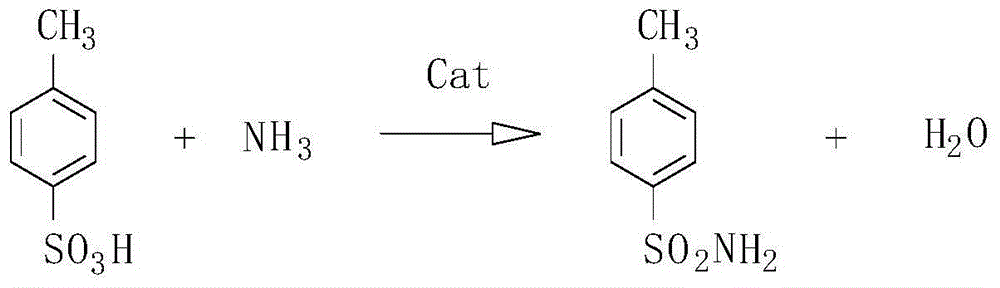
Method for preparing para toluene sulfonamide by directly amidating para-toluenesulfonic acid
A technology of p-toluenesulfonamide and p-toluenesulfonic acid is applied in the field of preparing p-toluenesulfonamide by catalytic sulfonamide reaction, and can solve the problems of environmental pollution, a large amount of waste acid and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1. Dissolve 3.444g (20mmol) of anhydrous p-toluenesulfonic acid and 0.172g of 2-bromophenylboronic acid in dichloromethane, add 5g of molecular sieve type 5A, and stir evenly at 0°C for 2h.
[0018] 2. Heat 20g of 25% ammonia water, and the generated ammonia gas is passed into the reaction solution after passing through the gas drying processor. Add 20g of ammonia water every 4h until 24h, adding 120g of ammonia water in total.
[0019] 3. After the molecular sieves were removed by suction filtration, the filtrate was washed once with 10 mL hydrochloric acid solution (0.5 mol / L concentration), 10 mL sodium hydroxide solution (0.5 mol / L concentration) and 10 mL saturated NaCl solution.
[0020] 4. After the organic phase was dried with anhydrous sodium sulfate, the desiccant was removed, and dichloromethane was recovered by distillation to obtain a crude product of p-toluenesulfonamide, which was washed with distilled water and dried to obtain a white crystal weighing 1....
Embodiment 2
[0023] 1. Dissolve 3.444g (20mmol) of anhydrous p-toluenesulfonic acid and 0.241g of 4-bromoboronic acid in dichloromethane, add 5g of molecular sieve type 5A, and stir evenly at 0°C for 2h.
[0024] 2. Heat 20g of 25% ammonia water, and the generated ammonia gas is passed into the reaction solution after passing through the gas drying processor. Add 20g of ammonia water every 4h until 24h, adding 120g of ammonia water in total.
[0025] 3. After the molecular sieves were removed by suction filtration, the filtrate was washed once with 10 mL of 0.5 mol / L hydrochloric acid solution, 0.5 mol / L sodium hydroxide solution and saturated NaCl solution respectively.
[0026] 4. Dry the organic phase with anhydrous sodium sulfate, remove the desiccant, distill and recover dichloromethane to obtain crude p-toluenesulfonamide, wash with distilled water and dry to obtain white crystals, weighing 0.53g
[0027] 5. Through liquid chromatography analysis, the active ingredient is 98.8%, the...
Embodiment 3
[0029] 2. 1. Dissolve 3.444g (20mmol) of anhydrous p-toluenesulfonic acid and 0.31g of 3-bromoboronic acid in dichloromethane, add 5g of molecular sieve type 5A, and stir evenly at 0°C for 2h.
[0030] 2. Heat 20g of 25% ammonia water, and the generated ammonia gas is passed into the reaction solution after passing through the gas drying processor. Add 20g of ammonia water every 4h until 24h, adding 120g of ammonia water in total.
[0031] 3. After the molecular sieves were removed by suction filtration, the filtrate was washed once with 10 mL of 0.5 mol / L hydrochloric acid solution, 0.5 mol / L sodium hydroxide solution and saturated NaCl solution respectively.
[0032] 4. After the organic phase was dried with anhydrous sodium sulfate, the desiccant was removed, dichloromethane was recovered by distillation, and the crude product of p-toluenesulfonamide was obtained, which was washed with distilled water and then dried to obtain a white crystal weighing 0.395 g.
[0033] 5. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting range | aaaaa | aaaaa |
| Melting range | aaaaa | aaaaa |
| Melting range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com