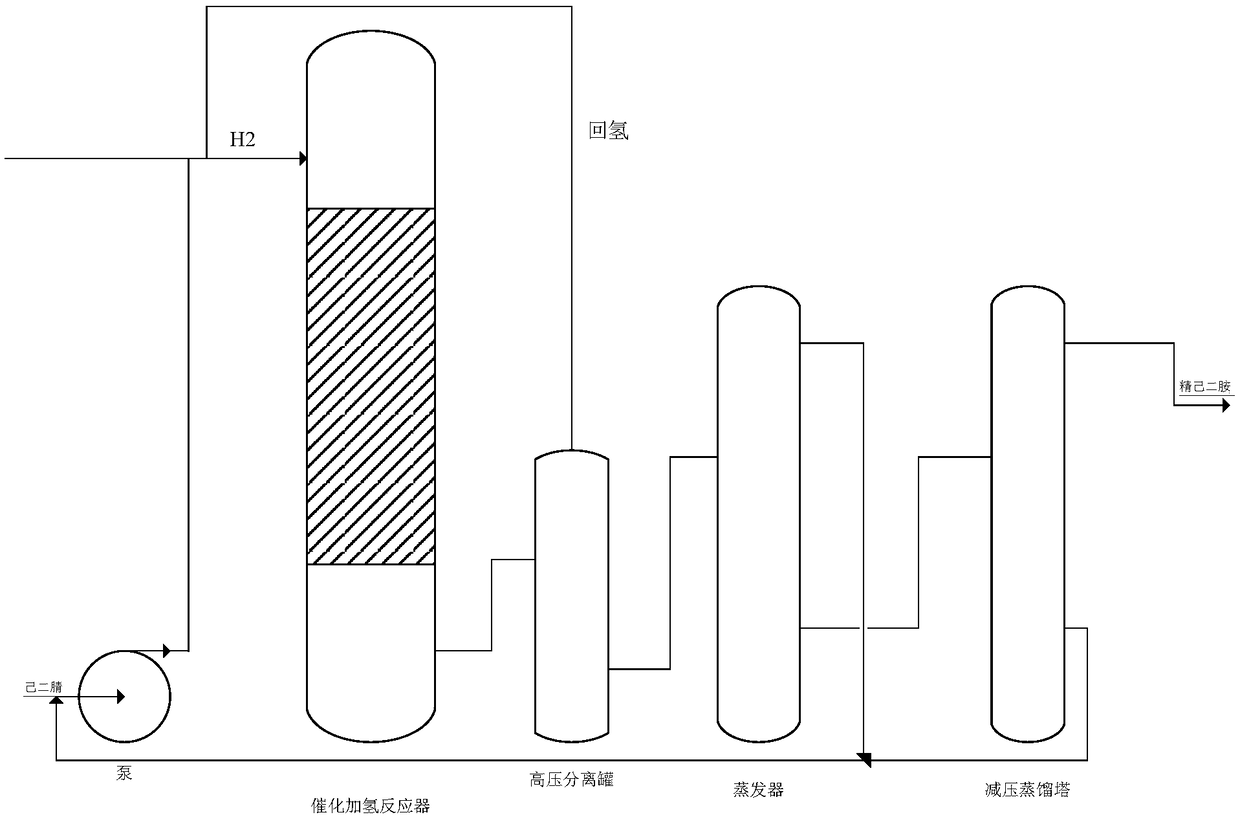
Method for preparing hexamethylene diamine by direct hydrogenation of adiponitrile under alkali-free conditions
A technology of adiponitrile and hexamethylene diamine, applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problems of corrosion, environmental pollution, poor mechanical properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 NiLa / MgO-Al 2 o 3 Catalyst preparation
[0016] According to the metering ratio of the catalytically active metal Ni to the oxide carrier, a certain mass of nickel nitrate and aluminum nitrate, and relative metering ratios of magnesium nitrate and lanthanum nitrate are dissolved in deionized water, and the concentration of aluminum ions is about 1mol / L. mixture. Then take anhydrous sodium carbonate with a stoichiometric ratio of 1.1 times relative to the metal ion and dissolve it in a certain amount of deionized water to prepare a sodium carbonate solution with pH=11. Then the above two clear solutions were dripped into a 250mL three-necked flask filled with 5-10mL deionized water at a rate of 1mL / min, that is, 3s / drop. During the dropwise addition, an oil bath was used to maintain a constant temperature of 45°C and vigorously stirred, at which point a light green precipitate gradually appeared. After the dropwise addition, the pH of the test mixture was 6...
Embodiment 2
[0017] Embodiment 2NiCe / BaO-Al 2 o 3 Catalyst preparation
[0018] According to the metering ratio of catalyst active metal Ni to the oxide carrier, a certain mass of nickel nitrate and aluminum nitrate, and relative metering ratio of barium nitrate and cerium nitrate are dissolved in deionized water, and the concentration of aluminum ions is about 1mol / L. mixture. Then take anhydrous sodium carbonate with a stoichiometric ratio of 1.1 times relative to the metal ion and dissolve it in a certain amount of deionized water to prepare a sodium carbonate solution with pH=11. Then the above two clear solutions were dripped into a 250mL three-necked flask filled with 5-10mL deionized water at a rate of 1mL / min, that is, 3s / drop. During the dropwise addition, an oil bath was used to maintain a constant temperature of 45°C and vigorously stirred, at which point a light green precipitate gradually appeared. After the dropwise addition, the pH of the test mixture was 6-7, and the pH...
Embodiment 3
[0019] Embodiment 3NiZr / SrO-Al 2 o 3 Catalyst preparation
[0020] According to the metering ratio of the catalytically active metal Ni to the oxide carrier, a certain mass of nickel nitrate and aluminum nitrate, and relative metering ratios of zirconium nitrate and strontium nitrate are dissolved in deionized water, and the concentration of aluminum ions is about 1mol / L. mixture. Then take anhydrous sodium carbonate with a stoichiometric ratio of 1.1 times relative to the metal ion and dissolve it in a certain amount of deionized water to prepare a sodium carbonate solution with pH=11. Then the above two clear solutions were dripped into a 250mL three-necked flask filled with 5-10mL deionized water at a rate of 1mL / min, that is, 3s / drop. During the dropwise addition, an oil bath was used to maintain a constant temperature of 45°C and vigorously stirred, at which point a light green precipitate gradually appeared. After the dropwise addition, the pH of the test mixture was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com