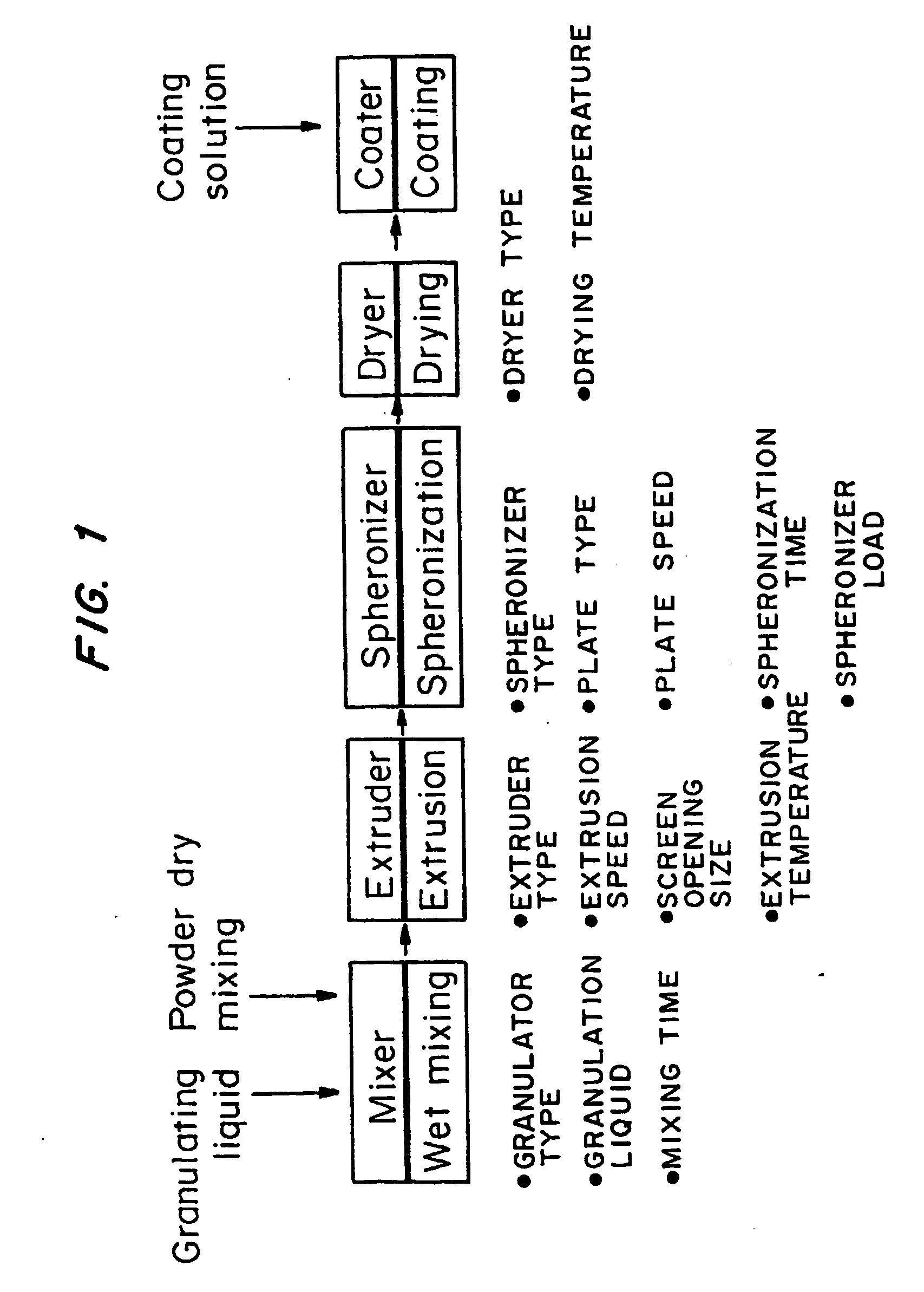
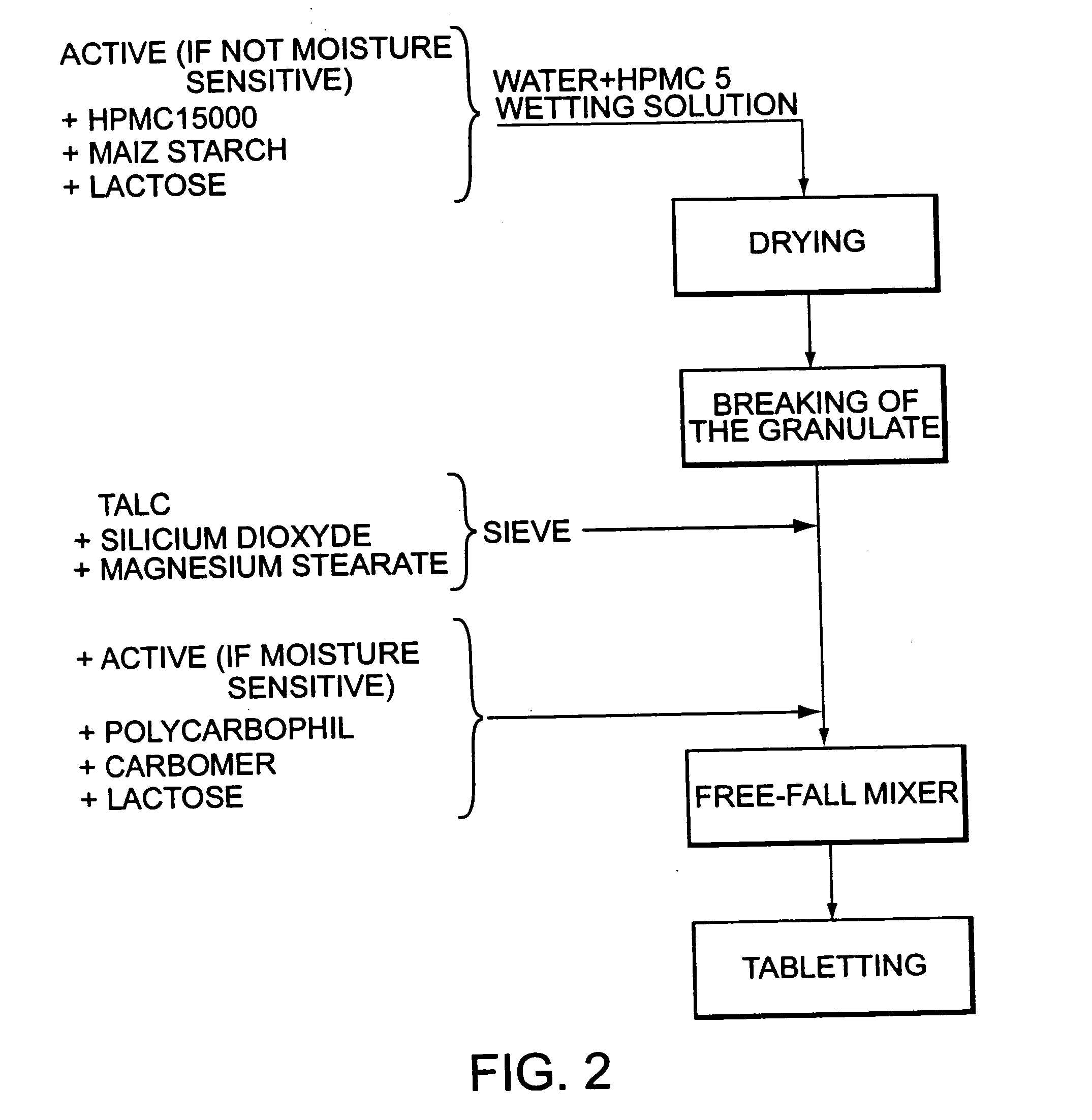
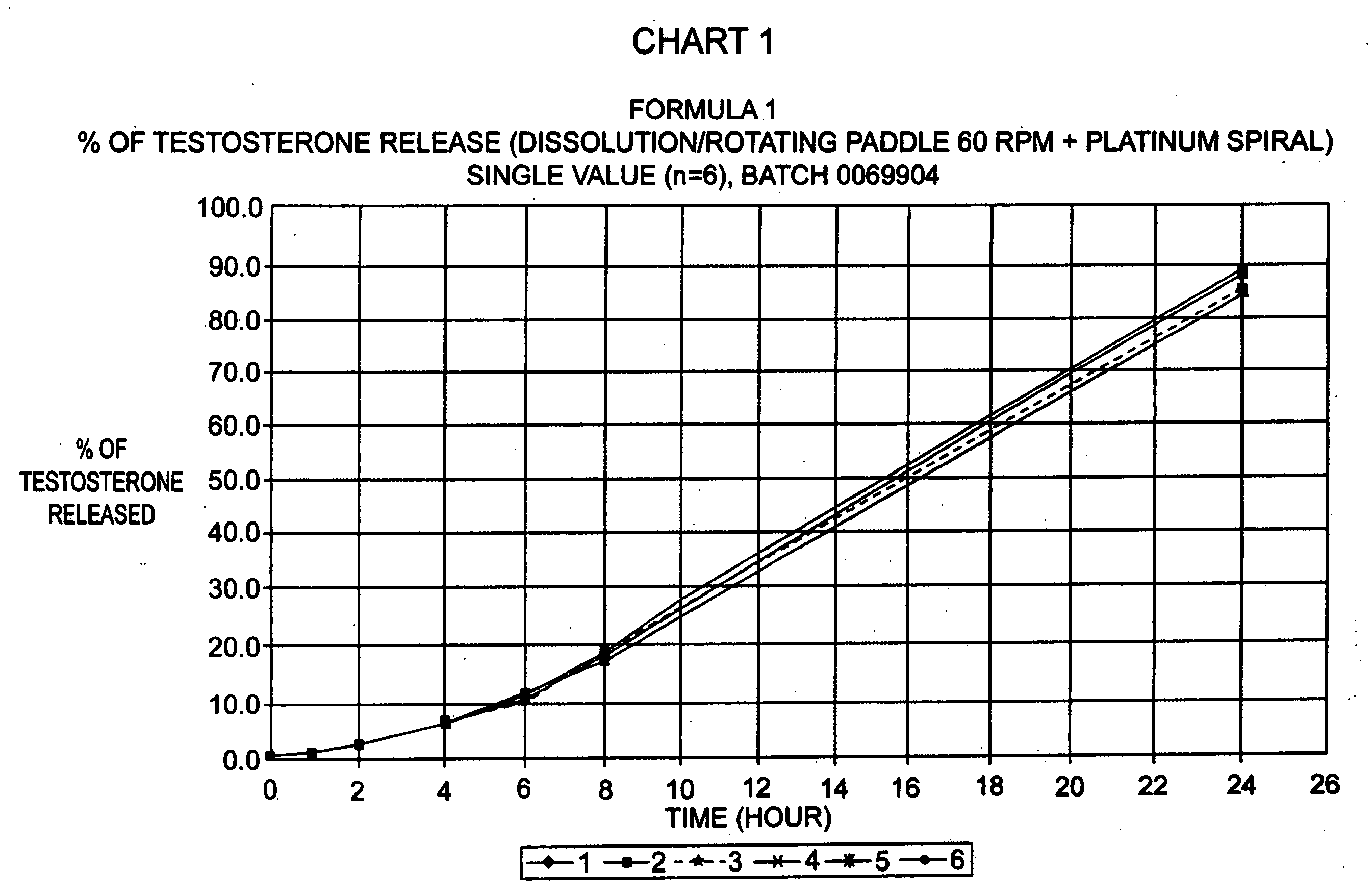
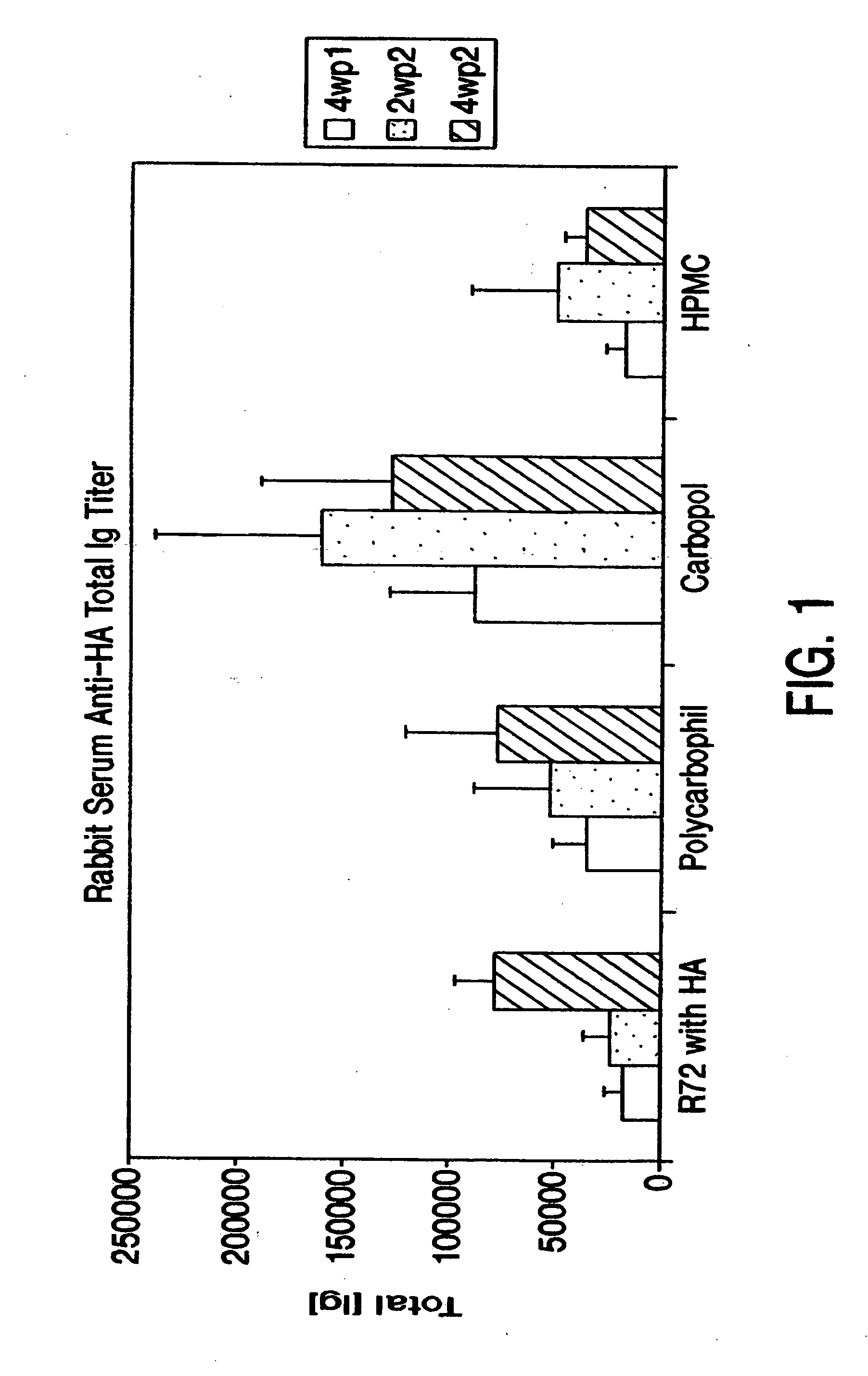
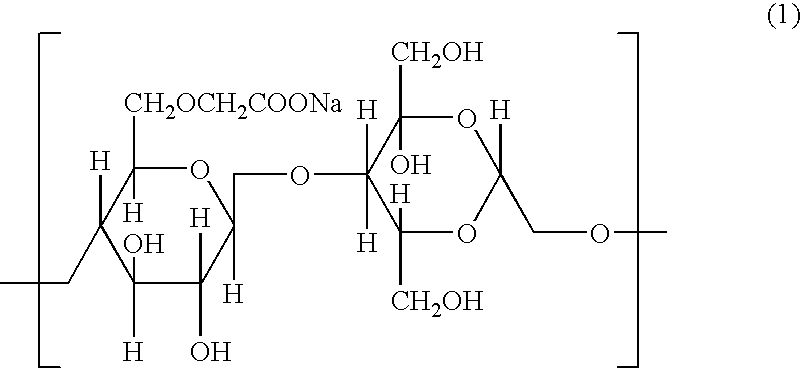
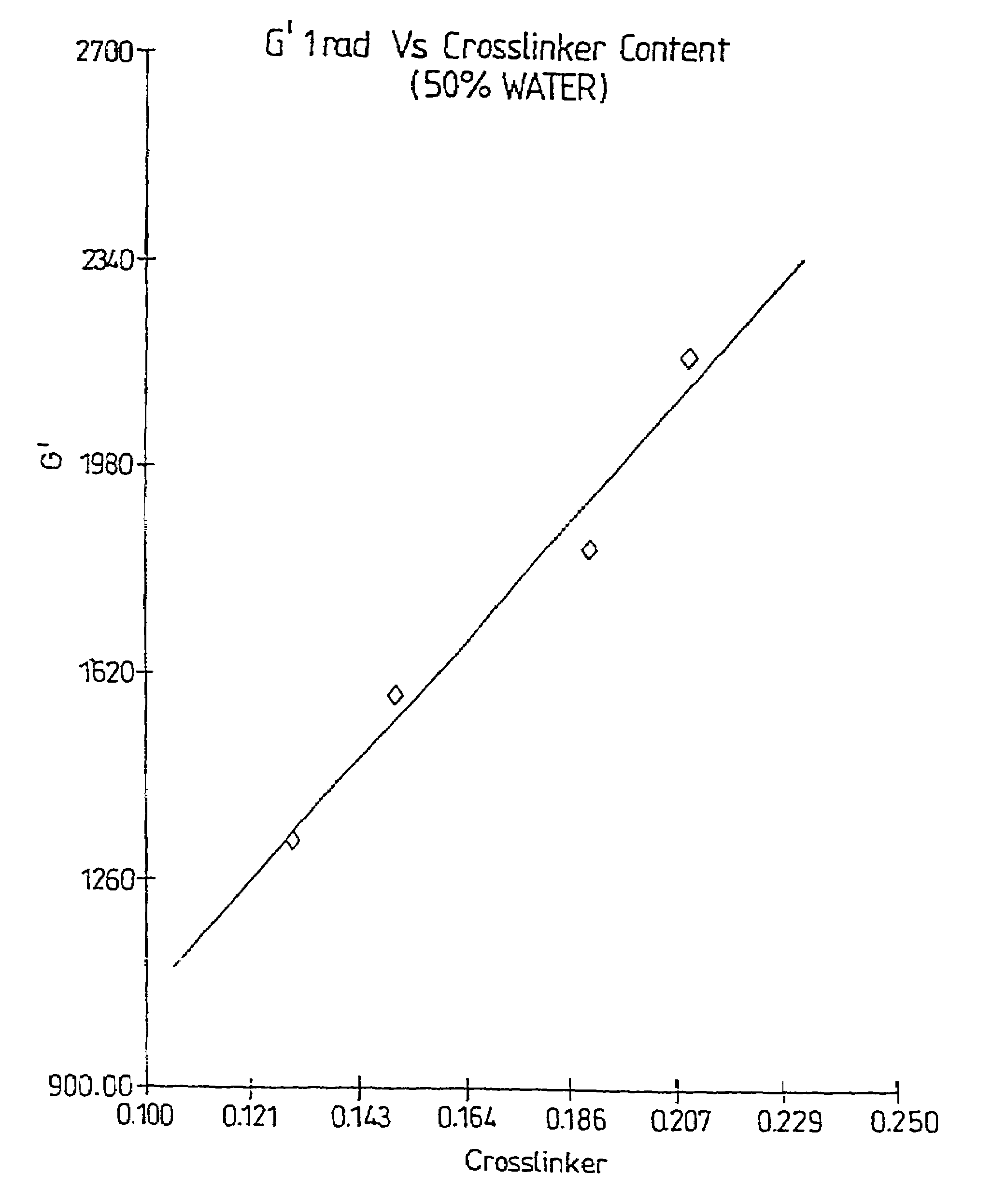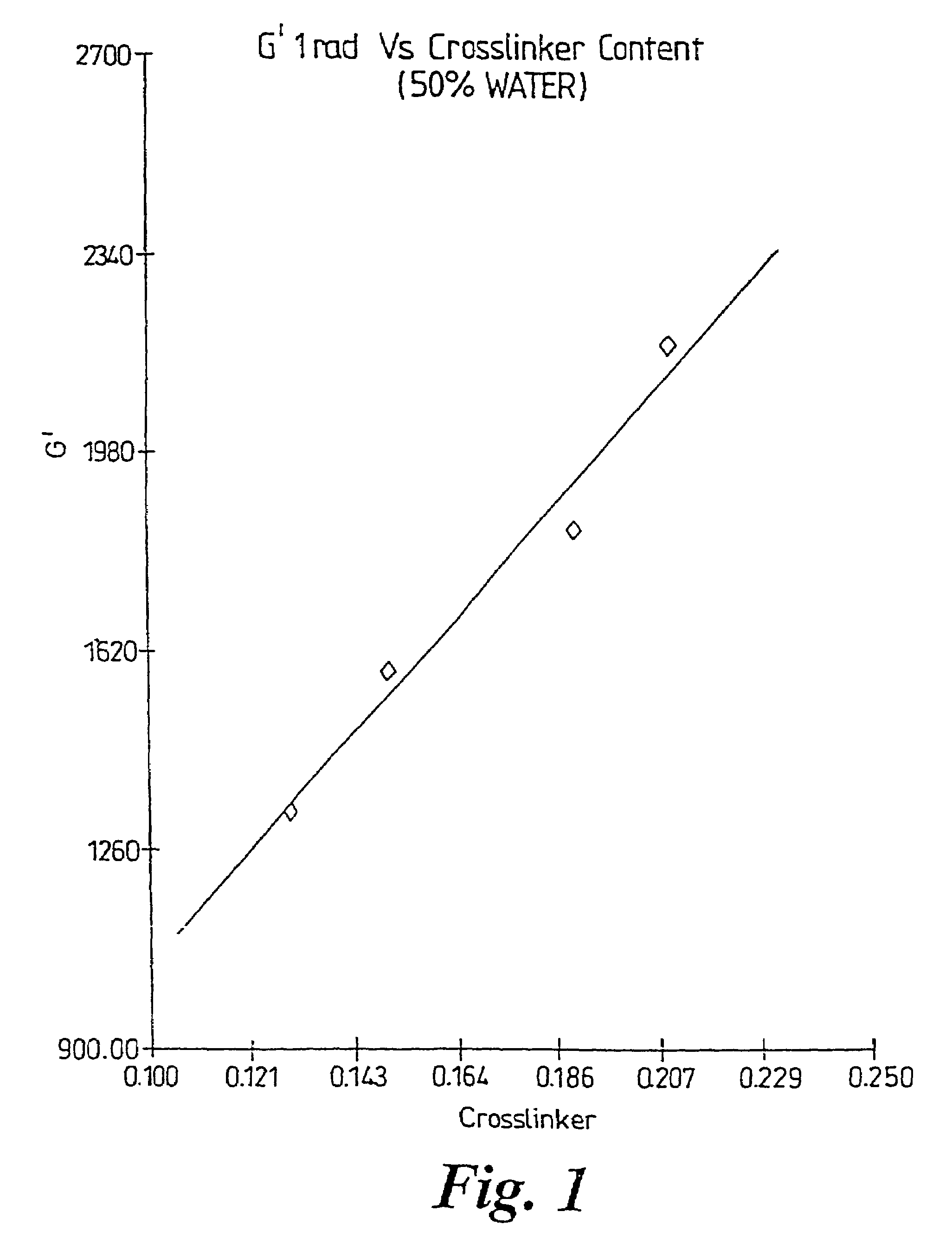Patents
Literature
348 results about "Bioadhesive" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bioadhesives are natural polymeric materials that act as adhesives. The term is sometimes used more loosely to describe a glue formed synthetically from biological monomers such as sugars, or to mean a synthetic material designed to adhere to biological tissue.
Self-supporting, shaped, three-dimensional biopolymeric materials and methods
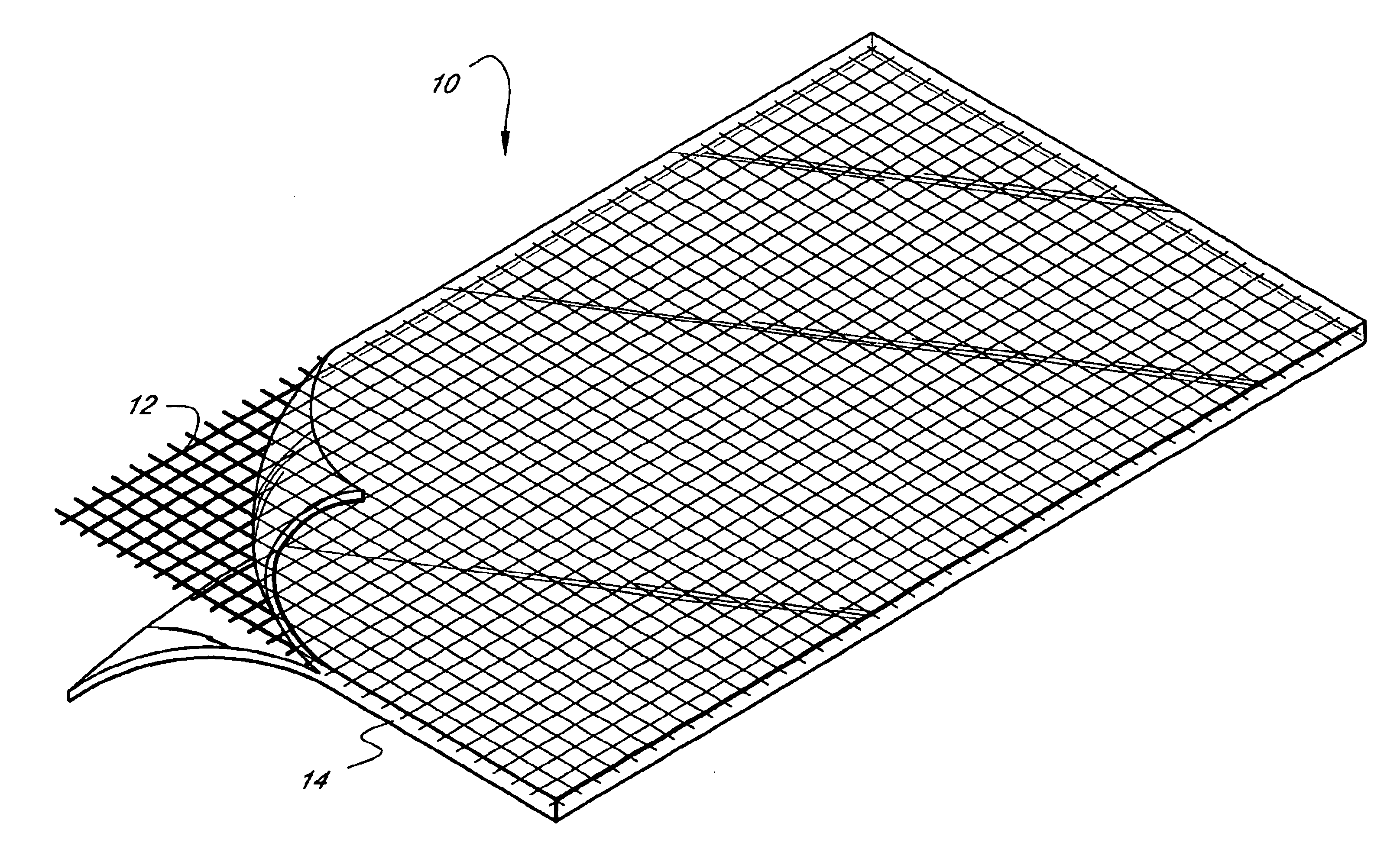
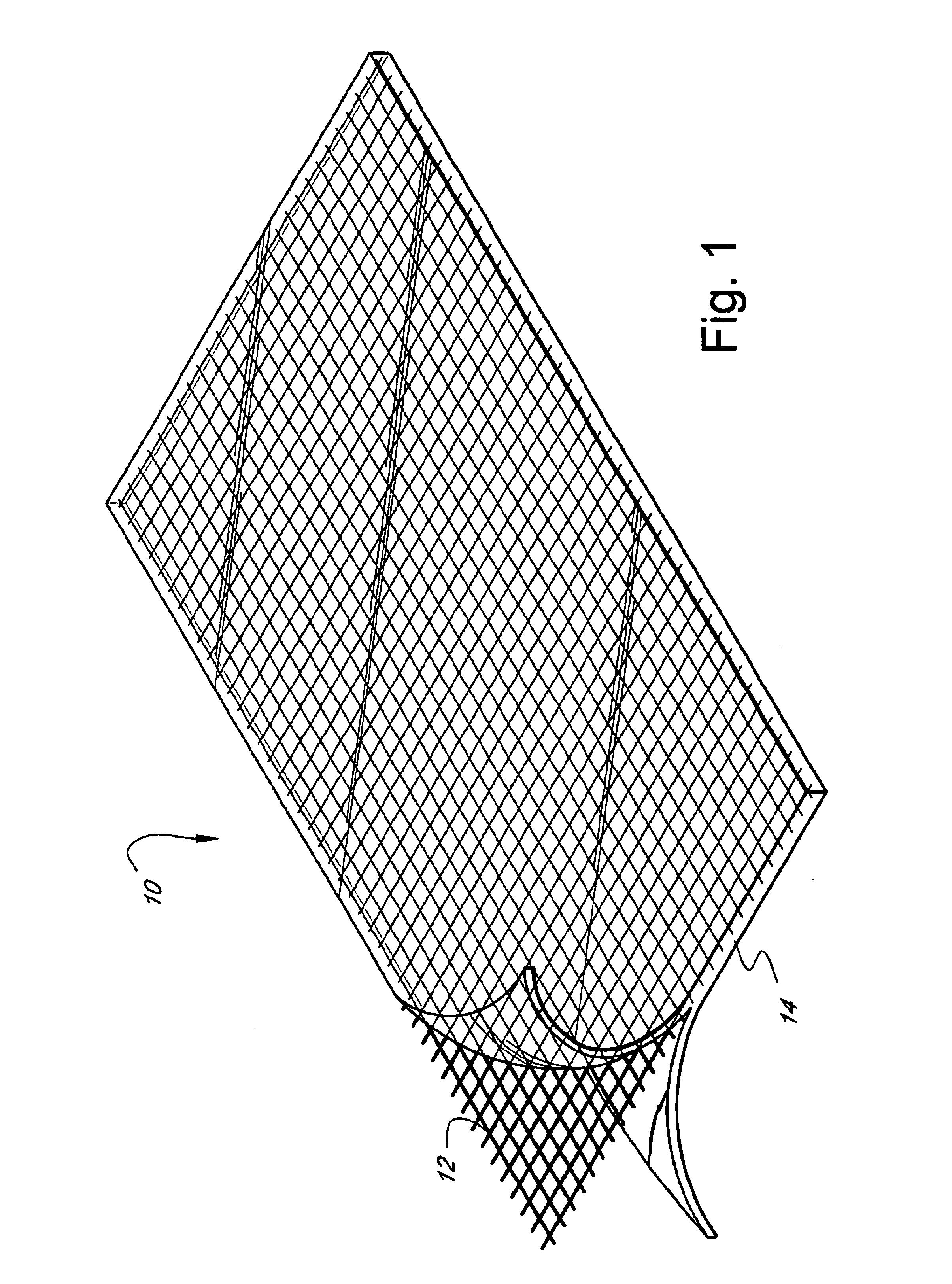
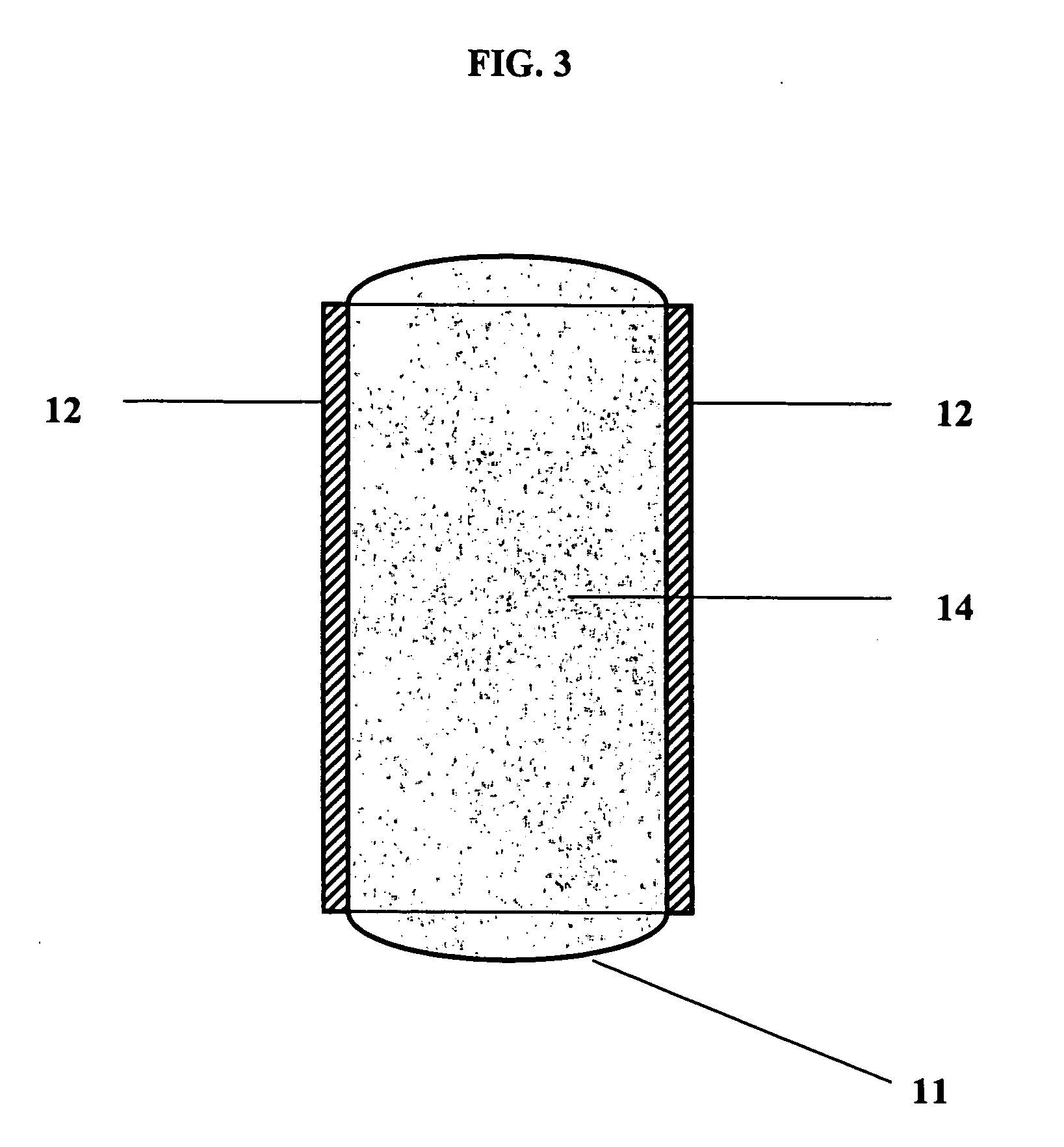
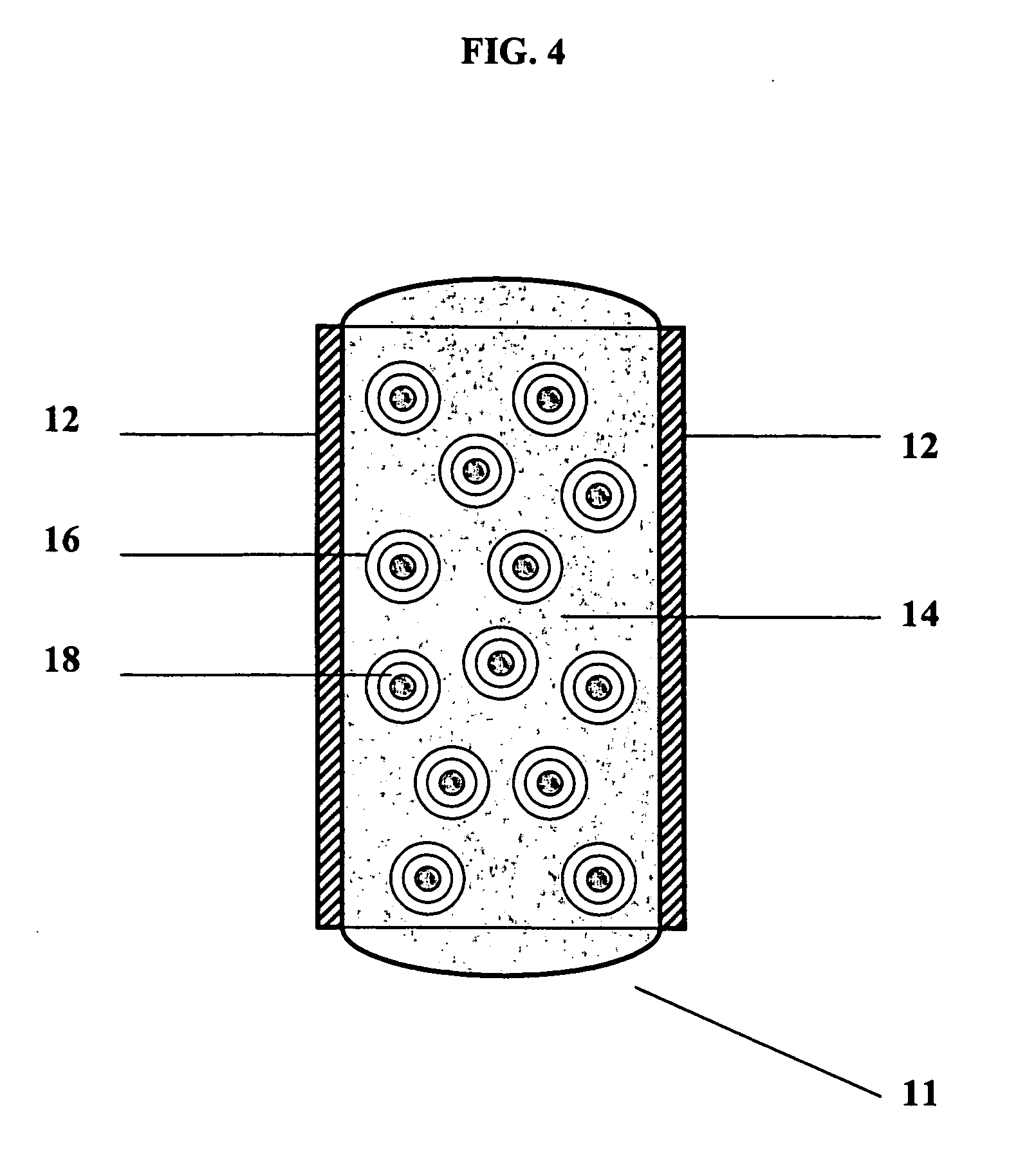
Self-supporting, shaped, three-dimensional cross-linked proteinaceous biopolymeric materials that may be implanted in vivo, and methods of making such materials are disclosed. The biopolymeric materials most preferably include reinforcing media, such as biocompatible fibrous or particulate materials. In use, the preformed, shaped biopolymeric materials may be applied to tissue in need of repair and then sealed around its edges with a liquid bioadhesive. In such a manner, repaired tissue which is capable of withstanding physiological pressures may be provided.
Owner:CRYOLIFE
External mixer assembly
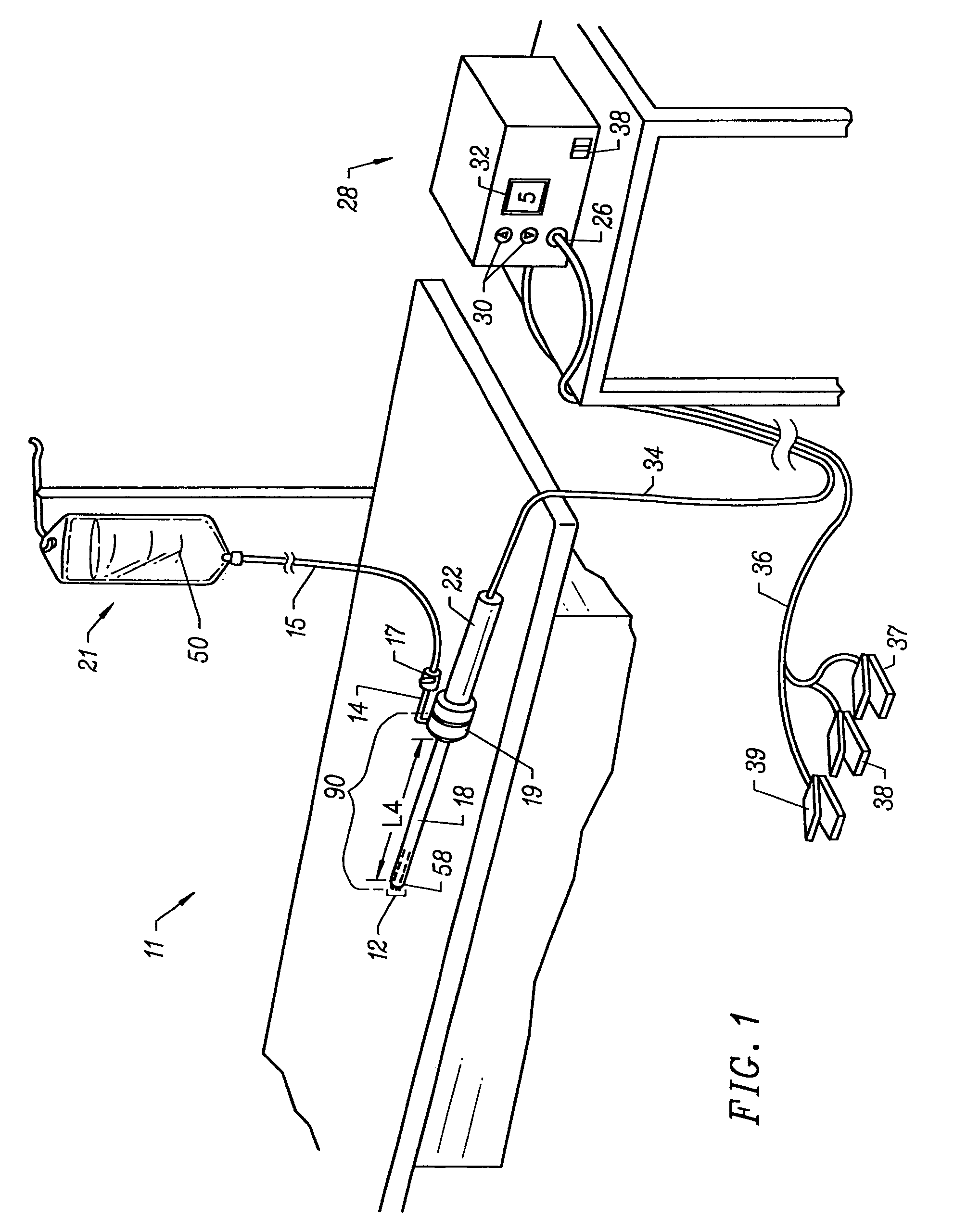
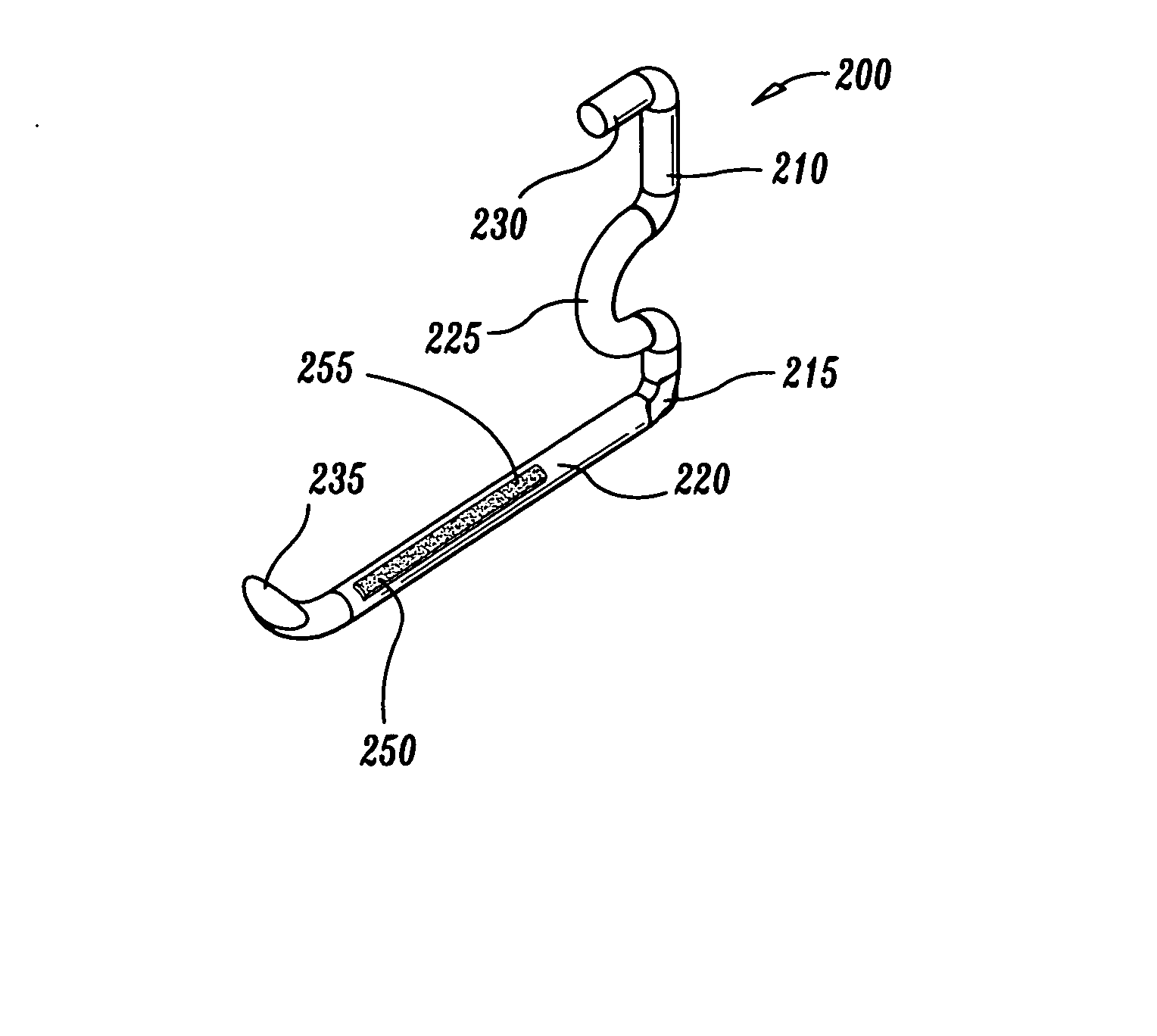
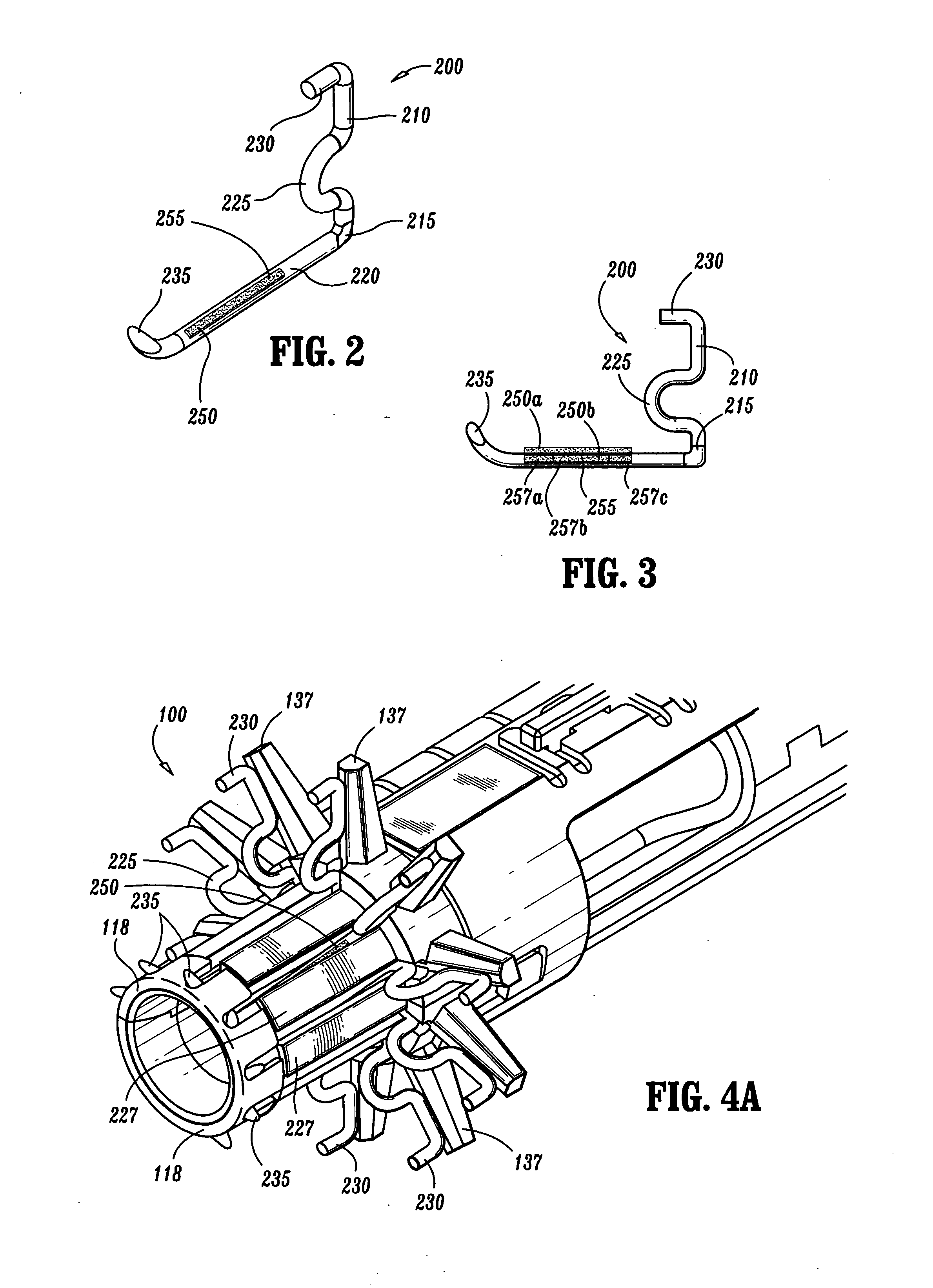
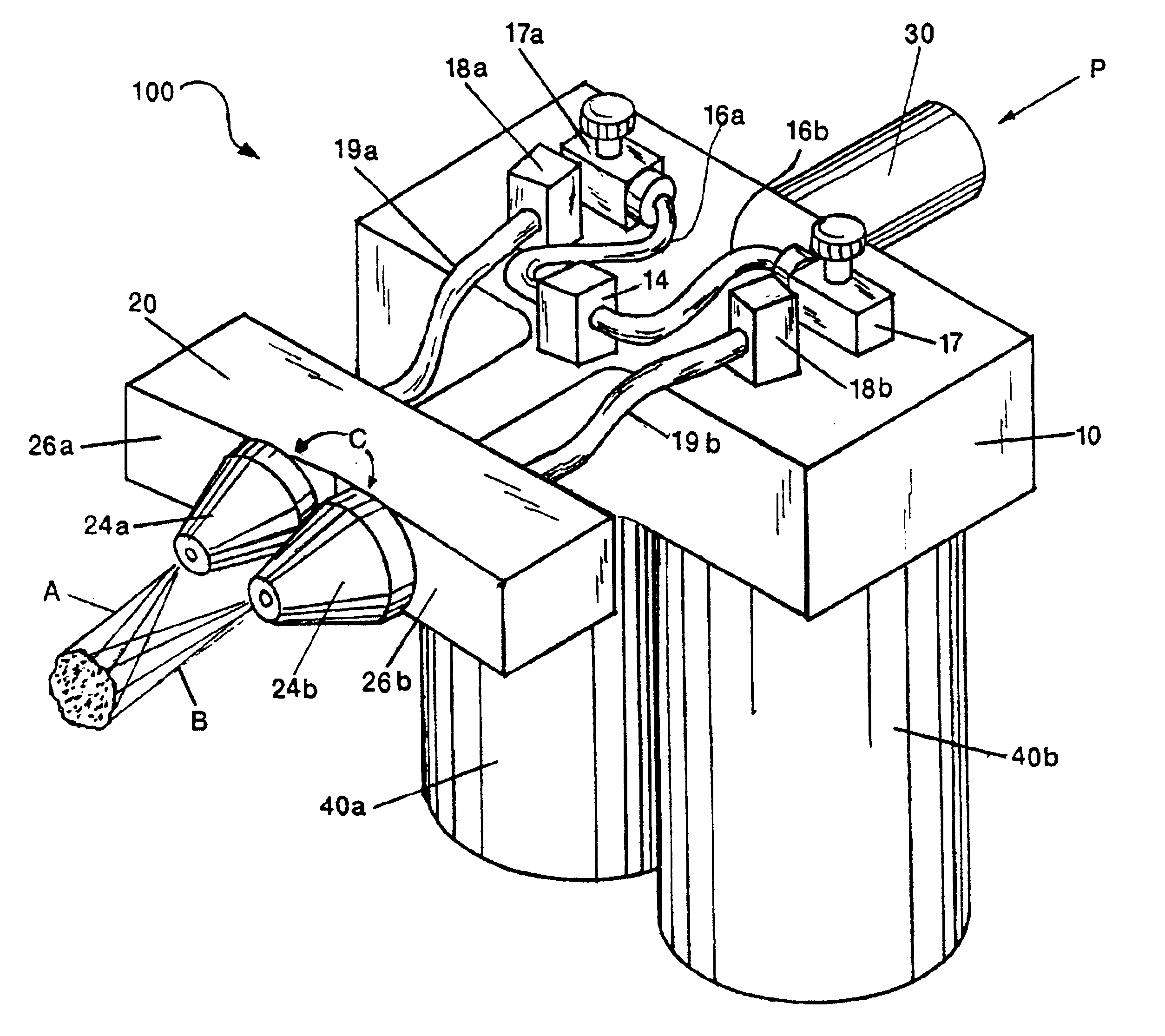
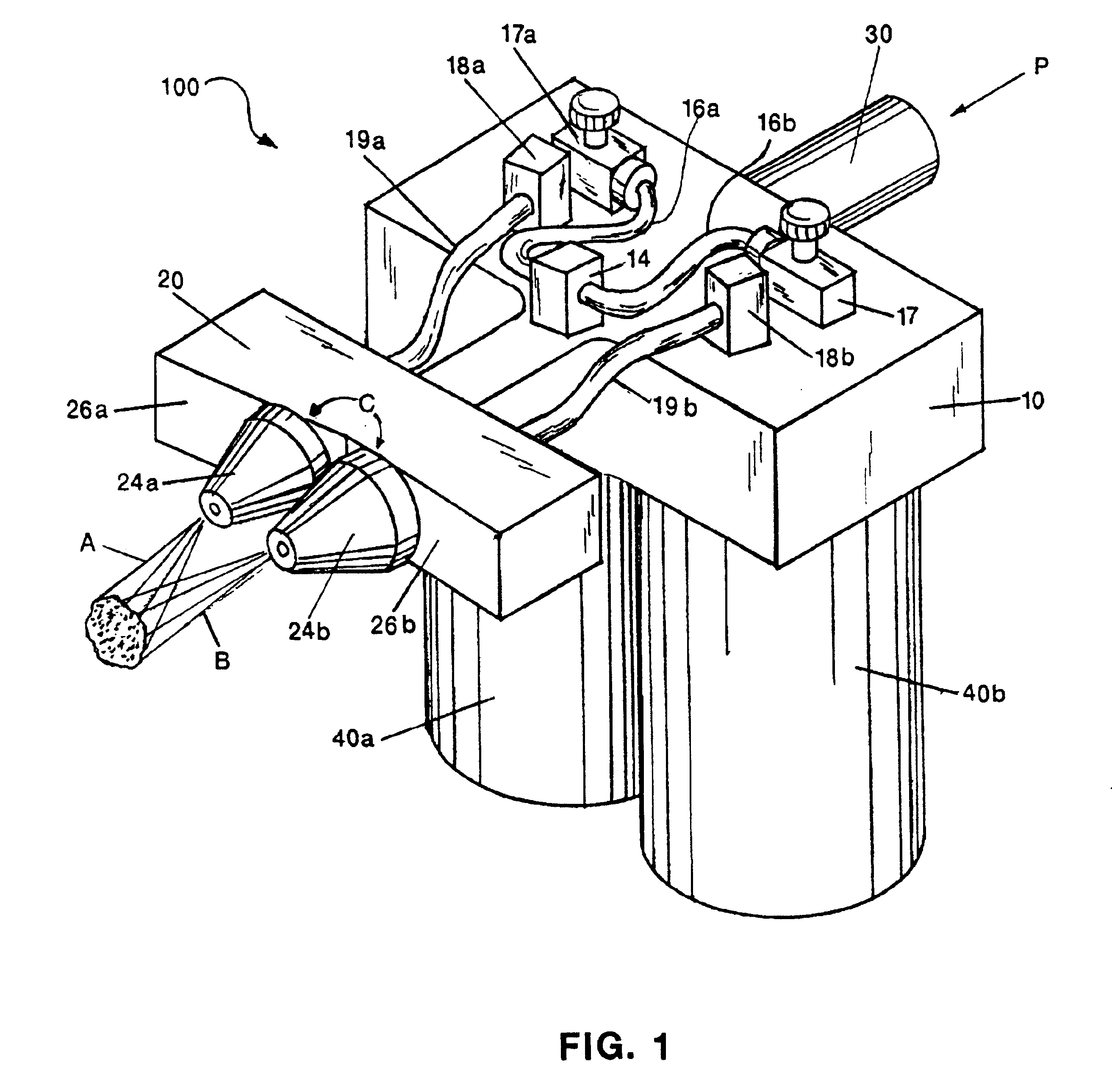
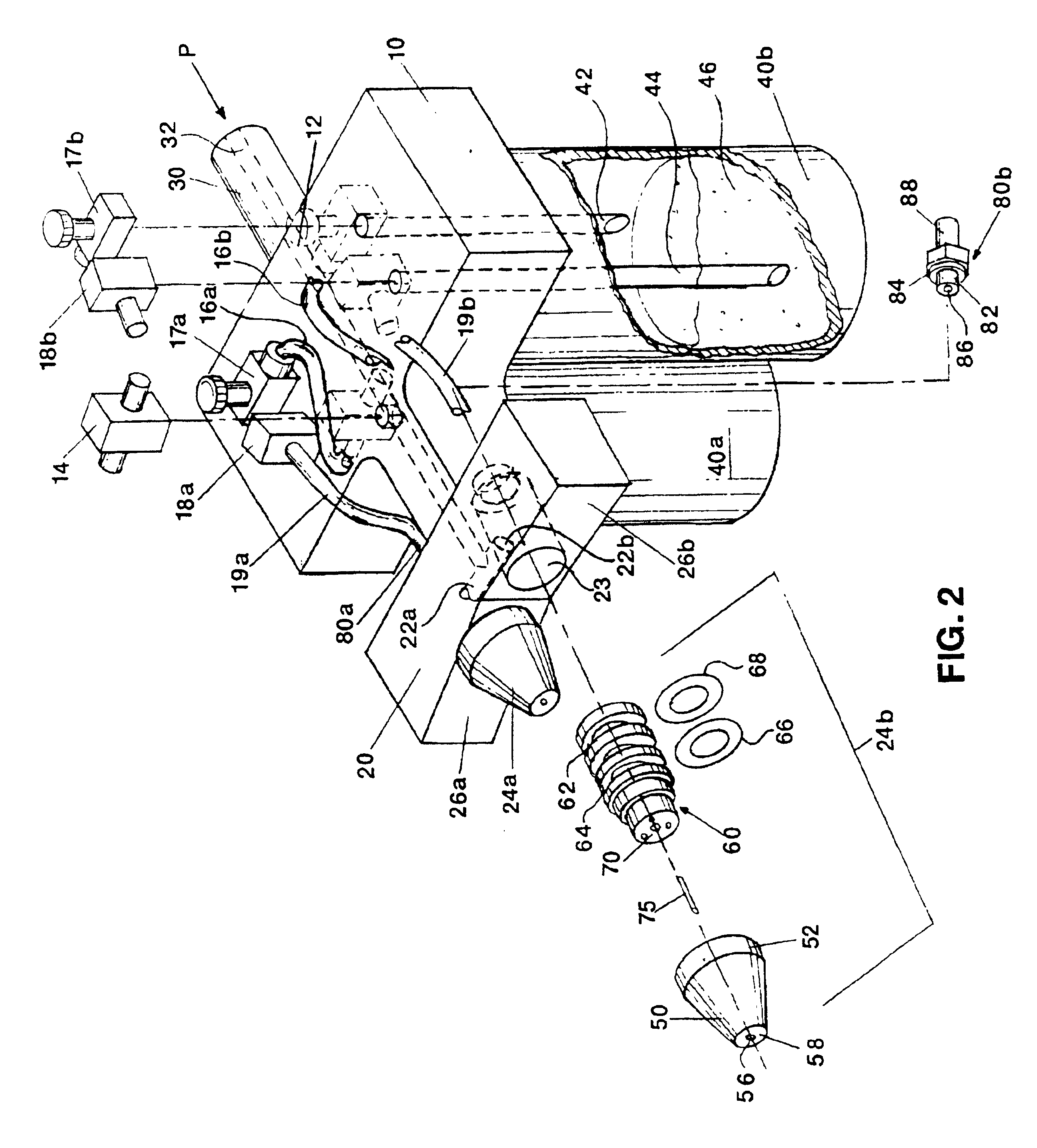
An external mixer assembly is provided which externally mixes and delivers a first and a second component of a biological adhesive to tissues or organs for sealing wounds, stopping bleeding and the like. The first and second components are mixed immediately after exiting from separate outlet ports disposed in fluid communication with component reservoirs. In on embodiment, the external mixer assembly includes a housing having a housing head for enclosing therein a first reservoir containing the first component, and a second reservoir containing the second component. The housing further includes a discharge nozzle defining a longitudinal axis for enclosing therein a conduit assembly having a first and a second conduit in communication with the first and second reservoir, respectively. A deflector assembly is connected to the discharge nozzle. The deflector assembly includes a deflector plate to provide a space for initial mixing of the first and second components. The deflector plate is oriented in generally parallel juxtaposed relation distal to the distal face of the discharge nozzle. The first and second components are preferably fibrinogen and thrombin which intermix to form a fibrin sealant.
Owner:TYCO HEALTHCARE GRP LP
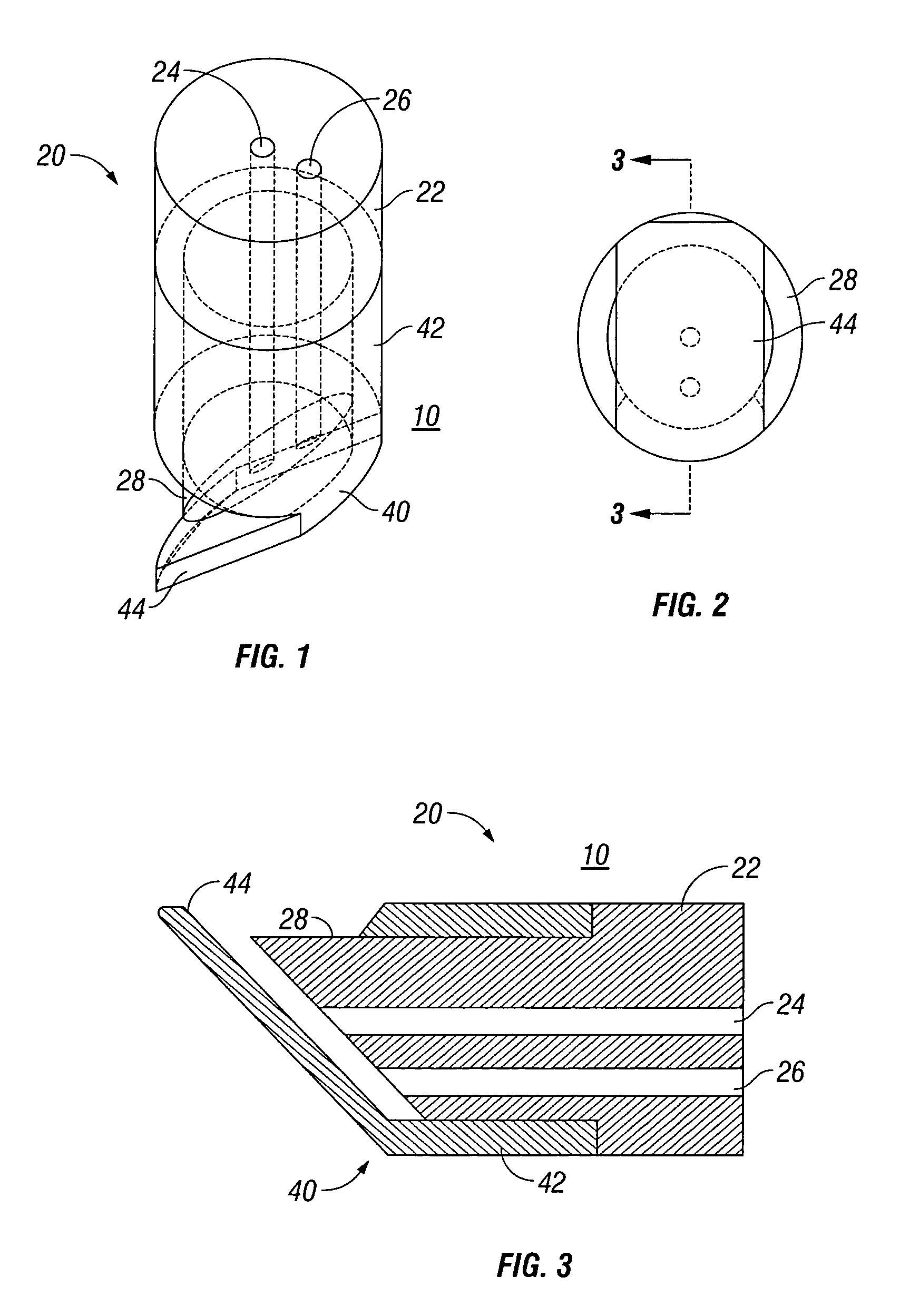
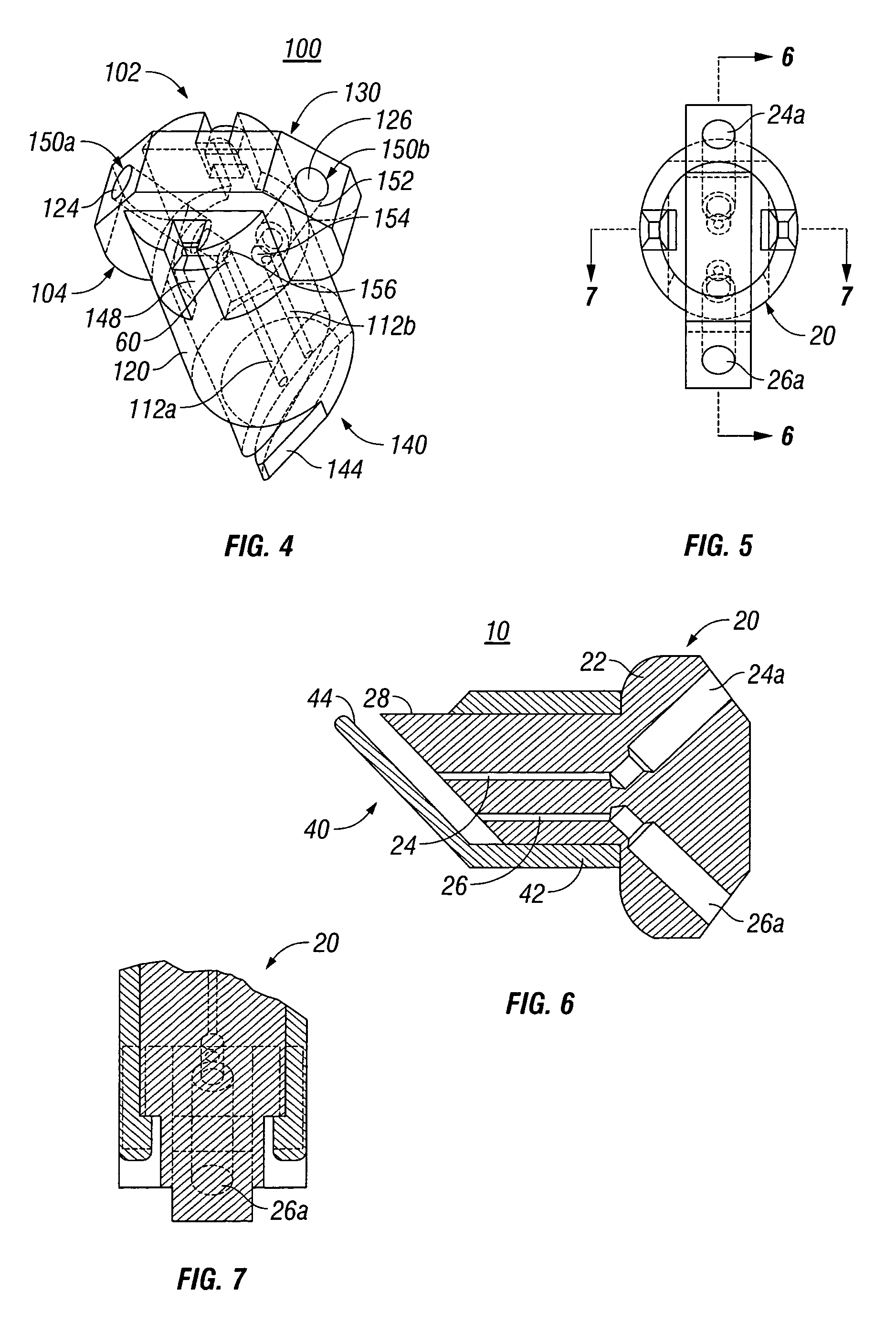
Anastomotic staple with capillary which expels a bonding agent upon deformation
InactiveUS8636191B2Efficient and accurate deliveryPromote better anastomosesSuture equipmentsStapling toolsBioadhesiveEngineering
Owner:COVIDIEN LP
Method for treating obstructive sleep disorder includes removing tissue from the base of tongue
InactiveUS7090672B2Stiffening the surrounding tissue structureUseful in treatmentSuture equipmentsHeart valvesThermal energyTongue root
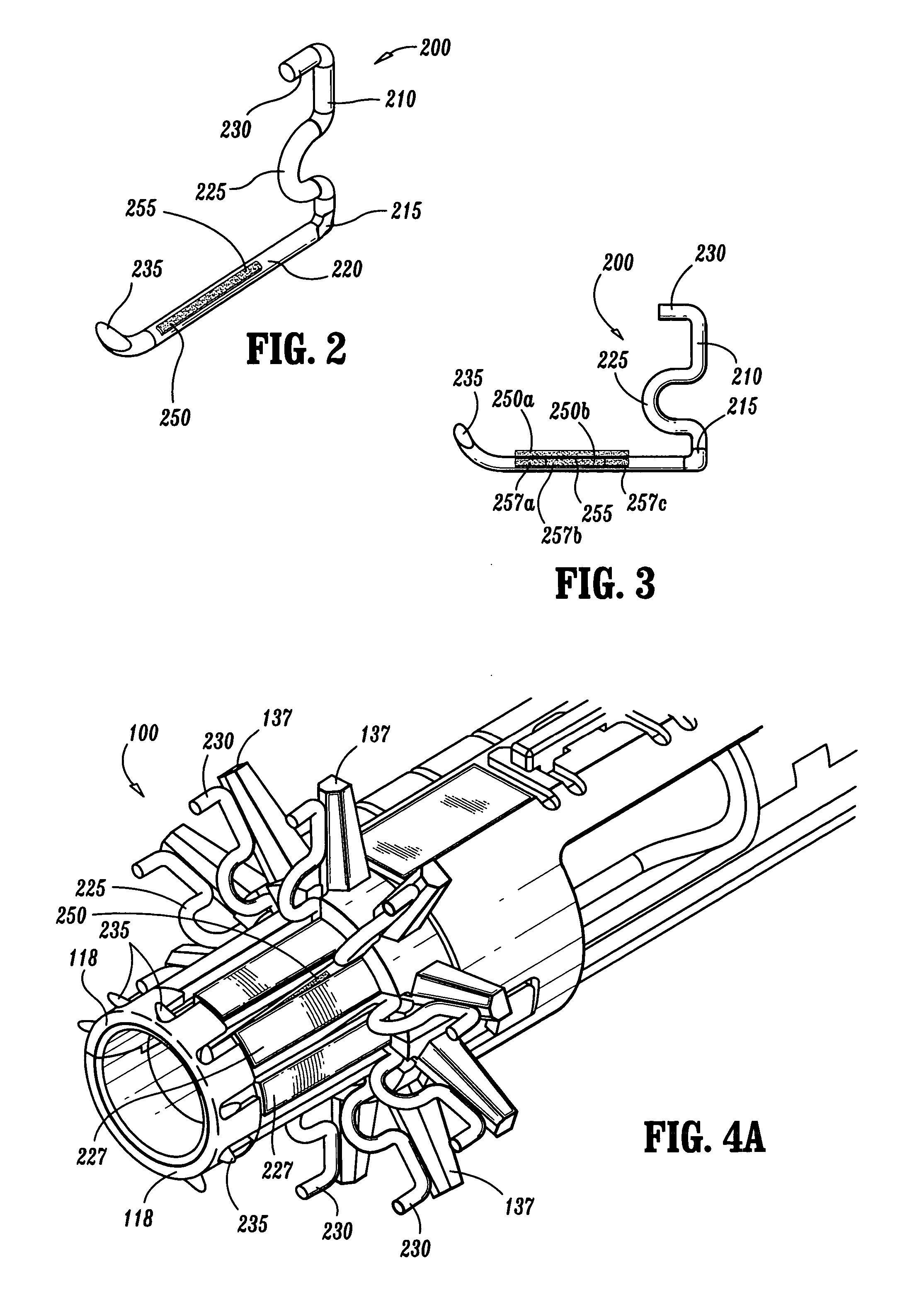
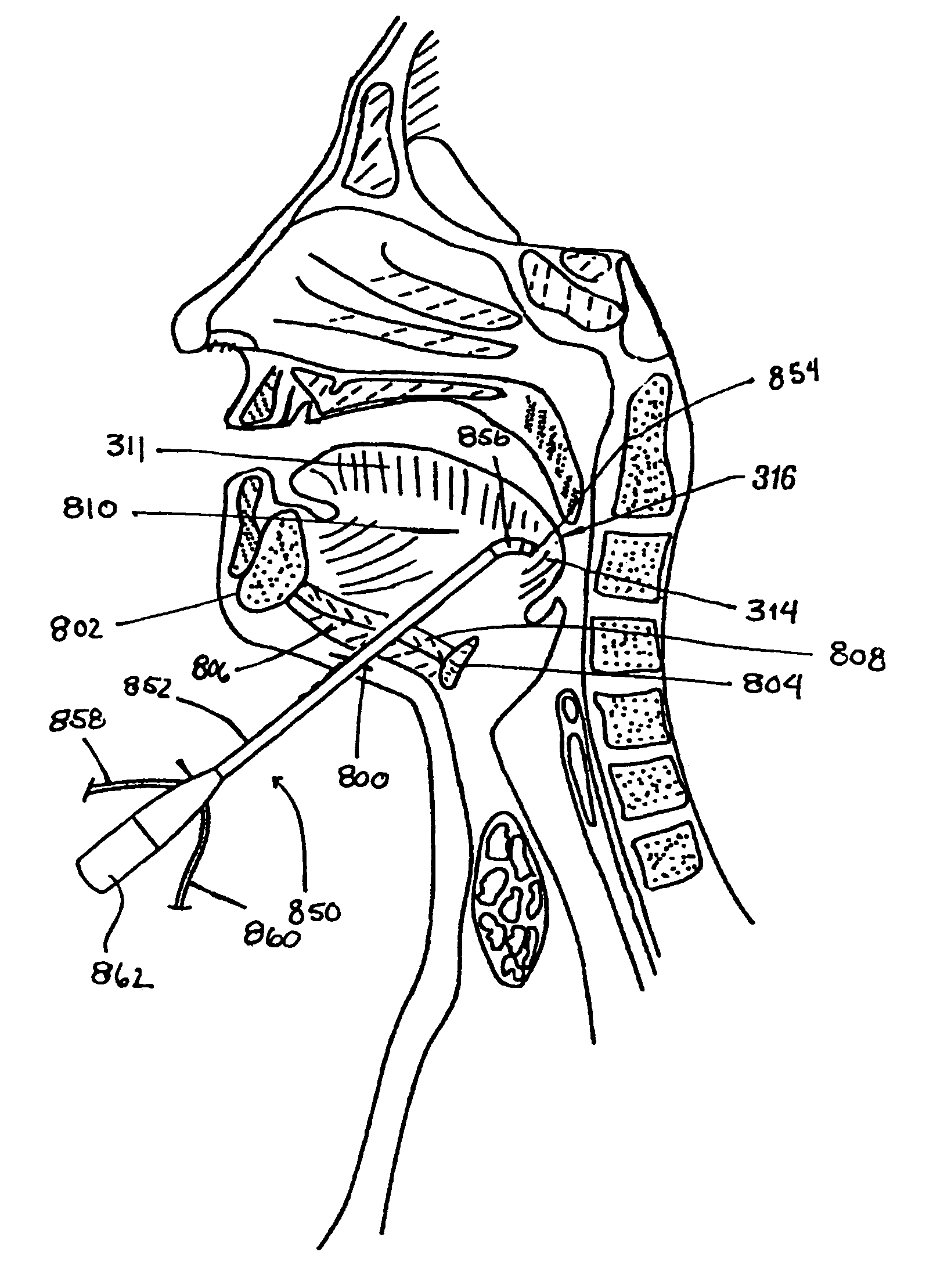
A method for treating obstructive sleep disorders includes accessing the interior of the tongue through an incision made in the skin in the vicinity of the jaw of a patient; advancing an instrument through the incision into the interior of the tongue; and removing an amount of tissue from the interior of the base of the tongue with the instrument. The present invention includes forming a cavity or plurality of channels in the tongue. The cavity may be collapsed using a suture, fastener or bioadhesive. An emplaced suture may be provided to hold the cavity in a collapsed position thereby reducing the degree of obstruction. Additionally, thermal energy may be applied to the tissue surface immediately surrounding the channels to cause thermal damage to the tissue surface, thereby creating hemostasis and stiffening the surrounding tissue structure.
Owner:ARTHROCARE
Anastomotic staple with capillary which expels a bonding agent upon deformation
InactiveUS20070066981A1Efficient and accurate deliveryReduce leakageStaplesNailsBioadhesiveEngineering
A surgical fastener for use with an anastomosis of two tissues includes a base leg and an upright leg. The base leg is selectively deformable and includes a traumatic tip for piecing tissue. The surgical fastener also includes at least one capillary disposed on the base leg which has a reservoir defined therein for retaining a liquid, e.g., bioadhesive, bonding agent, medicament, etc. Each of the capillaries is ruptureable upon deformation of the surgical fastener to dispense the liquid to the anastomosis site.
Owner:TYCO HEALTHCARE GRP LP
Antibiotic kit and composition and uses thereof
The present invention relates to a therapeutic kit to provide a safe and effective dosage of an antibiotic agent, including an aerosol packaging assembly including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam, wherein the pressurized product comprises a foamable composition including: an antibiotic agent; at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, an organic polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight, a surface-active agent, about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent, water; and liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Method for treating obstructive sleep disorder includes removing tissue from base of tongue
InactiveUS20050234439A1Stiffening surrounding tissue structureUseful in treatmentDiagnosticsSurgical instruments for heatingTongue rootThermal energy
A method for treating obstructive sleep disorders includes accessing the interior of the tongue through a sublingual incision; advancing an instrument through the incision into the interior of the tongue; and instantaneously removing an amount of tissue from the interior of the base of the tongue with the instrument. The present invention includes forming a cavity or plurality of channels in the tongue. The cavity may be collapsed using a suture, fastener or bioadhesive. An emplaced suture may be provided to hold the cavity in a collapsed position thereby reducing the degree of obstruction. Additionally, thermal energy may be applied to the tissue surface immediately surrounding the channels to cause thermal damage to the tissue surface, thereby creating hemostasis and stiffening the surrounding tissue structure.
Owner:ARTHROCARE
Bioadhesive compositions and biomedical electrodes containing them
InactiveUS7076282B2Good bioadhesionHigh mechanical strengthElectrocardiographySurgical adhesivesWound dressingHydrophobic polymer
Owner:FIRST WATER
Method for treating obstructive sleep disorder includes removing tissue from base of tongue
InactiveUS7491200B2Stiffening the surrounding tissue structureUseful in treatmentDiagnosticsSurgical instruments for heatingTongue rootThermal energy
A method for treating obstructive sleep disorders includes accessing the interior of the tongue through a sublingual incision; advancing an instrument through the incision into the interior of the tongue; and instantaneously removing an amount of tissue from the interior of the base of the tongue with the instrument. The present invention includes forming a cavity or plurality of channels in the tongue. The cavity may be collapsed using a suture, fastener or bioadhesive. An emplaced suture may be provided to hold the cavity in a collapsed position thereby reducing the degree of obstruction. Additionally, thermal energy may be applied to the tissue surface immediately surrounding the channels to cause thermal damage to the tissue surface, thereby creating hemostasis and stiffening the surrounding tissue structure.
Owner:ARTHROCARE
Bioadhesive polymers with catechol functionality
InactiveUS20050201974A1Good bioadhesionExtended stayNervous disorderPill deliveryArameHydrophobic polymer
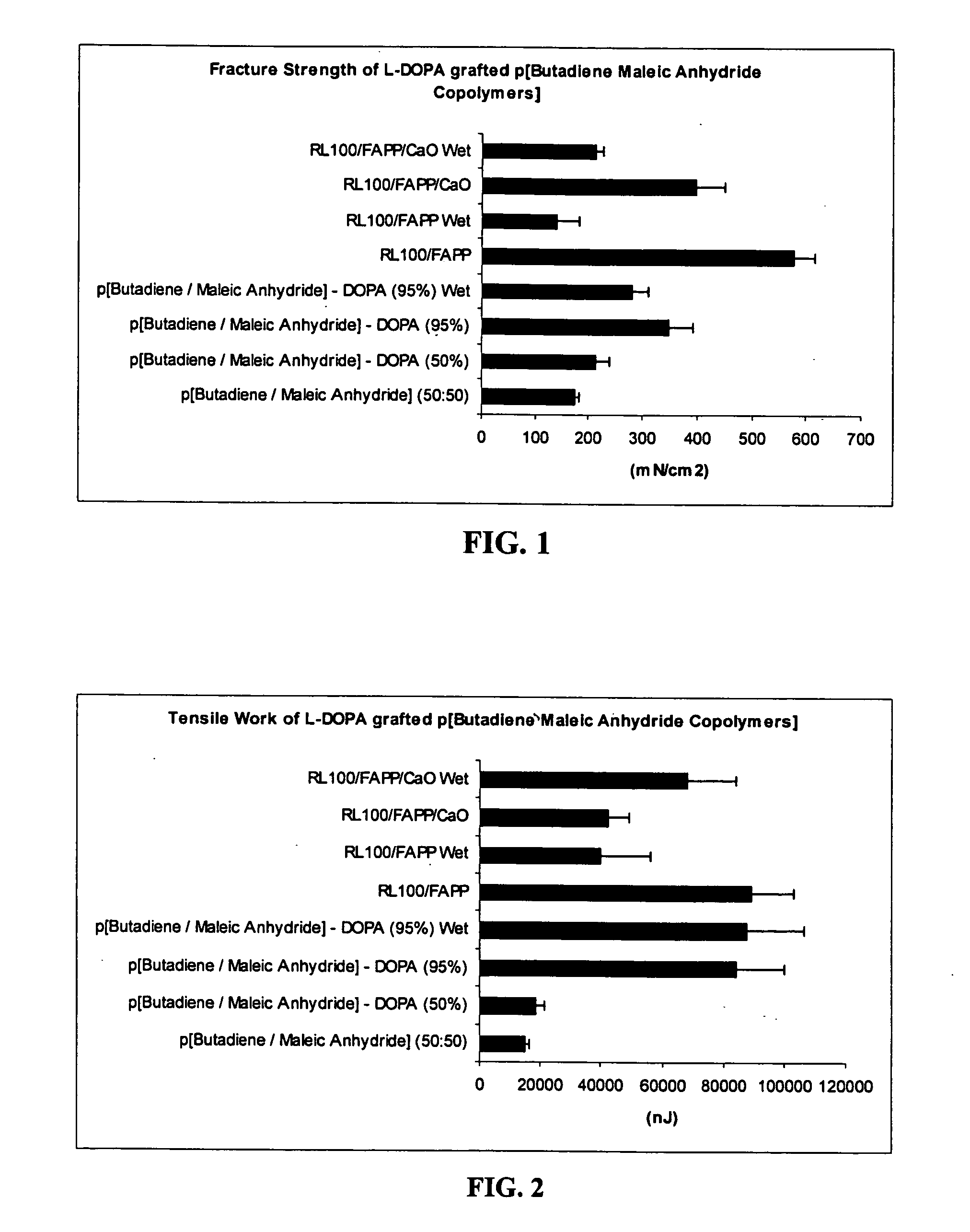
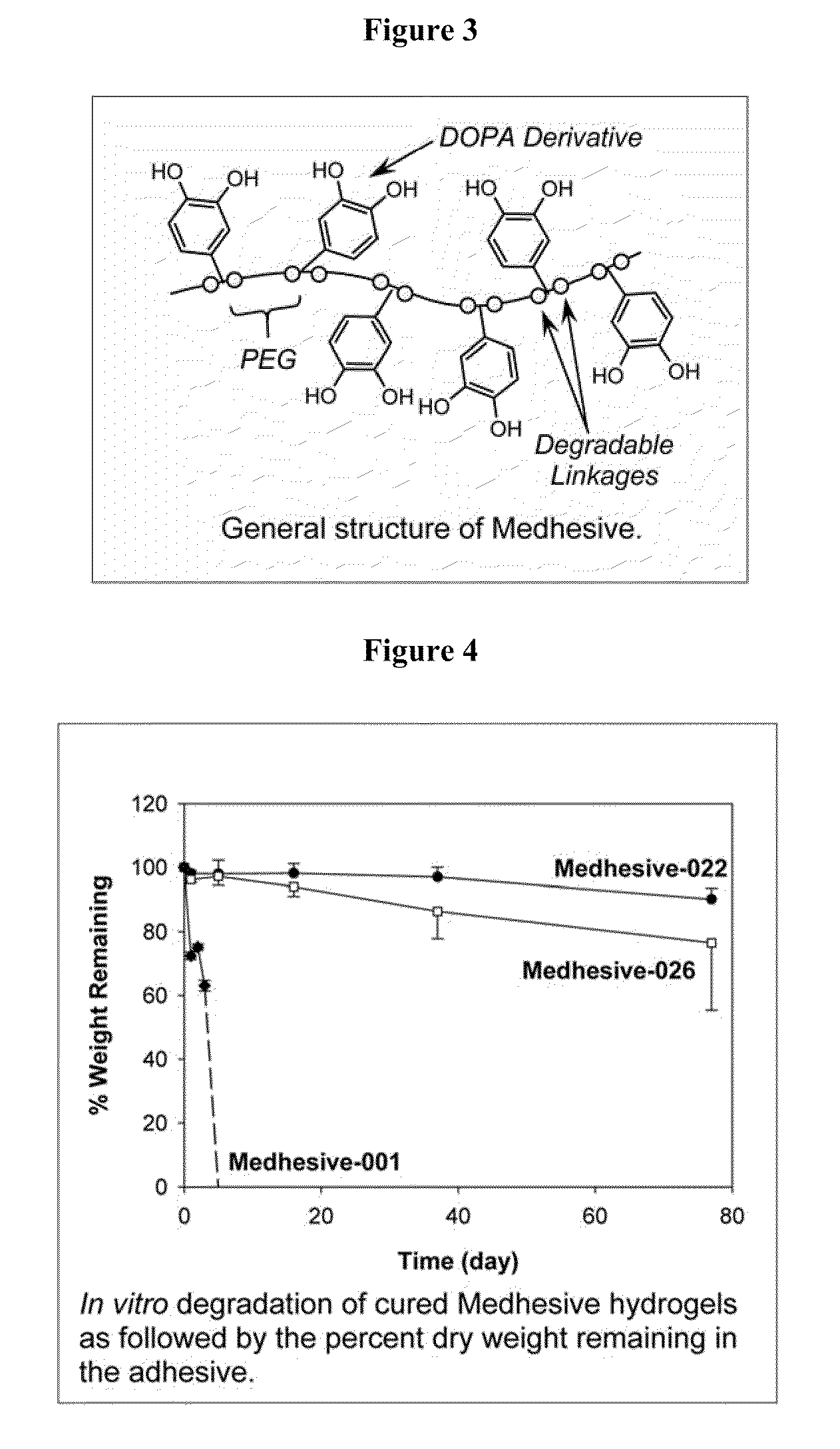
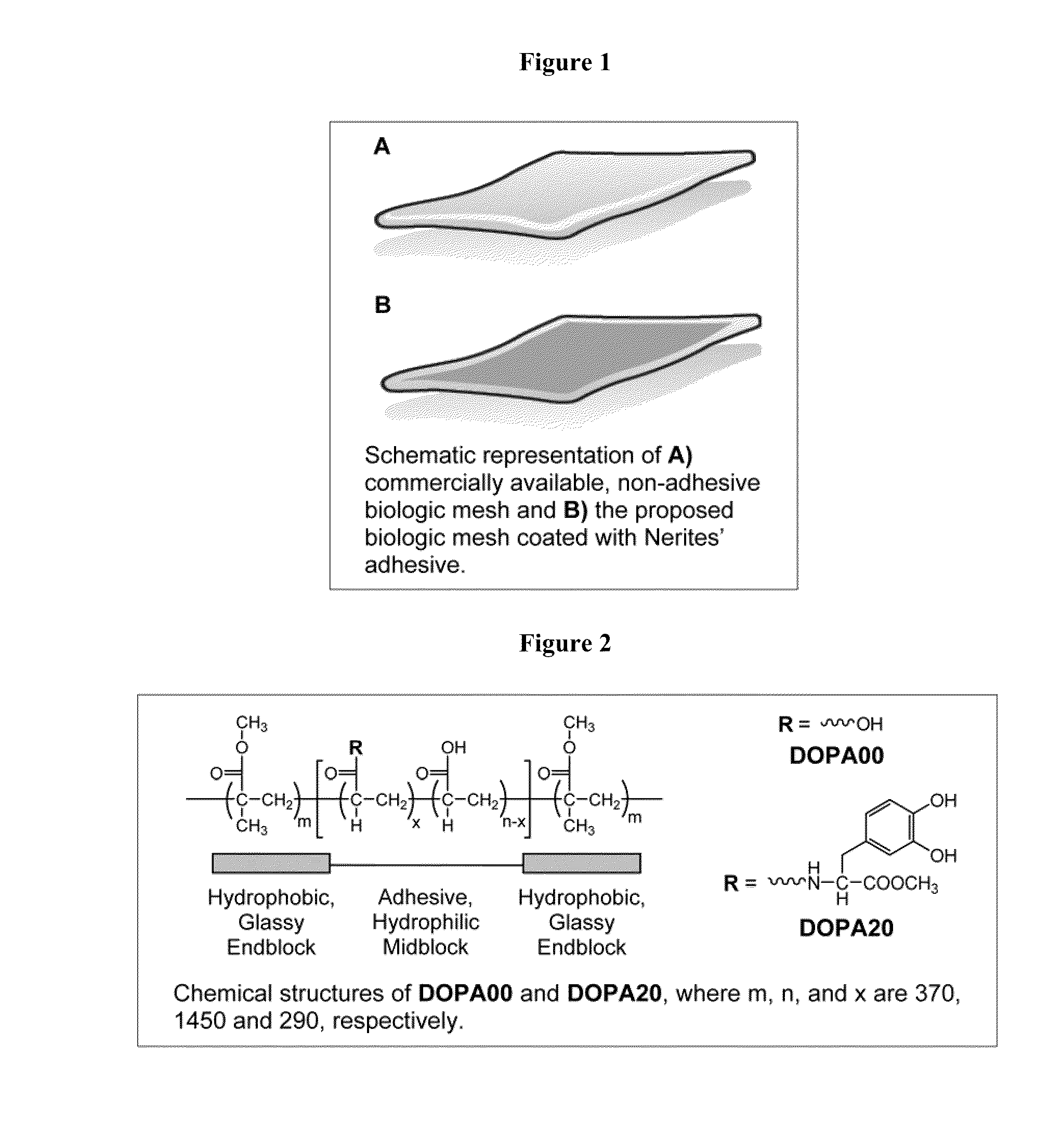
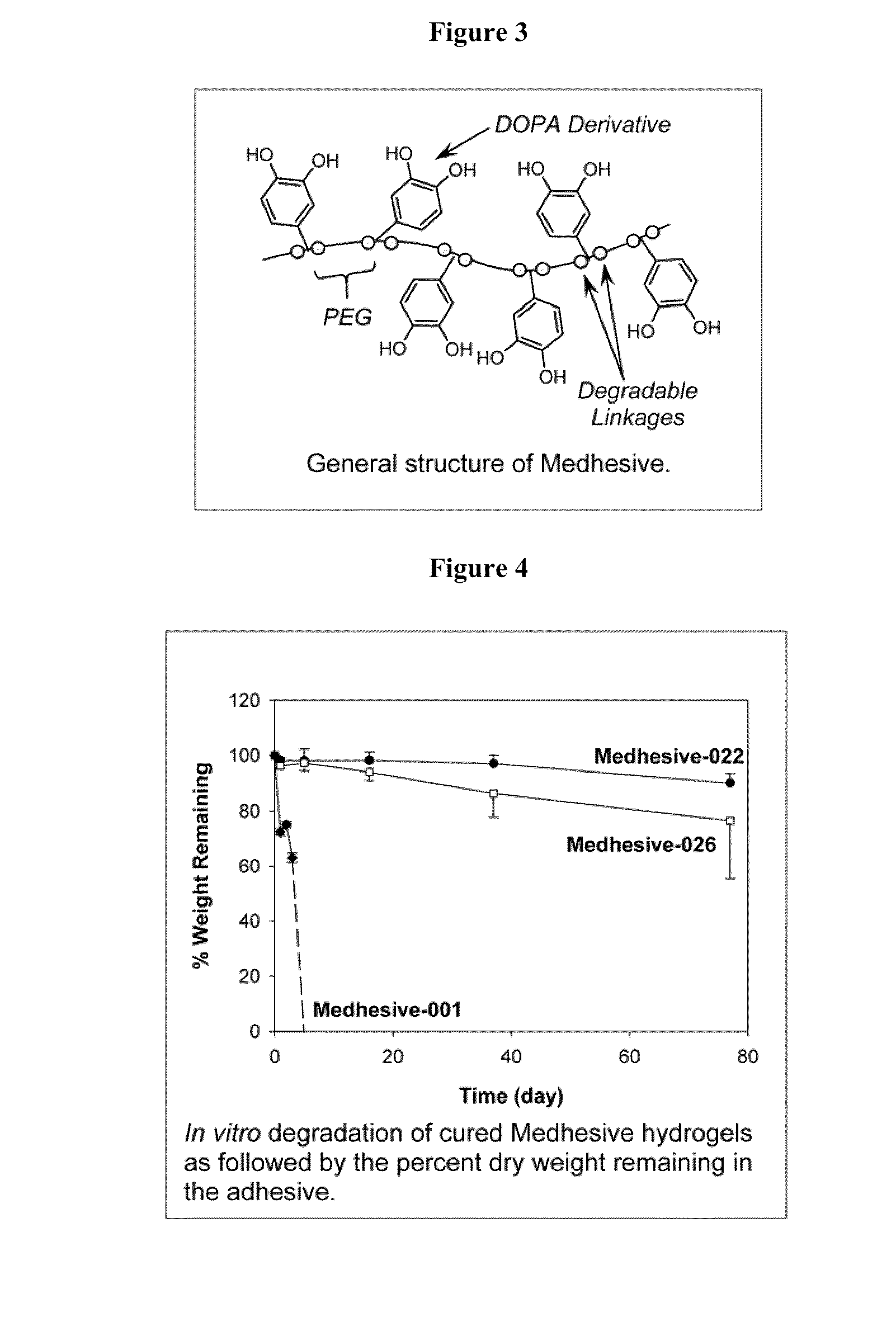
Polymers with improved bioadhesive properties and methods for improving bioadhesion of polymers have been developed. A compound containing an aromatic group which contains one or more hydroxyl groups is grafted onto a polymer or coupled to individual monomers. In one embodiment, the polymer is a biodegradable polymer. In another embodiment, the monomers may be polymerized to form any type of polymer, including biodegradable and non-biodegradable polymers. In some embodiments, the polymer is a hydrophobic polymer. In the preferred embodiment, the aromatic compound is catechol or a derivative thereof and the polymer contains reactive functional groups. In the most preferred embodiment, the polymer is a polyanhydride and the aromatic compound is the catechol derivative, DOPA. These materials display bioadhesive properties superior to conventional bioadhesives used in therapeutic and diagnostic applications. These bioadhesive materials can be used to fabricate new drug delivery or diagnostic systems with increased residence time at tissue surfaces, and consequently increase the bioavailability of a drug or a diagnostic agent. In a preferred embodiment, the bioadhesive material is a coating on a controlled release oral dosage formulation and / or forms a matrix in an oral dosage formulation.
Owner:SPHERICS
Method of preparing polymeric adhesive compositions utilizing the mechanism of interaction between the polymer components
InactiveUS20050113510A1Easy to handleReduce leakageCosmetic preparationsImpression capsPolymer scienceBioadhesive
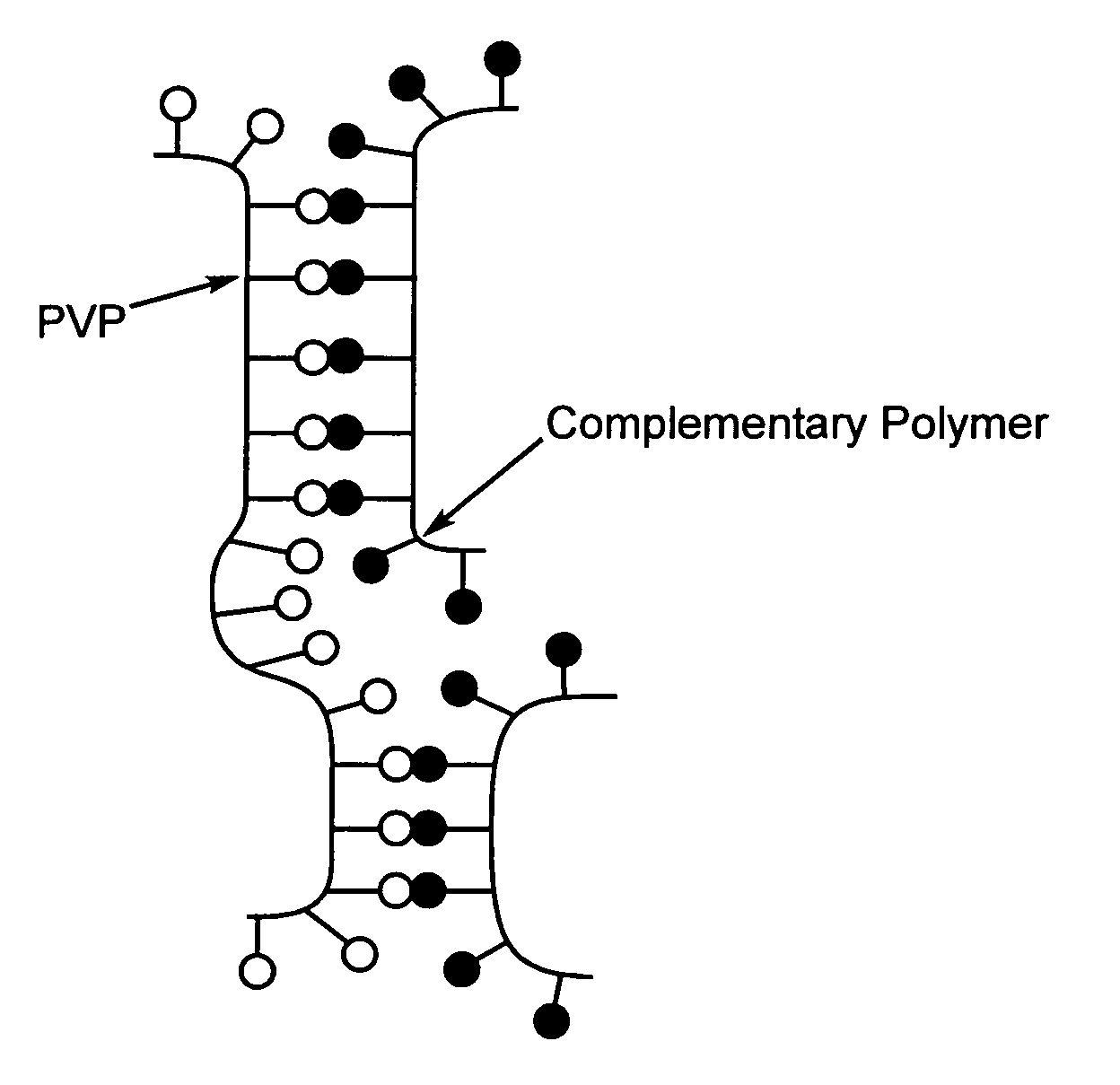
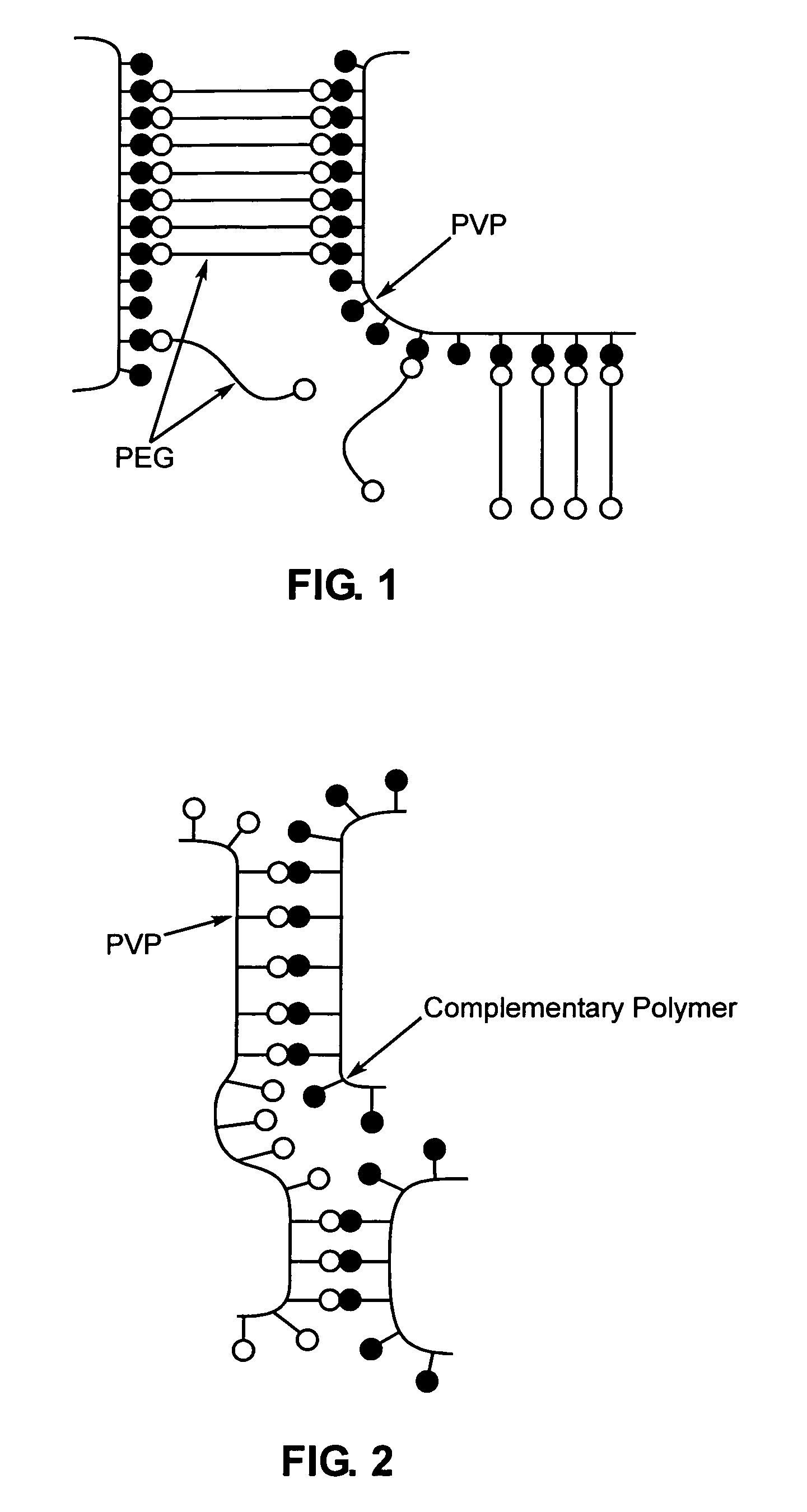
A method of selecting components for use in water-absorbing pressure-sensitive adhesive compositions is provided. The method involves selecting a film-forming polymer, a ladder-like non-covalent crosslinker that is capable of forming a ladder-like interpolymer complex with the film-forming polymer selected, and selecting a carcass-like non-covalent crosslinker that is capable of forming a carcass-like complex with at least one of the film-forming polymer selected or the ladder-like non-covalent crosslinker selected. The adhesive hydrogels provide high adhesion in a swollen state and bridge the gap between conventional pressure sensitive adhesives and bioadhesives. Methods for preparing and using the resulting compositions are also disclosed.
Owner:A V TOPCHIEV INST OF PETROCHEM +1
Biocompatible polymer compositions for dual or multi staged curing
ActiveUS20050197422A1Increase ratingsHigh viscosityCosmetic preparationsImpression capsWound healingBioadhesive
The present invention provides a biocompatible polymer composition for use in biomedical applications comprising a base molecule, a linker molecule and at least one initiator compound, said base molecule having at least two differing functionalities, and said linker molecule having a functionality reactive with at least one functionality of said base molecule, the first of said at least two functionalities of said base molecule enabling a first curing stage of said polymer composition by reaction with said linker molecule, and the second and any further functionality of said base molecule enabling second and further curing stages of said polymer composition, said first, second and any further curing stages being capable of activation simultaneously or independently of each other as required. The invention further provides uses of the polymer compositions of the invention in biomedical applications such as tissue engineering, dry delivery, as a bioadhesive in wound healing, as bone substitutes or scaffolds, as cements in dental and periodontal applications and as anti-adhesives or protective barriers.
Owner:POLYNOVO BIOMATERIALS PTY LTD
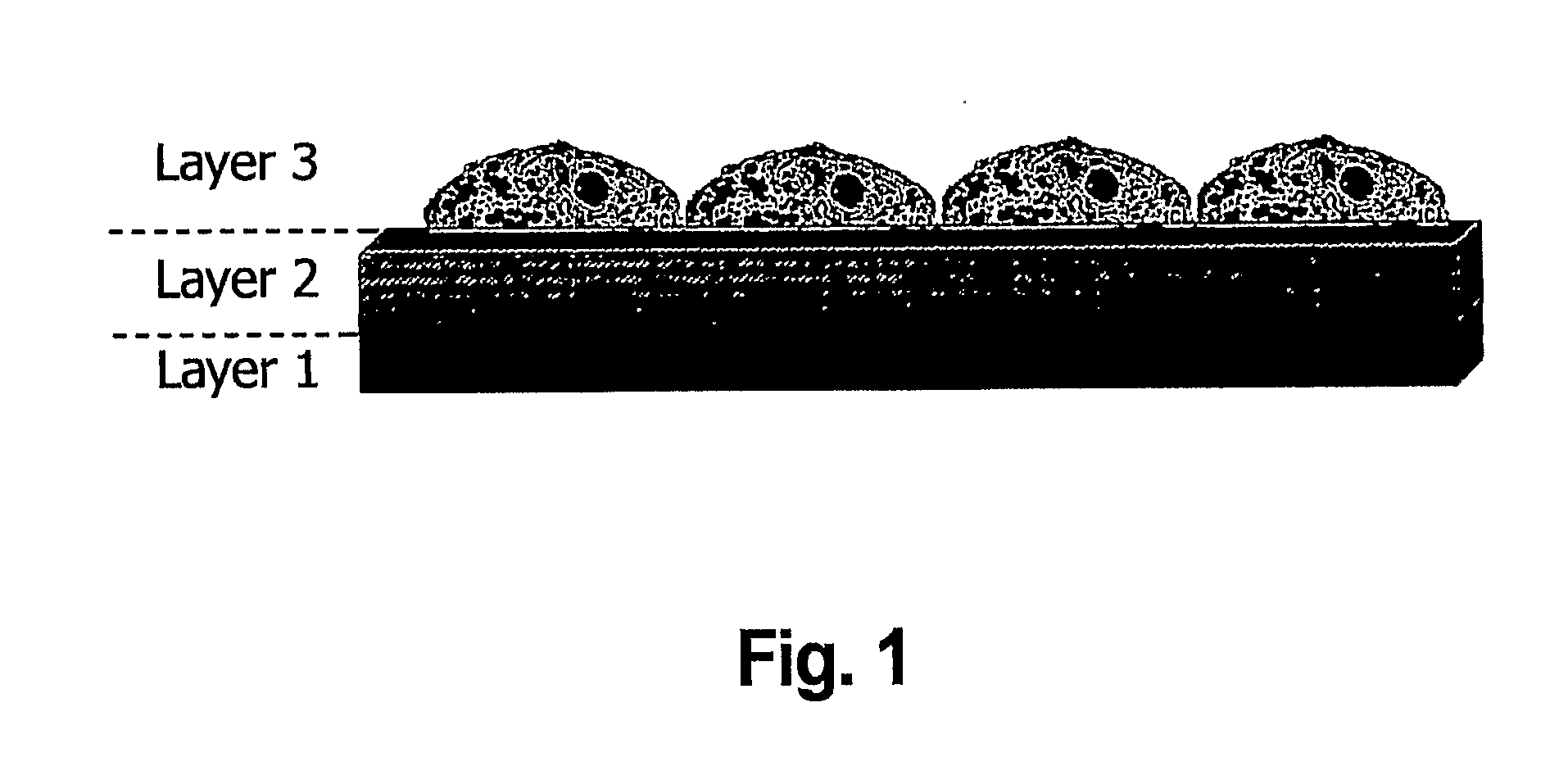
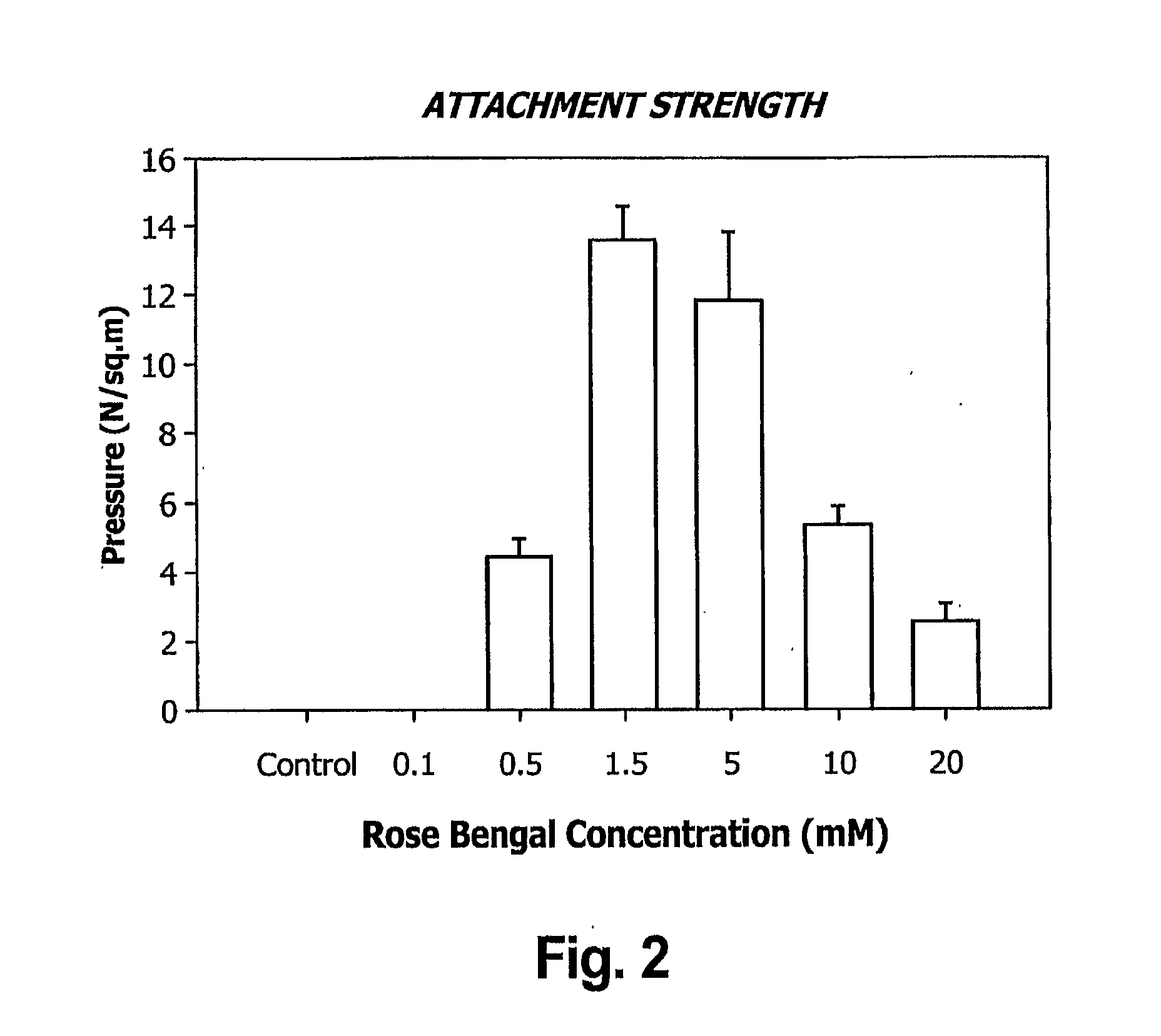
Layered bio-adhesive compositions and uses thereof
The invention generally provides compositions and methods for promoting and enhancing wound closure and healing. Specifically, the invention provides a biologic composition which comprises a support layer which serves as transport scaffold, for example made of gelatin, which is coated or impregnated with a bio-adhesive molecule such as rose bengal or glyceraldehyde. The composition can also comprise an artificial or biological matrix, optionally processed (i.e. cleaned and coated with extracellular matrix proteins) to enhance cell attachment and survival. The composition can further comprise a monolayer of epithelial, endothelial cells or mesenchymal cells. The invention provides methods for using the compositions for treating wounds due to disease, trauma or surgery. Specific methods for treating ocular wounds are provided.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Bioadhesive film
Bioadhesive films for delivery of active agent to the mucosa are disclosed. Particularly, bioadhesive films for treating the vaginal mucosa are disclosed.
Owner:WYETH LLC
Absorbent multilayer hydrogel wound dressings
ActiveUS20060200063A1Acceptable water uptake speedGood flexibilityAbsorbent padsBandagesPolymer scienceHydrophilic polymers
The invention provides wound dressings comprising an absorbent (porous) hydrogel composition comprising a foam portion which comprises a flexible plasticised hydrophilic polymer matrix having an internal cellular structure, and a continuous portion which comprises a flexible plasticised hydrophilic polymer matrix having relatively continuous internal structure. The continuous portion of the hydrogel composition includes apertures providing fluid flow communication through the continuous portion between an external surface of the continuous portion and the foam portion whereby the foam portion can take up external water or other fluid into the cellular structure through the apertures of the continuous portion. The continuous portion of the hydrogel composition may be tacky to the skin, allowing its use as a bioadhesive.
Owner:KCI USA
Insulative products having bio-based binders
InactiveUS20110223364A1Readily availableLow costStarch adhesivesStarch derivtive adhesivesFiberWater soluble polysaccharides
Fibrous insulation products have an aqueous binder composition that includes a carbohydrate and a crosslinking agent. In exemplary embodiments, the carbohydrate-based binder composition may also include a catalyst, a coupling agent, a process aid, a crosslinking density enhancer, an extender, a moisture resistant agent, a dedusting oil, a colorant, a corrosion inhibitor, a surfactant, a pH adjuster, and combinations thereof. The carbohydrate may be natural in origin and derived from renewable resources. Additionally, the carbohydrate polymer may have a dextrose equivalent (DE) number from 2 to 20. In at least one exemplary embodiment, the carbohydrate is a water-soluble polysaccharide such as dextrin or maltodextrin and the crosslinking agent is citric acid. Advantageously, the carbohydrates have a low viscosity and cure at moderate temperatures. The environmentally friendly, formaldehyde-free binder may be used in the formation of insulation materials and non-woven chopped strand mats. A method of making fibrous insulation products is also provided.
Owner:OWENS CORNING INTELLECTUAL CAPITAL LLC
Bioadhesive drug delivery system with enhanced gastric retention
InactiveUS20050064027A1Prolonged gastric retention timeHigh retention rateBiocideCosmetic preparationsWhole bodyRetention time
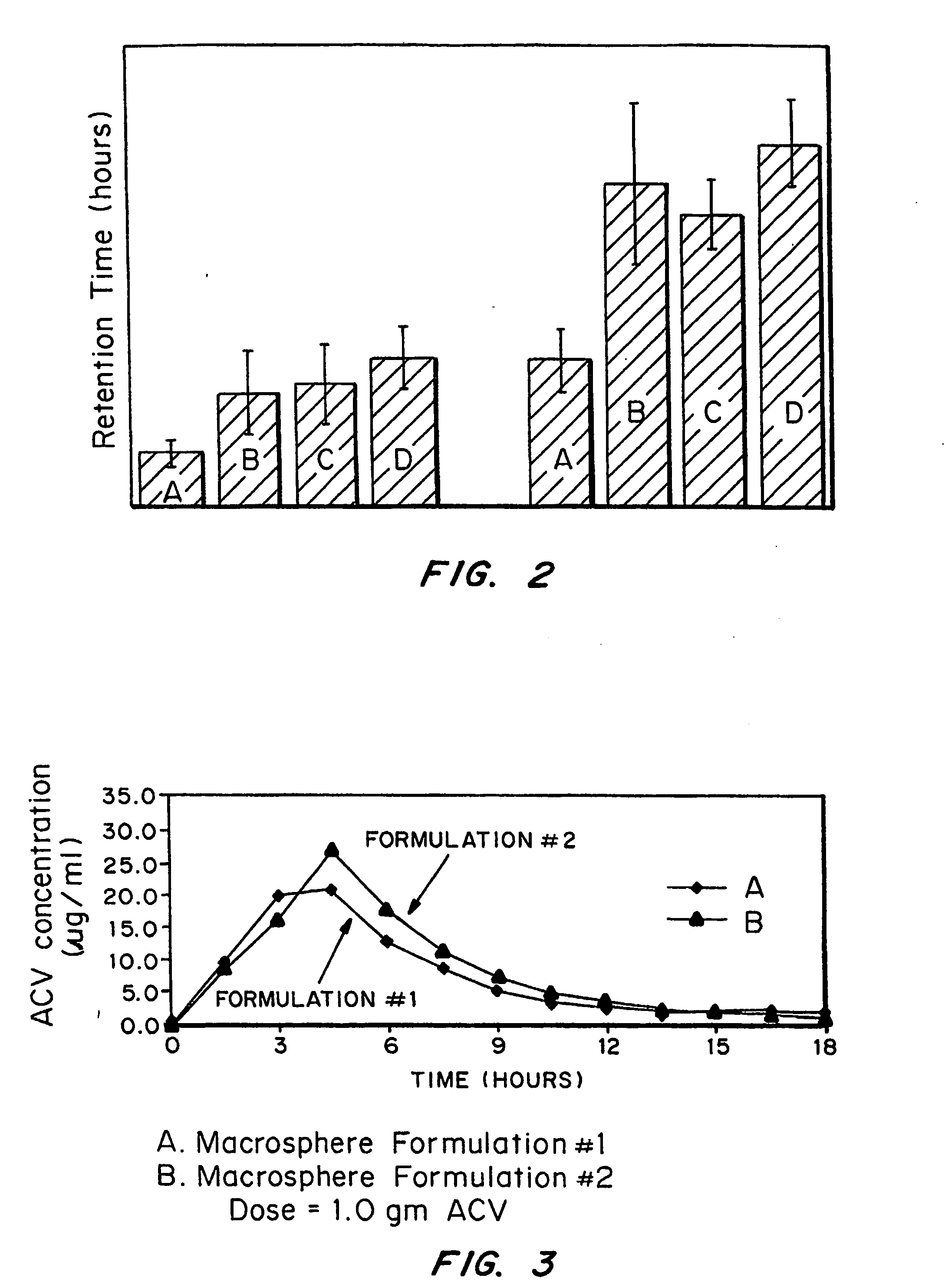
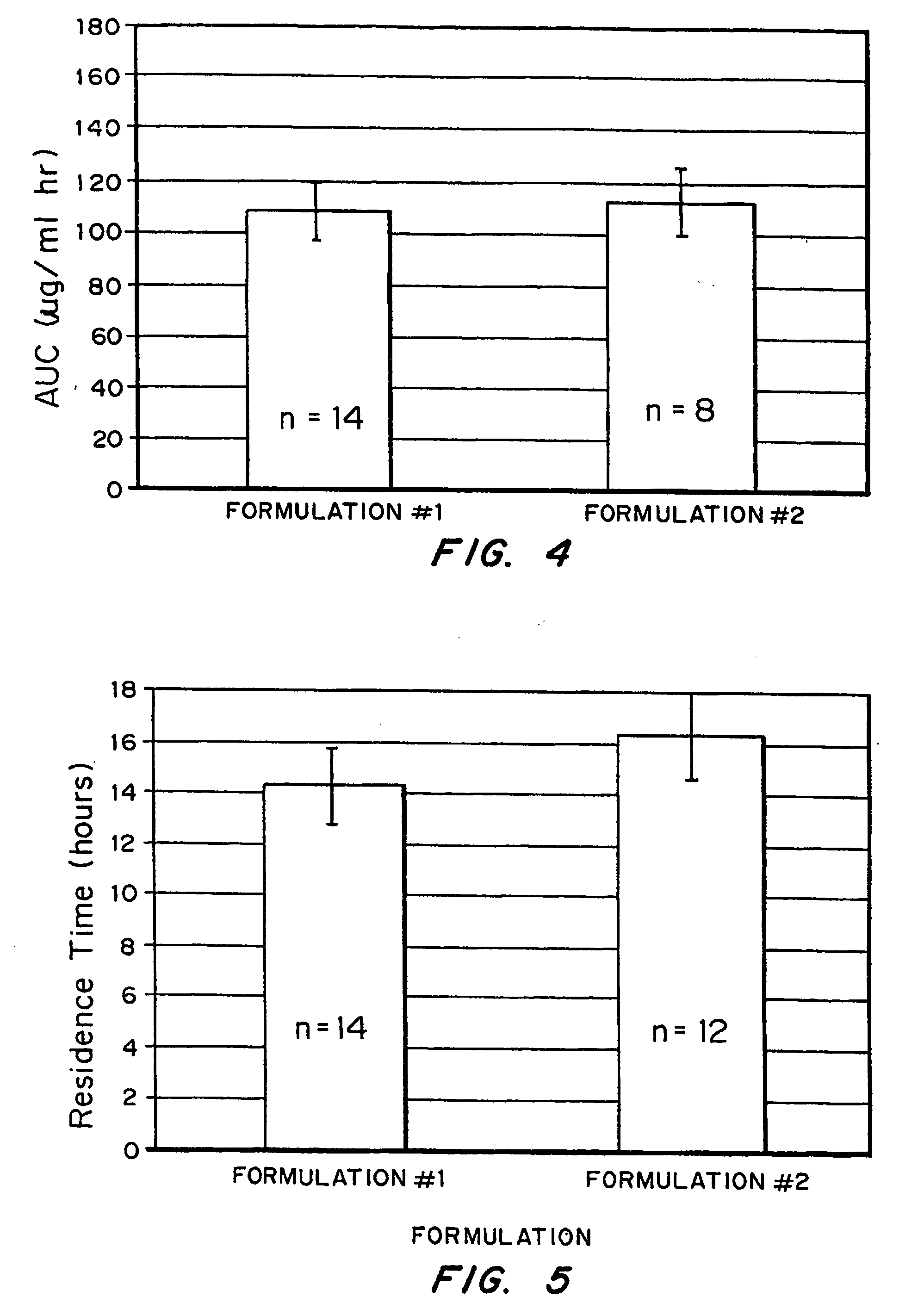
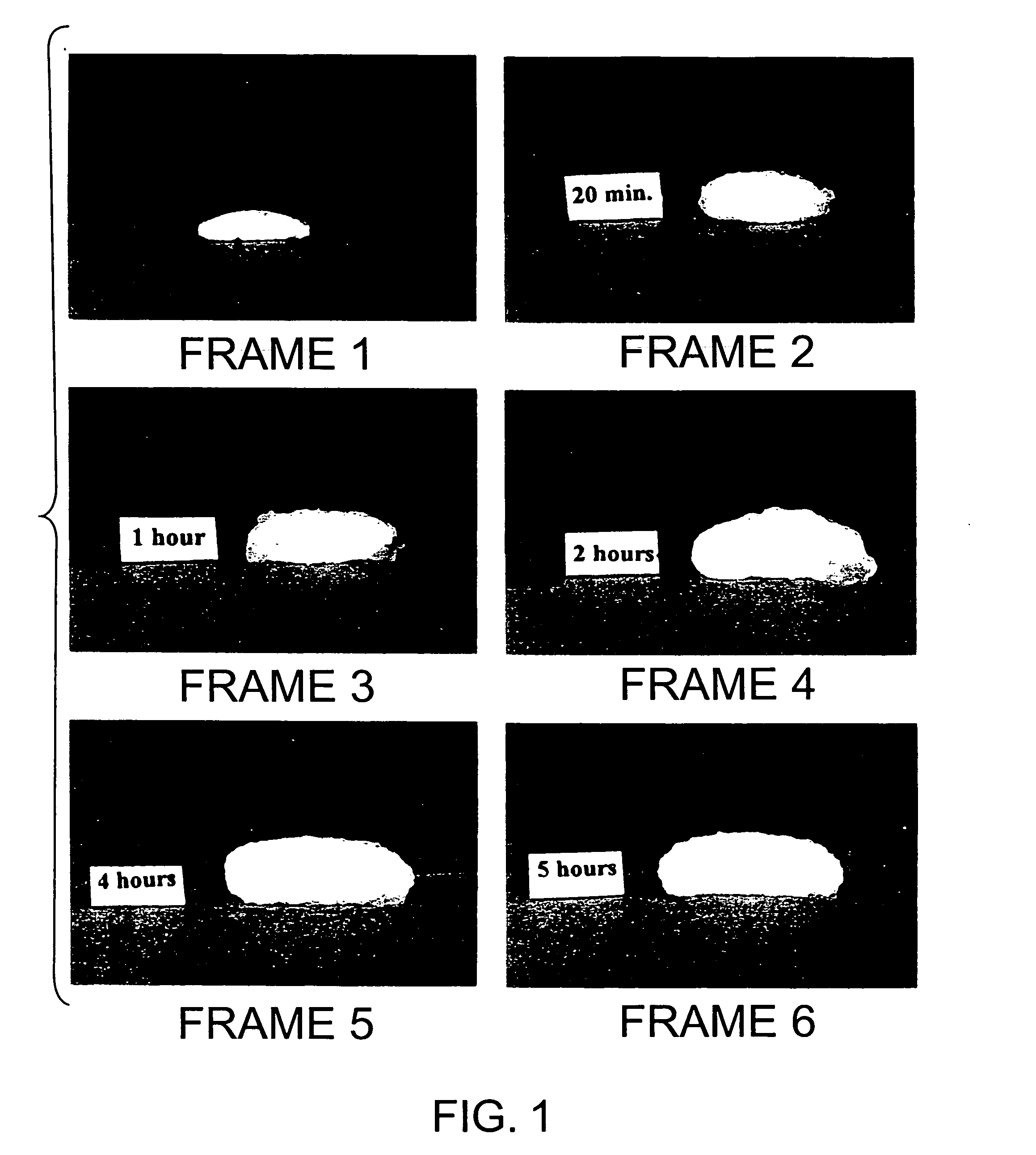
Bioadhesive macrosphere delivery systems (“BDDS”) having prolonged gastric retention time due to bioadhesion rather than physical density or size are described. In general, the macrospheres have diameters that are greater than 200 microns, more preferably greater than 500 microns. The bioadhesive macrospheres are released in the stomach where they reside in close proximity to the gastric mucosa for a prolonged period of time. Increased residence of BDDS in the upper GI can lead to increased systemic absorption of drug in the preferred site of systemic absorption, namely the upper GI tract (upper to mid-jejunum). The BDDS may be engineered either as a capsule with drug delivery controlled by a diffusion-limited membrane or degradable shell, or as a solid matrix system with drug delivery controlled by a combination of diffusion and polymer degradation kinetics.
Owner:SPHERICS
Bioadhesive progressive hydration tablets
InactiveUS20070031491A1Facilitated releasePharmaceutical delivery mechanismBioadhesiveBioavailability
A bioadhesive controlled, extended release progressive hydration composition wherein the active ingredient may be protected from water or the surrounding environment, thereby protecting it from metabolism or from other degradation caused by moisture, enzymes, or pH effects, and making it bioavailable only at a controlled rate. The active ingredient may be protected from moisture during the manufacturing process, as necessary or desired, and more importantly may be protected from moisture and the immediate septic environment until well after the patient has applied the composition, and then only at a slow and controlled rate. It is by this process of progressive hydration that the active ingredient remains protected for many hours after administration. It is also by the process of progressive hydration that controlled and sustained release is achieved because only that part of the active ingredient that is the hydrated (aqueous) fraction of the composition is available for absorption (bioavailable).
Owner:JUNIPER PHARMA INC
Use of bioadhesives and adjuvants for the mucosal delivery of antigens
InactiveUS20050281843A1Efficient methodImproving immunogenicitySsRNA viruses negative-senseBacterial antigen ingredientsAntigenAdjuvant
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Medical prosthetic devices and implants having improved biocompatibility
Disclosed are medical prosthetic devices or medical implants which exhibit improved biocompatitibly. The devices or implants include a metal material, e.g. titanium, in which the metal surface parts are coated with a corresponding hydride material that contains one or more biomolecule substance. This biomolecule substance may contain one or more biologically active molecules, e.g. bio-adhesives, biopolymers, blood proteins, enzymes, extra cellular matrix proteins, extra cellular matrix biomolecules, growth factors and hormones, peptide hormones, deoxyribonucleic acids, ribonucleic acids, receptors, enzyme inhibitors, drugs, biologically active anions and cations, vitamins, adenosine monophosphate (AMP), adenosine diphosphate (ADP), adenosine triphosphate (ATP), marker biomuolecules, amino acids, fatty acids, nucleotides (RNA and DNA bases), or sugars.
Owner:STRAUMANN HLDG AG
Double-Layered Absorbable Solid Compositions for the Topical Treatment of Oral Mucosal Disorders
Bioadhesive sticker tablets which are applied directly to vaginal, rectal and / or oral mucosa are described herein. In one embodiment, the sticker tablets are applied directly to ulcers or lesions in the oral cavity. The compositions adhere immediately upon administration, swell over time, and remain adherent to the ulcer or lesion for at least 60 minutes. The compositions can be in the form of single layer, double layer, or multilayer sticker tablets. The compositions provide immediate pain relief to the patient and promote rapid healing of the ulcer or lesion. The sticker tablet compositions contain one or more bioadhesive polymers. In one embodiment, the polymers are crosslinked polycarboxylic acids and polyols. The compositions contain at least one herbal agent and / or irritating compound, and optionally, a non-herbal active agent. The compositions can deliver an non-irritating effective dose of the agent for at least 60 minutes. The compositions described herein are stable upon storage for six months or longer.
Owner:AXIOMEDIC
Bioadhesive Composition Formed Using Click Chemistry
The present disclosure provides compositions which may be utilized as adhesives or sealants in medical and surgical applications. The compositions may, in embodiments, be formed from the cycloaddition reaction of a first component possessing at least one azide group with a second component possessing at least one alkyne group.
Owner:TYCO HEALTHCARE GRP LP
Bioadhesive progressive hydration tablets and methods of making and using the same
InactiveUS6248358B1Protect environmentOrganic active ingredientsNervous disorderBioadhesiveBULK ACTIVE INGREDIENT
Owner:COLUMBIA LAB BERMUDA
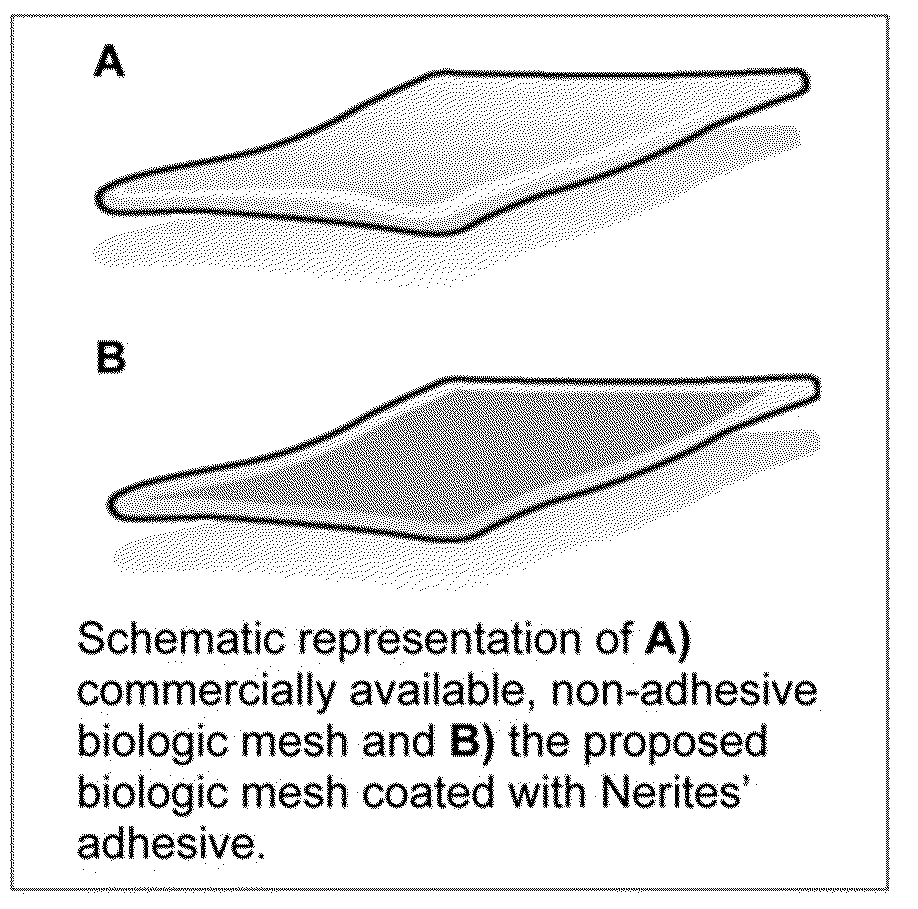
Bioadhesive constructs
ActiveUS20100137902A1Reduce and eliminate needAvoid nerve damageSurgical adhesivesSynthetic polymeric active ingredientsBioadhesiveProsthesis
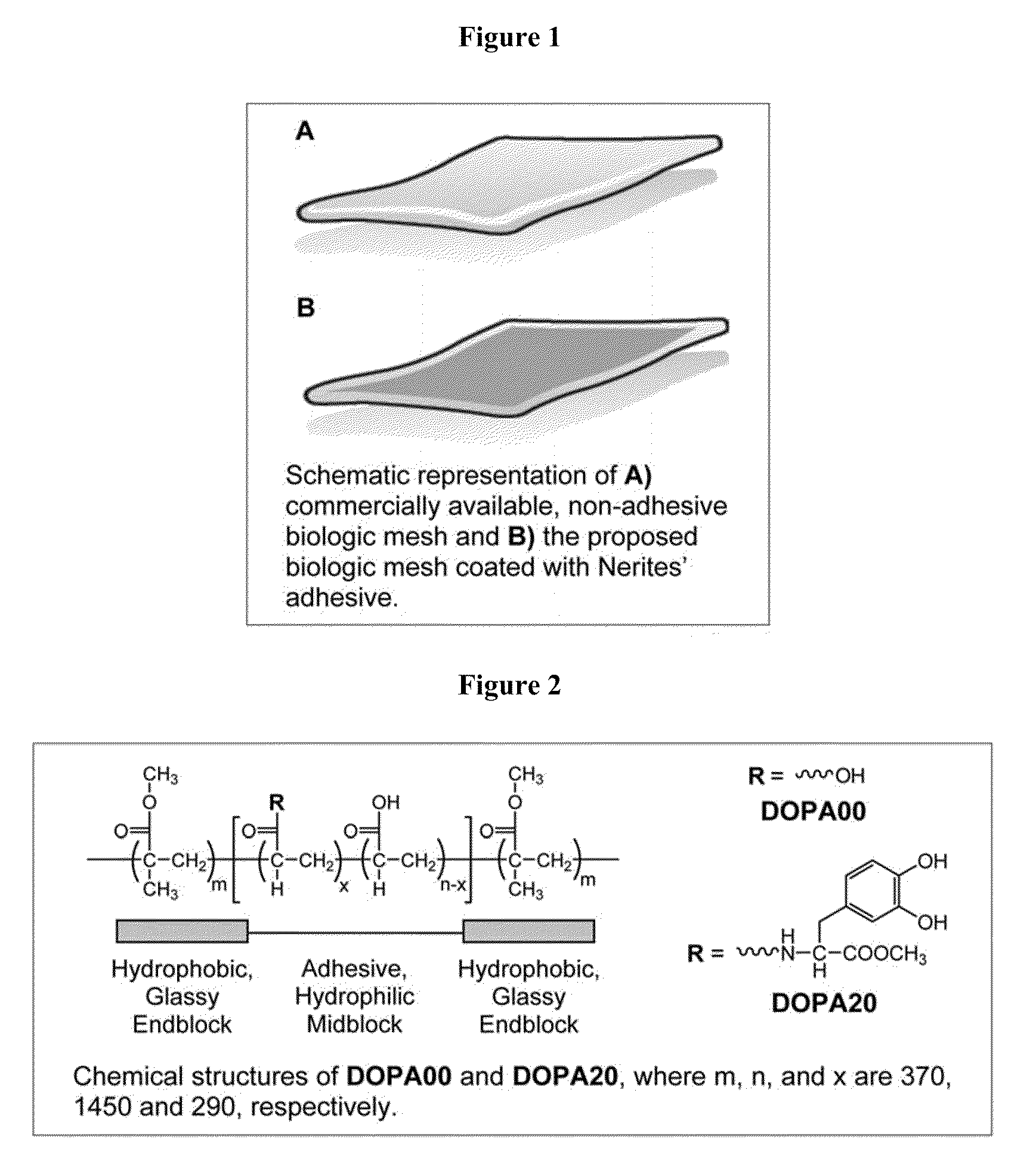
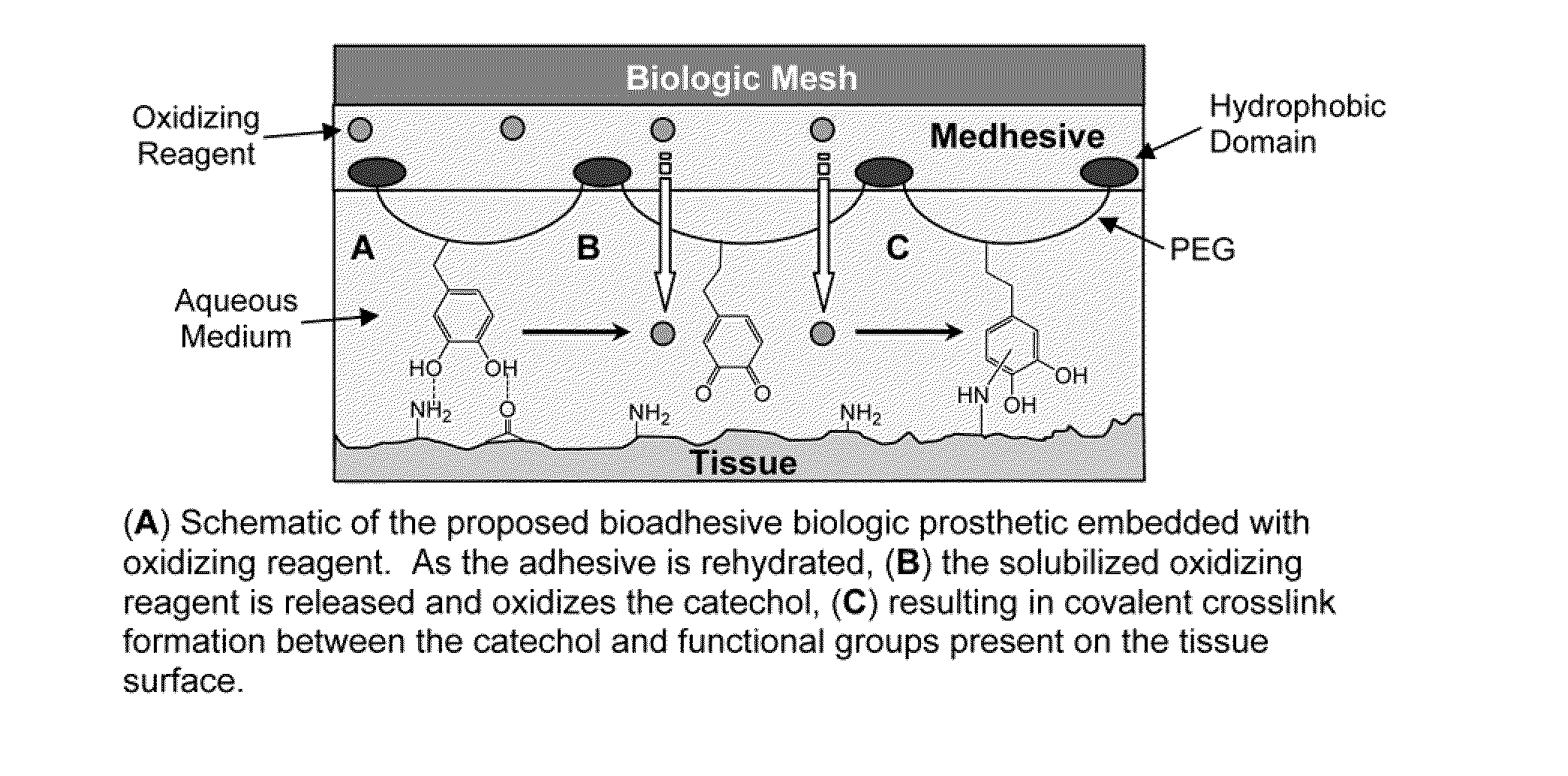
The invention describes substrates, such as prosthetics, films, nonwovens, meshes, etc. that are treated with a bioadhesive. The bioadhesive includes polymeric substances that have phenyl moieties with at least two hydroxyl groups. The bioadhesive constructs can be used to treat and repair, for example, hernias and damaged tendons.
Owner:DSM IP ASSETS BV
Mussel mucoprotein liquid product as well as preparation method and application thereof
InactiveCN103520766AImprove stabilityHigh molecular weightSurgical adhesivesPeptide preparation methodsLiquid productProtein molecules
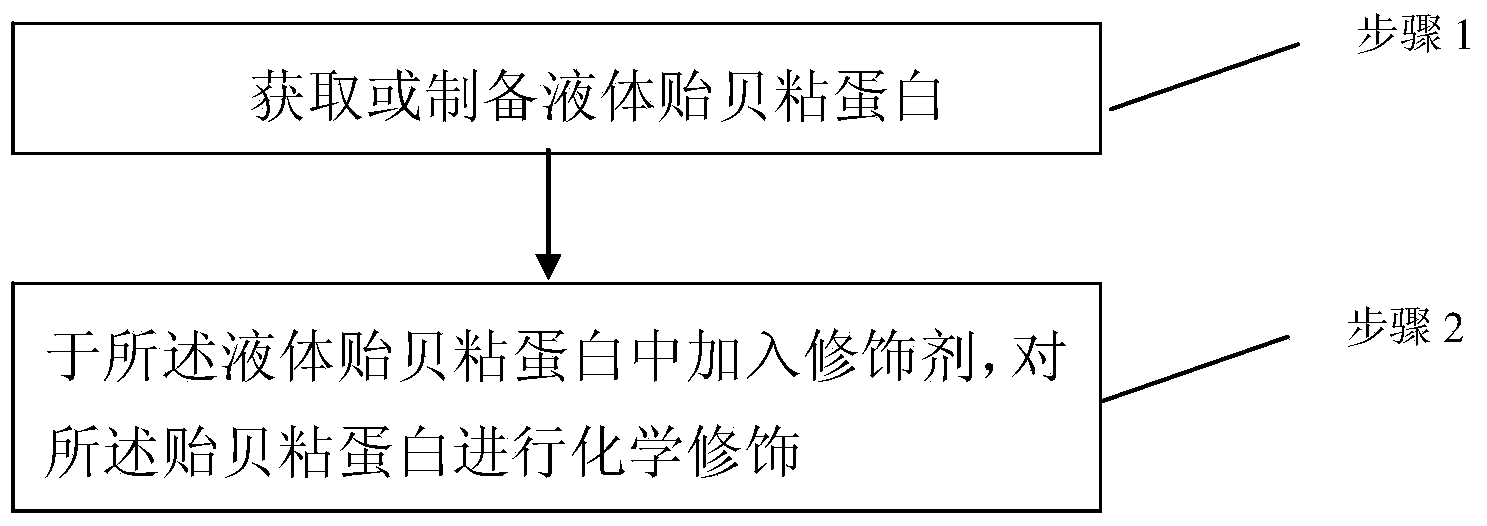
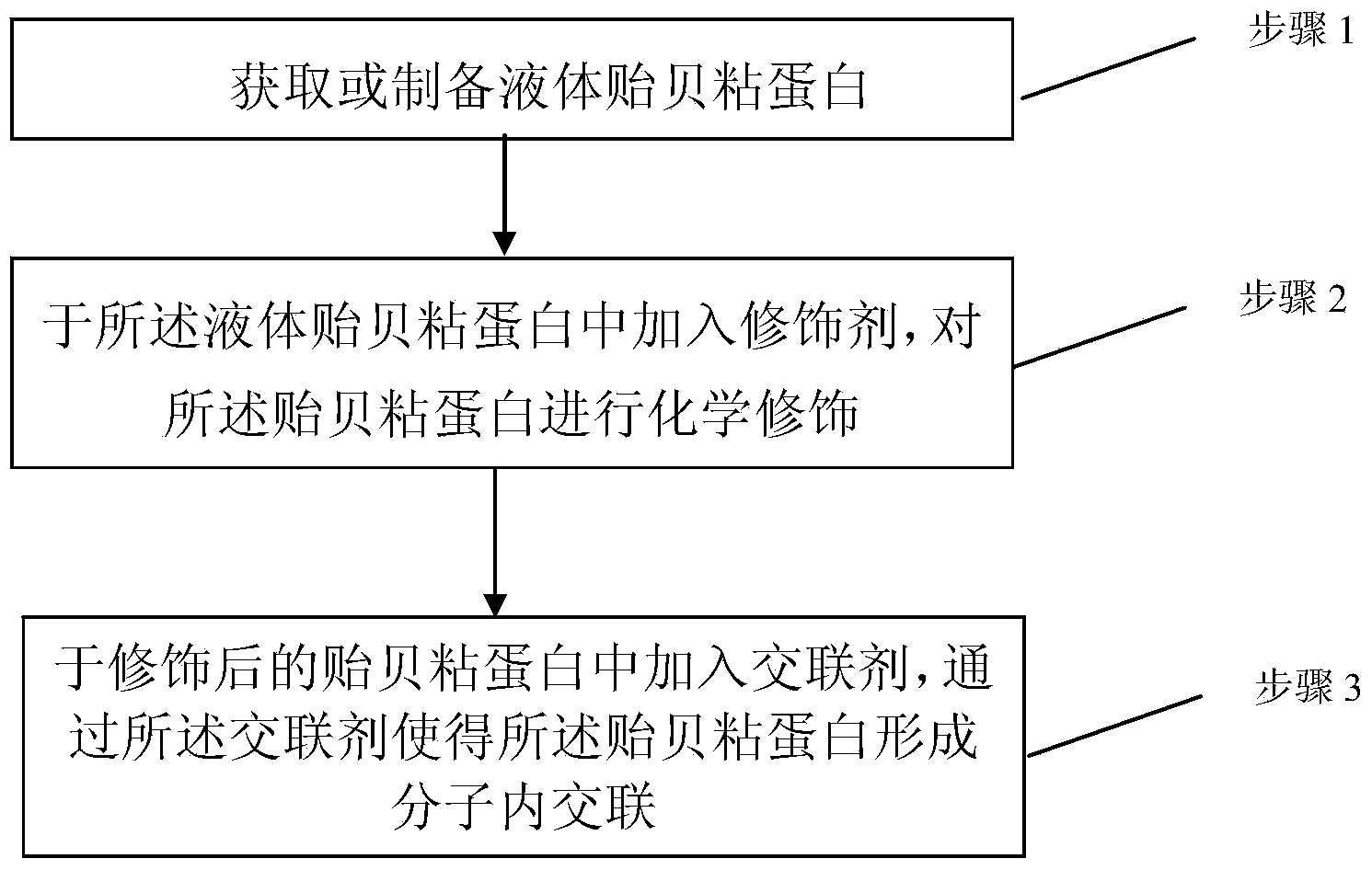
The invention discloses a mussel mucoprotein liquid product as well as a preparation method and an application thereof. One or more of a modifier, an additive and a crosslinker are added into mussel mucoprotein to form a new product which can serve as a wound repair product, a wound protection product, a medical biological bonder product, a medical coating product, an industrial coating product, a biochemical reagent product or the like. According to the mussel mucoprotein liquid product and the preparation method of the mussel mucoprotein liquid product, the modifier, the additive and the crosslinker are added into the mussel mucoprotein, so that the stability of a protein structure is enhanced, the protein molecular weight is increased, and the mussel mucoprotein liquid product is widely applied in various fields.
Owner:高敏
Bioadhesive constructs
InactiveUS20100137903A1Facilitate physiological reformationPromote crosslinking of the multihydroxy phenyl groupsOrganic active ingredientsSurgical adhesivesProsthesisBioadhesive
The invention describes substrates, such as prosthetics, films, nonwovens, meshes, etc. that are treated with a bioadhesive. The bioadhesive includes polymeric substances that have phenyl moieties with at least two hydroxyl groups. The bioadhesive constructs can be used to treat and repair, for example, hernias and damaged tendons.
Owner:KNC NER ACQUISITION SUB
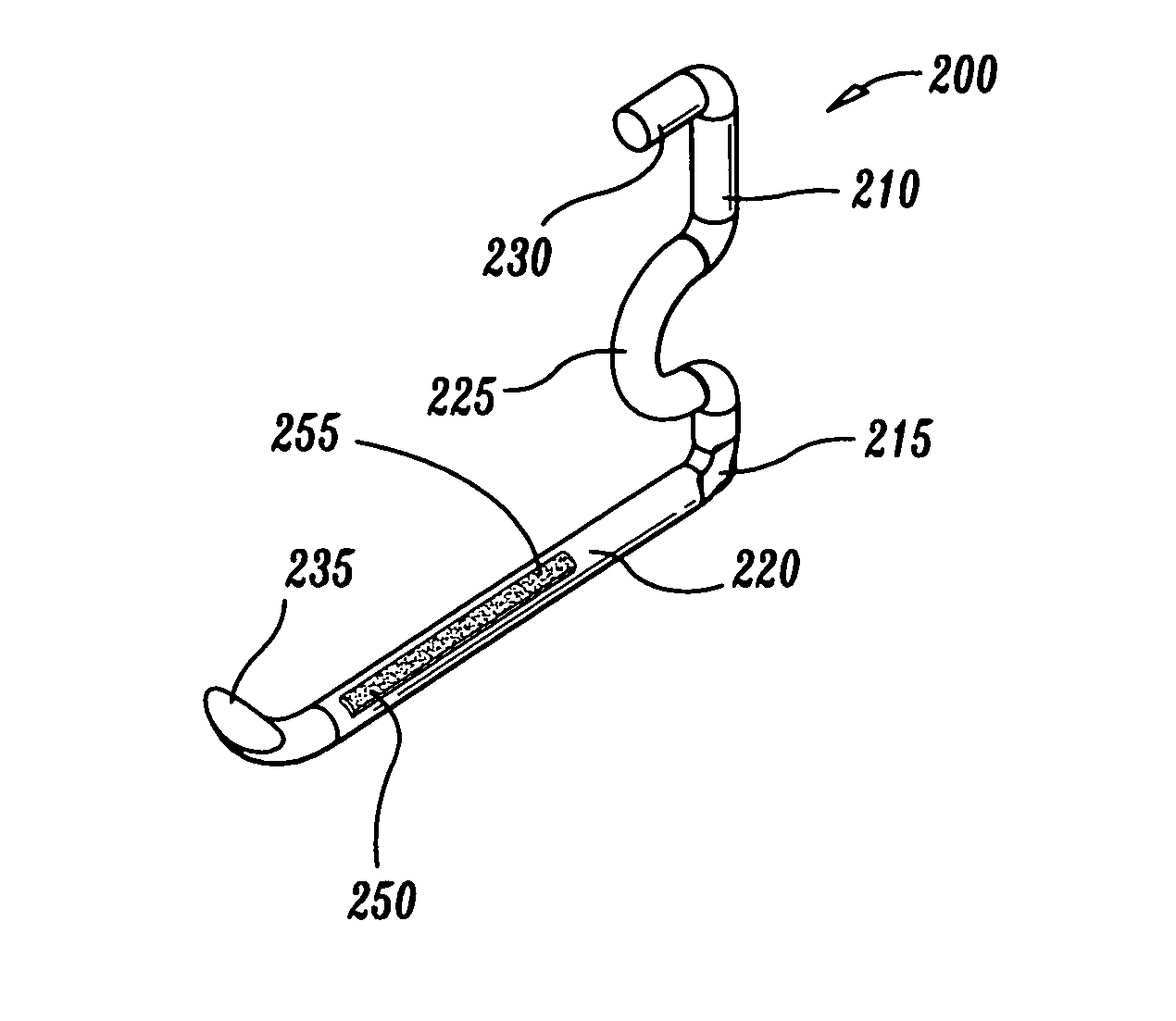
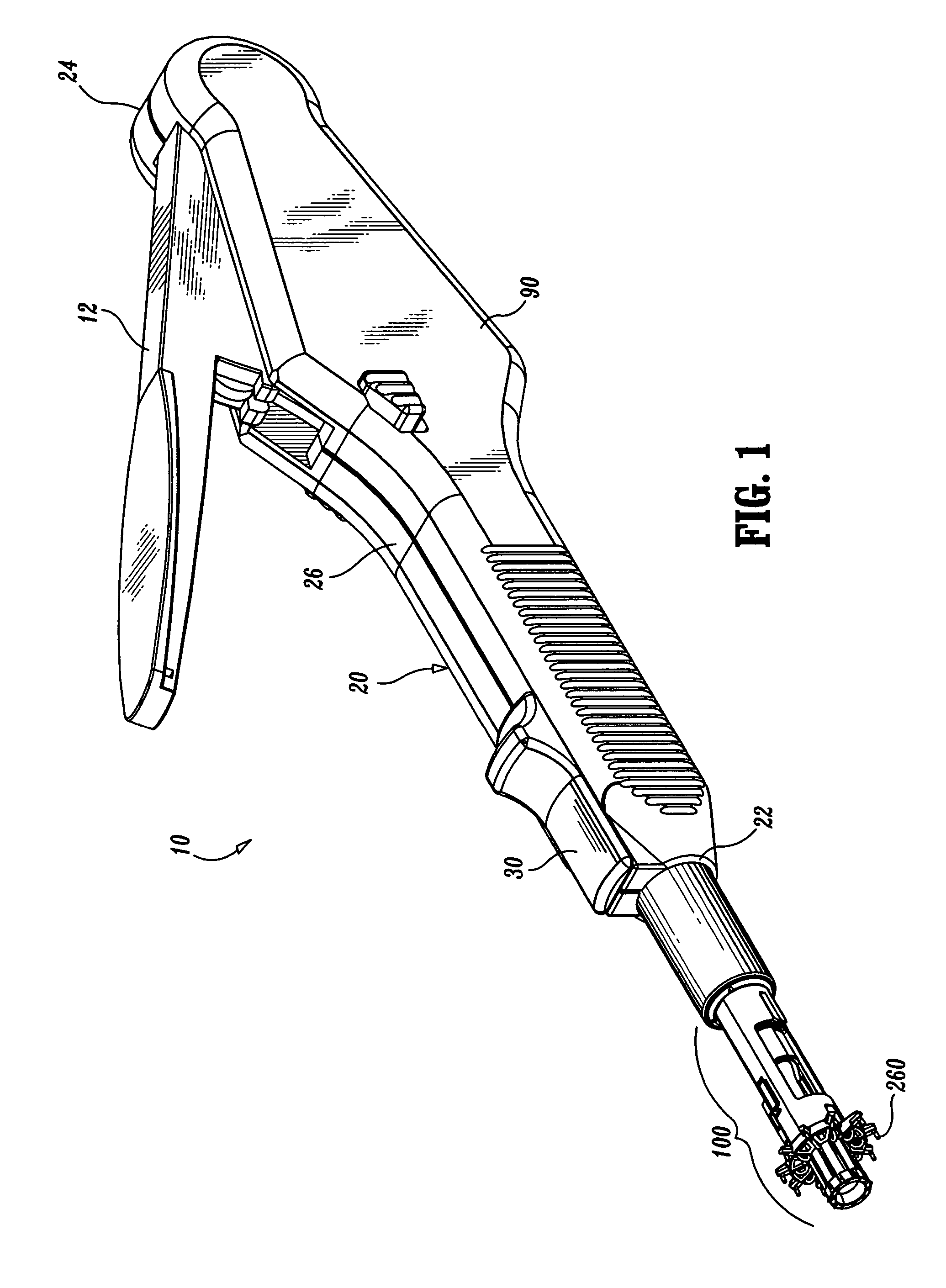
Fibrin applicator pistol
InactiveUS6863660B2Reliable deliveryNanotechPeptide/protein ingredientsSuspended particlesFibrin glue
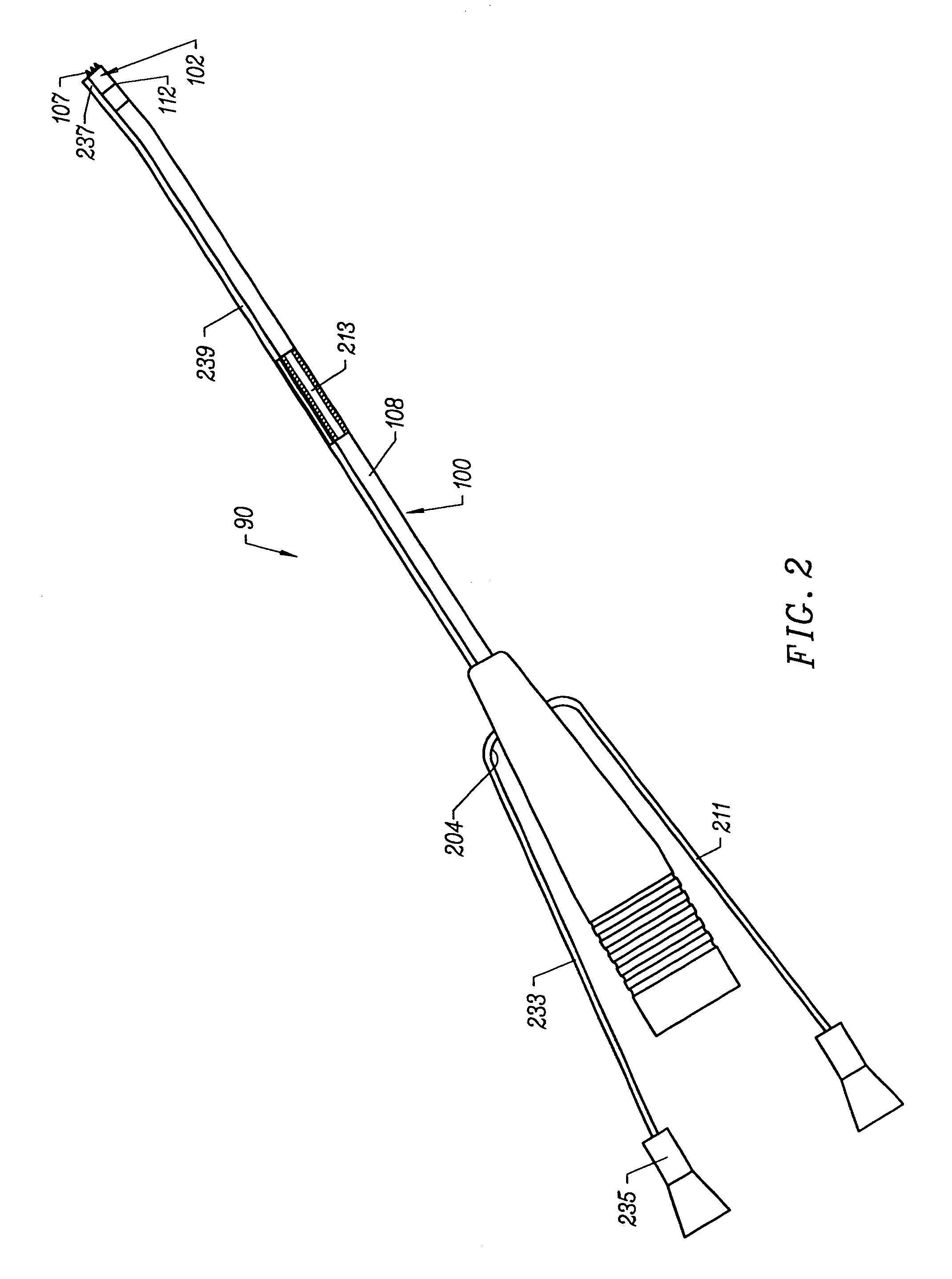
An applicator for dispensing a first and second component of a biological adhesive, such as fibrin glue. At least one of said components may contain a suspension of fibrin microbeads (FMB) or a suspension of cells. The present invention uses a single supply of pressurized gas to force the components from the applicator using positive fluid pressure and to atomize them into a convergent spray. Another embodiment of the present invention also provides for the endoscopic application of the biological adhesive directly to tissue defects. The application of positive pressure allows precise metering of the components and application of the adhesive, prevents internal coagulation of the fibrin or clogging by suspended particles and reduces waste and contamination of the components.
Owner:HAPTO BIOTECH
Hydrophilic ampholytic polymer
InactiveCN1334830ACosmetic preparationsOrganic detergent compounding agentsHydrophilic monomerPersonal care
A novel hydrophilic ampholytic polymer synthesized by reacting polymerizable amino and carboxy functional ethylenically unsaturated monomers, together with a non-ionic hydrophilic monomer, to provide a polymer having a glass transition temperature (Tg) above about 50 DEG C, and optionally hydrophobic monomer(s), and cross-linking monomer(s). The copolymer is precipitated from a polymerization media which includes a suitable organic solvent. The resulting copolymer is in the form of a fine powder, with submicron particle size. As such it is suitable for use as a thickener or rheology modifier in personal care formulations, such as shampoo, conditioner, and the like, as a bioadhesive, and for other pharmaceutical applications.
Owner:PMD控股公司
Drug formulations for oral transmucosal delivery to pediatric patients
InactiveUS20090010992A1Easy to manageEffective drug administrationBiocideNervous disorderPediatric patientBioadhesive
Improved compositions, methods and systems for oral transmucosal administration of small volume bioadhesive drug dosage forms to pediatric subjects are provided. The drug dosage form is easily administered and may be delivered using a single dose applicator or a device.
Owner:ACEIRX PHARM INC
Biologic partial meniscus and method of preparation
InactiveUS20130304209A1Provide biomechanical strengthMetal rolling stand detailsSurgeryFibrin glueAdhesive
Biologic partial menisci (biologic partial meniscal replacements / constructs) used to replace at least a part of a meniscus, and methods of forming such biologic partial menisci. Meniscal cartilage is employed to form a moldable allograft paste. A sterile mold that replicates a meniscus (for example, the medial or lateral meniscus) is provided in various sizes and is used as a biologic mold to recreate the anatomic shape of the meniscus. The moldable paste is inserted (for example, injected) into the mold and allowed to set into a stable, anatomically shaped meniscus (the biologic partial meniscus). Fibrin glue or other biologic adhesives or strengtheners may be optionally added to provide further biomechanical strength. Once the biologic partial meniscus is removed from the mold, it is provided at the surgical site and attached to the excised meniscus (placed into the correct anatomical shape) to complete the meniscal repair.
Owner:ARTHREX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com