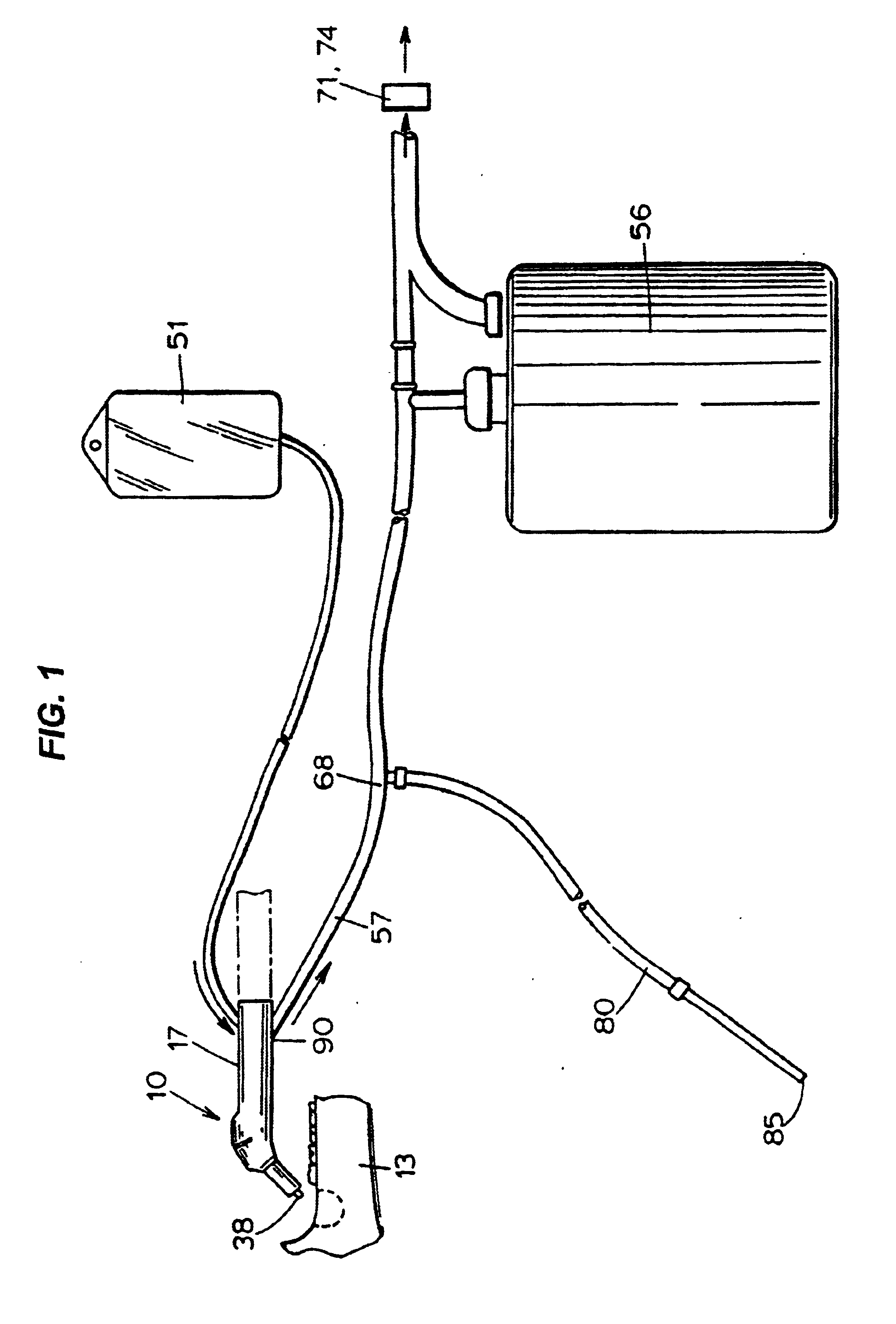
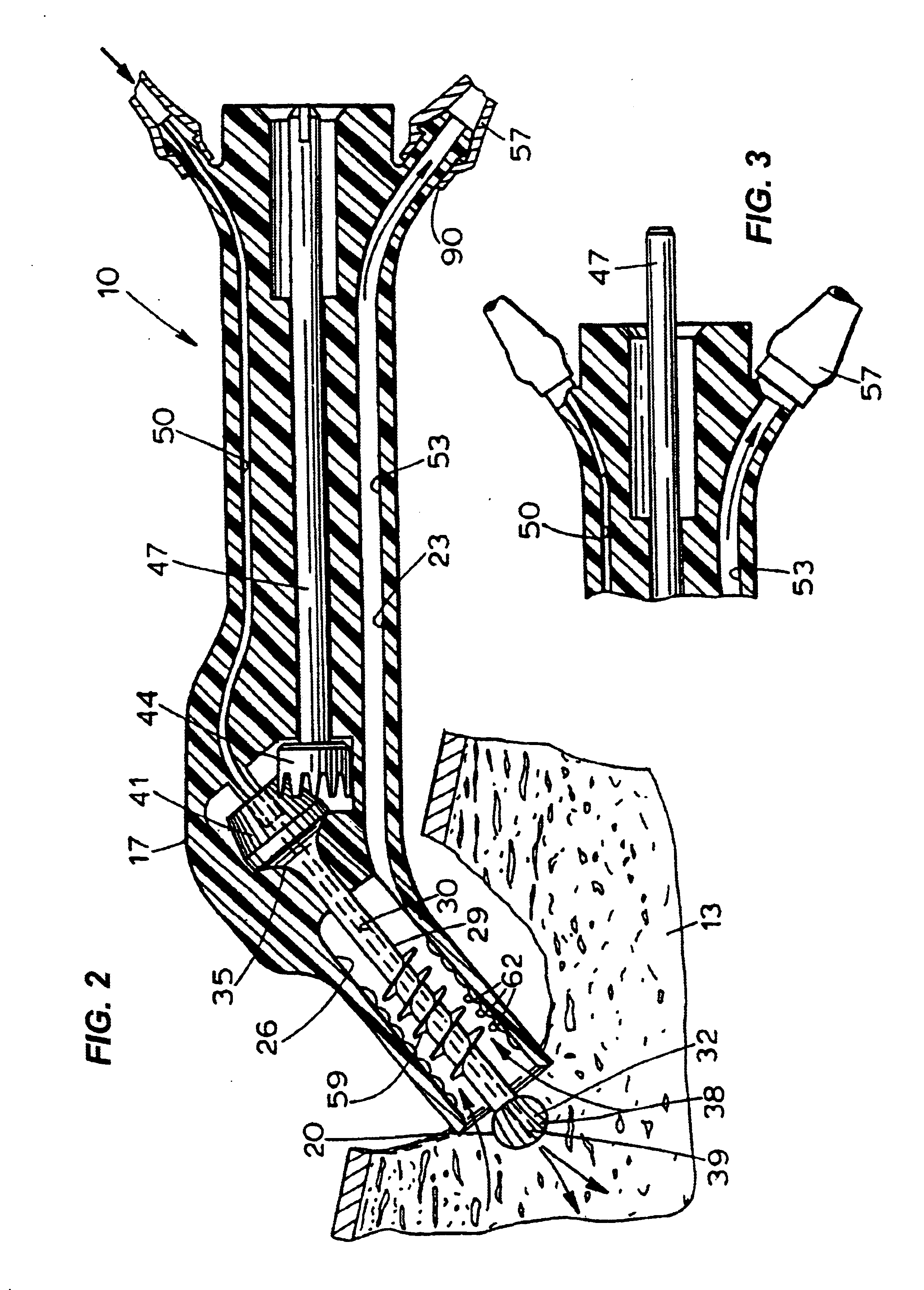
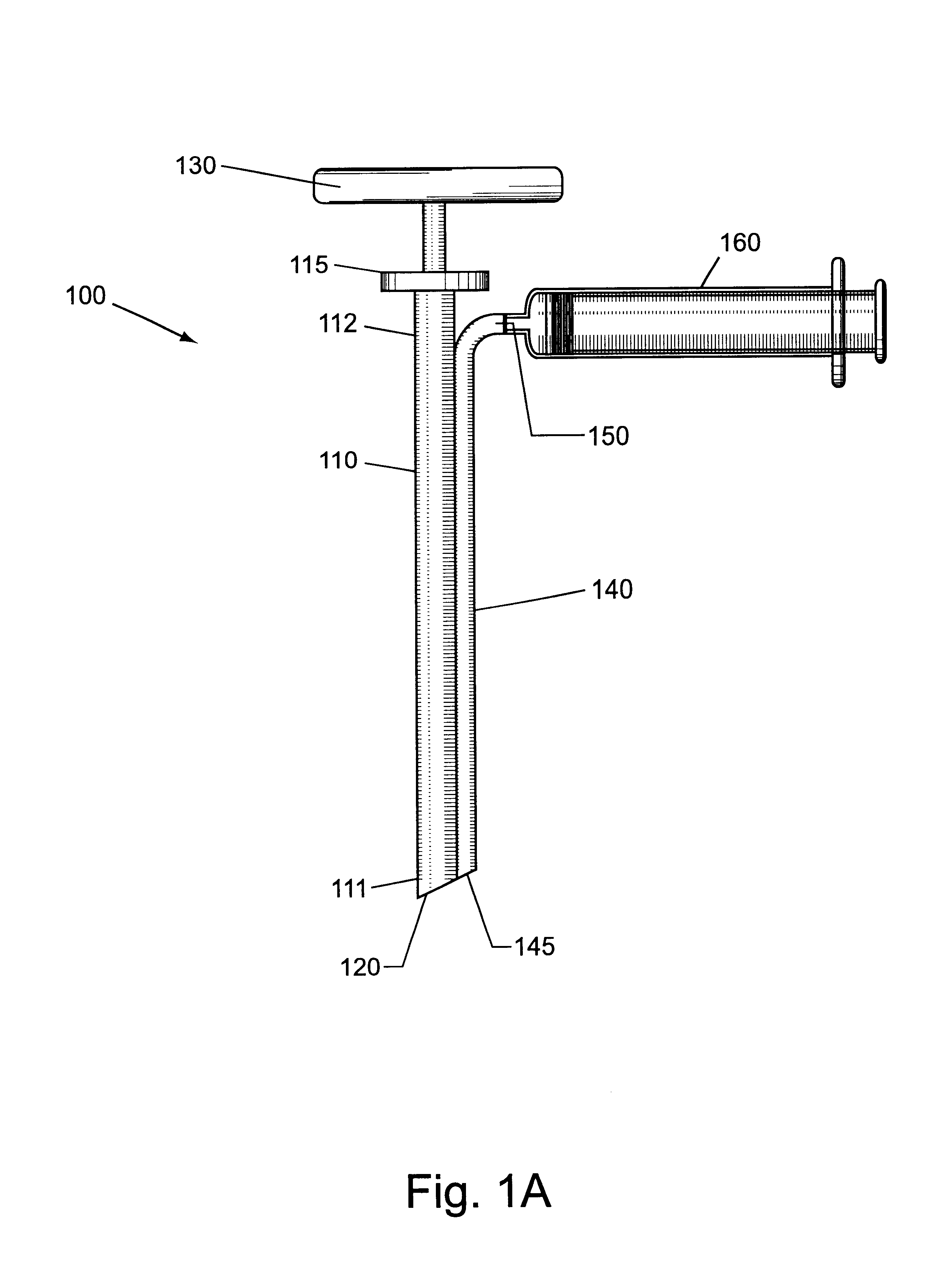
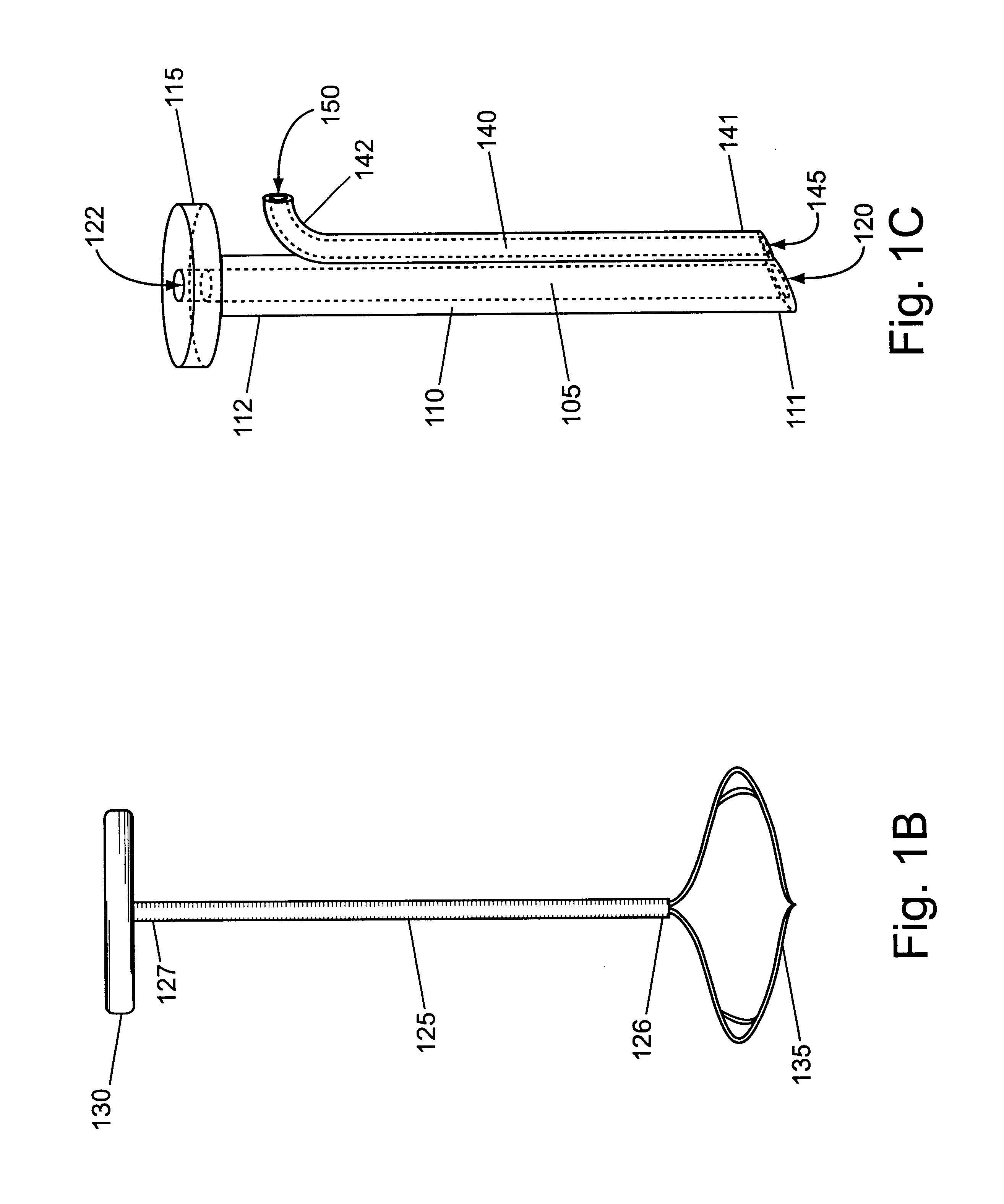
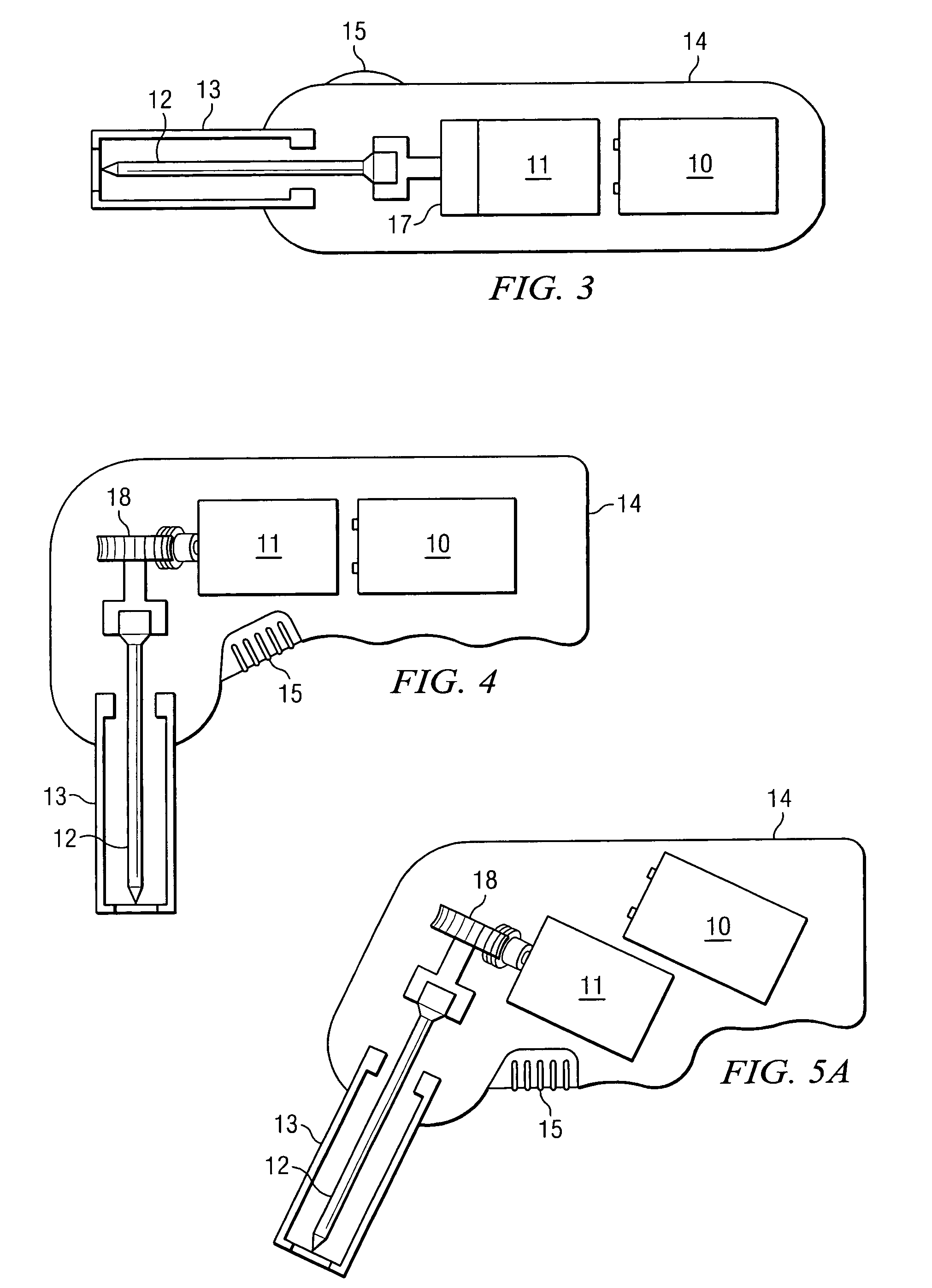
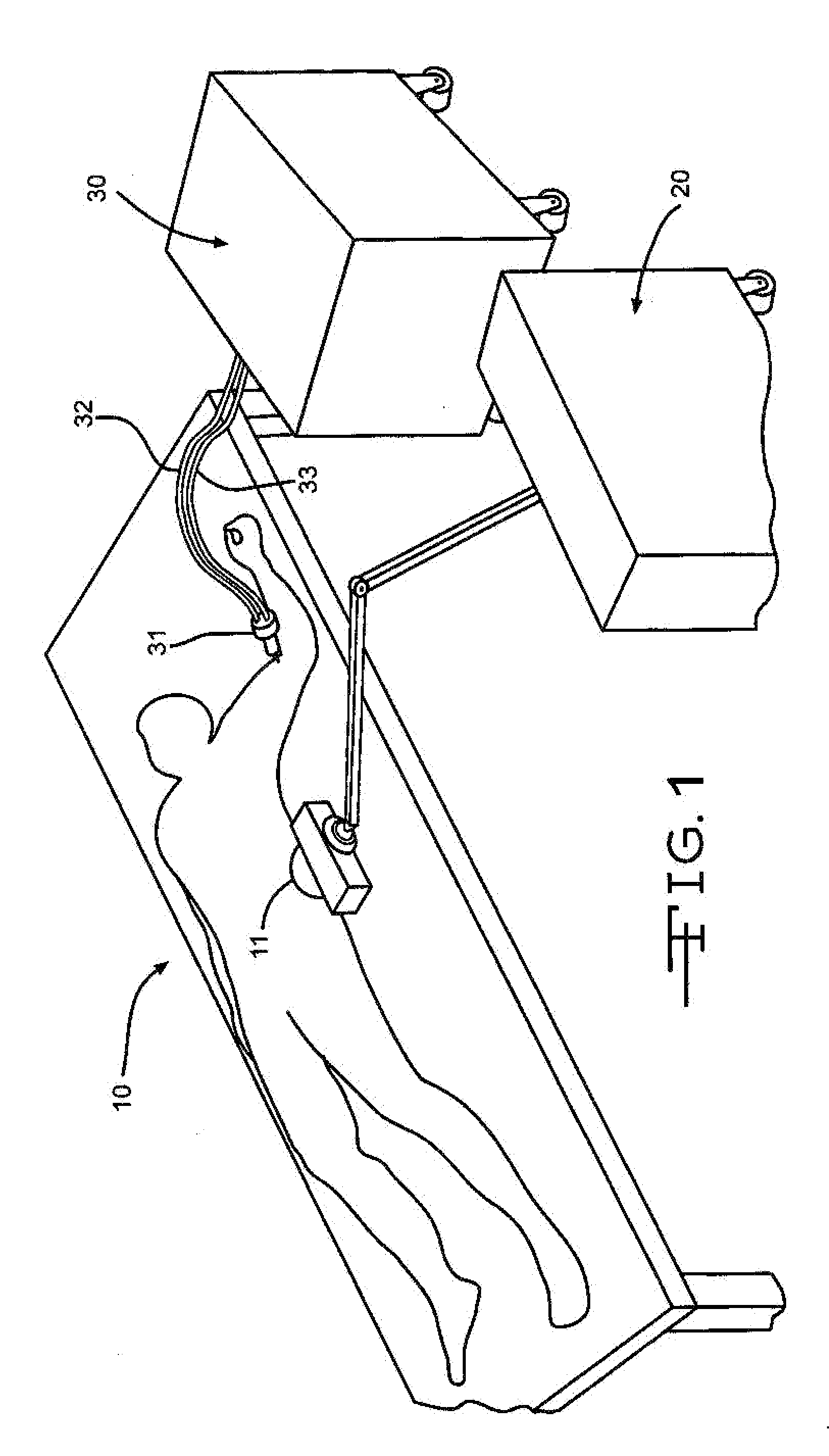
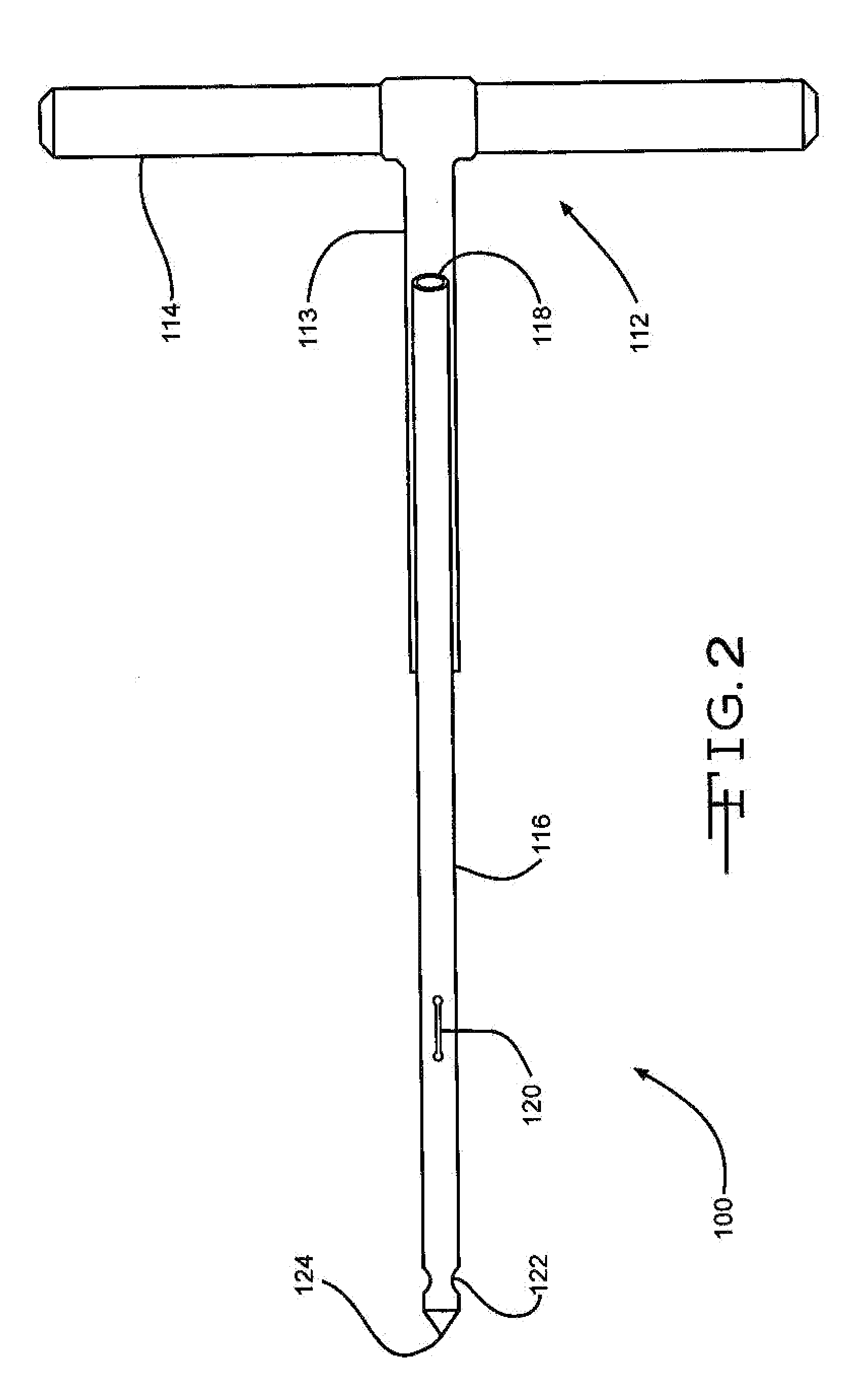
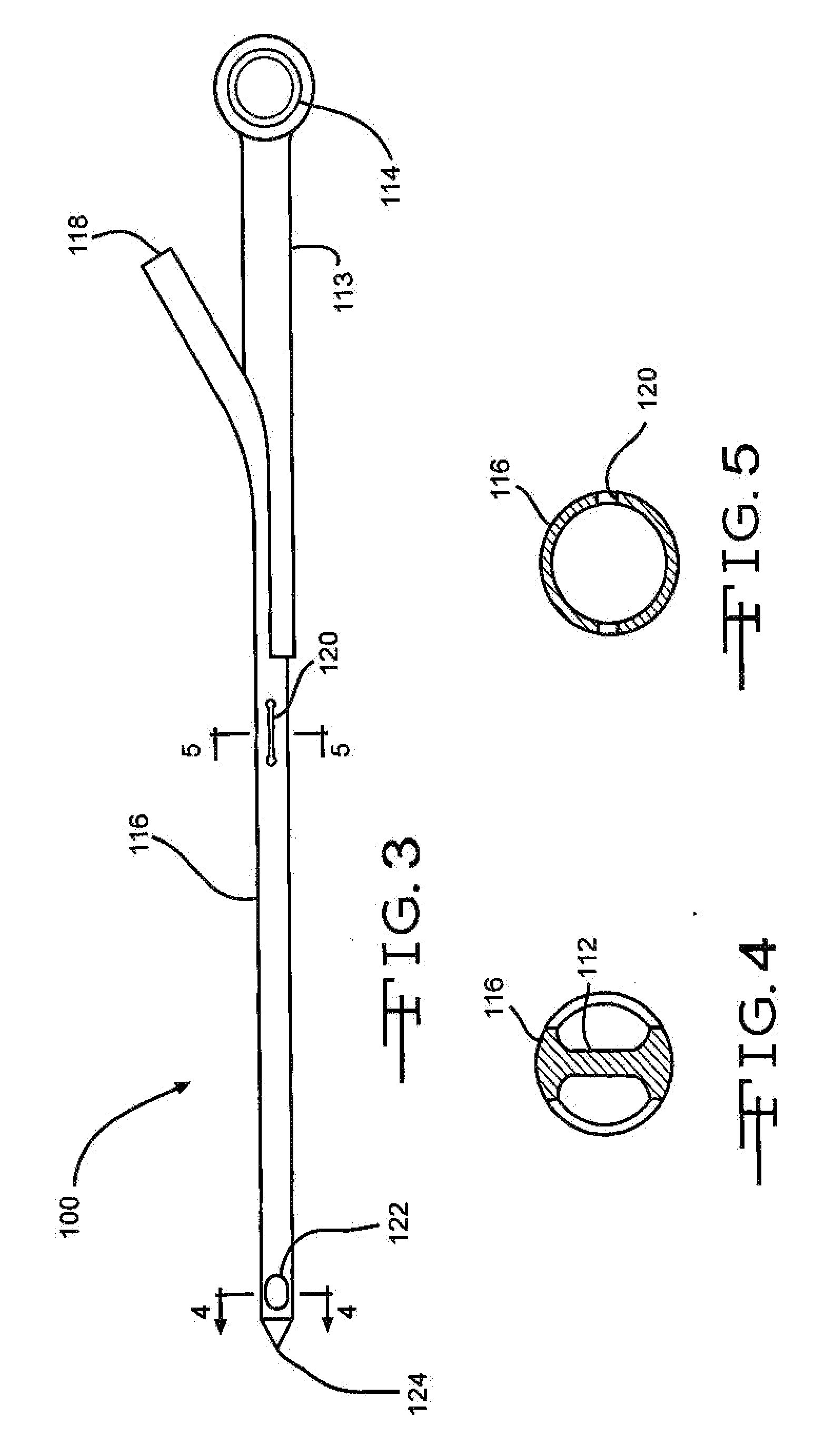
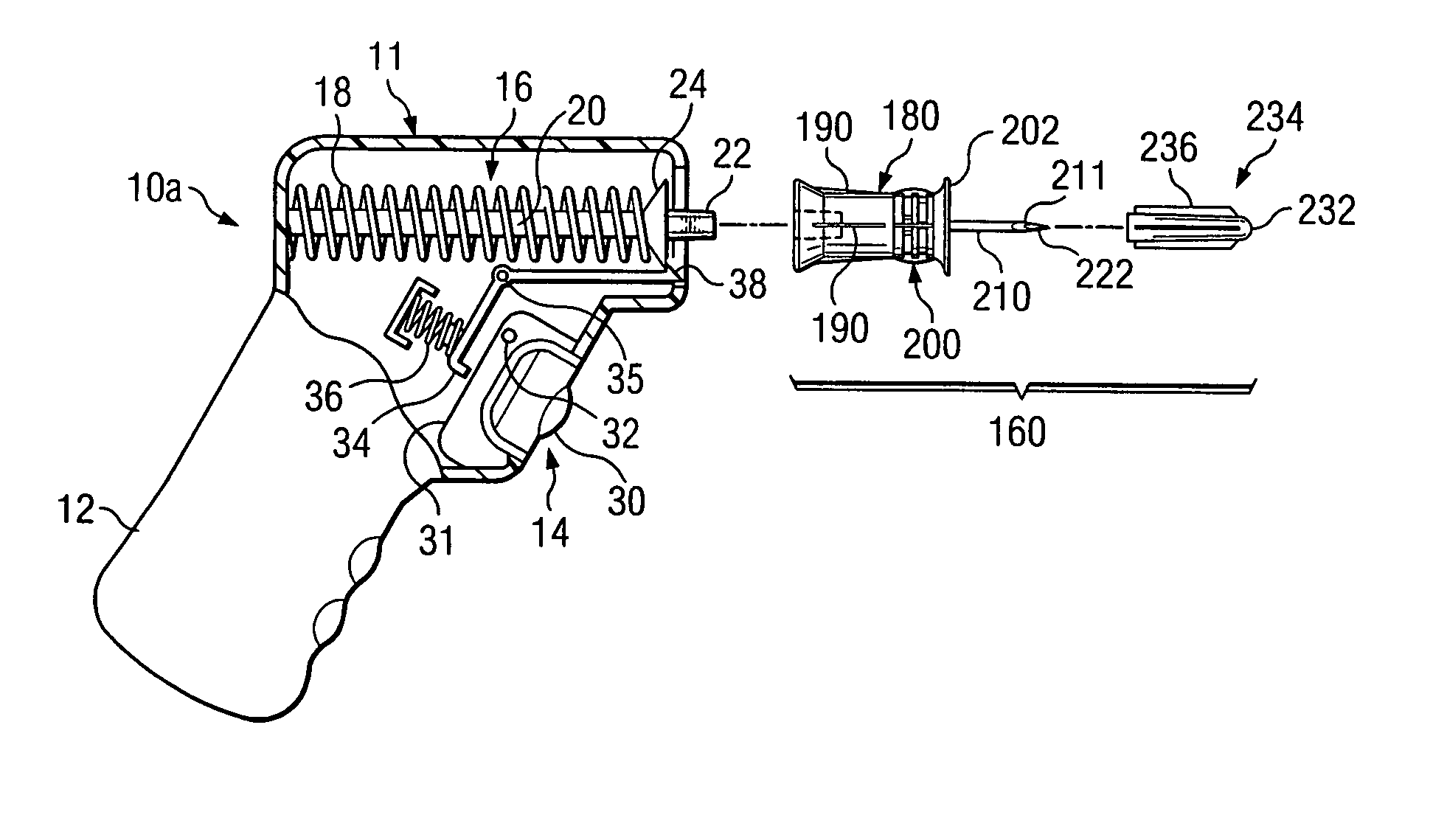
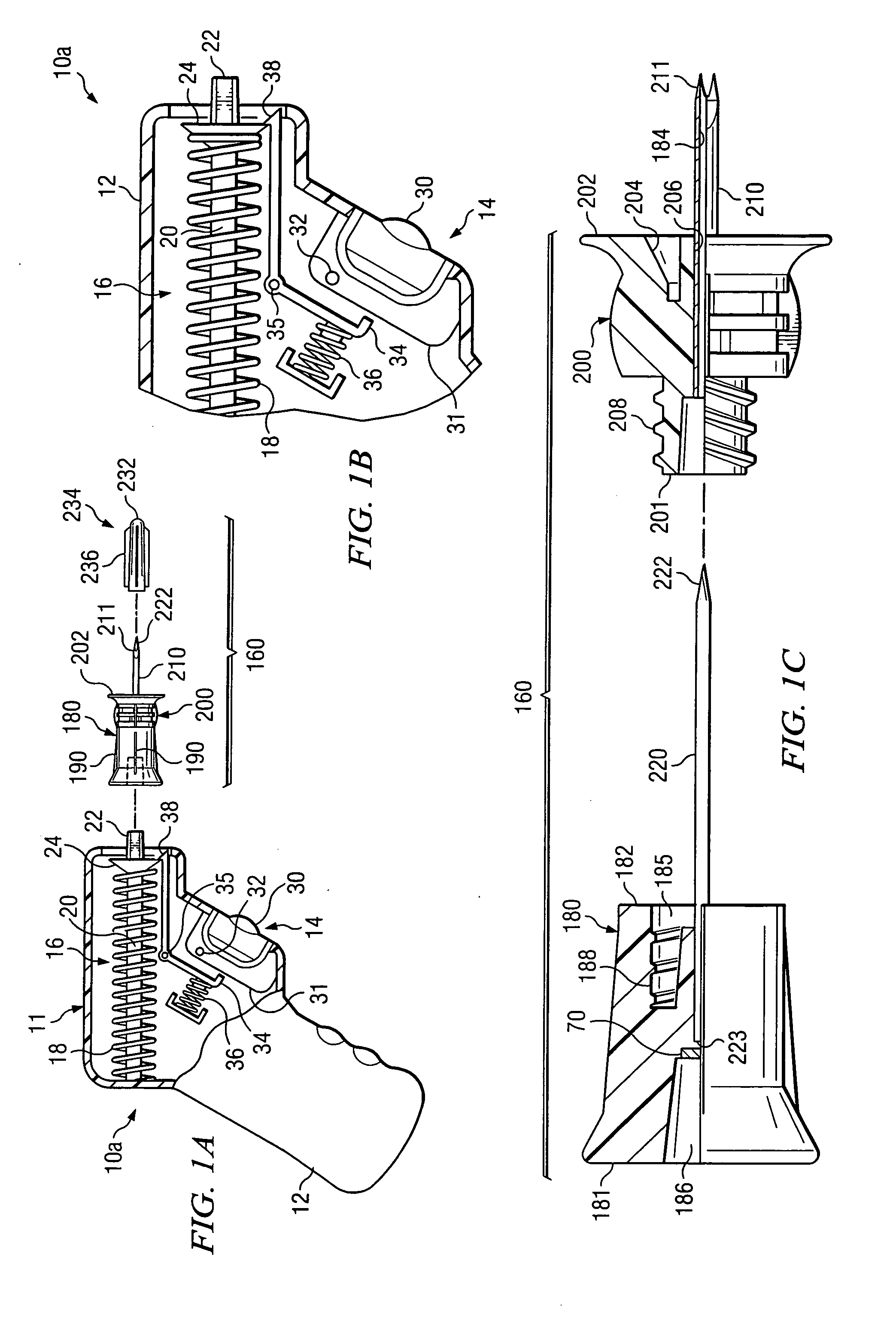
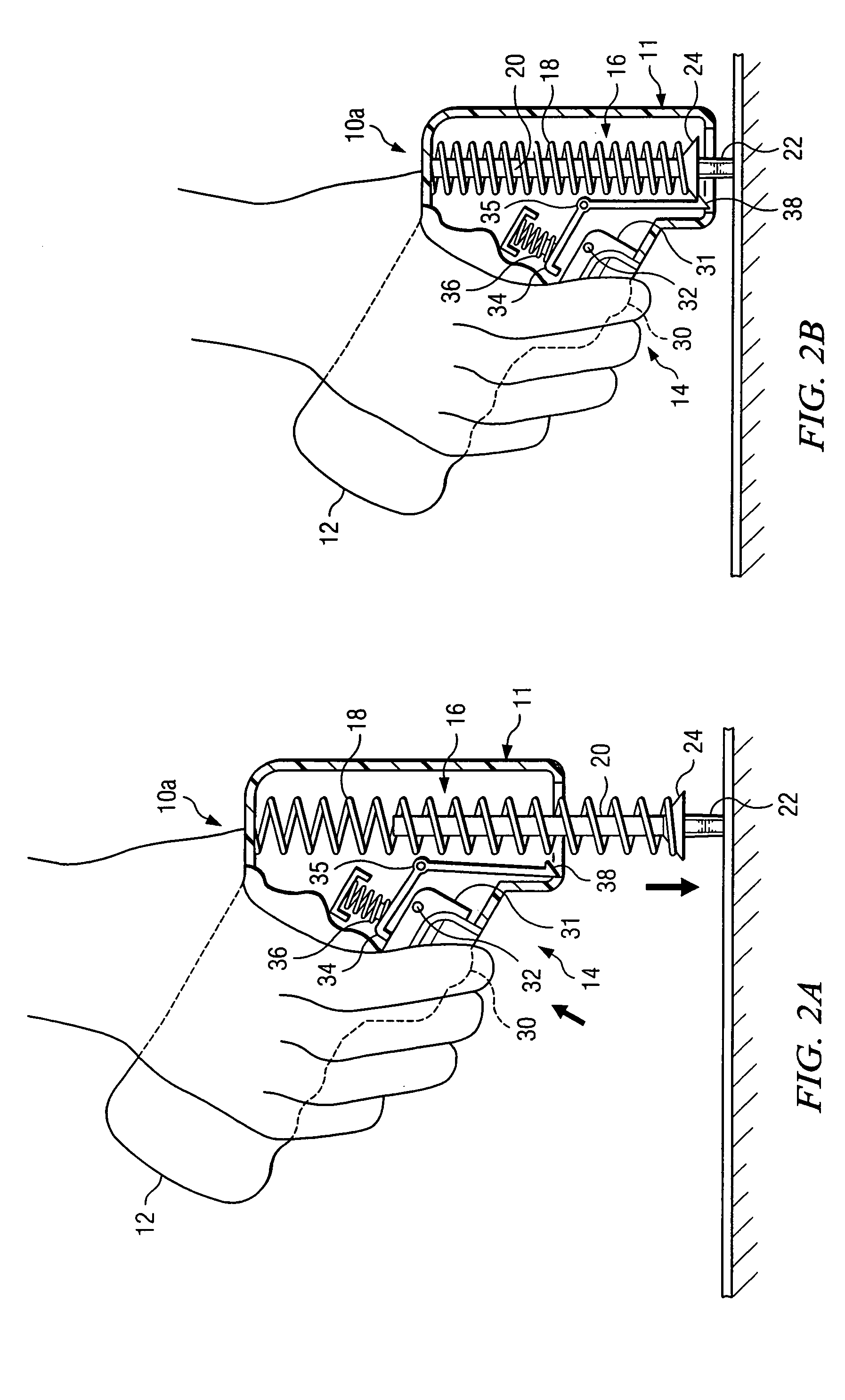
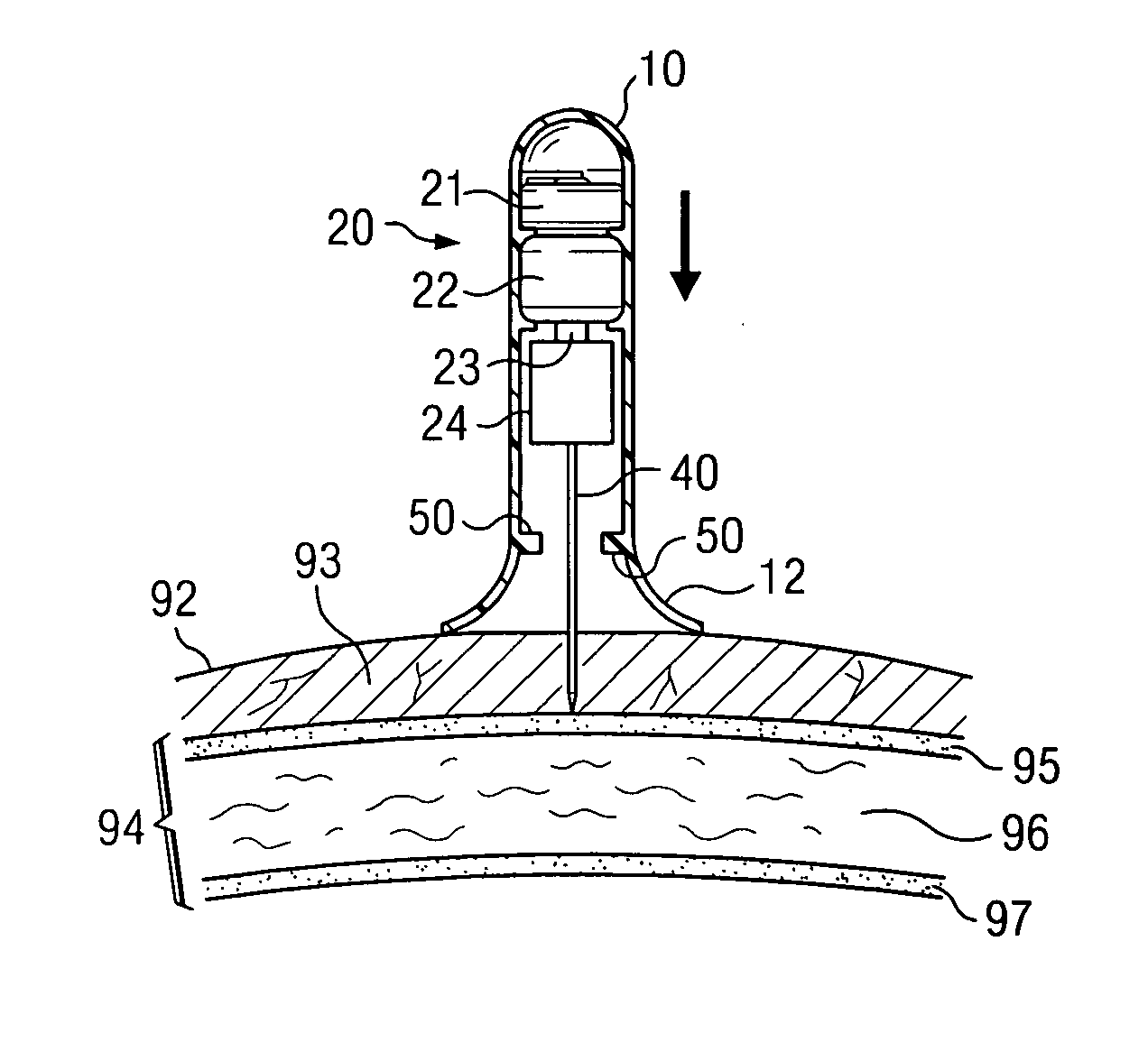
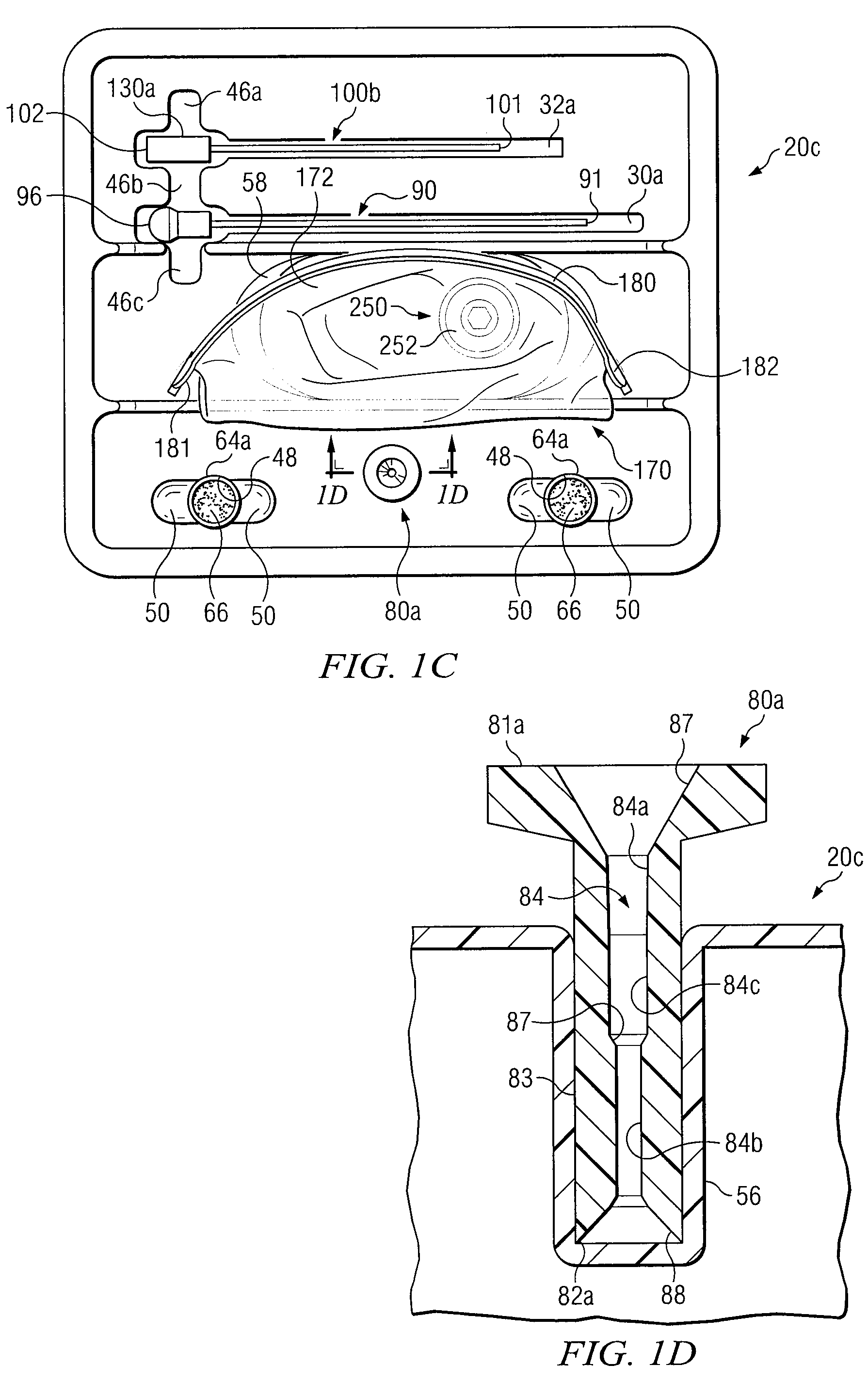
Bone marrow aspirator
InactiveUS6916292B2Facilitate single-handed useFacilitate manipulationSurgical needlesVaccination/ovulation diagnosticsEngineeringBiomedical engineering
A bone marrow aspiration device is provided having a handle adapted to facilitate grasping thereof, and an elongate penetrating element coupled to the handle and having a proximal inlet port and a distal piercing tip for penetrating into tissue and bone. The device is particularly effective in that the configuration of the handle and the elongate penetrating element allows a person to grasp and manipulate the handle while accessing the inlet port to aspirate fluid therethrough. The device is also effective to facilitate penetrating of the device into bone due to the configuration of the distal penetrating tip. A person having ordinary skill in the art will appreciate that, while the device is described for use as a bone marrow aspiration device, the device can be used to withdraw or inject fluid.
Owner:DEPUY ACROMED INC
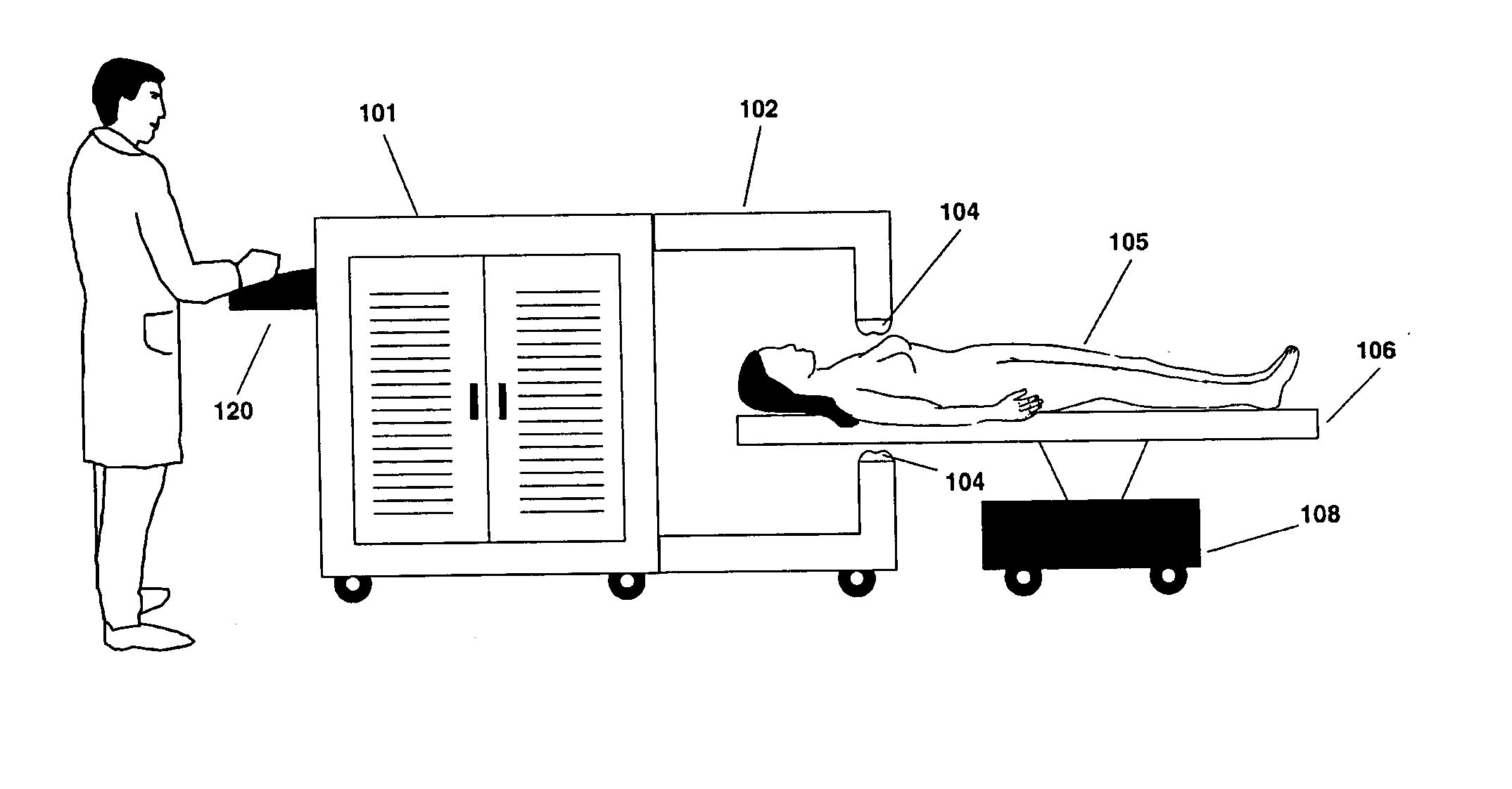
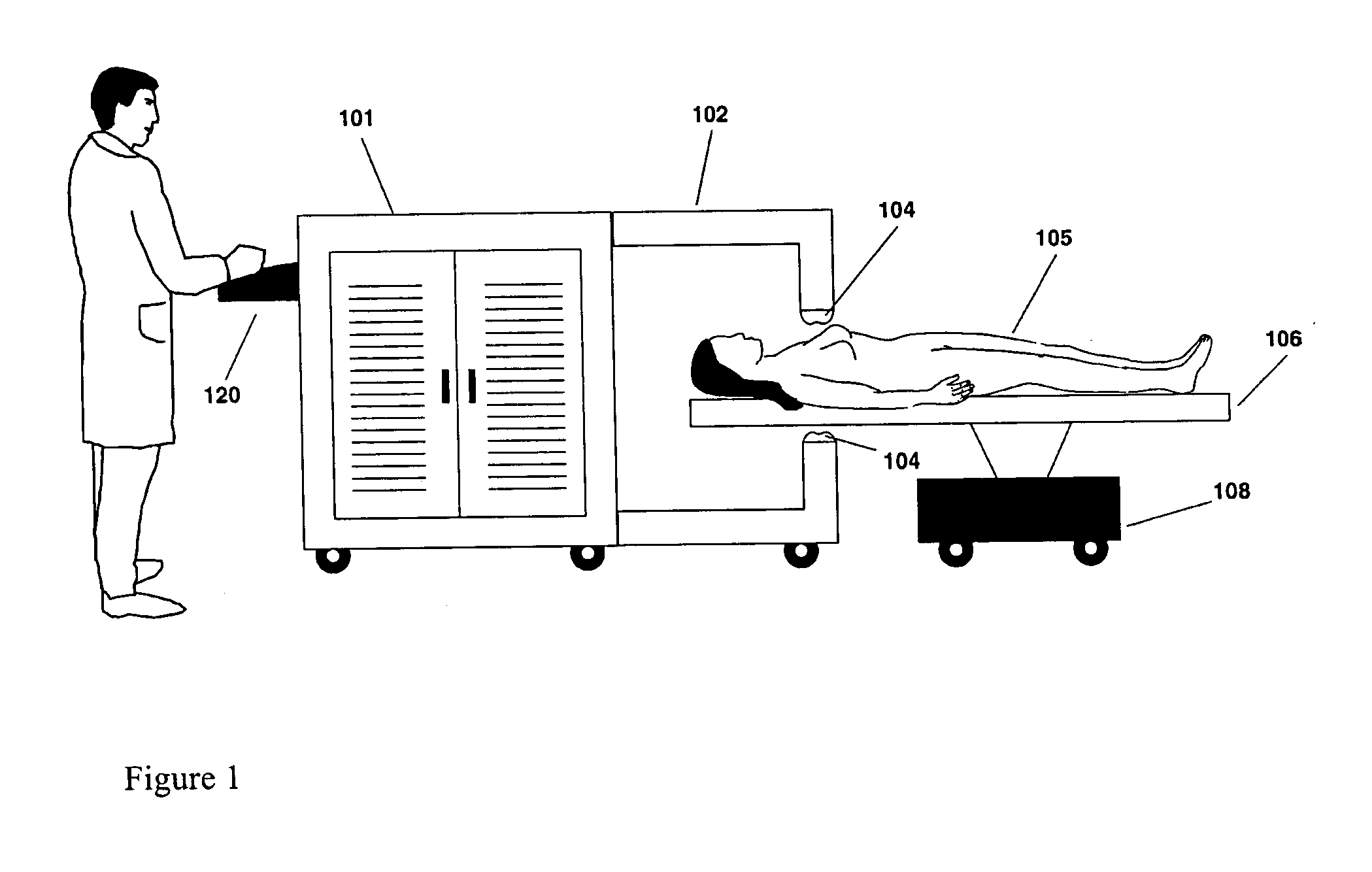
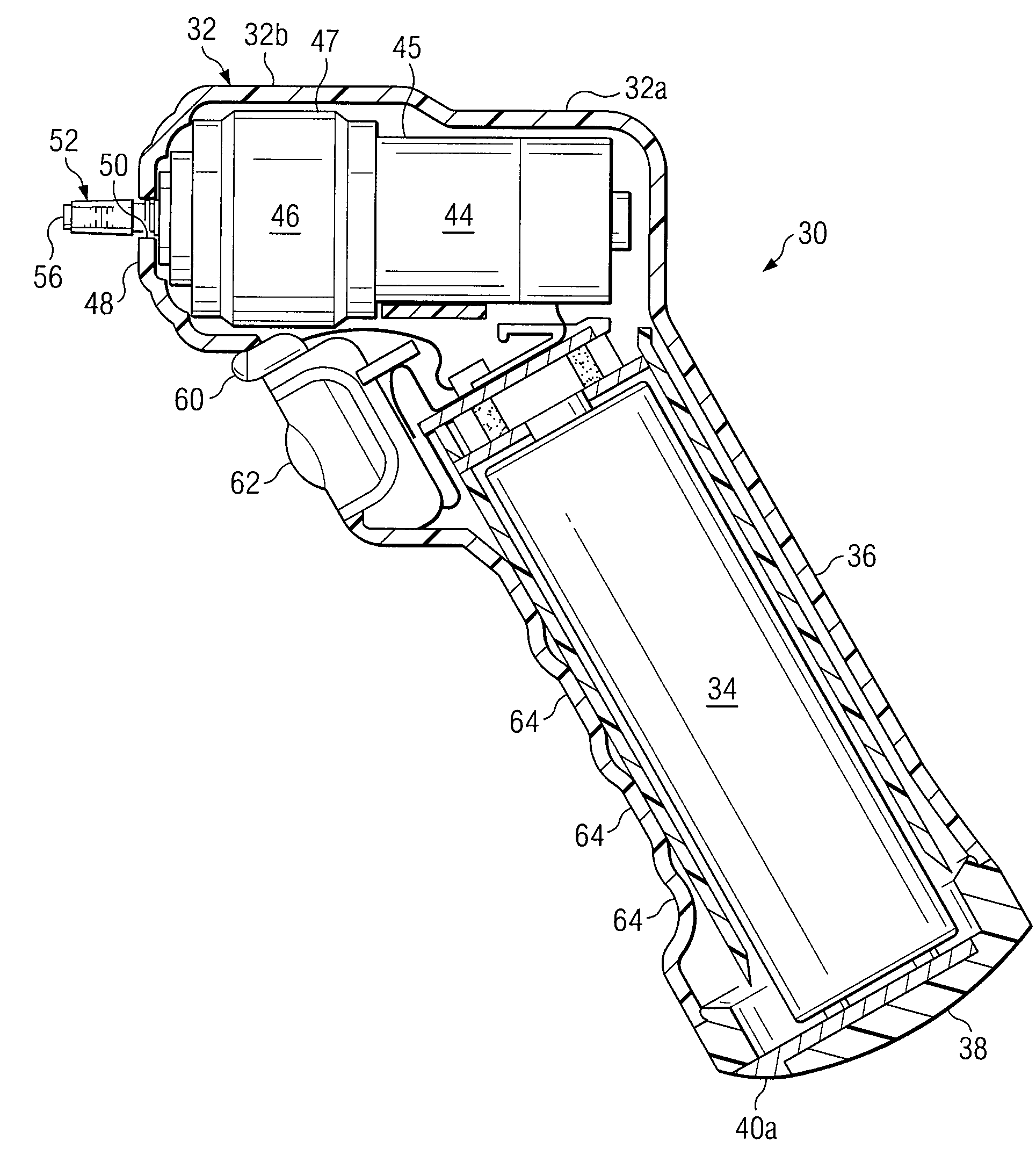
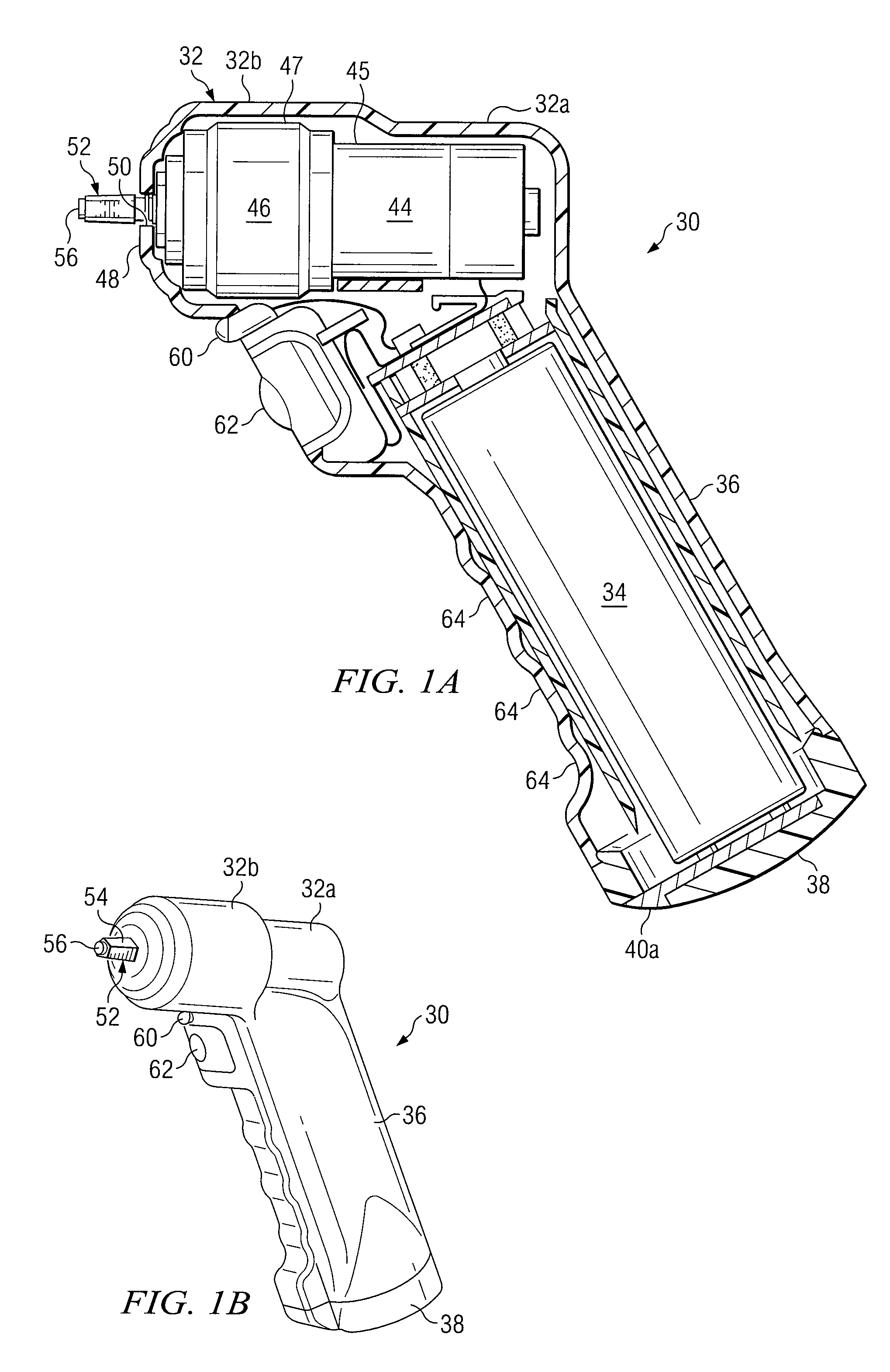
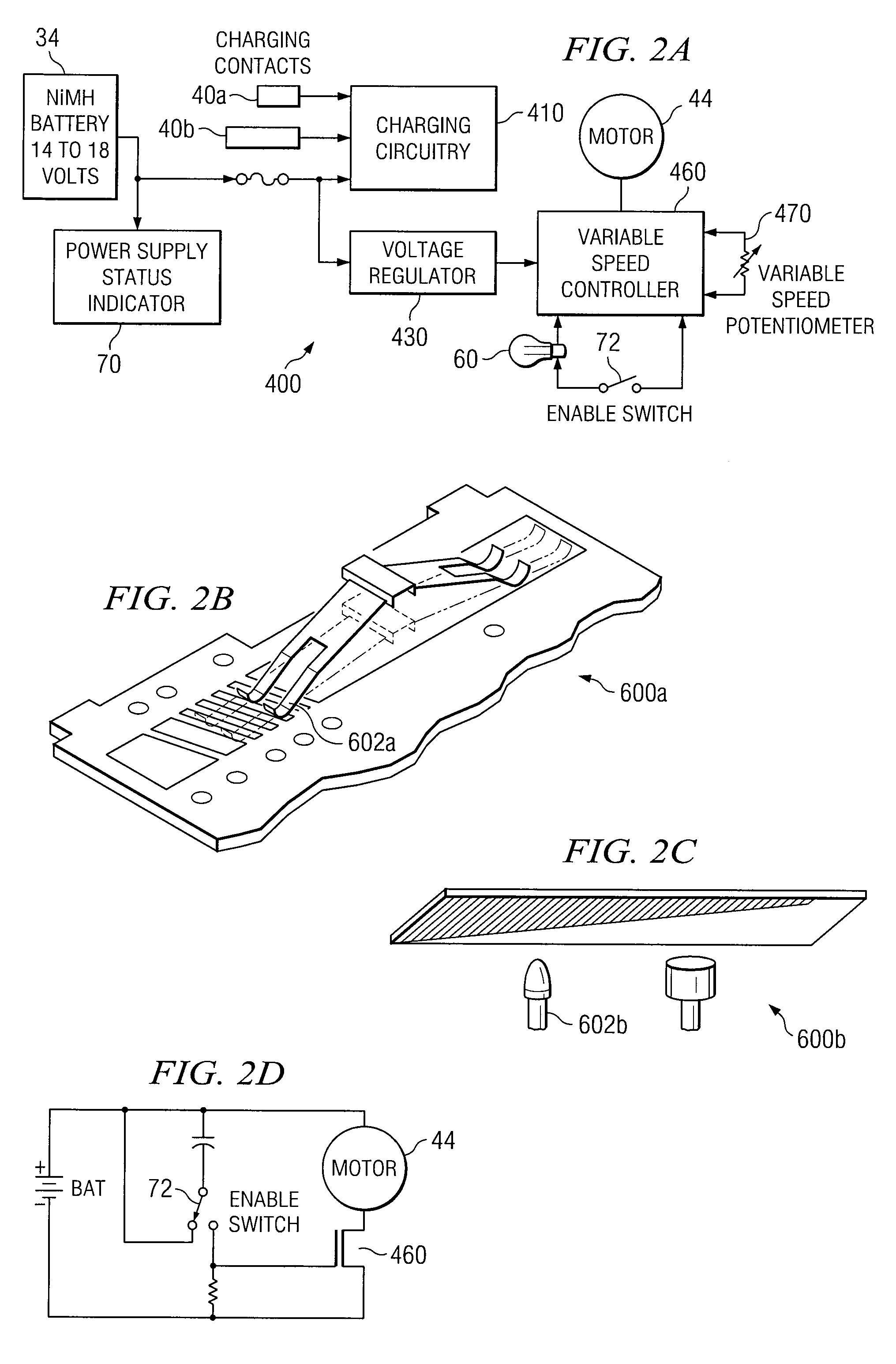
Powered Drivers, Intraosseous Devices And Methods To Access Bone Marrow
Apparatus and methods are provided to penetrate a bone and associated bone marrow using a powered driver. The powered driver may include a housing having a gear assembly, a motor and a power supply disposed therein. A penetrator assembly may be releasably engaged with one end of a drive shaft extending from the housing. The powered driver may include a light operable to illuminate an insertion site for the penetrator assembly. An indicator may be provided to show status of an associated power supply. An indicator may also be provided to show status of penetrating bone and / or associated bone marrow using a penetrator assembly. The apparatus may include an enable switch to prevent undesired operation of the powered driver.
Owner:TELEFLEX LIFE SCI LTD
Method and apparatus for extracting bone marrow
InactiveUS6846314B2Surgical needlesVaccination/ovulation diagnosticsExtraction siteExtraction methods
Methods and apparatus are presented for extracting and collecting bone material from an extraction site of a patient. The method and apparatus further provides a readily accessible, and easily harvested, source of bone material without the drawbacks of current extraction methods.
Owner:SHAPIRA IRA L
Devices and methods for extraction of bone marrow
InactiveUS6849051B2Disruption can be efficiently accuratelyRotation can be efficiently accuratelyElectrotherapySurgical needlesMedical deviceBiomedical engineering
Owner:STEMSOURCE LLC
Apparatus and method to provide emergency access to bone marrow
An apparatus and method for penetrating the bone marrow is provided. The apparatus includes a housing, a penetrator assembly, operable to penetrate the bone marrow, a connector operable to releasably attach the penetrator assembly to a drill shaft, the drill shaft operable to connect the penetrator assembly to a gear assembly, a gear assembly operable to engage and rotate the drill shaft, a motor operable to engage the reduction gear assembly and drive the penetrator into the bone marrow by rotation of the drill shaft, and a power supply and associated circuitry operable to power the motor. The apparatus and method may be adapted to insert a probe through the skull and into the brain.
Owner:TELEFLEX LIFE SCI LTD
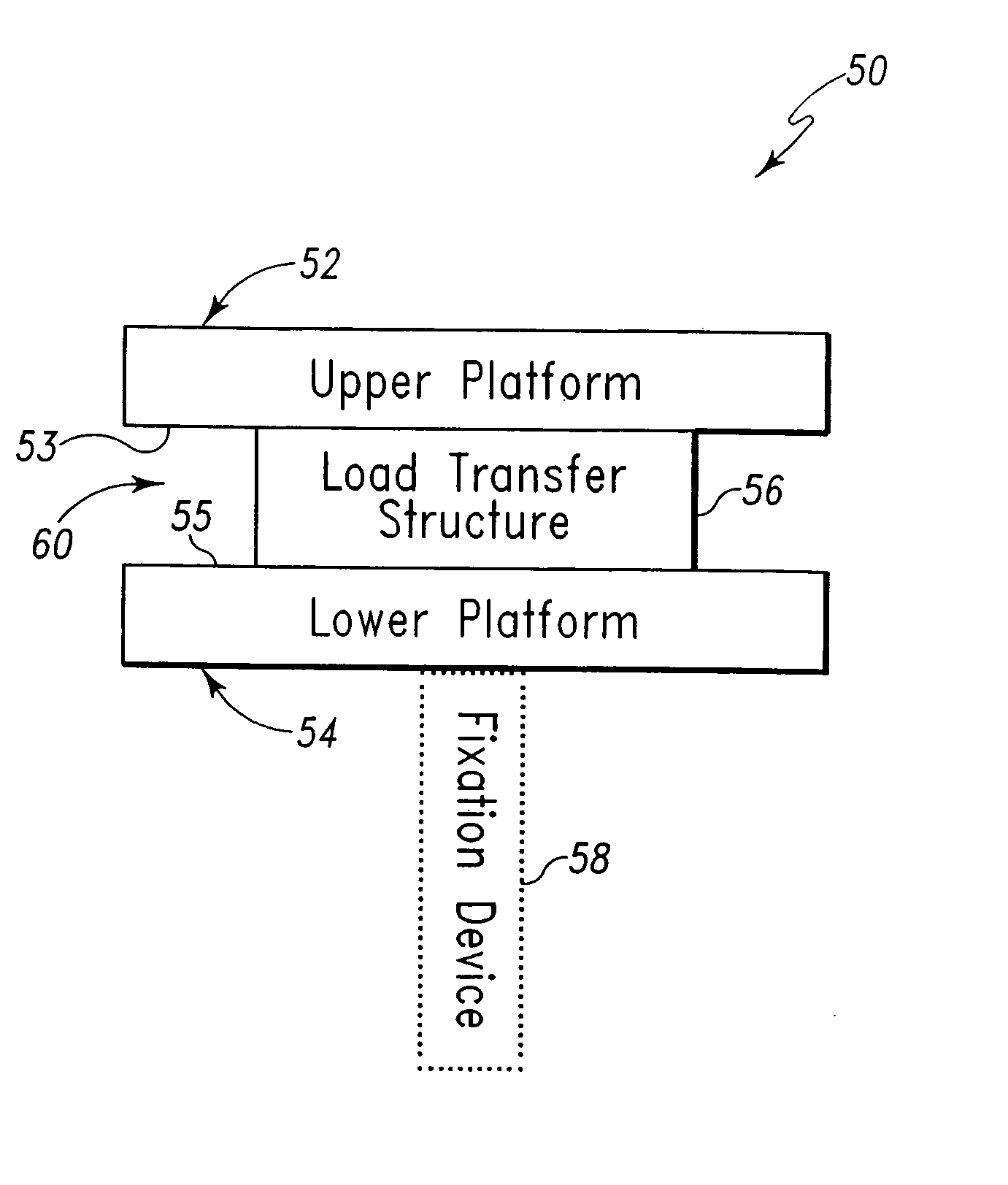
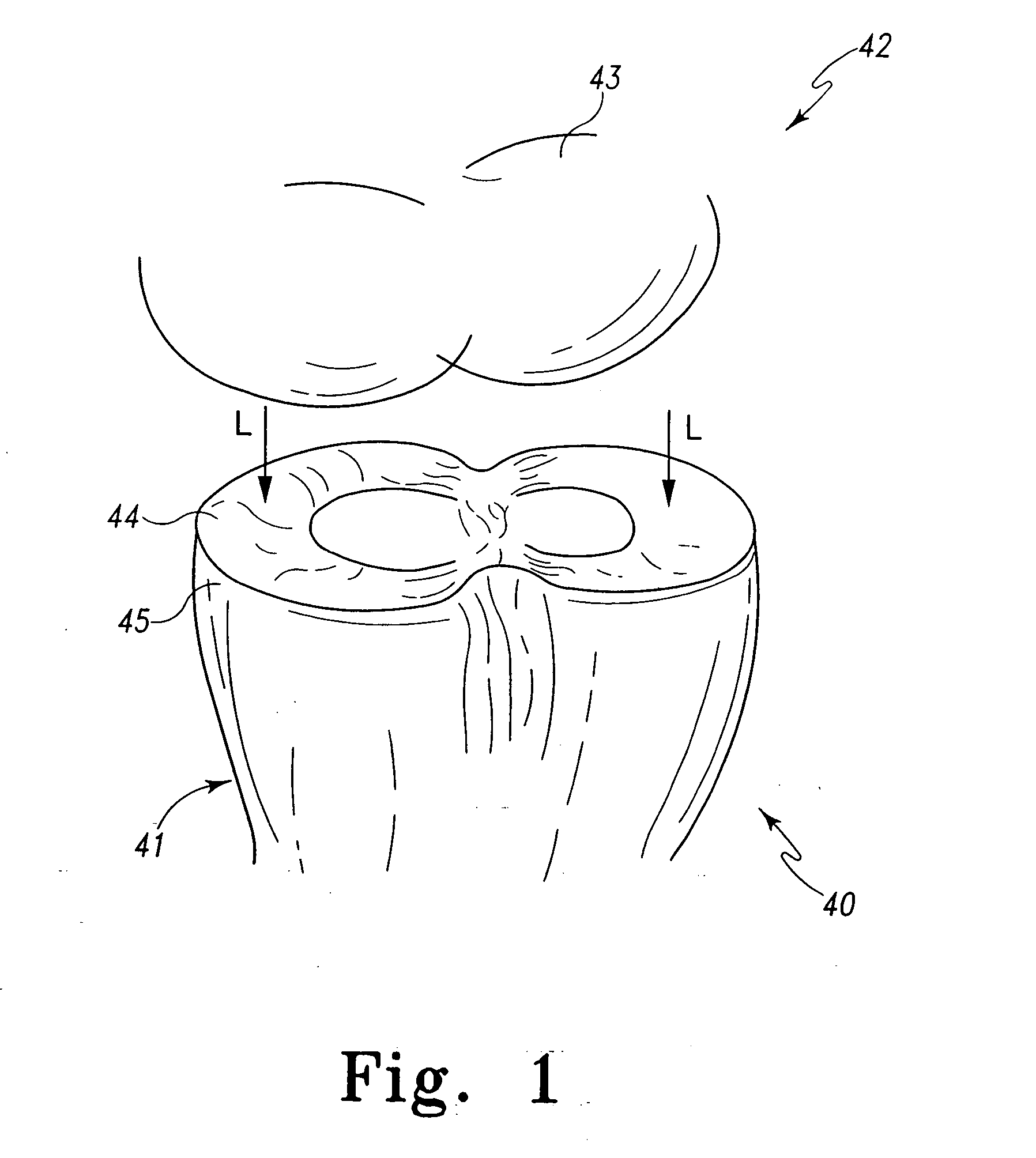
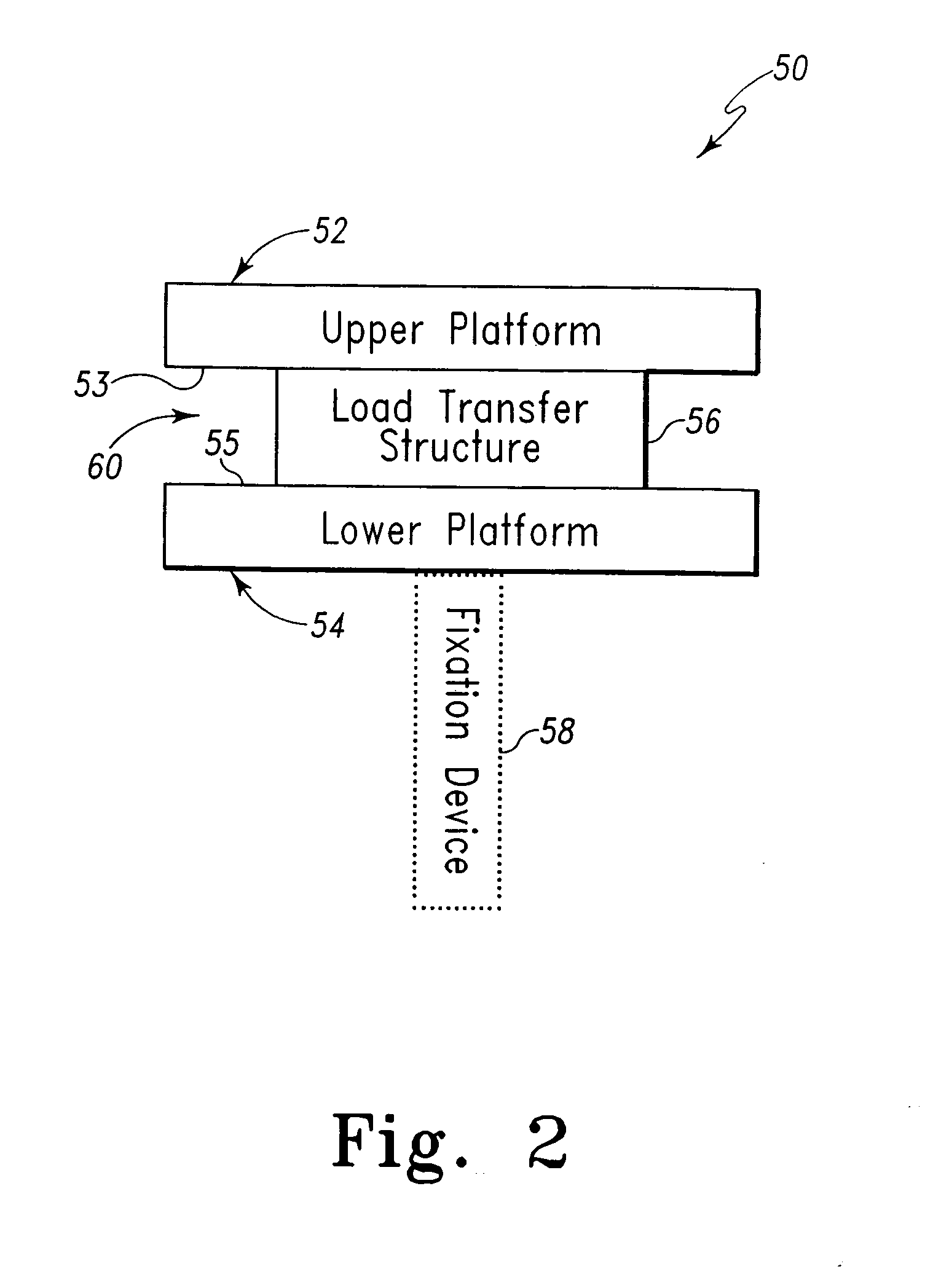
Implant device for cartilage regeneration in load bearing articulation regions
An implant device for cartilage regeneration in loading-bearing regions uses the osteochondral defect model. The implant is formed of resorbable polymeric materials. The implant is designed such that load is transmitted from the articulating surface of the bone platform through the implant to the entire area of subchondral bone of the bone platform. Application of load in this manner results in reduced subchondral bone resorption, leading to joint stabilization and maintenance of normal joint biomechanics. The implant allows for the incorporation therein of a resorbable scaffold or matrix material. The present implant solves the current inability to regenerate cartilage in load-bearing articulating surfaces using engineered scaffold devices.
Owner:DEPUY PROD INC
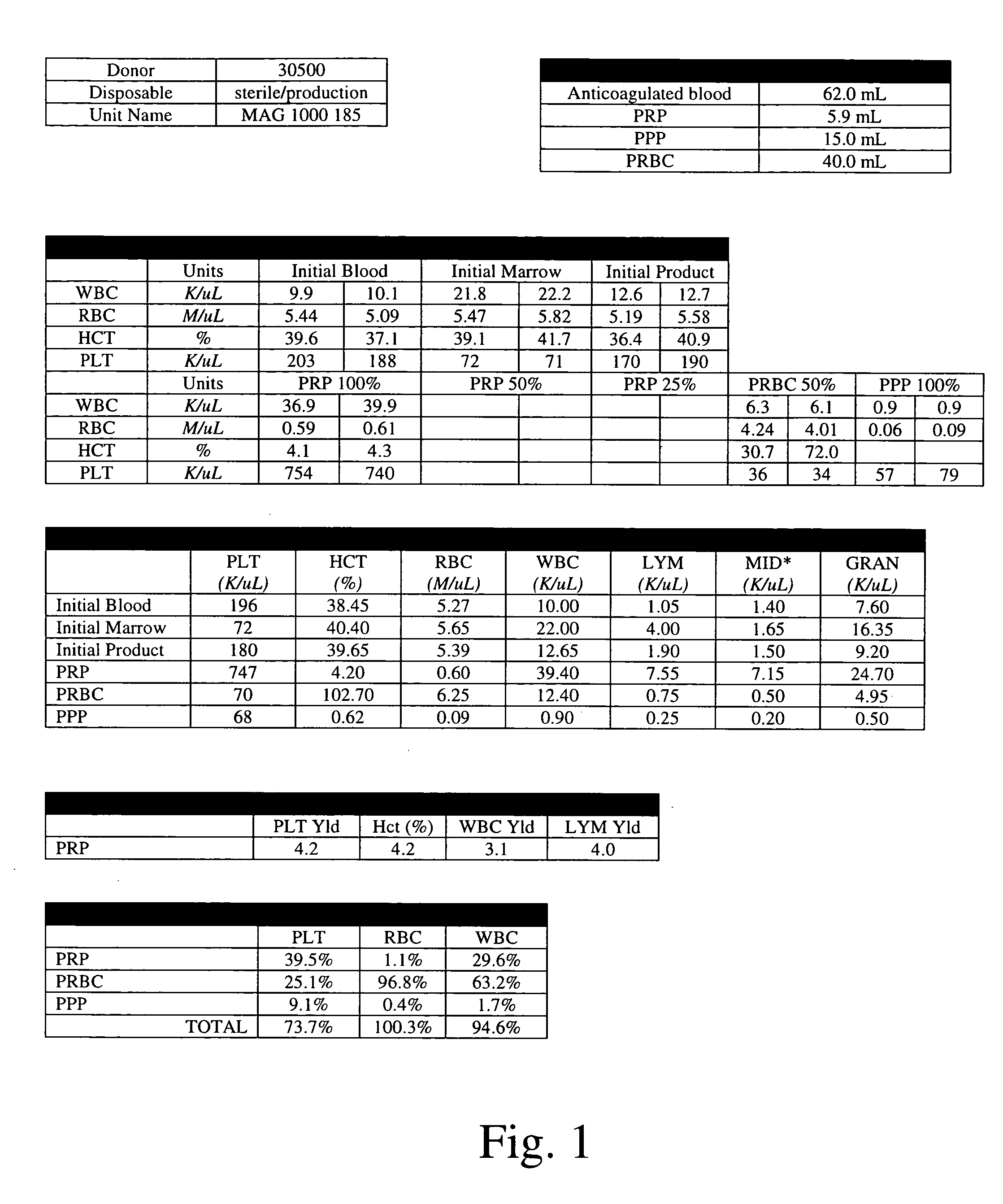
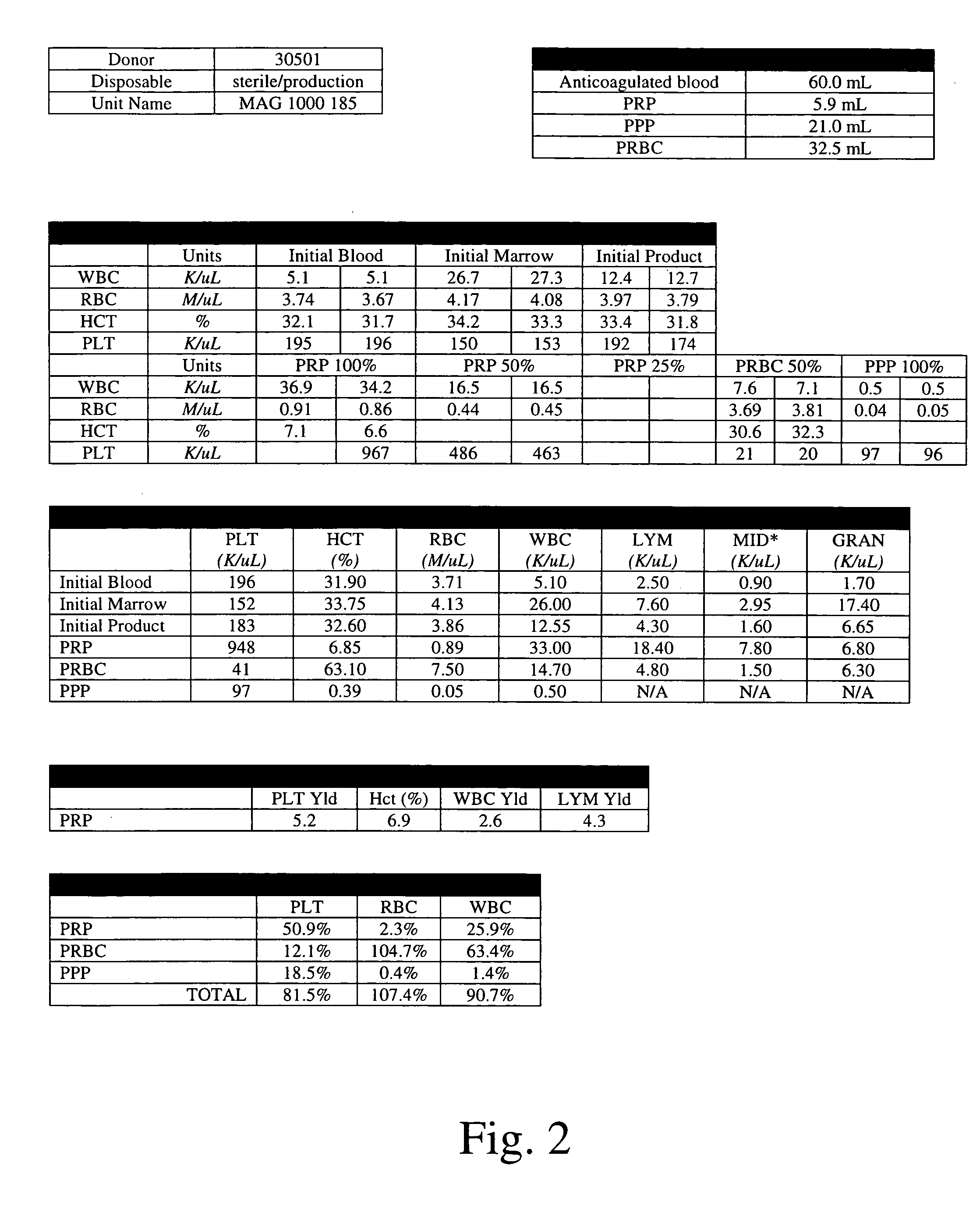
Method of determining a complete blood count and a white blood cell differential count
ActiveUS20090269799A1Improve accuracyLow costBioreactor/fermenter combinationsTelevision system detailsWhite blood cellBody fluid
Systems and methods analyzing body fluids such as blood and bone marrow are disclosed. The systems and methods may utilize an improved technique for applying a monolayer of cells to a slide to generate a substantially uniform distribution of cells on the slide. Additionally aspects of the invention also relate to systems and methods for utilizing multi color microscopy for improving the quality of images captured by a light receiving device.
Owner:ROCHE DIAGNOSTICS HEMATOLOGY INC
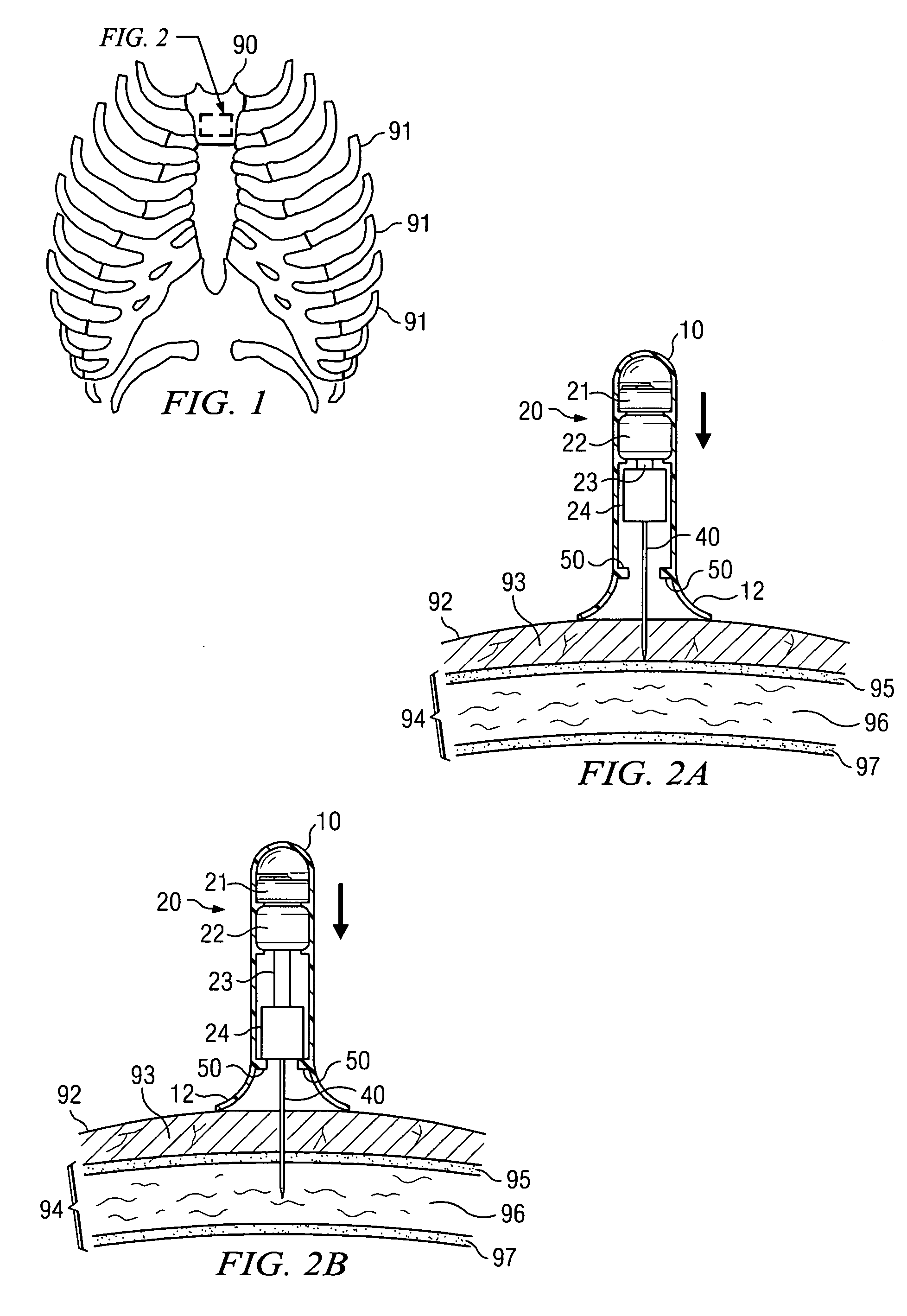
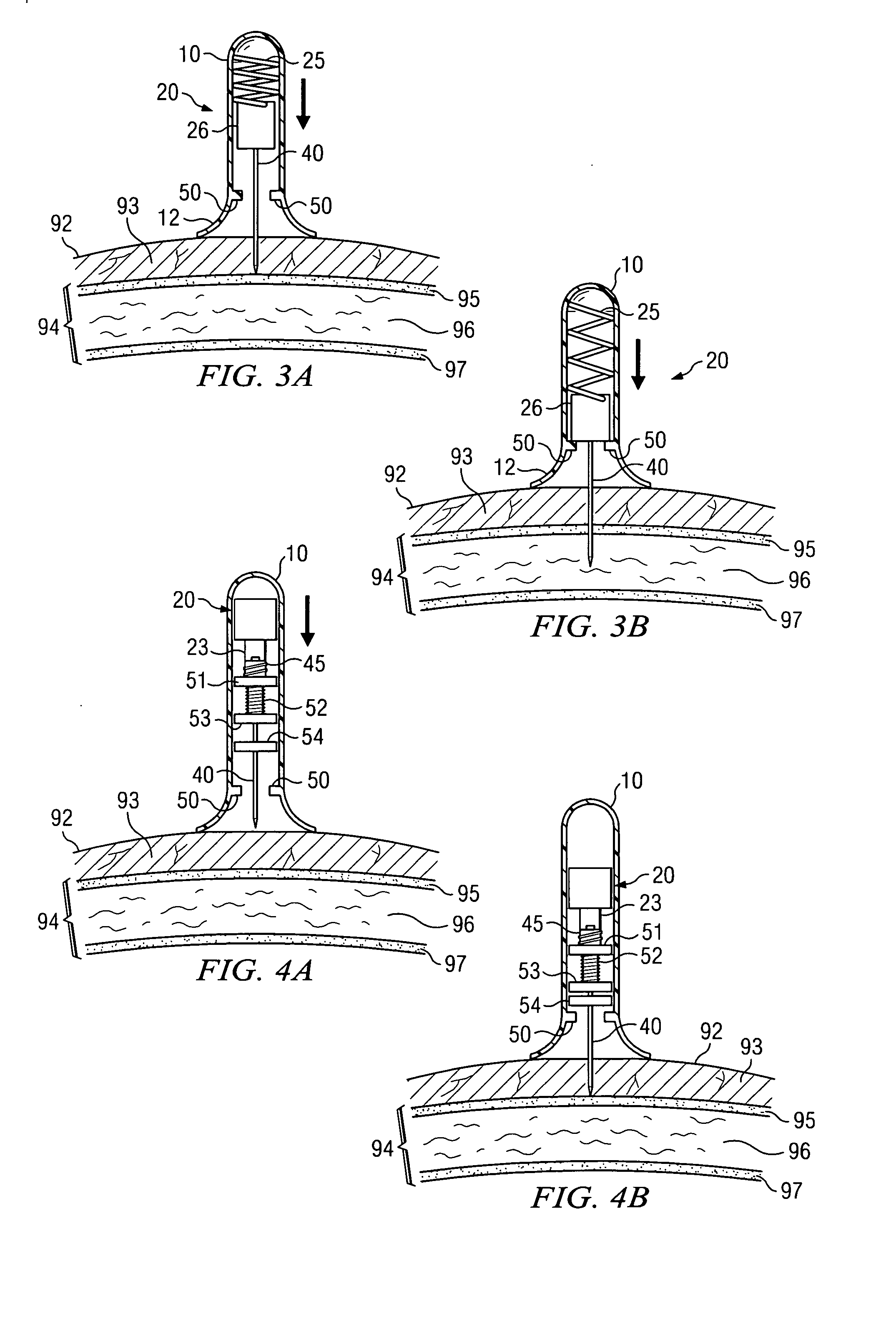
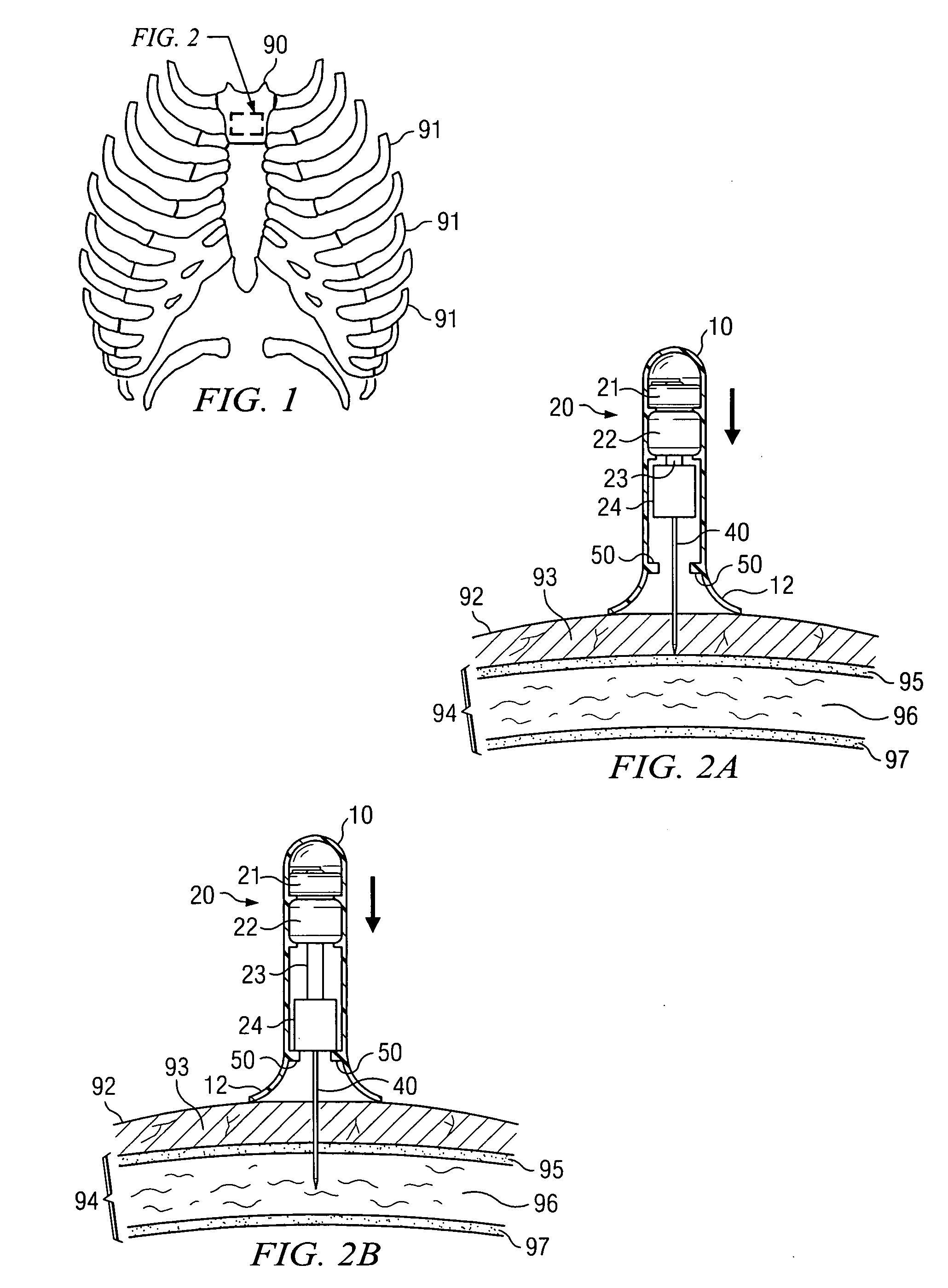
Apparatus and method for accessing the bone marrow of the sternum
ActiveUS20050131345A1Easy accessSurgical needlesVaccination/ovulation diagnosticsThoracic boneBiomedical engineering
An apparatus and method for accessing the bone marrow of a human's sternum is provided. The apparatus includes a tissue penetrator configured to penetrate the sternum, a trigger mechanism configured to engage when the tissue penetrator contacts the sternum and a depth control mechanism operable to limit the penetration of the tissue penetrator into the sternum to a pre-selected depth.
Owner:TELEFLEX LIFE SCI LTD
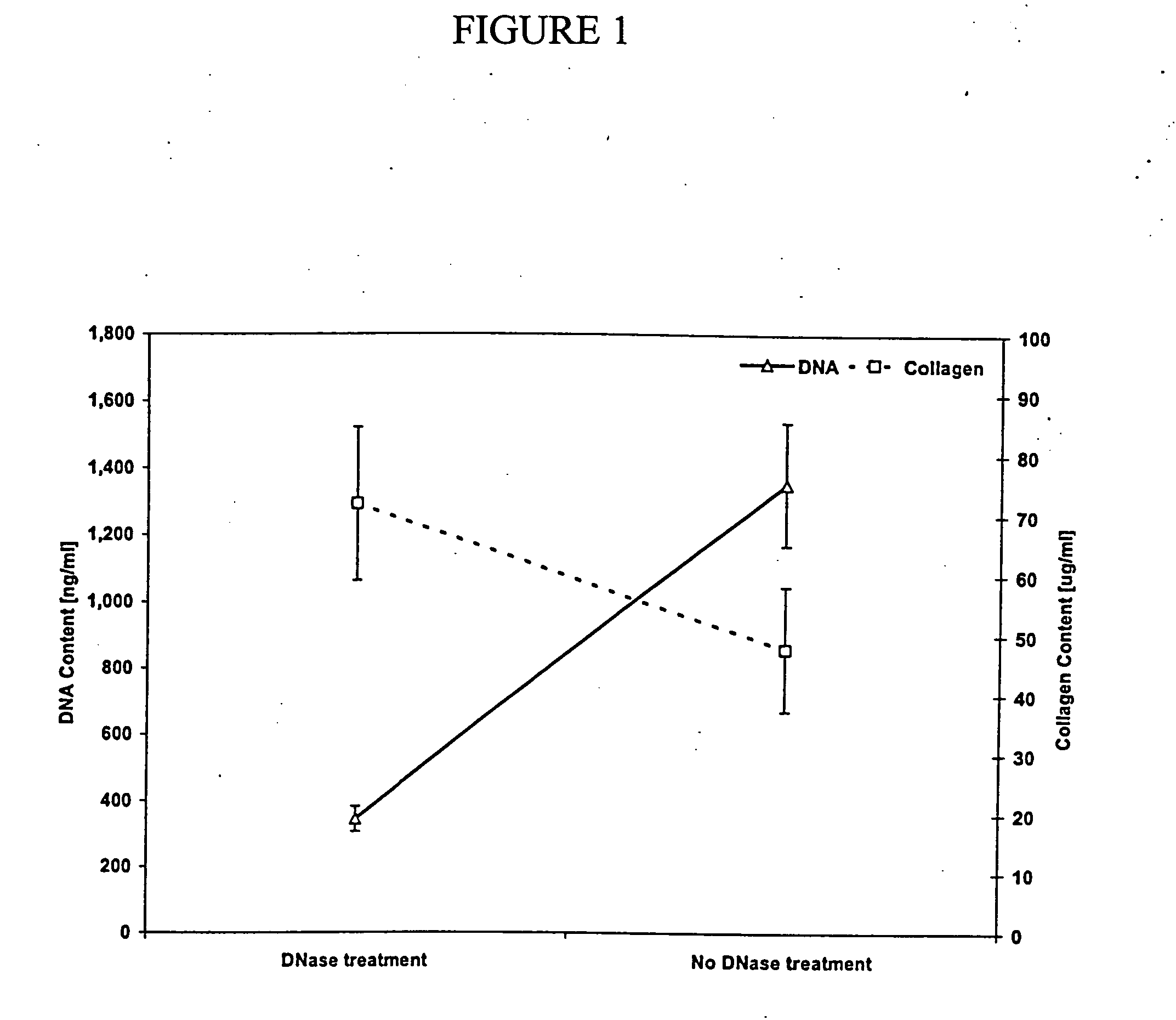
Isolation of bone marrow fraction rich in connective tissue growth components and the use thereof to promote connective tissue formation
InactiveUS20050130301A1Peptide/protein ingredientsSkeletal disorderConnective tissue fiberTissue defect
A bone marrow isolate rich in one or more connective tissue growth components, methods of forming the isolate, and methods of promoting connective tissue growth using the isolate are described. A biological sample comprising bone marrow is centrifuged to separate the sample into fractions including a fraction rich in connective tissue growth components. The fraction rich in connective tissue growth components is then isolated from the separated sample. The isolate can be used directly or combined with a carrier and implanted into a patient at a tissue (e.g., bone) defect site. The biological sample can comprise bone marrow and whole blood. The isolate can be modified (e.g., by transfection with a nucleic acid encoding an osteoinductive polypeptide operably linked to a promoter) prior to application to the tissue defect site. The isolate can be made and applied to the tissue defect site in a single procedure (i.e., intraoperatively).
Owner:MCKAY WILLIAM F +1
Femoral intramedullary rod system
InactiveUS20060122600A1Easy to compressFacilitate distractionInternal osteosythesisJoint implantsFemoral boneIntramedullary rod
Owner:ORTHODYNE
Ultrasound Therapy Resulting in Bone Marrow Rejuvenation
A method and system for treating a patient to repair damaged tissue which includes exposing a selected area of bone marrow of a patient to ultrasound waves or ultra shock waves so that cells comprising stem cells, progenitor cells or macrophages are generated in the area of the bone marrow of the patient due to the ultrasound, converting the cells from the bone marrow of the patient and reducing the damaged tissue in the bone marrow of the patient by repairing the damaged tissue.
Owner:JOHNSON LANNY L
Bone Matrix Compositions and Methods
ActiveUS20070098756A1High activityEasy to addPeptide/protein ingredientsBone implantActive agentOSTEOINDUCTIVE FACTOR
An osteoinductive composition, corresponding osteoimplants, and methods for making the osteoinductive composition are disclosed. The osteoinductive composition comprises osteoinductive factors, such as may be extracted from demineralized bone, and a carrier. The osteoinductive composition is prepared by providing demineralized bone, extracting osteoinductive factors from the demineralized bone, and adding the extracted osteoinductive factors to a carrier. Further additives such as bioactive agents may be added to the osteoinductive composition. The carrier and osteoinductive factors may form an osteogenic osteoimplant. The osteoimplant, when implanted in a mammalian body, can induce at the locus of the implant the full developmental cascade of endochondral bone formation including vascularization, mineralization, and bone marrow differentiation. Also, in some embodiments, the osteoinductive composition can be used as a delivery device to administer bioactive agents.
Owner:WARSAW ORTHOPEDIC INC
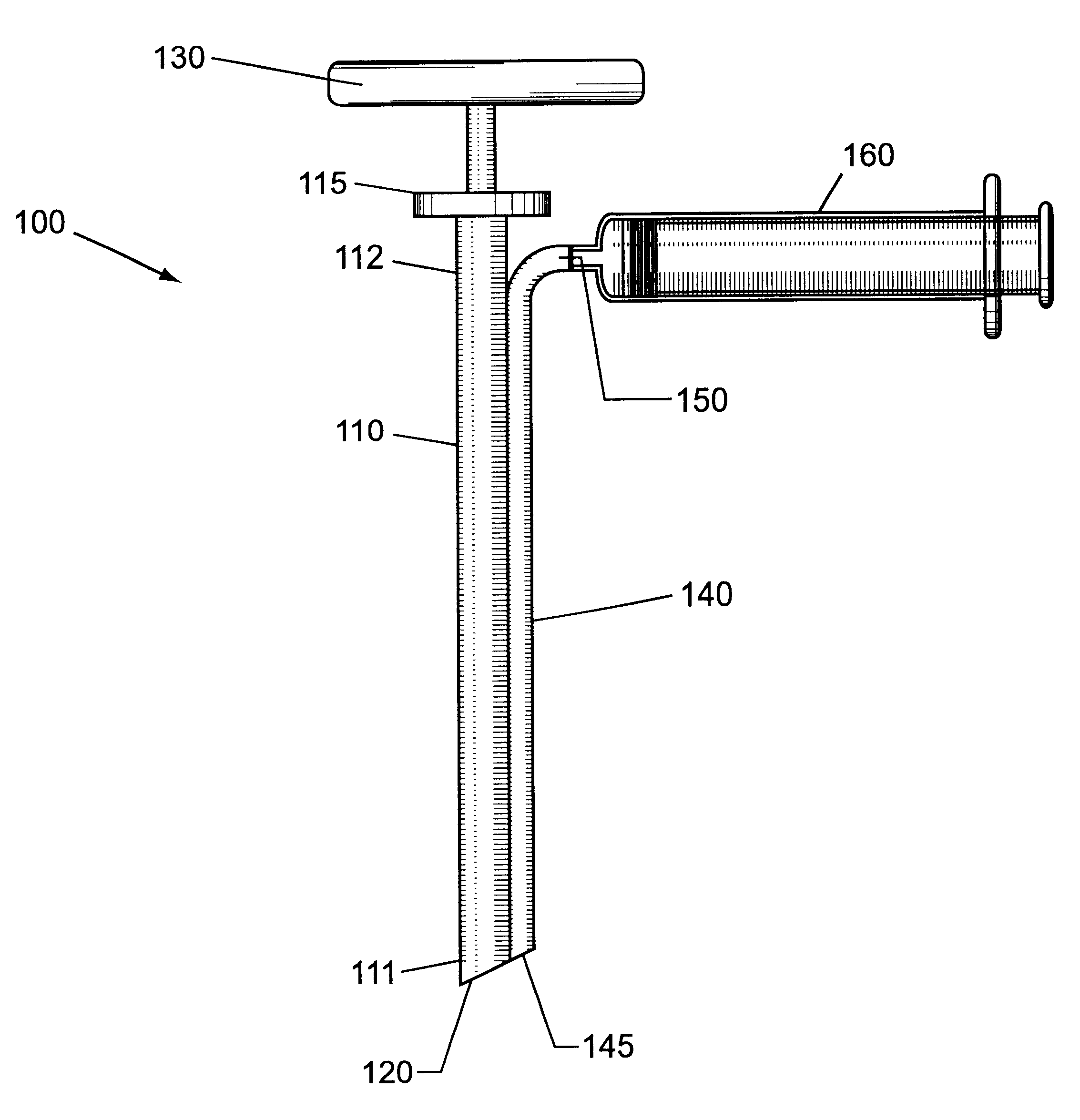
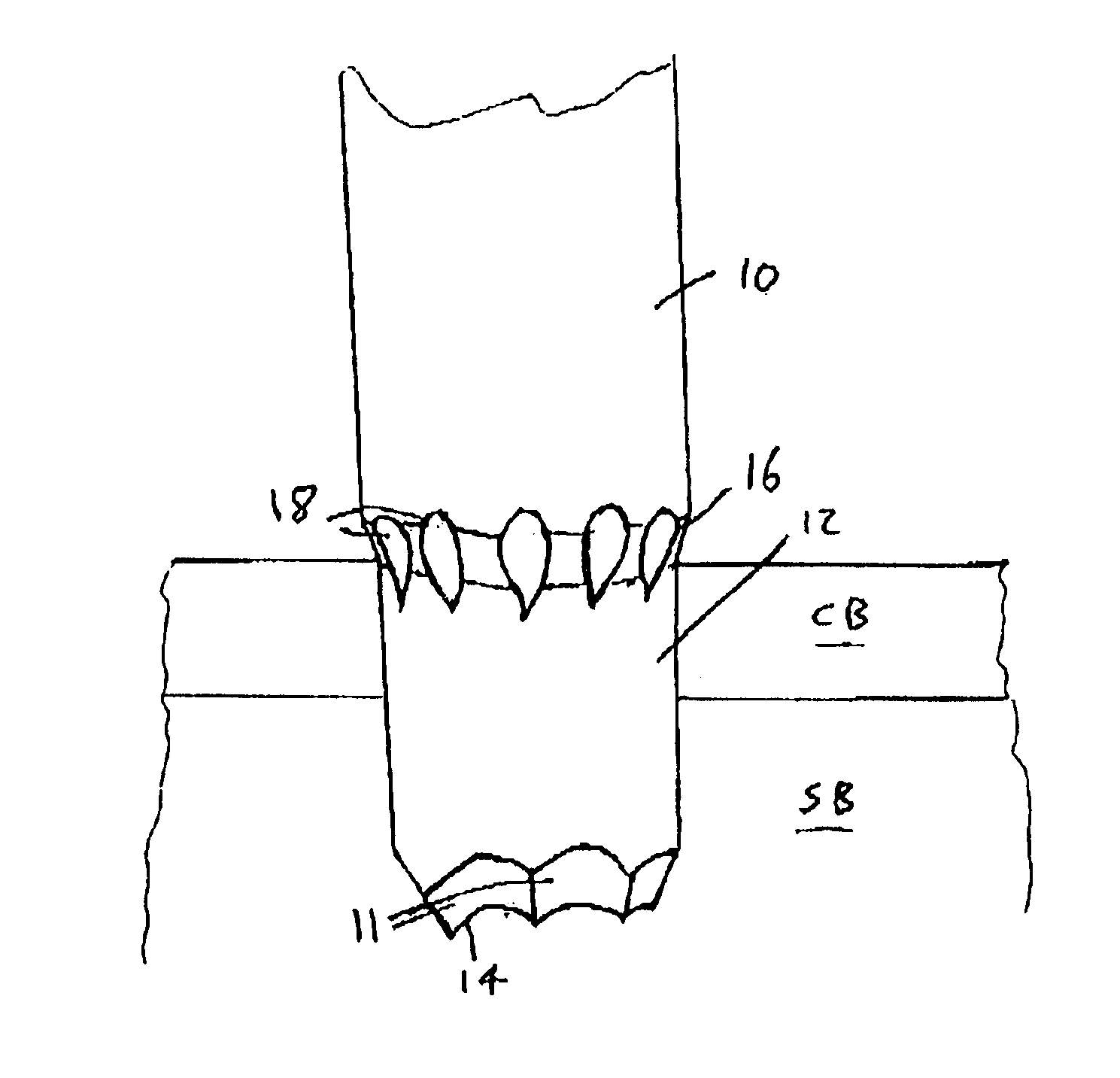
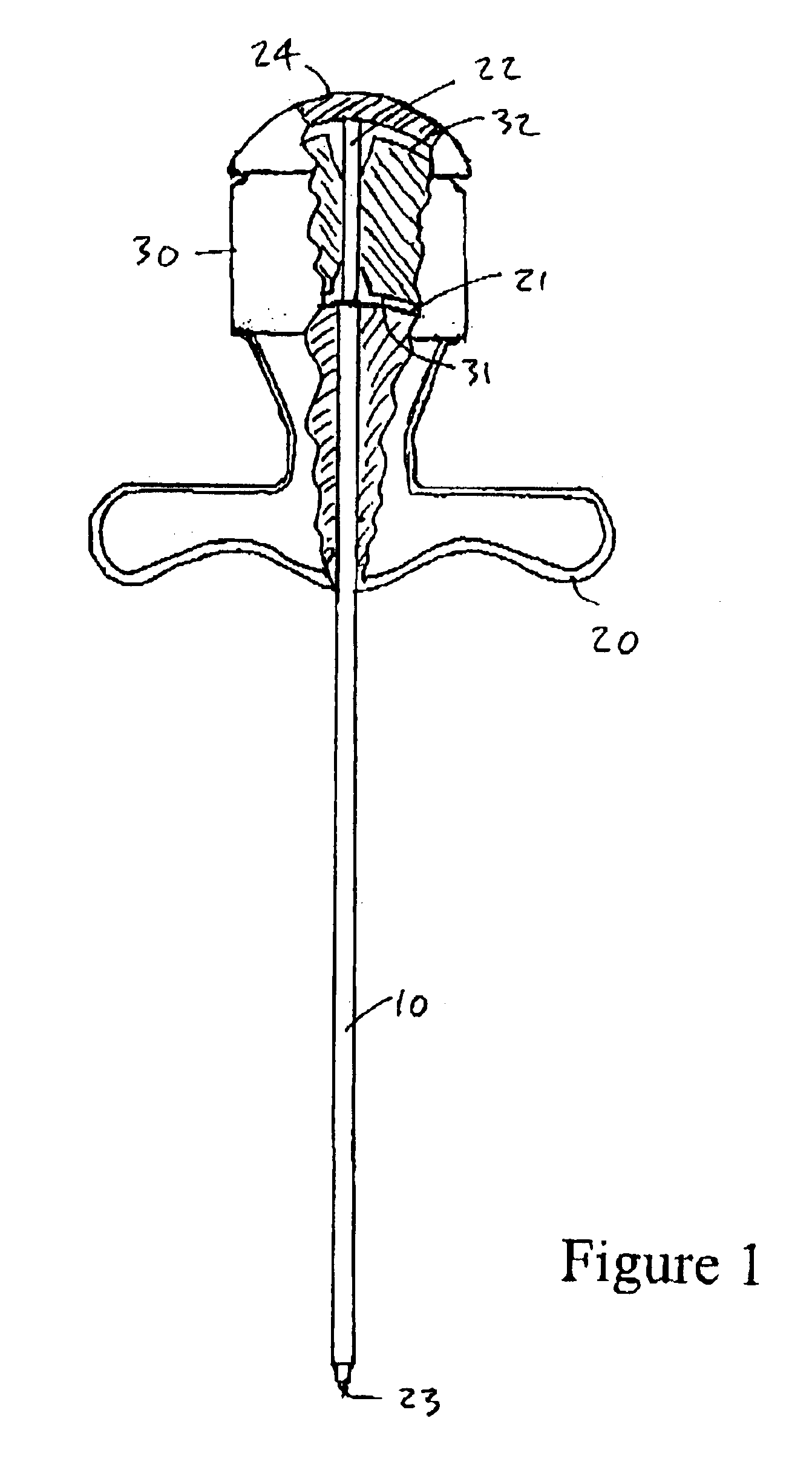
Bone marrow biopsy needle
InactiveUS6890308B2Without any resistanceLess-expensive to manufactureSurgical needlesVaccination/ovulation diagnosticsBone CortexSurgery
A needle for use in taking a bone marrow biopsy comprises a hollow tube having a front end portion formed to a reduced diameter by swaging. The front end is tapered by means of a number of circumferentially-spaced facets, forming a cutting edge. A tapering transition portion, between the main portion of the hollow tube and its reduced-diameter front end portion, is formed with a series of flutes which help in the needle cutting through the cortical bone. A spacer is provided for use in pushing the sample rearwardly out of the hollow tube, the spacer having a through-passage through which a trocar needle is passed and serving for accurate alignment of the distal ends of the hollow tube and trocar needle.
Owner:ISLAM ABUL BASHAR MOHAMMED ANWARUL
Process and composition for cleaning soft tissue grafts optionally attached to bone and soft tissue and bone grafts produced thereby
InactiveUS6024735AEfficient removalEfficient formationSurgical needlesVaccination/ovulation diagnosticsPresent methodAdditive ingredient
The invention relates to compositions effective for the cleansing of mammalian soft tissue optionally attached to bones, and particularly the removal of blood deposits and bone marrow therefrom. The compsotions are composed of an aqueous solution containing as its essential ingredients a detergent having a functionality of the nature of a polyoxyethylene-23-lauryl either, a detergent having a functionality of the nature of exyethylated alkylphenol, and water, where the compositions are free from any membrane stabilizers. The present invention is also directed to a method and composition for cleaning cadaveric soft tissue optionally attached to bone to produce soft tissue grafts optionally attached to bone suitable for transplantation into a human. The present method involves removing bone marrow elements, blood deposits and any bacteria, virus or fungi contamination, from the donor bone and / or associated soft tissues.
Owner:LIFENET HEALTH
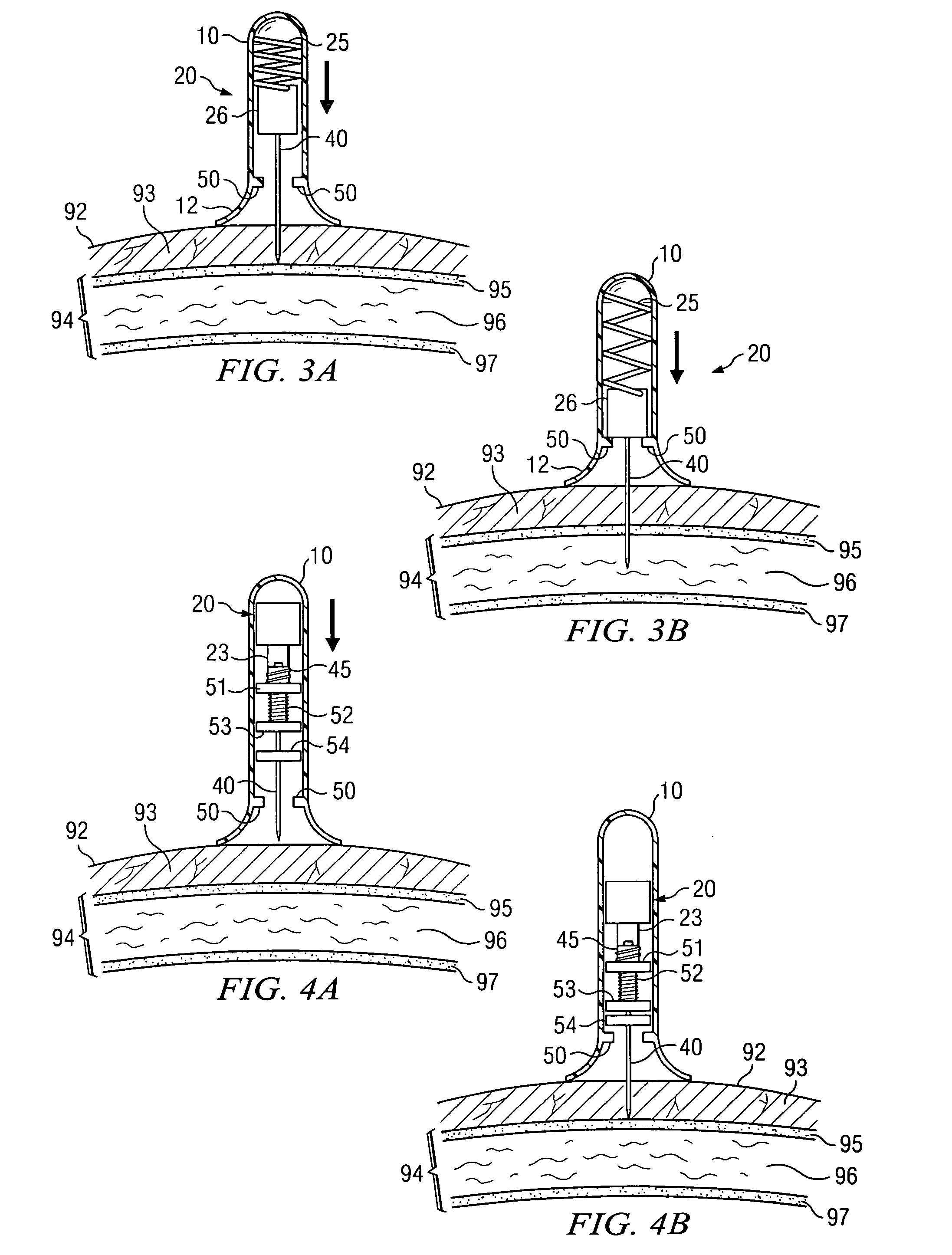
Impact-driven intraosseous needle
An apparatus for penetrating a bone marrow of a bone is provided. The apparatus includes a housing comprising a handle and a trigger mechanism, a spring-loaded assembly comprising a rod and a shaft; and a connector comprising a first end operable to connect to the drive shaft and a second end operable to attach to a penetrator hub. The penetrator hub includes a penetrator operable to access the bone marrow.
Owner:TELEFLEX LIFE SCI LTD
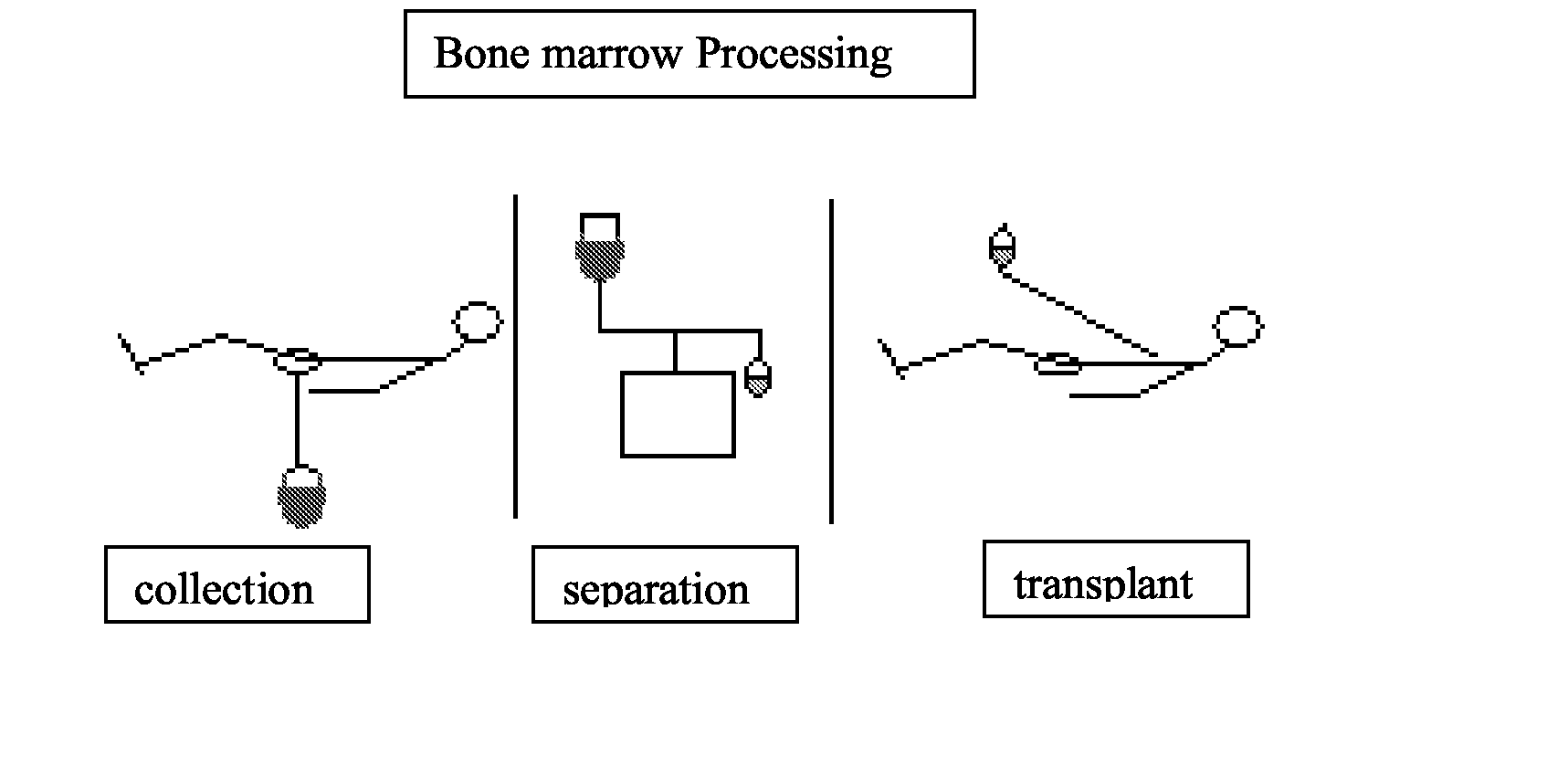
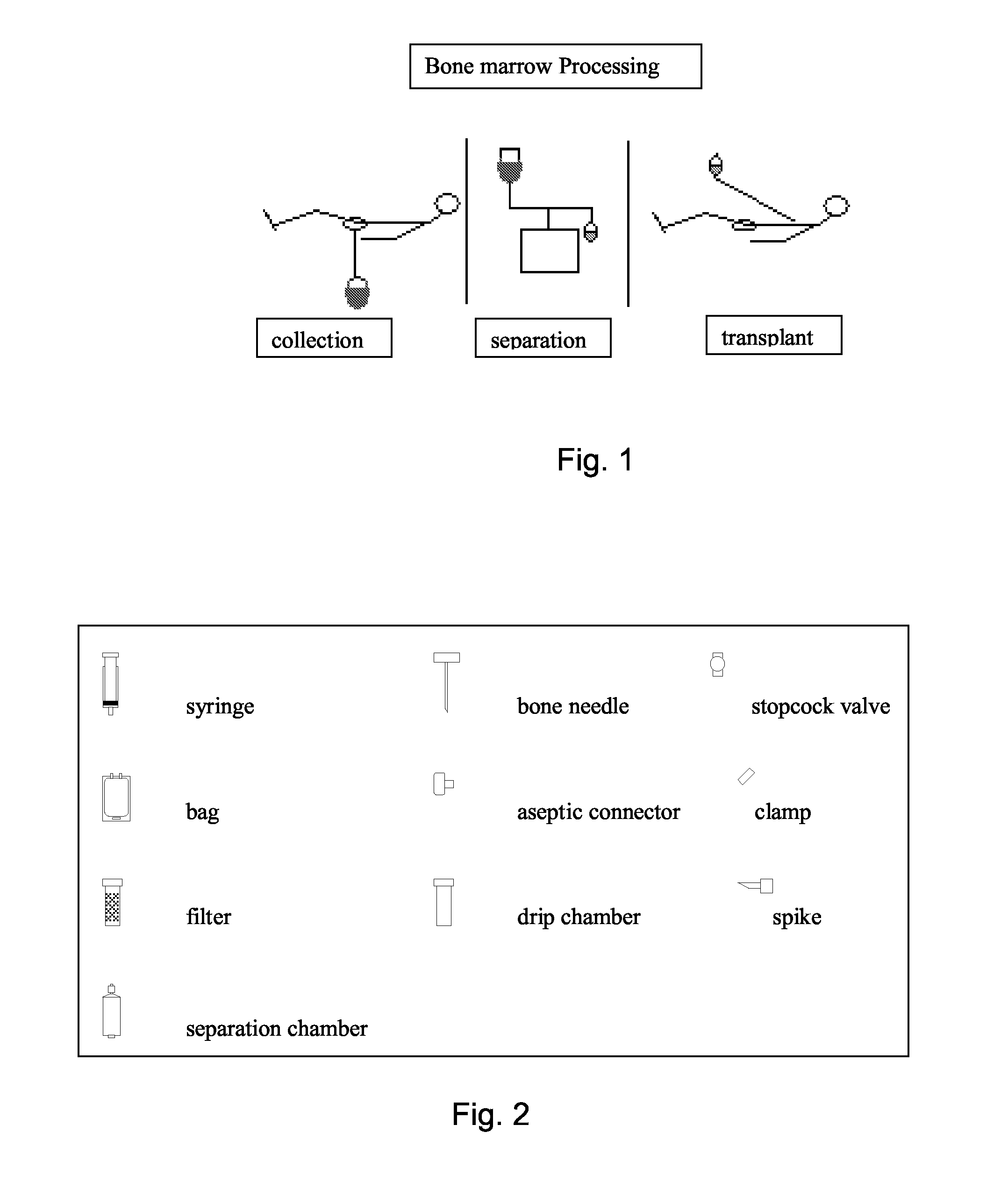
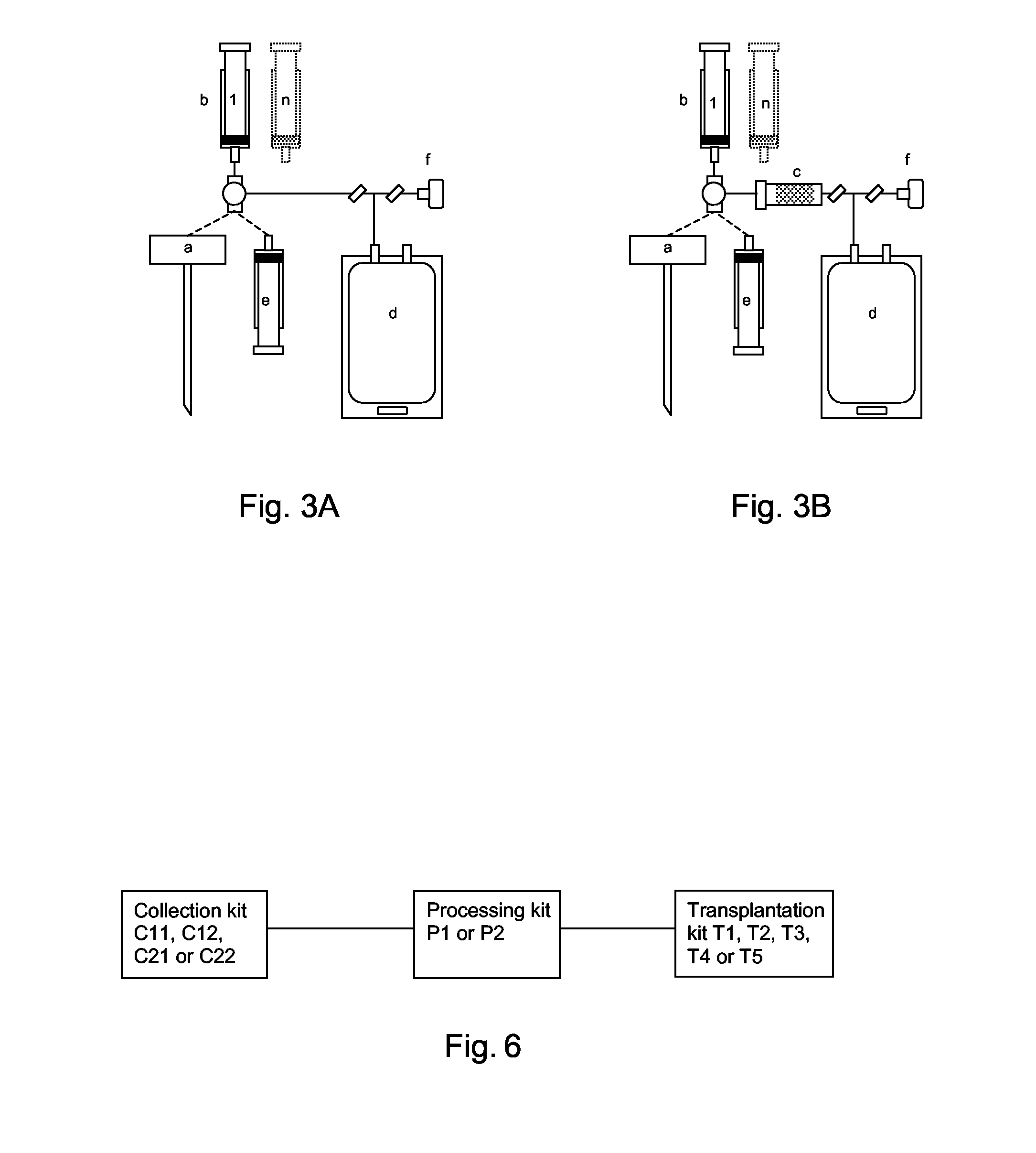
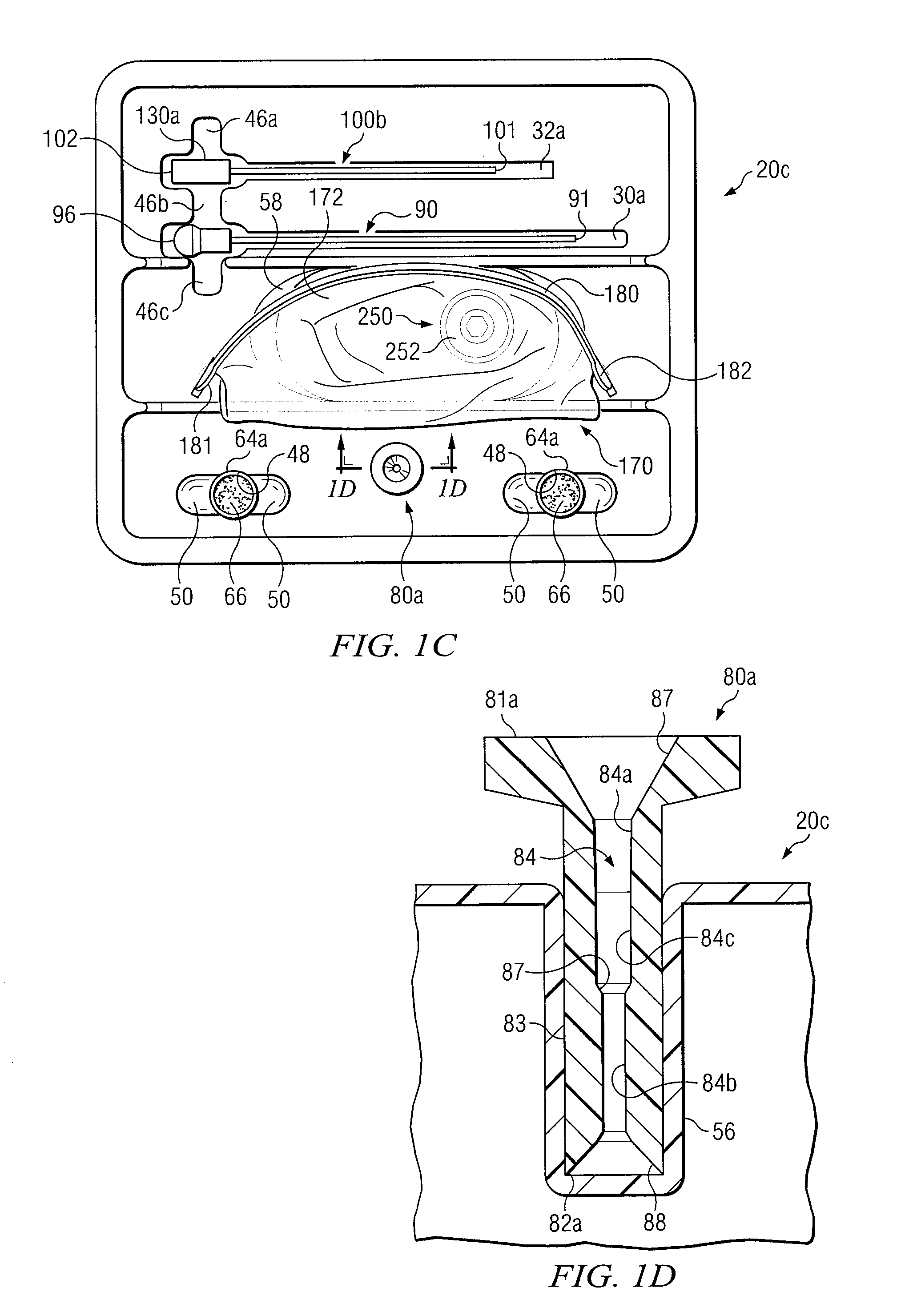
Integrated System for Collecting, Processing and Transplanting Cell Subsets, Including Adult Stem Cells, for Regenerative Medicine
InactiveUS20080171951A1Accurate collectionMinimize risk of contaminationBioreactor/fermenter combinationsNervous disorderFluid transportTissue repair
A system for the extraction, collection, processing and transplantation of cell subsets, including adult stem cells and platelets, in particular for tissue repair in regenerative medicine, comprises a set of disposable fluid-transport elements that are pre-connected or that include aseptic connectors for making interconnections between them in an aseptic manner or are adapted to be aseptically connected. The set usually includes three kits of disposable sterile elements, a collection kit, a processing kit, and a transplantation kit packaged in a blister pack on a support such as a tray, having one compartment for receiving each inter-connectable kit of the set. The set includes an extracting device, for example including a needle for bone puncture or vein puncture, for extracting bone marrow or other sources of cell subsets from a patient.
Owner:BIOSAFE SA
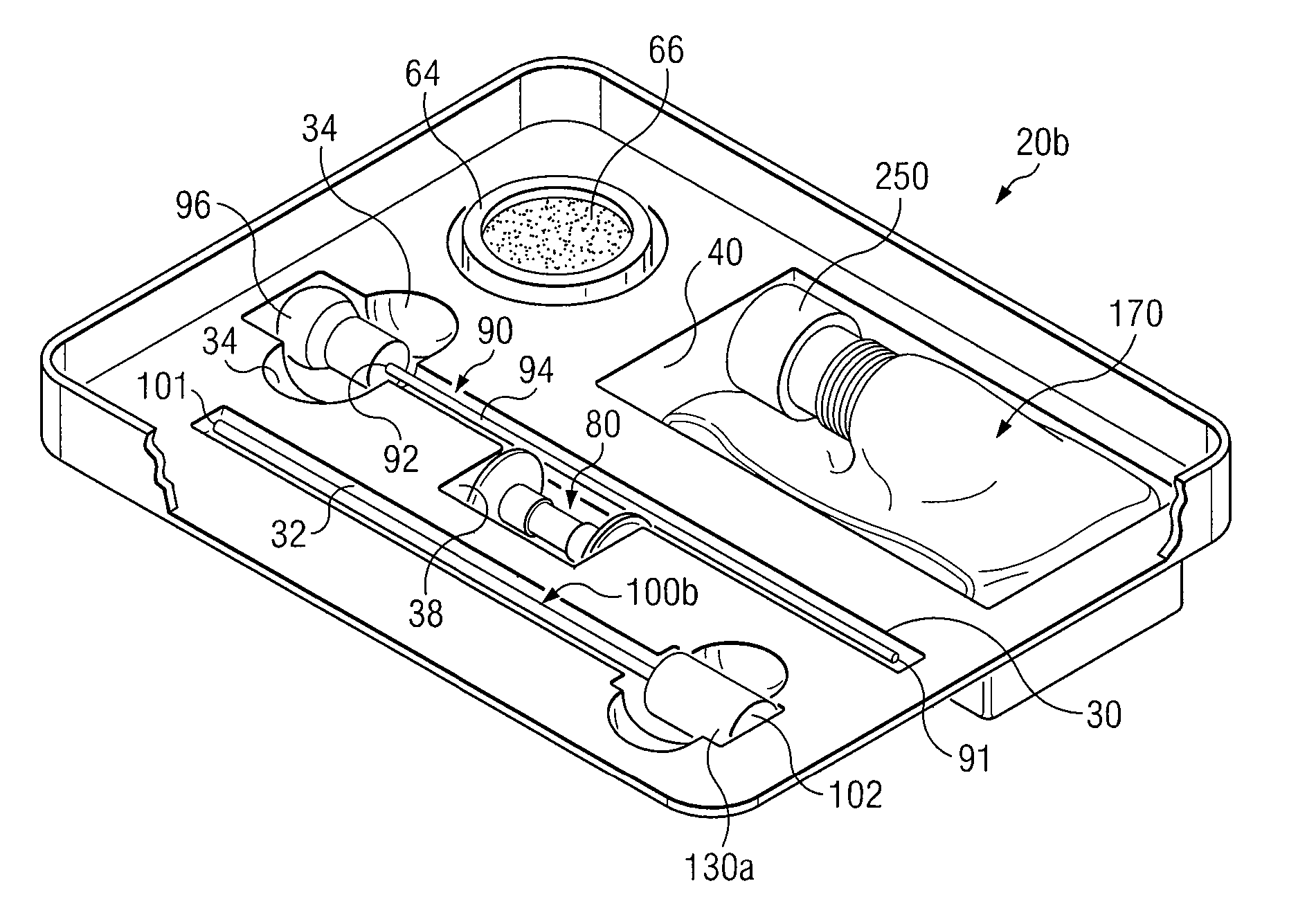
Medical Procedures Trays And Related Methods
ActiveUS20080045861A1Reduced inside diameterEasy to captureSurgical furnitureDispensing apparatusTransplant ProcedureSurgery
Medical procedure trays and related methods are provided to accommodate joining a first non-sterile medical device with a second sterile medical device and maintaining required sterilization of the second sterile medical device to perform an associated medical procedure. One example of such medical procedures includes biopsy of a bone and / or associated bone marrow using a non-sterile powered drive and a sterile biopsy needle or biopsy needle set. Medical procedure trays and related methods may also be provided for use during aspiration of bone marrow and / or stem cell transplant procedures. Each medical procedure tray may include a containment bag or sterile sleeve. A coupler assembly, one or more sharps protectors, a biopsy sample ejector and / or associated ejector funnel may also be included. Some medical procedure trays may allow engaging a non-sterile powered driver with one end of a coupler assembly and sealing the non-sterile powered driver in a sterile sleeve or containment bag without compromising sterility of other components in the medical procedure tray.
Owner:TELEFLEX LIFE SCI LTD
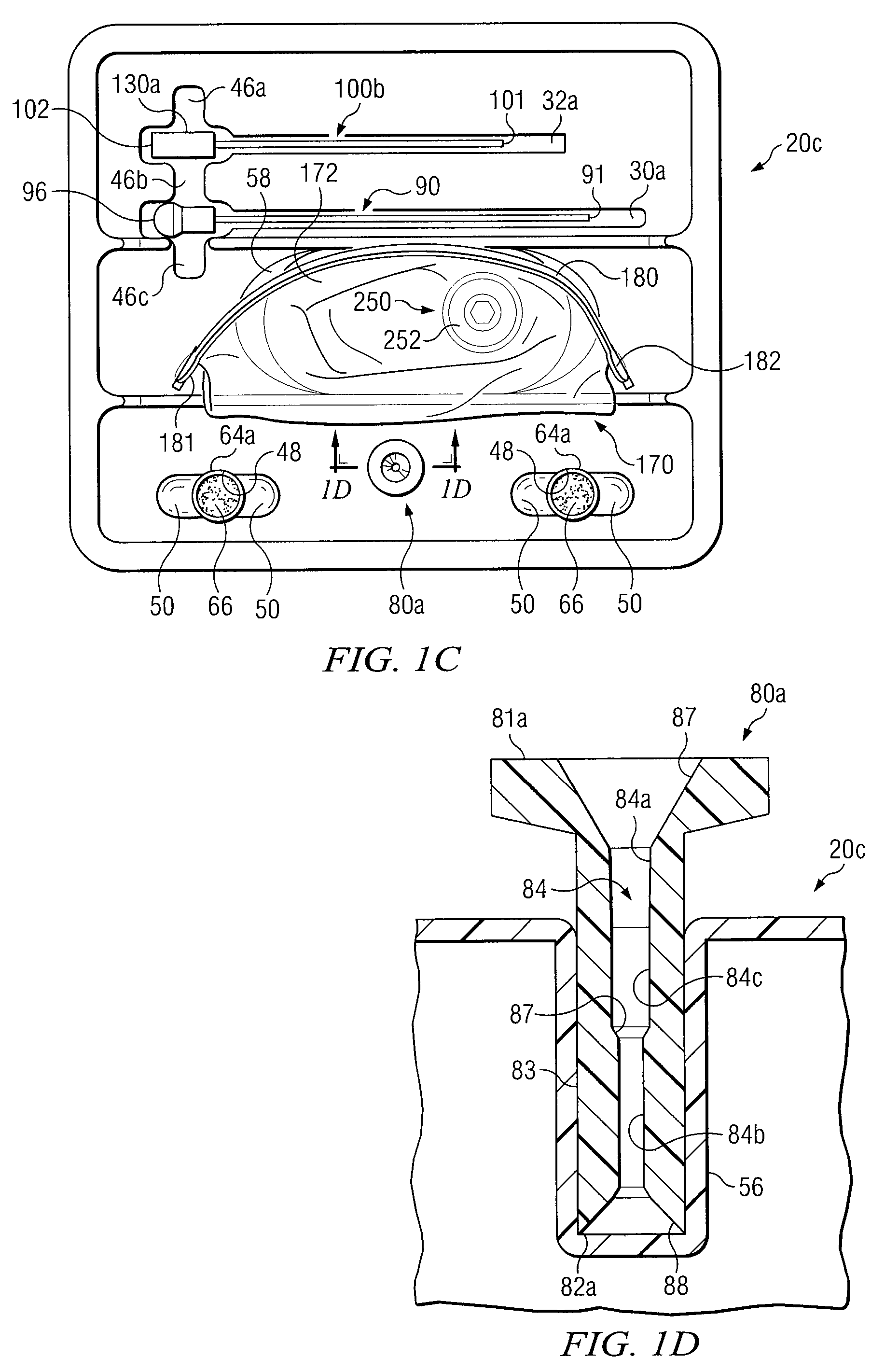
Biopsy Devices and Related Methods
InactiveUS20080045860A1Reduced inside diameterEasy to captureSurgical furnitureSurgical needlesBiopsy deviceCancellous bone
Apparatus and methods provided to remove biopsy specimens from bone and / or associated bone marrow. A powered driver may rotate a biopsy needle at an optimum speed to obtain the biopsy specimen. A thread or a groove may be disposed on interior portions of the biopsy needle. The thread or groove may engage a biopsy specimen and enhance removal of a bone marrow core from cancellous bone. Manufacturing procedures are provided for bonding a single helical thread with interior portions of the biopsy needle. The apparatus may also include a biopsy sample ejector and / or ejector funnel. A biopsy needle set may include a cannula and a trocar with respective tips having optimum configurations, dimensions and / or orientations relative to each other to optimize penetration of a bone and / or bone marrow with minimum patient trauma and enhanced reliability of obtaining a biopsy specimen.
Owner:TELEFLEX LIFE SCI LTD
Decellularized bone marrow extracellular matrix
ActiveUS20050013872A1Minimizes and avoids immune responsePeptide/protein ingredientsSkeletal disorderCell-Extracellular MatrixInsertion stent
The invention is directed to compositions comprising decellularized bone marrow extracellular matrix and uses thereof. Methods for repairing or regenerating defective, diseased, damaged or ischemic tissues or organs in a subject, preferably a human, using the decellularized bone marrow extracellular matrix of the invention are also provided. The invention is further directed to a medical device, preferably a stent or an artificial heart, and biocompatible materials, preferably a tissue regeneration scaffold, comprising decellularized bone marrow extracellular matrix for implantation into a subject.
Owner:BOSTON SCI SCIMED INC
Apparatus and method for accessing the bone marrow
ActiveUS20050148940A1Easy accessSurgical needlesVaccination/ovulation diagnosticsBreast boneBiomedical engineering
An apparatus and method for accessing the bone marrow of a human's sternum is provided. The apparatus includes a tissue penetrator configured to penetrate the sternum, a trigger mechanism configured to engage when the tissue penetrator contacts the sternum and a depth control mechanism operable to limit the penetration of the tissue penetrator into the sternum to a pre-selected depth.
Owner:TELEFLEX LIFE SCI LTD
Biopsy devices and related methods
InactiveUS7850620B2Easy to captureIncrease speedSurgical furnitureSurgical needlesBiopsy deviceCancellous bone
Apparatus and methods provided to remove biopsy specimens from bone and / or associated bone marrow. A powered driver may rotate a biopsy needle at an optimum speed to obtain the biopsy specimen. A thread or a groove may be disposed on interior portions of the biopsy needle. The thread or groove may engage a biopsy specimen and enhance removal of a bone marrow core from cancellous bone. Manufacturing procedures are provided for bonding a single helical thread with interior portions of the biopsy needle. The apparatus may also include a biopsy sample ejector and / or ejector funnel. A biopsy needle set may include a cannula and a trocar with respective tips having optimum configurations, dimensions and / or orientations relative to each other to optimize penetration of a bone and / or bone marrow with minimum patient trauma and enhanced reliability of obtaining a biopsy specimen.
Owner:TELEFLEX LIFE SCI LTD
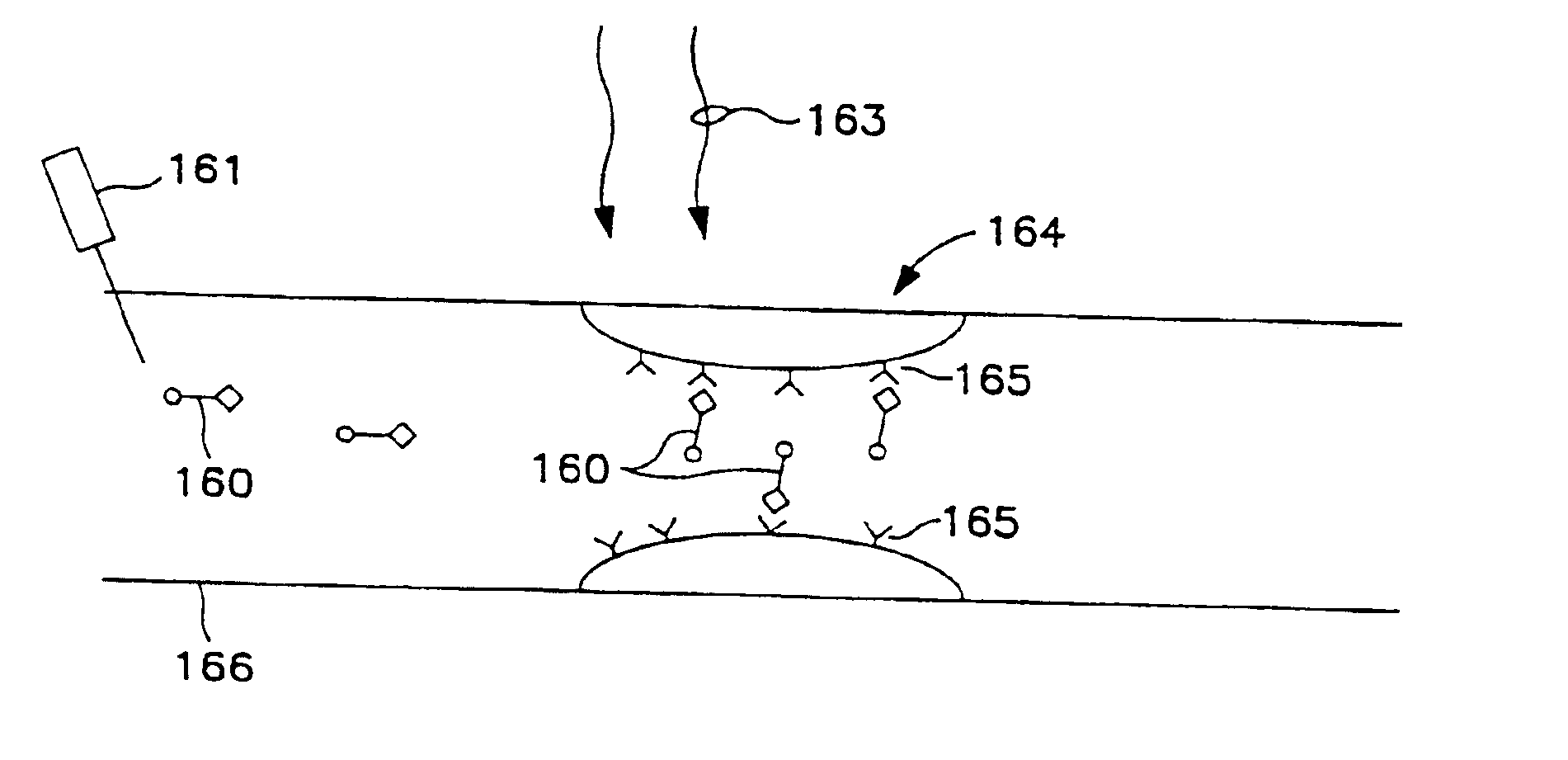
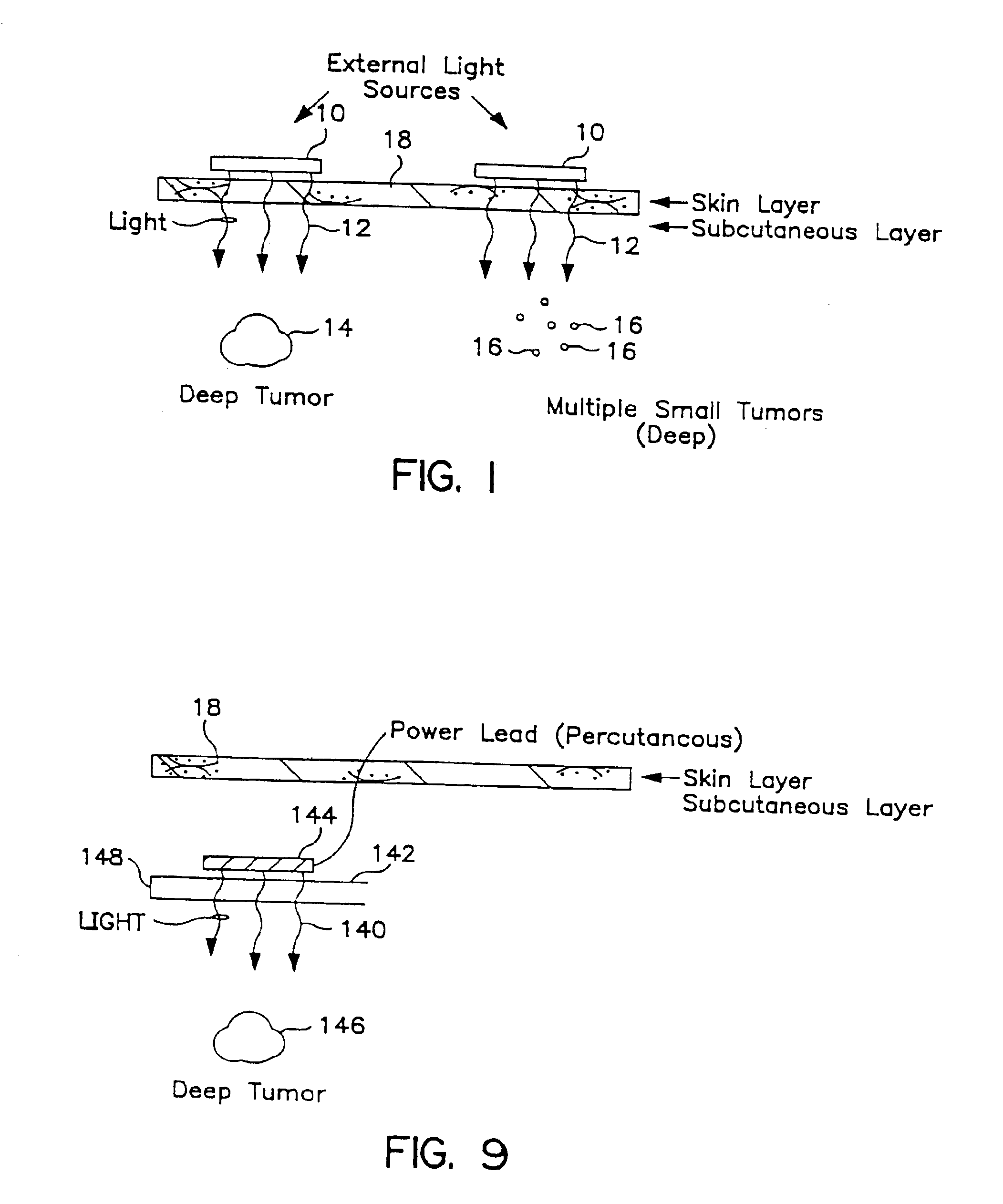
Transcutaneous photodynamic treatment of targeted cells
The present invention is drawn to methods and compounds for photodynamic therapy (PDT) of a target tissue or compositions in a mammalian subject, using a light source that preferably transmits light to a treatment site transcutaneously. The method provides for administering to the subject a therapeutically effective amount of a targeted substance, which is either a targeted photosensitizing agent, or a photosensitizing agent delivery system, or a targeted prodrug. This targeted substance preferably selectively binds to the target tissue. Light at a wavelength or waveband corresponding to that which is absorbed by the targeted substance is then administered. The light intensity is relatively low, but a high total fluence is employed to ensure the activation of the targeted photosensitizing agent or targeted prodrug product. Transcutaneous PDT is useful in the treatment of specifically selected target tissues, such as vascular endothelial tissue, the abnormal vascular walls of tumors, solid tumors of the head and neck, tumors of the gastrointestinal tract, tumors of the liver, tumors of the breast, tumors of the prostate, tumors of the lung, nonsolid tumors, malignant cells of the hematopoietic and lymphoid tissue and other lesions in the vascular system or bone marrow, and tissue or cells related to autoimmune and inflammatory disease.
Owner:LIGHT SCI ONCOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com