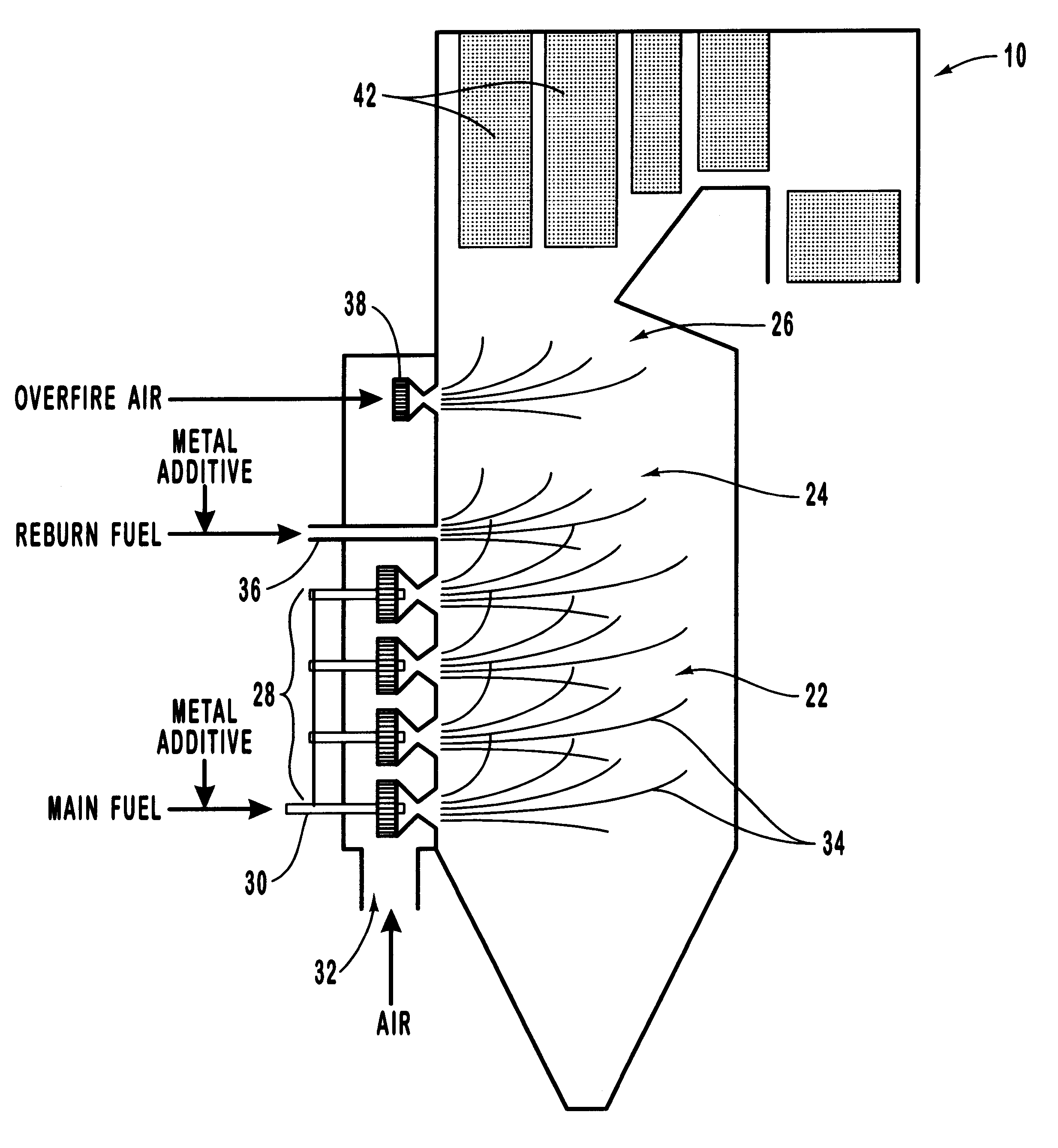
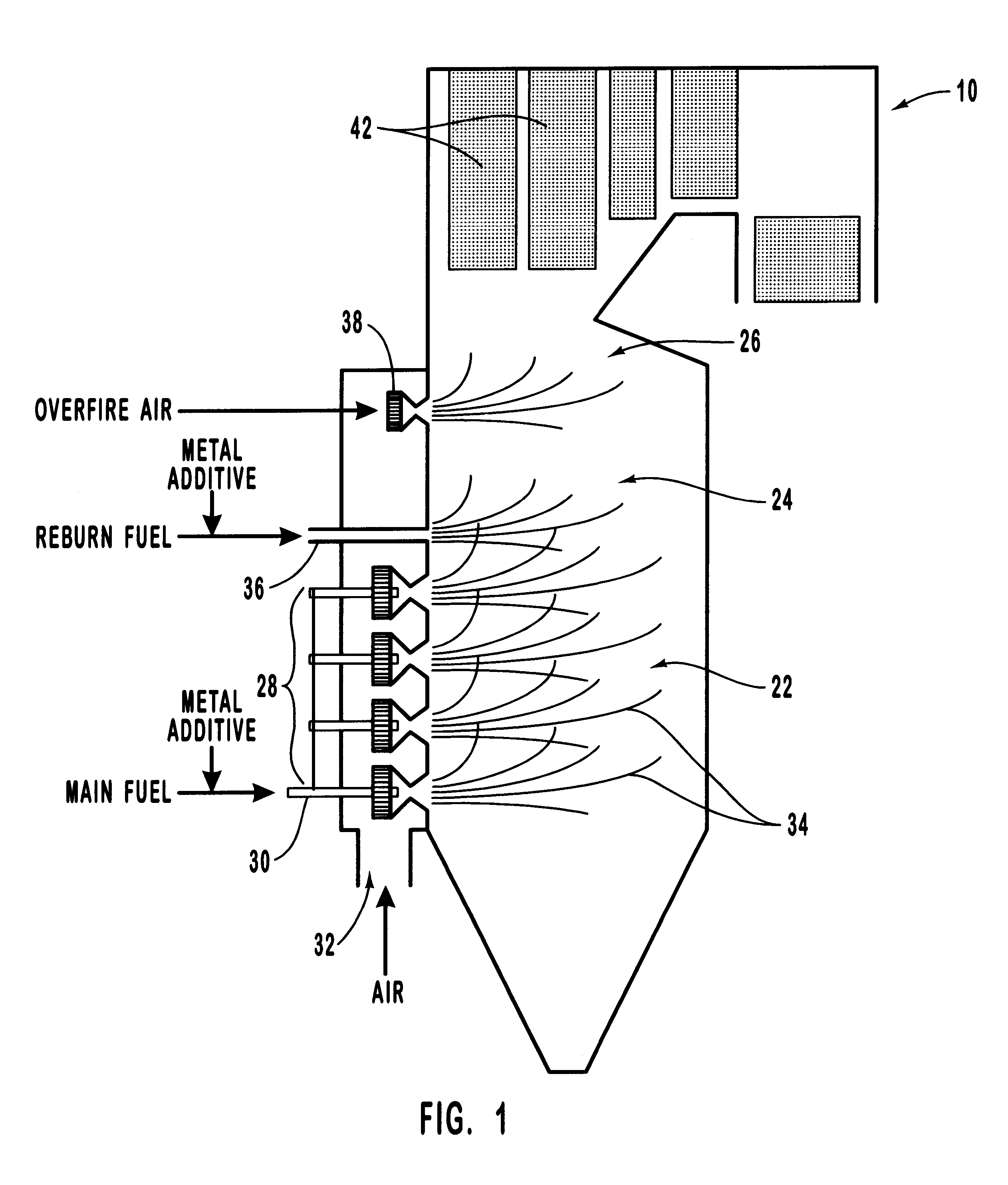
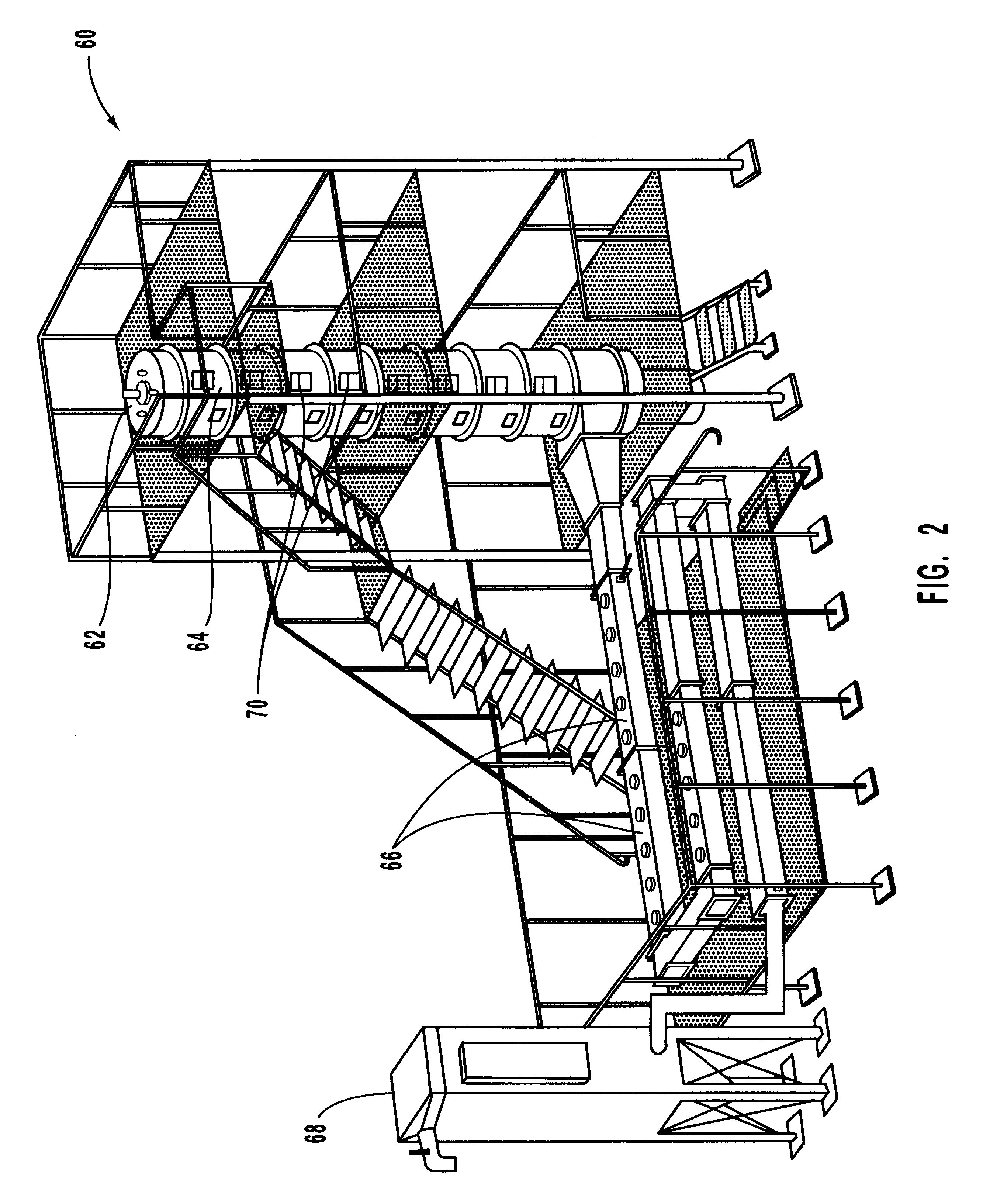
Method for reducing NOX in combustion flue gas using metal-containing additives
a technology of additives and combustion flue gas, which is applied in the direction of combustion types, separation processes, lighting and heating apparatus, etc., can solve the problems of low capital cost, near zero operating cost, and growing concern for nitrogen oxides, and achieves enhanced no.sub.x reduction, increased load on particulate control devices, and high surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 2-10
below describe various test results obtained using the BSF described above. In the Examples that follow, "SR.sub.1 ", "SR.sub.2 " and "SR.sub.3 " indicate the stoichiometric ratio of fuel to oxygen in the main combustion zone, the reburning zone, and the burnout zone, respectively.
example 2
Sodium and calcium-containing additives were co-injected with the main fuel in the presence and absence of reburning. 100 ppm of sodium was added as Na.sub.2 CO.sub.3, and 100 ppm of calcium as Ca(CH.sub.3 COO).sub.2. The BSF conditions used were as follows:
Main Fuel: Natural Gas @ 712,500 BTU / hr SR.sub.1 : 1.10 SR.sub.2 : 0.90 w / N.sub.2 SR.sub.3 : 1.15 NO.sub.x : 600 ppm as measured Reburn Fuel: natural gas (18% heat input) OFA: 2150.degree. F.
Air and bottled nitrogen were used as transport media for natural gas injection. FIG. 3 shows the percent reduction of NO.sub.x for both metal-containing additives, in the presence and in the absence of reburning. Injection of metal compounds in the absence of reburning resulted in 16% to 21% NO.sub.x reduction. Reburning itself provided a 48% and 66% NO.sub.x reduction with air and nitrogen transport, respectively. Injection of 100 ppm of metal compounds with the main fuel provided an additional 4-11 percentage points of NO.sub.x reduction. ...
example 3
The experiment of Example 2 was repeated, using sodium- and potassium-containing metal additives co-injected with the main fuel, as a function of additive concentration. Sodium was added at concentrations ranging from 0 ppm to about 1150 ppm in the form of Na.sub.2 CO.sub.3, and potassium was added from 0 ppm to about 550 ppm in the form of K.sub.2 CO.sub.3. The results shown in FIG. 4 indicate that addition of sodium and potassium compounds to the main fuel have similar effects on NO.sub.x reduction. In the absence of reburning, up to approximately 28% NO.sub.x reduction was achieved at 500-550 ppm of Na or K. The additives also improved the efficiency of reburning by 11 percentage points with N.sub.2 transport and 18 percentage points with air transport. Thus, results presented in FIG. 4 illustrate that metal additives, upon being added into the main combustion zone, are capable of reducing NO.sub.x emissions in the presence and in the absence of reburning, over a range of additiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com