Preparation process of high-purity phosphorus pentafluoride
A phosphorus pentafluoride and preparation technology, applied in the direction of phosphorus halide/oxyhalide, etc., can solve the problems of product performance degradation, safety hazards, separation difficulties, etc., and achieve the effect of simple method, effective operation, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
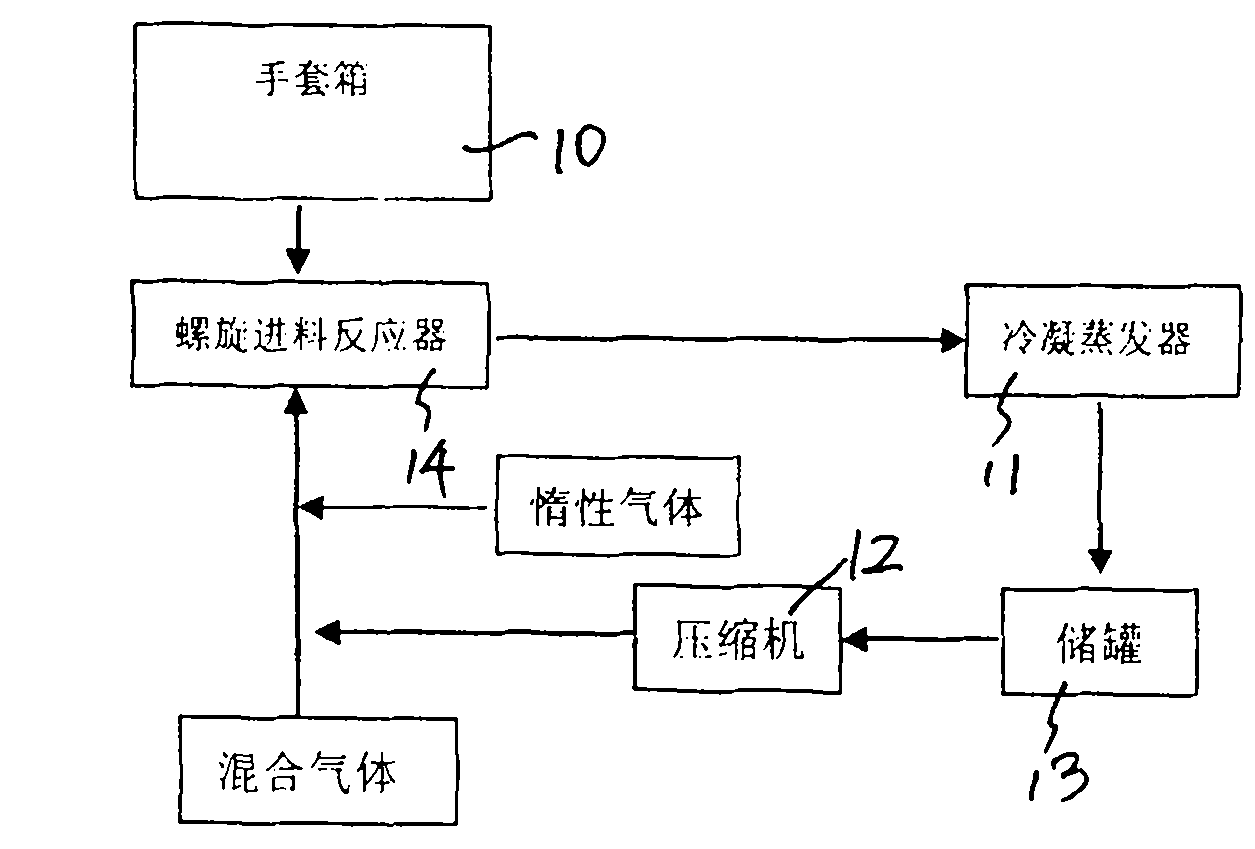
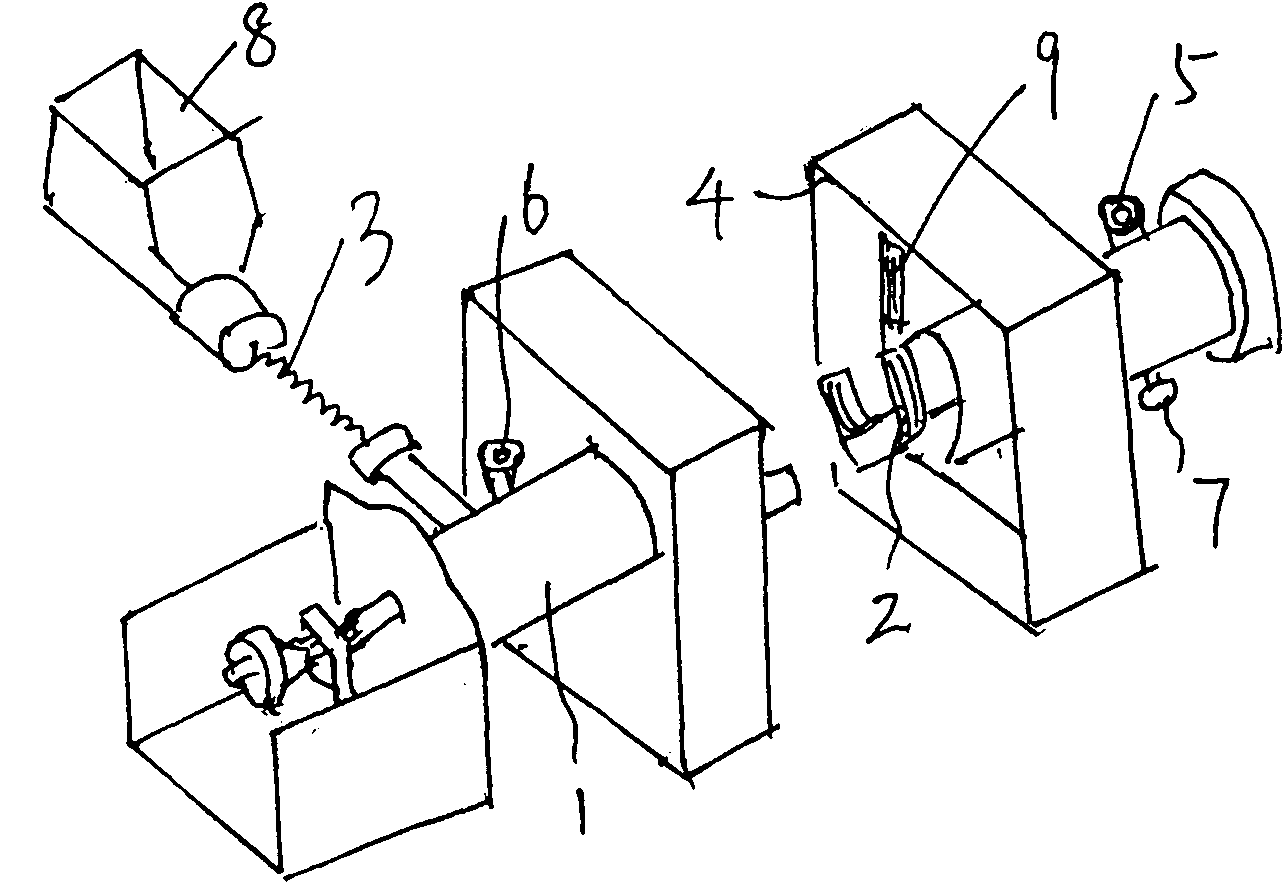
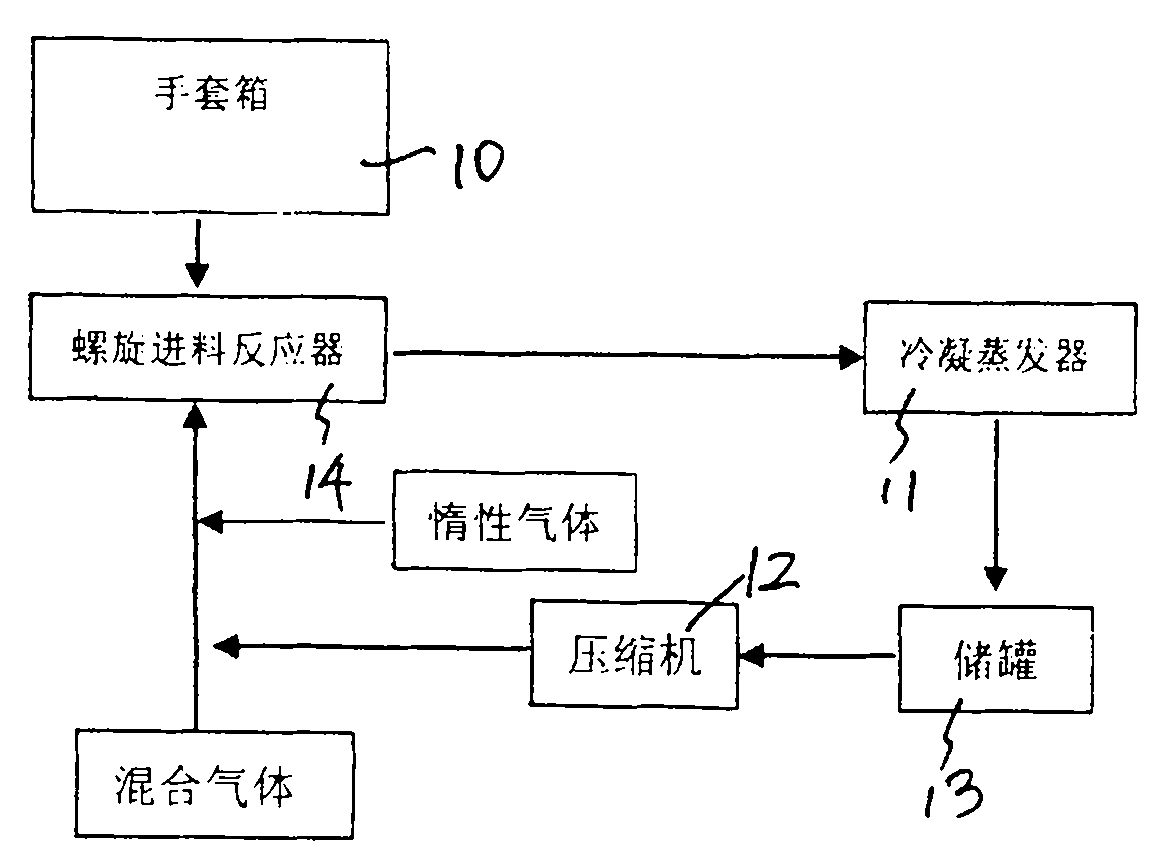
[0026] First, add 10 kg of phosphorus pentachloride to the feeding funnel of the spiral stirring reaction furnace in the glove box 10 in advance to evacuate the gas circulation loop, and then feed 0.06MPa high-purity argon into the gas circulation loop to make the condensing evaporator 11 is cooled to -180 to -140°C, and then the compressor 12 is started to circulate the gas in the gas circulation loop. Start the screw feeder of the spiral stirring reaction furnace, push phosphorus pentachloride into the reaction furnace cylinder of the spiral stirring reaction furnace, and at the same time slowly feed the mixed gas of fluorine gas and hydrogen fluoride into the gas circulation loop, and control the supply of the screw feeder. The rotation frequency of phosphorus pentachloride is 5-8 rpm, the temperature of the gas flow at the outlet of the spiral stirring reactor is controlled at 60°C-165°C, and the pressure in the gas circulation loop is controlled to be less than 0.15MPa. A...
Embodiment 2
[0031] First add 15 kg of phosphorus pentachloride to the feeding funnel of the spiral stirring reaction furnace in the glove box, vacuumize the gas circulation loop, and then pass 0.07MPa high-purity argon into the gas circulation loop to cool the condensing evaporator to -150 to -120°C, and then start the compressor to circulate the gas in the gas circulation loop. Start the screw feeder of the spiral stirring reaction furnace, push phosphorus pentachloride into the reaction furnace cylinder of the spiral stirring reaction furnace, and at the same time slowly feed the mixed gas of fluorine gas and hydrogen fluoride into the gas circulation loop, and control the supply of the screw feeder. The rotation frequency of phosphorus pentachloride is 10-12 rpm, the temperature of the gas flow at the outlet of the spiral stirring reactor is controlled at less than 400°C, and the pressure in the gas circulation loop is controlled to be less than 0.25 MPa. After the reaction, the phosph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| critical temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com