Patents
Literature
71 results about "Partial thromboplastin time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
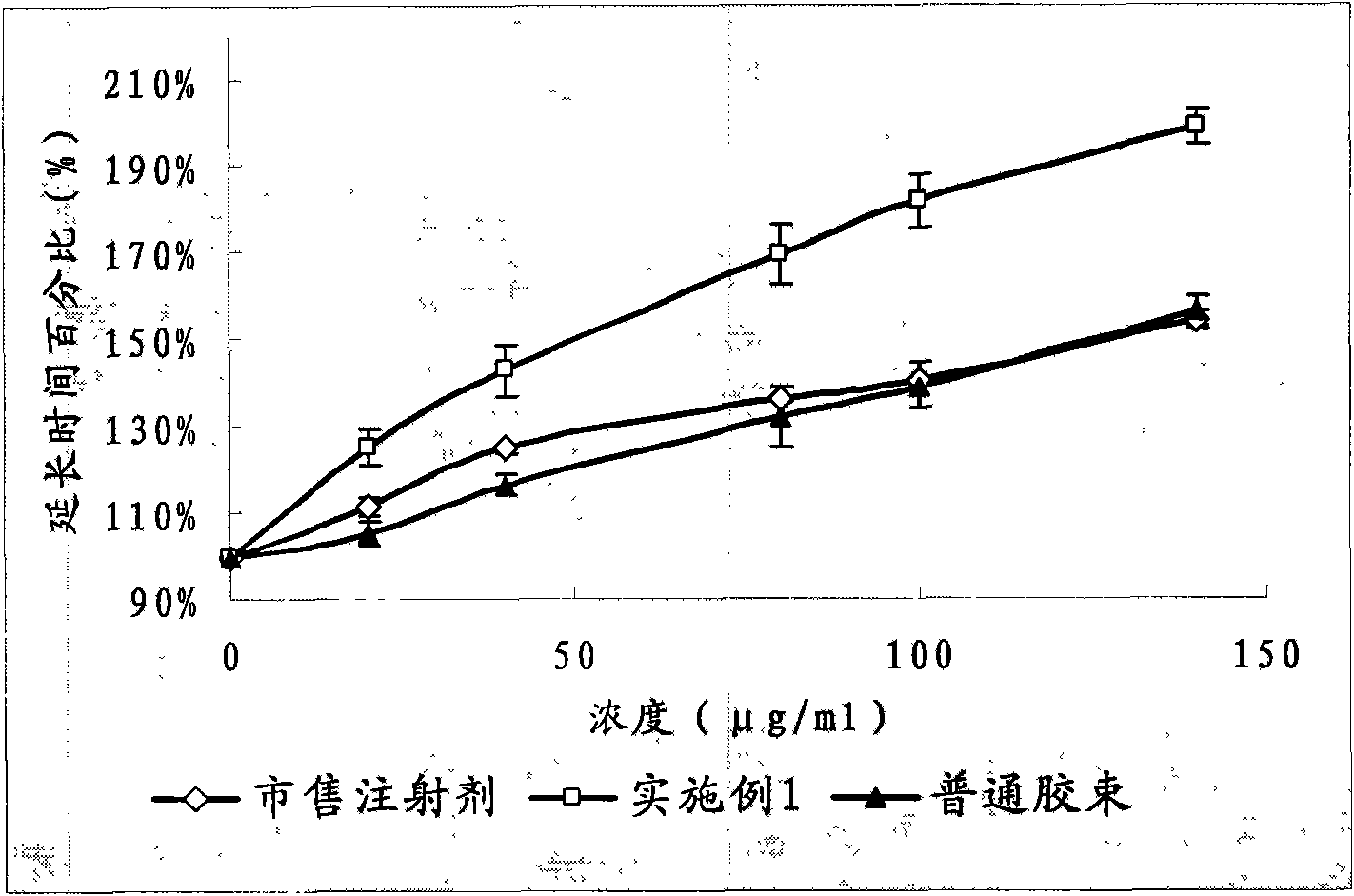
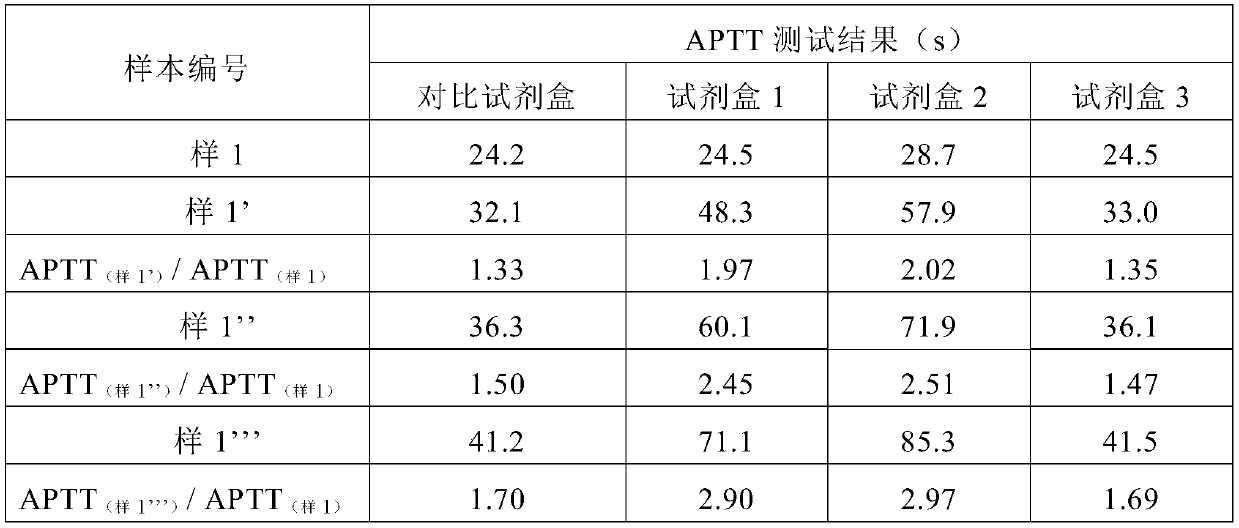
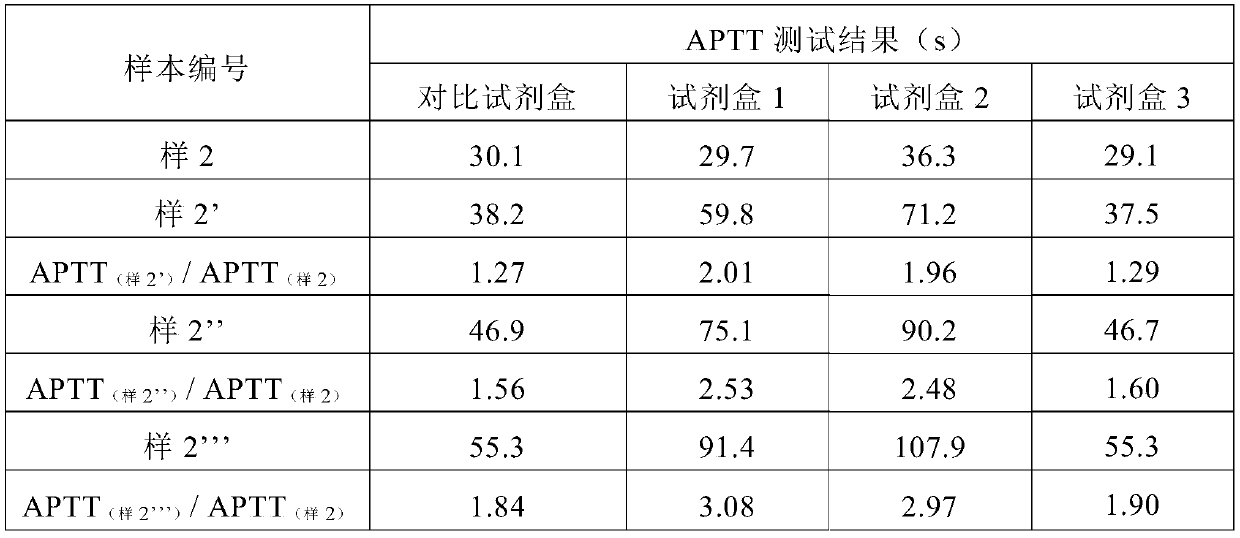
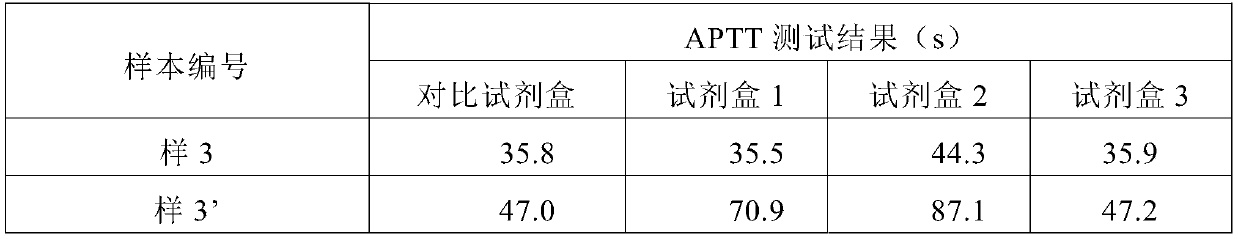
The partial thromboplastin time (PTT) or activated partial thromboplastin time (aPTT or APTT) is a blood test that characterizes coagulation of the blood. A historical name for this measure is the kaolin-cephalin clotting time (KCCT), reflecting kaolin and cephalin as materials historically used in the test. Apart from detecting abnormalities in blood clotting, partial thromboplastin time is also used to monitor the treatment effect of heparin, a widely prescribed drug that reduces blood's tendency to clot.
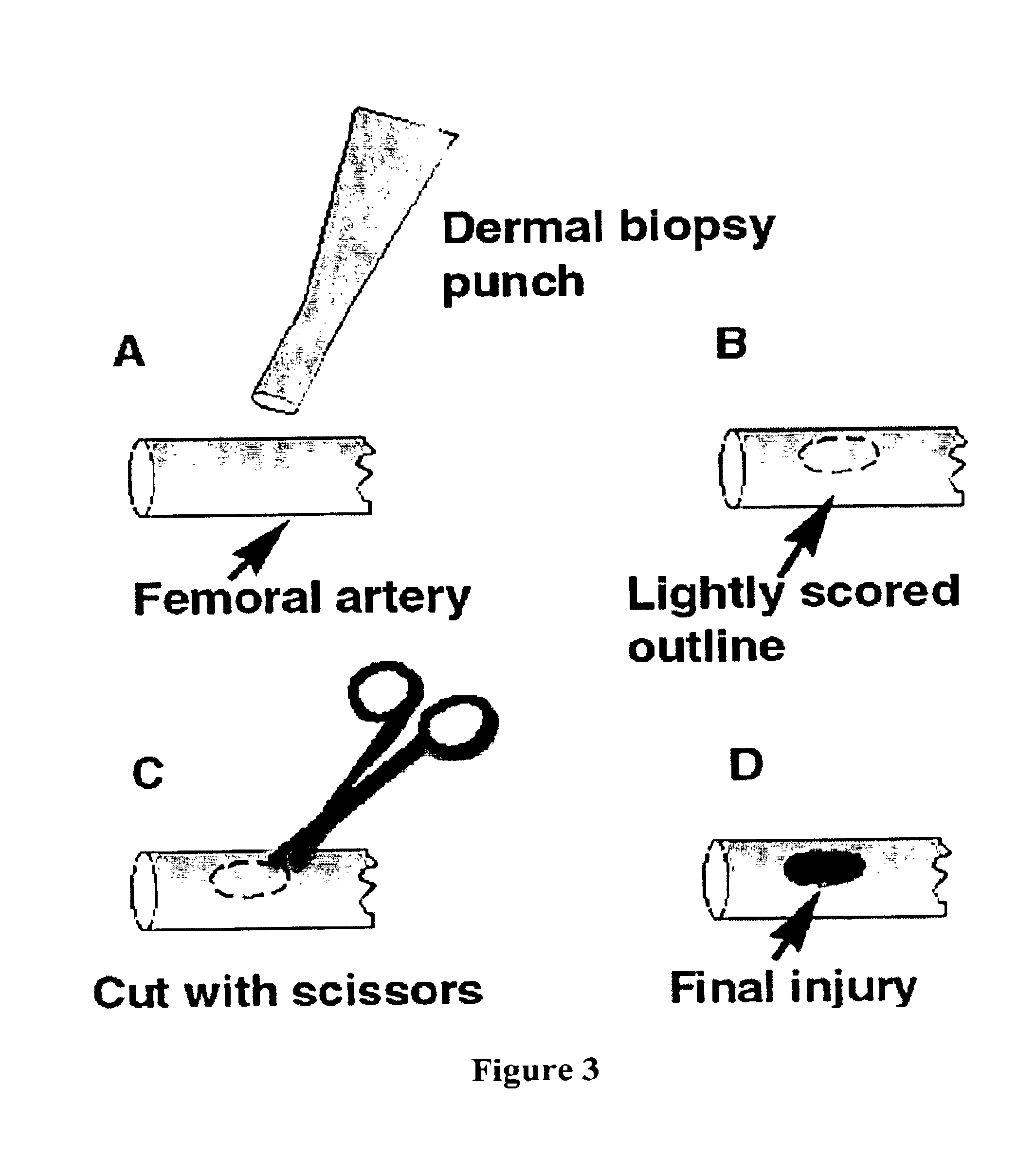
Fibrinogen bandages and arterial bleeding models and methods of making and using thereof
InactiveUS6891077B2Reduce the amount of solutionIncreasing of rate of clotSurgeryBaby linensBlood platelet countsClot formation
Disclosed herein are wound dressings comprising fibrinogen and at least one procoagulant such as propyl gallate in a therapeutic amount. Also disclosed are methods of treating wounds, increasing an amount of or rate of coagulation of blood from a wound, increasing an amount of or rate of clot formation over a wound, increasing blood platelet counts, activating a coagulation system, increasing the plasma concentration of fibrinogen, and decreasing the activated partial thromboplastin time. Also disclosed are an arterial bleeding model and methods of studying arterial bleeding.
Owner:UNITED STATES ARMY U S ARMY MEDICAL RES & MATERIEL COMMAND
External diagnostic reagent kit used for measuring activated partial thromboplastin time
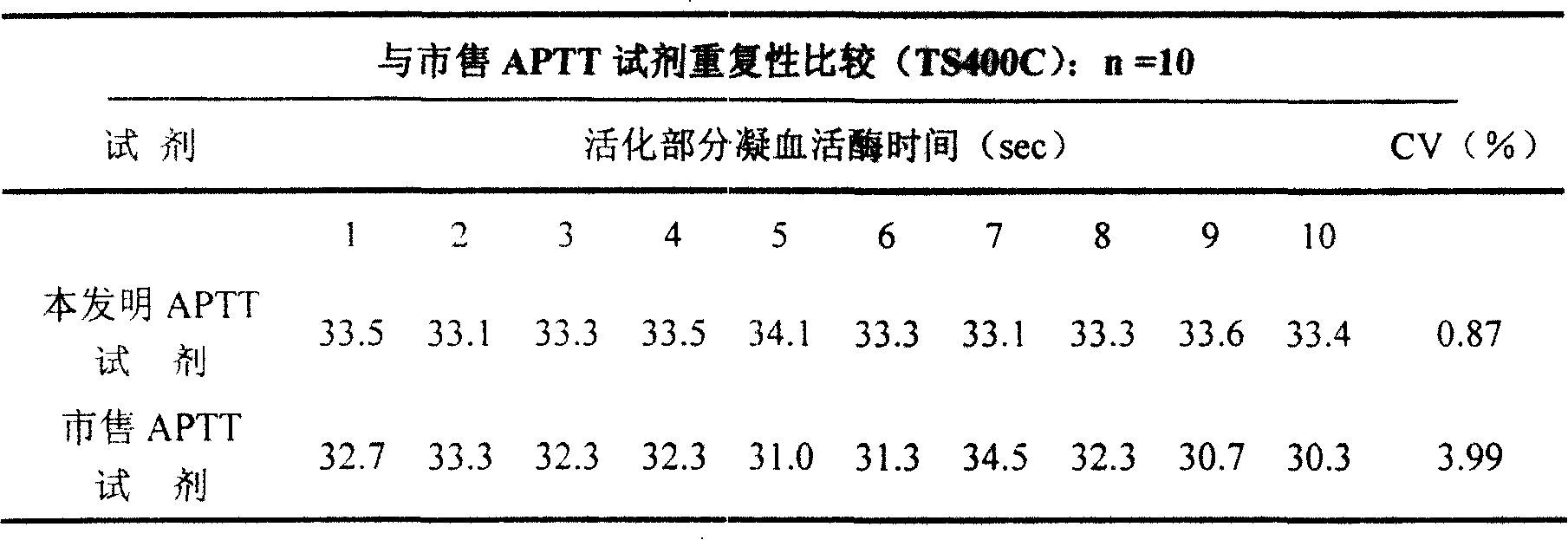
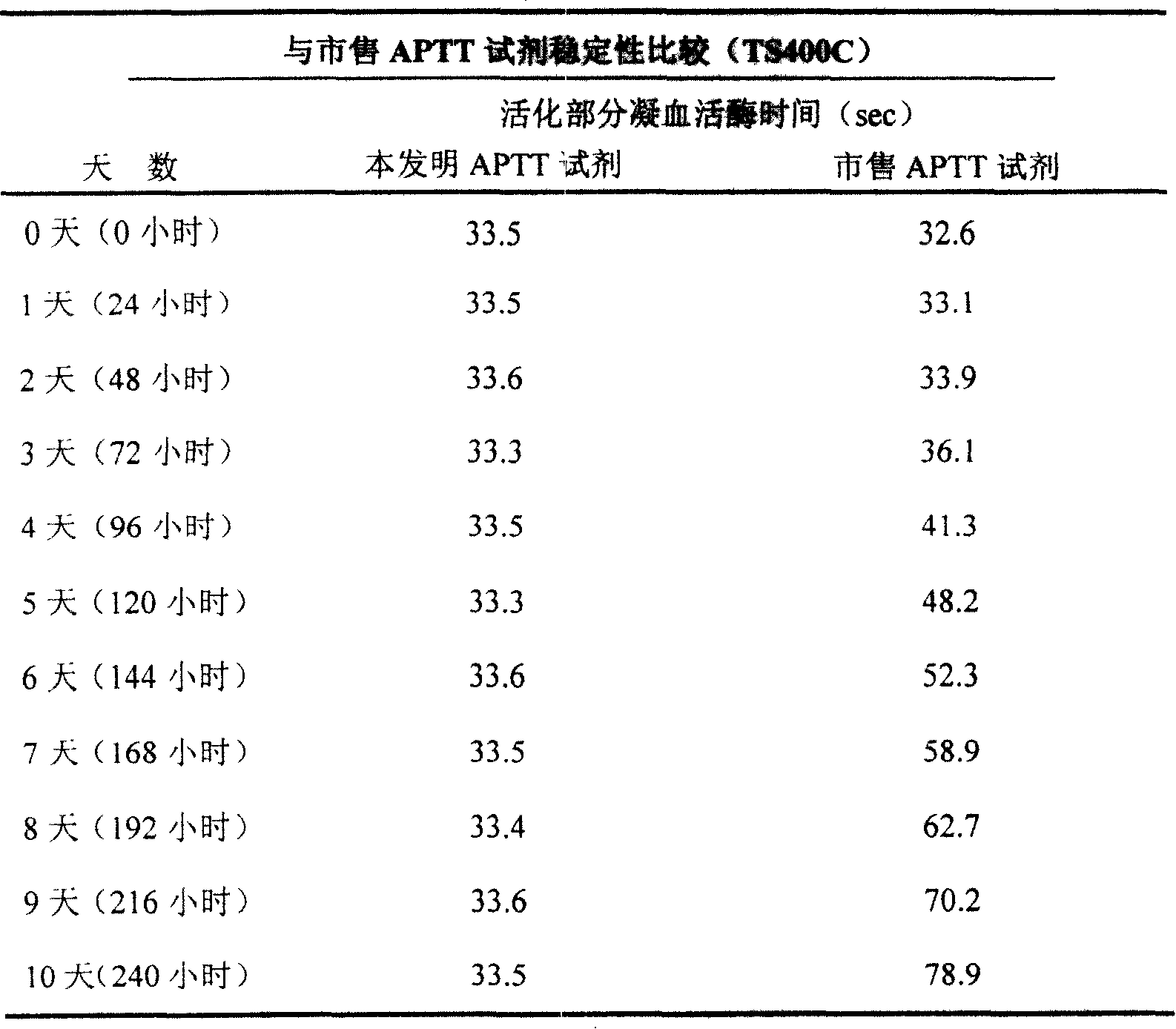
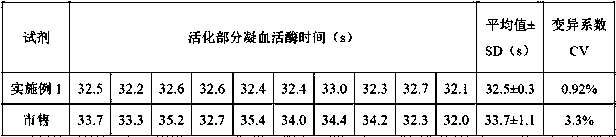
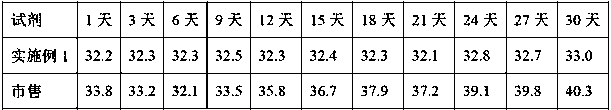
ActiveCN101221189AImprove stabilityGood repeatabilityMicrobiological testing/measurementBiological testingDisease causeAPTT - reference
The invention relates to an in vitro diagnostic kit for the determination of activated partial thromboplatin time (APTT) in clinical test. The invention consists of a partial thromboplatin reagent and a calcium chloride solution, which is used for the detection of the defects of the intrinsic coagulation pathway factors and the screening test of the related inhibitors, and the invention is also a primary means for the current coagulation factor and heparin anticoagulant treatment and the detection of lupus anticoagulant. The invention has the advantages of long stability time and good repeatability of the partial thromboplatin reagent after a re-dissolution, at the same time, the invention has better consistency of the measurement results of a blood coagulation analyzer by using an optical method and a magnetic bead method, therefore, the invention is applicable to large, medium and small hospitals, and the test results of different hospitals have comparability, therefore the invention has important meaning for implementing the one general report of test reports, provides the reliable experimental data for the clinical diagnosis and the treatment of diseases and improves the efficiency and value of the basic studies of thrombosis and hemostasis.
Owner:SHANGHAI SUNBIO TECH
Agent for activation sector cruor activating-enzyme time (APTT)
The invention relates to a detection agent for hemostasis and thrombus belonging to medical clinical detection technical field, in particular to a detection agent for measuring activation partial thromboplastin time (APTT) of blood plasma. The invention is characterized in that the agent is prepared from 0.05-0.5g / L agent solid activator, 20-200mg / L phosphatide, 0.1-10mmol / L divalent metal ion salt, 0.1-0.5g / L stabilizer, 0.1-10g / L polyethylene glycol PEG, 0.05-4.0g / L buffer agent, 2.0-5.0g / L revival agent, while the left is water. The invention provides a high-stability standard agent for meeting APTT measurement, with simple operation, suitable price, short activation time and high sensitivity.
Owner:CHANGAN UNIV
Method and reagent kit for measuring activated partial thromboplastin time
InactiveCN104076156APrevent precipitationLowering assayMicrobiological testing/measurementBiological material analysisBlood plasmaEnzyme
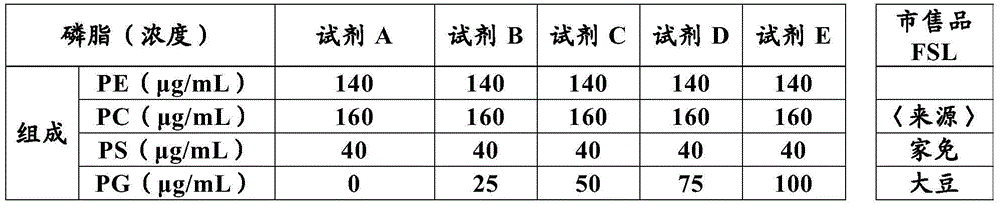
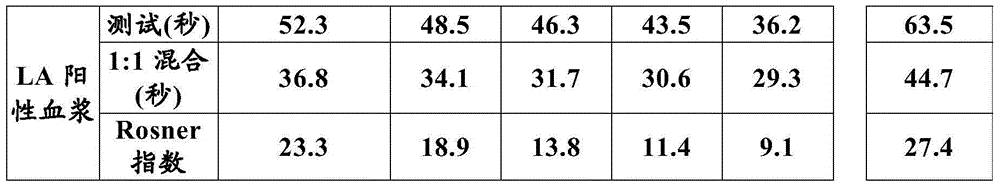
The present invention provides a method for measuring activated partial thromboplastin time. The method comprises: a first mixing step of mixing a blood plasma with a first reagent, wherein the first reagent comprises an activator and phosphatidylglycerol at a concentration equal to or greater than 25 µg / mL; a second mixing step of mixing a sample obtained in the first mixing step with a second reagent comprising a calcium salt; and a step of measuring coagulation time of the sample obtained in the second mixing step.
Owner:SYSMEX CORP
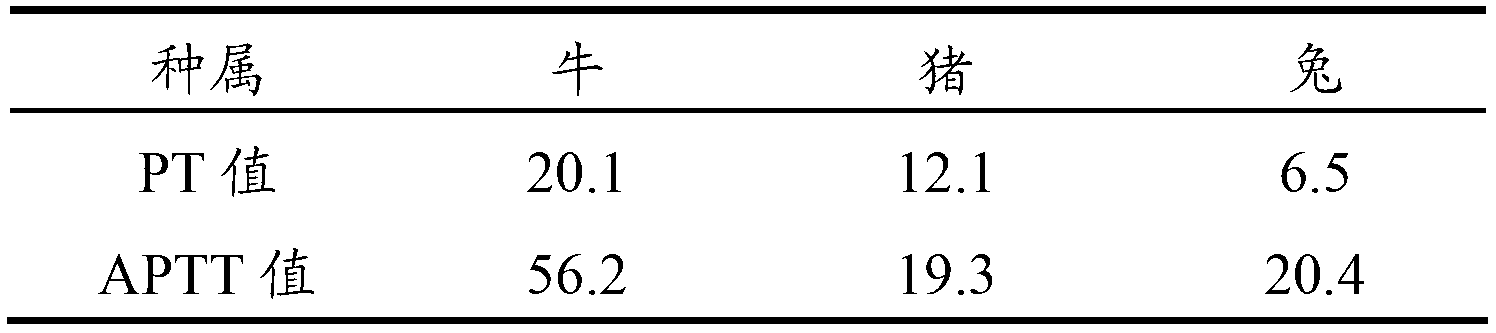
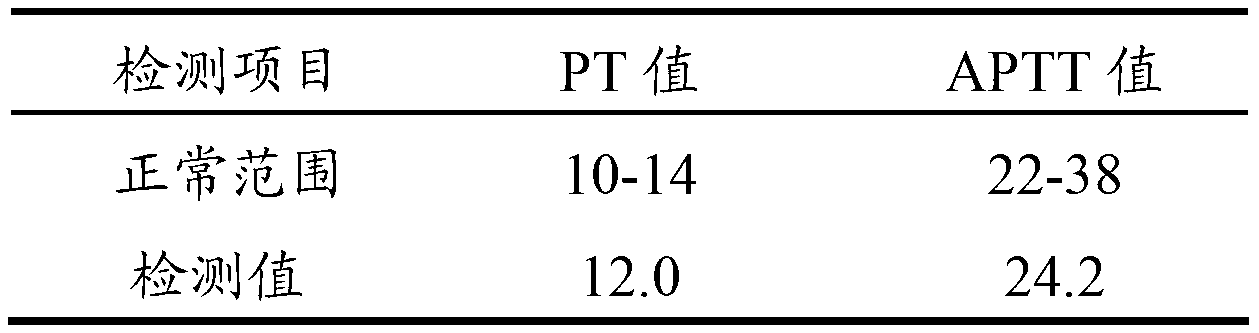
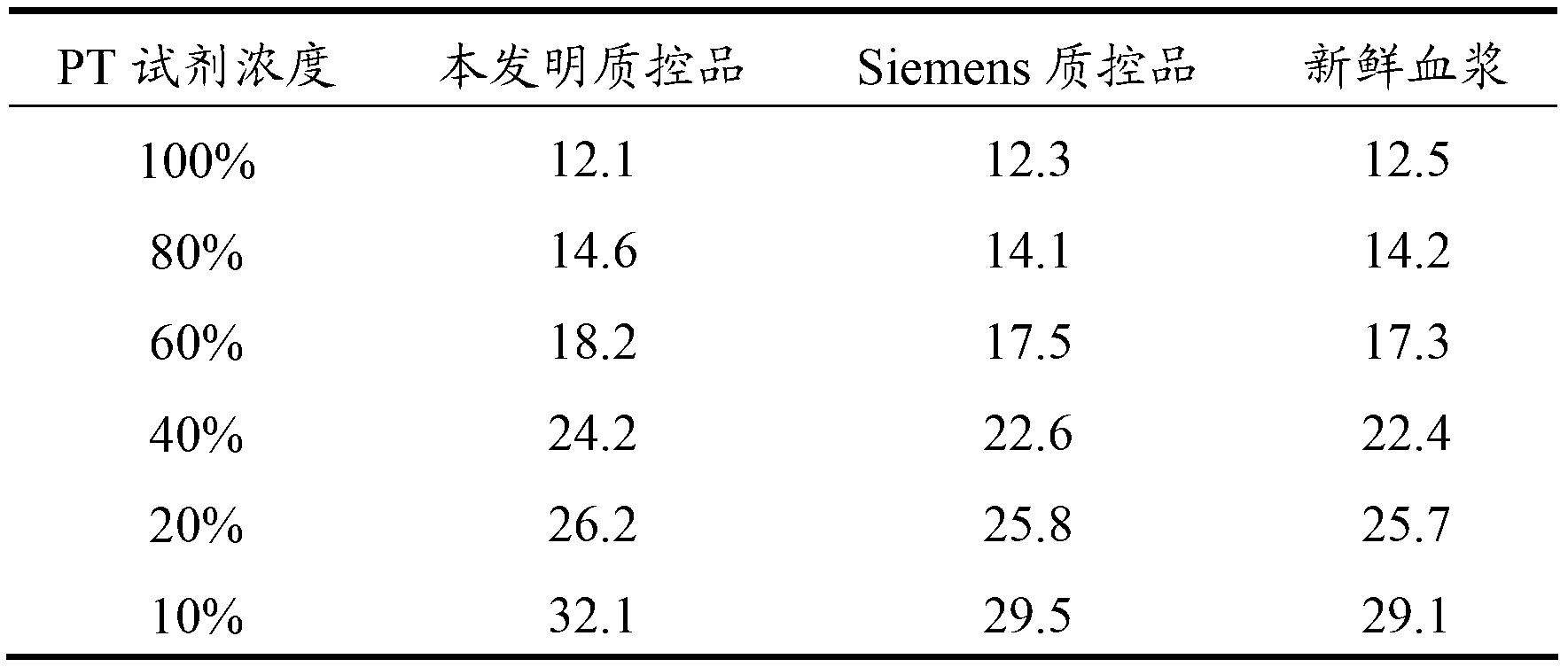
Blood coagulation quality control product and preparing method thereof
The invention relates to the field of quality control of a clinical blood coagulation inspection item, and in particular provides to a method for preparing a quality control product which can be used for simultaneously carrying out PT (Prothrombin Time), APTT (activated partial thromboplastin time) and FIB (fibrinogen) blood coagulation inspection items through animal blood plasma. The method comprises the steps of mixing the animal blood plasma according to an appropriate rate, adding or removing the FIB, adding proper amount of blood plasma buffer solution contacting a stabilizing agent so as to enable detecting results of mixed blood plasma PT, APTT and FIB to be in the range of a blood coagulation indicator of a normal person, drying and cooling, thus obtaining the product. The blood coagulation quality control product prepared by the method provided by the invention has good sensibility to variation of a detection reagent, has high stability and meets the quality control requirement of the clinical blood coagulation inspection item.
Owner:SHANGHAI SUNBIO TECH
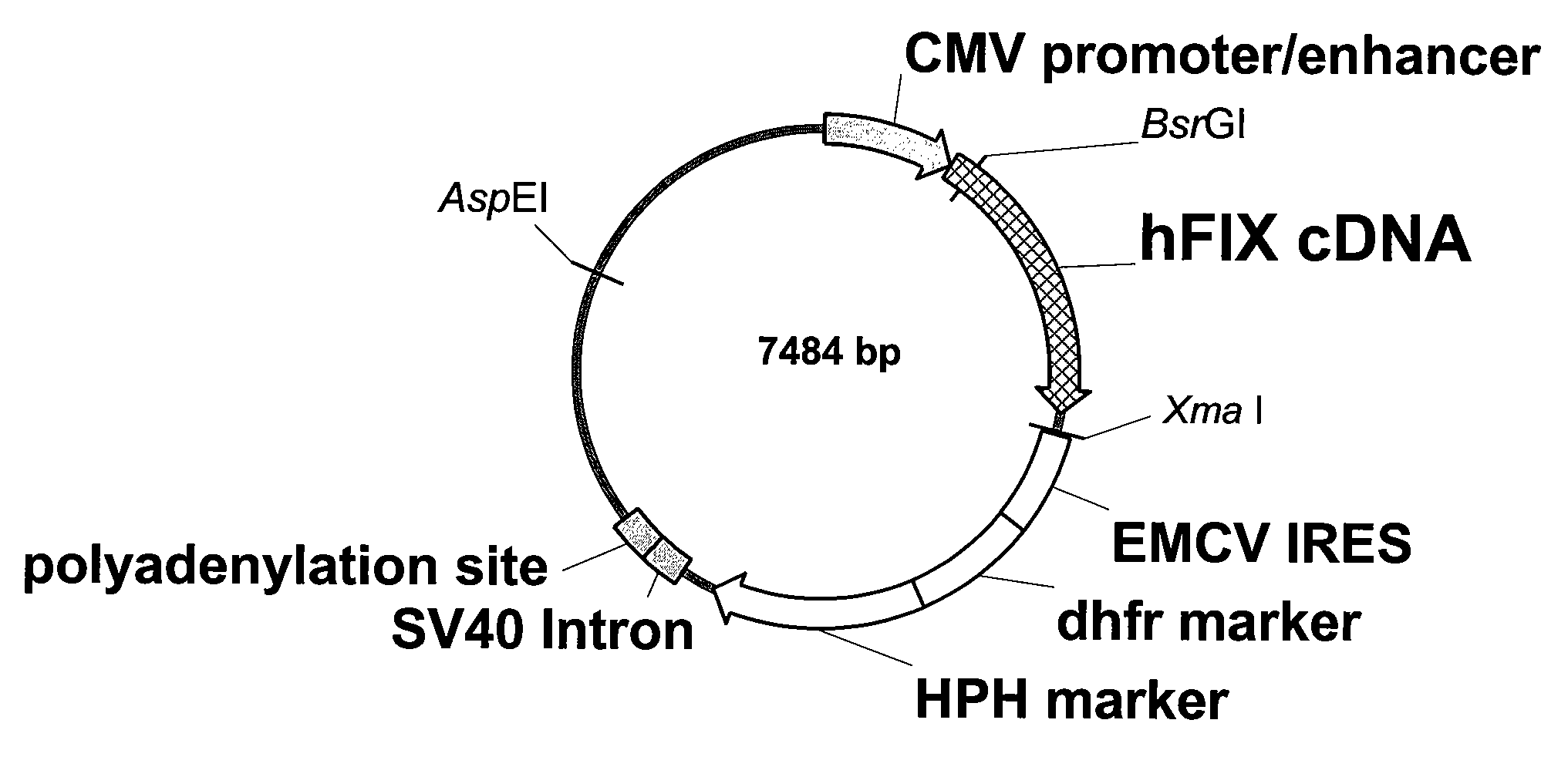
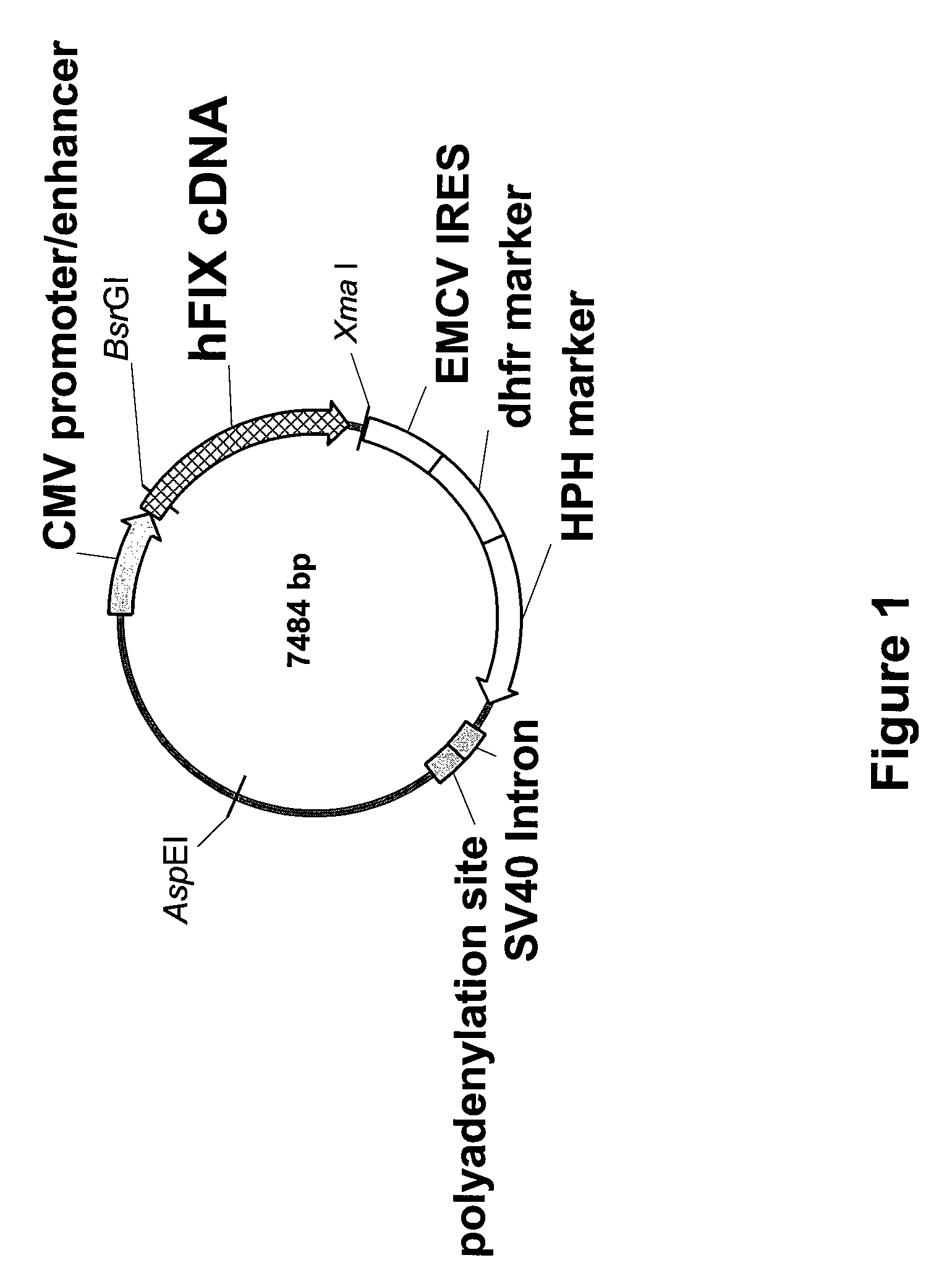
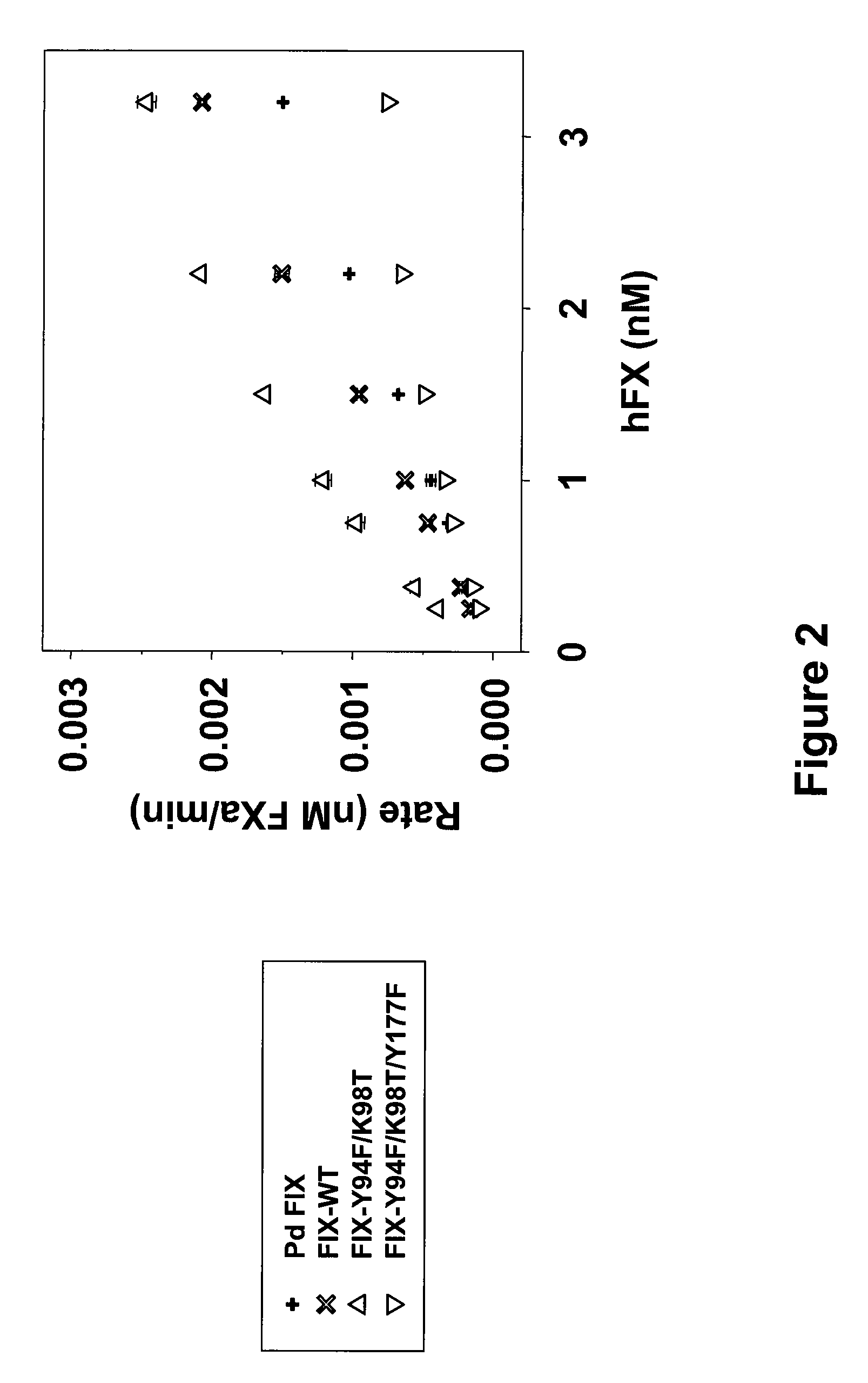
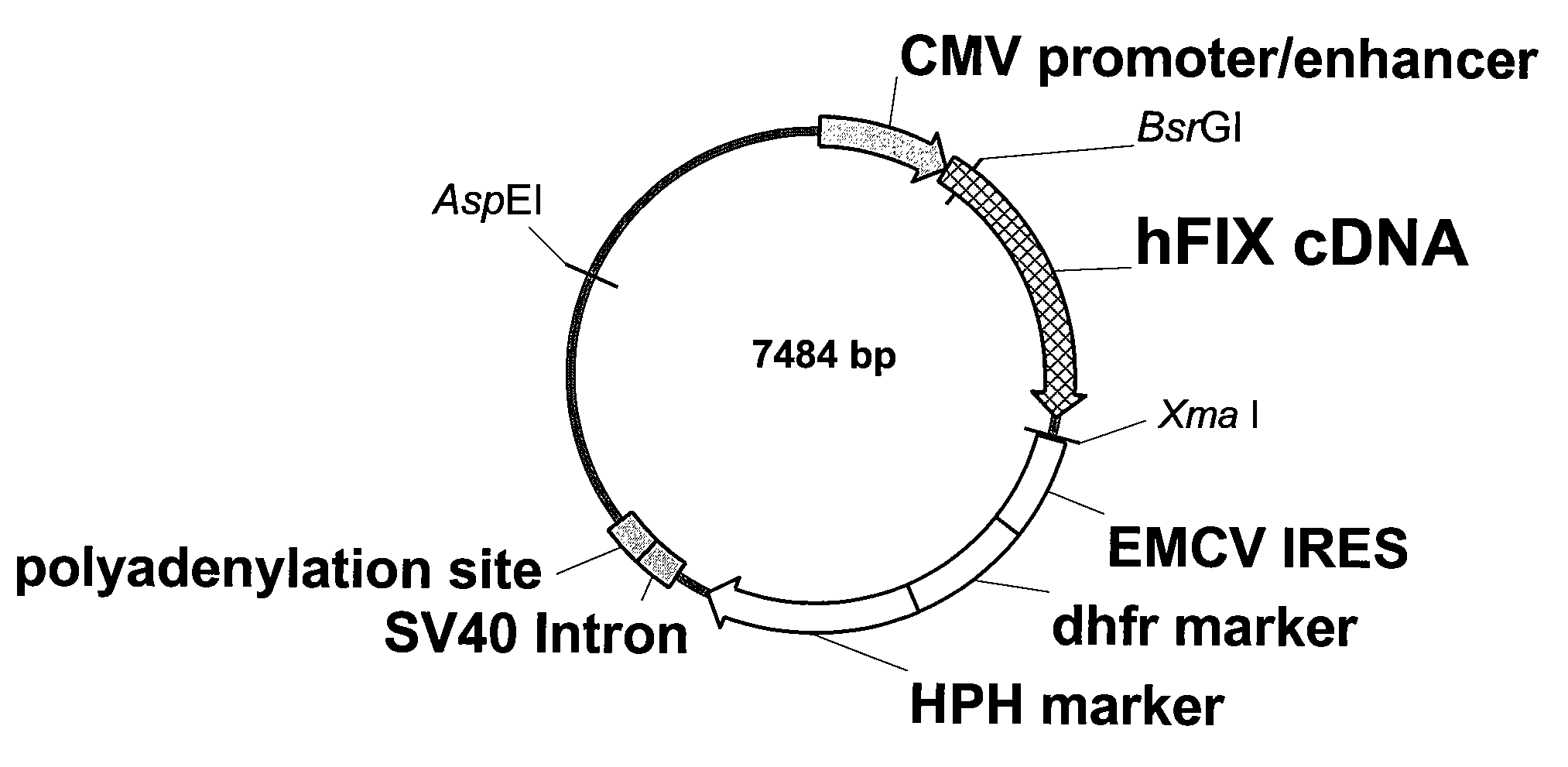
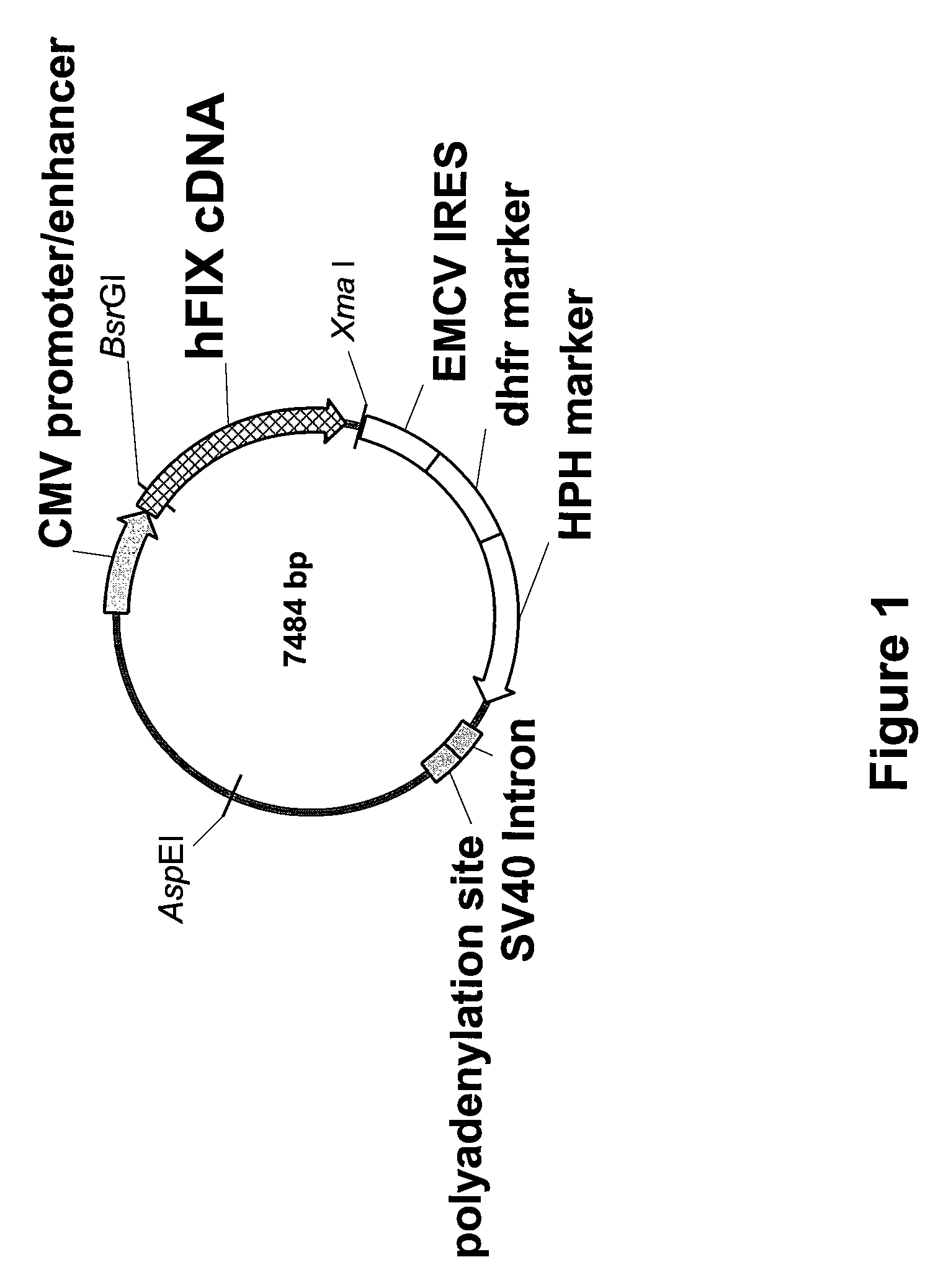
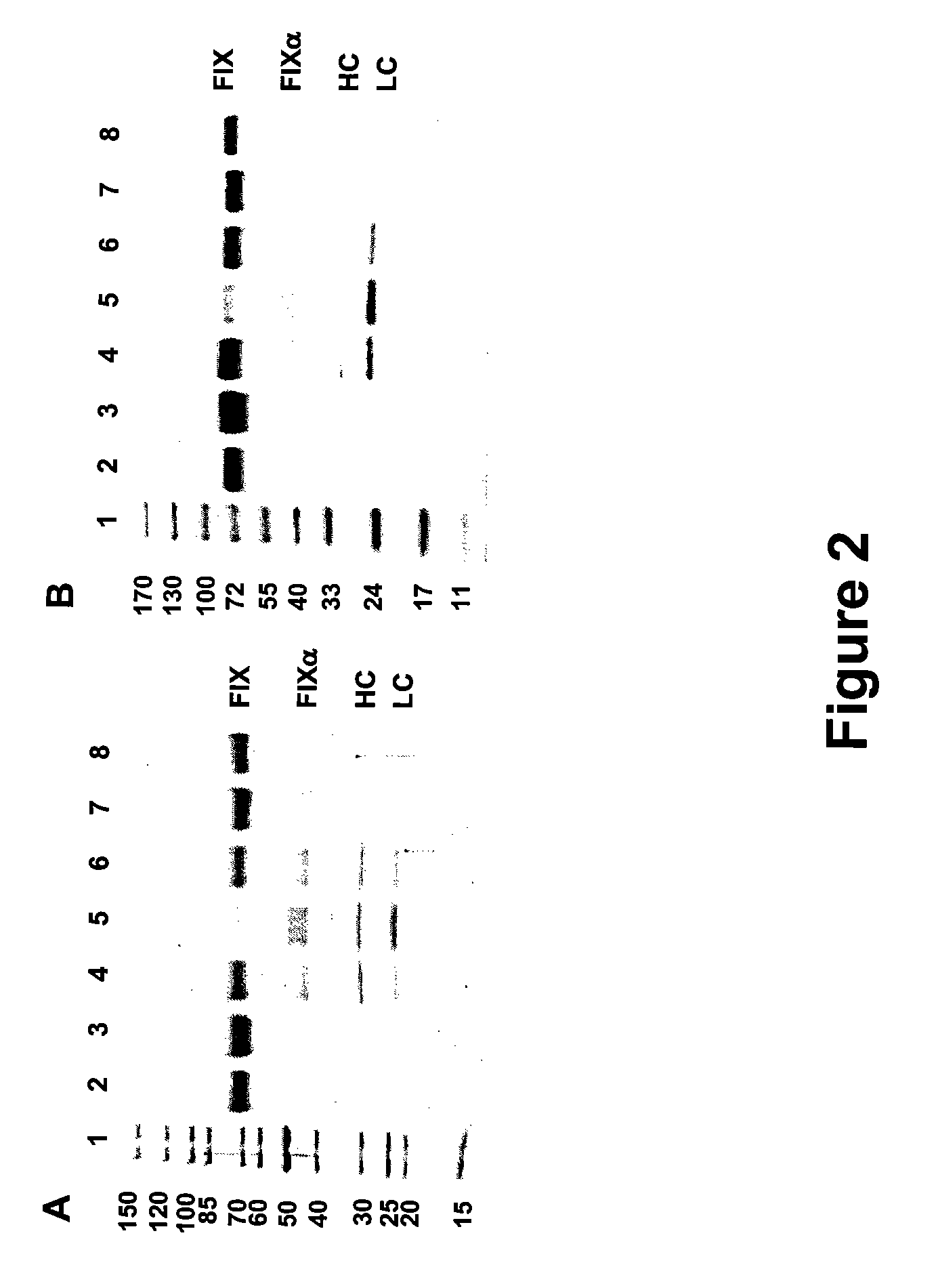
FIX-Mutant Proteins for Hemophilia B Treatment
InactiveUS20080214462A1Improved clot activityHigh activityPeptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsDisease
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having improved FIX clotting activity. Three full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assays in FIX-depleted plasma. Two mutant proteins had increased specific FIX activity. Furthermore, a pre-activated FIX protein had an increased activity in FIX-depleted plasma. Therefore these FIX mutants can be used for the treatment of FIX associated bleeding disorders.
Owner:BAXTER INT INC +1
A starch composite hemostatic dressing of mesoporous silica microspheres
InactiveCN106806931AHas a hemostatic effectThe hemostatic effect is achievedAbsorbent padsBandagesWound dressingMicrosphere
The invention relates to a starch composite hemostatic dressing of mesoporous silica microspheres, which adopts a sol-gel method and uses a three-block copolymer pluronic as a template agent to synthesize mesoporous silica microspheres with a pore diameter of about 5 nm. It is compounded with starch to prepare a mesoporous silicon oxide microsphere / starch composite hemostatic dressing, and the hemostatic performance of the composite material is observed through animal experiments. The mesoporous silica microsphere / starch composite material not only has strong water absorption performance, but also has obvious in vitro coagulation performance, which can significantly shorten the partial thromboplastin time and prothrombin time. The mesoporous silica microsphere / starch composite material can prevent the bleeding of the rabbit's back skin and liver injury and shorten the bleeding time, and has obvious hemostatic effect.
Owner:TIANJIN YIYAO SCI & TECH
FVIII-Independent FIX-Mutant Proteins for Hemophilia A Treatment
ActiveUS20080214461A1Peptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsMutated protein
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having factor VIII (FVIII) independent factor X (FX) activation potential. Five full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assay in FVIII-depleted plasma as well as in FVIII-inhibited patient plasma. In FVIII-depleted plasma functional activity of the FIX mutants was calculated as increased FVIII equivalent activity. The mutant proteins had increased FVIII equivalent activity. In FVIII-inhibited patient plasma the FEIBA equivalent activity was calculated for analysis of FVIII independent FX activation potential. The proteins had also increased FEIBA equivalent activity. Furthermore, the pre-activated FIX proteins had an increased activity in FIX-depleted plasma containing FVIII inhibitors. Therefore these FIX mutants are alternatives as bypassing agents for treatment of FVIII inhibitor patients.
Owner:TAKEDA PHARMA CO LTD
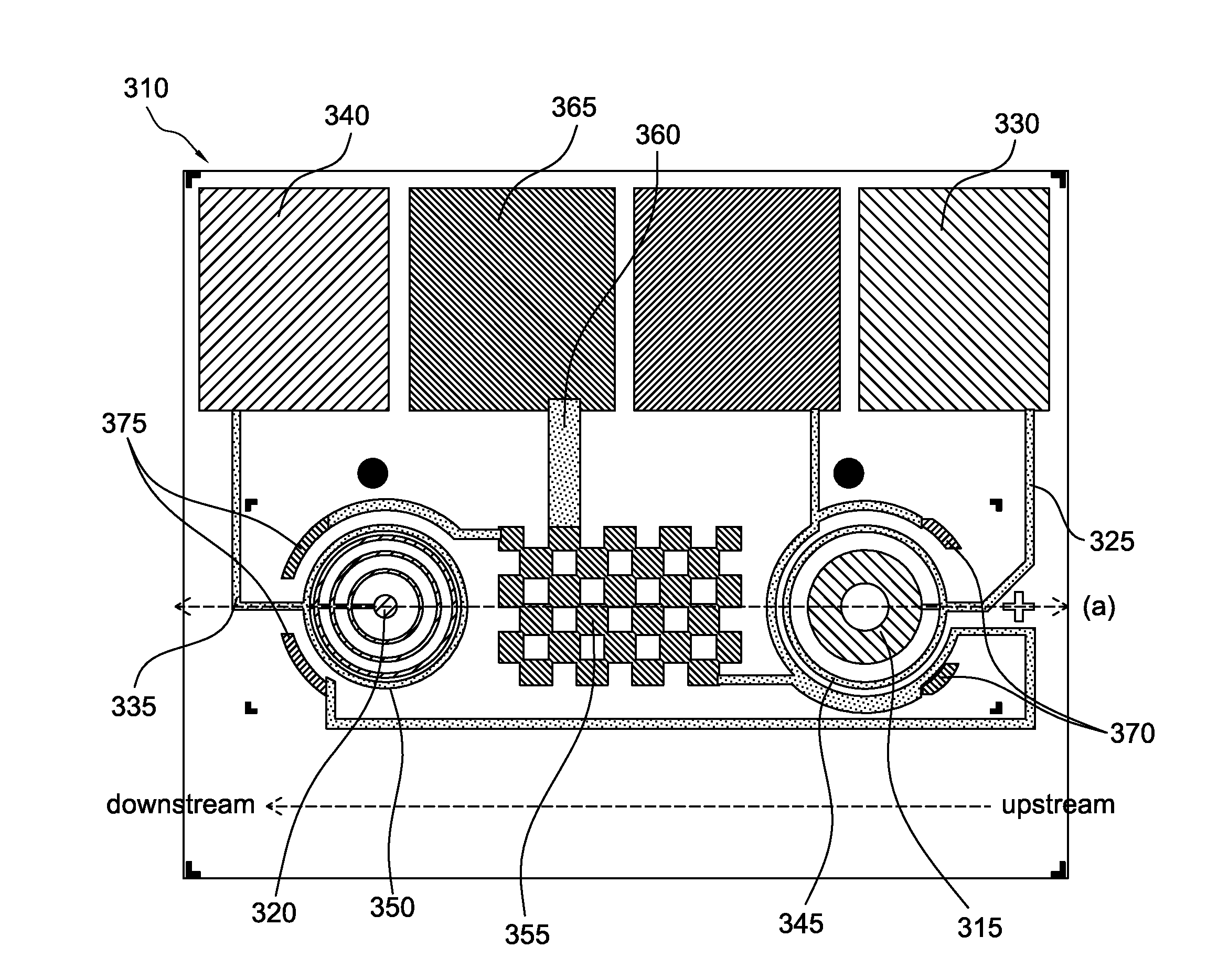
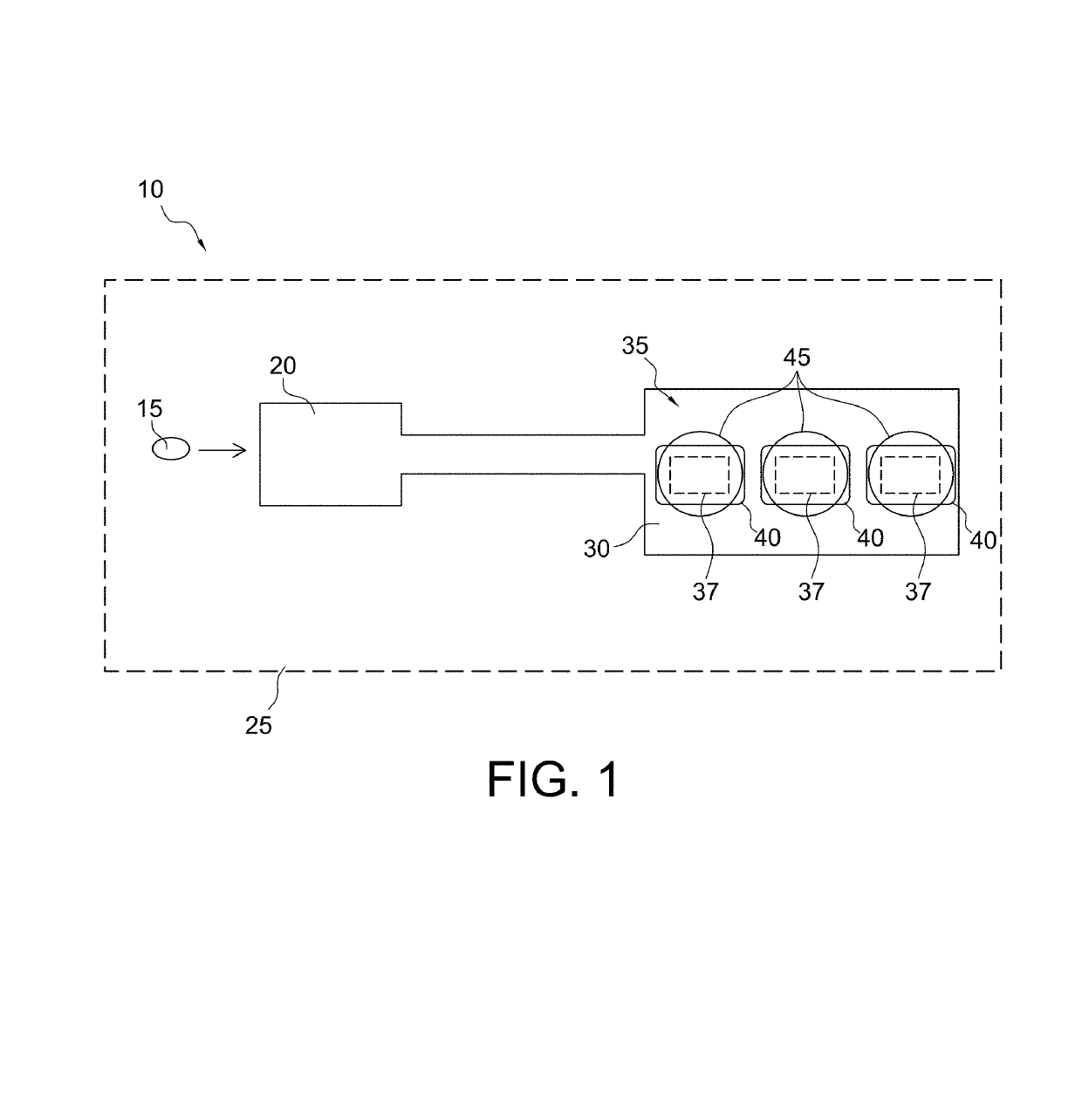
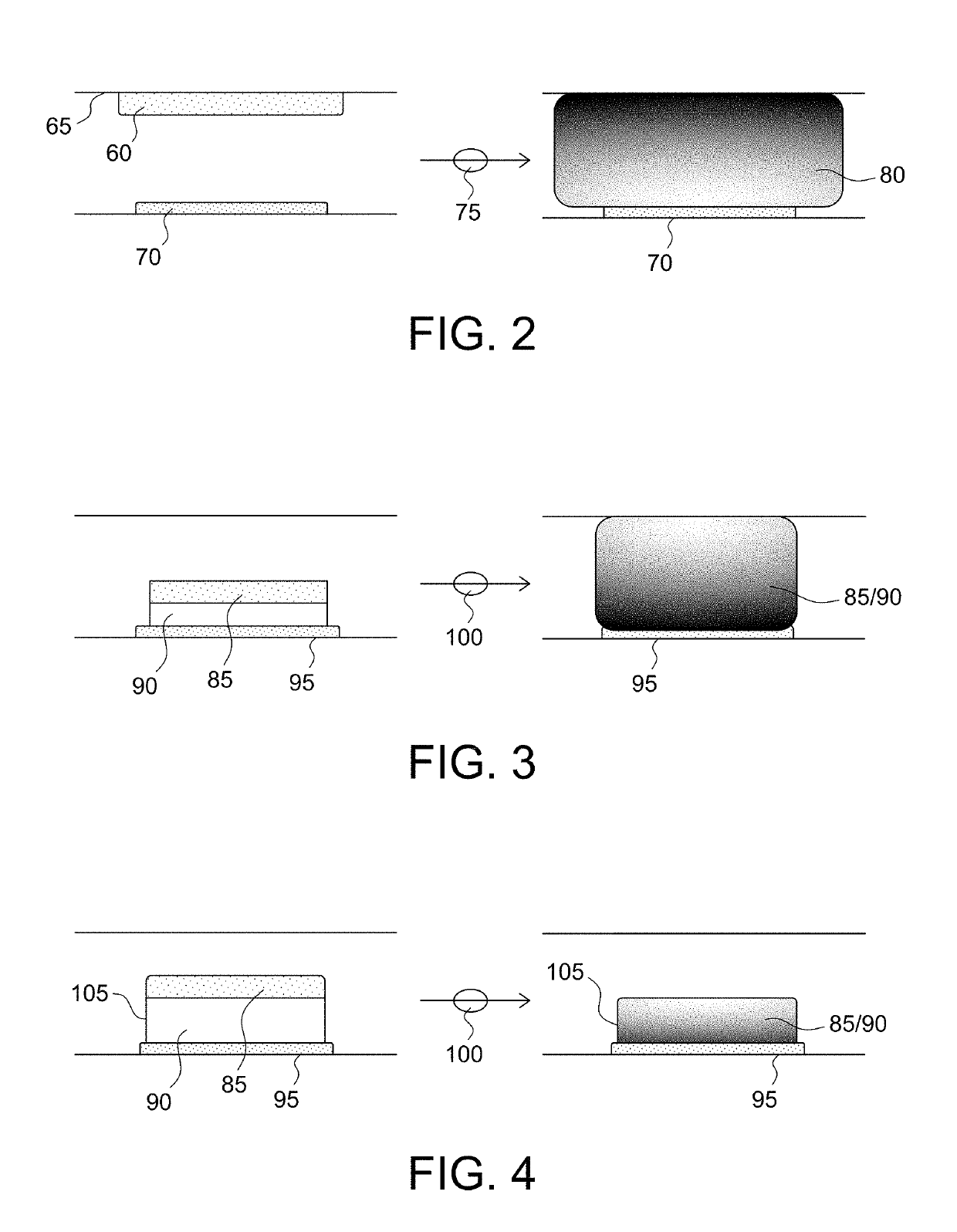
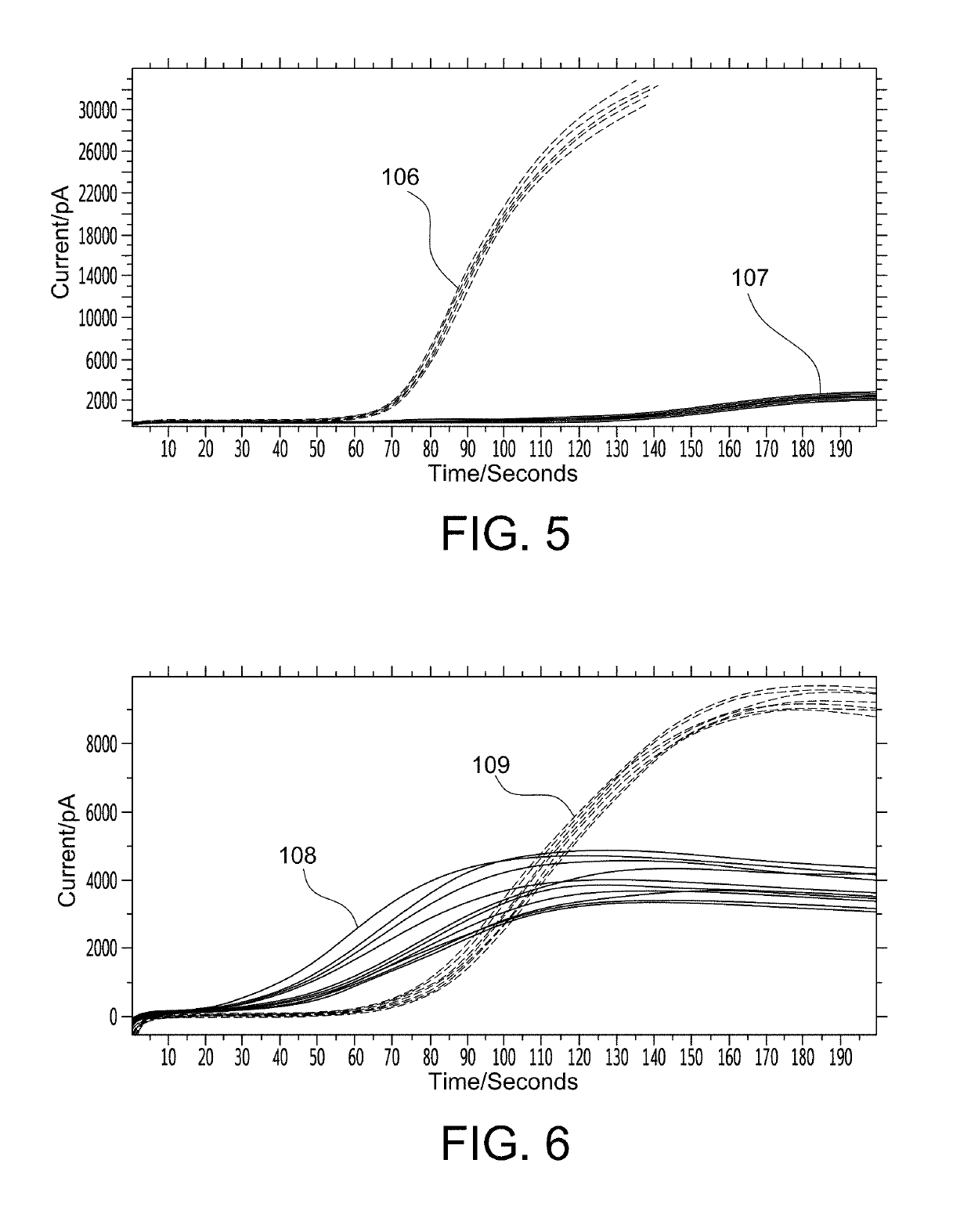
Microfabricated device with micro-environment sensors for assaying coagulation in fluid samples
ActiveUS20160091508A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careEngineering
The present invention relates to sample analysis cartridges comprising micro-environment sensors and methods for assaying coagulation in a fluid sample applied to the micro-environment sensors, and in particular, to performing coagulation assays using micro-environment sensors in a point of care sample analysis cartridge. For example, the present invention may be directed to a sample analysis cartridge including an inlet chamber configured to receive a biological sample, and a conduit fluidically connected to the inlet chamber and configured to receive the biological sample from the inlet chamber. The conduit may include a micro-environment prothrombin time (PT) sensor, and a micro-environment activated partial thromboplastin time (aPTT) sensor.
Owner:ABBOTT POINT CARE
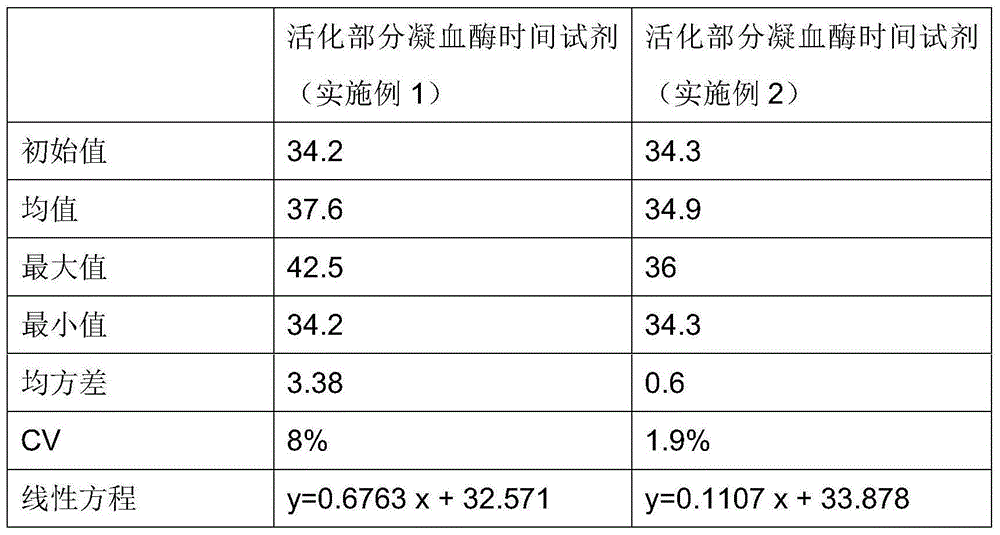
Testing reagent for activated partial thromboplastin time and preparation method of testing reagent
ActiveCN105203777AImprove production safetyImprove stabilityBiological testingPhospholipidBuffer solution
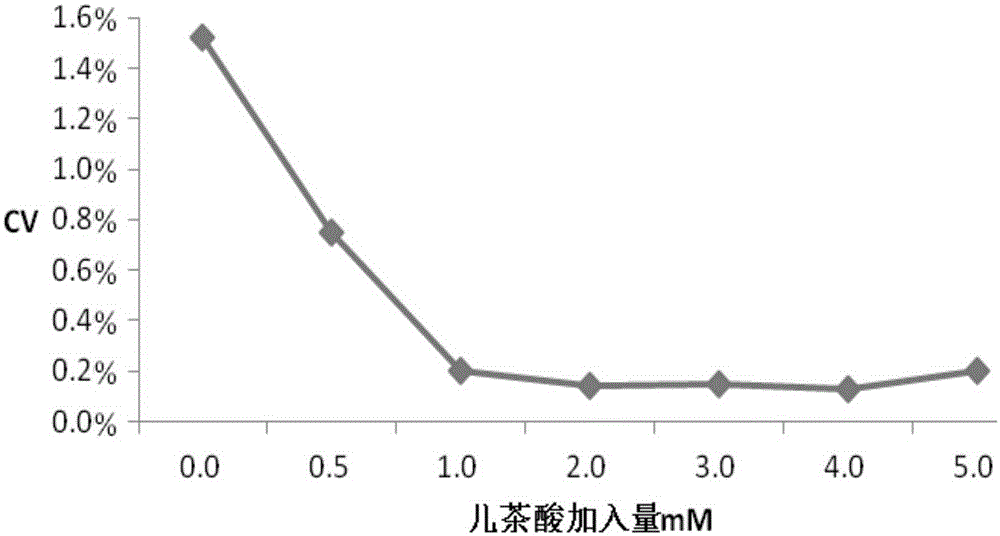
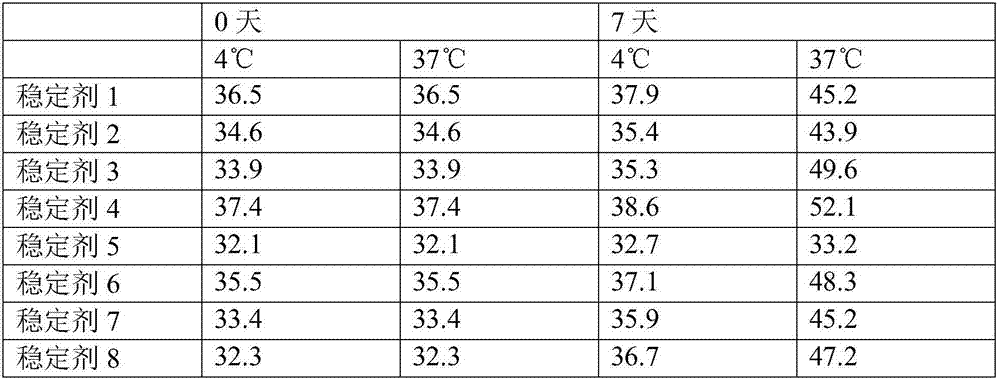
The invention relates to a testing reagent for activated partial thromboplastin time. The testing reagent is prepared from phospholipids, activator and stabilizer. The stabilizer is prepared from, by mass, 1%-4% of alanine and 0.5-5 mM of catechin. A preparation method of the testing reagent includes the steps that an HEPES buffer solution is prepared, the pH value of the buffer solution is adjusted to be neutral, and sodium chloride is added; the activator is added to the buffer solution, phospholipids and other components are added after stirring and even mixing are conducted, and then the testing reagent for APTT is obtained after even mixing, stirring and filtering are conducted. According to the testing reagent for activated partial thromboplastin time, stabilizer components with high toxicity are removed, so that production and use processes are safer; meanwhile, the stability and detection accuracy of the testing reagent for APTT are greatly improved.
Owner:QINGDAO GUGAO BIOTECH CO LTD
Detection reagent, application thereof and kit containing reagent
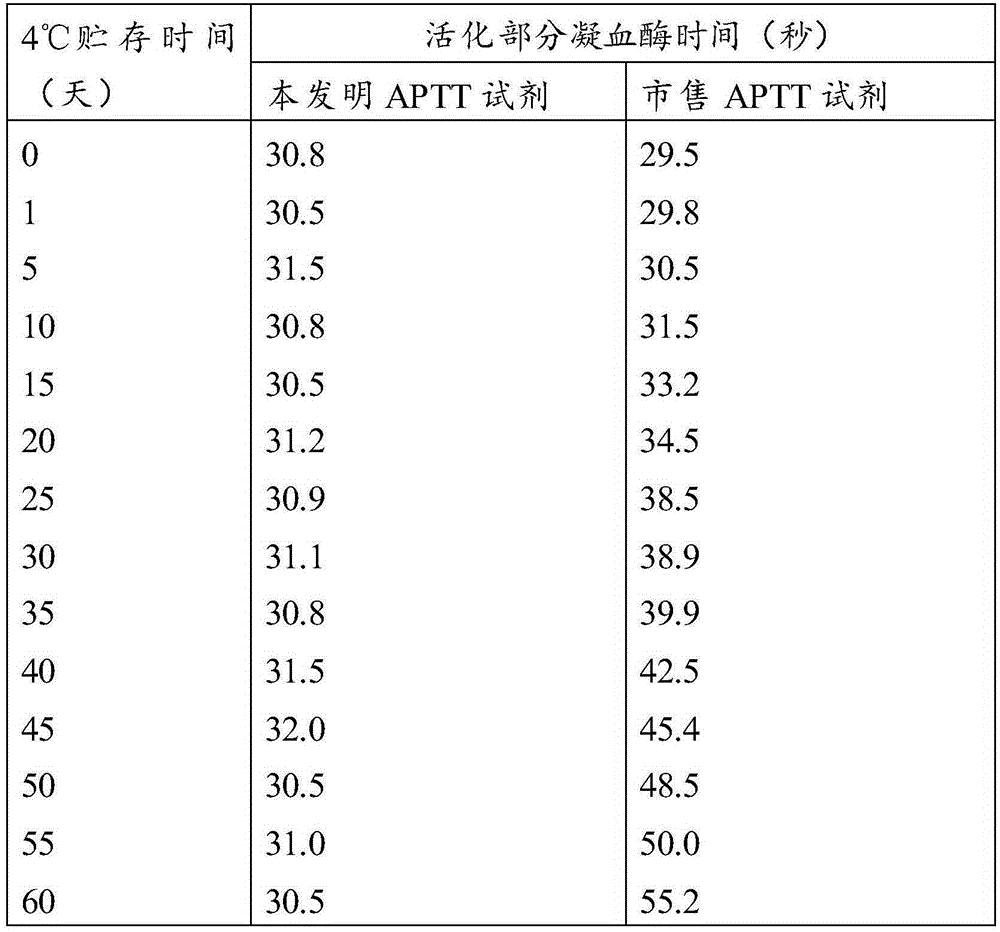
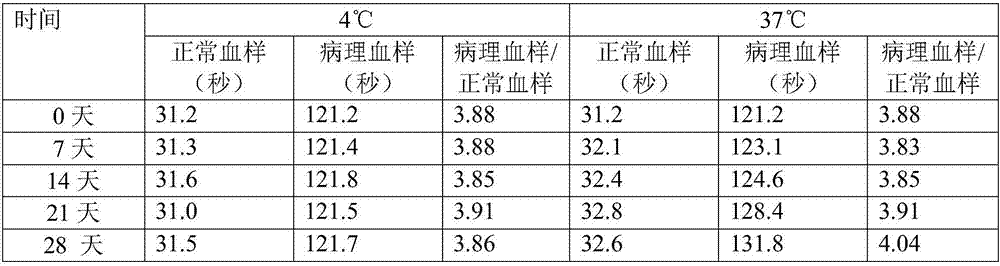
The invention relates to the technical field of biology, and especially relates to an activated partial thromboplastin time (APTT) detection reagent, application thereof and a kit containing the reagent. The detection reagent comprises kaolin, cephalin, a buffer, bovine serum albumin (BSA), glycine, glucan and NaN3. Compared with the prior art, the technical scheme comprises the following advantages: by adjusting the compositions and the proportions of a protective solution of the APTT kit, the APTT reagent stability is extremely good, is stable within 18 months at 4 DEG C, is stable within 15 days at 37 DEG C, and is stable within 50 days at 4 DEG C after being opened. The product stability is substantially (P<0.05) better than common commercially-available products, cost is saved for hospitals and extremely good reagent source is provided.
Owner:BEIJING MDC NEW SPRING MEDICAL DEVICES
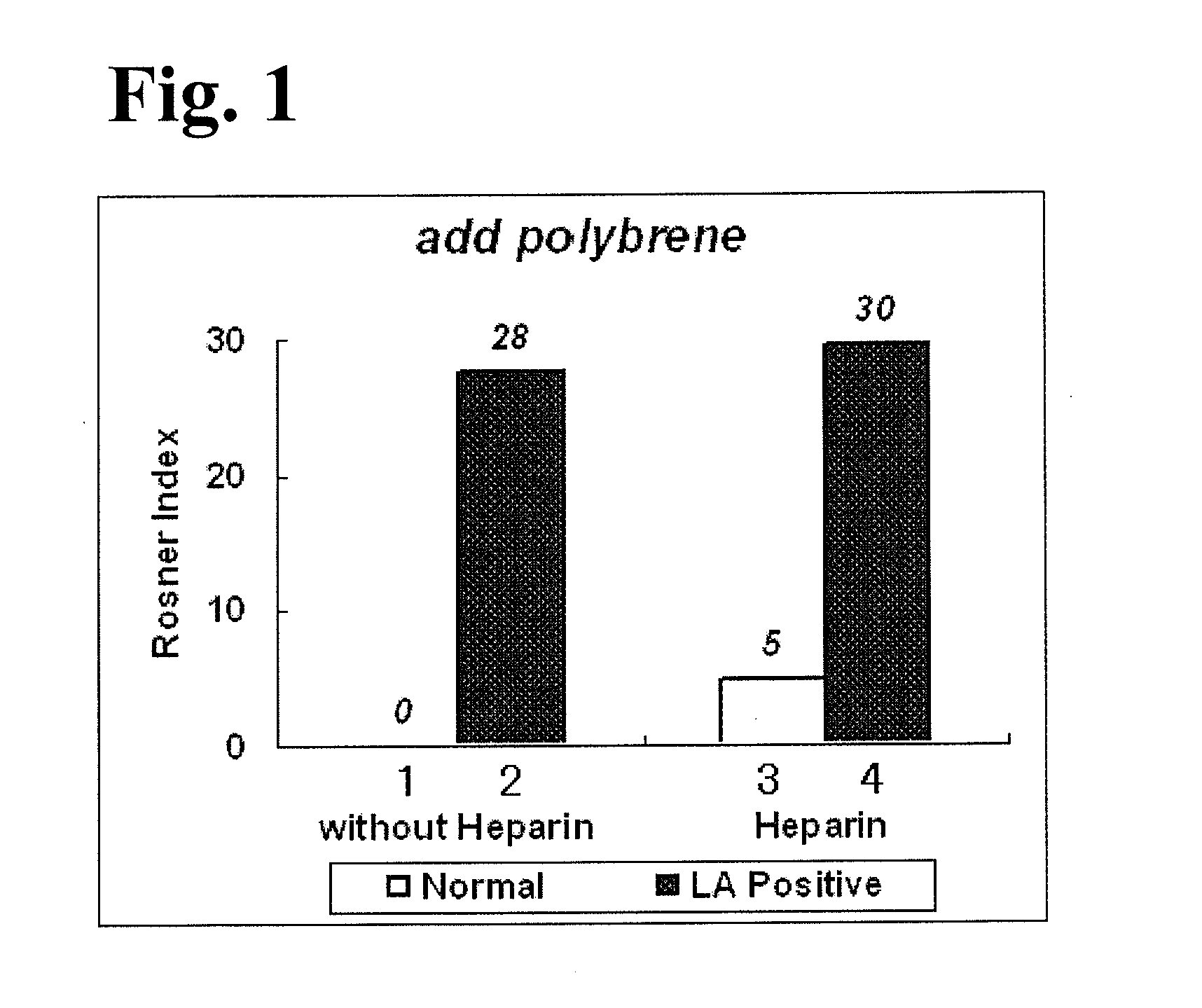
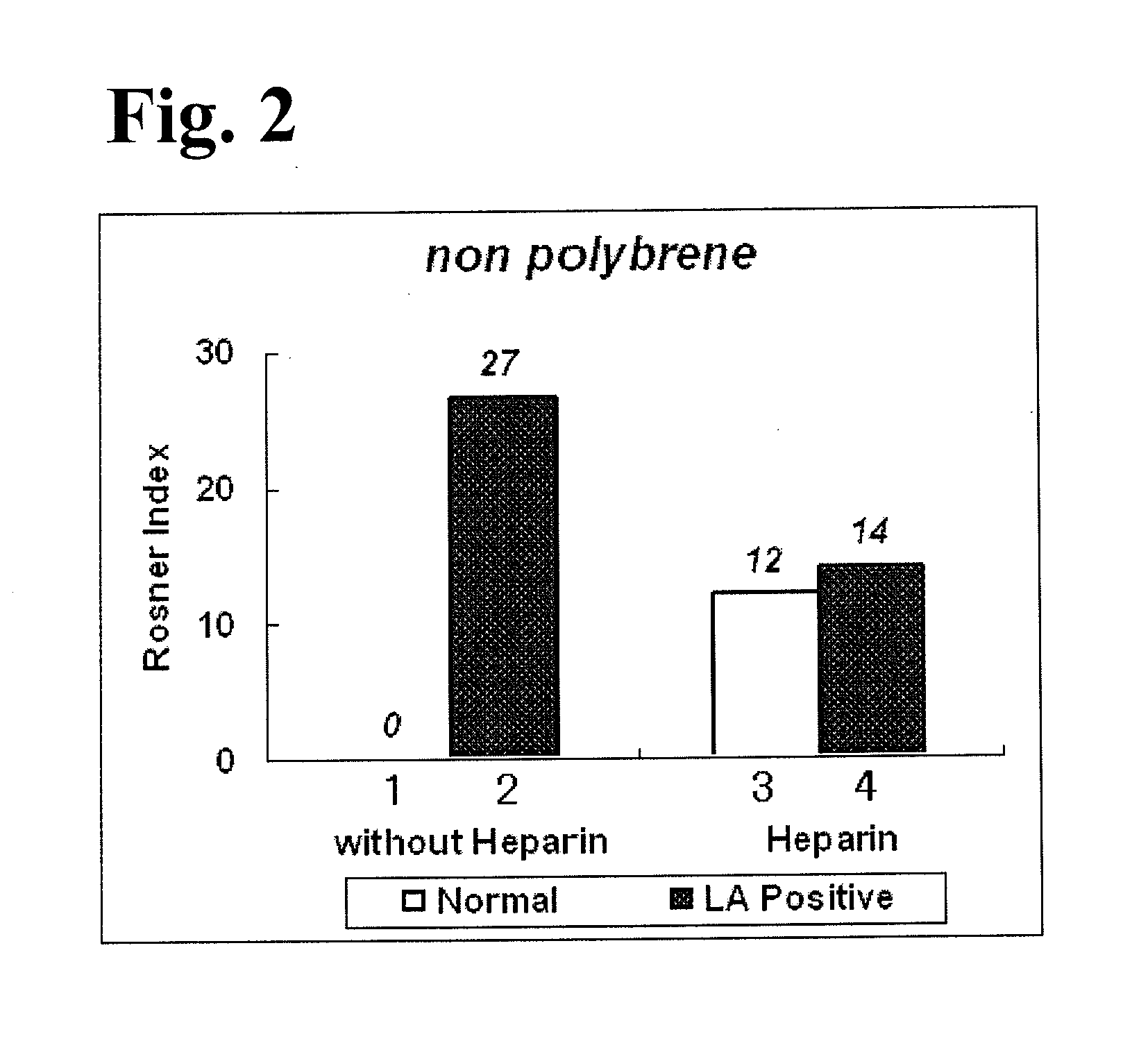
Activated partial thromboplastin time measuring reagent, activated partial thromboplastin time measuring method, and determination method for determining presence or absence of blood coagulation inhibitor
InactiveUS20110159597A1Organic chemistryOrganic compound preparationPartial thromboplastin timeThromboplastin
An activated partial thromboplastin time measuring reagent, comprising a heparin neutralizer is disclosed. An activated partial thromboplastin time measuring method, and a determination method for determining a presence or absence of a blood coagulation inhibitor are also disclosed.
Owner:SYSMEX CORP
Method for preparing anticoagulant composite coating
InactiveCN107456611AExcellent anticoagulant propertiesIncreased anticoagulationSurgeryPharmaceutical delivery mechanismBiocompatibility TestingPre treatment
The invention discloses a method for preparing an anticoagulant composite coating. The method for preparing the anticoagulant composite coating comprises the steps of first using a coupling agent to process the surface of a pre-processed biomedical base material to obtain a hydrophilic surface, using a specific anticoagulant composite coating to modify the biomedical base material, and obtaining a biomedical material having good anticoagulation property after drying. Studies have shown that the biomedical material modified by the anticoagulant composite coating is greatly improved in hydrophilicity and shows excellent biocompatibility. The modified material can not only maintain its original physical and mechanical properties, but also greatly extend activated partial thromboplastin time (APTT) in the coagulation test to show excellent anticoagulant properties.
Owner:BEIJING UNIV OF CHEM TECH
Activated partial thromboplastin time detection reagent and detection method
ActiveCN108226539AIncrease reaction strengthAccurate detectionDisease diagnosisBiological testingAntioxidantDivalent metal ions
The invention relates to the technical field of biology and especially relates to an activated partial thromboplastin time detection reagent and a detection method. The detection reagent is composed of: an activator, phospholipid, a bivalent metal ion salt, a buffer reagent, a surfactant, an antioxidant, and a stabilizer. The reagent can accurately and quickly detect the APTT and is good in stability and is stable even after preservation at 37 DEG C for 15 days without influence on detection effect. Compared with a control group without the surfactant, the detection reagent can accurately detect a sample in low fibrinogen content, so that detection limit is reduced and sensitivity is increased. The reagent is high in precision in detection on various samples, cv value being lower than 3%.
Owner:SINOCARE
Method and reagent kit for measuring activated partial thromboplastin time
InactiveUS20140295470A1Less LA-induced impactOccurrence of preventedMicrobiological testing/measurementBiological material analysisBlood plasmaReagent
The present invention provides a method for measuring activated partial thromboplastin time. The method comprises: a first mixing step of mixing a blood plasma with a first reagent, wherein the first reagent comprises an activator and phosphatidylglycerol at a concentration equal to or greater than 25 μg / mL; a second mixing step of mixing a sample obtained in the first mixing step with a second reagent comprising a calcium salt; and a step of measuring coagulation time of the sample obtained in the second mixing step.
Owner:SYSMEX CORP
A kit for measuring activated partial thromboplastin time (APTT)
ActiveCN107748267AAvoid Bottle-to-Bottle VariationsGuaranteed stabilityDisease diagnosisBiological testingFreeze-dryingChloride
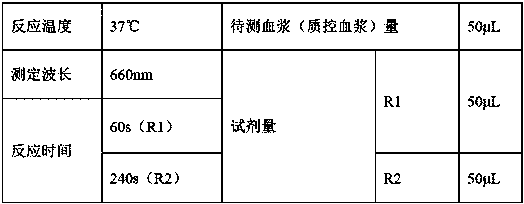
A kit for measuring activated partial thromboplastin time (APTT) is provided. The kit includes R1 including an activator, partial thromboplastin, a stabilizer, an aseptic and a buffer liquid; and R2 that is a calcium chloride solution. The stabilizer includes dextran. In the R1, the concentration of the activator is 0.05-0.2 mM, the content of the partial thromboplastin is 0.1-0.5 wt%, the contentof the aseptic is 0.01-0.02 wt% and the content of the dextran is 2-4 wt%. All agents in the kit are liquid and stable so that reagent differences among bottles caused by freeze-drying and redissolving processes in preparation and application processes, thus avoiding large differences among measurement results. Stability of the reagents can be ensured without the need of freeze drying, and optimum validity of the reagents after bottle opening is at least a month, thus avoiding large differences among experiment results, reducing using amounts of the reagents, achieving instant use after bottle opening, and making operation rapider and simpler.
Owner:山东艾科达生物科技有限公司
Liquid ready-to-use activated partial thromboplastin time detection reagent
InactiveCN107356769AImprove stabilitySmall difference between batchesBiological testingCholesterolReady to use
The invention discloses a liquid ready-to-use detection reagent for activated partial thromboplastin time, which comprises a buffer, a synthetic phospholipid, an activator, a stabilizer and phenol, and the synthetic phospholipid is composed of phosphatidylserine, phosphatidylcholine, phospholipid Ethanolamine and cholesterol composition, the activator is ellagic acid. The detection reagent of the invention has good stability, small difference between batches, easy quality control during production, and high sensitivity.
Owner:NINGBO ACCUTECH BIOSCI LTD
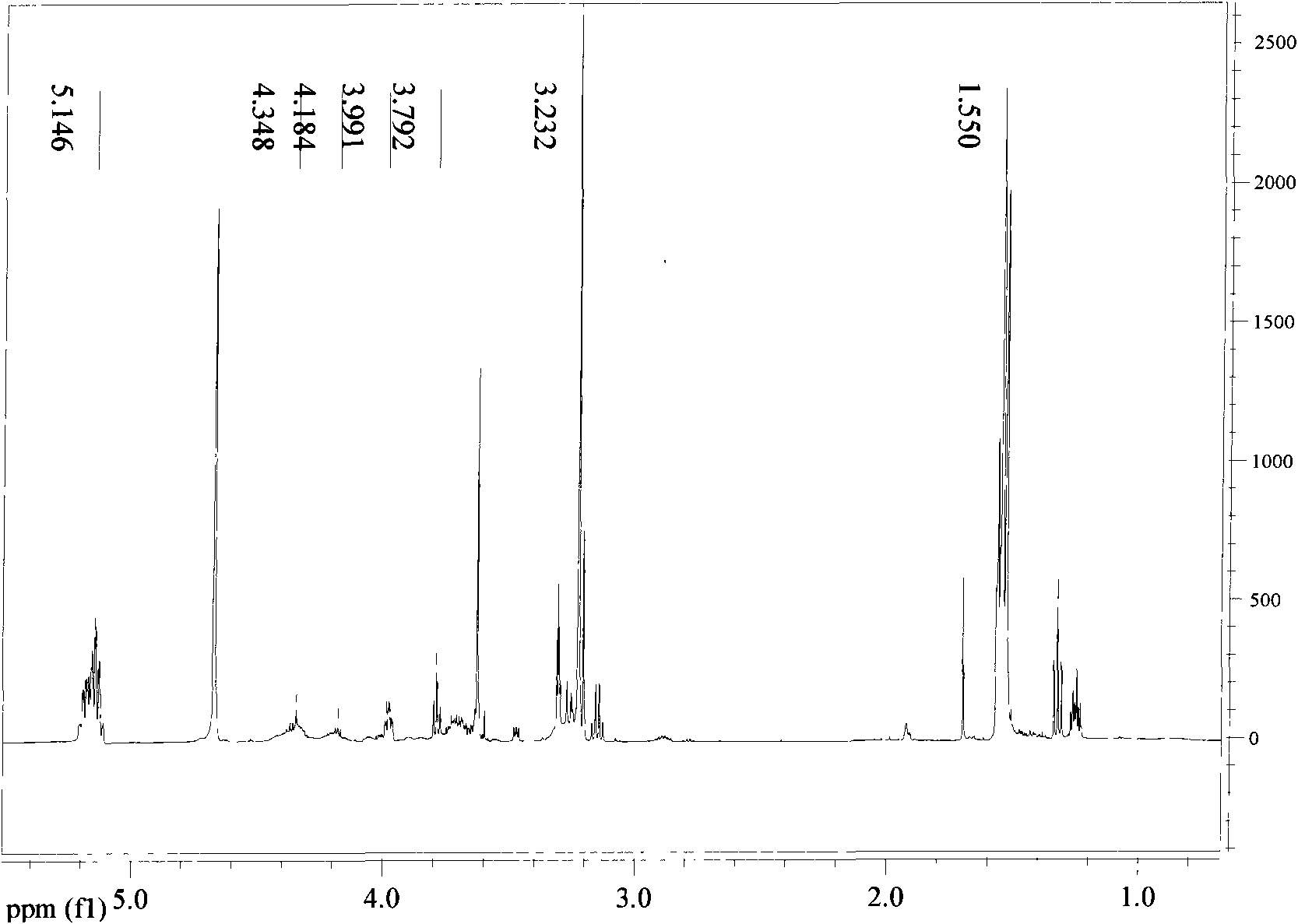
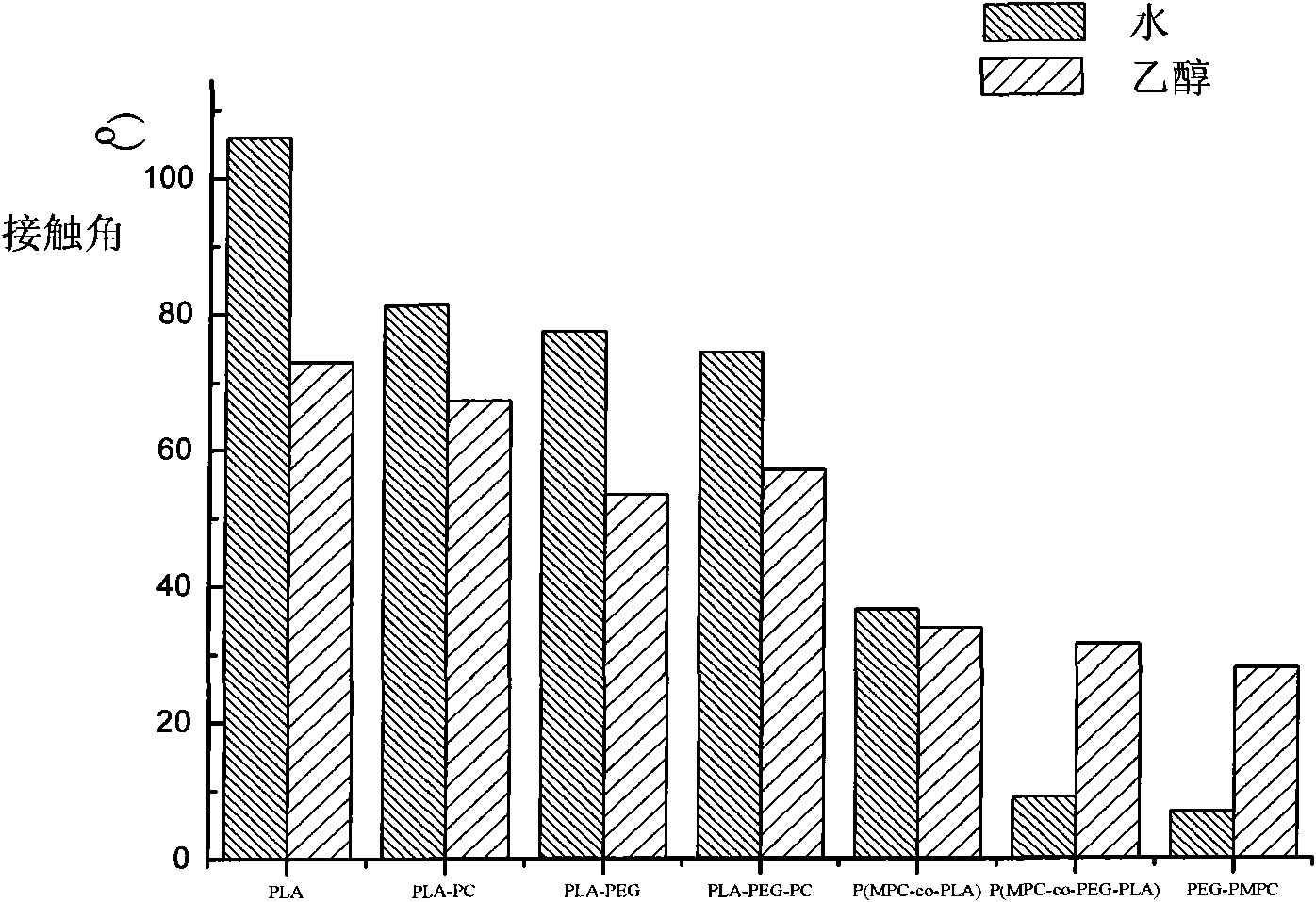
Biodegradable polymer containing phosphorylcholine and polyethylene glycol and synthetic method thereof
InactiveCN101538353AImprove hydrophilicityImprove anticoagulant performancePharmaceutical containersMedical packagingPolymer scienceLactide
The invention relates to a biodegradable polymer containing phosphorylcholine (PC) and polyethylene glycol (PEG) and a synthetic method thereof. The synthetic method comprises: MPC with the mass ratio of 1-99% and PEG-PLA which is connected with double linkage and has the mass ratio of 99-1% are dissolved in trichloromethane and then added with free radical polymerization initiator to react for 6-24h at the temperature of 0-80 DEG C, and the products are precipitated by methyl alcohol and dried in vacuum; the PEG-PLA which is connected with double linkage is prepared by ring opening polymerization of lactide initiated by the PEG that reacts with acryloyl chloride at a single end. The phosphorylcholine group which has positive and negative charges and is introduced on the PLA of the polymer can be seen according to the static contact angle result, and then the contact angle is obviously reduced, so that hydrophilicity is greatly improved. The higher the MPC content is, the smaller the contact angle is, and the better the wettability of the material is. Test results of anticoagulation prove that the PEG can reduce coagulation of blood platelet, affects prothrombin time (PT) and effectively avoids the activation of an extrinsic coagulation system; the high content PC group can effectively reduce the conglutination of the blood platelet and has influence on anginal partial thromboplastin time (APTT) of the activation part.
Owner:TIANJIN UNIV
Measuring reagent for activated partial thromboplastin time
ActiveCN108226538AImprove biological activityStrong superoxidantBiological testingHydrogenAntioxidant
The invention discloses a measuring reagent for an activated partial thromboplastin time. The measuring reagent is prepared from a buffer solution, an activator, phospholipid, an antioxidant and a stabilizer, wherein a pH (potential of Hydrogen) value is 7.0 to 7.5; the antioxidant is prepared from astaxanthin; the addition amount of the astaxanthin is 0.1g / L to 0.5g / L. The reagent adopts the astaxanthin as the antioxidant to be capable of effectively generating a reaction with a peroxy radical; thus, the peroxidation chain reaction of lipid is terminated, and the stabile performance of the reagent is further promoted.
Owner:GUANGZHOU WONDFO BIOTECH
Activated partial thromboplastin time detection reagent and activated partial thromboplastin time detection method
InactiveCN107942080AGood repeatabilityImprove stabilityBiological testingHeparin therapyClotting factor
The invention belongs to a clinical medicine detection technology, and particularly relates to an activated partial thromboplastin time detection reagent and an activated partial thromboplastin time detection method, wherein the detection reagent comprises a fresh rabbit brain impregnation liquid. According to the present invention, the method has advantages of precise detection and simple operation; and the safe, reliable and good-repeatability in vitro diagnostic reagent is provided for the endogenous blood coagulation factor deficiency and heparin therapy in clinical medicine.
Owner:北京众驰伟业科技发展有限公司
APTT (activated partial thromboplastin time) detecting agent stabilizer and APTT detecting agent
ActiveCN109444438AImprove stabilityImprove production safetyBiological material analysisBiological testingMedicineCHITOSAN OLIGOSACCHARIDE
The invention relates to an APTT detecting agent stabilizer and an APTT detecting agent and belongs to the technical field of biological detection. The APTT detecting agent is composed of, by weight percentage, 55.3-58.9% of chitosan oligosaccharide, 17.2-20.8% of glucosamine and auxiliary agents as balance. The dosage of the APTT detecting agent stabilizer in the APTT detecting agent containing gallogen and rabbit cephalin is 5.0-5.5%. The APTT detecting agent stabilizer is green and toxicity-free, safe in production process and good in stabilizing effects on the APTT detecting agent.
Owner:NINGBO RUI BIO TECH
Hirudin polyion micelle composition of targeted platelet
InactiveCN101810559AEvaluate targetingPeptide/protein ingredientsPharmaceutical delivery mechanismArginineThrombus
The invention relates to a hirudin polyion micelle composition of a targeted platelet. The compound contains hirudin, arginine-glycine-aspartic acid-polyethylene glycol grafted chitosan and a polyion initiator. The polyion micelle composition can be prepared by a simple method, has platelet targeting, enhances the hirudin anticoagulation and the thrombus proofing activity, effectively suppresses the platelet aggregation and obviously improves the partial thromboplasting time of plasma.
Owner:PEKING UNIV
Activated partial thromboplastin time measuring reagent and application thereof
The invention relates to an activated partial thromboplastin time measuring reagent and application thereof. The activated partial thromboplastin time measuring reagent is prepared from an activator,phospholipids, a buffering agent and a protective agent, wherein the activator is ellagic acid. The activated partial thromboplastin time measuring reagent not only has good sensitivity to heparin, but also has stable and reliable detection results, can be effectively used for clinical monitoring of heparin anticoagulant therapy, and has broad application prospects.
Owner:深圳优迪生物技术有限公司
Microfabricated device with micro-environment sensors for assaying coagulation in fluid samples
ActiveUS10247741B2Biological material analysisMaterial analysis by electric/magnetic meansPoint of careEngineering
The present invention relates to sample analysis cartridges comprising micro-environment sensors and methods for assaying coagulation in a fluid sample applied to the micro-environment sensors, and in particular, to performing coagulation assays using micro-environment sensors in a point of care sample analysis cartridge. For example, the present invention may be directed to a sample analysis cartridge including an inlet chamber configured to receive a biological sample, and a conduit fluidically connected to the inlet chamber and configured to receive the biological sample from the inlet chamber. The conduit may include a micro-environment prothrombin time (PT) sensor, and a micro-environment activated partial thromboplastin time (aPTT) sensor.
Owner:ABBOTT POINT CARE
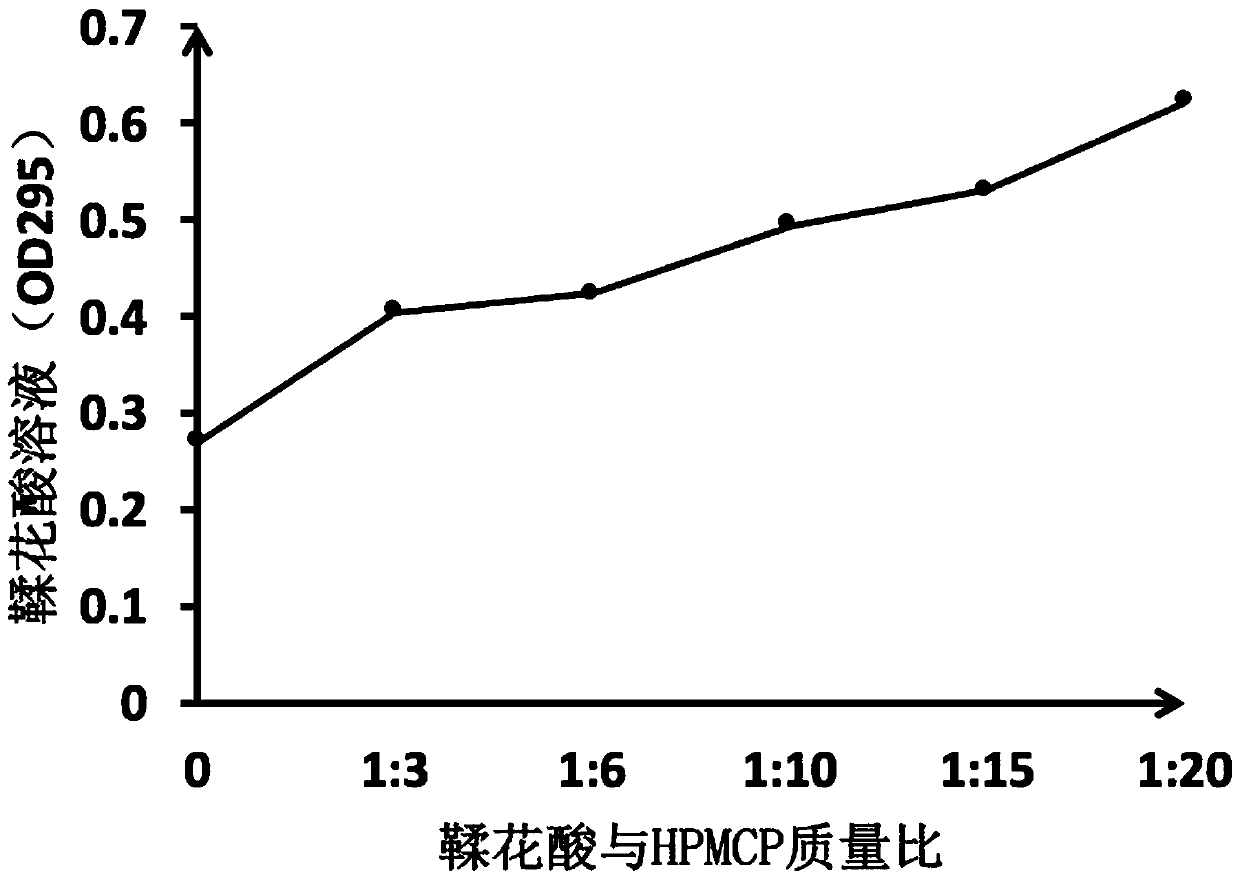
Novel ellagic acid compound and method for preparing activated partial thromboplastin time measuring reagent through ellagic acid compound
The invention belongs to the technical field of blood coagulation reagent preparation, and provides a novel ellagic acid compound aiming at the characteristic that ellagic acid is difficult to dissolve in water. The novel ellagic acid compound is prepared from the ellagic acid and a solid dispersant which are mixed according to a certain proportion. The solubility of the ellagic acid is improved,the problem that the ellagic acid is prone to being precipitated is solved, and the stability of the ellagic acid in products is guaranteed. The invention also provides a method for preparing an activated partial thromboplastin time measuring reagent through the ellagic acid compound. The reagent is prepared from liposomes, the ellagic acid compound, metal ions, a stabilizer and a preservative according to a certain proportion. According to the prepared reagent, through the ellagic acid solid compound, the problem that the ellagic acid is prone to being precipitated is solved, components of the liposomes are optimized, the inter assay variation between the activated partial prothrombin reagent products is lowered, the stability of the products is good, and the quality of the activated partial prothrombin reagent is guaranteed.
Owner:太原博奥特生物技术有限公司
Activated partial thromboplastin time detection reagent and kit
The invention relates to an activated partial thromboplastin time detection reagent and an activated partial thromboplastin time detection kit comprising the activated partial thromboplastin time detection reagent. The activated partial thromboplastin time detection reagent comprises phospholipid, a stabilizer, an activator and a buffer solution, and the stabilizer comprises peptone. According to the activated partial thromboplastin time detection reagent, peptone is added as a stabilizer, and the peptone contains more than 20 amino acids, so that the stability of an activating agent in liquid can be improved. Compared with a traditional APTT reagent, the activated partial thromboplastin time detection reagent is better in stability.
Owner:SHENZHEN DYMIND BIOTECH
Method for improving heparin sensitivity of activated partial thromboplastin time reagent and application
The invention discloses a method for improving the heparin sensitivity of an activated partial thromboplastin time reagent and application. The method provided by the invention comprises the followingstep: adding a soluble manganese ion and / or magnesium ion metal salt into a reaction system when the activated partial thromboplastin time reagent is used for detecting plasma; or adding the solublemanganese ion and / or magnesium ion metal salt into the activated partial thromboplastin time reagent in advance before application of the reagent to activate partial thromboplastin time detection. According to the method, the heparin sensitivity of the activated partial thromboplastin time reagent is improved by adding the metal salt of soluble manganese ions and / or magnesium ions, so the activated partial thromboplastin time reagent can more sensitively reflect the changes of a heparin concentration. The invention also discloses the application of the soluble manganese ion or magnesium ion metal salt to the preparation of the activated partial thromboplastin time reagent sensitive to heparin.
Owner:SHANGHAI LONG ISLAND BIOTEC CO LTD
Ellagic acid reagent and preparation method thereof as well as activated partial thromboplastin time (APTT) determination reagent and APTT kit
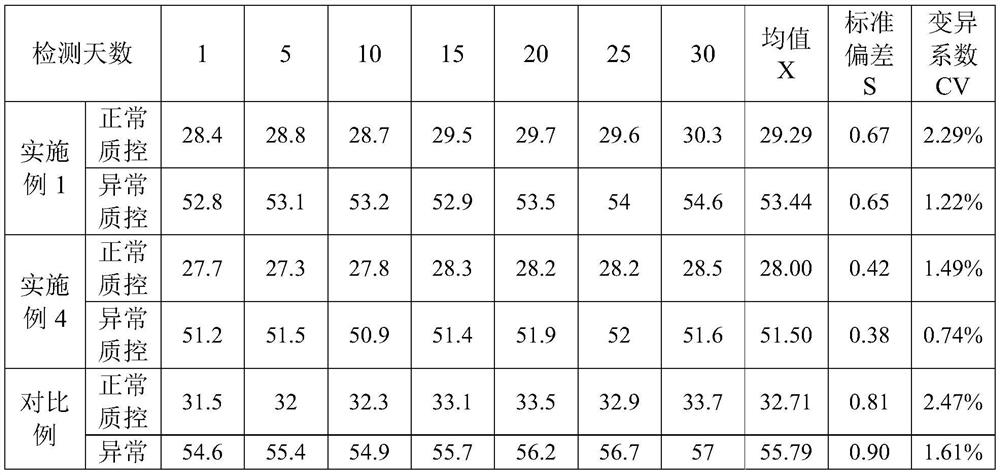
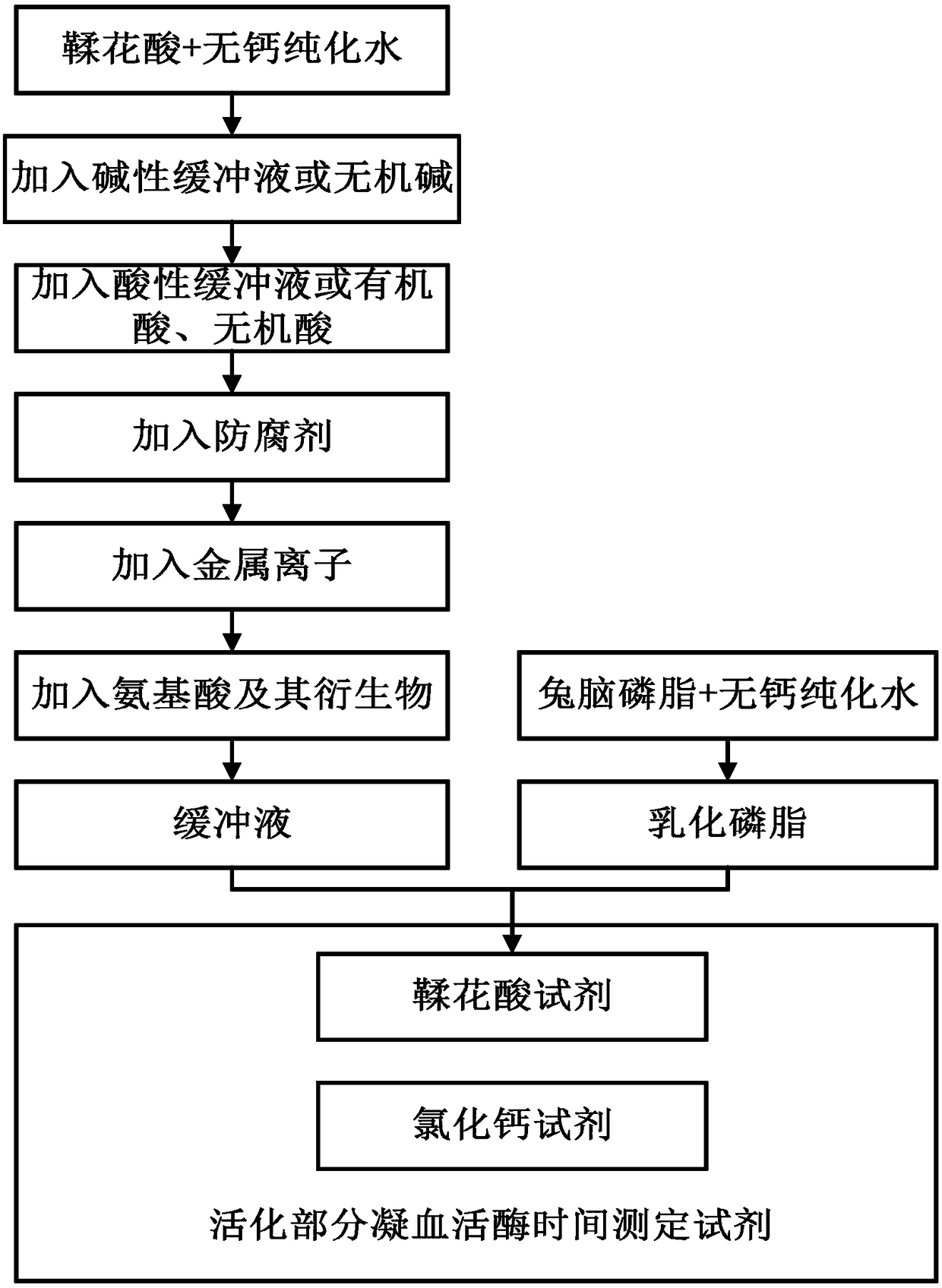
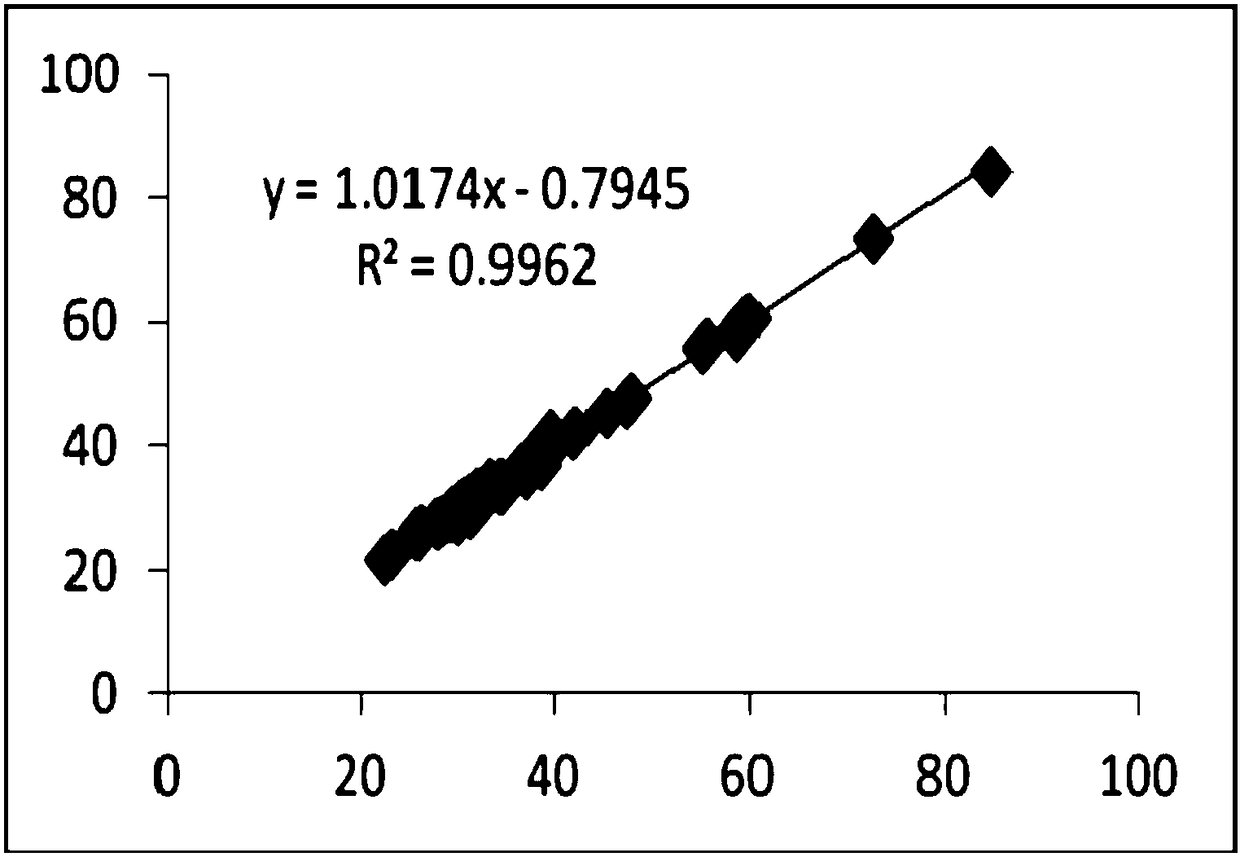
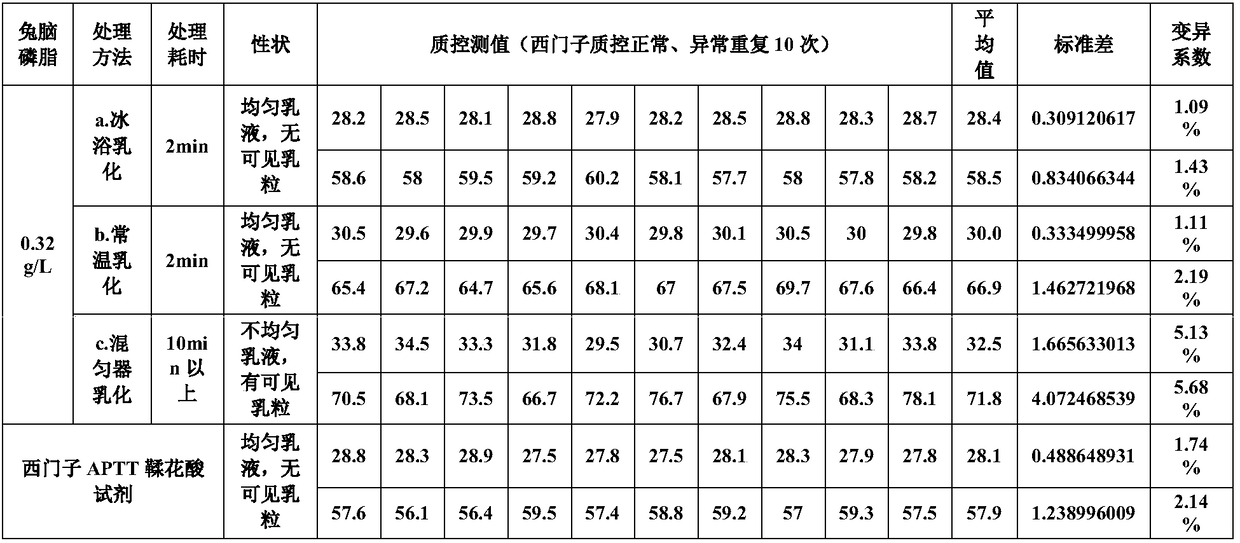
The invention provides an ellagic acid reagent and a preparation method thereof as well as an activated partial thromboplastin time (APTT) determination reagent and an APTT kit and relates to the field of clinical diagnosis reagents. The preparation method comprises the following steps: adding ellagic acid into calcium-free purified water and rapidly stirring to uniformly disperse the ellagic acid; adding an alkaline buffering solution or inorganic alkali; rapidly adding an acidic buffering solution or organic acid and inorganic acid within 10min after the ellagic acid is added; adding metal ions; adding amino acid and a derivative thereof to obtain a buffering solution; furthermore, adding the calcium-free purified water into rabbit brain phospholipid and uniformly mixing and emulsifyingto obtain emulsified phospholipid; adding the emulsified phospholipid into the buffering solution and rapidly stirring and uniformly mixing to obtain the ellagic acid reagent. The technology is simpleand rapid and has detailed descriptions. The activated partial thromboplastin time determination reagent comprises the ellagic acid reagent and a calcium chloride reagent and has good stability. TheAPTT kit containing the activated partial thromboplastin time determination reagent can be used for directly replacing an imported high-stability APTT kit.
Owner:WUHAN CHANGLI BIOLOGICAL TECH CO LTD
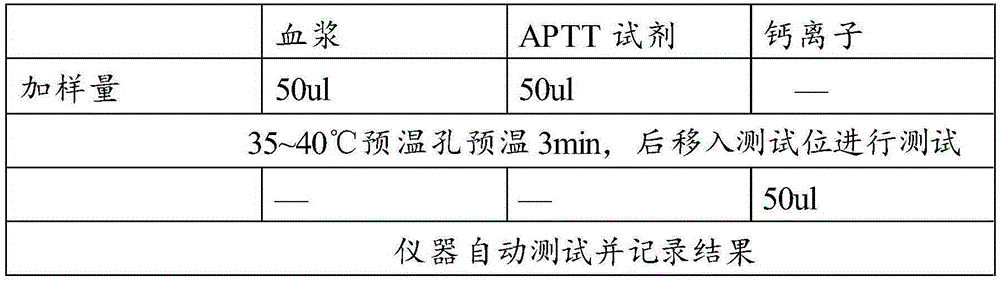
Device and method for testing activated partial thromboplastin time
The invention provides a device and a method for testing activated partial thromboplastin time (APTT) based on an optical method. The device is formed by a support, a chemical reagent and the like, wherein the support is formed by three parts: a sample adding area, a reacting area and a feeding control area. The chemical reagent is concretely prepared from an activating agent, phospholipid, CaCl2and other main components. The device for testing the APTT provided by the invention can be used for testing the APTT of fingertip blood or a non-coagulated blood sample, has the advantages of convenience, fastness, simplicity in operation and the like, is simple in preparation method, low in technical requirements on equipment and operating personnel, high in operability, and broad in applicationprospect in the fields such as clinic examination.
Owner:北京乐普诊断科技股份有限公司
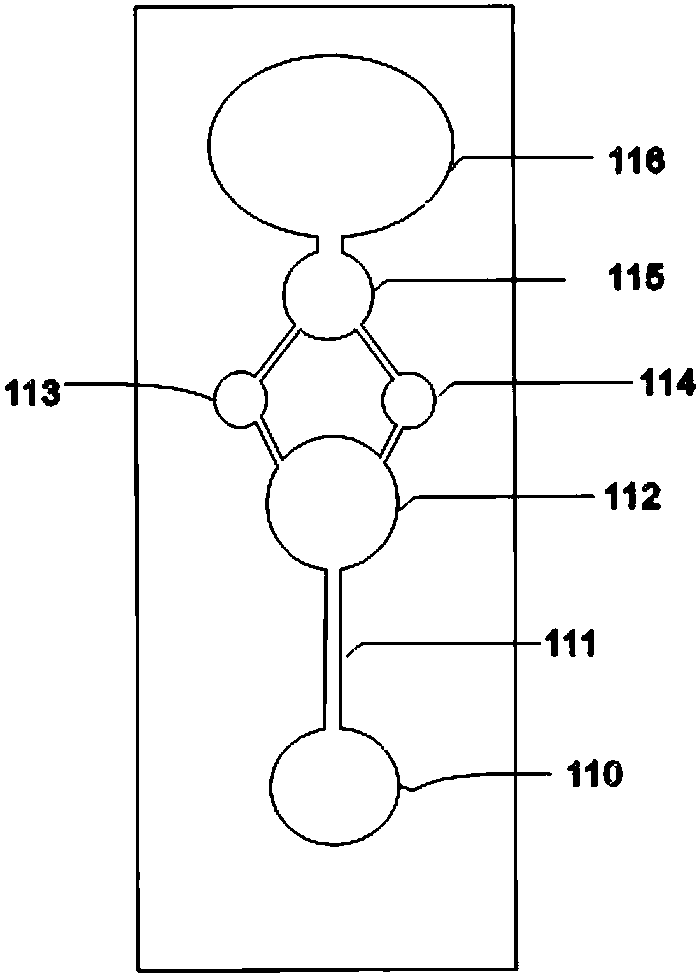
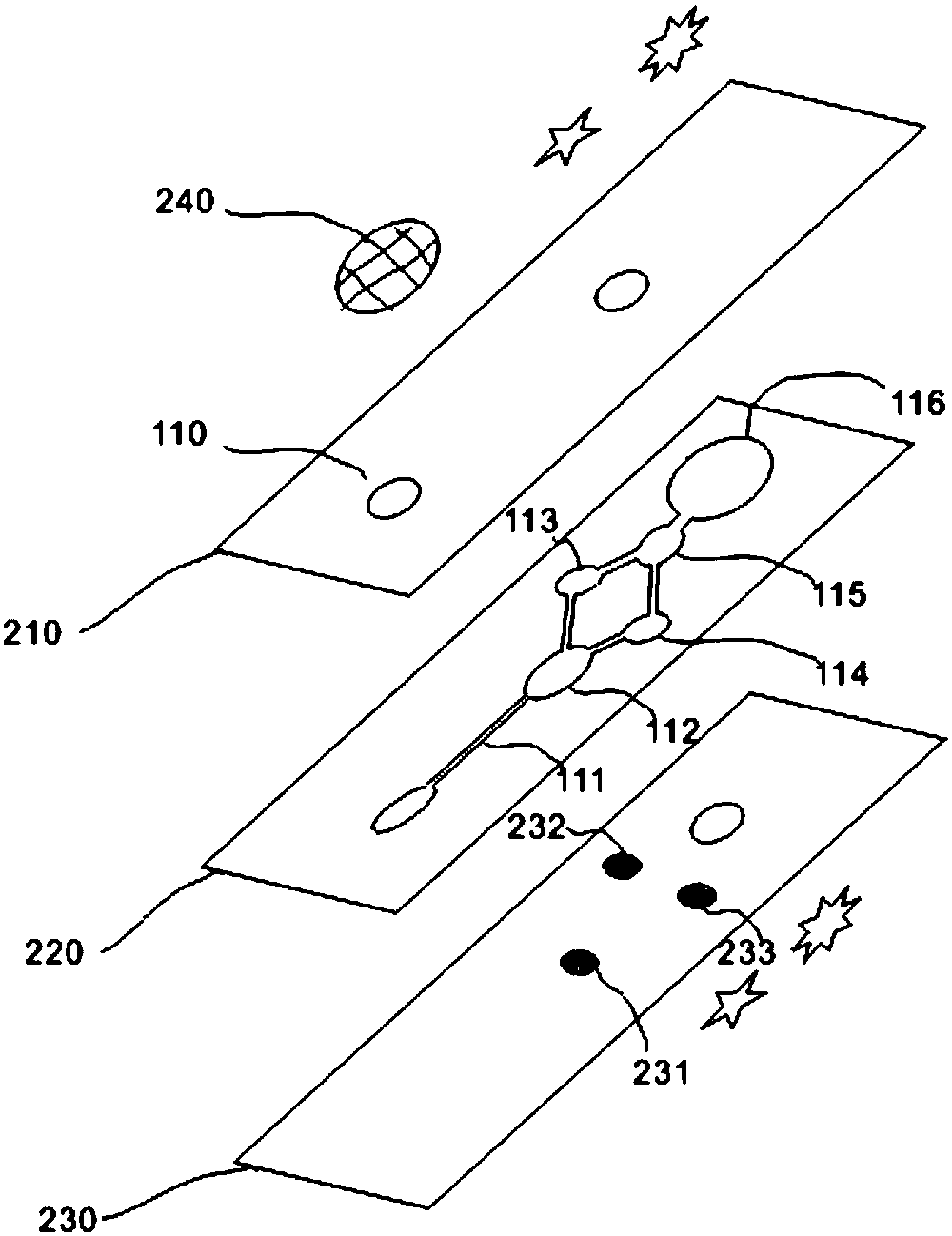
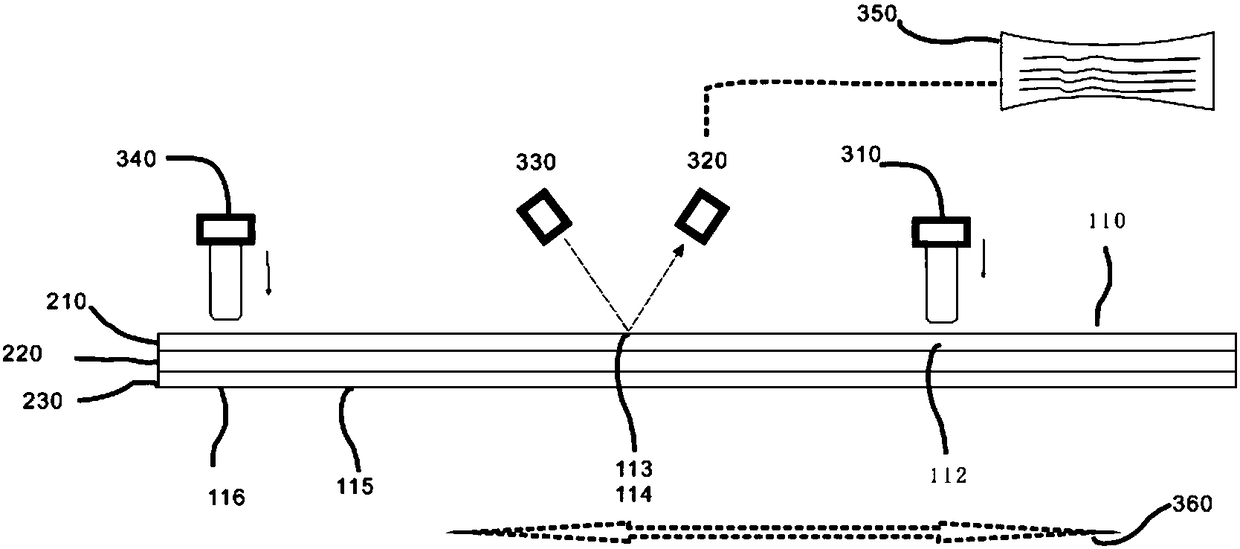
Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors
InactiveUS20130090494A1Group 5/15 element organic compoundsPhosphorous compound active ingredientsAntithrombotic AgentBlood plasma
There is proposed a chemical compound of 5,11,17,23-tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene and a sodium salt thereof, which can be used as antithrombotic agents. A highly specific inhibiting effect of the aforementioned calixarenes on the fibrin polymerization has been identified. It has been found that the addition of the aforementioned calixarenes to blood plasma leads to an increase of the prothrombin time and the activated partial thromboplastin time.
Owner:KOMISARENKO SERHII VASYLOVYCH +7
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors](https://images-eureka.patsnap.com/patent_img/40cd001c-2fc7-4d20-a12c-3d42ee869982/US20130090494A1-20130411-D00001.png)
![Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors](https://images-eureka.patsnap.com/patent_img/40cd001c-2fc7-4d20-a12c-3d42ee869982/US20130090494A1-20130411-D00002.png)
![Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors Tetrakis[bis(dihydroxyphosphoryl)methyl]calix[4]arene or its sodium salt thereof as fibrin polymerization inhibitors](https://images-eureka.patsnap.com/patent_img/40cd001c-2fc7-4d20-a12c-3d42ee869982/US20130090494A1-20130411-D00003.png)