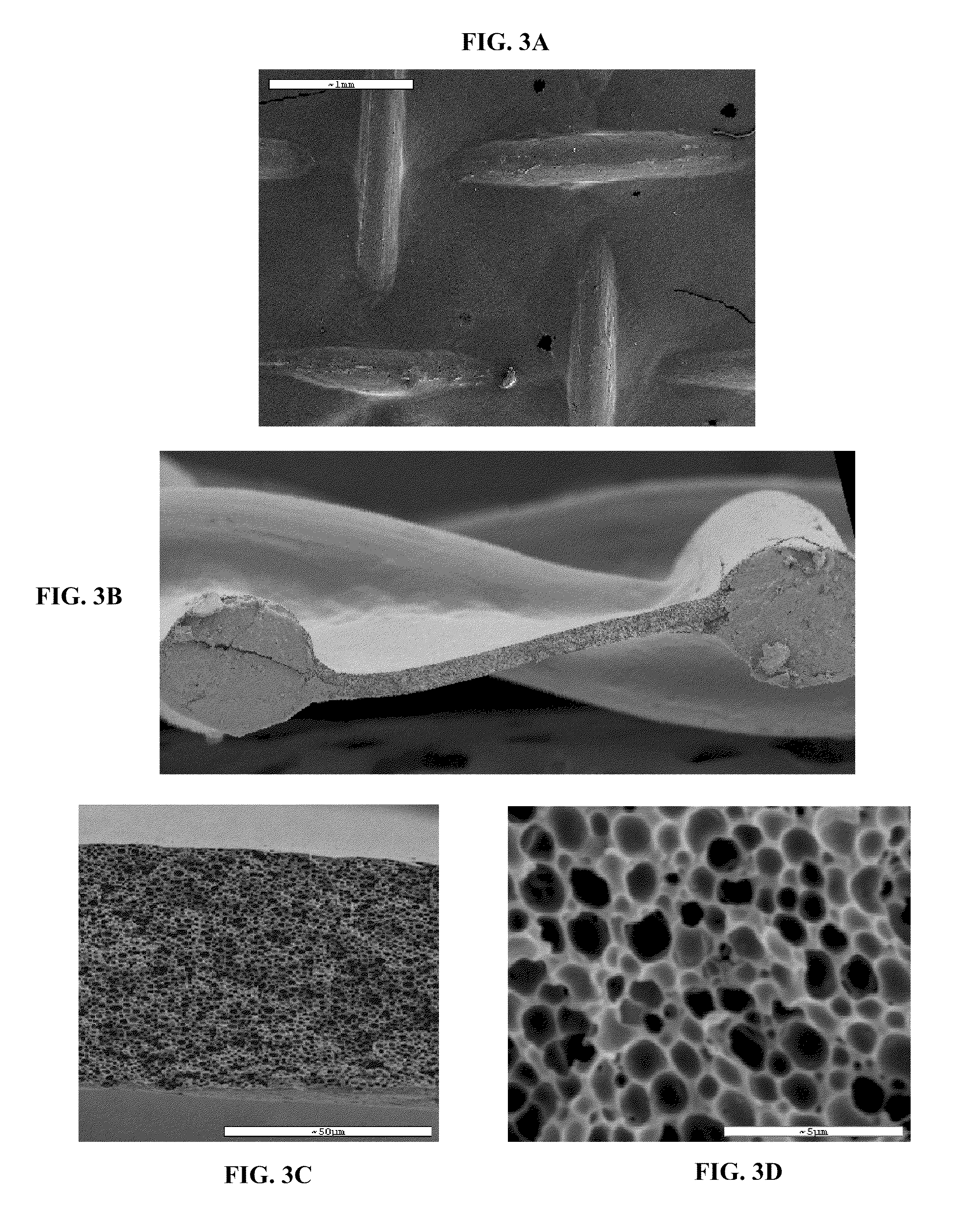
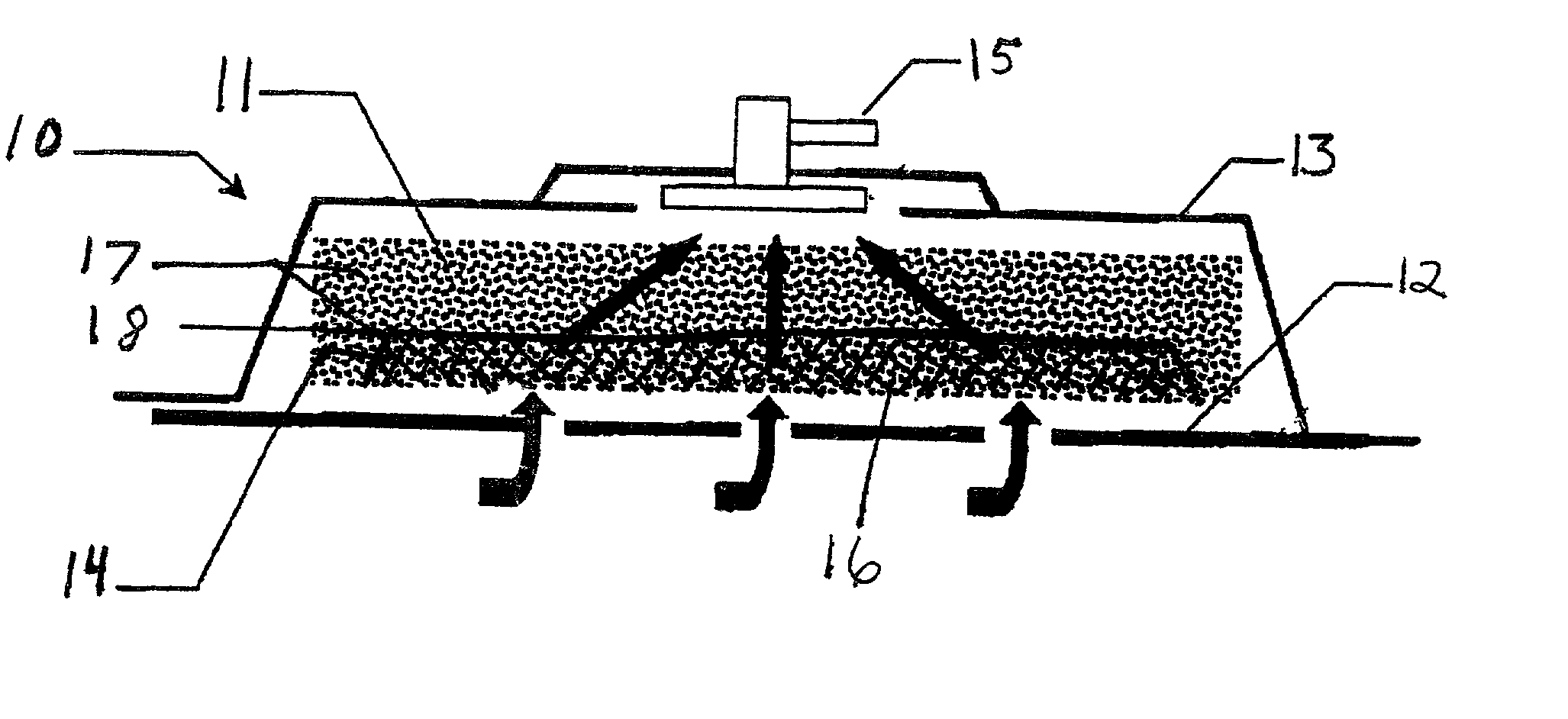
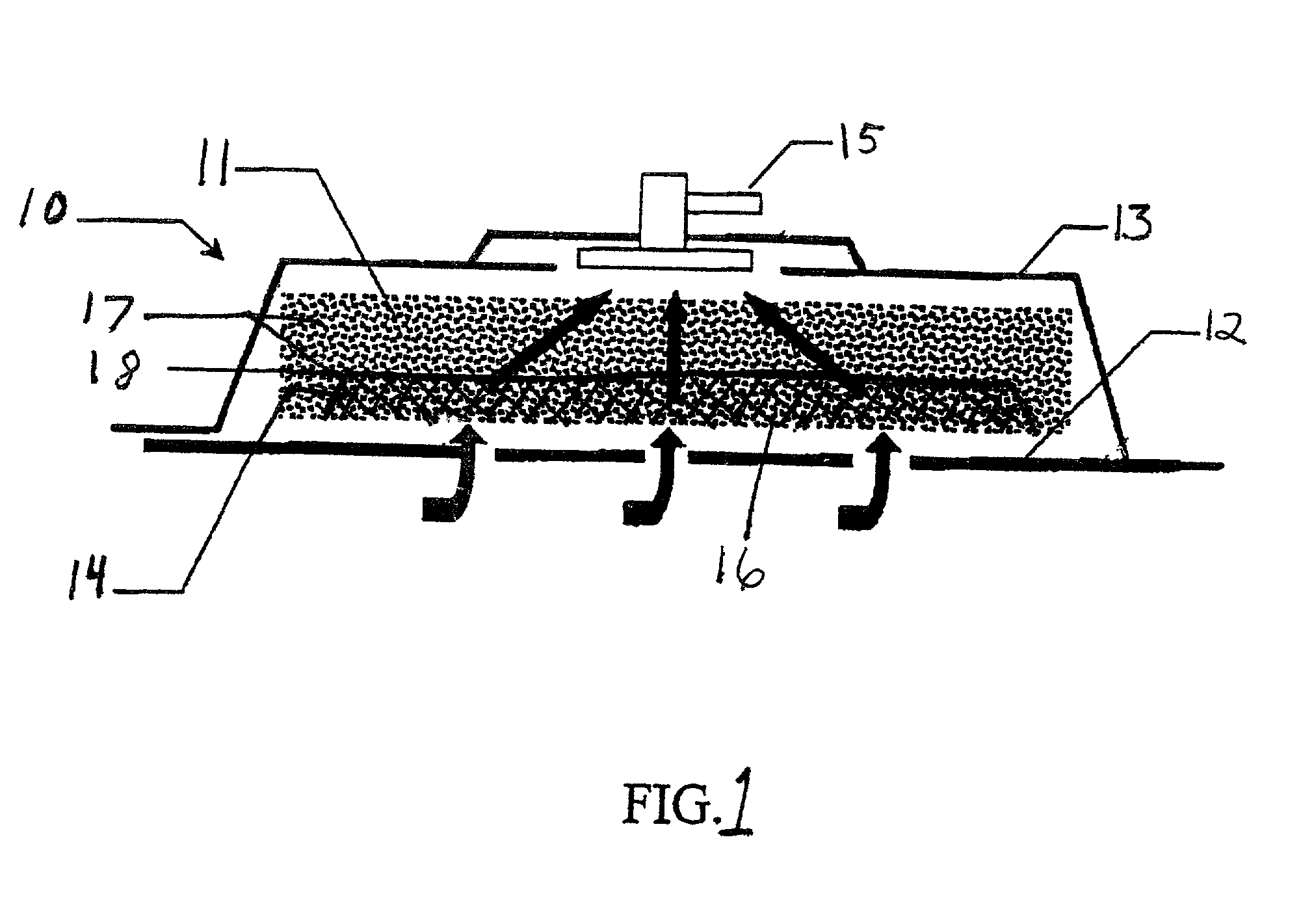
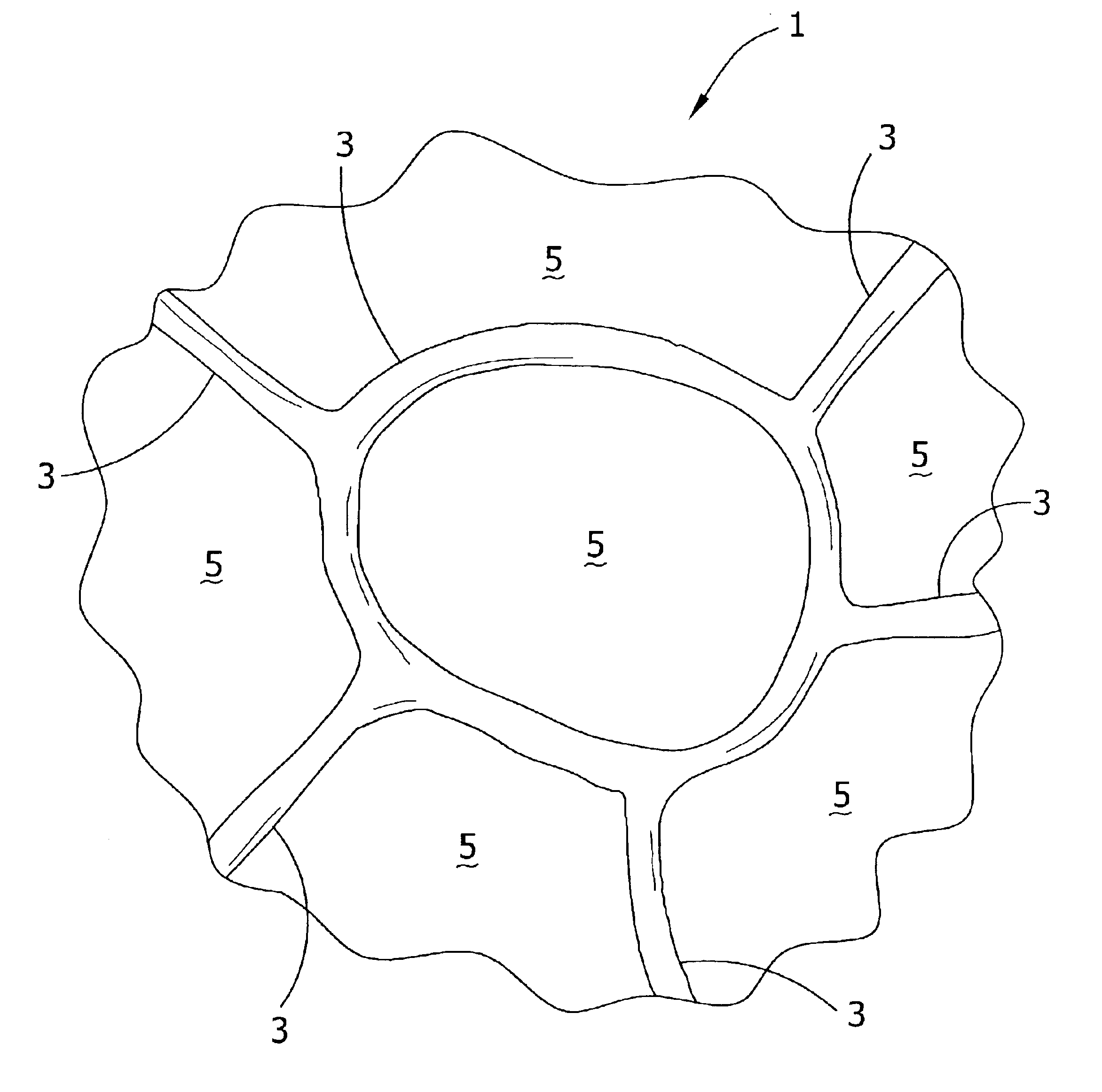
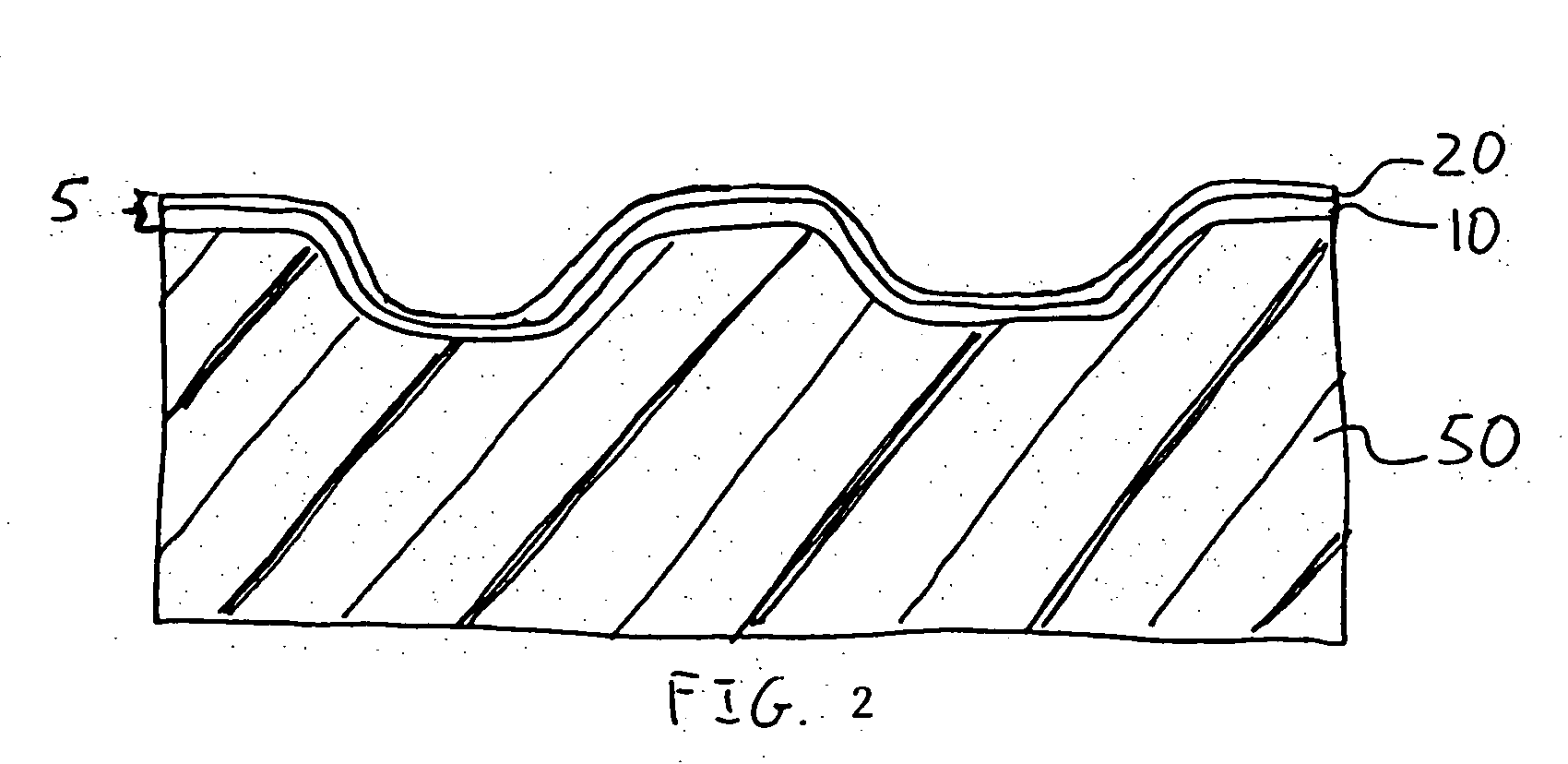
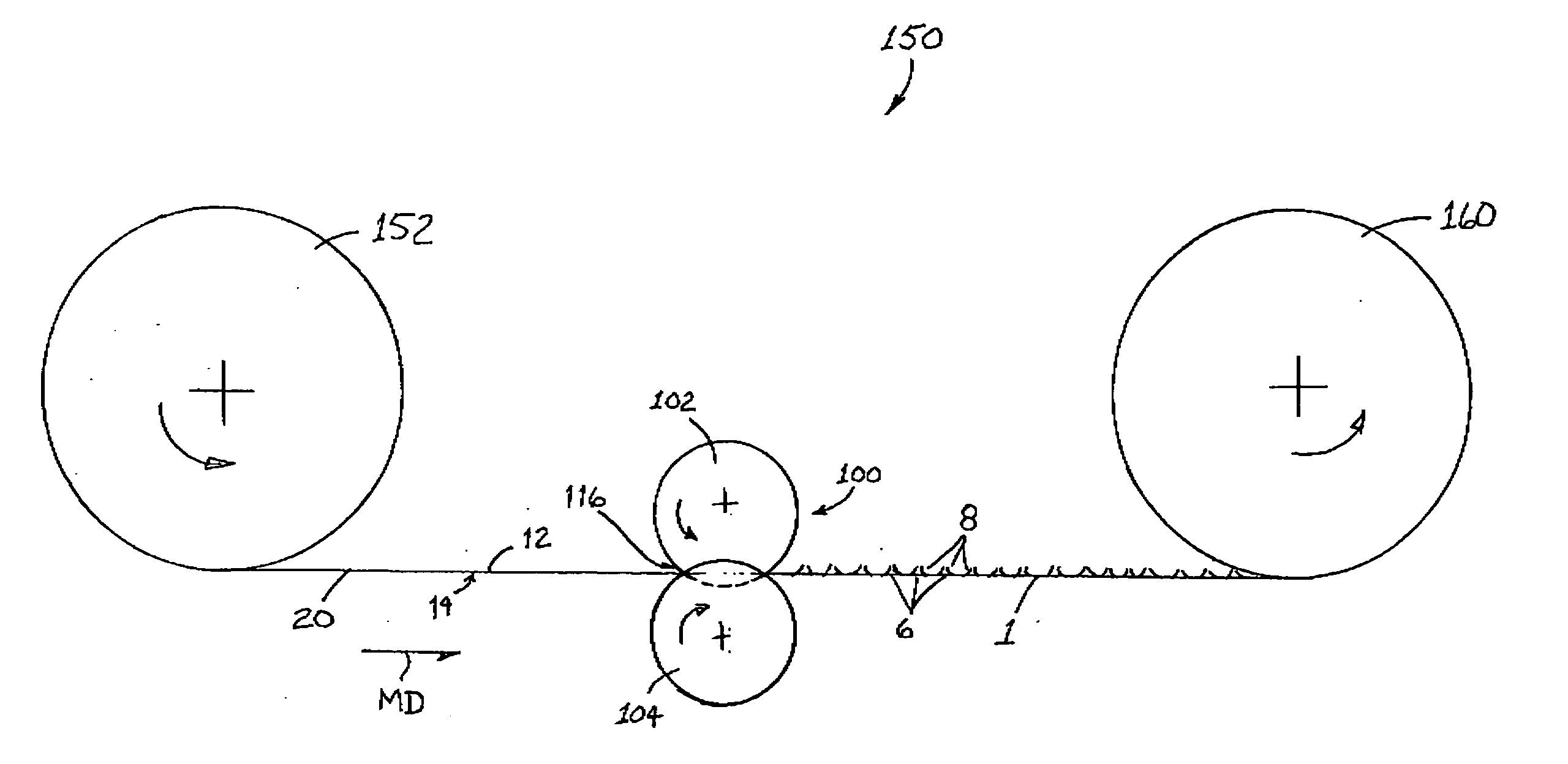
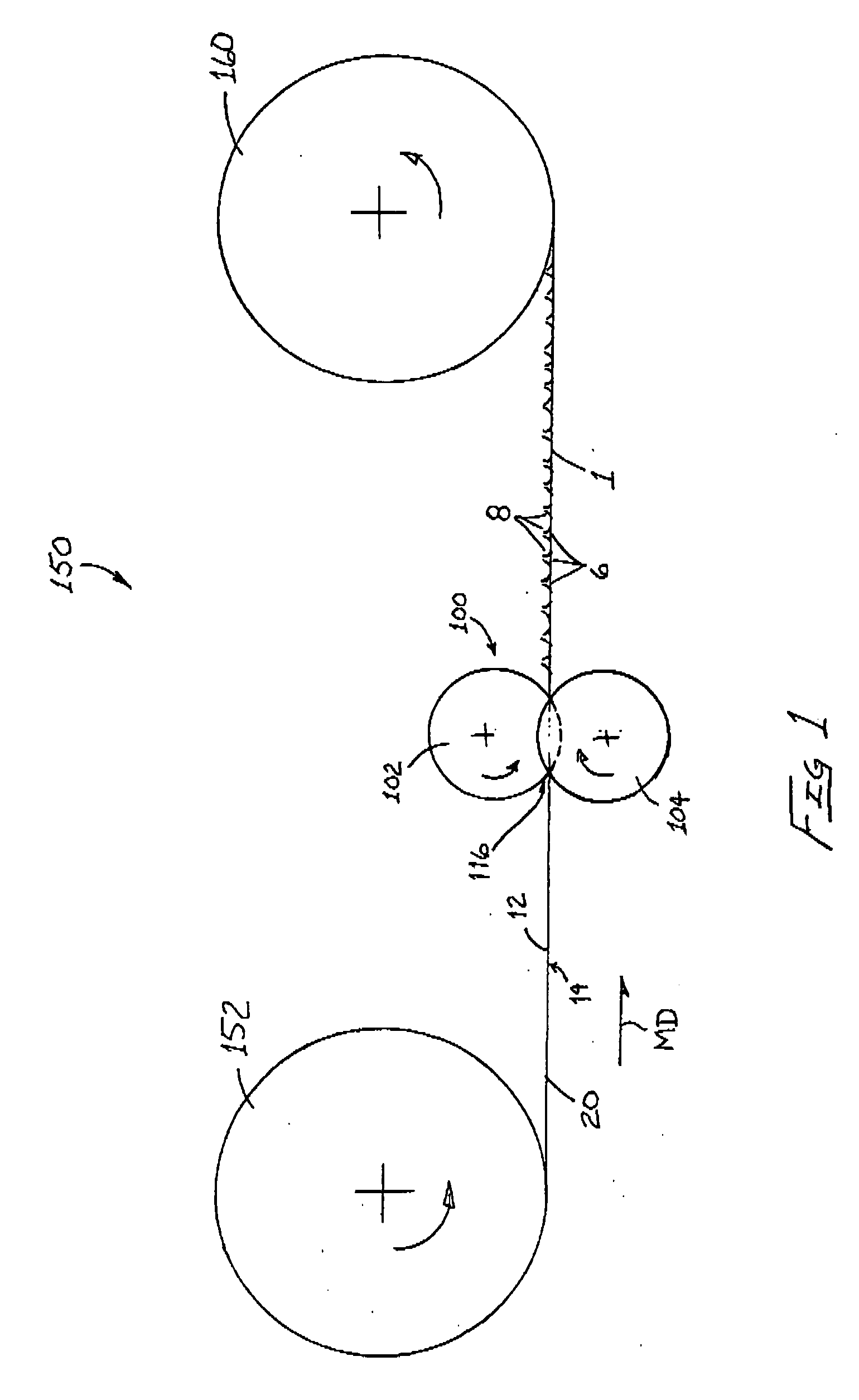
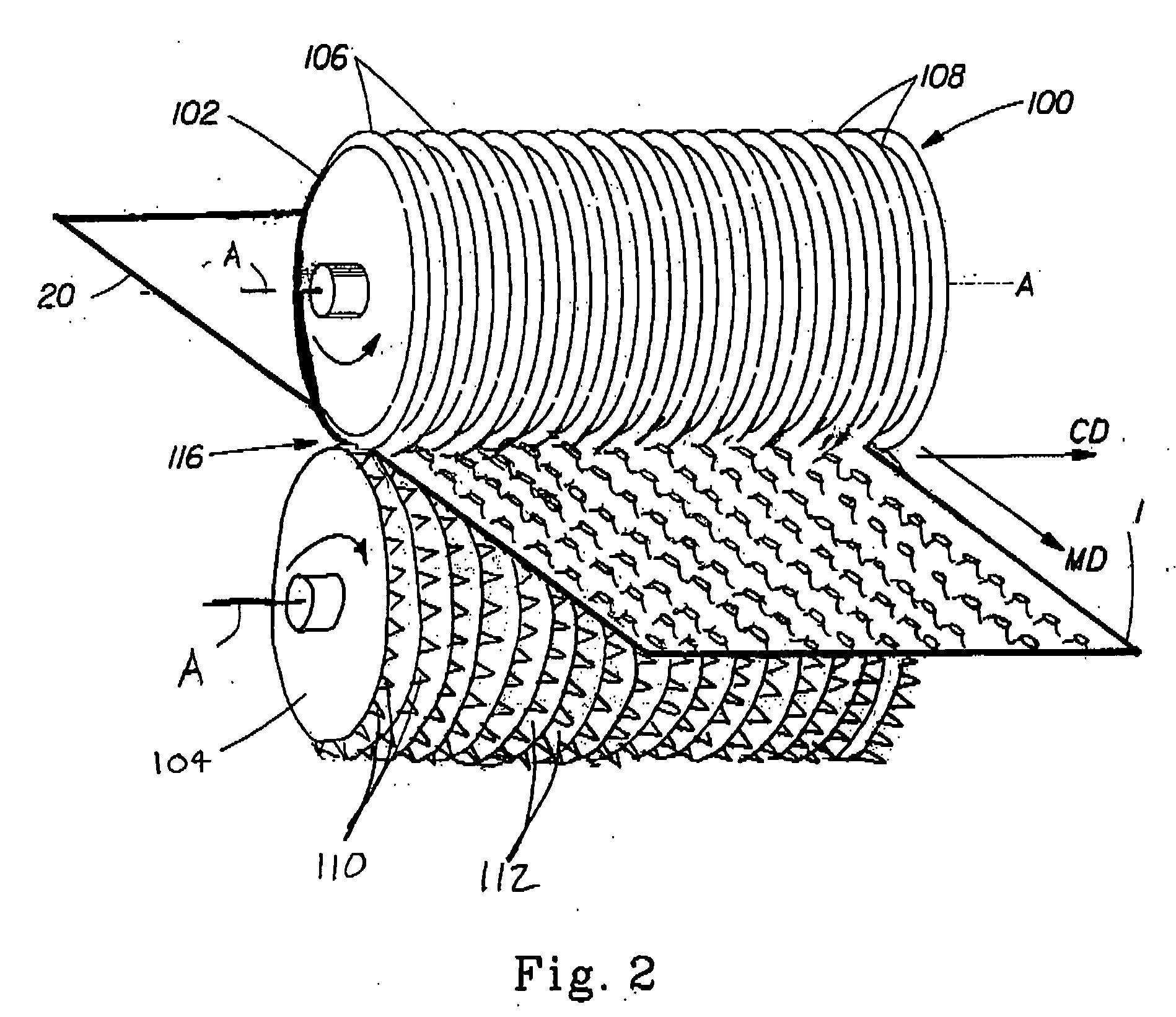
Patents
Literature
24162results about "Absorbent pads" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
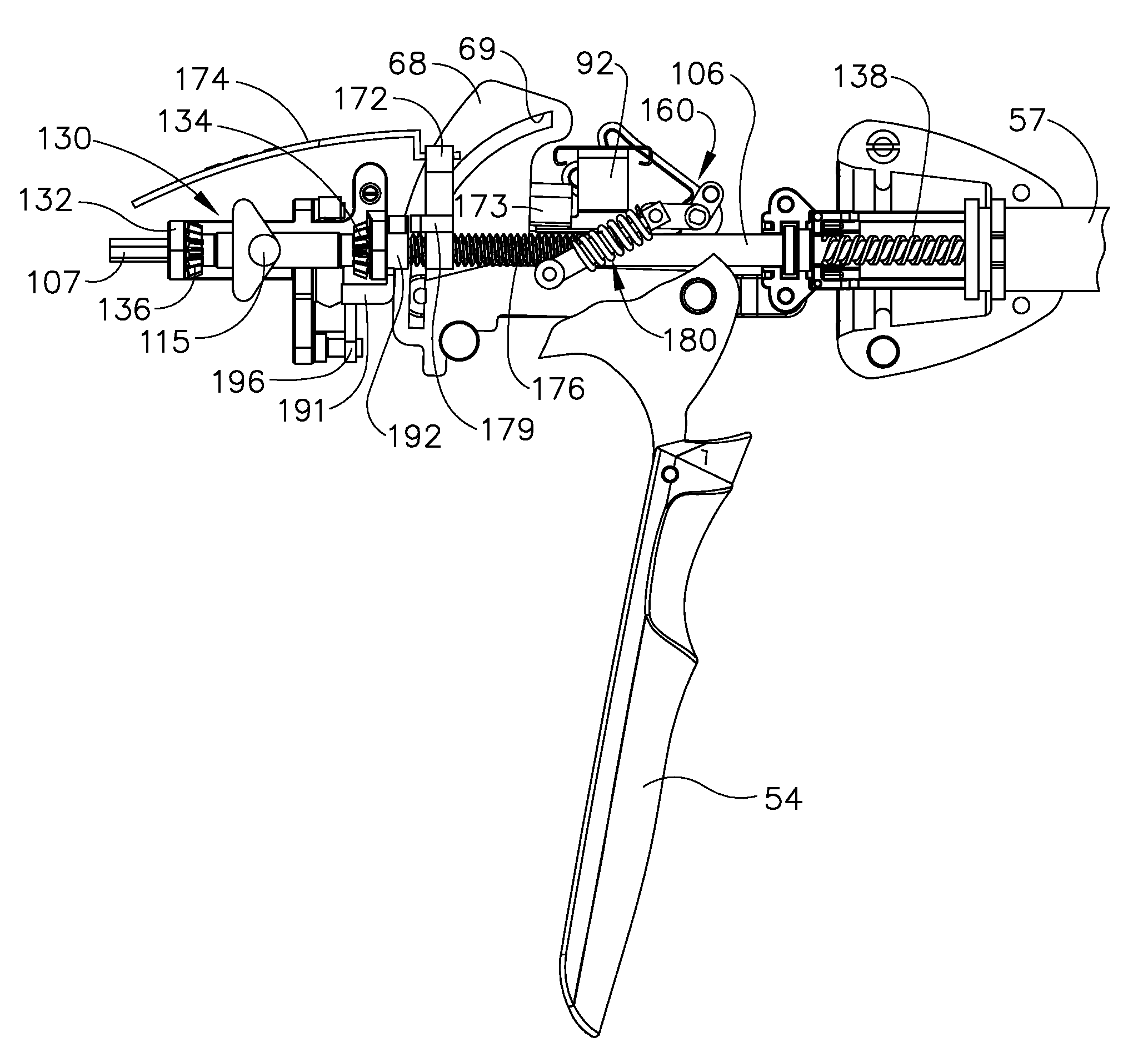
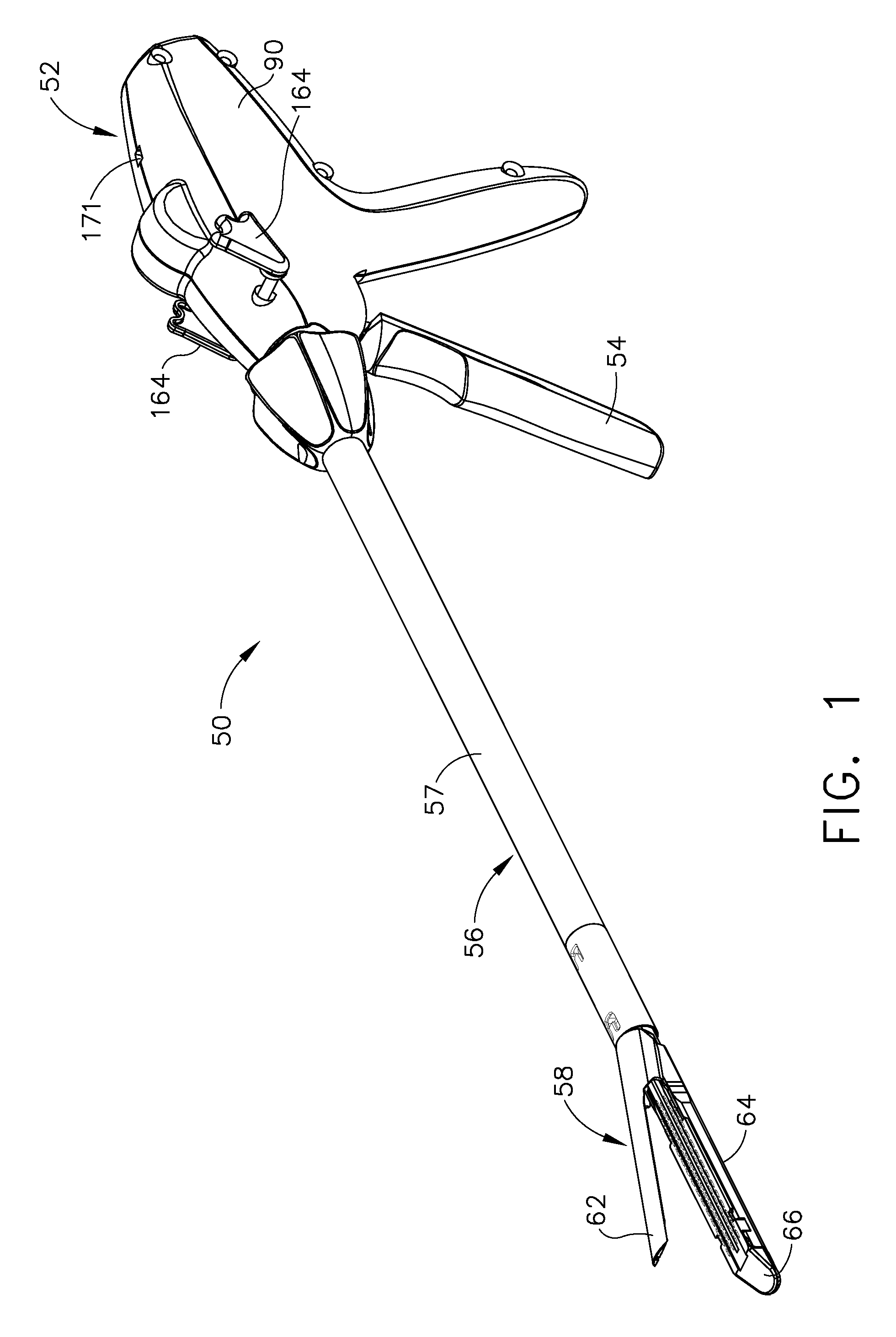
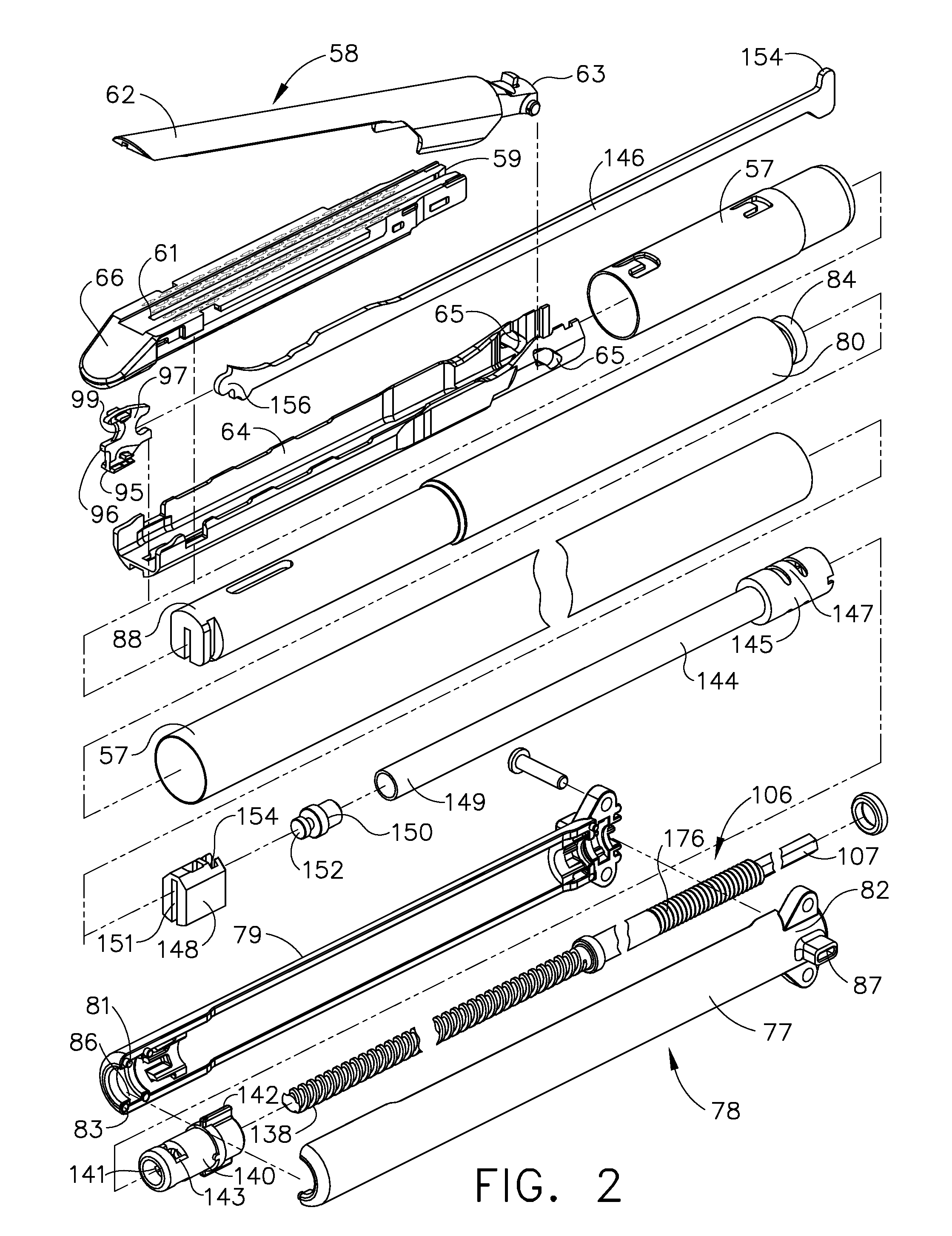
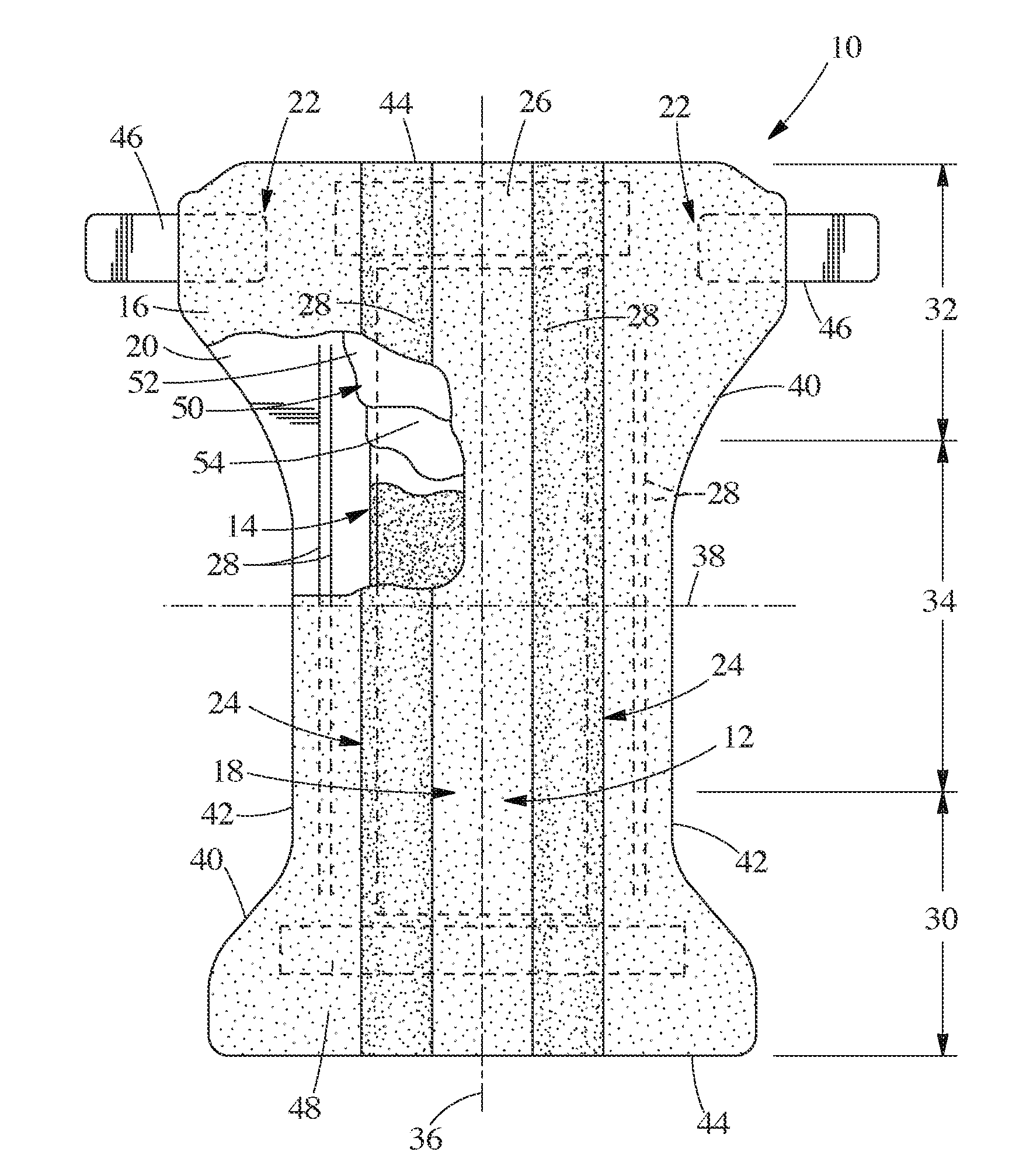
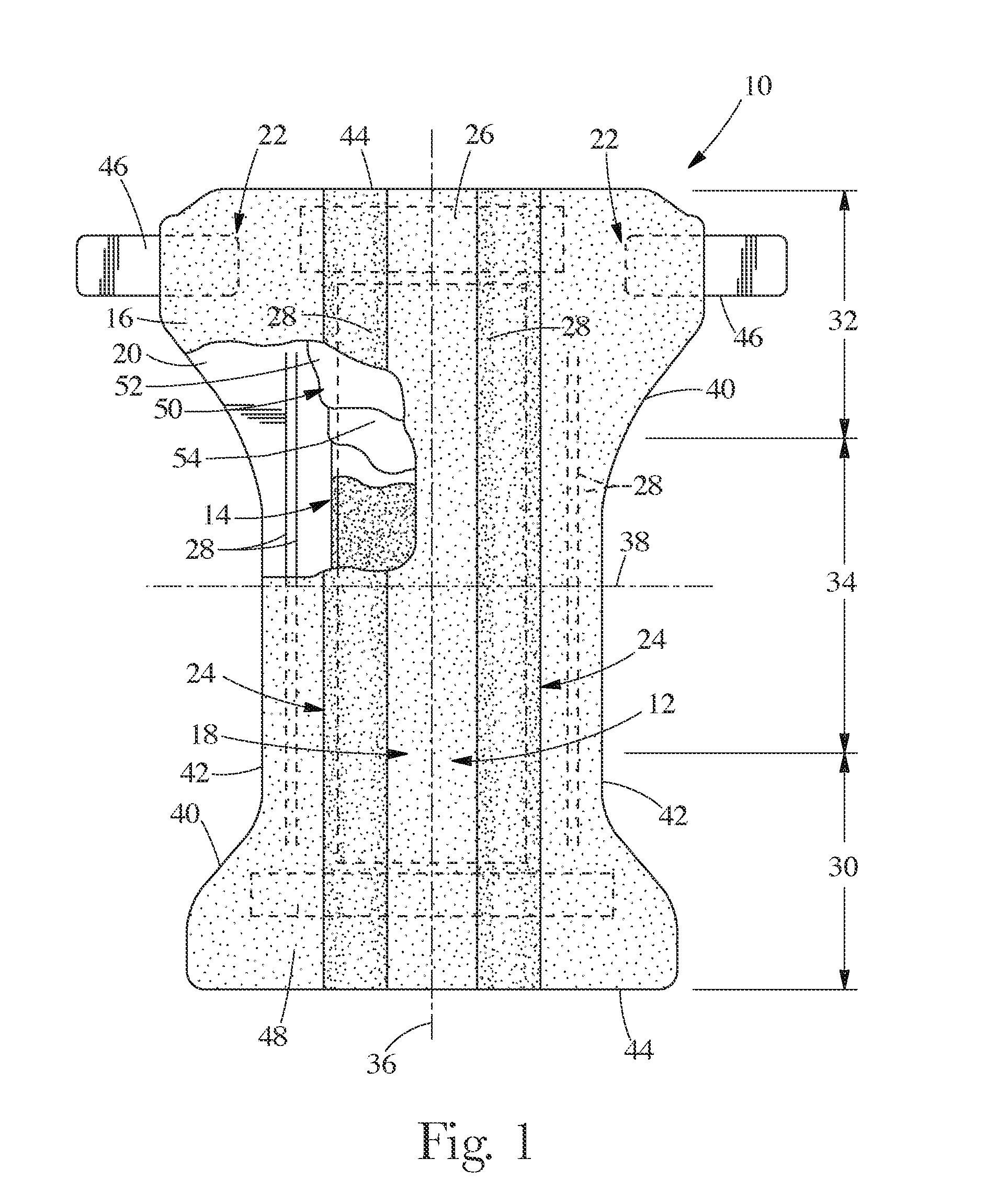
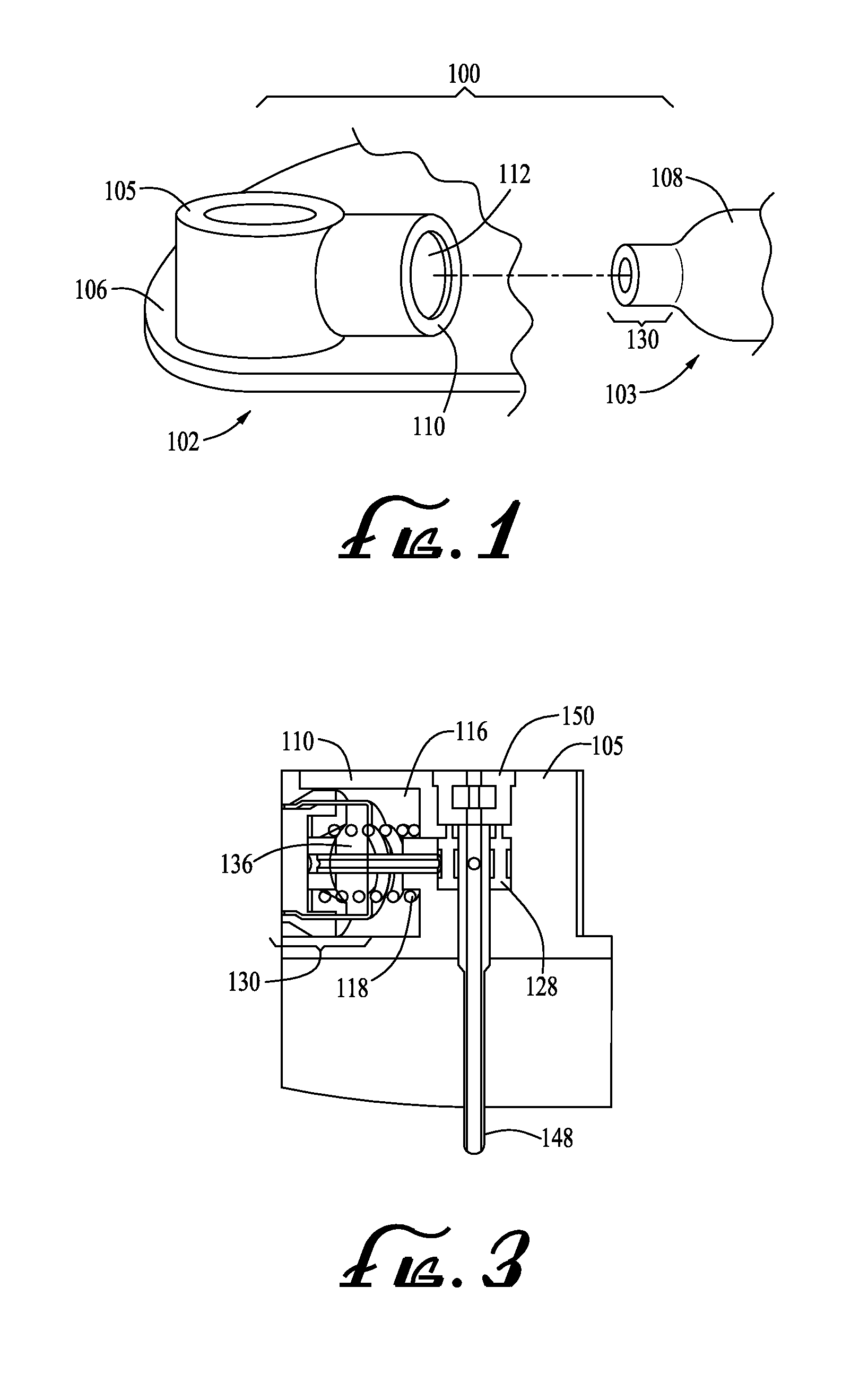
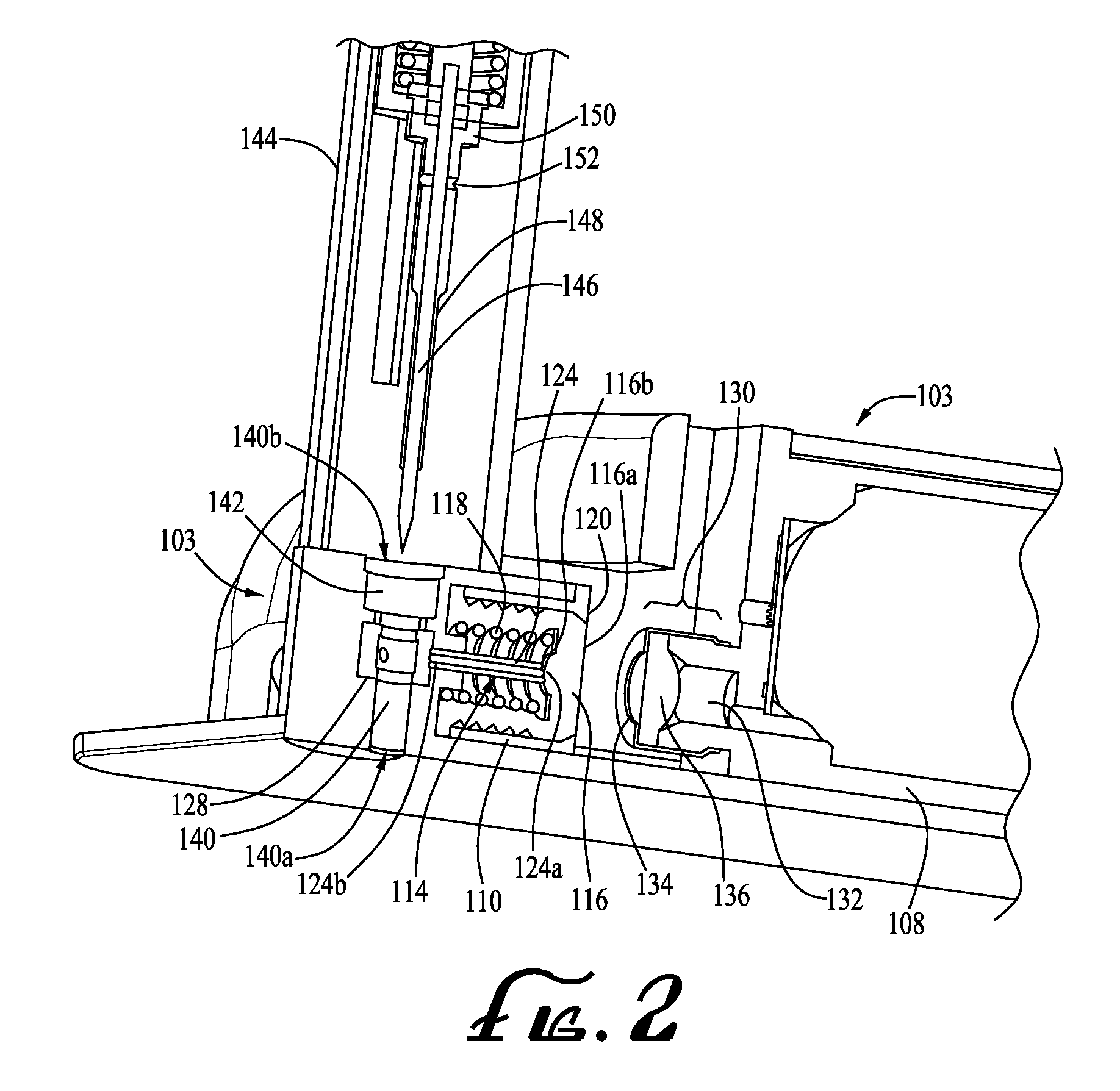
Surgical instrument having a common trigger for actuating an end effector closing system and a staple firing system
ActiveUS7819299B2Quickly and conveniently selectSuture equipmentsStapling toolsSurgical operationSurgical site
A surgical instrument including a trigger which can be configured to close a jaw member upon a first actuation of the trigger and advance a staple driver and / or cutting member upon a subsequent, or second, actuation of the trigger. Such a surgical instrument can allow a surgeon to position the surgical instrument in a surgical site and close the jaw member with an initial actuation of the trigger without deploying any staples into, or incising, the tissue. As a result, the surgeon can manipulate the position of the surgical instrument and then actuate the trigger a second time to deploy staples into, and / or incise, the tissue. In at least one such embodiment, the first actuation of the trigger which closes the jaw member can also unlock a firing drive configured to advance the staple driver and cutting member during the second actuation of the trigger.
Owner:CILAG GMBH INT
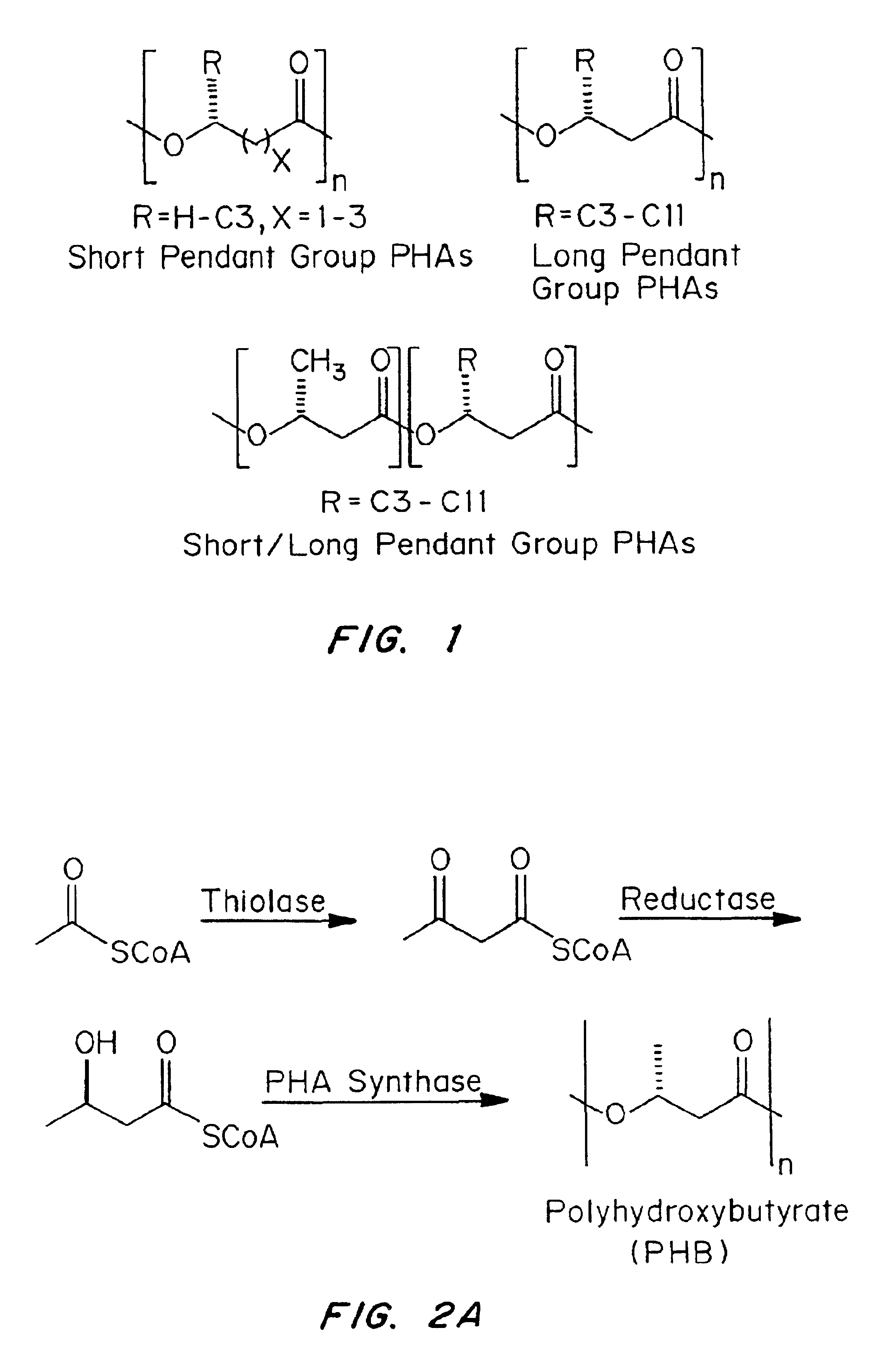
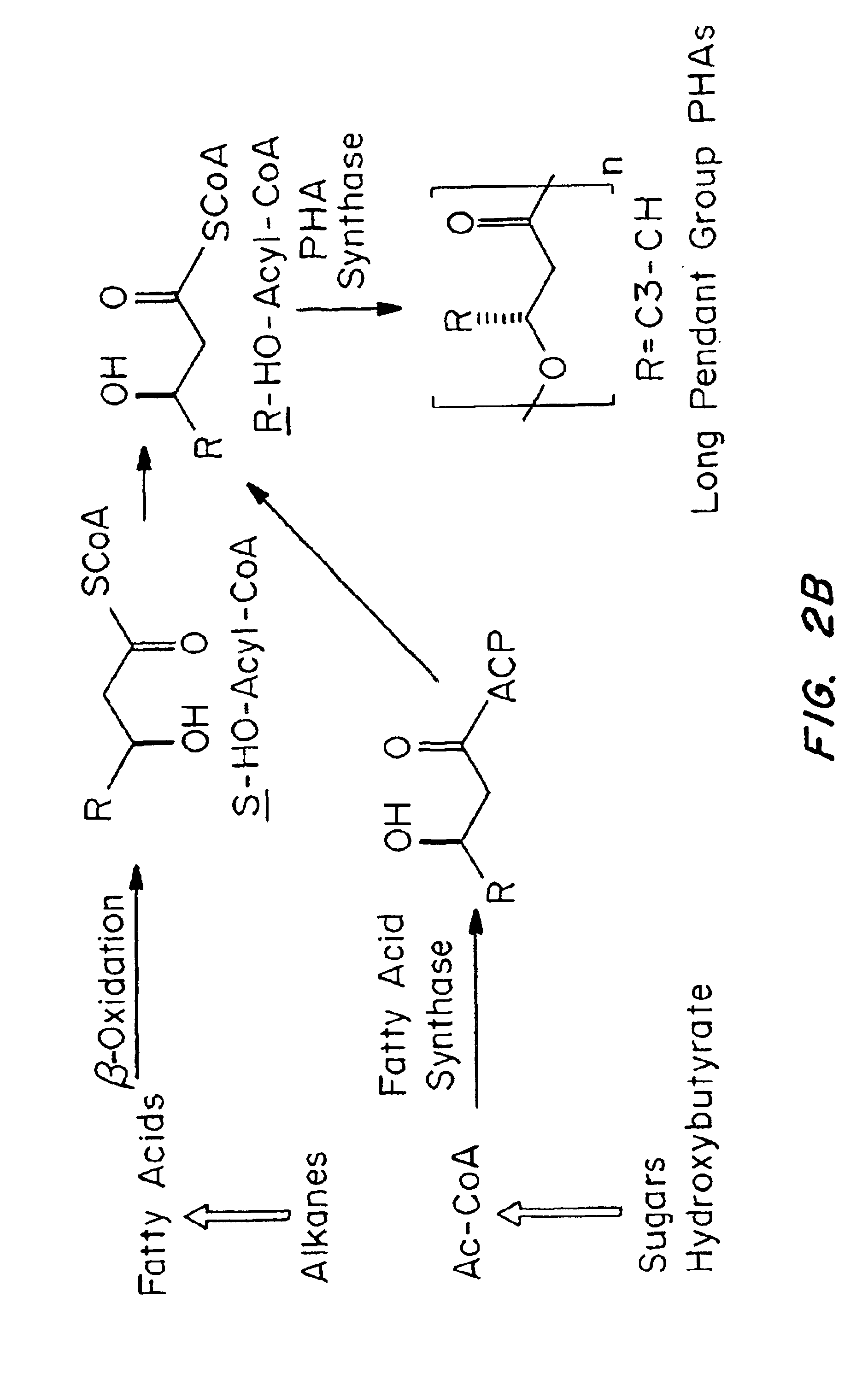
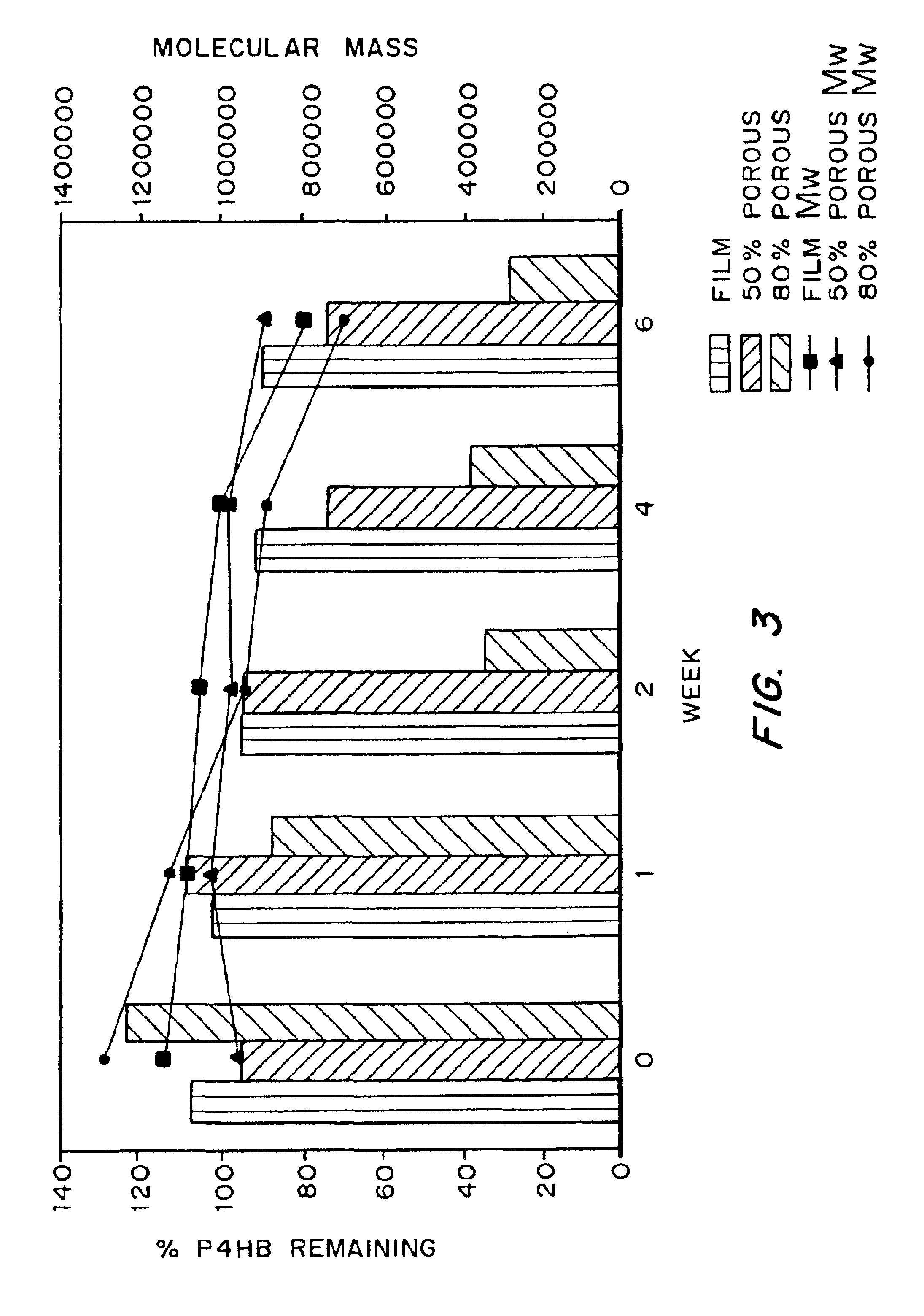
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Bioabsorbable wound dressing
ActiveUS7041868B2Promote wound healingEasy to useAdhesive dressingsNon-surgical orthopedic devicesCell adhesionAdhesion process
A wound dressing includes a first layer located adjacent the wound and which comprises a material that is bioabsorbable, porous and adapted for serving as a scaffold for cell attachment and proliferation; and a second layer which is in contact with the first layer and which comprises an absorbent, gel forming material adapted for serving as a barrier to cell adhesion and penetration. A method of treating a wound with the dressing is also disclosed.
Owner:AVENT INC
Dissolvable medical sealing device
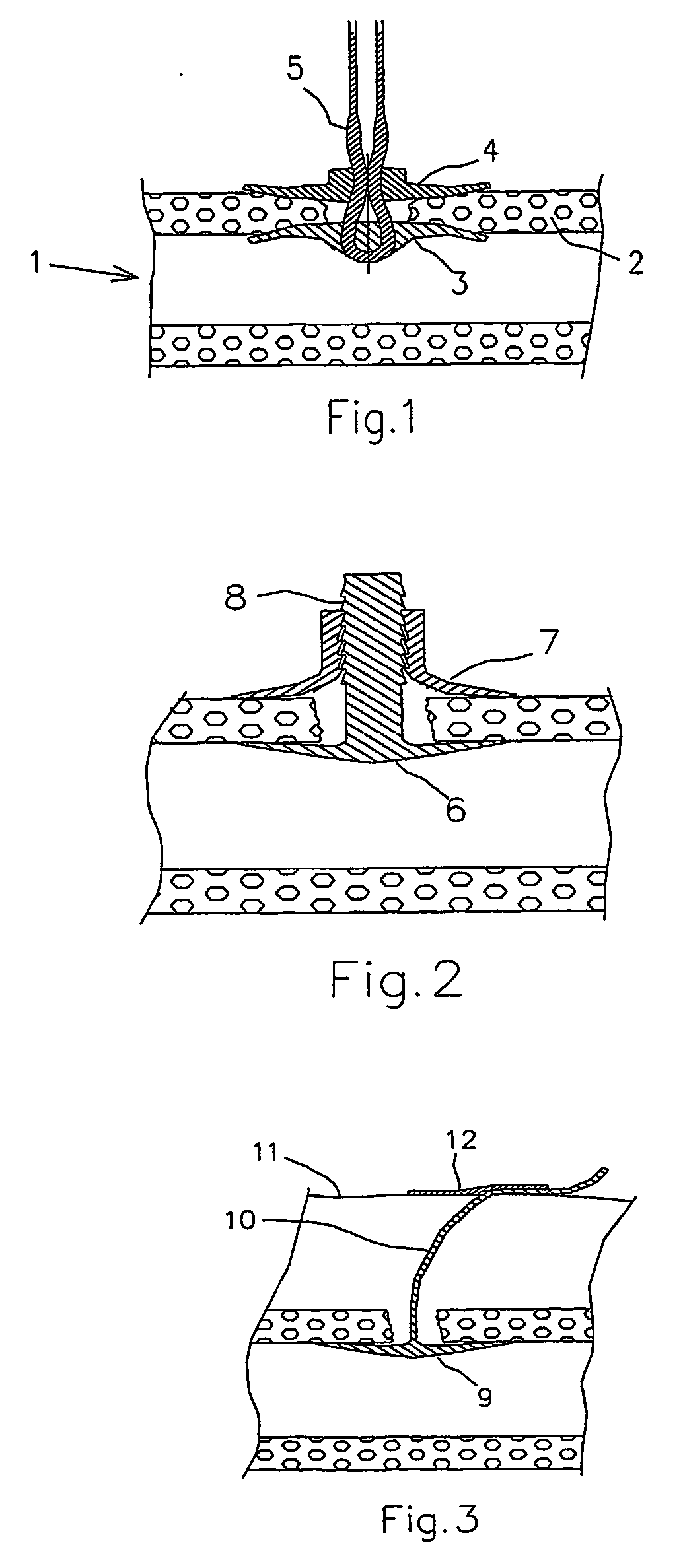
ActiveUS20050169974A1Shorten the timeSuture equipmentsPharmaceutical delivery mechanismOrganismal ProcessPolyvinyl alcohol
The present invention provides a dissolvable medical sealing device (3, 4; 6, 7; 9) for closing a wound in vessel. A sealing device (3, 4, 6, 7, 9) according to the invention is made of a material that dissolves by means of physical processes, rather than by means of chemical or biological processes. Such a sealing device (3, 4; 6, 7; 9) can be made of polyethylene glycol, polypropylene glycol, copolymers containing ethylene glycol and propylene glycol, polyvinyl alcohol or polyvinyl pyrolidone, or any combinations thereof.
Owner:TERUMO MEDICAL CORP
Method for preparing two-layer bicomposite collagen material for preventing post-operative adhesions
InactiveUS6596304B1Improve propertiesAvoid stickingPeptide/protein ingredientsSurgerySurgical operationPost operative
A bicomposite material based on collagen is prepared which has two closely bound layers and is biocompatible, non-toxic, hemostatic and biodegradable in less than a month, and can be used in surgery to achieve hemostasis and prevent post-surgical adhesion. To prepare the material, a solution of collagen or gelatin, which may contain glycerine and a hydrophilic additive such as polyethylene glycol or a polysaccharide, is poured onto an inert support to form a layer 30 .mu.m to less than 100 .mu.m thick. Then a polymeric porous fibrous layer is applied during gelling of the collagen or gelatin, and the resultant material is dried. The polymeric porous fibrous layer may be made of collagen or a polysaccharide, and have a density of not more than 75 mg / cm.sup.2, a pore size from 30 .mu.m to 300 .mu.m and a thickness of 0.2 cm to 1.5 cm.
Owner:IMEDEX BIOMATERIAUX CHAPONOST
Agents and devices for providing blood clotting functions to wounds
InactiveUS20070190110A1Promote healingShorten the timePhysical treatmentAntithrombogenic treatmentNitrogen dioxideMedicine
Hemostatic agents and devices are made from oxidized cellulose fiber, the oxidized cellulose having a carboxylation content increased by the action of nitrogen dioxide on virgin cellulose fiber. A composition may be incorporated into the oxidized cellulose fiber to cause a pharmacological effect on a wound to which the hemostatic agents and devices are applied. When applied, the oxidized cellulose fiber causes blood emanating from the wound to clot. The oxidized cellulose fiber can either be resorbed into the wound or removed from the wound after healing. A hemostatic bandage includes a pad of unwoven oxidized cellulose fibers mounted on a substrate. Methods of arresting a flow of blood emanating from a wound using such devices are also disclosed. Methods of fabricating oxidized cellulose are also disclosed.
Owner:PAMEIJER CORNELIS H +1
Hemostatic compositions for arresting blood flow from an open wound or surgical site
A hemostatic composition for stopping or decreasing blood flow from an open wound or medical or surgical procedure. Compositions of the invention comprise a mixture of a cationic polymer and a cation exchange material. In one embodiment, the composition comprises a mixture: (1) a high molecular weight copolymer of diallyl dimethyl ammonium chloride (DADMAC) and acrylamide [DADMAC copolymer], and (2) the hydrogen form of a crosslinked, sulfonated polystyrene (hydrogen resin). In an exemplified embodiment, a composition of the invention comprises the mixture of DADMAC copolymer and hydrogen resin provided in a dry powdered form. The compositions of the invention may be applied directly to a wound or treatment site, or they may be incorporated into a wound dressing, such as a bandage. The seal formed at a wound or treatment site treated with the present invention is adhesive and exhibits considerable toughness.
Owner:BIOLIFE
Hemostatic fibrous material
InactiveUS20120004636A1Promote blood clottingPromoting blood clottingBiocideDiagnosticsFiberMolecular materials
Owner:TELEFLEX LIFE SCI LTD
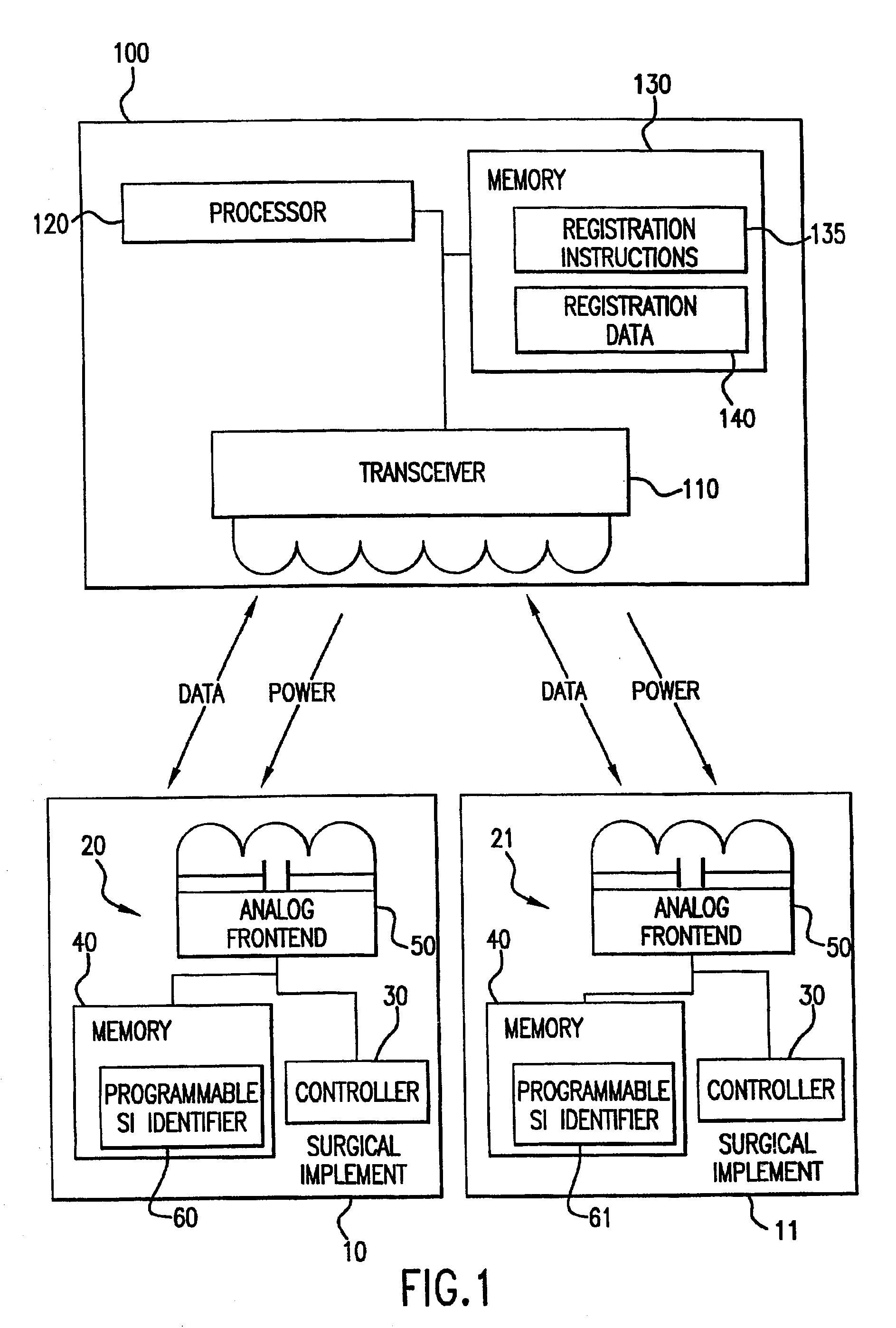
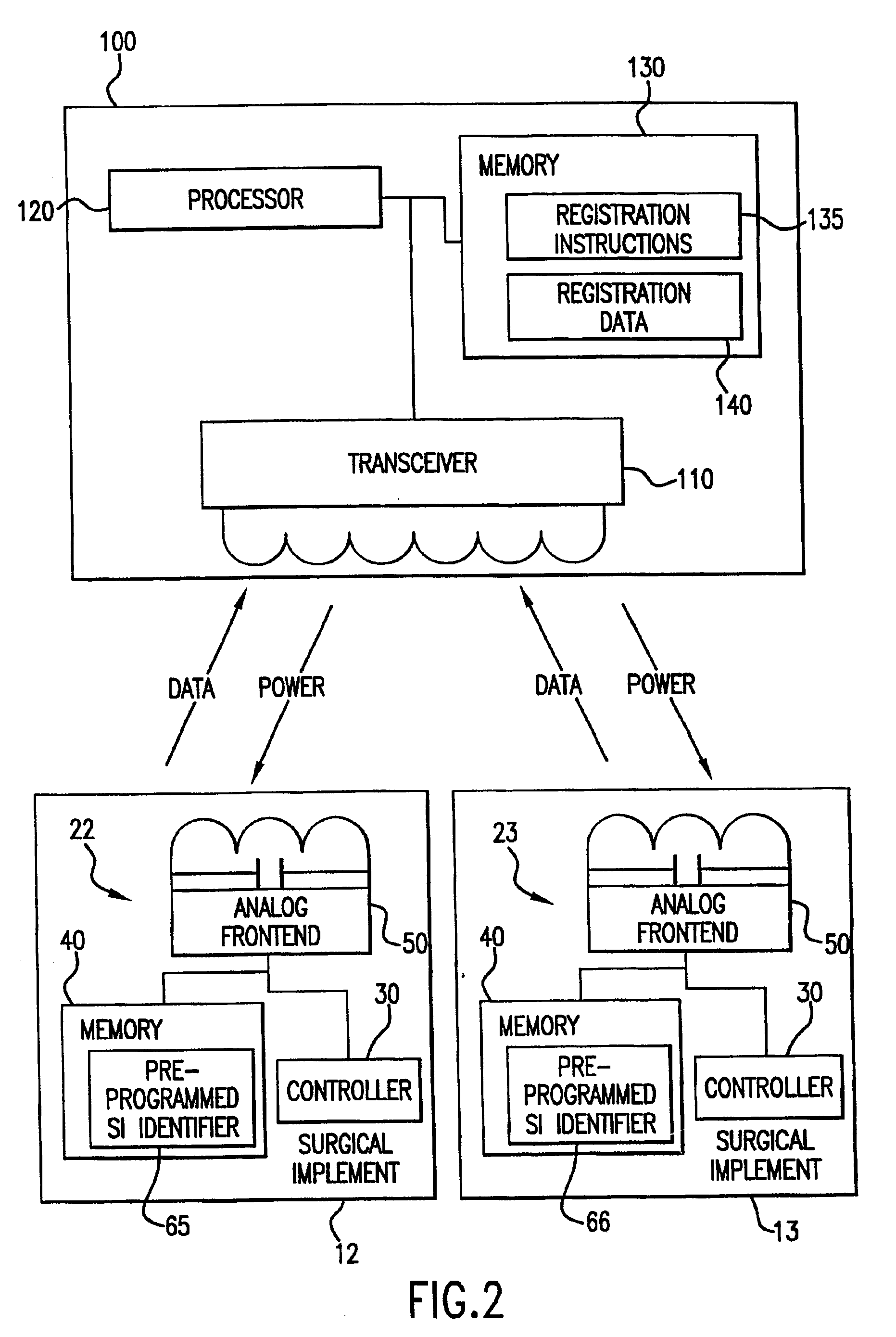
Tracking medical products with integrated circuits
A system and method of tracking medical products provides for associating a group of medical products with a group location based on a group radio frequency identification (RF ID) device signal, where the group includes a first unit and a second unit. The first unit is associated with a first remote location based on a first unit RF ID device signal. The method further provides for associating the second unit with a second remote location based on a second remote location based on a second unit RF ID device signal. The signals uniquely identify the units and the group.
Owner:OLIVE SHADE LLC
Absorbent articles comprising nanoparticles
Owner:NANO MET ZERO
Cartridge shipping aid
Owner:TYCO HEALTHCARE GRP LP
Drug-eluting medical devices
Owner:ZILBERMAN MEITAL
Apparatus and method for steam reprocessing flexible endoscopes
A system for reprocessing flexible endoscopes having lumen therein. The reprocessing system deploys steam to disinfect and / or sterilize the endoscopes, and designs, components, and methods for reducing or balancing the reprocessing cycle time and the effects of thermal expansion and contraction on the endoscopes.
Owner:MEDIVATORS INC
Electronic discount couponing method and apparatus for generating an electronic list of coupons
InactiveUS6035280AReduce the possibilityEliminate needPublic buildingsDigital computer detailsGraphicsGraphical user interface
A method and apparatus for distributing, generating, and redeeming discount Virtual Coupons TM , rebate or gift certificates or the like which may be used on conjunction with a frequency card program or the like. Virtual Coupons TM may be distributed electronically, for example, in the form of a diskette or CD-ROM software. Software on the diskette or CD-ROM may prompt a consumer to call a 1-800 number for a validation number or code. During the phone call, telemarketing personnel may request consumer demographic and or identification information which may be entered into a centralized database. Once the software is validated, a consumer may print out a list selected Virtual Coupons TM displayed on a Graphical User Interface (GUI). When a product is purchased, the UPC code of the product may be compared electronically with a list of Virtual Coupons TM authorized for a particular consumer. An appropriate coupon discount may then be applied and the Virtual Coupon TM may be considered "redeemed". Once redeemed, consumer ID information and Virtual Coupon TM information may be retrieved electronically and used to update a central database. Accurate data may then be produced illustrating which consumers or groups of consumers are redeeming which Virtual Coupons TM . Such data may be used for marketing purposes or to generated further diskettes for distribution targeting specific consumers or groups of consumers with specific classes of Virtual Coupon TM offerings. The use of Virtual Coupons TM eliminates or reduces fraud, and allows a frequency card discount to be applied only a limited number of times.
Owner:CATALINA MARKETING CORP
Drug-eluting medical devices
Composite structures composed of a device as a core structure, being a medical device or article, and a porous polymeric coat and designed capable of encapsulating bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures.
Owner:RAMOT AT TEL AVIV UNIV LTD
Biocompatible wound dressing
InactiveUS7070584B2Promote cell growthPrevent vacuum leakageWound drainsMedical applicatorsWound dressingWound site
A biocompatible wound dressing comprised of a pad for insertion substantially into a wound site and a wound drape for sealing enclosure of the foam pad at the wound site. The pad, comprised of a foam or other like material having relatively few open cells in contact with the areas upon which cell growth is to be encouraged so as to avoid unwanted adhesions, but having sufficiently numerous open cells so that drainage and negative pressure therapy may continue unimpaired, is placed in fluid communication with a vacuum source for promotion of fluid drainage, as known in the art. The pad is further comprised of an ultra-low density fused-fibrous ceramic, or a bioabsorbable branched polymer, or cell growth enhancing matrix or scaffolding.
Owner:KCI LICENSING INC
Method of making an absorbent composite
In a method of making an absorbent composite, a porous, stabilized structure is formed and impregnated with a flowable superabsorbent precursor. The flowable superabsorbent precursor is cross-linked to form a superabsorbent material within the stabilized structure. The surface area of one of the flowable superabsorbent precursor impregnated with the stabilized structure and the superabsorbent material formed within the structure is increased. In one embodiment, the surface is increased by freeze drying the impregnated structure.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Collagen biofabric and methods of preparation and use therefor
InactiveUS20040048796A1Improved biophysical propertyImprove featuresSenses disorderPeptide/protein ingredientsSurgical GraftWound dressing
The present invention relates to collagenous membranes produced from amnion, herein referred to as a collagen biofabric. The collagen biofabric of the invention has the structural integrity of the native non-treated amniotic membrane, i.e., the native tertiary and quaternary structure. The present invention provides a method for preparing a collagen biofabric from a placental membrane, preferably a human placental membrane having a chorionic and amniotic membrane, by decellularizing the amniotic membrane. In a preferred embodiment, the amniotic membrane is completely decellularized. The collagen biofabric of the invention has numerous utilities in the medical and surgical field including for example, blood vessel repair, construction and replacement of a blood vessel, tendon and ligament replacement, wound-dressing, surgical grafts, ophthalmic uses, sutures, and others. The benefits of the biofabric are, in part, due to its physical properties such as biomechanical strength, flexibility, suturability, and low immunogenicity, particularly when derived from human placenta.
Owner:CELLULAR THERAPEUTICS DIV OF CELGENE +1
Compressed high density fibrous polymers suitable for implant
An embodiment of the present invention may be made by the following steps: providing a mixture comprising a plurality of fibers, a lubricant, and a suspension fluid, with the suspension fluid filling a void space between said fibers and subjecting said mixture to at least one compressive force. The compressive force causes the migration and alignment of said fibers; and may remove substantially all of the suspension fluid from said mixture. The mixture may further comprise a biologically active agent, or a reinforcing agent.
Owner:DSM IP ASSETS BV
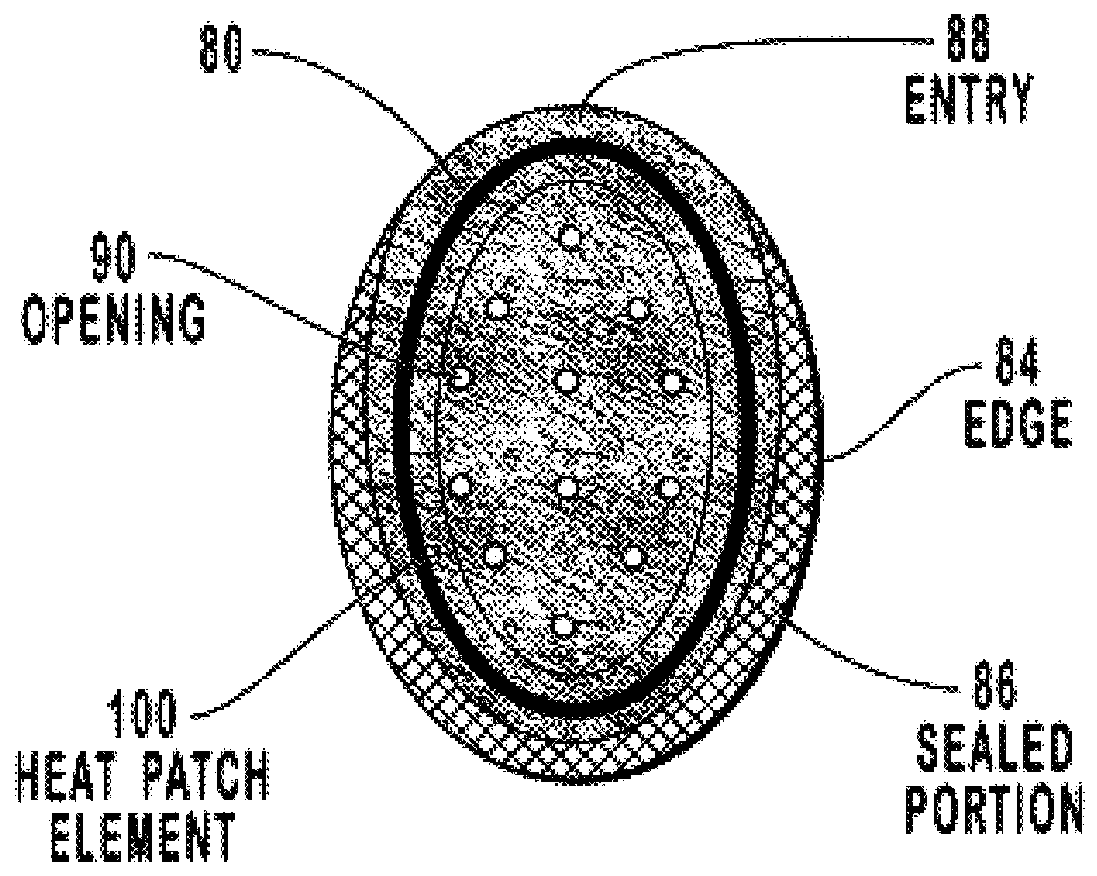
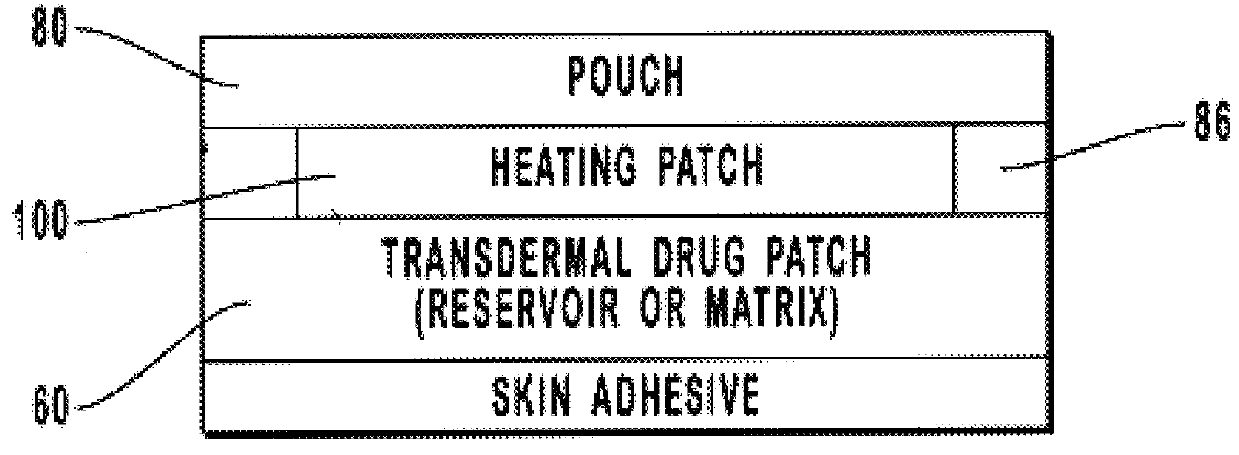
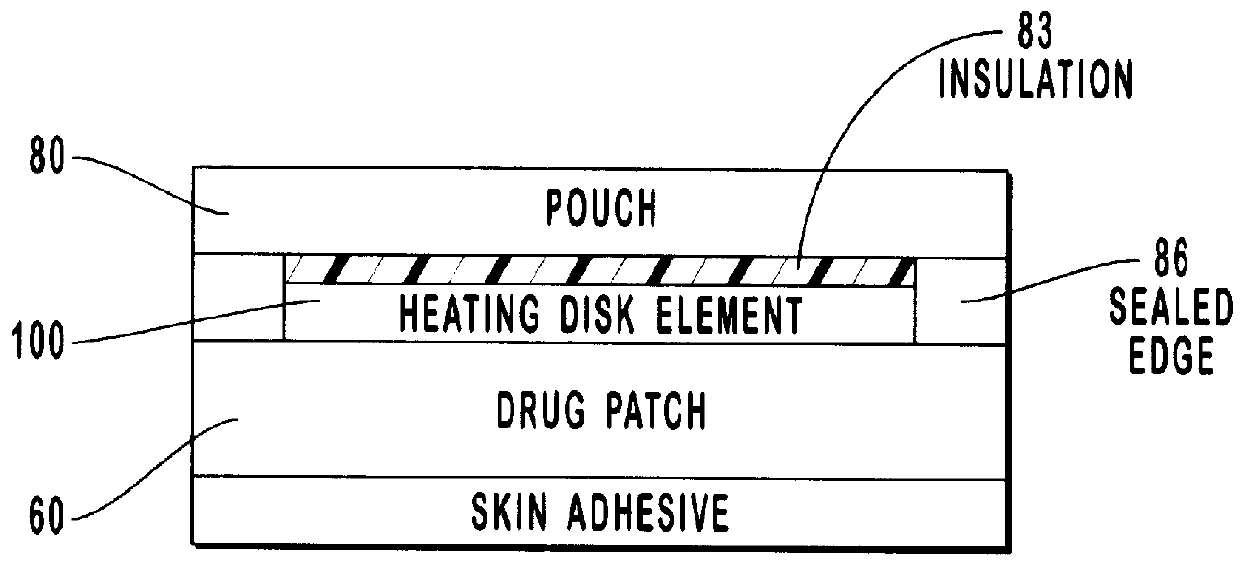
Transdermal drug patch with attached pocket for controlled heating device
InactiveUS6261595B1Shorten the timeEasy to replaceElectrotherapyMedical devicesTransdermal patchDrug administration
The present invention relates to a transdermal drug delivery system comprising a dermal drug delivery patch and a heating element compartment securable to the dermal drug delivery patch. A freely transferrable heating element is securable within the heating element compartment. A drug can be administered transdermally using the present invention by placing the dermal drug delivery patch upon a patient's skin at an administration site. A heating element compartment is secured to the dermal drug delivery patch and a freely transferrable heating element is placed within the heating element compartment. The heating element provides controlled heat to the dermal drug patch and the patient's skin aid thereby improves dermal drug administration.
Owner:ZARS INC
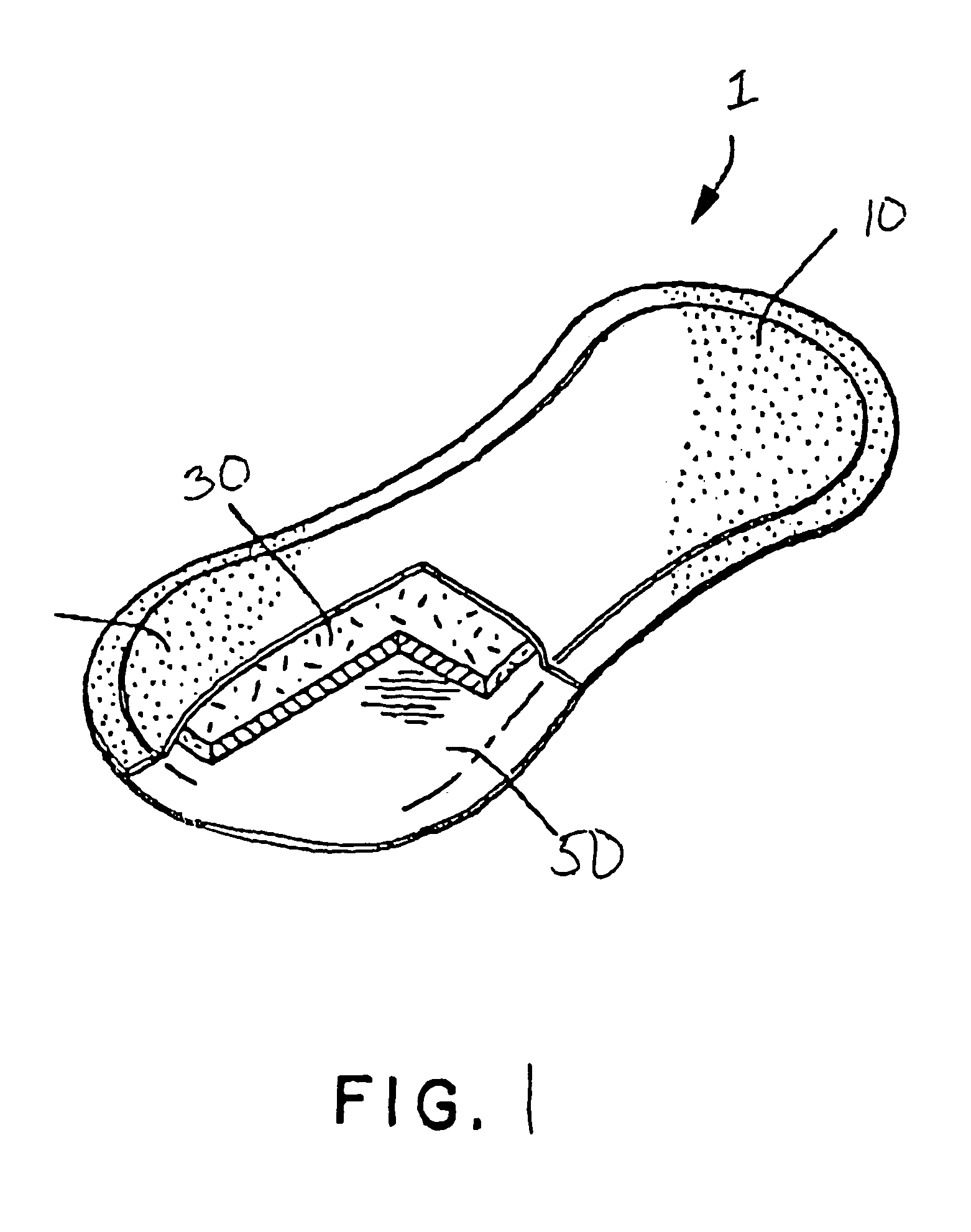
Removable wound closure
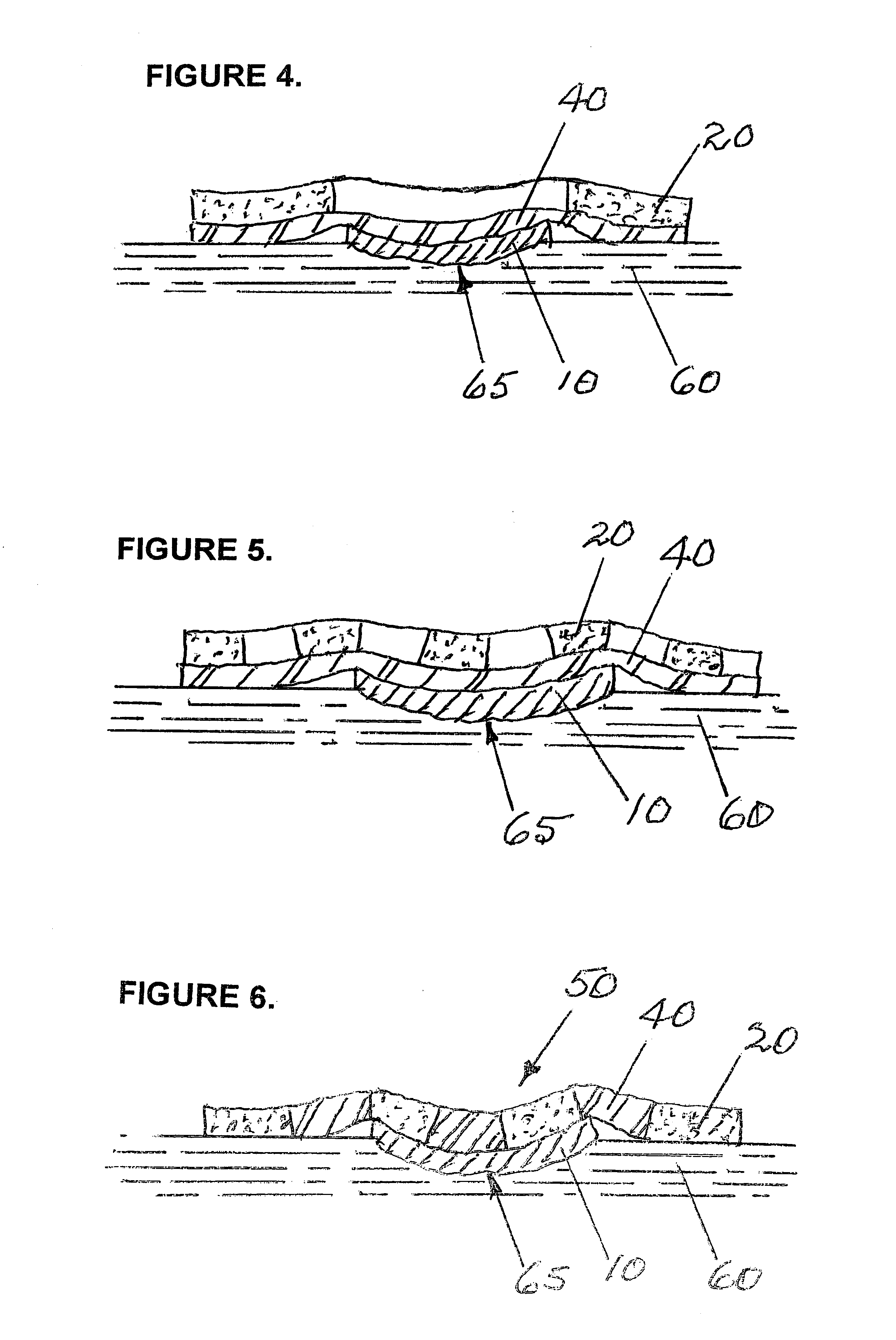
InactiveUS7381859B2Promote wound healingMinimizes adhesion formationNon-adhesive dressingsWound drainsElastomerPorosity
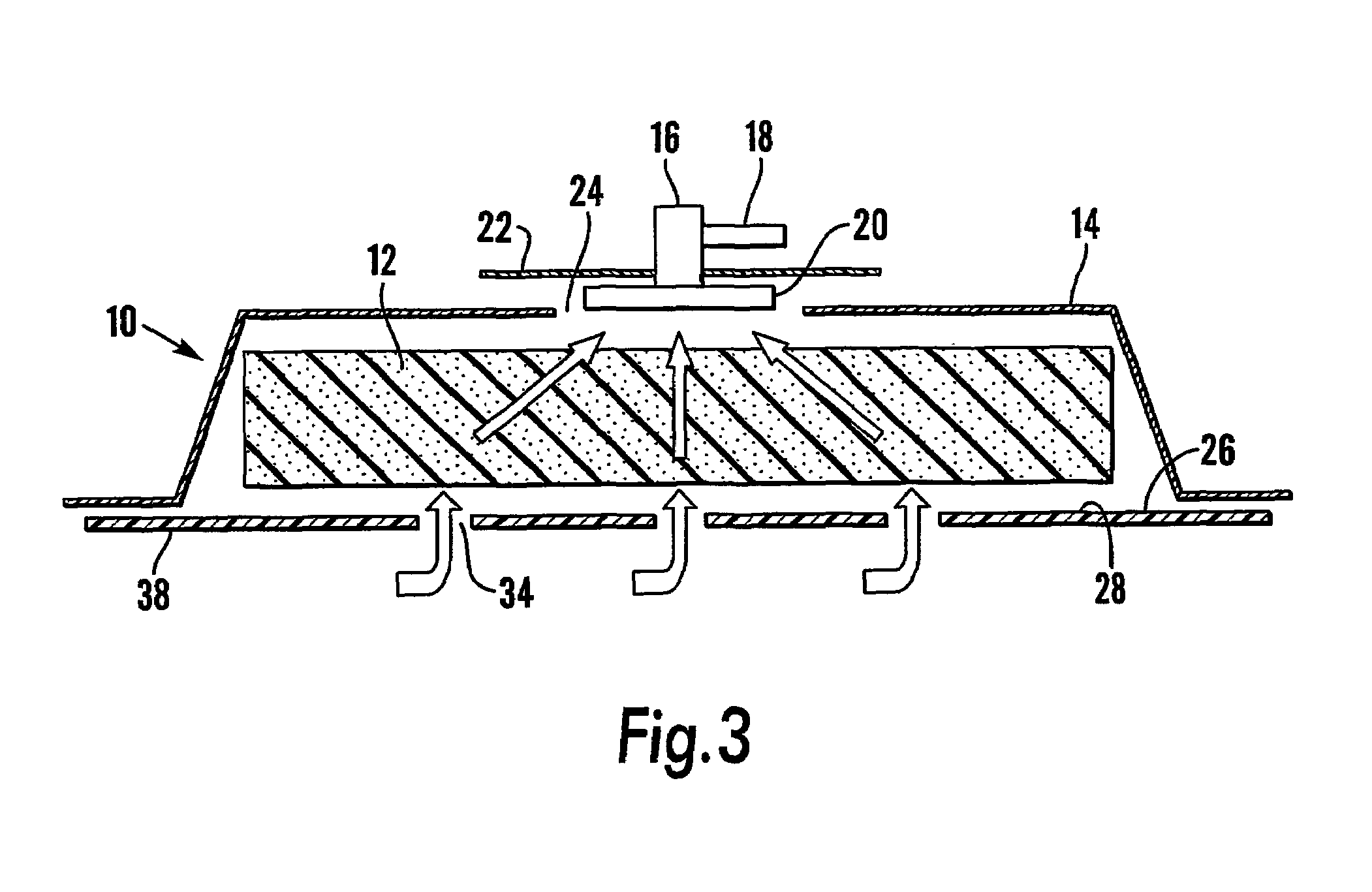
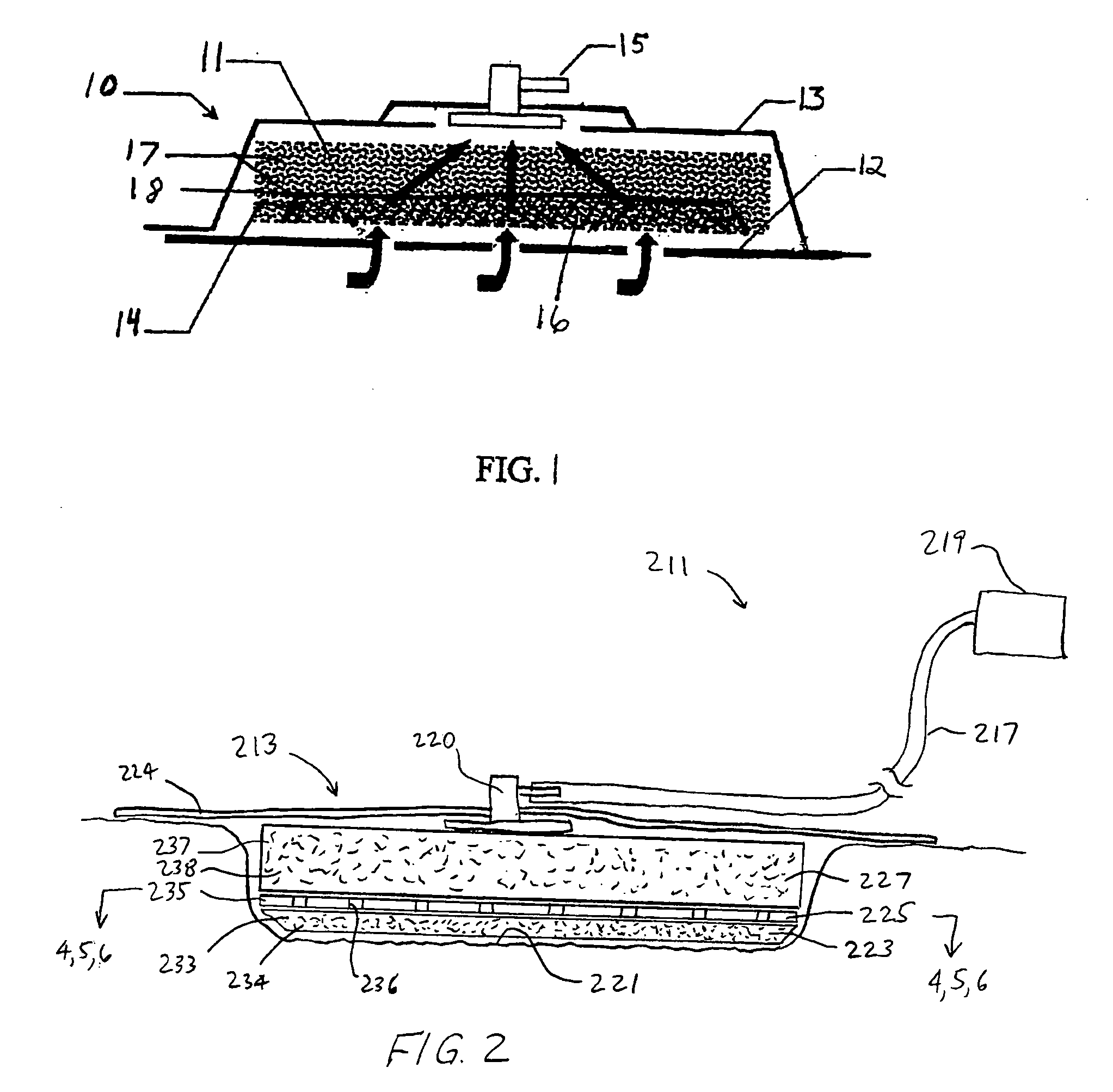
A system and method for the temporary closure of a wound, especially an abdominal wound, to facilitate re-entry, final closure, and long term healing of the wound. An abdominal wound dressing and methods of use are described that enable the application of negative pressure to the wound site in a site healing promoting manner while also limiting the formation of adhesions that would prevent the removal of the dressing. The dressing comprises a layer of porous foam material (36) enclosed by sheets of elastomeric material (38) punctuated by a number of appropriately placed holes (34). Multiple layers of porous foam may also be used. A suction tube connector (16) is provided on an upper surface of a layer of foam (12) for connection to a negative pressure source. At least one layer of foam is enclosed in elastomeric material and is placed in direct contact with the tissue within the open wound. Fluids are drawn by negative pressure through the holes positioned in the elastomeric envelope, and through the foam. If multiple foam layers are employed, the lower layer(s) of foam are of a finer porosity while the upper layer of foam is coarse. An adhesive elastomeric sheet (14) covers the entire wound dressing and seals the edges to the skin surrounding the wound. An appropriate vacuum device is attached to the suction tube connector.
Owner:KCI LICENSING INC
Biocompatible wound dressing
InactiveUS20070185426A1Reduce pressureReduced tissue treatmentNon-adhesive dressingsAdhesive dressingsWound dressingContact layer
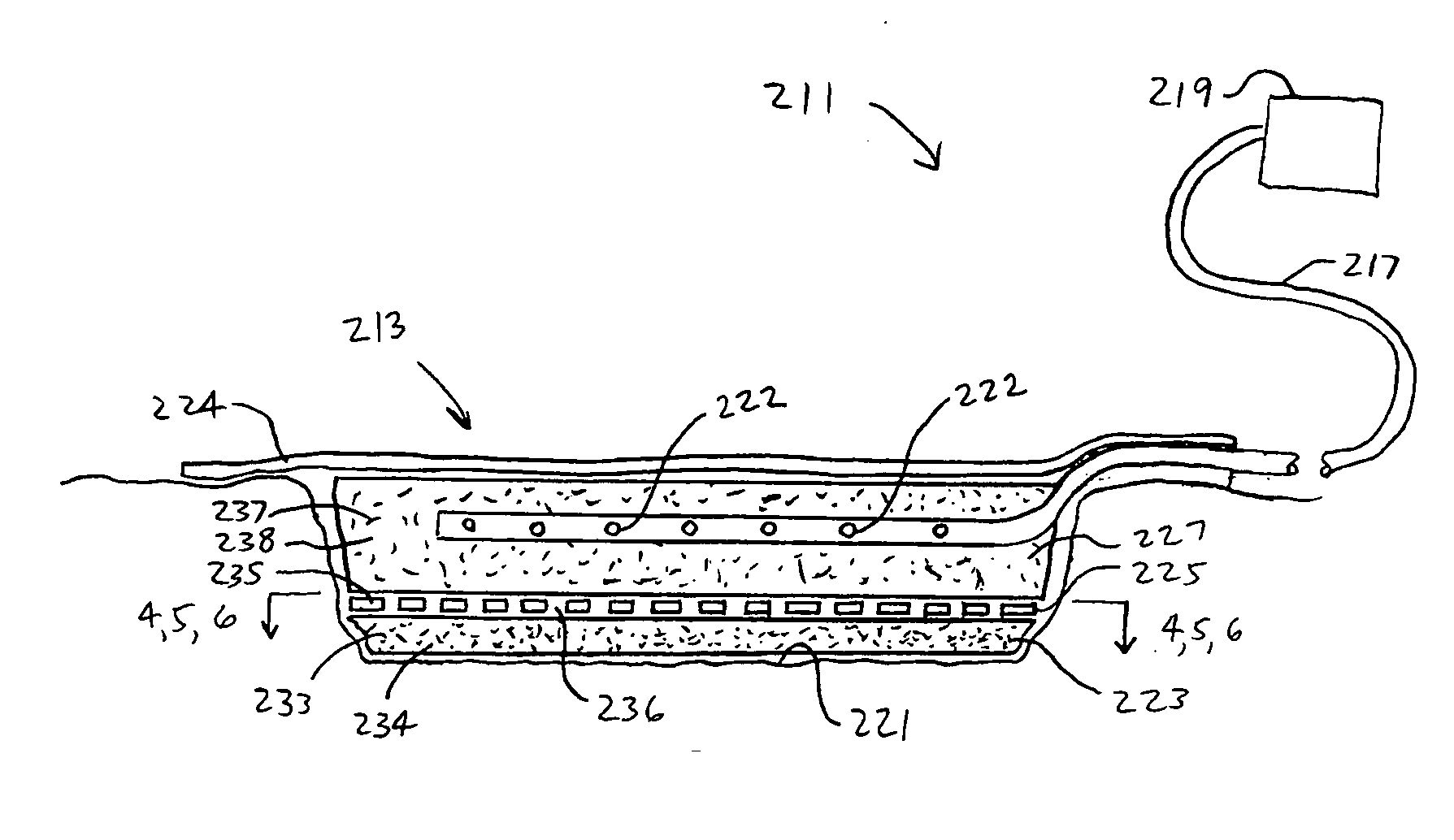
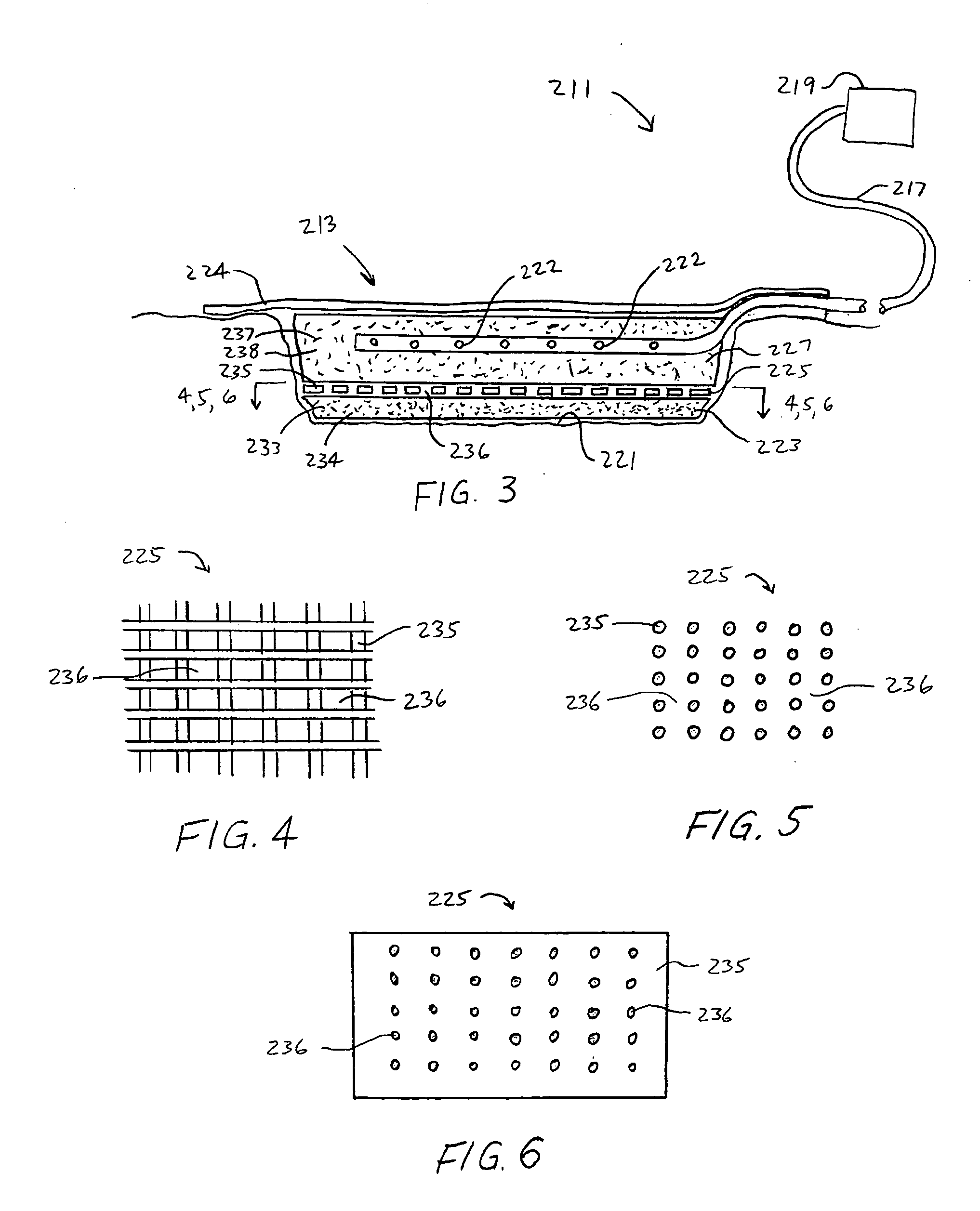
A multi-layer reduced pressure delivery apparatus is provided for applying reduced pressure tissue treatment to a tissue site. The multi-layer apparatus includes a tissue contact layer, a release layer, and a manifold layer. The tissue contact layer includes a scaffold adapted to contact the tissue site, the release layer includes a hydrogel-forming material and a plurality of flow channels, and the manifold layer includes a distribution manifold. The release layer is positioned between the tissue contact layer and the manifold layer to allow easy release of the manifold layer from the tissue contact layer following the administration of reduced pressure tissue treatment.
Owner:KCI LICENSING INC
Nonwoven Having Durable Hydrophilic Coating
InactiveUS20110268932A1Promote migrationLamination ancillary operationsDecorative surface effectsHydrophilic coatingTrace Amounts
A nonwoven material coated with an amine-polyether silicone. The coating composition may include a wetting agent, an acid, and / or a defoamer. The nonwoven may be incorporated into a disposable absorbent article. The disposable absorbent article may include at least trace amounts of a mineral oil. The coating of the nonwoven may be durable even in the presence of mineral oil.
Owner:THE PROCTER & GAMBLE COMPANY
Adhesive Patch Systems and Methods
ActiveUS20080269687A1High bonding strengthReduced adhesion strengthInfusion syringesFiltering accessoriesAdhesion strengthUltimate tensile strength
Various embodiments of the present invention are directed to patches for medical devices. In various embodiments, an adhesive patch of a medical device may have selective areas with adhesive material of varying adhesion strengths. In other embodiments, an adhesive patch of a medical device may include adhesive material that may be activated by a catalyst to increase or decrease the adhesion strength of the adhesive material. In further embodiments, a medical device may include a pierceable membrane containing an agent, the pierceable membrane positioned to be pierced by a needle and to cause some of the agent to be carried to the user-patient.
Owner:MEDTRONIC MIMIMED INC
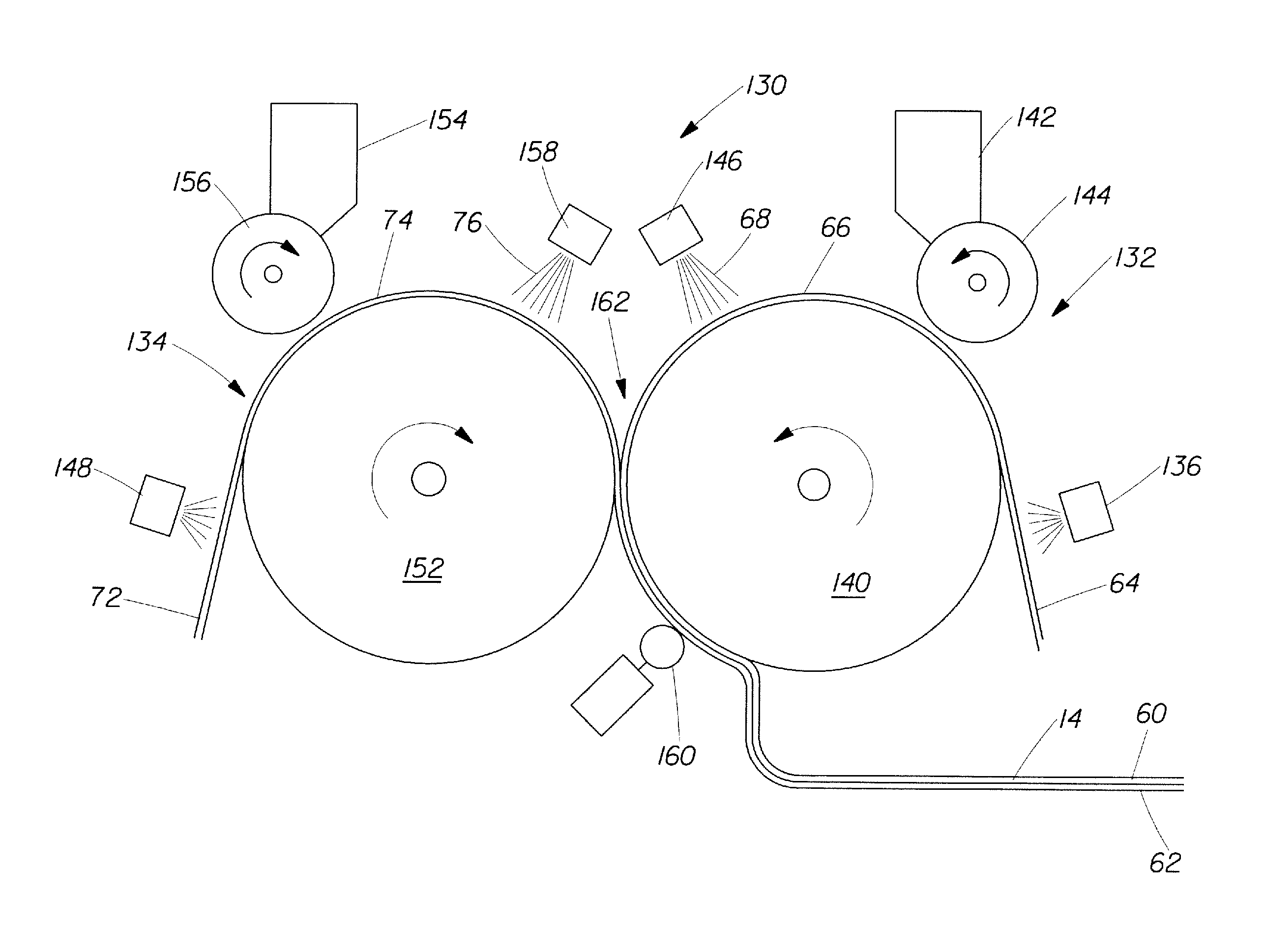
Method And Apparatus For Making Disposable Absorbent Article With Absorbent Particulate Polymer Material And Article Made Therewith
A method for making a disposable absorbent core comprises depositing absorbent particulate polymer material from a plurality of reservoirs in a printing roll onto a substrate disposed on a grid of a support which includes a plurality of cross bars extending substantially parallel to and spaced from one another so as to form channels extending between the plurality of cross bars. The plurality of reservoirs in the first peripheral surface are arranged in an array comprising rows extending substantially parallel to and spaced from one another. The support and printing roll are arranged such that the plurality of cross bars are substantially parallel to the rows of the plurality of reservoirs and the absorbent particulate polymer material is deposited on the substrate in a pattern such that the absorbent particulate polymer material collects in rows on the first substrate formed between the first plurality of cross bars. A thermoplastic adhesive material is deposited on the absorbent particulate polymer material and the substrate to cover the absorbent particulate polymer material on the substrate and form an absorbent layer. A disposable absorbent article and apparatus for making an absorbent article are also disclosed.
Owner:THE PROCTER & GAMBLE COMPANY
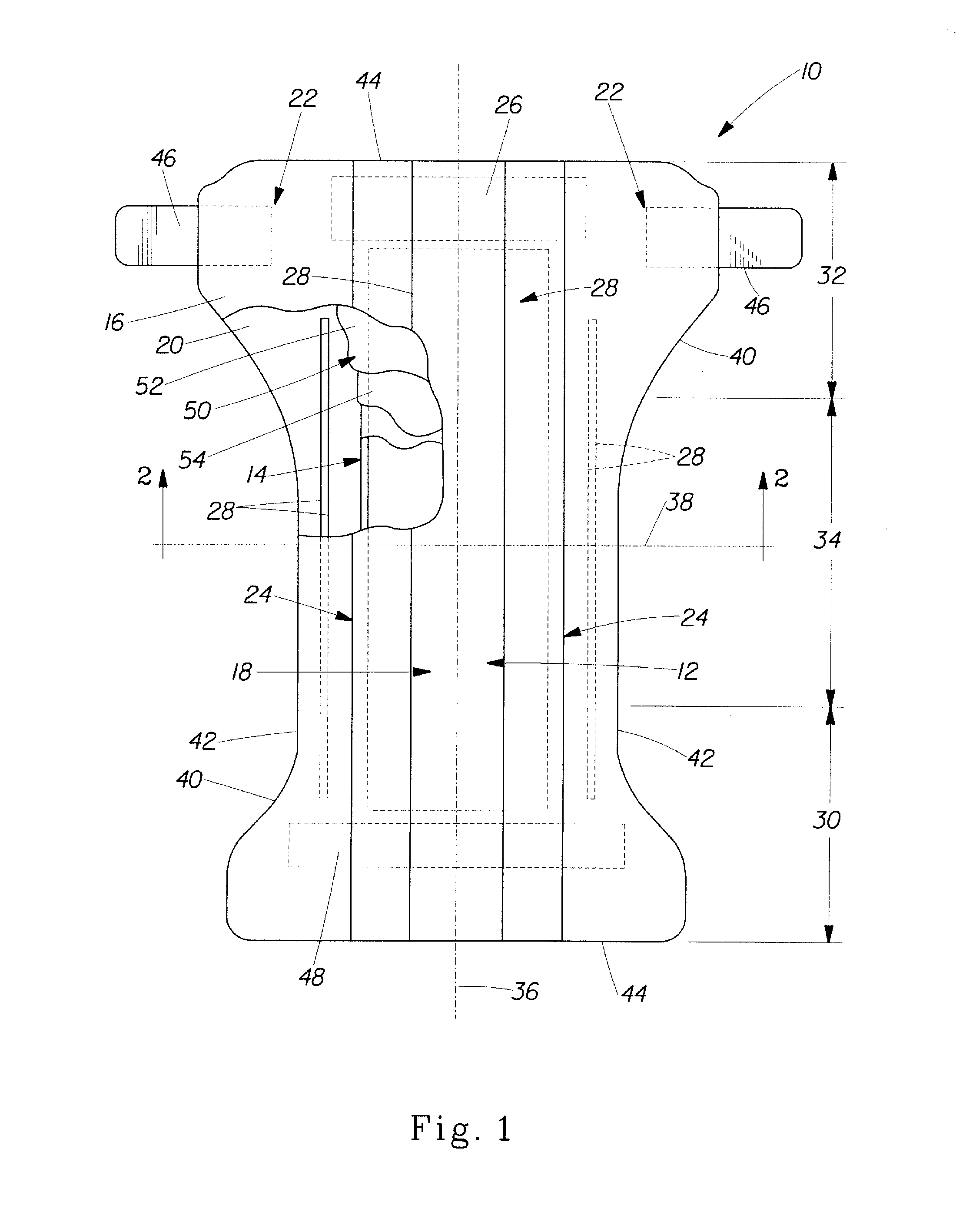
Assemblies for coupling intraosseous (IO) devices to powered drivers
ActiveUS8944069B2Easy to useDirect contact guaranteeSurgical furnitureSurgical needlesMultiple useBone marrow aspiration procedure
Medical devices, medical procedure trays, kits and related methods are provided for use to perform medical procedures that require access to the interior of a bone. The devices, trays and methods allow multiple use of non-sterile medical devices with sterile medical devices for performing medical procedures requiring sterile conditions. A coupler assembly, capable of releasably attaching to a non-sterile medical device at one end and releasably attaching to one or more sterile medical devices at another end, and further comprising a containment bag which allows maintaining sterility of a non-sterile medical device which may be used in conjunction with sterile medical devices and procedures. The devices, trays, kits and methods enable the performance of multiple medical procedures with a single insertion into bone. For example, a vertebral procedure such as a vertebroplasty may be performed along with biopsy and / or bone marrow aspiration procedures, thereby reducing patient trauma and costs.
Owner:TELEFLEX LIFE SCI LTD
Method of Making Prefastened Refastenable Disposable Absorbent Articles
The present disclosure relates to methods for manufacturing absorbent articles, and in particular, methods for making pre-fastened refastenable pant diapers. Aspects of the methods according to the present disclosure relate to the fabrication of refastenable pant diapers wherein discrete chassis are advanced in a machine direction such that the lateral axis is parallel with the machine direction. First side panels are then refastenably connected with the first waist region, and second side panels are permanently connected the second waist regions of the discrete chassis. The chassis are connected with discrete lengths of side panel material and / or connection zone material. The chassis are then folded, and the first and second side panels are subsequently bonded together. The article is then subjected to knife cut at or adjacent the bonded regions to create discrete, pre-fastened refastenable pant diapers.
Owner:THE PROCTER & GAMBLE COMPANY
Method of Making Prefastened Refastenable Disposable Absorbent Articles
InactiveUS20120061015A1Lamination ancillary operationsLaminationKnife cutsElectrical and Electronics engineering
Aspects of the methods according to the present disclosure relate to the fabrication of refastenable pant diapers wherein discrete chassis are advanced in a machine direction such that the lateral axis is parallel with the machine direction. First side panels are then refastenably connected with the first waist region, and second side panels are permanently connected the second waist regions of the discrete chassis. The methods disclosed herein connect chassis with discrete lengths of side panel material and / or connection zone material, and forms a continuous web of articles formed by intermittently spaced chassis and intermittently spaced side panels bridging the gap between the intermittently spaced chassis. The chassis are then folded in the cross direction parallel to a lateral centerline and the first and second side panels are subsequently bonded together. The article is then subjected to knife cut adjacent the bonded regions to create discrete, pre-fastened refastenable pant diapers.
Owner:THE PROCTER & GAMBLE COMPANY
Nanoparticle coatings for flexible and/or drawable substrates
ActiveUS20050287348A1Better able to withstandHigh glossPretreated surfacesRecord information storageNanometreMaterials science
Owner:PPG IND OHIO INC
Method and apparatus for making an apertured web
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com