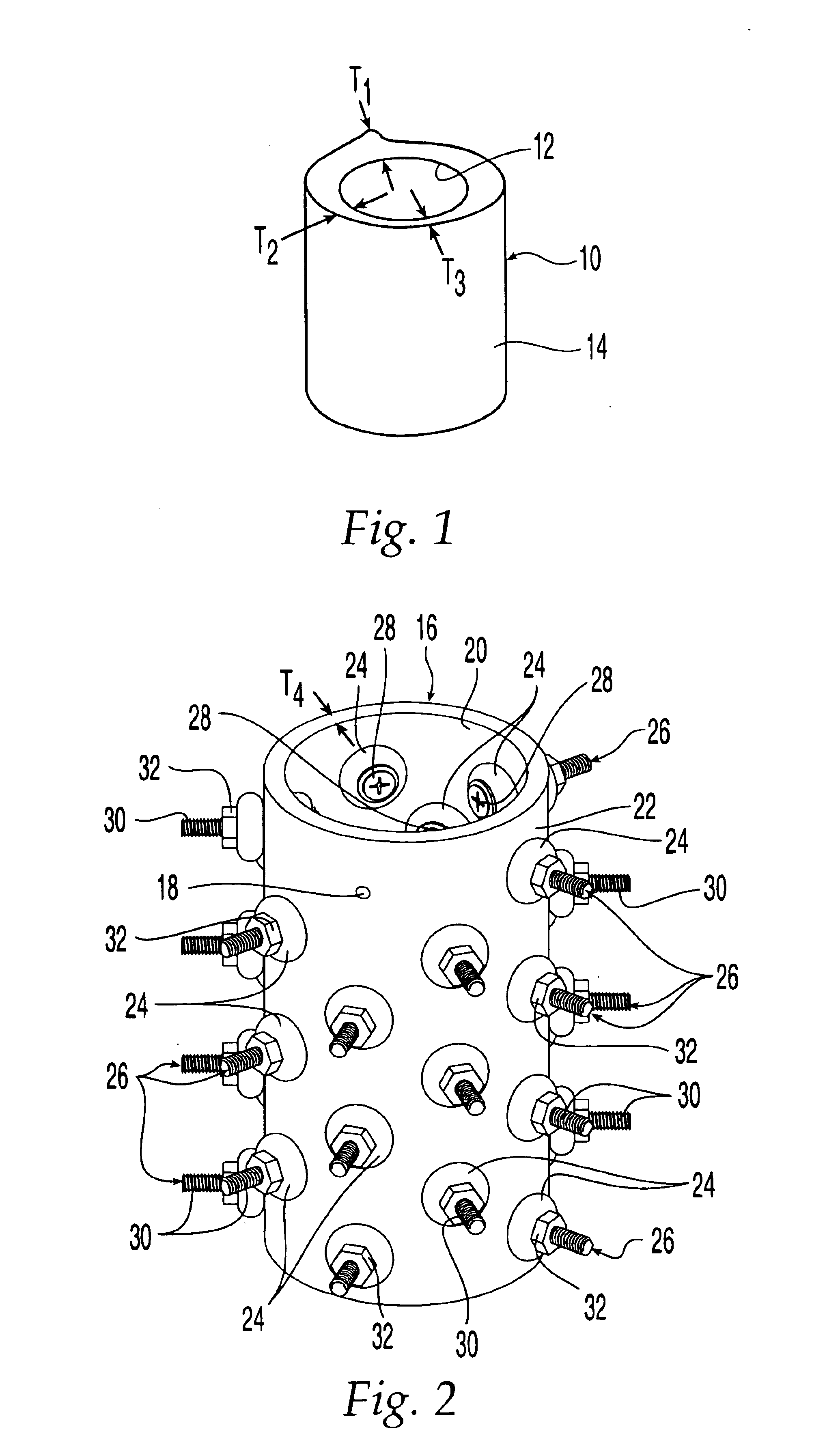
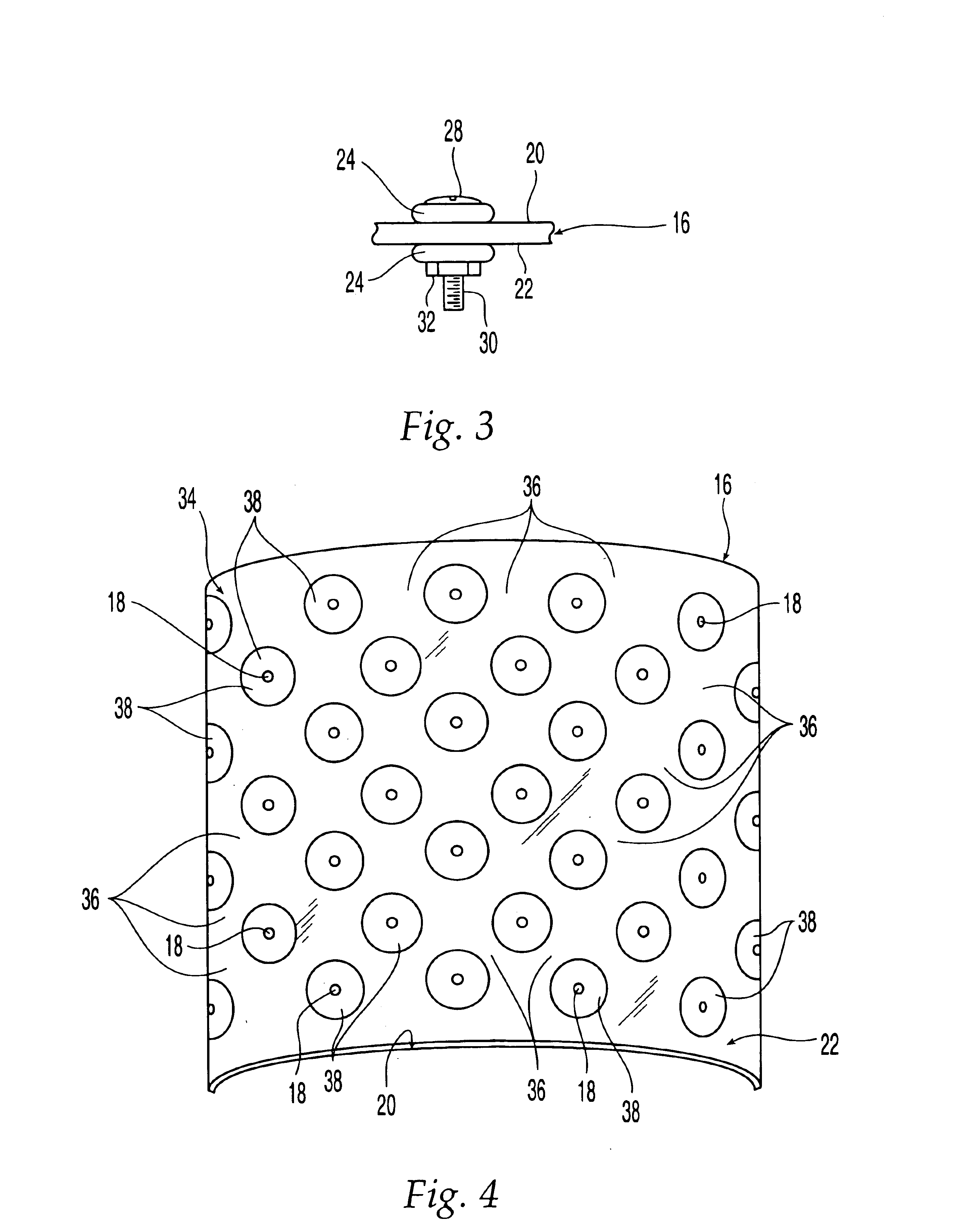
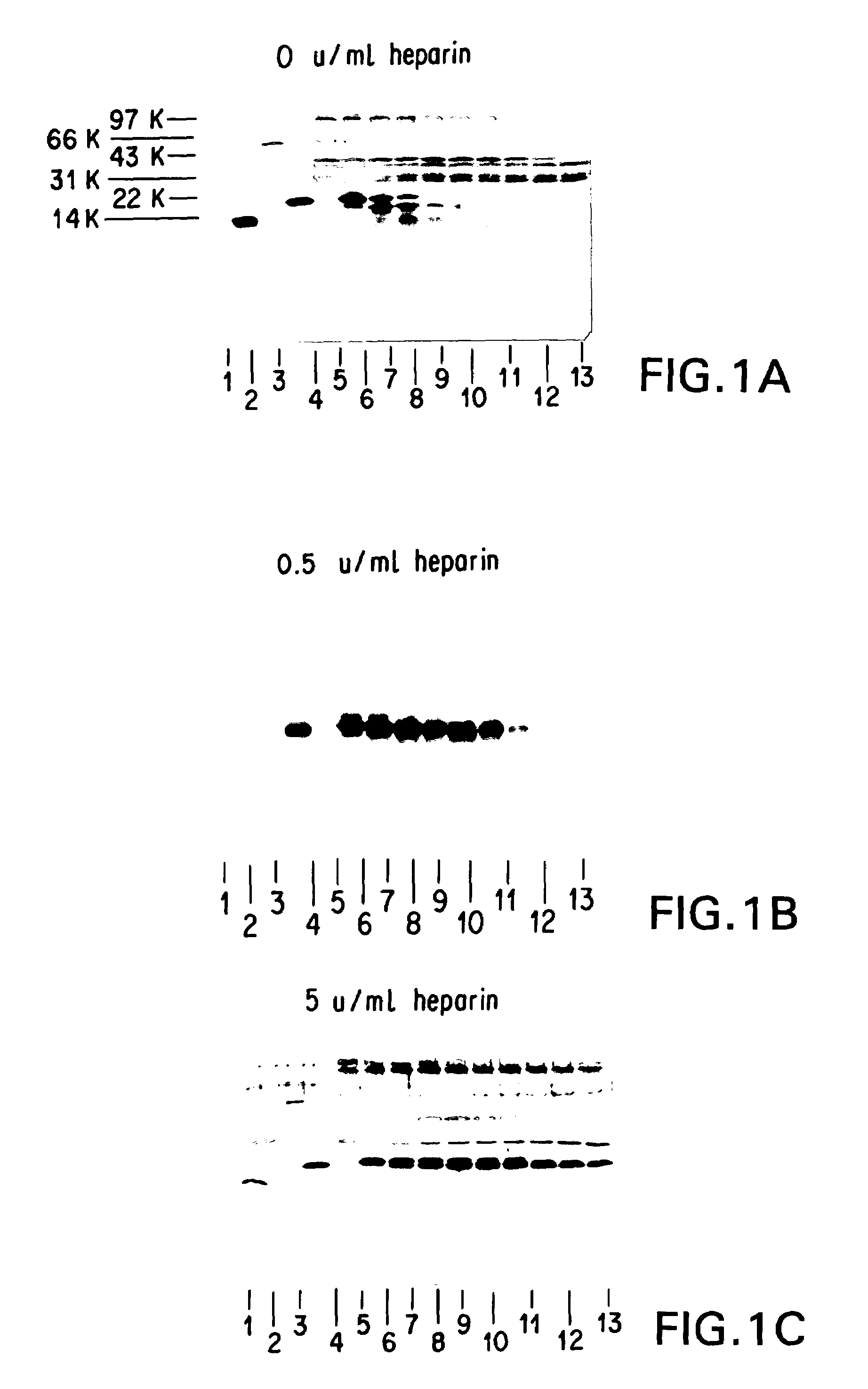
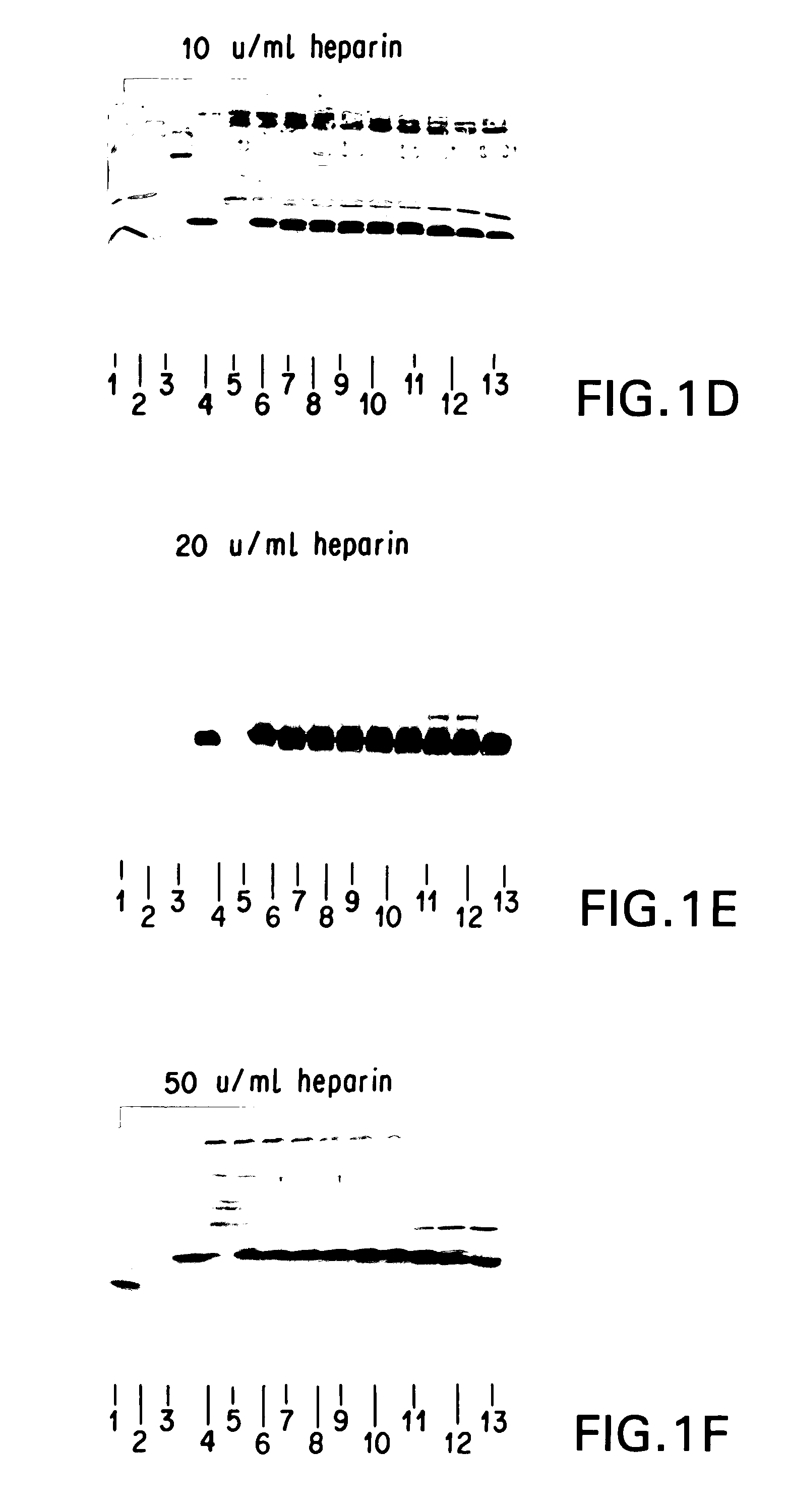
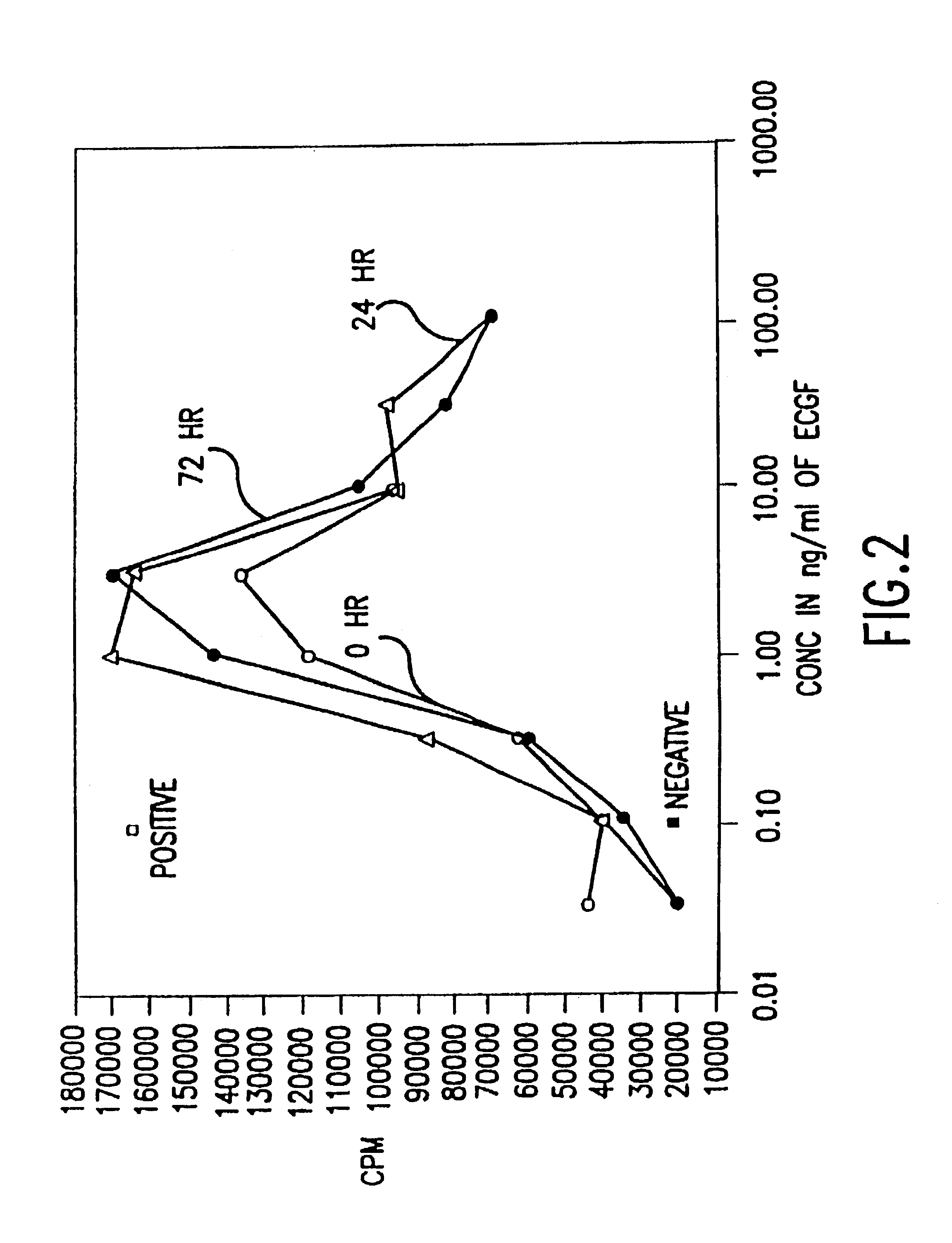
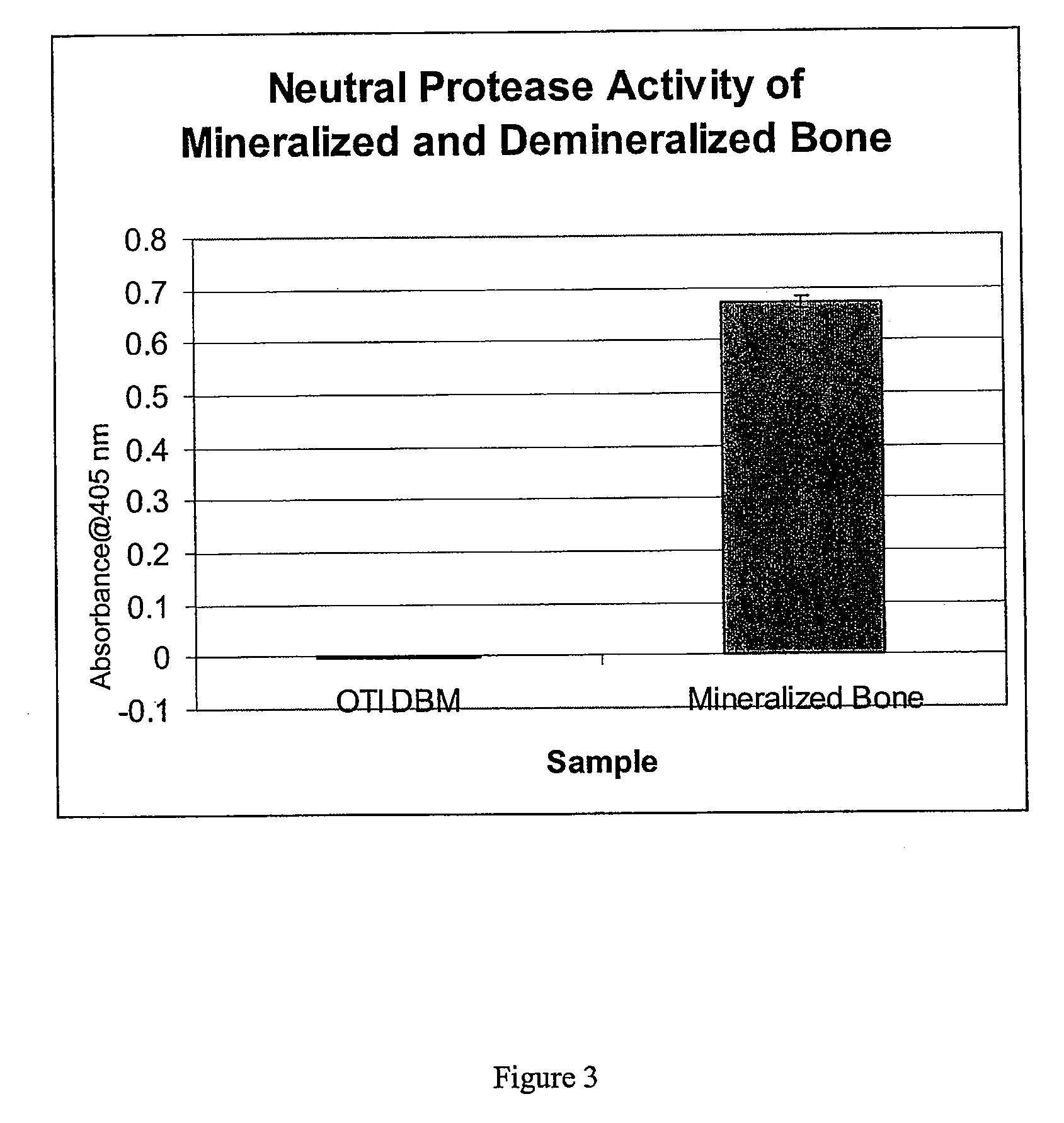
Patents
Literature
114 results about "Demineralized bone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
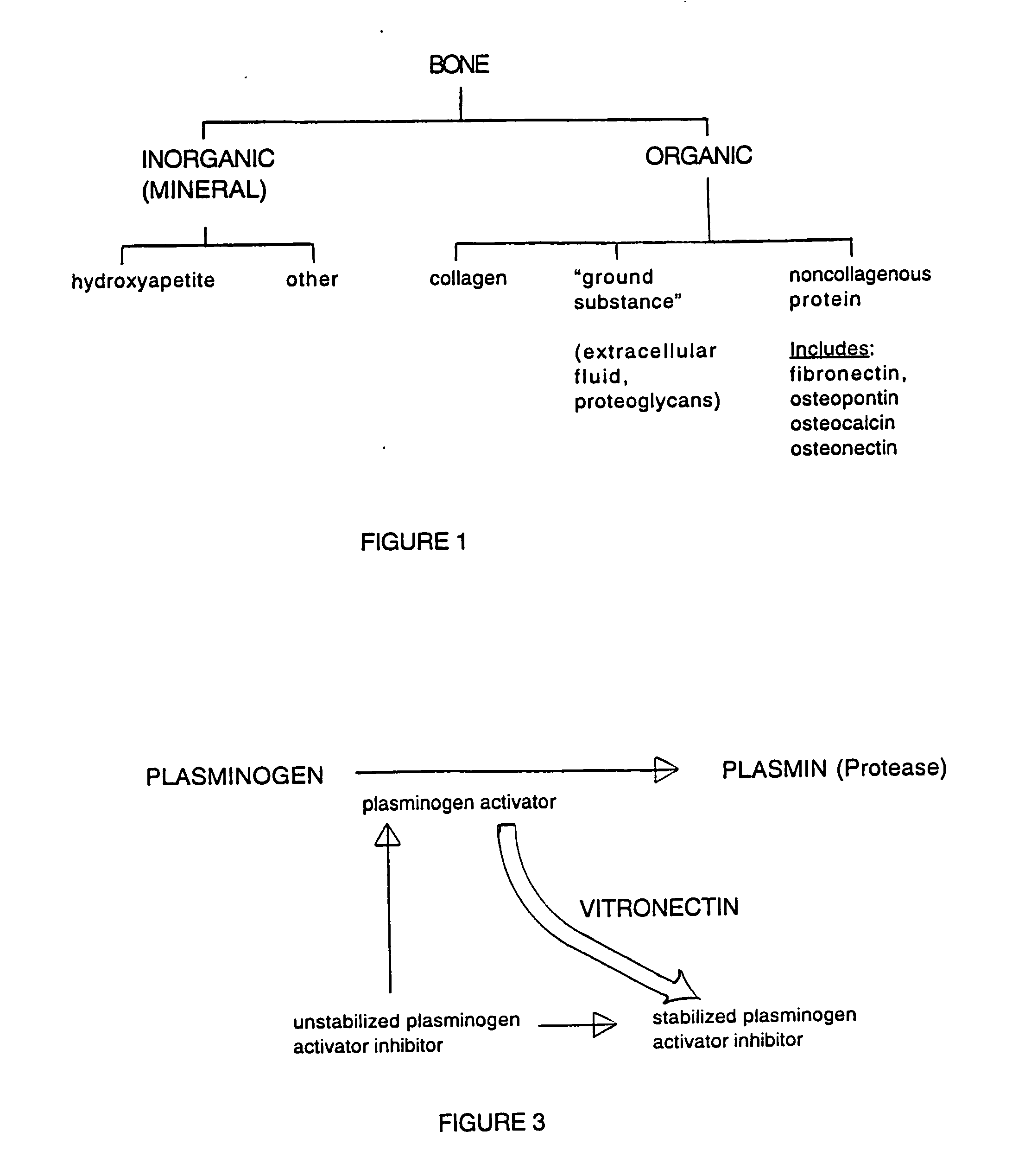
Demineralized Bone Definition. Demineralized bone is bone that has had the calcium removed and is used to make bone tissue more conducive to spinal fusion. Bone morphogenic proteins from demineralized bone are added to a polymer or glycerol substrate to form a product that enhances bone growth.
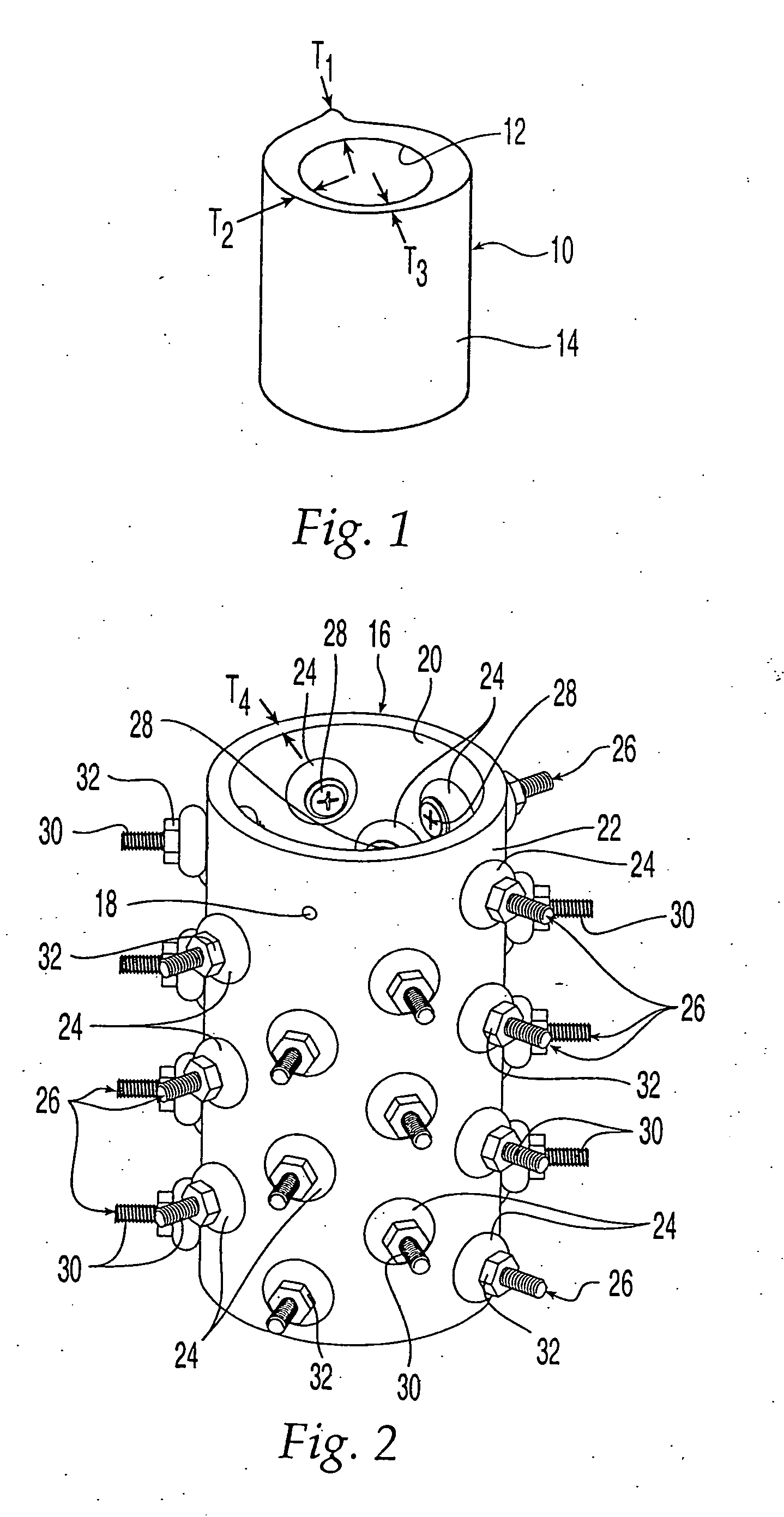
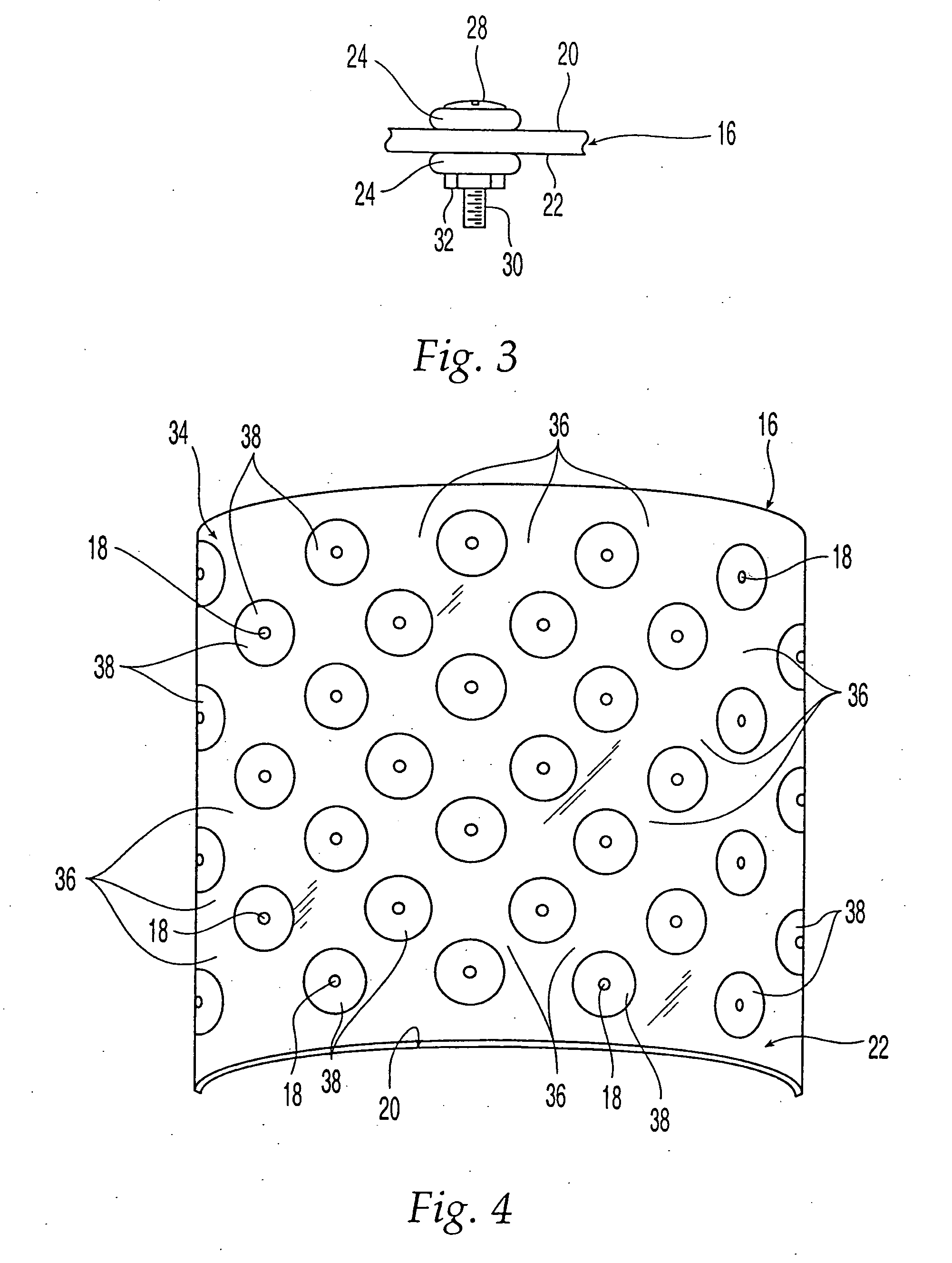
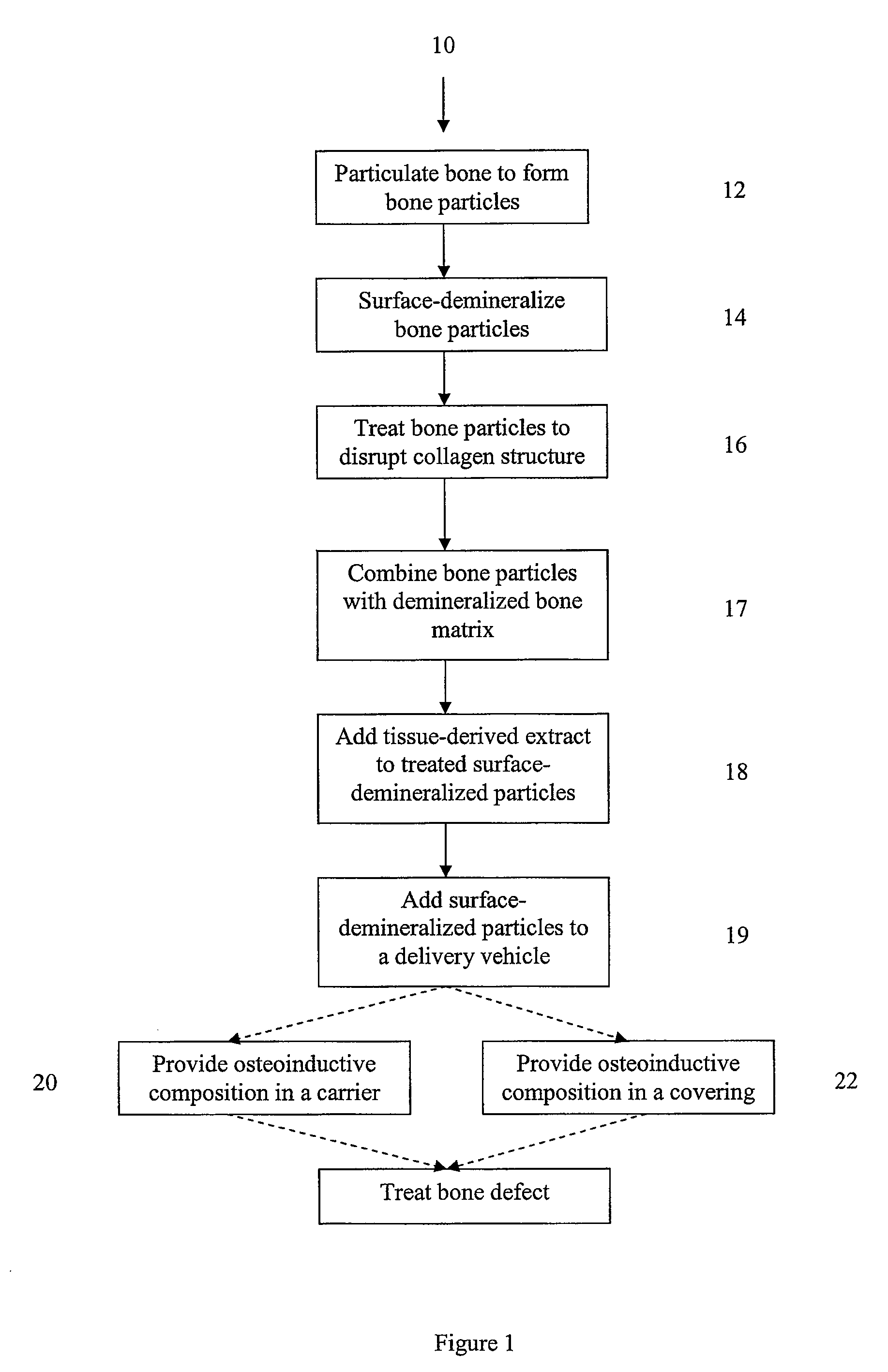
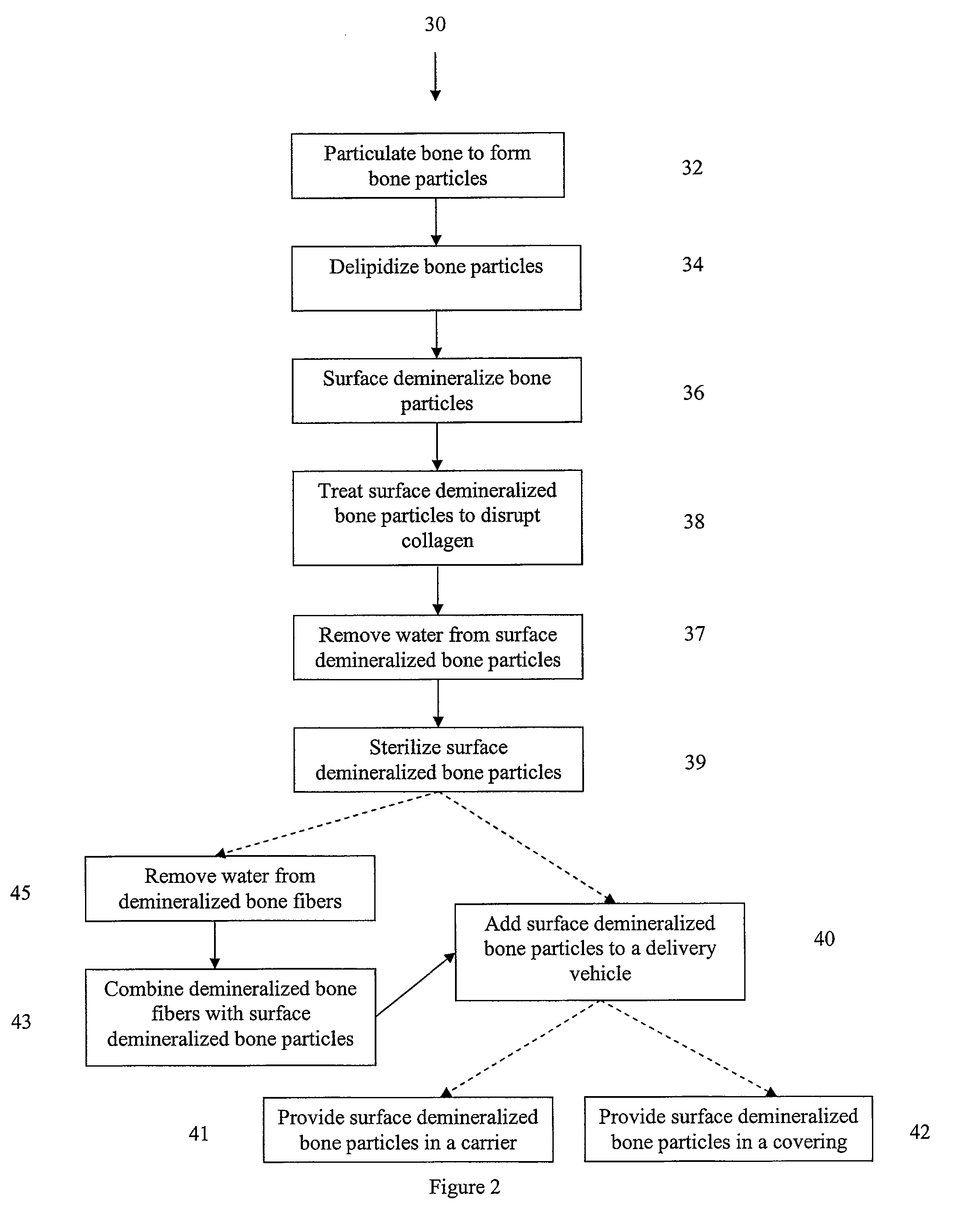
Bone matrix compositions and methods
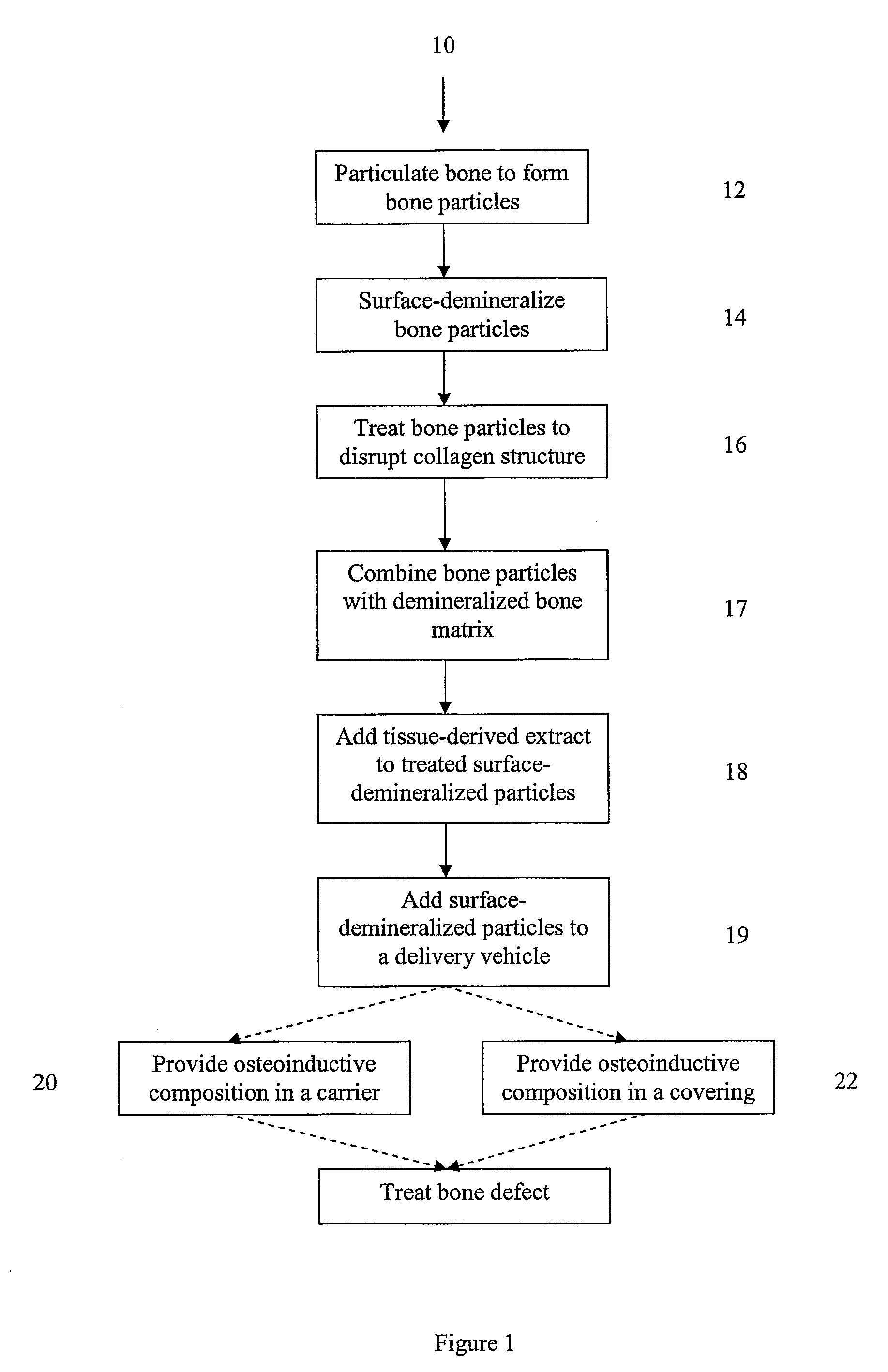
Osteoinductive compositions and implants having increased biological activities, and methods for their production, are provided. The biological activities that may be increased include, but are not limited to, bone forming; bone healing; osteoinductive activity, osteogenic activity, chondrogenic activity, wound healing activity, neurogenic activity, contraction-inducing activity, mitosisinducing activity, differentiation-inducing activity, chemotactic activity, angiogenic or vasculogenic activity, and exocytosis or endocytosis-inducing activity. In one embodiment, a method for producing an osteoinductive composition comprises providing partially demineralized bone, treating the partially demineralized bone to disrupt the collagen structure of the bone, and optionally providing a tissue-derived extract and adding the tissue-derived extract to the partially demineralized bone. In another embodiment, an implantable osteoinductive and osteoconductive composition comprises partially demineralized bone, wherein the collagen structure of the bone has been disrupted, and, optionally, a tissue-derived extract.
Owner:WARSAW ORTHOPEDIC INC
Demineralized bone-derived implants
Selectively demineralized bone-derived implants are provided. In one embodiment, a bone sheet for implantation includes a demineralized field surrounding mineralized regions. In another embodiment, a bone defect filler includes a demineralized cancellous bone section in a first geometry. The first geometry is compressible and dryable to a second geometry smaller than the first geometry, and the second geometry is expandable and rehydratable to a third geometry larger than the second geometry.
Owner:SYNTHES USA
Bone Matrix Compositions and Methods
ActiveUS20070098756A1High activityEasy to addPeptide/protein ingredientsBone implantActive agentOSTEOINDUCTIVE FACTOR
An osteoinductive composition, corresponding osteoimplants, and methods for making the osteoinductive composition are disclosed. The osteoinductive composition comprises osteoinductive factors, such as may be extracted from demineralized bone, and a carrier. The osteoinductive composition is prepared by providing demineralized bone, extracting osteoinductive factors from the demineralized bone, and adding the extracted osteoinductive factors to a carrier. Further additives such as bioactive agents may be added to the osteoinductive composition. The carrier and osteoinductive factors may form an osteogenic osteoimplant. The osteoimplant, when implanted in a mammalian body, can induce at the locus of the implant the full developmental cascade of endochondral bone formation including vascularization, mineralization, and bone marrow differentiation. Also, in some embodiments, the osteoinductive composition can be used as a delivery device to administer bioactive agents.
Owner:WARSAW ORTHOPEDIC INC
Composition for filling bone defects
InactiveUS7019192B2Low tensile strengthIncrease delayBiocideSurgical adhesivesSodium phosphatesDemineralized bone
The invention is directed toward a formable bone composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of demineralized lyophilized allograft bone particles. The particle size ranges from about 0.1 mm to about 1.0 cm and is mixed in a hydrogel carrier containing a sodium phosphate saline buffer, the hydrogel component of the carrier ranging from about 1.0 to 5.0% of the composition and a pH between 6.8–7.4 with one or more additives of a cellular material, growth factor, demineralized bone chips or mineralized bone chips.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
Expandable osteoimplant
InactiveUS20080091270A1Improve conformityIncrease contactBone implantSpinal implantsLamina terminalisIncrease size
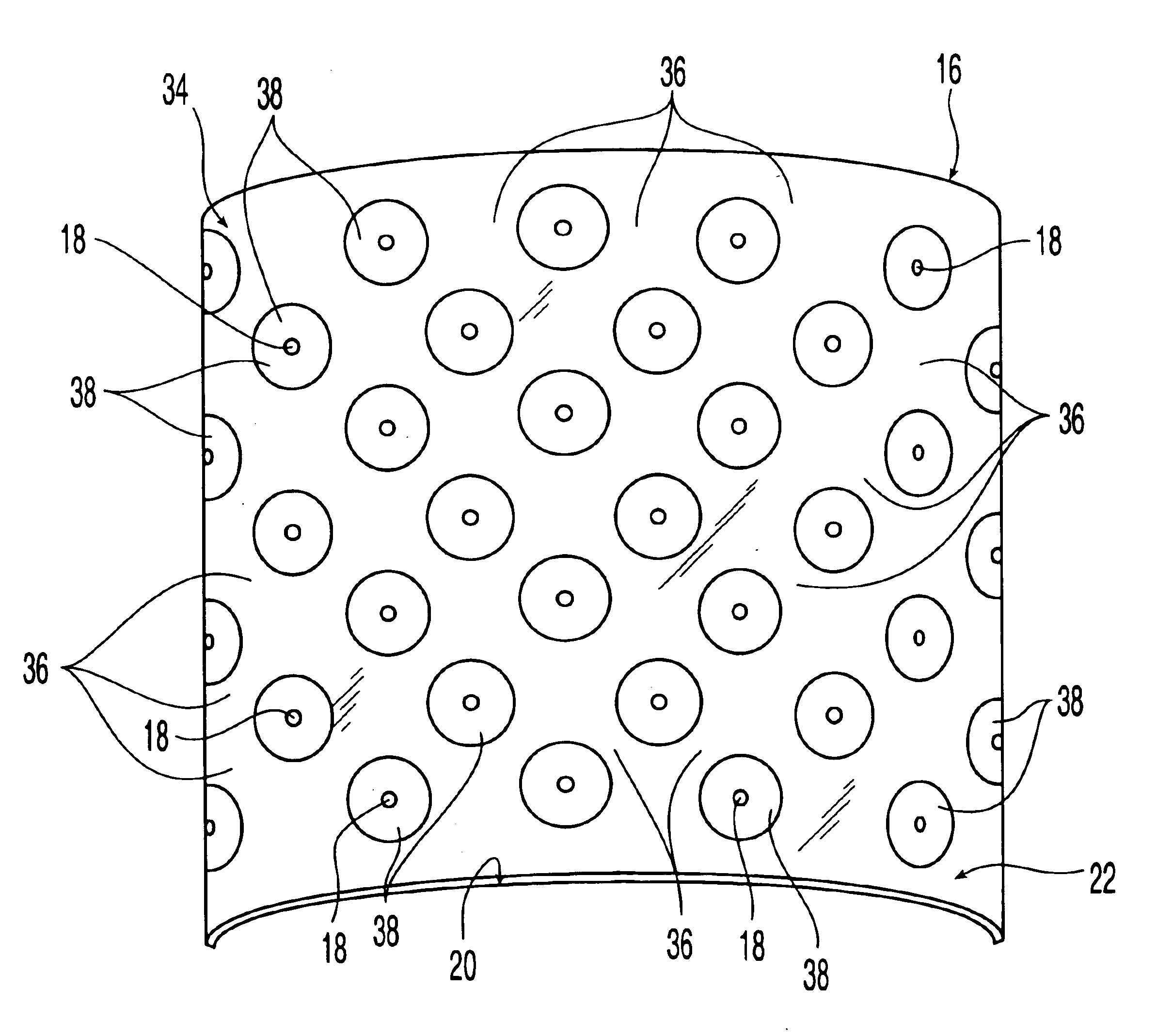
An osteoimplant comprising an expandable, biocompatible material. The expandable material may be demineralized bone particles such as demineralized cancelous chips or demineralized cortical fibers or may be another material such as a polymer. The osteoimplant has a first state and a second expanded state. The osteoimplant may be used with another device or on its own. The osteoimplant may be inserted into a device such as an intervertebral body fusion device in the compressed state. The osteoimplant may be rehydrated to expand to an increased size, for example as far as permitted by the confines of the intervertebral body fusion device and spinal endplates, thereby aiding in greater vertebral endplate contact and conformity in spinal surgery.
Owner:WARSAW ORTHOPEDIC INC
Composition for repair of defects in osseous tissues
ActiveUS7498041B2Pharmaceutical delivery mechanismUnknown materialsTissue repairConnective tissue fiber
Tissue repair compositions, particularly bone repair compositions, containing demineralized bone fragments and homogenized connective tissues. The compositions can be used in the form of an injectable gel, an injectable paste, a paste, a putty, or a rehydratable freeze-dried form.
Owner:LIFENET HEALTH
Method and apparatus for repairing bone
InactiveUS20060280803A1Not easily dislodgedIncrease resistanceBone implantUnknown materialsDemineralized boneAnimal subject
Formed compositions for application to a bone surface of a human or animal subject, comprising: a bone material; and a carrier comprising denatured demineralized bone, where the composition is formed into a shape suitable for administration to the bone. Methods are provided for making formed compositions for application to a bone surface of a human or animal subject comprise mixing a demineralized bone and water; heating the mixture to form a carrier; mixing the carrier with bone to form a moldable composition; and molding the moldable composition to produce a formed composition. Several apparatuses are provided in which to hydrate the formed bone composition. Methods of hydrating a formed bone composition are also provided.
Owner:BIOMET MFG CORP
Method and apparatus for engineered regenerative biostructures such as hydroxyapatite substrates for bone healing applications
An engineered regenerative biostructure (erb) for implantation into a human body as a bone substitute, which includes an internal microstructure, mesostructure and / or macrostructure to provide improved bone in-growth, and methods for making the erb. Under one aspect of the invention, the biostructure has resorbable and nonresorbable regions. Under another aspect of the invention, the biostructure is constructed of hydroxyapatite, tricalcium phosphate and / or demineralized bone. Under yet another aspect of the invention, the porous biostructure is partially or fully infused with a resorbable, nonresorbable or dissolvable material.
Owner:THEKEN SURGICAL LLC
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39321E1Decreasing thrombogenicityLow antigenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant dressing, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, antiinflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant dressing.
Owner:AMERICAN NAT RED CROSS
Bone Matrix Compositions and Methods
ActiveUS20070110820A1High activityEasy to addPeptide/protein ingredientsImmunoglobulinsOSTEOINDUCTIVE FACTORBone implant
Owner:WARSAW ORTHOPEDIC INC
Apparatus and methods for vertebral augmentation
ActiveUS20070093846A1Increase heightReduce compressionInternal osteosythesisJoint implantsSpinal columnSpinal curvature
Apparatuses and methods of treating a collapsed vertebra may include inserting cannulae into the damaged vertebral body and one or more adjacent vertebral bodies and repositioning the one or more adjacent vertebral bodies by manipulating one or more of the cannulae. Before or after repositioning the vertebral bodies, the method may further include passing through the cannula and into the damaged vertebra a disruption tool for fracturing the damaged vertebral body. Once the cortical bone is broken or otherwise disrupted, the height of the damaged vertebral body may be restored, for example by removing the disruption tool and inserting through the cannula another tool, implant, device and / or material to restore the height of the vertebral body and to restore normal spinal curvature in the affected area. Bone cement, bone chips, demineralized bone or other grafting material or filler may be added with or without an implanted device to augment the damaged vertebral body.
Owner:SYNTHES USA
Methods for treating wound tissue and forming a supplemented fibrin matrix
InactiveUS7196054B1Low antigenicityDecreasing thrombogenicityOrganic active ingredientsSurgical adhesivesTissue sealantVascular dilatation
Owner:AMERICAN NAT RED CROSS
Osteoinductive demineralized cancellous bone
ActiveUS20090155378A1Increase osteoinductive activityHigh activityBone implantUnknown materialsBone implantDemineralized bone
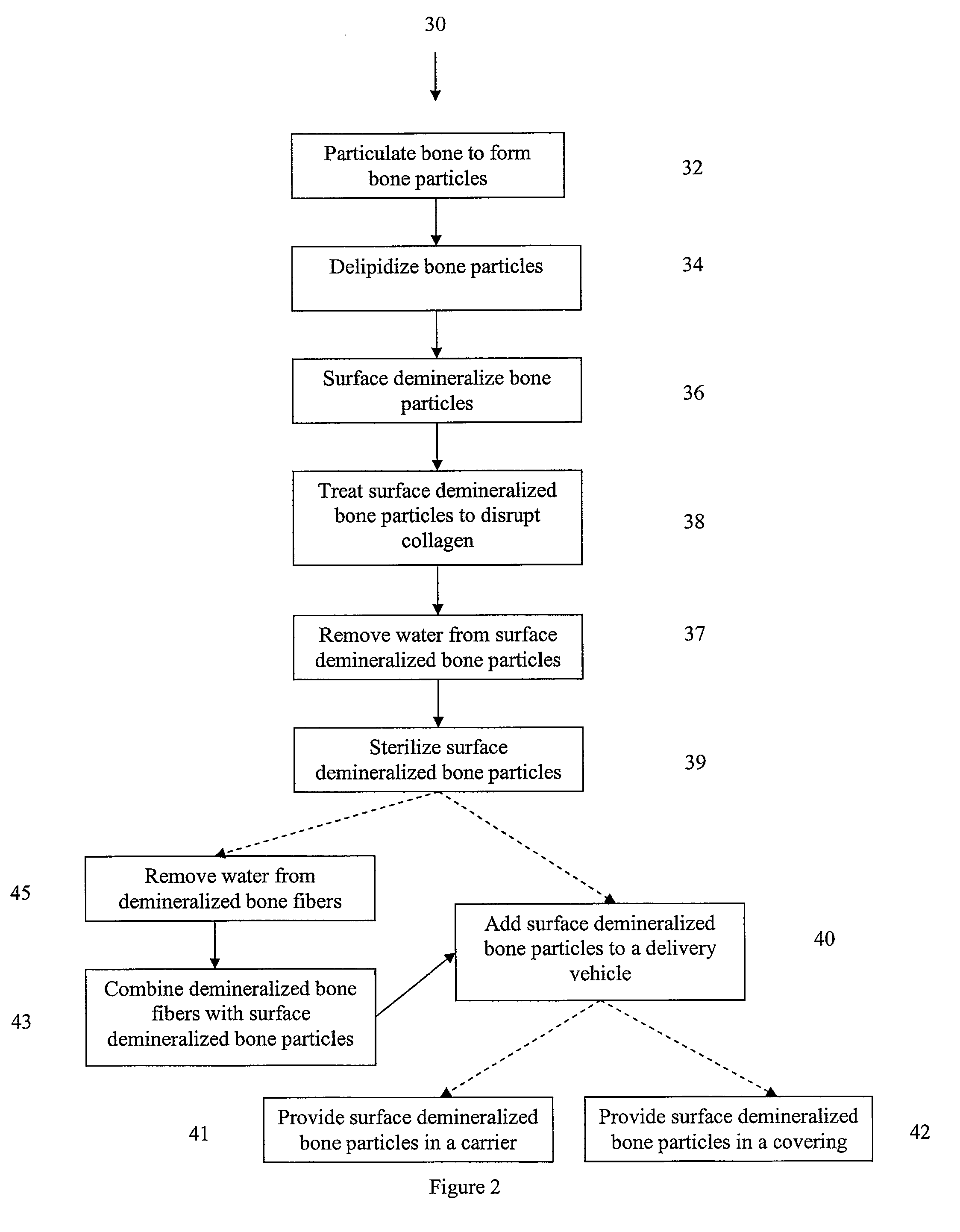
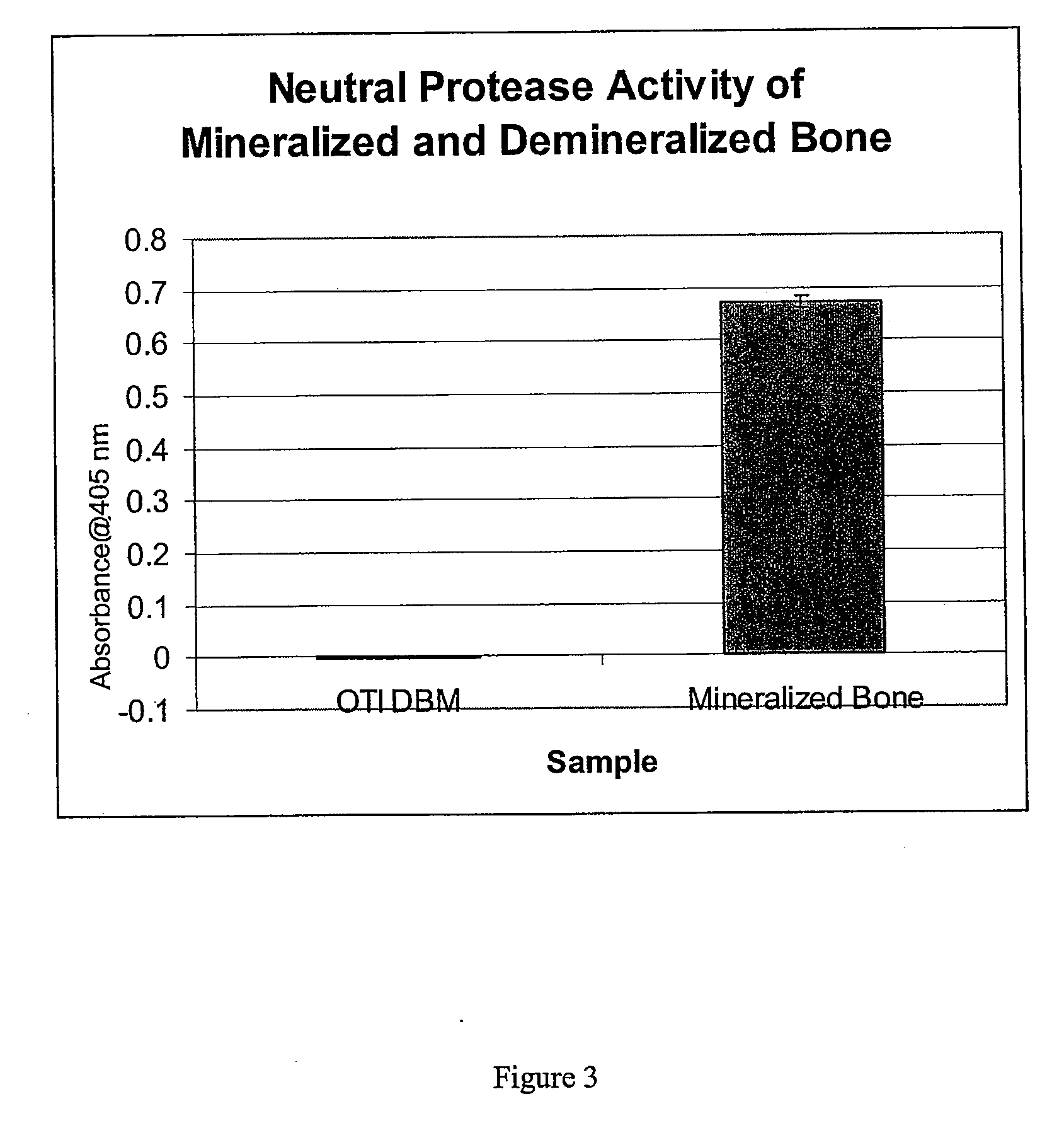
An osteoinductive demineralized bone matrix, corresponding osteoimplants, and methods for making the osteoinductive demineralized bone matrix are disclosed. The osteoinductive demineralized bone matrix may be prepared by providing demineralized bone and altering the collagenous structure of the bone. The osteoinductive demineralized bone matrix may also be prepared by providing demineralized bone and compacting the bone, for example via mechanical compaction, grinding into a particulate, or treatment with a chemical. Additives such as growth factors or bioactive agents may be added to the osteoinductive demineralized bone matrix. The osteoinductive demineralized bone matrix may form an osteogenic osteoimplant. The osteoimplant, when implanted in a mammalian body, may induce at the locus of the implant the full developmental cascade of endochondral bone formation including vascularization, mineralization, and bone marrow differentiation. The osteoinductive demineralized bone matrix may also be used as a delivery device to administer bioactive agents.
Owner:WARSAW ORTHOPEDIC INC
Resorbable bone graft materials
Ceramic materials operable to repair a defect in bone of a human or animal subject comprising a porous ceramic scaffold having a bioresorbable coating, and a carrier comprising denatured demineralized bone. The ceramic may contain a material selected from the group consisting of hydroxyapatite, tricalcium phosphate, calcium phosphates, calcium carbonates, calcium sulfates, and combinations thereof. The compositions may also contain a bone material selected from the group consisting of: bone powder, bone chips, bone shavings, and combinations thereof. The bioresorbable coating may be, for example, demineralized bone matrix, gelatin, collagen, hyaluronic acid, chitosan, polyglycolic acid, polylactic acid, polypropylenefumarate, polyethylene glycol, or mixtures thereof.
Owner:BIOMET MFG CORP
Cohesive demineralized bone compositions
ActiveUS7582309B2Keep shapeEnhance bone ingrowth and remodelingPowder deliverySurgical adhesivesFiberDemineralized bone matrix
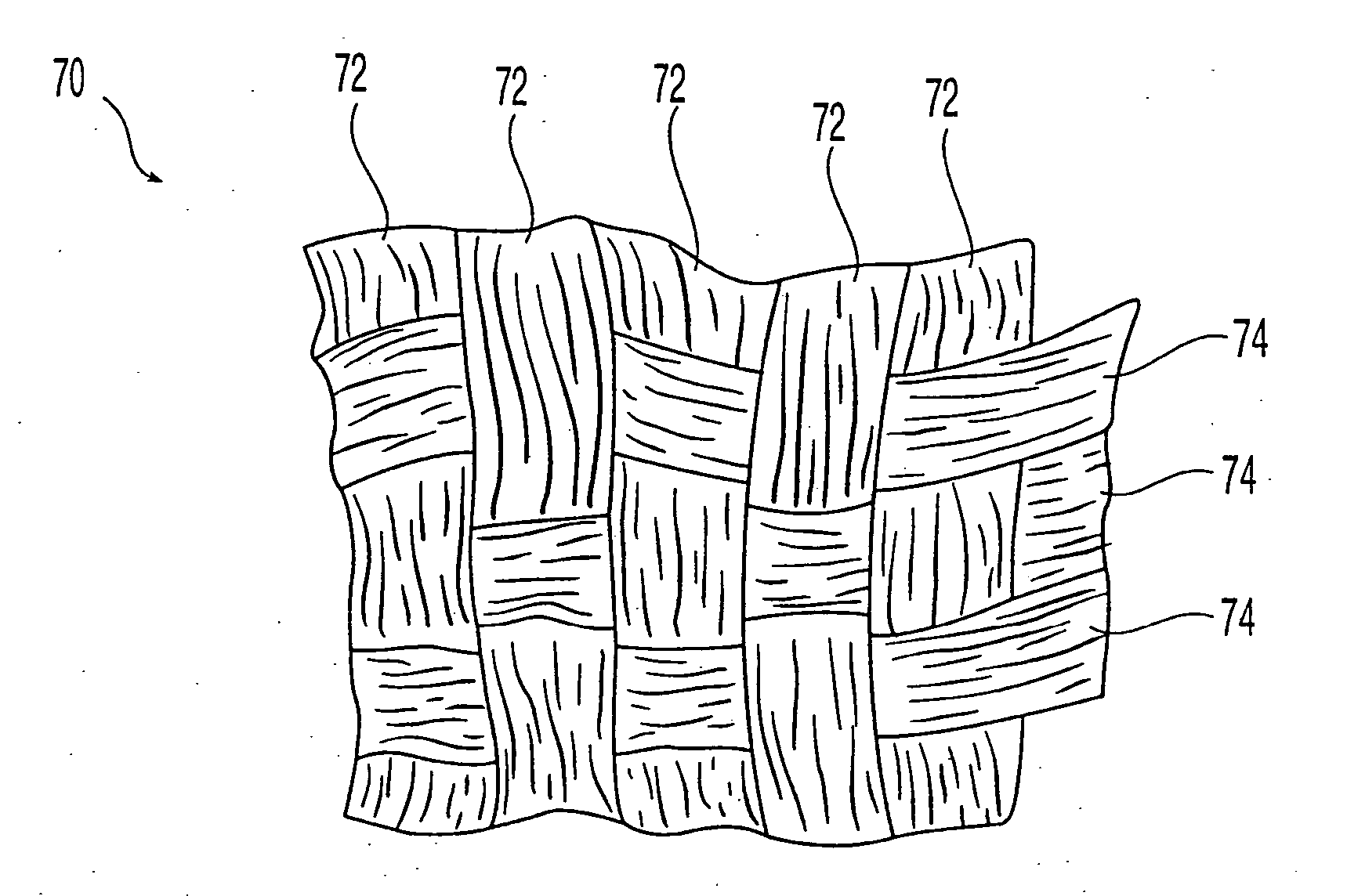
Demineralized bone matrix fibers and a demineralized bone matrix composition are provided. The demineralized bone matrix fibers have an average fiber length in the range from about 250 μm to about 2 mm and an aspect ratio of greater than about 4. The demineralized bone matrix composition includes demineralized bone matrix fibers and a biocompatible liquid in an amount to produce a coherent, formable mass. The formable mass retains its cohesiveness when immersed in a liquid. Methods for making the demineralized bone matrix fibers and composition are also provided.
Owner:ETEX
Method of making demineralized bone particles
InactiveUS7323193B2High yieldConnective tissue peptidesUnknown materialsDemineralized boneBone particle
Demineralized bone particles are obtained by demineralizing whole bone and thereafter subdividing the demineralized bone to provide the demineralized bone particles.
Owner:WARSAW ORTHOPEDIC INC
Tissue repair compositions and methods for their manufacture and use
An osteogenic composition is prepared by a process including the steps of subjecting demineralized bone to an extraction medium to produce an insoluble extraction product and a soluble extraction product, separating the insoluble extraction product and the soluble extraction product, drying the soluble extraction product to remove all or substantially all of the moisture in the soluble extraction product, and combining the dried soluble extraction product of step c) with demineralized bone particles. Preferably, the process does not involve heating.
Owner:ISOTIS ORTHOBIOLOGICS
Bone graft material incorporating demineralized bone matrix and lipids
InactiveUS6565884B2Good osteoinductivityEasy to operateBiocidePowder deliveryHydrophilic polymersVitamin C
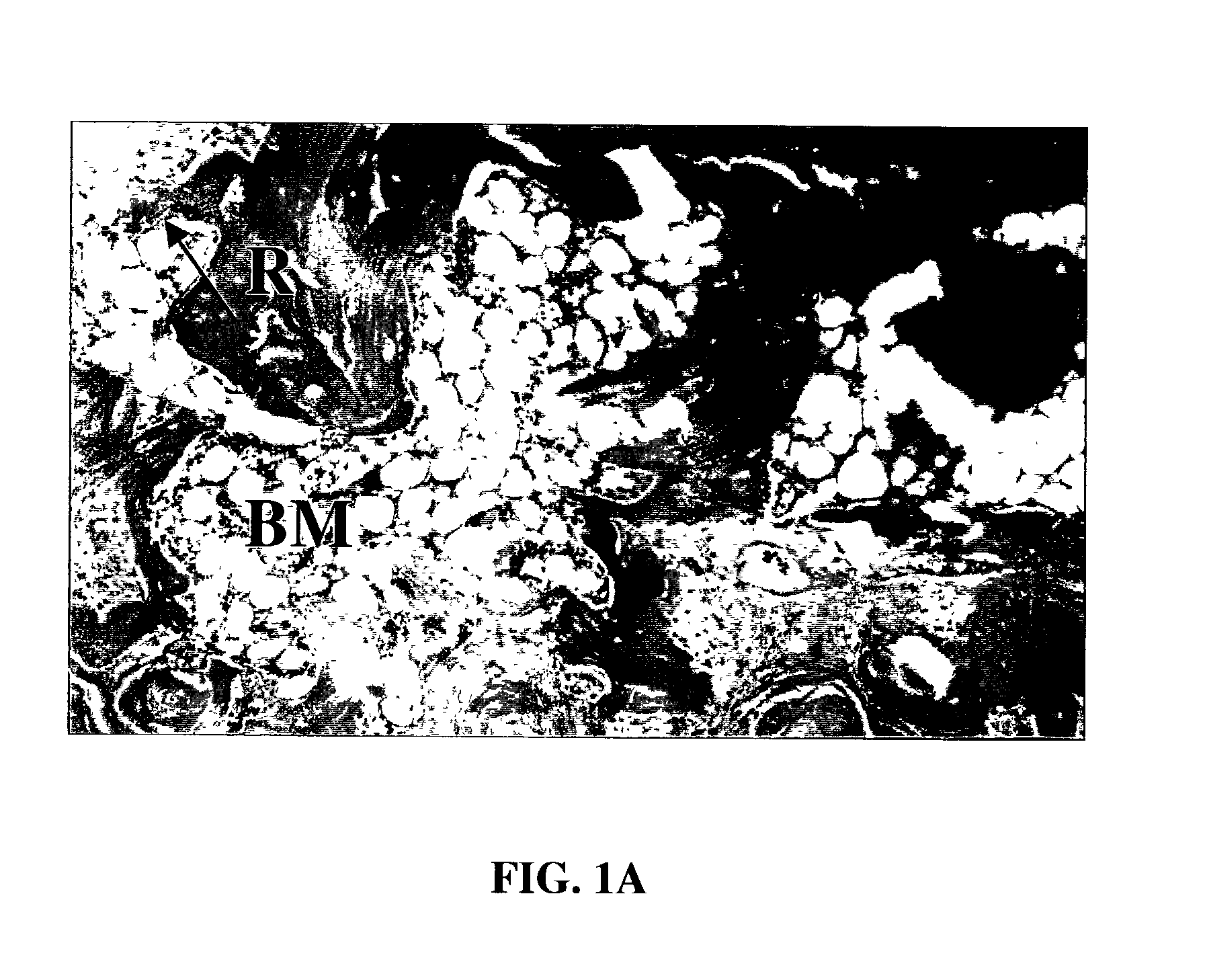
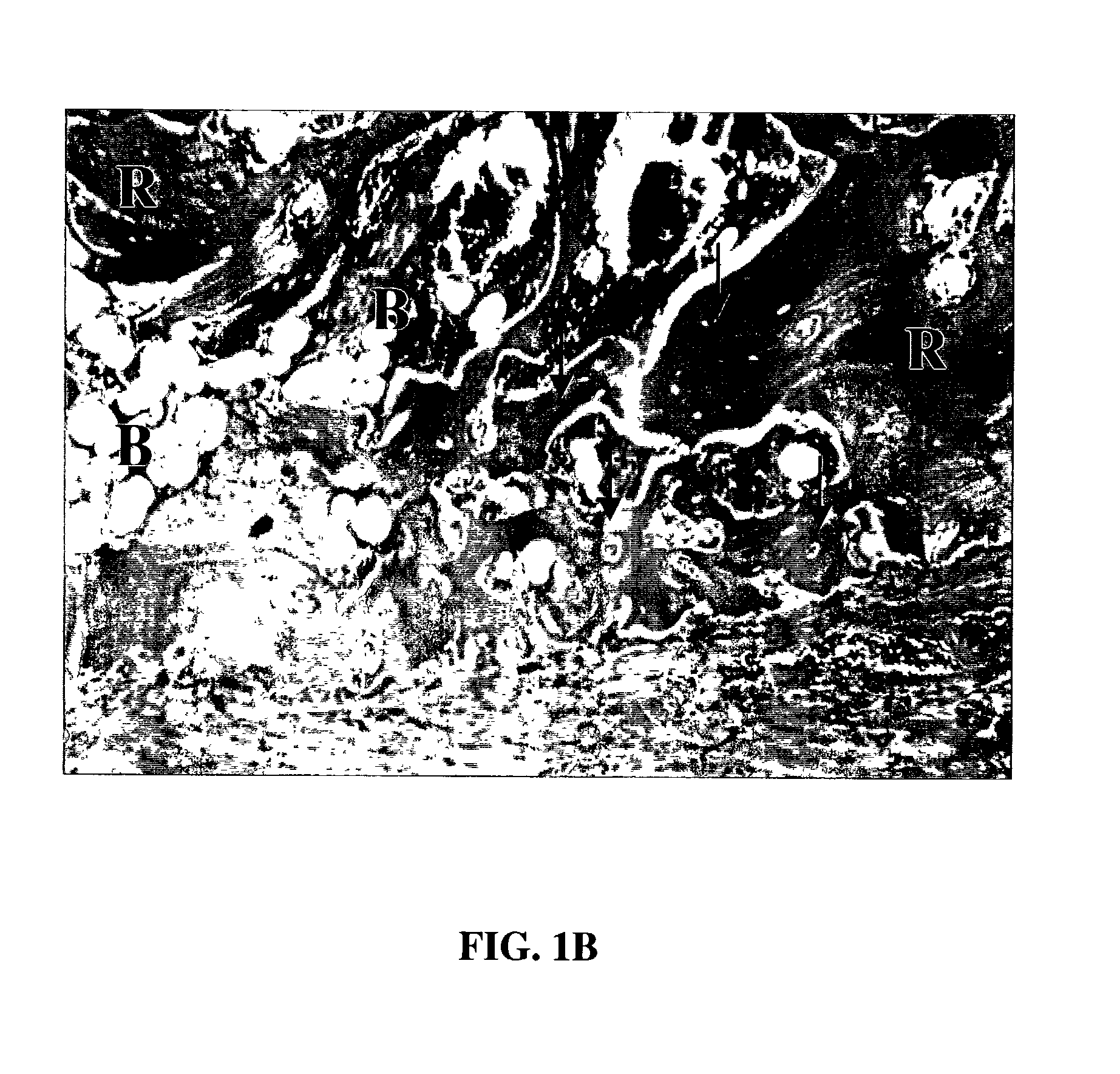
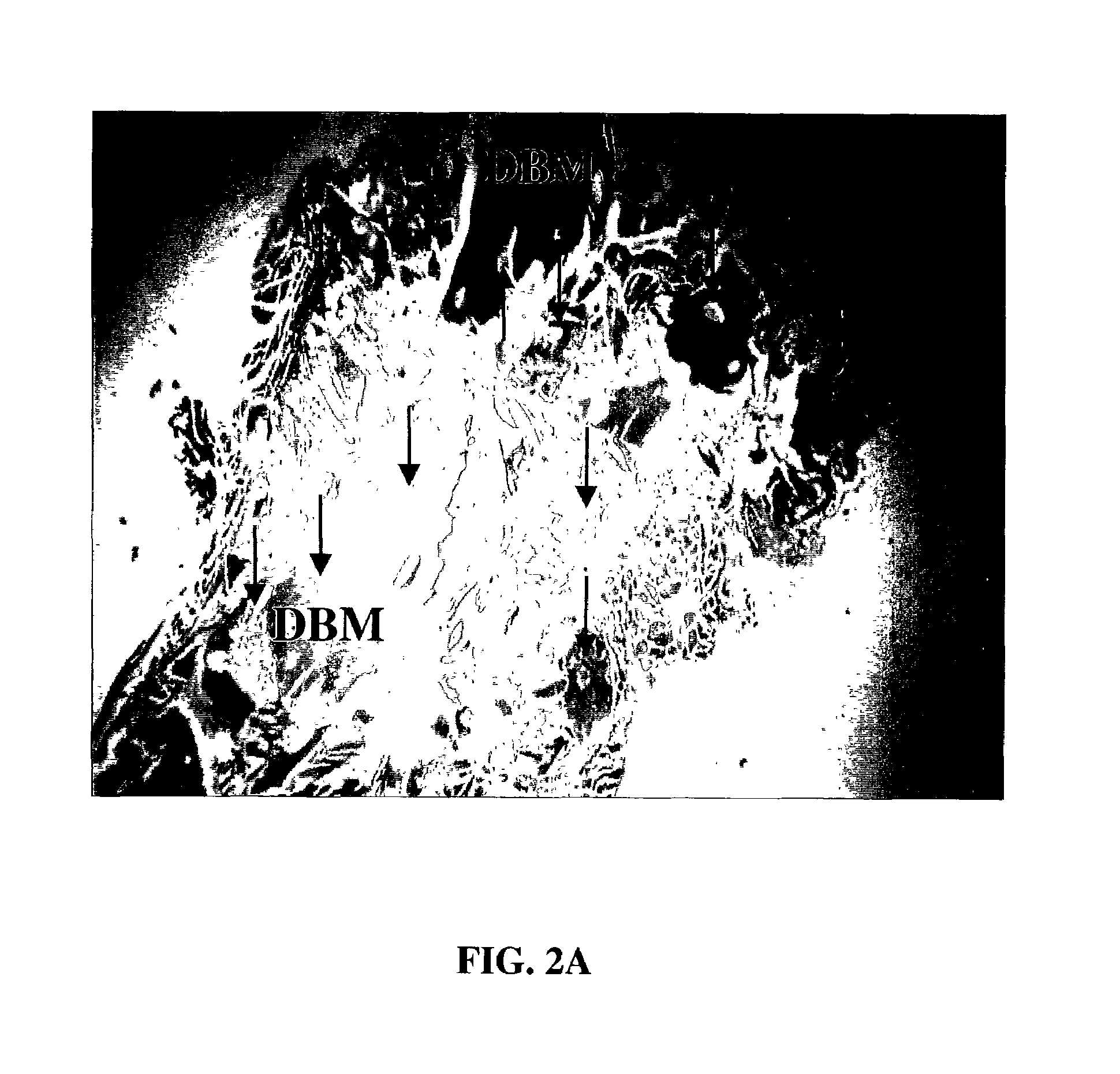
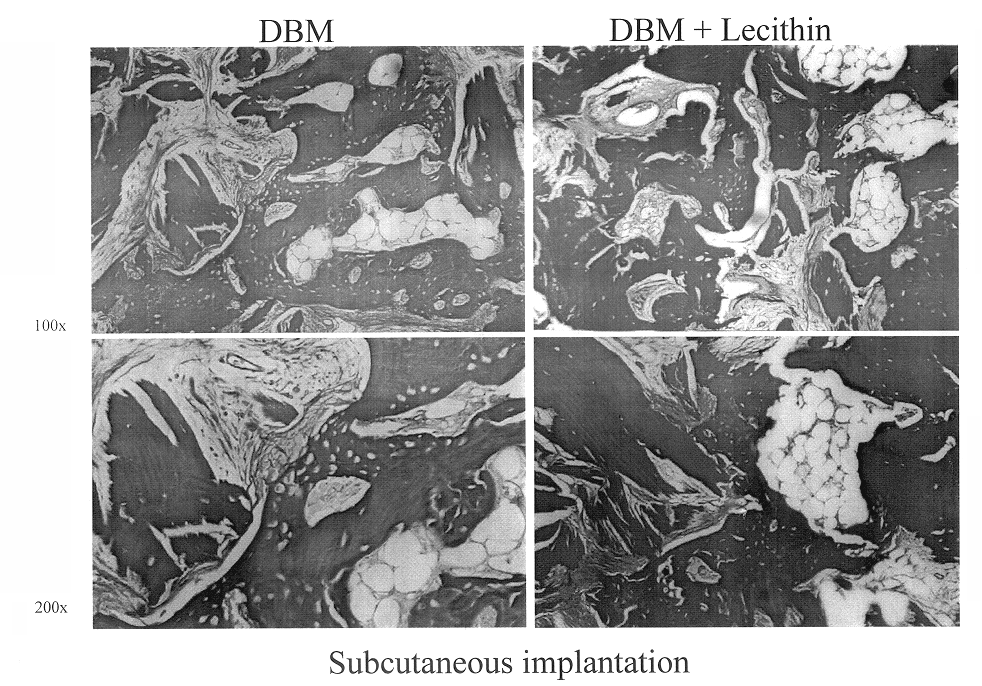
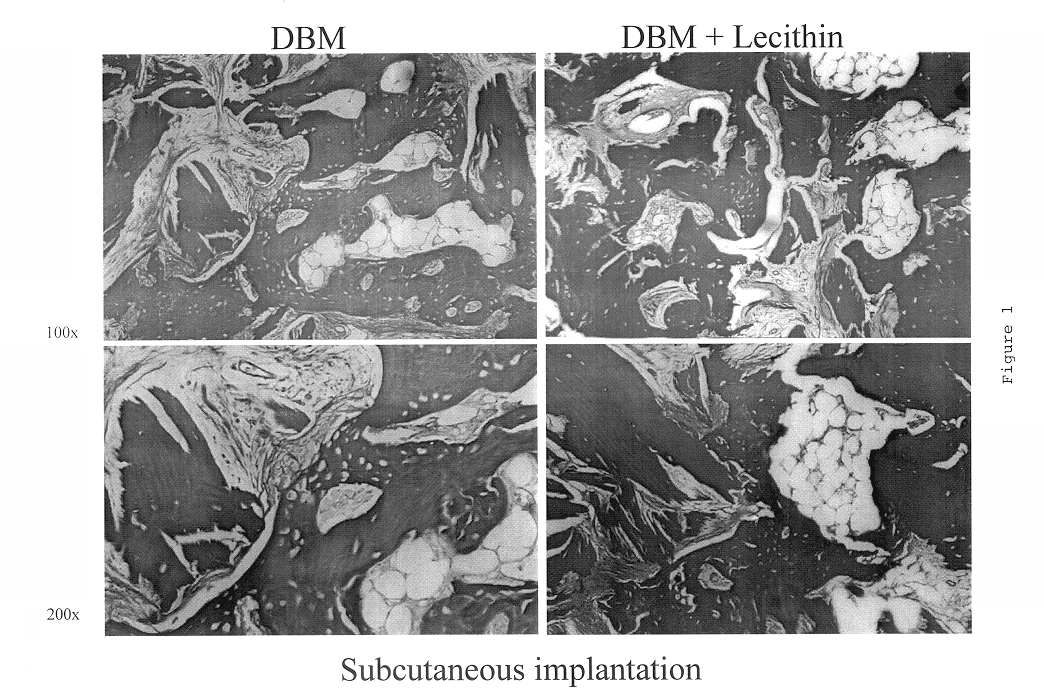
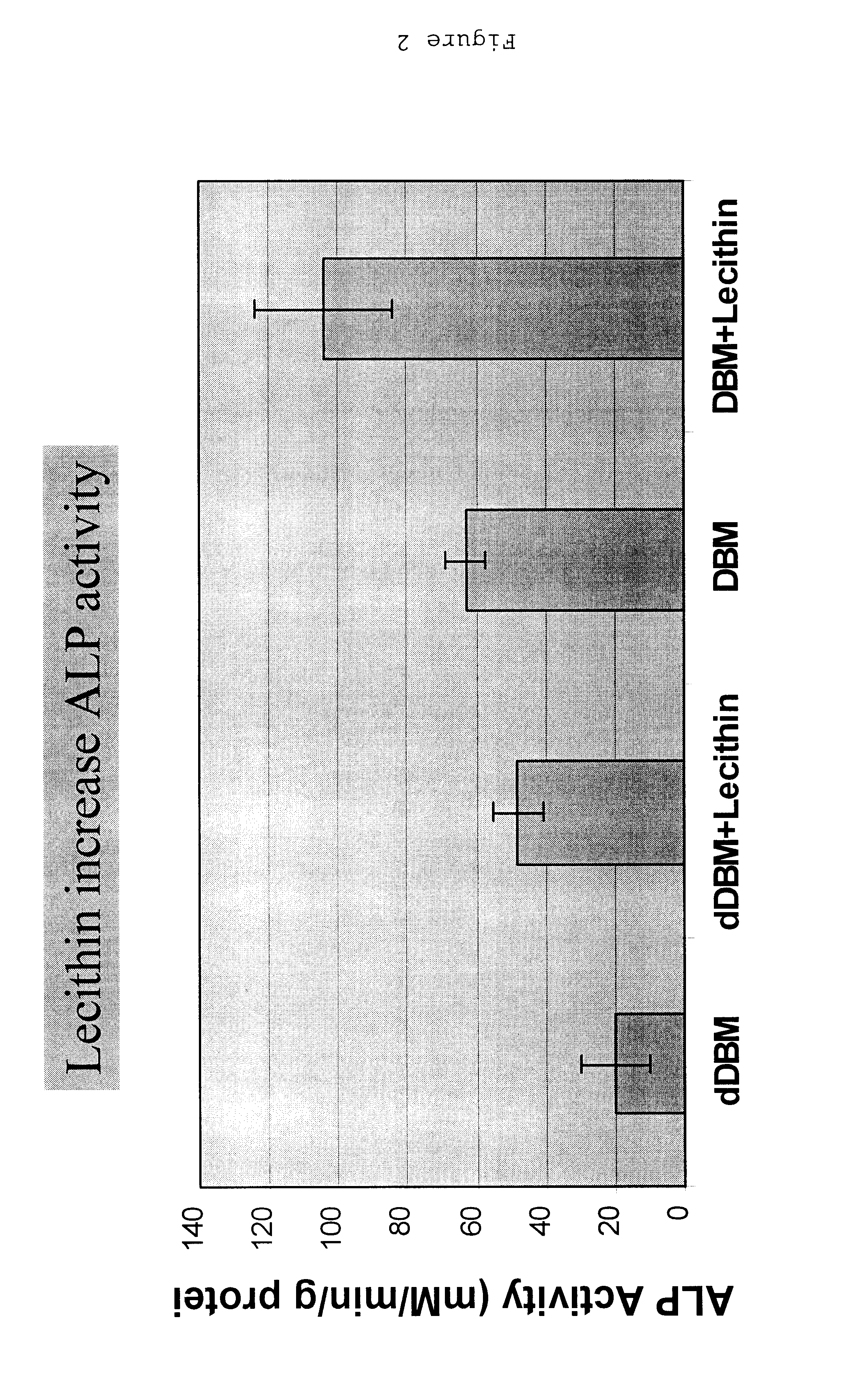
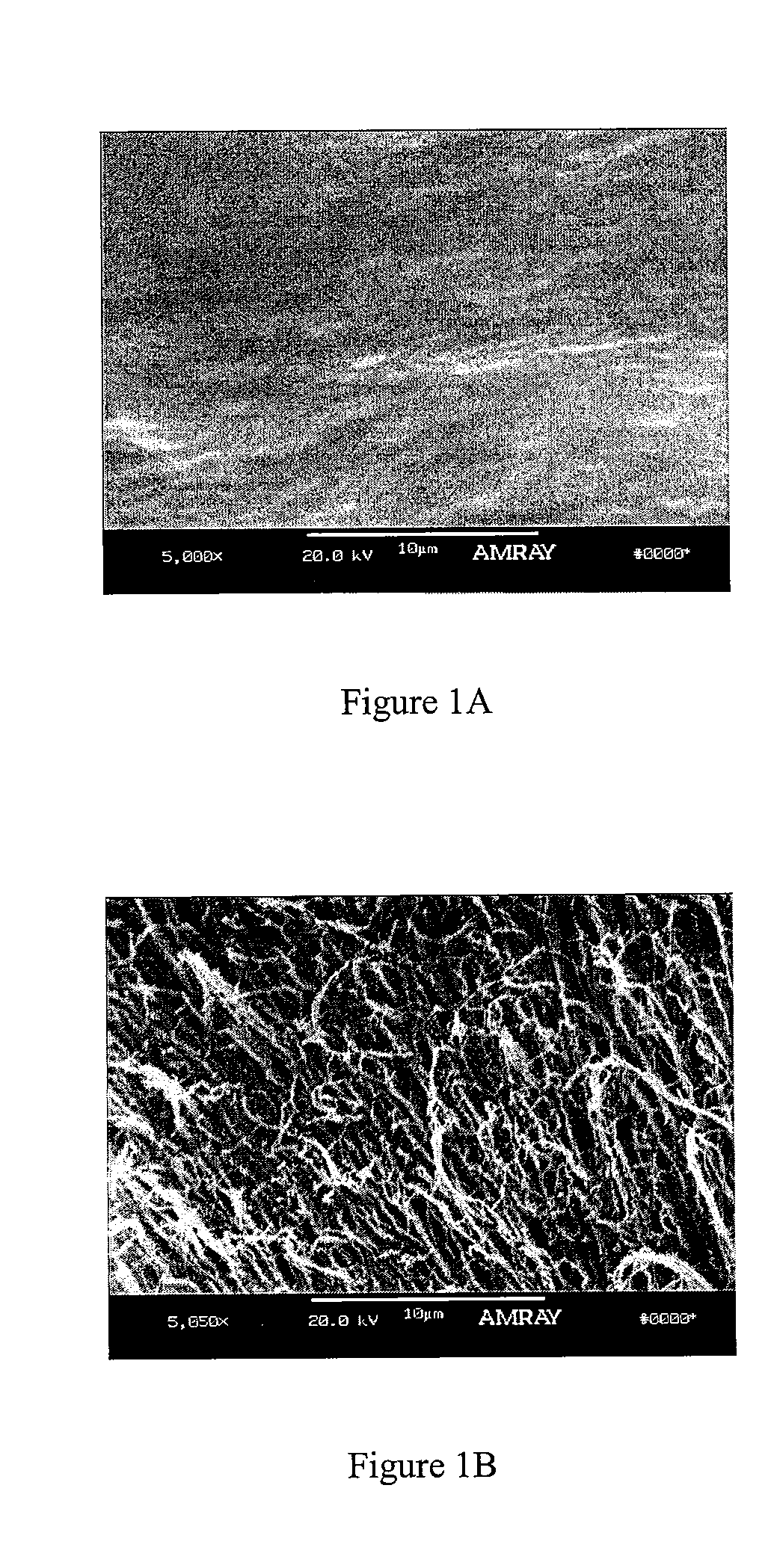
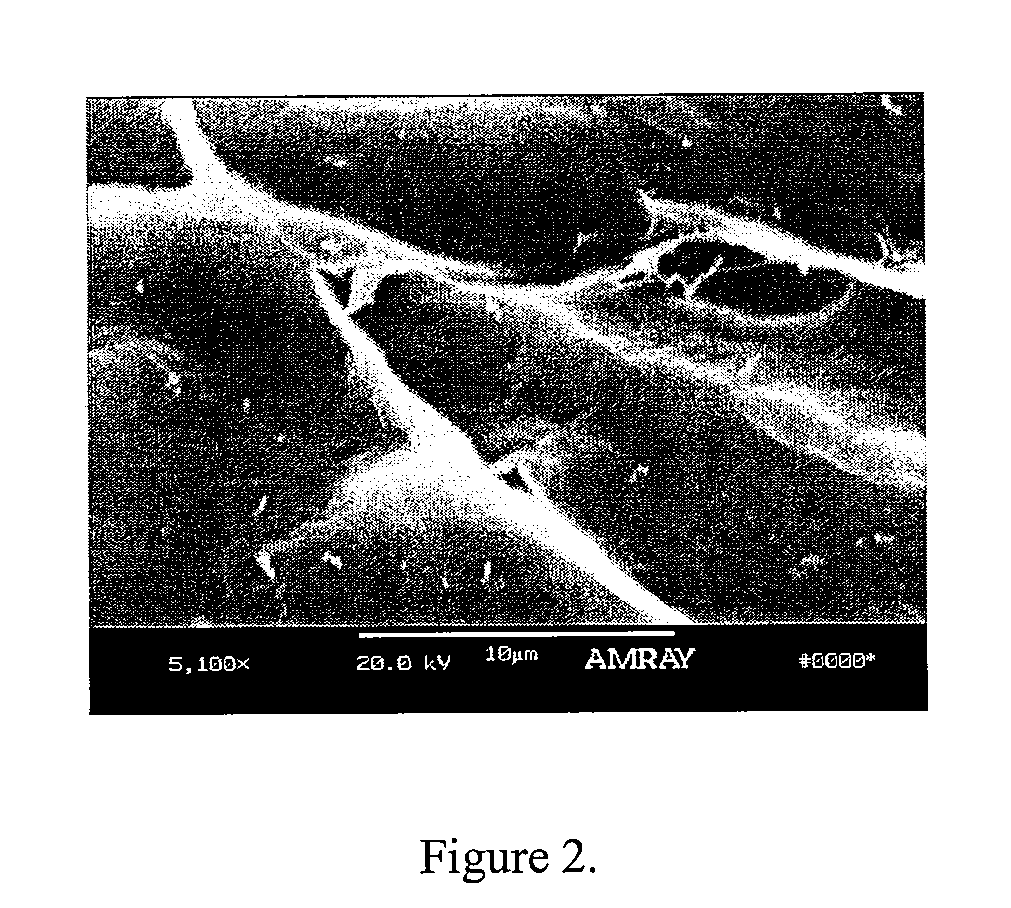
A demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, and soluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose or hydroxypropyl methylcellulose.
Owner:BIOMET MFG CORP
Bone matrix compositions having nanoscale textured surfaces
Bone matrix compositions having nanoscale textured surfaces and methods for their production are provided. In some embodiments, bone matrix is prepared for implantation and retains nanoscale textured surfaces. In other embodiments, nanostructures are imparted to bone matrix wherein collagen fibrils on the surface of the bone matrix have been compromised, thus imparting a nanoscale textured surface to the bone matrix. Generally, these methods may be applied to mineralized or demineralized bone including partially or surface demineralized bone.
Owner:WARSAW ORTHOPEDIC INC
Flexible implant using partially demineralized bone
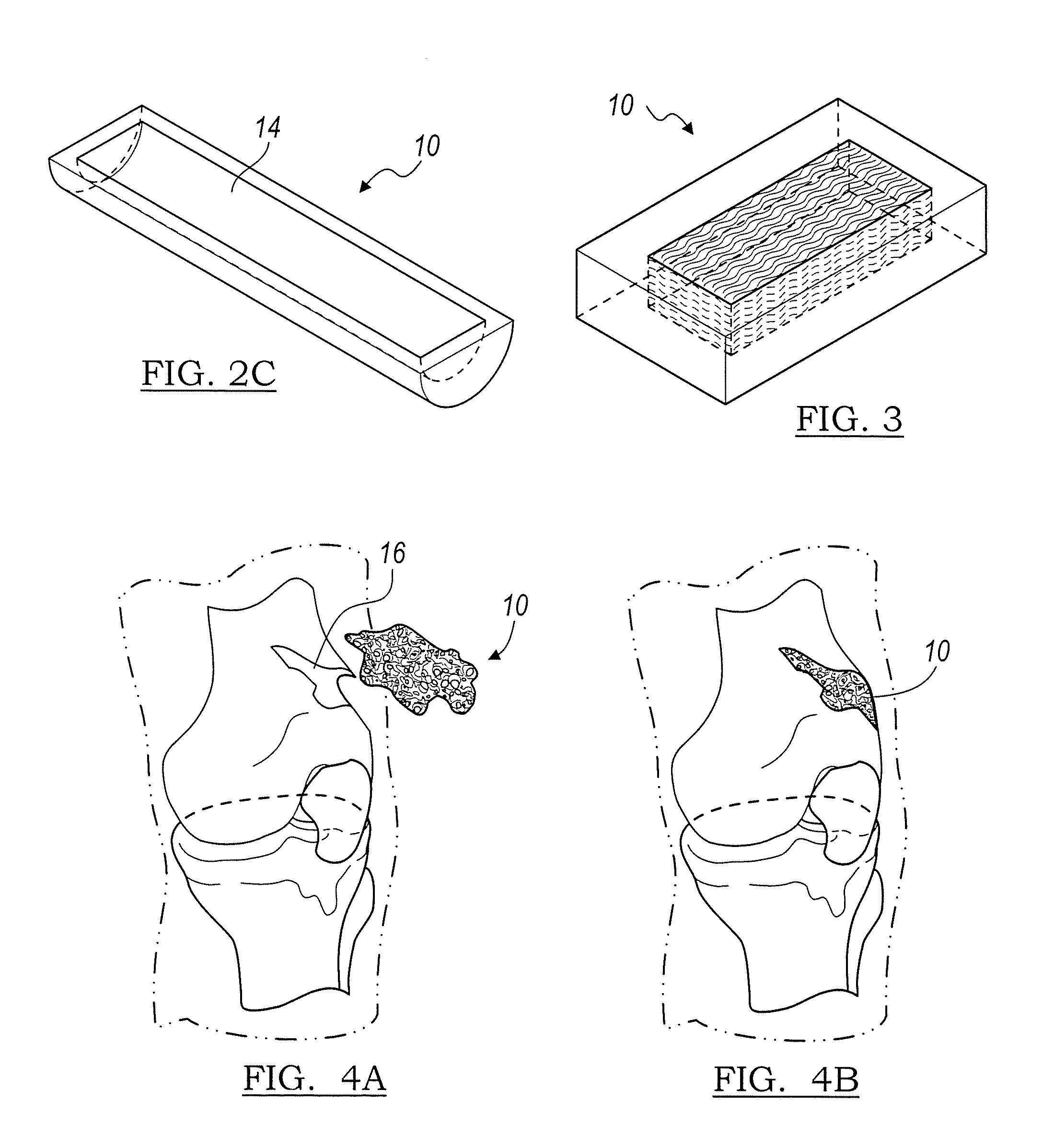
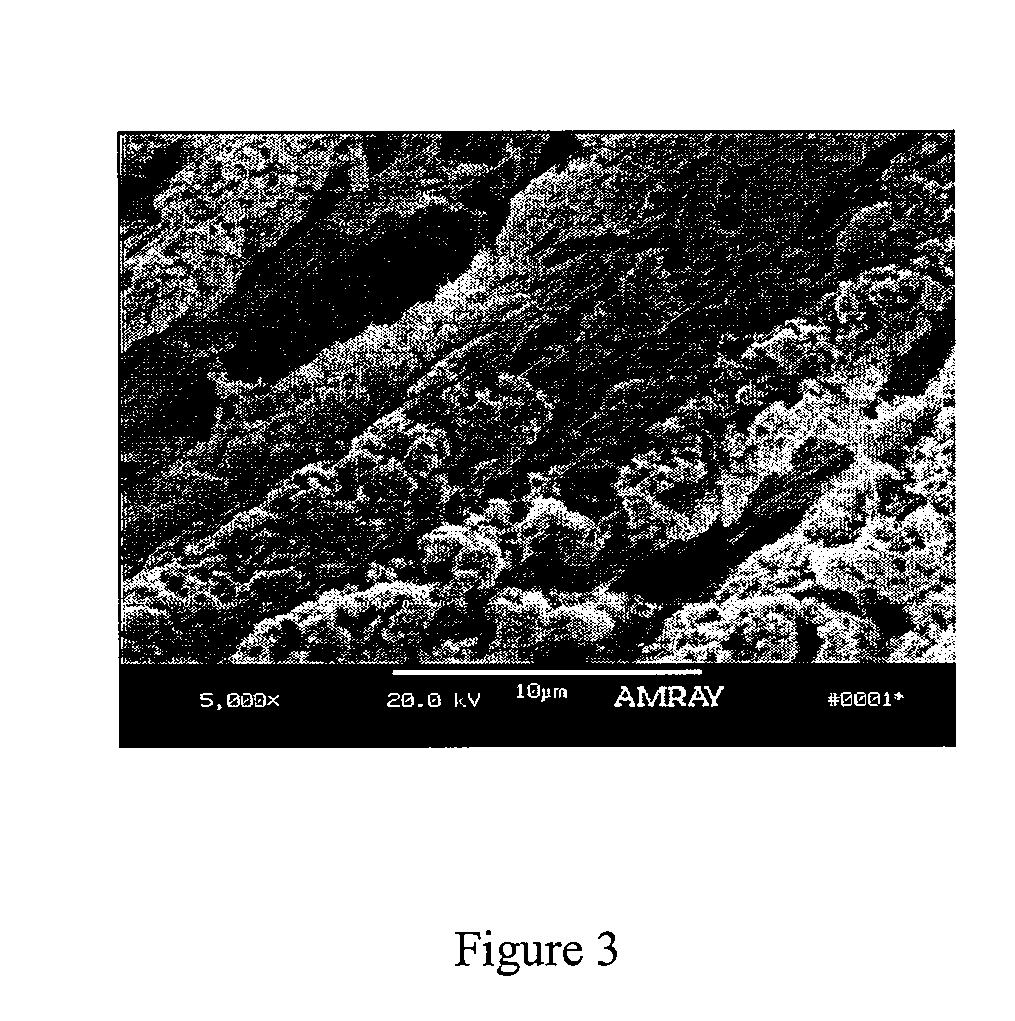
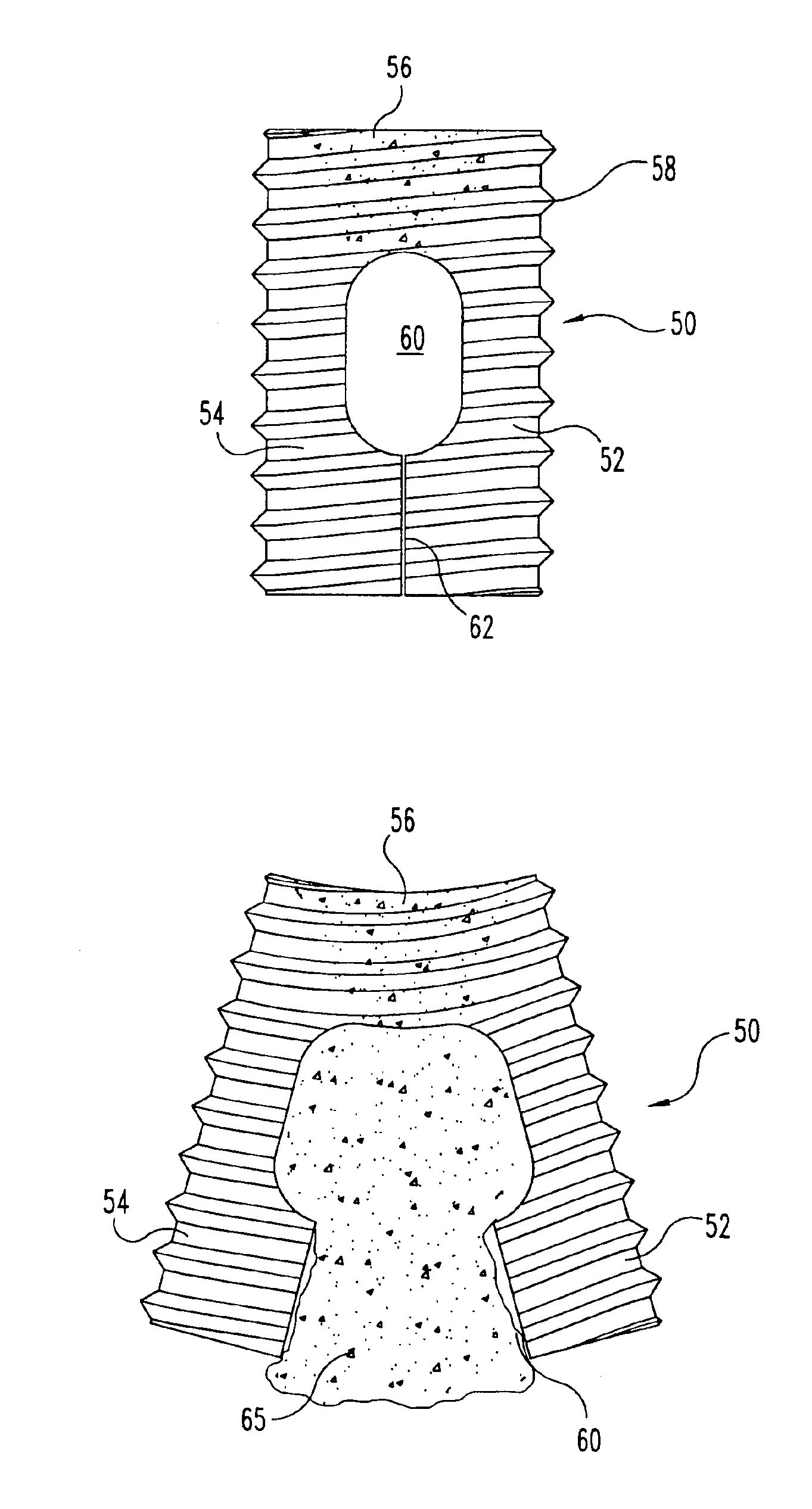
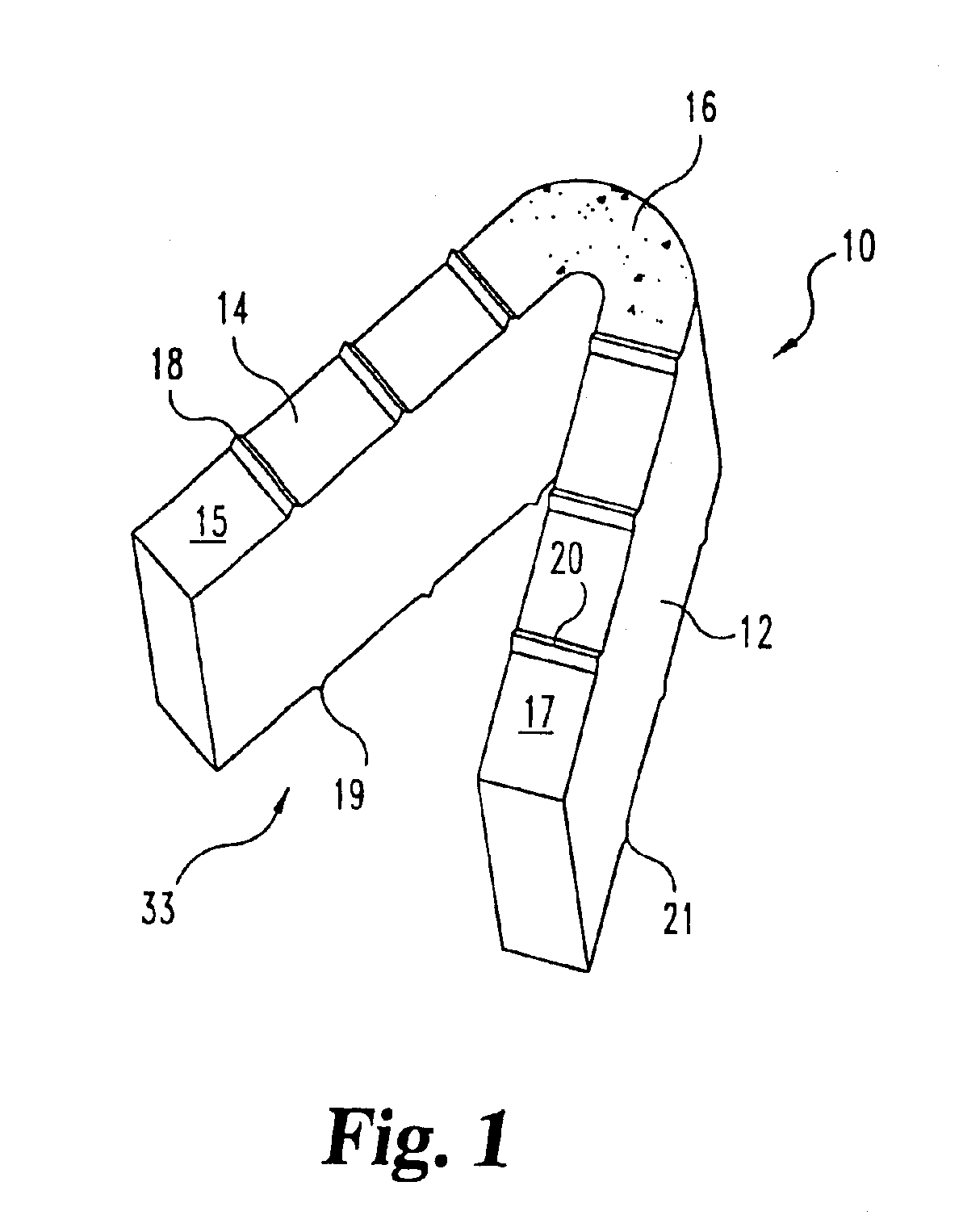
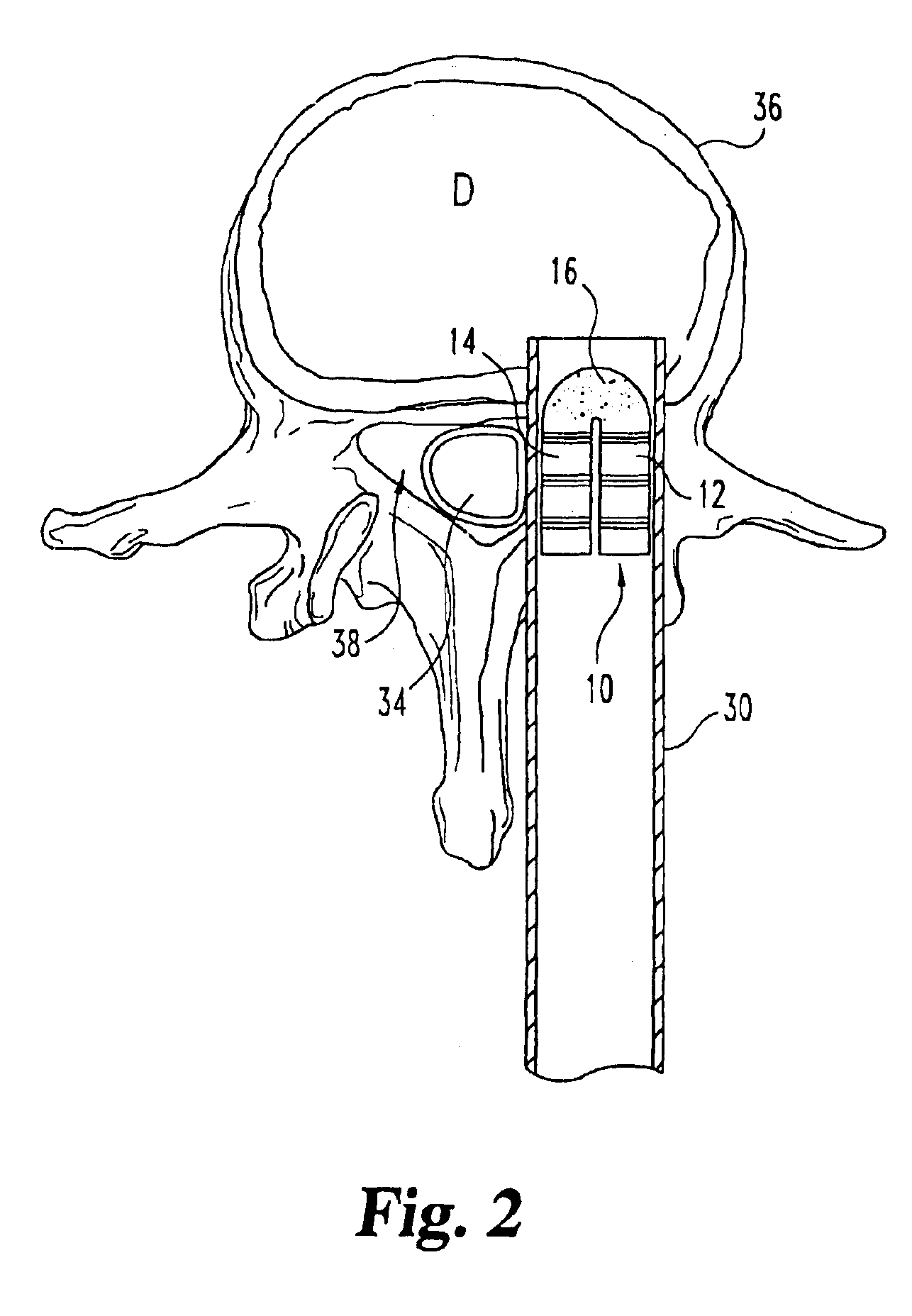
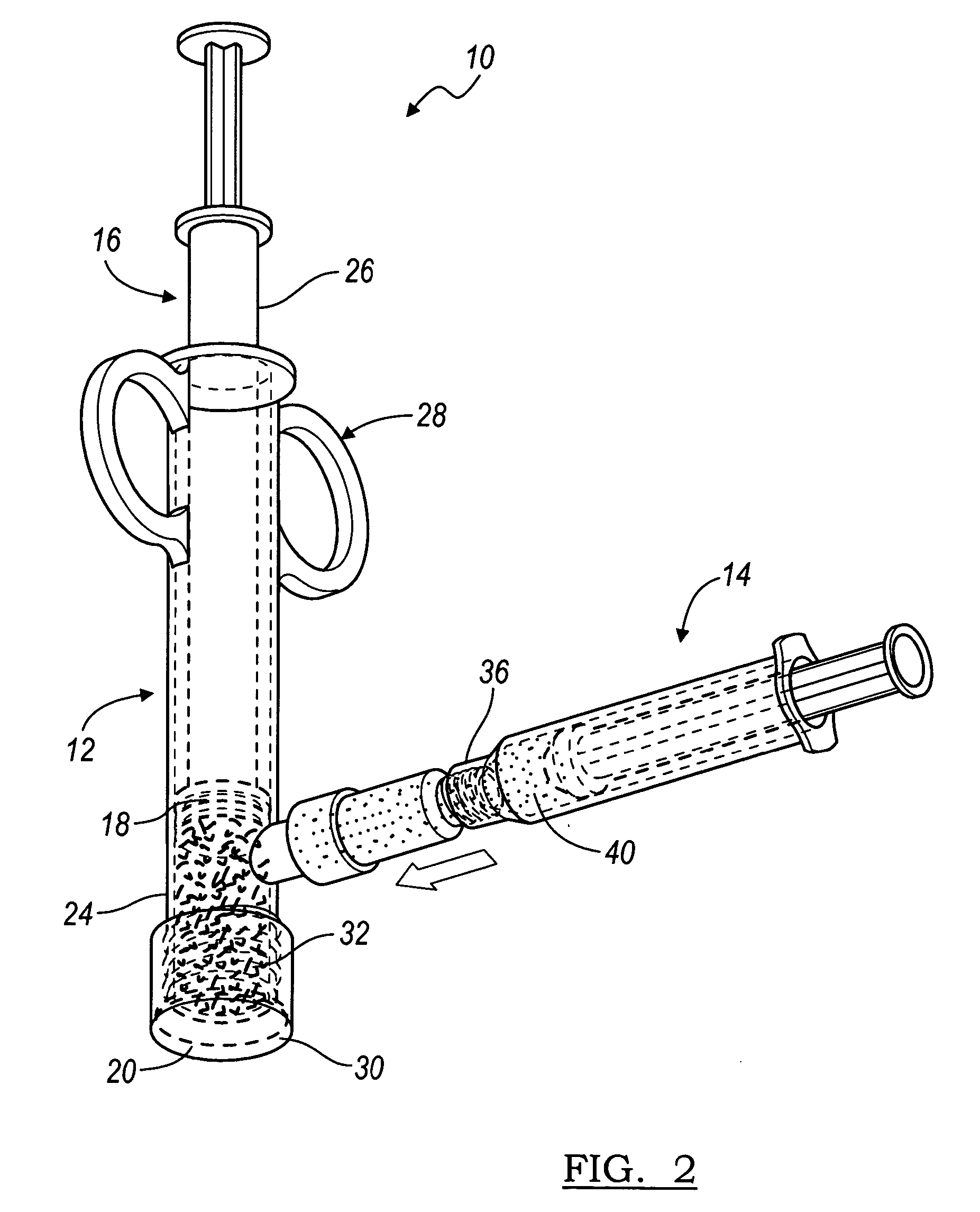
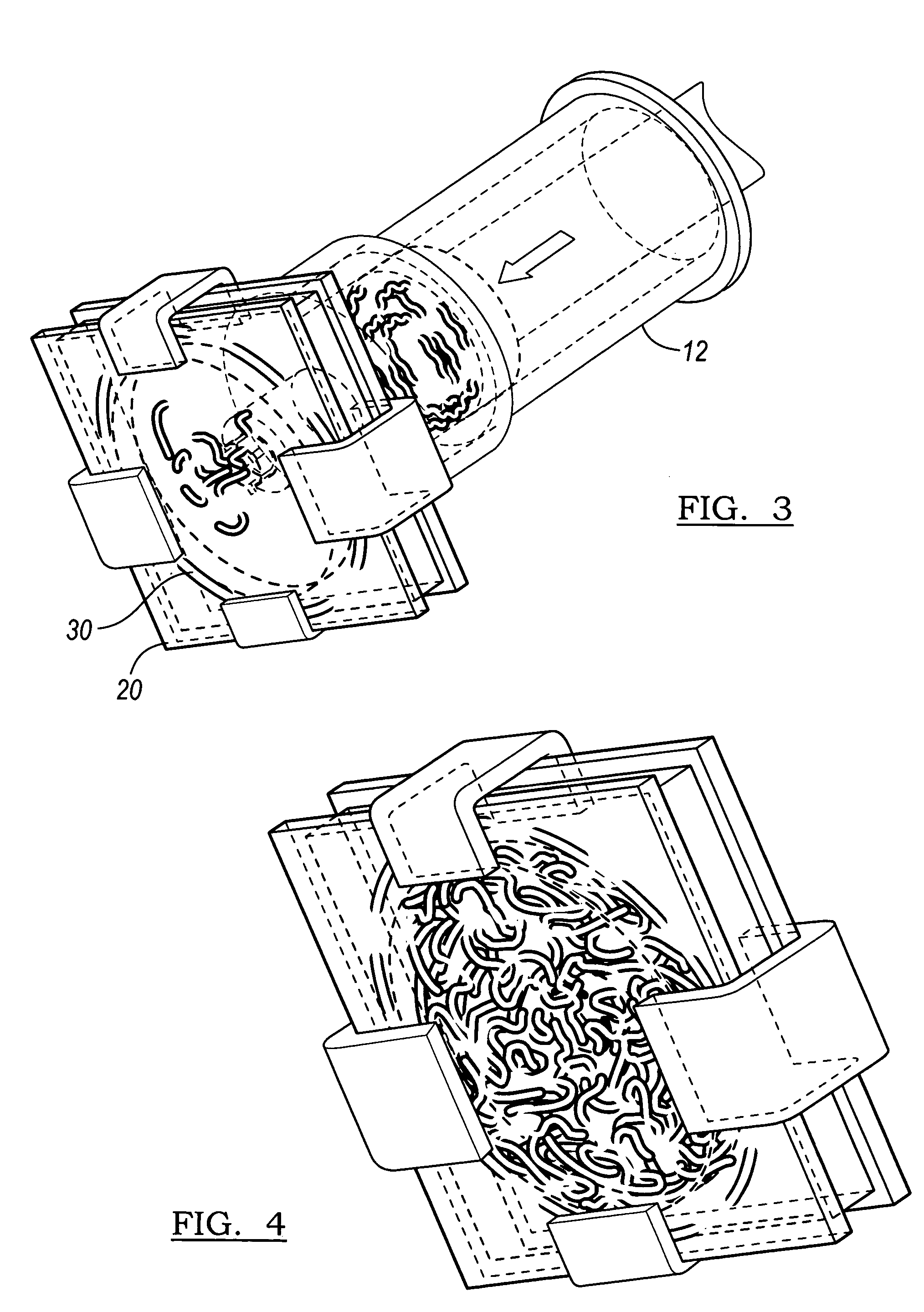
Implantable devices useful for creating bony fusion particularly in intervertebral spinal fusion. The device is formed of bone and has an at least partially demineralized portion between two rigid bone portions creating an area of flexibility. In one application, the area of flexibility may be used to move the device between a reduced size insertion configuration and an expanded implanted configuration. In another use, the area of flexibility may be useful to dampen shock applied to the implant. A method is also disclosed for making the implants and inserting the implants into an intervertebral disc space to promote interbody fusion.
Owner:SDGI HLDG
Method of making demineralized bone particles
InactiveUS7939108B2High yieldConnective tissue peptidesPeptide/protein ingredientsDemineralized boneBone particle
Demineralized bone particles are obtained by demineralizing whole bone and thereafter subdividing the demineralized bone to provide the demineralized bone particles.
Owner:WARSAW ORTHOPEDIC INC
Composition and method for bone regeneration
InactiveUS20050147645A1Good effectDipeptide ingredientsTripeptide ingredientsOsteoblastBone formation
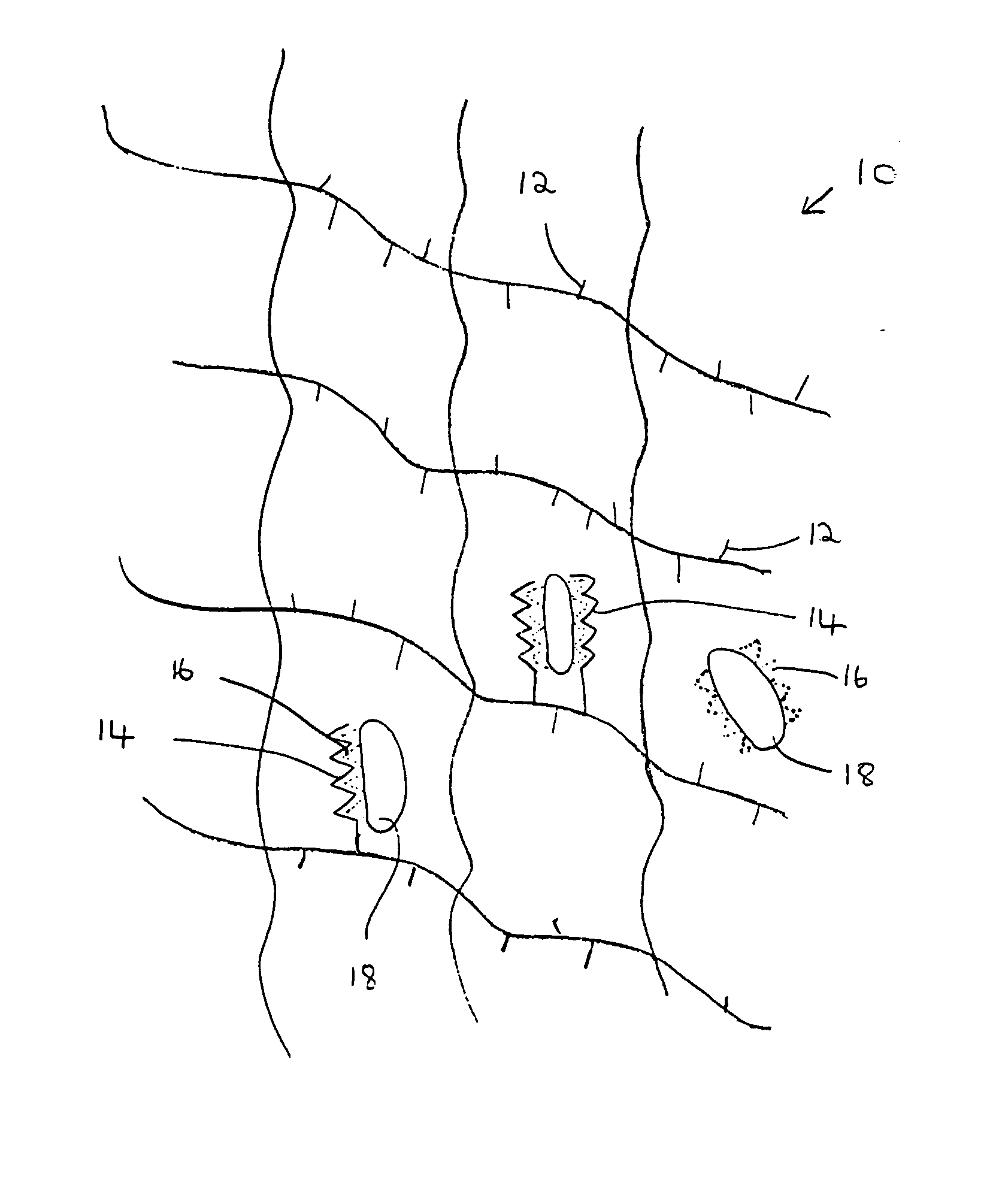
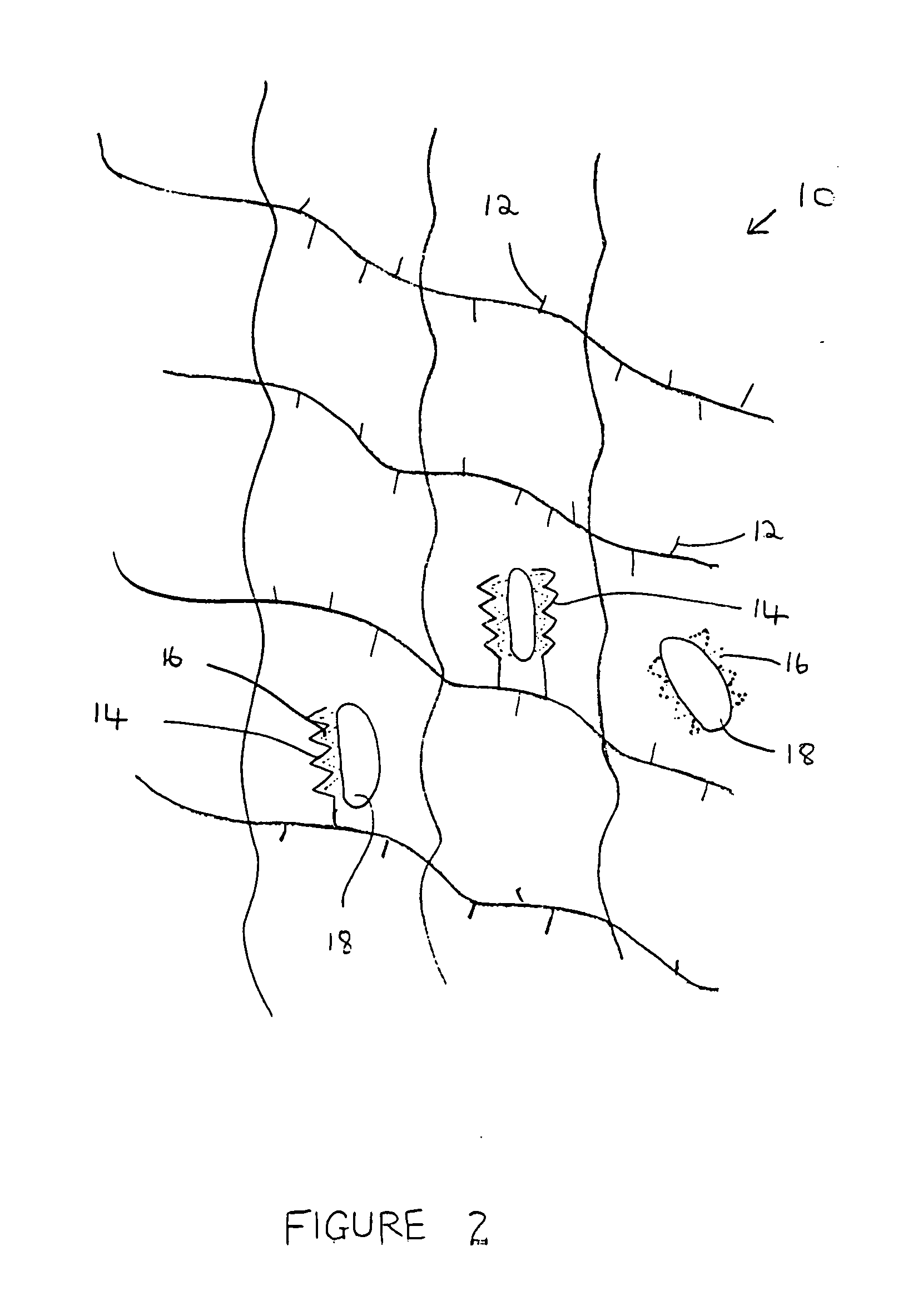
A composition for modulating bone regeneration composition comprises a matrix selected from the group consisting of glycolic acid, lactic acid, collagen, demineralized bone, or a combination thereof. A first biologically active molecule comprising a fibronectin is attached to a portion of the matrix, to facilitate osteoblast activity and for promoting an increase in bone formation. A second biologically active molecule comprising a vitronectin, selected for its ability to attract osteoclasts and produce an inhibiting effect on osteoclast activity to thereby promote a decrease in bone resorption, is also attached to a portion of the matrix.
Owner:BUDNY JOHN ARNOLD
Demineralized bone-derived implants
Selectively demineralized bone-derived implants are provided. In one embodiment, a bone sheet for implantation includes a demineralized field surrounding mineralized regions. In another embodiment, a bone defect filler includes a demineralized cancellous bone section in a first geometry. The first geometry is compressible and dryable to a second geometry smaller than the first geometry, and the second geometry is expandable and rehydratable to a third geometry larger than the second geometry.
Owner:DEPUY SYNTHES PROD INC
Bone matrix compositions and methods
Owner:WARSAW ORTHOPEDIC INC
Bone graft composition comprising a bone material and a carrier comprising denatured demineralized bone
InactiveUS7670384B2Easily placed into injury siteEfficiently hydrateBone implantUnknown materialsDemineralized boneAnimal subject
Formed compositions for application to a bone surface of a human or animal subject, comprising: a bone material; and a carrier comprising denatured demineralized bone, where the composition is formed into a shape suitable for administration to the bone. Methods are provided for making formed compositions for application to a bone surface of a human or animal subject comprise mixing a demineralized bone and water; heating the mixture to form a carrier; mixing the carrier with bone to form a moldable composition; and molding the moldable composition to produce a formed composition. Several apparatuses are provided in which to hydrate the formed bone composition. Methods of hydrating a formed bone composition are also provided.
Owner:BIOMET MFG CORP
Hemostatic bone graft
ActiveUS20080063671A1Reduce stepsInduce and osteoinductive activitySurgical adhesivesPharmaceutical delivery mechanismPolyethylene glycolTonicity
The present invention provides a hemostatic bone graft product and method. Hemostatic bone grafts may include demineralized bone matrix in combination with additives. In one embodiment, the graft comprises demineralized bone and polyethylene glycol. Methods for producing the hemostatic bone graft may include mixing demineralized bone with additives to facilitate protein precipitation, surface tension reduction in blood, and / or a cytolytic effect on cells at a bleeding site.
Owner:WARSAW ORTHOPEDIC INC
Method and apparatus for preparing bone
InactiveUS20060083769A1Not easily dislodgedHigh strengthUnknown materialsTissue regenerationDemineralized boneAnimal subject
Formed compositions for application to a bone surface of a human or animal subject, comprising: a bone material; and a carrier comprising denatured demineralized bone, where the composition is formed into a shape suitable for administration to the bone. Methods are provided for making formed compositions for application to a bone surface of a human or animal subject comprise mixing a demineralized bone and water; heating the mixture to form a carrier; mixing the carrier with bone to form a moldable composition; and molding the moldable composition to produce a formed composition. Apparatus are provided comprising a retaining tube and a hydrating tube. Methods of hydrating a formed bone composition are also provided.
Owner:BIOMET MFG CORP
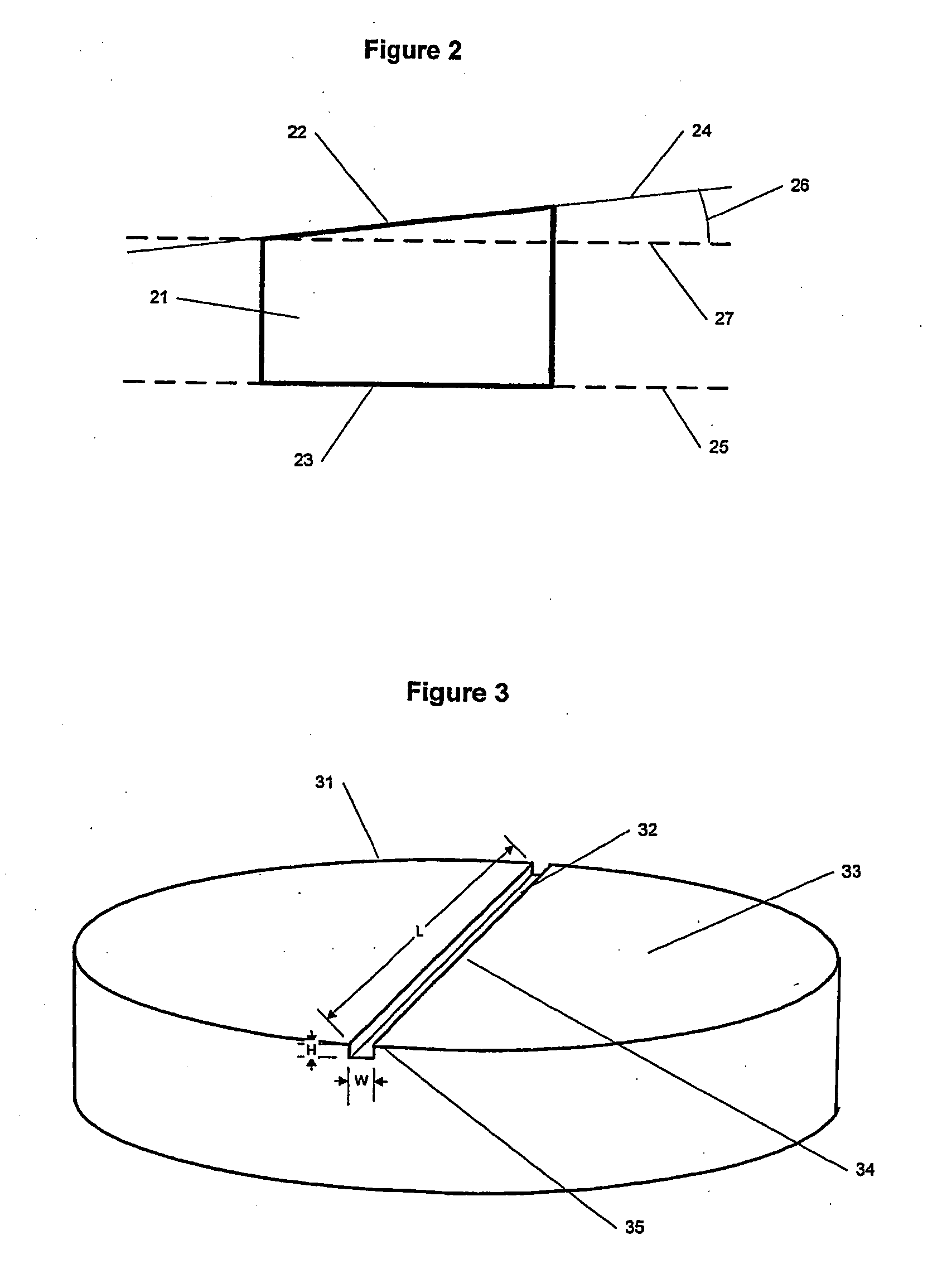
Spinal implants
InactiveUS20050015147A1Improve structural stabilityEasy to handleBone implantJoint implantsSurface layerDemineralized bone
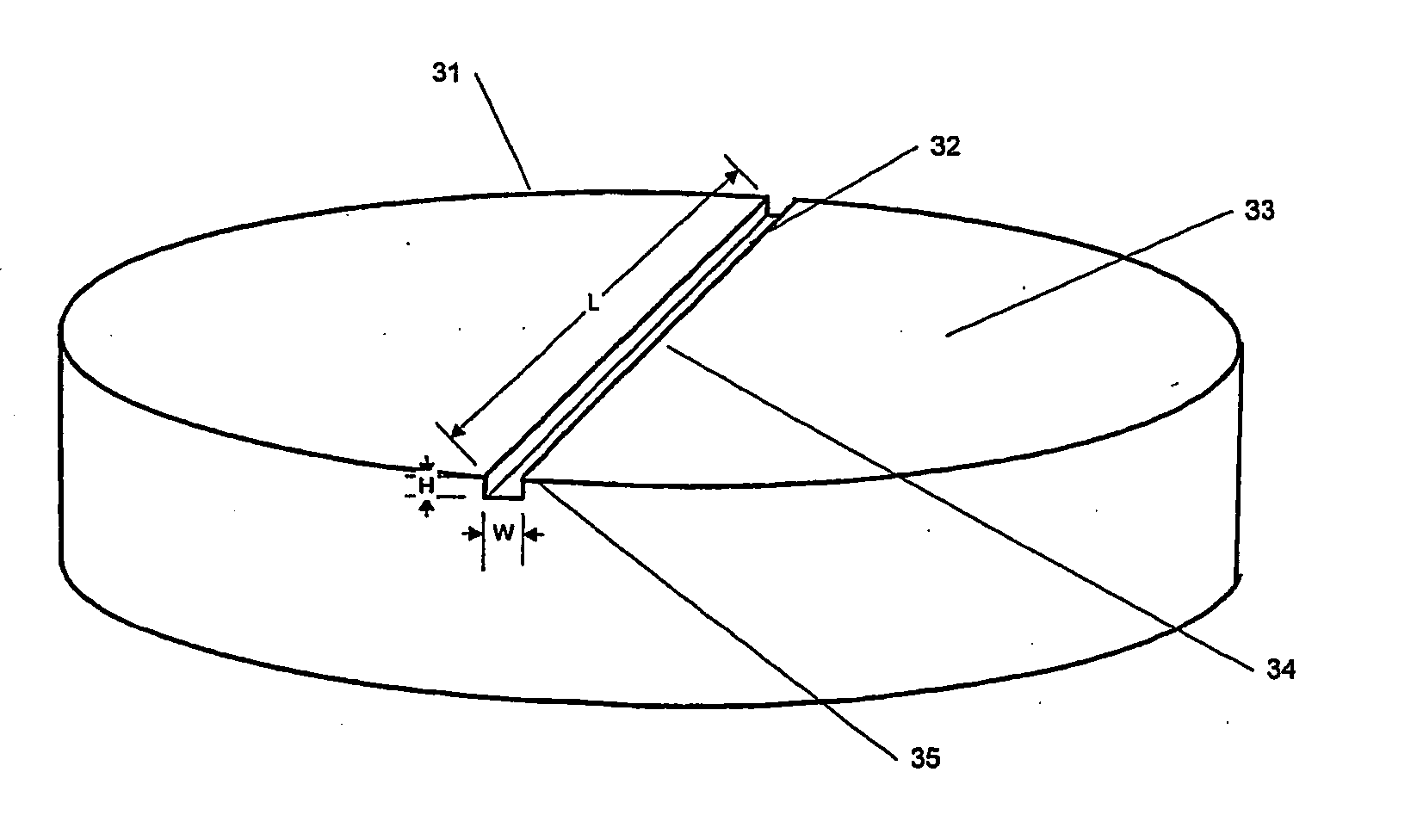
Spinal implants having at least one of several attributes, including a surface layer of demineralized bone, a beveled edge, and channels in the faces in contact with adjacent vertebral bodies. Preferably, the implant has a generally planar top surface, a generally planar bottom surface, and a side surface, wherein at least one of the top and bottom surfaces is demineralized to a depth of from 0.8 mm to 3 mm. Preferably, the top and bottom surfaces of the implant are textured. Also preferably, the implant is disk shaped and comprises an insertion side extending at least 10% of the circumference of the implant, wherein the edge formed by the insertion side and the top surface, and the edge formed by the insertion side and the bottom surface, are beveled.
Owner:EUROPEAN BIOINFORMATICS INSTITUTE
Hard tissue repairing material and its prepn
InactiveCN1403167APromote bone healingPromote absorptionSurgeryProsthesisAbsorbable polymersPolyester
The present invention relates to surgical material. The material includes biological absorbable polymer 10-85 wt%, un-sintered calcium phosphate salt 5-80 wt%, and demineralized bone 1-70 wt%. The biologically absorbable polymer includes polyester, chitosan, hondroitin sulfate, collagen, alginatel, etc.; and the calcium phosphate salt is un-sintered hydroxyapatite or tricalcium phosphate of granularity less than 100 microns. The hard tissue repairing material is prepared through mixing biologically absorbable polymer, un-sintered calcium phosphate salt and demineralized bone dispersed in solvent and freeze drying to obtain the hard tissue repairing material. The composition has the function of promoting bone healing and excellent biocompatibility.
Owner:SOUTHEAST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com