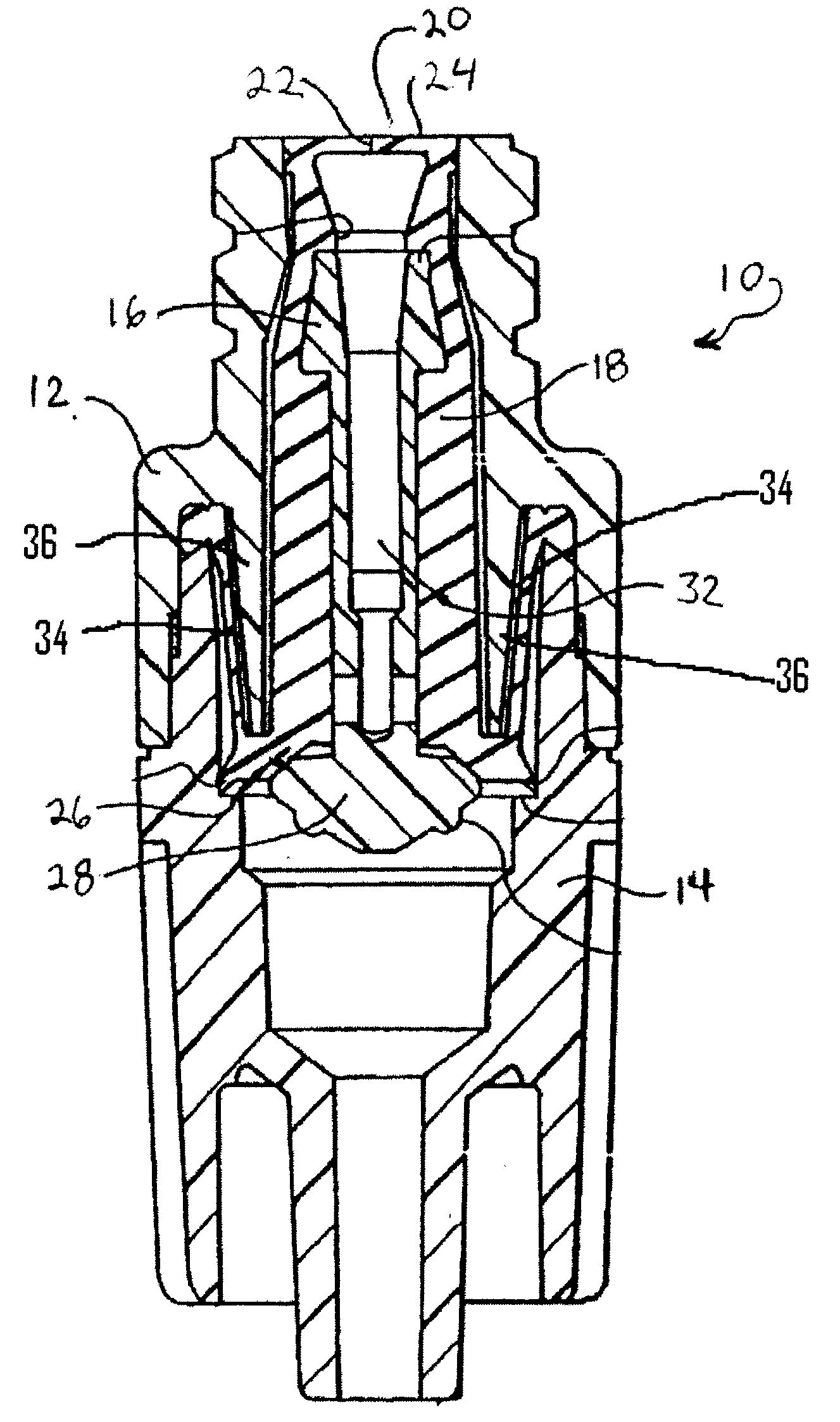
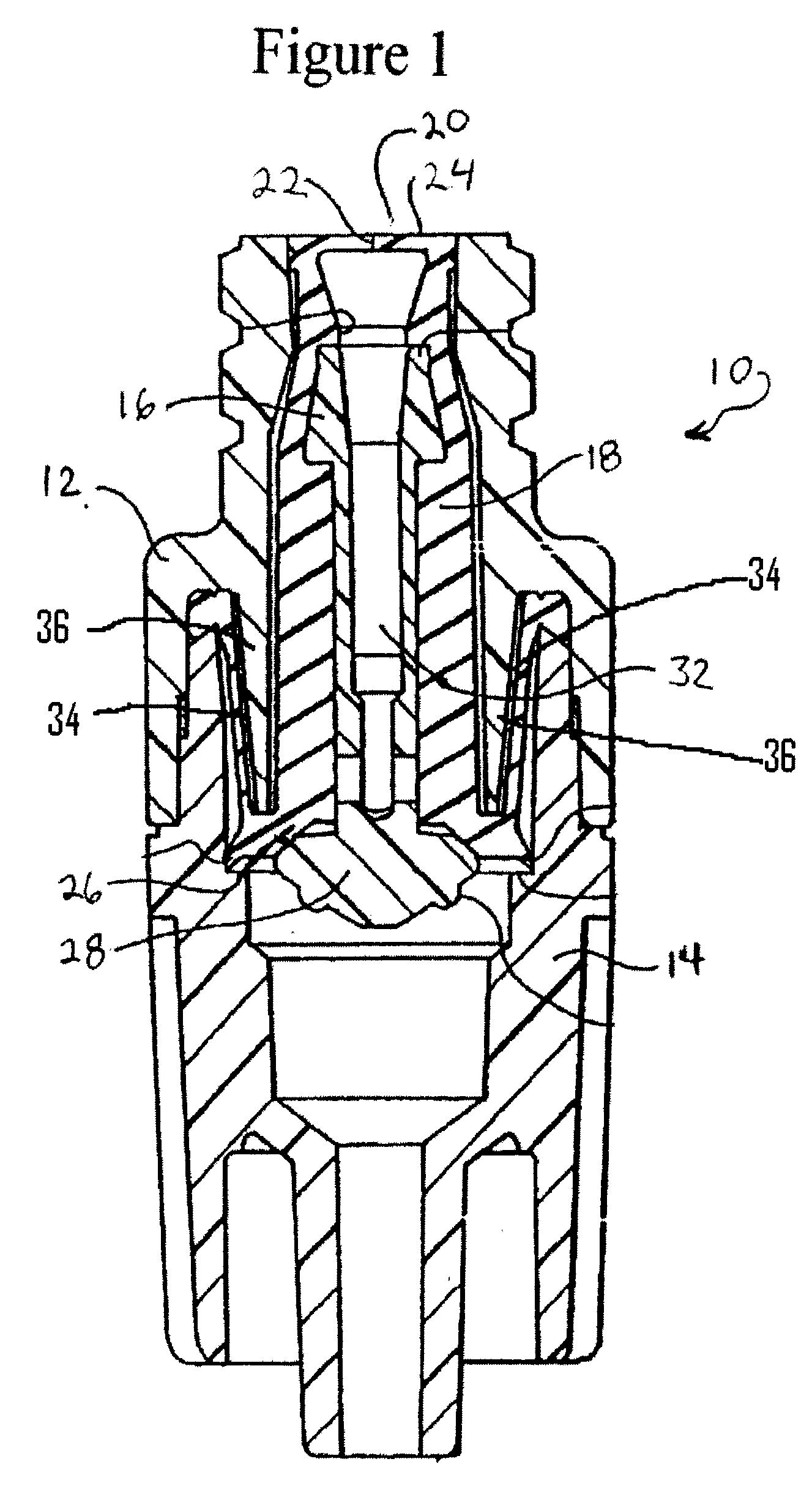
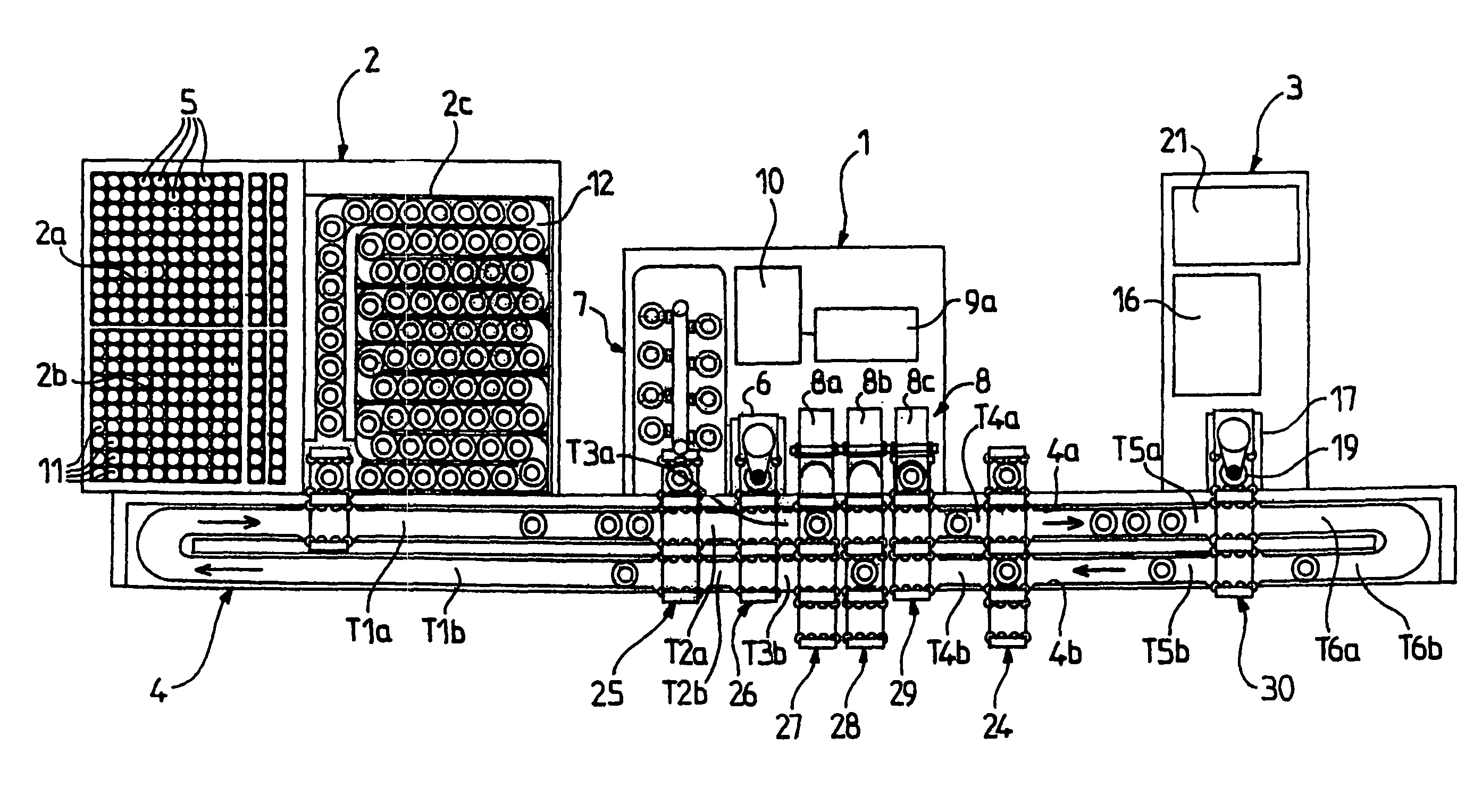
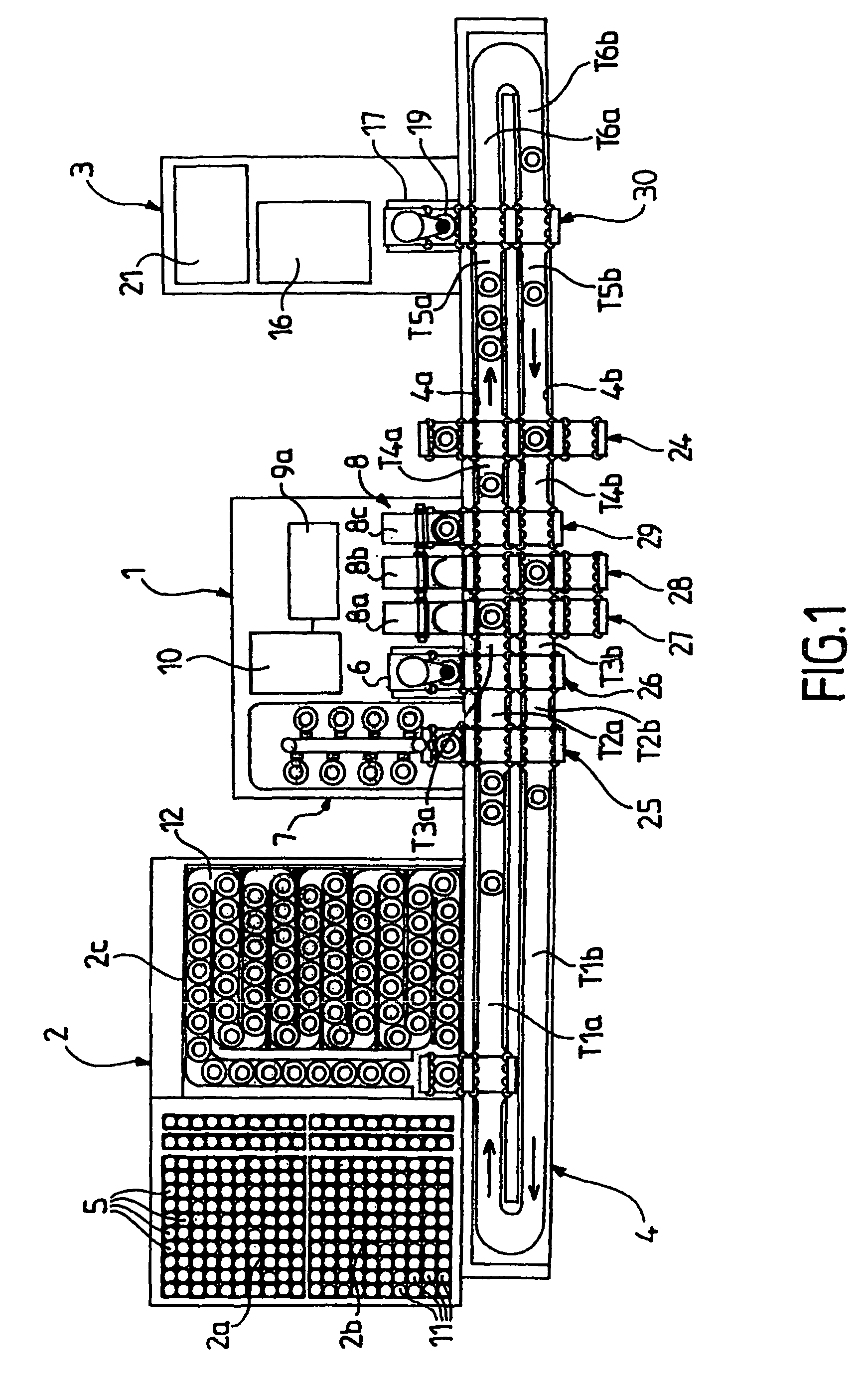
Patents
Literature
167 results about "Clot formation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Blood clot formation. Overview. Blood clotting normally occurs when there is damage to a blood vessel. Platelets immediately begin to adhere to the cut edges of the vessel and release chemicals to attract even more platelets. A platelet plug is formed, and the external bleeding stops.
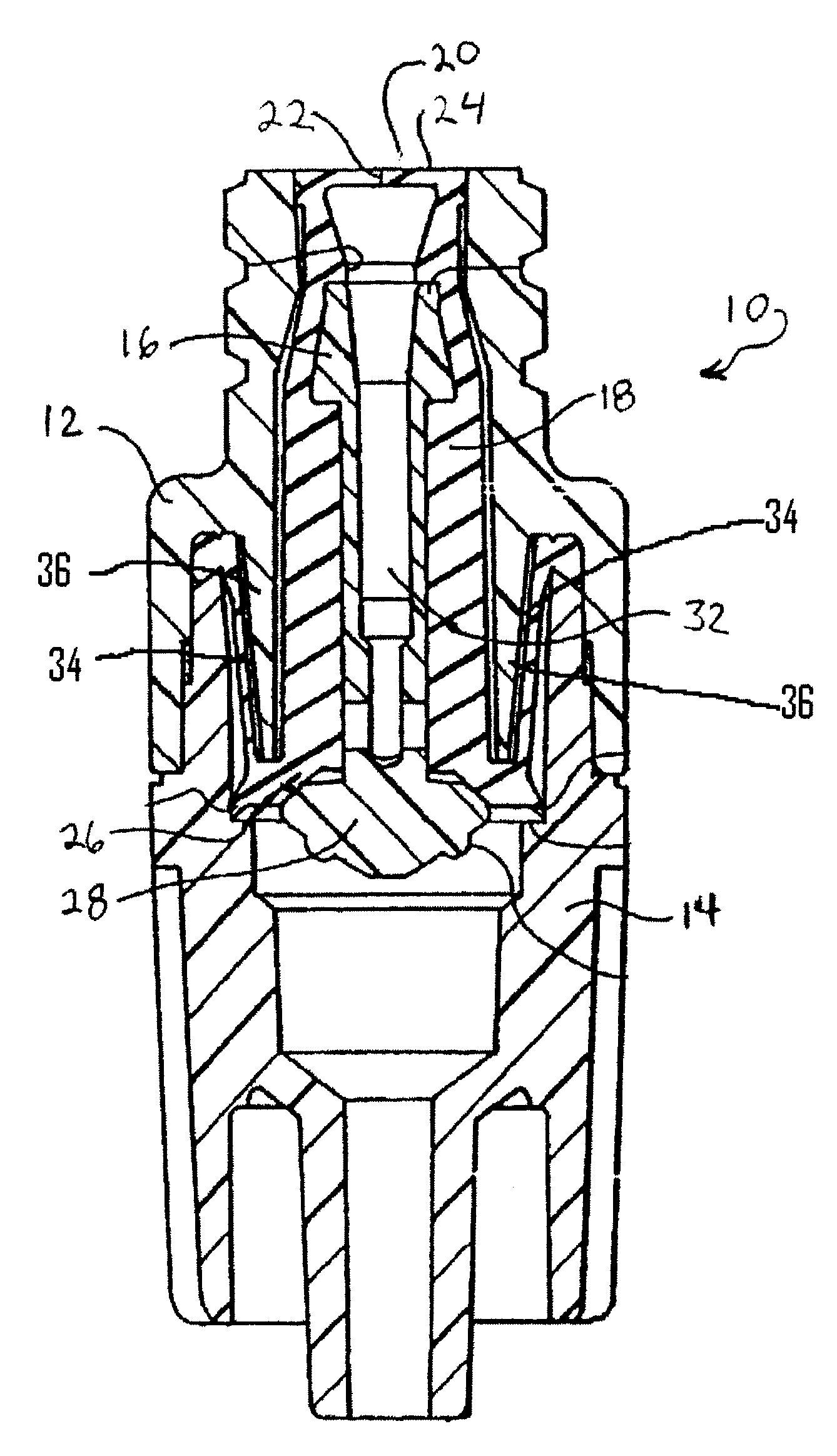
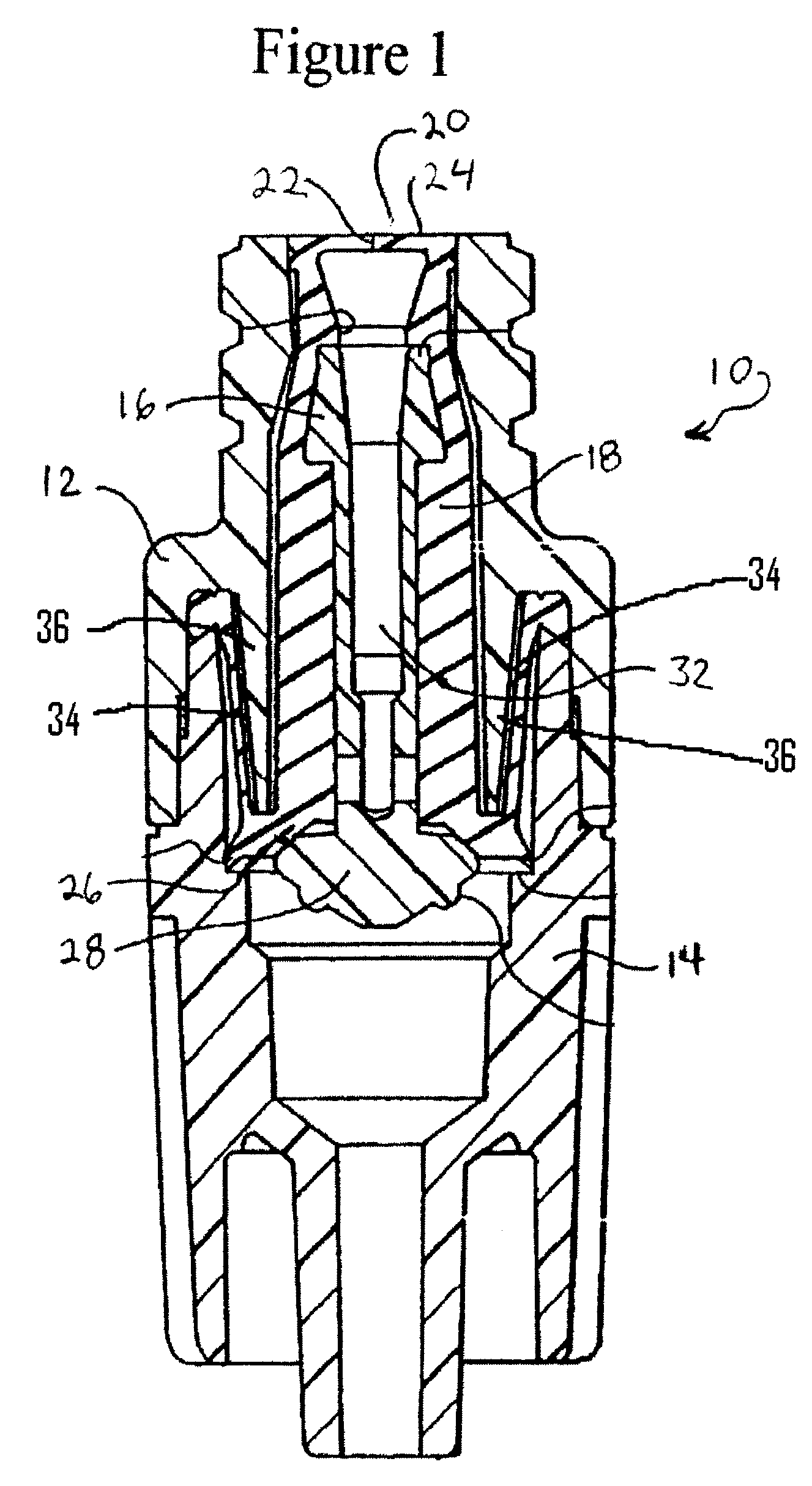
Wound dressing and method for controlling severe, life-threatening bleeding
ActiveUS7371403B2Stanching flowProhibiting flow of bloodBiocideSuspensory bandagesHydrophilic polymersWound dressing
This invention is directed to advanced hemorrhage control wound dressings, and methods of using and producing same. The subject wound dressing is constructed from a non-mammalian material for control of severe bleeding. The wound dressing for controlling severe bleeding is formed of a biomaterial comprising chitosan, a hydrophilic polymer, a polyacrylic polymer or a combination thereof. The kind of severe, life-threatening bleeding contemplated by this invention is typically of the type not capable of being stanched when a conventional gauze wound dressing is applied with conventional pressure to the subject wound. The wound dressing being capable of substantially stanching the flow of the severe life-threatening bleeding from the wound by adhering to the wound site, to seal the wound, to accelerate blood clot formation at the wound site, to reinforce clot formation at the wound site and prevent bleed out from the wound site, and to substantially prohibit the flow of blood out of the wound site.
Owner:PROVIDENCE HEALTH SYST OREGONDBA ST VINCENTMEDICAL CENT
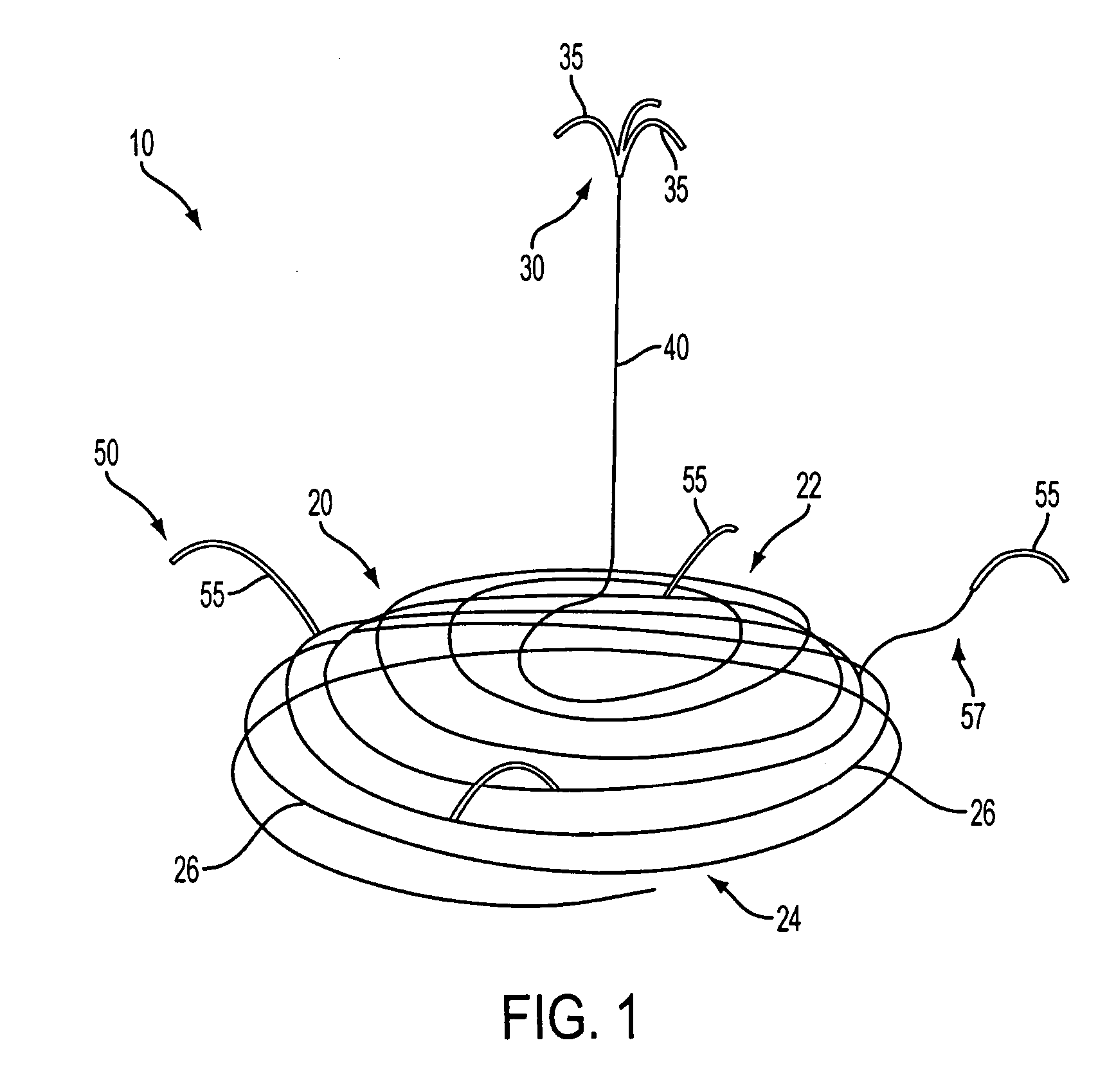
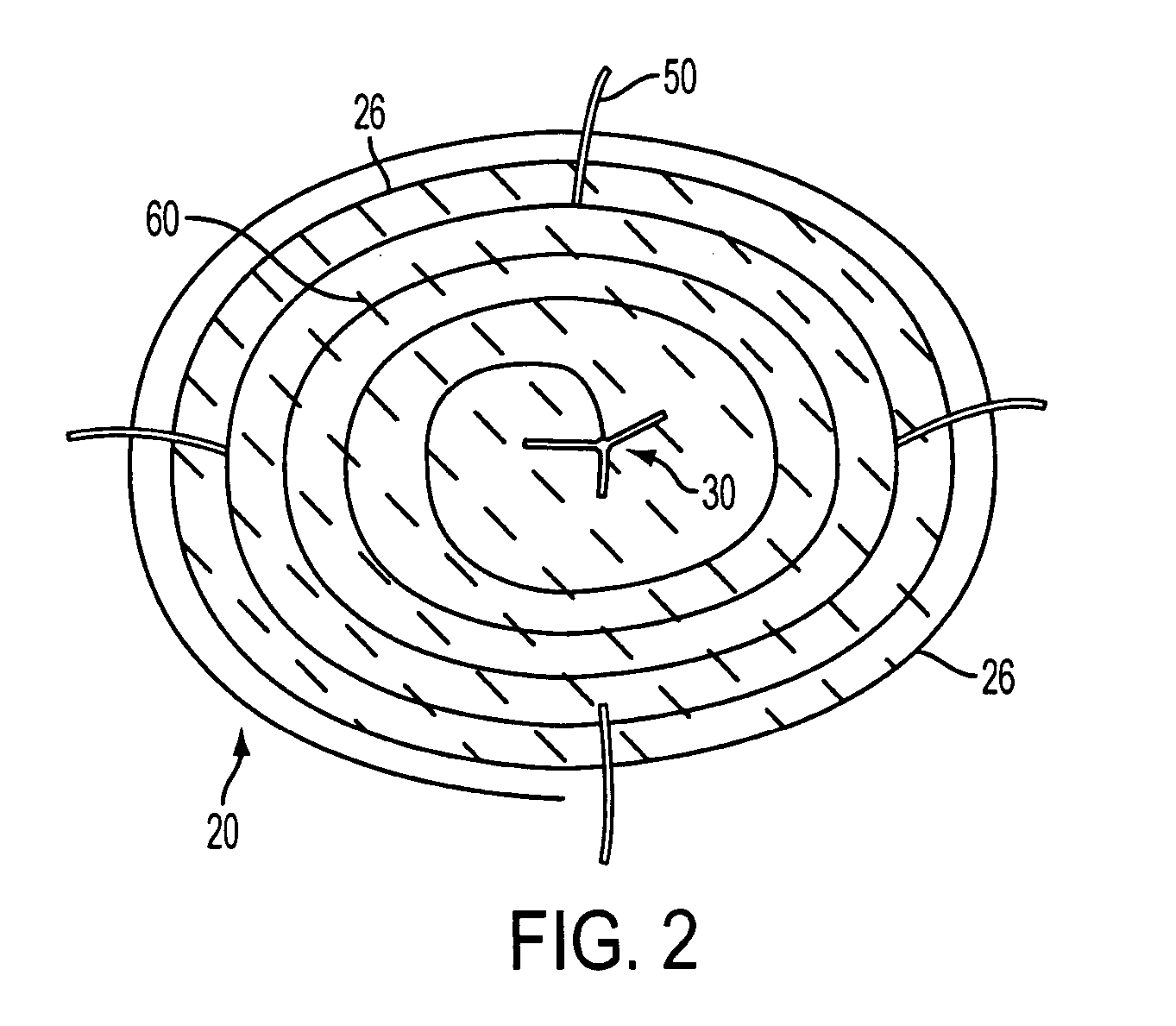
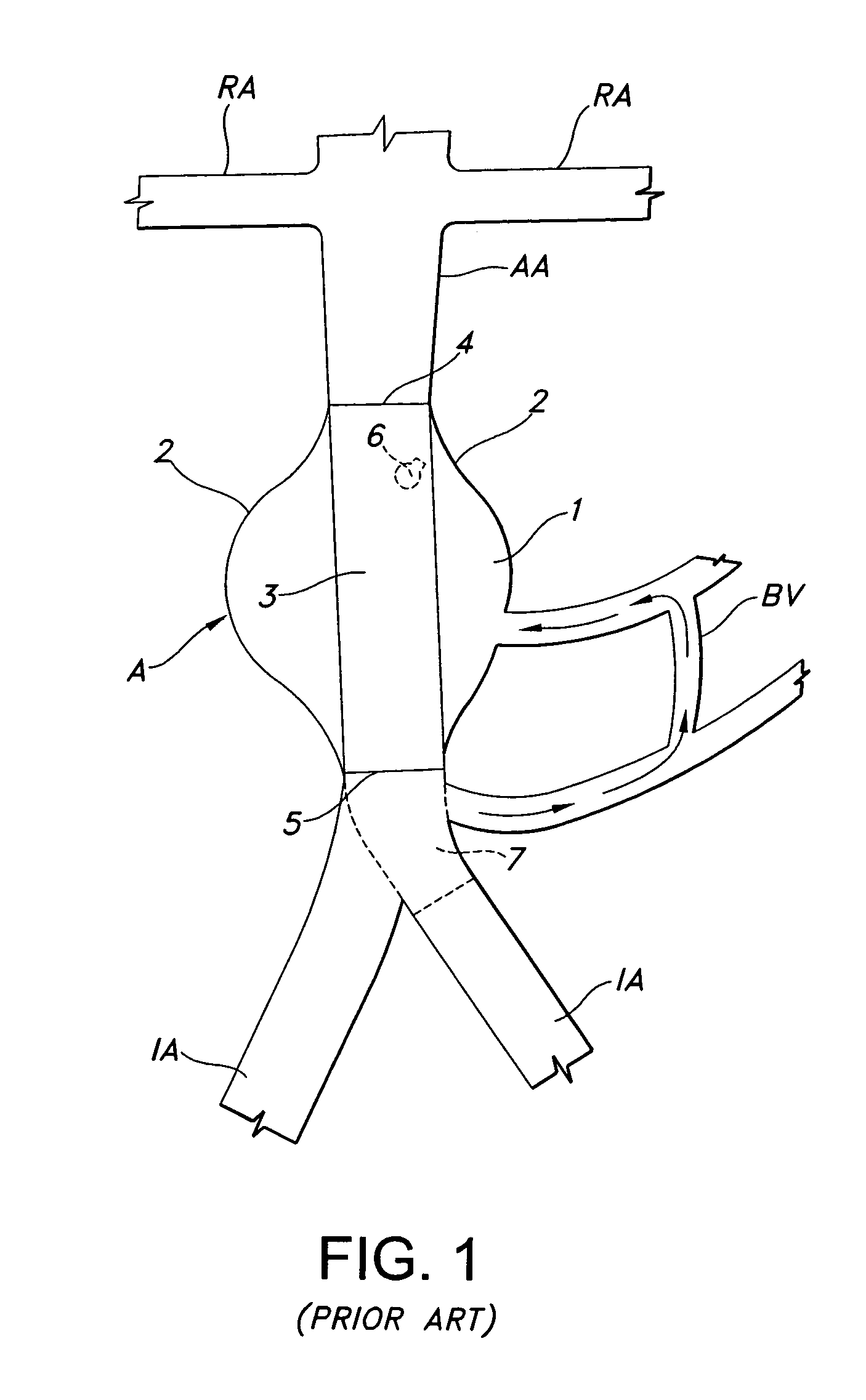
Left atrial appendage occlusion device
ActiveUS20070083230A1Reduce riskPrevention of clot formation and clot migrationOcculdersSurgical veterinaryClot formationLeft atrial appendage occlusion
The present invention provides devices and methods for occluding the left atrial appendage. The device includes an easily deployed wire structure of shape memory material sized to be appropriate for use in a patient. The device may be manufactured in various sizes for use in various patients. The device has a sheet of material attached for eclipsing the opening of the left atrial appendage and means for secured attachment substantially within the left atrial appendage. The method includes deploying the device within the left atrial appendage, securely anchoring the device, and ensuring that a sheet of material eclipses the opening of the left atrial appendage. These features are incorporated into the improved devices and methods in order to more effectively aid in the prevention of clot formation and clot migration into or out of the left atrial appendage to greatly decrease the risk of stroke in patients with atrial fibrillation.
Owner:JAVOIS ALEX
Wound dressing and method for controlling severe, life-threatening bleeding
InactiveUS20050137512A1Reduce the temperatureStanching flowPlastersAdhesive dressingsWound dressingClot formation
This invention is directed to advanced hemorrhage control wound dressings, and methods of using and producing same. The subject wound dressing is constructed from a non-mammalian material for control of severe bleeding. The wound dressing for controlling severe bleeding is formed of a biomaterial comprising chitosan, a hydrophilic polymer, a polyacrylic polymer or a combination thereof. The kind of severe, life-threatening bleeding contemplated by this invention is typically of the type not capable of being stanched when a conventional gauze wound dressing is applied with conventional pressure to the subject wound. The wound dressing being capable of substantially stanching the flow of the severe life-threatening bleeding from the wound by adhering to the wound site, to seal the wound, to accelerate blood clot formation at the wound site, to reinforce clot formation at the wound site and prevent bleed out from the wound site, and to substantially prohibit the flow of blood out of the wound site.
Owner:HEMCON
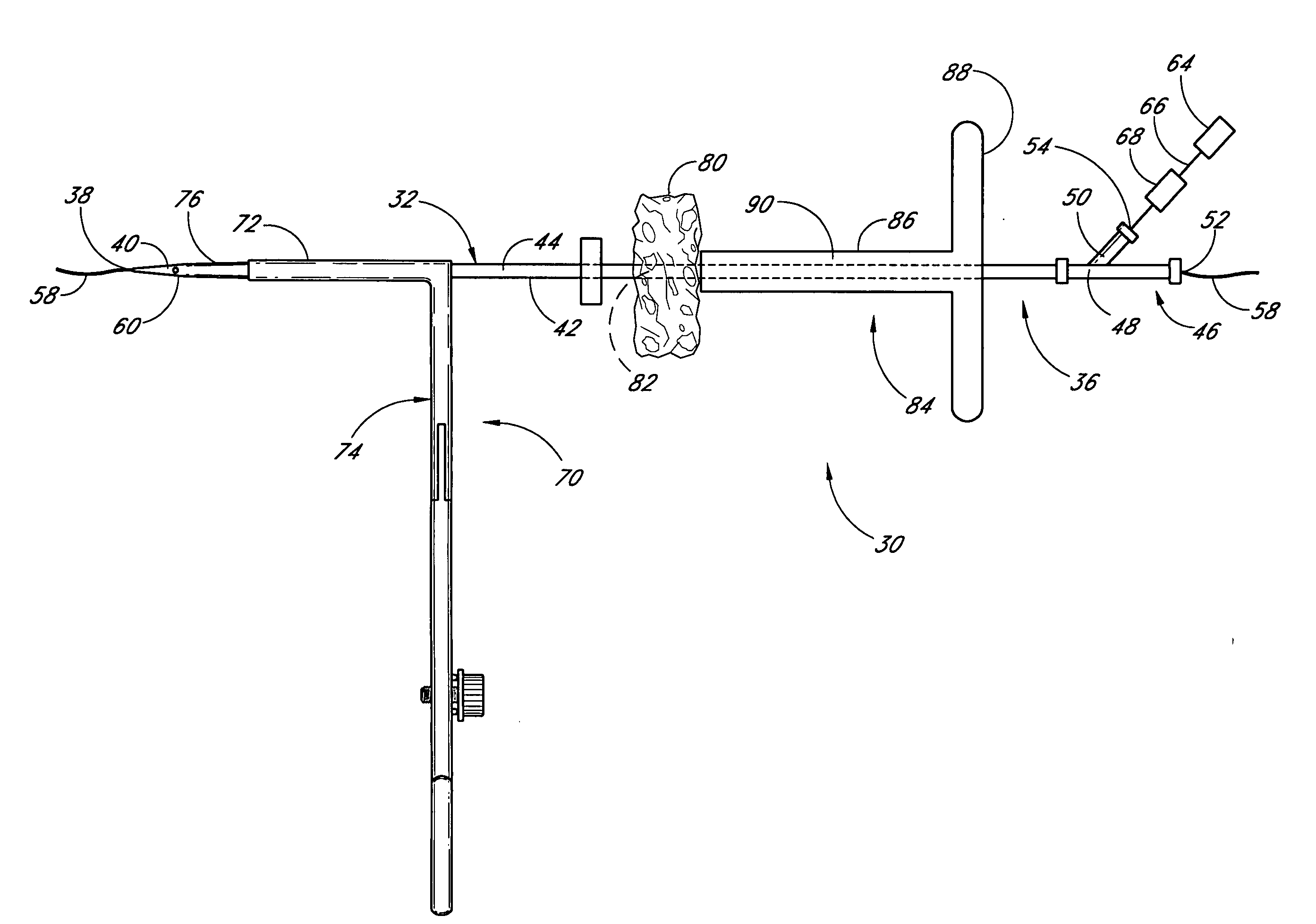
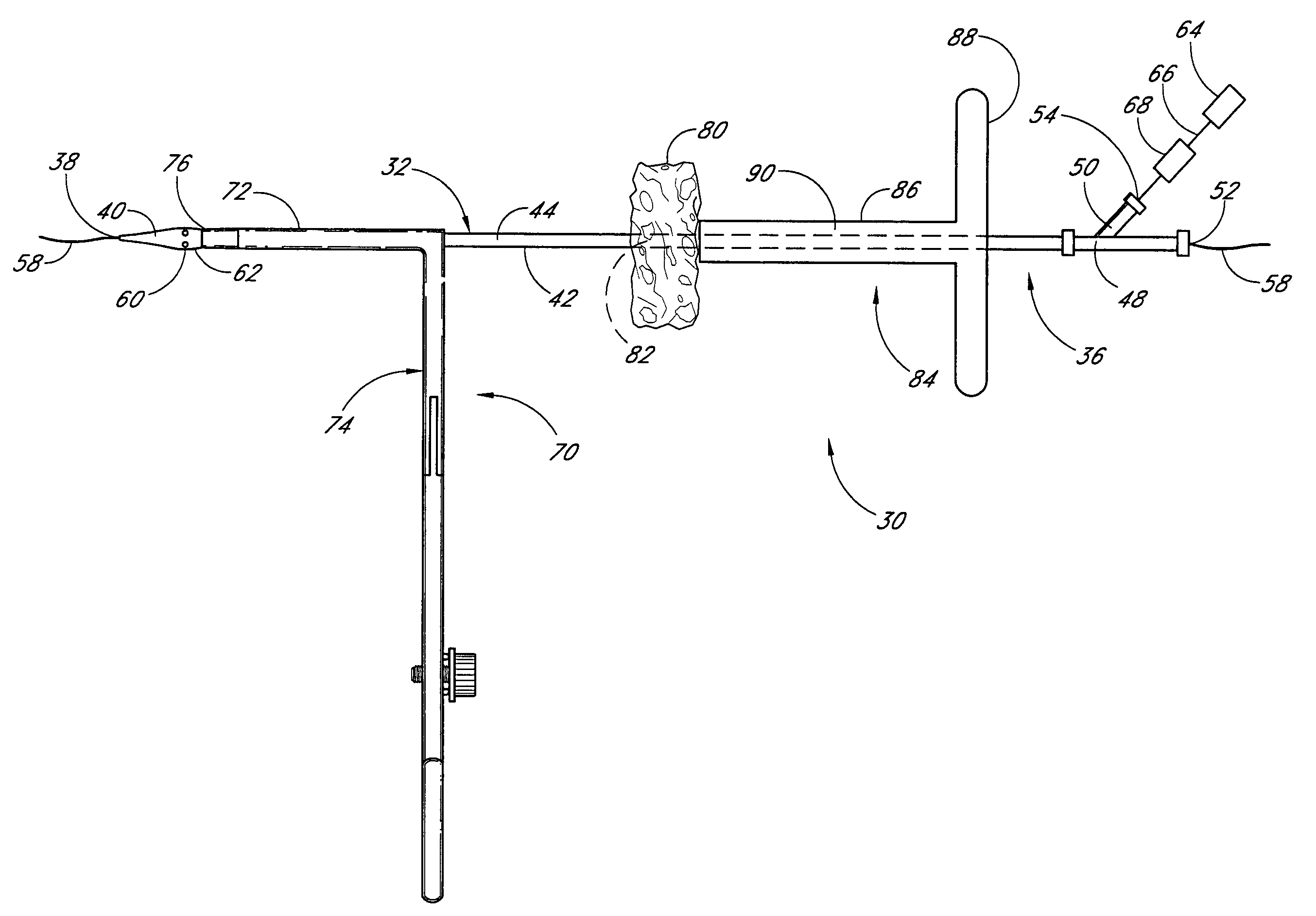
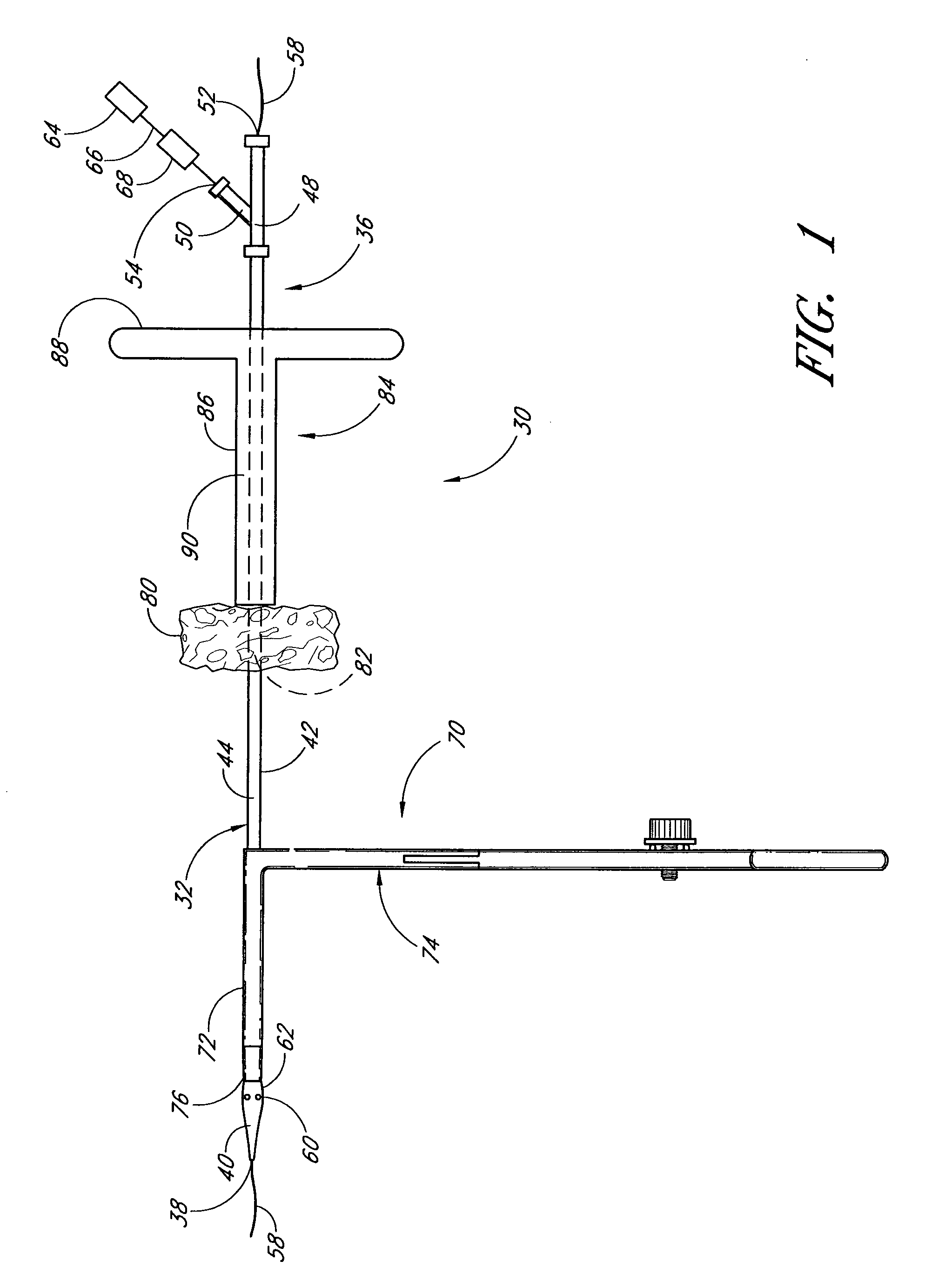
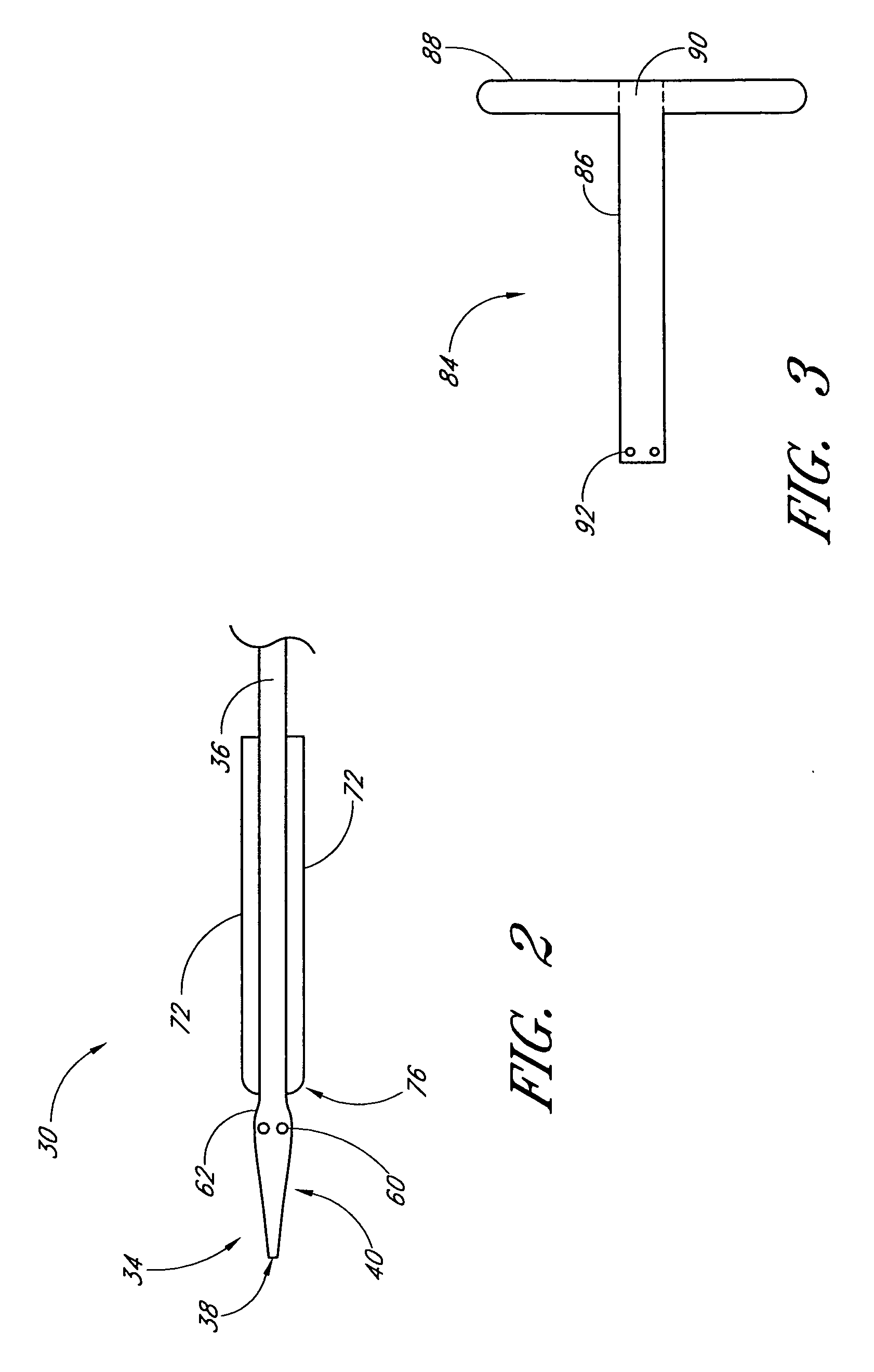
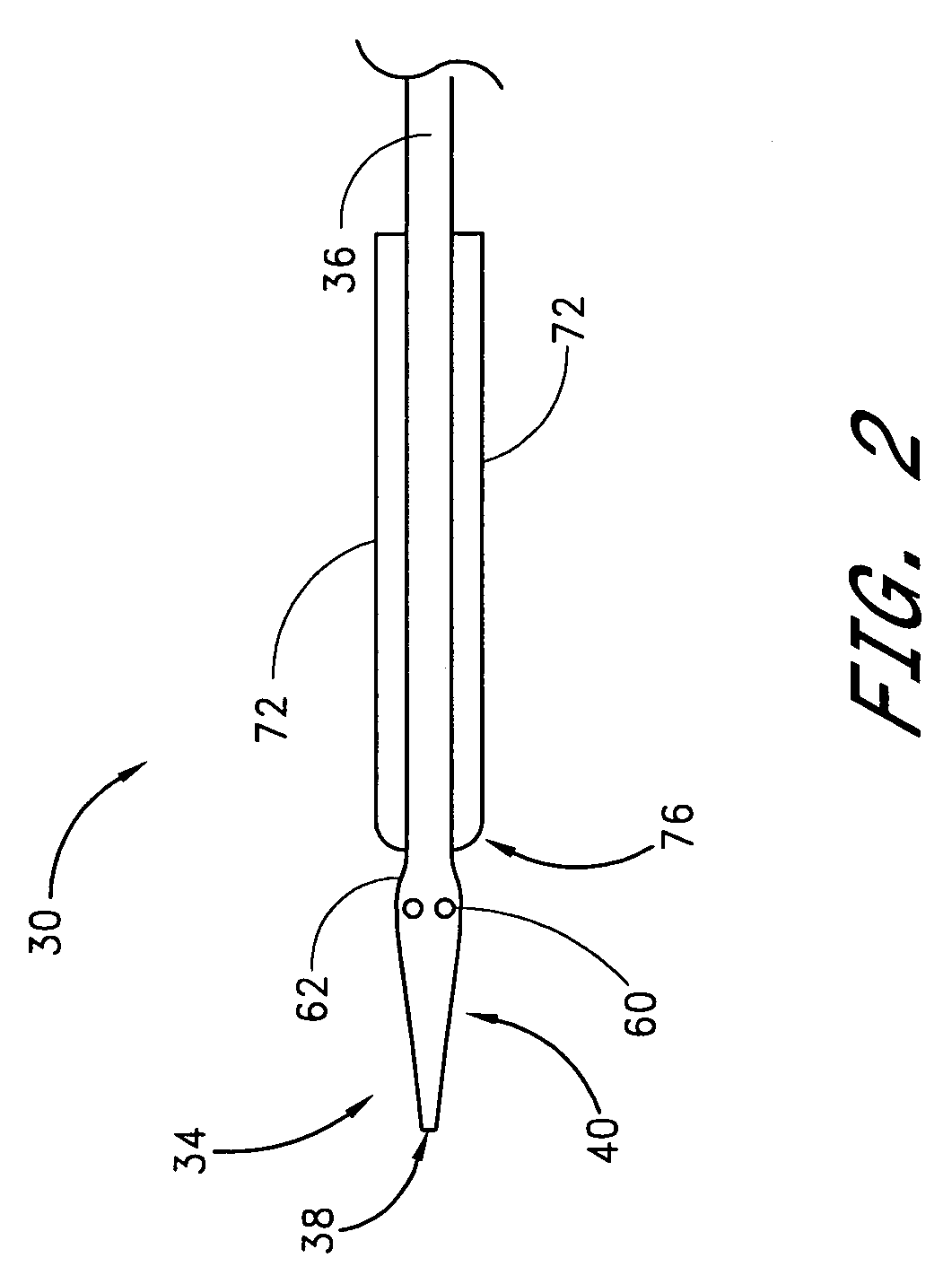
Vascular wound closure device and method
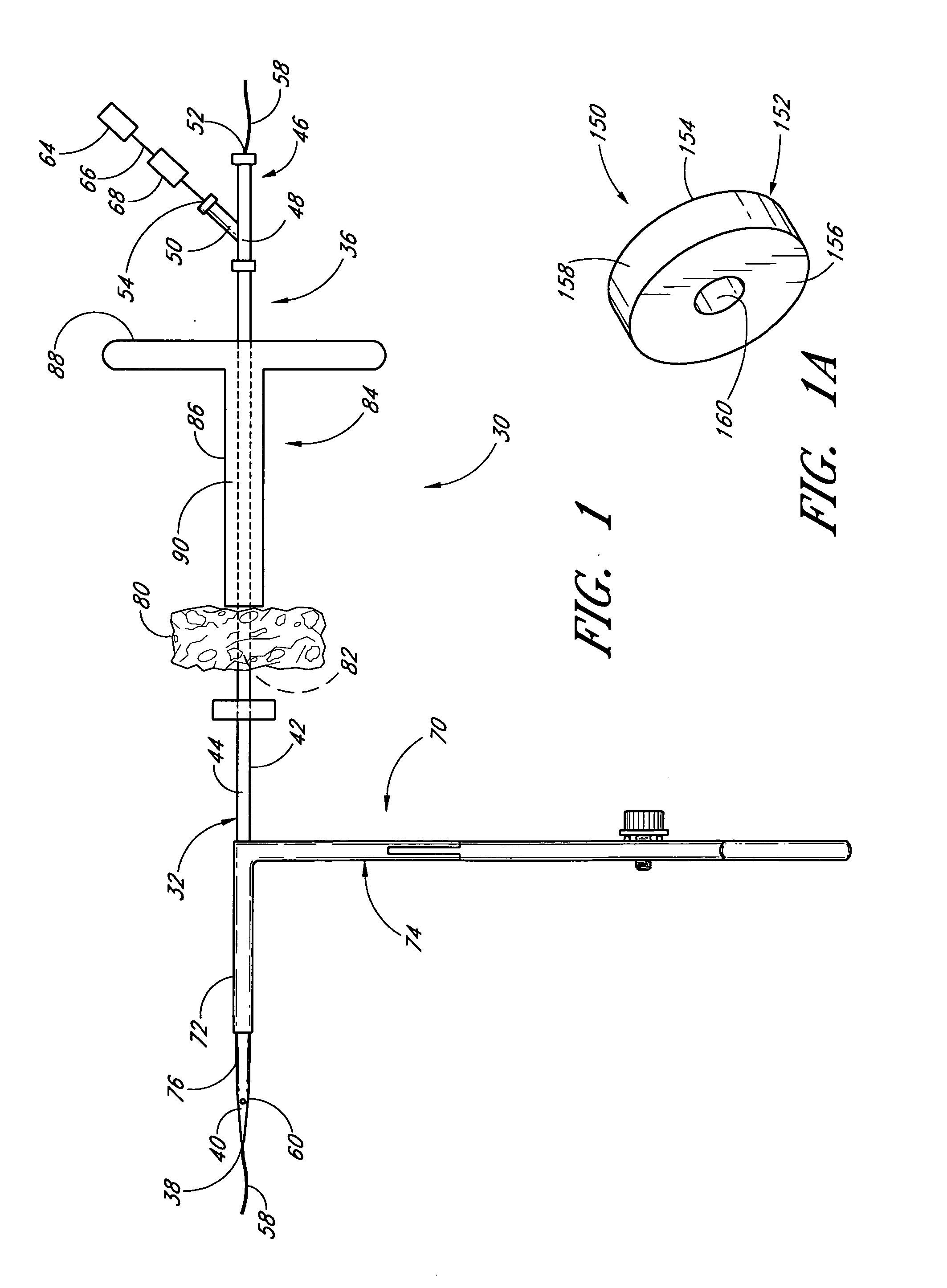
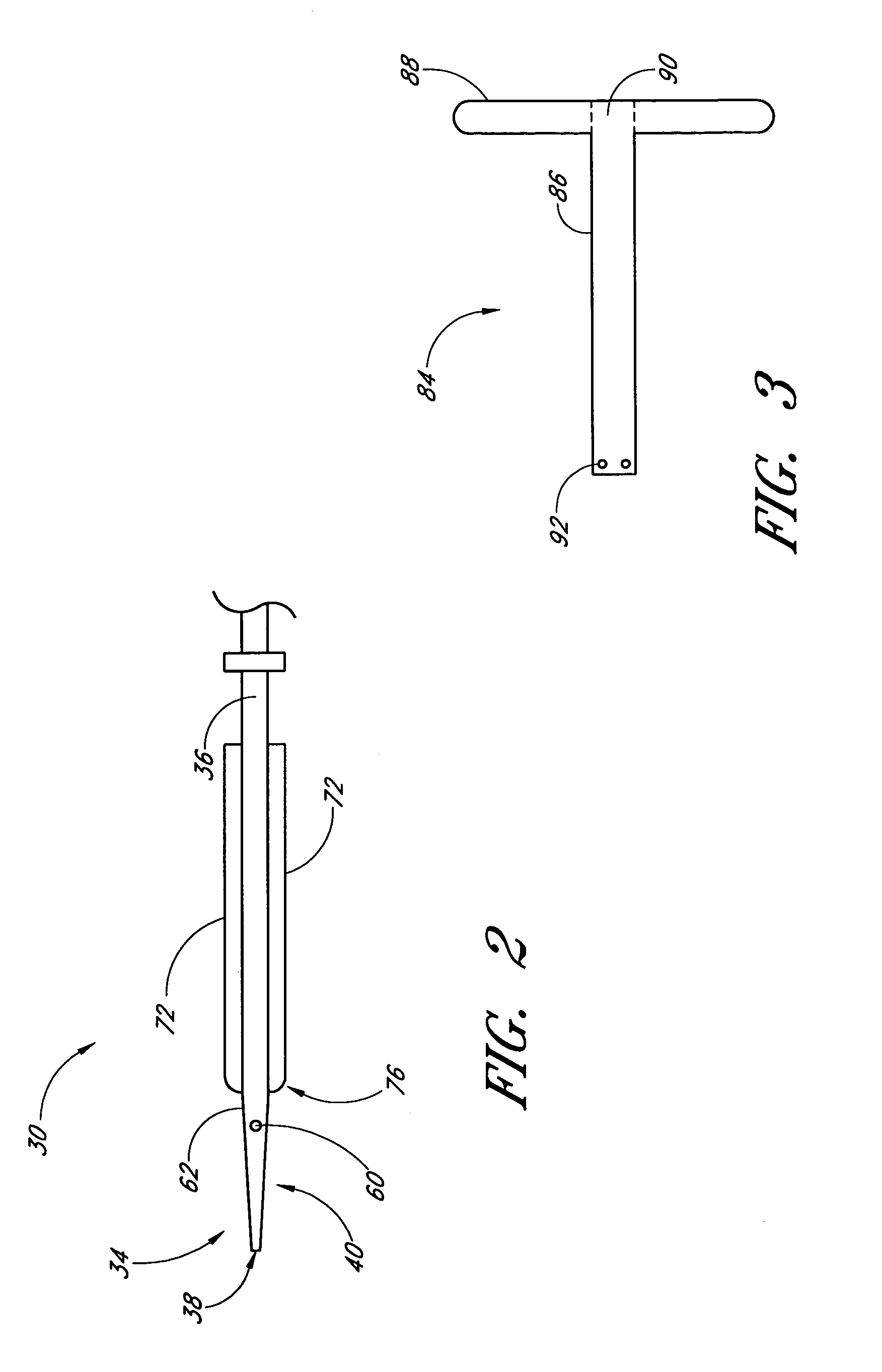
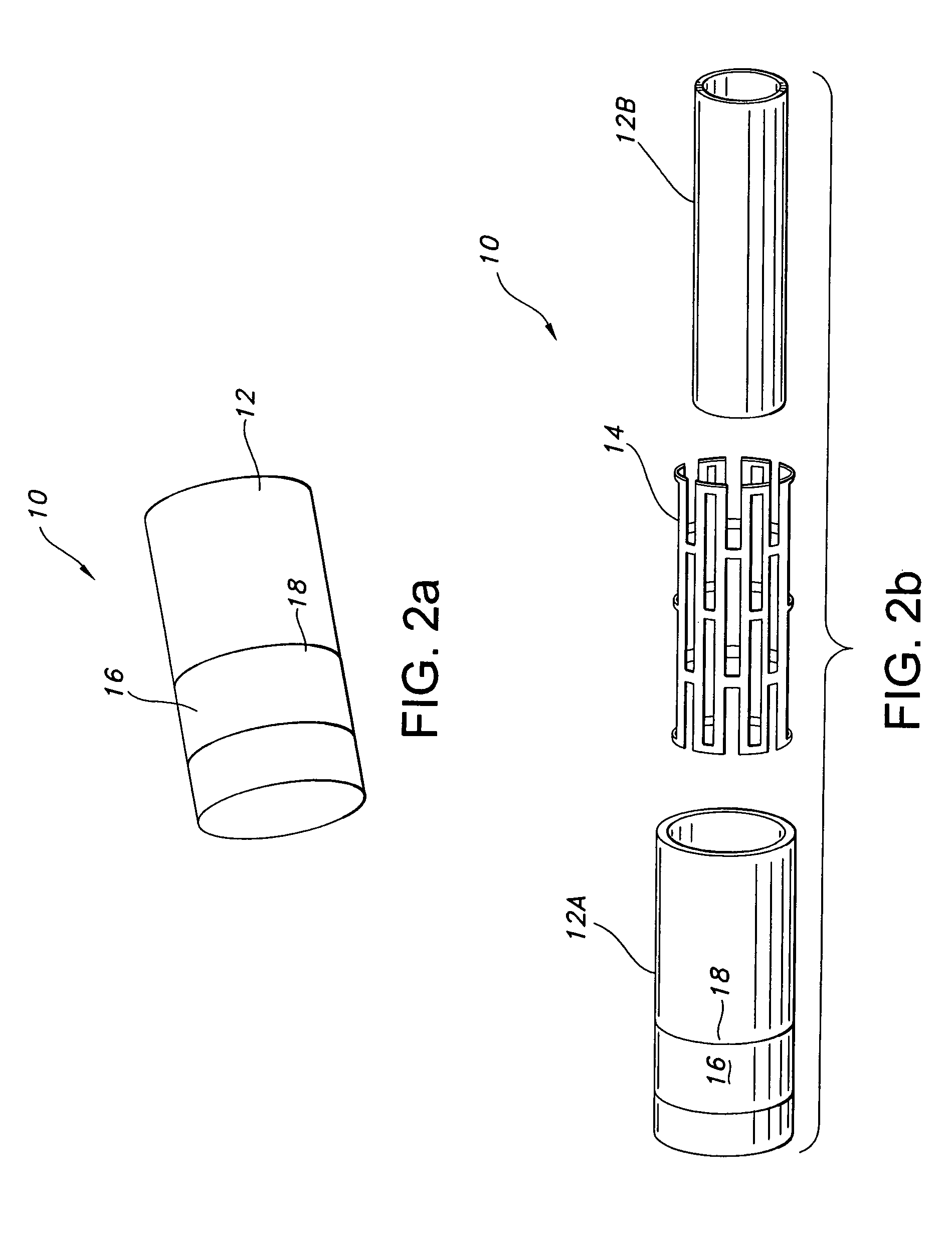
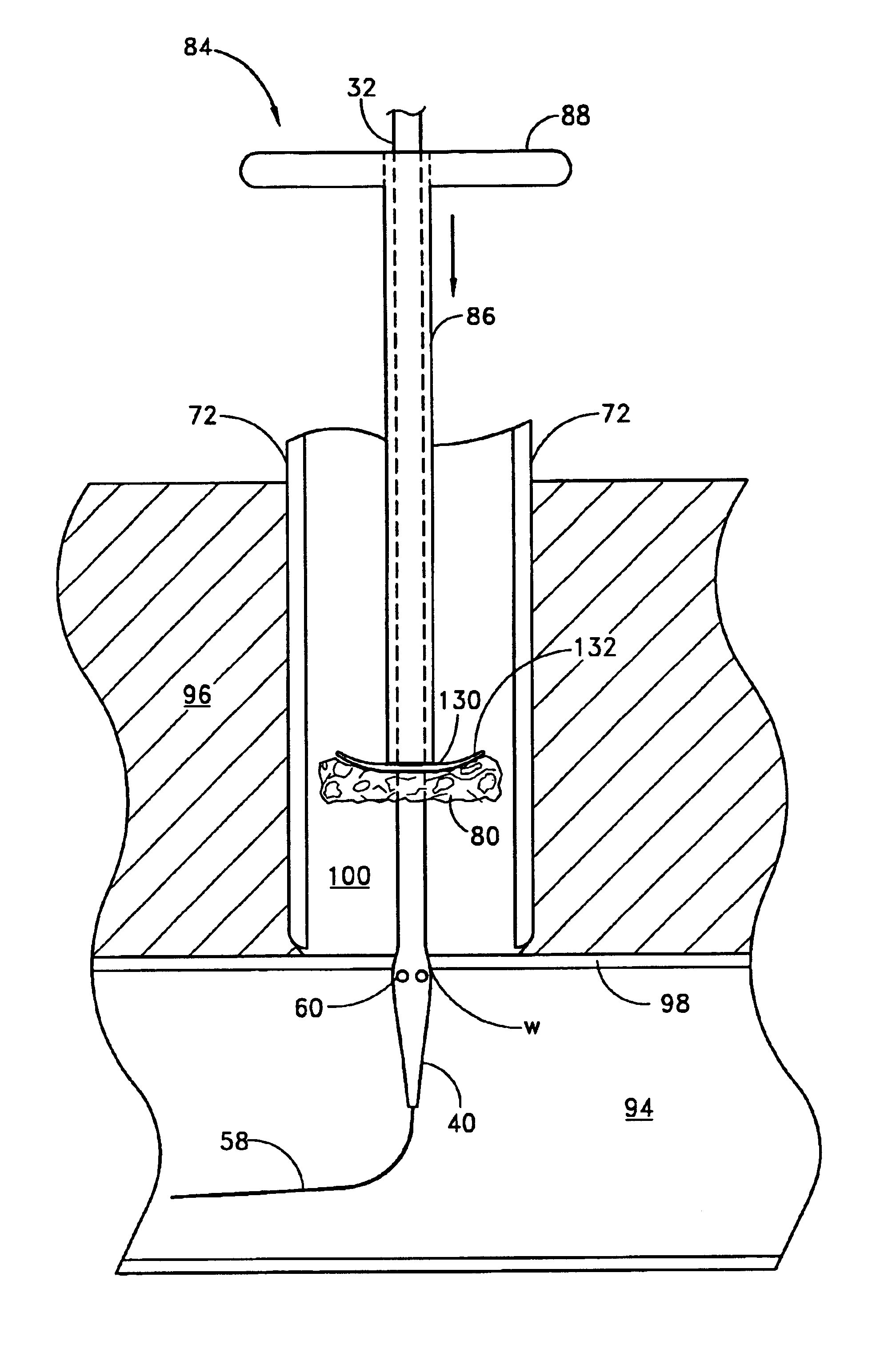
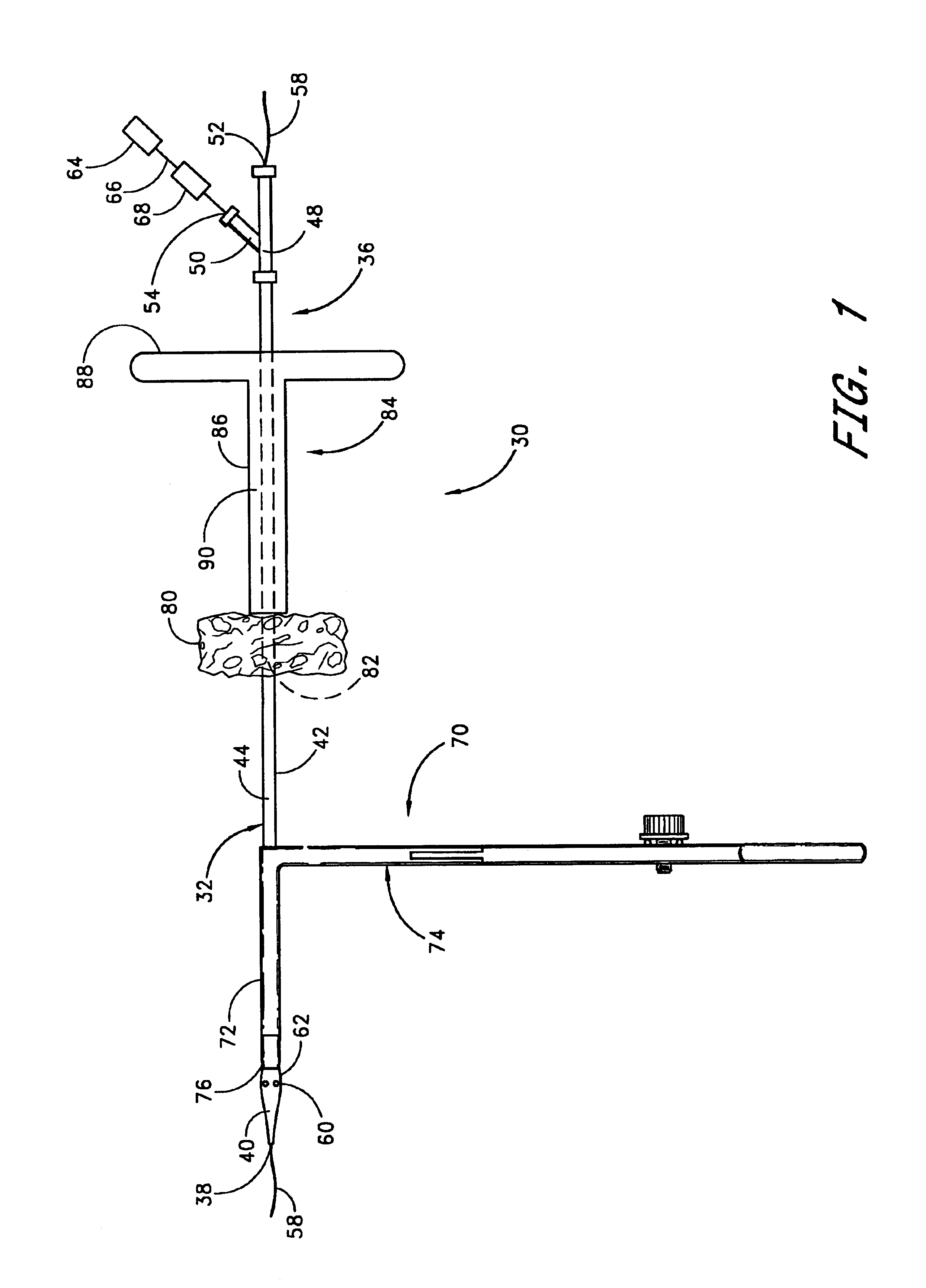
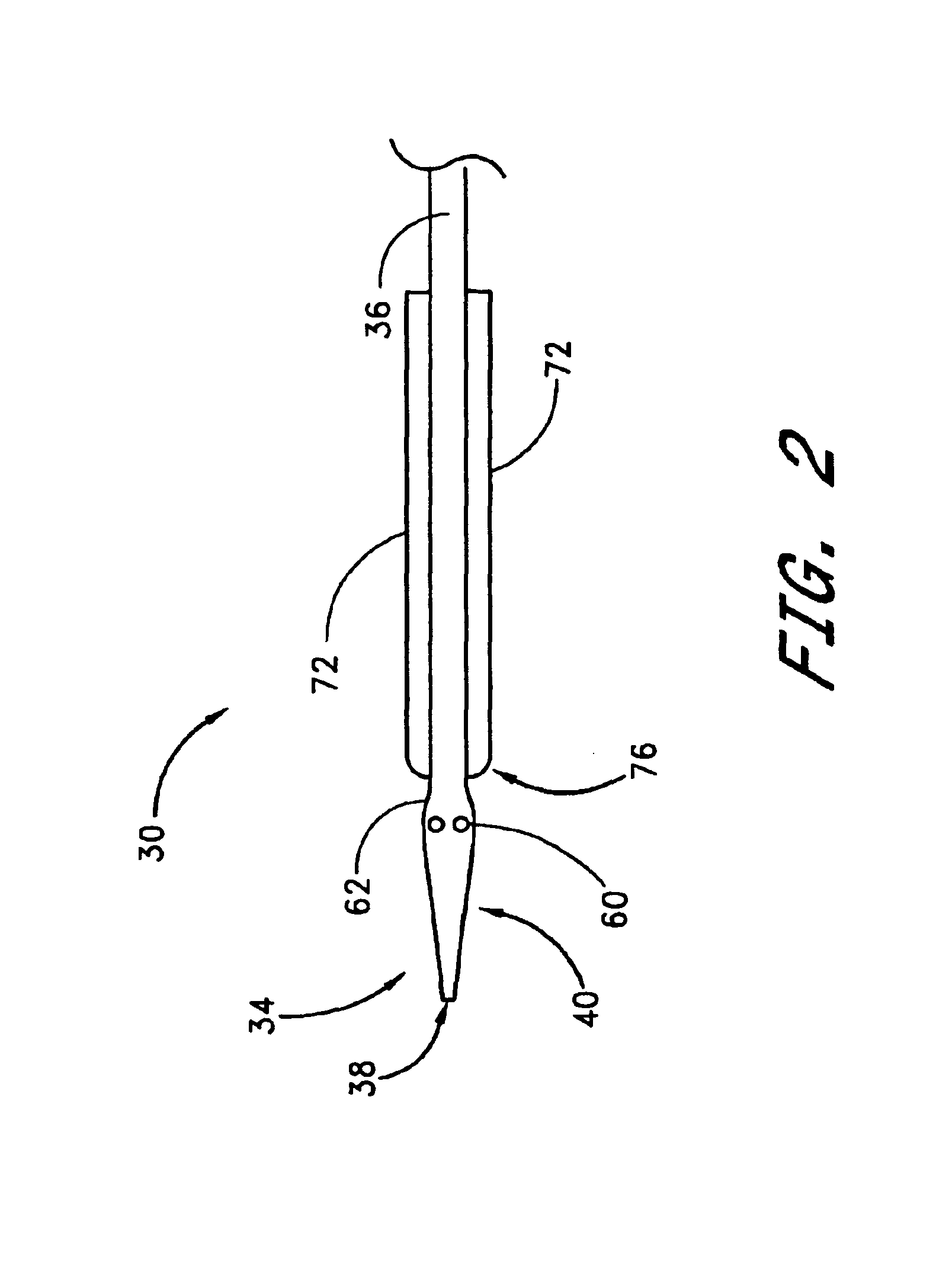
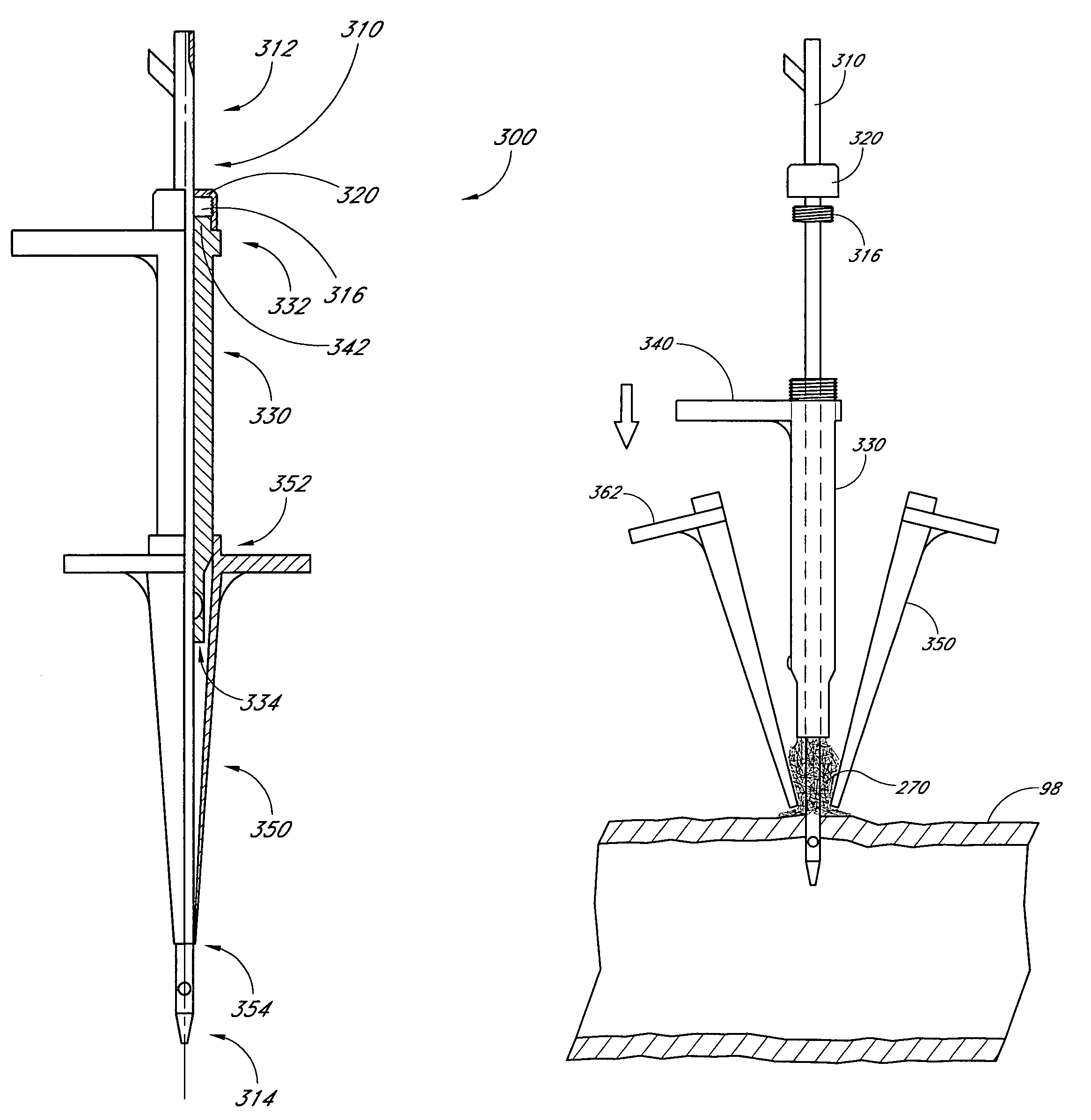
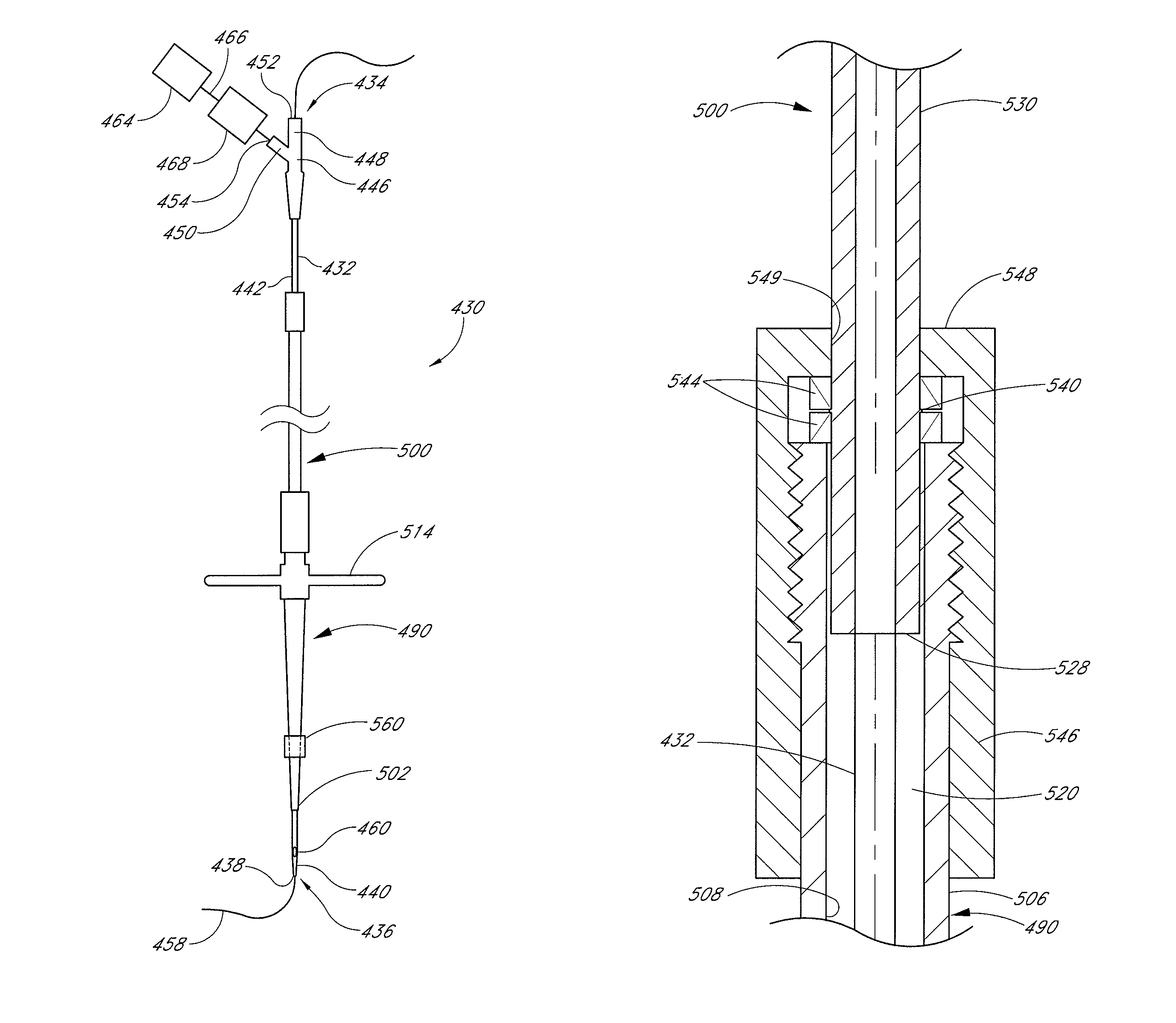
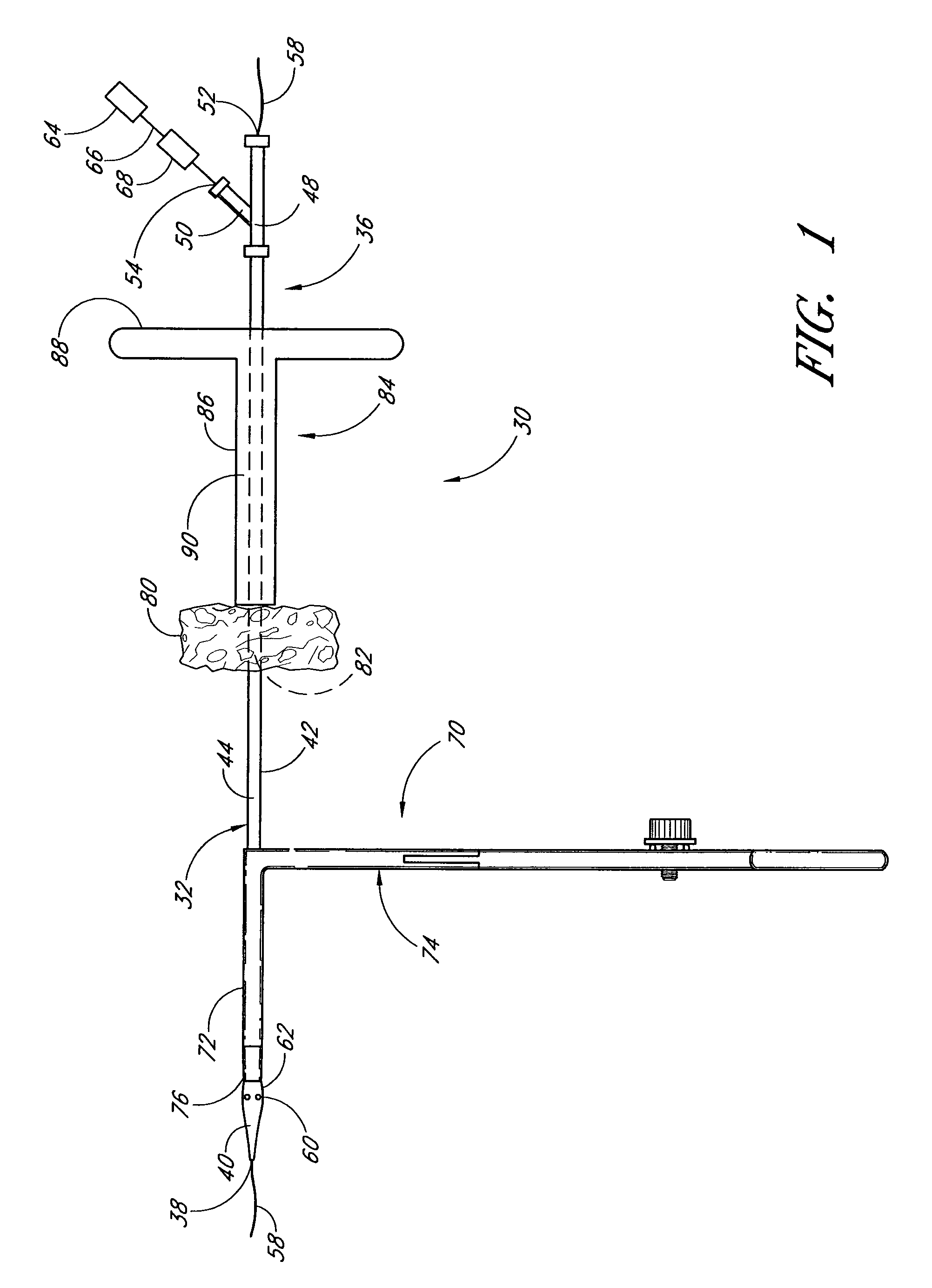
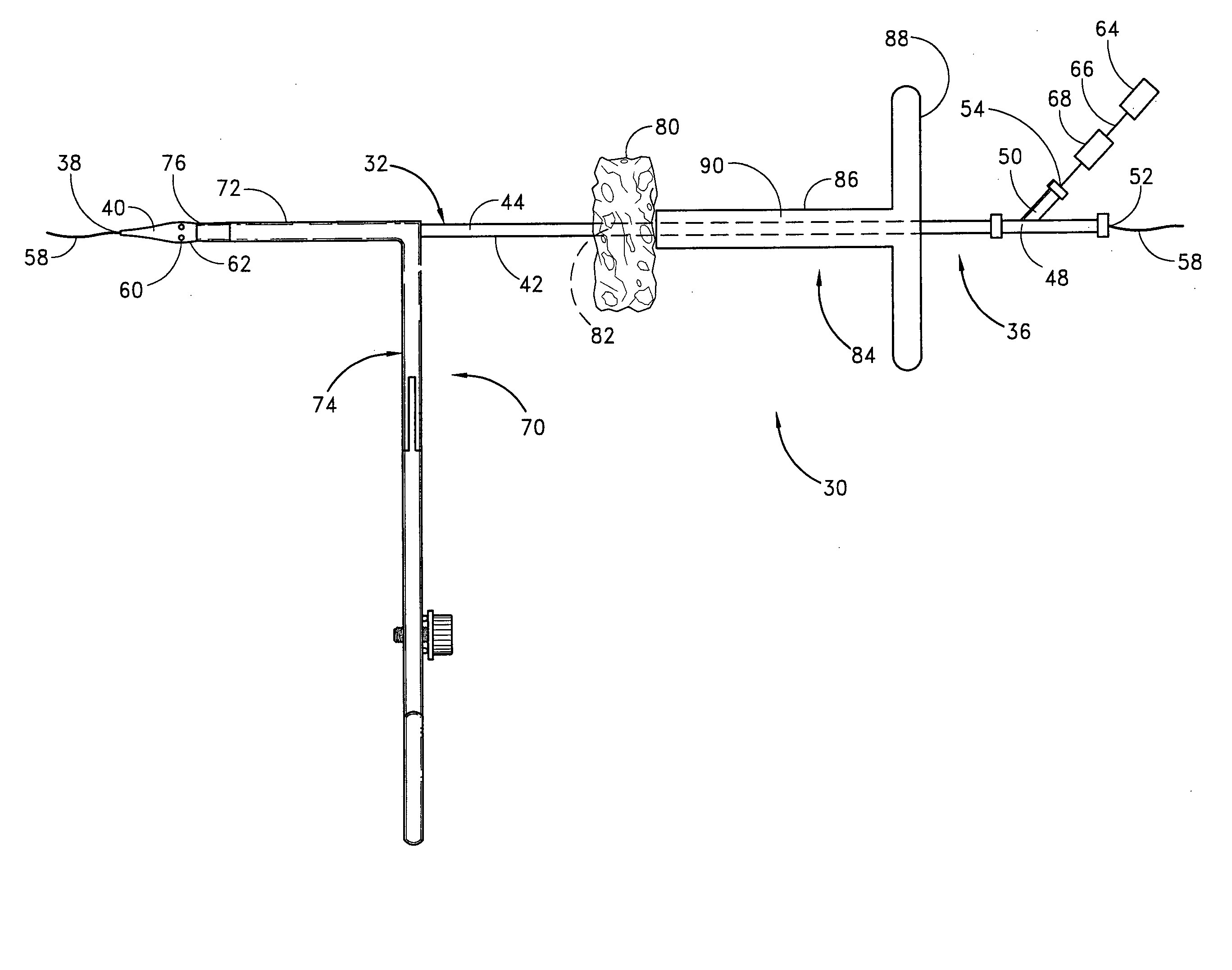
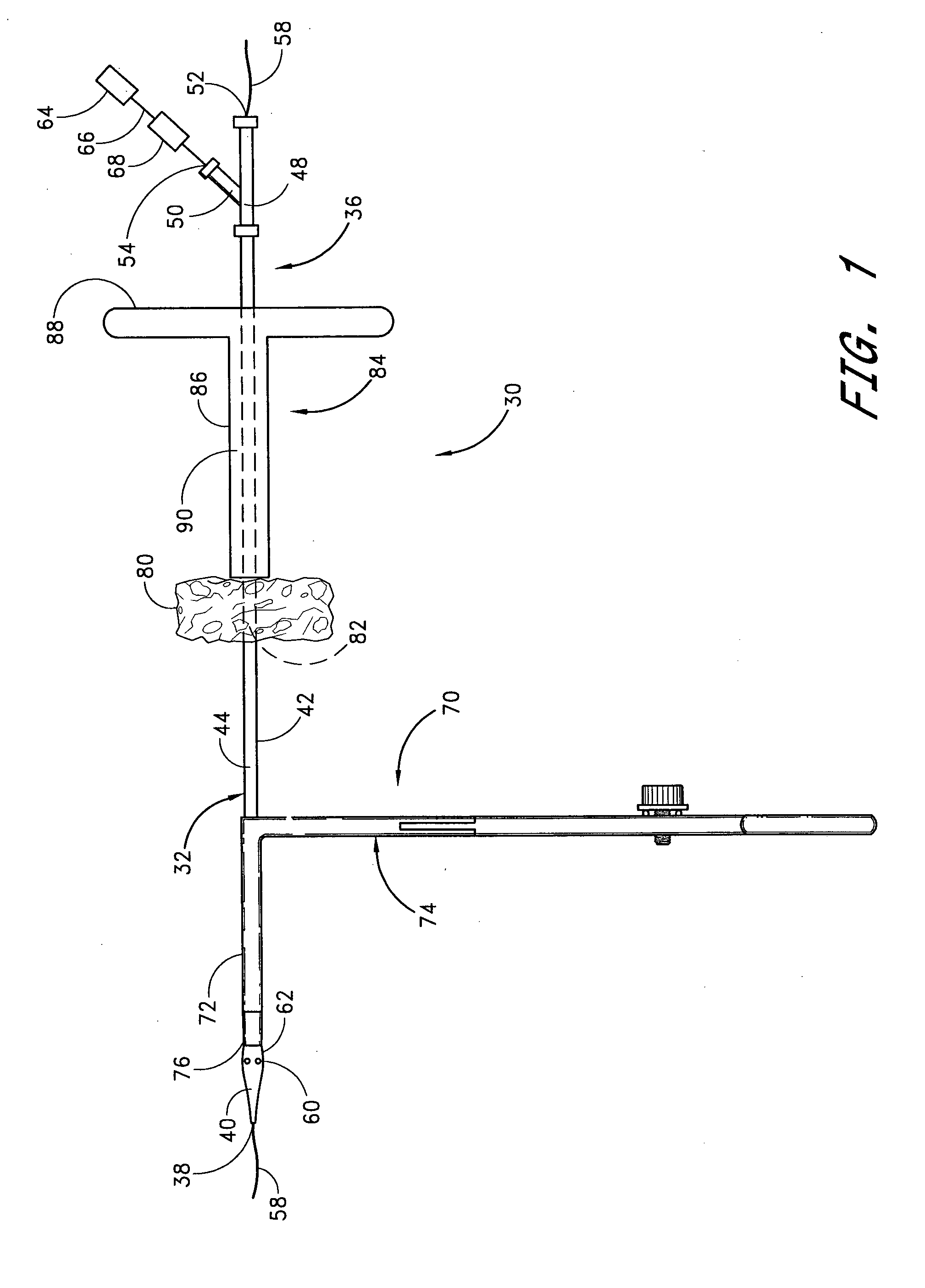
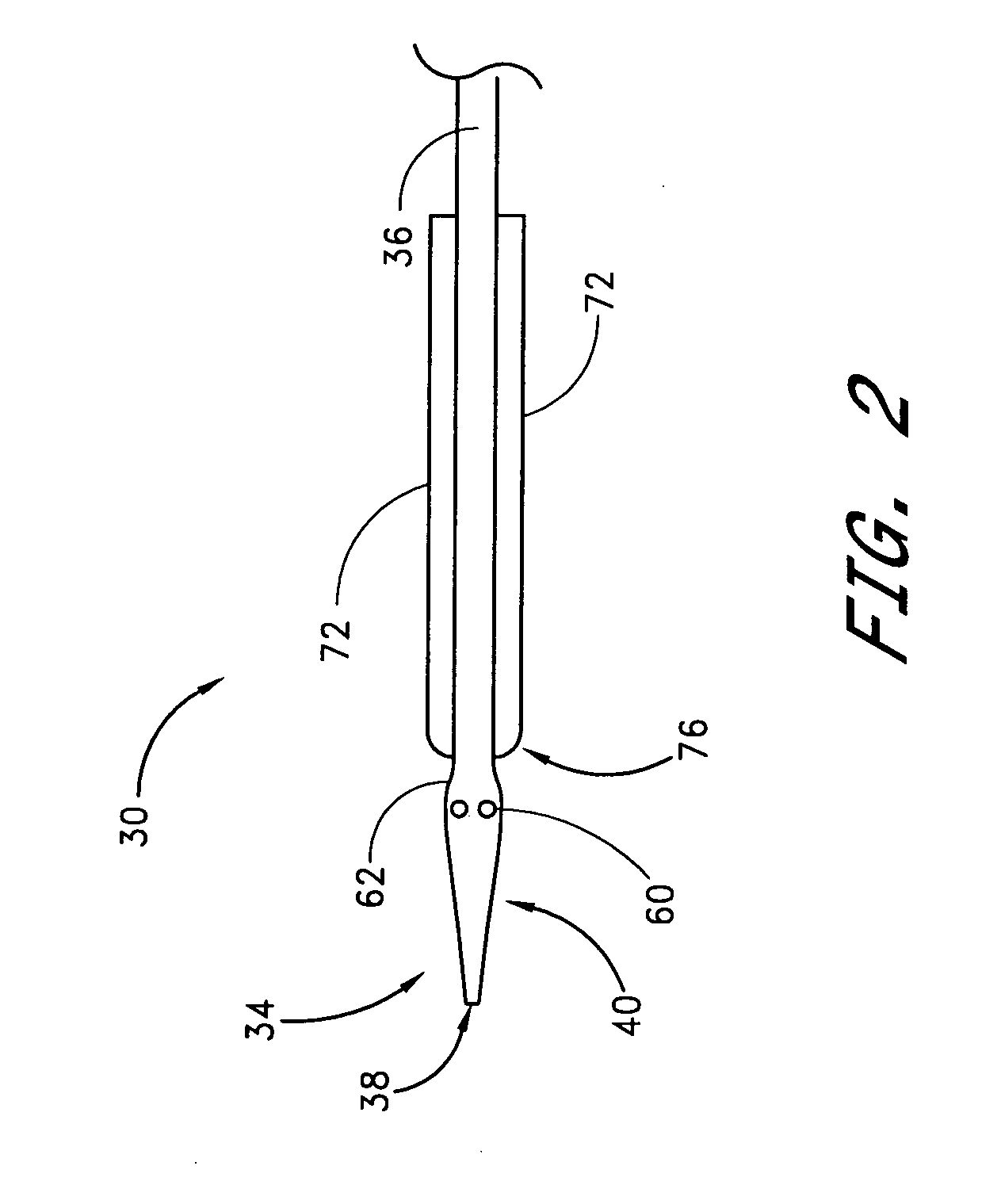
A method and apparatus for closing a vascular wound includes an apparatus that can be threaded over a guidewire into place at or adjacent the wound. The apparatus includes a chamber that encloses a hemostatic material therein. When the apparatus is positioned adjacent the wound as desired, the hemostatic material is deployed from the chamber. A blocking member distal of the hemostatic material functions as a barrier to prevent the hemostatic material from entering the wound. Blood contacts the hemostatic material, and blood clotting preferably is facilitated by a hemostatic agent within the material. Thus, the vascular puncture wound is sealed by blood clot formation.
Owner:LOMA LINDA UNIV MEDICAL CENT
Implantable prosthesis with displaceable skirt
An implantable prosthesis is provided having a radially-expandable tubular body and at least one skirt extending therefrom. The skirt terminates in a peripheral edge, wherein at least portions of the peripheral edge are free and displaceable to a greater diameter of the tubular body. Thus, with the implantable prosthesis being a stent-graft used to treat an aortic aneurysm (e.g., abdominal aortic aneurysm (“AAA”)), the skirt may be used to inhibit Type I endoleaks upon its selective displacement in response to irregular aortic shaping and / or aneurysm neck expansion. The skirt may actively inhibit Type I endoleaks by forming a physical barrier against flow between the tubular body and the aortic wall. In addition, the skirt may passively inhibit endoleak formation by sufficiently restricting blood flow to allow coagulation and clot formation, which would act as a barrier against endoleakage.
Owner:LIFESHIELD SCI
Method and apparatus for closing vascular puncture using hemostatic material
A method and apparatus for closing a vascular wound includes a guidewire and / or other surgical implement extending from the wound. A hemostatic material is advanced over the surgical implement and into contact with an area of the blood vessel surrounding the wound. The surgical implement is removed. Blood soaks the hemostatic material, and blood clotting is facilitated by the hemostatic agent within the material. A sealing layer of adhesive can be applied to the hemostatic material, confining the blood flow to the material. Thus, the vascular puncture wound is sealed by natural blood clot formation.
Owner:LOMA LINDA UNIVERSITY
Vascular wound closure device and method
A method and apparatus for closing a vascular wound includes an apparatus that can be threaded over a guidewire into place at or adjacent the wound. The apparatus includes a chamber that encloses a hemostatic material therein. When the apparatus is positioned adjacent the wound as desired, the hemostatic material is deployed from the chamber. Blood contacts the hemostatic material, and blood clotting preferably is facilitated by a hemostatic agent within the material. Thus, the vascular puncture wound is sealed by blood clot formation.
Owner:LOMA LINDA UNIV MEDICAL CENT
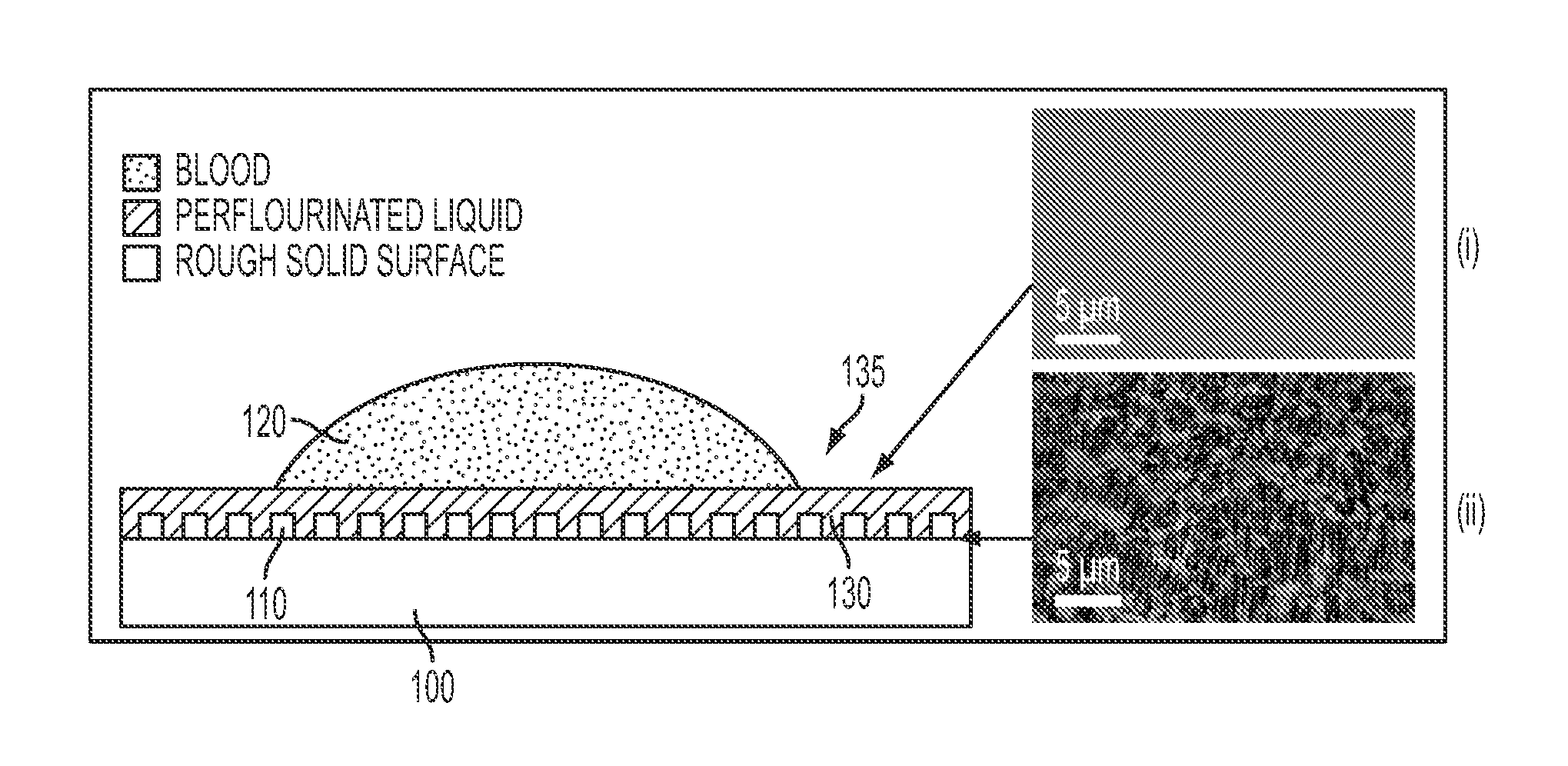
Slippery liquid-infused porous surfaces and biological applications thereof
ActiveUS20140187666A1Inhibit inflammationPrevents wound healingAntifouling/underwater paintsSurgical adhesivesSelf-healingHigh density
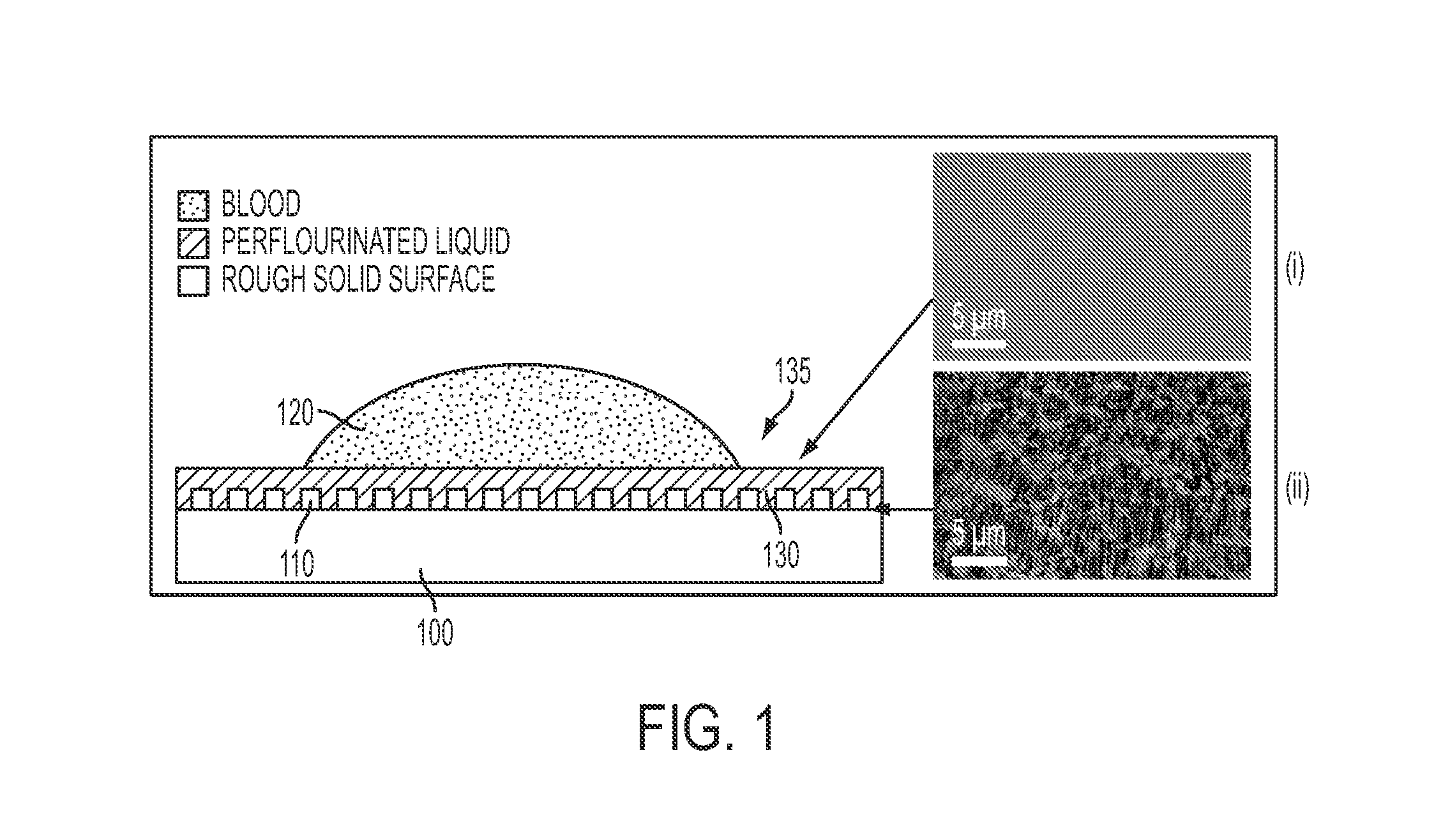
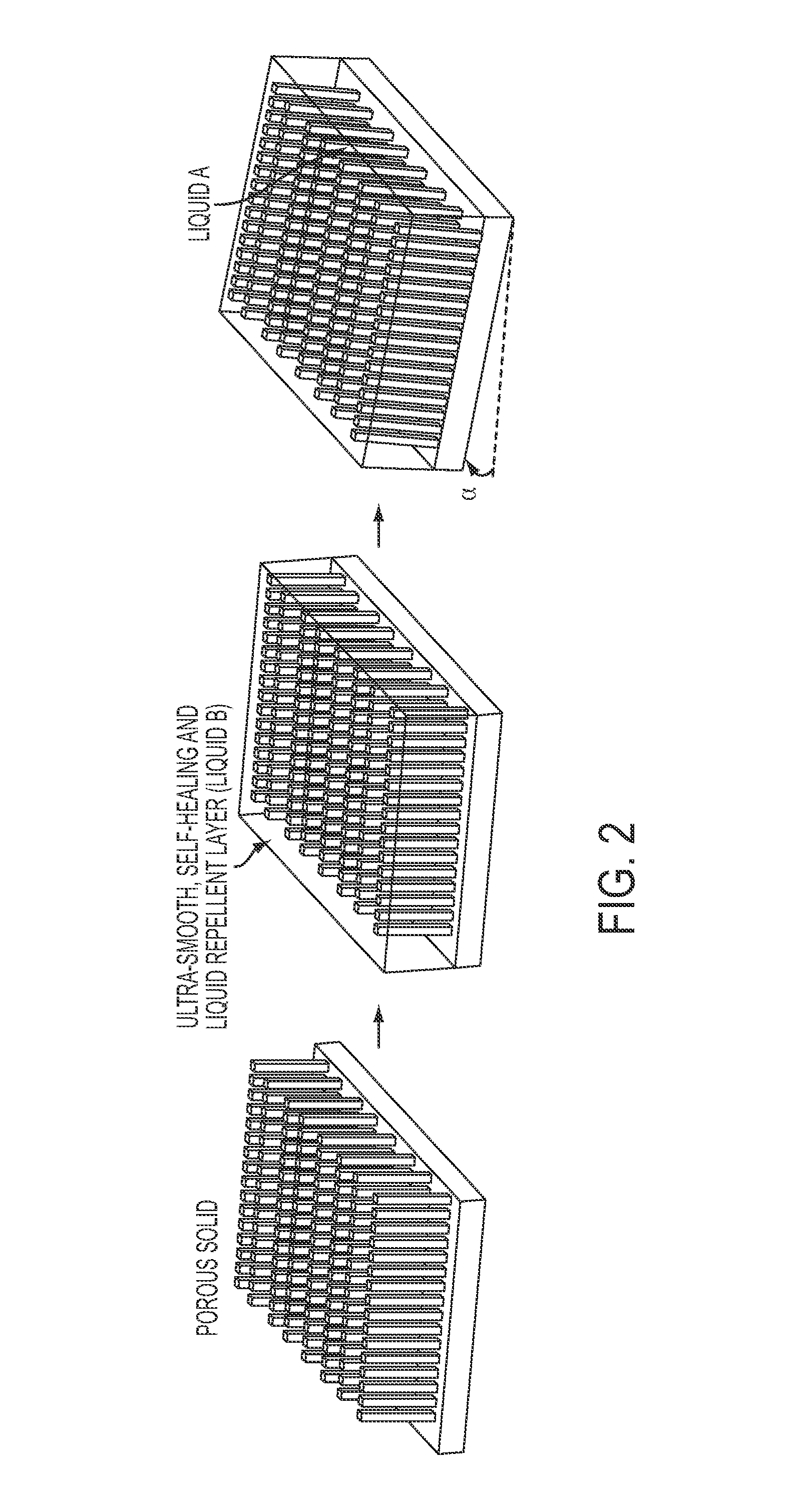
A self-healing, scratch resistant slippery surface that is manufactured by wicking a chemically-inert, high-density liquid coating over a roughened solid surface featuring micro and nanoscale topographies is described. Such a slippery surface shows anti-wetting properties, as well as exhibits significant reduction of adhesion of a broad range of biological materials, including particles in suspension or solution. Specifically, the slippery surfaces can be applied to medical devices and equipment to effectively repel biological materials such as blood, and prevent, reduce, or delay coagulation and surface-mediated clot formation. Moreover, the slippery surfaces can be used to prevent fouling by microorganisms such as bacteria.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Fibrin glue without fibrinogen and biosealant compositions and methods
InactiveUS6168788B1Stop blood flowPrevents unwantedSurgical adhesivesPeptide/protein ingredientsFibrin glueClot formation
The invention is a fibrin glue that avoids the use of fibrinogen and thus eliminates the need for premixing and premature clot formation. The fibrin glue of the invention comprises thrombin, thromboplastin and calcium and may have clotting Factors, VII, IX and X, and the like. The invention also comprises a biosealant for use with the fibrin glue without fibrinogen or for use alone. The biosealant is a two component mixture of gelatin / resorcinol and glyoxal / glutaraldehyde / 4-(p-maleimidophenyl) butyric acid. The two components are mixed on use.
Owner:WORTHAM LEON
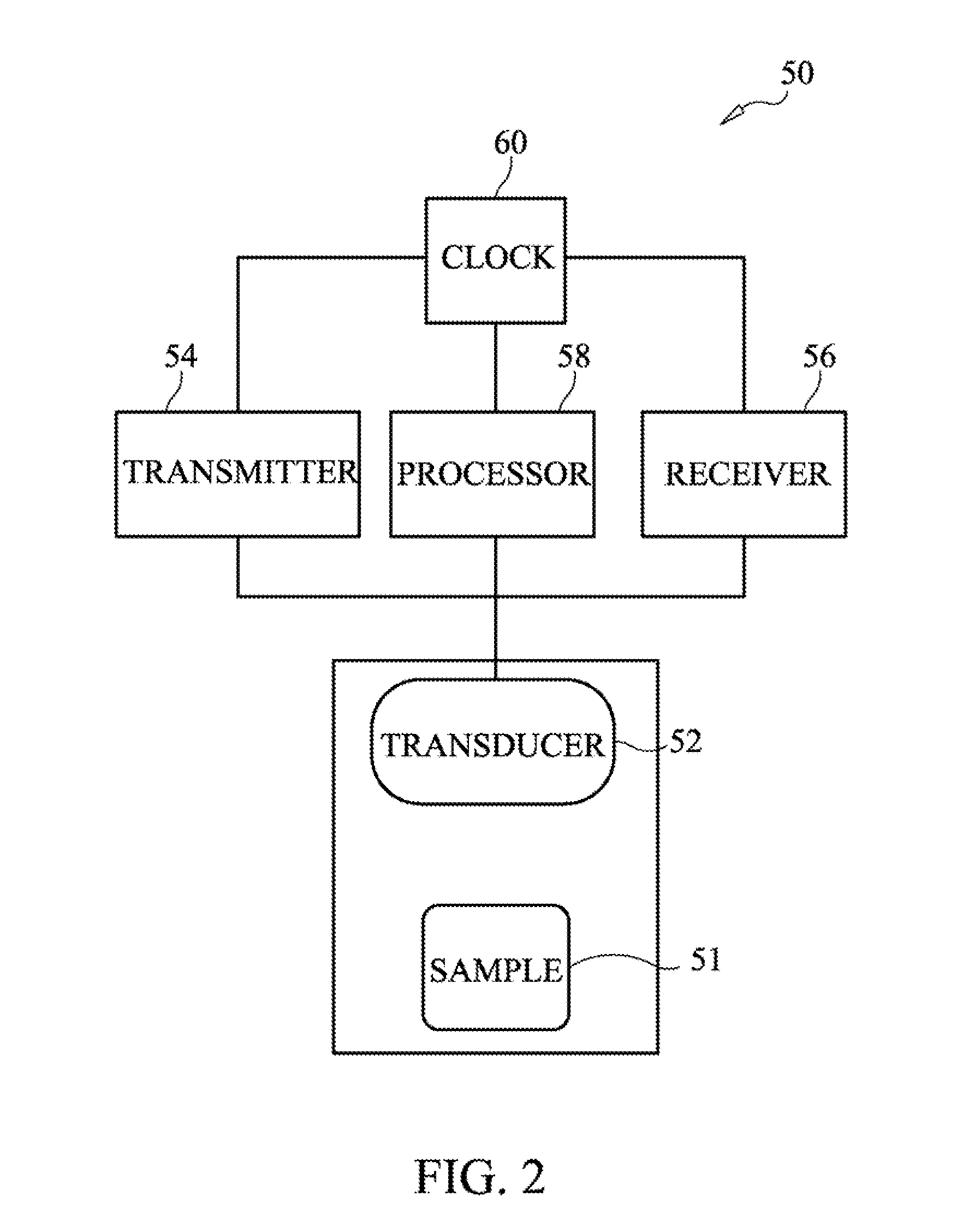
Method and apparatus for characterization of clot formation
ActiveUS20050148899A1Analysing solids using sonic/ultrasonic/infrasonic wavesUltrasonic/sonic/infrasonic wave generationHigh intensityClot formation
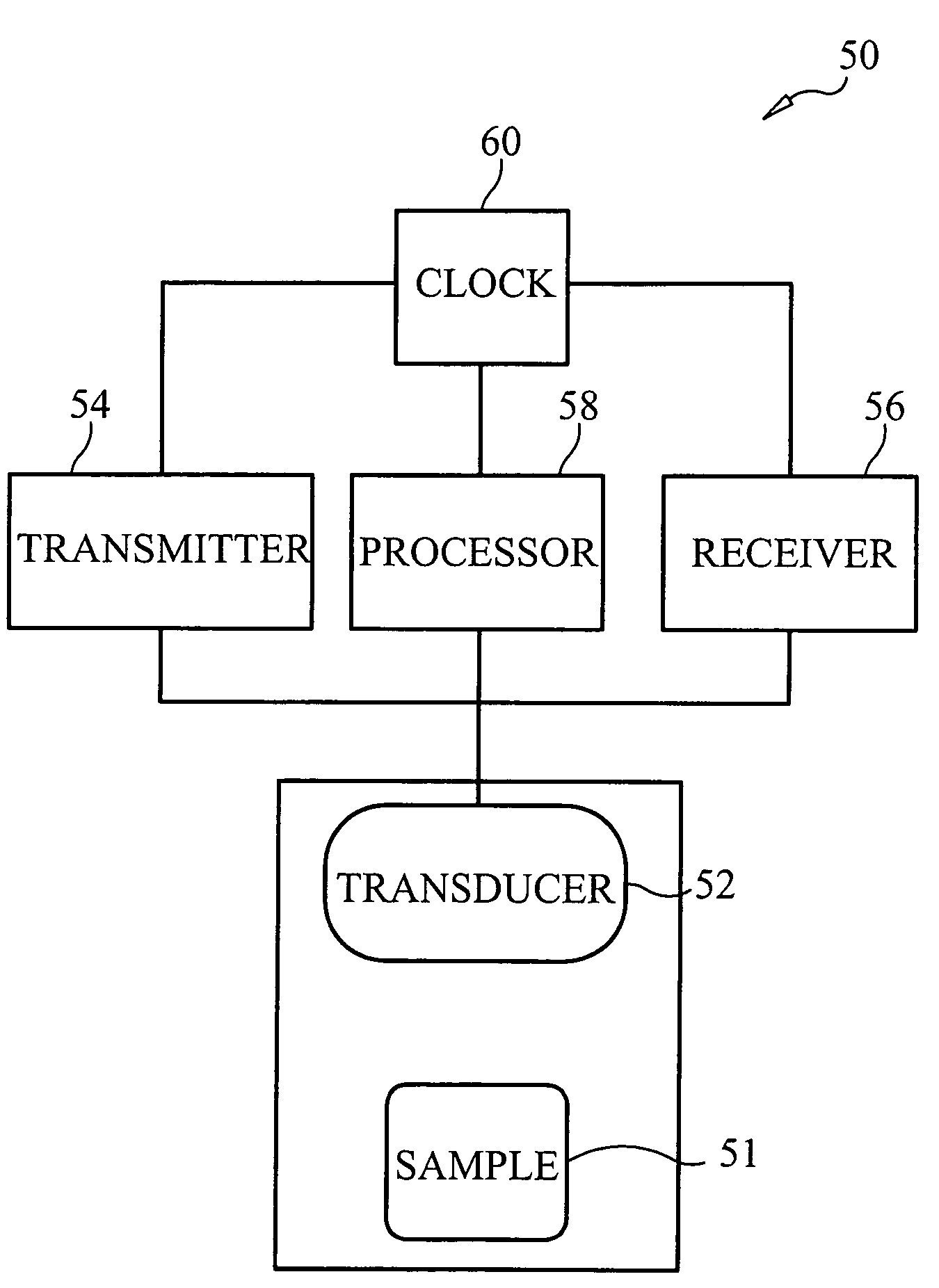
Methods, apparatus and systems for characterizing changes in at least one physical property of soft tissue. A series of acoustic pulses is generated and directed into the soft tissue such that at least one of the pulses is of sufficiently high intensity to induce physical displacement of the tissue. Waves reflected off the tissue, or a flexible member that moves with the tissue, are received and measured to estimate at least one characteristic of the physical displacement induced thereby. Repetition of the generating, receiving and estimating steps provides characterization of the at least one physical property over time. Methods, apparatus and systems for characterizing at least one physical property of blood, by generating a series of acoustic pulses and directing the series of pulses into the blood such that at least one of the pulses is of sufficiently high intensity to induce physical displacement of the blood. Acoustic pulses and / or optical waves reflected from the blood, or a flexible member in contact with the blood that moves with the blood, are received and measured to estimate at least one characteristic of the physical displacement induced thereby.
Owner:HEMOSONICS
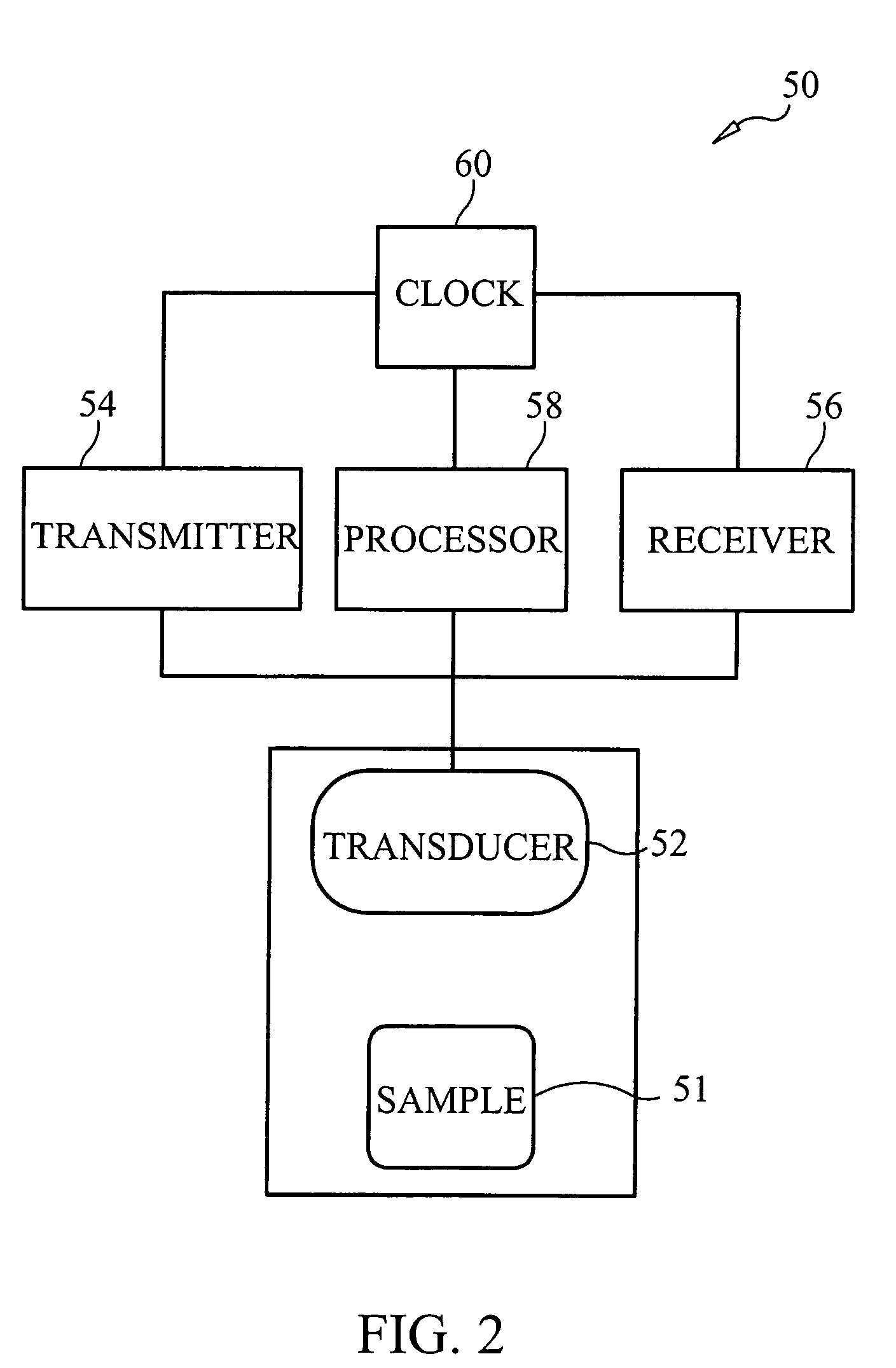
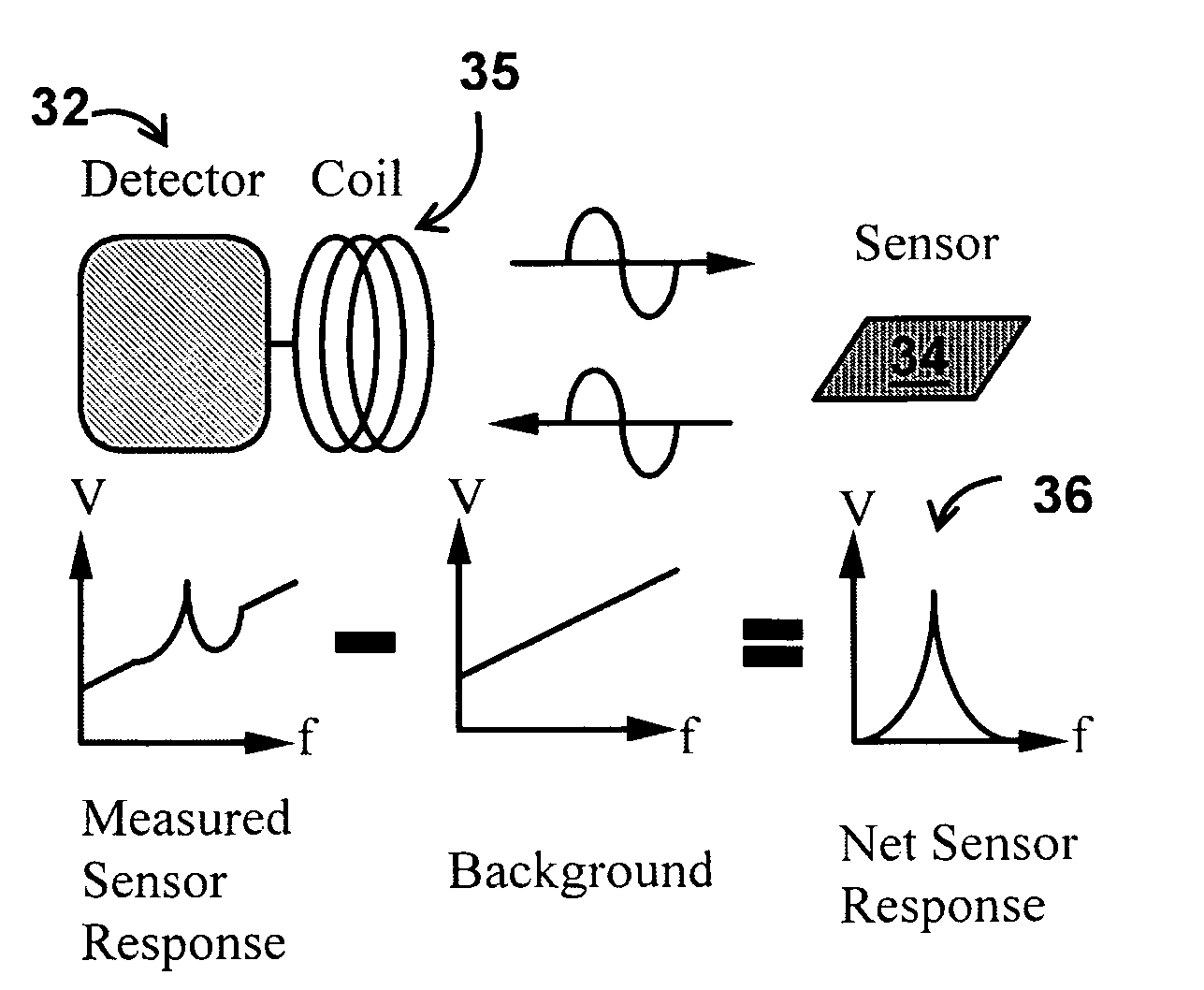
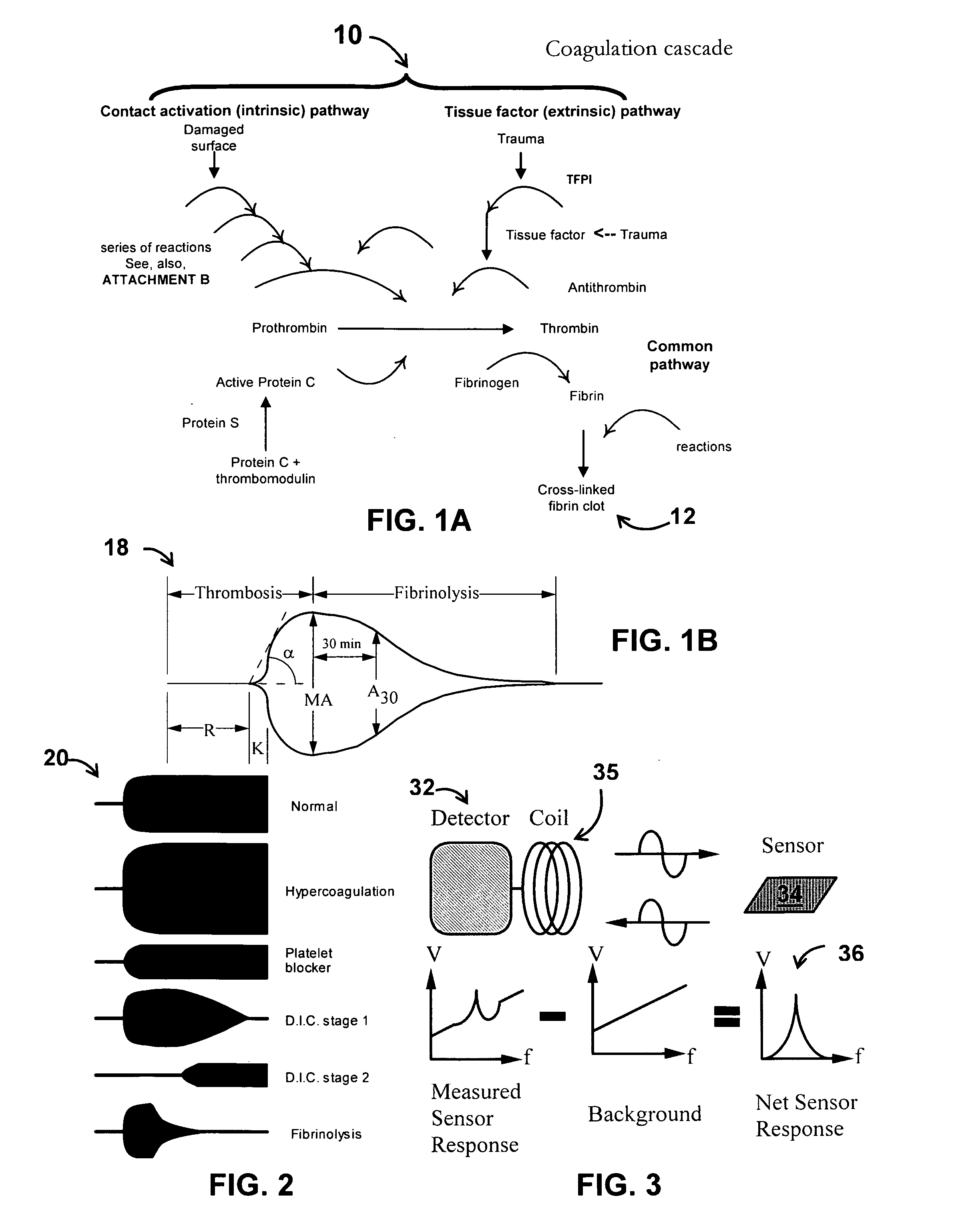
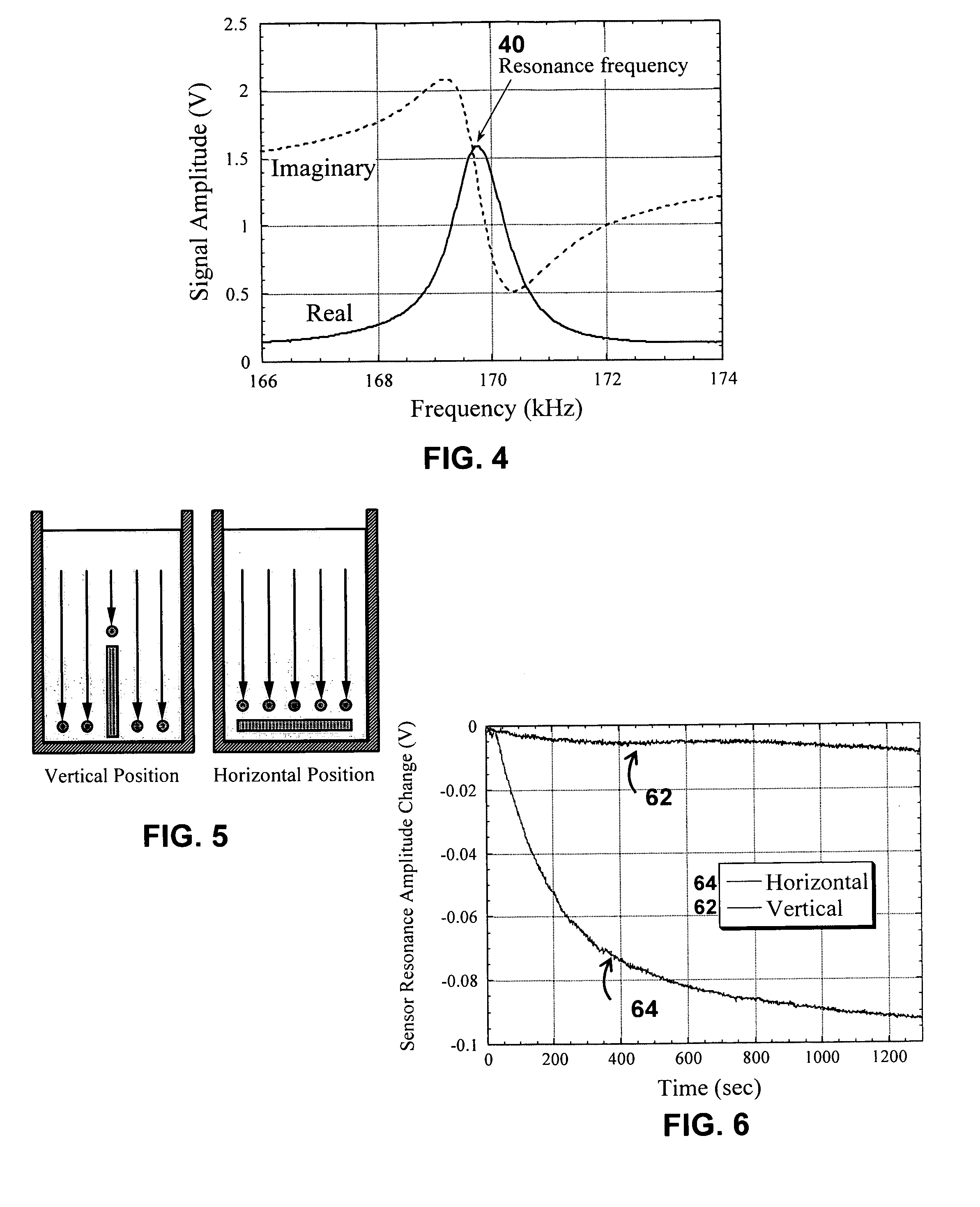
System/unit and method employing a plurality of magnetoelastic sensor elements for automatically quantifying parameters of whole blood and platelet-rich plasma
InactiveUS20080261261A1Quantifying platelet-fibrin clot strengthBioreactor/fermenter combinationsBiological substance pretreatmentsClot formationBlood plasma
A system / analyzer-unit and method / platform—using information obtained from at least one, adapted for a plurality of, magnetoelastic sensor elements in contact with one or more samples comprising blood from a patient—for automatically quantifying one or more parameters of the patient's blood. Information obtained from emissions measured from each of the sensor elements is uniquely processed to determine a quantification about the patient's blood, such as, quantifying platelet aggregation to determine platelet contribution toward clot formation; quantifying fibrin network contribution toward clot formation; quantifying platelet-fibrin clot interactions; quantifying kinetics of thrombin clot generation; quantifying platelet-fibrin clot strength; and so on. Structural aspects of the analyzer-unit include: a cartridge having at least one bay within which a sensor element is positioned; each bay in fluid communication with both (a) an entry port for injecting a first blood sample composed of blood taken from the patient (human or other mammal), and (b) a gas vent through which air displaced by injecting the first blood sample into the bay.
Owner:KMG2 SENSORS CORP
Wound dressing and method for controlling severe, life-threatening bleeding
InactiveUS20050038369A1Stanching flowAvoid bleedingBiocideNon-adhesive dressingsWound dressingClot formation
This invention is directed to advanced hemorrhage control wound dressings, and methods of using a producing same. The subject wound dressing is constructed from a non-mammalian material for control of severe bleeding. The wound dressing is formed of a biomaterial comprising chitosan for controlling severe bleeding. The kind of severe, life-threatening bleeding contemplated by this invention is typically of the type not capable of being stanched when a conventional gauze wound dressing is applied with conventional pressure to the subject wound. The wound dressing being capable of substantially stanching the flow of the severe life-threatening bleeding from the wound by adhering to the wound site, to seal the wound, to accelerate blood clot formation at the wound site, to reinforce clot information at the wound site and prevent bleed out from the wound site, and to substantially prohibit the flow of blood out of the wound site.
Owner:PROVIDENCE HEALTH SYST OREGON +1
Pharmaceutical Compositions of a 5-HT2A Serotonin Receptor Modulator Useful for the Treatment of Disorders Related Thereto
The present invention relates to certain pharmaceutical compositions of a 5-HT2A serotonin receptor modulator and methods for preparing pharmaceutical composition related thereto. The pharmaceutical compositions are useful in the treatment of platelet aggregation, coronary artery disease, myocardial infarction, transient ischemic attack, angina, stroke, atrial fibrillation, reducing the risk of blood clot formation, asthma or symptoms thereof, agitation or a symptom, behavioral disorders, drug induced psychosis, excitative psychosis, Gilles de la Tourette's syndrome, manic disorder, organic or NOS psychosis, psychotic disorder, psychosis, acute schizophrenia, chronic schizophrenia, NOS schizophrenia and related disorders, sleep disorders, diabetic-related disorders, progressive multifocal leukoencephalopathy and the like.
Owner:ARENA PHARMA
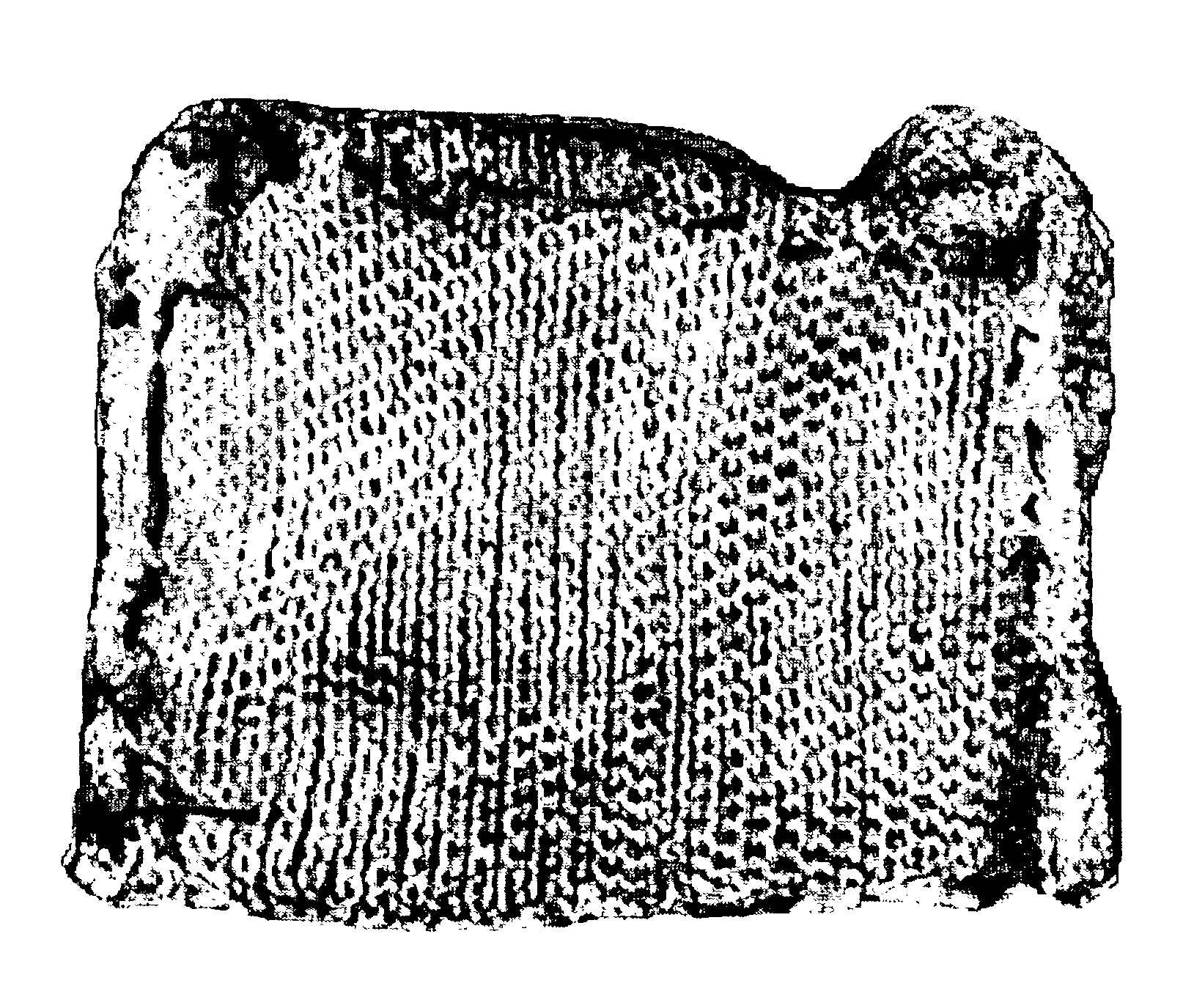
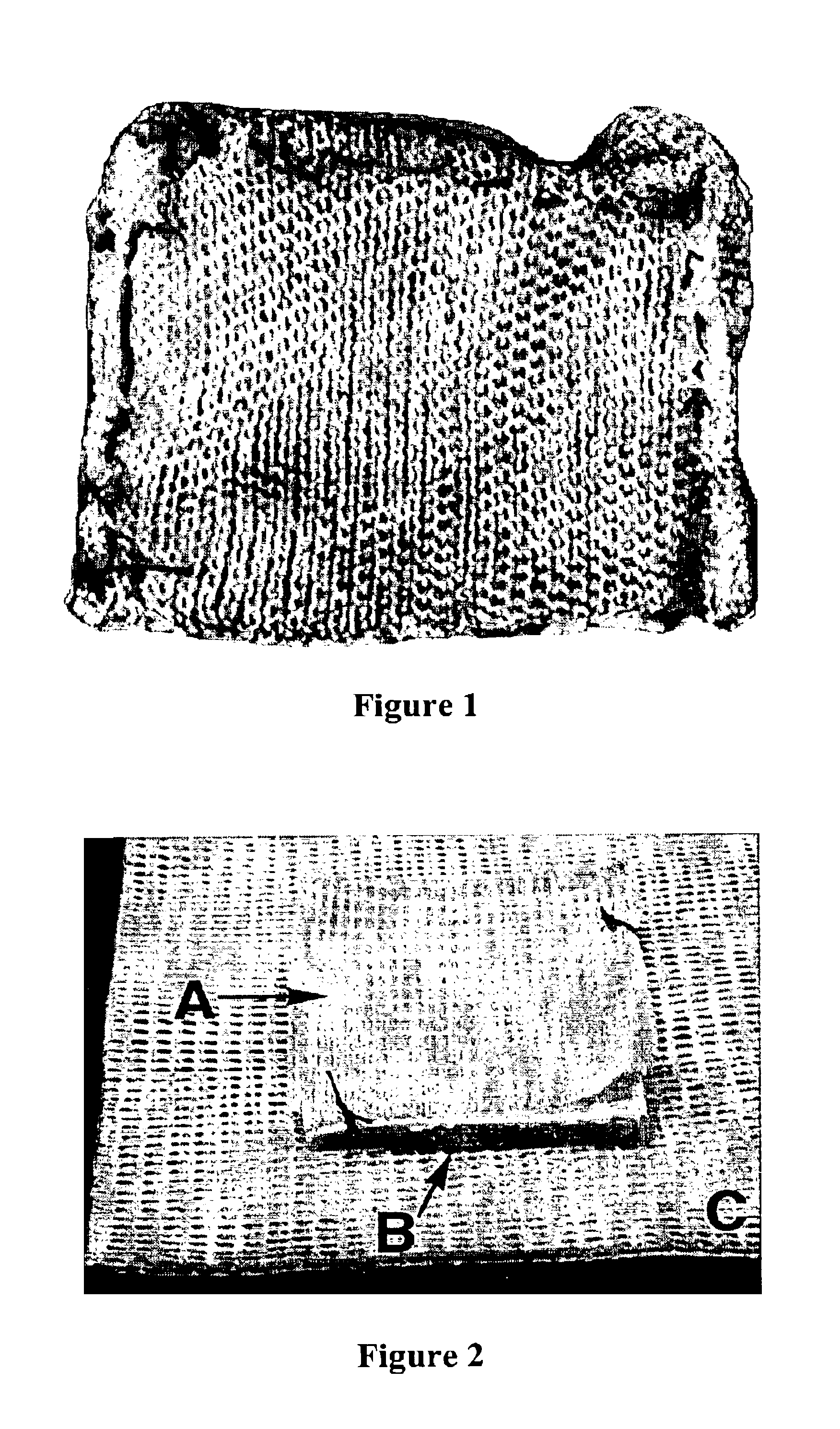
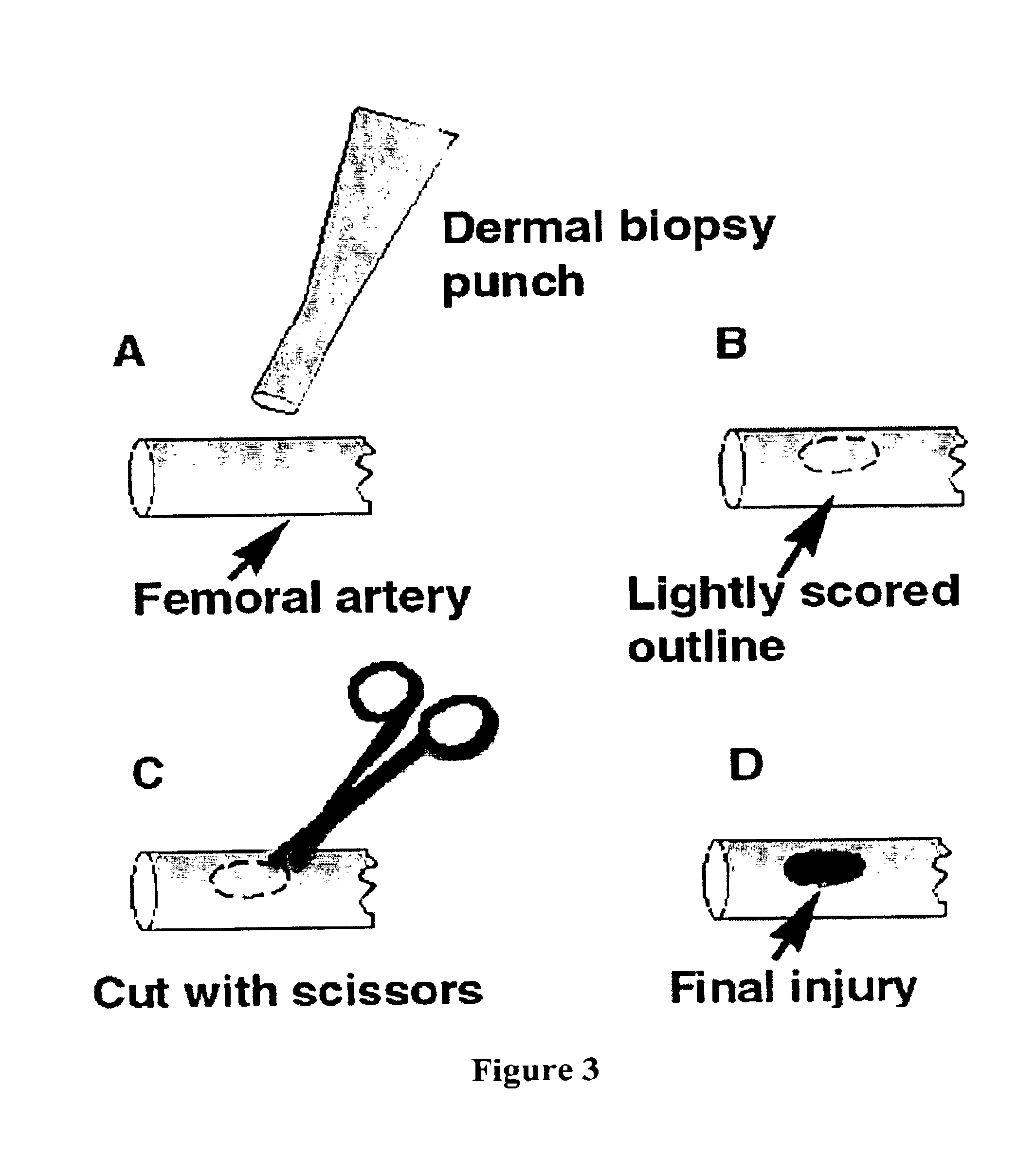
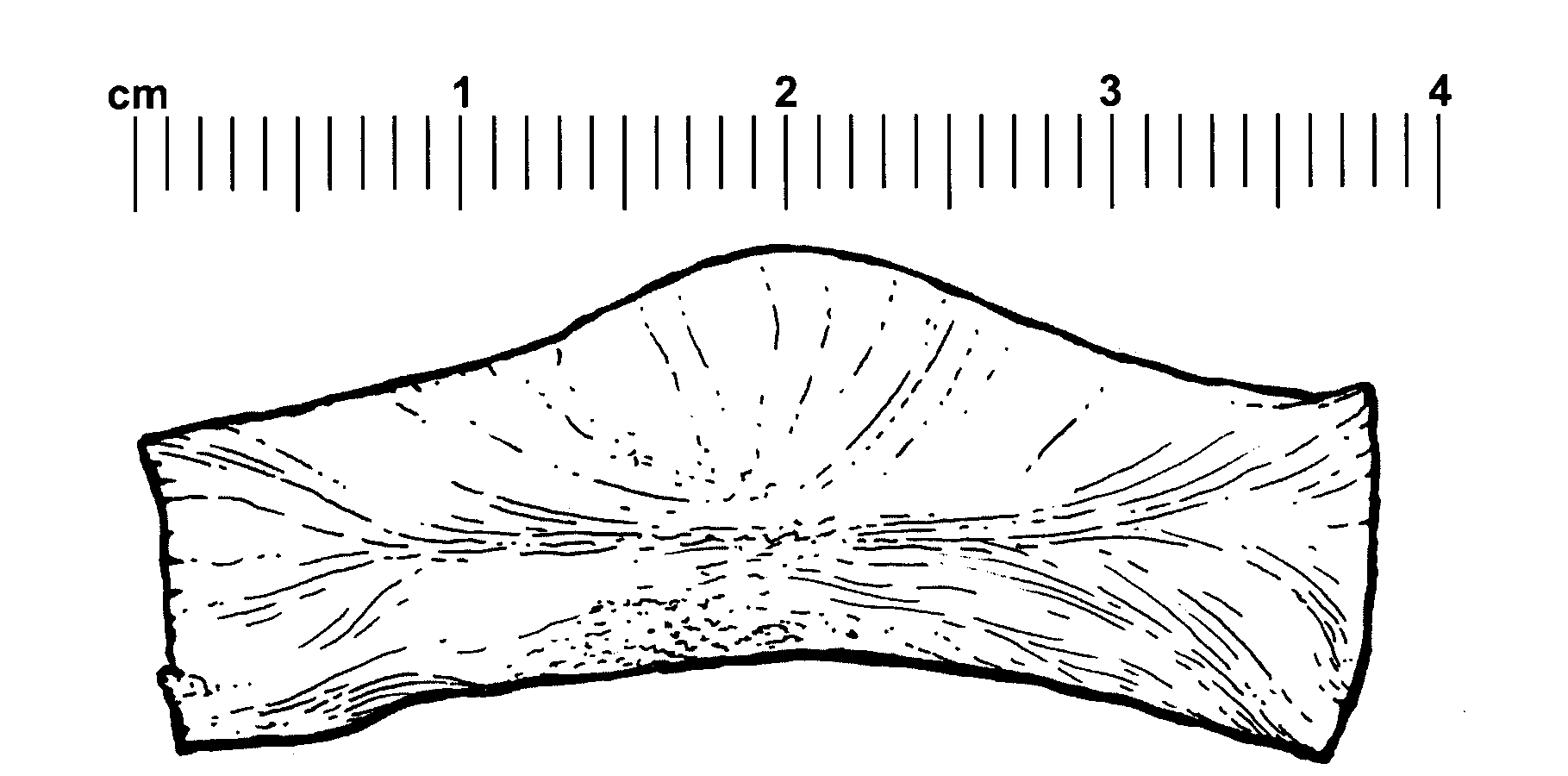
Fibrinogen bandages and arterial bleeding models and methods of making and using thereof
InactiveUS6891077B2Reduce the amount of solutionIncreasing of rate of clotSurgeryBaby linensBlood platelet countsClot formation
Disclosed herein are wound dressings comprising fibrinogen and at least one procoagulant such as propyl gallate in a therapeutic amount. Also disclosed are methods of treating wounds, increasing an amount of or rate of coagulation of blood from a wound, increasing an amount of or rate of clot formation over a wound, increasing blood platelet counts, activating a coagulation system, increasing the plasma concentration of fibrinogen, and decreasing the activated partial thromboplastin time. Also disclosed are an arterial bleeding model and methods of studying arterial bleeding.
Owner:UNITED STATES ARMY U S ARMY MEDICAL RES & MATERIEL COMMAND
Semi-synthetic platelet gel and method for the preparation thereof
A semi-synthetic platelet gel comprising a platelet-rich plasma, at least one platelet activator, and a biocompatible polymer selected from the group comprising carbomers, polyalkylene glycols, poloxamers, polyesters, polyethers, polyanhydrides, polyacrylates, polyvinyl acetates, polyvinyl pyrrolidones, polysaccharides, and derivatives thereof. A method for preparing a semi-synthetic platelet gel comprising the steps of (a) mixing a platelet-rich plasma with at least one platelet activator, and, before the start of clot formation, (b) adding the mixture thus obtained to a biocompatible polymer selected from the group comprising carbomers, polyalkylene glycols, poloxamers, polyesters, polyethers, polyanhydrides, polyacrylates, polyvinyl acetates, polyvinyl pyrrolidones, polysaccharides, and derivatives thereof.
Owner:LECTIO PHARMAENTWICKLUNGS UND VERW
Wound dressing and method for controlling severe, life-threatening bleeding
InactiveUS20080213344A1Stanching flowProhibiting flow of bloodBiocideOrganic active ingredientsWound dressingHydrophilic polymers
Owner:PROVIDENCE HEALTH SYST OREGON
Vascular wound closure device and method
A method and apparatus for closing a vascular wound includes a guidewire and / or other surgical implement extending from the wound. A hemostatic material is advanced over the surgical implement and into contact with an area of the blood vessel surrounding the wound. The surgical implement is removed. Blood soaks the hemostatic material, and blood clotting is facilitated by the hemostatic agent within the material. A sealing layer of adhesive can be applied to the hemostatic material, confining the blood flow to the material. Thus, the vascular puncture wound is sealed by natural blood clot formation.
Owner:LOMA LINDA UNIV MEDICAL CENT
Method and apparatus for characterization of clot formation
ActiveUS20110034805A1Analysing solids using sonic/ultrasonic/infrasonic wavesBlood flow measurement devicesTemporal changeHigh intensity
Owner:HEMOSONICS
Lubricious or/and wettable or/and anti-thrombin elastomeric gland materials in luer activated devices
An elastomeric gland is provided for a luer activating device (LAD). and comprises a unique lubricant and / or wetting agent and / or anti-clotting agent incorporated into the elastomer gland during raw material formulation, calendar blending / molding / curing to deliver the surface lubricity and / or wettability and / or avoid slit plane re-knitting and / or gland induced valve stick down of such devices Functional additive chemistries are selected in terms of generated functional performance level, thermal stability against processing, molecular migratability, molecular weight and elastomer substrate of interest. These additives could include lubricants like chemically modified silicone oils and / or wetting agents like silicone-based surfactant. Elastomer gland with wetting agent would ease fluid path priming and minimize micro air bubble adherence to gland surface. Additives may also include anti-clotting agents intended to reduce potential for clot formation within the fluid path and interstitial space of the valve during blood sampling and infusion.
Owner:BAXTER INT INC +1
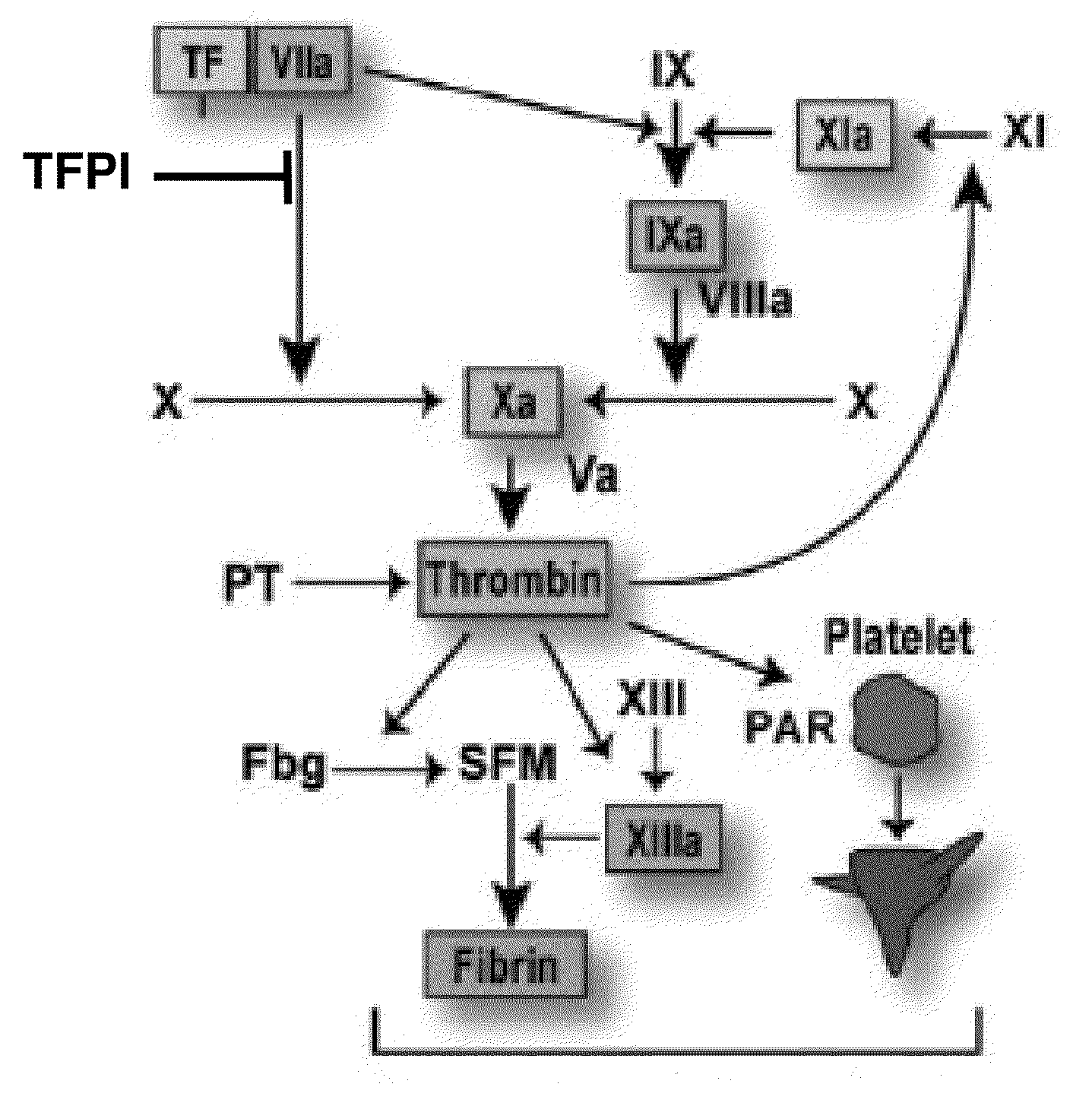
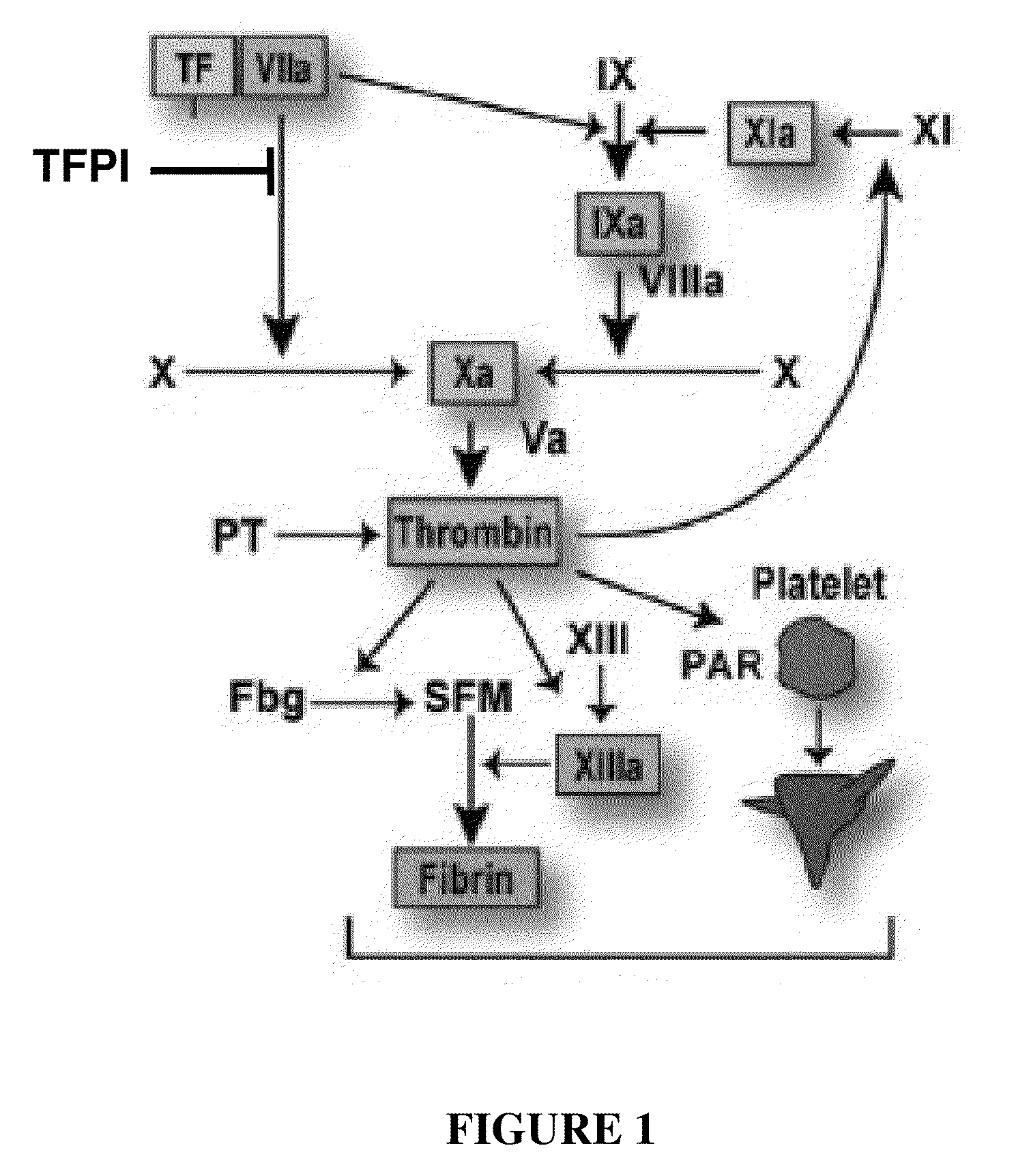
TFPI inhibitors and methods of use
ActiveUS20100173847A1Improves TFPI-regulated thrombin generationFactor VIIPeptide-nucleic acidsMedicineThrombin activity
The invention provides peptides that bind Tissue Factor Pathway Inhibitor (TFPI), including TFPI-inhibitory peptides, and compositions thereof. The peptides may be used to inhibit a TFPI, enhance thrombin formation in a clotting factor-deficient subject, increase blood clot formation in a subject, and / or treat a blood coagulation disorder in a subject.
Owner:TAKEDA PHARMA CO LTD
Hemostatic Wound Dressings
InactiveUS20100129427A1Improved hemorrhage control wound dressingStop the flowPharmaceutical delivery mechanismAbsorbent padsWound dressingWound site
Hemostatic wound dressings for substantially arresting the flow of severe, life threatening bleeding from a wound by rapidly adhering to the wound site, absorbing and concentrating and thickening the blood at the dressing blood interface and accelerating the natural clot formation beneath the dressing and finally, forming a strong seal that will substantially prohibits further flow of blood out of the wound site. These hemostatic wound dressings are formed of unique combinations of hemostatic dressing aspects which achieve wound seal strengths that are significantly higher than the sum of seal strengths expected from the individual aspects alone. Some embodiments also achieve these synergistic seal strengths by combining one hemostatic dressing with a non-hemostatic device.
Owner:BIOLIFE
Lubricious or/and wettable or/and Anti-thrombin elastomeric gland materials in luer activated devices
InactiveUS20070012893A1Improve wettabilityAvoid clotsMedical devicesCouplingsElastomerClot formation
An elastomeric gland is provided for a luer activating device (LAD). and comprises a unique lubricant and / or wetting agent and / or anti-clotting agent incorporated into the elastomer gland during raw material formulation, calendar blending / molding / curing to deliver the surface lubricity and / or wettability and / or avoid slit plane re-knitting and / or gland induced valve stick down of such devices Functional additive chemistries are selected in terms of generated functional performance level, thermal stability against processing, molecular migratability, molecular weight and elastomer substrate of interest. These additives could include lubricants like chemically modified silicone oils and / or wetting agents like silicone-based surfactant. Elastomer gland with wetting agent would ease fluid path priming and minimize micro air bubble adherence to gland surface. Additives may also include anti-clotting agents intended to reduce potential for clot formation within the fluid path and interstitial space of the valve during blood sampling and infusion.
Owner:BAXTER INT INC +1
Device for supplying blood tubes to a whole blood analyser
InactiveUS7858032B2Good flexibilityBioreactor/fermenter combinationsBiological substance pretreatmentsPre analyticalBlood tube
A device including a pre-analytical module provided with at least one tube stirrer for homogenizing blood and preventing clot formation and disposed on a line for single-tube transfer between an area for storing analyzable tubes, and at least one unitary operating whole blood analyzer and automatic tube-loading / unloading device arranged between the tube stirrer and the single-tube transfer line. The device is suitable for blood analyzers.
Owner:HORIBA ABX SAS
Vascular wound closure device and method
A method and apparatus for closing a vascular wound includes an apparatus that can be threaded over a guidewire into place at or adjacent the wound. The apparatus includes a chamber that encloses a hemostatic material therein. When the apparatus is positioned adjacent the wound as desired, the hemostatic material is deployed from the chamber. Blood contacts the hemostatic material, and blood clotting preferably is facilitated by a hemostatic agent within the material. Thus, the vascular puncture wound is sealed by blood clot formation.
Owner:LOMA LINDA UNIV MEDICAL CENT
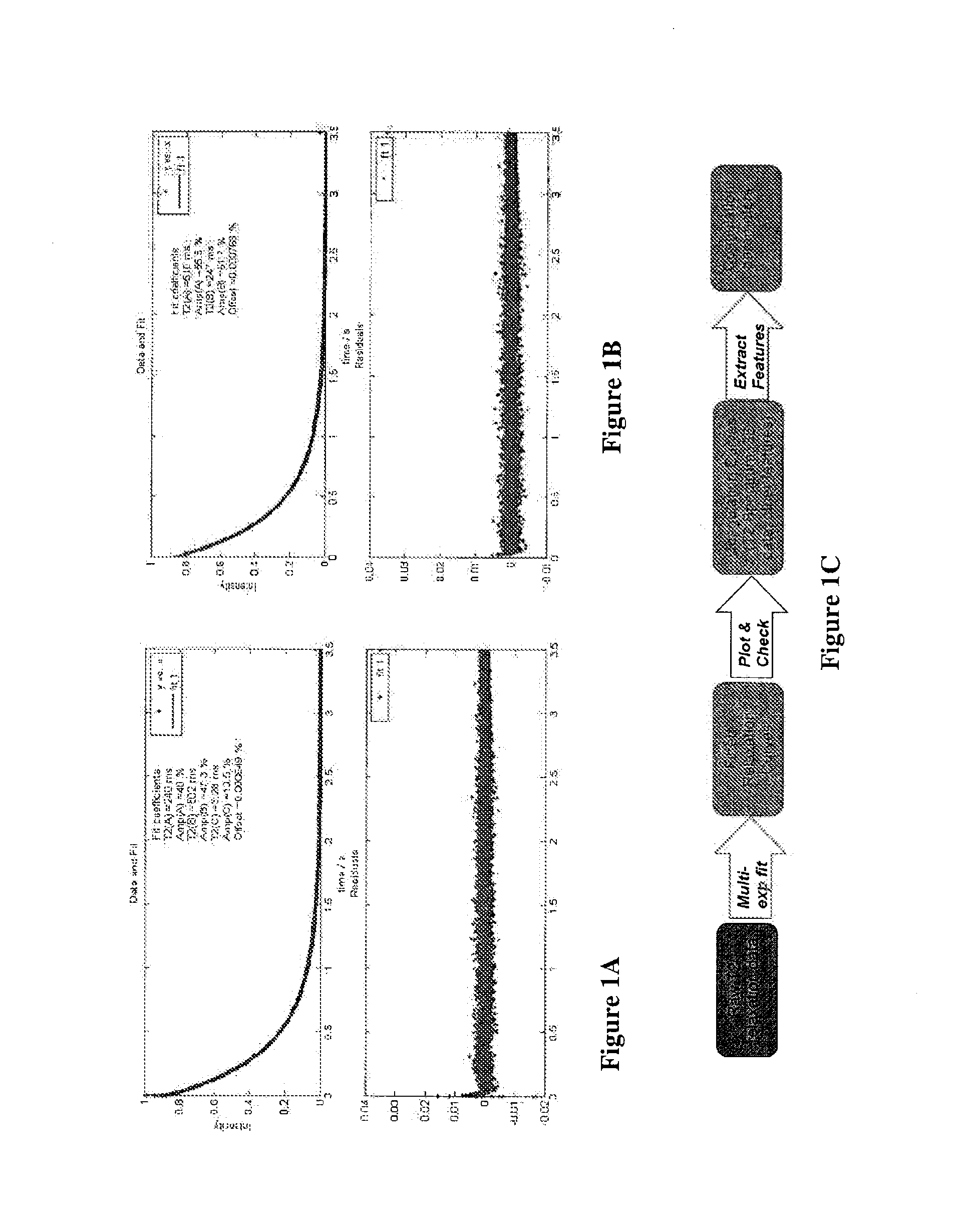
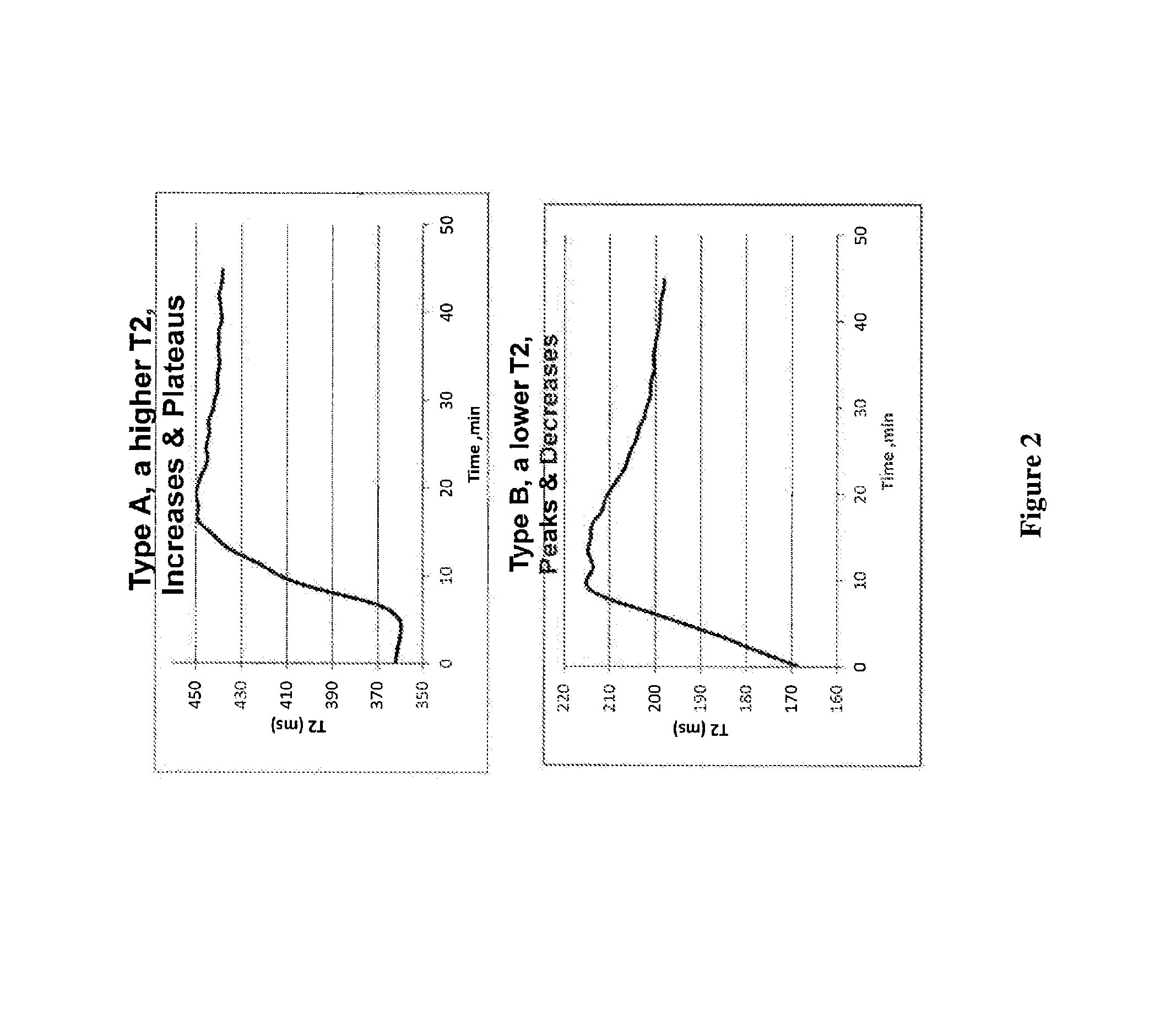
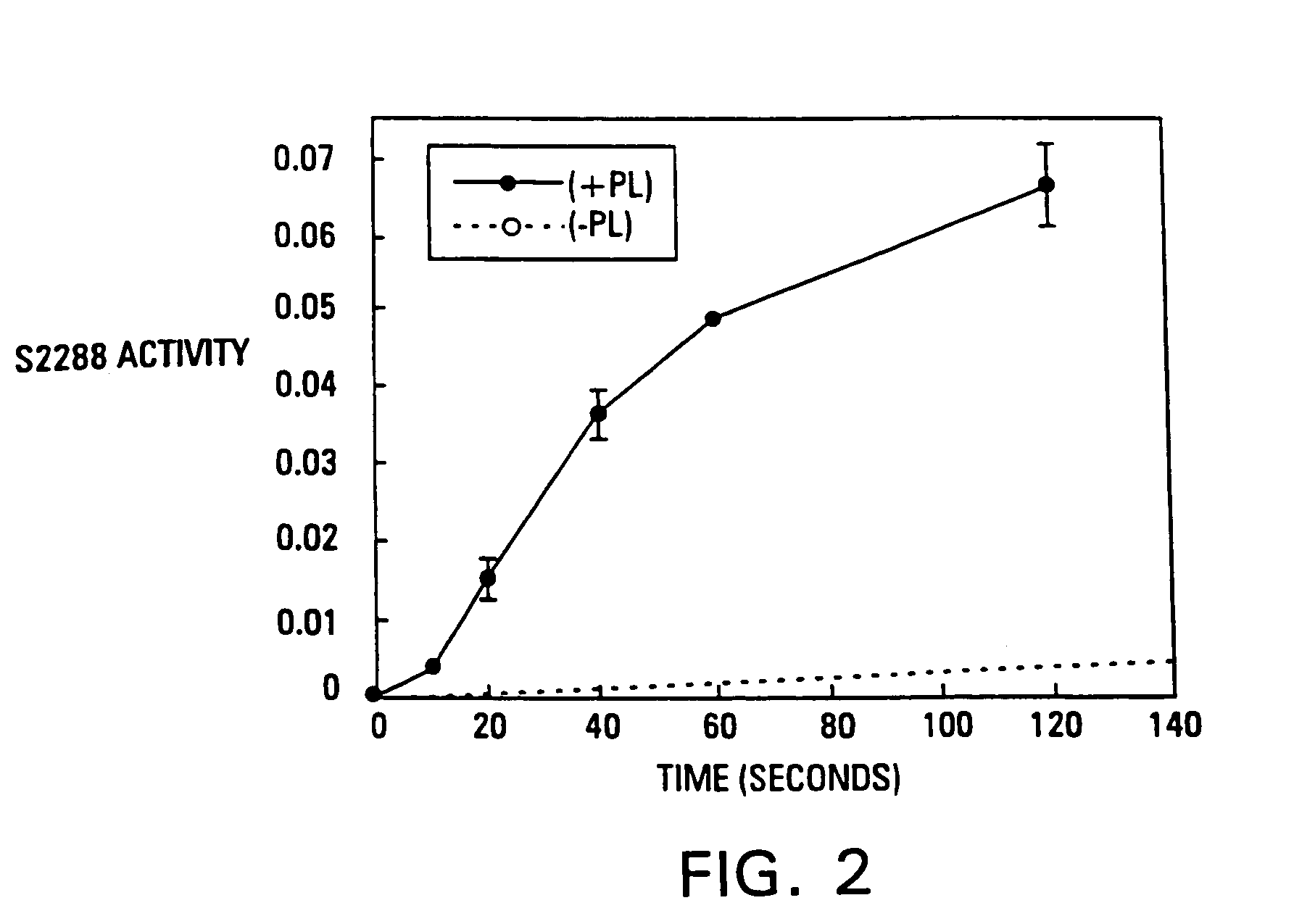
Nmr methods for monitoring blood clot formation
The invention features a method of monitoring a clotting process by measuring a signal characteristic of the NMR relaxation of water in a sample undergoing clotting to produce NMR relaxation data and determining from the NMR relaxation data a magnetic resonance parameter of water in the sample characteristic of the clots being formed.
Owner:T2 BIOSYST
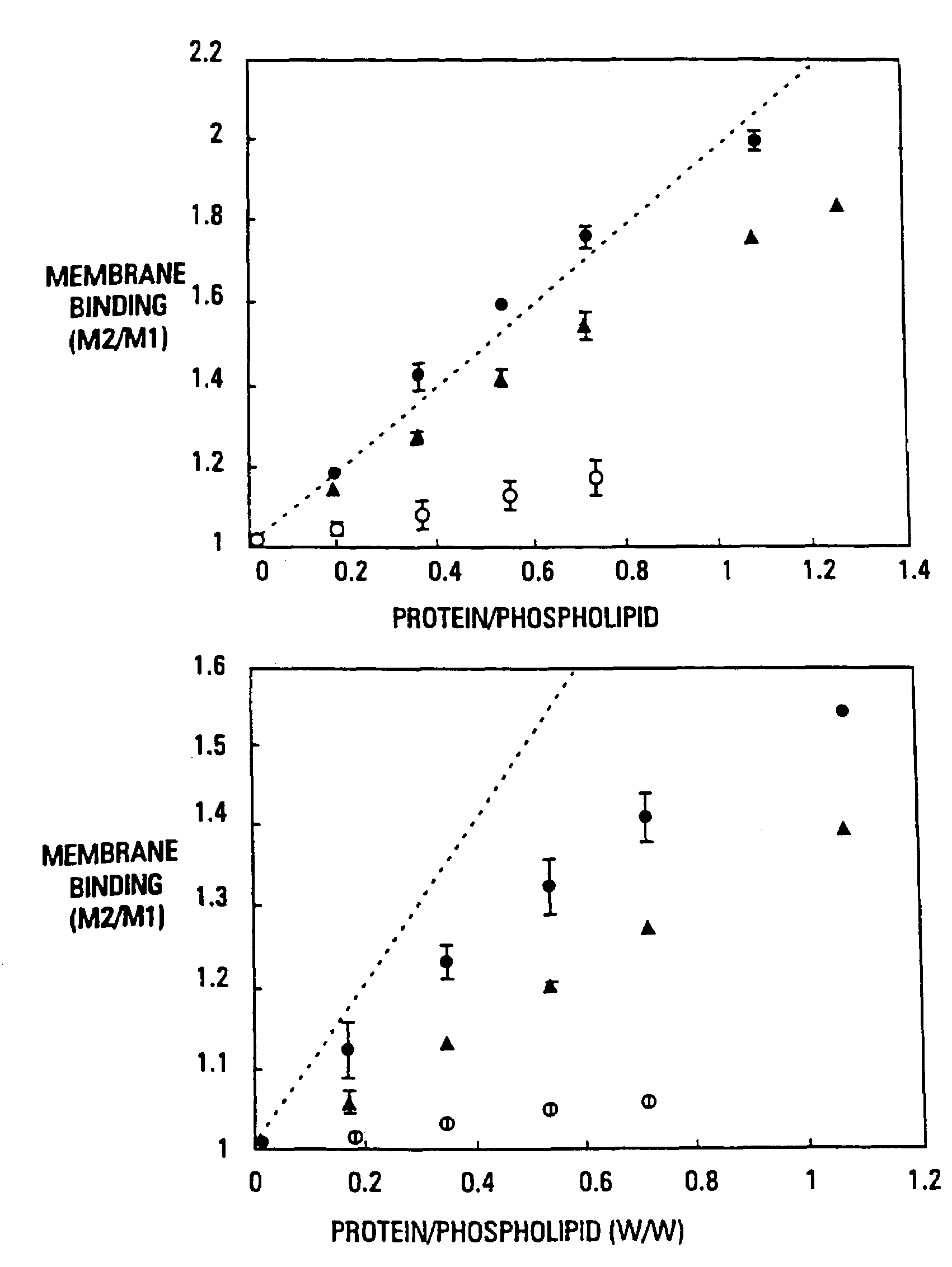
Modified vitamin K-dependent polypeptides
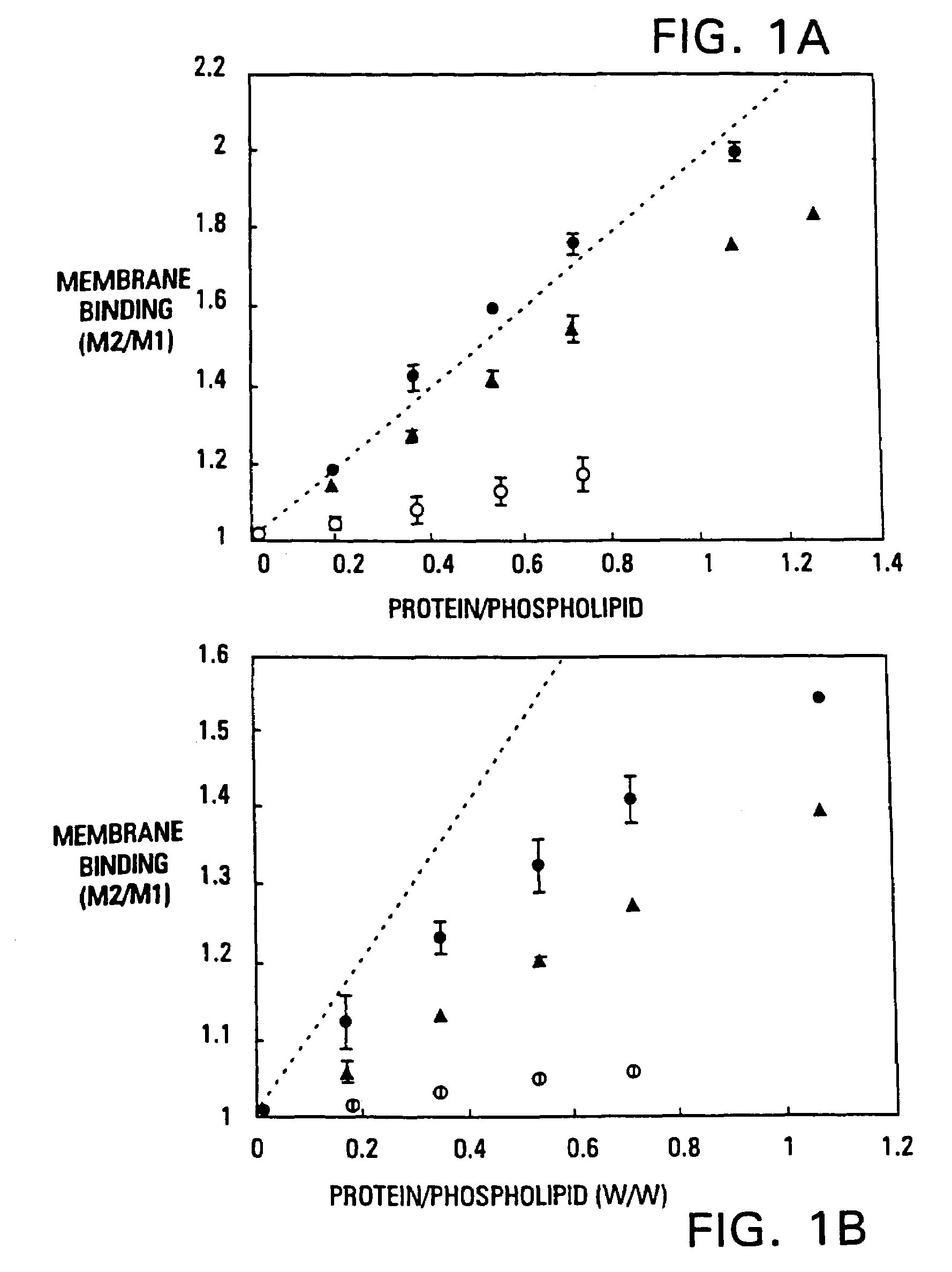
InactiveUS7314917B2High binding affinityHigh activityHydrolasesPeptide/protein ingredientsMammalClot formation
The invention provides vitamin K-dependent polypeptides with enhanced membrane binding affinity. These polypeptides can be used to modulate clot formation in mammals. Methods of modulating clot formation in mammals are also described.
Owner:RGT UNIV OF MINNESOTA
Modulators of the prostacyclin (PGI2) receptor useful for the treatment of disorders related thereto
The present invention relates to amide derivatives of Formula (XIIIa) and pharmaceutical compositions thereof that modulate the activity of the PGI2 receptor. Compounds of the present invention and pharmaceutical compositions thereof are directed to methods useful in the treatment of: pulmonary arterial hypertension (PAH); idiopathic PAH; familial PAH; PAH associated with a collagen vascular disease, a congenital heart disease, portal hypertension, HIV infection, ingestion of a drug or toxin, hereditary hemorrhagic telangiectasia, splenectomy, pulmonary veno-occlusive disease (PVOD) or pulmonary capillary hemangiomatosis (PCH); PAH with significant venous or capillary involvement; platelet aggregation; coronary artery disease; myocardial infarction; transient ischemic attack; angina; stroke; ischemia-reperfusion injury; restenosis; atrial fibrillation; blood clot formation in an angioplasty or coronary bypass surgery individual or in an individual suffering from atrial fibrillation; atherosclerosis; atherothrombosis; asthma or a symptom thereof; a diabetic-related disorder such as diabetic peripheral neuropathy, diabetic nephropathy or diabetic retinopathy; glaucoma or other disease of the eye with abnormal intraocular pressure; hypertension; inflammation; psoriasis; psoriatic arthritis; rheumatoid arthritis; Crohn's disease; transplant rejection; multiple sclerosis; systemic lupus erythematosus (SLE); ulcerative colitis; ischemia-reperfusion injury; restenosis; atherosclerosis; acne; type 1 diabetes; type 2 diabetes; sepsis; and chronic obstructive pulmonary disorder (COPD).
Owner:ARENA PHARMA
Microfluidic flow assay for measuring hemostatic phenotypes
ActiveUS20110223627A1Bioreactor/fermenter combinationsBiological substance pretreatmentsClot formationMicrofluidic channel
A microfluidic-based flow assay and methods of manufacturing the same are provided. Specifically, the microfluidic flow assay includes a micropatterned surface that induces clot formation and an array of microfluidic channels though which blood flows. The micropatterned surface contains two clotting stimuli, one for inducing platelet adhesion and another for inducing the coagulation cascade.
Owner:COLORADO SCHOOL OF MINES
Contrast enhancement agent for magnetic resonance imaging
InactiveUS6972122B2Peptide/protein ingredientsNMR/MRI constrast preparationsWound healingImaging agent
Imaging agents for magnetic resonance imaging are disclosed. Methods of monitoring angiogenesis and the growth and remission of tumor tissue are also disclosed. Methods of monitoring blood clot formation and dissolution are additionally disclosed. Methods of monitoring wound healing are further disclosed. A kit for obtaining an image of a biological structure is further disclosed.
Owner:DUKE UNIV +1
Apparatus for closing vascular puncture
A method and apparatus for closing a vascular wound includes a guidewire and / or other surgical implement extending from the wound. A hemostatic material is advanced over the surgical implement and into contact with an area of the blood vessel surrounding the wound. The surgical implement is removed. Blood soaks the hemostatic material, and blood clotting is facilitated by the hemostatic agent within the material. A sealing layer of adhesive can be applied to the hemostatic material, confining the blood flow to the material. The vascular puncture wound is sealed by natural blood clot formation.
Owner:LOMA LINDA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com