Caprolactam preparation method
A technology of caprolactam and cyclohexanone, which is applied in the field of organic chemical raw material production, can solve the problems of industrial application limitations, difficulty in meeting industrial requirements, and high production costs, and achieve the goals of shortening the production process, reducing ammonium sulfate by-products, and reducing energy consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
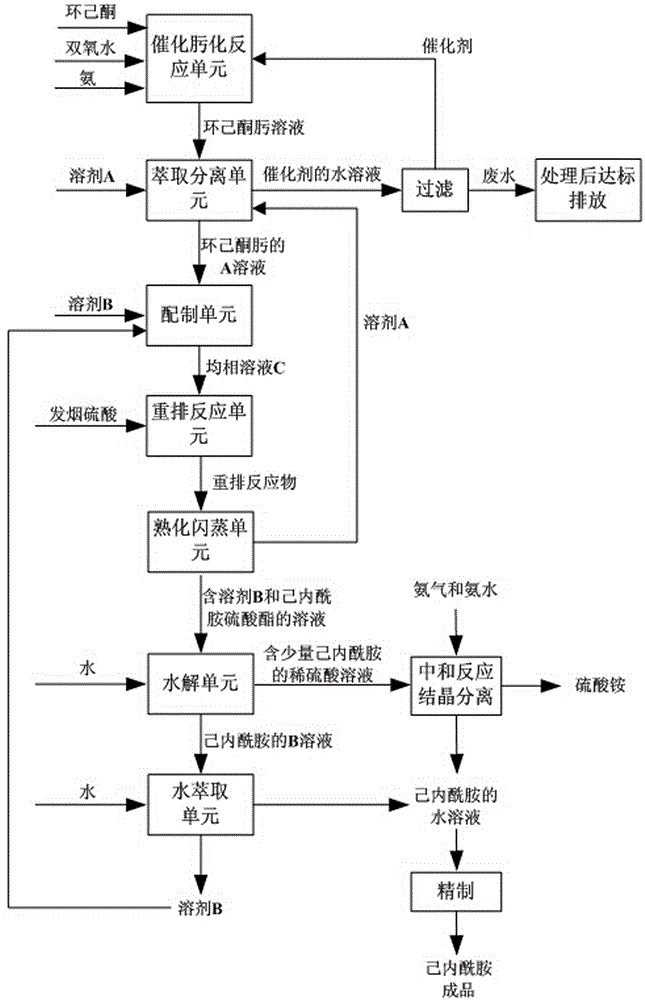
[0028] (1) In the catalytic oximation reaction unit, cyclohexanone, hydrogen peroxide, ammonia and the formed TS-1 catalyst are continuously added from the corresponding feed nozzles of the microreactor in the order of feed inlets from top to bottom, and continuous Catalyzed oximation reaction to obtain cyclohexanone oxime solution;
[0029] Among them, the particle size of the formed TS-1 catalyst used is 0.1 μm; the molar ratio of hydrogen peroxide and cyclohexanone is 1.0; the molar ratio of ammonia and cyclohexanone is 1.0; the mass fraction of hydrogen peroxide is 15%; the catalytic oximation reaction temperature is 70°C; the pressure is 200KPa (gauge pressure); the circulation volume of the circulating fluid is 600m3 / h; the mass fraction of the catalyst in the circulating fluid is 3.0%.
[0030] (2) In the extraction and separation unit, add solvent A to the cyclohexanone oxime solution obtained in step (1) and carry out extraction and separation, obtain the A solution o...
Embodiment 2
[0044] (1) In the catalytic oximation reaction unit, the raw powder after cyclohexanone, hydrogen peroxide, ammonia and TS-1 molecular sieve have changed the hydrophilicity is fed from the corresponding feeding ports of the microreactor in the order from top to bottom. The nozzle is continuously added, and the continuous catalytic oximation reaction is carried out to obtain the cyclohexanone oxime solution;
[0045] Among them, the particle size of the original powder after the TS-1 molecular sieve is used to change the hydrophilicity is 1.0 μm; the molar ratio of hydrogen peroxide and cyclohexanone is 1.1; the molar ratio of ammonia and cyclohexanone is 1.2; the concentration of hydrogen peroxide is 20% (wt %); the catalytic oximation reaction temperature is 80°C; the pressure is 250KPa (gauge pressure); the circulation volume of the circulating fluid is 700m3 / h; the catalyst concentration in the circulating fluid is 2.7% (wt%).
[0046] (2) In the extraction and separation u...
Embodiment 3
[0060] (1) In the catalytic oximation reaction unit, cyclohexanone, hydrogen peroxide, ammonia and the formed TS-1 catalyst are continuously added from the corresponding feed nozzles of the microreactor in the order of feed inlets from top to bottom, and continuous Catalytic oximation reaction obtains cyclohexanone oxime solution;
[0061] Among them, the particle size of the formed TS-1 catalyst used is 5.0 μm; the molar ratio of hydrogen peroxide to cyclohexanone is 1.2; the molar ratio of ammonia to cyclohexanone is 1.3; the concentration of hydrogen peroxide is 25% (wt%); The temperature is 85°C; the pressure is 300KPa (gauge pressure); the circulation volume of the circulating fluid is 800m3 / h; the catalyst concentration in the circulating fluid is 2.5% (wt%).
[0062] (2) In the extraction and separation unit, add solvent A to the cyclohexanone oxime solution obtained in step (1) and carry out extraction and separation, obtain the A solution of the organic phase cyclohex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com