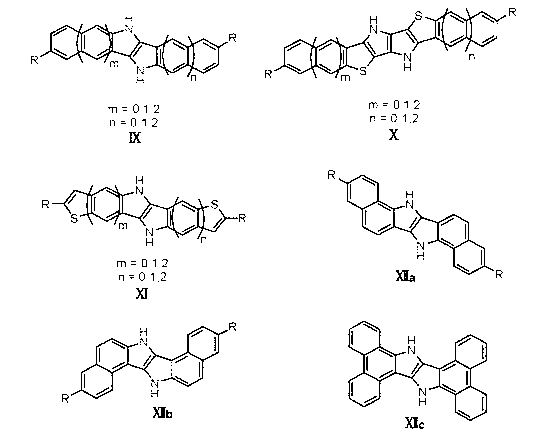
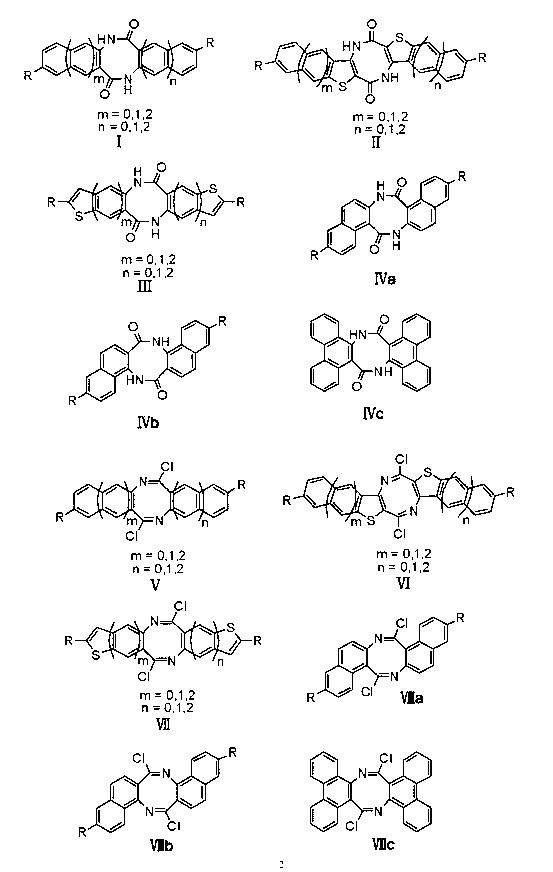
Patents
Literature
32 results about "Phosphorous pentachloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Conductive slurry for solar battery front side electrode and production method thereof
ActiveCN101295739AImprove photoelectric conversion efficiencyImprove adhesionFinal product manufactureNon-conductive material with dispersed conductive materialConductive pasteElectrical battery
The invention relates to a conductive paste for the surface electrode of a solar cell, which comprises conductive metal powders, organic carriers, adhesives, solvents and addition agent, wherein, the conductive paste also comprises additives which are selected from phosphorus pentachloride and one or more VIII group metal halide. The additive in the conductive paste of the invention can help improve the adhesive force between the conductive paste and silicon substrate, and lead adhesion between compounds obtained after electrode sintering and silicon substrate to be more firm; the formed compound does not have cracks and bubbles and the electrode surface is flat and smooth, thus providing the solar cell finally prepared with higher photoelectric conversion efficiency.
Owner:BYD CO LTD
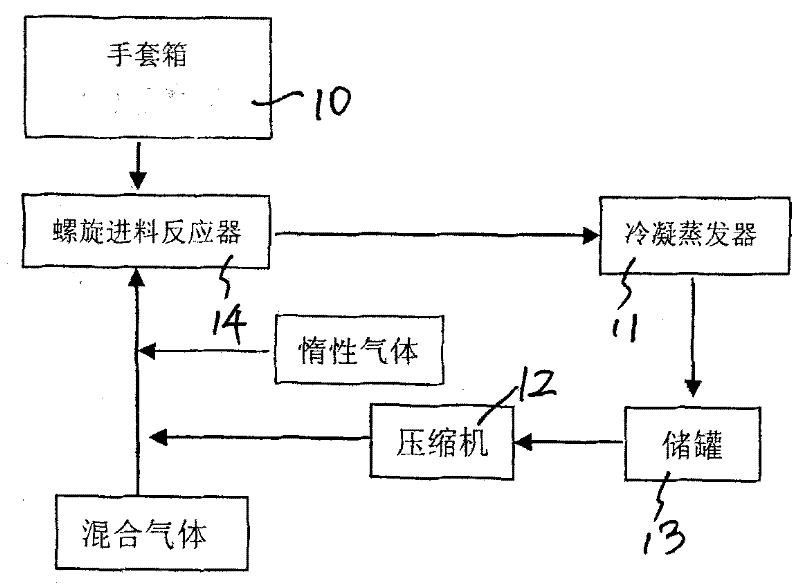
Preparation process of high-purity phosphorus pentafluoride
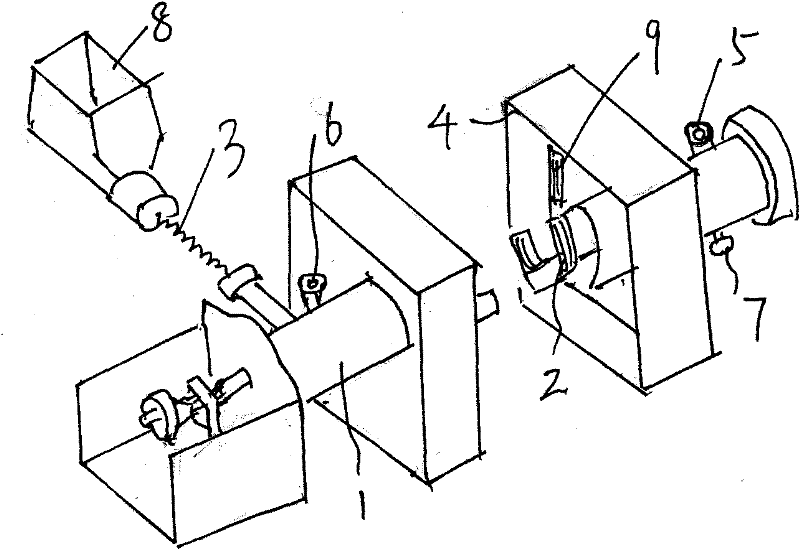
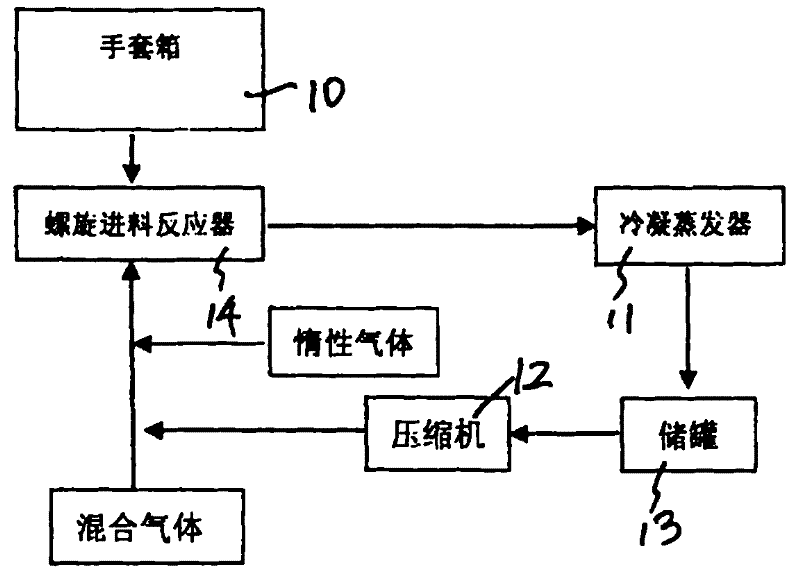
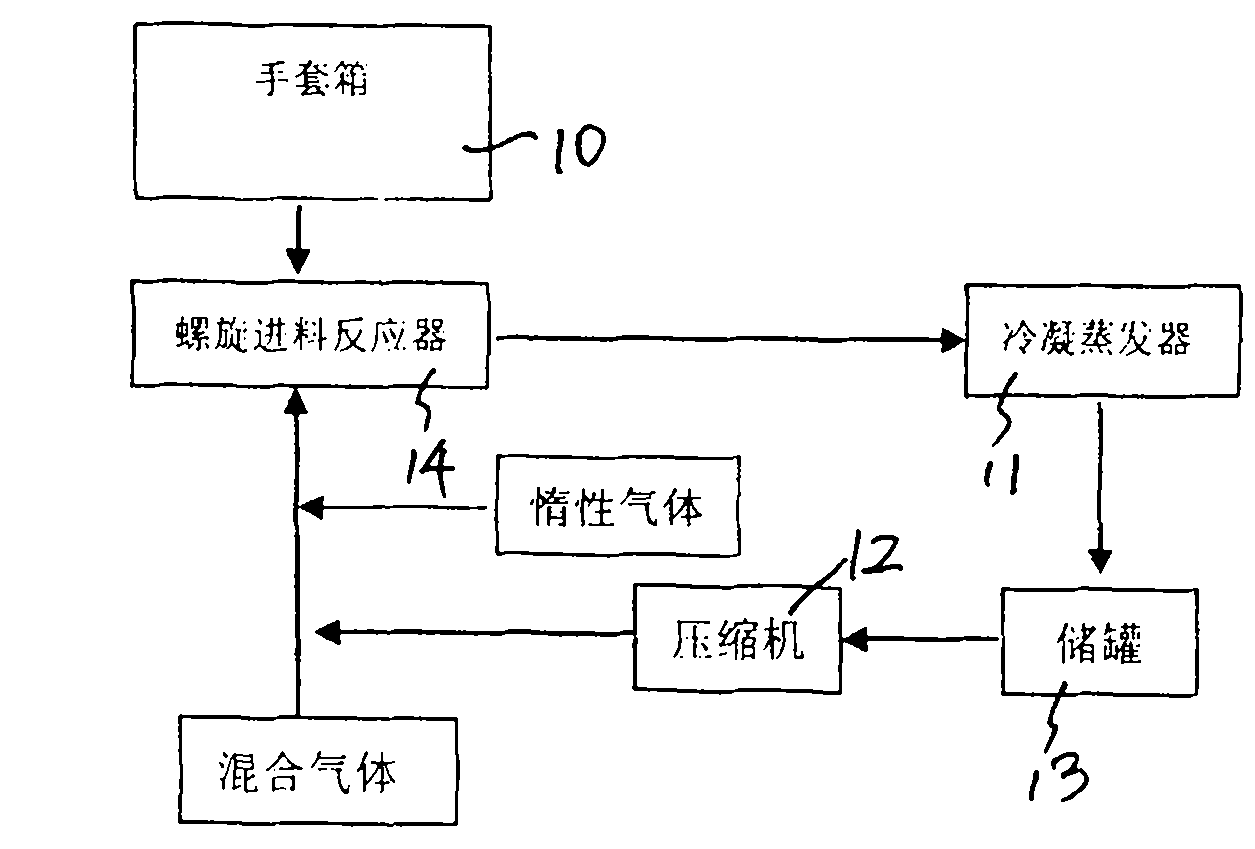
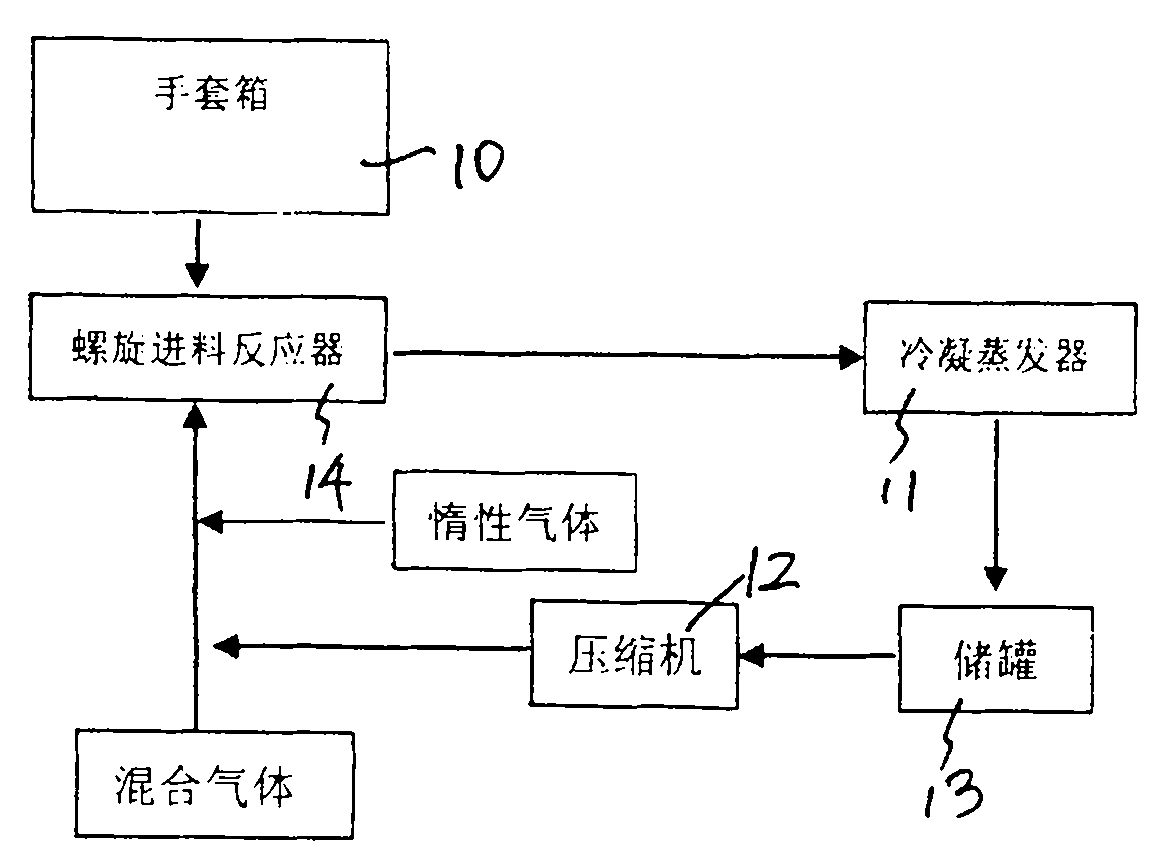
ActiveCN101844754AEffective control of feed rateControl feed ratePhosphorus halides/oxyhalidesPhysical chemistryHydrogen chloride
The invention discloses a preparation process of high-purity phosphorus pentafluoride, which comprises the following steps of: firstly adding phosphorous pentachloride into a feed hopper of a spiral feeder under the protection of dry gas in a glove box to assemble a gas circulation loop, and simultaneously adding inert gas into the gas circulation loop in advance; starting a spiral stirring reaction furnace, simultaneously injecting mixed gas of hydrogen fluoride and fluorine gas into the spiral stirring reaction furnace, and controlling the reaction process by controlling the rotation frequency of the phosphorous pentachloride in the spiral feeder, the temperature of gas flow at the outlet of the spiral stirring reaction furnace and the pressure of the gas circulation loop; freezing the phosphorus pentafluoride gas and the hydrogen fluoride gas generated in the reaction process and unreacted hydrogen fluoride gas by a condenser-evaporator, and collecting high-purity phosphorus pentafluoride gas after reaction. The method is simple and effective and can be operated easily.
Owner:JIANGSU JIUJIUJIU TECH
Method for preparing lithium hexafluorophosphate quickly
InactiveCN104555959AAdjust feed weight ratioEasy to operatePhosphorus halides/oxyhalidesDistillationMixed gas
The invention discloses a method for preparing lithium hexafluorophosphate quickly. The method comprises steps as follows: (1) performing distillation to obtain a hydrogen fluoride liquid with the purity higher than 99.99wt%; (2) reacting the hydrogen fluoride liquid with phosphorous pentachloride to obtain a mixed gas of phosphorus pentafluoride and hydrogen chloride; (3) feeding the mixed gas of the phosphorus pentafluoride and the hydrogen chloride into hydrogen fluoride and lithium fluoride to obtain a lithium hexafluorophosphate solution; (4) filtering the lithium hexafluorophosphate solution obtained in Step (3) to remove insoluble impurities, performing stirring crystallization on a filtrate at the stirring speed of 50-100 rpm, and performing rotary drying at the temperature of 120 DEG C-130 DEG C after stirring crystallization to directly obtain the lithium hexafluorophosphate product with the radius ranging from 100 meshes to 400 meshes. According to the method, the operation is convenient, the lithium hexafluorophosphate obtained through stirring crystallization is in uniform granule shape directly, repeated drying and crushing steps are omitted, and the crystallization time is shortened.
Owner:MORITA NEW ENERGY MATERIALS ZHANGJIAGANG CO LTD
Method for preparing novel organic field effect transistor material
InactiveCN103214490AEfficient synthesisThe synthetic method is simple and efficientOrganic chemistrySolid-state devicesOrganic field-effect transistorCombinatorial chemistry
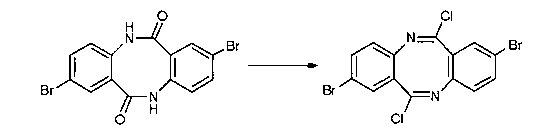
The present invention relates to a method for preparing a novel organic field effect transistor material of a pyrrolo[3,2-b]pyrrole fused ring compound. The method includes the main steps of: 1) producing 6,12-dichloro diazocine from a diazocine compound under the action of phosphorus pentachloride; and 2) producing the target pyrrolo[3,2-b]pyrrole fused ring compound through reductive coupling of 6,12-dichloro diazocine under the action of a zinc powder and acid. The invention is characterized in that: 1) compared with the conventional synthetic strategy by a C-N bond to build a key reaction, the synthetic route takes the C-C bond built reduction coupling reaction as a key step, and the mehtod is simple, efficient and wide in universality; 2) reaction raw materials and reagents are cheap and easily available, reaction operation is simple and easy for amplification and for industrial production; 3) the remaining pyrrolo[3,2-b]pyrrole fused ring compounds obtained by the invention are firstly synthesized except for DBPP which has been reported, and the method can provide a variety of novel organic semiconductor materials with potential application values.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Internal circulation reactor and method for continuously preparing phosphorus pentafluoride
InactiveCN102976298AStable concentrationIncrease production capacityPhosphorus halides/oxyhalidesAutomatic controlInternal circulation reactor
The invention discloses an internal circulation reactor and method for continuously preparing phosphorus pentafluoride. The internal circulation reactor comprises a cylinder body with a heat exchange jacket, wherein planar end closures are arranged at the upper and lower ends of the cylinder body, and a concentric inner cylinder body is arranged in the cylinder body; the upper end closure is provided with a mixed gas leading-out pipe; the lower end closure is provided with a three-way gas intake pipe; and a reaction solution eduction pipe and a feed liquid ingress pipe are arranged on the cylinder body. The process of preparing phosphorus pentafluoride comprises the following steps: blowing the reactor with an inert gas; creating a low temperature environment of the reactor with a refrigerant; adding a phosphorous pentachloride solution, anhydrous hydrogen fluoride and the inert gas to the reactor for reaction; and continuously leading out mixed gas containing phosphorus pentafluoride and part of a low-concentration phosphorous pentachloride solution, and at the same time continuously adding a phosphorous pentachloride concentrated solution, anhydrous hydrogen fluoride and the inert gas to realize continuous operation. The internal circulation reactor and method for continuously preparing phosphorus pentafluoride have the advantages that the concentration stability of reactants in the reactor can be maintained, and continuous production and automatic control of phosphorus pentafluoride are realized.
Owner:TIANJIN LONGER NEW MATERIAL SCI & TECH
Preparation method of chlorine/phosphorus-codoped carbon quantum dots
The invention discloses a preparation method of chlorine / phosphorus-codoped carbon quantum dots. The method comprises the following steps: mixing liquid alcohol or carboxylic acid with phosphorous pentachloride, repeatedly heating and cooling, and carrying out chromatography to obtain the purified chlorine / phosphorus-codoped carbon quantum dots. The preparation method has the advantages of mild reactions, high controllability, high carbon quantum dot yield and the like, and is suitable for mass production.
Owner:ZHONGBEI UNIV
Synthesis method of dimethylheptyl methylphosphonate
ActiveCN102796136AReduce usageAvoid Measuring DifficultiesGroup 5/15 element organic compoundsDimethyl methylphosphonateSynthesis methods
The invention relates to a synthesis method of dimethylheptyl methylphosphonate, and aims to solve the difficulty in raw material measurement and the safety problem caused by the use of the high-pressure reaction kettle in the existing technique, thereby lowering the production cost. The invention has the advantages of simple operating procedure, high output and high yield, and is suitable for industrial production. The method comprises the following steps: adding an acyl-chlorination reagent into a reaction vessel, wherein the acyl-chlorination reagent is thionyl chloride, phosphorous pentachloride or triphosgene; dropwisely adding dimethyl methyl phosphonate (DMMP) into the acyl-chlorination reagent at room temperature and adding catalytic amount of catalyst, wherein the consumption of the acyl-chlorination reagent is 2-5 times of the DMMP (0.6-1.5 times for triphosgene) on mol basis, and the catalyst is N,N-di-substituted-formamide or N-containing aromatic heterocyclic ring or N-substituted N-containing aromatic heterocyclic ring or tertiary amine; and uniformly stirring at room temperature.
Owner:洛阳市三诺化工有限公司
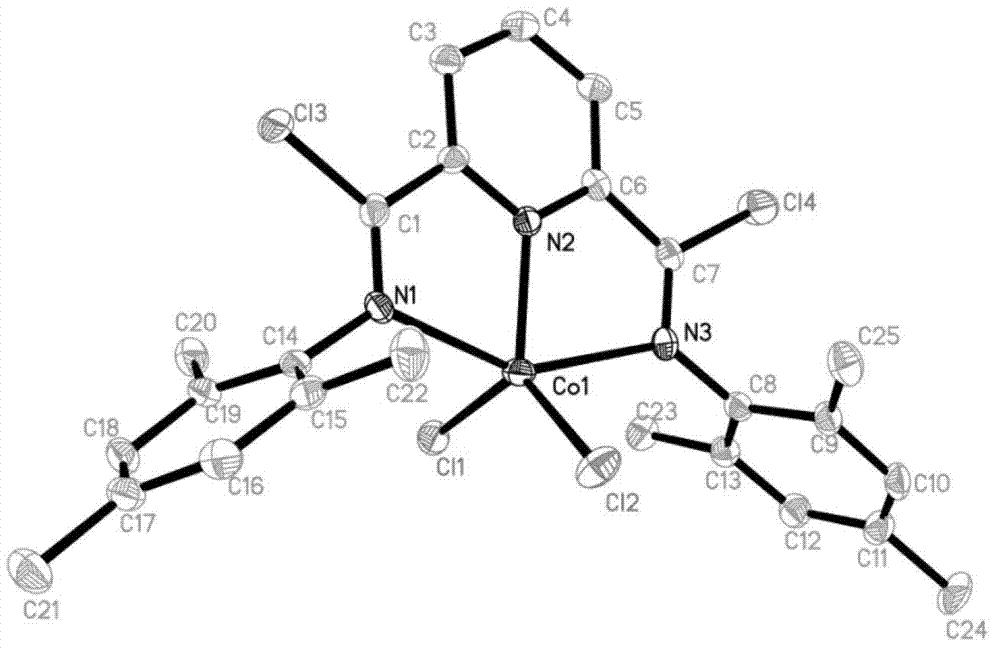
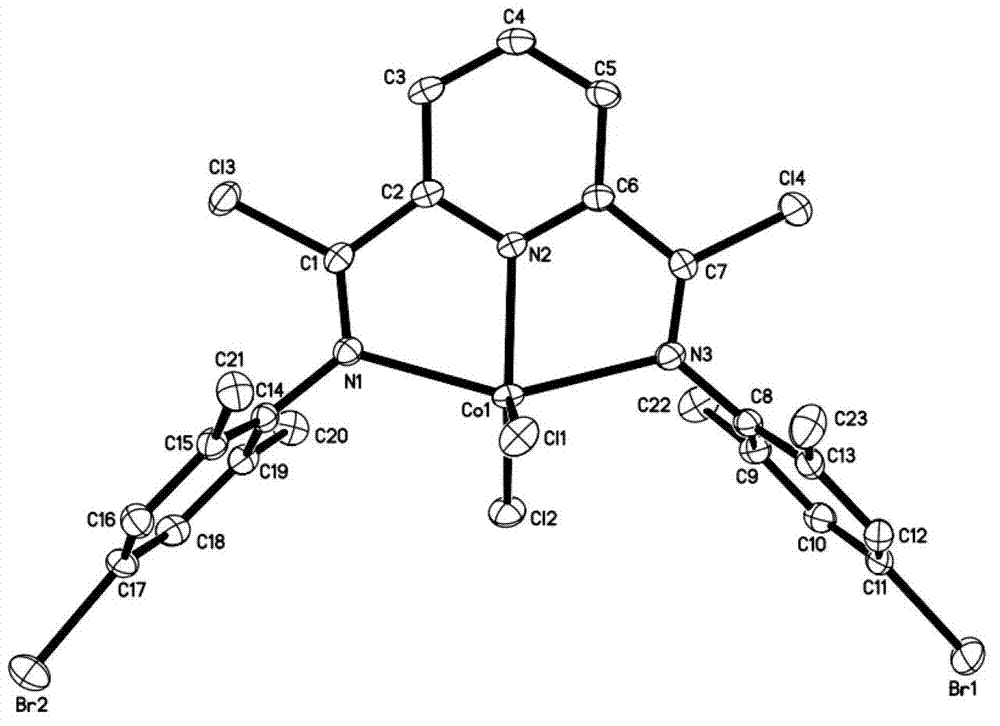
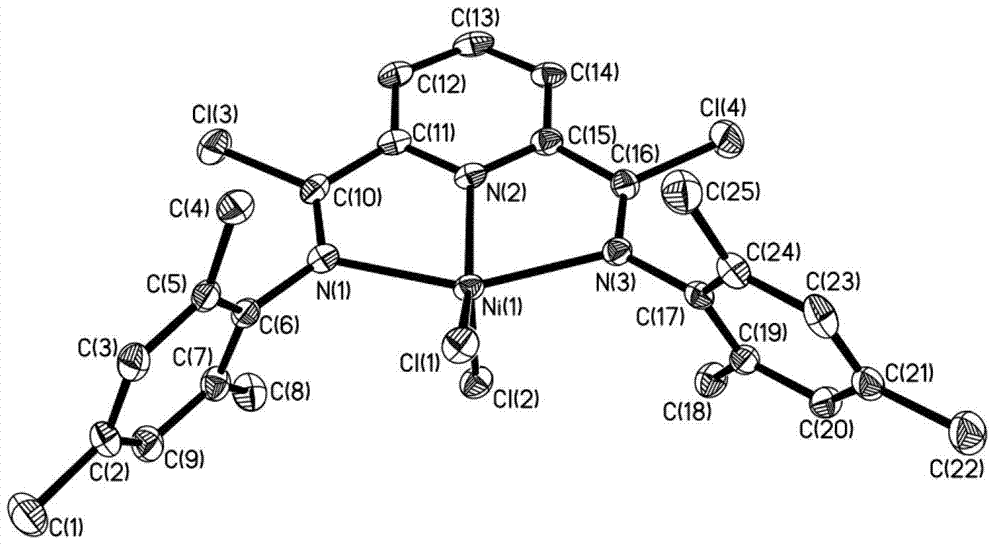
Post-transition metal complex, preparation method thereof and polyethylene preparation method
ActiveCN102775448AIncrease electropositivityStrong electron-withdrawing effectNickel organic compoundsCobalt organic compoundsOrganic solventAniline
The invention provides a post-transition metal complex having a structure shown in a formula (I). The invention also provides a preparation method of the post-transition metal complex, which comprises the following steps: performing first reaction on di(substituted phenylamine)-2,6-pyridine dicarboxamide and phosphorous pentachloride in a first organic solvent to obtain a ligand having a structure shown in a formula (II); and performing second reaction on the ligand and post-transition metal halide in a second organic solvent to obtain the post-transition metal complex having the structure shown in the formula (I). The invention also provides a polyethylene preparation method which comprises the following specific step: under the effects of a cocatalyst and the post-transition metal complex having the structure shown in the formula (I), performing polymerization reaction on ethylene in an organic solvent to obtain polyethylene. The post-transition metal complex provided by the invention has high catalytic activity in catalytic ethylene polymerization.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
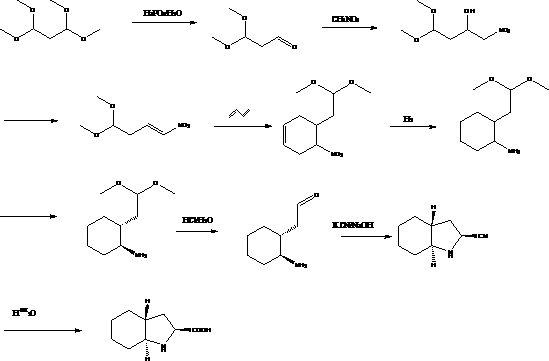
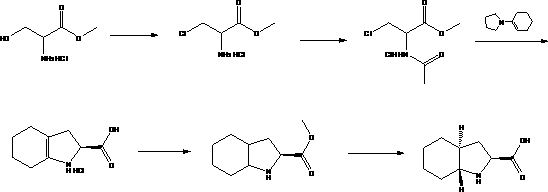
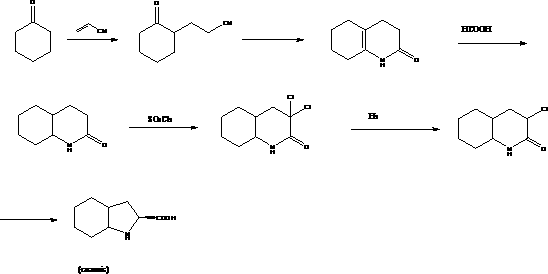
Preparation method of trandolapril midbody (2S, 3aR, 7aS)-octahydro-1H-indole-2-carboxylic acid
The invention discloses a preparation method of trandolapril midbody (2S, 3aR, 7aS)-octahydro-1H-indole-2-carboxylic acid, and the preparation method comprises the following steps of: preparing 3-chlorine-2-amino-propionic acid methyl ester hydrochloride by using phosphorous pentachloride and dichloromethane; preparing 3-chlorine-2-acetyl amino-propionic acid methyl ester hydrochloride by using the 3-chlorine-2-amino-propionic acid methyl ester hydrochloride, methylbenzene and acetyl chloride; preparing 2,3,4,5,6,7-hexahydro-1H-indole-2-carboxylic acid by using the 3-chlorine-2-acetyl amino-propionic acid methyl ester hydrochloride, DMF (Dimethyl Formamide) and 1-pyrrole cyclohexene; preparing (2S)-octahydro-1H-indole-2-methyl carboxylat by using the 2,3,4,5,6,7-hexahydro-1H-indole-2-carboxylic acid, methanol and palladium on carbon; preparing the (2S, 3aR, 7aS)-octahydro-1H-indole-2-carboxylic acid by using the (2S)-octahydro-1H-indole-2-methyl carboxylat and the methanol. Compared with the prior art, the preparation method of the trandolapril midbody (2S, 3aR, 7aS)-octahydro-1H-indole-2-carboxylic acid, which is disclosed by the invention, has the advantages of easiness and convenience for process, easiness for control, safety, reliability and low cost.
Owner:大连鼎燕医药化工有限公司 +1
Preparation method of cefozopran hydrochloride intermediate
InactiveCN102336772AEasy to getEasy to controlOrganic chemistryBulk chemical productionPyridazineCarboxyl radical
The invention discloses a preparation method of cefozopran hydrochloride intermediate, which comprises the following steps: (1) the chemical name of the cefozopran hydrochloride intermediate is 7-amino-2-carboxy-8-oxo-5-thia-1-aza-bicyclo[4.2.0]octyl-2-alkenyl-3-yl]methyl]imidazo-[1,2-b]pyridazine onium salt; (2) by using 7-phenylacetamido-3-chloromethylcephalosporanate (GCLE) as the initial raw material, activating C-3 site with iodine, potassium iodide or sodium iodide hydrate, reacting with imidazo-[1,2-b]pyridazine disclosed as Formula (III), and carrying out after-treatment to obtain a compound; and (3) hydrolyzing the compound obtained in the step (2) under the action of phosphorous pentachloride to remove C-7 site protective group, hydrolyzing under the action of phenol to remove C-4 site phenylacetyl protective group, and carrying out after-treatment to obtain the cefozopran hydrochloride intermediate. The invention has the advantages of cheap and accessible raw materials, mild and manageable reaction conditions, high production safety and low production cost.
Owner:SHANDONG CHENGCHUANG PHARMA R&D
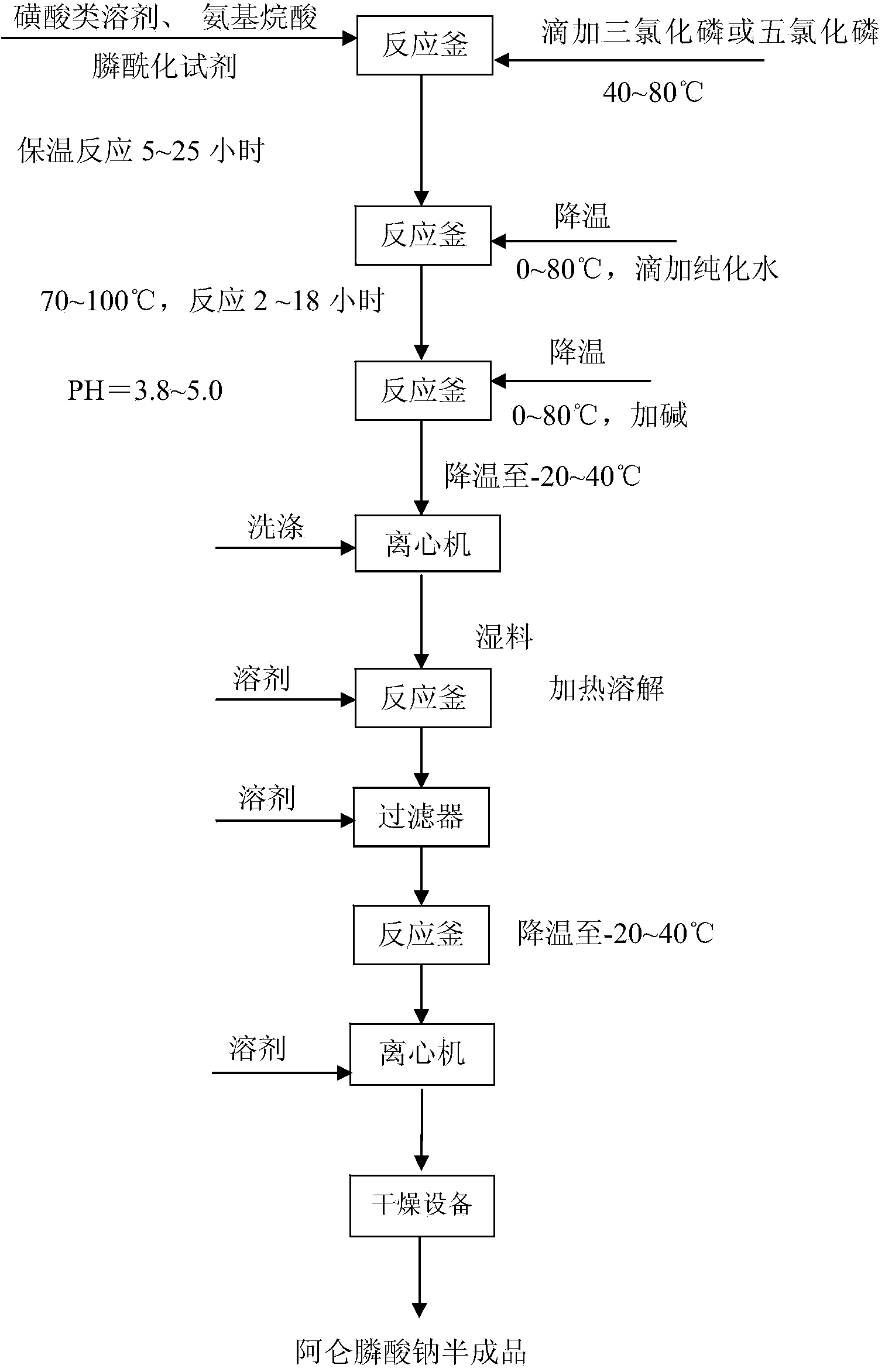
Synthetic method of diphosphonate medicine
InactiveCN104072538AImprove solubilityRelieve pressureGroup 5/15 element organic compoundsChemical synthesisPhosphorylation
The invention relates to a synthetic method of a diphosphonate medicine. The synthetic method comprises the following steps: 1.1) adding a sulfoacid liquid solvent, amino alkyl acid and a phosphorylation agent; stirring and heating, wherein the temperature range is 30-130 DEG C; fully dissolving; 1.2) dropping phosphorus trichloride or phosphorus pentachloride and preserving heat at 40-80 DEG C to react; 1.3) dropping water, then heating to warm, preserving heat at 70-100 DEG C and completing a hydrolysis reaction to obtain a solution in a diphosphonic acid structure; 1.4) kalifying to neutralize, cooling and devitrifying, and centrifugally washing to obtain a coarse product of diphosphonate; 2.1) returning the obtained coarse product of diphosphonate to a tank and adding a solvent, stirring and heating, and dissolving; 2.2) after fully dissolving, decoloring and press-filtering; and 2.3) cooling and devitrifying the solution, and centrifugally washing and drying the obtained diphosphonate crystals. The chemical synthetic method of the diphosphonate medicine provided by the invention solves the potential safety hazards such as a plenty of heat release and explosion and boiling in reaction on the premise of guaranteeing the yield.
Owner:陕西汉江药业集团股份有限公司
Method for synthesizing 2-chloropyridine
InactiveCN105669534AProcess production is safe and reliableOrganic chemistryDistillationToxic material
The invention discloses a method for synthesizing 2-chloropyridine. The steps of the method are to add pyridine and solvent into a 500mL container, control the pressure at normal pressure, stir for 10-20min, then add fluorine acetate dropwise, and control the temperature not to exceed 30°C; move the reaction mixture to a 500mL container, The temperature is raised to 50°C-70°C, vacuum distillation is carried out under the conditions of vacuum degree 0.07-0.09MPa, and after no liquid is evaporated, the remaining material is 2-chloropyridine. The chlorination of the inventive method does not need highly toxic substances such as chlorine gas, phosphorus trichloride, phosphorus pentachloride, phosphorus oxychloride and phosgene as the chlorination reagent, but uses dichloromethane as the chlorination reagent. It is safe and reliable; the reaction is carried out under normal temperature and pressure, and the yield of 2-chloropyridine reaches 80%.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Novel method for synthesizing and purifying phosphonitrilic chloride trimer
The invention discloses a novel method for synthesizing and purifying phosphonitrilic chloride trimer by taking Lewis acid as the catalyst and adopting the sublimation method. The method disclosed by the invention is characterized by comprising the following steps of: synthesizing phosphonitrilic chloride trimer in chlorobenzene as the solvent by selecting Lewis acid as the catalyst and taking ammonium chloride and phosphorus pentachloride as the raw materials, after removing chlorobenzene, dissolving phosphonitrilic chloride trimer by using normal hexane to eliminate linear polymer, and finally, purifying by adopting the sublimation method to obtain phosphonitrilic chloride trimer with the purity of above 99%, wherein the total yield is about 60%. Compared with the prior art, the catalytic system is optimized; the purity of the prepared phosphonitrilic chloride trimer is high; and the novel method disclosed by the invention is applied to industrialized mass production.
Owner:上海永鸿实业集团化学科技有限公司
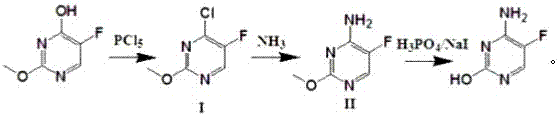
A kind of preparation method of 5-flucytosine suitable for industrialized production
ActiveCN105153041BLow costRaw materials are cheap and easy to getOrganic chemistryChemical synthesisAfter treatment
The invention relates to a 5-fluctyosine preparation method suitable for industrial production, belonging to the technical field of pharmaceutical chemical synthesis. The method comprises the following steps: by using 2-methoxy-5-fluorouracil as a raw material, chlorinating with phosphorous pentachloride to obtain 2-methoxy-4-chloro-5-fluoropyrimidine, and concentrating the acidic water phase obtained by after-treatment for later use; carrying out amination reaction on the 2-methoxy-4-chloro-5-fluoropyrimidine and an aminating agent to obtain 2-methoxy-4-amido-5-fluoropyrimidine; and carrying out acidic hydrolysis on the 2-methoxy-4-amido-5-fluoropyrimidine by using the concentrated acidic water phase recycled from chlorination to obtain the 5-fluctyosine. The 5-fluctyosine preparation method provided by the invention has the advantages of high yield, convenient after-treatment and fewer three-waste pollutants, solves the problems of high virulent product consumption, difficulty in acidic waste gas treatment, high pollution, high cost and the like in the traditional technique, and is suitable for industrial production application. The total mole yield is up to 70% or above.
Owner:ZHEJIANG XIANFENG TECH
Synthesis method of phosphorus pentachloride
InactiveCN107117591AEasy to makeLow costPhosphorus halides/oxyhalidesSynthesis methodsReaction temperature
The invention discloses a method for synthesizing phosphorus pentachloride. Phosphorus trichloride is injected into a reactor, chlorine gas is passed into the bottom of the reactor through a pipeline, and stirred at a stirring speed of 500 rpm and a pressure of 0.6kpa. Control the reaction temperature to 75-83°C, stop the reaction until no solid matter is generated, and obtain solid phosphorus pentachloride; then put the solid phosphorus pentachloride into a sublimation tank for decompression sublimation; collect the sublimation gas, and carry out Cool and recrystallize to obtain high-purity solid phosphorus pentachloride. Beneficial technical effects of the present invention: the present invention has the advantages of simple preparation, low cost and easy popularization.
Owner:XUZHOU JIANGHAIYUAN CHEM
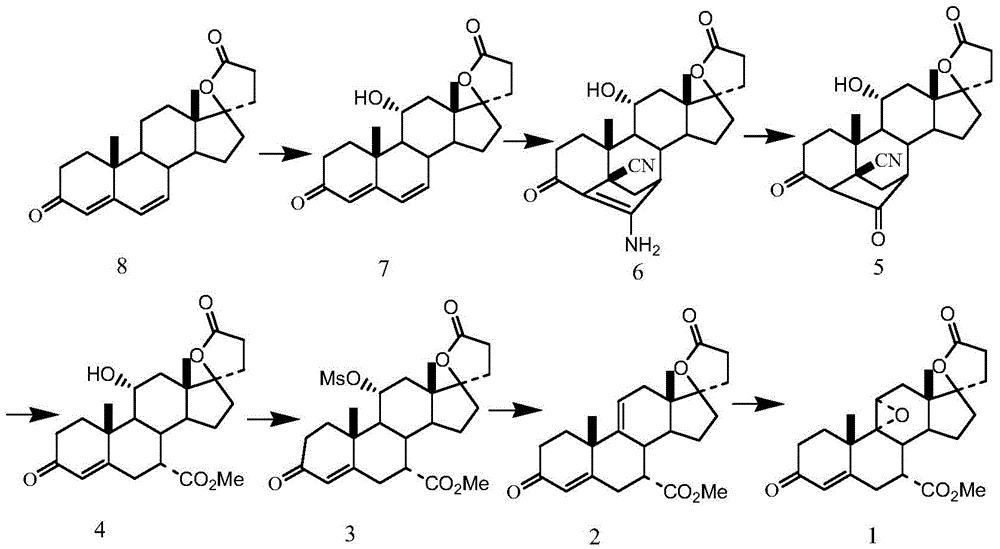
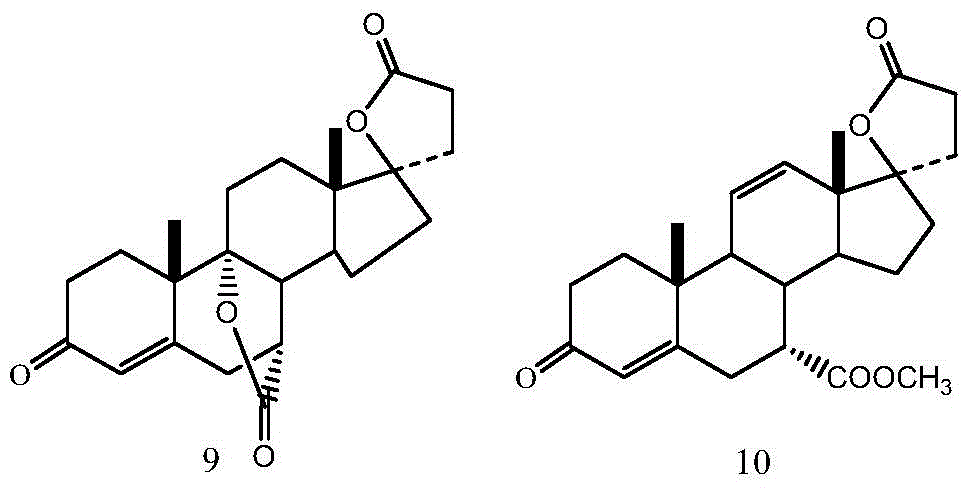
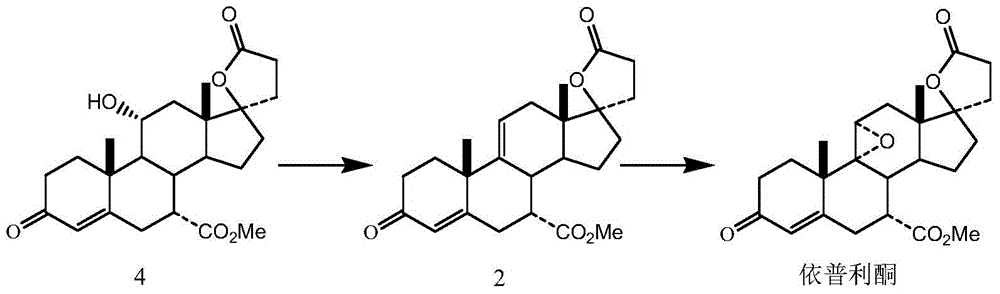
Preparation method of eplerenone
The invention belongs to the field of medicine, and in particular relates to a preparation method of eplerenone. Starting from 17α-pregna-4-ene-7α, 21-dicarboxylic acid-11α, 17β-dihydroxy-3-oxo-γ-lactone-7-methyl ester as starting materials, phosphorus pentachloride and Dehydration reaction occurs under the action of boron trihalide to obtain 17α-pregna-4,9(11)-diene-7α, 21-dicarboxylic acid-17β-hydroxy-3-oxo-γ-lactone, 7- Methyl ester, 17α-pregna-4,9(11)-diene-7α, 21-dicarboxylic acid-17β-hydroxy-3-oxo-γ-lactone, 7-methyl ester obtained by epoxidation Eplerenone. The preparation method has specificity, reduces the generation of impurities, and the obtained product has good quality and high yield, and the product purity reaches over 99.5%. The raw material is easy to obtain, the operation is simple, the reaction condition is mild, and industrial production is easy to realize.
Owner:SHANDONG XINHUA PHARMA CO LTD
Preparation method of chlorine phosphorus co-doped carbon quantum dots
The invention discloses a preparation method of chlorine / phosphorus-codoped carbon quantum dots. The method comprises the following steps: mixing liquid alcohol or carboxylic acid with phosphorous pentachloride, repeatedly heating and cooling, and carrying out chromatography to obtain the purified chlorine / phosphorus-codoped carbon quantum dots. The preparation method has the advantages of mild reactions, high controllability, high carbon quantum dot yield and the like, and is suitable for mass production.
Owner:ZHONGBEI UNIV
The synthetic method of dimethyl heptyl methylphosphonate
ActiveCN102796136BReduce usageAvoid Measuring DifficultiesGroup 5/15 element organic compoundsDimethyl methylphosphonateSynthesis methods
The invention relates to a synthesis method of dimethylheptyl methylphosphonate, and aims to solve the difficulty in raw material measurement and the safety problem caused by the use of the high-pressure reaction kettle in the existing technique, thereby lowering the production cost. The invention has the advantages of simple operating procedure, high output and high yield, and is suitable for industrial production. The method comprises the following steps: adding an acyl-chlorination reagent into a reaction vessel, wherein the acyl-chlorination reagent is thionyl chloride, phosphorous pentachloride or triphosgene; dropwisely adding dimethyl methyl phosphonate (DMMP) into the acyl-chlorination reagent at room temperature and adding catalytic amount of catalyst, wherein the consumption of the acyl-chlorination reagent is 2-5 times of the DMMP (0.6-1.5 times for triphosgene) on mol basis, and the catalyst is N,N-di-substituted-formamide or N-containing aromatic heterocyclic ring or N-substituted N-containing aromatic heterocyclic ring or tertiary amine; and uniformly stirring at room temperature.
Owner:洛阳市三诺化工有限公司
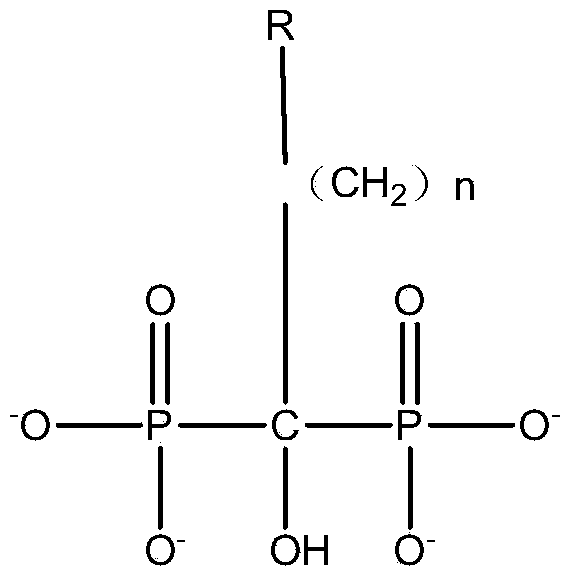
Phosphate as well as preparation method and application thereof
ActiveCN113206294AInhibit side effectsInhibition of contact responseFinal product manufactureSecondary cells servicing/maintenanceElectrolytic agentPhosphoric Acid Esters
The invention relates to the field of lithium batteries, and discloses phosphate as well as a preparation method and application thereof. In the phosphate, R1 is alkyl with the carbon atom number of 1-8, or fluorine-substituted alkyl with the carbon atom number of 1-8, or fluorine-substituted aryl or fluorine-free aryl; R2 is a hydrocarbon group having 1-8 carbon atoms and containing an alkenyl group or an alkynyl group; and R3 is a group containing a cyano group. The preparation method comprises the following steps: S1, reacting alcohol R1OH with phosphorus pentachloride in a solvent to obtain an intermediate; S2, reacting another alcohol R2OH with the intermediate to obtain a product or another intermediate; S3, reacting a third alcohol R3OH with the intermediate in the second step to obtain a product. The electrolyte prepared from the phosphonate can improve the cycling stability, safety performance and storage stability of the secondary battery.
Owner:湖州永兴锂电池技术有限公司
Synthetic method of linear low molecular alkoxy phosphonitrile compound
The invention discloses a synthetic method of linear low molecular alkoxy phosphonitrile compound, wherein phosphorous pentachloride and ammonium chloride are used as raw materials, 1,1,2,2-tetrachloroethanes is used as solvent for reaction under the condition of normal temperature to 150 deg.C, obtaining linear low molecular chlorine replaced phosphoric nitrile, and toluene is used as solvent, at the presence of tetrabutyl ammonium bromide, the linear low molecular chlorine replaced phosphoric nitrile reacts with alcohol sodium.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthesis method of N-cyclohexyl-5-(4-chlorobutyl)-1H-tetrazole
The invention discloses a synthesis method of N-cyclohexyl-5-(4-chlorobutyl)-1H-tetrazole. The synthesis method comprises the following steps: firstly, synthesizing N, N-dimethylformamide; the synthesis method comprises the following synthesis steps: taking 5-chlorovaleronitrile and cyclohexanol as raw materials, controlling the molar ratio of the 5-chlorovaleronitrile to the cyclohexanol to be (1: 1)-(1: 1.5) and the reaction temperature to be 5-55 DEG C, and carrying out a catalytic reaction for 1-6 hours by virtue of concentrated sulfuric acid, so as to obtain 5-chloro-N-cyclohexylvaleramide; wherein the molar ratio of the 5-chlorovaleronitrile to the concentrated sulfuric acid for catalysis is (1: 3)-(1: 10); the preparation method comprises the following steps: treating 1, 5-chloro-N-cyclohexylvaleric amide with phosphorus pentachloride; wherein the molar ratio of the 3, 5-chloro-N-cyclohexylvaleric amide to the phosphorus pentachloride is (1: 1)-(1: 1.5) and the molar ratio of the 2, 5-chloro-N-cyclohexylvaleric amide to the phosphorus pentachloride is (1: 1)-(1: 1.5); according to the invention, trimethyl silicon azide is used as a cyclization reagent instead of azoic acid or sodium azide, so that the synthesis method has the advantages of higher stability, no explosion and higher safety, the cost can be effectively reduced, the synthesis method is environment-friendly,and the purity of the obtained product is high.
Owner:上海立科化学科技有限公司
Preparation method of lithium hexafluorophosphate
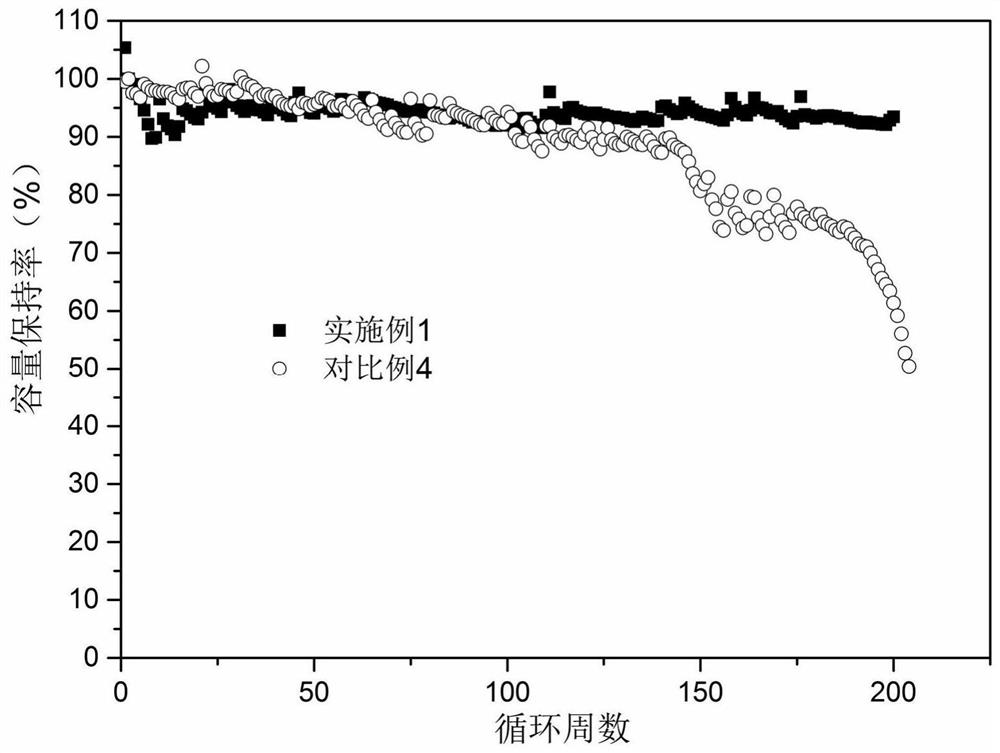
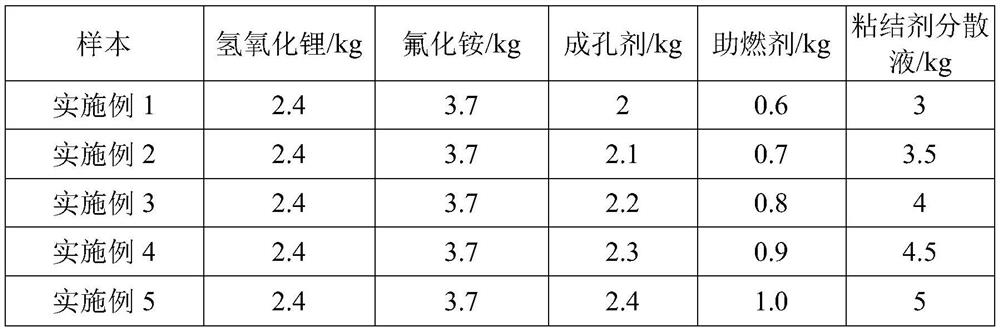
PendingCN114180602ALarge specific surface areaHigh reactivityLithium hexafluorophosphateLithium hydroxidePhysical chemistry
The invention relates to the technical field of battery production, and particularly discloses a preparation method of lithium hexafluorophosphate. The preparation method of the lithium hexafluorophosphate comprises the following steps: (1) uniformly mixing phosphorus pentachloride, triethylamine and a fluorinating agent to obtain a raw material solution; (2) mixing and uniformly stirring lithium hydroxide, ammonium fluoride, a pore-forming agent, a combustion improver and the binder dispersion liquid, and drying to obtain a lithium fluoride mixture; (3) calcining the lithium fluoride mixture to constant weight at 700-750 DEG C to obtain porous lithium fluoride; and (4) uniformly mixing the porous lithium fluoride with the raw material solution under the protection of nitrogen, keeping the temperature at 5-15 DEG C for 60-80 minutes, filtering and concentrating under reduced pressure to obtain a crude product lithium hexafluorophosphate, and purifying the crude product lithium hexafluorophosphate to obtain the lithium hexafluorophosphate. According to the porous lithium fluoride, the generation rate of lithium hexafluorophosphate can be increased, and the time required for producing the lithium hexafluorophosphate can be shortened.
Owner:SUZHOU HUAYI NEW ENERGY TECH CO LTD
Production method of diacetyl monoxime
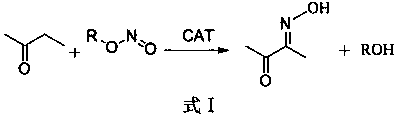
PendingCN110981751AReduce hydrolysisReduce dosagePhysical/chemical process catalystsChemical/physical/physico-chemical microreactorsDiacetyl monooximeO-Phosphoric Acid
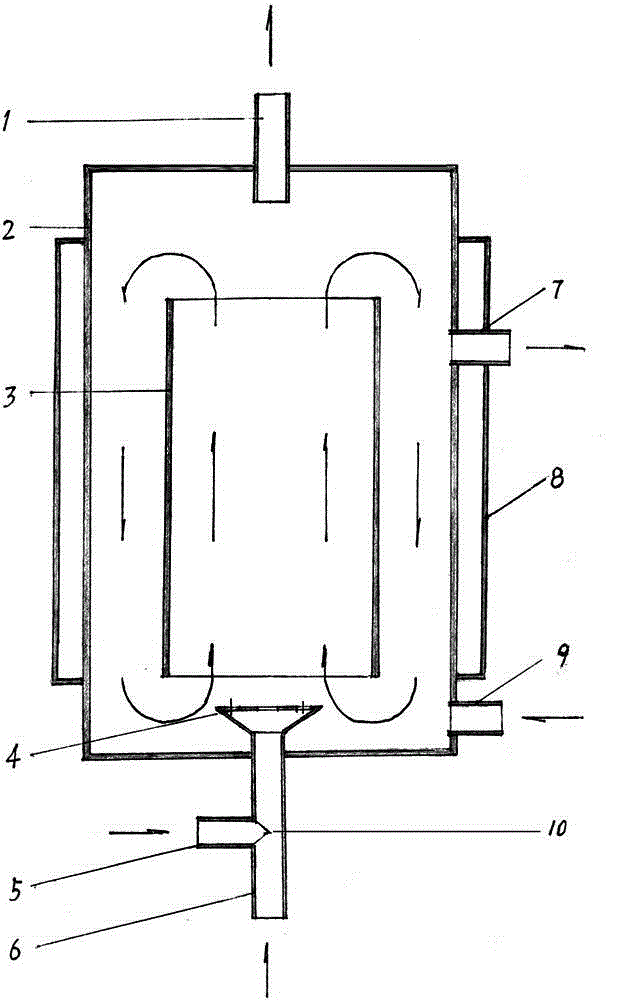
The invention discloses a production method of diacetyl monoxime, and relates to a method for producing diacetyl monoxime by reacting butanone with alkyl nitrite in a microtube reactor under the catalysis of a mixed catalyst system. . The method comprises the following steps: respectively adding premixed liquid of the catalyst and the butanone and the alkyl nitrite into the microtube reactor through a metering pump, wherein the mass ratio of the catalyst to the butanone is (0.1-0.2): 1, the molar ratio of the alkyl nitrite to the butanone is (1.01-1.1): 1, and the reaction pressure in the microtube reactor is 0.1-0.5 MPa, the reaction temperature is set to be 30-50 DEG C, the reaction time is 30-60 seconds and the catalyst is a mixture of concentrated hydrochloric acid, phosphorus pentachloride and concentrated phosphoric acid. The method has the beneficial effects that the moisture in the reaction system is reduced, the excessive use of raw materials is avoided, the production cost issaved, the production efficiency is improved and the pollution of excessive reactants to the environment is reduced and the reaction safety is improved; and the equipment investment is low, the occupied area is small and mass production is facilitated.
Owner:湖州柏特生物科技有限公司
Internal circulation reactor and method for continuously preparing phosphorus pentafluoride
InactiveCN102976298BStable concentrationIncrease production capacityPhosphorus halides/oxyhalidesAutomatic controlInternal circulation reactor
The invention discloses an internal circulation reactor and method for continuously preparing phosphorus pentafluoride. The internal circulation reactor comprises a cylinder body with a heat exchange jacket, wherein planar end closures are arranged at the upper and lower ends of the cylinder body, and a concentric inner cylinder body is arranged in the cylinder body; the upper end closure is provided with a mixed gas leading-out pipe; the lower end closure is provided with a three-way gas intake pipe; and a reaction solution eduction pipe and a feed liquid ingress pipe are arranged on the cylinder body. The process of preparing phosphorus pentafluoride comprises the following steps: blowing the reactor with an inert gas; creating a low temperature environment of the reactor with a refrigerant; adding a phosphorous pentachloride solution, anhydrous hydrogen fluoride and the inert gas to the reactor for reaction; and continuously leading out mixed gas containing phosphorus pentafluoride and part of a low-concentration phosphorous pentachloride solution, and at the same time continuously adding a phosphorous pentachloride concentrated solution, anhydrous hydrogen fluoride and the inert gas to realize continuous operation. The internal circulation reactor and method for continuously preparing phosphorus pentafluoride have the advantages that the concentration stability of reactants in the reactor can be maintained, and continuous production and automatic control of phosphorus pentafluoride are realized.
Owner:TIANJIN LONGER NEW MATERIAL SCI & TECH
Synthetic method of 5-(4'-methyl-[1,1'-biphenyl]-2-yl)-1hydro-tetrazole
The invention discloses a synthesis method of 5-(4'-methyl-[1,1'-biphenyl]-2-yl)-1-hydro-tetrazole, which comprises the following steps: by using 2-carboxy-4'-methyl biphenyl as the raw material, reacting with excessive thionyl chloride under reflux conditions, depressurizing to remove excess thionyl chloride, and carrying out acylation reaction on benzsulfamide to obtain a product III; reacting the product III with phosphorous pentachloride by using carbon tetrachloride as a solvent to obtain an imino acyl chloride derivative IV; carrying out substitution reaction on the imino acyl chloride derivative IV and hydrazine hydrate to obtain a product V; and finally, carrying out cyclization on the product V and sodium nitrate under acidic conditions, and carrying out benzenesulfonyl removal reaction to obtain the 5-(4'-methyl-[1,1'-biphenyl]-2-yl)-1-hydro-tetrazole. The method has the advantages of mild reaction conditions, high safety and high efficiency.
Owner:启东东岳药业有限公司 +1
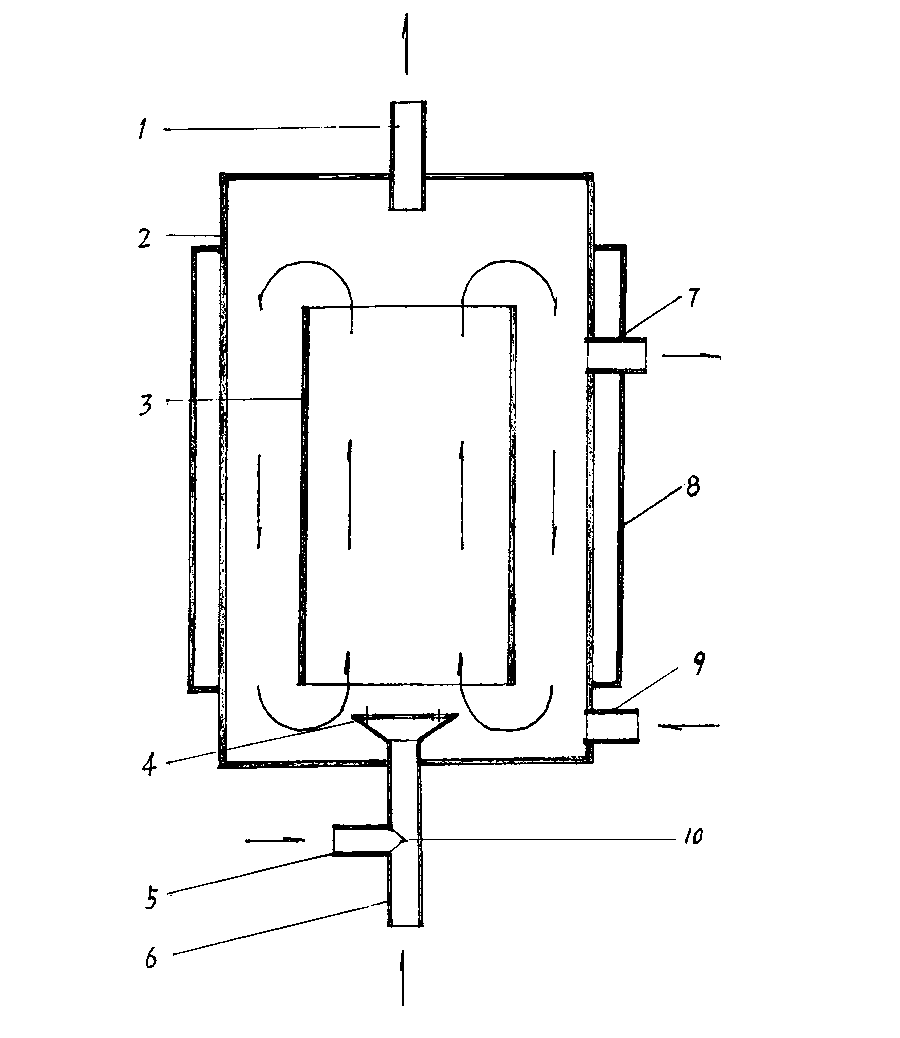
Process for manufacturing phosphorous trichloride, phosphorous pentachloride and cement
ActiveUS20110256040A1Less quantityEasy to processPhosphorus halides/oxyhalidesCeramic production plantsSilicon dioxidePhosphorous trichloride
A process for manufacturing phosphorous trichloride, phosphorous pentachloride and cement comprising of heating at elevated temperature of 1100° C. to 1500° C. pulverised mixture of phosphate ores, carbonaceous substances, silica and / or alumina and treating the resultant gaseous products with chlorine in the ratio of 1 to 5 moles of chlorine per mole of phosphorous oxide contained in the phosphate ores while maintaining the temperature between 400° C. to 1000° C. by cooling and thereafter firstly separating gaseous mixture of primarily phosphorous trichloride and phosphorous pentachloride, from cement and later separating phosphorous trichloride and phosphorous pentachloride, both separations by known methods.
Owner:GHARDA KEKI H
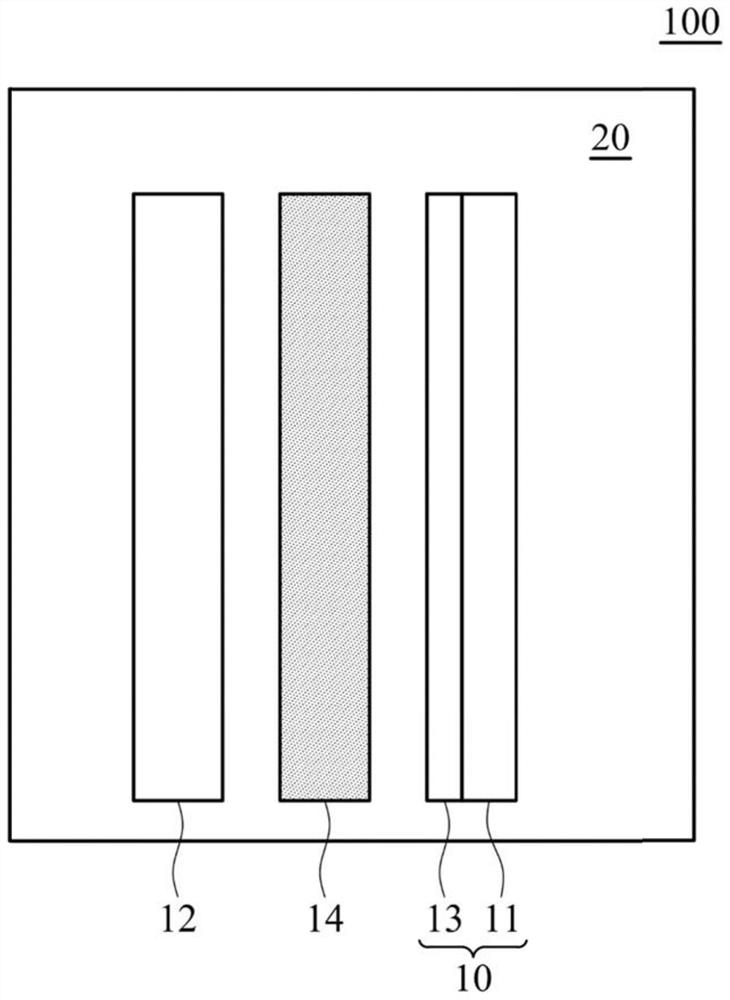
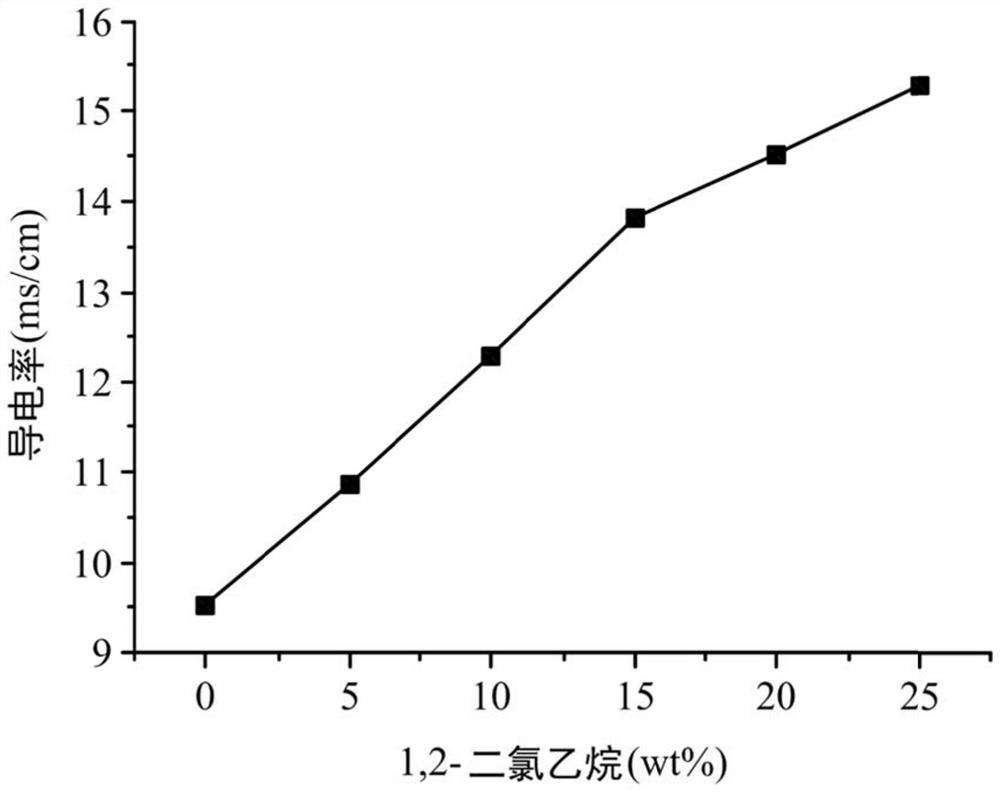
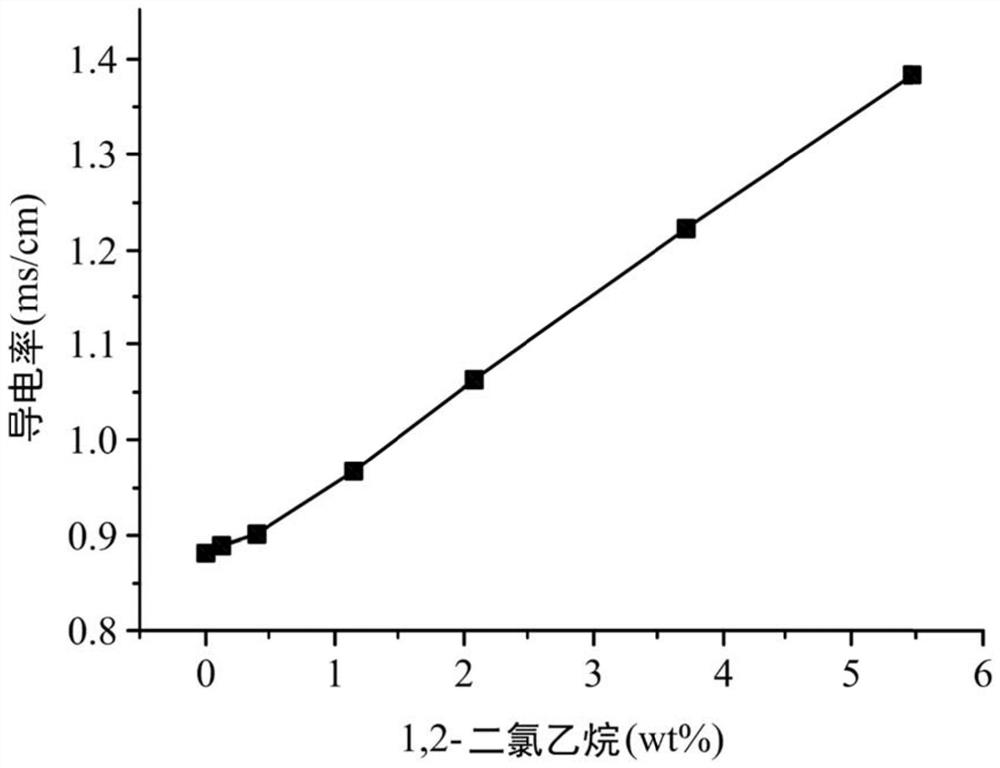
Electrolyte composition and metal ion battery comprising same
ActiveCN109962289BImprove conductivityIncrease capacityNon-aqueous electrolyte accumulatorsTrichloroethyleneMethyl palmoxirate
Owner:IND TECH RES INST
Preparation process of high-purity phosphorus pentafluoride
ActiveCN101844754BContinuous productionEasy to operatePhosphorus halides/oxyhalidesPhysical chemistryHydrogen chloride
The invention discloses a preparation process of high-purity phosphorus pentafluoride, which comprises the following steps of: firstly adding phosphorous pentachloride into a feed hopper of a spiral feeder under the protection of dry gas in a glove box to assemble a gas circulation loop, and simultaneously adding inert gas into the gas circulation loop in advance; starting a spiral stirring reaction furnace, simultaneously injecting mixed gas of hydrogen fluoride and fluorine gas into the spiral stirring reaction furnace, and controlling the reaction process by controlling the rotation frequency of the phosphorous pentachloride in the spiral feeder, the temperature of gas flow at the outlet of the spiral stirring reaction furnace and the pressure of the gas circulation loop; freezing the phosphorus pentafluoride gas and the hydrogen fluoride gas generated in the reaction process and unreacted hydrogen fluoride gas by a condenser-evaporator, and collecting high-purity phosphorus pentafluoride gas after reaction. The method is simple and effective and can be operated easily.
Owner:JIANGSU JIUJIUJIU TECH
Inorganic phosphates reversibly grafted with bioactive compounds and a method of their preparation
InactiveUS20070178124A1Prolong the action timeSurgical adhesivesPharmaceutical delivery mechanismProtonationDecomposition
Biologically active solid systems are disclosed, containing immobilized organic compounds, such as medicines, in the form of materials appropriate as bone fillers, implant coatings or other alike systems, of the common formula B-P-A, where B is an inorganic support consisting of calcium phosphate, calcium hydroxyphosphate (hydroxyapatite), their mixture, or some other biocompatible mixture containing these or other insoluble inorganic phosphates, -P- is a phosphoryl group -P-O-, and A is the residue of the immobilized, organic, biologically active compound, such as a medicine, containing unprotonated amine and / or hydroxide group(s), and a method of their preparation consisting of the treatment of inorganic phosphate with a grafting reagent and consequent reaction with organic compound. As a grafting reagent, phosphorus pentachloride is applied and all operations are completed in an aprotic solvent. The advantage of the above product is the extension of the time duration of release of the immobilized organic compound such as a medicine from the surface, the release of the organic compound in its initial, unaltered, fully biologically active form, in the absence of any toxic or alien side products of hydrolytic decomposition of the immobilized system.
Owner:DR MICHAEL GOLDFELD +1
Preparation method of diphosphorus ligand
ActiveCN114835749AEase of mass productionSimple processOrganic chemistry methodsGroup 5/15 element organic compoundsCombinatorial chemistryGrignard reaction
The invention relates to a preparation method of a diphosphorus ligand, which comprises the following steps: carrying out Grignard reaction on a compound (1) and magnesium metal to prepare a compound (2); carrying out first substitution reaction on the compound (3) and phosphorus pentachloride to prepare a compound (4); then carrying out second substitution reaction on the compound (2) and a compound (4) to prepare a compound (5); finally, the compound (5) is subjected to a reduction reaction, and the diphosphine ligand shown in the formula (6) is obtained. The process is simple, raw materials are simple and easy to obtain, the process conditions in the preparation process are easy to control, phosphorus-hydrogen intermediates do not need to be prepared, the diphosphine ligand can be prepared under the mild process conditions, and large-scale production of the diphosphine ligand is facilitated.
Owner:JIANGSU SINOCOMPOUND CATALYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
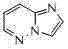
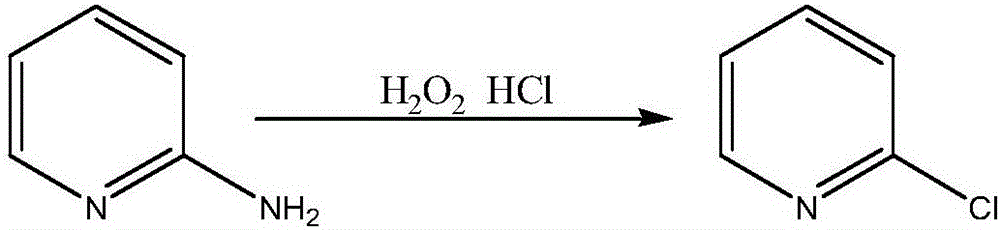

![Synthetic method of 5-(4'-methyl-[1,1'-biphenyl]-2-yl)-1hydro-tetrazole Synthetic method of 5-(4'-methyl-[1,1'-biphenyl]-2-yl)-1hydro-tetrazole](https://images-eureka.patsnap.com/patent_img/79895173-866c-422d-b983-b6c8d159ba64/BDA0000730611380000021.png)
![Synthetic method of 5-(4'-methyl-[1,1'-biphenyl]-2-yl)-1hydro-tetrazole Synthetic method of 5-(4'-methyl-[1,1'-biphenyl]-2-yl)-1hydro-tetrazole](https://images-eureka.patsnap.com/patent_img/79895173-866c-422d-b983-b6c8d159ba64/FDA0001182265080000011.png)