Oxidation Catalyst and Exhaust-Gas Purification System Using the Same
a technology of oxidation catalyst and purification system, which is applied in the direction of metal/metal-oxide/metal-hydroxide catalyst, machine/engine, arsenic compound, etc., can solve the problem of complex system, insufficient purification effect of nitrogen oxides and particulates, and so on. achieve the effect of efficient promotion of the reaction of a substan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
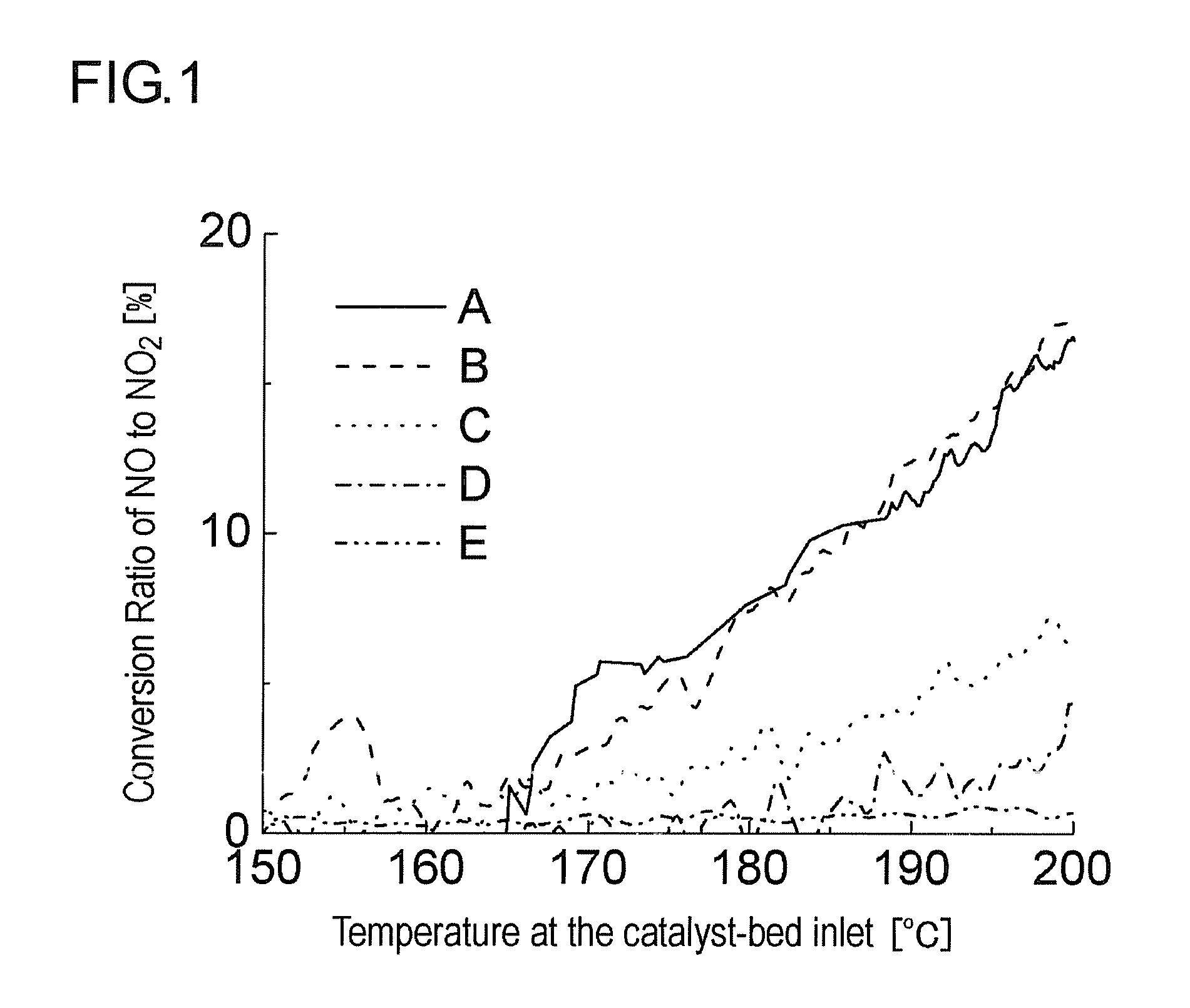
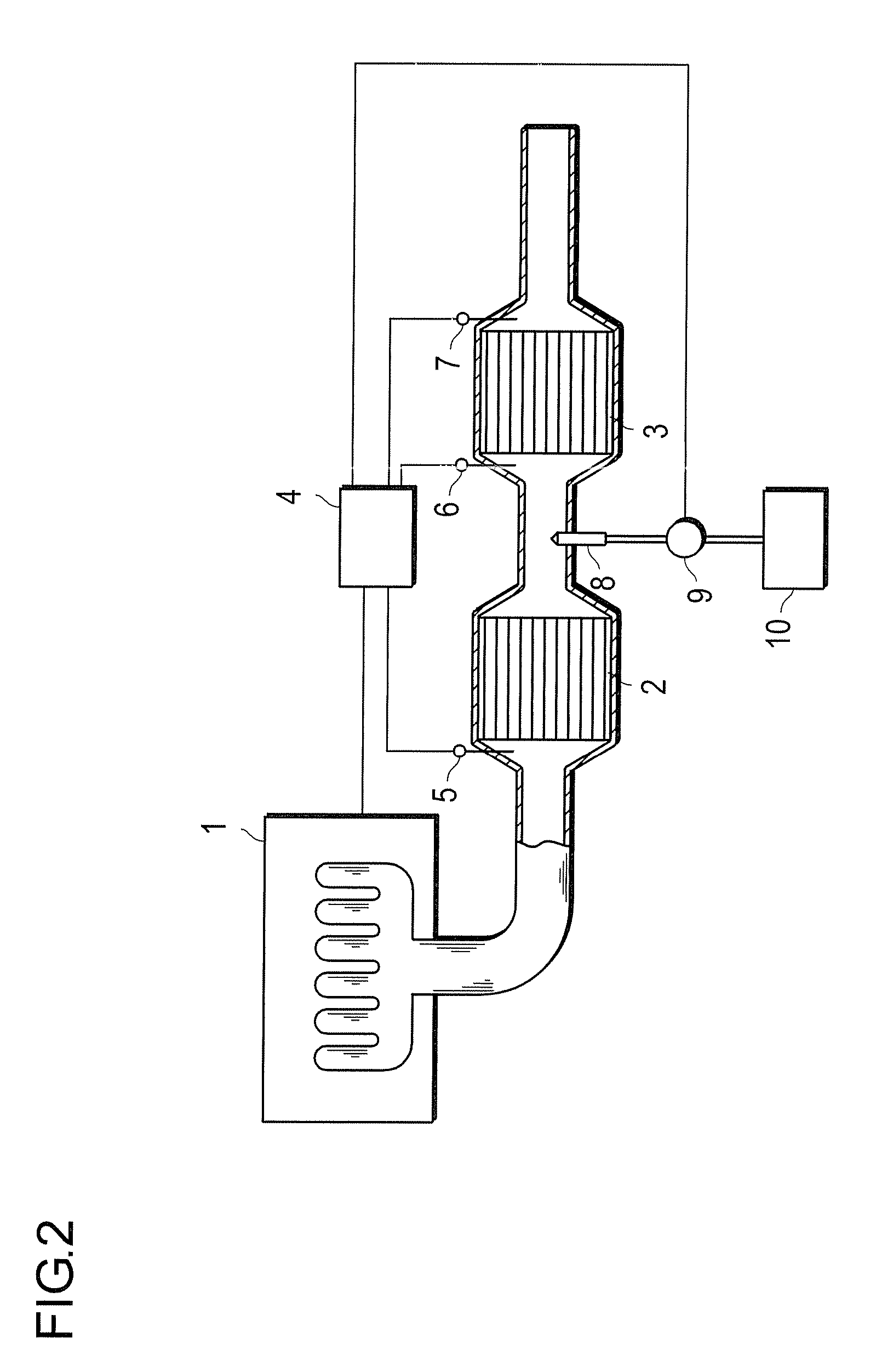
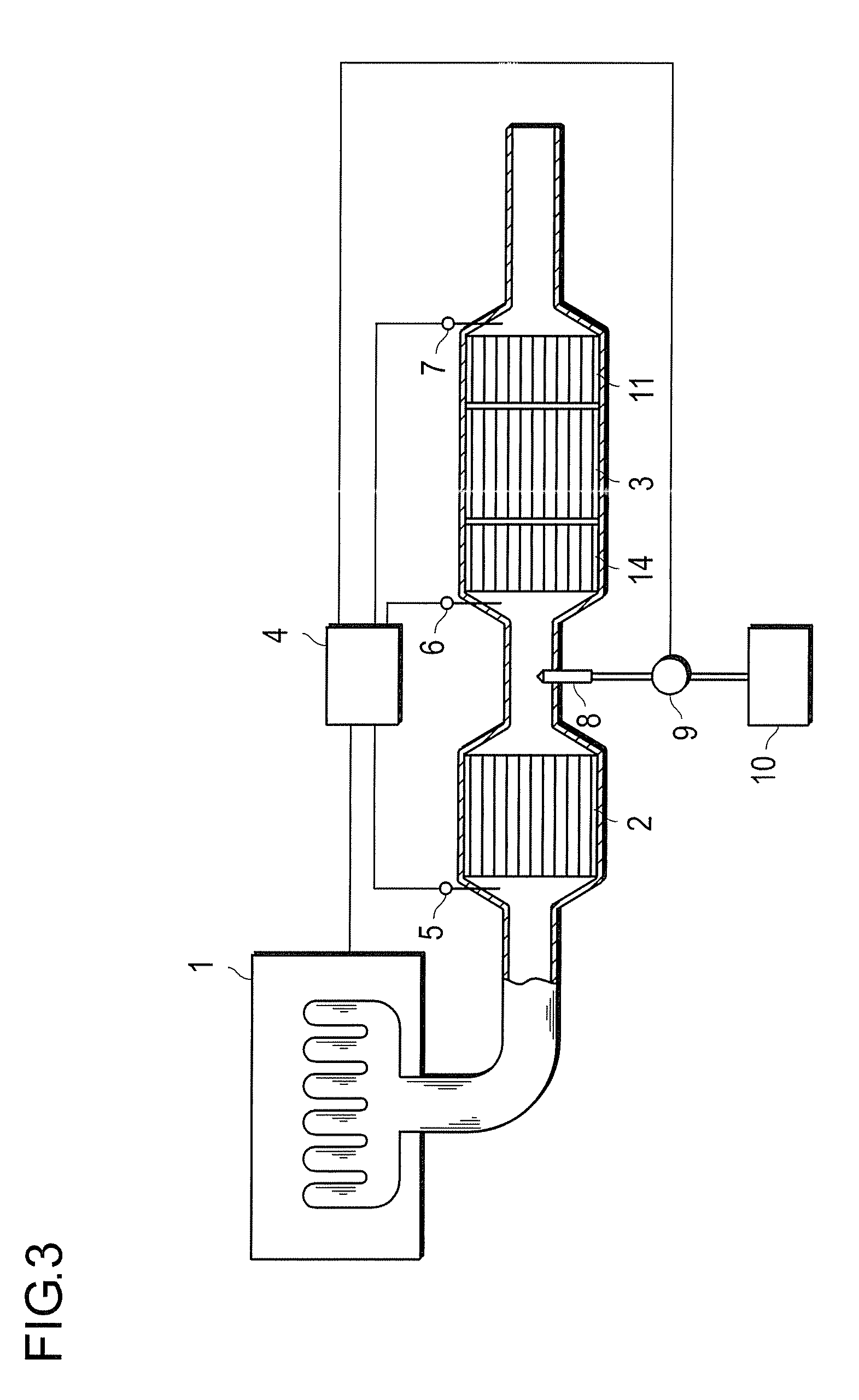
[0071]20.4 g of an aqueous solution of dinitrodiammine platinum corresponding to 2.32 g of platinum, 1.1 g of an aqueous solution of palladium nitrate corresponding to 0.15 g of palladium and 80 g of activated alumina (γ-Al2O3, BET specific surface area: 200 m2 / g, mean primary particle diameter: 6 μm) were subjected to wet milling by a ballmill to prepare 300 g in total of an aqueous slurry (A). A cylindrical honeycomb carrier made of cordierite of 24 mm in diameter and 50 mm in length having 400 cells per square inch of cross-sectional area was coated (wash coat) with this slurry, dried at 120° C. for 8 hours and then calcined at 500° C. for an hour to obtain catalyst A so that the sum of platinum, palladium and alumina is 82.47 q per liter of the honeycomb carrier.
example 2
[0072]19.8 g of an aqueous solution of dinitrodiammine platinum corresponding to 2.25 g of platinum, 1.57 g of an aqueous solution of palladium nitrate corresponding to 0.22 g of palladium and 80 g of activated alumina (γ-Al2O3, BET specific surface area: 200 m2 / g, mean primary particle diameter: 6 μm) were subjected to wet milling by a ballmill to prepare 300 g in total of an aqueous slurry (B). A cylindrical honeycomb carrier made of cordierite of 24 mm in diameter and 50 mm in length having 400 cells per square inch of cross-sectional area was coated (wash coat) with this slurry, dried at 120° C. for 8 hours and then calcined at 500° C. for an hour to obtain catalyst B so that the sum of platinum, palladium and alumina is 82.47 g per liter of the honeycomb carrier.
example 3
[0073]14.4 g of an aqueous solution of dinitrodiammine platinum corresponding to 1.64 g of platinum, 5.92 g of an aqueous solution of palladium nitrate corresponding to 0.83 g of palladium and 80 g of activated alumina (γ-Al2O3, BET specific surface area: 200 m2 / g, mean primary particle diameter: 6 μm) were subjected to wet milling by a ballmill to prepare 300 g in total of an aqueous slurry (C). A cylindrical honeycomb carrier made of cordierite of 24 mm in diameter and 50 mm in length having 400 cells per square inch of cross-sectional area was coated (wash coat) with this slurry, dried at 120° C. for 8 hours and then calcined at 500° C. for an hour to obtain catalyst C so that the sum of platinum, palladium and alumina is 82.47 g per liter of the honeycomb carrier.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com