Synthesis method for dehydroepiandrosterone
A technology of dehydroepiandrosterone and a synthesis method, applied in the directions of steroids, organic chemistry, etc., can solve the problems of high risk, cumbersome post-processing process, difficult reaction control, etc., and achieves low price, simple post-processing process, The effect of easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
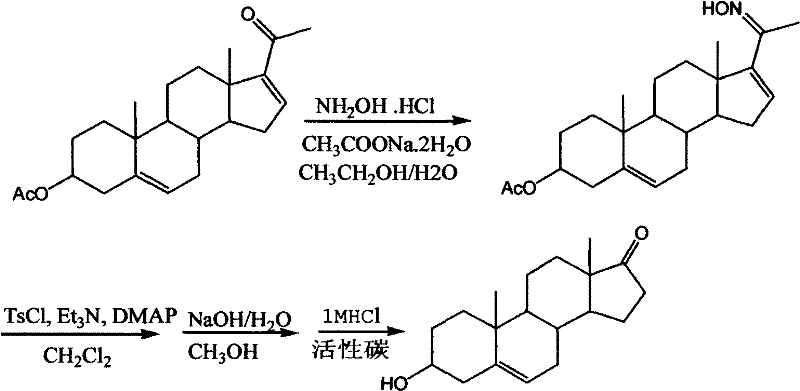
[0014] In a 1000-ml reaction flask equipped with a reflux tube and a thermometer, add 50 grams of gestational dienolone acetate and 400 mL of absolute ethanol (or 95% ethanol) and stir to dissolve, add 10.7 grams of hydroxylamine hydrochloride and 18.2 grams of dihydrate Sodium acetate was dissolved in 100 mL of aqueous solution, heated to reflux for 1 hour, cooled to room temperature, the reaction solution was slowly poured into 500 mL of ice water, a large amount of product precipitated, left standing for 1 hour, suction filtered, and dried to obtain 52 grams of the product.
[0015] Add 52 grams of acetic acid pregnant dienol ketoxime, 520 mL of dichloromethane (or toluene 600M1; or 450 mL of 1,2-dichloroethane) and 15.5 grams of triethylamine in a 1000 ml reaction bottle, stir at room temperature to dissolve, and cool down Add 29 g of p-toluenesulfonyl chloride (or 27.2 g of benzenesulfonyl chloride) and 17 mg of N,N-lutidine (DMAP) to -20°C, and react at -20°C for 1 hour. ...
Embodiment 2
[0018] In a 2000 ml reaction flask equipped with a reflux tube and a thermometer, add 100 grams of dienolone acetate and 800 mL of absolute ethanol (or 95% ethanol) and stir to dissolve, add 48 grams of hydroxylamine hydrochloride and 165 grams of dihydrate Sodium acetate was dissolved in 600mL of aqueous solution, heated to reflux for 5 hours, cooled to room temperature, the reaction solution was slowly poured into 14000mL of ice water, a large amount of product was precipitated, left standing for 2 hours, suction filtered, and dried to obtain 105 grams of the product.
[0019] Add 105 grams of acetic acid pregnant dienol ketoxime, 1050 mL of dichloromethane (or 1200 mL of toluene; or 900 mL of 1,2-dichloroethane) and 86 grams of triethylamine in a 2000 mL reaction bottle, stir and dissolve at room temperature, and cool down Add 108 g of p-toluenesulfonyl chloride (or 100 g of benzenesulfonyl chloride) and 3.5 g of N,N-lutidine (DMAP) to 25°C, and react at 25°C for 10 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com