Preparation method of trandolapril midbody (2S, 3aR, 7aS)-octahydro-1H-indole-2-carboxylic acid
A technology for trandolapril and intermediates, which is applied in the field of intermediates for the preparation of drugs, can solve problems such as difficult separation and purification processes, and achieve the effects of low cost, easy control, and simple process
Inactive Publication Date: 2013-09-11
大连鼎燕医药化工有限公司 +1
View PDF8 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
But the separation and purification process of the whole reaction process is more difficult to solve
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
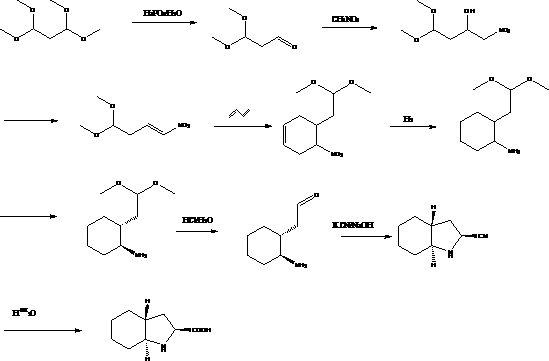
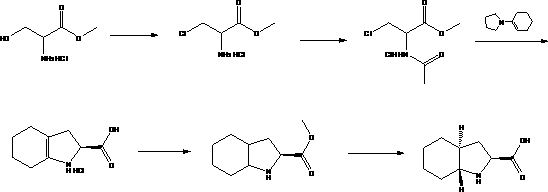
The invention discloses a preparation method of trandolapril midbody (2S, 3aR, 7aS)-octahydro-1H-indole-2-carboxylic acid, and the preparation method comprises the following steps of: preparing 3-chlorine-2-amino-propionic acid methyl ester hydrochloride by using phosphorous pentachloride and dichloromethane; preparing 3-chlorine-2-acetyl amino-propionic acid methyl ester hydrochloride by using the 3-chlorine-2-amino-propionic acid methyl ester hydrochloride, methylbenzene and acetyl chloride; preparing 2,3,4,5,6,7-hexahydro-1H-indole-2-carboxylic acid by using the 3-chlorine-2-acetyl amino-propionic acid methyl ester hydrochloride, DMF (Dimethyl Formamide) and 1-pyrrole cyclohexene; preparing (2S)-octahydro-1H-indole-2-methyl carboxylat by using the 2,3,4,5,6,7-hexahydro-1H-indole-2-carboxylic acid, methanol and palladium on carbon; preparing the (2S, 3aR, 7aS)-octahydro-1H-indole-2-carboxylic acid by using the (2S)-octahydro-1H-indole-2-methyl carboxylat and the methanol. Compared with the prior art, the preparation method of the trandolapril midbody (2S, 3aR, 7aS)-octahydro-1H-indole-2-carboxylic acid, which is disclosed by the invention, has the advantages of easiness and convenience for process, easiness for control, safety, reliability and low cost.
Description
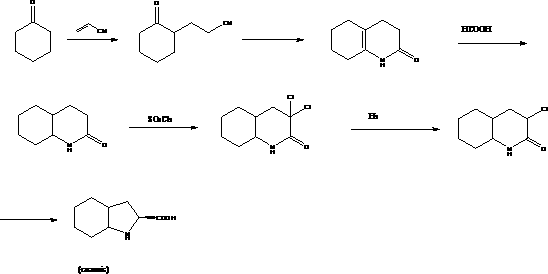
technical field The invention relates to an intermediate for the preparation of medicines, in particular to a method for preparing a trandolapril intermediate (2S, 3aR, 7aS)-octahydro-1H-indole-2-carboxylic acid. Background technique The chemical name of trandolapril is (2S, 3aR, 7aS)-1-[(2S)-2-[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxo Propyl]octahydro-1H-indole-2-carboxylic acid is a long-acting angiotensin-converting enzyme inhibitor (ACEI) developed by Roussel Uclaf Company in Germany. Clinical indications for hypertension, congestive heart failure and myocardial infarction. (2S, 3aR, 7aS)-octahydro-1H-indole-2-carboxylic acid is the most important intermediate for the preparation of trandolapril. Many multinational companies in the world have conducted in-depth research on its synthesis process. So far, it has There are 5 main process routes with their respective pros and cons as follows: Route 1: In U.S. Patent No. 6,599,318, 1,1,3,3,-tetramethoxypropa...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D209/42
Inventor 张勇鹿军汪洋范莉莉吴冬辉李艳凤孙立芹薛丽红李德龙
Owner 大连鼎燕医药化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com