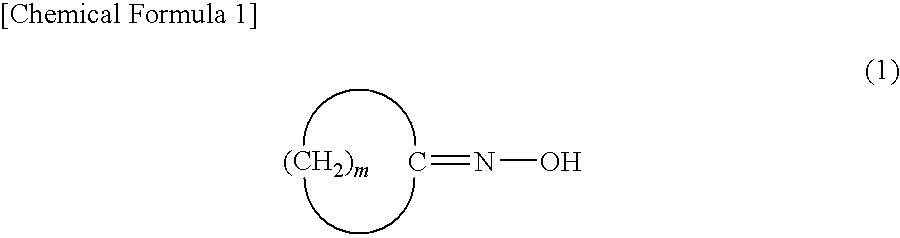Patents
Literature
726 results about "Rearrangement reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule.
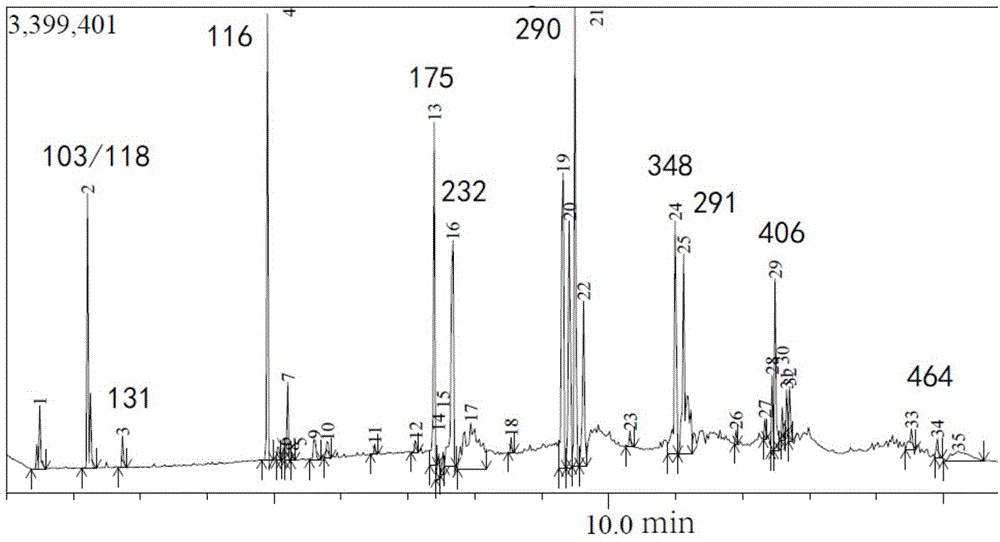
Green synthesis of high-content carvacrol capable of replacing natural origanum
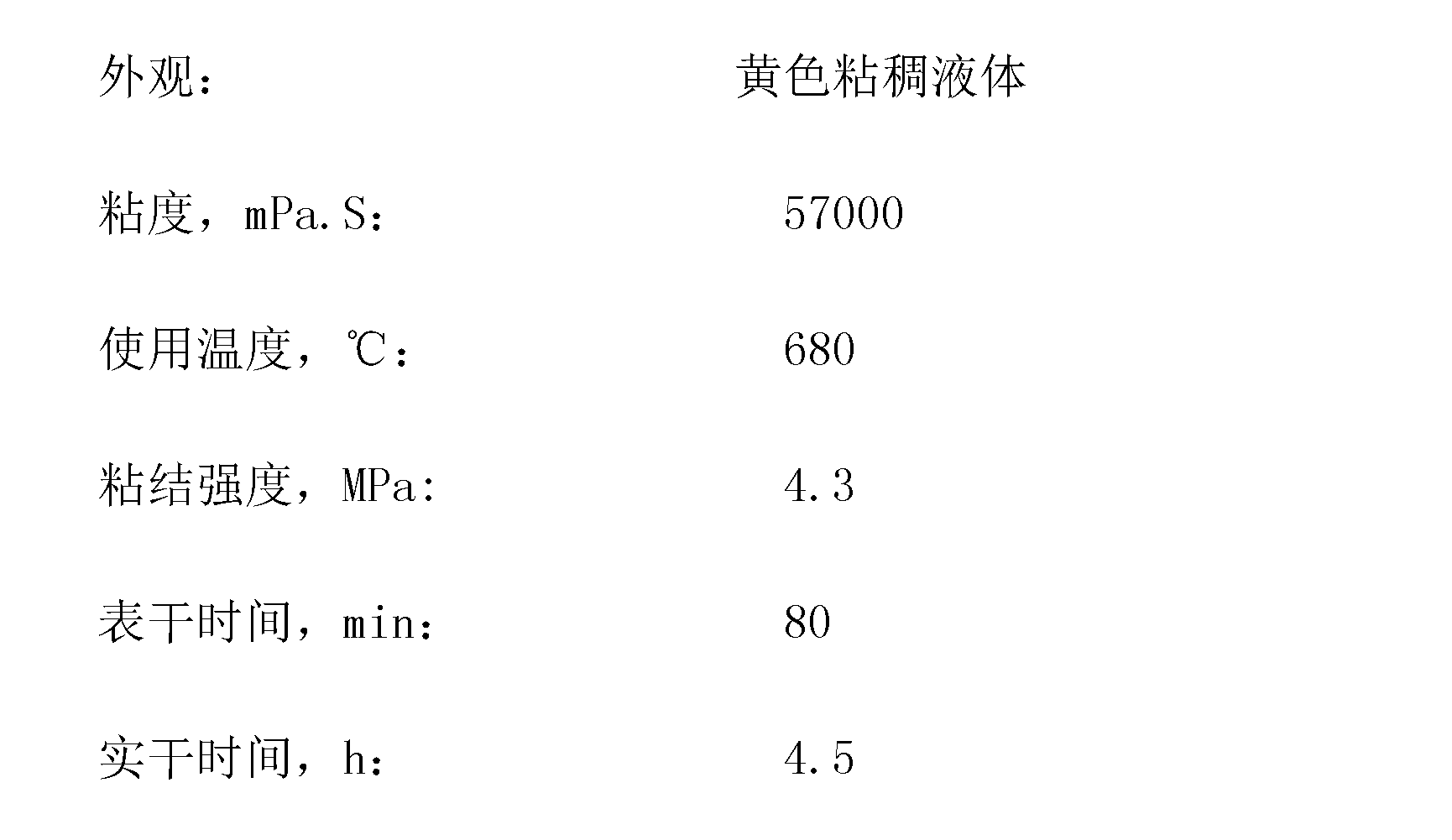
ActiveCN101475448AMild reaction conditionsEasy to operateOrganic chemistryOrganic compound preparationOrganic acidPEG 400
The invention discloses a green synthesis method for high-content carvacrol capable of replacing natural oregano. The method comprises the steps of taking food-grade L-carvone as raw material, adopting organic acid or inorganic acid as a main catalyst, taking PEG-400 or PEG-600 as an auxiliary catalyst and preparing the high-content carvacrol through intramolecular rearrangement reaction. The method adopts one step to synthesize a target product, and has the advantages of mild reaction conditions and convenient operation. The product is high in yield and good in quality, and can replace natural carvacrol. As no solvent harmful to human body and environment is added in reaction, the method is safe and friendly to environment.
Owner:HUAIAN WAN BANG SPICE IND CO LTD
Borosiloxane and preparation method thereof
The invention relates to borosiloxane and a preparation method thereof. According to the method, a hydrolysis solvent and a boron compound are mixed proportionally and added to a reactor, the temperature keeps from 10 DEG C to 35 DEG C, and a mixed monomer is slowly dropwise added under the stirring condition for a hydrolysis reaction; the dropwise-adding time is controlled in 3-6 hours, the reactor is slowly heated to the temperature ranging from 55 DEG C to 65 DEG C after the dropwise-adding is finished, a stirring reaction is performed for 1 hour, and still standing is performed; acid water on the lower layer is separated and neutralized to be neutral, a saturated NaCl solution is used for washing, and an oil phase material is obtained; reduced pressure distillation is performed to remove the solvent, a catalyst I is added, the mixture is heated to 110-130 DEG C, and the rearrangement reaction is performed for 3-5 hours; the mixture is heated to 150-160 DEG C, the splitting cyclization reaction is performed for 2-5 hours, the reaction is finished, the mixture is cooled, and a polyborosiloxane prepolymer is obtained; an end-capping reagent and a catalyst II are added to a system and heated to 110-130 DEG C, and react for 2-4 hours; and pressure is reduced to remove a low-boiling-point substance, and a polyborosiloxane resin is obtained. The borosiloxane and the preparation method have the advantages of good temperature resistance, ageing resistance, impact resistance, toughness and low-temperature flexibility, high adhesion and the like.
Owner:上海爱世博有机硅材料有限公司
Process for producing epsi-caprolactam
InactiveUS6265574B1High yieldSteadily producedLactams preparationMolecular sieve catalystsBeckmann rearrangementFluidized bed
A process for producing epsi-caprolactam is provided which comprises the steps of subjecting cyclohexanone oxime to a gaseous phase Beckmann rearrangement reaction in a fluidized bed system using a solid catalyst and re-generating the catalyst, wherein said process comprises a step of treating the catalyst with an oxygen-containing gas at an elevated temperature in a re-generation step so that the nitrogen content of the catalyst falls within a range of 10 ppm to 2,500 ppm on its way to the reaction step from the re-generating step. According to the present invention, epsi-caprolactam is produced with a high conversion or a high selectivity without interrupting the rearrangement reaction or the re-generation step.
Owner:SUMITOMO CHEM CO LTD
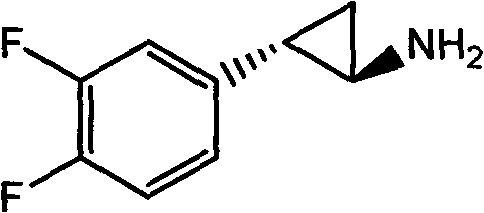
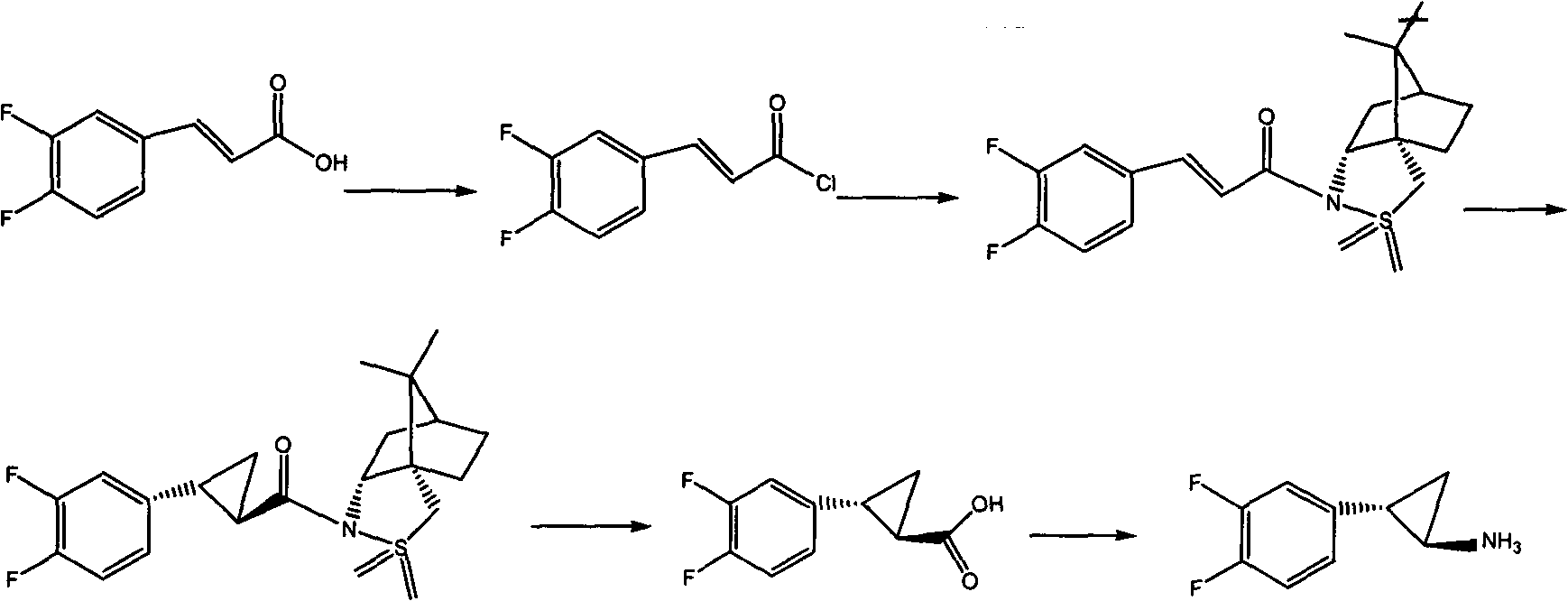
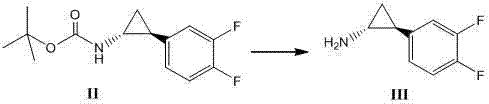
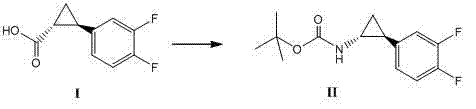
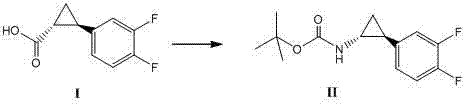
Method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine
InactiveCN102249929AReduce difficulty of reactionHigh reaction yieldOrganic compound preparationAmino compound preparationBiochemical engineeringTert-Butyloxycarbonyl protecting group
The invention relates to a method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine which is an intermediate for preparing an anticoagulation medicine Ticagrelor. The method provided by the invention mainly comprises the following steps of: synthesizing the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine protected by tertiarybutoxy carbonyl through carrying out a rearrangement reaction of DPPA (Diphenylphosphoryl Azide); then removing the protective group of the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine protected by the tertiarybutoxy carbonyl and then alkalifying to obtain the product. The whole reaction can be finished through a one-pot boiling synthetic method so that synthesizing steps and synthesizing time are greatly saved, the cost is effectively reduced and the yield is improved; and the method for synthesizing the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine has the very active meaning in the industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
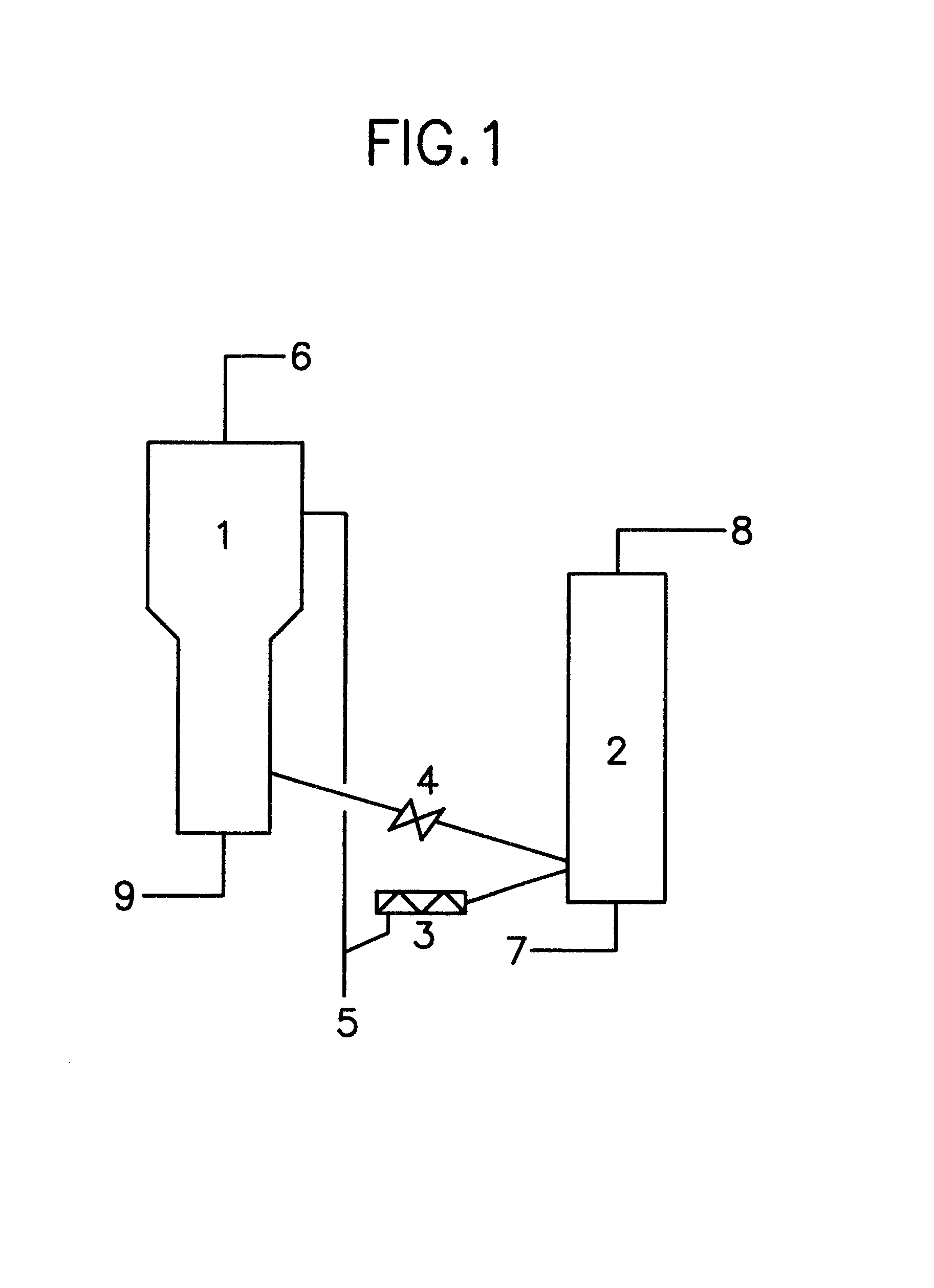
Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline
The invention discloses a method for synthesizing 4-(3-chloro-4-fluorophenylamino)-7-methoxy -6-[3-(4-morpholinyl)-propoxy] quinoline. The method comprises the following steps: 1) 3-hydroxide radical-4-methoxybenzaldehyde is used as a raw material to prepare 3-hydroxide radical-4-methoxy-benzonitrile; 2) the 3-hydroxide radical-4-methoxy-benzonitrile and 3- chloropropy morpholinehydrochloride are heated to have reflux reaction to obtain 4- methoxy-3-[3-(4- morpholinyl) propoxy] benzonitrile; 3) the 4- methoxy-3-[3-(4-morpholinyl) propoxy] benzonitrile is subjected to nitration to obtain 2- nitryl-4- methoxy -5-[3-(4-morpholinyl) propoxy] benzonitrile; 4) the 2- nitryl-4- methoxy -5-[3-(4-morpholinyl) propoxy] benzonitrile is subjected to reduction to obtain 2-amido-4- methoxy-5-[3-(4-morpholinyl) propoxy] benzonitrile; and 5) the 2-amido-4- methoxy-5-[3-(4-morpholinyl) propoxy] benzonitrile and an azomethine intermediate of 3-chloro-4-fluoroaniline have rearrangement reaction to obtain 4-(3-chloro-4-fluorophenylamino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline. The method has characteristics of environmental protection and high production rate.
Owner:浙江精进药业有限公司
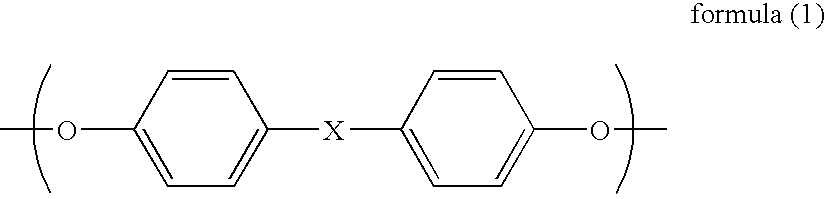
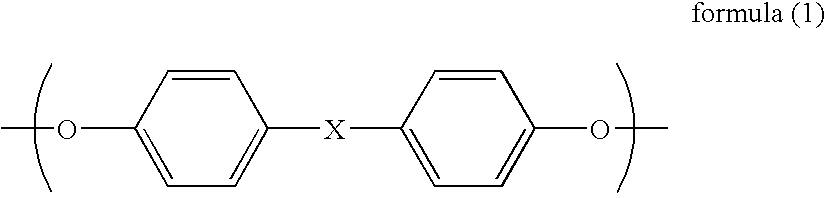
Aromatic polycarbonate, process for producing the same, polycarbonate composition, and hollow container obtained from the same
InactiveUS7084233B2Satisfactory color toneRetains intact melt characteristicEnvelopes/bags making machinerySynthetic resin layered productsTransesterificationPolystyrene
A subject for the invention is to provide a branched aromatic polycarbonate which is excellent in hue and in melt characteristics such as melt strength.The invention provides a branched aromatic polycarbonate having a viscosity-average molecular weight of 16,000 or higher obtained by the transesterification method, characterized in that the ratio of the weight-average molecular weight (Mw) to number-average molecular weight (Mn) as measured by gel permeation chromatography and calculated for standard polystyrene (Mw / Mn) is in the range of from 2.8 to 4.5 and that the proportion of the number of moles of all structural units yielded by a rearrangement reaction in the course of melt polymerization reaction to 1 mol of structural units having the framework of an aromatic dihydroxy compound used as a starting material is higher than 0.3 mol % and not higher than 0.95 mol.
Owner:MITSUBISHI ENG PLASTICS CORP +1
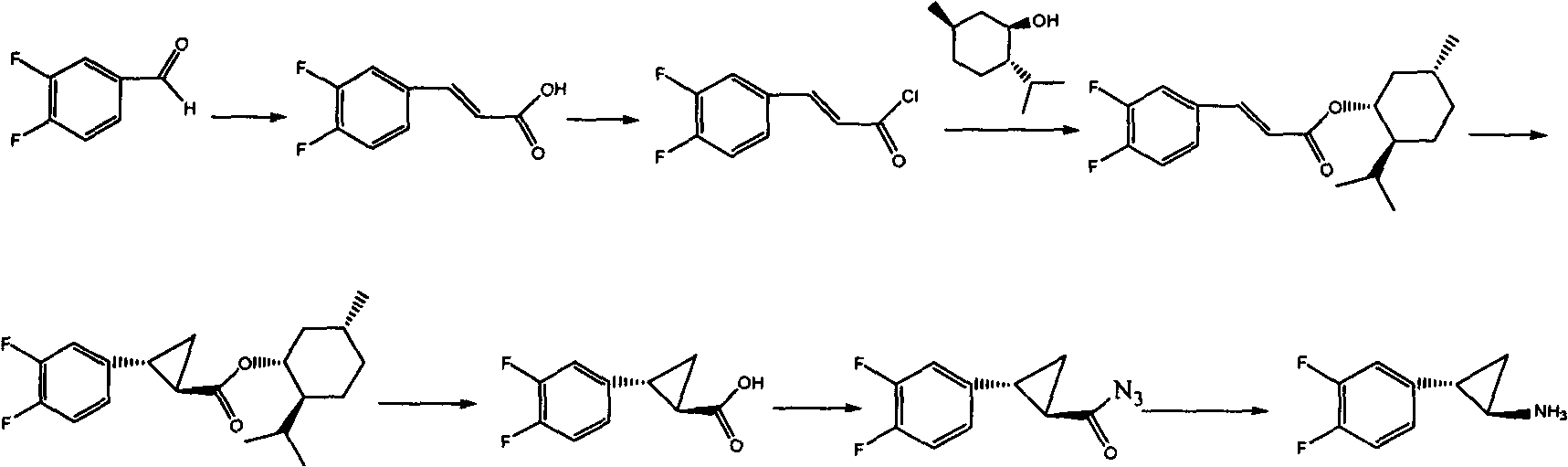
Preparation method of Ticagrelor intermediate
ActiveCN102796007AEasy to prepareEasy to operatePreparation by rearrangement reactionsChemical synthesisTicagrelor
The invention relates to the medicine chemical synthesis field, and especially discloses a preparation method of a Ticagrelor intermediate. The preparation method comprises the following steps: 1) taking 3,4-difluorobenzaldehyde (I) as an initial raw material, reacting with a phosphorus ylide material liquid to obtain (E)-3-(3,4-difluorophenyl)-2-acrylic acid ester (II); 2) performing a Simons-Smith asymmetric cyproteronethe reaction on the (E)-3-(3,4-difluorophenyl)-2-acrylic acid ester (II) to obtain trans-(1R,2R)-2-(3,4-difluorophenyl) cyclopropanecarboxylic acid ester (III); 3) performing aminolysis on the trans-(1R,2R)-2-(3,4-difluorophenyl) cyclopropanecarboxylic acid ester to obtain trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxamide (IV); and 4) performing a Huffman rearrangement reaction on the trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxamide (IV) to obatain the Ticagrelor intermediate (V). The method of the invention has the advantages of simple process, convenient operation, mild reaction condition and easy control, low cost and easy acquisition of raw material, high product yield and product purity, and is adapted to large scale industrial production.
Owner:JINAN RUIFENG PHARMA +2
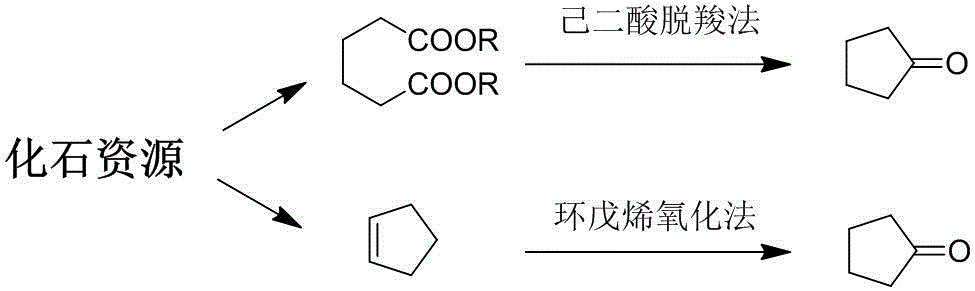
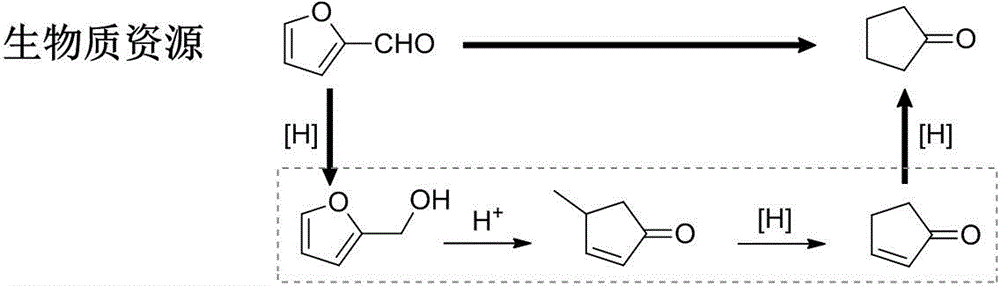
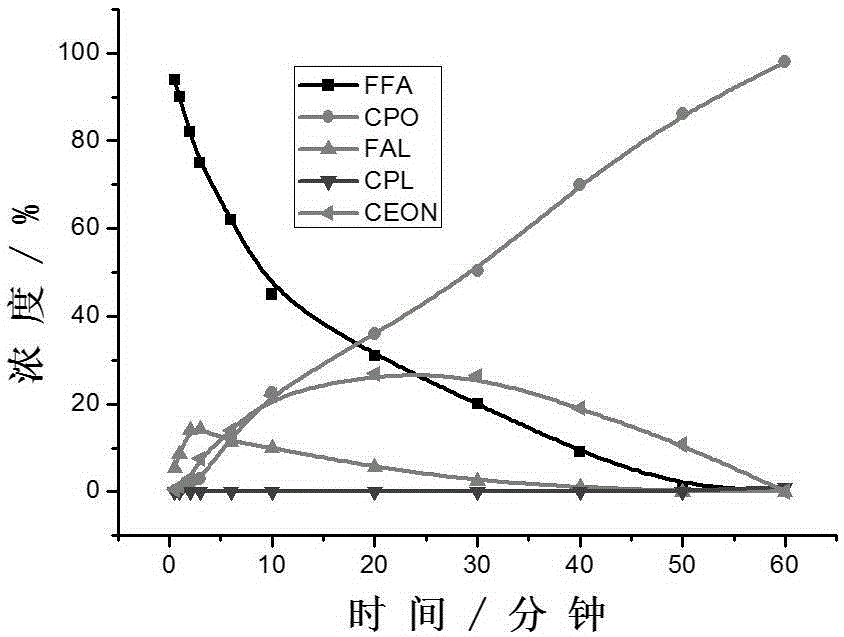
Method for preparing cyclopentanone by taking biomass resource as raw material
InactiveCN105330523AReduce yieldFew stepsMolecular sieve catalystsChemical industryFixed bedFurfural
The invention belongs to the technical field of biomass conversion utilization, and concretely relates to a method for preparing cyclopentanone by taking biomass resource as a raw material. The method comprises taking furfural coming from biomass as the raw material, taking a hydrogen-containing gas as a reducing agent, and in the presence of a metal supported type catalyst, performing hydrogenation rearrangement reaction in a high-pressure reaction kettle or a fixed bed reactor, so as to obtain cyclopentanone in one step, wherein the carrier of the metal supported type catalyst is selected from Nb2O5, H-ZSM-5 molecular sieve, HY molecular sieve, Fe2O3, ZrO2, Al2O3, SiO2, CeO2, MgO, active carbon, and TiO2 in various crystal forms, the active composition is selected from Au, Pt, Ru, Rh, Pd, Ir, Ni and Cu, and the active composition load capacity is 0.1-5% of the catalyst. The method is mild in reaction technological conditions, and cheap and easily-available in raw materials, is capable of realizing quantitative conversion of furfural to cyclopentanone, and belongs to an environment-friendly green chemical technology.
Owner:FUDAN UNIV
Method for preparing high-purity palbociclib and reaction intermediate of palbociclib
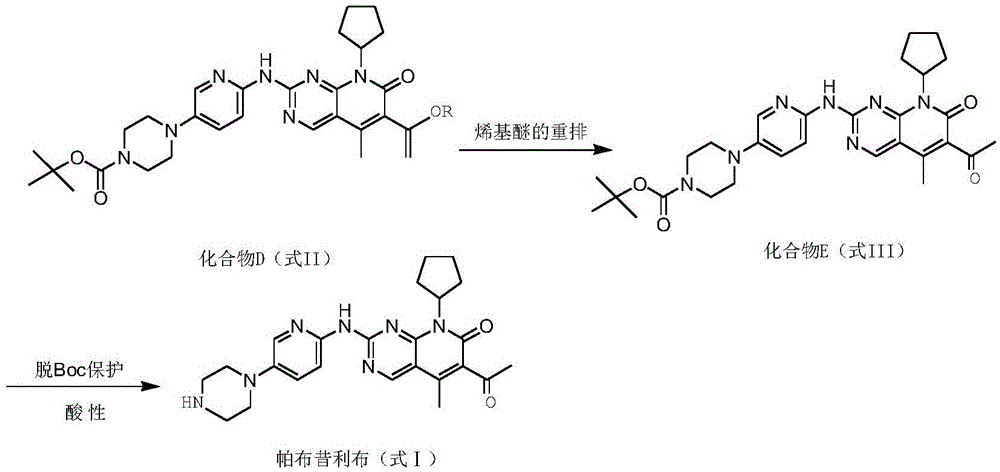
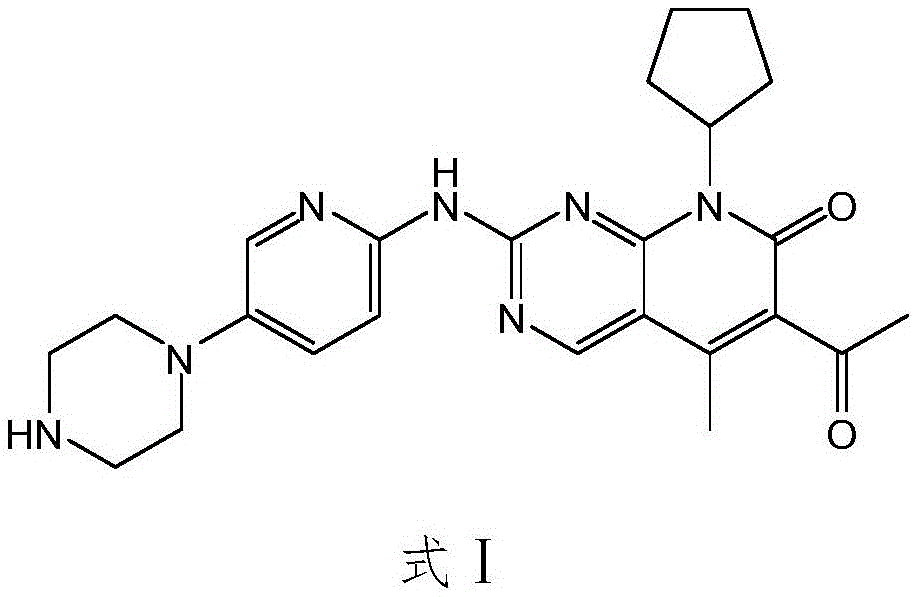
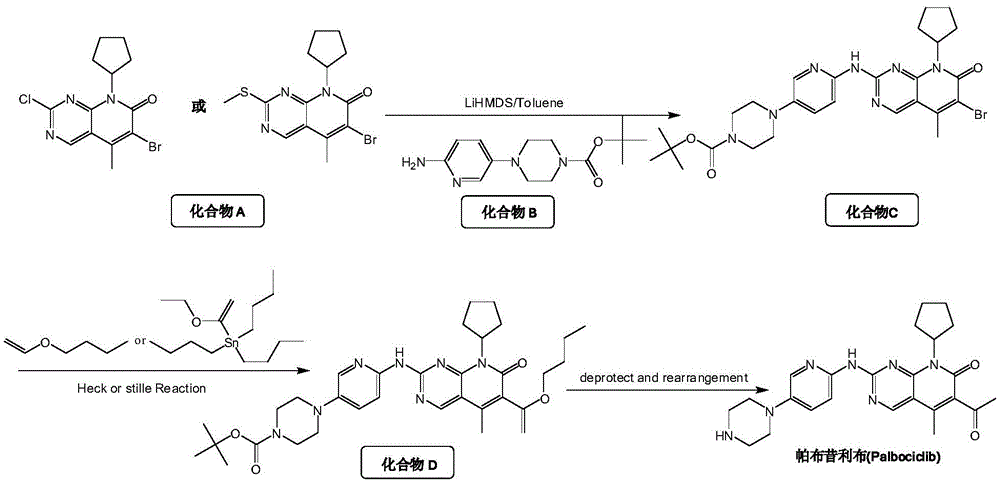
InactiveCN105418603AImprove solubilityReduce the difficulty of purificationOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupReaction intermediate
The invention relates to a method for preparing compound palbociclib (shown as the formula I). A compound D (shown as the formula II) serves as a raw material, and a compound E (shown as the formula III) is obtained through a rearrangement reaction of alkenyl ether; then the compound E is subjected to t-butyloxycarboryl protection group removal under the acidic condition, and target product palbociclib is obtained; an R group in a compound D is selected from any one of a C1-C6 alkyl group, a C1-C6 halogenate alkyl group, C1-C6 hydroxyalkyl and a C1-C6 naphthenic base. The process procedure and operation steps are easy and convenient, prepared palbociclib is high in purity, and good popularization prospects are achieved.
Owner:重庆莱美隆宇药业有限公司
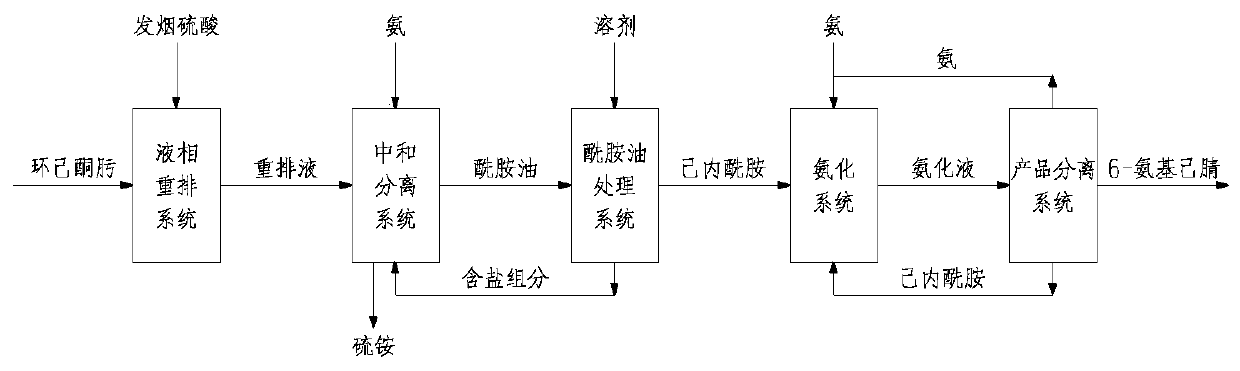
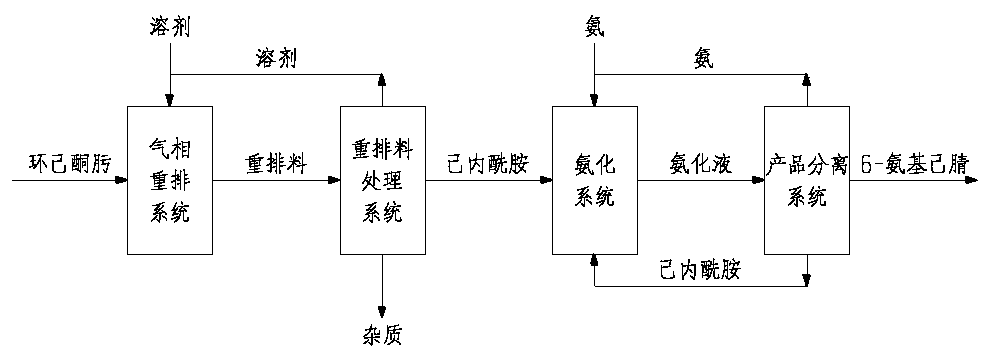
Method for preparing 6-aminocapronitrile from cyclohexanone-oxime
InactiveCN110835311AReduce consumptionReduce processing complexityLactams preparationLactams separation/purificationDistillationIon exchange
The invention discloses a method for preparing 6-aminocapronitrile from cyclohexanone-oxime. The method comprises the following steps: carrying out a rearrangement reaction on cyclohexanone-oxime to obtain a rearranged reaction material; and treating the rearranged reaction material to obtain caprolactam, carrying out an ammoniation dehydration reaction on the caprolactam to obtain an ammoniated dehydrated reaction material, and performing separation to obtain the 6-aminocapronitrile. Compared with the prior art, the method of the invention reduces complex separation and refining processes such as back extraction, ion exchange, hydrogenation, evaporation and distillation required after liquid phase rearrangement of the cyclohexanone-oxime, reduces the processes of crystallization, solventrefining and recovery needed after gas phase rearrangement, and organically combines the caproamide evaporation and rearrangement treatment processes in the ammoniation dehydration reaction process. The steam consumption can be reduced by 2.0-3.0 t / t 6-aminocapronitrile, and the cyclohexanone-oxime consumption is reduced by 20-50 kg / t 6-aminocapronitrile.
Owner:HUNAN BAILI ENGINEERING SCIENCE AND TECHNOLOGY CO LTD
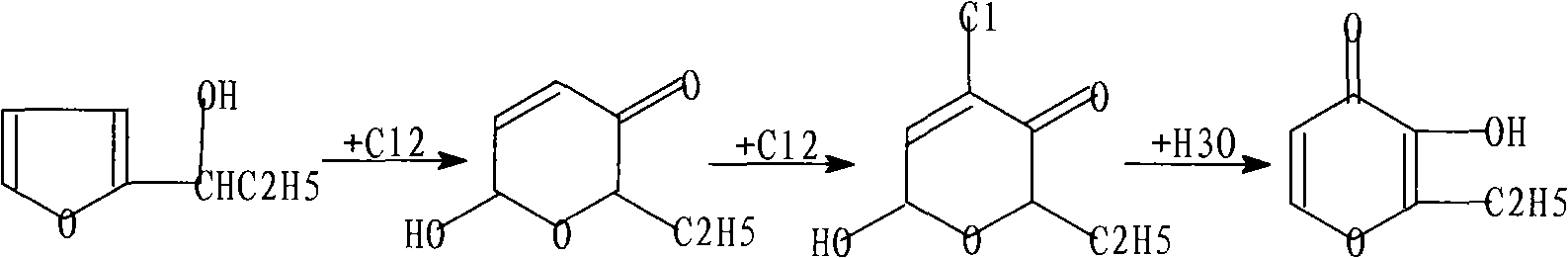
Ethyl maltol synthetic method
InactiveCN101585822AReduce concentrationReduce contentOrganic chemistryHydrolysateReaction temperature
The invention relates to an ethyl maltol synthetic method, which includes an alpha-furans propanol chlorination rearrangement reaction and a hydrolysis reaction to the product of the alpha-furans chlorination rearrangement reaction. Wherein, the hydrolysis reaction comprises: hydrolyzing the mixture obtained in the chlorination rearrangement reaction under the temperature of 95-140 degrees centigrade and the pressure of 1.9-8.5Mpa for 1.5-5.5 hours; cooling to below 100 degrees centigrade; separating out the by-product: chloromethanes gas; and obtaining the hydrolysis mixed liquor containing the product: ethyl maltol. The invention can achieves the synthesized ethyl maltol yield of 60% which is improved by 4-6% compared with conventional methods; enables the hydrogen chloride generated in the reaction process to react with solvent methanol to generate a large amount of recyclable by-product: chloromethanes; reduces the hydrochloric acid concentration in the hydrolysate by 75-80%; reduces the sodium hydroxide used amount in the subsequent ethyl maltol separating and refining process; and reduces the sodium chloride content in the technique waste water; thereby, reduces environmental pollution and production cost.
Owner:CHUZHOU UNIV
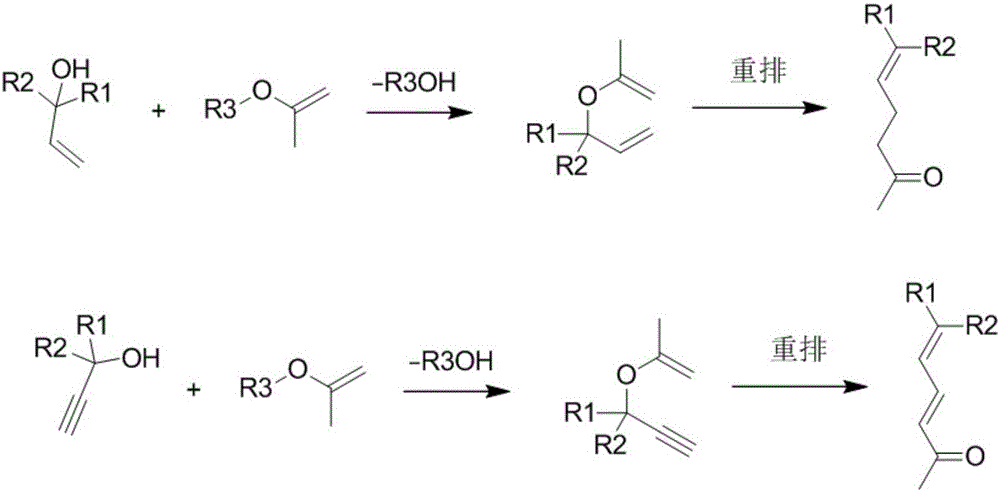
Method for synthesizing gamma, delta-nonsaturated ketones compound
ActiveCN106478514ANot easy to loseExtend your lifeOrganic compound preparationGroup 5/15 element organic compoundsAlcoholBoiling point
The invention discloses a method for synthesizing a gamma, delta-nonsaturated ketones compound. The method comprises the following steps: under protection of inert gas, nonsaturated alcohol and 2-alkyloxy propylene are taken as the raw materials, Bronsted acid functional ionic liquid is dissolved in a high boiling solvent as a catalyst, a rearrangement reaction is carried out, after the reaction is complete, the materials is subjected to post-treatment to obtain the gamma, delta-nonsaturated ketones compound and a catalyst solution. The used Bronsted acid functional ionic liquid catalyst has a plurality of physical and chemical properties which are similar with the conventional ionic liquid, the acid functional group is introduced, so that the catalyst has the advantages of high acid site density, adjustable acidity, uniform acidity distribution and difficult loss of acidity. An autoclave series mode is used for realizing the continuous synthesis of nonsaturated ketone and continuous recycling of the catalyst.
Owner:ZHEJIANG NHU CO LTD +2
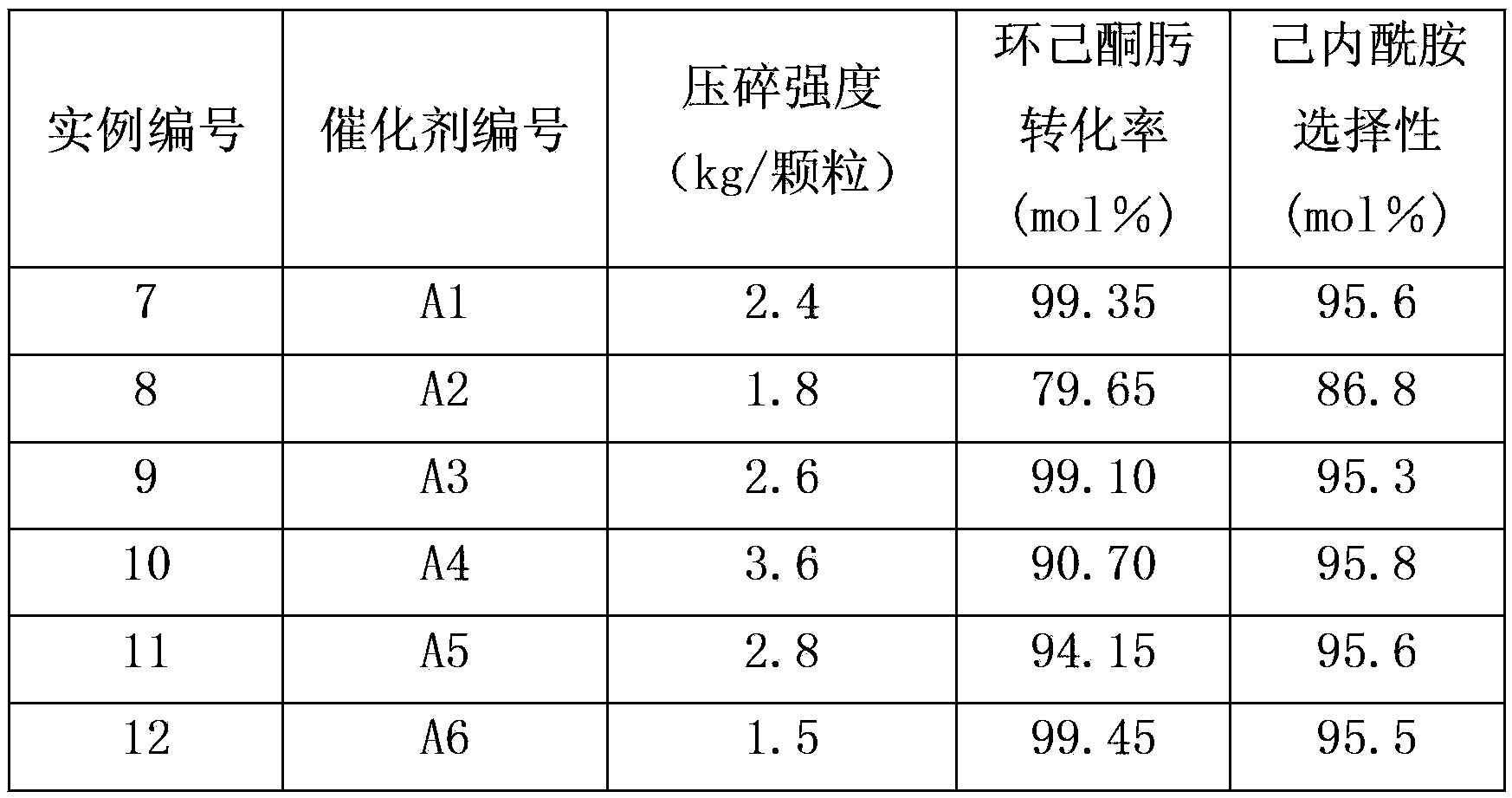
Method for preparing caprolactam through gas phase Beckmann rearrangement of cyclohexanone-oxime gas
ActiveCN103896839AImprove conversion rateHigh selectivityLactams preparationMolecular sieve catalystsBeckmann rearrangementMolecular sieve
The invention provides a method for preparing caprolactam through gas phase Beckmann rearrangement of cyclohexanone-oxime gas. According to the method, under the condition of rearrangement reaction, cyclohexanone-oxime is in contact with a catalyst, and the catalyst is obtained by the steps of forming a molecular screen with an aluminum-free MFI (Melt Flow Index) structure, and enabling the molecular screen to be in contact with an alkaline buffer solution of a nitrogen-containing compound. The method is high in cyclohexanone-oxime conversion rate, and is capable of realizing long-period and continuous production; the selectivity of caprolactam is capable of reaching 95.8 percent.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of high-throughput graphene oxide/polyimide mixed matrix membrane material
ActiveCN107551835AImprove throughputHigh selectivitySemi-permeable membranesChemical structureRearrangement reaction
The invention relates to a preparation method of high-throughput graphene oxide / polyimide mixed matrix membrane material. The high-throughput graphene oxide / polyimide mixed matrix membrane material isprepared by the steps of introducing graphene oxide into a polyimide matrix subjected to thermotropic rearrangement reaction through an in-situ method to prepare a composite material membrane, and processing the composite material membrane for at least 0.1h under inert atmosphere. The prepared mixed matrix gas separation membrane material has the characteristics of excellent seepage separation performance and stable chemical structure and can solve the inadequate problems of limited separation performance, poor anti-plasticizing capability, poor temperature tolerance and the like of the existing polymer separation membrane material.
Owner:UNIV OF SCI & TECH LIAONING +1
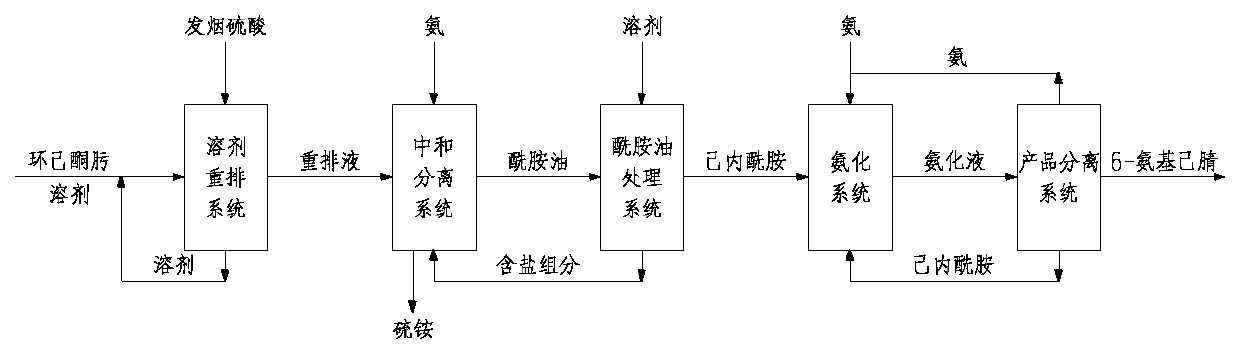
Method for preparing amide using nonhomogeneous phase oximation rearrangement
ActiveCN1762985ALow costGood economic valueOrganic compound preparationCarboxylic acid amides preparationBeckmann rearrangementReaction temperature
The present invention discloses oximation and rearrangement process of preparing amide with aliphatic and / or cyclic aliphatic ketone as material. The preparation process includes catalytic reaction of ketone, hydrogen peroxide solution and ammonia inside inert solvent to produce ketoxime solution, Beckmann rearrangement of the oil phase product under the action of fuming sulfuric acid, and hydrolysis to neutralize and produce amide. The present invention has shortened technological process, lowered cyclohexyl ketoxime rearranging reaction temperature, lowered sulfuric acid consumption and reduced side product.
Owner:HUBEI JINXIANGNING CHEM ENG TECHENOLOGY CO LTD
Selective hydrogenation desulfurization method for bastard gasoline
ActiveCN101173184AEliminate strong inhibitionAvoid generatingRefining to eliminate hetero atomsHydrodesulfurizationSulfide
The invention discloses a selective hydrodesulfurization method for poor quality gasoline, which is characterized in that, the method comprises the hydrodesulfurization treatment of poor quality gasoline material and the separation of the hydrodesulfurization product, wherein the hydrodesulfurization process of the poor quality gasoline material is as followings: the poor quality gasoline material, a hydrogen sulfide removing agent and hydrogen are contacted to a selective hydrodesulfurization catalyst, and selective hydrodesulfurization reaction is carried out under hydrodesulfurization reaction conditions. Compared with the method not using hydrogen sulfide removing agent, the invention has the advantages that, H2S is removed in the hydrodesulfurization reaction process, so not only the strong inhibition effect of the H2S to thiophene-type sulfide HDS is eliminated, the mercaptan generated by the rearrangement reaction between the H2S and olefin is also avoided; therefore, not only the hydrodesulfurization selectivity is increased, the subsequent deodorization facility can also be cancelled, and the operation flow is simplified; the method can produce clean gasoline having the sulfur content is not more than 50Mu g / g and the mercaptan sulfur content is not more than 10Mu g / g under the condition of low loss of octane number.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for synthesizing tantalum-containing SiC ceramic precursor
InactiveCN101186504AWide variety of sourcesControllable and adjustable contentFiberSynthesis methods
The invention discloses a synthesis method of an SiC-containing ceramics precursor, which comprises the steps that: 1. low molecular weight polymer with main chain containing silicon is sent into a three-mouth flask, and is added with 0.5wt% - 20wt% of tantalum organic compounds or chloride; 2. under the protection of Ar or N2 or compound of the Ar or N2, the temperature of the three-mouth flask is raised to 350 to 500 DEG C, and the temperature of a cracking column is controlled within 450 DEG C to 550 DEG C; then thermal decomposition rearrangement reaction is carried out for 0.5 to 25 hours, and then cooling is done; 3. the obtained rough product is dissolved by xylene and filtered; the filtrate solution is distilled by reducing temperature to between 250 to 390 DEG C, then the obtained material is cooled. The invention has the advantages of wide raw material source, reaction process being easy to be controlled, simple equipment, high purity of product, good formability, and good performance of super-high temperature and absorbing property; in addition, the ceramics fiber produced in the method has good oxidation resistance performance and is easy to realize large-scale industrial production.
Owner:NAT UNIV OF DEFENSE TECH
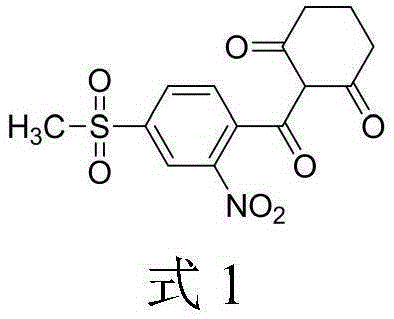
Composite method of triketone compound
ActiveCN101735119ALow toxicityHigh yieldOrganic chemistryOrganic compound preparationOrganic solventPurine
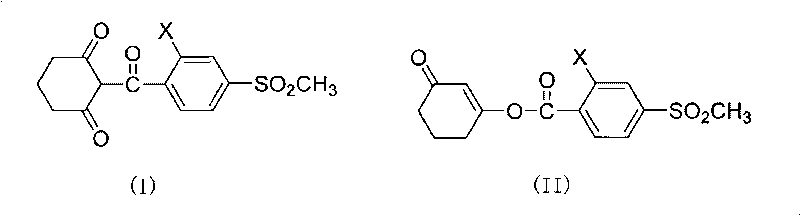
The invention discloses a composite method of triketone compound shown in the formula (I). In the formula (I), X is Cl or NO2. The composite method is as follows: enol ester shown in the formula (II) generates rearrangement reaction under the action of an alkaline reagent and a rearrangement catalyst in organic solvent; after reaction ends, the obtained reaction liquid is acidized and separated to obtain the triketone compound; the rearrangement catalyst is purine compound. The composite method of the triketone compound (sulcotrione and mesotrione) of the invention adopts the catalyst with small toxicity, protects environment, has high product yield and is suitable for industrial production.
Owner:ZHEJIANG UNIV OF TECH
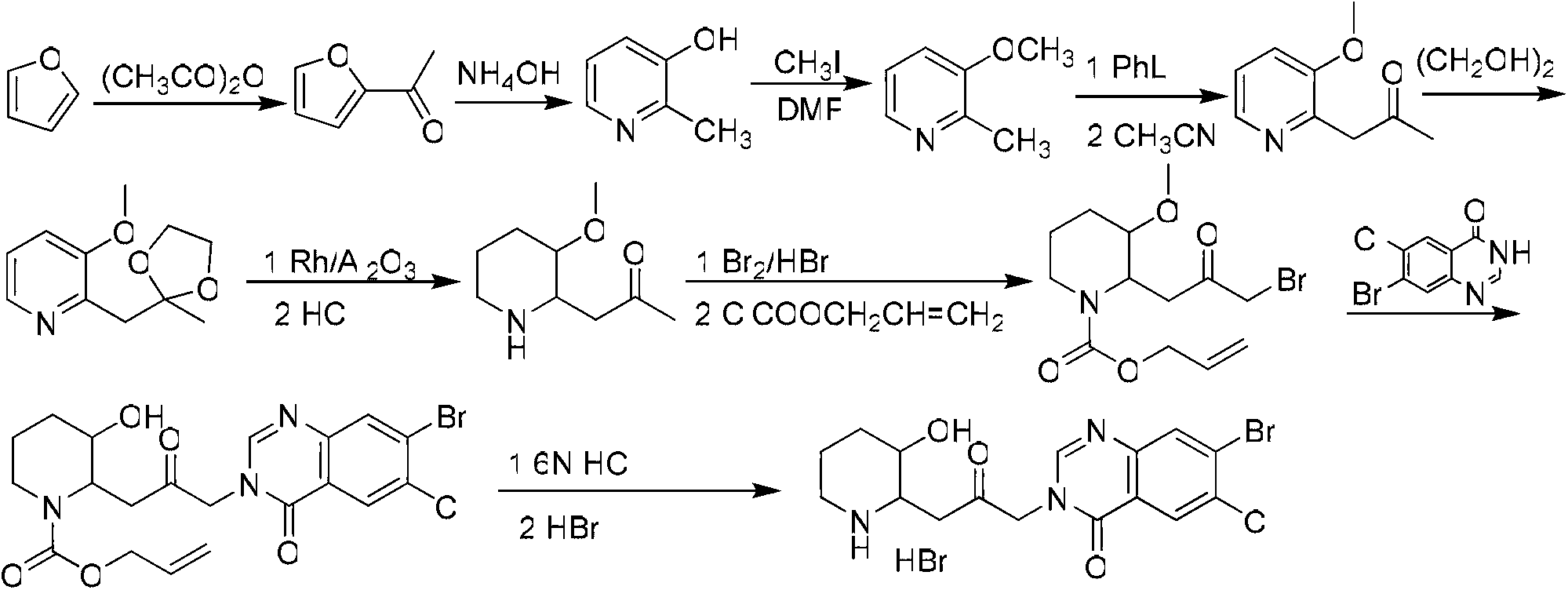
Method for preparing halofuginone hydrobromide
The invention discloses a method for preparing halofuginone hydrobromide, which comprises the following steps: by using N-benzyl-3-piperidone or hydrochloride thereof, organic or inorganic base, halide of alkali metal and beta, gamma-dihaloalkene or allyl haloalkane as initial raw materials, performing Steven rearrangement reaction to obtain an intermediate VIII; performing Von Braun reaction on the intermediate VIII to obtain an intermediate VII; performing reduction reaction on the intermediate VII to obtain an intermediate VI; obtaining an intermediate V after reaction of the intermediate VI and NBS (N-bromosuccinimide); obtaining an intermediate IV after the reaction of the intermediate V and a compound 7-bromine-6-chlorine-4(3H) quinazolinone; performing deprotection on the intermediate IV to obtain an intermediate III; performing backflow processing on the intermediate III in ethanol to obtain an intermediate II; and obtaining the halofuginone hydrobromide after the salt forming reaction of intermediate II. The invention develops an efficient and simple method for synthesizing the halofuginone hydrobromide. The total yield can reach over 50 percent, cost is greatly reduced and the product has high purity.
Owner:CHONGQING WEIPENG PHARMA
Method for continuously preparing 3-methyl-2-butenol
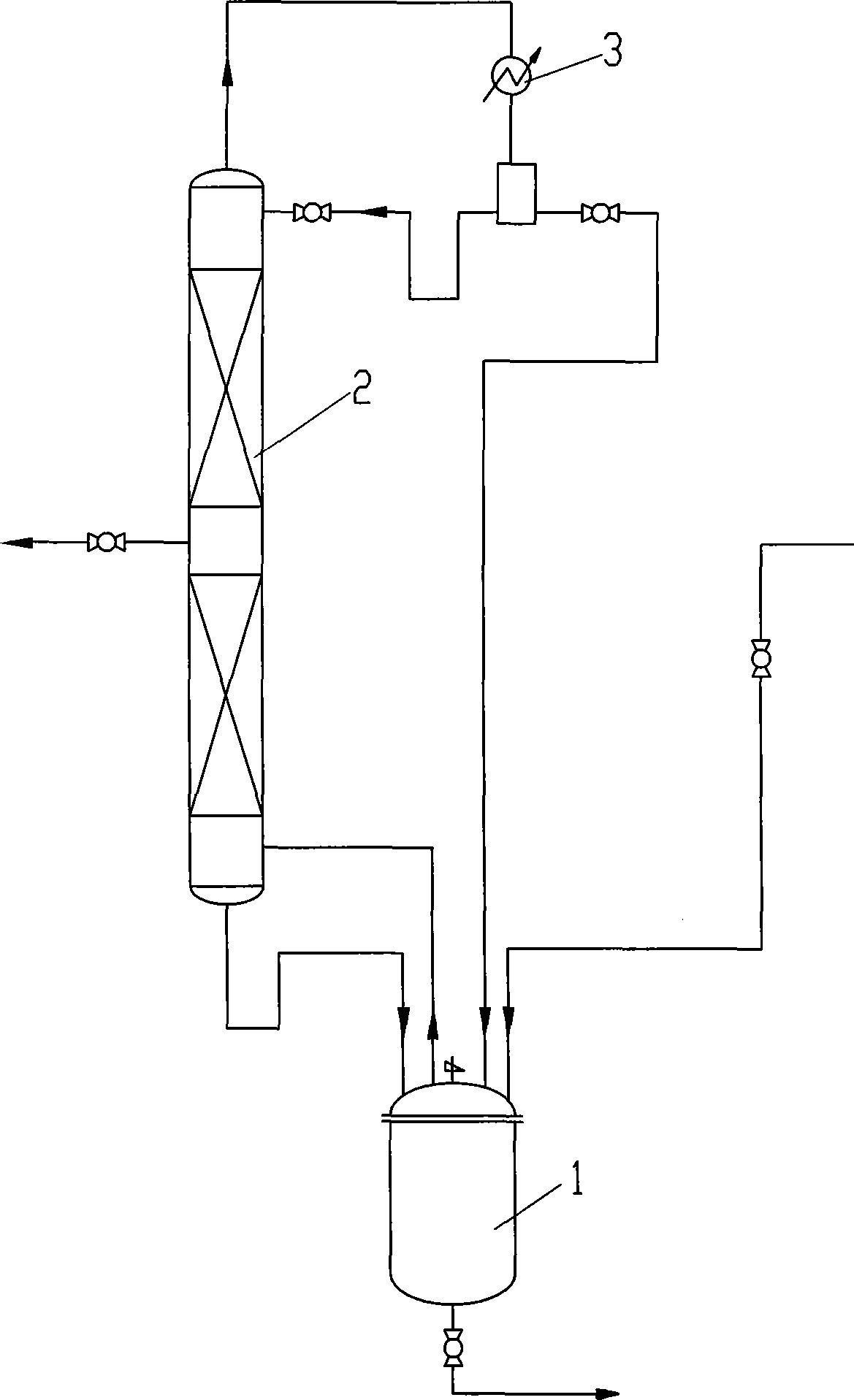
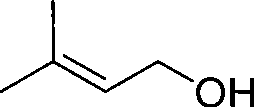
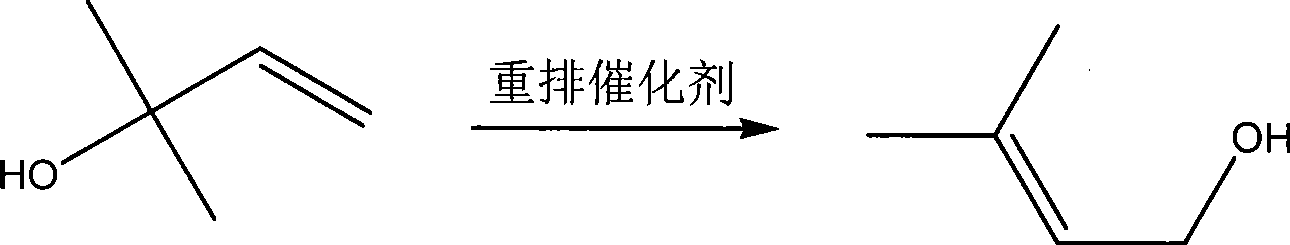
InactiveCN101381283AOperations that reduce recyclingRaw materials are easy to getPreparation by isomerisationButeneSolvent
The invention discloses a method for preparing intermediate 3-methyl-2-butenol. The prior method has the defects of rigorous conversion reaction condition, low conversion rate, difficult separation, and high technical requirement. The method has the following steps of: putting 2-methyl-3-butene-2-hydrin and rearrangement catalyst into a reaction kettle, and heating the 2-methyl-3-butene-2-hydrin and rearrangement catalyst to undergo a catalytical rearrangement reaction to obtain the mixture of the 2-methyl-3-butene-2-hydrin and the 3-methyl-2-butenol; separating the mixture through a rectification tower, recovering the 2-methyl-3-butene-2-hydrin at the top of the rectification tower and returning the 2-methyl-3-butene-2-hydrin to the reaction kettle for feeding, obtaining the crude product of the 3-methyl-2-butenol through discharging from a lateral line of the rectification tower, and continuously replenishing new 2-methyl-3-butene-2-hydrin into the reaction kettle. The method has the advantages of no use of solvent, mild reaction condition and easy control, and realizes the continuous production based on the continuous feeding and discharging.
Owner:ZHEJIANG NHU CO LTD +1
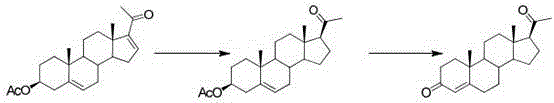
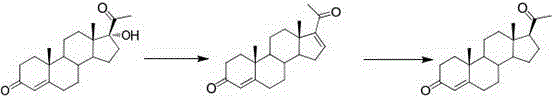
Method for preparing progesterone and derivatives thereof
InactiveCN106589037AReduce manufacturing costEasy to operateSteroidsProgesteronesRearrangement reaction
The invention discloses a method for preparing progesterone and derivatives thereof. A compound 1 serves as a starting material, and through an oxidation reaction and a rearrangement reaction, the progesterone and derivatives thereof, namely, compounds 3 are obtained, wherein the reaction formula is defined in the description. By means of the method, the finished product progesterone and derivatives thereof are obtained with the total yield being 75wt% or above; the method is low in cost, environmentally friendly and suitable for industrial production.
Owner:ZHEJIANG XIANJU PHARMA
Synthesis method of mesotrione
InactiveCN105254543AImprove conversion rateHigh yieldOrganic chemistryOrganic compound preparationSynthesis methodsEsterification reaction
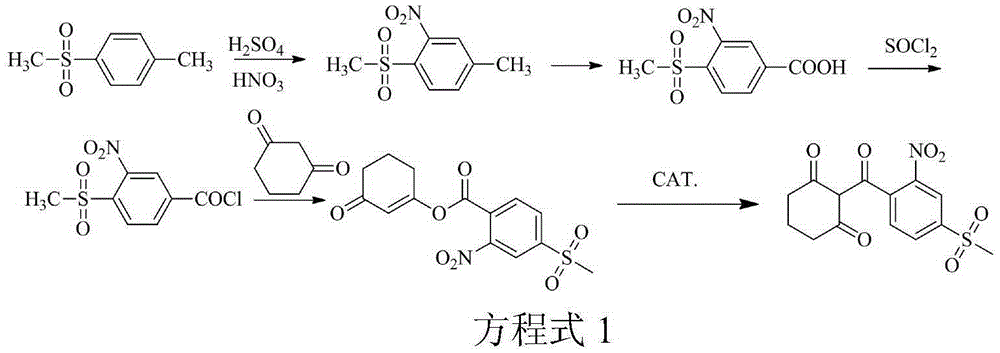
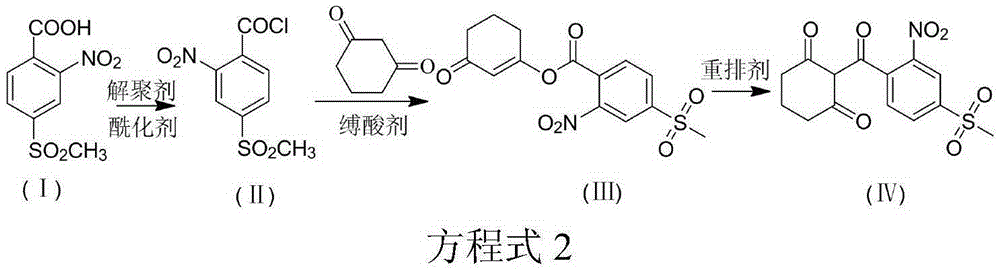
The invention discloses a synthesis method of mesotrione. The method includes the following steps that firstly, acylation is performed, wherein 2-nitro-4-methylsulfonylbenzoic acid and acylating agents are subjected to an acylation reaction under catalysis of depolymerizing agents, acylation reaction liquid is obtained, and the temperature of the acylation reaction is 10-70 DEG C; secondly, esterification is performed, wherein acid binding agents and 1,3-cyclohexanedione are added in esterification reaction liquid, an esterification reaction is performed, the esterification reaction liquid is obtained, the temperature of the esterification reaction is 30-65 DEG C, and the acid binding agents are inorganic acid; thirdly, rearrangement is performed, wherein rearrangement agents are added in the esterification reaction liquid so that a rearrangement reaction can be performed, and coarse product reaction liquid is obtained; fourthly, refining is performed, wherein the coarse product reaction liquid is subjected to alkali liquor reverse extraction, acidification and recrystallization, and mesotrione is obtained. The method is easy to operate, the raw materials are easy to obtain, the reactant conversion rate is high, reaction is thorough, the purity of the product is high, and the synthesis method is applicable to industrial production.
Owner:SHANGYU NUTRICHEM +2
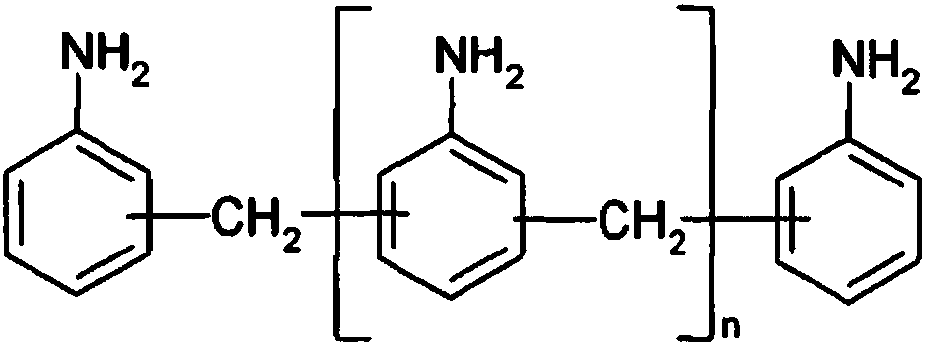
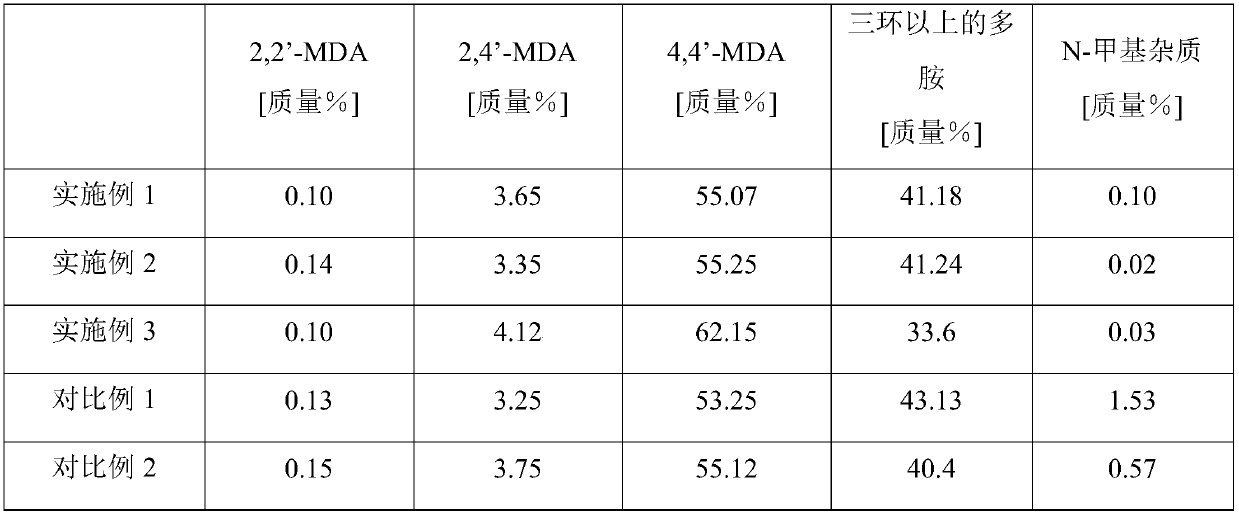
Method and device for preparing diphenylmethane series diamine and polyamine with low N-methyl impurity content and catalyst
ActiveCN107827756AReduce contentHigh selectivityMetal/metal-oxides/metal-hydroxide catalystsPreparation by rearrangement reactionsDiphenylmethaneFixed bed
The invention relates to a method and a device for preparing diphenylmethane series diamine and polyamine with low N-methyl impurity content and a catalyst. The method comprises the steps as follows:a) phenylamine and formaldehyde are subjected to a condensation reaction in the presence of an acid catalyst, and a reaction mixture containing polyamino benzylaniline salt is obtained; b) the reaction mixture from a) enters a fixed bed reactor loaded with the catalyst for a transposition rearrangement reaction, a mixture containing diphenylmethane series diamine salt and polyamine salt is obtained after the reaction, and active components of the transposition rearrangement reaction catalyst comprise one or more of vanadium phosphate oxide, a Nb2O5-La2O3 solid solution and a Pr2O3-Ce2O3 solidsolution. The reaction selectivity at the transposition rearrangement stage can be improved, so that the content of N-methyl MDA impurities in the product can be decreased, the product quality can besubstantially improved, and the N-methyl MDA content can be decreased to 0.01% or below.
Owner:WANHUA CHEM GRP CO LTD +1
Method for continuously preparing hexanolactam by using cyclohexanone-oxime Beckmann rearrangement reaction
InactiveCN101851203AHigh yieldImprove qualityLactams preparationBeckmann rearrangementReaction temperature
The invention discloses a method for continuously preparing hexanolactam by using a cyclohexanone-oxime Beckmann rearrangement reaction. A circular reaction system consists of a recycle pump, a cooler, a mixing reactor and a curing reactor, and the circular reaction system is characterized in that heat instantly released from the rearrangement reaction is dissipated by combining heat storage of materials with heat absorption in solvent vaporization. The combination of the heat storage of the materials with the heat absorption in the thermal inertia solvent vaporization can control a micro-reaction temperature, and meanwhile, the heat absorption in the thermal inertia solvent vaporization can prevent over-high temperature of the partial reaction, so a reaction product with high yield and high quality can be obtained, the use amount of fuming sulphuric acid is small, the energy consumption is low and the operation is stable.
Owner:XIANGTAN UNIV
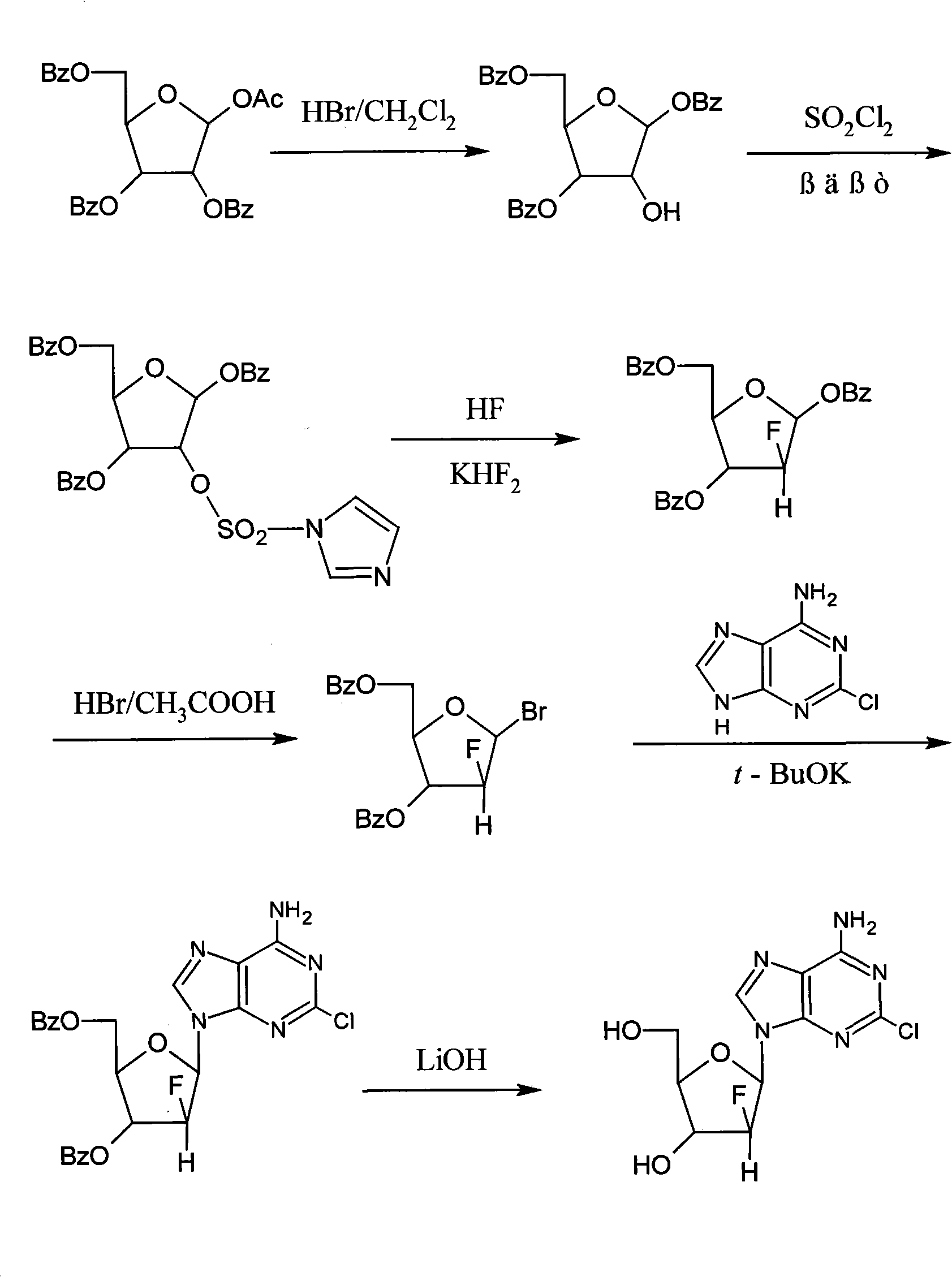
Method for synthesizing clofarabine
InactiveCN101265284AHigh selectivityHigh yieldSugar derivativesAntineoplastic agentsHydrobromidePhenacyl
The invention provides a preparation method of clofarabine, which includes allowing 1-acetyl-2,3,5-tri-o-benzoyl-Beta-D-ribofuranose as the initial raw material and dichloromethane solution of hydrobromide to perform the rearrangement reaction, reacting with sulfuryl chloride and imidazole, performing the fluoridation reaction in the presence of hydrogen fluoride aqueous solution and potassium hydrogen fluoride, and performing bromination reaction in acetic acid solution of hydrogen bromide, condensing with 2-chloro adenosine in alkaline condition, and removing benzoyl in the presence of lithium hydroxide to obtain clofarabine. Compared with prior art, the method has the advantages of high yield of each step, higher total yield, and easily realized industrialized production.
Owner:深圳万乐药业有限公司
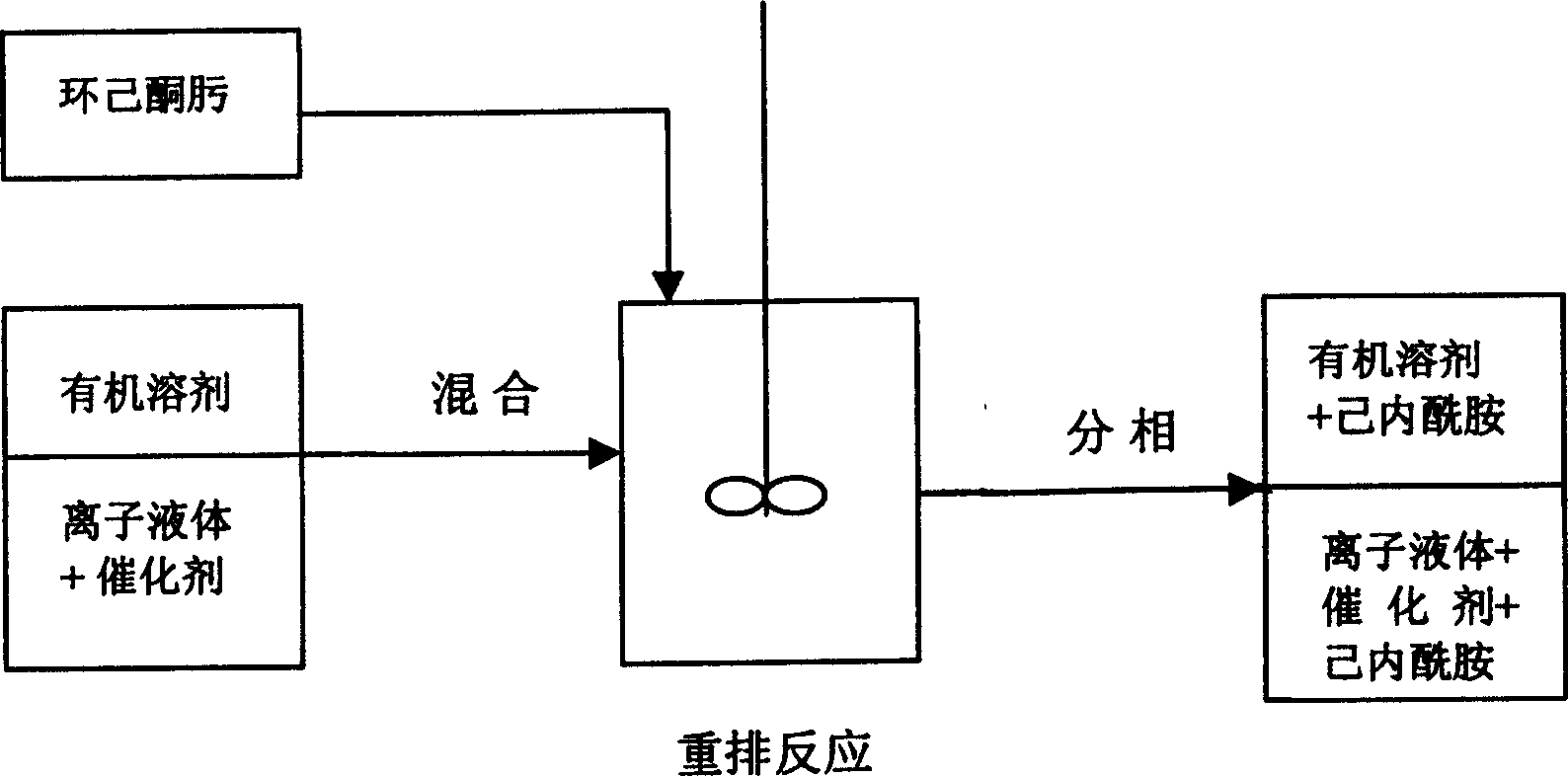
Method for separating Beckmann rearrangement reaction products from ion liquid
ActiveCN1670017ASolve the separation problemEfficient separationOrganic chemistryBeckmann rearrangementOrganic solvent
The invention relates to a method for separating Beckmann rearrangement reaction production from ion liquid, which comprises: selecting an organic dissolvant with the ion liquid used in cyclohexanone oxime Beckmann rearrangement reaction immiscible in the temperature of 10-60 Deg.C and miscible in the temperature of 50-150 Deg.C, mixing the organic dissolvant with the ion liquid with cyclohexanone oxime Beckmann rearrangement reaction production with the proportion in volume of 50 degree 1-1 degree 10 to form a immiscible two-phase system, stirring and lifting the temperature to 50-150 Deg.C to get the miscible mixture liquid of both organic dissolvents and ion liquid, holding 1-120 min in the temperature, then cooling the mixture liquid to 10-60 Deg.C to separate phase inactively.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for preparation of caprolactam
The invention discloses a process for preparation of caprolactam from cyclic ketones through liquid phase rearrangement reaction in ionic liquid and at the presence of phosphorus-containing compounds as the catalyst, wherein the ionic liquid, organic solvent non-dissolving to the ionic liquids and acidic phosphorus-containing compound catalyst are mixed under stirring condition, dissolving the catalyst into the ionic liquid phase, heating the obtained two phase catalytic system to a rearrangement reaction temperature, charging the cyclic ketones or solution into the catalytic system for reaction, then subjecting to stewing and phase-splitting.
Owner:CHINA PETROLEUM & CHEM CORP +1
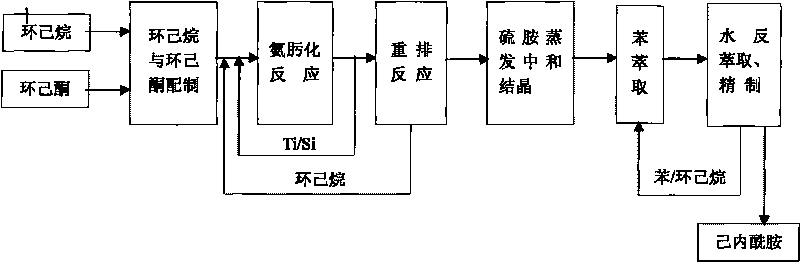
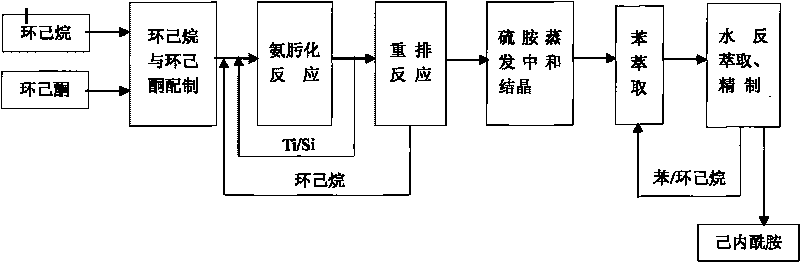
Process for preparing caprolactam
The invention discloses a process for preparing caprolactam, which comprises the following steps: after mixing cyclohexanone and cyclohexane evenly, carrying out an oxamidine reaction to obtain a cyclohexane solution of cyclohexanone oxime; enabling the cyclohexane solution of the cyclohexanone oxime and oleum to react to generate a caprolactam sulfate solution, curing the caprolactam sulfate solution, then carrying out a neutralization reaction on the cured caprolactam sulfate solution and ammonia, afterwards separating the two materials to obtain a crude product of caprolactam and then carrying out extraction and water back extraction respectively to prepare a finished product. Because of the existence of a cyclohexane inert solvent, rectification and separation do not need to be carried out after the oxamidine reaction, and only a rearrangement reaction needs to be carried out after the water phase of a catalyst is separated, thereby saving a rectification device and the steam consumption; as a result, a caprolactam enterprise with an annual yield of a hundred thousand tons can save energy worth approximately 60000000 yuan every year and can also save the one-off equipment investment totaling 60000000 yuan, the technological process of caprolactam is simplified, the procedures of water washing, extraction, distillation and the like are reduced, the production period is shortened, and the annual yield is enhanced.
Owner:河北美邦工程科技股份有限公司
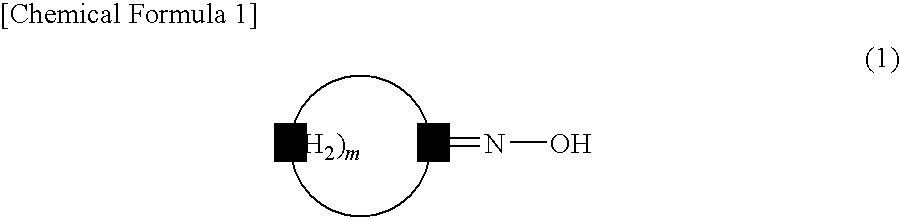
Method for producing lactam compound
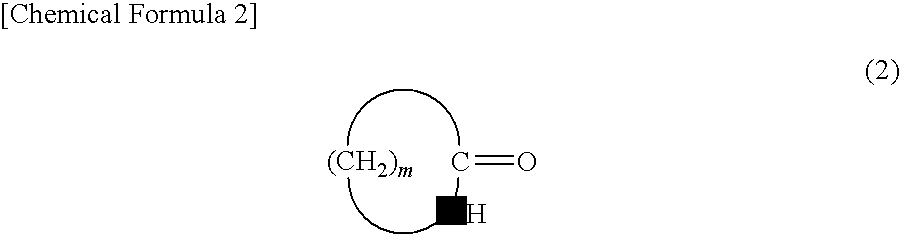
Disclosed is a method for industrially efficiently producing a lactam compound having 8 to 15 carbon atoms at low cost by allowing a rearrangement reaction of a cyclic oxime compound to proceed without causing large amounts of by-products such as ammonium sulfate.[Solving Means] Disclosed is a method for producing a lactam compound, which includes the step of rearranging a cyclic oxime compound in a nonpolar solvent B in the presence of an aromatic compound A to give the lactam compound, in which the aromatic compound A has a leaving group bonded to a carbon atom constituting its aromatic ring and contains, as an atom constituting the aromatic ring, a heteroatom, or a carbon atom bonded with an electron-withdrawing group, the cyclic oxime compound is represented by following Formula (1):wherein “m” denotes an integer of 7 to 14,and the lactam compound is represented by following Formula (2):wherein “m” is as defined above.
Owner:SHIBAMOTO AKIHIRO +2
Catalytic rearrangement preparation method of polycarbosilane
ActiveCN105273199AHigh synthetic yieldStrong Lewis acidityFibre chemical featuresSilanesReaction temperature
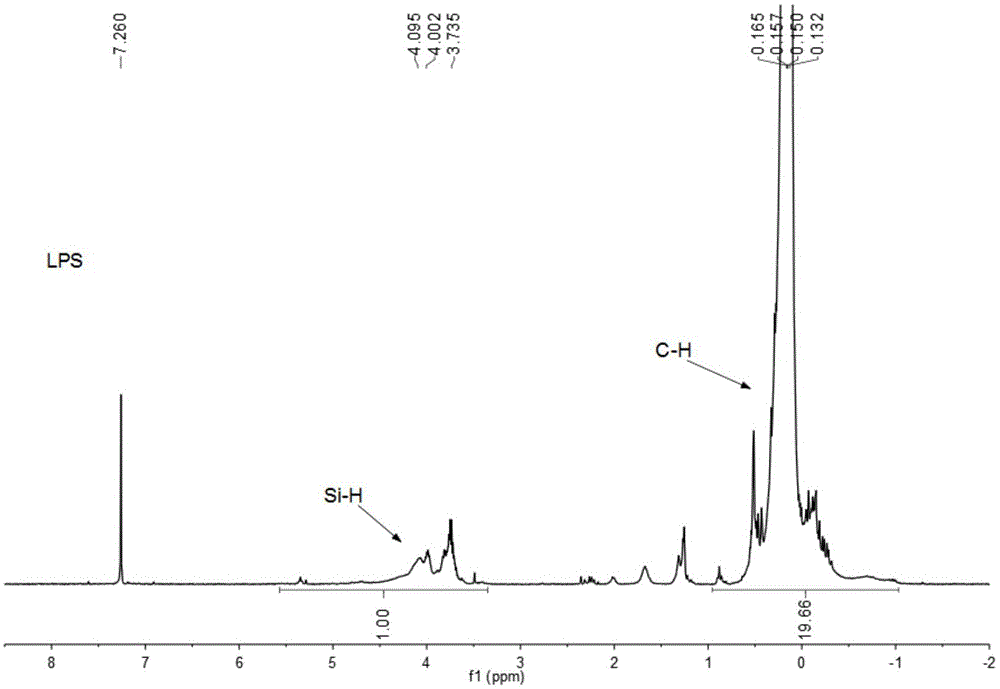
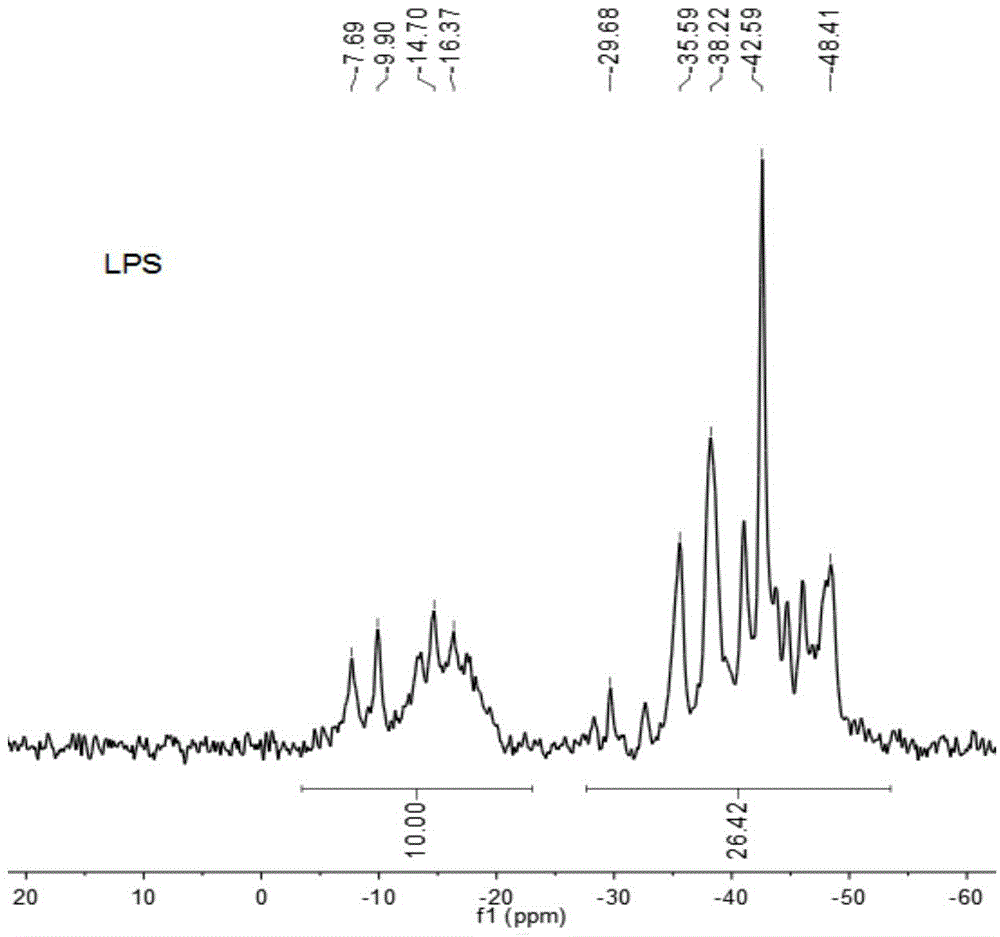
The invention relates to a method for preparing polycarbosilane through cracking rearrangement of a cyclic silane compound or chain polysilane under the catalytic effect of trace (lower than 1wt%) boron-containing catalyst. According to the invention, polydimethylsilane (PDMS) or a pyrolysis product thereof which is a liquid-state silane-carbosilane compound (LPS) is adopted as a raw material; lower than 1wt% (relative to the amount of the raw material) of the boron-containing catalyst is added; the temperature is gradually increased to a reaction temperature under a normal pressure or a high pressure, such that a pyrolysis / rearrangement reaction is carried out, and solid-state polycarbosilane (PCS) with relatively high ceramic yield is obtained. The method provided by the invention has the advantages of short reaction time, high synthesis yield, good product quality, simple equipment and safe operation. The prepared polycarbosilane is a SiC precursor polymer, and can be used in the preparations of SiC fiber and SiC-based composite materials.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
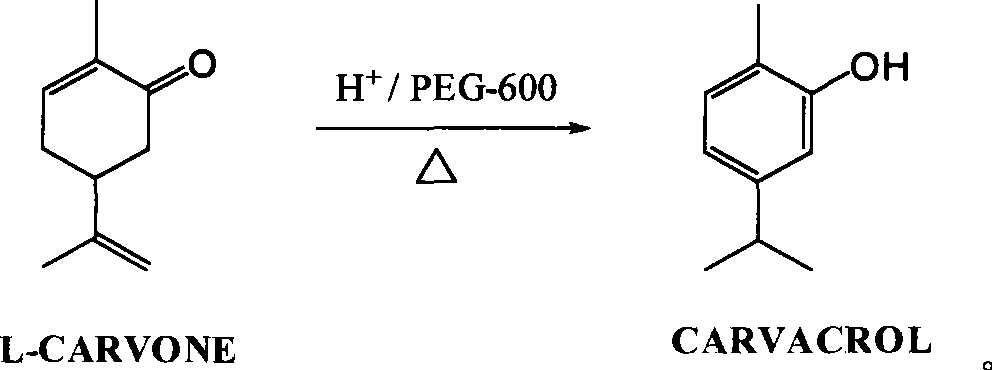
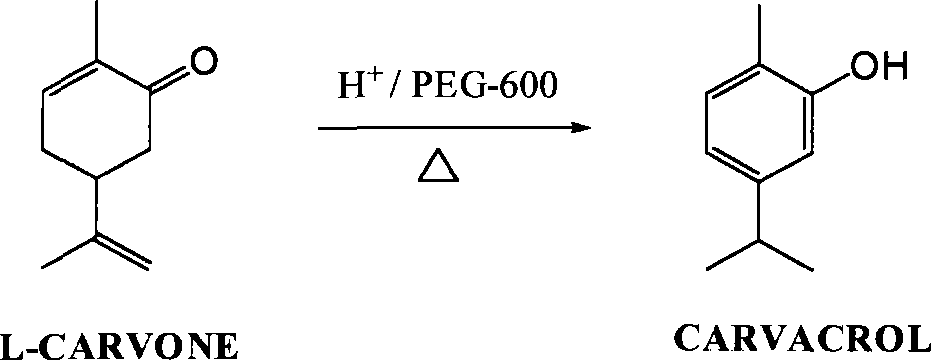


![Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline](https://images-eureka.patsnap.com/patent_img/37680de7-1f05-4d21-961b-fff7e39228d0/a20081012224200041.PNG)
![Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline](https://images-eureka.patsnap.com/patent_img/37680de7-1f05-4d21-961b-fff7e39228d0/a20081012224200042.PNG)
![Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline](https://images-eureka.patsnap.com/patent_img/37680de7-1f05-4d21-961b-fff7e39228d0/a20081012224200051.PNG)