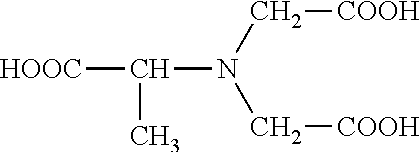
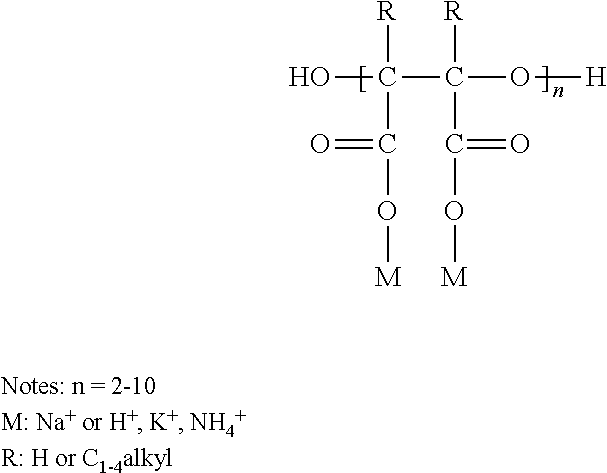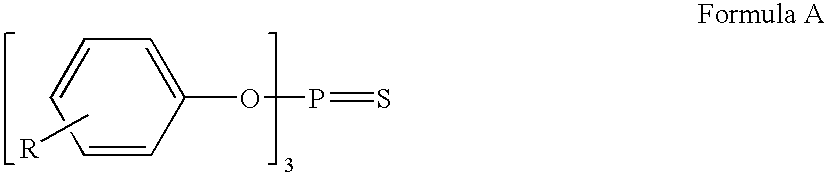Patents
Literature
6773 results about "Ascorbic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Defined media for stem cell culture
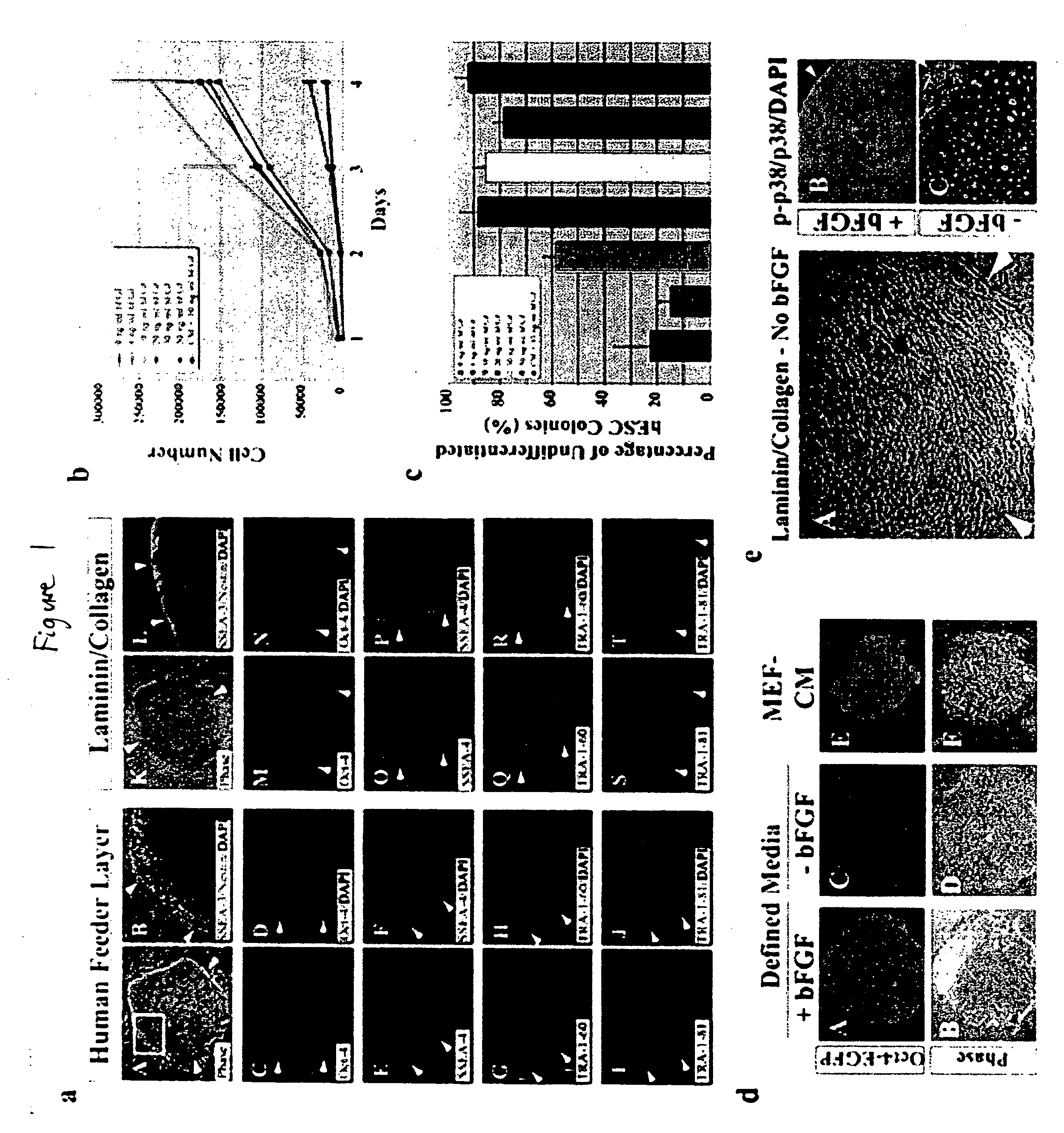
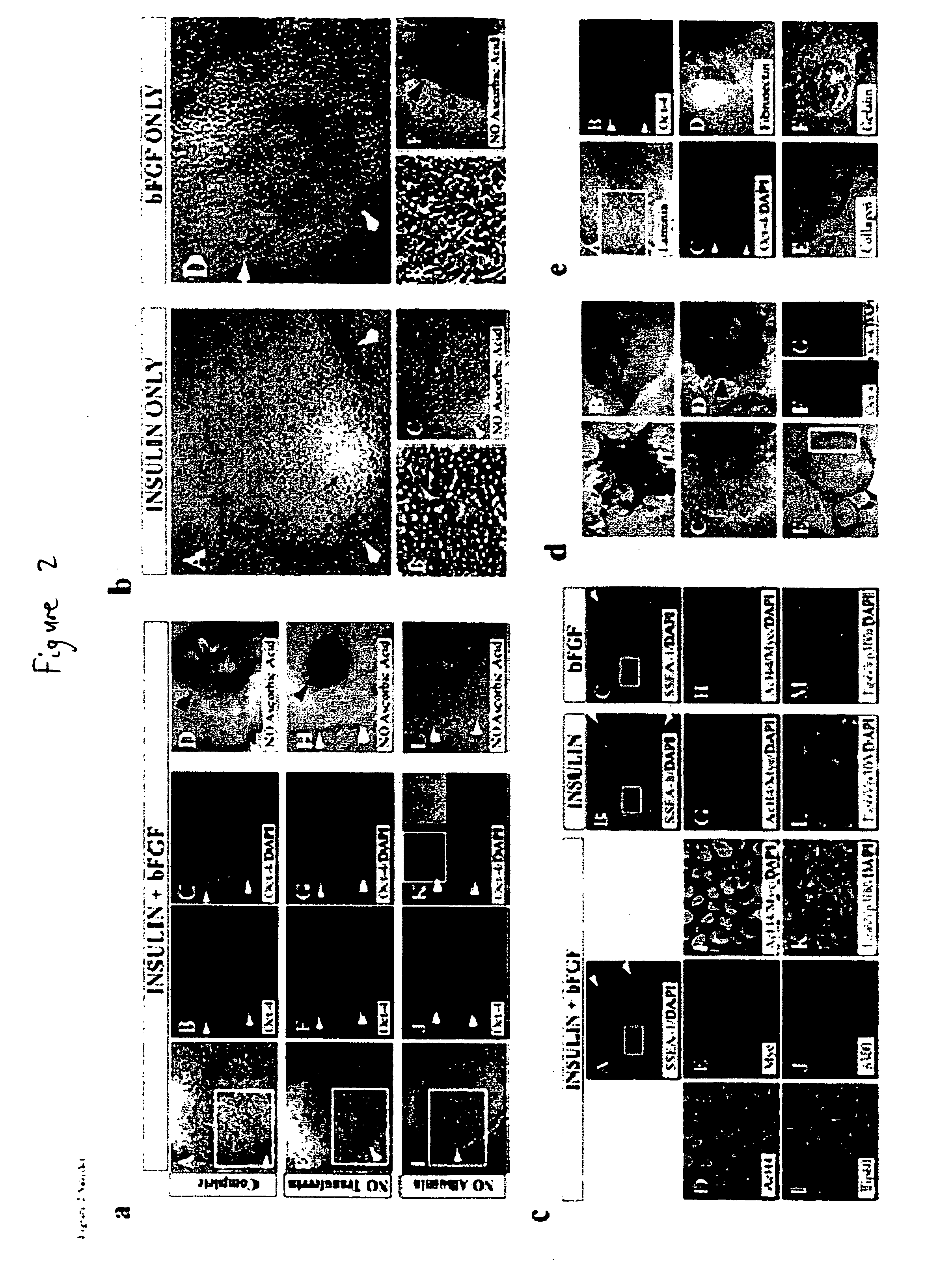
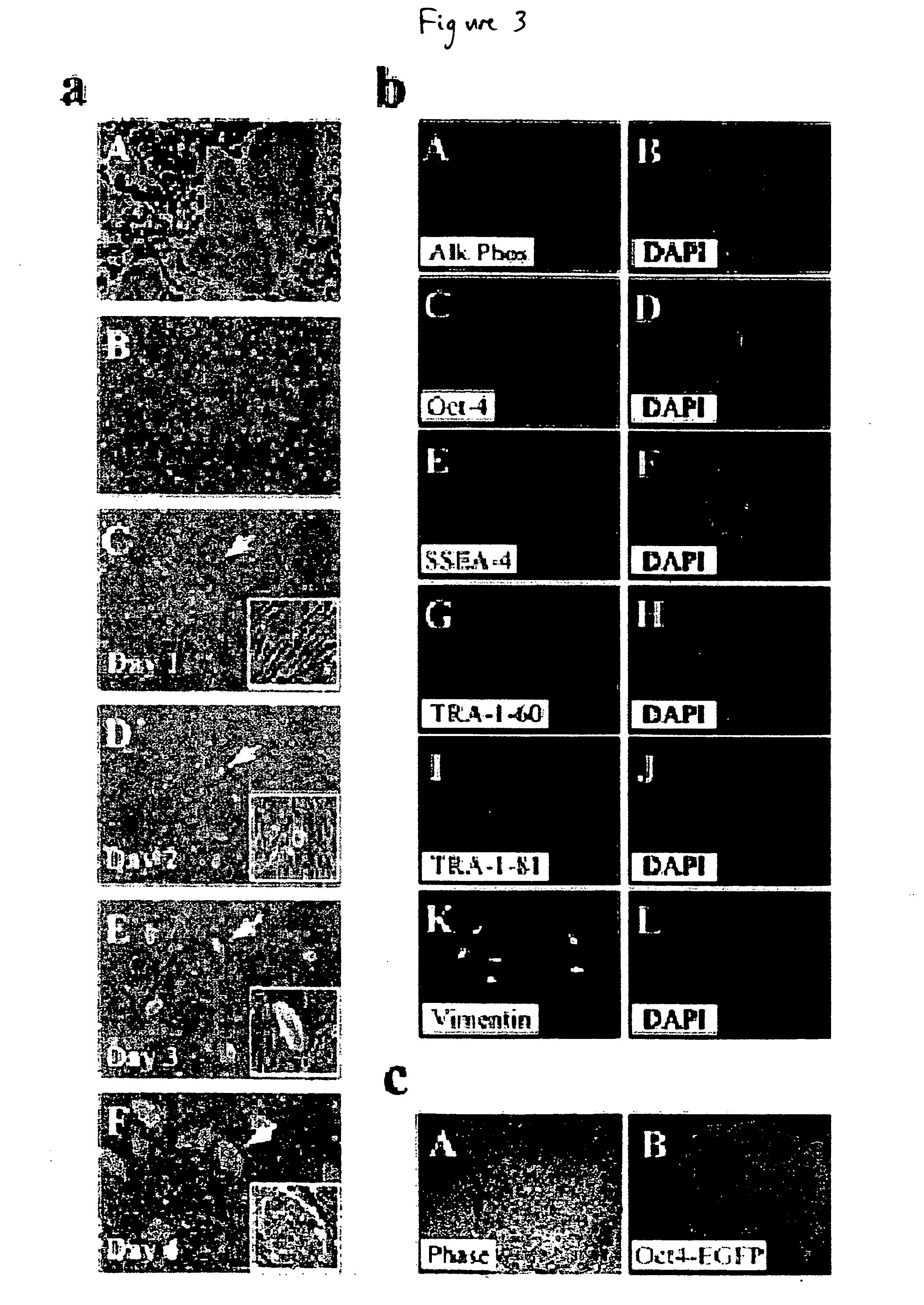
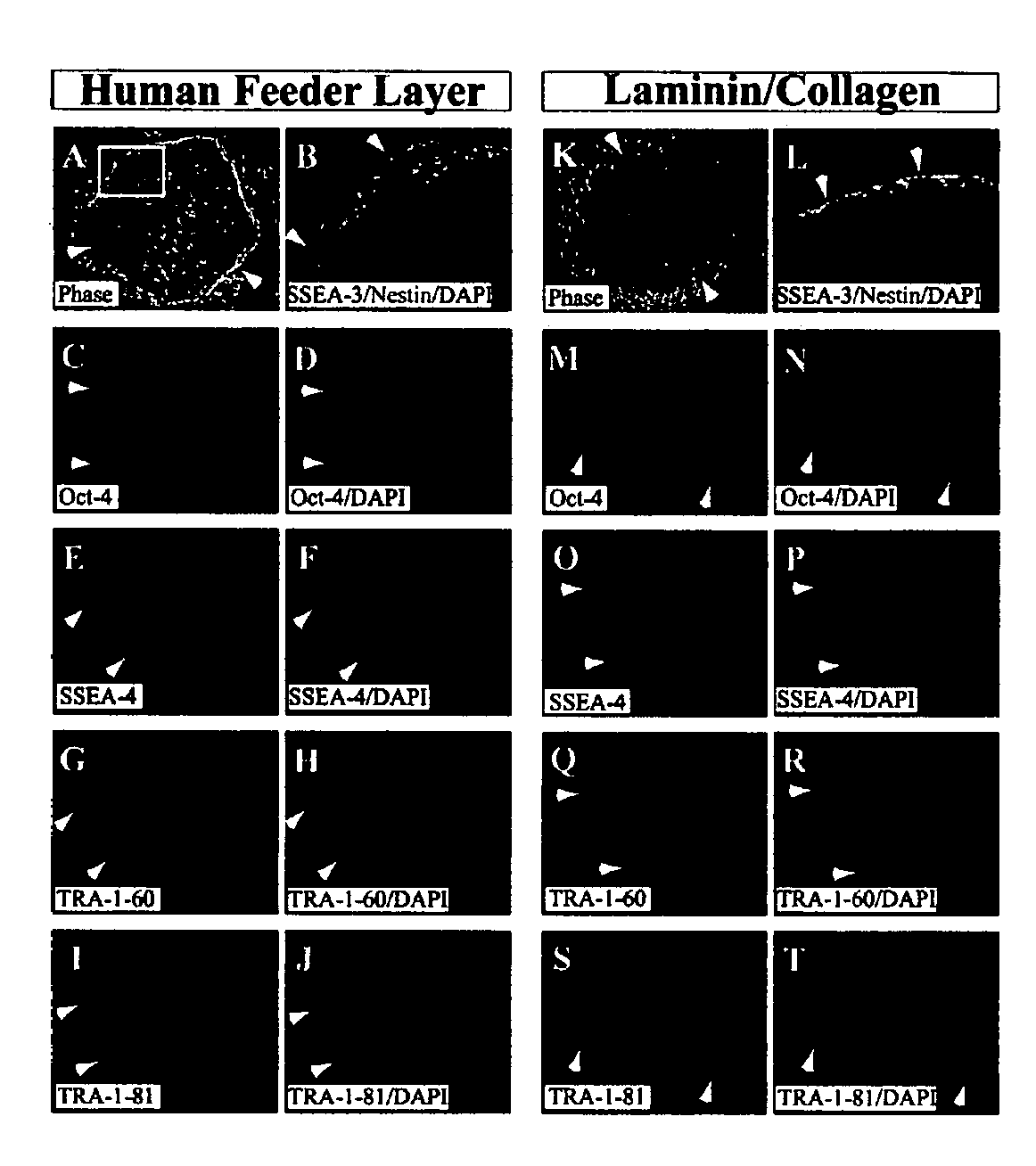
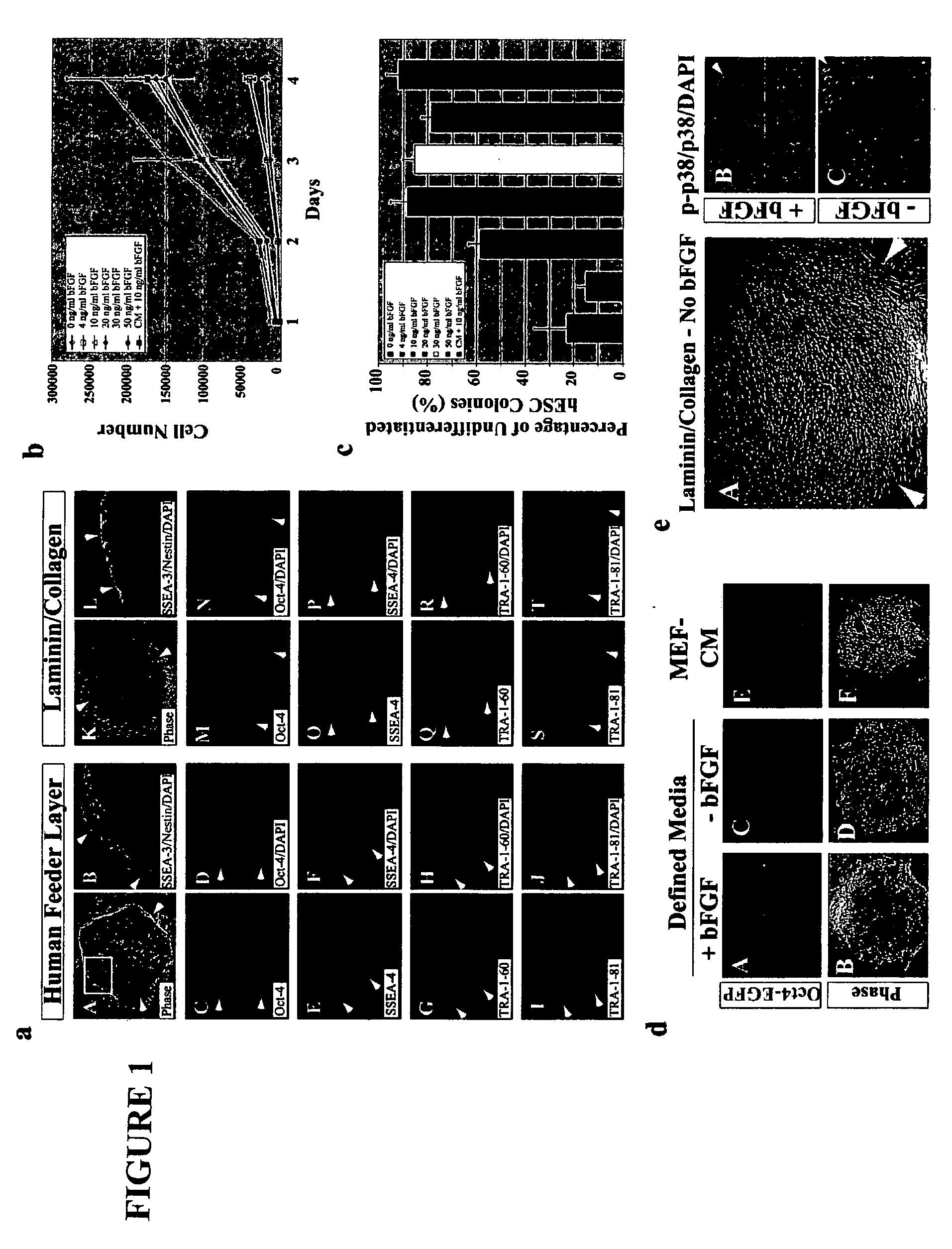
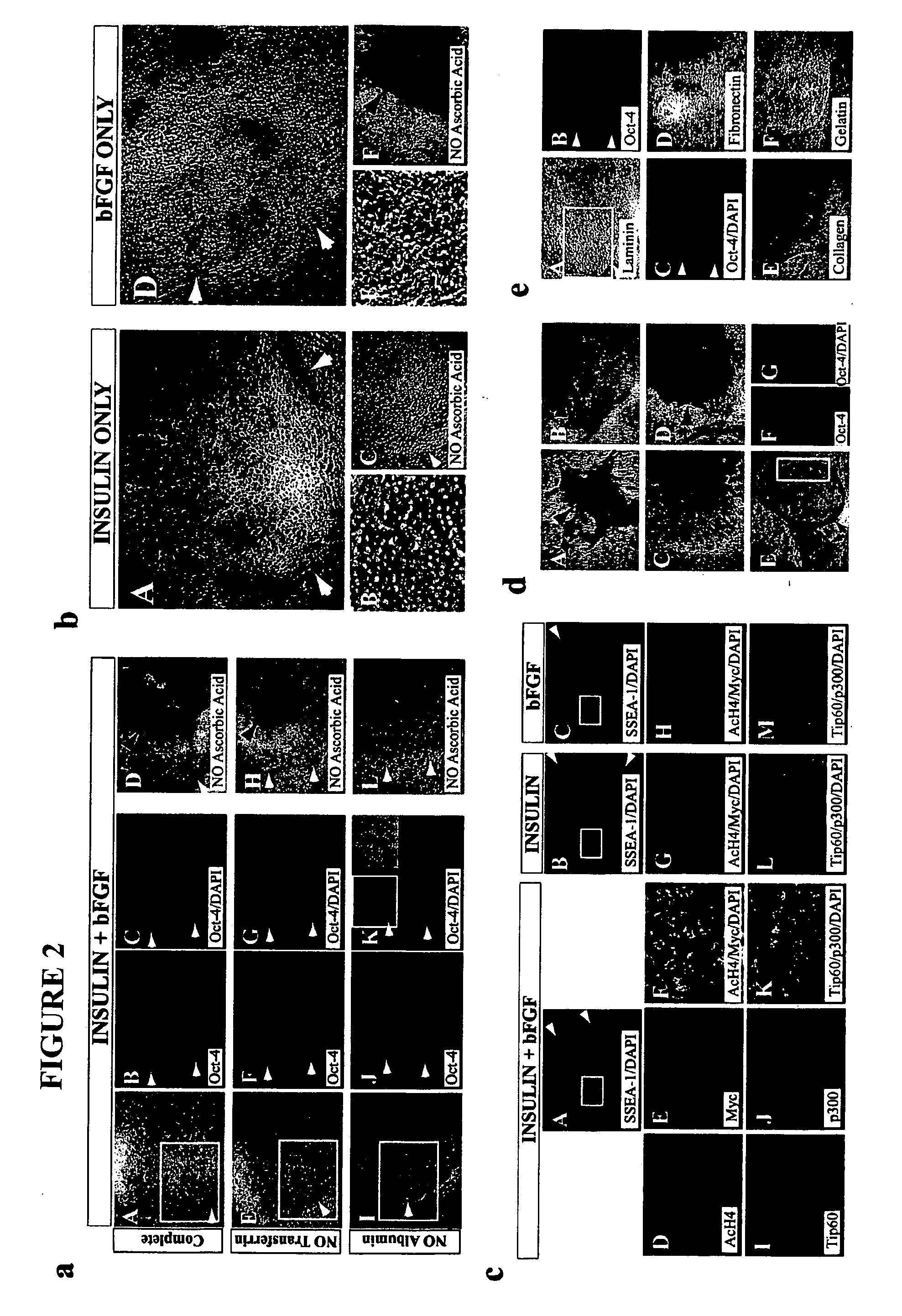
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Method of preparing kakadu plum powder
ActiveUS20050163880A1High viscosityImpede efficient processingBiocideUnknown materialsAbsorption capacityUltrafiltration
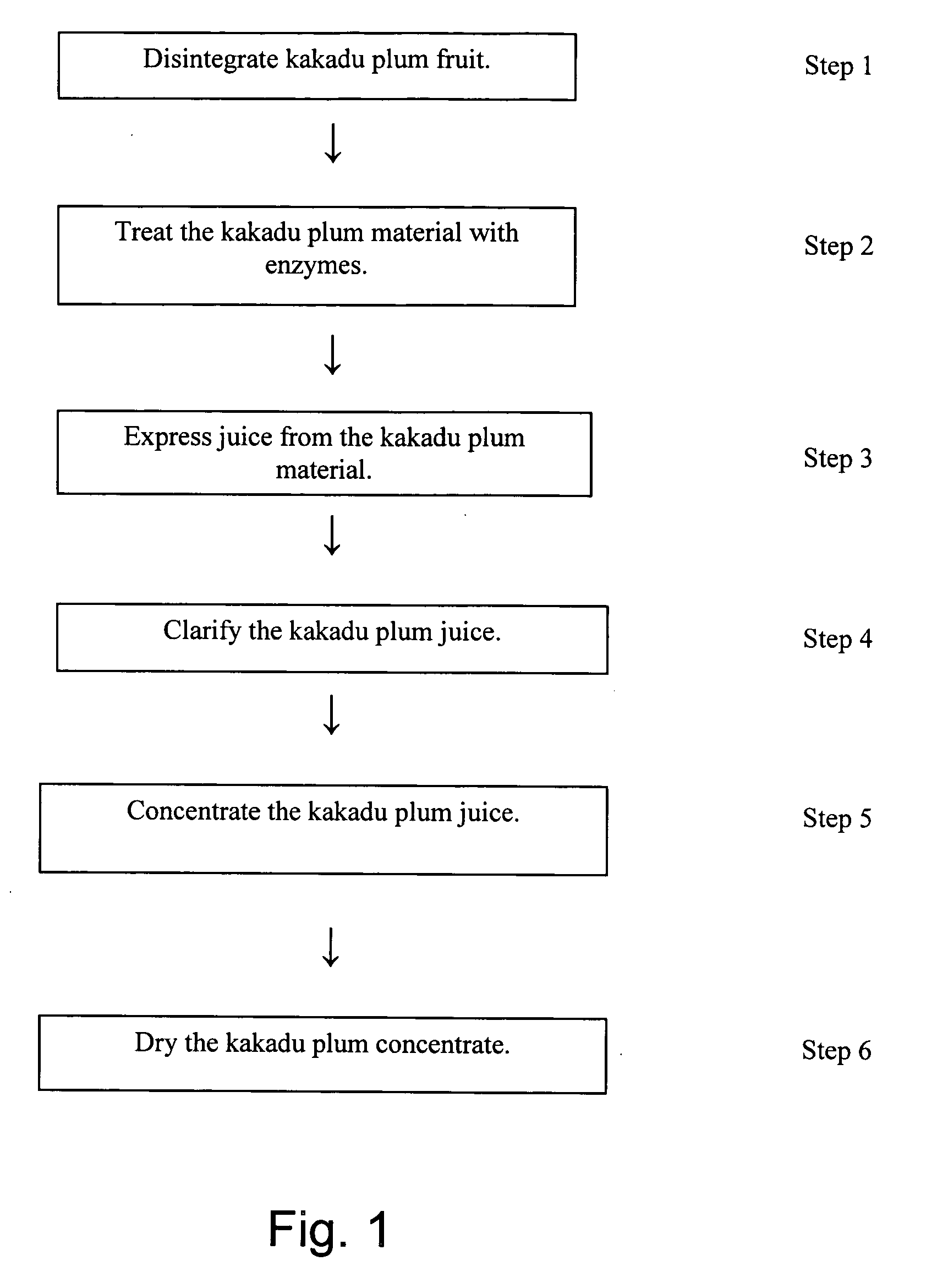
A process for producing a kakadu plum powder having an increased amount of naturally occurring ascorbic acid and high ORAC value. The process of preparing the extract includes the following: disintegrating kakadu plum fruit; treating the disintegrated kakadu plum material with enzymes to at least partially digest the material; juicing the kakadu plum material and drying the juice to produce a powder. In a preferred embodiment, the kakadu plum juice is further clarified with ultrafiltration and concentrated by performing reverse osmosis on the kakadu plum juice. The resultant kakadu plum powder has a natural ascorbic acid content of at least about 15% and a naturally occurring Oxygen Reduction Absorption Capacity value of at least 1500.
Owner:ACCESS BUSINESS GRP INT LLC +1
Defined media for pluripotent stem cell culture
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
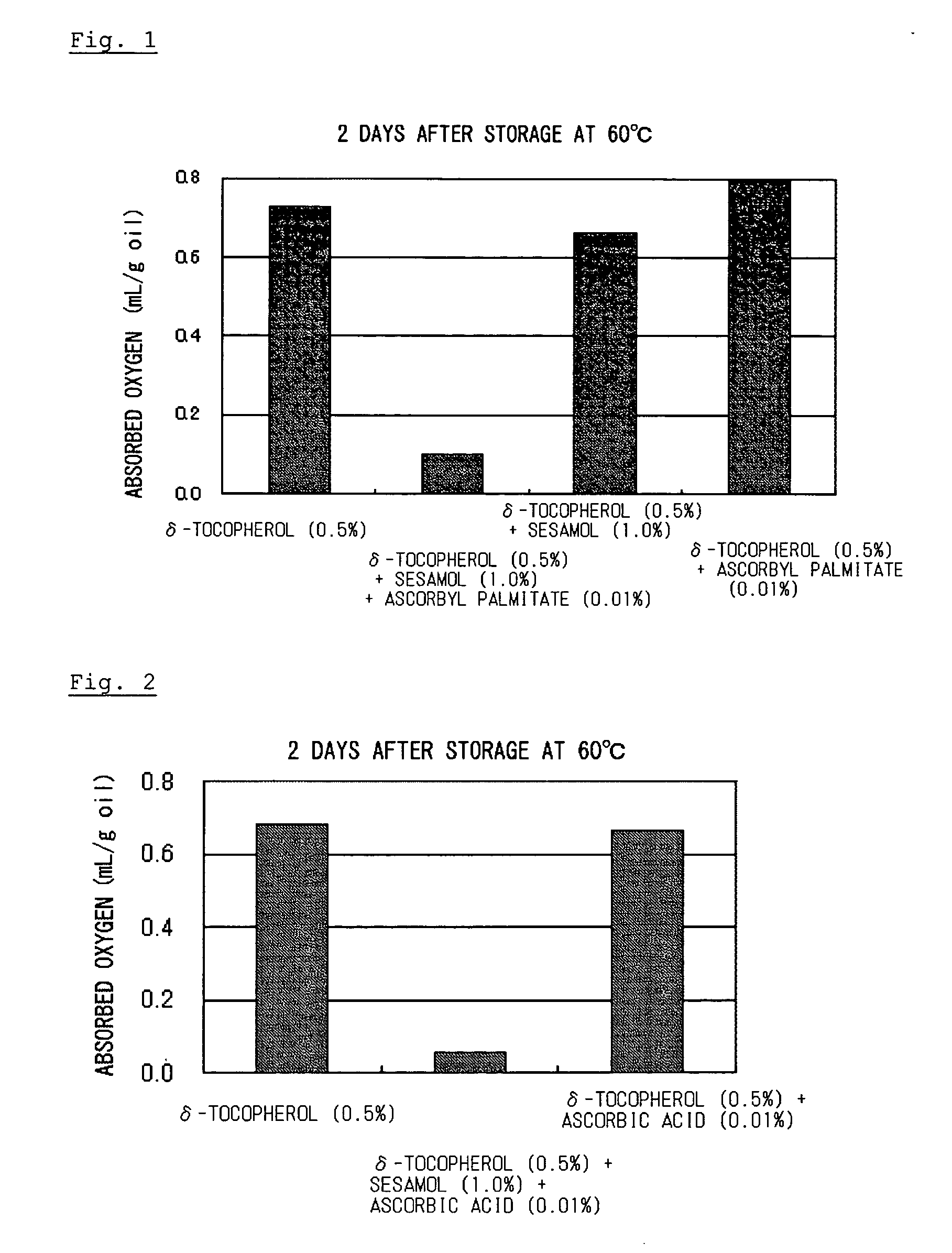
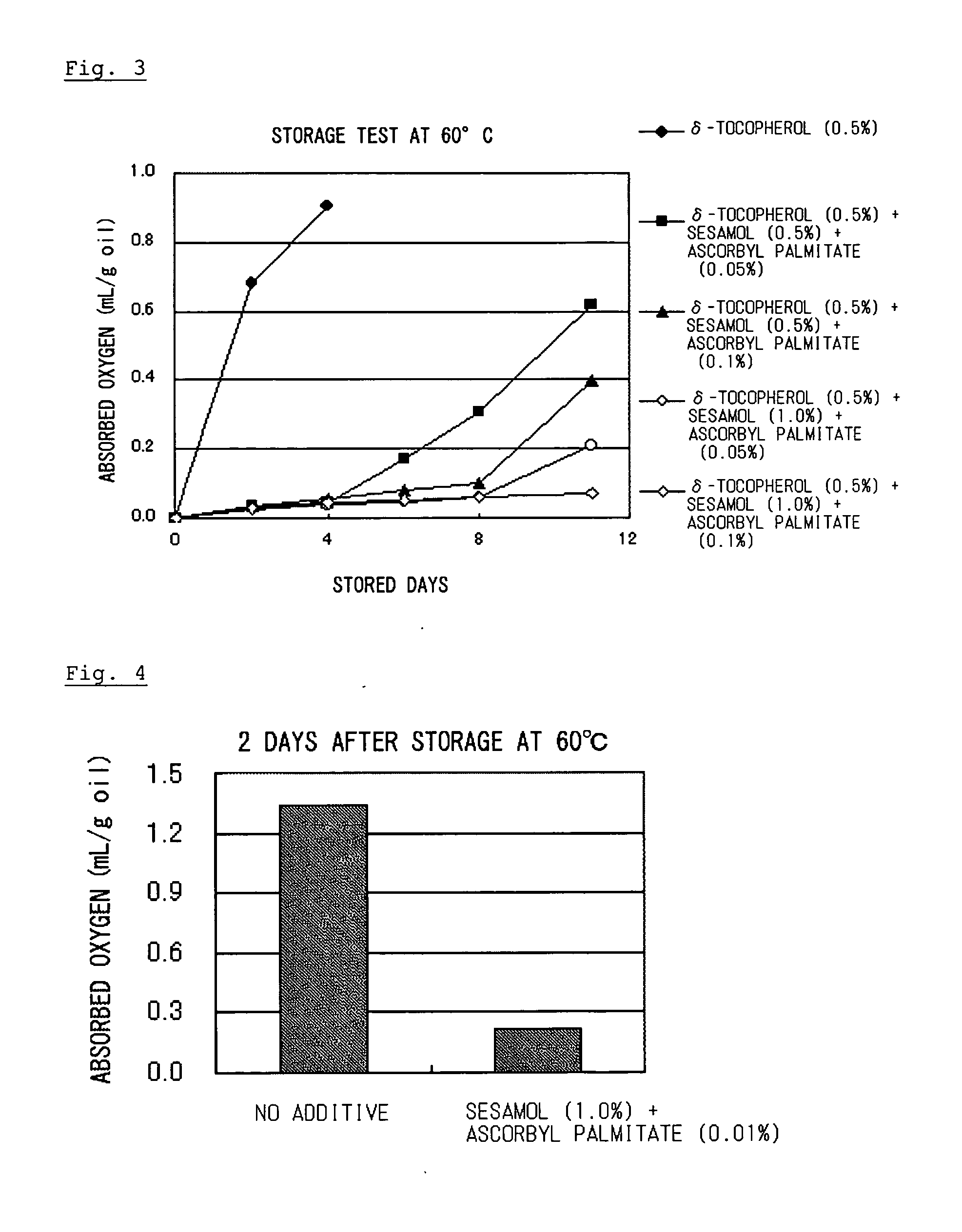
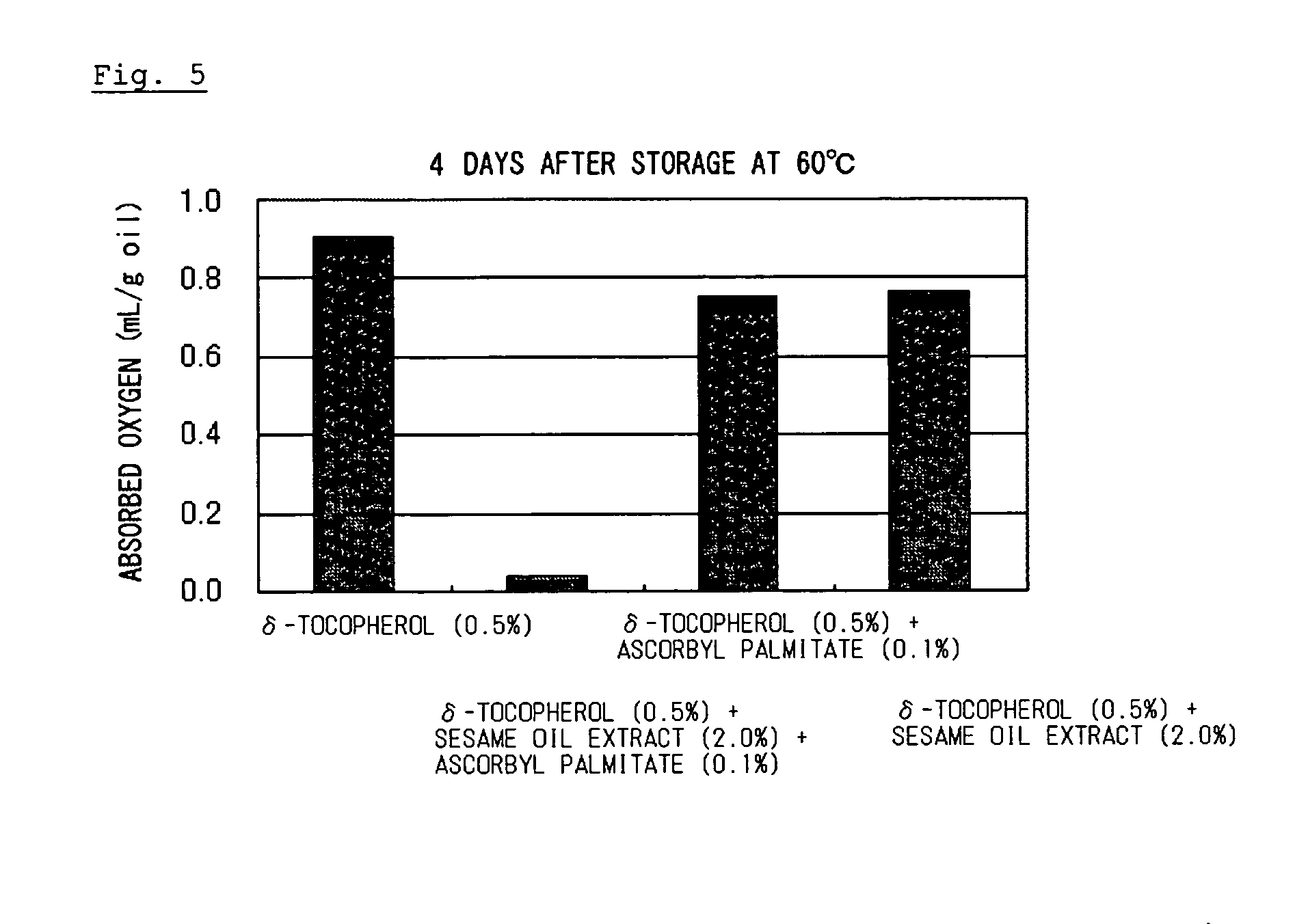
Composition containing organic substance having double bond with improved oxidative stability
ActiveUS20060134178A1Improve stabilitySafe antioxidantOrganic active ingredientsCosmetic preparationsSesamum orientaleGeneral purpose
To an organic substance having a double bond such as a polyunsaturated fatty acid was added an antioxidative component containing an antioxidative sesame component and ascorbic acid or an ascorbyl fatty acid ester. The above method provides a composition containing an organic substance having a double bond exhibiting enhanced oxidative stability. Particularly, it extremely improves oxidative stability of fat and oil which contains polyunsaturated fatty acid. General-purpose refined fish oil which is easy to handle can be provided for food, medicine or feed uses.
Owner:NIPPON SUISAN KAISHA LTD
Systems and methods for topical treatment with nitric oxide
InactiveUS7048951B1Reduce skin irritationBiocideInorganic active ingredientsSandwich likeTransdermal patch
A simple, biocompatible system and procedure for generating nitric oxide (NO) is described. A mixture of powdered sodium nitrite, ascorbic acid, and maleic acid (or another organic acid of adequate strength) immediately generates nitric oxide (NO) on treatment with water. To slow down the NO generation, one may prepare an ointment from a nonaqueous medium (petrolatum, Vaseline™) and the three powdered ingredients, which on being applied topically on the skin will release NO as water permeates through this medium; alternatively, one may convert the aqueous sodium nitrite solution into a gel with hydroxyethylcellulose (or other gel-forming compound) and combine this gel with another gel obtained from aqueous ascorbic and maleic acids with hydroxyethylcellulose for topical application (on intact skin, burns intra-cavity, burns, intra-cavity, etc.). The two gels may be admixed immediately before use (possibly from a single container with separate chambers and dual nozzle, via pushing or squeezing the two gels through the nozzle), or may be applied in sandwich-like fashion (possibly as a transdermal patch) for further slowing down the delivery of NO.
Owner:NITRIC SOLUTIONS
Method and composition to reduce cancer incidence
InactiveUS6090414AReduce distractionsInhibition formationBiocideTetrapeptide ingredientsHydroxybenzoate EthersS oxidation
The five component composition consisting essentially of: (1) Water soluble antioxidant vitamin C or ascorbic acid, or any of its forms or derivatives, or mixtures thereof. (2) Oil soluble antioxidant vitamin E or Alpha-tocophorol, or any of its forms or derivatives, or mixtures thereof. (3) The element selenium, or a chemical (or composition) containing it, or mixtures thereof. The most preferred chemical containing selenium is dimethyl selenide and mixtures thereof. The words "dimethyl selenide" here and hereinafter mean dimethyl selenide and / or it's oxidation products, including dimethyl selenoxide. (4) A sulfur amino acid, in any form, or a sulfur peptide, or a sulfur protein, or any of their derivatives, or mixtures thereof. The mixture of methionine and cysteine, which contains as impurities some seleno-methionine and some selenocysteine, is preferred,-the tripeptide glutathione containing cysteine is also preferred. (5) Another antioxidant, other than vitamin C and other than vitamin E, which is synthetic or natural and water soluble or oil soluble, or a mixture of such antioxidants, or a combination of such forms thereof. The mixtures of butylated hydroxyanisole and ethoxyquin is preferred.
Owner:LIFE SCI LAB
Biodegradable activators to gel silica sol for blocking permeability
ActiveUS20130292120A1Risk of damageDrilling compositionSealing/packingButanedioic acidSalicylic acid
A method of treating a treatment zone in a well to reduce the permeability of the treatment zone including the steps of: introducing into the treatment zone a water-based treatment fluid comprising: an aqueous silica sol; and a water-soluble chemical activator for gelling the silica sol, wherein the chemical activator is selected from the group consisting of: phytic acid, methylglycinediacetic acid, a water-soluble polyepoxysuccinic acid, salicylic acid, ascorbic acid, tannic acid, and an alkali metal salt or ammonium salt of any of the foregoing; and shutting in treatment zone for at least a sufficient time to allow the treatment fluid to in-situ form a solid gel at a design temperature for the method. Alternatively, a first treatment fluid including the aqueous silica sol and a second treatment fluid including the chemical activator can be introduced into the treatment zone separately, in any order.
Owner:HALLIBURTON ENERGY SERVICES INC
A combination of mitochondrial nutrients for relieving stress, preventing and improving stress-related disorders
InactiveUS20060257502A1Accelerated agingIncreasing oxidative metabolismBiocideCosmetic preparationsAlpha-TocopherolL-Carnosine
A dietary supplement of mitochondrial nutrients is designed for relieving stress, preventing and improving stress-related disorders, such as chronic fatigue syndrome, diabetes, age-associated cognitive dysfunction and diseases (Parkinson's and Alzheimer's disease). The supplement composition has the following nutrients: B vitamins (cyanocobalamin 2-1,000 ug, thiamin 1-1,000 mg, niacin 15-2,000 mg, pyridoxine 1-1,000 mg, Pantothenate 5-150 mg, folic acid 400-40,000 ug), alpha-tocopherol 10-800 mg, ascorbic acid 50-10,000 mg, calcium 20-2,000 mg, vitamin A 200-10,000 ug, alpha-lipoic acid 100-1,000 mg, N-acetyl cysteine 100-3,000 mg, L-carnosine 100-9,000 mg, tyrosine 100-9,000 mg, vanillin 10-100 mg, phosphatidylserine 10-800 mg, resveratrol 10-50 mg, dehydroepiandrosterone 1-50 mg, and melatonin 0.1-3 mg, all of which have been individually used experimentally or clinically for relieving stress, preventing and treating age- and stress-related disorders and diseases but no combination of these compounds has been used. Many embodiments also contain at least one adjunct ingredient such as coenzyme Q 10-200 mg, acetyl-L-carnitine 100-2,000 mg, choline 50-1,000 mg, and creatine 100-2,000 mg.
Owner:LIU JIANKANG
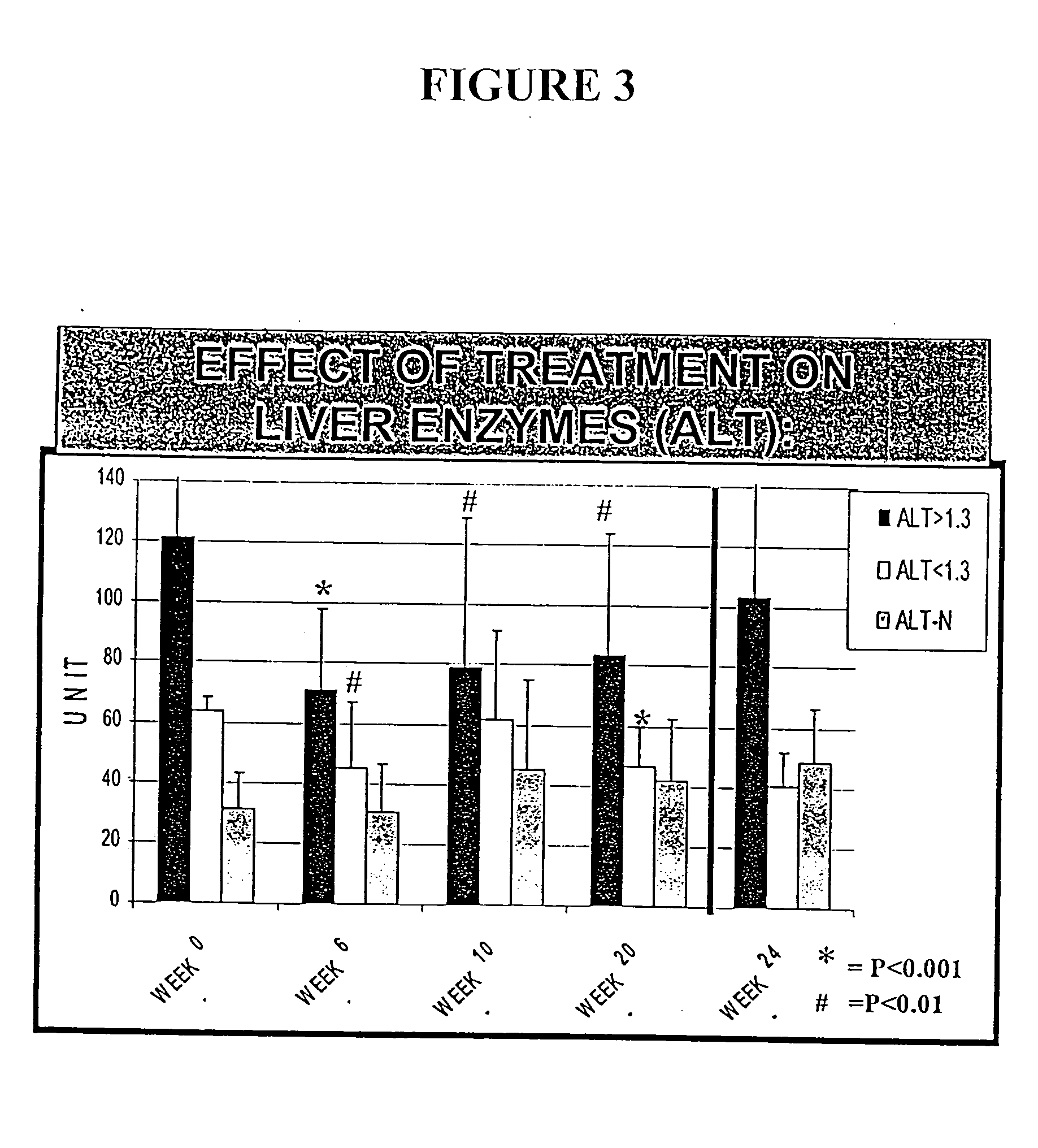
Compositions and methods useful for treating and preventing chronic liver disease, chronic HCV infection and non-alcoholic steatohepatitis
InactiveUS20050123628A1Reduce oxidative stressReduce lipid peroxidationBiocideDipeptide ingredientsChronic viral hepatitis CLipid peroxidation
The invention relates generally to compositions comprising antioxidants useful for reducing oxidative stress and lipid peroxidation, and treating chronic liver disease, chronic hepatitis C virus infection and non-alcoholic steatohepatitis. In particular, the invention relates to the preparation and oral administration of compositions comprising glycyrrhizin, schisandra, ascorbic acid, L-glutathione, silymarin, lipoic acid, and d-alpha-tocopherol. The invention also relates to the preparation and parenteral administration of compositions comprising glycyrrhizin, ascorbic acid, L-glutathione, and vitamin B-complex, preferably by infusion or intravenous injection. The invention further relates to methods of using the antioxidants, oral compositions and parenteral compositions.
Owner:ZABRECKY GEORGE
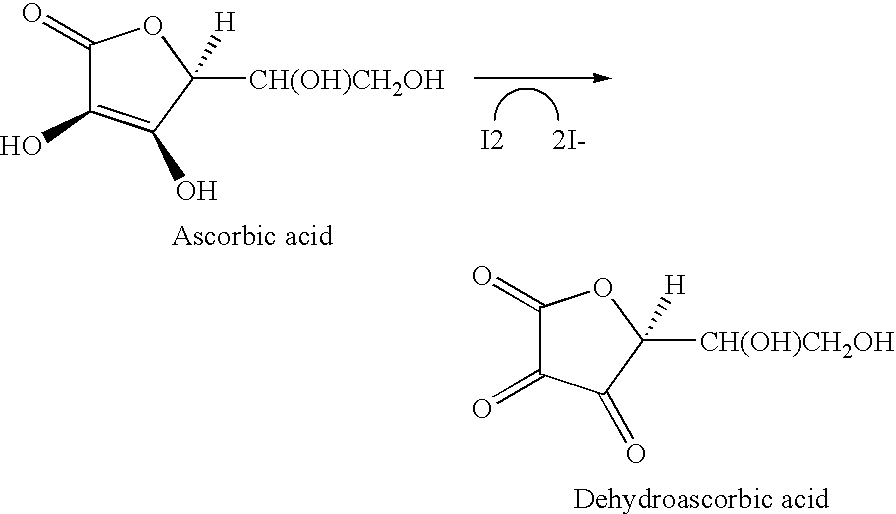
Stabilization of ascorbic acid, ascorbic acid derivatives and/or extracts containing ascorbic acid for topical use
InactiveUSRE38623E1Effective and stable mannerStable composition and pharmaceutical delivery systemBiocideCosmetic preparationsWrinkle skinSkin color
A stable composition is provided to treat and / or prevent photo-aged skin and related skin disorders such as sunburn, wrinkles, poor skin tone and skin discoloration by topically applying to the skin the treatment composition containing an effective amount of a compound such as ascorbic acid, derivatives of ascorbic acid and / or extracts containing ascorbic acid, in a pharmaceutically acceptable vehicle containing a substantially anhydrous base having no water added. The anhydrous base stabilizes the compound, so that the compound remains effective for an effective period of time, even in the presence of exposure to water.
Owner:TOPIX PHARMA INC
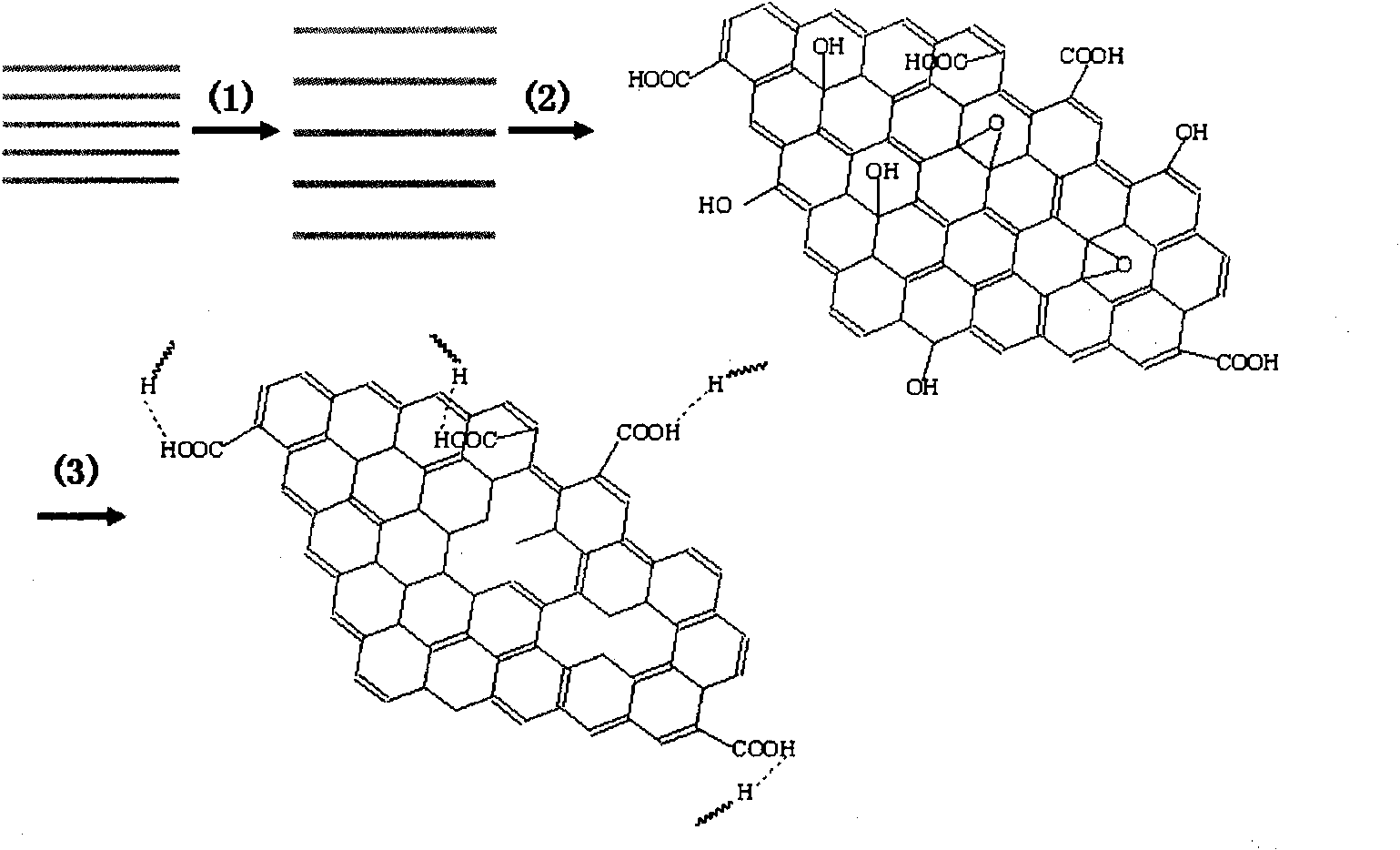
Preparation method of graphene based on ascorbic acid
ActiveCN101602504AEase of mass productionShort reaction timeNanostructure manufactureMaterials scienceReducing agent
The invention relates to a preparation method of graphene based on ascorbic acid, belonging to the technical field of nano materials. The preparation method comprises the following steps: preparing brown graphite suspending liquid; preparing luminous yellow graphite diluent; preparing graphite oxide solid; dispersing graphite oxide into deionized water, separating graphene oxide by ultrasonic separation treatment for 1 hour and preparing graphene oxide solution; and fully mixing the graphene oxide solution and ascorbic acid water solution, stewing and obtaining the graphene solution. The preparation adopts the ascorbic acid as a reducing agent, shortens reaction time under the condition that no stabilizing agent is added, obtains single-layer grapheme with the thickness of 0.8 to 1.2nm and is beneficial to batch production of the grapheme.
Owner:上海碳源汇谷新材料科技有限公司
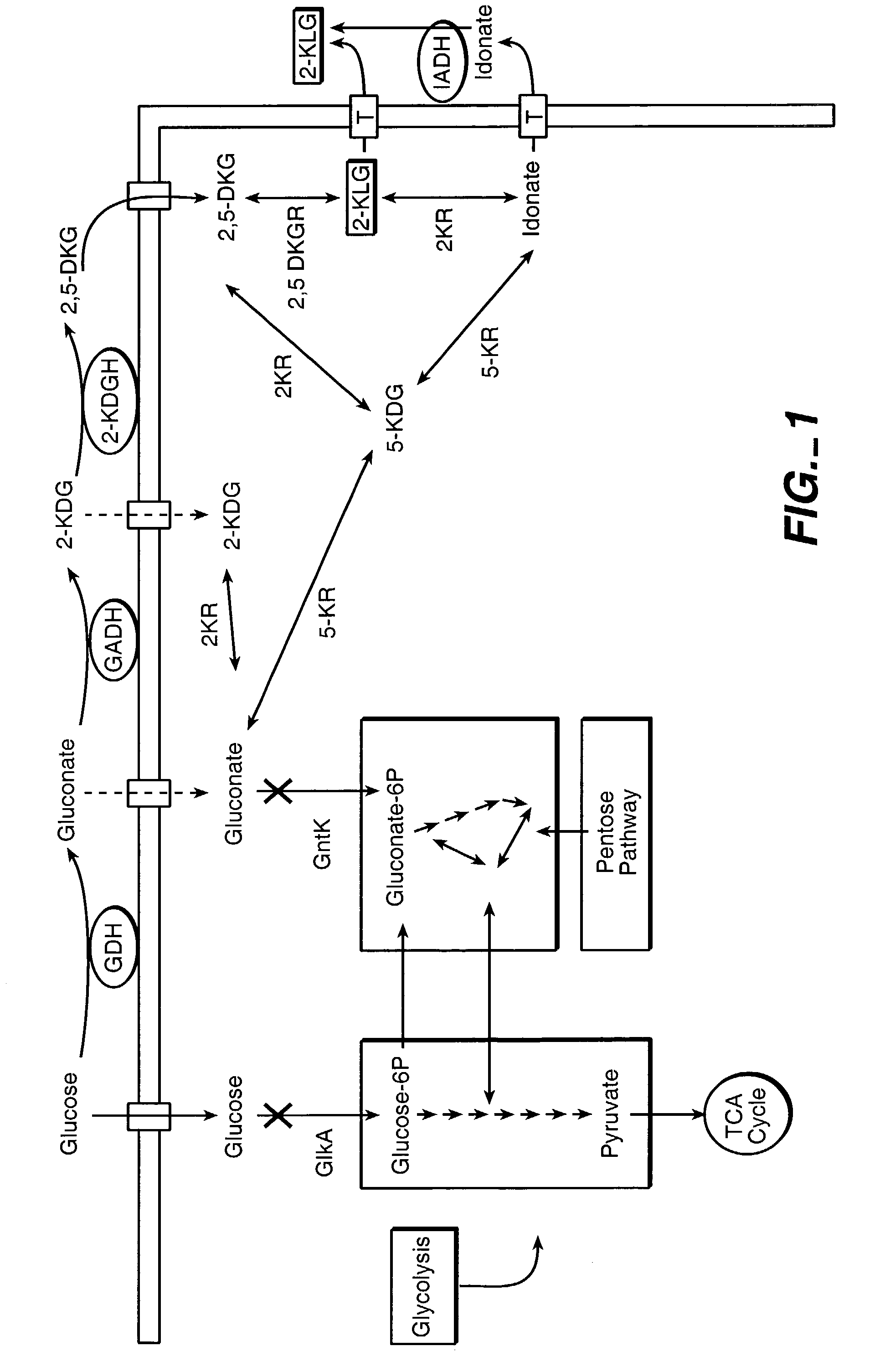
Method of uncoupling the catabolic pathway of glycolysis from the oxidative membrane bound pathway of glucose conversion
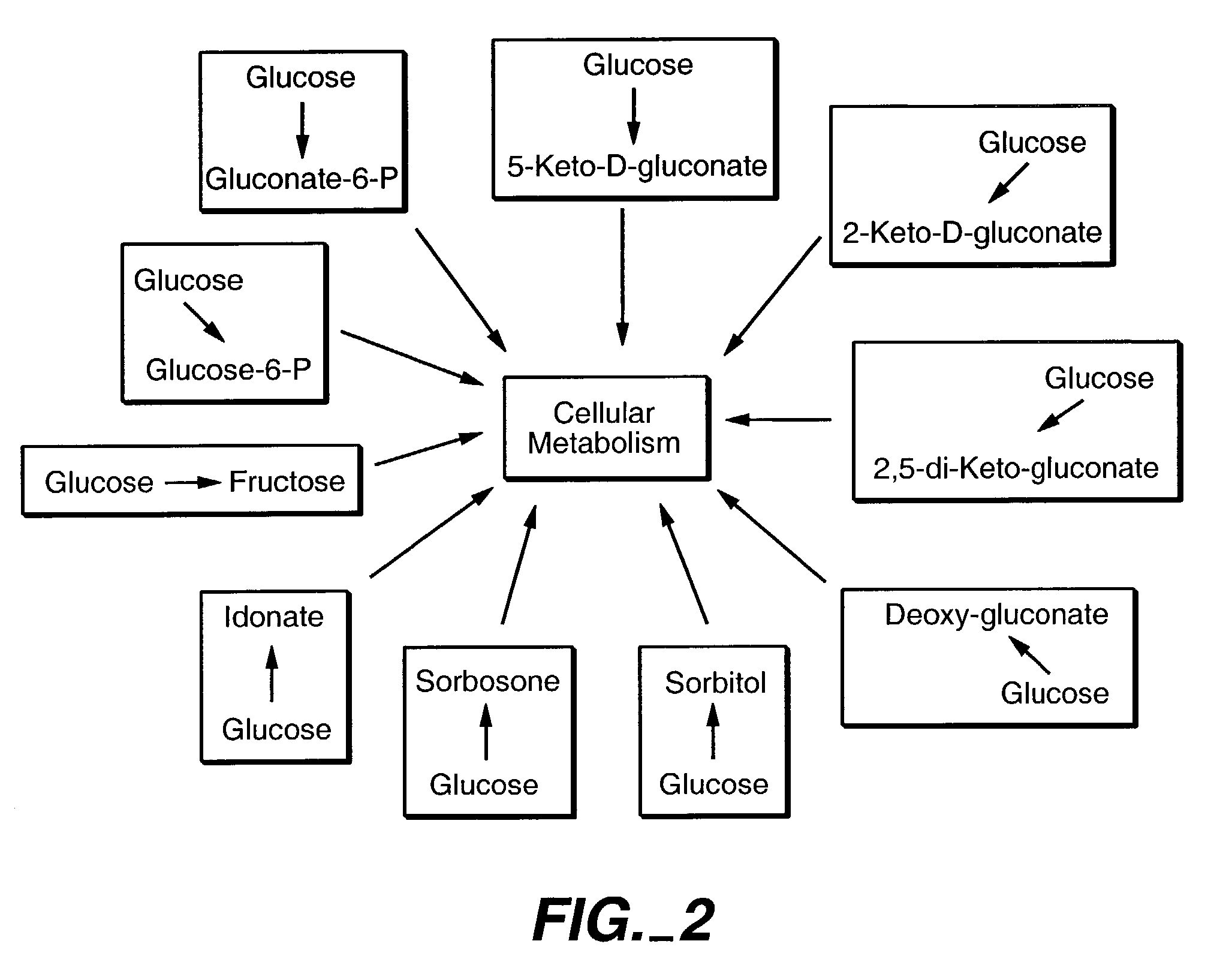
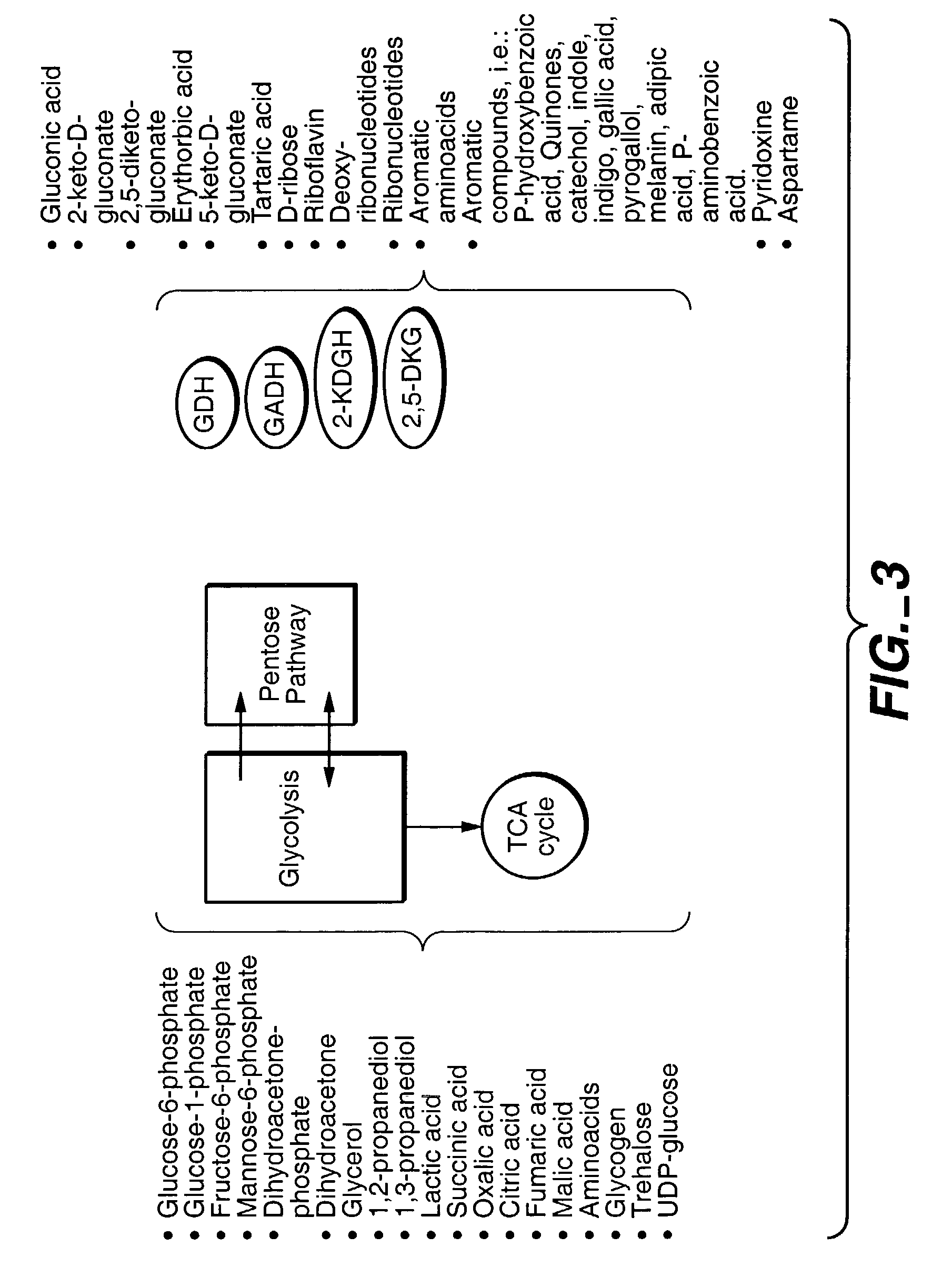
InactiveUS7241587B2Increase volumeMeet actual needsBacteriaSugar derivativesPhosphorylationGluconic acid
The invention provides methods for producing products comprising improved host cells genetically engineered to have uncoupled productive and catabolic pathways. In particular, the present invention provides host cells having a modification in nucleic acid encoding an endogenous enzymatic activity that phosphorylates D-glucose at its 6th carbon and / or a modification of nucleic acid encoding an enzymatic activity that phosphorylates D-gluconate at its 6th carbon. Such improved host cells are used for the production of products, such as, ascorbic acid intermediates. Methods for making and using the improved host cells are provided. Nucleic acid and amino acid sequences for glucokinase and gluconokinase are provided.
Owner:GENENCOR INT INC
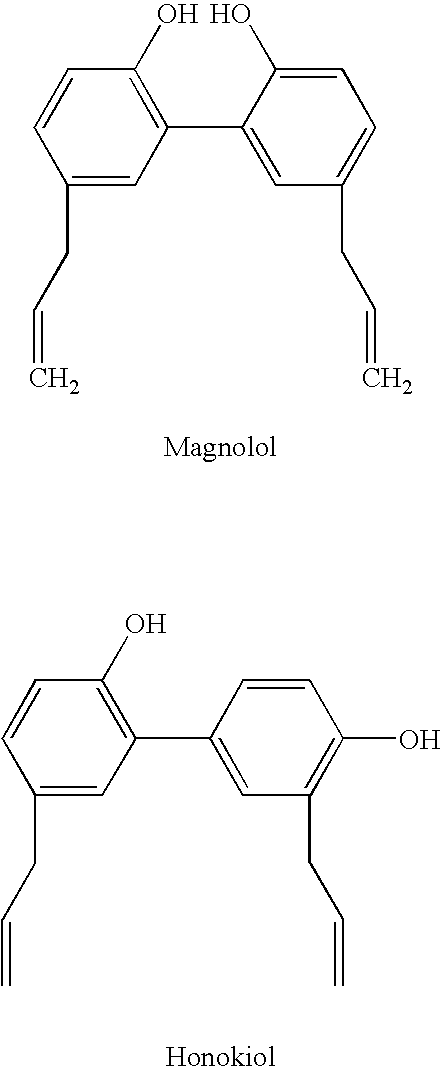
Magnolia extract containing compositions
ActiveUS20080260869A1Increase of collagen synthesisIncrease productionCosmetic preparationsBiocideCentella asiatica extractDipeptide
Disclosed is a composition comprising at least two of the following ingredients: Magnolia extract, honokiol, humulus lupulus extract, hesperidin methyl chalcone, gotu kola, dipeptide valyl-tryptophane, palmitoyl tetrapeptide-3, corylus avellana bud extract, centella asiatica extract, cucumis sativa extract, morus alba extract, hibiscus sabdariffa flower extract, vitis vinifera extract, ascorbyl glucoside, citrus medica limonum extract, avena sativa kernel extract, hydrolyzed soy protein, aniseed myrtle extract, tasmania lanceolata leaf extract, artemisia abrotanum extract, or citrus grandis fruit extract or any combination thereof. Also disclosed are methods of treating skin conditions by topically applying the composition to skin.
Owner:MARY KAY INC
Compositions and polymer composites prepared from the same
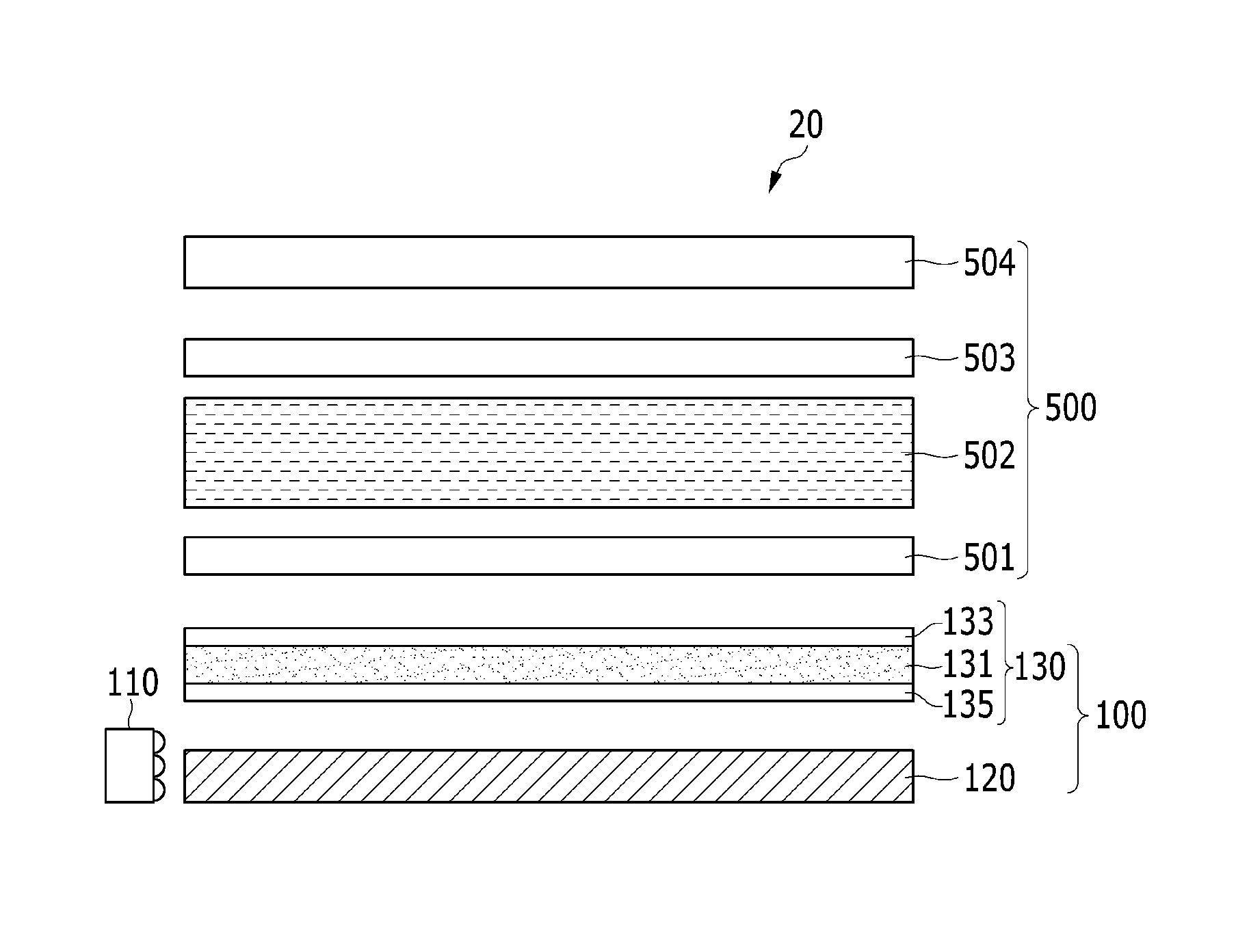
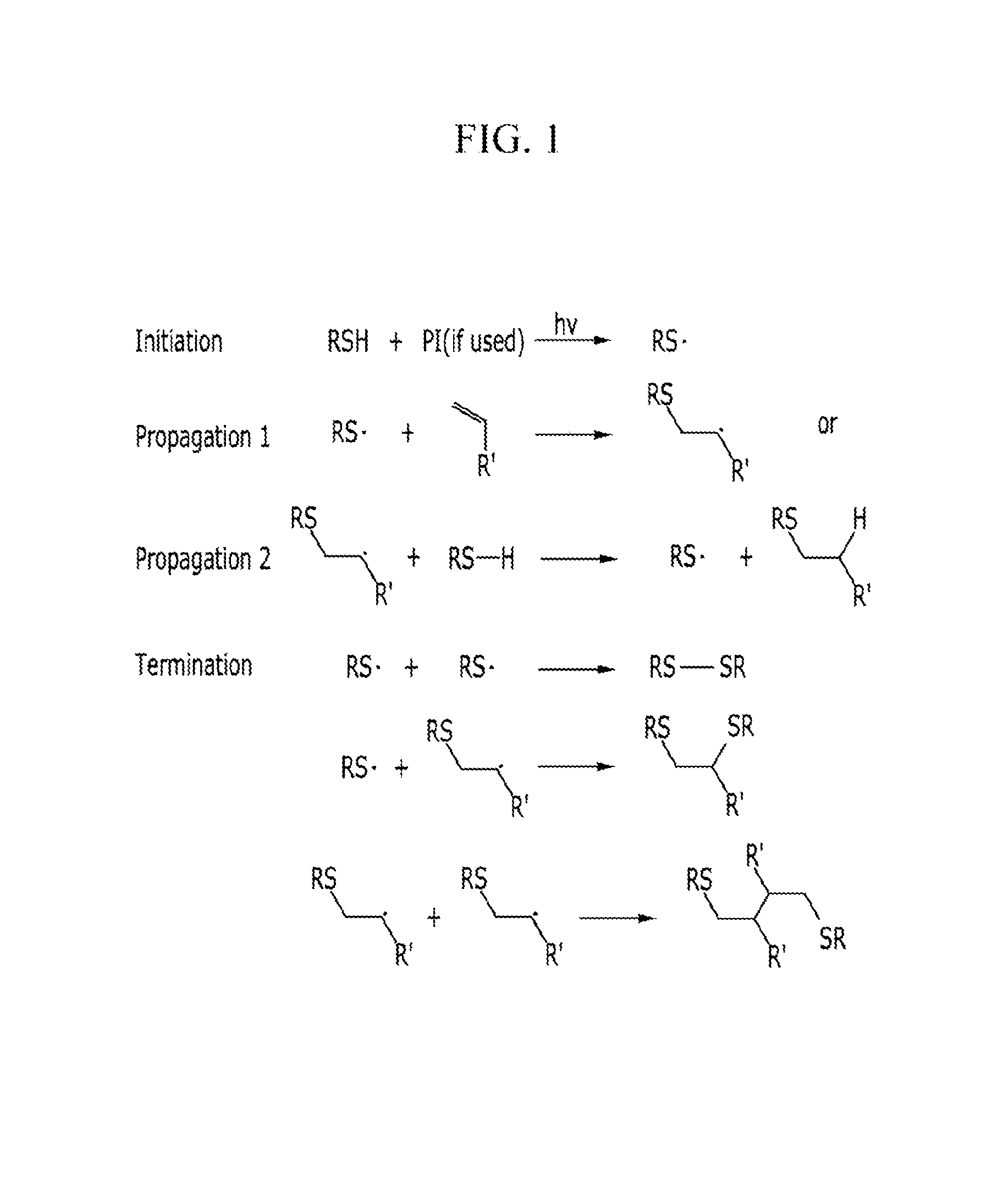
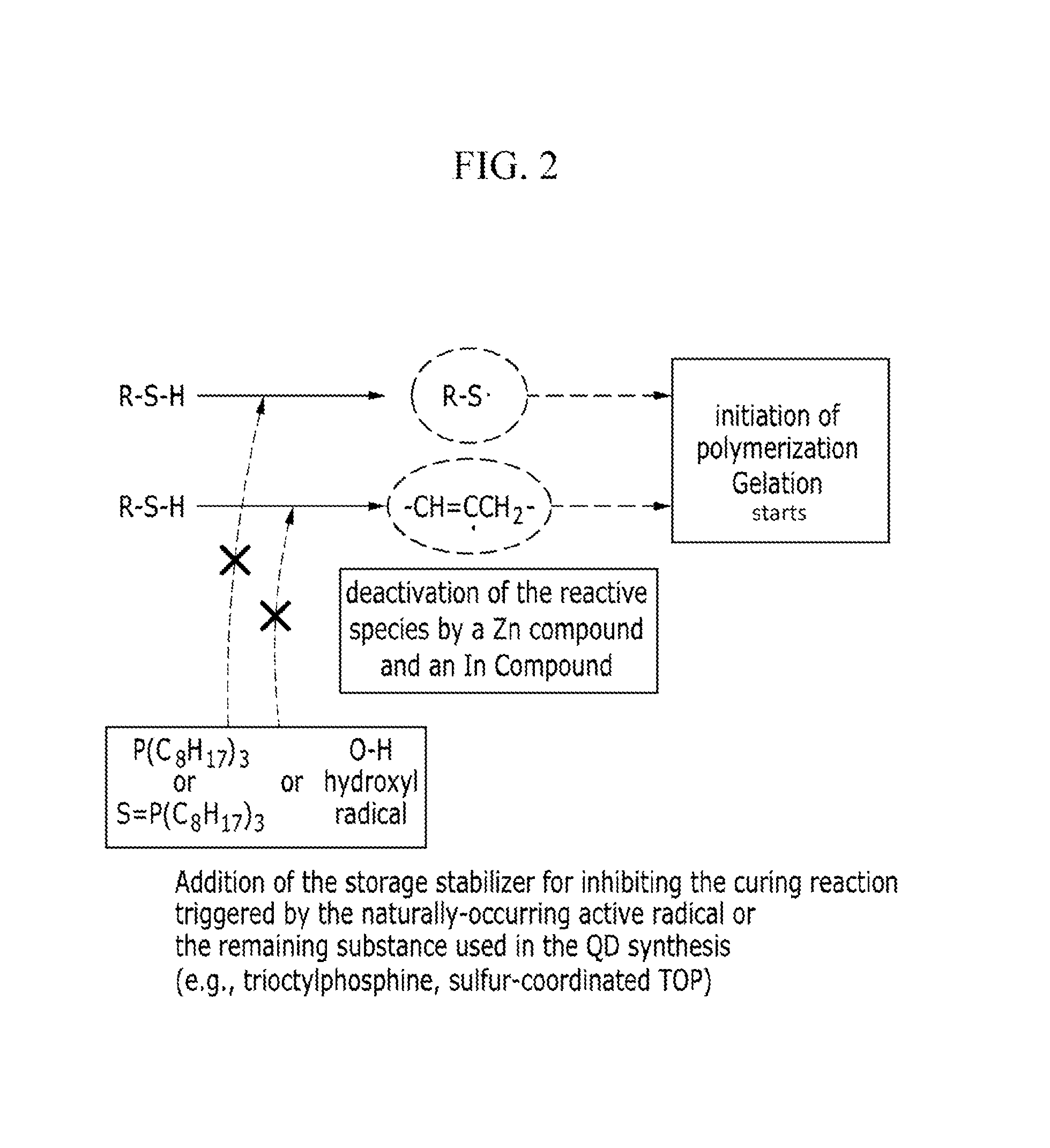
ActiveUS20160005932A1Good storage stabilityOther chemical processesSolid ballsZinc compoundsPolymer composites
A composition including: a monomer mixture including a first monomer having at least two thiol groups at its terminal end and a second monomer having at least two carbon-carbon unsaturated bond-containing groups at its terminal end; and at least one additive selected from a zinc compound, an indium compound, ascorbic acid or a salt thereof, citric acid or a salt thereof, a tocopherol, and a tocotrienol.
Owner:SAMSUNG ELECTRONICS CO LTD
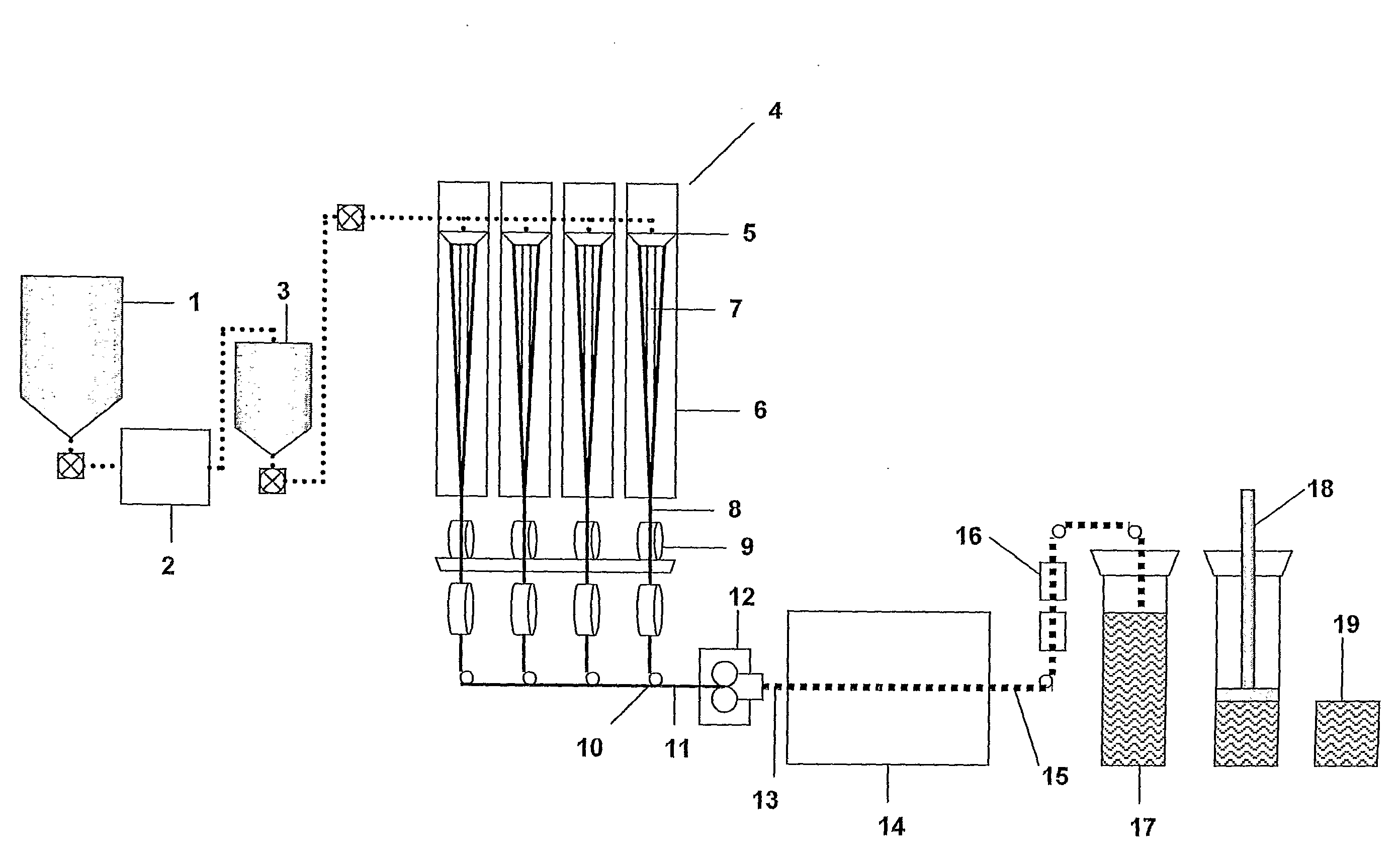
Method for preparing iron lithium phosphate by recovering water-system waste lithium-ion power battery
ActiveCN101916889AReduce manufacturing costSolving Recycling ProblemsWaste accumulators reclaimingBattery recyclingPower batteryIron salts
The invention discloses a method for preparing iron lithium phosphate by recovering water-system waste lithium-ion power batteries, comprising the following steps: 1) cutting and brushing a water-system waste lithium-ion power battery, processing by deionized water, sieving and drying to recover the mixture of an electrode material and a conductive agent; 2) adding inorganic acid to the dried mixture of the electrode material and the conductive agent to process, filtering to obtain acid solution containing Li+, Fe2+ and PO43-; 3) adding lithium salt or iron salt to the acid solution containing Li+, Fe2+ and PO43-, adding ascorbic acid and stirring, controlling the pH value to equal to 3-7, and filtering to obtain precipitation; and 4) adding the crude product LiFePO4 obtained in step 3) to a water solution of cane sugar for ball milling, drying and calcining to obtain a regenerative LiFePO4 material. The method has low cost, simple operation and no secondary pollution.
Owner:长春劲能科技集团有限公司
Oral hygiene products containing ascorbic acid and method of using the same
InactiveUS20080057007A1Efficient removalEffectively and efficiently protectCosmetic preparationsOrganic active ingredientsDiseaseAdditive ingredient
The present invention is directed to dental compositions, including dentifrices, containing ascorbic acid for removing and inhibiting dental biofilms which form plaque and tartar, and also for treating and preventing gingivitis and periodontitis. The ascorbic acid composition can contain may additional ingredients, including an enamel-strengthening component, and be used in many different forms, including breath spray, chewing gum, dental floss, dental powder, gargle, lozenge, mouth spray, mouth wash, tooth gel, tooth liquid, tooth paste and tooth strips. Also described in a method of using a dental composition containing ascorbic acid in order to treat plaque and tartar as well as gum disorders.
Owner:DENTECH
Compound biological antiseptic and preservation agent for food
The invention relates to a compound biological antiseptic and preservation agent for food. The compound biological antiseptic and preservation agent is prepared by evenly mixing pure biological solid preparations or products such as epsilon-polylysine, nisin, natamycin, lysozyme, bacillus subtilis, ascorbic acid, a toona sinensis leaf extract, a water-soluble resveratrol preparation, tea polyphenol and chitosan. The compound biological antiseptic and preservation agent has a strong inhibition effect on spoilage bacteria and pathogenic bacteria in various foods and good inhibition action on oxidative deterioration of the food. Compared with the existing various preservatives, the compound biological antiseptic and preservation agent has the characteristics of broad antibacterial spectrum, high safety, high efficiency and convenience in use.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
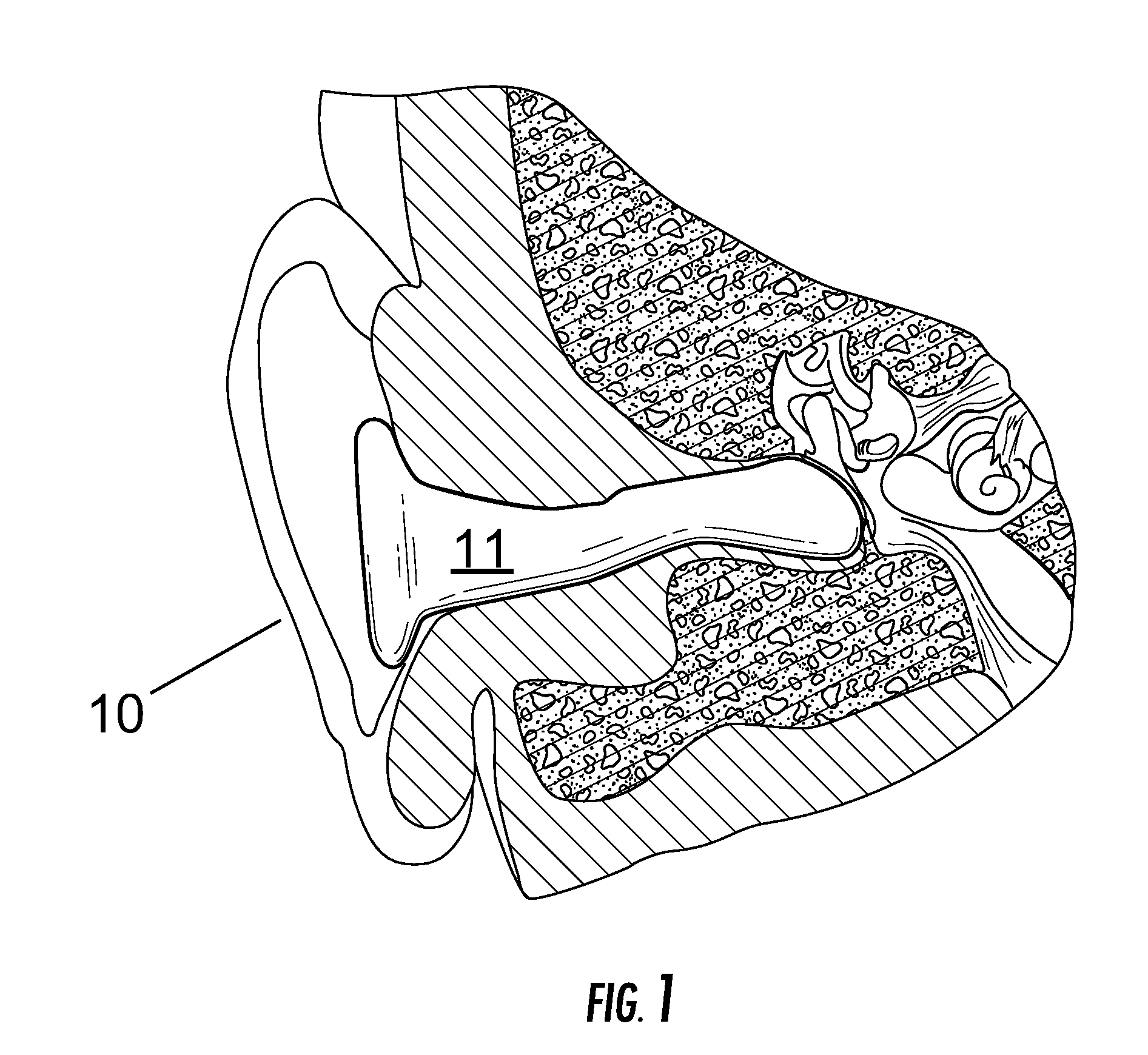
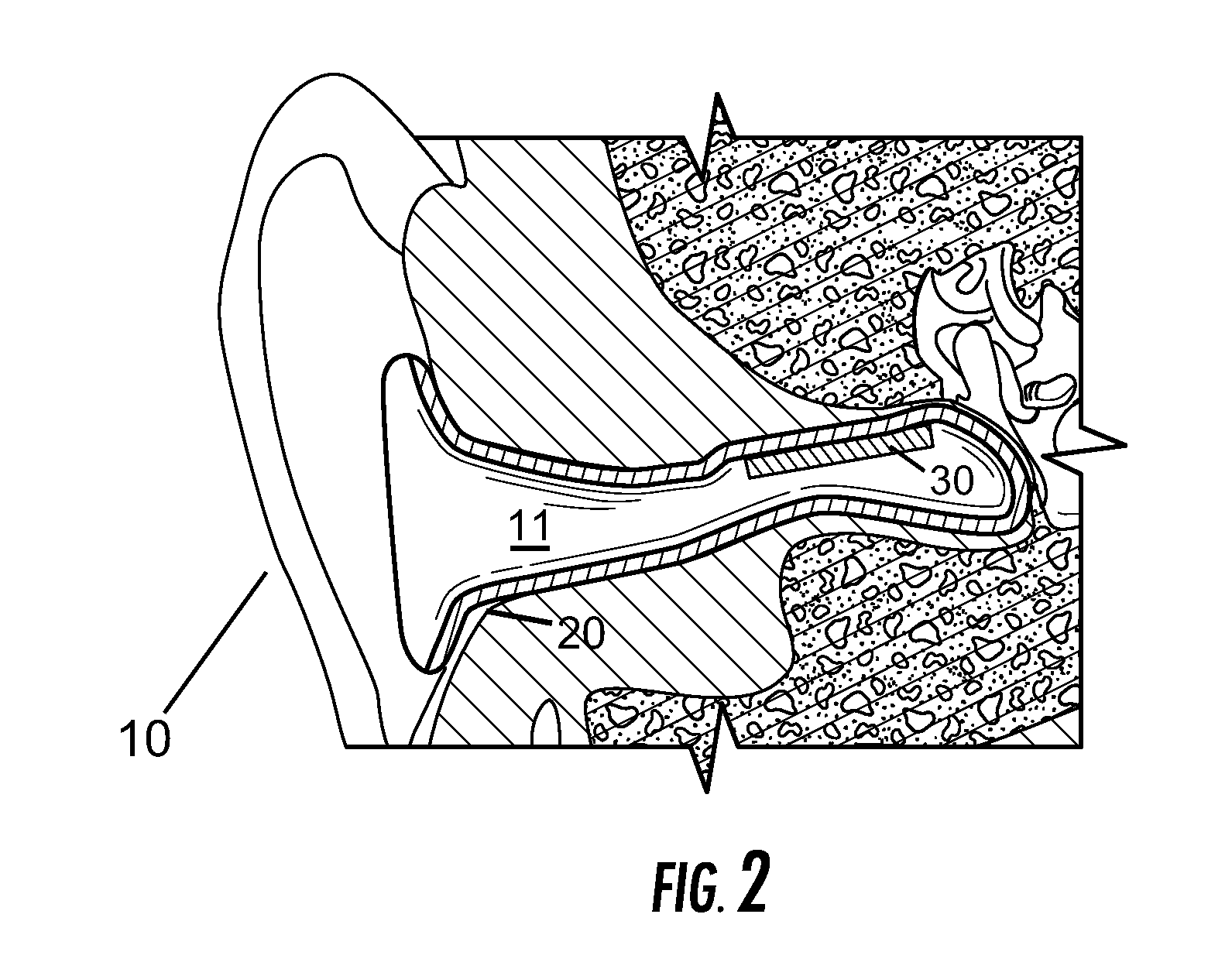
Vestibular stimulation apparatus and associated methods of use
ActiveUS20100211142A1Improve ventilationDevices for heating/cooling reflex pointsAdditive manufacturing apparatusHistamine ProductionVestibular system
A device and associated method for providing vestibular stimulation to an individual includes active elements positioned on or proximate an ear insert. The active elements include but are not limited to at least one electrode, at least one thermometer, and at least one thermoelectric transducer. The device includes a computerized control module regulating the active elements. The device incorporates an ear insert that allows the active elements to engage the individual's ear canal and therefore access the individual's vestibular system. Vestibular stimulation applied to the individual is customized for directly stimulating desired regions of the brain for therapeutic or diagnostic purposes. In a preferred embodiment, the device provides vestibular stimulation sufficient to promote physiological changes in the individual, the changes selected from the group consisting of circadian temperature cycle time shifts, ascorbic acid production, serotonin production, acetylcholine production, histamine production, and heat shock protein production.
Owner:SCION NEUROSTIM LLC
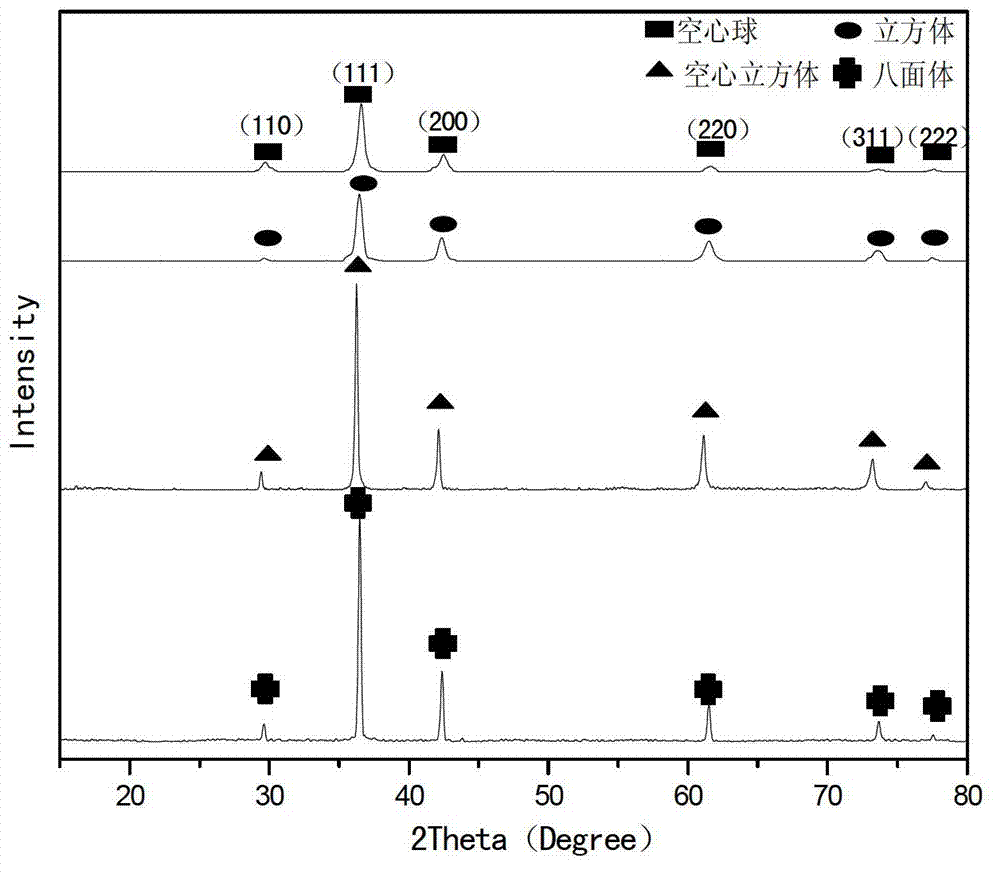
Preparation method of nano cuprous oxide
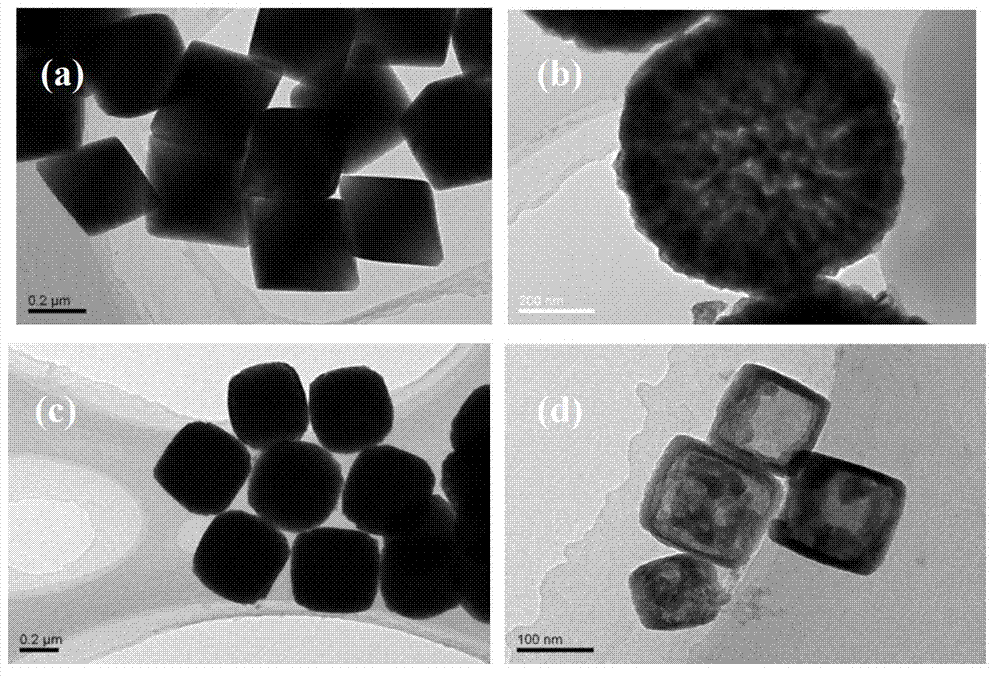
InactiveCN103172104AHigh purityNothing producedMaterial nanotechnologyCopper oxides/halidesOctahedronCopper nitrate
Owner:ZHEJIANG SCI-TECH UNIV
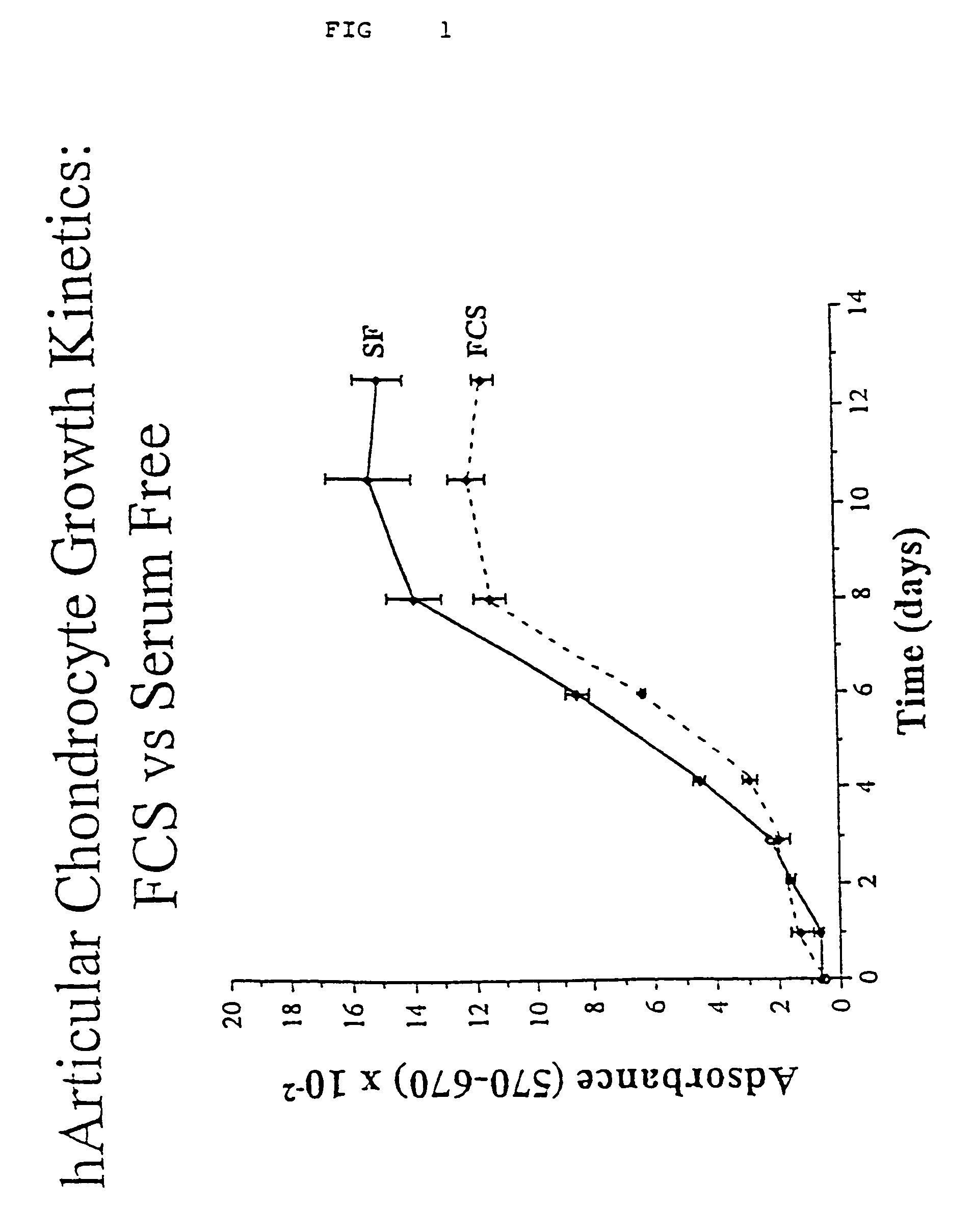
Serum-free medium for mesenchymal stem cells
Serum-free media for growth and proliferation of chondrocytes and mesenchymal stem cells in culture are provided. A serum-free medium for growth of chondrocytes includes a serum-free composition comprising FGF-2, linoleic acid, ascorbic acid, B-mercaptoethanol, transferrin and dexamethasone. Further, the composition comprises EGF, PDGFbb, insulin and albumin. A method for growing chondrocytes in a serum free medium comprising the compostion is also provided. Also provided for mesenchymal stem cell growth, is a serum-free medium which includes a composition comprising FGF-2, LIF, SCF, pantotenate, biotin and selenium and method, therefore.
Owner:CONSORZIO PER LA GESTIONE DEL CENT DI BIOTECNOLOGIA AVANZATA +1
Coating composition and article coated therewith
InactiveUS20050215670A1Improve corrosion resistancePlastic/resin/waxes insulatorsAnti-corrosive paintsEpoxyTriazole antifungals
A coating composition used for coating of a steel material and / or aluminum material comprises a corrosion inhibitor, a base resin and a curing agent. The corrosion inhibitor may be selected from cerium compounds, lanthanum compounds, molybdate salt compounds, gluconic acid derivative salts, porous base materials, triazole compounds, thiazole compounds, tetracyclines, and metal phosphate salt compounds of ascorbic acid. The base resin may include a xylene-formaldehyde-resin-modified amino-containing epoxy resins obtained by reacting an epoxy resin having an epoxy equivalent of from 180 to 2500 with a xylene formaldehyde resin and an amino-containing compound. The curing agent may be a blocked polyisocyanate compound obtained by blocking an isocyanate group of a polyisocyanate compound with a blocking agent.
Owner:KANSAI PAINT CO LTD
Compositions for Regulation of Hair Growth
InactiveUS20080254055A1Reduce frequencyImprove shaving efficiencyBiocideCosmetic preparationsPersonal carePhytic acid
Personal care composition comprising at least one hair growth regulating compound selected from the group consisting of glyceryl dilaurate, apigenin, tetrahydrocurcumin, oleanolic acid, azelaic acid, sulforaphane, canavanine, pyridoxal 5-phosphate, phytic acid, tannic acid, grape seed extract, NG-nitro-L-arginine-methyl ester, benzamidine, sodium butyrate, betulinic acid, polyornithine, polyarginine, fisetin, jasmonates, methyl-jasmonate, cis-jasmone, caffeic acid phenethyl ester, delphinidin, ethyl abietate, esculetin, sorbic acid methyl ester, canaline, N-formyl-methionine, N-formyl-alanine, taurine, palmitoyl carnitine, undecanol, undecylenic acid, rutin, fusidic acid, phenyl pyruvic acid, L-isoleucine, phenyl glycine, silibinin, silymarin, L-ascorbic acid-6-palmitate, N-undecylenoyl-L-phenylalanine, and salts, derivatives and mixtures of any of the foregoing; and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
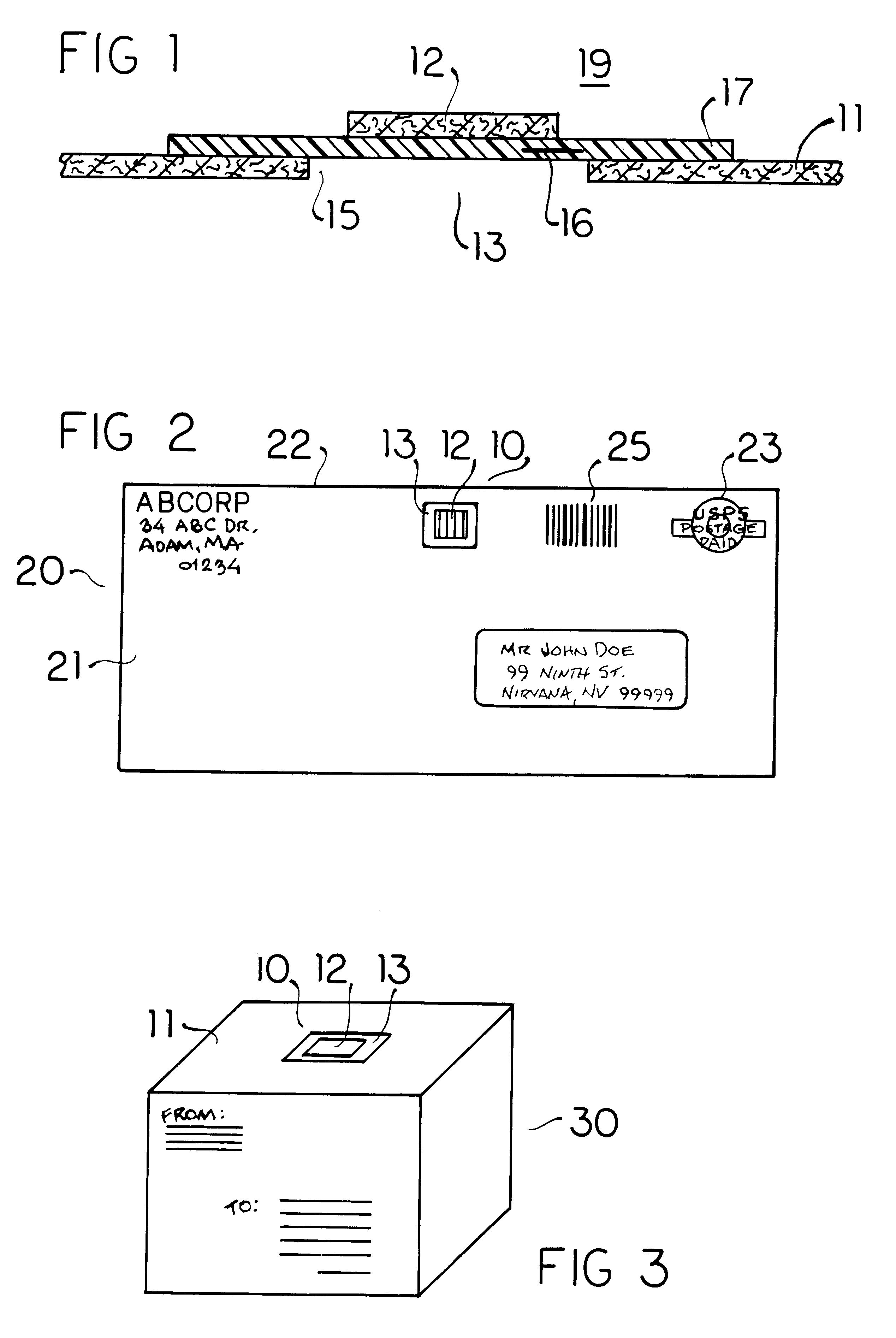
Biological toxin detection system for mailed materials
InactiveUS6524846B1Easy to manufactureReadily adheres to paperBioreactor/fermenter combinationsBiological substance pretreatmentsPorous substrateQuarantine
Envelopes and other containers intended for mail transportation possess a biological agent / toxin indicator operative upon the interior in detection of volatile bases including gaseous amines released from bacterial biological agents including Bacillus antracis, i.e. anthrax. The biological agent indicator has an acidic acid-base indicator compound that irreversibly changes color when neutralized by volatile bases. The compound is applied in liquid form to an appropriately porous substrate. A polymeric matrix is specifically suggested for this substrate. Irreversible indication of the presence of volatile bases including amines produced by live bacterial agents as toxins at temperate ambient conditions down to below freezing is provided. An indicator compound having a pH of 2-5 is recommended. Halogenated xanthene, sulphonated azo, and sulphonated hydroxy-functional triphenylmethane dyes are suggested. The presence of toxins produced by live bacterial biological agents within a package or container upon which the indicator is mounted is indicated by a slowly reversible color change. Primary public use envelopes and collection containers, secondary transportation and stationary quarantine enclosures, and articles worn inside a room with mail and when handling mail including a badge and gloves are specifically suggested.
Owner:ROBINSON JR WILLIAM L
Ascorbic acid, terephthalate and nitromethane stabilizers for fluoroolefins
ActiveUS8097181B2Improve stabilityAvoid instabilityHeat-exchange elementsNitromethaneAir conditioning
The present disclosure relates to compositions comprising at least one fluoroolefin and an effective amount of a stabilizer comprising at least one ascorbic acid, terephthalate, or nitromethane, or mixtures thereof. The stabilized compositions may be useful in cooling apparatus, such as refrigeration, air-conditioning, chillers and heat pumps, as well as in applications as foam blowing agents, solvents, aerosol propellants, fire extinguishants, and sterilants.
Owner:THE CHEMOURS CO FC LLC
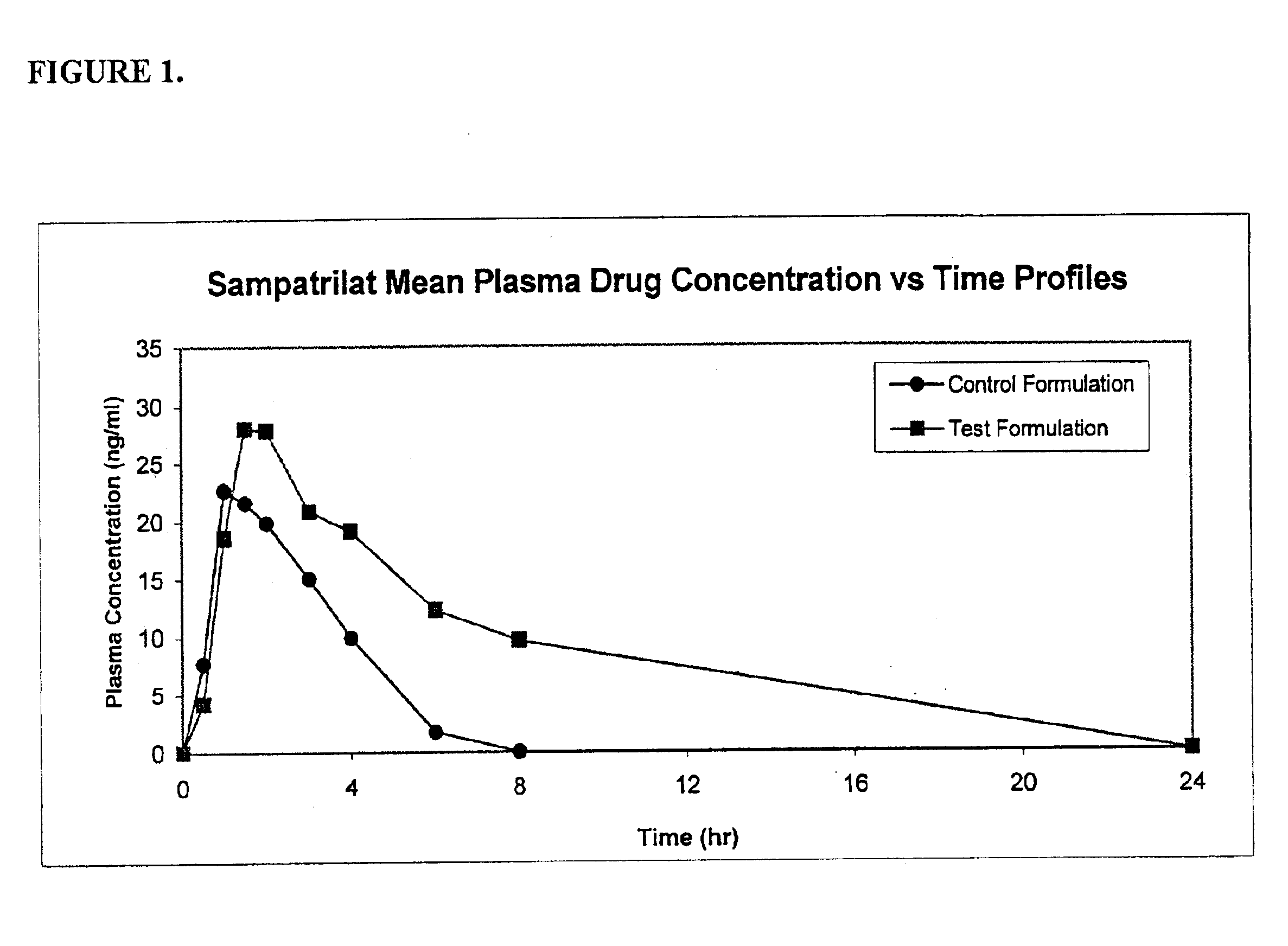
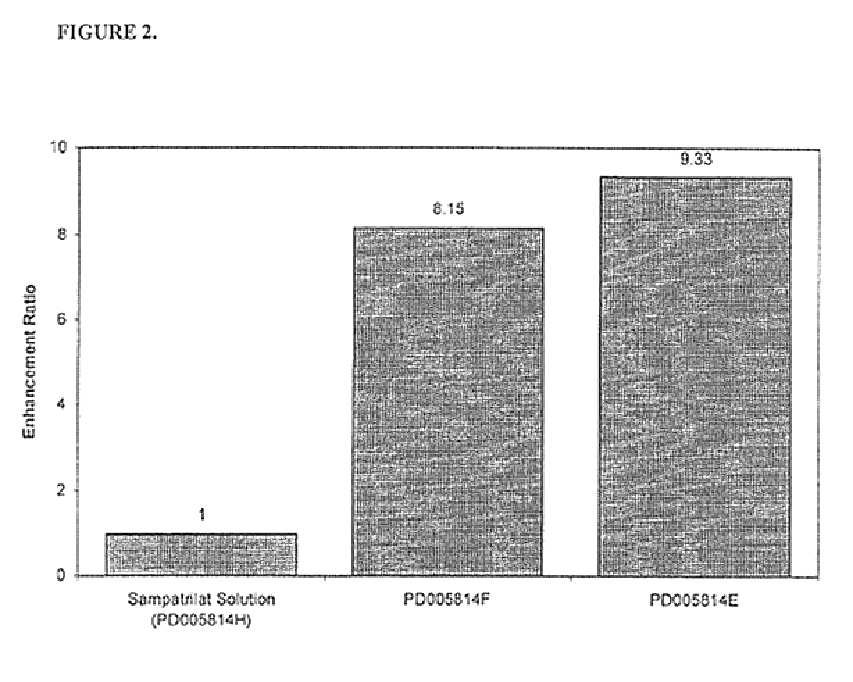
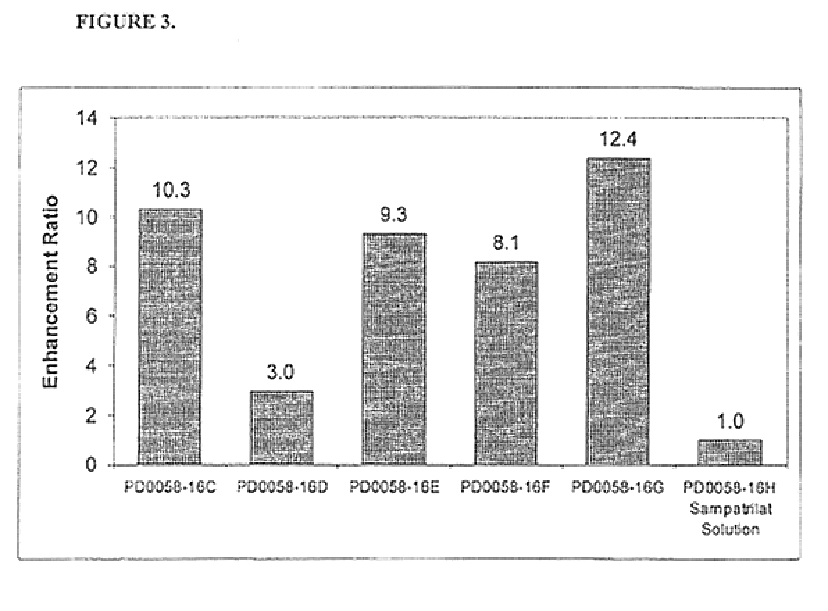
Pharmaceutical compositions including ACE/NEP inhibitors and bioavailability enhancers
InactiveUS6890918B2Enhance the oral bioabsorption of saidBiocideAngiotensinsAngiotensin-converting enzymeOrganic acid
A pharmaceutical composition comprising an inhibitor of angiotensin converting enzyme and neutral endopeptidase, such as sampatrilat, and at least one bioavailability enhancer such as an organic acid, e.g., ascorbic acid. Such a composition has improved systemic bioavailability.
Owner:SUPERNUS PHARM INC
Color changing skin sealant
Iodine is used in about 80 percent of surgeries in the US to remove some level of microbial load on the skin prior to making an incision. Skin sealants are applied over skin preps to seal the skin and hold any remaining bacteria in place. Iodine produces a characteristic orange-brown color on skin. A skin sealant is provided that has a decolorant that reacts with the iodine found in most skin preps, rendering the skin prep colorless. A skin sealant containing ascorbic acid (vitamin C), Indigo Carmine or Indigo will react with the iodine, thus visually indicating where the skin prep and sealant have been applied and allowing an unobstructed view of the incision.
Owner:KIMBERLY-CLARK WORLDWIDE INC
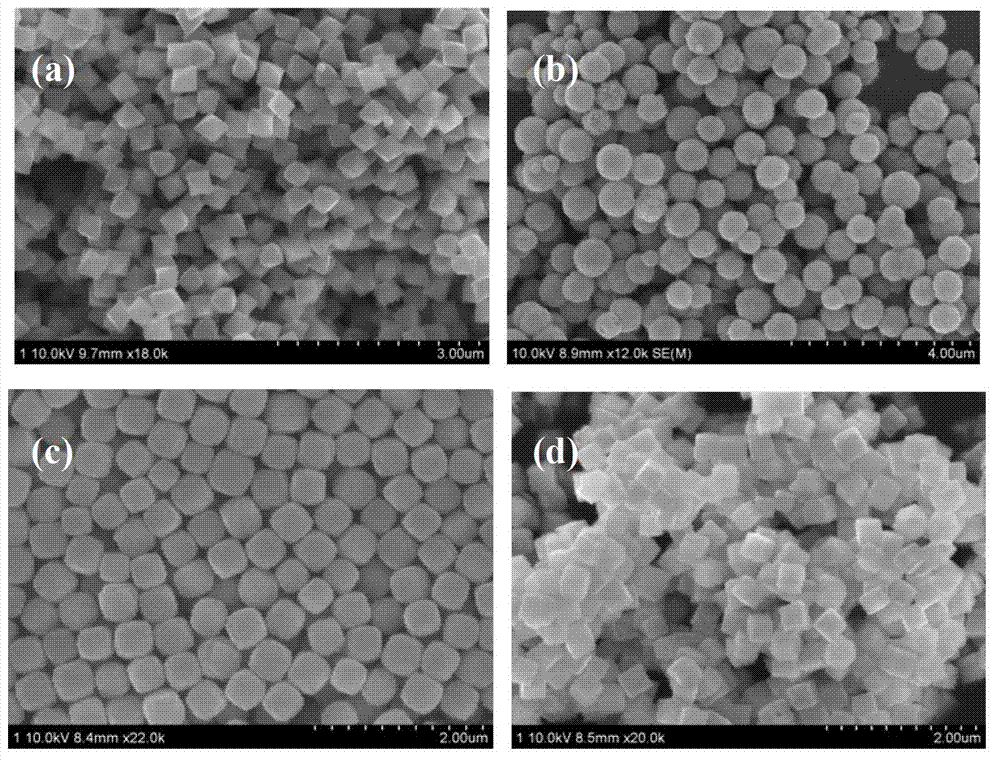
Preparation method of high dispersibility superfine silver powder with adjustable grain diameter
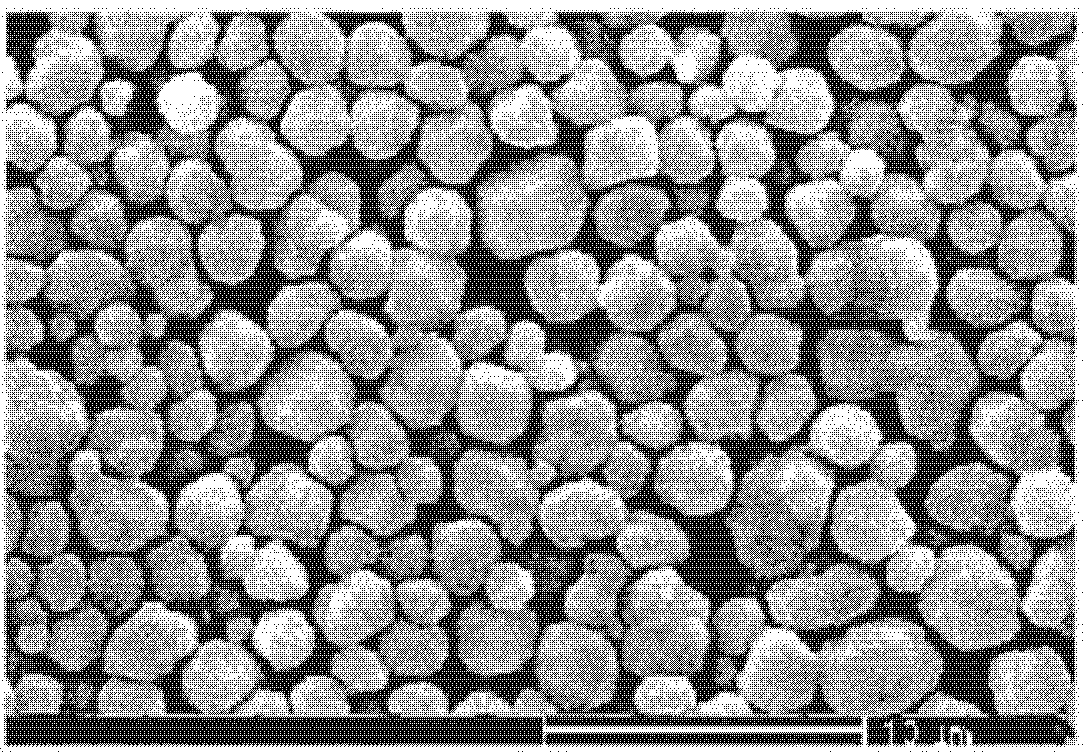
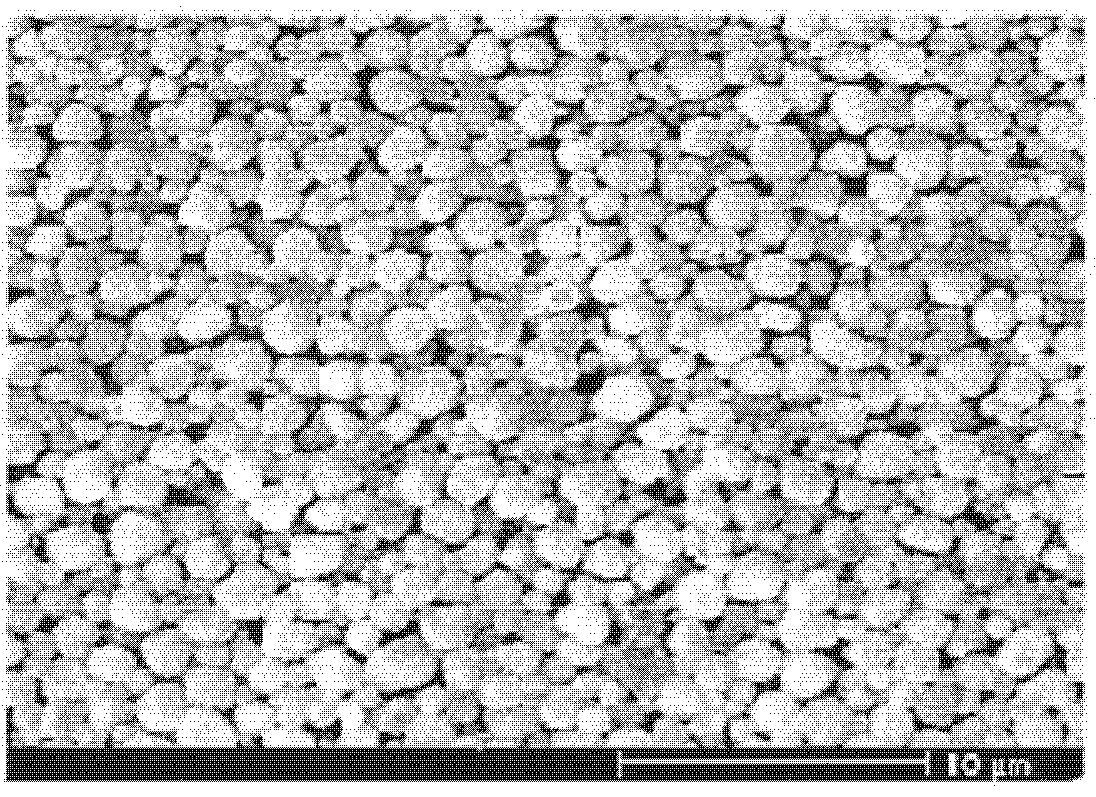
ActiveCN101733410AHigh tap densityImprove particle size uniformityVolumetric Mass DensitySURFACTANT BLEND
The invention relates to a preparation method of high dispersibility superfine silver powder with adjustable grain diameter, comprising the following steps: 1. dissolving silver nitrate and surfactant in water to prepare silver solution A; 2. dissolving ascorbic acid, PH regulator and surfactant in water to prepare reducing liquid B; 3. preparing alkaline aqueous solution C by alkaline PH regulator; dropwise adding alkaline aqueous solution C and silver solution A to reducing liquid B at the set time. PH value of the reducing liquid B and flow velocity of alkaline aqueous solution C are adjusted, so that silver power particle size can be easily adjusted and the silver power particles can grow up uniformly. The silver powder obtained by the method of the invention has the characteristics of polyhedral microcosmic appearance, good dispersibility, narrow size distribution, high tap density and good sintering character.
Owner:GUANGDONG FENGHUA ADVANCED TECH HLDG
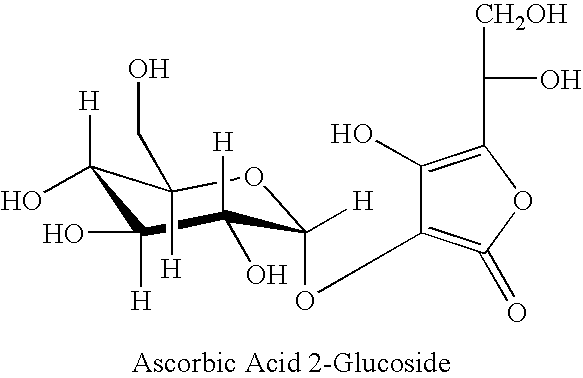
Ascorbic acid salts of organic bases with enhanced bioavailability for synergictic anti-aging and skin protective cosmetic compositions
This invention relates to in-situ preparation, and stable topical delivery systems of ascorbic acid salts of organic bases that provide skin beneficial properties, including reduction in signs of skin aging, anti-wrinkle, anti-oxidant, and photo-protection from UV and sunlight. The formulation avoids the use of oils, minimizes the importance of the pH of the formulation, allows the incorporation of an aqueous solution of ascorbic acid or alkali metal salts of ascorbic acid in the formulation, does not require packaging the formulation in air tight containers, allows the use of large amounts of ascorbic acid, its salts, and its derivatives, and does not require the use of expensive coatings. Moreover, several ascorbic acid derivatives of different chemical composition can be made in a stable topical formulation by the in-situ combination of readily available starting materials in a water solution, despite the understanding well known in the prior art that such compositions in water are inherently unstable. The in-situ method also permits the preparation of novel ascorbic acid derivatives that are not known in the prior art.
Owner:GUPTA SHYAM K
Process For Making Filter Tow
InactiveUS20080245376A1Consistent amountReduce concentrationCigar manufacturePaper/cardboard wound articlesCellulose acetateCyclodextrin
A method of preparing a crimped tow of cellulose acetate filaments comprising the steps of: a) providing cellulose acetate dope b) forming filaments (23) from the dope c) applying at least one additive to the filaments d) crimping the filaments to form a crimped tow wherein the at least one additive is capable of removing a component from cigarette smoke. Preferably, the component is a Hoffmann analyte. The additive may comprise a solution, liquid, emulsion or particulate material or combinations thereof. Preferably, the additive comprises an acidic compound or an alkaline compound. The additive may comprise malic acid, potassium carbonate, citric acid, tartaric acid, lactic acid, ascorbic acid, polyethyleneimine, cyclodextrin, sodium hydroxide, sulphamic acid, sodium sulphamate, polyvinyl acetate and carboxylated acrylate, carbon, silica, zeolite, clay, alumina, metal, molecular sieves or an ion exchange resin. The product tow can be processed on standard equipment to make efficient filter rods from which cigarette filter tips can be made which give significantly increased and selective retention of key smoke constituents.
Owner:CELANESE ACETATE LLC
Oxygen scavenging composition
InactiveUS20060069197A1Delayed action of absorptionPromote formationSynthetic resin layered productsConductive materialPolyesterAlloy
The present invention provides an Oxygen scavenging composition as a concentrate master batch to act as active and passive barrier, said composition comprising a resin carrier which is an alloy of PET with PTN, or PBN or PBT, mixed micron sized and nano-sized iron particles, an alkali metal bisulphate and ascorbate, a metal halide and additives. The iron particle mixture with a particle size in the range of 1 to 50 micron along with nano sized iron particles of 5 to 50 nanometers (nm) having an additional enhanced rate of oxygen absorption. The oxygen scavenging composition also includes metal halide and ascorbates and bisulphates of alkali metals. The oxygen scavenging composition of the present invention as a master batch in a polyester carrier resin containing barrier, color and U.V. light absorbing additives is used in food packaging applications of human consumption, especially those oxygen sensitive food items including alcoholic beverages, fruit beverages etc, where the oxygen permeation levels are to be maintained at very low levels.
Owner:TAMMAJI KULKARNY SANJAY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com