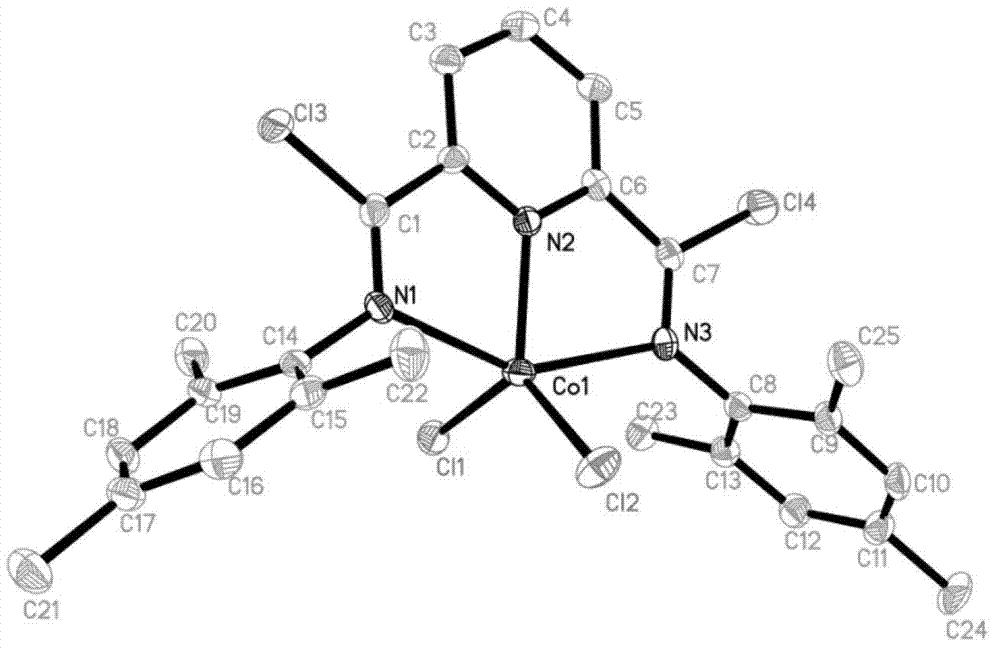
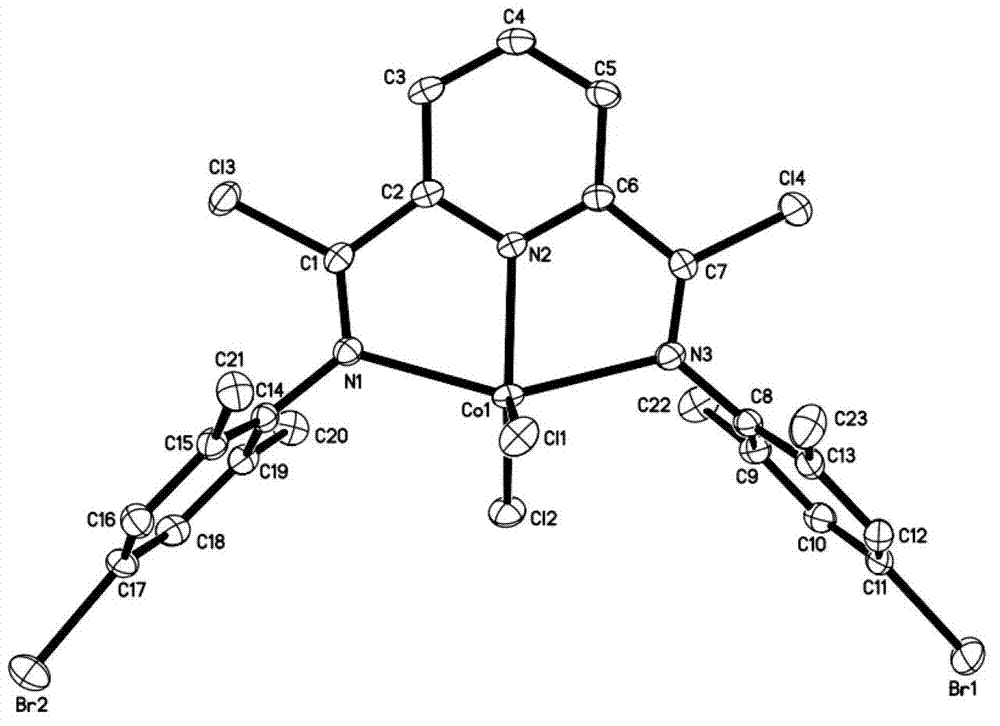
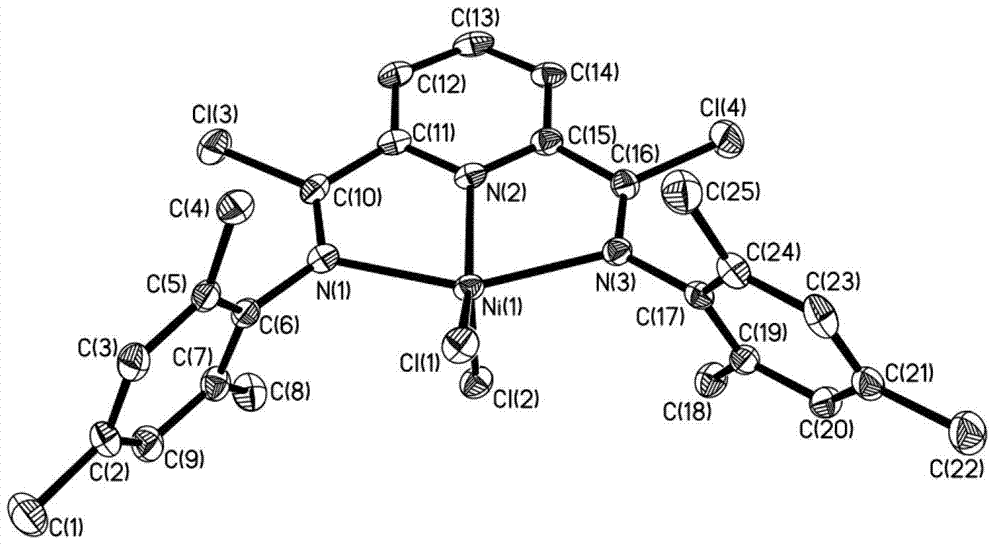
Post-transition metal complex, preparation method thereof and polyethylene preparation method
A late transition metal and complex technology, applied in the field of catalysts, can solve the problem of low catalytic activity of late transition metal complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The present invention also provides a preparation method of the late transition metal complex, which is specifically prepared according to the following method:
[0054]Reacting bis(substituted aniline)-2,6-pyridinedicarboxamide and phosphorus pentachloride in a first organic solvent for the first time to obtain a ligand with the structure of formula (II);
[0055] performing a second reaction between the ligand and a late transition metal halide in a second organic solvent to obtain a late transition metal complex having the structure of formula (I), the late transition metal being cobalt, iron or nickel;
[0056]
[0057] Among them, R 1 and R 1 Each independently selected from hydrogen or C 1 ~ C 10 alkyl;
[0058] R 3 is hydrogen, halogen or C 1~C 10 alkyl;
[0059] The process of obtaining the ligand with the structure of formula (II) is specifically:
[0060] Under the protection of nitrogen, bis(substituted aniline)-2,6-pyridinedicarboxamide and phosphoru...
Embodiment 1
[0076] Dissolve 8.11g (60mmol) of 2,4,6-trimethylaniline and 8.36ml (60mmol) of triethylamine in 50ml of CH 2 Cl 2 , 6.1 g (30 mmol) of pyridinedicarboxylic acid chloride was dissolved in 20 ml of CH 2 Cl 2 In, pyridine dicarboxylic acid chloride / CH 2 Cl 2 The solution was added dropwise to the substituted aniline / CH 2 Cl 2 After reflux for 4 hours, use 100ml of water and 100ml of 1.5M NaCO 3 The solution was washed, and a separatory funnel was used to obtain the lower CH 2 Cl 2 solution, adding MgSO 4 Dry overnight, filter and suck dry, then recrystallize with toluene, filter and dry to obtain the product (5a). Yield 91%. The product (5a) was analyzed by nuclear magnetic resonance, and the characterization results are as follows: 1 H NMR (400MHz, CDCl 3 , δ, ppm): 9.02 (s, 2H, NH), 8.51 (d, 2H, Pyr-H m ), 8.14 (t, 1H, Pyr-H p ), 6.95 (s, 4H, Ar-H), 2.30 (s, 6H, Ar-C p h 3 ), 2.26 (s, 12H, Ar-C o h 3 ); 13 C NMR (100MHz, CDCl 3 , δ, ppm): 161.6, 148.8, 139....
Embodiment 2
[0082] Dissolve 11.94 g (60 mmol) 4-bromo-2,6-dimethylaniline and 8.36 ml (60 mmol) triethylamine in 50 ml CH 2 Cl 2 , 6.1 g (30 mmol) of pyridinedicarboxylic acid chloride was dissolved in 20 ml of CH 2 Cl 2 In, pyridine dicarboxylic acid chloride / CH 2 Cl 2 The solution was added dropwise to the substituted aniline / CH 2 Cl 2 After reflux for 4 hours, use 100ml water and 100ml 1.5M NaCO 3 The solution was washed, and the lower layer of CH was obtained with a separatory funnel 2 Cl 2 solution, adding MgSO 4Dry overnight, filter and suck dry, then recrystallize with toluene, filter and dry to obtain the product (10a), the yield is 86.4%. The product (10a) was analyzed by nuclear magnetic resonance, and the characterization results are as follows: 1 H NMR (400MHz, CDCl 3 , δ, ppm): 8.98 (s, 2H, NH), 8.55 (d, 2H, Pyr-H m ), 8.19 (t, 1H, Pyr-H o ), 7.33 (s, 4H, Ar-H), 2.30 (s, 12H, -CH 3 ); 13 C NMR (100MHz, CDCl 3 , δ, ppm): 161.5, 148.6, 139.5, 137.4, 131.0, 128.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com