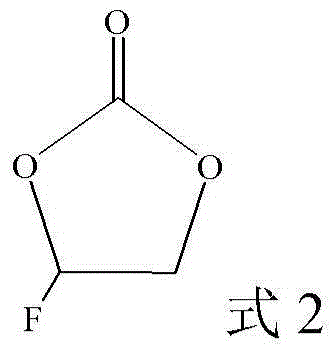
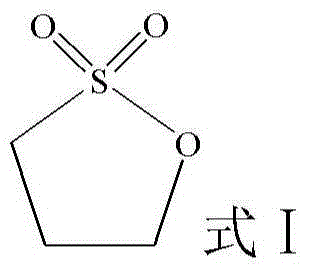
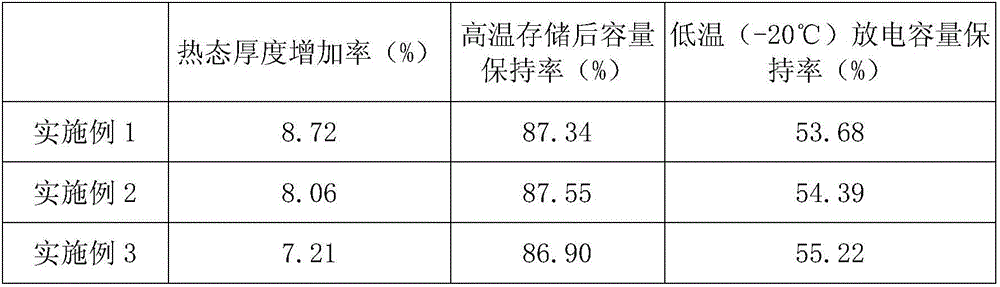
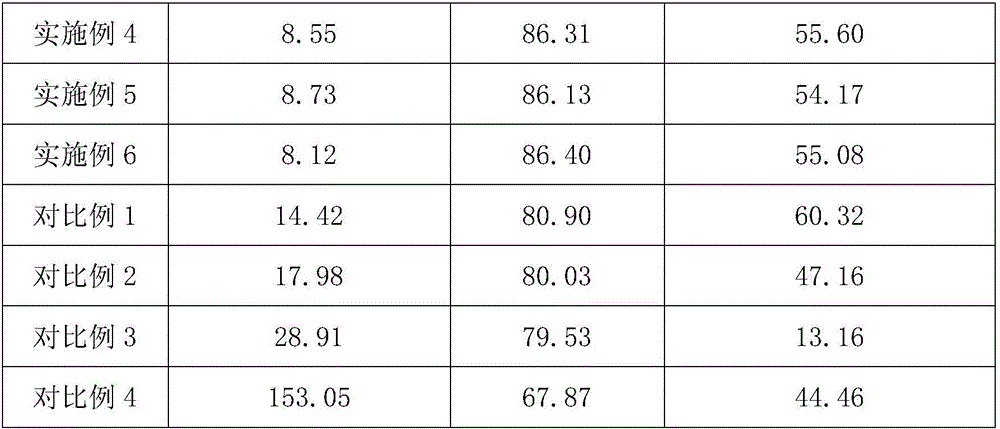
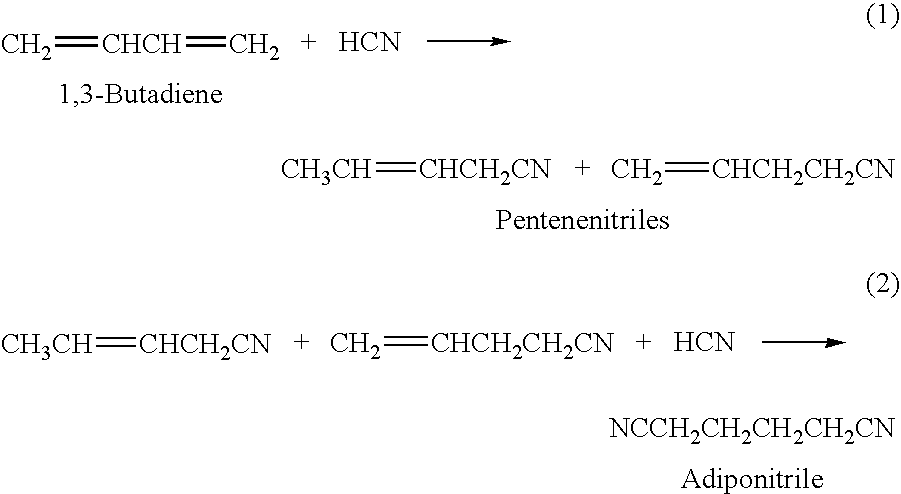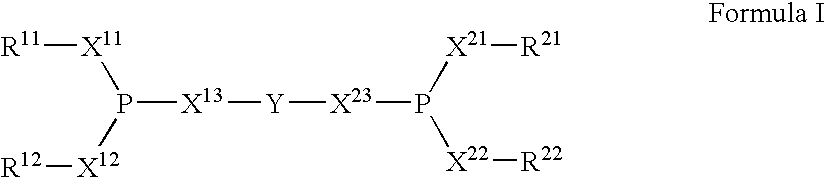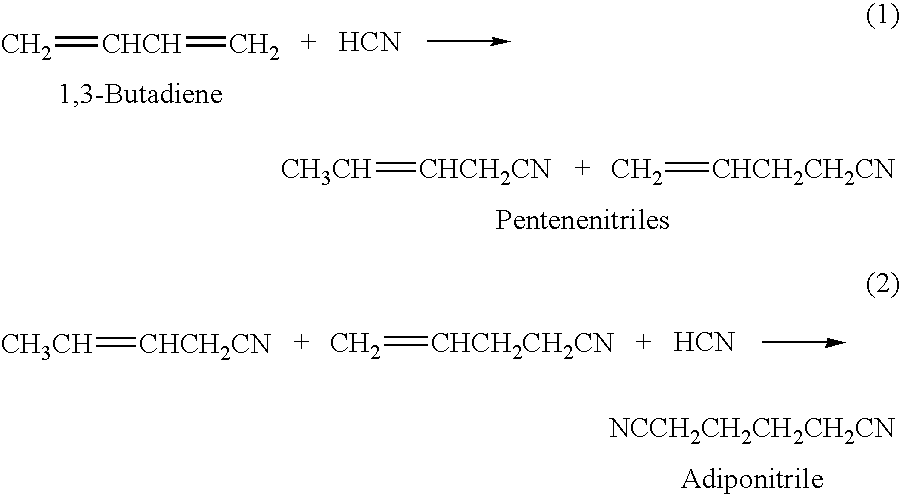Patents
Literature
172 results about "Adiponitrile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
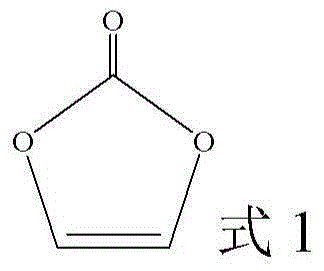
Adiponitrile is the organic compound with the formula (CH₂)₄(CN)₂. This dinitrile, a viscous, colourless liquid, is an important precursor to the polymer nylon-6,6. In 2005, about one billion kilograms were produced.
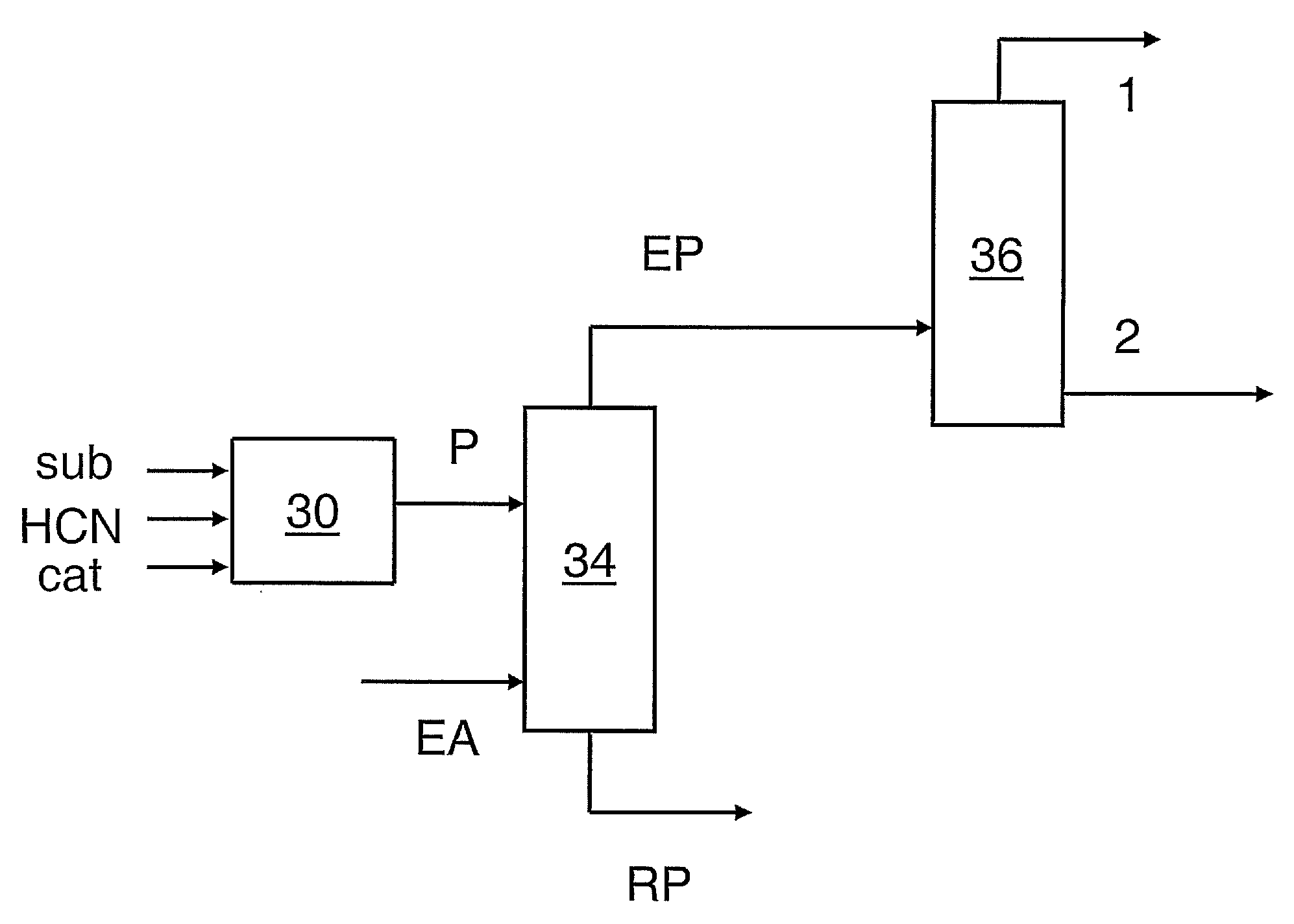
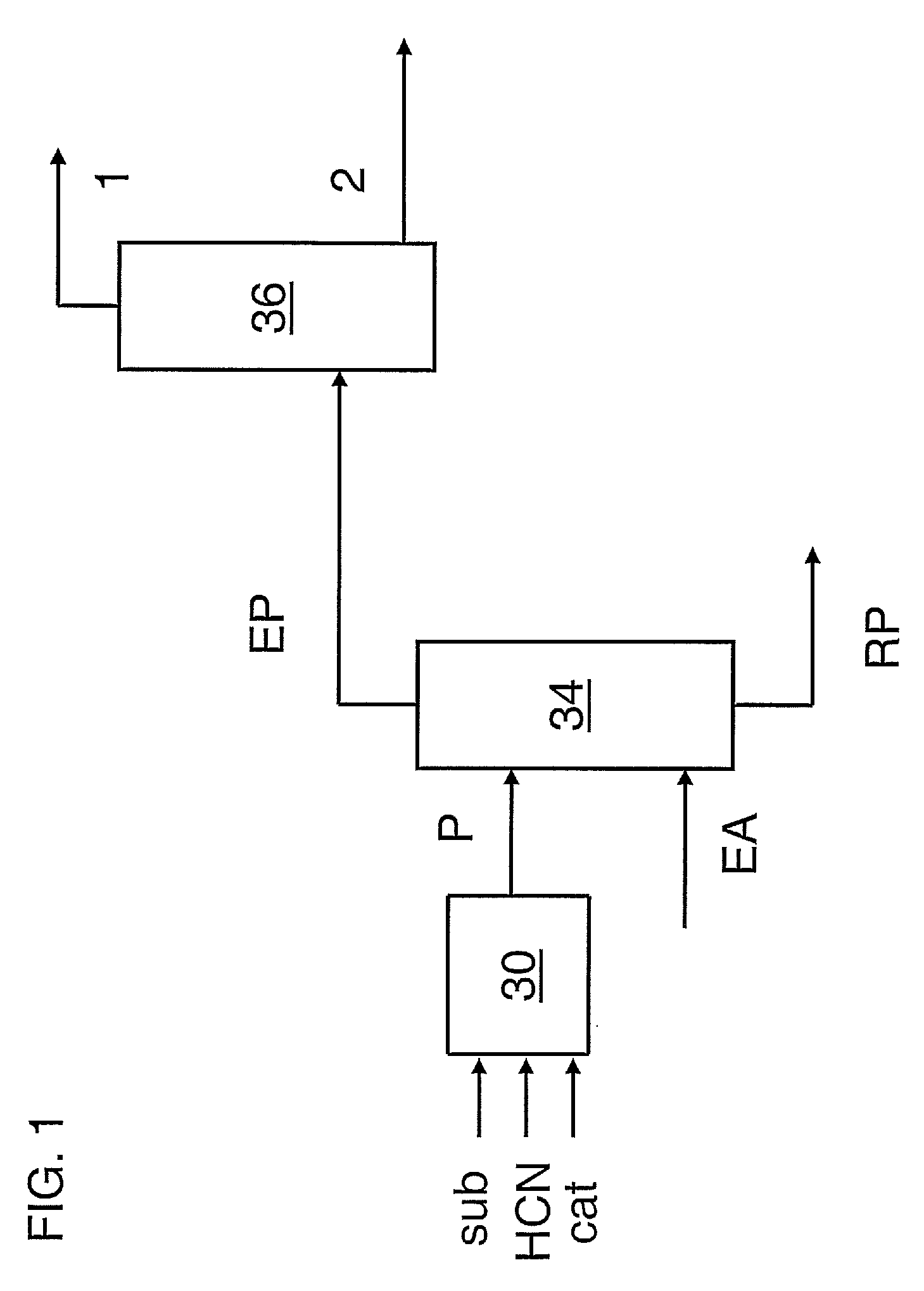
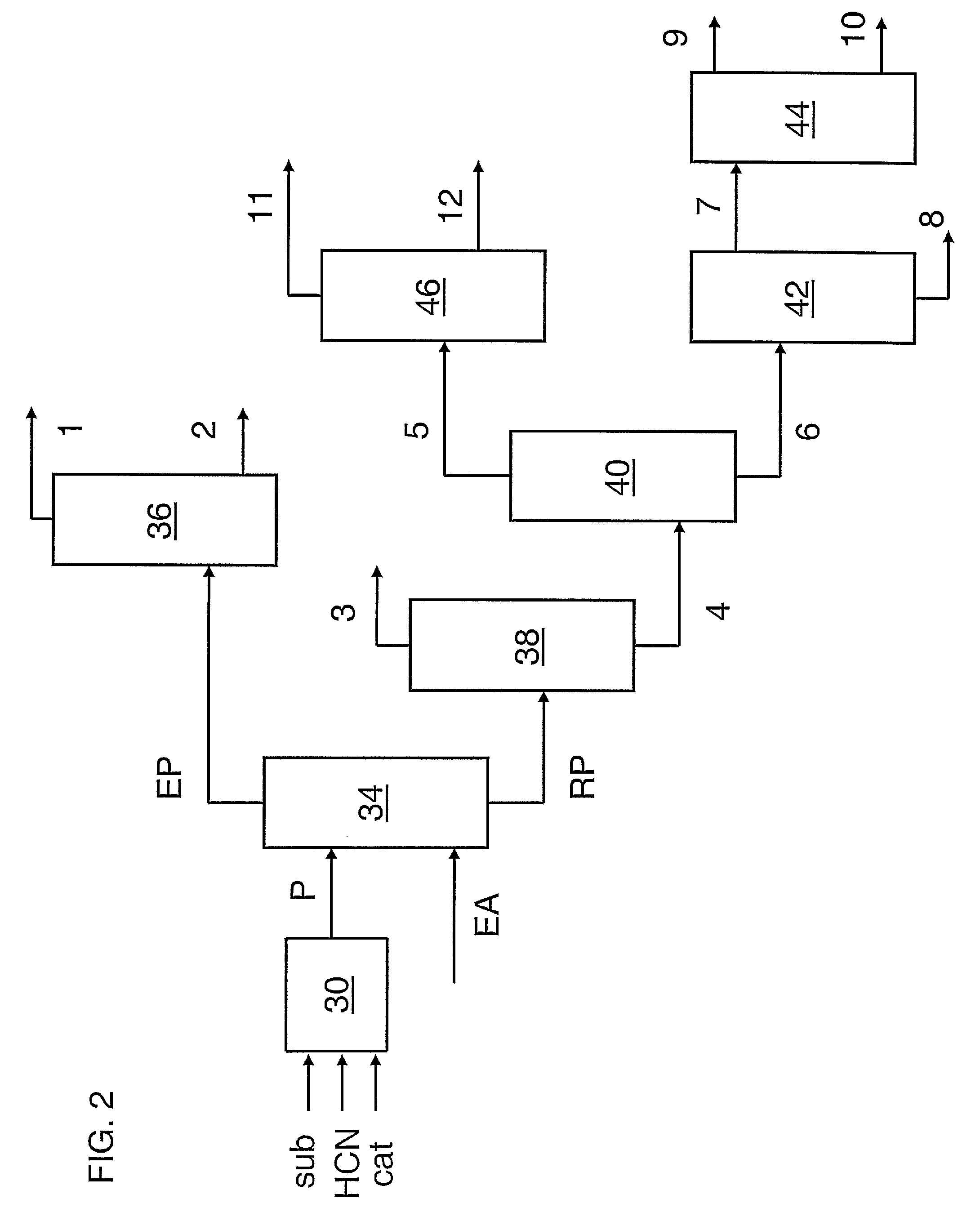
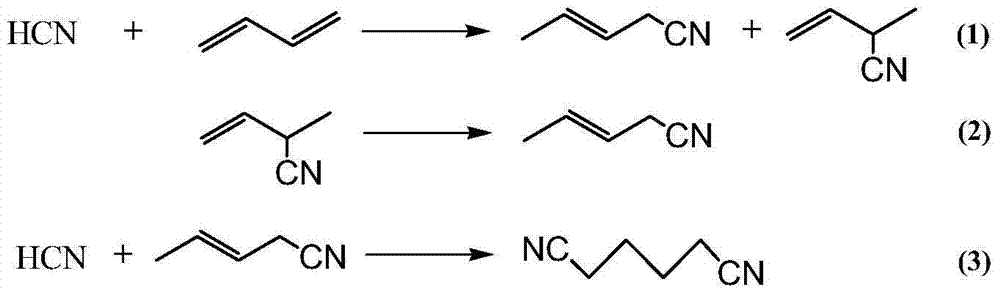
Hydrocyanation of pentenenitriles
ActiveUS20090182164A1Organic compound preparationPreparation by hydrogen cyanide additionHydrogenHydrocyanation
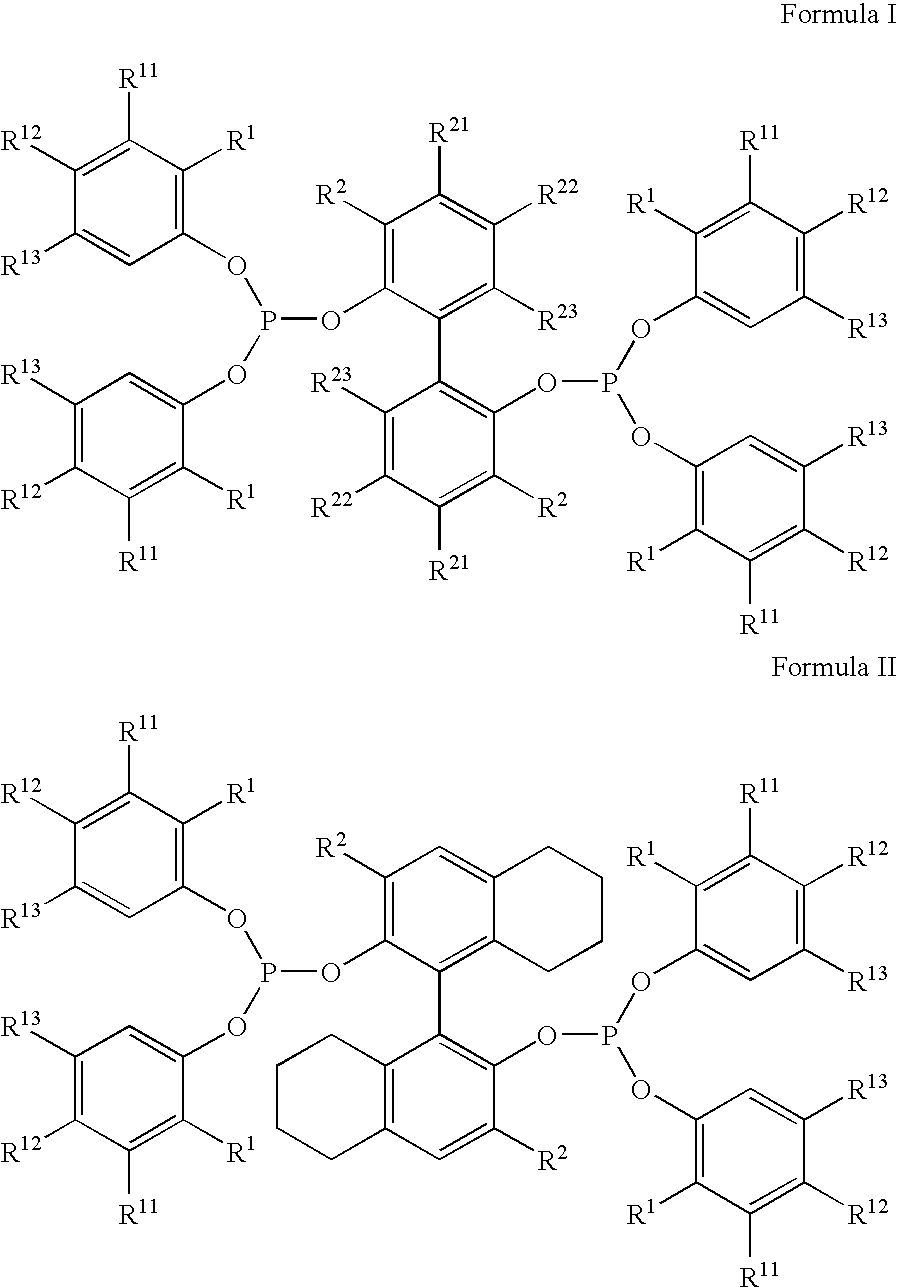
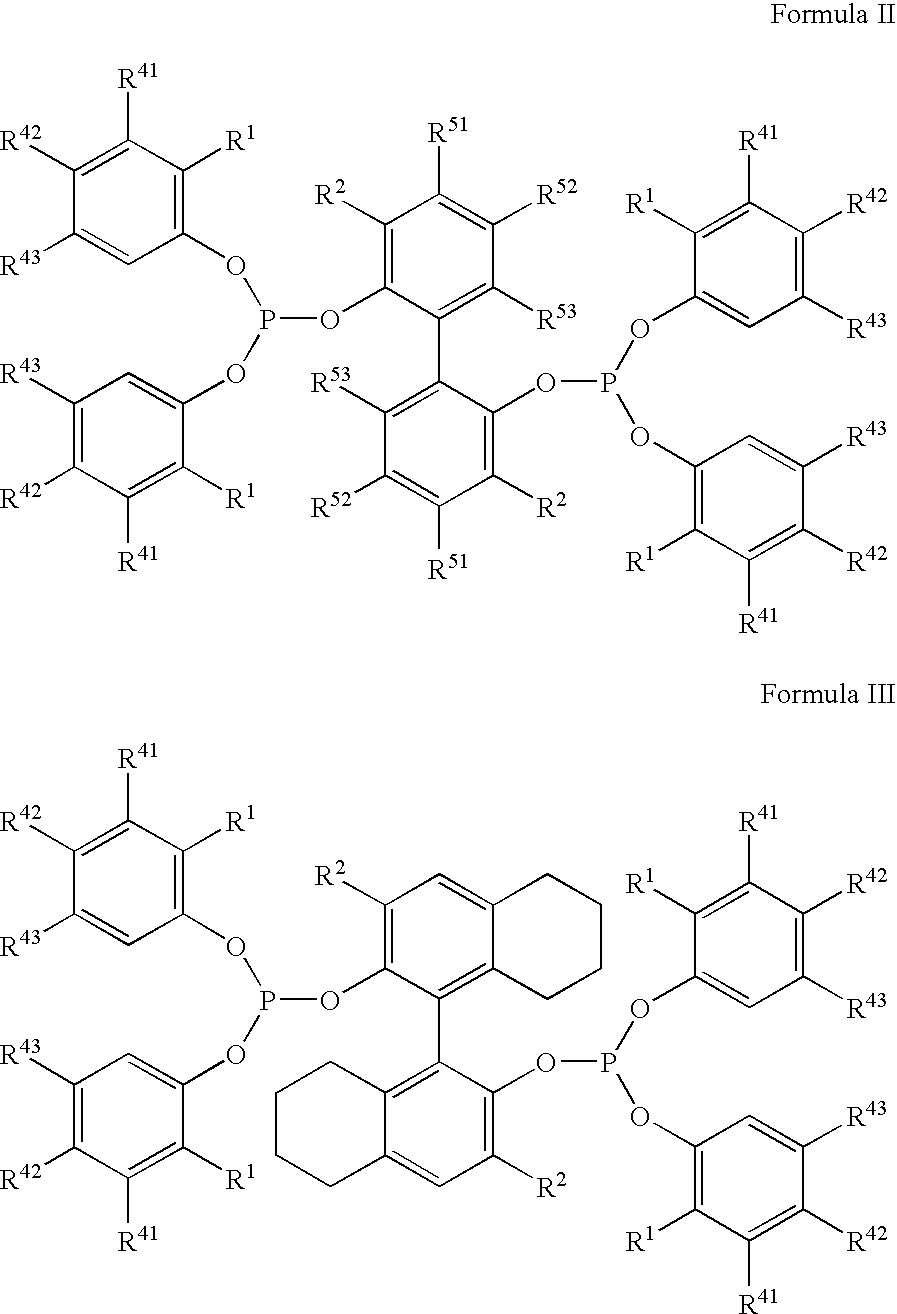
The invention provides a hydrocyanation process to produce adiponitrile and other dinitriles having six carbon atoms, in the presence of catalyst composition comprising a zero-valent nickel and at least one bidentate phosphorus-containing ligand wherein the bidentate phosphorus-containing ligand gives acceptable results according to at least one protocol of the 2-Pentenenitrile Hydrocyanation Test Method.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation process with reduced yield losses
ActiveUS20080015381A1Investment exemptionSelective and efficient and stableOrganic compound preparationPreparation by hydrogen cyanide additionNitriteHydrogen
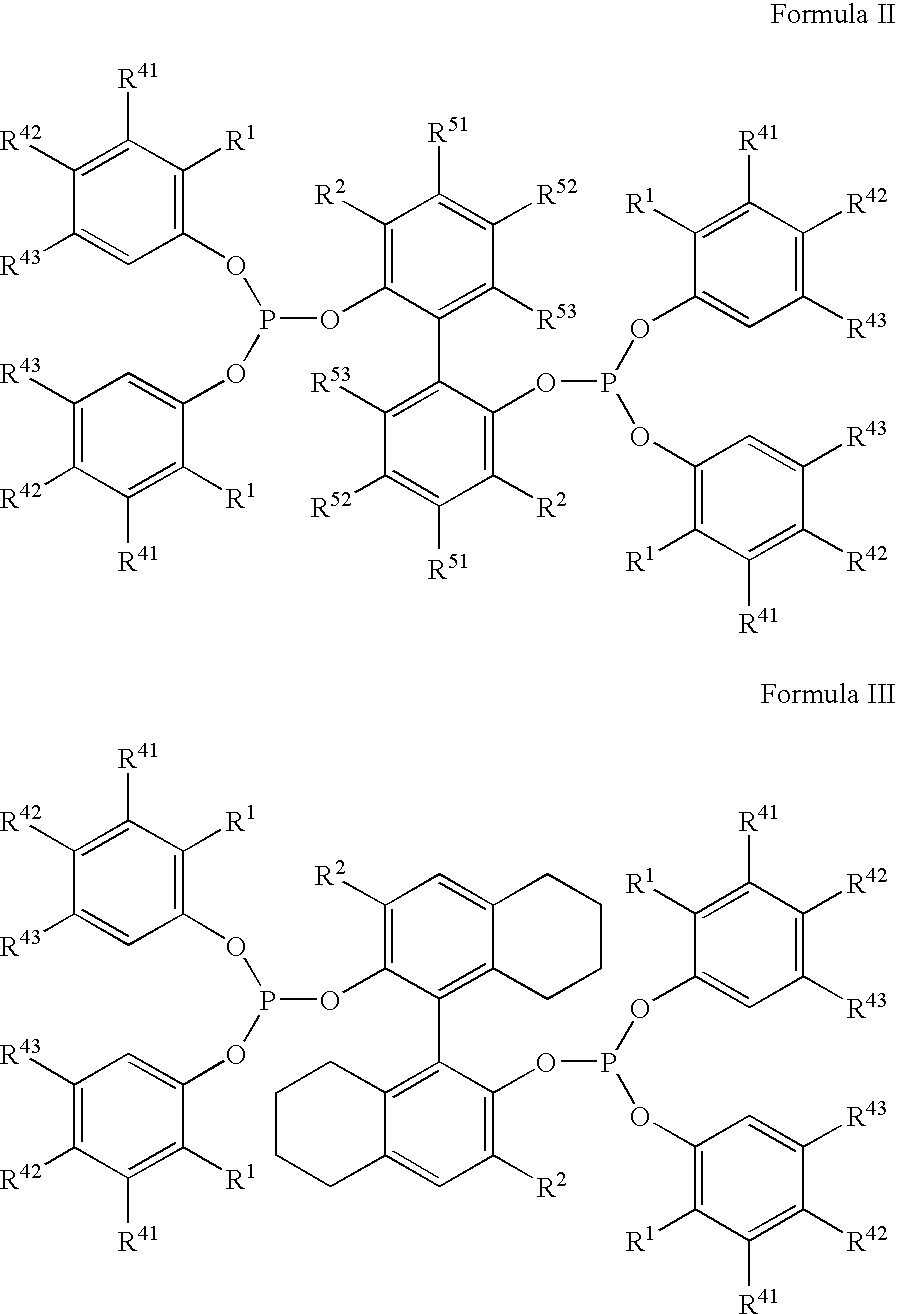
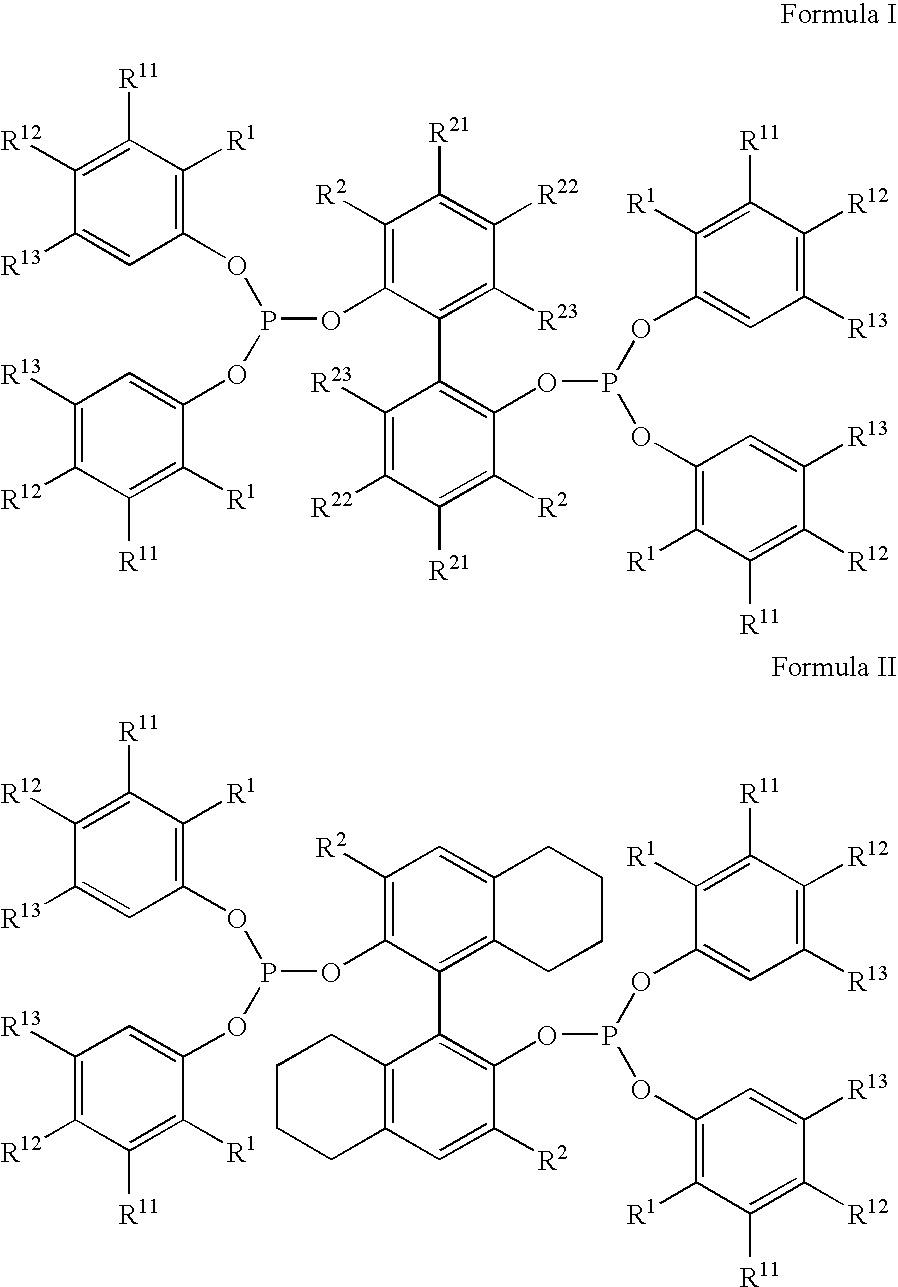
The invention provides a hydrocyanation process for the production of adiponitrile and other dinitriles having six carbon atoms, the process comprising:a) forming a reaction mixture in the presence of at least one Lewis acid, said reaction mixture comprising ethylenically unsaturated nitrites having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition, by continuously feeding ethylenically unsaturated nitrites, hydrogen cyanide, and a catalyst precursor composition;b) controlling X and Z, wherein X is the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and Z is the overall feed molar ratio of hydrogen cyanide to all unsaturated nitrites, by selecting a value for X in the range from about 0.001 to about 0.5, and a value for Z in the range from about 0.5 / 1 to about 0.99 / 1, such that the value of quotient Q, whereinQ=X[(moles3PN+4PNinthefeed)(molesallunsaturatednitrilesinthefeed)]-Zis in the range from about 0.2 to about 10, wherein 3PN is 3-pentenenitriles and 4PN is 4-pentenenitrile; andc) withdrawing a reaction product mixture comprising adiponitrile;wherein the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles in the reaction mixture is from about 0.2 / 1 to about 10 / 1;wherein the catalyst precursor composition comprises a zero-valent nickel and at least one bidentate phosphite ligand; andwherein the bidentate phosphite ligand is selected from a member of the group represented by Formulas I and 11 as described herein.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation process with reduced yield losses
ActiveUS20080015382A1Investment exemptionSelective and efficient and stableOrganic compound preparationPreparation by hydrogen cyanide additionHydrogenPhosphite ester
The invention provides a hydrocyanation process for the production of adiponitrile and other dinitriles having six carbon atoms, the process comprising:a) forming a reaction mixture in the presence of at least one Lewis acid, said reaction mixture comprising ethylenically unsaturated nitriles having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition, by continuously feeding ethylenically unsaturated nitriles, hydrogen cyanide, and a catalyst precursor composition;b) controlling X and Z, wherein X is the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and Z is the overall feed molar ratio of hydrogen cyanide to all unsaturated nitriles, by selecting a value for X in the range from about 0.001 to about 0.5, and a value for Z in the range from about 0.5 / 1 to about 0.99 / 1, such that the value of quotient Q, whereinQ=X[(moles3PN+4PNinthefeed) / (molesallunsaturatednitrilesinthefeed)]-Zis in the range from about 0.2 to about 10, wherein 3PN is 3-pentenenitriles and 4PN is 4-pentenenitrile; andc) withdrawing a reaction product mixture comprising adiponitrile;wherein the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles in the reaction mixture is from about 0.2 / 1 to about 10 / 1;wherein the catalyst precursor composition comprises a zero-valent nickel and at least one multidentate phosphorus-containing ligand;wherein the multidentate phosphorus-containing ligand is selected from the group consisting of a phosphite, a phosphonite, a phosphinite, a phosphine, and a mixed phosphorus-containing ligand or a combination of such members; andwherein the multidentate phosphorus-containing ligand gives acceptable results according to at least one protocol of the 2-Pentenenitrile Hydrocyanation Test Method.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation process with reduced yield losses
ActiveUS7659422B2Investment exemptionSelective and efficient and stableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenHydrogen cyanide
A hydrocyanation process produces adiponitrile and other dinitriles having six carbon atoms. The process involves forming a reaction mixture in the presence of at least one Lewis acid. The reaction mixture includes ethylenically unsaturated nitriles having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition. The reaction mixture is continuously fed while controlling the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and the overall feed molar ratio of hydrogen cyanide to all unsaturated nitriles. In the reaction product mixture, including adiponitrile, the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles is from about 0.2 / 1 to about 10 / 1. Included in the catalyst precursor composition is a zero-valent nickel and at least one bidentate phosphite ligand.
Owner:INV NYLON CHEM AMERICAS LLC
Rare-earth oxide modified high-selectivity catalyst for adiponitrile hydrogenation and hexylenediamine production, preparation method and application thereof
InactiveCN109647419AHigh mechanical strengthReduce pollutionOrganic compound preparationAmino compound preparationRare earthSolvent
The invention discloses a rare-earth oxide modified high-selectivity catalyst for adiponitrile hydrogenation and hexylenediamine production, a preparation method and application thereof. An alumina-supported nickel-based catalyst is used as an active component, and at 25-90 DEG C and through a parallel flow precipitation process, a low-content rare-earth oxide is used for the modification. A tankreactor is adopted at the low temperature and without an alkaline reagent to carry out a reaction at the pressure of 1-9 MPa for 1-6 h so as to achieve adiponitrile catalytic conversion for high-selectivity preparation of hexylenediamine. No alkaline reagent or NH3 is added during the reaction process to inhibit side reaction of cyclization. The environmental pollution is reduced; and the catalysthas high mechanical strength, the reaction post-treatment is simple and the reaction condition is relatively mild. According to the method and in part of the embodiments, the conversion rate of adiponitrile reaches up to 100% according to set reaction parameters, and selectivity of the product hexylenediamine can reach up to 90%. The invention has good economic benefit and industrial applicationprospects.
Owner:DALIAN UNIV OF TECH
Method for preparing hexamethylene diamine by direct hydrogenation of adiponitrile under alkali-free conditions
InactiveCN108084035AOrganic compound preparationCatalyst activation/preparationAlkali freeHexamethylenediamine
The invention discloses a method for preparing hexamethylene diamine by direct hydrogenation of adiponitrile under alkali-free conditions. The method comprises the steps of using an alkaline earth metal oxide or rare earth metal oxide prepared by a coprecipitation method for modifying an aluminium trioxide-supported metallic nickel catalyst under a condition of no need of adding alkali or ammoniawater, directly hydrogenating an adiponitrile ethanol solution at a temperature of 60 to 200 DEG C, under pressure of 3 to 8 MPa, and obtaining high-qualityhexamethylene diamine through vacuum distillation of a resulting product. The method is suitable for using in batch tank reactors and also in continuous fixed bed reactors.
Owner:DALIAN UNIV OF TECH
Rhodococcus ruber and method for preparing 5-cyanovaleramide by utilizing same
ActiveCN101619299ASimple separation and purification processHigh catalytic efficiencyBacteriaMicroorganism based processesSporeMicroorganism
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Process for making nitriles
ActiveUS20130211126A1Reduce nickel contentOrganic compound preparationChemical recyclingMethyl groupHydrogen cyanide
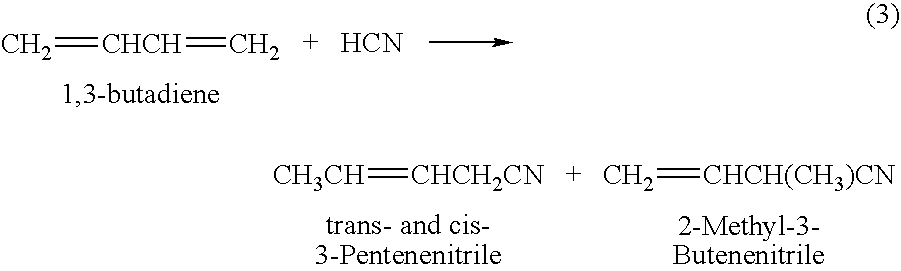
Adiponitrile is made by reacting 3-pentenenitrile with hydrogen cyanide. The 3-pentenenitrile is made by reacting 1,3-butadiene with hydrogen cyanide and by isomerizing 2-methyl-3-butenenitrile. The reaction of 1,3-butadiene with hydrogen cyanide to produce 3-pentenenitrile also produces small amounts of dinitrile compounds, including adiponitrile (ADN) and methylglutaronitrile (MGN). Methylglutaronitrile is removed to provide an adiponitrile-enriched stream, which is used in a catalyst purification step.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation of pentenenitriles
ActiveUS8088943B2Organic compound preparationPreparation by hydrogen cyanide additionHydrogenHydrocyanation
Owner:INV NYLON CHEM AMERICAS LLC
Electrolyte and application thereof
InactiveCN103000945AImprove cycle performanceHigh densitySecondary cellsElectrolytic agentOrganosolv
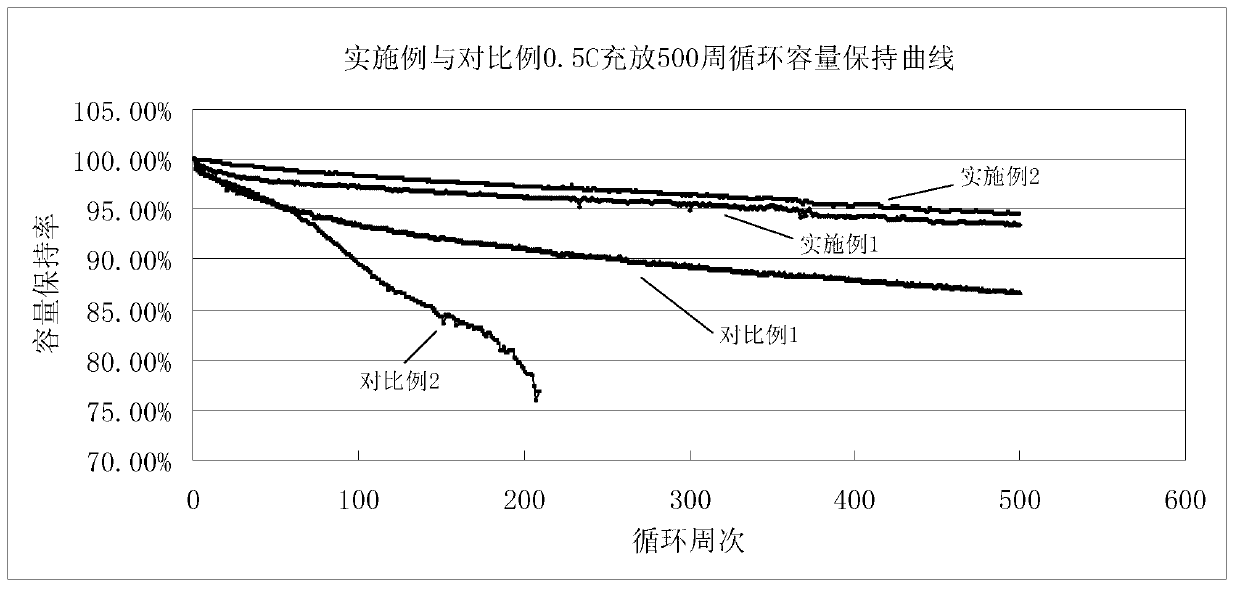
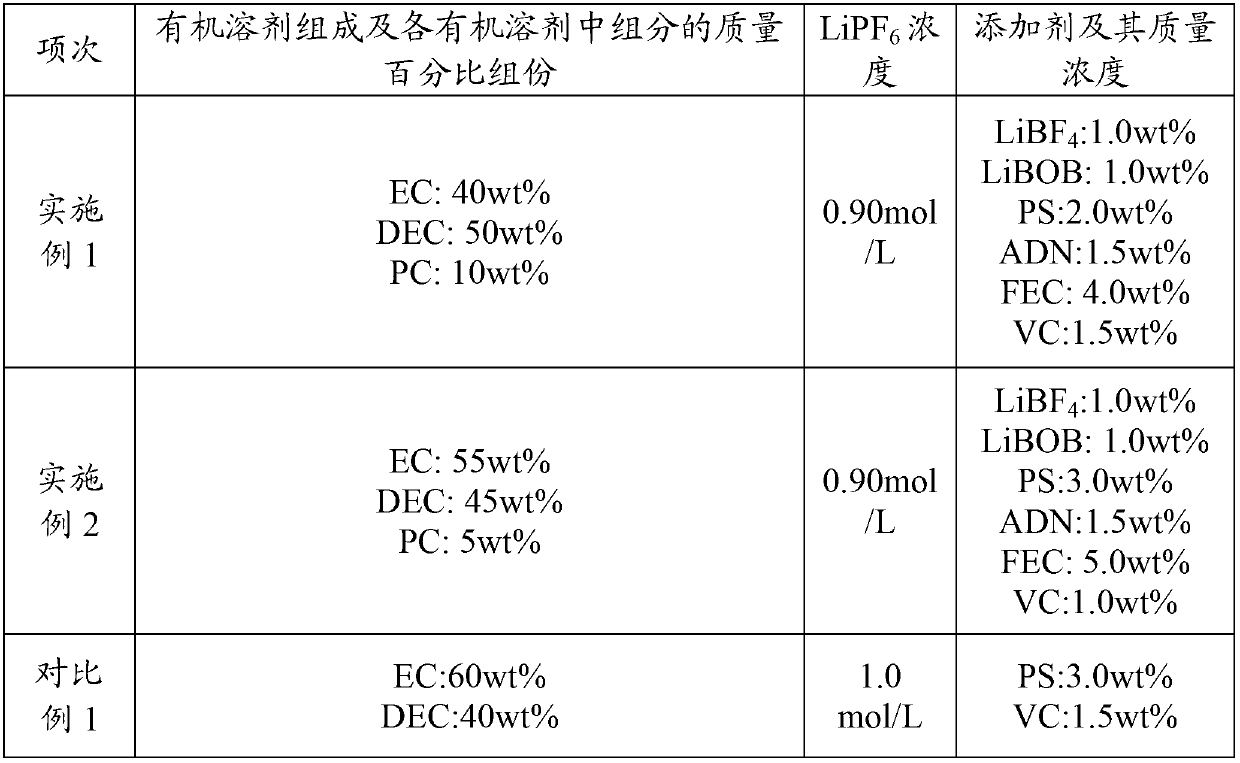
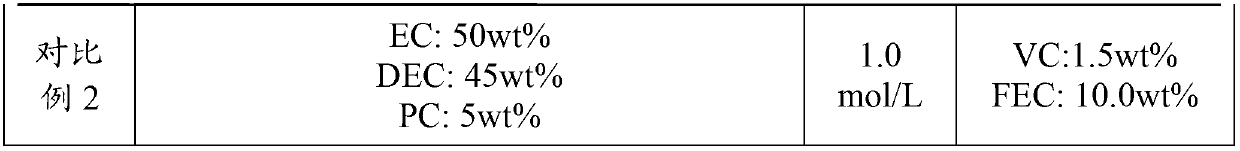
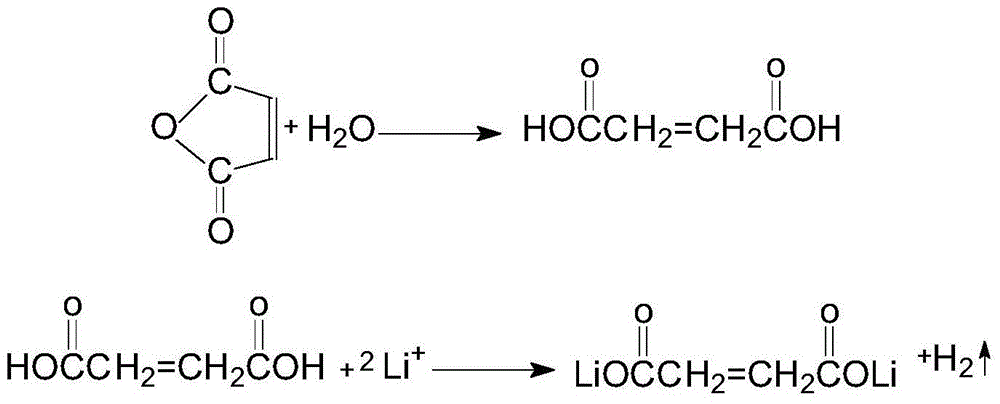
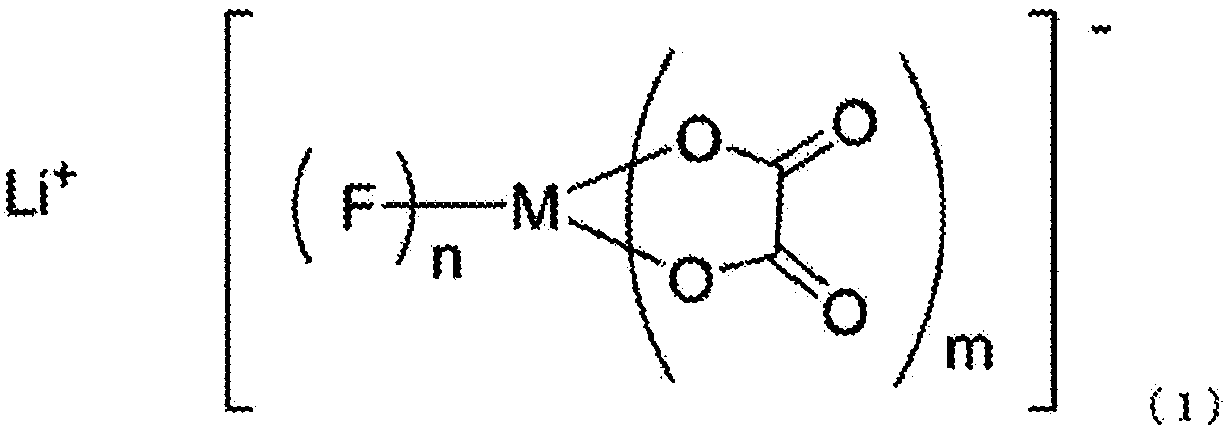
The invention relates to an electrolyte. The electrolyte comprises an organic solvent, lithium hexafluorophosphate and additives, wherein the additives comprise lithium tetrafluoroborate, 1,3-propane sultone, lithium bis(oxalate) borate, adiponitrile, vinylene carbonate and fluoroethylene carbonate, and in the electrolyte, the mass concentration of the lithium tetrafluoroborate is 0.5%-2%, the mass concentration of the 1,3-propane sultone is 1%-4%, the mass concentration of the lithium bis(oxalate) borate is 1%-5%, the mass concentration of the adiponitrile is 1%-3%, the mass concentration of the vinylene carbonate is 0.5%-2%, and the mass concentration of the fluoroethylene carbonate is 2%-5%. The electrolyte can be used for improving the cycle performance, the high-temperature storage performance and the initial energy density of a lithium-ion battery. The invention further provides an application of the electrolyte in the lithium-ion battery.
Owner:EVE HYPERPOWER BATTERIES INC +1
Method and equipment for production of adiponitrile from adipic acid
ActiveCN103896805ACatalyst content is stableNormal responsePreparation by ammonia-carboxylic acid reactionPhosphateGas phase
The invention relates to a method and equipment for production of adiponitrile from adipic acid, and mainly solves the problem of unstable product quality in the prior art for production of adiponitrile from adipic acid. The method employs materials of fusing adipic acid, ammonia, phosphate and a diluent. The production method is as below: mixing phosphoric acid with the diluent in a pipeline mixer; conducting neutralization of adipic acid and ammonia in a cyanation reactor; sending reaction products into the bottom of a separation tower for separation; recovering gas phase of light components from an outlet; sending the diluent recovered by a gas lifting plate into the pipeline mixer, mixing the diluent with phosphoric acid in the pipeline mixer, and sending the mixture into the cyanation reactor to dilute adipic acid; and sending a liquid phase to a scraper type evaporator. The employed equipment includes an adipic acid storage tank, an ammonia compressor, a cyanation reactor, a pipeline mixer, a separation tower, a tail gas removal tower, a phosphoric acid storage tank and a scraper type evaporator. The invention has the advantage of a good quality of the produced adiponitrile product.
Owner:ANSHAN GUORUI CHEM
Preparation method of hexamethylenediamine
InactiveCN107805203AReduce usageHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHexamethylenediamineOxide composite
The invention discloses a preparation method of hexamethylenediamine. Hexane dinitrile is used as a raw material, and a nano-nickel and magnesium oxide composite material is used as a catalyst to prepare the hexamethylenediamine through liquid phase hydrogenation. The preparation method of the hexamethylenediamine comprises the following steps: dipping a porous carrier by using nickel acetate andmagnesium nitrate aqueous solutions at different concentrations at first, and carrying out in-situ reduction to obtain an immobilized nickel and magnesium oxide based composite catalyst under different conditions; adding raw materials, the catalyst and a solvent in a reaction kettle in proportion, sealing the reaction kettle, removing air in the kettle by a hydrogen substitution method, starting aheating power supply, applying set pressure to hydrogen after the temperature in the kettle is raised to the set reaction temperature, then starting a magnetic stirrer, and beginning to time reaction; and after the reaction is finished, filtering out the catalyst, and distilling and separating under reduced pressure. The yield of a target product is high, the yield of the hexamethylenediamine isgreater than 70%, and the solvent and the catalyst are recyclable.
Owner:SHANGHAI INST OF TECH
Electrolyte of lithium ion battery taking lithium titanate as cathode
InactiveCN104466249AImprove high temperature storageImprove high temperature cycle performanceSecondary cellsElectrolytesCooking & bakingHigh temperature storage
The invention discloses electrolyte of a lithium ion battery taking lithium titanate as cathode. The electrolyte comprises the organic solvent, lithium salt and additive dissolved in the organic solvent; the additive comprises one or the combination of the adiponitrile, butanedinitrile, 1,3-propane sultone, wherein the additive further comprises one or the combination of the cyclic acid anhydride and / or the derivate thereof, the total weight of the additive is 1-10% of the weight of the electrolyte, the weight of the cyclic acid anhydride and / or the derivate thereof, is 0.01-1% of the weight of the electrolyte. The additive comprises the cyclic acid anhydride and / or the derivate which have chemical reaction with the water in the battery, the combination water in the lithium titanate material, the water, the hydroxy and other groups for generating one component of the electrolyte, the defect that the water cannot be removed totally in the conventional vacuum baking process is compensated. The catalytic reaction for the hydroxy can be effectively avoided while having reaction with the Ti-OH bond on the surface of the lithium titanate particle, and the high temperature storage performance and the high temperature circulation performance of the lithium titanate battery are improved.
Owner:东莞市天丰电源材料有限公司
Method for producing adiponitrile with adipic acid liquid-phase method
ActiveCN106146345AImprove dehydration ratePhysical/chemical process catalystsCarboxylic acid nitrile purification/separationSynthesis methodsDiluent
The invention discloses a method for producing adiponitrile with an adipic acid liquid-phase method. A solid phosphoric acid catalyst is adopted, adipic acid and a diluent are added into a reaction still, heating and raw material stirring are conducted during reaction, ammonia gas is supplied after temperature reaches a certain value, and the product is generated after reaction is conducted for a while. A novel environment-friendly adiponitrile synthesis method is adopted. By the adoption of the solid phosphoric acid catalyst, the defects of traditional catalysts are overcome, and catalysis effect is improved.
Owner:PETROCHINA CO LTD
Preparation method of adiponitrile and product thereof
ActiveCN108821997AHigh selectivityExtended operating cycleOrganic compound preparationCarboxylic compound preparationPhosphoric acidAdipic acid
The invention relates to a preparation method of adiponitrile. The preparation method comprises the following steps: firstly performing a reaction on adipic acid and ammonia at 155-200 DEG C to produce diammonium adipate, and then dehydrating under a catalytic reaction of phosphoric acid or ammonium phosphate to produce adiponitrile; a product can be further separated in a separation tower. The reaction is performed on the adipic acid and the ammonia gas at the low temperature of 155-200 DEG C to produce H4NOOC(CH2)4COONH4 first, and then high-temperature cyanation is performed, so that the opportunity of producing a by-product and a coke from the adipic acid at high temperature is effectively avoided, not only the yield is increased by 3-5%, but also the operation cycle of a production device is prolonged by 30-60 days, and the production per unit time is increased. Liquid phosphoric acid is used as a catalyst, firstly ammonium salt of the phosphoric acid is produced, and the ammoniumphosphate can be mixed with ammonium salt of the adipic acid very well, so that the catalytic performance is improved better and the selectivity of the adiponitrile is increased by more than 2%.
Owner:重庆华峰聚酰胺有限公司 +1
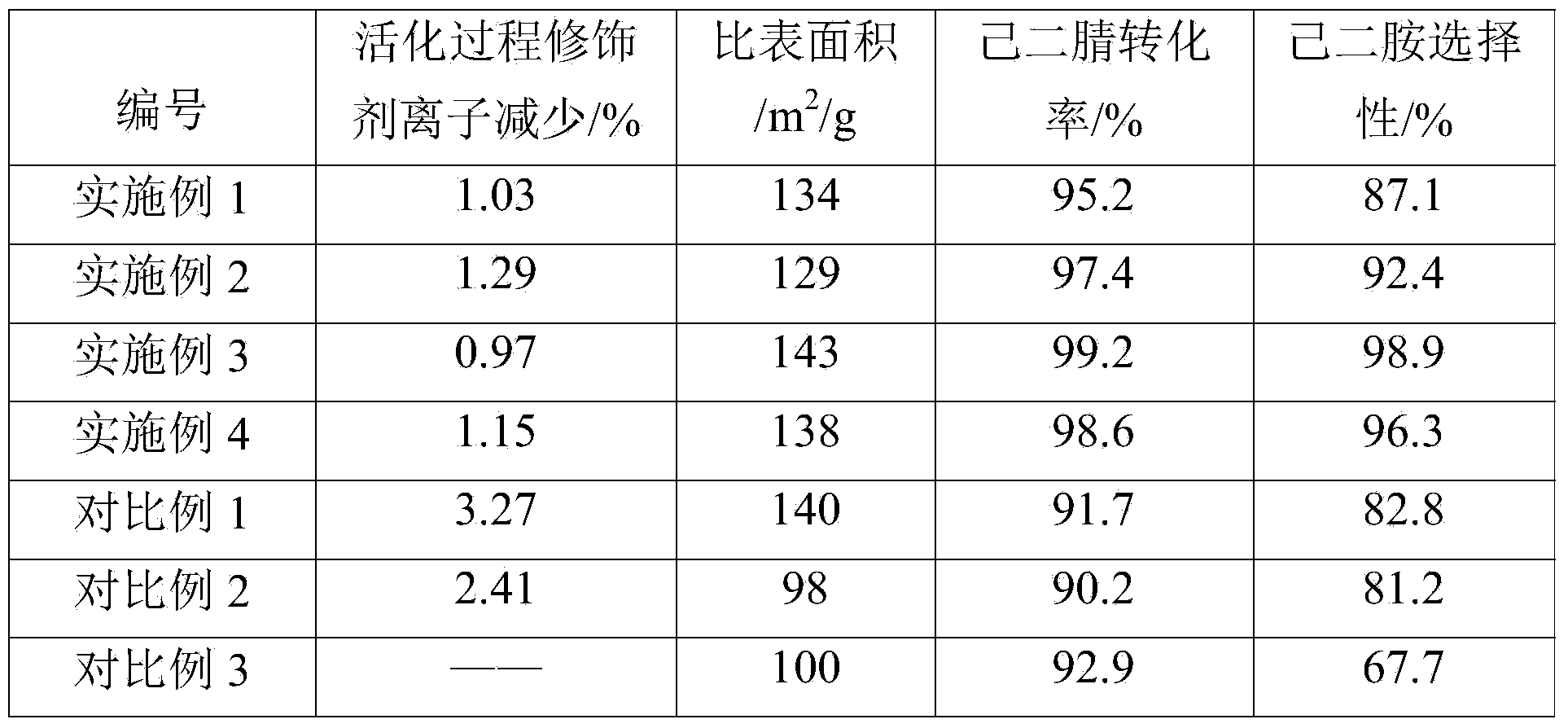
Method for activating adiponitrile hydrogenation catalyst
ActiveCN103977819AReduce churnActive ingredient retentionOrganic compound preparationCatalyst activation/preparationHexamethylenediamineManganese
The invention discloses a method for activating an adiponitrile hydrogenation catalyst. The method is characterized in that the catalyst is a modified raney nickel catalyst and is activated by the method comprising the following steps: preparing a mixing solution from iron, chromium, molybdenum, bismuth, manganese or tungsten soluble salts and NaOH respectively; slowly adding the modified raney nickel catalyst and ammonium salt; heating with microwave and reacting, and then washing; the modified raney nickel catalyst is prepared by the method comprises the following steps: grinding nickel-aluminum alloy into powder; adding to a mixing solution to dissolve part of aluminum, wherein the mixing solution is prepared through NaOH, ammonium salt and ammonium hydroxide in certain ratio; washing with deionized water and absolute ethyl alcohol; adding the powder to the mixing solution consisting of ferric nitrate and a modifier under a microwave condition and reacting to obtain a solid; drying and roasting the solid; and then charging hydrogen and reducing to obtain the modified raney nickel catalyst. With the adoption of the method for activating the adiponitrile hydrogenation catalyst, the prepared catalyst is relatively large in specific surface area, loss of the modifier is small, and high conversion rate of adiponitrile and high selectivity of hexamethylenediamine are achieved in the adiponitrile catalytic hydrogenation process.
Owner:CHINA TIANCHEN ENG +1
Electrolyte and lithium ion battery comprising same
InactiveCN105098244AImprove first-time efficiencyImprove cycle performanceCell electrodesSecondary cellsHigh temperature storageLithium-ion battery
The invention relates to an electrolyte and a lithium ion battery comprising the same. The electrolyte comprises an organic solvent, a lithium salt, vinylene carbonate, fluoroethylene carbonate and a combined additive, wherein the combined additive comprises the following raw materials by percentage: 0.1%-7% of propane sultone, 0.1%-7% of ethylene sulfate and 0.1%-9% of adiponitrile on the basis of total weight of the electrolyte. The electrolyte provided by the invention is applied to the lithium ion battery, so that the initial efficiency, the cycle performance, the high-temperature storage performance, the overcharge resistance performance and the safety performance of the lithium ion battery at high voltage of above 4.4V can be greatly improved.
Owner:NINGDE AMPEREX TECH
Electrolyte for lithium ion battery and lithium ion battery containing electrolyte
ActiveCN106025339AAvoid mixingReduce thickness expansionSecondary cellsMethyl carbonatePhysical chemistry
Owner:HIGHPOWER TECH HUIZHOU
Electrolyte of power lithium ion secondary battery
InactiveCN103700884AGood effectIncrease loopSecondary cellsOrganic electrolytesSolventLithium hexafluorophosphate
The invention discloses an electrolyte of a power lithium ion secondary battery, which is prepared from an electrolyte, a main solvent and an additive according to certain mass percents; the electrolyte of the invention is characterized in that the electrolyte comprises 5-15% of lithium hexafluorophosphate and 1-10% of lithium bis(oxalate)borate; the main solvent comprises 25.0%-35.0% of dimethyl carbonate (DMC), 5.0%-15.0% of ethyl methyl carbonate (EMC), 3.0%-10.0% of ethylene carbonate (EC), and 2.0%-8.0% of propylene carbonate (PC); the additive comprises 1%-3% of ethylene sulfite (ES), 1%-3% of vinylene carbonate (VC), 0.5%-2.0% of 1,3-propane sultone (PS), 0.1%-0.5% of anisole, 0.5%-2.0% of biphenyl (BP), 0.01%-2% of adiponitrile, 0.5%-2.0% of gamma-butyrolactone (GBL), and 0.5%-2.0% of cyclohexylbenzene (CHB). The electrolyte of the invention can significantly improve conductivity, wettability, and high temperature circularity, thus enlarging the application scope of the electrolyte.
Owner:JIANGXI CHC BATTERY
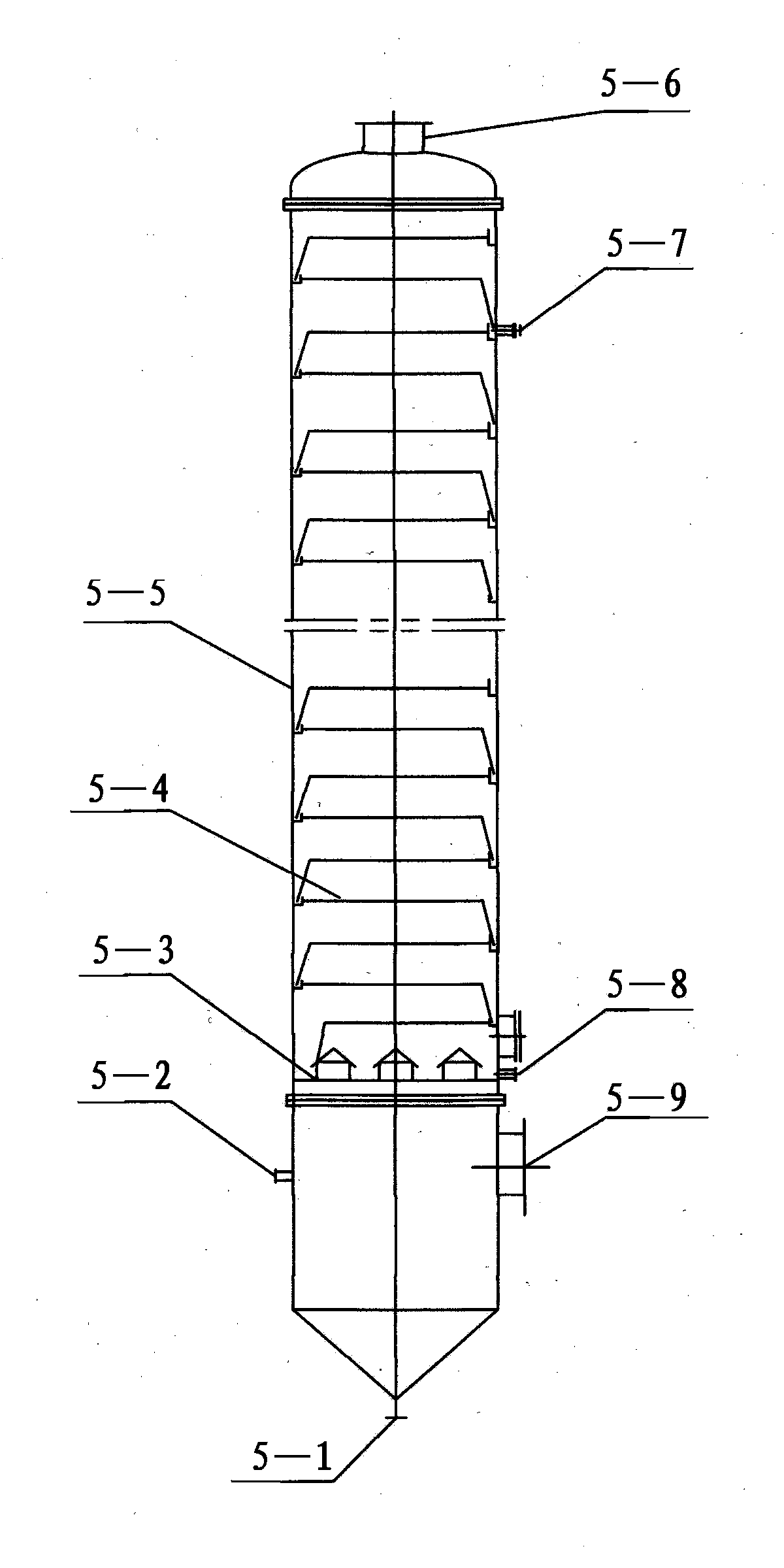
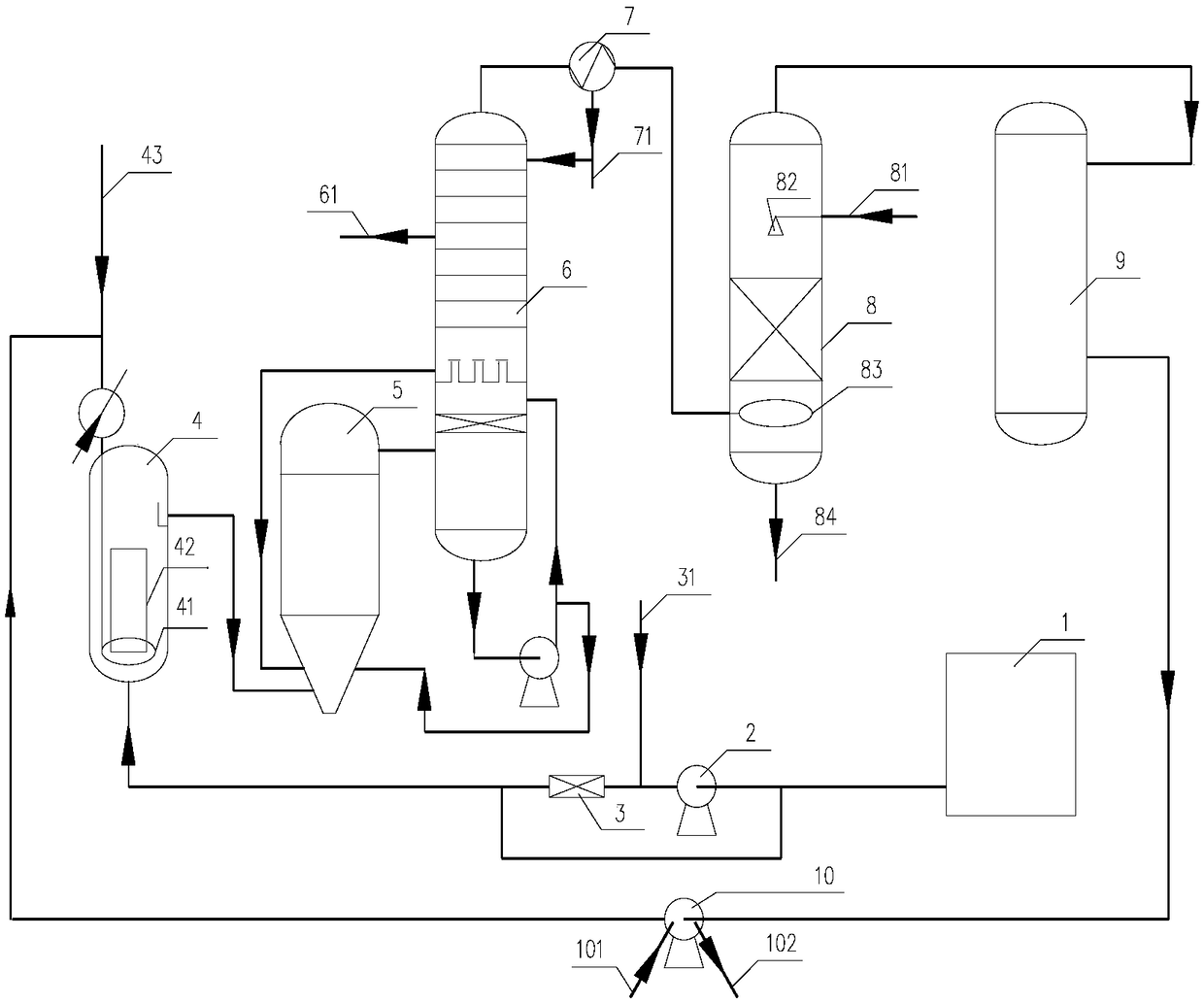
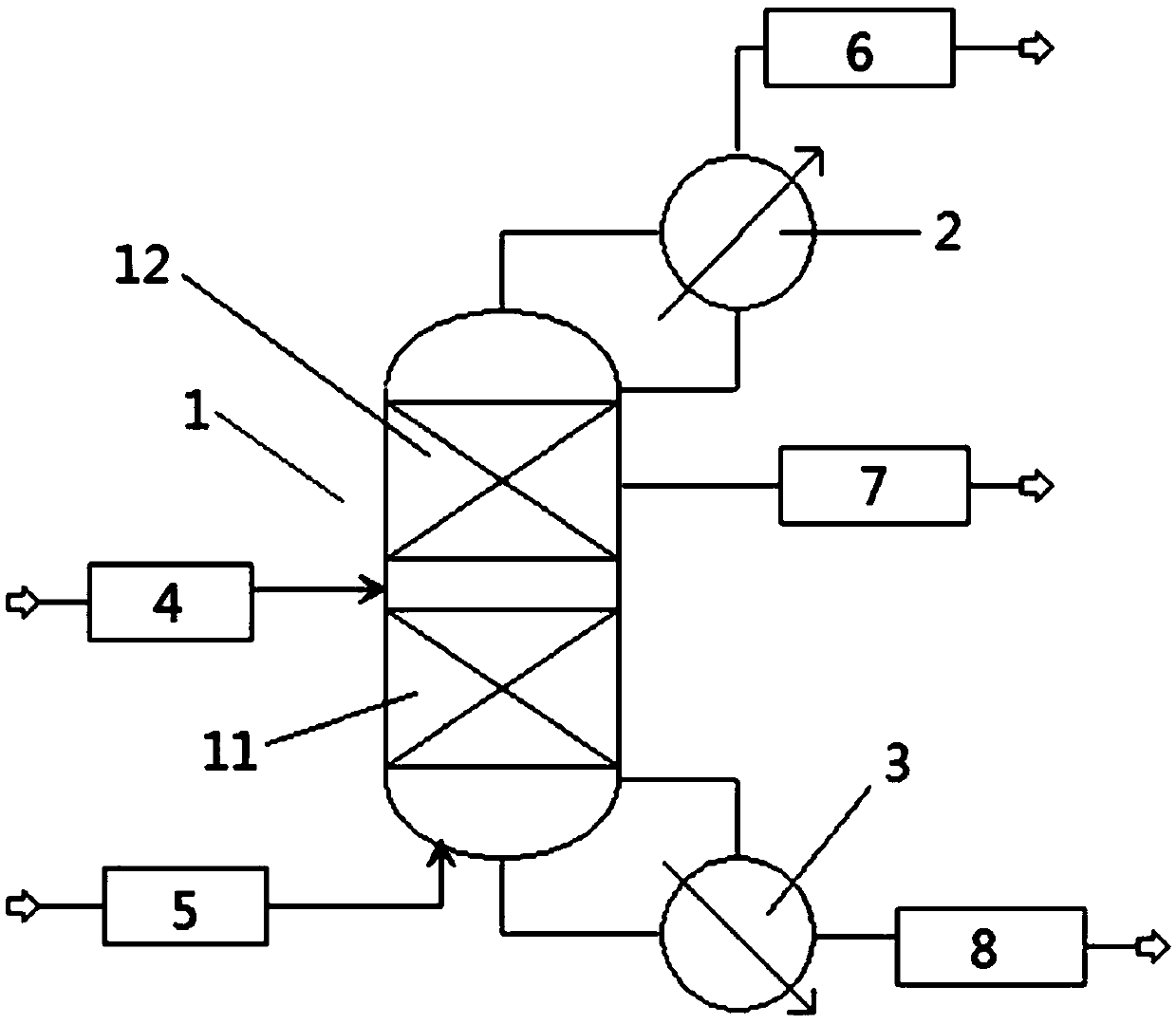
Reaction device and method for adiponitrile production
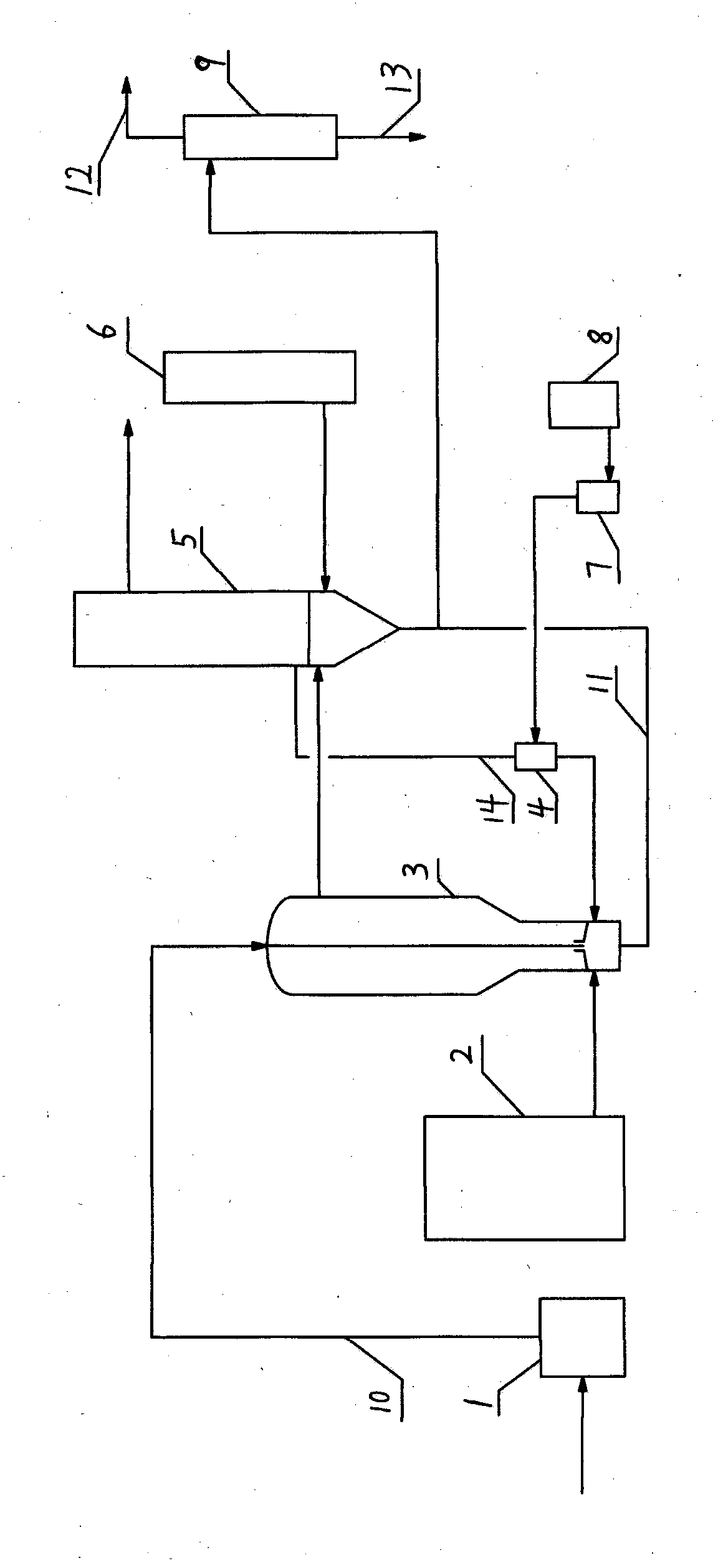
ActiveCN109678756AImprove conversion ratePreliminary separationChemical industryPreparation by ammonia-carboxylic acid reactionLateral lineLiquid phase
The invention relates to a reaction device and a method for adiponitrile production. The reaction device comprises a reactive distillation tower, a fractional condenser at the tower top and a reboilerat the tower bottom, wherein the reactive distillation tower comprises a reaction section and a distillation section located above the reaction section and is provided with two feeding ports and three discharging ports, a first feeding port is located between the reaction section and the distillation section, a second feeding port is located in the tower bottom, a first discharging port of the reactive distillation tower is located in the fractional condenser at the tower top, a second discharging port is a lateral line liquid phase discharging port and located in the middle of the distillation section, a third discharging port is a tower bottom liquid phase discharging port, and adiponitrile is produced from adipic acid as a raw material by the reaction device through liquid phase catalytic ammoniation. With the adoption of a reactive distillation mode, countercurrent sufficient contact of ammonia and adipic acid at the reaction section is realized, meanwhile, water as a reaction product can leave the reaction area in a vapor state, and accordingly, conversion rate of adipic acid and selectivity of adiponitrile are increased.
Owner:SHANGHAI JIAO TONG UNIV
Electrolytic solution used in electrolysis of acrylonitrile for preparing adiponitrile and method
InactiveCN105543888AReduce consumptionElectrode corrosion is smallElectrolysis componentsElectrolytic organic productionPhosphateAcrylonitrile
The invention discloses an electrolytic solution used in electrolysis of acrylonitrile for preparing adiponitrile and a method. The electrolytic solution consists of the following components in percentage by mass: 1%-5% of acrylonitrile, 0-10% of adiponitrile, 8%-15% of phosphate, 0.1%-2% of EDTA or EDTA salt, 1%-10% of borax, 0.1%-5% of biquaternary ammonium salt and the balance of water. The electrolytic solution is added into a non-membrane electrolytic cell, so that the adiponitrile is prepared by electrolysis. The biquaternary ammonium salt and the borax are added into the electrolytic solution, so that electrode corrosion during electrolysis is reduced, and the selectivity and the current efficiency of the acrylonitrile are higher than 90%; and moreover, the consumption of the biquaternary ammonium salt is reduced, so that the content of the propionitrile is kept at a relatively low level while the propionitrile is continuously used for 400 hours without being supplemented.
Owner:CHONGQING UNISPLENDOUR INT CHEM
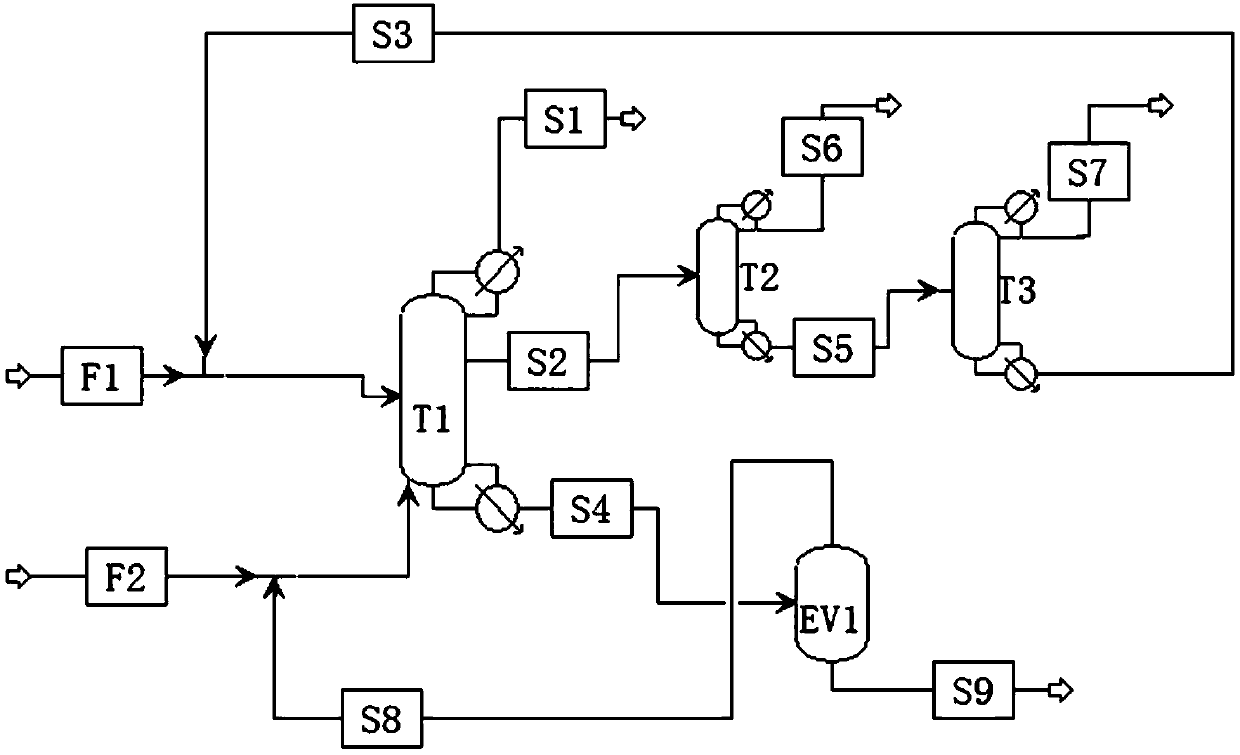
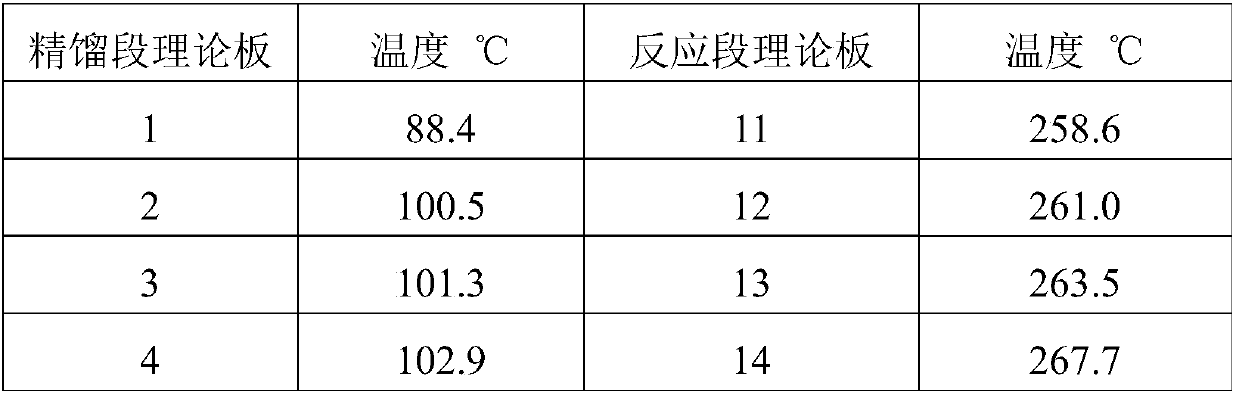
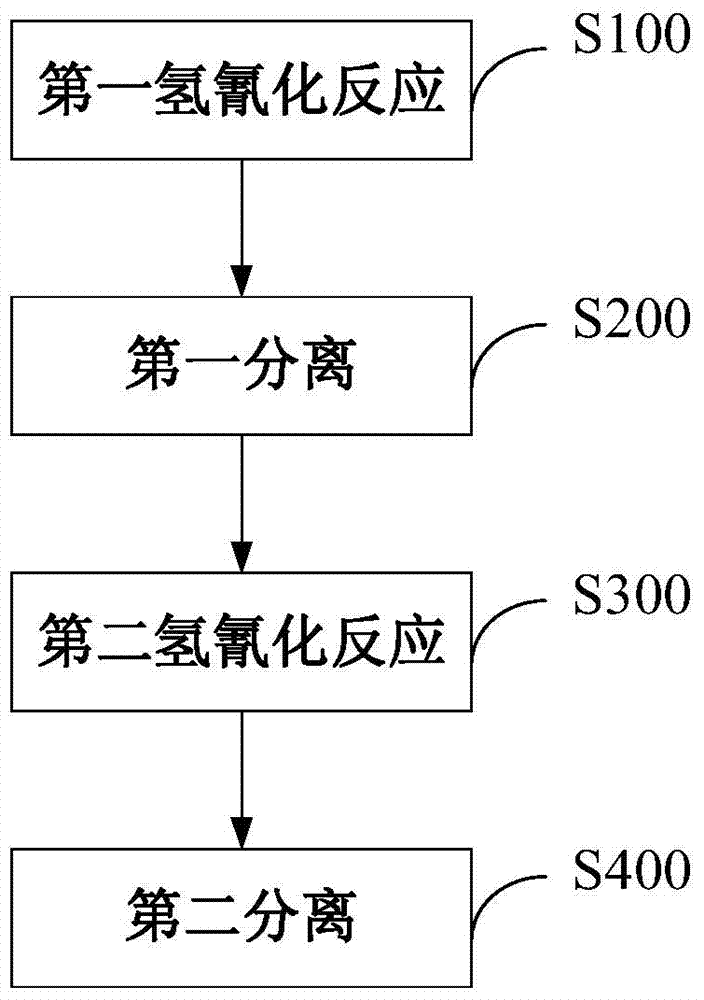
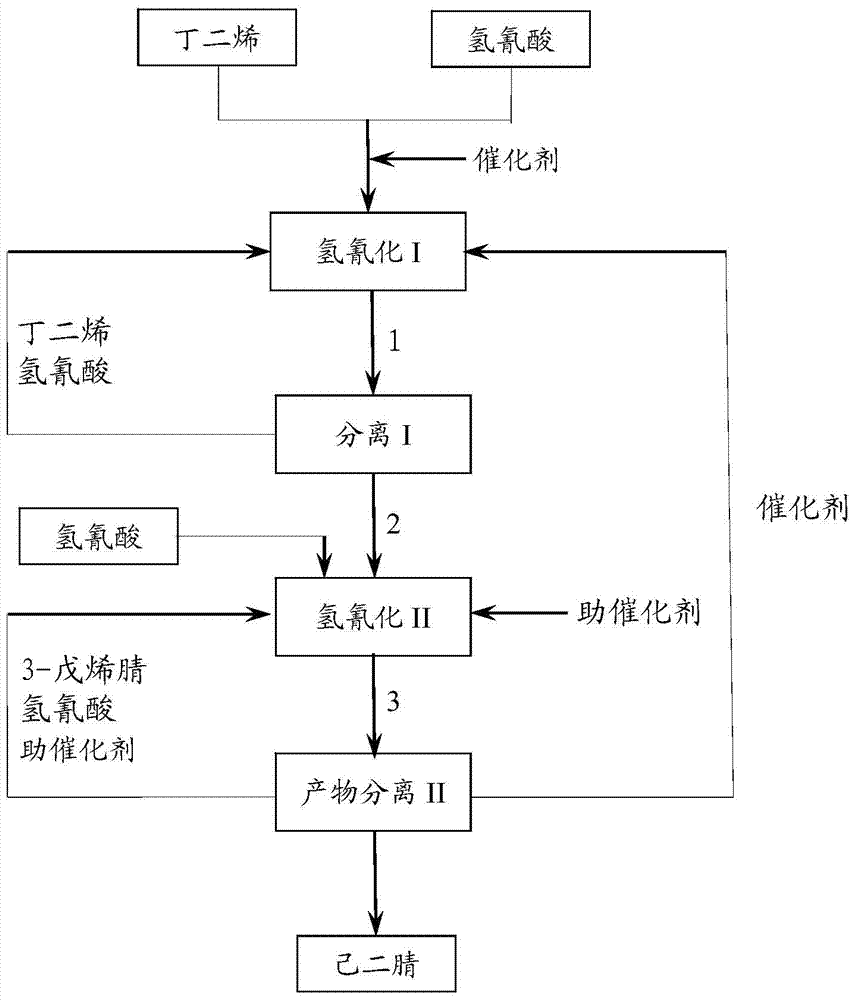
Method for preparing adiponitrile
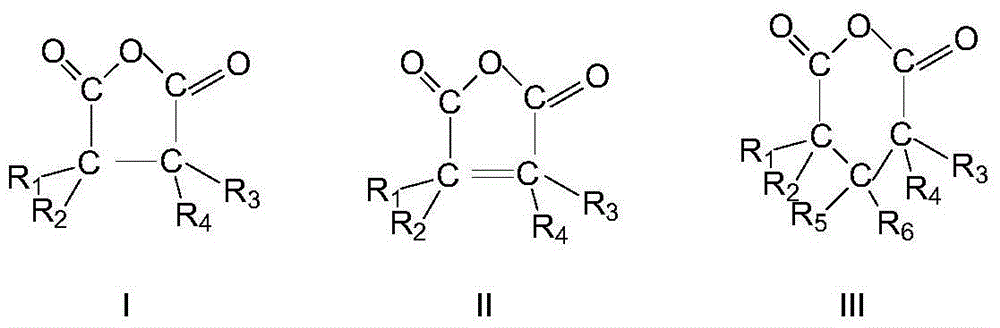
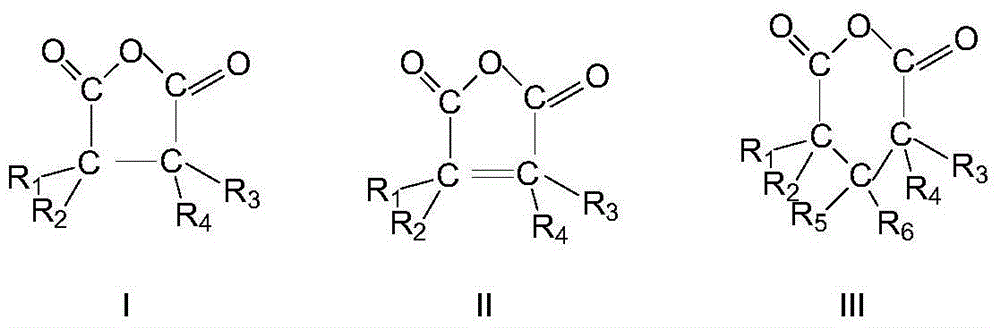
ActiveCN103664691AHigh purityQuality improvementPreparation by hydrogen cyanide additionIsomerizationPhosphine
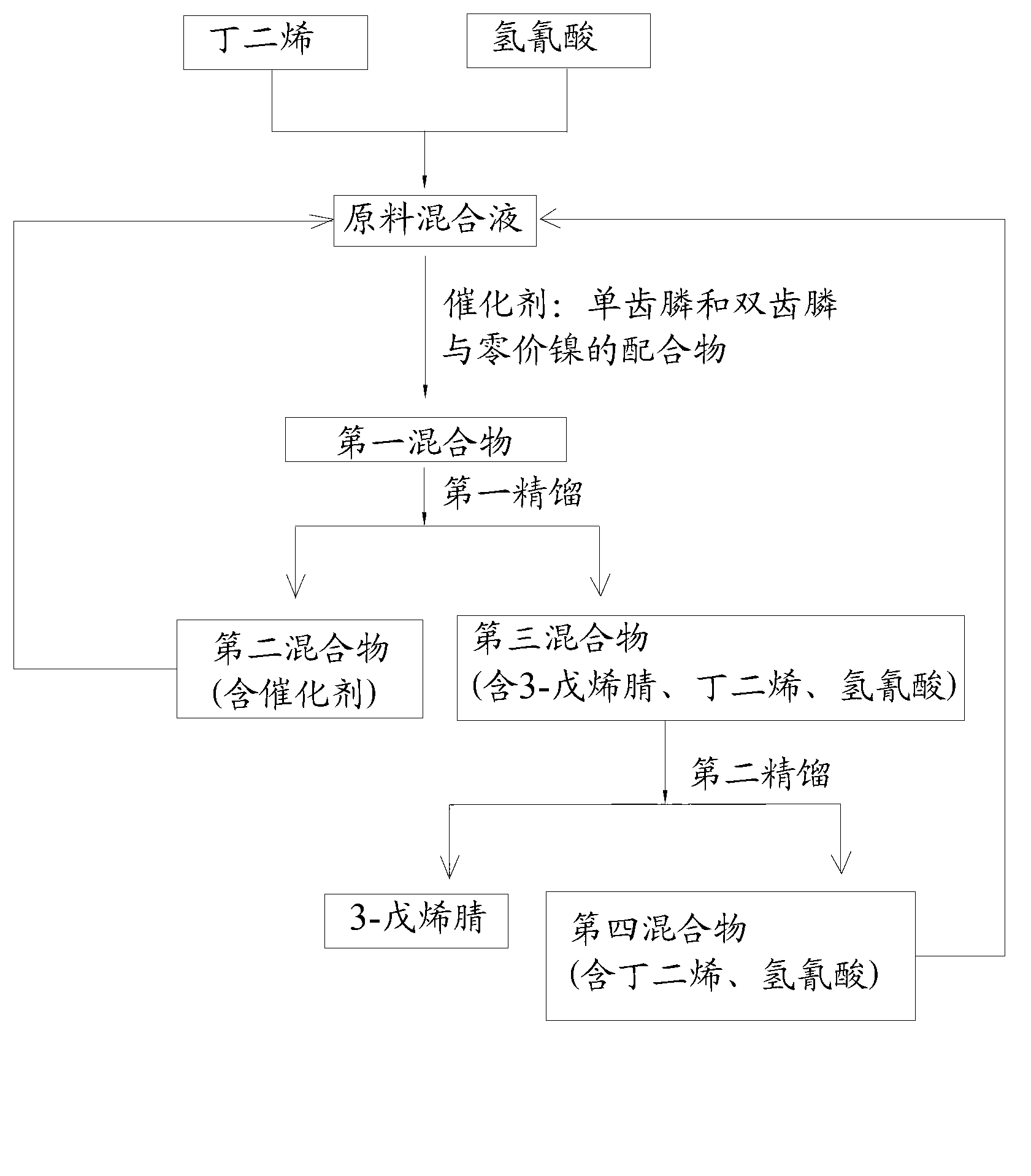
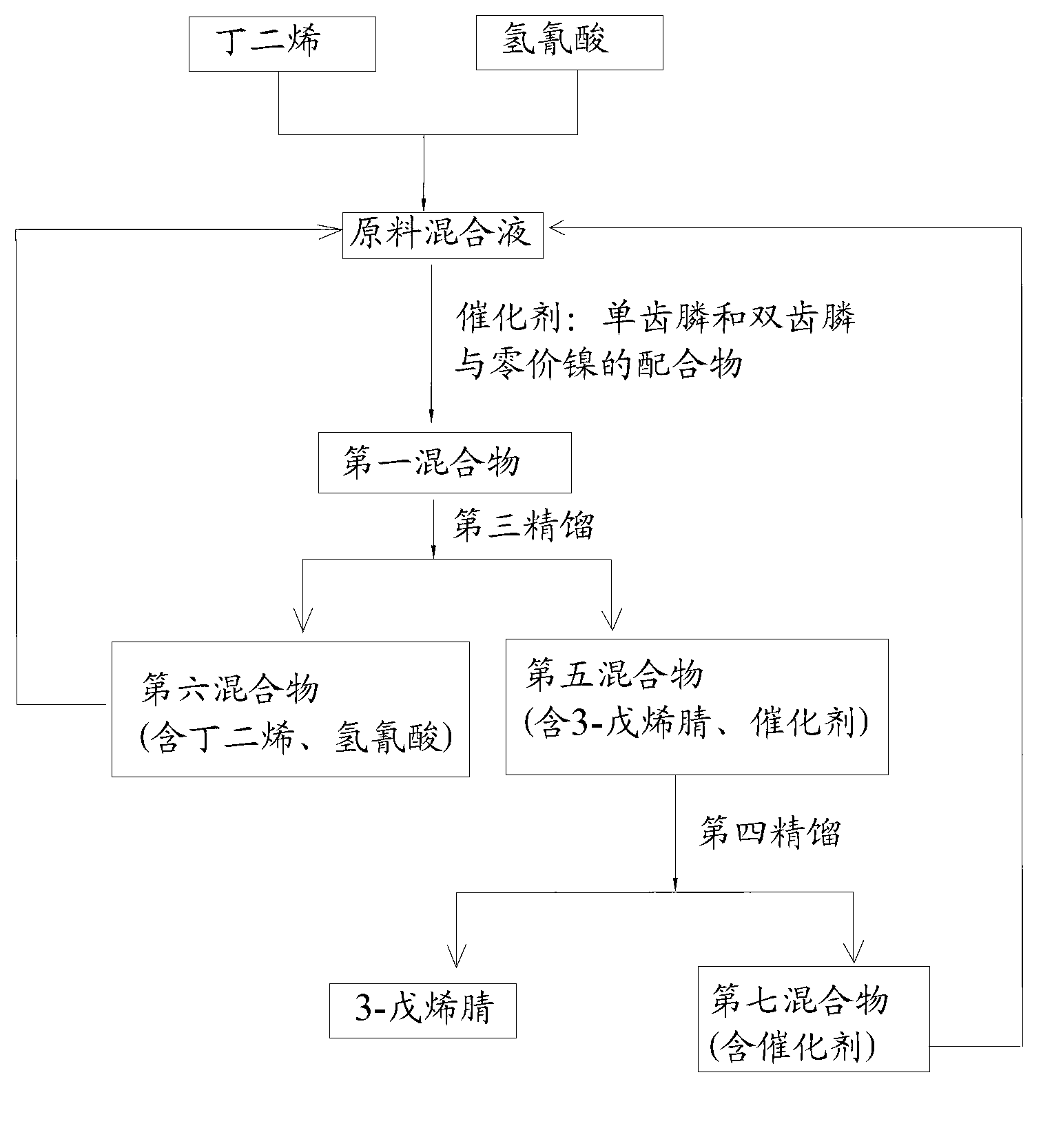
The invention discloses a method for preparing adiponitrile. The method comprises the following steps: performing a first hydrocyanation reaction on butadiene and first hydrocyanic acid under the action of a catalyst to obtain a first mixture; performing primary separation on each component in the first mixture to obtain a second mixture, residual butadiene and residual first hydrocyanic acid respectively; adding a co-catalyst into the second mixture, and introducing second hydrocyanic acid to obtain a third mixture containing the adiponitrile; performing secondary separation on the third mixture to obtain the adiponitrile, residual 3-pentenenitrile, residual second hydrocyanic acid, the reacted catalyst and the reacted co-catalyst, wherein the catalyst is a coordination compound consisting of a monodentate phosphine ligand, a bidentate phosphine ligand and zerovalent nickel, and the co-catalyst is Lewis acid. The method for preparing the adiponitrile has the advantages of small quantity of side products, no need of performing a 2M3BN isomerization reaction, simple process, low cost, high yield, good product quality, economical efficiency and environmental friendliness.
Owner:ANHUI ANQING SHUGUANG CHEM GRP +1
Nonaqueous electrolyte secondary battery
ActiveCN107851832ACompromise and improve durabilityCompromise and improve cycle durabilityCell seperators/membranes/diaphragms/spacersNegative electrodesElectrolysisSolvent
A nonaqueous electrolyte secondary battery, wherein: an electrolyte is configured to contain an electrolyte salt, a nonaqueous solvent into which the electrolyte salt is able to be dissolved, a firstadditive selected from among specific oxalate compounds and disulfonic acid ester compounds, and a second additive that has a reduction potential lower than the reduction potential of the first additive and is selected from among vinylene carbonate, fluoroethylene carbonate, vinyl ethylene carbonate, 1, 3-propane sultone, 1, 4-butane sultone, 1, 3-propene sultone, succinonitrile and adiponitrile;and 0.21 <= A * L / SSA <= 0.69 and 0.51 <= B * L / SSA <= 1.5 are satisfied, where SSA (m2 / g) is the BET specific area of the negative electrode active material, L is the ratio (liquid coefficient) of the electrolyte amount to the total void volume of the positive electrode, the negative electrode and the separator, A (mass%) is the ratio of the addition amount of the first additive to the total amount of the nonaqueous solvent and the electrolyte salt in the electrolyte, and B (mass%) is the ratio of the addition amount of the second additive to the total amount of the nonaqueous solvent and theelectrolyte salt in the electrolyte.
Owner:ENVISION AESC JAPAN LTD
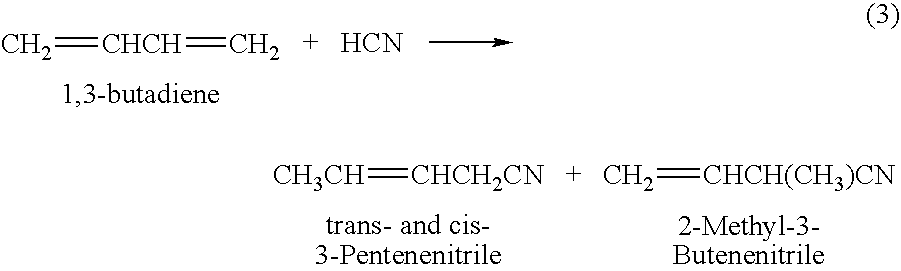
Preparation method of 3-pentenenitrile and preparation method of adiponitrile
ActiveCN103012197AEfficient preparationCheap manufacturingPreparation by hydrogen cyanide additionButadiene DioxidePhosphine
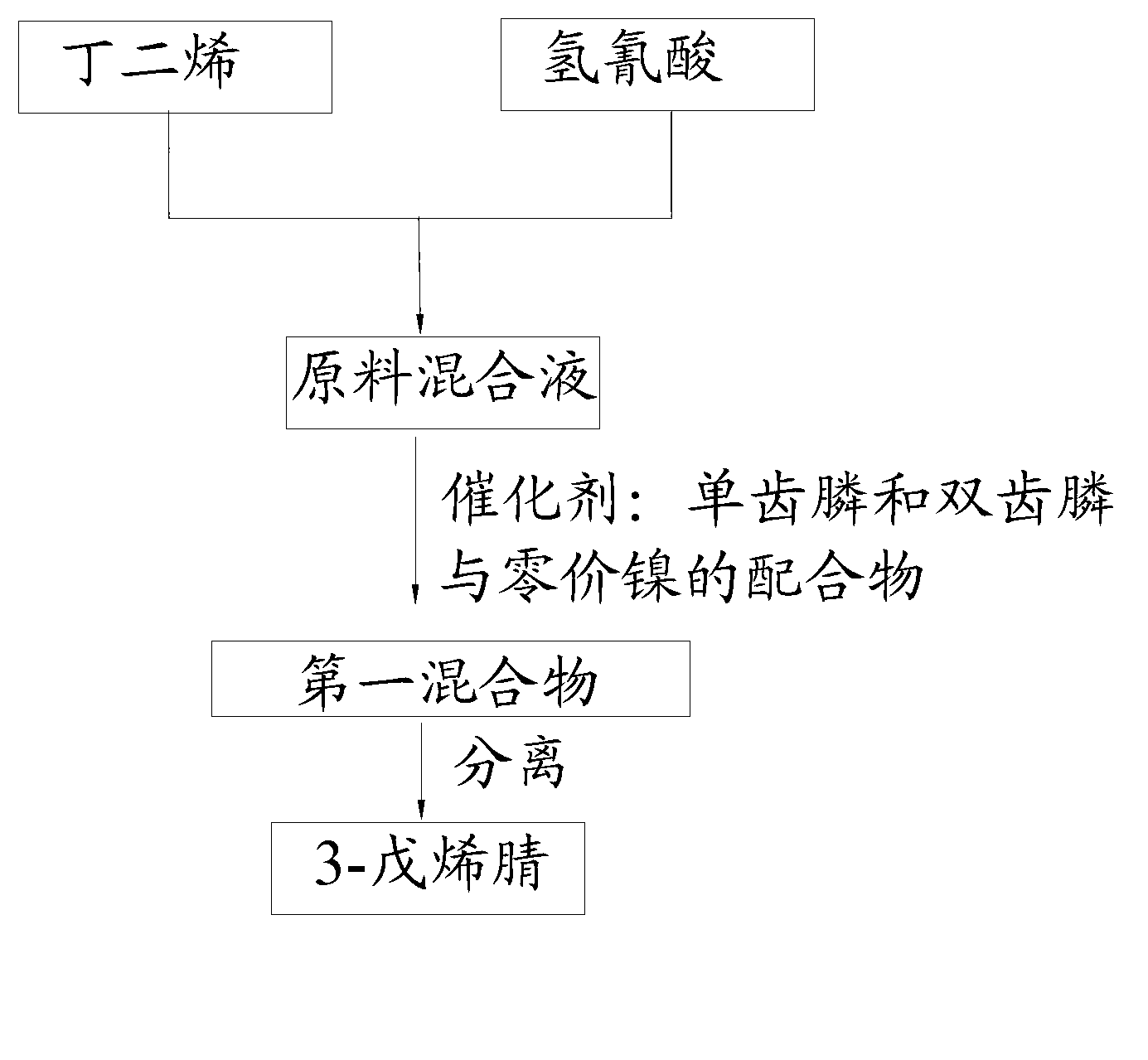
The invention provides a preparation method of 3-pentenenitrile and a preparation method of adiponitrile. The preparation method of 3-pentenenitrile comprises the following steps: 1, performing the hydrocyanation reaction on raw material mixed solution of butadiene and hydrocyanic acid under the action of a catalyst so as to obtain a first mixture containing the 3-pentenenitrile; and 2, carrying out separation on the first mixture to obtain the 3-pentenenitrile, wherein the catalyst is a coordination complex consisting of monodentate phosphine, bidentate phosphine and zero-valent nickel. By adopting the method, the 3-pentenenitrile can be effectively, efficiently and economically prepared by using the butadiene and the hydrocyanic acid as the raw materials; the integral process is safe and reliable; the flow is simple; and moreover, production cost and equipment investment can be obviously reduced.
Owner:ANHUI ANQING SHUGUANG CHEM GRP +1
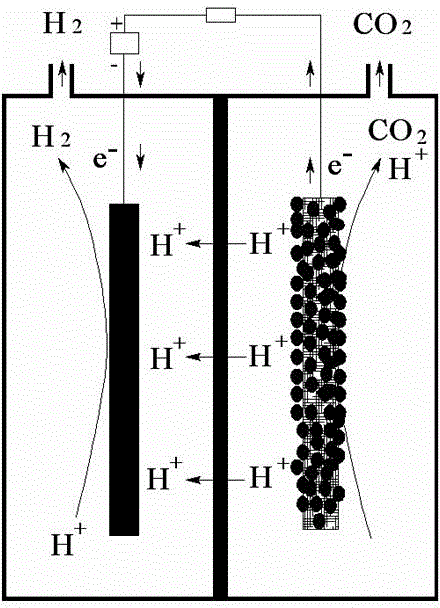
Apparatus and method for preparing adiponitrile by electrolyzing acrylonitrile assisted by electro-active microbes
ActiveCN103334118AAchieve recyclingEmission reductionElectrolysis componentsElectrolytic organic productionData acquisitionAcrylonitrile
The invention discloses an apparatus and a method for preparing adiponitrile by electrolyzing acrylonitrile assisted by electro-active microbes. The apparatus comprises a microbe electrolytic tank, a data acquisition system and a recording unit, wherein an anode electrode and a cathode electrode of the microbe electrolytic tank are made of electrically inert materials; the anode electrode and the cathode electrode are connected through a titanium wire, a constant potential rectifier and a resistor; the data acquisition system is connected with the resistor in parallel; and the recording unit is connected with the data acquisition system. The method comprises the steps of organic materials are metabolized by electro-active microbes in an anode chamber of the microbe electrolytic tank to produce electrons and protons; the produced electrons are transferred to the anode electrode directly or indirectly and then transferred to the cathode electrode along an external circuit under the effect of an applied voltage; at the same time, H<+> ions pass through an ion exchange membrane and are migrated to a cathode chamber of the microbe electrolytic tank; H<+> ions, the electrons and acrylonitrile are combined on the surface of the cathode electrode with relatively high hydrogen evolution over potential to generate adiponitrile. The apparatus and the method have the advantages of low energy consumption, no corrosions to the anode electrode, etc. Besides, the apparatus and the method can reduce discharge of organic wastes.
Owner:INNER MONGOLIA UNIV OF SCI & TECH
Hydrogenation catalyst, and preparation method and application thereof
ActiveCN107224977AFacilitated DiffusionReduce the impactOrganic compound preparationAmino compound preparationFluidized bedActive component
The invention discloses a hydrogenation catalyst, and a preparation method and application thereof, belonging to the field of hydrogenation of adiponitrile. The hydrogenation catalyst comprises the following components in percentage by mass: 60-90% of active component, 1-30% of aid and 1-10% of at least one metal selected from Group IIA metals. The preparation method comprises the following steps: preparing a mixed solution from the components, putting the mixed solution in an 80-110-DEG C environment, and performing reaction for 1-8 hours to obtain the hydrogenation catalyst. The hydrogenation catalyst is applied to reduction reaction. The hydrogenation catalyst has the advantages of small particle size, uniform particle size distribution (15-18nm), large specific area (up to 25-30 m<2> / g), high hydrogenation activity, favorable selectivity, low price, simple preparation technique, easy operation process and low requirements for processing equipment, and can be easily separated from the product after the reaction finishes. The hydrogenation catalyst is applicable to tank reactors, fluidized bed reactors and fixed bed reactors, and thus, has favorable adaptability to equipment.
Owner:SINOPEC YANGZI PETROCHEM +1
Preparation method and application of nitrogen-doped activated carbon supported alkali metal modified nickel-based catalyst
ActiveCN105921164ALow costIncrease costCarboxylic acid nitrile preparationOrganic compound preparationHexamethylenediamineHigh activity
The invention discloses a preparation method and application of a nitrogen-doped activated carbon supported alkali metal modified nickel-based catalyst. The preparation method includes the steps that firstly, acid activation or acid modification is conducted on a wooden charcoal material, nitrogen doping treatment is conducted on the wooden charcoal material, and then the wooden charcoal material is impregnated and supported with nickel and alkali metals. The nitrogen-doped activated carbon supported alkali metal modified nickel-based catalyst is applied to an adiponitrile hydrogenation process and can have high activity and high overall selectivity on 6-aminocapronitrile and hexamethylenediamine under relatively mild reaction conditions. Usage of a large number of precious metals serving as active components is avoided, equipment is not corroded (a large amount of additional ammonia or alkali metal hydroxide for inhibiting the formation of by-products is not needed), the cost is low, the catalyst is friendly to the environment, the preparation process of the catalyst is simple, the used active charcoal material is cheap and easy to obtain, and the preparation method is simple and has a very good application prospect.
Owner:XIANGTAN UNIV
Non-aqueous electrolyte secondary cell
InactiveCN102170016AWithout degrading load characteristicsDoes not degrade recycling propertiesNon-aqueous electrolyte accumulatorsCell electrodesPropionatePolyolefin
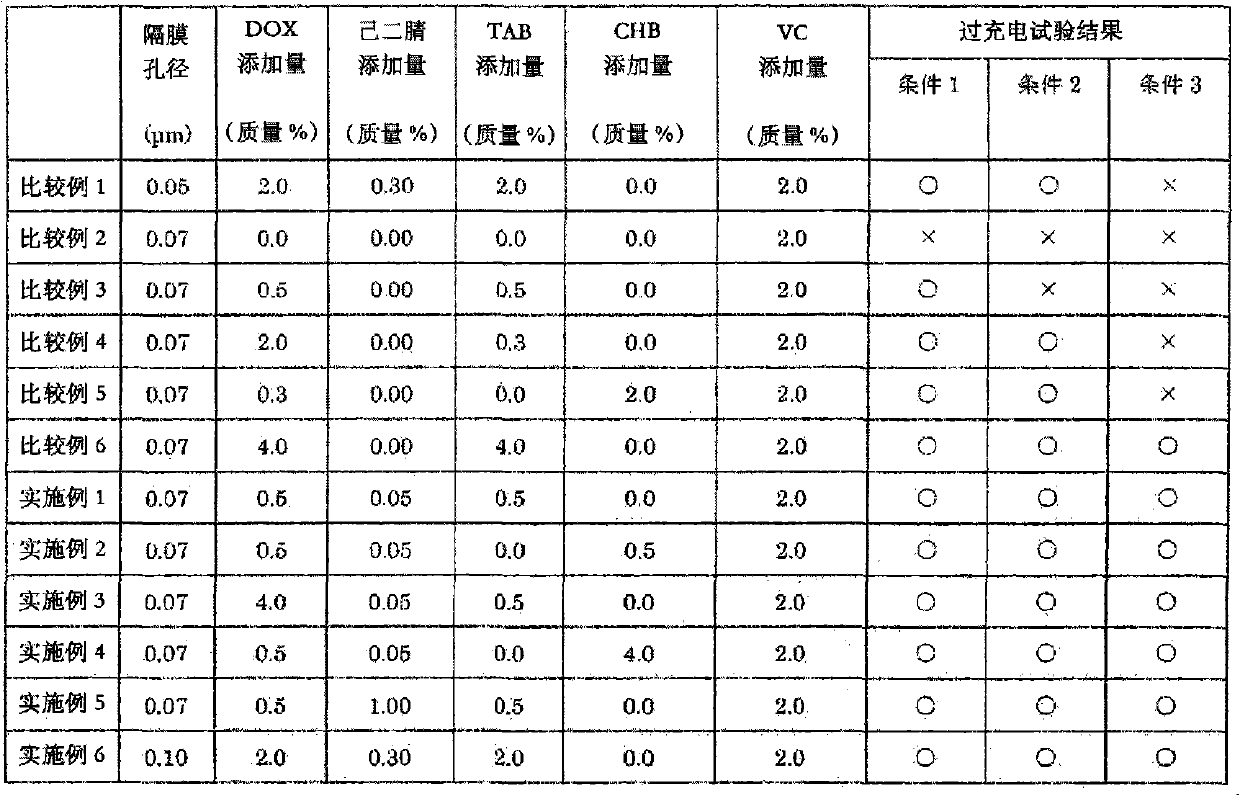
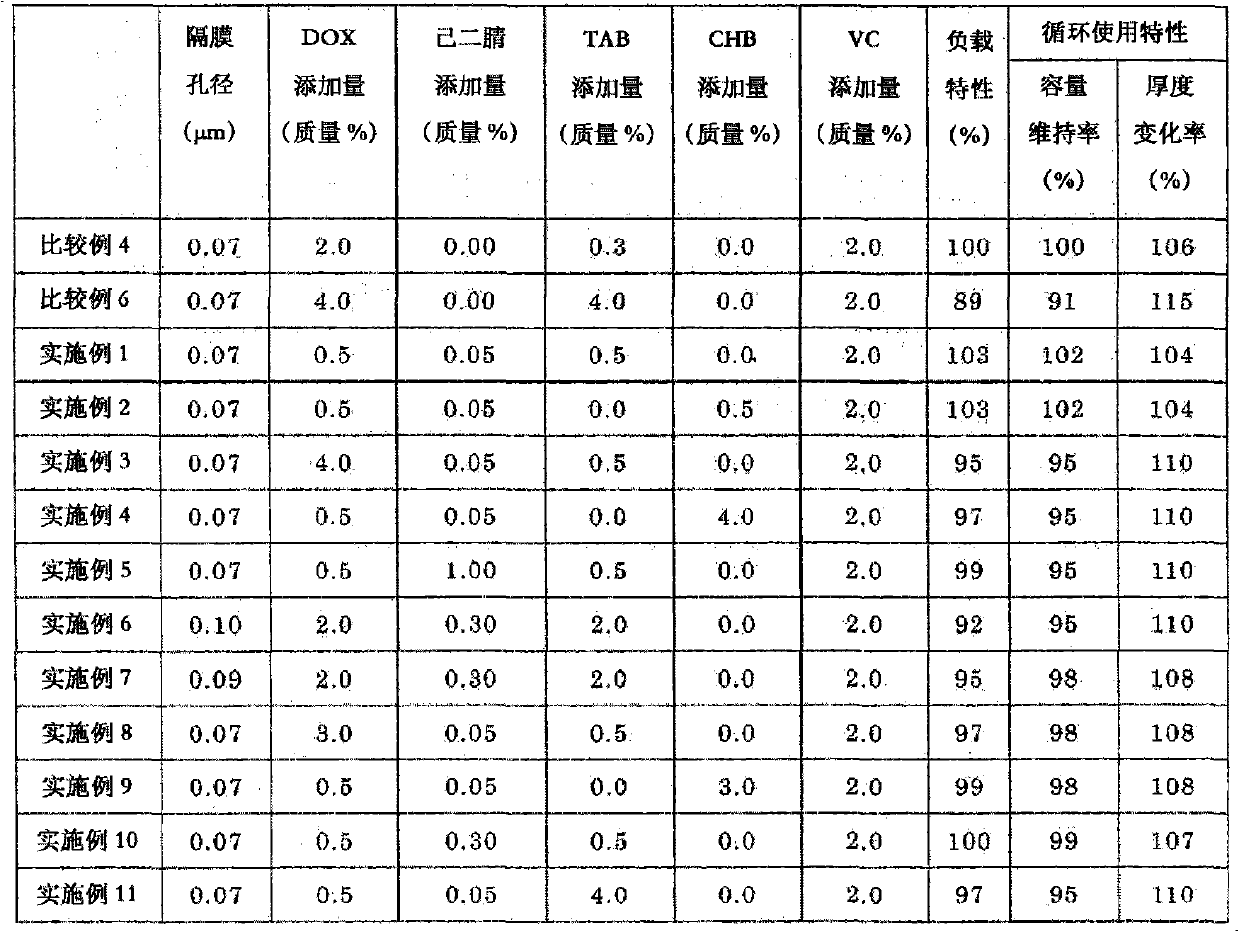
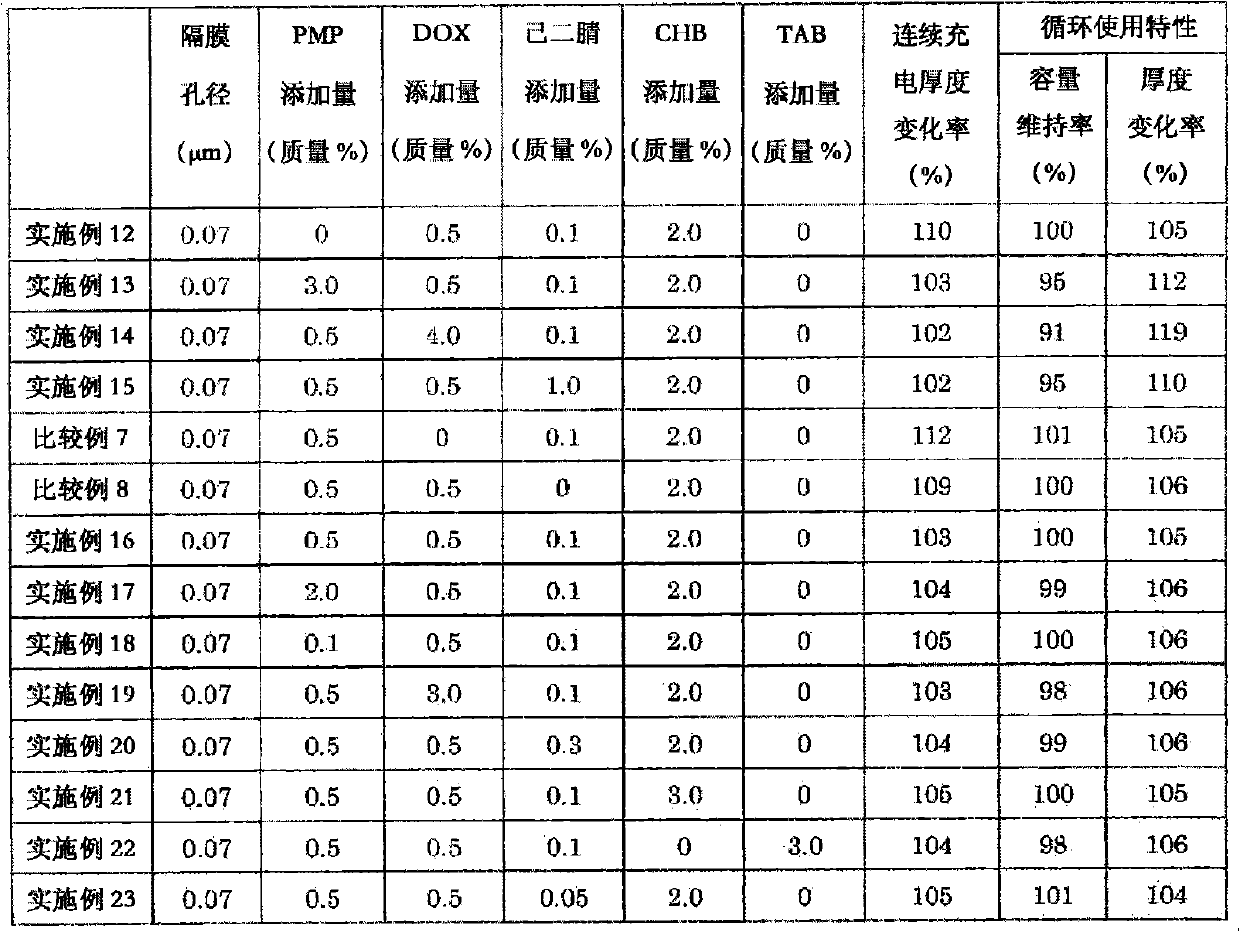
The object of the present invention is to provide a non-aqueous electrolyte secondary cell that excels in safety against overcharging and shows only a small increase in thickness during continuous charge. This object can be achieved by adopting the following configuration: a separator is used that is made of a microporous polyolefin membrane having an average pore diameter of 0.07 to 0.09 [mu]m; a non-aqueous electrolyte contains 0.5 to 3.0 mass % of 1,3-dioxane, 0.05 to 0.3 mass % of adiponitrile, and 0.5 to 3.0 mass % of cyclohexylbenzene and / or tert-amylbenzene relative to the mass of the non-aqueous electrolyte; and preferably the non-aqueous electrolyte further contains 0.5 to 5.0 mass % of a vinylene carbonate and 0.1 to 2.0 mass % of 2-propyn-1-yl 2-(methylsulfonyloxy) propionate.
Owner:SANYO ELECTRIC CO LTD
Process for making nitriles
ActiveUS20130211121A1Reduce nickel contentOrganic compound preparationChemical recyclingCatalyst degradationHydrogen cyanide
Owner:INV NYLON CHEM AMERICAS LLC
Solid phosphorus acid catalyst for producing adiponitrile and preparation method of solid phosphorus acid catalyst
ActiveCN106140234AImprove dehydration ratePhysical/chemical process catalystsPreparation by ammonia-carboxylic acid reactionCentrifugationSulfate
The invention discloses a solid phosphorus acid catalyst for producing adiponitrile and a preparation method of the solid phosphorus acid catalyst. The preparation method of the solid phosphorus acid catalyst comprises the steps as follows: diatomite is impregnated in a phosphoric acid and sulfate mixed solution, and a carrier is separated through centrifugation after impregnation balance, and then drying and roasting are performed, wherein the concentration of phosphoric acid is 55wt%-95wt%, the concentration of sulfate is 15wt%-25wt%, the roasting temperature is 300-700 DEG C, sediment is cooled and then subjected to preforming, particles between 10 meshes and 50 meshes are screened, and the solid phosphorus acid catalyst is formed. One novel green catalyst is developed for production of adiponitrile with an adipic acid liquid phase method, and the catalysis effect is improved while various defects caused by traditional catalysts are overcome.
Owner:PETROCHINA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com