Isotretinoin oral-mucosal formulations and methods for using same
A technology of tretinoin and mucoadhesion, which is applied in the direction of pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
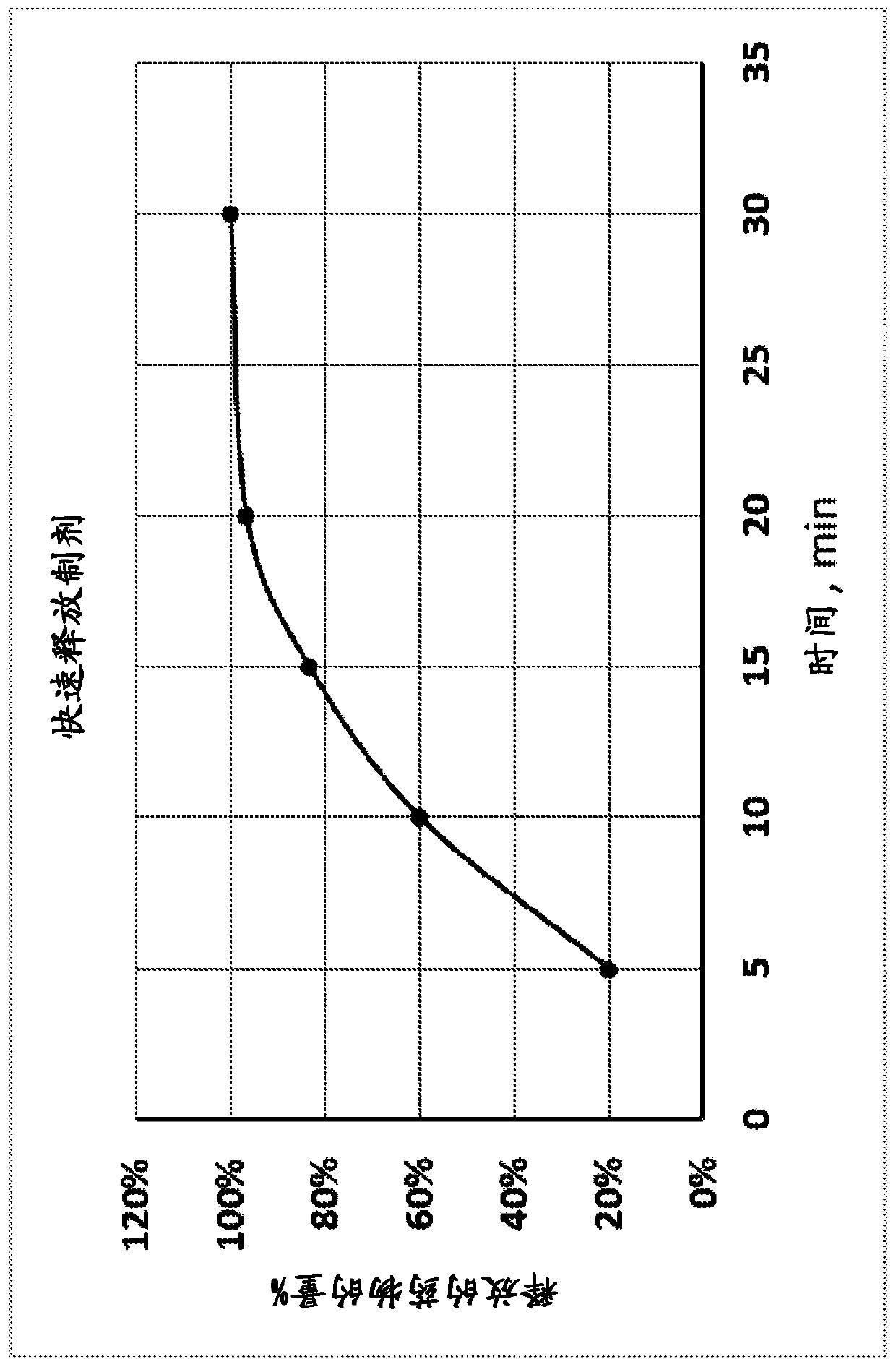
[0124] Example 1. Drug Dissolution Test Protocol
[0125] The aim of this study was to develop a drug dissolution (drug release) method for isotretinoin oral mucoadhesive films. The criterion to be achieved is a dissolution rate greater than 85% at the end of the dissolution.
[0126] The dissolution apparatus used was a Logan DISSO III-7 (USP 3 and 7 dissolution apparatus in one unit) manufactured by Logan Instruments, Somerset, NJ. The program has been set up to run the tests with a USP 3 instrument. Sampling was performed using a LoganDSC-37 System Controller / Logan SYP-8L Syringe Pump and a Logan SCR-160 SamplerCollector.
[0127] Drug assays in dissolution media were determined using an in-house method by an Agilent HPLC / UV system equipped with ChemStation. The medium used for dissolution studies was 1% (w / w) aqueous solution of N,N-dimethyldodecylamine-N-oxide (DDAO), prepared from 30% DDAO solution, supplied by Sigma-Aldrich. Other operating parameters were: one memb...
Embodiment 2
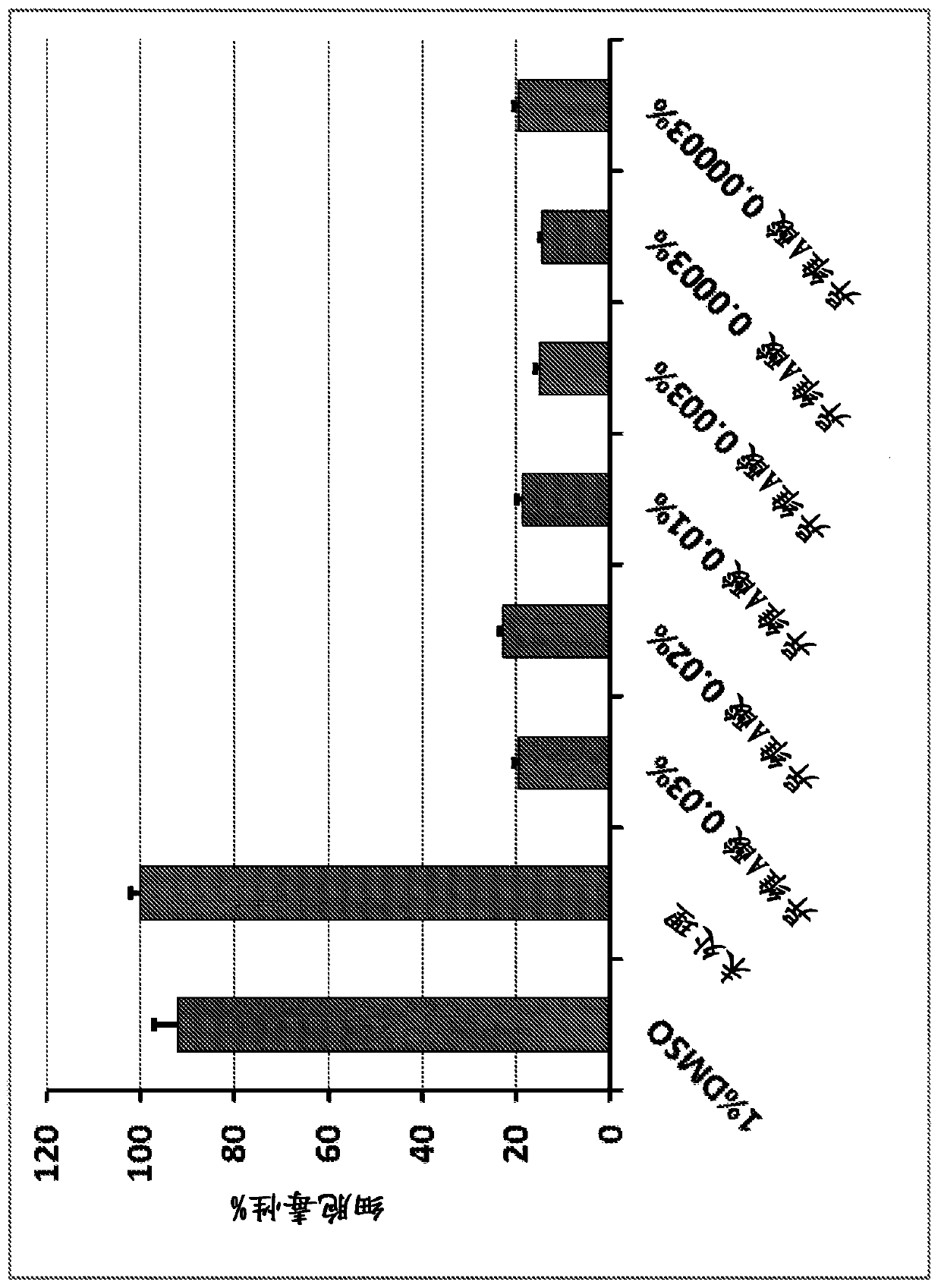
[0129] Example 2. Mucoadhesive formulation A
[0130] Formulation A was formulated for an orally dissolvable and erodible thin flexible film with and without a soft (EVA polymer) backing film. The formulation is designed for slow release of the drug.
[0131] Table 1. 0.2% Oromucosal Patch Formulations
[0132]
[0133] Dissolve or disperse all components in acetone / ethanol / water (v / v / v ratio is 24 / 26 / 42) mixed solvent, and cast directly on the EVA polymer backing film or release liner, and in Dry at 75°C for 15 minutes to produce a dry film. For oral discs with film backing, the cast / dried film was peeled from the release liner to obtain a freestanding film. These films were die cut into 1 inch x 1 inch unit dose discs and tested accordingly.
[0134] In vitro drug release studies were performed using Formulation A and using the drug dissolution protocol described in Example 1. The results of the study indicated that the drug was completely released in about 60 minute...
Embodiment 3
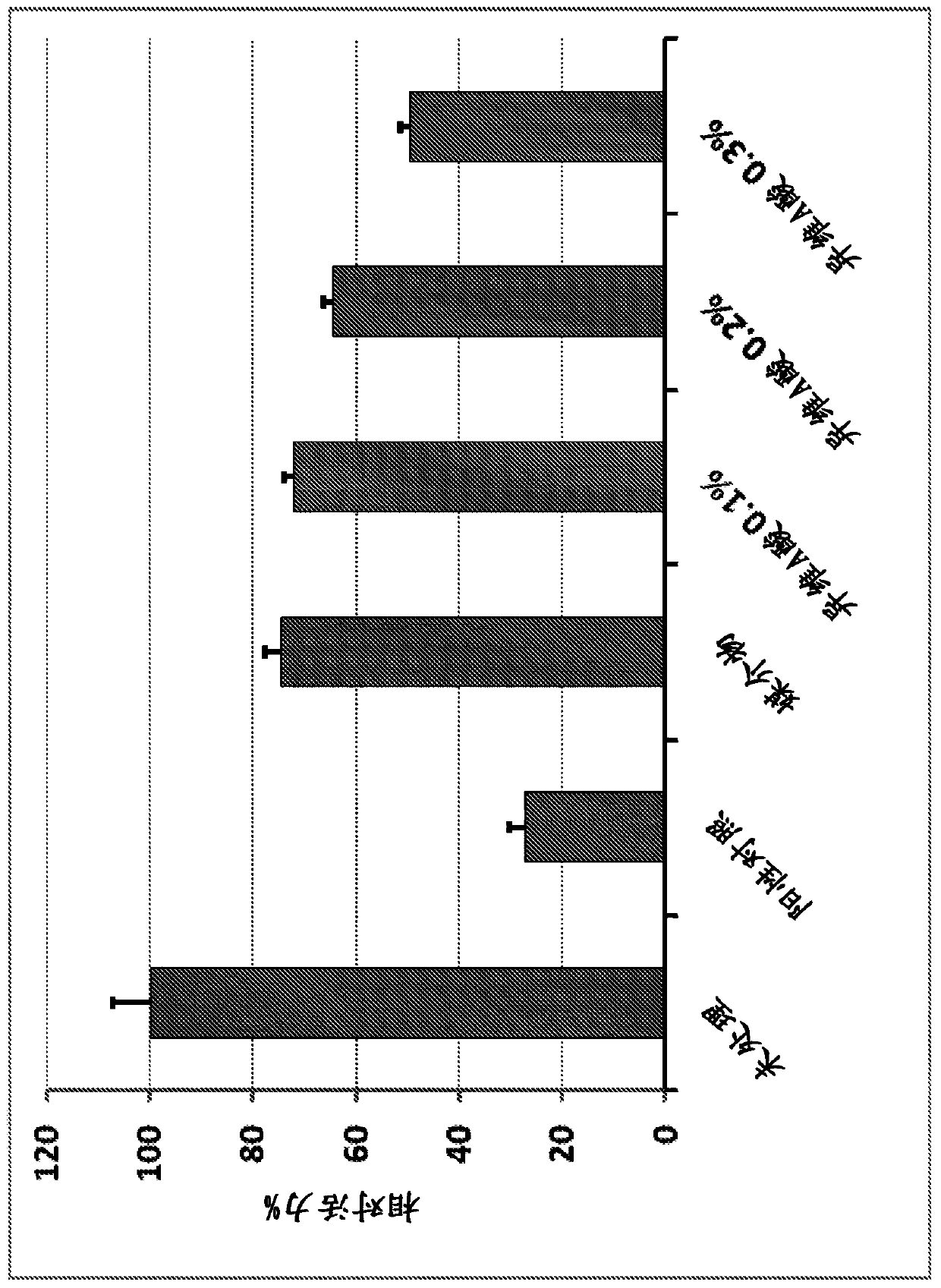
[0137] Example 3. Mucoadhesive formulation B
[0138] Mucoadhesive Formulation B was formulated to shorten the drug release time in the oral cavity and to replace the non-ingestable EVA film with a dissolvable or ingestible backing film. The bioadhesive polymer of Formulation A (ie, Noveon AAl (polycarbophil)) was replaced with a more water-soluble, polyvinylpyrrolidone (PVP)-based polymer. Formulation B comprises two layers: a mucoadhesive layer and an ingestible layer formulated from a polymethacrylate (Eudragit) based polymer (Evonik), which is typically used as a tablet film coat for oral solid dosage forms. Two-layer configurations were prepared by two casting processes.
[0139] Table 2. 0.3% Isotretinoin Formulations for Immediate Release
[0140] Element Wet grams dry part Dry% water 4 ethanol 35 Kollidon 90F 8 8 68.1% Kollidon VA64 2 2 17.0% Isotretinoin 0.04 0.04 0.3% PEG400 1.5 1.5 12.8% Prop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com