Processes for producing phosphorus pentafluoride and hexafluorophosphate
A technology of hexafluorophosphate and its manufacturing method, which is applied in lithium hexafluorophosphate, chemical instruments and methods, separation methods, etc., which can solve the problems of fast reaction speed, high manufacturing cost, violent reaction, etc., and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
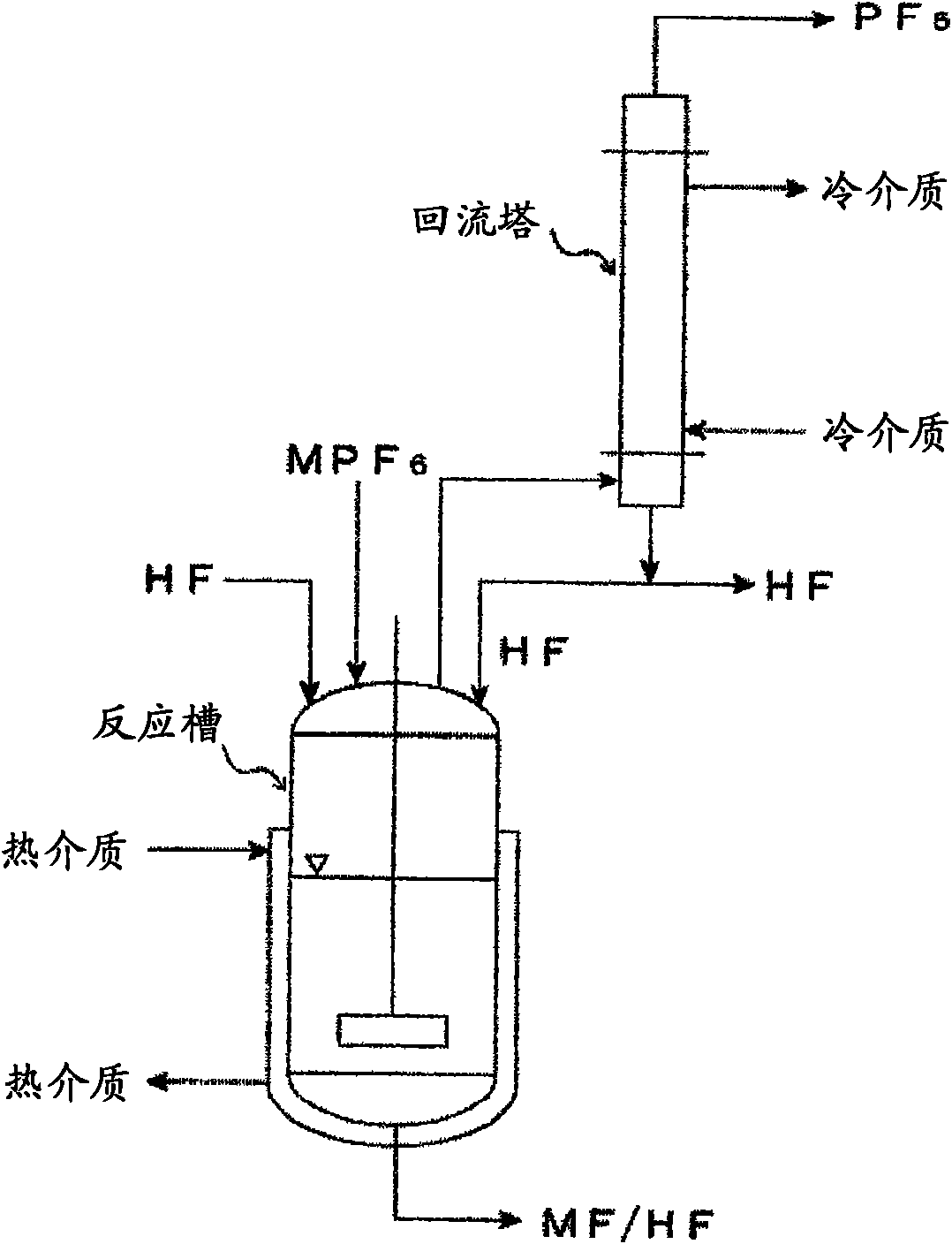
[0115] use figure 1 The apparatus shown performs the following implementations.
[0116] Commercially available potassium hexafluorophosphate (KPF 6 ) 50g and anhydrous hydrogen fluoride (HF) 2000g together with the rotor into a 5L reaction tank made of fluororesin (PFA), connected to a reflux tower made of SUS316 (20mm )superior. While heating the PFA reaction tank with a water bath so that the external temperature of the reaction tank reached 45°C, the reflux tower was cooled with -50°C brine. Furthermore, the reaction solution was stirred with a magnetic stirrer. As soon as the temperature of the water bath rises, the HF starts to reflux. At this time, the internal HF temperature was 21°C.
[0117] After 5 minutes, gas started to evolve from the reflux column, which was analyzed by FTIR. The result is that it is confirmed as PF 5 and a small amount of HF.
Embodiment 2
[0119] Lithium hexafluorophosphate (LiPF 6 ) 330g and anhydrous HF 2000g together with the rotor into the reaction tank made of 5LPFA, connected to the reflux tower made of SUS316 (20mm )superior. While heating the PFA reaction tank to 80°C with a water bath, the reflux tower was cooled with 0°C brine. Furthermore, the reaction solution was stirred with a magnetic stirrer. As soon as the temperature of the water bath rises, the HF starts to reflux. At this time, the internal HF temperature was 30°C.
[0120] The gas generated from the reflux tower was analyzed by FTIR and confirmed to be PF 5 and a small amount of HF. In addition, the gas generated at the same time was absorbed with pure water for 4 hours, the content of P in the absorption liquid was measured, and the generated PF was calculated. 5 The gas weight was 205g, and 75% was formed.
Embodiment 3
[0122] Ammonium hexafluorophosphate (NH 4 PF 6 ) 90g and anhydrous HF 2000g are added together with the rotor into a reaction tank made of 5LPFA, and the reaction solution is stirred with a magnetic stirrer. Embodiment 3 is carried out without setting the reflux tower. The generated gas was analyzed by FTIR while being absorbed by pure water. When the PFA reaction tank is heated to 65°C with a water bath, hydrogen fluoride evaporates and reacts violently with pure water as the absorbing liquid. The generated gas was analyzed by FTIR and confirmed to be PF 5 and lots of HF. Absorb with pure water for 6 hours, measure the content of P in the absorption liquid, and calculate the generated PF 5 The gas weight was 43g, and 62% was formed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com