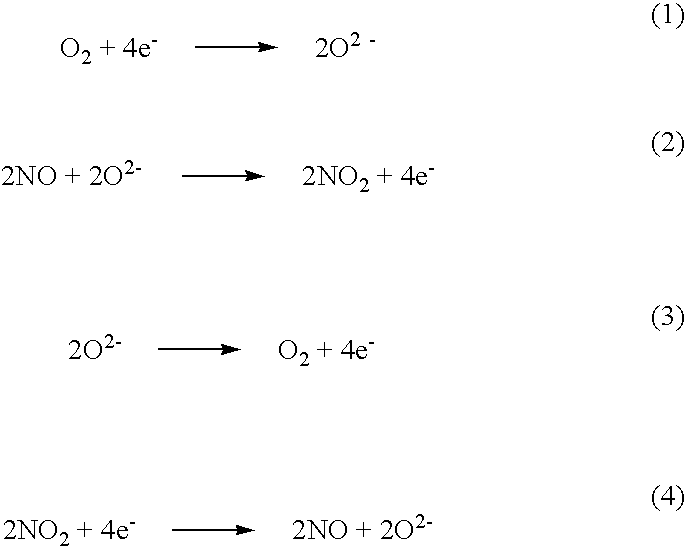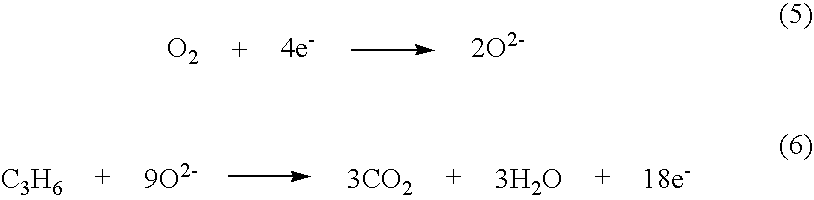Patents
Literature
5719 results about "Nitrogen oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
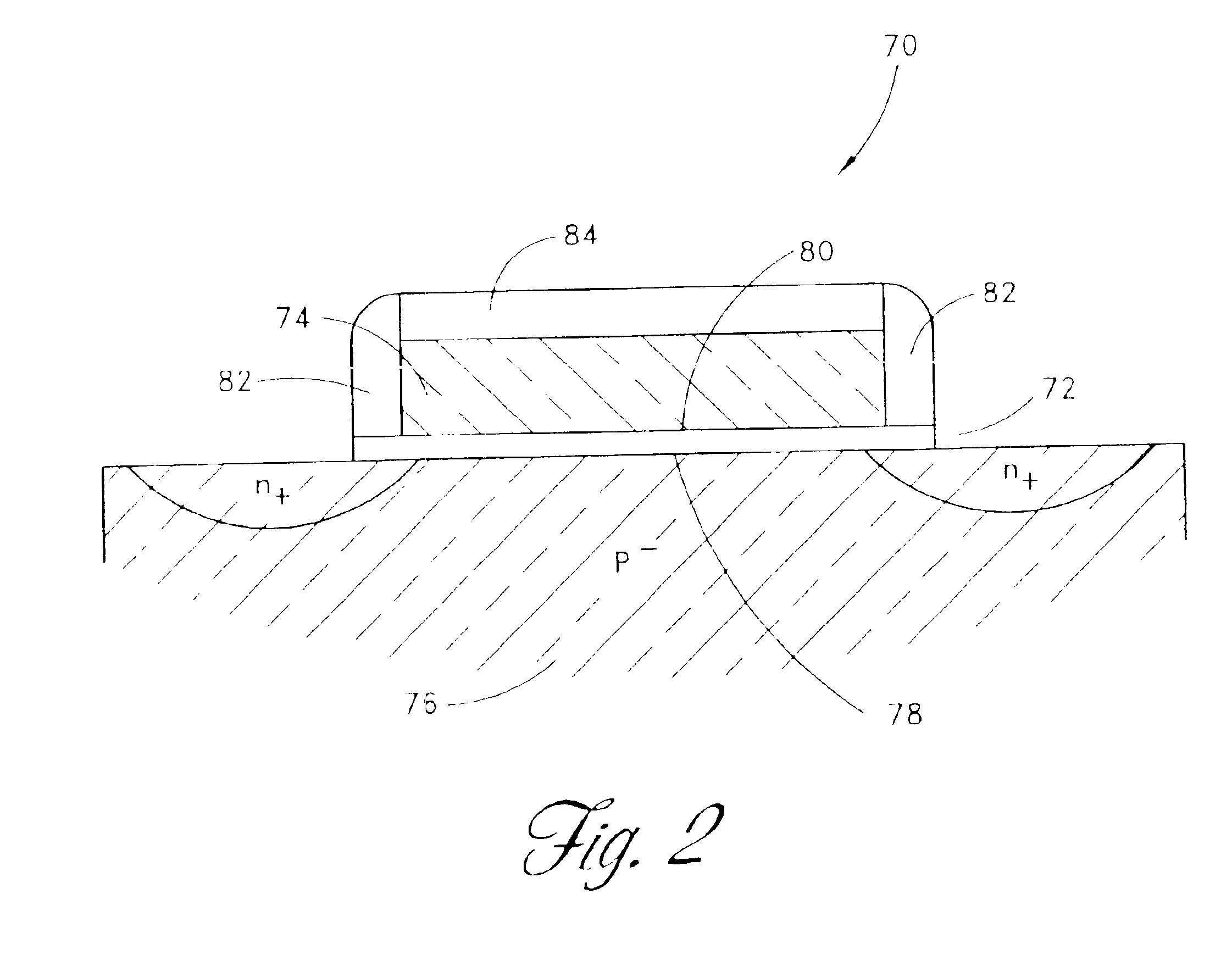
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Nitric oxide, also known as nitrogen monoxide, nitrogen oxide Nitrogen dioxide, nitrogen oxide Nitrous oxide, nitrogen oxide Nitrosylazide, nitrogen oxide Dinitrogen trioxide, nitrogen oxide Dinitrogen tetroxide, nitrogen oxide Dinitrogen pentoxide, nitrogen oxide Trinitramide, nitrogen oxide In addition, nitrogen oxides form several radicals. The most stable of these is the nitrate anion: Nitrate, nitrogen oxide but other nitrogen oxoanions include nitrite, peroxonitrite, trioxodinitrate, and nitroxylate. In atmospheric chemistry, air pollution, and related fields, nitrogen oxides refers specifically to NOₓ. Only the first three of these compounds can be isolated at room temperature. N₂O₃, N₂O₄, and N₂O₅ all decompose rapidly at room temperature. NO₃, N₄O, and N(NO₂)₃ are very reactive. N₂O is stable and rather unreactive at room temperature, while NO and NO₂ are quite reactive but nevertheless quite stable when isolated. Nitric oxide, NO Nitrogen dioxide, NO₂
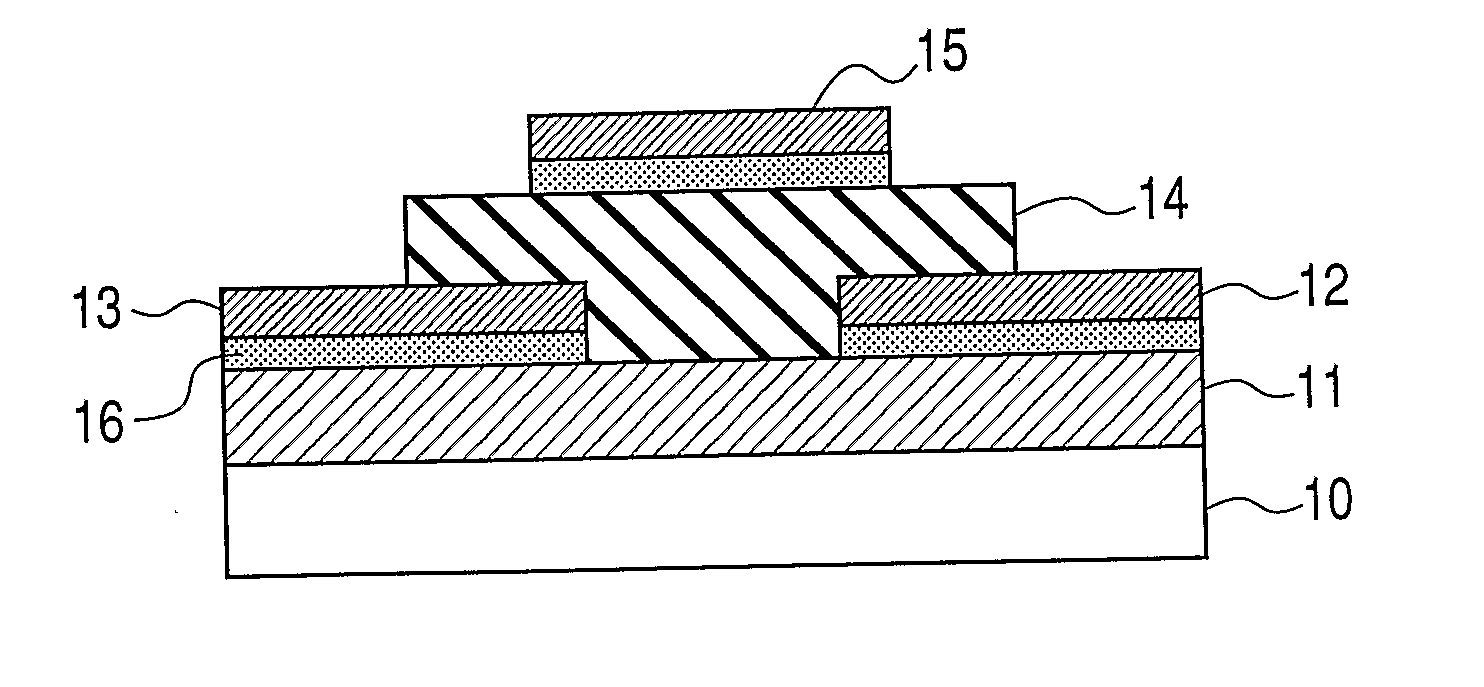
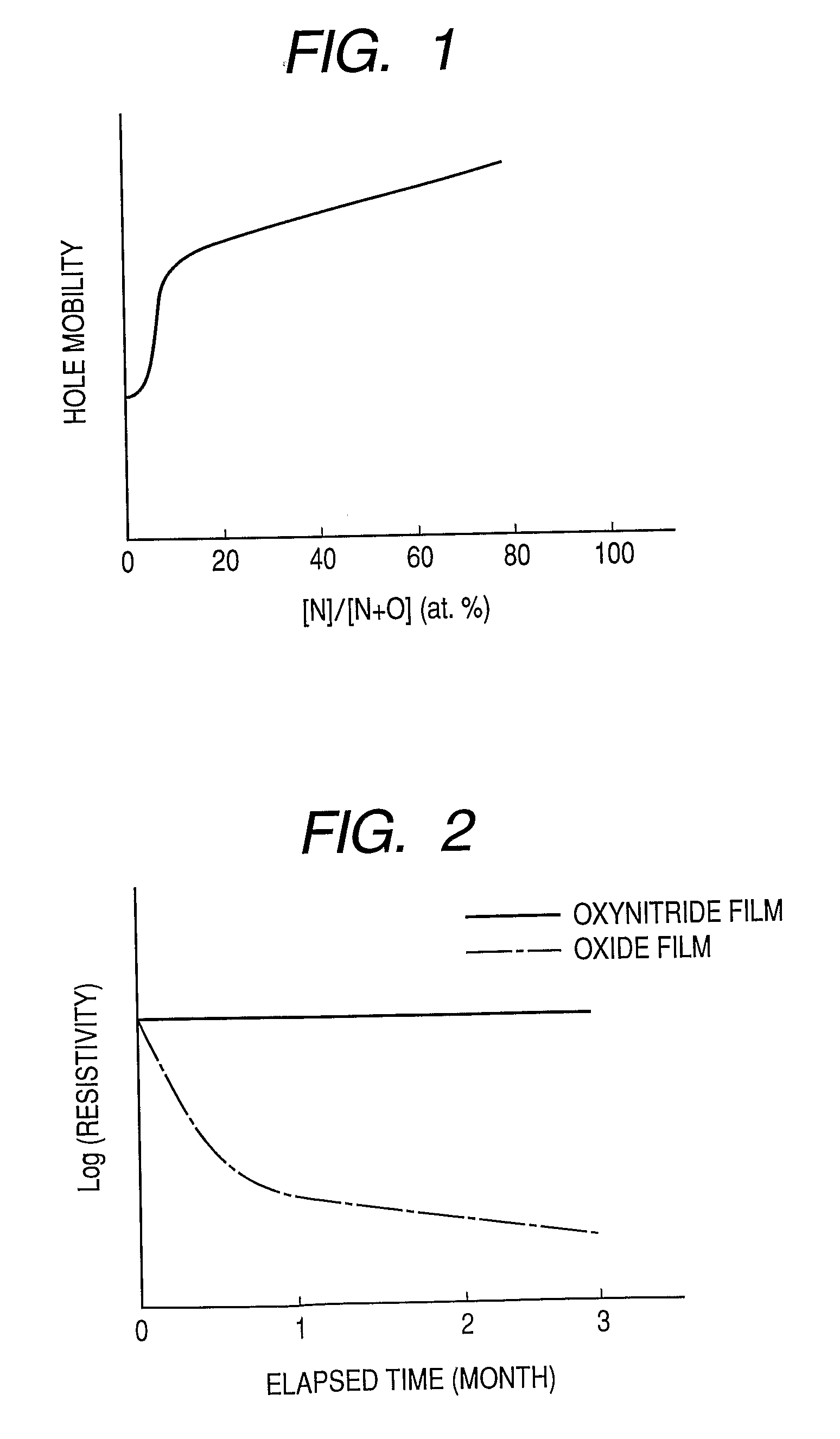
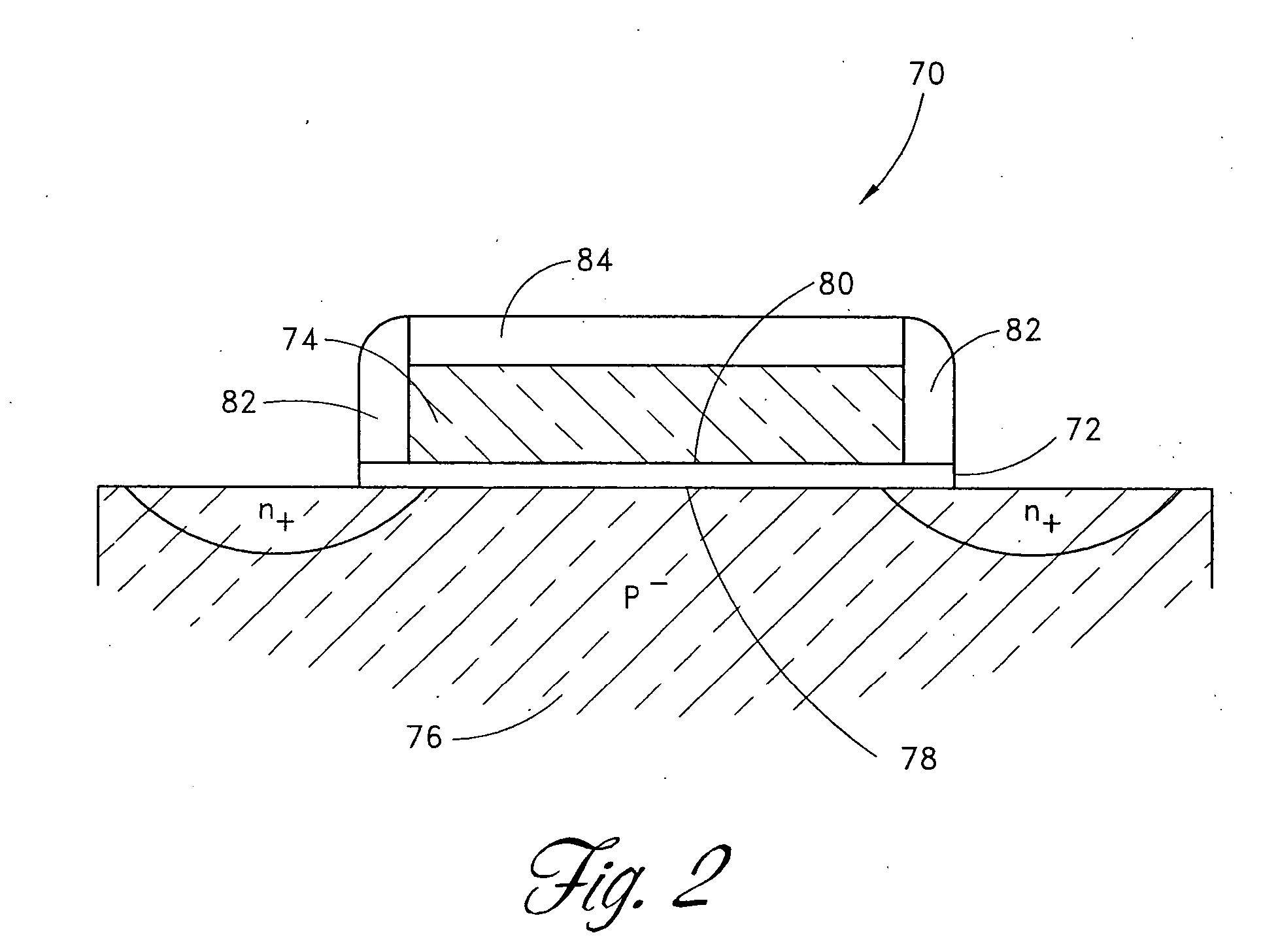
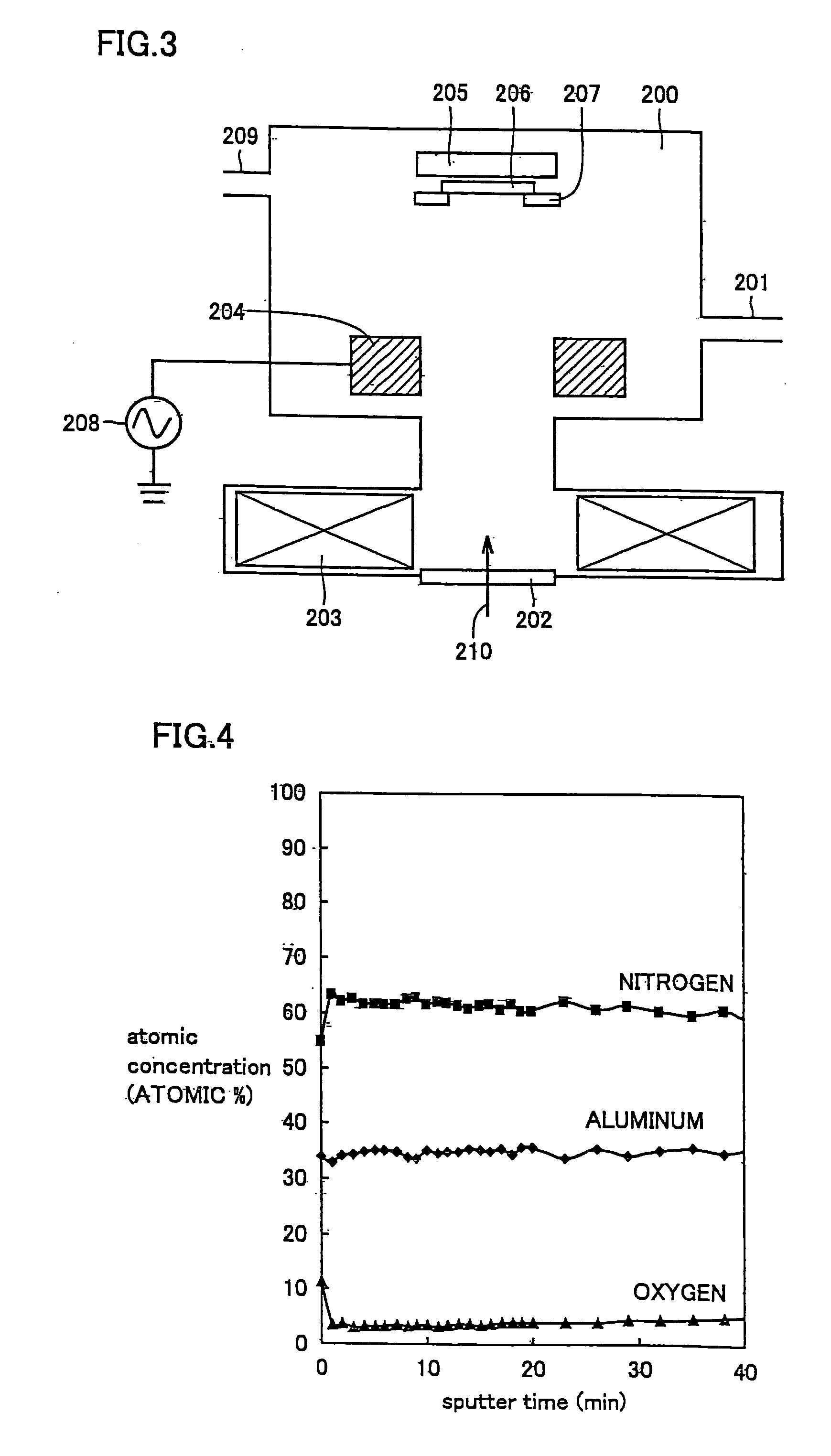
Oxynitride semiconductor
ActiveUS20100109002A1High mobility and environmental stabilityImprove mobilitySemiconductor devicesNitrogen oxideOxygen
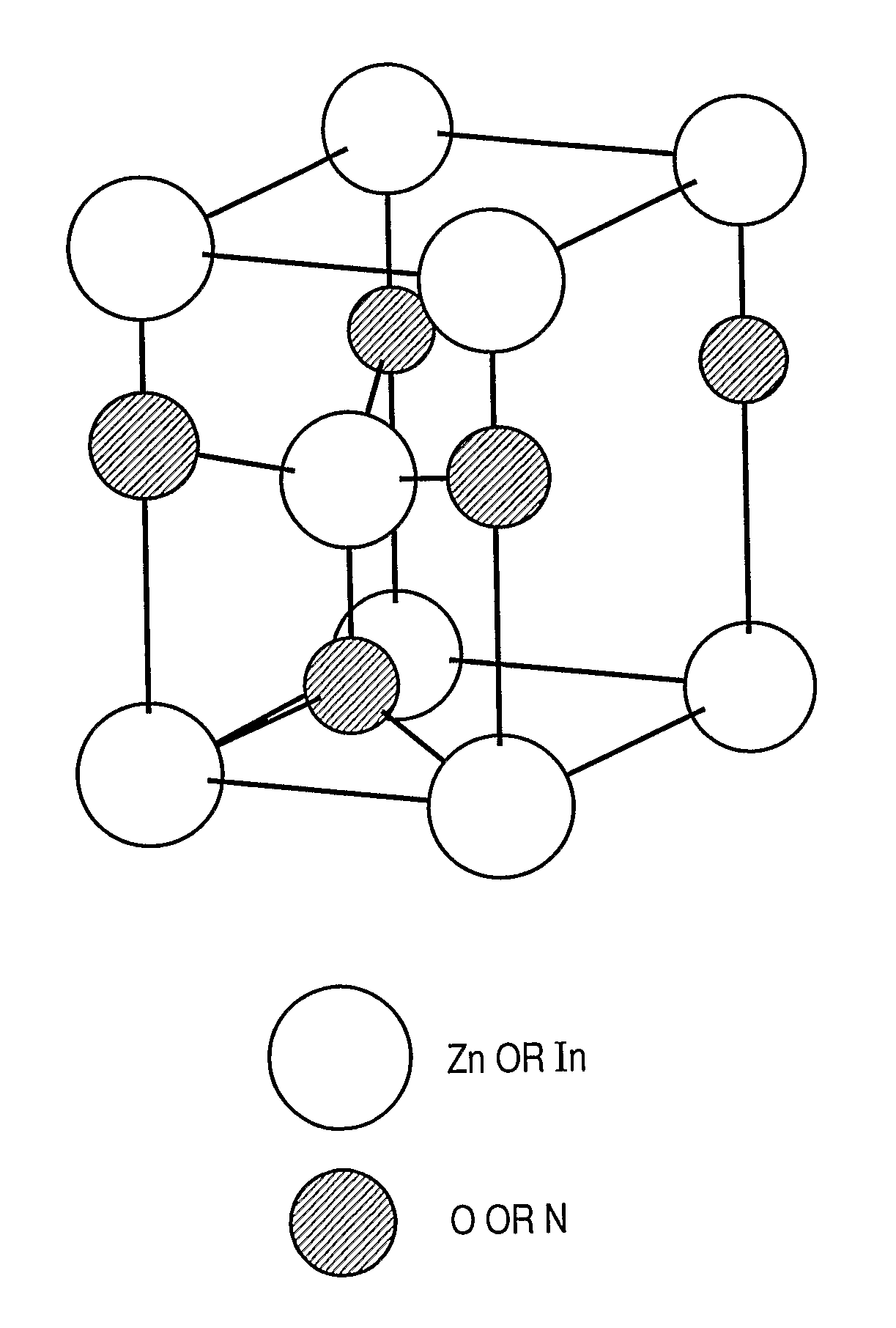
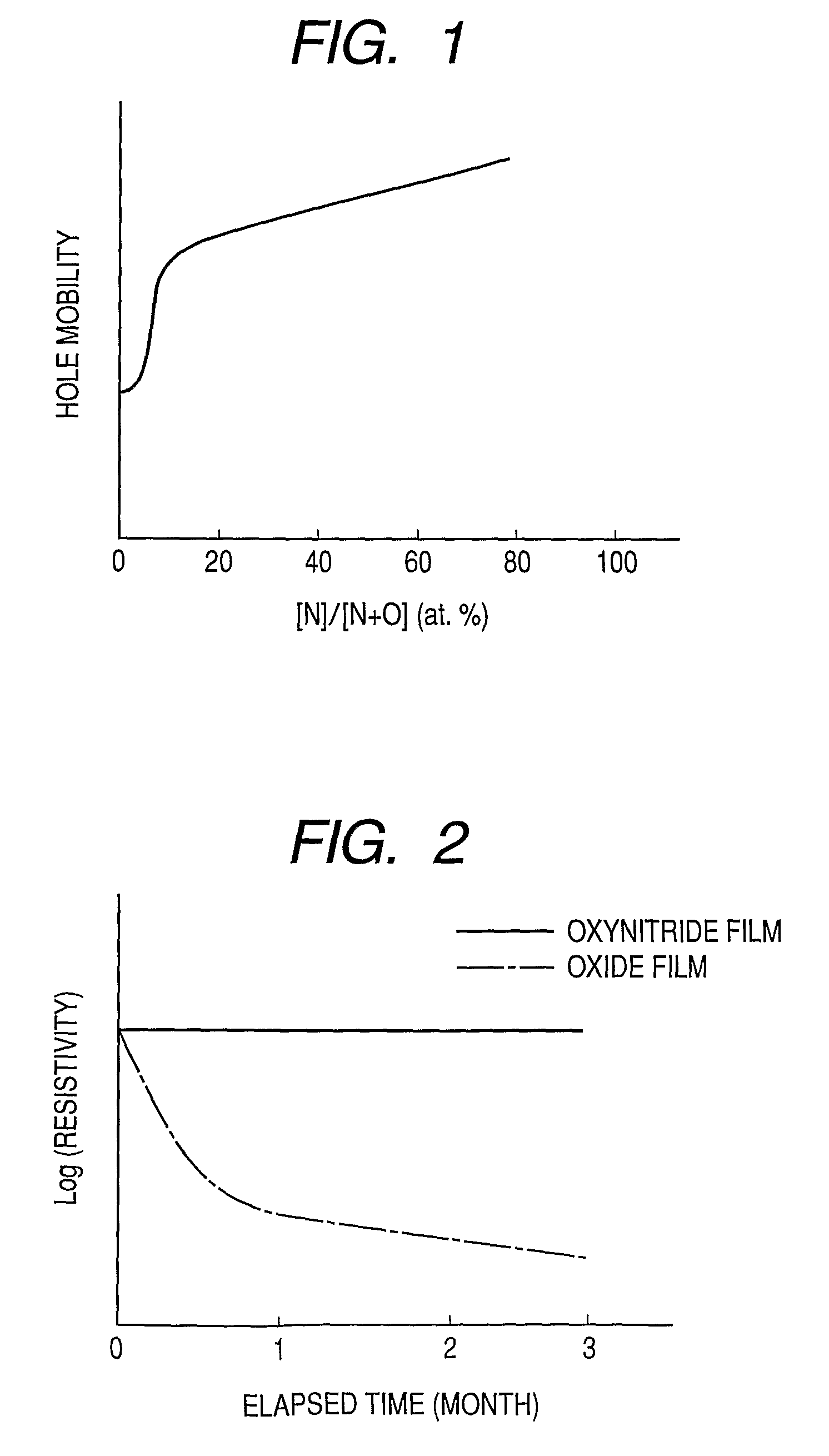
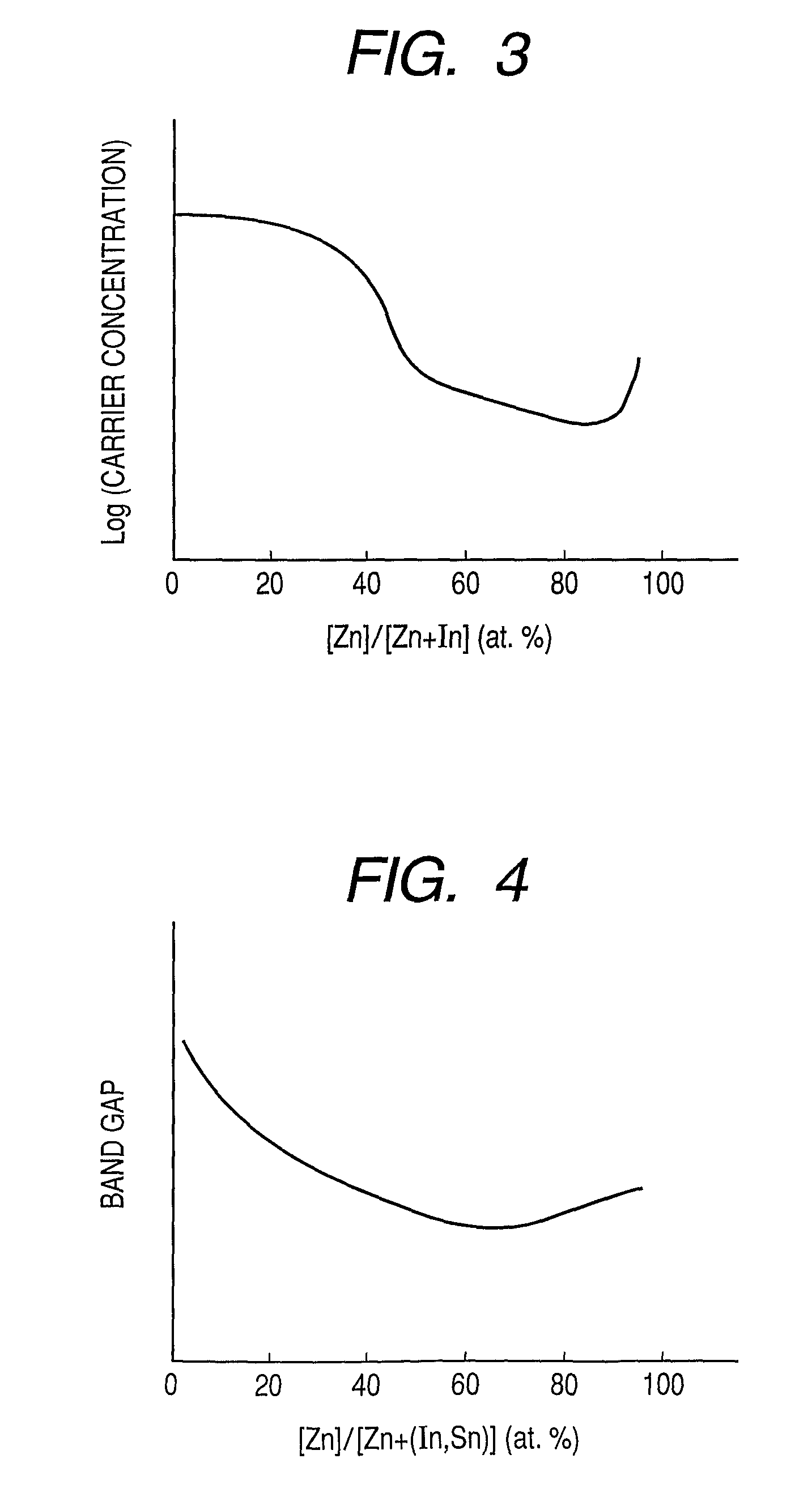
Provided is an oxynitride semiconductor comprising a metal oxynitride. The metal oxynitride contains Zn and at least one element selected from the group consisting of In, Ga, Sn, Mg, Si, Ge, Y, Ti, Mo, W, and Al. The metal oxynitride has an atomic composition ratio of N, N / (N+O), of 7 atomic percent or more to 80 atomic percent or less.
Owner:CANON KK
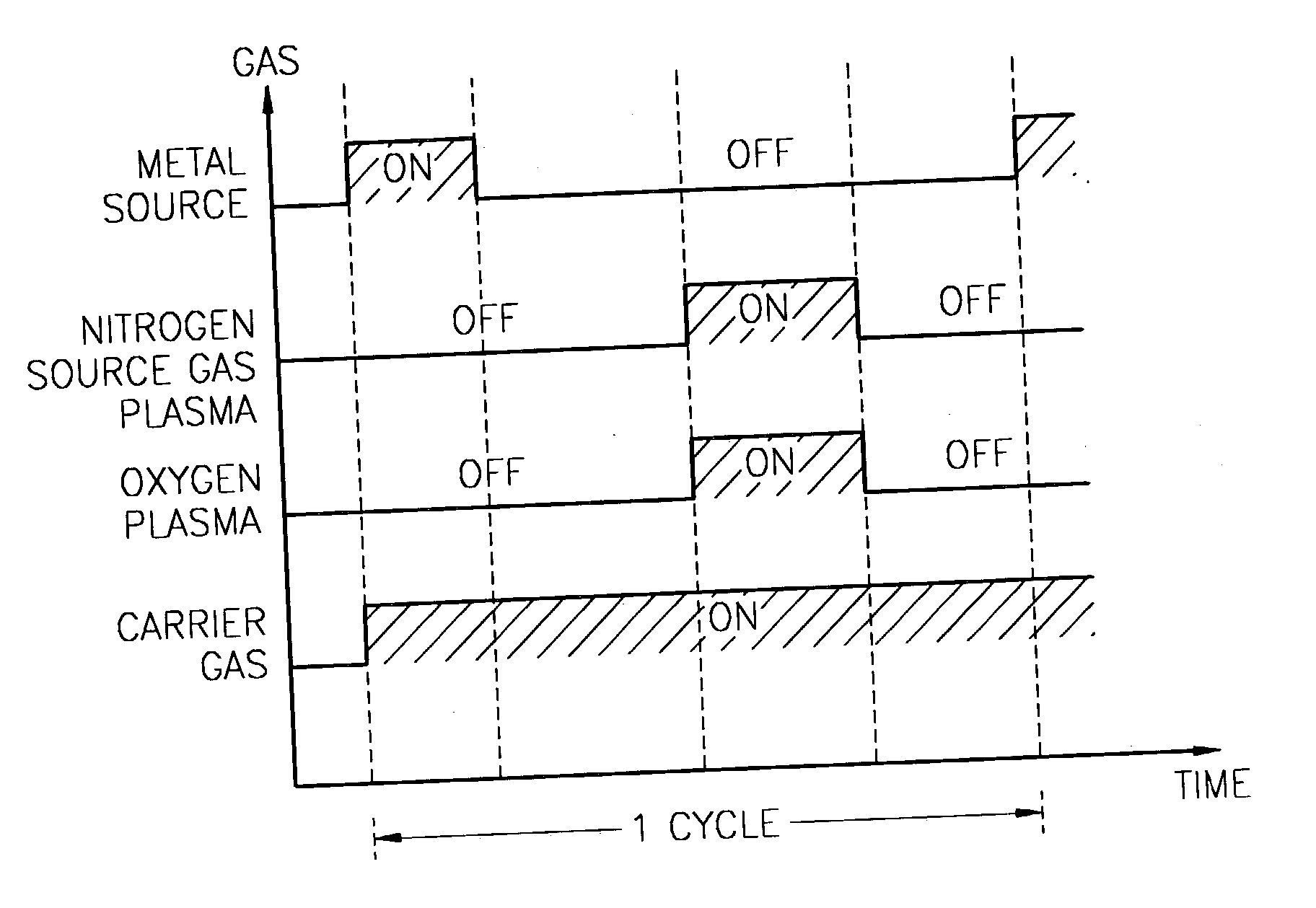
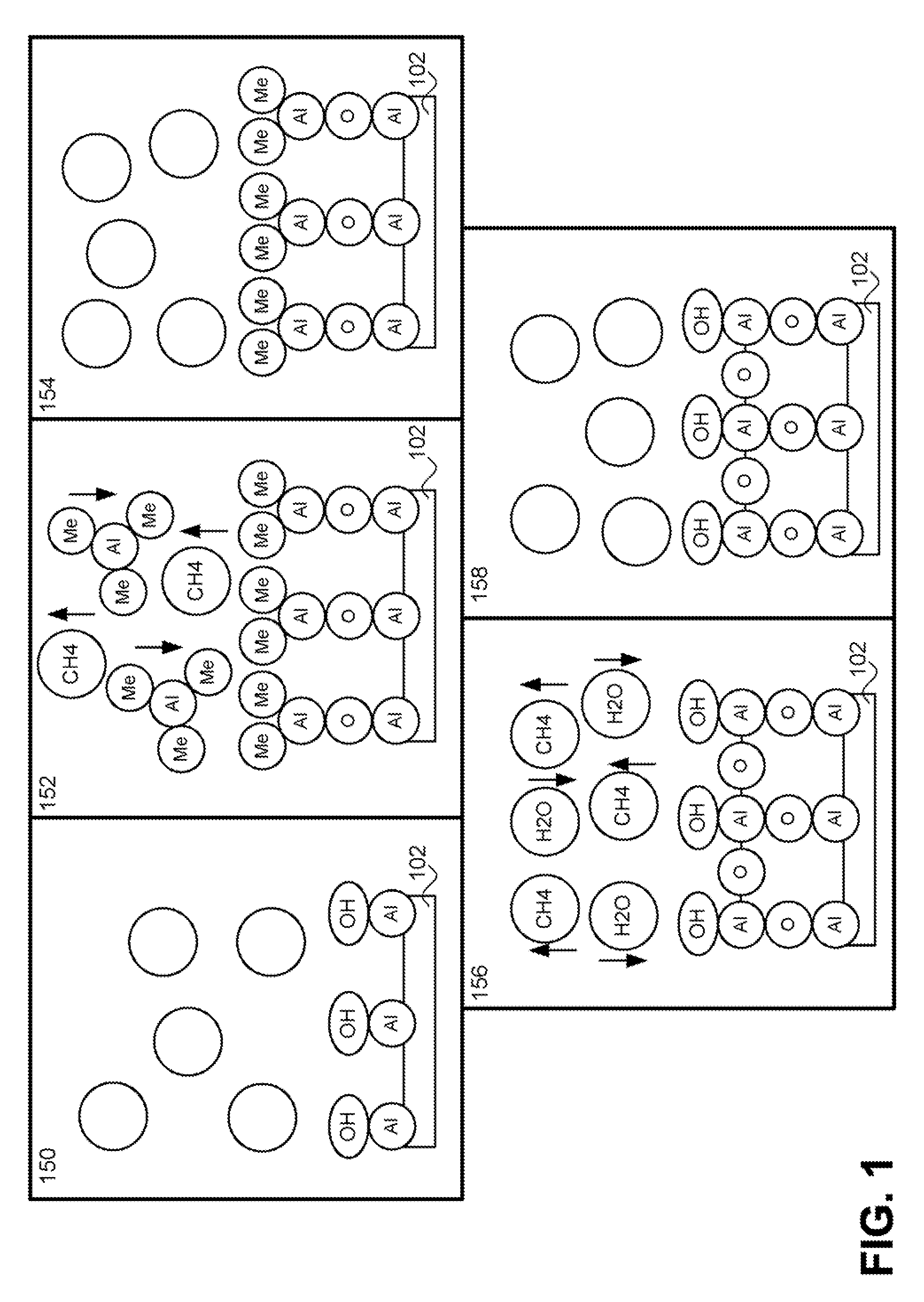
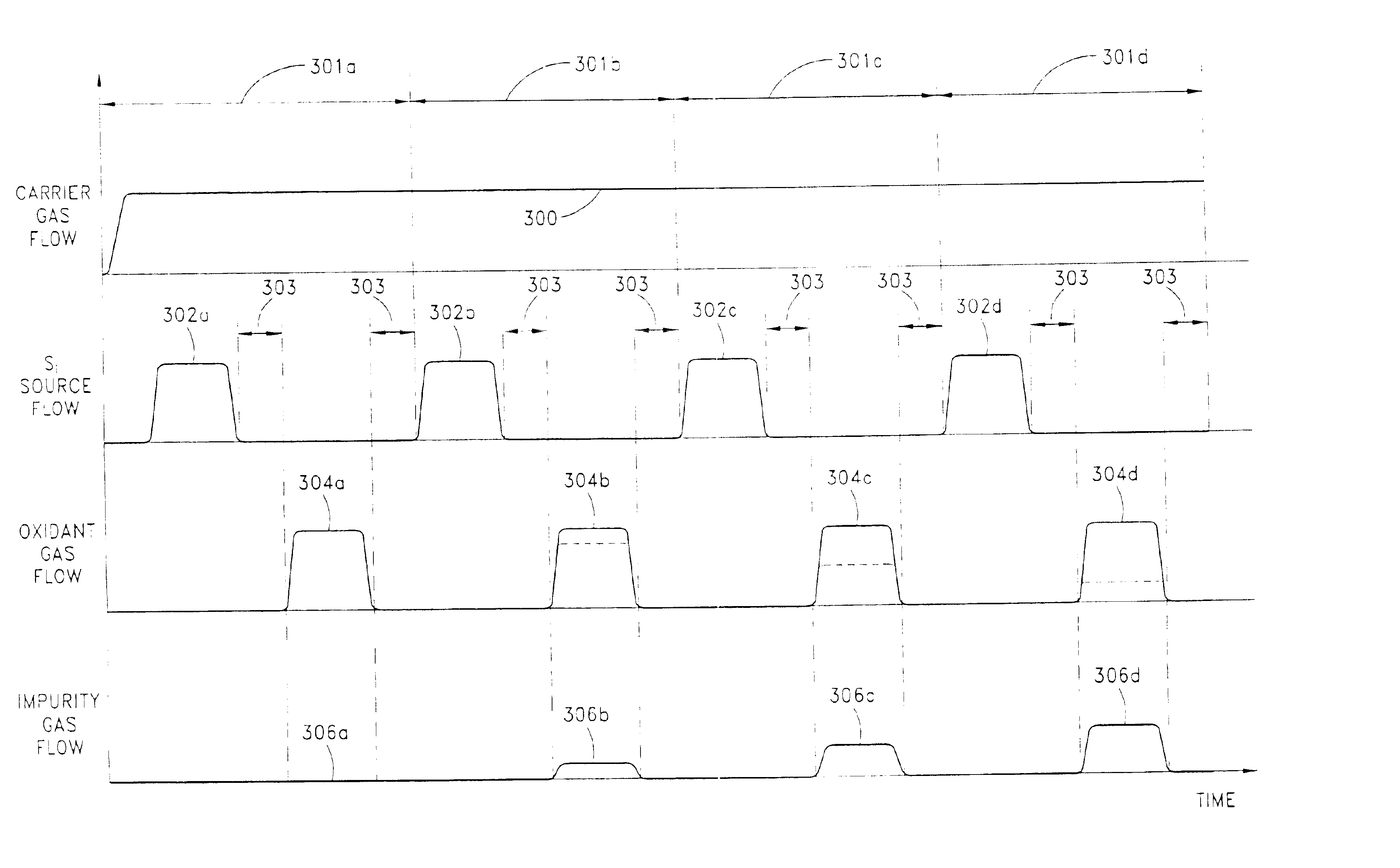
Method for forming introgen-containing oxide thin film using plasma enhanced atomic layer deposition
InactiveUS20040077182A1Density of thinThin rateSemiconductor/solid-state device manufacturingChemical vapor deposition coatingHigh rateNitrogen source
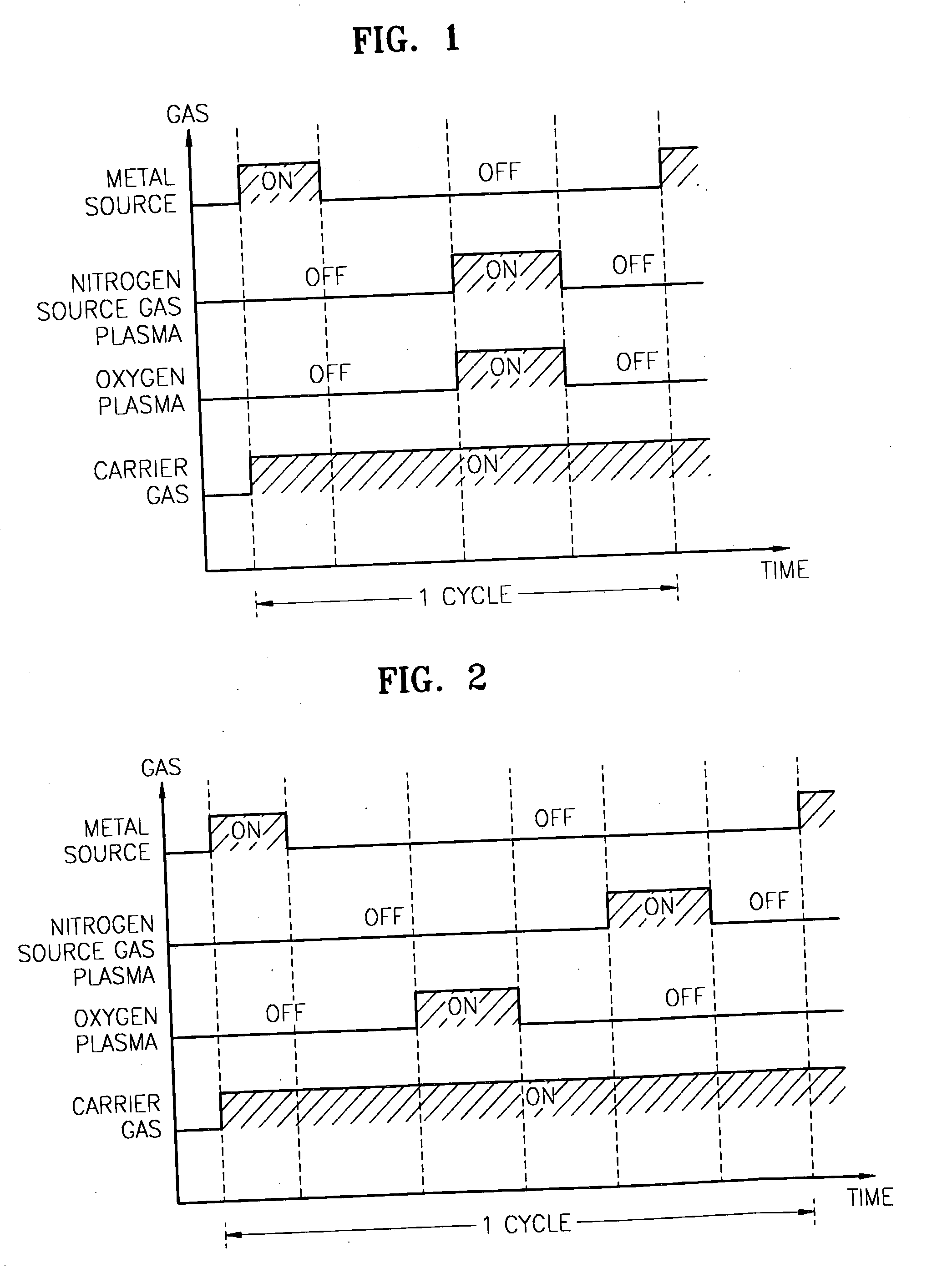
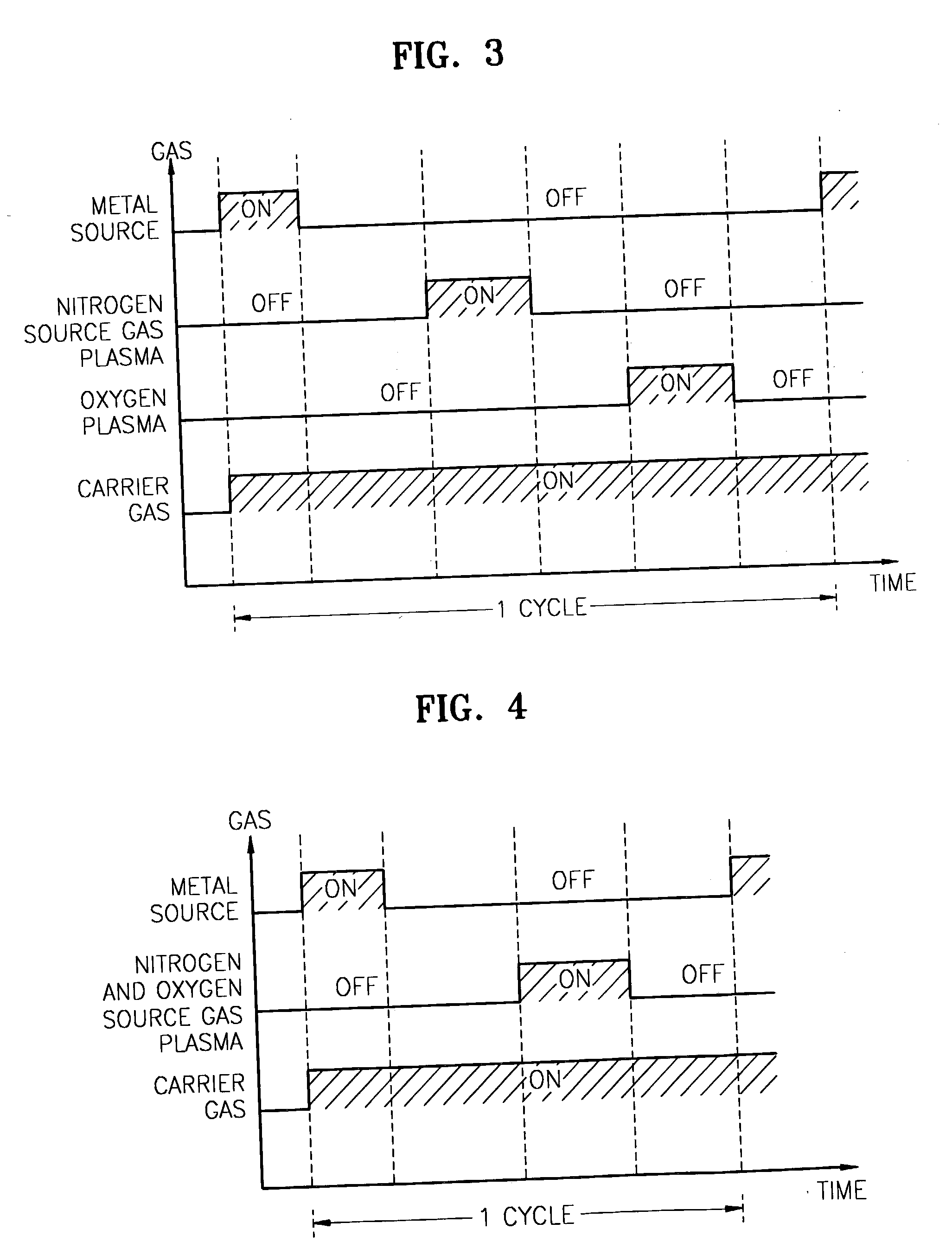
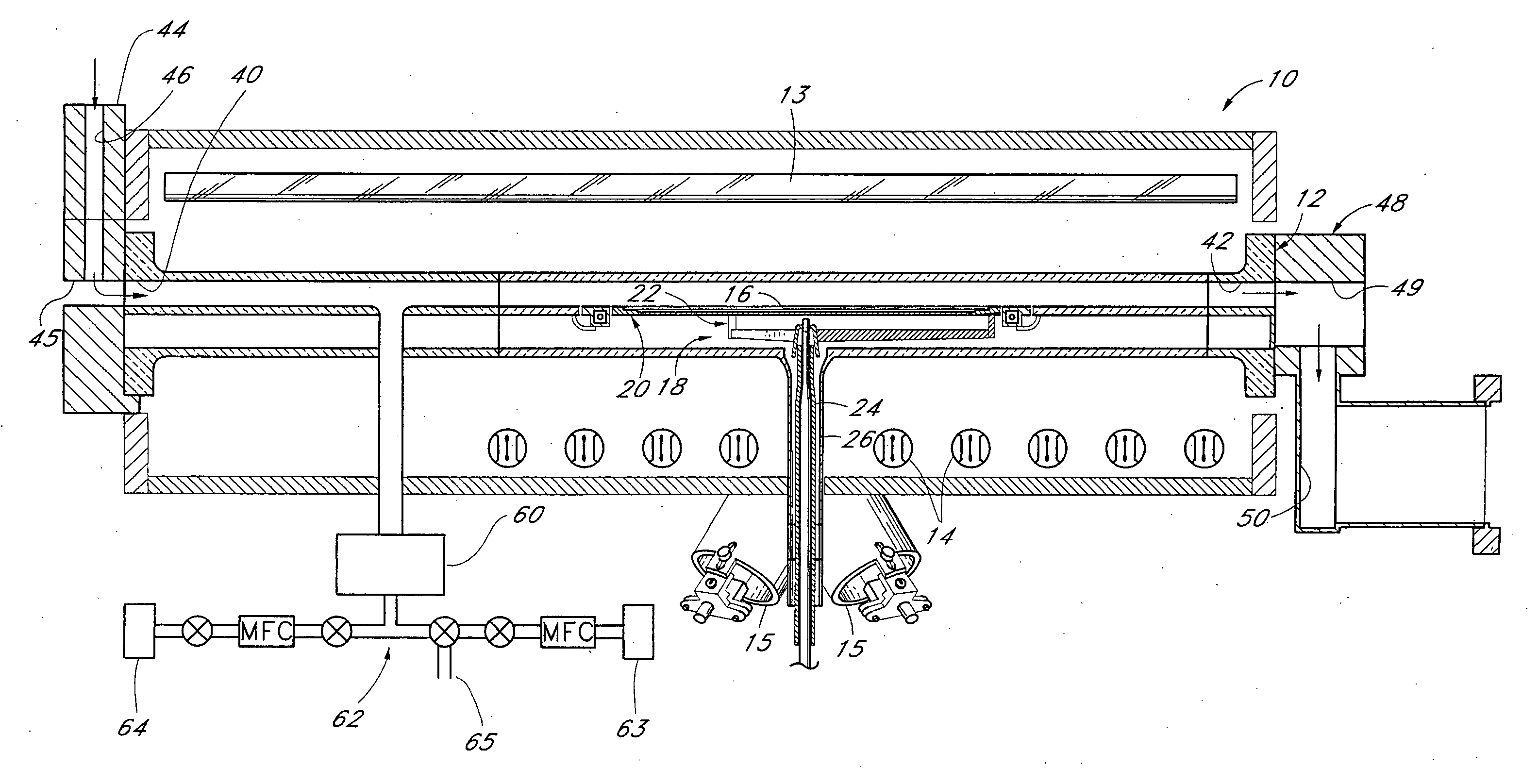
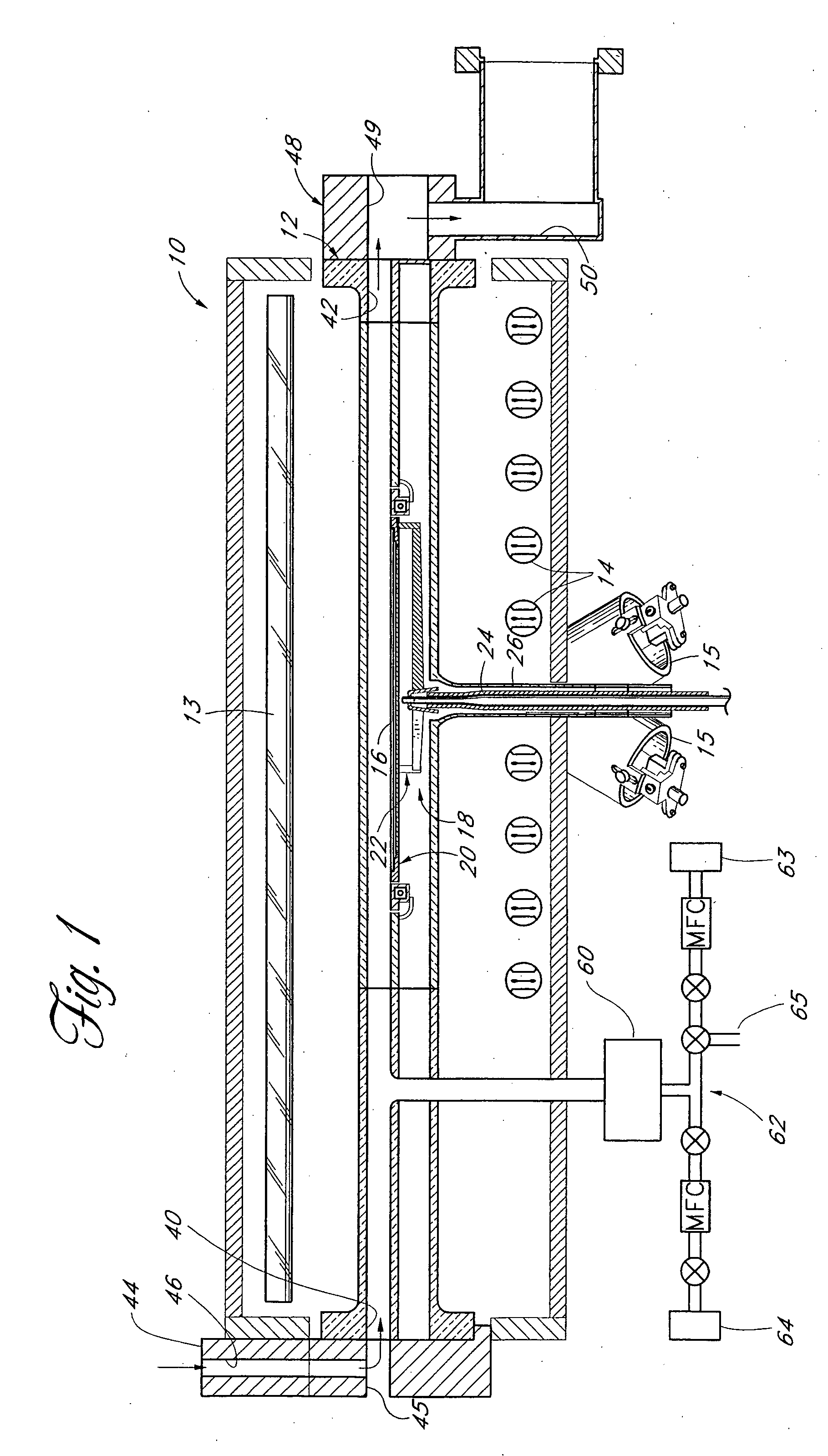
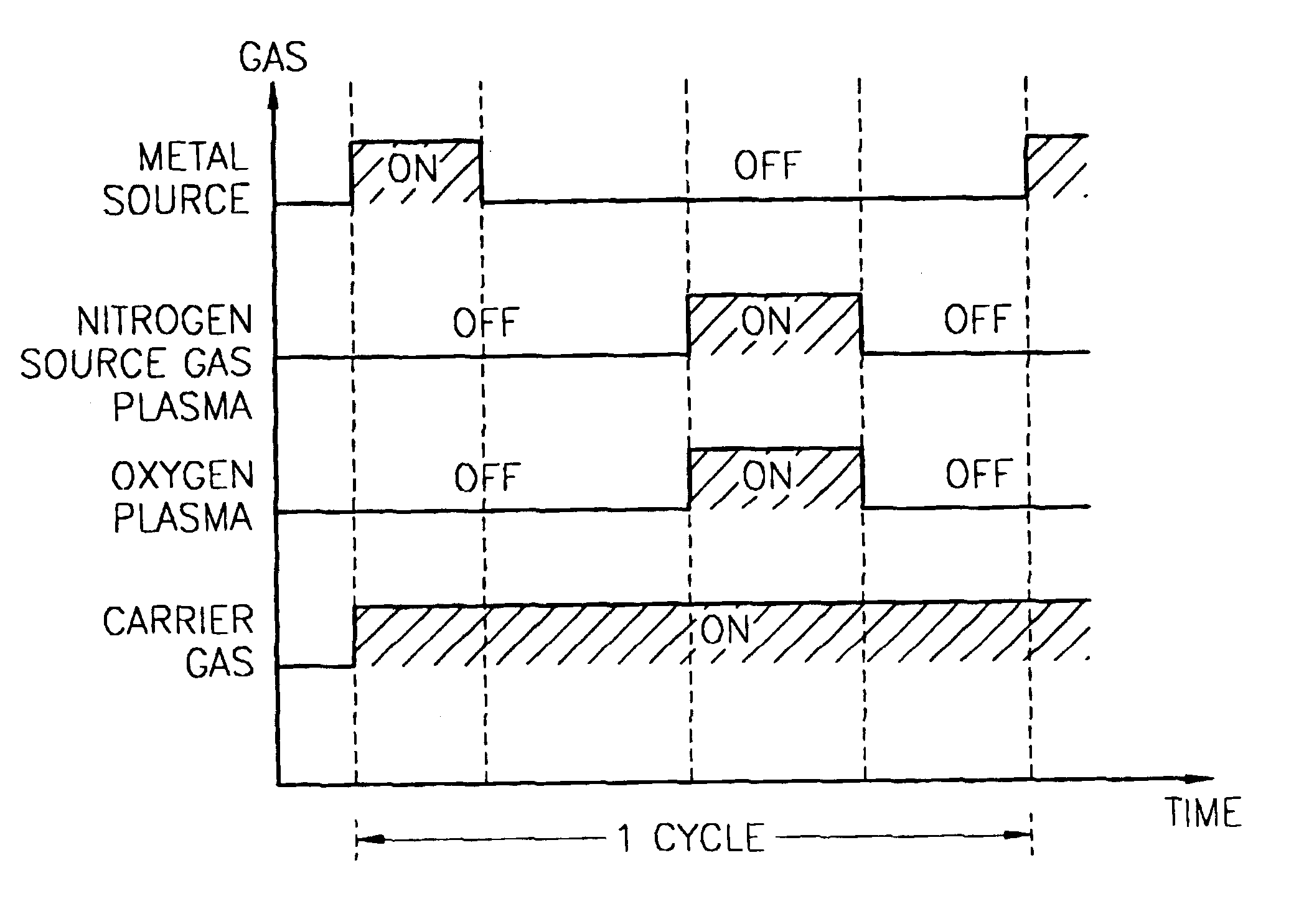
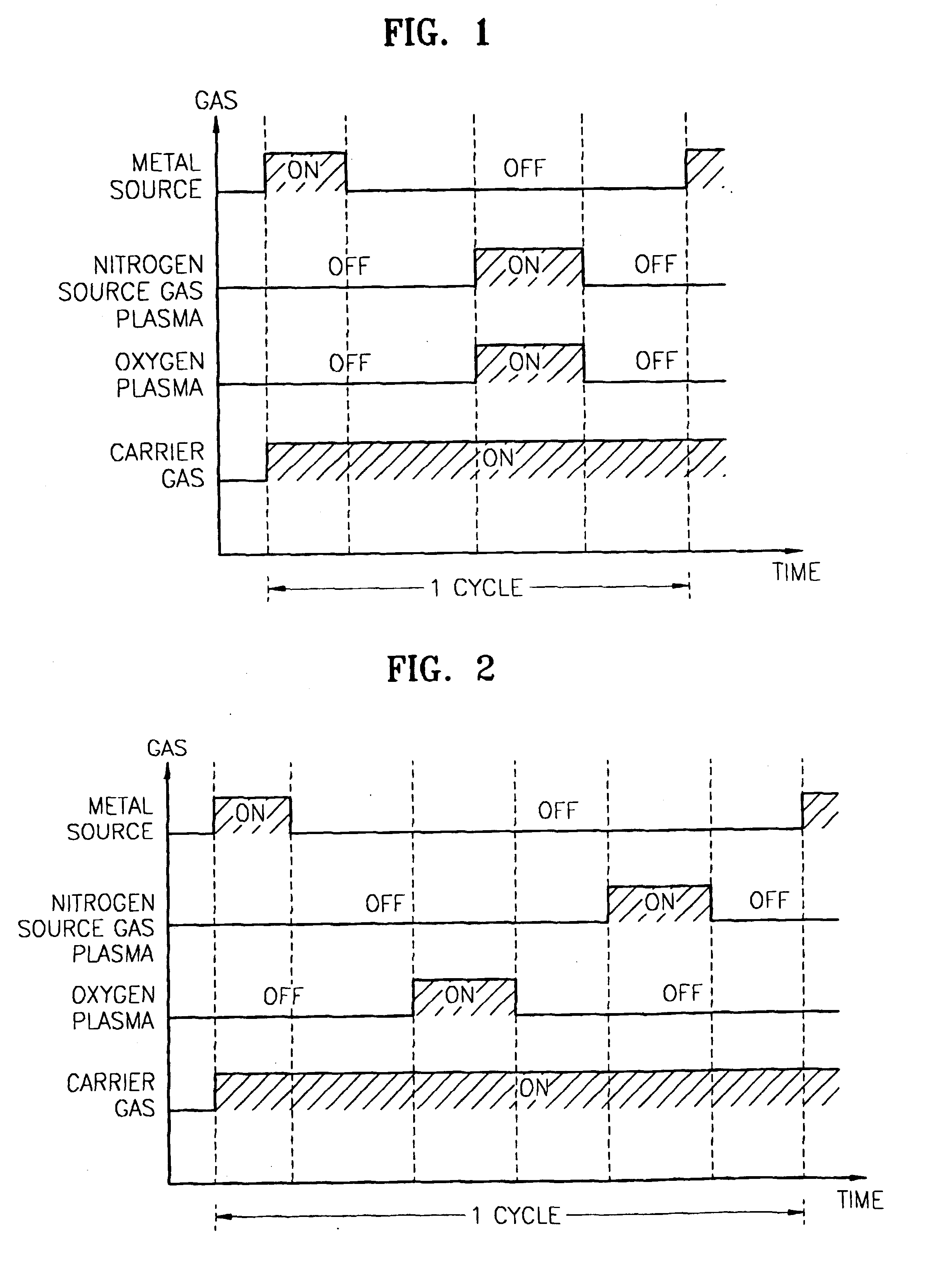
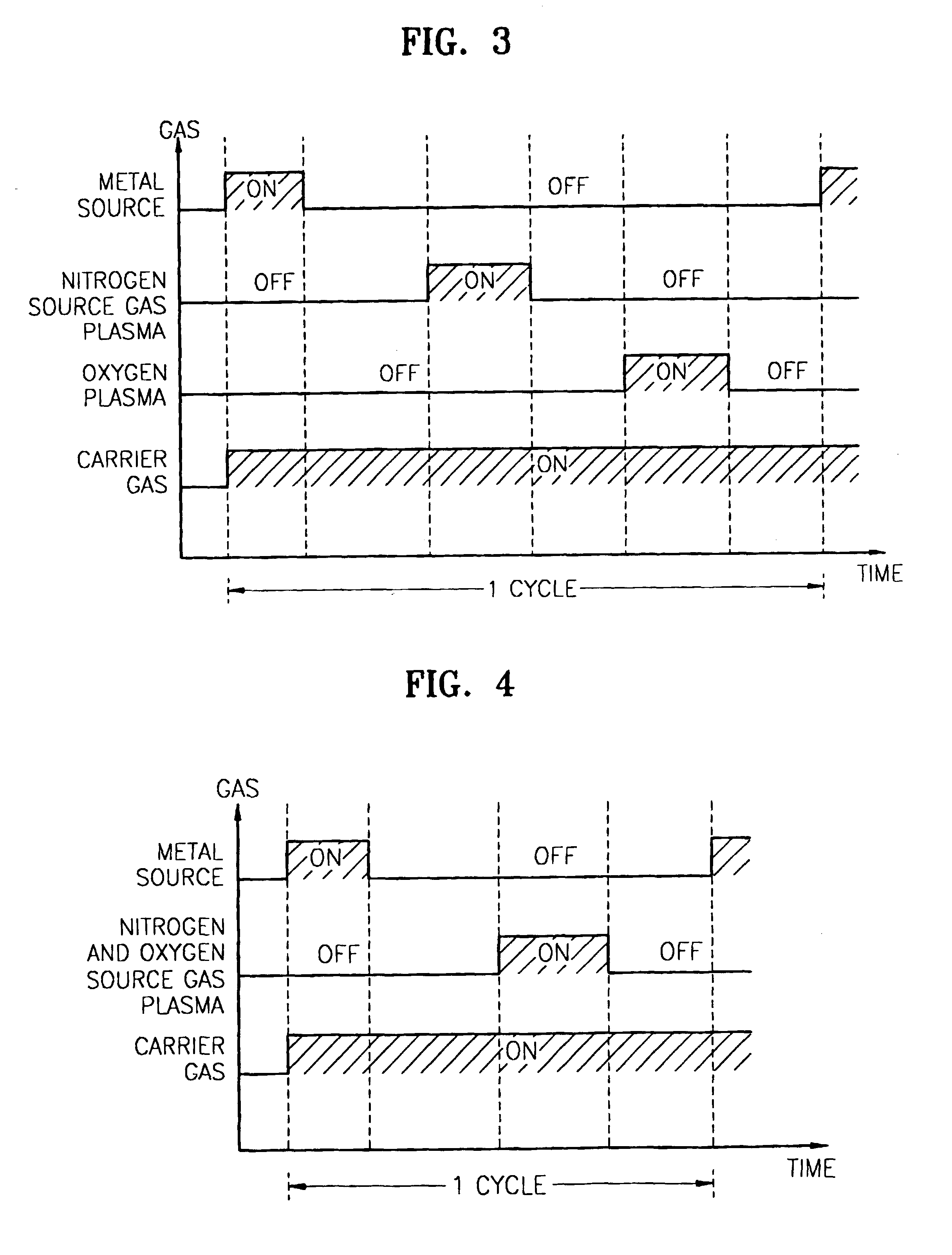
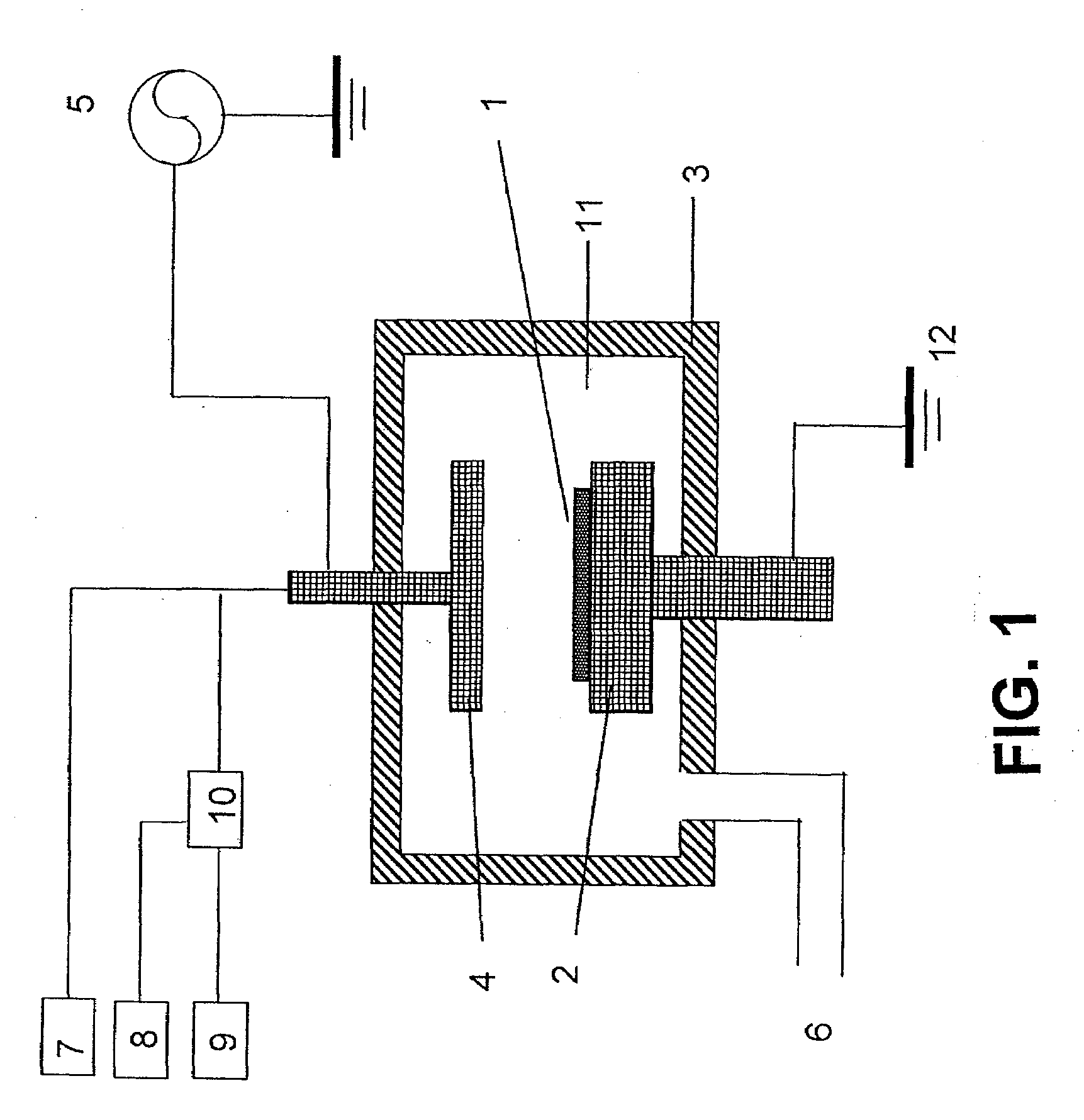
A method for forming a nitrogen-containing oxide thin film by using plasma enhanced atomic layer deposition is provided. In the method, the nitrogen-containing oxide thin film is deposited by supplying a metal source compound and oxygen gas into a reactor in a cyclic fashion with sequential alternating pulses of the metal source compound and the oxygen gas, wherein the oxygen gas is activated into plasma in synchronization of the pulsing thereof, and a nitrogen source gas is further sequentially pulsed into the reactor and activated into plasma over the substrate in synchronization with the pulsing thereof. According to the method, a dense nitrogen-containing oxide thin film can be deposited at a high rate, and a trace of nitrogen atoms can be incorporated in situ into the nitrogen-containing oxide thin film, thereby increasing the breakdown voltage of the film.
Owner:ELECTRONICS & TELECOMM RES INST
Thin films
InactiveUS20050181555A1Quality improvementHigh dielectric constantSolid-state devicesSemiconductor/solid-state device manufacturingGate dielectricSilicon oxide
Thin films are formed by formed by atomic layer deposition, whereby the composition of the film can be varied from monolayer to monolayer during cycles including alternating pulses of self-limiting chemistries. In the illustrated embodiments, varying amounts of impurity sources are introduced during the cyclical process. A graded gate dielectric is thereby provided, even for extremely thin layers. The gate dielectric as thin as 2 nm can be varied from pure silicon oxide to oxynitride to silicon nitride. Similarly, the gate dielectric can be varied from aluminum oxide to mixtures of aluminum oxide and a higher dielectric material (e.g., ZrO2) to pure high k material and back to aluminum oxide. In another embodiment, metal nitride (e.g., WN) is first formed as a barrier for lining dual damascene trenches and vias. During the alternating deposition process, copper can be introduced, e.g., in separate pulses, and the copper source pulses can gradually increase in frequency, forming a transition region, until pure copper is formed at the upper surface. Advantageously, graded compositions in these and a variety of other contexts help to avoid such problems as etch rate control, electromigration and non-ohmic electrical contact that can occur at sharp material interfaces. In some embodiments additional seed layers or additional transition layers are provided.
Owner:ASM INTERNATIONAL
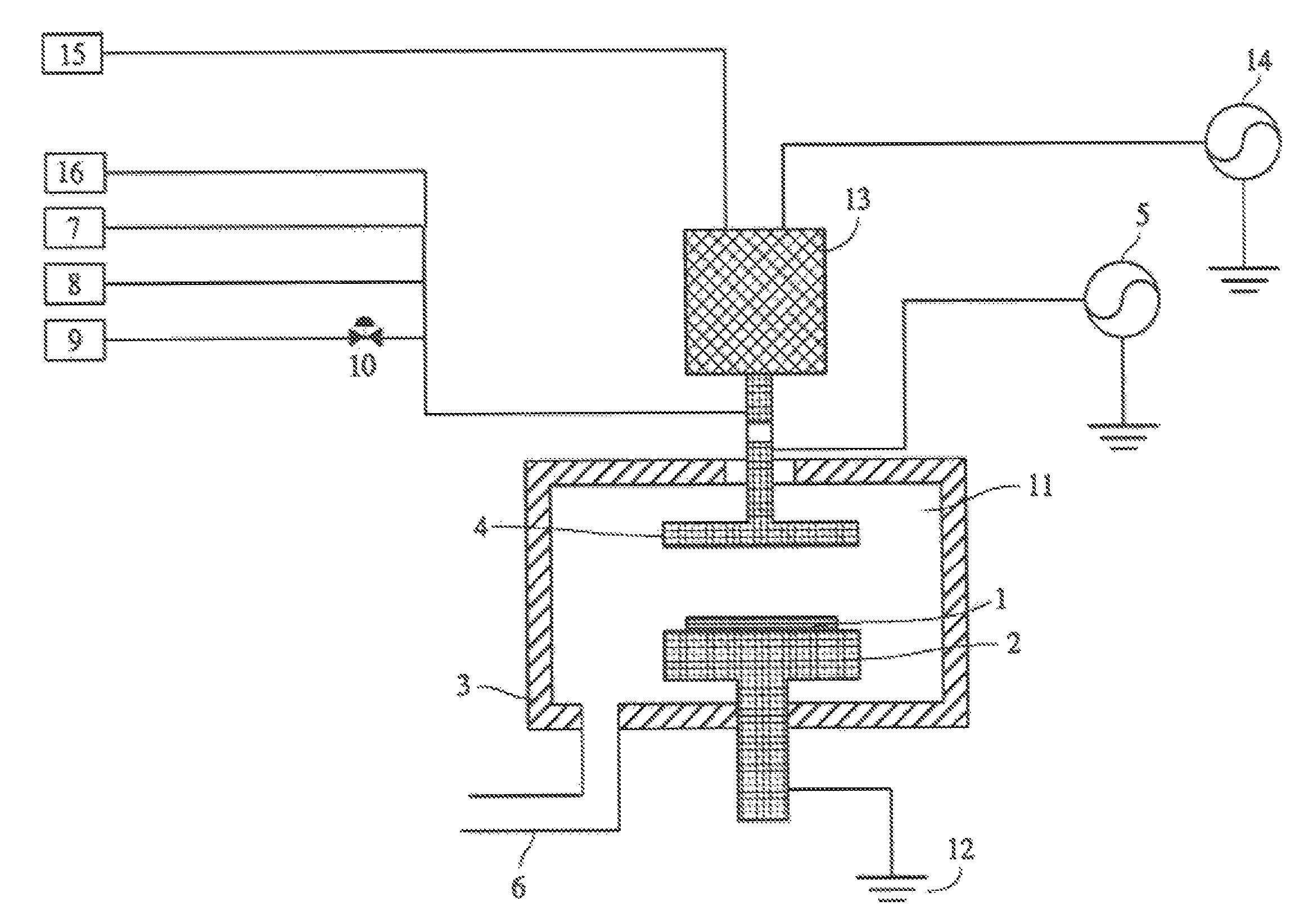
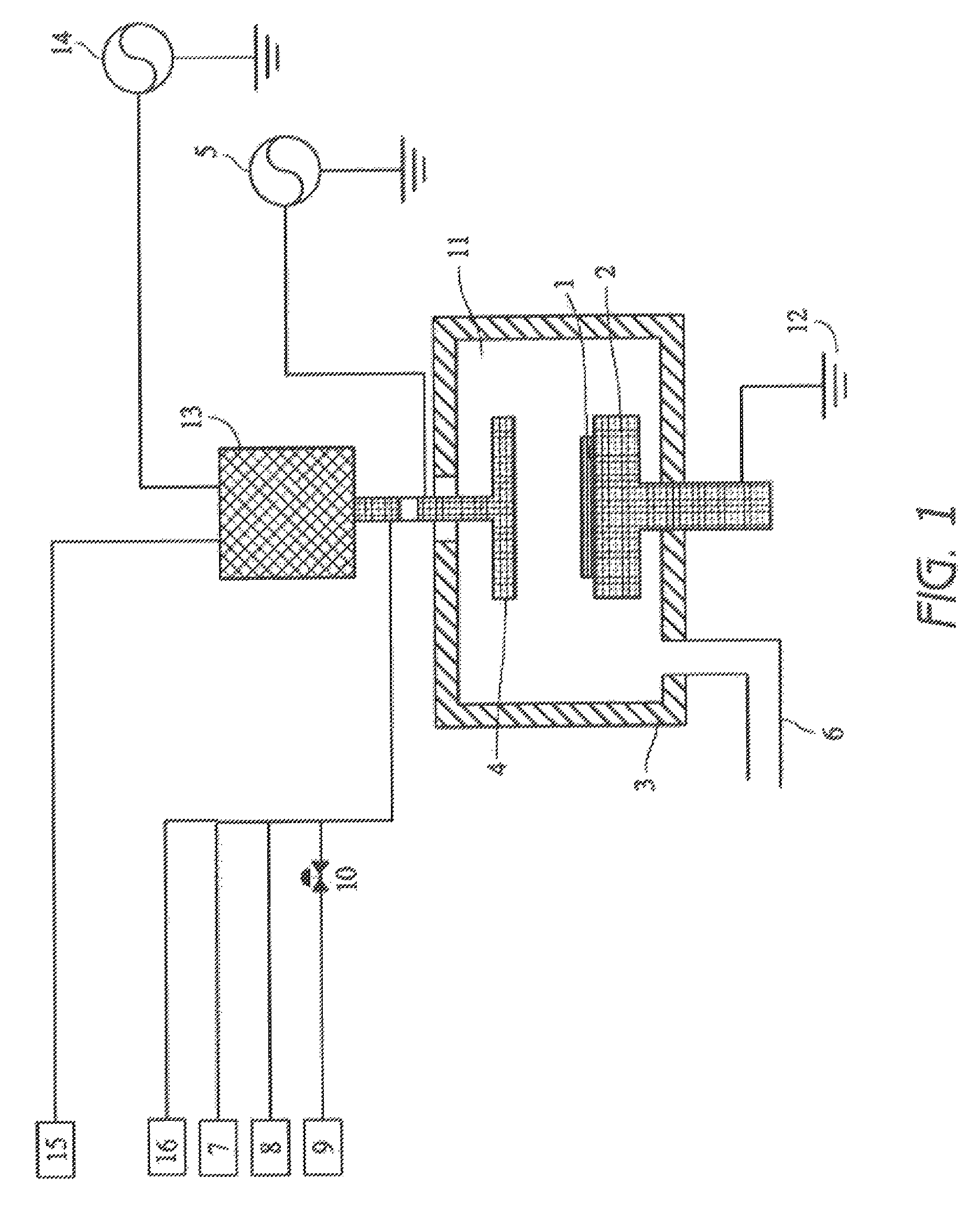
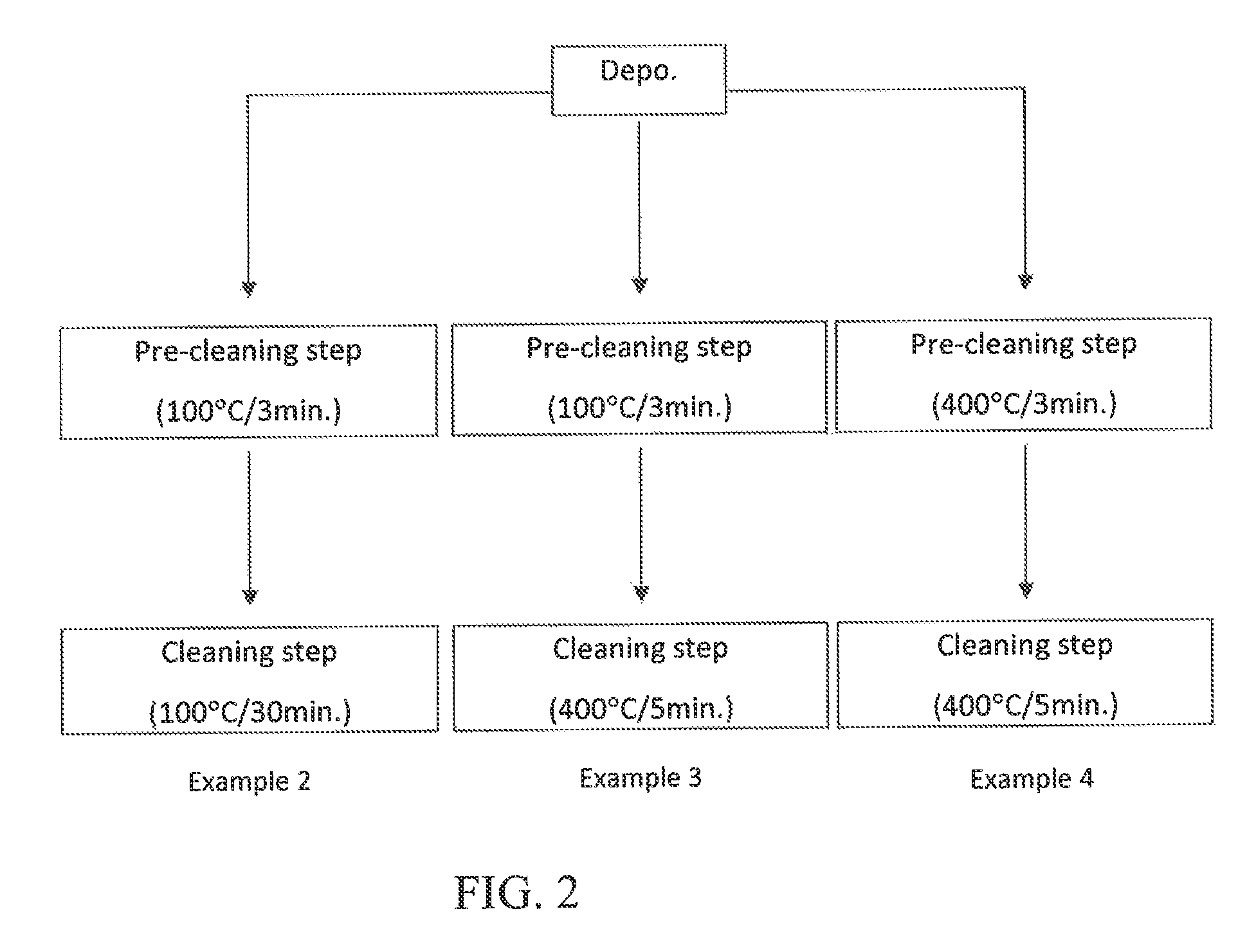
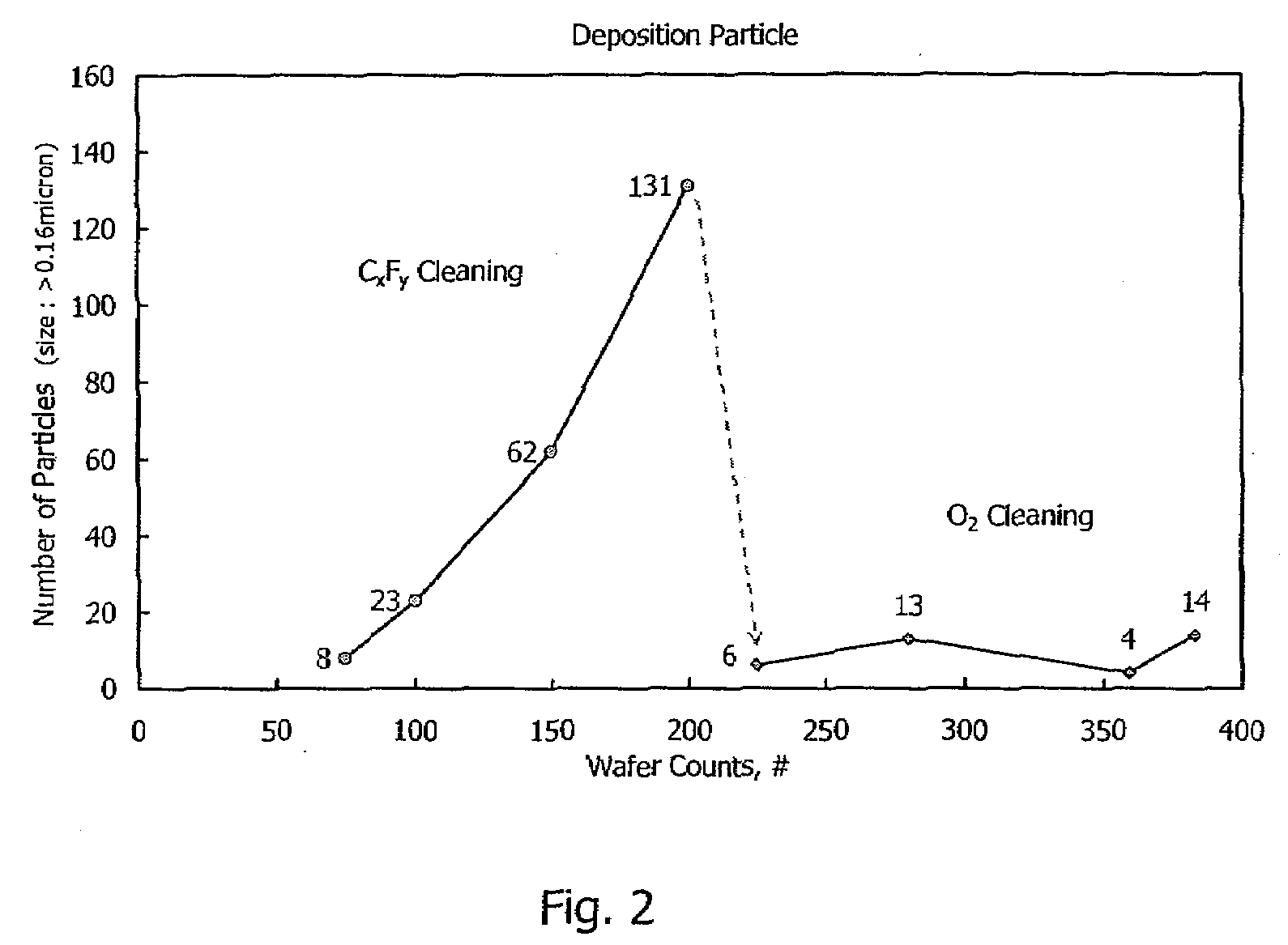
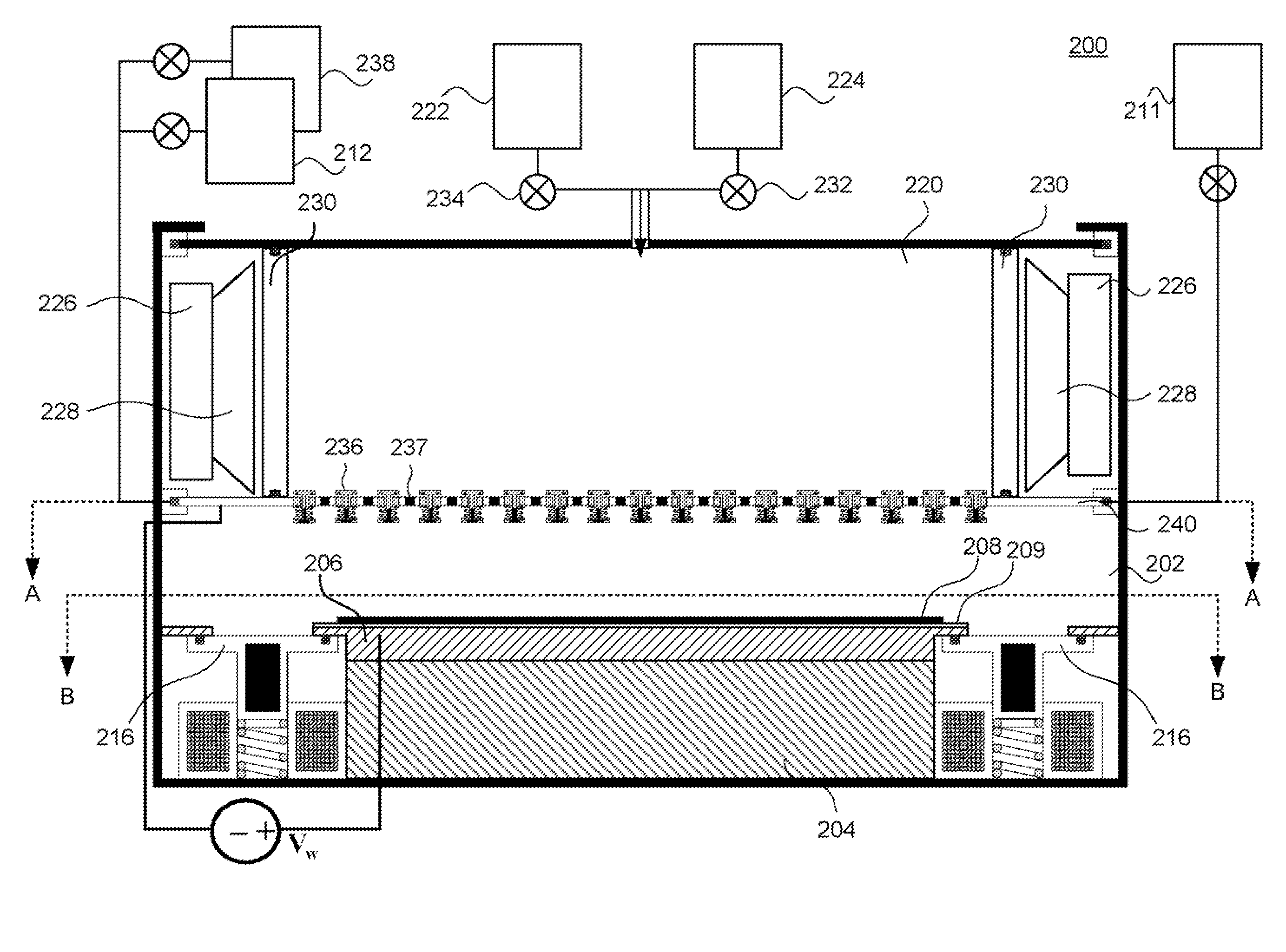
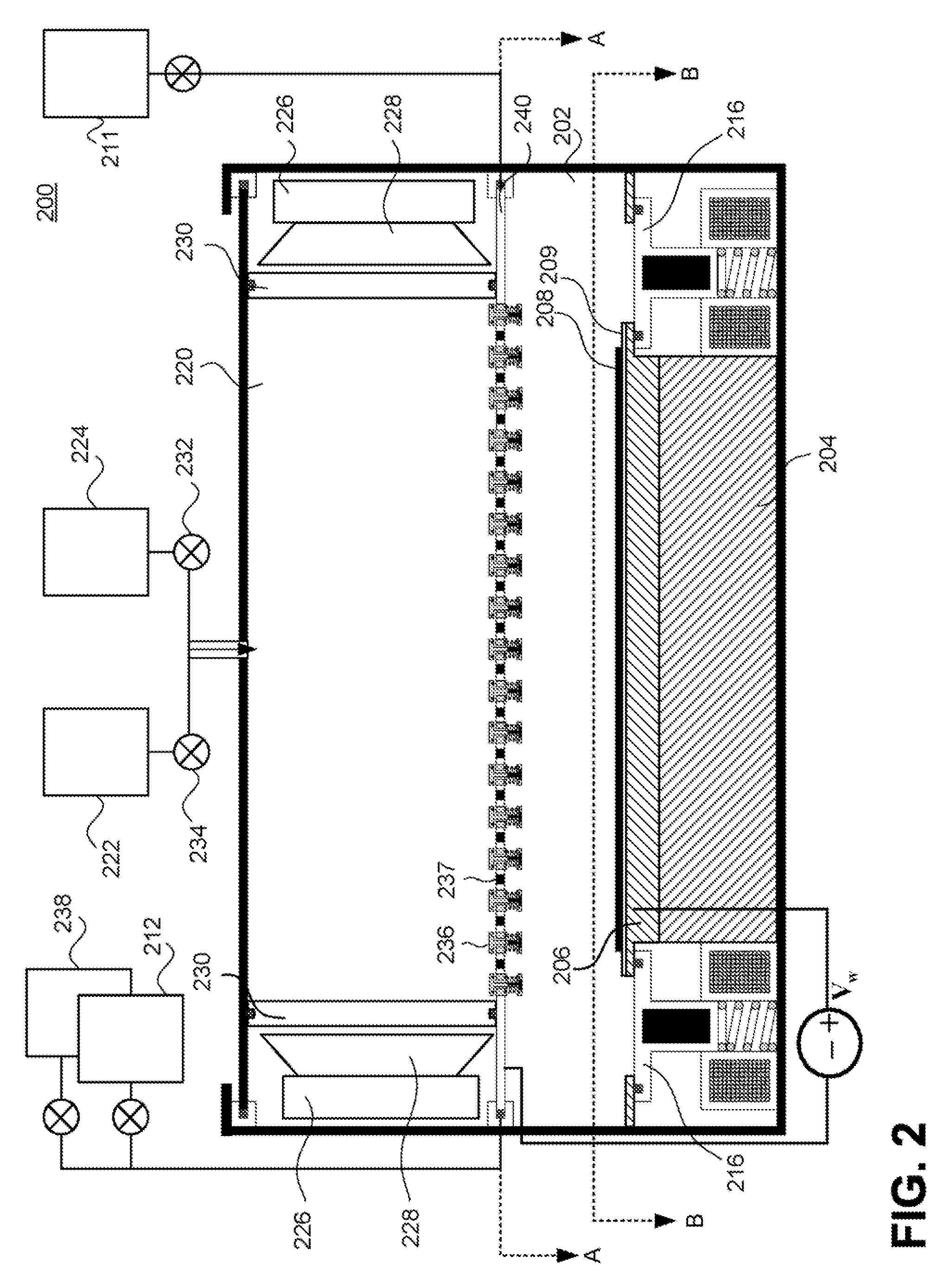
Method for cleaning reaction chamber using pre-cleaning process
ActiveUS9142393B2Improve efficiencyElectric discharge tubesHollow article cleaningNitrogenSilicon oxide
Owner:ASM IP HLDG BV
Method for forming nitrogen-containing oxide thin film using plasma enhanced atomic layer deposition
InactiveUS6723642B1Density of thinThin rateSemiconductor/solid-state device manufacturingChemical vapor deposition coatingNitrogen sourceNitrogen oxide
A method for forming a nitrogen-containing oxide thin film by using plasma enhanced atomic layer deposition is provided. In the method, the nitrogen-containing oxide thin film is deposited by supplying a metal source compound and oxygen gas into a reactor in a cyclic fashion with sequential alternating pulses of the metal source compound and the oxygen gas, wherein the oxygen gas is activated into plasma in synchronization of the pulsing thereof, and a nitrogen source gas is further sequentially pulsed into the reactor and activated into plasma over the substrate in synchronization with the pulsing thereof. According to the method, a dense nitrogen-containing oxide thin film can be deposited at a high rate, and a trace of nitrogen atoms can be incorporated in situ into the nitrogen-containing oxide thin film, thereby increasing the breakdown voltage of the film.
Owner:ELECTRONICS & TELECOMM RES INST
Method of self-cleaning of carbon-based film
InactiveUS20070248767A1Hollow article cleaningVacuum evaporation coatingNitrogen oxidePlasma reactor
A method of self-cleaning a plasma reactor upon depositing a carbon-based film on a substrate a pre-selected number of times, includes: (i) exciting oxygen gas and / or nitrogen oxide gas to generate a plasma; and (ii) exposing to the plasma a carbon-based film accumulated on an upper electrode provided in the reactor and a carbon-based film accumulated on an inner wall of the reactor.
Owner:ASM JAPAN
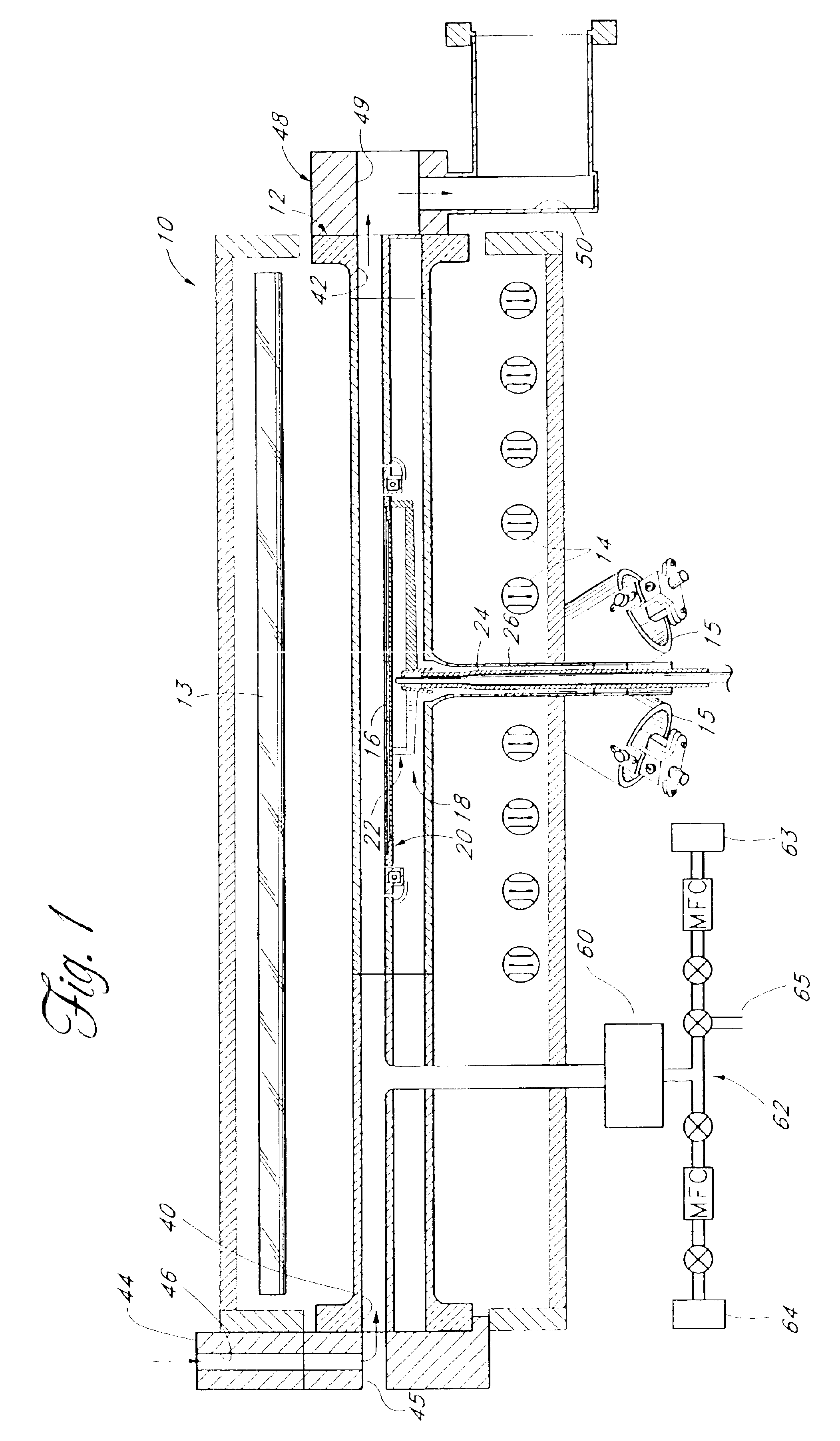
Atomic Layer Deposition of Oxides Using Krypton as an Ion Generating Feeding Gas
InactiveUS20070281105A1Improve abilitiesImprove efficiencyChemical vapor deposition coatingPlasma techniqueKryptonHigh density
An atomic layer deposition system and method utilizing radicals generated from a high-density mixed plasma for deposition is disclosed. A high-quality oxide or oxynitride can be deposited by exposing a substrate to a first precursor which is adsorbed onto the substrate during a first phase of one deposition cycle. After purging the deposition chamber, the substrate is exposed to a second precursor which includes oxygen radicals and krypton ions formed from the high-density mixed plasma. The ions and radicals are formed by introducing a radical generating feed gas (e.g., O2) and an ion generating feed gas into a plasma chamber and exciting the gases to form the high-density mixed plasma. The radicals and ions are then introduced to the substrate where they react with the first precursor to deposit a layer of the desired film. Krypton is preferably used as the ion generating feed gas because the metastable states of krypton lead to an efficient dissociation of oxygen into oxygen radicals when compared with other inert gases.
Owner:SANDISK TECH LLC
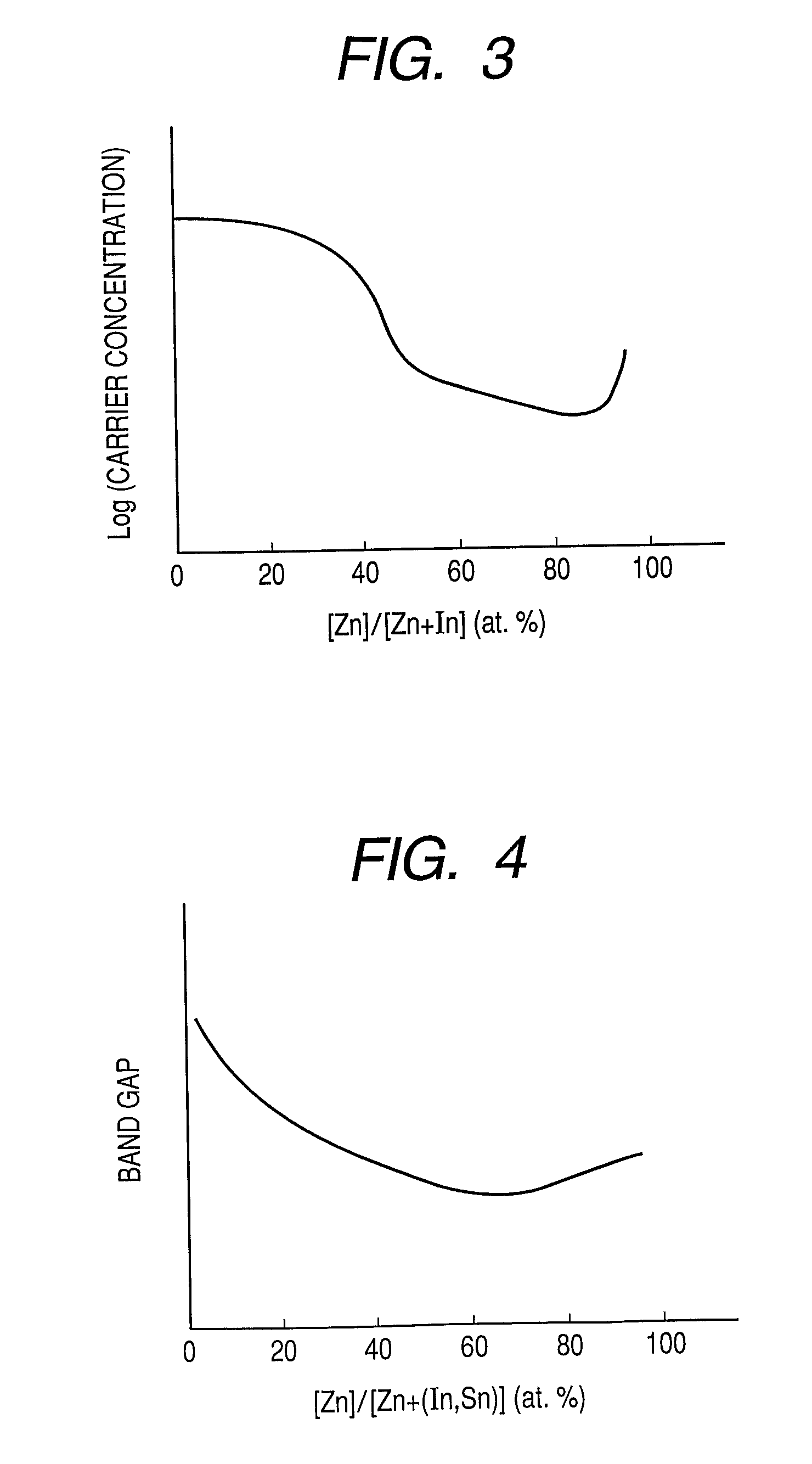
Metal oxynitride semiconductor containing zinc
ActiveUS8274078B2High mobility and environmental stabilityImprove mobilityPhotovoltaic energy generationSemiconductor devicesNitrogen oxideOxygen
Provided is an oxynitride semiconductor comprising a metal oxynitride. The metal oxynitride contains Zn and In and at least one element selected from the group consisting of Ga, Sn, Mg, Si, Ge, Y, Ti, Mo, W, and Al. The metal oxynitride has an atomic composition ratio of N, N / (N+O), of 7 atomic percent or more to 80 atomic percent or less.
Owner:CANON KK
Zero shrinkage smooth interface oxy-nitride and oxy-amorphous-silicon stacks for 3D memory vertical gate application
InactiveUS20130161629A1Reduce AlFx depositReduce AlFx buildingSolid-state devicesSemiconductor/solid-state device manufacturingDeposition temperatureAmorphous silicon
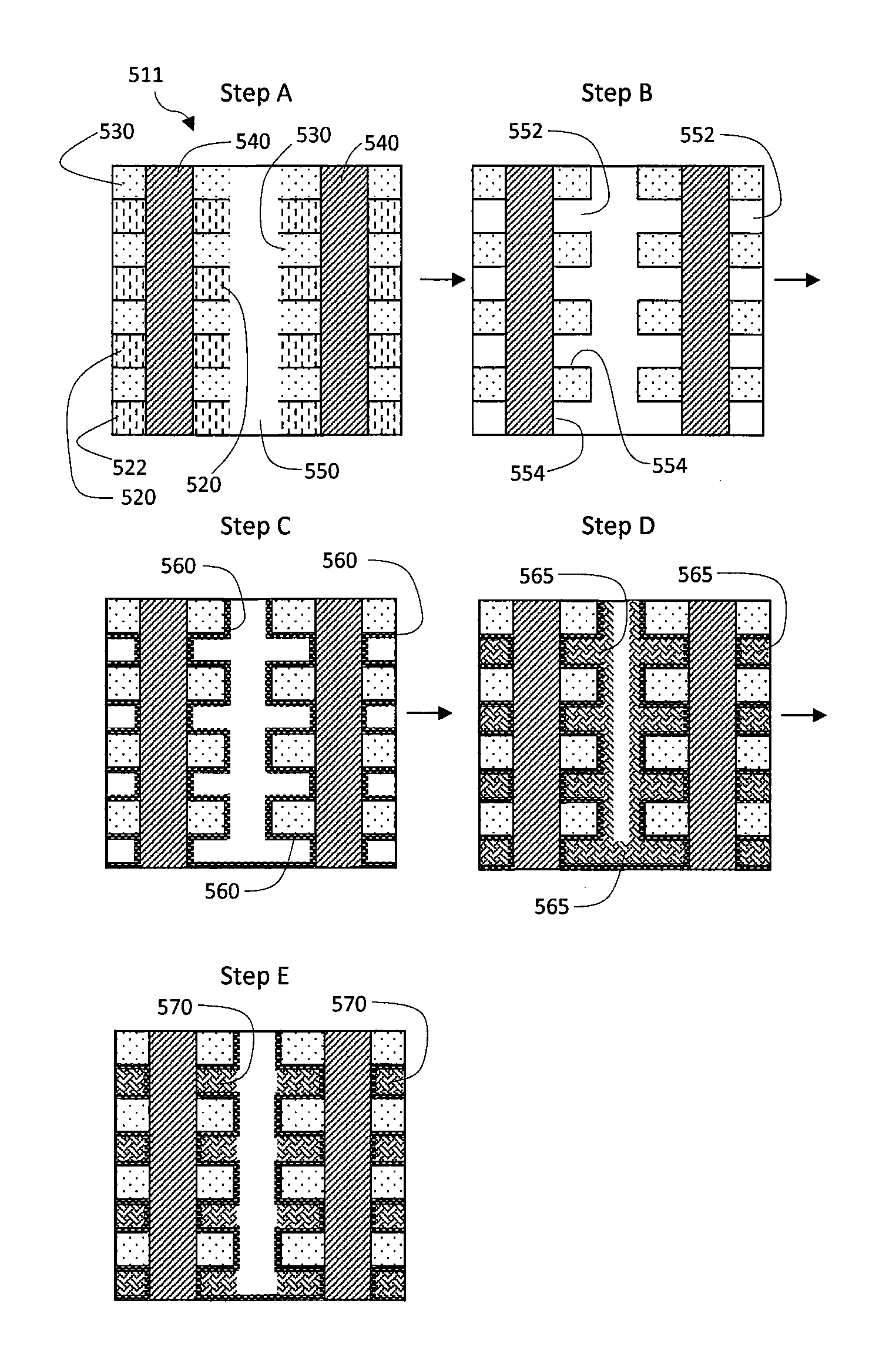
Methods are provided for depositing a stack of film layers for use in vertical gates for 3D memory devices, by depositing a sacrificial nitride film layer at a sacrificial film deposition temperature greater than about 550° C.; depositing an oxide film layer over the nitride film layer, at an oxide deposition temperature of about 600° C. or greater; repeating the above steps to deposit a film stack having alternating layers of the sacrificial films and the oxide films; forming a plurality of holes in the film stack; and depositing polysilicon in the plurality of holes in the film stack at a polysilicon process temperature of about 700° C. or greater, wherein the sacrificial film layers and the oxide film layers experience near zero shrinkage during the polysilicon deposition. Flash drive memory devices may also be made by these methods.
Owner:APPLIED MATERIALS INC
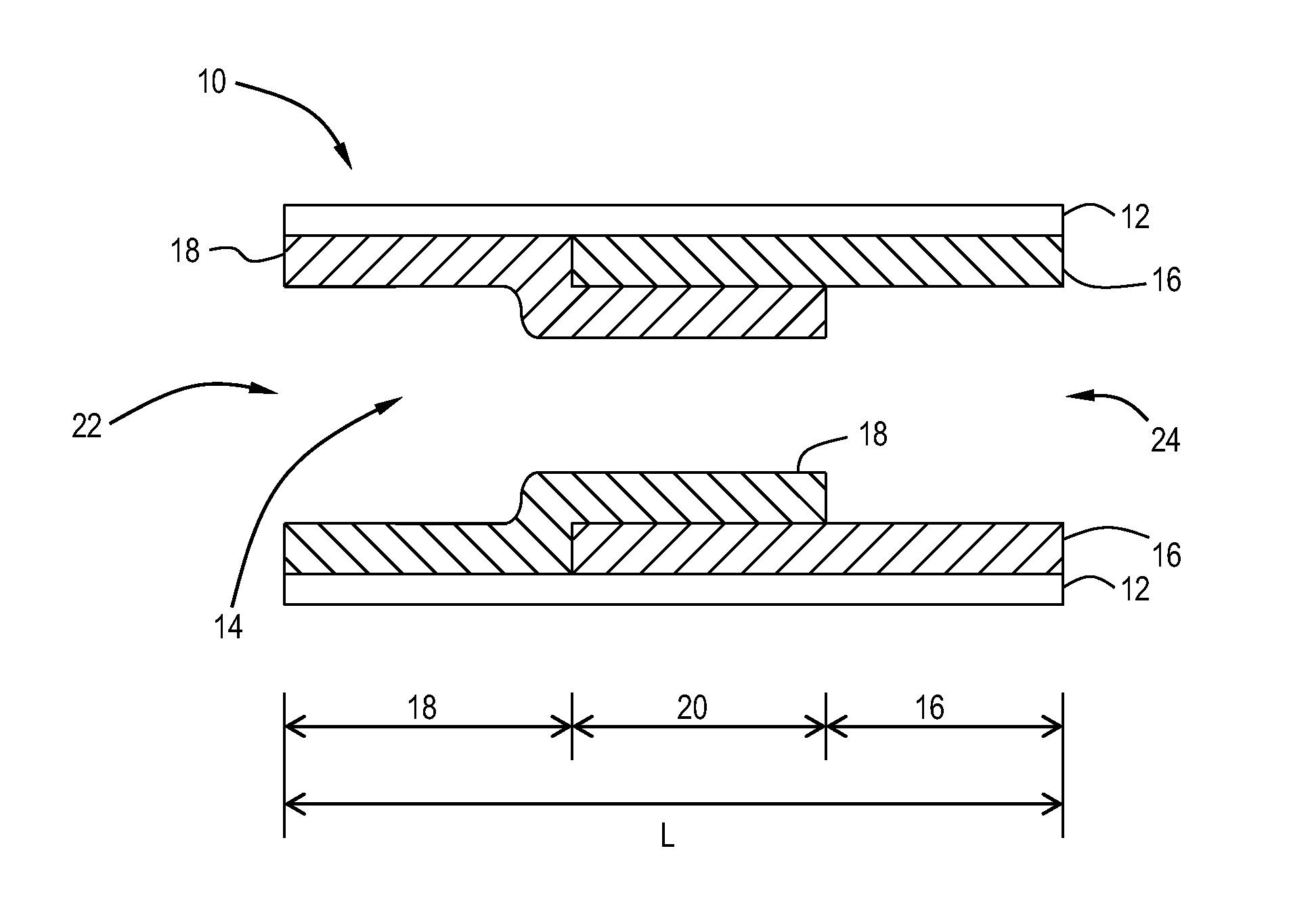
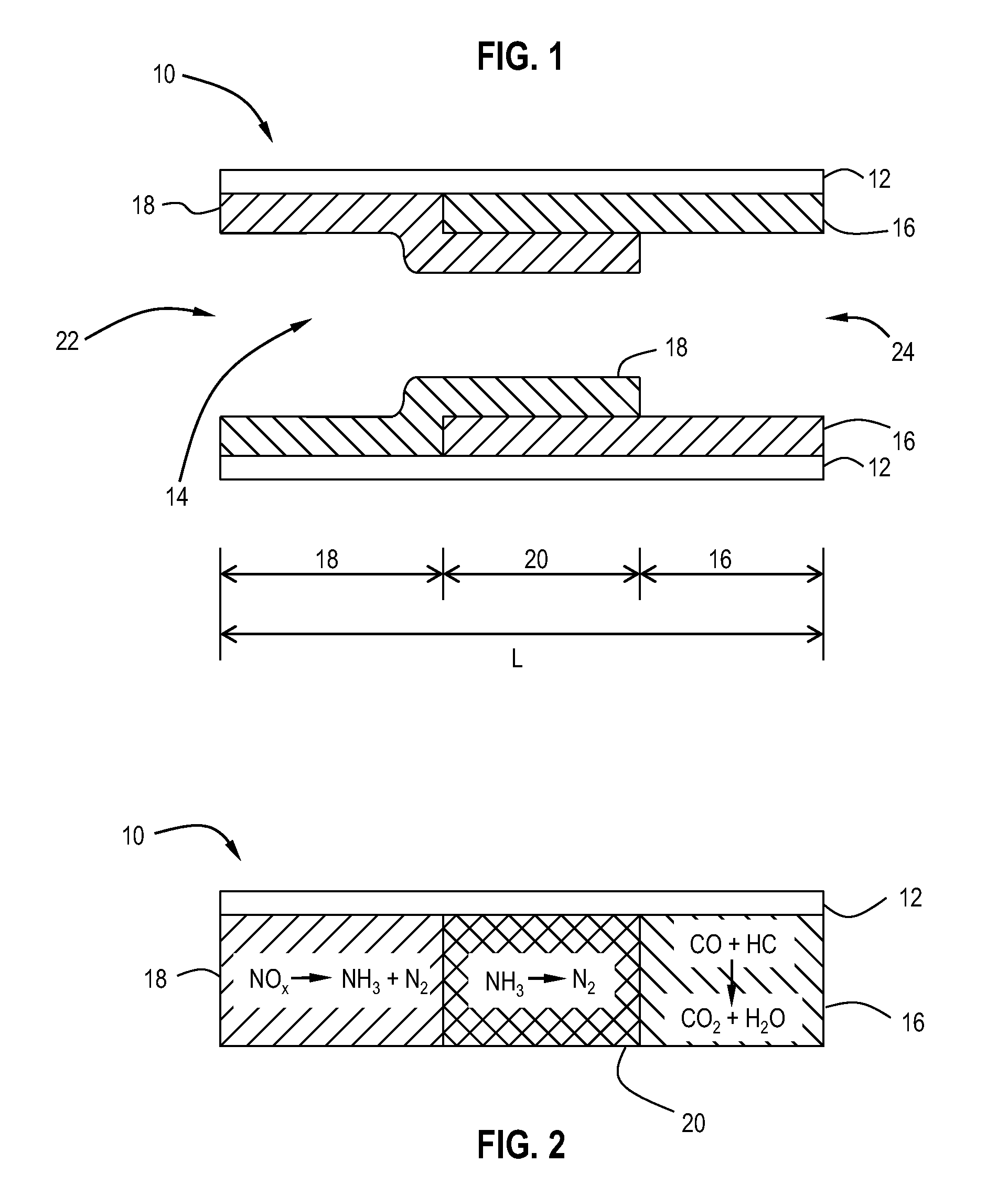
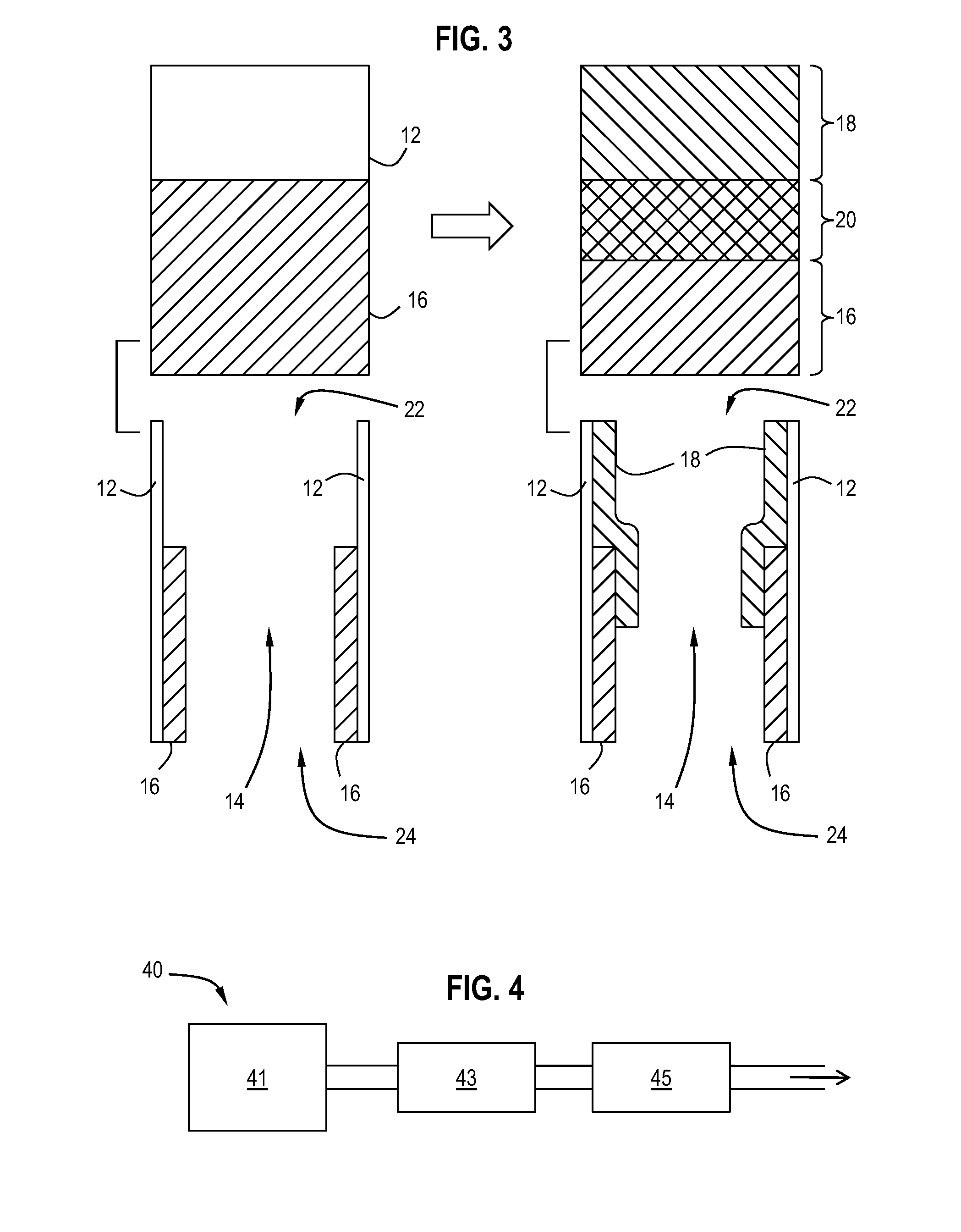
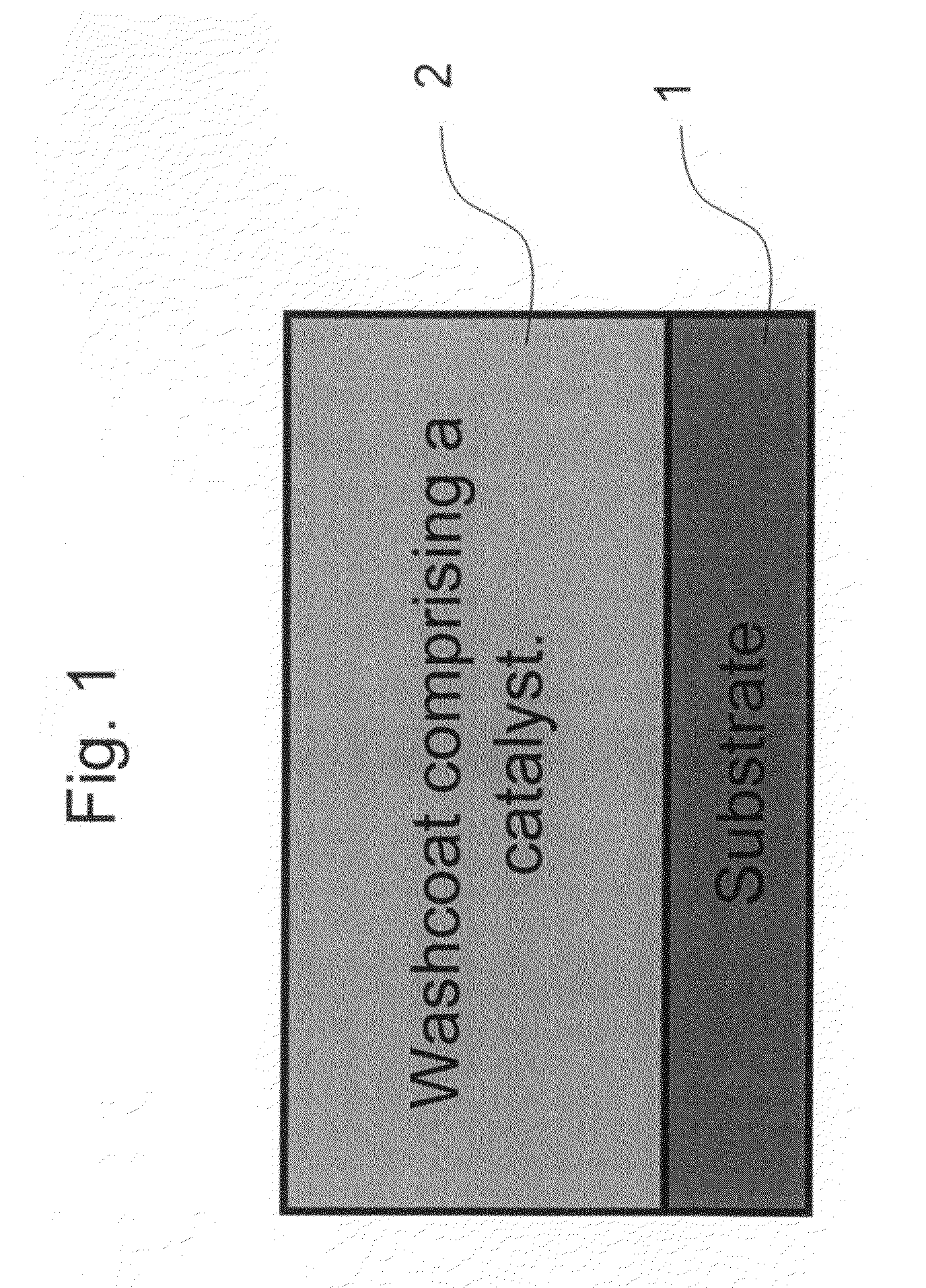
Pt-Pd diesel oxidation catalyst with CO/HC light-off and HC storage function
ActiveUS20080045405A1Nitrous oxide captureMolecular sieve catalystsExhaust gas emissionsNitrogen oxide
The present invention is directed to a diesel oxidation catalyst for the treatment of exhaust gas emissions, such as the oxidation of unburned hydrocarbons (HC), and carbon monoxide (CO) and the reduction of nitrogen oxides (NOx). More particularly, the present invention is directed to a novel washcoat composition comprising two distinct washcoat layers containing two distinctly different ratios of Pt:Pd.
Owner:BASF CATALYSTS LLC
Catalyzed SCR filter and emission treatment system
ActiveUS7229597B2Reduce the temperaturePromote regenerationCombination devicesLiquid degasification with auxillary substancesNitrogen oxideSoot
Provided is an emission treatment system and method for simultaneously remediating the nitrogen oxides (NOx), particulate matter, and gaseous hydrocarbons present in diesel engine exhaust streams. The emission treatment system has an oxidation catalyst upstream of a soot filter coated with a material effective in the Selective Catalytic Reduction (SCR) of NOx by a reductant, e.g., ammonia. Also provided is a method for disposing an SCR catalyst composition on a wall flow monolith that provides adequate catalyst loading, but does not result in unsuitable back pressures in the exhaust.
Owner:BASF CORP
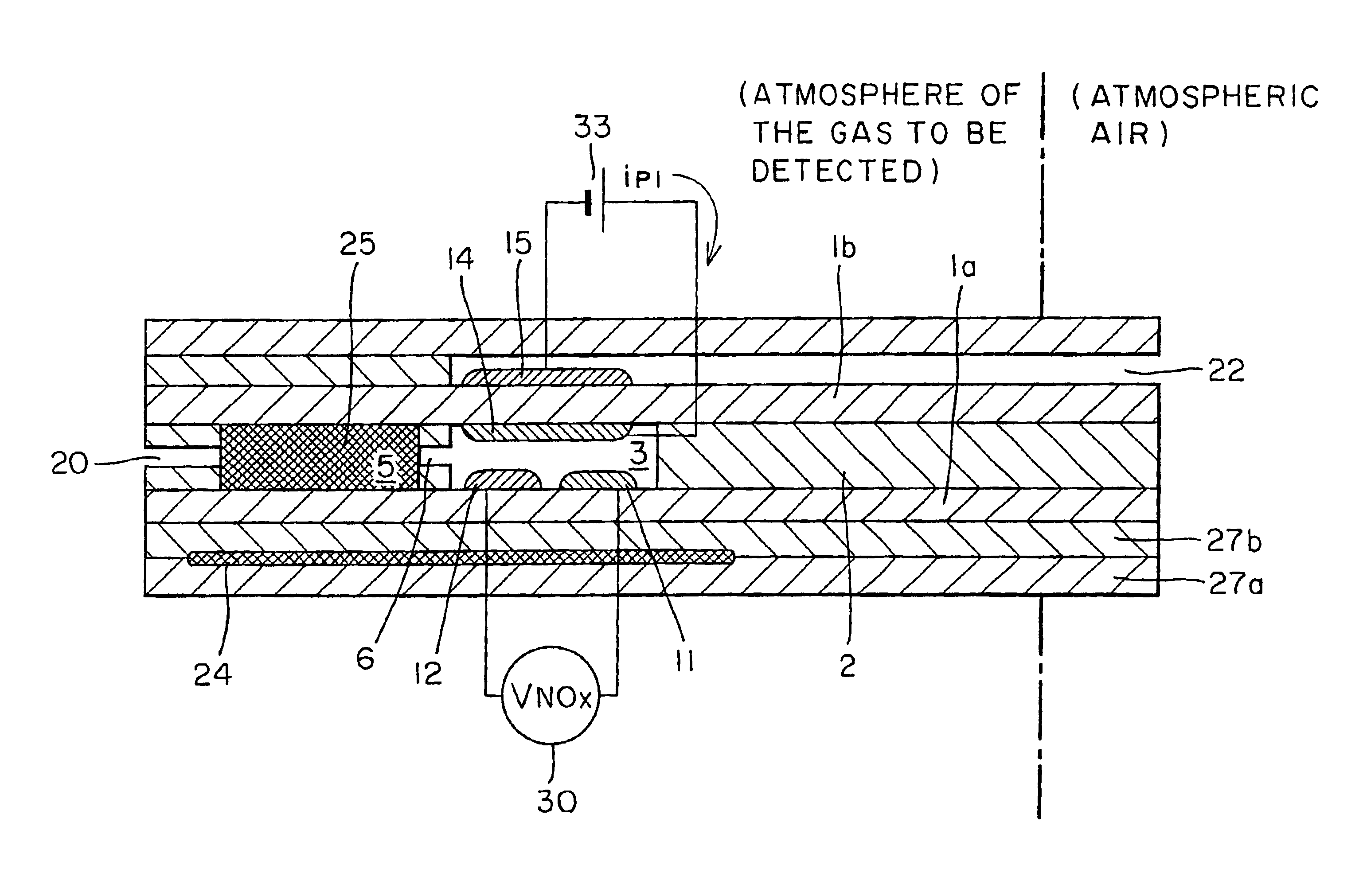
NOx sensor
InactiveUS6773565B2Avoid flowIncreasing active sitesWeather/light/corrosion resistanceVolume/mass flow measurementNitrogen oxideSingle component
Owner:RIKEN CO LTD
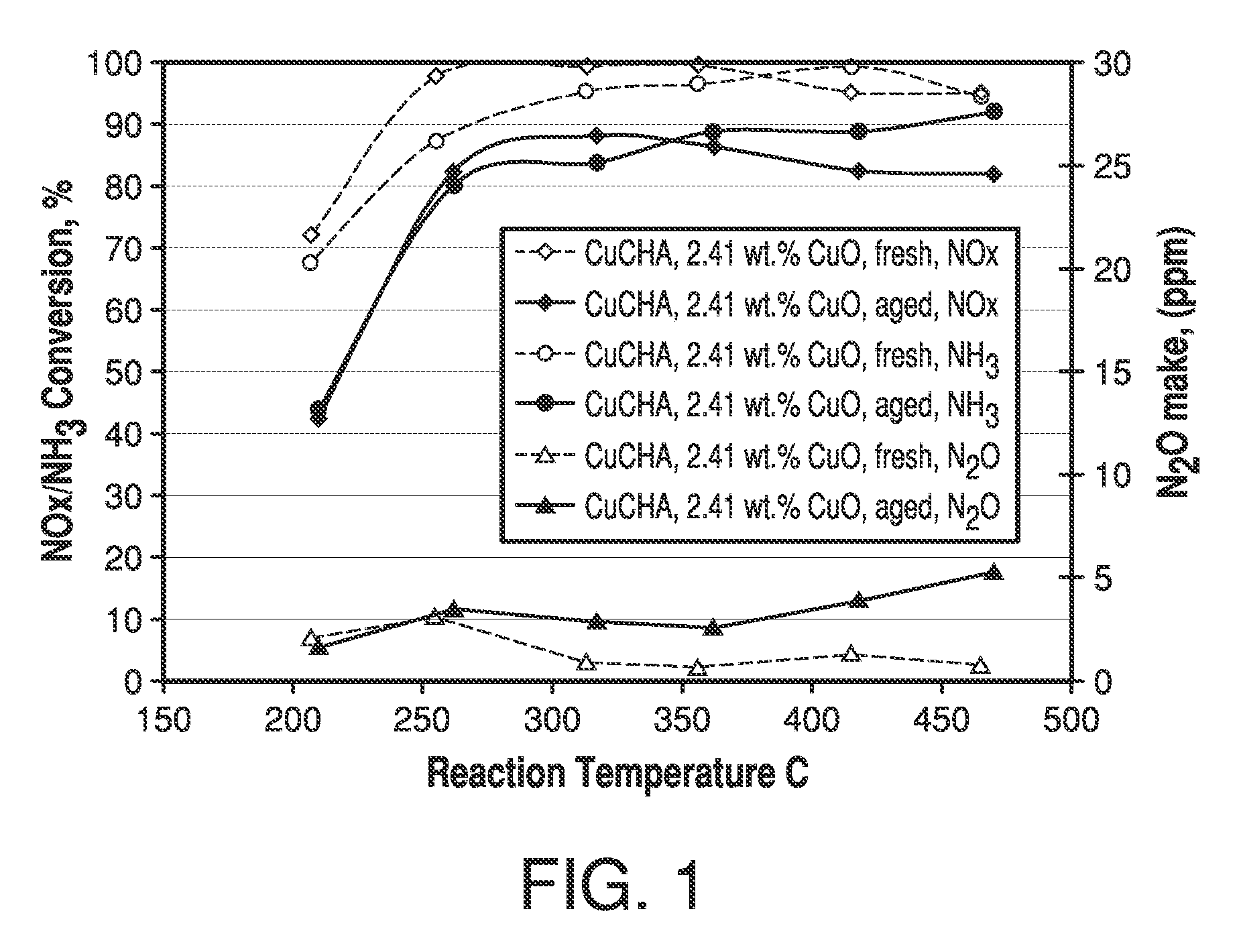
Copper CHA zeolite catalysts
ActiveUS7601662B2Good hydrothermal stabilityHigh catalytic activityCombination devicesAluminium compoundsReaction temperatureCrystal structure
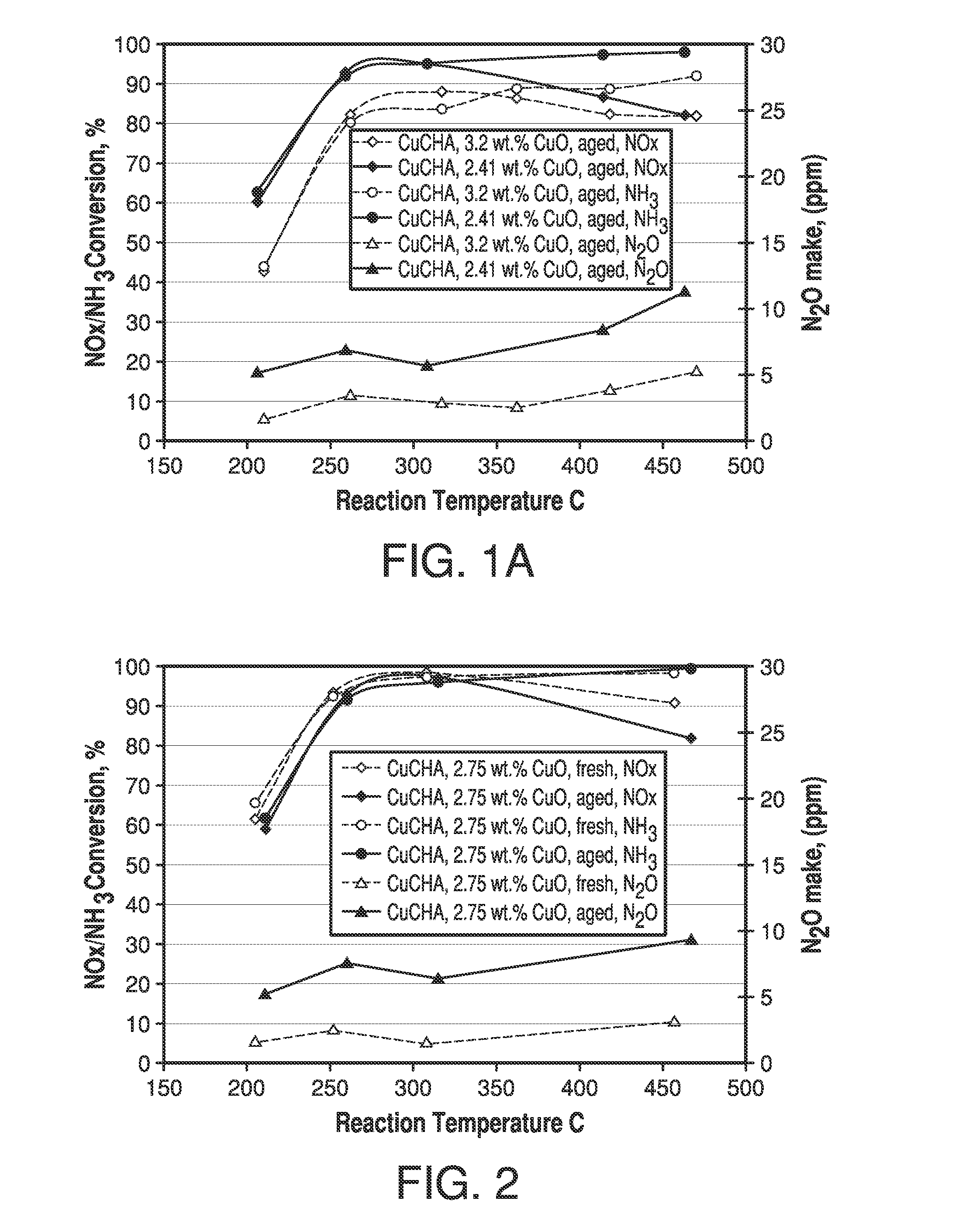
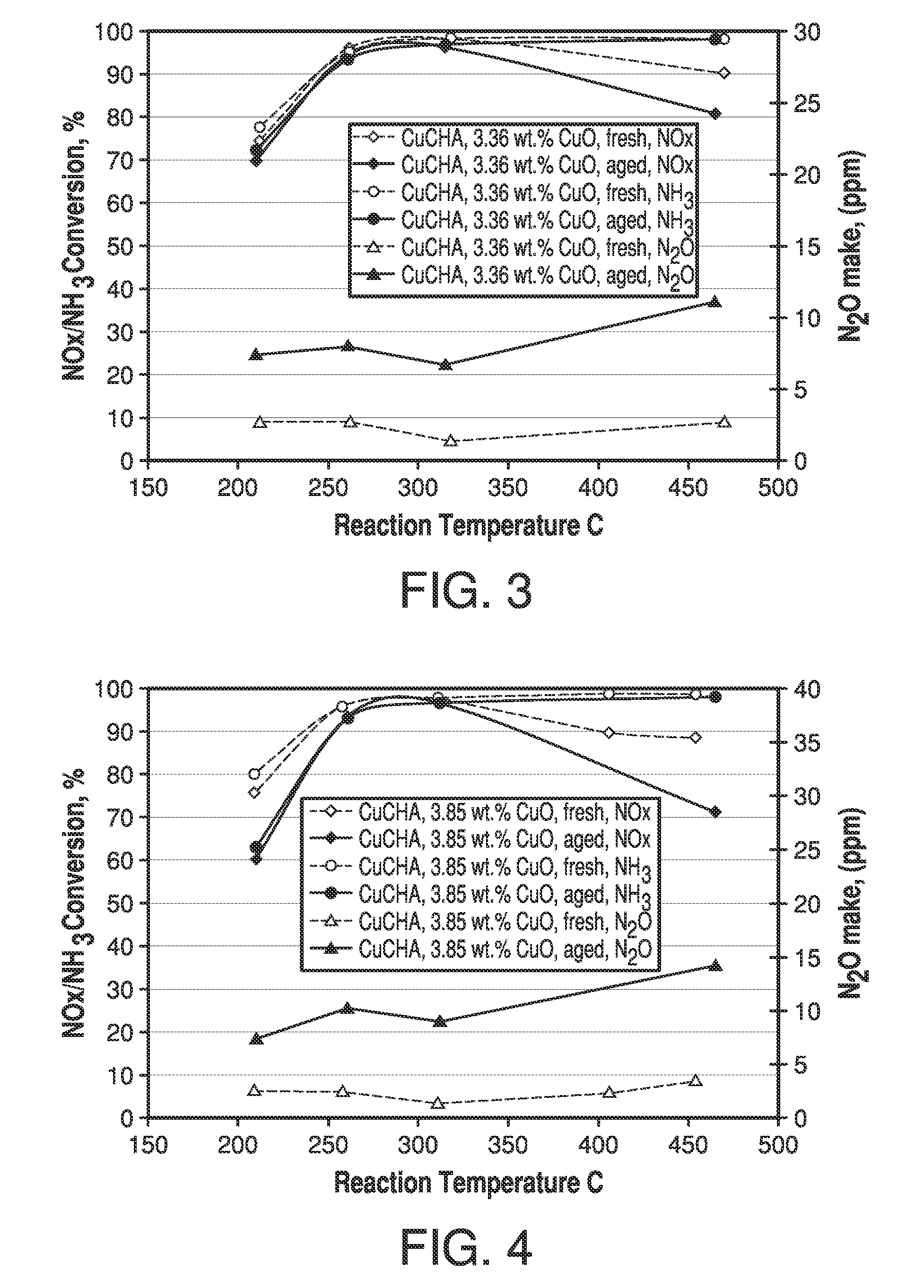
Zeolite catalysts and systems and methods for preparing and using zeolite catalysts having the CHA crystal structure are disclosed. The catalysts can be used to remove nitrogen oxides from a gaseous medium across a broad temperature range and exhibit hydrothermal stable at high reaction temperatures. The zeolite catalysts include a zeolite carrier having a silica to alumina ratio from about 15:1 to about 256:1 and a copper to alumina ratio from about 0.25:1 to about 1:1.
Owner:BASF CORP
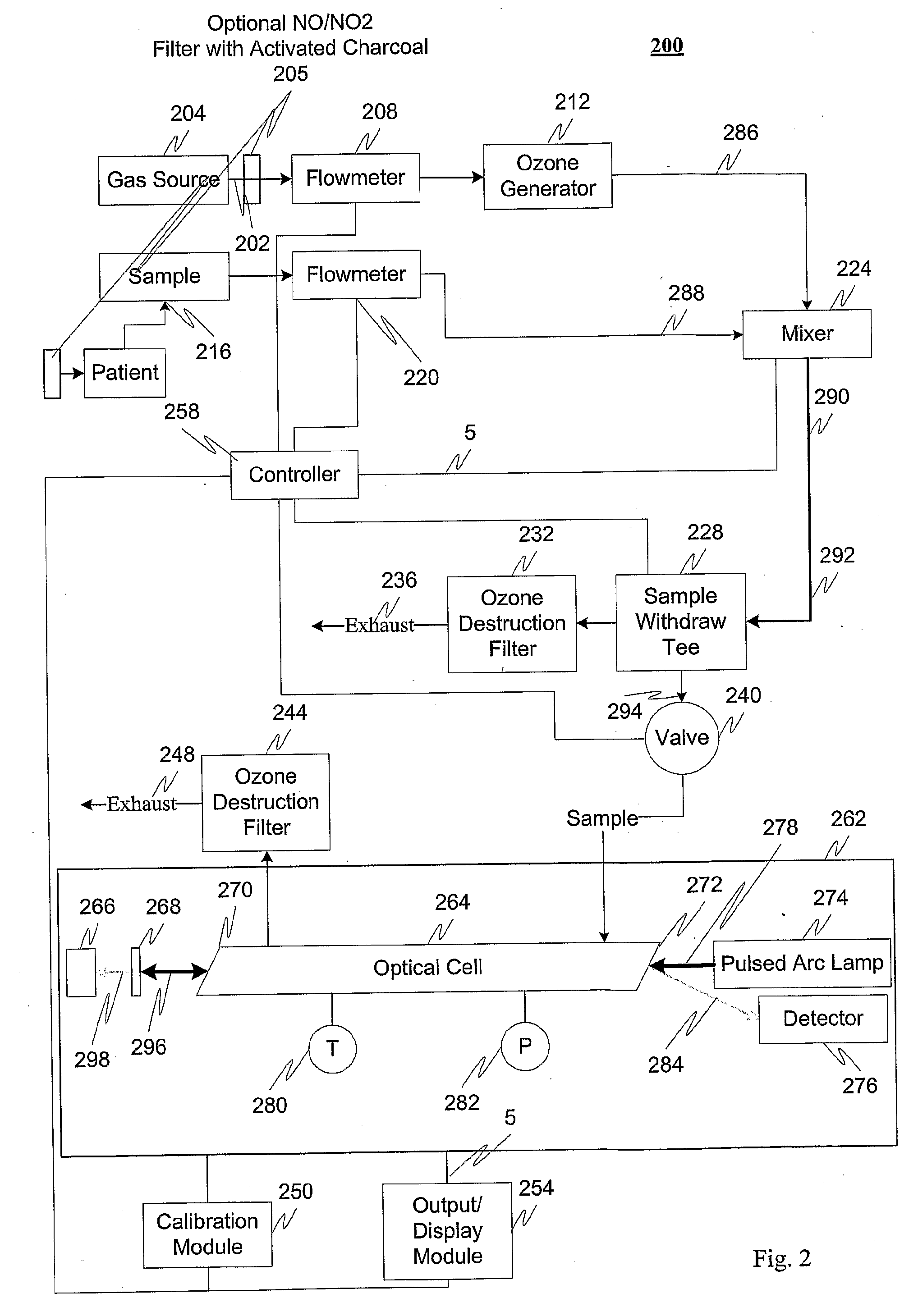
Measuring nitrogen oxides and other gases by ozone formation
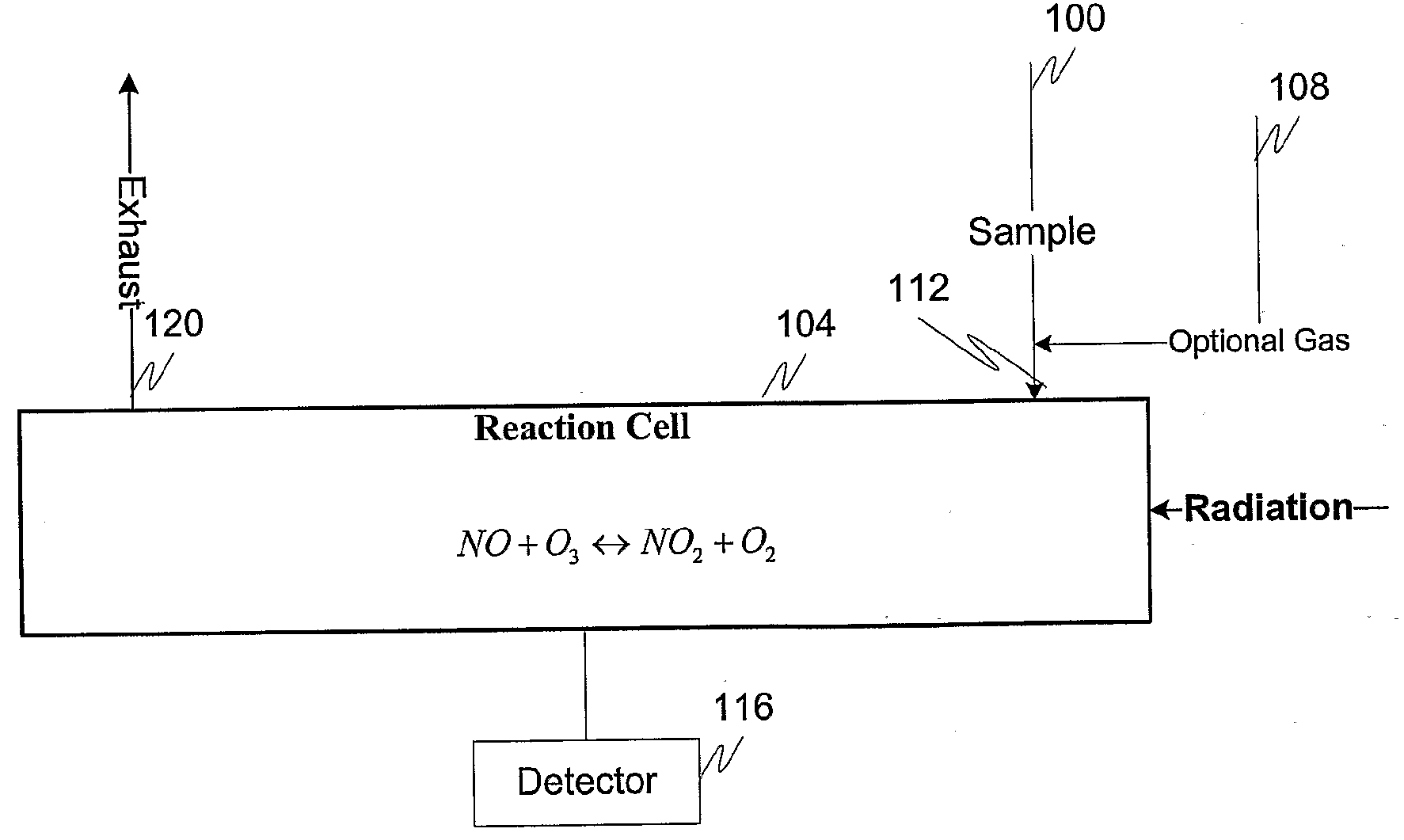
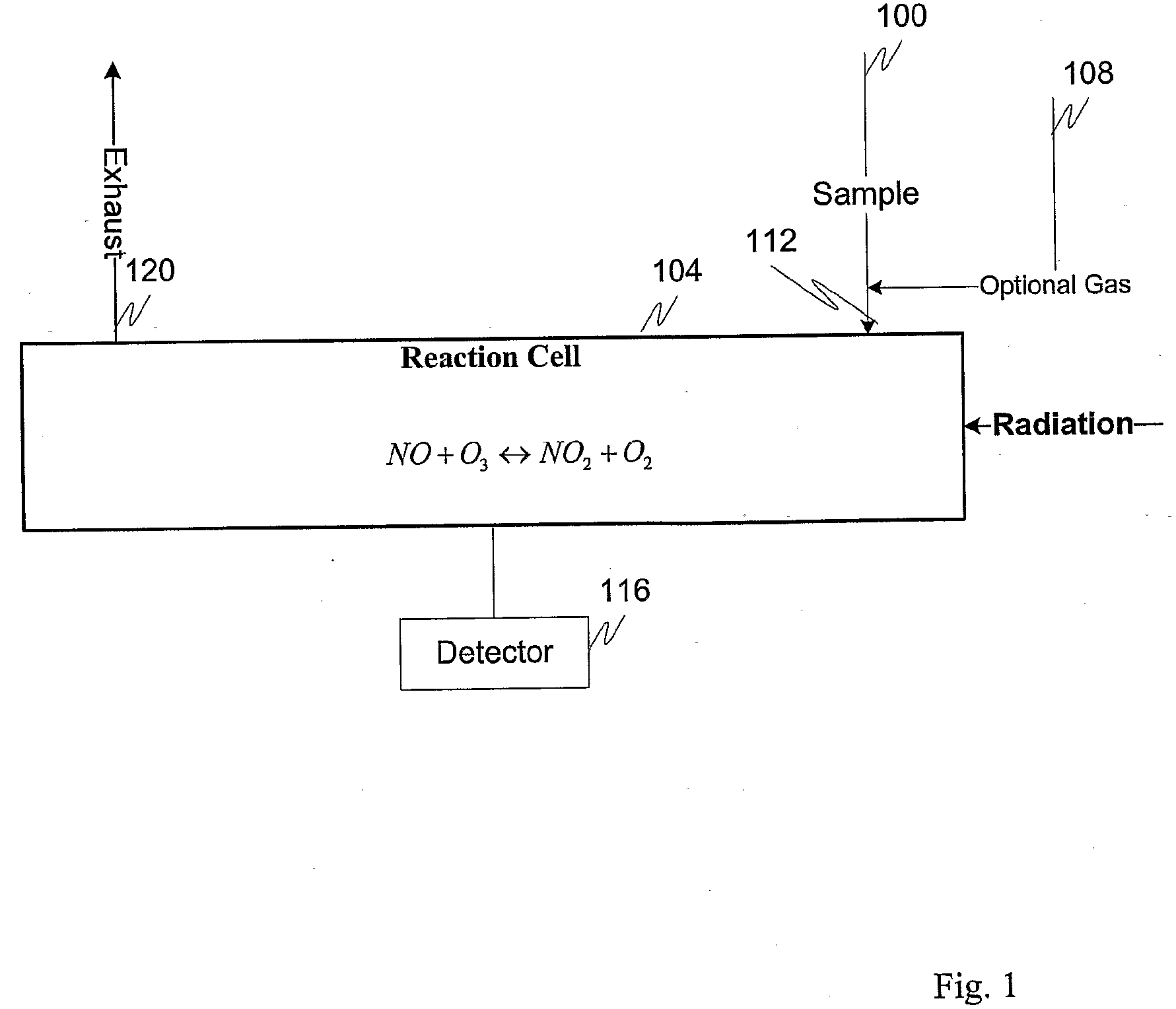
InactiveUS20090137055A1Improve signal-to-noise ratioRecalibration requirement is also reducedMaterial analysis by electric/magnetic meansMaterial analysis by optical meansNitrogen dioxideNitric oxide
A photochemical sensing system enables the measurement of nitrogen oxides (nitrogen dioxide and nitric oxide) by photolyzing nitrogen dioxide to form oxygen atoms which combine with oxygen molecules to form ozone. Ozone reacts with nitric oxide to for nitrogen dioxide-decreasing ozone. Changes in ozone concentration are measured as a surrogate for the nitrogen dioxide and nitric oxide. Any species which photolyzes to yield oxygen atoms may be measured by this technique. Additional specificity for nitrogen oxides is conferred by allowing the nitric oxide to react with the ozone to recreate the nitrogen dioxide. By periodically photolyzing the nitrogen dioxide (to form ozone), and then allowing the resulting nitric oxide to react with the ozone (thereby reducing ozone), a pulsed signal is obtained whose amplitude is proportional to the total amount of nitrogen dioxide and nitric oxide present. Medical applications include measuring nitric oxide concentrations in expired air samples.
Owner:BOGNAR JOHN A
Nitride semiconductor light emitting device and method of fabricating nitride semiconductor laser device
ActiveUS20080291961A1High output without impairing reliabilityImprove the level ofOptical wave guidanceLaser detailsNitrogen oxideLight emitting device
There is provided a nitride semiconductor light emitting device having a light emitting portion coated with a coating film, the light emitting portion being formed of a nitride semiconductor, the coating film in contact with the light emitting portion being formed of an oxynitride. There is also provided a method of fabricating a nitride semiconductor laser device having a cavity with a facet coated with a coating film, including the steps of: providing cleavage to form the facet of the cavity; and coating the facet of the cavity with a coating film formed of an oxynitride.
Owner:SHARP FUKUYAMA LASER CO LTD
Zirconium oxide and hafnium oxide etching using halogen containing chemicals
ActiveUS20050164479A1Effective meanSemiconductor/solid-state device manufacturingSemiconductor devicesGate dielectricHafnium
A method is described for selectively etching a high k dielectric layer that is preferably a hafnium or zirconium oxide, silicate, nitride, or oxynitride with a selectivity of greater than 2:1 relative to silicon oxide, polysilicon, or silicon. The plasma etch chemistry is comprised of one or more halogen containing gases such as CF4, CHF3, CH2F2, CH3F, C4F8, C4F6, C5F6, BCl3, Br2, HF, HCl, HBr, HI, and NF3 and leaves no etch residues. An inert gas or an inert gas and oxidant gas may be added to the halogen containing gas. In one embodiment, a high k gate dielectric layer is removed on portions of an active area in a MOS transistor. Alternatively, the high k dielectric layer is used in a capacitor between two conducting layers and is selectively removed from portions of an ILD layer.
Owner:TAIWAN SEMICON MFG CO LTD
Oxynitride laminate "blocking layer" for thin film semiconductor devices
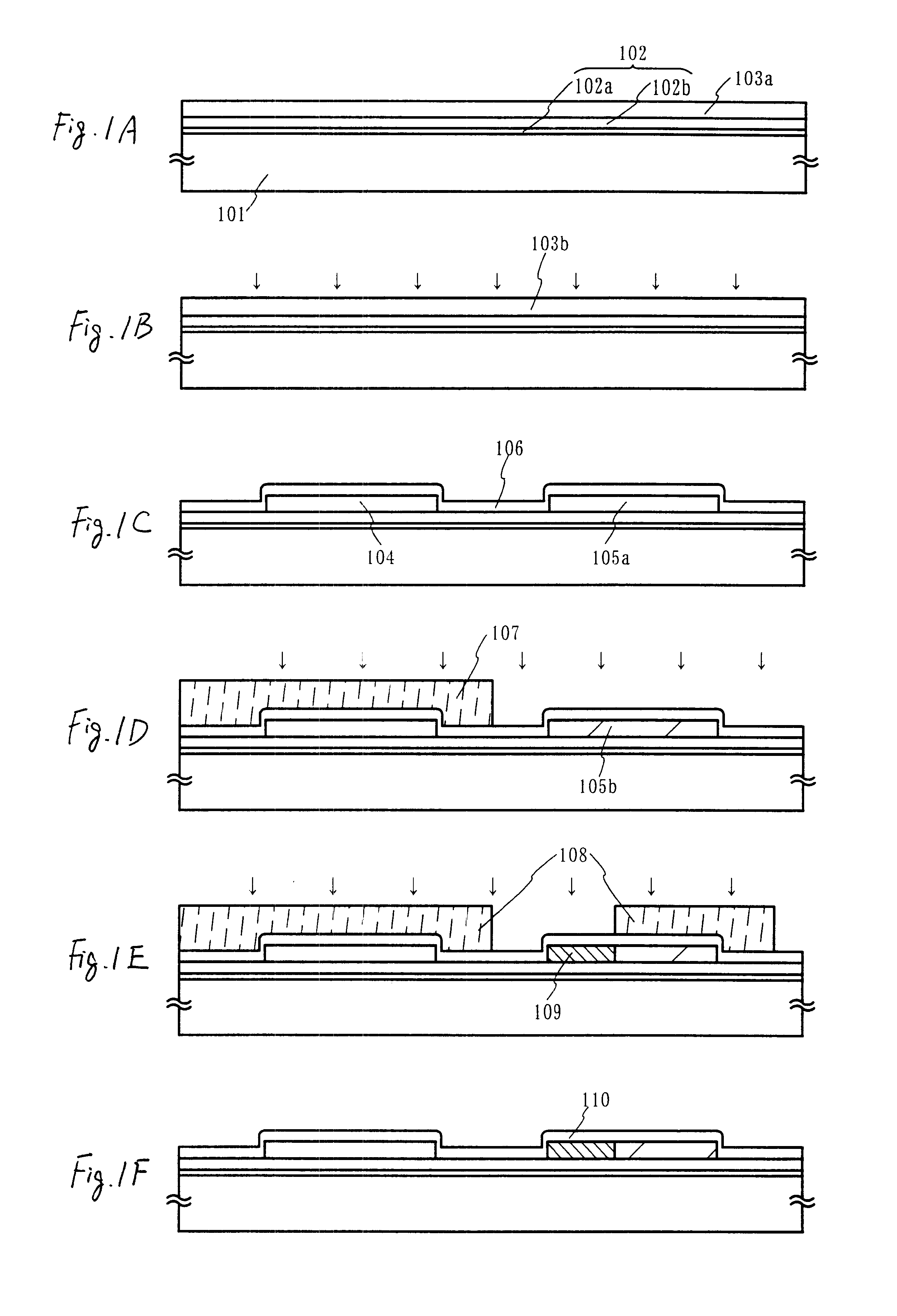
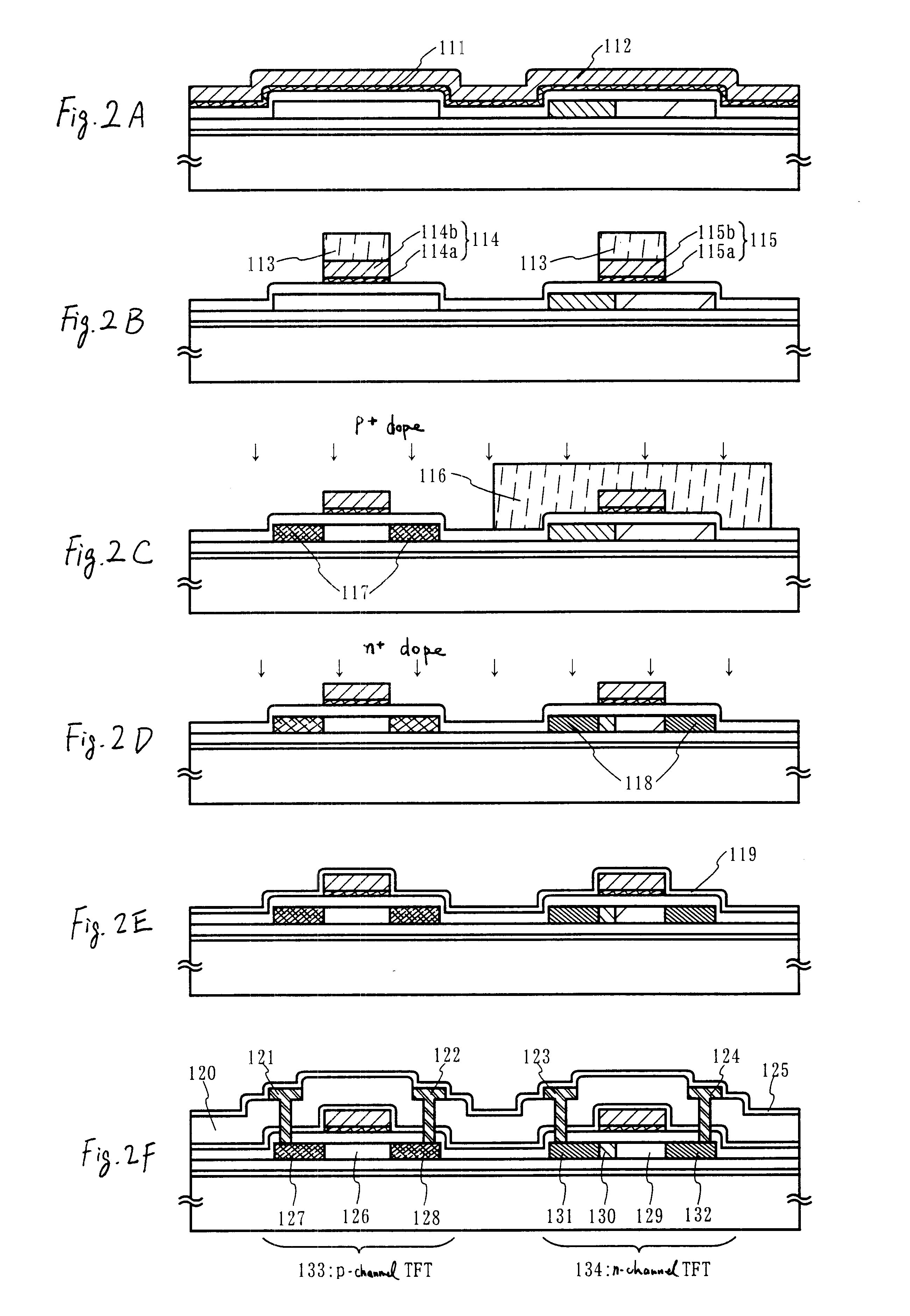
Channel doping is an effective method for controlling Vth, but if Vth shifts to the order of -4 to -3 V when forming circuits such as a CMOS circuit formed from both an n-channel TFT and a P-channel TFT on the same substrate, then it is difficult to control the Vth of both TFTs with one channel dope. In order to solve the above problem, the present invention forms a blocking layer on the back channel side, which is a laminate of a silicon oxynitride film (A) manufactured from SiH4, NH3, and N2O, and a silicon oxynitride film (B)manufactured from SiH4and N2O. By making this silicon oxynitride film laminate structure, contamination by alkaline metallic elements from the substrate can be prevented, and influence by stresses, caused by internal stress, imparted to the TFT can be relieved.
Owner:SEMICON ENERGY LAB CO LTD
Method for combined removal of mercury and nitrogen oxides from off-gas streams
InactiveUS7118720B1Promote oxidationProcess economyGas treatmentNitrogen compoundsNitrogen dioxideNitrogen oxide
A method for removing elemental Hg and nitric oxide simultaneously from a gas stream is provided whereby the gas stream is reacted with gaseous chlorinated compound to convert the elemental mercury to soluble mercury compounds and the nitric oxide to nitrogen dioxide. The method works to remove either mercury or nitrogen oxide in the absence or presence of each other.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Pt-Pd diesel oxidation catalyst with CO/HC light-off and HC storage function
ActiveUS7576031B2Nitrous oxide captureInternal combustion piston enginesExhaust gas emissionsNitrogen oxide
The present invention is directed to a diesel oxidation catalyst for the treatment of exhaust gas emissions, such as the oxidation of unburned hydrocarbons (HC), and carbon monoxide (CO) and the reduction of nitrogen oxides (NOx). More particularly, the present invention is directed to a novel washcoat composition comprising two distinct washcoat layers containing two distinctly different ratios of Pt:Pd.
Owner:BASF CATALYSTS LLC
Method of making an ultra thin silicon nitride film
InactiveUS6150286ASemiconductor/solid-state device manufacturingSemiconductor devicesSelf limitingThin oxide
Various methods of fabricating a circuit structure utilizing silicon nitride are provided. In one aspect, a method of fabricating a circuit structure is provided that includes forming a silicon nitride film on a silicon surface, annealing the silicon nitride film in an ammonia ambient and annealing the silicon nitride film in a nitrous oxide ambient to form a thin oxide layer at an interface between the silicon nitride film and the silicon surface. The process of the present invention enables the manufacture of thin silicon nitride films with highly uniform morphology for use as gate dielectrics or other purposes. The thin oxide film is self-limiting in thickness and improves differential mechanical stresses.
Owner:GLOBALFOUNDRIES INC
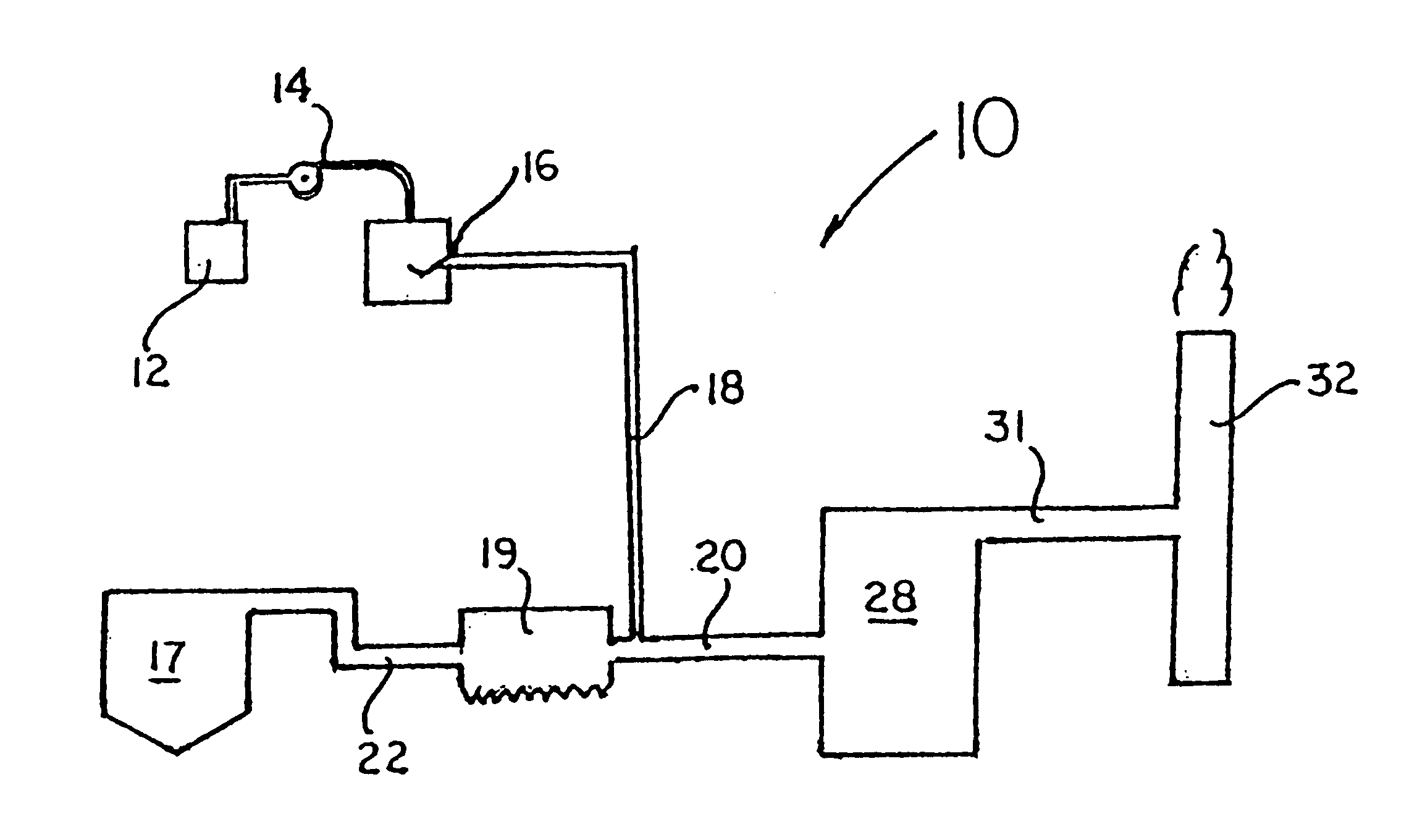
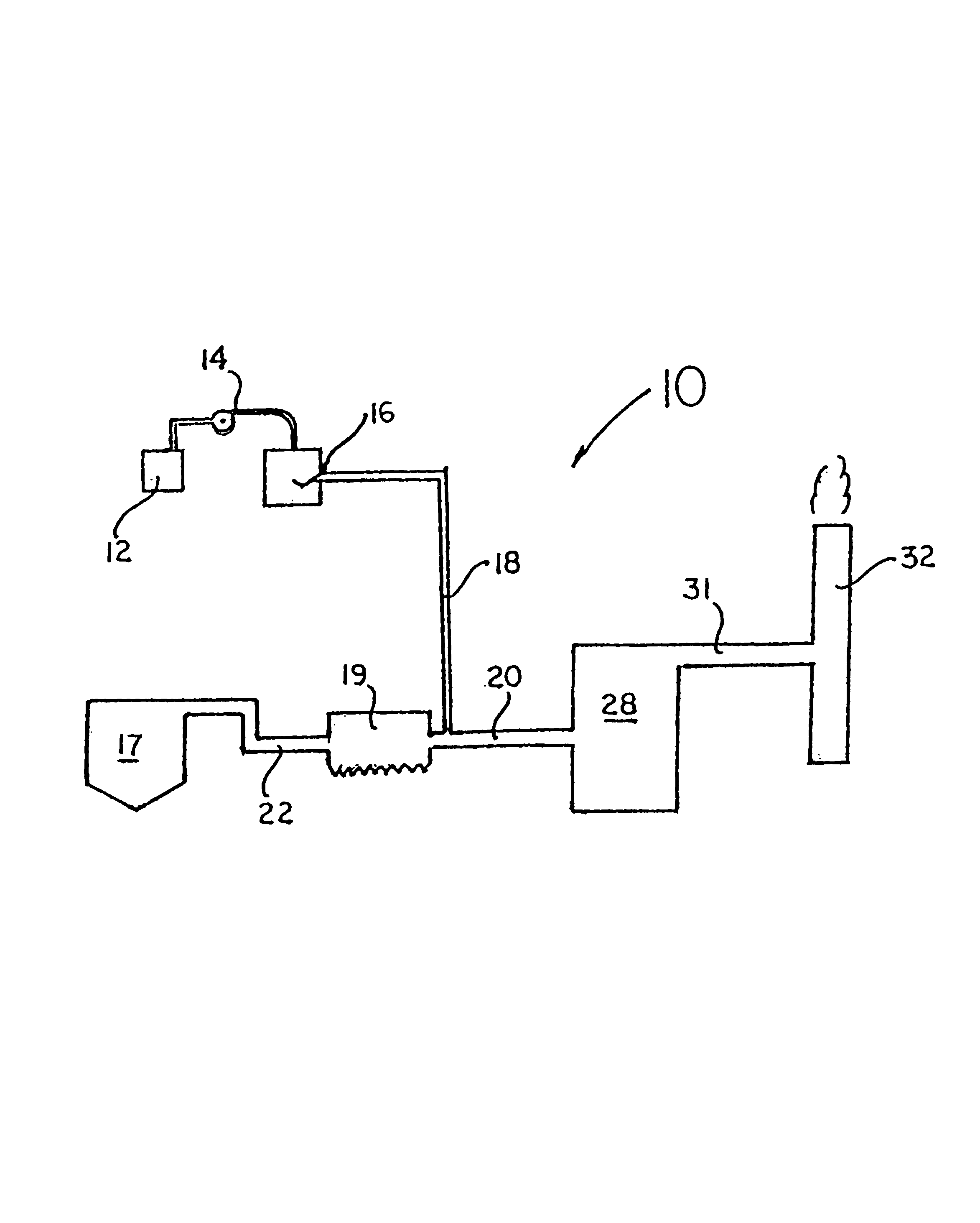
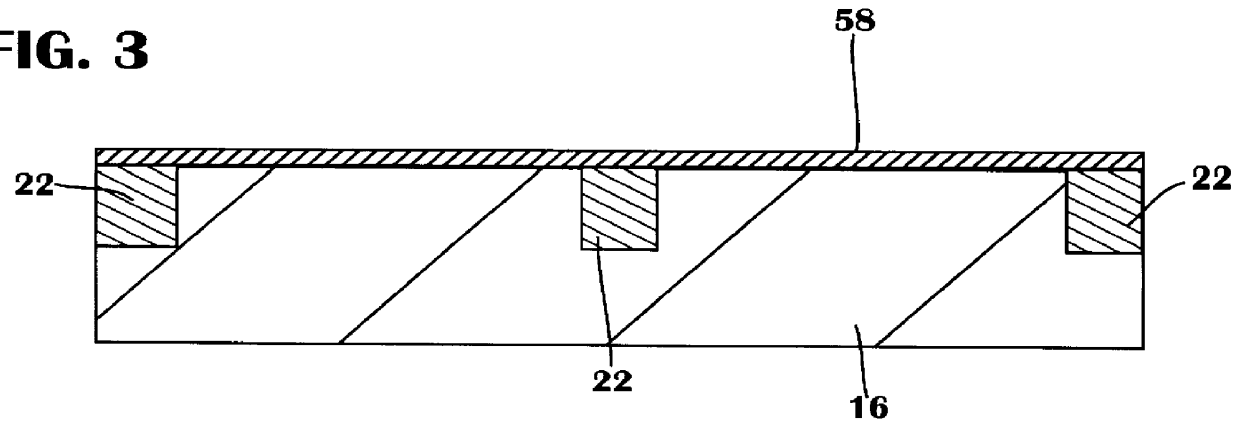
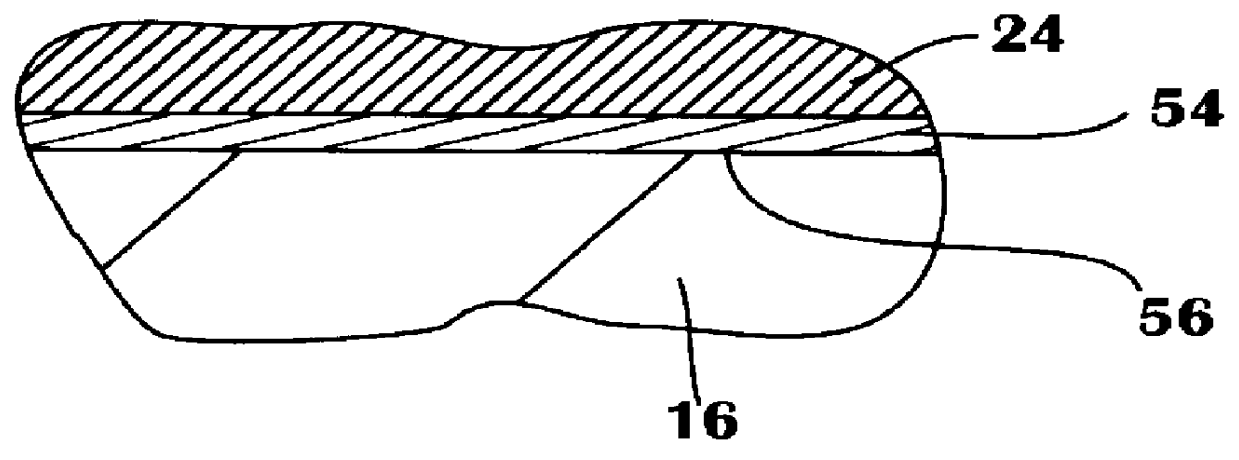
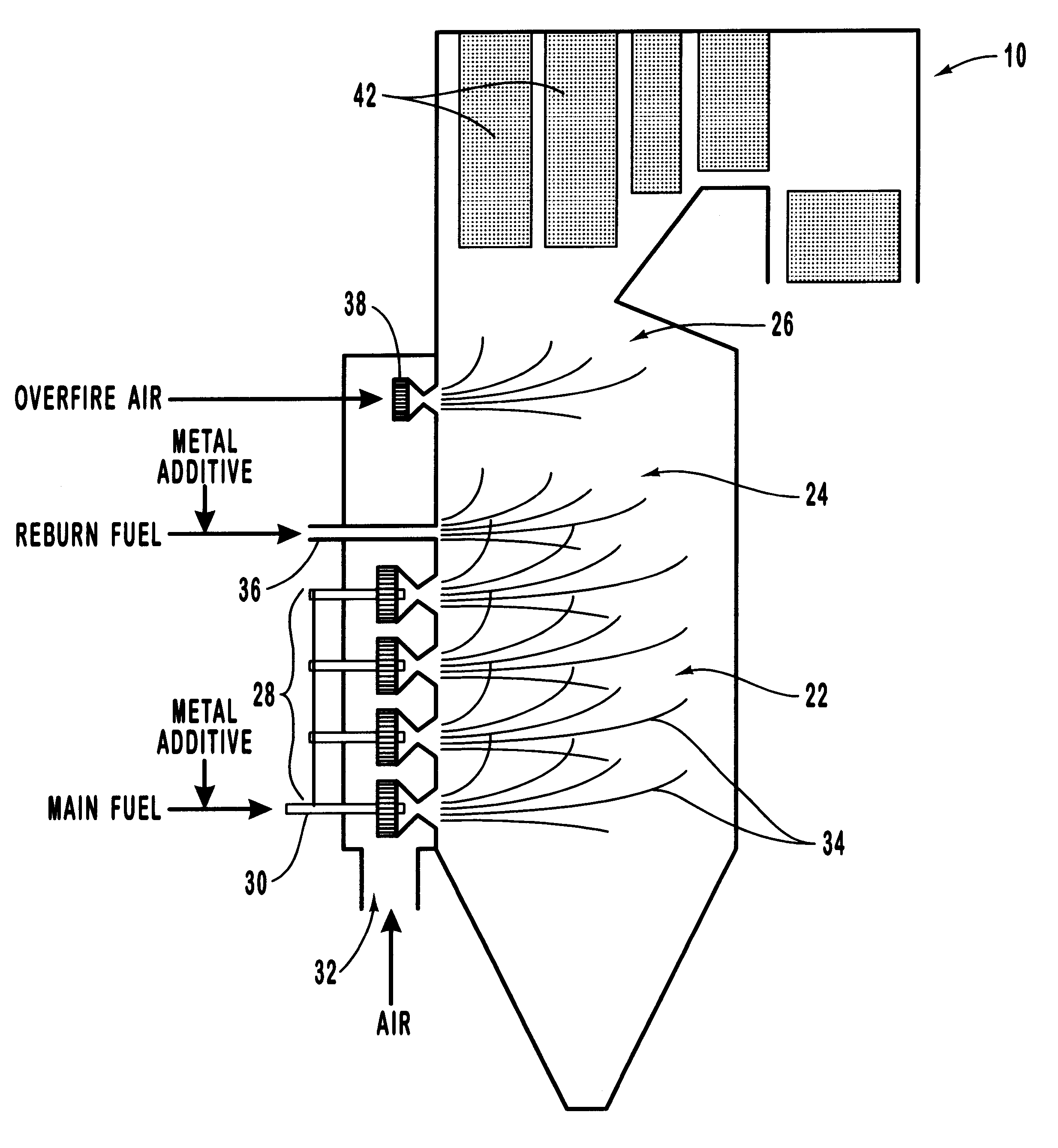
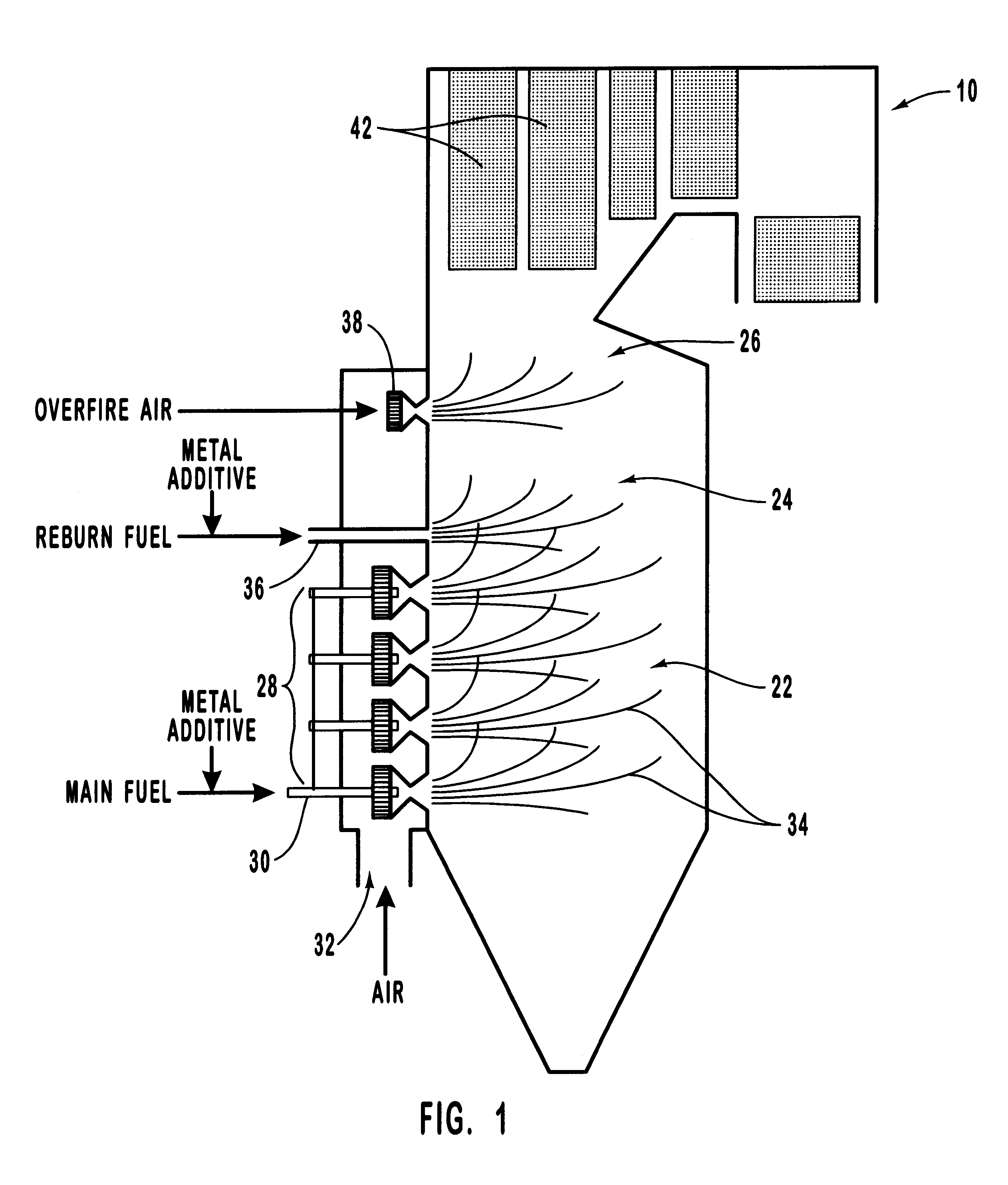
Method for reducing NOX in combustion flue gas using metal-containing additives
InactiveUS6206685B1Improved control deviceSure easyDispersed particle separationSolid fuel combustionAtmospheric airNitric oxide
Various methods for decreasing the amount of nitrogen oxides released to the atmosphere as a component of combustion gas mixtures are provided. The methods specifically provide for the removal of nitric oxide and nitrogen dioxide (NOx) from gas mixtures emitted from stationary combustion systems. In particular, methods for improving efficiency of nitrogen oxide reduction from combustion systems include injecting metal-containing compounds into the main combustion zone and / or the reburning zone of a combustion system. The metal containing compounds react with active combustion species, and these reactions change radical concentrations and significantly improve NOx conversion to molecular nitrogen. The metal-containing additives can be injected with the main fuel, in the main combustion zone, with secondary or reburning fuel addition, or at several locations in the main combustion zone and reburning zone. Optionally, nitrogenous reducing agents and / or overfire air can be injected downstream to further increase NOx reduction.
Owner:GE ENERGY & ENVIRONMENTAL RES
System and methods for improved emission control of internal combustion engines using pulsed fuel flow
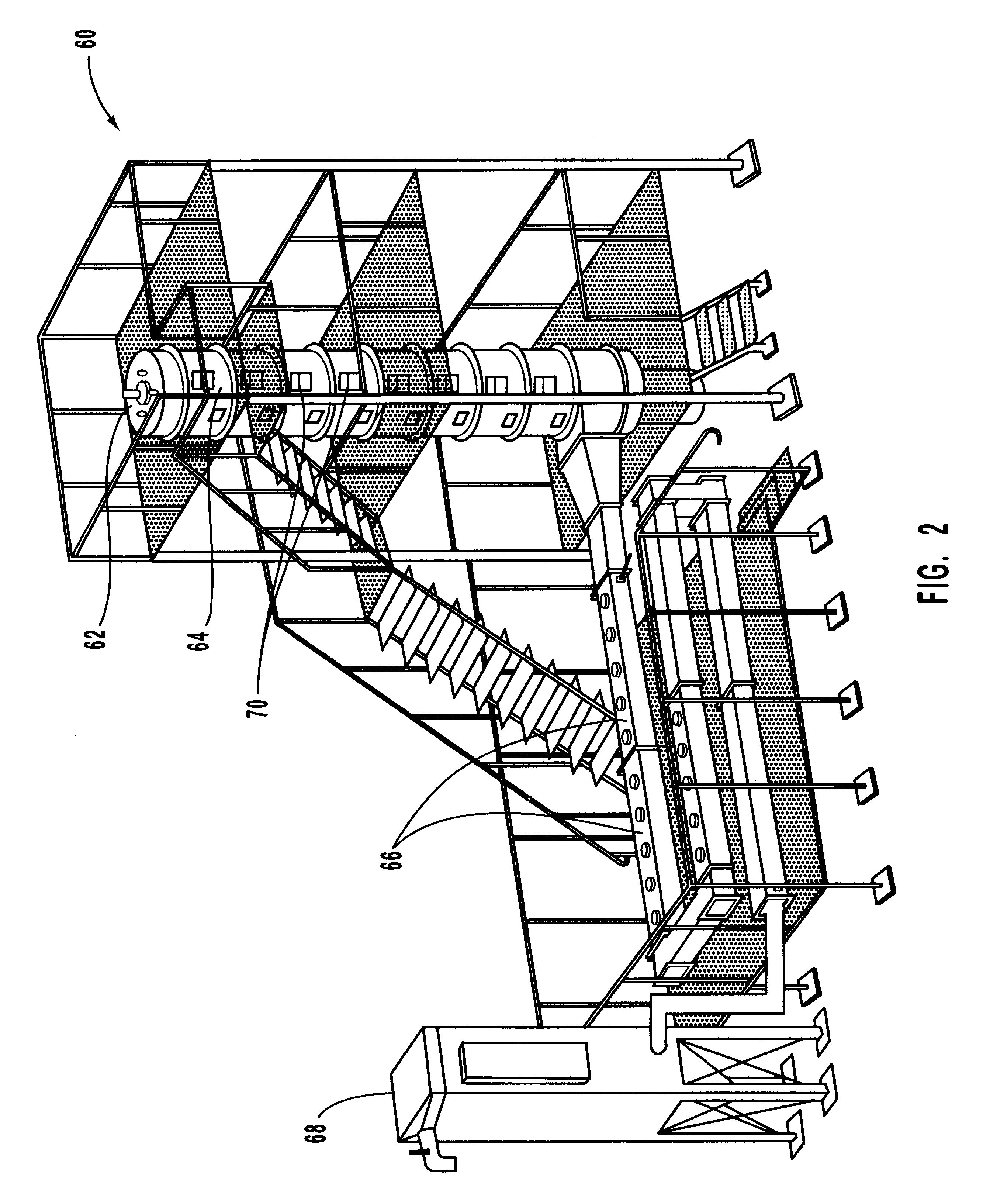
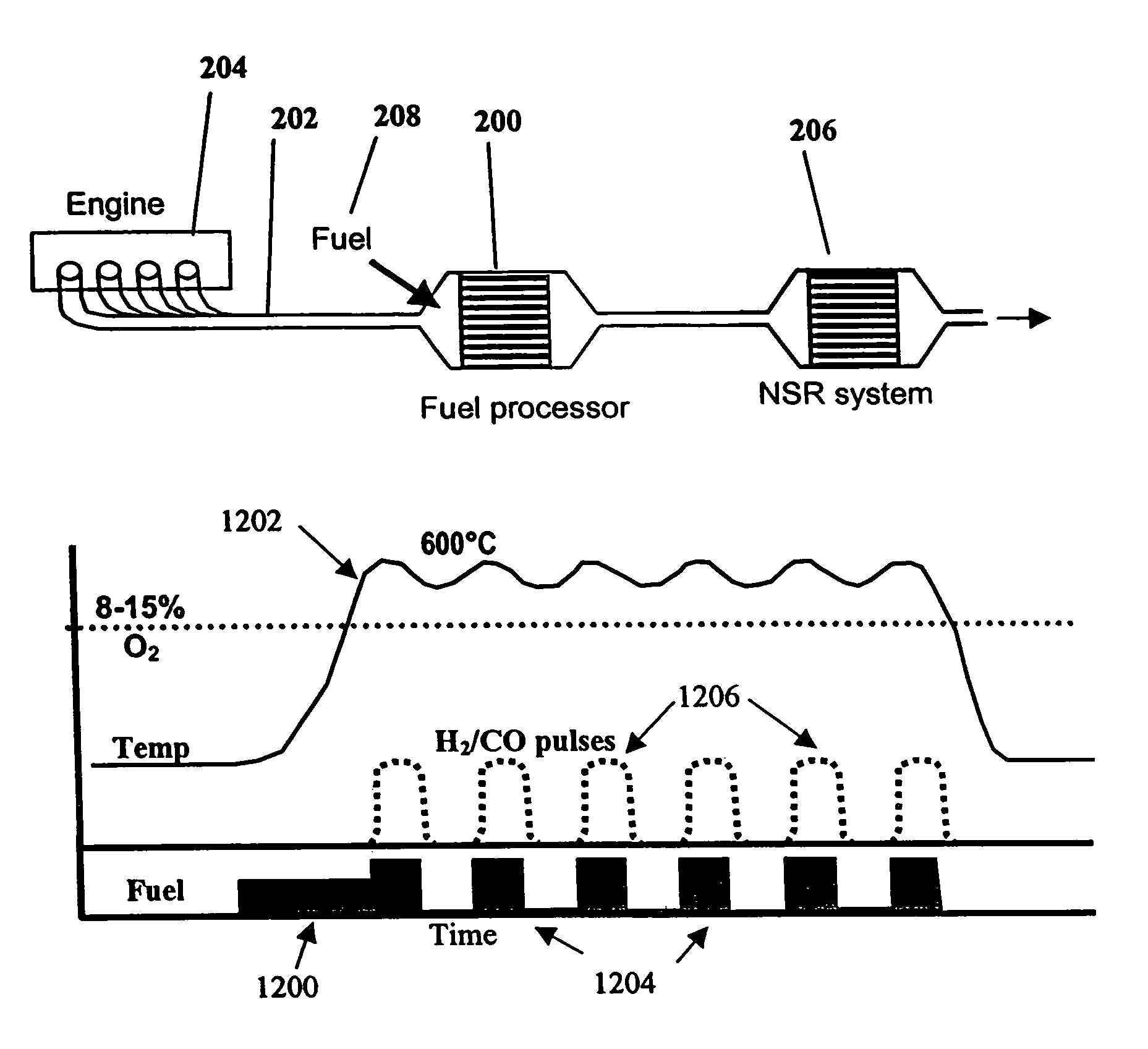
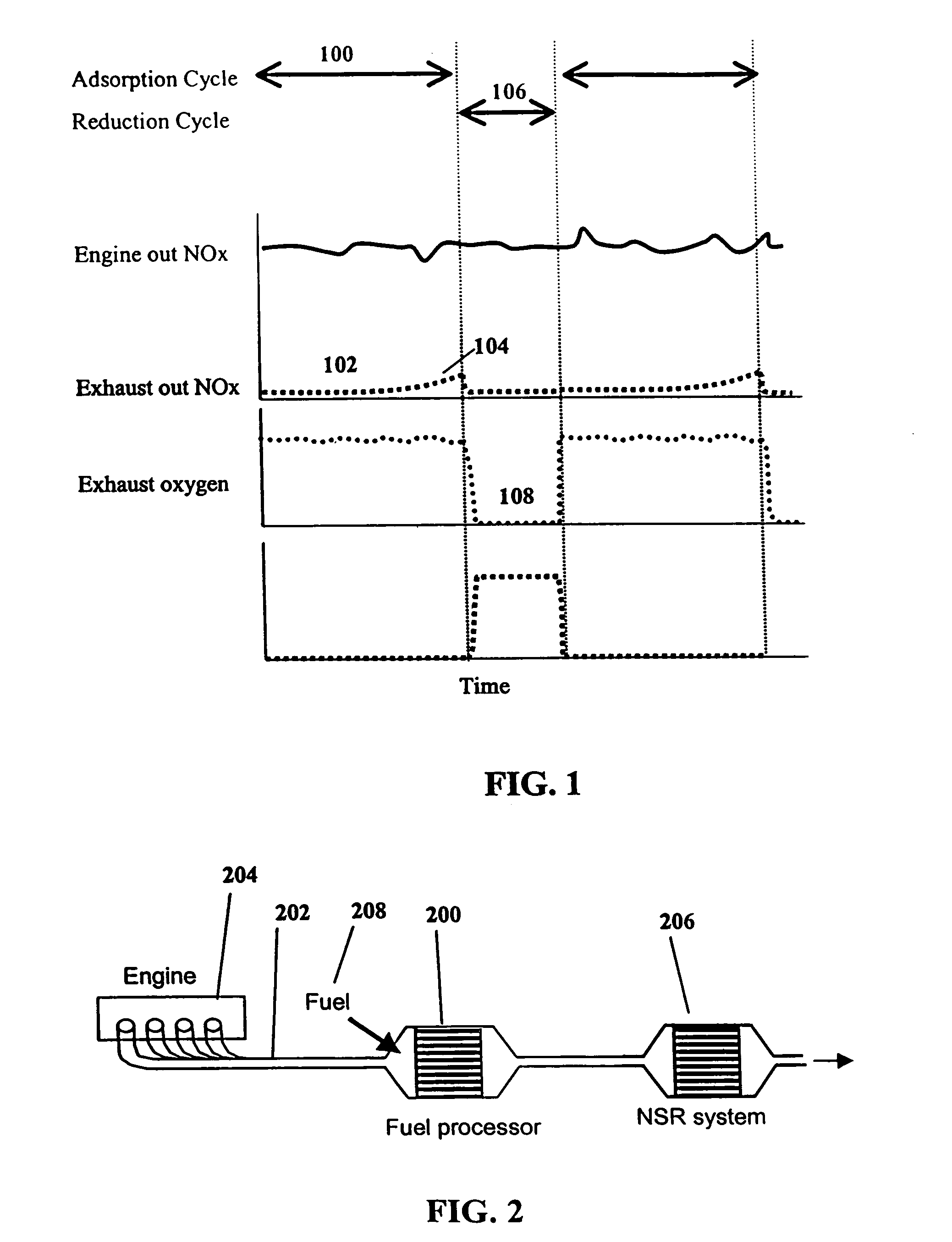
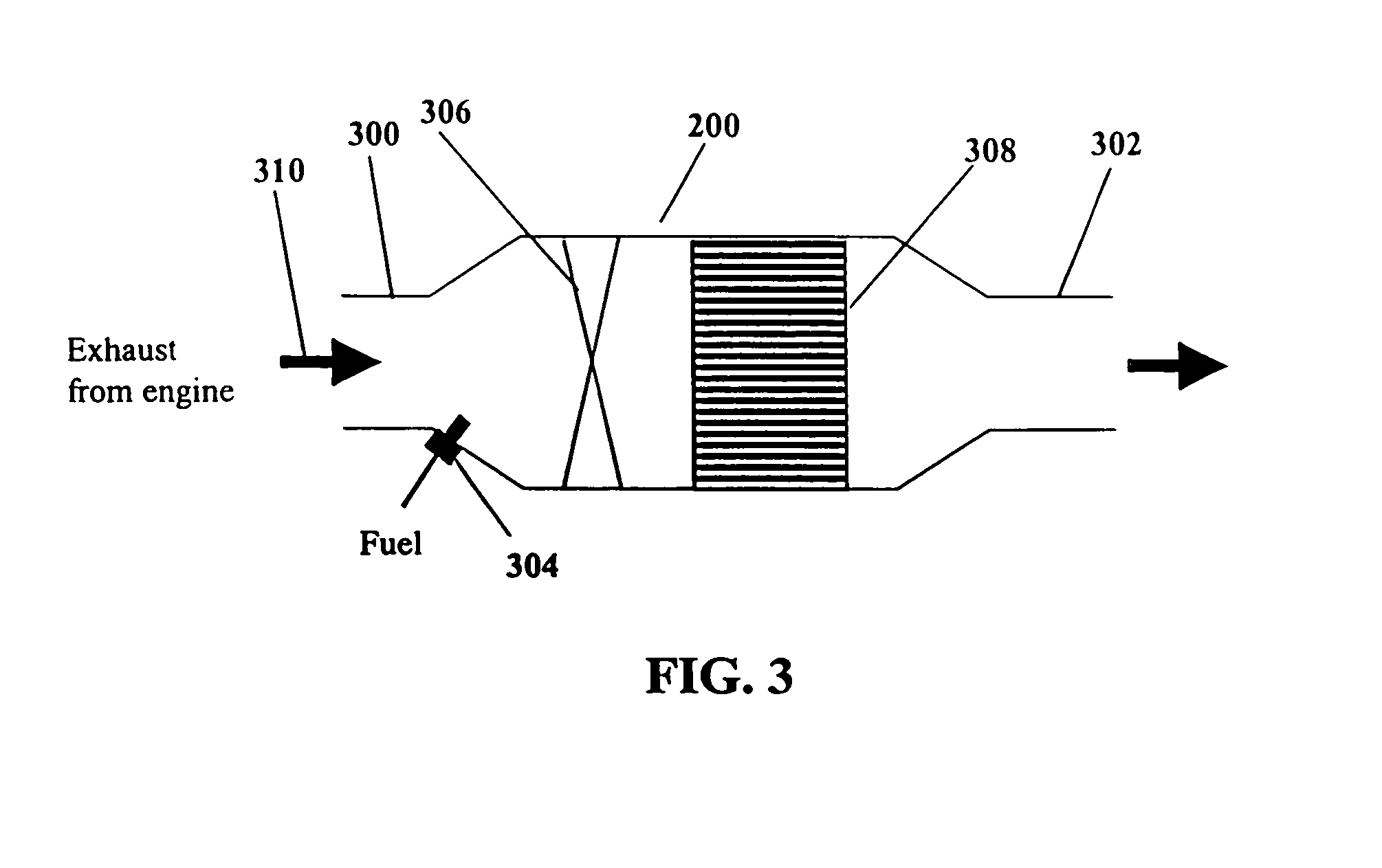
InactiveUS7082753B2Save oilReducing greenhouse gas emissionExhaust apparatusCombustion enginesPartial oxidationExternal combustion engine
The present invention provides systems and methods to improve the performance and emission control of internal combustion engines equipped with nitrogen oxides storage-reduction (“NSR”) emission control systems. The system generally includes a NSR catalyst, a fuel processor located upstream of the NSR catalyst, and at least one fuel injection port. The fuel processor converts a fuel into a reducing gas mixture comprising CO and H2. The reducing gas mixture is then fed into the NSR catalyst, where it regenerates the NSR adsorbent, reduces the NOx to nitrogen, and optionally periodically desulfates the NSR catalyst. The fuel processor generally includes one or more catalysts, which facilitate reactions such as combustion, partial oxidation, and / or reforming and help consume excess oxygen present in an engine exhaust stream. The methods of the present invention provide for NSR catalyst adsorbent regeneration using pulsed fuel flow. Control strategies are also provided.
Owner:INT ENGINE INTPROP CO LLC
Integrated SCR and AMOX Catalyst Systems
Catalysts and catalytic articles for treating exhaust gas streams are described. In one or more embodiments, a catalyst system includes a first zone to abate nitrogen oxides by selective catalytic reduction, a second zone to oxidize ammonia and a third zone to oxidize carbon monoxide and hydrocarbons. Methods for treating the exhaust gas stream are also provided. Methods of making and using such catalysts and catalytic articles are also described.
Owner:BASF CORP
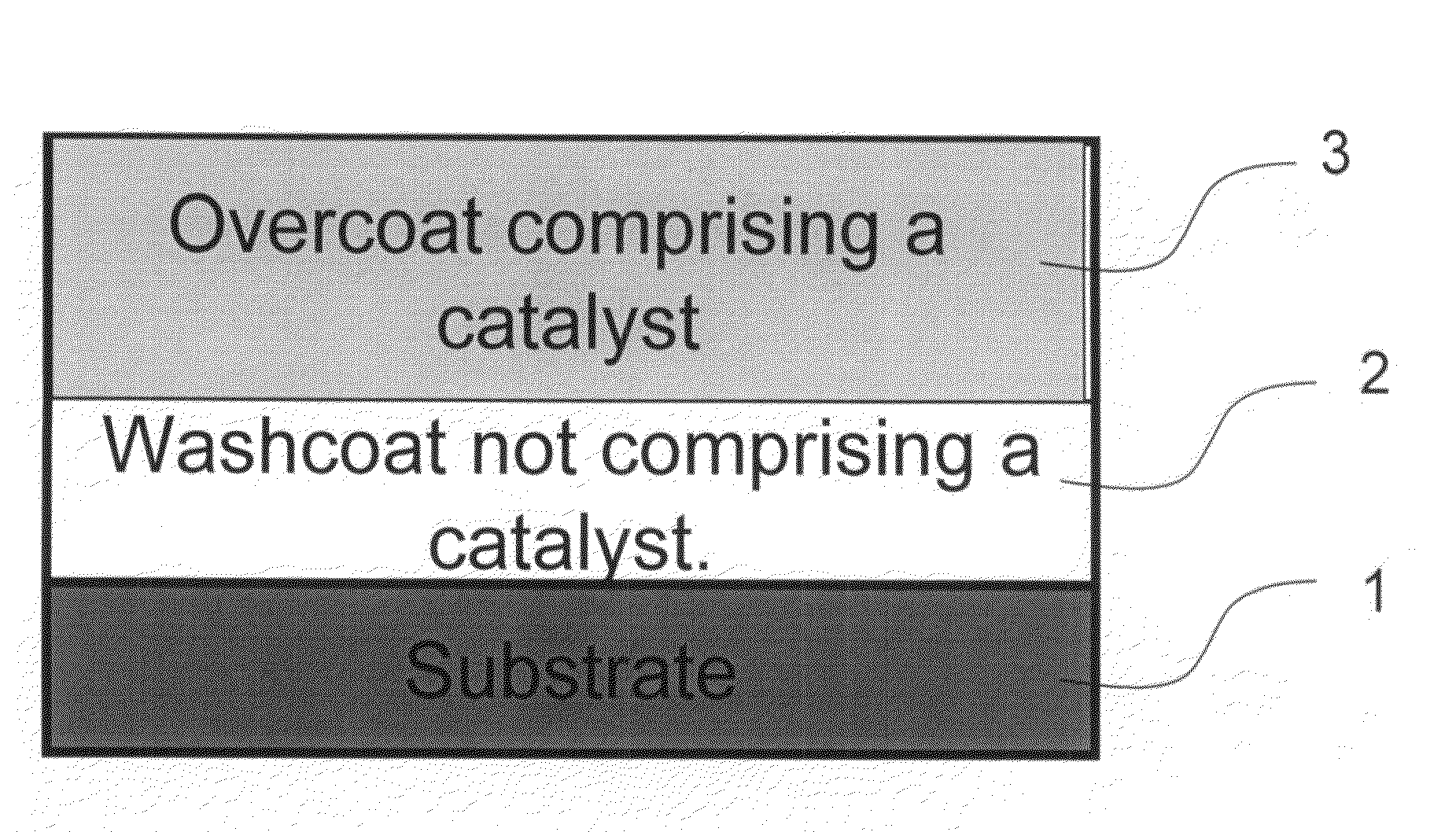
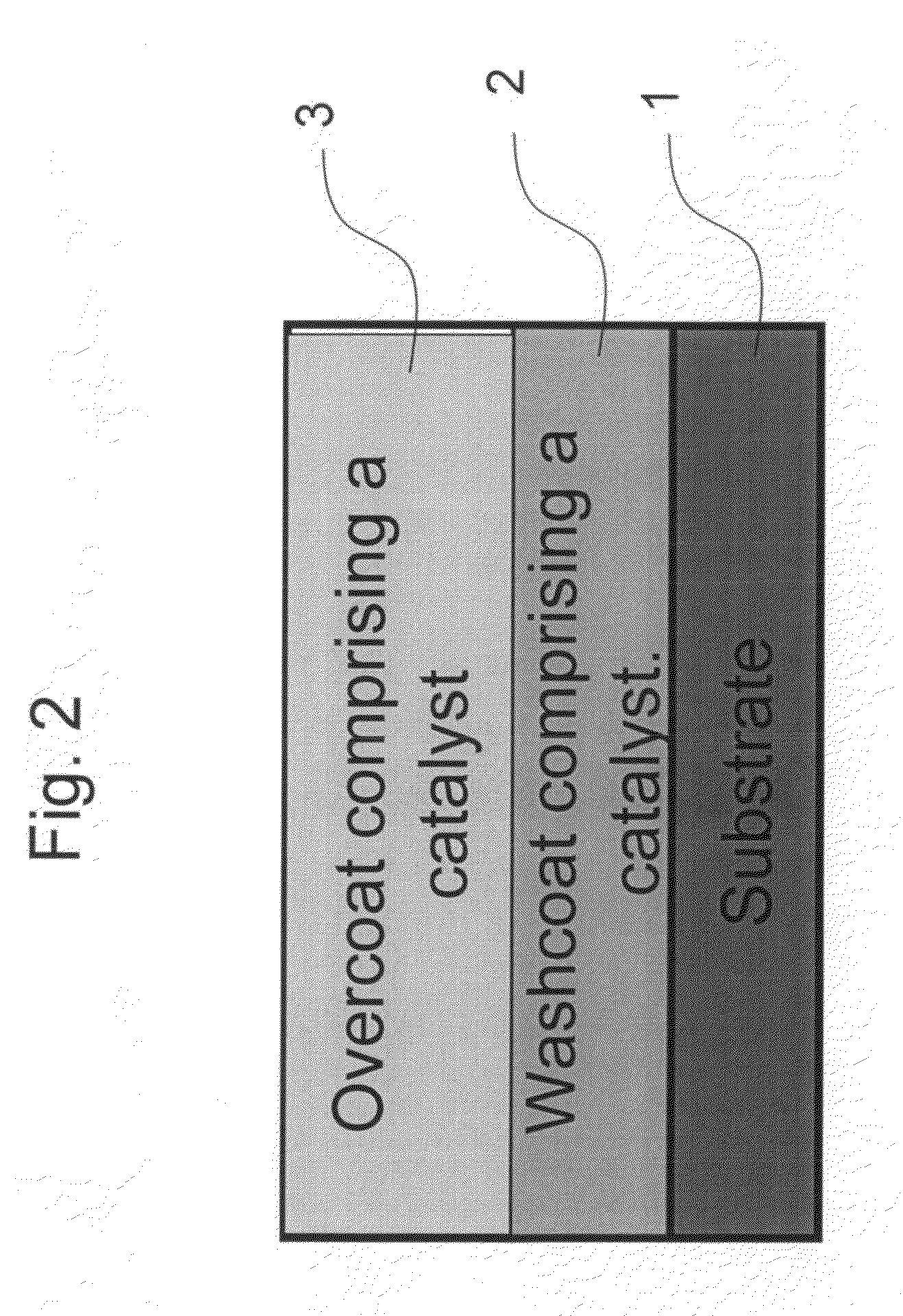
Zero platinum group metal catalysts
The present invention pertains to catalyst systems for nitrogen oxide, carbon monoxide, hydrocarbon, and sulfur reactions that are free or substantially free of platinum group metals. The catalyst system of the present invention comprise a substrate and a washcoat, wherein the washcoat comprises at least one oxide solid, wherein the oxide solid comprises one or more selected from the group consisting of a carrier material oxide, a catalyst, and mixtures thereof. The catalyst system may optionally have an overcoat, wherein the overcoat comprises at least one oxide solid, wherein the oxide solid comprises one or more selected from the group consisting of a carrier material oxide, a catalyst, and mixtures thereof. The catalyst comprises one or more selected from the group consisting of a ZPGM transition metal catalyst, a mixed metal oxide catalyst, a zeolite catalysts, or mixtures thereof.
Owner:CATALYTIC SOLUTIONS INC
Graded thin films
InactiveUS6933225B2Reduce the temperatureDesirable interface propertySolid-state devicesSemiconductor/solid-state device manufacturingGate dielectricSilicon oxide
Thin films are formed by atomic layer deposition, whereby the composition of the film can be varied from monolayer to monolayer during cycles including alternating pulses of self-limiting chemistries. In the illustrated embodiments, varying amounts of impurity sources are introduced during the cyclical process. A graded gate dielectric is thereby provided, even for extremely thin layers. The gate dielectric as thin as 2 nm can be varied from pure silicon oxide to oxynitride to silicon nitride. Similarly, the gate dielectric can be varied from aluminum oxide to mixtures of aluminum oxide and a higher dielectric material (e.g., ZrO2) to pure high k material and back to aluminum oxide. In another embodiment, metal nitride (e.g., WN) is first formed as a barrier for lining dual damascene trenches and vias. During the alternating deposition process, copper can be introduced, e.g., in separate pulses, and the copper source pulses can gradually increase in frequency, forming a graded transition region, until pure copper is formed at the upper surface. Advantageously, graded compositions in these and a variety of other contexts help to avoid such problems as etch rate control, electromigration and non-ohmic electrical contact that can occur at sharp material interfaces.
Owner:ASM INTERNATIONAL
System and method of improving emission performance of a gas turbine
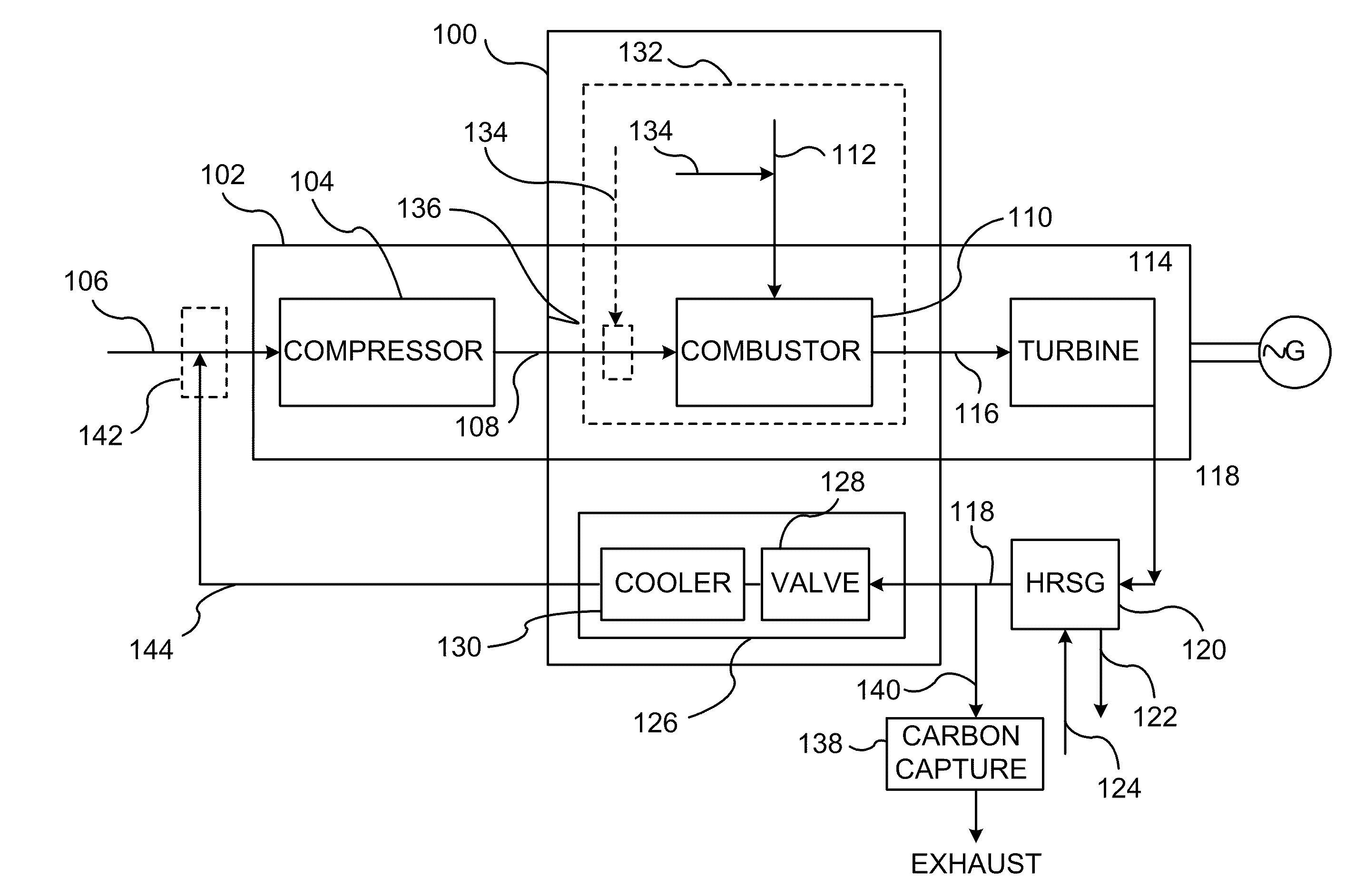
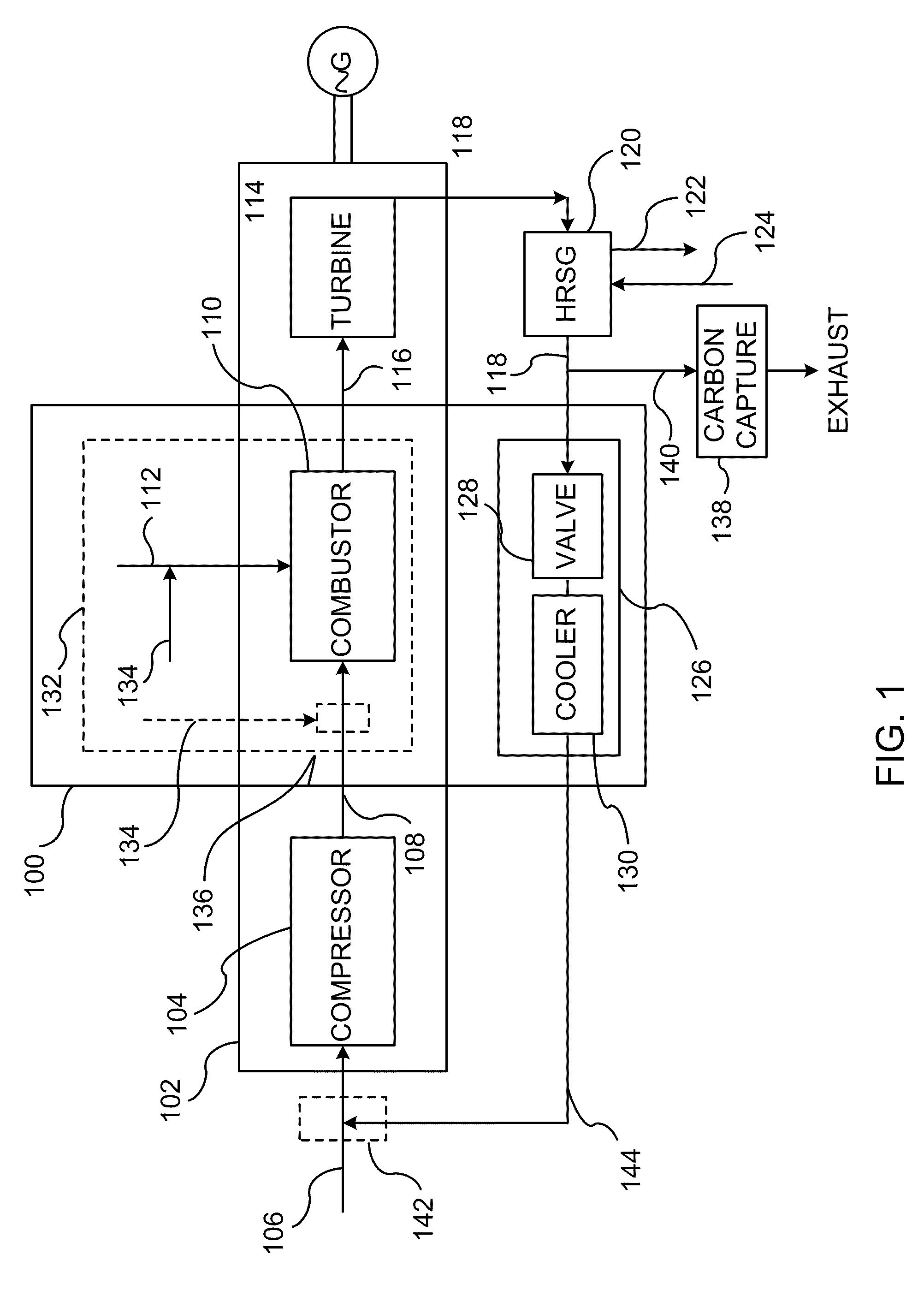
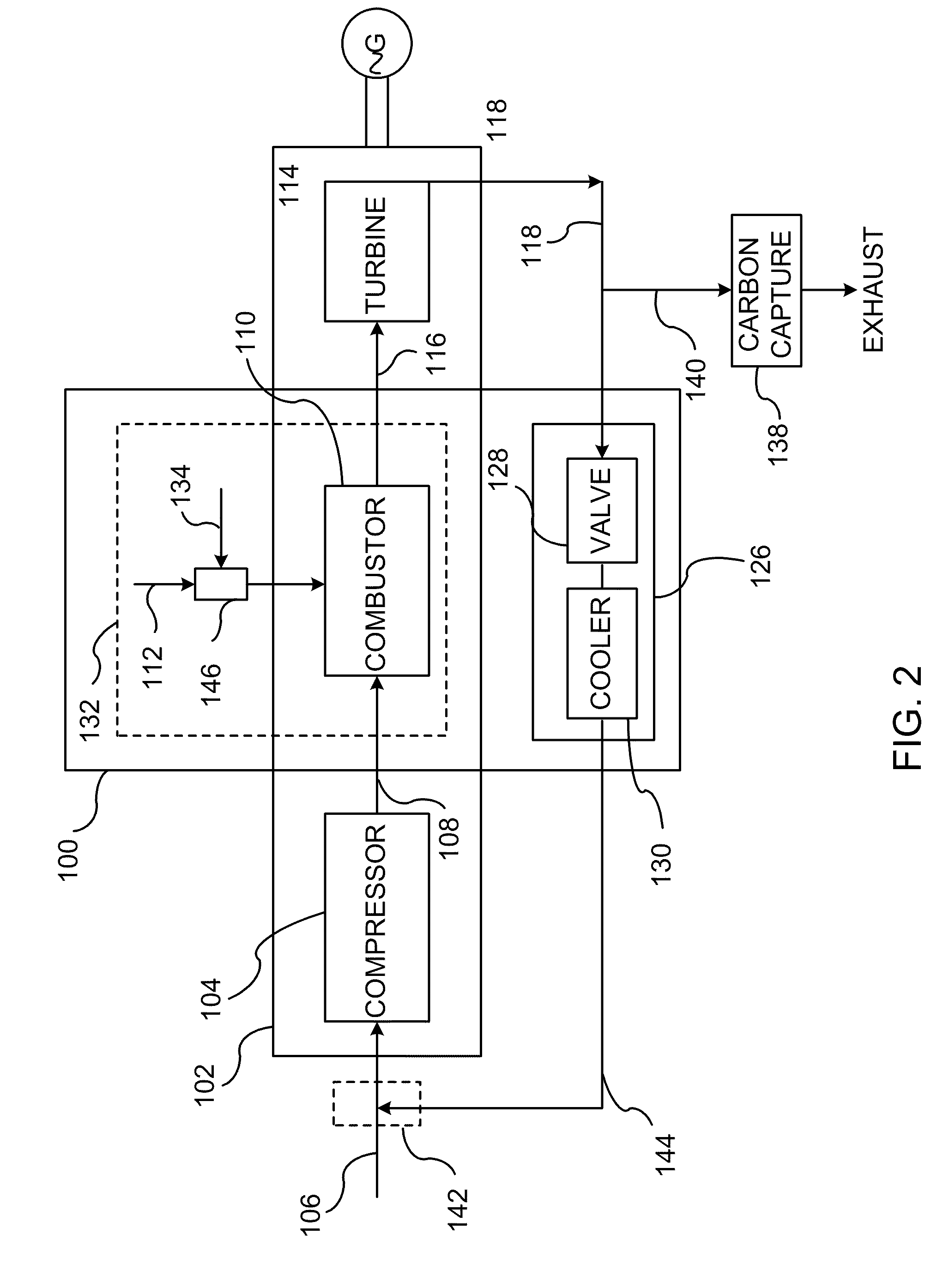
InactiveUS20110138766A1Improve emission effectReduce oxygen concentrationEngine fuctionsGas turbine plantsHigh pressureOxygen
A method of improving emission performance of a gas turbine is provided. The method includes recirculating a portion of an exhaust gas stream to a compressor of the gas turbine via an exhaust gas recirculating system, to reduce concentration of oxygen in a high pressure feed oxidant stream into a combustor of the gas turbine. The method further includes adding diluent to at least one of a fuel stream directed to the combustor or a low pressure feed oxidant stream directed to the compressor, to reduce concentration of oxides of nitrogen (NOx) and increase concentration of carbon dioxide in a resultant exhaust gas stream.
Owner:GENERAL ELECTRIC CO
Aluminum oxide-coated article
InactiveUS6333103B1Improve featuresReduced durabilityPigmenting treatmentRecord information storageX-rayCarbide
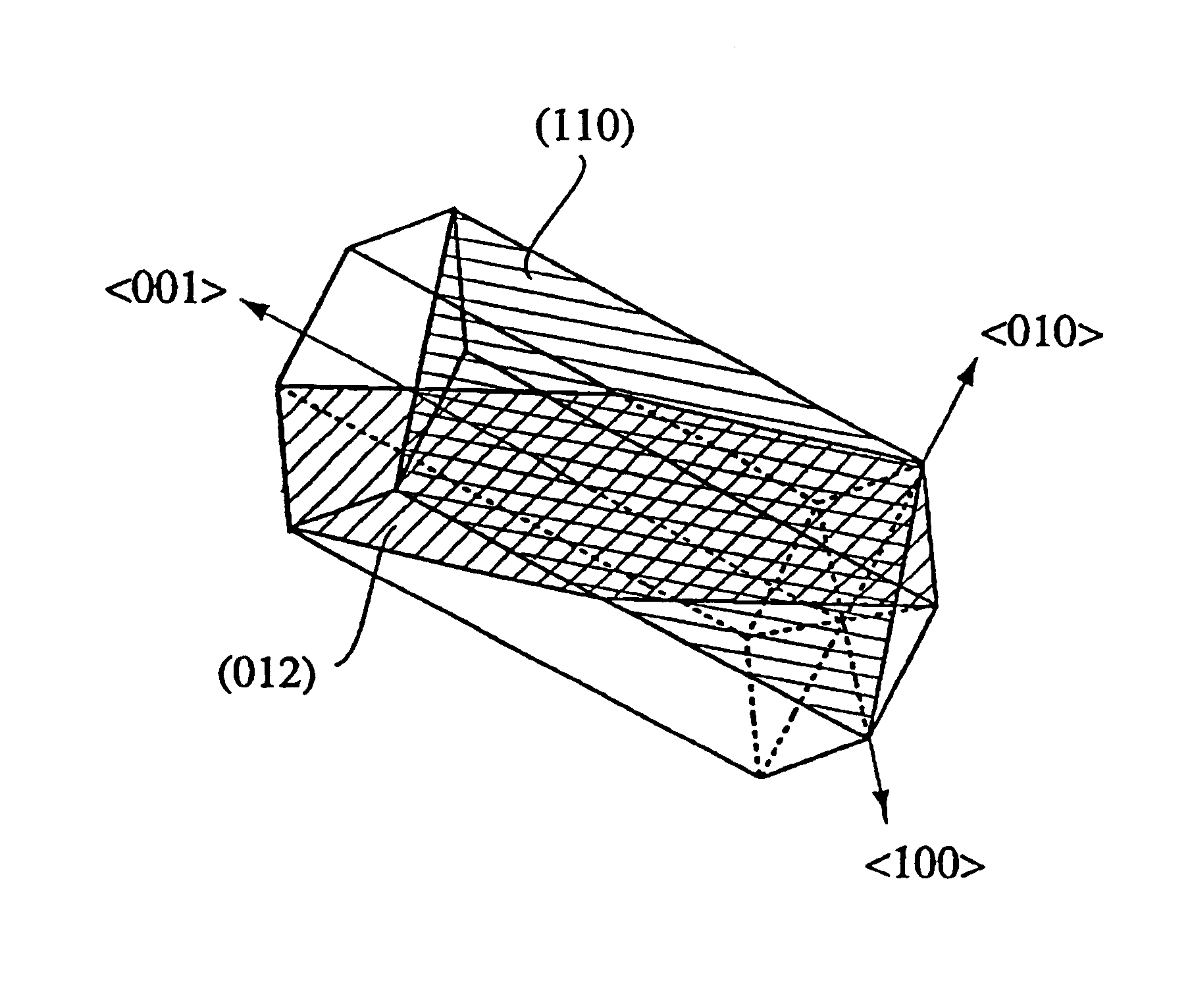
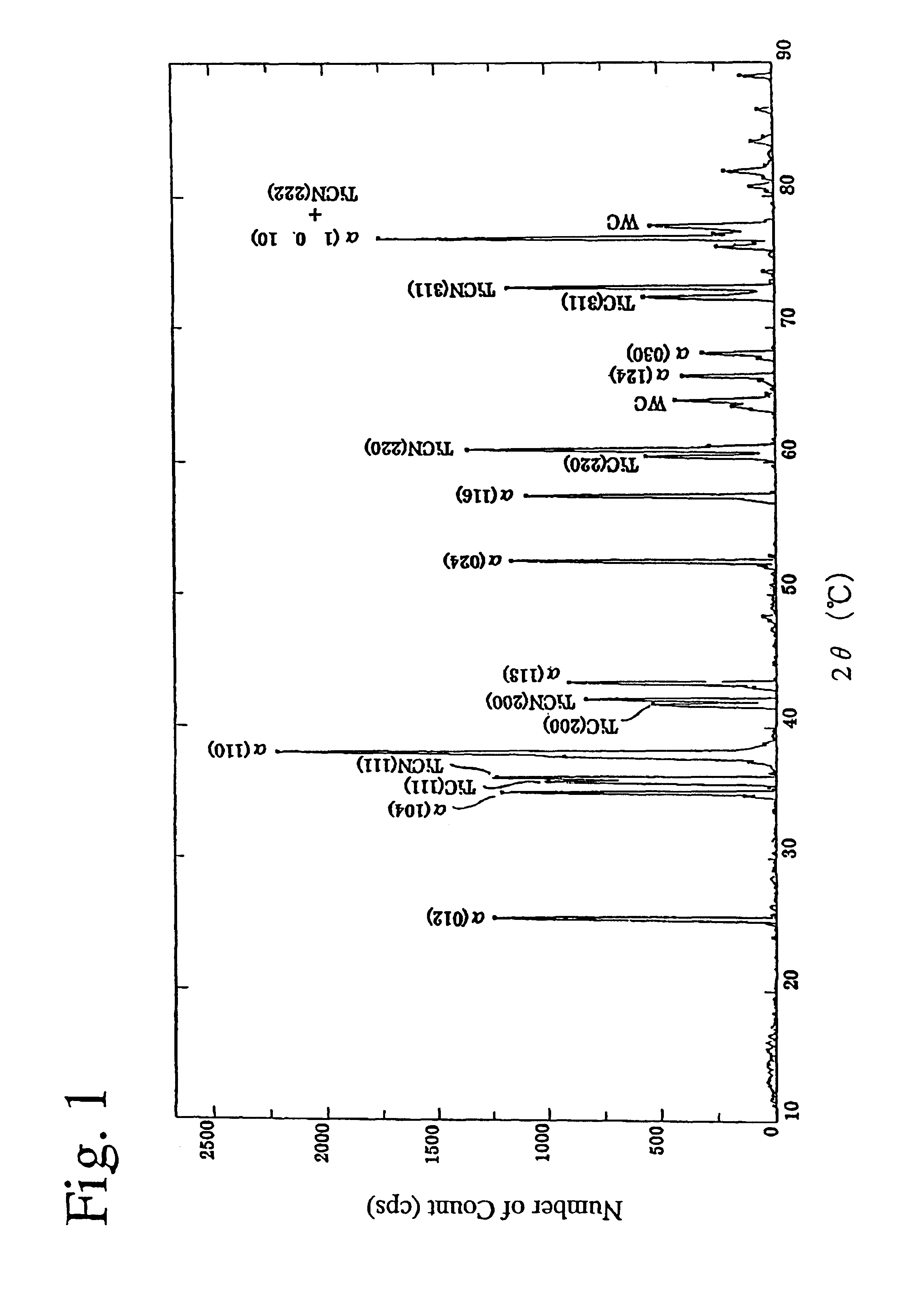
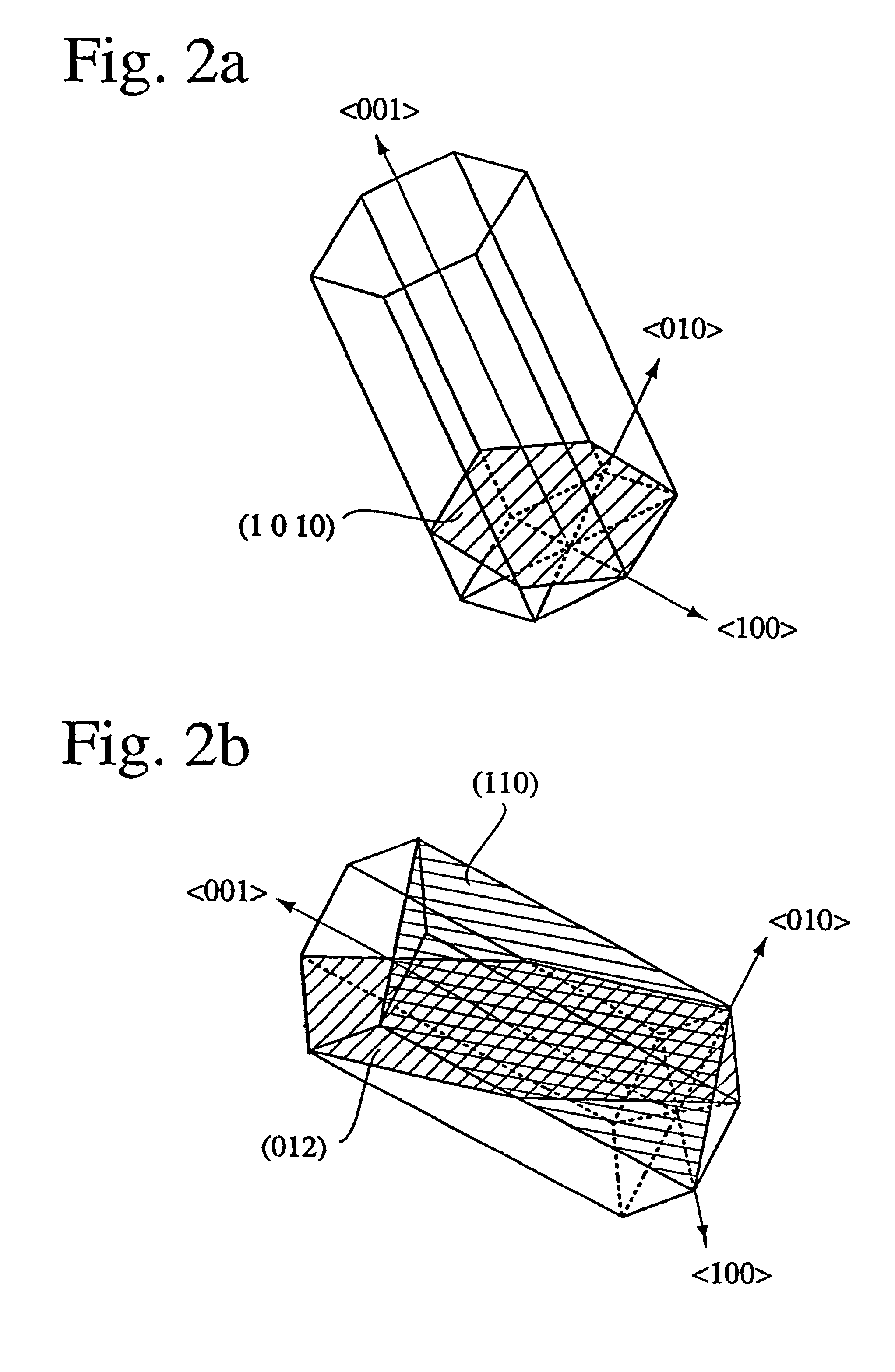
An aluminum oxde-coated article has a first coating layer and a second coating layer formed in this order on a substrate, the first coating layer having a single- or multi-layer structure and being made of at least one selected from the group consisting of carbides, nitrides, carbonitrides, oxides, oxycarbides, oxynitrides and oxycarbonitrides of metals in Groups IVa, Va and VIa of the Periodic Table, and the second coating layer being constituted by at least one alpha-aluminum oxide-based oxide layer, which has an equivalent X-ray diffraction peak ratio PR (1 0 10) of 1.3 or more in a (1 0 10) plane.
Owner:HITACHI TOOL ENG LTD
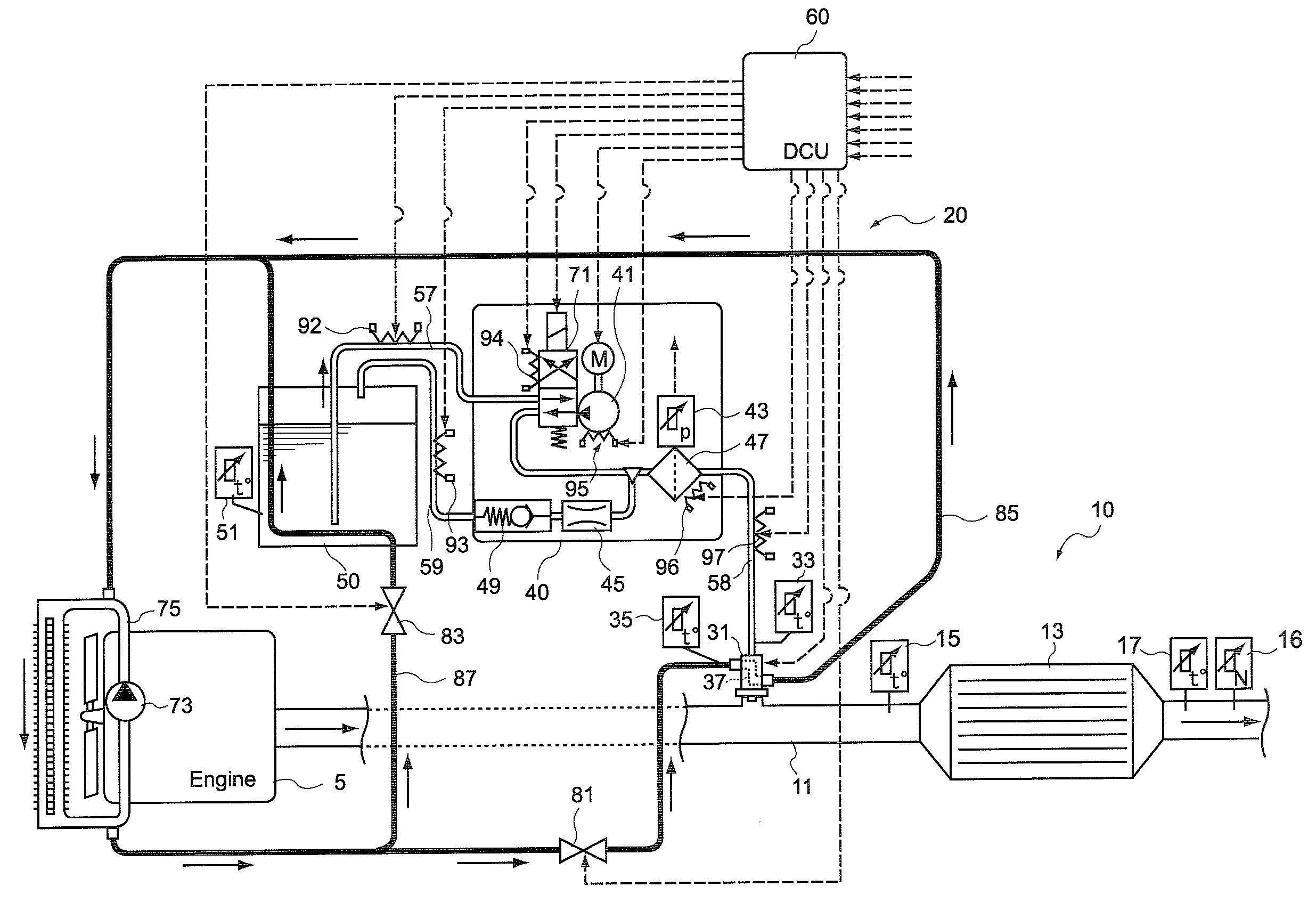
Control unit and control method for reductant supply device
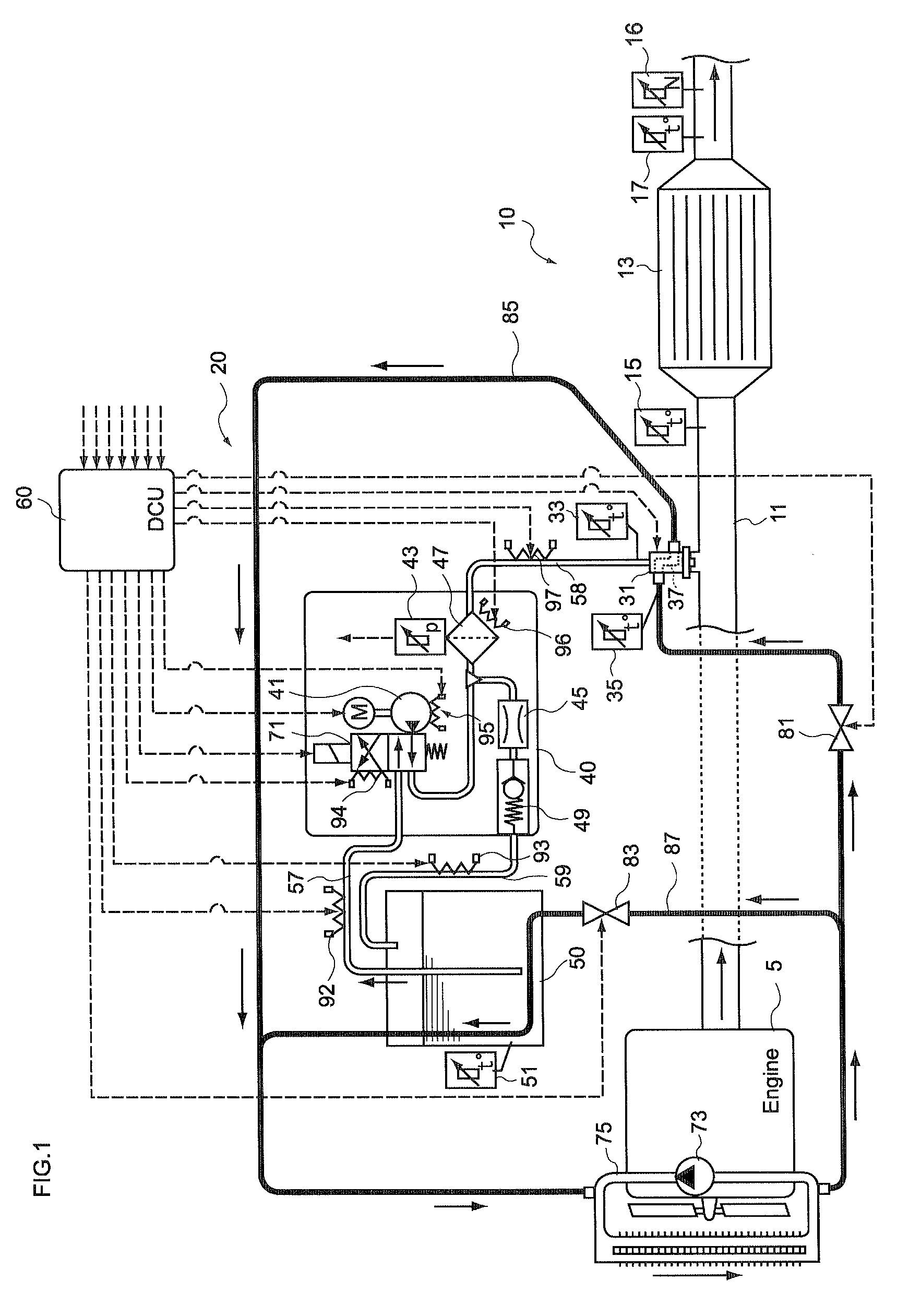
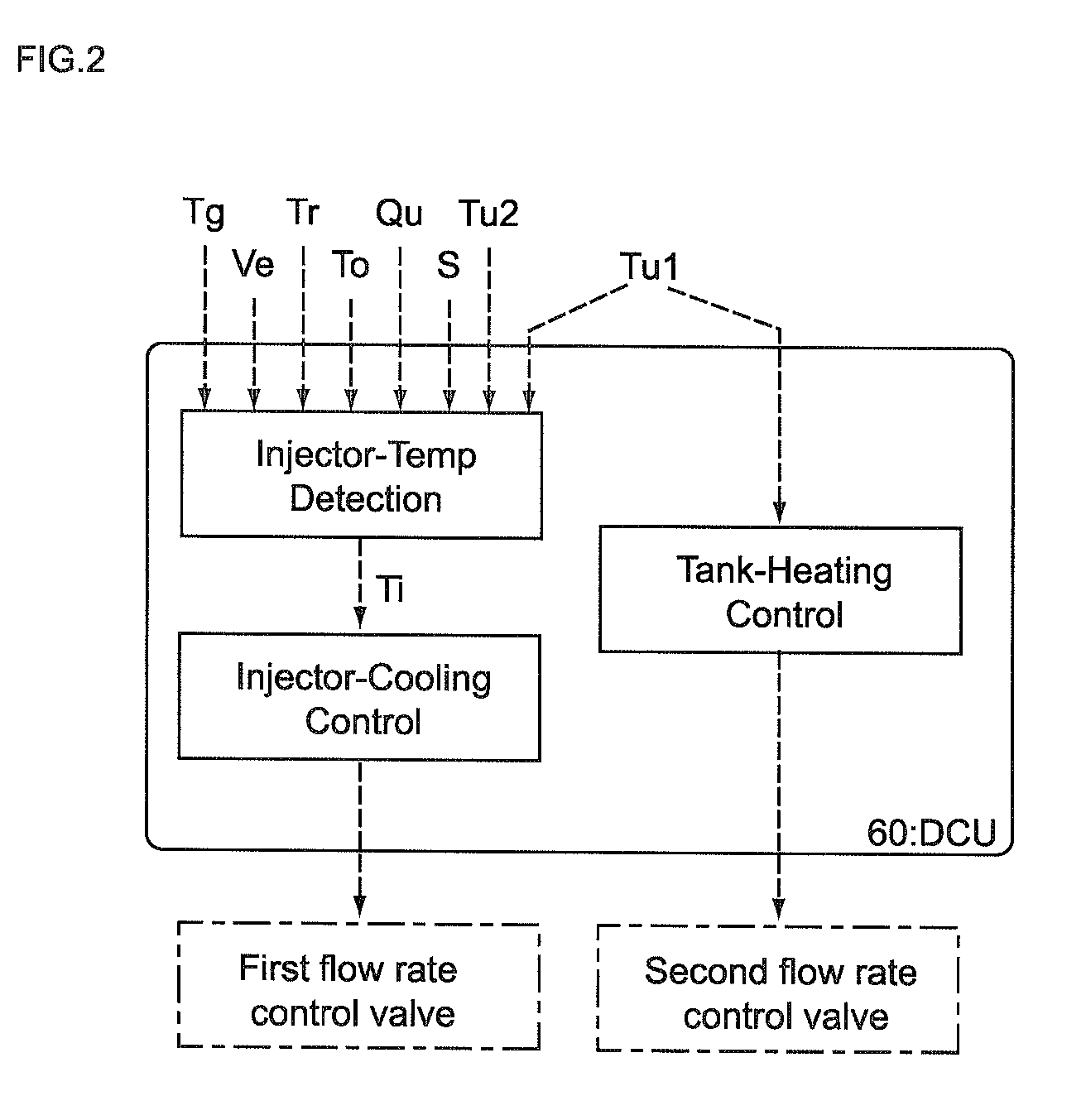
InactiveUS20100242439A1Prevent overcoolingImprove cooling effectLiquid coolingInternal combustion piston enginesEngineeringWater circulation
There are provided a reductant supply device and a control method for the reductant supply device, which can prevent heat damage of a reductant injection valve, and also prevent crystallization of urea solution due to excessive cooling of the solution reductant.The reductant supply device which is used in an exhaust gas purification device that injects and supplies, as a reductant, a urea solution to an exhaust gas upstream side of a reduction catalyst disposed in an exhaust gas passage of an internal combustion engine, and that reduces and purifies nitrogen oxides contained in exhaust gas using the reduction catalyst, the reductant supply device having a reductant injection valve that is fixed to an exhaust pipe on the exhaust gas upstream side of the reduction catalyst, includes: a cooling water circulation passage that circulates at least part of cooling water of the internal combustion engine to cool the reductant injection valve; flow rate control means for adjusting a flow rate of cooling water flowing through the cooling water circulation passage; temperature detection means for detecting a temperature of the reductant injection valve; and control means for controlling the flow rate control means based on the temperature of the reductant injection valve.
Owner:BOSCH CORP
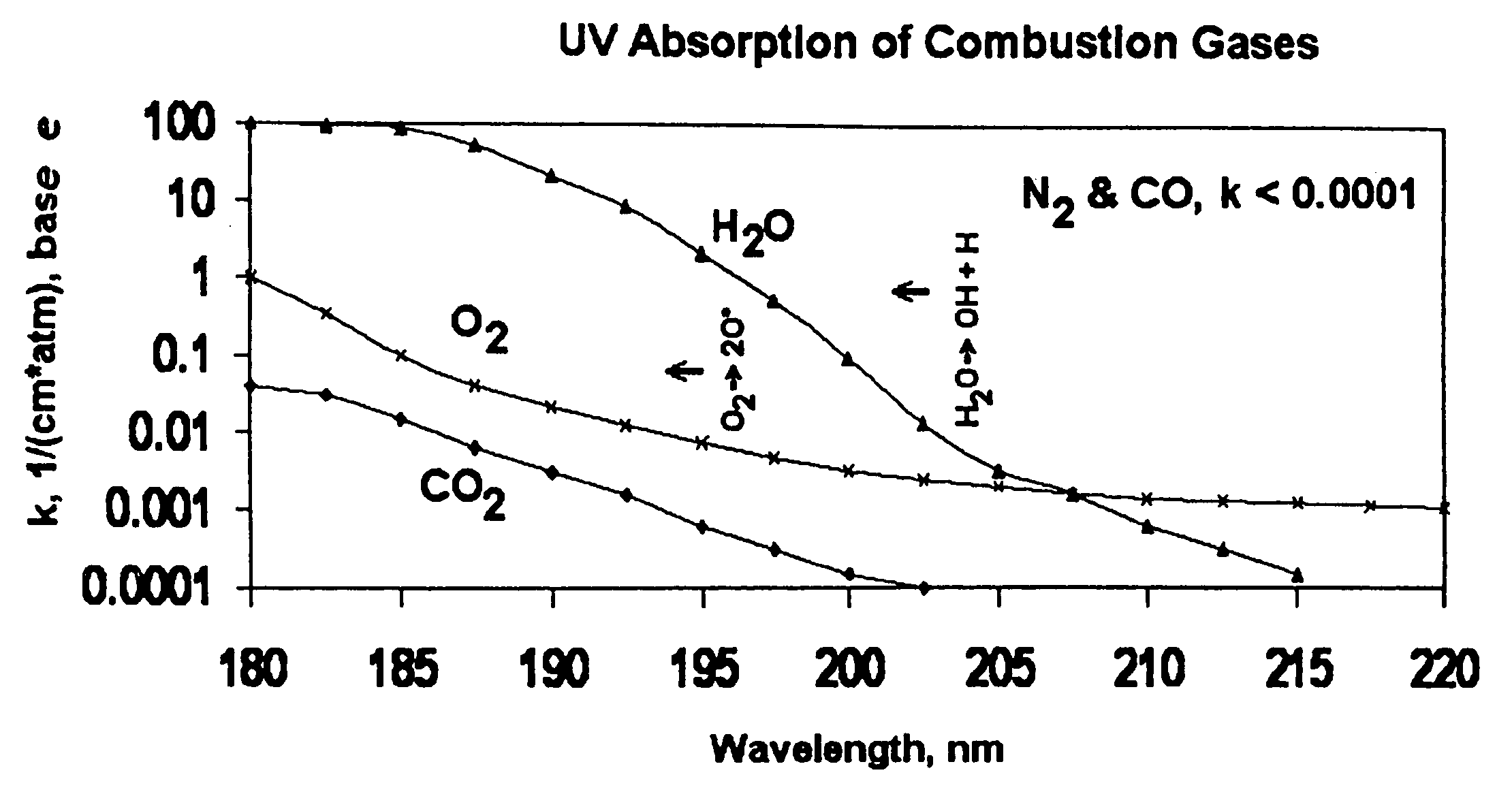
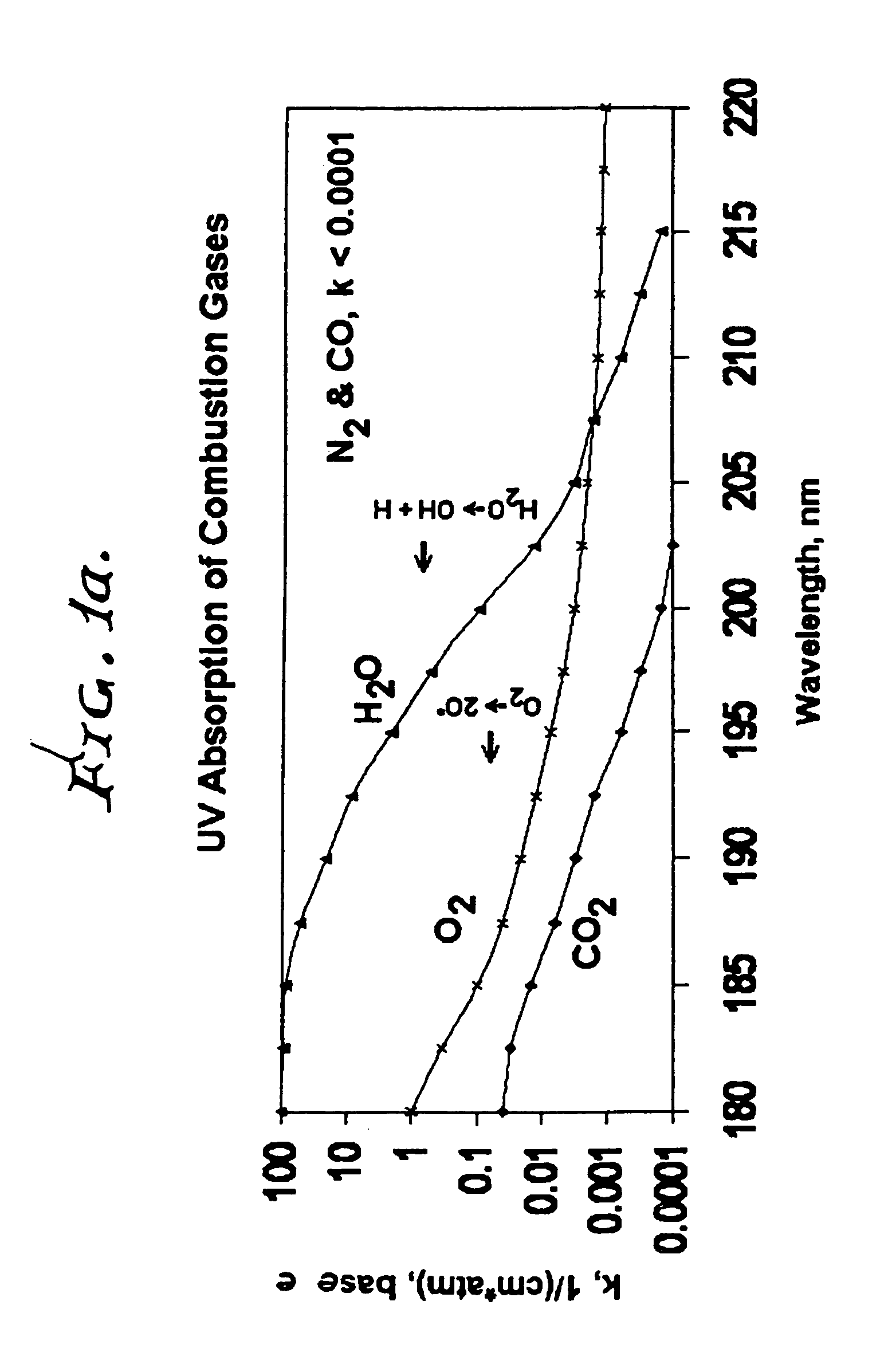
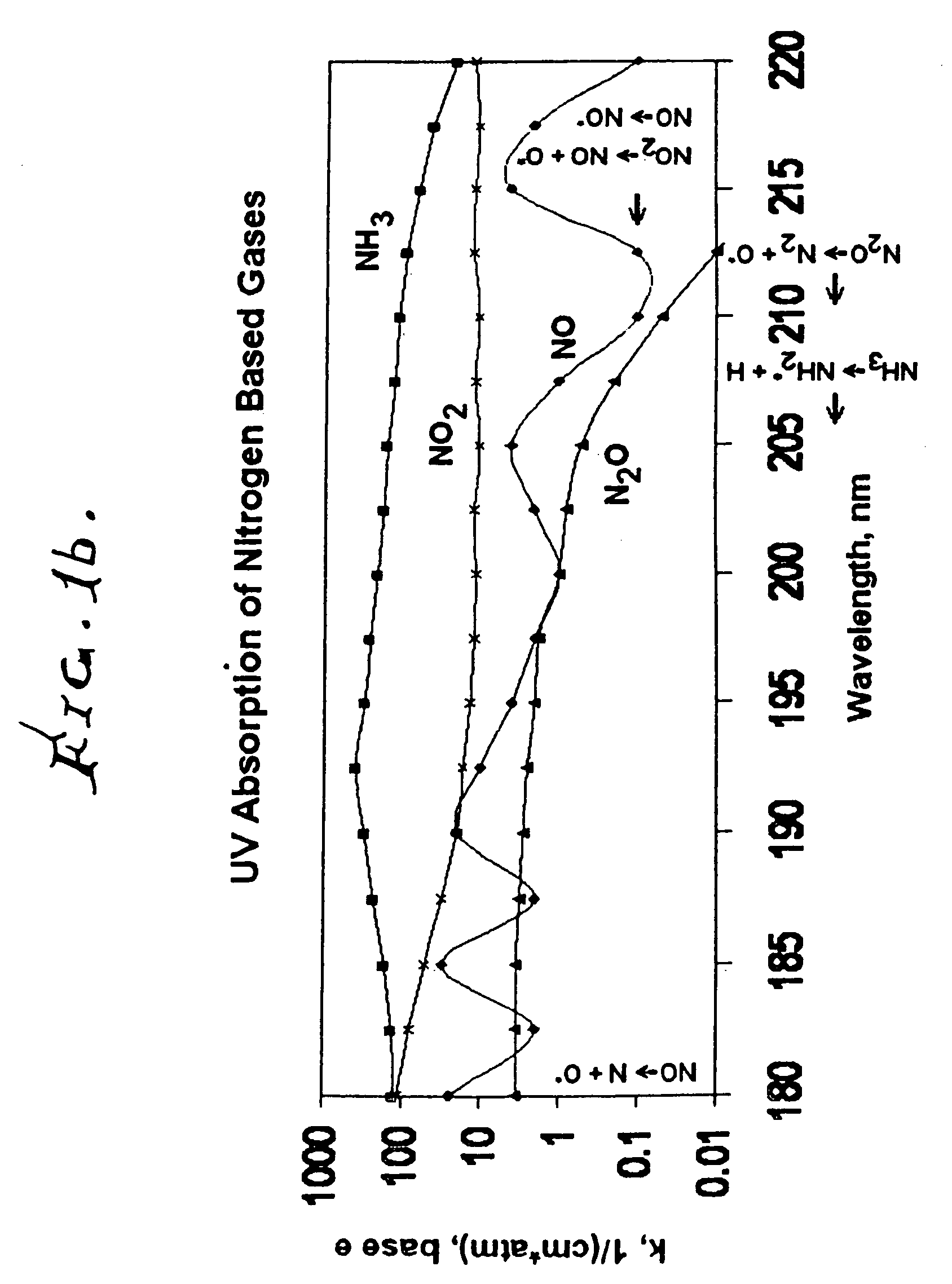
Controlled spectrum ultraviolet radiation pollution control process
InactiveUS7498009B2Cost effectiveEasy to adaptCombination devicesOrganic chemistryEnvironmental engineeringNitrogen oxide
A method for reducing or substantially eliminating oxides of nitrogen from an effluent gas stream, that includes providing a source of ultraviolet radiation with a precise wavelength, adding ammonia or an ammonia based reagent to the effluent stream, upstream of the ultraviolet radiation source, controllably operating the ultraviolet radiation source to irradiate the effluent stream flowing in the duct and substantially reducing or eliminating oxides of nitrogen by promotion a reaction of ammonia with the oxides of nitrogen to produce N2 and H2O, and also thereby destroying any surplus ammonia. This process can also be modified to oxidize carbon monoxide and VOC's to CO2 and H2O.
Owner:DANA UV
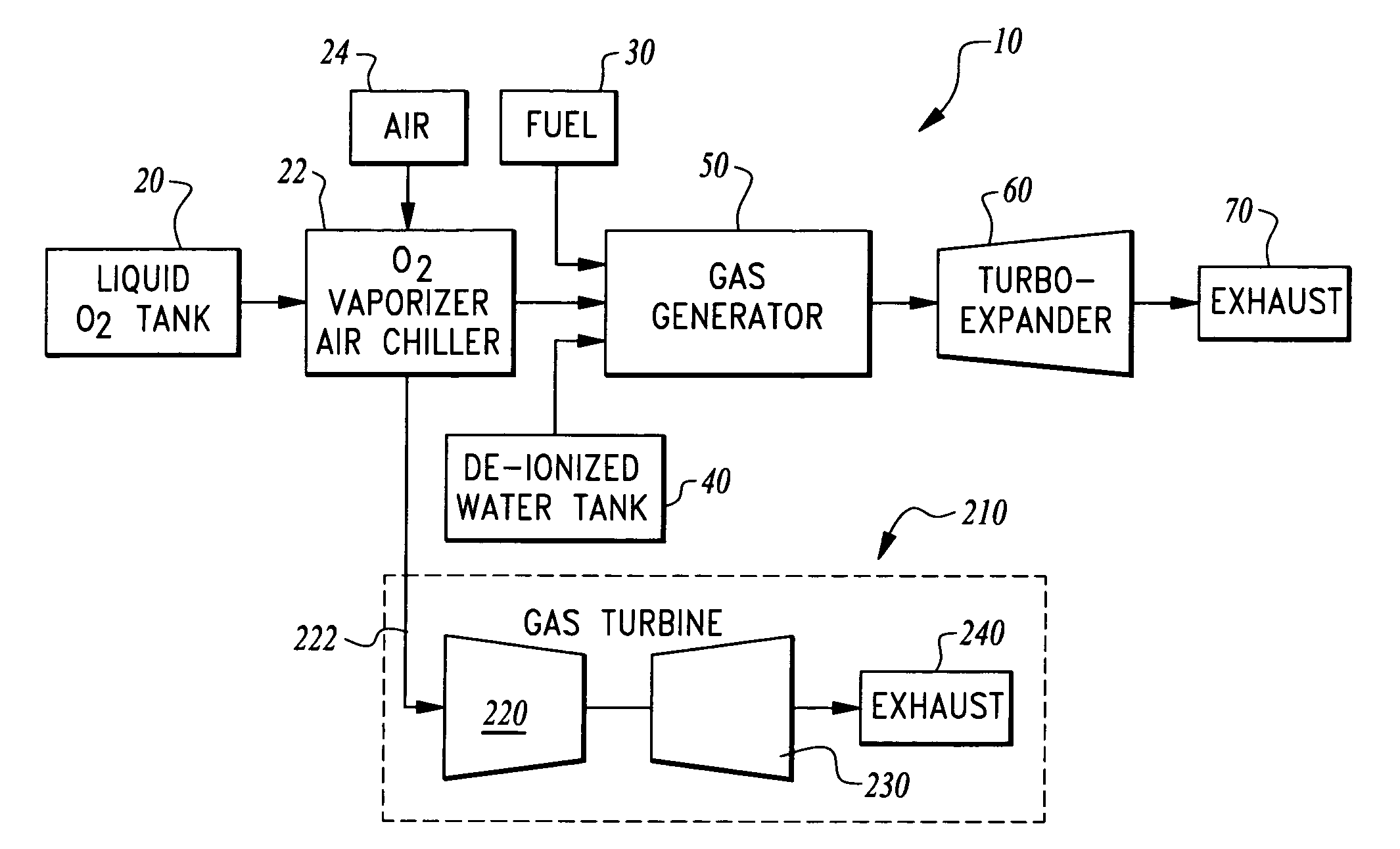
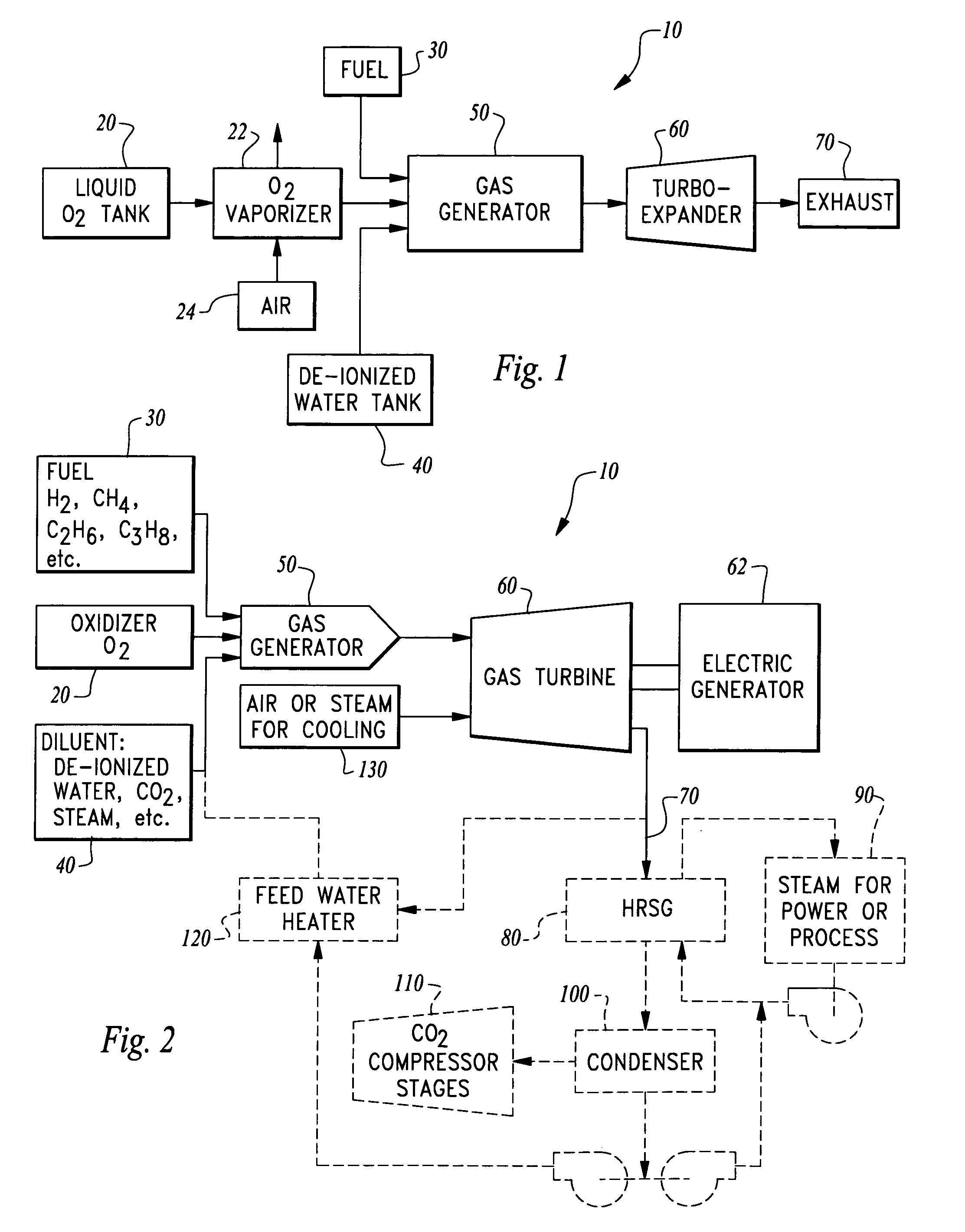
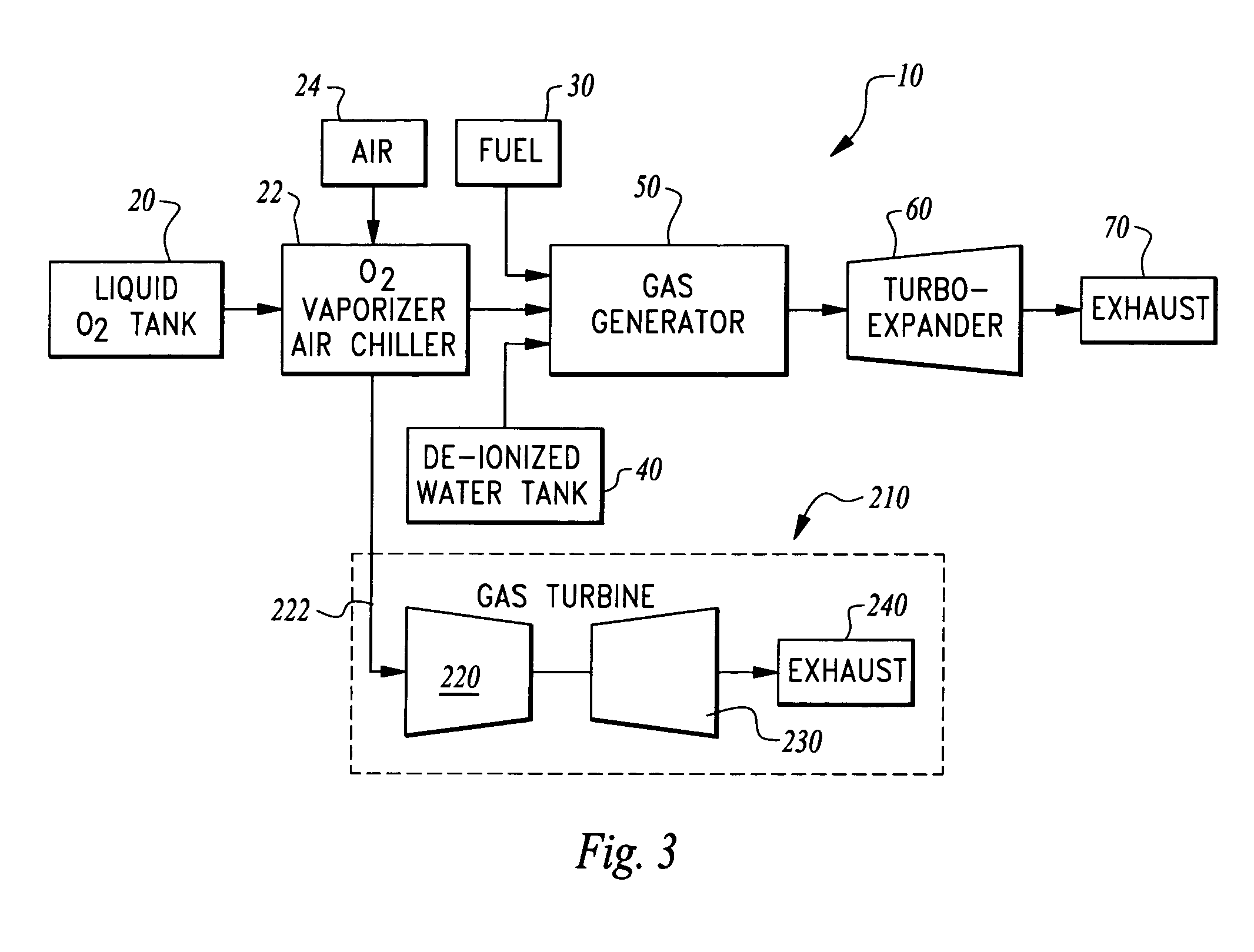
Ultra low emissions fast starting power plant
InactiveUS7827794B1Fast starting low emissionMinimal capital expenseTurbine/propulsion engine coolingGas turbine plantsNuclear engineeringLiquid fuel
The power plant combusts a hydrocarbon fuel with oxygen to produce high temperature high pressure products of combustion. These products of combustion are routed through an expander to generate power. The products of combustion are substantially free of oxides of nitrogen because the oxidizer is oxygen rather than air. To achieve fast starting, oxygen, fuel and water diluent are preferably stored in quantities sufficient to allow the power plant to operate from these stored consumables. The fuel can be a gaseous or liquid fuel. The oxygen is preferably stored as liquid and routed through a vaporizer before combustion in a gas generator along with the hydrocarbon fuel. In one embodiment, the vaporizer gasifies the oxygen by absorption of heat from air before the air is routed into a separate heat engine, such as a gas turbine. The gas turbine thus operates on cooled air and has its power output increased.
Owner:CLEAN ENERGY SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com