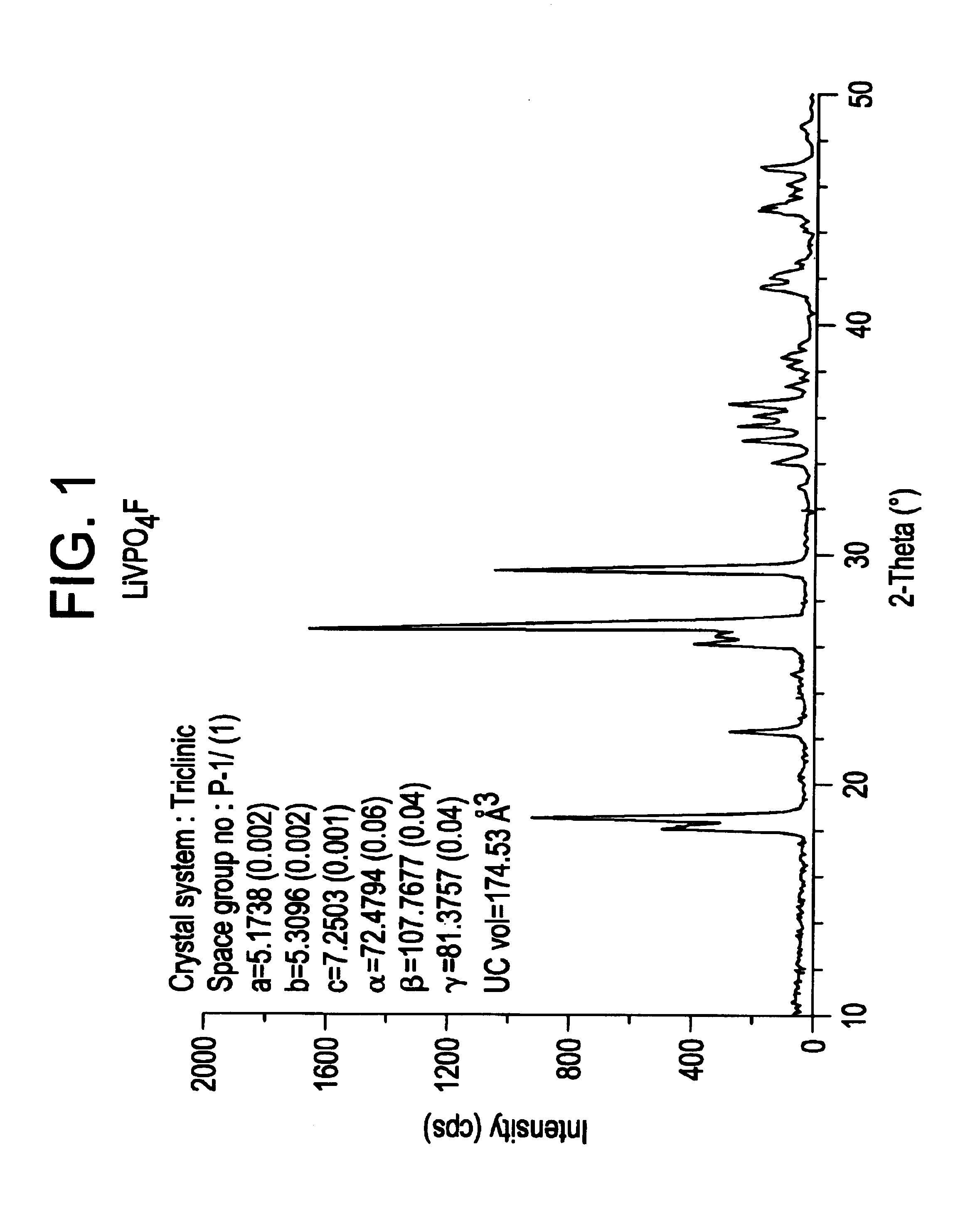
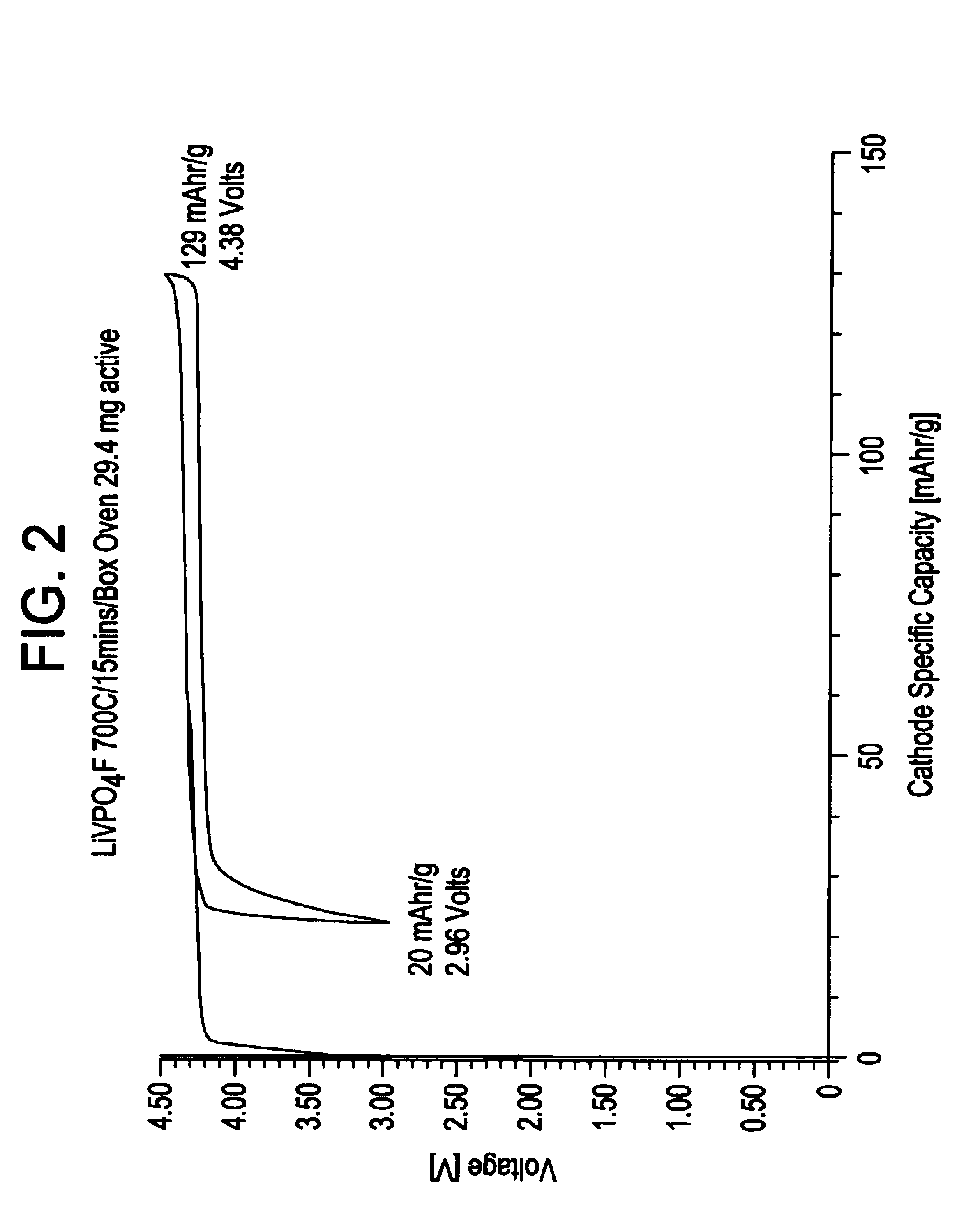
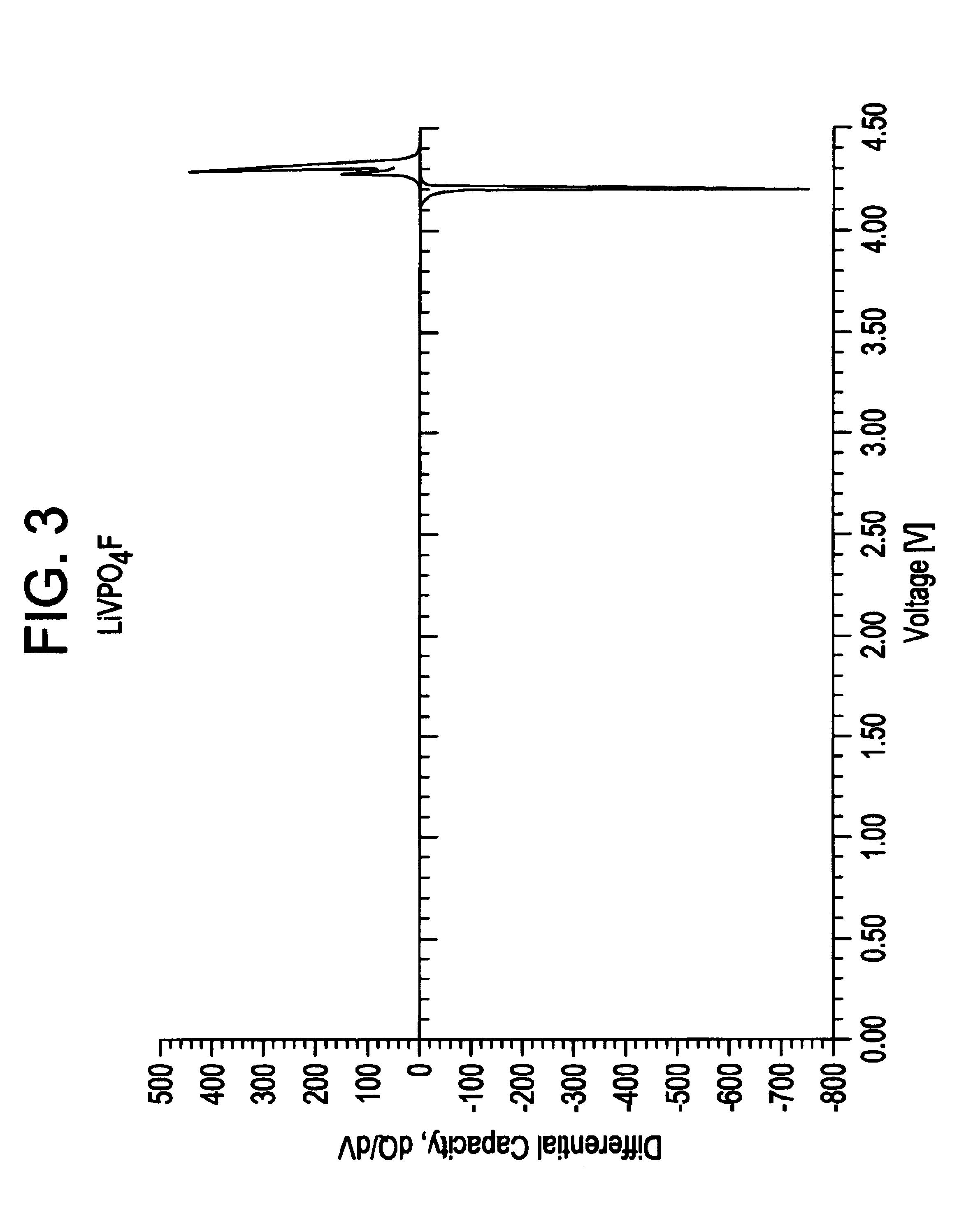
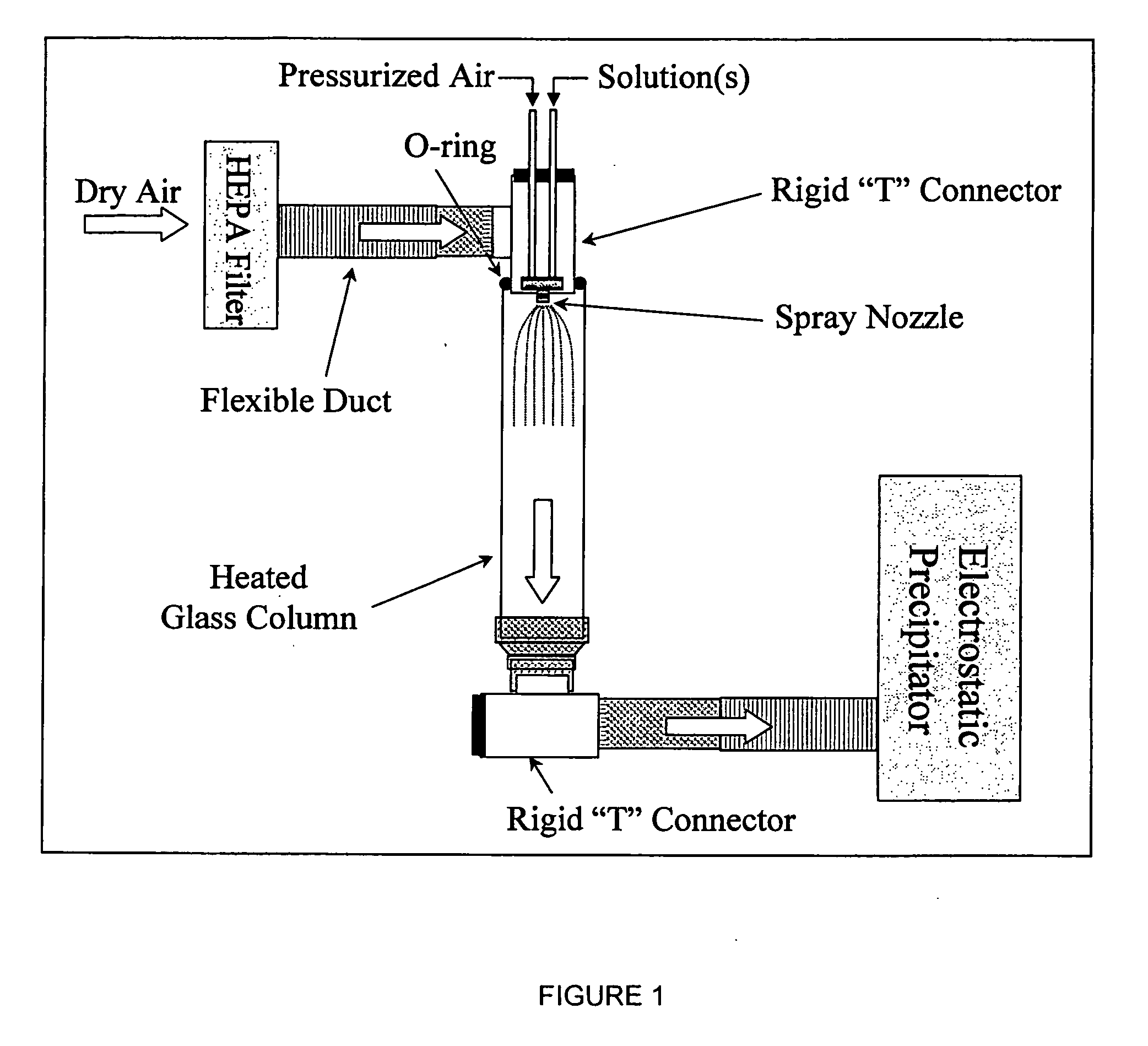
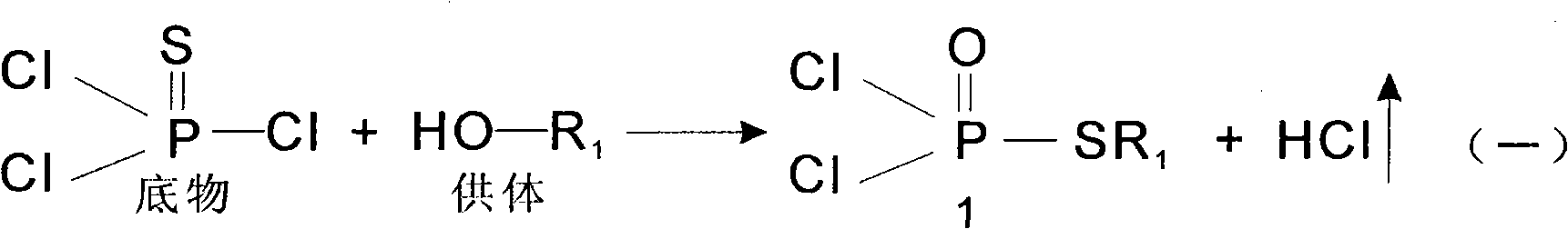
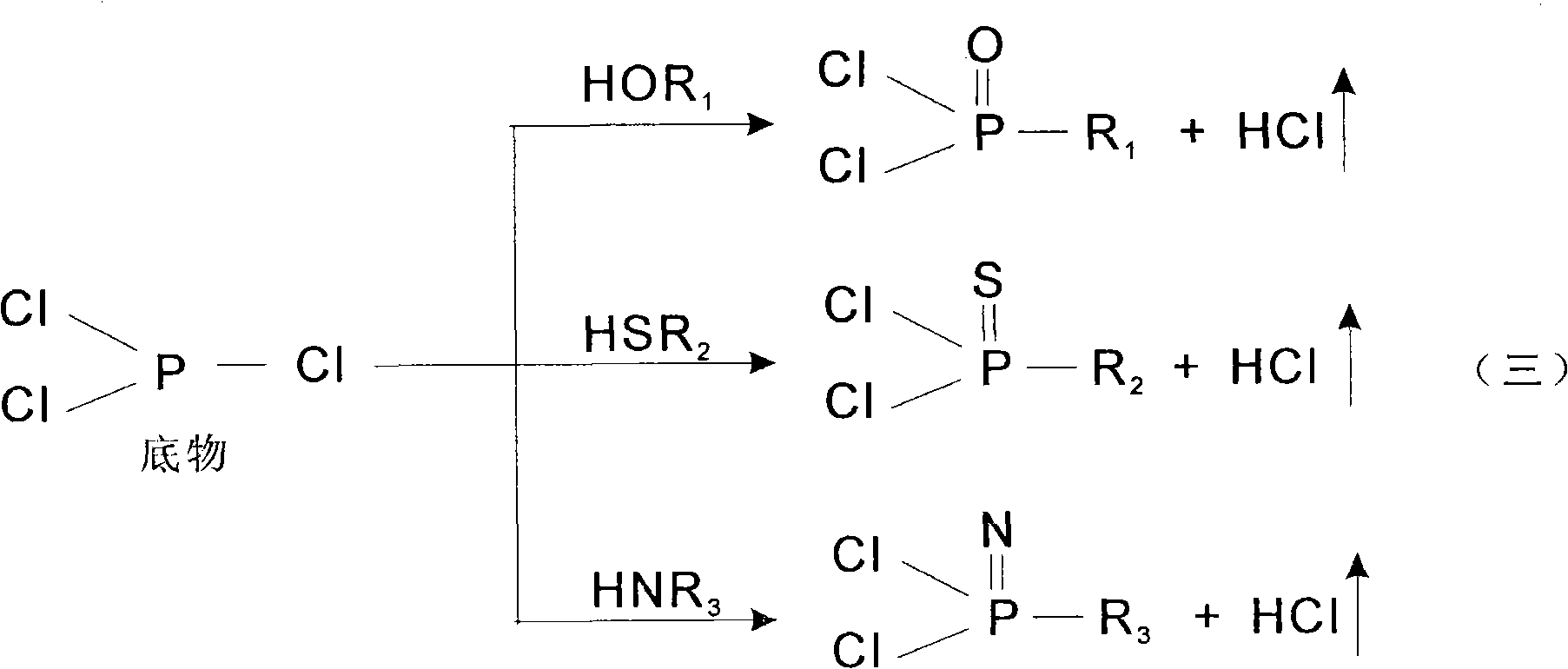
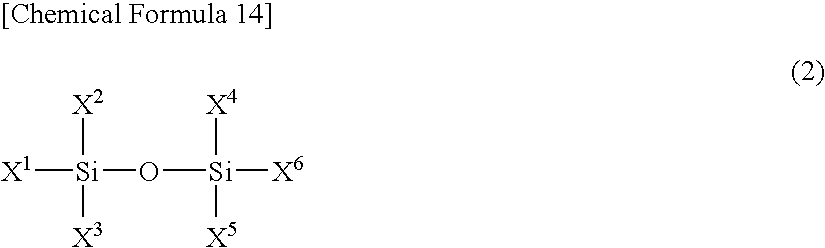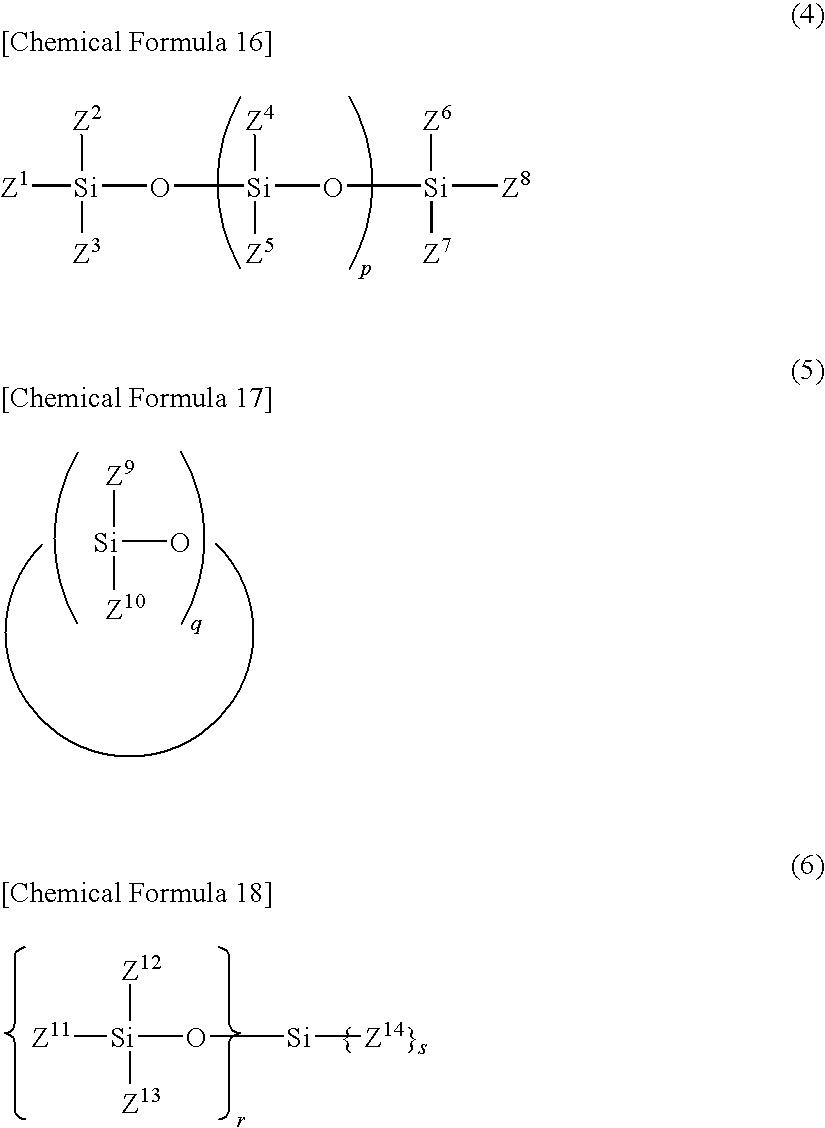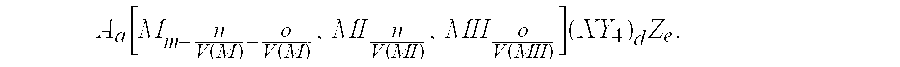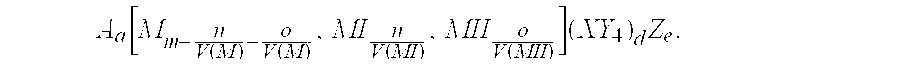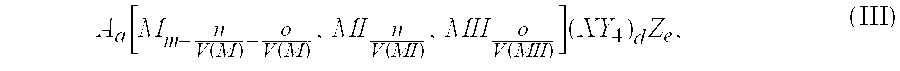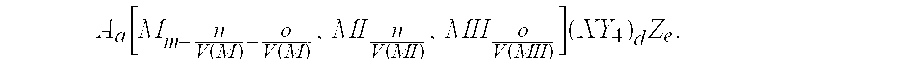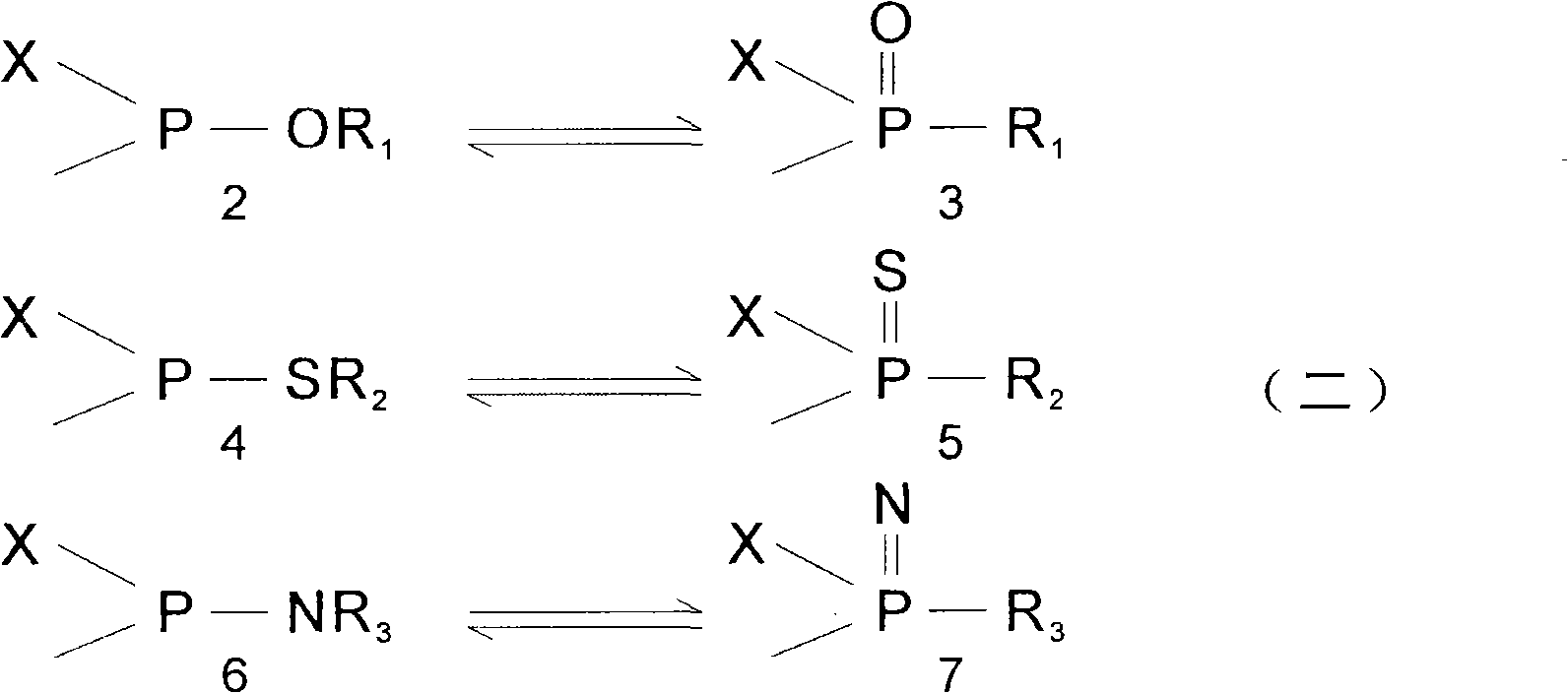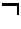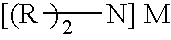Patents
Literature
304results about "Phosphorus halides/oxyhalides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method of hydrothermal liquid phase sintering of ceramic materials and products derived therefrom
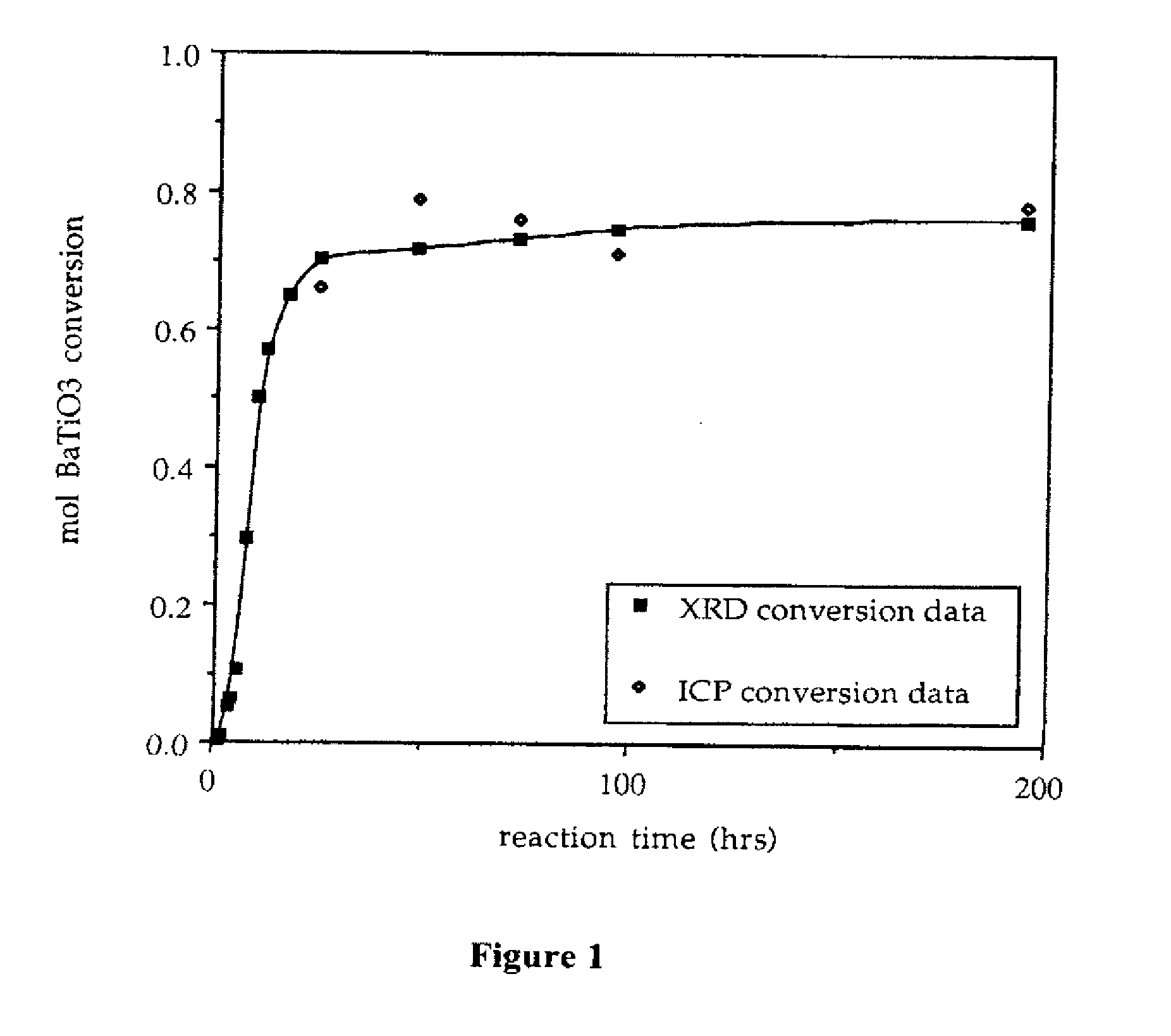
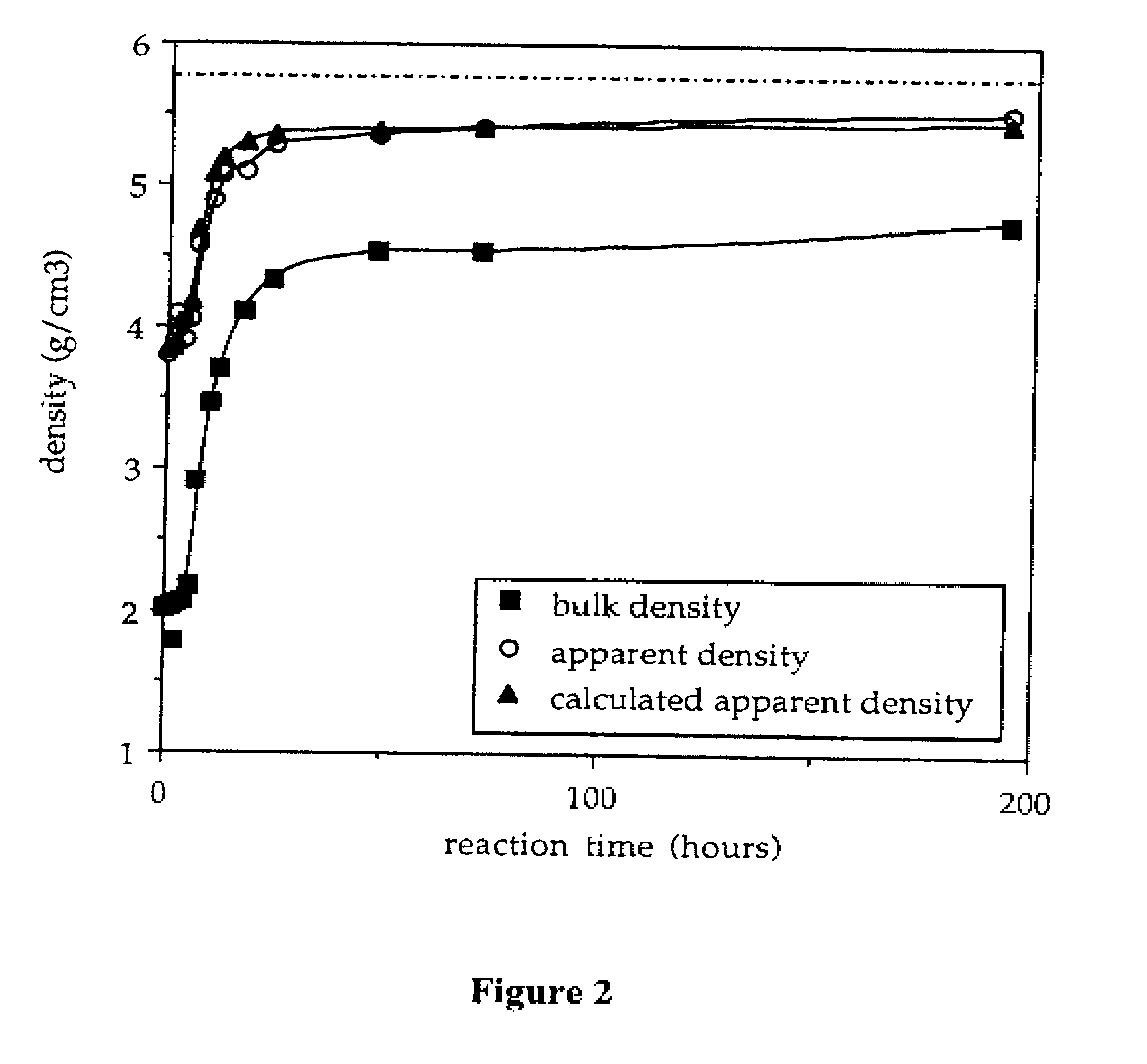
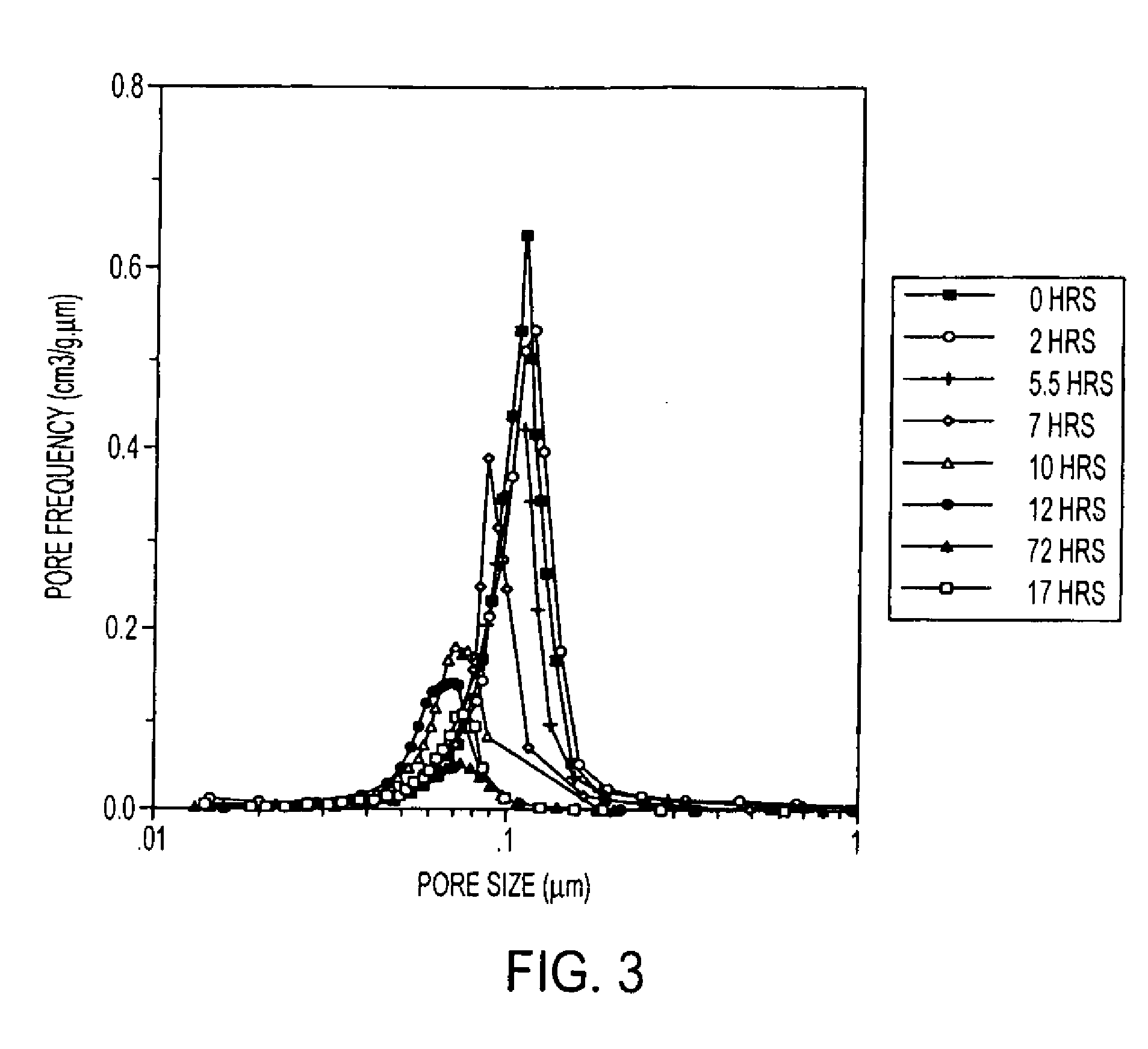
Provided here is a method of producing a monolithic body from a porous matrix, comprising: (i) providing a porous matrix having interstitial spaces and comprising at least a first reactant; (ii) contacting the porous matrix with an infiltrating medium that carries at least a second reactant; (iii) allowing the infiltrating medium to infiltrate at least a portion of the interstitial spaces of the porous matrix under conditions that promote a reaction between the at least first reactant and the at least second reactant to provide at least a first product; and (iv) allowing the at least first product to form and fill at least a portion of the interstitial spaces of the porous matrix, thereby producing a monolithic body, wherein the monolithic body does not comprise barium titanate.
Owner:RUTGERS THE STATE UNIV
Lithium difluorophosphate, electrolyte containing lithium difluorophosphate, process for producing lithium difluorophosphate, process for producing nonaqueous electrolyte, nonaqueous electrolyte, and nonaqueous electrolyte secondary battery containing the same
InactiveUS20090286155A1Good low temperatureExcellent cycle characteristicsPhosphorus halides/oxyhalidesCell electrodesBoiling pointSolvent
A difluorophosphate salt, which is expensive and not readily available, can be produced with a high purity readily and efficiently from inexpensive and readily available materials. A nonaqueous electrolyte secondary battery that exhibits low-temperature discharge and heavy-current discharge characteristics and high-temperature preservation and cycle characteristics without impairing the battery safety. A hexafluorophosphate salt is reacted with a compound having a bond represented by the following formula (1) in the molecule:Si—O—Si (1)A nonaqueous electrolyte used for nonaqueous electrolyte secondary batteries including a negative electrode and a positive electrode that can occlude and discharge ions, and a nonaqueous electrolyte is prepared from a mixture obtained by mixing at least one nonaqueous solvent, a hexafluorophosphate salt and a compound having a bond represented by the following formula (1), and removing low-boiling compounds newly formed in the system, the low-boiling compounds having a lower boiling point than that of the compound having the bond represented by the formula (1):Si—O—Si (1)
Owner:MITSUBISHI CHEM CORP
Porous material having hierarchical porous structure and preparation method thereof
Disclosed are porous ceramic balls with a hierarchical porous structure ranging in size from nanometers to micrometers, and preparation methods thereof. Self-assembly polymers and sol-gel reactions are used to prepare porous ceramic balls in which pores ranging in size from ones of nanometers to tens of micrometers are hierarchically interconnected to one another. This hierarchical porous structure ensures high specific surface areas and porosities for the porous ceramic balls. Further, the size and distribution of the pores can be simply controlled with hydrophobic solvent and reaction time. The pore formation through polymer self-assembly and sol-gel reactions can be applied to ceramic and transition metals. Porous structures based on bioceramic materials, such as bioactive glass, allow the formation of apatite therein and thus can be used as biomaterials of bioengineering, including bone fillers, bone reconstruction materials, bone scaffolds, etc.
Owner:KOREA INST OF MATERIALS SCI
Method for Producing Difluorophosphate, Nonaqueous Electrolyte Solution for Secondary Battery and Nonaqueous Electrolyte Secondary Battery
ActiveUS20080102376A1Phosphorus halides/oxyhalidesNon-aqueous electrolyte accumulatorsDifluorophosphateSource material
The present invention provides a simple method for producing a difluorophosphate from a source material, the difluorophosphate being useful as additives for nonaqueous electrolyte solutions for secondary batteries. In the method, a source material containing a carbonate and / or a borate is allowed to react with a source gas which contains P and F and which may further contain O as required. The source material may contain lithium carbonate. The source gas may be produced by decomposing LiPF6. The source gas may be produced in such a manner that LiPF6 and lithium carbonate are mixed and then subjected to reaction. The nonaqueous electrolyte solution contains the product obtained from the reaction.
Owner:MU IONIC SOLUTIONS CORP +1
Methods of making lithium metal cathode active materials
The invention provides a novel method for making lithium mixed metal materials in electrochemical cells. The lithium mixed metal materials comprise lithium and at least one other metal besides lithium. The invention involves the reaction of a metal compound, a phosphate compound, with a reducing agent to reduce the metal and form a metal phosphate. The invention also includes methods of making lithium metal oxides involving reaction of a lithium compound, a metal oxide with a reducing agent.
Owner:LITHIUM WERKS TECH BV
Nanostructured bioactive materials prepared by dual nozzle spray drying techniques
ActiveUS20060110306A1Easy to evaporatePromote formationMagnesium fluoridesImpression capsNanoparticleNanostructure
Nano-particles of calcium and phosphorous compounds are made in a highly pure generally amorphous state by spray drying a weak acid solution of said compound and evaporating the liquid from the atomized spray in a heated colunm followed by collection of the precipitated particles. Hydroxyapatite (HA) particles formed by such apparatus and methods are examples of particle manufacture useful in bone and dental therapies. Dual nozzle spraying etechniques are utilized for generally insoluble compounds.
Owner:ADA FOUND
Method for Producing Difluorophosphate, Non-Aqueous Electrolyte for Secondary Cell and Non-Aqueous Electrolyte Secondary Cell
ActiveUS20080305402A1Poor battery performanceExcellent coating agentPhosphorus halides/oxyhalidesNon-aqueous electrolyte accumulatorsDifluorophosphatePhysical chemistry
A difluorophosphate effective as an additive for a nonaqueous electrolyte for secondary battery is produced by a simple method from inexpensive common materials.The difluorophosphate is produced by reacting lithium hexafluorophosphate with a carbonate in a nonaqueous solvent. The liquid reaction mixture resulting from this reaction is supplied for providing the difluorophosphate in a nonaqueous electrolyte comprising a nonaqueous solvent which contains at least a hexafluorophosphate as an electrolyte lithium salt and further contains a difluorophosphate. Also provided is a nonaqueous-electrolyte secondary battery employing this nonaqueous electrolyte.
Owner:MU IONIC SOLUTIONS CORP +1
Mixed Lithium/Sodium Ion Iron Fluorophosphate Cathodes for Lithium Ion Batteries
InactiveUS20080153002A1Phosphorus halides/oxyhalidesSecondary cellsCarbon compositesLithium-ion battery
A compound having the formula LiaNa2−aFePO4F, wherein 0<a≦2 may be synthesized by exchanging lithium ions for sodium ions in Na2FePO4F. The compound may be used as a cathode material for a lithium ion battery. A battery may be comprised of an electrode active material having the formula Li2FePO4F, an anode; and an electrolyte. Na2FePO4F may be synthesized by flux reaction. Microcrystalline Na2FePO4F may be synthesized by a solution method. Na2FePO4F may be used as a cathode material for a lithium ion battery and may be carbon composite coated.
Owner:NAZAR LINDA FAYE +4
Method for Producing Lithium Difluorophosphate and Nonaqueous Electrolyte Battery Using the Same
ActiveUS20100323240A1Improve performanceLow costHybrid capacitor electrolytesPhosphorus halides/oxyhalidesSolventAqueous solution
[Problems] An object of the present invention is to provide: a production method for commercially advantageously producing lithium difluorophosphate or an electrolyte solution containing the lithium difluorophosphate, the lithium difluorophosphate serving as an additive useful for improving performance of a nonaqueous electrolyte battery; and a nonaqueous electrolyte battery employing the electrolyte solution for the nonaqueous electrolyte battery which solution contains the lithium difluorophosphate produced by the production method.[Means for Solving Problems] To provide an electrolyte solution for a nonaqueous electrolyte battery which solution contains lithium difluorophosphate, in such a manner as to produce lithium difluorophosphate in the electrolyte solution by reacting a halide other than a fluoride, LiPF6 and water in a nonaqueous solvent, the lithium difluorophosphate serving as an additive useful for improving performance of the nonaqueous electrolyte battery.
Owner:CENT GLASS CO LTD
Method of purifying lithium hexafluorosphate
InactiveUS6514474B1Phosphorus halides/oxyhalidesNon-aqueous electrolyte accumulatorsHydrogen fluoridePhosphor
A method of purifying lithium hexafluorosphate that allows to purify lithium hexafluorophosphate, useful as lithium secondary cell electrolyte, organic synthesis medium or the like, to an extremely high purity is provided. Lithium hexafluorophosphate containing harmful impurities such as oxyfluoride, lithium fluoride is purified by adding phosphoric chloride. The purification is performed in the presence of phosphoric chloride and hydrogen fluoride of the quantity equal or superior to the equivalent amount for reacting them, and then by converting lithium fluoride to lithium hexafluorophosphate with generated phosphor pentafluoride.
Owner:STELLA CHEMIFA CORP
Solid electrolyte
A solid electrolyte including an alkali metal element, phosphorous, sulfur and halogen as constituent components.
Owner:IDEMITSU KOSAN CO LTD
Secondary battery electrode active materials and methods for making the same
InactiveUS7008726B2Limit scopeIncrease capacitySilver accumulatorsPhosphatesElectrochemical cellBattery electrode
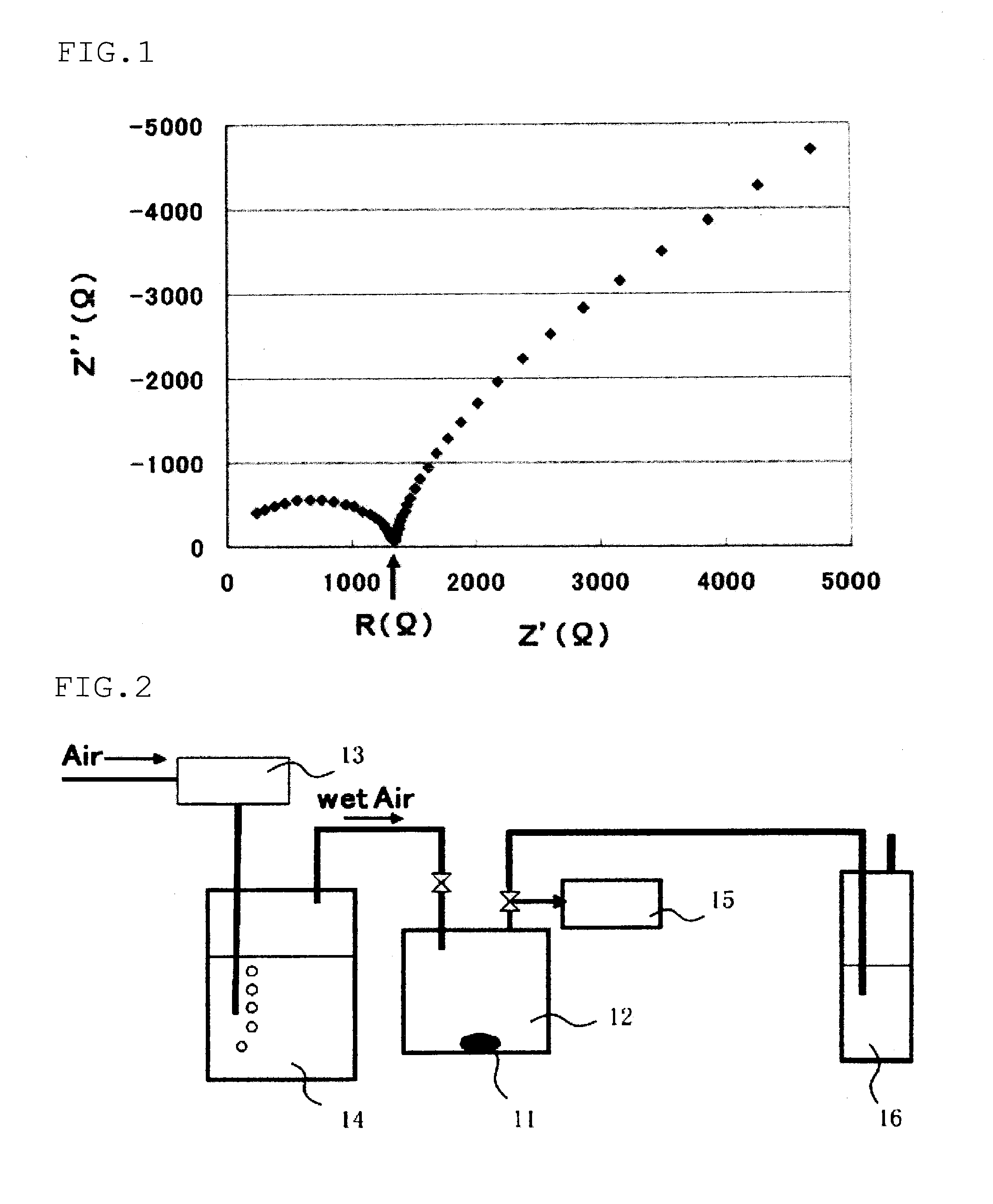
The invention provides an electrochemical cell which includes a first electrode and a second electrode which is a counter electrode to said first electrode, and an electrolyte material interposed there between. The first electrode comprises an electrode active material represented by the general nominal formula Aa[Mm,MIn,MIIo](XY4)dZe, wherein at least one of M, MI and MII is a redox active element, 0<m,n,o≦4, and ½[V(MI)+V(MII)]=V(M), wherein V(M) is the valence state of M, V(MI) is the valence state of MI, and V(MII) is the valence state of MII.
Owner:VALENCE TECH INC
Methods for preparing phosphorus pentafluoride gas and preparing lithium hexafluorophosphate using the gas
ActiveCN101353161AEasily hydrolyzedStrong moisture absorptionPhosphorus halides/oxyhalidesLithiumPhysical chemistry
A preparation method of phosphorus pentafluoride gas comprises a step of causing phosphorus pentachloride to react with anhydrous hydrogen fluoride, wherein, the reaction occurs in the presence of a solvent. A preparation method of lithium hexaflourophosphate comprises a contact reaction between solid lithium fluoride and phosphorus pentafluoride gas, wherein the phosphorus pentafluoride gas is prepared by the method of the invention. Compared with the preparation method of the lithium hexaflourophosphate with the phosphorus pentafluoride gas as the raw material in the prior art, the phosphorus pentafluoride gas prepared by the preparation method of the invention has higher purity and lower cost. The yield of the lithium hexaflourophosphate prepared by the method of the invention is higher than 93%, and the purity thereof is up to 99.95%.
Owner:BYD CO LTD
Preparation of phosphorus pentafluoride
A process for the preparation of anhydrous high purity phosphorus pentafluoride in high yield. The process uses an excess of hydrogen fluoride in a reaction with a phosphoric acid to form hexafluorophosphoric acid followed by reaction with a sulfur based acid reactant in a reaction medium containing an excess of hydrogen fluoride.
Owner:LITHDYNE
Secondary battery electrode active materials and methods for making the same
InactiveUS20050164084A1Limit scopeIncrease capacitySilver accumulatorsPhosphatesPhysical chemistryElectrochemical cell
The invention provides an electrochemical cell which includes a first electrode and a second electrode which is a counter electrode to said first electrode, and an electrolyte material interposed there between. The first electrode comprises an electrode active material represented by the general nominal formula Aa[Mm,MIn,MIIo](XY4)dZe, wherein at least one of M, MI and MII is a redox active element, 0<m,n,o≦4, and ½[V(MI)+V(MII)]=V(M), wherein V(M) is the valence state of M, V(MI) is the valence state of MI, and V(MII) is the valence state of MII.
Owner:VALENCE TECH INC
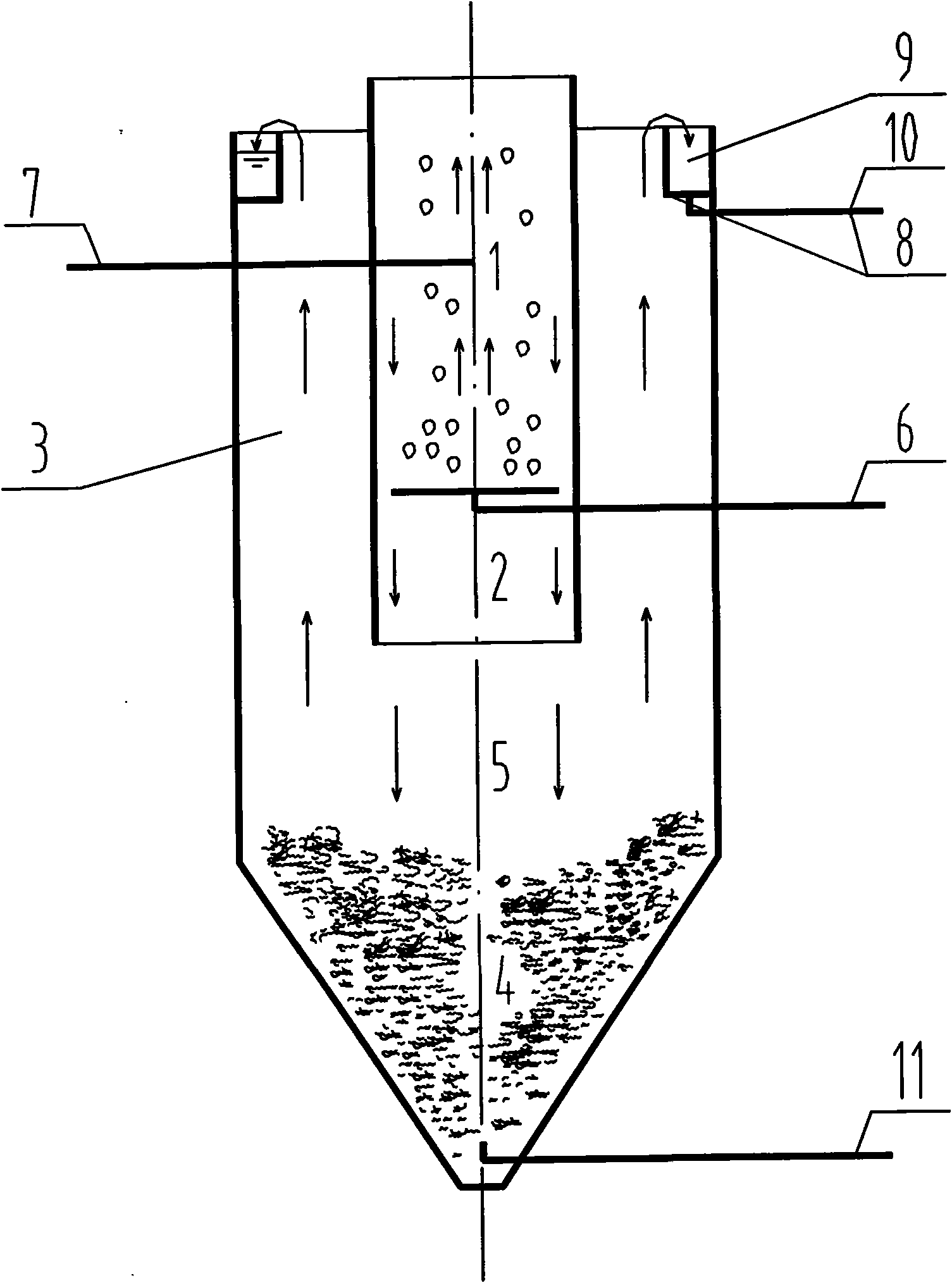
Phosphor recovery crystallization reactor and phosphor recovery method
ActiveCN101602535AHigh recycling efficiencyLow costPhosphorus halides/oxyhalidesWater/sewage treatmentRecovery methodHigh concentration
The invention relates to a phosphor recovery crystallization reactor and a phosphor recovery method. The reactor is in inner-outer dual barrel structure, wherein the inner barrel is disposed at the center position of the outer barrel. The reactor includes an aeration stripping area, a crystallization reaction area, a precipitation separation area, a high concentration phosphor mud area and a buffering area, wherein the crystallization reaction area communicates with the precipitation separation area through the upper portion of the buffering area, so that the sewage water after the crystallization reaction in the crystallization reaction area flows into a precipitation separation area without affecting the high concentration phosphor mud area. The invention fully utilizes effective ingredients in the anaerobic treated water of the high concentration waste water of domestic animal and poultry, enhances pH value of the anaerobic treated water by aeration stripping, removes phosphor in the form of crystal of ammoniomagnesium phosphate using relatively high concentration of phosphor and ammonian in the waste water of domestic animal and poultry. The total removal rate of the phosphor can reach 50-60% without any chemical agents added. Phosphor is recovered in form of crystals, which is beneficial to utilization of the recovered phosphor.
Owner:BEIJING MUNICIPAL RES INST OF ENVIRONMENT PROTECTION
Isomerization reaction
The invention discloses a highly selective novel reaction with an important application value in organic phosphorus synthesis methodology. The reaction can be applied in important pesticide commodities of acephate, glyphosate, glufosinate-ammonium, chloramine phosphorus, profenofos, and the like. With the reaction, innovative technical synthetic routes can be designed. With the reaction, standards of clean production technologies can be satisfied, and the production cost is greatly reduced compared to existing technical routes.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
In situ restoration of apatite-based chromatography resins
Methods and compositions are provided for treatment of an apatite-based resin from which retained solutes have been eluted by an elution buffer that contains an alkali metal salt with solutions of calcium ion, phosphate ion, and hydroxide separately from any sample loading and elution buffers. The treatment solutions restore the resin, reversing the deterioration that is caused by the alkali metal salt in the elution buffer.
Owner:BIO RAD LAB INC
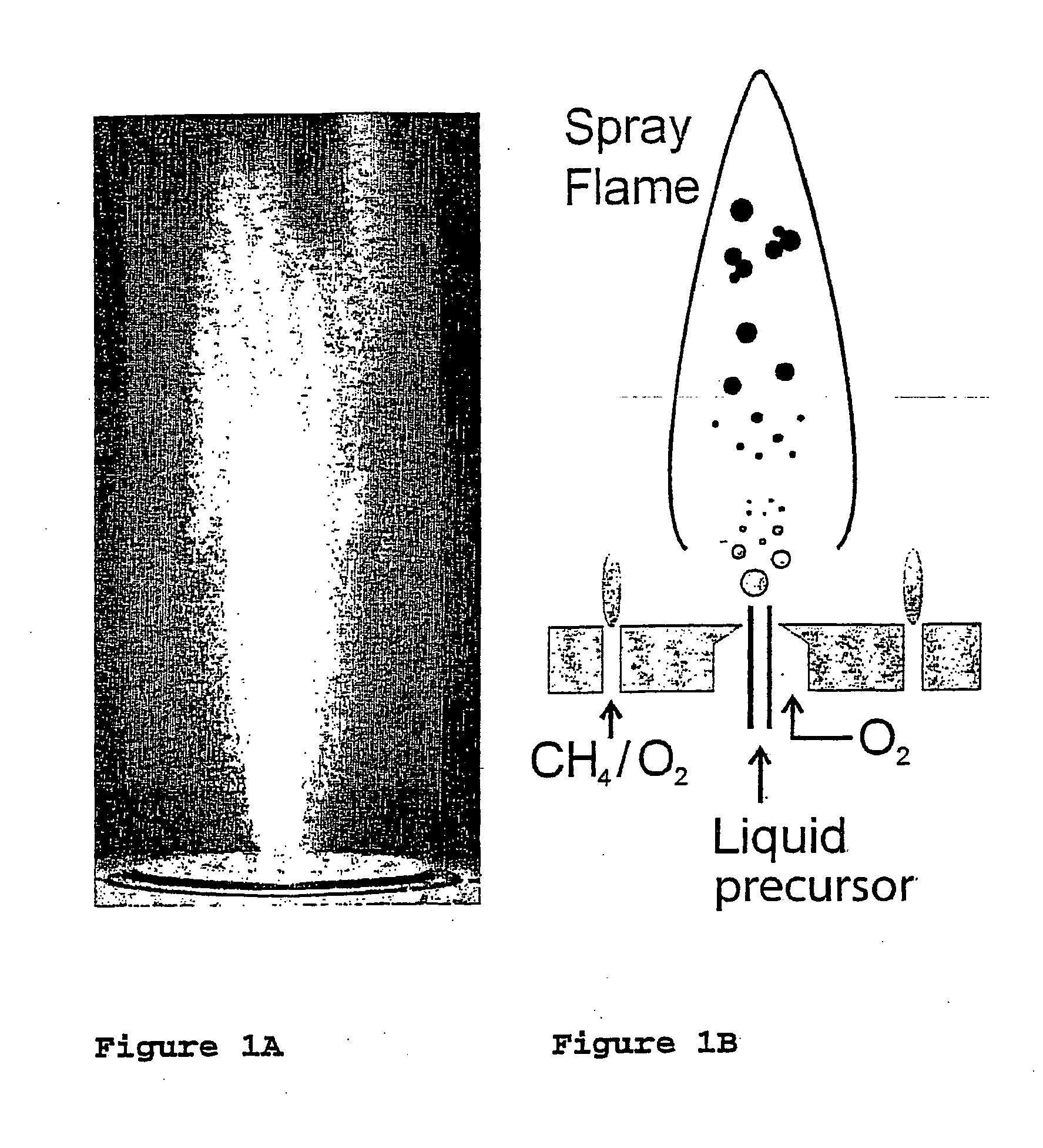
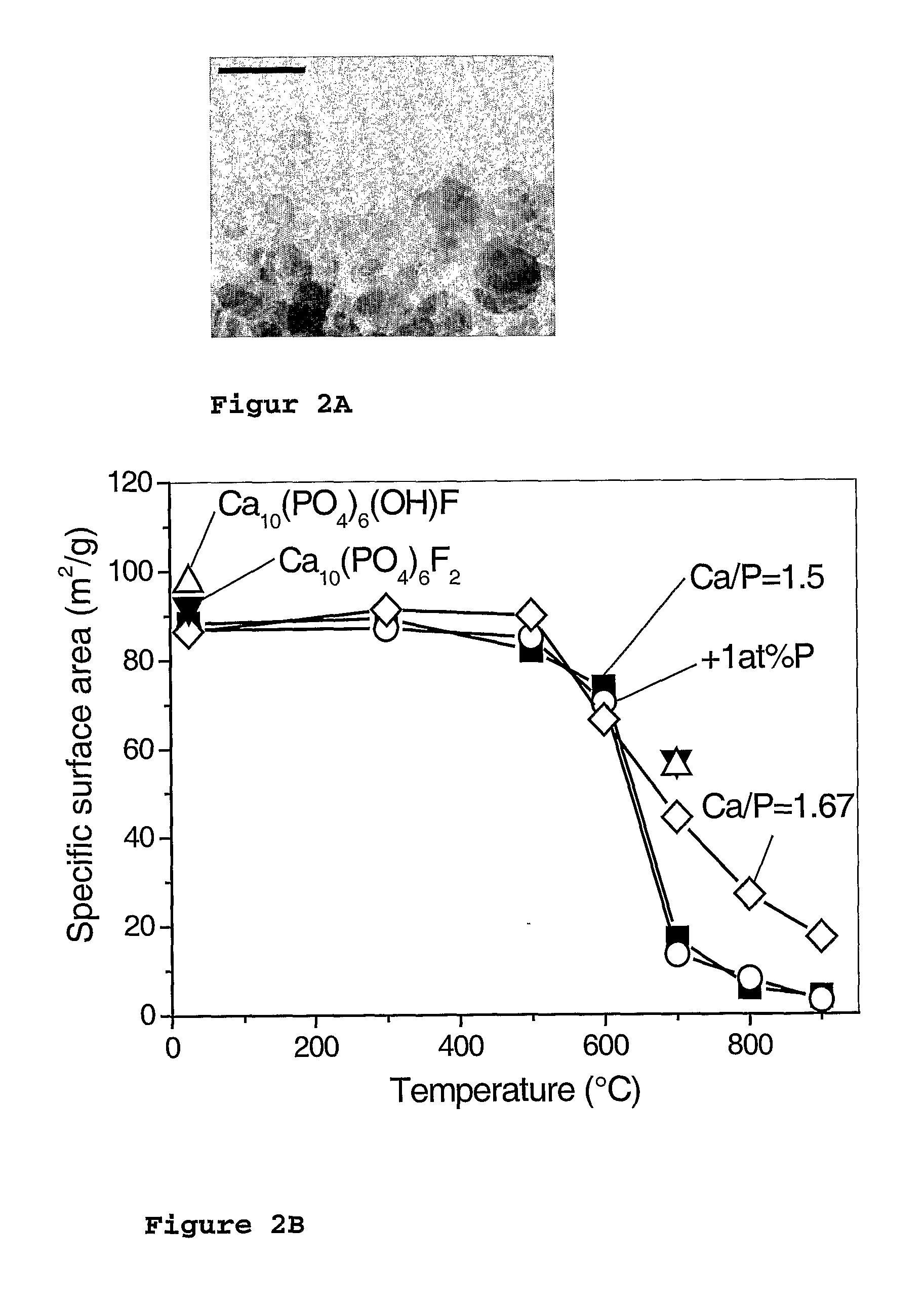
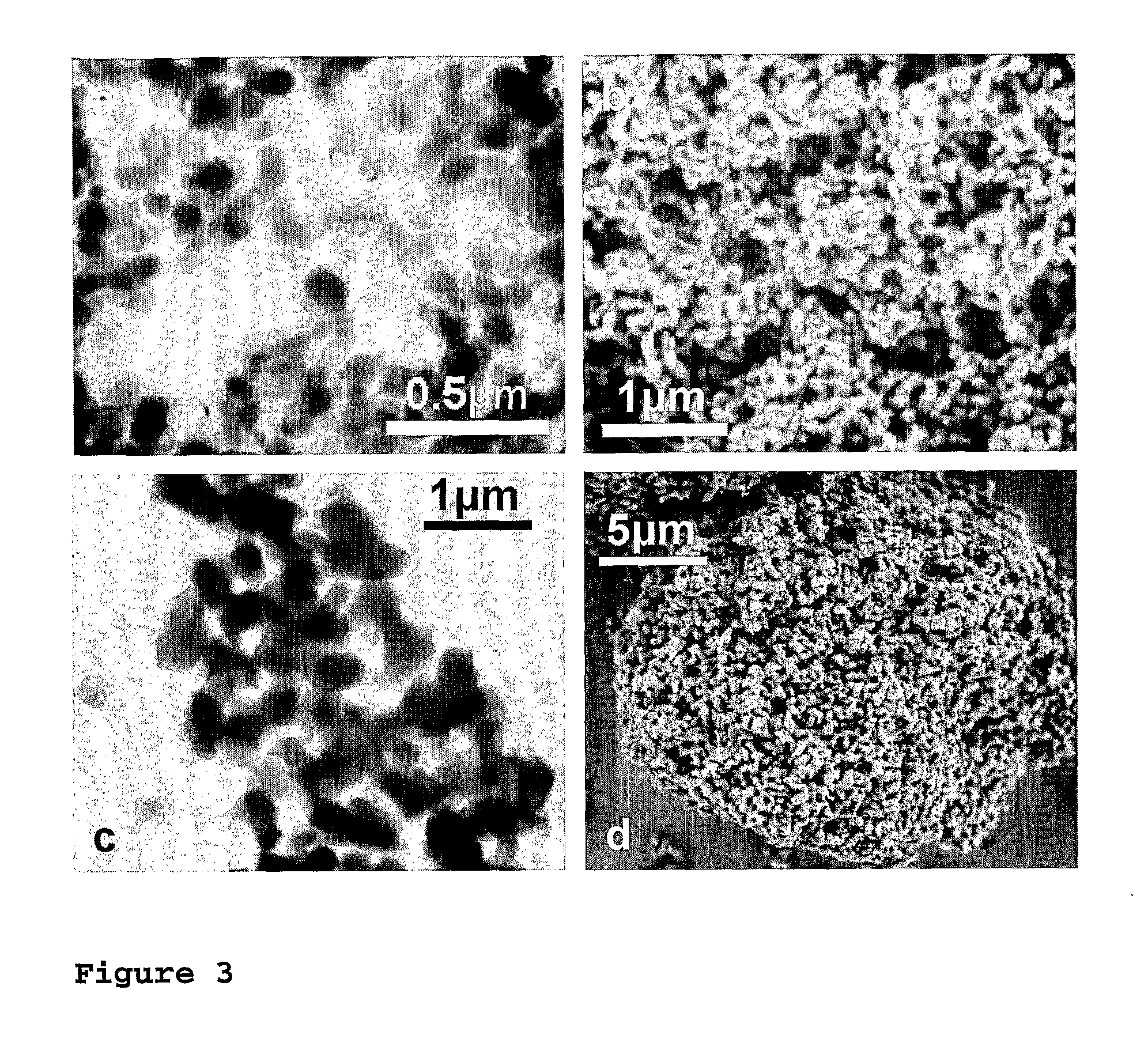
Flame synthesis of metal salt nanoparticles, in particular calcium and phosphate comprising nanoparticles
InactiveUS20070196259A1Increase chanceCalcium/strontium/barium carbonatesMaterial nanotechnologyBiocompatibility TestingMetallacarboxylic acid
Described is a method for the production of metal salts, wherein the cationic metal is preferably selected from Group I to IV metals and mixtures thereof and the anionic group is selected from phosphates, silicates, sulfates, carbonates, hydroxides, fluorides and mixtures thereof, and wherein said method comprises forming a mixture of at least one metal source that is a metal carboxylate with a mean carbon value per carboxylate group of at least 3 and at least one anion source into droplets and oxiding said droplets in a high temperature environment, preferably a flame. This method is especially suited for the production of calcium phosphate biomaterials such as hydroxyapatite (HAp,Cal0(P04)6(OH)2) and tricalcium phosphate (TCP,Ca3(P04)2) that exhibit excellent biocompatibility and osteoconductivity and therefore are widely used for reparation of bony or periodontal defects, coating of metallic implants and bone space fillers.
Owner:EIDENGOSSICHE TECHN HOCHSCHULE ZURICH
Method for producing inorganic compounds
InactiveUS20120007020A1Prevent oxidationElectrode manufacturing processesPhosphatesRare-earth elementAlkaline earth metal
Compounds (I) AaMm(YO4)yZz (I) are provided where A is at least one element selected from the alkaline metals, alkaline earth metals, a doping element and a hole, M being (T1-1TV), T being one or more transition metals and T′ being at least on element selected from Mg, Ca, Al, and the rare earths, 0≦t<1; Y is a least one element selected from S, Se, P, As, Si, or Ge and A1; Z is at least one element selected from F, O or OH; a, m, y, and z are whole numbers of zero or above such that the electric neutrality of the inorganic oxide of formula (I) is respected, a≧O; m>O; y>0; z≧O. The compounds (I) are obtained from precursors of the constituent elements by means of a method comprising the following steps: dispersion of said precursors in a liquid support comprising one or more ionic liquids made up of a cation and an anion the electric charges of which balance out to give a suspension of said precursors in said liquid, heating said suspension to a temperature of 25 to 380° C. and separation of said ionic liquid and the inorganic oxide of formula (I) from the reaction of said precursors.
Owner:CENT NAT DE LA RECHERCHE SCI +1
Methods and compositions for the treatment of cancer
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
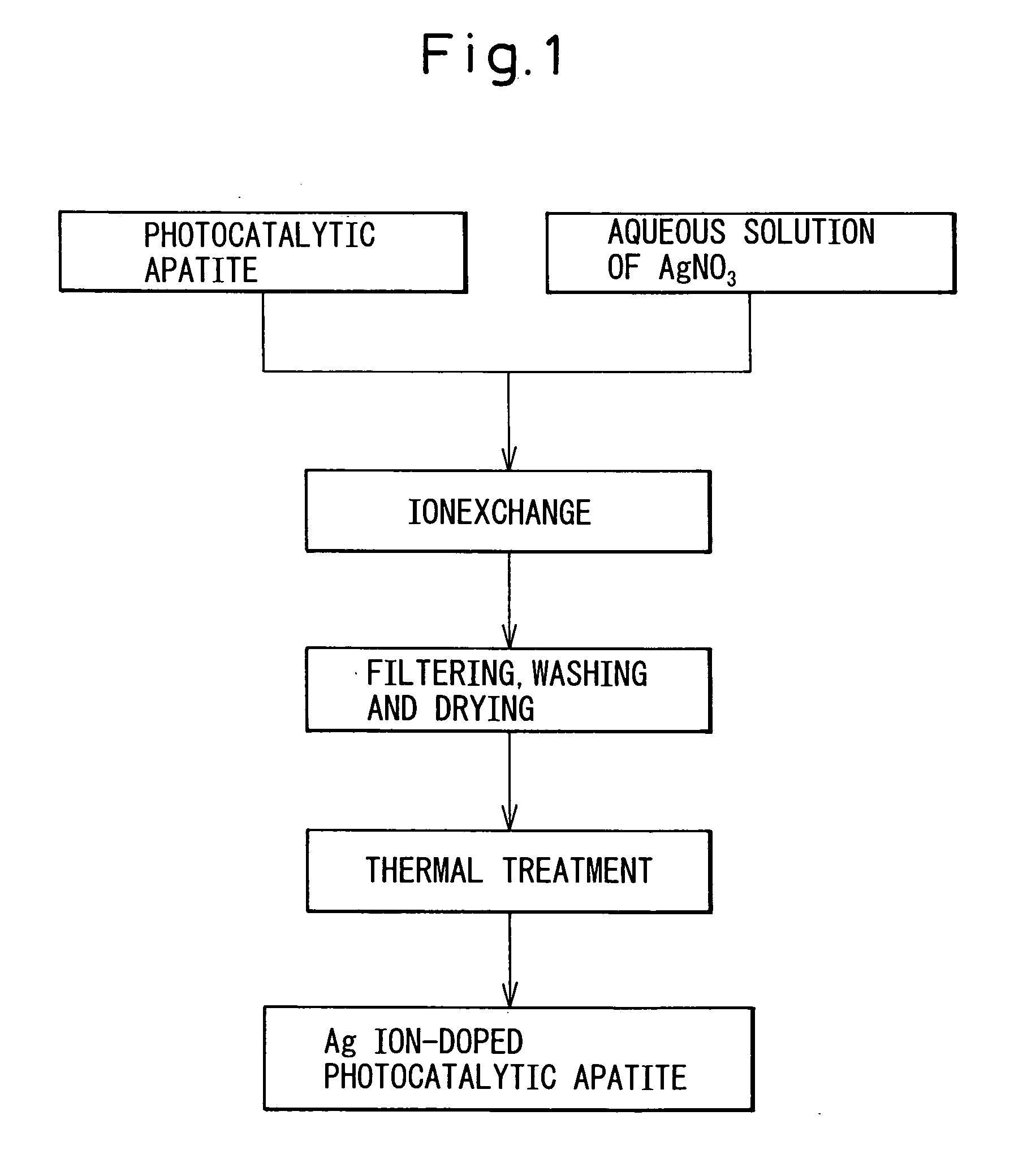
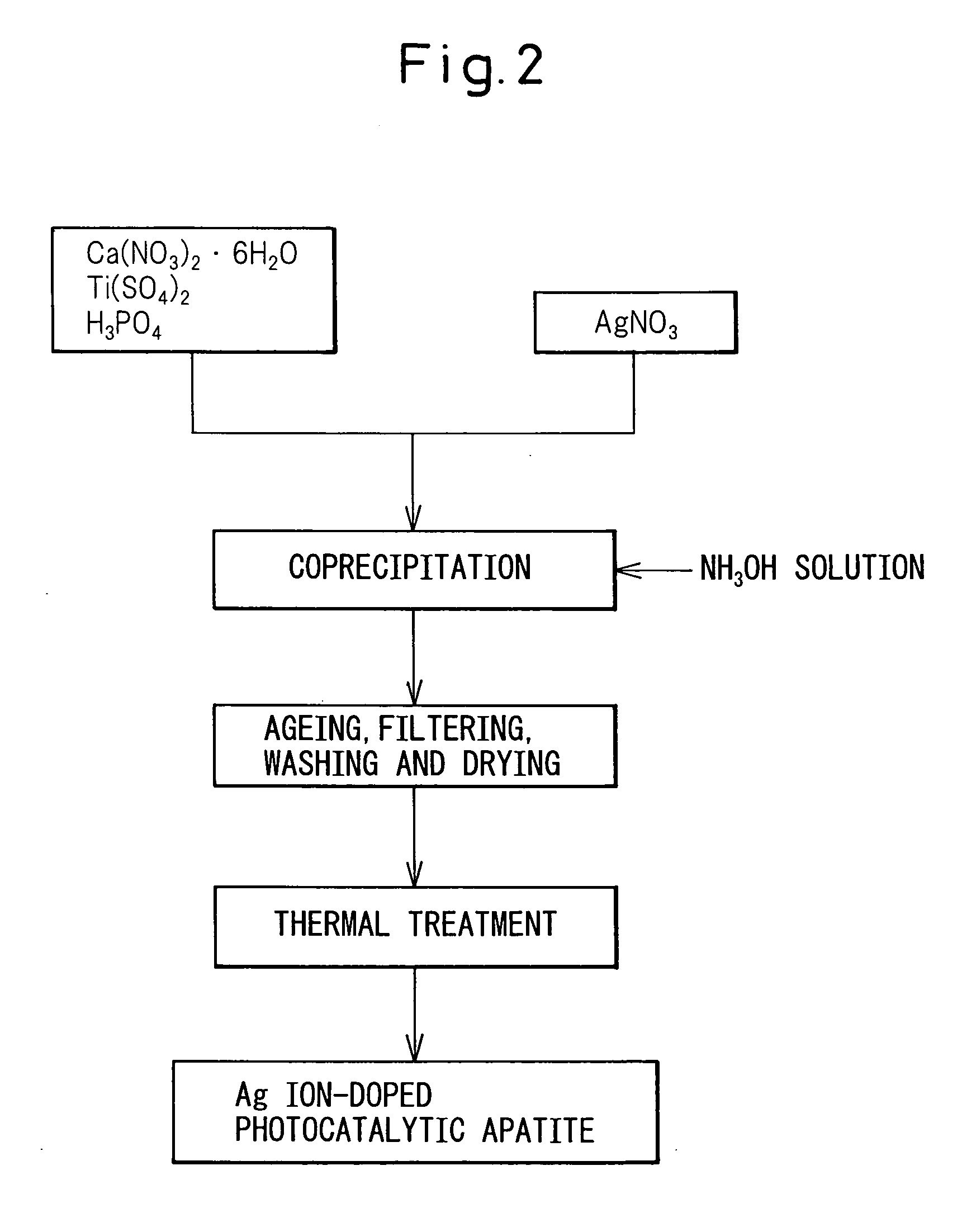
Photocatalytic apatite composition as well as production method and article thereof
ActiveUS20070232487A1Improve antibacterial propertiesPromote decompositionFireproof paintsCosmetic preparationsDecompositionCrystal structure
The photocatalytic apatite composition and its production method are disclosed. The photocatalytic apatite comprises a photocatalytic apatite having incorporated into the apatite crystal structure thereof a metal oxide having a photocatalytic action, such as titanium oxide, and a metal ion having an antimicrobial property, such as a silver ion or a copper ion. The photocatalyst apatite composition is capable of maintaining excellent decomposition and adsorption properties for various organic materials such as VOCs or specific adsorbing substances such as a virus for a long time and, at the same time, expressing an excellent antimicrobial property in a dark place as well as under daylight.
Owner:FUJITSU LTD
Fluoroalkyl phosphates for use in electrochemical cells
InactiveUS6841301B2Simple preparation processExtend and improve lifeHybrid capacitor electrolytesPhosphatesGalvanic cellAlkylphosphate
Owner:MERCK PATENT GMBH
Preparation process of high-purity phosphorus pentafluoride
ActiveCN101844754AEffective control of feed rateControl feed ratePhosphorus halides/oxyhalidesPhysical chemistryHydrogen chloride
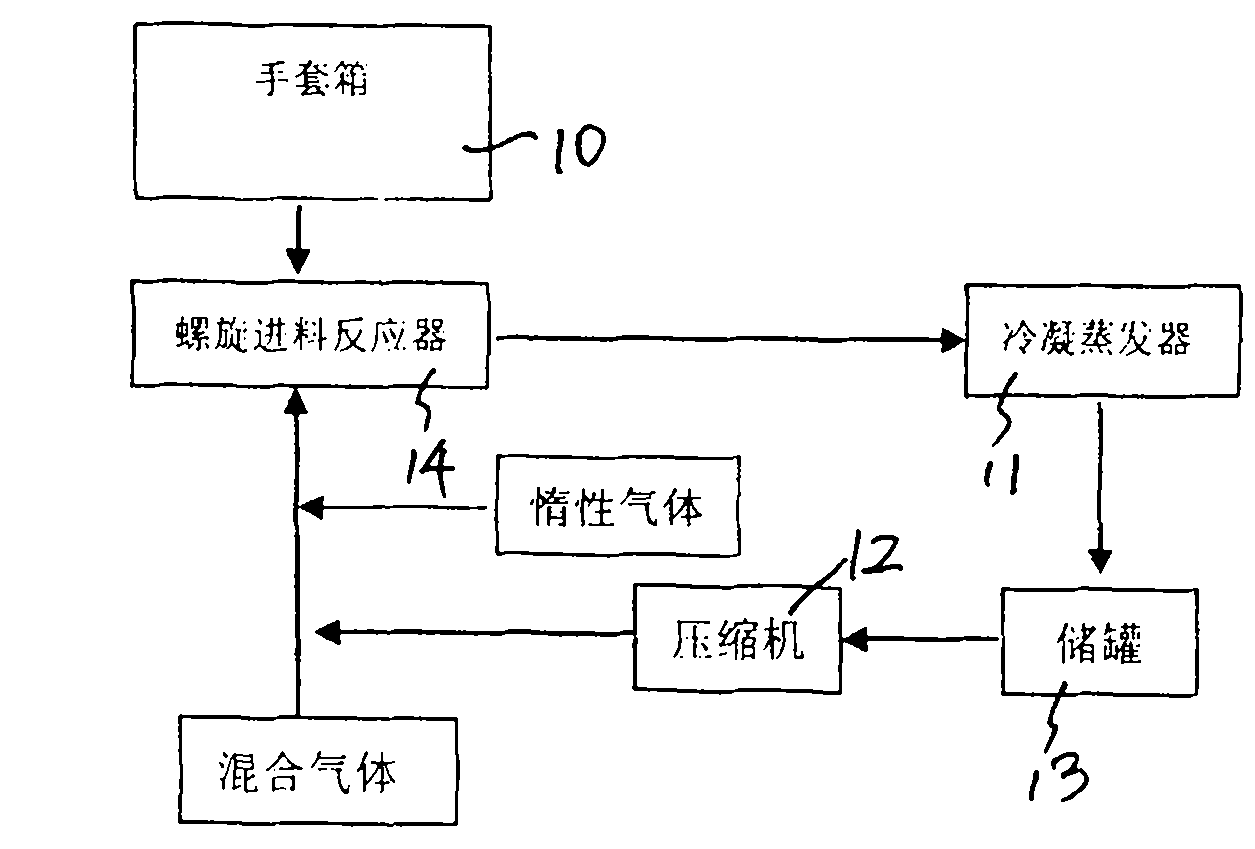
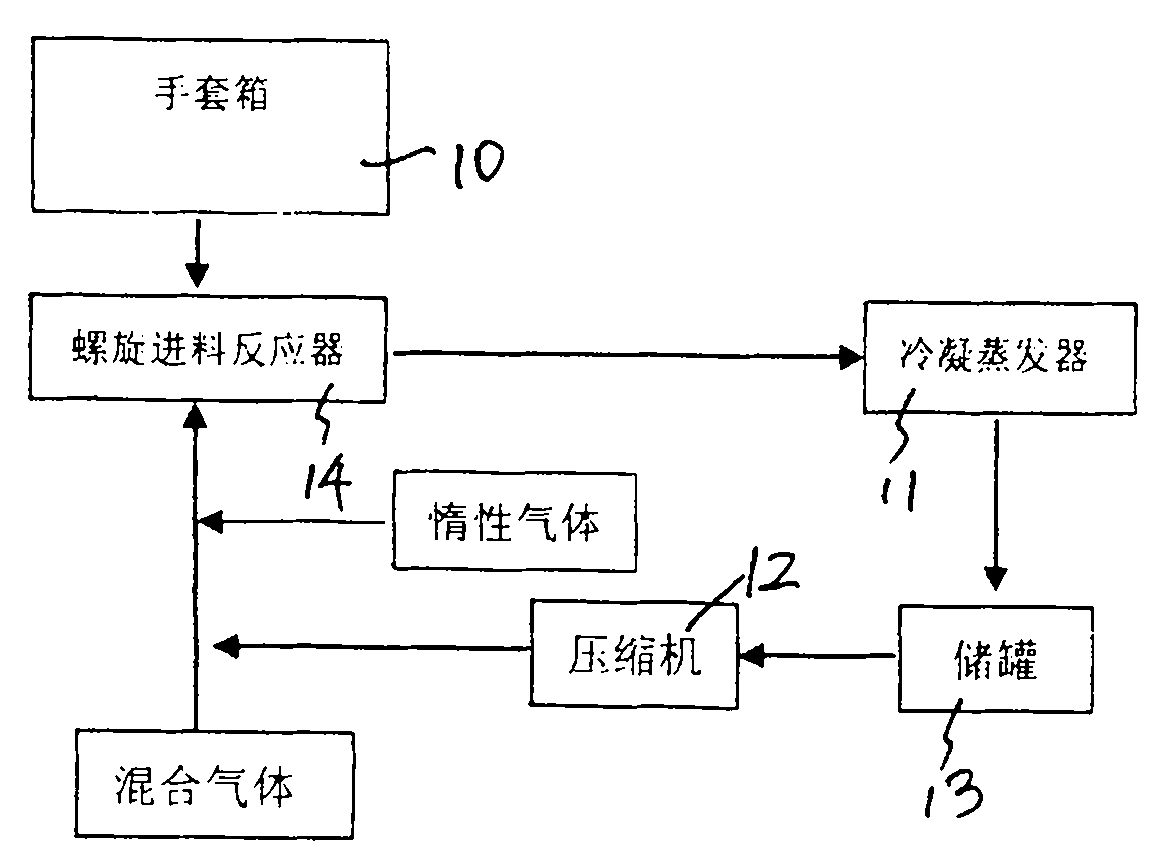
The invention discloses a preparation process of high-purity phosphorus pentafluoride, which comprises the following steps of: firstly adding phosphorous pentachloride into a feed hopper of a spiral feeder under the protection of dry gas in a glove box to assemble a gas circulation loop, and simultaneously adding inert gas into the gas circulation loop in advance; starting a spiral stirring reaction furnace, simultaneously injecting mixed gas of hydrogen fluoride and fluorine gas into the spiral stirring reaction furnace, and controlling the reaction process by controlling the rotation frequency of the phosphorous pentachloride in the spiral feeder, the temperature of gas flow at the outlet of the spiral stirring reaction furnace and the pressure of the gas circulation loop; freezing the phosphorus pentafluoride gas and the hydrogen fluoride gas generated in the reaction process and unreacted hydrogen fluoride gas by a condenser-evaporator, and collecting high-purity phosphorus pentafluoride gas after reaction. The method is simple and effective and can be operated easily.
Owner:JIANGSU JIUJIUJIU TECH
Preparation method of high purity phosphorus pentafluoride
ActiveCN104261369ALow costNo occupational health hazardPhosphorus halides/oxyhalidesPhosphoric acidSulfur trioxide
The invention provides a preparation method of high purity phosphorus pentafluoride. The preparation method comprises the following steps: step one, taking polyphosphoric acid and waterless hydrogen fluoride as the primary raw materials to prepare a hexafluorophosphoric acid water solution; step two, reacting the hexafluorophosphoric acid water solution obtained in the step one with sulfur trioxide so as to obtain a mixture of hexafluorophosphoric acid and sulfuric acid; step three, directly heating the mixture of the hexafluorophosphoric acid and sulfuric acid obtained in the step two without separation, condensing the generated phosphorus pentafluoride steam so as to obtain a coarse product of phosphorus pentafluoride; step four, refining the obtained coarse product so as to obtain high purity phosphorus pentafluoride with a purity more than 99.5%. The product prepared by the provided preparation method has a high purity and low purity content, wherein the phosphorus pentafluoride content is not less than 99.95%, the waterless hydrogen fluoride content is not more than 50 ppm, the water content is not more than 10 ppm, the metal ion content is not more than 1 ppm, and the content of other purities is not more than 5 ppm.
Owner:JIUJIANG TINCI ADVANCED MATERIALS CO LTD
System and method for preparing high-purity phosphorus pentafluoride
ActiveCN102757027AAvoid introducingAvoid secondary pollutionPhosphorus halides/oxyhalidesPhysical chemistryPhosphorus pentafluoride
The invention relates to a system and a method for preparing high-purity phosphorus pentafluoride. The high-purity phosphorus pentafluoride is prepared from raw materials comprising PCl3, Cl2 and anhydrous HF by performing on-line purification treatment on the raw materials, performing on-line direction transformation on the intermediate product PCl5 into the PF5, performing on-line purification on the crude PF5, and the like. Preparation of the high-purity phosphorus pentafluoride is realized by the economic, high-efficiency and conveniently-operated method. The system and the method are characterized in that the high-purity phosphorus pentafluoride is prepared by performing on-line purification on the crude PF5 by two-stage condensers; and the temperatures of the two-stage condensers are respectively -50 to 19 DEG C and -85 to -76 DEG C.
Owner:陕西延长石油集团氟硅化工有限公司
Processes for producing phosphorus pentafluoride and hexafluorophosphate
InactiveCN101605721AEasy to operateReduce synthesisPhosphorus halides/oxyhalidesDispersed particle separationPhosphatePhysical chemistry
Owner:STELLA CHEMIFA CORP
Processes for production of phosphorus pentafluoride and hexafluorophosphates
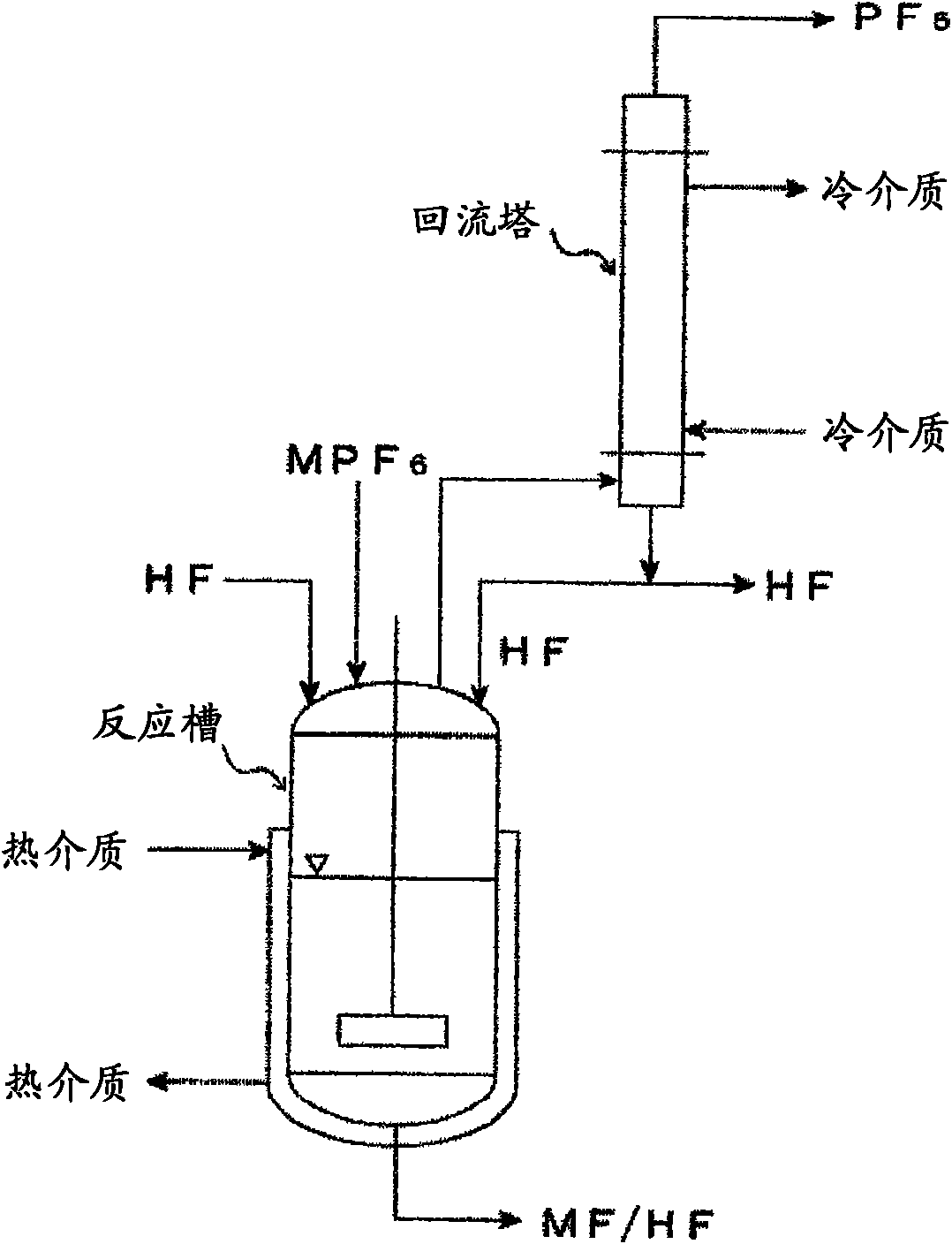
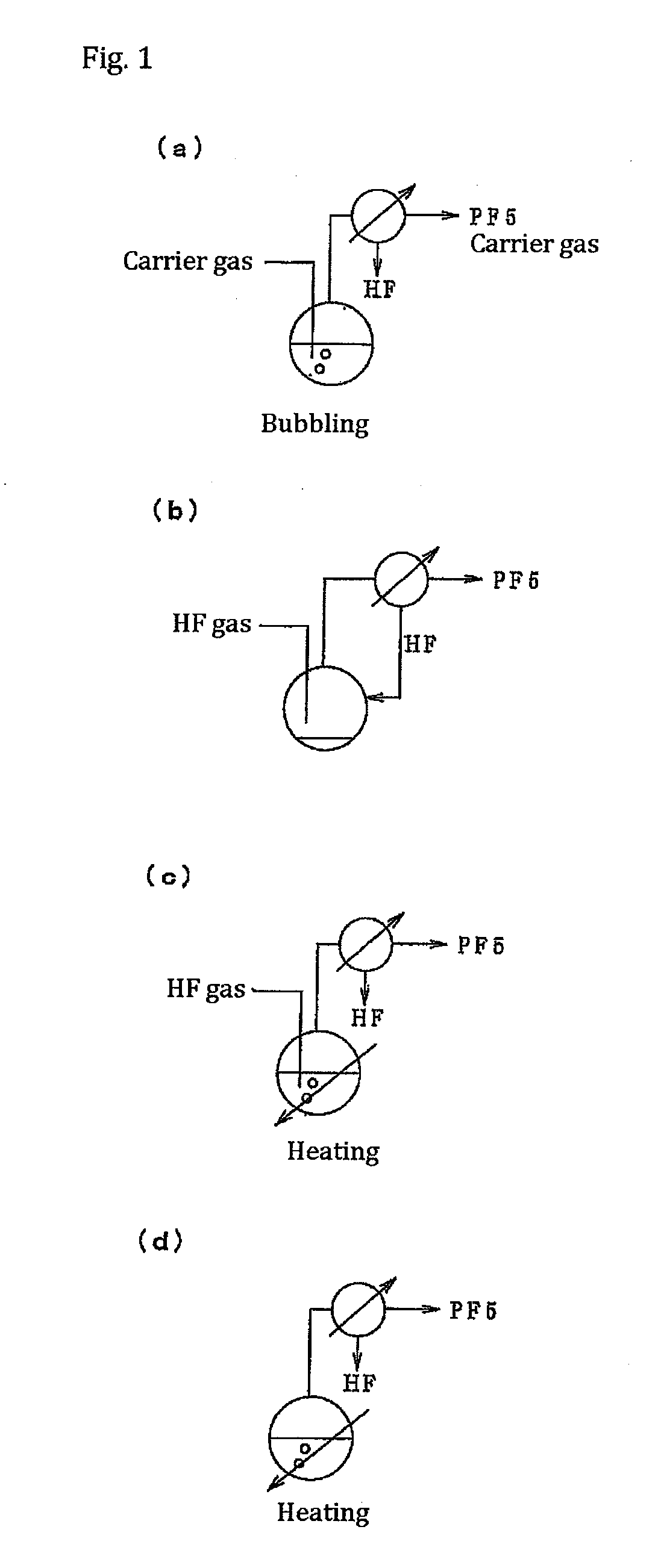
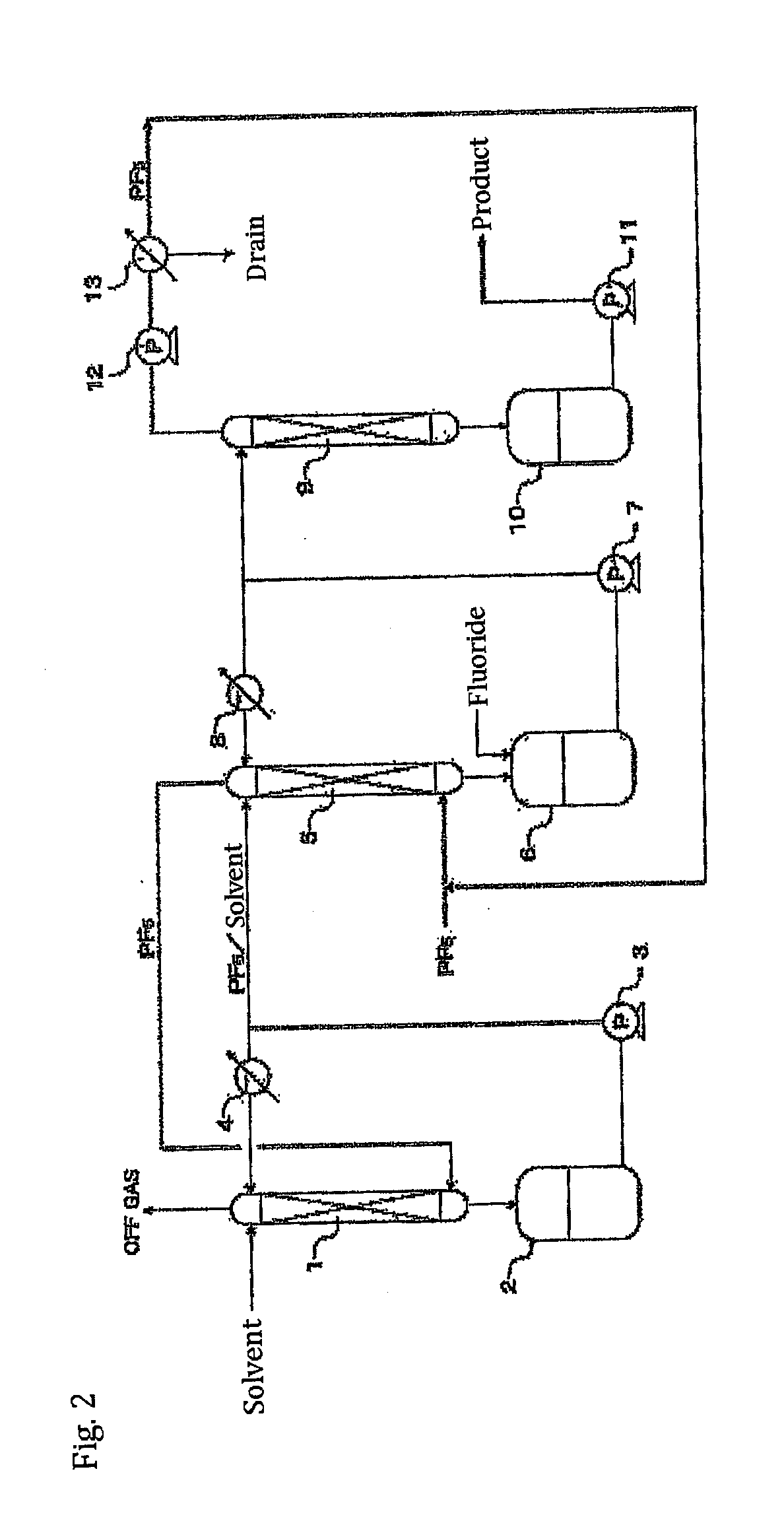
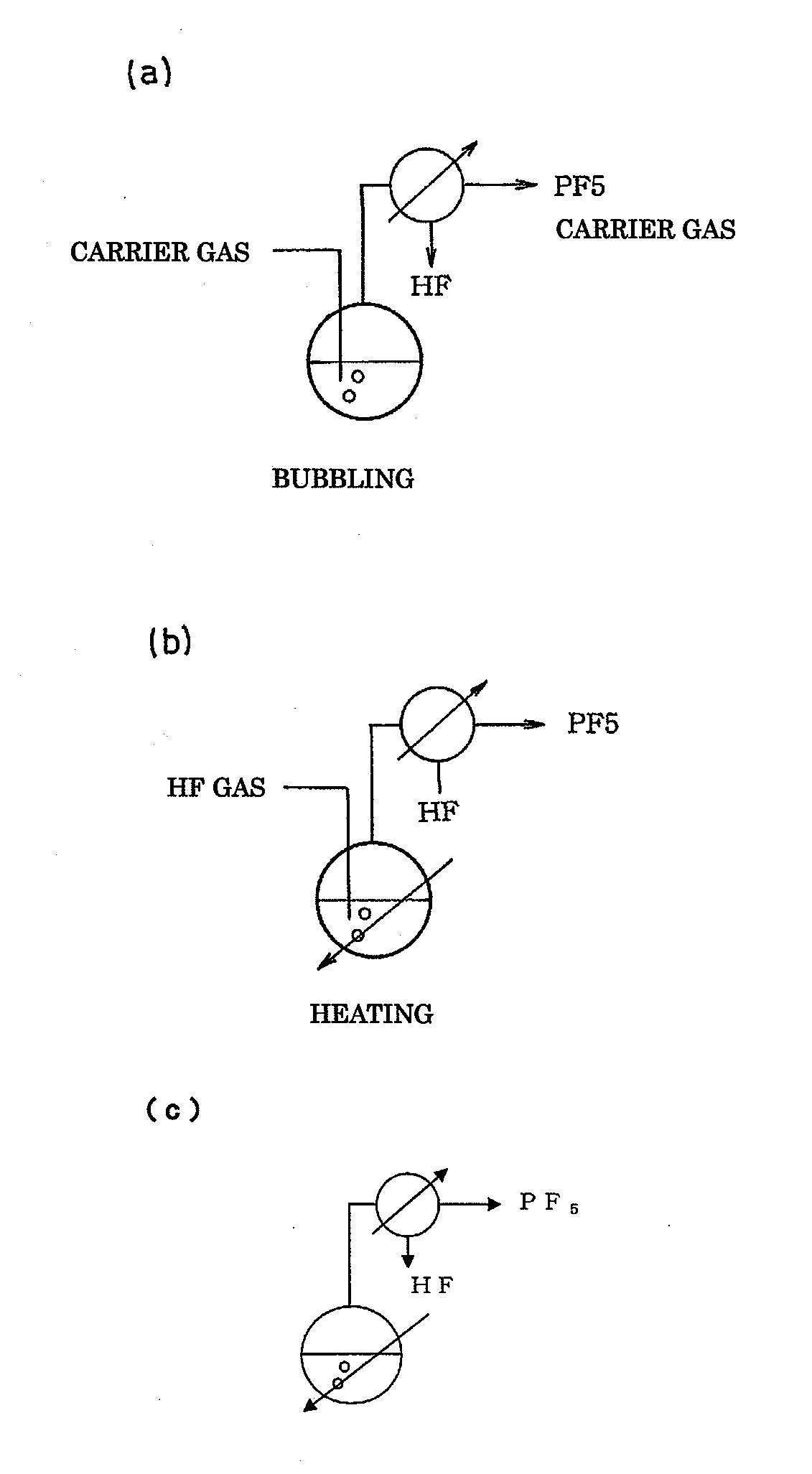
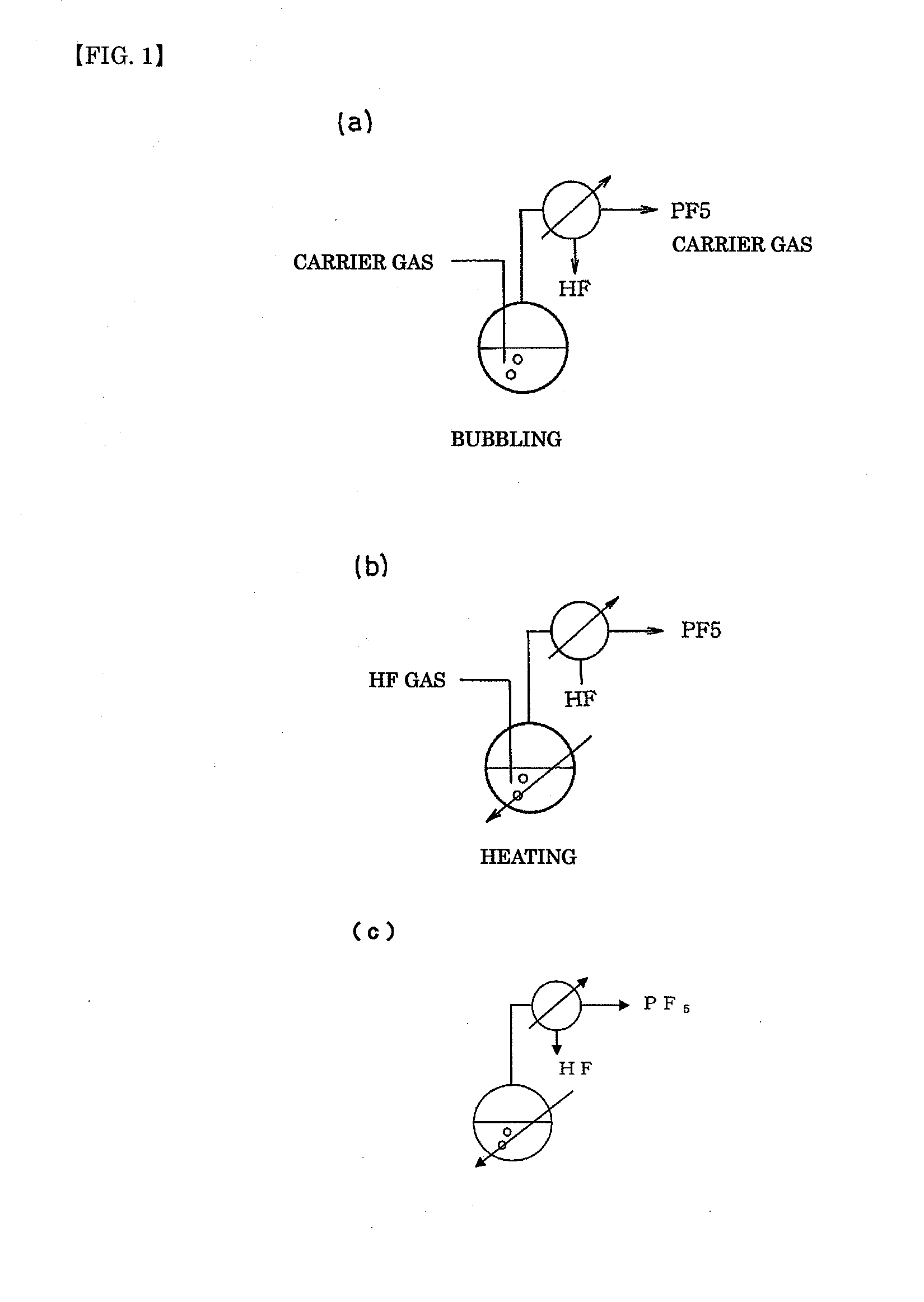
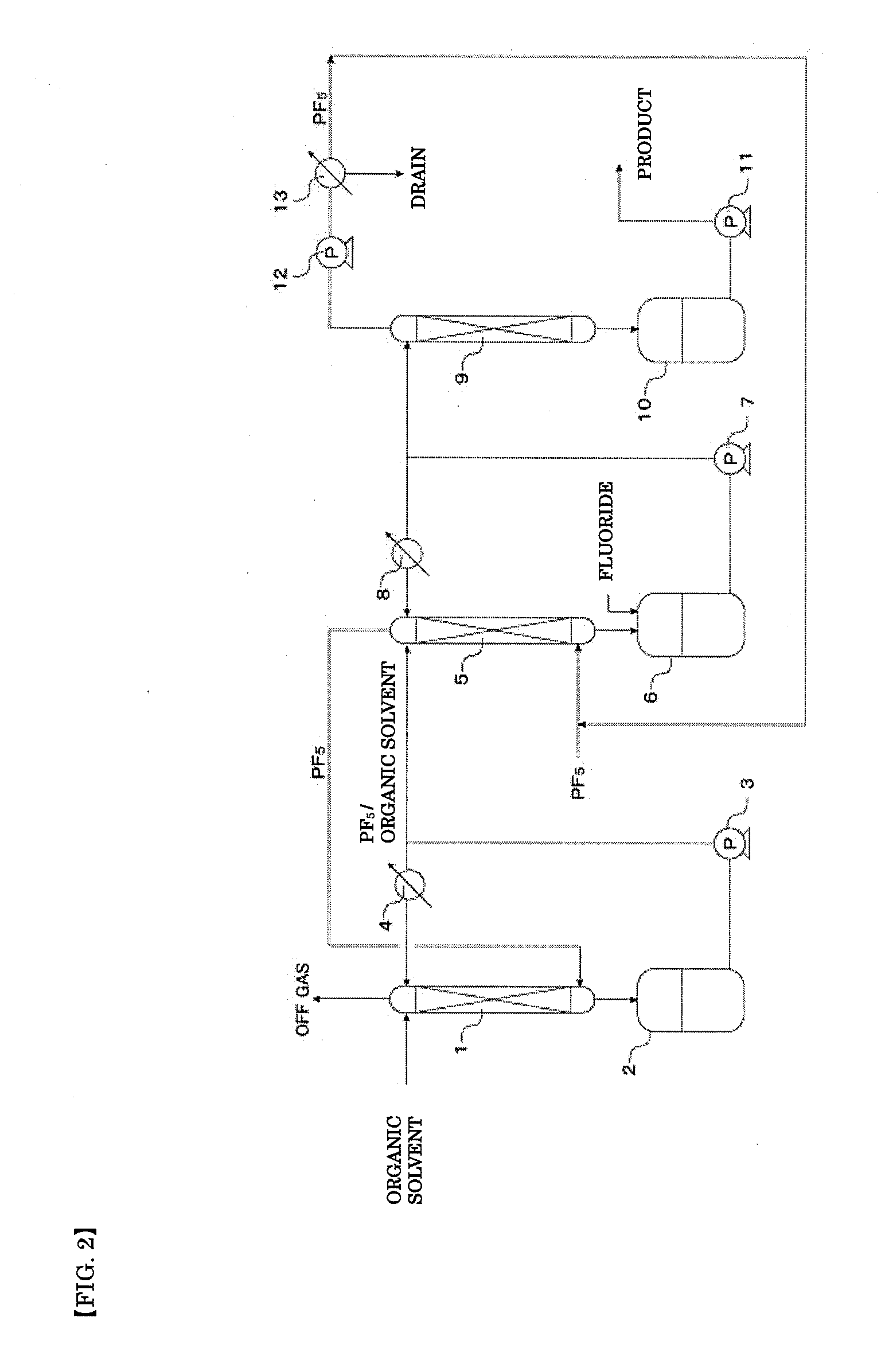
ActiveUS20110189538A1InexpensiveLow moisture concentrationPhosphorus halides/oxyhalidesLithium hexafluorophosphateChemical reactionPhysical chemistry
A method of manufacturing phosphorus pentafluoride and hexafluorophosphate can suppress the manufacturing cost and also can manufacture high-quality phosphorus pentafluoride from an inexpensive and low-quality raw material. The raw material for the method can include at least a phosphorus atom and a fluorine atom. These are brought into contact with a carrier gas, and a phosphorus pentafluoride is extracted and separated into the carrier gas. A method of manufacturing hexafluorophosphate includes reacting fluoride with the resulting phosphorus pentafluoride according to the following chemical reaction scheme: sPF5+AFs→A(PF6)s, in which s is in the range of 1≦s≦3, and A is at least one of the following: Li, Na, K, Rb, Cs, NH4, Ag, Mg, Ca, Ba, Zn, Cu, Pb, Al and Fe.
Owner:STELLA CHEMIFA CORP
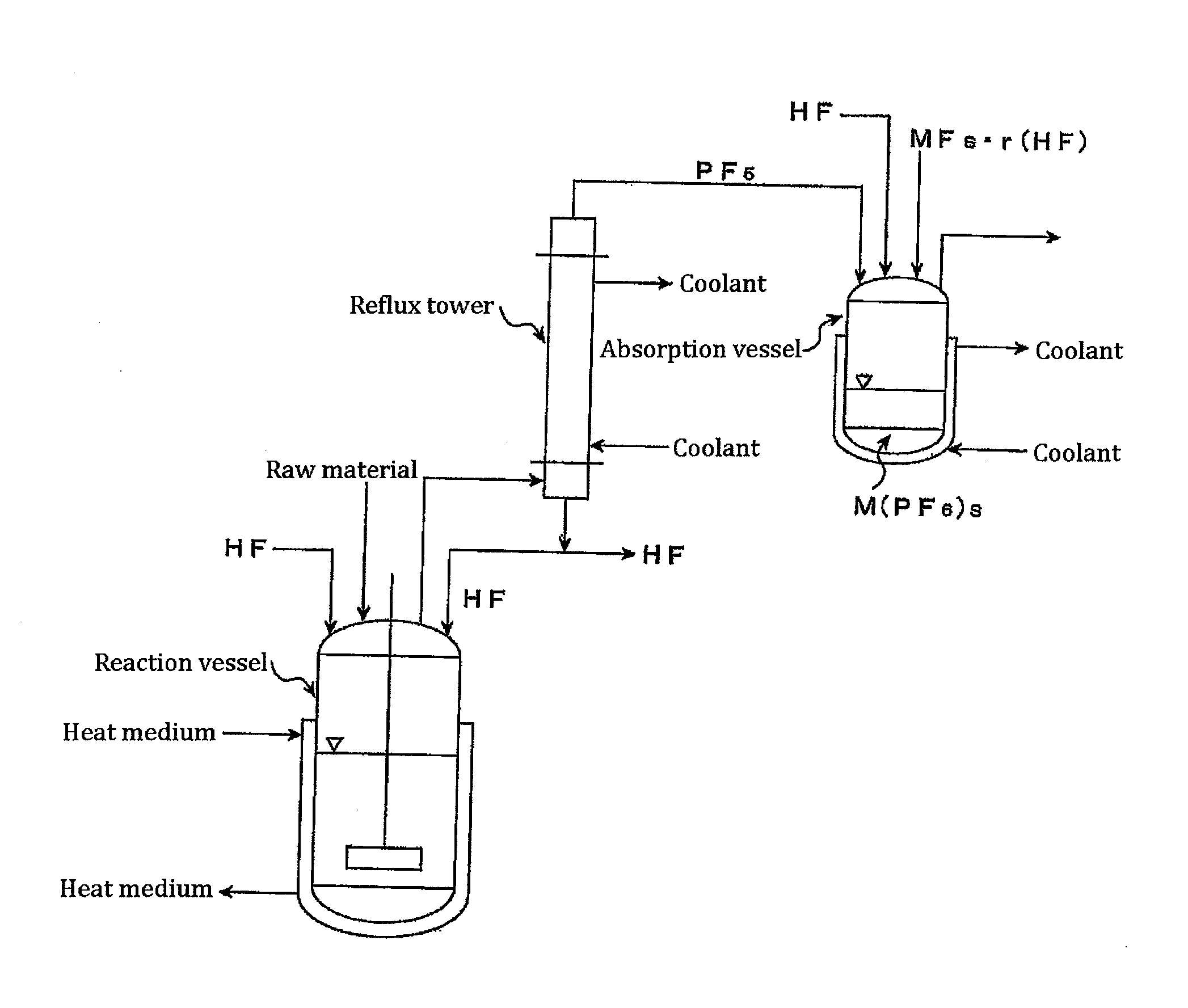
Processes for producing phosphorus tetrafluoride and phosphate hexafluoride
InactiveUS20110286905A1High purityPhosphorus halides/oxyhalidesLarge-sized flat cells/batteriesPhosphatePhotochemistry
An object the invention is to provide a phosphorus pentafluoride producing process wherein phosphorus pentafluoride is separated / extracted from a pentavalent phosphorus compound or a solution thereof, or a composition obtained by allowing the pentavalent phosphorus compound or the solution thereof to react with hydrogen fluoride, thereby producing phosphorus pentafluoride; and a phosphate hexafluoride producing process wherein the resultant phosphorus pentafluoride is used as raw material to produce a phosphate hexafluoride high in purity. The present invention relates to a process for producing phosphorus pentafluoride, wherein a carrier gas is brought into contact with either of the following one: a pentavalent phosphorus compound, a solution thereof, or a solution in which a composition obtained by allowing the pentavalent phosphorus compound or the solution thereof to react with hydrogen fluoride is dissolved, thereby a phosphorus pentafluoride is extracted into the career gas.
Owner:STELLA CHEMIFA CORP
Method for Producing Imidic Acid Salt
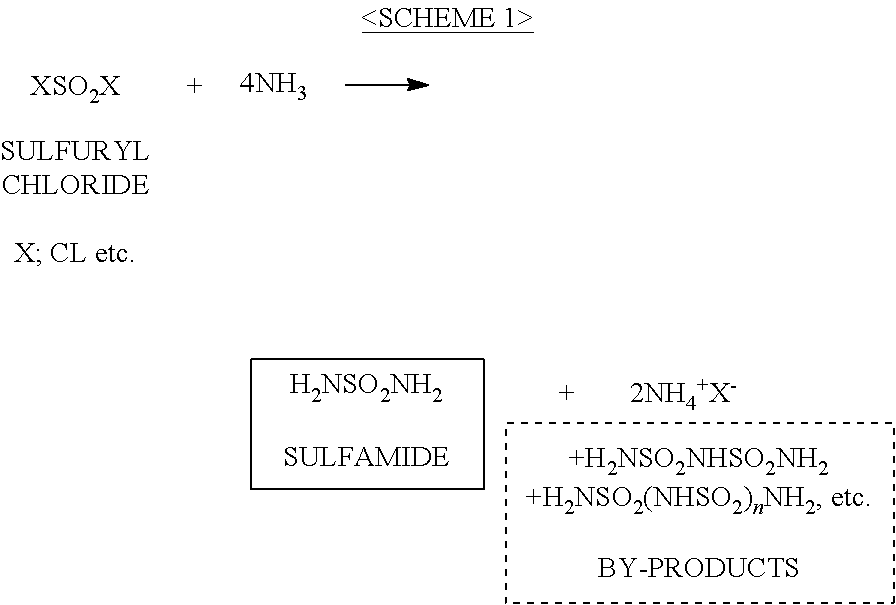
ActiveUS20120070358A1Easy to handleInhibition formationNitrosyl chloridePhosphorus halides/oxyhalidesAlkali metalHigh selectivity
To provide an imide salt represented by the formulawherein, R represents a halosulfonyl group (—SO2X1 where X1 is a halogen such as fluorine, chlorine, bromine and iodine) or dihalophosphoryl group (—POX2X3 where X2 and X3 are the same or different halogens such as fluorine, chlorine, bromine and iodine), and M represents an alkali metal;with high selectivity and high efficiency by using a low-cost starting material.In the production of an imide salt, an alkali metal fluoride, a sulfuryl halide or phosphoryl halide, and ammonia or an ammonium salt are reacted. According to this method, a desired imide salt can be produced with high yield, while greatly suppressing the production of a by-product.
Owner:CENT GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com