Patents
Literature
64 results about "Sjögren syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sjögren syndrome (SjS, SS) is a long-term autoimmune disease that affects the body's moisture-producing glands. Primary symptoms are a dry mouth and dry eyes. Other symptoms can include dry skin, vaginal dryness, a chronic cough, numbness in the arms and legs, feeling tired, muscle and joint pains, and thyroid problems. Those affected are at an increased risk (5%) of lymphoma.
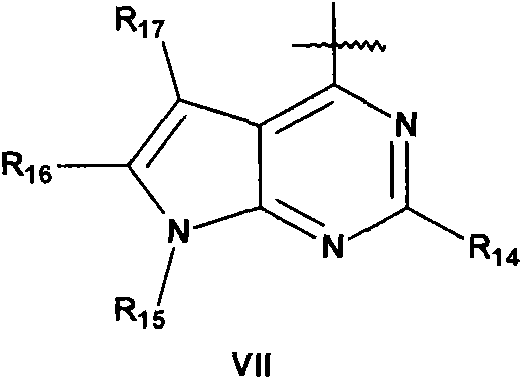
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
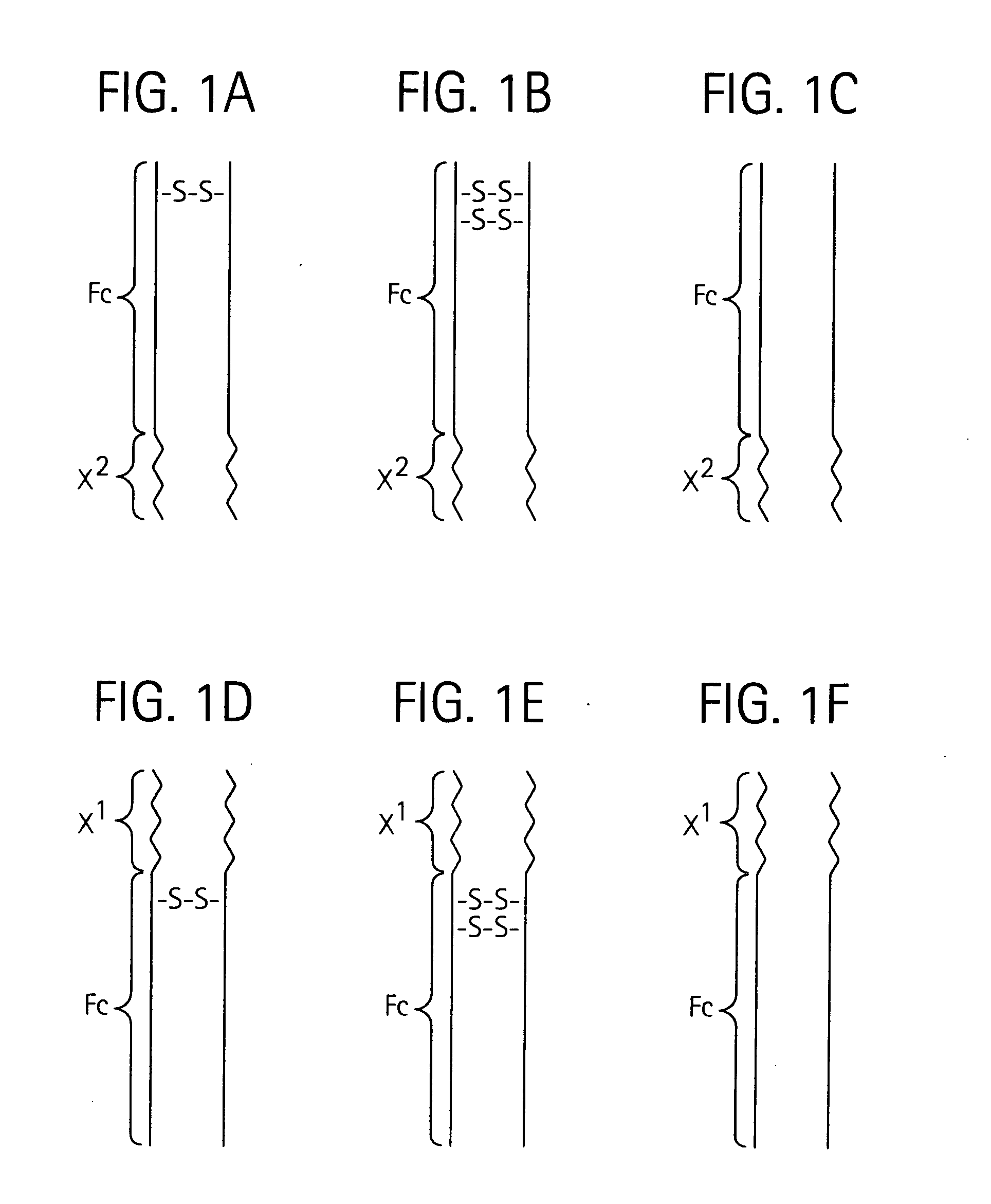
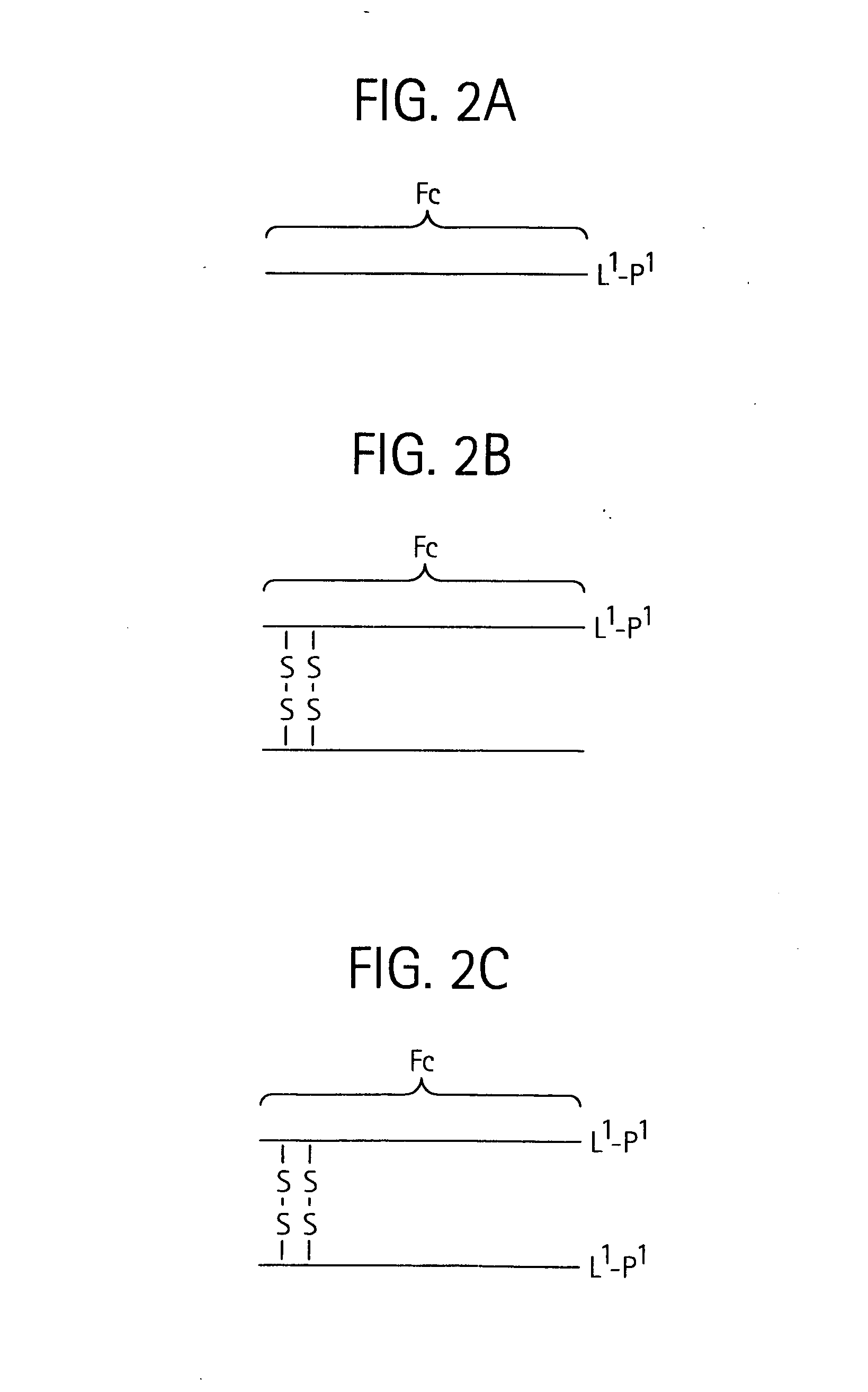
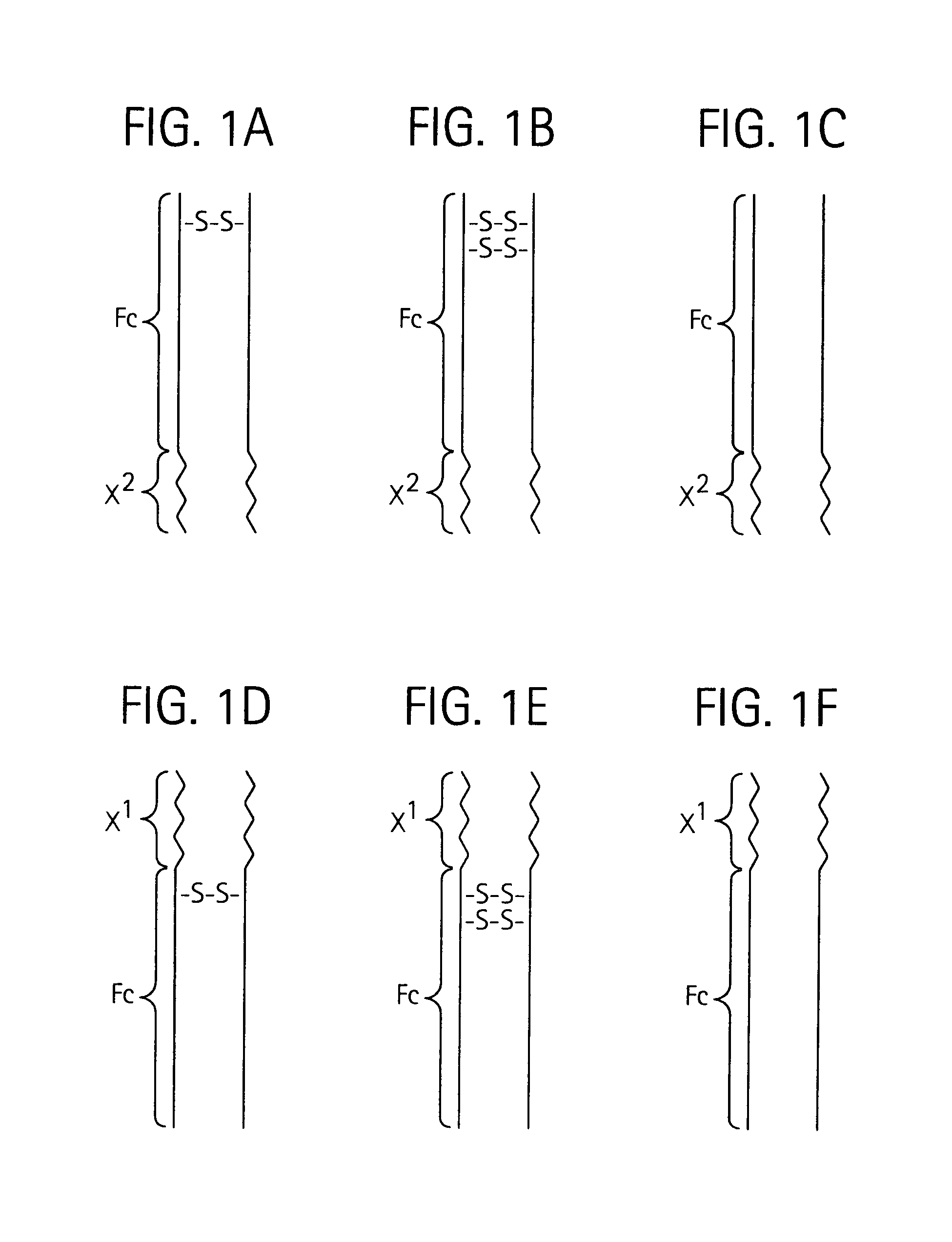
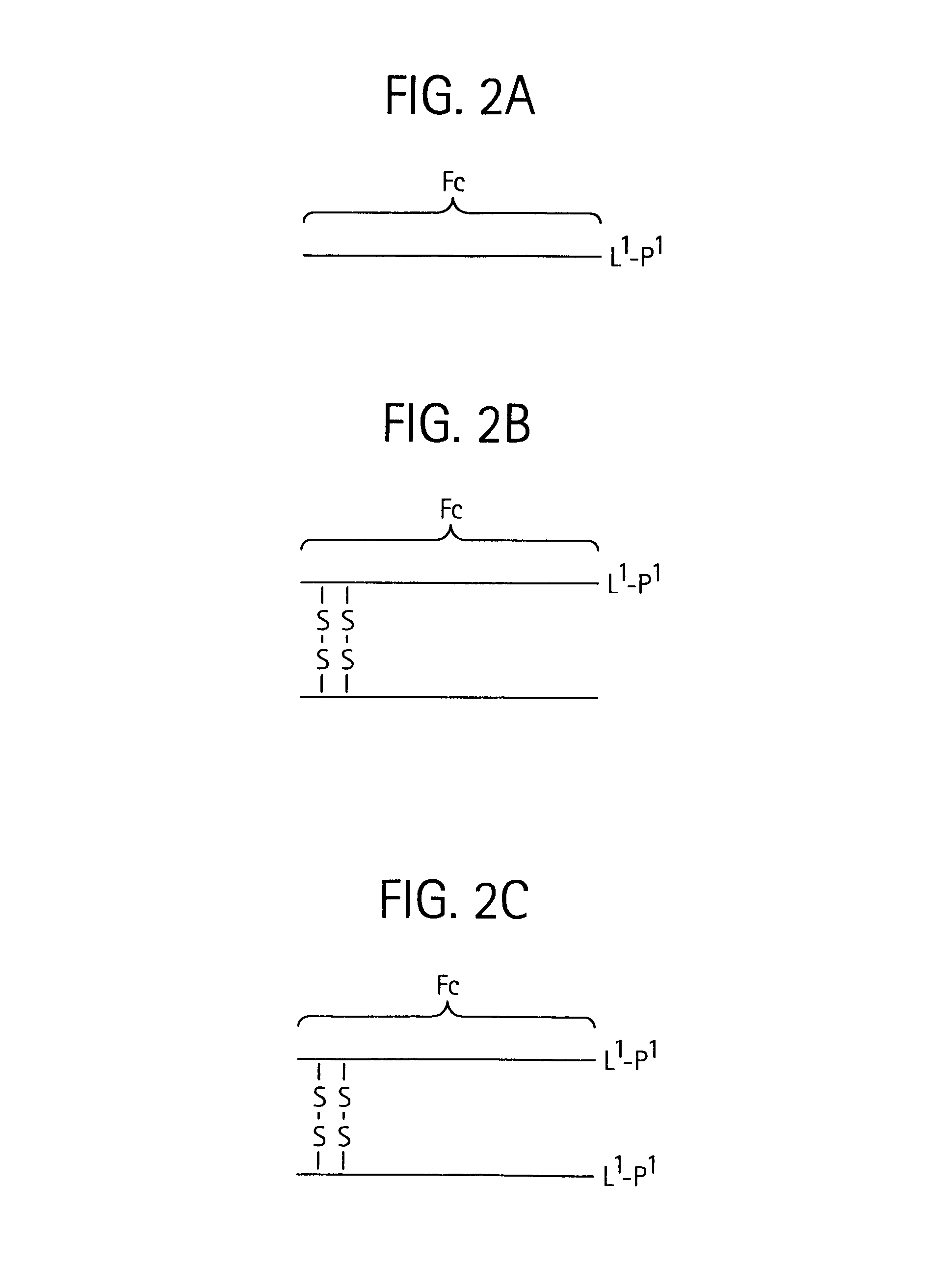
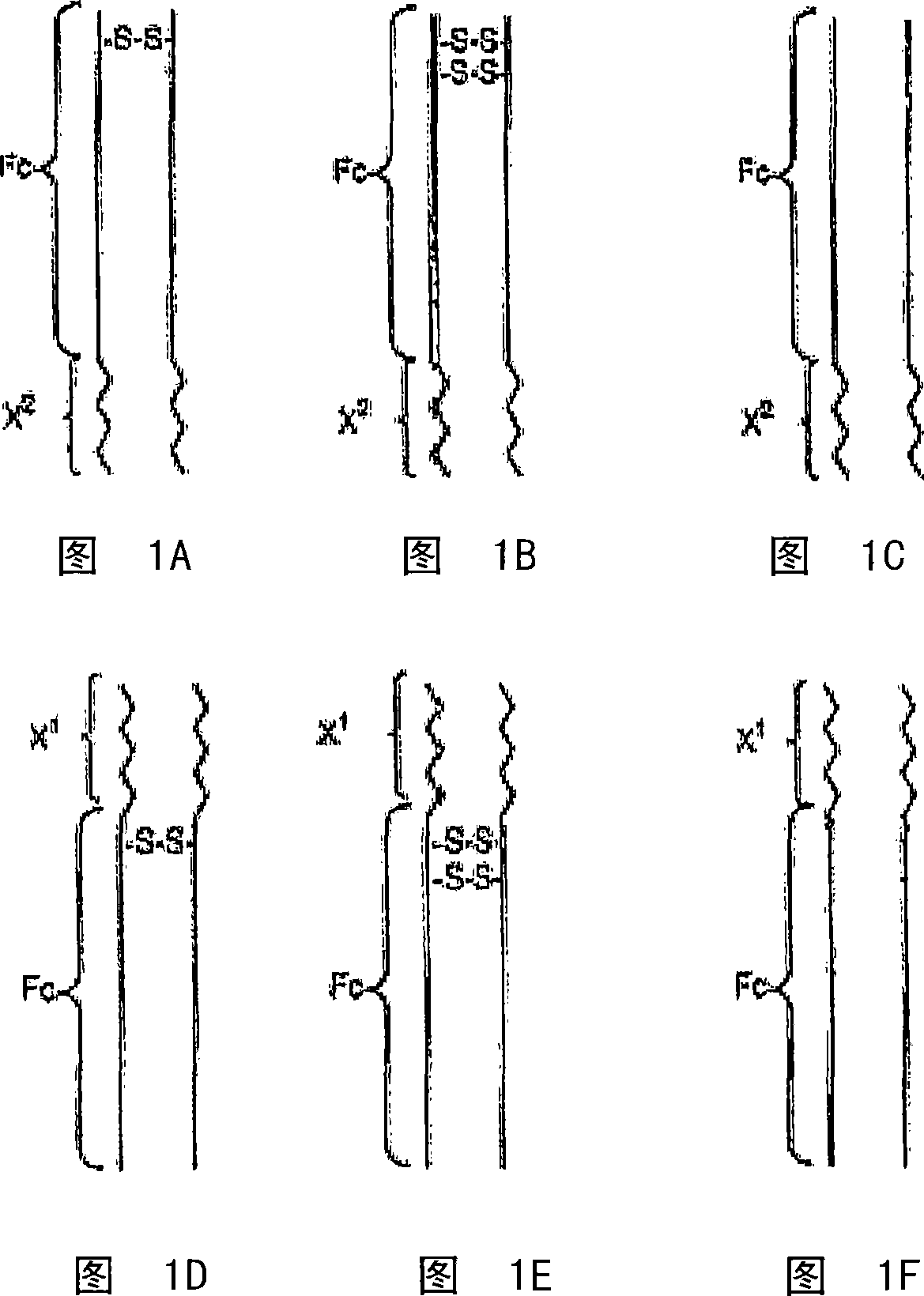
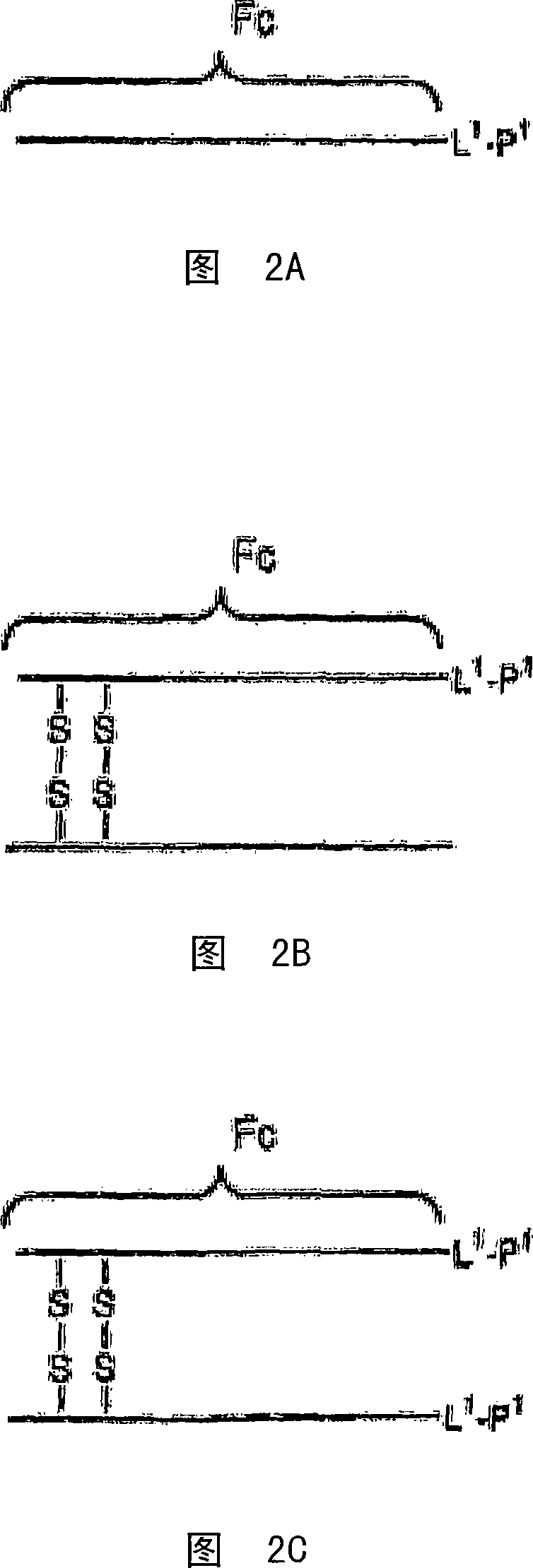
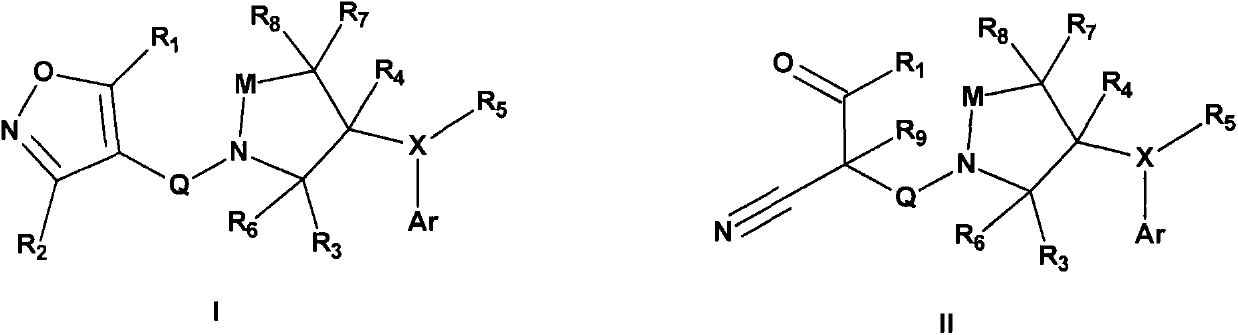
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Therapeutic Agent For Ophthalmic Diseases
A therapeutic agent for ophthalmic diseases containing Laennec (trade name) as an active ingredient. Laennec, the active ingredient, exhibits a therapeutic effect on a wide variety of ophthalmic diseases by increasing tears and the like and is highly safe even though it is an animal-derived component. Therefore, the therapeutic agent is applicable to the prevention and / or treatment of various types of ophthalmic diseases, particularly corneal disorders, dry eye, asthenopia, inflammatorily ophthalmic diseases (e.g., meibomian gland dysfunction, Stevens-Johnson syndrome, Sjogren syndrome, uveitis) and ophthalmic diseases caused by active oxygen (e.g., cataract, glaucoma, age-related macular degeneration, optic disc atrophy).
Owner:HIBINO SAWAKO
Topical Delivery System for Phytosterols
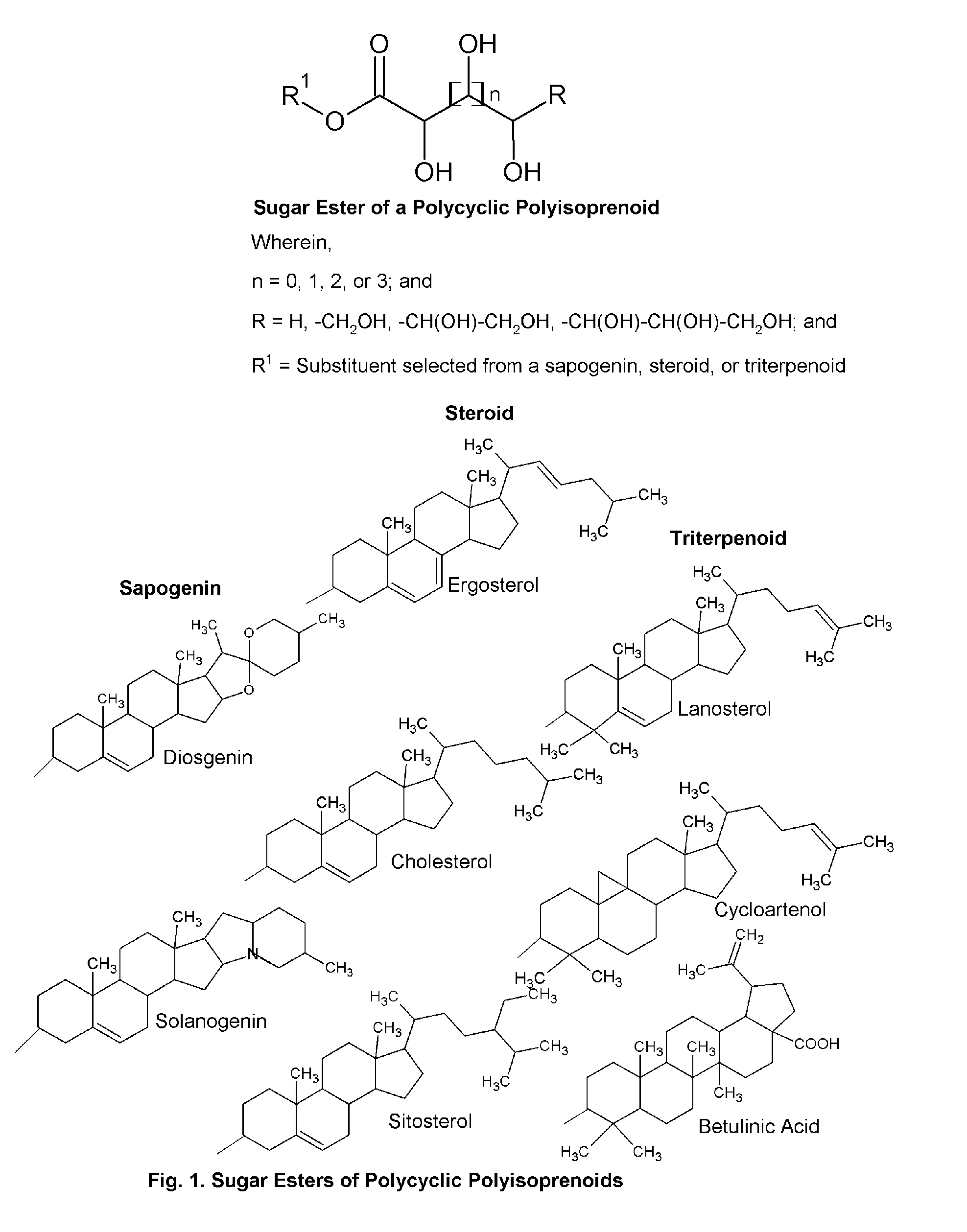
This invention relates to certain sugar esters of phytosterols of formula (I). These esters are useful for topical application, and for the treatment of skin condition, including age spots, acne, loss of cellular antioxidants, collagen loss, loss of skin pliability, loss of skin suppleness, skin wrinkles including fine lines, oxidation, damage from radiation, malfunction of matrix metalloproteases, malfunction of tyrosinases, damage from free radicals, damage from UV, dry skin, xerosis, ichthyosis, dandruff, brownish spots, keratoses, melasma, lentigines, liver spots, pigmented spots, dark circles under the eyes, skin pigmentation including darkened skin, blemishes, oily skin, warts, eczema, pruritic skin, psoriasis, inflammatory dermatoses, topical inflammation, disturbed keratinization, skin changes associated with aging, nail or skin requiring cleansers, conditioning or treatment, and hair or scalp requiring shampooing or conditioning, and combinations thereof;Wherein,n=0, 1, 2, or 3; andR=H, —CH2OH, —CH(OH)—CH2OH, —CH(OH)—CH(OH)—CH2OH; andR1=Substituent selected from a sapogenin, steroid, or terpenoid.
Owner:BIODERM RES
Toxin peptide therapeutic agents
ActiveUS7833979B2Preventing and mitigating relapseAvoid it happening againNervous disorderAntipyreticHalf-lifeFibrosis
Disclosed is a composition of matter of the formula(X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I)and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
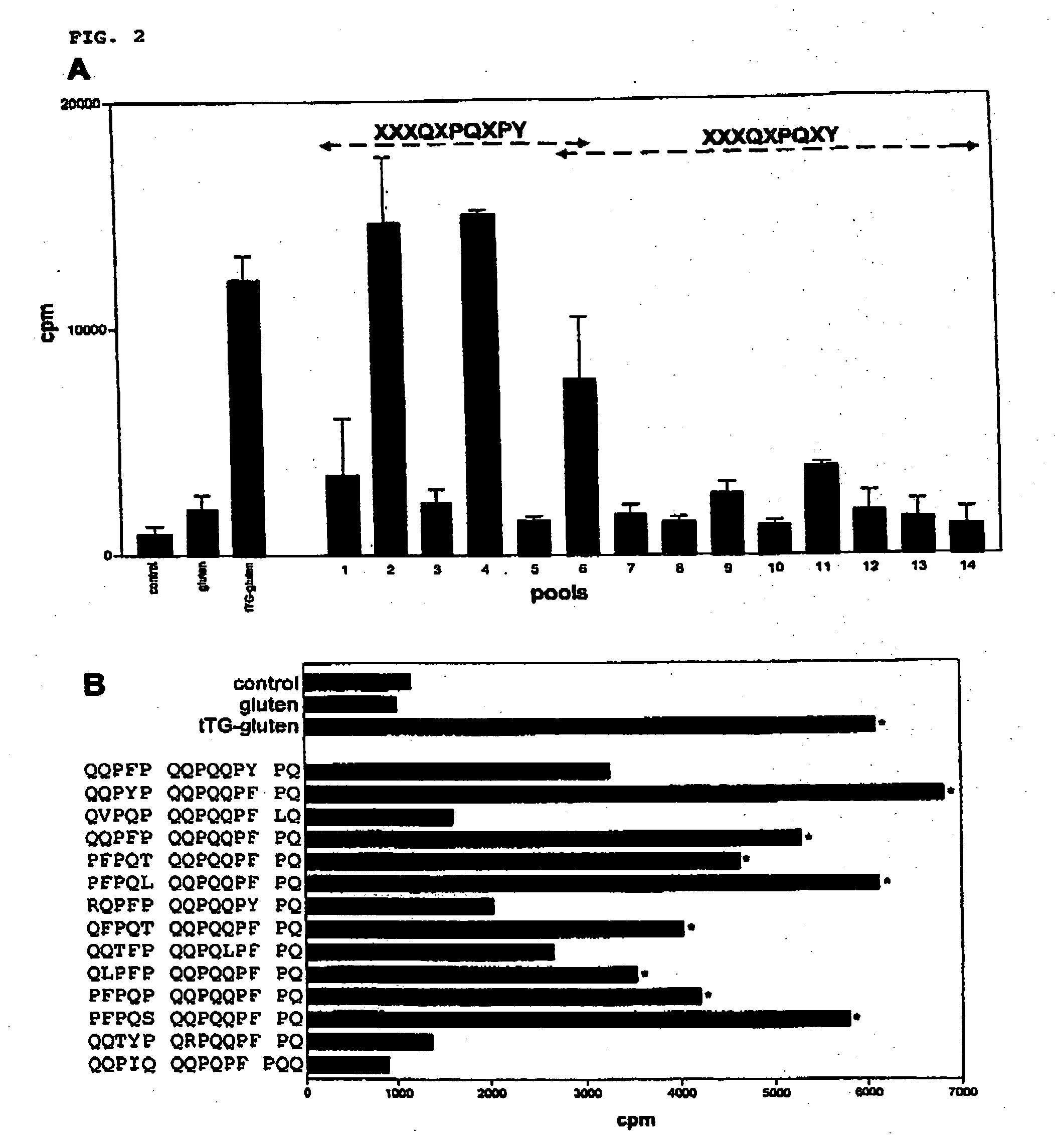
Novel epitopes for celiac disease and autoimmune diseases, methods for detecting those and novel non-antigenic food compounds
InactiveUS20050244823A1Peptide/protein ingredientsImmunoglobulins against plantsAutoimmune conditionSjögren syndrome
The invention describes the patterns of deamidation in gluten, and it is found that this is highly dependent on the spacing between the glutamine and proline residues. This knowledge can be used to predict novel T cell stimulatory gluten peptides. Several newly defined peptides and epitopes are provided. Also, the finding can explain the formation of neo-epitopes in autoimmune diseases such as RA (rheumatoid arthritis), MS (multiple sclerosis), SLE (systemic lupus erythomatosus), SS (Sjogren syndrome) and DB (diabetes). Several neo-epitopes and the peptides that are substrate for deamidation are provided. Further, the inventions provides for methods for detecting these peptides and epitopes and methods for making food more suitable for celiac disease patients.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Phenylpropionic acid derivatives
Specified phenylpropionic acid derivatives and analogues thereof have an antagonistic activity to α 4 integrin. They are used as therapeutic agents or preventive agents for various diseases concerning α 4 integrin, such as inflammatory diseases in which α 4 integrin-depending adhesion process participates in the pathology, rheumatoid arthritis, inflammatory bowel diseases, systemic lupus erythematosus, multiple sclerosis, Sjögren's syndrome, asthma, psoriasis, allergy, diabetes, cardiovascular diseases, arterial sclerosis, restenosis, tumor proliferation, tumor metastasis and transplantation rejection.
Owner:EA PHARMA CO LTD
Pharmaceutical composition for ophtalmic use
InactiveUS20030186931A1Treating and preventing ophthalmologic clinical symptom and signEffect andBiocideOrganic active ingredientsAdditive ingredientSjögren syndrome
An ophthalmic pharmaceutical composition comprising trehalose as an effective ingredient and a pharmaceutically-acceptable carrier. The pharmaceutical composition is a safe, long-term continuously-administrable, therapeutic and / or prophylactic agent for the ophthalmologic clinical symptoms and signs in Sjögren syndrome.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
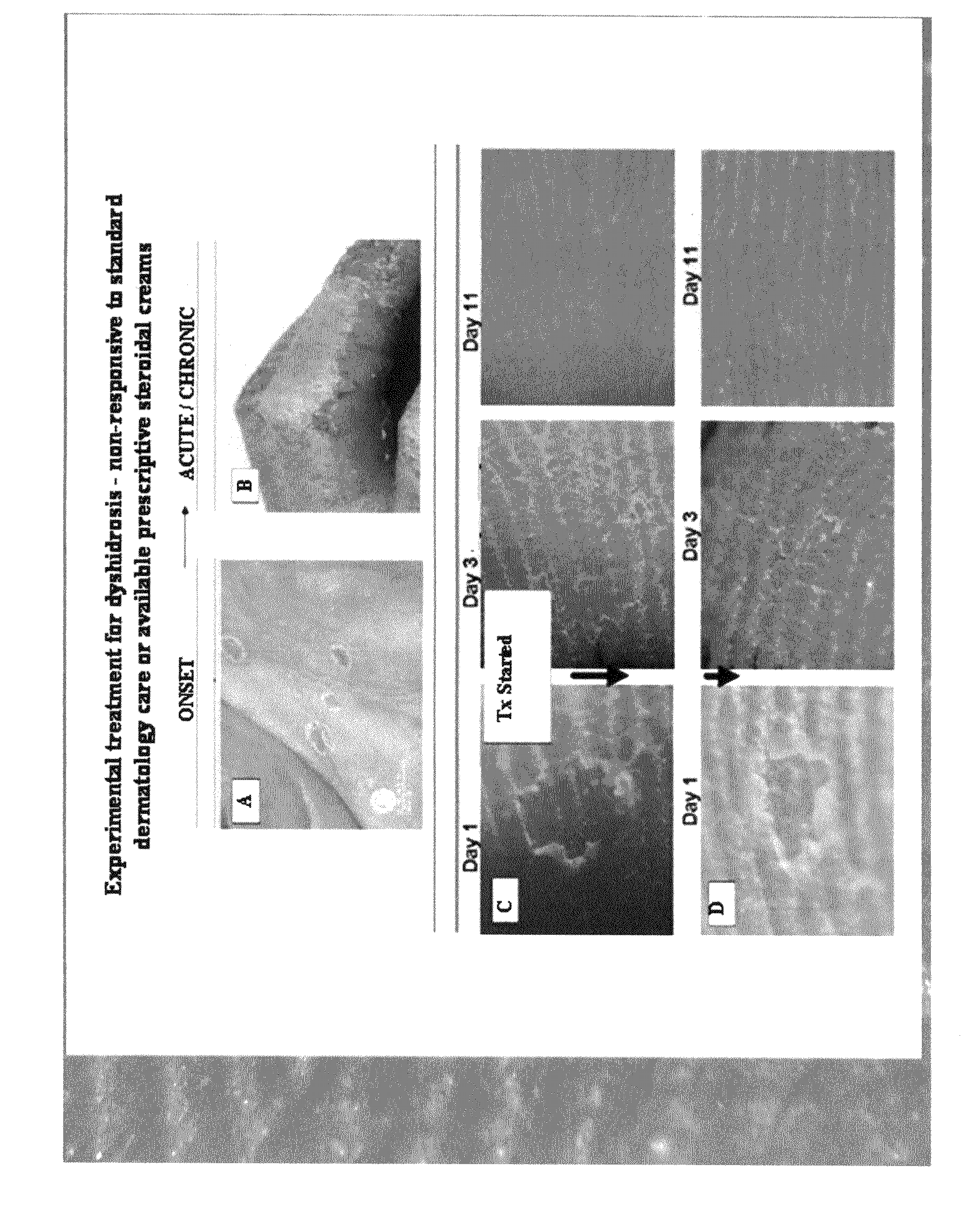
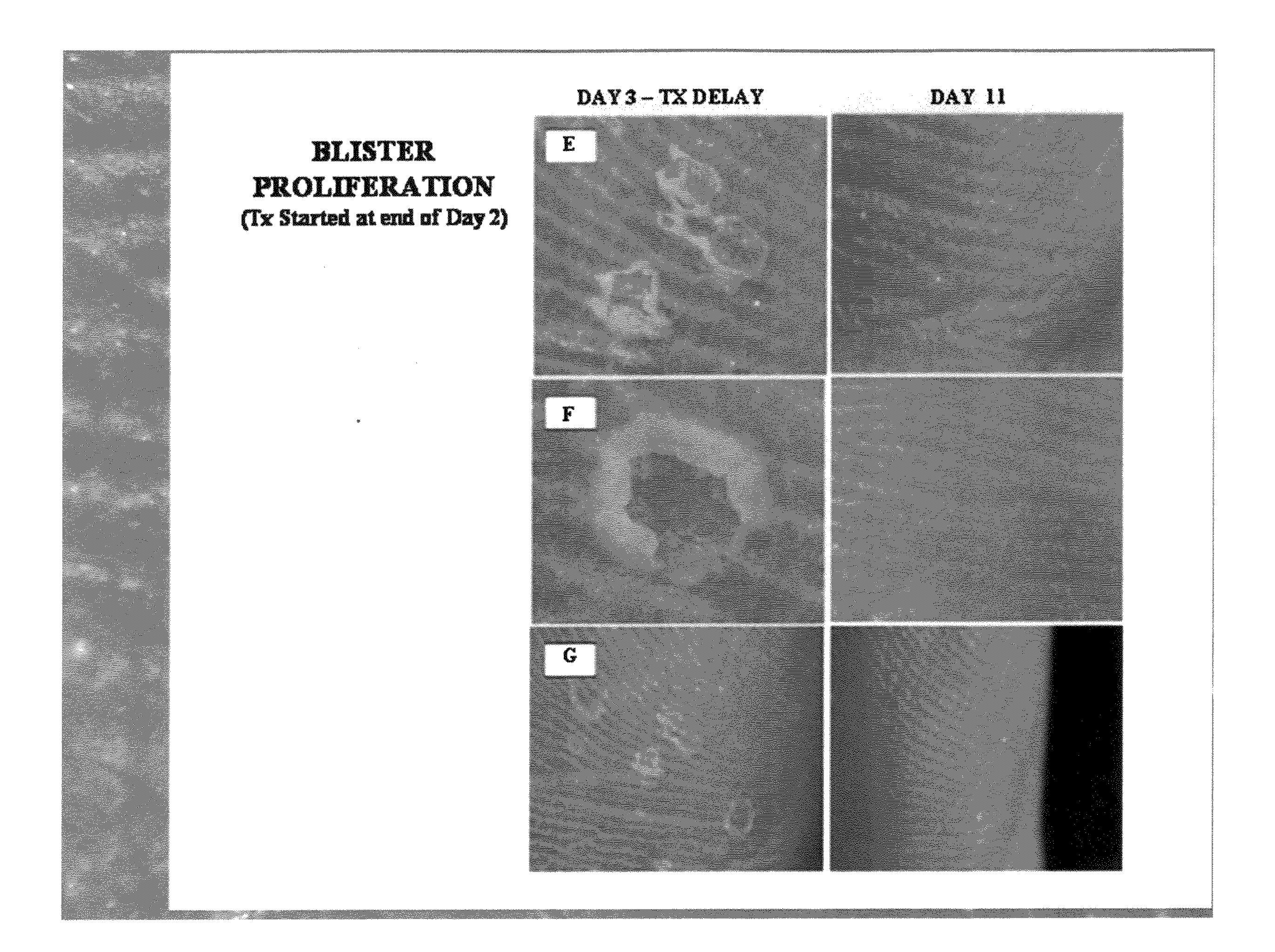
Method of treating dyshidrosis(pompholyx) and related dry skin disorders
This invention discloses a method of use for a topical herbal formulation alone or in combination with oral administration of niacin (preferably a flush preparation) to prevent and / or treat dyshidrosis (pompholyx) and related skin diseases. The formulation may also be used to treat contact dermatitis, eczema, palmoplantar pustulosis and skin infections incurred by invasive pathogens such as mold, fungus and bacteria. The formulation is comprised of plant extracts and niacin, that when combined yield an effective multi-faceted pharmaceutical approach to treating dry skin disorders. The active ingredients within the formula include a combination of dry, aqueous, acid and alcohol extracts of black walnut hull (Juglans nigra), wormwood (Artemisia absinthium), tumeric rhizome (Curcuma longa), garlic (Allium sativum), two or more herbal antibacterial agents from the group consisting of chamomile (Matricaria Chamomile), licorice root (Glycyrrhiza glabra), St Johns wort (Hypericum perforatum), clove (Syzygium aromaticum), nutmeg (Myristica fragans), ginger (Zingiber officinale), frankincense (Boswellia carteri) and myrrh (Commiphora molmol), further combined with aloe vera and niacin.
Owner:MAZZIO ELIZABETH ANNE +1
Topical base and active agent-containing compositions, and methods for improving and treating skin
InactiveUS20120100183A1Desirable tasteEasy to manageBiocideOrganic active ingredientsDiseaseIrritant dermatitis
The present invention provides unique, efficacious, inexpensive, safe, reliable, convenient, minimally bitter, skin protecting and penetrating, easy-to-administer base compositions and active agent-containing compositions, such as those including hydrocortisone, and related production and topical application methods, for treating the skin of mammals for a wide variety of different dermatologic conditions, disorders and diseases, such as inflammation, redness, cracking, insect bites, dryness, allergic reactions, trauma, irritant dermatitis, perleche, contact dermatitis, psoriasis, eczema, seborrheic dermatitis, acne excoriate, xerosis, eczema craquele, stasis dermatitis, disease related conditions and dryness from medications such as isotretinoin, acitretin and lipid lowering agents. This is effected by topically administering, or otherwise applying, effective amounts of the compositions thereto in forms that not only address the skin and mucosa of the mouth and lips, but also of the rest of the body and, in particular, areas where other topical balms containing hydrocortisone and other active ingredients have not been developed or marketed. Additionally, the flavoring addition to this product, and the base wherein the active ingredient(s) reside, affords a significantly better tasting, and less bitter, composition, thereby allowing a more pleasant experience and better compliance by patients. Larger sized stick formulation(s) allow for more applicability of the product, and more usefulness thereof, in various areas, and mucosal skin, of the body. The compositions include a unique formulation of FANCOL VB, Natunola Castor 1023, Finsolv TN, bees wax and, optionally, one or a plurality of plant or plant seed oils, fatty alcohols, fats and flavorings, in desirable weight percents thereof, in various forms, and preferably in the form of a solid roll-on stick present in a variety of sizes.
Owner:SCHLESSINGER JOEL +1
Microarray-ELISA detecting reagent kit for detecting autoimmunity disease relevant antibody spectrum
InactiveCN101063680AImprove throughputHigh parallelMaterial analysisDiffuse sclerodermaAutoimmune responses
This invention relates to one anti-extracting nuclear antigen spectrum array to ELISA test agent case for selecting for systematic lupus erythematosus, mixed connective tissue disease, Sjogren syndrome, systemic scleroderma, polymyositis and atrophic arthritis system self immune property antigen ENA spectrum micro array to enzyme immune agent case.
Owner:BEIJING BGI GBI BIOTECH +4
Application of norisoboldine in preparing medicament for treating autoimmune disease
InactiveCN101375850AOrganic active ingredientsImmunological disordersImmunologic disordersUlcerative colitis
The invention relates to the medicine filed, in particular to a medicinal purpose of norisoboldine. The medicinal purpose is characterized in that the norisoboldine can be used in the application of preparing drugs for the treatment of autoimmune diseases. The autoimmune diseases are: rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, multiple sclerosis, type-I diabetes, psoriasis, ulcerative colitis, Sjogren syndrome, scleroderma, polymyositis, chronic active hepatitis, mixed connective tissue disease, primary biliary cirrhosis, autoimmune hemolytic anemia and other diseases.
Owner:CHINA PHARM UNIV
Pharmaceutical composition for ophthalmic use
InactiveUS7732425B2Satisfied with the effectAdvantageous usefulness and safetyBiocideOrganic active ingredientsOphthalmologySjögren syndrome
An ophthalmic pharmaceutical composition comprising trehalose as an effective ingredient and a pharmaceutically-acceptable carrier. The pharmaceutical composition is a safe, long-term continuously-administrable, therapeutic and / or prophylactic agent for the ophthalmologic clinical symptoms and signs in Sjögren syndrome.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
Modeling method of Sjogren syndrome mouse model
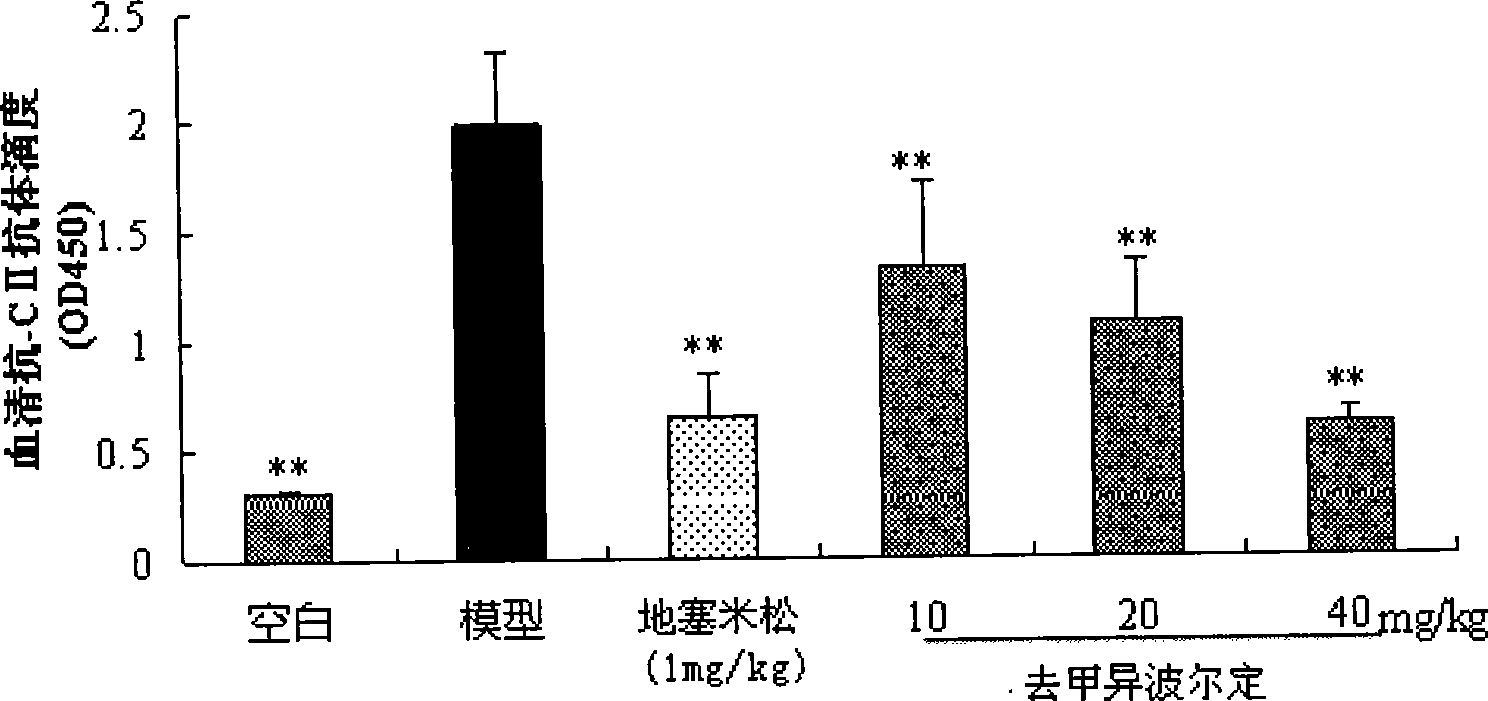
InactiveCN106110315ALow physiological stateLow mental stateAntibody medical ingredientsMulti siteSjögren syndrome
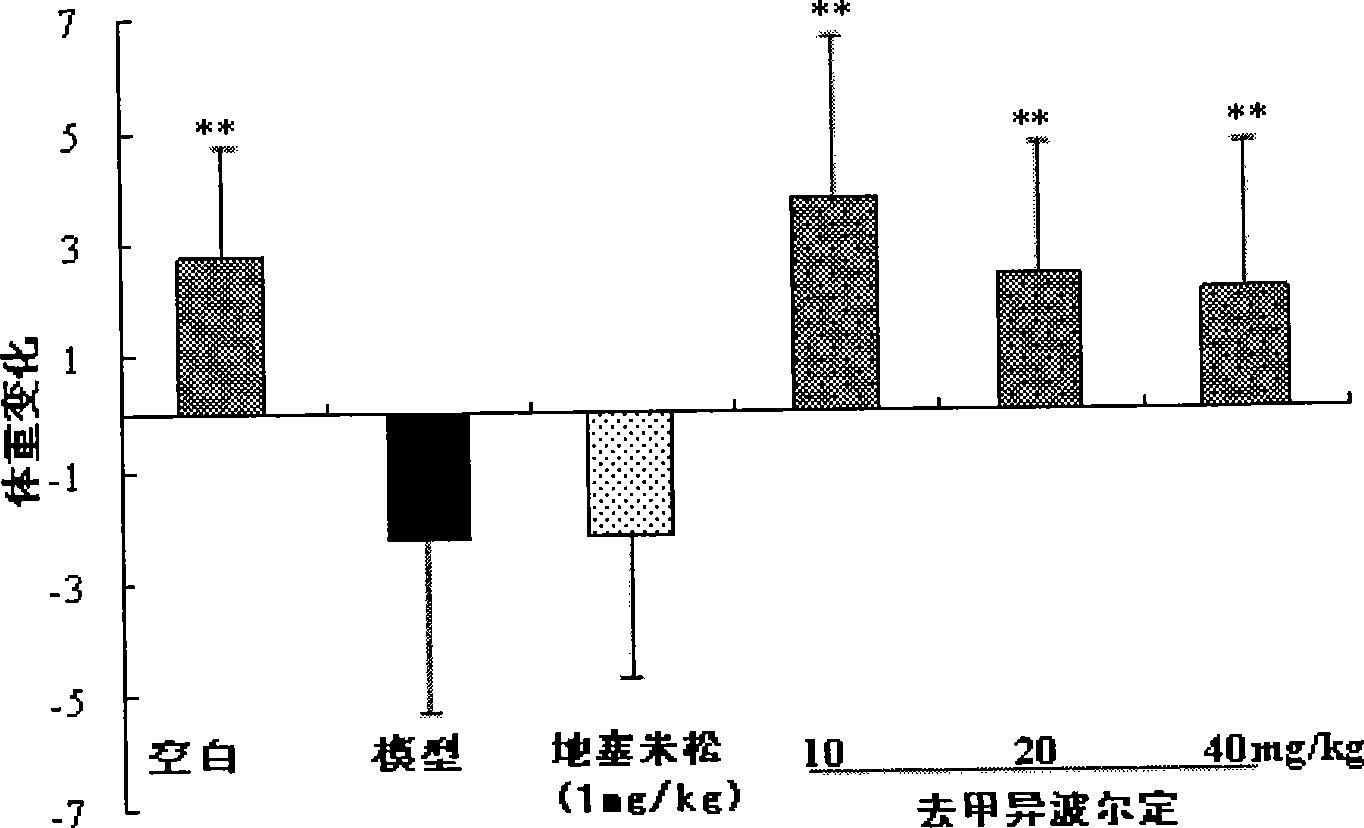
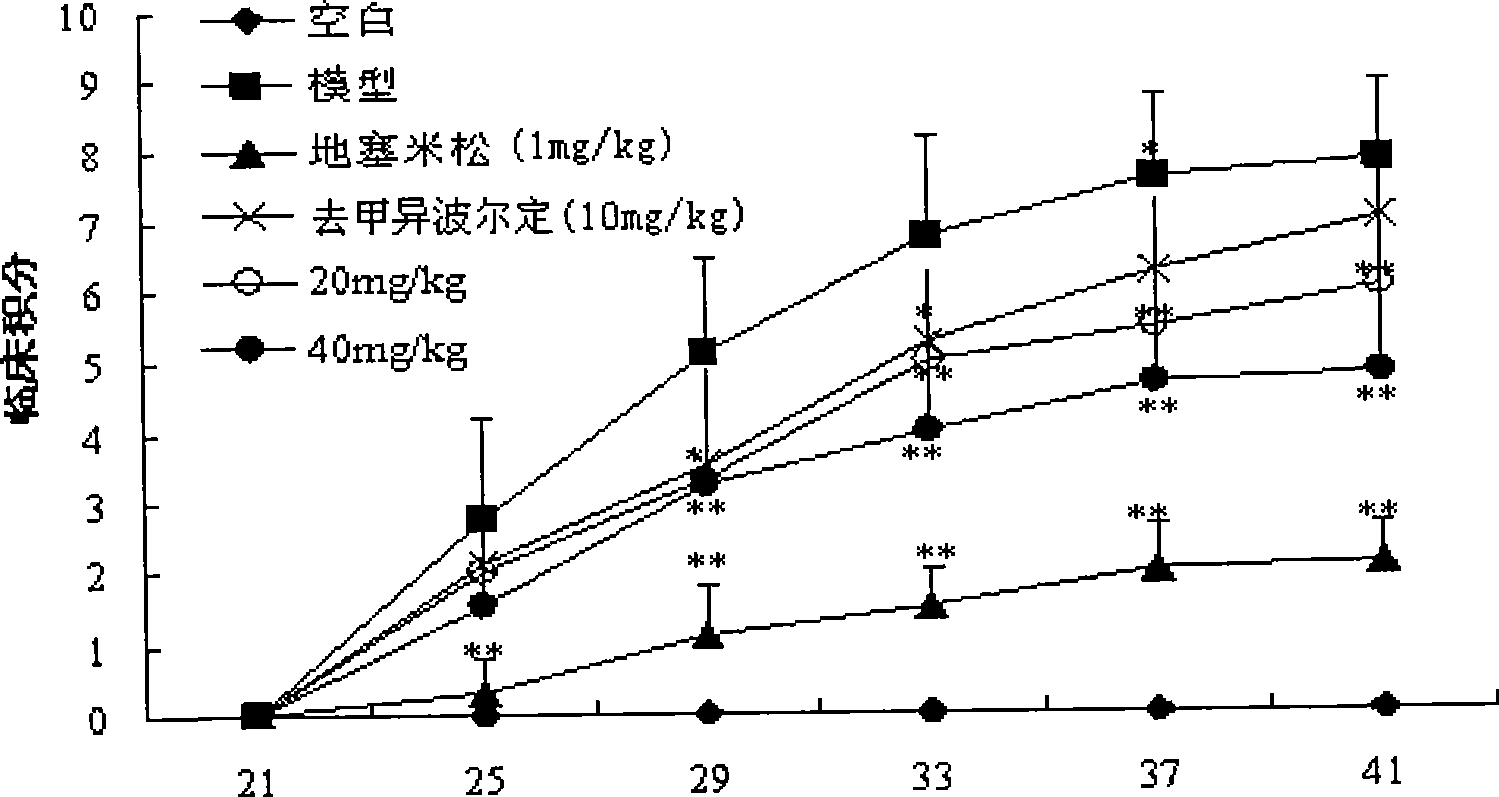
The invention discloses a modeling method of an Sjogren syndrome mouse model. The modeling method comprises the following steps: killing mice, taking out bilateral salivary glands and peeling off capsules and connective tissues; washing with PBS (Poly Butylenes Succinate); adding the PBS according to the amount of adding 0.5ml of the PBS into each salivary gland; shearing the salivary glands into pieces, and uniformly homogenizing and centrifuging in an ice bath; then taking supernatant and quantifying salivary gland antigens by adopting a BCA (Bicinchoninic Acid) protein quantifying method; adjusting the concentration of the salivary gland antigens to be 4mg / ml by utilizing the PBS; adding equal quantity of an FCA (Freund Complete Adjuvant) or an FIA (Freund Incomplete Adjuvant) and diluting the concentration to be 2mg / ml; repeatedly blowing and beating until two liquid phases are dissolved mutually to form an ivory color; randomly grouping C57BL / 6 mice and shaving off furs on the backs of the mice; carrying out intradermal multi-site injection of 2mg / ml mouse salivary gland antigens prepared by the FCA on the back and tails of the mice on the current day and the 7th day, wherein the injection amount is 0.1ml per mouse; injecting equal quantity of the salivary gland antigens prepared by the FIA on the 14th day through the same method; after modeling for about 6 weeks, detecting indexes and screening the successfully modeled mice. The modeling method disclosed by the invention is high in modeling efficiency and short in modeling time.
Owner:魏伟
Beta-fodrin antigen epitope polypeptide, and its screening method and use
InactiveCN1935837AAdvantages of value assessmentBiological testingAnimals/human peptidesEpitopeSerum ige
The invention relates beta-fordin antigen epitope polypeptide screening for gaining amino acid sequence corresponded with the optimum beta-fordin polypeptide. The beta-fordin antigent epitope polypeptide includes the following two that one is formed by the amino acid sequence showed in SEQ ID NO.1; another is derived from the above amino acid sequence by replacing, missing or adding one or many amino acid with antigen epitope function. It can used to specially test beta-fordin polypeptide IgG antibody for Sjogren syndrome patient. The invention also supplies the beta-fordin polypeptide IgG antibody immunology method, and its application used as antigen in Sjogren syndrome medicine preparation.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Traditional Chinese medicine composition for treating sjogren syndrome as well as preparation method and application thereof
InactiveCN102935177AReasonable prescription designStrict compatibilityImmunological disordersPlant ingredientsForsythia suspensaSjögren syndrome
The invention provides a traditional Chinese medicine composition for treating sjogren syndrome. The traditional Chinese medicine composition is prepared from the following raw materials in parts by weight: 20 to 30 parts of dogwood, 20 to 30 parts of rhizoma dioscoreae, 10 to 20 parts of rhizoma alismatis, 15 to 25 parts of rhizoma polygonati, 15 to 25 parts of fructus forsythiae, 10 to 20 parts of cistanche deserticola, 10 to 20 parts of radix paeoniae rubra, 10 to 20 parts of radix paeoniae alba, 10 to 20 parts of dendrobium nobile, 15 to 25 parts of cortex moutan, 15 to 25 parts of radix rehmanniae, 15 to 25 parts of poria cocos, 30 to 50 parts of radix ophiopogonis, 15 to 25 parts of eucommia ulmoides, 10 to 20 parts of cynanchum paniculatum, and 10 to 20 parts of liquorice. The traditional Chinese medicine composition has the effects of moistening lung and promoting secretion, so that the purpose of treating sjogren syndrome can be achieved. The invention also discloses a preparation method and application of the traditional Chinese medicine composition.
Owner:THE FIRST AFFILIATED HOSPITAL OF XINXIANG MEDICAL UNIV
Toxin peptides with extended blood halflife
InactiveCN101232903APeptide/protein ingredientsPharmaceutical non-active ingredientsInflammatory Bowel DiseasesHalf-life
Disclosed is a composition of matter of the formula (X<1>)a-(F<1>)d-(X<2>)b-(F<2>)e-(X<3>)c and multimers thereof, in which F<1> and F<2> are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X<1>, X<2>, and X<3> are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
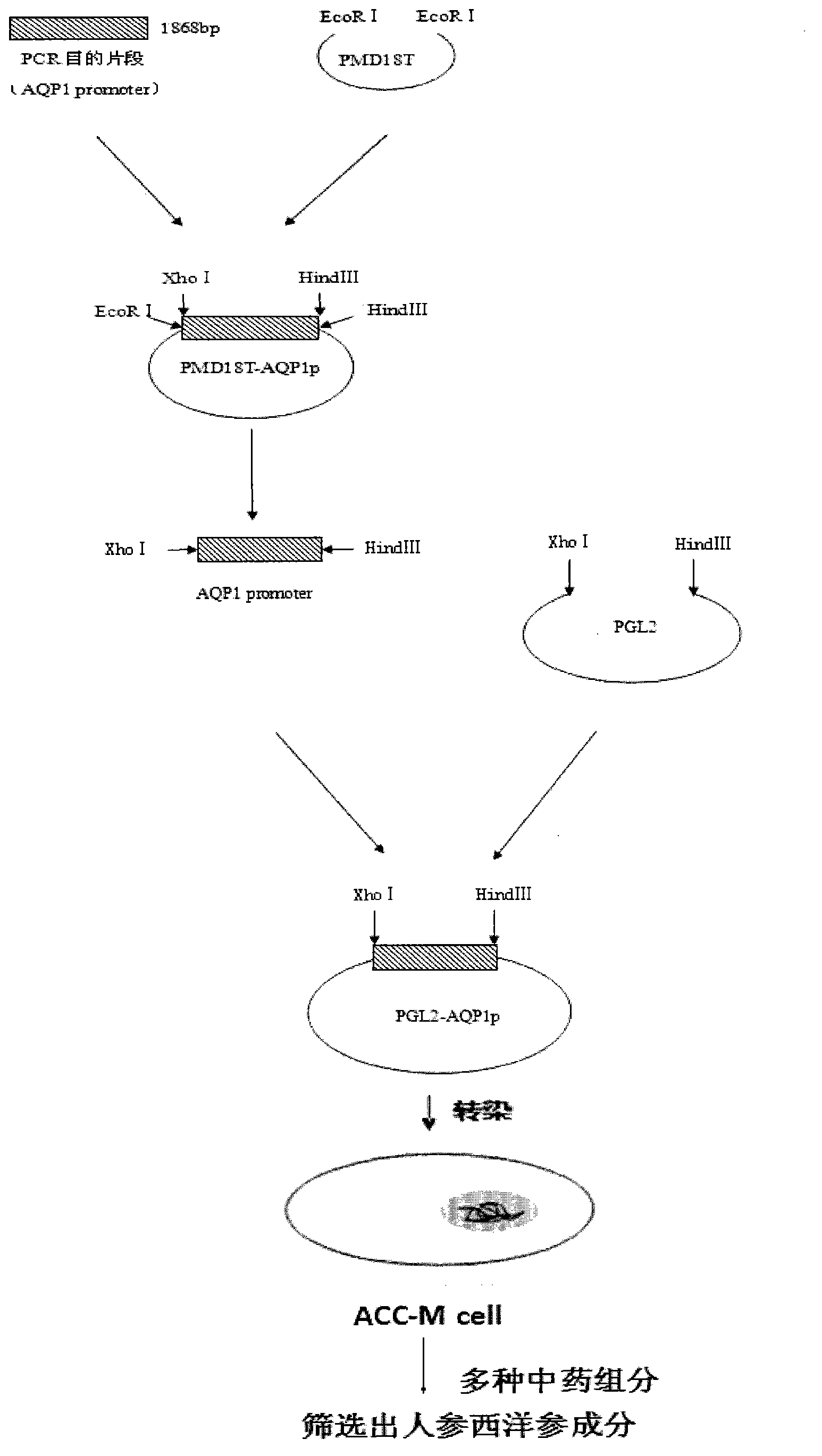
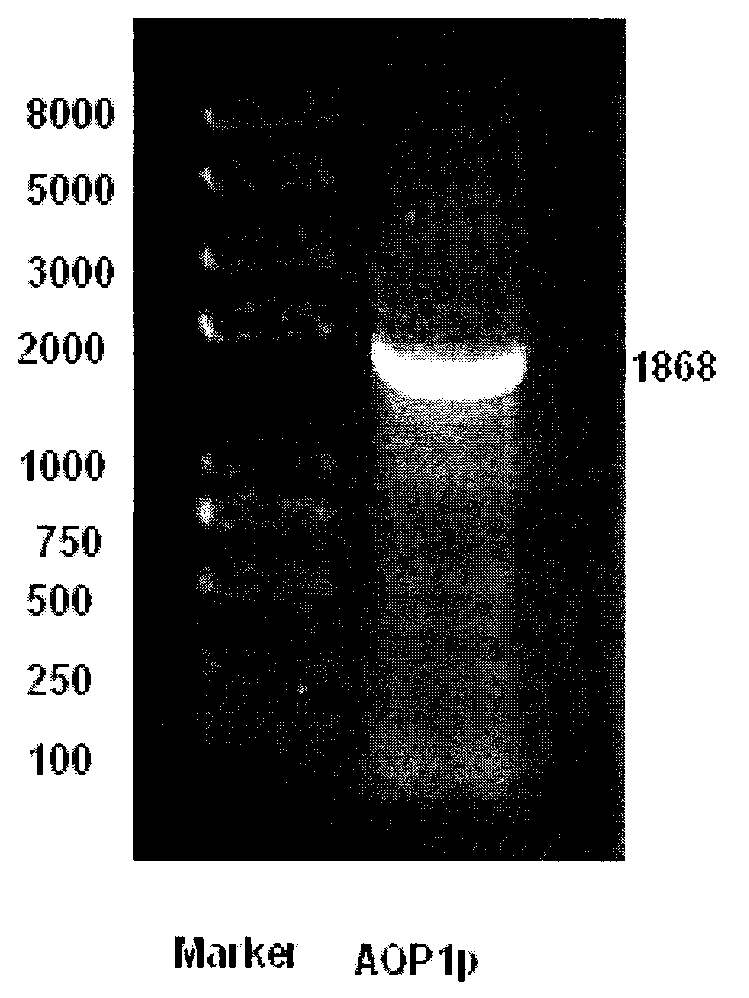
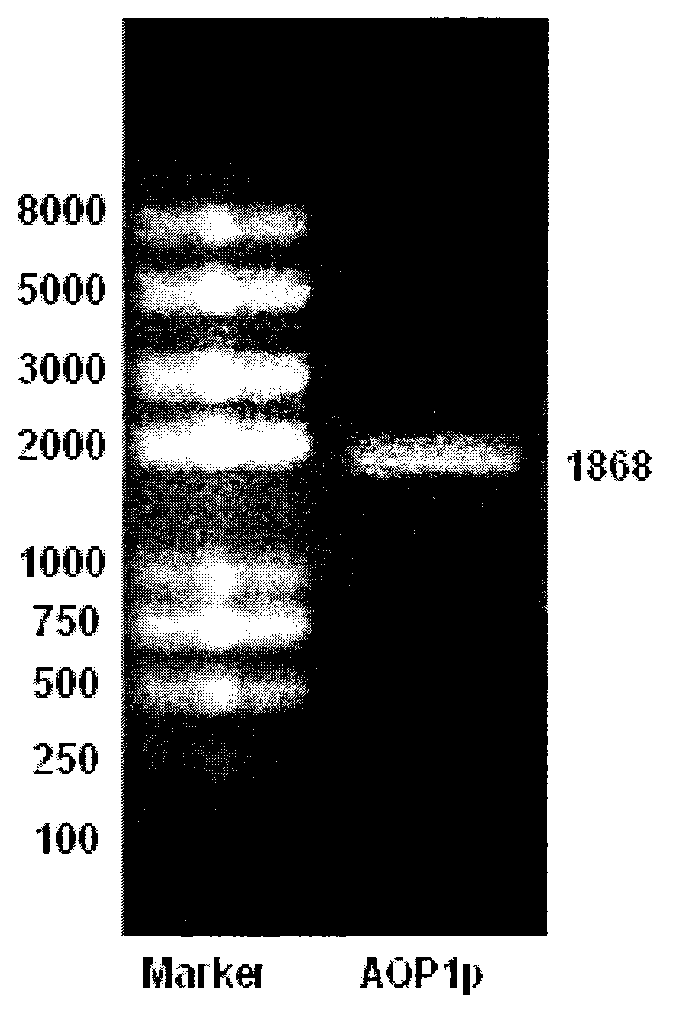
Recombination method and application of luciferase reporter gene with aquaporin 1 (AQP1) promoter to drug screening
InactiveCN103233031AMicrobiological testing/measurementVector-based foreign material introductionSjögren syndromeSalivary gland cell
The invention belongs to the technical field of bioengineering and in particular relates to a recombination method and application of a luciferase reporter gene with a human aquaporin 1 (AQP1) promoter to drug screening. The highly expressed AQP1 plays an important role in the salivary secretion process and is closely related to onset of Sjogren syndromes. In the invention, a 1868bp AQP1 promoter sequence is amplified from a personal genome by utilizing the gene engineering technology and is cloned onto a PGL2-luc vector to construct an AQP1 promoter-Luc expression vector. AQP1 expression is controlled by a promoter region. Constructing a salivary gland cell model with the AQP1 promoter is a key step for screening the traditional Chinese medicine ingredients which can treat the Sjogren syndromes. The drug screening cell model proves that ginseng and American ginseng ingredients at certain concentrations can promote transcription of the AQP1 promoter and the higher the concentrations are, the stronger the activity is, and can guide the drugs to be clinically applied to treat the Sjogren syndromes.
Owner:JILIN UNIV
Coptis chinensis and donkey-hide gelatin composition for treating sicca syndrome and application of coptis chinensis and donkey-hide gelatin composition
The invention discloses a coptis chinensis and donkey-hide gelatin composition for treating sicca syndrome and an application of the coptis chinensis and donkey-hide gelatin composition, and belongs to the field of traditional Chinese medicines. The traditional Chinese medicine composition is prepared from the following raw materials: coptis chinensis, donkey-hide glue, white paeony root, scutellaria baicalensis, spina date seeds, cinnamon and cortex albiziae. The prescription has the efficacies of nourishing yin, moistening dryness, clearing away heat and toxic materials, activating blood circulation and removing stasis, meanwhile, the treatment effect on a sjogren syndrome is relatively obvious; the recovery rate is 62.9%; the total effective rate is 95.2%; and the coptis chinensis and donkey-hide gelatin composition has the characteristics of being good in curative effect, short in treatment course, free of a side effect and the like. The traditional Chinese medicine composition disclosed by the invention is safe in clinical application, and free of adverse reaction after being used by patients, and is capable of effectively improving the mental state and the immune function of the patients; and no dependent reaction is generated after the medicine disclosed by the invention is stopped.
Owner:SHAN DONG DONG E E JIAO
Therapeutic agent for ophthalmic diseases
A therapeutic agent for ophthalmic diseases containing Laennec (trade name) as an active ingredient. Laennec, the active ingredient, exhibits a therapeutic effect on a wide variety of ophthalmic diseases by increasing tears and the like and is highly safe even though it is an animal-derived component. Therefore, the therapeutic agent is applicable to the prevention and / or treatment of various types of ophthalmic diseases, particularly corneal disorders, dry eye, asthenopia, inflammatorily ophthalmic diseases (e.g., meibomian gland dysfunction, Stevens-Johnson syndrome, Sjogren syndrome, uveitis) and ophthalmnic diseases caused by active oxygen (e.g., cataract, glaucoma, age-related macular degeneration, optic disc atrophy).
Owner:HIBINO SAWAKO
Medicinal pill for treating Qi and Yin deficiency-type sjogren syndrome and preparation method thereof
InactiveCN106902255AGood treatment effectSolve the problem of getting back to health soonerSenses disorderDigestive systemSjögren syndromeOphiopogon japonicus
The invention belongs to the field of traditional Chinese medicinal preparations, and aims to provide a medicinal pill for treating a Qi and Yin deficiency-type sjogren syndrome according to the cognitive mechanism of the traditional Chinese medicine about the Qi and Yin deficiency-type sjogren syndrome. The medicinal pill comprises the following components in proportions by mass: 30-180g of radix ginseng, 100-260g of raw radix astragali, 100-160g of radix adenophorae, 60-150g of radix ophiopogonis, 60-150g of radix angelicae sinensis, 50-120g of rhizoma chuanxiong, 100-260g of radix paeoniae alba, 30-120g of fructus aurantii, 60-180g of herba dendrobii, 100-180g of poria cocos and 80-180g of peach kernels. The medicinal pill provided by the invention adopts a very good traditional Chinese medicinal formula.
Owner:CHENGDU FENGMI BIOTECH CO LTD
Mixture ophthalmic strips
InactiveUS20150168380A1Microbiological testing/measurementDisease diagnosisXerophthalmiaBulbar conjunctiva
This invention is within the field of eye medicine, involving the preparation method of one type of cornea intravital staining. The above mentioned staining includes the mixed solution of fluorescein sodium aqueous solution and lissamine green aqueous solution. In the above fluorescein sodium aqueous solution, the concentration of fluorescein sodium is 0.5%-4.0% w / v; while in the above lissamine green aqueous solution, the concentration of lissamine green is 0.5%-4.0% w / v. And the volume ratio of the above mentioned fluorescein sodium aqueous solution and lissamine green aqueous solution is 1:0.25-1:3. The cornea intravital staining in this invention possesses some advantages like making the staining of cornea and bulbar conjunctiva proceed at the same time, completed by one time, little irritation to eye tissue and being susceptive for patients. And it is mainly used in diagnosing and evaluating xerophthalmia, keratohelcosis, keratitis (KCS), arborized corneal epithelium herpes, and early diagnosis of Sjogren syndrome etc.
Owner:WU LIANG
Medicinal composition for treating sjogren syndrome and preparation method thereof and application
ActiveCN111759940ARelieve symptomsVisible and long-lasting drynessSenses disorderAntipyreticSjögren syndromeFlos chrysanthemi
The invention discloses a medicinal composition for treating sjogren syndrome and a preparation method thereof and an application. The medicinal composition is prepared from components including the following raw materials; 15-25 parts by weight of radix rehmanniae, 20-30 parts by weight of radix scrophulariae, 15-25 parts by weight of radix ophiopogonis, 25-35 parts by weight of radix paeoniae alba, 5-15 parts by weight of radix glycyrrhizae, 15-25 parts by weight of mulberry leaves, 10-20 parts by weight of flos chrysanthemi, 10-20 parts by weight of fructus aurantii, 15-25 parts by weight of fructus ligustri lucidi, 25-35 parts by weight of radix puerariae, and 15-25 parts by weight of caulis sinomenii. The medicinal composition of the present invention has significant effect on treating sjogren syndrome.
Owner:BEIJING UNIV OF CHINESE MEDICINE
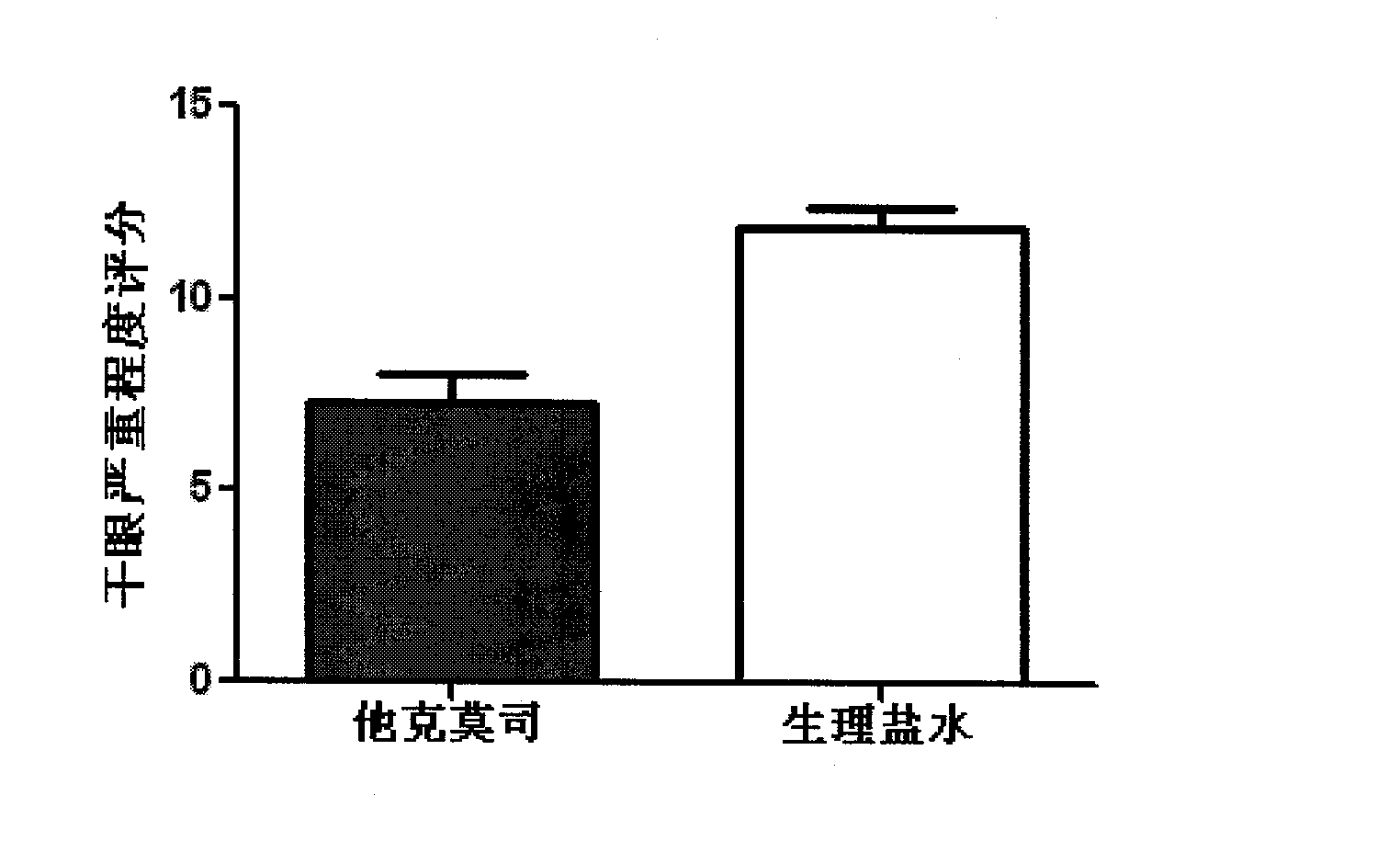
Tacrolimus eye drop for treatment of dry eyes
InactiveCN104042564ALittle side effectsSignificant effectOrganic active ingredientsSenses disorderSide effectSjögren syndrome
The invention relates to a novel treatment method for dry eyes, i.e. treatment of dry eyes by tacrolimus with a concentration of 0.02%-0.03%. Directed at the current situation of clinical absence of effective methods for treatment of dry eyes, the invention aims to provide an effective measure with low side effect to treat dry eyes. Specifically, tacrolimus with a concentration of 0.02%-0.03% is applied four times per day for one month continuously so as to treat chronic non-sjogren syndrome related dry eyes.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
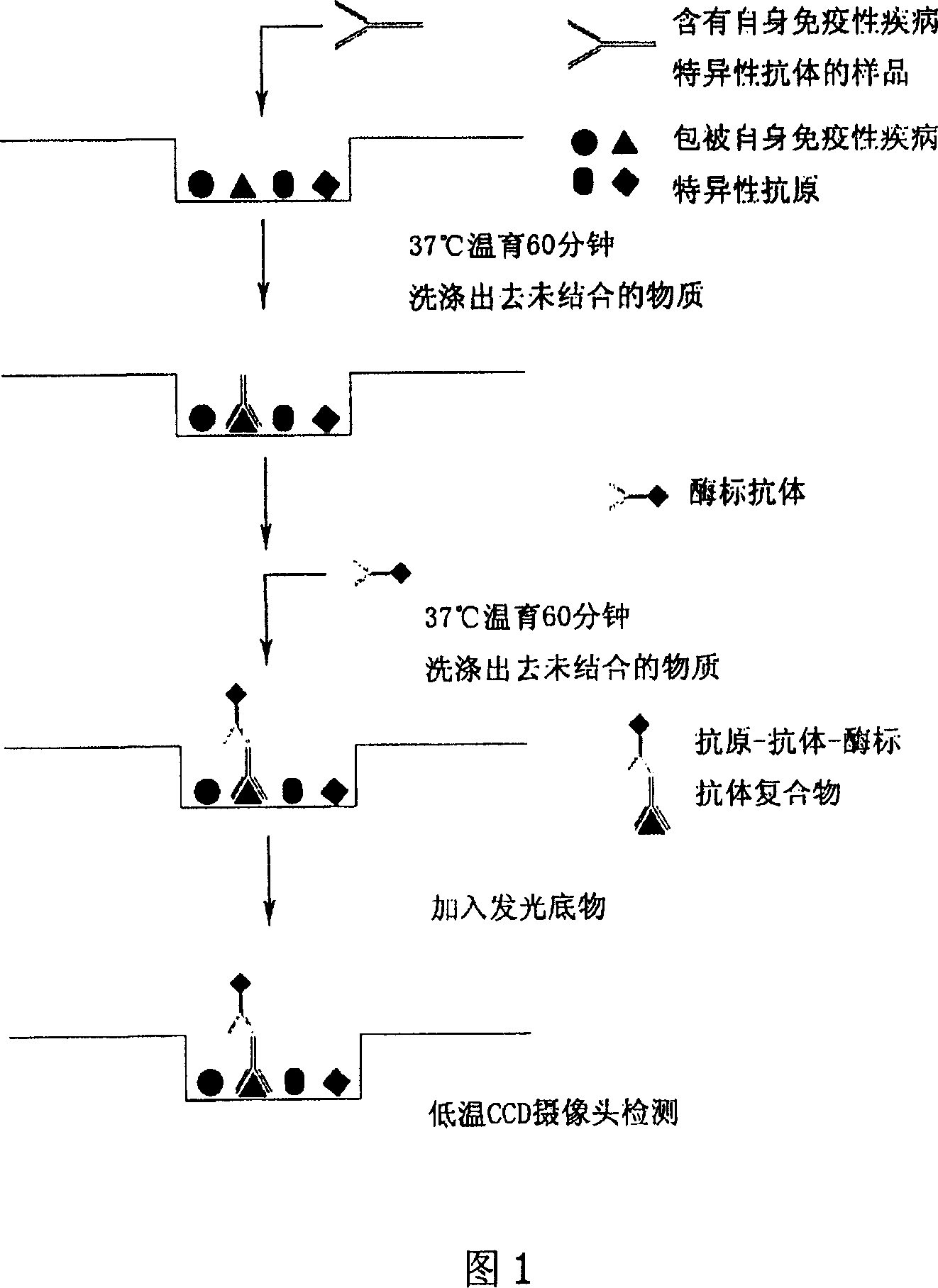
Sjogren syndrome specific autoantibody immunoblotting kit
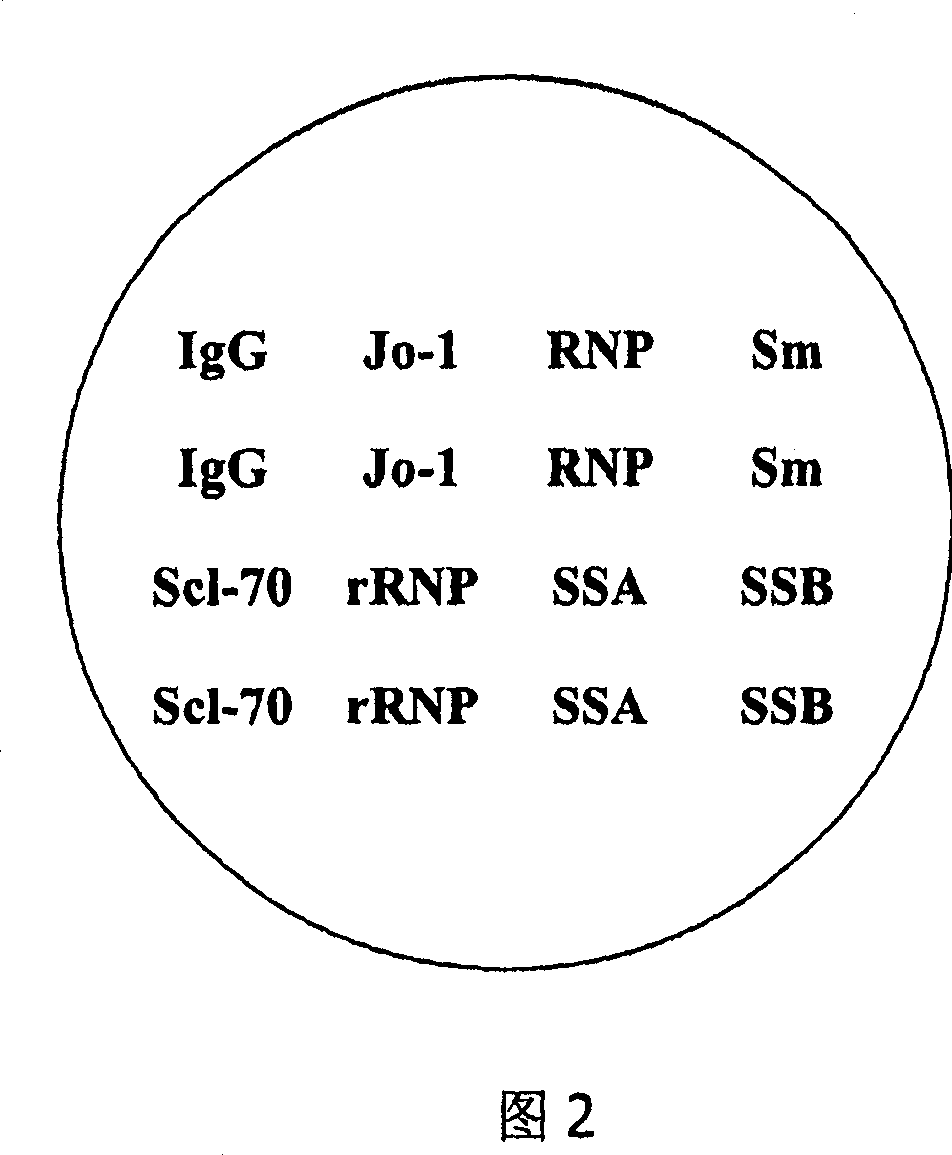
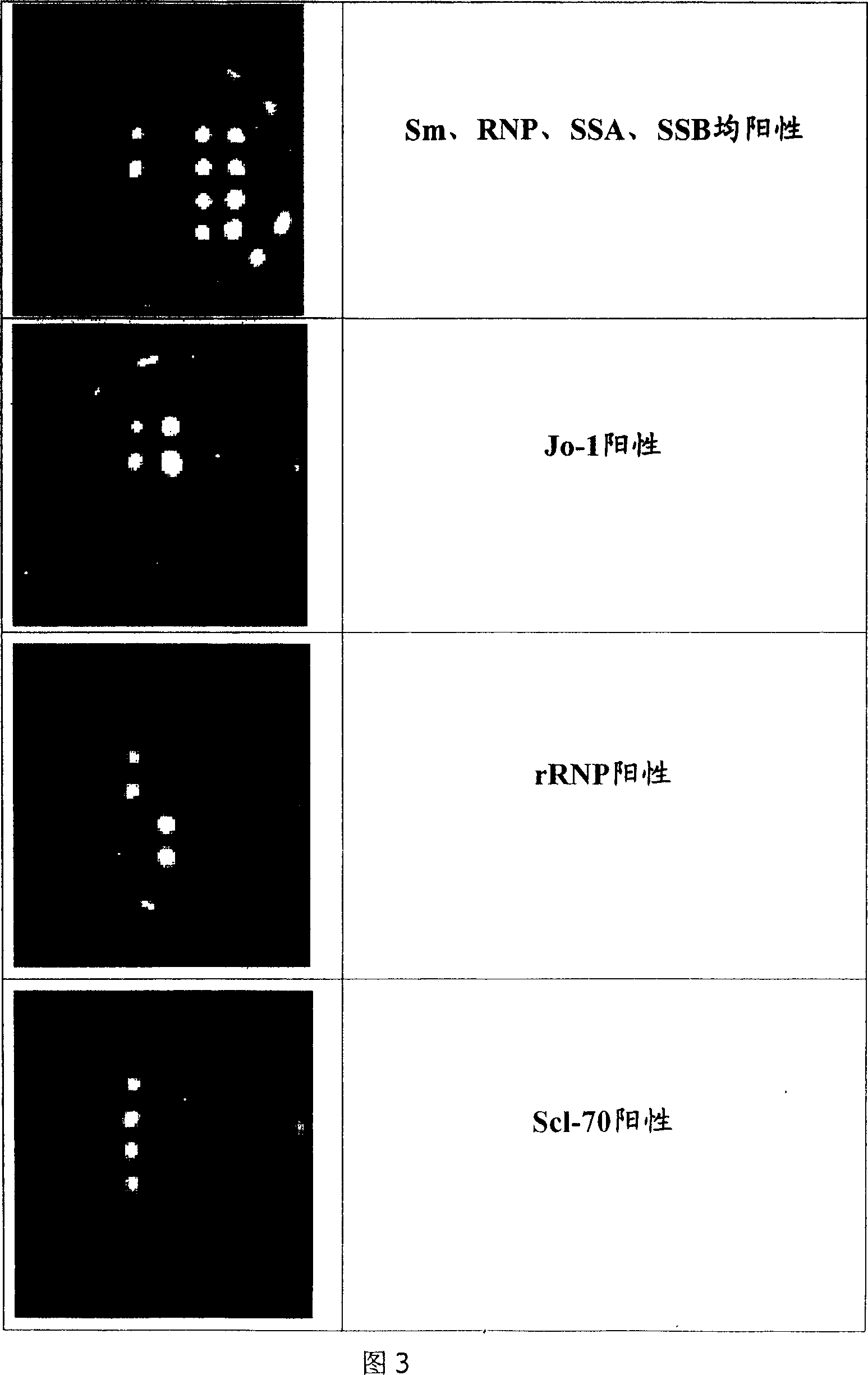
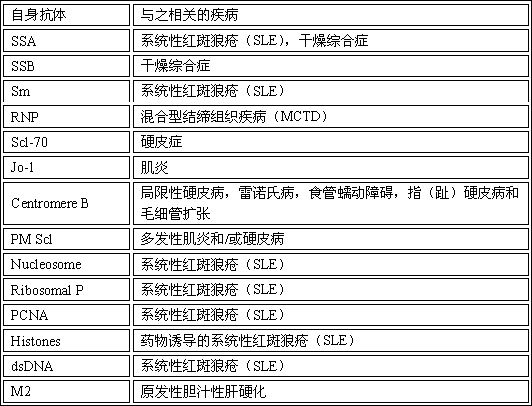
The invention discloses an immunoblotting kit for sjogren syndrome specific autoantibody detection. The kit includes: a blotting membrane strip placed in a reaction tank, an enzyme conjugate, a chromogenic reagent A, a chromogenic reagent B, a stop solution, a dilution solution, concentrated washing liquid, and a standard zone tape. The membrane strip is placed into the reaction tank to react with to-be-tested serum, if the to-be-tested serum contains a corresponding autoantibody, the autoantibody can combine with a corresponding antigen, then the enzyme conjugate and the chromogenic reagents are added, contrast with the standard zone tape is carried out to judge whether the serum contains the sjogren syndrome specific autoantibody: anti-SSA antibody (Ro), SSB (La), salivary gland protein 1 (SP1), carbonic anhydrase 6 (CA6), and parotid secretory protein (PSP). The existence of sjogren syndrome specific autoantibodies and the antibody species and quantity are of important clinical significance for sjogren syndrome diagnosis and differential diagnosis.
Owner:厦门敖依生物科技有限公司
New compounds for the prevention and treatment of various autoimmune diseases
Owner:ZHEJIANG DTRM BIOPHARMA
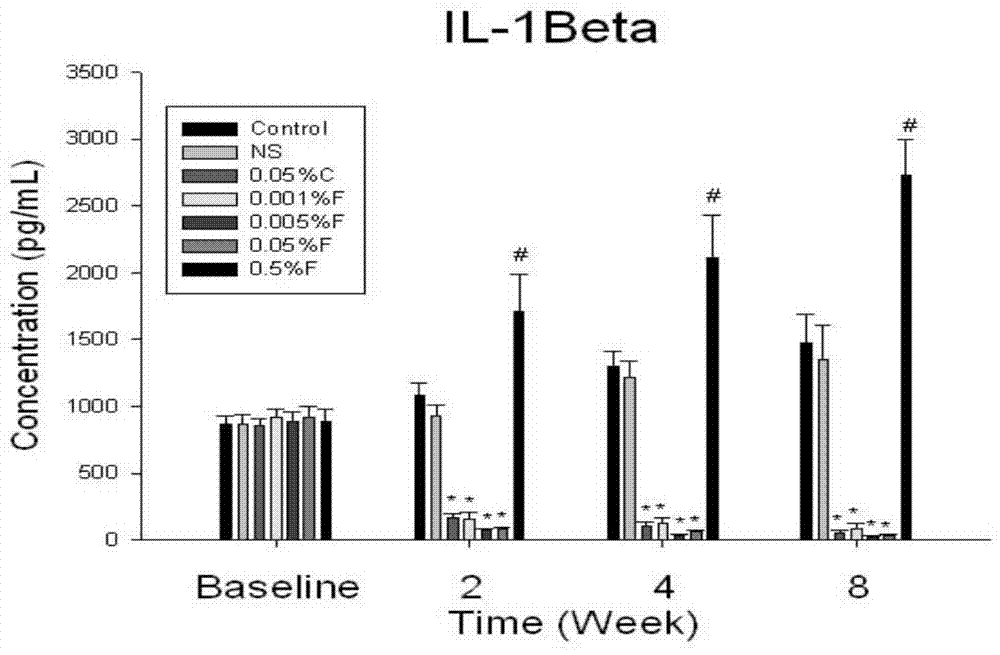
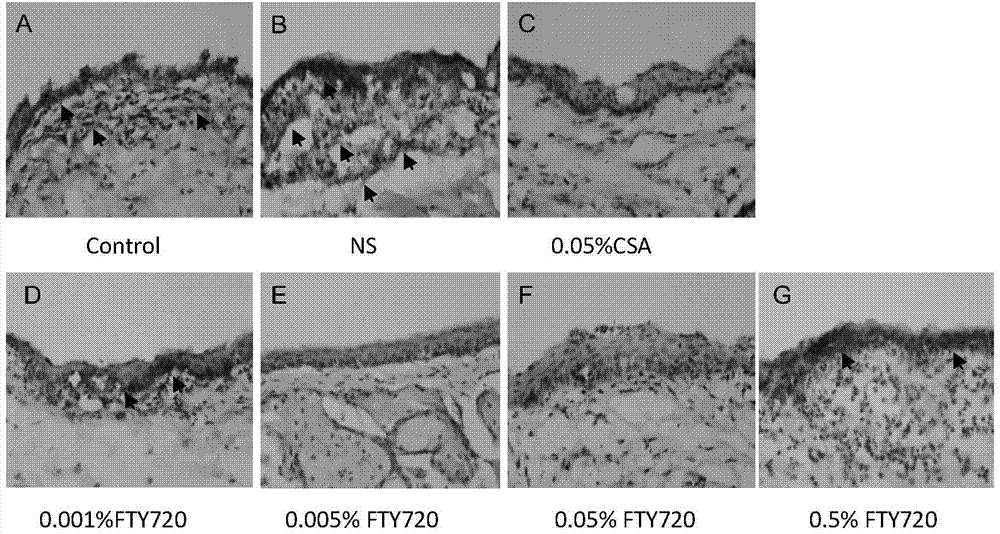
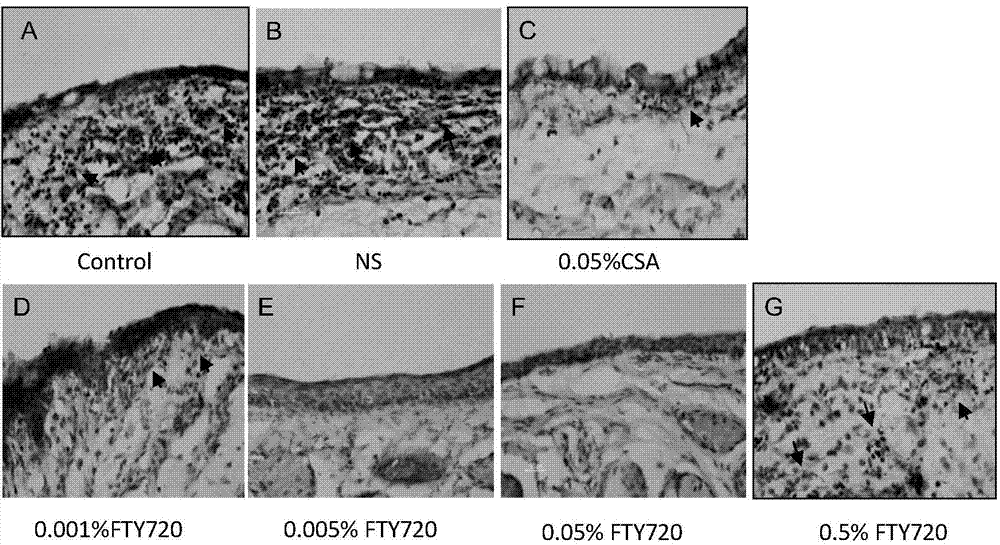
Uses of FTY720 in preparation of xerophthalmia treating drugs
The present invention belongs to the field of drug applications, and relates to new uses of FTY720 in pharmacy, particularly to uses of the FTY720 in preparation of xerophthalmia treating drugs, especially to new uses of the FTY720 in preparation of an ocular surface disease Sjogren syndrome drugs, wherein the FTY720 provides immunoregulation and inhibition effects for ocular surface autoimmunity inflammations adopting the Sjogren syndrome as the representative. According to the present invention, animal experiment results demonstrate that: after the diluted FTY720 is administered to the mouse ocular part in an ocular drop manner, the inflammation symptoms of the Sjogren syndrome of the treated NOS mouse are significantly relieved, the mass and the quality of tears are significantly improved, the tear film breaking time is significantly prolonged, and the proinflammatory factor level and the MMP level in the tear and the ocular surface tissue are significantly reduced. Further the FTY720 can be prepared into the drug for treating the ocular surface disease adopting the Sjogren syndrome as the representative, and is especially prepared into the ocular drop with the specific use concentration range.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Method of biomarker for identifying sjogren syndrome and detection kit for identifying sjogren syndrome
InactiveCN109406667AThe pre-processing process is simpleEasy to handleComponent separationSjögren syndromeHplc mass spectrometry
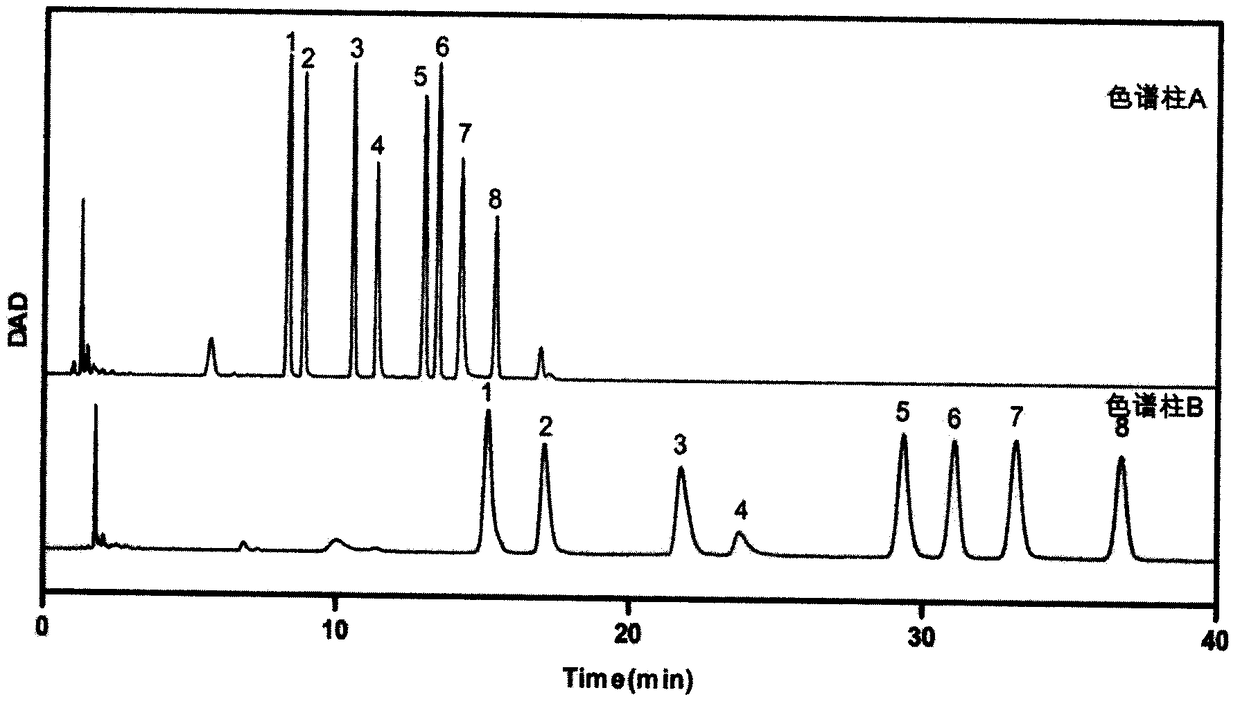
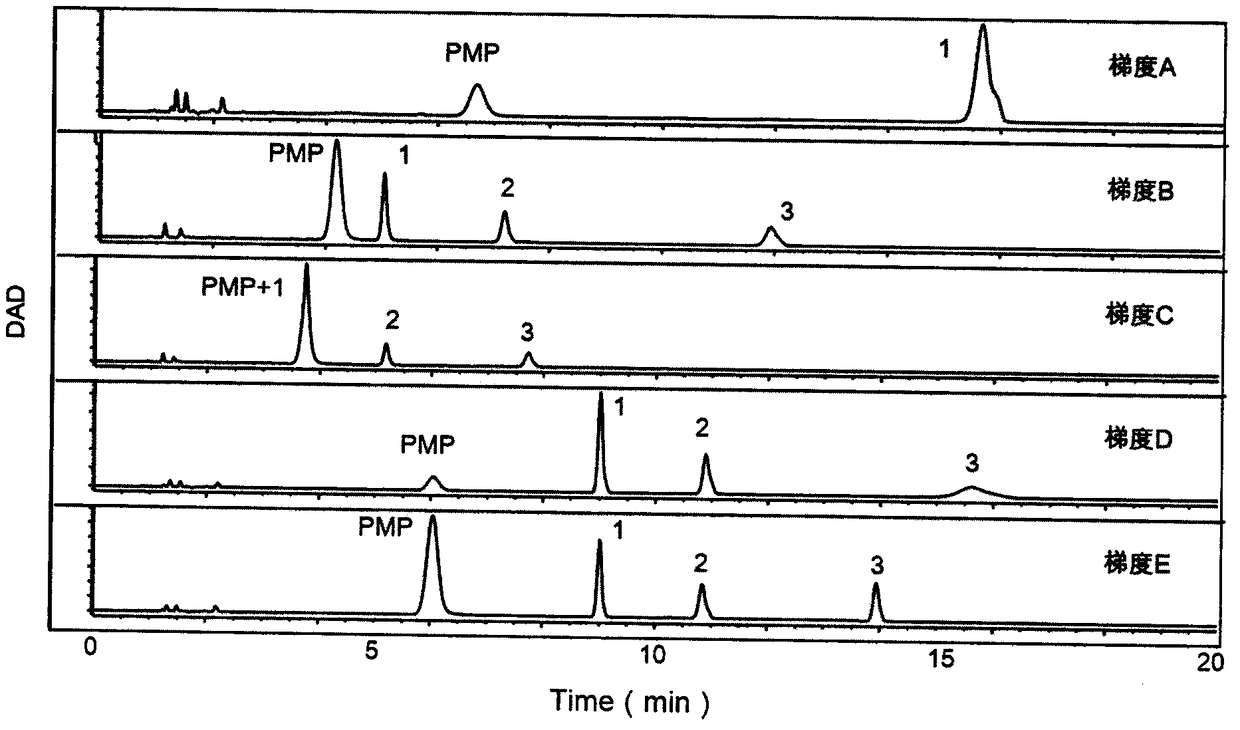
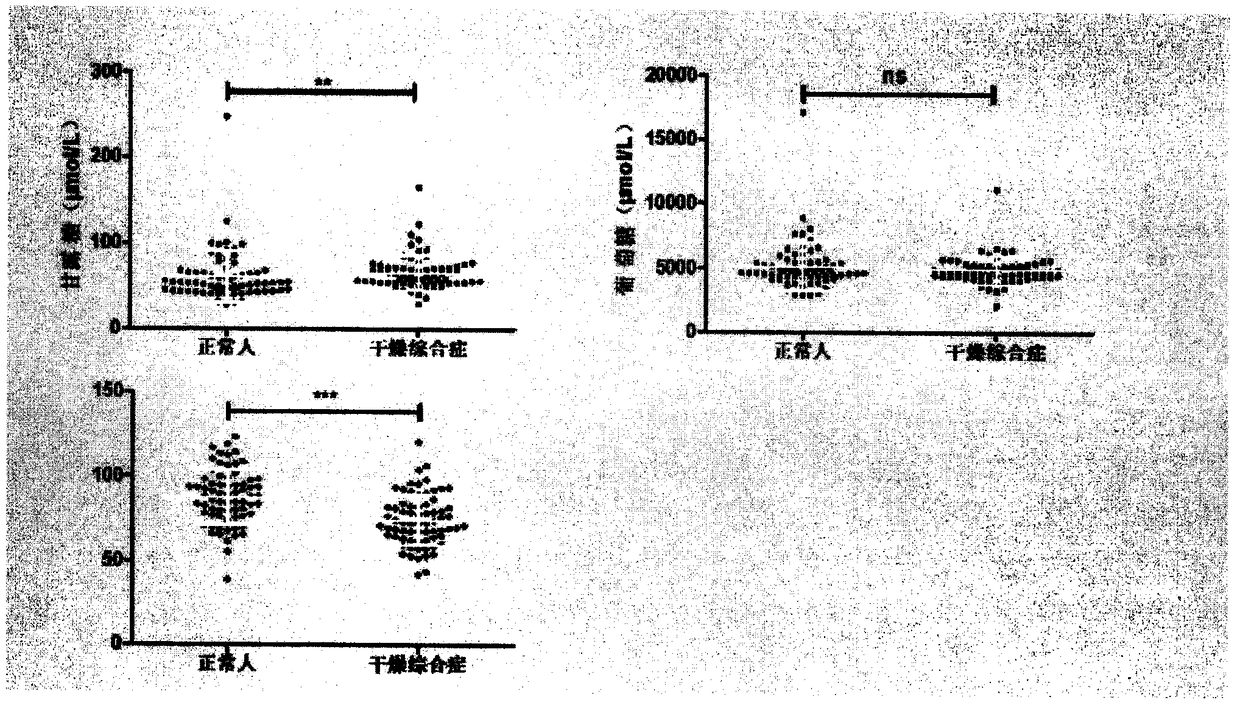
The invention provides a method of a biomarker for identifying sjogren syndrome and a detection kit for identifying the sjogren syndrome. The biomarker comprises free mannose and glucose which are obtained by high performance liquid chromatography derived from pre-column 1-phenyl-5-methyl pyrazolone (PMP) in serum. The detection method is a pre-column PMP derived high performance liquid chromatography method. According to the technical scheme, the method and the detection kit have the advantages that pretreatment is easy, the analysis time is short, the instrument price is reasonable, the method is compliance with conventional use, operation steps are simple and easyto learn, the accuracy of detection results is high, normal people can be distinguished from sjogren syndrome patients onlyby collecting, the amount of required serum is small, and the amount of the collected blood is less than 1 ml. The results show that the analytical method can rapidly quantify the free mannose and glucose in the serum of the sjogren syndrome patients, the method and the detection kit are of great significance for studying of the relationship between serum free mannose and glucose and sjogren syndrome, and seeking for new sjogren syndrome clinical detection markers.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Traditional Chinese medicine preparation for treating sjogren syndrome
InactiveCN104873878AStrong targeted curative effectQuick effectAnthropod material medical ingredientsImmunological disordersLiver and kidneyCurative effect
The invention belongs to the technical field of medicines, and particularly relates to a traditional Chinese medicine preparation for treating sjogren syndrome and a preparation method thereof. The traditional Chinese medicine preparation is prepared by the following medicines of prepared rhizome of rehmannia, dogwood, tuckahoe, cardamun, asarum, stiff silkworm, licorice root, ladybell root, radix scrophulariae, and flower of notoginseng. The traditional Chinese medicine preparation has the advantages that the compounding is reasonable, the preparation is easy, the obvious therapy effects of warming and reinforcing livers and kidneys, moistening dryness and eliminating dampness, and invigorating qi and nourishing yin are realized, and the traditional Chinese medicine preparation is an effective medicine for treating the sjogren syndrome, especially the yin deficiency and endogenous dryness type sjogren syndrome.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
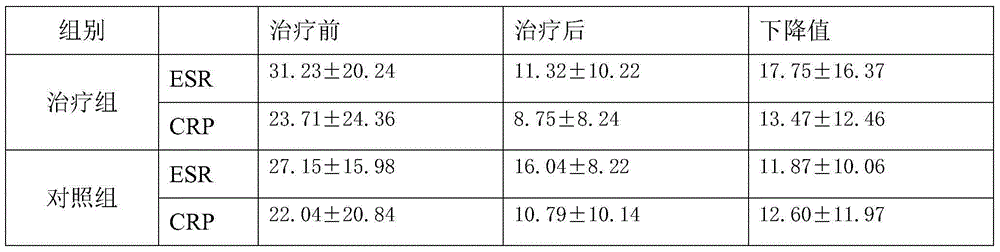
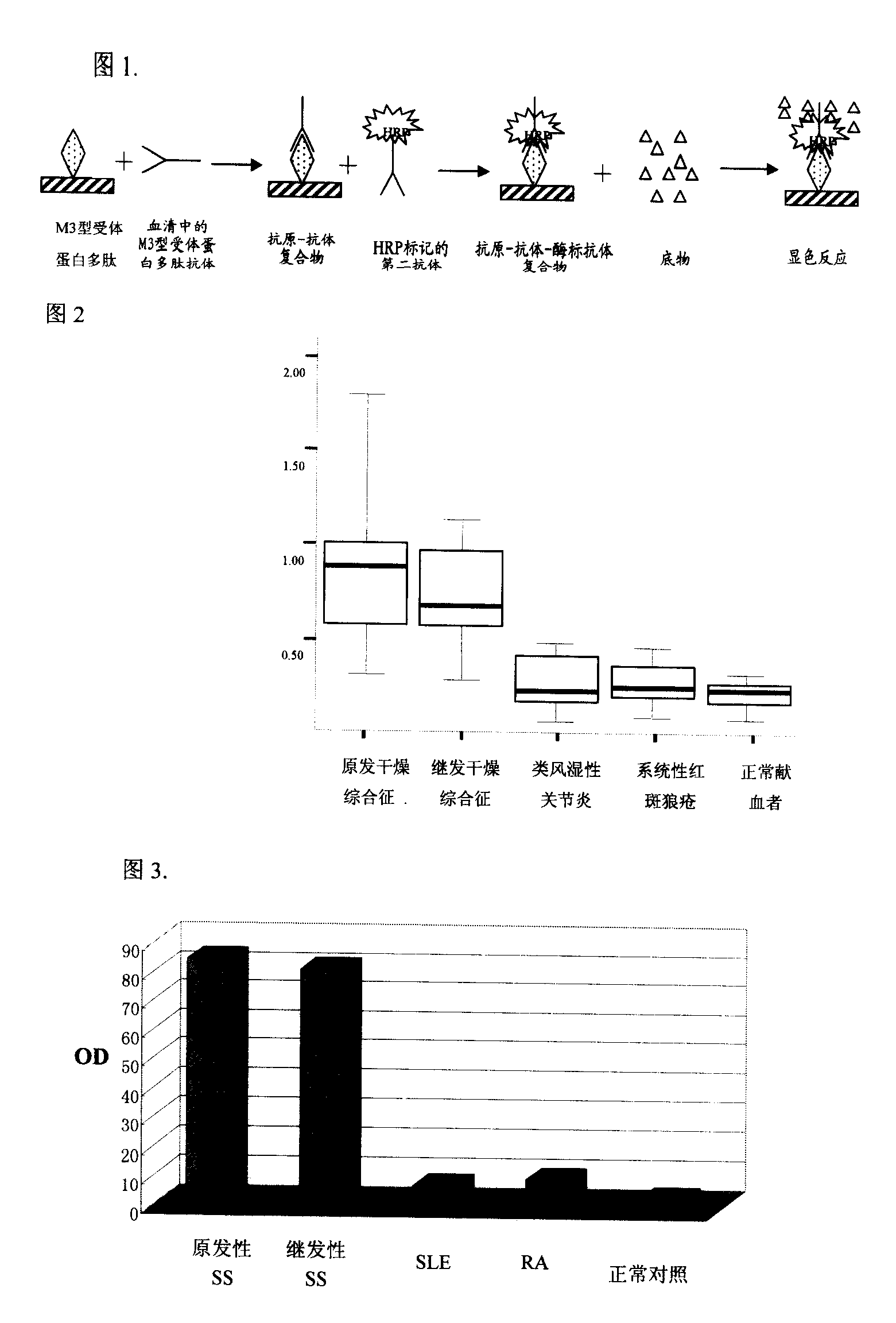
Application of M3 type receptor protein polypeptide resisting antibody measurement in Sjogren syndrome diagnosis
The invention provides a check of Sjogren syndrome by M3-type receptor protein polypeptide and analogues, wherein the peptide antigen can be specially combined with specific antibody in Sjogren syndrome patient, with high sensitivity and specificity.
Owner:PEOPLES HOSPITAL PEKING UNIV
Detection kit for antinuclear antibody repertoires
The invention discloses a detection kit for antinuclear antibody repertoires, and belongs to the technical field of detection kits. The detection kit comprises a composite micro bead suspension, a fluorescence indicator and a sample diluent, wherein a plurality of micro beads are contained in the composite micro bead suspension; a part of micro beads are coated with different types of autologous antigens respectively, a part of micro beads are coated with antigens prepared from HEP-2 cells, a part of micro beads are coated with antigens capable of detecting non-specific antibodies in patient samples, and the remaining micro beads are four types of standard control micro beads; the fluorescence indicator is phycoerythrin labeled goat-anti-human IgG (specific gamma chain); the sample diluentis a scrubbing solution. The detection for a plurality of autologous antibodies can be performed for one patient sample inside the same hole, so that auxiliary diagnosis is provided for various systemic autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, scleroderma, polymyositis, mixed connective tissue disease (MCTD), drug-induced SLE and sjogren syndrome.
Owner:宙斯生命科技(常州)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com