Method for preparing liquid porcellanite APTT reagent
A kind of white clay and reagent technology, applied in the direction of biological testing, material inspection products, etc., can solve the problems of unstable product quality, unstable test results, high price, etc., to expand the scope of screening, strong stability, strong compatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment one: the preparation of liquid kaolin APTT reagent
[0012] Weigh 1.0g of kaolin and dissolve it in 1000mL 20mM pH7.5 Tris-HCl buffer solution, stir for 30min until the whole liquid presents a uniform white suspension, then add 2.0g of phospholipid, mix well, keep warm at 37°C for 1h, and finally scale Add a stabilizer (prepared from 0.5% polyethylene glycol, 1% gelatin, and 0.1% sodium azide), and mix well to obtain a liquid kaolin APTT reagent. During the preparation process of the reagent, the holding time at 37°C and the composition of the stabilizer have a great influence on the stability of the reagent, and the addition of kaolin affects the sensitivity of the reagent.
[0013] The main technical indicators of preparing liquid kaolin APTT reagent are as follows:
[0014] 1. Appearance: Liquid kaolin APTT reagent is a white suspension, and the activated partial thromboplastin time value in normal plasma is 24.6-36.8s.
[0015] 2. Reagent repeatability:...
Embodiment 2
[0018] Embodiment two: Stability detection of liquid kaolin APTT reagent
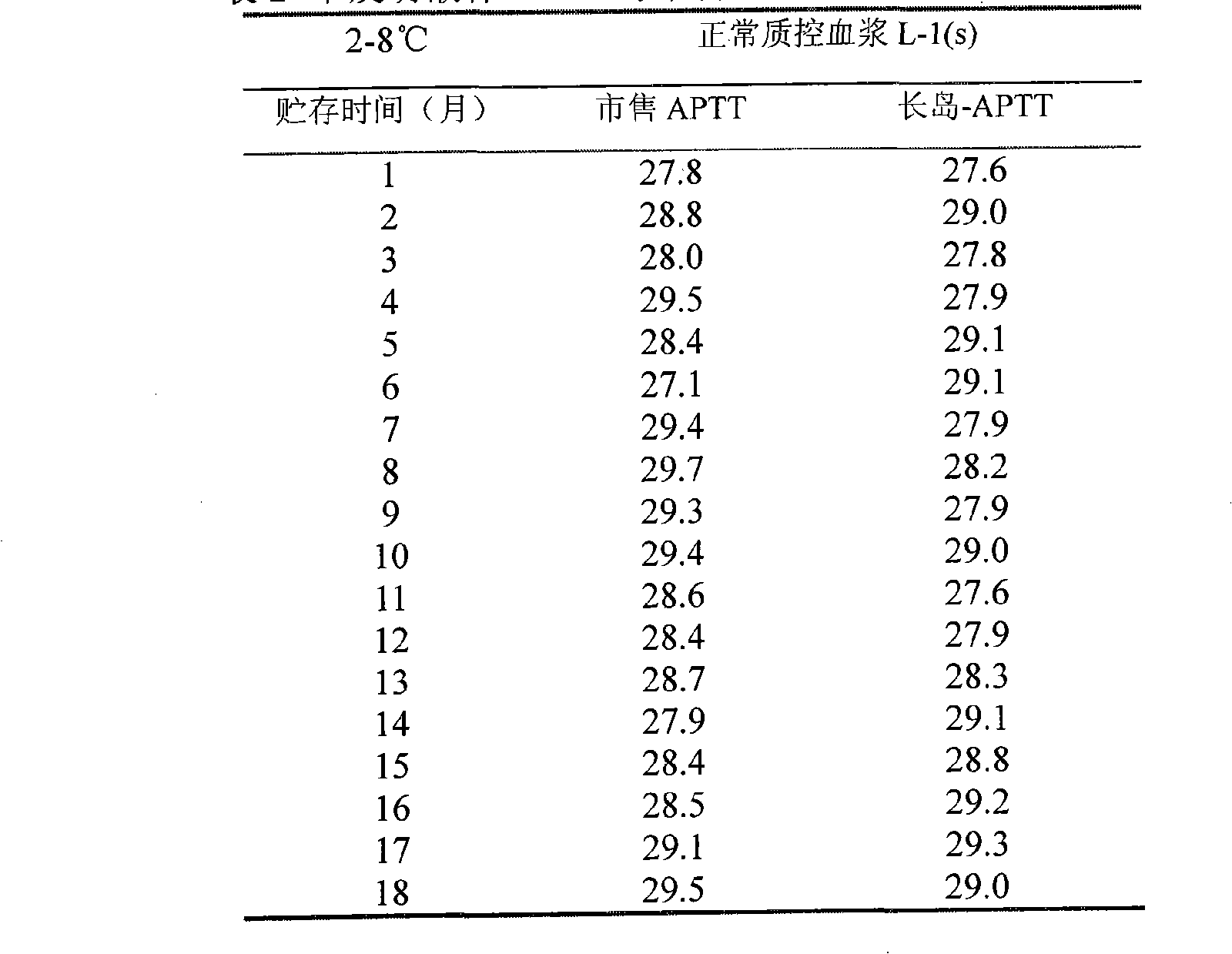
[0019] The stability test of the present invention includes stability after unsealing, stability under simulated transportation conditions, unopened accelerated destruction test, and long-term stability test.
[0020] The results of the stability test after opening showed that within 60 days, the measured value of the normal quality control plasma was within the normal range without much change, and the deviation of the abnormal quality control plasma was within 5%. After opening, it was stored at 25°C and 37°C Stable for more than 30 days.
[0021] Under high-temperature transportation conditions, the measured value of normal quality control plasma is within the normal range within 10 days; the deviation of abnormal quality control plasma is within 5%, which is equivalent to imported reagents.
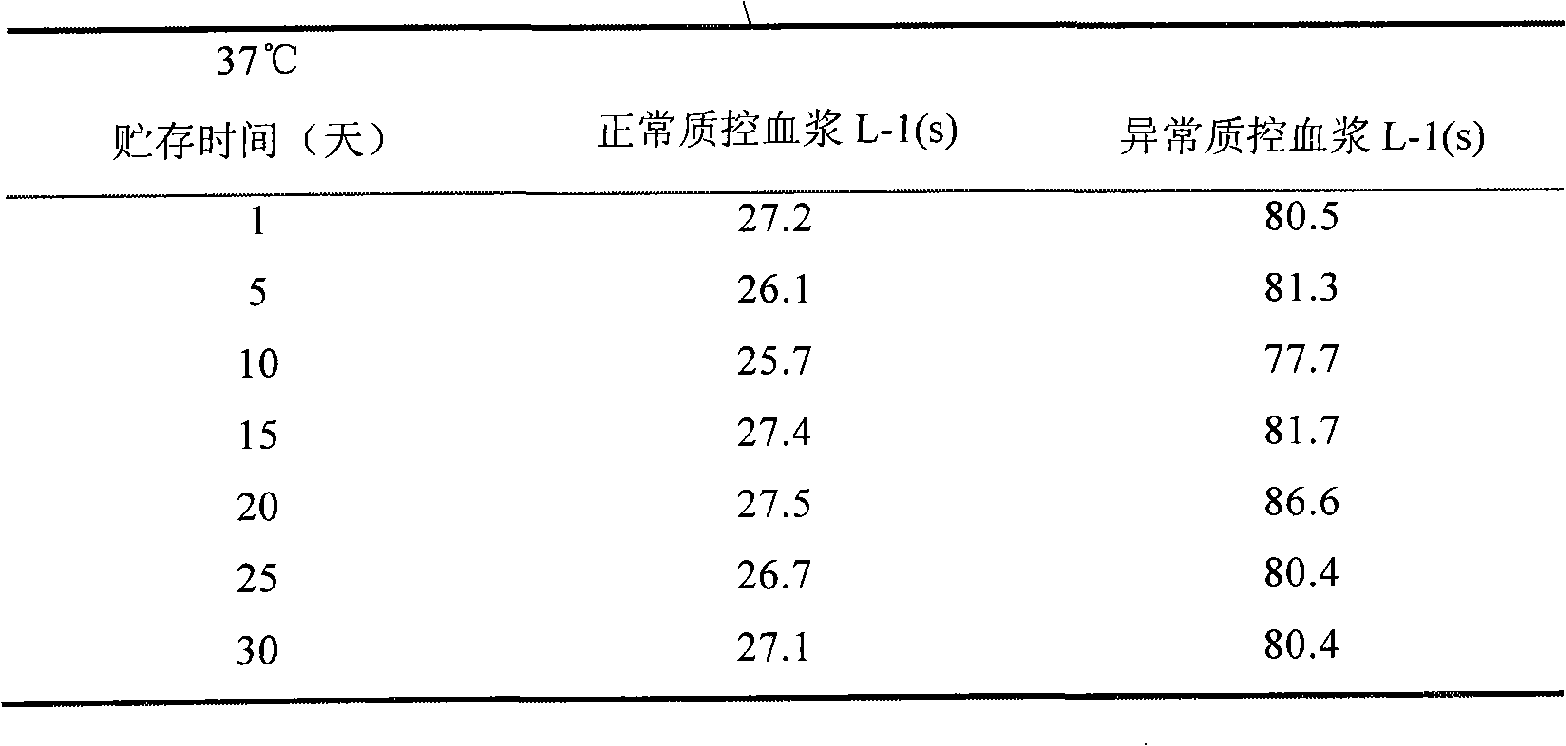
[0022] Under the condition of accelerated destruction at 37°C, the measured value of normal quality control...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com