Method for preparing p-toluenesulfonic acid by toluene sulfonation
A p-toluenesulfonic acid and toluene technology, applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems of many by-products, difficulty in ensuring purity, excessive acid residue, etc., and achieve short process, convenient operation, and high content of finished products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Process for preparing p-toluenesulfonic acid according to the present invention
[0020]
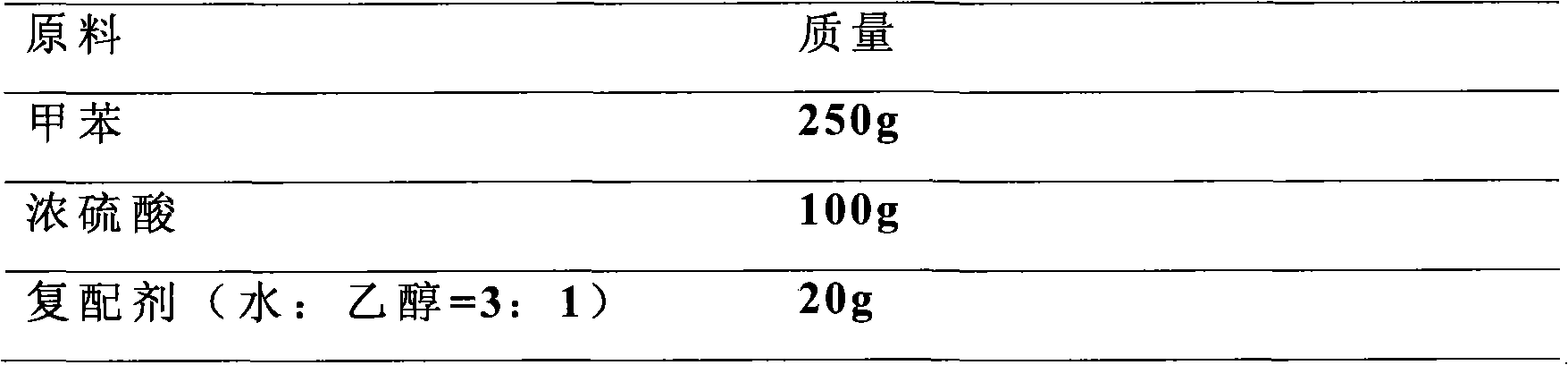
[0021] Add 250g of toluene to a four-necked flask with stirring, reflux, water separator and thermometer, heat up to increase the temperature, add 100g of concentrated sulfuric acid (98%) dropwise under the toluene reflux condition, add dropwise evenly within 30min, continue to reflux and heat, The water generated by the reaction and the water in the concentrated sulfuric acid are taken out with toluene vapor, the toluene is condensed and refluxed, and when water is no longer separated in the reflux liquid, the reaction is completed for 4 hours. Cool down and add 20 g of compounding agent (a mixture of water and alcohol) at a temperature of 50-70 °C to precipitate a crystalline compound, then carry out vacuum filtration and vacuum drying at 70 °C to obtain the product.
[0022] It was determined that the content of the finished product was 98.7%, the purity of p-tolue...
Embodiment 2
[0023] Example 2 The process for preparing p-toluenesulfonic acid according to the present invention and the comparison with the existing process
[0024]
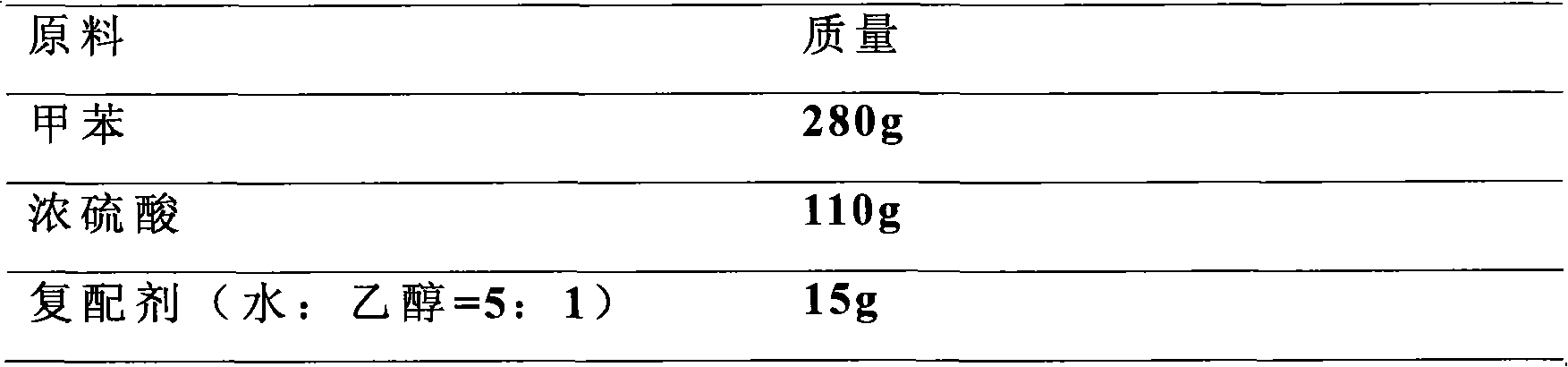
[0025] Add 280g of toluene to a four-necked flask with stirring, reflux, water separator, and thermometer, heat up the temperature, add 110g of concentrated sulfuric acid (98%) dropwise under the condition of toluene reflux, add dropwise evenly within 30min, continue to reflux and heat, The water generated by the reaction and the water in the concentrated sulfuric acid are taken out with toluene vapor, the toluene is condensed and refluxed, and when no water is separated from the reflux liquid, the reaction is continued for 1 hour to complete the reaction. Cool the temperature and add 15 g of compounding agent (a mixture of water and alcohol) at a temperature of 50-70 °C to separate out a crystalline compound, then carry out vacuum filtration and vacuum drying at 70 °C to obtain the product.
[0026] After the sulfonati...
Embodiment 3
[0030] Example 3 Process for preparing p-toluenesulfonic acid according to the present invention
[0031]
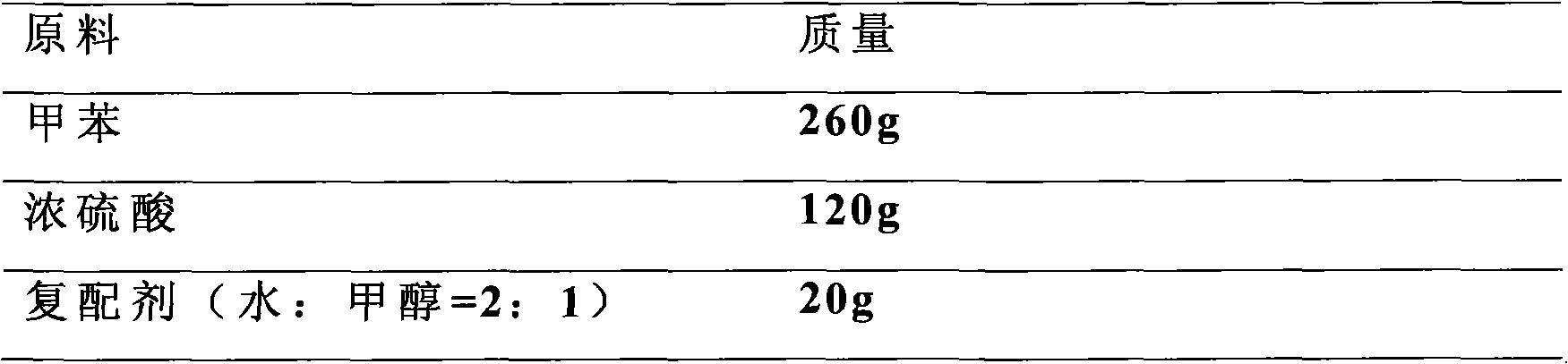
[0032] Add 260 g of toluene to a four-necked flask with stirring, reflux, water separator and thermometer, heat up to increase the temperature, add 120 g of concentrated sulfuric acid (98%) dropwise under the condition of toluene reflux, add dropwise evenly within 30 min, and continue to reflux for heating. The water generated by the reaction and the water in the concentrated sulfuric acid were taken out with toluene vapor, the toluene was condensed and refluxed, and when no water was separated from the reflux liquid, the reaction was completed for 5 hours. Cool down and add 20 g of compounding agent (a mixture of water and alcohol) at a temperature of 50-70 °C to precipitate a crystalline compound, then carry out vacuum filtration and vacuum drying at 70 °C to obtain the product.
[0033] It was determined that the content of the finished product was 98.9%, the purit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com