Method for preparing 6-aminocapronitrile from cyclohexanone-oxime
A technology of aminocapronitrile and cyclohexanone oxime, which is applied to the preparation of lactam, chemical instruments and methods, and dehydration of carboxylic acid amide, can solve the problems of high energy consumption, many wastes, and large investment, and achieve steam saving consumption, cyclohexanone oxime consumption reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
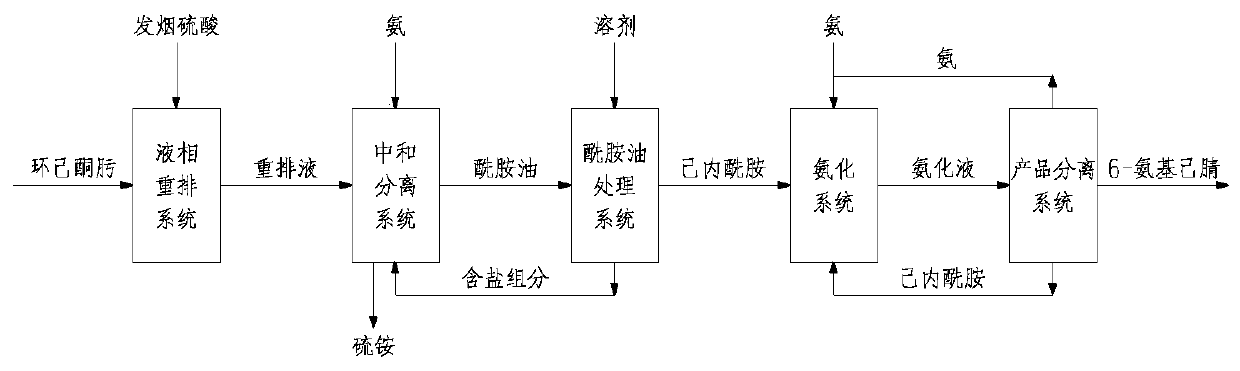
[0039] Preparation of 6-Aminocapronitrile by Liquid Phase Rearrangement of Cyclohexanone Oxime
[0040]70-80% of the total flow rate of cyclohexanone oxime is initially reacted with oleum at 85-105°C under normal pressure to obtain a rearrangement mixture containing ε-caprolactam. The reaction conditions are oleum:cyclohexane Ketoxime ratio is 1.5-1.8:1, SO in the rearrangement mixture after reaction 3 The content is 4%-5% (wt); continue to react 20-30% flow of cyclohexanone oxime with oleum at 85-105°C under normal pressure to obtain a rearrangement mixture containing ε-caprolactam.
[0041] The obtained rearrangement mixture containing ε-caprolactam is neutralized and crystallized with ammonia in a neutralization crystallization reactor to obtain amide oil containing 70% ε-caprolactam, and ammonium sulfate is produced as a by-product.
[0042] Extract ε-caprolactam from amide oil with benzene to obtain 15-25% ε-caprolactam benzene solution.
[0043] The extracted ε-caprola...
Embodiment 2
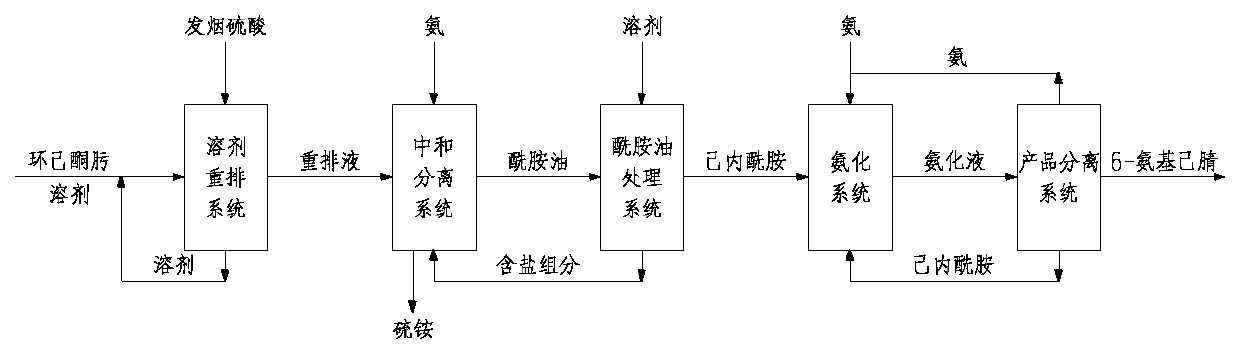
[0048] Others are the same as in Example 1, except that before the ε-caprolactam in the amide oil is extracted with benzene, the amide oil is washed and desalted with 4% NaOH solution, and the desalted amide oil is extracted with benzene.
[0049] Due to the use of alkali to elute the salt, the operating cycle of the caprolactam evaporator can be extended, and the selectivity of 6-aminocapronitrile is 97.2%. Save steam consumption by 2.0-3.0t / t 6-aminocapronitrile, and reduce cyclohexanone oxime consumption by 20-50kg / t 6-aminocapronitrile.
Embodiment 3
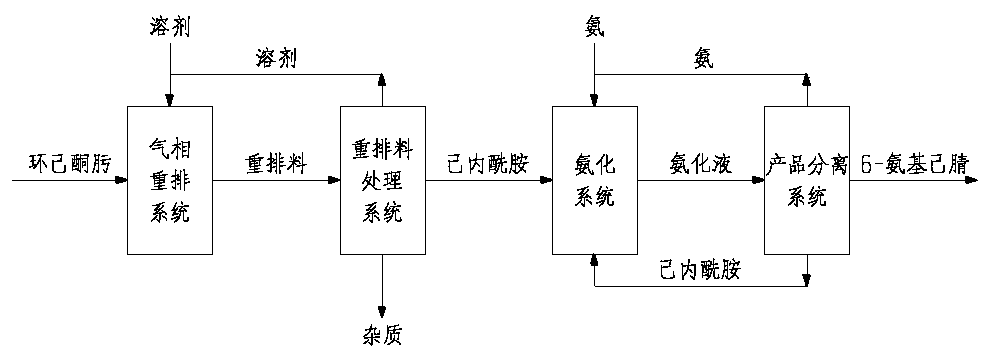
[0051] Preparation of 6-Aminocapronitrile by Vapor Phase Rearrangement of Cyclohexanone Oxime
[0052] In the presence of a molecular sieve catalyst with an MFI structure, cyclohexanone oxime in a gas phase is reacted in the presence of a carrier gas and a solvent. The conditions of the gas-phase Beckmann rearrangement reaction may include: the temperature is 320-450°C, preferably 370-400°C; the pressure is 0.05-0.5MPa, preferably 0.1-0.3MPa; the mass space velocity of cyclohexanone oxime is 0.1-5h -1 . The solvent can be low-carbon alcohol; the carrier gas is nitrogen and inert gas.
[0053] The reaction gas is cooled, and high-boiling components as impurities are separated from the cooled reaction liquid to obtain a mixed gas containing ε-caprolactam, lower alcohols and inert gases, and the crude ε-caprolactam mixture containing ε-caprolactam is subjected to Distillation separates light and heavy components.
[0054] The vacuum-state caprolactam vapor separated from the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com