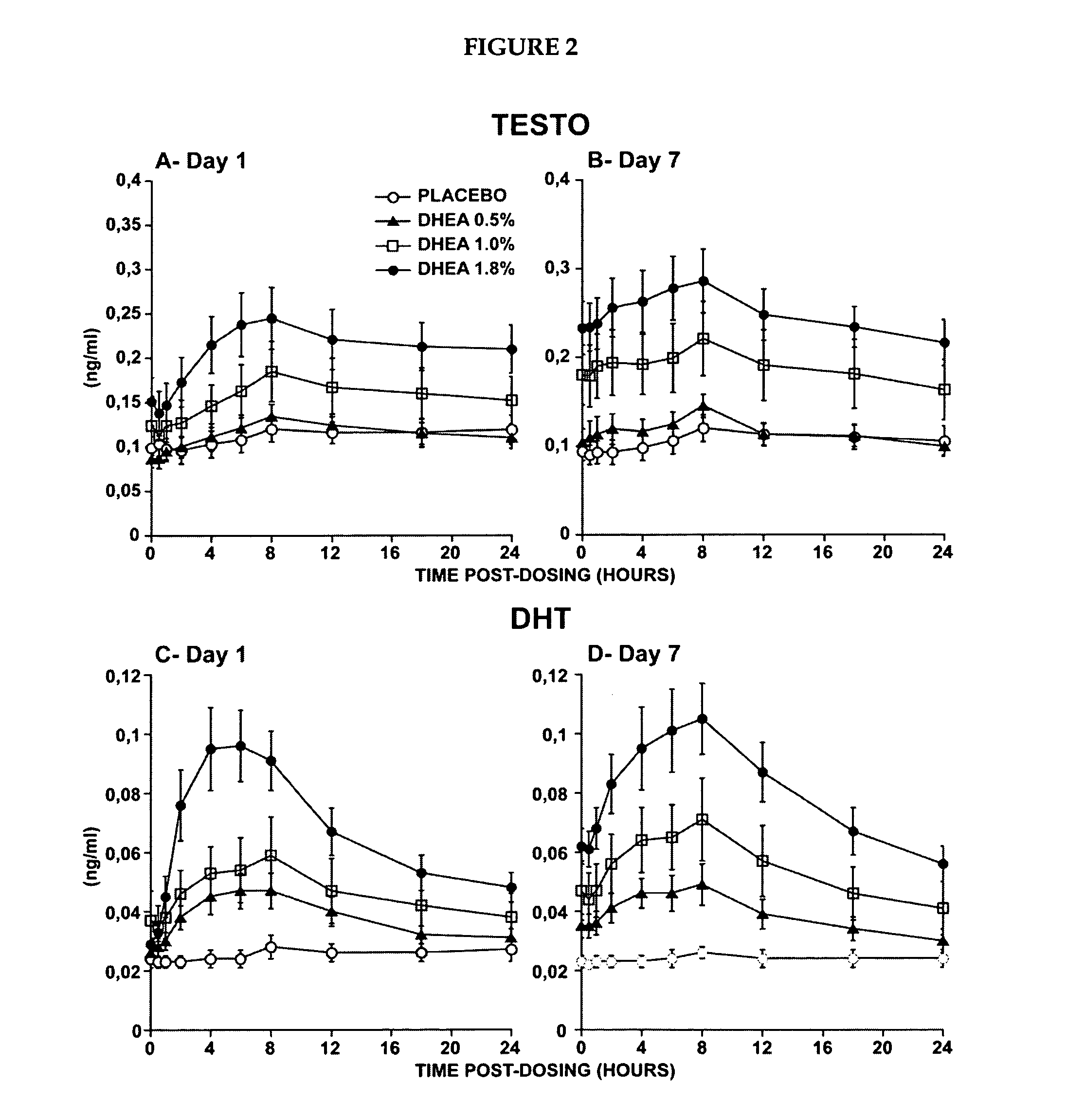
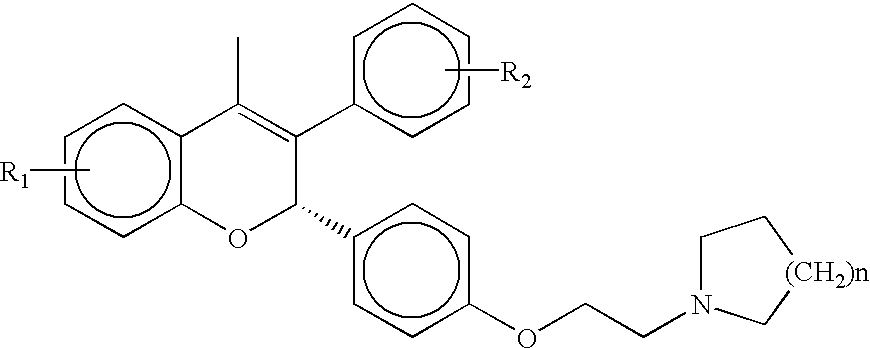
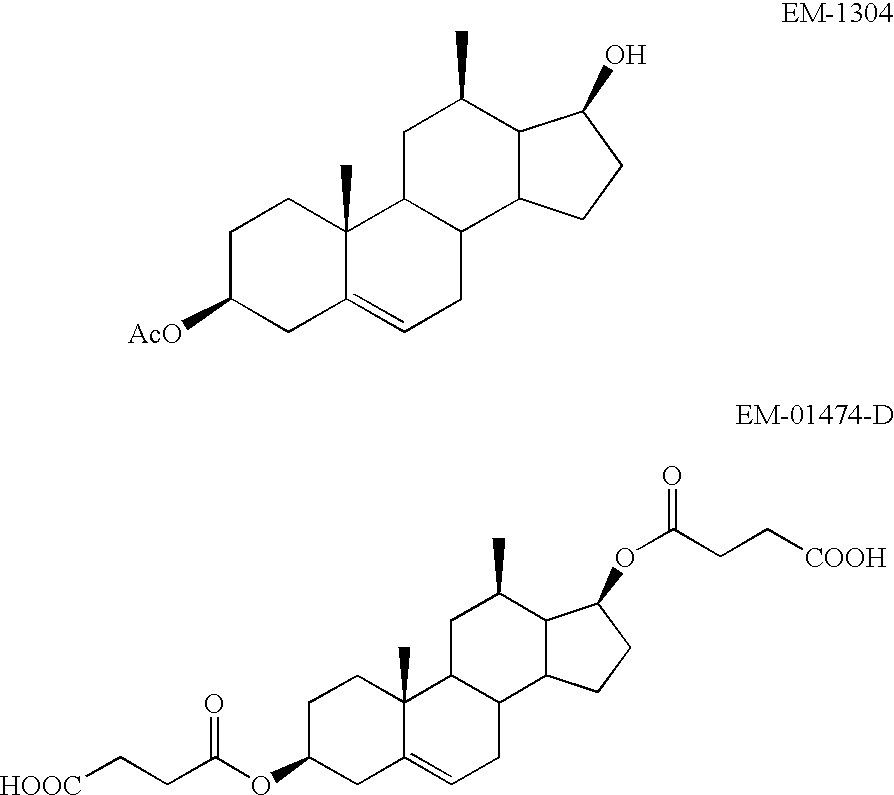
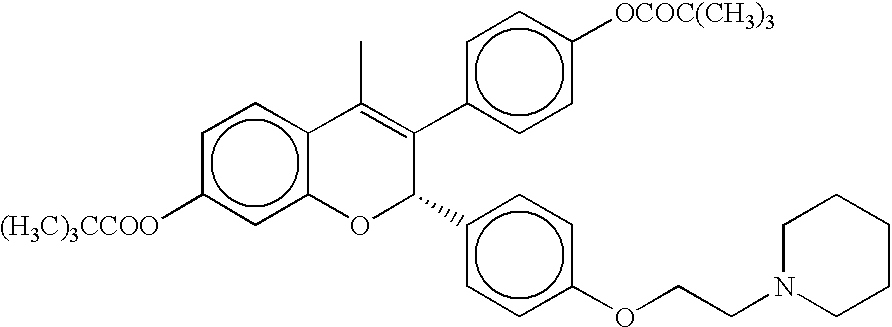
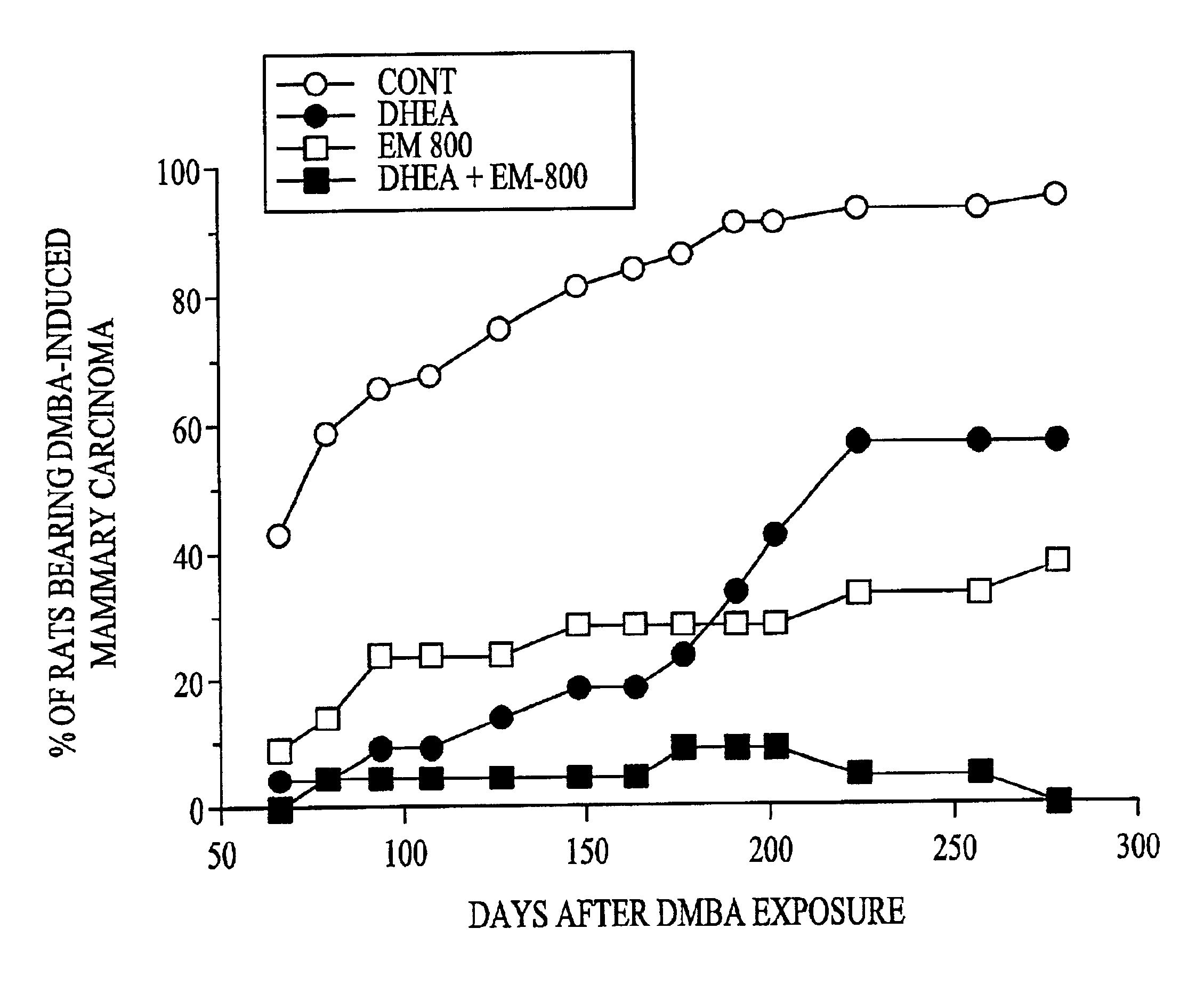
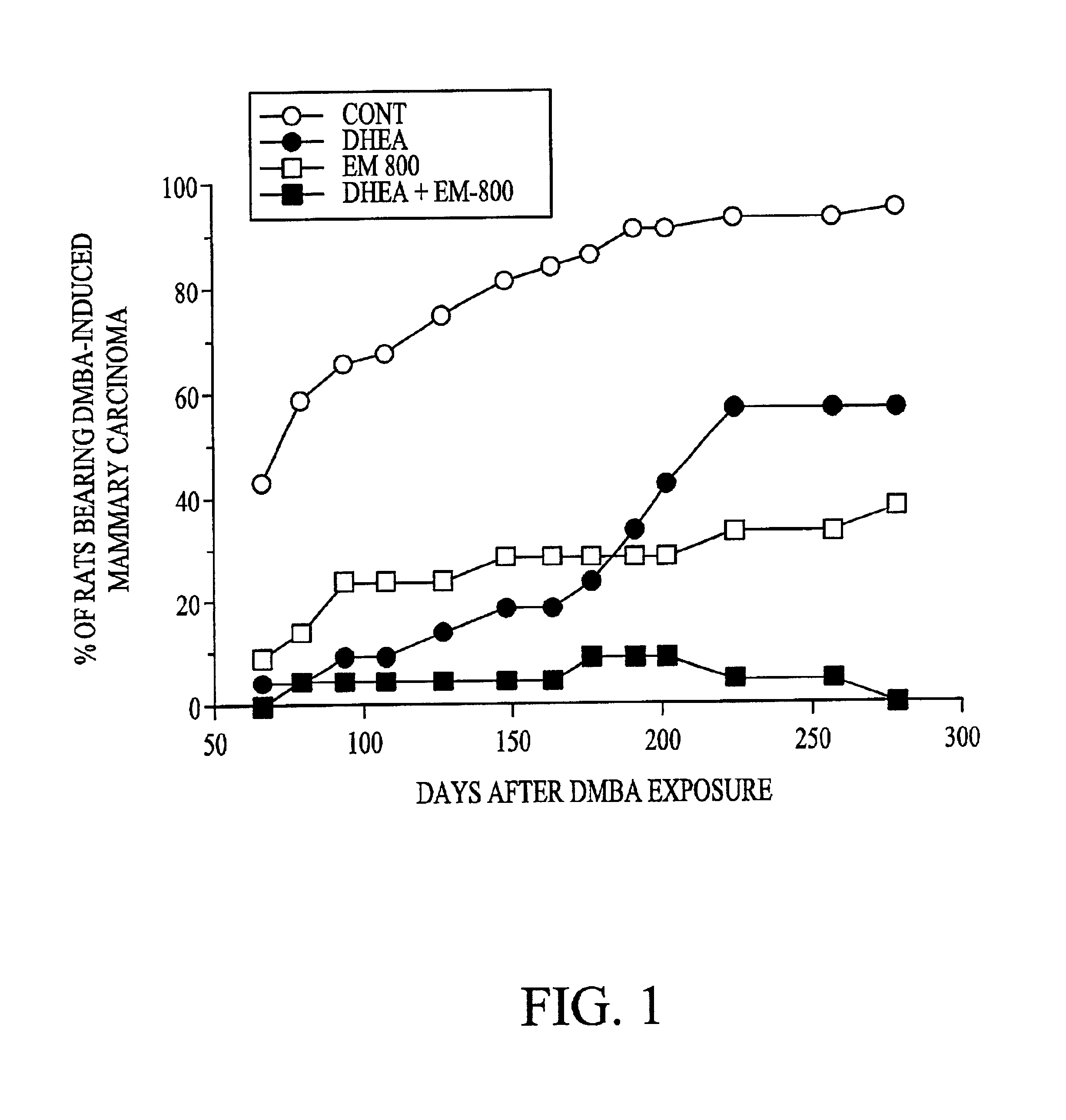
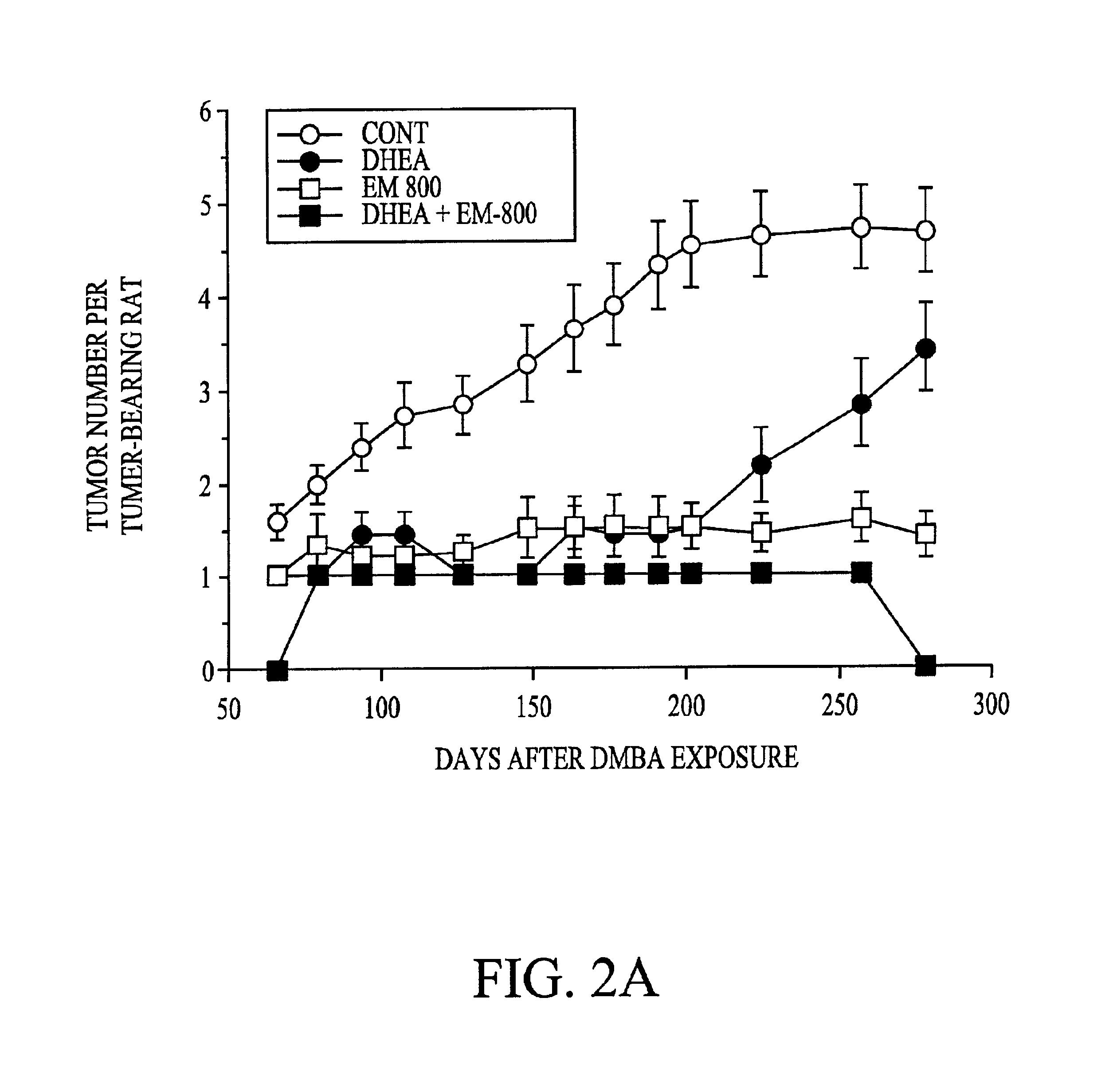
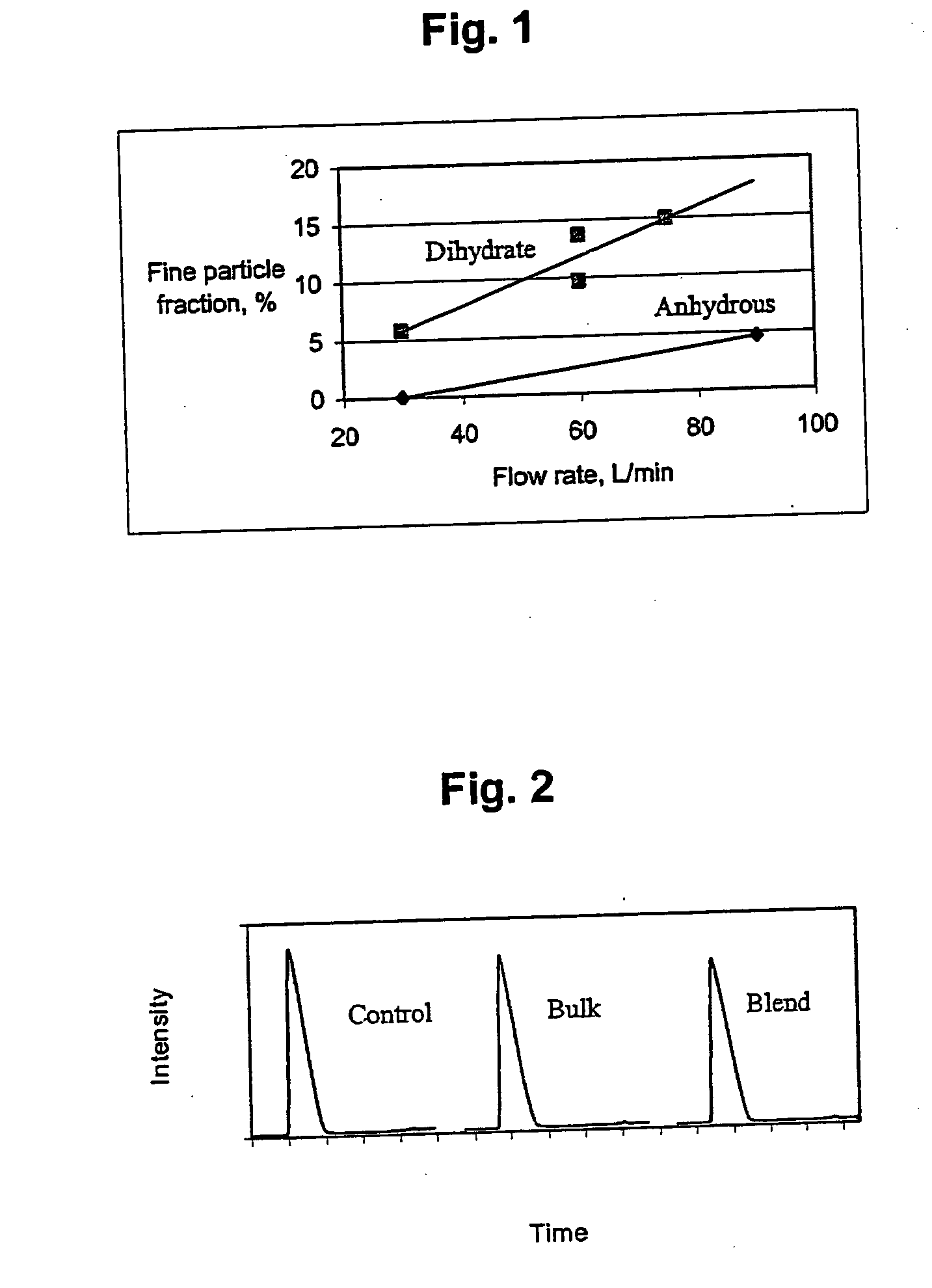
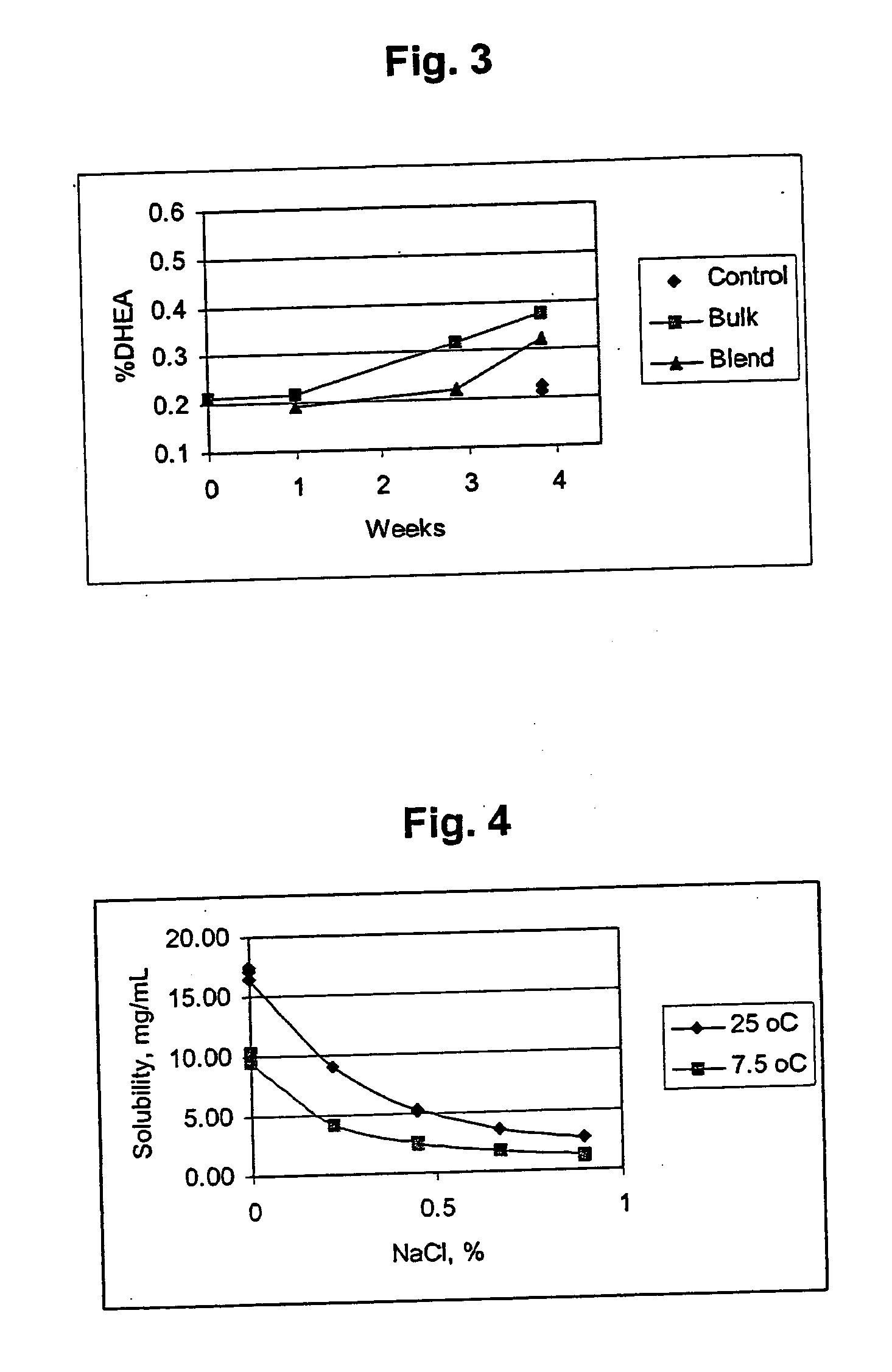
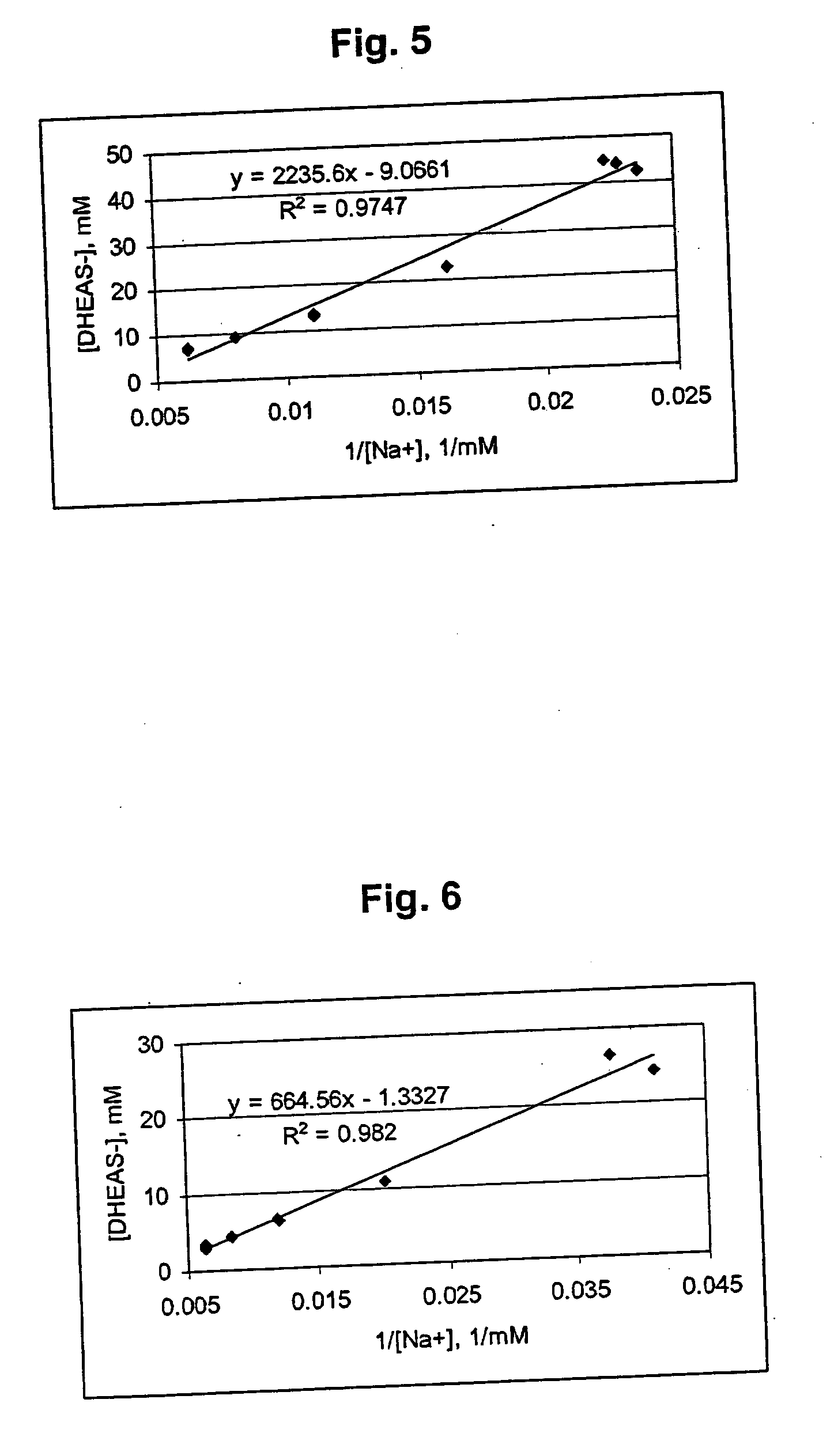
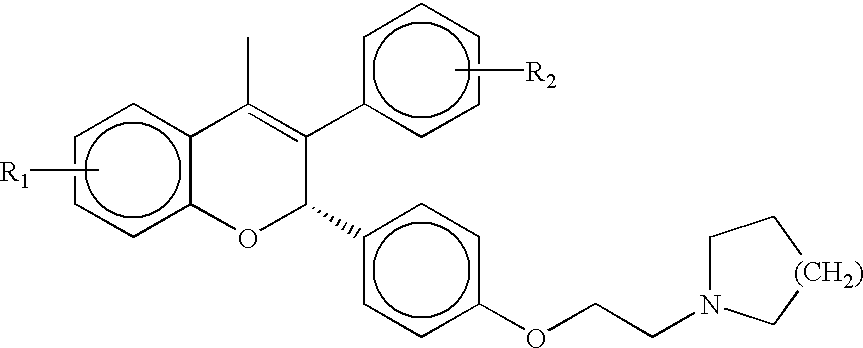
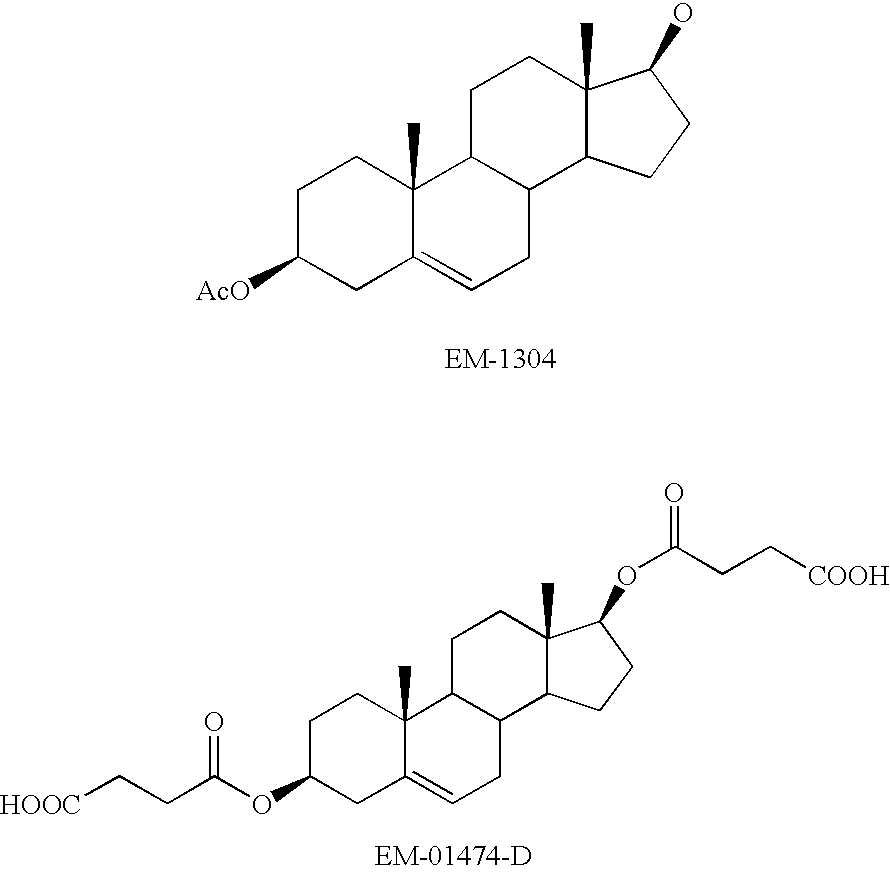
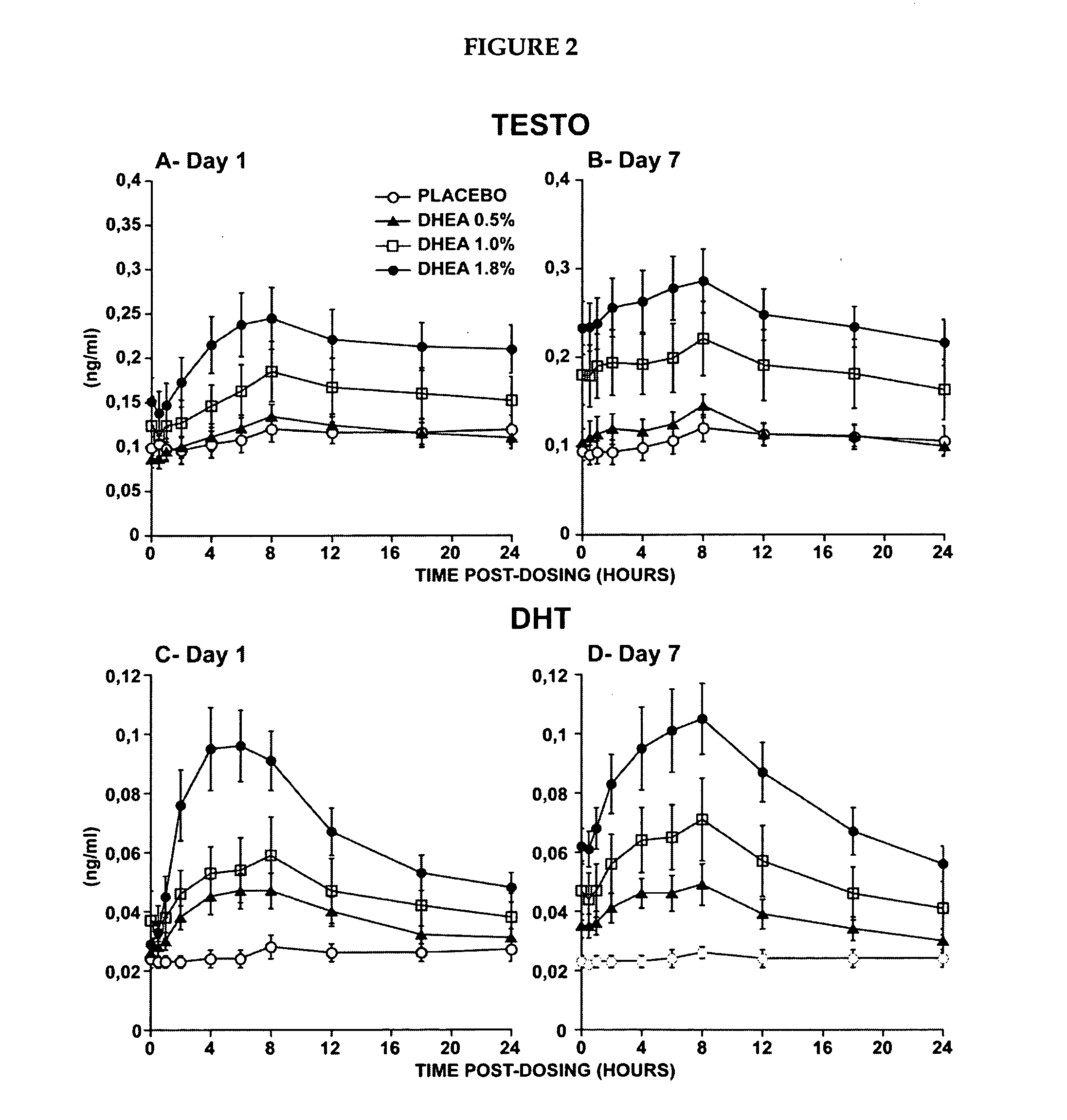
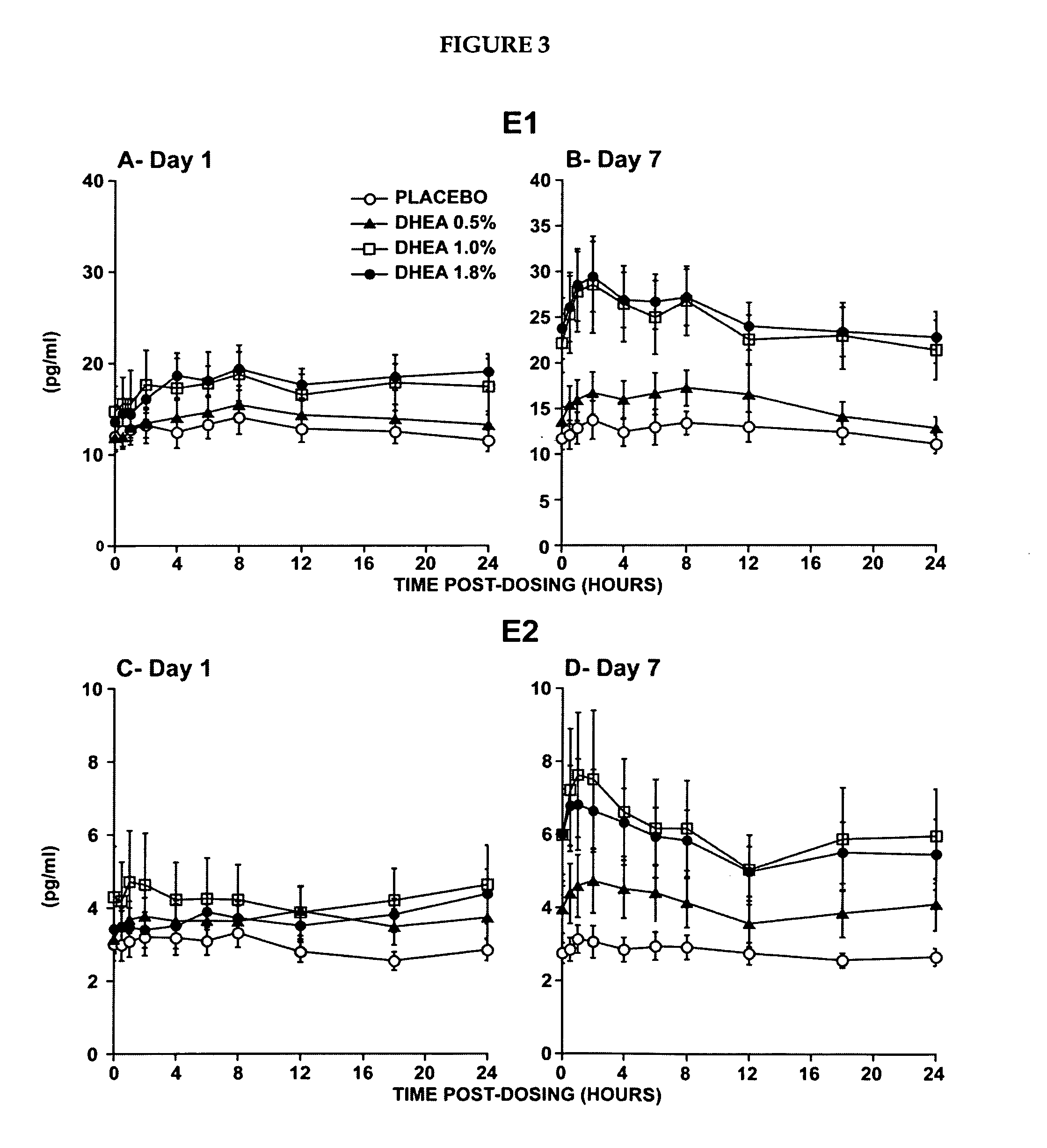
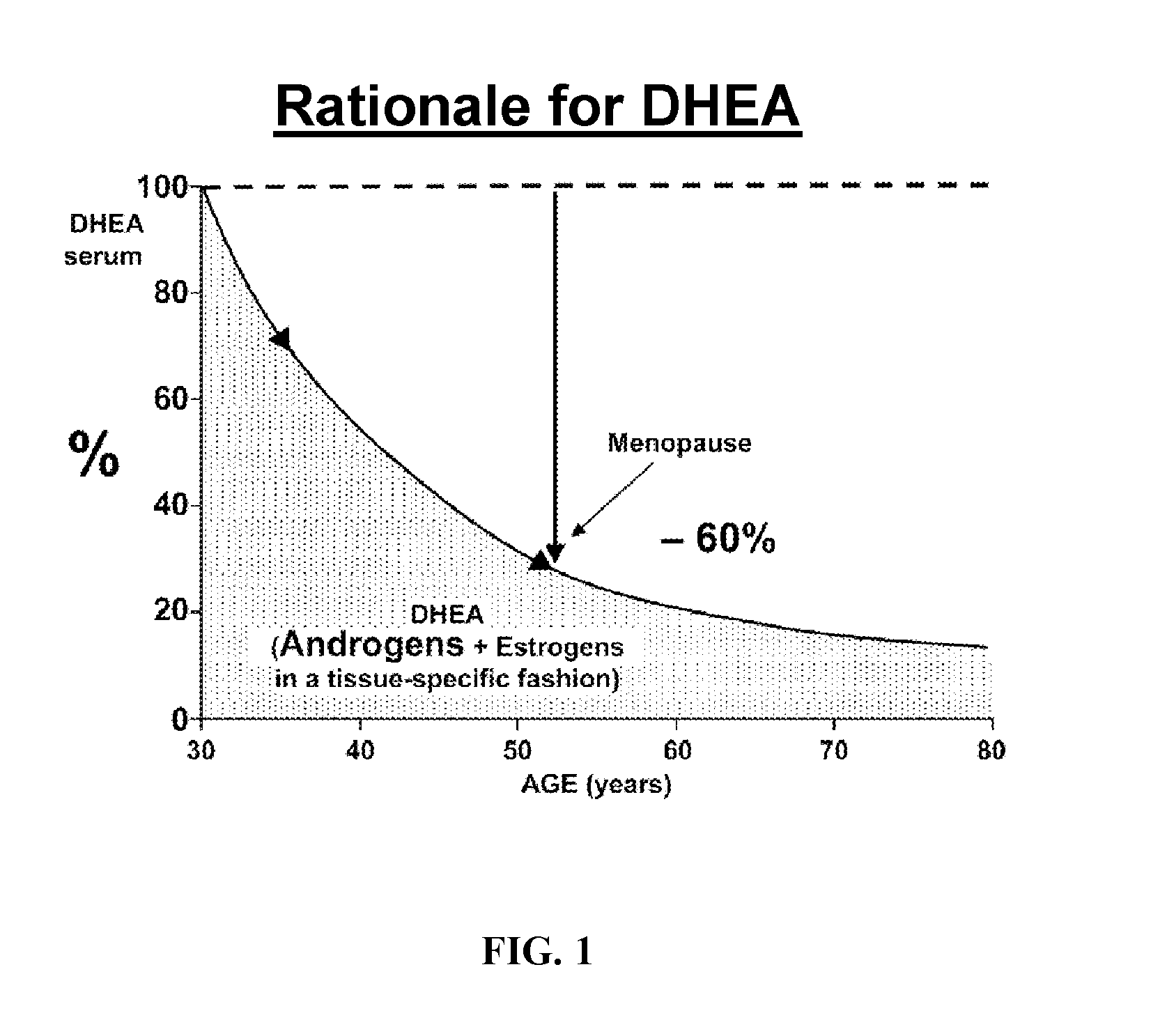
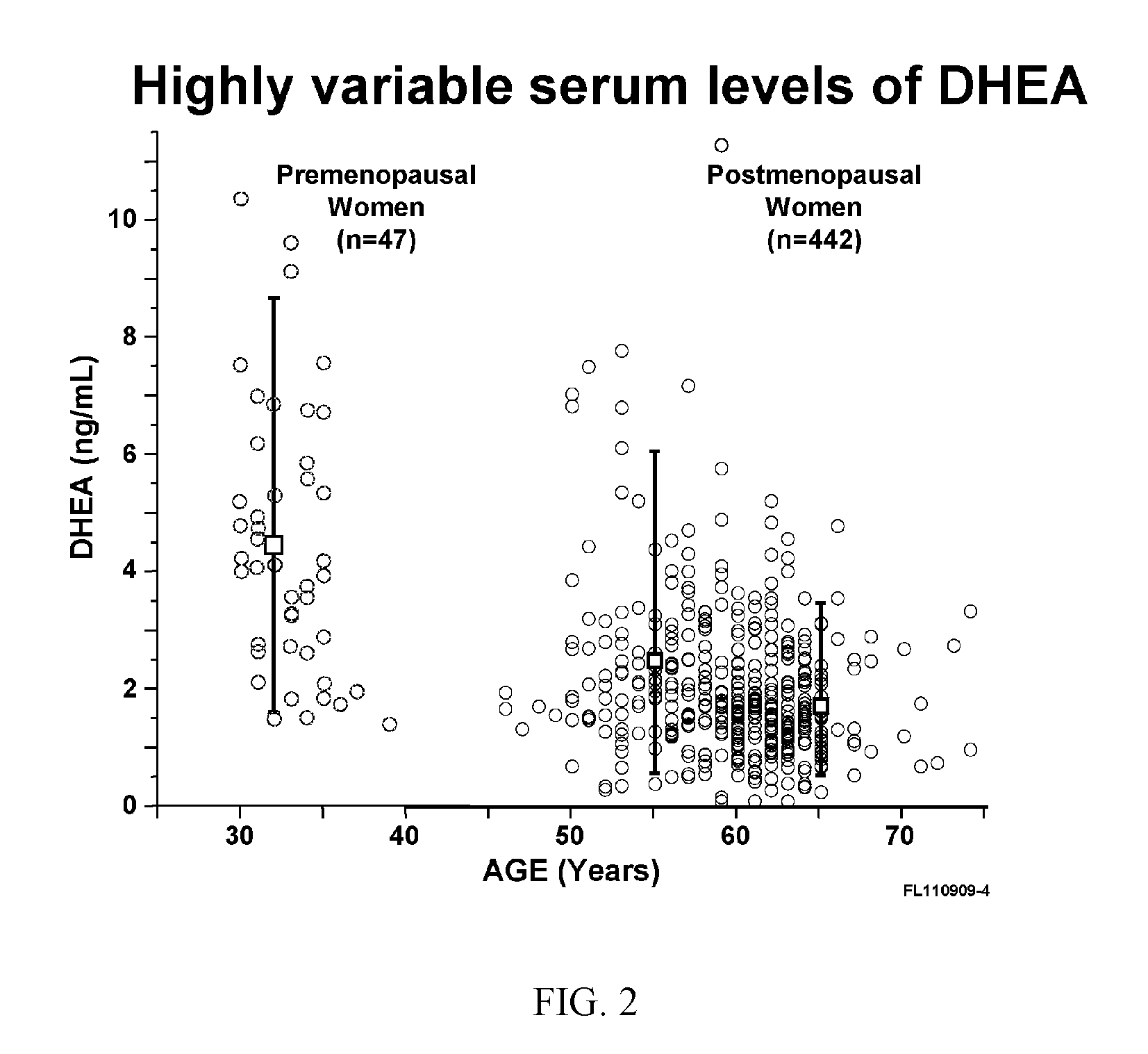
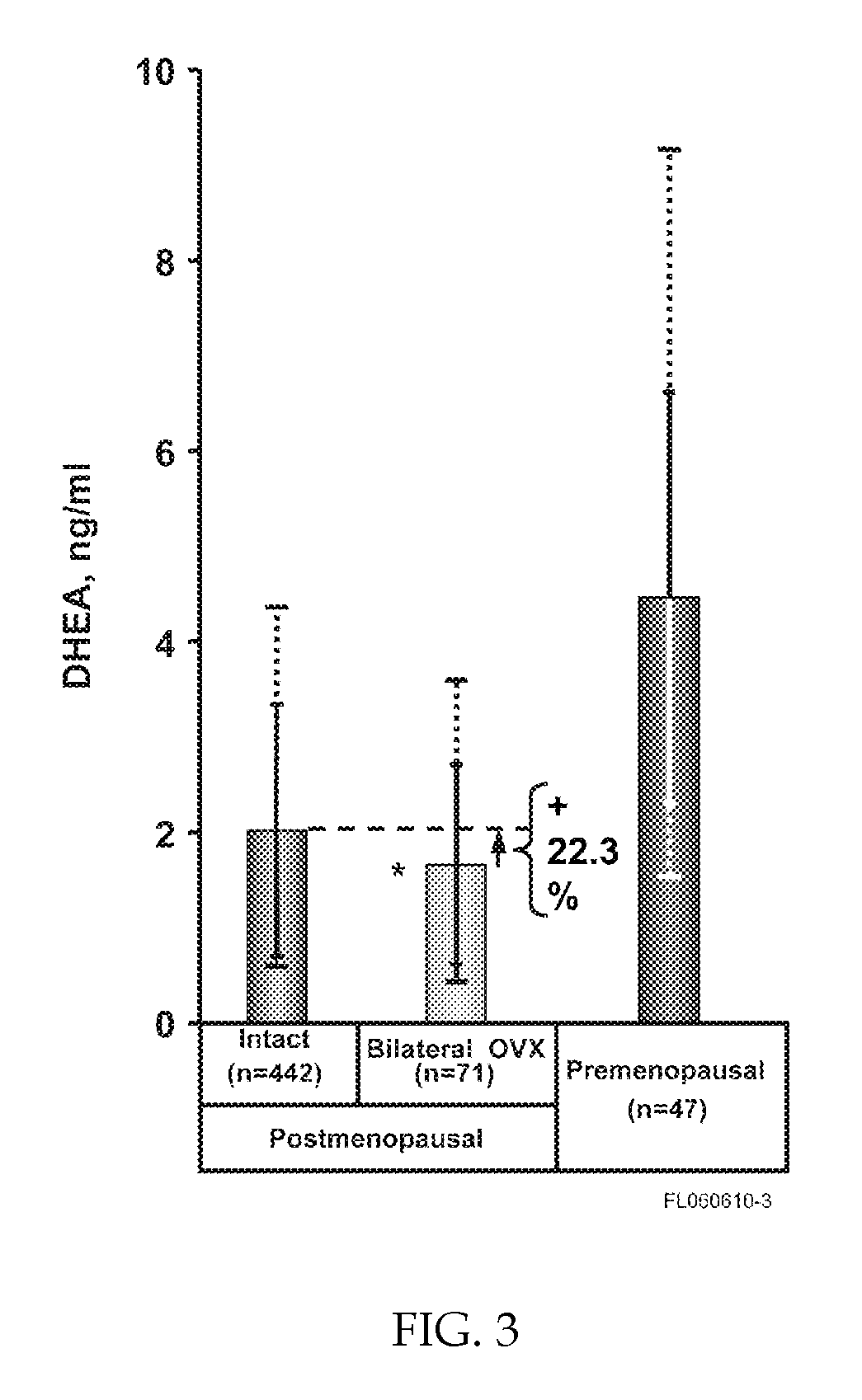
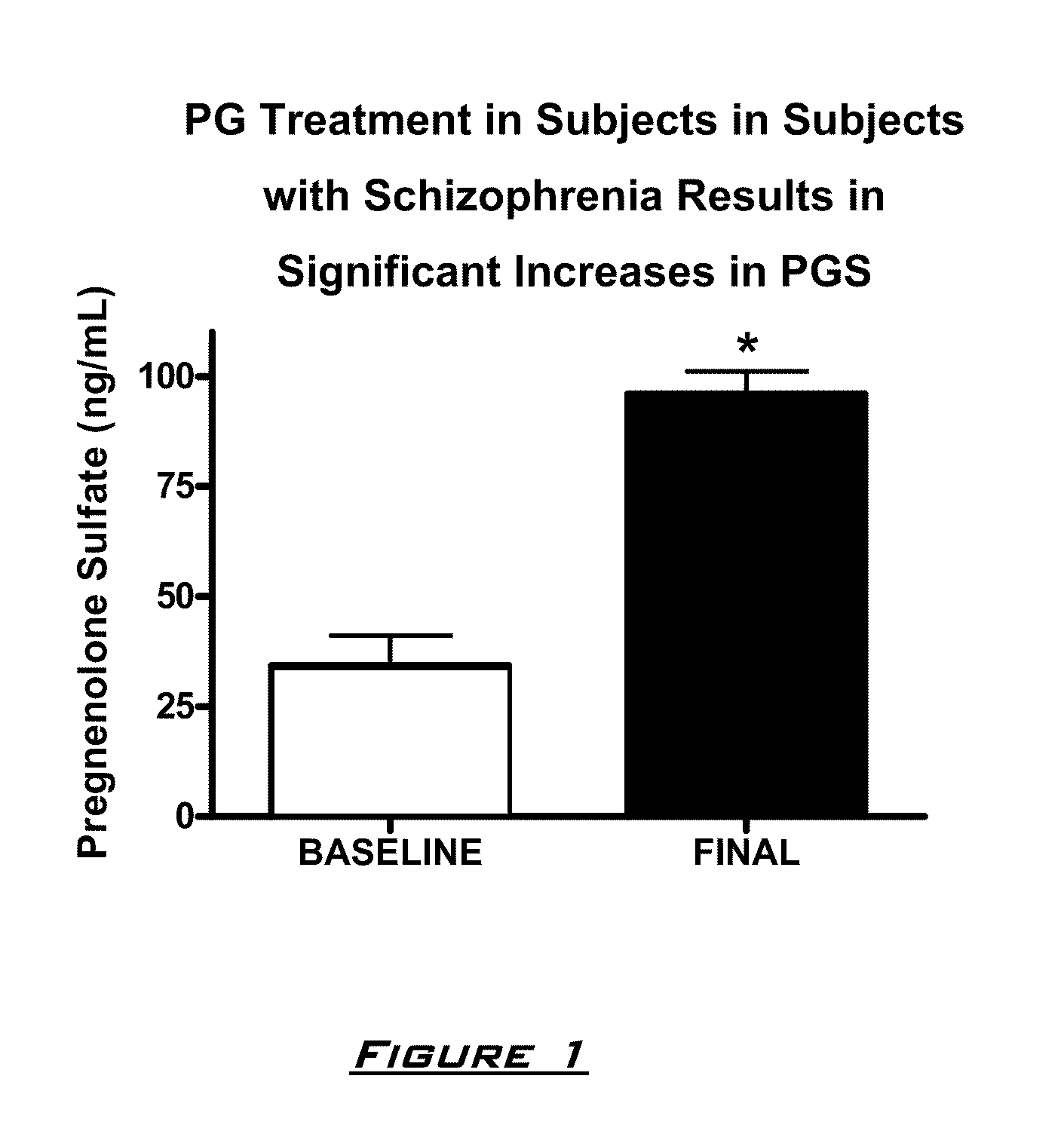
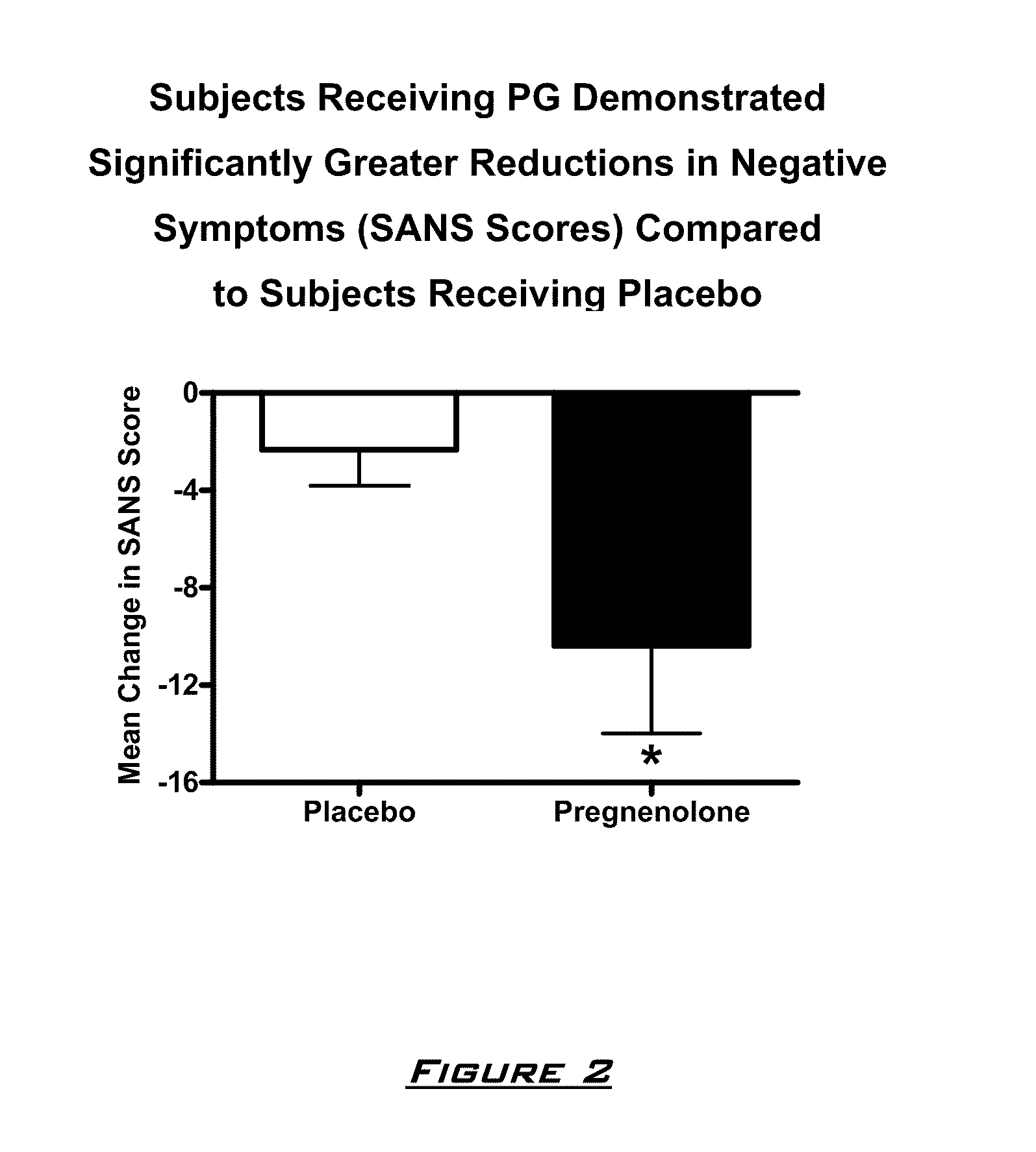
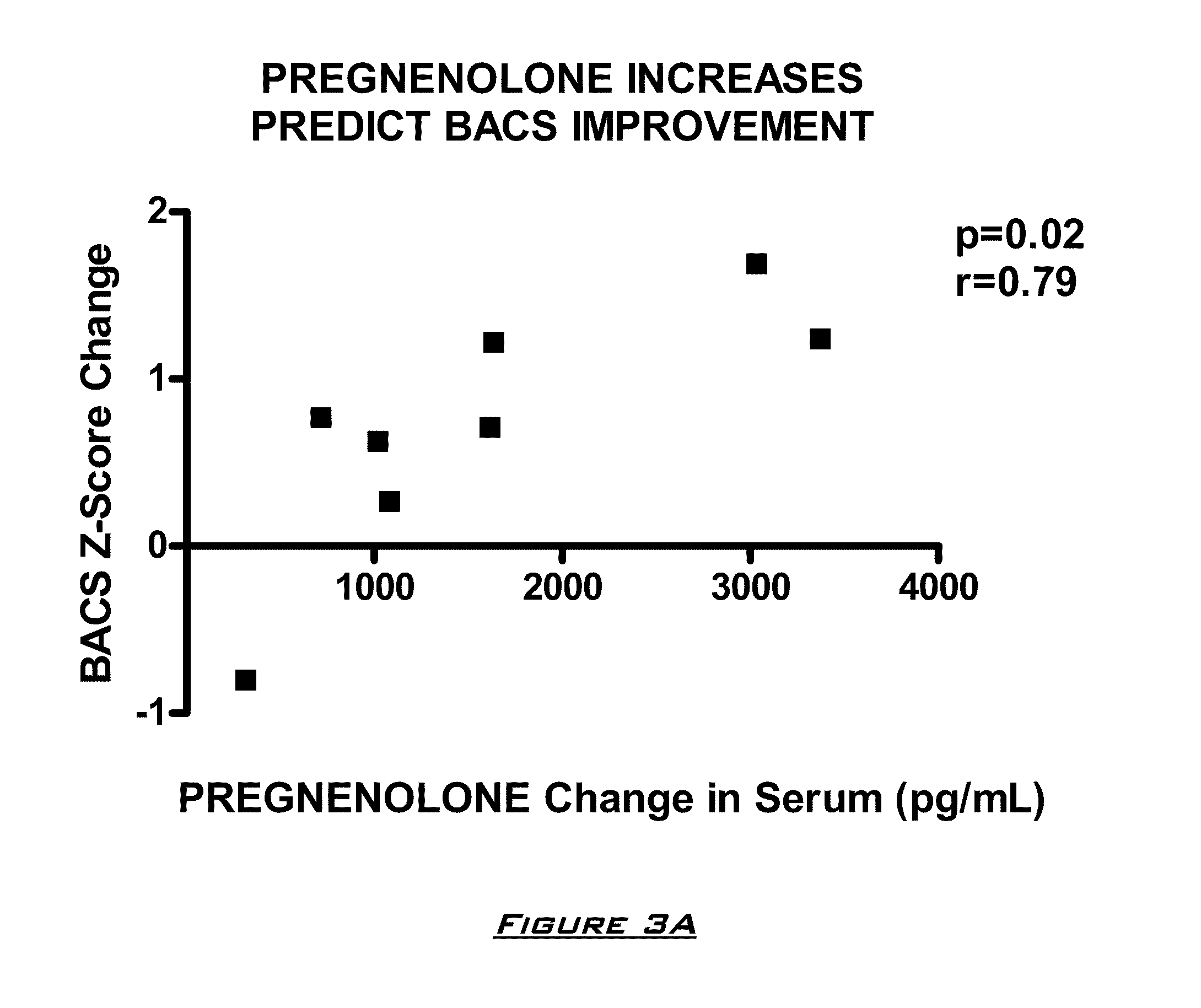
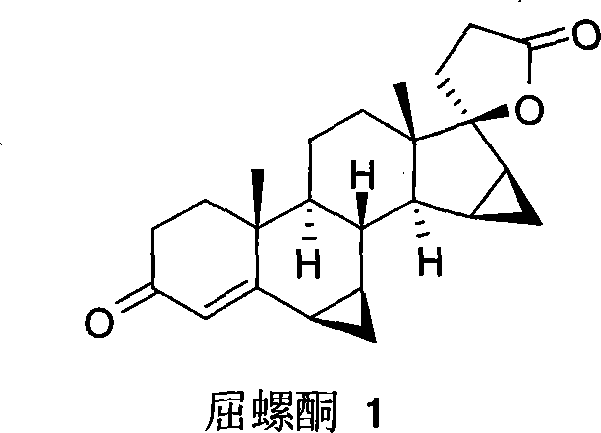
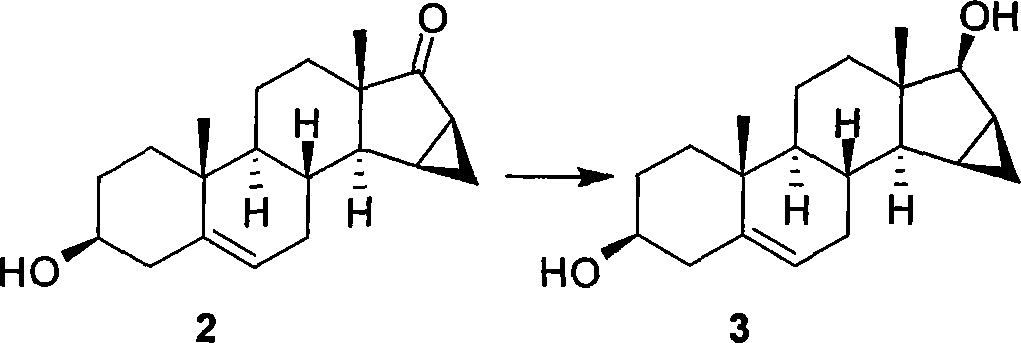
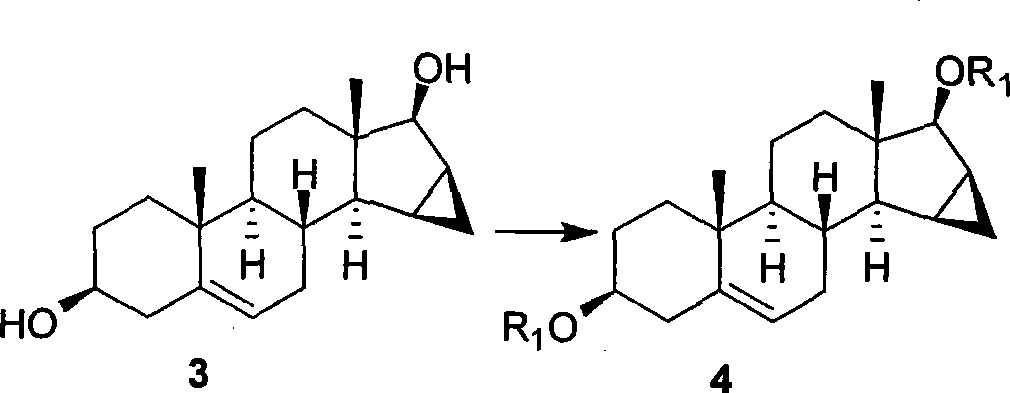
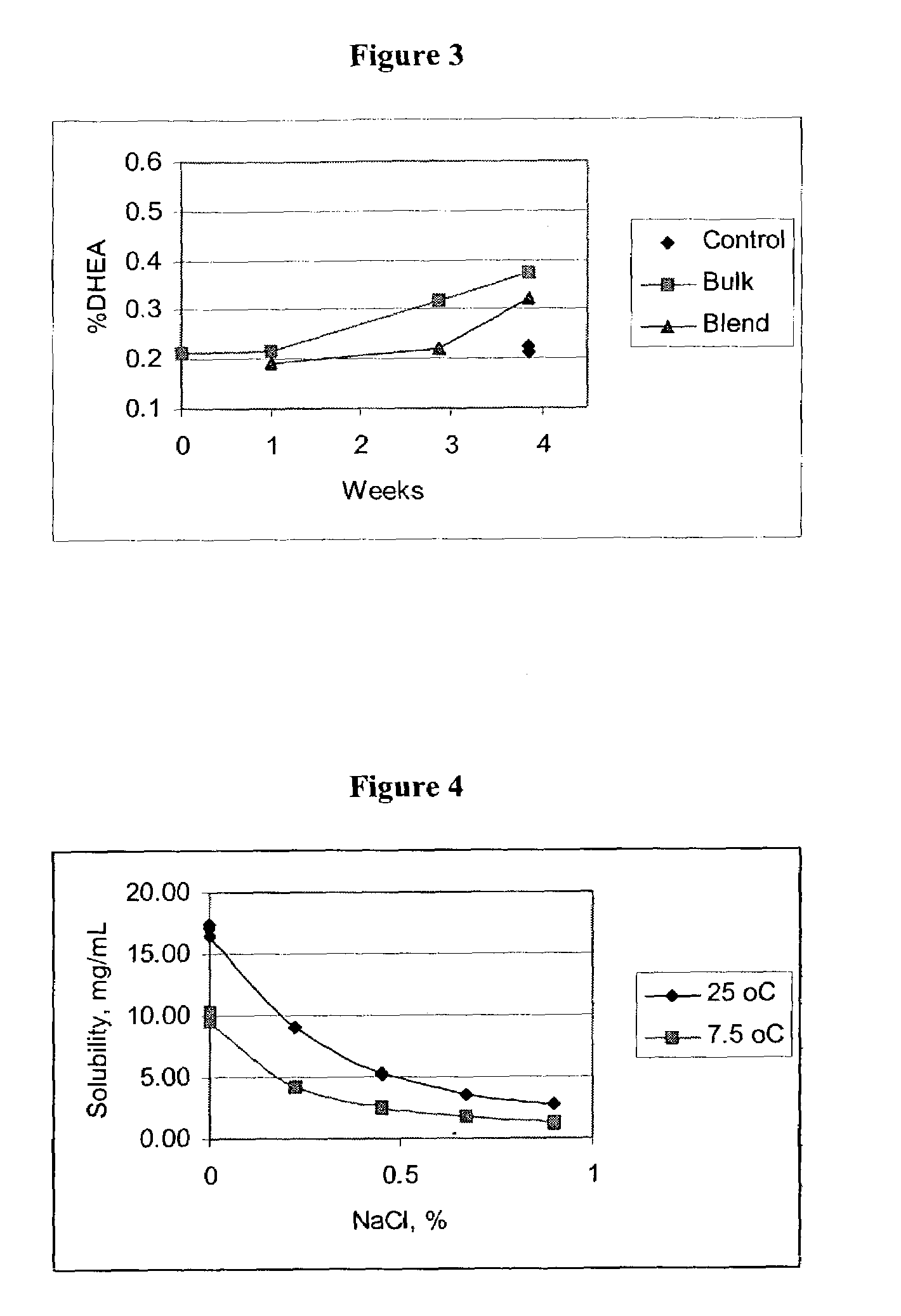
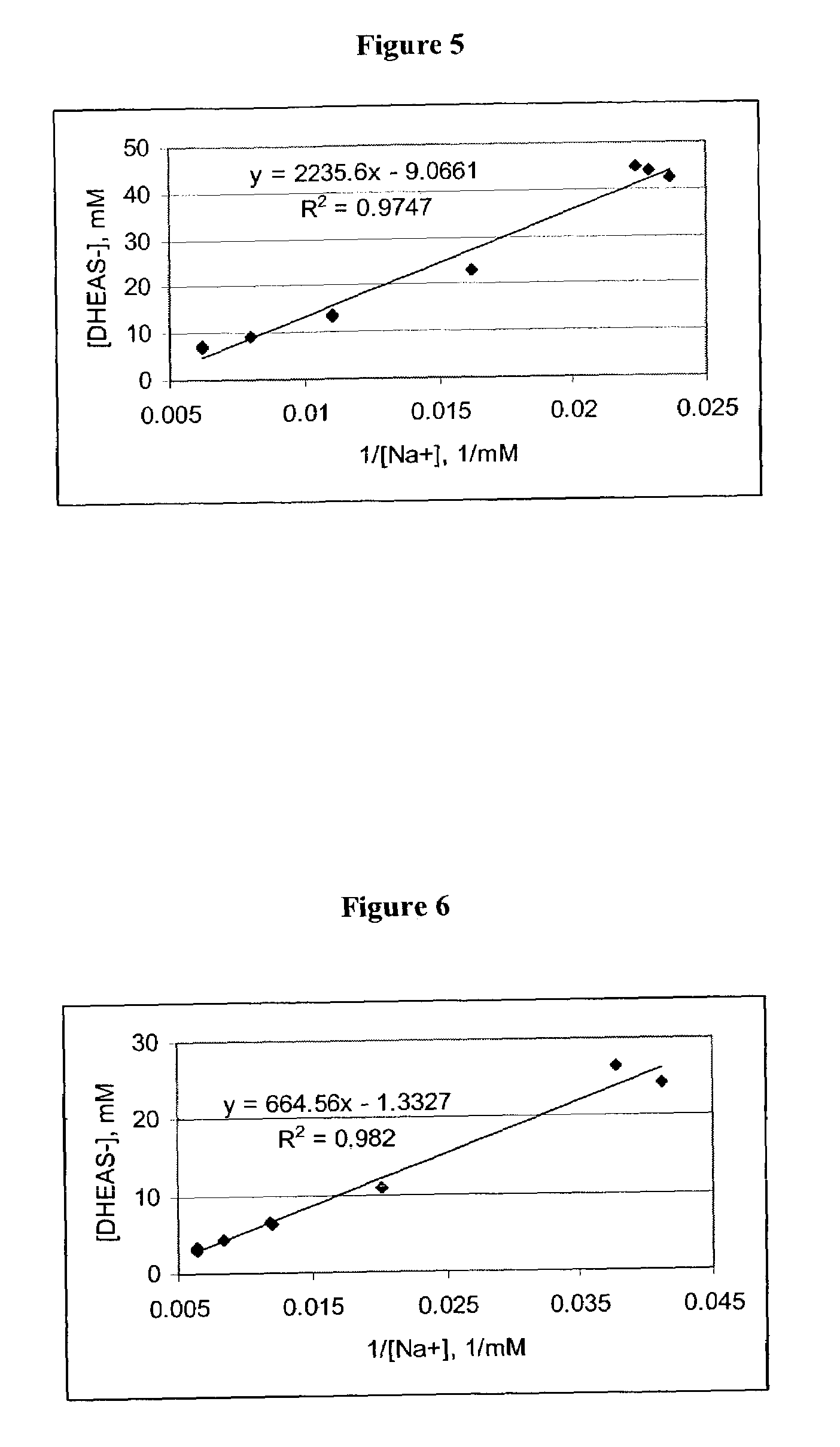
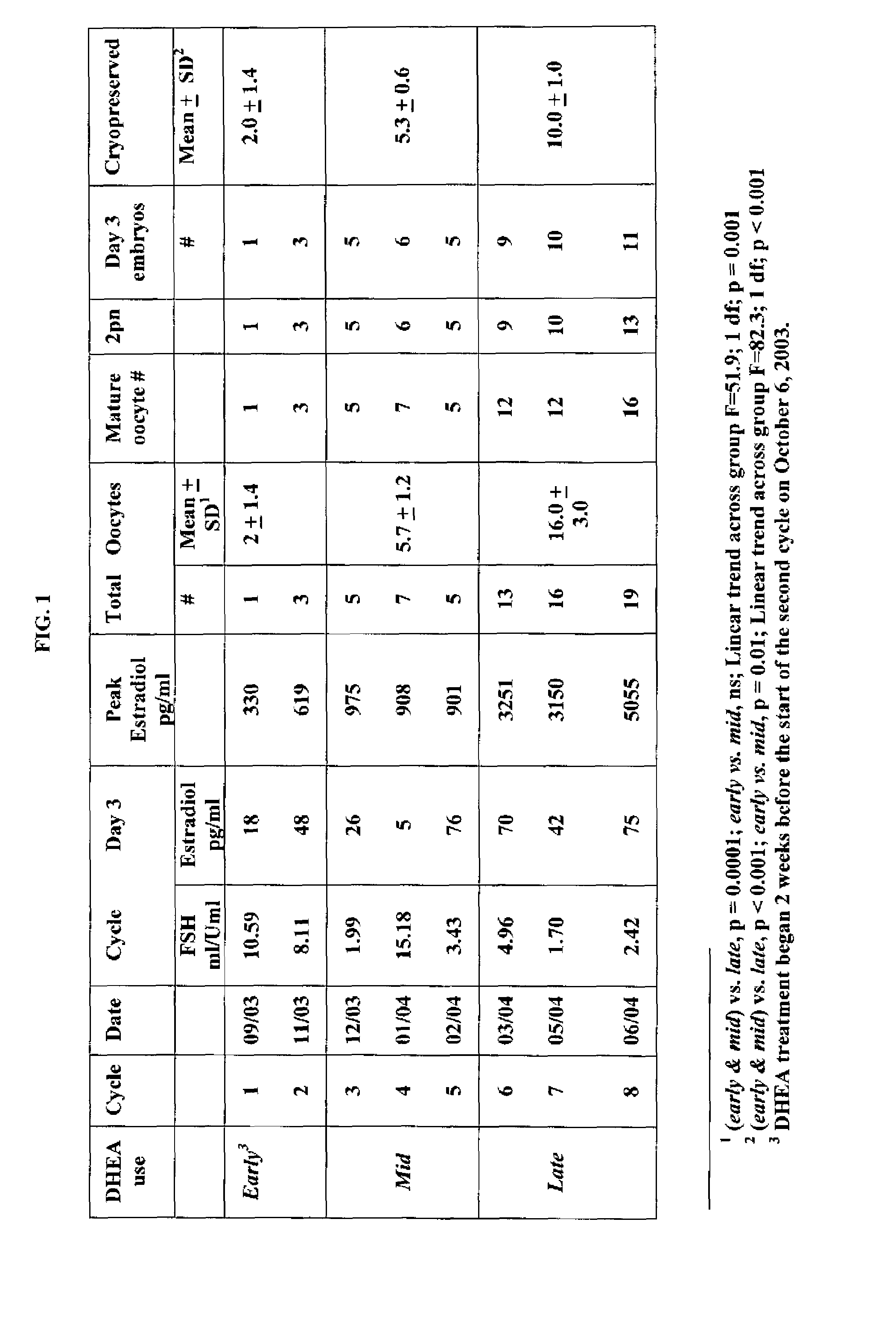
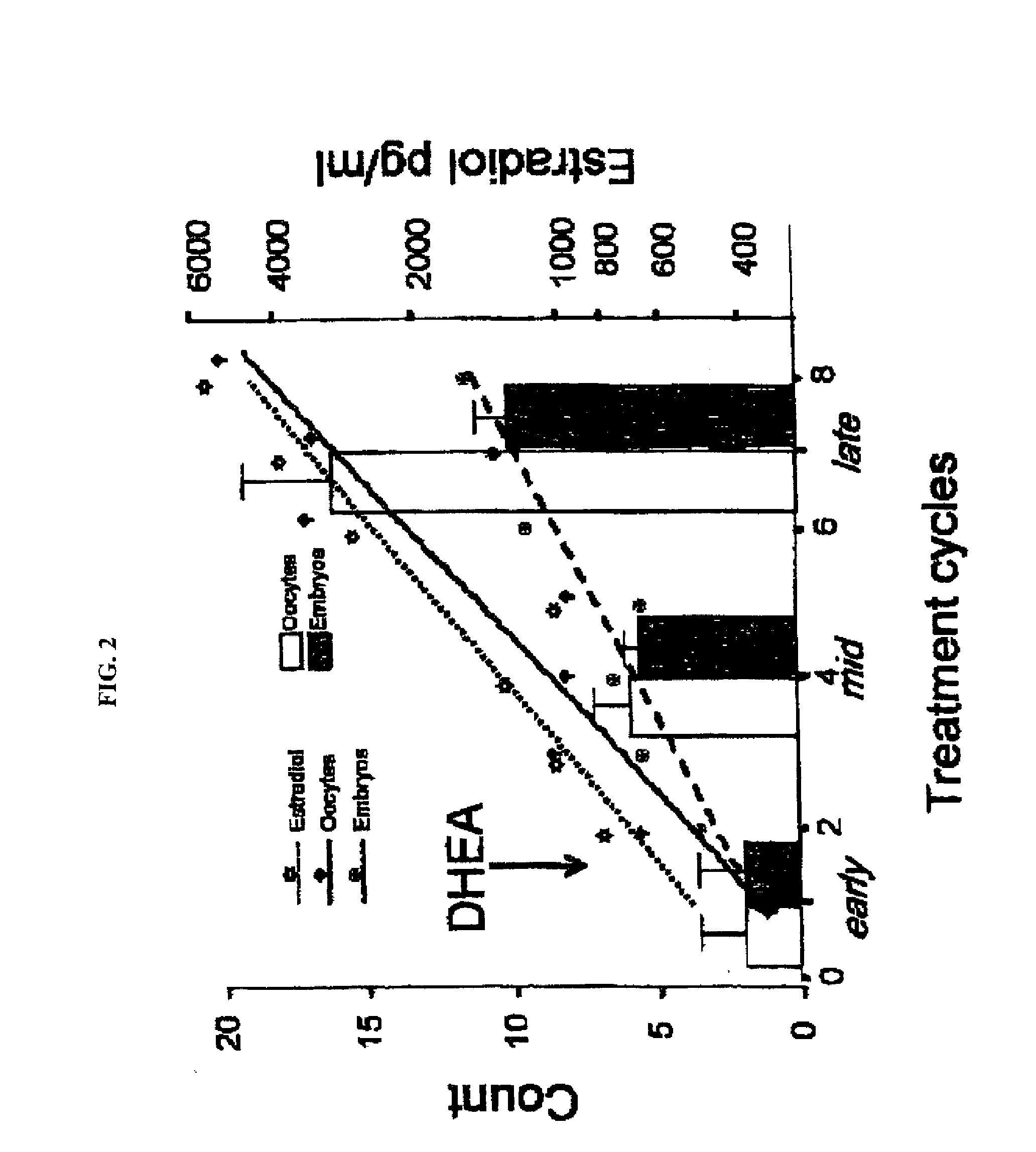
Patents
Literature
195 results about "Dehydroepiandrosterone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone. It is one of the most abundant circulating steroids in humans, in whom it is produced in the adrenal glands, the gonads, and the brain. It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues. However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors, and acting as a neurosteroid and modulator of neurotrophic factor receptors.
Method for reducing adenosine levels with a dehydroepiandrosterone and optionally a ubiquinone
A method and composition for reducing adenosine levels comprises administering a dehydroepiandrosterone, and optionally a ubiquinone.
Owner:EAST CAROLINA UNIVERISTY
Methods of treating and/or suppressing weight gain
Novel methods for the medical treatment and / or prevention of obesity, abdominal fat, and insulin resistance in susceptible warm-blooded animals including humans involves the administration of selective estrogen receptor modulators (SERMs). A combination of a SERM with an amount of estrogen or a sex steroid precursor selected from the group consisting of dehydroepiandrosterone, dehydroepiandrosterone sulfate, androst-5-ene-3b,17b-diol and compounds converted in vivo to one of the foregoing precursors or estrogen is also disclosed.
Owner:ENDORES & DEV
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
A combination of mitochondrial nutrients for relieving stress, preventing and improving stress-related disorders
InactiveUS20060257502A1Accelerated agingIncreasing oxidative metabolismBiocideCosmetic preparationsAlpha-TocopherolL-Carnosine
A dietary supplement of mitochondrial nutrients is designed for relieving stress, preventing and improving stress-related disorders, such as chronic fatigue syndrome, diabetes, age-associated cognitive dysfunction and diseases (Parkinson's and Alzheimer's disease). The supplement composition has the following nutrients: B vitamins (cyanocobalamin 2-1,000 ug, thiamin 1-1,000 mg, niacin 15-2,000 mg, pyridoxine 1-1,000 mg, Pantothenate 5-150 mg, folic acid 400-40,000 ug), alpha-tocopherol 10-800 mg, ascorbic acid 50-10,000 mg, calcium 20-2,000 mg, vitamin A 200-10,000 ug, alpha-lipoic acid 100-1,000 mg, N-acetyl cysteine 100-3,000 mg, L-carnosine 100-9,000 mg, tyrosine 100-9,000 mg, vanillin 10-100 mg, phosphatidylserine 10-800 mg, resveratrol 10-50 mg, dehydroepiandrosterone 1-50 mg, and melatonin 0.1-3 mg, all of which have been individually used experimentally or clinically for relieving stress, preventing and treating age- and stress-related disorders and diseases but no combination of these compounds has been used. Many embodiments also contain at least one adjunct ingredient such as coenzyme Q 10-200 mg, acetyl-L-carnitine 100-2,000 mg, choline 50-1,000 mg, and creatine 100-2,000 mg.
Owner:LIU JIANKANG
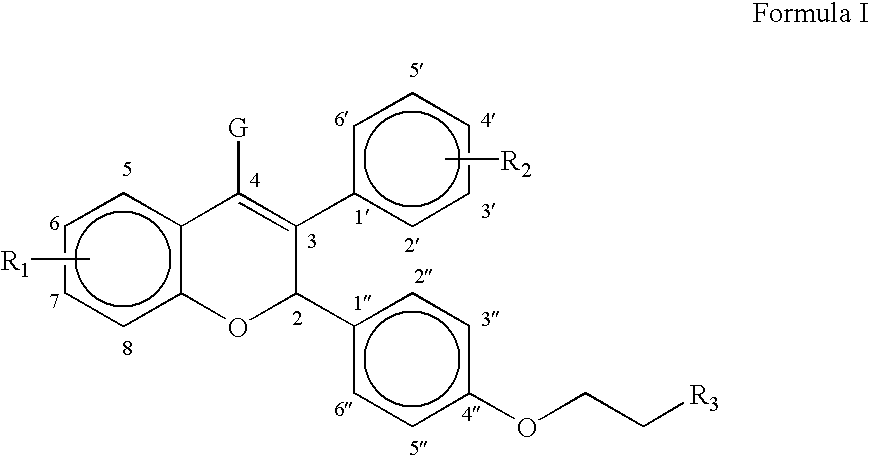
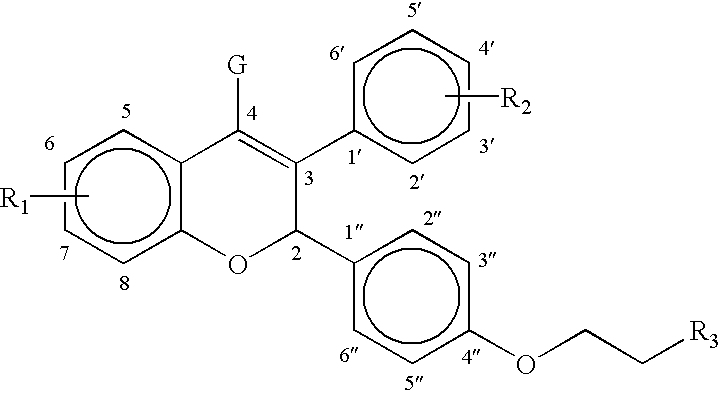
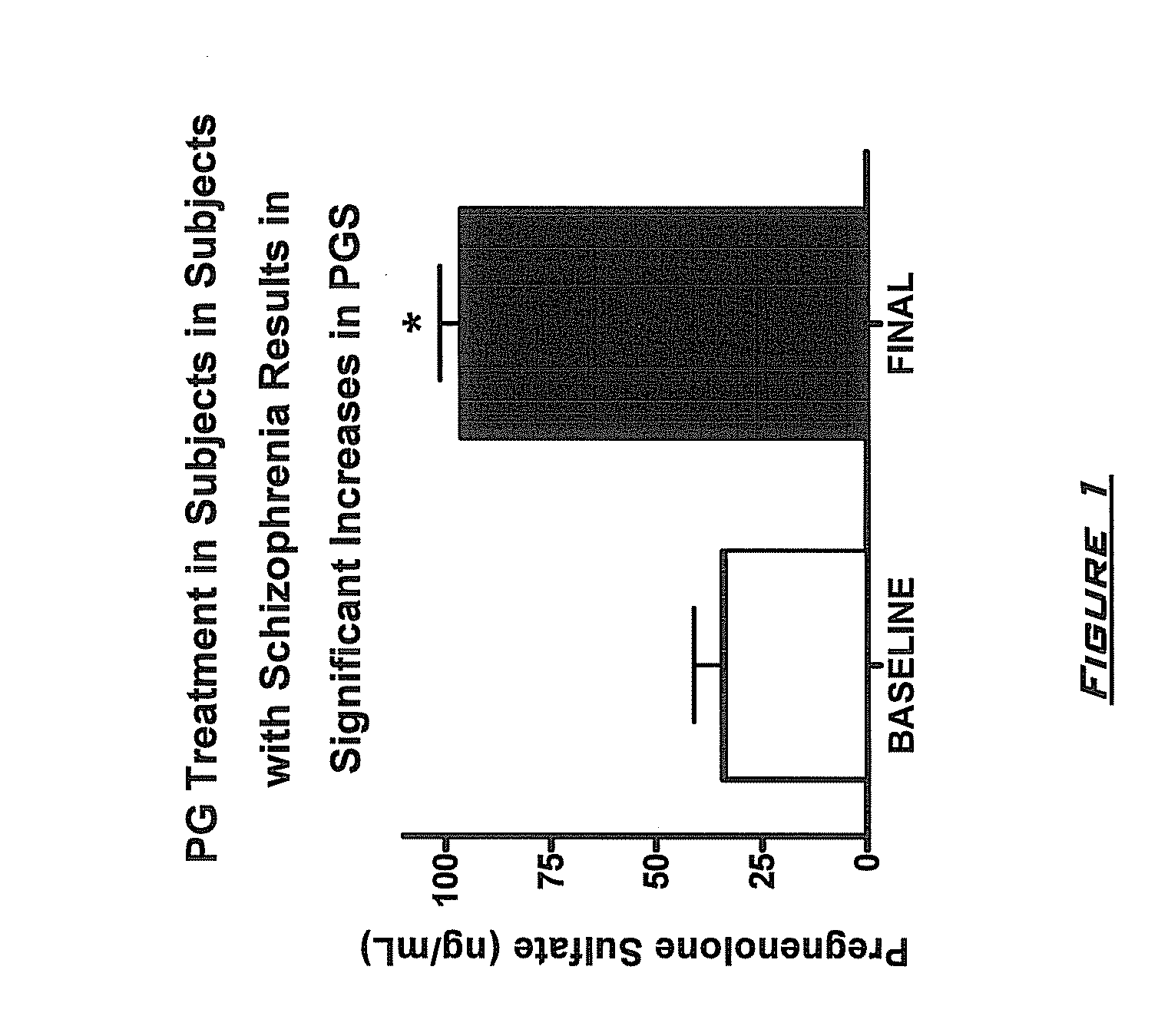
Neuroactive steroid compositions and methods of use therefor
InactiveUS20090203658A1Improve cognitive functionPromote more developedOrganic active ingredientsBiocideMetaboliteSchizo-affective type
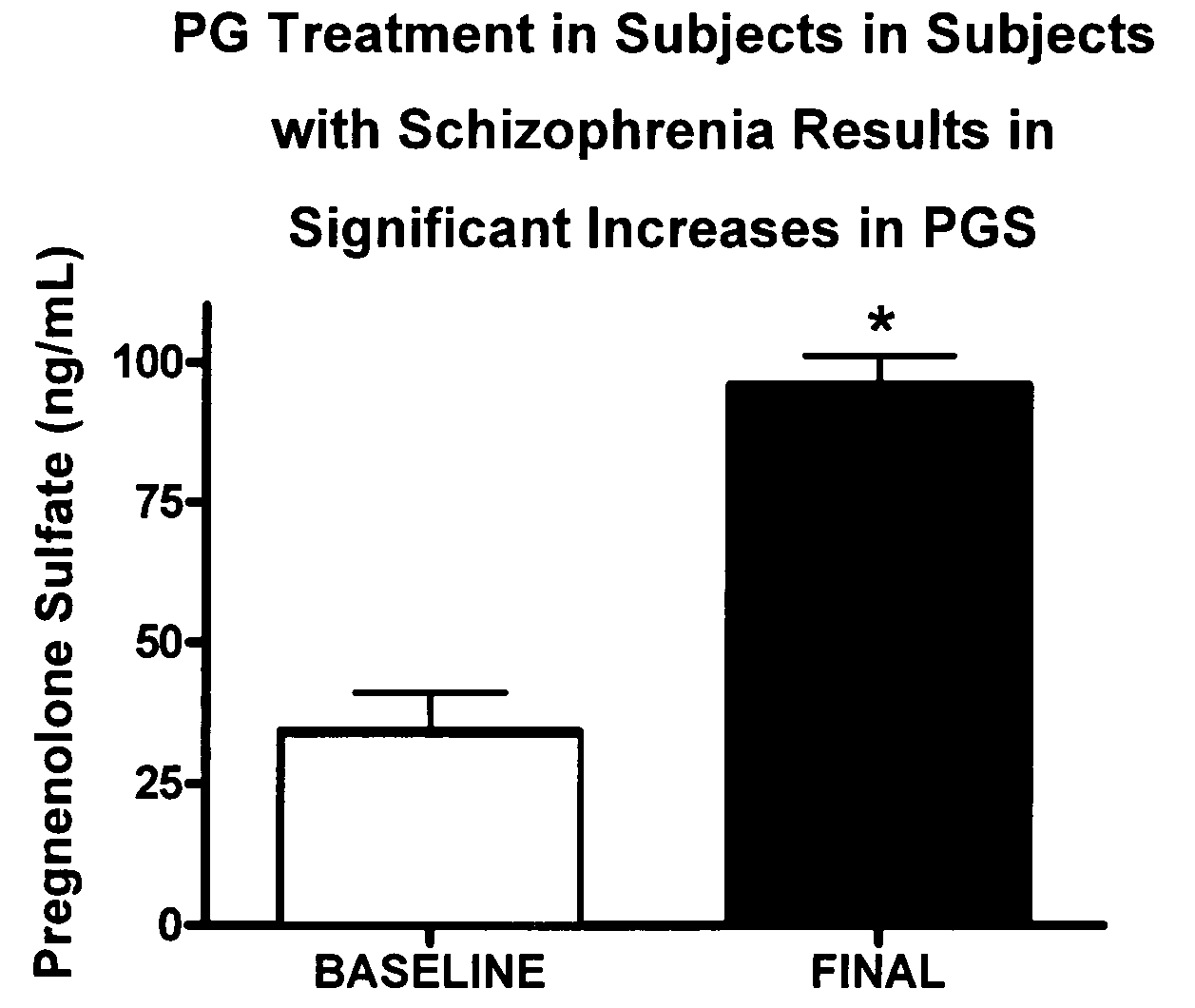
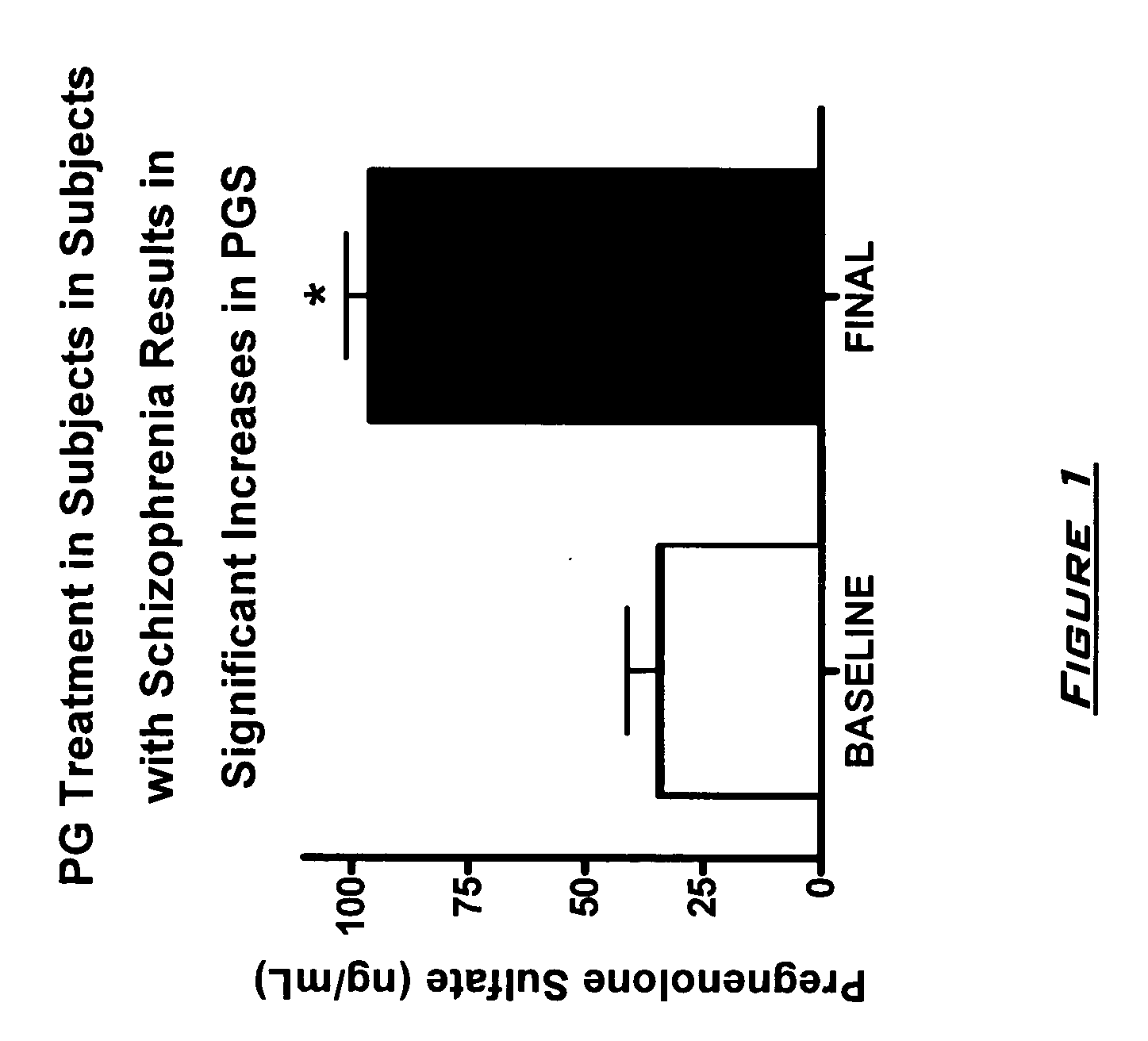
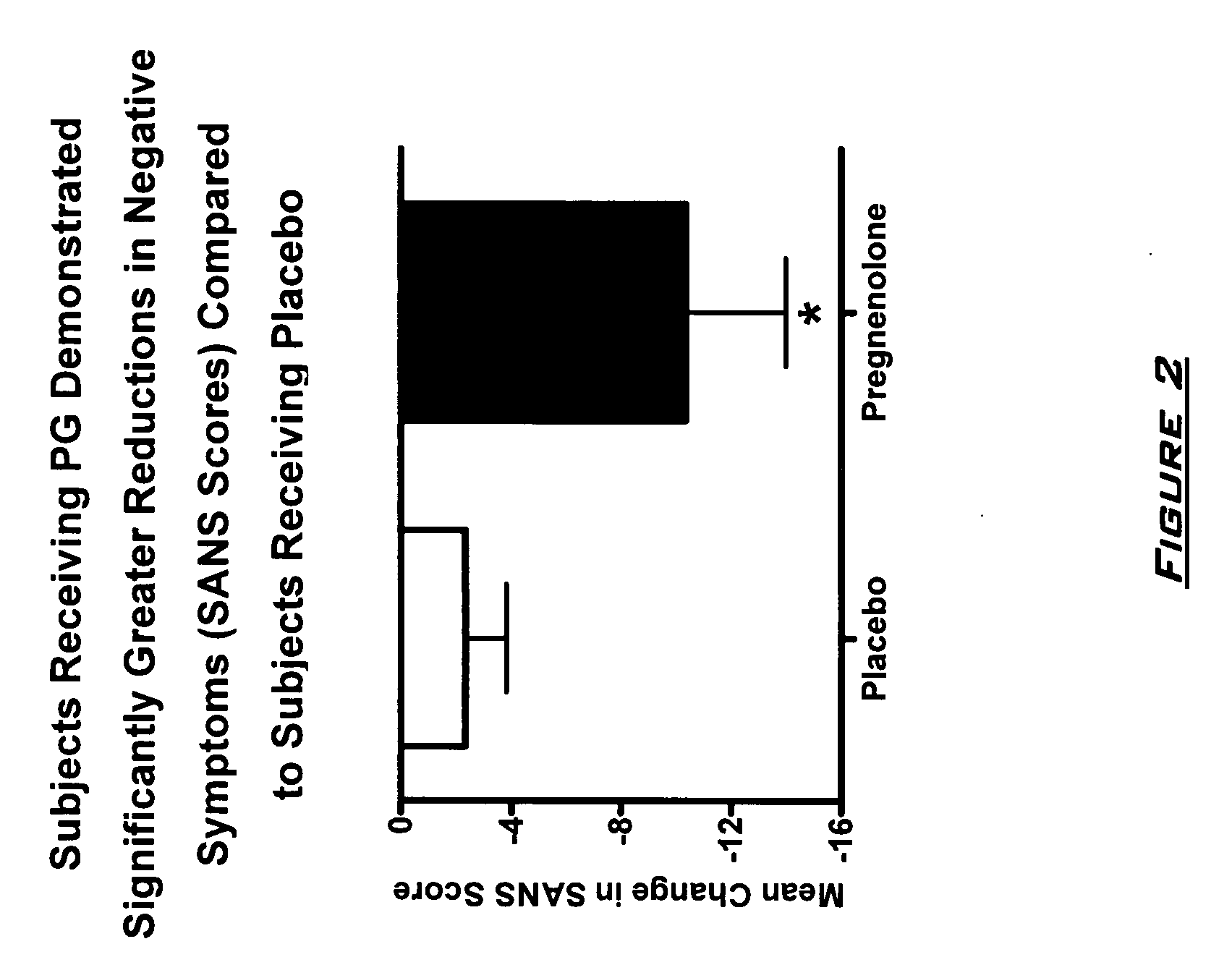
Provided are methods for ameliorating a symptom of a neuropsychiatric disorder in a subject. Also provided are methods for ameliorating at least one physical symptom or at least one psychological symptom resulting from tobacco cessation in a subject, methods for ameliorating a symptom of Alzheimer's disease or other cognitive disorder in a subject, methods for ameliorating a symptom of schizophrenia, schizoaffective disorder, or other psychotic disorder in a subject, methods for ameliorating a symptom of a depressive disorder in a subject, methods for ameliorating a symptom of bipolar disorder in a subject, methods for ameliorating a symptom of post-traumatic stress disorder or other anxiety disorder in a subject, methods for predicting a predisposition to suicide, suicidal ideation, suicidal behavior, or a combination thereof in a subject, methods for ameliorating a symptom of a pain disorder in a subject, methods for ameliorating a neurodegenerative disorder in a subject, methods for ameliorating a symptom of traumatic brain injury in a subject, methods for ameliorating a sleep disorder in a subject, and methods for improving cognitive functioning in a subject. In some embodiments, the methods include administering to a subject in need thereof an effective amount of a neuroactive steroid composition comprising pregnenolone (PG), allopregnanolone (ALLO), dehydroepiandrosterone (DHEA), progesterone (PROG), precursors thereof, metabolites thereof, pharmaceutically acceptable salts thereof, derivatives thereof, or combinations thereof.
Owner:DUKE UNIV
Use of Δ5-androstene-3β-ol-7,17-dione in the treatment of lupus erythematosus
Lupus erythematosus can be treated by administering therapeutic amounts of Δ5-androstene-3β-ol-7,17-dione and metabolizable precursors thereof, such as Δ5-androstene-3b-acetoxy-7,17-dione, which are readily metabolized in vivo to Δ5-androstene-3β-ol-7,17-dione but are not appreciably metabolizable in vivo to androgens, estrogens or dehydroepiandrosterone. Such treatment can be prophylactic, ameliorative or curative in nature.
Owner:INTERHEALTH NUTRACEUTICALS
Pharmaceutical compositions
ActiveUS8268806B2Achieve beneficial effectAvoid their undesirable side effectOrganic active ingredientsMuscular disorderEstrogenic EffectsInsulin resistance
Owner:MYRIEL PHARM LLC
Method of preventing or treating benign gynaecological disorders
ActiveUS8071576B2Few side-effectsLow recurrence rateBiocideOrganic active ingredientsDiseaseGynecological disorders
The present invention relates to a method of preventing or treating benign estrogen sensitive gynecological disorders in a female mammal, wherein the method comprises the administration to said female mammal of a combination of progestogen and androgen in an amount that is therapeutically effective to prevent or reduce the symptoms of these disorders. The present method is particularly suitable for preventing or treating disorders selected from the group consisting of endrometriosis, adenomyosis, uterine fibroids, dysmenorrhoea, menorrhagia and metrorrhagia. Another aspect of the invention relates to a pharmaceutical kit comprising a plurality of oral dosage units which comprise a progestogen in an amount equivalent to 3-500 μg levonorgestrel and either 5 to 250 mg dehydroepiandrosterone or 1 to 50 mg testosterone undecanoate.
Owner:PANTARHEI BIOSCI
Medical uses of a selective estrogen receptor modulator in combination with sex steroid precursors
InactiveUS6465445B1Reduce riskGood effectBiocideOrganic chemistrySelective progesterone receptor modulatorOsteopetrosis
Novel methods for the medical treatment and / or inhibition of the development of osteoporosis, breast cancer, hypercholesterolemia, hyperlipidemia or atherosclerosis in susceptible warm-blooded animals including humans involving administration of selective estrogen receptor modulator particularly compounds having the general structure:and an amount of a sex steroid precursor selected from the group consisting of dehydroepiandrosterone, dehydroepiandrosterone sulfate, androst-5-ene-3beta,17beta-diol and compounds converted in vivo to one of the foregoing presursor. Further administration of bisphosphonates in combination with selective estrogen receptor modulators and / or sex steroid precursor is disclosed for the medical treatment and / or inhibition of the development of osteoporosis. Pharmaceutical compositions for delivery of active ingredient(s) and kit(s) useful to the invention are also disclosed.
Owner:ENDORES & DEV
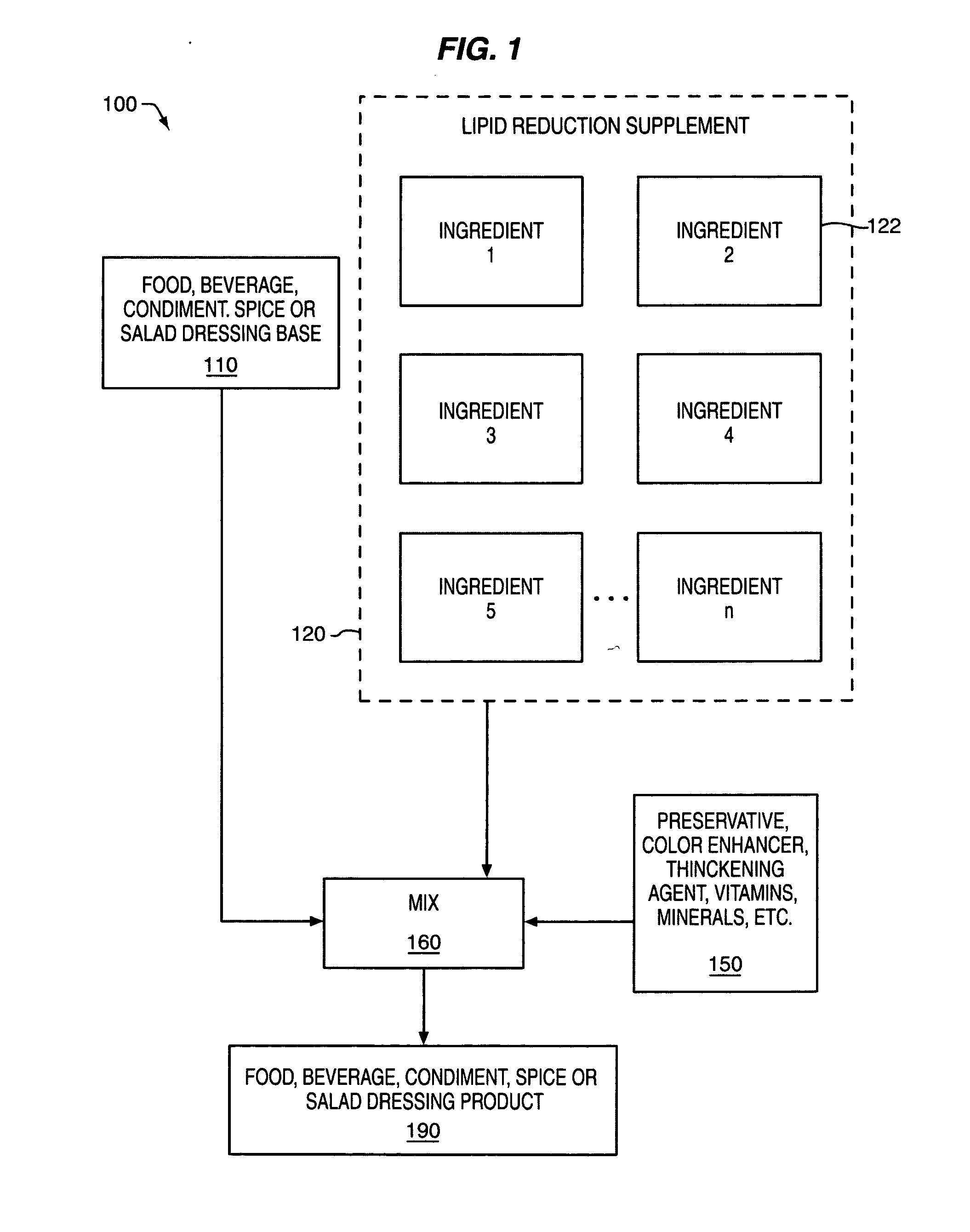
Composition and method for reducing lipid storage
InactiveUS20050095233A1Increasing fatty acid oxidationEnhancement of thermogenic futile cycleBiocidePeptide/protein ingredientsLipid storageAdditive ingredient
An orally or parenterally administered composition for reducing the storage of lipids in a human, the composition comprising: an effective amount of hydroxycitric acid; an effective amount of carnitine; an effective amount of biotin; an effective amount of one or more gluconeogenic substrates selected from the group consisting of: aspartate, lactate, glycerol, and a gluconeogenic amino acid or alphaketo analogue thereof; an effective amount of eicosapentanoic acid; and an effective amount of one or more ingredients selected from the group consisting of: medium chain triglycerides (MCT) with fatty acid backbones containing 6 to 14 carbon atoms or their individual fatty acid analogues or metabolic precursors, sesame seeds or derivative products, sesamin and / or its epimer episesamin, caffeine, forskolin, 7-keto dehydroepiandrosterone (7-keto DHEA), green tea extract containing epigallocatechingallate (EGCG), capsaicum, and 5-hydroxytryptophan (5-HTP). The supplement may also be combined with a food, beverage, condiment, spice or salad dressing base to provide a food, beverage, condiment, spice or salad dressing product designed to reduce lipid storage.
Owner:MCCLEARY EDWARD LARRY +2
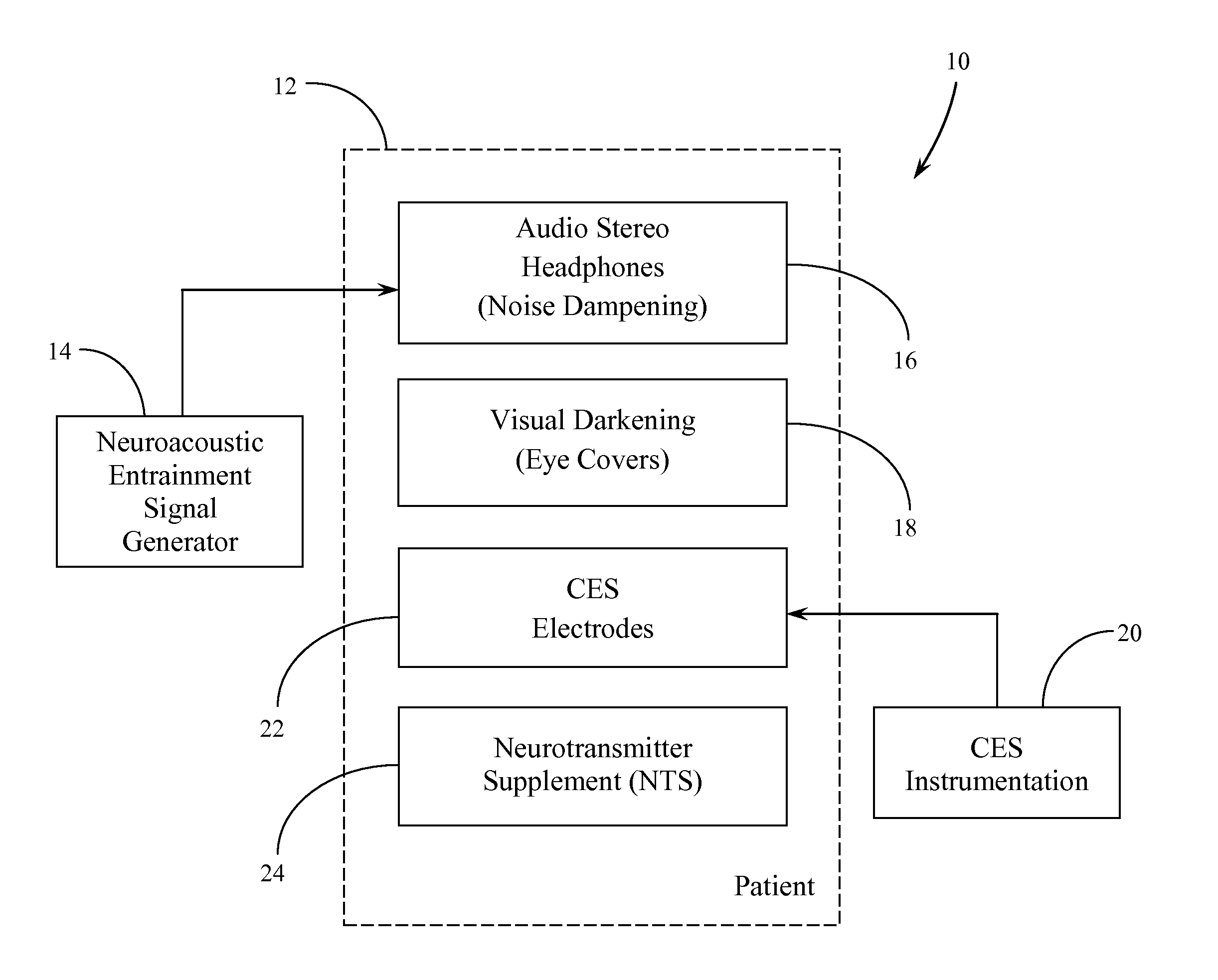
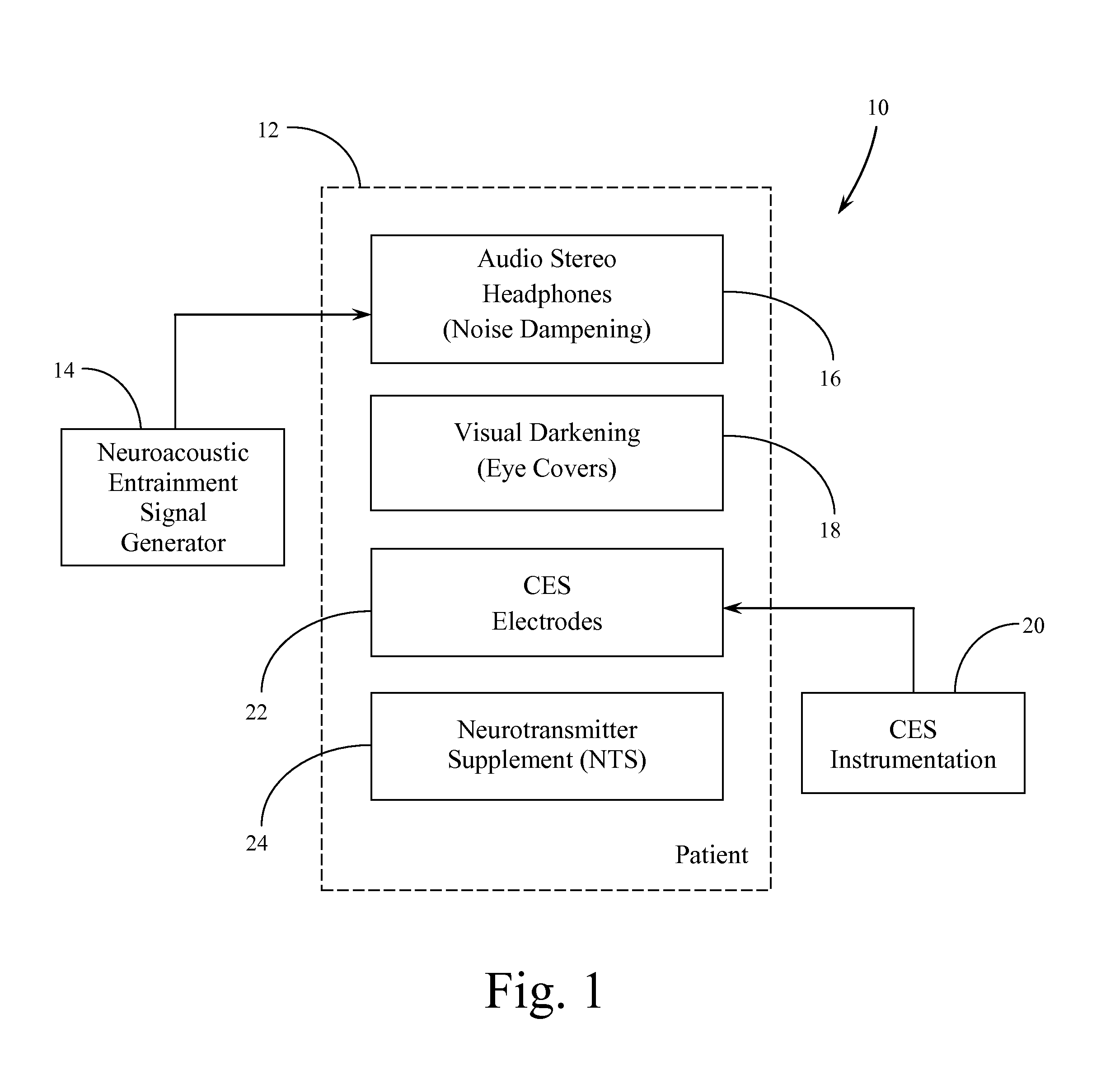
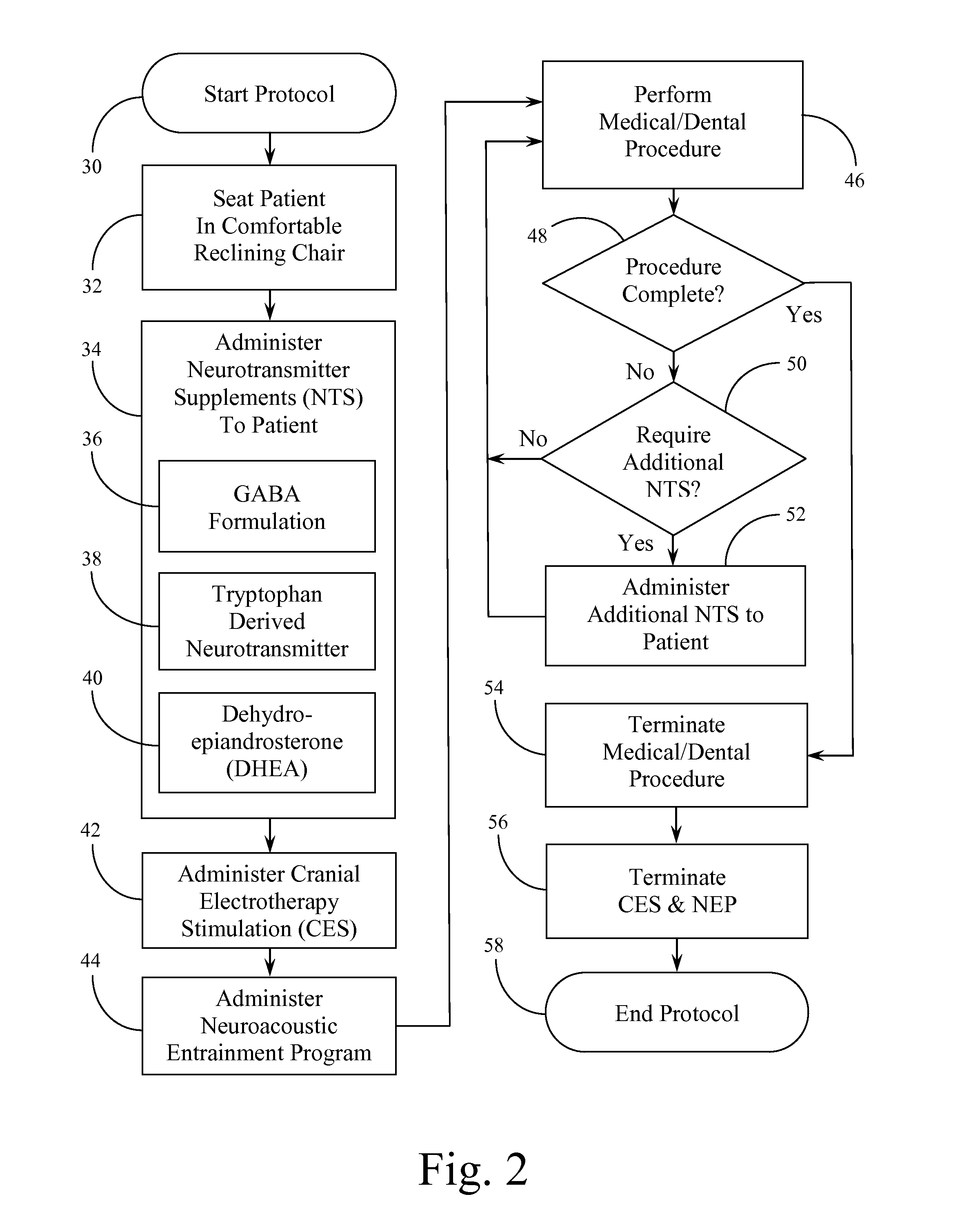
Systems and Methods for Balancing and Maintaining the Health of the Human Autonomic Nervous System
ActiveUS20120149973A1Promote recoveryOrganic active ingredientsElectrotherapyNervous systemBlack out
A protocol or procedure is provided for lowering sympathetic nervous system arousal in a person in order to prepare that person for a medical or dental procedure. First, a therapeutic dosage of one or more neurotransmitter supplements, such as a gamma aminobutyric acid formulation, a tryptophan-derived neurotransmitter precursor, and dehydroepiandrosterone are administered to the patient. Concomitantly, gelled electrodes are placed adjacent or below the mastoid. The gelled electrodes are connected to a cranial electrotherapy stimulation device that administers a sub-sensation level current to the patient. Also, a noise dampening headset is placed on the patient and a neuroacoustic entrainment recording or program is played. Next, light is blocked with black out glasses. Then, the medical or dental procedure is performed.
Owner:SOLACE LIFESCI INC
Preparation method of abiraterone acetate
ActiveCN102627681AReduce usageAvoid separation and purificationSteroidsAcetic anhydrideEthyl Chloride
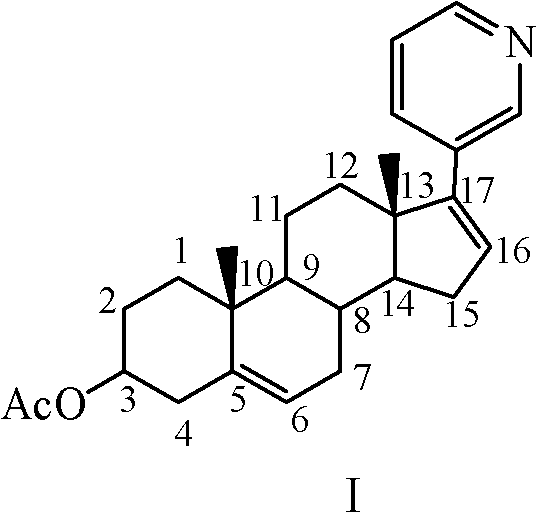
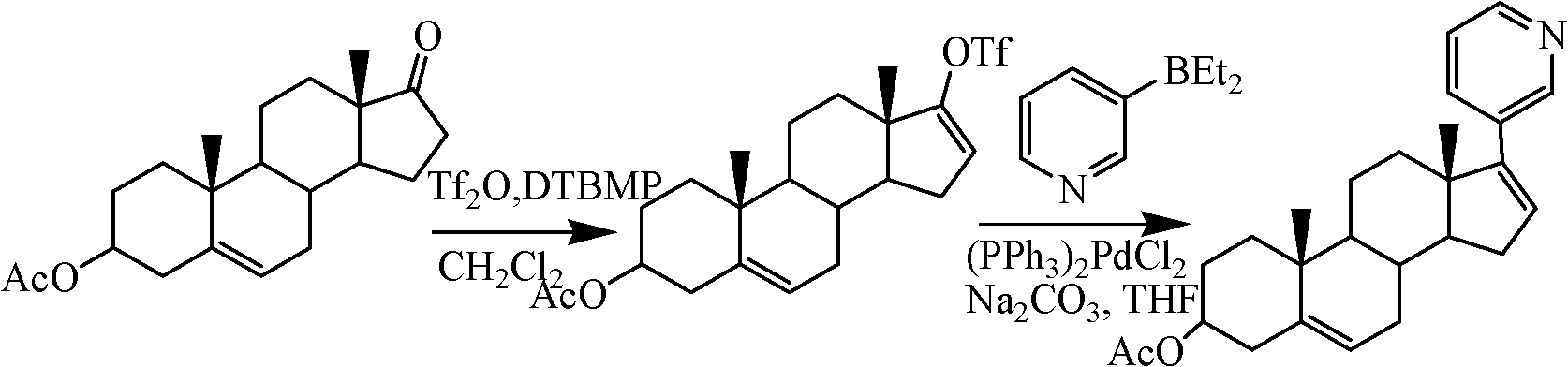
The invention relates to a preparation method of abiraterone acetate. The method comprises: taking dehydroepiandrosterone as the raw material, which successively reacts with hydrazine hydrate and idoine so as to obtain 17-iodo-adrost-5, 16-diene-3beta-ol, which then undergoes a Negishi coupling reaction with 3-pyridine zinc halide to obtain abiraterone, and finally conducting esterification with acetyl chloride or acetic anhydride, thus obtaining the target product abiraterone acetate.
Owner:SHANDONG NEWTIME PHARMA
Neuroactive steroid compositions and methods of use therefor
InactiveUS20090074677A1Improve cognitive functionPromote more developedOrganic active ingredientsBiocideSchizo-affective typePain disorder
Provided are methods for ameliorating a symptom of a neuropsychiatric disorder in a subject. Also provided are methods for ameliorating at least one physical symptom or at least one psychological symptom resulting from tobacco cessation in a subject, methods for ameliorating a symptom of Alzheimer's disease or other cognitive disorder in a subject, methods for ameliorating a symptom of schizophrenia, schizoaffective disorder, or other psychotic disorder in a subject, methods for ameliorating a symptom of a depressive disorder in a subject, methods for ameliorating a symptom of bipolar disorder in a subject, methods for ameliorating a symptom of post-traumatic stress disorder or other anxiety disorder in a subject, methods for predicting a predisposition to suicide, suicidal ideation, suicidal behavior, or a combination thereof in a subject, methods for ameliorating a symptom of a pain disorder in a subject, methods for ameliorating a neurodegenerative disorder in a subject, methods for ameliorating a symptom of traumatic brain injury in a subject, methods for ameliorating a sleep disorder in a subject, and methods for improving cognitive functioning in a subject. In some embodiments, the methods include administering to a subject in need thereof an effective amount of a neuroactive steroid composition comprising pregnenolone (PG), allopregnanolone (ALLO), dehydroepiandrosterone (DHEA), pharmaceutically acceptable salts thereof, derivatives thereof, or combinations thereof.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
DHEA composition and method
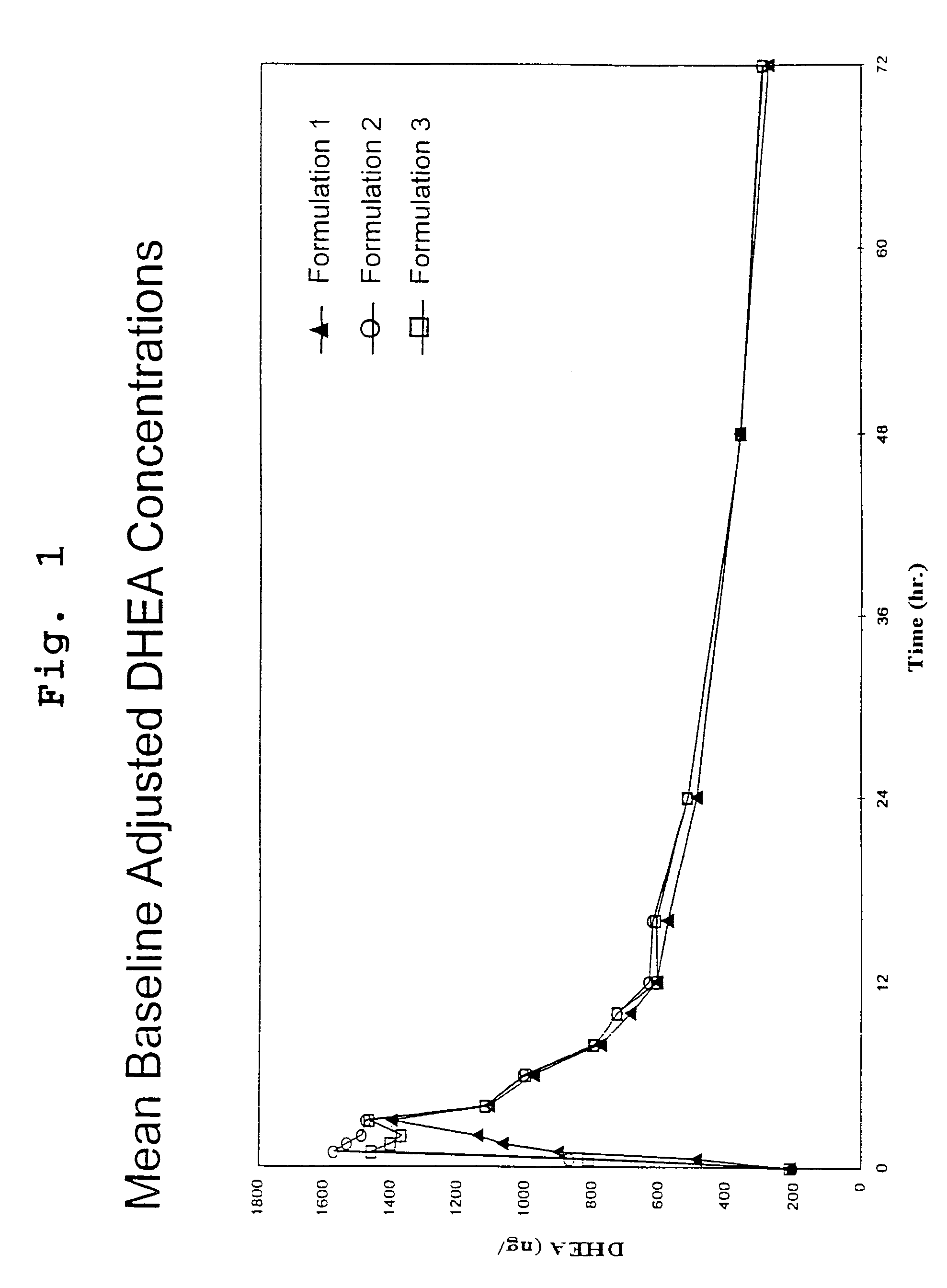
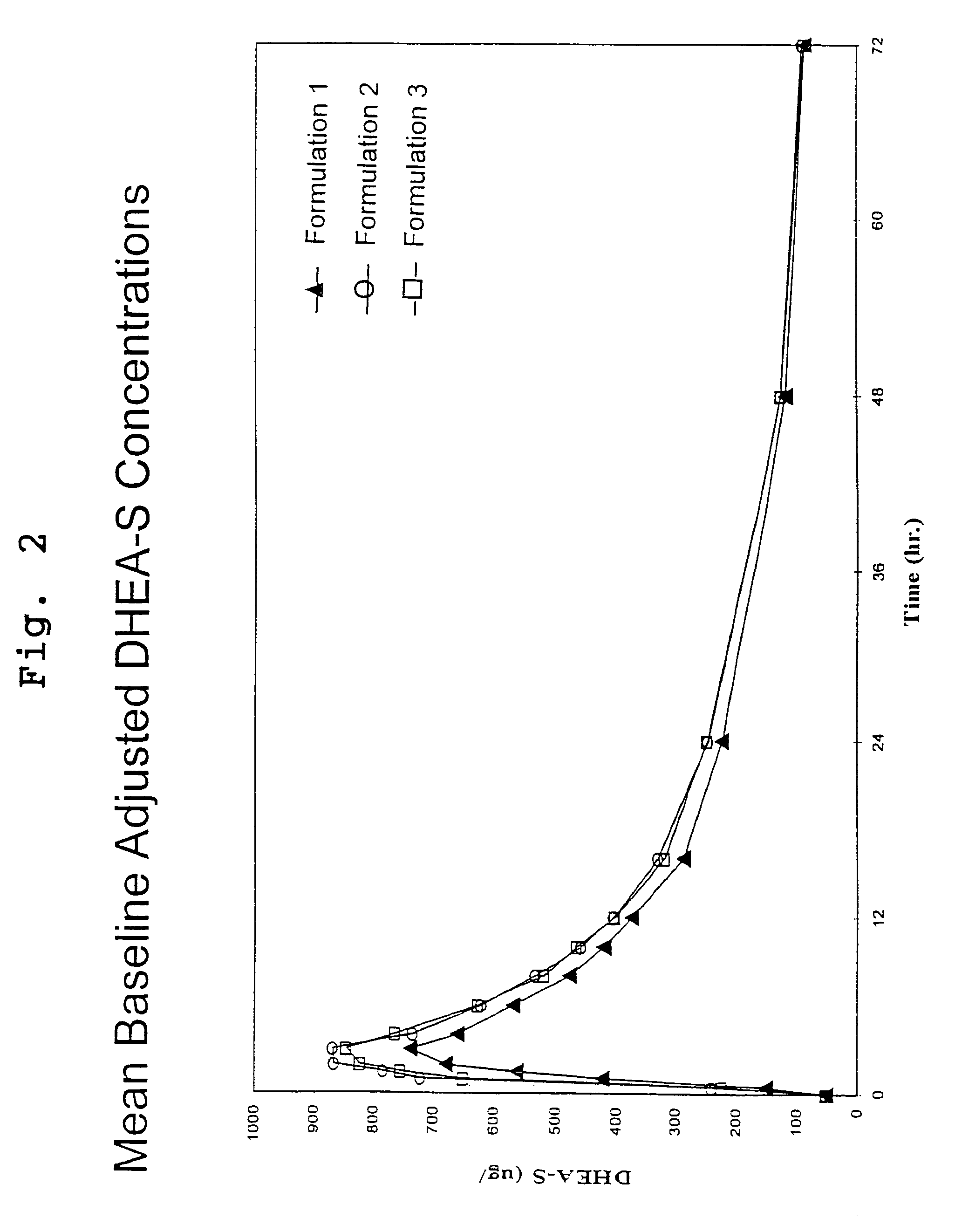
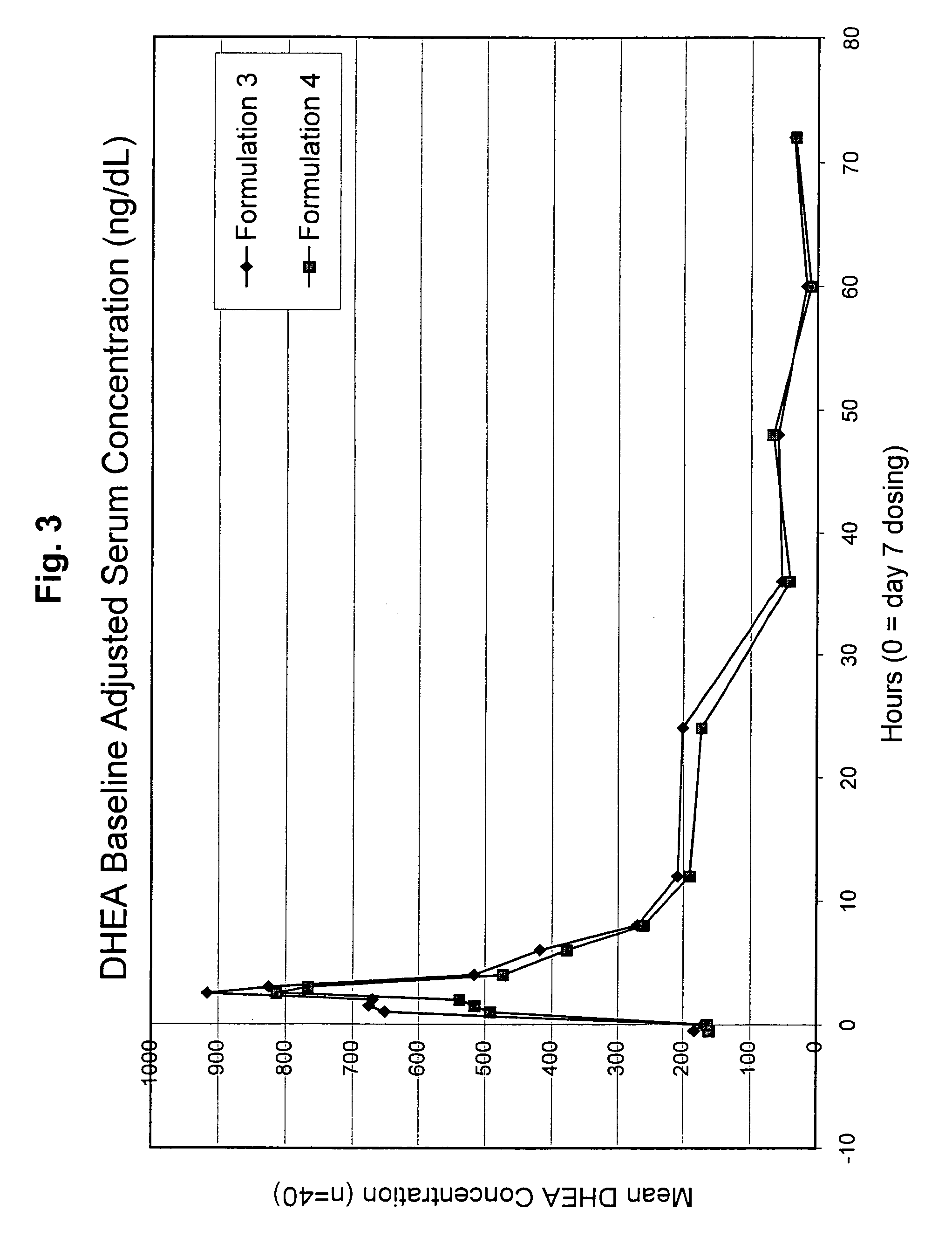
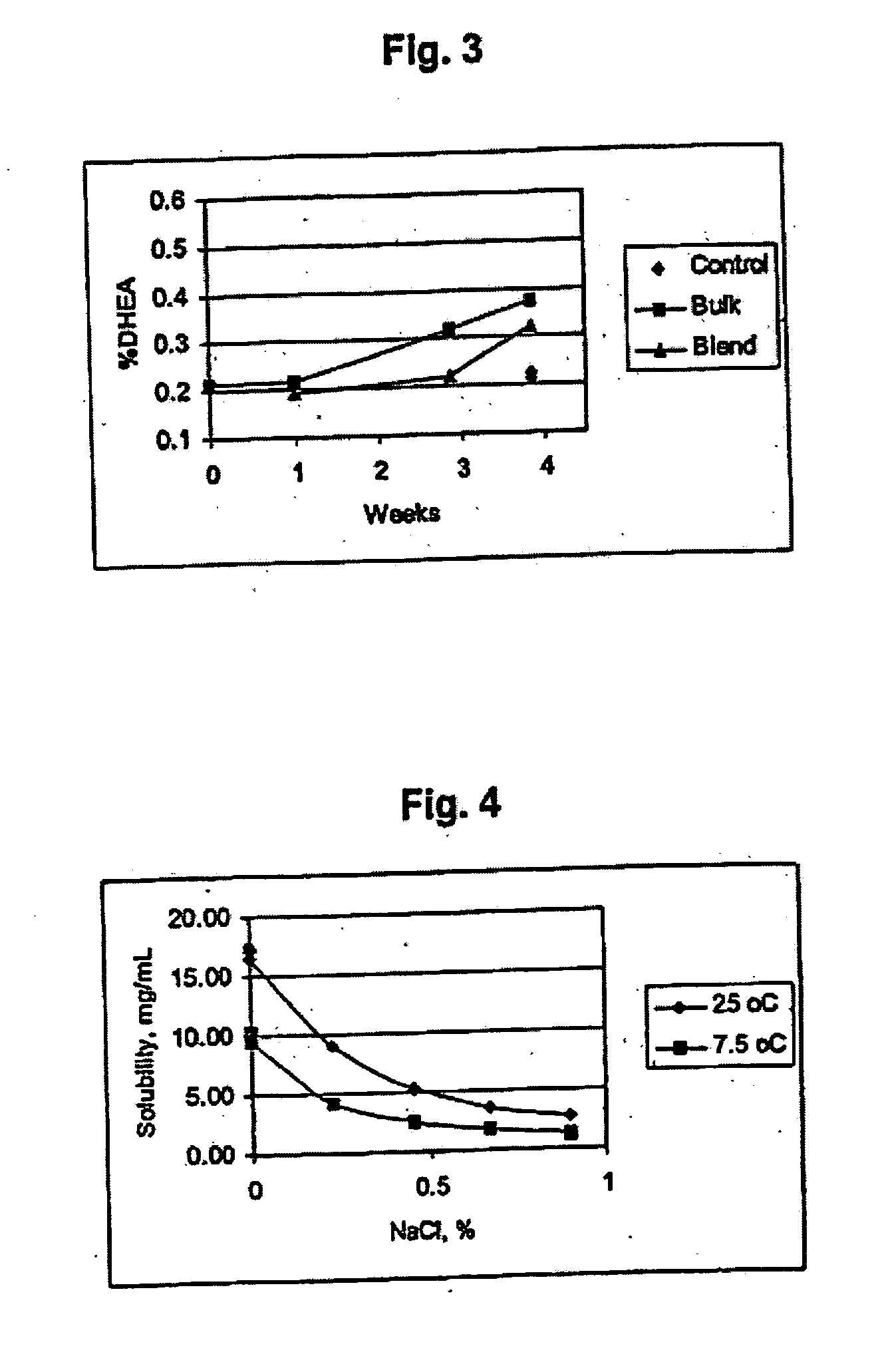
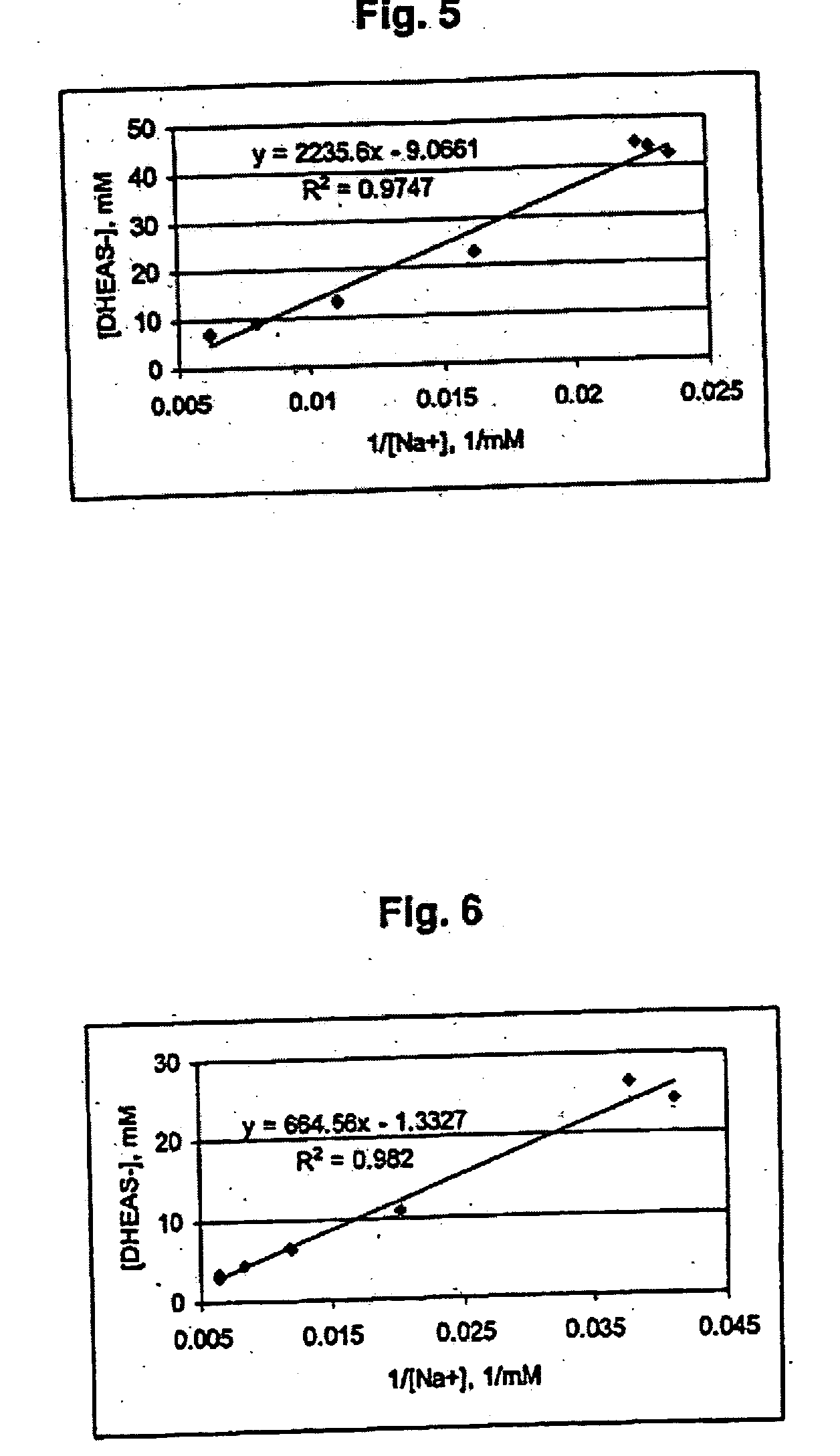
Disclosed are improved pharmaceutical formulations comprising dehydroepiandrosterone (DHEA), enriched in selected polymorphic forms, for therapeutic applications. In one embodiment, the formulation comprises, in solid form, DHEA, at least 85% of which is present as a single polymorph selected from the form I polymorph or the form II polymorph, and at least one pharmaceutical excipient. Methods for making and using such compositions are also disclosed.
Owner:GENELABS TECH INC
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a tyrosine kinase inhibitor, delta opioid receptor antagonist, neurokinin receptor antagonist, or VCAM inhibitor for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026850A1Alleviate different aspectConvenient treatmentBiocideOrganic active ingredientsDiseaseActive agent
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a tyrosine kinase inhibitor, delta opioid receptor antagonist, neurokinin receptor antagonist, or VCAM inhibitor for the treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases.
Owner:EPIGENESIS PHARMA LLC
Medical uses of a selective estrogen receptor modulator in combination with sex steroid precursors
InactiveUS7005428B1Efficient treatment methodMinimizing undesirable sideBiocidePhosphorous compound active ingredientsIn vivoBULK ACTIVE INGREDIENT
Novel methods for the medical treatment and / or inhibition of the development of hypercholesterolemia in susceptible warm-blooded animals including humans involving administration of selective estrogen receptor modulator particularly compounds having the general structure: and an amount of a sex steroid precursor selected from the group consisting of dehydroepiandrosterone, dehydroepiandrosterone sulfate, androst-5-ene-3β,17β-diol and compounds converted in vivo to one of the foregoing precursor. Further administration of bisphosphonates in combination with selective estrogen receptor modulators and / or sex steroid precursor is disclosed for the medical treatment and / or inhibition of the development of osteoporosis. Pharmaceutical compositions for delivery of active ingredient(s) and kit(s) useful to the invention are also disclosed.
Owner:ENDORES & DEV
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a methylxanthine derivative for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026848A1Alleviate different aspectConvenient treatmentOrganic active ingredientsBiocideDiseaseDehydroepiandrosterone sulfate
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a methylxanthine derivative for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
Medical uses of a selective estrogen receptor modulator in combination with sex steroid precursors
InactiveUS6670346B1Reduce riskBiocideOrganic chemistrySelective progesterone receptor modulatorIn vivo
Novel methods for the medical treatment and / or inhibition of the development of osteoporosis, breast cancer, hypercholesterolemia, hyperlipidemia or atherosclerosis in susceptible warm-blooded animals including humans involving administration of selective estrogen receptor modulator particularly compounds having the general structure:and an amount of a sex steroid precursor selected from the group consisting of dehydroepiandrosterone, dehydroepiandrosterone sulfate, androst-5-ene-3beta,17beta-diol and compounds converted in vivo to one of the foregoing presursor. Further administration of bisphosphonates in combination with selective estrogen receptor modulators and / or sex steroid precursor is disclosed for the medical treatment and / or inhibition of the development of osteoporosis. Pharmaceutical compositions for delivery of active ingredient(s) and kit(s) useful to the invention are also disclosed.
Owner:ENDORES & DEV
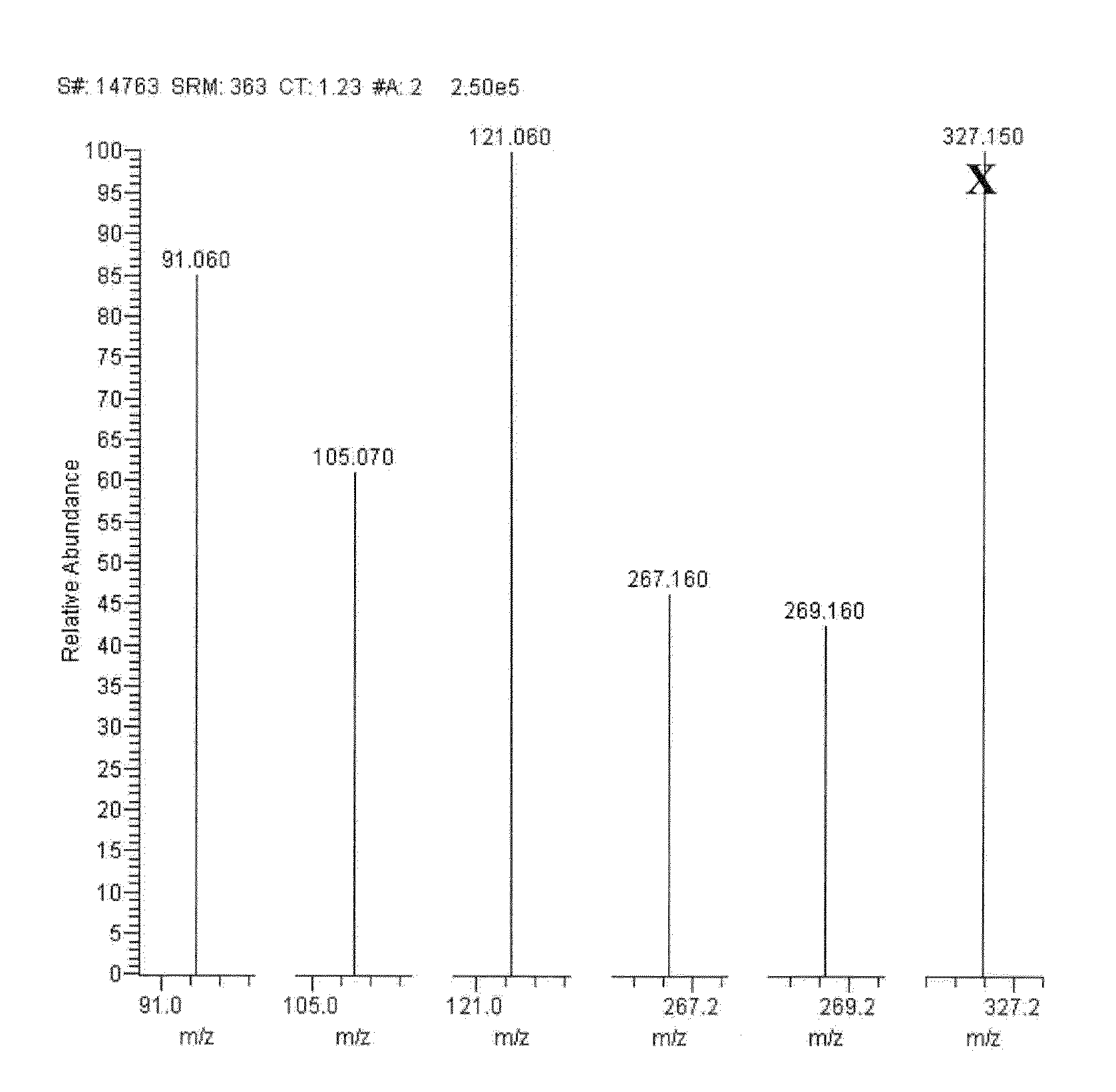
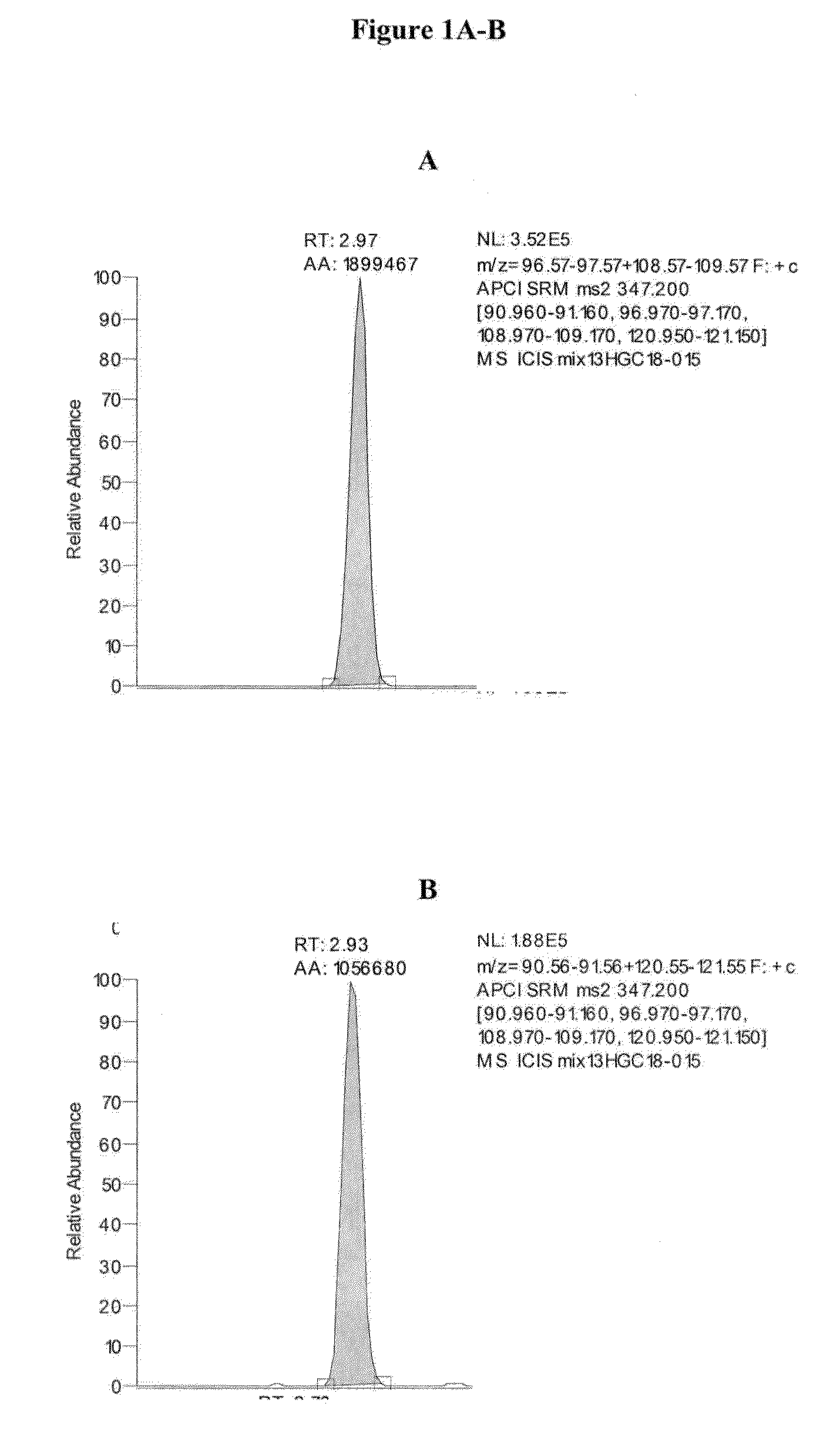
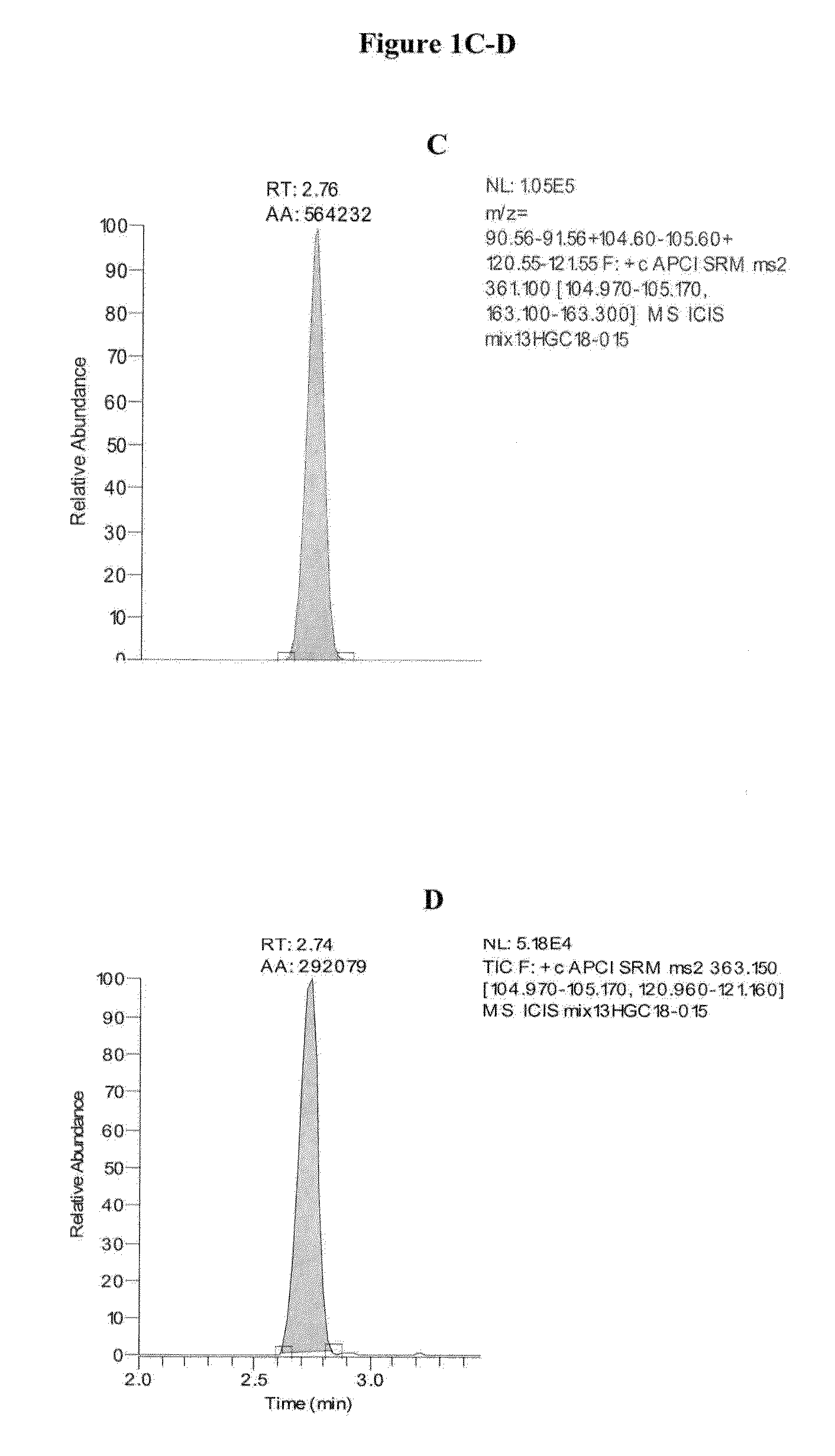
Mass spectrometry assay for congenital adrenal hyperplasia
ActiveUS20100155595A1Avoid condensationSolvent is evaporatedComponent separationFuel lighters11-DesoxycortisolHydrocortisone
Methods are provided for detecting the amount of one or more CAH panel analytes (i.e., pregnenolone, 17-OH pregnenolone, progesterone, 17-OH progesterone, dehydroepiandrosterone (DHEA), androstenedione, testosterone, deoxycorticosterone, 11-deoxycortisol, and cortisol) in a sample by mass spectrometry. The methods generally involve ionizing one or more CAH panel analytes in a sample and quantifying the generated ions to determine the amount of one or more CAH panel analytes in the sample. In methods where amounts of multiple CAH panel analytes are detected, the amounts of multiple analytes are detected in the same sample injection.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
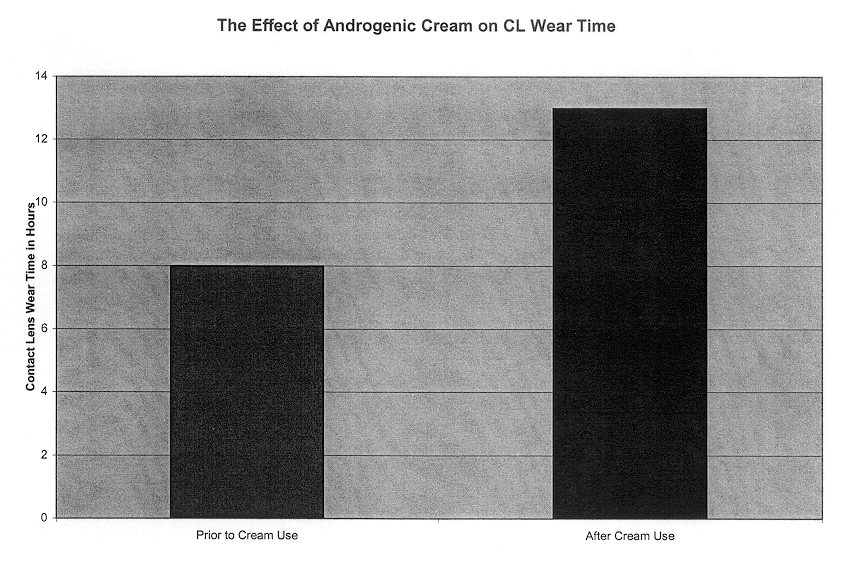
Method to use transdermal administration of androgens to the adnexa of the eye
InactiveUS6659985B2Increasing contact lens wear timeIncrease contact lens wear timeOrganic active ingredientsPharmaceutical delivery mechanismGynecologyAndrogen
This invention relates to a method for treating dry eye or increasing contact lens wear time through the transdermal delivery of androgenic hormones to the adnexa of the eye. More specifically, an androgenic hormone such as testosterone or dehydroepiandrosterone is solubilized in pharmaceutically effective carrier such as a facial cream or gel. The androgenic hormone in a pharmaceutically effective carrier is applied to the adnexa of the eye, which is the tissue adjacent to and surrounding the eyeball.
Owner:SOUTHERN COLLEGE OF OPTOMETRY
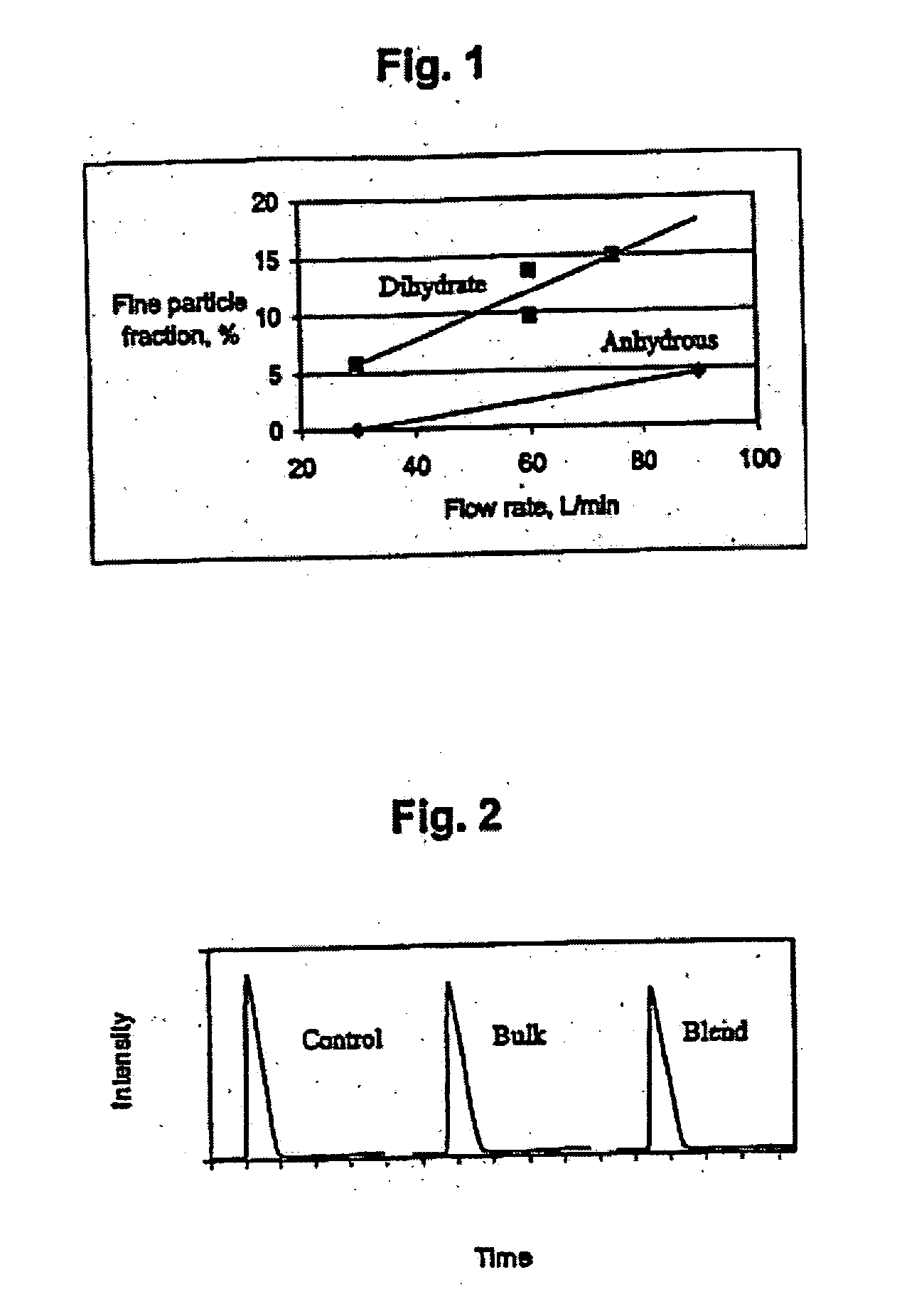
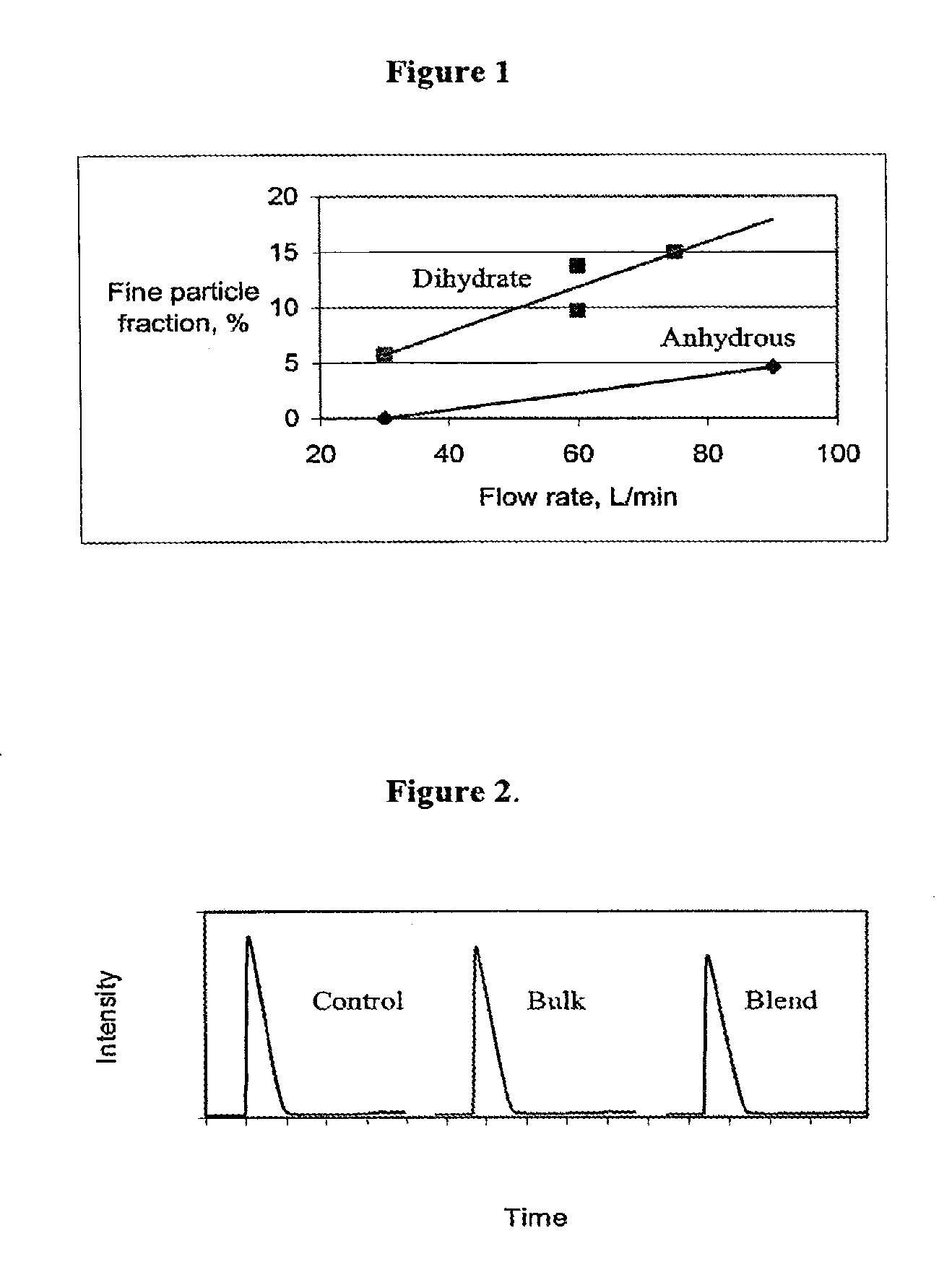
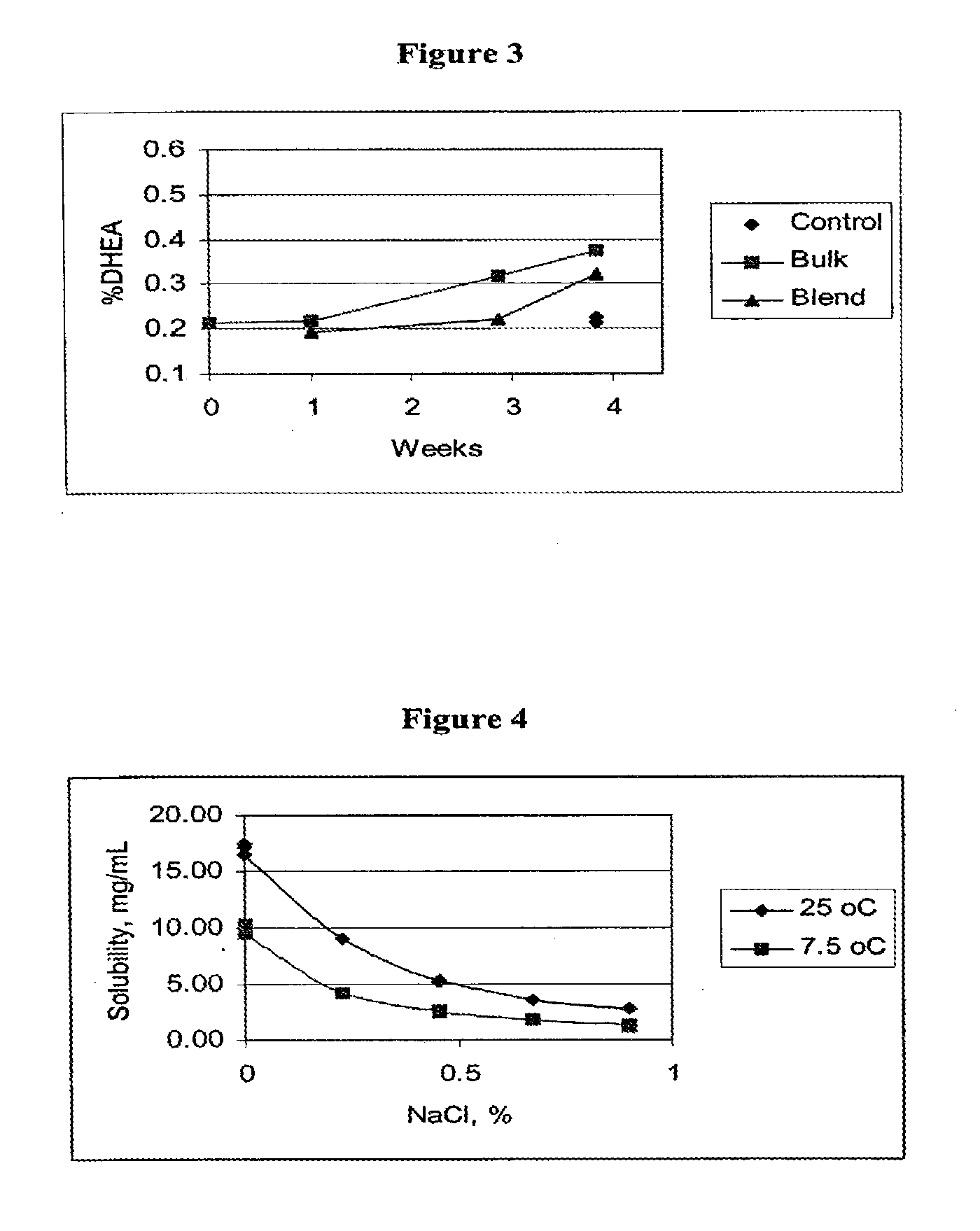
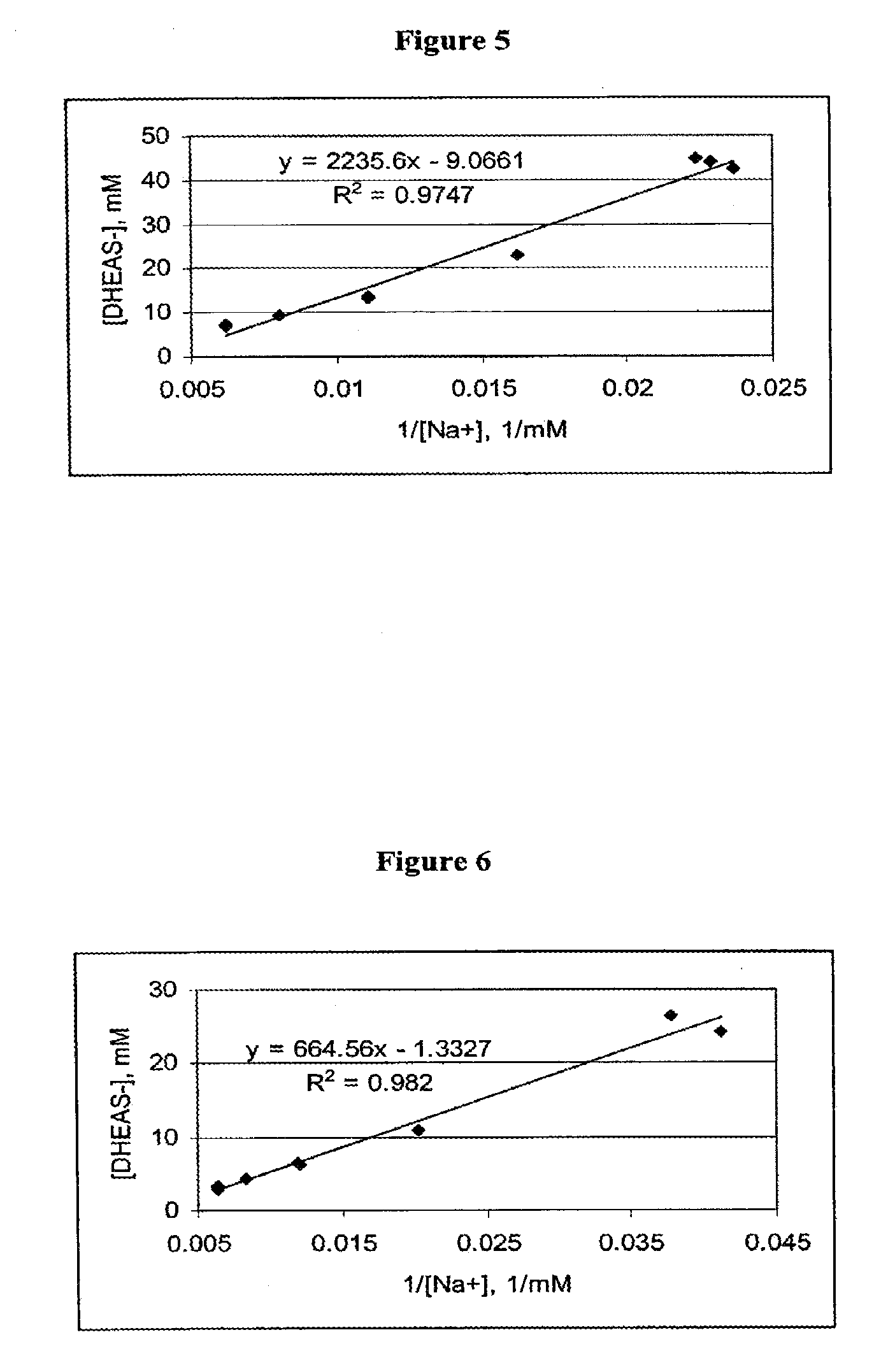
Dehydroepiandrosterone sulfate dihydrate inhalation compositions and methods
InactiveUS20090087389A1Reduce and deplete adenosine levelReducing and depleting adenosineAntibacterial agentsPowder deliveryCOPDDehydroepiandrosterone sulfate
The invention relates to inhalation compositions derived from dehydroepiandrosterone sulfate. The compositions of the invention involve liquid nebulizer formulations prepared from dehydroepiandrosterone sulfate dihydrate. The compositions of the invention comprise water and chloride ion. The liquid nebulizer formulations can be used to treat patients suffering from asthma or COPD.
Owner:EPIGENESIS PHARMA LLC
Edible fortified tea oil for pregnant woman and preparation method thereof
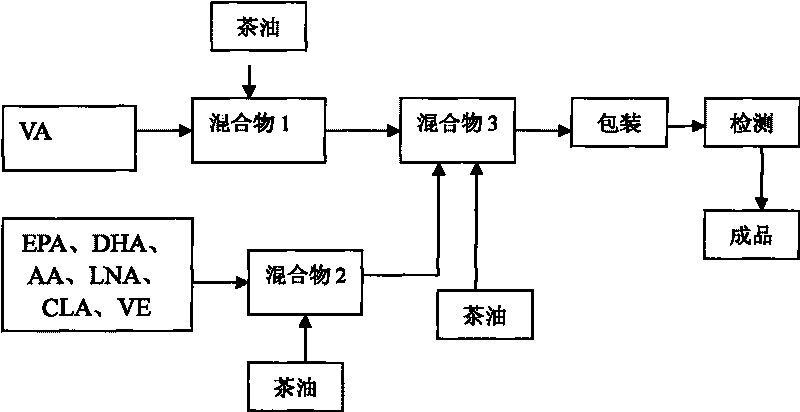
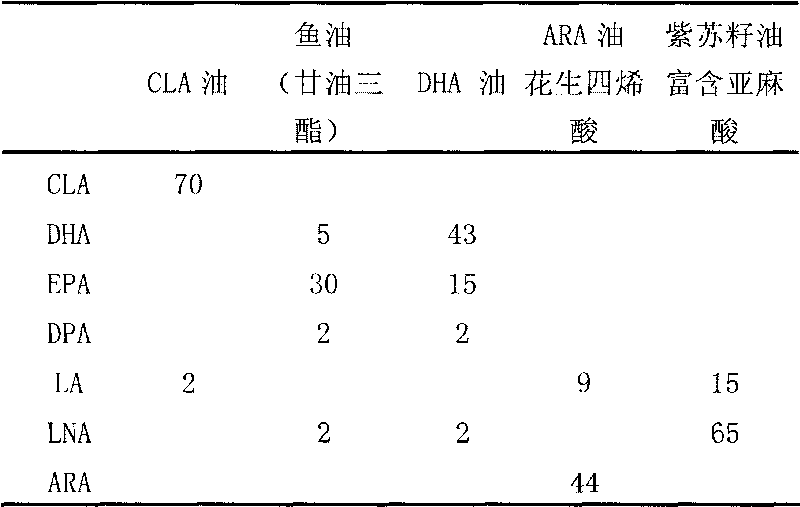
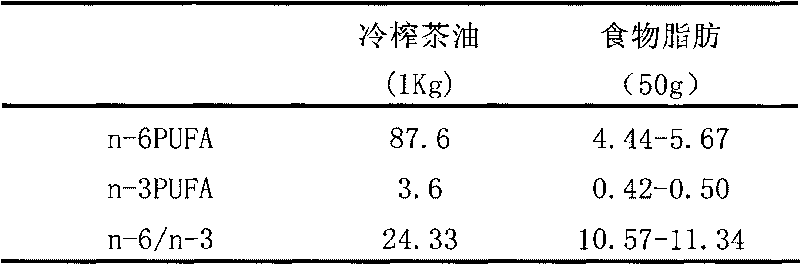
InactiveCN101755927ADelayed visual developmentEnhance memoryEdible oils/fatsCamellia oleiferaPesticide residue
The invention discloses edible fortified tea oil for pregnant women and a preparation method thereof. The oil is particularly designed for Chinese pregnant women based on selecting tea oil of the first degree refined with natural camellia oleosa seeds with high quality, fortifies DHA (dehydroepiandrosterone), EPA (eicosapentaenoic acid), arachidonic acid, Alpha-linolenic acid and vitamin A, and ensures that n-6 / n-3 fatty acid in food for pregnant women reaches the optimal 4:1. Fatty acids with different structures in the oil reach a theoretical balance, and can satisfy the requirement of pregnant women for vitamin A at the same time. The edible fortified tea oil for pregnant women contains no additive or flavor, and contacts no chemical solvent in processing and storage and contains no harmful trans-fatty acid or pesticide residue. The tea oil has a pure and delicate unique flavor and accords with state standards for edible tea oil.
Owner:HUBEI HUANGPAOSHAN GREEN PROD
Synthesis method for dehydroepiandrosterone
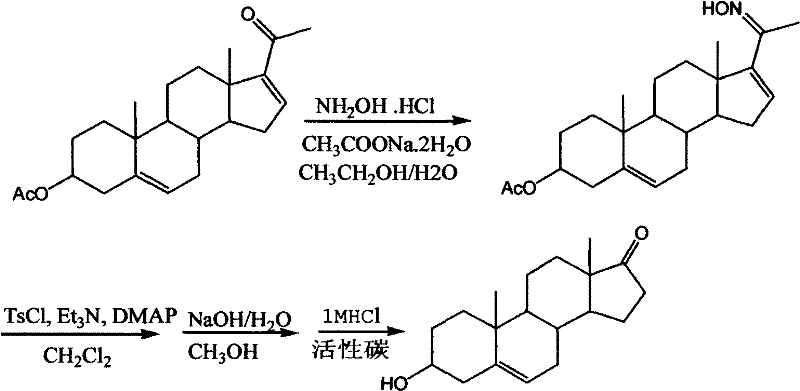
InactiveCN102212099AOvercoming reactivityOvercoming processingSteroidsBeckmann rearrangementSodium acetate
The invention belongs to a novel synthesis method for dehydroepiandrosterone, which comprises the following steps: oximation of 16-dehydropregnenolone acetate, Beckmann rearrangement, hydrolysis and refining to obtain a product. The synthesis method is characterized by carrying out oximation of ketone by using sodium acetate as a base and water and ethanol as solvent, carrying out Beckmann rearrangement, hydrolysis and one-pot refining reaction, reacting 16-dehydropregnenolone acetate oxime with p-toluenesulfonamide chloride, benzenesulfonyl chloride, triethylamine or N,N-dimethyl-pyridine (DMAP) in a dichloromethane solution, concentrating the solvent after reaction, adding methanol and a sodium hydroxide solution for refluxing hydrolysis, cooling and regulating the pH value to 7-8, adding activated carbon, then refluxing for 30-60 minutes, filtering, concentrating, crystallizing, and centrifuging to obtain the product. The synthesis method provided by the invention is simple to operate, and has characteristics of mild reaction conditions, high yield, low environmental pollution and the like.
Owner:HUNAN KEREY BIOTECH
Mass spectrometry assay for congenital adrenal hyperplasia
ActiveUS8153962B2Facilitate desorptionGood removal effectComponent separationFuel lighters11-DesoxycortisolHydrocortisone
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Pharmaceutical compositions
ActiveUS20090054383A1Achieve beneficial effectPrevent adverse side effectsBiocideOrganic active ingredientsDiseaseEstrogenic Effects
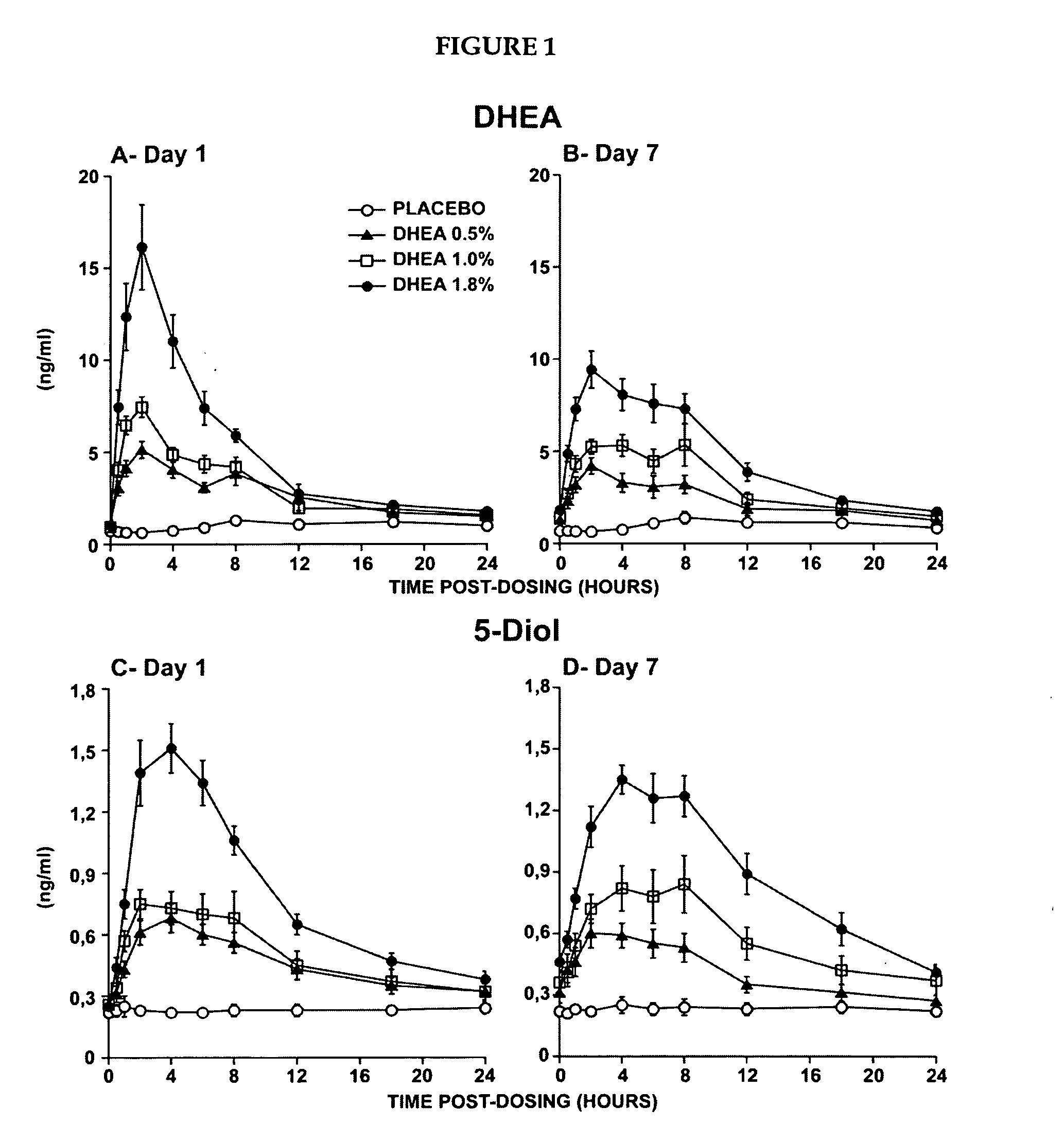
Novel methods for treating or reducing the likelihood of acquiring symptoms or diseases due to the menopause, in postmenopausal women, particularly osteoporosis, vaginal atrophy and dryness, hypogonadism, diminished libido, skin atrophy, connective tissue disease, urinary incontinence, breast, endometrial, ovarian and uterine cancers, hot flashes, loss of muscle mass, insulin resistance, fatigue, loss of energy, aging, physical symptoms of menopause, in susceptible warm-blooded animals including humans involving administration of a sex steroid precursor are disclosed. Said method comprising novel ways of administering and dosing dehydroepiandrosterone (DHEA) in order to take advantage of positive androgenic effects in the vaginal layers lamina propia and / or the layer muscularis, without undesirably causing systemic estrogenic effects in order to avoid the risk of breast and uterine cancer. Pharmaceutical compositions for delivery of active ingredient(s) useful to the invention are also disclosed.
Owner:MYRIEL PHARM LLC
Methods of treating or preventing estrogen-related diseases
ActiveUS20110312925A1Minimizing undesirable side effectEfficient treatment methodBiocideNervous disorderDiseaseGonadal Steroid Hormones
Methods for treating or reducing the likelihood of acquiring estrogen-related (e.g. estrogen-exacerbated) diseases including endometriosis include administering to a patient a selective estrogen receptor modulator (SERM), in combination with inhibiting ovarian secretions, e.g., by administering an LHRH agonist or antagonist. In some embodiments, a precursor of sex steroids, said precursor being selected from the group consisting of dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), androst-5-ene-3β,17β-diol (5-diol), and androstenedione or a compound transformed into one of these, is also administered
Owner:ENDORES & DEV
Neuroactive steroid compositions and methods of use therefor
InactiveUS20110288059A1Improve cognitive functionPromote more developedBiocideOrganic active ingredientsSchizo-affective typePain disorder
Provided are methods for ameliorating a symptom of a neuropsychiatric disorder in a subject. Also provided are methods for ameliorating at least one physical symptom or at least one psychological symptom resulting from tobacco cessation in a subject, methods for ameliorating a symptom of Alzheimer's disease or other cognitive disorder in a subject, methods for ameliorating a symptom of schizophrenia, schizoaffective disorder, or other psychotic disorder in a subject, methods for ameliorating a symptom of a depressive disorder in a subject, methods for ameliorating a symptom of bipolar disorder in a subject, methods for ameliorating a symptom of post-traumatic stress disorder or other anxiety disorder in a subject, methods for predicting a predisposition to suicide, suicidal ideation, suicidal behavior, or a combination thereof in a subject, methods for ameliorating a symptom of a pain disorder in a subject, methods for ameliorating a neurodegenerative disorder in a subject, methods for ameliorating a symptom of traumatic brain injury in a subject, methods for ameliorating a sleep disorder in a subject, and methods for improving cognitive functioning in a subject. In some embodiments, the methods include administering to a subject in need thereof an effective amount of a neuroactive steroid composition comprising pregnenolone (PG), allopregnanolone (ALLO), dehydroepiandrosterone (DHEA), pharmaceutically acceptable salts thereof, derivatives thereof, or combinations thereof.
Owner:MARX CHRISTINE E +1
Novel method for stereo-selective chemosynthesis of drospirenone
The invention relates to a new method of stereo selectivity synthesis drospirenone and main intermediate in the synthesizing method; 15 Beta, 16 Beta-methylene dehydroepiandrosterone (3 Beta-hydroxy-15Beta, 16Beta-methylene -androstane -5-ene-17-ketone, 2) are adopted as starting raw material for the methods and the drospirenone is synthesized via fourteen steps. The invention has the advantages of higher yield and good stereo selectivity.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Nebulizer formulations of dehydroepiandrosterone and methods of treating asthma or chronic obstructive pulmonary disease using compositions thereof
InactiveUS7405207B2Reduce and deplete adenosine levelReducing and depleting adenosineOrganic active ingredientsPowder deliveryDiseaseNebulizer
This invention relates to a sealed container containing a powder formulation comprising a dehydroepiandrosterone, its analogue(s) or salt(s) by itself or with a pharmaceutically or veterinarily acceptable carrier or diluent, and having a particle size of about 0.1 μm to about 100 μm. The formulation can be used to treat or prevent asthma, chronic obstructive pulmonary disease, lung inflammation, and other respiratory diseases or conditions. The formulation may be prepared by jet milling, and may be delivered through the respiratory tract or other routes using a nebulizer. The sealed container is provided in a device and / or a therapeutic kit.
Owner:EPIGENESIS PHARMA LLC
Androgen treatment in females
The present invention is directed to a method of improving ovarian reserve in a human female with diminished ovarian reserve as measured by the female's anti-Müllerian hormone level. The method may include evaluating a first anti-Müllerian hormone level of the female, administering dehydroepiandrosterone to the female for at least about one month, and evaluating a second anti-Müllerian hormone level of the female, wherein the second anti-Müllerian hormone level is greater than the first anti-Müllerian hormone level.
Owner:AMERICAN INFERTILITY OF NEW YORK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com