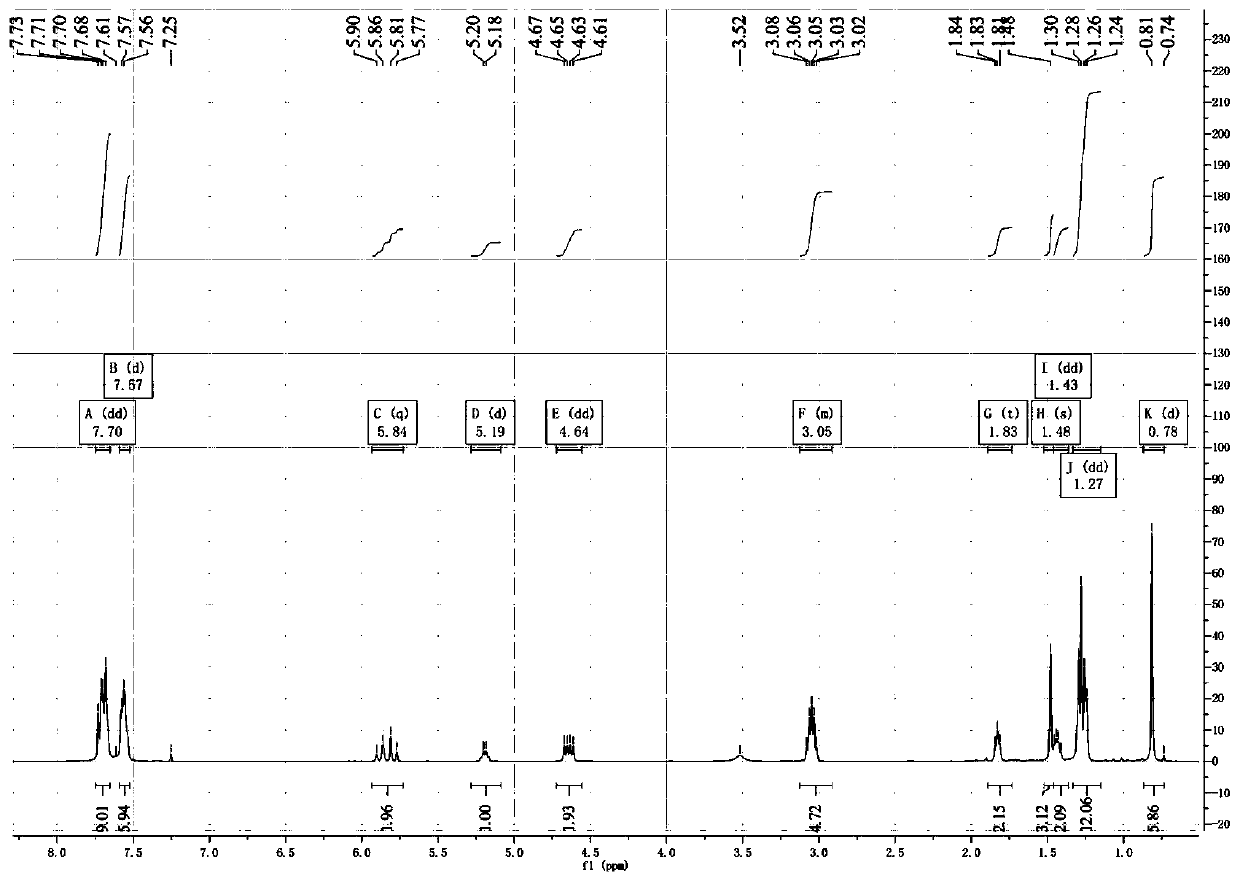
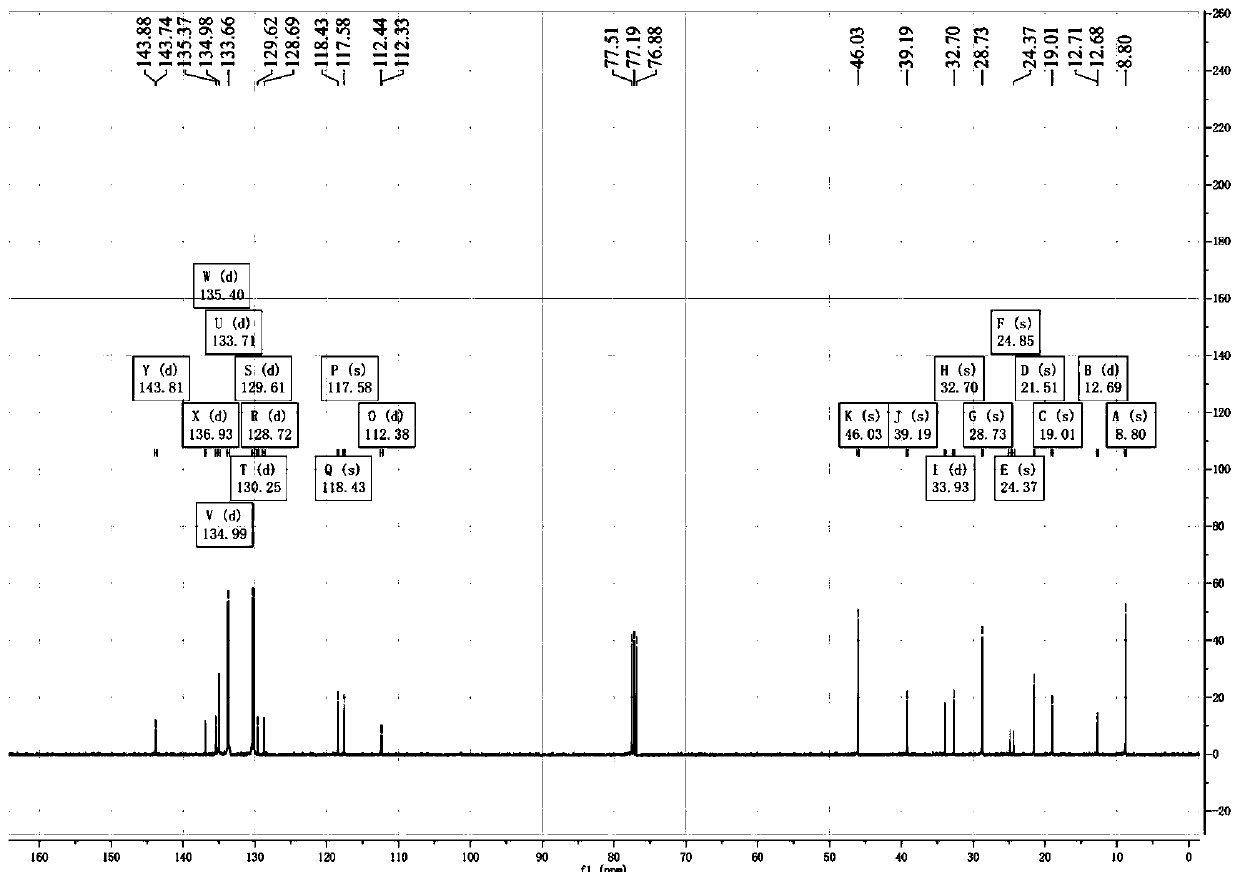
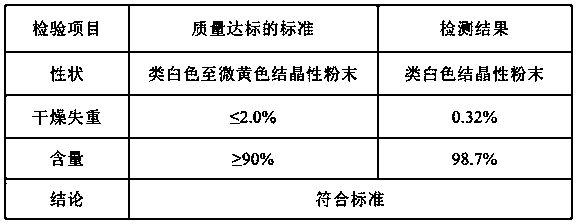
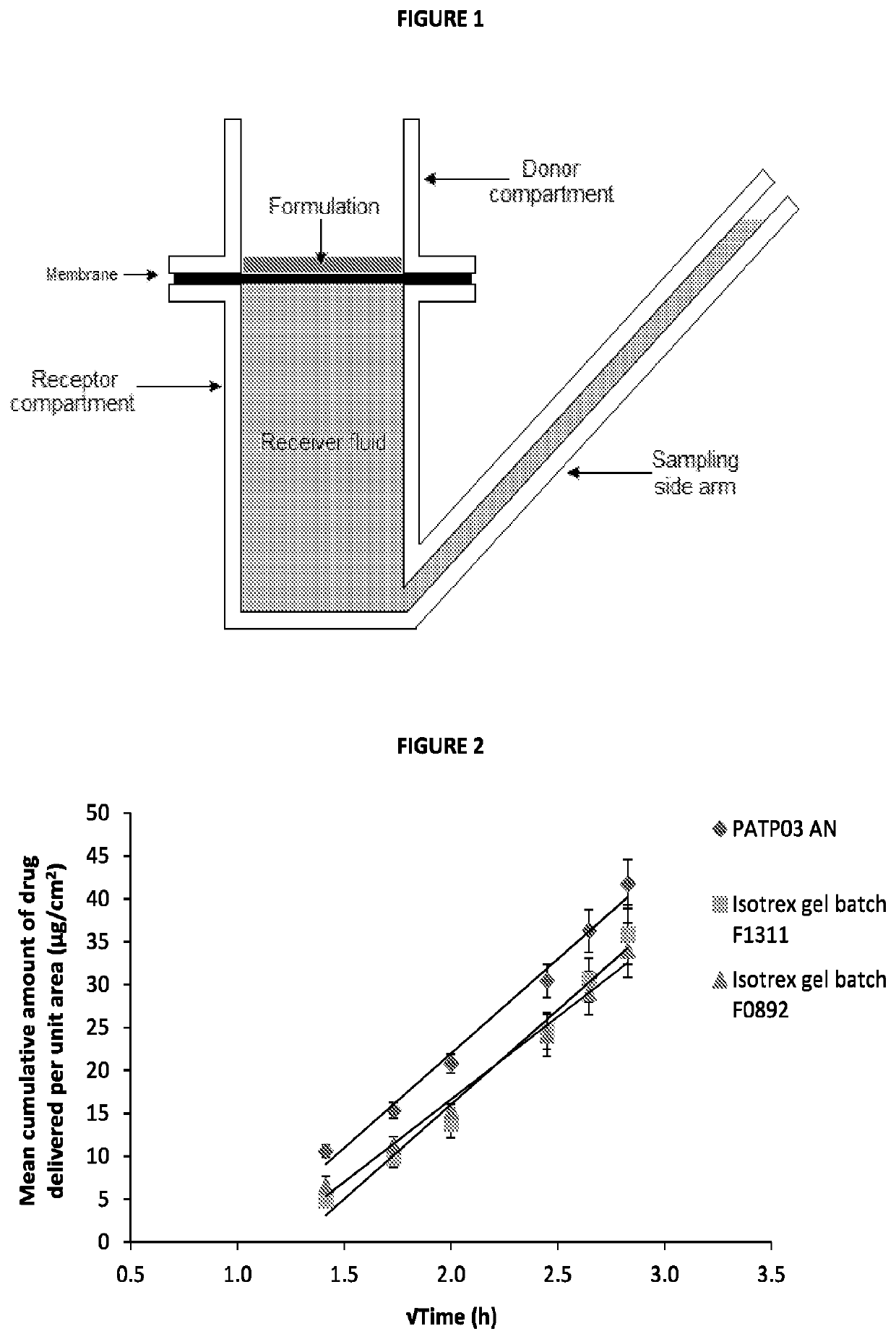
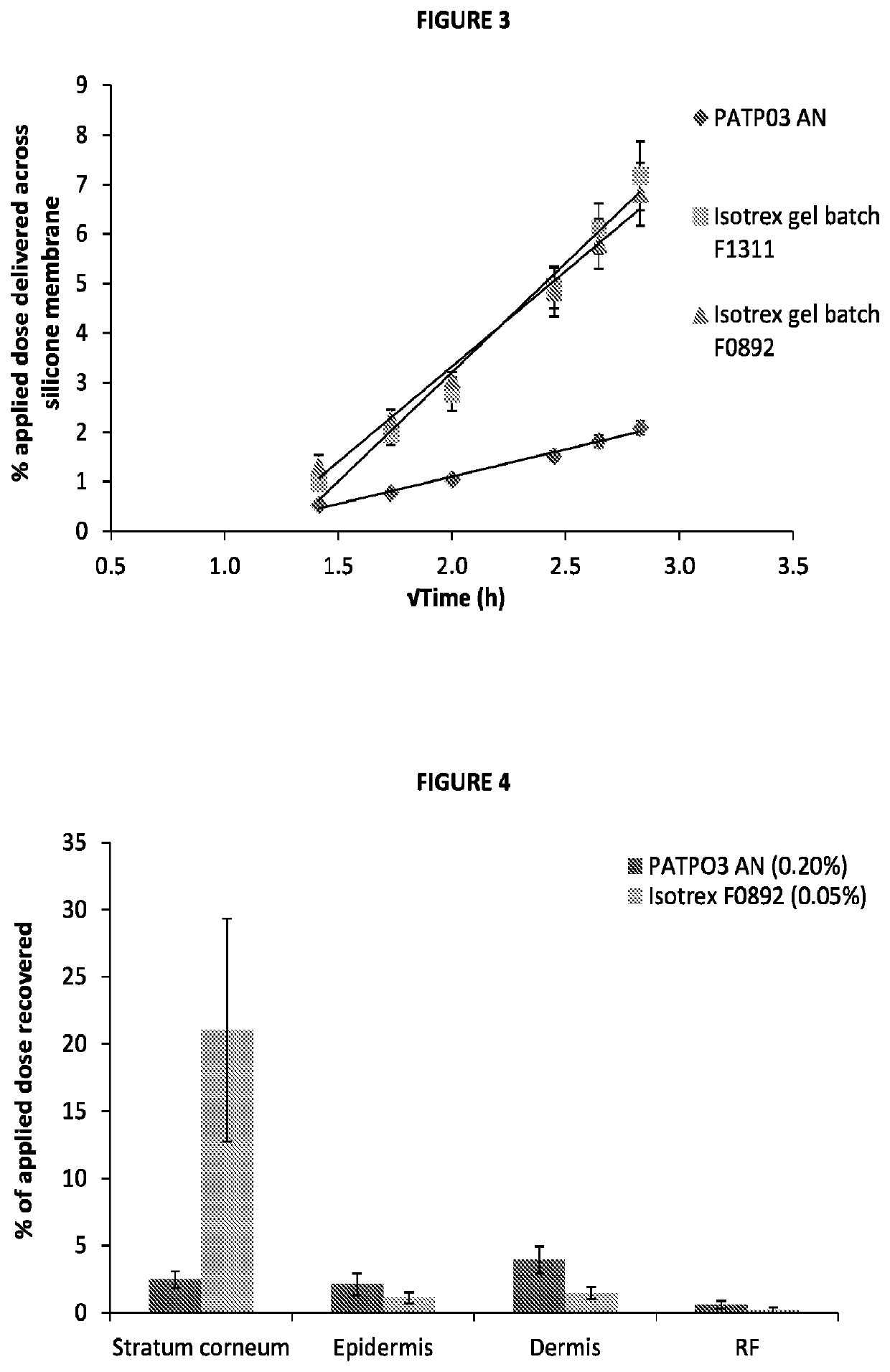
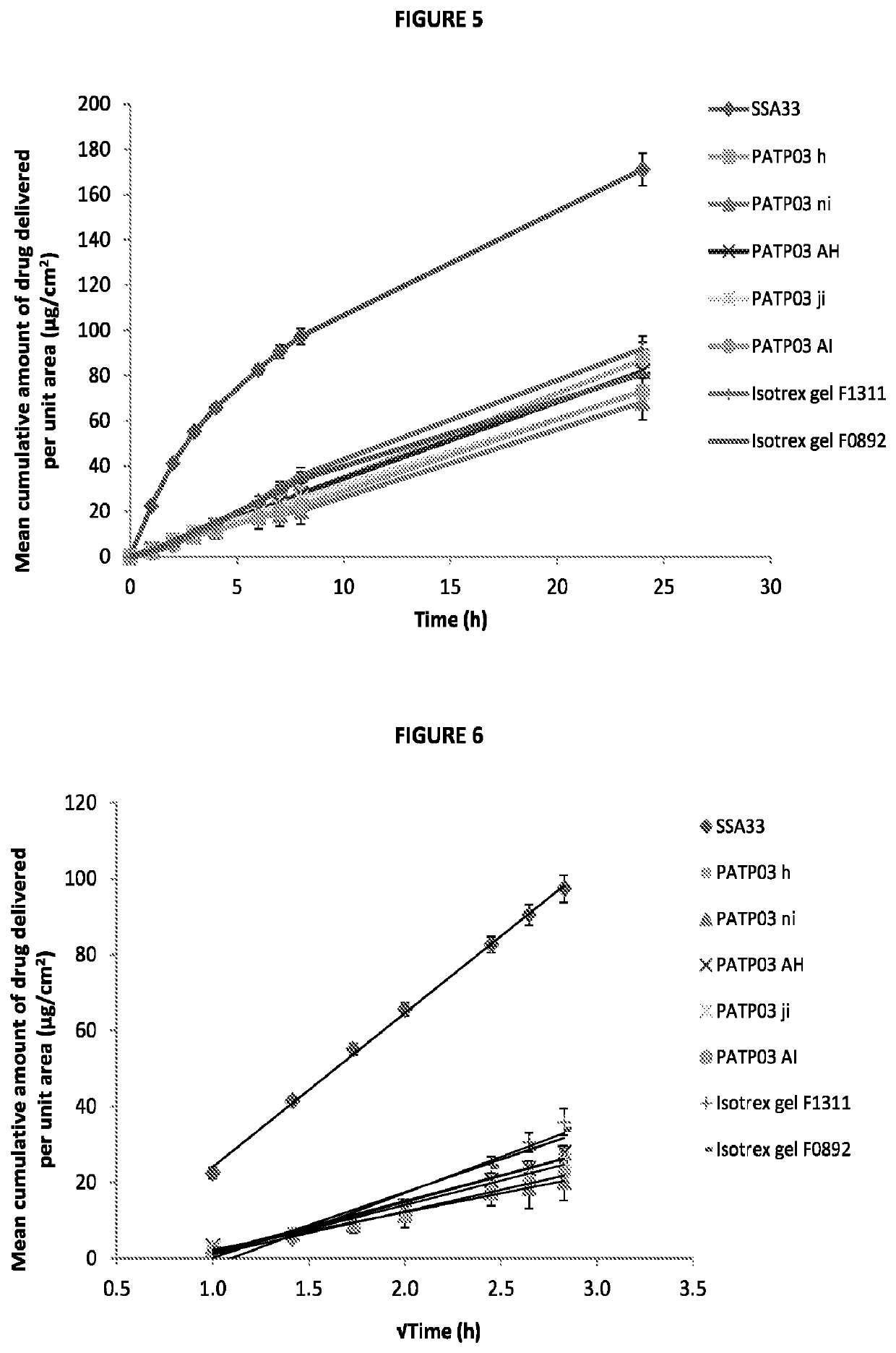
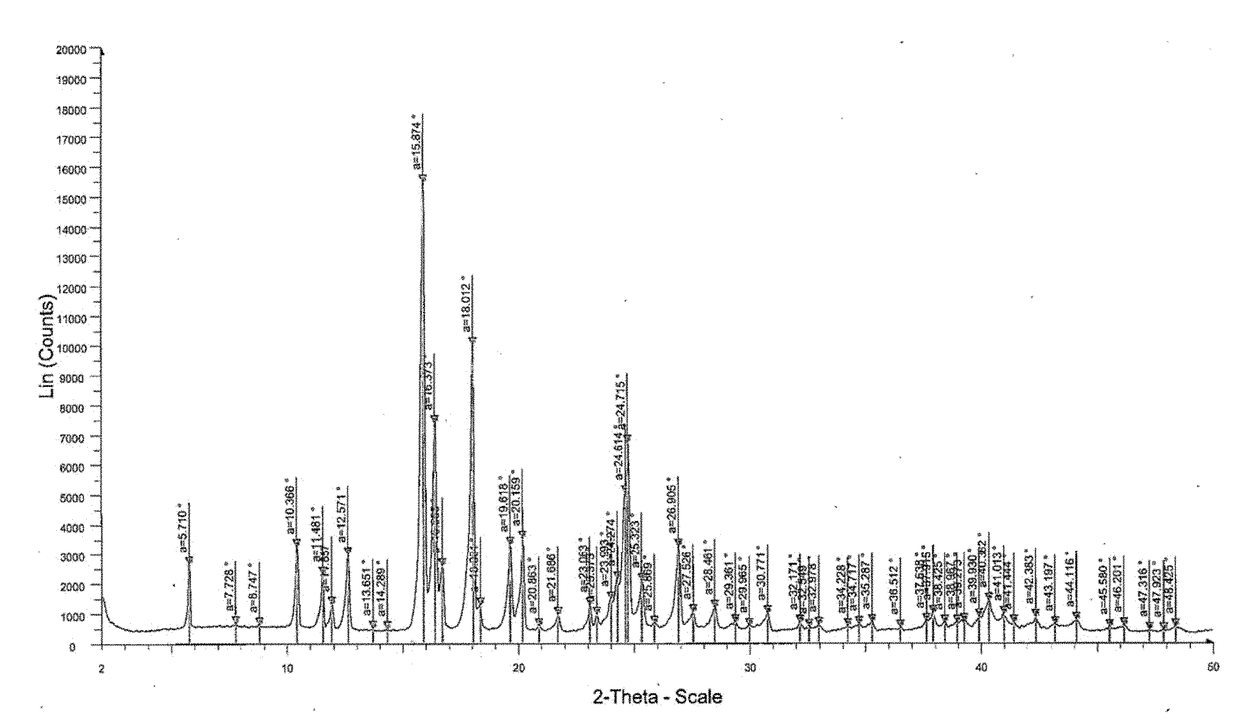
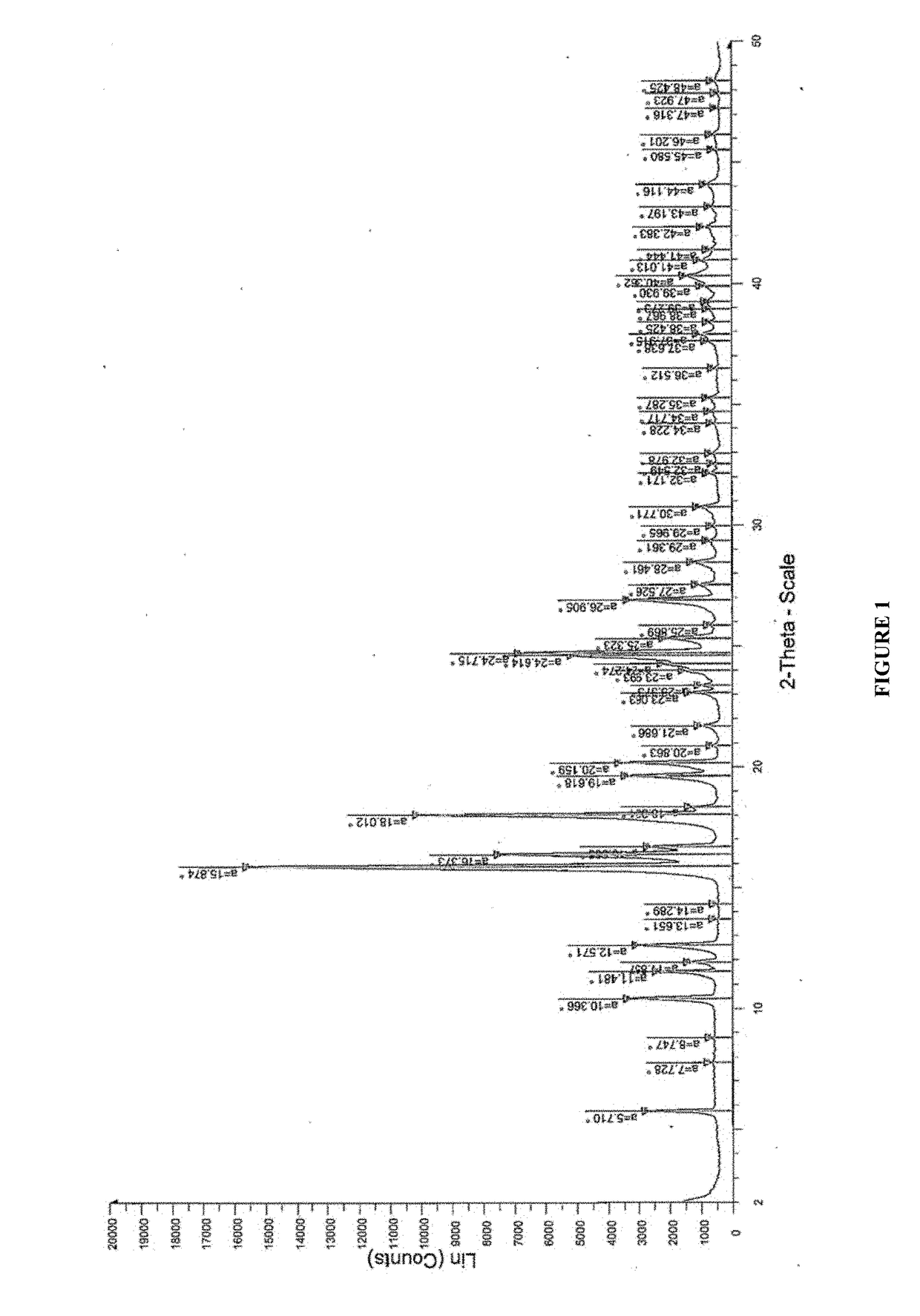
Patents
Literature
58 results about "Isotretinoin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
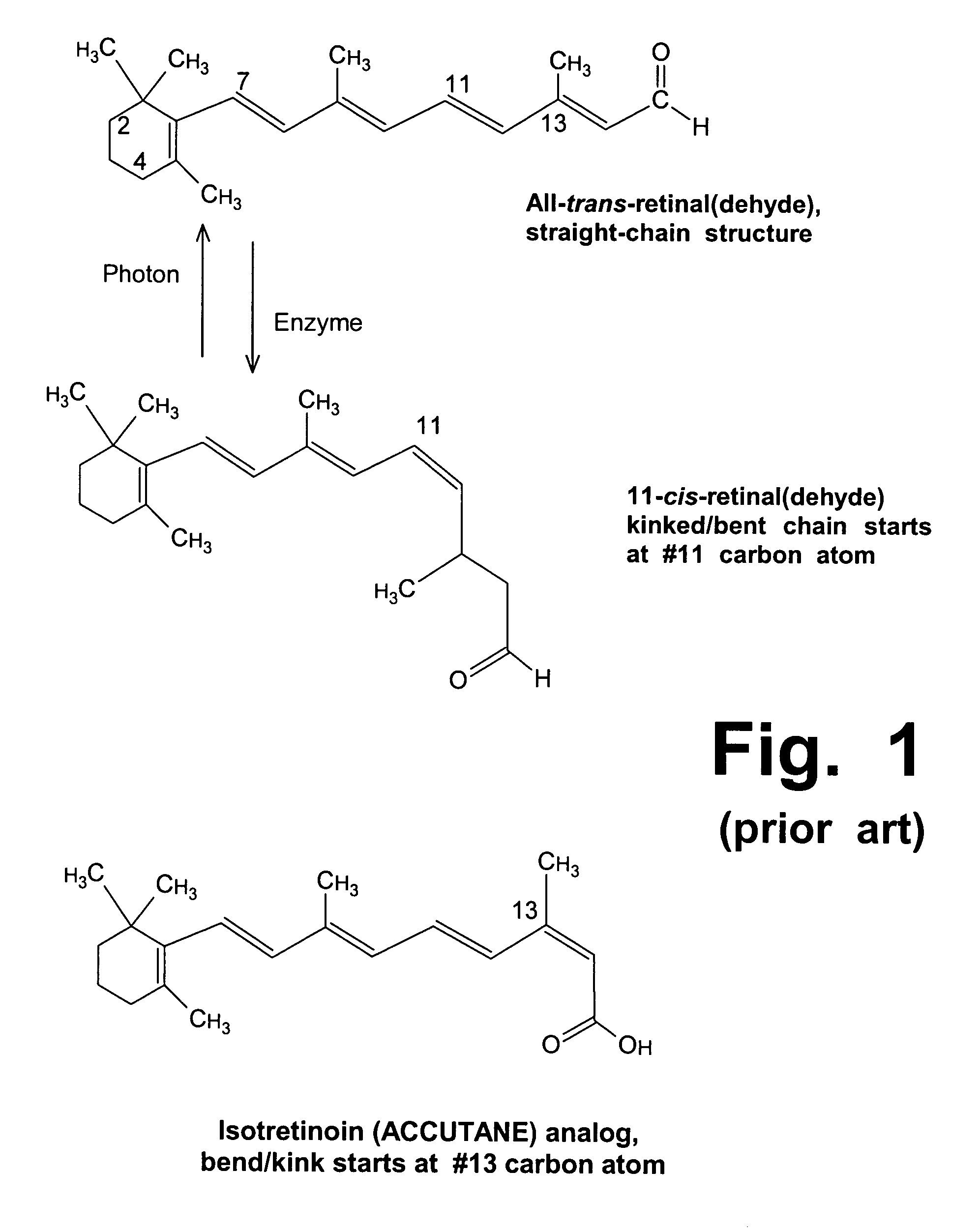
This medication is used to treat severe cystic acne (also known as nodular acne) that has not responded to other treatment (e.g., benzoyl peroxide or clindamycin applied to the skin or tetracycline or minocycline taken by mouth).
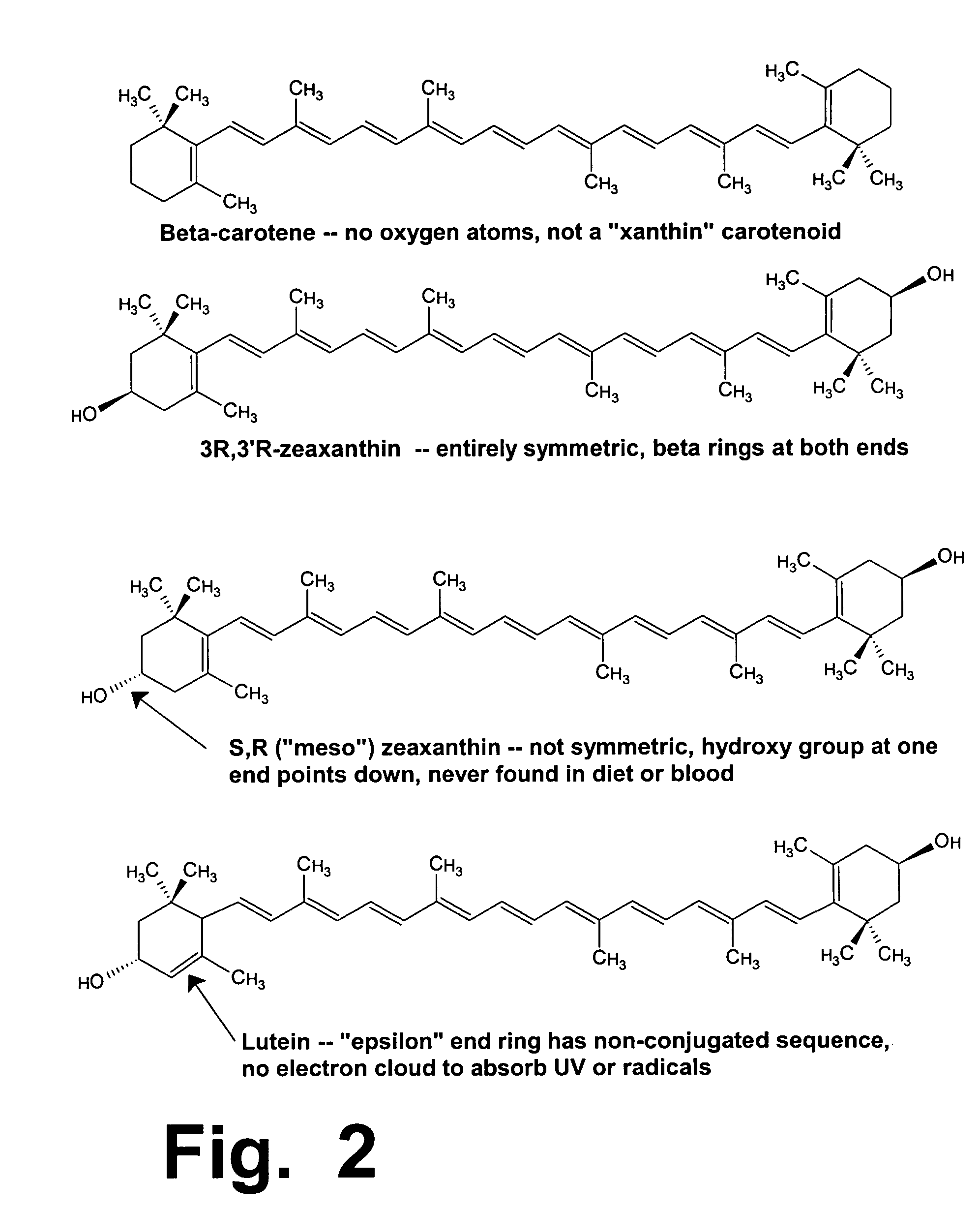
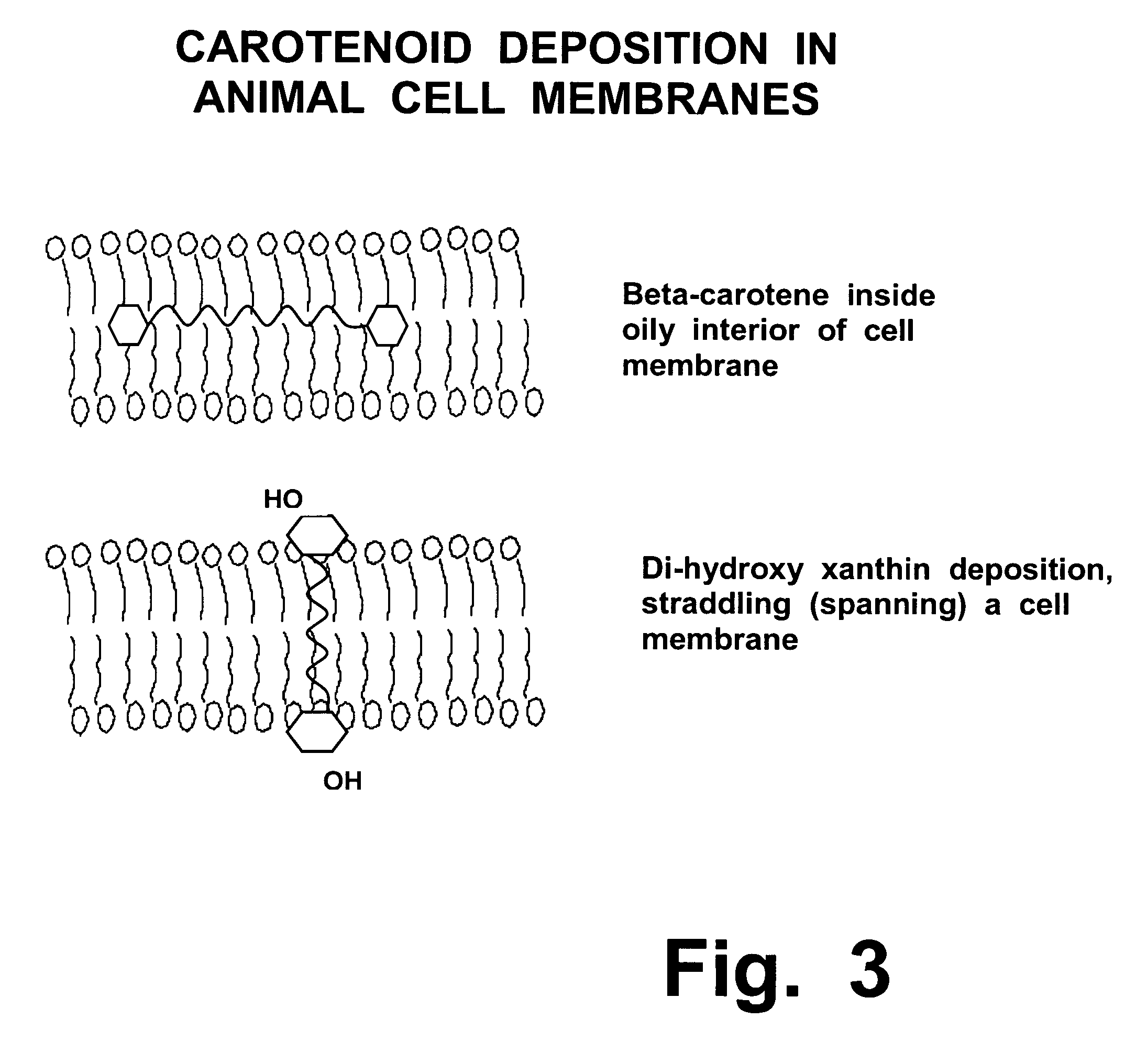
Treatment of Stargardt's disease and other lipofuscin disorders with combined retinaldehyde inhibitor and zeaxanthin
InactiveUS20060089411A1Slow downImprove abilitiesBiocideHydroxy compound active ingredientsMetaboliteRetinoid
Zeaxanthin, a natural carotenoid, can improve and increase the ability of enzyme inhibitors that can slow down certain enzymes that are contributing to toxic metabolite accumulation in people who suffer from Stargardt's disease or other lipofuscin disorders. Such enzyme inhibitors include retinoid analogs such as isotretinoin, commonly known by the trademark, ACCUTANE. This drug binds to and inhibits at least two retinal enzymes, known as RPE65 and short chain dehydrogenase, which create surplus metabolites that feed into a pathway that eventually creates toxic metabolites in people with Stargardt's disease. However, isotretinoin treatment alone is not highly effective; therefore, use of zeaxanthin as an adjunctive treatment can improve the efficacy and outcomes of such treatments.
Owner:ZEAVISION LLC
Topical base and active agent-containing compositions, and methods for improving and treating skin
InactiveUS20120100183A1Desirable tasteEasy to manageBiocideOrganic active ingredientsDiseaseIrritant dermatitis
The present invention provides unique, efficacious, inexpensive, safe, reliable, convenient, minimally bitter, skin protecting and penetrating, easy-to-administer base compositions and active agent-containing compositions, such as those including hydrocortisone, and related production and topical application methods, for treating the skin of mammals for a wide variety of different dermatologic conditions, disorders and diseases, such as inflammation, redness, cracking, insect bites, dryness, allergic reactions, trauma, irritant dermatitis, perleche, contact dermatitis, psoriasis, eczema, seborrheic dermatitis, acne excoriate, xerosis, eczema craquele, stasis dermatitis, disease related conditions and dryness from medications such as isotretinoin, acitretin and lipid lowering agents. This is effected by topically administering, or otherwise applying, effective amounts of the compositions thereto in forms that not only address the skin and mucosa of the mouth and lips, but also of the rest of the body and, in particular, areas where other topical balms containing hydrocortisone and other active ingredients have not been developed or marketed. Additionally, the flavoring addition to this product, and the base wherein the active ingredient(s) reside, affords a significantly better tasting, and less bitter, composition, thereby allowing a more pleasant experience and better compliance by patients. Larger sized stick formulation(s) allow for more applicability of the product, and more usefulness thereof, in various areas, and mucosal skin, of the body. The compositions include a unique formulation of FANCOL VB, Natunola Castor 1023, Finsolv TN, bees wax and, optionally, one or a plurality of plant or plant seed oils, fatty alcohols, fats and flavorings, in desirable weight percents thereof, in various forms, and preferably in the form of a solid roll-on stick present in a variety of sizes.
Owner:SCHLESSINGER JOEL +1
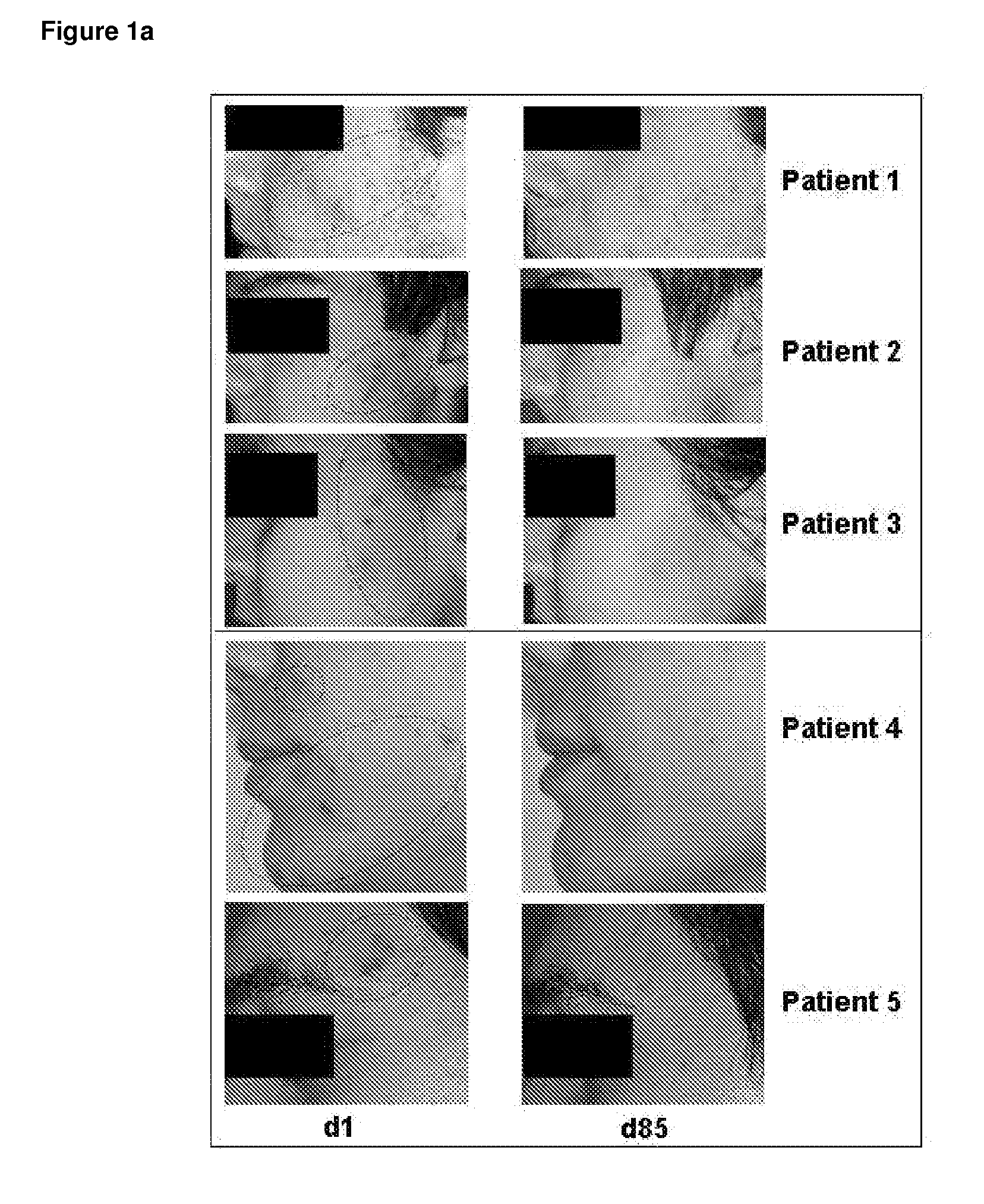
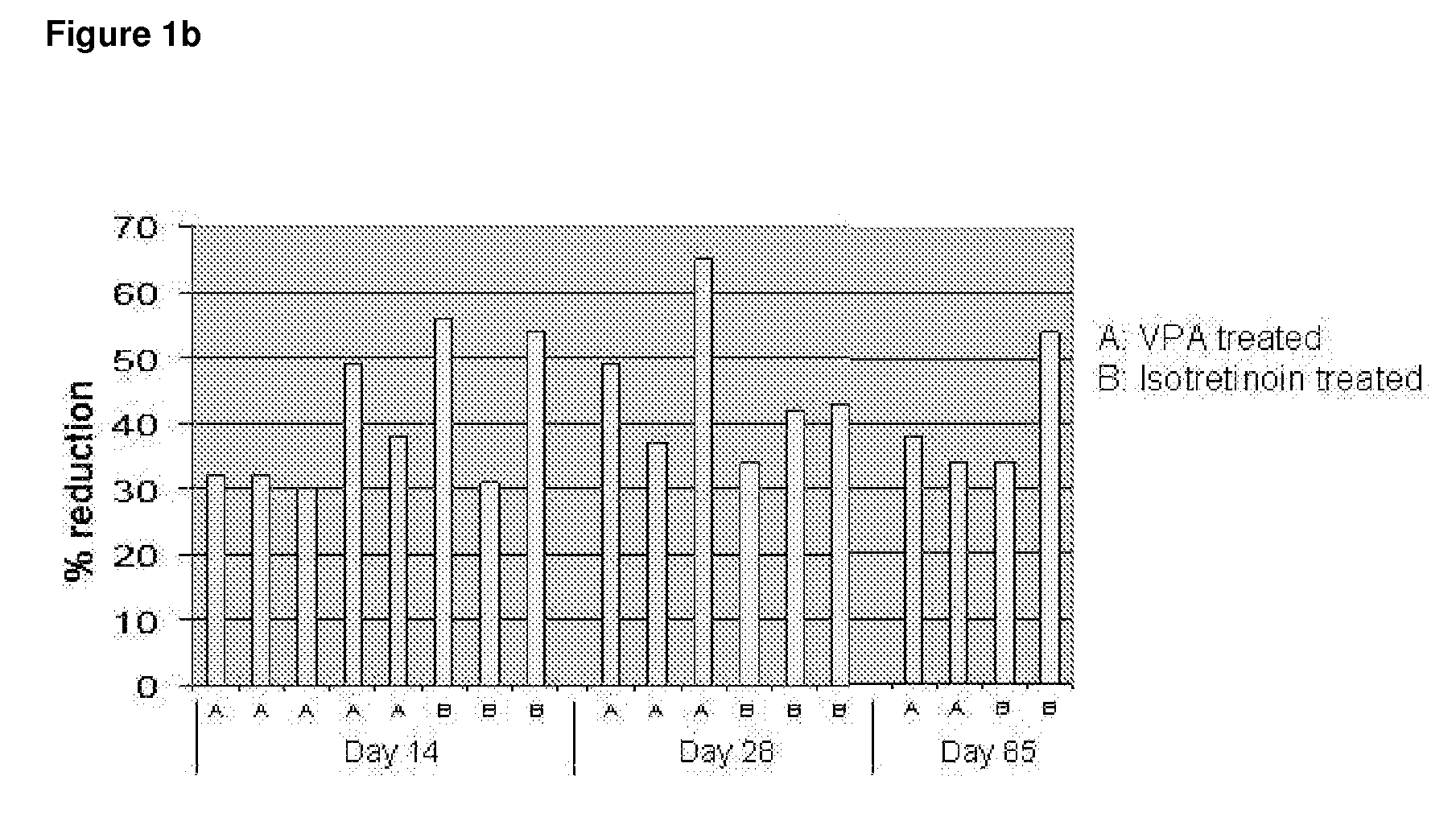
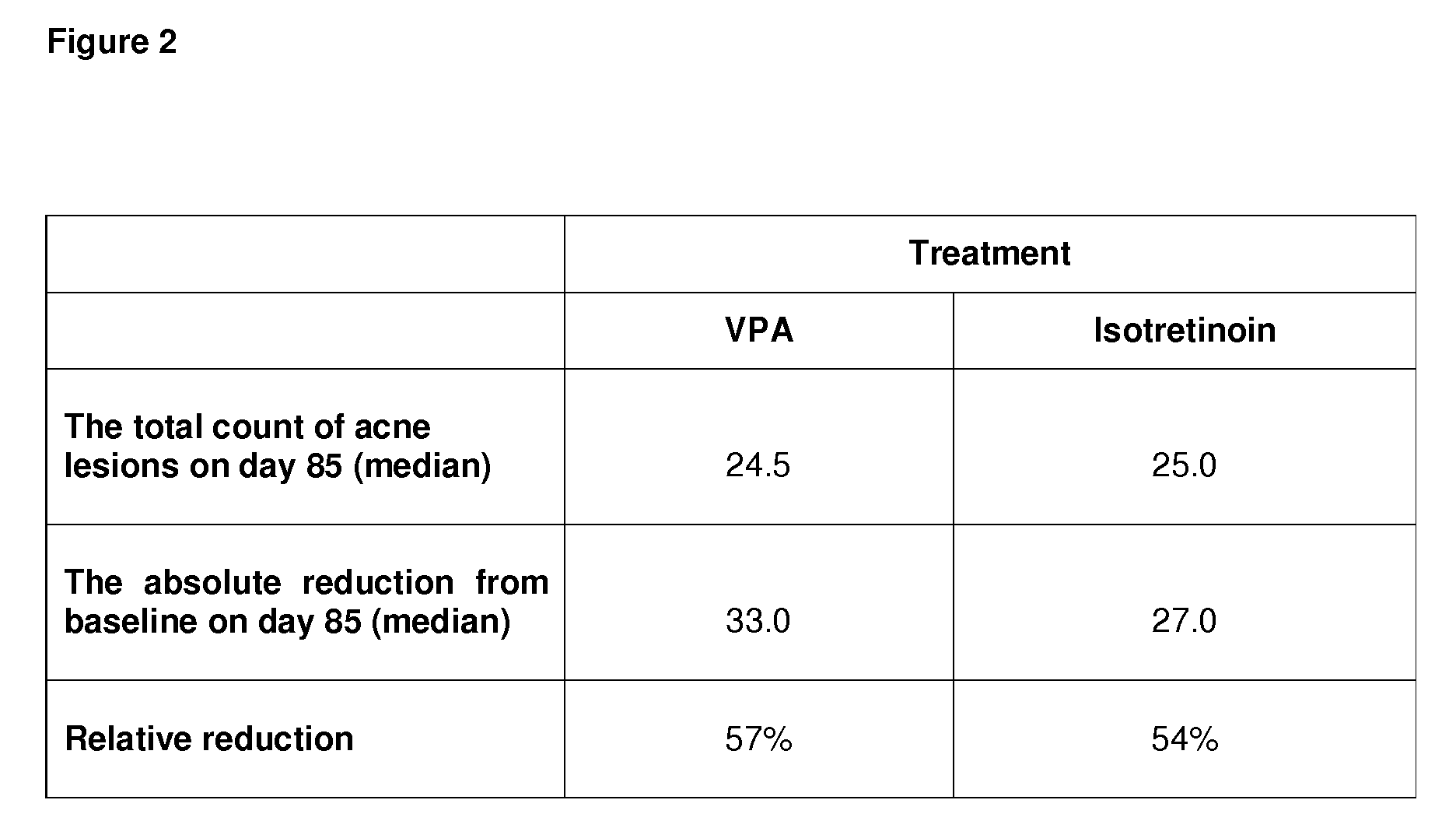
Use of valproic acid for the topical treatment of mild to moderate acne vulgaris
InactiveUS20090186809A1Improve toleranceGood local tolerabilityAntibacterial agentsBiocideClinical efficacyValproic Acid
The present invention provides a surprising therapeutic beneficial use for the topical application of valproic acid as a single agent therapy for patients suffering from mild to moderate acne vulgaris. Topically applied VPA has a clinical efficacy comparable to that of the marketed standard medication for this indication, isotretinoin. Furthermore, topically applied VPA is on average well to very well tolerated. The invention relates to the topical medical use of VPA for the treatment of acne vulgaris and comprises the topical application of VPA or of a pharmaceutically acceptable salt thereof.
Owner:TOPOTARGET GERMANY AG
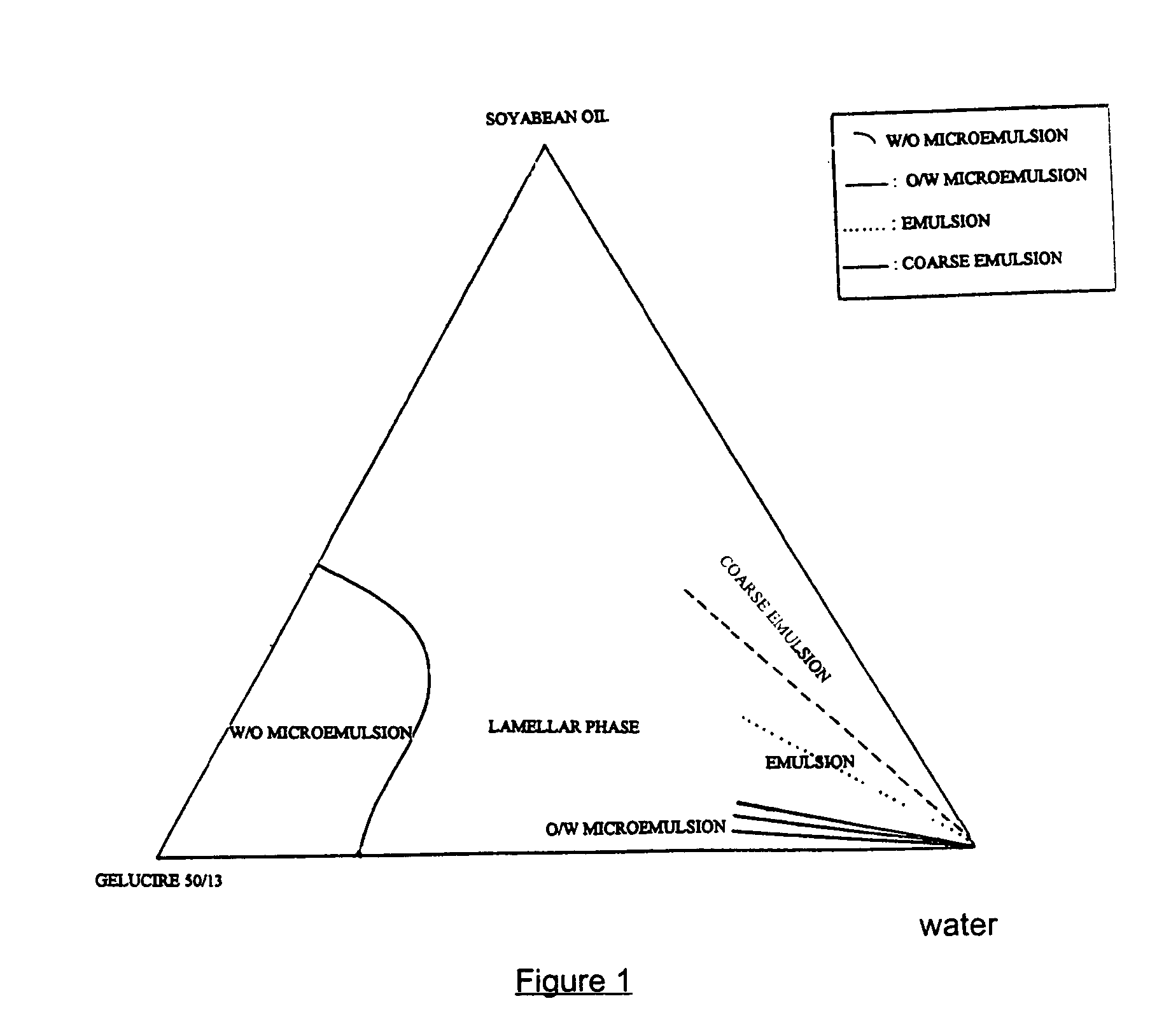
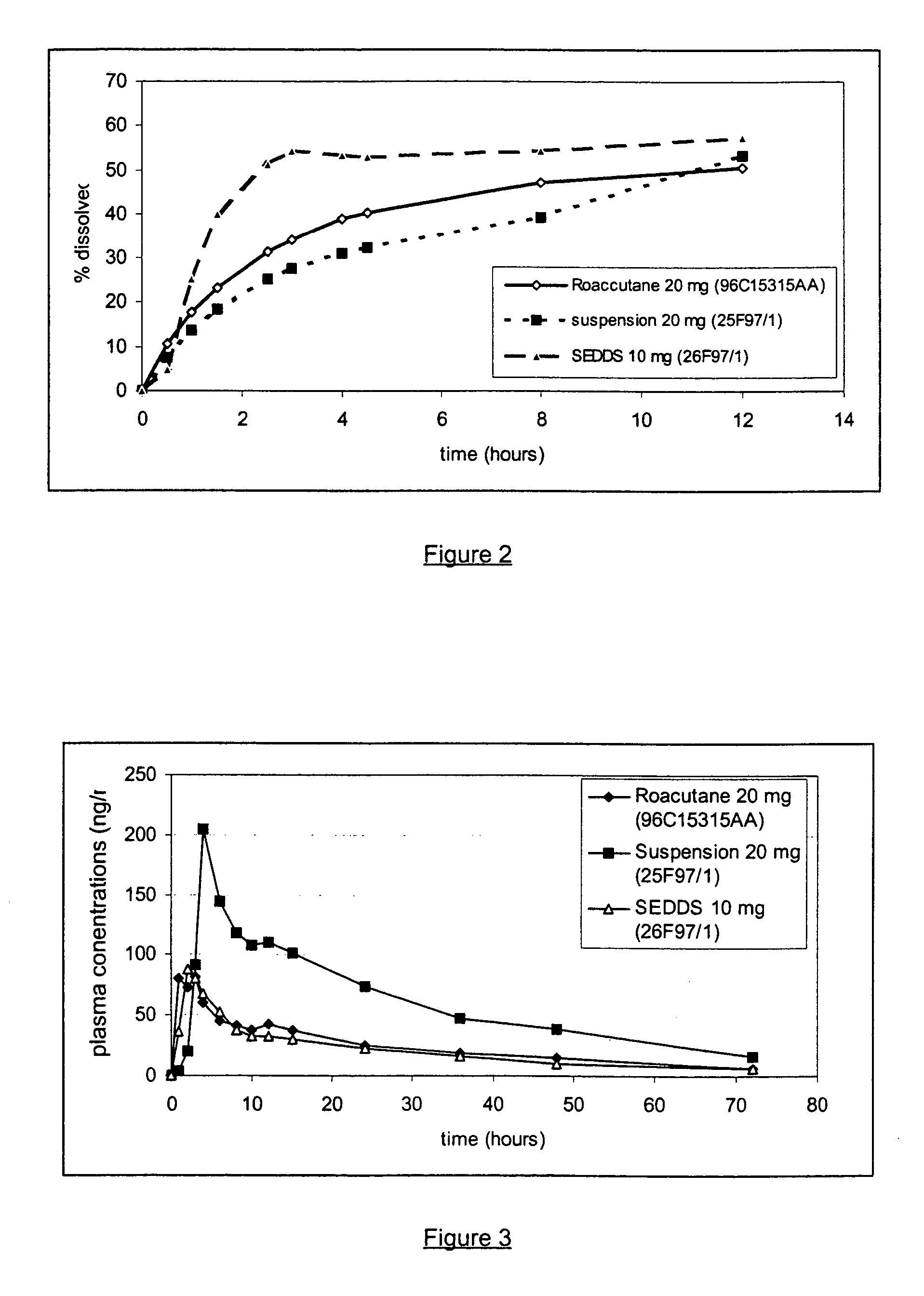
Pharmaceutical semi-solid composition of isotretinoin
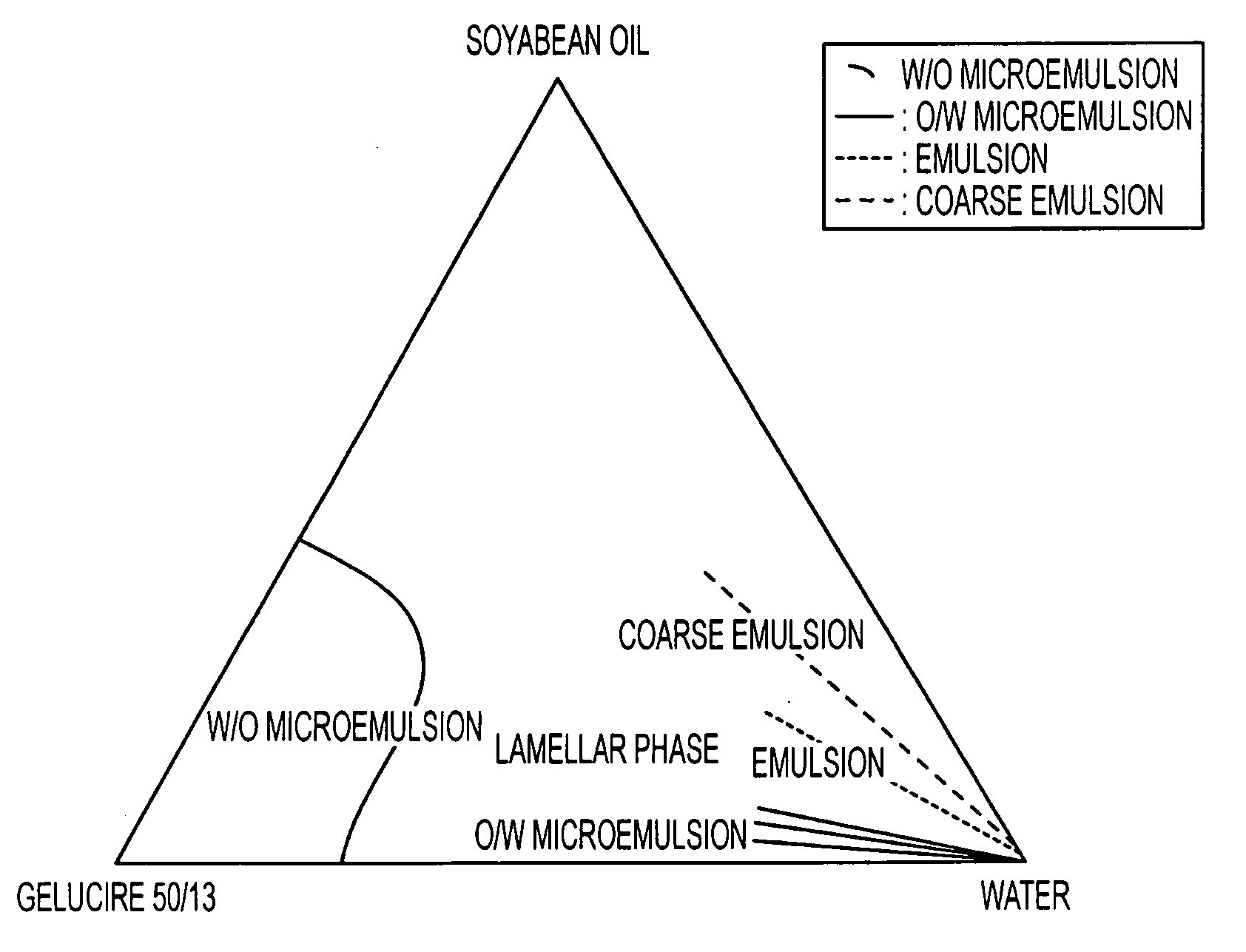
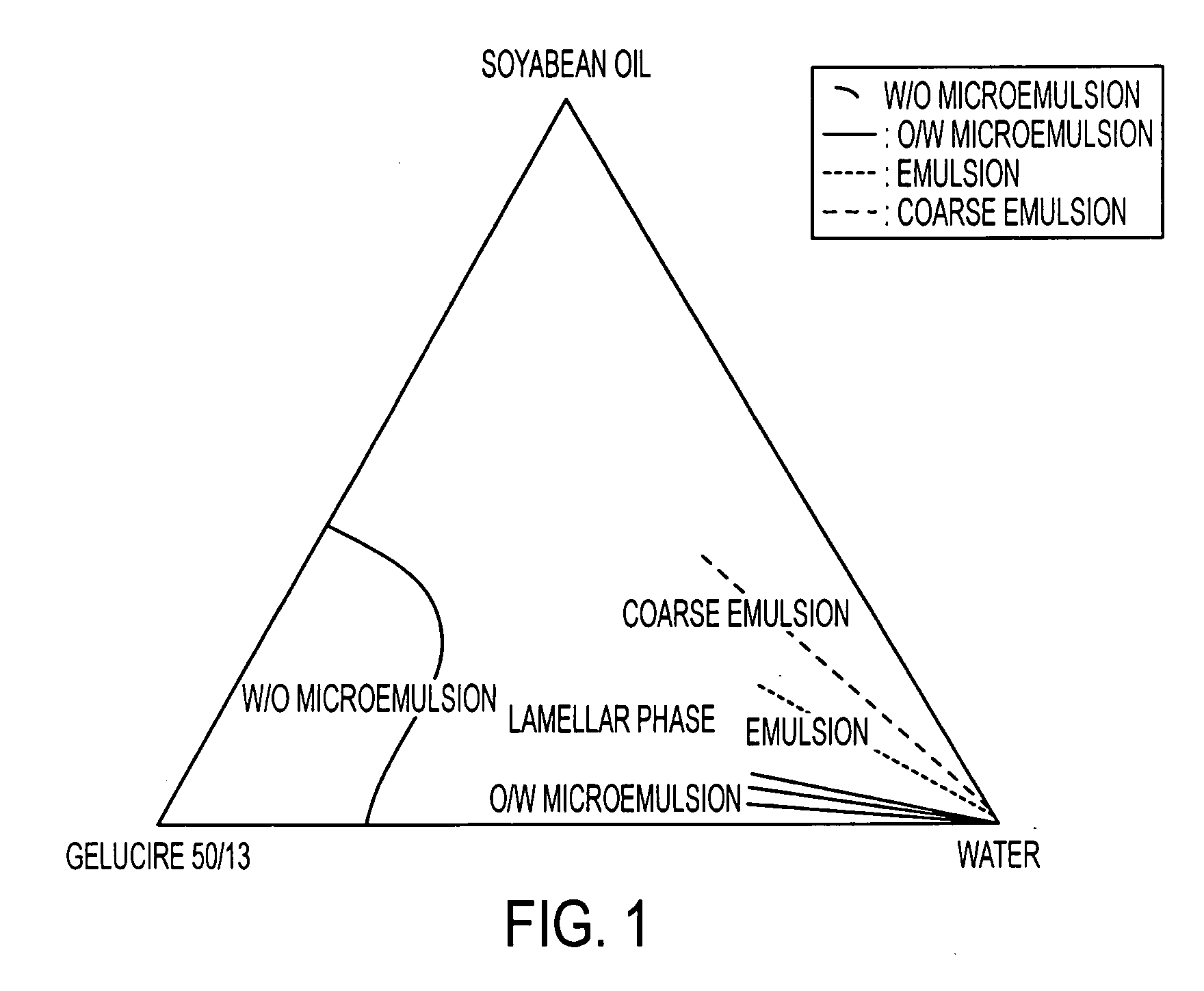
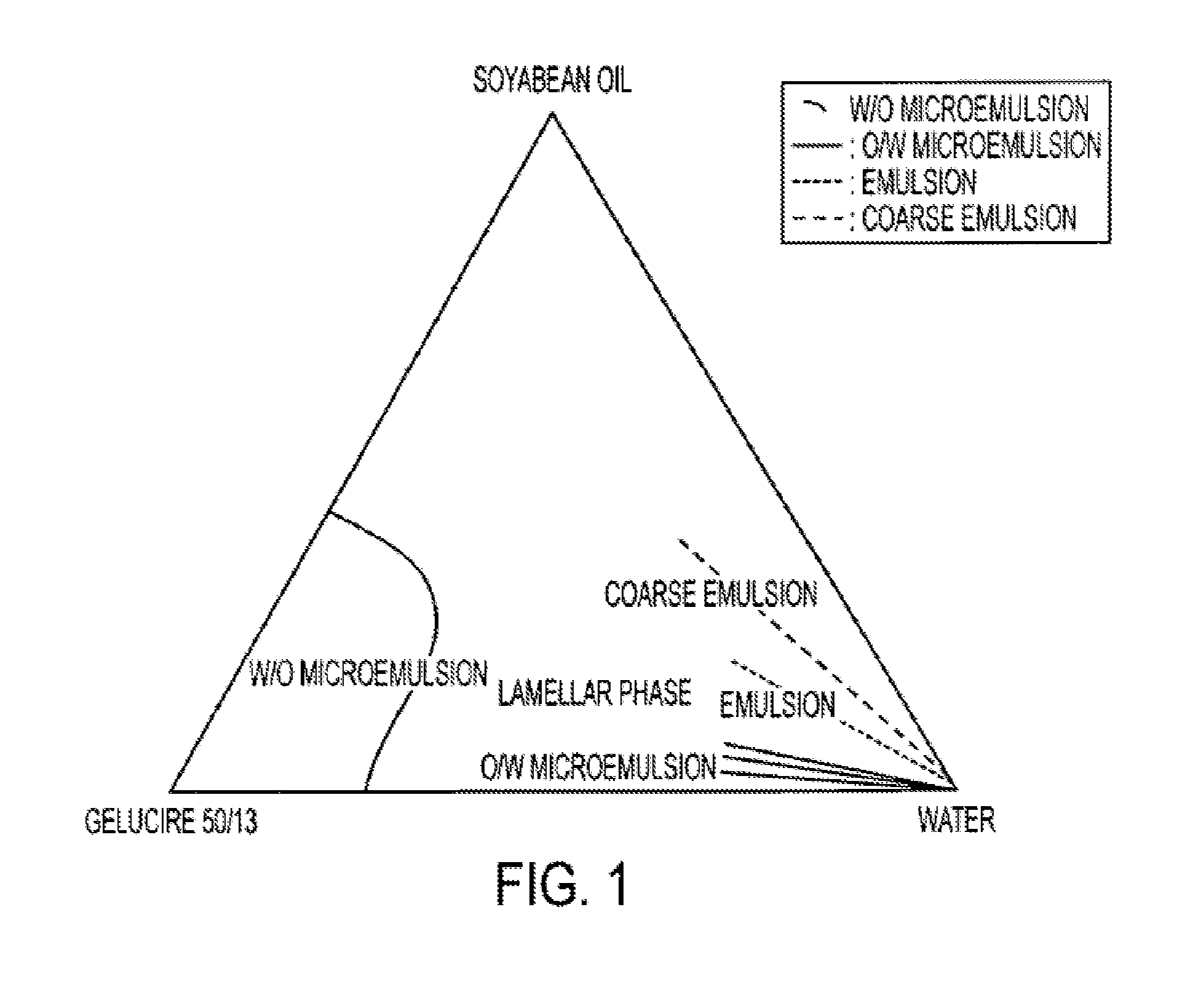
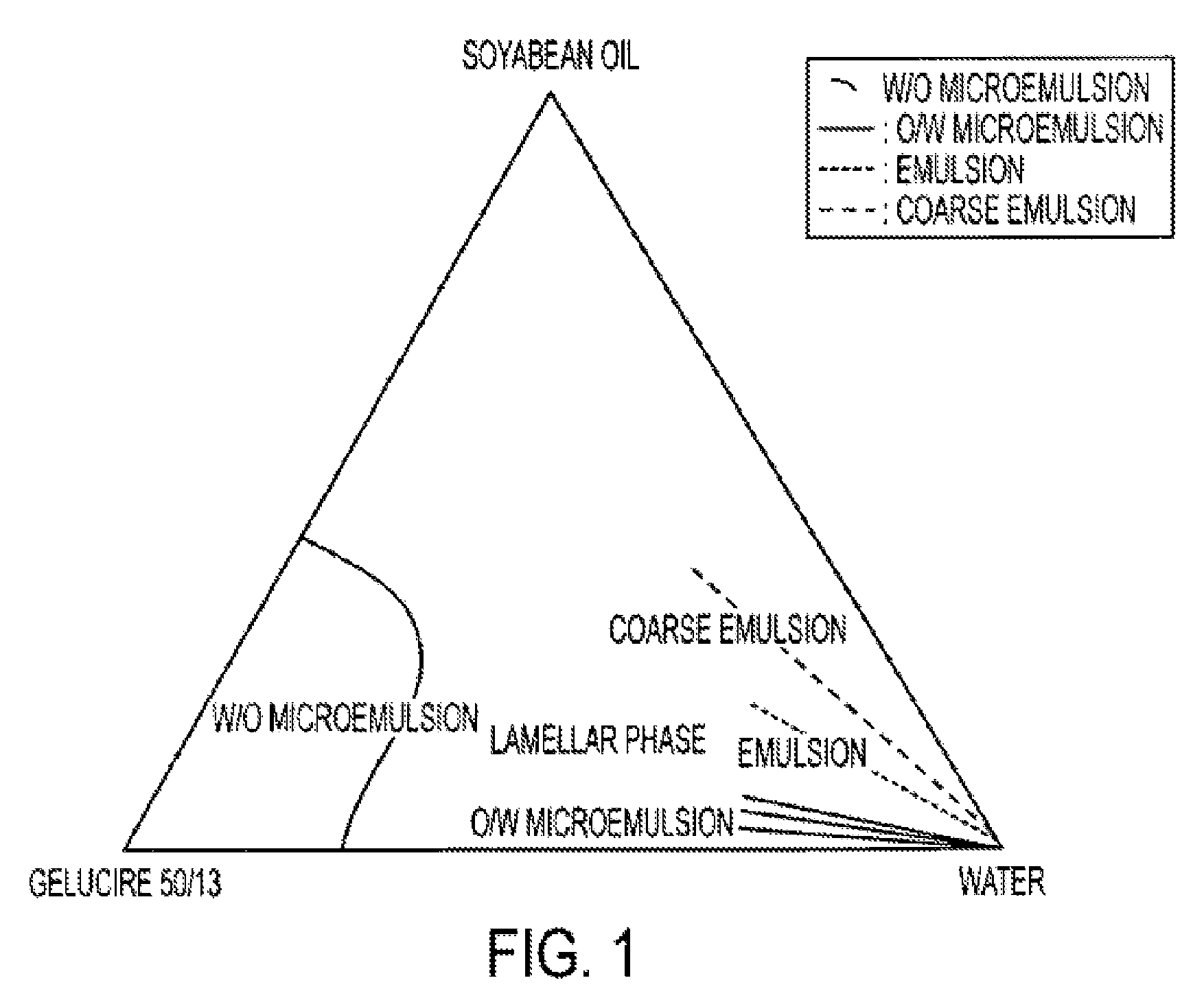
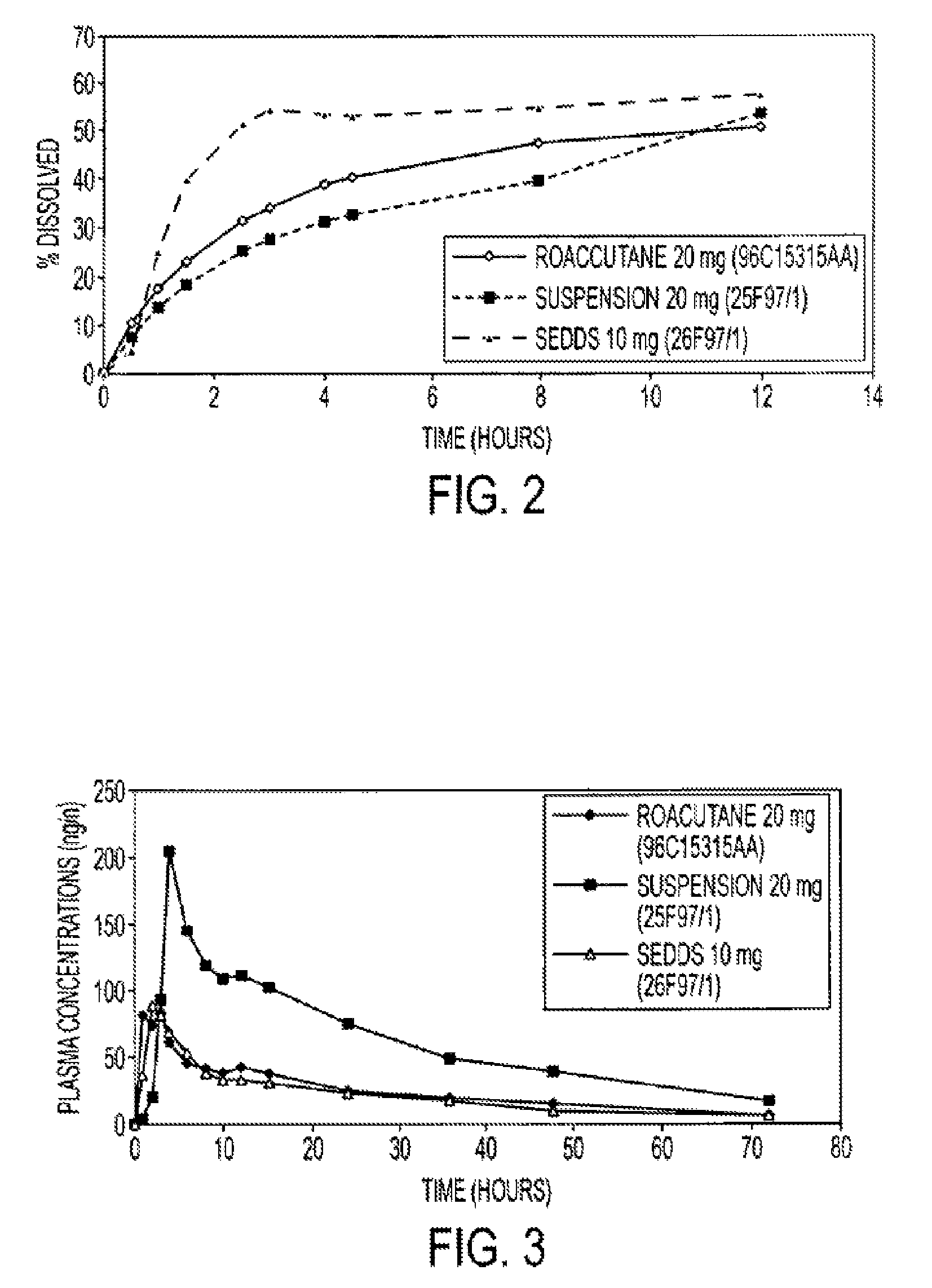
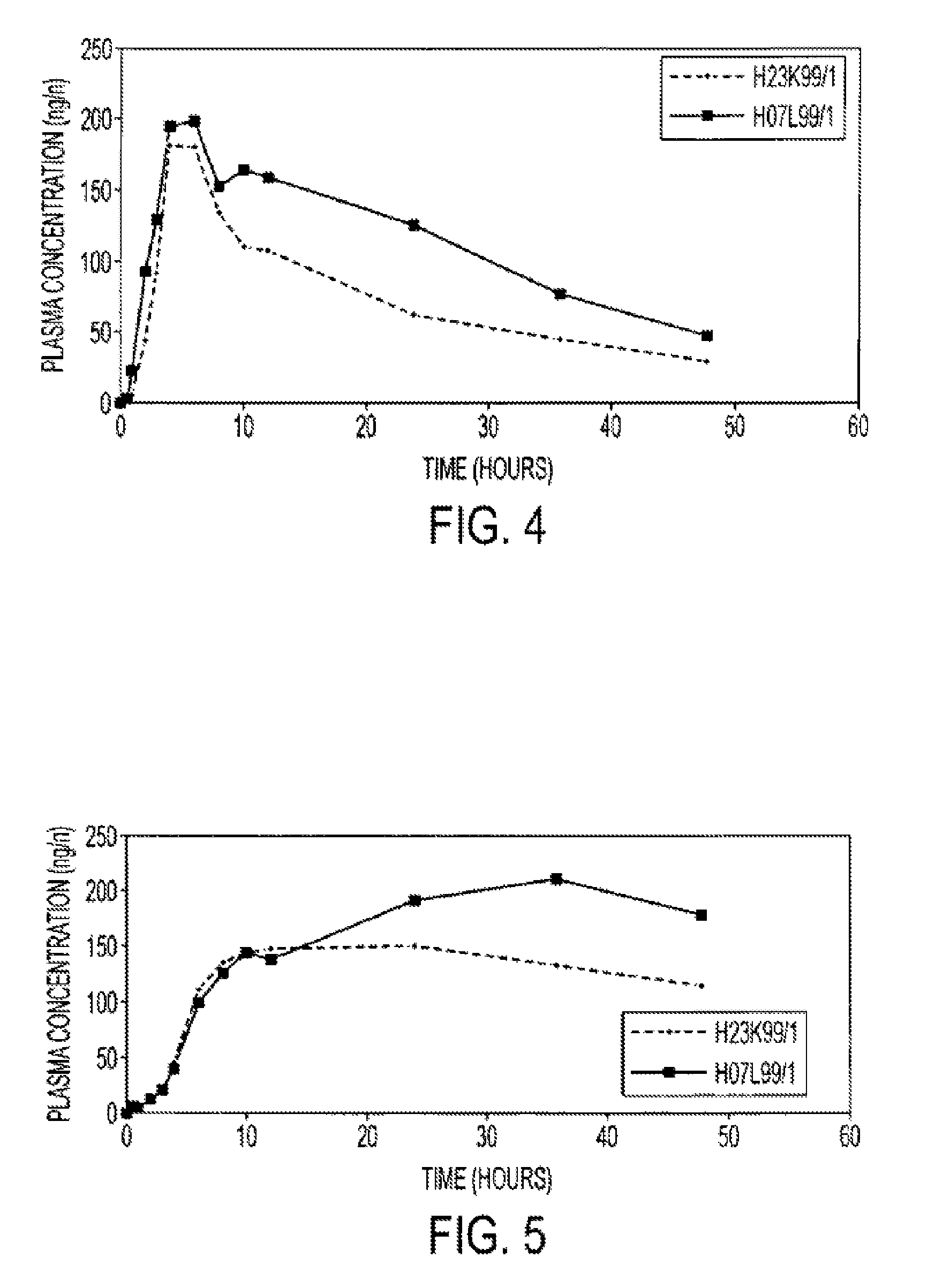
An oral pharmaceutical composition of isotretinoin containing at least two lipidic excipients, one of them being hydrophilic (i.e. having an HLB value superior or equal to 10), the other being an oily vehicle
Owner:GALEPHAR PHARMA RES
Liquid dosage forms of isotretinoin
Owner:SUN PHARMA INDS
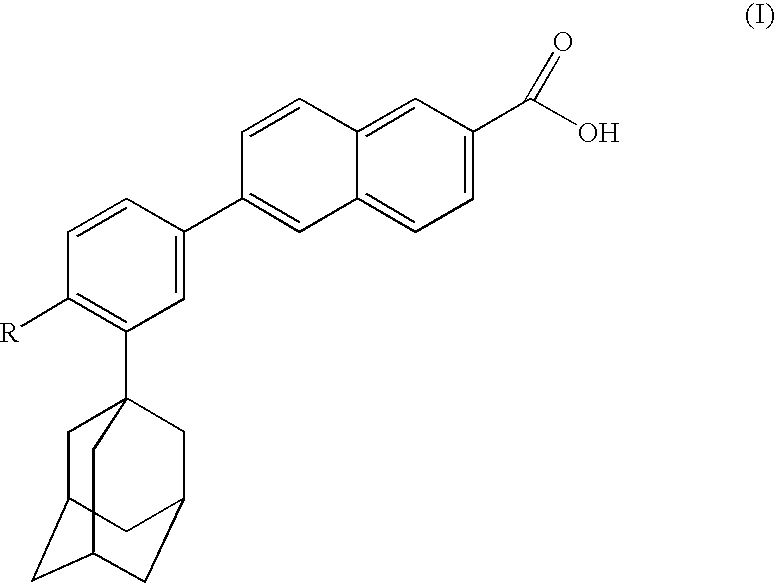
Dermatological compositions comprising at least one retinoid compound, an Anti-irritant compound and benzoyl peroxide
InactiveUS20100160439A1Improve toleranceOvercome problemsCosmetic preparationsBiocideRetinoidIsotretinoin
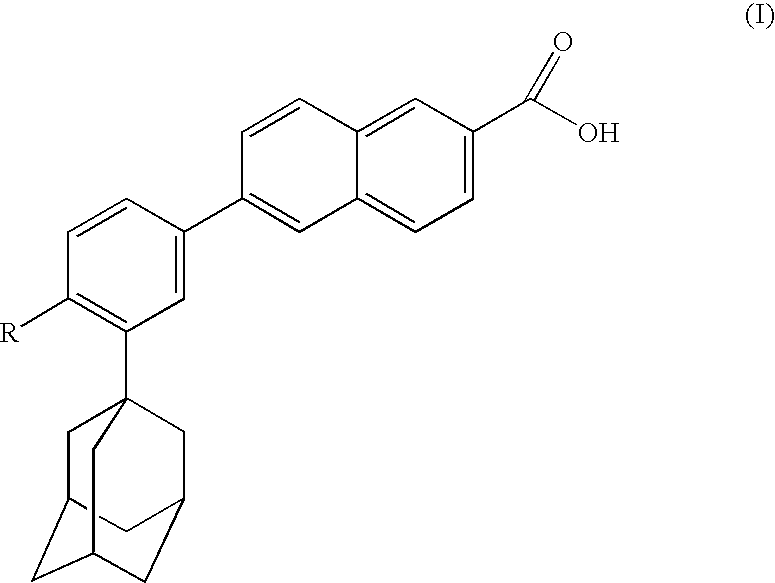
Dermatological compositions contain, formulated into a physiologically acceptable medium, at least one retinoid compound selected from among all-trans retinoic acid, isotretinoin, motretinide, and naphthoic acid compounds of formula (I), and salts and esters thereof:wherein R is a hydrogen atom, a hydroxyl radical, a branched or unbranched alkyl radical having from 1 to 4 carbon atoms, an alkoxy radical having from 1 to 10 carbon atoms, or a cycloaliphatic radical which is substituted or unsubstituted, and benzoyl peroxide, and also at least one anti-irritant compound selected from among 18β-glycyrrhetinic acid, and its salts and derivatives thereof.
Owner:GALDERMA RES & DEV SNC
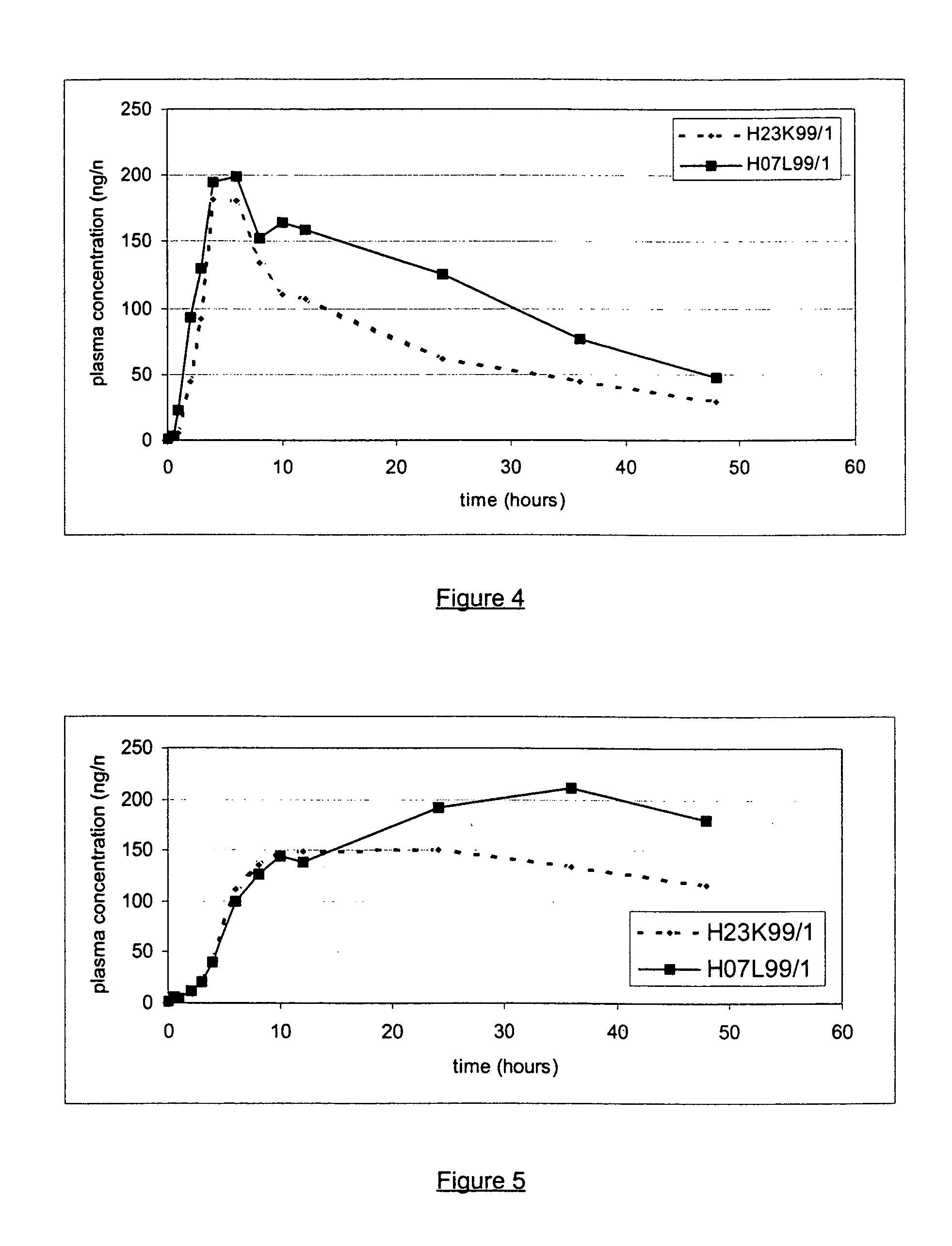
Pharmaceutical semi-sold composition of isotretinoin
InactiveUS20080171084A1Improve oral bioavailabilityHigh dissolution rateCosmetic preparationsBiocideIsotretinoinExcipient
An oral pharmaceutical composition of isotretinoin containing at least two lipidic excipients, one of them being hydrophilic (i.e. having an HLB value superior or equal to 10), the other being an oily vehicle
Owner:GALEPHAR PHARMA RES
Pharmaceutical semi-solid composition of isotretinoin
InactiveUS20140107203A1Improve solubilityPromote absorptionBiocideHydroxy compound active ingredientsCholic acidFOOD EFFECT
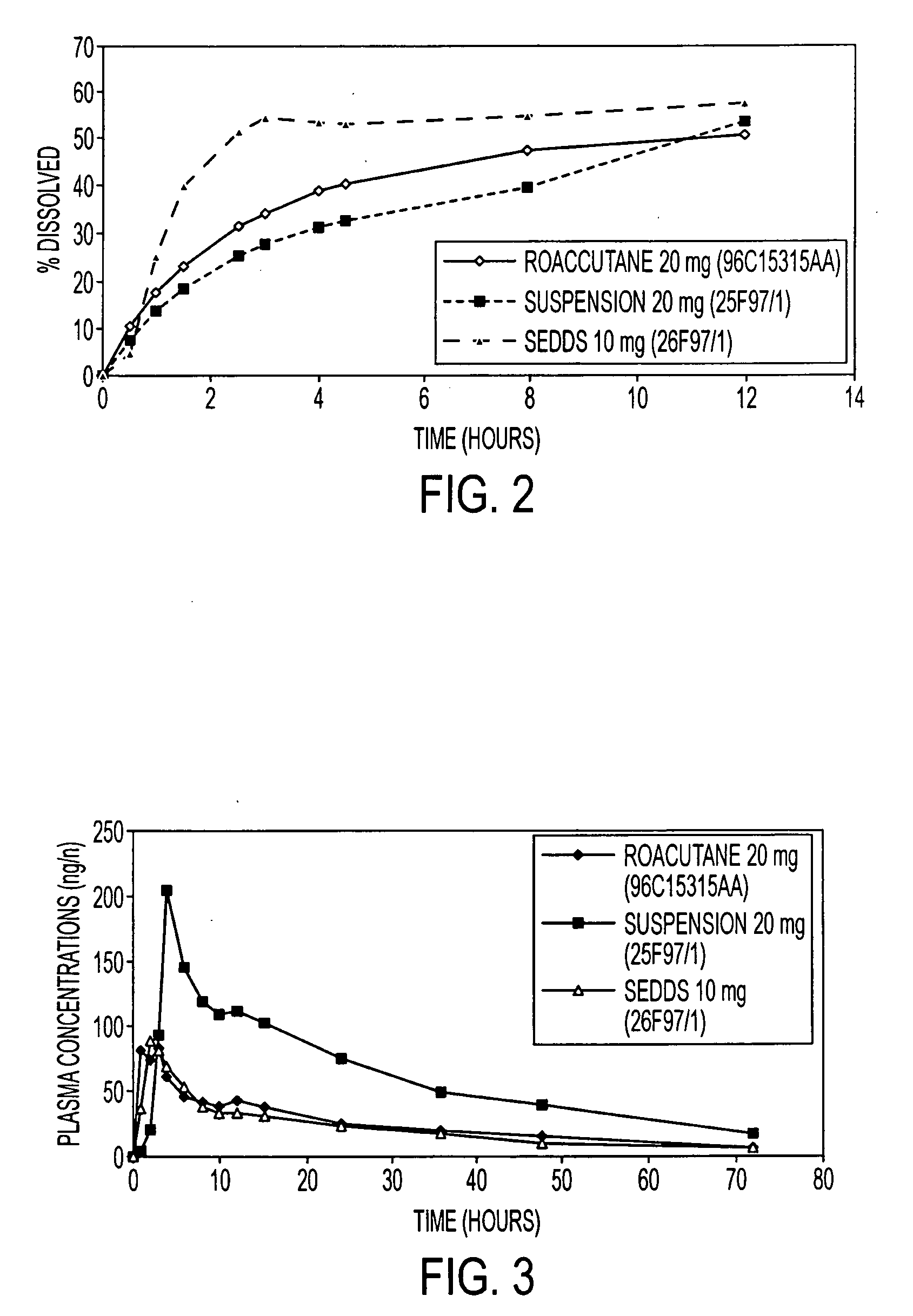
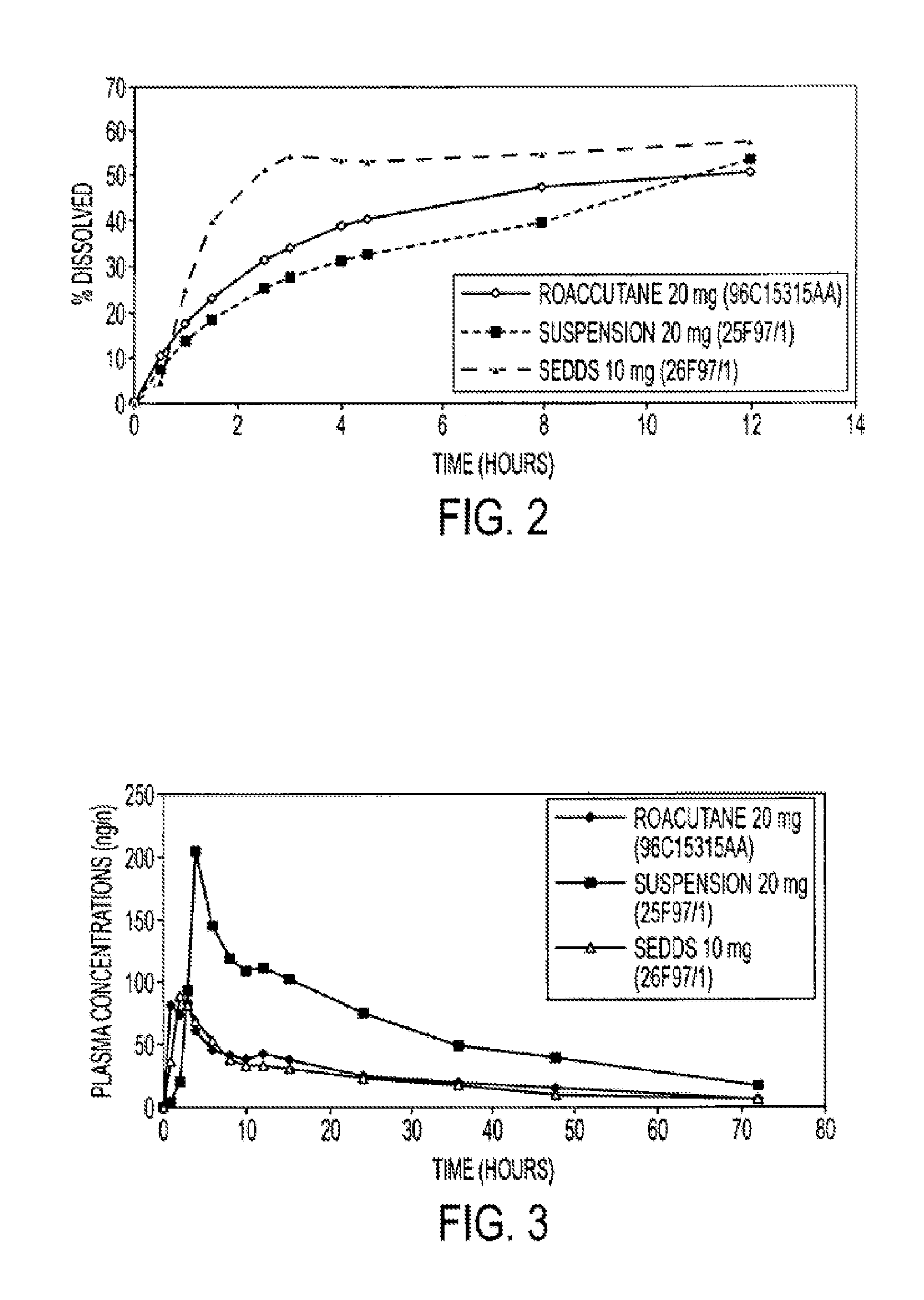
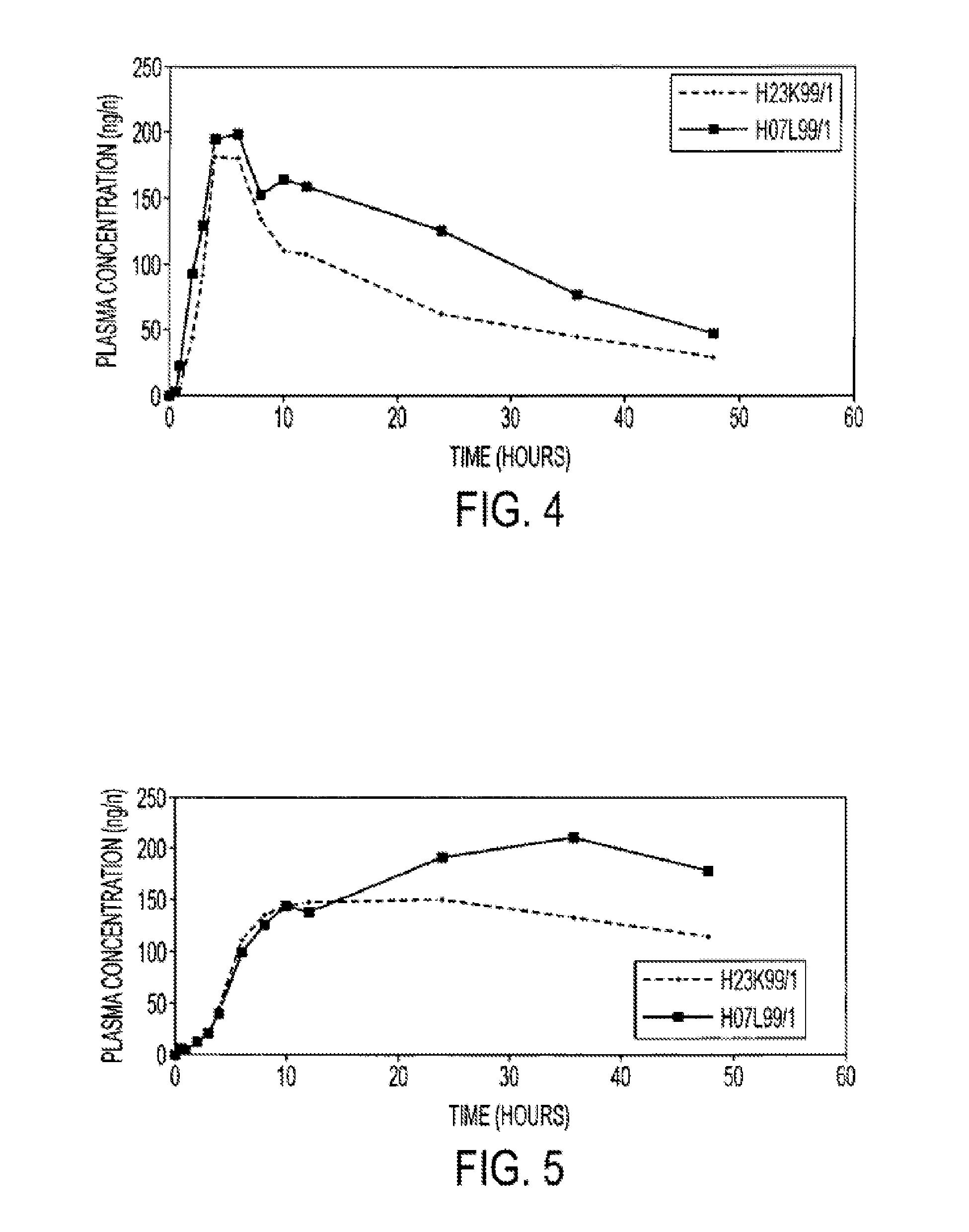
The invention relates to an oral pharmaceutical composition of isotretinoin at least two excipients, one of the excipients being a hydrophilic excipient having an HLB value greater than or equal to 10 and the other excipient being an oily vehicle. The oral pharmaceutical composition is substantially devoid of food effect as characterized by a dissolution profile wherein at least 70% of the oral pharmaceutical composition is dissolved after about four hours in a USP2 dissolution apparatus at a paddle speed of 100 rpm, and a dissolution media composed of 900 mL of pH 7.5 buffer containing 0.11% pancreatin, 4.7% cholic acid, 0.14% sodium dihydroxide phosphate and 0.5% sodium hydroxide at 37° C.
Owner:GALEPHAR PHARMA RES
Whelk treating medicine composition and its preparation method
InactiveCN1557326AEasy to useQuick resultsOrganic active ingredientsAerosol deliveryDexamethasoneSide effect
The acne treating medicine composition consists of Ethinylestradiol, compound sulfamethoxazole, promethazine, chlorpheniramine, dexamethasone, isotretinoin, chloromycetin, vitamin E and vanishing cream. The present invention can heal acne radically according to the pathogenesis, has unique acne healing effect, and has the functions of eliminating scar, nourishing skin, beautifying, etc. when used regularly. The present invention has fast effect and no toxic side effect.
Owner:陈开明
Oral contraceptive and acne medication combination and treatment of acne with reduced side effects
InactiveUS20070254025A1Prevented from conceivingControlling the riskBiocideHydroxy compound active ingredientsSide effectTreatment acne
The present invention provides pharmaceutical compositions for the treatment of acne comprising co-administering a therapeutically effective amount of isotretinoin and a contraceptive in a contraceptively effective dosage wherein the contraceptive does not contain 100 wt % progesterone. Methods of treating acne and unit dosage delivery systems are also provided.
Owner:CRONK PETER
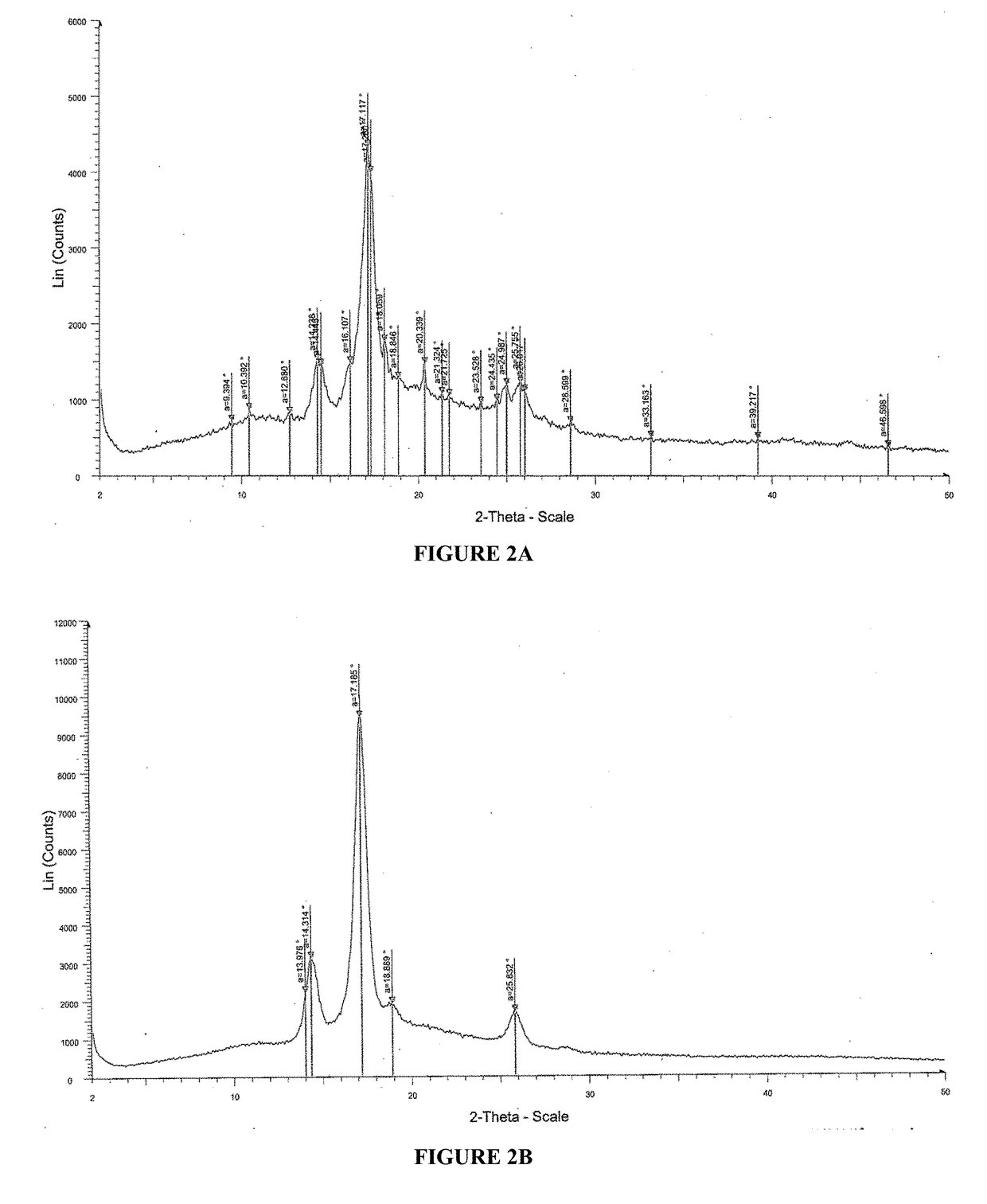
Novel crystal form of isotretinoin as well as preparation method and application thereof
InactiveCN104447459AImprove stabilityHave storageOrganic chemistryHydroxy compound active ingredientsAlcoholPhysical chemistry
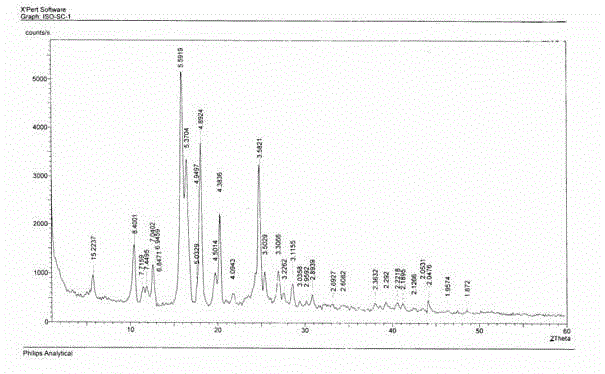
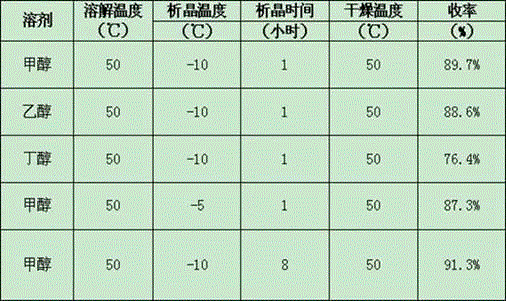
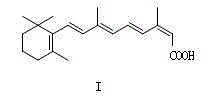
The invention relates to the field of medicinal chemistry, in particular to a crystal form preparation process and application of isotretinoin. A crystal form A takes positions 2theta of diffraction peaks as spectrogram feature parameters, wherein the positions 2theta sequentially comprise 5.8+ / -0.2, 10.52+ / -0.2, 11.46+ / -0.2, 11.87+ / -0.2, 12.56+ / -0.2, 15.83+ / -0.2, 16.49+ / -0.2, 18.11+ / -0.2, 19.70+ / -0.2, 20.24+ / -0.2, 21.68+ / -0.2, 24.83+ / -0.2, 25.40+ / -0.2, 26.94+ / -0.2, 28.62+ / -0.2, and 30.87+ / -0.2. A specific preparation method comprises the steps that an isotretinoin raw material is dissolved with organic alcohol to obtain an isotretinoin organic alcohol solution, crystallization is conducted with a temperature differential method, solid-liquid separation is conducted, a solid is dried and then the crystal form A is obtained; the crystal form A is put under light in air and the stability of the crystal form A is obviously better than that of a raw material medicine.
Owner:CHONGQING HUAPONT PHARMA
Test method of isotretinoin soft shell capsules
InactiveCN101063675ARaise quality standardsHigh practical valueComponent separationColor/spectral properties measurementsPreservativeIsotretinoin
This invention provides one different dimensional A acid capsule solvent degree and its anti-oxidant and antiseptic identification method, which improves the capsule quality standard helpful for monitor product initial period and effective storage period with large application value.
Owner:SHANGHAI SINE WANXIANG PHARMA
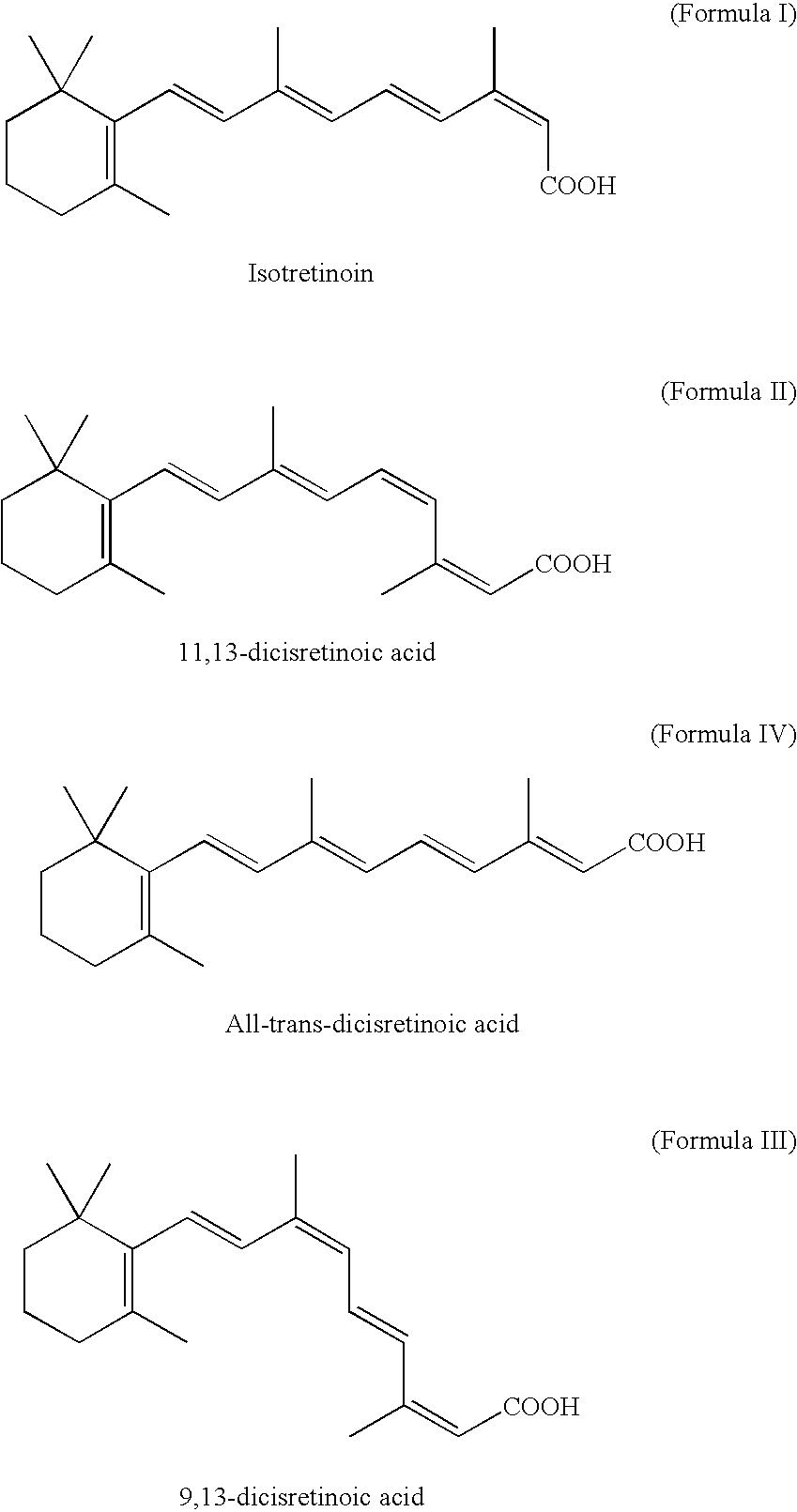
Method for preparing 13-cis isotretinoin
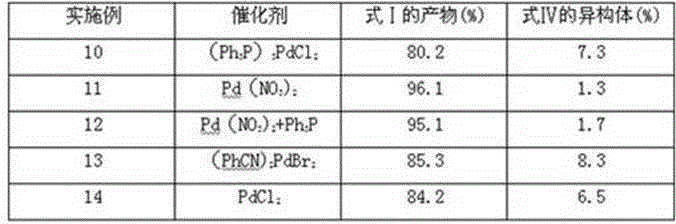
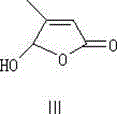
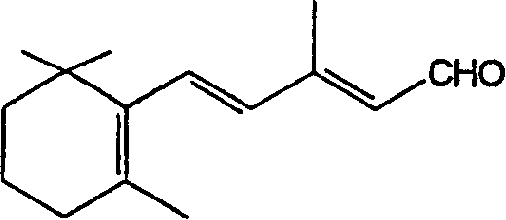
The invention discloses a method for preparing 13-cis isotretinoin. The method comprises the following steps: directly adding an acid to a solution containing a compound represented by formula I and a compound represented by formula IV and generated through a WITTIG reaction to adjust the pH value to 5-10, adding a palladium compound or a rhodium compound, and carrying out an isomerization reaction to obtain a product with the configuration represented by formula I. The 13-cis isotretinoin preparation is completed in a one step mode through a series of reactions comprising the WITTIG reaction and the isomerization reaction without hydrolysis, extraction or distillation after the WITTIG reaction, so the method has the advantages of step simplification, yield increase, total yield reaching 90%, low cost, and suitableness for industrial production.
Owner:CHONGQING HUABANGSHENGKAI PHARM
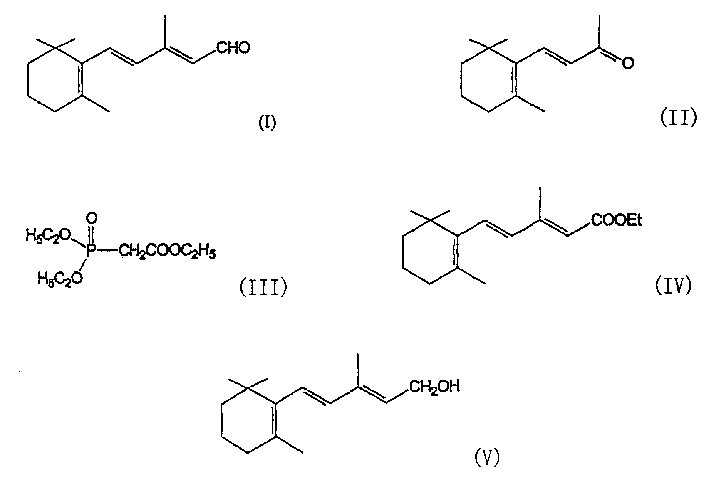
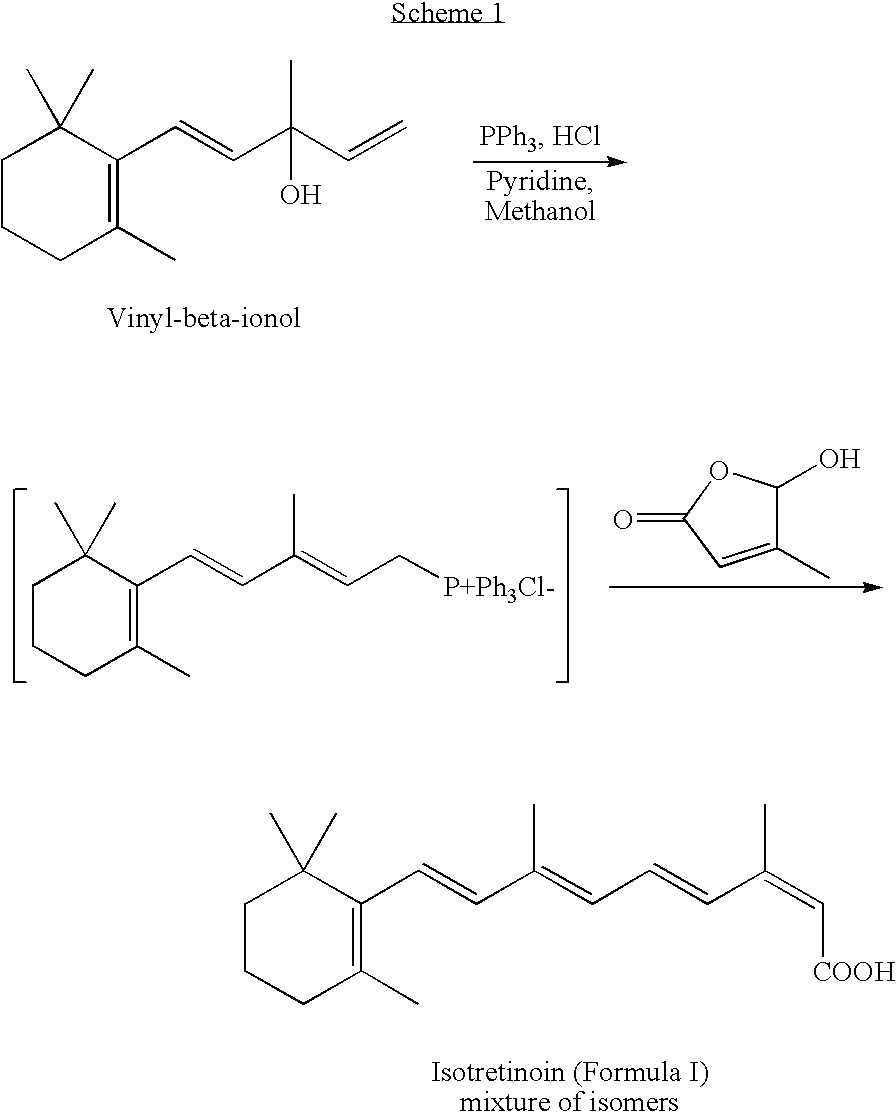
Process for the preparation of beta-ionylideneacetaldehyde
InactiveCN1612854AOrganic compound preparationOrganic chemistry methodsTreatment acneStructural formula
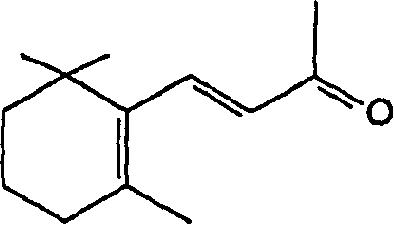
The present invention relates to an industrially advantageous method for producing β-Pyroxylin acetaldehyde of structural formula I, which is a key intermediate for synthesizing vitamin A and related compounds such as tretinoin and isotretinoin. These compounds have a wide range of biological activities. For example, isotretinoin can inhibit the function and keratinization of sebaceous glands and is used to treat skin diseases such as acne. Isotretinoin also has anticancer activity.
Owner:RANBAXY LAB LTD
Pharmaceutical semi-solid composition of isotretinoin
InactiveUS9078925B2Improve oral bioavailabilityLow absolute bioavailabilityHydroxy compound active ingredientsCapsule deliveryCholic acidFOOD EFFECT
The invention relates to an oral pharmaceutical composition of isotretinoin at least two excipients, one of the excipients being a hydrophilic excipient having an HLB value greater than or equal to 10 and the other excipient being an oily vehicle. The oral pharmaceutical composition is substantially devoid of food effect as characterized by a dissolution profile wherein at least 70% of the oral pharmaceutical composition is dissolved after about four hours in a USP2 dissolution apparatus at a paddle speed of 100 rpm, and a dissolution media composed of 900 mL of pH 7.5 buffer containing 0.11% pancreatin, 4.7% cholic acid, 0.14% sodium dihydroxide phosphate and 0.5% sodium hydroxide at 37° C.
Owner:GALEPHAR PHARMA RES
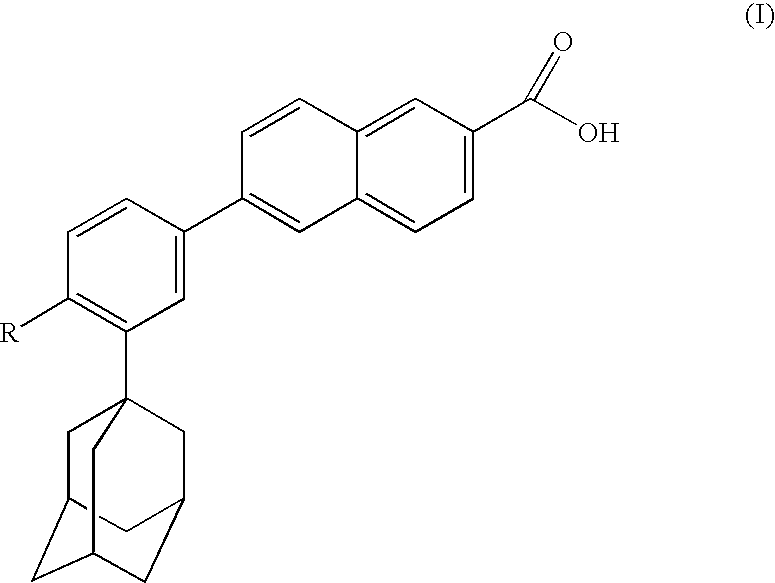
Dermatological compositions comprising at least one retinoid compound, an Anti-irritant compound and benzoyl peroxide
InactiveUS20100183741A1Improve toleranceOvercome problemsCosmetic preparationsBiocideRetinoidHydrogen atom
Dermatological compositions contain, formulated into a physiologically acceptable medium, at least one retinoid compound selected from among all-trans retinoic acid, isotretinoin, motretinide, and naphthoic acid compounds of formula (I), and salts and esters thereof:wherein R is a hydrogen atom, a hydroxyl radical, a branched or unbranched alkyl radical having from 1 to 4 carbon atoms, an alkoxy radical having from 1 to 10 carbon atoms or a cycloaliphatic radical which is substituted or unsubstituted, and at least one anti-irritant compound and benzoyl peroxide.
Owner:GALDERMA RES & DEV SNC
Isotretinoin amido derivative, preparation method thereof and applications thereof
ActiveCN103319365BIncrease medication optionsOvercome the technical difficulty of greatly reducing the acylation reaction activityOrganic active ingredientsOrganic compound preparationBenzoic acidDisease
The invention discloses an isotretinoin amido alkyl benzoate derivative, a preparation method thereof and applications thereof. The derivative has stronger inhibition and differentiation regulating effects than isotretinoin on psoriasis, acne and epithelial cell tumors including, but not limited to skin squamous epithelial cell carcinoma, stomach cancer, lung cancer, and cervical cancer; the derivative has less influence on normal tissue cells, has a certain targeting effect on inhibiting proliferating cells, and has very less side effects than isotretinoin; and the derivative has wide promising prophylaxis and treatment applications such as cornification abnormality diseases and cell abnormal proliferation including tumors, psoriasis, acne, and other cornification abnormality dermatopathy.
Owner:CHUGOKU IGAKU KAGAKUIN HIFUBIYOU KENKYUSHO
Process for preparation of highly pure isotretinoin
The present invention relates to a process for preparation of isotretinoin and more specifically, to a purification process for obtaining highly pure isotretinoin that is useful as a keratolytic agent, particularly useful for the treatment of acne. The process involves treating isotretinoin containing metal contamination and / or other impurities with a base in a suitable solvent to form a solution of isotretinoin, followed by adsorption, precipiation, and filtration or centrifugation
Owner:IPCA LAB LTD
Isotretinoin amido derivative, preparation method thereof and applications thereof
ActiveCN103319365AEnhanced inhibitory effectEasy to adjustOrganic active ingredientsOrganic compound preparationDiseaseSide effect
The invention discloses an isotretinoin amido alkyl benzoate derivative, a preparation method thereof and applications thereof. The derivative has stronger inhibition and differentiation regulating effects than isotretinoin on psoriasis, acne and epithelial cell tumors including, but not limited to skin squamous epithelial cell carcinoma, stomach cancer, lung cancer, and cervical cancer; the derivative has less influence on normal tissue cells, has a certain targeting effect on inhibiting proliferating cells, and has very less side effects than isotretinoin; and the derivative has wide promising prophylaxis and treatment applications such as cornification abnormality diseases and cell abnormal proliferation including tumors, psoriasis, acne, and other cornification abnormality dermatopathy.
Owner:CHUGOKU IGAKU KAGAKUIN HIFUBIYOU KENKYUSHO
Drug for retinal degenerative disease associated with photoreceptor degeneration
PendingUS20200330415A1Increase the number ofSymptoms improvedCompounds screening/testingSenses disorderAcitretinPharmacology
An object of the present invention is to provide a medicine that can simply treat and / or prevent a retinal degenerative disease associated with photoreceptor degeneration, including retinitis pigmentosa. The solution is to provide an agent for treating and / or preventing a retinal degenerative disease associated with photoreceptor degeneration, containing a compound having a retinoic acid receptor agonistic activity (for example, tamibarotene, tamibarotene methyl ester, tamibarotene ethyl ester, tazarotene, tazarotenic acid, adapalene, palovarotene, retinol, isotretinoin, alitretinoin, etretinate, acitretin or bexarotene) or a salt thereof.
Owner:DAIICHI SANKYO CO LTD
Isotretinoin oral-mucosal formulations and methods for using same
PendingCN111032017AHydroxy compound active ingredientsInorganic non-active ingredientsDiseaseMucosal disease
Disclosed herein, in part, is a pharmaceutical formulation comprising isotretinoin or a pharmaceutically acceptable salt thereof, and a mucoadhesive polymer. A method of treating a mucosal disease comprising administering a disclosed pharmaceutical formulation to a subject in need thereof is also provided herein.
Owner:SKYLINE BIOSCI LLC
Topical solution of isotretinoin
InactiveUS20160338985A1Hydroxy compound active ingredientsPharmaceutical delivery mechanismIsotretinoinDermatology
The present invention relates to a topical solution comprising a retinoid or its pharmaceutically acceptable salts thereof and process of preparing it.
Owner:SUN PHARMA INDS
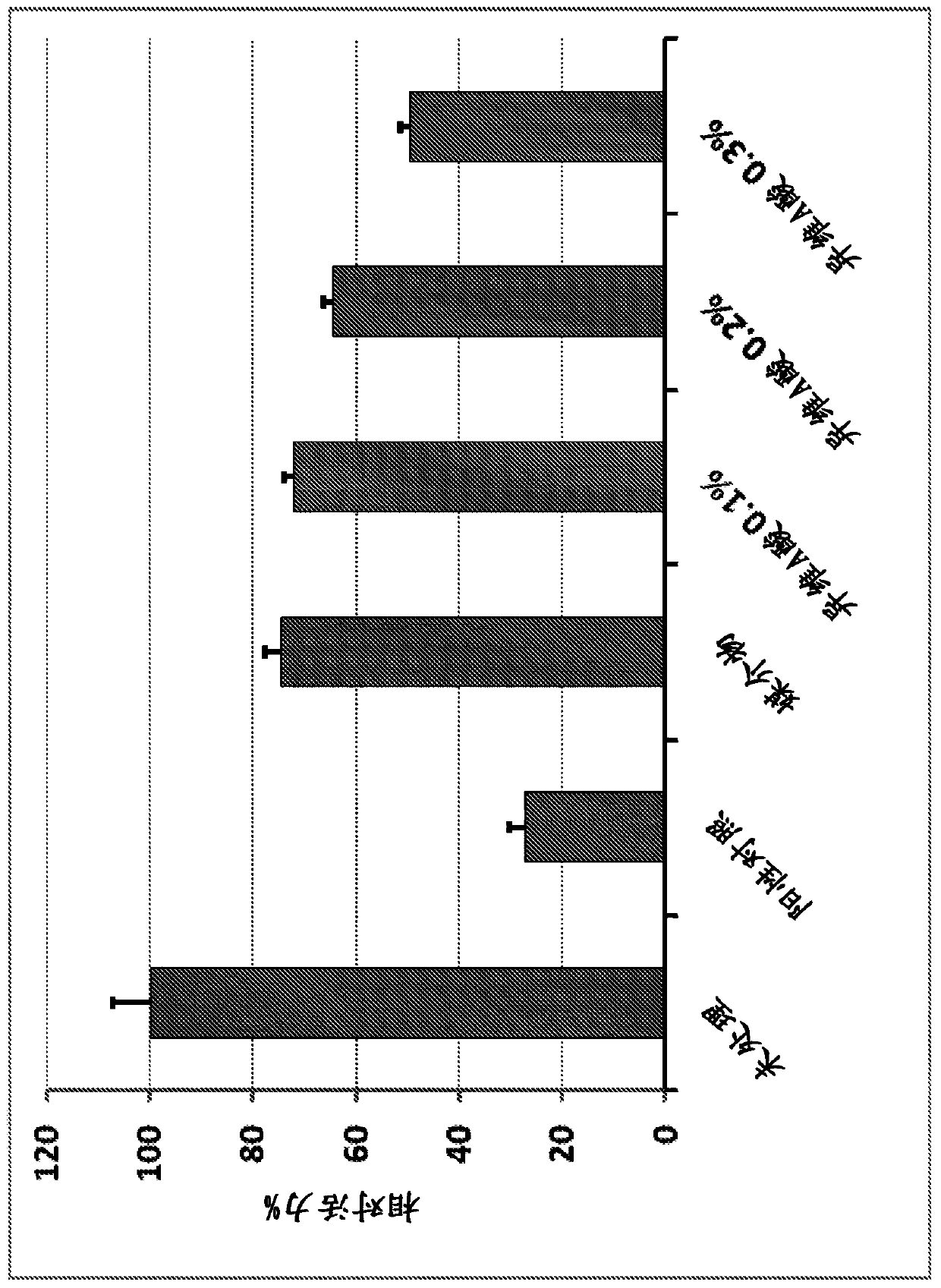
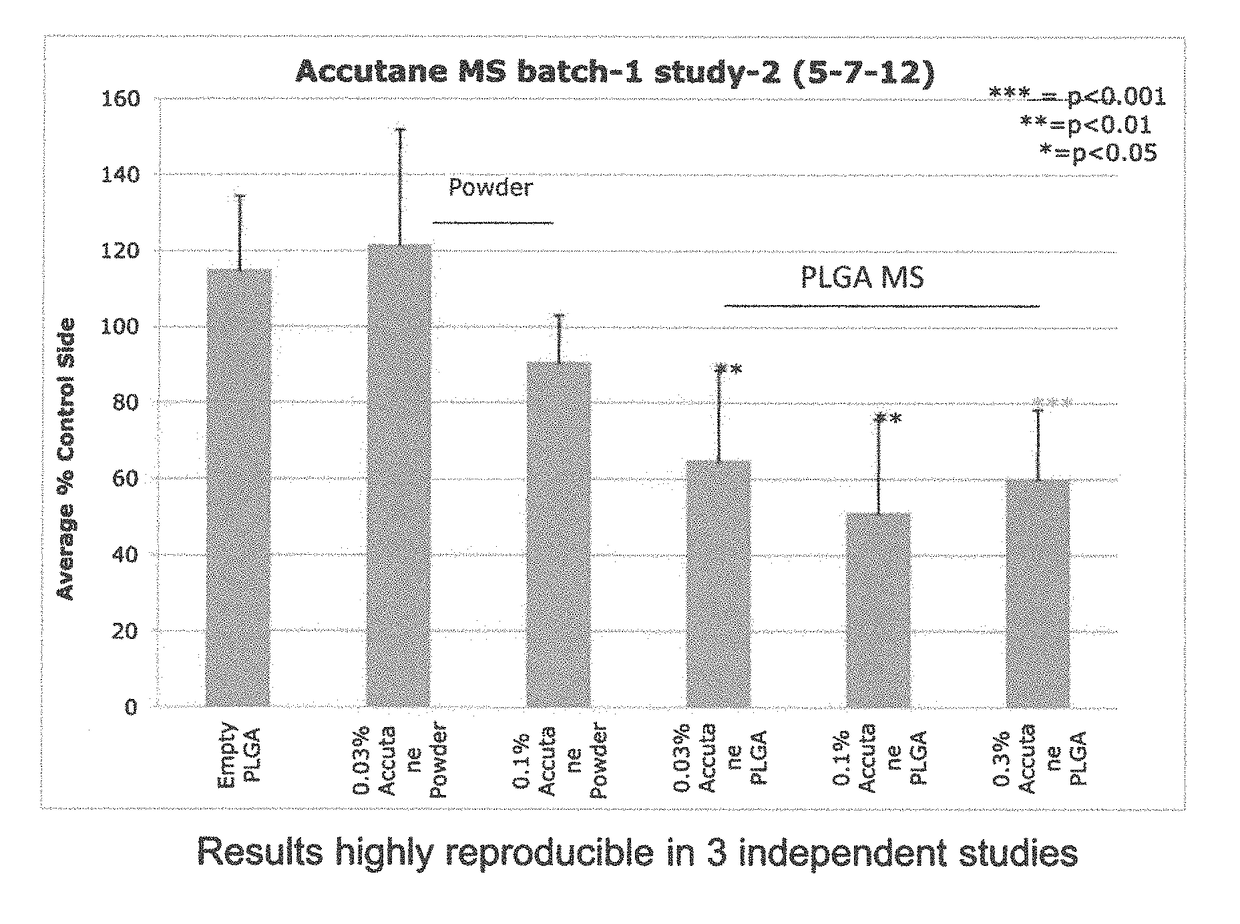
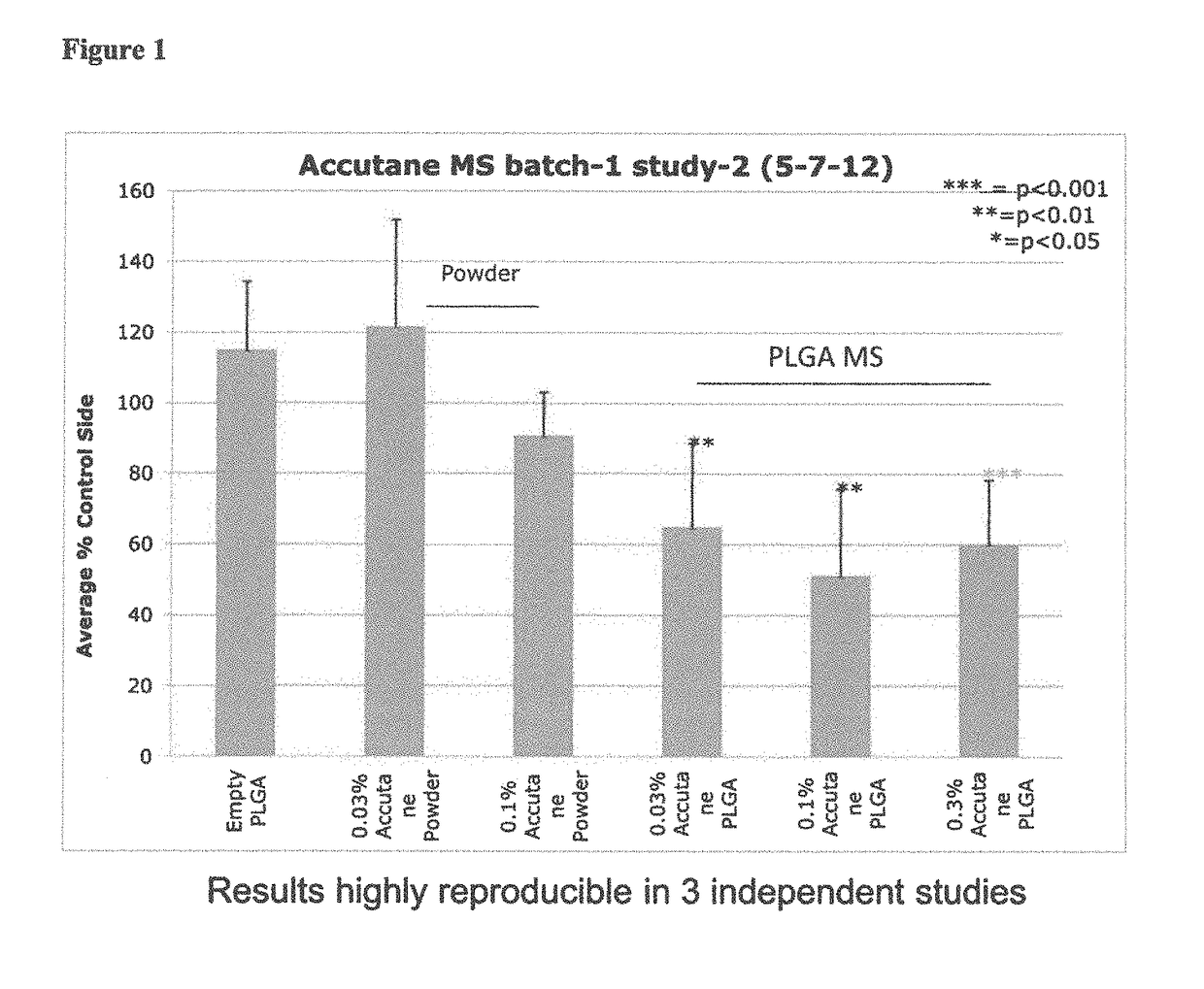
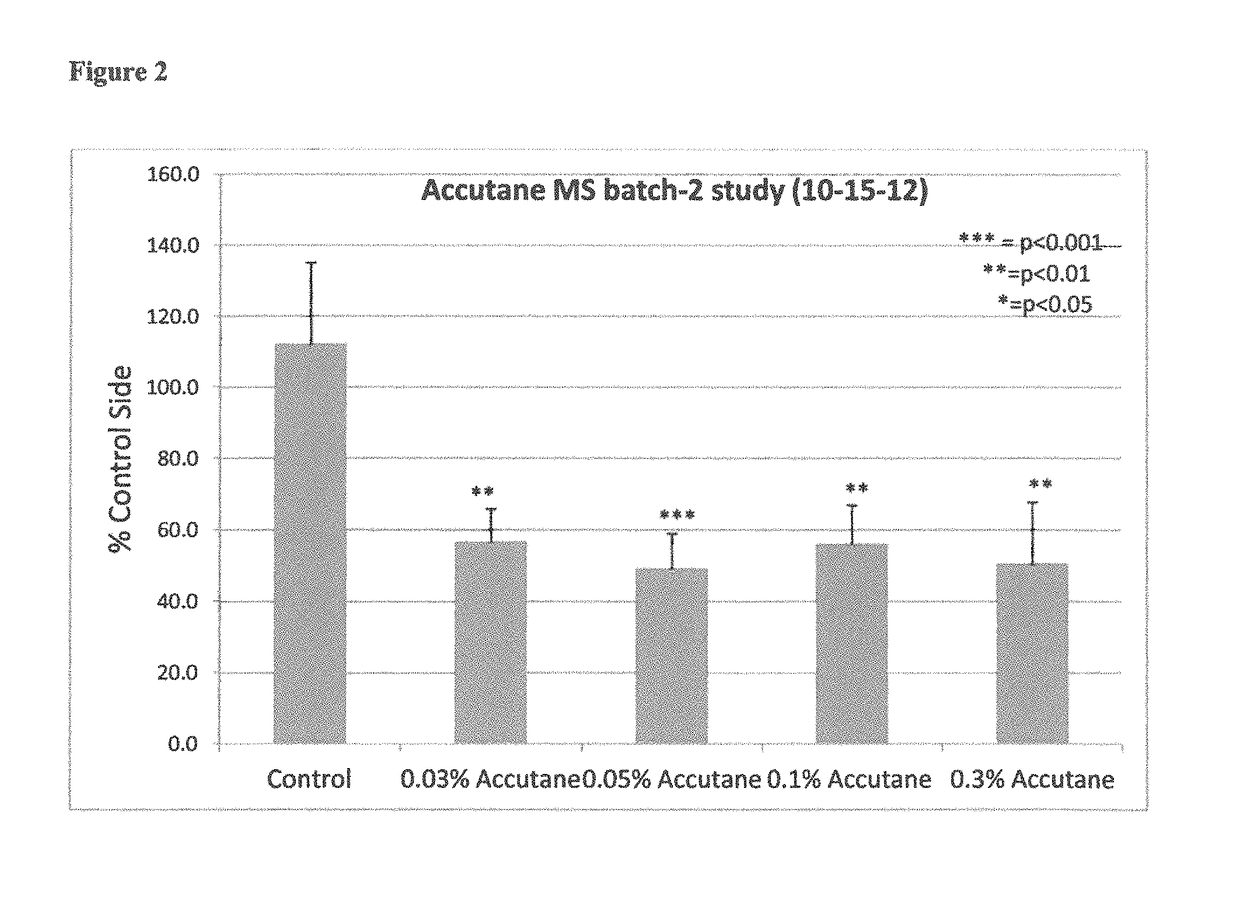
Compositions including encapsulated isotretinoin and methods for use thereof
ActiveUS10123988B2Reduced activityEliminate side effectsHydroxy compound active ingredientsAerosol deliveryMicrosphereIsotretinoin
The present invention provides topical dermal compositions including microsphere encapsulated isotretinoin. Pharmaceutically acceptable salts, esters, or amides of isotretinoin are also contemplated for use in the practice of the invention. The compositions of the invention are useful for treating a variety of conditions associated with excess sebum production, such as, for example, acne.
Owner:ALLERGAN INC
Nutritional Supplement Without Vitamin A
This invention relates to a dietary supplement that is specifically designed for use in three categories of patient type; patients undergoing treatment with oral or topical forms of retinoids such as Isotretinoin, Tretinoin and Retinoic Acid; patients with medical conditions / treatments that are aggravated by the consumption of Vitamin A; individuals in the prenatal period wishing to avoid the consumption of Vitamin A in supplements.The dietary supplement is not limited by the inclusion of specific vitamins, minerals or other nutrients, but rather the exclusion specifically of Vitamin A and / or its precursors for the purpose outlined above.
Owner:CIAVARELLA APRILLE +2
Process for preparation of isotretinoin
InactiveCN1371362AOrganic compound preparationCarboxylic compound preparationIsotretinoinMedicinal chemistry
The invention relates to a single-step method for preparing the 13-cis isomer of retinoic acid (commonly known as isotretinoin).
Owner:RANBAXY LAB LTD
Isotretinoin C15-triphenylphosphine chloride as well as preparation method and application thereof
ActiveCN111499662AHigh purityHigh yieldAntipyreticGroup 5/15 element organic compoundsDistillationEthyl acetate
The invention provides isotretinoin C15-triphenylphosphine chloride and a preparation method and application thereof. The preparation method comprises the steps of: stirring and mixing vinyl-beta-ionol, triethylamine and methyl alcohol to prepare a feed liquid I; feeding nitrogen to expel oxygen, then under a vacuum condition, preparing a mixed solution of methanol, CP hydrochloric acid, 2, 6-di-tert-butyl-p-cresol and triphenylphosphine, and carrying out condensation reflux and stirring on the mixed solution to obtain a feed liquid II; dropwise adding the feed liquid II into the feed liquid Iat 30-52 DEG C to obtain a mixture; repeatedly adding ethyl acetate into the mixture, and then carrying out reduced pressure distillation to obtain a final product precursor; and carrying out cooling, cold precipitation, centrifugation and drying treatment on the final product precursor to obtain a target product. The isotretinoin C15-triphenylphosphine chloride obtained by the preparation methoddisclosed by the invention is relatively high in purity, relatively high in yield, mild in reaction condition and easy for industrial production.
Owner:SHANGHAI NEW HUALIAN PHARMA
Isotretinoin formulations and uses and methods thereof
ActiveUS10933018B2Enhanced targeted local deliveryLow amountHydroxy compound active ingredientsAntipyreticPharmaceutical drugIsotretinoin
Provided herein are novel isotretinoin formulations that provide an enhanced targeted dermal delivery system for the drug isotretinoin with improved thermodynamic activity using no to a small level of ethanol relative to existing isotretinoin gel products, and methods for treatment of ichthyosis and other skin conditions using the same.
Owner:LEO PHARMA AS
Pharmaceutical compositions for treating acne
ActiveUS20170340594A1Reduced food effectPowder deliveryHydroxy compound active ingredientsIsotretinoinRetinoic acid
The present application relates to pharmaceutical compositions comprising retinoic acid or its derivatives such as isotretinoin and processes for preparing thereof. It also provides methods for treating acne by administering such pharmaceutical composition to a patient in need thereof.
Owner:DR REDDYS LAB LTD
Active ingredient combination of a retinoid and a hormone combination with contraceptive action as medicament for treatment of skin diseases
InactiveUS20140249117A1Effective treatmentRisk minimizationOrganic active ingredientsBiocideRetinoidSeborrhoea
The present invention relates to a medicament, the active ingredient combination of which consists of a retinoid selected from the group consisting of acitretin [9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoic acid], etretinate [ethyl 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoate], isotretinoin [3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid] and tretinoin [3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)nona-2,4,6,8-tetraenoic acid] and of a hormone combination with contraceptive action of an oestrogen component and a gestagen component, and to a dosage form consisting of at least 28 daily units, of which the final 7-3 daily units contain only the retinoid as active ingredient and the other daily units also contain the hormone-containing active ingredient combination, and to the use thereof for treating acne, seborrhoea or psoriasis.
Owner:RICHTER GEDEON NYRT
Ointment for treating verruca plana and preparation method thereof
InactiveCN106215060ALess irritatingPromote healthy growthAerosol deliveryOintment deliveryImmunocompetenceBarrier function
Owner:WANNAN MEDICAL COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com