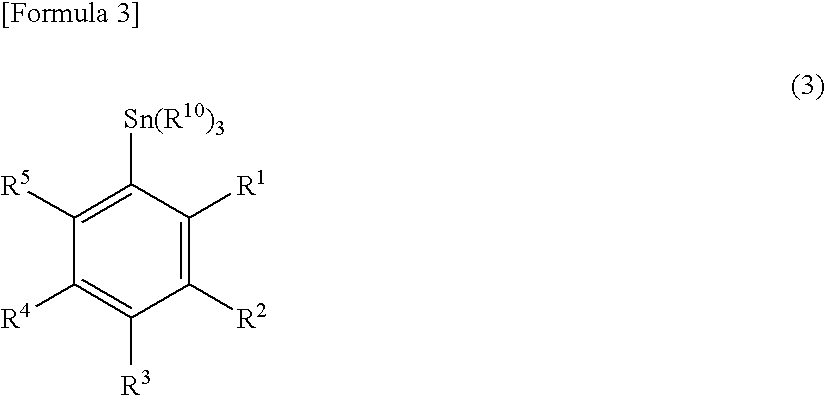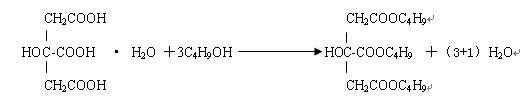Patents
Literature
57 results about "Tosylic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
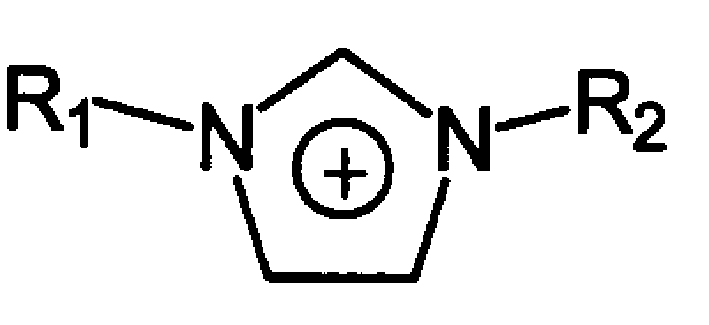
Room temperature ionic liquid containing unsaturated double bond and its prepn and application
InactiveCN1417407AGroup 5/15 element organic compoundsPulping with organic solventsSulfate radicalsTriflic acid
The room temperature ionic liquid containing unsaturated double bond has the general expressino of A+B-, where A+ contains R1 being hydroxyl with 1-4 carbon atoms and R2 containing 2-20 carbon atoms and at least one double bond; and B- is one of anions, including chlorate radical, bromate radical, iodate radical, acetate radical, sulfate radical, nitrate radical, tetrafluorobromate radical, etc. Its preparation is to mixture and react olefin halide R1X and N-alkyl imidazole to obtain ionic liquid dialkyl imidazolium halide. The present invention also relates to the application of the ionic liquid in dissolving cellulose and preparing cellulose derivative.
Owner:山东中科恒联生物基材料有限公司
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
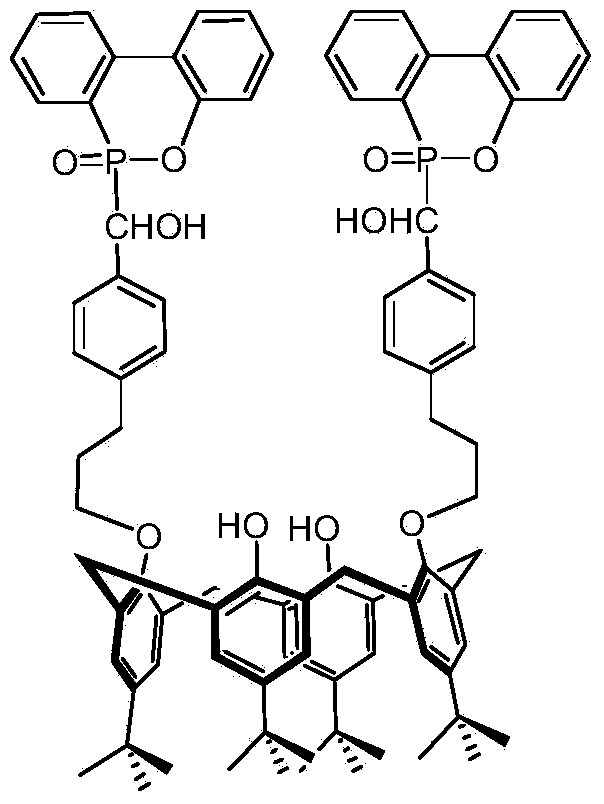
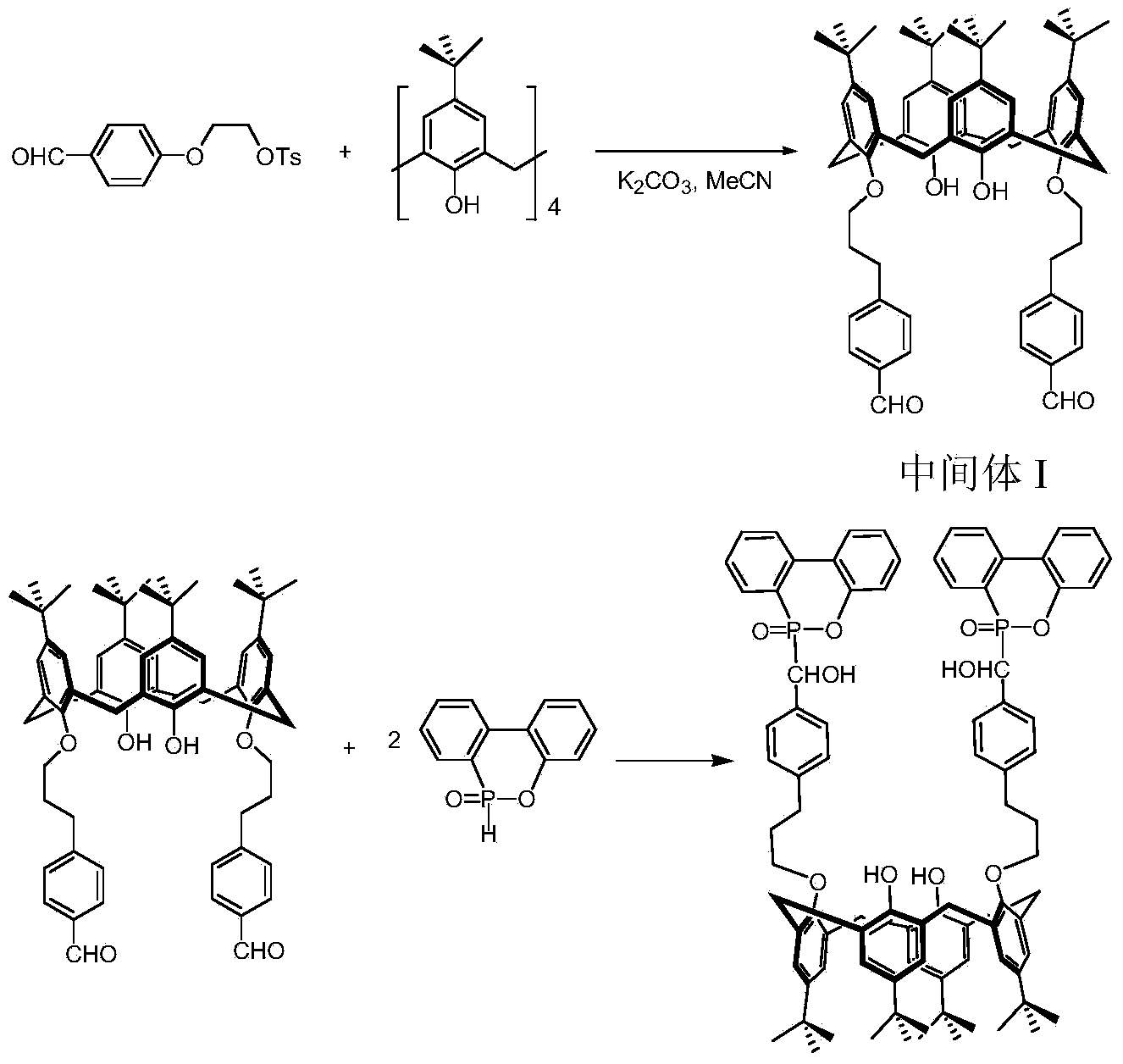
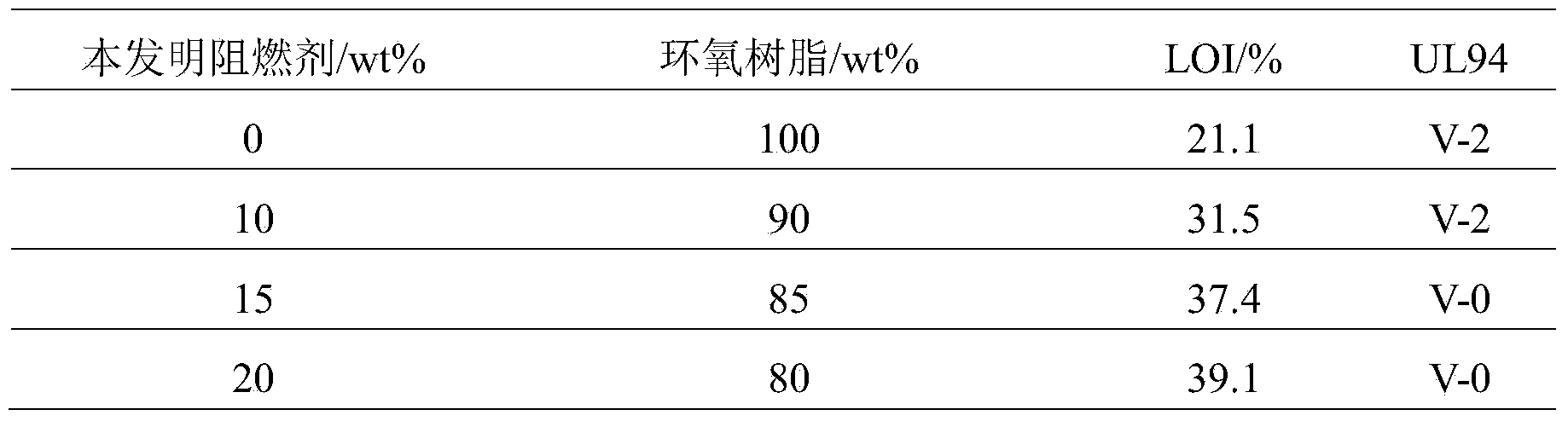
Oxa-phosphaphenanthrene fire retardant as well as preparation method and application of oxa-phosphaphenanthrene fire retardant
ActiveCN104231309AEasy to operateAdvanced technologyGroup 5/15 element organic compoundsEpoxyPolymer science
The invention discloses an oxa-phosphaphenanthrene fire retardant as well as a preparation method and application of the oxa-phosphaphenanthrene fire retardant. The fire retardant has a calyx[4]arene structure; p-tert-butyl calyx[4]arene and p-tosylate ethoxy p-benzaldehyde are prepared into an intermediate I in the presence of K2CO3 and acetonitrile, and the intermediate I reacts with DOPO to obtain a target compound; the compound is white in appearance, has the melting point of 198-200 DEG C and the purity of 98.5% and is good in thermal stability and high in flame retarding rate; the used raw materials are easily available, and a process is advanced and easy for industrialized production. The flame retardant not only can serve as a reactive flame retardant to be used in thermosetting resin such as epoxy resin and polyurethane but also can serve as an additive flame retardant to be used for engineering plastics with relatively high requirement on the heat resistance of the flame retardant.
Owner:湖北同广和新材料有限公司
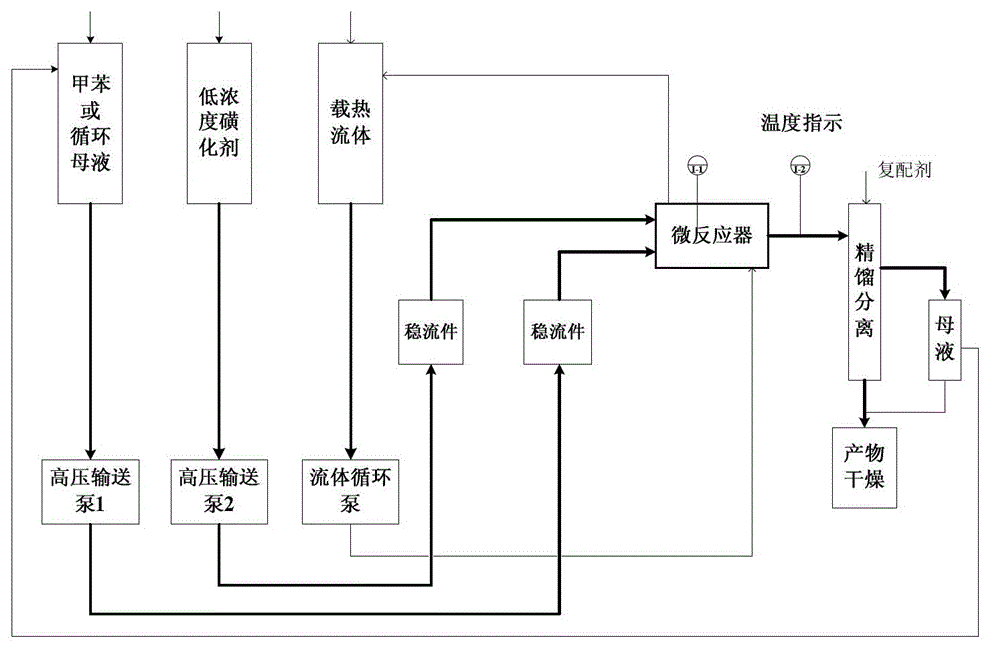
Method for preparing p-toluenesulfonic acid through toluene sulfonation
ActiveCN103936636APrecise control of reaction temperatureProcess Safety ContinuityChemical recyclingSulfonic acid preparationHigh concentrationTosylic acid
The invention discloses a method for preparing p-toluenesulfonic acid through toluene sulfonation in a micro-reactor. The method is characterized in that a low-concentration liquid SO3 is taken as a sulfonating agent, an excess feeding ratio of toluene to SO3 is employed, and in the sulfonation technology, a high-concentration toluene is used for feeding and a product mother liquor (filtrate) is cycled. Also, a micro-channel in-situ heat exchange technology is employed for precisely regulating the sulfonation reaction temperature. The method has the advantages that liquid sulfur trioxide is taken as the sulfonating agent, no waste acid is basically generated in products, separation is easy to operate, the concentration of the main product p-toluenesulfonic acid is 97%, and the micro-reaction sulfonation technology is safe and continuous in process and high in efficiency.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
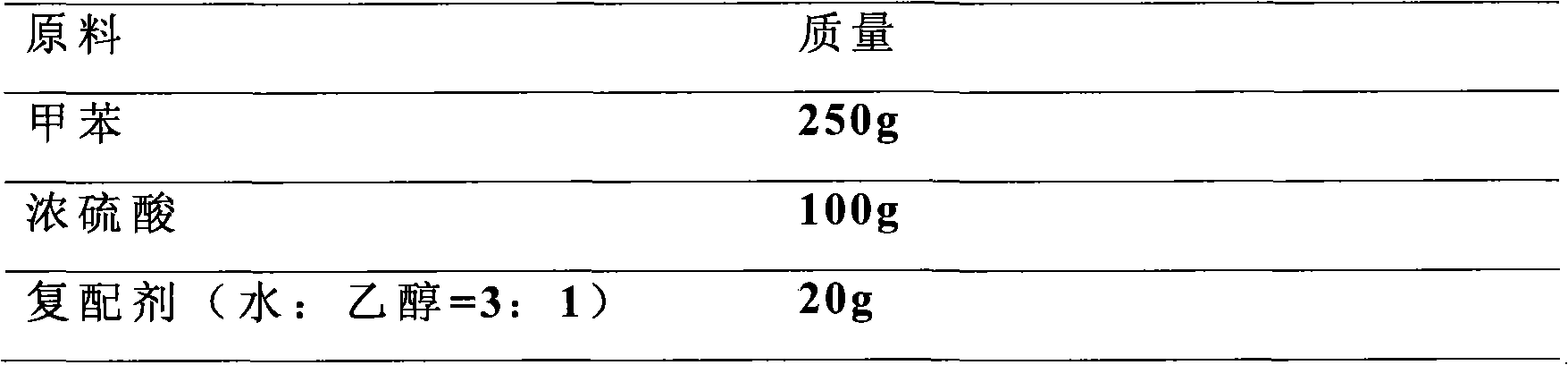
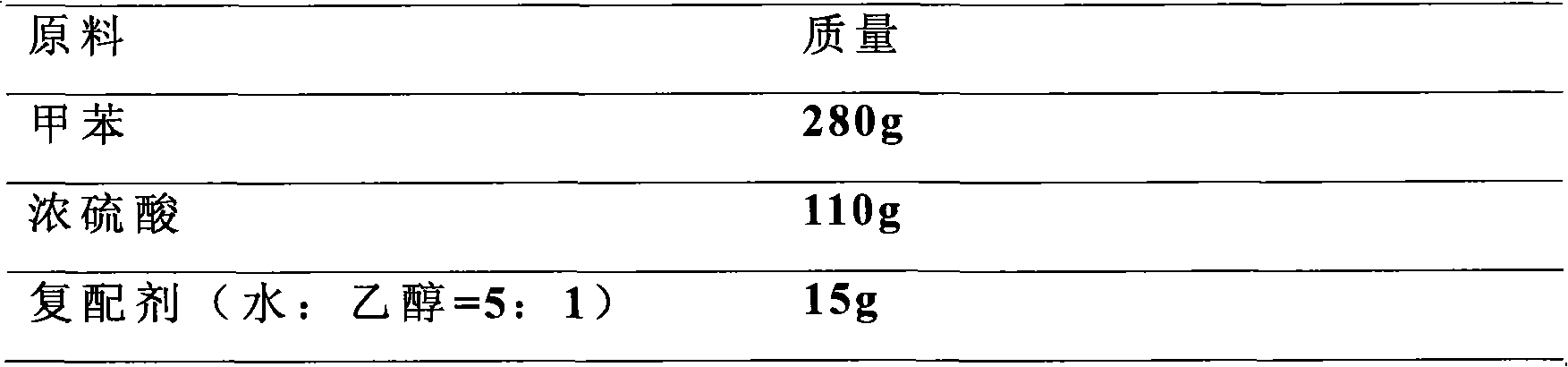
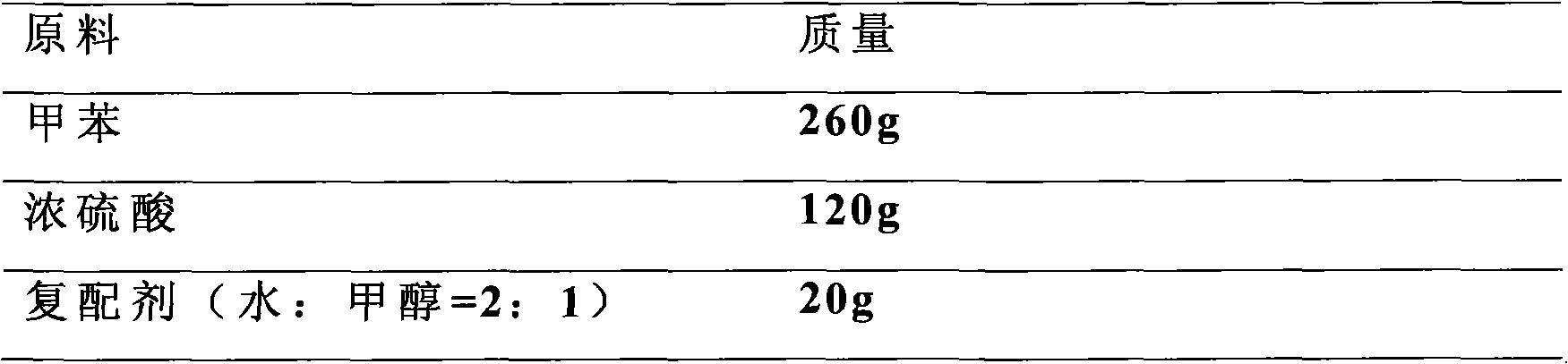
Method for preparing p-toluenesulfonic acid by toluene sulfonation
ActiveCN101845004AHigh purityNot easy to agglomerateSulfonic acid preparationTosylic acidP-Toluenesulfonic acid
The invention discloses a method for preparing p-toluenesulfonic acid by toluene sulfonation, which is characterized by comprising a step of adding a compound agent into a reaction product to perform crystallization after toluene and sulfonating agent produce sulfonation reaction, wherein the compound agent consists of at least two different p-toluenesulfonic acid solvents. A p-toluenesulfonic acid finished product prepared by the method has high content and has high purity of the p-toluenesulfonic acid; and the finished product content is more than or equal to 98.5 percent and the purity of the p-toluenesulfonic acid is more than or equal to 99.5 percent through detection, and the finished product is difficult to form blocks after placing. Because the synthesis and refining are finished in the same reaction system, the method has the advantages of short flow, convenient operation, improved production efficiency and reduced labor intensity.
Owner:SUZHOU XINGYE CHEM
Preparation method for amphoteric hydroxypropyl guar gum derivative
The invention discloses a preparation method for an amphoteric hydroxypropyl guar gum derivative. The preparation method is characterized by comprising the following steps: (1) performing esterification reaction on hydroxypropyl guar gum and toluene sulfochloride to obtain hydroxypropyl guar gum tosylate; (2) employing an amination reagent to perform nucleophilic substitution on hydroxypropyl guar gum tosylate, removing tosylate group, so as to obtain an amino guar gum derivative; and (3) reacting the amino guar gum derivative under the catalysis of an acid, so as to obtain an amphoteric polymer containing both amino and carboxyl. The preparation method is a new method for preparing the amphoteric guar gum derivative in the field, and the method is simple and practicable, and the reactions are easy to control.
Owner:昆山京昆油田化学科技有限公司
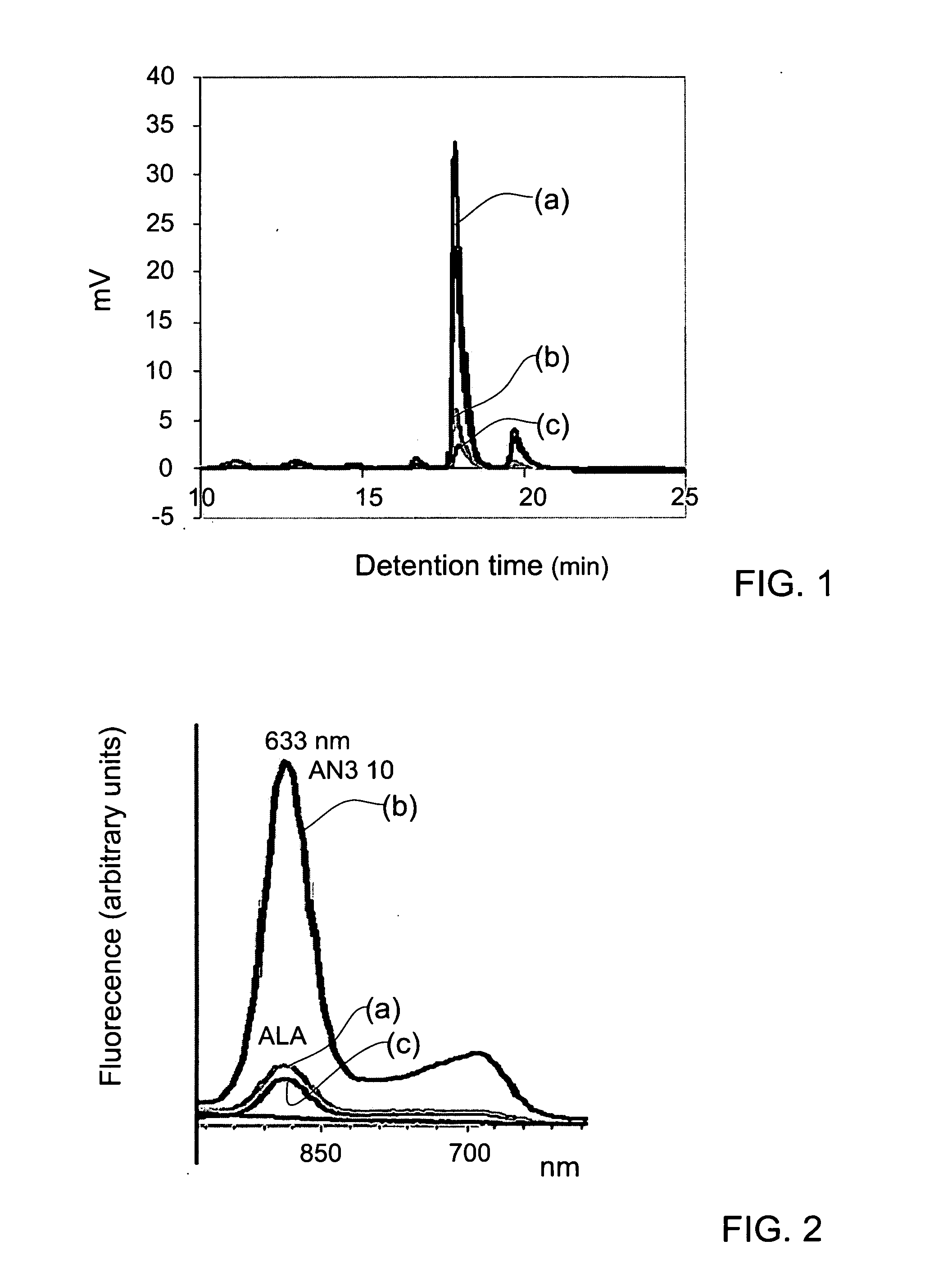
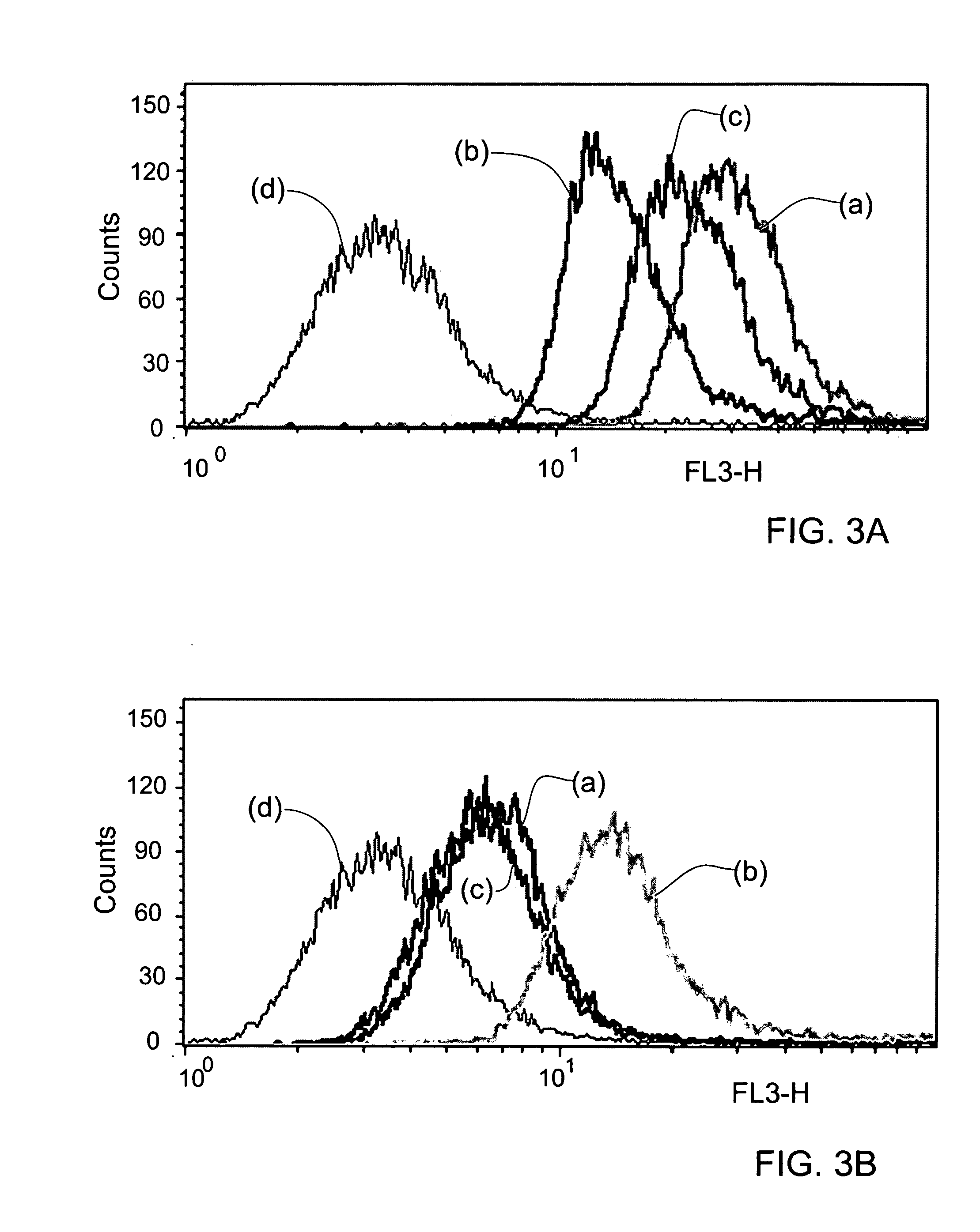
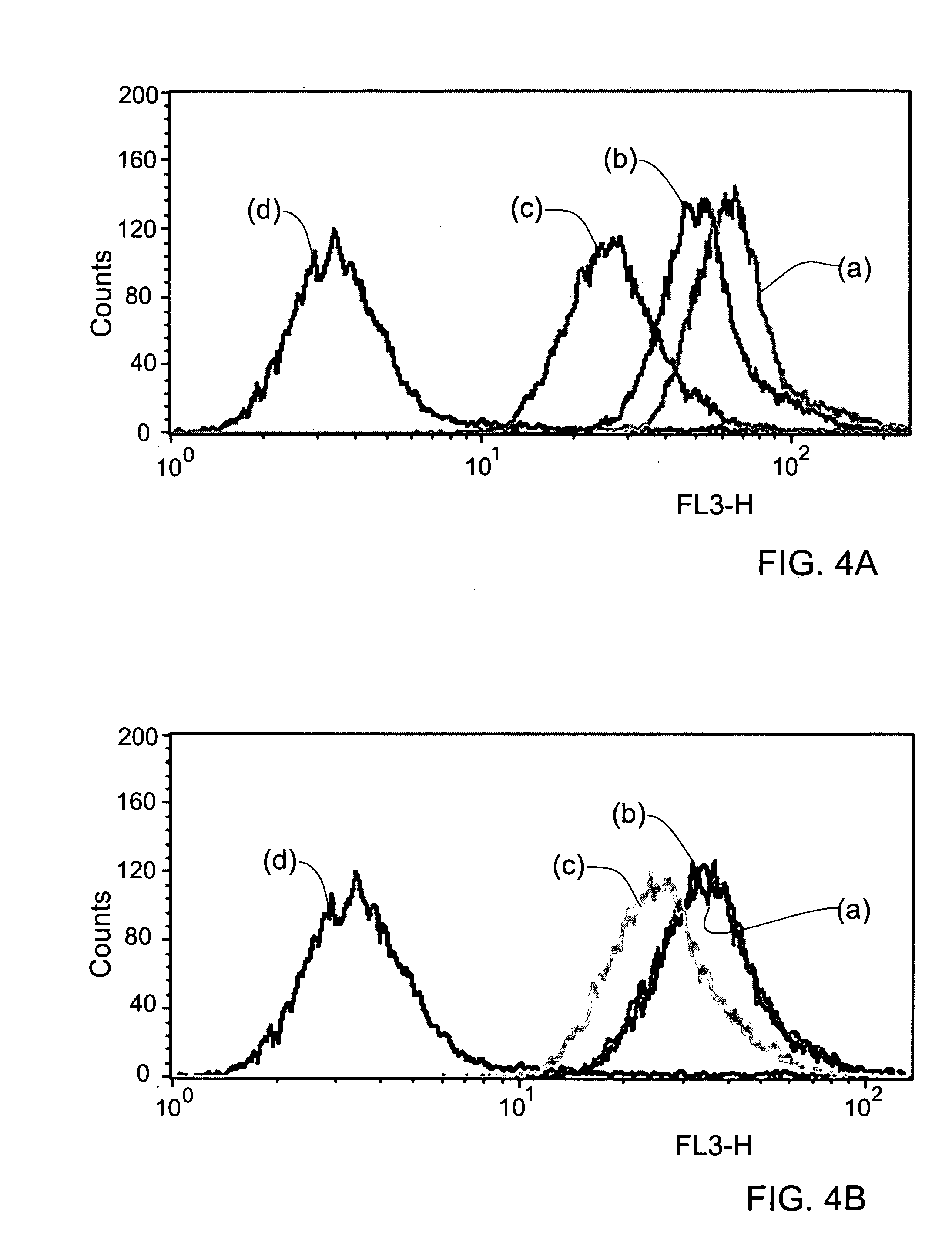
5-Aminolevulinic acid salts and their use
The invention provides salts of 5-Aminolevulinic acid (ALA) of formula (I): wherein RY is an organic acid moiety; Y is selected from the group consisting of a sulfonic acid residue, mono- or di-phosphoric acid residue, mono- or di-carboxylic acid residue and R is selected from the group consisting of saturated, unsaturated, straight or branched C2-C20 chains, aryl, aralkyl or naphthyl. In preferred embodiments, RY is selected from benzenesulfonic acid (besylate), 2-naphthalene sulfonic acid (napsyate), p-toluenesulfonic acid (tosylate), diethyl phosphate, dibenzyl phosphate, di-(2-ethylhexyl) phosphate, caproic or stearic acids. The invention also provides methods for preparing the ALA salts of the invention, pharmaceutical compositions containing the ALA salts of the invention, and use of the ALA salts of the invention in photodynamic therapy (PDT).
Owner:BAR ILAN UNIV +1
Esterified acrylie-ester high-polymer, its synthesis and use
An acrylic ester polymer and its synthesis are disclosed. The process is carried out by heat polymerizing for mixture of thermal initiator benzoyl peroxide and acrylic ester under agitation and nitrogen protection to obtain acrylic ester polymer, adding into acrylic acid, taking tosylate as catalyst and hydroquinone as inhibitor, reacting at 100-110 degree, removing water, decompressing, and removing solvent and inactive raw material to obtain final product. It can be used to machine building material cyanamide paper, and improve coating and substrate performances and adhesion.
Owner:扬州雅致达板饰有限公司
Method for synthesizing radioactive ligand having 18f-labeled fluorobenzene ring
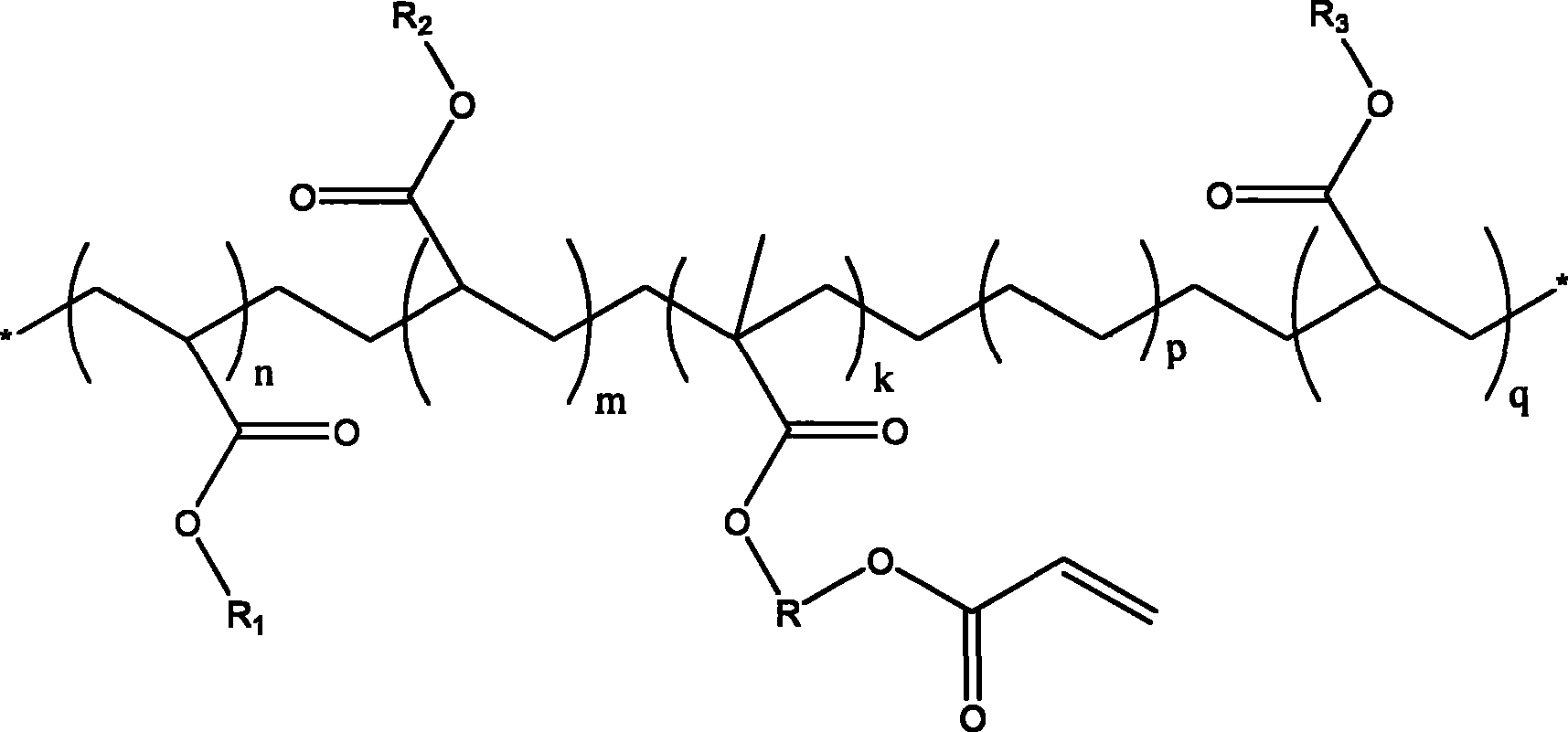
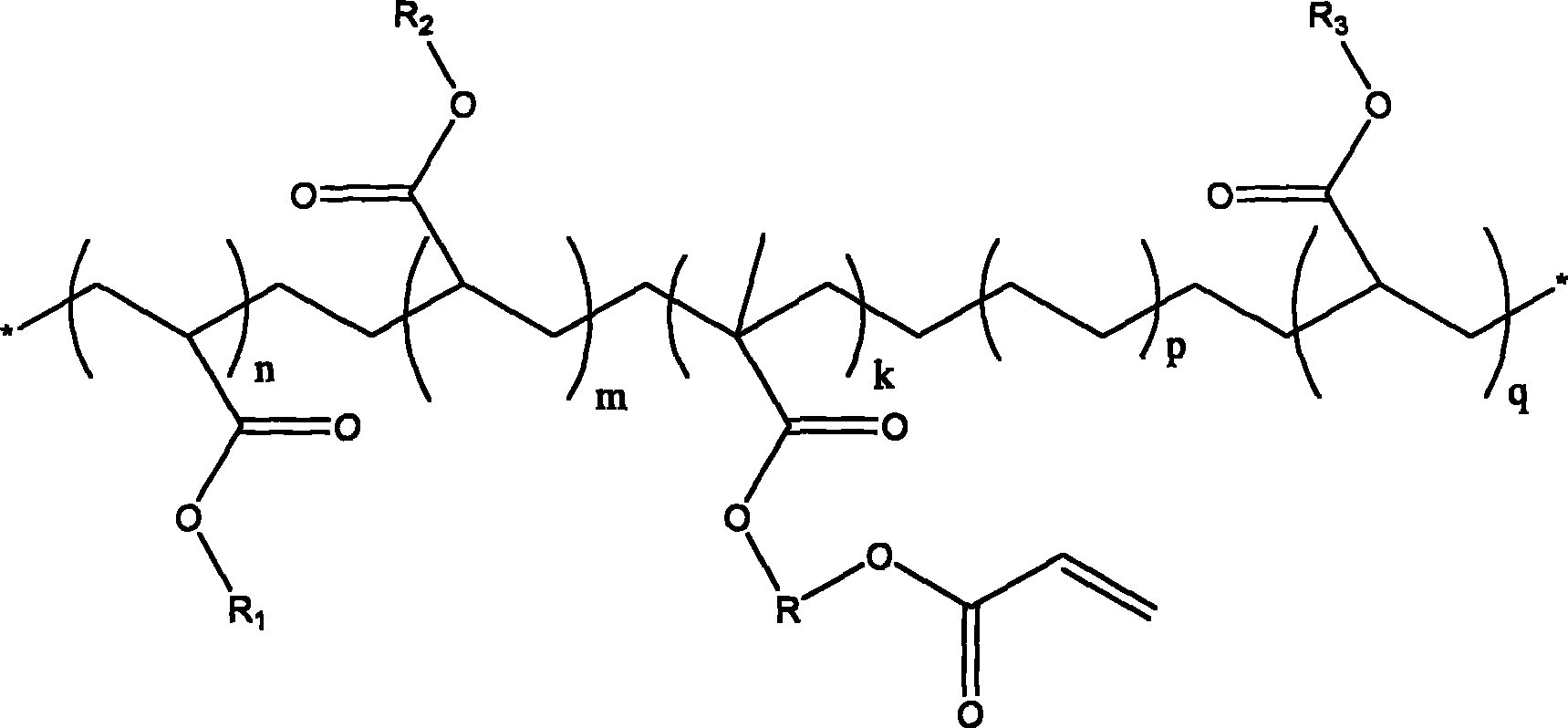
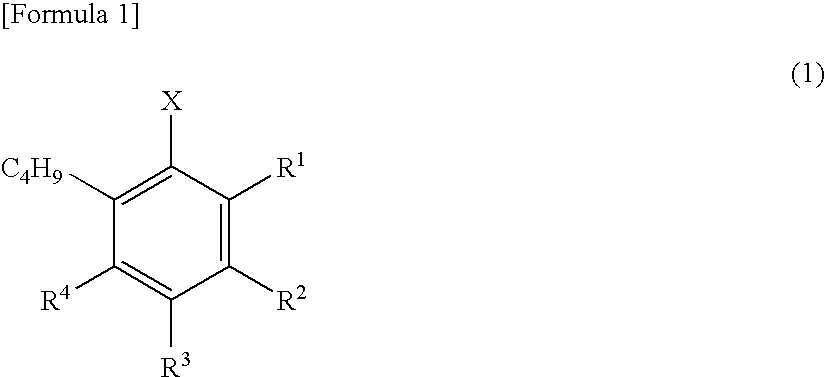
InactiveUS20090069592A1High yieldShort timeBiological testingIsotope introduction to acyclic/carbocyclic compoundsTosylic acidFluorobenzene
A phenyl tin compound is synthesized by using a derivative having various functional groups and a bromo- or iodo-benzene ring as a labeling material of a radioactive ligand. On the other hand, a novel hydroxytosyl iodobenzene compound having an electron-donating group is obtained by oxidizing iodobenzene having one or more electron-donating groups and reacting it with tosylic acid. Then, a diphenyliodonium salt which is a labeling precursor is synthesized by reacting the resulting compound with various phenyl tin compounds. Finally, a 18F-labeled ligand having various functional groups and a [18F] fluorobenzene ring is synthesized by reacting the resulting diphenyliodonium salt with [18F]F−.
Owner:NAT INST FOR QUANTUM & RADIOLOGICAL SCI & TECH
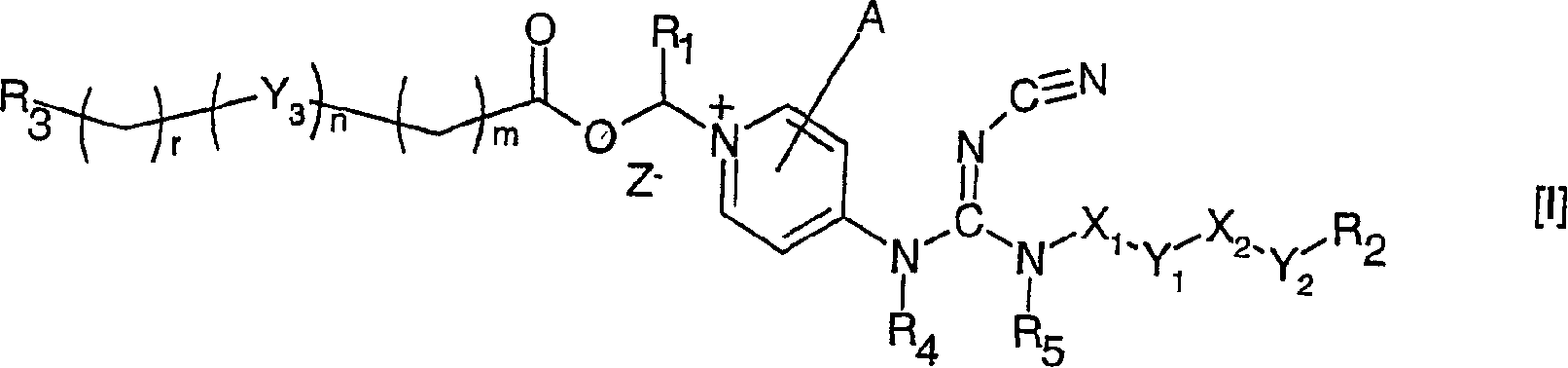
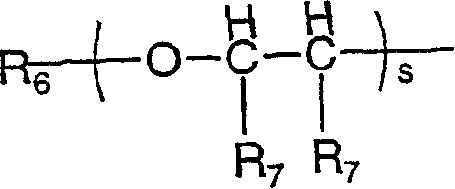
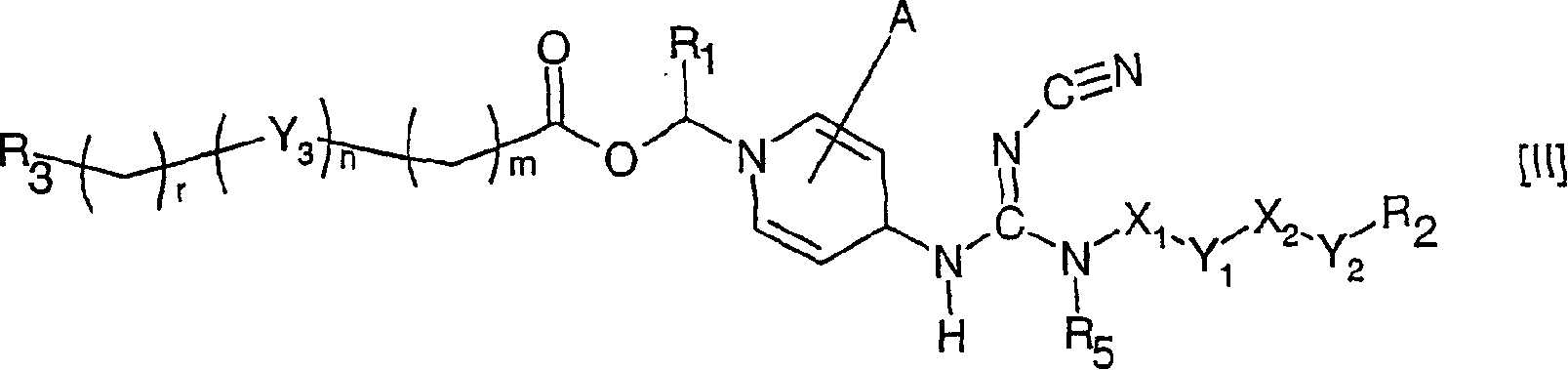
Cyanoguanidine prodrugs
The invention relates to compounds of formula (I), wherein X1 and X2 independently represent a bond; a straight, branched and / or cyclic hydrocarb on diradical, optionally substituted with one or more hydroxy, halogen, nitro, amino, cyano, aminosulfonyl, alkylsulfonylamino, alkylcarbonyl, formyl, aminocarbonyl or alkylcarbonylamino; a heteroarylene or non-aromatic heterocyclic hydrocarbon diradical, all of which are optionally substituted with one or more straight, branched and / or cyclic non-aromatic hycrocarbon radical, hydroxyl, halogen, amino, nitro, cyano, aminosulfonyl, alkylsulfonylamino, alkylcarbonyl, formyl, aminocarbonyl or alkylcarbonylamino; Y1 and Y2 independently represent a bond, an ether diradical (R'-O-R''), an amine diradical (R'-N-R''), O, S, S(O), S(O)2, C(O) , NH-CO, CO-NH, SO2-N(R'), methylene or N(R')-SO2 wherein R' et R'' independently represent straight or branched hydrocarbon diradicals containi ng up to 4 carbon atoms; Y3 represents O, O-C(O), C(O)-O, N(R8), R8 being hydrogen or C1-4alkyl; R1 represents hydrogen or straight, branched and / or cyclic alkyl, optionally substituted with phenyl; or an aromatic hydrocarbon radical; R2 represents aryl, heteroaryl or a non-aromatic heterocyclic hydrocarbon radical, all of which are optionally substituted; tetrahydropyranyloxy, di-(C1-4 alkoxy)phosphinoyloxy or C1-4 alkoxycarbonylamino; R3 represents hydrogen; a straight, branched and / or cyclic hydrocarbon radical, optionally substituted with one or more amino, hydroxy, carboxy, halogen, nitro, cyano, alcoxy, aminocarbonyl, C1- 4alkoxycarbonyl, C1-4alkoxycarbonylamino, sulfo, hydroxysulfonyloxy, dihydroxyphosphinoyloxy, phosphono, sulfamino, aminosulfonyl, aminoacylamino or dialkoxyphosphinoyl; heteroaryl or a non-aromatic heterocyclic hydrocarbo n radical, all of which are optionally substituted by one or more group.
Owner:LEO PHARMA AS
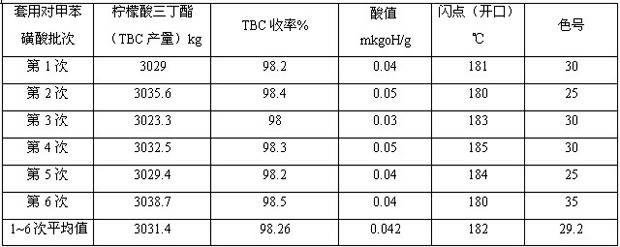
Synthesis of citric acid ether ester plasticizer
InactiveCN105085980AOrganic compound preparationCarboxylic acid esters preparationTosylic acidTwo step
The present invention discloses a method for synthesis of a new green environmentally friendly citric acid ether ester plasticizer from citric acid, a monobasic acid, and a dibasic alcohol as main raw materials by two-step esterification reaction, neutralizing, water washing, decolorizing, and vacuum distillation purification, wherein p-toluenesulfonic acid is used as a catalyst, toluene is used as a water-carrying agent, the raw materials are rich in sources, the reaction process is simple, safe and efficient, the relative molecular mass of the new citric acid ether ester is 500-2000g / mol, by increasing of the number of ester groups and the introduction of an ether bond, and the new citric acid ether ester exhibits better performance in anti-migration, cold resistance and flexibility, and can become a green environmentally friendly plasticizer with better performance.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Synthesis method of p-toluene sulfonic acid pleuromutilin ester
InactiveCN103450057AGood for healthLow priceSulfonic acid esters preparationTosylic acidDistillation
The invention relates to a synthesis method of p-toluene sulfonic acid pleuromutilin ester. Especially, the method includes: under catalysis of three inorganic alkalis, i.e. sodium hydroxide, potassium hydroxide or sodium carbonate, reacting pleuromutilin with p-toluenesulfonyl chloride for 0.5-1.5h in a methyl isobutyl ketone solvent at 50-65DEG C, conducting washing and distillation of the solvent, thus obtaining the p-toluene sulfonic acid pleuromutilin ester with yield of 95-96.5% and chromatographic purity of 97-98.1%. The method has the advantages of environmental-friendliness, worker health promotion, low production cost, short synthesis reaction time and high yield.
Owner:大英九合药业有限公司
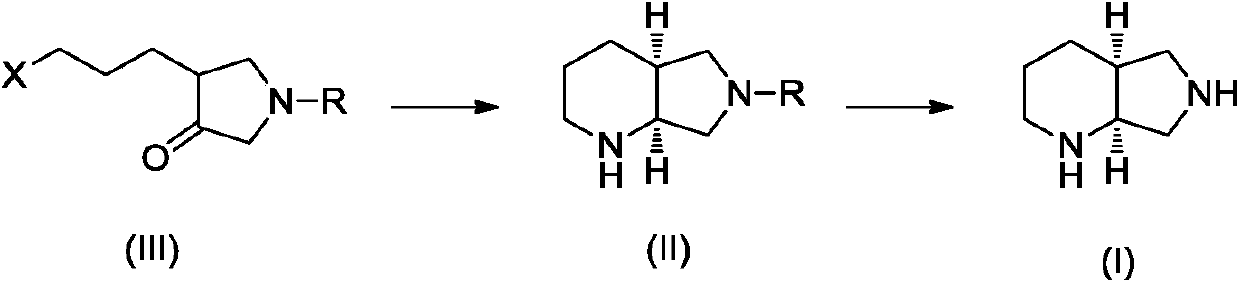
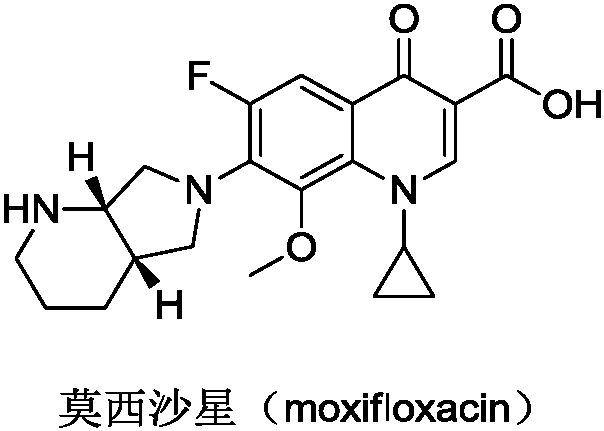
Preparation method of moxifloxacin intermediate compound
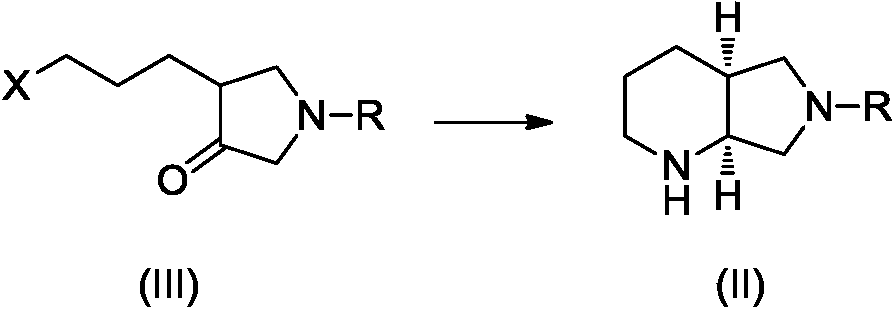
The invention discloses a preparation method of a moxifloxacin intermediate compound. The preparation method comprises the following step of under the actions of omega-transaminase and / or immobilizingtype thereof and ammonia donor, performing the following ammonia conversion reaction on a compound shown in a formula (III) in a solvent, so as to prepare a compound shown in a formula (II), whereinan amino acid sequence of the omega-transaminase is shown in SEQ ID NO.1, SEQ ID NO.2 or SEQ ID NO.3 in a sequence table; X is chloride, bromine, iodine, methanesulfonate or tosylate; R is C1-4 carbalkoxy, carbobenzoxy or benzyl. The preparation method has the advantage that the cost is low, the fewer steps are required, the operation is simple, the ee value of a product reaches 99% or above, andthe preparation method is more suitable for industrialization production. (The formulas are shown in the attached figures.).
Owner:SHANGHAI PUYI CHEM CO LTD
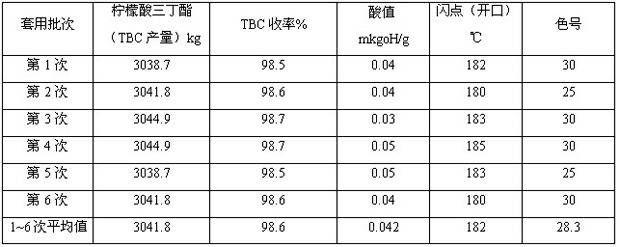
Method for catalytic synthesis of tributyl citrate by utilizing immobilized p-toluenesulfonic acid
ActiveCN102531899ANot obviously corrosiveEasy to recycleOrganic compound preparationCarboxylic acid esters preparationTosylic acidToluene
The invention provides a method for catalytic synthesis of tributyl citrate by utilizing immobilized p-toluenesulfonic acid, comprising the steps of taking the immobilized p-toluenesulfonic acid as a catalyst, carrying out esterification reaction on citric acid and n-butyl alcohol and synthetizing the tributyl citrate. The method provided by the invention has the advantages that: the immobilized p-toluenesulfonic acid adopted as the catalyst has no obvious corrosivity and is easy to recover and repeatedly utilize, the activity of the recovered catalyst basically remains unchanged; the esterification reaction efficiency is increased and the product yield can be up to 97.2-99.6%; adopting the reactant n-butyl alcohol as a water-carrying agent not only avoids the use of toxic solvents such as toluene and the like but also simplifies the aftertreatment process; the esterification reaction is mild in condition and simple in process, and the method is suitable for industrial production.
Owner:BENGBU BBCA MEDICINE SCI DEV
Method for preparing p-toluenesulfonic acid through toluene sulfonation
InactiveCN109232327AImprove conversion rateImprove efficiencySulfonic acid preparationTosylic acidAcetic acid
The invention discloses a method for preparing p-toluenesulfonic acid through toluene sulfonation. The method comprises the following steps of using methylbenzene as raw materials; using a multitubular membrane sulfonation reactor; introducing mixed gas of SO3 / nitrogen gas into the reactor for sulfonation reaction; obtaining the p-toluenesulfonic acid, wherein positioning agents are mixed in the methylbenzene, and are mixtures of N-fluorobenzenesulfonimide, acetic acid and xylene sulfone. The preparation method provided by the invention has the advantages that the positioning agents are added;the conversion rate from methylbenzene to p-toluenesulfonic acid is effectively improved; meanwhile, unreacted methylbenzene can be reused; the resource use rate is improved.
Owner:浙江工业大学上虞研究院有限公司
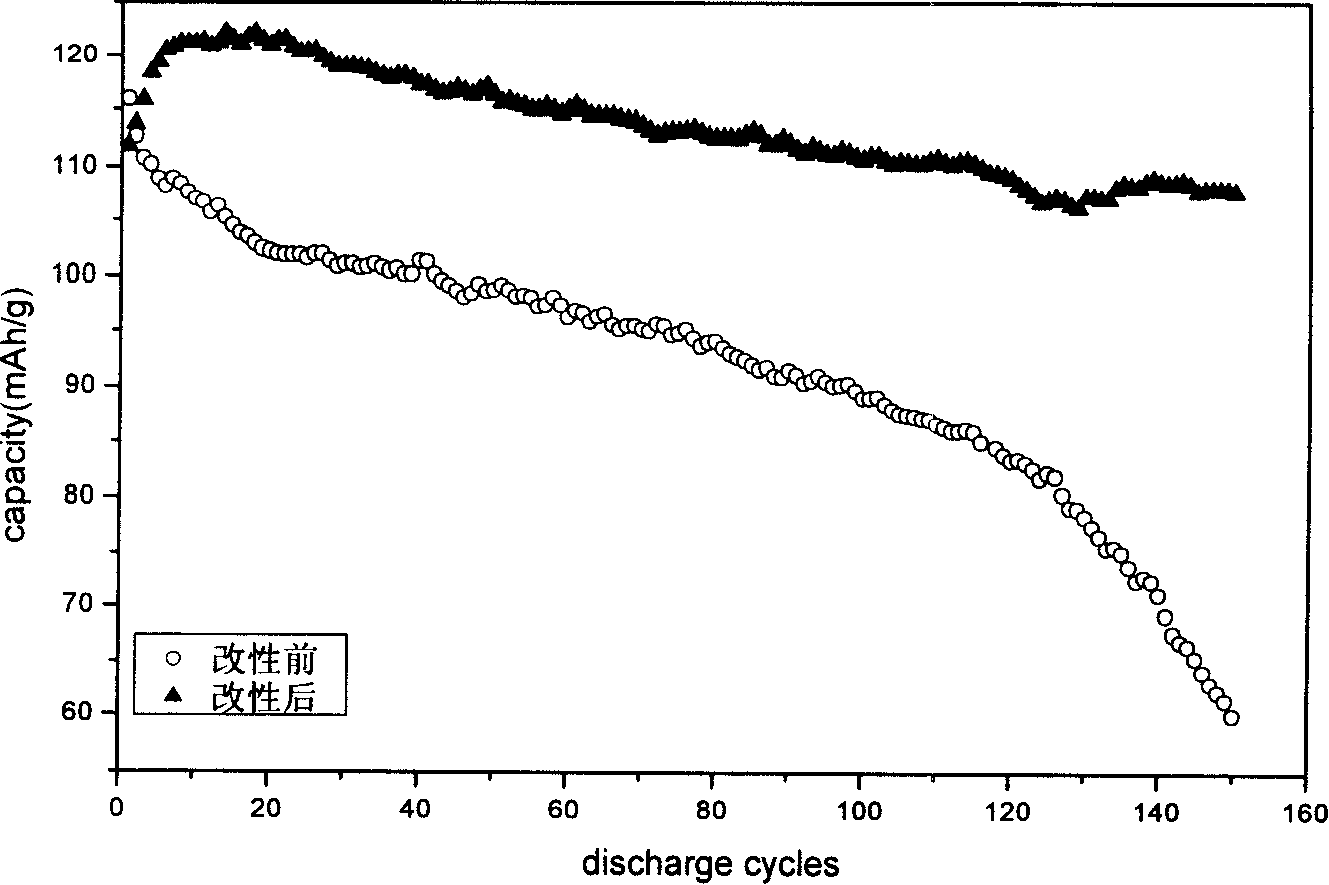
Method for modifying lithium ion battery cathode
InactiveCN1700490AInhibition of capacity fadingImprove cycle performanceElectrode manufacturing processesSecondary cellsTosylic acidPolypyrrole
This invention relates to lithium ion battery negative modifying method, which comprises the following steps: solving sodium sulfo benzoate, tosylic acid and pyrrole into water to process mixture; then mixing the lithium battery negative active materials, conductive carbon and adherent agent to process mixture and adding 1-cymene and 2-pyrrolidone to process plasm; finally coating plasm on the flow aluminum foil and after drying to process the lithium ion negative electrode to dip into mixture liquid and washing out of the impurity in ion water with flow density of 0.1-1.0mA / cm2 for 1-300 seconds polymerizing under temperature of 60-300deg C for heating for 1-48 hours.
Owner:XI AN JIAOTONG UNIV
Preparation process of fatty acid isooctyl ester
ActiveCN104232323AImprove conversion rateHigh yieldFatty acid esterificationTosylic acidIsooctyl alcohol
The invention relates to a preparation process of fatty acid isooctyl ester. The preparation process comprises the step of carrying out a reaction on fatty acid and isooctanol in the presence of a catalyst, wherein the catalyst is selected from acid sulfate, supported acid sulfate and supported p-toluenesulfonic acid as well as combination thereof. The process provided by the invention at least has one of the advantages of being high in reaction conversion rate, low in use level of isooctanol and catalyst, mild in reaction condition, free from or few in side reactions, simple in post-treatment process and environmentally friendly.
Owner:丰益油脂科技(连云港)有限公司
Method for determining content and associated substances of sorafenib tosylate in high-performance liquid phase chromatography
InactiveCN105181844AEffective controlStrict and effective controlComponent separationTosylic acidFluoroacetic acid
The invention discloses a method for determining content and associated substances of sorafenib tosylate. The method comprises the following steps: taking a reverse high-performance liquid phase chromatography, preparing a test solution and a reference solution, respectively measuring 10 micro liters of the test solution and 10 micro liters of reference solution, injecting the solution into a chromatograph, recording a chromatogram, calculating at a peak area according to an external standard method, and drying to complete the test on the content of the sorafenib tosylate and other substances. The method has the advantages that the blank that no method for testing and analyzing the content and associated substances of the sorafenib tosylate is provided at present is filled, the sorafenib tosylate and impurities can be separated by virtue of an acetonitrile-trifluoroacetic acid aqueous solution, the research development and production requirement can be met, and the associated substances in a sorafenib tosylate active ingredient can be more strictly and effectively controlled.
Owner:JIANGSU SINOBIOPHARMA
Synthetic method of active bagasse xylan o-toluic acid esterification-g-AM
InactiveCN109400757AGood biocompatibilityImprove biological activityAntibacterial agentsAntineoplastic agentsSolubilityCross-link
The invention discloses a synthetic method of active bagasse xylan o-toluic acid esterification-g-AM. The synthetic method adopts natural macromolecular biological active bagasse xylan as a main raw material, adopts ammonium persulfate and sodium hydrogen sulfite as an initiation system, adopts N,N'-methylene bisacrylamide as a cross-linking agent to prepare bagasse xylan-g-AM; and then the synthetic method adopts the bagasse xylan-g-AM as a raw material, adopts o-toluic acid as an esterification agent, and adopts toluenesulfonic acid as a catalyst to prepare the bagasse xylan o-toluic acid esterification-g-AM in the N,N-dimethylacetamide (DMA) solvent. A product prepared in the synthetic method of the invention performs the esterification reaction on the basis of grafting, and a final synthesized product of bagasse xylan o-toluic acid esterification-g-AM not only solves the problem that the bagasse xylan is poor in solubility and enlarges the application range, but also further improves the biological activity of the bagasse xylan by introducing the active groups of acrylamide (AM) and o-toluic acid.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Hydrogen sulfide fluorescent probe as well as preparation method and application thereof
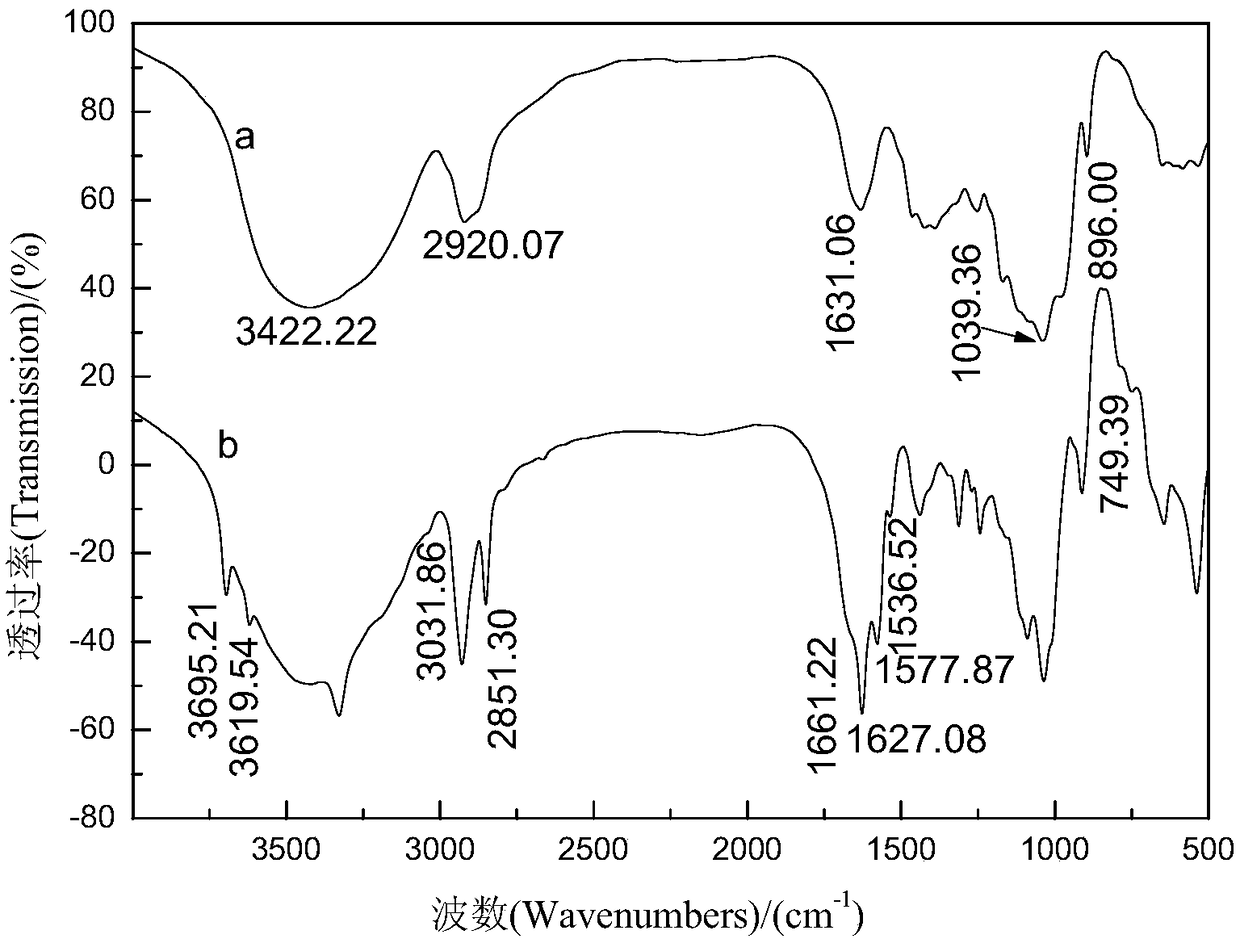
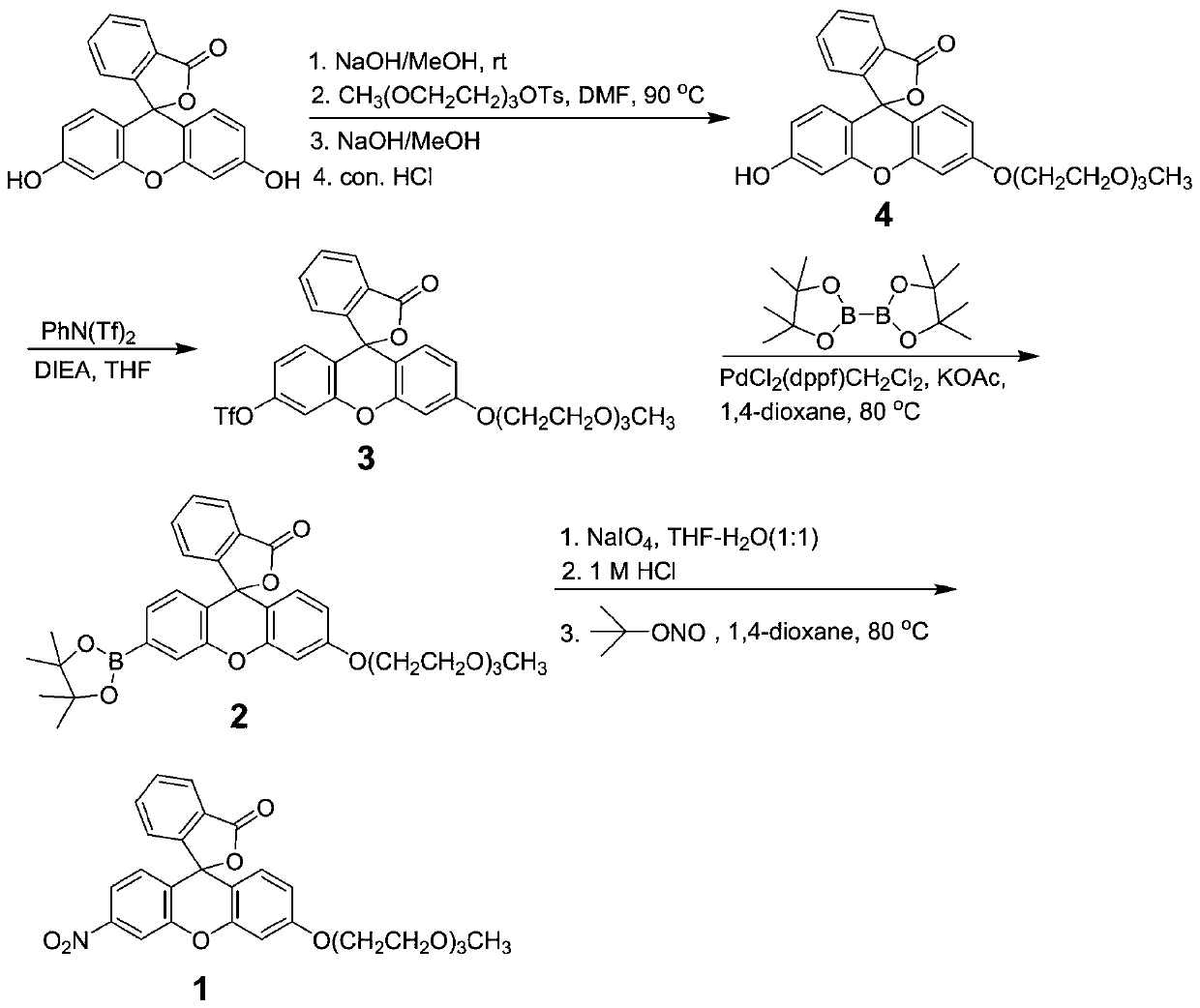
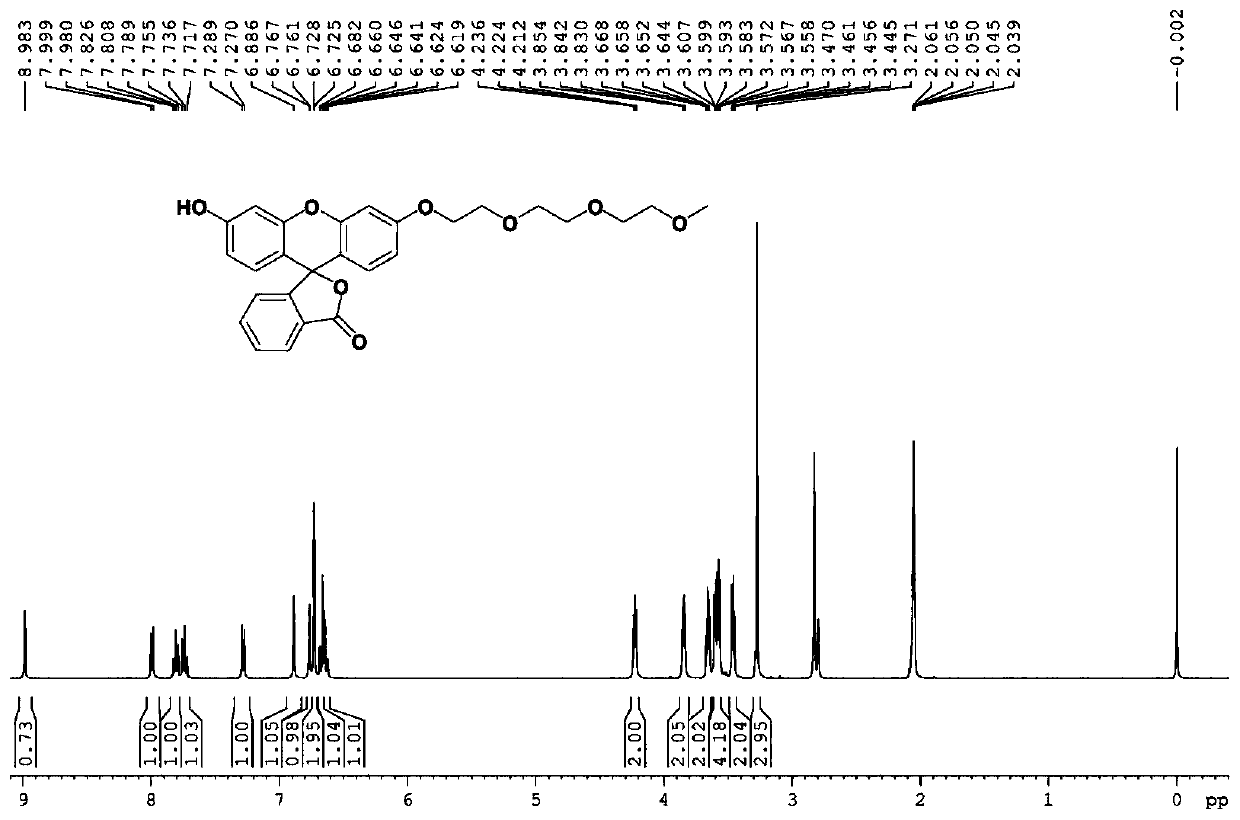
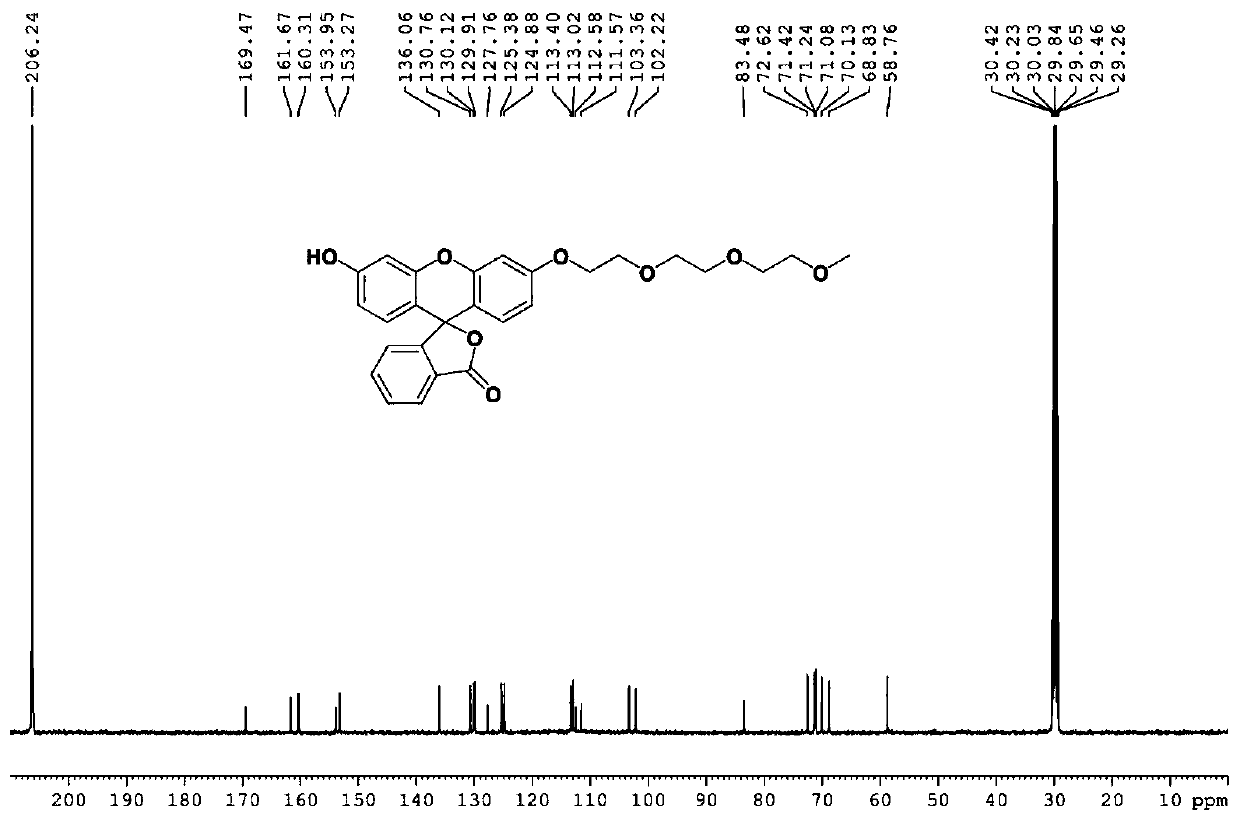
InactiveCN110734450AHigh selectivityOrganic chemistryFluorescence/phosphorescenceFluoProbesTosylic acid
The invention relates to a hydrogen sulfide fluorescent probe as well as preparation and application thereof. The molecular formula of the fluorescent probe is C27H25NO9; the preparation method of thefluorescent probe comprises the following steps: reacting a fluorescein with sodium hydroxide; reacting an obtained product with 2-(2-(2-methoxyethoxy)ethoxy)ethyl 4-methylbenzenesulfonate to obtaina compound 4, reacting the compound 4 with N-Phenylbis(trifluoromethanesulphonimide) to obtain a compound 3, reacting the compound 3 with bis (pinacolato) diboron to obtain a compound 2, hydrolyzing the compound 2, and reacting an obtained product with tert-butyl nitrite to obtain the hydrogen sulfide fluorescent probe. The probe shows relatively high selectivity on detection of hydrogen sulfide in a pure water solution.
Owner:HEZHOU UNIV
Method for preparing fatty acid methyl ester by using mixed fatty acid
InactiveCN101186574AReduce pollutionAvoid pollutionOrganic compound preparationCarboxylic acid esters preparationMixed fatty acidTosylic acid
The invention relates to a method for using mixed fatty acid to prepare fatty acid methyl ester, which comprises that arranges and mixes uniformly mixed fatty acid and methanol in a reactor, adds tosylic acid into the reactor to react, removes water to obtain crude fatty acid methyl ester after the reaction, arranges the crude fatty acid methyl ester and methanol in the reactor, adds tosylic acid into the reactor, depressurizes and distills to remove methanol in the solution after the reaction to obtain refine fatty acid methyl ester. Compared with prior art, the invention has low cost, high product conversion rate, simple post-treatment, and little pollution, with significant economic and practical benefits.
Owner:SHAANXI UNIV OF SCI & TECH
Preparation process for plasticizer-tributyl citrate
InactiveCN102627561AHigh reaction conversion rateReduce unit consumptionOrganic compound preparationCarboxylic acid esters preparationTosylic acidOil phase
The invention belongs to the field of fine chemical synthesis, and particularly relates to a synthesis process for tributyl citrate. The synthesis process comprises the steps as follows: (1) mixing citric acid monohydrate with n-butyl alcohol, then adding p-toluenesulfonic acid and active carbon, and adding nitrogen for performing esterification reaction to obtain an esterified substance; (2) dealcoholizing the esterified substance for the first time until the flash point is not less than 162 DEG C; (3) dissolving and recovering the p-toluenesulfonic acid in the esterified substance after dealcoholization by water, and adding the separated water phase into the preparation step (1) of next batch for recycling; (4) adding an alkali solution into an oil phase for neutralizing, removing the water phase, and washing the oil phase to be neutral by water; (5) adding the active carbon into the esterified substance after washing, and dealcoholizing again until the flash point is not less than 180 DEG C so as to obtain raw ester; and (6) filtering the raw ester to obtain the tributyl citrate as the industrial product. According to the synthesis process, the esterification reaction conversion rate reaches up to above 99.0%, the yield of the tributyl citrate can reach above 98%, and the unit consumption and the cost are reduced; and the catalyst, namely the p-toluenesulfonic acid has a good catalytic effect, the recovery rate reaches above 75% after the esterification reaction is ended, the p-toluenesulfonic acid can be repeatedly used as the catalyst, the pollution discharge is reduced, and the COD (Chemical Oxygen Demand) and BOD (Biochemical Oxygen Demand) in wastewater are reduced.
Owner:东营金明工贸有限公司
Salicylic acid and chitin-2-6-bit graft and its production
Salicylic acid and chitose-2,6 site grafter and its production are disclosed. The process is carried out by putting salicylic acid and chitose into reactor in proportion of 10:1-1:2, adding to 5-50(W / V) time dispersant of hydrochloric ether, ether, benzene, homolog or ono-protonic polar solvent, swelling for chitose by methane sulfonic acid, benzene monosulfonic acid and toluene sulfonic acid, taking pyridine or organic amine as proton acceptor, reacting at 0-30degree for 0.5-3hrs, adding into acetone to deposit reactant, neutralizing by alkali, washing by water, washing by alcohol, filtering and centrifugal separating to obtain the final product. It has better performance and effect.
Owner:河南省科学院河南省发展计划委员会地理研究所
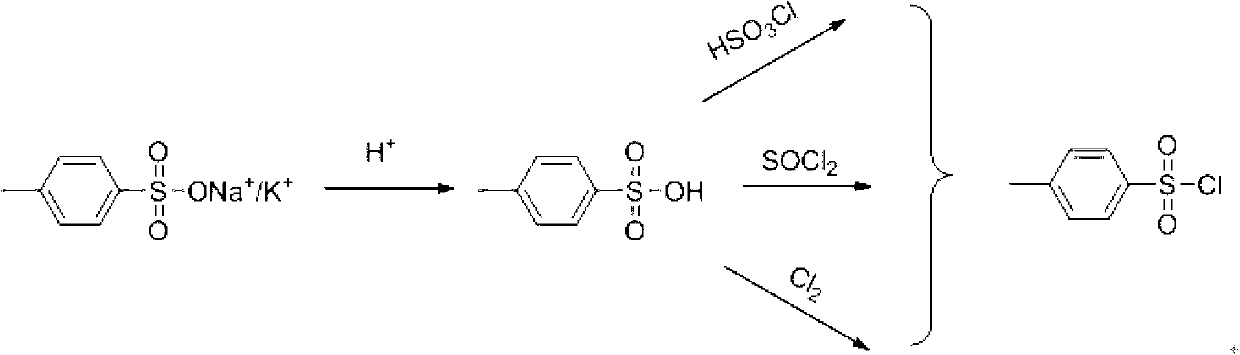
Method for recovering paratoluensulfonyl chloride from waste water generated by producing aryloxy phenoxy propionic acid herbicide
InactiveCN102746199AReduce COD contentIncrease incomeSulfonic acid preparationTosylic acidPropanoic acid
The invention discloses a method for recovering paratoluensulfonyl chloride from waste water generated by producing an aryloxy phenoxy propionic acid herbicide, which comprises the following steps of: adding acid to a solution containing tosylate to be acidized, performing decolorizing treatment, then removing water, dissolving obtained p-toluenesulfonic acid in a polar solvent, and adding an acyl chlorination reagent at a certain temperature to react to obtain the paratoluensulfonyl chloride. The purity of the paratoluensulfonyl chloride obtained by using the recovering method is more than 98%, and the recovery rate is more than 95%. The recovering method is successfully researched, so that the recycling of raw materials is realized, the production cost is reduced, and the COD (Chemical Oxygen Demand) content of the waste water is reduced. The method is suitable for large-scale industrial recycling.
Owner:JIANGSU FENGSHAN GROUP
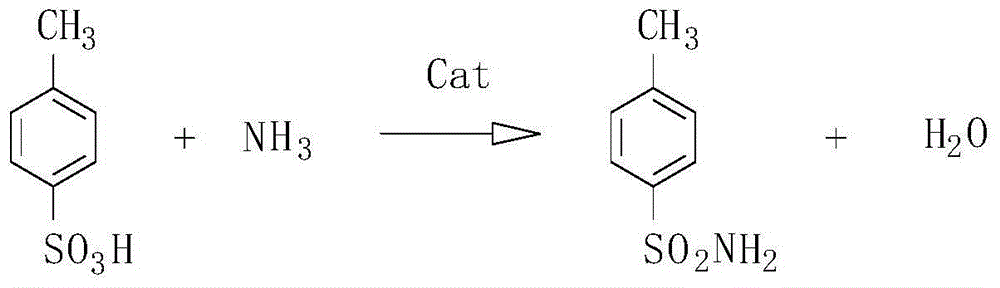
Method for preparing para toluene sulfonamide by directly amidating para-toluenesulfonic acid
ActiveCN104945288AHigh reactivityIncrease electropositivityOrganic compound preparationSulfonic acid amide preparationTosylic acidFiltration
A method for preparing para toluene sulfonamide by directly amidating para-toluenesulfonic acid comprises the following steps: (1) anhydrous para-toluenesulfonic acid is dissolved in dichloromethane, a catalyst, namely organic boronic acid, and a 5A molecular sieve are added in a mixed solution, and the mixed solution is uniformly stirred for a certain time under the condition that the temperature is controlled to be -10 to 0 DEG C; (2) an ammonia gas is introduced at the -10 to 0 DEG C for reaction; (3) after the reaction is finished, suction filtration is conducted on the reaction liquid to remove the molecular sieve, and then washing is respectively performed once with an acid solution, an alkali solution and a salt solution; (4) an organic phase is dried through anhydrous sodium sulfate, then a drying agent is removed, a dichloromethane solvent is subjected to distillation recovery, and crude para toluene sulfonamide is obtained; (5) weighing is performed after washing with distilled water and drying, and the product purity is analyzed through liquid chromatography. The complexation is generated between organic boric acid energy and oxygen on a sulfonic acid molecule, the reaction activity of the para-toluenesulfonic acid is improved, ammonia molecules are easily combined with sulfur to generate amide, the whole reaction energy consumption is low, no waste acid is discharged, and the yield of para toluene sulfonamide is about 40%.
Owner:浙江嘉福新材料科技有限公司
Environment-friendly production method for preparing paratoluenesulfonic acid sodium salt from waste sulfuric acid
InactiveCN106748900ASimple process equipmentLow heating temperatureOrganic compound preparationSulfonic acids salts preparationTosylic acidSodium-p-toluenesulfinate
The invention discloses an environment-friendly production method for preparing paratoluenesulfonic acid sodium salt from waste sulfuric acid. The environment-friendly production method comprises the following steps: (1) concentrating the waste sulfuric acid until weight percentage concentration of the sulfuric acid is greater than 70%, and adding methylbenzene into the sulfuric acid in a weight ratio of (1.2 to 2.0) : 1, and heating to 100-160 DEG C to react, thereby obtaining p-toluenesulfonic acid; (2) enabling a solution containing the p-toluenesulfonic acid to perform neutral reaction with a sodium hydroxide aqueous solution, thereby obtaining paratoluenesulfonic acid sodium; and (3) discoloring the solution containing paratoluenesulfonic acid sodium through active carbon, performing crystal separation, and drying to obtain a paratoluenesulfonic acid sodium product. The environment-friendly production method is used to solve the problem that the waste sulfuric acid is difficult to treat and provide a new idea for a paratoluenesulfonic acid sodium preparation process, and has great innovativeness and common applicability for a preparation process for the paratoluenesulfonic acid sodium salt.
Owner:四川省中明环境治理有限公司
Isostearic acid preparation method
InactiveCN103881821AHigh yieldHigh purityFatty acid hydrogenationFatty acid esterificationTosylic acidDistillation
The invention discloses an isostearic acid preparation method. The method comprises the following steps: 1, using vegetable oil acid to produce dimeric acid and obtain a byproduct monomer acid; 2, carrying out normal pressure esterification on the monomer acid and lower alcohol at 95DEG C with paratoluenesulfonic acid and zinc oxide as a catalyst to generate monomer acid ester; 3, carrying out catalytic hydrogenation on the monomer acid ester to obtain fatty acid ester with a low iodine number; 4, saponifying the fatty acid ester with a low iodine number, and acidifying to obtain a fatty acid mixture with a low iodine number; and 5, dissolving the fatty acid mixture with a low iodine number in solvent oil, cooling for crystallization, filtering, carrying out reduced pressure distillation of the obtained filtrate, and collecting an isostearic acid fraction to obtain a finished product. The method has the advantages of simple process, realization of less equipment investment and safe operation by selecting a proper catalyst and adopting normal pressure esterification, and realization of the high yield and the high purity of the finished product after improving the process steps.
Owner:QINGDAO SHUAIWANG OIL CHEM
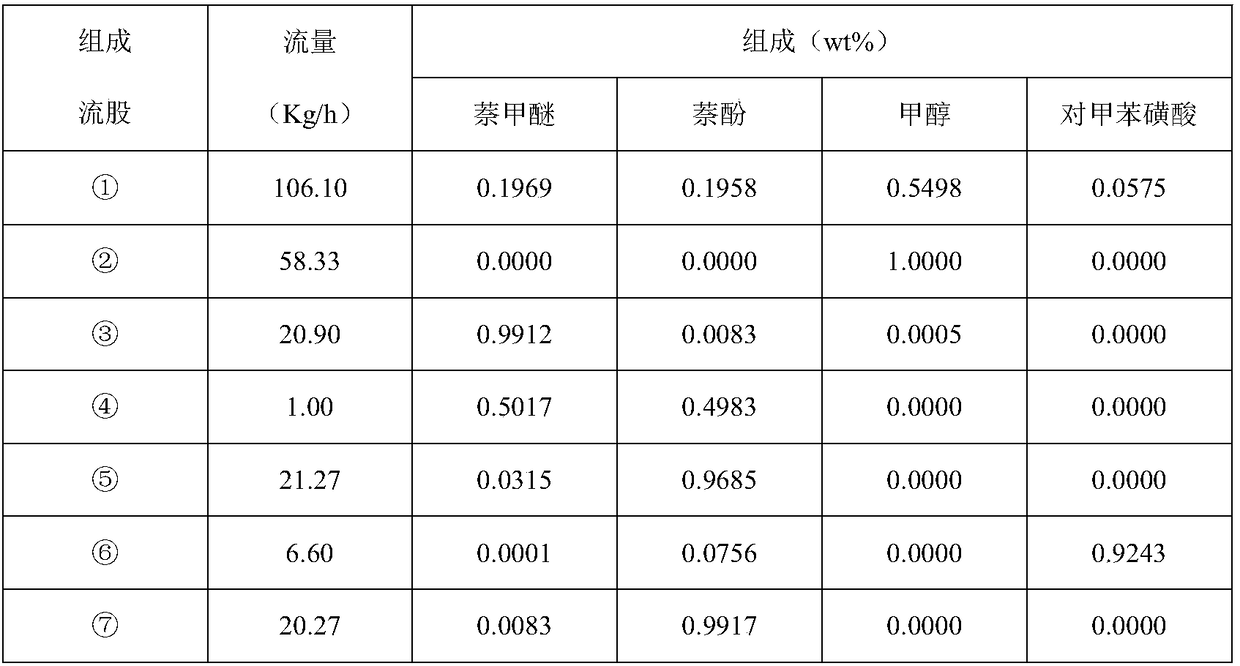
Device and method for extracting 2-naphthol methyl ether from 2-naphthol, methyl alcohol, 2-naphthol methyl ether and p-toluenesulfonic acid and recovering components
ActiveCN108569957AHigh purityNothing producedEther separation/purificationOrganic compound preparationTosylic acidAlcohol
The invention discloses a device and a method for extracting 2-naphthol methyl ether from 2-naphthol, methyl alcohol, 2-naphthol methyl ether and p-toluenesulfonic acid and recovering components. Thedevice comprises a double-sidestream rectifying tower and a stripping tower connected therewith. The preparation method thereof comprises the following steps: adding a 2-naphthol, methyl alcohol, 2-naphthol methyl ether and p-toluenesulfonic acid mixture; after performing purification in the double-sidestream rectifying tower, obtaining p-toluenesulfonic acid at a tower bottom, obtaining methyl alcohol at a tower top, and obtaining 2-naphthol methyl ether through a 2-naphthol methyl ether sidestream discharging collector; after delivering 2-naphthol distillate to the stripping tower for refining through a 2-naphthol sidestream discharging collector, obtaining 2-naphthol at a tower bottom, and circulating a product obtained at a tower top to the double-sidestream rectifying tower. The device and the method have the significant advantages that when the device is adopted to extract 2-naphthol methyl ether from the mixture, the purity and the yield are high, and no by-products is producedin the whole reaction process.
Owner:NANJING NORMAL UNIVERSITY
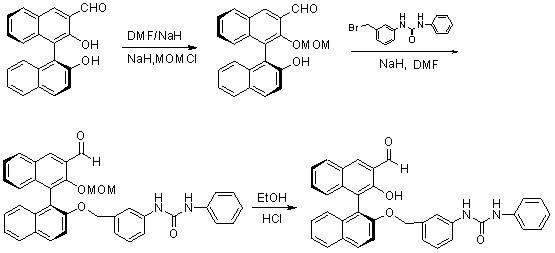
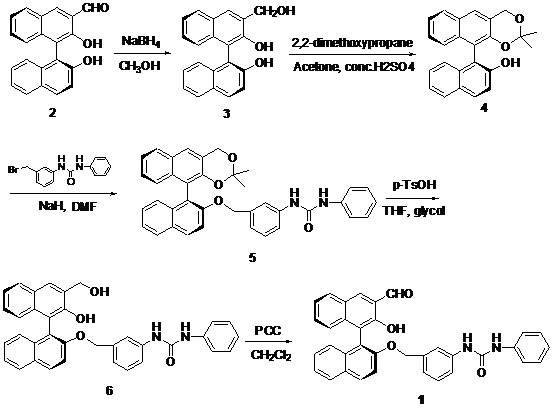
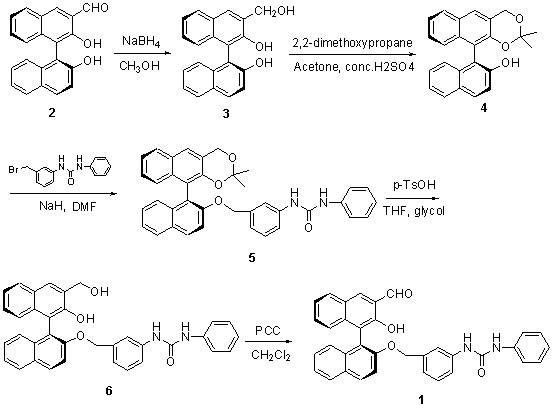
Method for synthesizing (S)-2-hydroxy-2'-(3-phenylureaphenyl)-1,1'-binaphthyl-3-formaldehyde
InactiveCN102070492ARich sourcesEasy to getUrea derivatives preparationOrganic compound preparationTosylic acidPtru catalyst
The invention discloses a method for synthesizing (S)-2-hydroxy-2'-(3-phenylureaphenyl)-1,1'-binaphthyl-3-formaldehyde, which uses widely available raw materials, makes operation easy, has high yield and produces little pollution. The method comprises the following process steps: dissolving 3-formoxyl-1,1'-binaphthol serving as a raw material in methanol, cooling in an ice bath, adding sodium borohydride, and reducing to obtain 3-hydroxymethyl-1,1'-binaphthol; dissolving 3-hydroxymethyl-1,1'-binaphthol in acetone serving as a solvent, adding 2,2'-dimethoxypropane to perform a reaction in the presence of a catalyst, extracting with an organic solvent, and obtaining (S)-2-hydroxy-1-(2,2-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)-naphthalene by column chromatograpic separation; dissolving (S)-2-hydroxy-1-(2,2-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)-naphthalene in dry dimethyl formamide (DMF) serving as a solvent, adding sodium hydride under an ice bath condition, reacting for 0.5 and 1 hours, adding 3-(4-methoxyphenyl)-ureido-benzyl bromide, reacting and obtaining (S)-2-(3-phenylureidobenzoxy)-1-(2,2-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)- naphthalene by column chromatograpic separation; dissolving the (S)-2-(3-phenylureidobenzoxy)-1-(2,2-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)- naphthalene in glycol serving as a solvent, heating to remove a protective group under the action of p-toluenesulfonic acid and reacting to obtain (S)-2-hydroxy-3-hydroxymethyl-2'-(3-tetrahydroanthracene)-1,1'-binaphthyl; and finally, oxidizing the (S)-2-hydroxy-3-hydroxymethyl-2'-(3-tetrahydroanthracene)-1,1'-binaphthyl in dry dichloromethane by pyridinium chlorochromate, and after reaction, filtering the reaction product by kieselguhr, concentrating and performing column chromatograpic separation to obtain the target product.
Owner:BOHAI UNIV
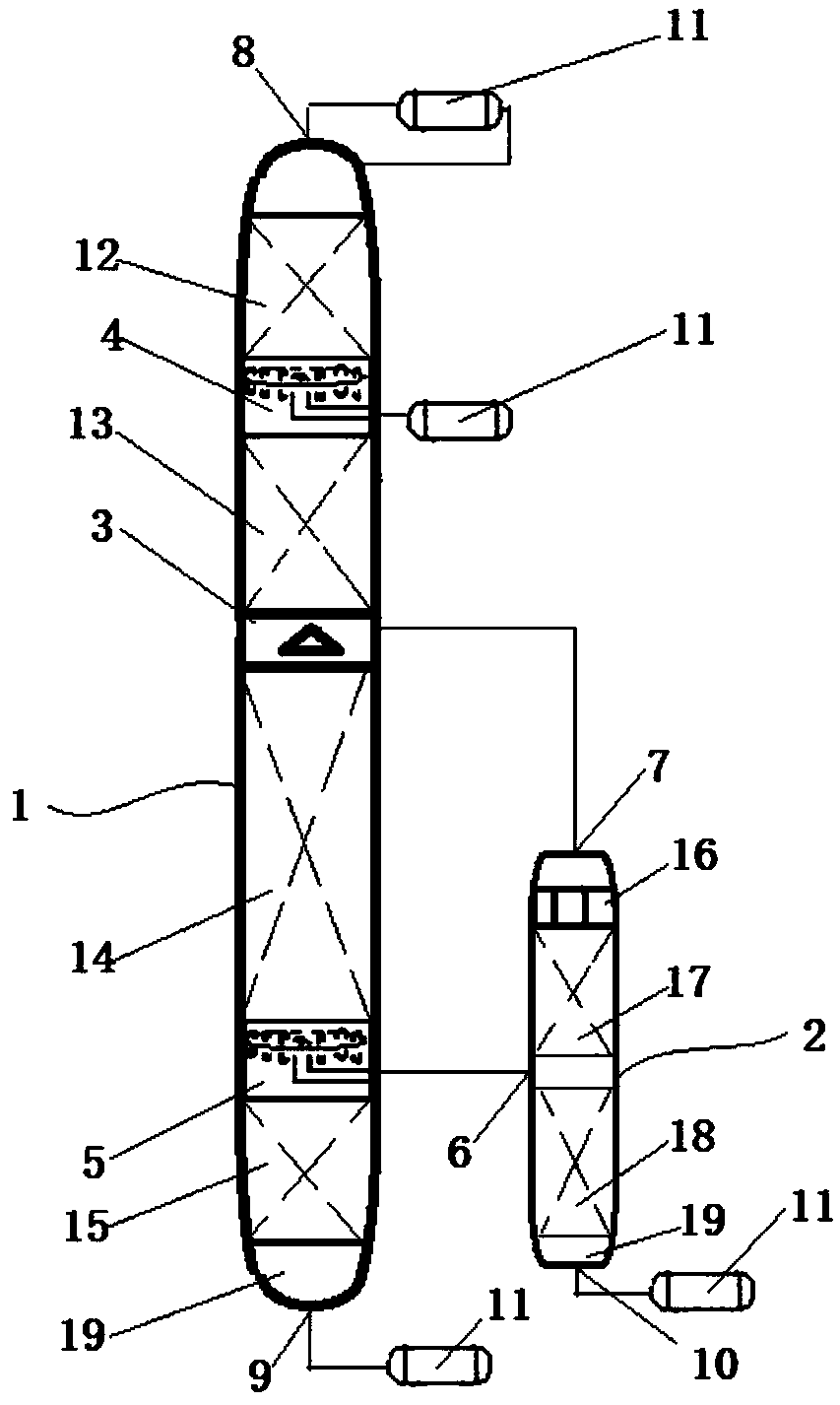
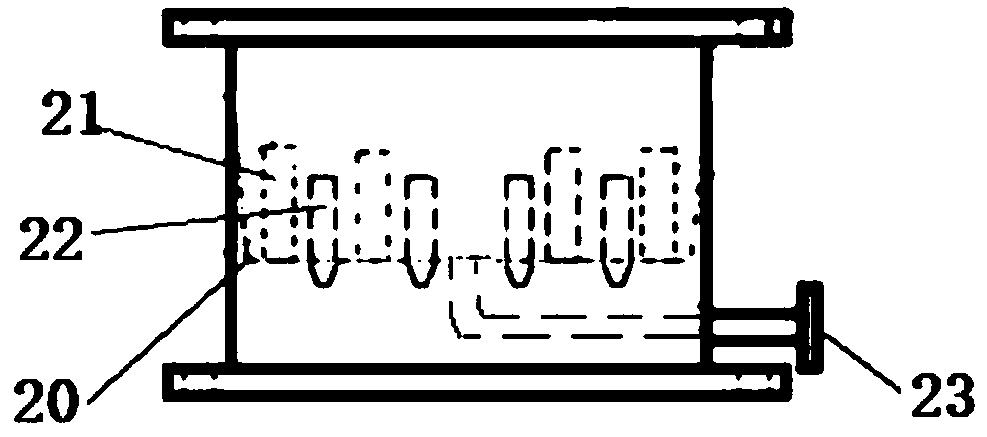
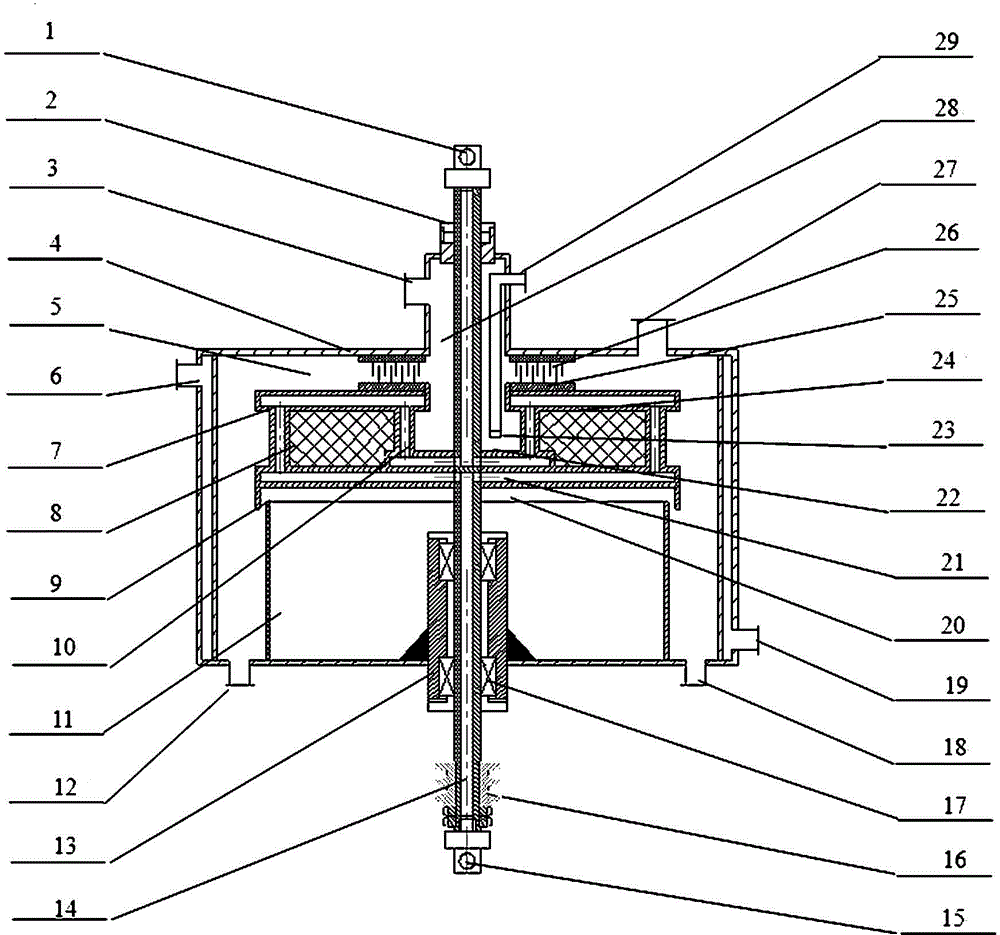
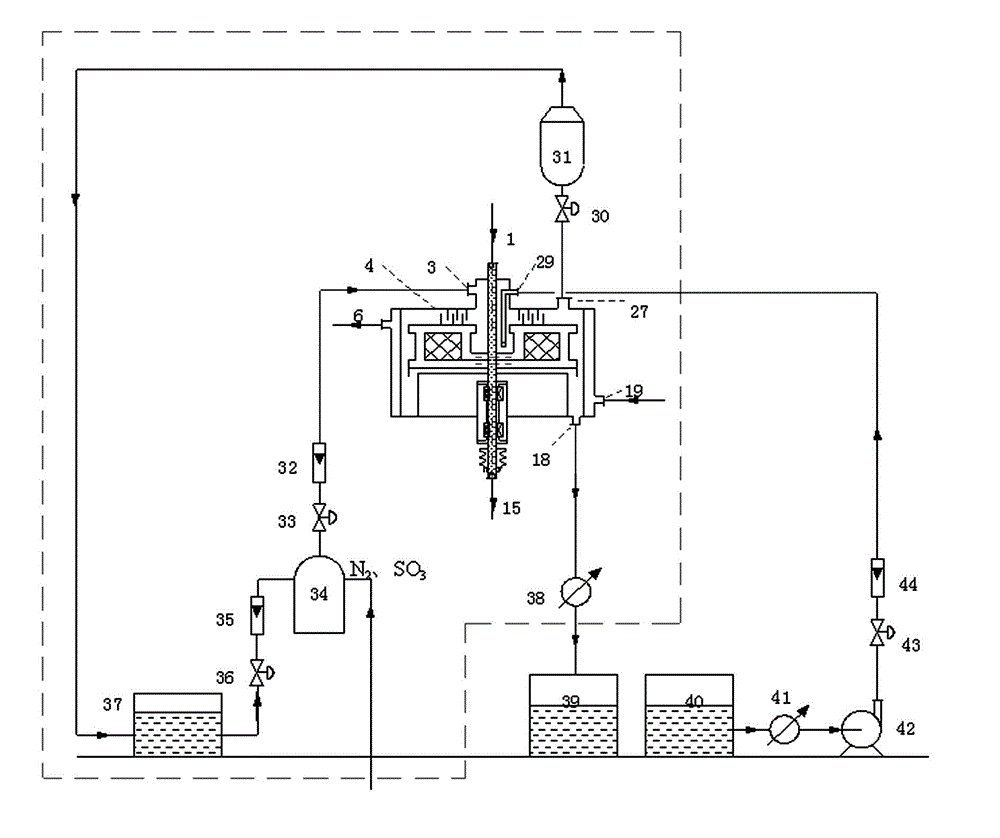
Sulfonation equipment and method for preparing p-toluenesulfonic acid by using gaseous sulfur trioxide sulfonated toluene
ActiveCN104437336AQuick contact mixIncrease contact areaProcess control/regulationSulfonic acid preparationTosylic acidGas phase
The invention belongs to the technical field of production of p-toluenesulfonic acid, and provides sulfonation equipment and a method for preparing p-toluenesulfonic acid by using gaseous sulfur trioxide sulfonated toluene for solving the problems of methods for preparing the p-toluenesulfonic acid by using the gaseous sulfur trioxide as a sulfonating agent for sulfonating toluene in the prior art. The sulfonation reaction is completed at a time by the efficient mass transfer characteristics of a rotary packed bed, a liquid phase product is not circulated to inhibit side reaction, and the rotary packed bed is characterized in that a heat transfer structure is arranged at a packing rotor of the rotary packed bed to quickly remove heat generated in the reaction and ensure that the reaction is carried out within a regulated temperature range, so that the side reaction is inhibited, the purity of the p-toluenesulfonic acid is improved, the yield of the p-toluenesulfonic acid is increased and the purposes of improving the production efficiency, reducing the energy consumption and reducing the environmental pollution are achieved.
Owner:ZHONGBEI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)