Patents
Literature
37 results about "Thromboplastin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thromboplastin (TPL) or thrombokinase is a mixture of both phospholipids and tissue factor found in plasma aiding blood coagulation through catalyzing the conversion of prothrombin to thrombin. It is a complex enzyme that is found in brain, lung, and other tissues and especially in blood platelets and that functions in the conversion of prothrombin to thrombin in the clotting of blood.
Fibrin glue without fibrinogen and biosealant compositions and methods
InactiveUS6168788B1Stop blood flowPrevents unwantedSurgical adhesivesPeptide/protein ingredientsFibrin glueClot formation
The invention is a fibrin glue that avoids the use of fibrinogen and thus eliminates the need for premixing and premature clot formation. The fibrin glue of the invention comprises thrombin, thromboplastin and calcium and may have clotting Factors, VII, IX and X, and the like. The invention also comprises a biosealant for use with the fibrin glue without fibrinogen or for use alone. The biosealant is a two component mixture of gelatin / resorcinol and glyoxal / glutaraldehyde / 4-(p-maleimidophenyl) butyric acid. The two components are mixed on use.
Owner:WORTHAM LEON
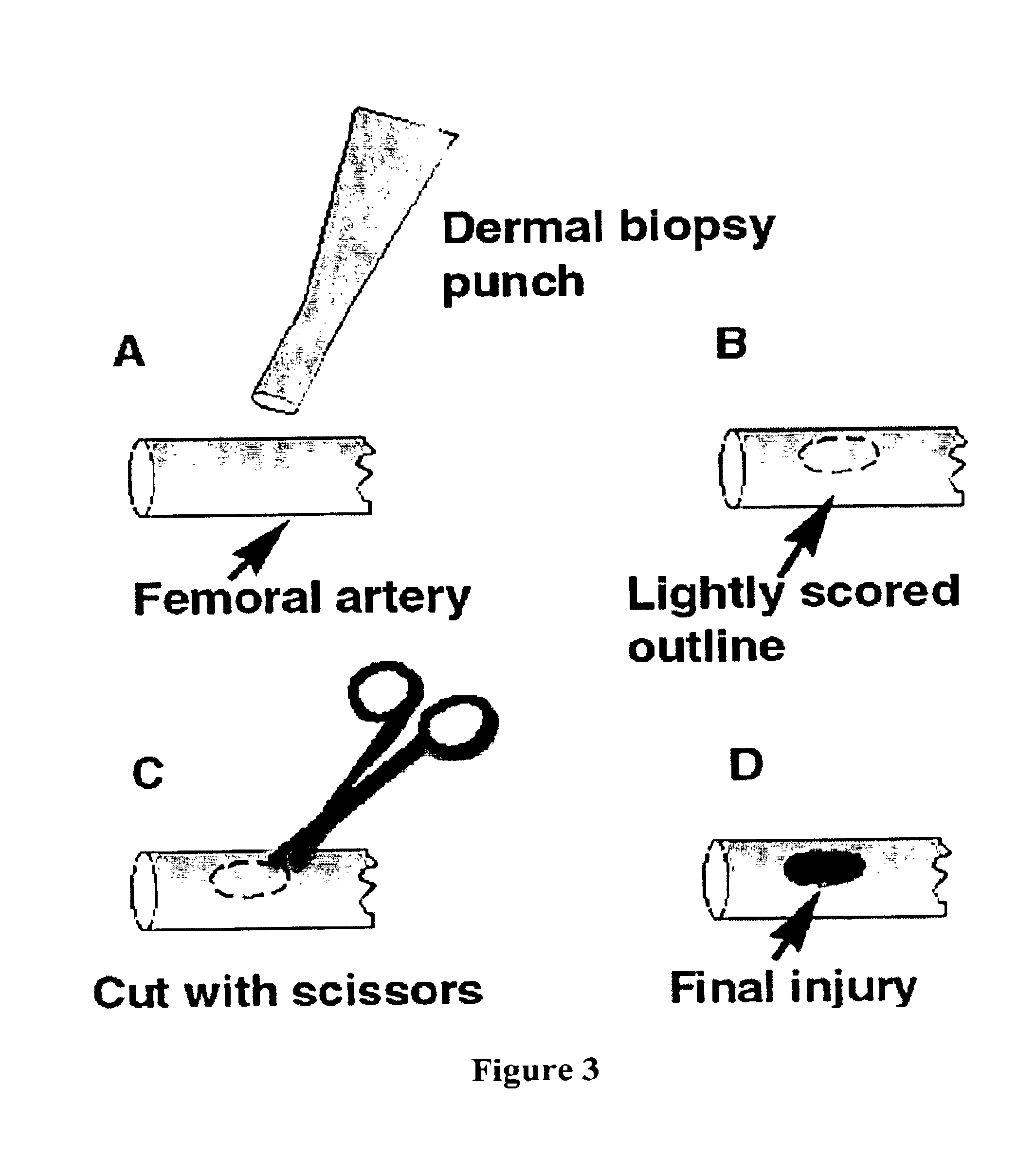
Fibrinogen bandages and arterial bleeding models and methods of making and using thereof
InactiveUS6891077B2Reduce the amount of solutionIncreasing of rate of clotSurgeryBaby linensBlood platelet countsClot formation
Disclosed herein are wound dressings comprising fibrinogen and at least one procoagulant such as propyl gallate in a therapeutic amount. Also disclosed are methods of treating wounds, increasing an amount of or rate of coagulation of blood from a wound, increasing an amount of or rate of clot formation over a wound, increasing blood platelet counts, activating a coagulation system, increasing the plasma concentration of fibrinogen, and decreasing the activated partial thromboplastin time. Also disclosed are an arterial bleeding model and methods of studying arterial bleeding.
Owner:UNITED STATES ARMY U S ARMY MEDICAL RES & MATERIEL COMMAND
Cordyceps militaris health-care beverage prepared by liquid submerged fermentation and preparation method thereof
ActiveCN103330258AHigh temperature resistantLong foam suppression timeFood preparationHorticultureCordycepsSubmerged fermentation
The invention discloses a cordyceps militaris health-care beverage prepared by liquid submerged fermentation and a preparation method thereof. The preparation method comprises the following steps: sequentially performing culture activation, shake culture, seeding tank cultivation and fermentation tank cultivation on cordyceps militaris, then achieving high-temperature extraction of effective components and thromboplastin killing to obtain fermentation liquor, and finally conducting filtering, blending, sterilizing, subpackaging and other procedures to obtain the health-care beverage product. The cordyceps militaris health-care beverage is prepared by adopting the liquid submerged fermentation technology, is simple in process and free from influences of seasons and weather, meets the large-scale periodic production requirement, is short in producting cycle, low in cost, and excellent and stable in product quality, and has unique heavy fragrance, efficiency and properties of cordyceps militaris, thereby obtaining very wide market prospect.
Owner:黄雄
Thromboplastin reagents
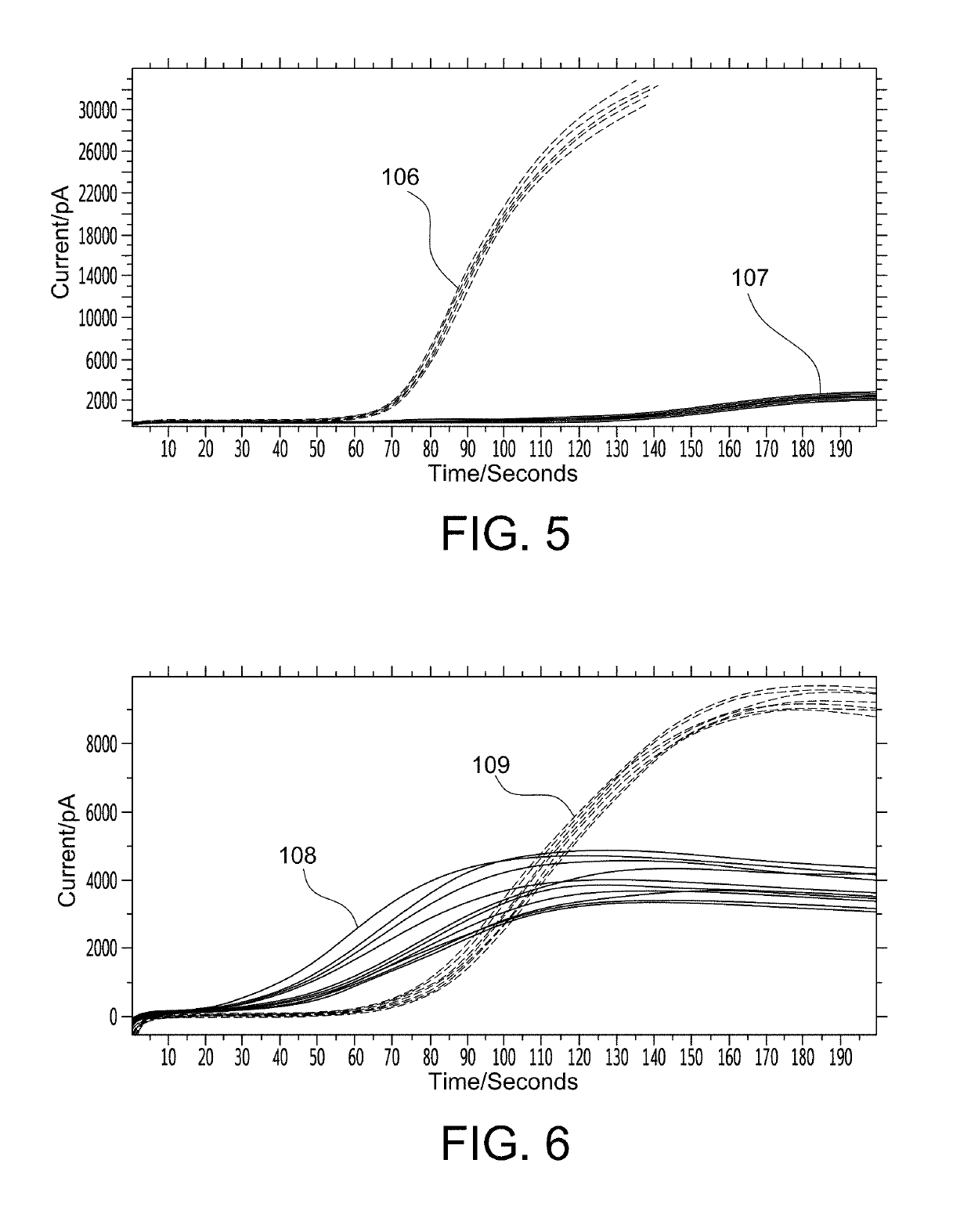
A thromboplastin reagent includes tissue factor, Factor VIIa, and a net negatively charged phospholipid. The thromboplastin reagent is a synthetic thromboplastin reagent, and is in dried form.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
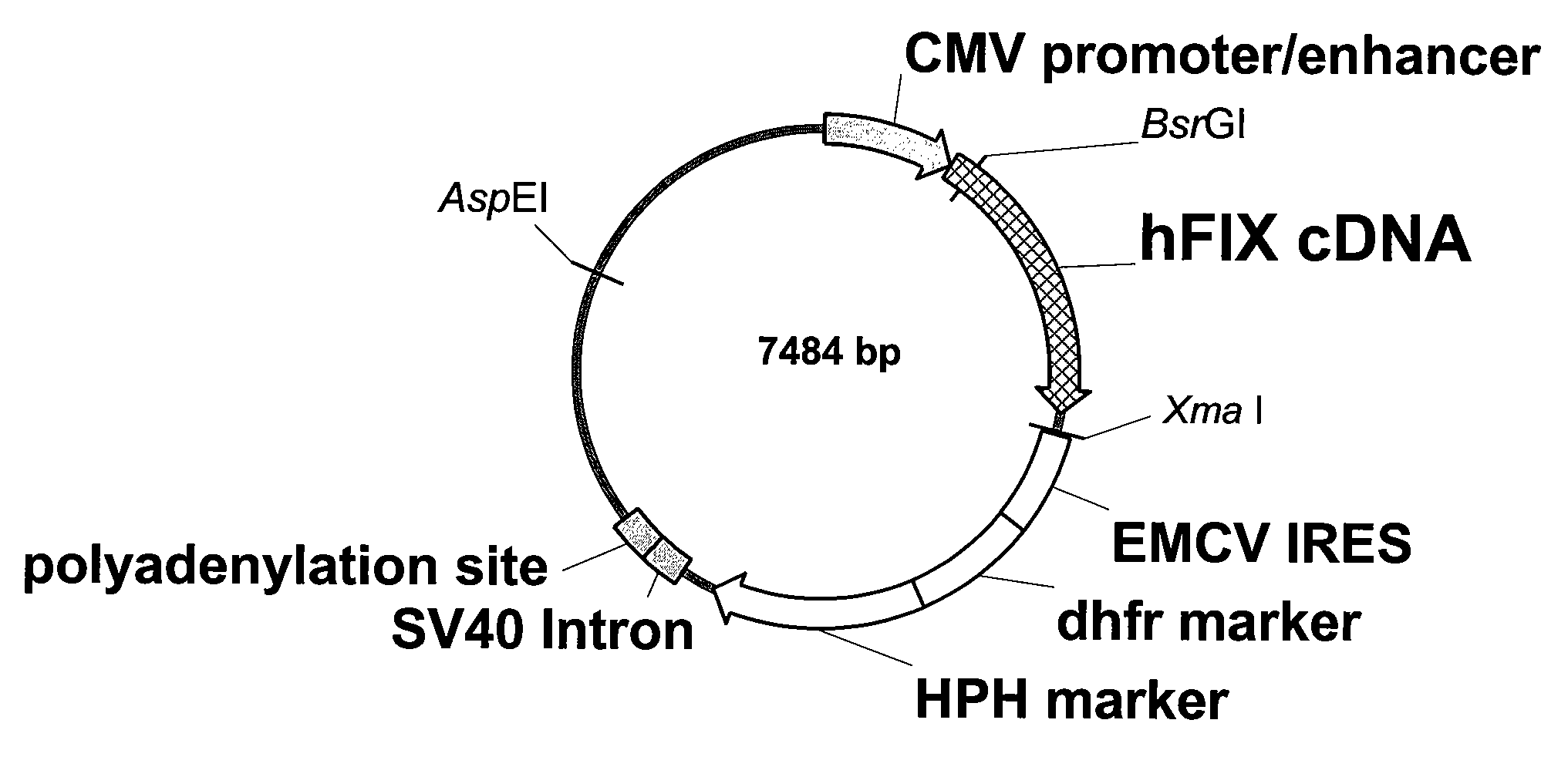
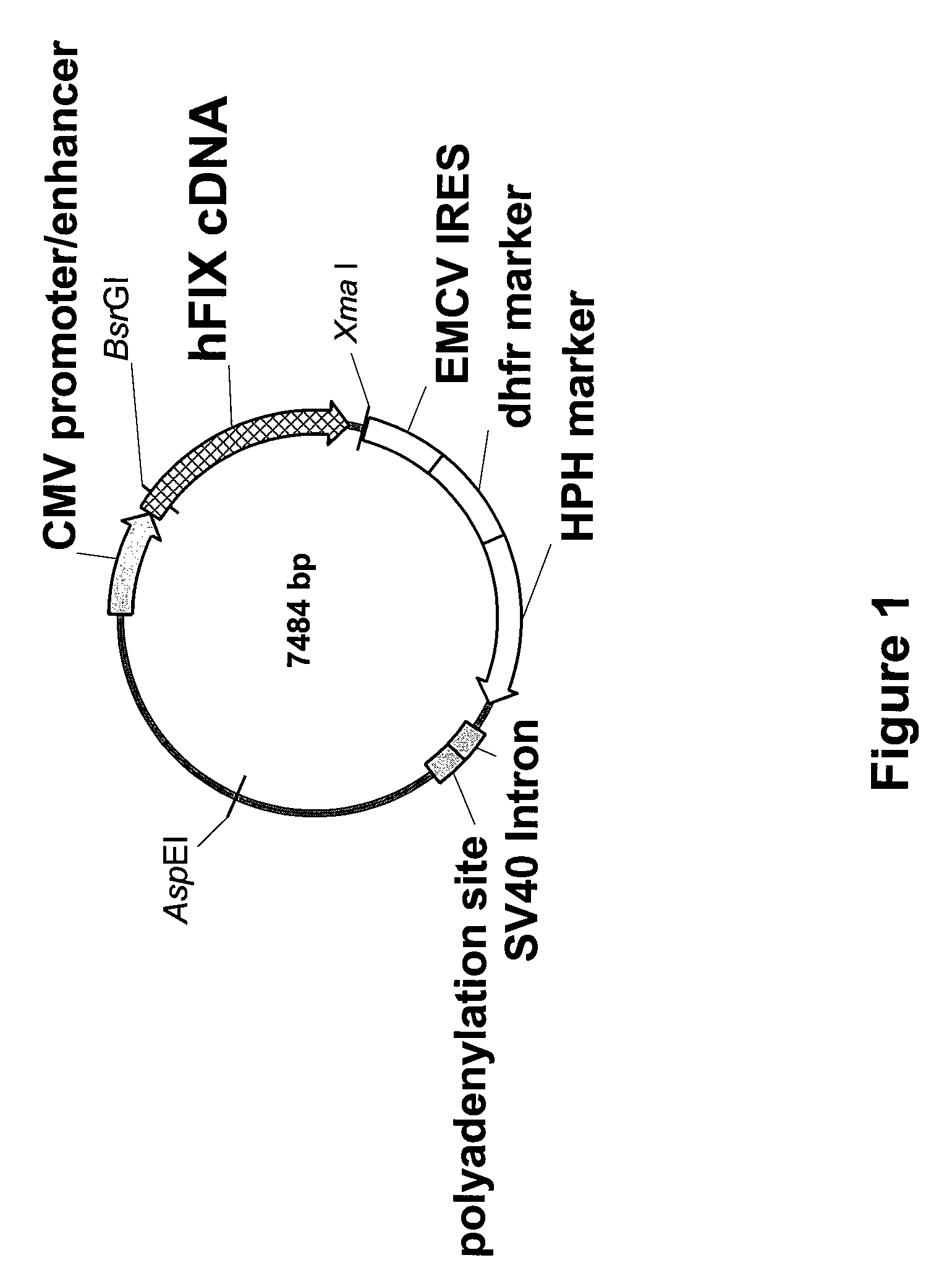
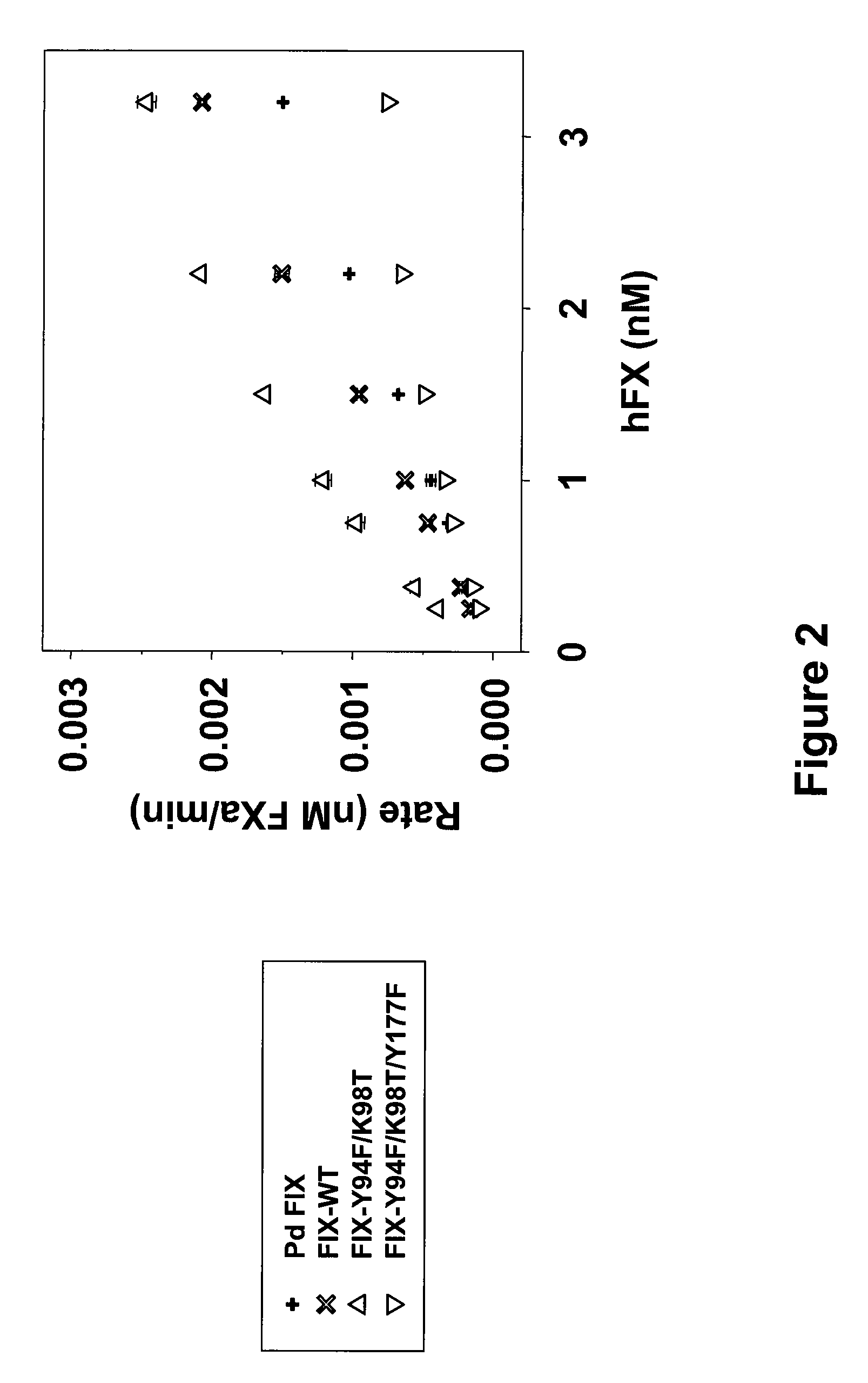
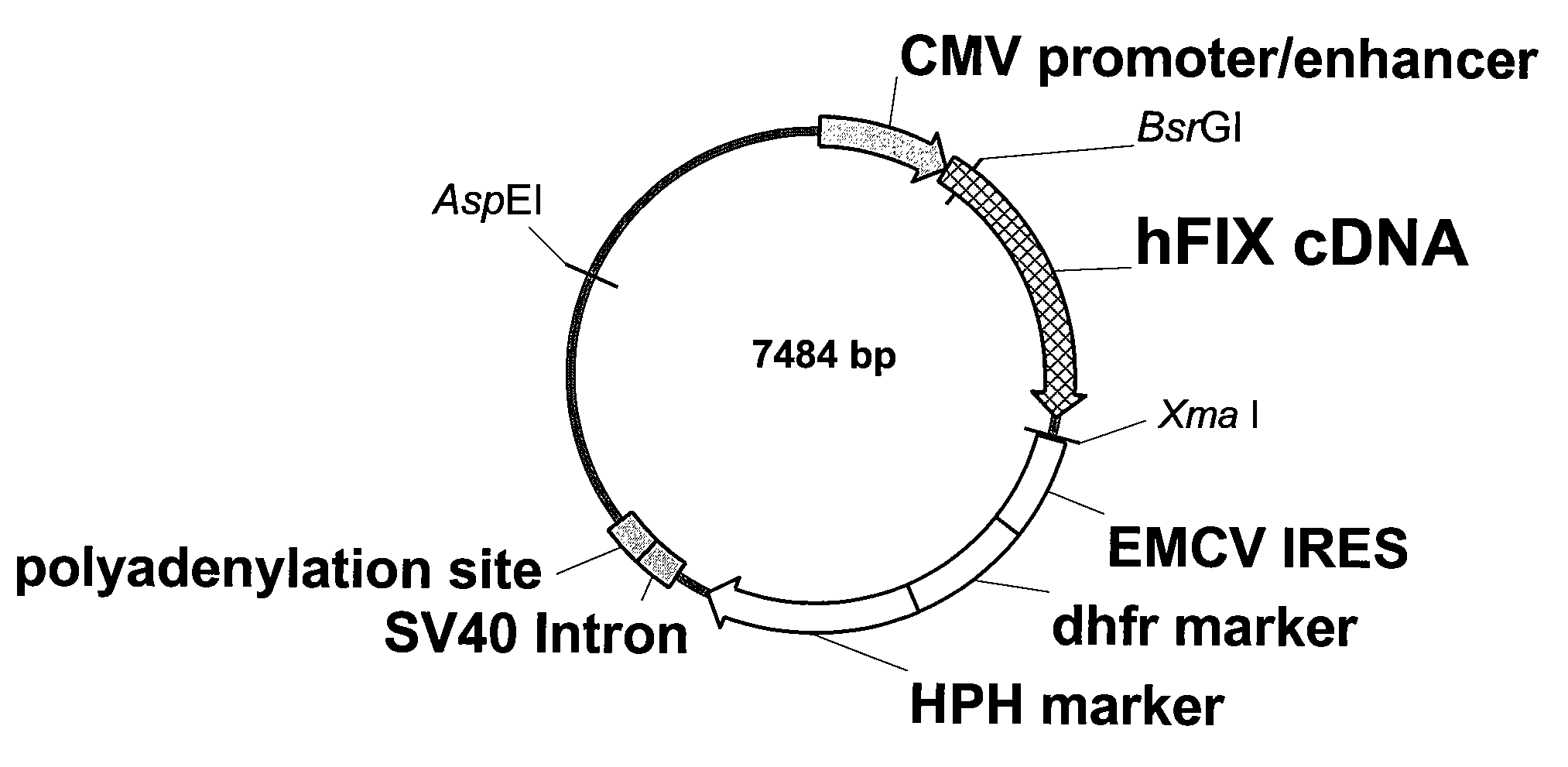
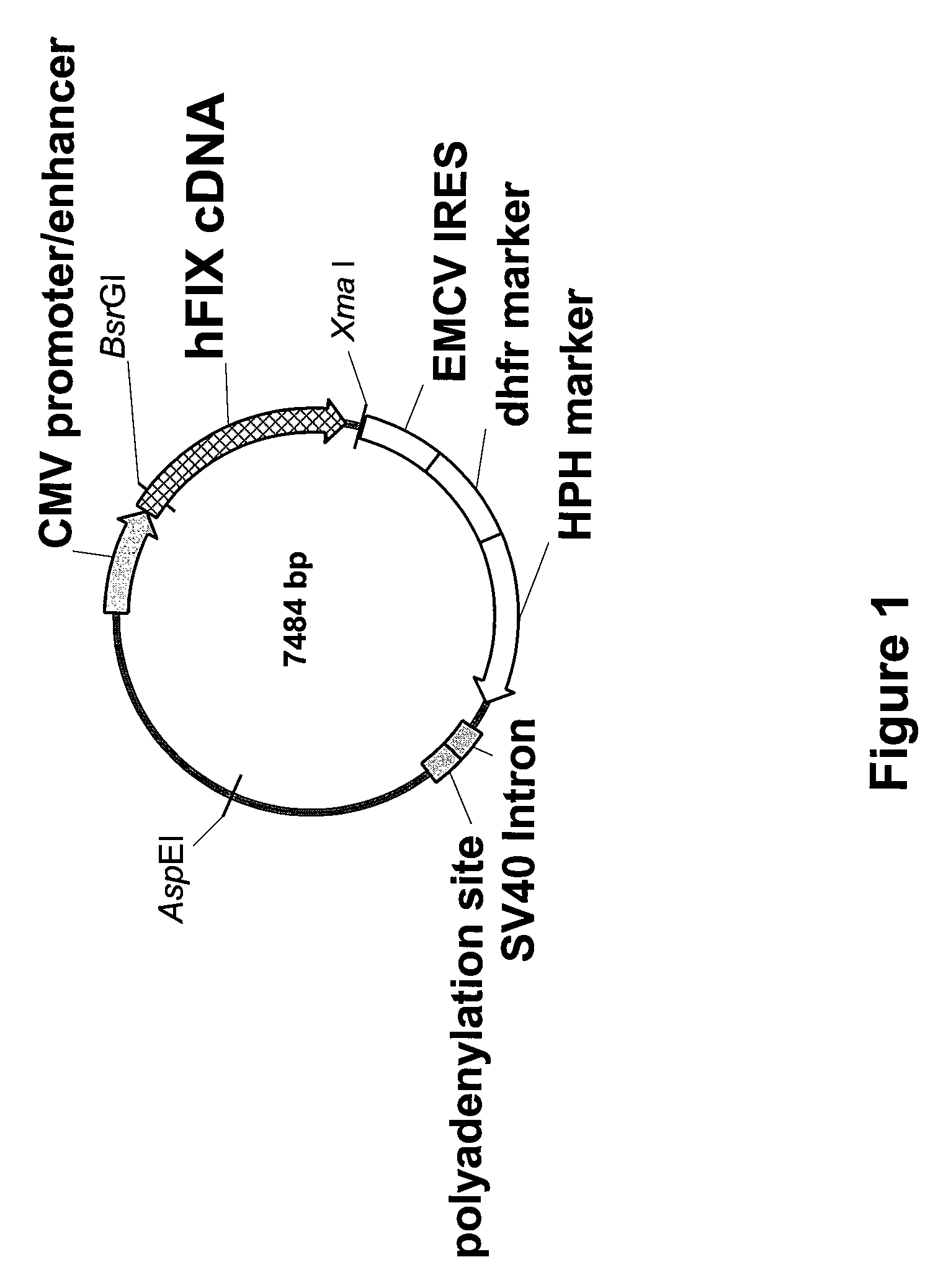
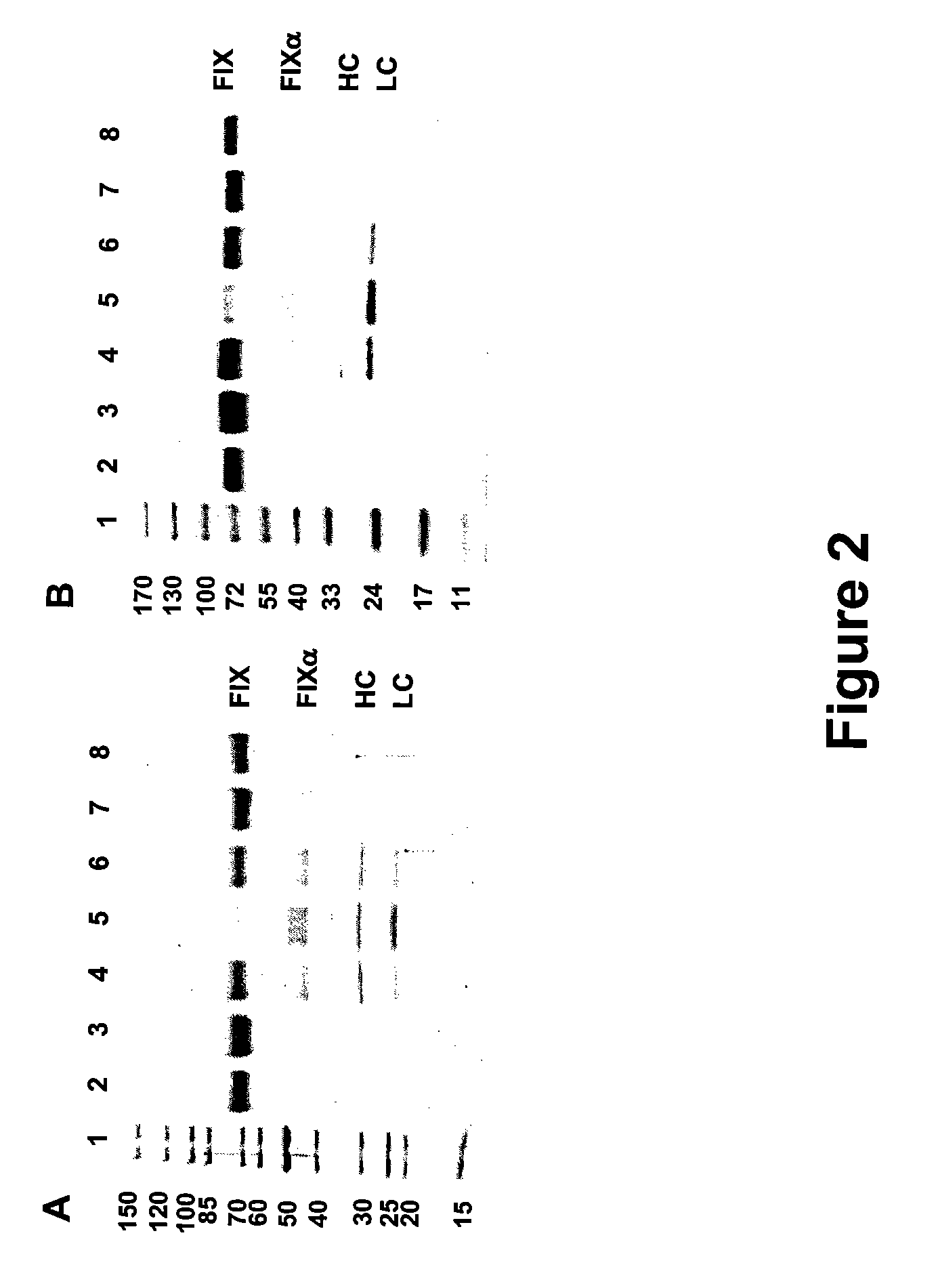
FIX-Mutant Proteins for Hemophilia B Treatment
InactiveUS20080214462A1Improved clot activityHigh activityPeptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsDisease
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having improved FIX clotting activity. Three full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assays in FIX-depleted plasma. Two mutant proteins had increased specific FIX activity. Furthermore, a pre-activated FIX protein had an increased activity in FIX-depleted plasma. Therefore these FIX mutants can be used for the treatment of FIX associated bleeding disorders.
Owner:BAXTER INT INC +1
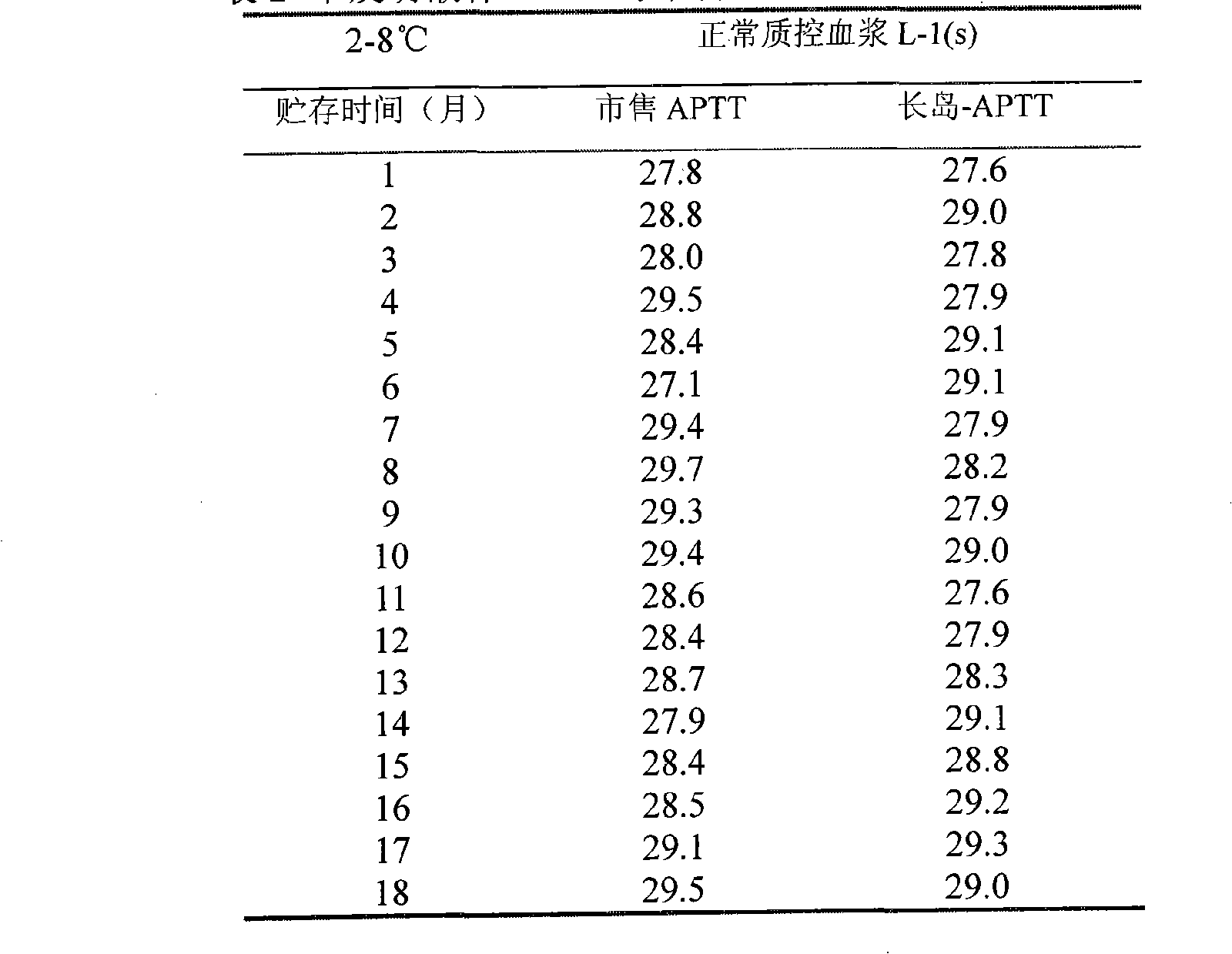
Method for preparing liquid porcellanite APTT reagent
ActiveCN101526538AImprove stabilityImprove compatibilityBiological testingSystemic lupus erythematosusPorcellanite
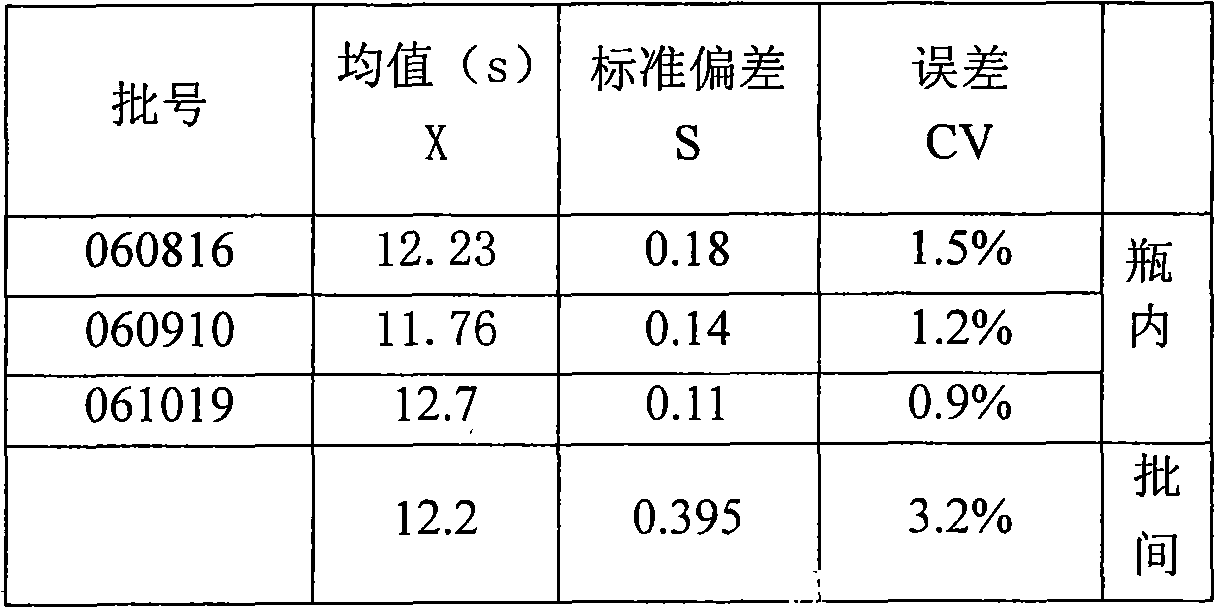
The invention relates to a method for preparing a liquid porcellanite activating partial thromboplastin (APTT) reagent. The reagent is mainly used for monitoring coagulation factor deficiency of endogenous coagulation systems and carrying out screening test of related inhibitors, and used for detecting coagulation factors, heparin anticoagulation treatment and lupus anticoagulation substances as the current main means. Taking porcellanite as an activating agent, the method improves the sensitivity to the coagulation factors; a novel stable system solves the problem of keeping the stability of the reagent at a liquid state; and the method has the advantages of high sensitivity, convenient use, small experimental error, strong stability, good compatibility, low cost and the like.
Owner:SHANGHAI LONG ISLAND BIOTEC CO LTD
FVIII-Independent FIX-Mutant Proteins for Hemophilia A Treatment
ActiveUS20080214461A1Peptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsMutated protein
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having factor VIII (FVIII) independent factor X (FX) activation potential. Five full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assay in FVIII-depleted plasma as well as in FVIII-inhibited patient plasma. In FVIII-depleted plasma functional activity of the FIX mutants was calculated as increased FVIII equivalent activity. The mutant proteins had increased FVIII equivalent activity. In FVIII-inhibited patient plasma the FEIBA equivalent activity was calculated for analysis of FVIII independent FX activation potential. The proteins had also increased FEIBA equivalent activity. Furthermore, the pre-activated FIX proteins had an increased activity in FIX-depleted plasma containing FVIII inhibitors. Therefore these FIX mutants are alternatives as bypassing agents for treatment of FVIII inhibitor patients.
Owner:TAKEDA PHARMA CO LTD
Activated partial thromboplastin time measuring reagent, activated partial thromboplastin time measuring method, and determination method for determining presence or absence of blood coagulation inhibitor
InactiveUS20110159597A1Organic chemistryOrganic compound preparationPartial thromboplastin timeThromboplastin
An activated partial thromboplastin time measuring reagent, comprising a heparin neutralizer is disclosed. An activated partial thromboplastin time measuring method, and a determination method for determining a presence or absence of a blood coagulation inhibitor are also disclosed.
Owner:SYSMEX CORP
Method and reagent kit for measuring activated partial thromboplastin time
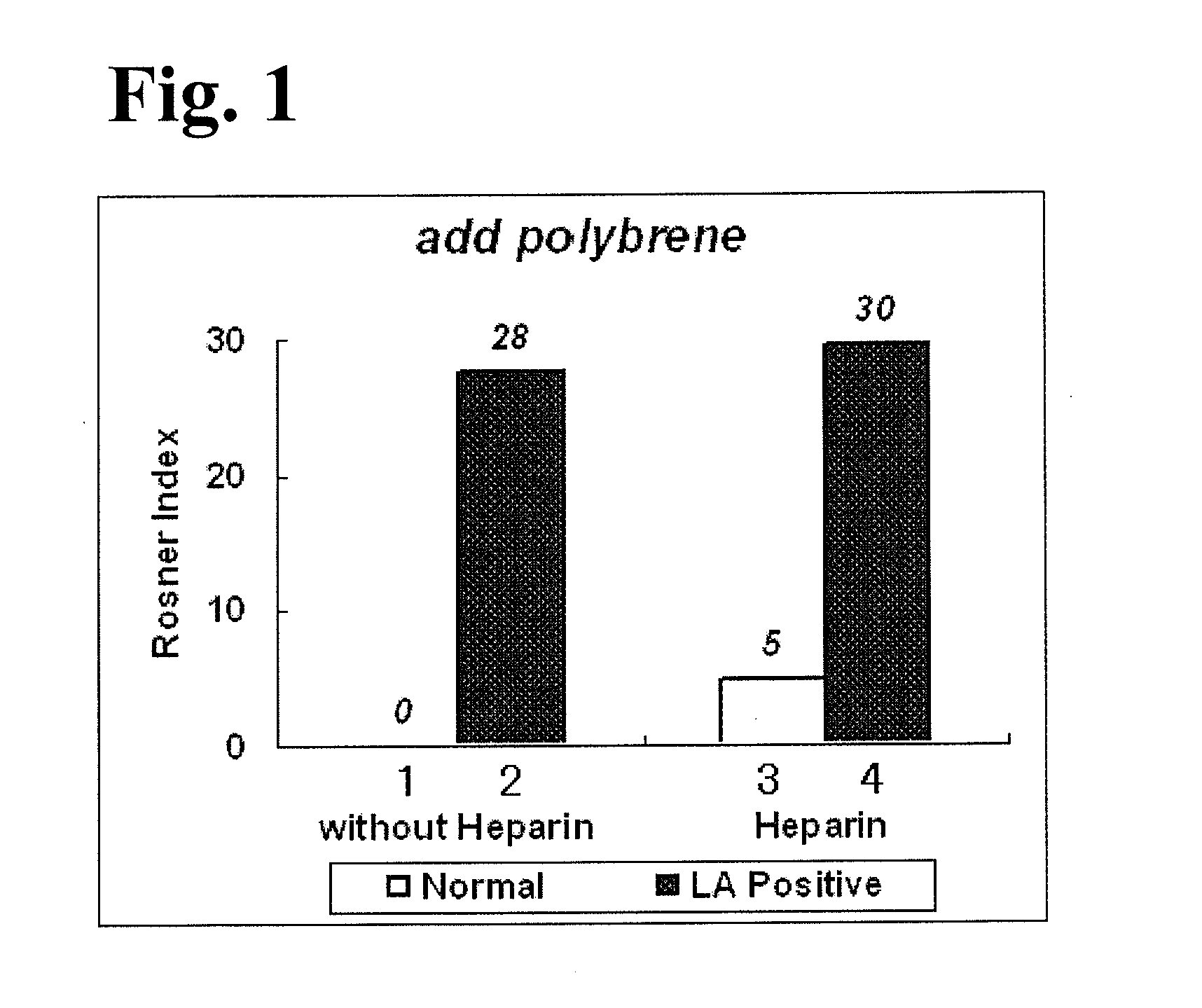
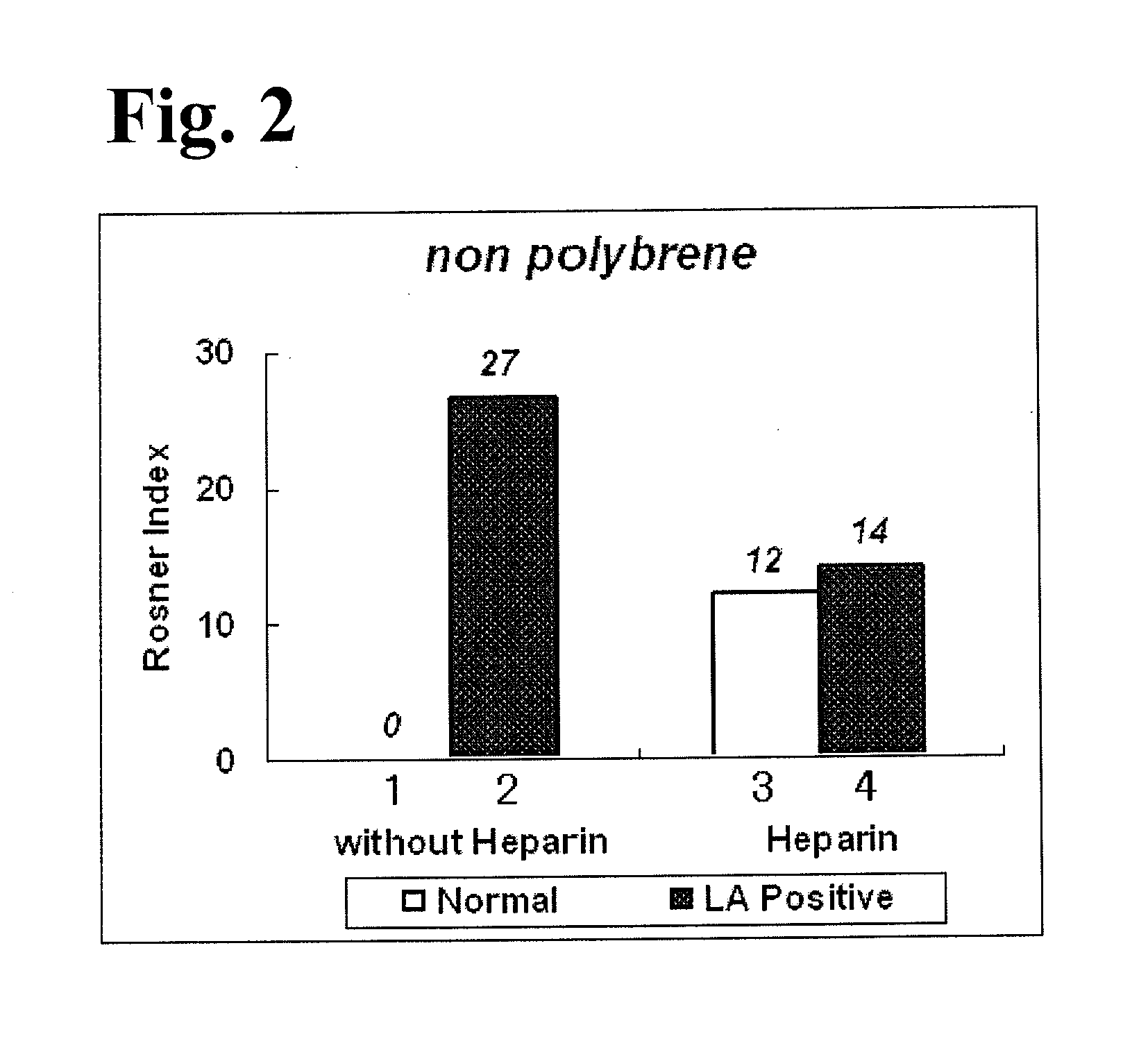
InactiveUS20140295470A1Less LA-induced impactOccurrence of preventedMicrobiological testing/measurementBiological material analysisBlood plasmaReagent
The present invention provides a method for measuring activated partial thromboplastin time. The method comprises: a first mixing step of mixing a blood plasma with a first reagent, wherein the first reagent comprises an activator and phosphatidylglycerol at a concentration equal to or greater than 25 μg / mL; a second mixing step of mixing a sample obtained in the first mixing step with a second reagent comprising a calcium salt; and a step of measuring coagulation time of the sample obtained in the second mixing step.
Owner:SYSMEX CORP
Thromboplastin reagents
A thromboplastin reagent includes tissue factor, Factor VIIa, and a net negatively charged phospholipid. The thromboplastin reagent is a synthetic thromboplastin reagent, and is in dried form.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
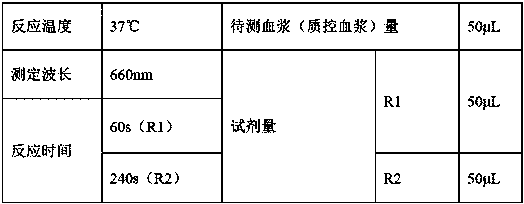
A kit for measuring activated partial thromboplastin time (APTT)
ActiveCN107748267AAvoid Bottle-to-Bottle VariationsGuaranteed stabilityDisease diagnosisBiological testingFreeze-dryingChloride
A kit for measuring activated partial thromboplastin time (APTT) is provided. The kit includes R1 including an activator, partial thromboplastin, a stabilizer, an aseptic and a buffer liquid; and R2 that is a calcium chloride solution. The stabilizer includes dextran. In the R1, the concentration of the activator is 0.05-0.2 mM, the content of the partial thromboplastin is 0.1-0.5 wt%, the contentof the aseptic is 0.01-0.02 wt% and the content of the dextran is 2-4 wt%. All agents in the kit are liquid and stable so that reagent differences among bottles caused by freeze-drying and redissolving processes in preparation and application processes, thus avoiding large differences among measurement results. Stability of the reagents can be ensured without the need of freeze drying, and optimum validity of the reagents after bottle opening is at least a month, thus avoiding large differences among experiment results, reducing using amounts of the reagents, achieving instant use after bottle opening, and making operation rapider and simpler.
Owner:山东艾科达生物科技有限公司
Prepn. of blood coagulation factor IX compound
InactiveCN1336178AHigh recovery rateReduce contentPeptide/protein ingredientsMammal material medical ingredientsPhosphateDEAE-Sepharose
The present invention relates to the preparation of plasma thromboplastin antecedent IX complex phosphate buffer solution or citrate buffer solution with 4-7 milli mole concertration and pH 5.5-6.5 is used to balance the gel used for adsorption, with fresh frozen human plasmia being added. DEAE-sepharose Fast Flow is used to proceed ion exchange adsorption separation, then same buffer solution with pH 5.5-7.0 is used to wash adsorption gel. further same buffer solution with pH 6-8 is used to elute adsorption gel, collect plasma thromboplastin antecedent IX complex containing factor II, VII, IX and X.
Owner:四川高维系统工程技术有限公司 +2
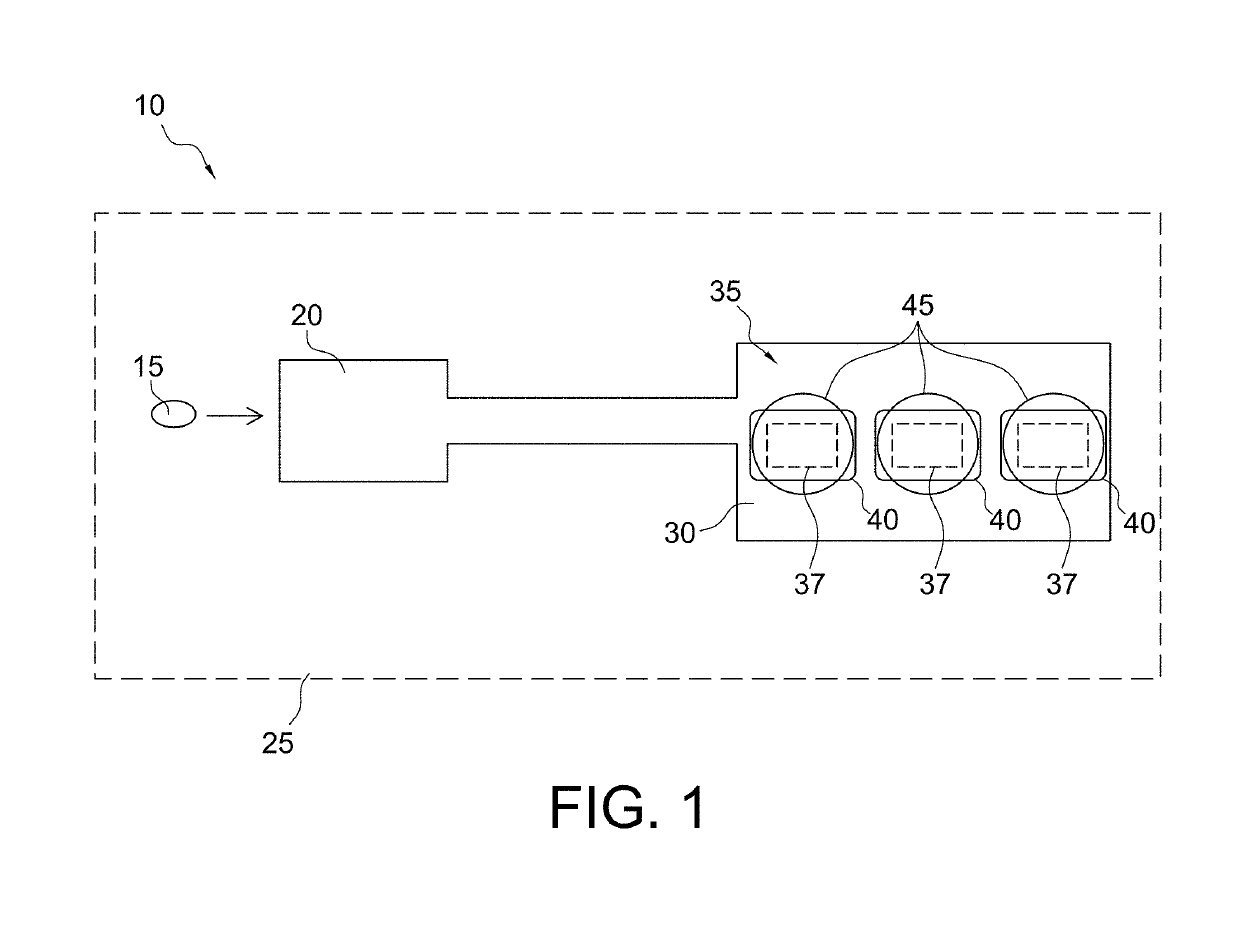
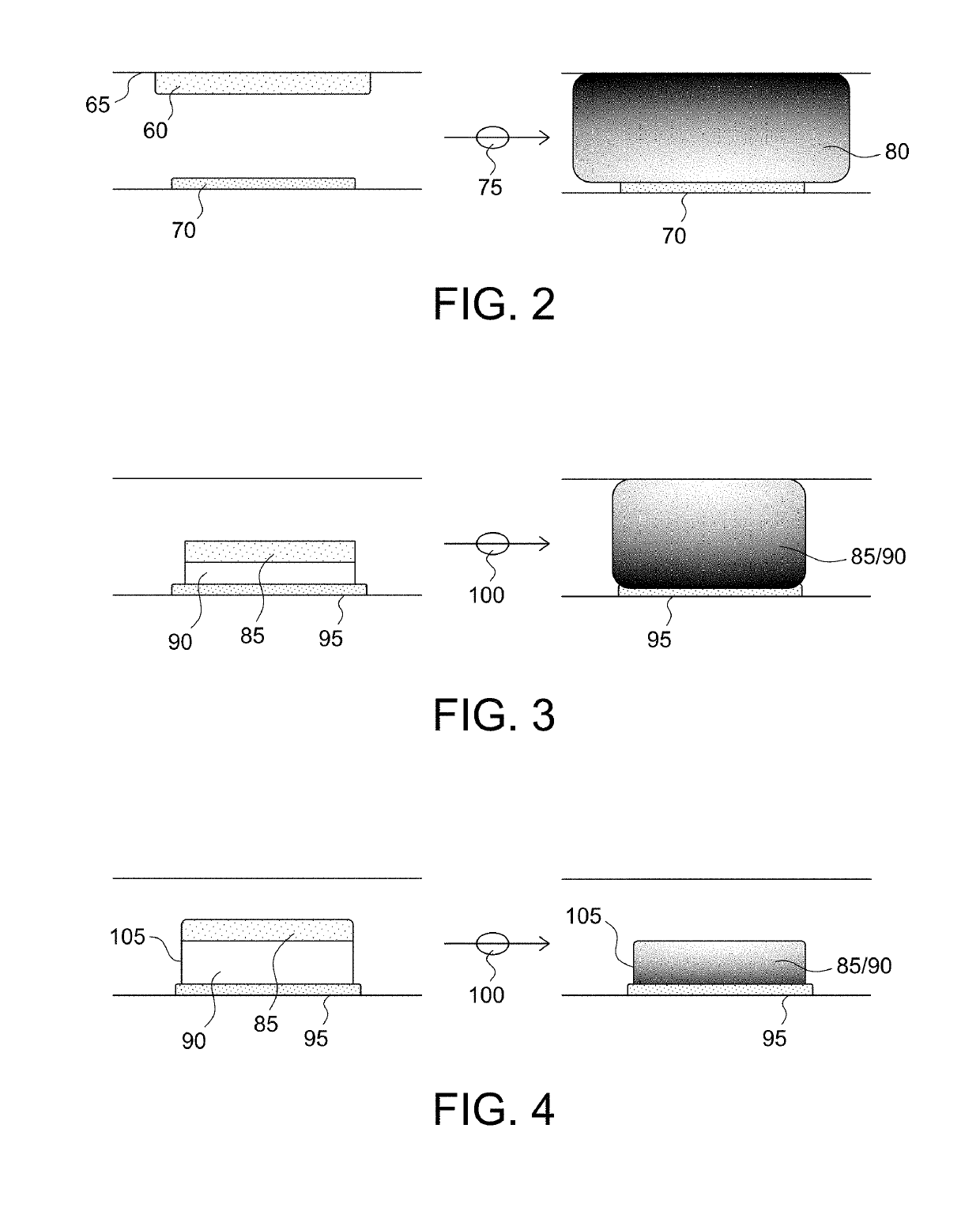
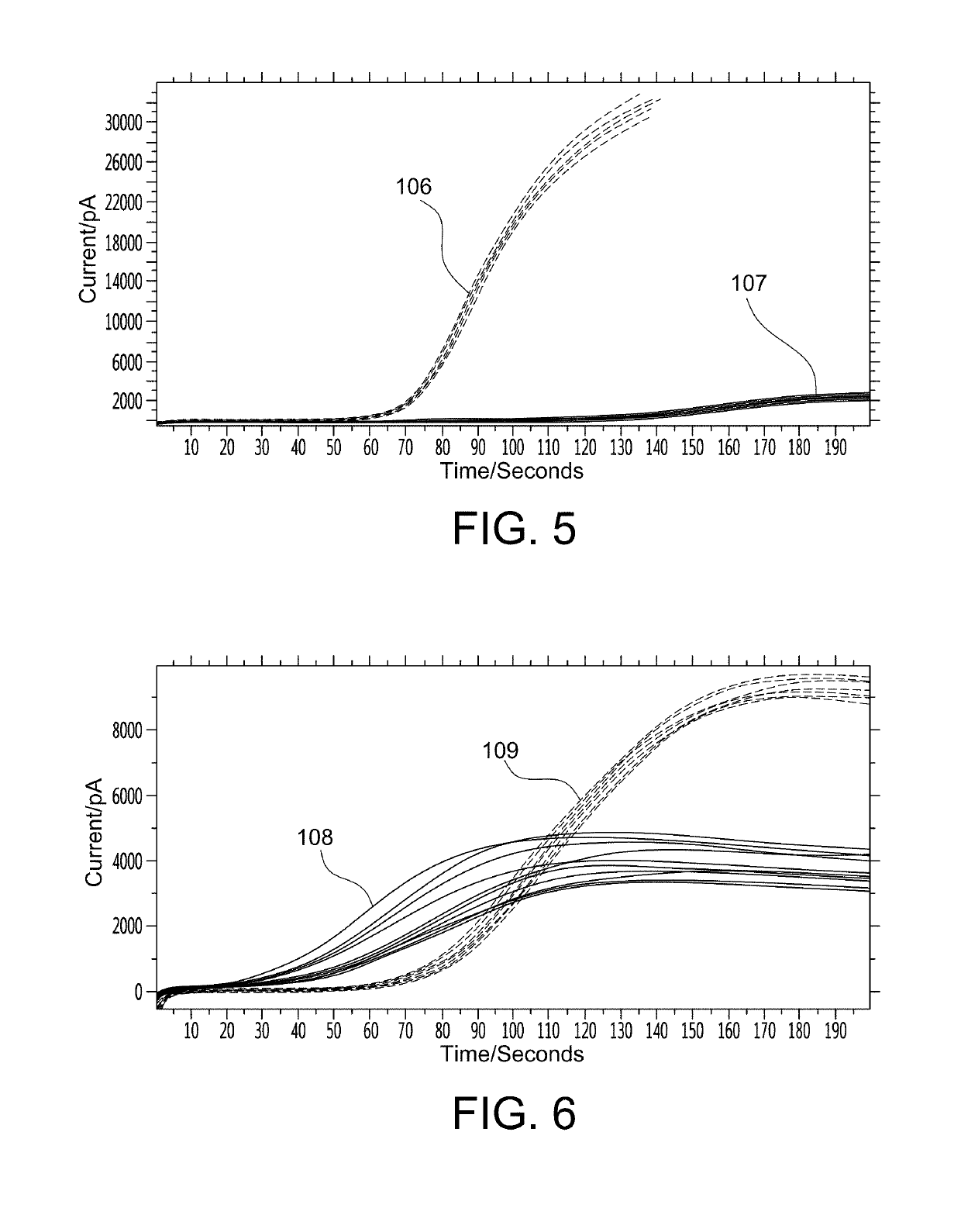
Microfabricated device with micro-environment sensors for assaying coagulation in fluid samples
ActiveUS10247741B2Biological material analysisMaterial analysis by electric/magnetic meansPoint of careEngineering
The present invention relates to sample analysis cartridges comprising micro-environment sensors and methods for assaying coagulation in a fluid sample applied to the micro-environment sensors, and in particular, to performing coagulation assays using micro-environment sensors in a point of care sample analysis cartridge. For example, the present invention may be directed to a sample analysis cartridge including an inlet chamber configured to receive a biological sample, and a conduit fluidically connected to the inlet chamber and configured to receive the biological sample from the inlet chamber. The conduit may include a micro-environment prothrombin time (PT) sensor, and a micro-environment activated partial thromboplastin time (aPTT) sensor.
Owner:ABBOTT POINT CARE
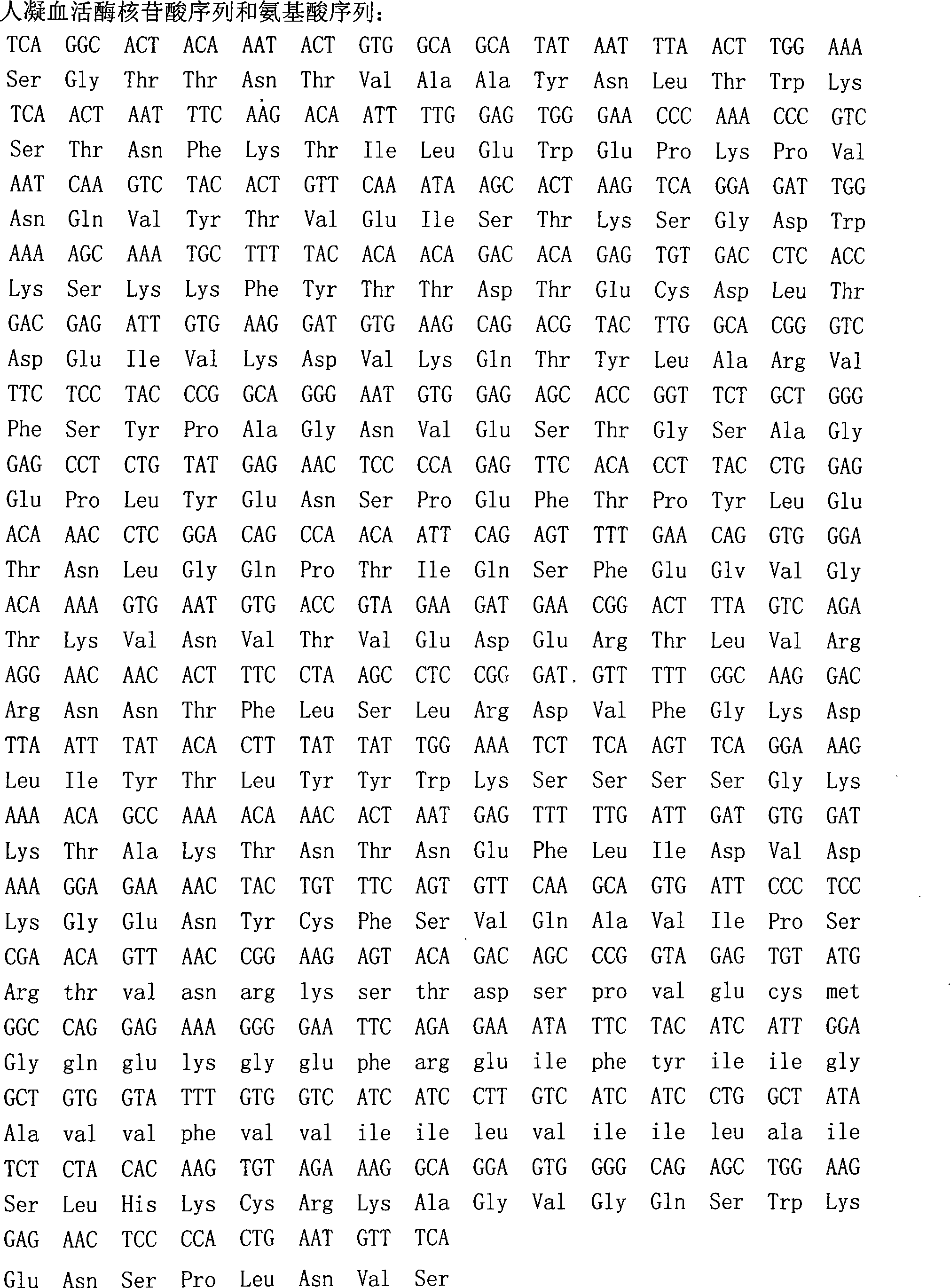
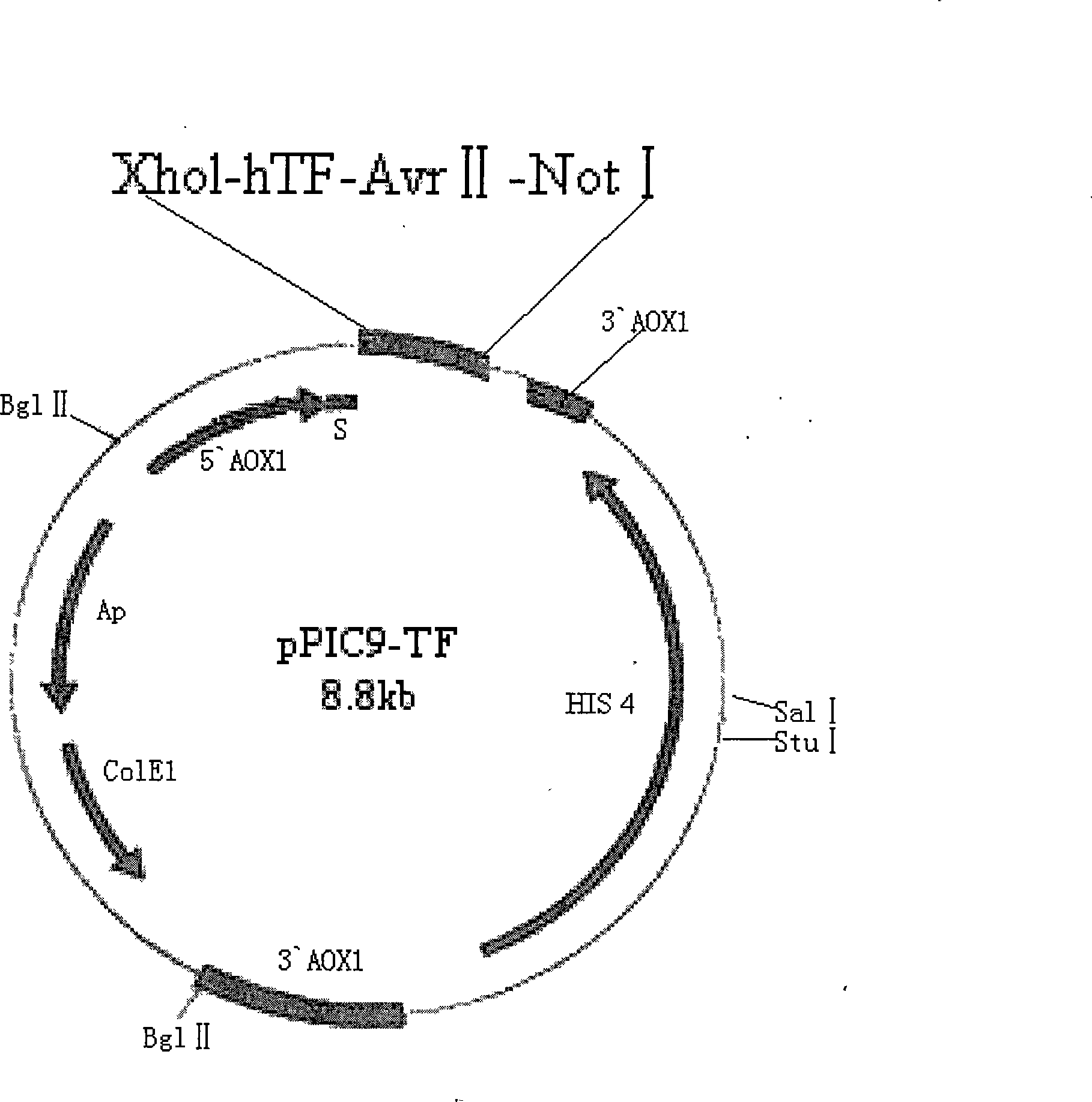
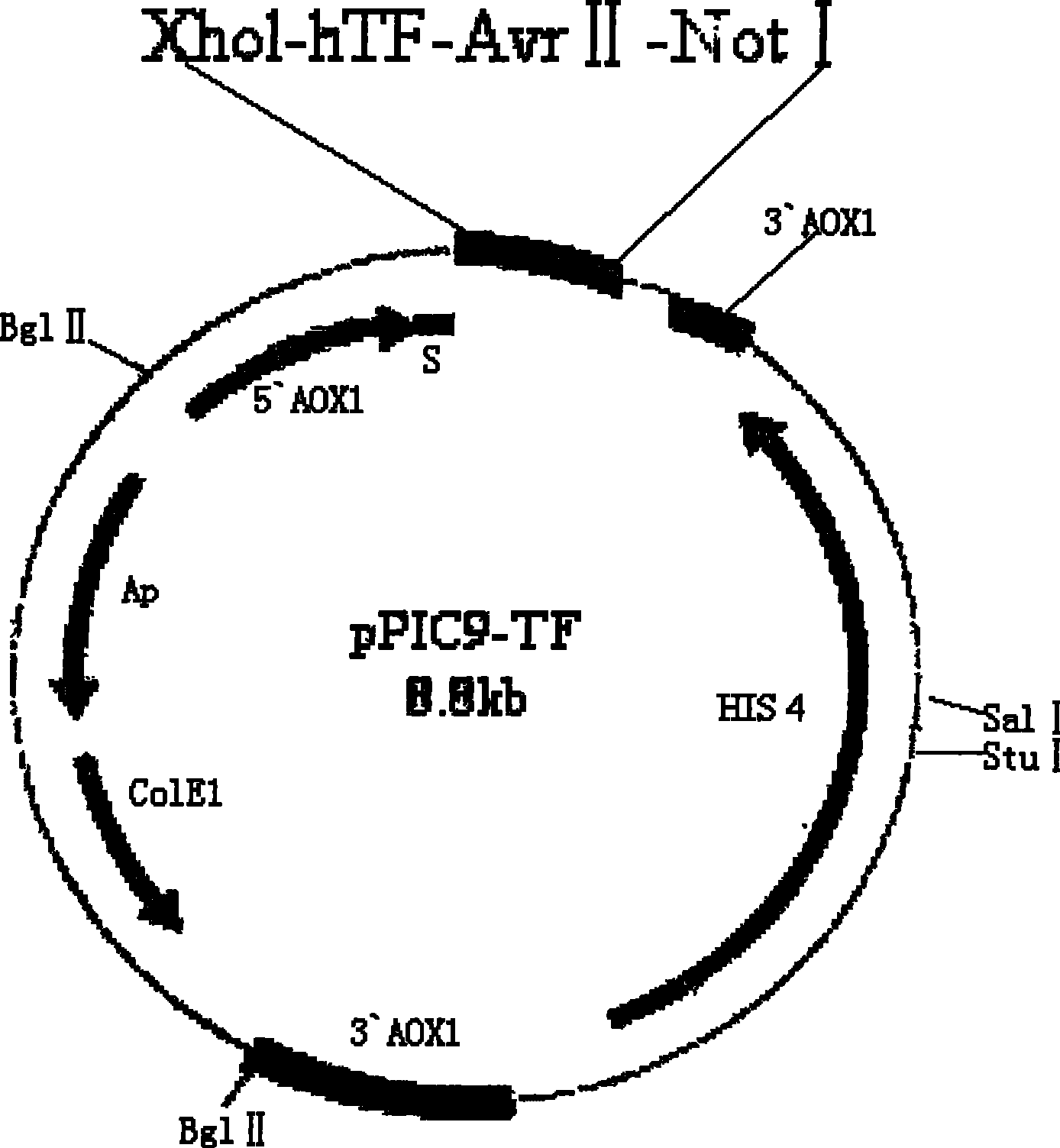
Method for preparing liquid type PT agent based on human recombinant thrombokinase
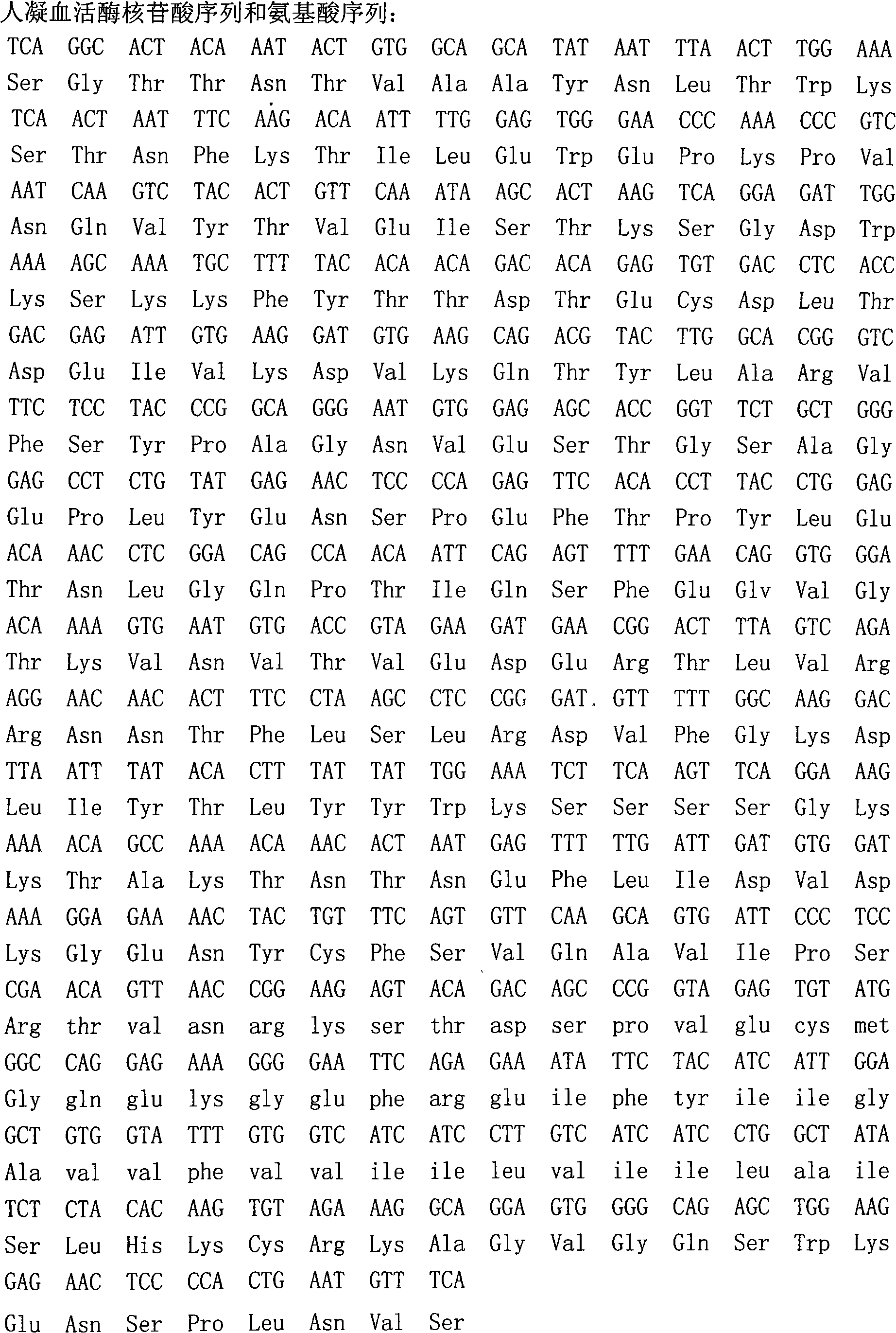
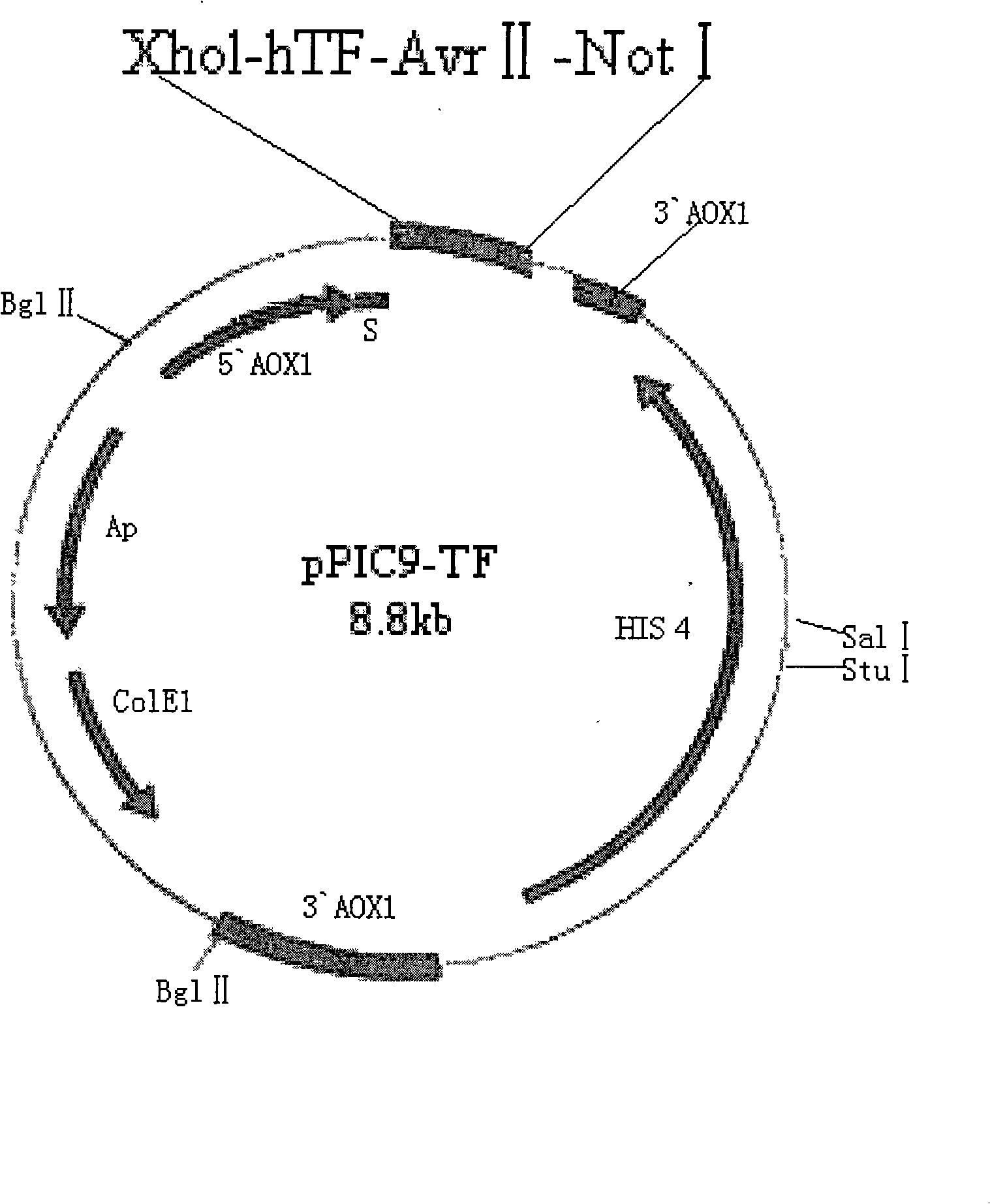
ActiveCN101294163AHigh purityHigh activityFungiMicrobiological testing/measurementBiotechnologyProtein target
The invention belongs to the field of biotechnology and aims to solve clinic standardization problem for PT determination. The quality of a PT reagent is a key factor influencing PT test, and the effective method for reducing the difference of determination results in different laboratories is to unify the chemical composition and biochemical characterics of thromboplastin in reagent. The recombined thromboplastin prepared by genetic engineering method has stable molecular structure and uniform protein, and can improve test sensitivity, precision and accuracy. The full-length human thromboplastin expressed by yeast cell can achieve high purity and target protein with high activity by one-step purification. The invention also provides a method for preparing liquid PT reagent based on recombined thromboplastin. The reagent has good sensitivity and stability and qualified each technical index, and test shows that the reagent is suitable for clinic application.
Owner:SHANGHAI LONG ISLAND BIOTEC CO LTD
Coagulation and fibrinolytic cascades modulator
InactiveCN101184775APeptide/protein ingredientsMicrobiological testing/measurementFactor iiPolyphosphate
The invention provides a thromboplastin reagent comprising: tissue factor, a phospholipid and a polyphosphate that acts as a blocker of tissue factor pathway inhibitor (TFPI). Also provided are a composition for promoting clotting comprising polyphosphate, and a reagent for a clotting assay also comprising polyphosphate together with an activator of clotting. Methods for stopping or slowing wound bleeding and fibrinolysis using compositions comprising polyphosphate are also disclosed.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Fibrin glue without fibrinogen and biosealant compositions and methods
InactiveUS20010016204A1Stop the flowPrevents unwanted and premature clot formationCosmetic preparationsSurgical adhesivesFibrin glueClot formation
The invention is a fibrin glue that avoids the use of fibrinogen and thus eliminates the need for premixing and premature clot formation. The fibrin glue of the invention comprises thrombin, thromboplastin and calcium and may have clotting Factors, VII, IX and X, and the like. The invention also comprises a biosealant for use with the fibrin glue without fibrinogen or for use alone. The biosealant is a two component mixture of gelatin / resorcinol and glyoxal / glutaraldehyde / 4-(p-maleimidophenyl) butyric acid. The two components are mixed on use.
Owner:WORTHAM LEON
Sterilizing agent for livestock breeding as well as preparation method and application thereof
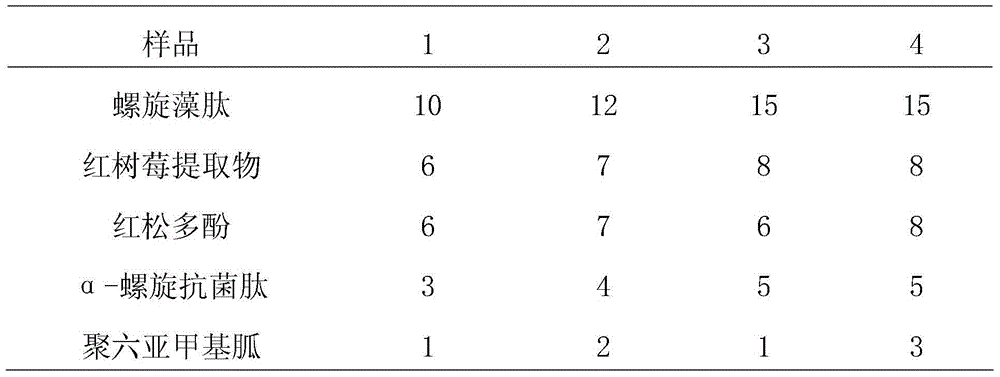
InactiveCN104957190AEfficient killingImprove the bactericidal effectBiocideFungicidesPinus koraiensisPolyphenol
The invention relates to the field of animal hygiene, and particularly relates to a sterilizing agent for livestock breeding. The sterilizing agent comprises the following raw materials in parts by weight: 10 to 15 parts of spirulina peptide, 6 to 8 parts of red raspberry extract, 6 to 8 parts of pinus koraiensis polyphenol, 3 to 5 parts of alpha-spiral antibacterial peptide, 1 to 3 parts of polyhexamethyleneguanidine and 61 to 74 parts of deionized water. The sterilizing agent is used for livestock breeding. Compared with the prior art, the sterilizing agent has the following advantages: (1) marine active thromboplastin and plant extract are used as sterilizing components, so that colibacillus, staphylococcus aureus and candida albicans can be effectively killed, the sterilizing effect is good, and the sterilizing spectrum is wide; (2) the function is stable not susceptible to the influence of temperature, organic matters and acidity and alkality, capable of penetrating the organic matters and cracks and capable of rapidly killing the bacteria and virus; (3) the sterilizing agent is harmless and non-irritable to the human beings, animals and poultry, and the drug has no corrosion after being diluted by water.
Owner:荆永正
Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors
InactiveUS20130090494A1Group 5/15 element organic compoundsPhosphorous compound active ingredientsAntithrombotic AgentBlood plasma
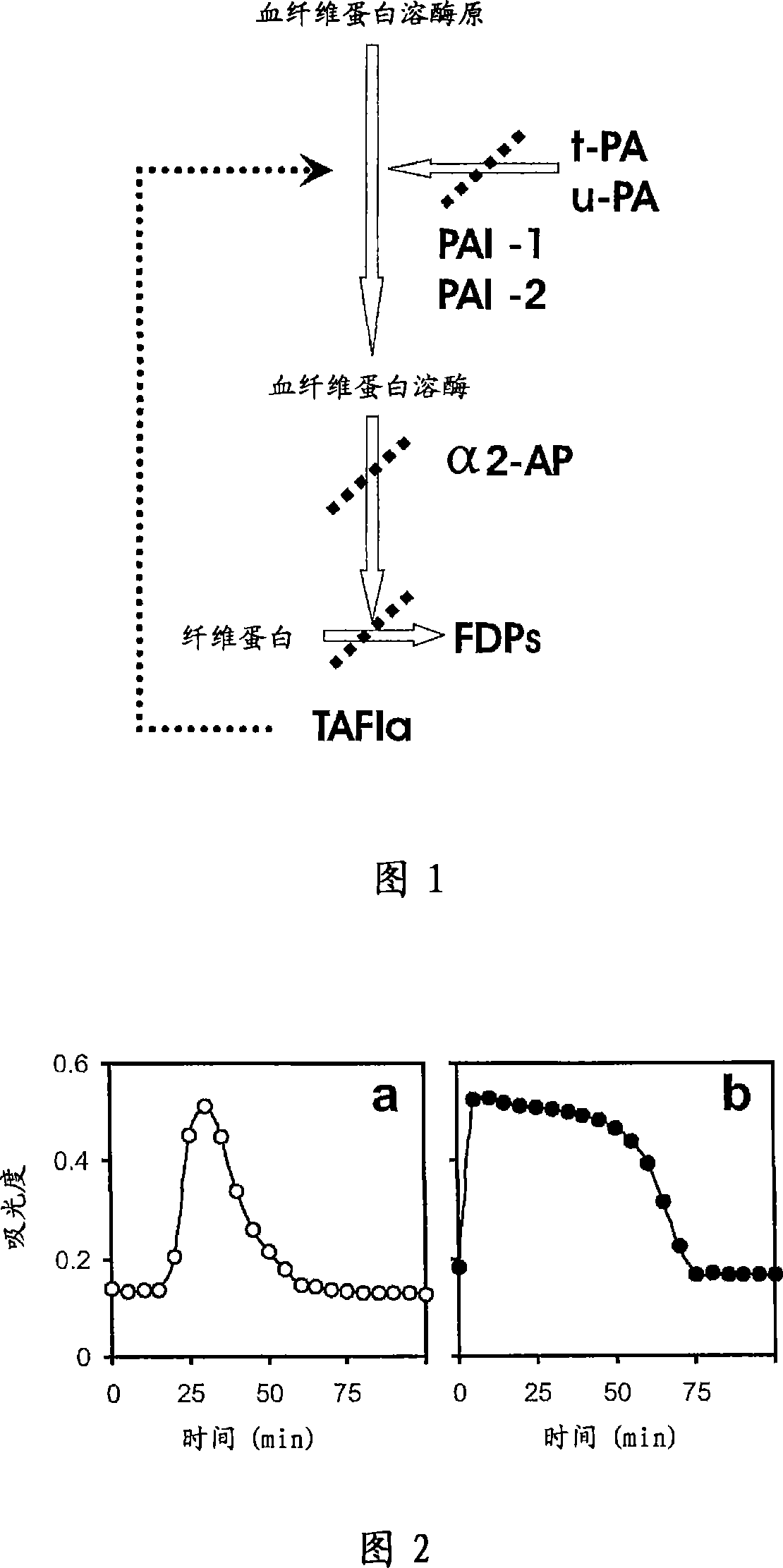
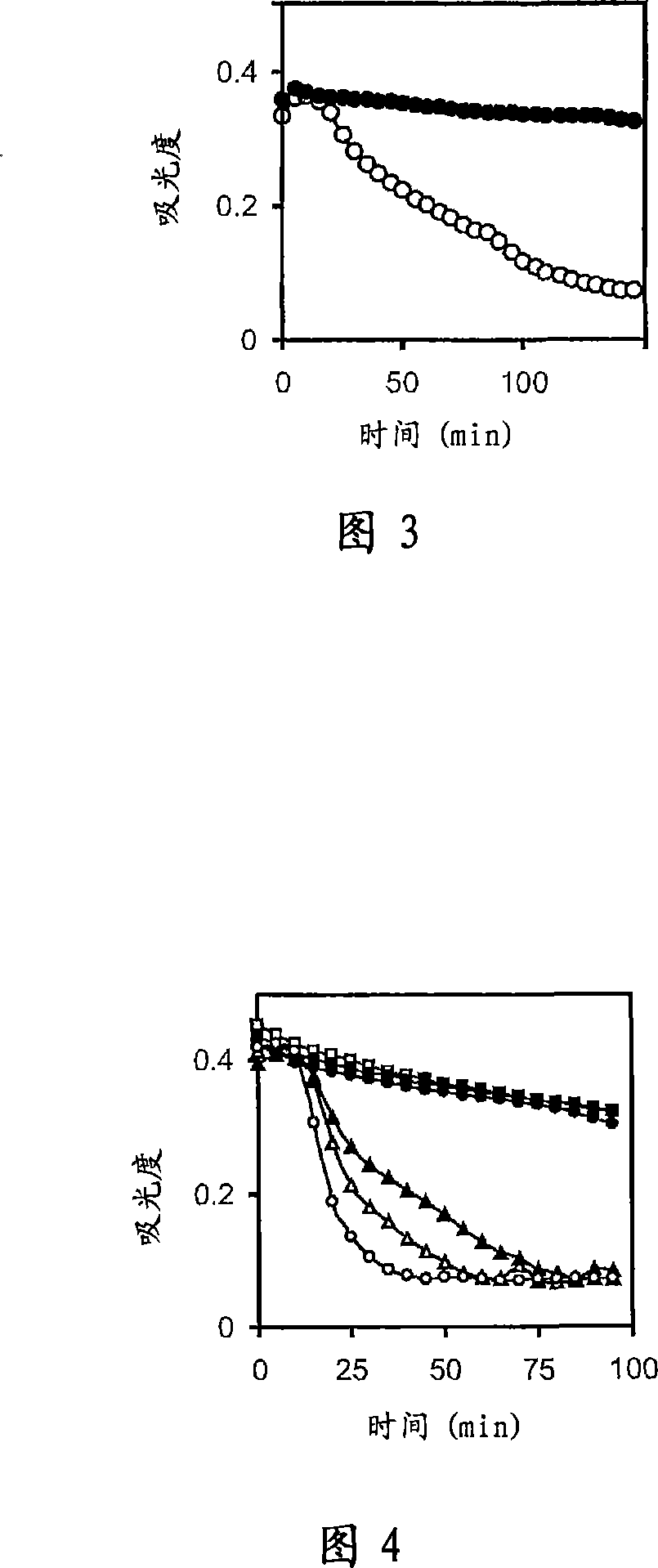
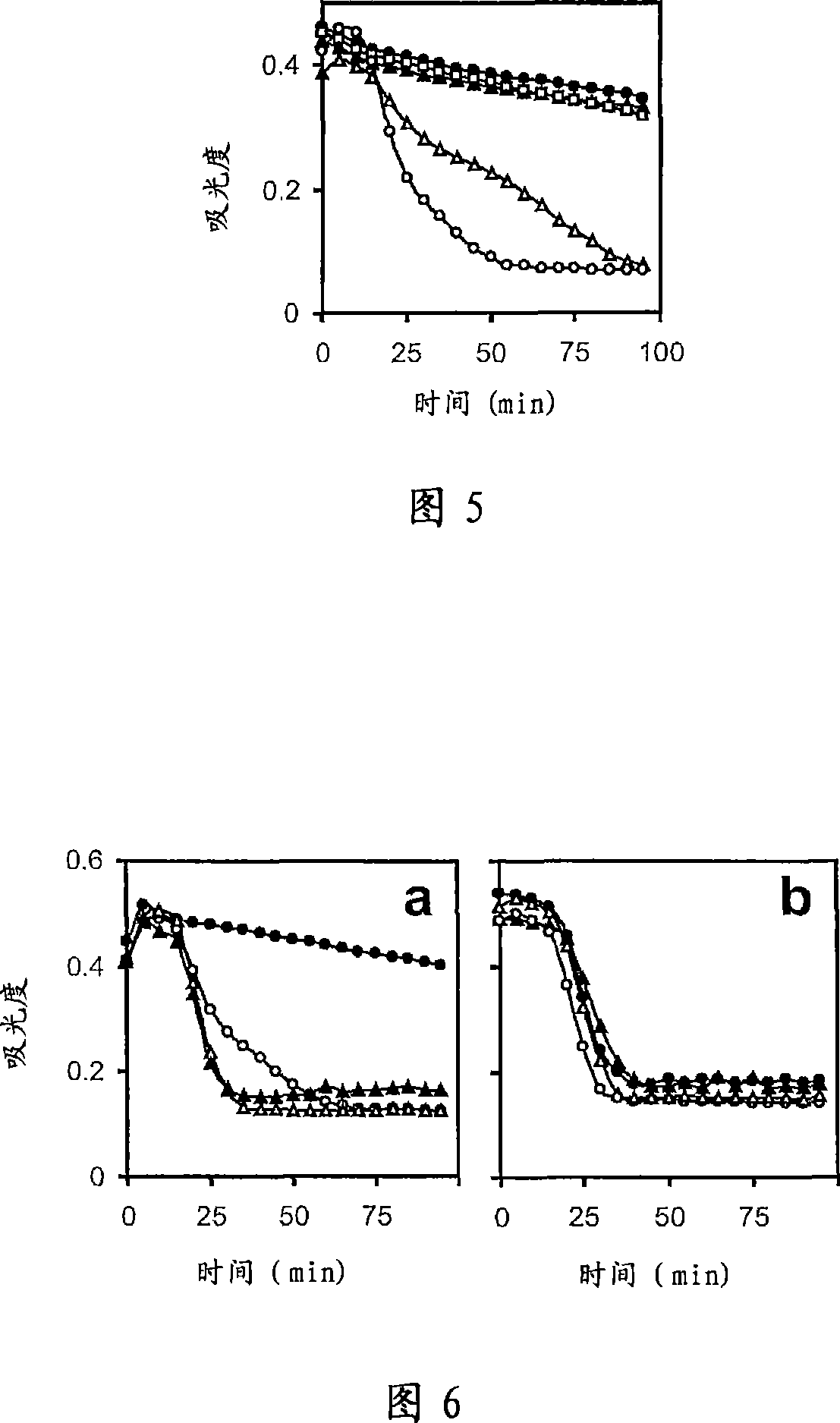
There is proposed a chemical compound of 5,11,17,23-tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene and a sodium salt thereof, which can be used as antithrombotic agents. A highly specific inhibiting effect of the aforementioned calixarenes on the fibrin polymerization has been identified. It has been found that the addition of the aforementioned calixarenes to blood plasma leads to an increase of the prothrombin time and the activated partial thromboplastin time.
Owner:KOMISARENKO SERHII VASYLOVYCH +7
Method for preparing liquid type PT agent based on human recombinant thrombokinase
ActiveCN101294163BHigh purityHigh activityFungiMicrobiological testing/measurementProtein targetChemical composition
The present invention provides a method for preparing liquid PT reagent by using recombined thromboplastin. The invention belongs to the field of biotechnology and aims to solve clinic standardization problem for PT determination. The quality of a PT reagent is a key factor influencing PT test, and the effective method for reducing the difference of determination results in different laboratories is to unify the chemical composition and biochemical characterics of thromboplastin in reagent. The recombined thromboplastin prepared by genetic engineering method has stable molecular structure and uniform protein, and can improve test sensitivity, precision and accuracy. The full-length human thromboplastin expressed by yeast cell can achieve high purity and target protein with high activity by one-step purification. The invention also provides a method for preparing liquid PT reagent based on recombined thromboplastin. The reagent has good sensitivity and stability and qualified each technical index, and test shows that the reagent is suitable for clinic application.
Owner:SHANGHAI LONG ISLAND BIOTEC CO LTD
Preparation method of lyophilization type determination PT reagent
InactiveCN110346559ASolve lossLong validity periodPreparing sample for investigationZymogenPhospholipid
The invention provides a preparation method of a lyophilization type determination PT reagent. Raw materials comprise HEPES buffer solution, synthetic phospholipids, a recombinant sheep tissue factor,a lyoprotectant and a preservative. The preparation method comprises the following steps: adding the lyoprotectant into prepared HEPES buffer solution, dissolving an appropriate amount of synthetic phospholipids in HEPES buffer solution containing 1% triton X-100, dissolving a sheep tissue factor in an appropriate amount of PBS buffer solution to form 55[mu]g / ml tissue factor solution, adding therecombinant sheep tissue factor into phospholipid solution, finally adding the preservative and lyoprotectant solution into phospholipid tissue factor solution, and performing lyophilization on the obtained supernate by virtue of the conventional technology, thus the PT reagent product is obtained. The preparation method provided by the invention optimizes a formula of a prepared thromboplastin lyoprotectant on the basis of existing components, the problem of loss of thromboplastin in an existing prothrombin detection reagent lyophilizing process is solved, sensitivity is relatively high, a validity period of the reagent is prolonged, and production is easy. The reagent has high clinical application value and potential market benefit.
Owner:山东艾科达生物科技有限公司
Buffer composition for purifying tissue factor, purification preparation, PT detection composition and PT detection kit
ActiveCN110777137AImprove stabilityHigh sensitivityHydrolasesBiological material analysisDiseaseWarfarin
The invention relates to the field of detection, in particular to a buffer composition for purifying a tissue factor, a purification preparation, a PT (prothrombin time) detection composition and a PTdetection kit. A key raw material, namely tissue thromboplastin, is prepared by a genetic engineering mode; high-purity r-TF (recombinant tissue factor) can be obtained on a large scale by a fermentation technology and a purification technology, and the problems that tissues such as rabbit brains and placentas are difficult in source, big in difference between batches and low in sensitivity are solved. A high-sensitivity prothrombin PT diagnosis kit based on r-TF liquid type has high stability, small difference between batches and high sensitivity, is mainly applied to screening of a clinicalextrinsic coagulation pathway, monitoring of oral warfarin medicines and auxiliary diagnosis of diseases such as liver disease, and can be traced back to an international standard product PT reagent,so that detection comparability of reagents among different reference laboratories and different manufacturers is achieved.
Owner:BEIJING SICCEEDER TECH CO LTD
Methods, compositions and kits for reducing tissue adhesions
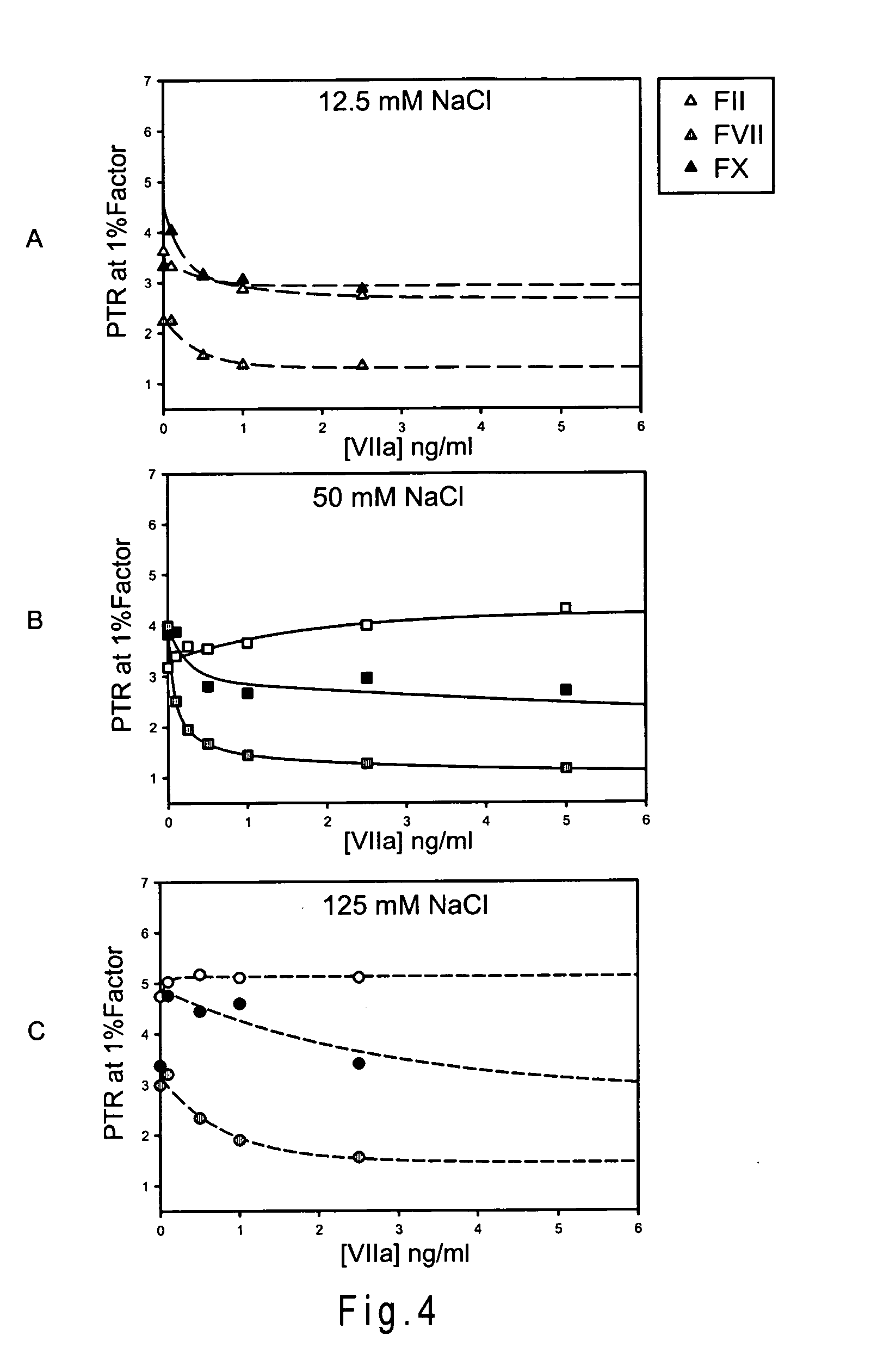
The present invention provides a composition, comprising: proaccelerin (factor V) and at least one factor selected from the group consisting of: prothrombin (factor II), proconvertin (factor VII), andStuart-Prower factor (factor X), wherein an amount of the proaccelerin in the composition is between 75% to 750% compared to an amount of proaccelerin in a blood plasma, and wherein an amount of theat least one factor is from 150% to 3000% compared to an amount of the at least one factor in the blood plasma; wherein the amount of proaccelerin and the at least one factor in the composition is determined by extrapolating an observed activity of the composition at a concentration of 500mM NaCl, using a prothrombin complementation assay using a standard curve constructed using the blood plasma.
Owner:EIO BIOMEDICAL LTD
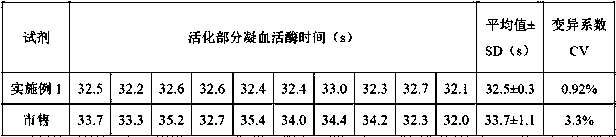
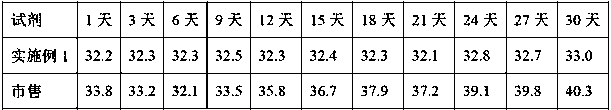
A kind of thromboplastin and its extraction method and pt reagent
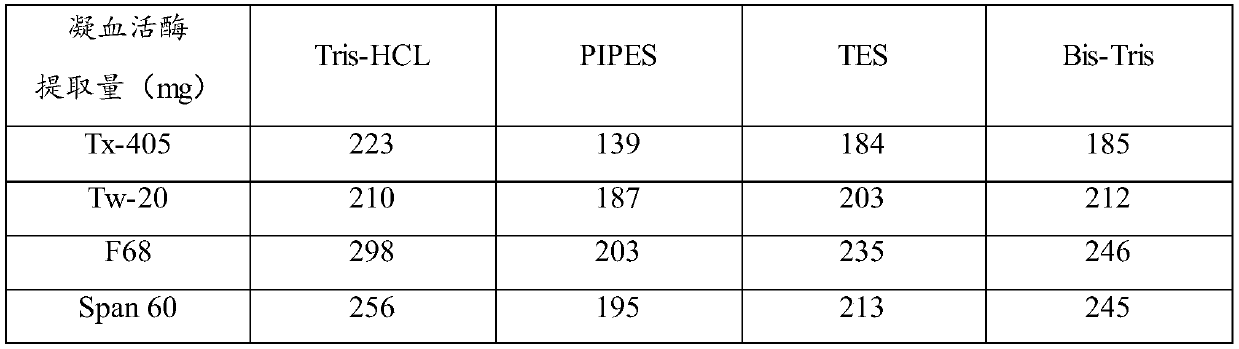
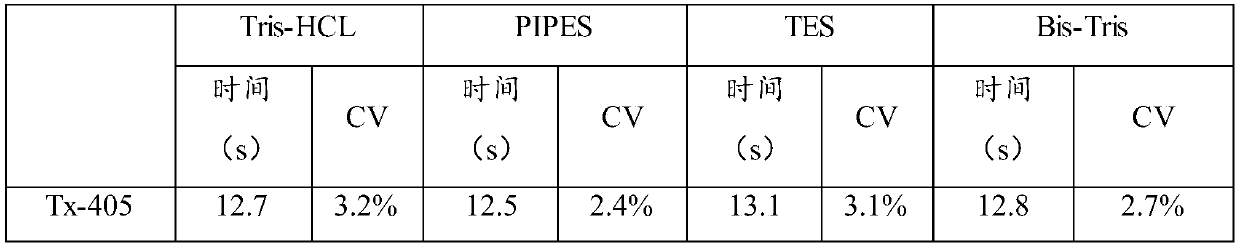
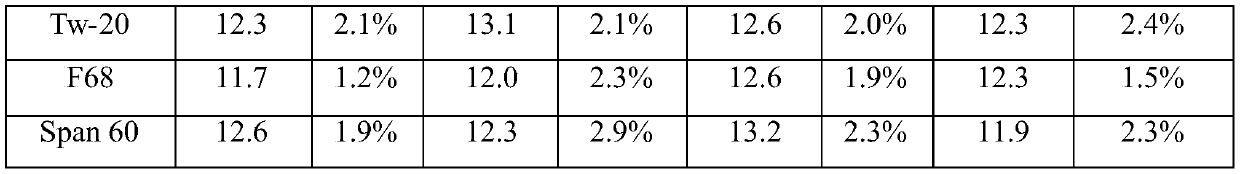
ActiveCN106591267BTime consumingMuch extractionHydrolasesBiological testingActive agentSurface-active agents
The invention relates to the technical field of biochemical detection and discloses thromboplastin, an extraction method thereof and a PT reagent. According to the extraction method, a buffering solution low in metal chelating capacity and a non-ionic surface active agent are added into rabbit brain powder, and oscillating extraction is performed; and then centrifuging is performed, and obtained supernate is the thromboplastin. According to the extraction method, in the process of extracting the thromboplastin with the rabbit brain powder as raw materials, the specific buffering solution and the non-ionic surface active agent are selected for performing extraction, the extracted thromboplastin consumes short time, and the extraction amount is large; and when PT is detected, it is only needed to incubate to-be-detected blood plasma for 1 min, and compared with long incubation time of an existing PT reagent, the thromboplastin can improve the detection efficiency.
Owner:SINOCARE
Method for synthesizing (R)-2-(t-butyloxycarboryl)-4-(2-pyridyl) butyric acid
InactiveCN107573282ASave raw materialsOrganic chemistryTert-Butyloxycarbonyl protecting groupSynthesis methods
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
Microfabricated device with micro-environment sensors for assaying coagulation in fluid samples
ActiveUS20190154710A1Biological material analysisMaterial analysis by electric/magnetic meansAssayMicrofabrication
Owner:ABBOTT POINT CARE
Method for measuring blood coagulation time
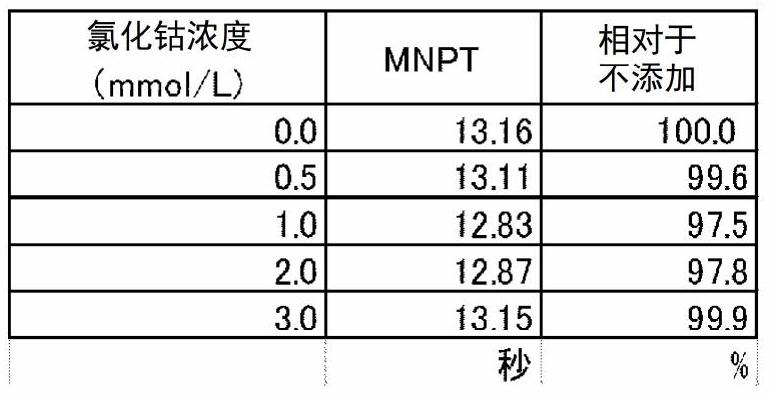
PendingCN112740034AGood storage stabilityBiological material analysisBiological testingBiomedical engineeringAnalytical chemistry
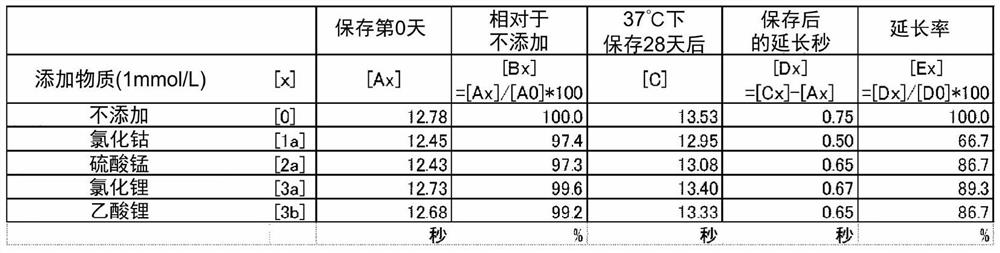
There is a demand for a method for controlling MNPT. According to the present invention, MNPT can be controlled and adjusted to a desired time by causing a specific metal ion to coexist with tissue thromboplastin. In addition, when the specific metal ion is caused to coexist with tissue thromboplastin, the stability of a thromboplastin reagent can be improved when the thromboplastin reagent is stored in a solution state.
Owner:SEKISUI MEDICAL CO LTD
Reagent for determination of coagulation time, production method therefor, reagent kit, and method for determination of coagulation time
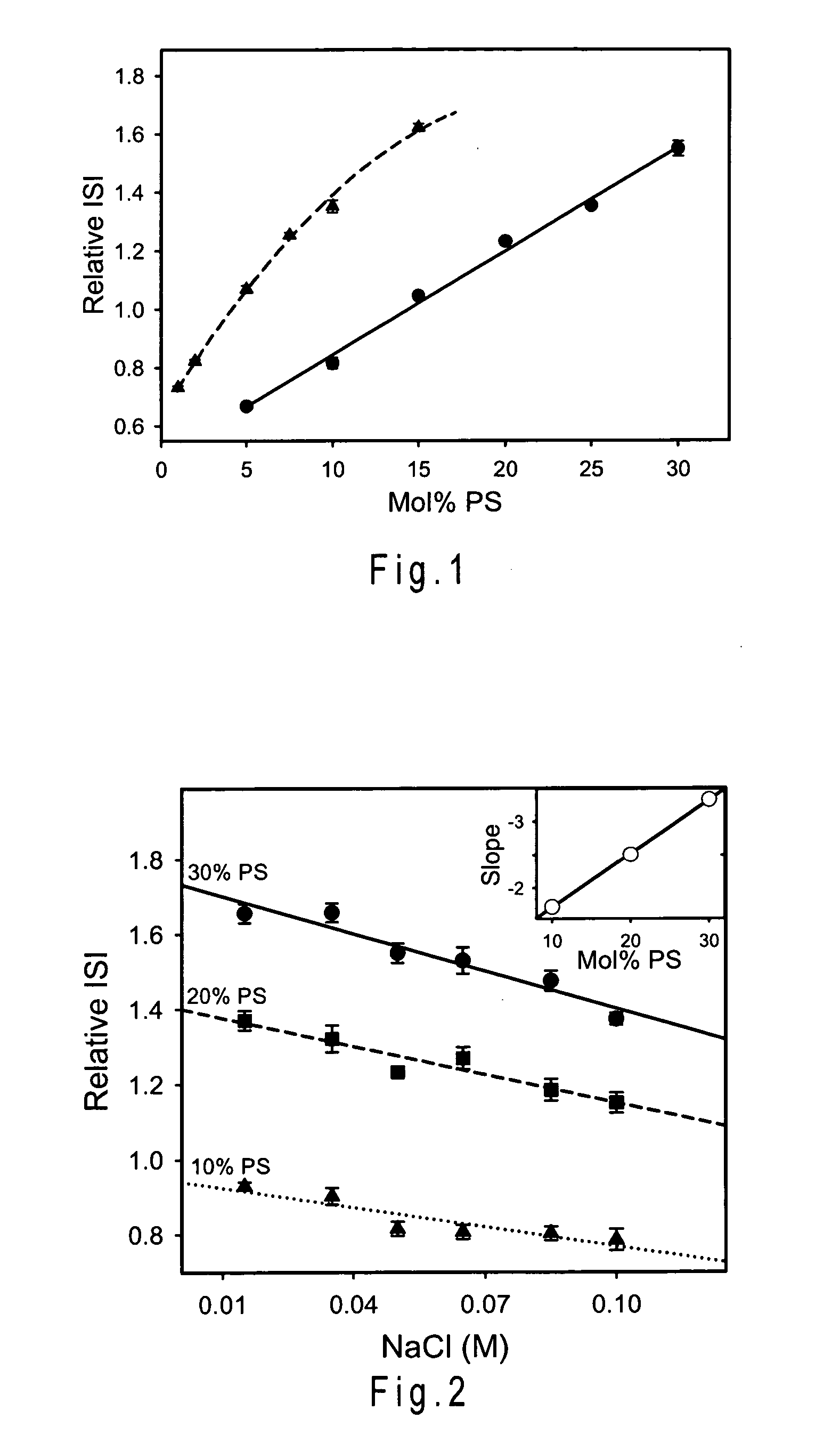
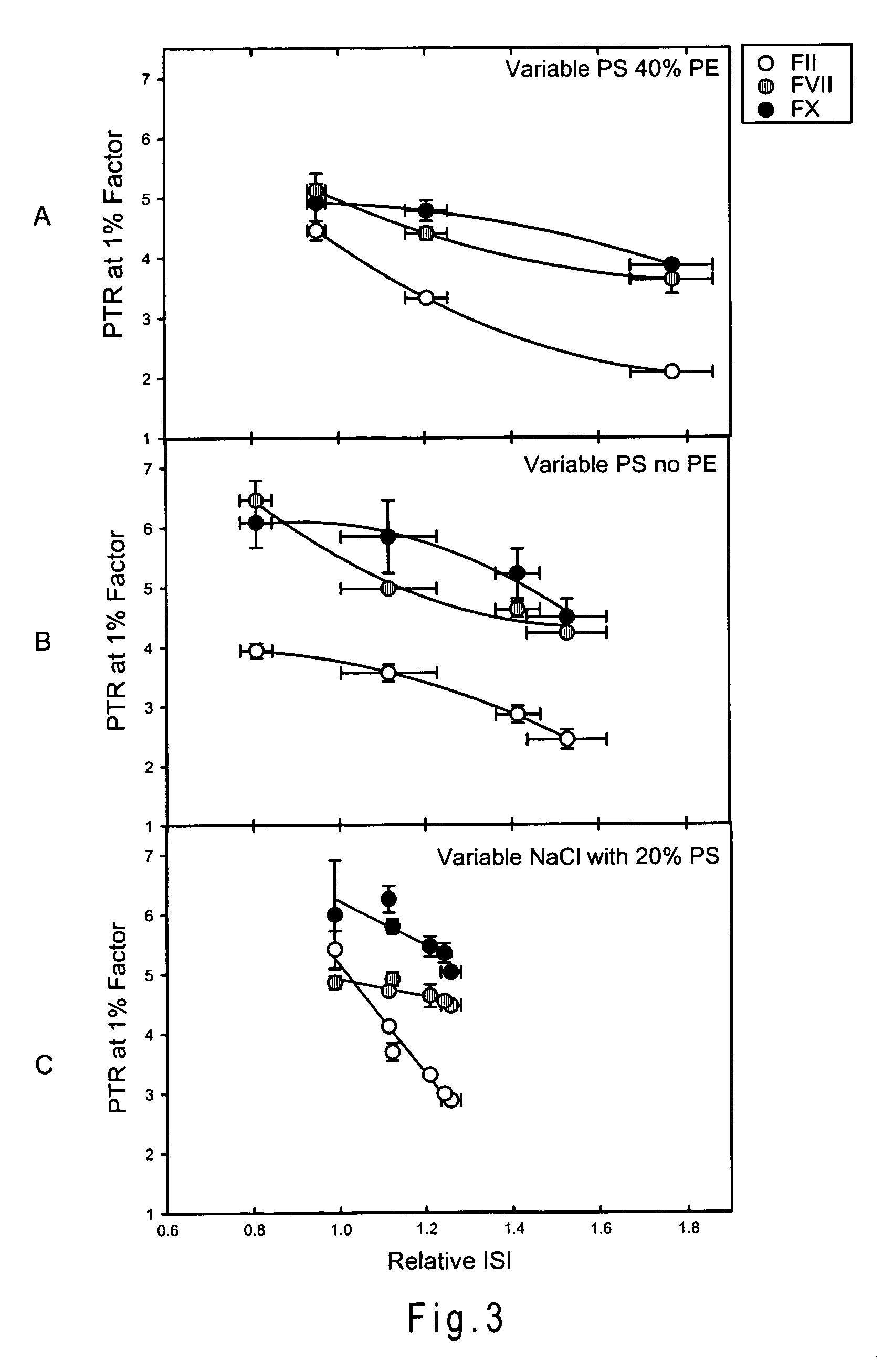
ActiveUS20200103421A1High sensitivityImprove responseMicrobiological testing/measurementBiological material analysisCoagulation timesPhosphatidylethanolamine
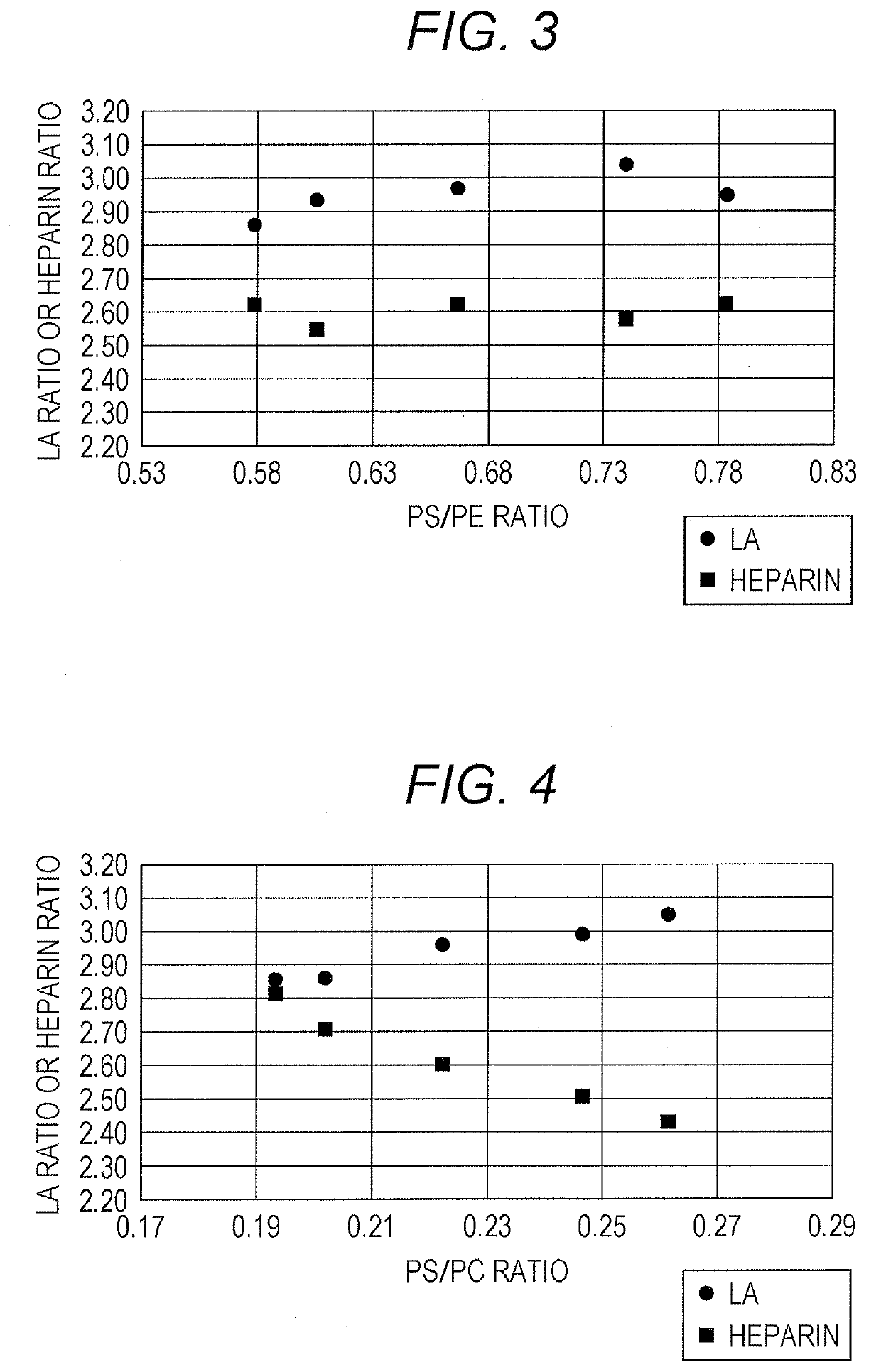
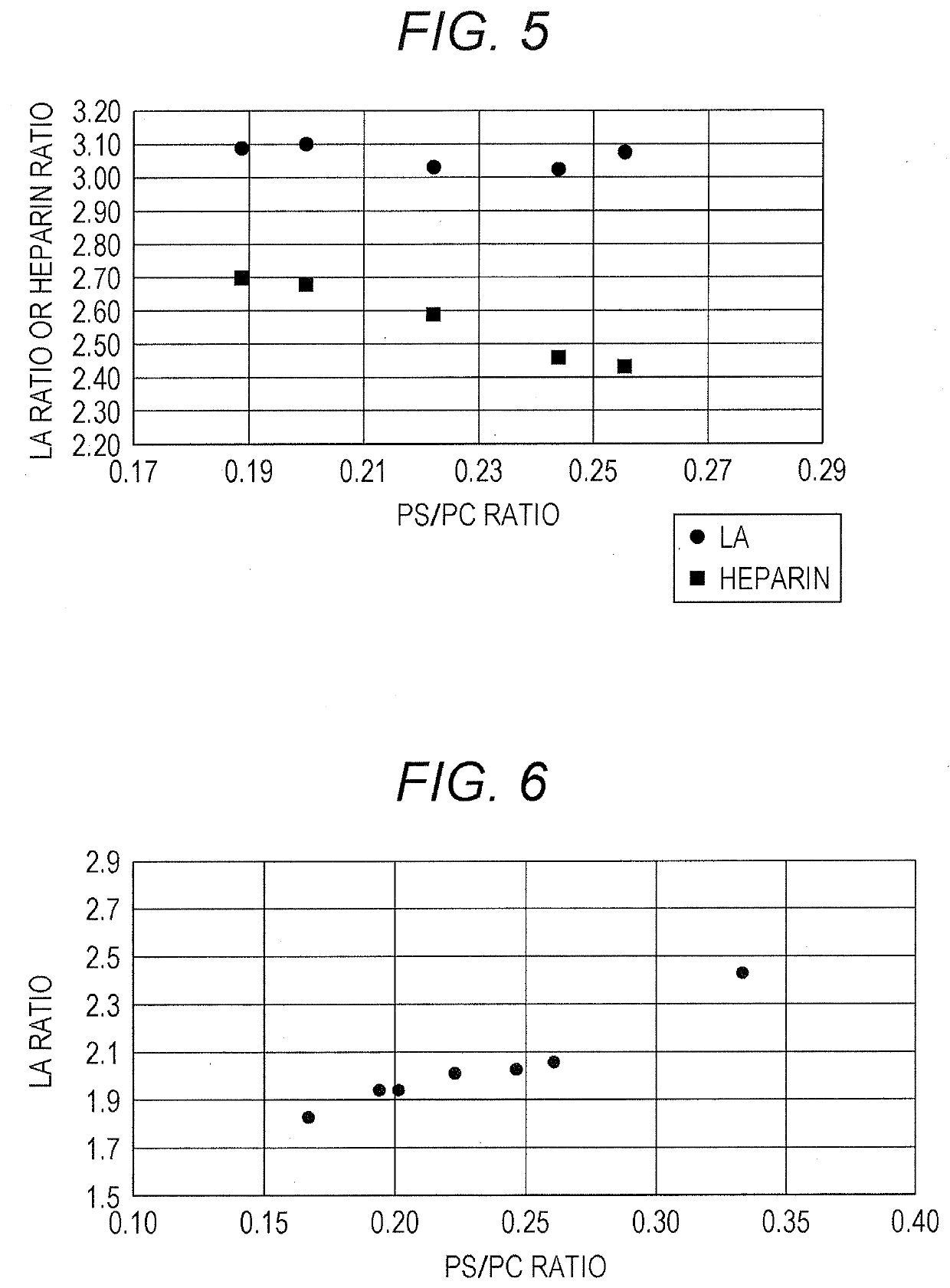
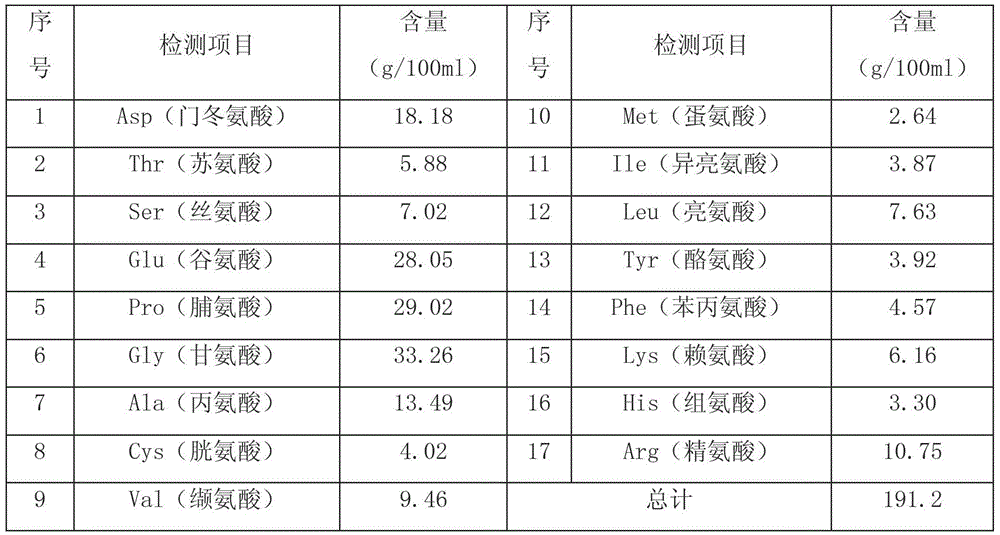
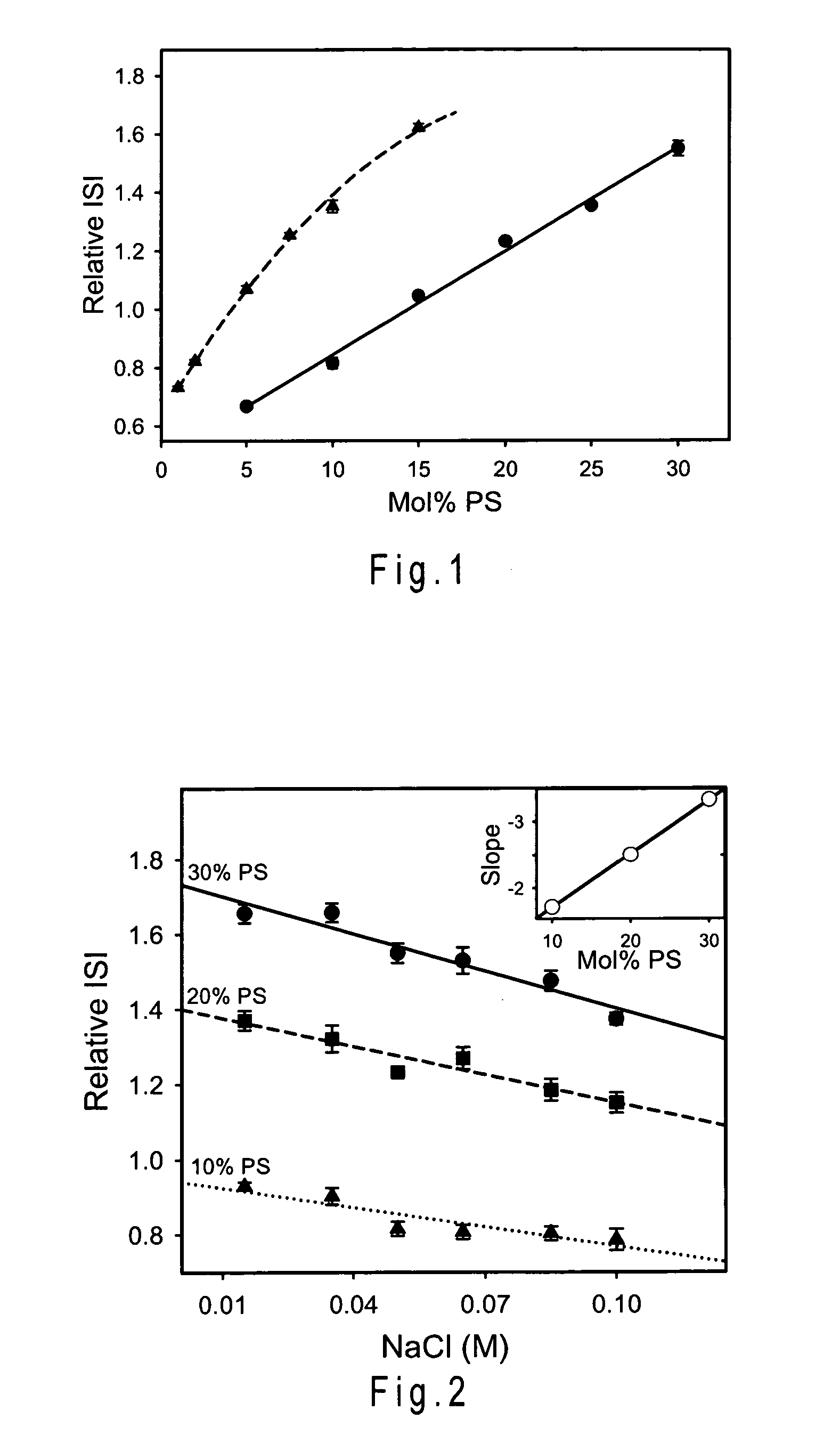
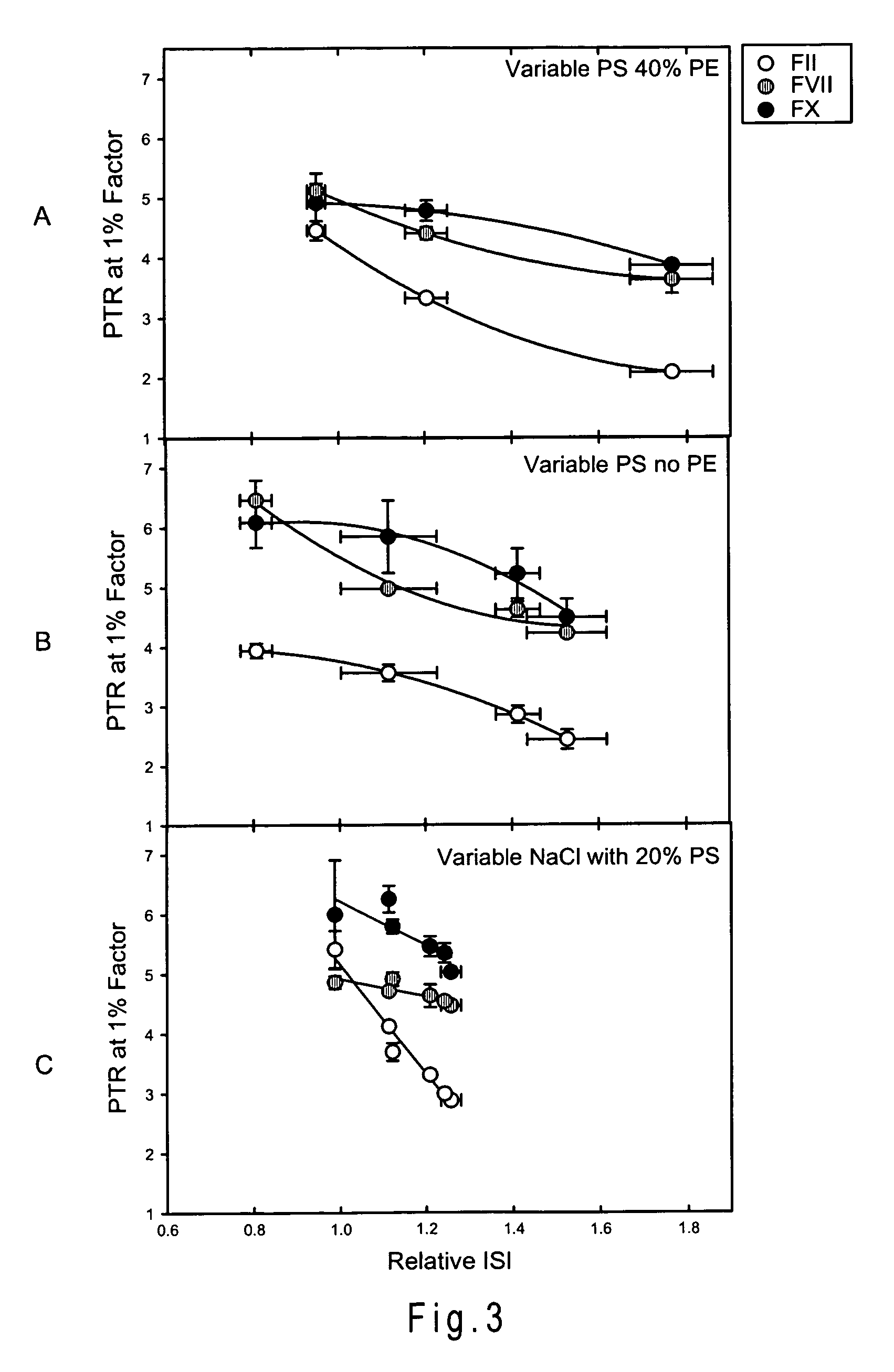
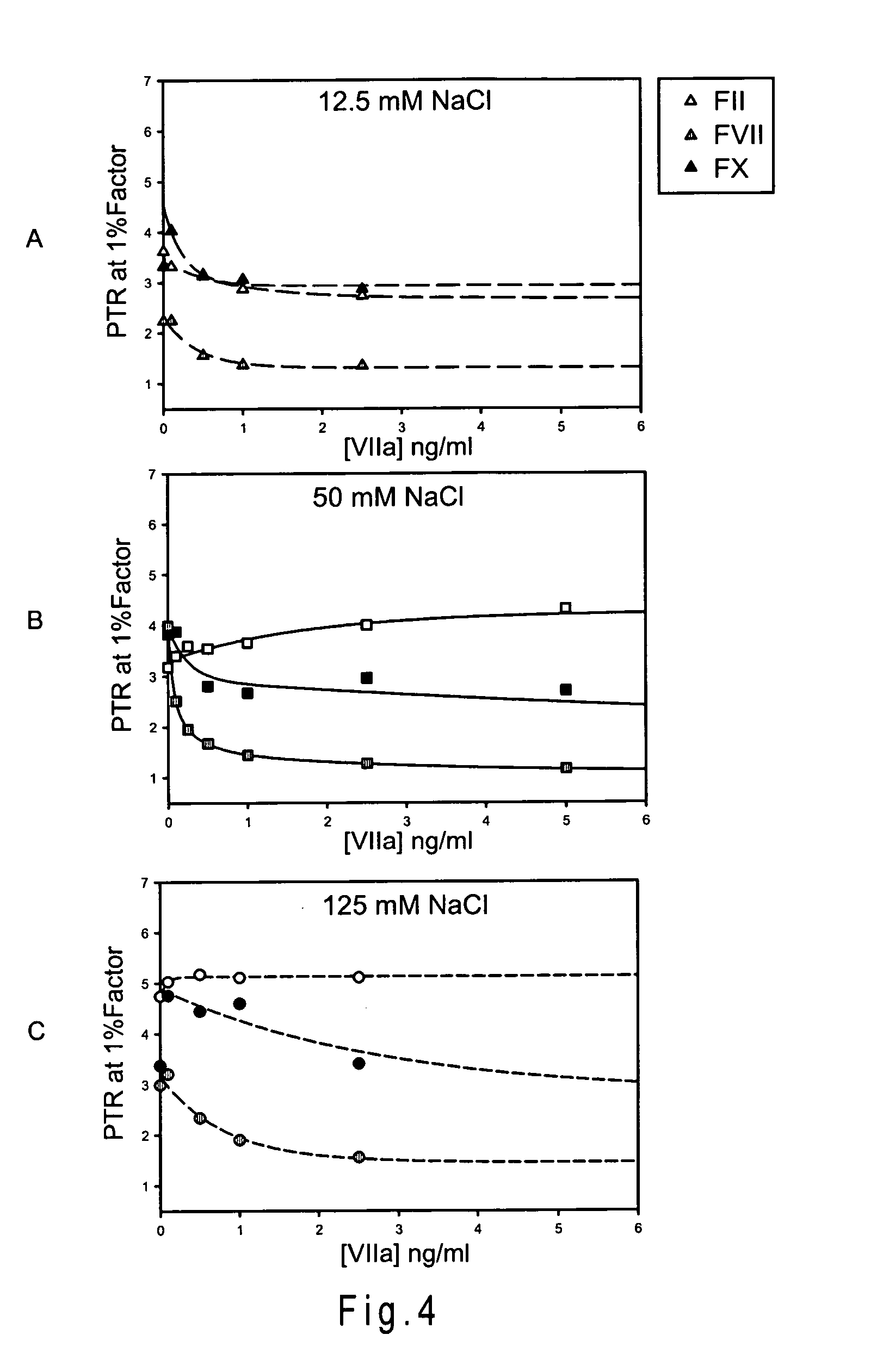
Disclosed is a reagent for determination of activated partial thromboplastin time, comprising: a phosphatidylcholine (PC); a phosphatidylserine (PS); and a phosphatidylethanolamine (PE), wherein a concentration ratio of the PS relative to the PC is not less than 0.16 and not more than 0.25, and a concentration of the PS is not less than 7 μg / mL and not more than 13 μg / mL.
Owner:SYSMEX CORP
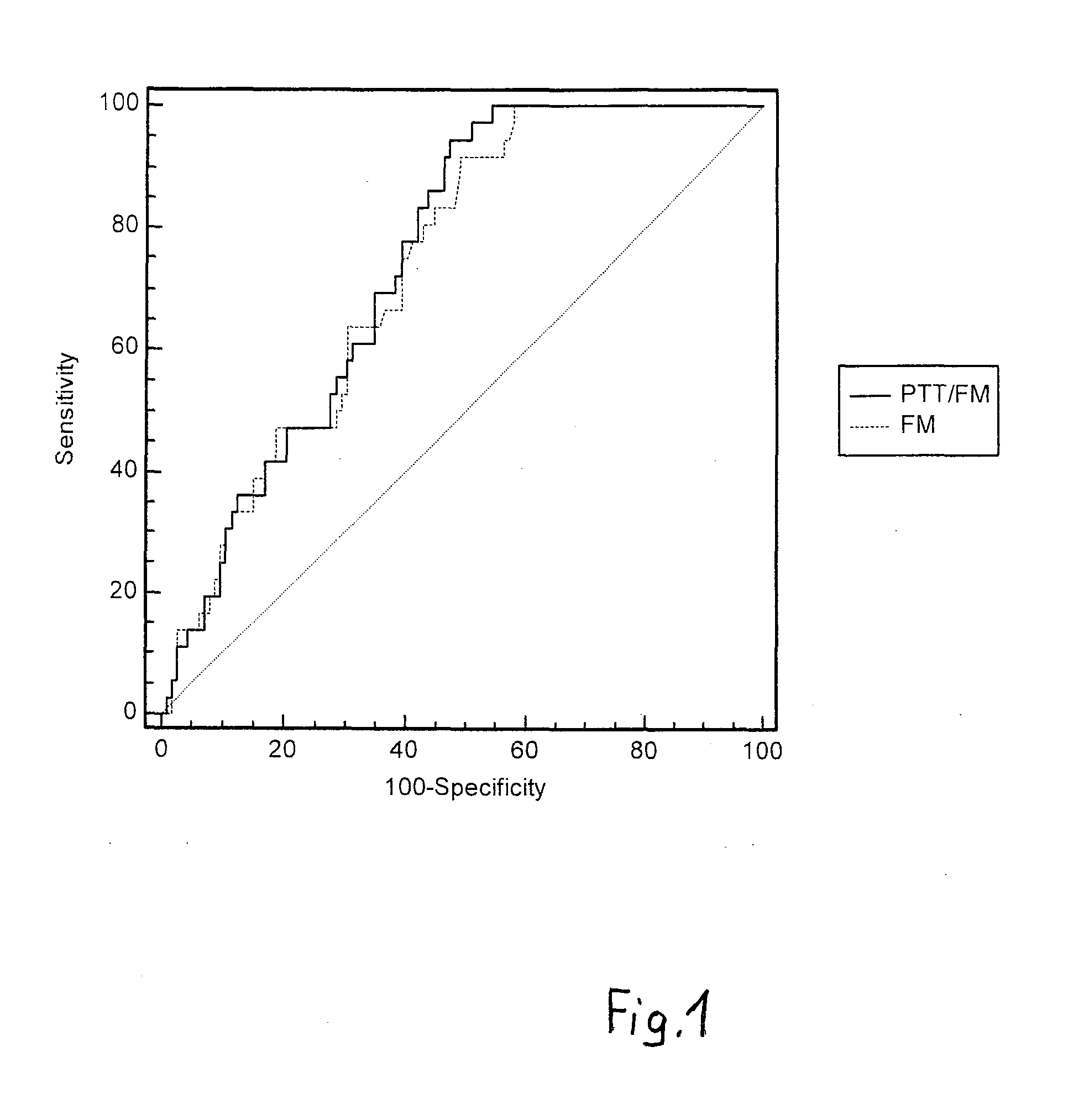
Method for the preoperative determination of the intraoperative risk of bleeding of a patient
InactiveUS20120064551A1High diagnostic sensitivityStrong specificityMicrobiological testing/measurementDisease diagnosisPlasma samplesFibrin Monomer
In order to determine the intraoperative risk of bleeding preoperatively, both the content of fibrin monomer (FM) and the partial thromboplastin time (PTT) are determined in a blood or plasma sample.
Owner:ZENT FUR LABORMEDIZIN
Method for preparing liquid porcellanite APTT reagent
ActiveCN101526538BIncreased sensitivityEasy to useBiological testingSystemic lupus erythematosusAnti coagulation
The invention relates to a method for preparing a liquid porcellanite activating partial thromboplastin (APTT) reagent. The reagent is mainly used for monitoring coagulation factor deficiency of endogenous coagulation systems and carrying out screening test of related inhibitors, and used for detecting coagulation factors, heparin anticoagulation treatment and lupus anticoagulation substances as the current main means. Taking porcellanite as an activating agent, the method improves the sensitivity to the coagulation factors; a novel stable system solves the problem of keeping the stability of the reagent at a liquid state; and the method has the advantages of high sensitivity, convenient use, small experimental error, strong stability, good compatibility, low cost and the like.
Owner:SHANGHAI LONG ISLAND BIOTEC CO LTD
Buffer composition for purifying tissue factor, purification preparation, pt detection composition and pt detection kit
ActiveCN110777137BImprove stabilityHigh sensitivityHydrolasesBiological material analysisDiseaseWarfarin
Owner:BEIJING SICCEEDER TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors](https://images-eureka.patsnap.com/patent_img/40cd001c-2fc7-4d20-a12c-3d42ee869982/US20130090494A1-20130411-D00001.png)
![Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors](https://images-eureka.patsnap.com/patent_img/40cd001c-2fc7-4d20-a12c-3d42ee869982/US20130090494A1-20130411-D00002.png)
![Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors](https://images-eureka.patsnap.com/patent_img/40cd001c-2fc7-4d20-a12c-3d42ee869982/US20130090494A1-20130411-D00003.png)