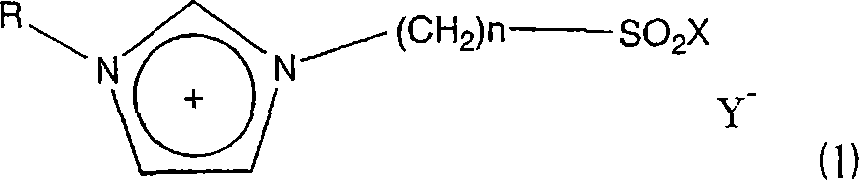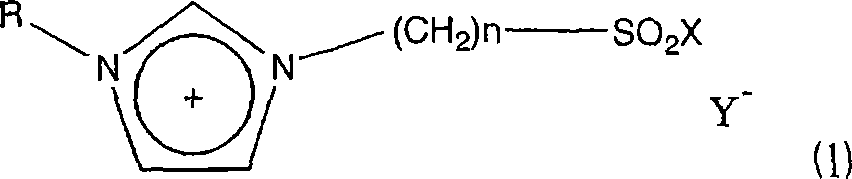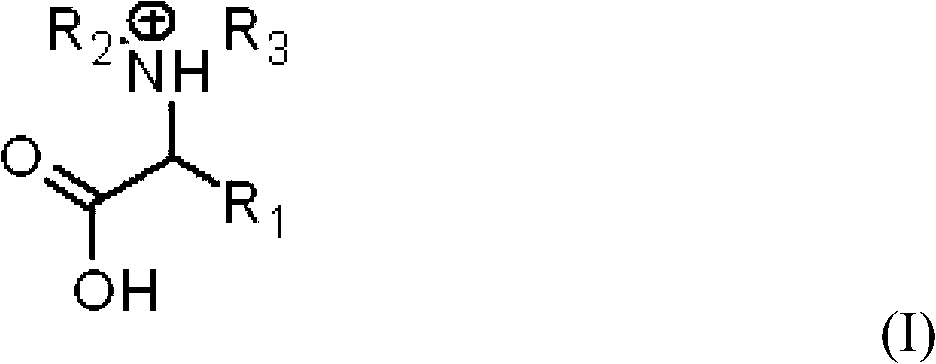Patents
Literature
230 results about "Beckmann rearrangement" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successful performed on haloimines and nitrones. Cyclic oximes and haloimines yield lactams.
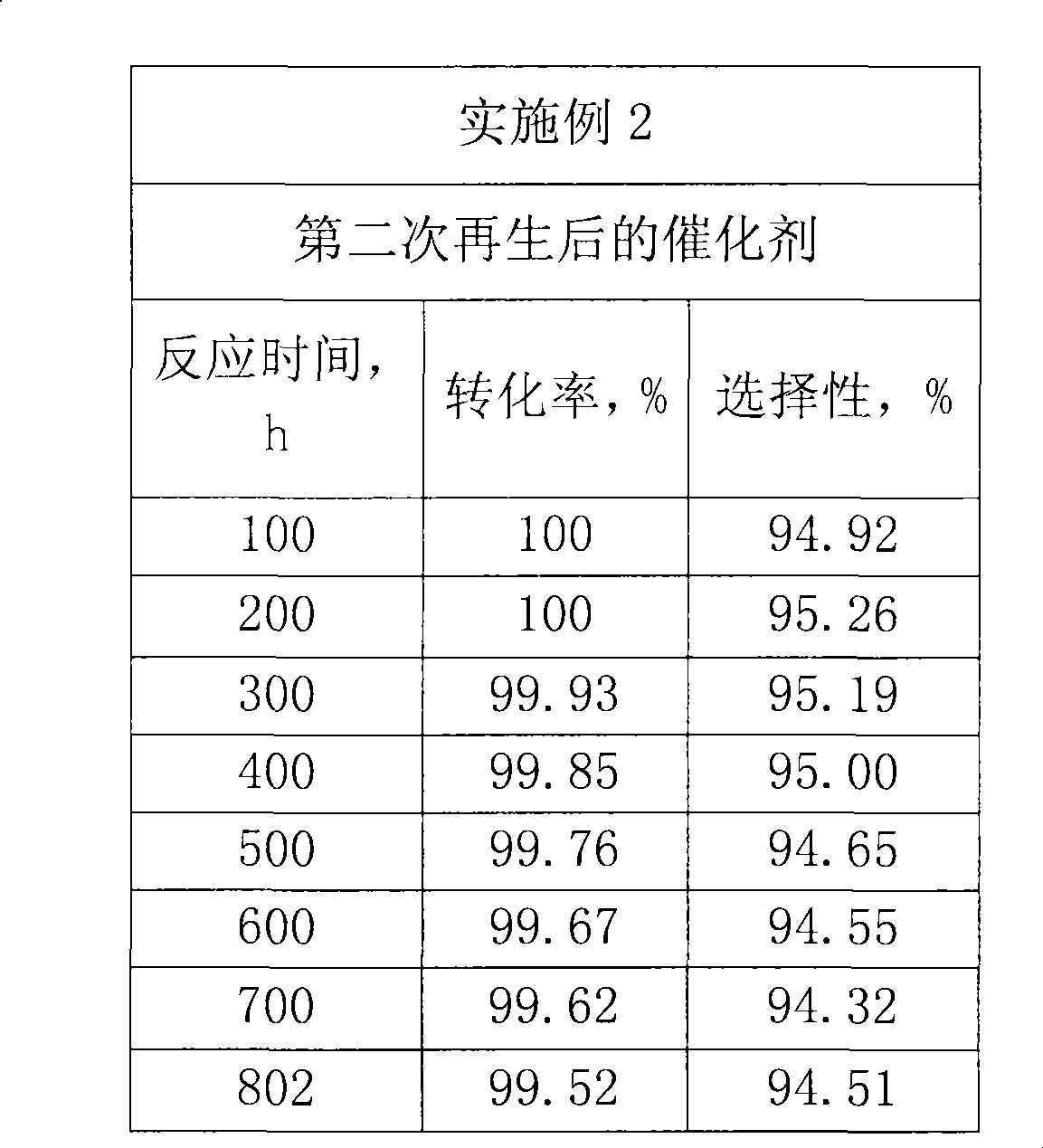
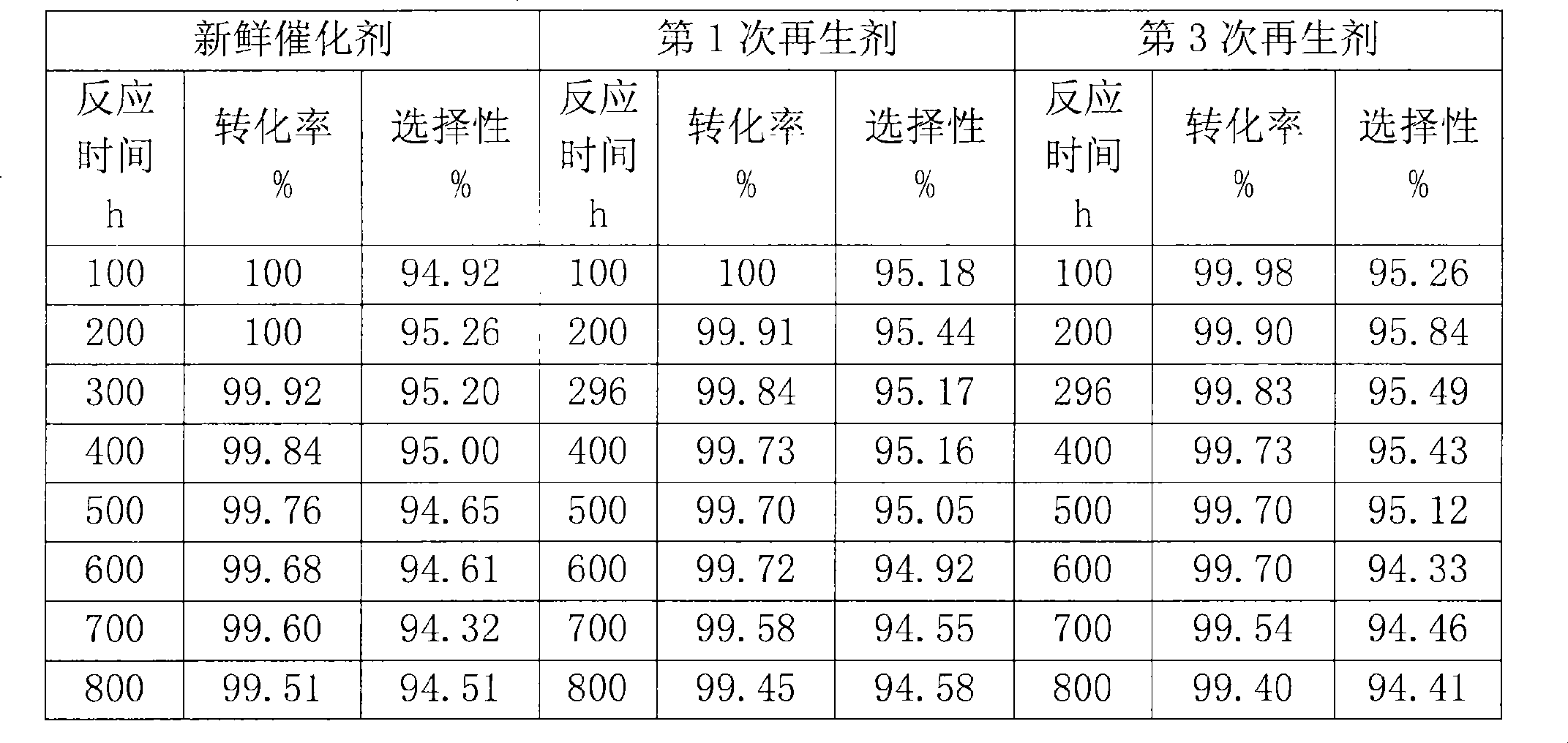
Method for preparing catalyst of containing MFI structured molecular sieve
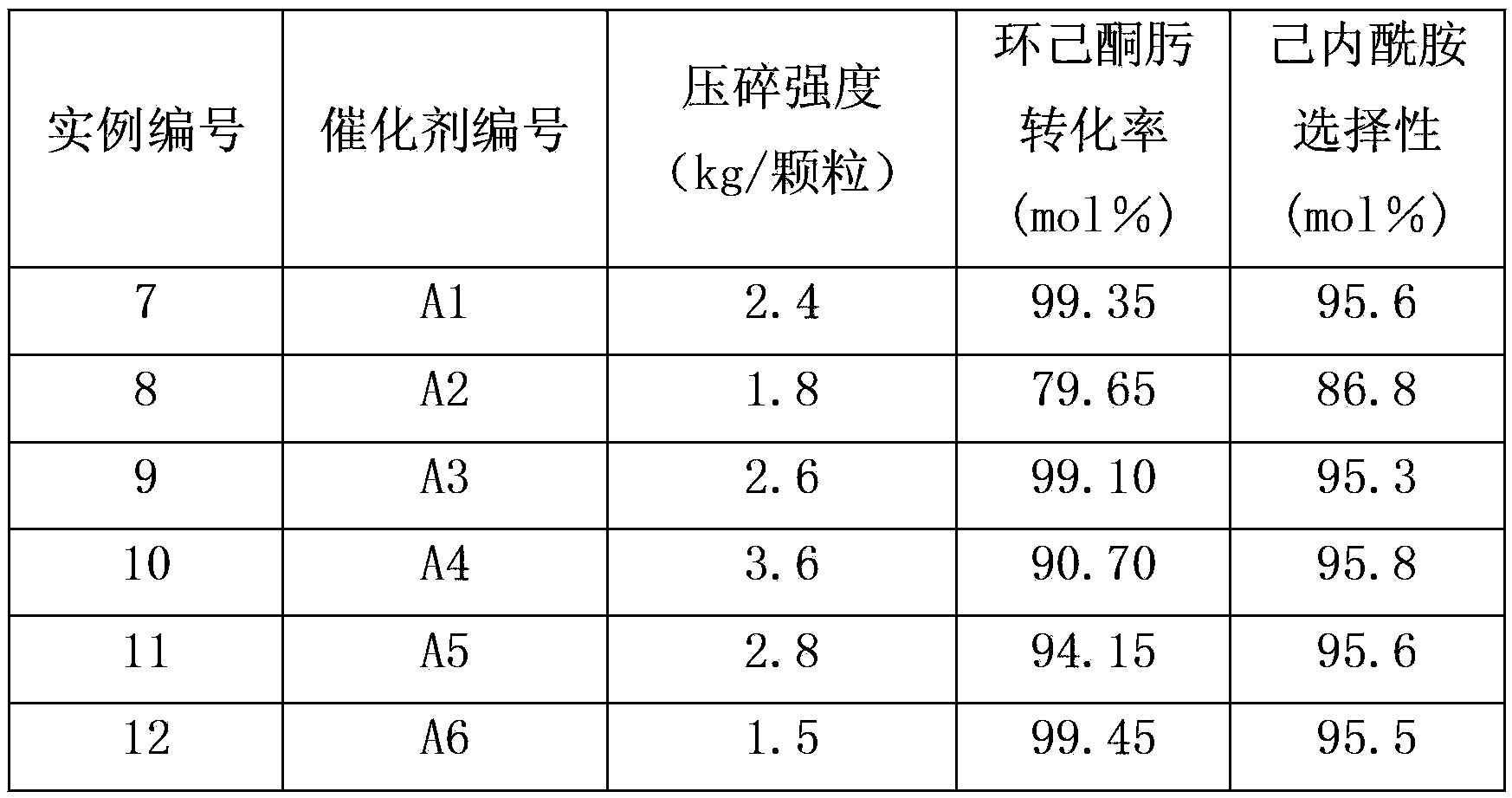
ActiveCN1600428APromote regenerationImprove conversion rateLactams preparationMolecular sieve catalystsBeckmann rearrangementMolecular sieve
A MFI-molecular sieve catalyst for preparing caprolactam from cyclohexanone oxime by gas-phase Beckmann rearrangement is prepared through proportionally mixing MFI-molecular sieve with alkaline silica gel, shaping, drying and calcining. It has high conversion rate and selectivity.
Owner:CHINA PETROLEUM & CHEM CORP +1
Ionic liquid and method of reaction using the same
InactiveCN1852898ASuppress generationCatalystsHydrocarbon preparation catalystsBeckmann rearrangementAlkyl transfer
A novel acidic ionic liquid which is useful as a catalyst for alkylation, nitration, Beckmann rearrangement, etc. and is stable to air and water. It is an ionic liquid represented by the following formula (1): (1) wherein X represents halogeno or hydroxy; Y<-> represents CF3SO3<->, BF4<->, PF6<->, CH3COO<->, CF3COO<->, (CF3SO2)2N<->, (CF3SO2)3C<->, F<->, Cl<->, Br<->, or I<->; n is an integer of 2 to 16; and R represents methyl, allyl, or vinyl. This ionic liquid not only functions as a BrDnsted acid or a Lewis acid but is a liquid insoluble in many organic solvents. The liquid is hence useful as a catalyst or solvent for Friedel-Crafts reaction, nitration, and Beckmann rearrangement. It can be easily separated from the reaction mixture and reused.
Owner:SUMITOMO CHEM CO LTD +1
Process for producing epsi-caprolactam
InactiveUS6265574B1High yieldSteadily producedLactams preparationMolecular sieve catalystsBeckmann rearrangementFluidized bed
A process for producing epsi-caprolactam is provided which comprises the steps of subjecting cyclohexanone oxime to a gaseous phase Beckmann rearrangement reaction in a fluidized bed system using a solid catalyst and re-generating the catalyst, wherein said process comprises a step of treating the catalyst with an oxygen-containing gas at an elevated temperature in a re-generation step so that the nitrogen content of the catalyst falls within a range of 10 ppm to 2,500 ppm on its way to the reaction step from the re-generating step. According to the present invention, epsi-caprolactam is produced with a high conversion or a high selectivity without interrupting the rearrangement reaction or the re-generation step.
Owner:SUMITOMO CHEM CO LTD
Process for preparing caprolactam by cyclohexanone-oxime gas phase rearrangement
ActiveCN1621405AHigh yieldReduce consumptionLactams preparationBeckmann rearrangementMolecular sieve
The process of preparing caprolactam with cyclohexanone oxime includes the vapor Beckmann rearrangement reaction of cyclohexanone oxime inside one first fixed bed reactor in the presence of MFI structure molecular sieve catalyst; the decomposition and conversion of the reaction side product O-alkyl-epsilon-caprolactim into caprolactam inside one second fixed bed reactor in the presence of MFI structure molecular sieve catalyst and water; and the separation and purification of the reaction effluent. Compared with single vapor Beckmann rearrangement reaction process of cyclohexanone oxime, the present invention has 1-3 % raised caprolactam yield.
Owner:CHINA PETROLEUM & CHEM CORP +1
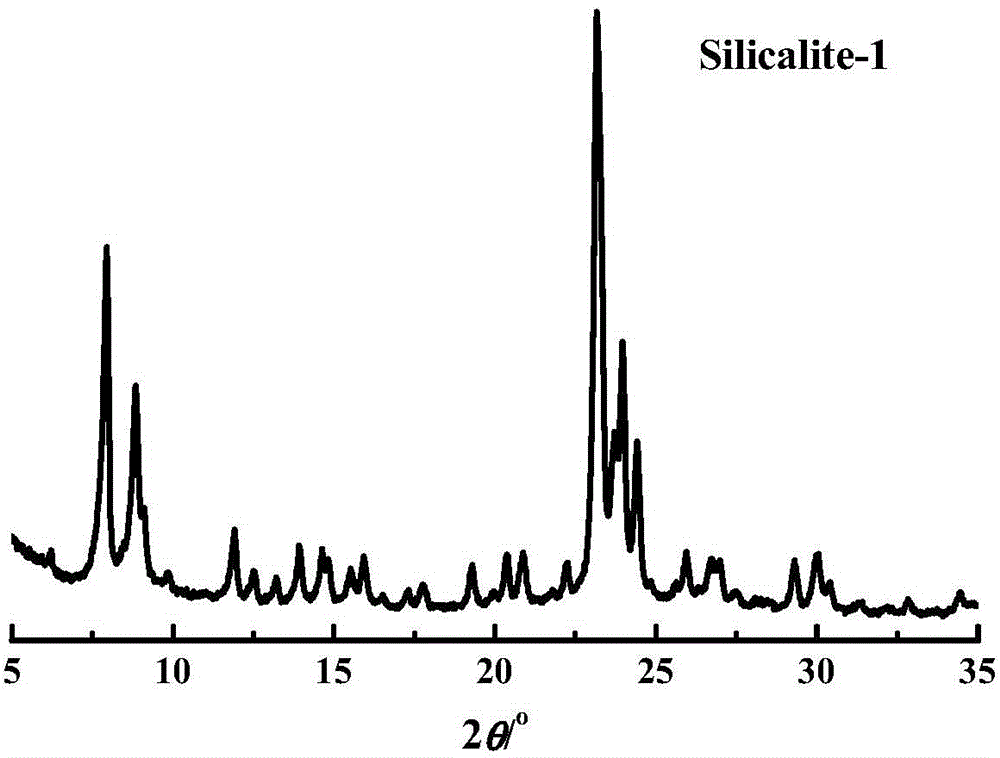
Nanometer all-silicon molecular sieve and its preparation method and use
InactiveCN102432032AHigh crystallinityRegular shapeLactams preparationMolecular sieve catalystsBeckmann rearrangementMass number
The invention provides a nanometer all-silicon molecular sieve. The nanometer all-silicon molecular sieve is prepared from tetrapropylammonium hydroxide, a silicon source, water and amino acids. A mole ratio of the silicon source to tetrapropylammonium hydroxide to water is 1: [0.05 to 0.50]: [15 to 65]. The mass of the used amino acids is 0.5 to 5% of that of the silicon source. A mole number and a mass number of silica represent a mole number and a mass number of the silicon source. The invention also provides a preparation method of the nanometer all-silicon molecular sieve, and a use of the nanometer all-silicon molecular sieve in a cyclohexanone-oxime gas phase beckmann rearrangement reaction. The nanometer all-silicon molecular sieve has a high degree of crystallization, regular morphology and adjustable particle sizes of 40 to 160 nanometers. In a cyclohexanone-oxime gas phase beckmann rearrangement reaction, the nanometer all-silicon molecular sieve shows excellent catalytic activity, selectivity and stability.
Owner:HUNAN UNIV
Method of preparing amide from ketoximes by Beckmann rearrangement
InactiveCN1919834AWill not corrodeNo pollutionOrganic compound preparationCarboxylic acid amides preparationDielectricBeckmann rearrangement
The invention discloses an amide preparing method of indoor temperature ionic liquid catalytic ketoximes through Beckmann rearrangement reaction, which is characterized by the following: possessing indoor ionic liquid with chlorosulfonylation functional group as reacting dielectric; transmitting into functional group under mild reacting temperature and shorter reacting time; recycling the ionic liquid catalytic system.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
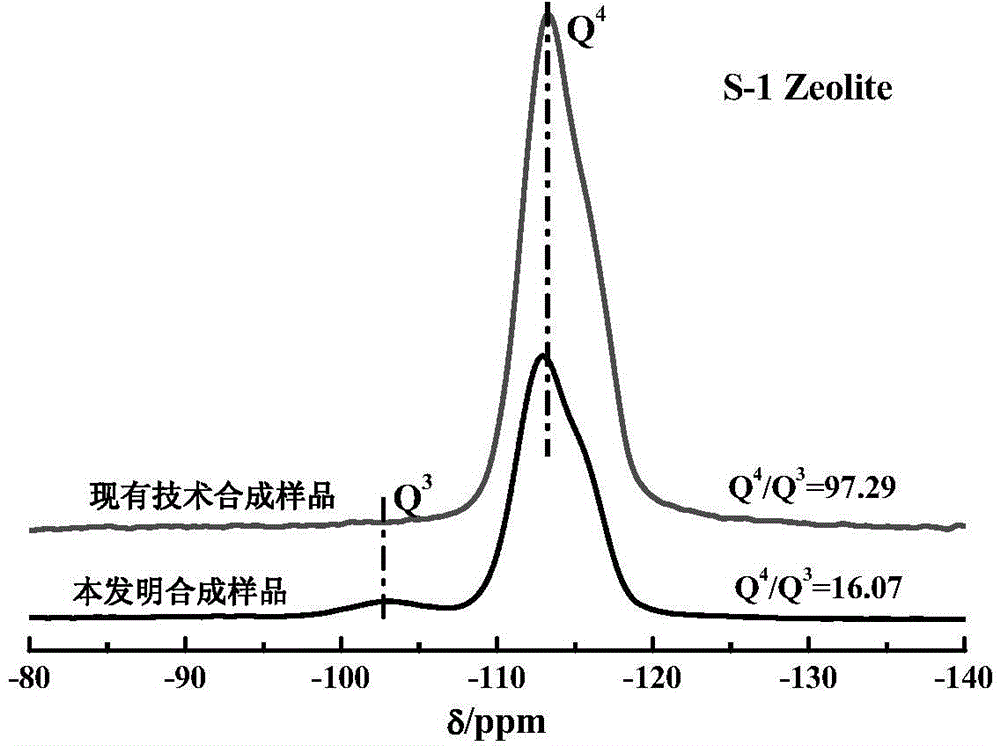
All-silicon molecular sieve and synthetic method thereof
ActiveCN104556087AHigh catalytic activityFlexible adjustment of particle sizeLactams preparationSilicaMolecular sieveBeckmann rearrangement
The invention provides an all-silicon molecular sieve and a synthetic method thereof. Q4 / Q3 of the all-silicon molecular sieve is (10-90):1, wherein Q4 is the peak intensity expressed by the peak height at the chemical shift of -112+ / -2ppm in a 29SiNMR spectrogram of the all-silicon molecular sieve, and Q3 is the peak intensity expressed by the peak height at chemical shift of -103+ / -2ppm in the 29SiNMR spectrogram of the all-silicon molecular sieve. The synthetic method of the all-silicon molecular sieve comprises the following steps: mixing a template agent, an organic silicon source, an inorganic ammonium source and water to be subjected to hydrolytic alcohol removal, aging the obtained product, mixing the aging product with a solid silicon source, crystallizing the mixture in a closed reaction kettle, and recovering the all-silicon molecular sieve. The all-silicon molecular sieve provided by the invention has higher activity in cyclohexanone-oxime beckmann rearrangement.
Owner:CHINA PETROLEUM & CHEM CORP +1
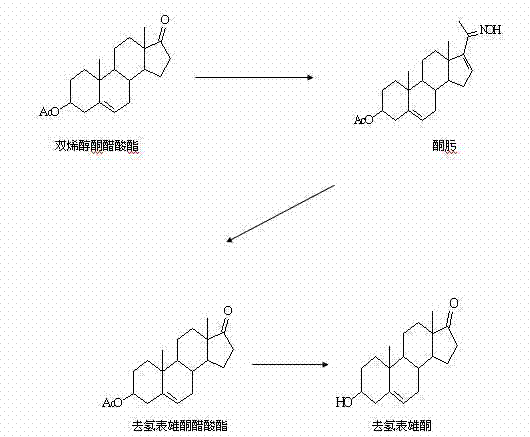
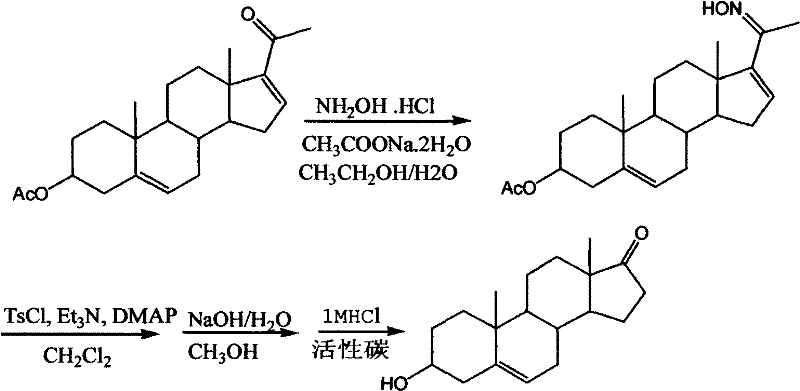
Preparation method of dehydroepiandrosterone
The invention discloses a preparation method of dehydroepiandrosterone, belongs to the technical field of medicine intermediate preparation, and is mainly used for solving the problems of higher production cost, low product yield and more serious pollution existing in the traditional dehydroepiandrosterone synthesis method. The preparation method comprises the following process steps of: (1) oximation reaction: subjecting 16-dehydropregnenolone acetate, sodium acetate and absolute ethyl alcohol with the ratio of 1:(1.2-1.5):(4-4.5) to oximation reaction to obtain 16-dehydropregnenolone acetate oxime; (2) Beckmann rearrangement hydrolysis reaction: charging the 16-dehydropregnenolone acetate oxime, chloroform, phosphorus pentoxide, hydrochloric acid and water with the ratio of 1:(6-6.5):(0.8-1.0):(0.5-0.8):(6-7.0) to obtain dehydroepiandrosterone acetate; and (3) hydrolysis reaction: charging a crude dehydroepiandrosterone acetate, methanol, potassium carbonate, hydrochloric acid, methanol and active carbon with the ratio of 1:(7.0-8.0):(0.4-0.5):(0.3-0.4):(5.0-6.0):(0.4-0.5) to obtain dehydroepiandrosterone. The preparation method has the characteristics of easiness in reaction condition control, simplicity in operation, little pollution and high yield and is mainly used for preparing dehydroepiandrosterone.
Owner:YICHENG GOTO PHARMA
Method for preparing caprolactam through gas phase Beckmann rearrangement of cyclohexanone-oxime gas
ActiveCN103896839AImprove conversion rateHigh selectivityLactams preparationMolecular sieve catalystsBeckmann rearrangementMolecular sieve
The invention provides a method for preparing caprolactam through gas phase Beckmann rearrangement of cyclohexanone-oxime gas. According to the method, under the condition of rearrangement reaction, cyclohexanone-oxime is in contact with a catalyst, and the catalyst is obtained by the steps of forming a molecular screen with an aluminum-free MFI (Melt Flow Index) structure, and enabling the molecular screen to be in contact with an alkaline buffer solution of a nitrogen-containing compound. The method is high in cyclohexanone-oxime conversion rate, and is capable of realizing long-period and continuous production; the selectivity of caprolactam is capable of reaching 95.8 percent.
Owner:CHINA PETROLEUM & CHEM CORP +1
Synthesis method for dehydroepiandrosterone
InactiveCN102212099AOvercoming reactivityOvercoming processingSteroidsBeckmann rearrangementSodium acetate
The invention belongs to a novel synthesis method for dehydroepiandrosterone, which comprises the following steps: oximation of 16-dehydropregnenolone acetate, Beckmann rearrangement, hydrolysis and refining to obtain a product. The synthesis method is characterized by carrying out oximation of ketone by using sodium acetate as a base and water and ethanol as solvent, carrying out Beckmann rearrangement, hydrolysis and one-pot refining reaction, reacting 16-dehydropregnenolone acetate oxime with p-toluenesulfonamide chloride, benzenesulfonyl chloride, triethylamine or N,N-dimethyl-pyridine (DMAP) in a dichloromethane solution, concentrating the solvent after reaction, adding methanol and a sodium hydroxide solution for refluxing hydrolysis, cooling and regulating the pH value to 7-8, adding activated carbon, then refluxing for 30-60 minutes, filtering, concentrating, crystallizing, and centrifuging to obtain the product. The synthesis method provided by the invention is simple to operate, and has characteristics of mild reaction conditions, high yield, low environmental pollution and the like.
Owner:HUNAN KEREY BIOTECH
Preparation method of aminocaprolactam
ActiveCN101117327AAchieve recyclingHigh selectivityLactams preparationBeckmann rearrangementAcetic anhydride
The invention discloses a preparation process for caprolactam which is characterized in that the acetic anhydride acts as the catalyzer to make a Beckmann rearrangement reaction of cyclohexanone oxime in ionic liquid homogeneous system , then the orgainic solvent is used to extract the ionic liquid and fully transfer the cyclohexanone oxime and the catalyzer into the organic solvent, finally the separation of the cyclohexanone oxime and the catalyzer is realized by the extraction to the solvent.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing amide using nonhomogeneous phase oximation rearrangement
ActiveCN1762985ALow costGood economic valueOrganic compound preparationCarboxylic acid amides preparationBeckmann rearrangementReaction temperature
The present invention discloses oximation and rearrangement process of preparing amide with aliphatic and / or cyclic aliphatic ketone as material. The preparation process includes catalytic reaction of ketone, hydrogen peroxide solution and ammonia inside inert solvent to produce ketoxime solution, Beckmann rearrangement of the oil phase product under the action of fuming sulfuric acid, and hydrolysis to neutralize and produce amide. The present invention has shortened technological process, lowered cyclohexyl ketoxime rearranging reaction temperature, lowered sulfuric acid consumption and reduced side product.
Owner:HUBEI JINXIANGNING CHEM ENG TECHENOLOGY CO LTD
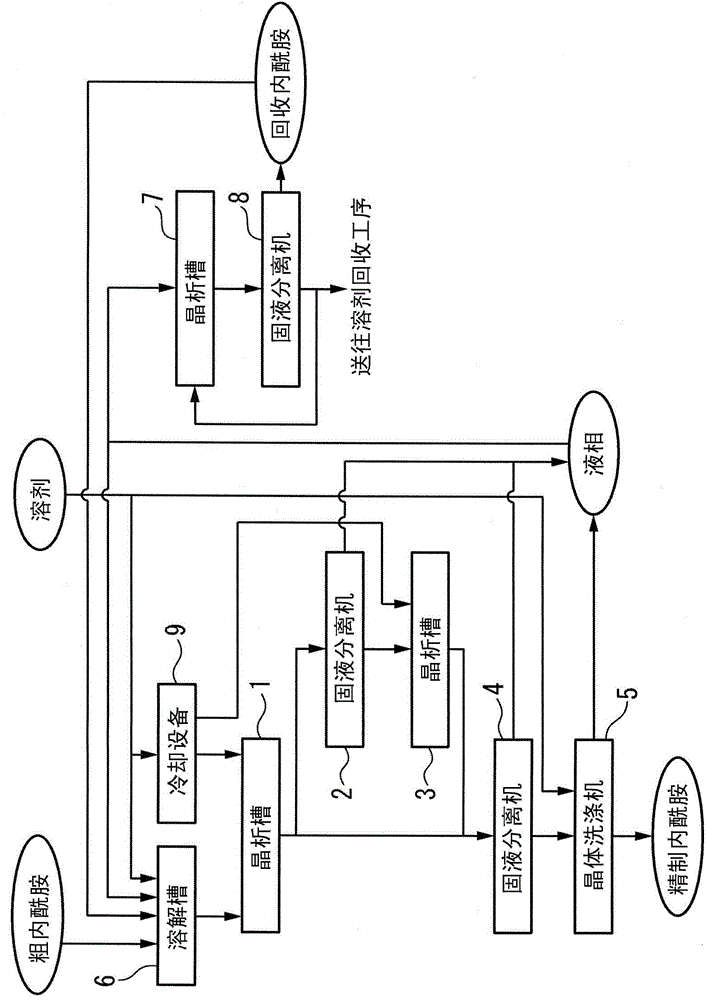
Method for purifying epsilon-caprolactam and method for preparing epsilon-caprolactam
ActiveCN102452982AFully removedNo foulingLactams preparationLactams separation/purificationBeckmann rearrangementMolecular sieve
The invention provides a method for purifying a crude epsilon-caprolactam product, which comprises the following steps of: dissolving the crude epsilon-caprolactam product in halogenated hydrocarbon to obtain a halogenated hydrocarbon solution of epsilon-caprolactam; and performing evaporative crystallization on the solution at the temperature of between -10 and 50 DEG C under reduced pressure to separate an epsilon-caprolactam crystal. The invention also provides a method for preparing the epsilon-caprolactam, which comprises the following steps of: performing Beckmann rearrangement reaction on gas-phase cyclohexanone-oxime in the presence of a molecular sieve catalyst with a melt flow index (MFI) structure, and distilling a reaction product to obtain the crude epsilon-caprolactam product; and performing crystallization purification by the method for purifying the crude epsilon-caprolactam product, and performing hydrogenation reaction on the epsilon-caprolactam crystal obtained after purification in the presence of a hydrogenation catalyst. By the method for purifying the crude epsilon-caprolactam product, the epsilon-caprolactam which meets the industrial product requirement can be obtained, and the phenomenon of scale formation in the crystallization process is avoided.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for continuously preparing hexanolactam by using cyclohexanone-oxime Beckmann rearrangement reaction
InactiveCN101851203AHigh yieldImprove qualityLactams preparationBeckmann rearrangementReaction temperature
The invention discloses a method for continuously preparing hexanolactam by using a cyclohexanone-oxime Beckmann rearrangement reaction. A circular reaction system consists of a recycle pump, a cooler, a mixing reactor and a curing reactor, and the circular reaction system is characterized in that heat instantly released from the rearrangement reaction is dissipated by combining heat storage of materials with heat absorption in solvent vaporization. The combination of the heat storage of the materials with the heat absorption in the thermal inertia solvent vaporization can control a micro-reaction temperature, and meanwhile, the heat absorption in the thermal inertia solvent vaporization can prevent over-high temperature of the partial reaction, so a reaction product with high yield and high quality can be obtained, the use amount of fuming sulphuric acid is small, the energy consumption is low and the operation is stable.
Owner:XIANGTAN UNIV
Method for separating Beckmann rearrangement reaction products from ion liquid
ActiveCN1670017ASolve the separation problemEfficient separationOrganic chemistryBeckmann rearrangementOrganic solvent
The invention relates to a method for separating Beckmann rearrangement reaction production from ion liquid, which comprises: selecting an organic dissolvant with the ion liquid used in cyclohexanone oxime Beckmann rearrangement reaction immiscible in the temperature of 10-60 Deg.C and miscible in the temperature of 50-150 Deg.C, mixing the organic dissolvant with the ion liquid with cyclohexanone oxime Beckmann rearrangement reaction production with the proportion in volume of 50 degree 1-1 degree 10 to form a immiscible two-phase system, stirring and lifting the temperature to 50-150 Deg.C to get the miscible mixture liquid of both organic dissolvents and ion liquid, holding 1-120 min in the temperature, then cooling the mixture liquid to 10-60 Deg.C to separate phase inactively.
Owner:CHINA PETROLEUM & CHEM CORP +1
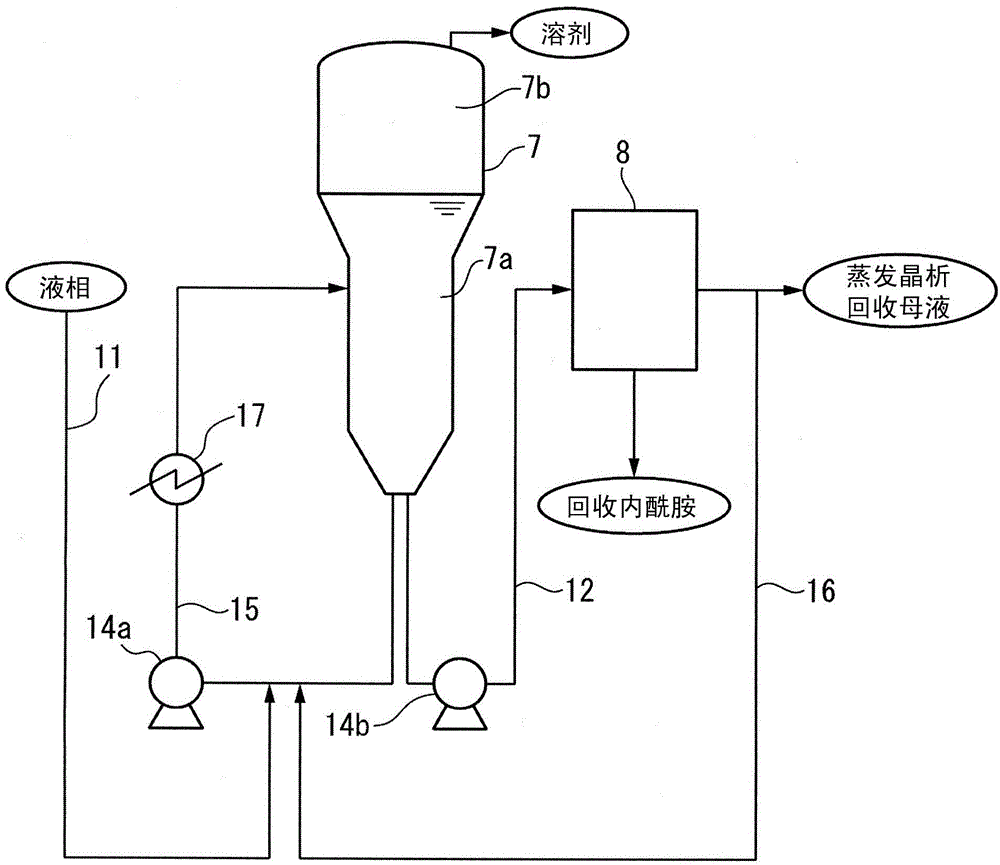
Method for producing high-quality epsilon-caprolactam
ActiveCN104024221APrevent scalingImprove qualityOrganic chemistry methodsLactams separation/purificationBeckmann rearrangementOrganic solvent
A method for producing high-quality epsilon-caprolactam, comprising: a purification step of allowing epsilon-caprolactam to be crystallized from a mixed solution that is prepared by mixing crude epsilon-caprolactam produced by the Beckmann rearrangement of cyclohexanoneoxime with an organic solvent and then subjecting the resultant solution to solid / liquid separation to produce high-quality epsilon-caprolactam and a drop crystallization-collected mother liquor; and a collection step of evaporating an evaporative crystallization mother liquor containing the drop crystallization-collected mother liquor to cause the crystallization of epsilon-caprolactam and then subjecting the resultant solution to solid / liquid separation to produce collected epsilon-caprolactam and an evaporative crystallization-collected mother liquor. In the method, prior to the collection step, the following steps are involved: a step of mixing the drop crystallization-collected mother liquor with at least a portion of the evaporative crystallization-collected mother liquor, at least a portion of an evaporative crystallization mother liquor that is removed from a vessel in which evaporative crystallization is to be carried out, or both of at least a portion of the evaporative crystallization-collected mother liquor and at least a portion of the evaporative crystallization mother liquor to prepare a mixed solution; and a step of introducing the mixed solution into the vessel and mixing the mixed solution with the evaporative crystallization mother liquor that is stored in the vessel.
Owner:SUMITOMO CHEM CO LTD
Method of preparing amide from ketoximes by Beckmann rearrangement
InactiveCN1919833ANo pollutionHigh conversion rate of rearrangement reactionOrganic compound preparationCarboxylic acid amides preparationBeckmann rearrangementChloride
The invention discloses an amide preparing method of indoor temperature ionic liquid catalytic ketoximes through Beckmann rearrangement reaction, which is characterized by the following: selecting catalyst from acylated-chloride compound; transmitting into amide under mild reacting temperature and shorter reacting time; extracting product through water; recycling the ionic liquid catalytic system.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing amide
InactiveCN101684076BNo generationSuitable for industrial mass productionLactams preparationOrganic compound preparationBeckmann rearrangementReaction temperature
The invention relates to a method for preparing amide, wherein an amino acid ionic liquid is used as a reaction medium and a catalyst for catalyzing the Beckmann rearrangement reaction of ketoxime to generate the amide. At mild reaction temperature and within ultrashort reaction time, the amino acid ionic liquid with an asymmetric characteristic is used for catalyzing the ketoxime to be rearranged to generate the amide without adding other catalysts, such as concentrated sulfuric acid. The method has the advantages of no pipeline equipment corrosion and high ketoxime conversion rate and amideselection rate.
Owner:CHINA PETROCHEM DEVMENT
Catalytic system for Beckmann rearrangement of oxime
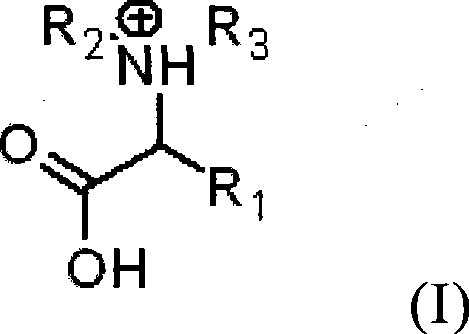
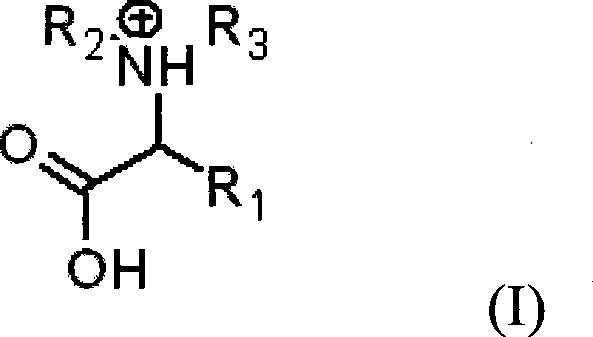
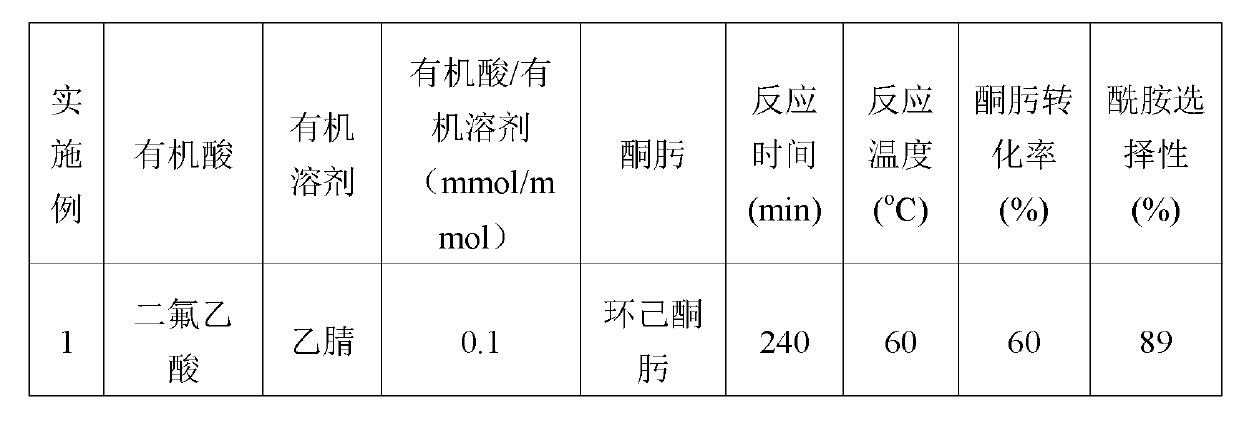
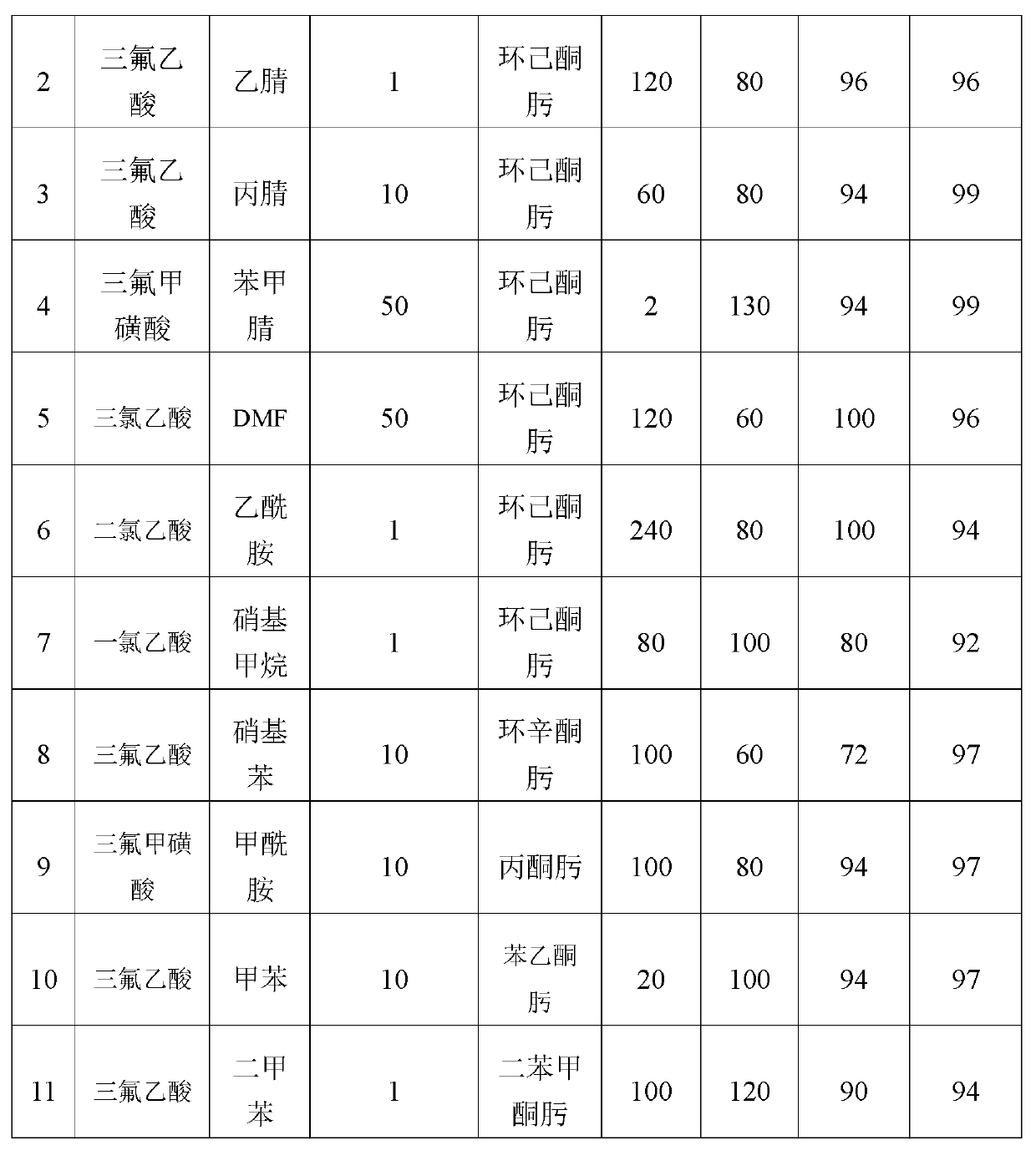
ActiveCN102895996AEasy to handleGood choiceLactams preparationOrganic compound preparationOrganic acidBeckmann rearrangement
The invention discloses a catalytic system for Beckmann rearrangement of oxime, characterized in that: the catalytic system is a homogeneous system comprising organic acid and organic solvent; the catalytic system is used as a catalyst in the Beckmann rearrangement of oxime for preparing amide, the reaction temperature is 60-130 DEG C, and the reaction time is 2-240 min; and the molar ratio of the organic acid to the organic solvent is 0.1-50. The catalytic system can efficiently convert oxime into a corresponding amide-type compound under relatively mild reaction conditions. According to the invention, the catalytic system can be recycled and has high conversion rate and selectivity, the reaction system while use is simple, and the reaction conditions are mild; the Beckmann rearrangement of oxime has high conversion rate and good selectivity; the product after the Beckmann rearrangement is easily processed, thus the recycling of the organic solvent and the catalyst can be realized.
Owner:TSINGHUA UNIV
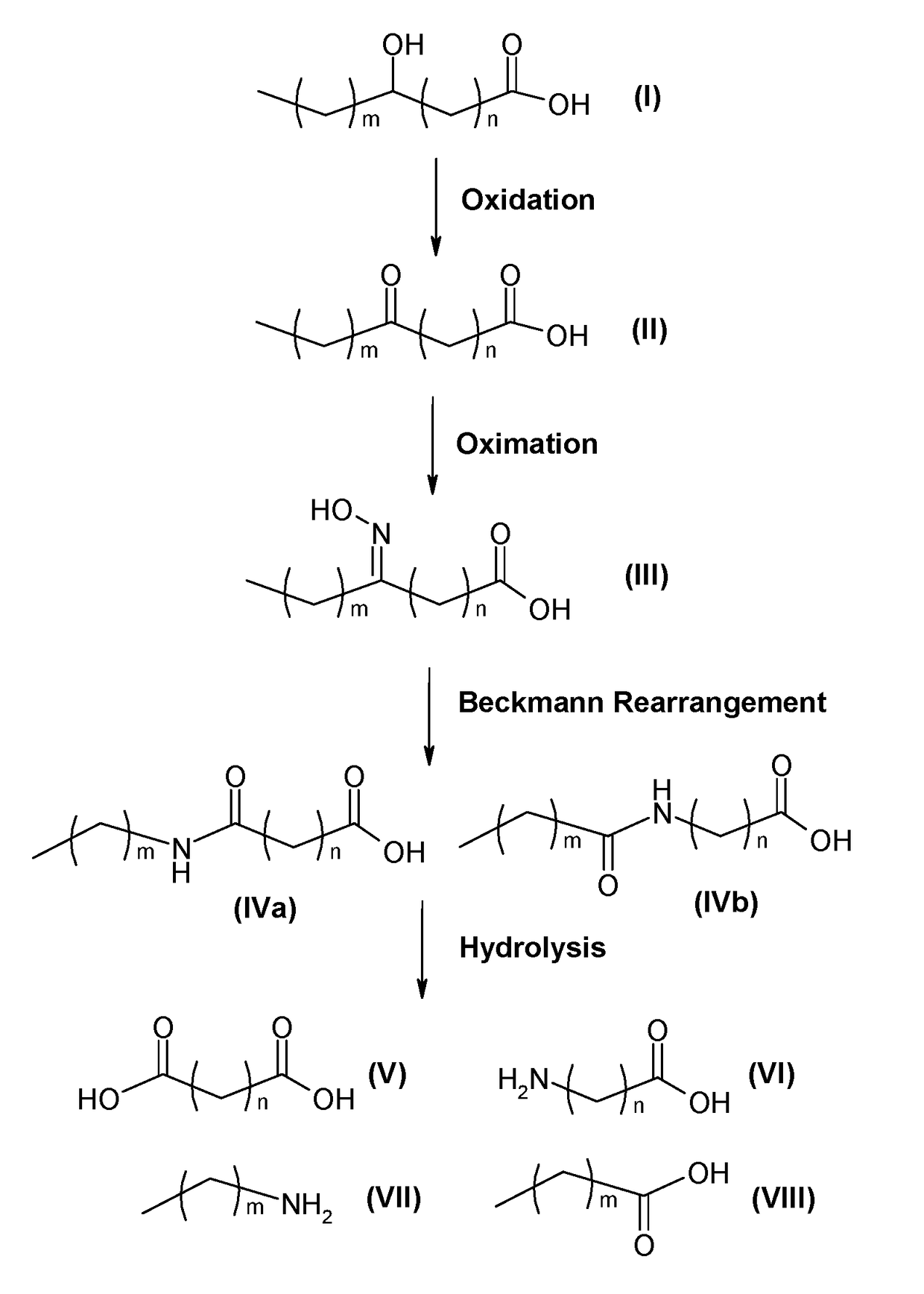
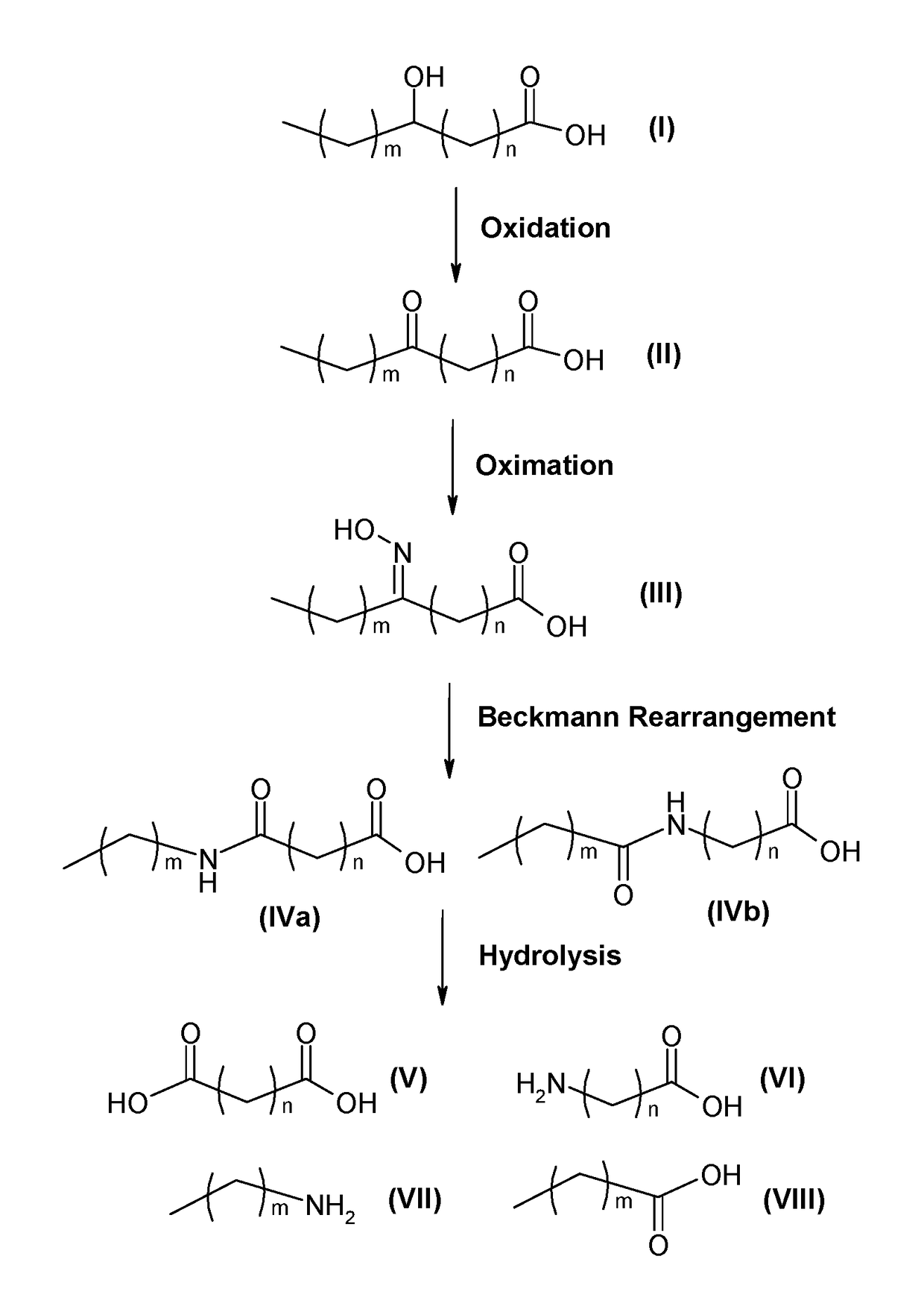
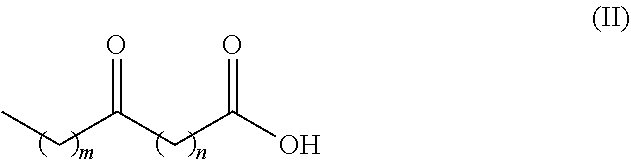
Process for producing long chain amino acids and dibasic acids
ActiveUS10065921B1Organic compound preparationCarboxylic acid amides preparationBeckmann rearrangementIodo fatty acid
There is disclosed a process for the production of long chain amino acid and long chain dibasic acid, comprising: (1) reacting long chain keto fatty acid with hydroxylamine or subjecting keto fatty acid to an ammoximation reaction to yield an oxime fatty acid; (2) subjecting the oxime fatty acid to the Beckmann rearrangement to yield a mixture of two amide fatty acids; (3) hydrolyzing the mixed amide fatty acids to produce long chain amino acid, long chain dibasic acid, short chain alkylamine, and alkanoic acid.
Owner:VITAWORKS IP LLC
Process for producing epsi-caprolactam
InactiveUS6252068B1Low costQuality improvementLactams separation/purificationBeckmann rearrangementHydrogen
A high purity epsi-caprolactam is prepared by crystallizing an epsi-caprolactam from a hydrocarbon solution containing a crude epsi-caprolactam, and allowing the crystallized epsi-caprolactam in contact with hydrogen in the presence of a hydrogenation catalyst. This process can effectively remove impurities from a crude epsi-caprolactam, which is obtained by subjecting cyclohexanone oxime to the Beckmann rearrangement, and provide a high purity epsi-caprolactam.
Owner:SUMITOMO CHEM CO LTD
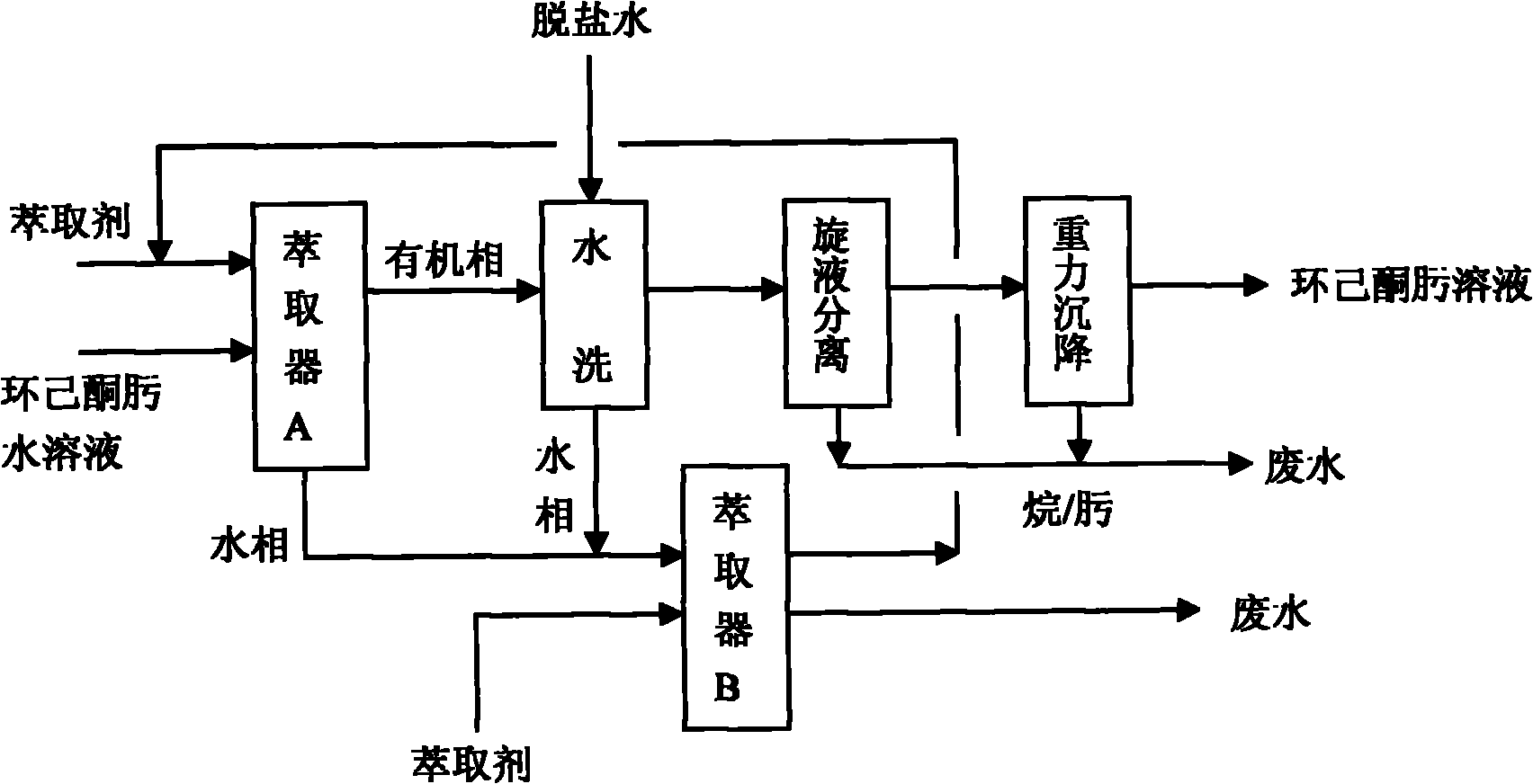
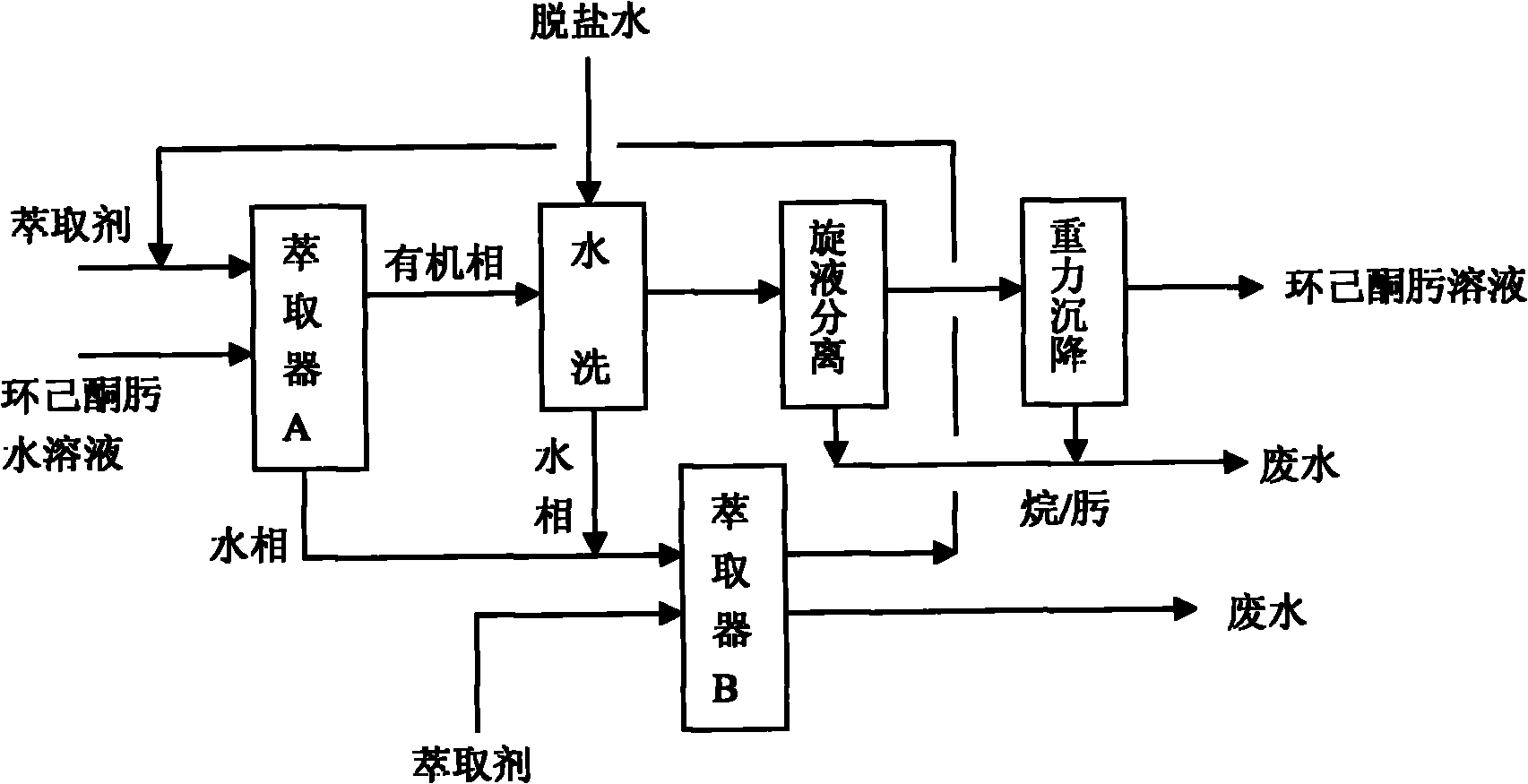
Process for extracting and washing cyclohexane oxime
ActiveCN101955445ASimple refining processFlexible refining processOximes preparationCycloneBeckmann rearrangement
The invention discloses a process for extracting and washing cyclohexane oxime. The process comprises the following steps of: extracting an extractant and aqueous solution of cyclohexane oxime in a weight part ratio of 1:1 to 2:1 at the temperature of between 45 and 55 DEG C; washing an organic phase obtained by the extraction with desalted water when the mass concentration of the cyclohexane oxime is less than 1 percent so as to remove salt ions in the cyclohexane oxime solution; mixing a water phase obtained after washing and a water phase obtained by extraction, and extracting the mixture by using a cyclohexane or hexane extractant in a weight part ratio of 0.5:1 at the temperature of between 45 and 55 DEG C so as to recycle the cyclohexane oxime in the mixture; and sequentially performing hydraulic cyclone separation and gravity settling separation on the washed organic phase when the mass concentration of the salt ions in the organic phase is less than 0.0001 percent so as to obtain the cyclohexane oxime. The process does not need a rectification process; the purity of the obtained cyclohexane oxime is high; beckmann rearrangement can be directly performed in the next process so as to prepare caprolactam; and for 100,000 t / a of cyclohexanone oxime devices, the process can save one-time equipment investment by about 20 to 30 million and can reduce energy consumption such as water, electricity, steam and the like by about 30 to 40 million.
Owner:河北美邦工程科技股份有限公司
Benzenesulfonic acid catalyst supported on silica gel, as well as preparation and application thereof
InactiveCN102302948AHigh bond energyIncrease loading capacityLactams preparationOrganic compound preparationBeckmann rearrangementChlorosulfuric acid
The invention discloses a benzenesulfonic acid catalyst supported on silica gel, which is prepared by adopting silica gel to react with thionyl chloride, phenol and chlorosulfonic acid sequentially. In the catalyst, the silica gel and benzenesulfonic acid are bonded in a covalent bond mode, so that the supporting capacity is stable, and the supporting capacity of the benzenesulfonic acid is about 2.25mmol / g in the benzenesulfonic acid catalyst supported on silica gel through analysis and calculation. Experiments prove that: the prepared catalyst has efficient catalytic activity to two kinds of atom economic reactions, namely Beckmann rearrangement reaction and Claisen rearrangement reaction, is non-toxic, harmless and noncorrosive, is easily separated, can be recycled, and accords with green and environmentally-friendly national industrial policy.
Owner:NORTHWEST NORMAL UNIVERSITY
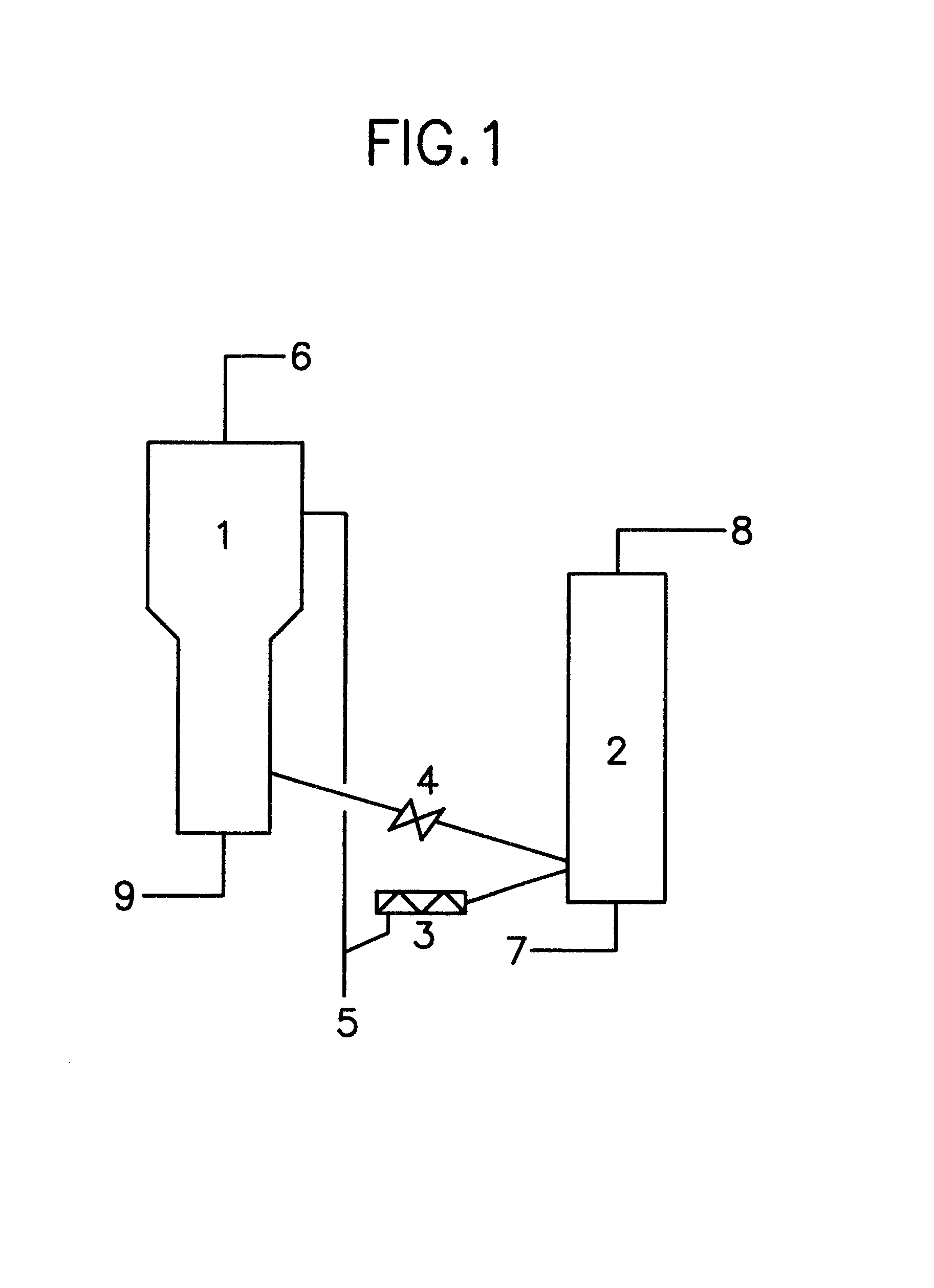
Method and equipment for continuously producing epsilon-caprolactam
The invention provides a method and equipment for continuously producing epsilon-caprolactam. The method orderly comprises the following steps: a) inputting i) fuming sulphuric acid and / or ii) cyclohexanone-oxime into a beckmann rearrangement reaction zone; b) discharging the generated mixture containing epsilon-caprolactam from the beckmann rearrangement reaction zone; c) adding ammonia to the mixture containing the epsilon-caprolactam; d) separating out ammonium sulfate from the mixture containing the epsilon-caprolactam; e) extracting the epsilon-caprolactam from the mixture containing the epsilon-caprolactam in an organic solvent; and f) adding water to remove an organic solvent from the extracted epsilon-caprolactam in a distillation zone by using distillation. The method is characterized in that at least one part of reaction heat generated in the beckmann rearrangement reaction zone is exchanged to the distillation zone. The invention also provides equipment for executing the method and the epsilon-caprolactam obtained according to the method.
Owner:CAP III
Tulathromycin intermediate, preparation method of tulathromycin intermediate and preparation method of tulathromycin
InactiveCN103772459AAvoid residueSimple and safe operationSugar derivativesSugar derivatives preparationBeckmann rearrangementHydrolysis
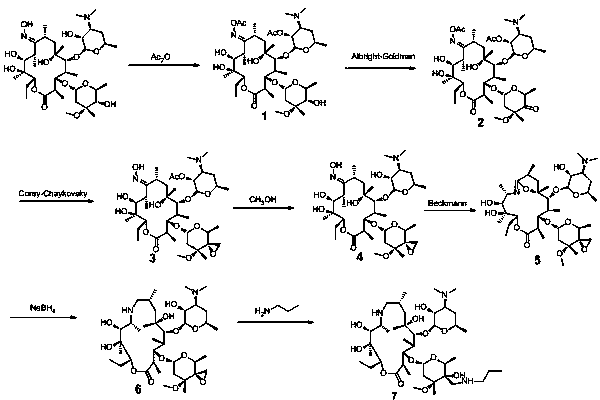
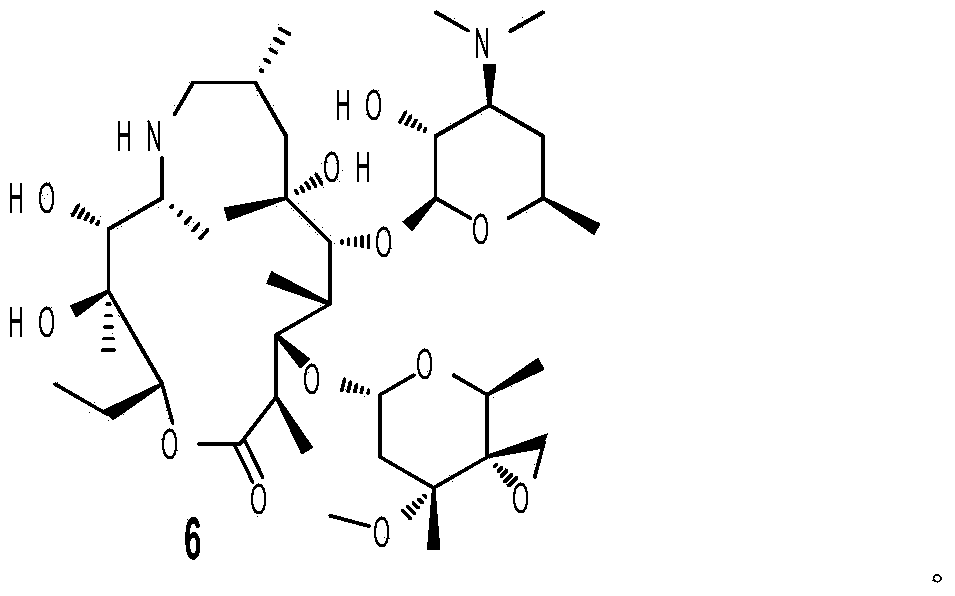
The invention provides a tulathromycin intermediate, a preparation method of the tulathromycin intermediate and a preparation method of tulathromycin. According to the preparation method, Erythromycin A (E) oxime used as a starting material is subjected to acetyl protection, Albright-Goldman oxidation, Corey-Chaykovsky epoxidation, deprotection, Beckmann rearrangement, reduction and hydrolysis, and n-propylamine ring opening to synthesize tulathromycin. The preparation method has mild conditions, has the advantages of convenient operation, high yield and low cost and is beneficial to industrial production, and the raw materials are easily available.
Owner:QINGDAO VLAND BIOTECH INC
Caprolactam preparation method
InactiveCN104910071AShort production processReduce consumptionLactams preparationBeckmann rearrangementOleum
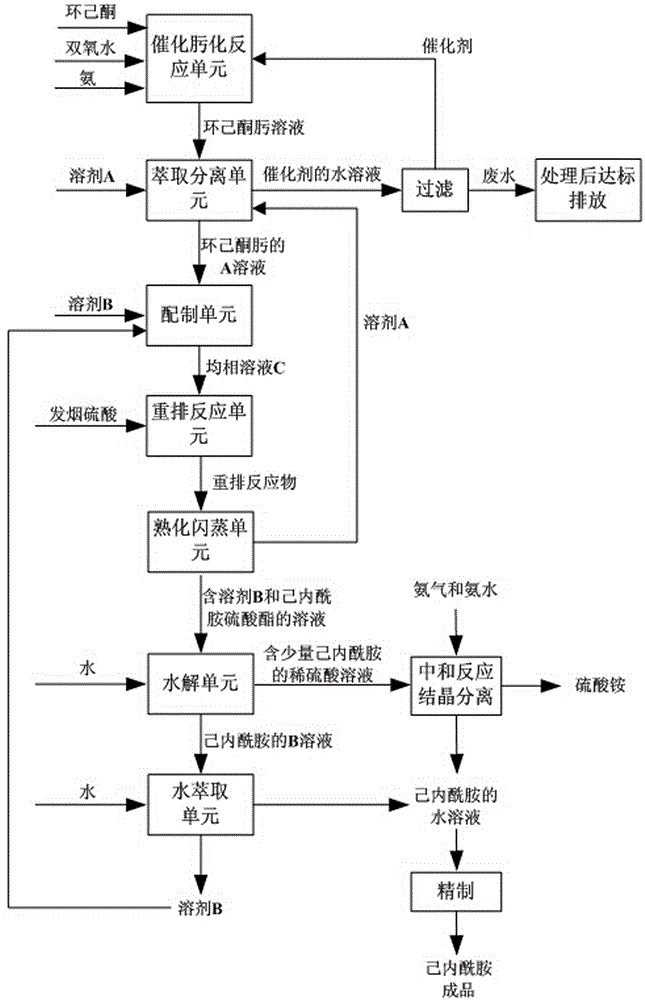
The invention discloses a caprolactam preparation method. The method is characterized in that caprolactam is prepared from cyclohexanone through oximation and rearrangement, and the method comprises the following steps: carrying out a catalytic reaction on cyclohexanone, hydrogen peroxide and ammonia to generate cyclohexanone oxime, extracting and separating by using a solvent A, adding a solvent B to the above obtained oil phase, carrying out a Beckmann rearrangement reaction under the action of oleum, carrying out slaking flash evaporation separation on the obtained product, hydrolyzing, extracting by using water, and carrying out original process refining to prepare finished caprolactam. The method shortens the caprolactam production process flow, reduces the consumption of raw materials and energy, generates less ammonium sulfate than original technologies, and improves the quality of the caprolactam product.
Owner:河北美邦工程科技股份有限公司
Method for preparing catalyst containing molecular sieve of MFI structure
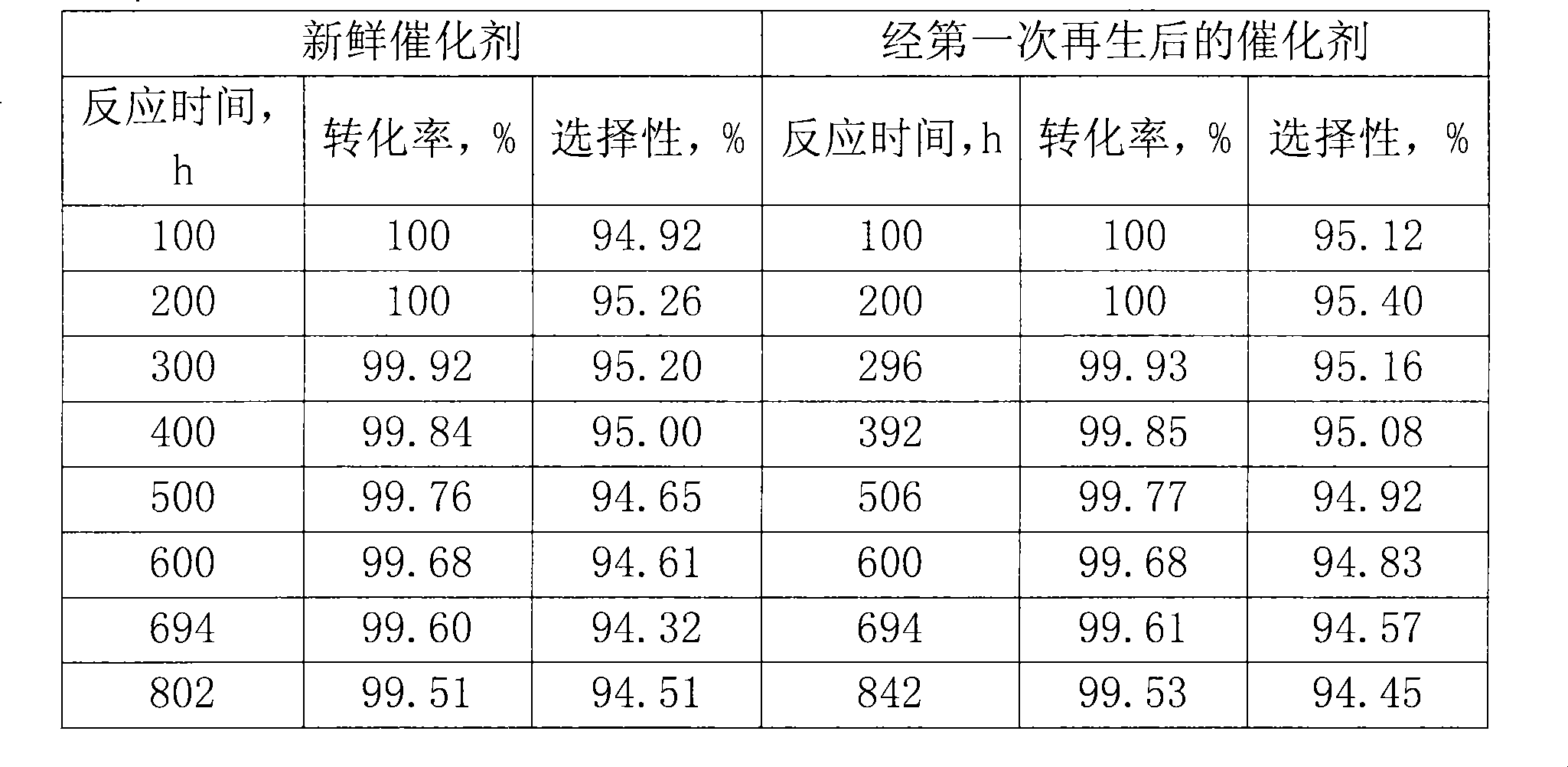
ActiveCN101468319AIncreased crush strengthImprove conversion rateLactams preparationMolecular sieve catalystsMolecular sieveBeckmann rearrangement
The invention provides a method for preparing a catalyst containing MFI-structure molecular sieves. The method comprises the steps of molding, drying and roasting a mixture containing the MFI-structure molecular sieves and a SiO2 carrier, wherein the method also comprises the step of allowing a product obtained after roasting to be in contact with an alkaline buffer solution of nitrogen-containing compounds. The catalyst containing MFI-structure molecular sieves, which is prepared by the method has high crushing strength, and can be used for a fixed bed process for preparing caprolactam by cyclohexanone-oxime gas phase Beckmann rearrangement. In addition, when the catalyst prepared by the method is used to perform the gas-phase Beckmann rearrangement reaction of cyclohexanone-oxime, both the conversion rate of cyclohexanone-oxime and the selectivity of caprolactam are high.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for producing Epsilon-hexanolactam with cyclohexanone oxime gas-phase beckmann rearrangement
ActiveCN101429149AHigh yieldHigh catalytic activityLactams preparationChemical recyclingBeckmann rearrangementGas phase
The invention discloses a method for preparing epsilon-caprolactam through cyclohexanone-oxime gas-phase Beckmann rearrangement. In nitrogen atmosphere, a low-carbon alcoholic solution of cyclohexanone-oxime is in contact with a catalyst bed for rearrangement reaction, and a product is recovered. The method is characterized in that the method is carried out in a fixed-bed reactor; when the conversion rate of the cyclohexanone-oxime is lower than 99.0 percent and / or when the BET specific surface area of a catalyst decreases to be or lower than 70 percent of that of a fresh catalyst, the low-carbon alcoholic solution of the cyclohexanone-oxime is changed into a low-carbon alcohol treatment catalyst bed; the low-carbon alcohol treatment catalyst bed is swept at a temperature between 350 and 550 DEG C by use of oxygen-containing atmosphere; and after surface coking matter of catalysts is burnt out and the BET specific surface area of the catalysts is basically recovered, the low-carbon alcohol treatment catalyst bed is in contact with a buffer solution of nitrogen-containing compound at a temperature between 50 and 120 DEG C, and is washed and dried, and then reaction continues to be performed.
Owner:CHINA PETROLEUM & CHEM CORP +1
Production of hexyl lactam in ion liquid
ActiveCN1778796AReduce lossesReduce interactionLactams preparationBulk chemical productionBeckmann rearrangementOrganic solvent
Production of caprolactam in ion liquid is carried out by Beckmann rearrangement reacting of cyclohexanone-oxime in ion liquid and organic solvent two-phase system, extracting ion liquid by organic solvent, transferring caprolactam into organic solvent, separating caprolactam from catalyst by solvent extraction. It has higher conversion rate and selectivity, no by products and circulating use.
Owner:CHINA PETROLEUM & CHEM CORP +1
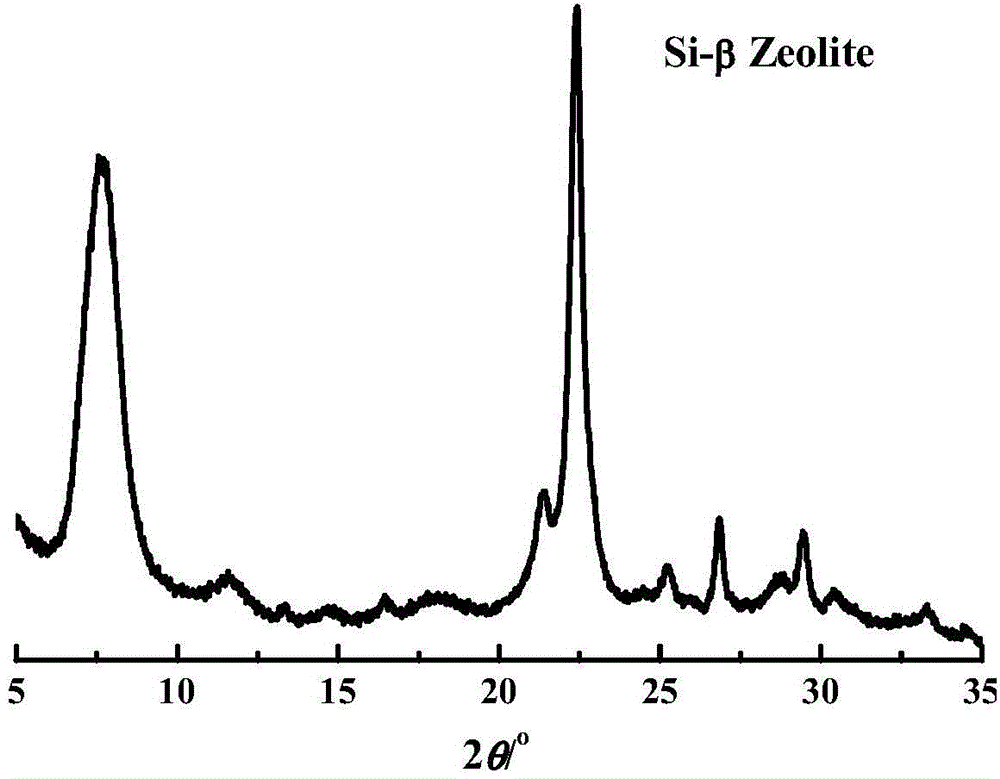
Method of producing epsilon -caprolactam
InactiveUS6051706ALactams preparationMolecular sieve catalystsBeckmann rearrangementCyclohexanone oxime
A method is disclosed of producing epsilon -caprolactam by the Beckmann rearrangement of cyclohexanone oxime using (B) beta zeolites as catalyst.
Owner:DEGUSSA AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com