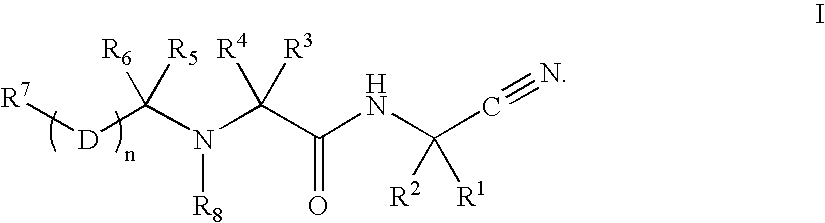
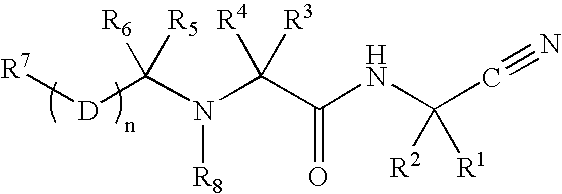
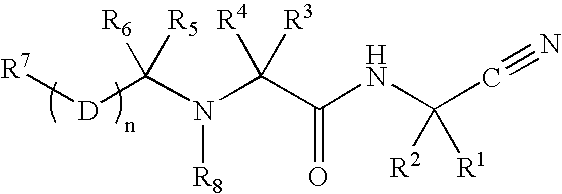
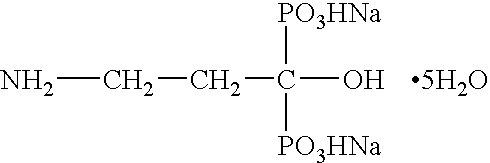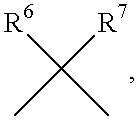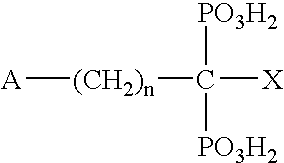Patents
Literature
474 results about "Bone resorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bone resorption is resorption of bone tissue, that is, the process by which osteoclasts break down the tissue in bones and release the minerals, resulting in a transfer of calcium from bone tissue to the blood.
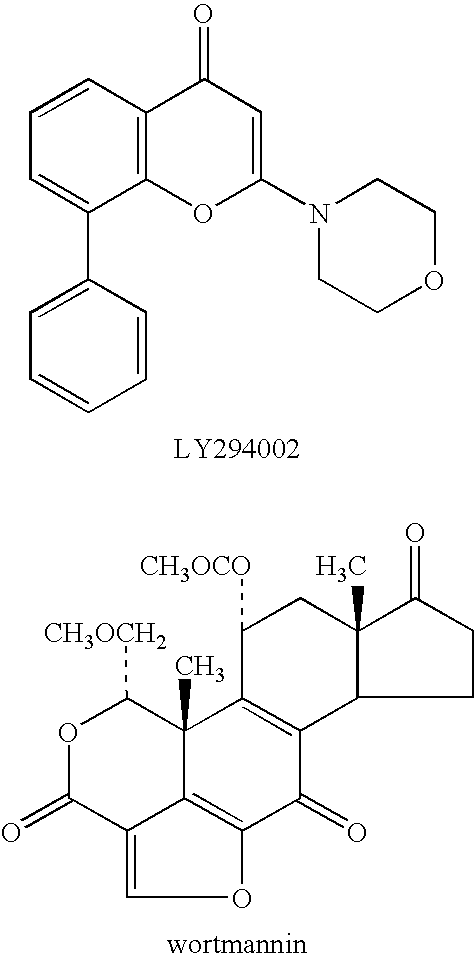
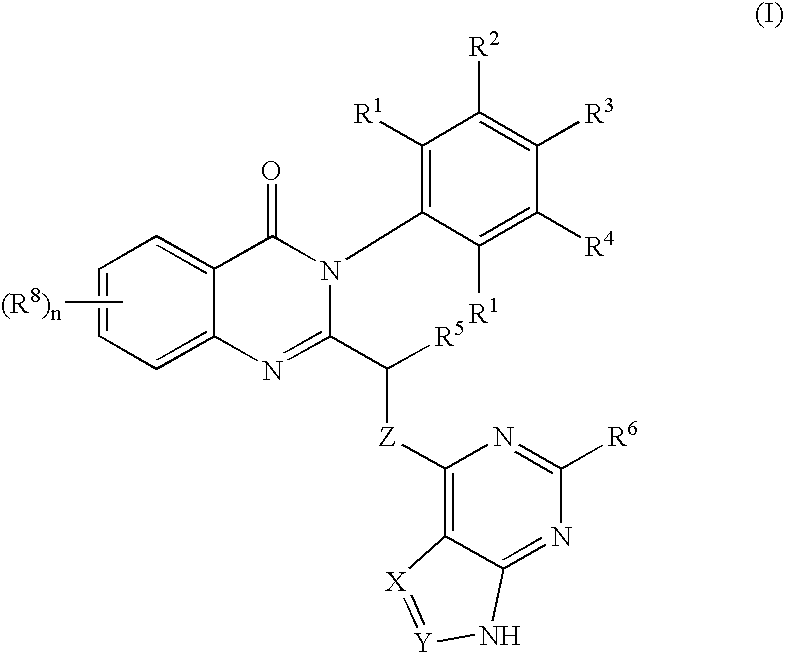
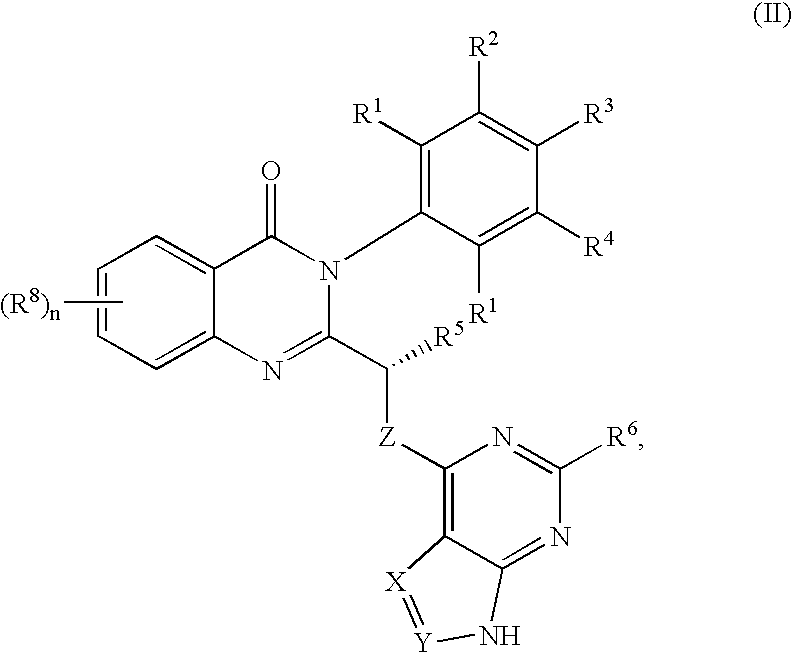
Quinazolinones as inhibitors of human phosphatidylinositol 3-kinase delta
ActiveUS7932260B2Inhibit growth and proliferationInhibit growthBiocideSenses disorderLeukocyte functionWhite blood cell
The invention provides a class of substituted quinazolinone compounds and methods of treating diseases mediated by PI3Kδ activity. The disclosed compounds are useful in treating diseases such as bone-resorption disorders; and cancer, especially hematopoietic cancers, lymphomas, multiple myelomas and leukemia. The compounds are also useful in disrupting or inhibiting cellular processes such as leukocyte function or accumulation, neutrophils function, lymphocyte proliferation, and endogenous immune responses.
Owner:ICOS CORP
Spinal fusion instrumentation, implant and method
InactiveUS20080065222A1Simple methodSmall sizeBone implantSpinal implantsSpinal columnSurgical instrumentation
A system and method includes surgical instrumentation, implants, bone graft material, and measurement equipment to enable a spine fusion procedure to proceed more accurately, efficiently and safely by allowing precision measurement of the characteristics of the intervertebral space, selection of and provision of new implants, placement of an intervertebral implant, and to help overcome bone resorption all where improved healing thereafter takes place.
Owner:HAMADA JAMES S
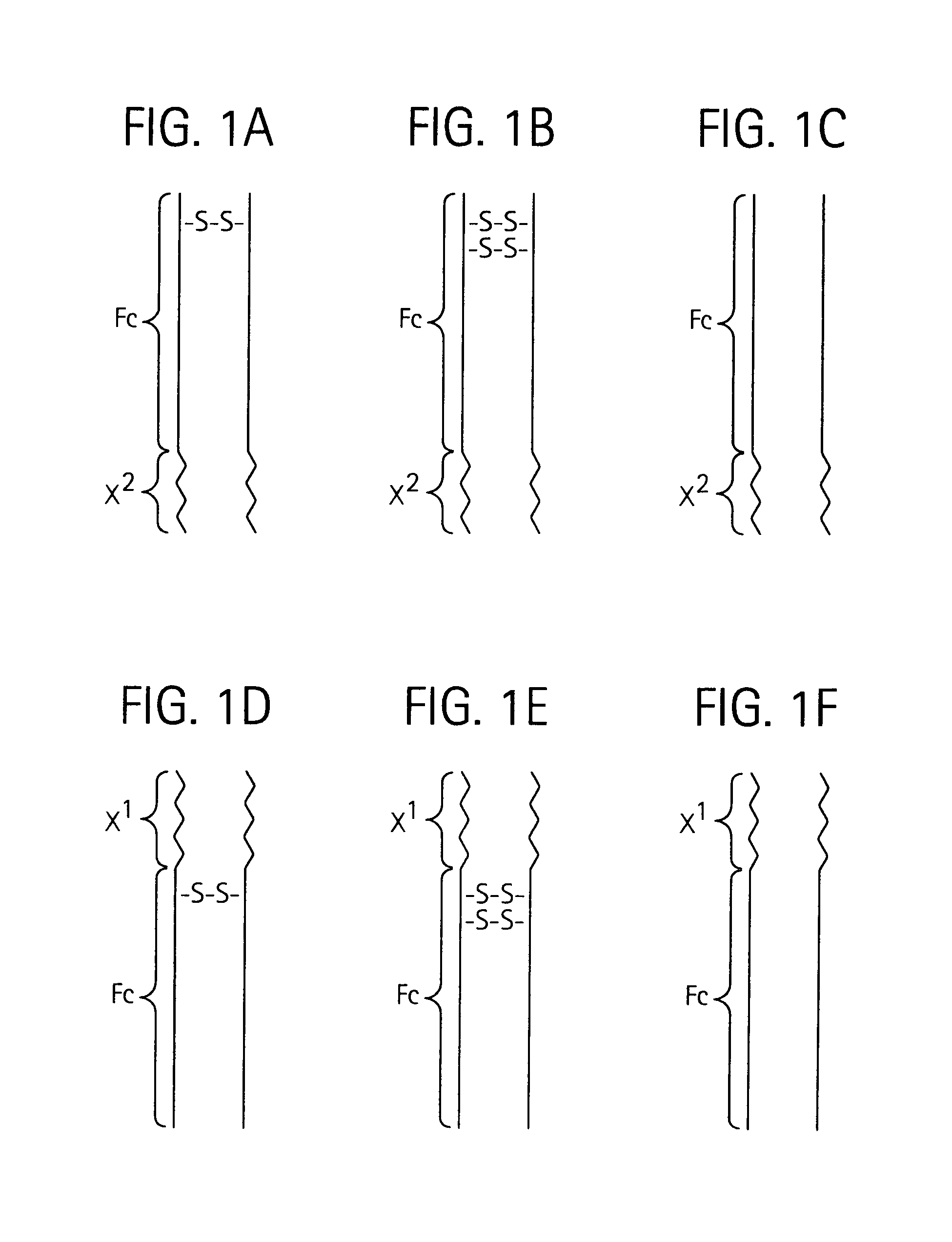
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Nanoparticulate bisphosphonate compositions
InactiveUS20060210639A1Avoid gastrointestinal irritationLow water solubilityBiocidePowder deliveryNanoparticleMedicine
Nanoparticulate bisphosphonate compositions, having an effective average particle size of less than 2000 nm, are described. The compositions are useful in treating bone resorption in a mammal.
Owner:ALKERMES PHARMA IRELAND LTD
Method for inhibiting bone resorption
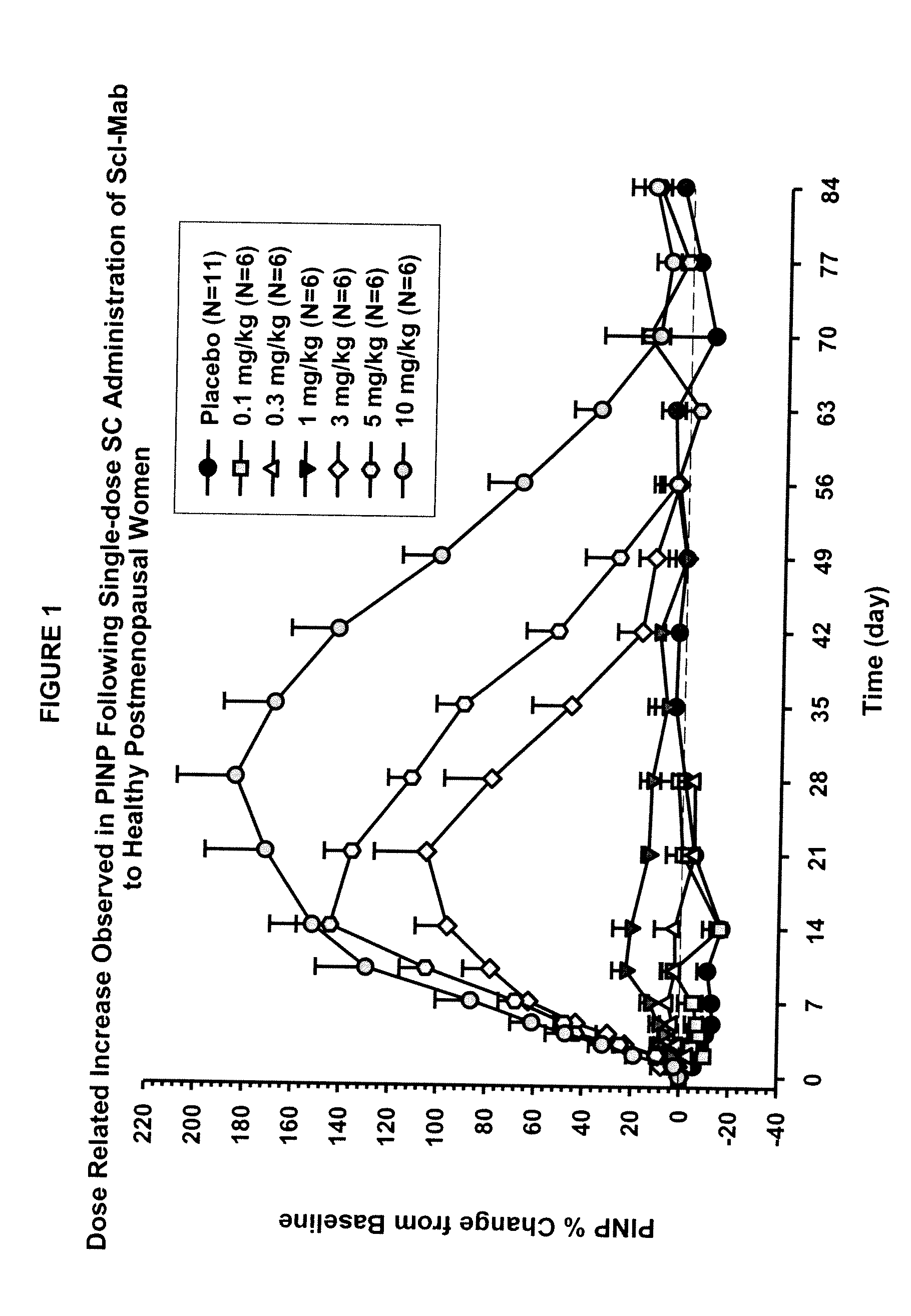
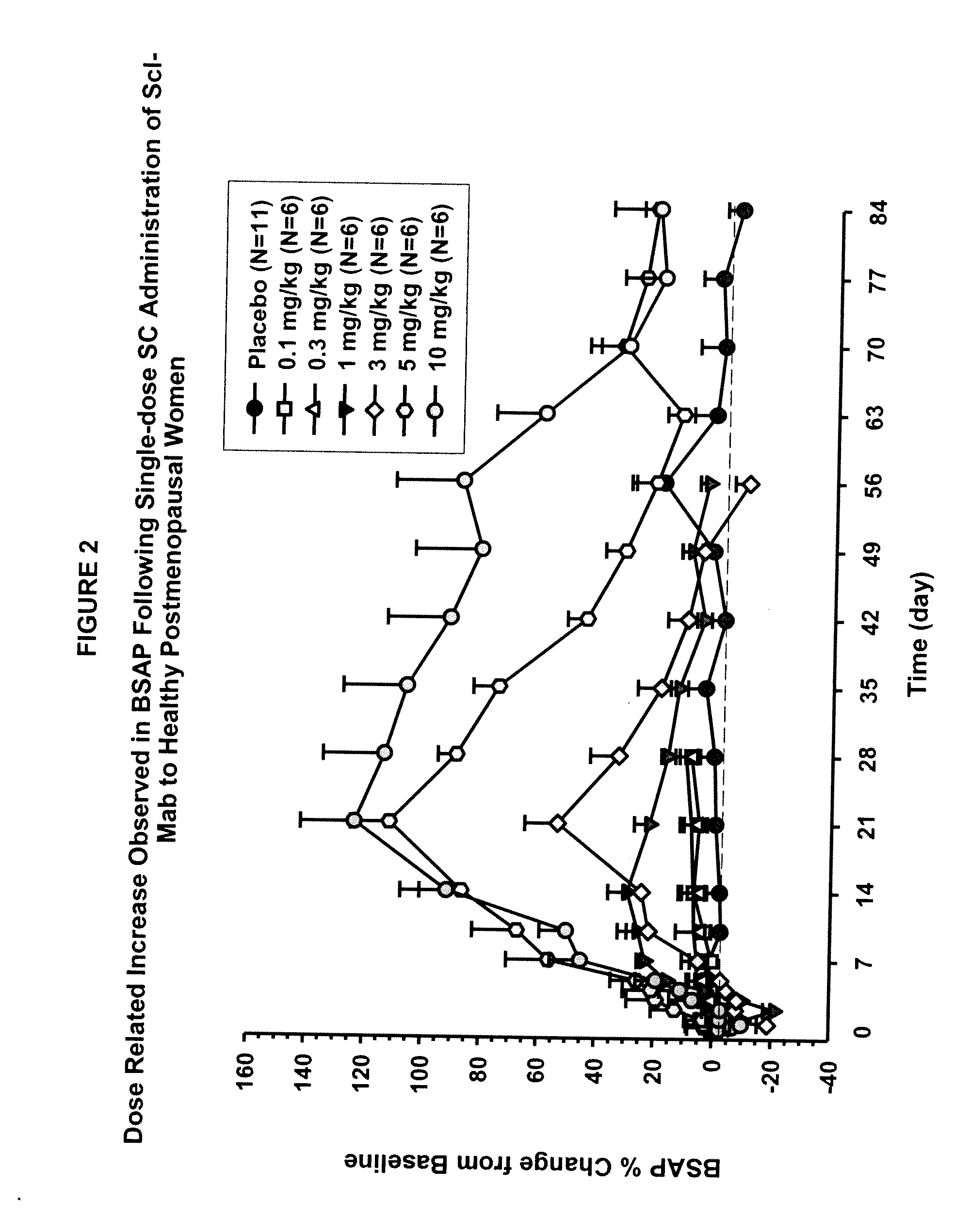
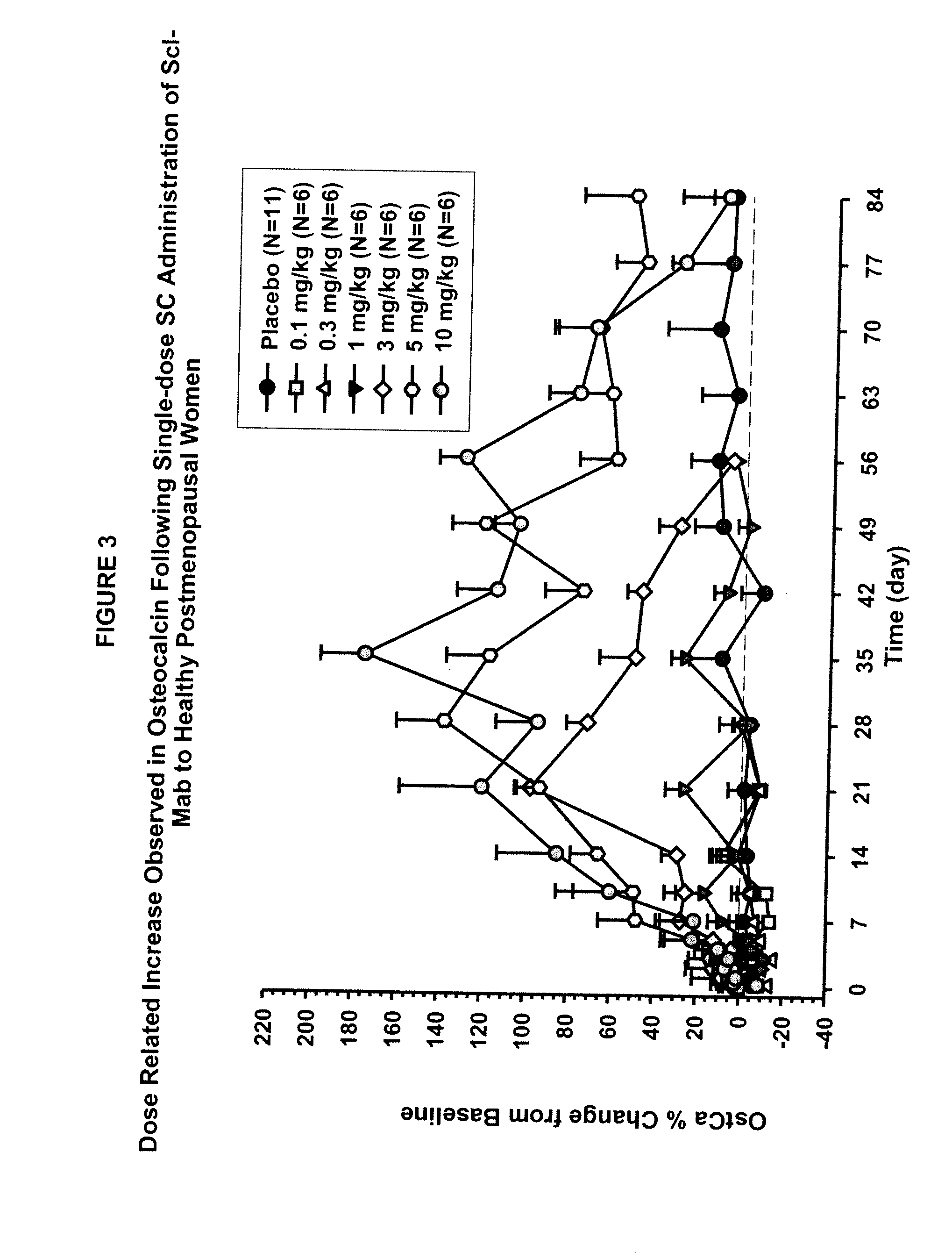
ActiveUS20090074763A1Inhibiting bone resorptionLower Level RequirementsAntibacterial agentsNervous disorderBone densityIncreased bone mineral density
The invention is directed to a method of inhibiting bone resorption. The method comprises administering to a human an amount of sclerostin inhibitor that reduces a bone resorption marker level for at least 2 weeks. The invention also provides a method of monitoring anti-sclerostin therapy comprising measuring one or more bone resorption marker levels, administering a sclerostin binding agent, then measuring the bone resorption marker levels. Also provided is a method of increasing bone mineral density; a method of ameliorating the effects of an osteoclast-related disorder; a method of treating a bone-related disorder by maintaining bone density; and a method of treating a bone-related disorder in a human suffering from or at risk of hypocalcemia or hypercalcemia, a human in which treatment with a parathyroid hormone or analog thereof is contraindicated, or a human in which treatment with a bisphosphonate is contraindicated.
Owner:AMGEN INC
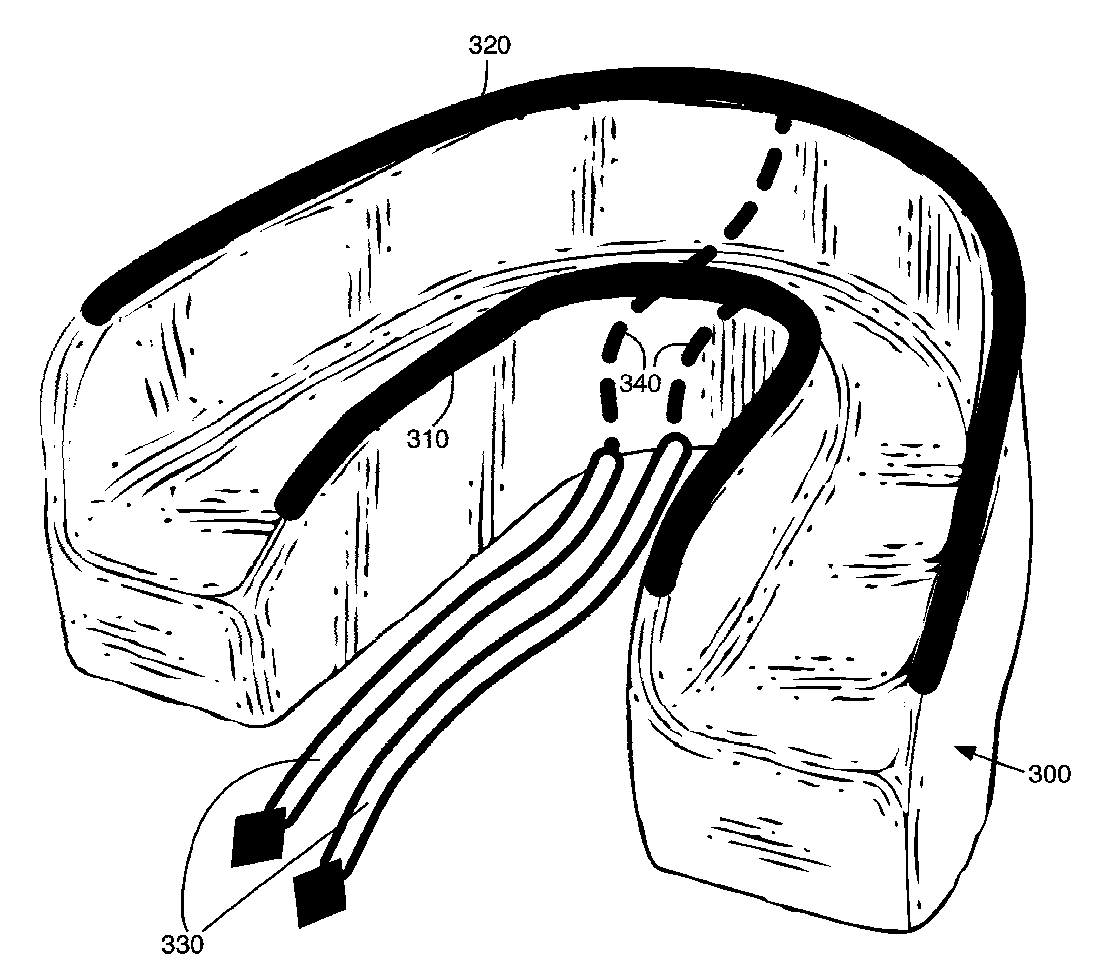
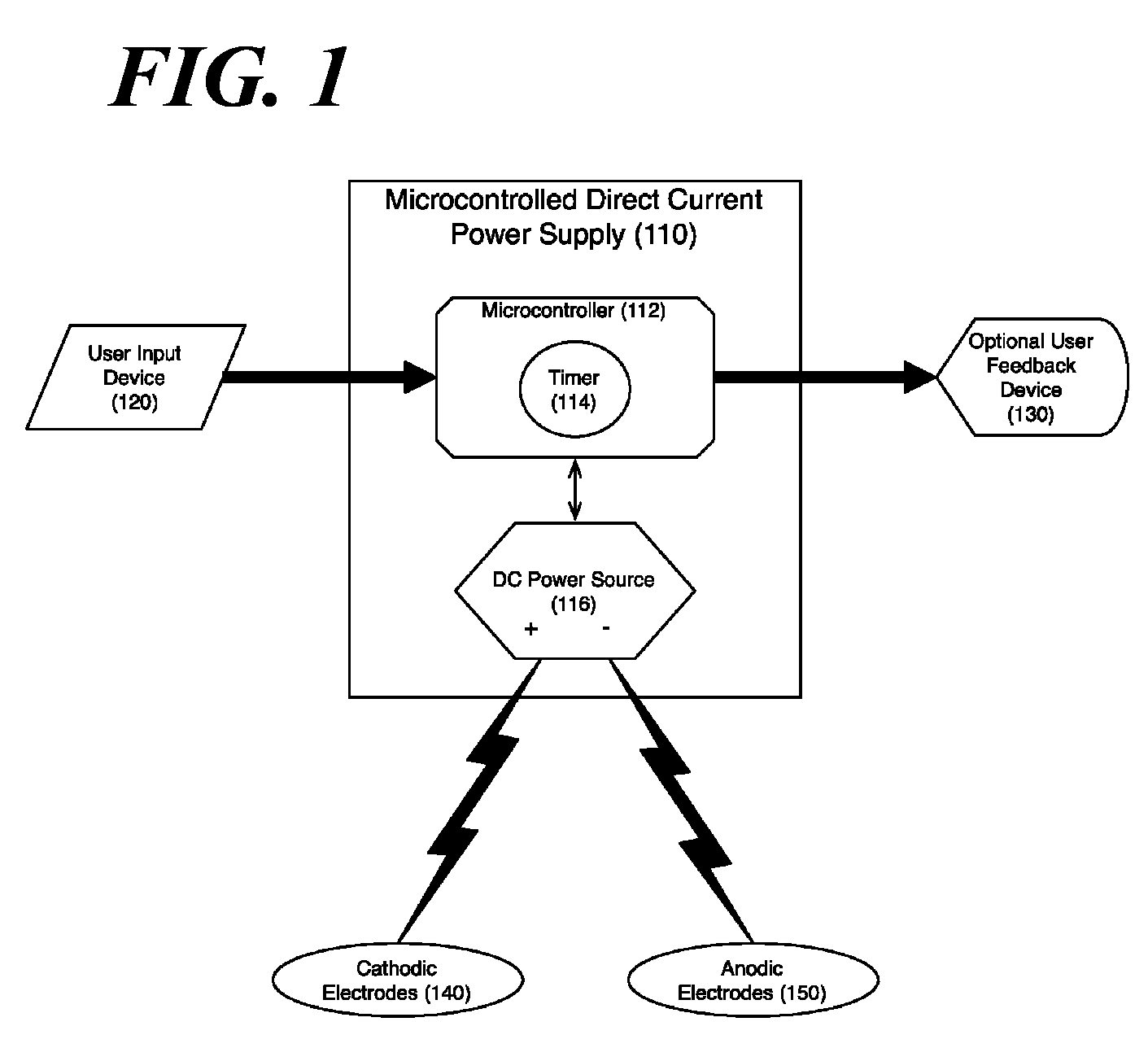
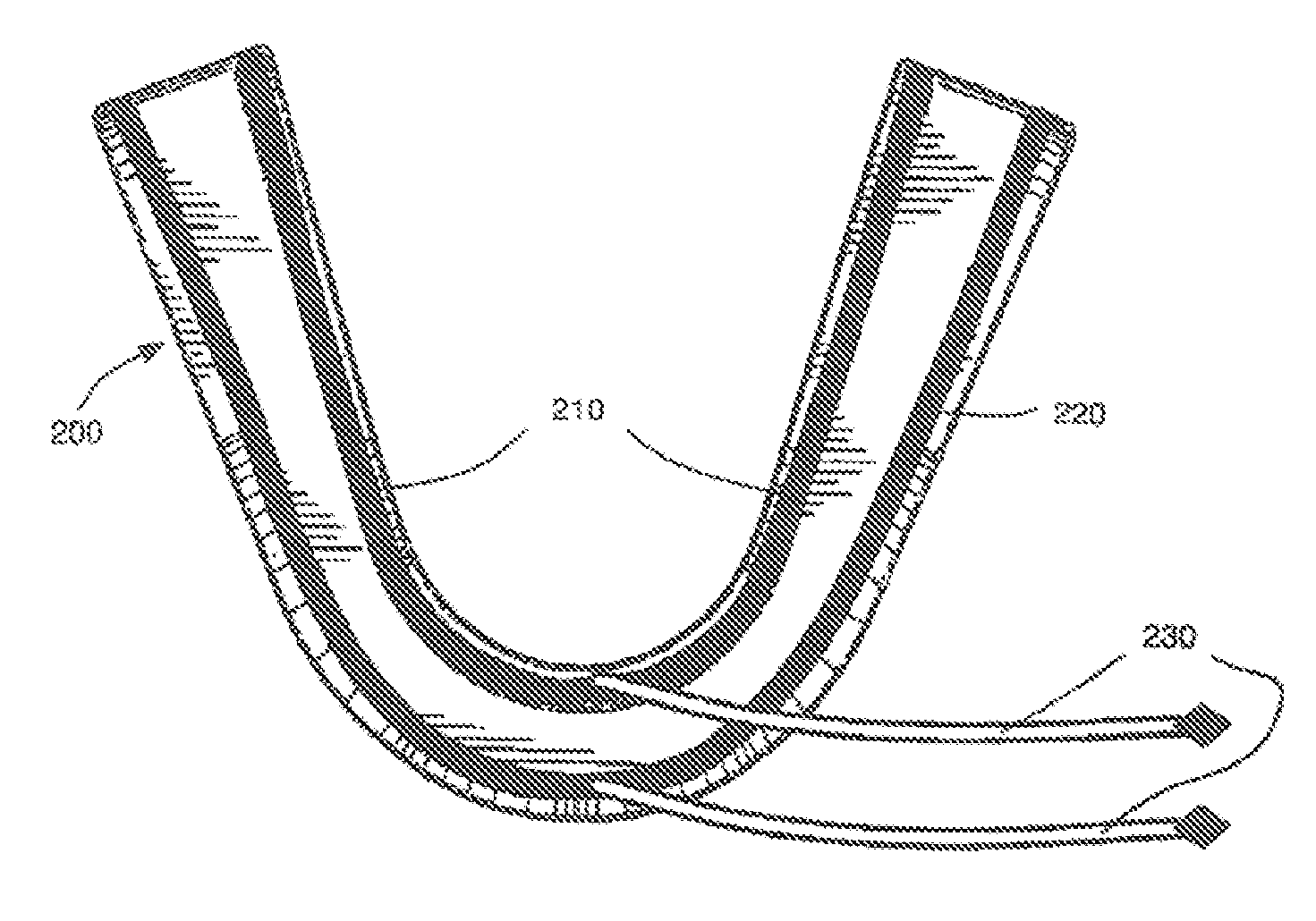
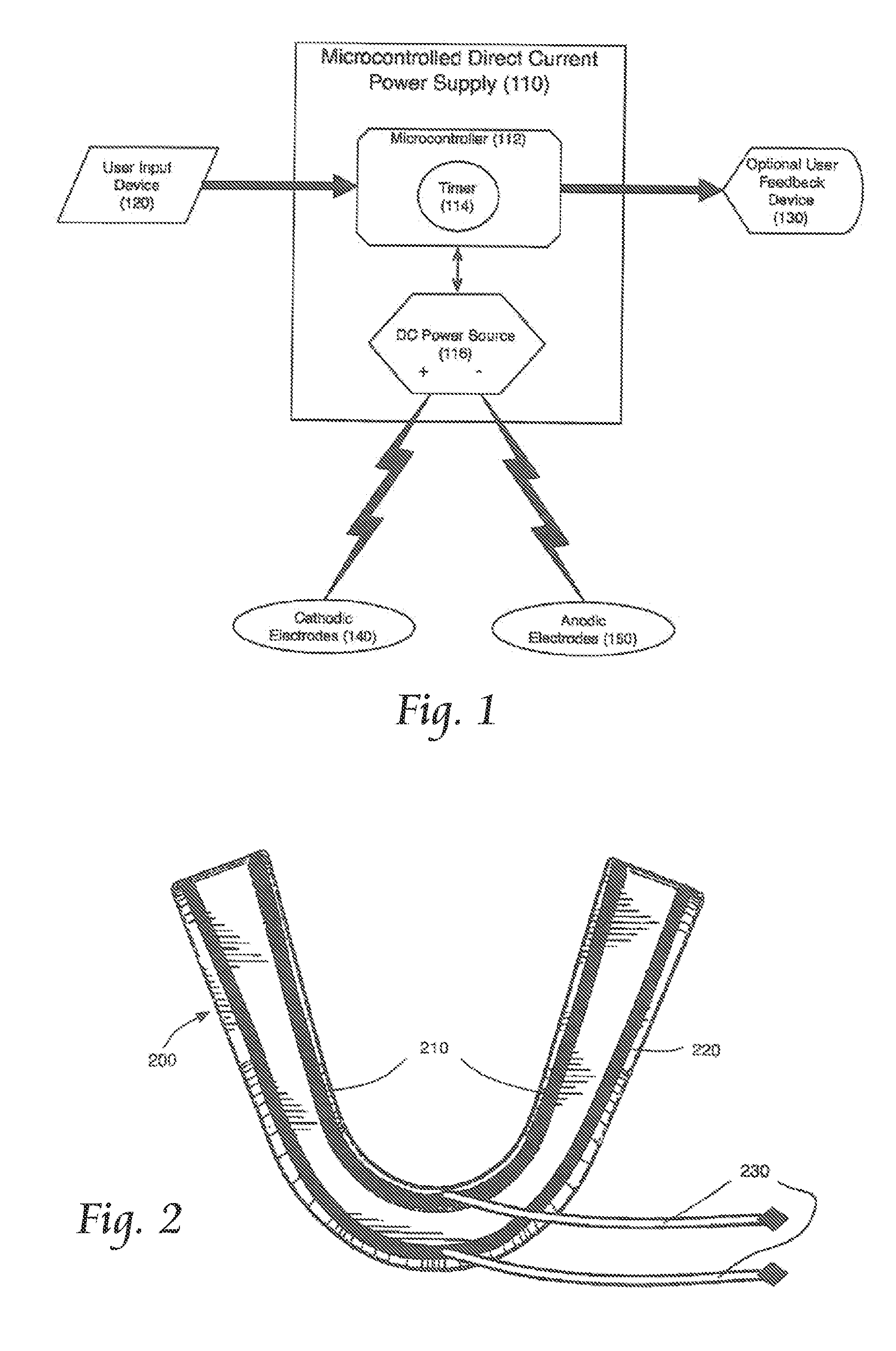
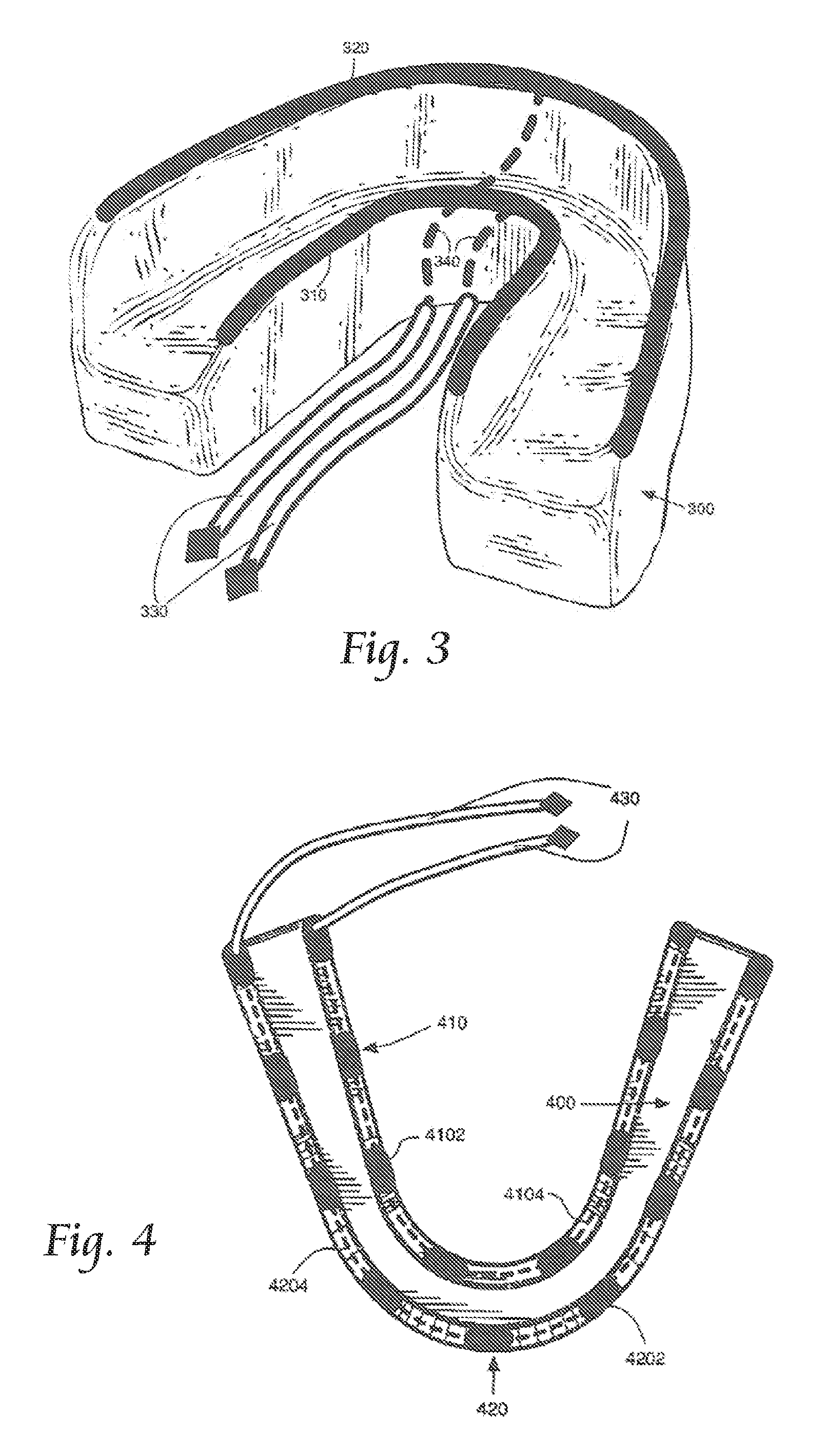
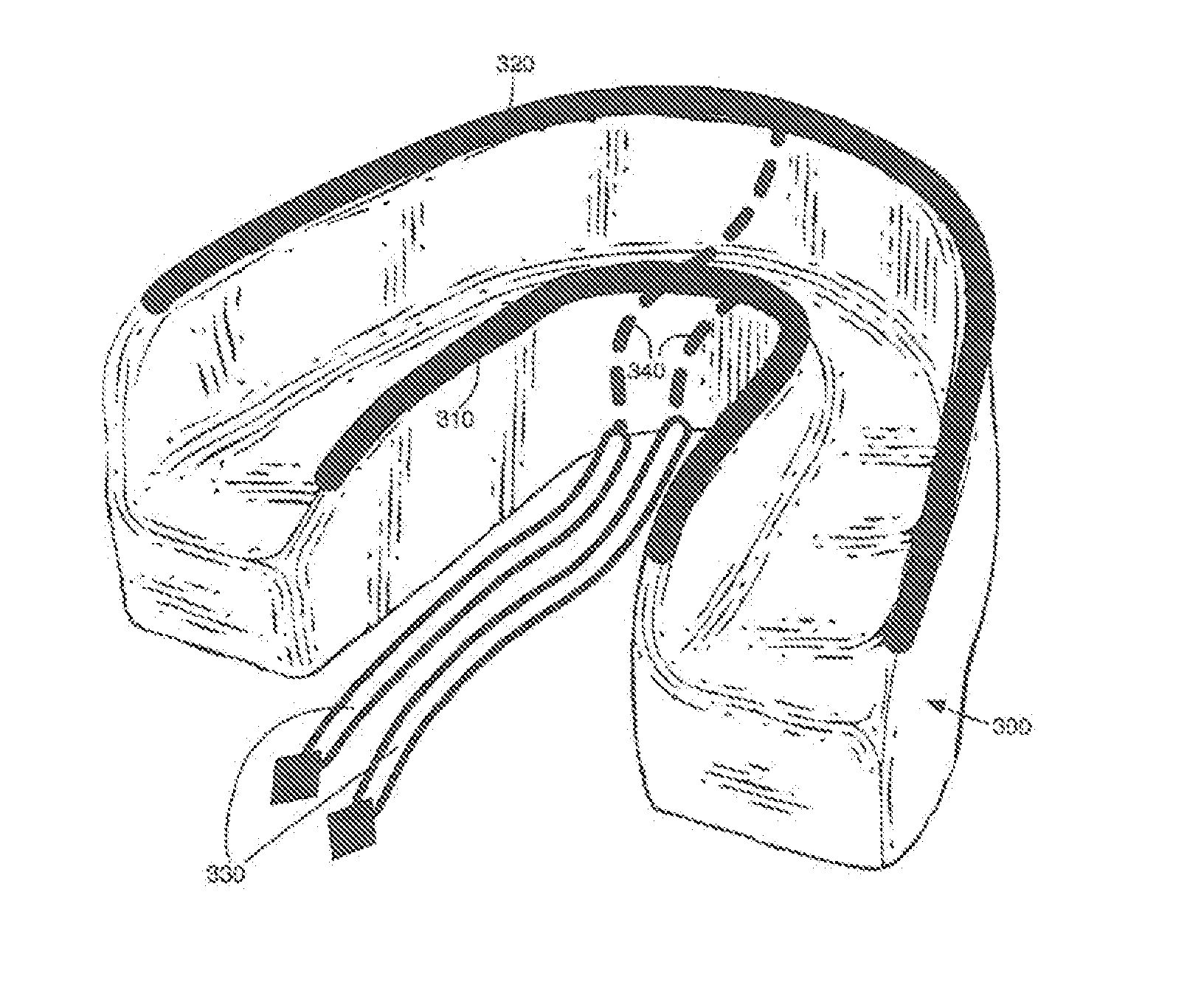
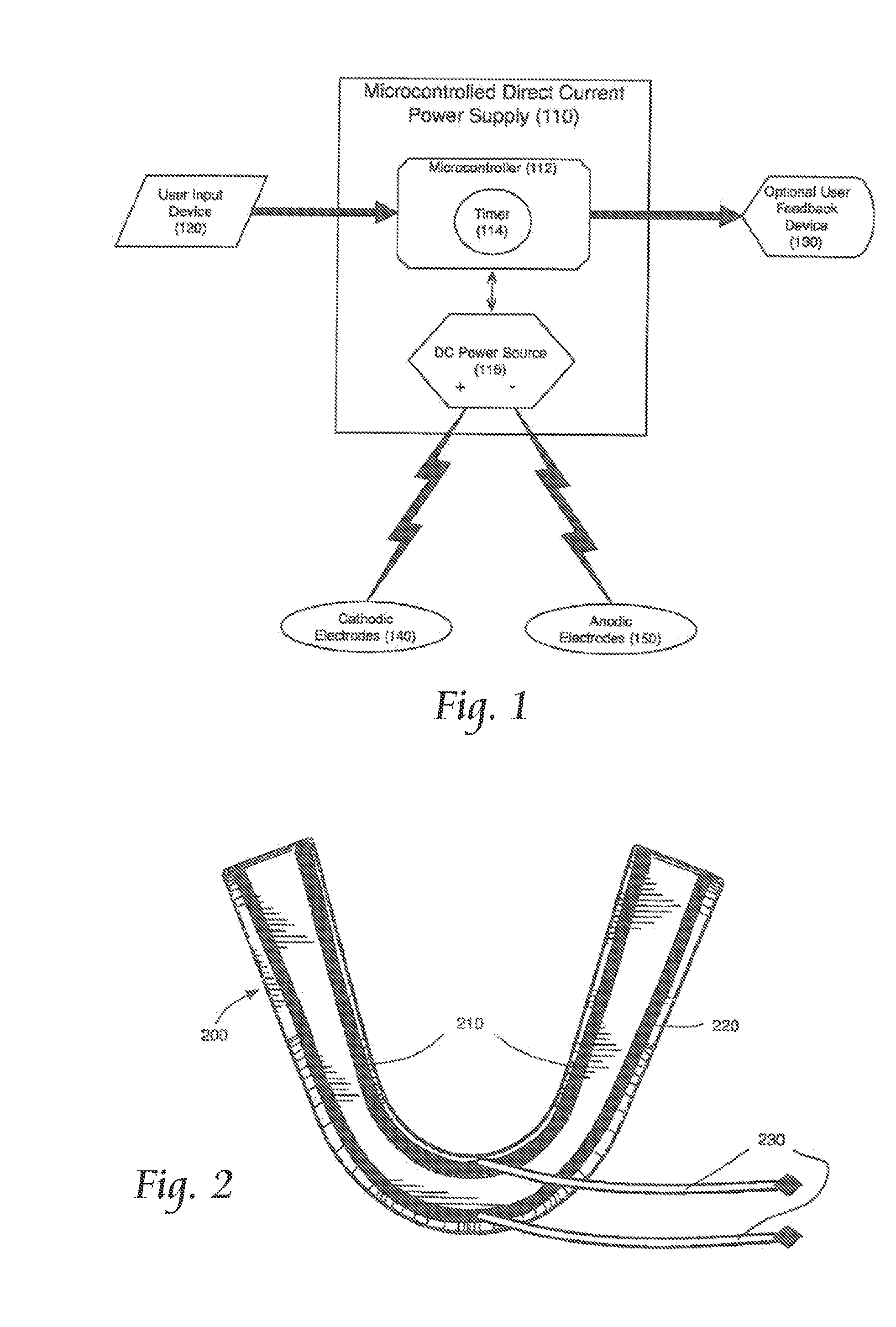
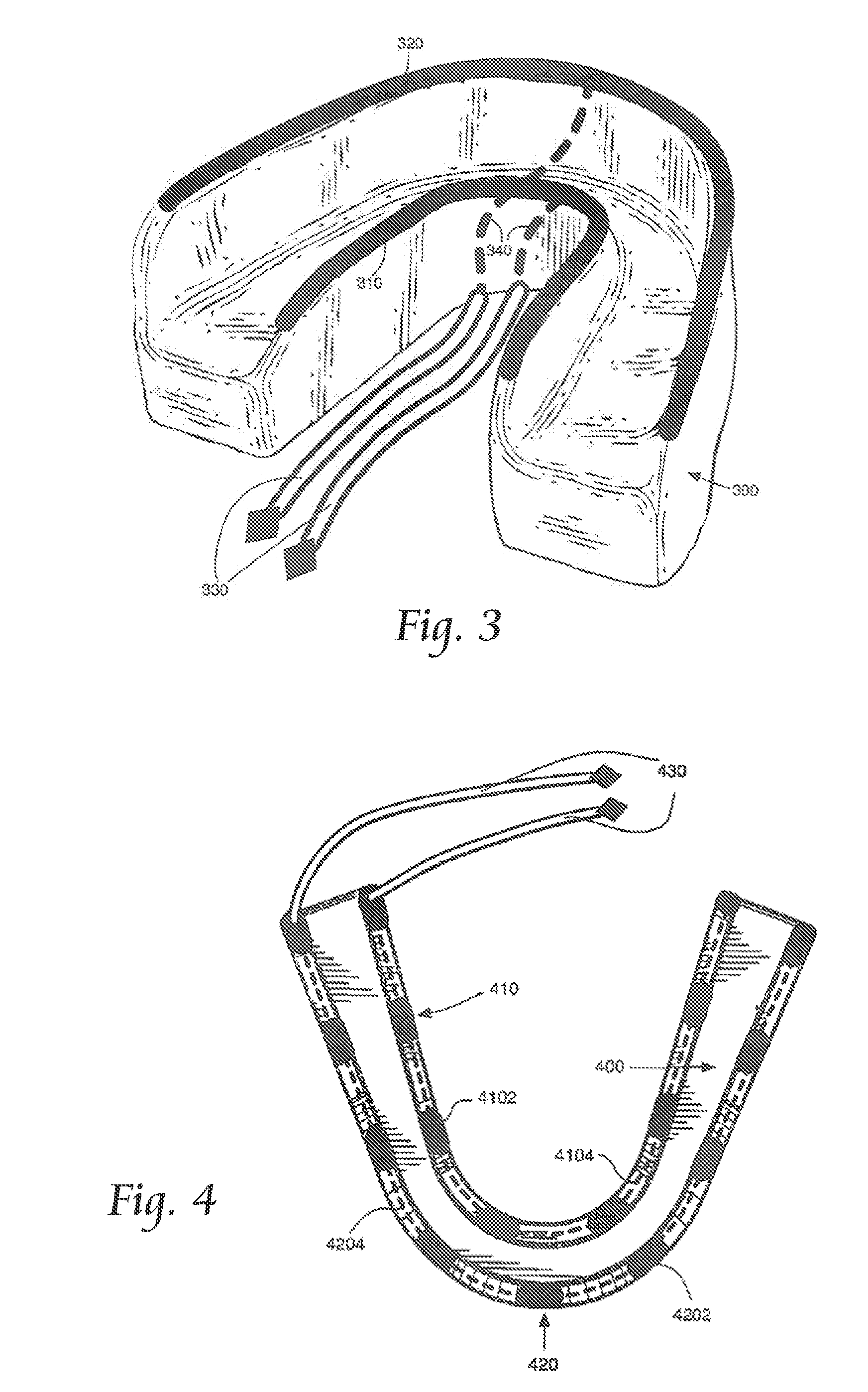
Concurrent treatment of oral maladies using direct current electricity
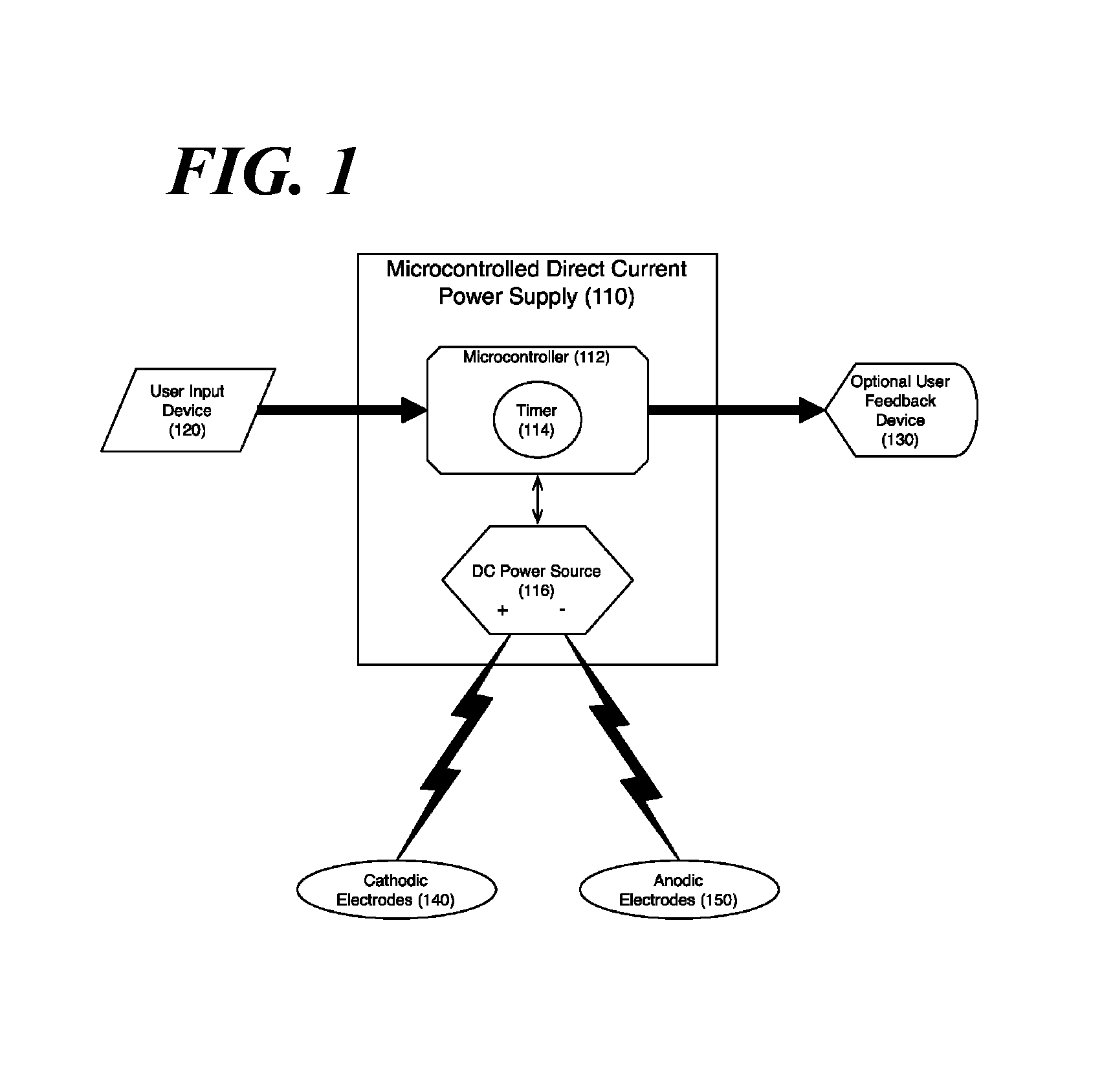
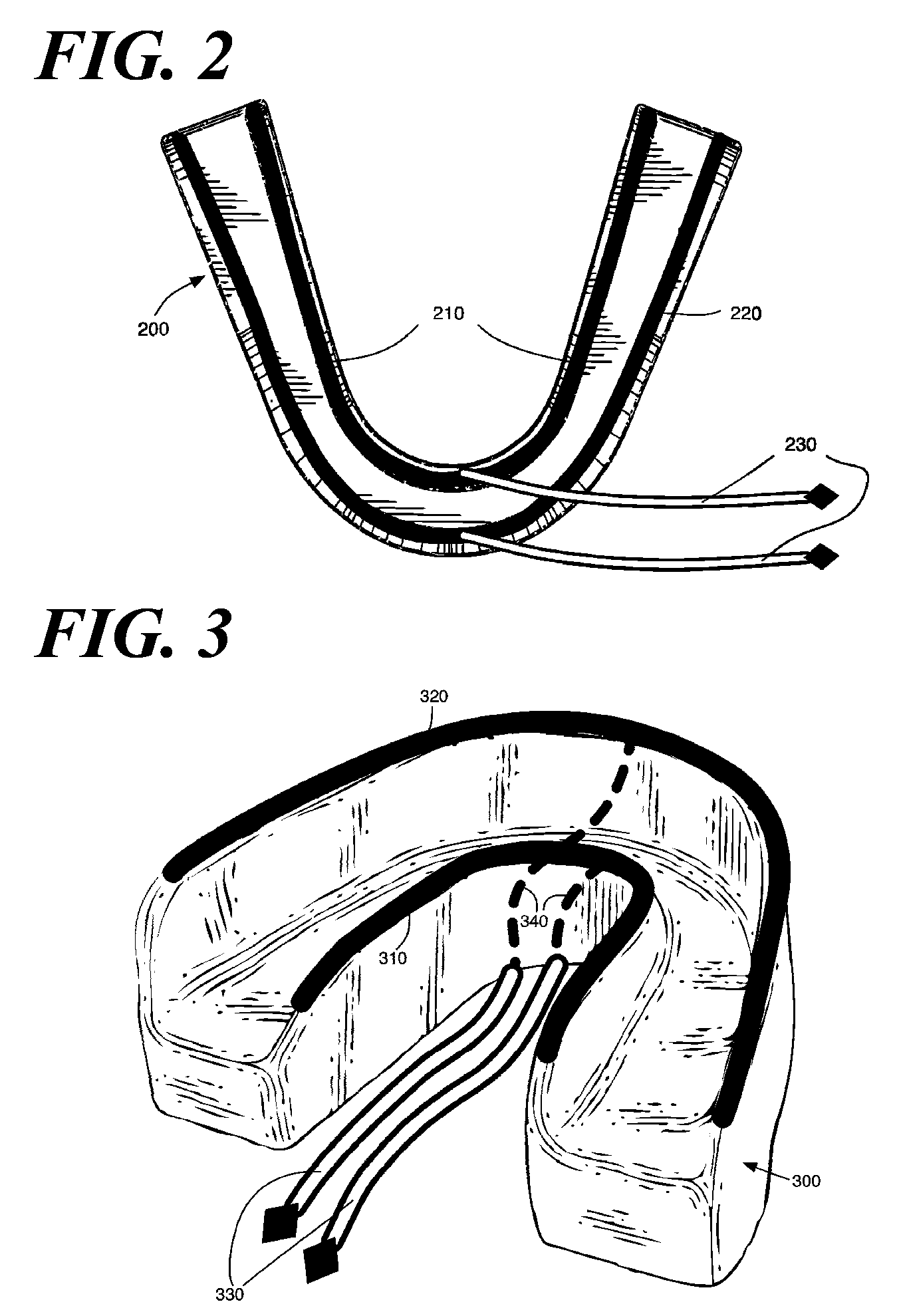
ActiveUS20090117513A1Preventing tooth decay and dental cariesSpeed up the flowElectrotherapyGum massageDiseaseGingival tissue
A method and apparatus for the concurrent treatment of multiple oral diseases and defects while promoting general oral hygiene utilizing direct current electricity. Electrodes are used to deliver a direct current to the gingival tissues of a mouth in order to achieve a number of therapeutic, prophylactic, and regenerative benefits. These benefits include killing oral microbes, increasing oral vasodilation, improving oral blood circulation, reversing oral bone resorption, promoting oral osteogenesis, treating gum recession, and fostering gingival regeneration. Other benefits include the treatment of gingivitis, perdiodontitis, and oral malodor while also promoting general oral hygiene.
Owner:BIOLECTRICS
Spinal fusion instrumentation, implant and method
InactiveUS7918888B2Small sizePromote bone growthBone implantSpinal implantsSurgical instrumentationIntervertebral space
A system and method includes surgical instrumentation, implants, bone graft material, and measurement equipment to enable a spine fusion procedure to proceed more accurately, efficiently and safely by allowing precision measurement of the characteristics of the intervertebral space, selection of and provision of new implants, placement of an intervertebral implant, and to help overcome bone resorption all where improved healing thereafter takes place.
Owner:HAMADA JAMES S
Compositions and methods for inhibiting bone resorption
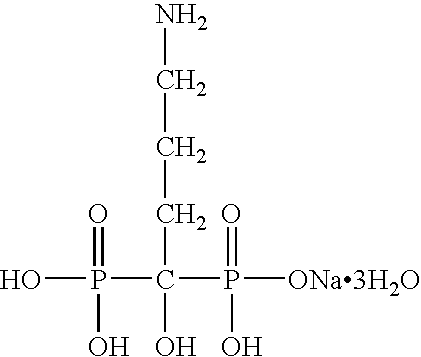
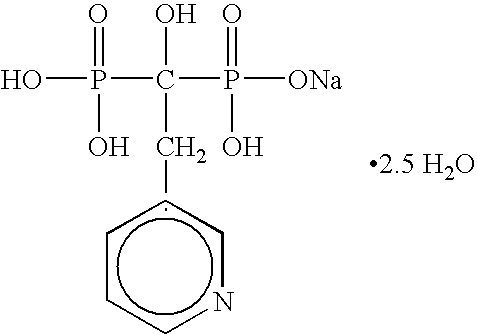
InactiveUS20050261250A1Reduce moisture contentPreventing and reducing and inhibiting and treating metabolic bone diseaseBiocidePhosphorous compound active ingredientsDiseaseNormal bone
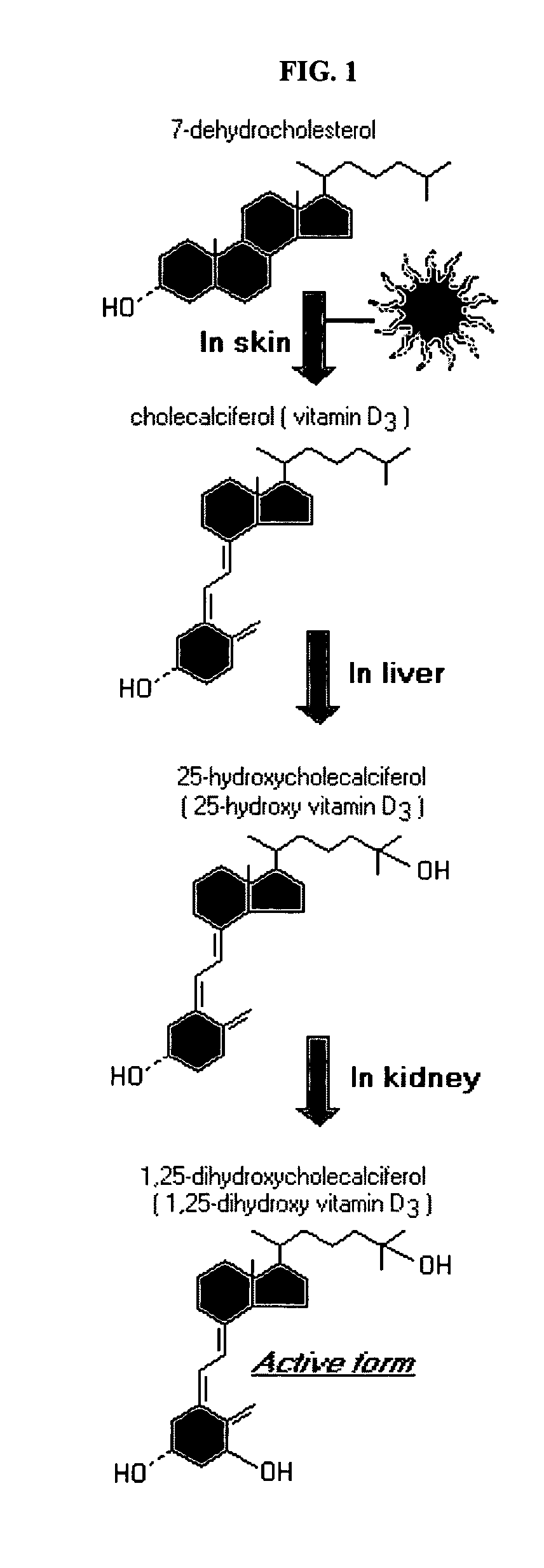
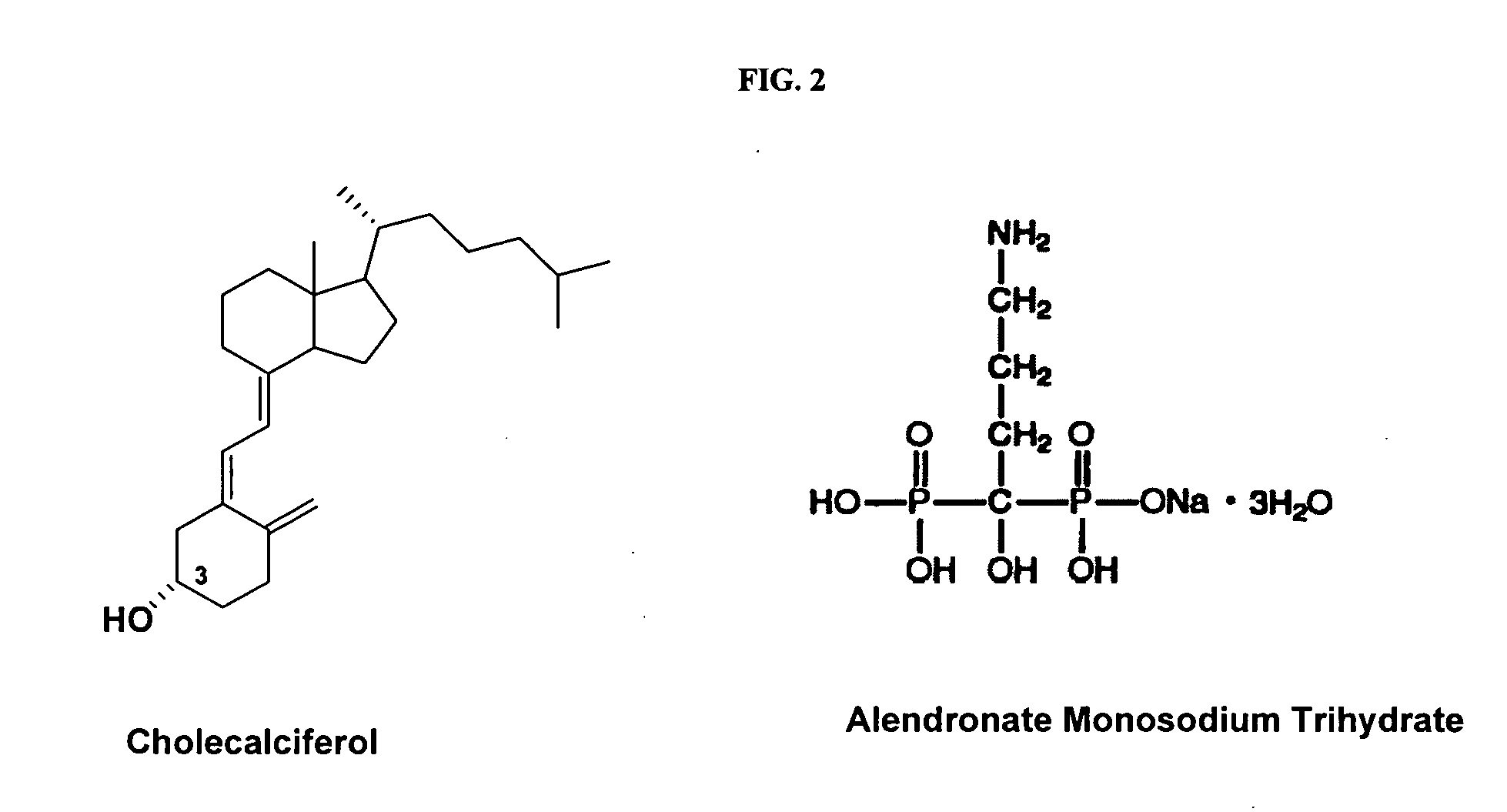
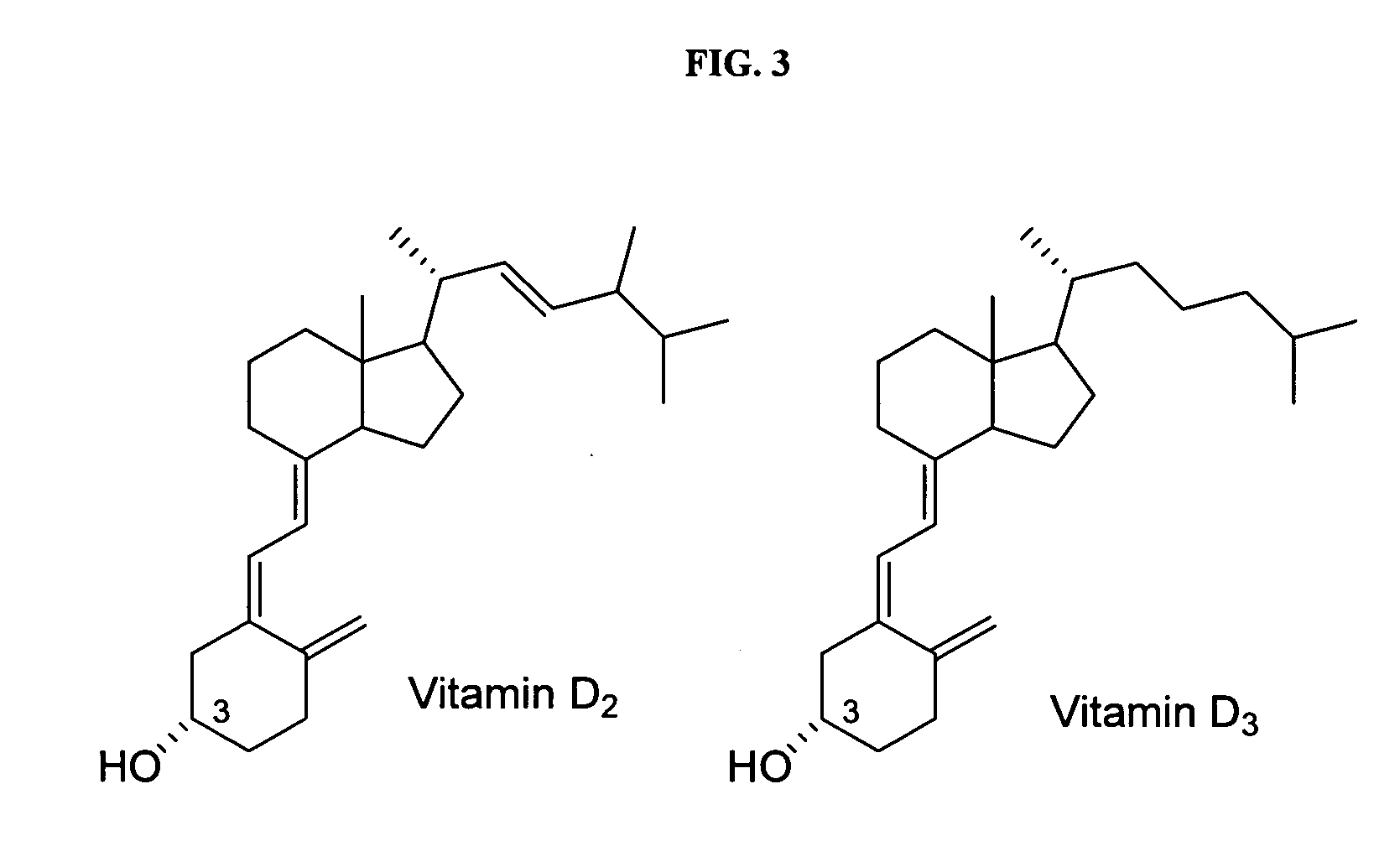
Disclosed are compositions and methods for preventing, inhibiting, reducing and treating conditions and diseases associated with abnormal bone resorption in mammals, including for example osteoporosis. Embodiments of compositions of the invention comprise a pharmaceutically effective amount of alendronate and vitamin D3 suitable for once-weekly dosing. Compositions and methods of the invention provide vitamin D nutrition during bisphosphonate treatment to facilitate normal bone formation and mineralization while minimizing the occurrence of or potential for the complications associated with vitamin D insufficiency, such as hypocalcaemia and osteomalacia. Also disclosed are methods for manufacturing compositions of the present invention, for measuring stability and degradation of those compositions, and for measuring blood plasma levels of vitamin D.
Owner:MERCK & CO INC
Concurrent treatment of oral maladies using direct current electricity
ActiveUS8660669B2Promote osteogenesisIncrease blood flowElectrotherapyGum massageDiseaseOral Cavity Disorder
A method and apparatus for the concurrent treatment of multiple oral diseases and defects while promoting general oral hygiene utilizing direct current electricity. Electrodes are used to deliver a direct current to the gingival tissues of a mouth in order to achieve a number of therapeutic, prophylactic, and regenerative benefits. These benefits include killing oral microbes, increasing oral vasodilation, improving oral blood circulation, reversing oral bone resorption, promoting oral osteogenesis, treating gum recession, and fostering gingival regeneration. Other benefits include the treatment of gingivitis, perdiodontitis, and oral malodor while also promoting general oral hygiene.
Owner:BIOLECTRICS
Composition and method for bone regeneration
InactiveUS20050147645A1Good effectDipeptide ingredientsTripeptide ingredientsOsteoblastBone formation
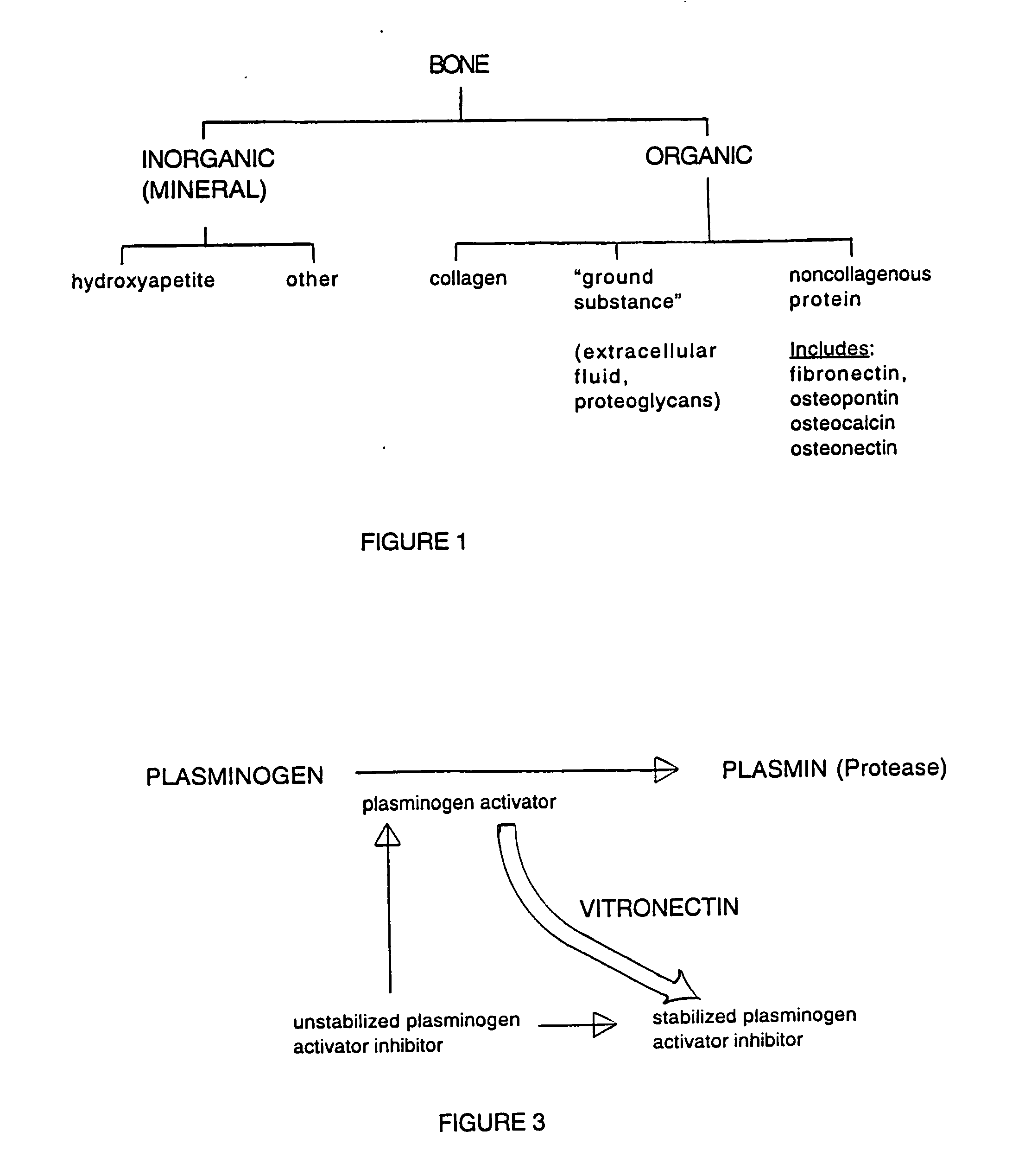
A composition for modulating bone regeneration composition comprises a matrix selected from the group consisting of glycolic acid, lactic acid, collagen, demineralized bone, or a combination thereof. A first biologically active molecule comprising a fibronectin is attached to a portion of the matrix, to facilitate osteoblast activity and for promoting an increase in bone formation. A second biologically active molecule comprising a vitronectin, selected for its ability to attract osteoclasts and produce an inhibiting effect on osteoclast activity to thereby promote a decrease in bone resorption, is also attached to a portion of the matrix.
Owner:BUDNY JOHN ARNOLD
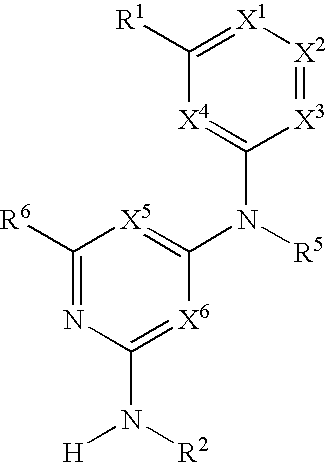
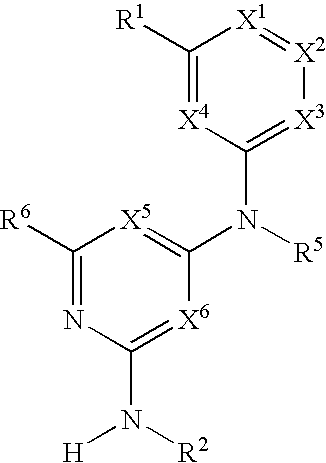
Substituted heterocyclic compounds and methods of use
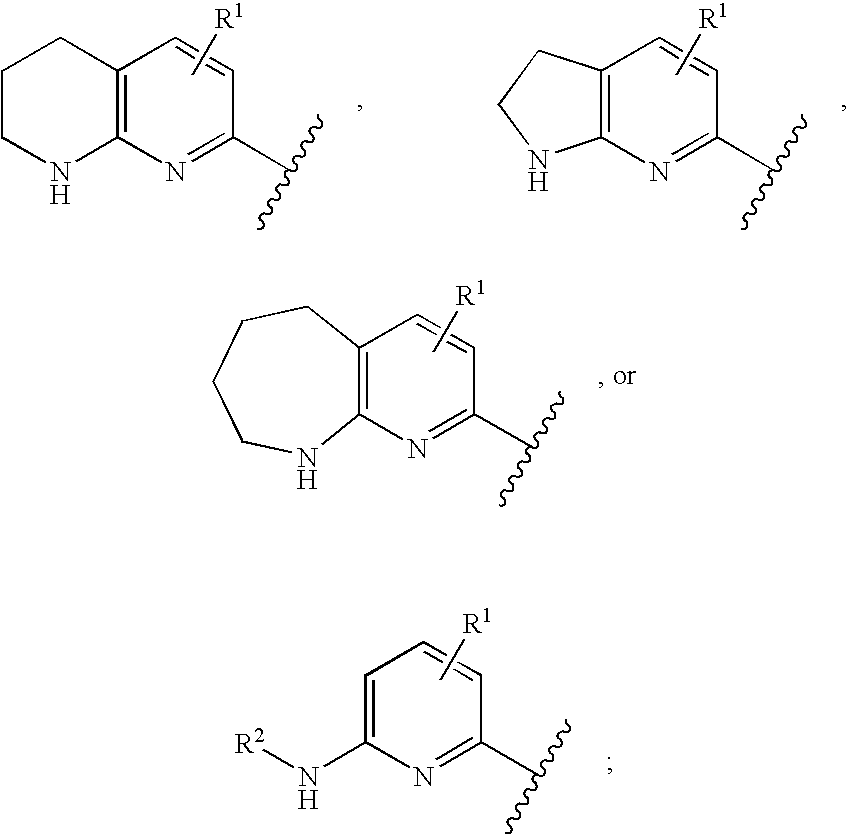
The present invention relates to pyridines, pyrimidines and derivatives thereof, and pharmaceutically acceptable salts thereof. Also included is a method of treatment of inflammation, rheumatoid arthritis, Pagets disease, osteoporosis, multiple myeloma, uveititis, acute or chronic myelogenous leukemia, pancreatic β cell destruction, osteoarthritis, rheumatoid spondylitis, gouty arthritis, inflammatory bowel disease, adult respiratory distress syndrome (ARDS), psoriasis, Crohn's disease, allergic rhinitis, ulcerative colitis, anaphylaxis, contact dermatitis, asthma, muscle degeneration, cachexia, Reiter's syndrome, type I diabetes, type II diabetes, bone resorption diseases, graft vs. host reaction, Alzheimer's disease, stroke, myocardial infarction, ischemia reperfusion injury, atherosclerosis, brain trauma, multiple sclerosis, cerebral malaria, sepsis, septic shock, toxic shock syndrome, fever, myalgias due to HIV-1, HIV-2, HIV-3, cytomegalovirus (CMV), influenza, adenovirus, the herpes viruses or herpes zoster infection in a mammal comprising administering an effective amount a compound as described above.
Owner:AMGEN INC
Concurrent Treatment of Oral and Systemic Maladies Using Direct Current Electricity
ActiveUS20130209964A1Lower F0Reduce the amount requiredHead electrodesGum massageWhole bodyGingival tissue
A method and apparatus for the concurrent treatment of multiple oral diseases and defects while promoting general oral hygiene utilizing direct current electricity. Electrodes are used to deliver a direct current to the gingival tissues of a mouth in order to achieve a number of therapeutic, prophylactic, and regenerative benefits. These benefits include killing oral microbes, increasing oral vasodilation, reducing oral biofilm, improving oral blood circulation, reversing oral bone resorption, promoting oral osteogenesis, treating gum recession, and fostering gingival regeneration. Other benefits include the treatment of gingivitis, periodontitis, and oral malodor, and other systemic diseases correlated with oral pathogens.
Owner:BIOLECTRICS
Toxin peptide therapeutic agents
ActiveUS7833979B2Preventing and mitigating relapseAvoid it happening againNervous disorderAntipyreticHalf-lifeFibrosis
Disclosed is a composition of matter of the formula(X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I)and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Sulfonylamino acid derivatives
InactiveUS6177466B1Easy to useLow toxicityBiocideOrganic chemistryAutoimmune conditionWhite blood cell
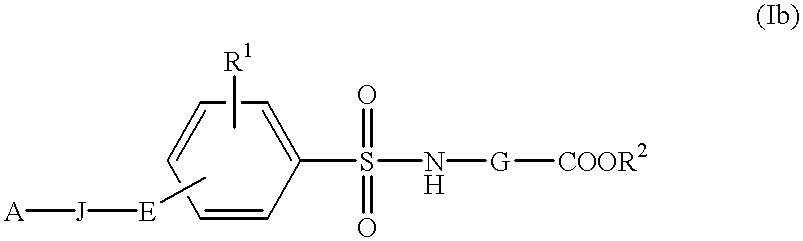
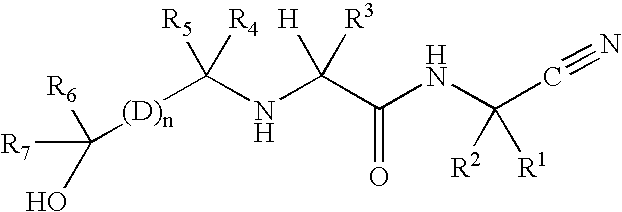
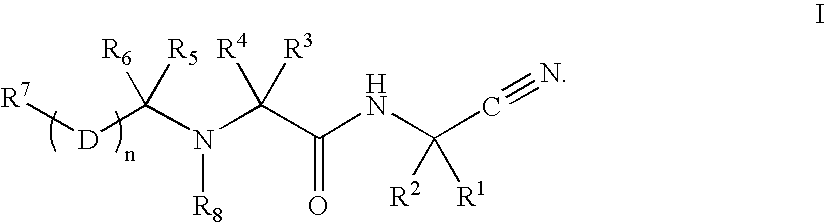
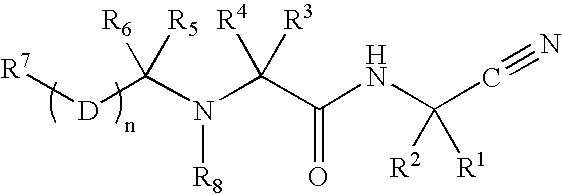
The present invention is related to:(i) matrix metalloproteinase inhibitors containing sulfonylamino acid derivatives of the formula (Ia):wherein R1 is hydrogen, C1-4 alkyl; R is hydrogen, C1-8 alkyl etc.; E is -CONR3-, in which R3 is hydrogen, C1-4 alkyl etc., -NR3CO-, -CO-O-, -O-CO- etc.; A is hydrogen, C1-8 alkyl, C3-7 cycloalkyl, or Ar; J is bond, C2-4 alkylene etc.; G is -(CH2)m- in which m is 2, 3 or 4, orin which R6 and R7 is hydrogen, C1-8 alkyl etc.; and non-toxic salts thereof,(ii) novel sulfonylamino acid derivatives of the formula (Ib):wherein all the symbols are the same meaning as (i); and non-toxic salts thereof, and(iii) process for the preparation of the compound of the formula (Ib). The compounds of the formula (Ia) are useful for prevention and / or treatment of diseases induced by overexpression and excess activity of MMP. The diseases such as above, for example, are rheumatoid, arthrosteitis, unusual bone resorption, osteoporosis, periodontitis, interstitial nephritis, arteriosclerosis, pulmonary emphysema, cirrhosis, cornea injury, metastasis of, invasion of or growth of tumor cell, autoimmune disease (Crohn's disease, Sjogren's syndrome etc.), disease caused by vascular emigration or infiltration of leukocytes, arterialization.
Owner:ONO PHARMA CO LTD
Calcium phosphate-based materials containing zinc, magnesium, fluoride and carbonate
ActiveUS20090068285A1Promotes bone formationMinimize and prevent bone resorptionBiocideInorganic phosphorous active ingredientsDiseaseAntioxidant
The present invention provides novel biomaterials comprising one or more of Mg, Zn and F ions in a carbonate-containing biphasic calcium phosphate (BCP) system. The biomaterial may contain Mg, Zn, F, Mg and Zn, Mg and F, Zn and F, or Mg, Zn and F. The biomaterial may be substantially similar in composition to bone mineral (a carbonate apatite). The biomaterial may feature slow release of Mg, Zn, F, Ca, and P ions. The biphasic calcium phosphate, BCP, may be a mixture of unsubstituted hydroxyapatite (HA) and unsubstituted .-TCP, Ca3(PO4)2. BCP of varying HA / .-TCP ratios may be produced by sintering calcium-deficient apatite, for instance having a Ca / P<1.5, 1.6, 1.67, 1.75 or 1.8 that has been prepared either by a precipitation or by a hydrolysis method or by a solid-state reaction. The amount of each component (by weight %) present in the biomaterials may be as follows: Mg 0.5 to 12 wt %, Zn 1 to 12 wt %, F 0.1 to 4 wt %, calcium 20 to 40 wt %, phosphate 10 to 20 wt %, and carbonate (CO3) 1 to 20 wt %. The biomaterial may further comprise one or more other ion such as strontium, manganese, copper, boron or silicate, or one or more other organic moiety such as a protein, a peptide, or a nutraceutical which may provide antioxidant, anti-bacterial or anti-inflammatory properties. The invention also provides methods of inhibiting bone resorption, methods of treating osteoporosis or delaying the onset of osteoporosis, methods of treating a bone fracture, and methods of inhibiting osteoclast activity. Further, the invention provides methods of treating or reversing bone deficiencies such as bone loss, similar to osteoporosis, caused all or in part by a mineral deficient diet, a disease such as cancer or osteopenia, a treatment such as steroid therapy or radiation therapy, or a physical condition such as immobilization.
Owner:NEW YORK UNIV
Cathepsin cysteine protease inhibitors
ActiveUS20060111440A1Avoid adjustmentImprove bioavailabilityBiocideSenses disorderDiseaseCathepsin O
This invention relates to a novel class of compounds which are cysteine protease inhibitors, including but not limited to, inhibitors of cathepsins K, L, S and B. These compounds are useful for treating diseases in which inhibition of bone resorption is indicated, such as osteoporosis.
Owner:MERCK CANADA
Injectable composite bone cement of hydroxyapatite-PMMA containing strontium, preparation method and application thereof
InactiveCN101934097AImprove biological activityGood biocompatibilityTissue regenerationProsthesisApatiteBiomechanics
The invention discloses an injectable composite bone cement of hydroxyapatite-PMMA containing strontium, consisting of a powder and a liquid monomer with the weight ratio of 1.5 to 2.5:1, wherein the main components of the powder are 10 to 30% of hydroxyapatite containing strontium and 70 to 90% of PMMA and / or PMMA / styrene copolymer in percentage by weight, and the main components of the liquid monomer are 98 to 99.9% of MMA and 0.1 to 2% of DMPT in percentage by weight. The bone cement has the advantages of safety, no toxity, convenient operation and molding, and low polymerization temperature. The bone cement has good biomechanics intensity, biological activity and biocompatibility, and can promote the growth of the bone, suppress the bone resorption and be used in radiological imaging because of containing the metallic element of strontium.
Owner:马文
Cathepsin cysteine protease inhibitors
ActiveUS20050240023A1Treating and preventing cathepsin dependent conditionBiocideOrganic chemistryCathepsin KCathepsin
This invention relates to a novel class of compounds which are cysteine protease inhibitors, including but not limited to, inhibitors of cathepsins K, L, S and B. These compounds are useful for treating diseases in which inhibition of bone resorption is indicated, such as osteoporosis.
Owner:AXYX PHARMA INC +1
Methods for decreasing osteoclast formation or bone resorption using an antibody to osteoprotegerin binding protein
A novel polypeptide, osteoprotegerin binding protein, involved in osteolcast maturation has been identified based upon its affinity for osteoprotegerin. Nucleic acid sequences encoding the polypeptide, or a fragment, analog or derivative thereof, vectors and host cells for production, methods of preparing osteoprotegerin binding protein, and binding assays are also described. Compositions and methods for the treatment of bone diseases such as osteoporosis, bone loss due to arthritis or metastasis, hypercalcemia, and Paget's disease are also provided.
Owner:AMGEN INC
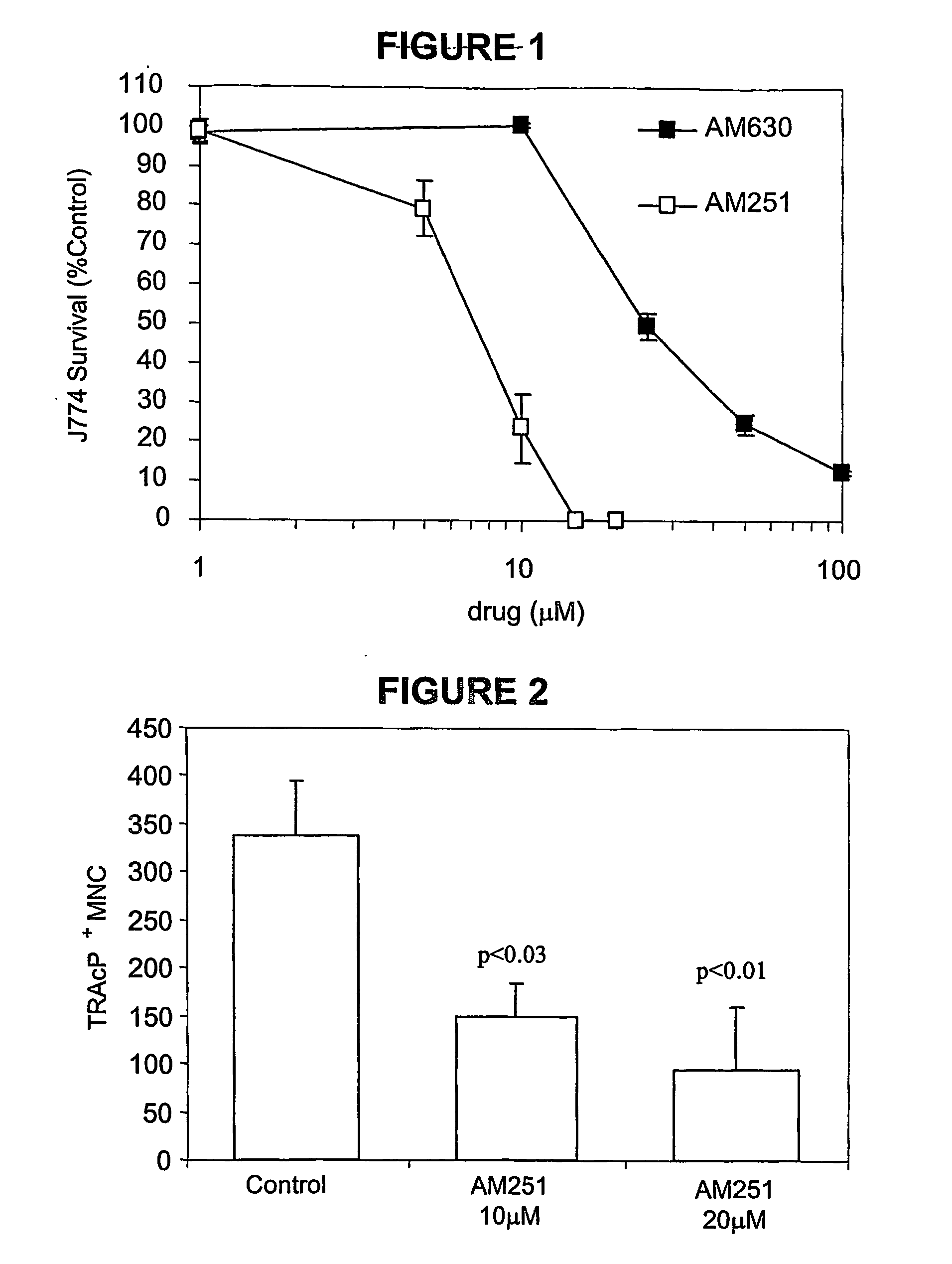
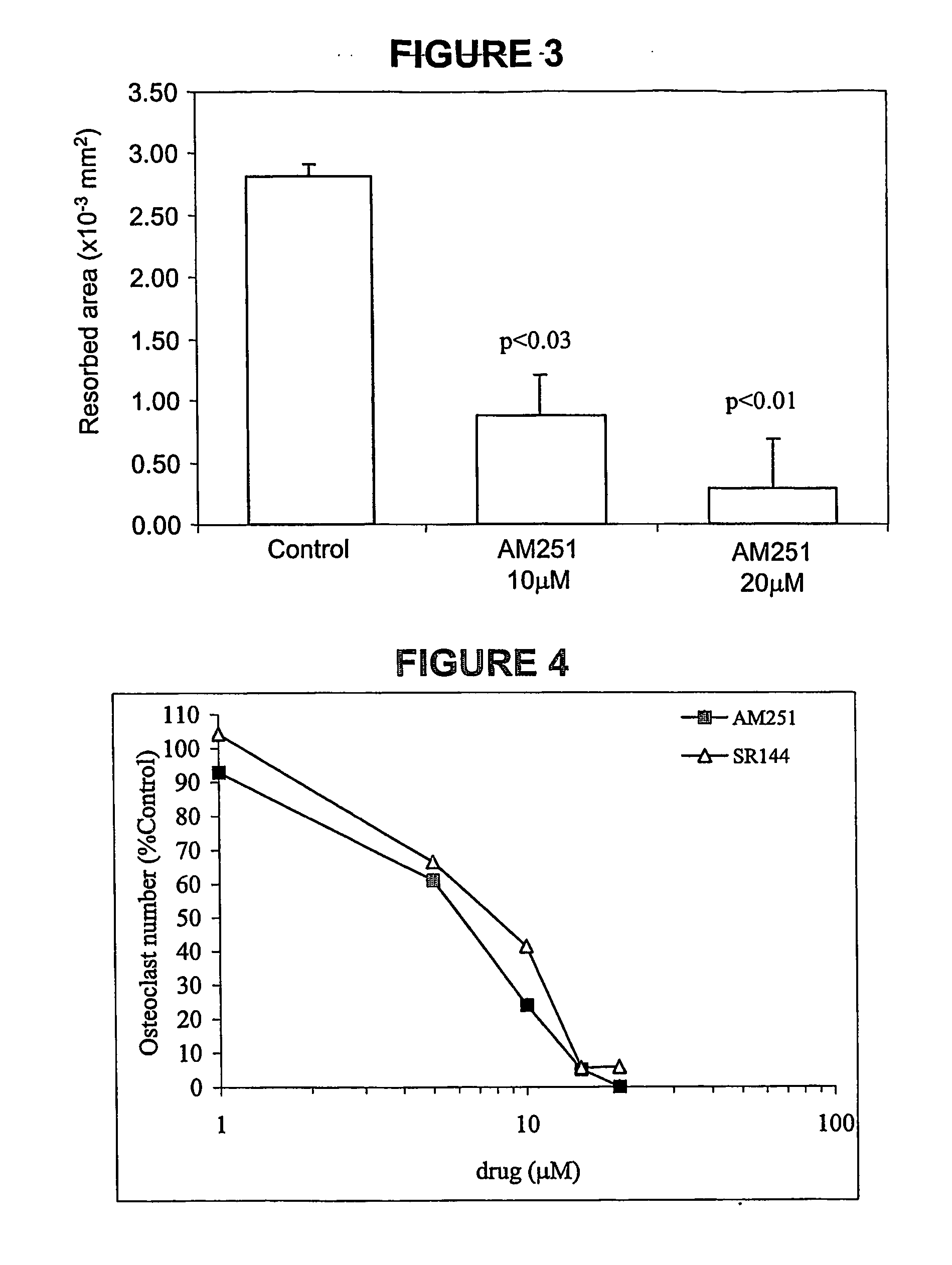
Cannabinoid receptor inverse agonists and neutral antagonists as therapeutic agents for the treatment of bone disorders
The present invention pertains to cannabinoid (CB) receptor inverse agonists and neutral antagonists, and especially CB1 and CB2 inverse agonists and neutral antagonists; such as, for example, certain pyrazole compounds; their use in the inhibition of osteoclasts (for example, the inhibition of the survival, formation, and / or activity of osteoclasts), and / or in the inhibition of bone resorption; their use in connection with treatment of bone disorders, such as conditions mediated by osteoclasts (e.g., increased osteoclast activity) and / or characterised by (e.g., increased) bone resorption, such as osteoporosis (e.g., osteoporosis not associated with inflammation; e.g., osteoporosis associated with a genetic predisposition, sex hormone deficiency, or ageing), cancer associated bone disease, and Paget's disease of bone.
Owner:ABERDEEN THE UNIV COURT OF THE UNIV OF
Prosthetic implant having segmented flexible stem
InactiveUS6887278B2Reduced and varying stiffnessHigh mechanical stiffnessJoint implantsFemoral headsHigh stiffnessTransverse groove
A prosthetic implant having a fixation stem with varying stiffness is disclosed. The stem comprises an elongated core and segments extending outward from the core. The segments are spaced apart so as to define transverse grooves surrounding the core between adjacent segments. The longitudinal length of the grooves, and the materials used for the core and the segments are selected such that the stiffness of the stem varies from the proximal end to the distal end. Typically, the stiffness of the stem will be lower at the distal end such that the distal end of the stem bears less force when loaded and thereby transfers more load to the proximal end of the stem, which has a higher stiffness. As a result, stress shielding, in which the load bypasses the proximal end of the stem, is minimized and bone resorption adjacent the proximal end of the stem is decreased.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
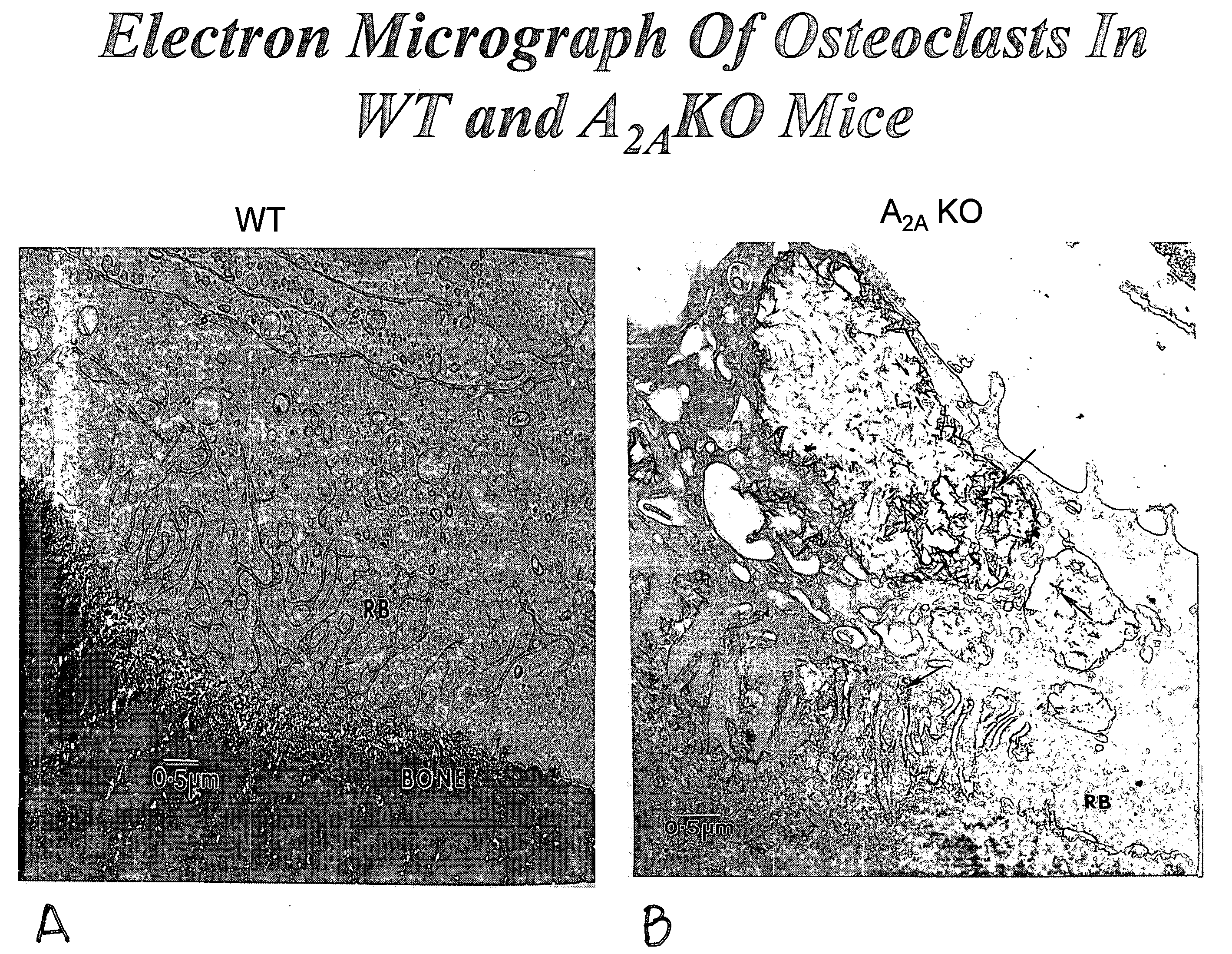
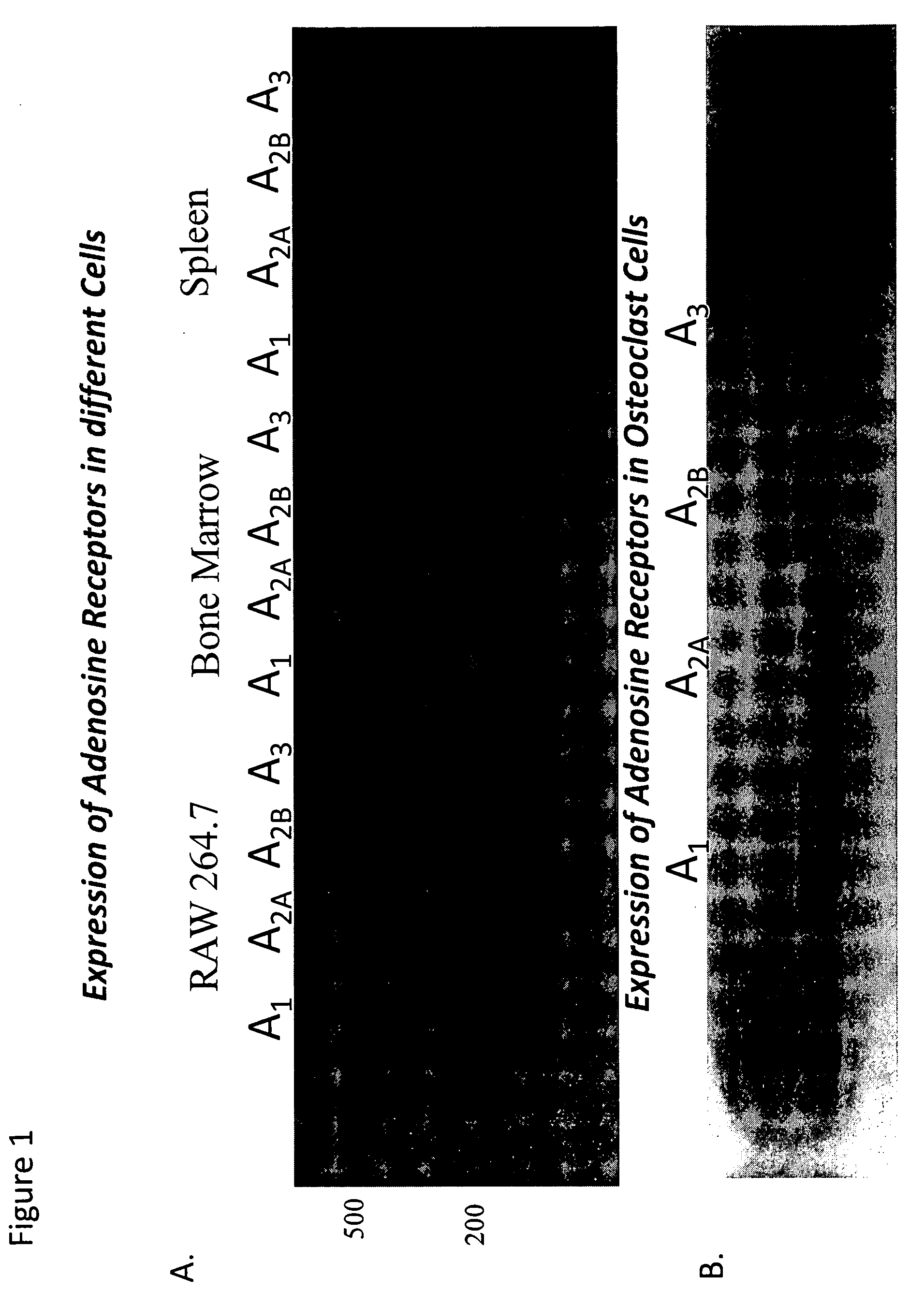
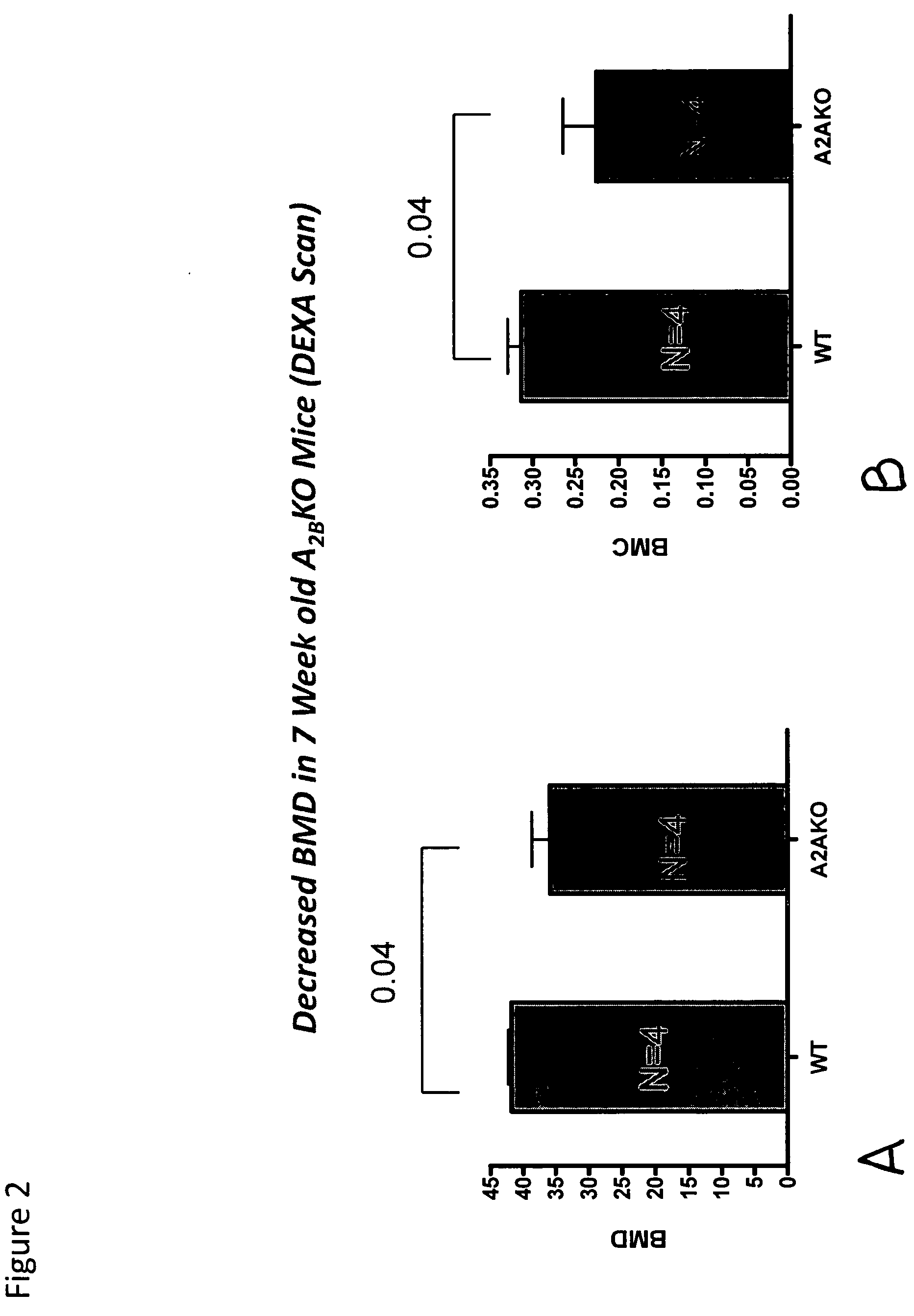
Medical implants containing adenosine receptor agonists and methods for inhibiting medical implant loosening
ActiveUS20090123510A1Potential damageEliminate side effectsBiocideSugar derivativesProsthesisAdenosine a
The invention provides methods and compositions for reducing or inhibiting bone resorption, osteoclast differentiation and stimulation and the loosening of medical prostheses by administering a compound or agent that modulates an adenosine receptor such as the adenosine A2A receptor, in particular, an agonist of an adenosine A2A receptor. The invention also extends to pharmaceutical compositions comprising such an agent that modulates an adenosine receptor such as an adenosine A2A agonist and to prosthetic devices containing such an agent that modulates an adenosine receptor such as an A2A agonist on one or more surfaces or within the prosthetic device such as, for example, suspended in the cement forming the prosthetic device.
Owner:NEW YORK UNIV
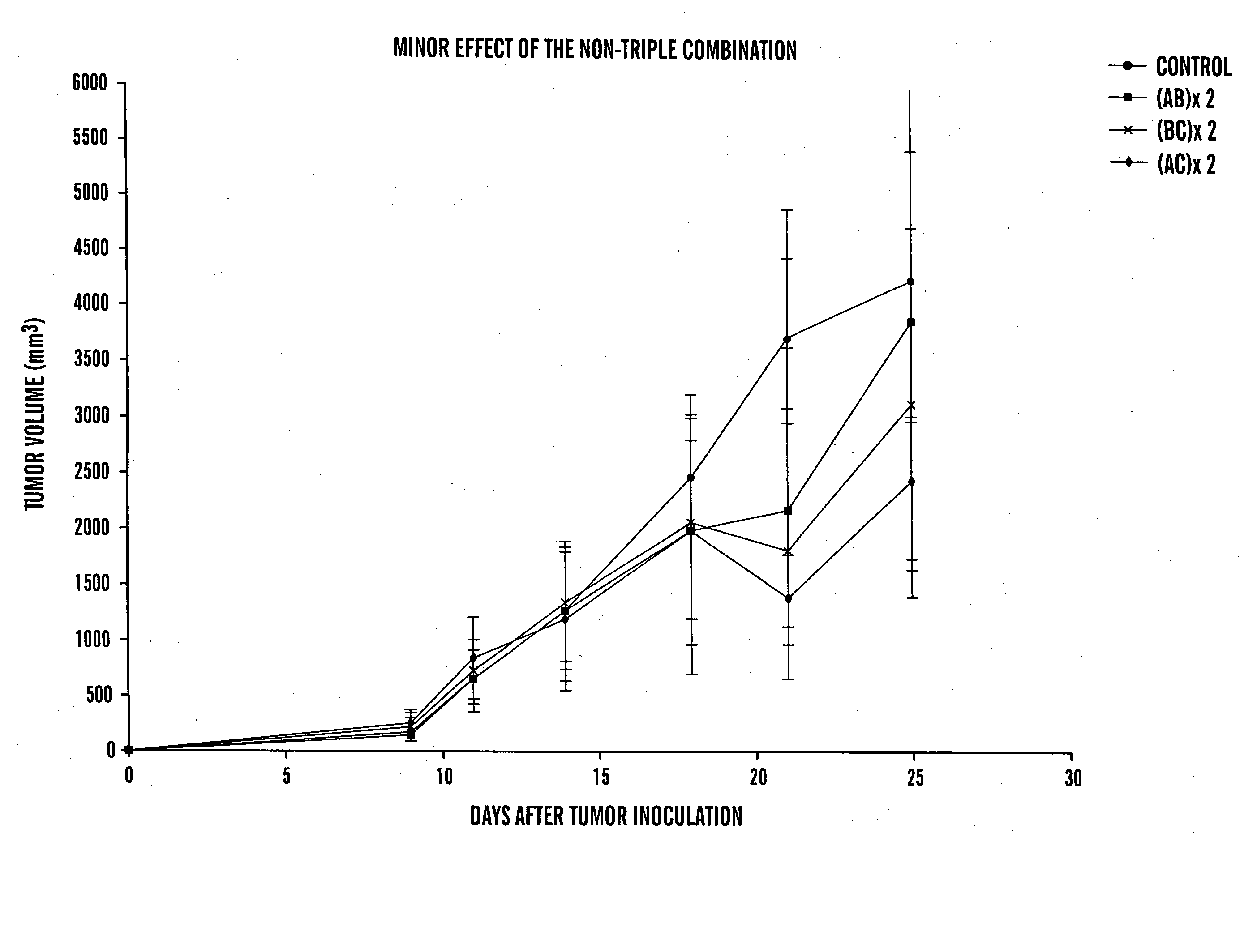
Anti-cancer combination and use thereof
InactiveUS20050148521A1Synergistic anti-cancer compositionReduce dosageBiocidePeptide/protein ingredientsSide effectCytotoxicity
The present invention relates to the surprising discovery that the combination of several agents, each well known for its established role in treating cancer, inflammation, hemostasis, bone resorption or serving as a solubilizing vehicle, results in a synergistic anti-cancer composition. Furthermore, the combination of at least three agents allows the cytotoxic agent, such as cyclophosphamide, to be used at a lower dosage than when administered alone. One predicted consequence of this treatment, therefore, is a highly desirable reduction in toxic side effects due to the cytotoxic agent.
Owner:TILTAN PHARMA LTD
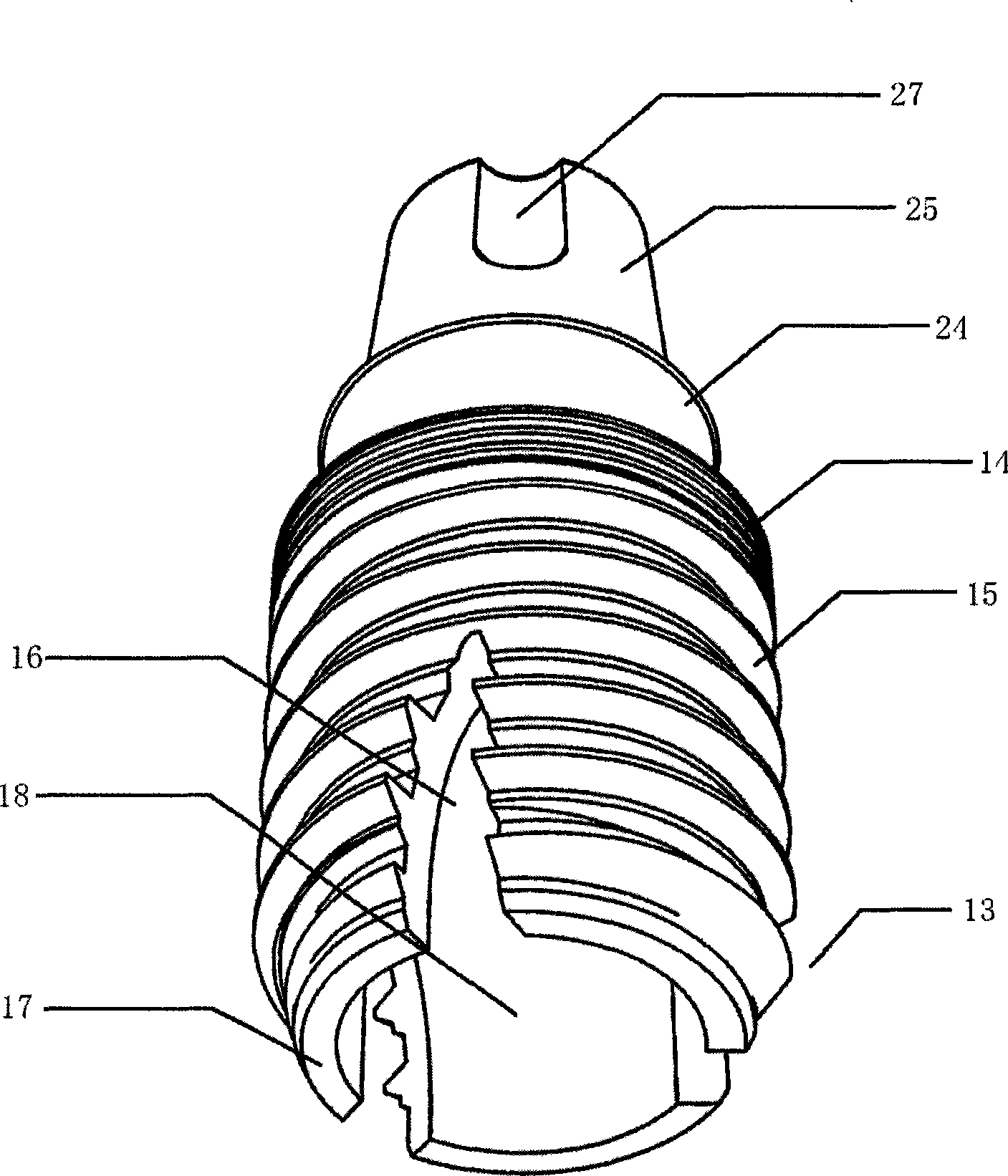
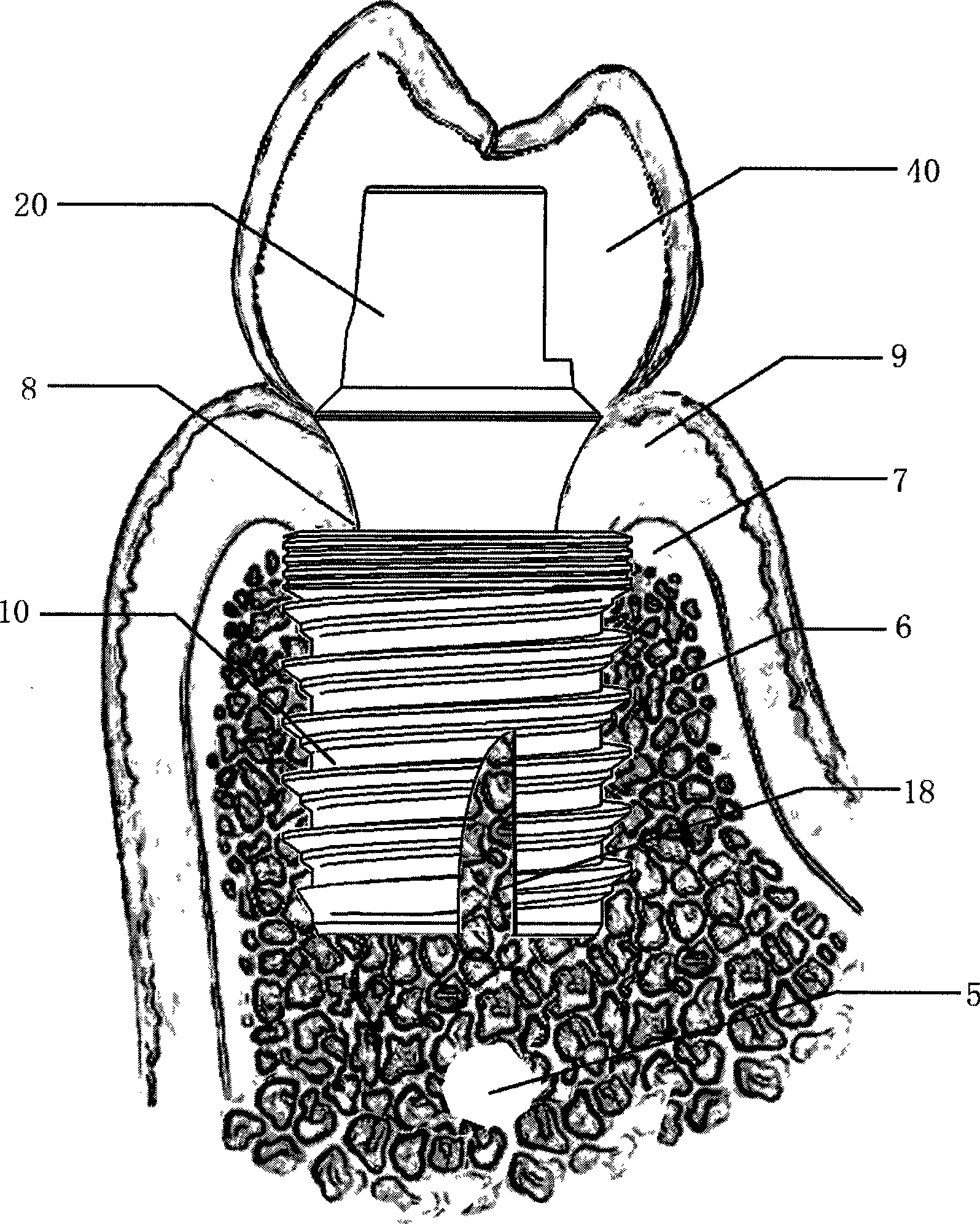
Hollow short grow body of oral cavity tooth grow
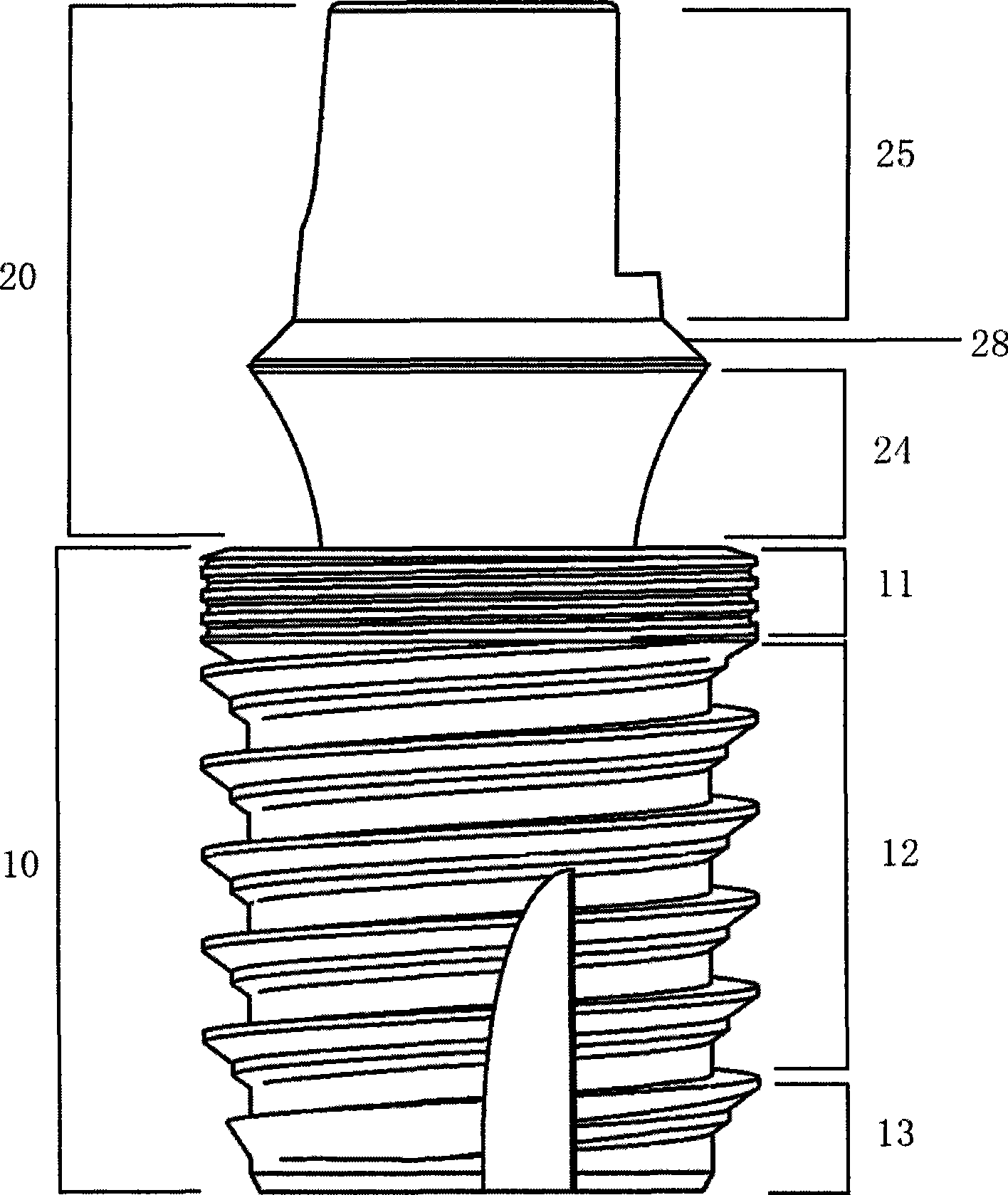
The invention relates to a hollow short implant of a dental implant, which comprises an one-stage structure and a two-stage structure, wherein the one-stage implant part is connected with an abutment part into a whole; the two-stage structure comprises an implant, a bridge adapter ring and a central bolt; peripheral cylinder spirochaeta of the implant part is divided into three stages, saw-tooth double thread in the main part of the implant can bear heavier load; the hollow structural design of the implant part keeps live bone column with a base and blood supply so as to enhance the supporting strength of the bone and the implant; a self-tapping socket can make bone tissue of the internal and external of the implant grow through and heal; a platform transfer design allows the epithelial cuff of the gum around the implant more reliable to avoid bone resorption caused by micro moving and micro leakage. The short hollow implant realizes combination of the internal and external bone, increases the combination area and supporting strength for a short or a long term, can be implanted rapidly with high strength, better retention in the early stage, high success rate in a long term, and solves the problem that the upper and lower jaws abrase the dental area in the conventional oral implant technology.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Concurrent Treatment of Oral and Systemic Maladies in Animals Using Electrical Current
ActiveUS20140093832A1Reducing oral biofilmsIncrease blood flowHead electrodesDentistryOral Cavity DisorderGingival tissue
A method and apparatus for the concurrent treatment of multiple oral diseases and defects while promoting general oral hygiene utilizing electricity are provided for non-human animals. Electrodes are used to deliver an electrical current to the gingival tissues of a mouth in order to achieve a number of therapeutic, prophylactic, and regenerative benefits. These benefits include killing oral microbes, increasing oral vasodilation, reducing oral biofilm, improving oral blood circulation, reversing oral bone resorption, promoting oral osteogenesis, treating gum recession, and fostering gingival regeneration. Other benefits include the treatment of gingivitis, periodontitis, and oral malodor, and other systemic diseases correlated with oral pathogens.
Owner:BIOLECTRICS
Cathepsin cysteine protease inhibitors
Owner:AXYX PHARMA INC +1
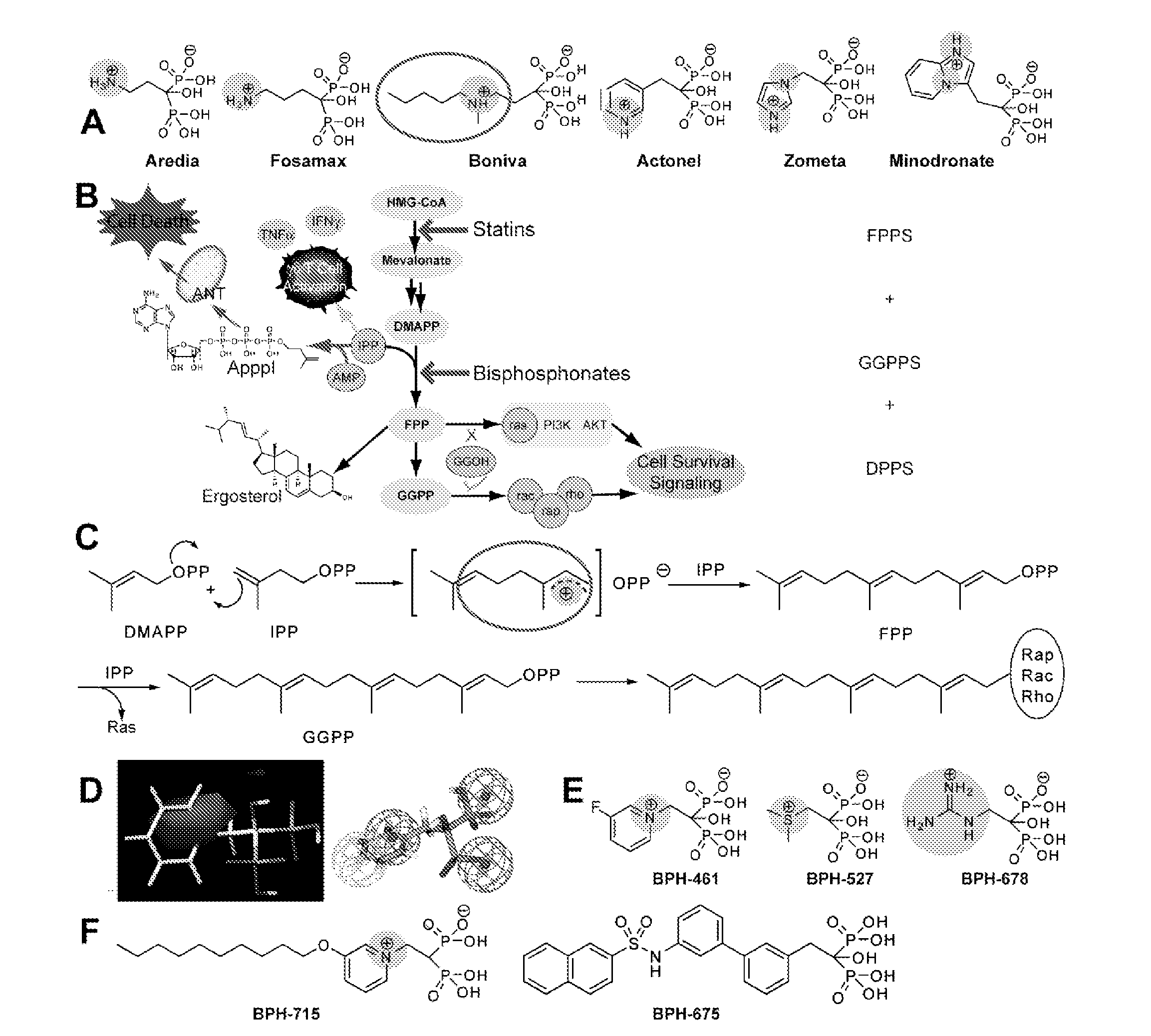
Bisphosphonate Compounds and Methods with Enhanced Potency for Multiple Targets including FPPS, GGPPS, AND DPPS
InactiveUS20080255070A1High activityGood effectBiocideMicrobiological testing/measurementCancer cellDiphosphonates
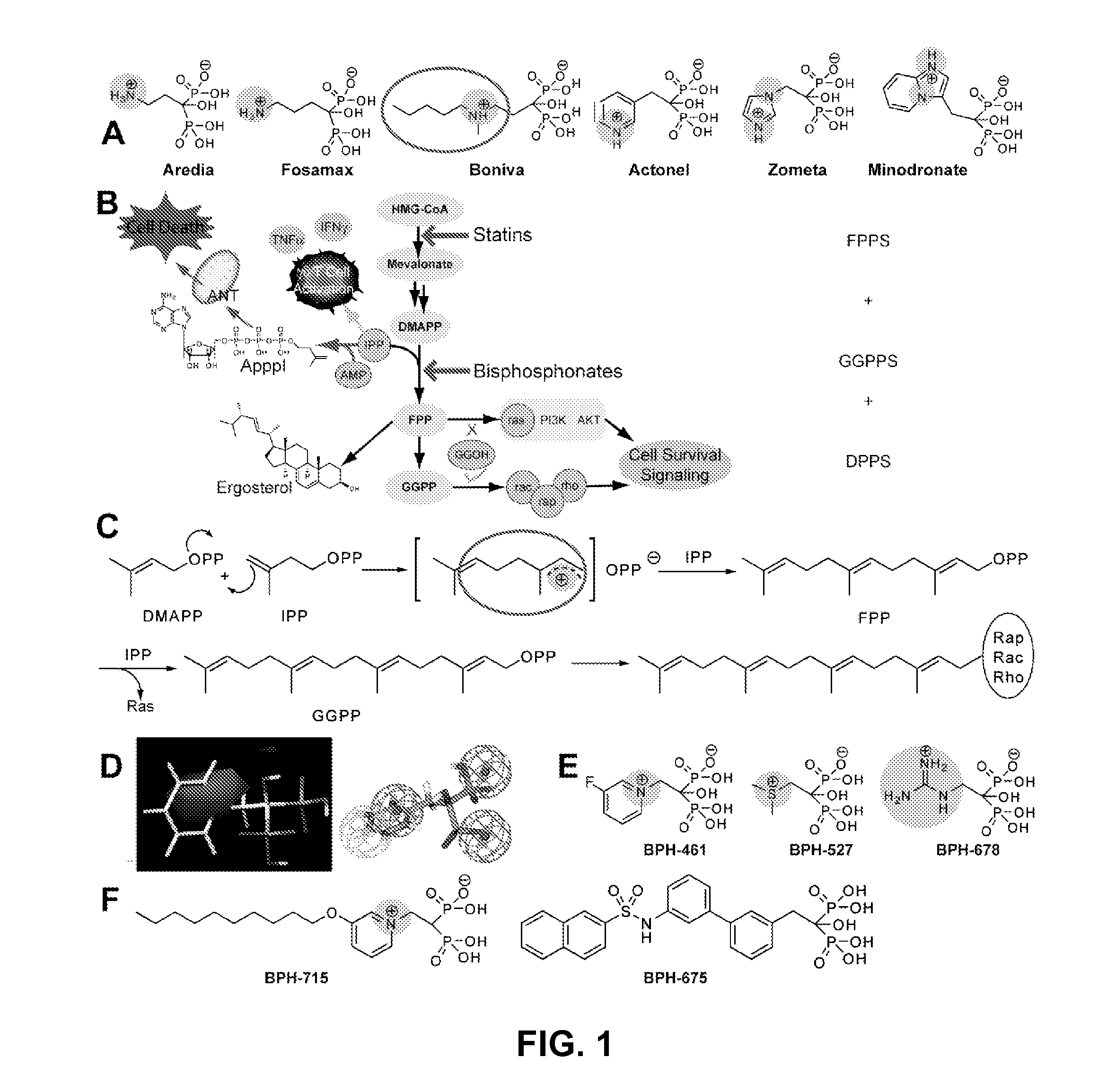
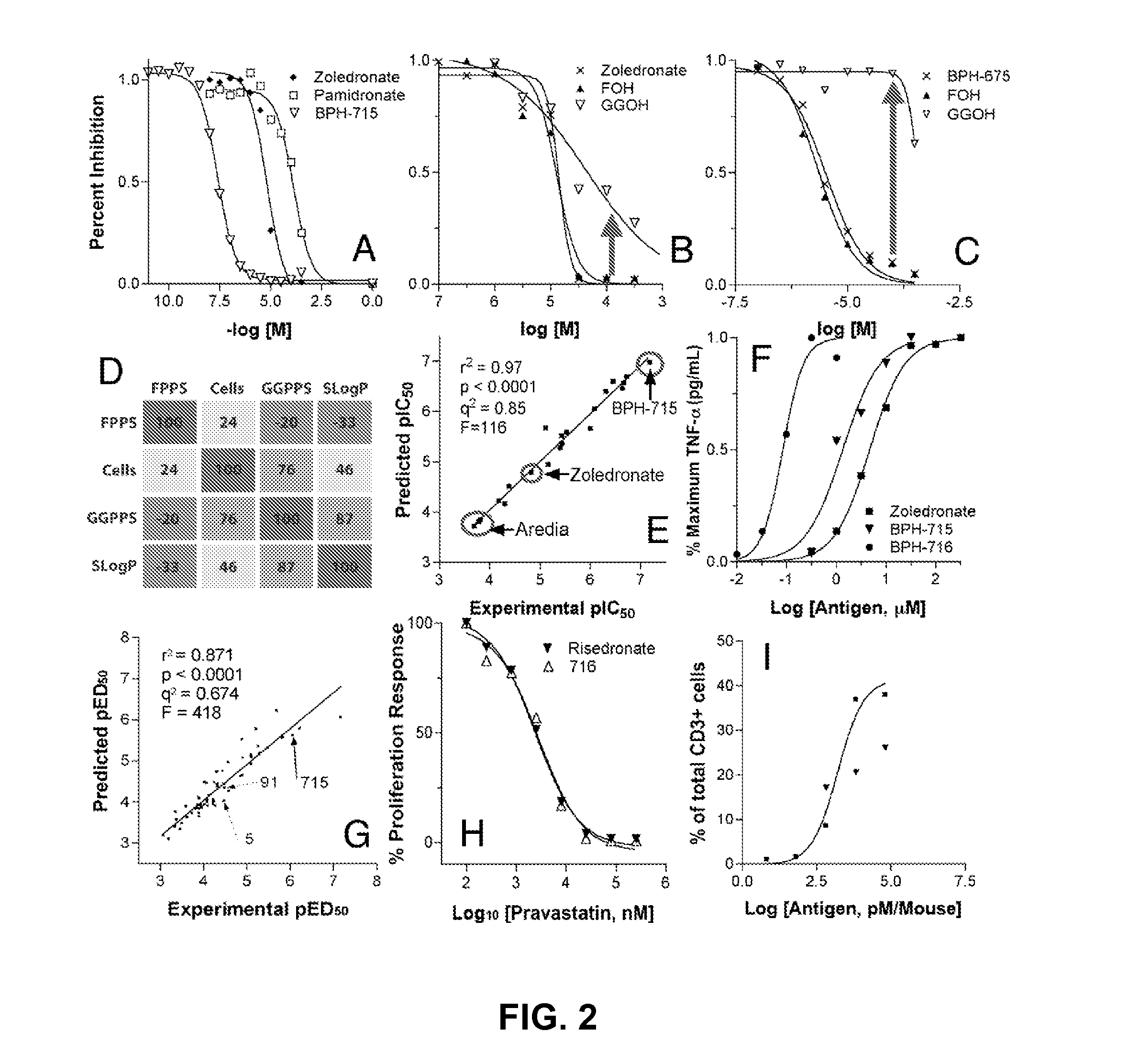
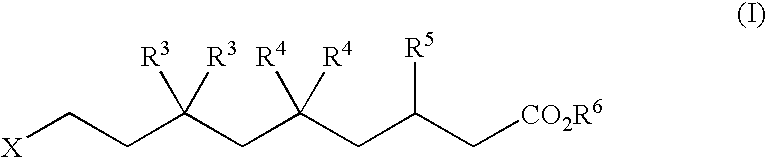
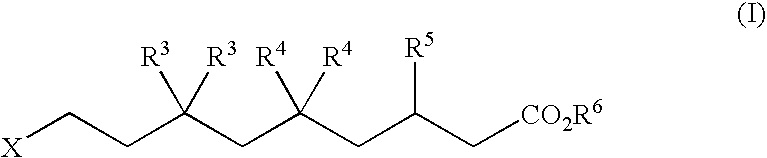
The disclosure provides, inter alia, novel bisphosphonate compounds and methods of making and using such compounds. In certain embodiments, compounds of the invention include bisphosphonates that are capable of selectively inhibiting one or more of farnesyl diphosphate synthase (FPPS), geranylgeranyl diphosphate synthase (GGPPS), and decaprenyl pyrophosphate synthase (DPPS). In preferred embodiments, compounds of the invention are capable of selectively inhibiting two or more of FPPS, GGPPS, and DPPS. In embodiments, compounds and methods of the invention demonstrate superior activity levels, such as in the anti-cancer context, immunostimulation context, and other contexts, which in several cases exceed the activity levels of previous generation bisphosphonate drugs by orders of magnitude. In embodiments, the invention provides compounds and methods in connection with research and therapeutic applications, e.g., for tumor or cancer cell growth inhibition, activation of gammadelta T cells, inhibition of certain enzymes related to the mevalonate metabolic pathway, bone resorption diseases, cancer, immune disorders, immunotherapy, and infectious diseases.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Alpha v integrin receptor antagonists
The present invention relates to novel chain-fluorinated alkanoic acid derivatives thereof, their synthesis, and their use as αv integrin receptor antagonists. More particularly, the compounds of the present invention are antagonists of the integrin receptors αvβ3 and / or αvβ5 and are useful for inhibiting bone resorption, treating and preventing osteoporosis, and inhibiting vascular restenosis, diabetic retinopathy, macular degeneration, angiogenesis, atherosclerosis, inflammation, inflammatory arthritis, viral disease, cancer, and metastatic tumor growth.
Owner:MERCK SHARP & DOHME CORP
Agent inducing increase in bone mass
InactiveUS20060173009A1Excellent osteoblast differentiation promoting activityEasy to operateBiocideAntipyreticMetabolic bone diseaseBone resorption
Osteoporosis and the like metabolic bone diseases have a high frequency of causing lumbago and the like pains and bone fracture, due to lowering of the bone strength caused by the reduction of bone mass. Accordingly, great concern has been directed toward the creation of a drug which can increase the bone mass and bone strength by controlling the whole bone metabolism. The pharmaceutical composition or combination product of the invention comprising a non-living body-derived non-peptide osteoblast differentiation promoting compound and a bisphosphonate having a bone resorption inhibitory action is useful as a bone mass increasing inducer which can increase the bone mass and / or bone strength by controlling the bone metabolism.
Owner:ASTELLAS PHARMA INC
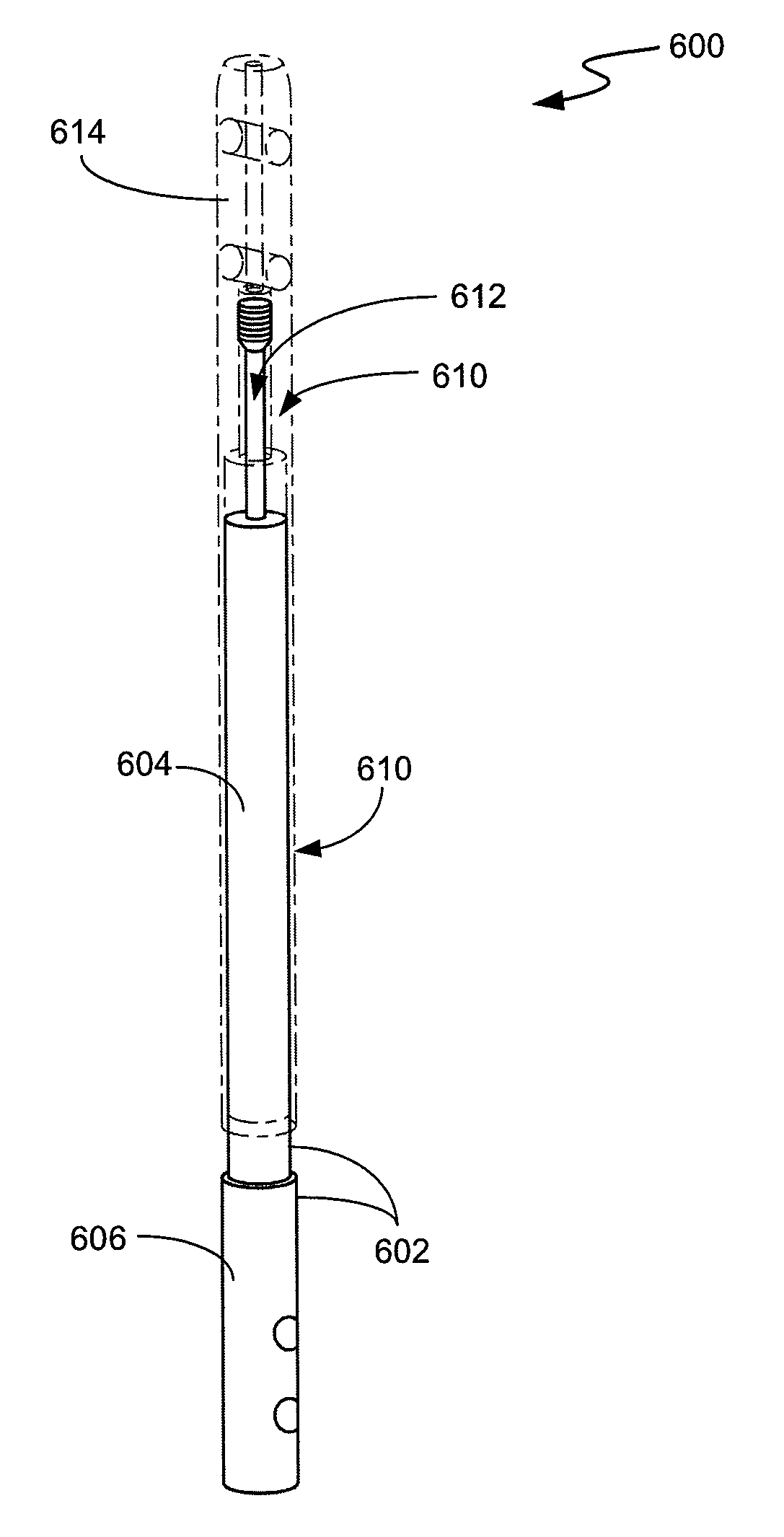
Intramedullary medical device and methods of use and manufacture
Intramedullary medical devices (e.g., intramedullary nails) and methods for their use and manufacture are described herein. The intramedullary medical devices described herein may provide sustained compressive forces across a bone fusion site despite bone resorption processes. Through various embodiments, the intramedullary medical devices described herein may provide non-linear force curves relative to displacement. Intramedullary medical devices are described with multiple elements made of different materials. Examples of intramedullary medical devices are described with shape memory alloys.
Owner:MEDSHAPE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com