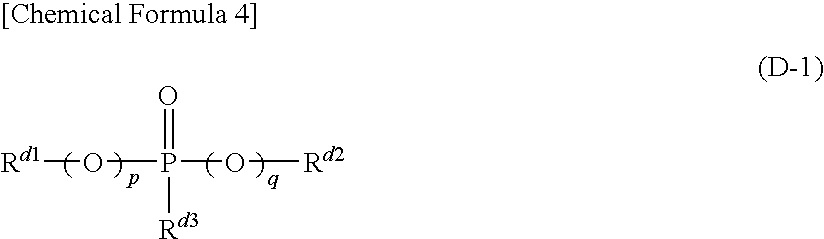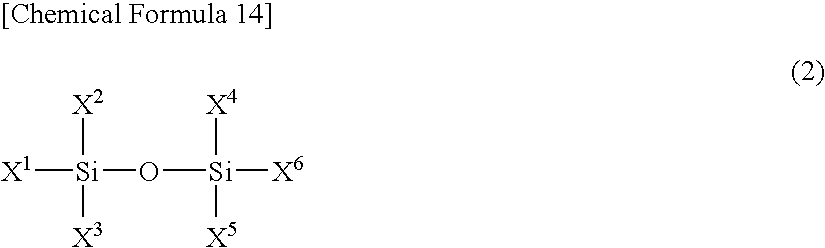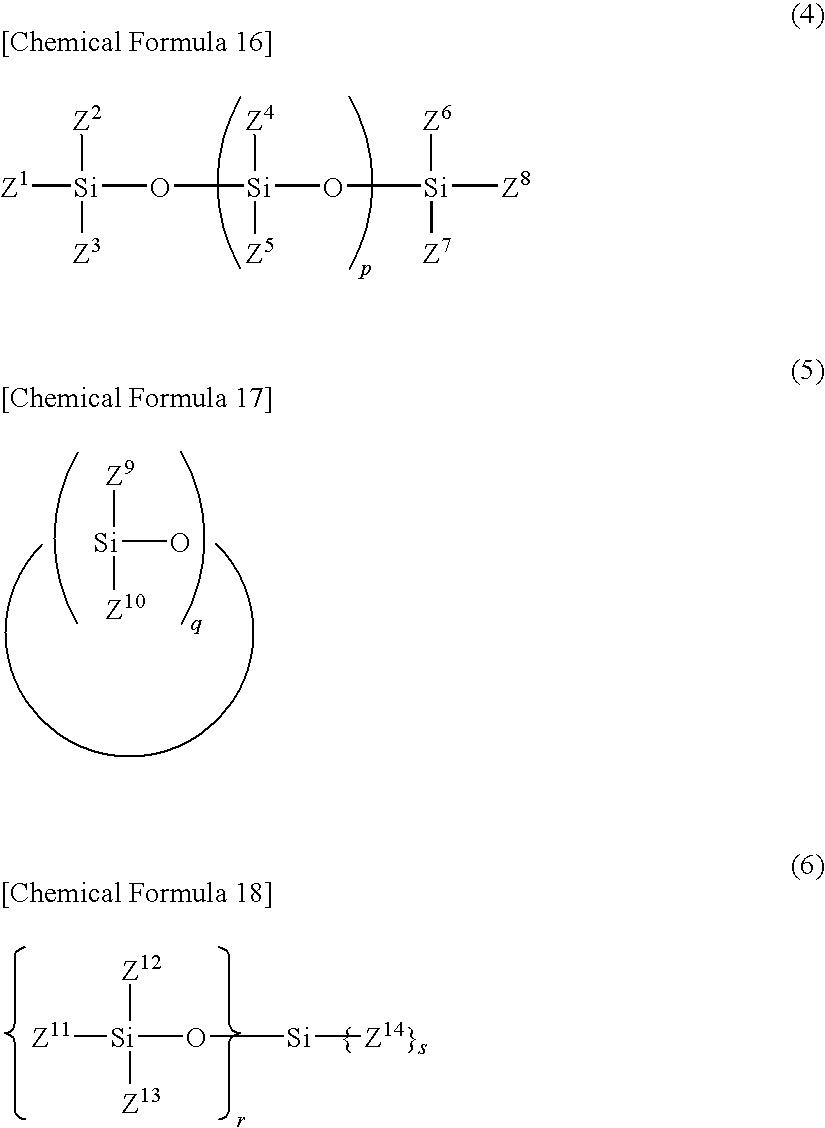Patents
Literature
5762results about "Organic electrolytes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
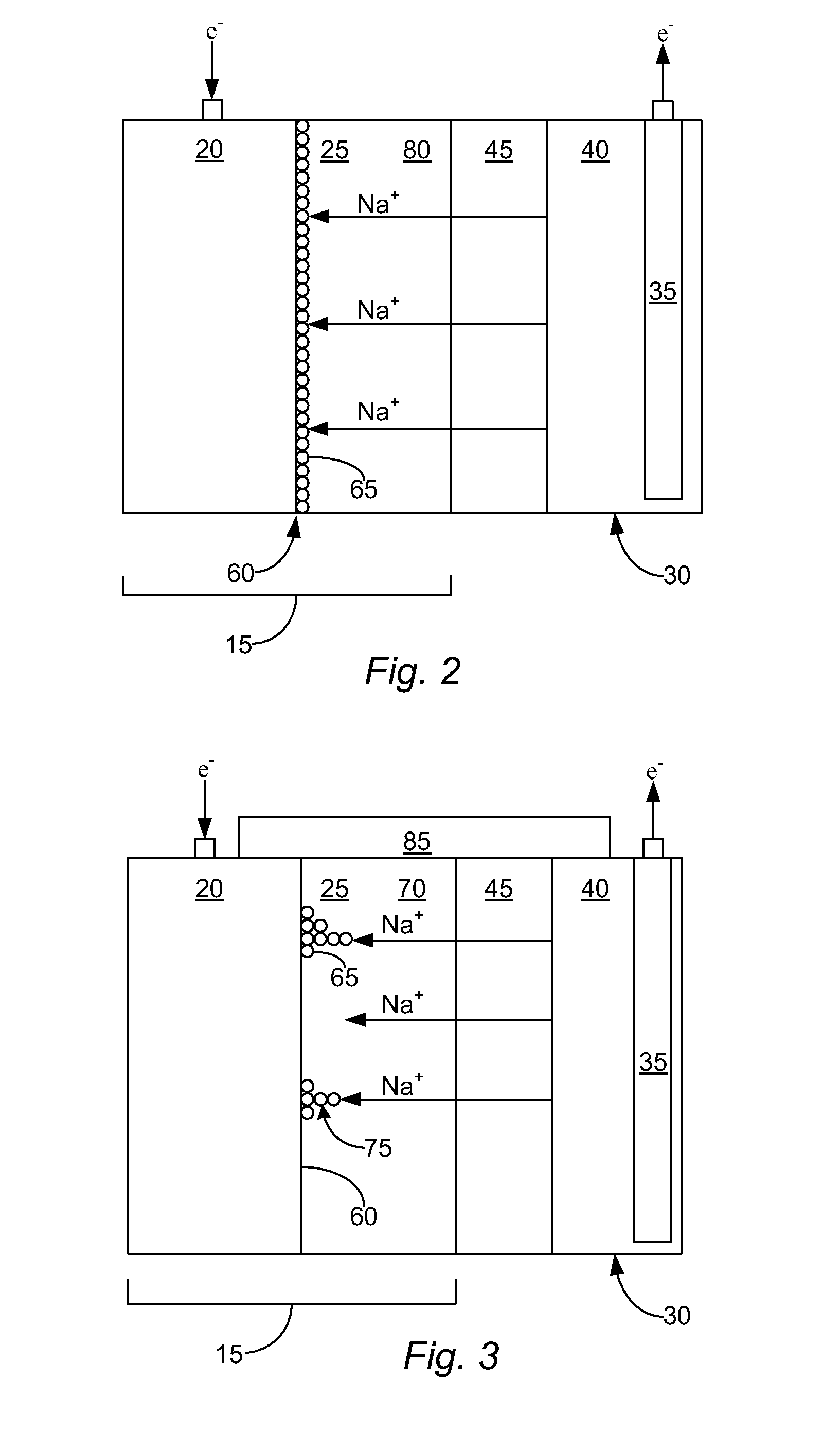
Li/air non-aqueous batteries
ActiveUS20070117007A1Improve battery performanceLarge capacityFuel and primary cellsFuel and secondary cellsLithiumOxygen
Non-aqueous alkali metal (e.g., Li) / oxygen battery cells constructed with a protected anode that minimizes anode degradation and maximizes cathode performance by enabling the use of cathode performance enhancing solvents in the catholyte have negligible self-discharge and high deliverable capacity. In particular, protected lithium-oxygen batteries with non-aqueous catholytes have this improved performance.
Owner:POLYPLUS BATTERY CO INC
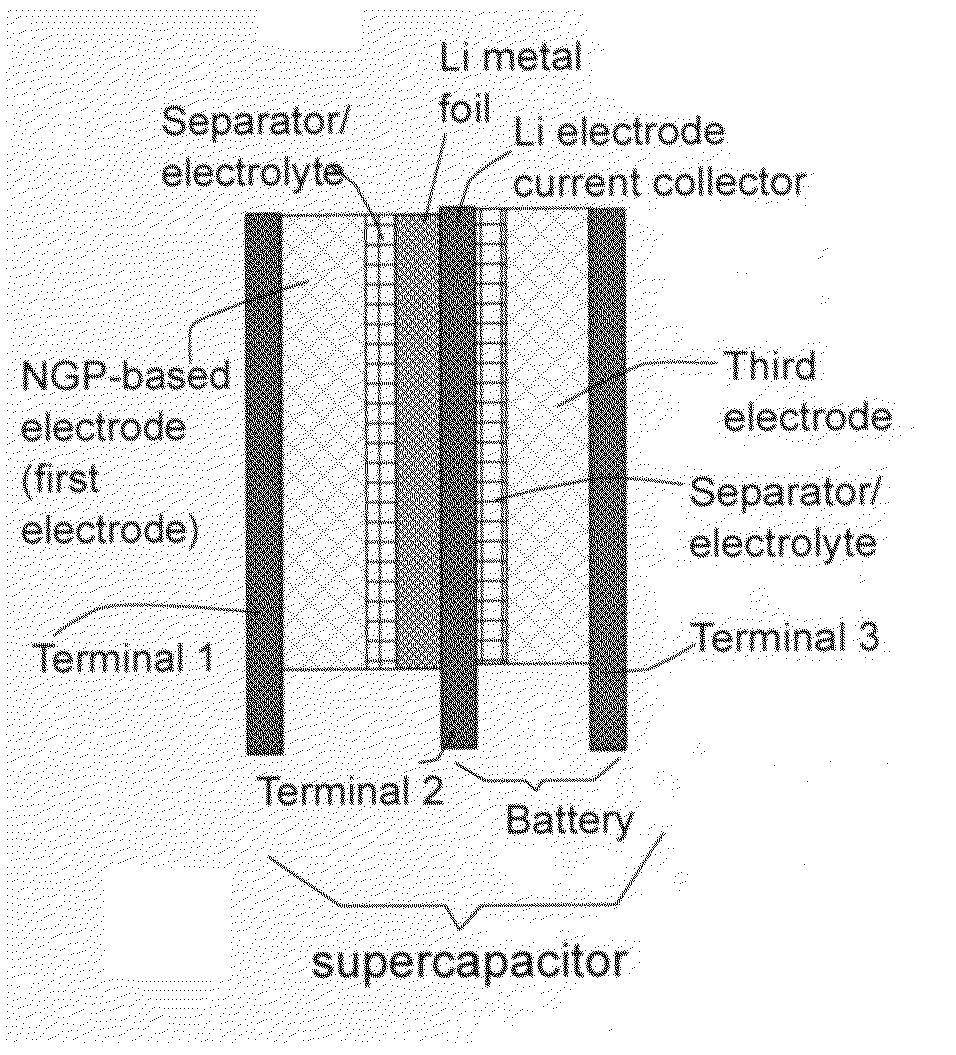
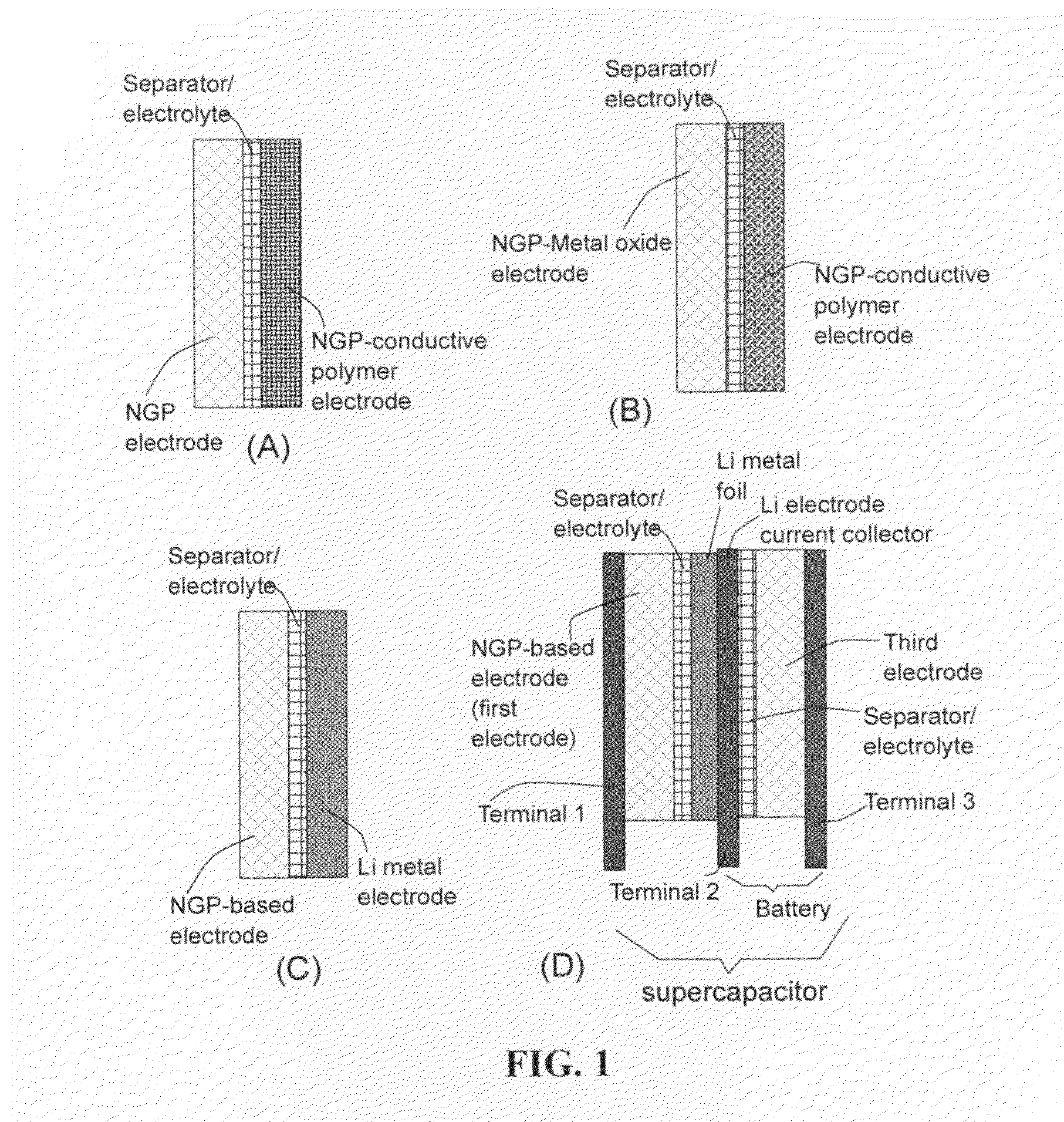
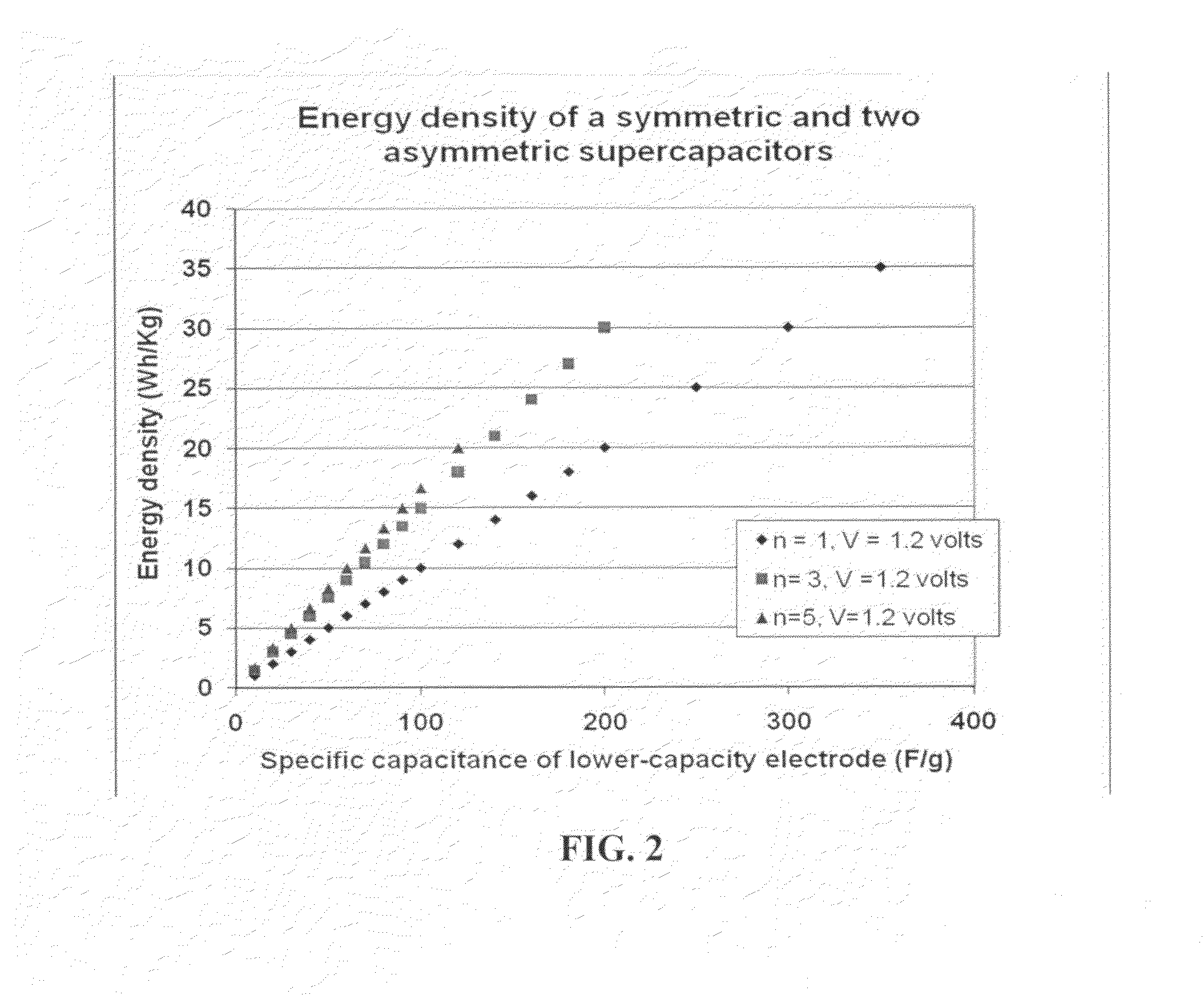
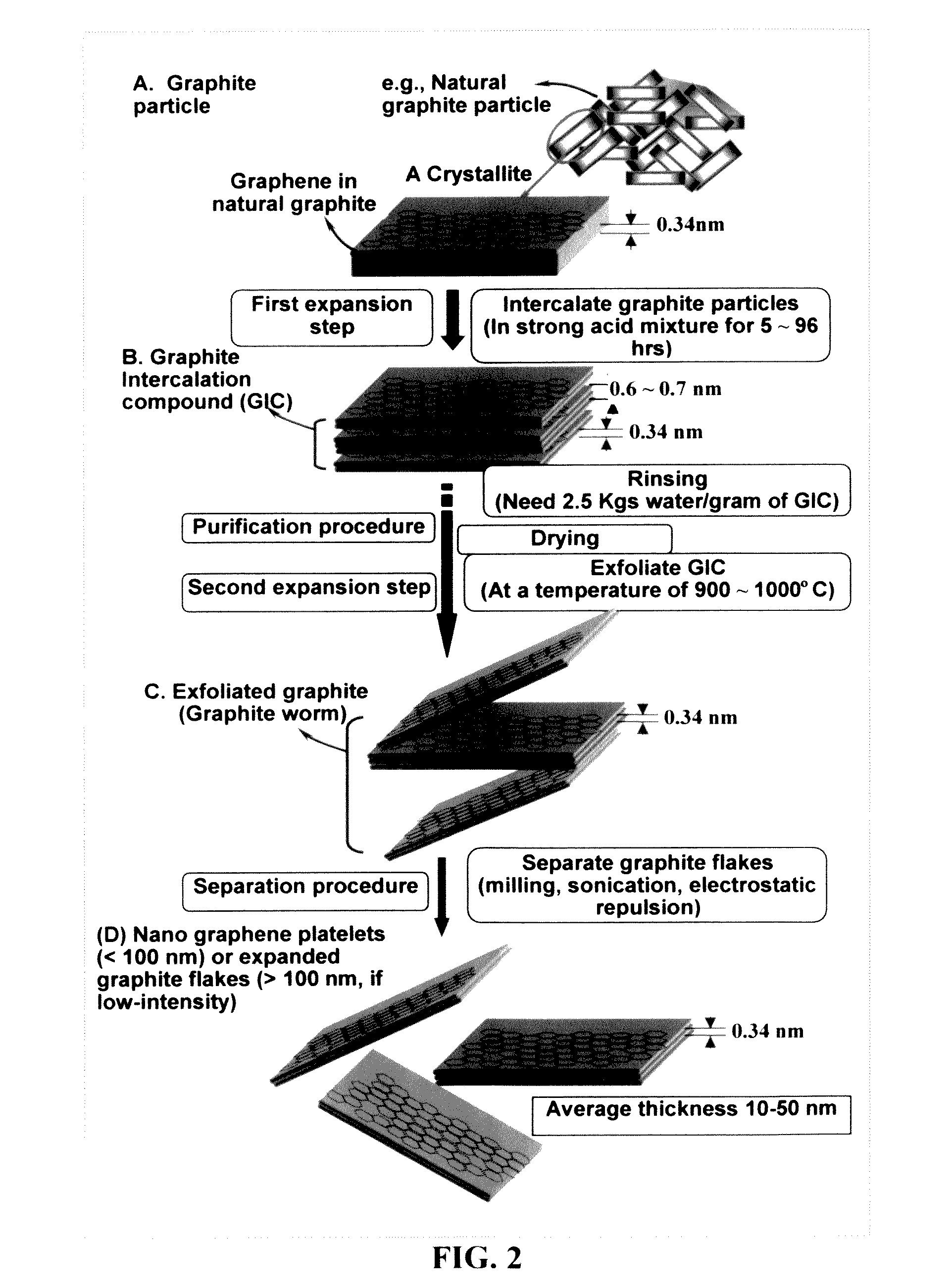
Flexible asymmetric electrochemical cells using nano graphene platelet as an electrode material
InactiveUS20110183180A1Excellent specific capacitanceLarge specific surface areaElectrochemical generatorsHybrid capacitor separatorsPlateletGraphene
A flexible, asymmetric electrochemical cell comprising: (A) A sheet of graphene paper as first electrode comprising nano graphene platelets having a platelet thickness less than 1 nm, wherein the first electrode has electrolyte-accessible pores; (B) A thin-film or paper-like first separator and electrolyte; and (C) A thin-film or paper-like second electrode which is different in composition than the first electrode; wherein the separator is sandwiched between the first and second electrode to form a flexible laminate configuration. The asymmetric supercapacitor cells with different NGP-based electrodes exhibit an exceptionally high capacitance, specific energy, and stable and long cycle life.
Owner:NANOTEK INSTR GRP LLC
Nonaqueous electrolyte for secondary battery and nonaqueous-electrolyte secondary battery employing the same
ActiveUS20100119956A1Reduce impactLower performance requirementsCell electrodesOrganic electrolyte cellsHigh current densityDifluorophosphate
An object is to provide a nonaqueous electrolyte and a nonaqueous-electrolyte secondary battery which have excellent discharge load characteristics and are excellent in high-temperature storability, cycle characteristics, high capacity, continuous-charge characteristics, storability, gas evolution inhibition during continuous charge, high-current-density charge / discharge characteristics, discharge load characteristics, etc. The object has been accomplished with a nonaqueous electrolyte which comprises: a monofluorophosphate and / or a difluorophosphate; and further a compound having a specific chemical structure or specific properties.
Owner:MU IONIC SOLUTIONS CORP +1
Nonaqueous electrolyte
ActiveUS20050221188A1Excellent characteristicsNon-aqueous electrolyte accumulatorsElectrode carriers/collectorsLithiumMaterials science
A nonaqueous electrolyte battery includes a positive electrode containing an active material, a negative electrode, and a nonaqueous electrolyte, the negative electrode including a current collector and a negative electrode active material supported by the current collector, the negative electrode active material having a Li insertion potential not lower than 0.2V (vs. Li / Li+) and an average primary particle diameter not larger than 1 μm, and a specific surface area of the negative electrode, excluding a weight of the current collector, as determined by the BET method falls within a range of 3 to 50 m2 / g.
Owner:KK TOSHIBA
Separator for nonaqueous electrolyte batteries, nonaqueous electrolyte battery using it, and method for manufacturing separator for nonaqueous electrolyte batteries
InactiveUS6511774B1High tear strengthEnhanced penetration strengthNon-aqueous electrolyte accumulatorsCell seperators/membranes/diaphragms/spacersHigh energyEngineering
The present invention provides a separator for non-aqueous electrolyte batteries which neither breaks nor slips off at the time of fabrication of battery, gives excellent battery fabricability, causes no internal short-circuit caused by contact between electrodes even if the electrodes are externally short-circuited, can inhibit ignition of battery and produces high energy density and excellent cycle life, and further provides a non-aqueous electrolyte battery using the separator and a method for manufacturing the separator. That is, the present invention relates to a separator for non-aqueous electrolyte batteries which comprises a porous base containing at least one member selected from a porous film, a woven fabric or nonwoven fabric containing an organic fiber and a paper and an organometallic compound applied to the porous base; a method for the manufacture of the separator for non-aqueous electrolyte batteries which comprises allowing said porous base to contact with a solution of organometallic compound by impregnation, coating or spraying, followed by drying or curing with heating to apply the organometallic compound to the porous base; and a non-aqueous electrolyte battery using the separator.
Owner:MITSUBISHI PAPER MILLS LTD
Novel composite cathodes, electrochemical cells comprising novel composite cathodes, and processes for fabricating same
The present invention pertains to composite cathodes suitable for use in an electrochemical cell, said cathodes comprising: (a) an electroactive sulfur-containing cathode material, wherein said electroactive sulfur-containing cathode material, in its oxidized state, comprises a polysulfide moiety of the formula —Sm—, wherein m is an integer equal to or greater than 3; and, (b) an electroactive transition metal chalcogenide composition, which encapsulates said electroactive sulfur-containing cathode material, and which retards the transport of anionic reduction products of said electroactive sulfur-containing cathode material, said electroactive transition metal chalcogenide composition comprising an electroactive transition metal chalcogenide having the formula MjYk(OR)l wherein: M is a transition metal; Y is the same or different at each occurrence and is oxygen, sulfur, or selenium; R is an organic group and is the same or different at each occurrence; j is an integer ranging from 1 to 12; k is a number ranging from 0 to 72; and l is a number ranging from 0 to 72; with the proviso that k and l cannot both be 0. The present invention also pertains to methods of making such composite cathodes, cells comprising such composite cathodes, and methods of making such cells.
Owner:SION POWER CORP
Zinc Ion-Exchanging Energy Storage Device
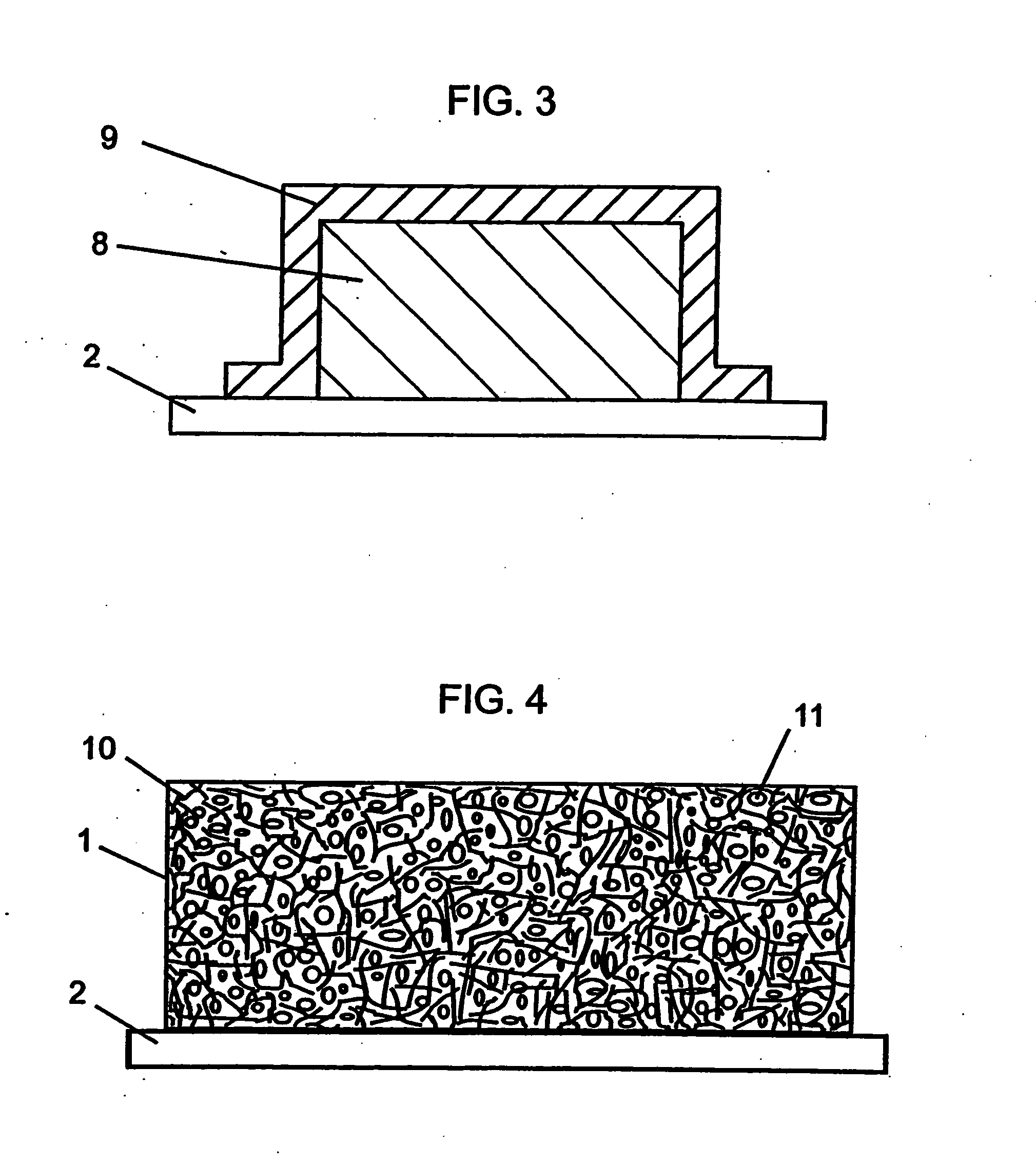
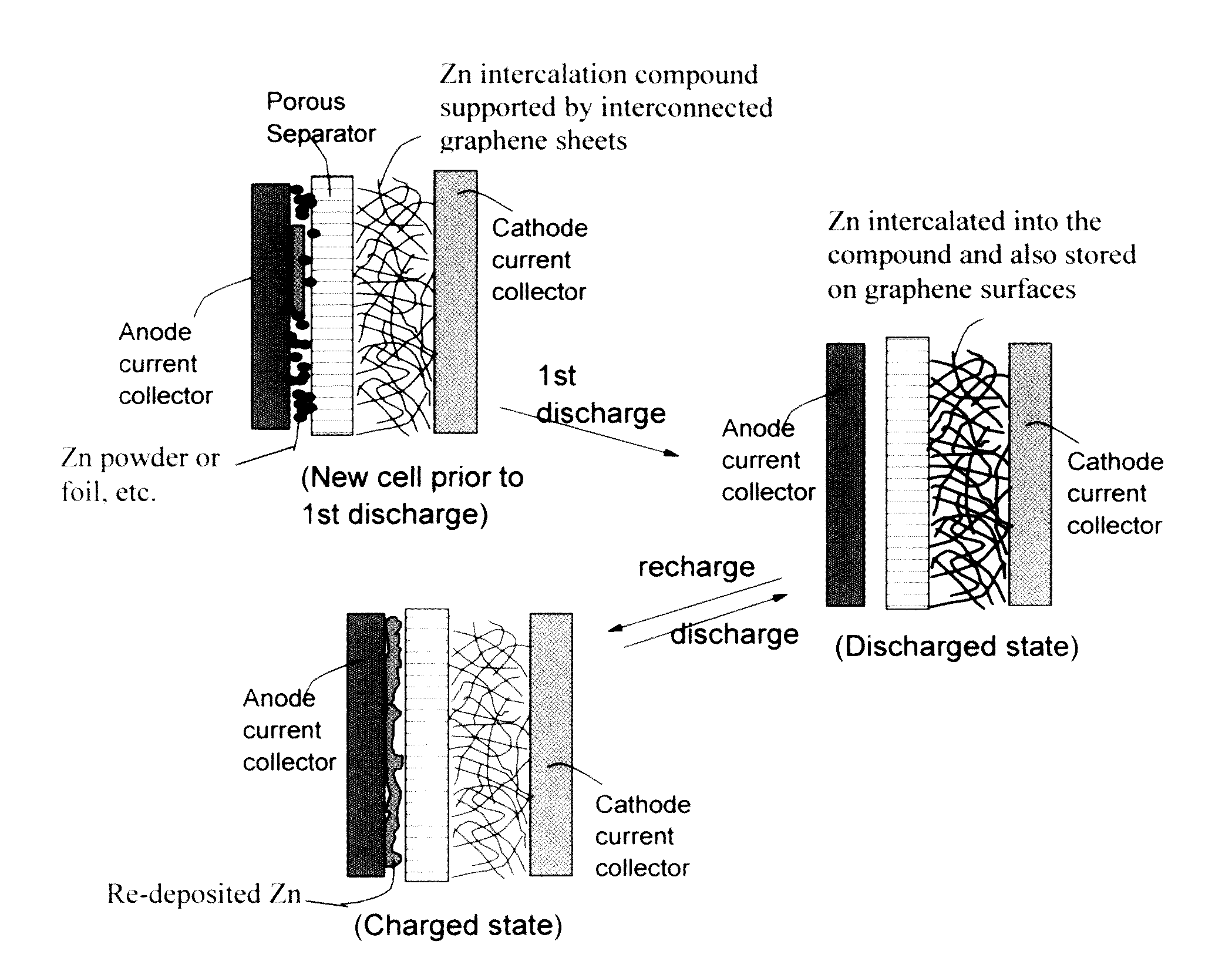
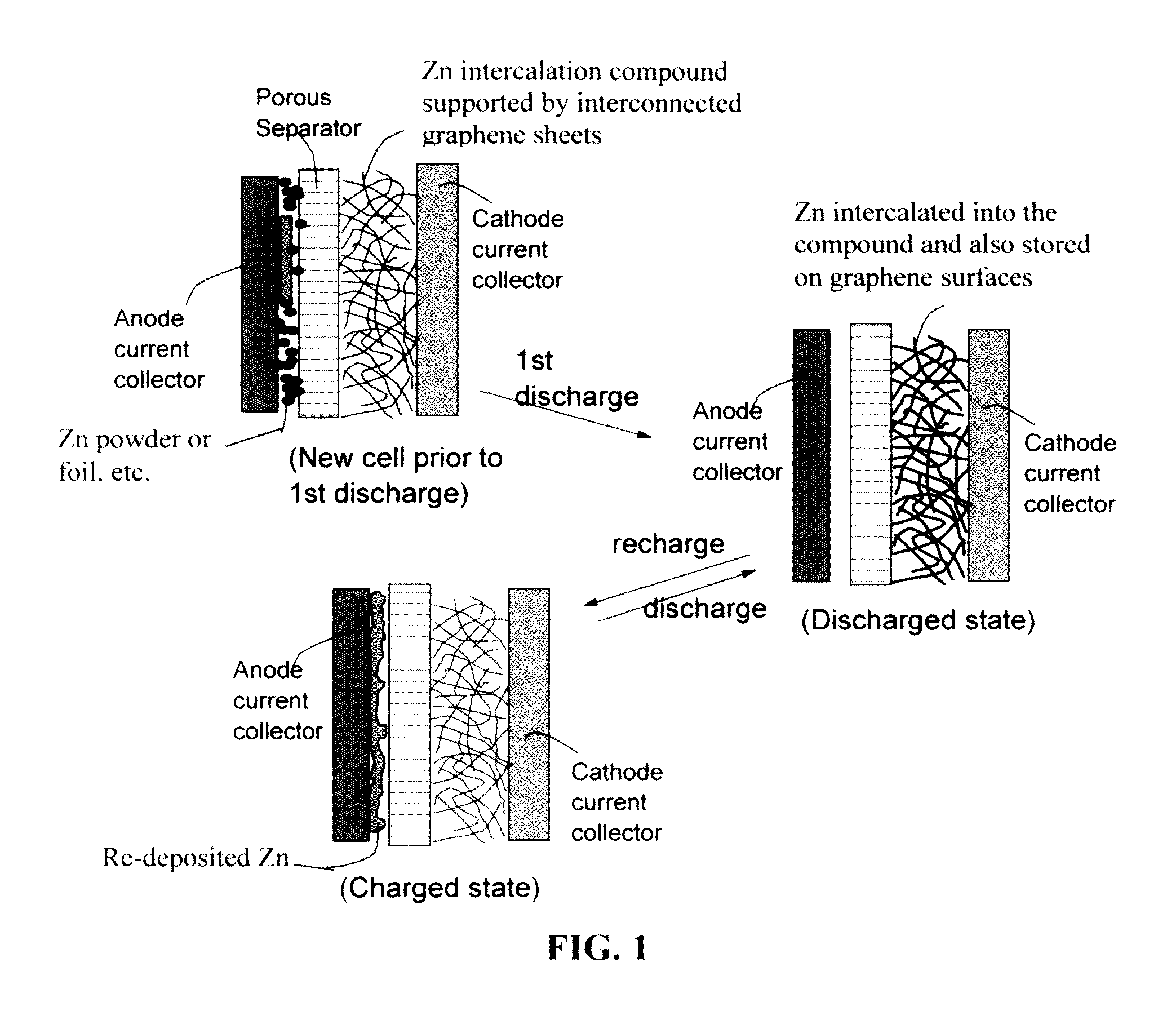
ActiveUS20160301096A1Quick releaseRapid depositionHybrid capacitor electrolytesAlkaline accumulatorsChemical treatmentZinc metal
A zinc ion-exchanging battery device comprising: (A) a cathode comprising two cathode active materials (a zinc ion intercalation compound and a surface-mediating material); (B) an anode containing zinc metal or zinc alloy; (C) a porous separator disposed between the cathode and the anode; and (D) an electrolyte containing zinc ions that are exchanged between the cathode and the anode during battery charge / discharge. The zinc ion intercalation compound is selected from chemically treated carbon or graphite material having an expanded inter-graphene spacing d002 of at least 0.5 nm, or an oxide, carbide, dichalcogenide, trichalcogenide, sulfide, selenide, or telluride of niobium, zirconium, molybdenum, hafnium, tantalum, tungsten, titanium, vanadium, chromium, cobalt, manganese, iron, nickel, or a combination thereof. The surface-mediating material contains exfoliated graphite or multiple single-layer sheets or multi-layer platelets of a graphene material.
Owner:GLOBAL GRAPHENE GRP INC
Redox shuttle for rechargeable lithium-ion cell
InactiveUS20050221196A1Excellent repeated overcharge stabilityNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsLithiumAlkoxy group
A redox chemical shuttle comprising an aromatic compound substituted with at least one tertiary carbon organic group and at least one alkoxy group (for example, 2,5-di-tert-butyl-1,4-dimethoxybenzene) provides repeated overcharge protection in rechargeable lithium-ion cells.
Owner:3M INNOVATIVE PROPERTIES CO
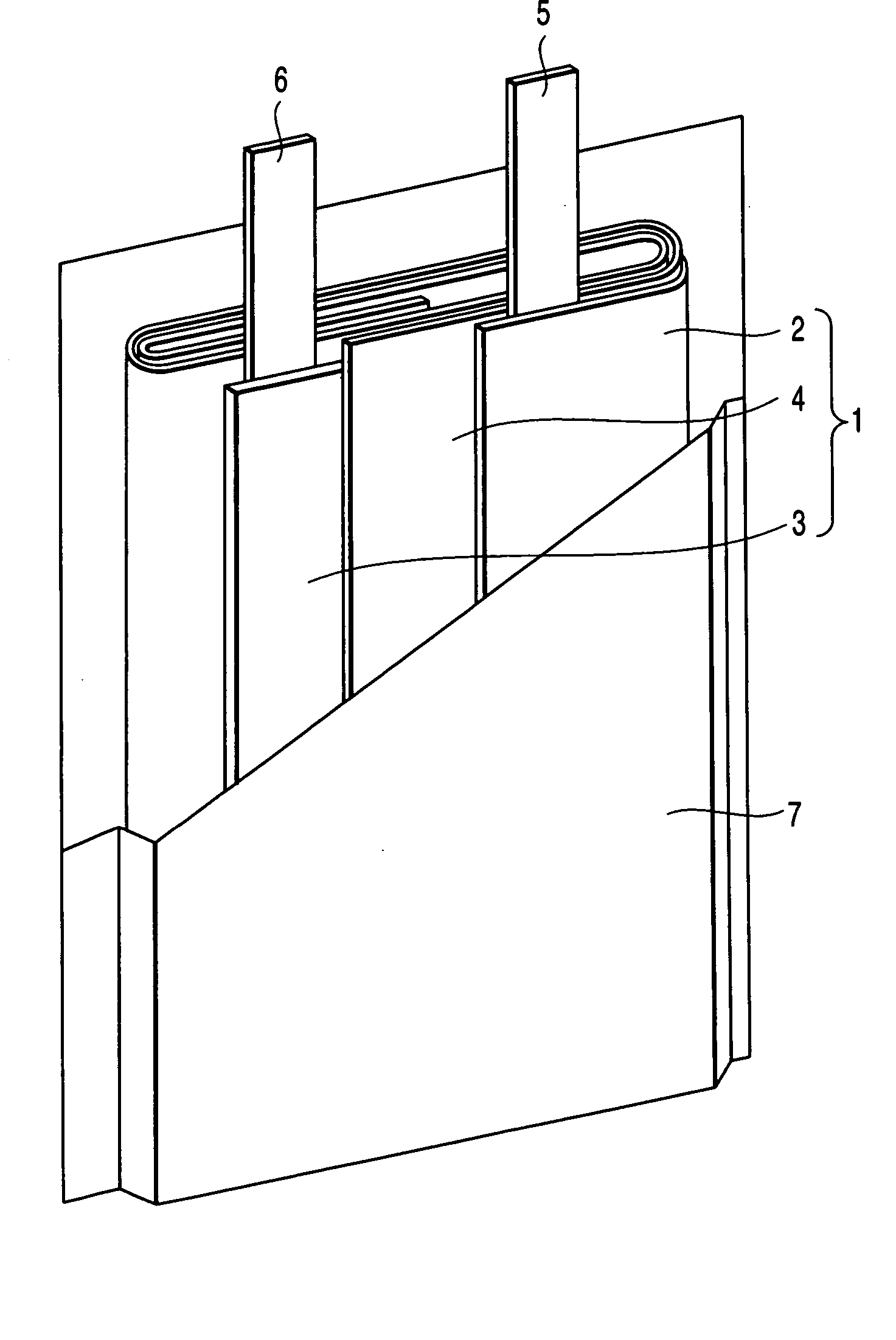
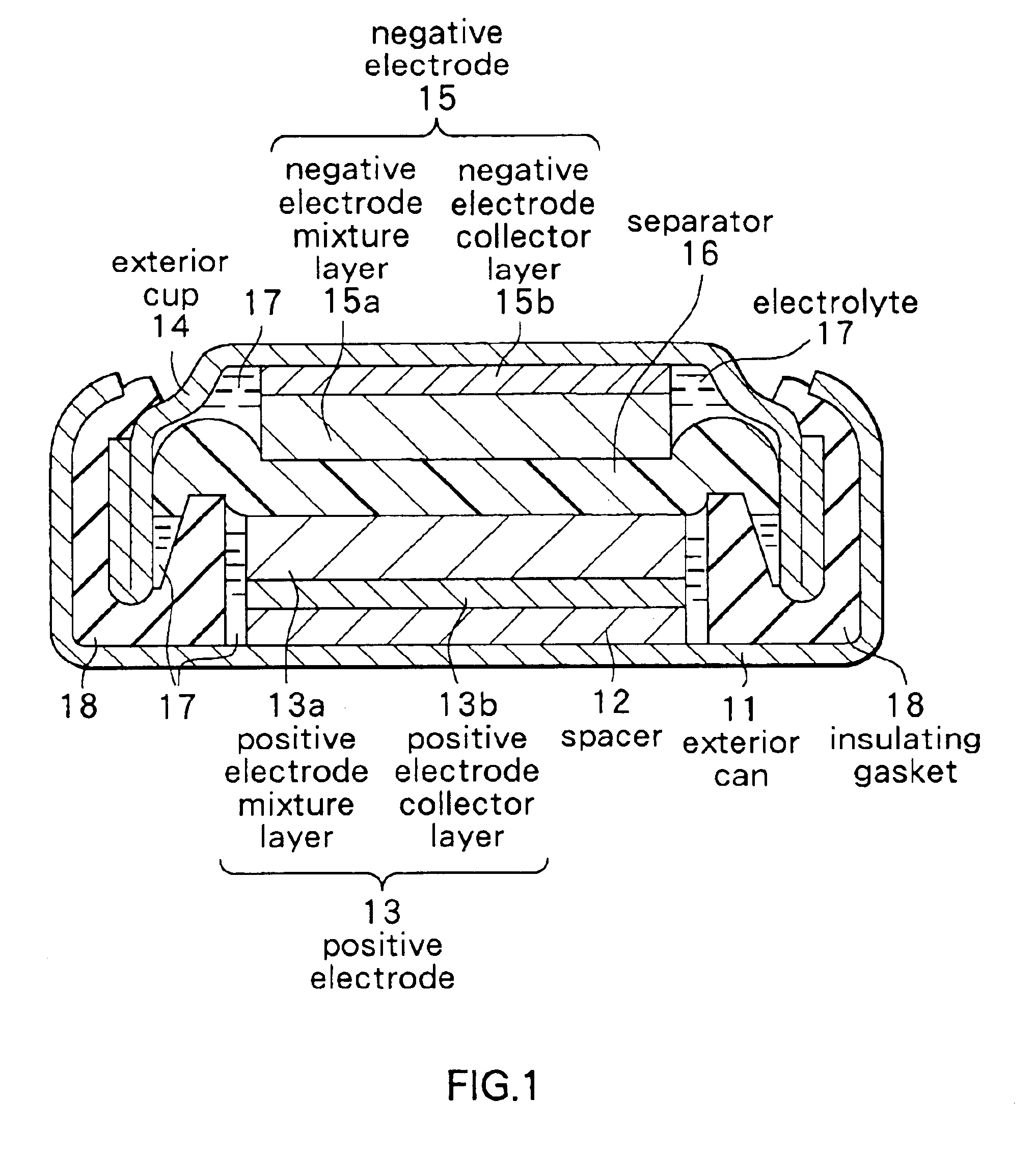
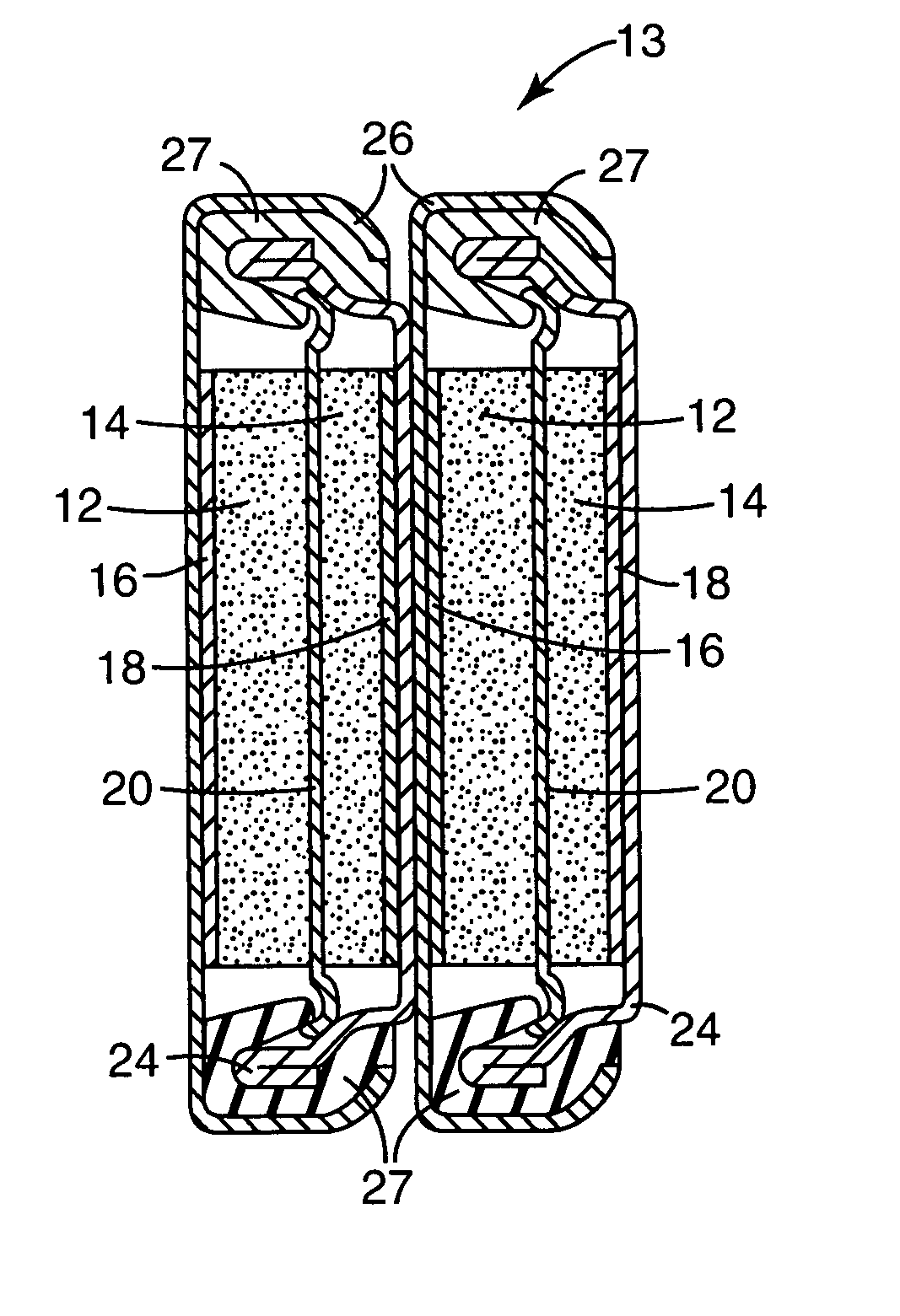
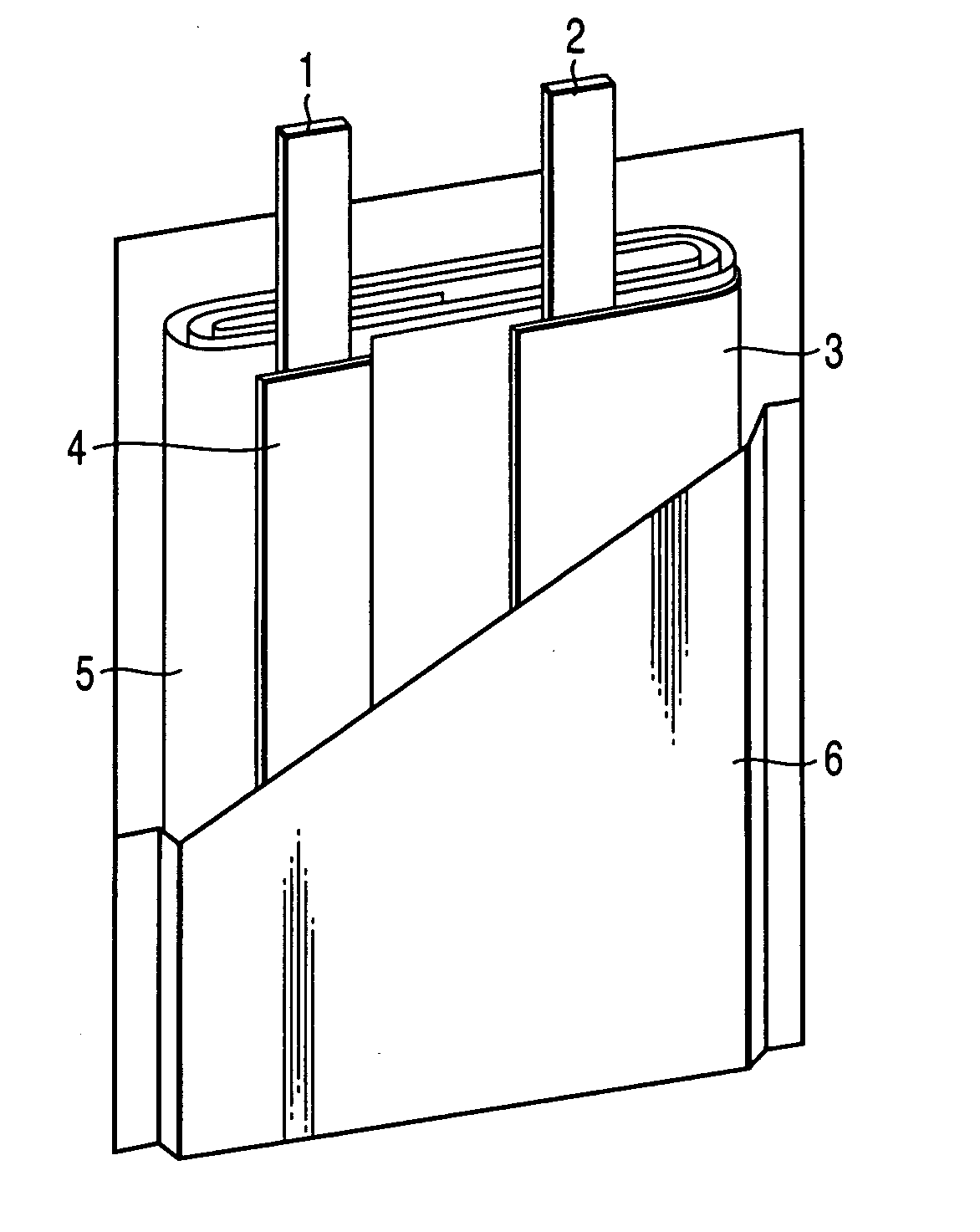
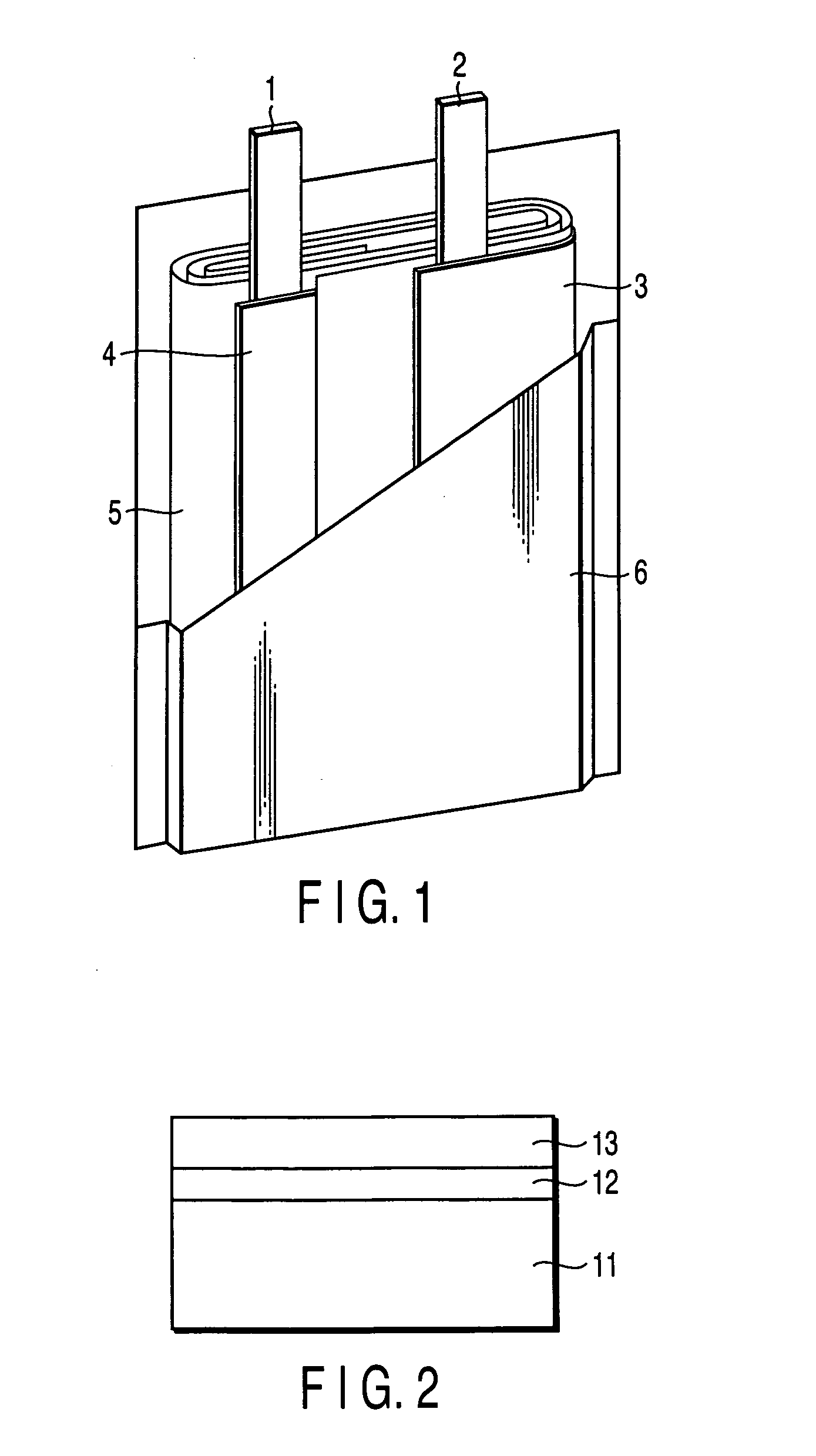
Secondary battery
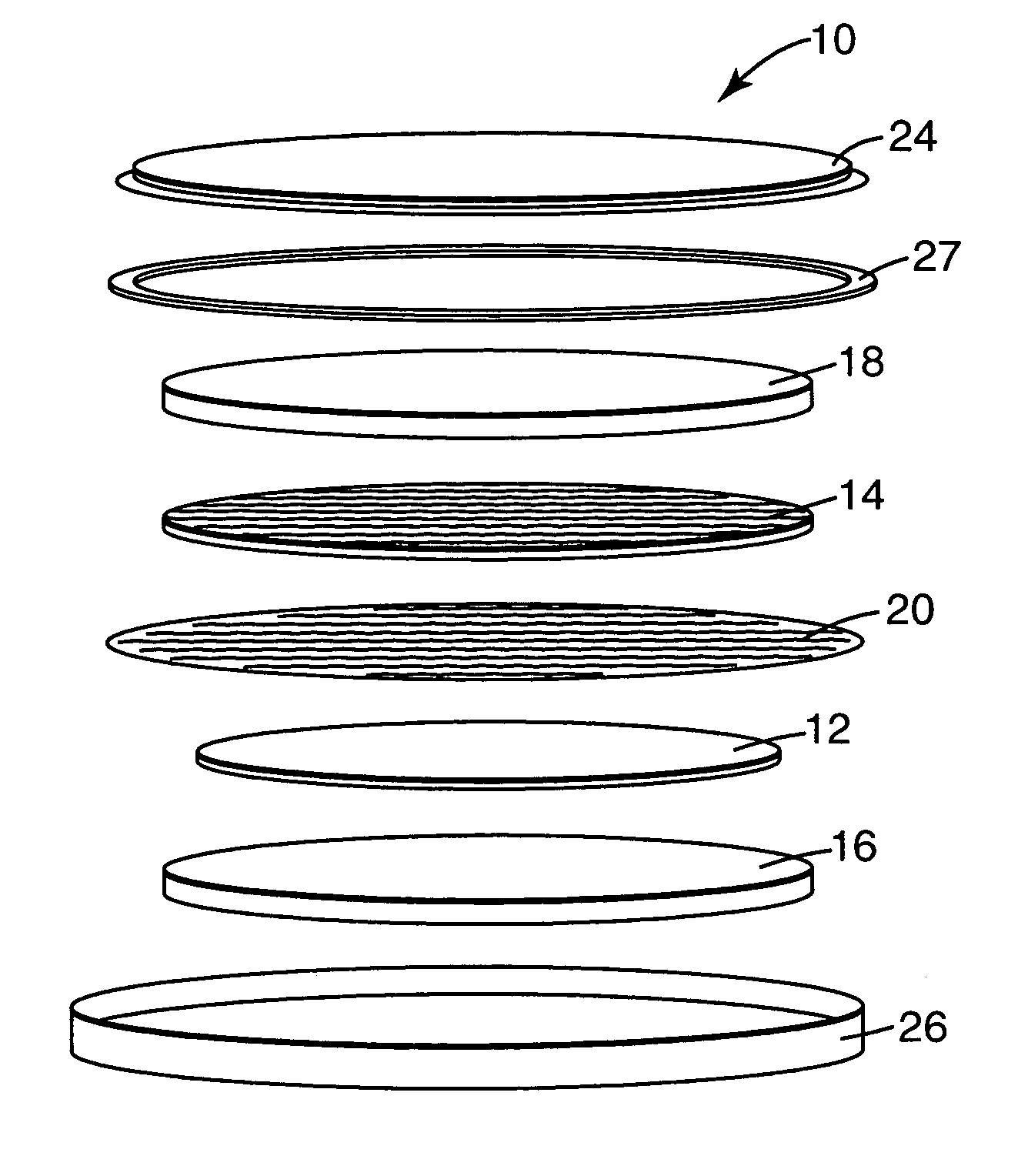
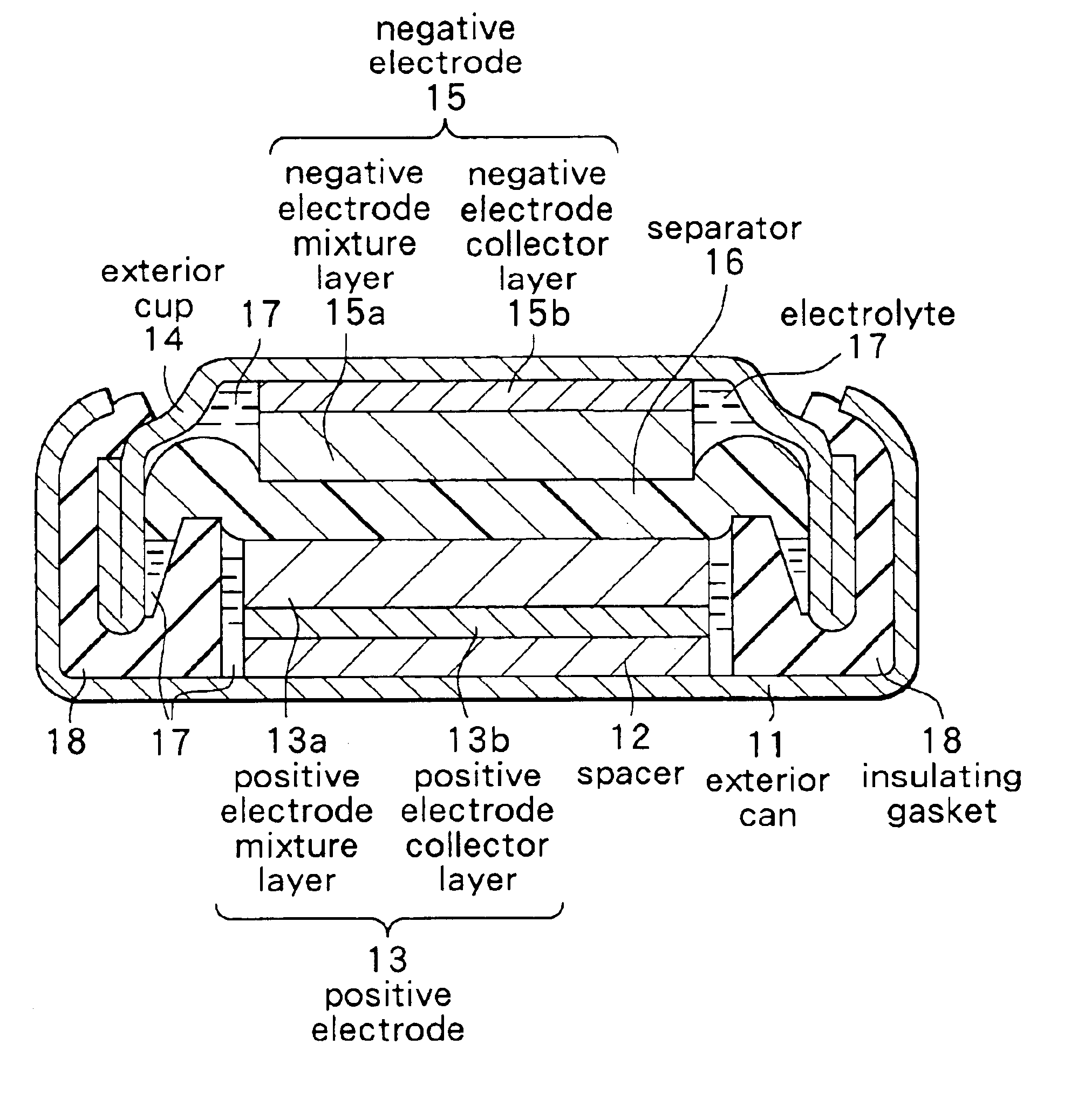
Provided is a secondary battery in which high energy density can be obtained and charging / discharging cycle characteristic can be improved. A positive electrode (13) and a negative electrode (15) are stacked with a separator (16) interposed therebetween, and are enclosed inside an exterior can (11) to which an electrolyte is injected. The negative electrode (15) contains a negative electrode material capable of occluding / releasing lithium in an ionic state. Thereby, lithium metal precipitates in the negative electrode (15) in a state where the open circuit voltage is lower than the overcharge voltage. In other words, lithium is occluded in an ionic state in a negative electrode material capable of occluding / releasing lithium in the beginning of charging, and then lithium metal precipitates on the surface of the negative electrode material thereafter during charging. The amount of precipitation of lithium metal is preferable to be from 0.05 to 3.0 times, both inclusive, the ability of charging capacity of the negative electrode material capable of occluding / releasing lithium. Thereby, a high energy density and an excellent cycle characteristic can be obtained.
Owner:MURATA MFG CO LTD
Substituted phenothiazine redox shuttles for rechargeable lithium-ion cell
InactiveUS20060263697A1Non-aqueous electrolyte accumulatorsElectrode carriers/collectorsLithiumRedox
A rechargeable lithium-ion cell contains a positive electrode, negative electrode, charge-carrying electrolyte containing charge carrying medium and lithium salt, and an N-substituted or C-substituted phenothiazine compound dissolved in or dissolvable in the electrolyte. The substituted phenothiazine compound has an oxidation potential above the positive electrode recharged potential and serves as a cyclable redox chemical shuttle providing cell overcharge protection.
Owner:3M INNOVATIVE PROPERTIES CO
Lithium secondary battery
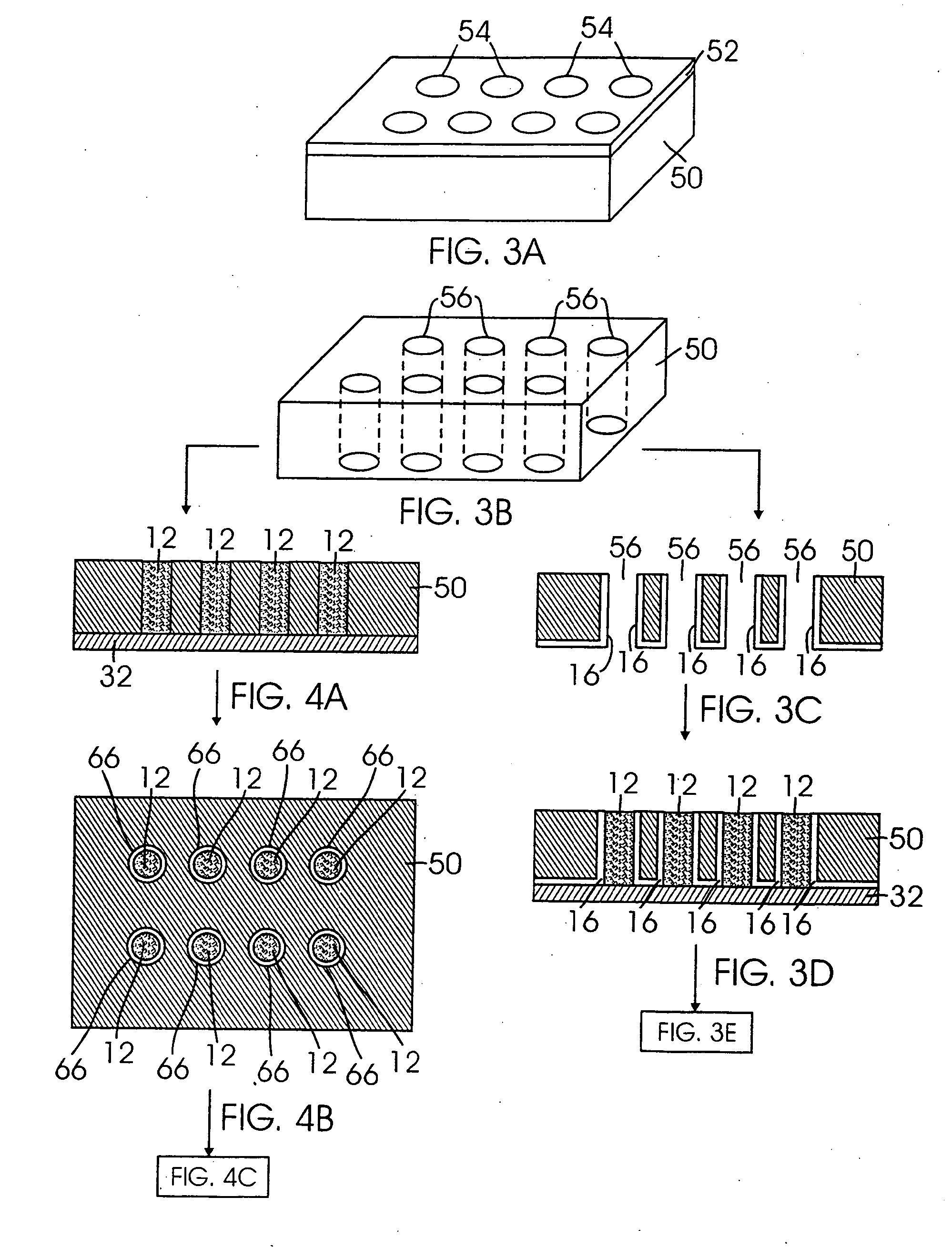
InactiveUS20050008939A1Improve featuresElectrochemical processing of electrodesFinal product manufactureLithiumPhosphate
A lithium secondary battery comprising a positive electrode, a negative electrode, and a nonaqueous electrolyte, wherein the positive electrode or the negative electrode is an electrode obtained by depositing a thin film of active material capable of lithium storage and release on a current collector, the thin film is divided into columns by gaps formed therein in a manner to extend in its thickness direction and the columnar portions are adhered at their bottoms to the current collector, and the nonaqueous electrolyte contains at least one selected from phosphate ester, phosphite ester, borate ester and carboxylic ester having a fluoroalkyl group.
Owner:SANYO ELECTRIC CO LTD
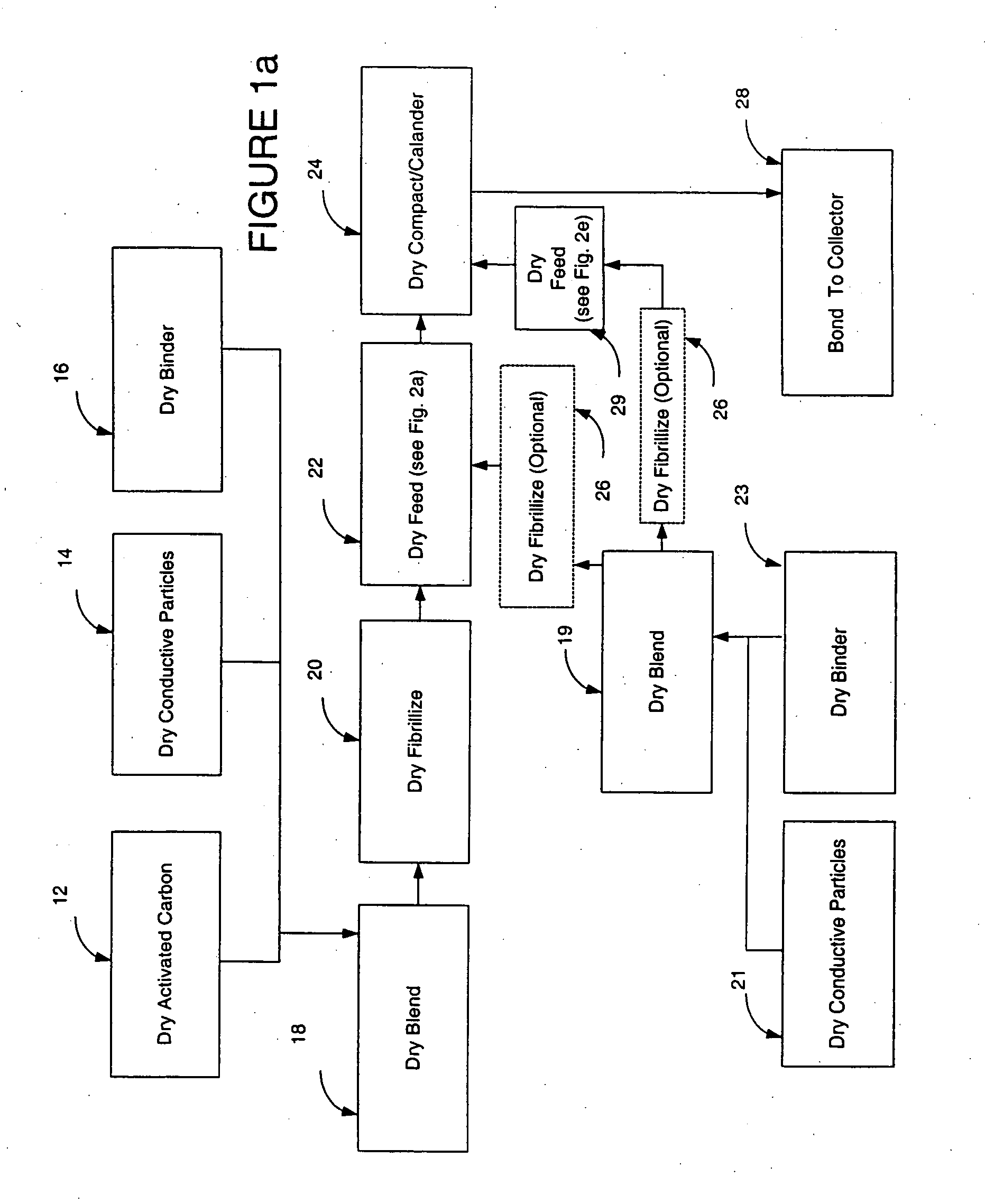
Dry particle based electro-chemical device and methods of making same
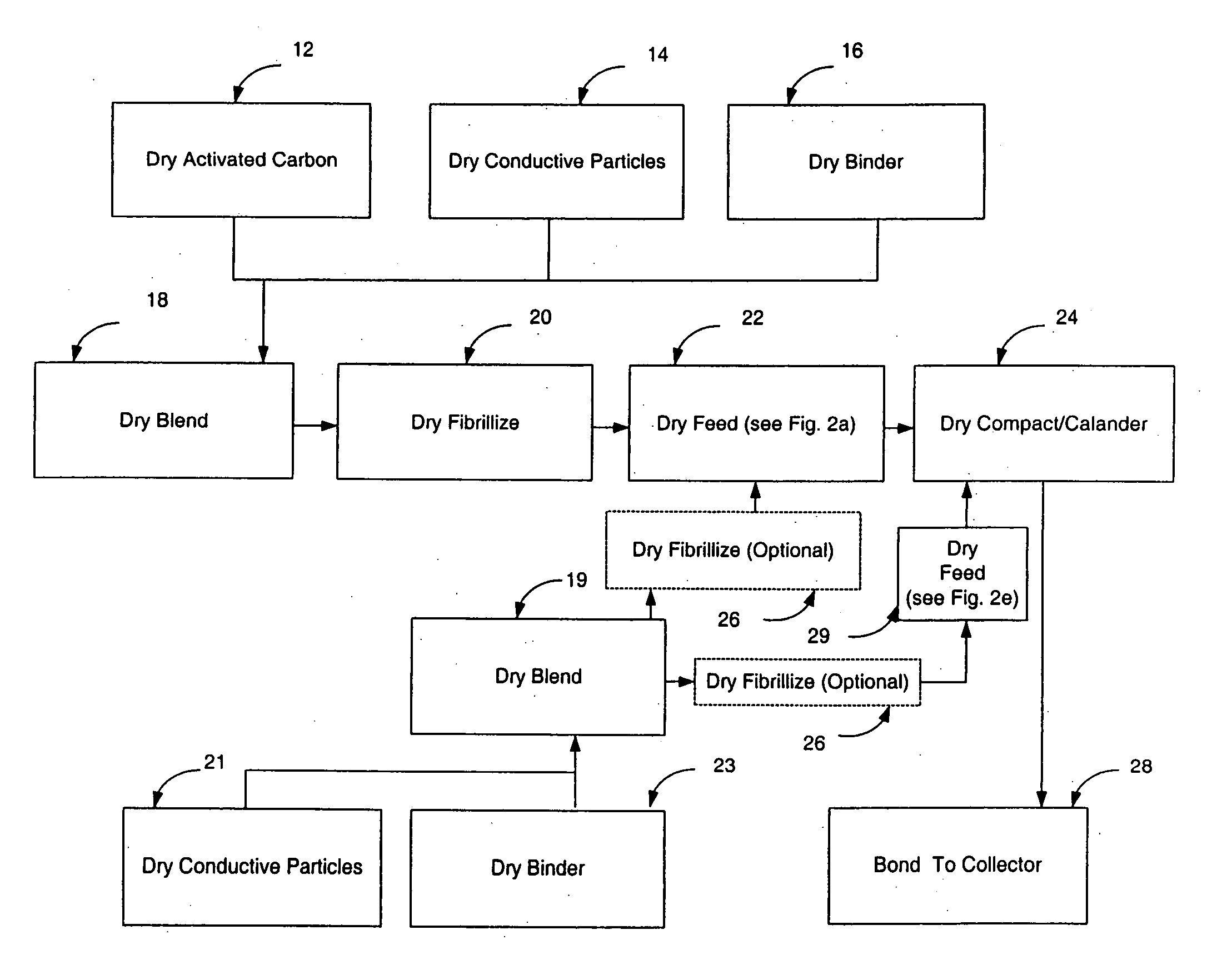
InactiveUS20050266298A1Reduce usageHigh yieldElectrode rolling/calenderingFinal product manufactureFuel cellsBiochemical engineering
A dry process-based electro-chemical device and method for making a self-supporting dry electrode film for use therein is disclosed. Cost effective manufacture of electrochemical devices such as batteries, capacitors, and fuel cells is enabled.
Owner:TESLA INC
Redox shuttle for overdischarge protection in rechargeable lithium-ion batteries
InactiveUS20050221168A1Wide windowLarge capacityNon-aqueous electrolyte accumulatorsElectrolytic capacitorsElectrode potentialCapacity loss
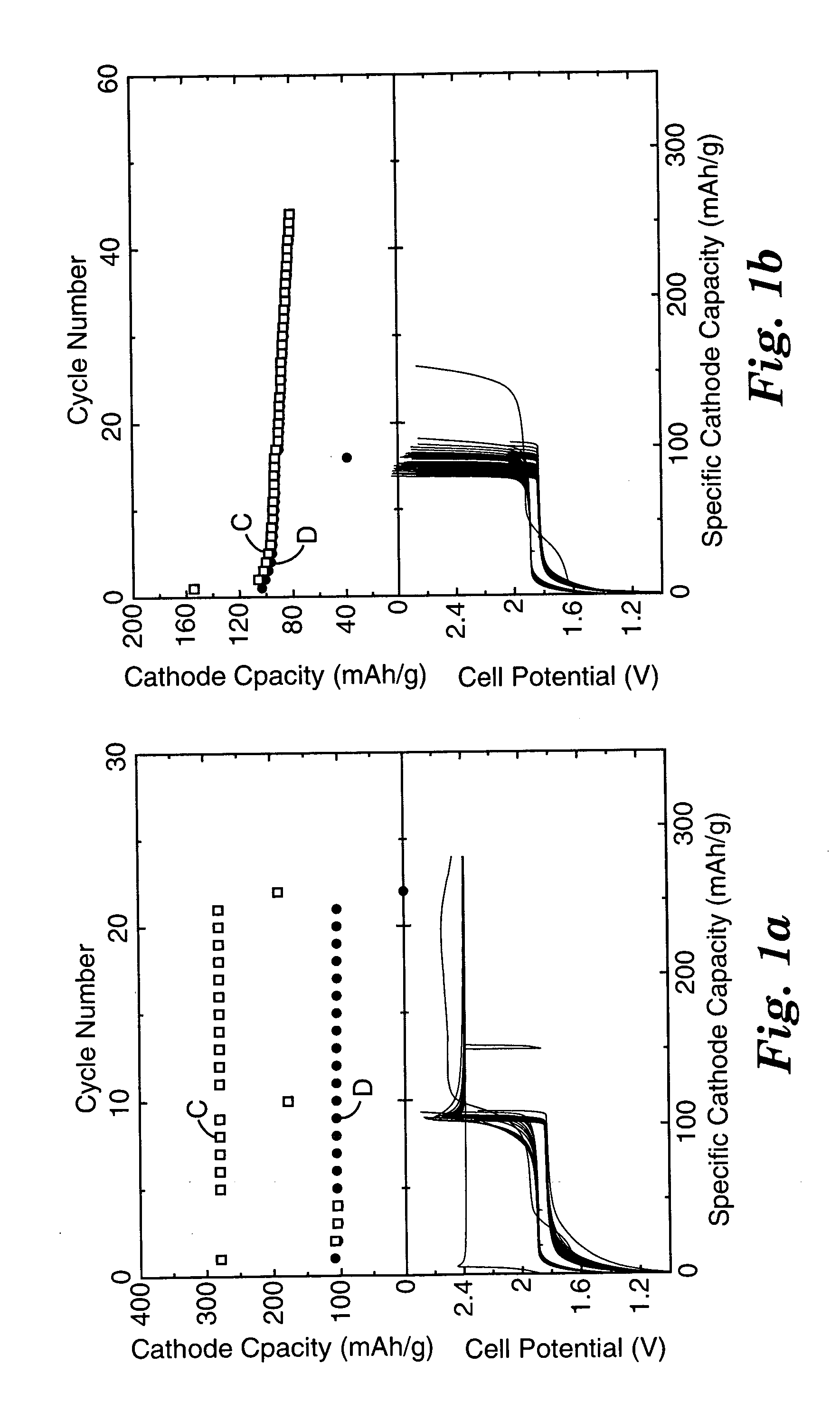
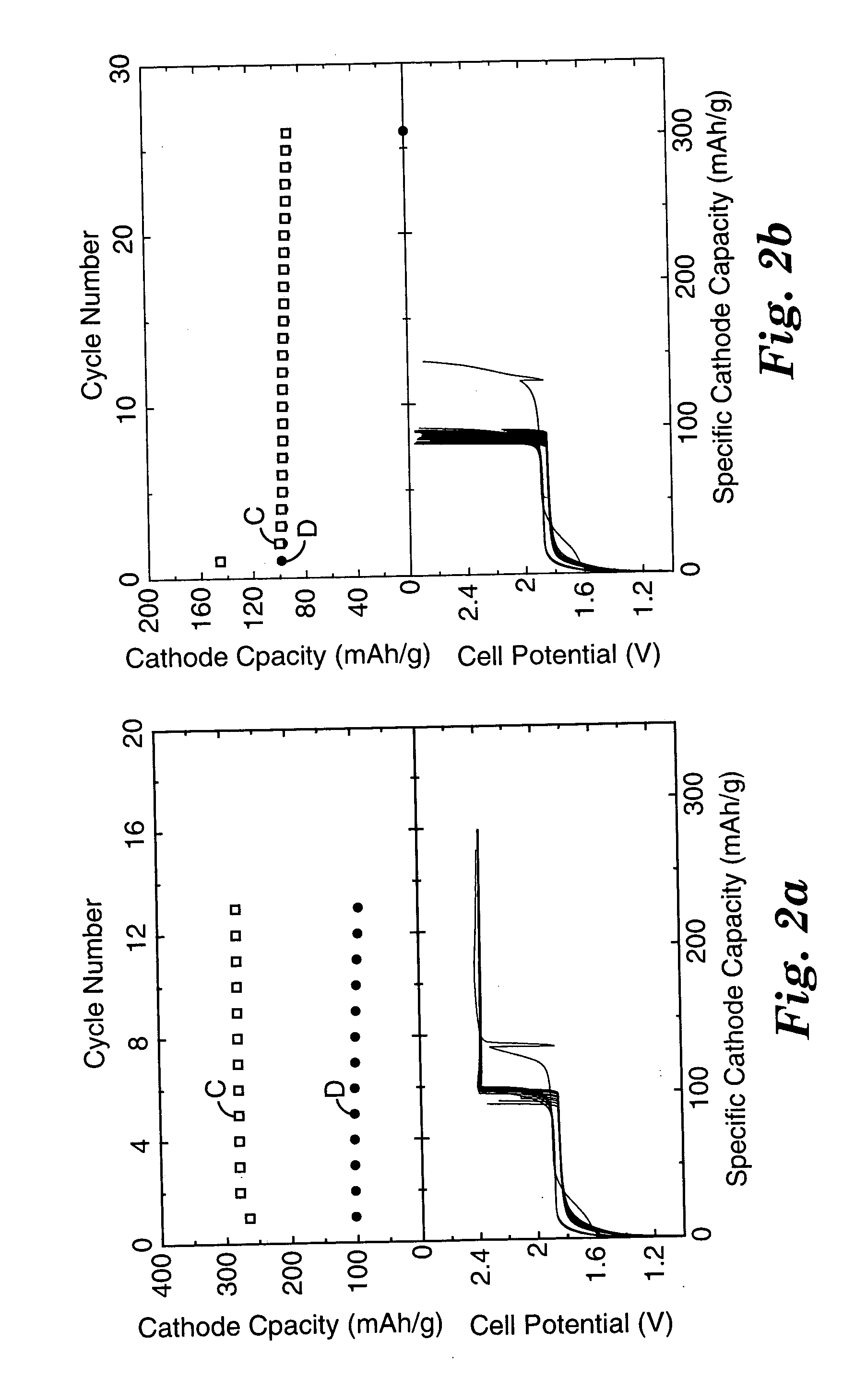
A battery of series-connected rechargeable lithium ion cells each of which contains a negative electrode; a negative electrode current collector; a positive electrode; a positive electrode current collector; and an electrolyte comprising charge carrying medium, lithium salt and cyclable redox chemical shuttle. The negative electrode has a larger irreversible first cycle capacity loss than that of the positive electrode, and is driven to a potential above that of the positive electrode if the cell is discharged to a state of cell reversal. The shuttle has an electrochemical potential above the positive electrode maximum normal operating potential, and prevents the negative electrode potential from reaching even higher and more destructive positive values during overdischarge. The current collector has a lithium alloying potential below the negative electrode minimum normal operating potential. The battery chemically limits or eliminates cell damage due to repeated overdischarge, and may operate without electronic overdischarge protection circuitry.
Owner:3M INNOVATIVE PROPERTIES CO
Electrolyte mixtures useful for li-ion batteries
InactiveUS20090286163A1Safer and thermally stable systemImprove compatibilityElectrode carriers/collectorsSolid electrolyte cellsDisplay devicePotassium
The present invention provides for the preparation of ionic liquid-lithium salt-low molecular weight liquid polymer mixtures. The mixture is useful as an electrolytic solution. Thus, the mixture is suitable as an electrolyte in batteries and supercapacitors as well as an active material for solid state light-emitting devices or polymer light-emitting displays or an electro deposition of alkali metals such as lithium, sodium, or potassium in the field of research or industry. The present invention further provides for a method making the mixture. Additionally, the present invention provides for a lithium battery comprising the mixture and a method of making the lithium battery.
Owner:RGT UNIV OF CALIFORNIA
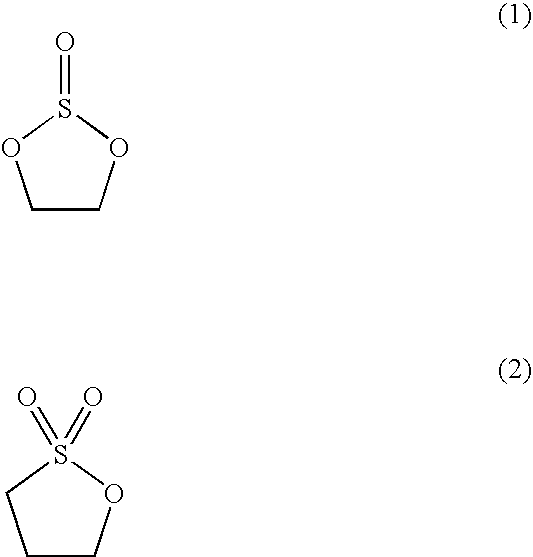
Non-aqueous liquid electrolyte and non-aqueous liquid electrolyte secondary battery
InactiveUS20090325065A1Improve charging capacityMaintain good propertiesAlkaline accumulatorsElectrode manufacturing processesLithiumCarbonate
A non-aqueous liquid electrolyte secondary battery using negative-electrode active material having Si, Sn and / or Pb, with high charge-capacity, superior characteristics including discharge-capacity retention rate over long is provided. Its non-aqueous liquid electrolyte contains carbonate having unsaturated bond and / or halogen and compounds like LiPF6 and / or LiBF4 (first lithium salt) and lithium salt different from said first one, represented by formula below (second lithium salt).Lil(αmXan)
Owner:MITSUBISHI CHEM CORP
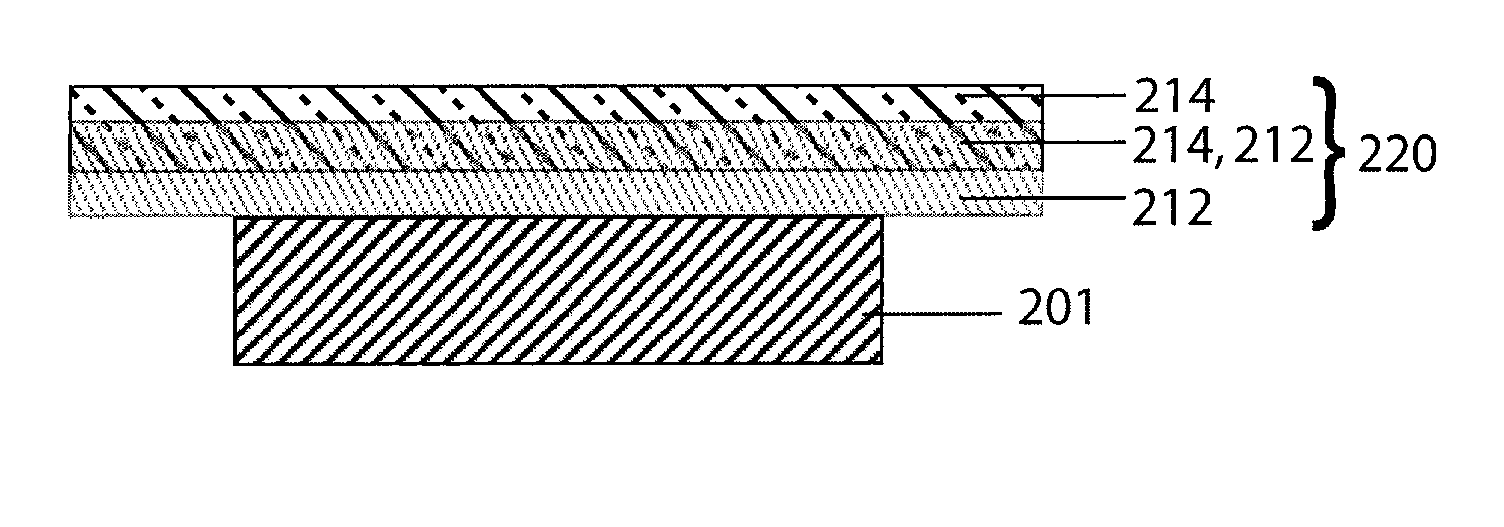
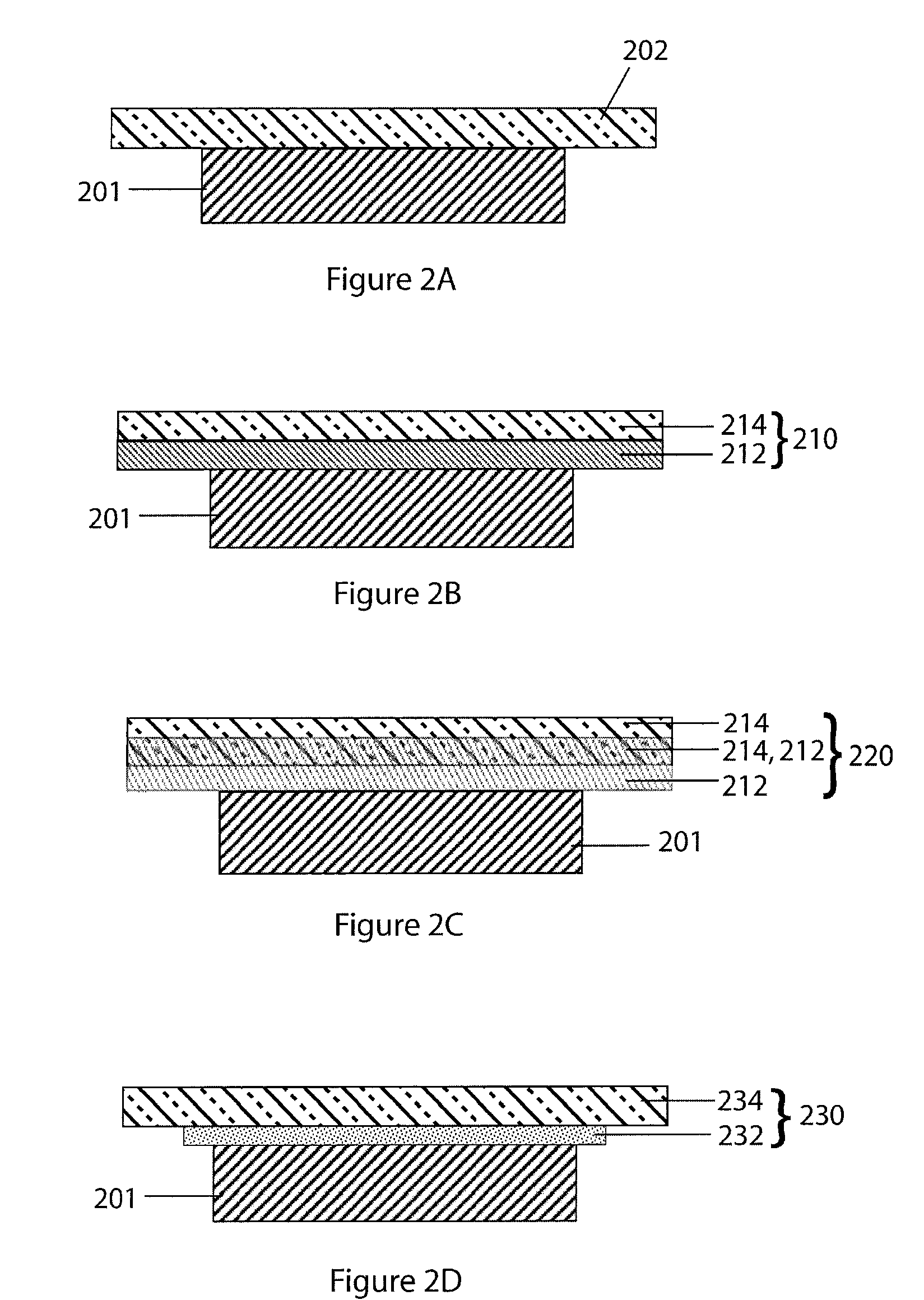
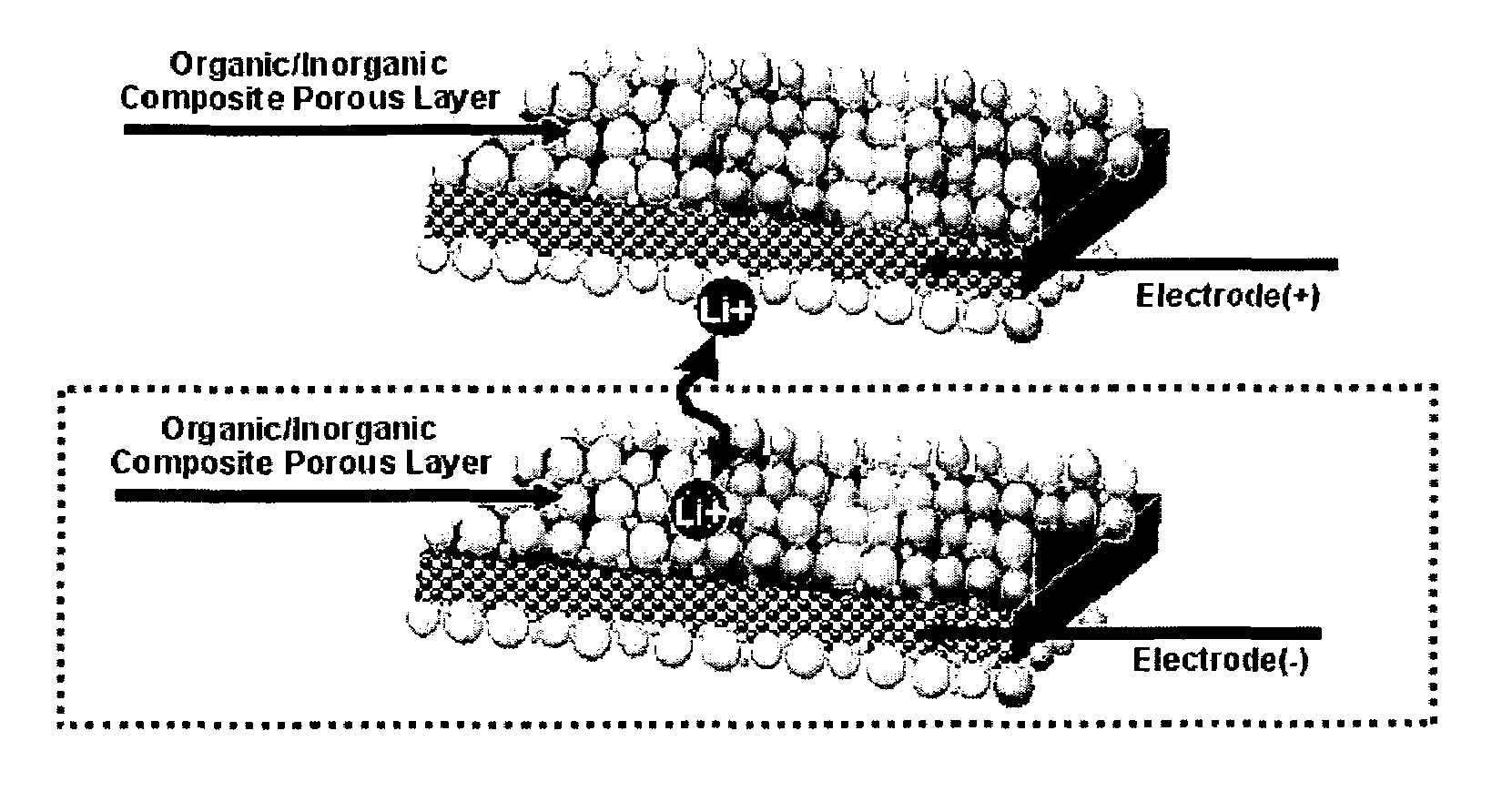
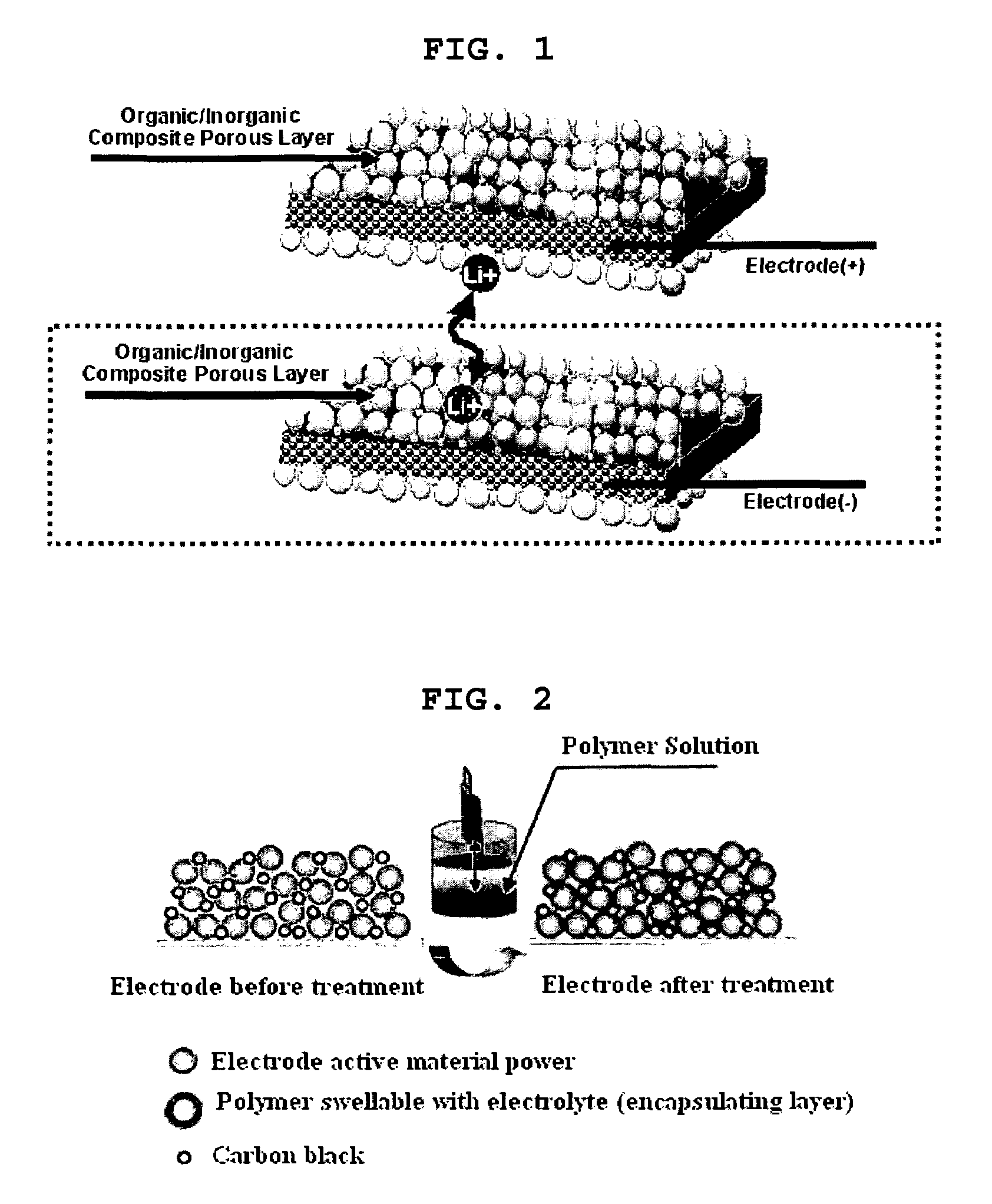
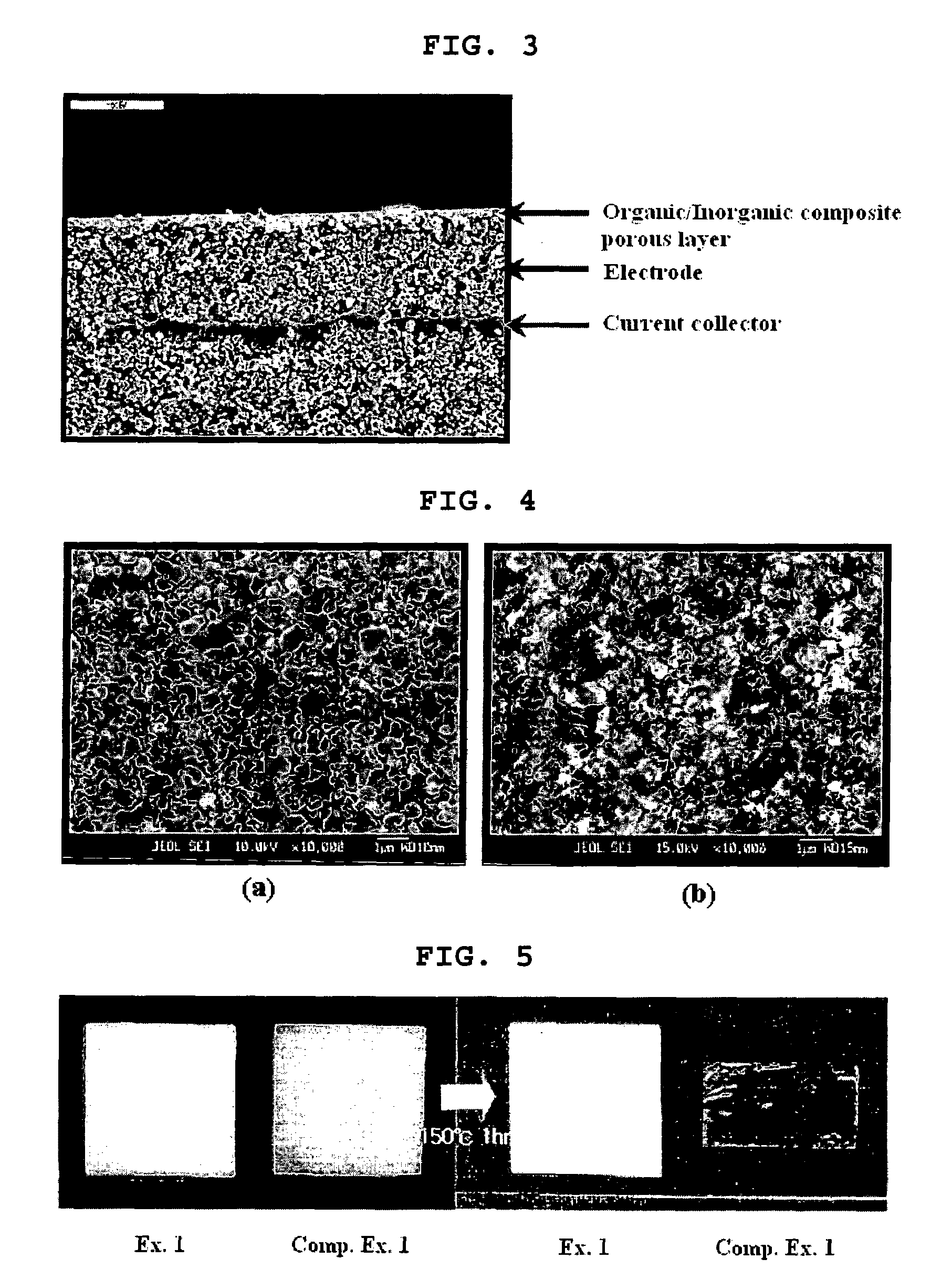
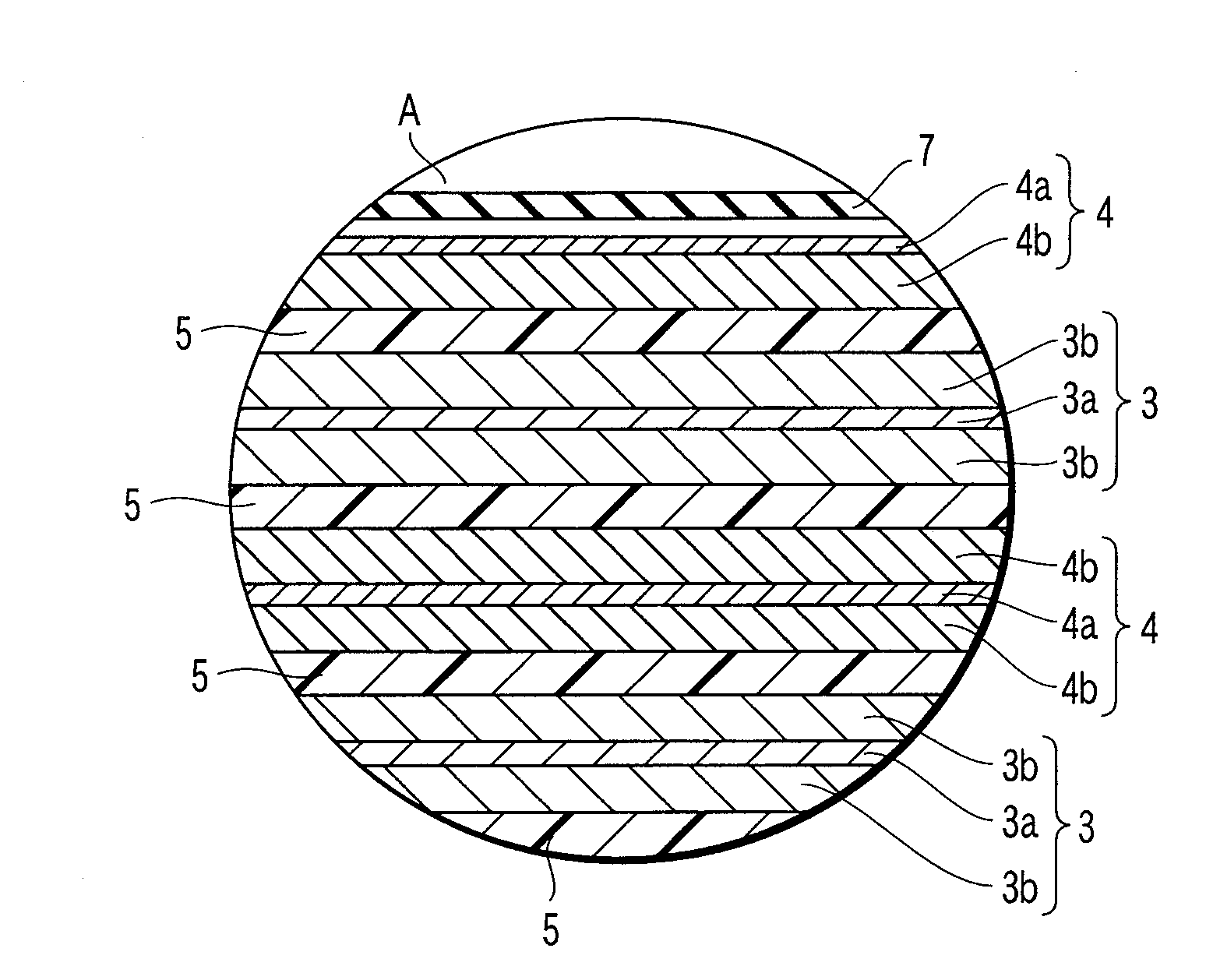
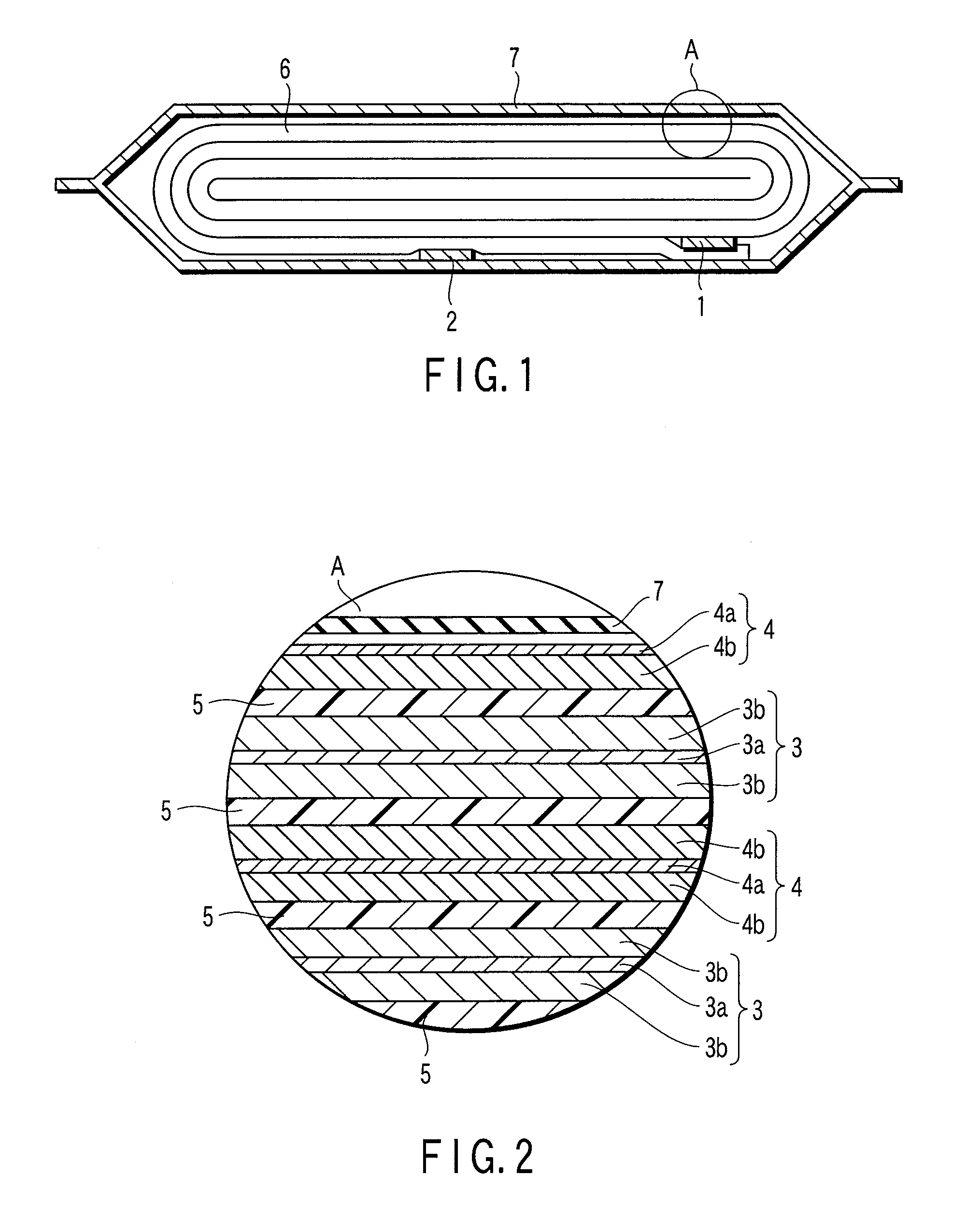
Organic/inorganic composite porous layer-coated electrode and electrochemical device comprising the same
ActiveUS7682740B2Improve performanceImprove securityElectrode rolling/calenderingNon-aqueous electrolyte accumulator electrodesPorous coatingInorganic particle
Disclosed is an electrode comprising a first organic / inorganic composite porous coating layer formed on its surface, wherein the first coating layer includes inorganic particles and a binder polymer for interconnecting and fixing the inorganic particles, and has micropores formed by interstitial volumes among the inorganic particles. An electrochemical device including the same electrode is also disclosed. Further, disclosed is a method for manufacturing an electrode having an organic / inorganic composite porous coating layer on the surface thereof, comprising the steps of: (a) coating a current collector with slurry containing an electrode active material and drying it to provide an electrode; and (b) coating the surface of electrode obtained from step (a) with a mixture of inorganic particles with a binder polymer. A lithium secondary battery including the electrode shows improved safety and minimized degradation in battery performance.
Owner:LG ENERGY SOLUTION LTD
Nonaqueous electrolytic solution and nonaqeuous-electrolyte secondary battery
ActiveUS20120308881A1Improve conductivityReduce resistanceElectrode carriers/collectorsNon-aqueous electrolyte accumulator electrodesPhysical chemistryFluorosulfonic acid
An object of the invention is to provide a nonaqueous electrolytic solution which is capable of bringing about a nonaqueous-electrolyte secondary battery improved in initial charge capacity, input / output characteristics, and impedance characteristics. The invention relates to a nonaqueous electrolytic solution which comprises: a nonaqueous solvent; LiPF6; and a specific fluorosulfonic acid salt, and to a nonaqueous-electrolyte secondary battery containing the nonaqueous electrolytic solution.
Owner:MU IONIC SOLUTIONS CORP +1
Non-aqueous electrolyte and lithium secondary battery using the same
InactiveUS6413678B1Satisfactory battery characteristicDeterioration of characteristicOrganic electrolyte cellsActive material electrodesPhysical chemistrySolvent
A non-aqueous electrolyte comprising a non-aqueous solvent and a lithium salt dissolved therein, and a cyclic carbonate, linear carbonate, and vinylene carbonate having a chlorine content of 100 ppm or less, and a lithium secondary battery using the same.
Owner:UBE IND LTD
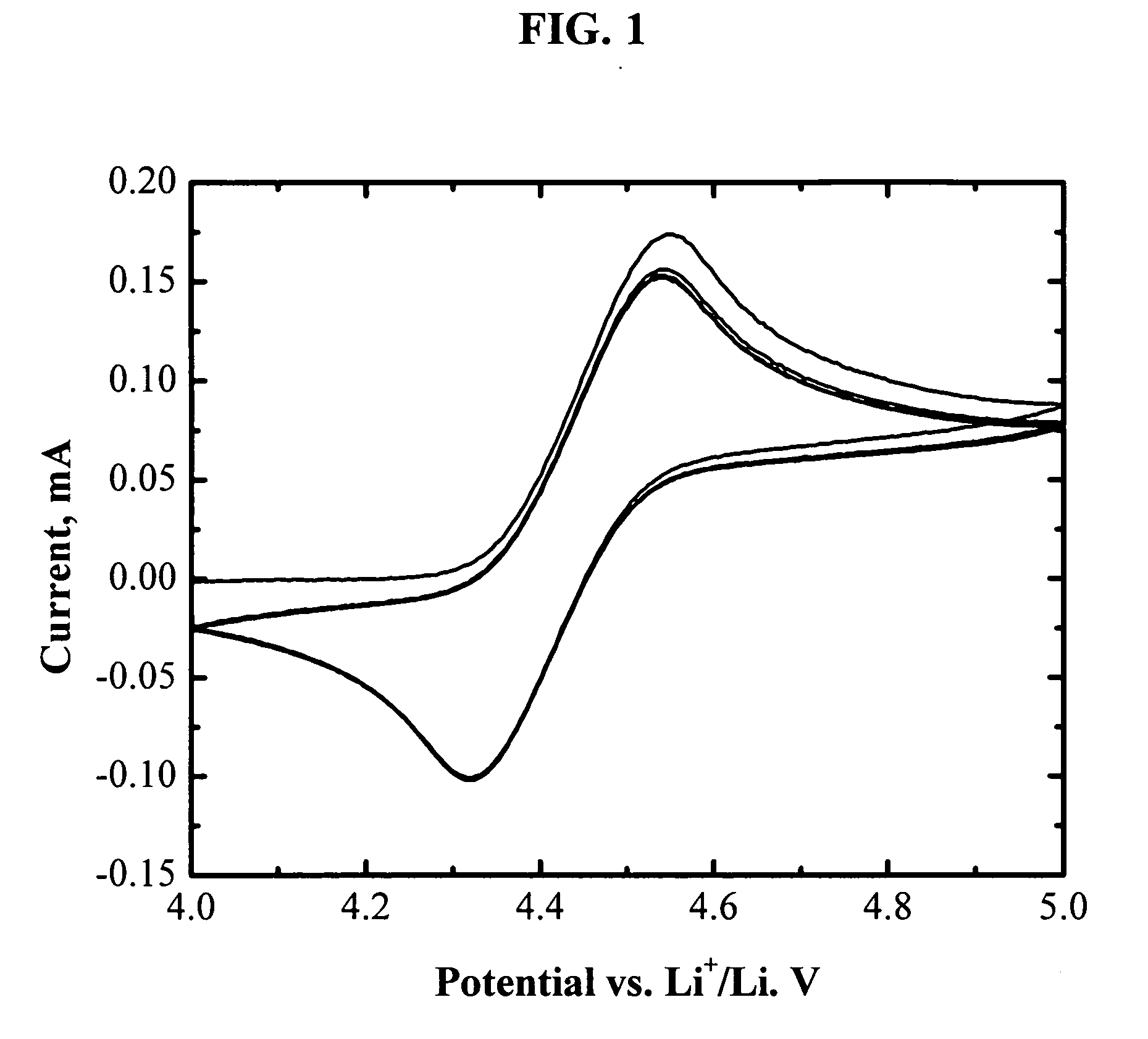
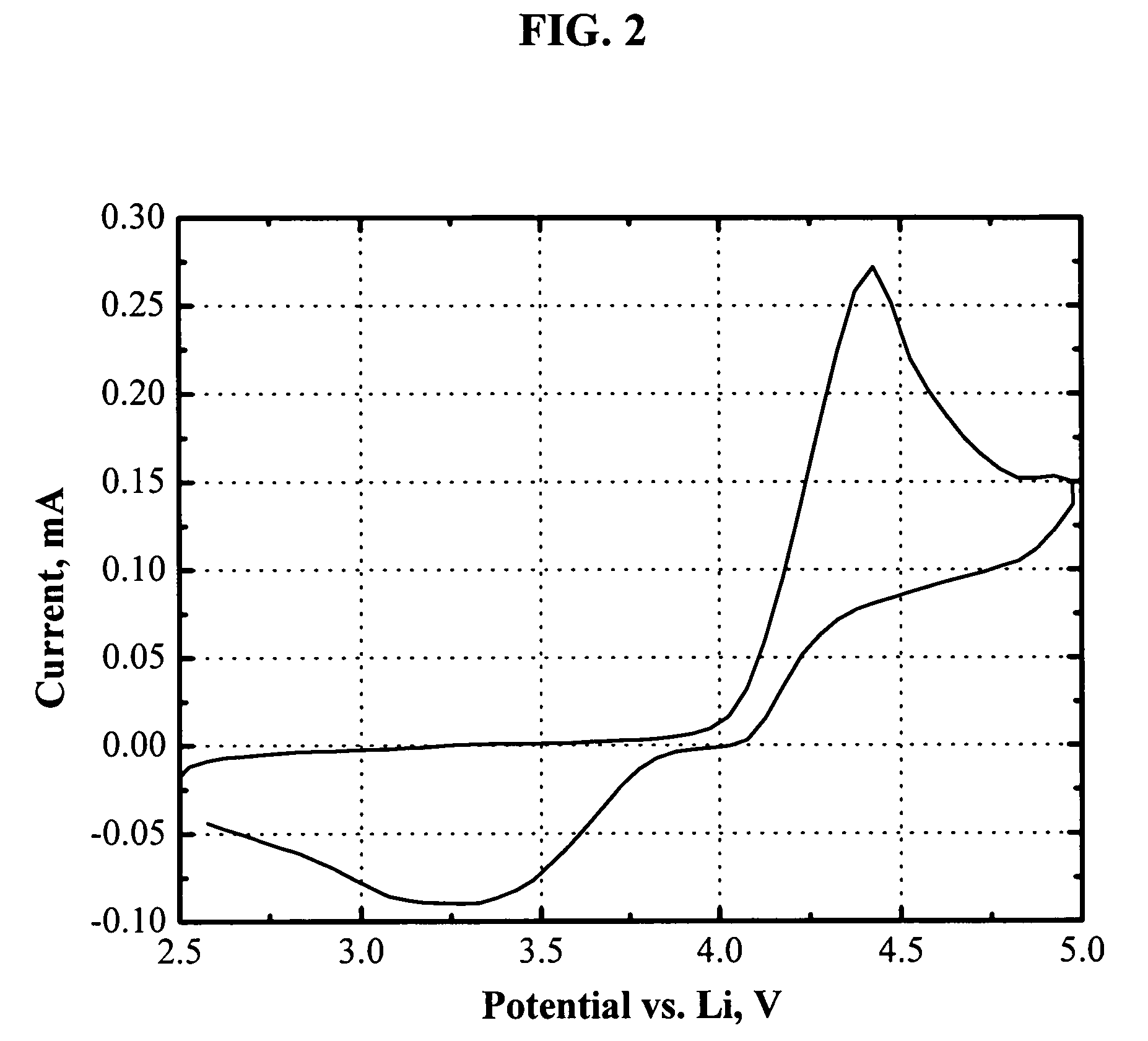
Novel redox shuttles for overcharge protection of lithium batteries
The present invention is generally related to electrolytes containing novel redox shuttles for overcharge protection of lithium-ion batteries. The redox shuttles are capable of thousands hours of overcharge tolerance and have a redox potential at about 3-5.5 V vs. Li and particularly about 4.4-4.8 V vs. Li. Accordingly, in one aspect the invention provides electrolytes comprising an alkali metal salt; a polar aprotic solvent; and a redox shuttle additive that is an aromatic compound having at least one aromatic ring with four or more electronegative substituents, two or more oxygen atoms bonded to the aromatic ring, and no hydrogen atoms bonded to the aromatic ring; and wherein the electrolyte solution is substantially non-aqueous. Further there are provided electrochemical devices employing the electrolyte and methods of making the electrolyte.
Owner:UCHICAGO ARGONNE LLC
Nonaqueous electrolyte battery, battery pack and vehicle
A nonaqueous electrolyte battery includes a negative electrode and a nonaqueous electrolyte. The negative electrode comprises an active material having a lithium ion insertion potential of 0.4V (vs Li / Li+) or more, a conductive agent and a current collector. The nonaqueous electrolyte contains first sultones having an unsaturated hydrocarbon group. A diameter distribution of pores of the negative electrode when measured by mercury porosimetry has a first peak having a mode diameter of 0.01 to 0.2 μm and a second peak having a mode diameter of 0.003 to 0.02 μm. A volume of pores having a diameter of 0.01 to 0.2 μm and a volume of pores having a diameter of 0.003 to 0.02 μm, which are measured by the mercury porosimetery, are 0.05 to 0.5 mL and 0.0001 to 0.02 mL, respectively, per g of the negative electrode excluding the current collector.
Owner:KK TOSHIBA
Metal-air cell with performance enhancing additive
ActiveUS20110281184A1Improves oxygen reduction thermodynamicsImproved kineticsFuel and primary cellsFuel and secondary cellsOxygenElectrochemical cell
Systems and methods drawn to an electrochemical cell comprising a low temperature ionic liquid comprising positive ions and negative ions and a performance enhancing additive added to the low temperature ionic liquid. The additive dissolves in the ionic liquid to form cations, which are coordinated with one or more negative ions forming ion complexes. The electrochemical cell also includes an air electrode configured to absorb and reduce oxygen. The ion complexes improve oxygen reduction thermodynamics and / or kinetics relative to the ionic liquid without the additive.
Owner:ARIZONA STATE UNIVERSITY
Lithium difluorophosphate, electrolyte containing lithium difluorophosphate, process for producing lithium difluorophosphate, process for producing nonaqueous electrolyte, nonaqueous electrolyte, and nonaqueous electrolyte secondary battery containing the same
InactiveUS20090286155A1Good low temperatureExcellent cycle characteristicsPhosphorus halides/oxyhalidesCell electrodesBoiling pointSolvent
A difluorophosphate salt, which is expensive and not readily available, can be produced with a high purity readily and efficiently from inexpensive and readily available materials. A nonaqueous electrolyte secondary battery that exhibits low-temperature discharge and heavy-current discharge characteristics and high-temperature preservation and cycle characteristics without impairing the battery safety. A hexafluorophosphate salt is reacted with a compound having a bond represented by the following formula (1) in the molecule:Si—O—Si (1)A nonaqueous electrolyte used for nonaqueous electrolyte secondary batteries including a negative electrode and a positive electrode that can occlude and discharge ions, and a nonaqueous electrolyte is prepared from a mixture obtained by mixing at least one nonaqueous solvent, a hexafluorophosphate salt and a compound having a bond represented by the following formula (1), and removing low-boiling compounds newly formed in the system, the low-boiling compounds having a lower boiling point than that of the compound having the bond represented by the formula (1):Si—O—Si (1)
Owner:MITSUBISHI CHEM CORP
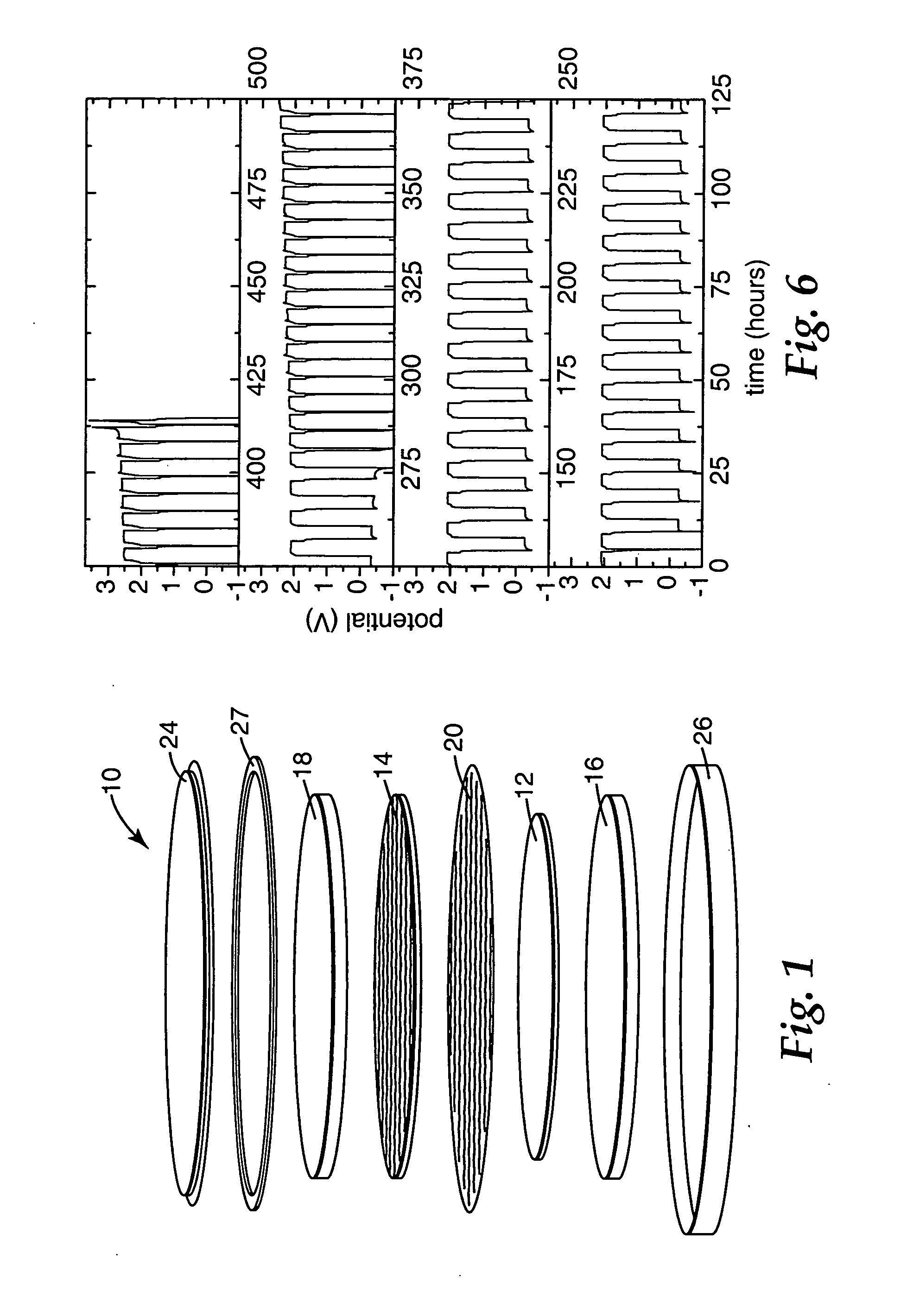
Solid-state sodium-based secondary cell having a sodium ion conductive ceramic separator
ActiveUS20110104526A1Non-aqueous electrolyte accumulatorsCell electrodesRechargeable cellRoom temperature
The present invention provides a solid-state sodium-based secondary cell (or rechargeable battery). While the secondary cell can include any suitable component, in some cases, the secondary cell comprises a solid sodium metal negative electrode that is disposed in a non-aqueous negative electrolyte solution that includes an ionic liquid. Additionally, the cell comprises a positive electrode that is disposed in a positive electrolyte solution. In order to separate the negative electrode and the negative electrolyte solution from the positive electrolyte solution, the cell includes a sodium ion conductive electrolyte membrane. Because the cell's negative electrode is in a solid state as the cell functions, the cell may operate at room temperature. Additionally, where the negative electrolyte solution contains the ionic liquid, the ionic liquid may impede dendrite formation on the surface of the negative electrode as the cell is recharged and sodium ions are reduced onto the negative electrode.
Owner:FIELD UPGRADING USA INC
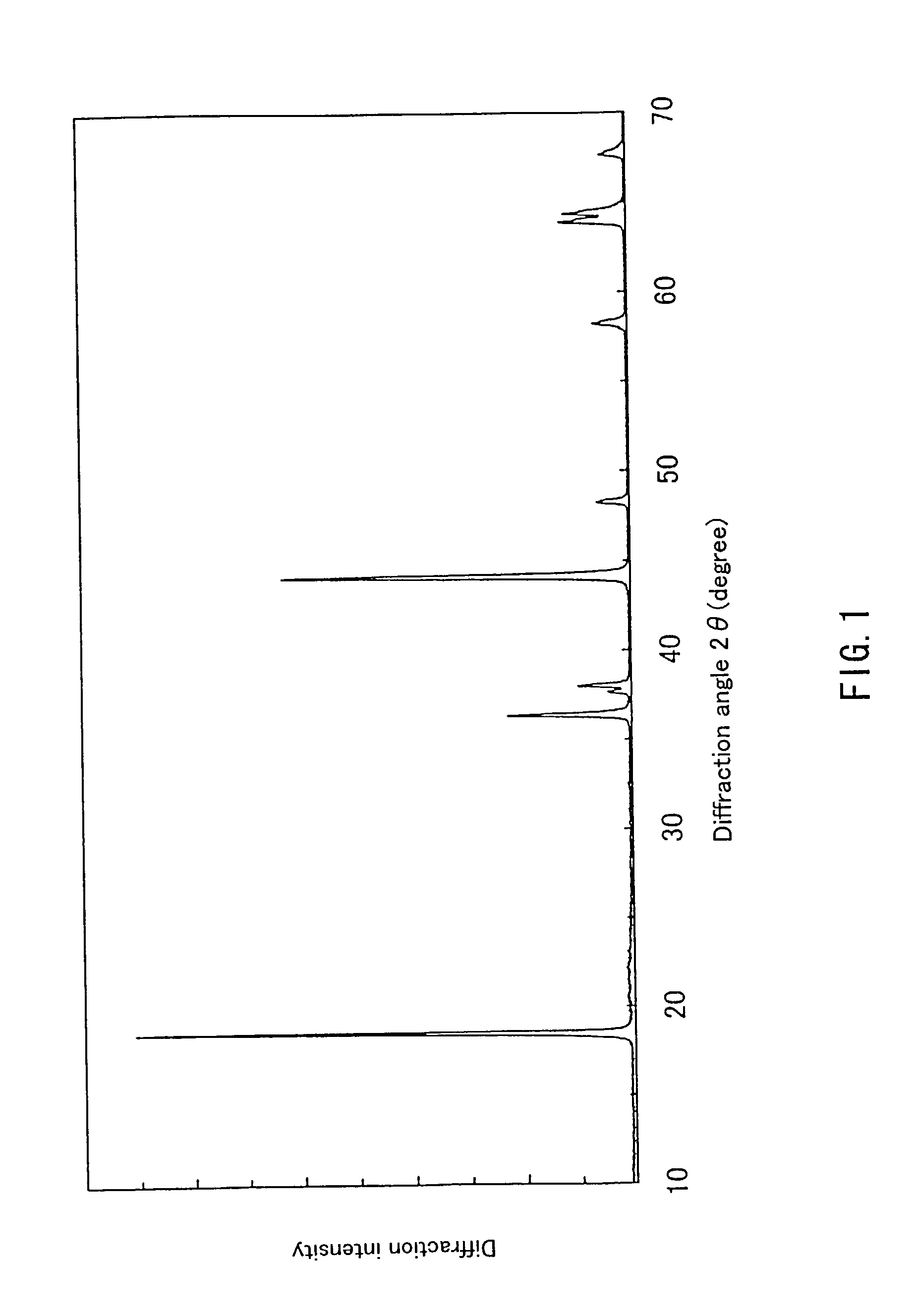
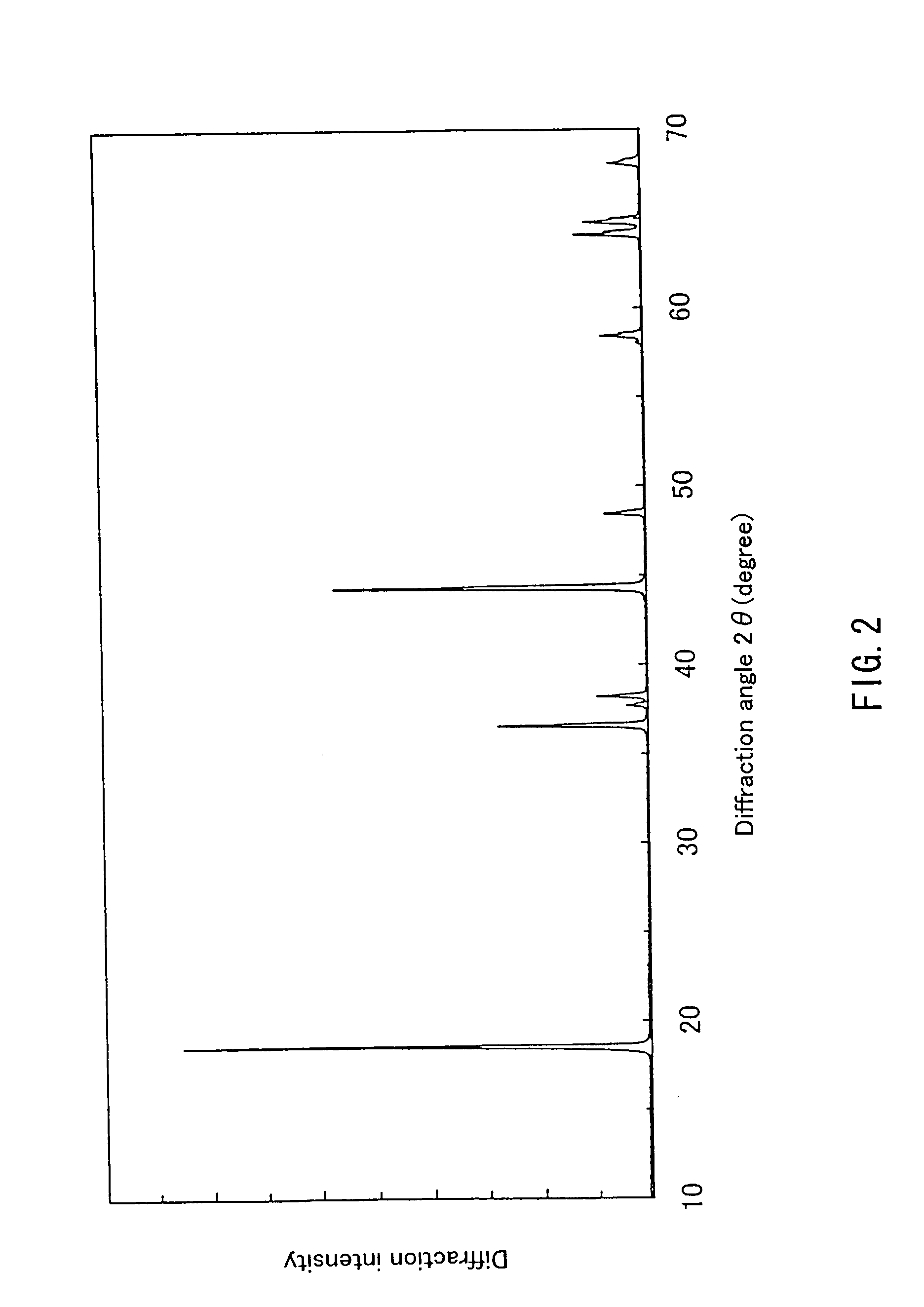
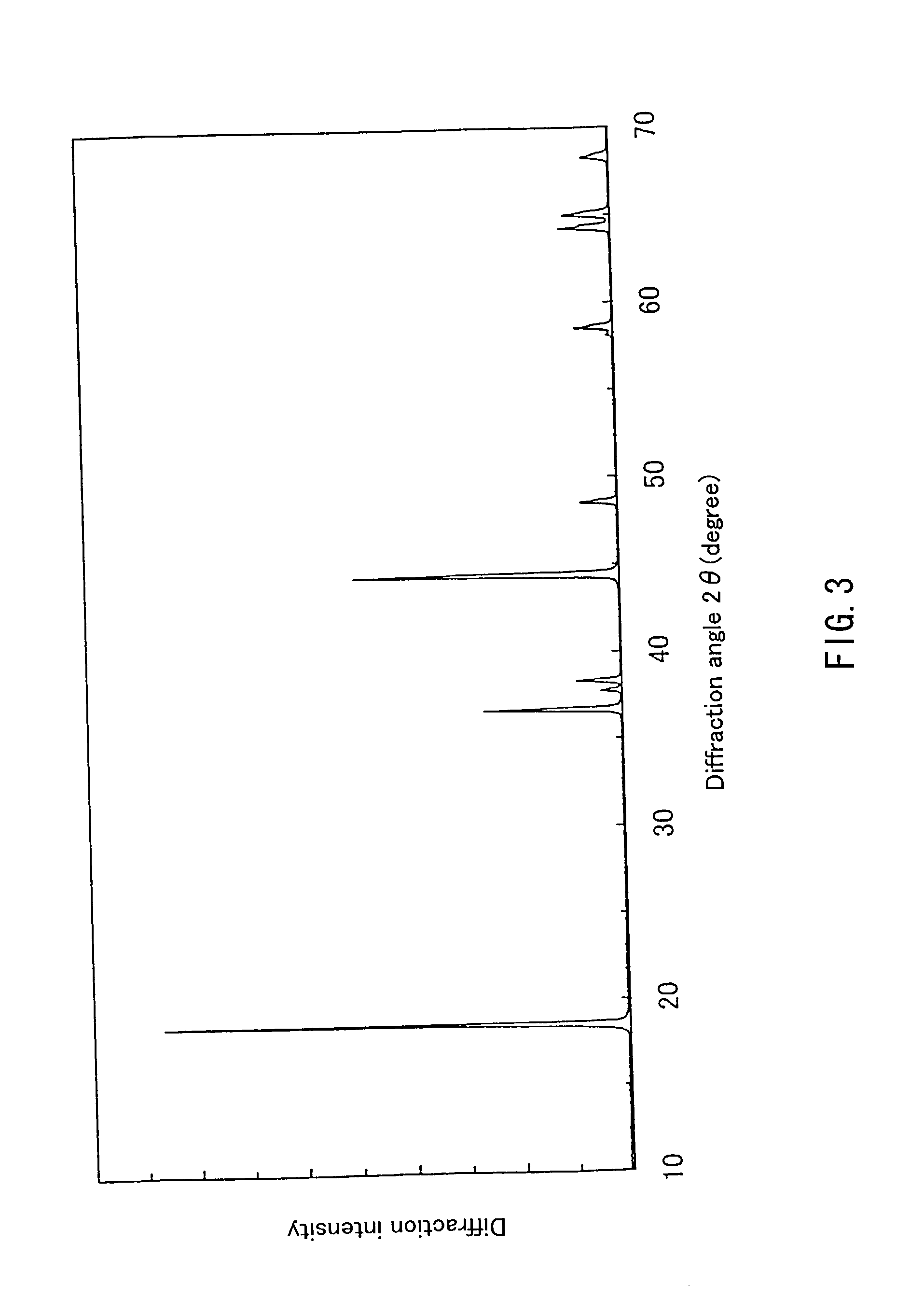
Lithium-containing composite oxide and nonaqueous secondary cell using the same, and method for manufacturing the same
InactiveUS20030082452A1Stable structureHigh energy density per volumeElectrode thermal treatmentOrganic electrolyte cellsLithiumHigh density
Because of the composition represented by General Formula: Li1+x+alphaNi(1-x-y+delta) / 2Mn(1-x-y-delta) / 2MyO2 (where 0<=x<=0.05, -0.05<=x+alpha<=0.05, 0<=y<=0.4; -0.1<=delta<=0.1 (when 0<=y<=0.2) or -0.24<=delta<=0.24 (when 0.2<=y<=0.4); and M is at least one element selected from the group consisting of Ti, Cr, Fe, Co, Cu, Zn, Al, Ge and Sn), a high-density lithium-containing complex oxide with high stability of a layered crystal structure and excellent reversibility of charging / discharging can be provided, and a high-capacity non-aqueous secondary battery excellent in durability is realized by using such an oxide for a positive electrode.
Owner:MAXELL HLDG LTD
Synthesis of bis(fluorosulfonyl)imide
The present invention provides methods for producing bis(fluorosulfonyl) compounds of the formula:F—S(O)2—Z—S(O)2—F Iby contacting a nonfluorohalide compound of the formula:X—S(O)2—Z—S(O)2—Xwith bismuth trifluoride under conditions sufficient to produce the bis(fluorosulfonyl) compound of Formula I, where Z and X are those defined herein.
Owner:SES HLDG PTE LTD
Alkali metal or Alkali-Ion batteries having high volumetric and gravimetric energy densities
ActiveUS20170077546A1Increase energy densityImprove power densityElectrode carriers/collectorsSecondary cellsAlkali ionsFluid electrolytes
Provided is an alkali metal-ion battery, comprising: (a) an anode having an anode active material dispersed in a first liquid electrolyte disposed in pores of a 3D porous anode current collector having at least 80% by volume of pores; (b) a cathode having a cathode active material dispersed in a second liquid electrolyte disposed in pores of a 3D porous cathode current collector wherein the cathode thickness-to-current collector thickness ratio is from 0.8 / 1 to 1 / 0.8; (c) a separator disposed between the anode and the cathode; wherein the anode or cathode active material loading is greater than 10 mg / cm2, the anode and cathode active materials combined exceeds 40% by weight of the battery, and / or the 3D porous anode and / or cathode current collector has a thickness no less than 200 μm (preferably greater than 500 μm and more preferably greater than 700 μm) and is in physical contact with the separator.
Owner:GLOBAL GRAPHENE GRP INC
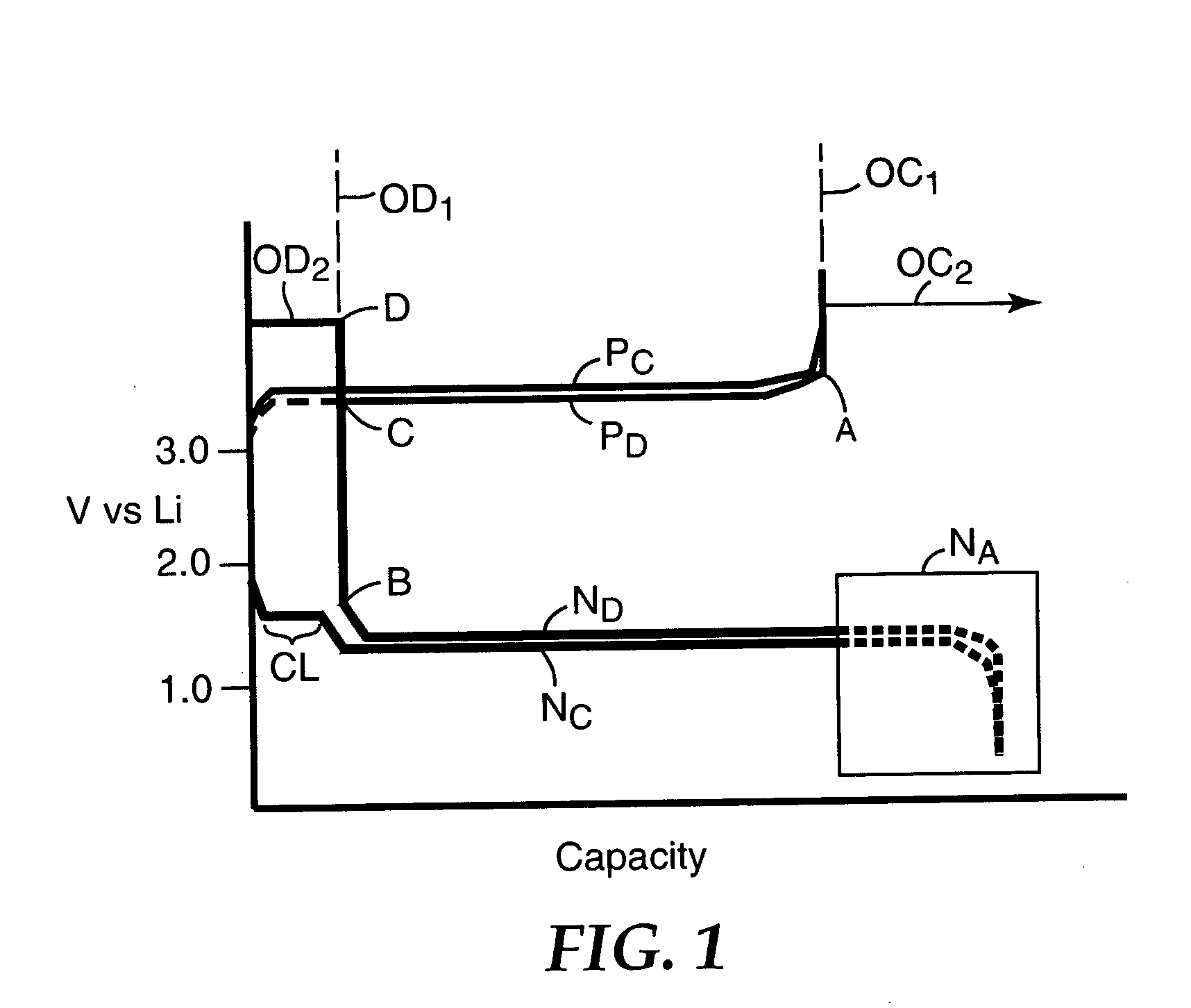
Overcharge protection for electrochemical cells
InactiveUS20070072085A1Extending overcharge capacity of cellMinimize impactNon-aqueous electrolyte accumulatorsFinal product manufactureAlkaline earth metalBoric acid
The invention relates to an improvement in a cell which is normally susceptible to damage from overcharging comprised of a negative electrode, a positive electrode, and an electrolyte comprised of an overcharge protection salt carried in a carrier or solvent. Representative overcharge protection salts are embraced by the formula: MaQ where M is an electrochemically stable cation selected from the group consisting of alkali metal, alkaline earth metal, tetraalkylammonium, or imidazolium groups, and Q is a borate or heteroborate cluster and a is the integer 1 or 2.
Owner:AIR PROD & CHEM INC
Three dimensional Battery Architectures and Methods of Making Same
InactiveUS20110171518A1Electrode manufacturing processesFinal product manufactureConductive materialsThree dimensional electrode
A three-dimensional electrode structure for use in a battery comprising a porous three-dimensional substrate formed from a first electrically conductive material, an ion-conducting dielectric material disposed on the porous three dimensional substrate, and a second electrically conductive material disposed on the ion-conducting dielectric material, wherein the ion-conducting dielectric material separates the first electrically conductive material from the second electrically conductive material.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +1
Nonaqueous electrolyte secondary battery
ActiveUS20050221187A1Excellent high rate characteristicsNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsEngineeringLi element
A nonaqueous electrolyte secondary battery includes a case, a nonaqueous electrolyte provided in the case and containing a linear sulfite, a positive electrode provided in the case and capable of absorbing-releasing Li element or Li ions, and a negative electrode provided in the case and containing a lithium titanium oxide and a conductive agent that includes a carbonaceous material.
Owner:KK TOSHIBA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com