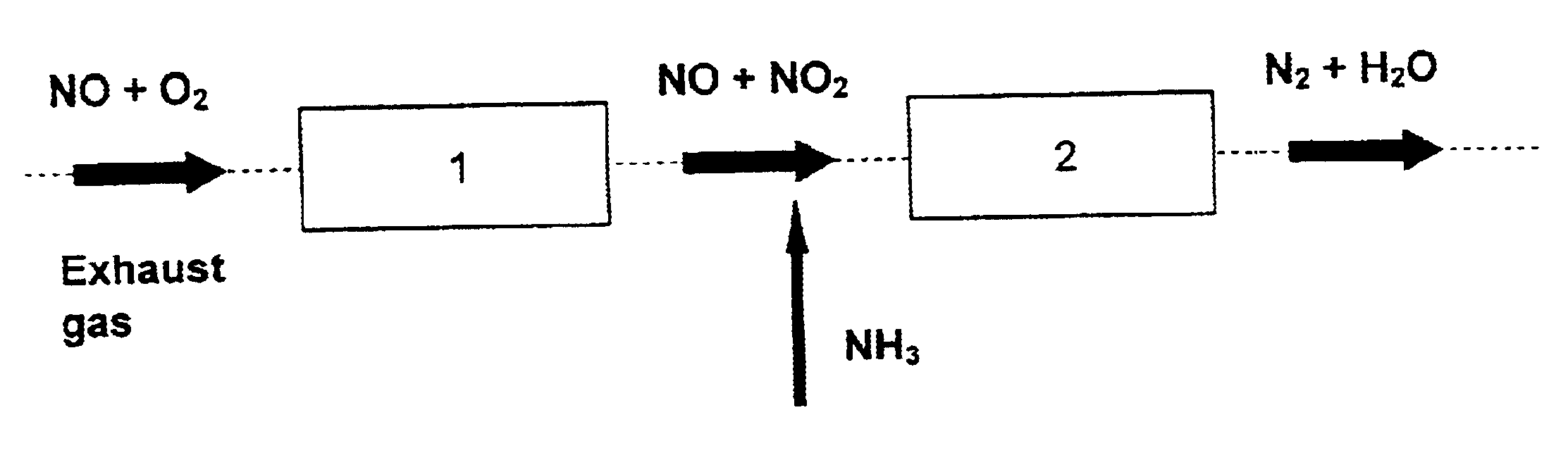
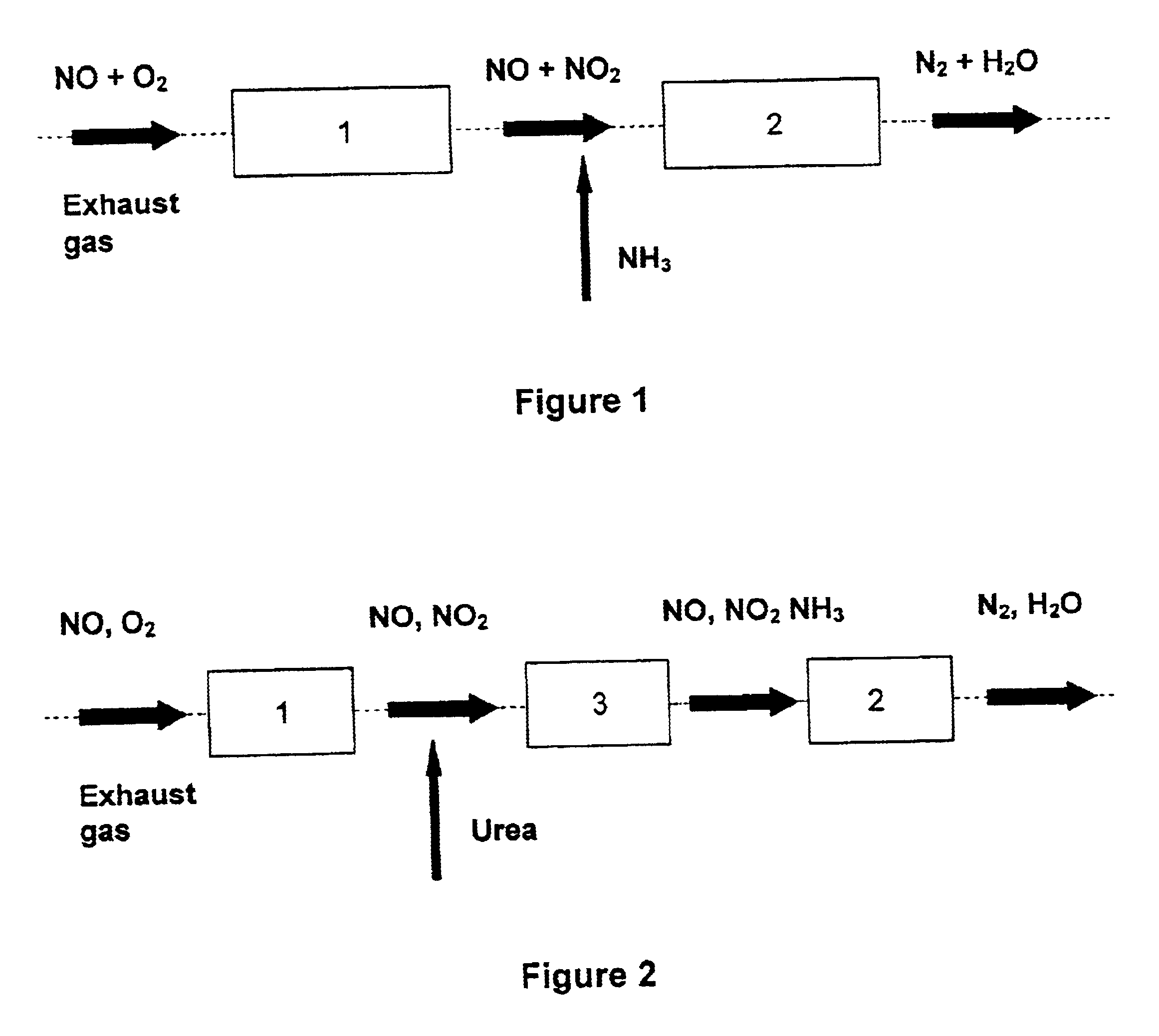
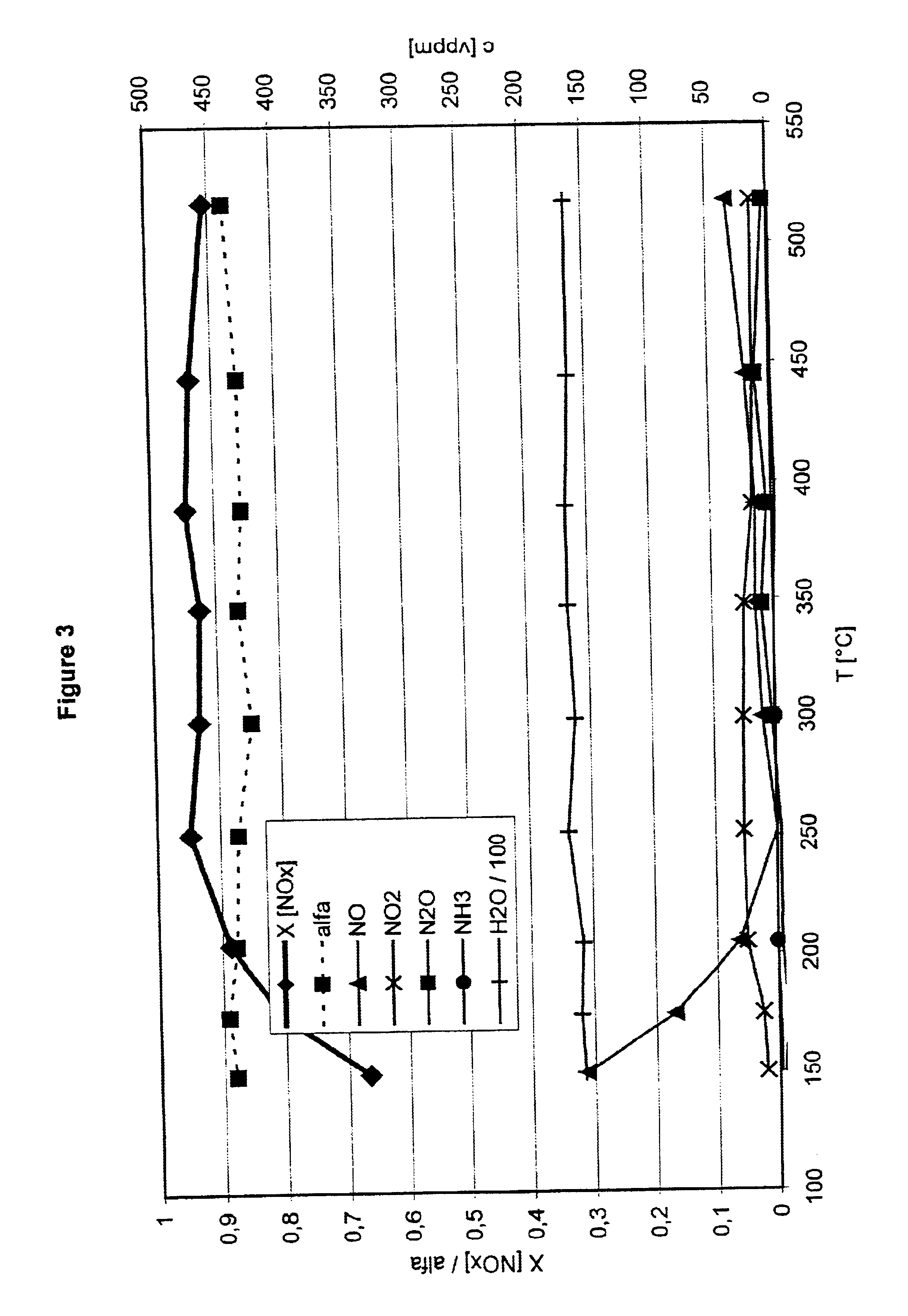
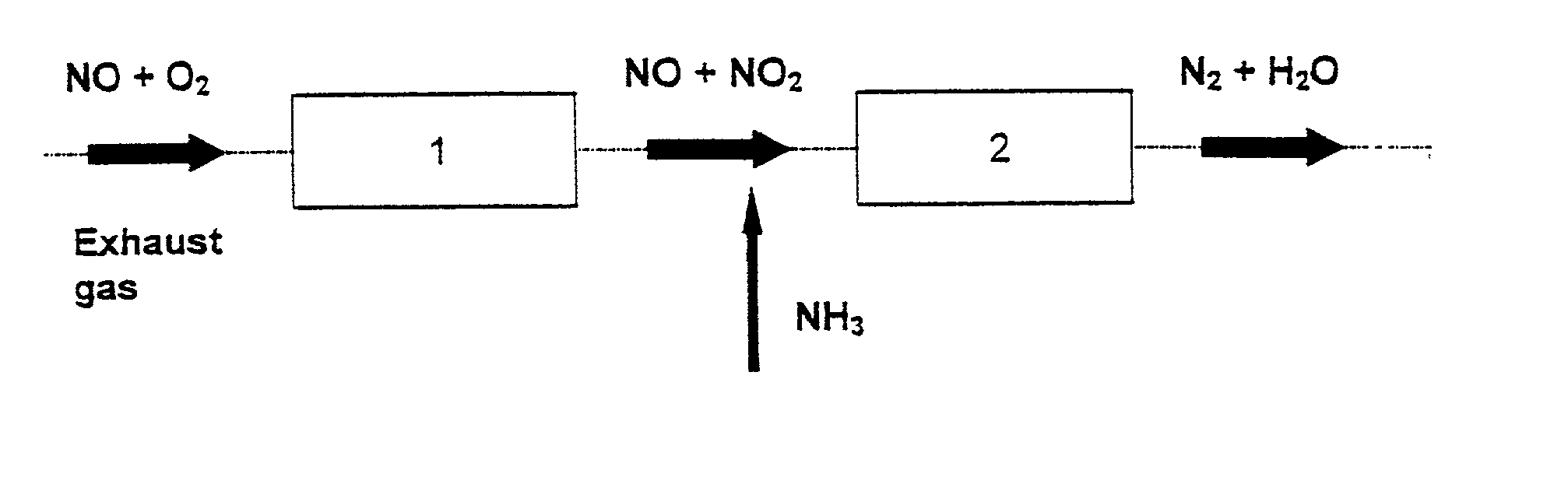
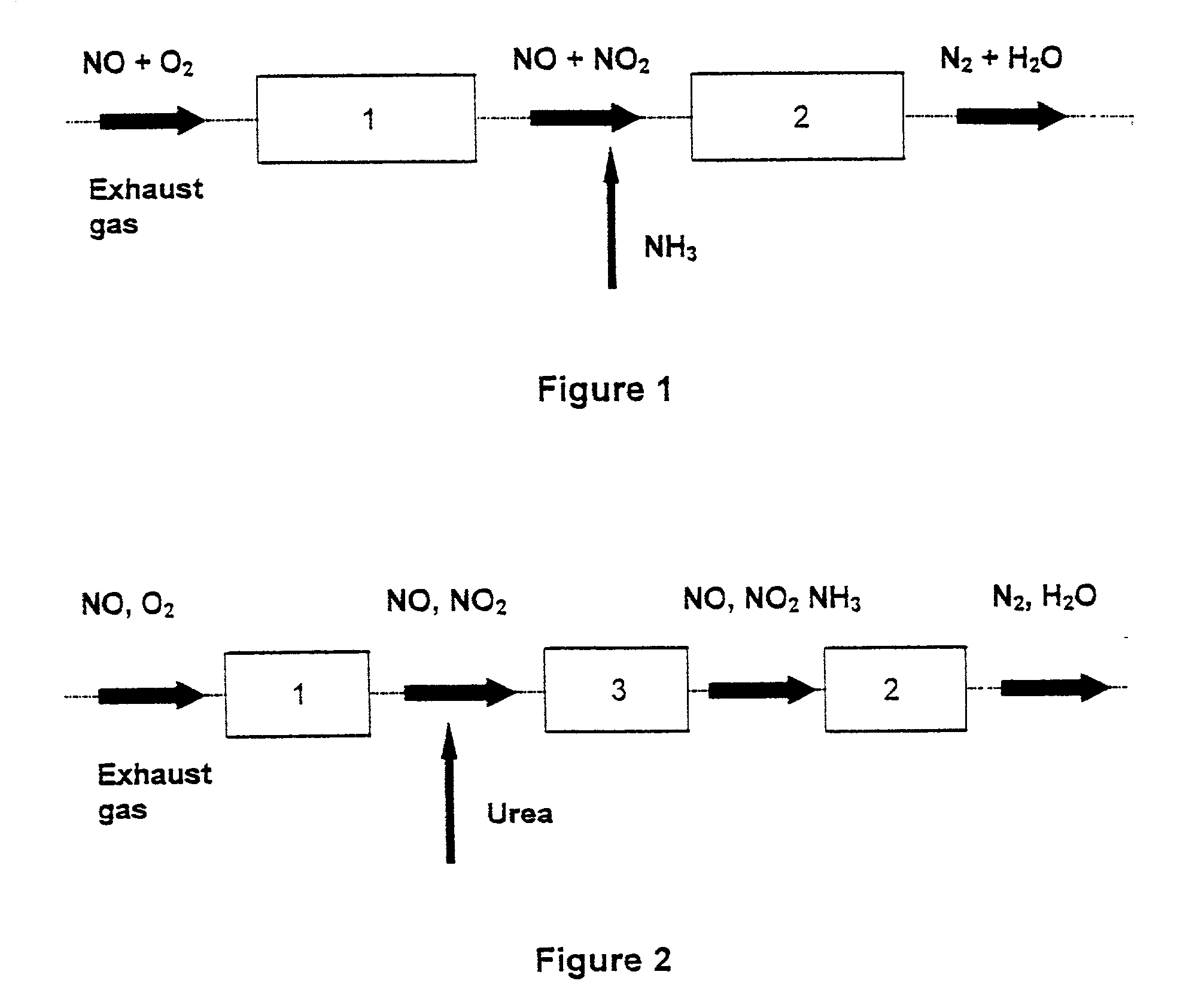
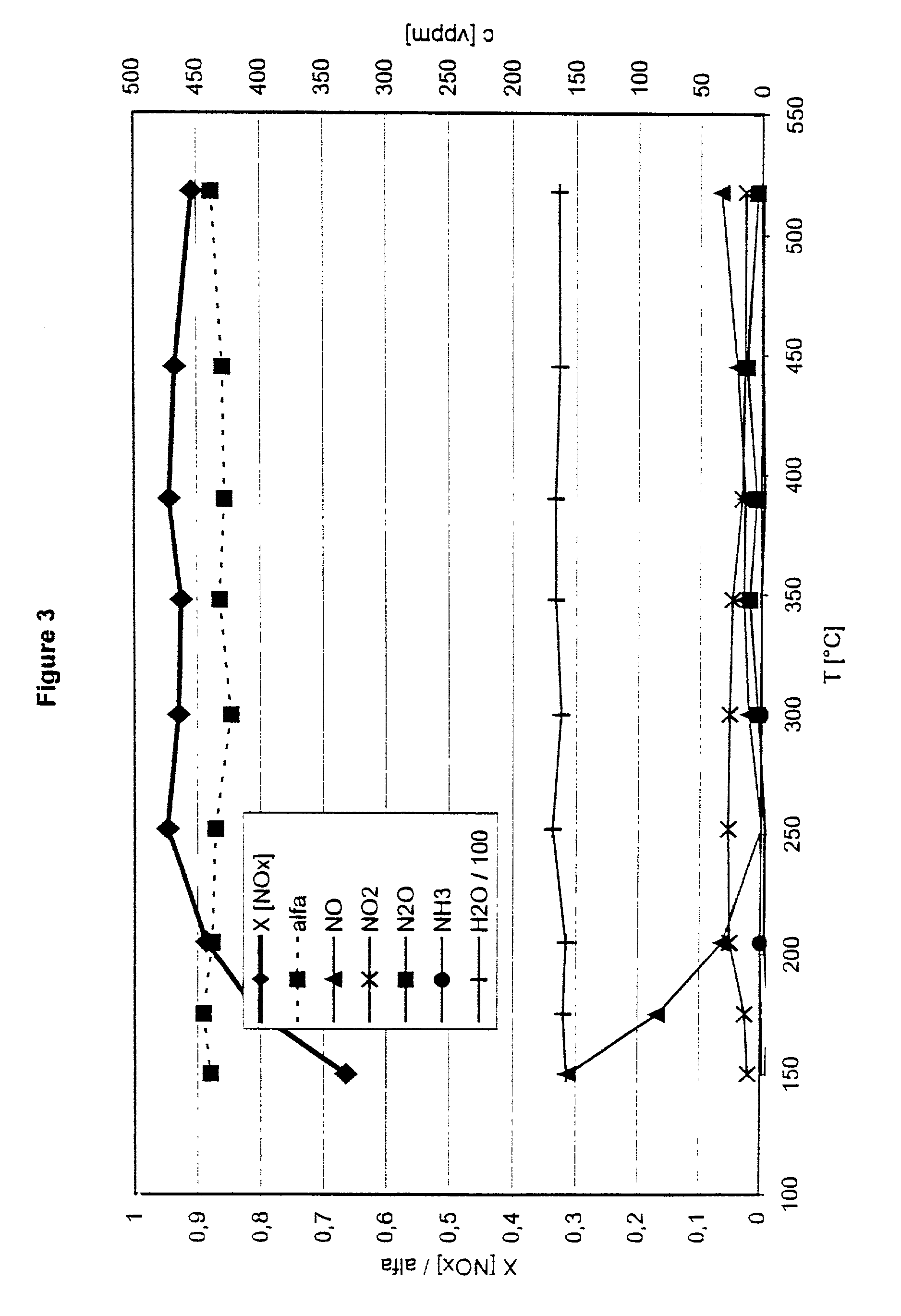
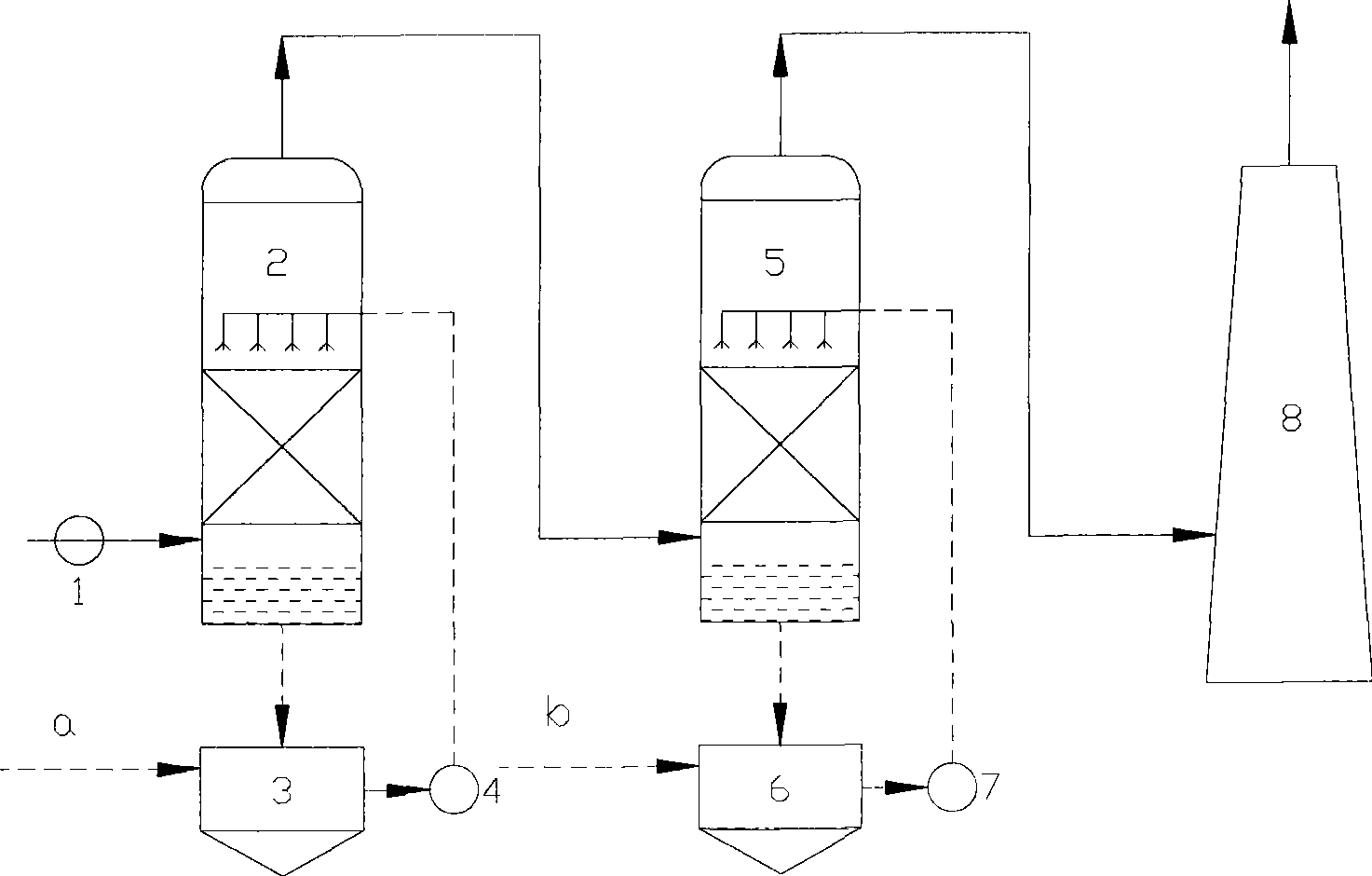
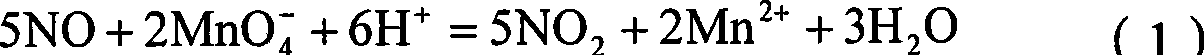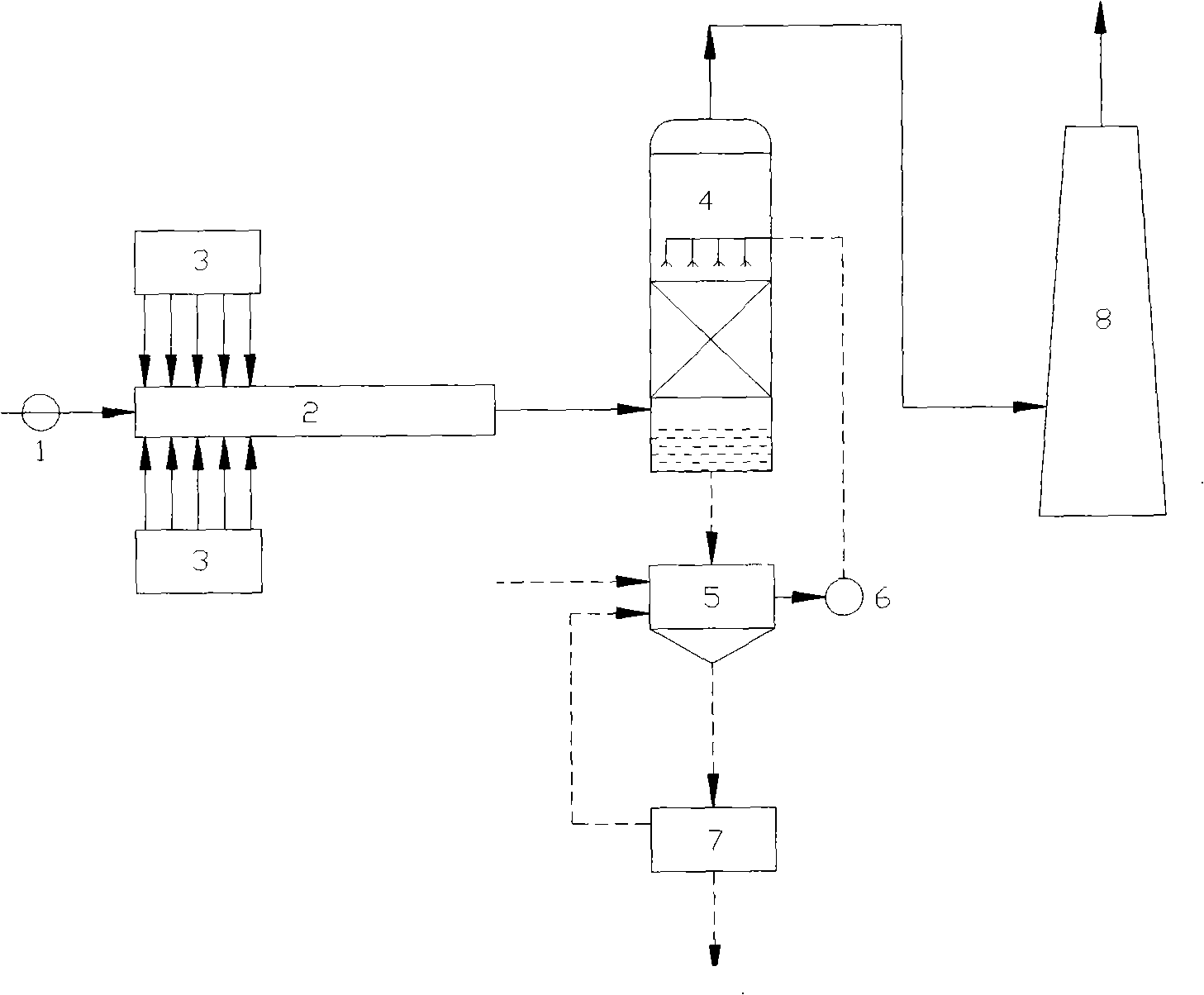Patents
Literature
1184 results about "Nitrogen dioxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nitrogen dioxide is the chemical compound with the formula NO₂. It is one of several nitrogen oxides. NO₂ is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year which is used primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas that has a characteristic sharp, biting odor and is a prominent air pollutant. Nitrogen dioxide is a paramagnetic, bent molecule with C₂ᵥ point group symmetry.
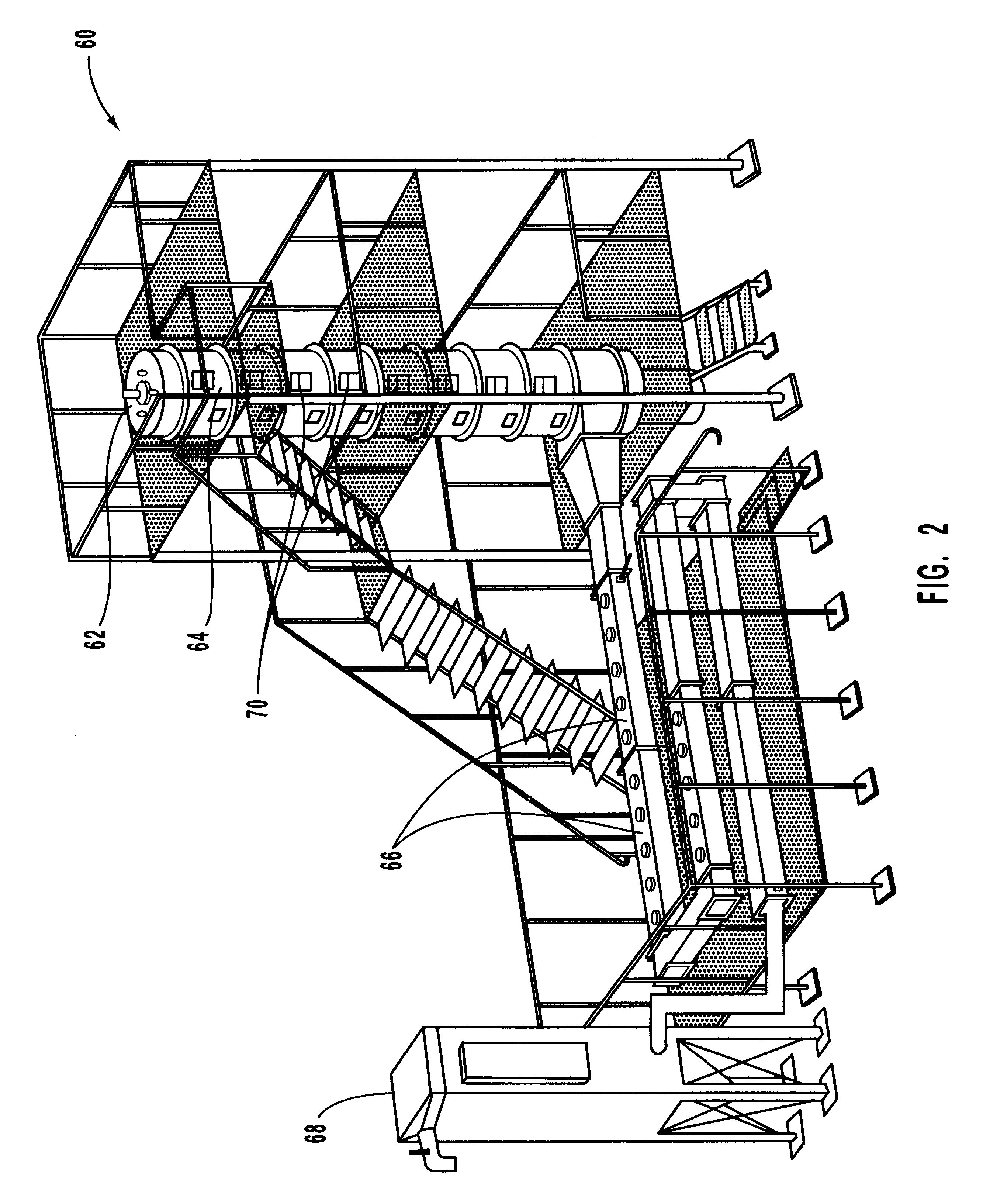
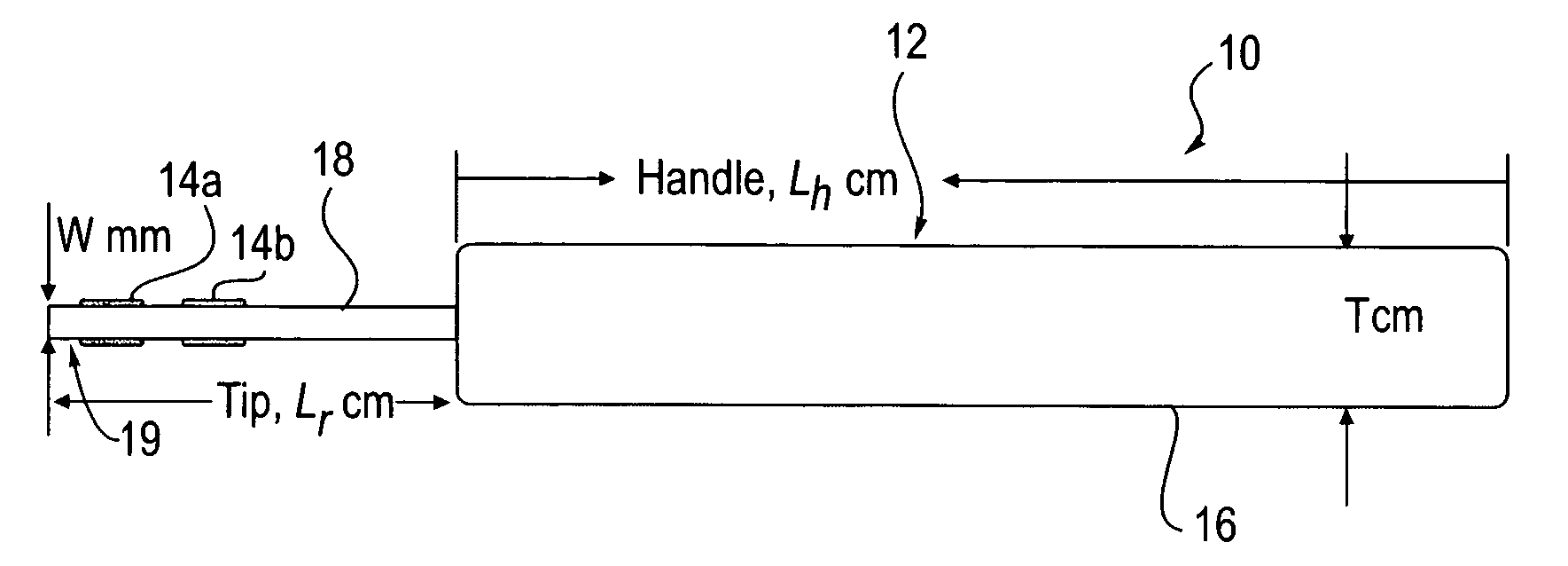
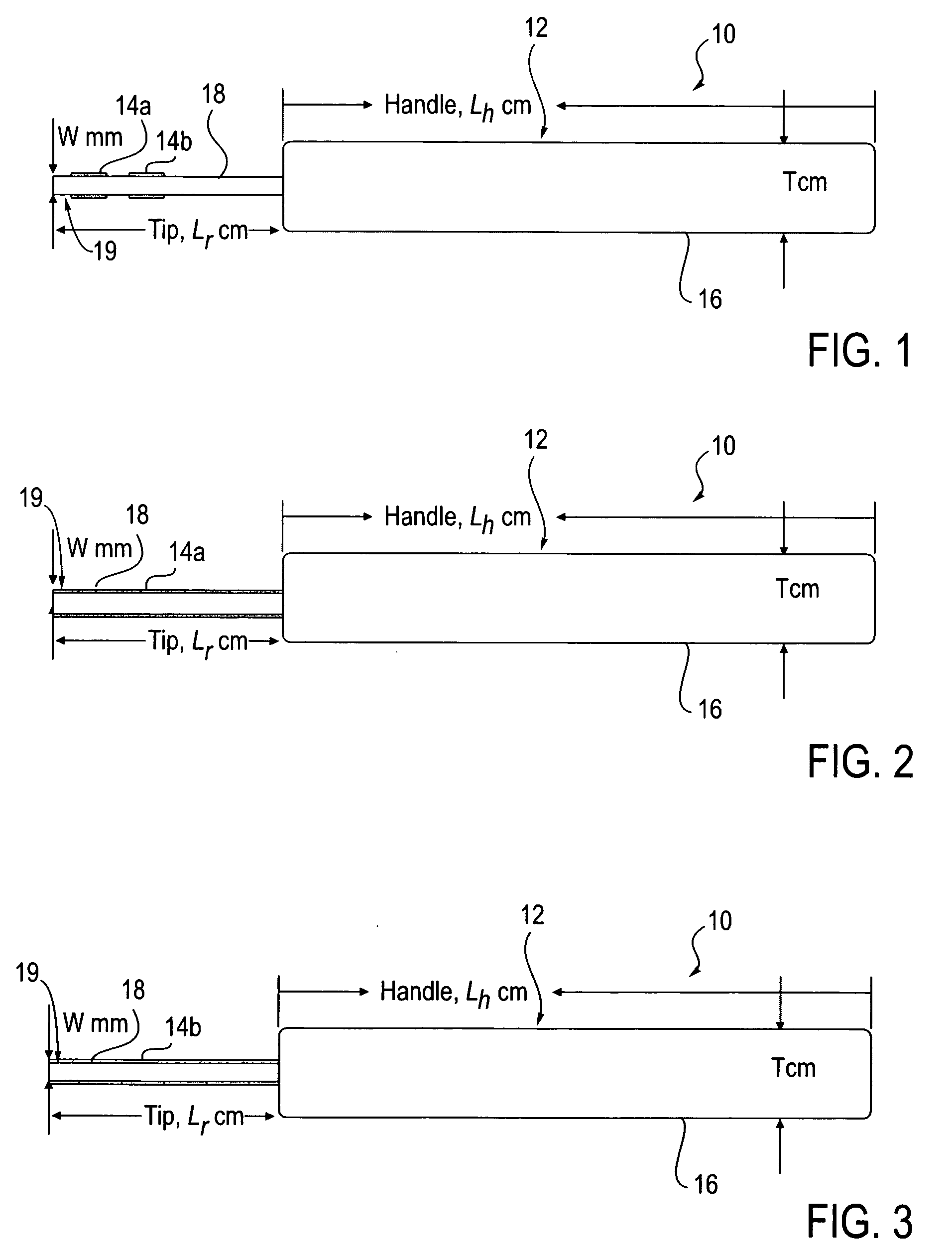
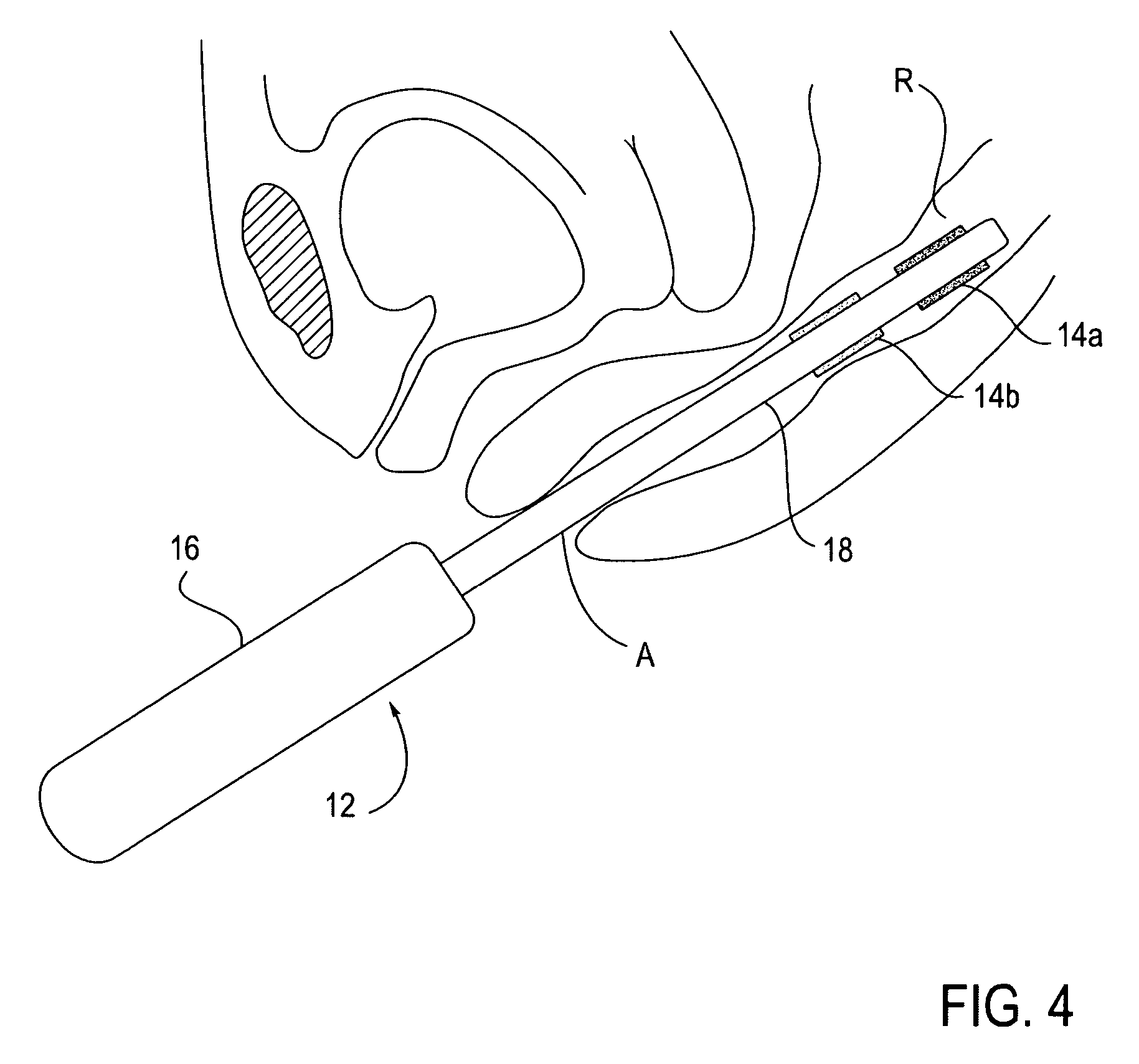
Agents and devices for providing blood clotting functions to wounds
InactiveUS20070190110A1Promote healingShorten the timePhysical treatmentAntithrombogenic treatmentNitrogen dioxideMedicine
Hemostatic agents and devices are made from oxidized cellulose fiber, the oxidized cellulose having a carboxylation content increased by the action of nitrogen dioxide on virgin cellulose fiber. A composition may be incorporated into the oxidized cellulose fiber to cause a pharmacological effect on a wound to which the hemostatic agents and devices are applied. When applied, the oxidized cellulose fiber causes blood emanating from the wound to clot. The oxidized cellulose fiber can either be resorbed into the wound or removed from the wound after healing. A hemostatic bandage includes a pad of unwoven oxidized cellulose fibers mounted on a substrate. Methods of arresting a flow of blood emanating from a wound using such devices are also disclosed. Methods of fabricating oxidized cellulose are also disclosed.
Owner:PAMEIJER CORNELIS H +1
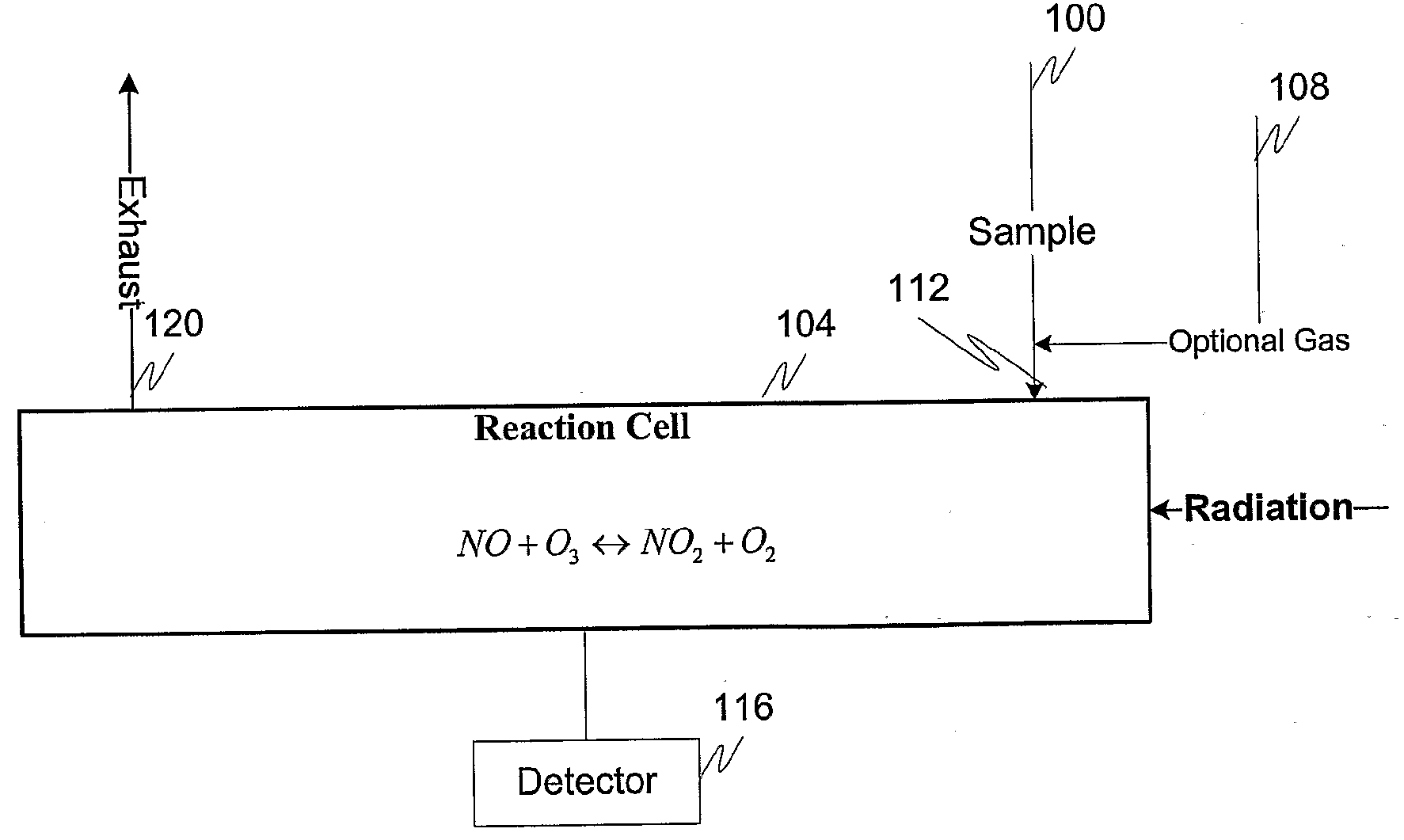
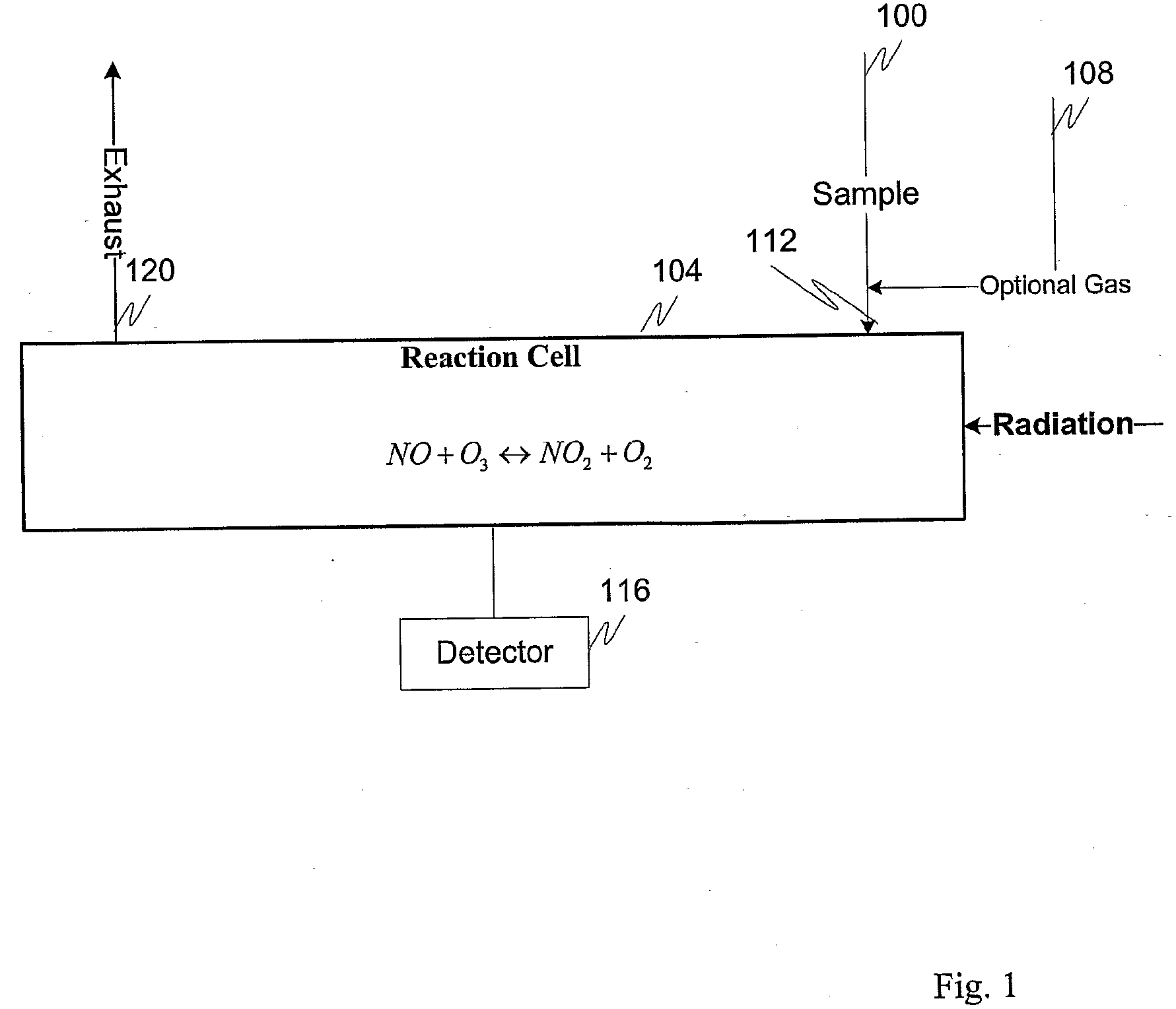
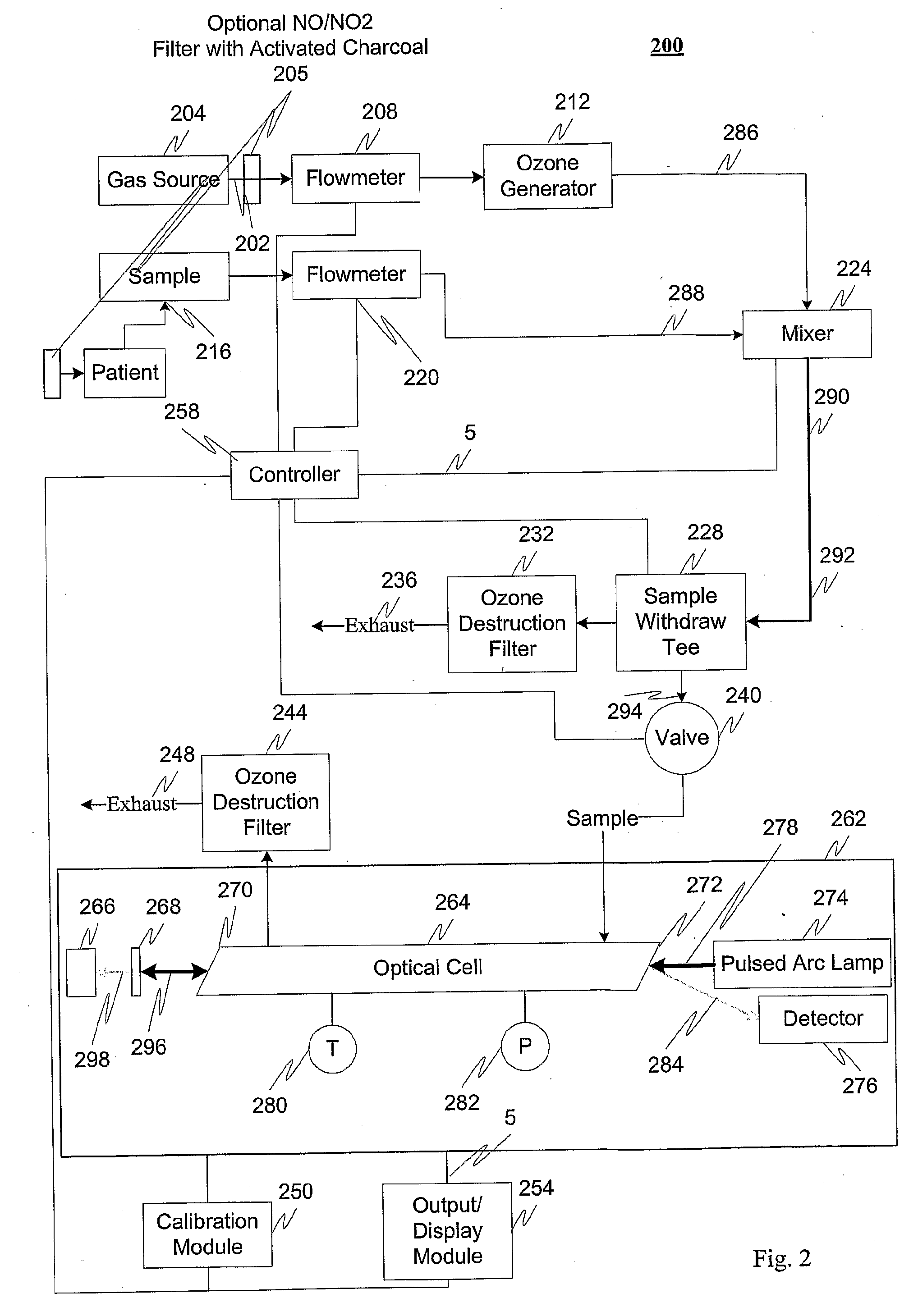
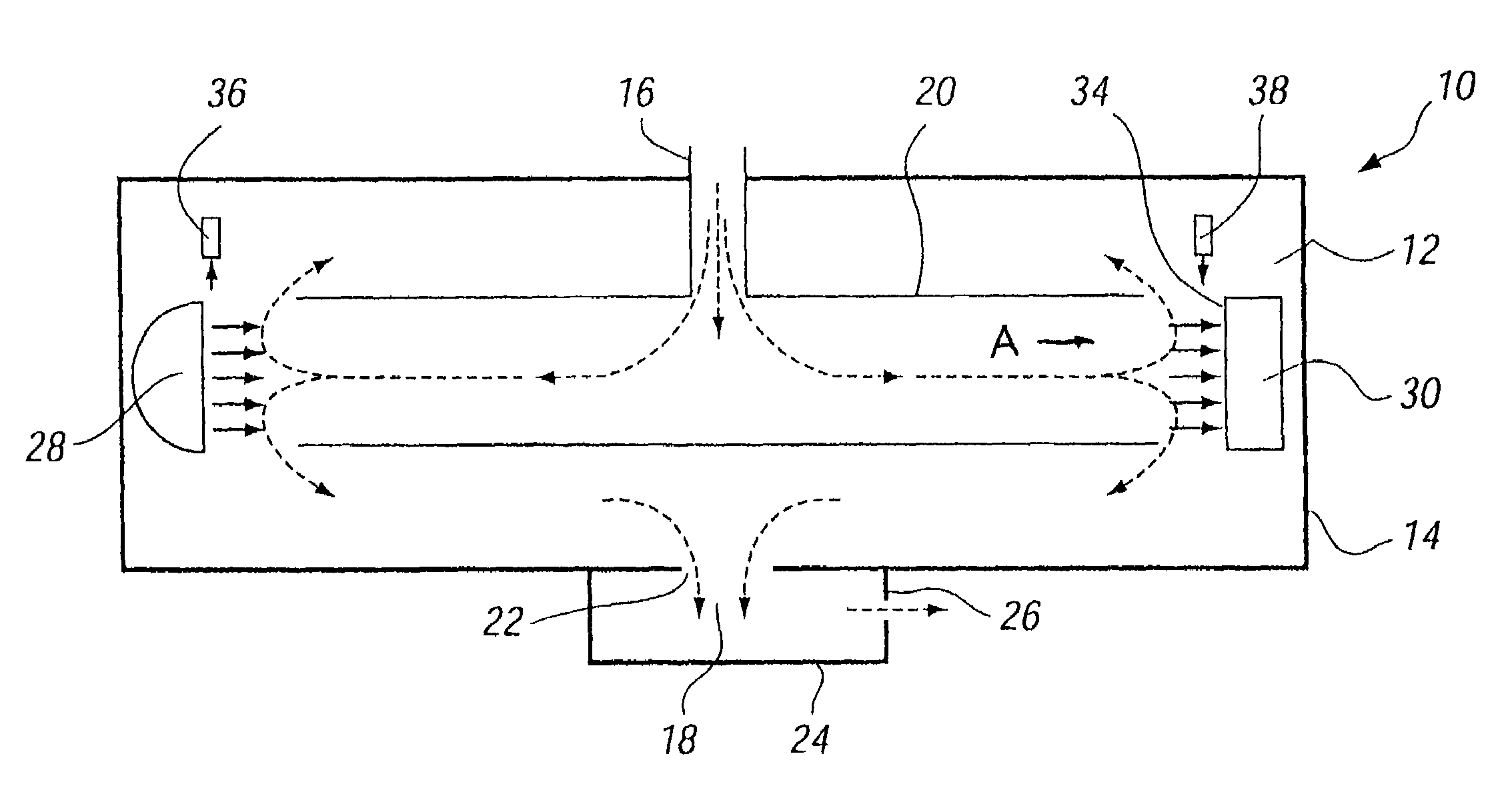
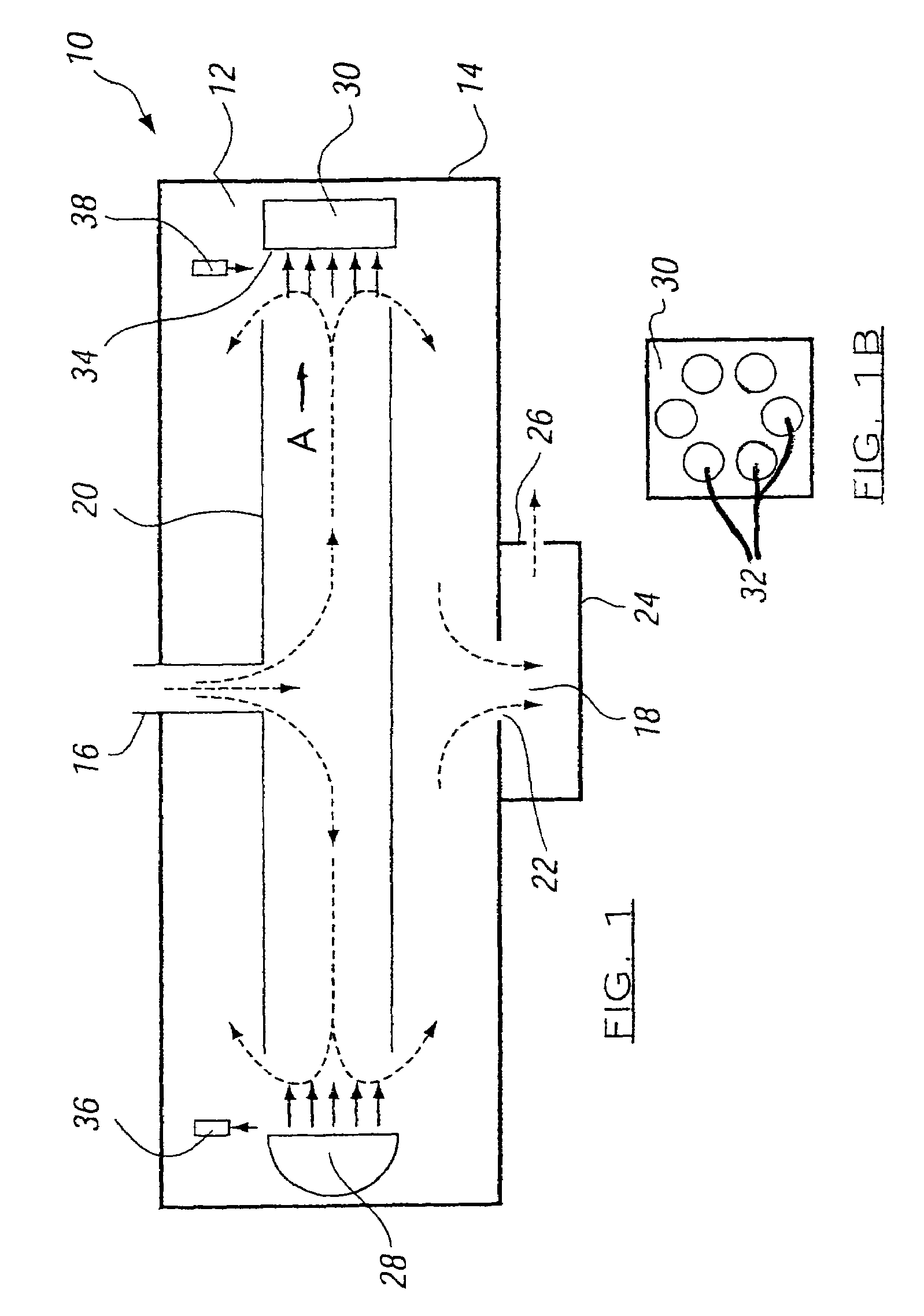
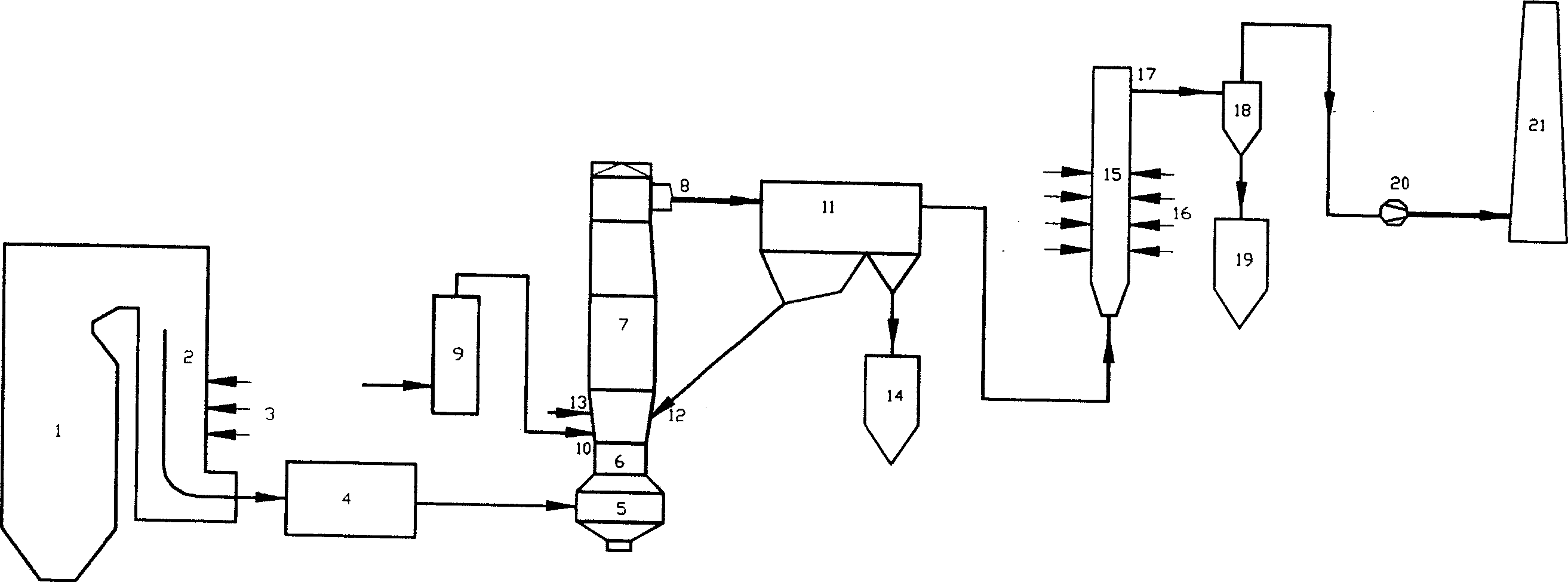
Measuring nitrogen oxides and other gases by ozone formation
InactiveUS20090137055A1Improve signal-to-noise ratioRecalibration requirement is also reducedMaterial analysis by electric/magnetic meansMaterial analysis by optical meansNitrogen dioxideNitric oxide
A photochemical sensing system enables the measurement of nitrogen oxides (nitrogen dioxide and nitric oxide) by photolyzing nitrogen dioxide to form oxygen atoms which combine with oxygen molecules to form ozone. Ozone reacts with nitric oxide to for nitrogen dioxide-decreasing ozone. Changes in ozone concentration are measured as a surrogate for the nitrogen dioxide and nitric oxide. Any species which photolyzes to yield oxygen atoms may be measured by this technique. Additional specificity for nitrogen oxides is conferred by allowing the nitric oxide to react with the ozone to recreate the nitrogen dioxide. By periodically photolyzing the nitrogen dioxide (to form ozone), and then allowing the resulting nitric oxide to react with the ozone (thereby reducing ozone), a pulsed signal is obtained whose amplitude is proportional to the total amount of nitrogen dioxide and nitric oxide present. Medical applications include measuring nitric oxide concentrations in expired air samples.
Owner:BOGNAR JOHN A
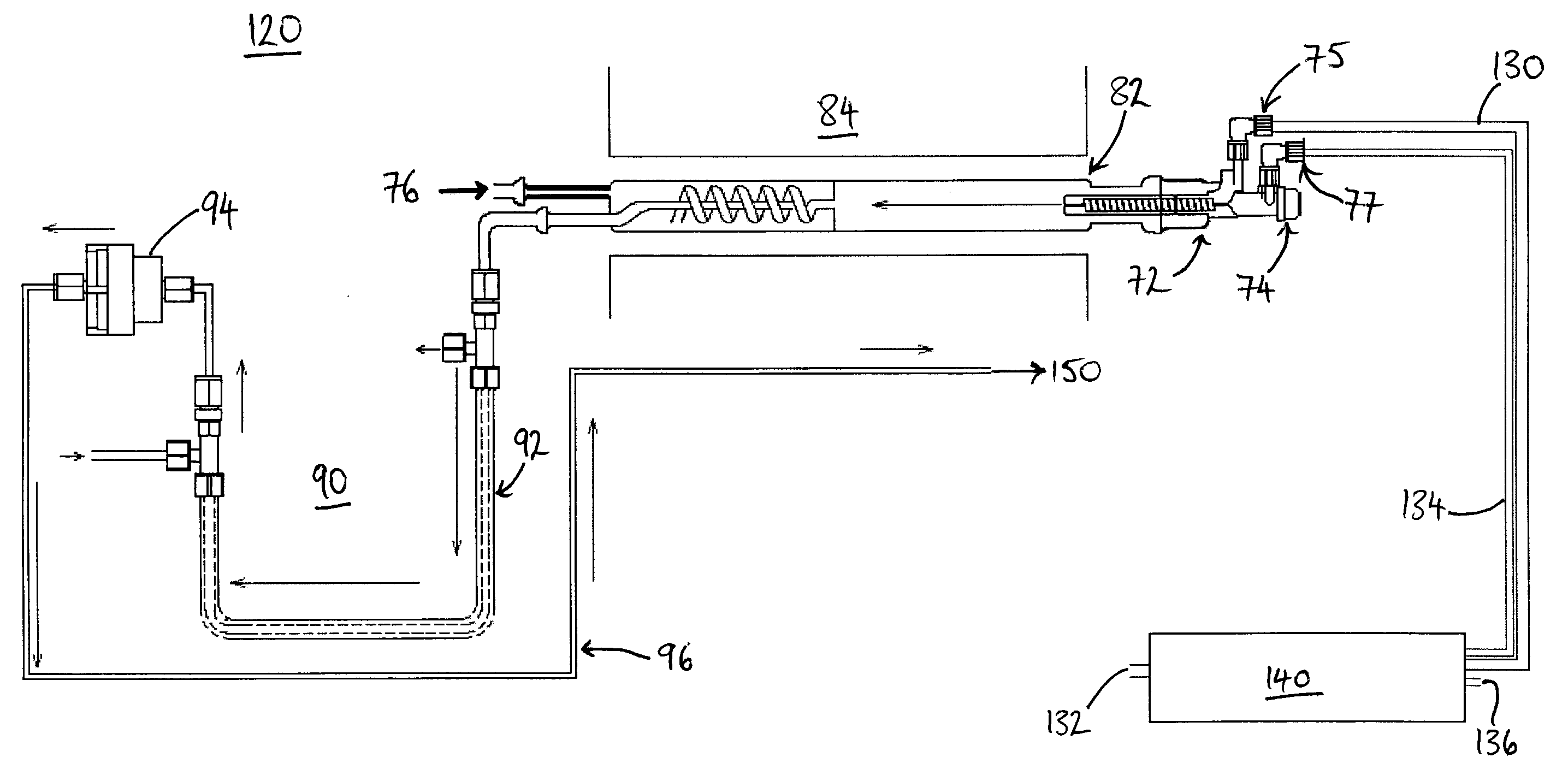
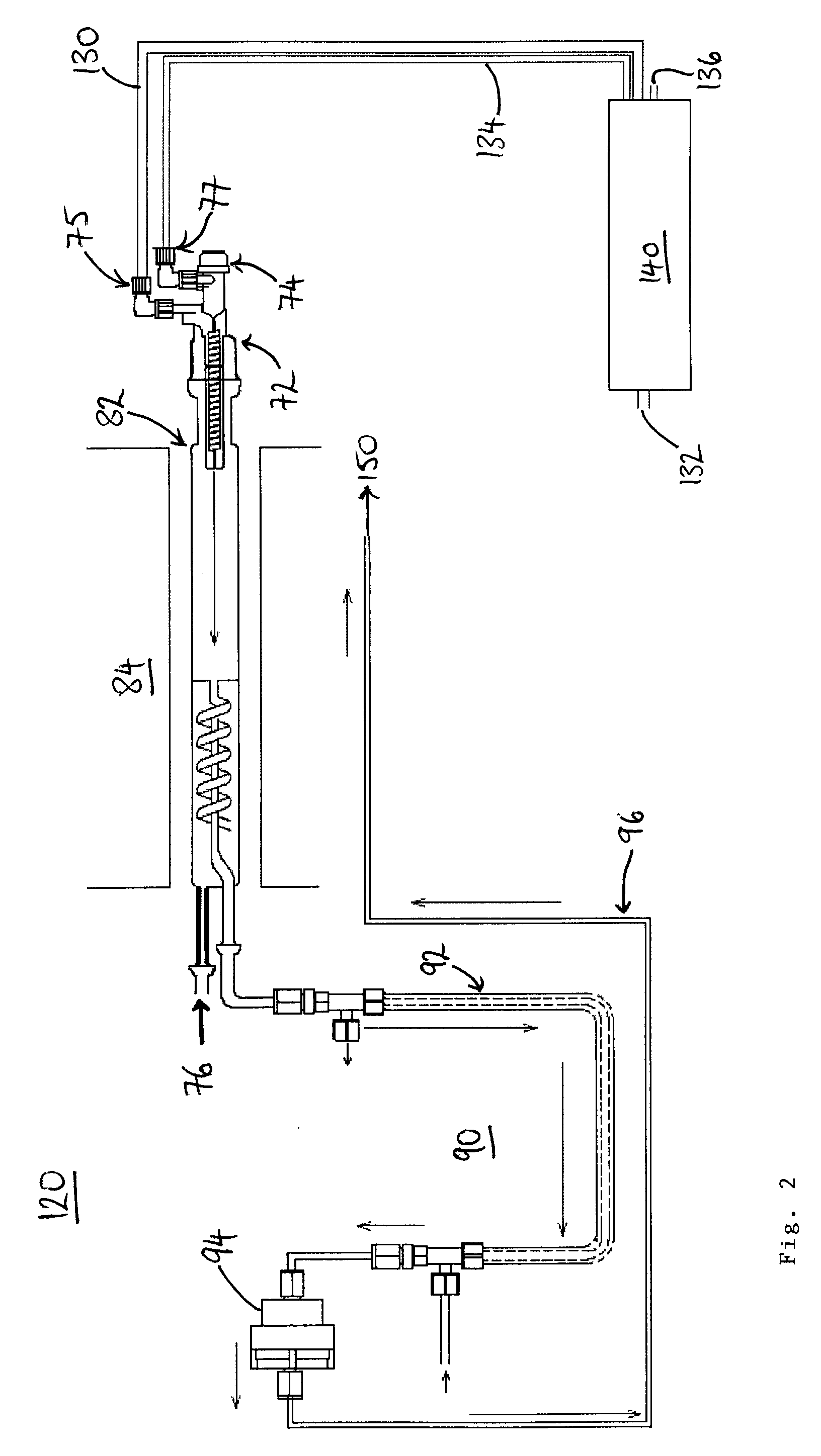
Apparatus and method for generating nitrogen oxides
InactiveUS20080176335A1Possible to useIncrease productionChemical analysis using combustionNitrogen compoundsCombustion chamberWorking temperature
A combustion analyzer apparatus and method for combustion analysing a sample, the analyzer comprising a combustion chamber (82) for receiving a sample for combustion therein to form combustion products, and a fluid supply apparatus for supplying fluid(s) into the chamber. The fluid supply apparatus (130-140) comprises a nitrogen oxides (NOx) generating apparatus (140,190,210,240) and is arranged to supply NOx into the combustion chamber. A yield of sulphur dioxide in the combustion products may thereby be improved. The NOx generating apparatus may be operated at a raised working temperature. The NOx generating apparatus may be provided by an ozonator with a supply of nitrogen and oxygen. A Venturi tube arrangement (246) may draw the generated NOx into a (carrier or oxygen) gas line to the combustion chamber. Ozone may be supplied to the combustion products to convert nitrogen monoxide therein to nitrogen dioxide. The NOx and ozone may be supplied by a single device (210,240).
Owner:THERMO ELECTRON MFG
Method for combined removal of mercury and nitrogen oxides from off-gas streams
InactiveUS7118720B1Promote oxidationProcess economyGas treatmentNitrogen compoundsNitrogen dioxideNitrogen oxide
A method for removing elemental Hg and nitric oxide simultaneously from a gas stream is provided whereby the gas stream is reacted with gaseous chlorinated compound to convert the elemental mercury to soluble mercury compounds and the nitric oxide to nitrogen dioxide. The method works to remove either mercury or nitrogen oxide in the absence or presence of each other.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
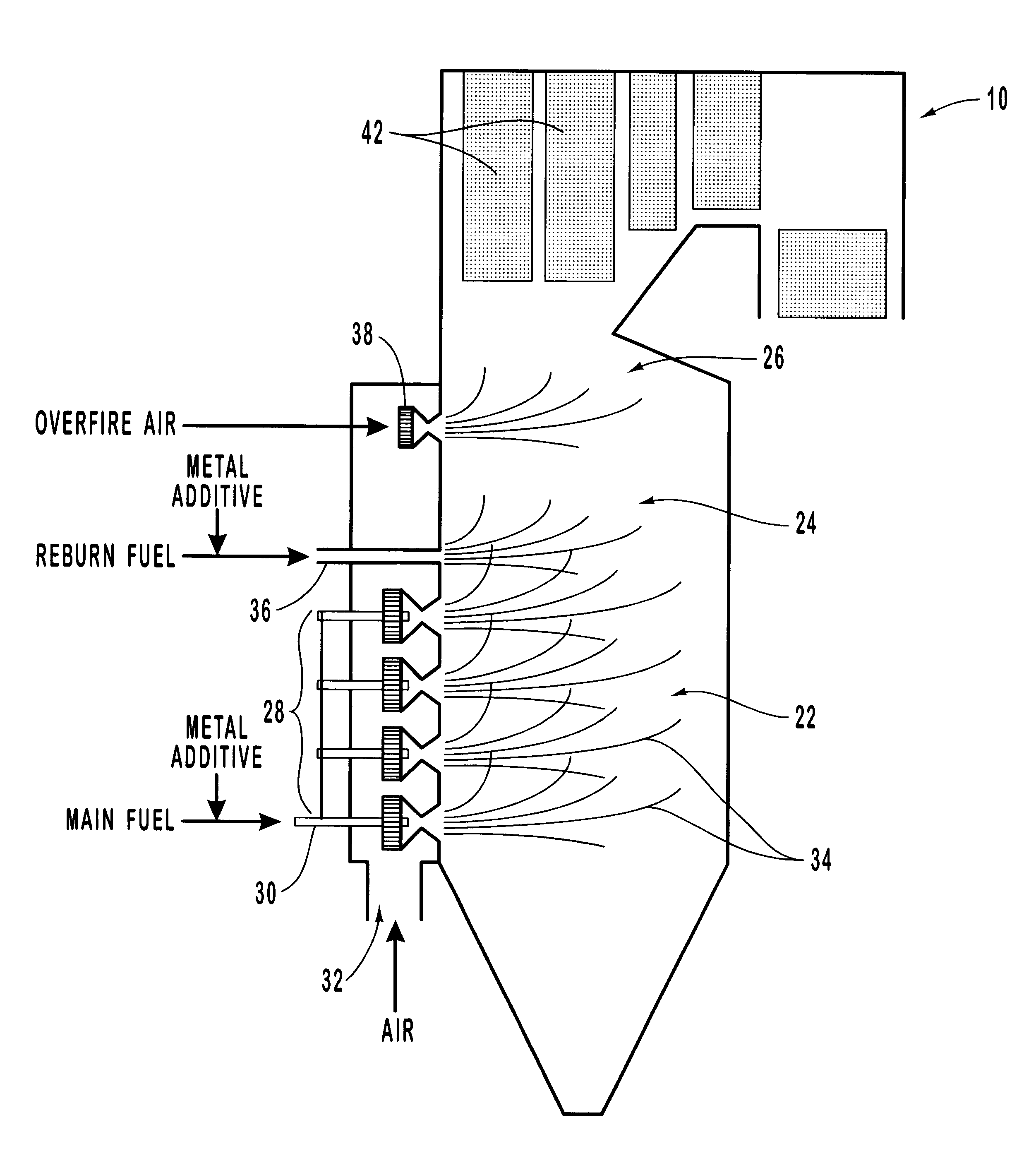
Method for reducing NOX in combustion flue gas using metal-containing additives
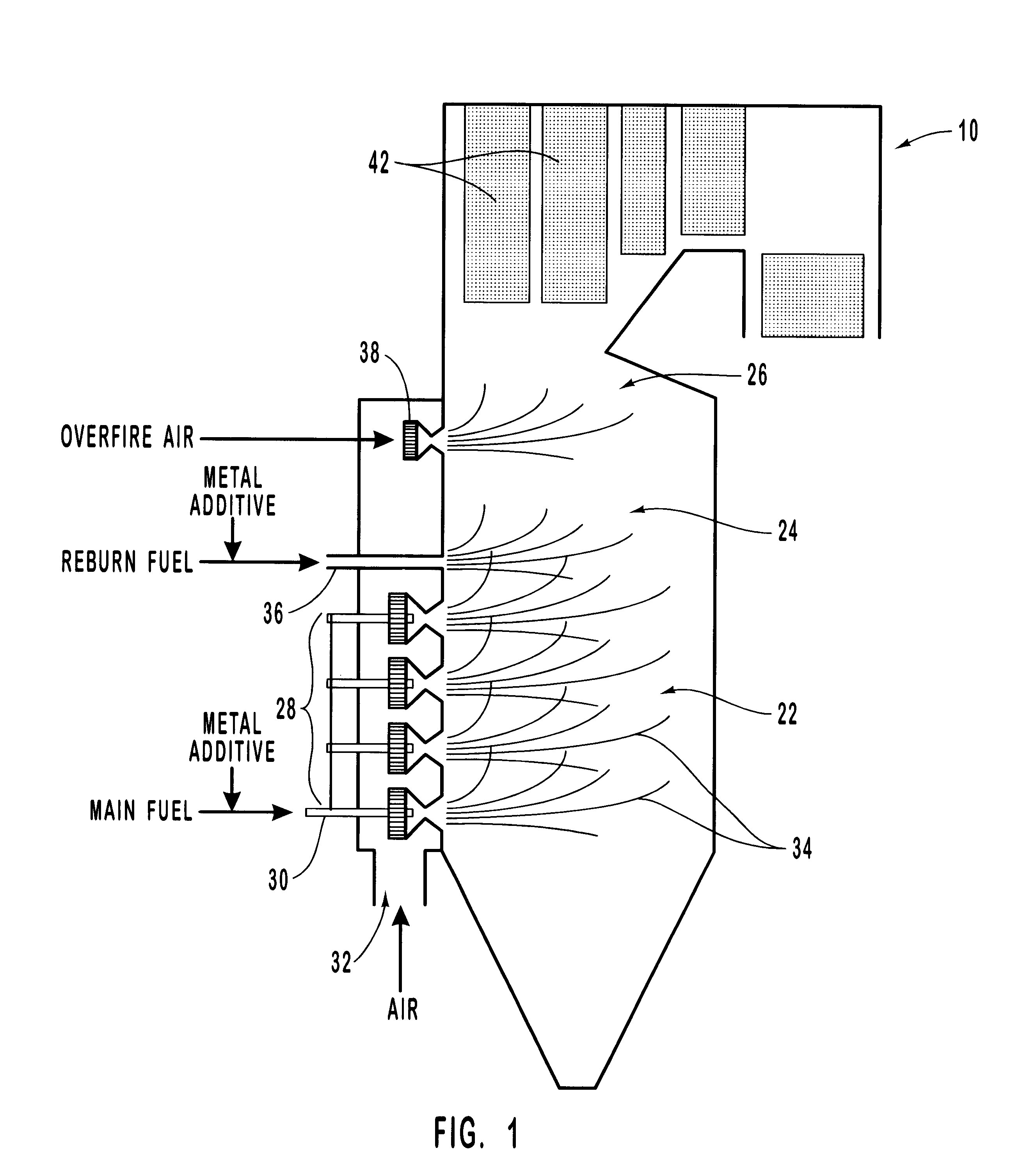
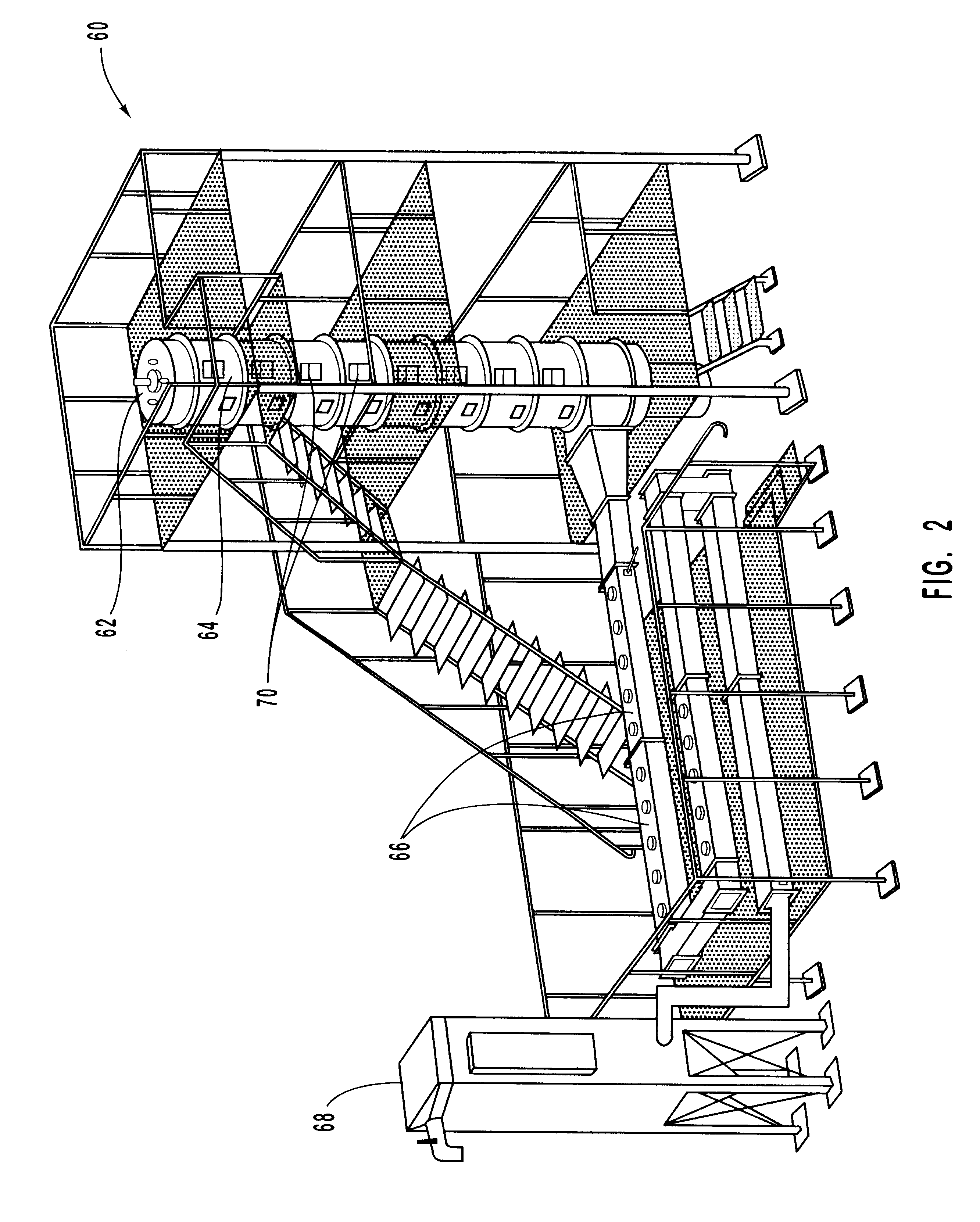
InactiveUS6206685B1Improved control deviceSure easyDispersed particle separationSolid fuel combustionAtmospheric airNitric oxide
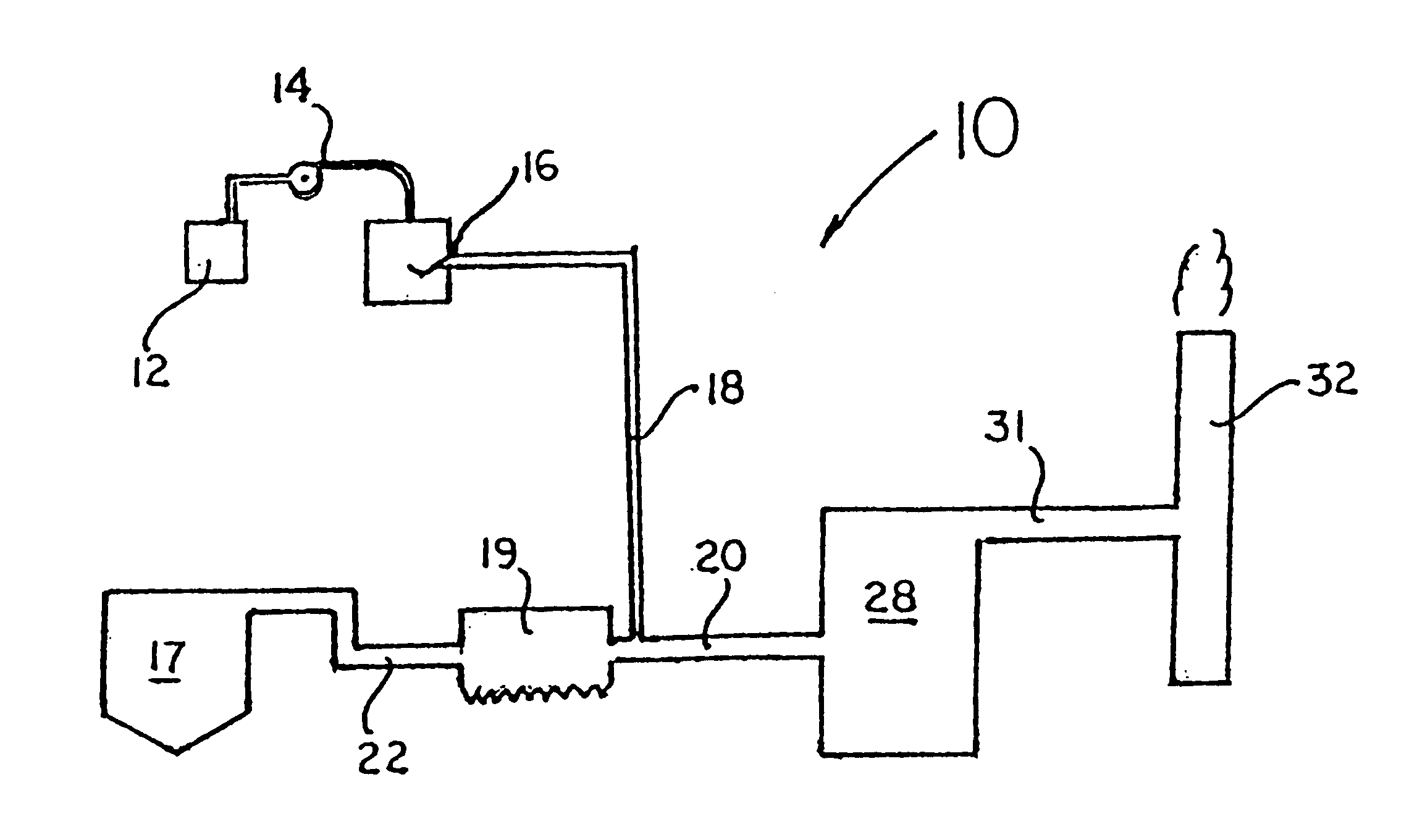
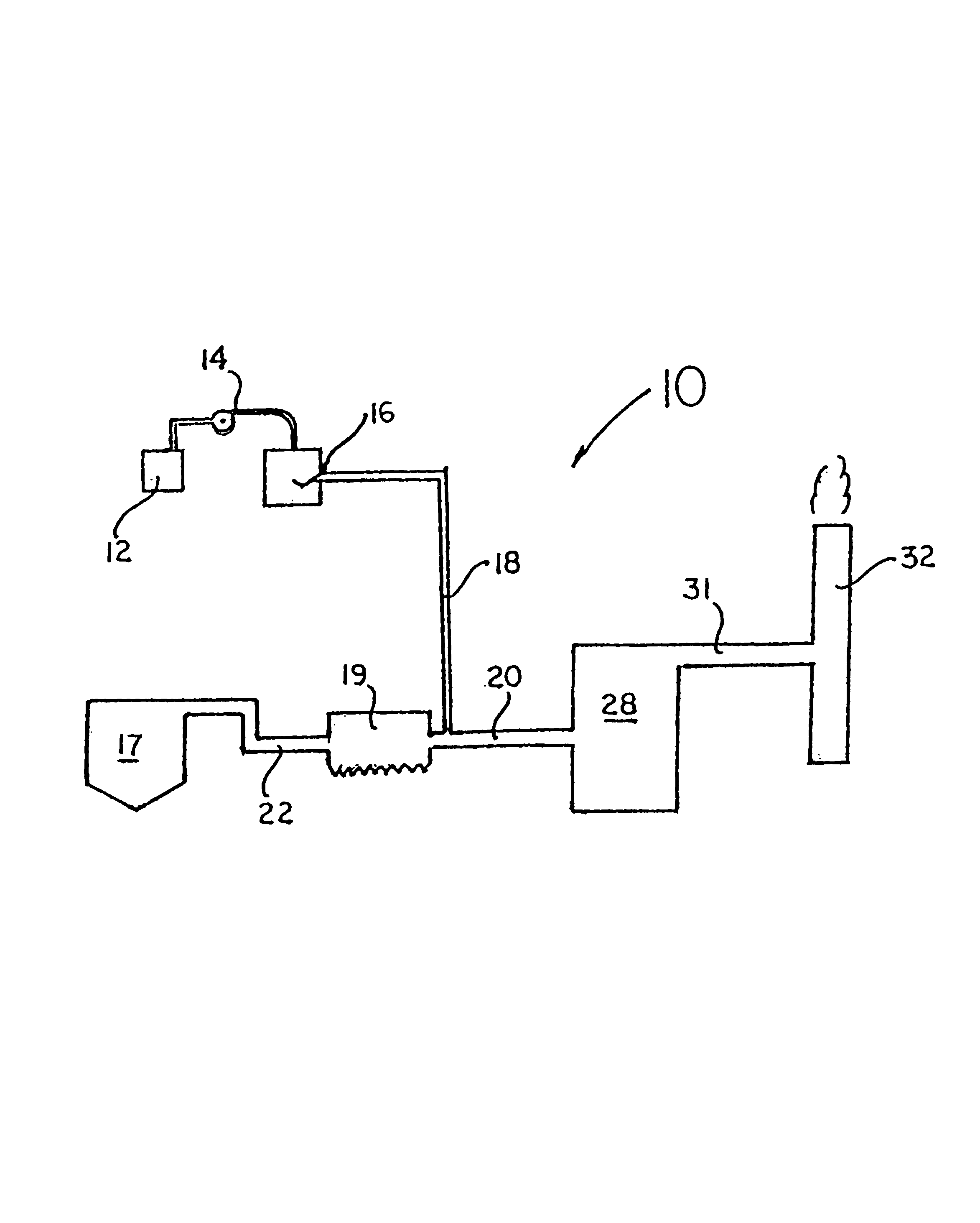
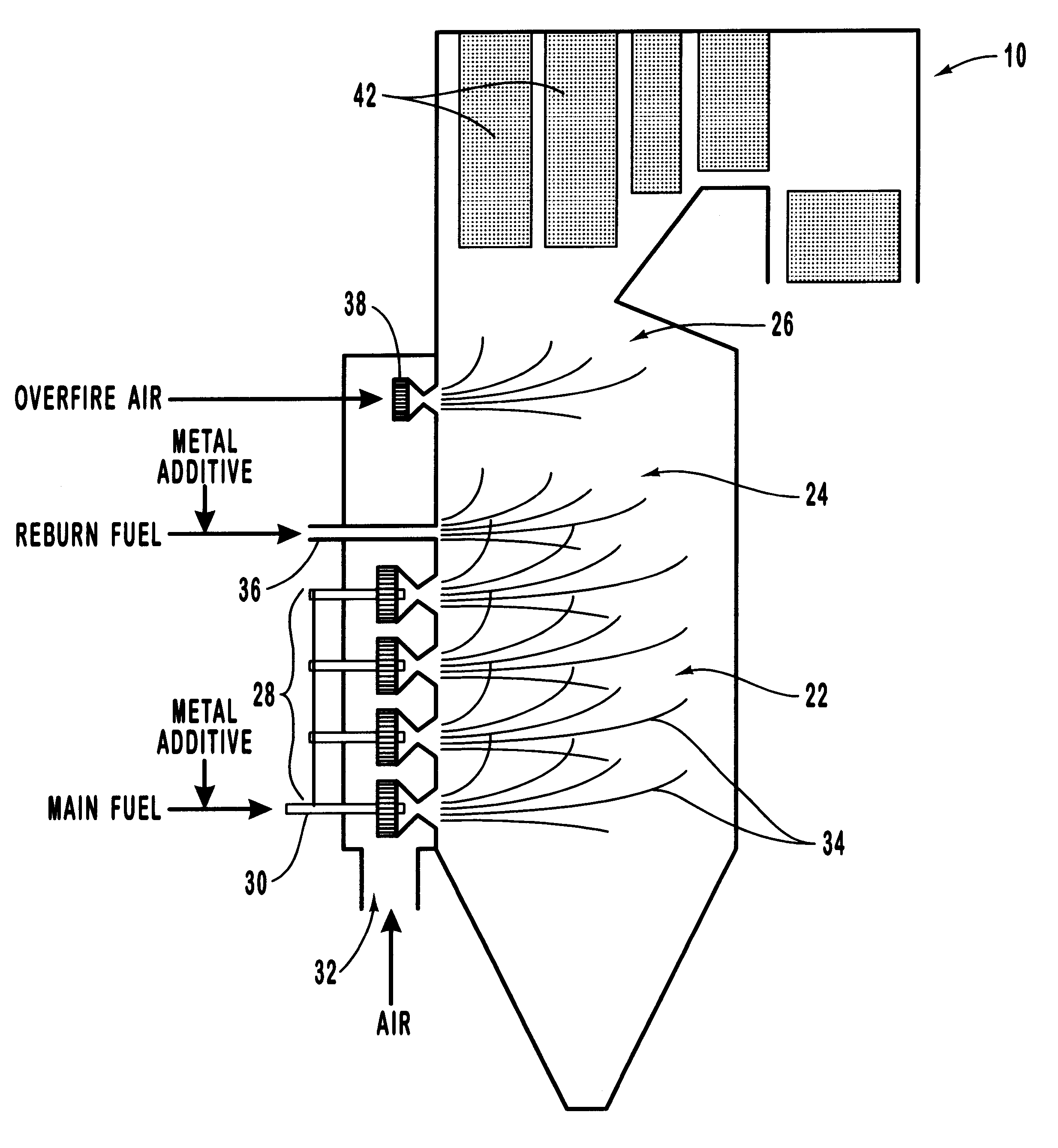
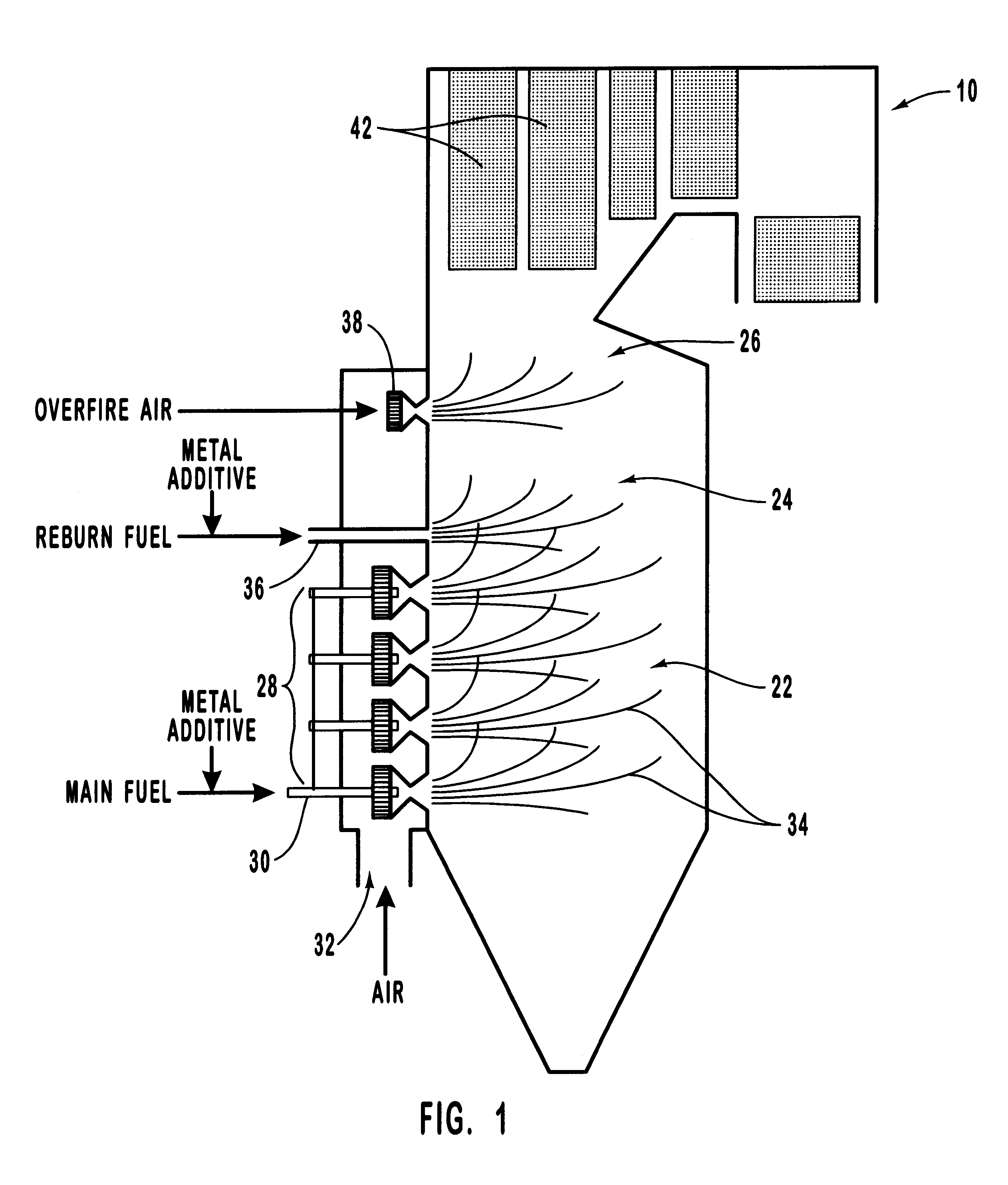
Various methods for decreasing the amount of nitrogen oxides released to the atmosphere as a component of combustion gas mixtures are provided. The methods specifically provide for the removal of nitric oxide and nitrogen dioxide (NOx) from gas mixtures emitted from stationary combustion systems. In particular, methods for improving efficiency of nitrogen oxide reduction from combustion systems include injecting metal-containing compounds into the main combustion zone and / or the reburning zone of a combustion system. The metal containing compounds react with active combustion species, and these reactions change radical concentrations and significantly improve NOx conversion to molecular nitrogen. The metal-containing additives can be injected with the main fuel, in the main combustion zone, with secondary or reburning fuel addition, or at several locations in the main combustion zone and reburning zone. Optionally, nitrogenous reducing agents and / or overfire air can be injected downstream to further increase NOx reduction.
Owner:GE ENERGY & ENVIRONMENTAL RES
Multi-component removal in flue gas by aqua ammonia
InactiveUS7255842B1Regeneration process is less-costlyIncrease load capacityGas treatmentNitrogen compoundsNitric oxideSlurry
A new method for the removal of environmental compounds from gaseous streams, in particular, flue gas streams. The new method involves first oxidizing some or all of the acid anhydrides contained in the gas stream such as sulfur dioxide (SO2) and nitric oxide (NO) and nitrous oxide (N2O) to sulfur trioxide (SO3) and nitrogen dioxide (NO2). The gas stream is subsequently treated with aqua ammonia or ammonium hydroxide which captures the compounds via chemical absorption through acid-base or neutralization reactions. The products of the reactions can be collected as slurries, dewatered, and dried for use as fertilizers, or once the slurries have been dewatered, used directly as fertilizers. The ammonium hydroxide can be regenerated and recycled for use via thermal decomposition of ammonium bicarbonate, one of the products formed. There are alternative embodiments which entail stoichiometric scrubbing of nitrogen oxides and sulfur oxides with subsequent separate scrubbing of carbon dioxide.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
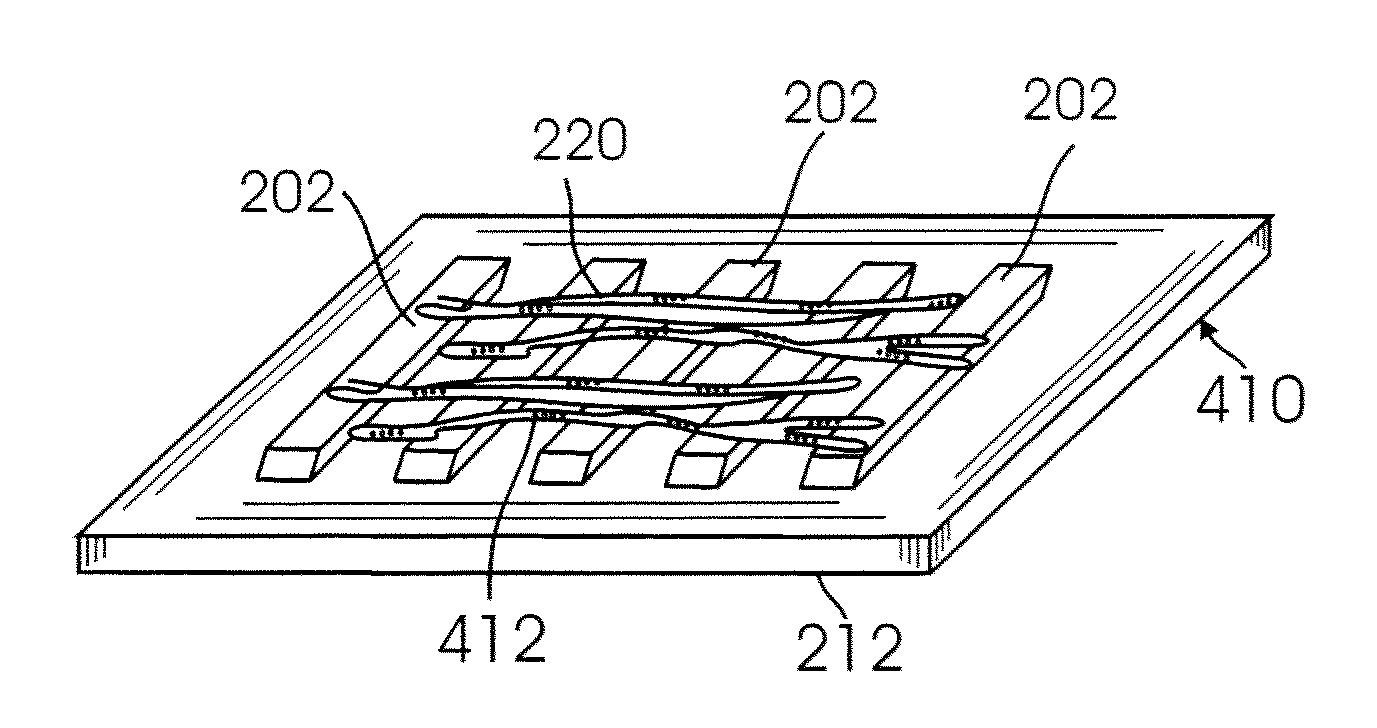
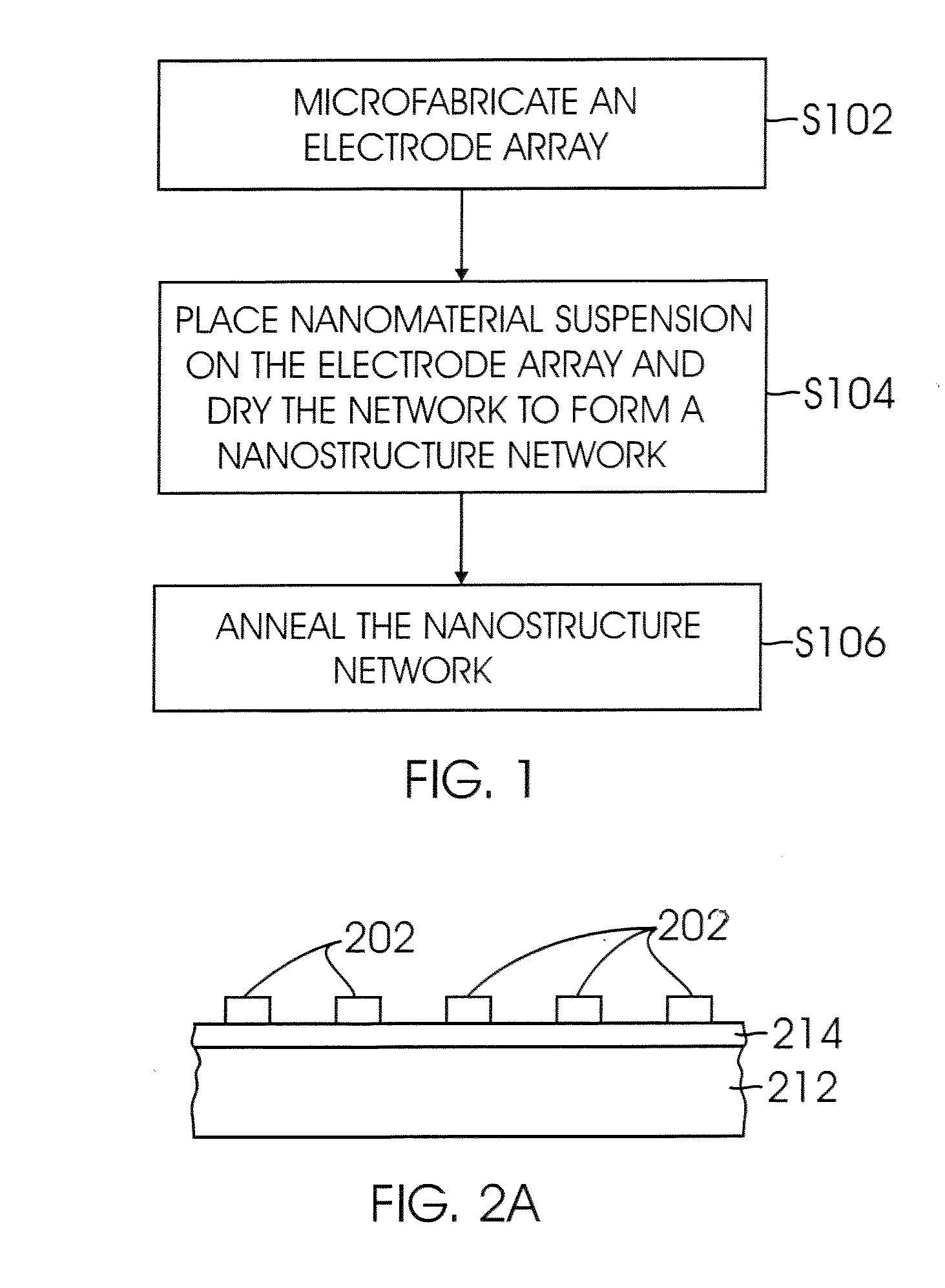
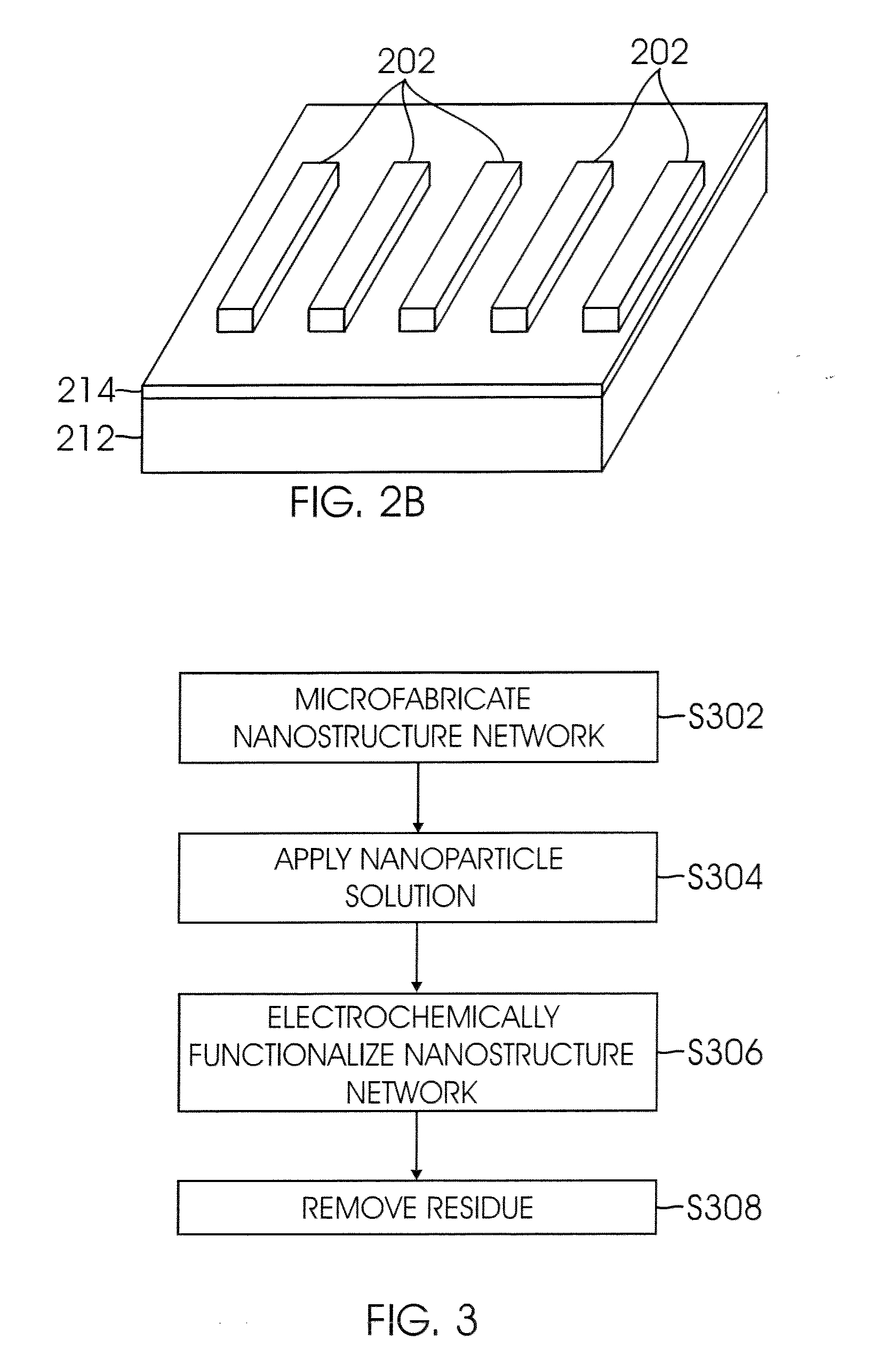
Nanomaterial-based gas sensors
InactiveUS20100089772A1High sensitivityLow detection limitWeather/light/corrosion resistanceVolume/mass flow measurementWater vaporCarbon nanotube
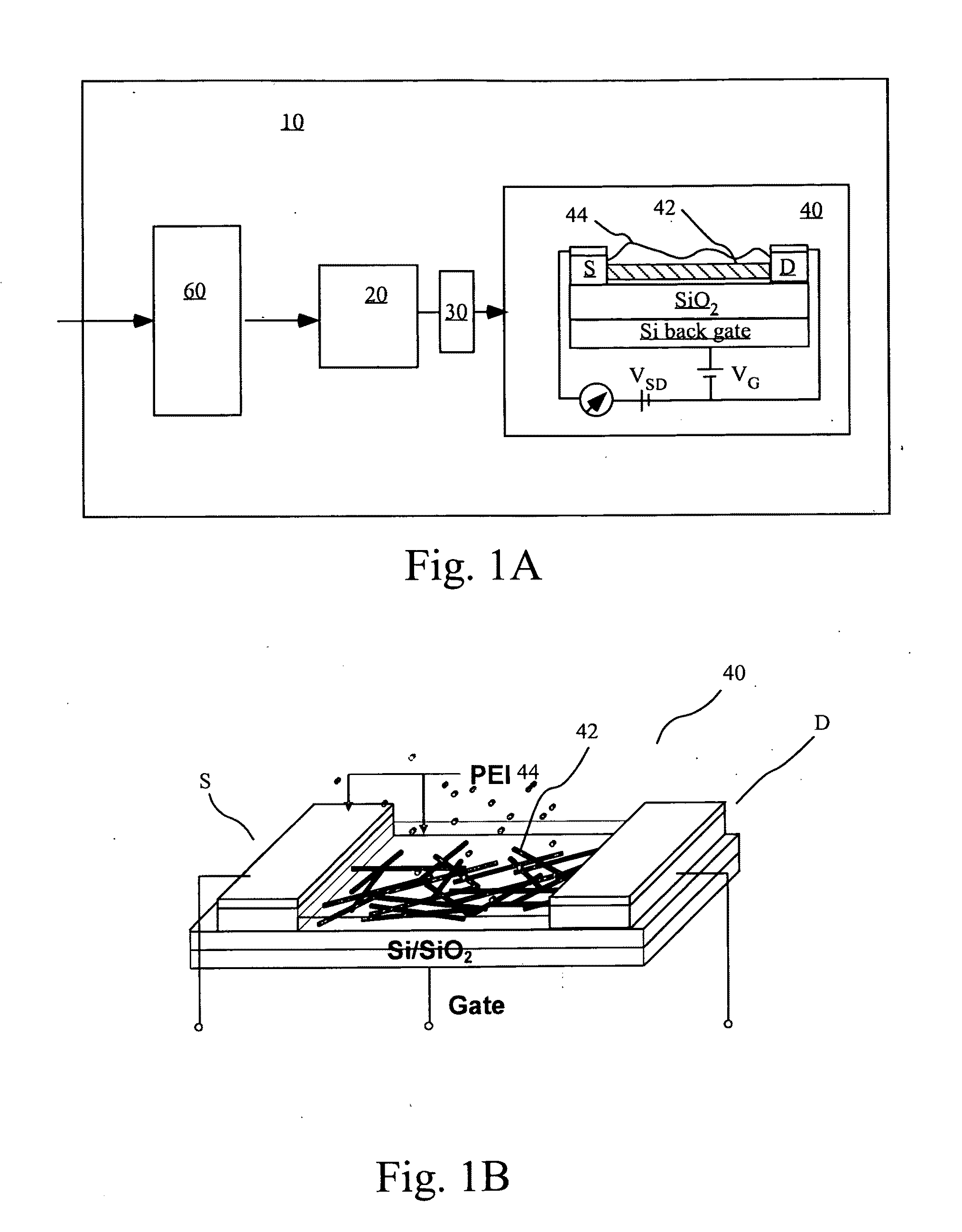
A gas sensing device (nanosensor) includes a substrate with at least a pair of conductive electrodes spaced apart by a gap, and an electrochemically functionalized semiconductive nanomaterial bridging the gap between the electrodes to form a nanostructure network. The nanomaterial may be single-walled carbon nanotubes (SWNTs) functionalized by the deposition of nanoparticles selected from the group consisting of an elemental metal (e.g., gold or palladium), a doped polymer (e.g., camphor-sulfonic acid doped polyaniline), and a metal oxide (e.g. tin oxide). Depending on the nanoparticles employed in the functionalization, the nanosensor may be used to detect a selected gas, such as hydrogen, mercury vapor, hydrogen sulfide, nitrogen dioxide, methane, water vapor, and / or ammonia, in a gaseous environment.
Owner:RGT UNIV OF CALIFORNIA
Methods for reducing NOx in combustion flue gas using metal-containing additives
InactiveUS6471506B1Simple and inexpensive methodReduce penetrationDispersed particle separationIncinerator apparatusAtmospheric airMolecular nitrogen
Various methods for decreasing the amount of nitrogen oxides released to the atmosphere as a component of combustion gas mixtures are provided. The methods specifically provide for the removal of nitric oxide and nitrogen dioxide (NOx) from gas mixtures emitted from stationary combustion systems. In particular, methods for improving efficiency of nitrogen oxide reduction from combustion systems include injecting metal-containing compounds into the main combustion zone and / or the reburning zone of a combustion system. The metal containing compounds react with active combustion species, and these reactions change radical concentrations and significantly improve NOx conversion to molecular nitrogen. The metal-containing additives can be injected with the main fuel, in the main combustion zone, with secondary or reburning fuel addition, or at several locations in the main combustion zone and reburning zone. Optionally, nitrogenous reducing agents and / or overfire air can be injected downstream to further increase NOx reduction.
Owner:GE ENERGY & ENVIRONMENTAL RES
Apparatus for monitoring engine exhaust
ActiveUS7696501B2Promote absorptionHigh sensitivityInternal-combustion engine testingRadiation pyrometryUltrasound attenuationNitrogen dioxide
An apparatus for monitoring the exhaust of an engine includes a flow-through chamber for receiving exhaust, a source of electromagnetic radiation and a detector. The source provides electromagnetic radiation in a range comprising the infrared, visible and ultraviolet wavelengths. The source and a detector are arranged so that radiation passing through the chamber is incident on the detector. An electronic circuit is connected to the detector to provide a signal indicative of the attenuation of the radiation by particles in the exhaust in the chamber. The detector provides respective measures of radiation which it receives for at least two different wavelengths of the radiation, and the electronic circuit provides corresponding electrical signals indicating the attenuation of the two different wavelengths by particles within the exhaust in the chamber. The wavelengths are selected to be those for which the attenuation caused by nitrogen dioxide in the exhaust is substantially the same.
Owner:HARTRIDGE L
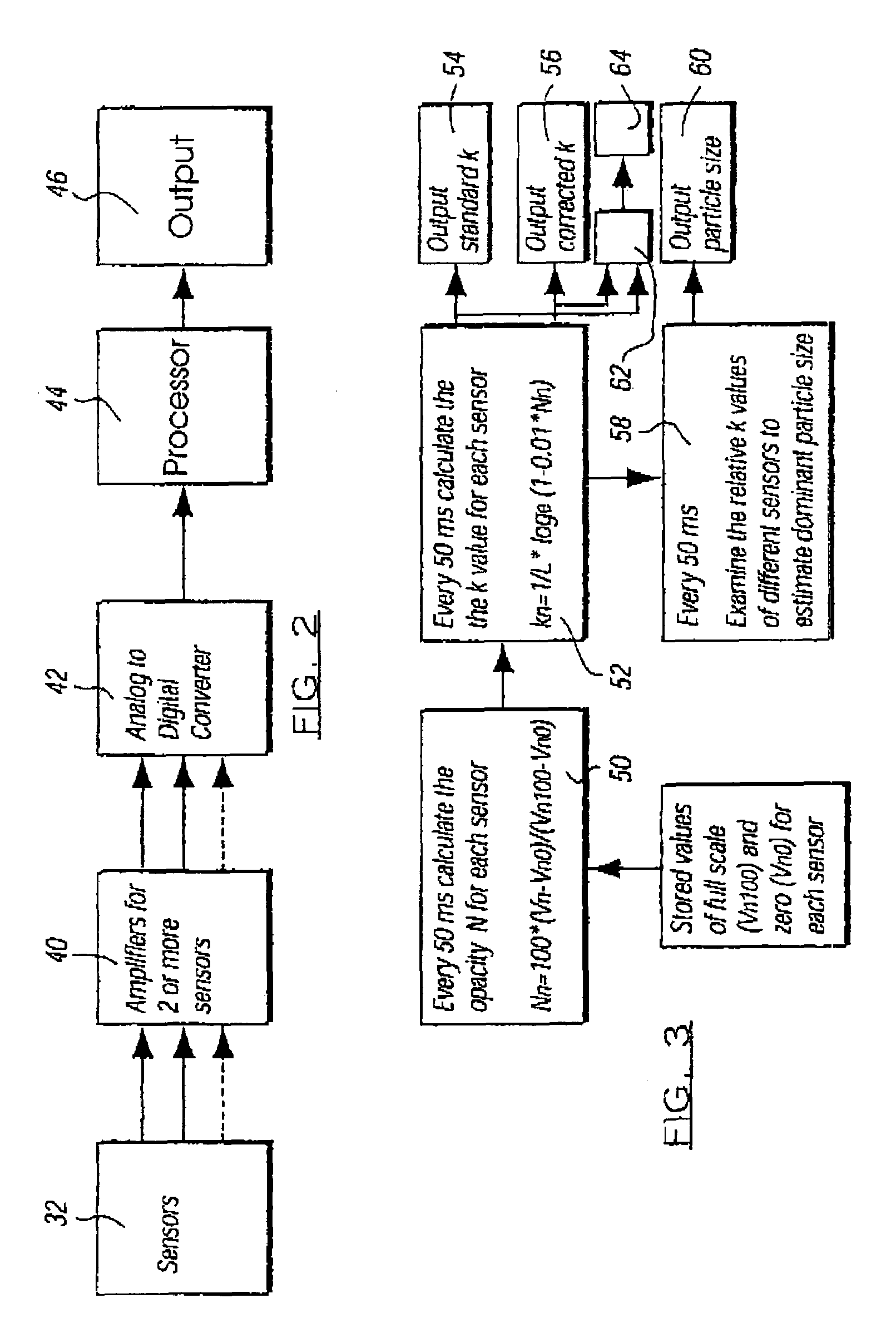
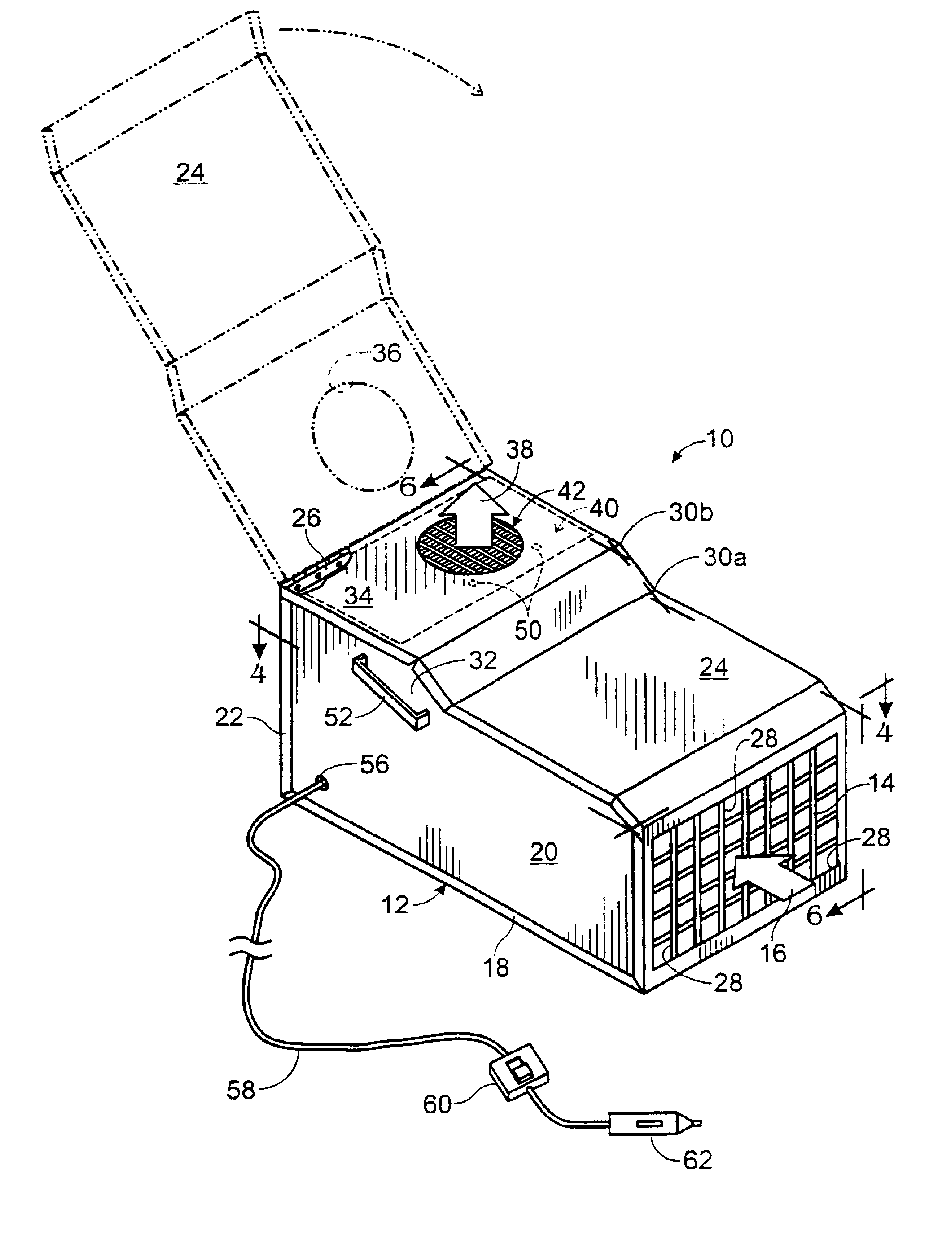
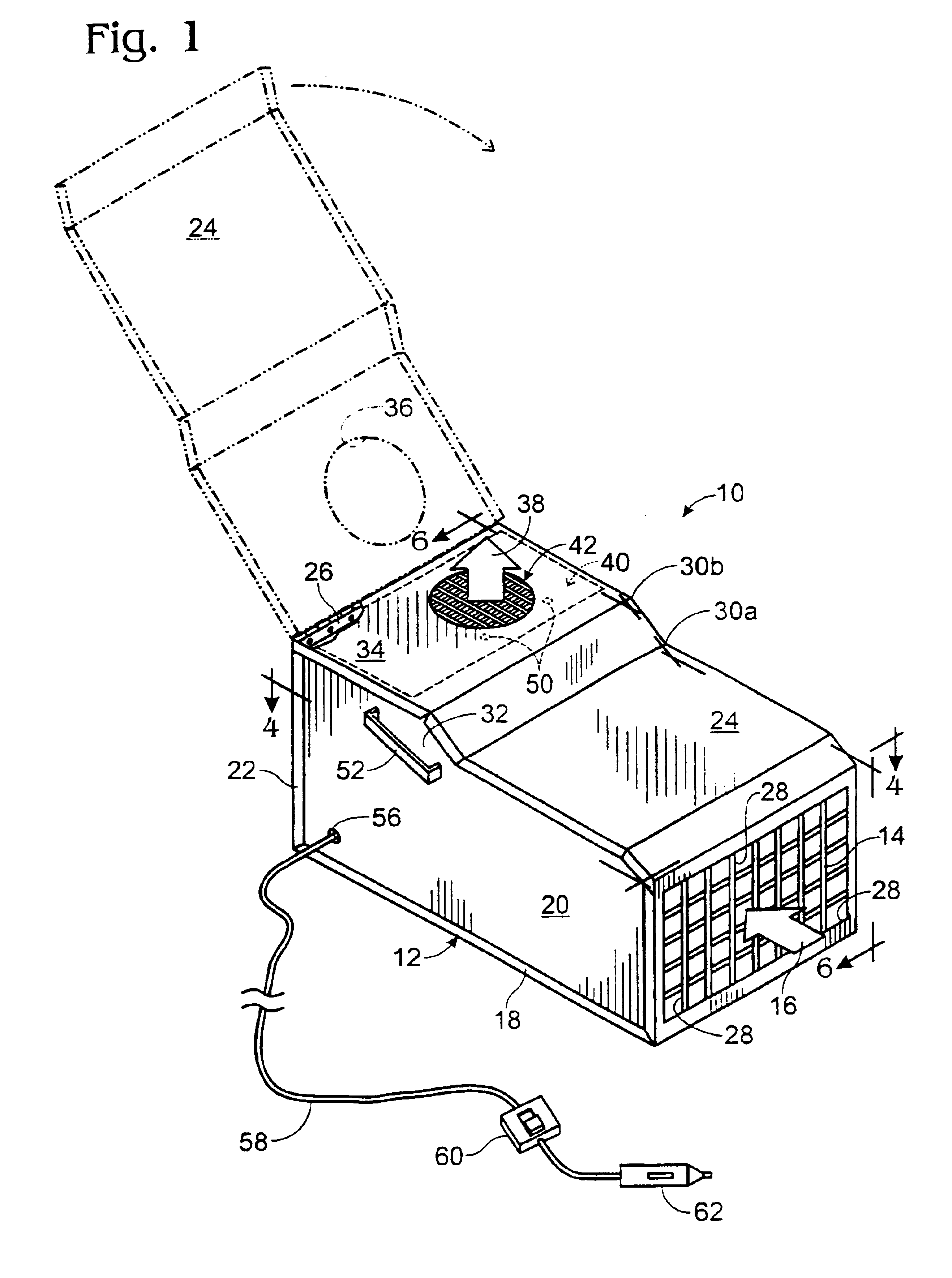
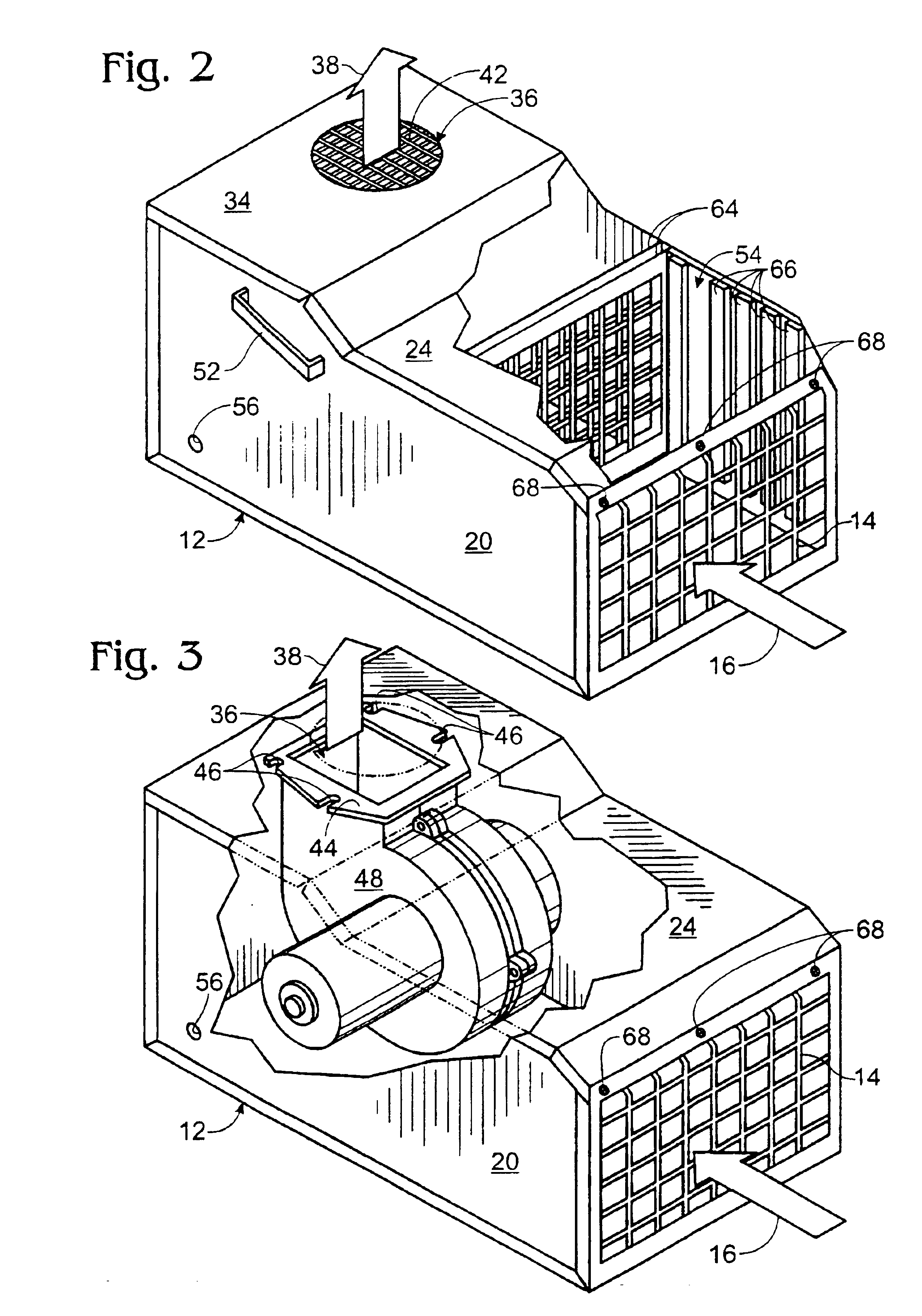
Portable motor vehicle cabin air purifier
InactiveUS6773477B2Improve comfortEasy to moveHuman health protectionCombination devicesHEPAAir cycle
A portable air purifier for reducing pollutants in the passenger cabin of a vehicle to concentrations at least as low as the US-EPA National Ambient Air Quality Standards for: carbon monoxide, ozone, nitrogen dioxide, sulfur dioxide, lead, and particulate matter; plus benzene to a European ambient air standard. The purifier includes an air inlet and air outlet in communication with the vehicle cabin, with air circulation provided by a DC electric motor / blower attachable to a vehicle power plug. The filter assembly includes a specified series of filter media packets and a HEPA filter. The machine's preferred location is in the center of a rear seat where it can serve as an armrest / console and be secured by a seat belt.
Owner:ZELLER MARIE DEHARPPORT
Cellulose oxidation by nitrogen dioxide in a perfluorinated tertiary amine solvent
ActiveUS7645874B2Efficiently oxidizedReduce usageBiocideOrganic active ingredientsAqueous alcoholNitrogen dioxide
This invention relates to a process for preparing bioabsorbable oxidized cellulose comprising combining cellulose material, with nitrogen dioxide and a nonaqueous solvent chosen from the class of perfluorinated tertiary amines. This invention also relates to a method of oxidizing cellulose material comprising introducing a solvent into the vessel, circulating the solvent through the cellulose material, adding nitrogen dioxide to said vessel containing the solvent and cellulose in the required amounts, circulating the solution for 7 to 24 hours while controlling the reaction temperature, and isolating the oxidized material. Preferably, isolation of the oxidized product is followed by first washing the oxidized cellulose material with cold water, then washing the oxidized cellulose material with an aqueous alcohol solution several times, then washing the material with 100% alcohol several times, and finally drying the oxidized material.
Owner:DEPUY SYNTHES PROD INC
Process and catalyst for reducing nitrogen oxides
InactiveUS6843971B2High activityDelay agingCyanogen compoundsInternal combustion piston enginesInternal combustion engineChemistry
A process for reducing the nitrogen oxides present in a lean exhaust gas from an internal combustion engine by selective catalytic reduction on a reduction catalyst using ammonia, wherein a fraction of the nitrogen monoxide present in the exhaust gas is oxidized to nitrogen dioxide before the exhaust gas, together with ammonia, is passed over the reduction catalyst. The reduction catalyst contains a zeolite exchanged with transition metals and oxidation of the nitrogen monoxide is performed in such a way that the exhaust gas contains 30 to 70 vol. % of nitrogen dioxide before contact with the reduction catalyst.
Owner:UMICORE AG & CO KG
Process and catalyst for reducing nitrogen oxides
InactiveUS20020039550A1High activityDelay agingInternal combustion piston enginesExhaust apparatusNitric oxideInternal combustion engine
A process for reducing the nitrogen oxides present in a lean exhaust gas from an internal combustion engine by selective catalytic reduction on a reduction catalyst using ammonia, wherein a fraction of the nitrogen monoxide present in the exhaust gas is oxidized to nitrogen dioxide before the exhaust gas, together with ammonia, is passed over the reduction catalyst. The reduction catalyst contains a zeolite exchanged with transition metals and oxidation of the nitrogen monoxide is performed in such a way that the exhaust gas contains 30 to 70 vol. % of nitrogen dioxide before contact with the reduction catalyst.
Owner:UMICORE AG & CO KG
Liquid-phase oxidation-absorption two-stage wet method flue-gas denitration technique
ActiveCN101385942ALow investment costLow running costDispersed particle separationPartial oxidationGas phase
The invention discloses a wet method smoke gas denitration technology of two segments of liquid phase oxidation and absorption, which adopts solution or mixtures of one or more of potassium permanganate, sodium chlorite, sodium hypochlorite, calcium hypochlorite, oxyful and chlorine dioxide as oxidizing agents to ensure nitrogen oxide in smoke gas contact and react with the oxidizing agents. After the nitrogen oxide is partially oxidized into nitrogen dioxide, oxidized nitrogen oxide in the smoke gas is absorbed by alkali liquid to generate corresponding nitrite. The technology adopts liquid phase oxidation to replace gas phase oxidation so as to reduce the investment and the running cost, simplify the technical process and the system structure, and enhance the operability. Compared with a method that oxidation and absorption are simultaneously carried out in the liquid phase, the two-segment technology not only can increase the removal efficiency, avoid secondary pollution caused by incomplete absorption of NO2, but also can achieve the purposes of selectively generating and recovering the nitrite in the absorption stage by controlling the oxidation degree of the oxidation stage.
Owner:ZHEJIANG TIANLAN ENVIRONMENTAL PROTECTION TECH
Cellulose oxidation by nitrogen dioxide in a perfluorinated tertiary amine solvent
ActiveUS20070054880A1Efficiently oxidizedReduce usageBiocideOrganic active ingredientsNitrogen dioxideAqueous alcohol
This invention relates to a process for preparing bioabsorbable oxidized cellulose comprising combining cellulose material, with nitrogen dioxide and a nonaqueous solvent chosen from the class of perfluorinated tertiary amines. This invention also relates to a method of oxidizing cellulose material comprising introducing a solvent into the vessel, circulating the solvent through the cellulose material, adding nitrogen dioxide to said vessel containing the solvent and cellulose in the required amounts, circulating the solution for 7 to 24 hours while controlling the reaction temperature, and isolating the oxidized material. Preferably, isolation of the oxidized product is followed by first washing the oxidized cellulose material with cold water, then washing the oxidized cellulose material with an aqueous alcohol solution several times, then washing the material with 100% alcohol several times, and finally drying the oxidized material.
Owner:DEPUY SYNTHES PROD INC
Tunable quantum cascade lasers and photoacoustic detection of trace gases, TNT, TATP and precursors acetone and hydrogen peroxide
ActiveUS20080159341A1High rejectionShorten the timeMaterial analysis by optical meansOptical resonator shape and constructionQuantum cascade laserPeroxide
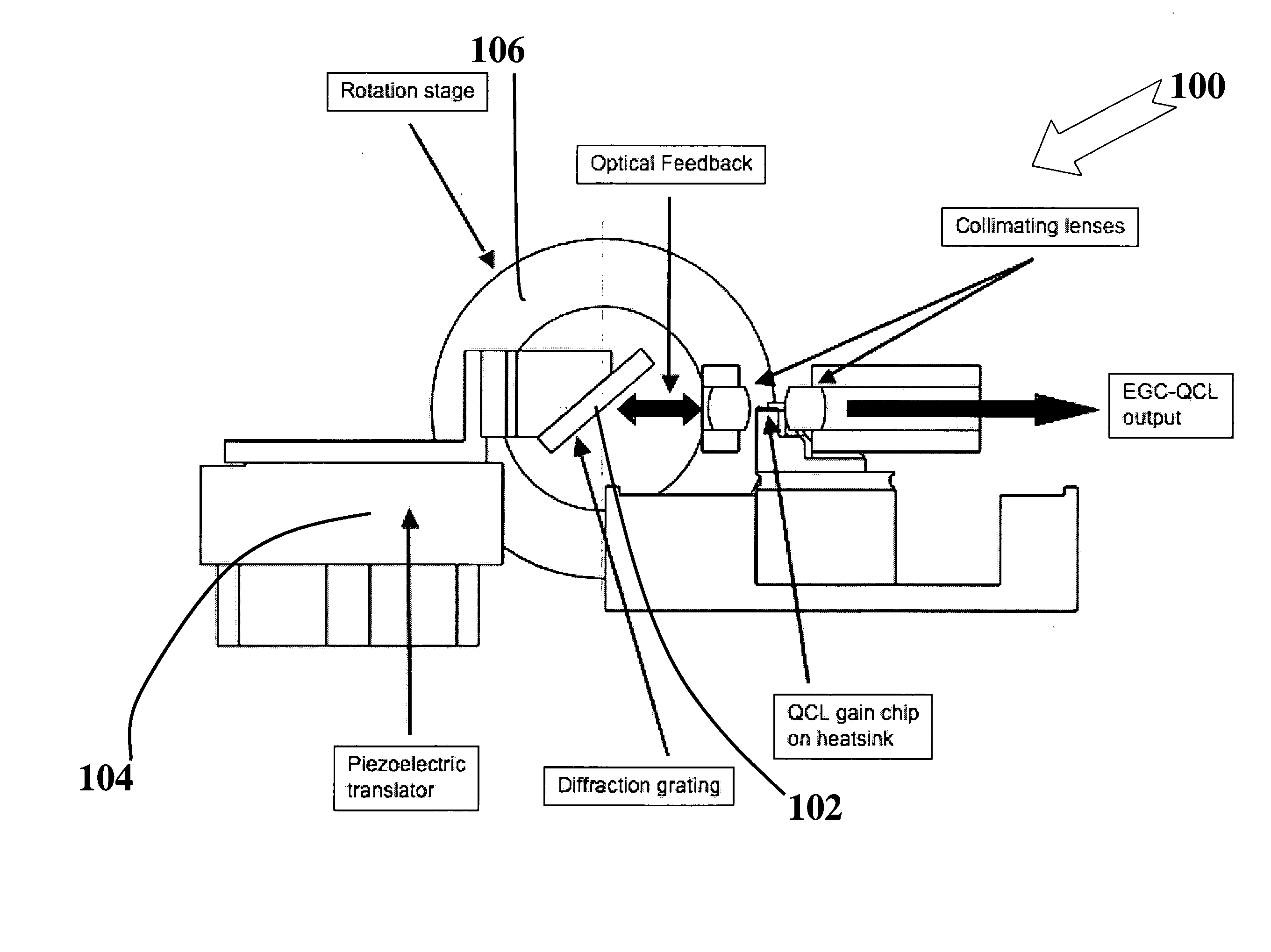
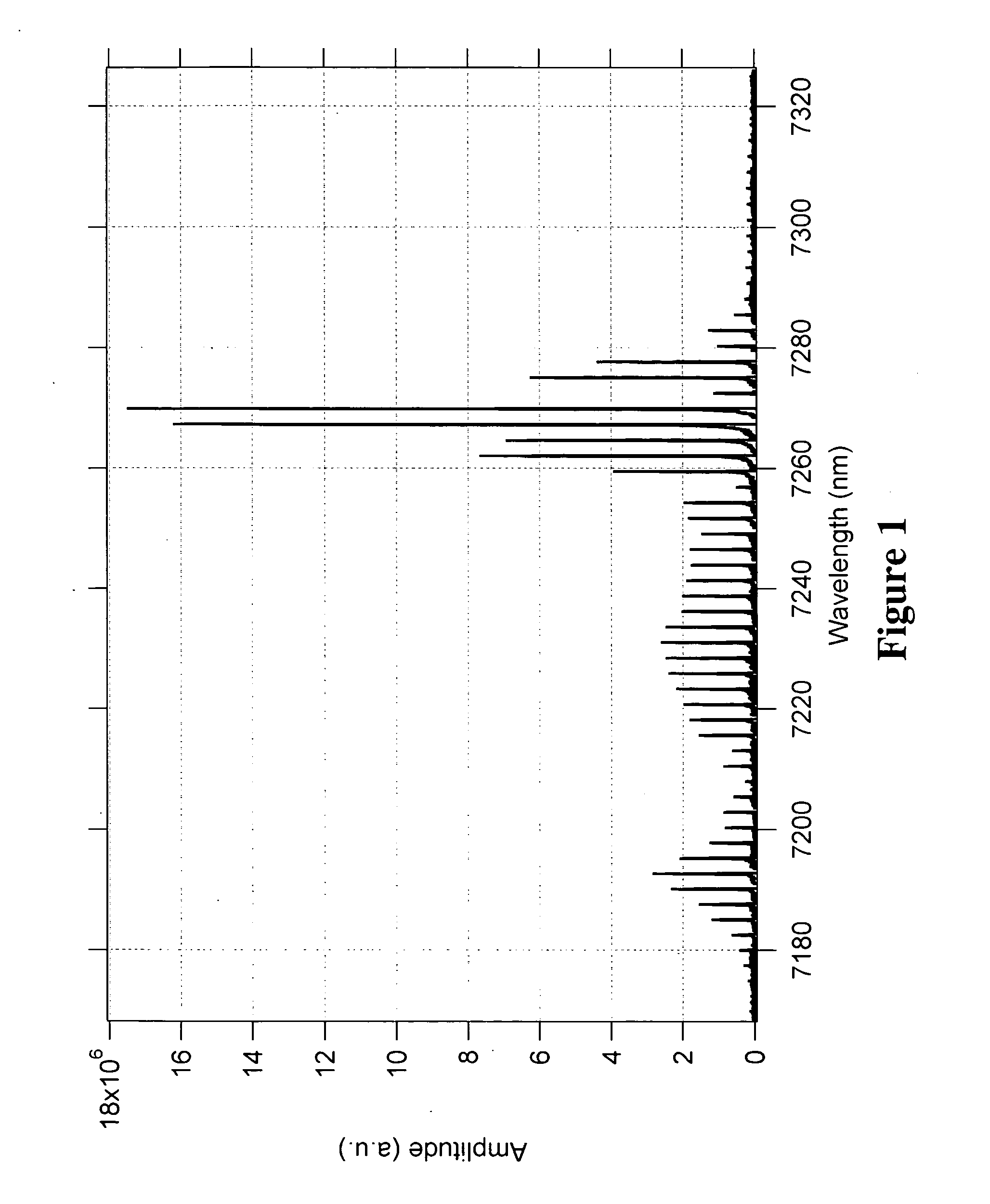
Methods and apparatus for broad tuning of single wavelength quantum cascade lasers and the use of light output from such lasers for highly sensitive detection of trace gases such as nitrogen dioxide, acetylene, and vapors of explosives such as trinitrotoluene (TNT) and triacetone triperoxide (TATP) and TATP's precursors including acetone and hydrogen peroxide. These methods and apparatus are also suitable for high sensitivity, high selectivity detection of other chemical compounds including chemical warfare agents and toxic industrial chemicals. A quantum cascade laser (QCL) system that better achieves single mode, continuous, mode-hop free tuning for use in L-PAS (laser photoacoustic spectroscopy) by independently coordinating gain chip current, diffraction grating angle and external cavity length is described. An all mechanical method that achieves similar performance is also described. Additionally, methods for improving the sensor performance by critical selection of wavelengths are presented.
Owner:DAYLIGHT SOLUTIONS
Flue gas catalytic oxidation denitration technique and catalyst thereof
ActiveCN101352645ALow costImprove efficiencyDispersed particle separationCatalyst activation/preparationNitriteResource utilization
The invention provides a smoke catalysis and oxidation denitration process which takes a catalyst using TiO2 or ZrO2-TiO2 as a carrier and Co as active ingredient, uses the oxygen contained in the smoke to oxidate the NO as NO2 which is easy to be dissolved in water, utilizes the alkali solution to absorb the NO2 and remove the NOx. The process of the invention has high denitration efficiency and low cost, can selectively recover the nitrite in the denitration outgrowth and realize the resource utilization of the outcome after controlling the content of the NO2 in the oxidated smoke.
Owner:ZHEJIANG TIANLAN ENVIRONMENTAL PROTECTION TECH
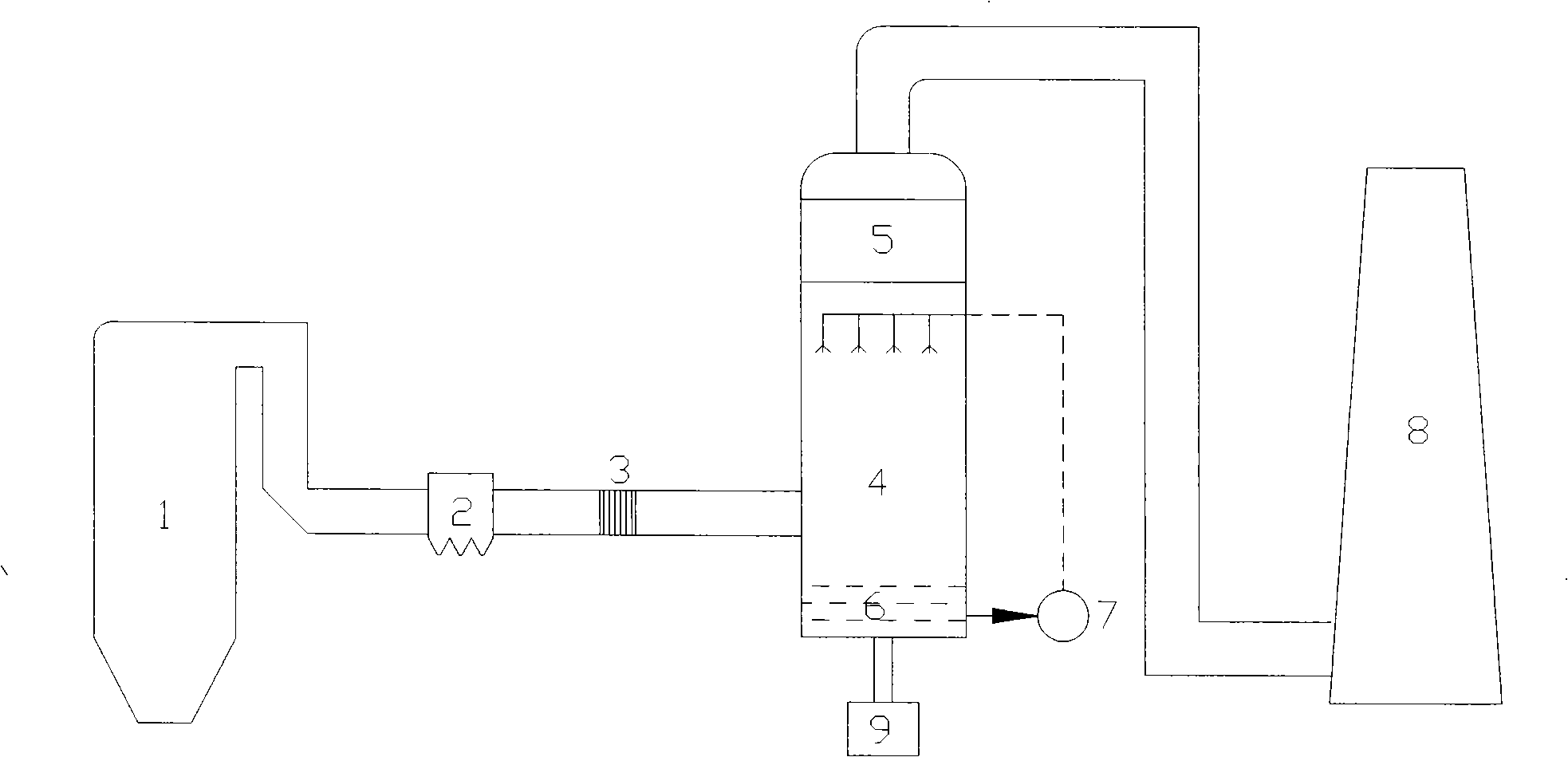
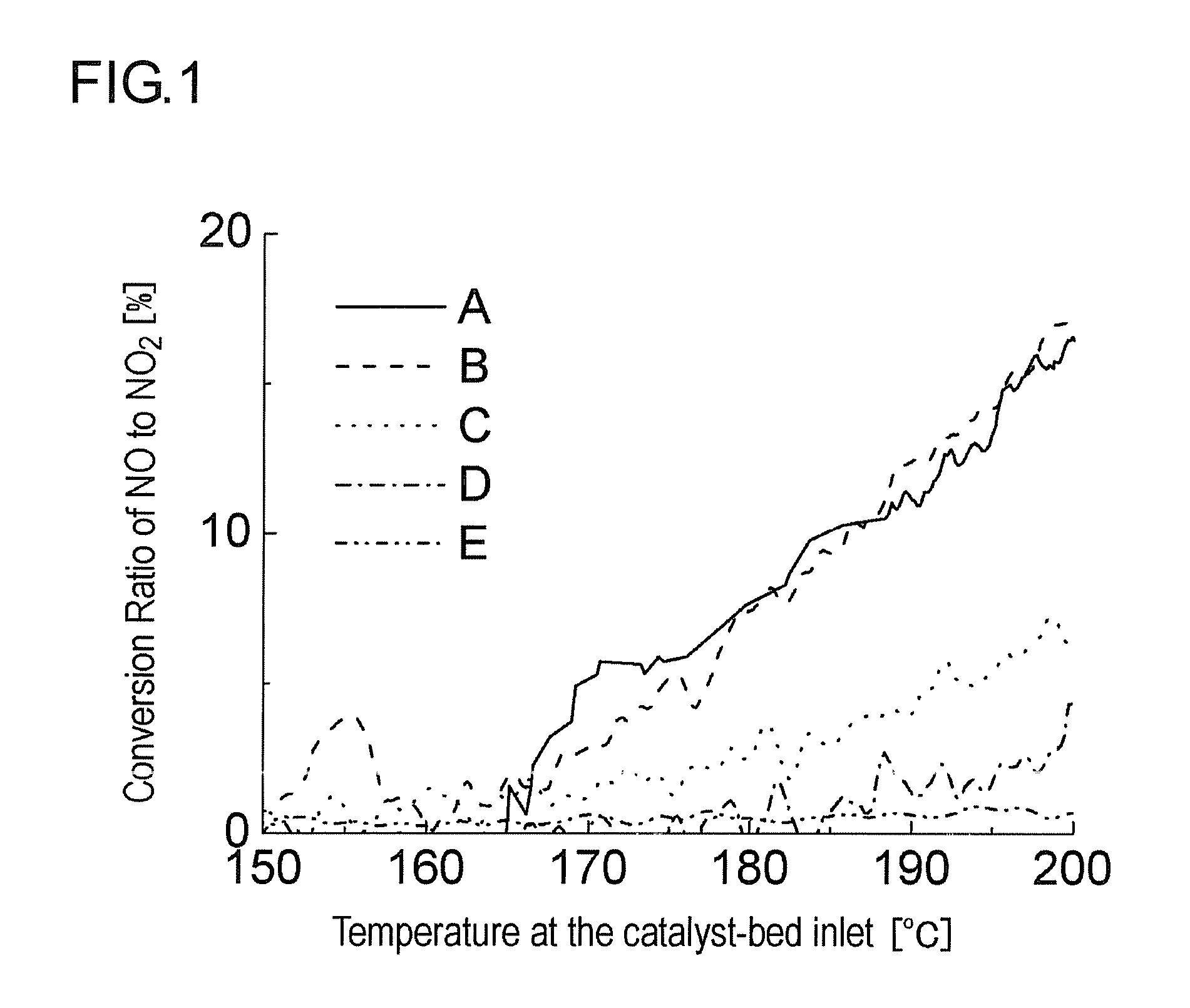
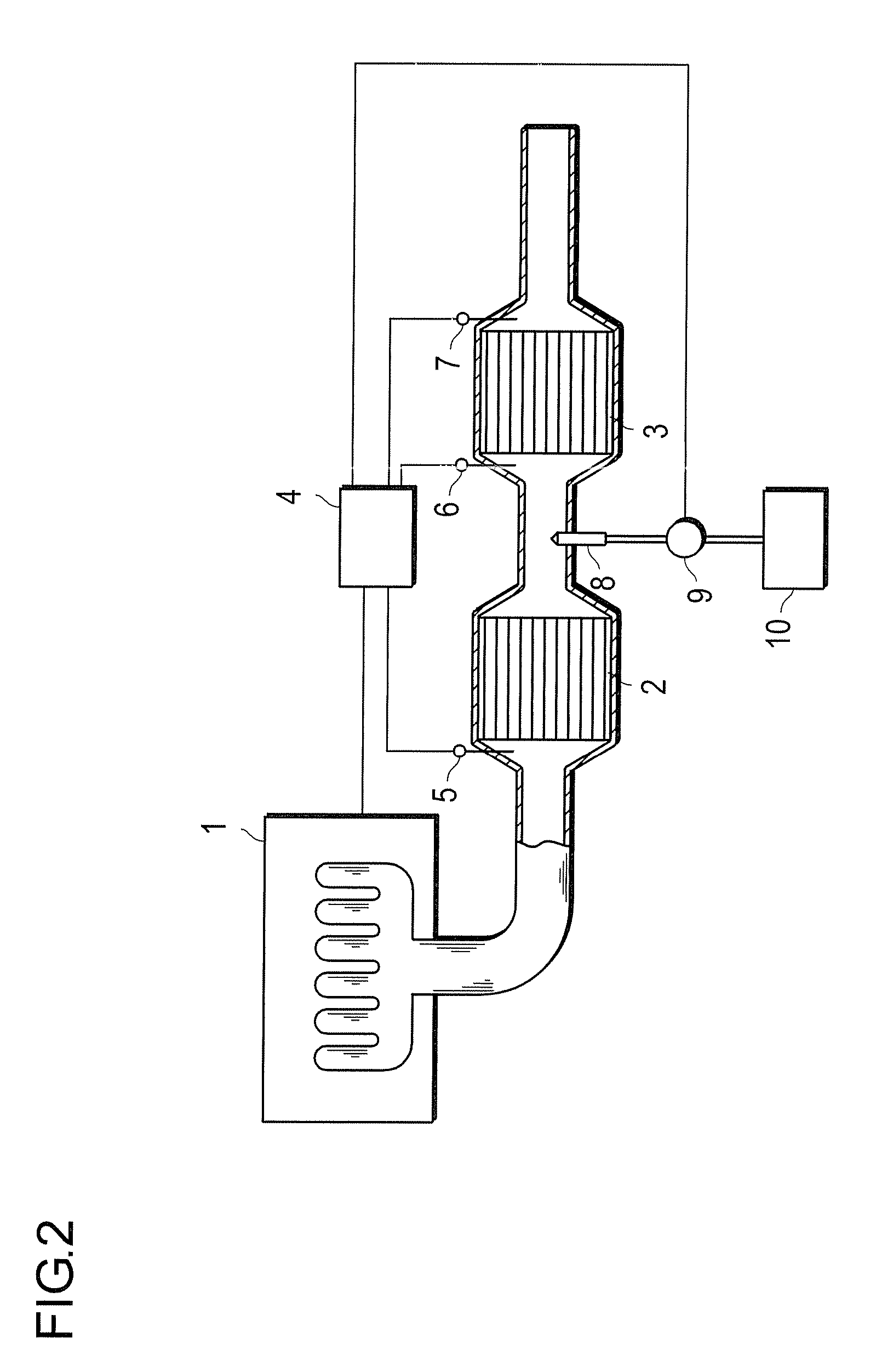
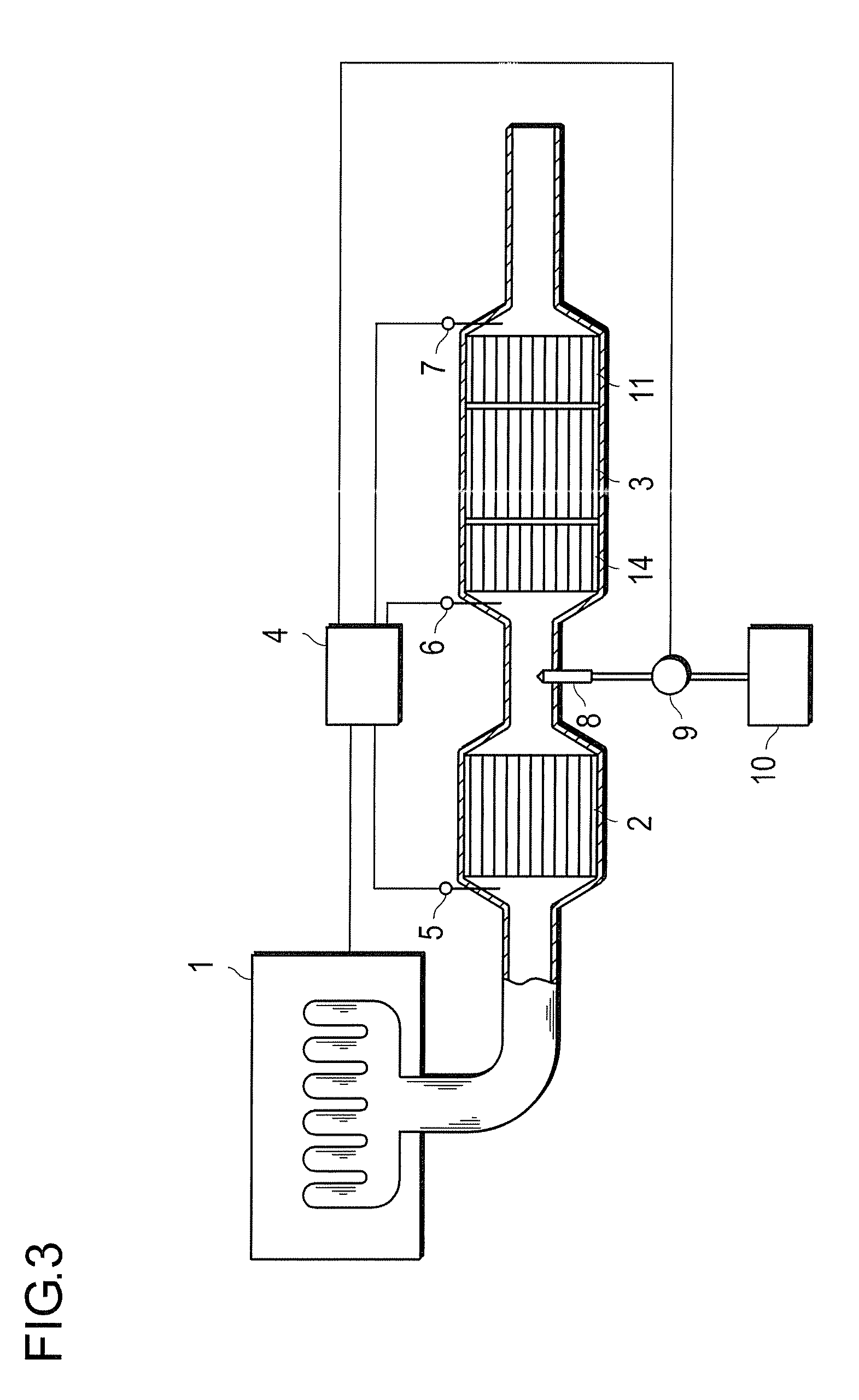
Oxidation Catalyst and Exhaust-Gas Purification System Using the Same
InactiveUS20080124264A1Promote effectiveEfficient removalCombination devicesNitrogen compoundsPlatinumNitrogen monooxide
An oxidation catalyst that efficiently promotes oxidation of NO to NO2 even in a low temperature range, and an exhaust-gas purification system and method that efficiently removes exhaust-gas components even in a low temperature range are provided. This invention provides an oxidation catalyst comprising platinum and palladium as catalytically active components, which promotes oxidation of nitrogen monoxide to nitrogen dioxide, wherein the oxidation catalyst comprises 1 to 55% by weight of the palladium relative to 100% by weight of the platinum.
Owner:ICT CO LTD +1
Wet flue gas denitration technique for nitrite recovery
ActiveCN101352644AHigh economic valueIncrease profitDispersed particle separationAir quality improvementNitriteResource utilization
The invention discloses a wet-method smoke denitration process used for recovering nitrite, comprising the steps as follows: hydrogen peroxide or ozone is taken as oxidant; the oxidant is uniformly sprayed into smoke disposed by pre-dedusting and desulfurizing so as to carry out gas oxidation reaction and lead the NO in the smoke to be oxidated as NO2; subsequently, alkali liquid is taken as absorbent so as to absorb the mixture of the oxidated NO and NO2 and generate the nitrite; the absorbent is concentrated after absorbing the NO and NO2 and cooled and crystallized; after centrifuging separation, the nitrite crystal is gained. The process of the invention can effectively remove the NOx in the smoke, gains the nitrite with high economic values at the same time, and realizes the resource utilization of denitration outgrowth.
Owner:ZHEJIANG TIANLAN ENVIRONMENTAL PROTECTION TECH
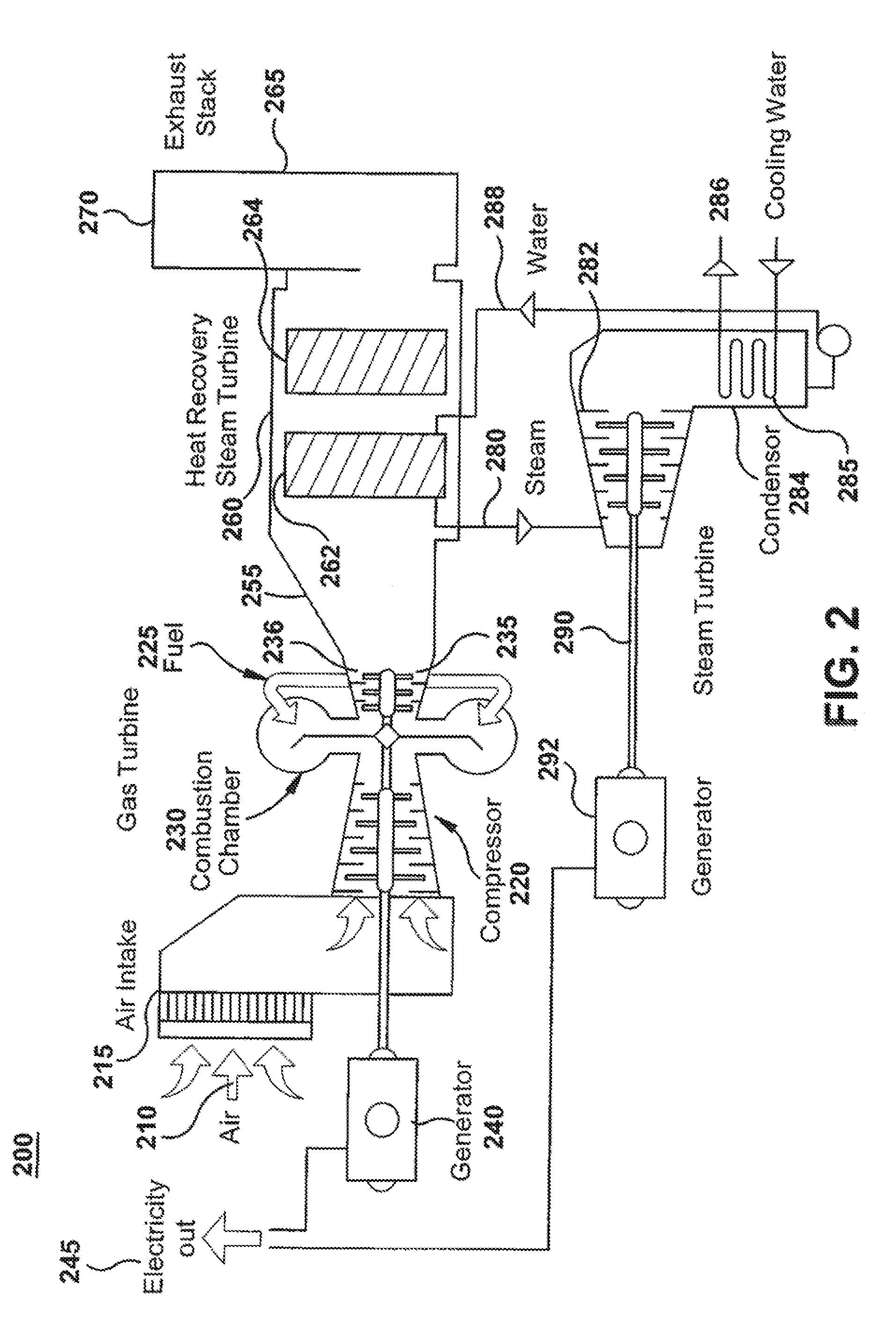
Method and apparatus for operation of co/voc oxidation catalyst to reduce no2 formation for gas turbine
ActiveUS20100215558A1Production be limitedGas treatmentNitrogen compoundsHydrocotyle bowlesioidesOxide
A power generating apparatus including a gas turbine engine combusting a fuel in air to produce shaft power and producing a flow of exhaust gases including oxides of nitrogen (NOx), carbon monoxide (CO) and hydrocarbons (HC). An emissions treatment apparatus includes in the exhaust gas flowpath a CO oxidation catalyst disposed at a location with an exhaust gas temperature for which the CO oxidation catalyst advantageously limits NO2 production. The emissions treatment apparatus further includes an ammonia injection apparatus, a mixing section, and a selective catalytic reduction element disposed downsteam of the ammonia injection apparatus and adapted for reduction of NOx.
Owner:GENERAL ELECTRIC CO
Nitric oxide generator and inhaler
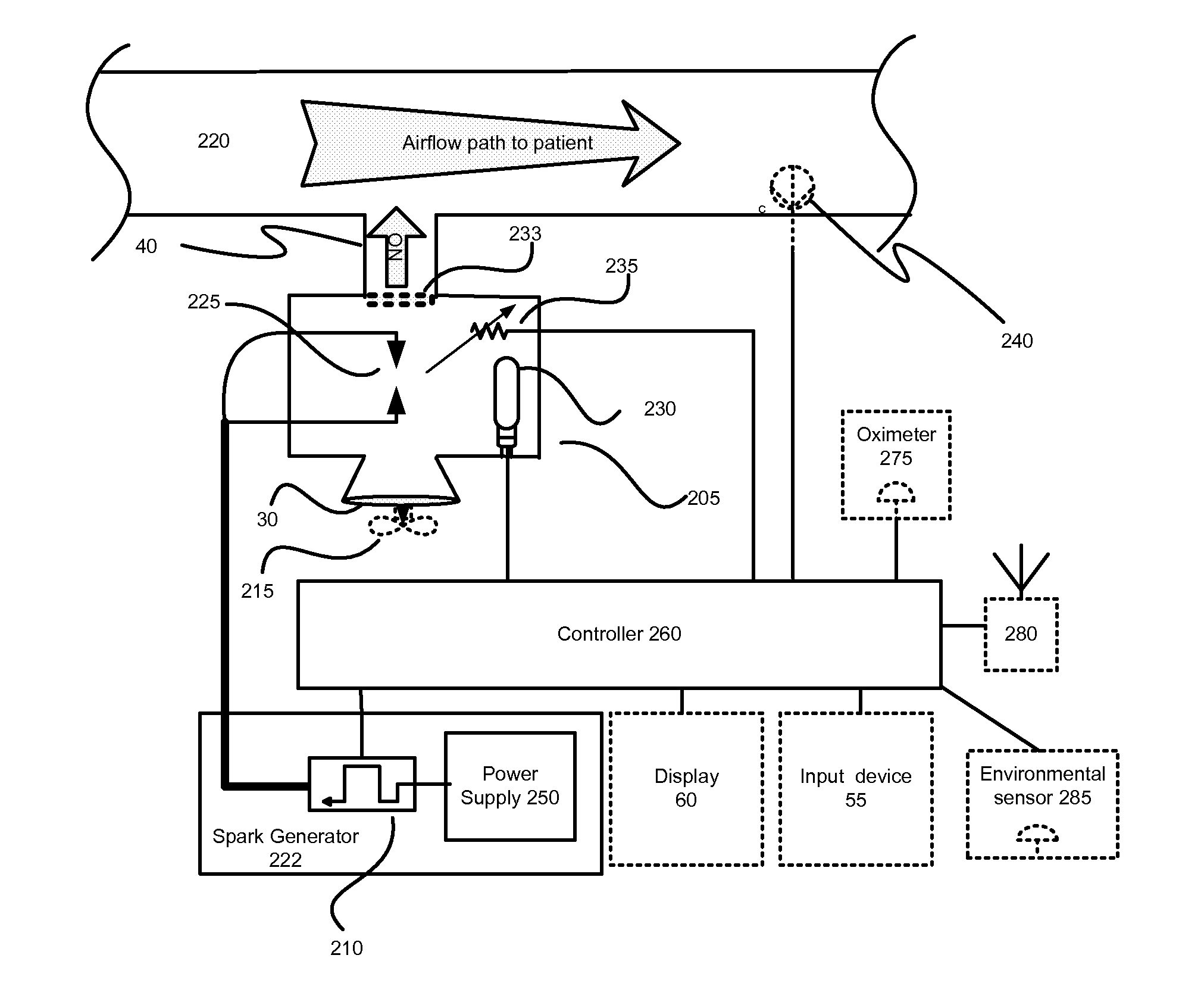
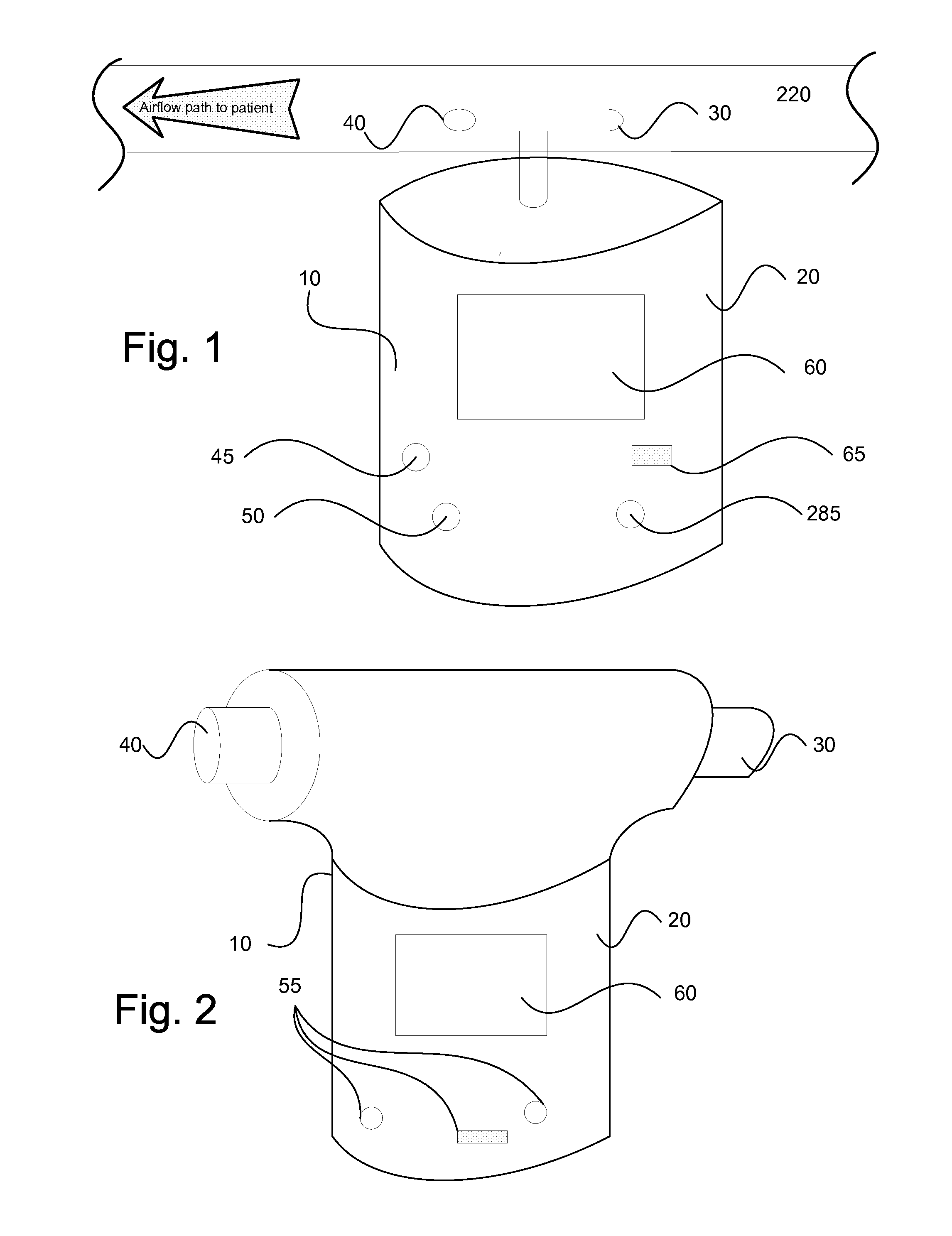
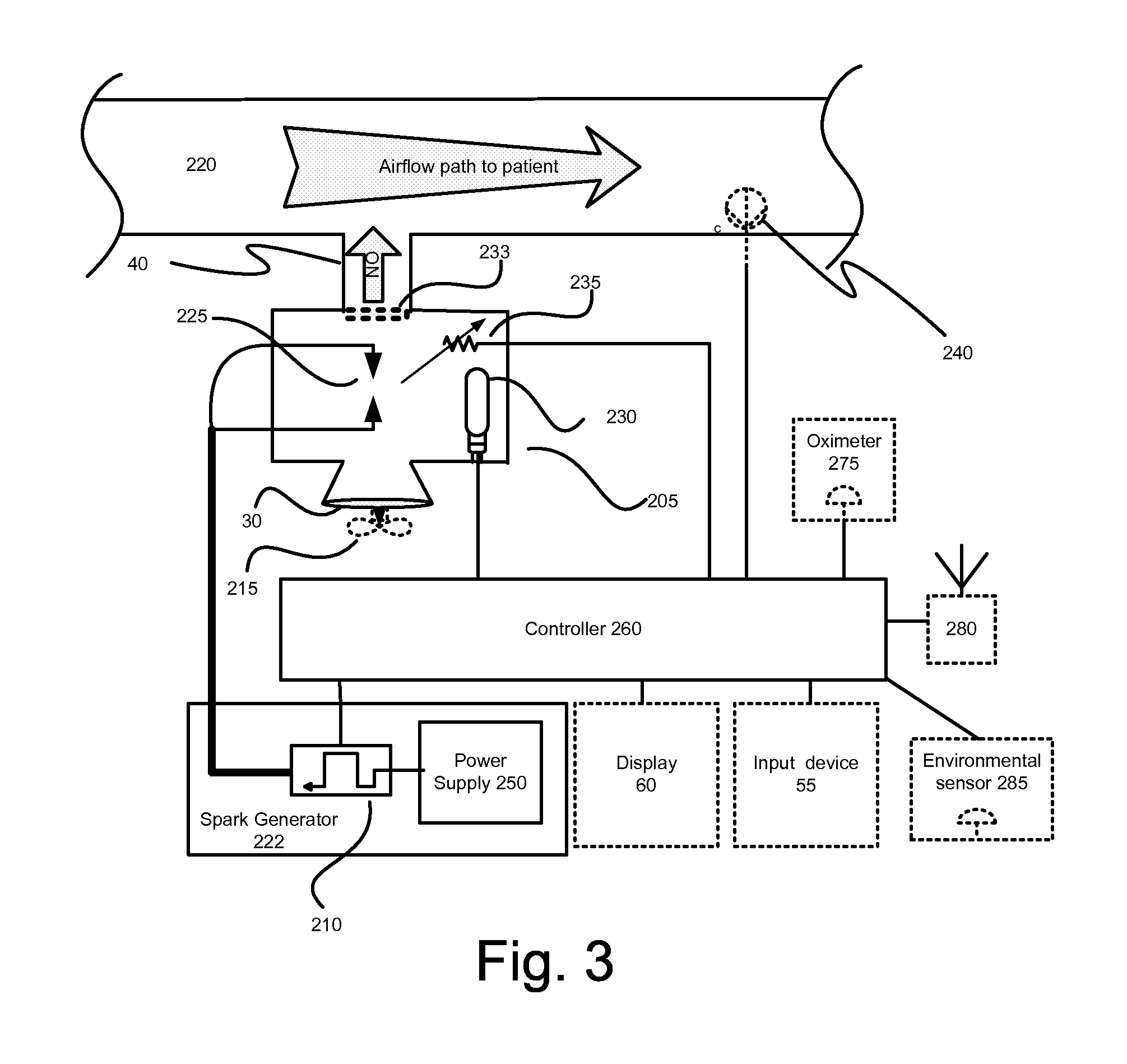
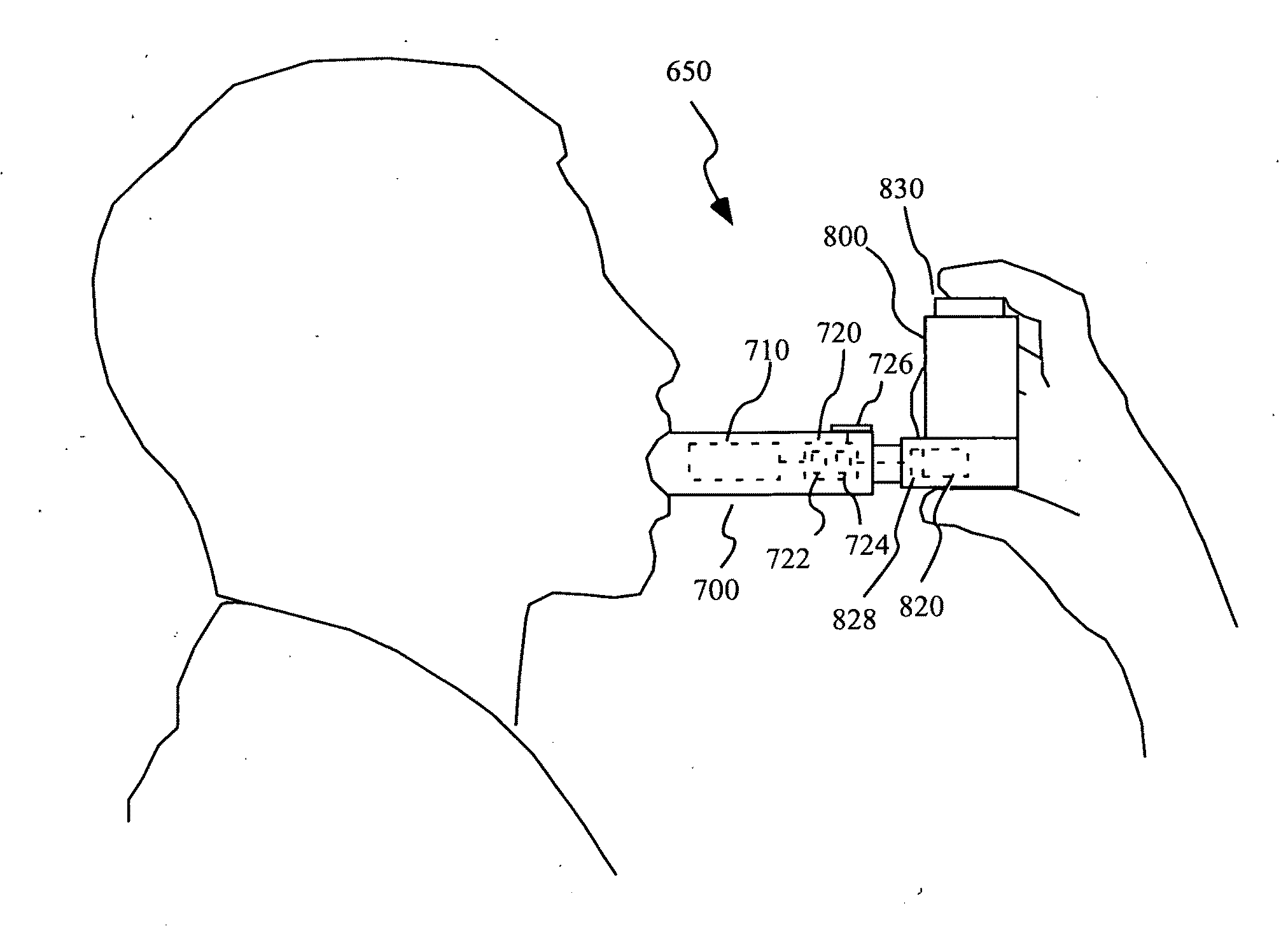
InactiveUS20150090261A1Little and no consumables useAffordable and reliableGas treatmentRespiratory masksNitrogen dioxideNitric oxide
Several embodiments of a Nitric Oxide Inhaler that uses an electrical spark to produce Nitric Oxide from Air, optimized to maximize the production of Nitric Oxide and minimize the production of Nitrogen Dioxide through hardware and control system. Further disclosed is a system to control such inhalers
Owner:CROSBIE DAVID BRUCE
Detection of nitric oxide
InactiveUS20100282245A1Reduce humidityWithdrawing sample devicesMaterial analysis by electric/magnetic meansNitrogen dioxideNitric oxide
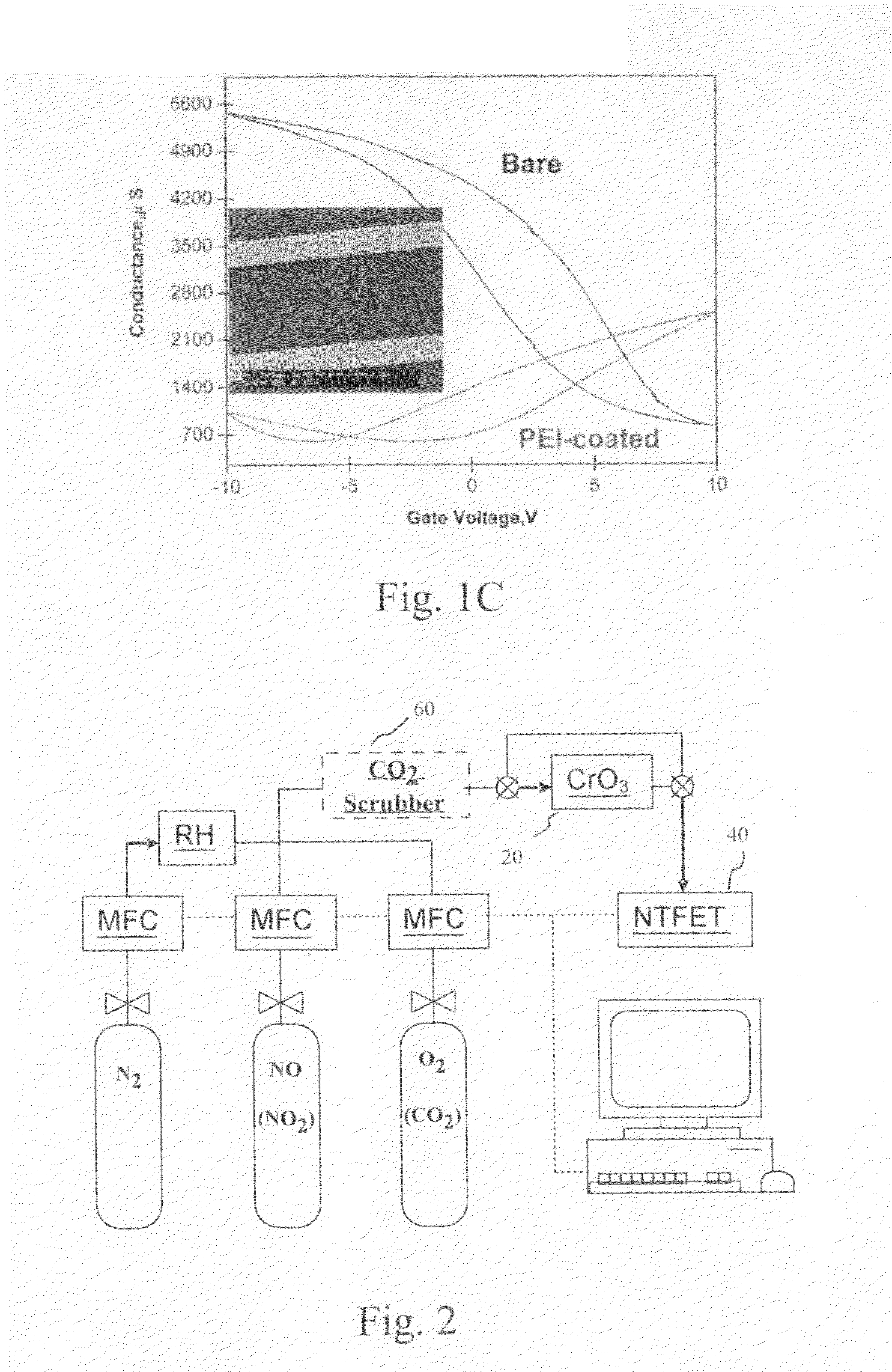
A system for the detection of nitric oxide in a gas sample includes a converter for oxidation of nitric oxide to nitrogen dioxide, a nitrogen dioxide sensor including nanostructures and a filtering device to remove at least carbon dioxide from the gas sample positioned upstream of the converter. The nitrogen dioxide sensor can, for example, include a recognition layer on the nanostructures adapted to enhance sensitivity to nitrogen dioxide. A method for detecting nitric oxide in exhaled breath includes detecting nitric oxide in the exhaled breath using a nitric oxide sensor including nanostructures. Another method for detecting nitric oxide in exhaled breath includes filtering the breath to remove at least carbon dioxide from the breath, oxidizing nitric oxide in the exhaled breath to nitrogen dioxide and detecting the nitrogen dioxide using a nitrogen dioxide sensor including nanostructures.
Owner:UNIVERSITY OF PITTSBURGH
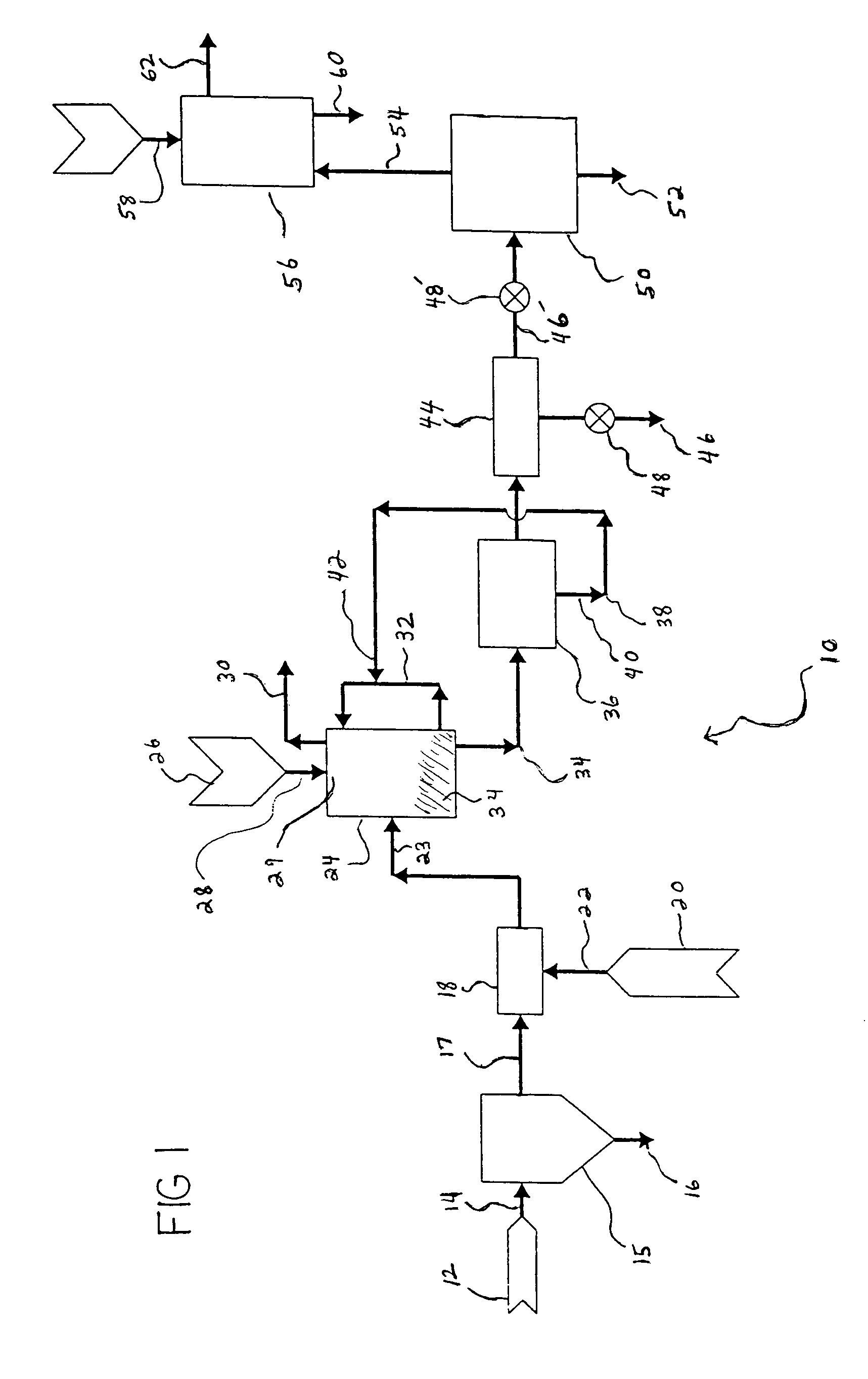
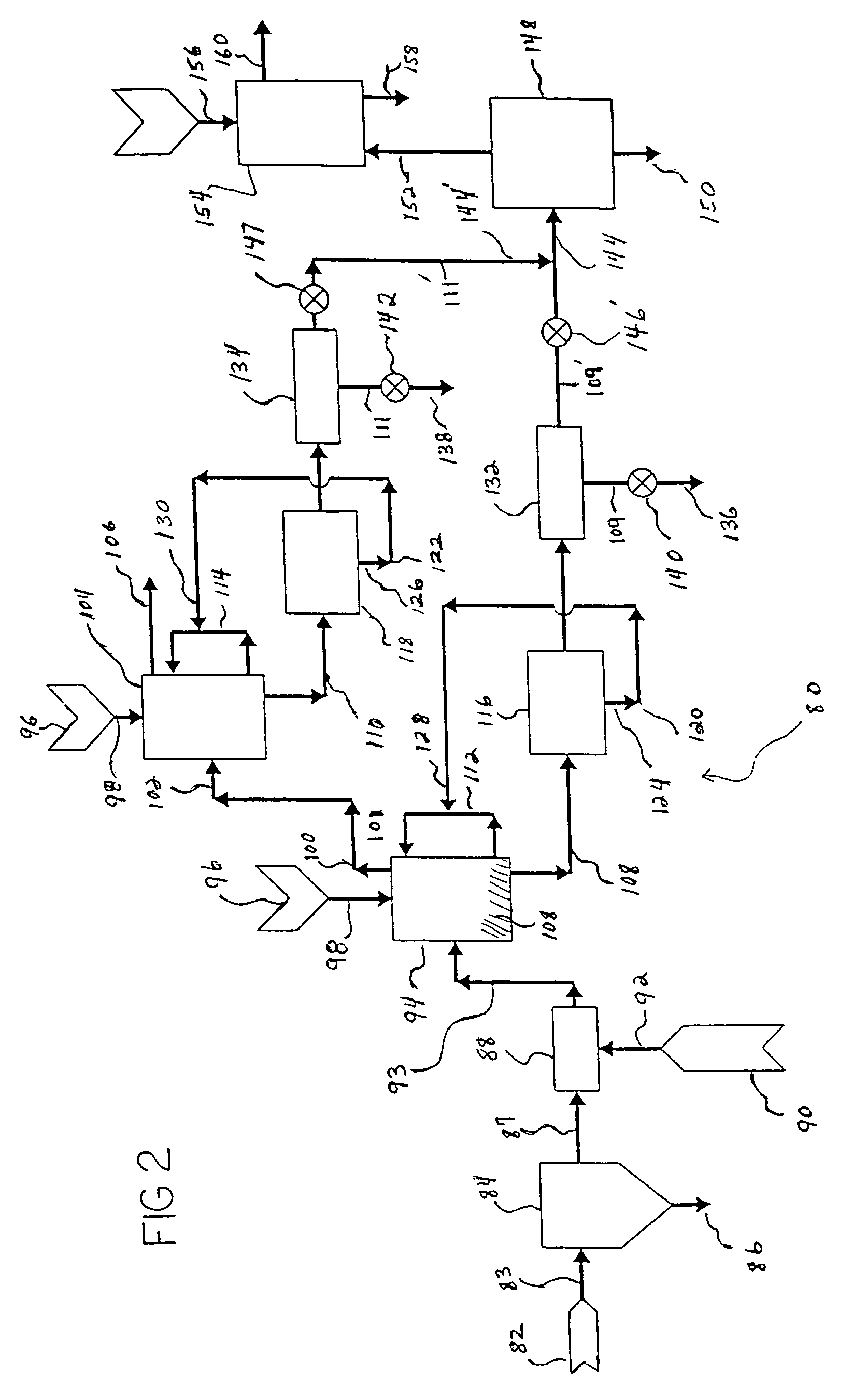
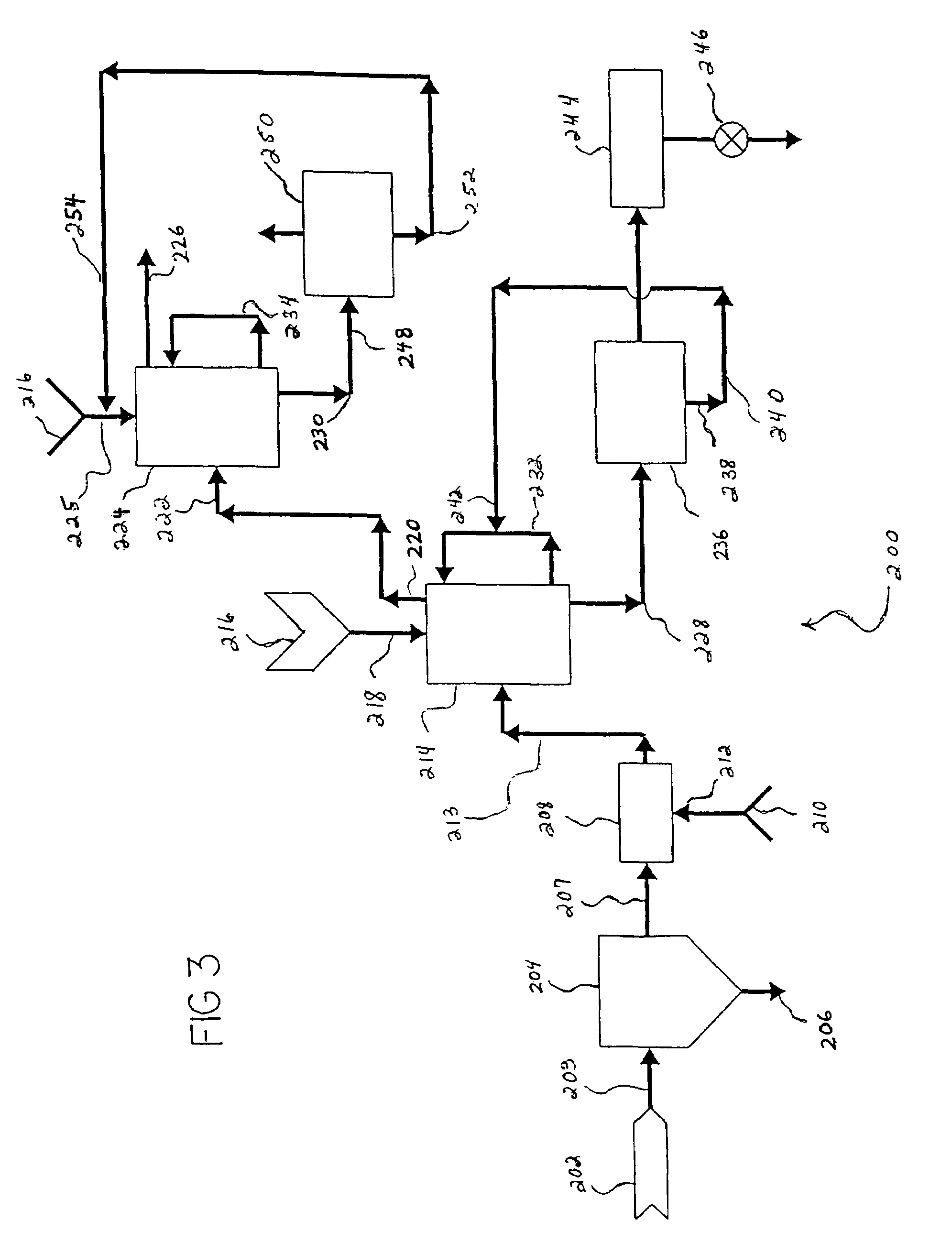
Process for producing saleable liquids from organic material
InactiveUS6919488B2Improve processing yieldReduce exhaust emissionsHydroxy compound preparationWaste based fuelNitrogen dioxideWater vapor
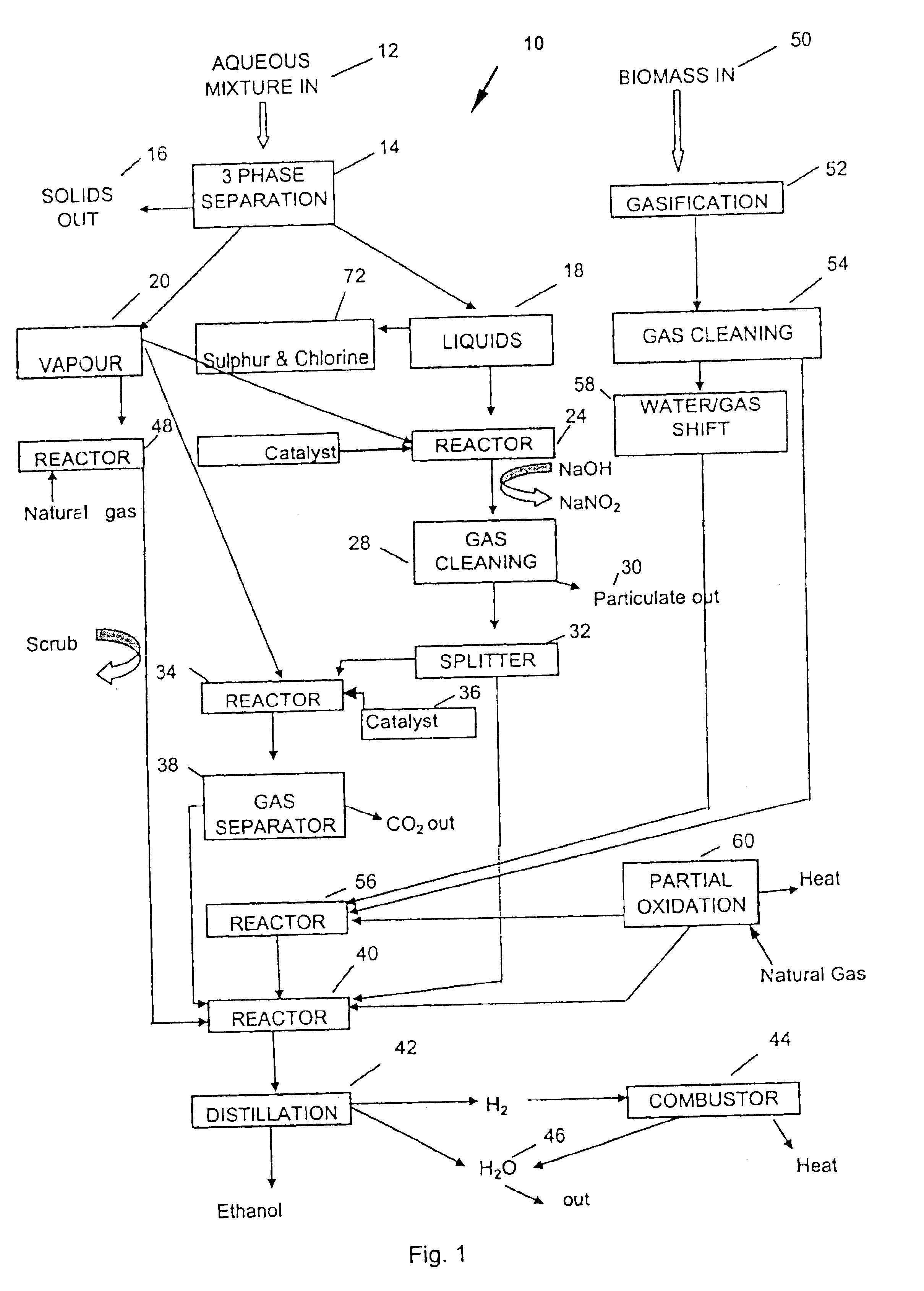
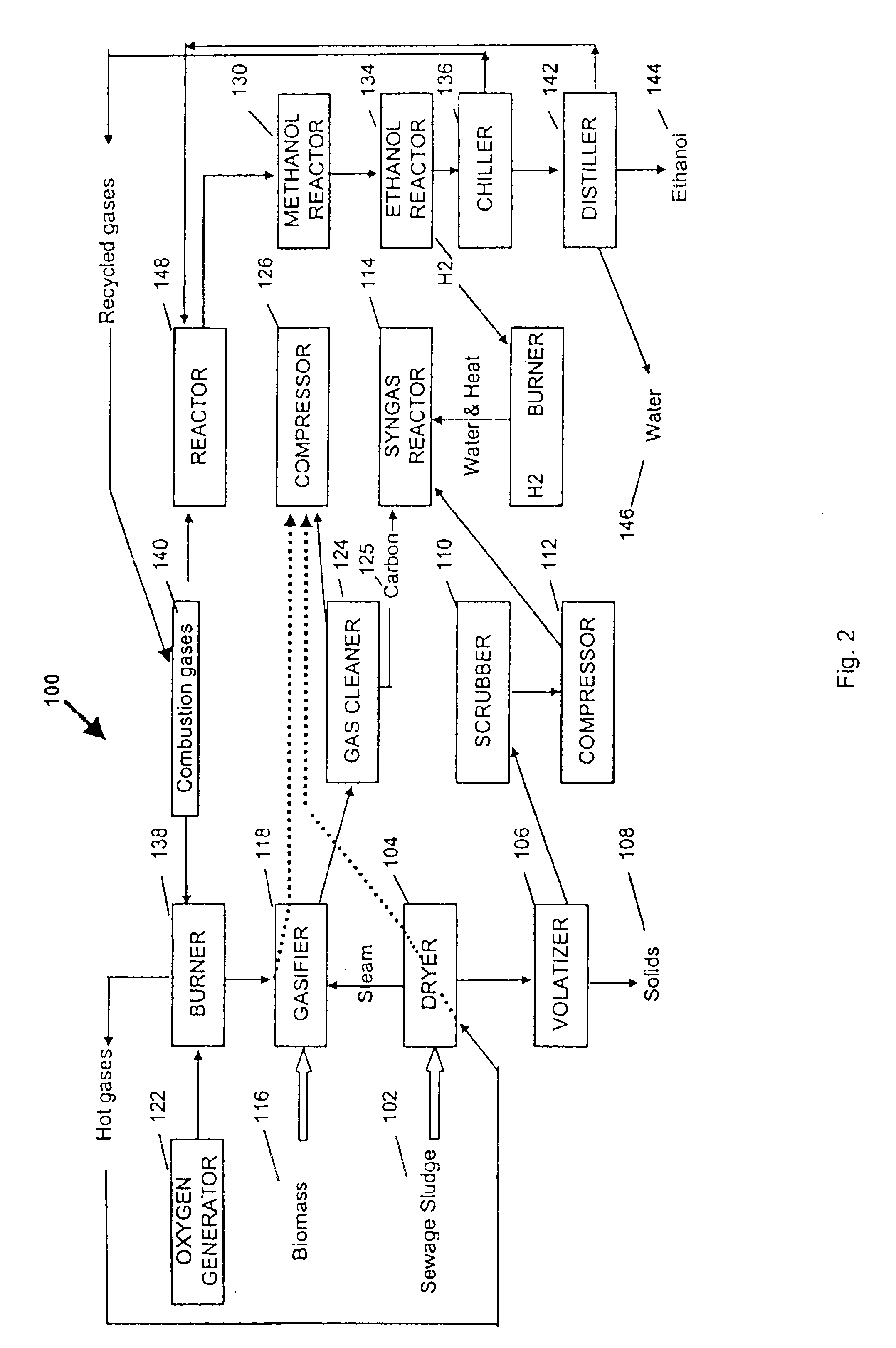
A process for producing saleable liquids from organic material comprising the following steps. Providing organic material and separating it into solids, liquids and vapor. Reacting the liquids, combining it with water vapor and producing a volatized gas stream. Removing nitrogen dioxide from the gas stream to produce a scrubbed volatized gas stream. Reacting the scrubbed volatized gas stream with water vapor to produce a combined volatized gas stream. Removing carbon dioxide from the combined volatized gas stream to produce a subtracted volatized gas stream. Reacting the subtracted volatized gas stream with methanol to produce an enhanced volatized gas stream. Distilling the enhanced volatized gas stream to produce ethanol.
Owner:WOODLAND BIOFUELS
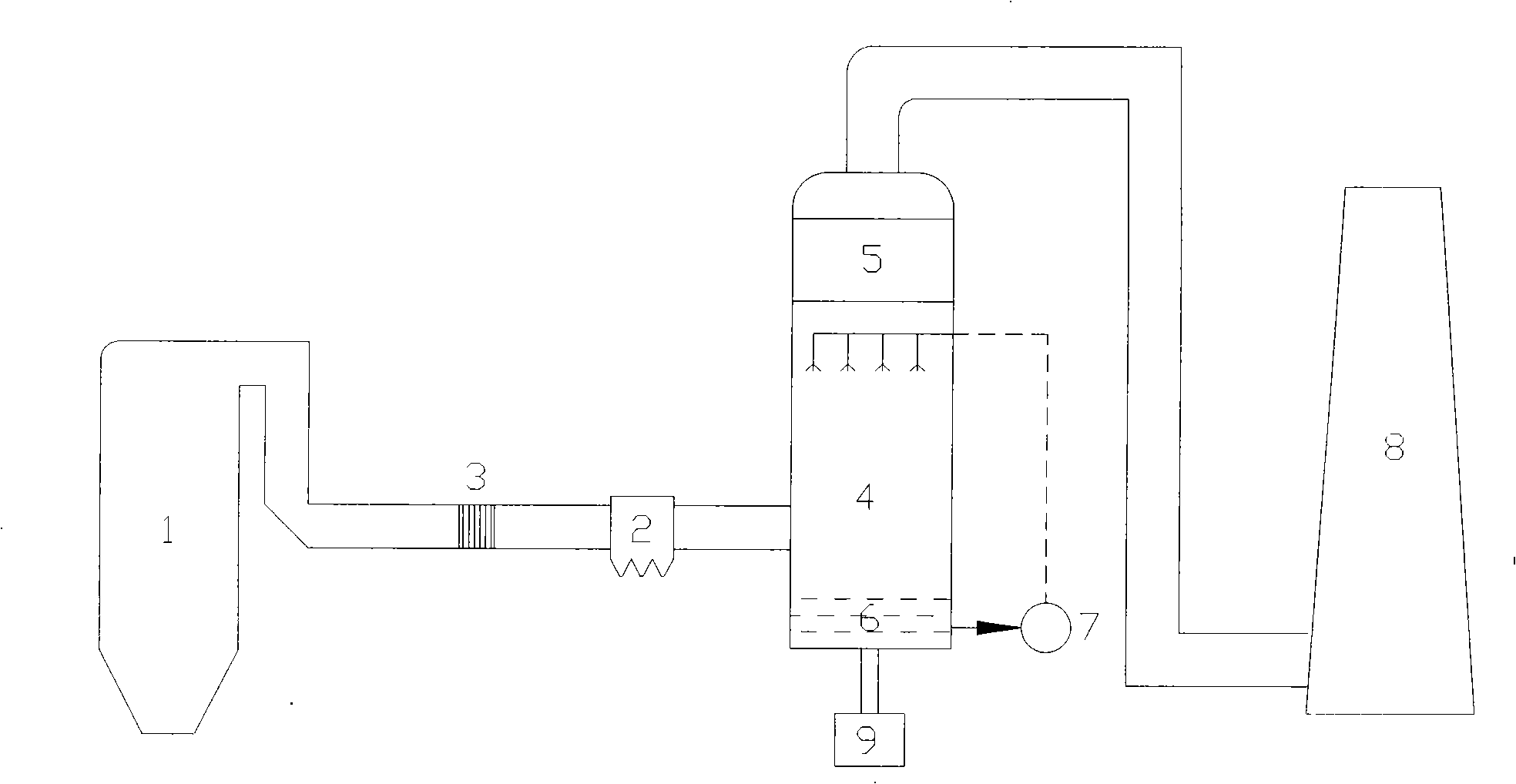
Purification technology and equipment of waste gas containing high concentration nitrogen oxide
ActiveCN1830526AEnhanced mass transferImprove responseDispersed particle separationHigh concentrationNitrogen dioxide
A process for cleaning the waste gas containing high-concentration NOx includes such steps as gathering the fume, coming in rotary filler bed, diffusing into rotary filler, spraying the absorptive liquid to filler, countercurrent absorption, mixing the treated gas with oxidant to oxidize NO to NO2, coming in rotary filler bed, absorbing by absorptive liquid, defrosting, and exhausting. Said absorptive liquid can be cyclically used. Its apparatus is also disclosed.
Owner:ZHONGBEI UNIV
Dual catalyst NOx reduction system for exhaust from lean burn internal combustion engines
ActiveUS20100000202A1Remove loadGas treatmentInternal combustion piston enginesMolecular nitrogenInternal combustion engine
A method and apparatus for reducing the percentage of nitrogen dioxide and nitrogen monoxide in an exhaust gas stream of an internal combustion engine, comprising the steps of injecting a hydrocarbon compound and optionally hydrogen into the exhaust gas stream; passing the exhaust gas through a first catalyst for selective reduction of a portion of the nitrogen oxides to nitrogen, ammonia, and N-containing species; passing the exhaust gas through a second catalyst for selective reduction of a portion of the nitrogen oxides with ammonia to molecular nitrogen; sensing ammonia concentration in the exhaust gas stream after passage through either or both of the first and second catalysts; and controlling by a controller in a feedback loop the injecting to an amount of hydrocarbon that will produce a predetermined concentration of ammonia and nitrogen oxides at the sensor that will lead to high NOx conversion.
Owner:DELPHI TECH IP LTD
Devices and methods of screening for neoplastic and inflammatory disease
InactiveUS20060036138A1Reduce controlReduce inflammationSensorsBlood characterising devicesAbnormal tissue growthFluorescence
Methods and devices are provided for evaluating the presence of disease in a patient. In particular, methods and devices are provided for screening patients for neoplastic and / or inflammatory disease. Such diseases are often indicated by the elevated level of a chemical compound associated with disease, such as nitric oxide (NO) and / or nitrogen dioxide (NO2). Through measuring and / or estimating the chemical compound-concentration, such as by change in fluorescence, absorbance or reflectance, the methods and tools provided distinguish between patients who require further testing and / or treatment and those who do not. The methods and tools also provide information about the effectiveness of treatment, such as treatment to reduce inflammation or control of the growth of malignant tumors. These methods and devices are relatively inexpensive, easy to use, and provide other advantages.
Owner:HELLER ADAM
Dry smoke cleaning process for desulfurizing and denitrating simultaneously and its system
InactiveCN1589954ASimplify stand-alone removal systemsSimple processDispersed particle separationSprayerNitric oxide
A dry method for simultaneously desulfurizing and denitrating fume includes using H2O2 or methanol to convert the NO in fume to NO2, using Ca-base particles as desulfurizing agent to react with SO2 to obtain calcium sulfate, and using ammonia water or urea as denitrating agent to react with NO2 to obtain ammonium nitrate and clean fume. Its apparatus is composed of additive sprayer, desulfurizing system and denitrating system.
Owner:WUHAN KAIDI ELECTRIC POWER CO LTD
Method of on-board diagnostic catalyst monitoring
InactiveUS20070234708A1Direct relationshipInternal combustion piston enginesExhaust apparatusHydrogenNitrogen dioxide
A method of on-board diagnostic (OBD) catalyst monitoring. Vehicle OBD exhaust systems often include a catalyst, a pre-catalyst exhaust gas oxygen sensor, and a post-catalyst exhaust gas oxygen sensor. A method is provided of monitoring the catalyst which includes the steps of measuring hydrogen or nitrogen dioxide generation by the catalyst, and correlating changes in hydrogen or nitrogen dioxide generation to changes in catalytic conversion efficiency. Because OBD legislation defines catalyst deterioration or malfunction in terms of hydrocarbon or nitrogen oxide emission levels, the method uses hydrogen or nitrogen dioxide generation as a metric for OBD monitoring of the catalyst and offers the advantage of a more direct relationship to catalyst health than conventional methods.
Owner:JONES JAMES PEYTON +1
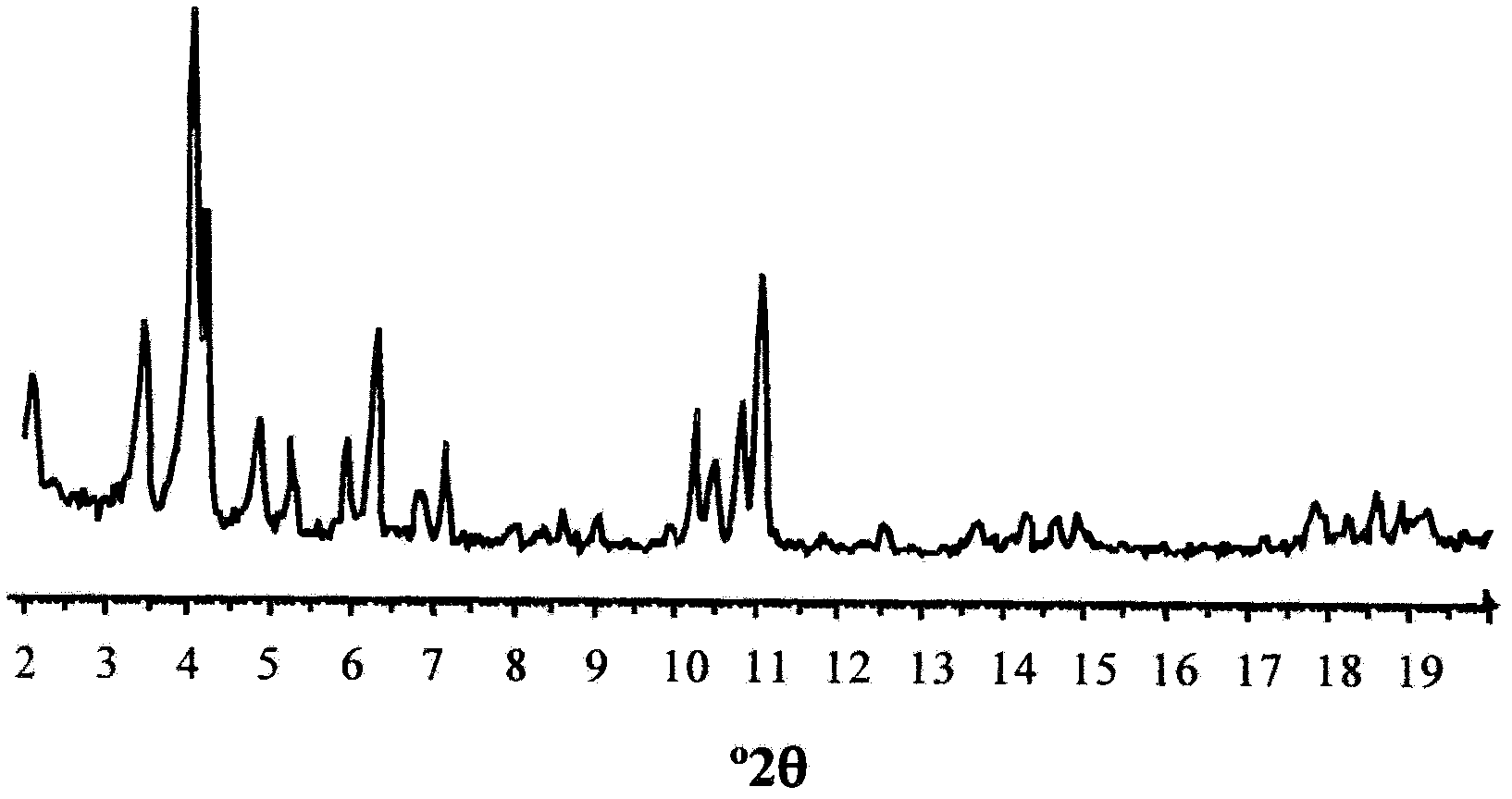
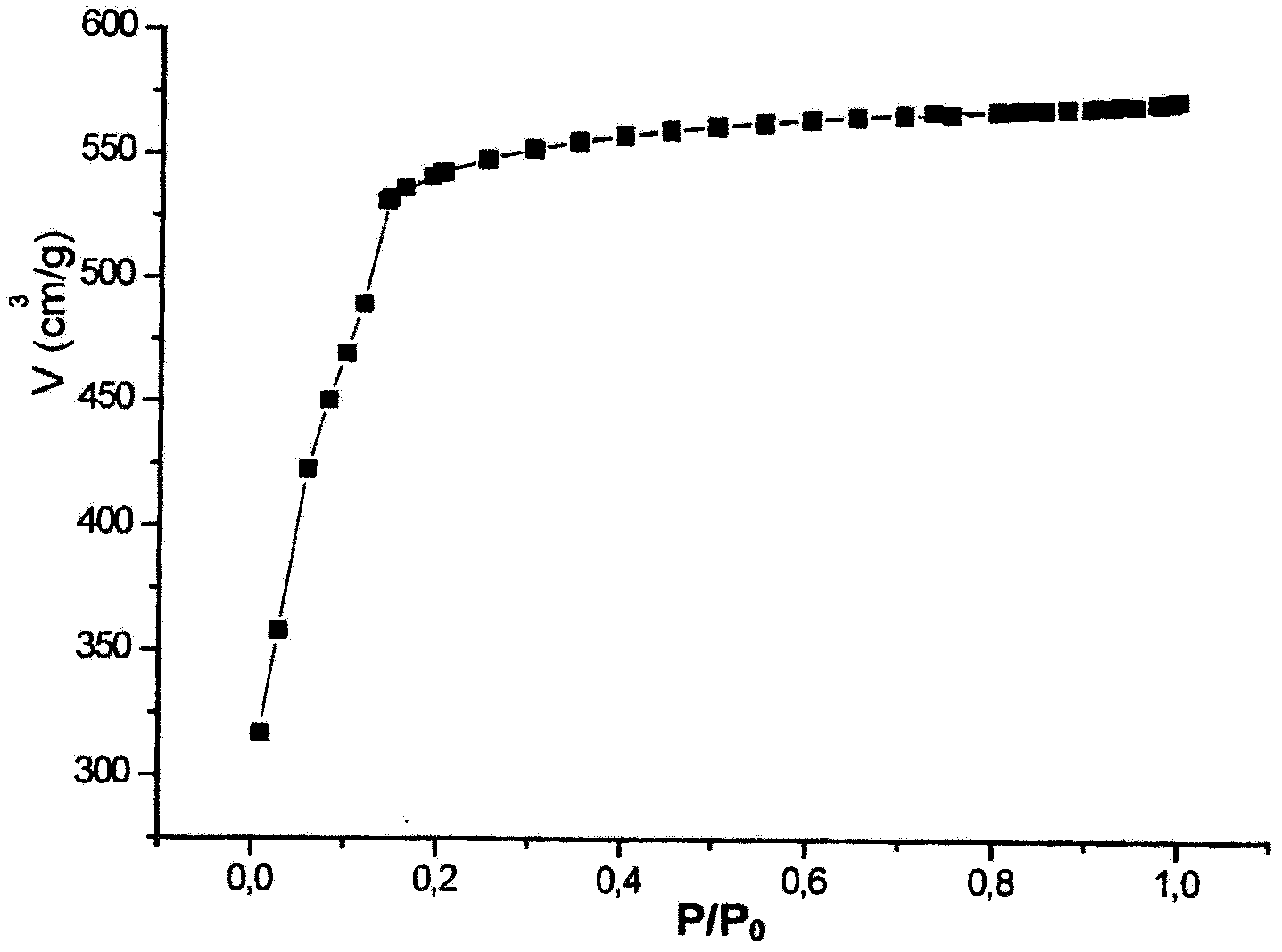
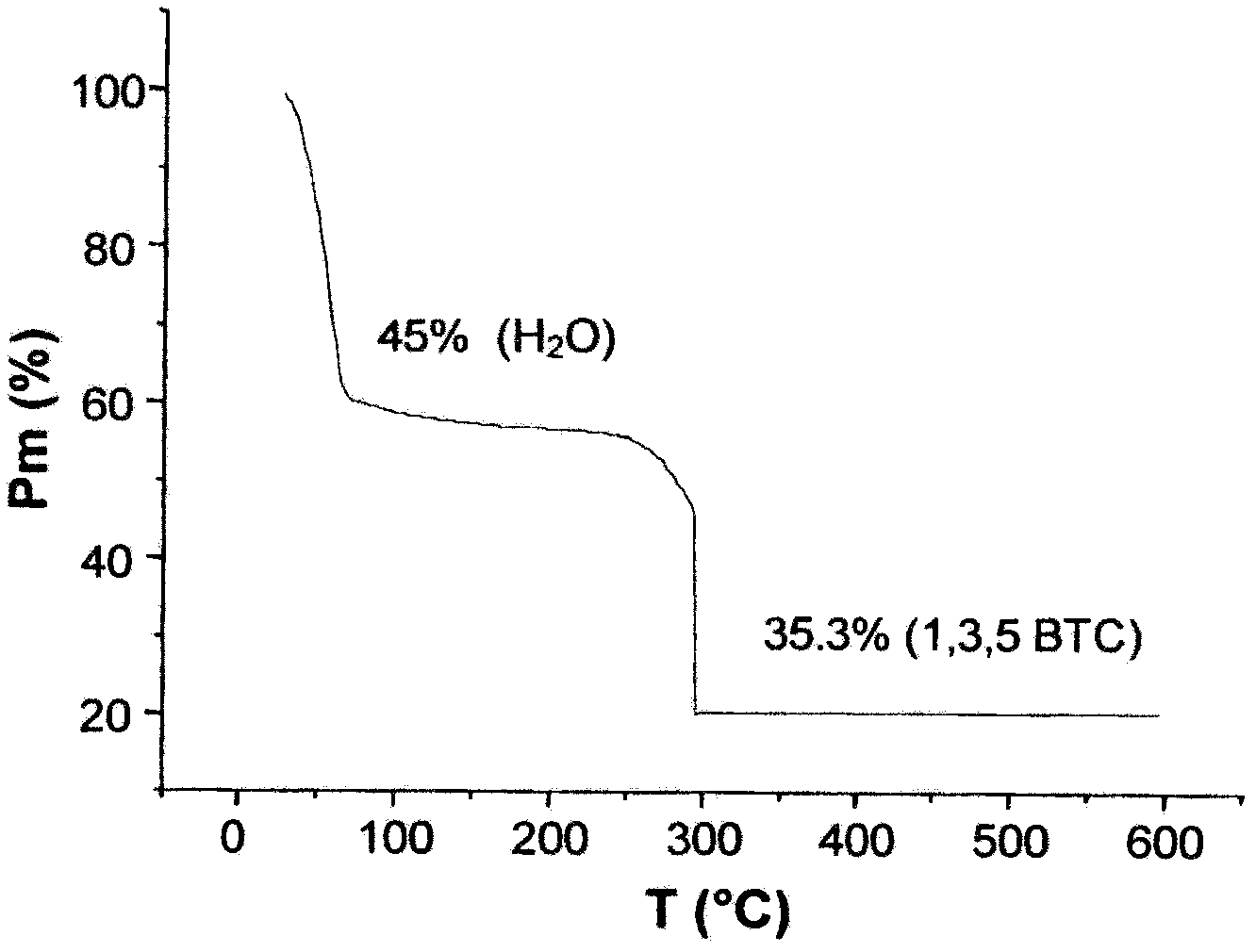
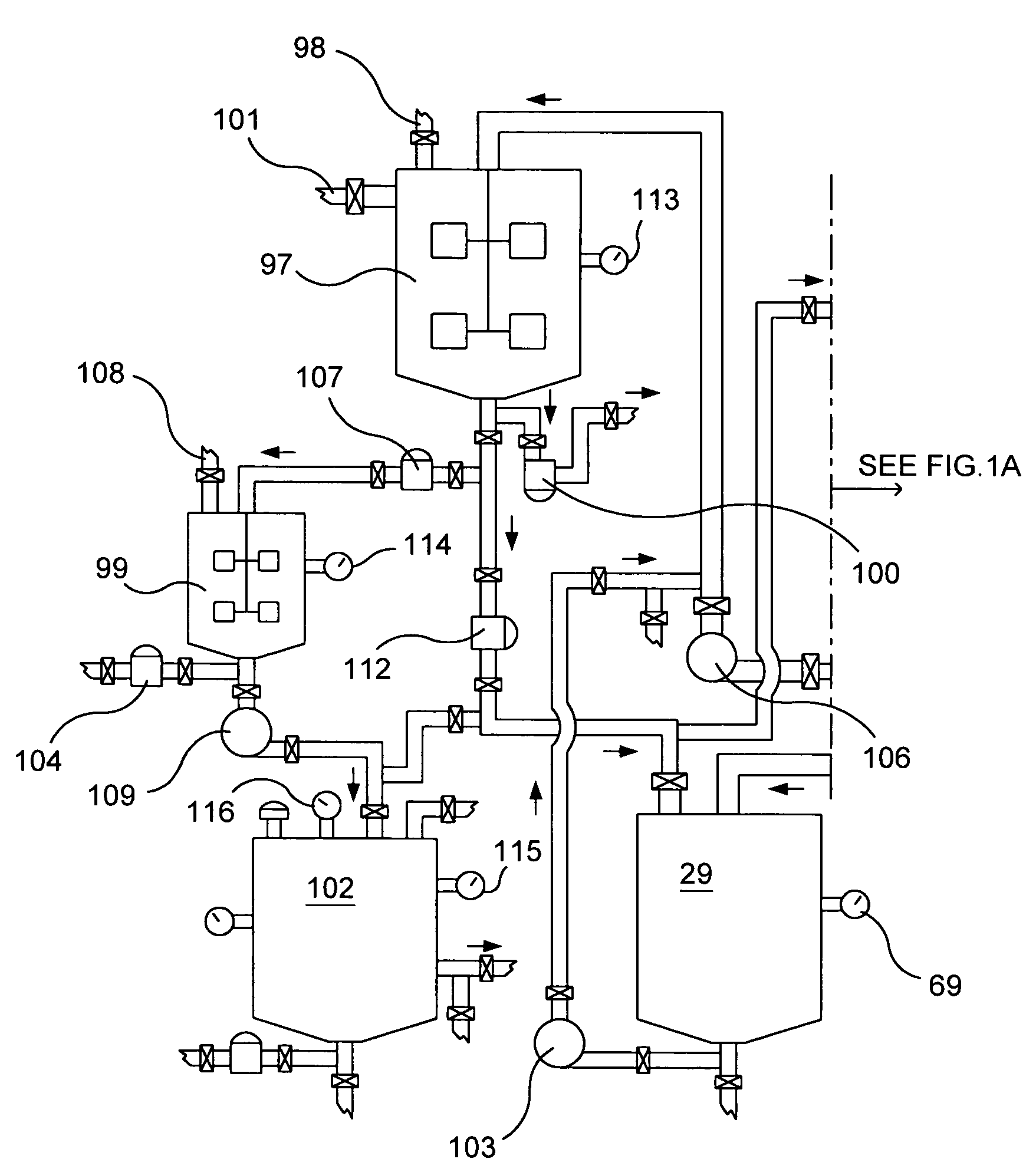
Use Of A Porous Crystalline Hybrid Solid As A Nitrogen Oxide Reduction Catalyst And Devices
ActiveCN102481558AEfficient removalLow costNitrous oxide captureOrganic-compounds/hydrides/coordination-complexes catalystsMetal-organic frameworkReducing agent
The present invention relates to the use of solids consisting of a metal-organic framework (MOF) and having the units of the following formula (I): MmOkXILp as a nitrogen-oxide catalyst. The present invention also relates to devices for enabling the implementation of said use. The nitrogen oxides in question are nitrogen monoxide and nitrogen dioxide, collectively referred to as NOx. The MOF solids of the present invention are advantageously capable of removing nitrogen oxides from a liquid or gaseous effluent, for example from water, from the exhaust gases of a vehicle, factory, workshop, laboratory, stored products, urban air vents, etc., without any reducing agent and at a low temperature. The DeNOx catalysis is a major issue for our societies. The invention can be used for reducing or even avoiding the consequences for public health of the toxic NOx gases resulting from human activity.
Owner:CENT NAT DE LA RECHERCHE SCI +3
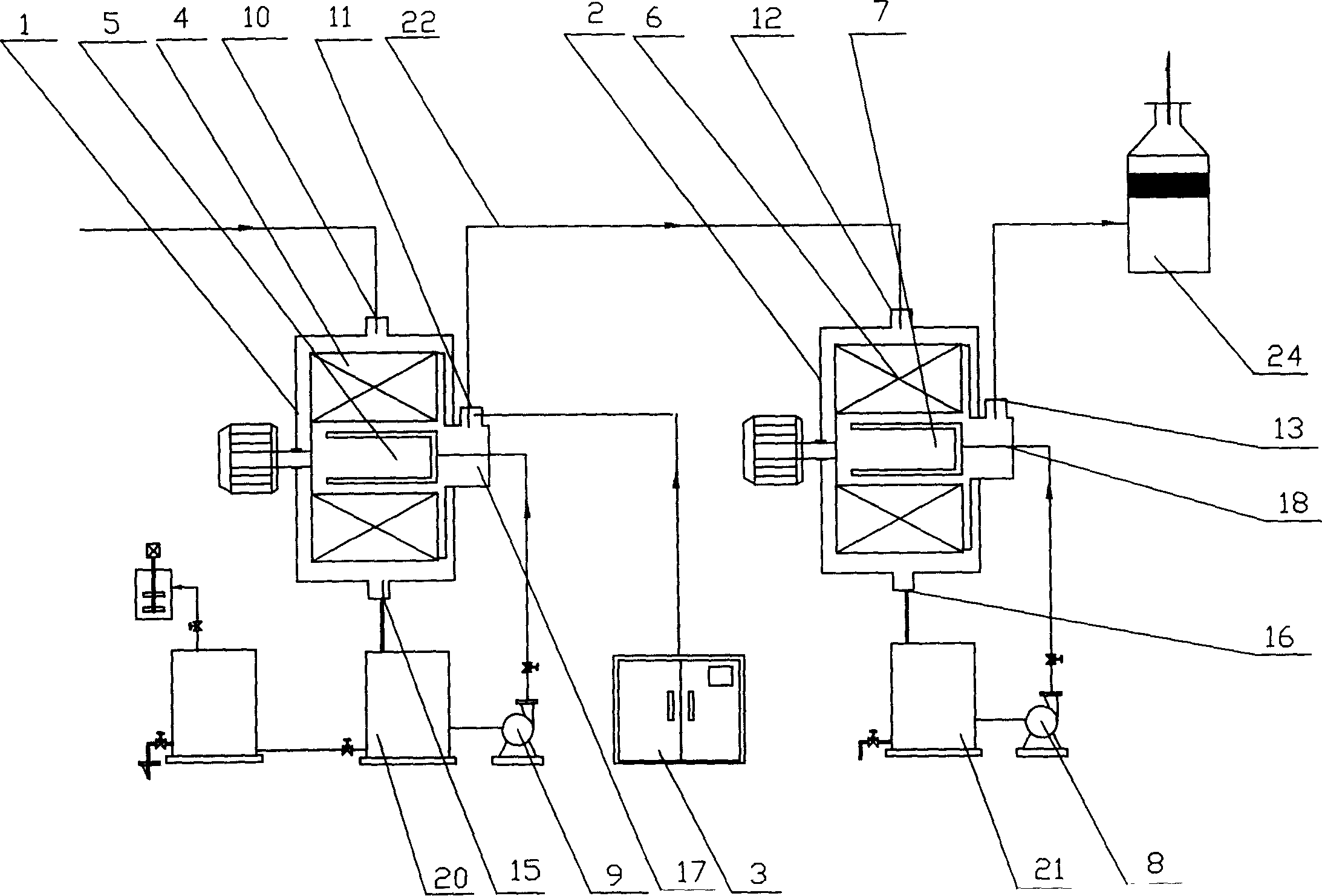
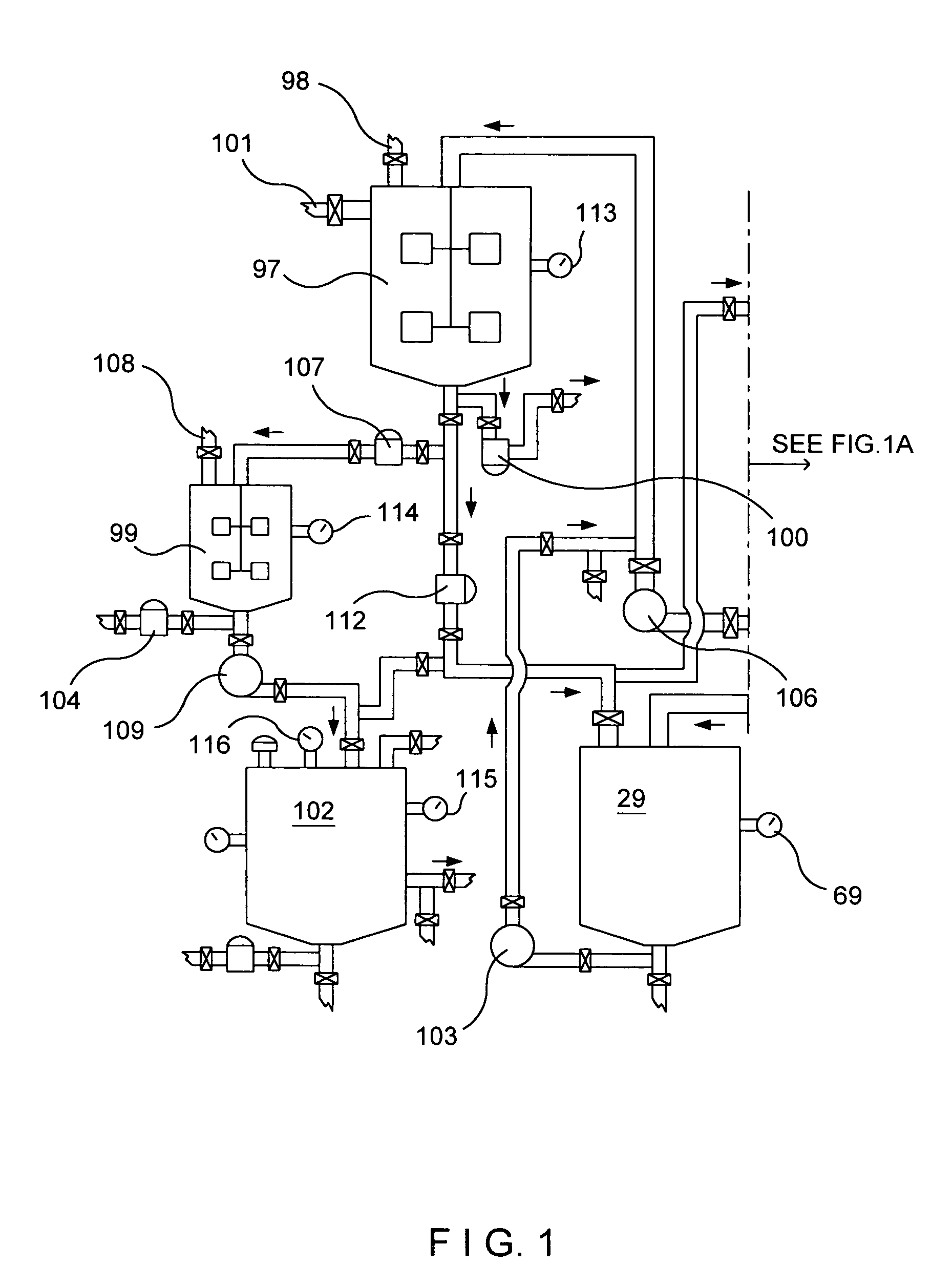
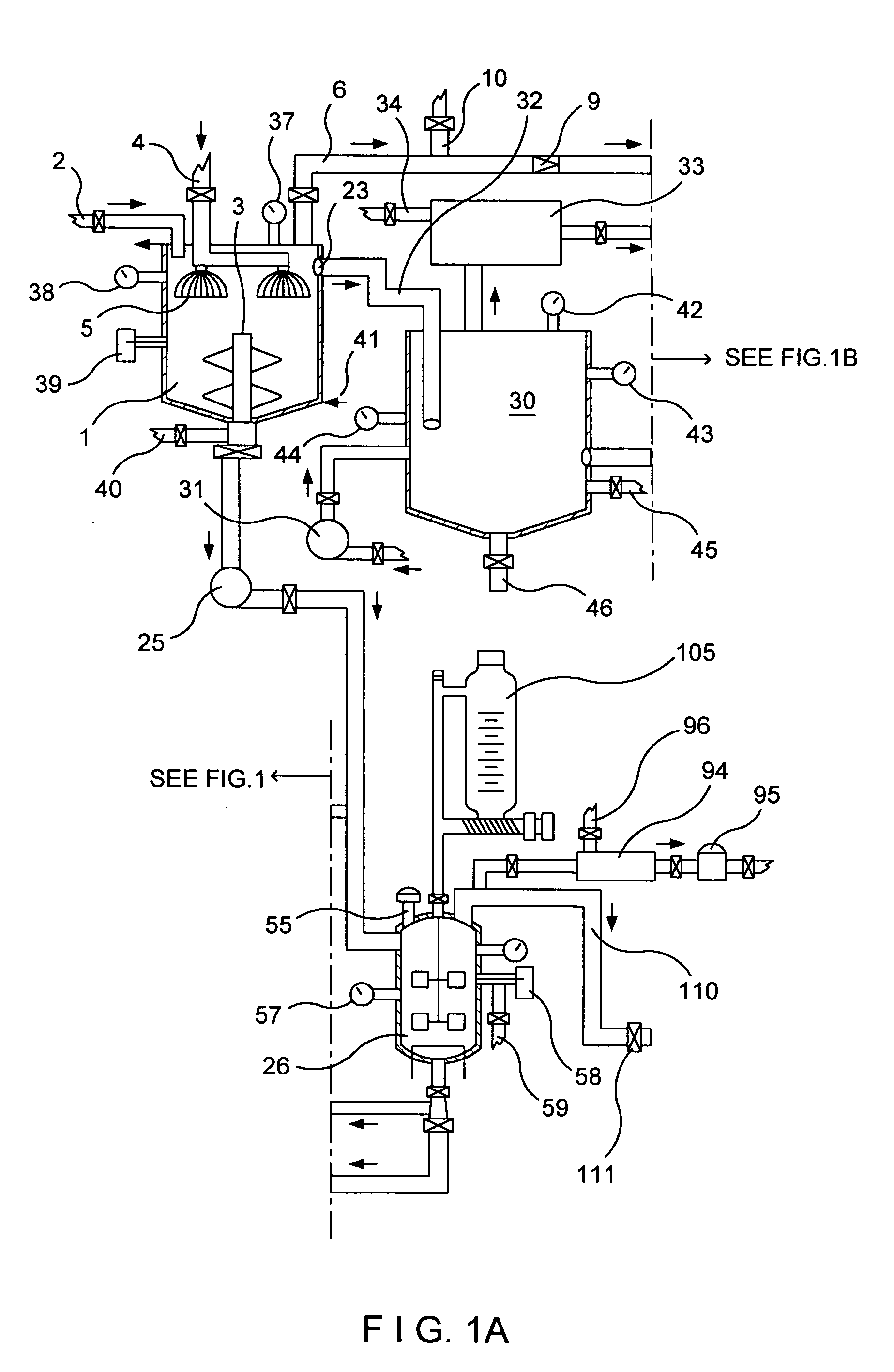
Reverse Kleiner method for manufacturing nitrogen dioxide, nitric oxide, nitric acid, metallic ascorbates and alkyl ascorbates of vitamin C
In this invention new chemical reactions, new chemical processes are established, and these chemical reactions, chemical processes can be used with the designed system to produce nitrogen dioxide, nitric oxide and calcium ascorbate or calcium isoascorbate.The reaction vessel contains the aqueous ascorbic acid solution or aqueous isoascorbic acid solution. The temperature in the reaction vessel at the start of the reaction is at temp. 55° C. Into this solution is injected calcium nitrite dissolved in water. The gases generated by the chemical reactions are collected in the gas vessel.Here two sets of chemical reactions take place; one on the surface of the solution that produces the bulk of the gas mixture. In the liquid phase, the reactions go slow; and it gives nitric acid, calcium ascorbate and nitric oxide. Instead of calcium ascorbate one can use isoascorbate, in that case the chemical reactions will go somewhat slower.
Owner:KLEINER BELA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com