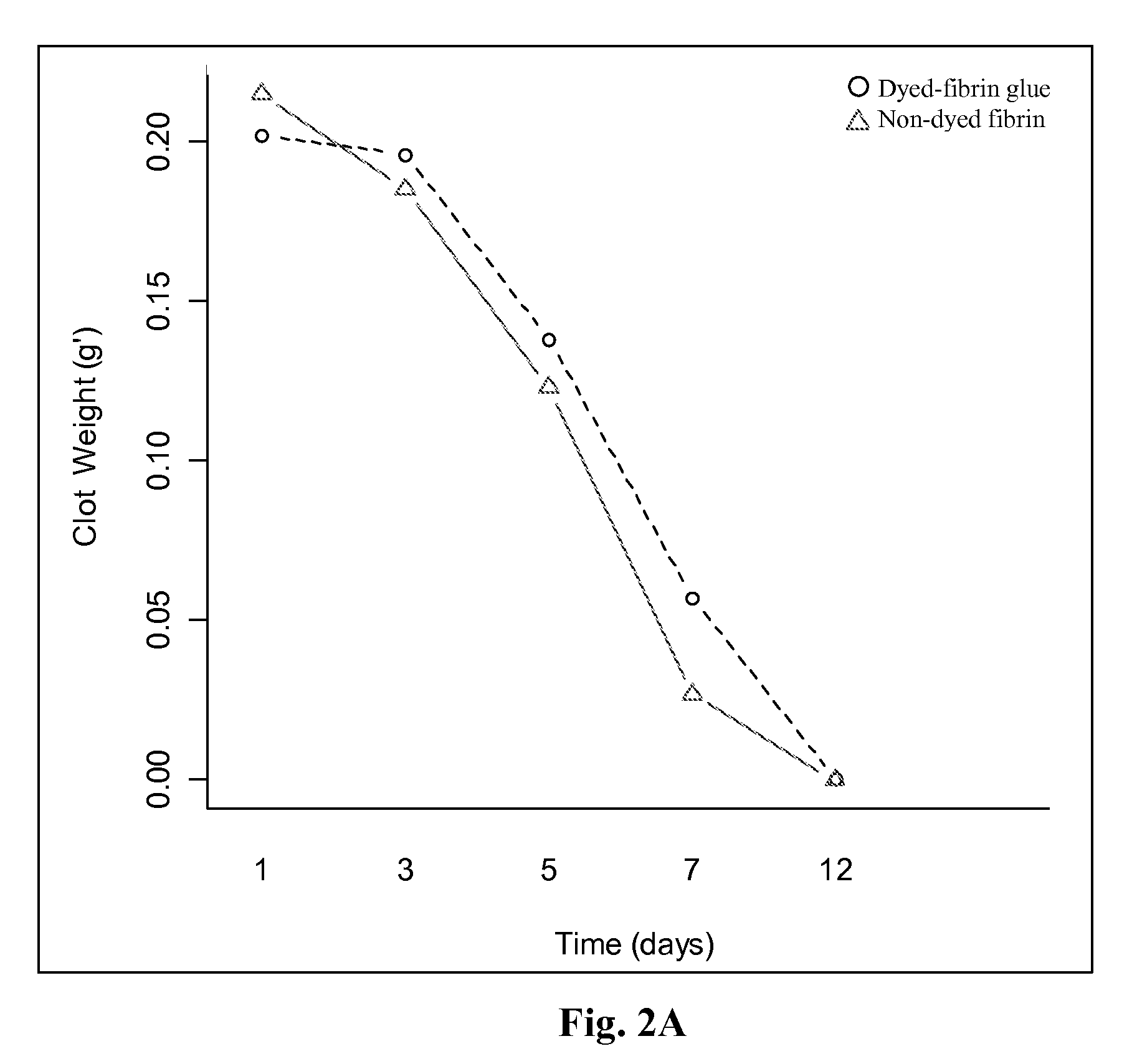
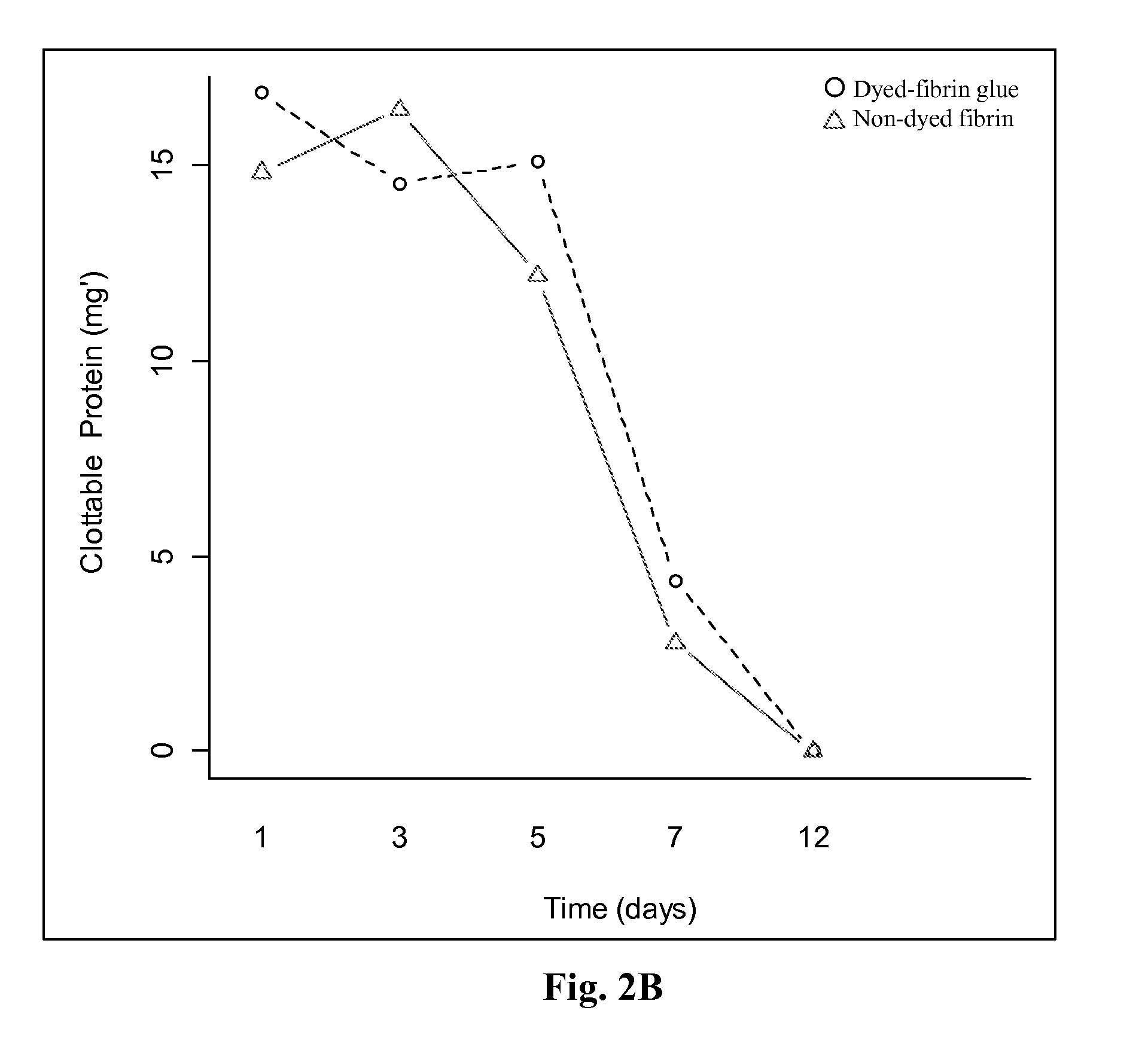
Patents
Literature
175 results about "Fibrin glue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fibrin glue (also called fibrin sealant) is a surgical formulation used to create a fibrin clot for hemostasis or wound healing.
Systems and methods for preparing autologous fibrin glue
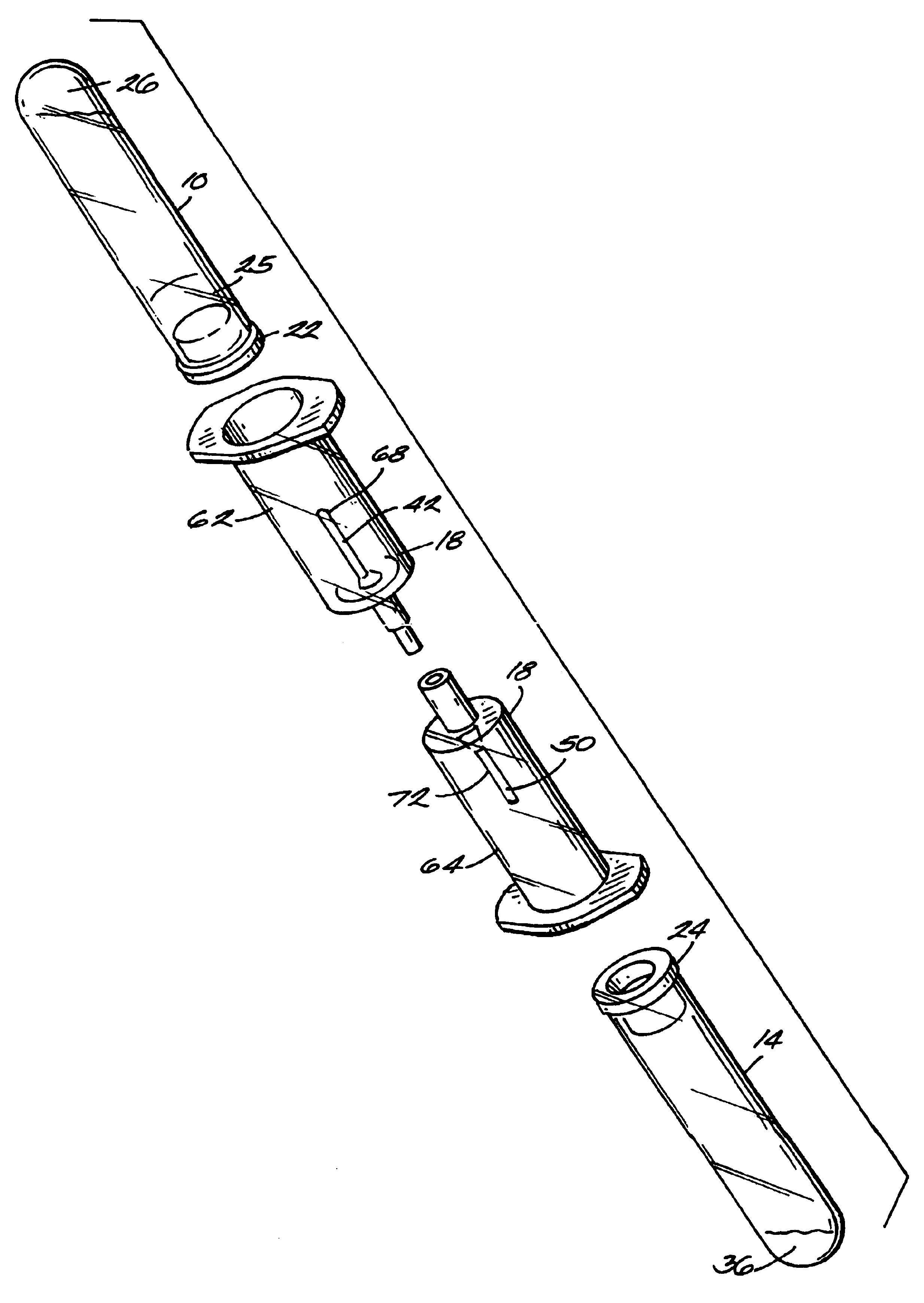
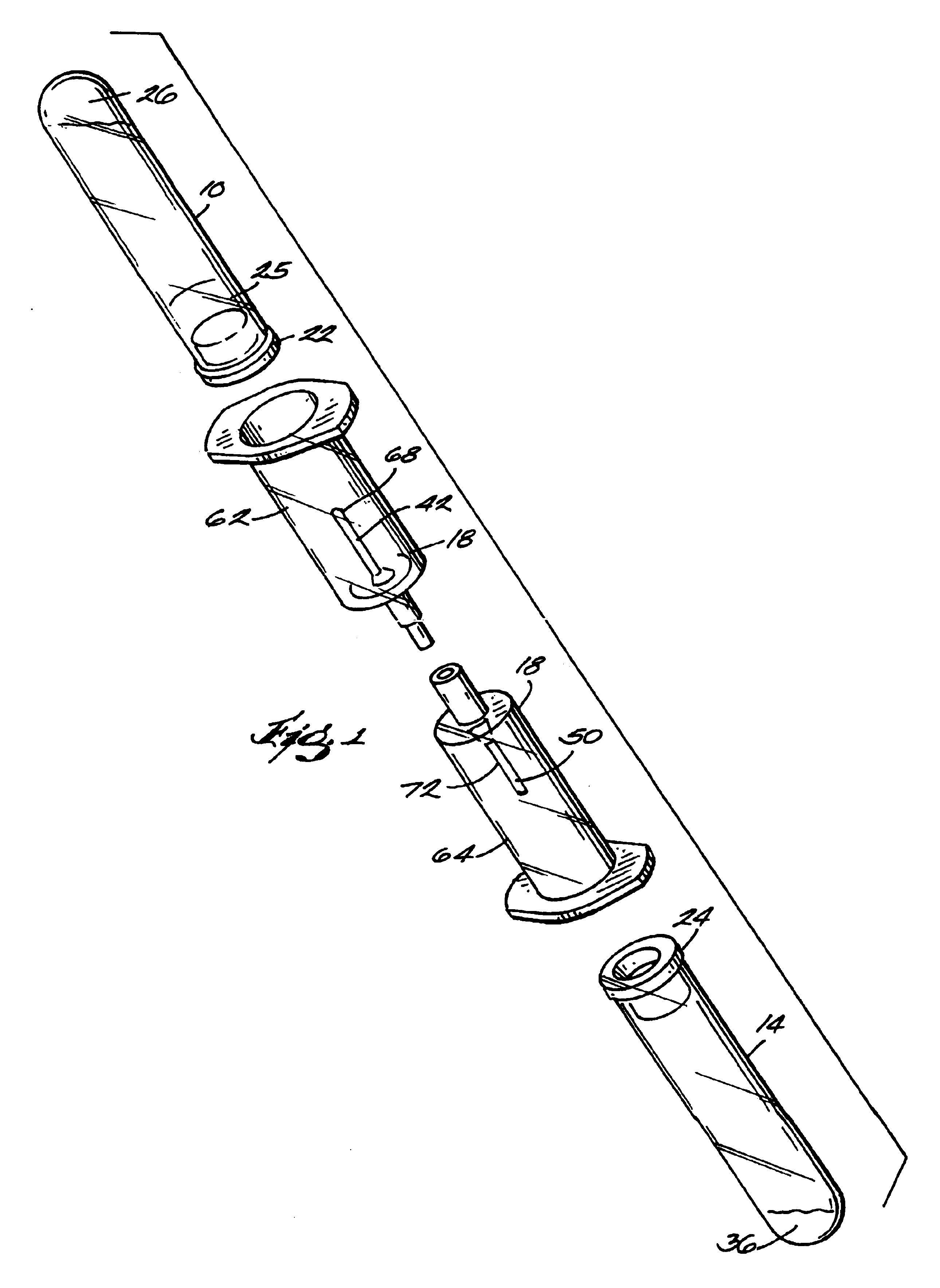
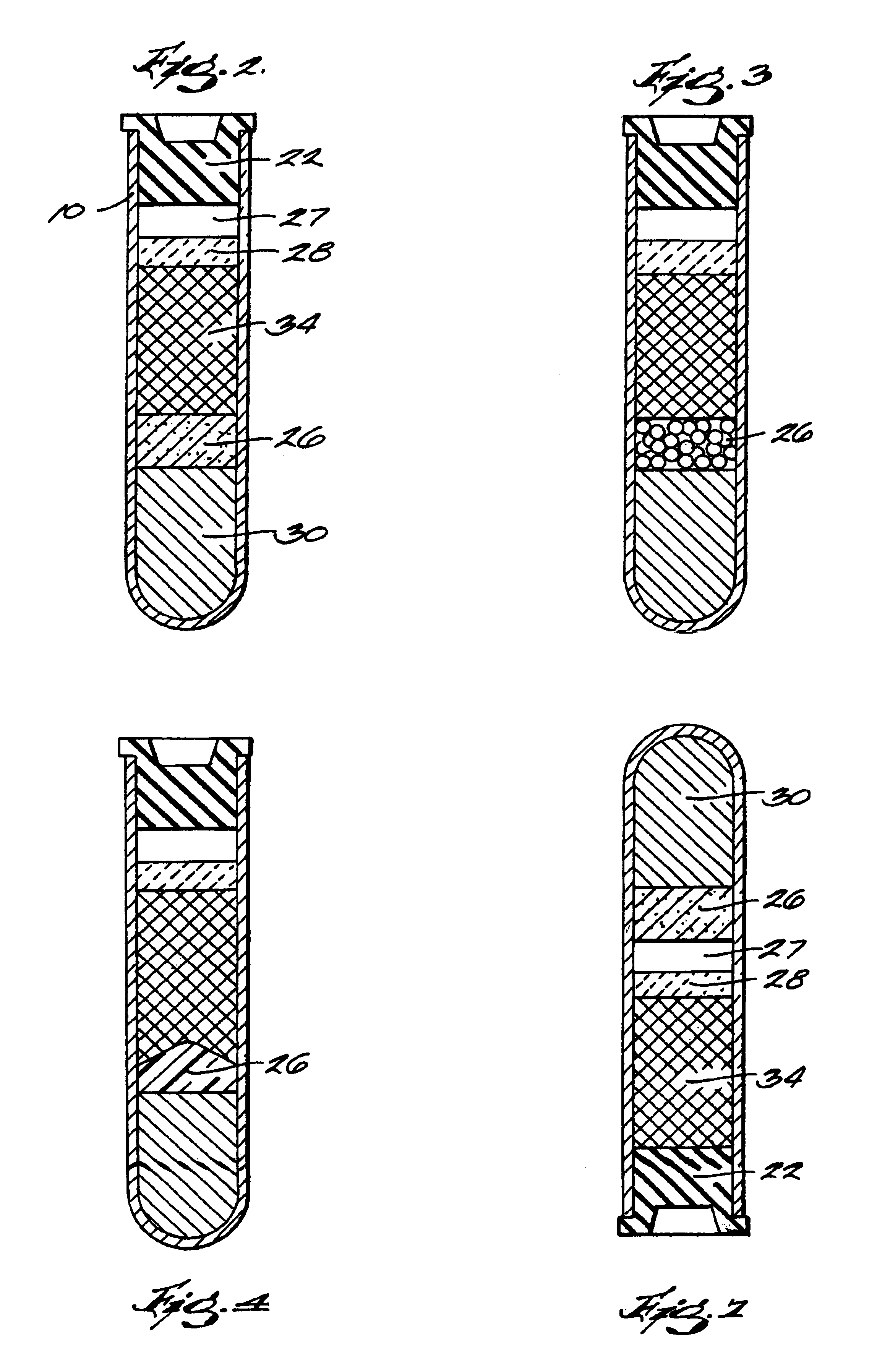
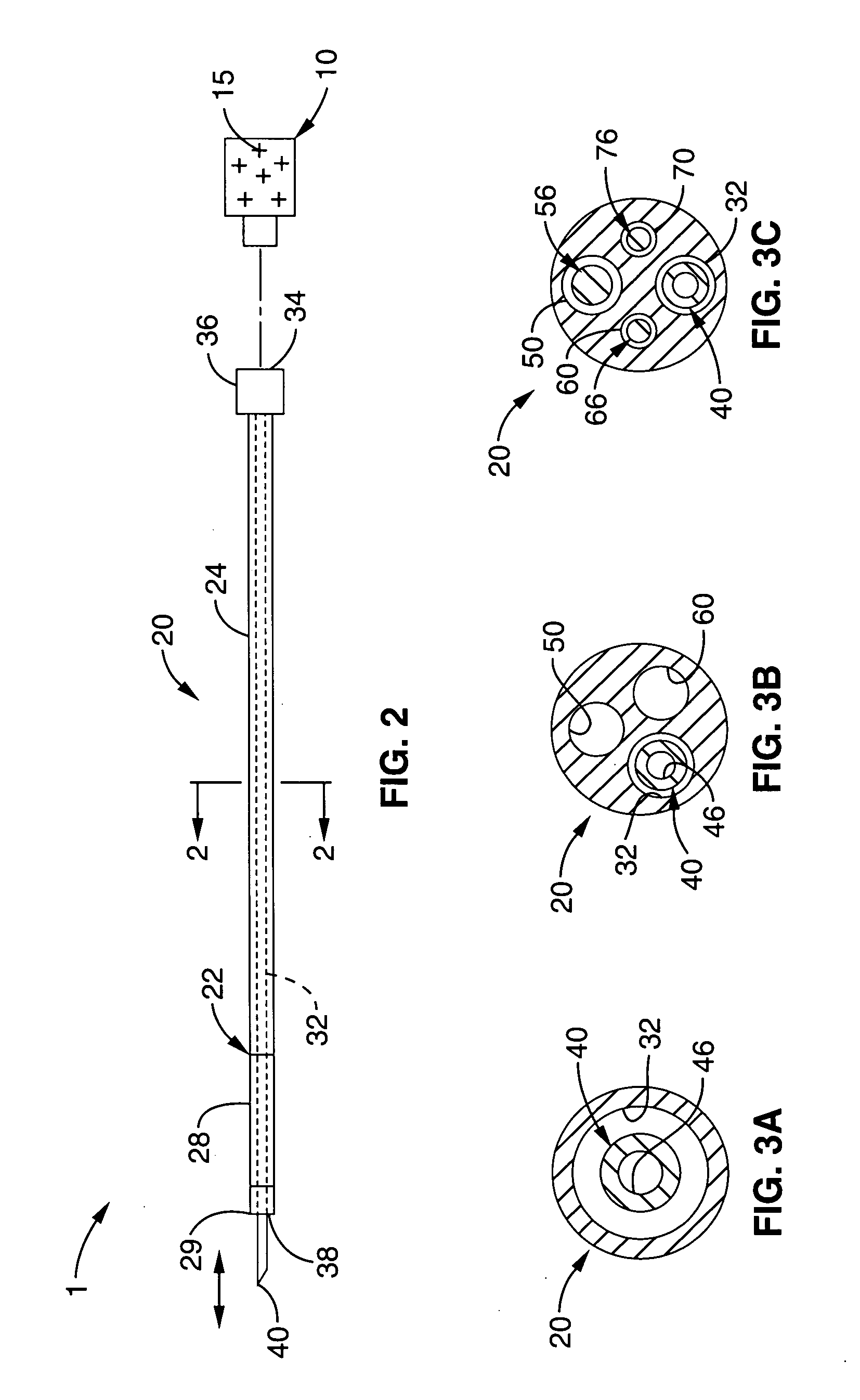
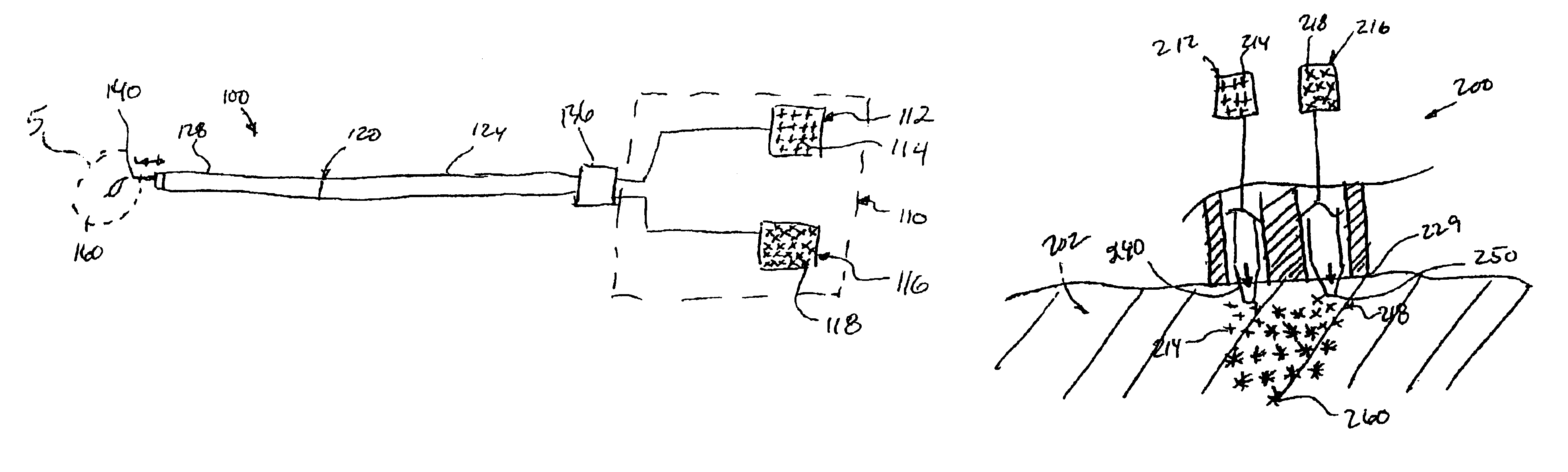
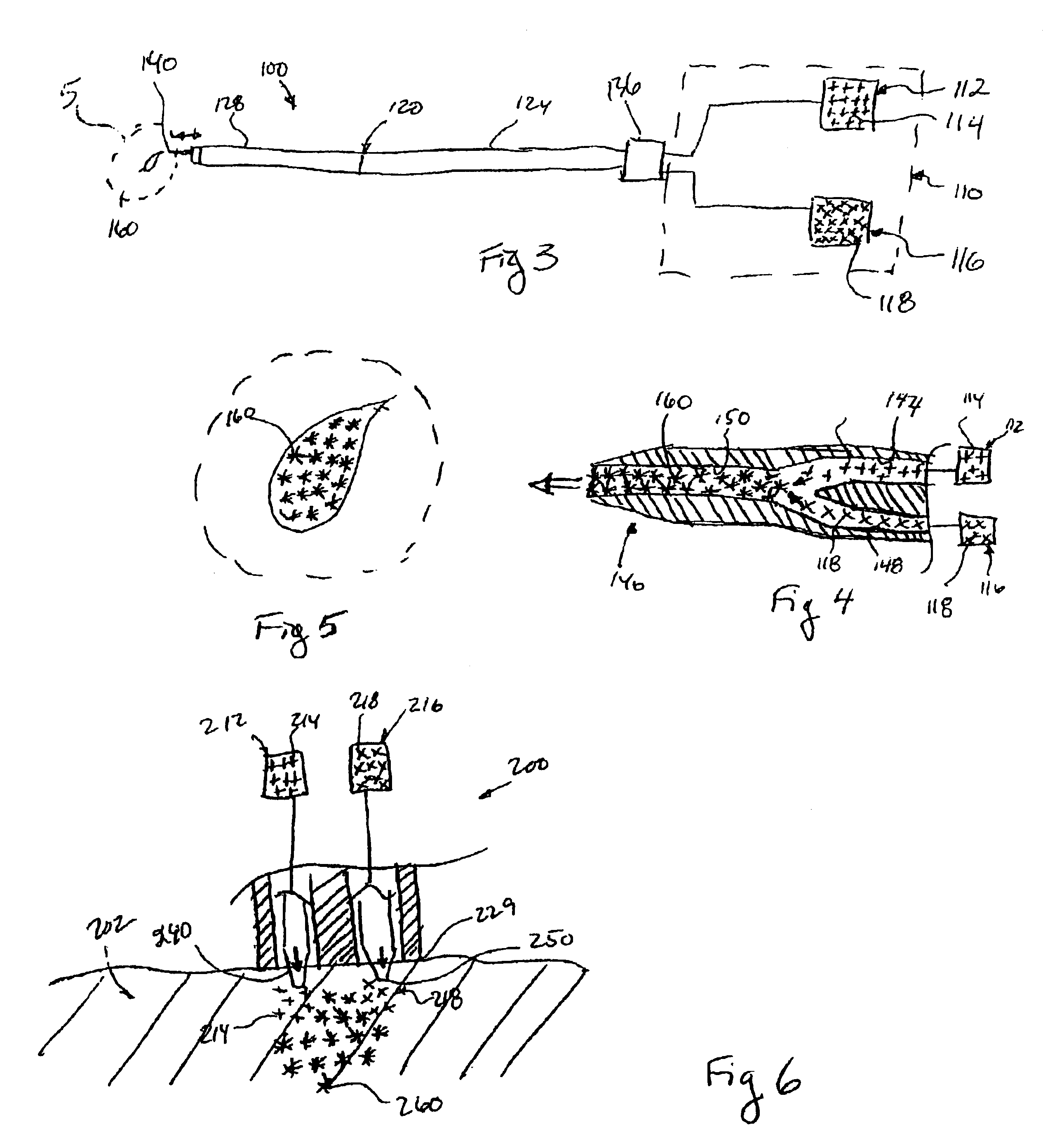
The invention provides a system for preparing an autologous solid-fibrin web suitable for regenerating tissue in a living organism. The system includes a sealed primary container containing a separation medium and a low-density high-viscosity liquid. The separation medium separates red blood cells from plasma when the container contains blood and is centrifuged, and the primary container has a first pressure. The system further includes a sealed secondary container containing a calcium-coagulation activator. The secondary container has a second pressure that is less than the first pressure. The system also comprises a transfer device including a cannula having a first end and a second end. The first and second ends puncture the sealed primary and secondary containers in order to provide fluid communication between the first and second containers. The low-density high-viscosity liquid of the primary container blocks flow through the cannula upon entering therein.
Owner:CASCADE MEDICAL ENTERPRISES
Fibrin glue without fibrinogen and biosealant compositions and methods
InactiveUS6168788B1Stop blood flowPrevents unwantedSurgical adhesivesPeptide/protein ingredientsFibrin glueClot formation
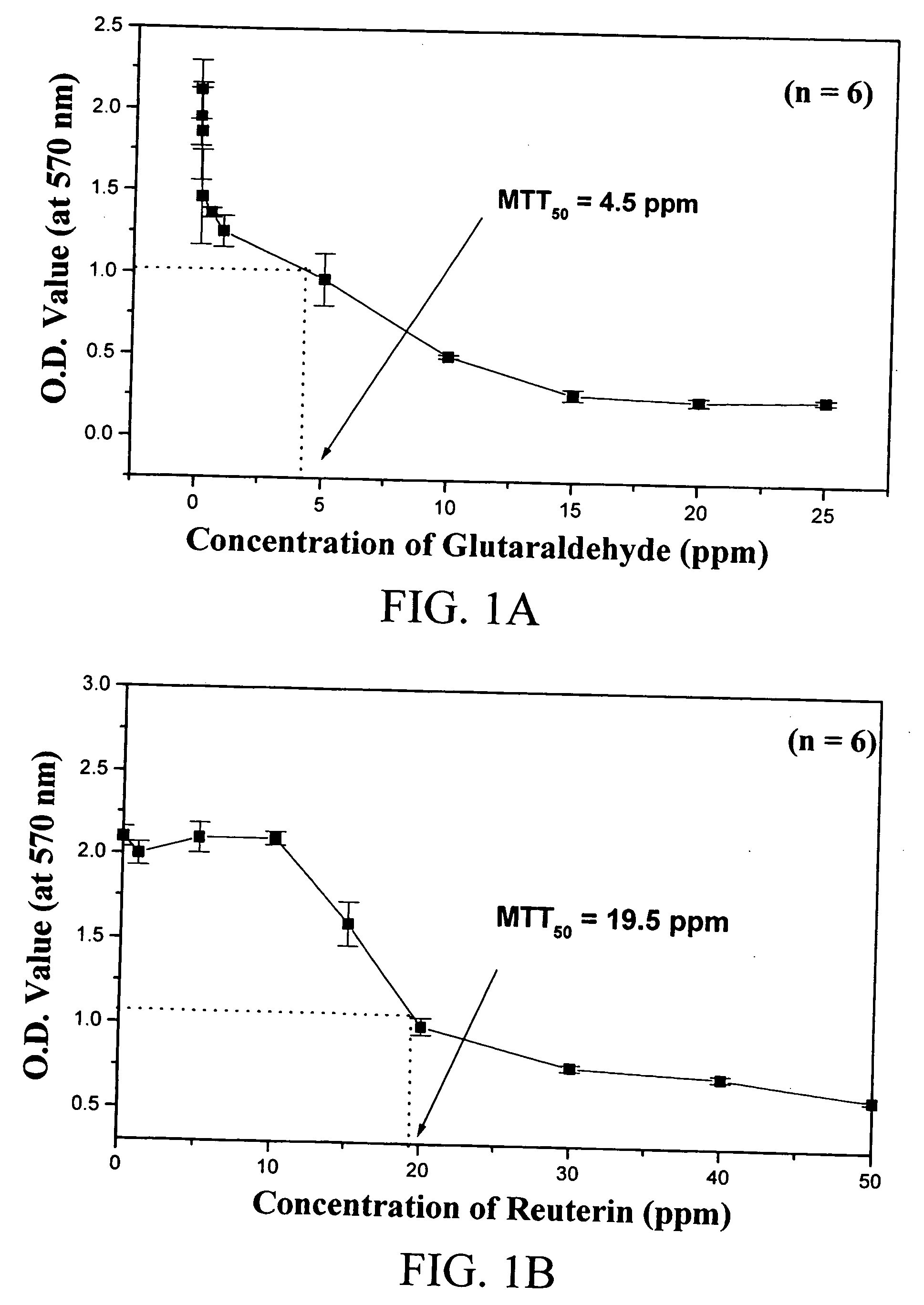
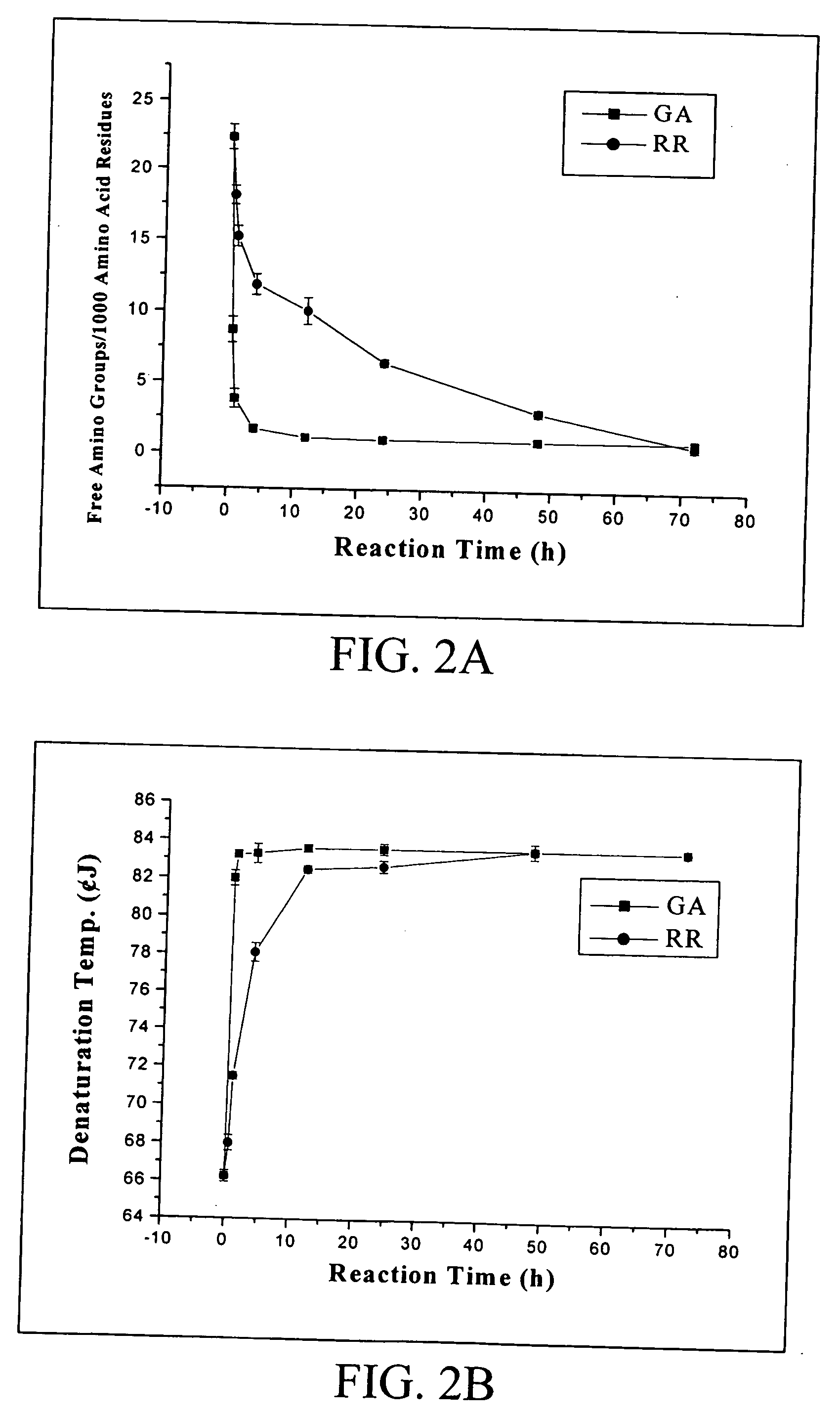
The invention is a fibrin glue that avoids the use of fibrinogen and thus eliminates the need for premixing and premature clot formation. The fibrin glue of the invention comprises thrombin, thromboplastin and calcium and may have clotting Factors, VII, IX and X, and the like. The invention also comprises a biosealant for use with the fibrin glue without fibrinogen or for use alone. The biosealant is a two component mixture of gelatin / resorcinol and glyoxal / glutaraldehyde / 4-(p-maleimidophenyl) butyric acid. The two components are mixed on use.
Owner:WORTHAM LEON
Material compositions and related systems and methods for treating cardiac conditions
InactiveUS20050271631A1Preventing negative remodelingHigh retention rateBiocideSurgical adhesivesInjectable polymersFibrin glue
A medical condition associated with a cardiac structure is treated by injecting an injectable polymer agent into the cardiac structure such that a therapeutic mechanical scaffolding is formed within the cardiac structure itself. In particular, the injectable scaffolding agent is a fibrin glue agent and is injected into regions of damaged myocardium such as ischemic tissue or infarct. LV wall dysfunction may also be treated by injecting the scaffolding agent into the LV wall. Cell therapy may be combined with the injection of fibrin glue or other injectable polymer scaffold agent. The polymeric forms of the agent may be injectable as precursor materials that polymerize as a scaffold in-situ within the cardiac structure. In other modes, polymer agents are injected in order to provide therapeutic angiogenesis, or to induce deposition of cells within the injected area, such as by providing the polymer with fragment E or RDG binding sites, respectively.
Owner:RGT UNIV OF CALIFORNIA
Biocolloid hemostatic prepared by aldehyde-modified sodium alginate and amine-modified gelatine
InactiveCN101716366AEasy to prepareRapid hemostasisAbsorbent padsBandagesFibrin glueBiocompatibility Testing
The invention relates to a biocolloid hemostatic prepared by aldehyde-modified sodium alginate and amine-modified gelatine. Sodium alginate and gelatine as raw materials; according to ratio, aldehyde-modified sodium alginate solution with the concentration of 5-25% and the same volume of amine-modified gelatine solution with the concentration of 5-40% are mixed; sodium alginate is modified by aldehyde through sodium periodate oxidation; and then gel is obtained by mixing with gelatine modified by ethanediamine. The adopted gelatine and sodium alginate are safe and stable, have good biocompatibility and can be absorbed and degraded by organism. Gel is rapidly formed through Schiff base reaction of aldehyde-modified sodium alginate and amine-modified gelatine, and the gel has the same gel-forming time as fibrin glue but better adhesion property and adhesion strength, can be acted on any part of body through simple local spraying or injection, and can be used as substitute product of fibrin glue.
Owner:TIANJIN UNIV
Method of preparing a collagen sponge, a device for extracting a part of a collagen foam, and an elongated collagen sponge
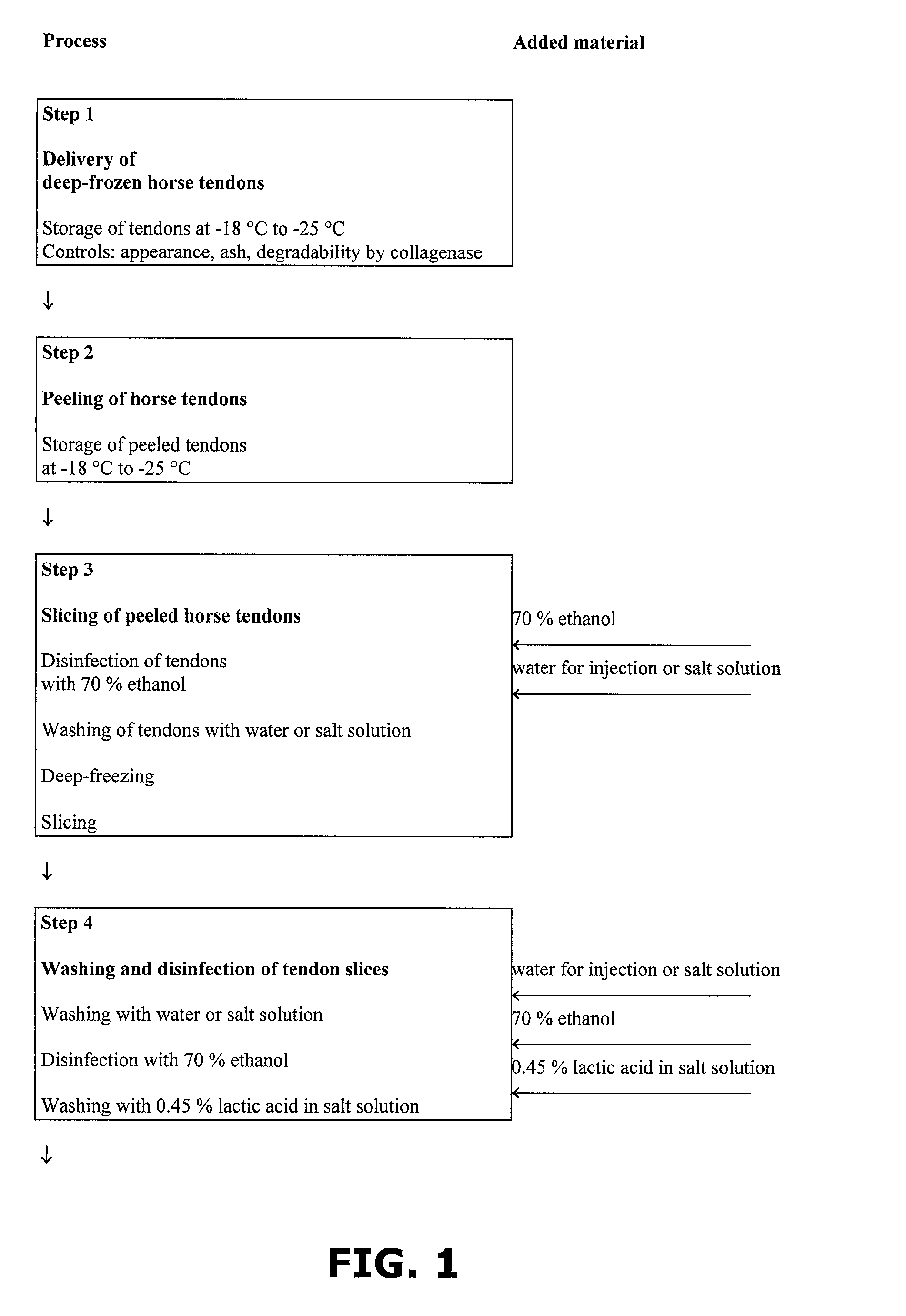
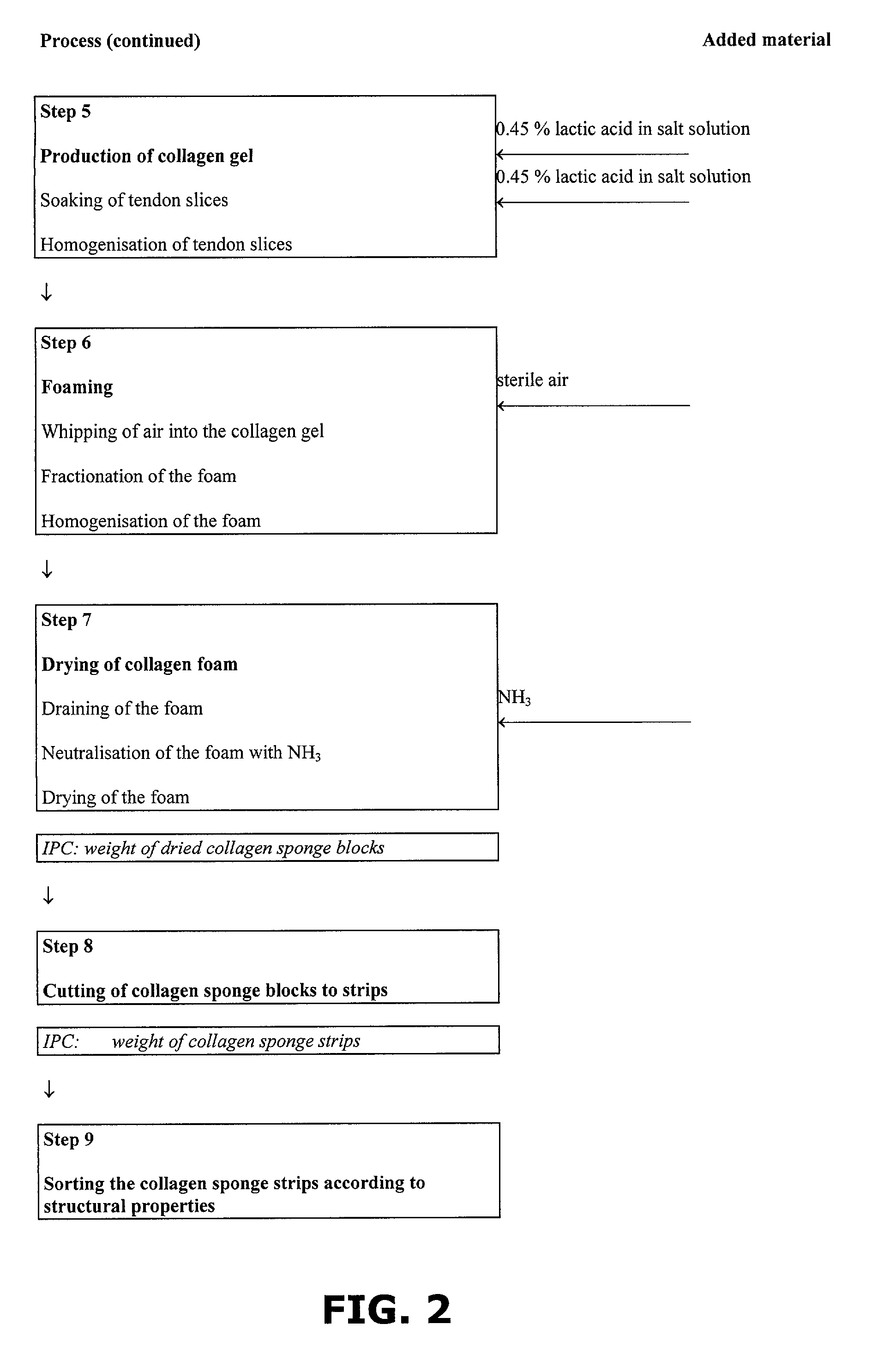
InactiveUS7098315B2Improve featuresHigh densitySurgical adhesivesPeptide/protein ingredientsAprotininFibrin glue
A method of preparing a collagen sponge comprises mixing air into a collagen gel, so as to obtain a collagen foam which is dried. From the dried product thereby obtained, collagen sponge is obtained by isolating parts of sponge with a chamber diameter of more than 0.75 mm and less than 4 mm, or parts with an average chamber diagonal dimension of 3 mm. The collagen sponge may be used as a material for sealing wounds, possibly with a coating comprising a fibrin glue, such as a combination of fibrinogen, thrombin and aprotinin. A device for extracting a part of a collagen foam and for degenerating another part of the collagen foam to a collagen gel is disclosed. An elongated collagen sponge having a through-going hole or bore and a flexible wall may be used for re-establishing walls in a mammalian gastrointestinal funnel or trachea system.
Owner:TOPAZ INVESTMENT AS
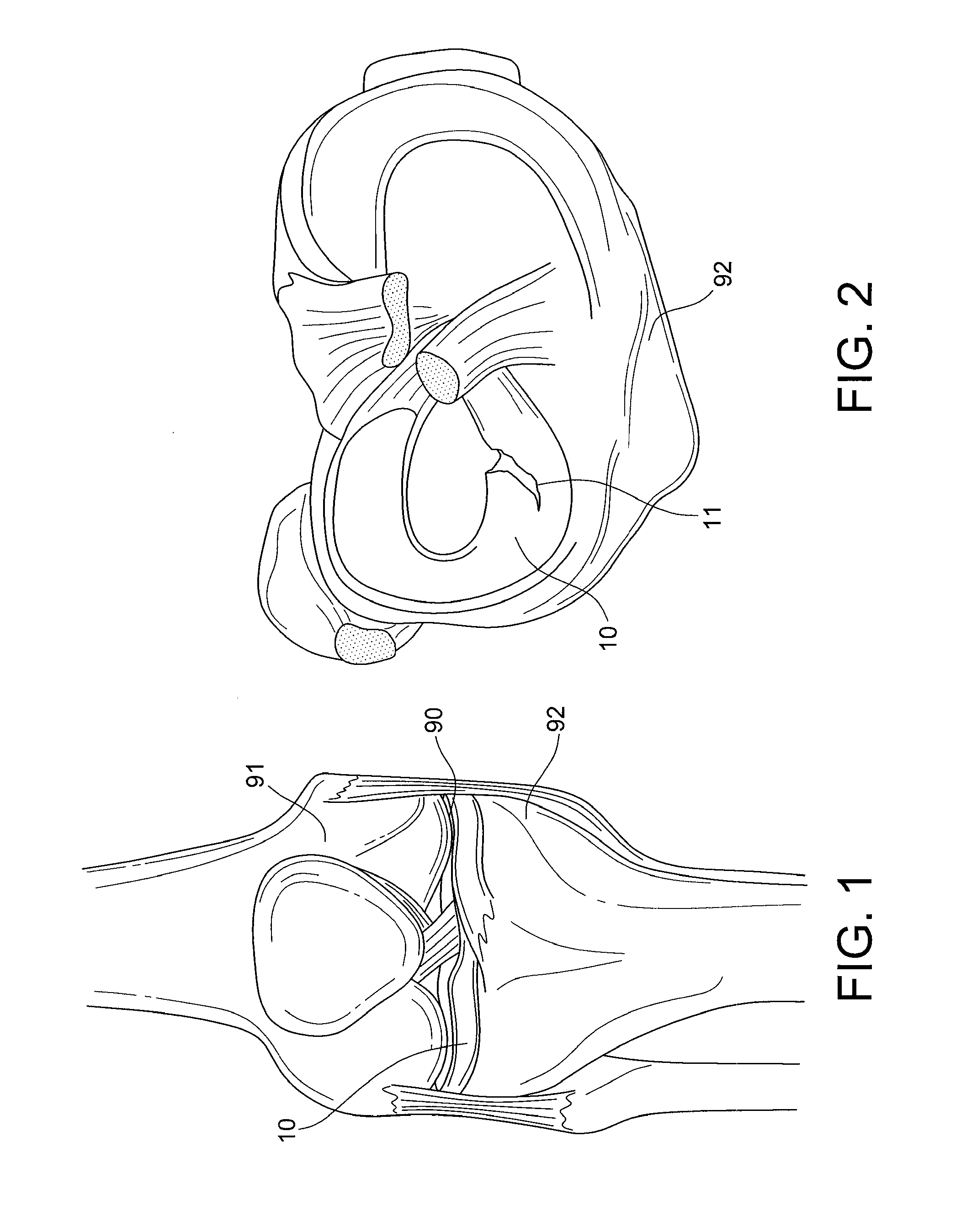
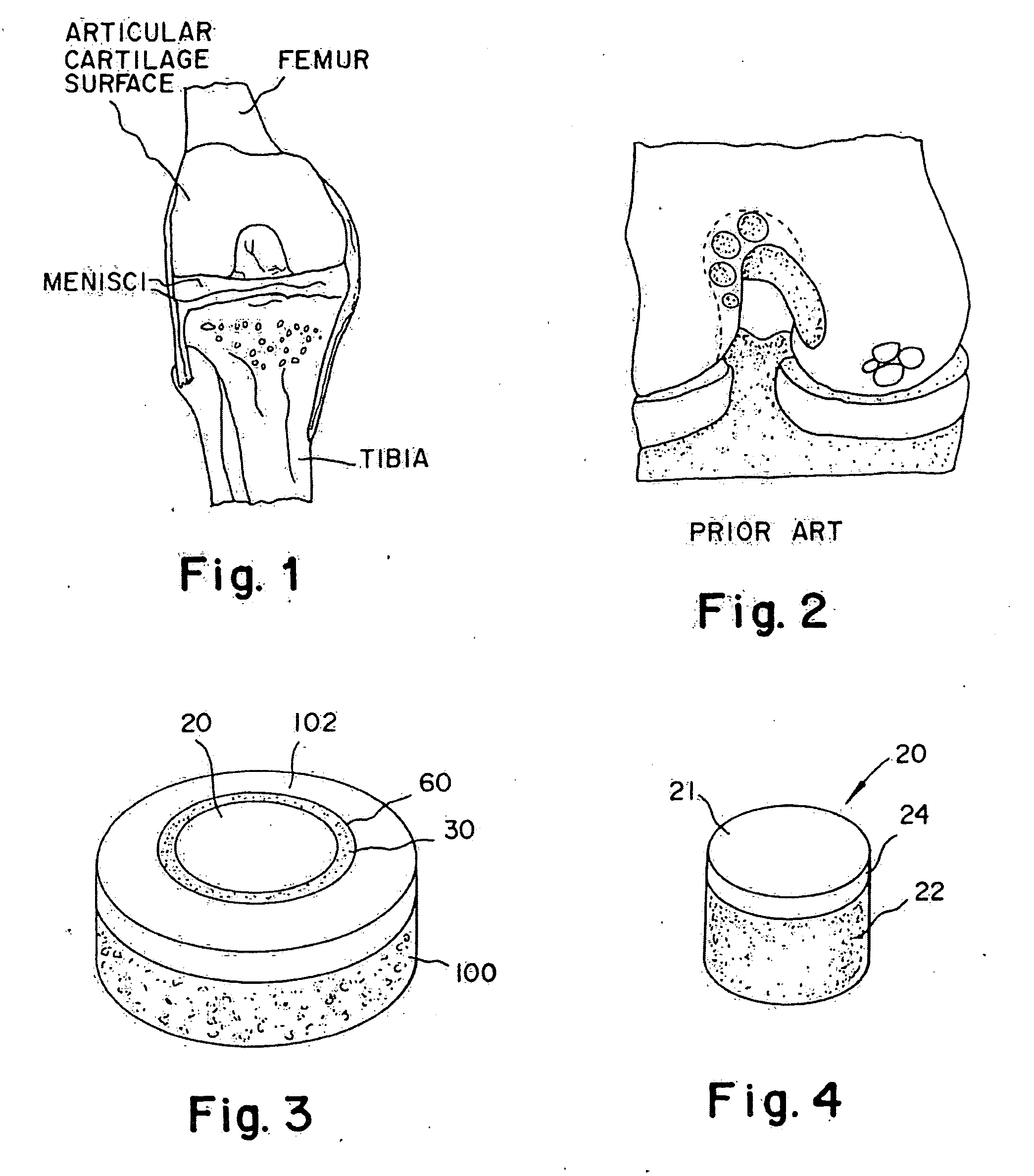
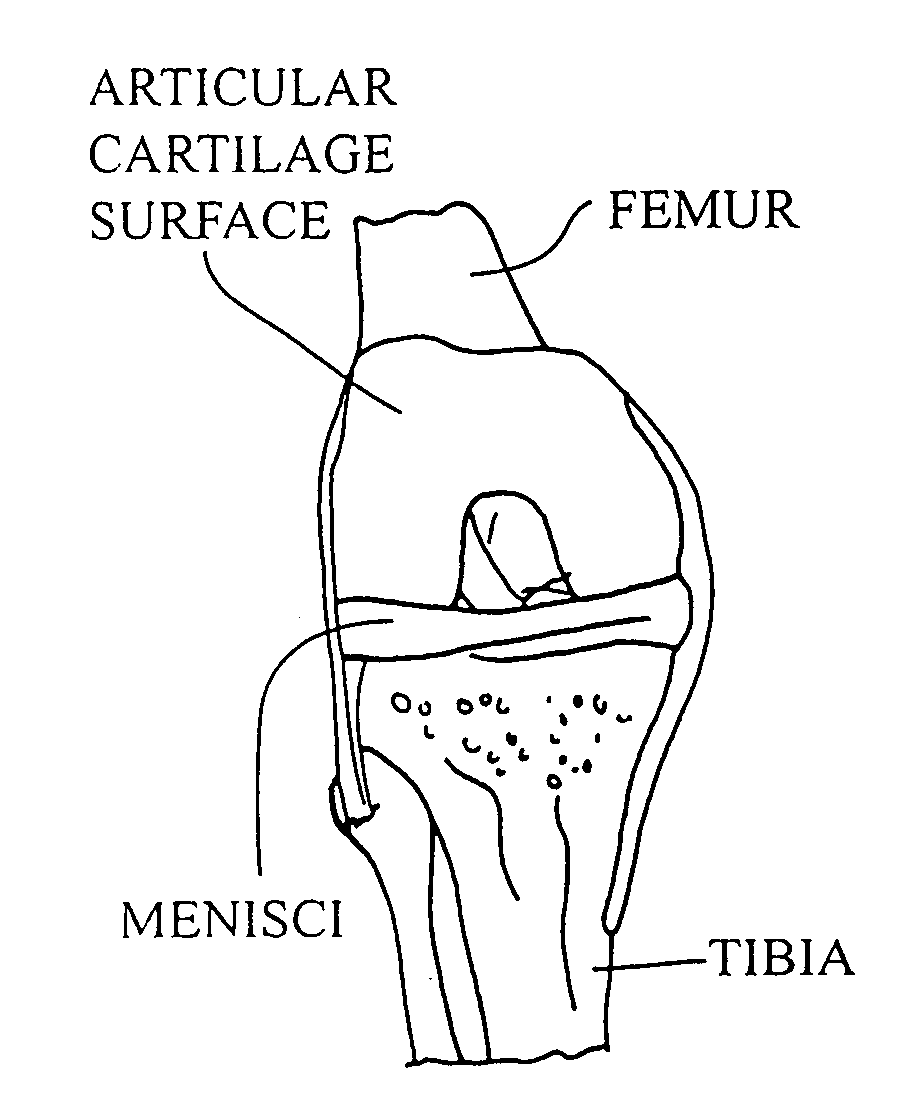
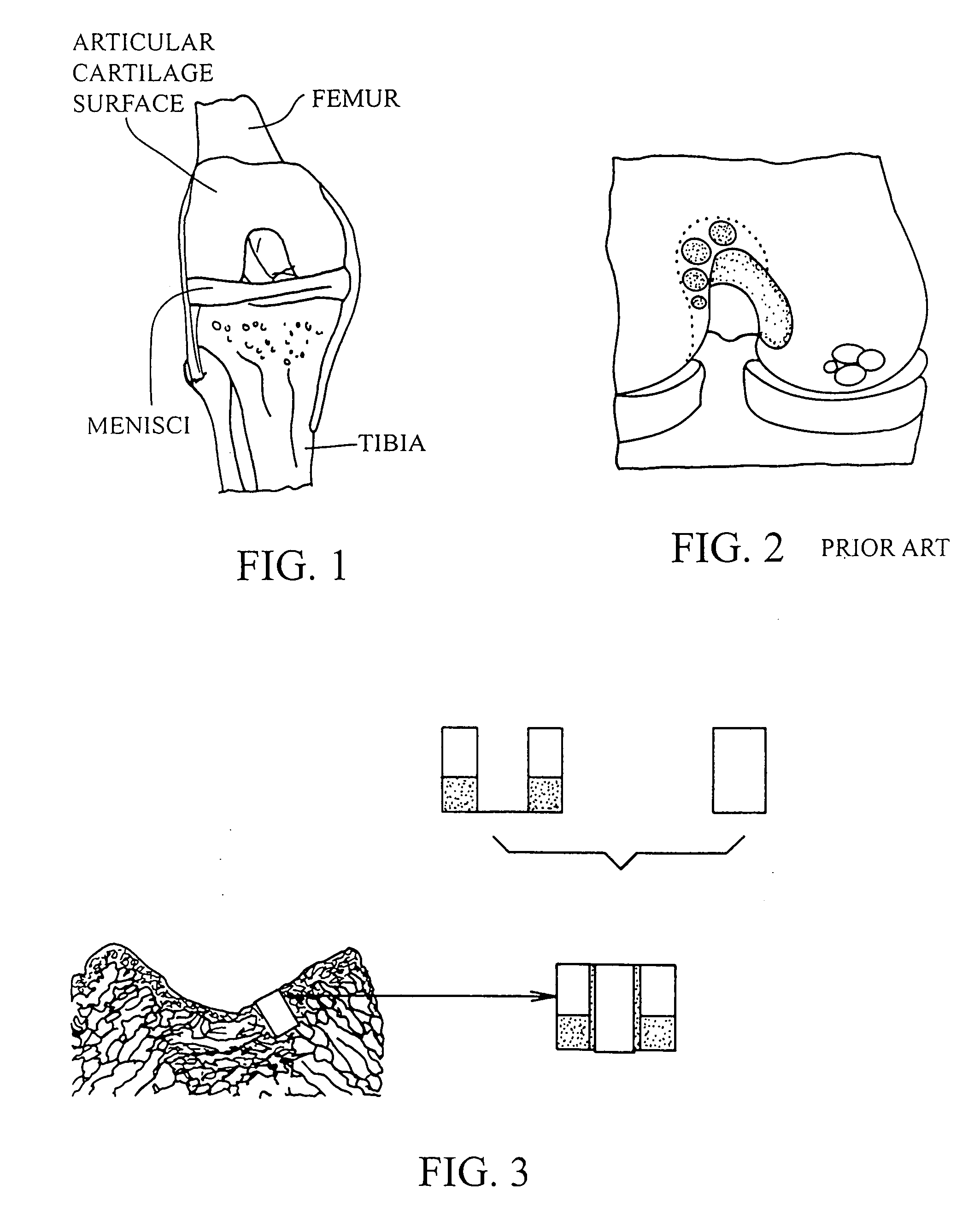
Articular cartilage, device and method for repairing cartilage defects
InactiveUS20100211173A1Easy to useSimple structureSuture equipmentsInternal osteosythesisYarnCartilage cells
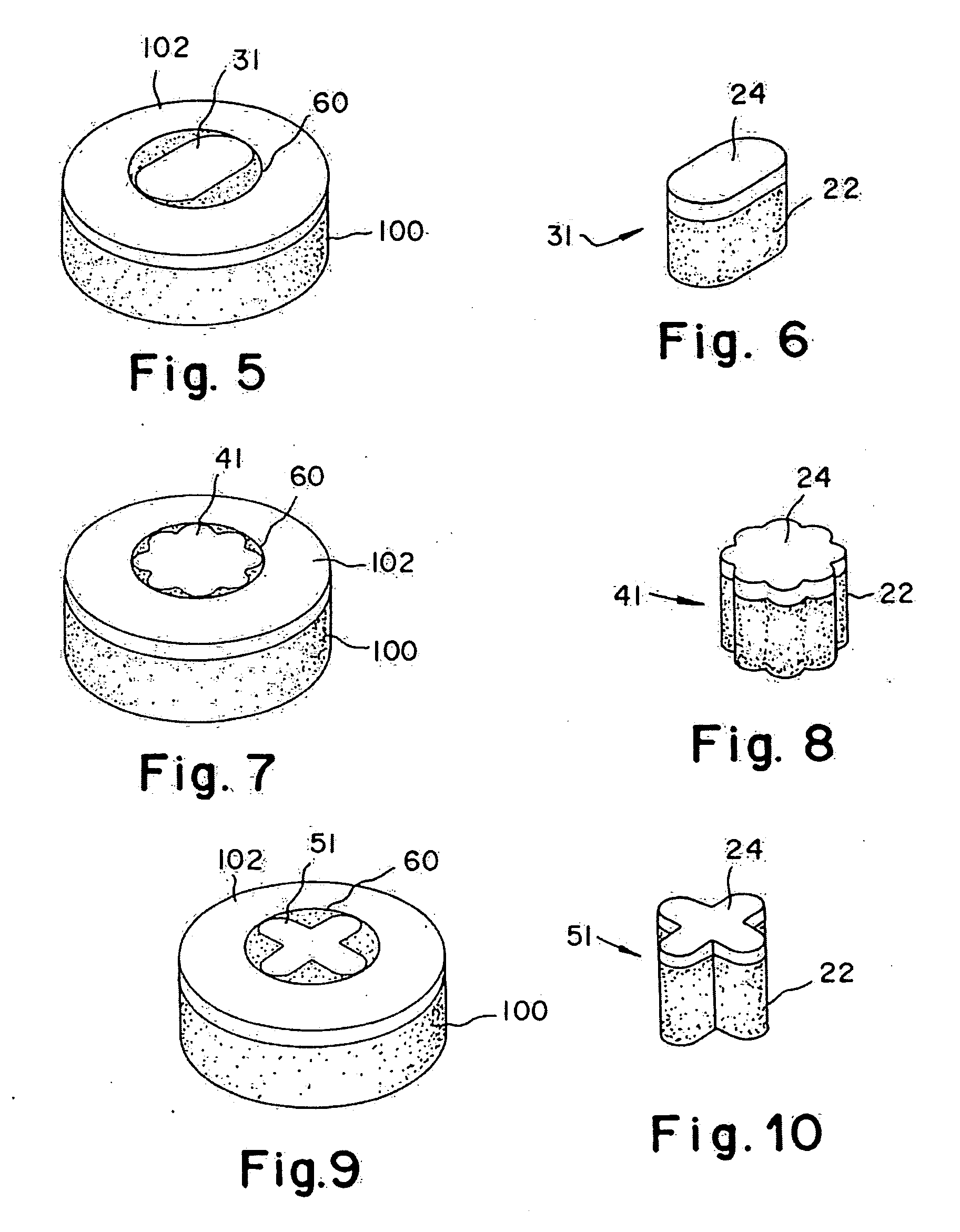
The articular cartilage according to the invention is made of pure cartilage and is provided with incisions (12) on the surface facing the bone. The cartilage cells are preferably seeded on the surface provided with incisions (12). The method for producing the articular cartilage comprises the step of collecting cartilage from joints, wherein pure cartilage is collected without bone, and incisions are made on the surface of the cartilage intended to face the bone. It is preferably fresh frozen until use. The device for harvesting articular cartilage, comprises handle and cutting blade, wherein the cutting blade (4) is curvilinear and is provided with spacer elements (5), meanwhile the device for producing incisions in articular cartilages comprises handle (2) and a bridge (3) connected to said handle (2) and being provided with one or more cutting blade(s) (4). During the method for applying the articular cartilage the articular cartilage is fixed by thin surgical yarn stitches, by fibrin glue or by small anchors (FIG. 8).
Owner:PECSI TUDOMANYEGYETEM
Fibrin applicator pistol
InactiveUS6863660B2Reliable deliveryNanotechPeptide/protein ingredientsSuspended particlesFibrin glue
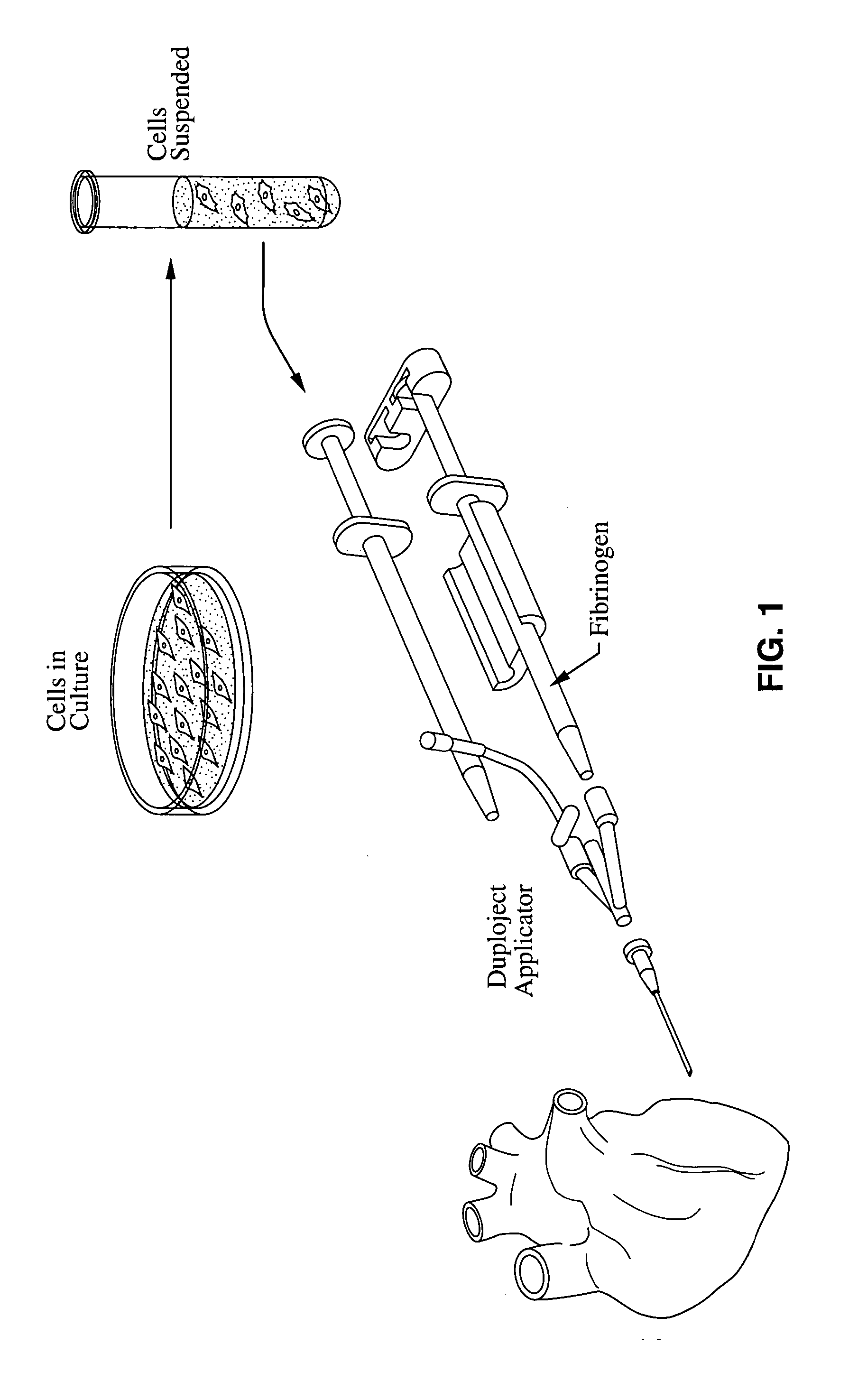
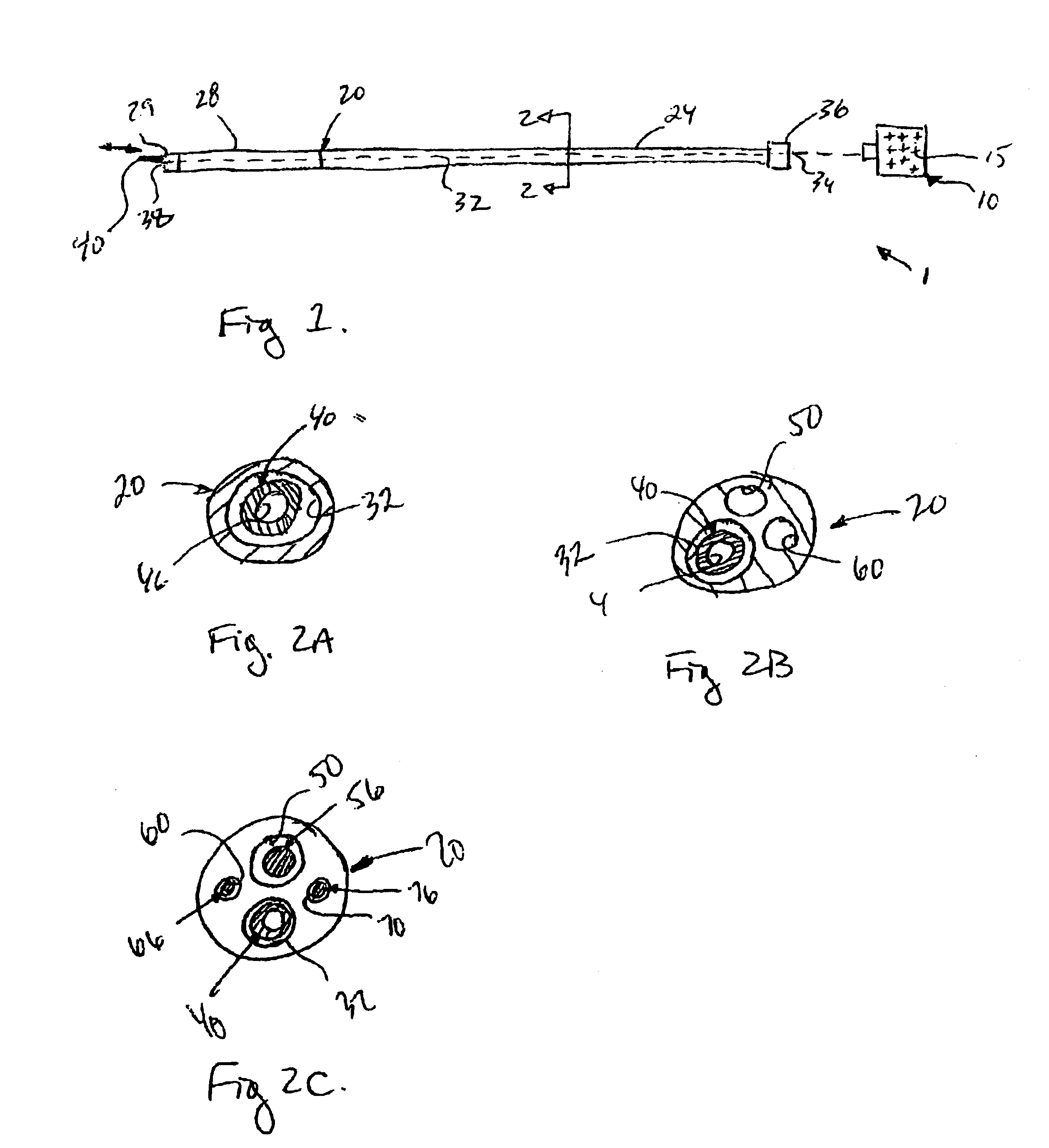
An applicator for dispensing a first and second component of a biological adhesive, such as fibrin glue. At least one of said components may contain a suspension of fibrin microbeads (FMB) or a suspension of cells. The present invention uses a single supply of pressurized gas to force the components from the applicator using positive fluid pressure and to atomize them into a convergent spray. Another embodiment of the present invention also provides for the endoscopic application of the biological adhesive directly to tissue defects. The application of positive pressure allows precise metering of the components and application of the adhesive, prevents internal coagulation of the fibrin or clogging by suspended particles and reduces waste and contamination of the components.
Owner:HAPTO BIOTECH
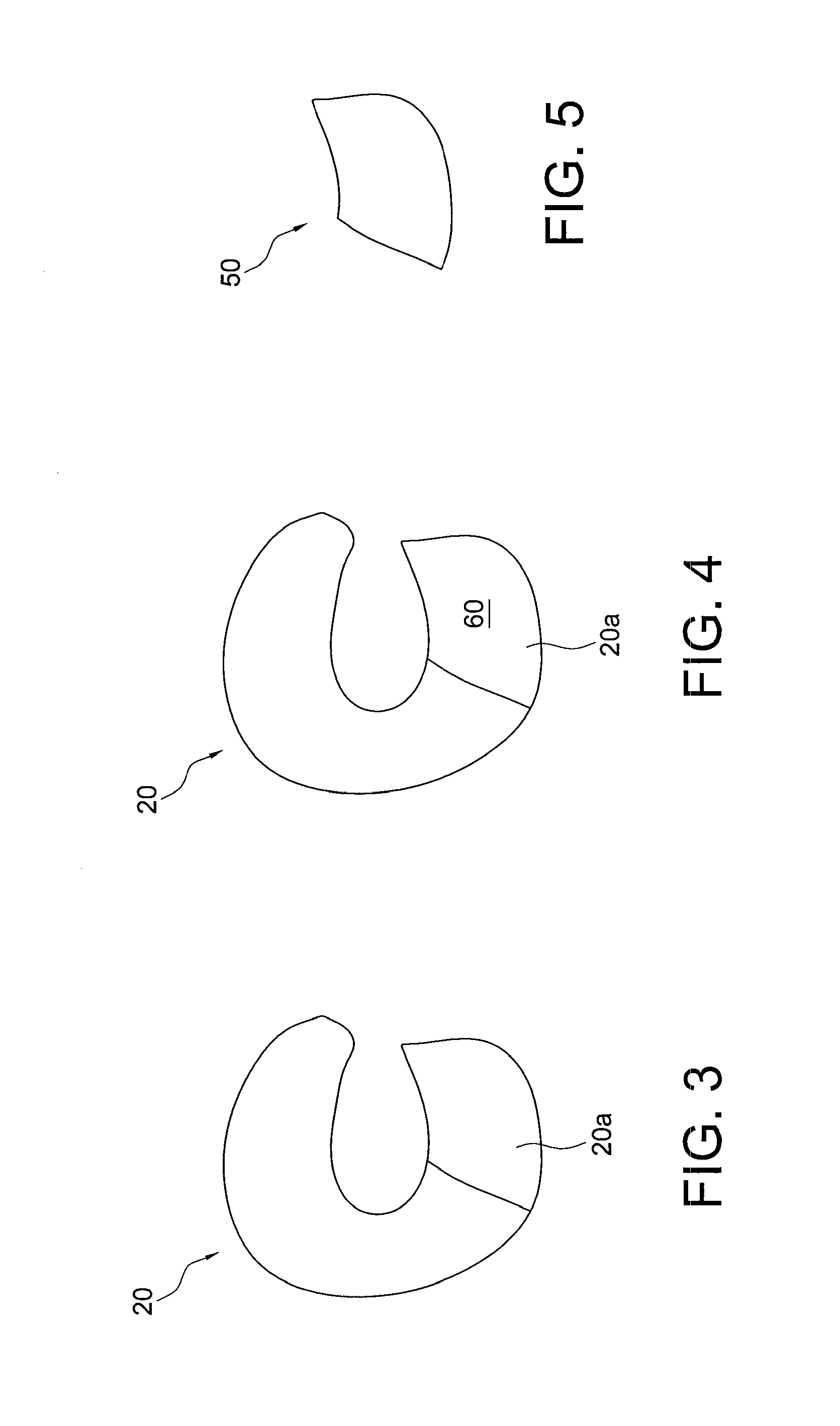
Biologic partial meniscus and method of preparation
InactiveUS20130304209A1Provide biomechanical strengthMetal rolling stand detailsSurgeryFibrin glueAdhesive
Biologic partial menisci (biologic partial meniscal replacements / constructs) used to replace at least a part of a meniscus, and methods of forming such biologic partial menisci. Meniscal cartilage is employed to form a moldable allograft paste. A sterile mold that replicates a meniscus (for example, the medial or lateral meniscus) is provided in various sizes and is used as a biologic mold to recreate the anatomic shape of the meniscus. The moldable paste is inserted (for example, injected) into the mold and allowed to set into a stable, anatomically shaped meniscus (the biologic partial meniscus). Fibrin glue or other biologic adhesives or strengtheners may be optionally added to provide further biomechanical strength. Once the biologic partial meniscus is removed from the mold, it is provided at the surgical site and attached to the excised meniscus (placed into the correct anatomical shape) to complete the meniscal repair.
Owner:ARTHREX
Cartilage implant plug with fibrin glue and method for implantation
Owner:MASSACHUSETTS INST OF TECH +1
Systems and methods for preparing autologous fibrin glue
Owner:CASCADE MEDICAL ENTERPRISES +1
Method of arthroscopic osteochondral resurfacing using PRP strengthened with fibrin glue
Methods of arthroscopic resurfacing of a joint utilizing a biological component strengthened with fibrin glue. The biological component is selected from the group consisting of PRP, bone marrow aspirate (BMA) and autologous conditioned plasma (ACP). The biological component / fibrin glue composition may be inserted (by injection or by employing a biologic resurfacing mold, for example) into a transosseous tunnel in the vicinity of the defect to be repaired. Upon insertion at the defect site, the biological component / fibrin glue composition is designed to coagulate and solidify within few minutes, to advance the healing of the damaged tissue and tissue growth. The biological component / fibrin glue composition may optionally comprise components such as growth factors, antiseptic chemicals and / or antibiotics and / or electrolytes, or hormones or site-specific hybrid proteins, among others.
Owner:ARTHREX
Cartilage implant plug with fibrin glue and method for implantation
InactiveUS20080274157A1Guaranteed functionEasy to placeSurgical adhesivesPeptide/protein ingredientsSubchondral boneFibrin glue
The invention is directed toward a cartilage repair assembly comprising a shaped structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that said shaped bone and cartilage cap when centered in the bore does not engage the side wall of the bore and is positioned from the side wall of the bone a distance ranging from 10 microns to 1000 microns and is surrounded by milled cartilage and a fibrin thrombin glue. A method for inserting the assembly into a cartilage defect area is disclosed.
Owner:MASSACHUSETTS INST OF TECH +1
Suspension comprising fibrinogen, thrombin and alcohol, a method for preparing such a suspension, a method for coating a carrier with such a suspension, a method of drying a coating of a carrier, and a coated collagen sponge
InactiveUS20050214277A1Easy to identifySatisfy fixationSurgical adhesivesFibrinogenFiberMean diameter
A suspension of fibrinogen, thrombin, alcohol and optionally aprotinin is obtained by mixing fibrinogen in alcohol with thrombin in alcohol. The suspension contains fibrinogen and thrombin particles with a Folk Ward mean diameter of 25-100 μm. The thrombin may be human, bovine or recombinant. The fibrinogen may be human or recombinant. A method for coating a carrier, such as a collagen sponge, with the suspension, and a method for drying the coating is disclosed. The coated collagen carrier may be used as a ready-to-use absorbable composition for tissue gluing, tissue sealing and hemostasis wherein the carrier is coated with solidly fixed components of fibrin glue, i.e. fibrinogen and thrombin.
Owner:SCHAUFLER ALFRED
Double-component medical adhesive based on glucan and chitosan and preparation method of double-component medical adhesive
ActiveCN107496974ALow priceGood biocompatibilitySurgical adhesivesPharmaceutical delivery mechanismEthylenediamineFibrin glue
The invention relates to a double-component medical adhesive based on glucan and chitosan and a preparation method of the double-component medical adhesive. The double-component medical adhesive comprises formylated glucan and aminated carboxymethyl chitosan, wherein the mass ratio of the formylated glucan to the aminated carboxymethyl chitosan is (1.67-3.33):1. The preparation method includes: adding a sodium periodate solution into a glucan solution under a dark condition to perform dialysis, and performing freeze drying to obtain the formylated glucan; mixing carboxymethyl chitosan, ethylenediamine and water-soluble carbodiimide, and performing dialysis and freeze drying to obtain the aminated carboxymethyl chitosan; mixing the formylated glucan and aminated carboxymethyl chitosan which are identical in volume, and performing Schiff base reaction to form hydrogel to obtain the medical adhesive. The double-component medical adhesive and the preparation method thereof have the advantages that the raw materials of the double-component medical adhesive are nontoxic and odorless, cheap and good in biocompatibility; the preparation method is simple; the prepared medical adhesive is good in adhesive strength and capable of shortening gelation time, the medical adhesive can act on any body parts through simple local spraying or injection, and the medical adhesive is an ideal replacement product of commercially available fibrin glue.
Owner:DONGHUA UNIV
Methods for making and delivering rho-antagonist tissue adhesive formulations to the injured mammalian central and peripheral nervous systems and uses thereof
InactiveUS7141428B2Avoid crackingReduce deliveryOrganic active ingredientsPowder deliveryNervous systemFibrin glue
The present invention provides methods for making, delivering and using formulations that combine a therapeutically active agent(s) (such as for example a Rho antagonist(s)) and a flowable carrier component capable of forming a therapeutically acceptable matrix in vivo (such as for example tissue adhesives), to injured nerves to promote repair and regeneration and regrowth of injured (mammalian) neuronal cells, e.g. for facilitating axon growth at a desired lesion site. Preferred active agents are known Rho antagonists such as for example C3, chimeric C3 proteins, etc. or substances selected from among known trans-4-amino(alkyl)-1-pyridylcarbamoylcyclohexane compounds or Rho kinase inhibitors. The system for example may deliver an antagonist(s) in a tissue adhesive such as, for example, a fibrin glue or a collagen gel to create a delivery matrix in situ. A kit and methods of stimulating neuronal regeneration are also included.
Owner:BIOAXONE BIOSCI +1
System and method for forming a non-ablative cardiac conduction block
A system forms a cardiac conduction block at a location in a heart of a patient without substantially ablating cardiac tissue. The system includes a delivery system coupled to a source of material that is substantially non-ablative with respect to cardiac tissue. The delivery system delivers the material to the location, and the material at the location forms a conduction block without ablating the cardiac cells there. The material may include living cells, such as for example skeletal myocytes, and / or may include a non-living matter such as biopolymers such as a fibrin glue agent, or collagen agents. An expandable member with needle assembly is used to deliver the material so as to form a non-ablative circumferential conduction block at a location where a pulmonary vein extends from an atrium.
Owner:RGT UNIV OF CALIFORNIA
Water gel fast forming process based on bionic process
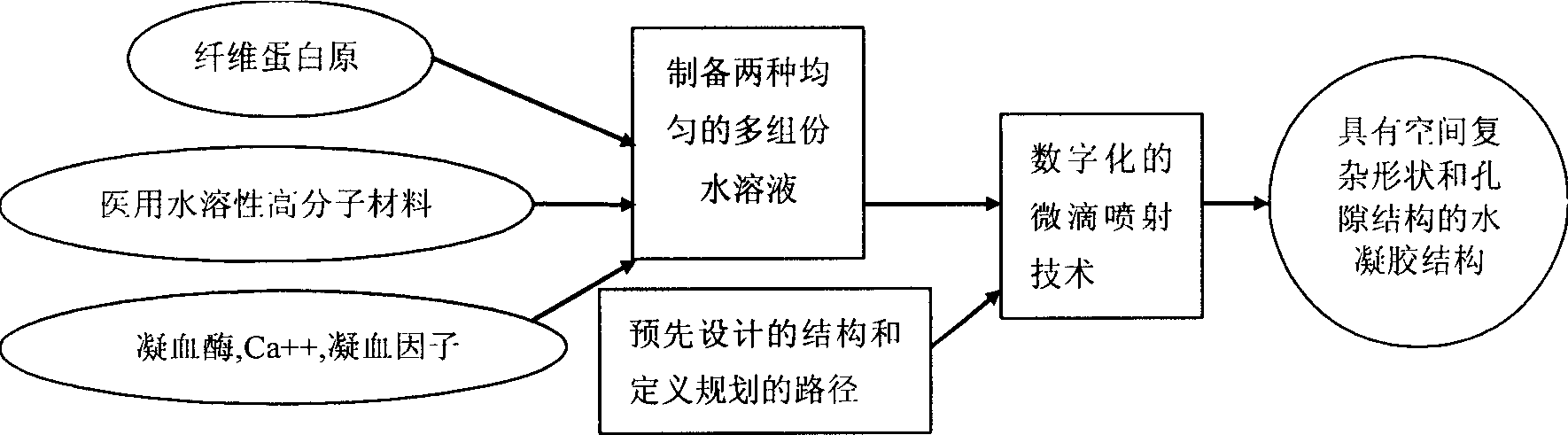
The fast bionic water gel forming process simulates human blood coagulation process to produce fibrin gel. Fibrinogen and medical water soluble polymer material, as well as thrombin, Ca ion and blood coagulation factor are prepared into two multiple-component water solutions; and the two water solutions are made to mix and accumulate in certain spatial position by means of digital micro dropping and jetting technology while producing enzyme reaction to form water gel with fibrin as matrix, so as to make 3D water gel structure with complicated spatial shape and porous structure. The coagulation process has complicated and effective regulating and controlling mechanism, and the water gel structure has excellent mechanical and biological performance, stable structure and effectively regulated degradation speed. The present invention is suitable for forming tissue engineering sample, medicine slow releasing carrier, etc.
Owner:TSINGHUA UNIV
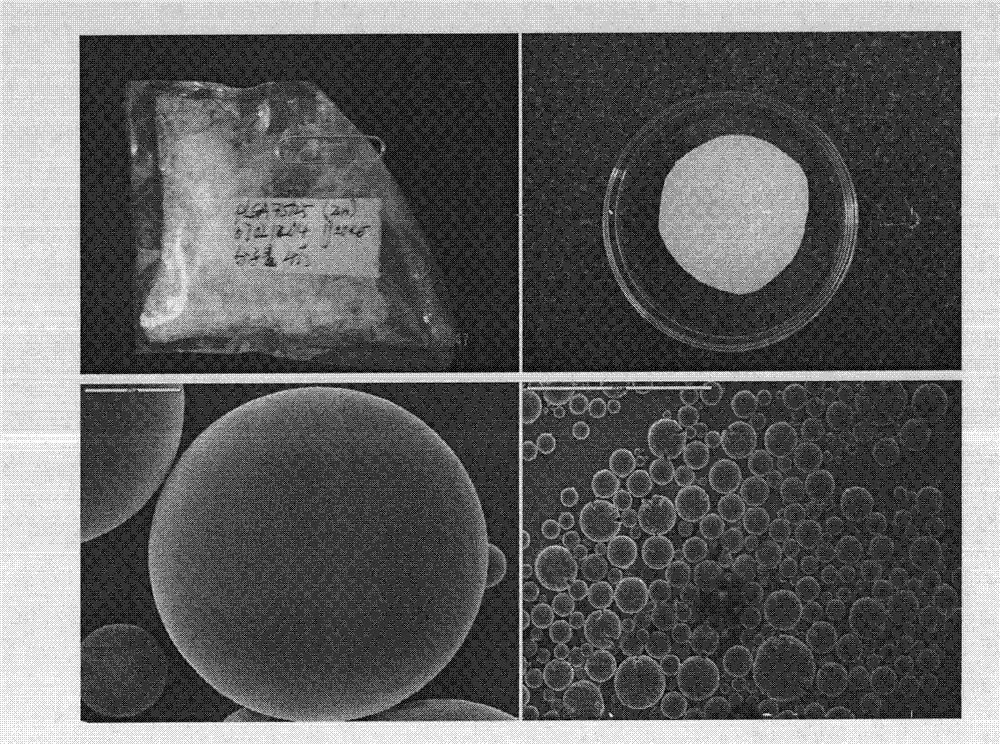
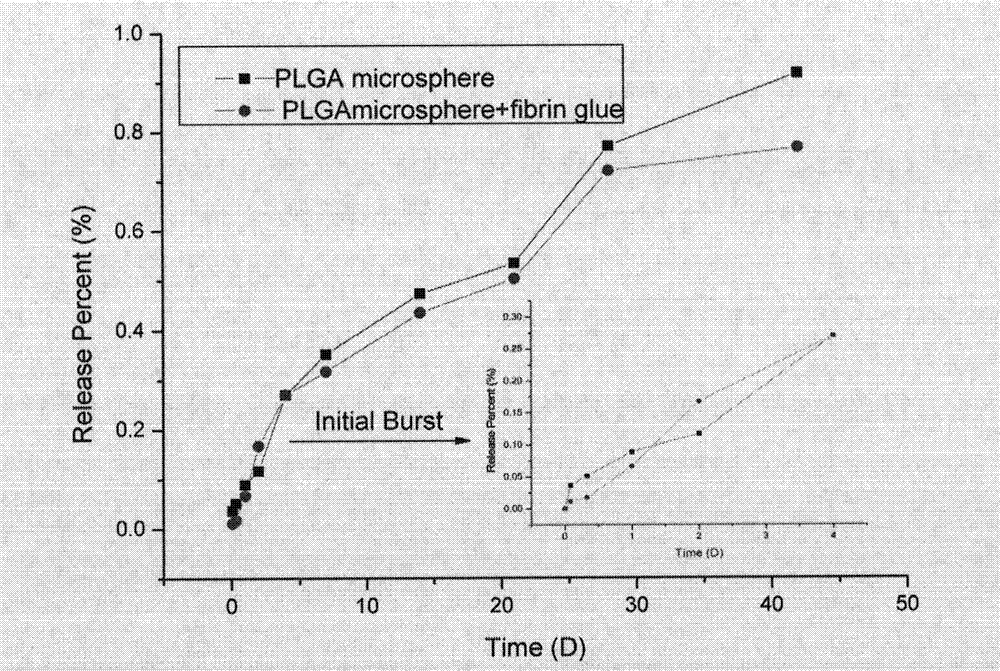
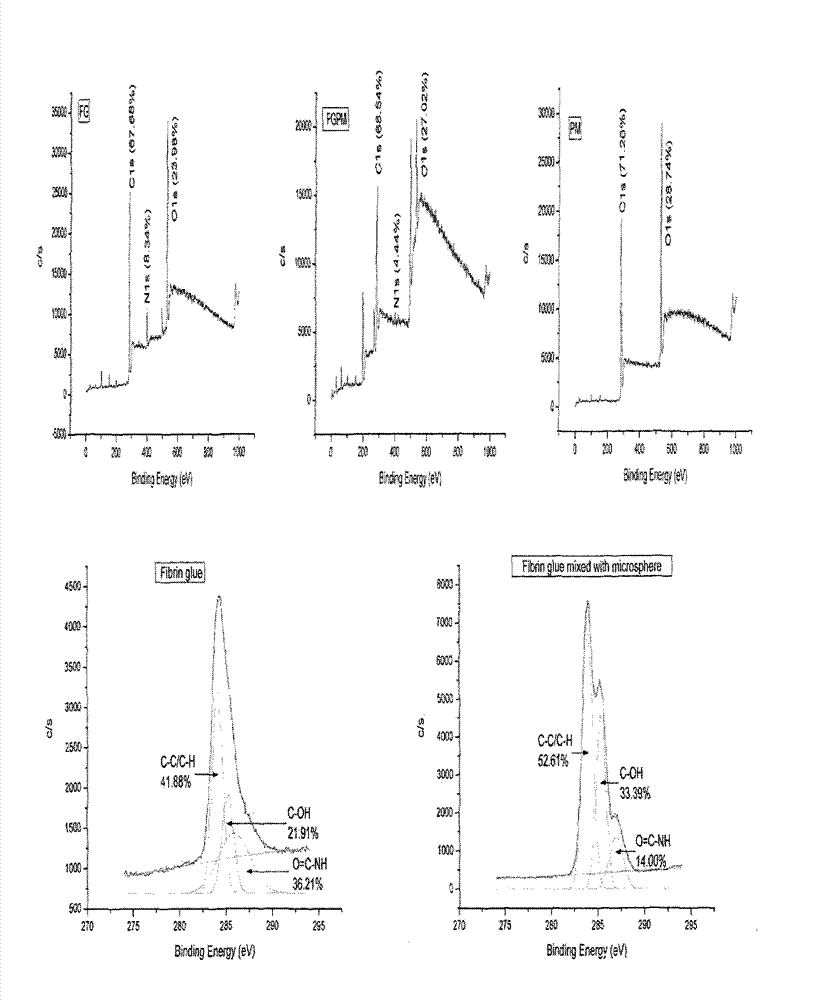
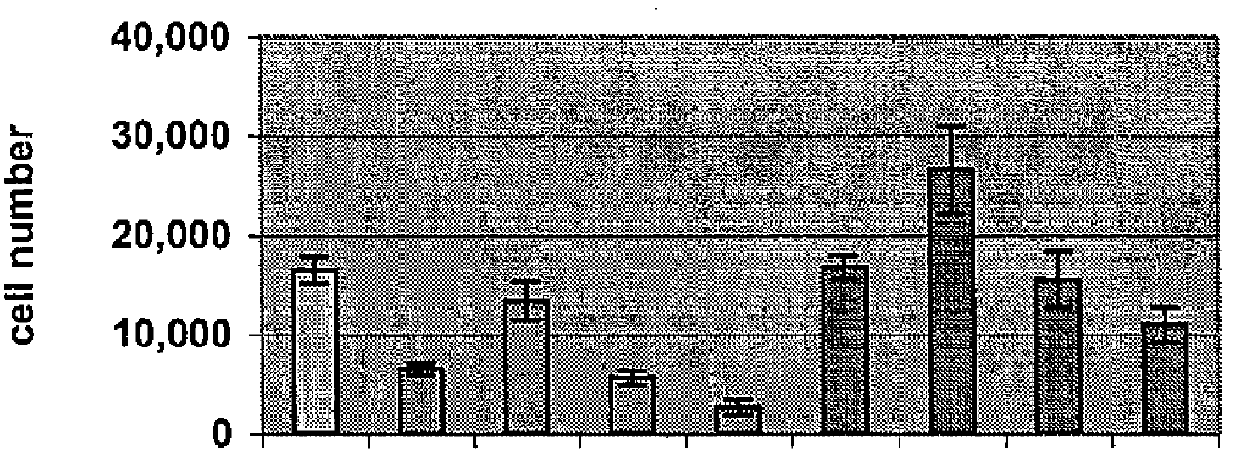
Preparation method and application of fibrin glue composite recombinant human bone morphogenetic protein-2 (rhBMP-2) microsphere
The invention relates to a preparation method and application of a fibrin glue composite recombinant human bone morphogenetic protein-2 (rhBMP-2) microsphere. The preparation method comprises the steps of: preparing a slow release microsphere with a proper particle diameter; and then constructing an rhBMP-2 / PLGA microsphere / fibrin glue composite material. The rhBMP-2 / PLGA microsphere fibrin glue composite material can be used through local injection, surgical trauma can be reduced, a healing process of bone fracture and nonunion is accelerated by continuously supplementing the local bone morphogenetic protein, thus the fibrin glue composite recombinant human bone morphogenetic protein-2 is a bone repair material with excellent degradability and osteogenic activity.
Owner:姚琦 +2
Treatment of peripheral vascular disease using umbilical cord tissue-derived cells
Compositions and methods of using cells derived from umbilical cord tissue, to stimulate and support angiogenesis, to improve blood flow, to regenerate, repair, and improve skeletal muscle damaged by a peripheral ischemic event, and to protect skeletal muscle from ischemic damage in peripheral vascular disease patients are disclosed. In particular, methods of treating a patient having a peripheral vascular disease with umbilical derived cells and fibrin glue are disclosed.
Owner:DEPUY SYNTHES PROD INC
Method for producing compound frame of injection type polyester micro-carrier and fibrin gel
The invention discloses a method for preparing a composite support of injection polyester micro carrier and fibrin gel, wherein the composite support is formed by composed fibrin gel and polyester micro carrier. The micro carrier supply physical strength and space for cell growth and expansion, the fibrin gel simulates the cell epimatrix of cartilage cell, to support the transmission and original injection shaping of micro carrier, and accelerate the transfer, increment and differentiation of cartilage cell, and accelerate the healing of cartilage cell. The inventive composite support can effectively load micro carrier from free movement in body and loss in plant process, with better biological compatibility, simple preparation, wide resource, high producing efficiency and wide application.
Owner:ZHEJIANG UNIV
Collagen-chitosan / fibrin glue asymmetric bracket and the preparing method and the application thereof
The invention discloses a collagen-chitose or fibrin glue asymmetric support, preparing method and application, which is characterized by the following: incorporating collagen-chitose porous material layer and fibrin glue layer; arranging the fibrin glue layer on the surface of collagen-chitose porous material layer evenly; forming interface between the fibrin glue layer and the surface of collagen-chitose porous material layer. This product possesses good cell compatibility, which is fit for complexing skin construction. This invention also discloses the preparing method of the asymmetric support and application.
Owner:韩春茂
Medical adhesive
The invention discloses a medical adhesive, comprising the following substances in parts by weight: 65-80 parts of alpha-N-butylcyanoacrylate, 5-8 parts of hydroxypropyl methacrylate, 3-5 parts of sulfur dioxide, 1-2 parts of hydroquinone, 0.5-2 parts of polyurethane, 0.5-1.5 parts of agar, 0.8-1.2 parts of fibrin glue, 2-3 parts of water soluble phenol-formaldehyde resin, and 20-25 parts of deionized water. The medical adhesive has the beneficial effects that the medical adhesive provided by the invention is low in thrill, and excellent in bonding persistence; compared with the traditional common adhesive, the persistence of the medical adhesive can be up to 1.5-3 times; and animal experiments show that the toxic and side effects are small.
Owner:台山市弘毅医疗用品有限公司
Tumor drug-loaded microparticle preparation and preparation method thereof
ActiveCN108042805AAvoid side effectsSmall toxicityOrganic active ingredientsPharmaceutical non-active ingredientsCell vesicleSide effect
The invention provides a tumor drug-loaded microparticle preparation and a preparation method thereof. The drug-loaded microparticle preparation includes tumor stem cell apoptosis released cell vesicles and a chemotherapeutic drug wrapped in the cell vesicles as an effective component; and is prepared by mixed culture of a three-dimensional soft fibrin glue and tumor cells in a medium. The tumor drug-loaded microparticle provided by the invention is more beneficial to high enrichment in tumor tissue and deep penetration of tumors and can achieve effective uptake by common tumor cells and tumorstem cells more easily, can improve the killing power of the chemotherapeutic drug to common tumor cells and tumor stem cells, can solve the problem that common tumor cell derived drug-loaded microparticles cannot permeate to the depth parts of tumors to kill a lot of tumor stem cells, and at the same time can reduce the toxic and side effect of the chemotherapeutic drug on the body.
Owner:HUAZHONG UNIV OF SCI & TECH
Wound healing agent and radix astragali composite freeze-dried platelet gel kit for preparing wound healing agent
InactiveCN102319425ATo achieve the effect of wound healingPeptide/protein ingredientsMammal material medical ingredientsWater bathsFibrin glue
A wound healing agent and a radix astragali composite freeze-dried platelet gel kit for preparing the wound healing agent. The kit comprises a radix astragali extract solution which is stored separately, freeze-dried thrombin and freeze-dried platelets. The preparation method of the wound healing agent mainly comprises the following steps: step one, mixing freeze-dried platelets and sterile injection water, placing the mixture in a water bath with a temperature of 30-37 DEG C, fully dissolving the freeze-dried platelets to obtain a freeze-dried platelet solution; adding freeze-dried thrombin into the radix astragali extract solution to obtain a composite thrombin solution; step two, well mixing the freeze-dried platelet solution and the composite thrombin solution to obtain the wound healing agent. In the invention, after the radix astragali extract solution is mixed with thrombin, the mixture has the effect of activating fibrinogen to become fibrin glue in vitro, and thus reaches theeffect of wound healing.
Owner:霍红梅 +1
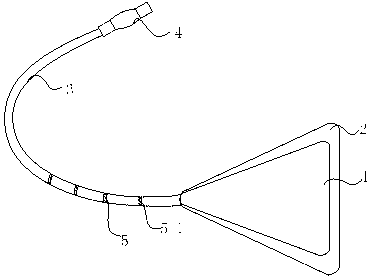
Inductive uterine slow release system
The invention discloses an inductive uterine slow release system. The incidence of uterine adhesion after an induced abortion operation is high, the re-adhesion is serious after a uterine adhesion operation, and no good solution is available at present. The system comprises a uterus-shaped elastic balloon stent, a slow release drug film, a catheter and a one-way inflation valve, wherein the uterus-shaped elastic balloon stent is a hollow isosceles triangle structural body; waists of the isosceles triangle are 3.5cm; the bottom edge of the isosceles triangle is 3cm; one end of the catheter is connected and communicated with the vertex angle end of the uterus-shaped elastic balloon stent; the other end of the catheter is connected with the one-way inflation valve; a plurality of annular scales are arranged on the outer side face of one end, close to the uterus-shaped elastic balloon stent, of the catheter; and the one-way inflation valve comprises an inflation valve and a pressure sensor. The uterus-shaped elastic balloon stent is coated with the slow release drug film, and the slow release drug film is made of fibrin glue and contains a slow release drug. The system is simple in structure and easy to operate, does not injure a uterine cavity easily, and can effectively avoid the uterine adhesion.
Owner:杭州安体科技有限公司
Autologous living cell soft tissue filling gel and preparation method thereof
The invention discloses autologous living cell soft tissue filling gel, which comprises autologous fibroblasts cultivated and amplified to 8*108, platelet-rich plasma of which the platelet concentration is 8 times that of normal blood concentration and a thrombin-calcium agent. The preparation method comprises the following steps of: amplifying the extracted fibroblasts to 8*108 in vitro; adding into the prepared platelet-rich plasma; activating with the thrombin-calcium agent; and coagulating fibrinogens in the plasma into fibrin glue with a netlike structure to obtain the living cell soft tissue filling gel, wherein culture media used in primary passage and passage of fibroblasts are prepared from a serum-free culture medium and 5 percent of platelet-rich plasma. The preparation method has the advantages that: the problems of in-vitro amplification and culturing of fibroblasts, collection and feedback, keeping of a cell healthy state in an in-vivo survival multiplication process, survival after feedback, nutrition supply of cell multiplication, space release of cell multiplication, and the like are solved, and high survival rate of cells is ensured.
Owner:侯强 +4
Kits, formulations and solutions having enzymatically- permissive amounts of visualization agents and uses thereof
InactiveUS20100203033A1Speed up recoveryImproves application targeting qualitySurgical adhesivesPeptide/protein ingredientsFibrin glueFibrinogen
The invention relates to a proteolytic enzyme which is capable of forming fibrin when it reacts with fibrinogen, a fibrin-glue kit and a fibrin-glue formulation comprising an enzymatically-permissive concentration of a visualization agent and to their use in methods for prevention and / or reduction of adhesions and / or methods for promotion of blood coagulation sealing or filling body surfaces.
Owner:OMRIX BIOPHARM
Technology for building small-caliber vascular stent
InactiveCN101756756APromote remodelingSolve the problem of low long-term patency rateStentsProsthesisFibrin glueElectrospinning
The invention relates to a technology for building a small-caliber vascular stent, which comprises the following steps: (1) manufacturing the inner wall of a vessel with fibrin glue by a stent building technology, (2) building the outer wall of the vessel by an electrospinning technology and (3) drying, sterilizing and preserving the vascular stent. Compared with the prior art, the invention has the advantages of simple technology, convenient operation, easy implementation of industrialization and high industrialization value.
Owner:SHANGHAI P & P BIOTECH
A safe and efficient freeze-dried fibrin sealant and preparation method thereof
InactiveCN102258770AEnsure activityNon-lipid enveloped virusSurgical adhesivesPeptide/protein ingredientsFibrin glueFreeze-drying
The invention discloses a safe and efficient freeze-dried fibrin sealant and a preparation method thereof. The freeze-dried fibrin sealant is composed of independent components in the following parts by weight: 12-15 parts of freeze-dried fibrinogen powder , 3 to 5 parts of freeze-dried thrombin powder. The invention provides a freeze-dried fibrin sealing agent that can be treated by a heat-resistant method by adopting an effective fibrinogen and thrombin stabilizer and a reasonable formula. The heat treatment of the preparation can not only ensure the inactivation of lipid-enveloped and non-lipid-enveloped viruses, but also allow the preparation to be quickly reconstituted at room temperature, which greatly facilitates clinical use, especially for fibrin glue used in first aid. significance.
Owner:SHANGHAI LIKANGRUI BIOLOGICAL ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com